
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nephrol., 05 October 2022
Sec. Clinical Research in Nephrology
Volume 2 - 2022 | https://doi.org/10.3389/fneph.2022.1011964
This article is part of the Research TopicSocial Determinants of Kidney Health: A Global PerspectiveView all 7 articles
In many cases the social determinants of health need to be assessed through their interaction with environmental factors. This review looks at the impact of physical location and occupation of individuals on their kidney health. It examines the effect of living at high altitude on kidney function and the relationship between extreme cold or hot temperatures and the incidence of kidney injury. It reviews as well the many occupations that have been linked to kidney disease in high-income and low-and-middle-income countries. As a conclusion, this overview proposes preventive recommendations that could be individualized based on weather, altitude, socio-economic level of the country and occupation of the individual.
In 2005, the World Health Organization (WHO) launched the commission on Social Determinants of Health with the aim to address the impact of social hazards on health and health inequities (1). Progressively, the global nephrology community started to show interest in social determinants of kidney health which have been assembled under five categories: first the economic stability including employment, income and food, second education, third health and healthcare access, fourth social, community and context, the fifth category being neighborhood and built environment (2). In fact, social determinants of health and their impact on health and disease are better analyzed through their interaction with other domains that influence individual health. Therefore, a multisectoral approach has been suggested to treat the complexity of the fundamental social, environmental, political and economic causes of kidney health. This paper aims to review the impact of physical location and occupation of individuals on their kidney health.
The first section of this review analyzes the physical environment that may affect kidney health with a focus on altitude, weather and other atmospheric variables such as humidity. There is evidence that the incidence, prevalence, level of care and outcomes of chronic kidney disease (CKD) patients are variable according to the geographical location (3). This review will look at the kidney health at high altitude in the world regions that include the highest summits. On the other hand, it will examine the evidence beyond the association between cold temperatures, the increase in blood pressure levels and whether this could indirectly affect the kidney function. It aims also to overview climate change and global warming as causes for excessive heat, dehydration and acute kidney injury. Finally, it gives a brief about other atmospheric variables that may impact kidney health like air pollution and humidity and their role in the exacerbation of some kidney diseases such as lupus nephritis flares.
The second section reviews the evidence behind the impact of occupation on kidney health of workers. In this part, occupational kidney disease is analyzed based on five main socio-economic, temporal and/or geographical subsections. First, it summarizes the old occupational nephrotoxic hazards, heavy metals, infections and pesticides that were well-known before the first report of El Salvador in 2002. Second, it reviews the occupational kidney disease or CKD of unknown etiology (CKDu) in Sri Lanka among agricultural workers exposed to contaminated water. Third, it looks at the evidence of heat stress in the Mesoamerican nephropathy among sugarcane workers. Fourth it overviews the types of occupational kidney disease reported in low- and middle-income countries (LMICs) other than Sri Lanka and Central America. Finally, it reviews the different causes of occupational kidney disease in high-income countries.
A search for articles was performed in PubMed by combining the following terms under “Title/Abstract”: “Kidney OR Renal” AND “Altitude” NOT “ALTITUDE Trial”. The filter human was added and this search led to 271 abstracts that were reviewed and 40 articles were selected.
A search for articles was performed in PubMed by combining the following terms under “Title/Abstract”: “Kidney OR Renal” AND “Seasonal variation OR Extreme temperatures”. The filter “humans” was added. Seventeen out of 120 abstracts were selected and papers reviewed.
A search for articles was performed in PubMed by combining the following terms under “Title/Abstract”: “Kidney OR Renal” AND “Cold weather OR Cold temperature” NOT “Transplant OR Transplantation”. The filter “humans” was added. This search led to 19 abstracts and 11 papers were selected and reviewed.
A search for articles was performed in PubMed by combining the following terms under “Title/Abstract”: “Kidney OR Renal” AND “Heat OR hot temperature” NOT “Heat stroke” NOT “Heat shock protein OR Heat-shock protein”. 36 out of 198 articles were selected and reviewed.
A search for articles was performed in PubMed by combining the following terms under “Title/Abstract”: “Kidney OR Renal” AND “Occupation OR Occupational OR Job” NOT “Cancer” NOT “Stone OR Lithiasis”. Filters added to this search were “humans” and timeline “1990 until July 2022”. This search led to 1388 results. These studies’ abstracts were reviewed to extract all types of clinical studies that described or analyzed occupational kidney diseases. Studies that covered only the kidney toxicity of heavy metals including lead, cadmium, arsenic, mercury and uranium were not included in the final tables but the most recent and relevant were discussed in the text. Among the 87 studies reporting infectious diseases as an occupational risk factor for kidney disease, thirty studies about hantavirus, schistosomiasis, brucellosis, malaria or dengue were not added to the tables. A total of 57 studies on leptospirosis were included because it was the most frequently reported infectious disease associated with occupational renal failure. Finally, 968 abstracts were reviewed, reviews and systematic reviews were not included in the final tables but summarized in the text. A total of 150 articles about occupational risk factors for kidney health were finally selected and divided into those conducted in low and middle-income countries (100 studies) or in high-income countries (50 studies). Data extracted from these articles included the study design, number of participants, occupation, occupational exposure, health outcomes and kidney outcomes.
Living at high altitude has been defined as living at ≥2500 meters (m) above sea level. It is globally estimated that 81.6 million at ≥2500 m (4). The highest summit in the world is Mount Everest in Nepal located at 8848 m in the Himalayas’ mountains. Other summits exist in Asia, located in India and Indonesia. In Africa, the Eastern African mountains are located in Tanzania with Kilimanjaro being the highest summit of 5895 m, extending along Tanzania’s northern borders with Kenya. Also in Africa, the highest peak in Ethiopia is Ras Dejen (4533 m). In Europe, the Alps’ highest summit is Mont Blanc (4809 m) between France and Italy. Running along the western edge of South America, we find the Andean mountains with the highest summit in Chile at 6140 m. The Andes extend over seven countries: Argentina, Chile, Bolivia, Peru, Ecuador, Colombia and Venezuela. Ethiopia has the highest absolute number of inhabitants living at ≥1,500 m and ≥2,500 m, China has the highest at ≥3,500 m (4).
High altitude induces physiological responses in the human body and adaptation of several organs to hypoxia which make it an important public health issue.
A summary of the effects of altitude on kidney function is illustrated in Figure 1. One of the first experimental studies that analyzed the cause of albuminuria at high altitude was published in 1987 and it showed that albuminuria after acclimatization at 4846 m is due to increased glomerular capillary permeability (5). A systematic review by Palubiski et al. studied the kidney physiological response to high altitude and highlighted the integrated response between the kidney, the lungs and the cardiovascular system when adapting to hypoxia at high altitude (6). They differentiated between acute and chronic kidney response to high altitude and hypoxia. The acute response starts with hyperventilation, leading to respiratory alkalosis; this latter is compensated by the role of the kidneys in eliminating the excess of bicarbonate (6, 7). There is an increase in diuresis and hemoconcentration that return to baseline at 2100m. This seems to precede the increase in erythropoietin secretion. In chronic high-altitude states > 2100 m, the erythropoietin increases and this is the most solid evidence revealed by the literature; however, there are no studies at altitudes lower than 2100 m (6). When looking at the glomerular filtration rate (GFR), an acute exposure to altitude is associated with a decrease in GFR, which is secondary to the decrease in renal plasma flow which in turn is due to hemoconcentration and hyperviscosity. When the exposure to altitude is chronic, GFR returns to a normal level despite the persistence of a diminished renal plasma flow probably due to sympathetic nerve hyperactivity starting at day 6. This leads to an increase in post-glomerular resistance and an increase in the filtration fraction (6, 8–10). The details of the mechanism that maintain GFR at chronic high-altitude exposure despite the tissue hypoxia, the hyperviscosity, the erythropoietin secretion and the affected blood flow are still unclear. It is also uncertain if the increase in blood pressure is omnipresent at high altitude, if it is dependent on high erythropoietin levels or not and if hypertension affects the GFR. The increase in blood pressure seems to be related in the first days to an acute secretion in norepinephrine. Later, the effects of renin, aldosterone and vasopressin on top of norepinephrine contribute to explain the mechanism of hypertension. Finally intrarenal tissue hypoxia seems to be reversible after the individual returns to lower altitude (6).
The high-altitude renal syndrome is a term that includes all the renal manifestations after chronic exposure to high altitude. Historically, three summits in the world are among the eldest places where humans have lived at > 2400 m: the Ethiopian summit in the North African highlands, the Andes mountains in South America and the Tibet in the Himalayan mountains in Asia (11). The common kidney manifestations in the three regions following high altitude are polycythemia (increase in erythropoietin secretion), hyperuricemia, elevated blood pressure, decreased renal blood flow with preserved GFR, glomerulomegaly and albuminuria. Despite their genetic adaptation to high altitude, the residents of these three regions have different characteristics. Compared to Andeans, the Tibetans historically had the longest exposure to high altitude thus they less erythrocytosis and less chronic mountain sickness. The Andeans have low birth weight newborns because of the hypoxia but to a lesser degree than some Europeans living at high altitude for shorter historical periods of time. In addition, some parameters of adaptation are still underreported in the Ethiopians like the prevalence of the chronic mountain sickness or Monge’s disease. In fact, the chronic mountain sickness does not increase with increasing altitude but it is rather linked to shorter adaptation and also to heavy metal toxicity like cobalt and nickel that induce an increase in erythropoietin and are found in the mining communities in Chile (2800 m) (11).
The impact of high altitude on the kidney has been studied since the 70s with one of the first interventional trials done on 7 climbers in East African mountains (12). They studied the kidney response and acclimatization in climbers who developed transient proteinuria. During the last four decades, most of the clinical studies come from Tibet, Nepal, Peru, China and India (5, 12–40). They are summarized in Table 1 and Figure 2. Several case reports described as well the kidney injury after a rapid ascent to very high altitudes. One of the cases comes from China in a previously healthy man who was suddenly exposed to an altitude of 5200 m and developed AKI (16). Another case of a 30-year-old healthy man who climbed the Everest at 5300 m in 11 days was reported with hypertensive crisis and reversible acute kidney injury (18).
People living at high altitude might have chronic kidney disease (CKD) similarly to the general population. However, there is paucity of data about the effect and consequences of high altitude on the progression of chronic kidney disease and on patients on dialysis or kidney transplant recipients. Luks et al. discussed the possibility of kidney function deterioration secondary to systemic hypoxemia at high altitude and the risk of acute motion sickness that CKD patients might experience (41). Because CKD patients have insufficient production of erythropoietin, they develop less increase in hemoglobin secondary to hypoxia at high altitude, this would decrease their oxygen delivery to tissues and induce dyspnea (41). Metabolic acidosis of chronic kidney disease might protect patients against the acute mountain sickness and the alkalosis induced by high altitude (41). Hemodialysis and peritoneal dialysis patients might be at a higher risk of volume overload when exposed to high altitude (40, 42). Bravo-Jaimes et al. showed that the mortality of hemodialysis patients in Peru was not different whether they lived at low or high altitude (>2000 m) (37). Studies from Europe and Nepal showed that kidney transplant recipients have higher hemoglobin levels in these patients when exposed to high altitude especially when treated with tacrolimus. They also have a higher level of arteriolar hyalinosis on biopsies, a less immunological protective reaction to physical exercise but stability of immunosuppressant levels (17, 20, 28, 29).
People working at high altitude in the space like flight crews or frequent flying passengers are increasing in number. This exposure to high altitude can impact their health because of the physiological changes of the body secondary to the decrease in atmospheric pressure. Moreover, there is a decrease in the partial pressure of oxygen which might lead to acute hypobaric hypoxia (43). This state will cause a redistribution of the blood to the main organs, the heart and the brain, inducing tachycardia and a decrease of the kidney perfusion as well as peripheral cyanosis (43). This is different from another entity called decompression sickness which is secondary to the formation of nitrogen bubbles in tissues and blood when exposed to an acute decrease in environmental pressure (43). Nitrogen accumulation leads to tissue ischemia, dyspnea, chest pain and sometimes to cardiac arrest.
Luks et al. made some suggestions regarding medications’ intake in CKD patients when exposed to short journeys at high altitude. There is no evidence of the need to change any chronic medication in these patients (41). Because of the risk of fluid retention in the context of acute motion sickness, patients susceptible to fluid retention should monitor their weight and adapt their diuretics (41). Chronic antihypertensive medications that inhibit the renin-angiotensin system are preferred over dihydropyridine calcium channel blockers because of the highest risk of proteinuria at high altitude. In this group of patients, anemia of these patients at high altitude seems to be corrected with lower doses of erythropoietin compared to patients at sea level but they have higher risk of hypertension and thrombotic events (41). Finally, medications used for acute mountain sickness need to be adjusted according to the kidney function. The best way to avoid mountain sickness for chronic kidney disease patients remains slow ascent and descent.
Weather and climate both describe atmospheric conditions like temperature, air pressure, precipitation, wind and humidity. Weather refers to the short-term atmospheric conditions or specific daily state of the atmosphere. Climate is the average of weather conditions of a region, throughout the year, sometimes averaged over a series of years, 30 years or more. The climate is classified as tropical, dry, polar, temperate or continental. The climate change globally is leading to a composite of warmer temperatures even in cold climates. This doesn’t mean that snow and extreme cold temperatures will become mild, on the opposite higher temperatures might lead to more precipitations and storms. This is why it is very important to study extreme temperatures and their impact on health.
Seasonality is an important emerging factor that has been globally addressed. Several studies have assessed the effect of seasonality on the variation in blood pressure, kidney function, cardiovascular outcomes and/or laboratory biomarkers. Although cold exposure has not been extensively studied as heat exposure, several reports demonstrated the deleterious impact of very cold temperatures on health. A study from Belgium by Demoury et al. analyzed 307,859 natural deaths between 2010 and 2015 (44). They found out that, compared to the normal temperature of 23.1°C, being exposed to cold (-1.7°C 1st percentile and 2.3°C 5th percentile) or high (26.7°C 95th percentile and 31.3°C 99th percentile) temperature significantly increased the mortality risk by 1.32 and 1.21 respectively (44). In this study, despite adjusting to exposure to humidity and fine particulate matter (<2.5 micrometers in diameter, PM2.5), the results remained significant. Moreover, women were more vulnerable to heat than men and stronger effects of cold were reported in highly-educated municipalities (44). Not only in Europe, but also in Taiwan/Asia, an analysis of emergency room visits between 2000 and 2014 revealed an association between extreme low temperature and hypertensive events whereas very high temperature increased the risk of visits for kidney disease and ischemic heart disease (45). Higher levels of blood pressure have been associated with cold temperature. In 2003 in Nigeria, Isezuo showed that hypertension-related hospital admissions were significantly more prevalent during the cold season (46). When it comes to chronic kidney disease patients, only one study from China of 109 CKD patients highlighted the seasonal variation of blood pressure and the prevalence of higher blood pressure levels in winter (47). A review of the studies that reported deleterious impact of cold and heat temperatures on population’s cardiovascular health suggested that the damage induced by extreme temperatures might be associated with the activation of the sympathetic nervous system and renin-angiotensin system (48).
Concerning acute kidney injury, there is a need to study seasonal variation of this disease in both developed and developing countries (49). This seasonal variation of acute kidney injury was highlighted in one study from Taiwan (50), two large studies from South Korea (51, 52) and a small Japanese study of 102 patients that analyzed the variation of eGFR in hypertensive patients with CKD (53).
A study from Rome, Italy that analyzed AKI cases between 2010 and 2014 showed a well-defined seasonal pattern with a significant increase of AKI incidence in winter (54) but they demonstrated a significant association with higher humidity levels rather than cold temperature.
On top of heat, cold and humidity, a high atmospheric PM2.5 level could increase lung edema in CKD stage 5 non-dialysis based on a study including 317 patients from Taiwan (55).
Extreme temperature is also important to address in the vulnerable population of children (56) who are mostly susceptible to dehydration during heat and can also have higher morbidity during extreme cold weather.
The study of Obermeyer et al. from USA including 4.8 million individuals showed variation of urea, creatinine and urine specific gravity according to different daily temperatures (57). A recent small study from Ghana showed the seasonal variation of serum creatinine and urea with a significant increase during the hot season (58). Surprisingly, a study from Japan of 903 individuals showed higher levels of urine specific gravity in spring than in summer (59).
In 2016, a public health initiative “ The Peer Kidney Care Initiative” drew attention on the seasonality of cardiovascular outcomes among chronic kidney disease patients and called to address these differences (60). In a Mediterranean climate country, Croatia, authors assessed 135 anuric hemodialysis patients and showed a seasonal variation in most of laboratory parameters like urea, albumin, phosphate, cholesterol and glucose (61).
Among nephropathies related to systemic diseases, lupus nephritis was the most related to seasonality (62–65). A study of 41 systemic lupus erythematosus (SLE) patients from Southern France found strong positive correlations between extreme low or high temperatures and renal lupus flares (62). This French group showed significant increase in flares during the spring season whereas patients treated with antimalarials had more flares in the sunny season (62). A review from China highlighted the importance of geographic distribution of SLE and the effect of ultraviolet radiation, climate and altitude on the activity of the disease (63). They reviewed the role of inflammatory mediators, apoptosis and epigenetic factors that are triggered by ultraviolet radiation or cold or humidity and would lead to the inflammatory lesions of systemic lupus erythematosus and in some cases aggravation of lupus nephritis (63). A study of 129 patients in China found a U-shaped relation between the incidence of lupus nephritis flares and environmental temperatures with significant higher cases of membranous nephropathy in December and January (64). A study from the USA of kidney biopsies of 179 SLE patients between 1992 and 2002 showed a significantly higher prevalence of membranous lupus nephritis in winter and spring (65).
Renal exposure to cold was first experimented during the 60s and 70s in studies from Sweden (66, 67). Eight healthy volunteers exposed to cold showed a decrease in circulating plasma volume, an increase of cardiac output and an increase in the capillary hydrostatic pressure in the renal vessels leading to a reduction in tubular sodium reabsorption and a rise in natriuresis hence hypovolemia and high blood viscosity (67). A study from Israel including 21 healthy volunteers marching at an altitude of 1700 m and temperature of 0°C showed that regular water intake would prevent the dehydration in these conditions (68).
The cold-induced vasoconstriction is due to the activation of the peripheral alpha(2C)-adrenergic receptors (69). Despite the multitude of reports of AKI being more incident in the hot climate, two studies from UK and Japan demonstrate the opposite. The UK study showed a higher trend of AKI and AKI-related mortality during the winter compared to other seasons of the year (70). This trend was observed in community-acquired and in-hospital acute kidney injury incidents (70). Another study including 81,279 AKI patients from Japan showed a higher AKI and severity of AKI cases in January (71).
A Japanese study highlighted the more significant impact of cold on increasing noradrenaline, heart rate and eGFR in normotensive individuals compared to patients with mildly elevated blood pressure. And they suggested to follow the out-of-office blood pressure of these patients to further understand the seasonal change (72). On the opposite, a recent study from Poland evaluated the central blood pressure of 56 patients exposed to cold (10 minutes of -10°C) and found out that the central aortic pressure was higher in hypertensive patients with CKD than in normotensive patients (73). When it comes to hemodialysis patients and seasonal variation, two cohorts from Taiwan showed that hemodialysis patients may have an increase in fluid accumulation during the cold season if they had a lower fractional weight loss (74, 75). Finally, few studies addressed the effect of indoor cold temperatures. Independently of outdoor temperatures, indoor cold temperature can increase nocturia in elderly patients as shown by Saeki et al. from Japan (76). Figure 3 illustrates the impact of cold on kidney function and blood pressure.
Fifteen years ago, when the terms “heat” and “kidney” were combined, heatstroke and renal failure from rhabdomyolysis were the first diagnoses to come to mind, more frequently described in men who were exposed to intense exertional activity with few case reports among women (77, 78). But with climate change and global warming, attention has been drawn to the effect of heat waves on chronic kidney injury. A large study from Australia in 2008 showed an increase of kidney disease during the “exposure to excessive natural heat” (79). In 2012, the Thai Cohort Study of 17402 men and 20414 women revealed an association between self-reported kidney disease and self-reported occupational heat exposure (80). In another case-control study from Taiwan, patients followed after 13 years of heat injury revealed an increased risk of CKD in these patients compared to controls (81).
In the first studies from Nicaragua and El Salvador in 2002 about the emerging kidney disease in sugarcane workers, heat exposure was not highlighted. It was a few years later that heat stress and the pathophysiology of chronic recurrent dehydration and hyperuricemia started to be evaluated (82, 83). A total of 189 sugarcane cutters from El Salvador were evaluated for dehydration markers during their strenuous work under very hot temperatures between 39 and 42°C at noon (82).
It is now well believed that heat-related kidney disease could be multifactorial with a higher incidence among disadvantaged population like vulnerable elderly and children, or socio-economically disadvantaged communities (84, 85). In a large Australian study, risk factors for acute kidney injury diagnosed in the emergency room following heat exposure were CKD, heart failure, age>64, male gender, diabetes and hypertension (86). Social and environmental factors seem to be both enhancing the kidney disease of undetermined origin that first emerged from central America and became a topic of research in other parts of the world. Schlader et al. and Chapman et al. have recently reviewed the kidney pathophysiology during heat stress (85, 87). Mostly based on data from animal models, Schlader et al. described the multifactorial mechanism of kidney injury when exposed to heat; heat could induce ischemia and hypoxia at the renal tubular level with ATP depletion which leads to oxidative stress and inflammation and a high risk for acute kidney injury (87). Chapman et al. defined heat stress as the net heat load to which an individual is exposed and results from the interaction of three factors, the environmental heat, the degree of the person’s physical activity that leads to metabolic heat production and the compensatory ability of the body to respond to heat by sweating (85). The combination of exercise and hot temperature can lead to proteinuria that is less likely present during passive heat stress (85). Chapman et al. emphasized two categories of populations exposed to heat stress, those with occupational heat stress like the Mesoamerican nephropathy in young male workers and those exposed to non-occupational heat like the elderly exposed to short days of unusual hot weather (85). The first group will be discussed further in the occupational section and was reported from several countries in the world not only Central American countries like Costa Rica, Guatemala (88–91),, Sri Lanka (92) but also from other developing countries like Thailand, Lebanon, Indonesia and Saudi Arabia (93–96) and even from developed countries mainly the USA (97–100).
Regarding the elderly, the topic is becoming a major public health issue because of the aging of populations globally, the global warming and increase in incidence of heat waves and the susceptibility of elderly kidneys to dehydration (85, 101). Even in countries with northern climate like Canada, a higher risk of acute kidney injury related to heat periods was found in a case-control study including >220,000 older adults of a mean age of 80 (101). In South Korea, an analysis of 21,656 cases of acute kidney injury admitted at the emergency department between 2010 and 2014 showed an increase in risk of AKI with every 1°C of increase in temperature (102).
Some studies could not demonstrate a higher incidence of AKI when heat stress was combined with non-steroidal inflammatory drugs and this association remains controversial (103, 104). A randomized controlled trial that included 40 cyclists with a mean age of 52 years in the US showed a significant increase in serum creatinine and decrease in fractional excretion of urinary sodium before and after one hour of endurance cycling in the heat, but ibuprofen intake did not worsen AKI (103). Another randomized cross-over trial from the US compared ten active males running in the heat versus temperate conditions, both conditions elevated their serum creatinine kinase but urinary neutrophil gelatinase-associated lipocalin (NGAL) was only increased in hot conditions which led to the conclusion that exercising in the heat increases the risk of mild acute kidney injury (105). Passive heat stress might not affect the glomerular filtration rate but the trials are controversial on whether heat acclimation improves or not GFR; a trial from the US showed that permissive dehydration with heat acclimation does not induce an increase in urinary NGAL, another trial from the UK showed that heat acclimatization can reduce AKI incidence but this was not consistent in another study from the US (106–109). Another interesting risk factor for acute kidney injury during heat stress is the consumption of soft drinks during and following exercise (110). This was demonstrated by Chapman et al. in a clinical trial of 12 healthy individuals that were exposed to heat, dehydration and who elevated their uric acid, serum copeptin and urinary NGAL levels after drinking a soft drink compared to water (110).
Few studies have assessed the risk of extreme heat events on patients with established chronic kidney disease. In a large study from the USA that included 7445 end-stage kidney disease patients with a mean age of 61 years, extreme heat was associated with higher mortality and morbidity and this association varied between geographic regions (111). A review of 58,330 hospital admissions during the warm season in Vietnam showed a higher incidence of hospitalization of kidney disease patients and urolithiasis (112). The same was demonstrated in California after assessing all hospitalizations between 1999 and 2009 (113).
Consequently, apart from the recurrent acute kidney injury secondary to heat stress, it is important to shed light on the risk of kidney stone in hot temperatures because it is expected to substantially increase in the future with the global warming (112, 114). Figure 4 depicts the different consequences of heat on kidney function.
The Occupational Safety and Health (OSH) has been a crucial component of the International Labour Organization (ILO) since its foundation in 1919. At the beginning, lead toxicity was one of the most important occupational risks that has been addressed. In 1950, after World War II and the awareness about emerging toxic substances, a joint commission ILO/World Health Organization (WHO) convened and defined occupational health (115).
Occupational health should aim at “the promotion and maintenance of the highest degree of physical, mental, and social well-being of workers in all occupations; the prevention among workers of departures from health caused by their working conditions; the protection of workers in their employment from risks resulting from factors adverse to health; the placing and maintenance of the worker in an occupational environment adapted to his physiological and psychological equipment and, to summarize: the adaptation of work to man and of each man to his job” (115).
Occupational health is a discipline that involves many stakeholders, it is adopted differently among countries worldwide and the regulations of OSH are not well followed and implemented in the majority of developing countries. When it comes to occupational-related kidney injury, there are no kidney health-specific recommendations but there are more general recommendations targeting the risk of exposure to heavy metals, infectious agents, chemicals and the environment (116). In addition, there is scarce data at the global level about mortality attributed to occupational-related kidney disease.
In most studies evaluating socioeconomic disparities among kidney disease patients, the socioeconomic status (SES) was considered as a combined measure of three indicators, education, income and occupation. A meta-analysis of 43 articles by Zeng et al. showed significant association between CKD prevalence and lower combined SES (117). Lower levels of occupation were also demonstrated to be associated with end-stage renal disease (117). Another meta-analysis by Tao et al. analyzed 14 studies including hemodialysis patients (118). Lower socioeconomic status (SES) indicators were significantly associated with higher mortality in dialysis (118). Moreover, a review of occupational nephropathies highlighted how disadvantaged populations and workers are more exposed to nephrotoxic agents and how poverty is contributing to occupational kidney disease like CKDu in agriculture workers in Central America (119). Finally, a study of 2011 on UK population showed an association between low SES and decreased eGFR. However, the significance of this association was attenuated when SES was adjusted to the components of the metabolic syndrome (120). Figure 5 illustrates the distribution of different social and environmental occupational factors that cause acute and chronic kidney disease.
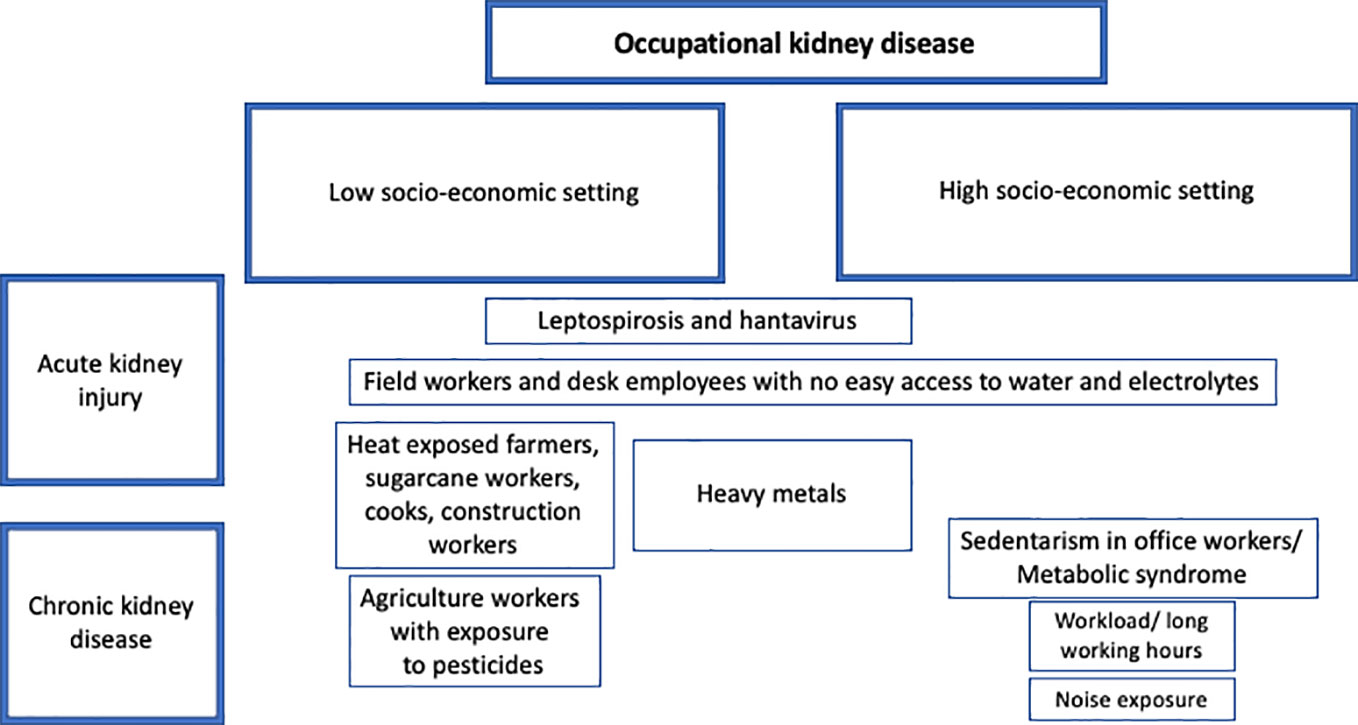
Figure 5 Distribution of occupational factors causing kidney disease across different socio-economic settings.
Before 2002, the occupational and environmental risk factors that have been linked to kidney disease were mainly heavy metals such as lead, mercury, arsenic, cadmium, uranium and organ solvents such as silica, infectious agents mainly leptospirosis and hantavirus and finally pesticides (121, 122). After the first report about CKD of unknown etiology (CKDu) in agriculture workers in El Salvador in 2002 (123), more than a hundred papers were published about the Mesoamerican nephropathy and heat exposure as a major risk factor. At the same time, several reports from Sri Lanka started to shed light on CKDu in agriculture workers where pesticides seem to be the main risk factor.
Lead and cadmium nephrotoxicity were first described in the 19th century; Lead, cadmium, arsenic and mercury are listed by the World Health Organization as four of 10 chemicals of major public health concern (124). They both cause tubular injury, Fanconi syndrome and may lead to chronic kidney disease (121, 122). Lead causes microcytic anemia, neurologic impairment, gout and kidney disease (122). Most of the first cases of cadmium were described in industrial countries like Belgium and Japan (122). The disease combining anemia, osteomalacia and kidney injury following the ingestion of contaminated rice by cadmium of the mines was called Itai-Itai disease (122). The concurrent toxicity of the two metals lead and cadmium has also been studied with growing evidence about their association with kidney disease, cancer and hypertension (125). Arsenic is found in pesticides and some studies linked kidney injury to urinary arsenic levels, mercury causes tubular and glomerular damage (122). Gold and mercury may also cause auto-immunity (126).
During the last 50 years, the international efforts and the enforcement of strict regulations in developed countries succeeded in minimizing the exposure of communities to lead, cadmium and other heavy metals (127). However, recent studies still report nephrotoxicity of heavy metals despite low levels. A recent analysis of Cadmium accumulation showed a correlation with albuminuria level (128). In 2021, a meta-analysis of 43 studies that analyzed the association of blood lead levels with renal function showed that high BUN is a valuable prognostic test for lead-associated kidney disease (129). A study on selenium from Taiwan and another on mercury from Colombia link the renal toxicity of these metals to genetic predisposition (130, 131).
The long-term toxicity of low levels of heavy metals is still controversial, especially in recent large longitudinal cohorts from high-income countries. Evans et al. studied 10,303 lead-exposed workers followed for 20 years and found no significant association between lead exposure and ESRD (132). Similarly, Steenland et al. found no significant association between higher lead exposure and ESRD in 58307 workers exposed to lead in the USA (133).
Golden et al. reviewed the outcome of 12,400 workers of uranium facilities in the USA between 1930 until 2017 and they found no increased renal mortality (134).
In addition to heavy metals, organic or solvent agents like silica or beryllium have also been evaluated as occupational hazards that may increase the risk of chronic kidney disease (135). High albuminuria was more observed in exposed workers to different solvents such as toluene, hydrocarbons, tetrachloroethene than in non-exposed controls (136). Silica nephropathy is a tubulointerstitial disease mainly, sometimes associated with immune-mediated systemic disease and could lead to end-stage renal disease (137). Occupations exposed to silica are miners, sandblasters, glass manufacturers, masons, ceramic and quarry workers (137).
Concerning infectious diseases, farmers from all over the globe suffered of hantavirus and leptospirosis. In a systematic analysis of 42 studies about the prevalence of Hantaviruses in farmers and forestry workers, the seroprevalence was estimated at 3.7% and 3.8% in these two occupational groups which is significantly higher than the general population (138). Cases of occupational leptospirosis and acute renal failure have been described in low-income countries, in Thailand, India, Bulgaria, Cuba and Argentine (139–143) but also more frequently in high-income countries, in farmers, abattoir workers in Barbados, Taiwan, Japan, Netherlands, Denmark, New Zealand, USA, in a sewage drain worker in Germany and among the French army in France (144–158). Studies on hantavirus and leptospirosis in the Mesoamerican countries were not conclusive even negative (159, 160).
Since the 1990s, chronic kidney disease of unknown etiology was described in agriculture workers in Sri Lanka. Table 2 summarizes most of the studies that reported occupational kidney disease in Sri Lanka. The first reports revealed several risk factors for CKDu in these male farmers such as age, drinking contaminated well water, being exposed to pesticides and snake bites (161, 162, 168). More recent studies highlighted as well the role of arsenic and the association between urinary herbicide level and biomarkers of kidney injury (165, 166). In 2016, a series of kidney biopsies in 59 patients with CKDu in Sri Lanka revealed chronic tubulointerstitial lesions with glomerular scarring (163). In 2017, de Silva et al. performed mixed methods research and revealed an association of CKDu with the poorest of the poor marginalized social category in agricultural settlements (164). In 2021, Kulasooriya compared 475 villagers including agriculture workers from endemic and non-endemic regions and demonstrated a significant higher level of heat stress index and urinary neutrophil gelatinase-associated lipocalin (NGAL) in workers from endemic CKDu regions (92). In 2022, Ekanayake et al. studied 188 paddy farmers, factory workers, plantation workers, they showed that Kidney Injury Molecule (KIM-1) is the best urinary biomarker to characterize renal injury in these occupational groups that suffer from CKDu (167).
In 2016, a systematic review of all studies on CKDu conducted in Sri Lanka could not identify a definitive cause and concluded that it was multifactorial, with variable geographic distribution, related to agriculture practices, use of agrochemicals, possible water contamination with heavy metals (169). Another review in 2020 revealed that the CKDu accounted for 70% of CKD cases in endemic areas and it was associated with being a farmer, exposed to heavy metals but found limited data on heat stress (170).
Several authors attempted a comparison of the “chronic interstitial nephritis in agriculture communities (CINAC)” that occurred in Sri Lanka, Central America and other tropical countries (171–173). A systematic review of 26 CKDu studies published in 2016 showed that most of the studies were conducted in Sri Lanka and Mesoamerican countries (171). Other countries reporting CKDu cases were Mexico, India, Tanzania, Tunisia, Japan, USA, Sweden, and Australia. The common risk factors were male sex, agriculture occupation and exposure to heavy metals. In Sri Lanka, agrochemical use was the additional risk factor whereas it was altitude and temperature in the Mesoamerican (171). A deep review by Jayasumana et al. of the CINAC endemic also described the common characteristics of CINAC, such as working in a poor agriculture community, being exposed to agrochemicals, having tubulointerstitial disease with low or absent proteinuria (172). Jayasumana et al. shed light as well at the different main triggers reported so far in the literature, the pesticide and heavy metal exposure from one side and the heat stress on the other (172). They argued that the absence of CINAC in some other hot areas of Sri Lanka, Cuba and Myanmar and the presence of this disease in women and children could not support heat stress and dehydration as a main trigger but it may be an additive on occupational and environmental toxins (172).
Before the era of CKDu and Mesoamerican nephropathy, heat exposure during work was considered as a risk factor for kidney stones. Warm climates at work, insufficient fluid intake and improper access to restrooms and bathrooms could lead to nephrolithiasis. In a recent review on occupational kidney stones, Malieckal et al. highlighted the several causes such as dehydration while working outdoors in hot temperatures, the risk of metabolic syndrome and uric acid stones in sedentary jobs and risk of calcium mobilization from bone in astronauts when working without gravity (174).
A growing attention in the last two decades took place towards the physical exertion of sugarcane cutters in very hot temperatures during the harvest season in El Salvador, Nicaragua, Guatemala and Costa Rica. Although the first reviews from El Salvador emphasized the role of pesticides (123), papers that were later published analyzed all possible causative factors of CKD in agriculture workers and specifically sugarcane workers in the four countries of Central America (82, 88, 90, 175–218). Table 3 summarizes these findings. The first research workshop on Mesoamerican nephropathy took place in 2012 in Costa Rica and paved the path of coordination between regional and international initiatives in order to further elucidate the cause of the disease and address it with interventional actions (219). In addition to research collaboration, in 2009, facing the high burden of farmers on dialysis in El Salvador (50.7%), El Salvador’s Ministry of Health collaborated with Cuba’s Ministry of Public Health and the Pan American Health Organization (PAHO) to launch a cooperative initiative to face the endemic kidney disease (220). Among 29 papers from Nicaragua, 13 from el Salvador, 4 from Costa Rica and 4 from Guatemala, sugarcane workers in the hottest areas suffered from kidney injury. Hansson et al. showed that, in comparison to banana, rice, coffee cultivation, the sugarcane cultivation had the highest burden of CKD (218). Kidney biopsies from patients in Nicaragua and El Salvador confirmed the chronic tubulointerstitial lesions and glomerular ischemia (185, 205, 207). Raines et al. found low eGFR in half of 151 male agriculture workers in Nicaragua, proteinuria of 300mg in <10%. Risk factors were pesticide exposure, long working hours and sugarcane chewing (179). Several studies demonstrated also a significant association between heat, dehydration during the harvest season and biomarkers of kidney injury in Nicaragua (178, 180, 183–185, 189, 192, 197, 199), El Salvador (82, 211), Guatemala (213) and Cost Rica (88, 215–218).
Jayasumana et al. argued that heat stress is not the major trigger of kidney disease in sugarcane cutters (174). Ordunez et al. agreed on that point because they found high CKD mortality trends between 1997 and 2003 in Central American countries among women and children in addition to men (221). In addition, González-Quiroz M et al. analyzed 25 epidemiological studies and found no association with heat stress or pesticide exposure with CKDu in Meso-America (222). However, Wesseling et al. exposed all the evidence that primarily links heat stress to this endemic kidney disease (223). They explained that strong-design studies demonstrated a decline in kidney function across the harvest due to heat and workload and that interventions implementing water, rest and shade (WRS) halted the decline in kidney function in these workers in Nicaragua (223). Other studies highlighted the risk of occupational heat strain on kidney health of workers (224, 225). A systematic review of 111 studies performed in 30 countries included 447 million workers with 40 different jobs, defined heat stress as wet-bulb globe temperature above 22°C or 24.8°C and showed that working under heat stress increased by 4 times the risk of heat strain; 15% of these individuals experienced kidney injury or disease (225). A review of farmworkers across the globe showed how heat stress affects all aspects of health and specifically kidney health in agriculture workers (226). Hansson et al. suggested in a detailed review the pathophysiology of heat stress causing kidney disease in agriculture workers (227). They discussed how systemic and kidney inflammation triggers such as endotoxemia, sub-rhabdomyolysis muscle damage, sugar intake, hyperuricemia and decreased renal blood flow could contribute to the acute kidney injury (228).
In addition to Central America and Sri Lanka, hundreds of occupational renal disease studies were reported after 1990 -excluding heavy metals- in other low and middle-income countries (Table 4), like India, Thailand, China, Indonesia and Nepal in Asia, Egypt, Kenya, Malawi, Ghana, Nigeria, Morocco and South Africa in the African continent, Lebanon, Turkey and Iran in the Middle-East, Bulgaria in Europe, Mexico in North America and Cuba, Argentine and Brazil in South America (22, 80, 94, 95, 233–261).
In 2014, Almaguer et al. reviewed countries that reported CKDu in agriculture workers and added India and Egypt to the list of Mesoamerican countries and Sri Lanka. They found that these countries have spots of high prevalence of chronic tubulointerstitial disease in men exposed to pesticides and dehydration (262). A review of CKDu in India found fragmented reports without clear characterization of the disease. It seems different from the Mesoamerican and Sri Lanka nephropathy as it affects older patients and it is associated with low levels of proteinuria and mild hypertension (263). But data evolved differently lately. In 2020, Mohanty et al. screened 2978 individuals in India and found a prevalence of 48% of CKD in agriculture workers and 49% were drinking from wells (231). The same year, Farag et al. found a CKD prevalence of 32.2% among 1201 participants and the “Uddanam nephropathy” was strongly associated with the farmer occupation (232). Venugopal et al. extensively studied as well he link between occupational heat exposure, kidney stones or kidney dysfunction in heat-exposed workers in the South of India (228, 233, 234). In the North of India, there has been a report about kitchen workers and higher albuminuria in those exposed to indoor air hydrocarbons in commercial kitchens (230). Papers from Indonesia reported the risk of kidney damage in shoe factory workers and rice farmers (22, 95). In Thailand, the Thai Cohort Study of 37816 included workers showed an association between occupational heat stress and self-reported kidney disease (80). And in Nepal, there is a serious concern about the high incidence of kidney disease among Nepali workers who migrated to work in the Gulf countries or Malaysia, which is still under research (238). In China, the reports on occupational kidney disease are different, do not include agriculture workers but they highlighted in 1713 adults the strong association between low SES and CKD prevalence (242). Another experimental paper from China studied the molecular alterations caused by the most used herbicide, glyphosate. Glyphosate, by altering metabolic pathways and increasing oxidative stress can cause various diseases like cancers, Parkinson’s disease and renal dysfunction (264).
In the African continent, reports from Malawi and Ghana ruled out the association between CKD, occupation, agriculture and socioeconomic status (247, 248). Morocco showed a high association between agriculture work and CKD (251). Nigeria reported in a review of 1278 patients 43% prevalence of unskilled workers among dialysis patients (250). In Kenya, workers in ceramics, bricks and tiles factory, silica-exposed had subclinical kidney injury (246).
In the Middle-East, a multicenter case-control study of 476 patients in Lebanon showed that heat-exposed cooks and construction workers were at high risk for end-stage kidney disease (94).
In the Americas, Mexico is also another hotspot for CKDu as studies from Tierra Blanca have shown 25% prevalence of CKD and a majority of non-traditional etiology (257, 258, 265). CKDu in Mexico seems to be more prevalent in poor communities (266).
Papers from Brazil emphasized the association between kidney disease and pesticides (261). A systematic review of Prudente et al. showed a high risk of renal injury in workers exposed to organophosphates and herbicides (267). Another interesting paper from Brazil showed how indoor foundry workers under extreme heat have a greater decline in their glomerular filtration rate compared to controls (260).
High-income countries that reported cases of occupational renal disease between 1990 and 2022 are USA and Barbados in North America, New Zealand, Australia, Japan, South Korea, Taiwan in the Western Pacific, UK, Ireland, France, Germany, Spain, Italy, Poland, Netherlands, Norway and Denmark (97, 98, 268–300) (Table 5).
In the USA, a great interest grew in the Mesoamerican experience with the aim to prevent CKD in US agriculture population (301). Several studies in agricultural workers of Hispanic origin and residing in California and Florida confirmed the recurrent AKI due to heat stress and dehydration during the work shift (265, 271–273, 276, 277). Smith et al. assessed 50 patients, undocumented workers (coming from Mexico), who received frequent emergent-hemodialysis and found out that they had high-risk occupations with heat stress and exposure to pesticides and lead paint fumes in construction (97). A large study in the USA of 23,692 citizens showed that lower neighborhood SES was associated with higher risk of CKD (278).
In Japan and South Korea, the occupational kidney disease was studied from another perspective. Tsurugano et al. from Japan showed an association between job stress, high blood pressure and kidney disease in 1231 male office workers (281). In 2020, an analysis of 20,851 workers from South Korea revealed an association between long working hours and decreased eGFR (284). Kim et al. in South Korea showed that occupational noise in females is associated with high CKD prevalence and this could be related to the increase in the sympathetic nervous system activity (285). In Taiwan, taxi drivers have higher albuminuria and metabolic syndrome (286). Another interesting review from high-income countries is about the role of newer halogenated hydrocarbons and their risk of DNA injury and altered renal function. Anesthesiologists are at risk of chronic exposure if air-conditioning systems are not protective and the best solution would be to use intravenous anesthetics (302).
After reviewing the impact of altitude on kidney health we can suggest the following measures:
-Healthy patients should climb to high altitude on several days to allow the acclimatization or acclimation process and to avoid acute mountain sickness and a severe decline in glomerular filtration rate.
-Hypertensive patients should monitor their blood pressure at high altitude.
-Patients with pre-established CKD should be aware of the risk of pulmonary edema; guidelines do not recommend a different hemoglobin target than those at sea-level. In case of acute mountain sickness, CKD patients should be aware of an adjustment of dosage of medications prescribed.
-Transplant patients do not need to change their immunosuppressant medications’ dose at high altitude.
-Living at high altitude may predispose to albuminuria and hypertension and renin-angiotensin inhibitors are the preferred drugs in these cases.
-People working in the space should be aware of the side effects of acute hypobaric hypoxia and avoid abrupt exposure to low atmospheric pressure.
Although seasonal variation is not extensively studied, climate change is expected to lead to an increase in extreme temperatures. Consequently, the following points are important to highlight:
-Hypertensive patients should be aware of the risk of exacerbation of hypertension during extreme cold weather.
-Vulnerable populations like elderly and children should be protected and well hydrated during heat waves to avoid acute kidney injury.
-Lupus erythematosus patients should be aware of the seasonal variation of renal flares and the impact of humidity, cold and ultraviolet radiation.
Despite all the evidence behind occupational renal risk, screening for occupational kidney disease is still not universally recommended. Several challenges may hinder screening and should be considered (303). One should assess first if reduction of exposure is possible, second if the evidence of using one biomarker for screening reflects well a preclinical stage of kidney disease, third if diagnostics are available in certain places, fourth if workers would accept screening, finally it is important to figure out whether screening will be followed by an existing effective intervention.
-In highly endemic regions or communities, screening is important and applying preventive interventions is crucial (304–306).
-Working in heat -whether outdoor in agriculture or construction or even as athletes, or indoor in kitchens or foundries-, should be recognized as an occupational kidney hazard and preventive interventions should be applied: hydration should be emphasized (307), it should include water and electrolytes and should be sugar-free; rest periods are very important as well as proper access to healthcare and ensuring as much as possible shade in outdoor activities and proper air conditioning in indoor jobs to avoid maintaining the dehydration and kidney injury.
-Gender disparities among different occupational kidney diseases need to be further evaluated in the future. Reports showed higher prevalence of agriculture-related kidney disease in men and occupational noise-related kidney disease in women. However, two recent reviews by García et al. and Swartling et al. found important sex differences in the detection and prevalence of kidney disease with lower diagnosis of chronic kidney disease and lower SES in women globally (308, 309).
-Despite the long history of heavy metals and pesticides, examples from the most vulnerable groups in the world show that there is insufficient awareness and vigilance about their nephrotoxicity. There is inconsistent coordination between governments, researchers, decision makers to prevent the high-risk exposure of agricultural and industrial workers and to mitigate the increase in kidney disease cases.
-It goes without saying that health is as important as productivity.
The experience from Sri Lanka and Mesoamerica showed the challenges that emerge when efforts are fragmented, the decision-makers are not committed and the different stakeholders are not well coordinating (310, 311). Involvement of international experts, mobilizing funds for research and preventive interventions are also needed. Occupational Safety and Health has a major role in preventing kidney disease worldwide with a need to update guidelines such as adding electrolytes to water during hydration, such as putting kidney health on the bigger agenda. Occupational Safety and Health can also ensure trainings in workplaces that are at high risk of exposure to nephrotoxic hazards (312).
Evidence-based research is very important to build relevant health policies and implement effective and efficient interventions. However, challenges are numerous when conducting research in vulnerable populations.
We can learn from the challenges of researchers in Mesoamerica. Three themes were hindering the progress of research (311). The first one is the influence of government and industries’ interests, the second is the scarce human and financial resources and third the pragmatic challenges of undergoing research projects.
Researchers from North America also faced challenges when conducting occupational studies in Mesoamerica (313). The lessons learned are: clear definition of roles and responsibilities and of mutual expectations, good discussion of research outcomes, setting realistic goals and timelines, active listening especially in a multilingual environment, sharing personal experience with humility, not presuming that expertise entails trust, creating a sense of community.
Finally, standardization of terms and research protocols is crucial when focusing on a hot health topic. In this present review, several publications on occupational kidney disease were missed because authors did not mention the term “occupation” or “occupational” in their abstract or keywords. As an example of standardized protocol, “The Disadvantaged Populations eGFR Epidemiology (DEGREE) Study” protocol was published in 2017 (314). The DEGREE project aims to characterize the distribution of eGFR in multiple LMICs in a standardized way that helps make international comparisons. This protocol has been a model implemented and still being implemented in several countries.
As a summary, environmental factors, socioeconomic status and occupation are all independent risk factors for kidney disease but they often act synergistically. Living in extreme temperatures and low socioeconomic neighborhood, even in high-income countries, is adding on the burden of global kidney disease. These weather and occupational conditions are widening the chasm in kidney health between the wealthy and disadvantaged populations. Altitude and cold-related kidney disease are also two topics that require more research and evidence especially with the climate change and the risk of extreme temperatures. Seasonality has important impact on the practice of nephrology and hypertension management. And most of all, the workplace should ensure safety to our kidney health and international efforts from scientists, ministries of health, nephrology societies should be gathered to address with standard policies and protocols the old and newly identified hazards that may jeopardize the kidney’s well-being.
MA contributed to the conceptualization and literature search. MA contributed to the first draft. DC revised the manuscript. Both authors approved the final version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Irwin A, Valentine N, Brown C, Loewenson R, Solar O, Brown H, et al. The commission on social determinants of health: Tackling the social roots of health inequities. PloS Med (2006) 3(6):e106. doi: 10.1371/journal.pmed.0030106
2. Hall YN. Social determinants of health: Addressing unmet needs in nephrology. Am J Kidney Dis (2018) 72(4):582–91. doi: 10.1053/j.ajkd.2017.12.016
3. McClellan AC, Plantinga L, McClellan WM. Epidemiology, geography and chronic kidney disease. Curr Opin Nephrol Hypertens (2012) 21(3):323–8. doi: 10.1097/MNH.0b013e3283521dae
4. Tremblay JC, Ainslie PN. Global and country-level estimates of human population at high altitude. Proc Natl Acad Sci U S A (2021) 118(18):e2102463118. doi: 10.1073/pnas.2102463118
5. Winterborn MH, Bradwell AR, Chesner IM, Jones GT. The origin of proteinuria at high altitude. Postgrad Med J (1987) 63(737):179–81. doi: 10.1136/pgmj.63.737.179
6. Palubiski LM, O'Halloran KD, O'Neill J. Renal physiological adaptation to high altitude: A systematic review. Front Physiol (2020) 1611:756. doi: 10.3389/fphys.2020.00756
7. Goldfarb-Rumyantzev AS, Alper SL. Short-term responses of the kidney to high altitude in mountain climbers. Nephrol Dial Transplant (2014) 29(3):497–506. doi: 10.1093/ndt/gft051
8. Thron CD, Chen J, Leiter JC, Ou ,LC. Renovascular adaptive changes in chronic hypoxic polycythemia. Kidney Int (1998) 54:2014–20. doi: 10.1046/j.1523-1755.1998.00186.x
9. Denton KM, Shweta A, Anderson ,WP. Preglomerular and postglomerular resistance responses to different levels of sympathetic activation by hypoxia. J Am Soc Nephrol (2002) 13:27–34. doi: 10.1681/ASN.V13127
10. Bestle MH, Olsen NV, Poulsen TD, Roach R, Fogh-Andersen N, Bie P. Prolonged hypobaric hypoxemia attenuates vasopressin secretion and renal response to osmostimulation in men. J Appl Physiol (2002) 92:1911–22. doi: 10.1152/japplphysiol.00936.2001
11. Arestegui AH, Fuquay R, Sirota J, Swenson ER, Schoene RB, Jefferson JA, et al. High altitude renal syndrome (HARS). J Am Soc Nephrol (2011) 22(11):1963–8. doi: 10.1681/ASN.2010121316
12. Pines A. High-altitude acclimatization and proteinuria in East Africa. Br J Dis Chest (1978) 72(3):196–8. doi: 10.1016/0007-0971(78)90041-4
13. Singh MV, Salhan AK, Rawal SB, Tyagi AK, Kumar N, Verma SS, et al. Blood gases, hematology, and renal blood flow during prolonged mountain sojourns at 3500 and 5800 m. Aviat Space Environ Med (2003) 74(5):533–6.
14. Cumbo TA, Braude D, Basnyat B, Rabinowitz L, Lescano AG, Shah MB, et al. Higher venous bicarbonate concentration associated with hypoxemia, not acute mountain sickness, after ascent to moderate altitude. J Travel Med (2005) 12(4):184–9. doi: 10.2310/7060.2005.12403
15. Chen W, Liu Q, Wang H, Chen W, Johnson RJ, Dong X, et al. Prevalence and risk factors of chronic kidney disease: A population study in the Tibetan population. Nephrol Dial Transplant (2011) 26(5):1592–9. doi: 10.1093/ndt/gfq608
16. Yijiang Z, Jianhua Z, Feili L. Acute kidney injury at high altitude. High Alt Med Biol (2013) 14(2):183–5. doi: 10.1089/ham.2012.1123
17. Suh KS, Kim T, Yi NJ, Hong G. Preparation for high altitude expedition and changes in cardiopulmonary and biochemical laboratory parameters with ascent to high altitude in transplant patients and live donors. Clin Transplant (2015) 29(11):1013–20. doi: 10.1111/ctr.12623
18. Gilbert-Kawai E, Martin D, Grocott M, Levett D. High altitude-related hypertensive crisis and acute kidney injury in an asymptomatic healthy individual. Extrem Physiol Med (2016) 14:5:10. doi: 10.1186/s13728-016-0051-3
19. Zhang L, Wang Z, Chen Y, Wang X, Chen Z, Feng B, et al. Prevalence and risk factors associated with chronic kidney disease in adults living in 3 different altitude regions in the Tibetan plateau. Clin Chim Acta (2018) 481:212–7. doi: 10.1016/j.cca.2018.03.025
20. Phelan B, Mather L, Regmi N, Starling J, Twillmann D, McElwee M, et al. Ambulatory blood pressure at Sea level and high altitude in a climber with a kidney transplant and hypertension. High Alt Med Biol (2019) 20(3):307–11. doi: 10.1089/ham.2018.0118
21. Zhao L, Wang X, Wang T, Fan W, Ren H, Zhang R, et al. Associations between high-altitude residence and end-stage kidney disease in Chinese patients with type 2 diabetes. High Alt Med Biol (2020) 21(4):396–405. doi: 10.1089/ham.2020.0076
22. Fitria L, Prihartono NA, Ramdhan DH, Wahyono TYM, Kongtip P, Woskie S. Environmental and occupational risk factors associated with chronic kidney disease of unknown etiology in West Javanese rice farmers, Indonesia. Int J Environ Res Public Health (2020) 17(12):4521. doi: 10.3390/ijerph17124521
23. Wei H, Luo Z. Ci-dan-yang-zong; bai-ma-yang-jin [Analysis of clinical characteristics of henoch-schonlein purpura patients from different altitudes in plateau areas]. Beijing Da Xue Xue Bao Yi Xue Ban (2021) 53(6):1072–7. doi: 10.19723/j.issn.1671-167X.2021.06.011
24. Li X, Wu X, Zhang M, Xu L, Li G, Wen Y, et al. Pregnancy-related acute kidney injury at high altitude: A retrospective observational study in a single center. BMC Nephrol (2021) 22(1):215. doi: 10.1186/s12882-021-02418-7
25. Hamilton SA, Jarhyan P, Fecht D, Venkateshmurthy NS, Pearce N, Venkat Narayan KM, et al. Environmental risk factors for reduced kidney function due to undetermined cause in India: An environmental epidemiologic analysis. Environ Epidemiol (2021) 5(5):e170. doi: 10.1097/EE9.0000000000000170
26. Wang H, Tang C, Dang Z, Yong A, Liu L, Wang S, et al. Clinicopathological characteristics of high-altitude polycythemia-related kidney disease in Tibetan inhabitants. Kidney Int (2022) 102(1):196–206. doi: 10.1016/j.kint.2022.03.027
27. Ghahramani N, Ahmed F, Al-Laham A, Lengerich EJ. The epidemiological association of altitude with chronic kidney disease: Evidence of protective effect. Nephrol (Carlton) (2011) 16(2):219–24. doi: 10.1111/j.1440-1797.2010.01332.x
28. Königsrainer I, Löffler M, Bühler S, Walter M, Schafbuch L, Beckert S, et al. Impact of endotoxin exposure after exhausting exercise on the immune system in solid organ transplant recipients. Exerc Immunol Rev (2012) 18:177–83.
29. Cippà PE, Grebe SO, Fehr T, Wüthrich RP, Mueller TF. Altitude and arteriolar hyalinosis after kidney transplantation. Nephrol (Carlton) (2016) 21(9):782–4. doi: 10.1111/nep.12734
30. Biollaz J, Buclin T, Hildebrandt W, Décosterd LA, Nussberger J, Swenson ER, et al. No renal dysfunction or salt and water retention in acute mountain sickness at 4,559 m among young resting males after passive ascent. J Appl Physiol (1985) 130(1):226–36. doi: 10.1152/japplphysiol.00382.2020
31. Perico N, Plata R, Anabaya A, Codreanu I, Schieppati A, Ruggenenti P, et al. Strategies for national health care systems in emerging countries: The case of screening and prevention of renal disease progression in Bolivia. Kidney Int Suppl (2005) 97):S87–94. doi: 10.1111/j.1523-1755.2005.09715.x
32. O'Donnell JK, Tobey M, Weiner DE, Stevens LA, Johnson S, Stringham P, et al. Prevalence of and risk factors for chronic kidney disease in rural Nicaragua. Nephrol Dial Transplant (2011) 26(9):2798–805. doi: 10.1093/ndt/gfq385
33. Gonzales GF, Tapia V. Increased levels of serum γ-glutamyltransferase and uric acid on metabolic, hepatic and kidney parameters in subjects at high altitudes. J Basic Clin Physiol Pharmacol (2015) 26(1):81–7. doi: 10.1515/jbcpp-2013-0162
34. Harhay MN, Harhay MO, Coto-Yglesias F, Rosero Bixby L. Altitude and regional gradients in chronic kidney disease prevalence in Costa Rica: Data from the Costa Rican longevity and healthy aging study. Trop Med Int Health (2016) 21(1):41–51. doi: 10.1111/tmi.12622
35. Hurtado-Arestegui A, Plata-Cornejo R, Cornejo A, Mas G, Carbajal L, Sharma S, et al. Higher prevalence of unrecognized kidney disease at high altitude. J Nephrol (2018) 31(2):263–9. doi: 10.1007/s40620-017-0456-0
36. Carrillo-Larco RM, Miranda JJ, Gilman RH, Narvaez-Guerra O, Herrera-Enriquez K, Medina-Lezama J, et al. Urbanization and altitude are associated with low kidney function in Peru. High Alt Med Biol (2019) 20(2):133–40. doi: 10.1089/ham.2018.0106
37. Bravo-Jaimes K, Loescher VY, Canelo-Aybar C, Rojas-Camayo J, Mejia CR, Schult S, et al. Effect of altitude on mortality of end-stage renal disease patients on hemodialysis in Peru. Clin Kidney J (2020) 14(3):998–1003. doi: 10.1093/ckj/sfaa056
38. Gutierrez-Peña M, Zuñiga-Macias L, Marin-Garcia R, Ovalle-Robles I, García-Díaz AL, Macías-Guzmán MJ, et al. High prevalence of end-stage renal disease of unknown origin in aguascalientes Mexico: Role of the registry of chronic kidney disease and renal biopsy in its approach and future directions. Clin Kidney J (2021) 14(4):1197–206. doi: 10.1093/ckj/sfaa229
39. Steele AR, Tymko MM, Meah VL, Simpson LL, Gasho C, Dawkins TG, et al. Global REACH 2018: volume regulation in high-altitude andeans with and without chronic mountain sickness. Am J Physiol Regul Integr Comp Physiol (2021) 321(3):R504–12. doi: 10.1152/ajpregu.00102.2021
40. Vizcarra-Vizcarra CA, Alcos-Mamani AL. High altitude pulmonary edema in a chronic kidney disease patient-is peritoneal dialysis a risk factor? High Alt Med Biol (2022) 23(1):96–9. doi: 10.1089/ham.2021.0136
41. Luks AM, Johnson RJ, Swenson ER. Chronic kidney disease at high altitude. J Am Soc Nephrol (2008) 19(12):2262–71. doi: 10.1681/ASN.2007111199
42. Mairbaurl H, Schobersberger W, Hasibeder W, Knapp E, Hopferwieser T, Humpeler E, et al. Exercise performance of hemodialysis patients during short-term and prolonged exposure to altitude. Clin Nephrol (1989) 32:31–9.
43. Ercan E. Effects of aerospace environments on the cardiovascular system. Anatol J Cardiol (2021) 25(Suppl 1):3–6. doi: 10.5152/AnatolJCardiol.2021.S103
44. Demoury C, Aerts R, Vandeninden B, Van Schaeybroeck B, De Clercq EM. Impact of short-term exposure to extreme temperatures on mortality: A multi-city study in Belgium. Int J Environ Res Public Health (2022) 19(7):3763. doi: 10.3390/ijerph19073763
45. Lin YK, Zafirah Y, Ke MT, Andhikaputra G, Wang YC. The effects of extreme temperatures on emergency room visits-a population-based analysis by age, sex, and comorbidity. Int J Biometeorol (2021) 65(12):2087–98. doi: 10.1007/s00484-021-02166-1
46. Isezuo SA. Seasonal variation in hospitalisation for hypertension-related morbidities in sokoto, north-western Nigeria. Int J Circumpolar Health (2003) 62(4):397–409. doi: 10.3402/ijch.v62i4.17583
47. Bi SH, Cheng LT, Zheng DX, Wang T. Seasonal changes in blood pressure in chronic kidney disease patients. Clin Nephrol (2010) 73(3):216–20. doi: 10.5414/cnp73216
48. Liu C, Yavar Z, Sun Q. Cardiovascular response to thermoregulatory challenges. Am J Physiol Heart Circ Physiol (2015) 309(11):H1793–812. doi: 10.1152/ajpheart.00199.2015
49. Cerdá J, Lameire N, Eggers P, Pannu N, Uchino S, Wang H, et al. Epidemiology of acute kidney injury. Clin J Am Soc Nephrol (2008) 3(3):881–6. doi: 10.2215/CJN.04961107
50. Lin YK, Wang YC, Ho TJ, Lu CA. Temperature effects on hospital admissions for kidney morbidity in Taiwan. Sci Total Environ (2013) 443:812–20. doi: 10.1016/j.scitotenv.2012.10.108
51. Lim YH, So R, Lee C, Hong YC, Park M, Kim L, et al. Ambient temperature and hospital admissions for acute kidney injury: A time-series analysis. Sci Total Environ (2018) 616-617:1134–8. doi: 10.1016/j.scitotenv.2017.10.207
52. Kim E, Kim H, Kim YC, Lee JP. Association between extreme temperature and kidney disease in south Korea, 2003-2013: Stratified by sex and age groups. Sci Total Environ (2018) 642:800–8. doi: 10.1016/j.scitotenv.2018.06.055
53. Masugata H, Senda S, Inukai M, Himoto T, Murao K, Hosomi N, et al. Seasonal variation in estimated glomerular filtration rate based on serum creatinine levels in hypertensive patients. Tohoku J Exp Med (2011) 224(2):137–42. doi: 10.1620/tjem.224.137
54. Lombardi G, Gambaro G, Pertica N, Naticchia A, Bargagli M, Ferraro PM. Seasonality of acute kidney injury in a tertiary hospital academic center: An observational cohort study. Environ Health (2021) 20(1):8. doi: 10.1186/s12940-021-00691-5
55. Chiu PF, Chang CH, Wu CL, Chang TH, Tsai CC, Kor CT, et al. High particulate matter 2.5 levels and ambient temperature are associated with acute lung edema in patients with nondialysis stage 5 chronic kidney disease. Nephrol Dial Transplant (2019) 34(8):1354–60. doi: 10.1093/ndt/gfy144
56. Xu Z, Etzel RA, Su H, Huang C, Guo Y, Tong S. Impact of ambient temperature on children's health: A systematic review. Environ Res (2012) 117:120–31. doi: 10.1016/j.envres.2012.07.002
57. Obermeyer Z, Pope D. Variation in common laboratory test results caused by ambient temperature. Med (N Y) (2021) 2(12):1314–1326.e2. doi: 10.1016/j.medj.2021.11.003
58. Ephraim RKD, Asamoah CA, Abaka-Yawson A, Kwadzokpui PK, Adusei S. Climate change causes changes in biochemical markers of kidney disease. BMC Nephrol (2020) 21(1):542. doi: 10.1186/s12882-020-02186-w
59. Tanaka S, Fujishiro M, Watanabe K, Imatake K, Suzuki Y, Abe M, et al. Seasonal variation in hydration status among community-dwelling elderly in Japan. Geriatr Gerontol. Int (2020) 20(10):904–10. doi: 10.1111/ggi.14010
60. Wetmore JB, Gilbertson DT, Collins AJ. Shaping public health initiatives in kidney diseases: The peer kidney care initiative. Blood Purif (2016) 41(1-3):151–8. doi: 10.1159/000441316
61. Kovacic V, Kovacic V. Seasonal variations of clinical and biochemical parameters in chronic haemodialysis. Ann Acad Med Singap (2004) 33(6):763–8.
62. Chiche L, Jourde N, Ulmann C, Mancini J, Darque A, Bardin N, et al. Seasonal variations of systemic lupus erythematosus flares in southern France. Eur J Intern Med (2012) 23(3):250–4. doi: 10.1016/j.ejim.2011.12.006
63. Pan Q, Chen J, Guo L, Lu X, Liao S, Zhao C, et al. Mechanistic insights into environmental and genetic risk factors for systemic lupus erythematosus. Am J Transl Res (2019) 11(3):1241–54.
64. Szeto CC, Mok HY, Chow KM, Lee TC, Leung JY, Li EK, et al. Climatic influence on the prevalence of noncutaneous disease flare in systemic lupus erythematosus in Hong Kong. J Rheumatol (2008) 35(6):1031–7.
65. Schlesinger N, Schlesinger M, Seshan SV. Seasonal variation of lupus nephritis: high prevalence of class V lupus nephritis during the winter and spring. J Rheumatol (2005) 32(6):1053–7.
66. Atterhög JH, Carlens P, Granberg PO, Wallenberg LR. Cardiovascular and renal responses to acute cold exposure in water-loaded man. Scand J Clin Lab Invest (1975) 35(4):311–7. doi: 10.3109/00365517509095746
68. Dann EJ, Gillis S, Burstein R. Effect of fluid intake on renal function during exercise in the cold. Eur J Appl Physiol Occup Physiol (1990) 61(1-2):133–7. doi: 10.1007/BF00236707
69. Kanagy NL. Alpha(2)-adrenergic receptor signalling in hypertension. Clin Sci (Lond) (2005) 109(5):431–7. doi: 10.1042/CS20050101
70. Phillips D, Young O, Holmes J, Allen LA, Roberts G, Geen J, et al. Seasonal pattern of incidence and outcome of acute kidney injury: A national study of welsh AKI electronic alerts. Int J Clin Pract (2017) 71(9):10. doi: 10.1111/ijcp.13000
71. Iwagami M, Moriya H, Doi K, Yasunaga H, Isshiki R, Sato I, et al. Seasonality of acute kidney injury incidence and mortality among hospitalized patients. Nephrol Dial Transplant (2018) 33(8):1354–62. doi: 10.1093/ndt/gfy011
72. Hattori T, Munakata M. Blood pressure measurement under standardized indoor condition may mask seasonal blood pressure variation in men with mildly elevated blood pressure. Clin Exp Hypertens (2015) 37(4):317–22. doi: 10.3109/10641963.2014.960975
73. Masajtis-Zagajewska A, Pawłowicz E, Nowicki M. Effect of short-term cold exposure on central aortic blood pressure in patients with CKD. Nephron (2021) 145(1):20–6. doi: 10.1159/000510365
74. Hwang JC, Wang CT, Chien CC. Effect of climatic temperature on fluid gain in hemodialysis patients with different degrees of overhydration. Blood Purif (2007) 25(5-6):473–9. doi: 10.1159/000112481
75. Woods HF, Cheng CT, Lim PS. Climate and volume and blood pressure trends in Taiwanese hemodialysis patients. Blood Purif (2008) 26(5):404–6. doi: 10.1159/000141957
76. Saeki K, Obayashi K, Kurumatani N. Indoor cold exposure and nocturia: A cross-sectional analysis of the HEIJO-KYO study. BJU Int (2016) 117(5):829–35. doi: 10.1111/bju.13325
77. Pattison ME, Logan JL, Lee SM, Ogden DA. Exertional heat stroke and acute renal failure in a young woman. Am J Kidney Dis (1988) 11(2):184–7. doi: 10.1016/s0272-6386(88)80210-5
78. Donham BP, Frankfurt SB, Cartier RA, O'Hara SM, Sieg VC. Low incidence of death and renal failure in united states military service members hospitalized with exertional heat stroke: A retrospective cohort study. Mil Med (2020) 185(Suppl 1):362–7. doi: 10.1093/milmed/usz214
79. Hansen AL, Bi P, Ryan P, Nitschke M, Pisaniello D, Tucker G. The effect of heat waves on hospital admissions for renal disease in a temperate city of Australia. Int J Epidemiol (2008) 37(6):1359–65. doi: 10.1093/ije/dyn165
80. Tawatsupa B, Lim LL, Kjellstrom T, Seubsman SA, Sleigh A, Thai Cohort Study Team. Association between occupational heat stress and kidney disease among 37,816 workers in the Thai cohort study (TCS). J Epidemiol (2012) 22(3):251–60. doi: 10.2188/jea.je20110082
81. Tseng MF, Chou CL, Chung CH, Chen YK, Chien WC, Feng CH, et al. Risk of chronic kidney disease in patients with heat injury: A nationwide longitudinal cohort study in Taiwan. PloS One (2020) 15(7):e0235607. doi: 10.1371/journal.pone.0235607
82. García-Trabanino R, Jarquín E, Wesseling C, Johnson RJ, González-Quiroz M, Weiss I, et al. Heat stress, dehydration, and kidney function in sugarcane cutters in El Salvador–a cross-shift study of workers at risk of mesoamerican nephropathy. Environ Res (2015) 142:746–55. doi: 10.1016/j.envres.2015.07.007
83. Roncal-Jimenez CA, García-Trabanino R, Wesseling C, Johnson RJ. Mesoamerican nephropathy or global warming nephropathy? Blood Purif (2016) 41(1-3):135–8. doi: 10.1159/000441265
84. de Lorenzo A, Liaño F. High temperatures and nephrology: The climate change problem. altas temperaturas y nefrología: A propósito del cambio climático. Nefrologia (2017) 37(5):492–500. doi: 10.1016/j.nefro.2016.12.008
85. Chapman CL, Johnson BD, Parker MD, Hostler D, Pryor RR, Schlader Z. Kidney physiology and pathophysiology during heat stress and the modification by exercise, dehydration, heat acclimation and aging. Temp (Austin) (2020) 8(2):108–59. doi: 10.1080/23328940.2020.1826841
86. Xu Z, Hu X, Tong S, Cheng J. Heat and risk of acute kidney injury: An hourly-level case-crossover study in queensland, Australia. Environ Res (2020) 182:109058. doi: 10.1016/j.envres.2019.109058
87. Schlader ZJ, Hostler D, Parker MD, Pryor RR, Lohr JW, Johnson BD, et al. The potential for renal injury elicited by physical work in the heat. Nutrients (2019) 11(9):2087. doi: 10.3390/nu11092087
88. Crowe J, Rojas-Valverde D, Rojas-Garbanzo M, Gutiérrez-Vargas R, Ugalde-Ramírez JA, Ledezma-Rojas JP, et al. Kidney function in rice workers exposed to heat and dehydration in Costa Rica. Int J Environ Res Public Health (2022) 19(9):4962. doi: 10.3390/ijerph19094962
89. Rojas-Valverde D, Martínez-Guardado I, Sánchez-Ureña B, Timón R, Scheer V, Pino-Ortega J, et al. Outpatient assessment of mechanical load, heat strain and dehydration as causes of transitional acute kidney injury in endurance trail runners. Int J Environ Res Public Health (2021) 18(19):10217. doi: 10.3390/ijerph181910217
90. Sorensen CJ, Krisher L, Butler-Dawson J, Dally M, Dexter L, Asensio C, et al. Workplace screening identifies clinically significant and potentially reversible kidney injury in heat-exposed sugarcane workers. Int J Environ Res Public Health (2020) 17(22):8552. doi: 10.3390/ijerph17228552
91. Butler-Dawson J, Krisher L, Yoder H, Dally M, Sorensen C, Johnson RJ, et al. Evaluation of heat stress and cumulative incidence of acute kidney injury in sugarcane workers in Guatemala. Int Arch Occup Environ Health (2019) 92(7):977–90. doi: 10.1007/s00420-019-01426-3
92. Kulasooriya PN, Jayasekara KB, Nisansala T, Kannangara S, Karunarathna R, Karunarathne C, et al. Utility of self-reported heat stress symptoms and NGAL biomarker to screen for chronic kidney disease of unknown origin (CKDu) in Sri Lanka. Int J Environ Res Public Health (2021) 18(19):10498. doi: 10.3390/ijerph181910498
93. Luangwilai T, Robson MG, Siriwong W. Effect of heat exposure on dehydration and kidney function among sea salt workers in Thailand. Rocz Panstw Zakl Hig (2021) 72(4):435–42. doi: 10.32394/rpzh.2021.0186
94. Doueihy C, Chelala D, Ossaili H, Hachem GE, Zeidan S, Ghoul BE, et al. Occupational heat exposure as a risk factor for end-stage kidney disease: A case-control study. J Occup Environ Med (2022) 64(3):e103–8. doi: 10.1097/JOM.0000000000002458
95. Nainggolan G, Soemarko D, Siregar P, Sutranto AL, Bardosono S, Prijanti AR, et al. Diagnostic role of urine specific gravity to detect kidney impairment on heat-exposed workers in a shoe factory in Indonesia: A cross-sectional study. BMJ Open (2021) 11(9):e047328. doi: 10.1136/bmjopen-2020-047328
96. Al-Bouwarthan M, Quinn MM, Kriebel D, Wegman DH. Risk of kidney injury among construction workers exposed to heat stress: A longitudinal study from Saudi Arabia. Int J Environ Res Public Health (2020) 17(11):3775. doi: 10.3390/ijerph17113775
97. Smith DJ, Mac V, Thompson LM, Plantinga L, Kasper L, Hertzberg VS. Using occupational histories to assess heat exposure in undocumented workers receiving emergent renal dialysis in Georgia. Workplace Health Saf (2022) 70(5):251–8. doi: 10.1177/21650799211060695
98. Shi DS, Weaver VM, Hodgson MJ, Tustin AW. Hospitalised heat-related acute kidney injury in indoor and outdoor workers in the USA. Occup Environ Med (2022) 79(3):184–91. doi: 10.1136/oemed-2021-107933
99. Chapman CL, Hess HW, Lucas RAI, Glaser J, Saran R, Bragg-Gresham J, et al. Occupational heat exposure and the risk of chronic kidney disease of nontraditional origin in the united states. Am J Physiol Regul Integr Comp Physiol (2021) 321(2):R141–51. doi: 10.1152/ajpregu.00103.2021
100. Smith DJ, Pius LM, Plantinga LC, Thompson LM, Mac V, Hertzberg VS. Heat stress and kidney function in farmworkers in the US: A scoping review. J Agromed (2022) 27(2):183–92. doi: 10.1080/1059924X.2021.1893883
101. McTavish RK, Richard L, McArthur E, Shariff SZ, Acedillo R, Parikh CR, et al. Association between high environmental heat and risk of acute kidney injury among older adults in a northern climate: A matched case-control study. Am J Kidney Dis (2018) 71(2):200–8. doi: 10.1053/j.ajkd.2017.07.011
102. Kim SE, Lee H, Kim J, Lee YK, Kang M, Hijioka Y, et al. Temperature as a risk factor of emergency department visits for acute kidney injury: A case-crossover study in Seoul, south Korea. Environ Health (2019) 18(1):55. doi: 10.1186/s12940-019-0491-5
103. McDermott BP, Smith CR, Butts CL, Caldwell AR, Lee EC, Vingren JL, et al. Renal stress and kidney injury biomarkers in response to endurance cycling in the heat with and without ibuprofen. J Sci Med Sport (2018) 21(12):1180–4. doi: 10.1016/j.jsams.2018.05.003
104. Mangoni AA, Kholmurodova F, Mayner L, Hakendorf P, Woodman RJ. The concomitant use of diuretics, non-steroidal anti-inflammatory drugs, and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (Triple whammy), extreme heat, and in-hospital acute kidney injury in older medical patients. Adv Ther (2017) 34(11):2534–41. doi: 10.1007/s12325-017-0629-1
105. Li Z, McKenna Z, Fennel Z, Nava RC, Wells A, Ducharme J, et al. The combined effects of exercise-induced muscle damage and heat stress on acute kidney stress and heat strain during subsequent endurance exercise. Eur J Appl Physiol (2022) 122(5):1239–48. doi: 10.1007/s00421-022-04919-1
106. Haroutounian A, Amorim FT, Astorino TA, Khodiguian N, Curtiss KM, Matthews ARD, et al. Change in exercise performance and markers of acute kidney injury following heat acclimation with permissive dehydration. Nutrients (2021) 13(3):841. doi: 10.3390/nu13030841
107. Ravanelli N, Barry H, Schlader ZJ, Gagnon D. Impact of passive heat acclimation on markers of kidney function during heat stress. Exp Physiol (2021) 106(1):269–81. doi: 10.1113/EP088637
108. Pryor RR, Pryor JL, Vandermark LW, Adams EL, Brodeur RM, Schlader ZJ, et al. Acute kidney injury biomarker responses to short-term heat acclimation. Int J Environ Res Public Health (2020) 17(4):1325. doi: 10.3390/ijerph17041325
109. Omassoli J, Hill NE, Woods DR, Delves SK, Fallowfield JL, Brett SJ, et al. Variation in renal responses to exercise in the heat with progressive acclimatisation. J Sci Med Sport (2019) 22(9):1004–9. doi: 10.1016/j.jsams.2019.04.010
110. Chapman CL, Johnson BD, Sackett JR, Parker MD, Schlader ZJ. Soft drink consumption during and following exercise in the heat elevates biomarkers of acute kidney injury. Am J Physiol Regul Integr Comp Physiol (2019) 316(3):R189–98. doi: 10.1152/ajpregu.00351.2018
111. Remigio RV, Jiang C, Raimann J, Kotanko P, Usvyat L, Maddux FW, et al. Association of extreme heat events with hospital admission or mortality among patients with end-stage renal disease. JAMA Netw Open (2019) 2(8):e198904. doi: 10.1001/jamanetworkopen.2019.8904
112. Chu L, Phung D, Crowley S, Dubrow R. Relationships between short-term ambient temperature exposure and kidney disease hospitalizations in the warm season in Vietnam: A case-crossover study. Environ Res (2022) 209:112776. doi: 10.1016/j.envres.2022.112776
113. Malig BJ, Wu XM, Guirguis K, Gershunov A, Basu R. Associations between ambient temperature and hepatobiliary and renal hospitalizations in California, 1999 to 2009. Environ Res (2019) 177:108566. doi: 10.1016/j.envres.2019.108566
114. Kaufman J, Vicedo-Cabrera AM, Tam V, Song L, Coffel E, Tasian G. The impact of heat on kidney stone presentations in south Carolina under two climate change scenarios. Sci Rep (2022) 12(1):369. doi: 10.1038/s41598-021-04251-2
115. Joint ILO/WHO Committee on Occupational Health, World Health Organization & International Labour Organization. Joint ILO/WHO committee on occupational health : Second report [of a meeting held in Geneva from 6 to 12 October 1952]. Geneva: World Health Organization ([amp]]lrm;1953). Available at: https://apps.who.int/iris/handle/10665/40212.
116. World Health Organization and the International Labour Organization. Caring for those who care: Guide for the development and implementation of occupational health and safety programmes for health workers. Geneva: World Health Organization (2022). Licence: CC BY-NC-SA 3.0 IGO.
117. Zeng X, Liu J, Tao S, Hong HG, Li Y, Fu P. Associations between socioeconomic status and chronic kidney disease: A meta-analysis. J Epidemiol Community Health (2018) 72(4):270–9. doi: 10.1136/jech-2017-209815
118. Tao S, Zeng X, Liu J, Fu P. Socioeconomic status and mortality among dialysis patients: A systematic review and meta-analysis. Int Urol. Nephrol (2019) 51(3):509–18. doi: 10.1007/s11255-019-02078-5
119. Said S, Hernandez GT. Environmental exposures, socioeconomics, disparities, and the kidneys. Adv Chronic. Kidney Dis (2015) 22(1):39–45. doi: 10.1053/j.ackd.2014.09.003
120. Al-Qaoud TM, Nitsch D, Wells J, Witte DR, Brunner EJ. Socioeconomic status and reduced kidney function in the Whitehall II study: Role of obesity and metabolic syndrome. Am J Kidney Dis (2011) 58(3):389–97. doi: 10.1053/j.ajkd.2011.04.017
121. Soderland P, Lovekar S, Weiner DE, Brooks DR, Kaufman JS. Chronic kidney disease associated with environmental toxins and exposures. Adv Chronic. Kidney Dis (2010) 17(3):254–64. doi: 10.1053/j.ackd.2010.03.011
122. Scammell MK, Sennett CM, Petropoulos ZE, Kamal J, Kaufman JS. Environmental and occupational exposures in kidney disease. Semin Nephrol (2019) 39(3):230–43. doi: 10.1016/j.semnephrol.2019.02.001
123. Trabanino RG, Aguilar R, Silva CR, Mercado MO, Merino RL. Nefropatía terminal en pacientes de un hospital de referencia en El Salvador [End-stage renal disease among patients in a referral hospital in El Salvador]. Rev Panam Salud Publica (2002) 12(3):202–6. doi: 10.1590/s1020-49892002000900009
124. World Health Organization. Ten chemicals 10 of major public health concern; public environment. Geneva, Switzerland: WHO (2020). Available at: https://www.who.int/news-room/photo-story/photo-story-detail/10-chemicals-of-public-health-concern.
125. Glicklich D, Frishman WH. The case for cadmium and lead heavy metal screening. Am J Med Sci (2021) 362(4):344–54. doi: 10.1016/j.amjms.2021.05.019
126. Bigazzi PE. Metals and kidney autoimmunity. Environ Health Perspect (1999) 107 Suppl 5(Suppl 5):753–65. doi: 10.1289/ehp.99107s5753
127. Usuda K, Kono K, Ohnishi K, Nakayama S, Sugiura Y, Kitamura Y, et al. Toxicological aspects of cadmium and occupational health activities to prevent workplace exposure in Japan: A narrative review. Toxicol Ind Health (2011) 27(3):225–33. doi: 10.1177/0748233710386404
128. Feng X, Zhou R, Jiang Q, Wang Y, Yu C. Analysis of cadmium accumulation in community adults and its correlation with low-grade albuminuria. Sci Total Environ (2022) 15834:155210. doi: 10.1016/j.scitotenv.2022.155210
129. Kuraeiad S, Kotepui M. Blood lead level and renal impairment among adults: A meta-analysis. Int J Environ Res Public Health (2021) 18(8):4174. doi: 10.3390/ijerph18084174
130. Chen TH, Huang JJ, Lee HY, Kung WS, Luo KH, Lu JY, et al. The association of renal function and plasma metals modified by EGFR and TNF-α gene polymorphisms in metal industrial workers and general population. Int J Environ Res Public Health (2021) 18(17):8965. doi: 10.3390/ijerph18178965
131. Sánchez Rodríguez LH, Medina Pérez OM, Rondón González F, Rincón Cruz G, Rocha Muñoz L, Flórez-Vargas O. Genetic polymorphisms in multispecific transporters mitigate mercury nephrotoxicity in an artisanal and small-scale gold mining community in Colombia. Toxicol Sci (2020) 178(2):338–46. doi: 10.1093/toxsci/kfaa142
132. Evans M, Discacciati A, Quershi AR, Åkesson A, Elinder CG. End-stage renal disease after occupational lead exposure: 20 years of follow-up. Occup Environ Med (2017) 74(6):396–401. doi: 10.1136/oemed-2016-103876
133. Steenland K, Barry V. Chronic renal disease among lead-exposed workers. Occup Environ Med (2020) 77(6):415–7. doi: 10.1136/oemed-2019-106363
134. Golden AP, Milder CM, Ellis ED, Anderson JL, Boice JD Jr, Bertke SJ, et al. Cohort profile: four early uranium processing facilities in the US and Canada. Int J Radiat Biol (2021) 97(6):833–47. doi: 10.1080/09553002.2021.1917786
136. Voss JU, Roller M, Brinkmann E, Mangelsdorf I. Nephrotoxicity of organic solvents: biomarkers for early detection. Int Arch Occup Environ Health (2005) 78(6):475–85. doi: 10.1007/s00420-005-0611-0
138. Riccò M, Peruzzi S, Ranzieri S, Magnavita N. Occupational hantavirus infections in agricultural and forestry workers: A systematic review and metanalysis. Viruses (2021) 13(11):2150. doi: 10.3390/v13112150
139. Ittyachen AM, Krishnapillai TV, Nair MC, Rajan AR. Retrospective study of severe cases of leptospirosis admitted in the intensive care unit. J Postgrad Med (2007) 53(4):232–5. doi: 10.4103/0022-3859.37510
140. Tangkanakul W, Tharmaphornpil P, Plikaytis BD, Bragg S, Poonsuksombat D, Choomkasien P, et al. Risk factors associated with leptospirosis in northeastern Thailand, 1998. Am J Trop Med Hyg (2000) 63(3-4):204–8. doi: 10.4269/ajtmh.2000.63.204
141. Christova I, Tasseva E, Manev H. Human leptospirosis in Bulgaria, 1989-2001: epidemiological, clinical, and serological features. Scand J Infect Dis (2003) 35(11-12):869–72. doi: 10.1080/00365540310016709
142. Roura Carrasco J, Pila Pérez R, Caveda Estela O, Pila Peláez R. Estudio clínico de la leptospirosis humana. a propósito de 215 casos [A clinical study of human leptospirosis. apropos 215 cases]. Rev Clin Esp (1992) 190(8):389–92.
143. Vanasco NB, Schmeling MF, Lottersberger J, Costa F, Ko AI, Tarabla HD. Clinical characteristics and risk factors of human leptospirosis in Argentina (1999-2005). Acta Trop (2008) 107(3):255–8. doi: 10.1016/j.actatropica.2008.06.007
144. Gale DA, Everard CO, Carrington DG, Everard JD. Leptospiral antibodies in patients from a barbadian general practice. Eur J Epidemiol (1990) 6(2):150–5. doi: 10.1007/BF00145787
145. Kupferman T, Coffee MP, Eckhardt BJ. Case report: A cluster of three leptospirosis cases in a new York city abattoir and an unusual complication in the index case. Am J Trop Med Hyg (2017) 97(6):1679–81. doi: 10.4269/ajtmh.17-0545
146. Vickery B, Flynn SA, Calder L, Freebairn RC. Leptospirosis presenting to an intensive care unit in provincial new Zealand: A case series and review. Crit Care Resusc (2006) 8(3):192–9.
147. Yamashita H, Furusu A, Nishino T, Obata Y, Miyazaki M, Ichinose H, et al. Two patients who developed leptospirosis-associated acute renal failure within the same season. Intern Med (2010) 49(12):1143–7. doi: 10.2169/internalmedicine.49.2920
148. Yang HY, Hsu PY, Pan MJ, Wu MS, Lee CH, Yu CC, et al. Clinical distinction and evaluation of leptospirosis in Taiwan–a case-control study. J Nephrol (2005) 18(1):45–53.
149. Wang HK, Lee MH, Chen YC, Hsueh PR, Chang SC. Factors associated with severity and mortality in patients with confirmed leptospirosis at a regional hospital in northern Taiwan. J Microbiol Immunol Infect (2020) 53(2):307–14. doi: 10.1016/j.jmii.2018.05.005
150. Ragnaud JM, Morlat P, Buisson M, Longy-Boursier M, Monlun E, Wone C, et al. Aspects épidémiologiques, cliniques, biologiques et évolutifs de la leptospirose: à propos de 30 observations recueillies en aquitaine [Epidemiological, clinical, biological and developmental aspects of leptospirosis: Apropos of 30 cases in aquitaine]. Rev Med Interne (1994) 15(7):452–9. doi: 10.1016/s0248-8663(05)81469-9
151. Schillinger F, Babeau N, Montagnac R, Milcent T. Les Formes rénales graves de la leptospirose. a propos de six cas recueillis en quinze ans dans un même service [Severe renal forms of leptospirosis. apropos of 6 cases seen in 15 years at one center]. Nephrologie (1999) 20(2):81–6.
152. Strady C, Penalba C, Baumard S, Brodard V, Wynckel A, Auvray C, et al. Nephropathia epidemica and leptospirosis in champagne-ardenne, France: comparison of clinical, biological and epidemiological profiles. Eur J Clin Microbiol Infect Dis (2009) 28(7):825–9. doi: 10.1007/s10096-009-0716-6
153. Miailhe AF, Mercier E, Maamar A, Lacherade JC, Le Thuaut A, Gaultier A, et al. Severe leptospirosis in non-tropical areas: A nationwide, multicentre, retrospective study in French ICUs. Intensive Care Med (2019) 45(12):1763–73. doi: 10.1007/s00134-019-05808-6
154. Gentile G, Tong C, Renaud C, Menoud N, Casanova L, Blatteau JE, et al. Incidence of leptospirosis in the French armed forces from 2004 to 2018: Retrospective analysis. Travel Med Infect Dis (2021) 39:101951. doi: 10.1016/j.tmaid.2020.101951
155. Bek M, Köppl H, Schwarzkopf G, Freudenberg N. Weil'sche erkrankung und nekrotisierende herpespneumonie als todesursache bei einem 39 jahre alt gewordenen kanalarbeiter [Weil's disease and necrotizing herpes pneumonia as the cause of death of a 39-year-old sewage drain worker]. Pathologe (1996) 17(6):471–6. doi: 10.1007/s002920050190
156. Olszyna DP, Jaspars R, Speelman P, van Elzakker E, Korver H, Hartskeerl RA. Leptospirose in nederland 1991-1995 [Leptospirosis in the Netherlands, 1991-1995]. Ned. Tijdschr. Geneeskd (1998) 142(22):1270–3.
157. Kolwijck E, Dofferhoff AS, van de Leur J, Meis JF. Leptospirosis in a Dutch catfish farm. Neth. J Med (2011) 69(4):201–4.
158. Rönsholt FF, Seidelin JB, Villumsen S. Samtidig leptospirose og salmonellainfektion [Concurrent leptospirosis and salmonella infection]. Ugeskr Laeger (2009) 171(19):1607–9.
159. Riefkohl A, Ramírez-Rubio O, Laws RL, McClean MD, Weiner DE, Kaufman JS, et al. Leptospira seropositivity as a risk factor for mesoamerican nephropathy. Int J Occup Environ Health (2017) 23(1):1–10. doi: 10.1080/10773525.2016.1275462
160. Yih WK, Kulldorff M, Friedman DJ, Leibler JH, Amador JJ, López-Pilarte D, et al. Investigating possible infectious causes of chronic kidney disease of unknown etiology in a Nicaraguan mining community. Am J Trop Med Hyg (2019) 101(3):676–83. doi: 10.4269/ajtmh.18-0856
161. Wanigasuriya KP, Peiris-John RJ, Wickremasinghe R, Hittarage A. Chronic renal failure in north central province of Sri Lanka: An environmentally induced disease. Trans R Soc Trop Med Hyg (2007) 101(10):1013–7. doi: 10.1016/j.trstmh.2007.05.006
162. Jayasumana C, Paranagama P, Agampodi S, Wijewardane C, Gunatilake S, Siribaddana S. Drinking well water and occupational exposure to herbicides is associated with chronic kidney disease, in padavi-sripura, Sri Lanka. Environ Health (2015) 14:6. doi: 10.1186/1476-069X-14-6
163. Badurdeen Z, Nanayakkara N, Ratnatunga NV, Wazil AW, Abeysekera TD, Rajakrishna PN, et al. Chronic kidney disease of uncertain etiology in Sri Lanka is a possible sequel of interstitial nephritis! Clin Nephrol (2016) 86(13):106–9. doi: 10.5414/CNP86S115
164. de Silva MW, Albert SM, Jayasekara JM. Structural violence and chronic kidney disease of unknown etiology in Sri Lanka. Soc Sci Med (2017) 178:184–95. doi: 10.1016/j.socscimed.2017.02.016
165. Gobalarajah K, Subramaniam P, Jayawardena UA, Rasiah G, Rajendra S, Prabagar J. Impact of water quality on chronic kidney disease of unknown etiology (CKDu) in thunukkai division in mullaitivu district, Sri Lanka. BMC Nephrol (2020) 21(1):507. doi: 10.1186/s12882-020-02157-1
166. Abdul KSM, De Silva PMCS, Ekanayake EMDV, Thakshila WAKG, Gunarathna SD, Gunasekara TDKSC, et al. Occupational paraquat and glyphosate exposure may decline renal functions among rural farming communities in Sri Lanka. Int J Environ Res Public Health (2021) 18(6):3278. doi: 10.3390/ijerph18063278
167. Ekanayake EMDV, Gunasekara TDKSC, De Silva PMCS, Jayasinghe S, Chandana EPS, Jayasundara N. Applicability of novel urinary biomarkers for the assessment of renal injury in selected occupational groups in Sri Lanka: A comparative study with conventional markers. Int J Environ Res Public Health (2022) 19(9):5264. doi: 10.3390/ijerph19095264
168. Faleel RA, Jayawardena UA. Progression of potential etiologies of the chronic kidney disease of unknown etiology in Sri Lanka. J Environ Sci Health C Toxicol Carcinog (2020) 38(4):362–83. doi: 10.1080/26896583.2020.1852012
169. Rajapakse S, Shivanthan MC, Selvarajah M. Chronic kidney disease of unknown etiology in Sri Lanka. Int J Occup Environ Health (2016) 22(3):259–64. doi: 10.1080/10773525.2016.1203097
170. Kaur P, Gunawardena N, Kumaresan J. A review of chronic kidney disease of unknown etiology in Sri Lanka, 2001-2015. Indian J Nephrol (2020) 30(4):245–52. doi: 10.4103/ijn.IJN_359_18
171. Lunyera J, Mohottige D, Von Isenburg M, Jeuland M, Patel UD, Stanifer JW. CKD of uncertain etiology: A systematic review. Clin J Am Soc Nephrol (2016) 11(3):379–85. doi: 10.2215/CJN.07500715
172. Jayasumana C, Orantes C, Herrera R, Almaguer M, Lopez L, Silva LC, et al. Chronic interstitial nephritis in agricultural communities: A worldwide epidemic with social, occupational and environmental determinants. Nephrol Dial Transplant (2017) 32(2):234–41. doi: 10.1093/ndt/gfw346
173. Orantes-Navarro CM, Herrera-Valdés R, Almaguer-López M, López-Marín L, Vela-Parada XF, Hernandez-Cuchillas M, et al. Toward a comprehensive hypothesis of chronic interstitial nephritis in agricultural communities. Adv Chronic. Kidney Dis (2017) 24(2):101–6. doi: 10.1053/j.ackd.2017.01.001
174. Malieckal DA, Goldfarb DS. Occupational kidney stones. Curr Opin Nephrol Hypertens (2020) 29(2):232–6. doi: 10.1097/MNH.0000000000000581
175. Torres C, Aragón A, González M, et al. Decreased kidney function of unknown cause in Nicaragua: A community-based survey. Am J Kidney Dis (2010) 55(3):485–96. doi: 10.1053/j.ajkd.2009.12.012
176. Sanoff SL, Callejas L, Alonso CD, et al. Positive association of renal insufficiency with agriculture employment and unregulated alcohol consumption in Nicaragua. Ren Fail (2010) 32(7):766–77. doi: 10.3109/0886022X.2010.494333
177. Laux TS, Bert PJ, Barreto Ruiz GM, et al. Nicaragua Revisited: evidence of lower prevalence of chronic kidney disease in a high-altitude, coffee-growing village. J Nephrol (2012) 25(4):533–40. doi: 10.5301/jn.5000028
178. Ramirez-Rubio O, Brooks DR, Amador JJ, Kaufman JS, Weiner DE, Scammell MK. Chronic kidney disease in Nicaragua: A qualitative analysis of semi-structured interviews with physicians and pharmacists. BMC Public Health (2013) 13:350. doi: 10.1186/1471-2458-13-350
179. Raines N, González M, Wyatt C, et al. Risk factors for reduced glomerular filtration rate in a Nicaraguan community affected by mesoamerican nephropathy. MEDICC Rev (2014) 16(2):16–22. doi: 10.37757/MR2014.V16.N2.4
180. Laws RL, Brooks DR, Amador JJ, et al. Changes in kidney function among Nicaraguan sugarcane workers. Int J Occup Environ Health (2015) 21(3):241–50. doi: 10.1179/2049396714Y.0000000102
181. Ramírez-Rubio O, Amador JJ, Kaufman JS, et al. Urine biomarkers of kidney injury among adolescents in Nicaragua, a region affected by an epidemic of chronic kidney disease of unknown aetiology. Nephrol Dial Transplant (2016) 31(3):424–32. doi: 10.1093/ndt/gfv292
182. Laws RL, Brooks DR, Amador JJ, et al. Biomarkers of kidney injury among Nicaraguan sugarcane workers. Am J Kidney Dis (2016) 67(2):209–17. doi: 10.1053/j.ajkd.2015.08.022
183. Wesseling C, Aragón A, González M, et al. Kidney function in sugarcane cutters in Nicaragua–a longitudinal study of workers at risk of mesoamerican nephropathy. Environ Res (2016) 147:125–32. doi: 10.1016/j.envres.2016.02.002
184. Wesseling C, Aragón A, González M, et al. Heat stress, hydration and uric acid: A cross-sectional study in workers of three occupations in a hotspot of mesoamerican nephropathy in Nicaragua [published correction appears in BMJ open. BMJ Open (2016) 6(12):e011034. doi: 10.1136/bmjopen-2016-011034
185. Wijkström J, González-Quiroz M, Hernandez M, et al. Renal morphology, clinical findings, and progression rate in mesoamerican nephropathy. Am J Kidney Dis (2017) 69(5):626–36. doi: 10.1053/j.ajkd.2016.10.036
186. Kupferman J, Ramírez-Rubio O, Amador JJ, et al. Acute kidney injury in sugarcane workers at risk for mesoamerican nephropathy. Am J Kidney Dis (2018) 72(4):475–82. doi: 10.1053/j.ajkd.2018.04.014
187. Gonzalez-Quiroz M, Smpokou ET, Silverwood RJ, et al. Decline in kidney function among apparently healthy young adults at risk of mesoamerican nephropathy. J Am Soc Nephrol (2018) 29(8):2200–12. doi: 10.1681/ASN.2018020151
188. Gallo-Ruiz L, Sennett CM, Sánchez-Delgado M, et al. Prevalence and risk factors for CKD among brickmaking workers in la paz centro, Nicaragua. Am J Kidney Dis (2019) 74(2):239–47. doi: 10.1053/j.ajkd.2019.01.017
189. Hansson E, Glaser J, Weiss I, et al. Workload and cross-harvest kidney injury in a Nicaraguan sugarcane worker cohort. Occup Environ Med (2019) 76(11):818–26. doi: 10.1136/oemed-2019-105986
190. Petropoulos ZE, Laws RL, Amador JJ, et al. Kidney function, self-reported symptoms, and urine findings in Nicaraguan sugarcane workers. Kidney360 (2020) 1(10):1042–51. doi: 10.34067/KID.0003392020
191. Ferguson R, Leatherman S, Fiore M, et al. Prevalence and risk factors for CKD in the general population of southwestern Nicaragua. J Am Soc Nephrol (2020) 31(7):1585–93. doi: 10.1681/ASN.2019050521
192. Fischer RSB, Unrine JM, Vangala C, Sanderson WT, Mandayam S, Murray KO. Evidence of nickel and other trace elements and their relationship to clinical findings in acute mesoamerican nephropathy: A case-control analysis. PloS One (2020) 15(11):e0240988. doi: 10.1371/journal.pone.0240988
193. Smpokou ET, González-Quiroz M, Martins C, et al. Environmental exposures in young adults with declining kidney function in a population at risk of mesoamerican nephropathy. Occup Environ Med (2019) 76(12):920–6. doi: 10.1136/oemed-2019-105772
194. Glaser J, Hansson E, Weiss I, et al. Preventing kidney injury among sugarcane workers: promising evidence from enhanced workplace interventions. Occup Environ Med (2020) 77(8):527–34. doi: 10.1136/oemed-2020-106406
195. Leibler JH, Ramirez-Rubio O, Velázquez JJA, et al. Biomarkers of kidney injury among children in a high-risk region for chronic kidney disease of uncertain etiology. Pediatr Nephrol (2021) 36(2):387–96. doi: 10.1007/s00467-020-04595-3
196. Petropoulos ZE, Ramirez-Rubio O, Scammell MK, et al. Climate trends at a hotspot of chronic kidney disease of unknown causes in Nicaragua, 1973-2014. Int J Environ Res Public Health (2021) 18(10):5418. doi: 10.3390/ijerph18105418
197. Stallings TL, Riefkohl Lisci A, McCray NL, et al. Dysuria, heat stress, and muscle injury among Nicaraguan sugarcane workers at risk for mesoamerican nephropathy. Scand J Work Environ Health (2021) 47(5):377–86. doi: 10.5271/sjweh.3963
198. Pacheco-Zenteno F, Glaser J, Jakobsson K, Weiss I, Arias-Monge E, Gyllensten K. The prevention of occupational heat stress in sugarcane workers in Nicaragua-an interpretative phenomenological analysis. Front Public Health (2021) 9:713711. doi: 10.3389/fpubh.2021.713711
199. Hansson E, Wegman DH, Wesseling C, et al. Markers of kidney tubular and interstitial injury and function among sugarcane workers with cross-harvest serum creatinine elevation. Occup Environ Med (2022) 79(6):396–402. doi: 10.1136/oemed-2021-107989
200. Keogh SA, Leibler JH, Sennett Decker CM, et al. High prevalence of chronic kidney disease of unknown etiology among workers in the mesoamerican nephropathy occupational study. BMC Nephrol (2022) 23(1):238. doi: 10.1186/s12882-022-02861-0
201. Andersson A, Hansson E, Ekström U, et al. Large Difference but high correlation between creatinine and cystatin c estimated glomerular filtration rate in mesoamerican sugarcane cutters. Occup Environ Med (2022) 79(7):497–502. doi: 10.1136/oemed-2021-107990
202. Gracia-Trabanino R, Domínguez J, Jansà JM, Oliver A. Proteinuria e insuficiencia renal crónica en la costa de El Salvador: Detección con métodos de bajo costo y factores asociados [Proteinuria and chronic renal failure in the coast of El Salvador: Detection with low cost methods and associated factors]. Nefrologia (2005) 25(1):31–8.
203. Orantes CM, Herrera R, Almaguer M, et al. Chronic kidney disease and associated risk factors in the bajo lempa region of El Salvador: Nefrolempa study, 2009. MEDICC Rev (2011) 13(4):14–22. doi: 10.37757/MR2011V13.N4.5
204. Peraza S, Wesseling C, Aragon A, et al. Decreased kidney function among agricultural workers in El Salvador. Am J Kidney Dis (2012) 59(4):531–40. doi: 10.1053/j.ajkd.2011.11.039
205. Wijkström J, Leiva R, Elinder CG, et al. Clinical and pathological characterization of mesoamerican nephropathy: A new kidney disease in central America. Am J Kidney Dis (2013) 62(5):908–18. doi: 10.1053/j.ajkd.2013.05.019
206. Vela XF, Henríquez DO, Zelaya SM, Granados DV, Hernández MX, Orantes CM. Chronic kidney disease and associated risk factors in two Salvadoran farming communities, 2012. MEDICC Rev (2014) 16(2):55–60. doi: 10.37757/MR2014.V16.N2.9
207. López-Marín L, Chávez Y, García XA, et al. Histopathology of chronic kidney disease of unknown etiology in Salvadoran agricultural communities. MEDICC Rev (2014) 16(2):49–54. doi: 10.37757/MR2014.V16.N2.8
208. Orantes CM, Herrera R, Almaguer M, et al. Epidemiology of chronic kidney disease in adults of Salvadoran agricultural communities [published correction appears in MEDICC rev. MEDICC Rev (2014) 16(2):23–30. doi: 10.37757/MR2014.V16.N2.5
209. Herrera Valdés R, Orantes CM, Almaguer López M, et al. Clinical characteristics of chronic kidney disease of non-traditional causes in women of agricultural communities in El Salvador. Clin Nephrol (2015) 83(7 Suppl 1):56–63. doi: 10.5414/cnp83s056
210. Orantes Navarro CM, Herrera Valdés R, López MA, et al. Epidemiological characteristics of chronic kidney disease of non-traditional causes in women of agricultural communities of El Salvador. Clin Nephrol (2015) 83(7 Suppl 1):24–31. doi: 10.5414/cnp83s024
211. Wegman DH, Apelqvist J, Bottai M, et al. Intervention to diminish dehydration and kidney damage among sugarcane workers. Scand J Work Environ Health (2018) 44(1):16–24. doi: 10.5271/sjweh.3659
212. Laux TS, Barnoya J, Cipriano E, et al. Prevalence of chronic kidney disease of non-traditional causes in patients on hemodialysis in southwest Guatemala. Rev Panam Salud Publica (2016) 39(4):186–93.
213. Butler-Dawson J, Krisher L, Asensio C, et al. Risk factors for declines in kidney function in sugarcane workers in Guatemala. J Occup Environ Med (2018) 60(6):548–58. doi: 10.1097/JOM.0000000000001284
214. Butler-Dawson J, Barnoya J, Brindley S, et al. Cross-sectional study examining the accuracy of self-reported smoking status as compared to urinary cotinine levels among workers at risk for chronic kidney disease of unknown origin in Guatemala. BMJ Open (2021) 11(10):e050374. doi: 10.1136/bmjopen-2021-050374
215. Crowe J, Wesseling C, Solano BR, et al. Heat exposure in sugarcane harvesters in Costa Rica. Am J Ind Med (2013) 56(10):1157–64. doi: 10.1002/ajim.22204
216. Crowe J, Nilsson M, Kjellstrom T, Wesseling C. Heat-related symptoms in sugarcane harvesters. Am J Ind Med (2015) 58(5):541–8. doi: 10.1002/ajim.22450
217. Wesseling C, van Wendel de Joode B, Crowe J, et al. Mesoamerican nephropathy: Geographical distribution and time trends of chronic kidney disease mortality between 1970 and 2012 in Costa Rica. Occup Environ Med (2015) 72(10):714–21. doi: 10.1136/oemed-2014-102799
218. Hansson E, Mansourian A, Farnaghi M, Petzold M, Jakobsson K. An ecological study of chronic kidney disease in five mesoamerican countries: Associations with crop and heat. BMC Public Health (2021) 21(1):840. doi: 10.1186/s12889-021-10822-9
219. Wesseling C, Crowe J, Hogstedt C, et al. Resolving the enigma of the mesoamerican nephropathy: A research workshop summary. Am J Kidney Dis (2014) 63(3):396–404. doi: 10.1053/j.ajkd.2013.08.014
220. Herrera-Valdés R, Almaguer-López MA, Orantes-Navarro CM, et al. Epidemic of chronic kidney disease of nontraditional etiology in El Salvador: Integrated health sector action and south-south cooperation. MEDICC Rev (2019) 21(4):46–52. doi: 10.37757/MR2019.V21.N4.8
221. Ordunez P, Nieto FJ, Martinez R, et al. Chronic kidney disease mortality trends in selected central America countries, 1997-2013: clues to an epidemic of chronic interstitial nephritis of agricultural communities. J Epidemiol Community Health (2018) 72(4):280–6. doi: 10.1136/jech-2017-210023
222. González-Quiroz M, Pearce N, Caplin B, Nitsch D. What do epidemiological studies tell us about chronic kidney disease of undetermined cause in meso-America? a systematic review and meta-analysis. Clin Kidney J (2018) 11(4):496–506. doi: 10.1093/ckj/sfx136
223. Wesseling C, Glaser J, Rodríguez-Guzmán J, et al. Chronic kidney disease of non-traditional origin in mesoamerica: A disease primarily driven by occupational heat stress. Rev Panam Salud Publica (2020) 44:e15. doi: 10.26633/RPSP.2020.15
224. Levi M, Kjellstrom T, Baldasseroni A. Impact of climate change on occupational health and productivity: A systematic literature review focusing on workplace heat. Med Lav (2018) 109(3):163–79. doi: 10.23749/mdl.v109i3.6851
225. Flouris AD, Dinas PC, Ioannou LG, et al. Workers' health and productivity under occupational heat strain: A systematic review and meta-analysis. Lancet Planet Health (2018) 2(12):e521–31. doi: 10.1016/S2542-5196(18)30237-7
226. El Khayat M, Halwani DA, Hneiny L, Alameddine I, Haidar MA, Habib RR. Impacts of climate change and heat stress on farmworkers' health: A scoping review. Front Public Health (2022) 8:782811. doi: 10.3389/fpubh.2022.782811
227. Hansson E, Glaser J, Jakobsson K, et al. Pathophysiological mechanisms by which heat stress potentially induces kidney inflammation and chronic kidney disease in sugarcane workers. Nutrients (2020) 12(6):1639. doi: 10.3390/nu12061639
228. Venugopal V, Latha PK, Shanmugam R, et al. Risk of kidney stone among workers exposed to high occupational heat stress - a case study from southern Indian steel industry. Sci Total Environ (2020) 722:137619. doi: 10.1016/j.scitotenv.2020.137619
229. Singh S, Kumar V, Thakur S, et al. DNA Damage and cholinesterase activity in occupational workers exposed to pesticides. Environ Toxicol Pharmacol (2011) 31(2):278–85. doi: 10.1016/j.etap.2010.11.005
230. Singh A, Kamal R, Mudiam MK, et al. Heat and PAHs emissions in indoor kitchen air and its impact on kidney dysfunctions among kitchen workers in lucknow, north India. PloS One (2016) 11(2):e0148641. doi: 10.1371/journal.pone.0148641
231. Mohanty NK, Sahoo KC, Pati S, Sahu AK, Mohanty R. Prevalence of chronic kidney disease in cuttack district of odisha, India. Int J Environ Res Public Health (2020) 17(2):456. doi: 10.3390/ijerph17020456
232. Farag YMK, Karai Subramanian K, Singh VA, Tatapudi RR, Singh AK. Occupational risk factors for chronic kidney disease in andhra pradesh: 'Uddanam nephropathy'. Ren Fail (2020) 42(1):1032–41. doi: 10.1080/0886022X.2020.1824924
233. Venugopal V, Latha PK, Shanmugam R, et al. Epidemiological evidence from south Indian working population-the heat exposures and health linkage. J Expo Sci Environ Epidemiol (2021) 31(1):177–86. doi: 10.1038/s41370-020-00261-w
234. Venugopal V, Lennqvist R, Latha PK, et al. Challenges in conducting epidemiological field studies evaluating associations between heat stress and renal health among workers in informal sectors: experiences from India. Environ Res (2021) 200:111343. doi: 10.1016/j.envres.2021.111343
235. Honda R, Swaddiwudhipong W, Nishijo M, et al. Cadmium induced renal dysfunction among residents of rice farming area downstream from a zinc-mineralized belt in Thailand. Toxicol Lett (2010) 198(1):26–32. doi: 10.1016/j.toxlet.2010.04.023
236. Mueangkhiao P, Siviroj P, Sapbamrer R, et al. Biological variation in kidney injury and kidney function biomarkers among farmers in lamphun province, Thailand. Environ Sci pollut Res Int (2020) 27(11):12386–94. doi: 10.1007/s11356-020-07661-3
237. Arphorn S, Manothum A, Santiwung K, Pangunta K, Hara K, Ishimaru T. Working conditions and urinalysis dipstick testing among female rice farmers: A preliminary cross-sectional study. Int J Environ Res Public Health (2021) 18(17):8942. doi: 10.3390/ijerph18178942
238. Devkota HR, Bhandari B, Adhikary P. Perceived mental health, wellbeing and associated factors among Nepali male migrant and non-migrant workers: A qualitative study. J Migr Health (2020) 4:S100013. doi: 10.1016/j.jmh.2020.100013
239. Yang HY, Wang JD, Lo TC, Chen PC. Occupational kidney disease among Chinese herbalists exposed to herbs containing aristolochic acids. Occup Environ Med (2011) 68(4):286–90. doi: 10.1136/oem.2010.058594
240. Wang T, Jia G, Zhang J, et al. Renal impairment caused by chronic occupational chromate exposure. Int Arch Occup Environ Health (2011) 84(4):393–401. doi: 10.1007/s00420-010-0569-4
241. Ou S, He H, Qu L, Wu W, Gan L, Liu J. Renal pathology in patients with occupational exposure to carbon disulphide: A case series. Nephrol (Carlton) (2017) 22(10):755–60. doi: 10.1111/nep.12853
242. Shen Q, Jin W, Ji S, Chen X, Zhao X, Behera TR. The association between socioeconomic status and prevalence of chronic kidney disease: A cross-sectional study among rural residents in eastern China. Med (Baltimore) (2019) 98(11):e14822. doi: 10.1097/MD.0000000000014822
243. Zhang S, Wang Y, Zhu Y, Li X, Song Y, Yuan J. Rotating night shift work, exposure to light at night, and glomerular filtration rate: Baseline results from a Chinese occupational cohort. Int J Environ Res Public Health (2020) 17(23):9035. doi: 10.3390/ijerph17239035
244. Yang X, Wu H, Li H. Dehydration-associated chronic kidney disease: A novel case of kidney failure in China. BMC Nephrol (2020) 21(1):159. doi: 10.1186/s12882-020-01804-x
245. Mourad BH, Ashour YA. Demonstration of subclinical early nephrotoxicity induced by occupational exposure to silica among workers in pottery industry. Int J Occup Environ Med (2020) 11(2):85–94. doi: 10.34172/ijoem.2020.1886
246. Mwangi DM, Njagi LJ, Mcligeyo SO, et al. Subclinical nephrotoxicity associated with occupational silica exposure among male Kenyan industrial workers. East Afr Med J (2009) 86(5):244–50. doi: 10.4314/eamj.v86i5.54198
247. Hamilton SA, Nakanga WP, Prynn JE, et al. Prevalence and risk factors for chronic kidney disease of unknown cause in Malawi: A cross-sectional analysis in a rural and urban population. BMC Nephrol (2020) 21(1):387. doi: 10.1186/s12882-020-02034-x
248. Adjei DN, Stronks K, Adu D, et al. Cross-sectional study of association between socioeconomic indicators and chronic kidney disease in rural-urban Ghana: The RODAM study. BMJ Open (2019) 9(5):e022610. doi: 10.1136/bmjopen-2018-022610
249. Ngajilo D, Ehrlich R. Rhabdomyolysis with acute tubular necrosis following occupational inhalation of thinners. Occup Med (Lond) (2017) 67(5):401–3. doi: 10.1093/occmed/kqx048
250. Okaka EI, Unuigbe EI. Eight year review of hemodialysis: Treated patients in a tertiary center in southern Nigeria. Ann Afr Med (2014) 13(4):221–5. doi: 10.4103/1596-3519.142295
251. Ben Khadda Z, Berni I, Sqalli Houssaini T. Prevalence and risk factors associated with chronic kidney disease in Moroccan rural communes: Fez-meknes region. Nephrol Ther (2022) 18(2):121–8. doi: 10.1016/j.nephro.2021.11.005
252. Balali-Mood M, Shademanfar S, Rastegar Moghadam J, et al. Occupational lead poisoning in workers of traditional tile factories in mashhad, northeast of Iran. Int J Occup Environ Med (2010) 1(1):29–38.
253. Aksoy N, Şelimen D. Investigation of the causes and risk factors of previous end-stage renal disease in kidney transplant recipients. Transplant Proc (2020) 52(1):140–5. doi: 10.1016/j.transproceed.2019.11.019
254. González-Yebra AL, Kornhauser C, Wrobel K, Pérez-Luque EL, Wrobel K, Barbosa G. Occupational exposure to toluene and its possible causative role in renal damage development in shoe workers. Int Arch Occup Environ Health (2006) 79(3):259–64. doi: 10.1007/s00420-005-0001-7
255. Christensen DL, Espino D, Infante-Ramírez R, et al. Normalization of elevated cardiac, kidney, and hemolysis plasma markers within 48 h in Mexican tarahumara runners following a 78 km race at moderate altitude. Am J Hum Biol (2014) 26(6):836–43. doi: 10.1002/ajhb.22607
256. Pérez-Herrera N, Díaz de León-Martínez L, Flores-Ramírez R, et al. Evaluation of benzene exposure and early biomarkers of kidney damage in children exposed to solvents due to precarious work in ticul, yucatán, méxico. Ann Glob Health (2019) 85(1):94. doi: 10.5334/aogh.2482
257. Aguilar-Ramirez D, Raña-Custodio A, Villa A, et al. Decreased kidney function and agricultural work: A cross-sectional study in middle-aged adults from tierra blanca, Mexico. Nephrol Dial Transplant (2021) 36(6):1030–8. doi: 10.1093/ndt/gfaa041
258. López-Gálvez N, Wagoner R, Canales RA, Ernst K, Burgess JL, de Zapien J, et al. Longitudinal assessment of kidney function in migrant farm workers. Environ Res (2021) 202:111686. doi: 10.1016/j.envres.2021.111686
259. Díaz de León-Martínez L, Ortega-Romero MS, Barbier OC, et al. Evaluation of hydroxylated metabolites of polycyclic aromatic hydrocarbons and biomarkers of early kidney damage in indigenous children from ticul, yucatán, Mexico. Environ Sci pollut Res Int (2021) 28(37):52001–13. doi: 10.1007/s11356-021-14460-x
260. Nerbass FB, Moist L, Clark WF, Vieira MA, Pecoits-Filho R. Hydration status and kidney health of factory workers exposed to heat stress: A pilot feasibility study. Ann Nutr Metab (2019) 74 Suppl 3:30–7. doi: 10.1159/000500373
261. Prudente IRG, Souza BRS, Nascimento LC, et al. Nephrotoxic effects caused by occupational exposure to agrochemicals in a region of northeastern Brazil: A cross-sectional study. Environ Toxicol Chem (2021) 40(4):1132–8. doi: 10.1002/etc.4962
262. Almaguer M, Herrera R, Orantes CM. Chronic kidney disease of unknown etiology in agricultural communities. MEDICC Rev (2014) 16(2):9–15. doi: 10.37757/MR2014.V16.N2.3
263. John O, Gummudi B, Jha A, et al. Chronic kidney disease of unknown etiology in India: What do we know and where we need to go. Kidney Int Rep (2021) 6(11):2743–51. doi: 10.1016/j.ekir.2021.07.031
264. Zhang Q, Liu X, Gao M, et al. The study of human serum metabolome on the health effects of glyphosate and early warning of potential damage. Chemosphere (2022) 298:134308. doi: 10.1016/j.chemosphere.2022.134308
265. Aguilar DJ, Madero M. Other potential CKD hotspots in the world: The cases of Mexico and the united states. Semin Nephrol (2019) 39(3):300–7. doi: 10.1016/j.semnephrol.2019.02.008
266. Garcia-Garcia G, Gutiérrez-Padilla A, Perez-Gomez HR, et al. Chronic kidney disease of unknown cause in Mexico: The case of poncitlan, jalisco. Clin Nephrol (2020) 93(1):42–8. doi: 10.5414/CNP92S107
267. Prudente IRG, Cruz CL, Nascimento LC, Kaiser CC, Guimarães AG. Evidence of risks of renal function reduction due to occupational exposure to agrochemicals: A systematic review. Environ Toxicol Pharmacol (2018) 63:21–8. doi: 10.1016/j.etap.2018.08.006
268. Steenland NK, Thun MJ, Ferguson CW, Port FK. Occupational and other exposures associated with male end-stage renal disease: A case/control study. Am J Public Health (1990) 80(2):153–7. doi: 10.2105/ajph.80.2.153
269. Vupputuri S, Parks CG, Nylander-French LA, Owen-Smith A, Hogan SL, Sandler DP. Occupational silica exposure and chronic kidney disease. Ren Fail (2012) 34(1):40–6. doi: 10.3109/0886022X.2011.623496
270. Sponholtz TR, Sandler DP, Parks CG, Applebaum KM. Occupational exposures and chronic kidney disease: Possible associations with endotoxin and ultrafine particles. Am J Ind Med (2016) 59(1):1–11. doi: 10.1002/ajim.22541
271. Moyce S, Joseph J, Tancredi D, Mitchell D, Schenker M. Cumulative incidence of acute kidney injury in california's agricultural workers. J Occup Environ Med (2016) 58(4):391–7. doi: 10.1097/JOM.0000000000000668
272. Moyce S, Mitchell D, Armitage T, Tancredi D, Joseph J, Schenker M. Heat strain, volume depletion and kidney function in California agricultural workers. Occup Environ Med (2017) 74(6):402–9. doi: 10.1136/oemed-2016-103848
273. Mix J, Elon L, Vi Thien Mac V, et al. Hydration status, kidney function, and kidney injury in Florida agricultural workers. J Occup Environ Med (2018) 60(5):e253–60. doi: 10.1097/JOM.0000000000001261
274. Murray KE, Buul A, Aden R, et al. Occupational health risks and intervention strategies for US taxi drivers. Health Promot Int (2019) 34(2):323–32. doi: 10.1093/heapro/dax082
275. Chapman CL, Johnson BD, Vargas NT, Hostler D, Parker MD, Schlader ZJ. Both hyperthermia and dehydration during physical work in the heat contribute to the risk of acute kidney injury. J Appl Physiol (1985) (2020) 128(4):715–28. doi: 10.1152/japplphysiol.00787.2019
276. Moyce S, Armitage T, Mitchell D, Schenker M. Acute kidney injury and workload in a sample of California agricultural workers. Am J Ind Med (2020) 63(3):258–68. doi: 10.1002/ajim.23076
277. Moyce S, Mitchell D, Vega A, Schenker M. Hydration choices, sugary beverages, and kidney injury in agricultural workers in California. J Nurs Scholarsh (2020) 52(4):369–78. doi: 10.1111/jnu.12561
278. Boyle SM, Zhao Y, Chou E, Moore K, Harhay MN. Neighborhood context and kidney disease in Philadelphia. SSM Popul Health (2020) 12:100646. doi: 10.1016/j.ssmph.2020.100646
279. Shrestha D, Picciotto S, LaValley MP, Liu S, Hammond SK, Weiner DE, et al. End-stage renal disease and metalworking fluid exposure. Occup Environ Med (2022) 79(1):24–31. doi: 10.1136/oemed-2020-106715
280. Rao N, Bendall A, Lanteri M. And IgA nephropathy linked to silica exposure. Occup Med (Lond) (2020) 70(6):445–8. doi: 10.1093/occmed/kqaa122
281. Tsurugano S, Nakao M, Takeuchi T, Nomura K, Yano E. Job stress strengthens the link between metabolic risk factors and renal dysfunction in adult men. Tohoku J Exp Med (2012) 226(2):101–8. doi: 10.1620/tjem.226.101
282. Noborisaka Y, Ishizaki M, Yamada Y, et al. Distribution of and factors contributing to chronic kidney disease in a middle-aged working population. Environ Health Prev Med (2013) 18(6):466–76. doi: 10.1007/s12199-013-0343-9
283. Lee J, Lee JP, An JN, et al. Factors affecting the referral time to nephrologists in patients with chronic kidney disease: A prospective cohort study in Korea. Med (Baltimore) (2016) 95(19):e3648. doi: 10.1097/MD.0000000000003648
284. Lee DW, Lee J, Kim HR, Jun KY, Kang MY. Long work hours and decreased glomerular filtration rate in the Korean working population. Occup Environ Med (2020) 77(10):699–705. doi: 10.1136/oemed-2020-106428
285. Kim YJ, Choi WJ, Ham S, Kang SK, Lee W. Association between occupational or environmental noise exposure and renal function among middle-aged and older Korean adults: A cross-sectional study. Sci Rep (2021) 11(1):24127. doi: 10.1038/s41598-021-03647-4
286. Chen SC, Chang JM, Lin MY, et al. Association of metabolic syndrome and albuminuria with cardiovascular risk in occupational drivers. Int J Mol Sci (2013) 14(11):21997–2010. doi: 10.3390/ijms141121997
287. Wu CF, Peng CY, Liu CC, et al. Ambient melamine exposure and urinary biomarkers of early renal injury. J Am Soc Nephrol (2015) 26(11):2821–9. doi: 10.1681/ASN.2014121233
288. Chuang KJ, Pan CH, Su CL, et al. Urinary neutrophil gelatinase-associated lipocalin is associated with heavy metal exposure in welding workers. Sci Rep (2015) 5:18048. doi: 10.1038/srep18048
289. Pai P, Stevenson A, Mason H, Bell GM. Occupational hydrocarbon exposure and nephrotoxicity: A cohort study and literature review. Postgrad Med J (1998) 74(870):225–8. doi: 10.1136/pgmj.74.870.225
290. Al-Qaoud TM, Nitsch D, Wells J, Witte DR, Brunner EJ. Socioeconomic status and reduced kidney function in the Whitehall II study: Role of obesity and metabolic syndrome. Am J Kidney Dis (2011) 58(3):389–97. doi: 10.1053/j.ajkd.2011.04.017
291. Jacob S, Héry M, Protois JC, Rossert J, Stengel B. New insight into solvent-related end-stage renal disease: Occupations, products and types of solvents at risk. Occup Environ Med (2007) 64(12):843–8. doi: 10.1136/oem.2007.032482
292. Scheer V. Severe kidney injury after a 110-km trail race. Cureus (2020) 12(4):e7814. doi: 10.7759/cureus.7814
293. Nuti M, Amaddeo D, Crovatto M, et al. Infections in an alpine environment: Antibodies to hantaviruses, leptospira, rickettsiae, and borrelia burgdorferi in defined Italian populations. Am J Trop Med Hyg (1993) 48(1):20–5. doi: 10.4269/ajtmh.1993.48.20
294. Kreuzer M, Deffner V, Schnelzer M, Fenske N. Mortality in underground miners in a former uranium ore mine–results of a cohort study among former employees of wismut AG in Saxony and thuringia. Dtsch Arztebl Int (2021) 118(4):41–8. doi: 10.3238/arztebl.m2021.0001
295. Adjei DN, Stronks K, Adu D, et al. Relationship between educational and occupational levels, and chronic kidney disease in a multi-ethnic sample- the HELIUS study. PloS One (2017) 12(11):e0186460. doi: 10.1371/journal.pone.0186460
296. Spinder N, Bergman JEH, van Tongeren M, Boezen HM, Kromhout H, de Walle HEK. Maternal occupational exposure to endocrine-disrupting chemicals and urogenital anomalies in the offspring. Hum Reprod (2021) 37(1):142–51. doi: 10.1093/humrep/deab205
297. Brattebø G, Wisborg T, Fredriksen K. Crush injuries in arctic off-shore fisheries: Initial treatment to prevent acute renal failure. Arctic Med Res (1991) 50 Suppl 6:104–7.
298. Martin-Reina J, Casanova AG, Dahiri B, et al. Adverse health effects in women farmers indirectly exposed to pesticides. Int J Environ Res Public Health (2021) 18(11):5909. doi: 10.3390/ijerph18115909
299. Wrońska-Nofer T, Pisarska A, Trzcinka-Ochocka M, et al. Scintigraphic assessment of renal function in steel plant workers occupationally exposed to lead. J Occup Health (2015) 57(2):91–9. doi: 10.1539/joh.14-0115-OA
300. Canney M, Leahy S, Scarlett S, et al. Kidney disease in women is associated with disadvantaged childhood socioeconomic position. Am J Nephrol (2018) 47(5):292–9. doi: 10.1159/000488362
301. Chicas R, Mix J, Mac V, et al. Chronic kidney disease among workers: A review of the literature. Workplace Health Saf (2019) 67(9):481–90. doi: 10.1177/2165079919843308
302. Irwin MG, Trinh T, Yao CL. Occupational exposure to anaesthetic gases: A role for TIVA. Expert Opin Drug Saf (2009) 8(4):473–83. doi: 10.1517/14740330903003778
303. Rocskay AZ, Robins TG. Assessment of a screening protocol for occupational renal disease. J Occup Med (1994) 36(10):1100–9. doi: 10.1097/00043764-199410000-00012
304. Glaser J, Lemery J, Rajagopalan B, et al. Climate change and the emergent epidemic of CKD from heat stress in rural communities: The case for heat stress nephropathy. Clin J Am Soc Nephrol (2016) 11(8):1472–83. doi: 10.2215/CJN.13841215
305. Glaser J, Wegman DH, Arias-Monge E, et al. Workplace intervention for heat stress: Essential elements of design, implementation, and assessment. Int J Environ Res Public Health (2022) 19(7):3779. doi: 10.3390/ijerph19073779
306. Pacheco-Zenteno F, Glaser J, Jakobsson K, Weiss I, Arias-Monge E, Gyllensten K. The prevention of occupational heat stress in sugarcane workers in Nicaragua-an interpretative phenomenological analysis. Front Public Health (2021) 9:713711. doi: 10.3389/fpubh.2021.713711
307. Nerbass FB, Pecoits-Filho R, Calice-Silva V. The environmental role of hydration in kidney health and disease. Contrib Nephrol (2021) 199:252–65. doi: 10.1159/000517712
308. García GG, Iyengar A, Kaze F, Kierans C, Padilla-Altamira C, Luyckx VA. Sex and gender differences in chronic kidney disease and access to care around the globe. Semin Nephrol (2022) 42(2):101–13. doi: 10.1016/j.semnephrol.2022.04.001
309. Swartling O, Yang Y, Clase CM, et al. Sex differences in the recognition, monitoring, and management of CKD in health care: An observational cohort study. J Am Soc Nephrol (2022) ASN.2022030373. doi: 10.1681/ASN.2022030373
310. Kumaresan J, Seneviratne R. Beginning of a journey: Unraveling the mystery of chronic kidney disease of unknown aetiology (CKDu) in Sri Lanka. Global Health (2017) 13(1):43. doi: 10.1186/s12992-017-0268-y
311. Wright EM. Researchers' perceived challenges in studying chronic kidney disease of nontraditional etiology in mesoamerica. MEDICC Rev (2018) 20(3):45–51. doi: 10.37757/MR2018.V20.N3.9
312. Kiefer M, Rodríguez-Guzmán J, Watson J, van Wendel de Joode B, Mergler D, da Silva AS. Worker health and safety and climate change in the americas: Issues and research needs. Rev Panam Salud Publica (2016) 40(3):192–7.
313. Scammell MK. Trust, conflict, and engagement in occupational health: North American epidemiologists conduct occupational study in communities affected by chronic kidney disease of unknown origin (CKDu). Curr Environ Health Rep (2019) 6(4):247–55. doi: 10.1007/s40572-019-00244-6
Keywords: Social Determinants of Kidney Health, Altitude, Cold weather, Heat, Hypertension, Occupation, High-income and low- and middle-income countries, Seasonality
Citation: Aoun M and Chelala D (2022) Where do you live and what do you do? Two questions that might impact your kidney health. Front. Nephrol. 2:1011964. doi: 10.3389/fneph.2022.1011964
Received: 04 August 2022; Accepted: 13 September 2022;
Published: 05 October 2022.
Edited by:
Guillermo Garcia Garcia, Universidad de Guadalajara, MexicoReviewed by:
Jonathan Samuel Chávez-Iñiguez, University of Guadalajara, MexicoCopyright © 2022 Aoun and Chelala. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mabel Aoun, YW91bm1hYmVsQHlhaG9vLmZy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.