- Unidad Académica Desarrollo Biotecnológico, Instituto de Higiene, Facultad de Medicina, Universidad de la República, Montevideo, Uruguay
Saponins are natural glycosides found in many plant species; they have a hydrophobic region, consisting of a steroid or triterpenoid skeleton called an aglycone, and a hydrophilic region, consisting of sugar chains attached to the aglycone through ether or ester linkages. This combination of polar and nonpolar elements endows saponins with soap-like behaviour in aqueous solutions. Owing to their structural characteristics, the amphiphilic nature of saponins is responsible for their foaming properties, as well as other biological functions, including their haemolytic activity. The adjuvant properties of saponins were known many years ago, but only in recent years have saponins been approved for human vaccine use in this manner. Saponins from Quillaja saponaria bark are the only source of approved preparations for human use, but a related species, Quillaja brasiliensis, also contains similar saponin compositions that can be obtained from leaves. In this work, we describe the different preparations of saponins used for adjuvants and the purification methods used to obtain each saponin.
1 Introduction
Saponins are secondary metabolites found in many plant species. The name derives from the Latin word “sapo,” meaning soap, owing to their ability to produce foam in water (Sparg et al., 2004; Vincken et al., 2007). They can be found in the bark, leaves, stems, roots, and even flowers of plants (Moghimipour and Handali, 2015; Rai et al., 2021). Saponins are divided into two main classes, namely, triterpenoids and steroid glycosides, with specific structures characterized by the number and position of the attached sugar units (Sparg et al., 2004; Vincken et al., 2007; Jolly et al., 2024). These natural glycosides have both a hydrophobic region, consisting of a steroid or triterpenoid skeleton called an aglycone, and a hydrophilic region, consisting of a sugar chain containing glucose, glucuronic acid, xylose, rhamnose or methyl pentose attached to the aglycone through ether or ester linkages (Supplementary Figure S1) (Sparg et al., 2004; Vincken et al., 2007; Moghimipour and Handali, 2015). This combination of polar and nonpolar elements endows saponins with soap-like behaviour in aqueous solutions (Sparg et al., 2004; Vincken et al., 2007). Saponins from Quillaja saponaria (QSap) contain a triterpenic aglycone, most frequently quillaic acid, which is glycosylated at the C-3 and C-28 positions of the aglycone (Fleck et al., 2019). The specific limitation of the use of saponins is their cytotoxicity, which correlates with their haemolytic activity, which is influenced by the affinity of the aglycone to cholesterol in cell membranes (Sparg et al., 2004; Lorent et al., 2014). However, saponins are particularly useful as vaccine adjuvants (Kensil, 1996; Sun et al., 2009; Shen et al., 2023).
Adjuvants, substances that aid in the effectiveness of vaccines, have greatly increased the protection provided by vaccine immunizations. Adjuvants play important roles in the type, duration and effectiveness of immune responses to vaccines (Awate et al., 2013; Shi et al., 2019; Verma et al., 2023; Zhao et al., 2023). In 1925, Ramon was the first to describe immunological adjuvants as substances that, when combined with a specific antigen, produce a stronger immune response than produced by the antigen alone. He reported increased yields of tetanus and diphtheria antitoxins produced in horses when the animals developed an abscess at the injection site (Awate et al., 2013; Verma et al., 2023; Chippaux, 2024). By injecting starch, breadcrumbs or tapioca with inactivated toxin, sterile abscesses were induced at the site of injection, increasing antitoxin production. He confirmed that substances able to induce local inflammation at the injection site were also able to enhance the immune response (Di Pasquale et al., 2015; Verma et al., 2023).
Despite numerous advancements in the isolation of antigens and vaccine production, only a limited number of adjuvants have been approved for use in humans. Aluminium salts, which were developed nearly 100 years ago, are still the most widely used adjuvant in licenced human vaccines (Di Pasquale et al., 2015; Shi et al., 2019).
The adjuvant effect of saponins was reported in the initial work of Ramon in 1925; in fact, the adjuvant effects of breadcrumbs, tapioca and starch oil were probably due to the presence of saponins. Years later, in 1951, Espinet used a crude commercially available Quillaja saponaria saponin preparation to increase the potency of foot-and-mouth disease vaccines (Espinet, 1951; Barr et al., 1998).
After Espinet’s work in foot-and-mouth disease vaccines, in 1974, Dalsgaard successfully isolated the commercially available saponin Quil A® from the cortex of the South American tree Quillaja saponaria Molina. He reported that the addition of Quil A® to vaccine preparations stimulated both humoral and cellular immunity and induced differential antibody isotypes (Dalsgaard, 1974; Dalsgaard, 1977). Unfortunately, saponins also have strong haemolytic effects, increasing adverse reactions.
Therefore, efforts have focused on purifying a defined saponin molecule that maintains the adjuvant effect with less haemolytic effects. In 1991, Kensil and coworkers patented QS-21 (Kensil and Marciani, 1991). They further purified saponins by reverse chromatography, and 22 fractions were obtained. The most predominant fractions (QAs 7, 18, 19, and 21) had adjuvant activity (Kensil and Marciani, 1991; Kensil et al., 1991; Wang et al., 2019; Wang, 2021). QA21 (now QS-21) was the fraction that exhibited a better balance between adjuvant activity and low toxicity (Kensil and Marciani, 1991; Kensil, 2001).
Morein and coworkers reported that the formulation of saponins into lipidic nanoparticles, called immune-stimulating complexes (ISCOMs), decreased the haemolytic activity, whereas the adjuvant activity was unaltered (Morein et al., 1984; Barr and Mitchell, 1996). ISCOMs are spherical, open cage-like structures approximately 40 nm in size (Barr and Mitchell, 1996). The formulation of nanoparticles removes the need for complicated purification processes and allows for the safe use of defined mixes of saponins (Barr and Mitchell, 1996). If an antigen is included in a nanoparticle formulation with cholesterol, phospholipids and saponins, the resulting particle is called ISCOM. It is also feasible to create nanoparticles without antigens and combine the nanoparticles and antigens later before delivery. In this case, the nanoparticles are called the ISCOM matrix or simply the matrix (Lövgren Bengtsson et al., 2011). Many studies have shown that adjuvant activity is conserved independently of the use of ISCOM or ISCOM Matrices plus antigen (Fossum et al., 1990; Stertman et al., 2023).
In 1995, Cox, Morein and coworkers patented an ISCOM particle (ISCOM Matrix) that included fractions A and C of saponins from QSap; within the next years, this product was named Matrix-M (Cox et al., 1995). The Matrix-M adjuvant is the third generation of ISCOM technology and consists of two different types of physically stable nanoparticles mixed at a defined ratio (85% Matrix-A + 15% Matrix-C) (Cox et al., 1995; Lövgren Bengtsson et al., 2011; Bai et al., 2024). Matrix-A (nanoparticle of fraction A) and Matrix-C™ (nanoparticle of fraction C) contain different QSap molecules with complementary properties. According to structural analysis, fraction C is composed mainly of QS-21. Compared with those from fraction C, fraction A saponins have weaker adjuvant activity but are less reactive and more easily form nanoparticles. The combination of the two types of particles reduces the reactogenicity while preserving the adjuvant activity (Cox et al., 1995; Lövgren Bengtsson et al., 2011).
Currently, there are two main QSap adjuvant preparations licenced for human use. AS01 and AS02 from GlaxoSmithKline combine QS-21 with other immunostimulatory molecules (Vandepapeliere, 2013). AS01 contains 3-O-desacyl-4′-monophosphoryl lipid A (MPL), and QS-21 is formulated as a liposome, whereas AS02 contains MPL combined with QS-21 in an oil-in-water emulsion; both are designed to induce strong humoral and T-cell-mediated responses (Didierlaurent et al., 2017; Garçon and Di Pasquale, 2017).
AS01 was designed to strengthen the CD8+ response and is included in two licenced human vaccines (Shingrix™ for herpes virus and Mosquirix™ for malaria); it is also currently used in a candidate HIV and tuberculosis vaccine (Garçon and Di Pasquale, 2017; Lacaille-Dubois, 2019; Alving et al., 2020). AS02 has been evaluated in vaccines targeting complex pathogens that require a strong T-cell response and induce strong humoral and cellular immune responses (e.g., against hepatitis B, malaria, and HIV) (Garçon and Di Pasquale, 2017).
Matrix-M (Novavax) is also used in licenced human vaccines such as the Novavax COVID-19 vaccine (Nuvaxovid™) and the antimalarian R21/Matrix-M in collaboration with Oxford University and the Serum Institute of India (Stertman et al., 2023). Nuvaxovid is a recombinant protein vaccine composed of SARS-CoV-2 spike trimer nanoparticles formulated with Matrix-M™ adjuvant (Underwood et al., 2023; Lenart et al., 2024). Matrix-M is also being evaluated in clinical trials for new influenza vaccines (Pedersen et al., 2014; Shinde et al., 2022).
Although QS-21 exhibits limited haemolytic activity in vitro, higher doses may lead to side effects. Saponins can disrupt cell membranes, leading to haemolysis, as demonstrated by pore formation in erythrocyte membranes (Petrovsky, 2015). To reduce this toxicity, saponins can be encapsulated in lipid nanoparticles. This formulation strategy has been shown to reduce saponin-related adverse effects (Ragupathi et al., 2011; Petrovsky, 2015; Bigaeva et al., 2016).
Adverse reactions reported for licensed products, such as QS-21 or ISCOMs, indicate a low incidence of side effects comparable to those of other adjuvants. Saponin-based adjuvants have been associated with local adverse effects, including pain, redness, swelling, and erythema, as well as mild systemic effects such as fever and flu-like symptoms (Ragupathi et al., 2011; Petrovsky, 2015; Bigaeva et al., 2016).
However, owing to overexploitation of the QSap bark of Chilean forests, which has caused important ecological damage and resulted in the scarcity of available supplies, considerable efforts have been undertaken to discover sources of new saponins with improved adjuvant activity and reduced toxicity (Schlotterbeck et al., 2015; Fleck et al., 2019). Another species that belongs to the family Quillajaceae, Quillaja brasiliensis (now Quillaja lancifolia D. Don), a native tree distributed in southern Brazil, northern Uruguay, northeastern Argentina and eastern Paraguay, has been shown to be a better source of saponins because the saponins can be purified from leaves (Luebert, 2013; Schlotterbeck et al., 2015; Fleck et al., 2019). At the laboratory scale, saponins from Quillaja brasiliensis (QBr) presented characteristics and adjuvant activity similar to those of saponins extracted from QSap, opening a new avenue for the development of saponin-based adjuvants (Cibulski et al., 2016b; Cibulski et al., 2022; Fleck et al., 2019; Magedans et al., 2019; Rivera-Patron et al., 2021).
The precise mechanism of action of QSap or QBr saponins in their role as an adjuvant remains unclear. However, recent investigations have begun to delineate potential modes of action and signalling pathways. Saponins possess the capacity to induce both pro-inflammatory Th1/Th2 and anti-inflammatory Th2 immune responses (Marciani, 2018; Wang, 2021). Structure-activity relationship studies have elucidated that the presence of imine-forming carbonyl groups within the saponin structure is indispensable for T cell activation and the subsequent induction of Th1/Th2 responses. While saponins with diverse triterpenoid aglycons and oligosaccharide chains can activate dendritic cells (DCs) to induce both Th1 and Th2 responses, the presence of fucopyranosyl residues within their oligosaccharide chains can bias DC activation towards a Th2-dominant response through engagement of the Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin (DC-SIGN) receptor (Guo et al., 2004; Aguilar and Rodríguez, 2007; Chea et al., 2012; Marciani, 2018; Marciani, 2022; Marciani, 2024). Glycosides like QS-21 can interact separately with T cells and dendritic cells. T cells are co-stimulated by the glycoside’s aldehyde, while dendritic cells are activated by interactions with the triterpene group and fucosyl residue, respectively (Soltysik et al., 1995; Liu et al., 2002; Ragupathi et al., 2011; Marciani, 2018; Marciani, 2024).
Lacalle-Dubois propose that QS-21 mechanism of action could be synthetized as follow: stimulation of Th2 humoral and Th1 cell-mediated immune responses through action on antigen presenting cells (APCs) and T cells; release of Th1 cytokines participating in the elimination of intracellular pathogens; activation of the NLRP3 inflammasome and the release of caspase-1 dependent cytokines IL 1β and IL-18 (Marty-Roix et al., 2016; Coccia et al., 2017; Lacaille-Dubois and Wagner, 2017; Marciani, 2018; Lacaille-Dubois, 2019). A growing body of evidence is continually revealing the mechanisms of action of QSap saponins, indicating their complex interactions with multiple signalling pathways.
In this review, we focus on the most common purification methods and formulations of saponins for vaccine adjuvants.
2 Saponin purification
2.1 QSap Quil-A (Quil-A®)
In 1970, commercially available saponins were used as adjuvants in some foot-and-mouth disease (FMD) vaccines. These preparations were introduced by Espinet in 1951 and have since gained considerable popularity among producers of FMD vaccines because some have pronounced adjuvant effects (Espinet, 1951).
In 1973, Dalsgaard published a series of articles concerning the improvement of the FMD vaccine using saponins from Q. saponaria as an adjuvant. Previously, poorly defined saponin extracts were used with good results; therefore, Dalsgaard focused on the characterization and possible standardization of saponins for use in FMD vaccines (Dalsgaard, 1974; Dalsgaard, 1977).
Supplementary Figure S2 shows the purification process of Quil-A® proposed by Dalsgaard from an aqueous extract of the cortex of Quillaja saponaria Molina (Dalsgaard, 1974). The dialyzed aqueous extract was first separated on an ion exchange DEAE cellulose column equilibrated with 0.1 Tris-HC (pH 7.5). Elution was performed with buffer containing 0.2 M NaCl. The elution peak was subsequently subjected to gel exclusion chromatography on a Sephadex G50 column equilibrated with phosphate buffer at pH 7.5, resulting in 3 peaks. The peak eluted in the second position (middle) was reintroduced into an ion exchange DEAE cellulose column equilibrated with 0.1 Tris-HC (pH 7.5) and was eluted with a linear NaCl gradient increasing from 0 to 1 M, with 2 peaks obtained. Peak F seemed to be a pure substance in gel filtration and thin-layer chromatography analysis. Furthermore, peak F maintained the adjuvant effect at a similar level of activity as the dialyzed aqueous extract and was called Quil-A.
2.2 QSap QS-21
The Quil-A® preparation was a definite improvement over the previously available commercial saponins, although it still showed considerable heterogeneity. Further analysis using high-pressure liquid chromatography revealed that Quil-A® was in fact a heterogeneous mixture of structurally related compounds. However, not all these saponins were active as adjuvants.
The four predominant purified Qsap saponins were QS-7, QS-17, QS-18, and QS-21 (Kensil and Marciani, 1991; Kensil et al., 1991; Kensil, 1996; Kensil, 2001). These saponins were purified by HPLC and low-pressure silica chromatography and were found to be adjuvant-active, although they differed in their biological activities, such as haemolysis and toxicity, in mice. In particular, QS-21 and QS-7 were found to be the least toxic in mice. Owing to its potent adjuvant activity and low toxicity, QS-21 (commercially available as the “Stimulon®” adjuvant) has been identified as a useful immunological adjuvant (Kensil and Marciani, 1991; Kensil, 2001; Lv et al., 2024).
QS-21 is a complex triterpene glycoside of quillaic acid that is glycosylated at triterpene carbon 3, triterpene carbon 28, and carbon 5 of the second fatty acyl unit in a fatty acid domain (Ragupathi et al., 2011; Marciani, 2024). More recently, QS-21 was further purified using hydrophilic interaction chromatography (HILIC) and resolved into two peaks, QS-21-V1 and QS-21-V2, which have been shown to be chemically different compounds. In C57BL/6 mice immunized with vaccines consisting of ovalbumin and either QS-21 or both the individual components, QS-21-V1, or QS-21-V2, the individual components were comparable in adjuvant effects to that of the original QS-21 peak (containing a mixture of 3:2 QS-21-V1 and QS-21-V2), boosting the IgG subclasses IgG1, IgG2b, and IgG2c, as well as the total IgG titre (Kensil, 2001).
Given its longstanding success as an adjuvant, efforts have been made to improve the extraction and purification processes in order to increase purity and yields.
Baig et al. patented a method for purifying QSap saponins to a purity of at least 93% of QS-21, with impurity peaks outside the QS-21 group below 1% by UV absorbance at 214 nm. The method begins with a crude aqueous extract of QSap bark, typically containing 1–2.8 g/L of QS-21. The method includes three steps: polyvinylpyrrolidone (PVPP) adsorption, diafiltration, and reverse-phase chromatography (Baig et al., 2019).
The first step involves treating the QSap extract with PVPP resin. Typically, the extract is agitated with the resin and subsequently separated from the PVPP resin, along with adsorbed impurities, by filtration. This step of the process generally removes polyphenolic impurities such as tannins. The next step involves purifying the solution by diafiltration, ultrafiltration, or dialysis, preferably diafiltration with a 30 kDa membrane cut-off. This step typically removes salts, sugars, and other low molecular weight materials. The final step involves purifying the solution by reverse-phase chromatography using a polystyrene resin. This step removes non-saponin material and enriches the desired saponins. Alternatively, reverse-phase chromatography can be performed using a phenyl resin (Baig et al., 2019).
In the same way, Qui and Fox presents a novel two-step chromatographic process for purifying the molecular adjuvant QS-21 from QSap bark extract. This method involves a polar reversed-phase (RP) chromatography step followed by hydrophilic interaction chromatography (HILIC). This orthogonal approach significantly improves the purification efficiency, resulting in a product with > 97% purity and high yield (Qi and Fox, 2021).
Moreover, Gao et al. proposed an efficient ultrasound-assisted enzymatic method for extracting the QS-21 from QSap bark. The method utilizes a combination of ultrasound treatment and enzymatic hydrolysis to enhance the extraction efficiency before chromatography purification. They found that the type of enzyme used and the particle size of the QSap bark greatly affect the reaction yield. The best conditions found are using the tannase enzyme and a particle size smaller than 48 µm. The optimized process resulted in a significant increase in QS-21 yield compared to traditional methods, while maintaining high purity (Gao et al., 2022).
2.3 QSap fractions A, B, and C for ISCOM/matrix formulation
Fractions A and C are used by Novavax in ISCOM matrix formulations for human vaccines, and their flow purification is shown in Figure 1. A crude aqueous extract of QSap is pretreated on a C18 column (Sep-Pak). After the column is washed with 10% acetonitrile, lipophilic substances, including saponins, are eluted with 70% acetonitrile. The lipophilic fraction is further fractionated using an HPLC semipreparative C8 column and eluted with an acetonitrile gradient from 25% to 60%. Fractions A, B and C are eluted at approximately 39, 47% and 49% acetonitrile, respectively (Cox et al., 1995; Barr et al., 1998; Lövgren Bengtsson et al., 2011). Fraction A has very high ISCOM-forming activity and low haemolytic activity but medium adjuvant activity. Conversely, fraction C has medium ISCOM-forming activity and high haemolytic activity and adjuvant activity. It has been reported that the ratio of 7 parts of fraction A to 3 parts of fraction C provides very high adjuvant activity, easily forms ISCOMs and has low haemolytic activity (Cox et al., 1995).
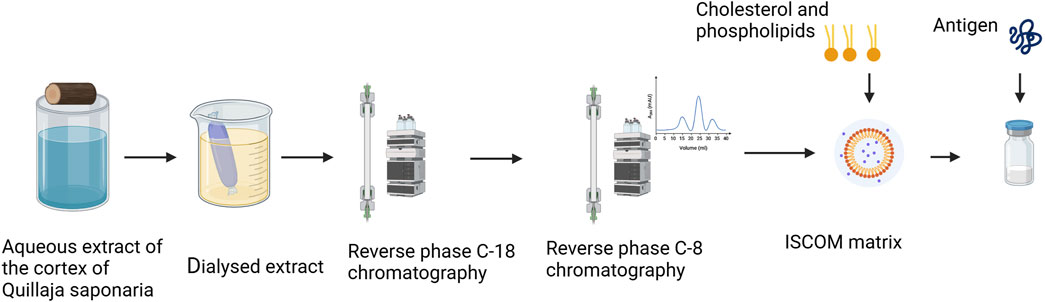
Figure 1. Saponin purification for Matrix M production. Created in BioRender. https://BioRender.com/i70i138.
2.4 QBr QB-90
The use of saponin fractions of QBr began in 2000. Fleck and colleagues were the first to evaluate the adjuvant power of the QBr saponin-enriched fraction (Fleck et al., 2006). The saponin fraction of this species is remarkably similar to that of the bark of QSap (Magedans et al., 2019). One of the most common protocols for saponin purification was published by Yendo and coworkers in 2017 and is shown in Figure 2 (Yendo et al., 2017). In this case, leaves are used for obtaining aqueous extracts. Further purification includes extraction of the most polar compounds using ethyl acetate and the precipitation of tannins with gelatine. The last step includes fraction purification chromatography with a C18 column and methanol elution. Although fraction QB-90 (eluted in 90% methanol) is the most commonly used fraction, the QB-80 and QB-100 fractions (eluted in 80% and 100% methanol, respectively) also show saponin adjuvant activity (Yendo et al., 2017).

Figure 2. Saponins fractions from Quillaja brasiliensis obtained by Yendo and coworkers’ process. Created in BioRender. https://BioRender.com/e44w417.
In recent years, the QB-90 fraction alone or in combination with ISCOM or ISCOM matrices has been proven to be effective in many vaccine candidates against influenza, herpes and other viruses at the laboratory scale with notable success (Cibulski et al., 2016a; Cibulski et al., 2016b; Cibulski et al., 2018).
2.5 QBr QB1
Owing to the large similarity between QSap and QBr, efforts have been made to purify QS-21 analogues from QBr. In 2022, Wallace and coworkers purified QB1, which is structurally similar to QS-21 (Wallace et al., 2022). They first purified an immunoadjuvant preparation (named fraction B) from the aqueous extract of QBr leaves by fractionation on a C18 column. Then, fraction B was further fractionated by consecutive separations with silica flash MPLC and reverse-phase C18 HPLC. Two compounds were isolated, and their structures were elucidated using a combination of NMR spectroscopy and mass spectrometry. One of these compounds was triterpene saponin (Qb1), which is an isomer of QS-21 (Wallace et al., 2022). The adjuvant activity of this compound has not yet been determined.
3 ISCOM/matrices formulation
There are two classic methods for the preparation of these nanoparticles: dialysis and centrifugation. The protocols are identical for the formulations of the ISCOMs and matrix except that the latter does not introduce the antigen into the nanoparticle; the antigen is added later. Among the two methods, dialysis has gained favour over centrifugation because of its simplicity and ease of scaling up, and currently, it is the widely accepted method for ISCOM/matrix formulation (Barr and Mitchell, 1996). The main important factor in the formation of optimal ISCOMs is the correct ratio of the various components that are combined at the start. According to Barr and Michell, the optimal weight ratios of cholesterol, phospholipid and QSap saponin should be 1:1:5 for the matrix and ratios of 1:1:5:0.1 to 1:1:5:1 when the antigen is included. The dialysis protocol is very simple: solubilized proteins (in the case of ISCOMs) are added to cholesterol and phospholipid dissolved in a nonionic detergent (MEGA-10 is the most commonly used), and saponins are added and mixed. The detergent is subsequently removed from the mixture by extensive dialysis or diafiltration (Barr and Mitchell, 1996; Barr et al., 1998; Rivera-Patron et al., 2022). Compared to other process, dialysis produces more homogeneous formulations with a narrow particle size distribution (Lendemans et al., 2005; Myschik et al., 2006).
Conversely, the centrifugation method is more complex and includes a sucrose gradient and ultracentrifugation, and it is rarely used (Barr and Mitchell, 1996; Hu et al., 1998). Other methods, such as ether or ethanol injection and lipid-film hydration, are described but are not as popular as the dialysis method (Bangham et al., 1965; Batzri and Korn, 1973; Pons et al., 1993; Copland et al., 2005; Myschik et al., 2006; Pham et al., 2006; Demana et al., 2010; Lendemans et al., 2010).
4 Conclusion
Saponins in combination with other immunostimulants or formulated as lipid nanoparticles have become new protagonists in human vaccine adjuvants (Kensil, 2001; Garçon and Di Pasquale, 2017; Stertman et al., 2023; Marciani, 2024). Table 1 presents the most commonly used saponin preparations as vaccine adjuvants. Adjuvants formulated with saponins have the advantage of generating a strong and balanced immune response, including strong cellular responses that make them suitable for use in viral vaccines (Sjölander et al., 1997; Cibulski et al., 2016a; Verma et al., 2023; Zhao et al., 2023).
With respect to saponin purification for human vaccines, improvements have been made in two different ways. First, the purification or purification/modification of defined saponin molecules can achieve a strong immune response with few side effects (Kensil, 1996; Garçon and Di Pasquale, 2017; Marciani, 2024). Second, the purification of a defined mixture of saponins that can be formulated into nanoparticles maintains immune activity and decreases side effects (Cox et al., 1995; Stertman et al., 2023).
The primary challenges in saponin production for adjuvant use are the sustainability of Q. saponaria tree cultivation and the need for better characterized and purified compounds that retain adjuvant potency while minimizing adverse effects (Ragupathi et al., 2011; Fleck et al., 2019).
The extraction of saponins from Q. brasiliensis leaves has proven to be the most efficient and environmentally friendly method. Nevertheless, alternative sustainable methods are currently under investigation. Lv and coworkers, reports the successful production of QS-21, through plant cell culture. The study demonstrates that plant cell culture can provide a sustainable and scalable alternative for producing QS-21 with comparable chemical and biological properties to the bark-derived product (Lv et al., 2024).
In this way, Martin et al. propose the complete biosynthesis of QS-21 saponin. The study successfully reconstituted the entire 20-step pathway in tobacco, demonstrating the production of QS-21 in a heterologous expression system (Martin et al., 2024).
In relation with the molecular structure, Marciani emphasizes the need to view adjuvants, particularly saponins like QS-21, as a distinct class of drugs with specific immunopharmacological properties shaped by their functional groups (Marciani, 2024). Specifically, QS-21 has been shown to induce a pro-inflammatory Th1 response through its aldehyde group, challenging the misconception that its adjuvanticity is solely due to its particulate nature (Soltysik et al., 1995; Aguilar and Rodríguez, 2007). The fucose residue is also critical, influencing the type of immune response elicited, although it is often mischaracterized as merely structural. Research into synthetic analogues could help clarify the roles of these components, as changes in sugar moieties may shift immune response (Ragupathi et al., 2011; Marciani, 2024). To fully understand the mechanisms of action of complex adjuvants, it is essential to identify the specific cell receptors they target. Understanding these interactions and their immunological effects can lead to improved adjuvant design (Borriello et al., 2022; Marciani, 2024).
Author contributions
VM: Conceptualization, Visualization, Writing–original draft, Writing–review and editing. NS: Writing–original draft, Writing–review and editing. FS: Conceptualization, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by PEDECIBA, Química.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fntpr.2024.1524624/full#supplementary-material
References
Aguilar, J. C., and Rodríguez, E. G. (2007). Vaccine adjuvants revisited. Vaccine 25, 3752–3762. doi:10.1016/J.VACCINE.2007.01.111
Alving, C. R., Peachman, K. K., Matyas, G. R., Rao, M., and Beck, Z. (2020). Army Liposome Formulation (ALF) family of vaccine adjuvants. Expert Rev. Vaccines 19, 279–292. doi:10.1080/14760584.2020.1745636
Awate, S., Babiuk, L. A., and Mutwiri, G. (2013). Mechanisms of action of adjuvants. Front. Immunol. 4, 114. doi:10.3389/FIMMU.2013.00114
Bai, Z., Wan, D., Lan, T., Hong, W., Dong, H., Wei, Y., et al. (2024). Nanoplatform based intranasal vaccines: current progress and clinical challenges. ACS Nano 18, 24650–24681. doi:10.1021/acsnano.3c10797
Baig, A. T., Denet, F. G. C., Díaz García, J. J., Farrenburgl, C. A., Lawrence, L. L., Myers, K. R., et al. (2019). Saponin purification World Intellectual Property Organization (WIPO), Patent Cooperation Treaty (PCT) 2019106192A1.
Bangham, A. D., Standish, M. M., and Watkins, J. C. (1965). Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 13, 238–IN27. doi:10.1016/S0022-2836(65)80093-6
Barr, I. G., and Mitchell, G. F. (1996). ISCOMs (immunostimulating complexes): the first decade. Immunol. Cell. Biol. 74, 8–25. doi:10.1038/ICB.1996.2
Barr, I. G., Sjölander, A., and Cox, J. C. (1998). ISCOMs and other saponin based adjuvants. Adv. Drug Deliv. Rev. 32, 247–271. doi:10.1016/S0169-409X(98)00013-1
Batzri, S., and Korn, E. D. (1973). Single bilayer liposomes prepared without sonication. Biochimica Biophysica Acta (BBA) - Biomembr. 298, 1015–1019. doi:10.1016/0005-2736(73)90408-2
Bigaeva, E., van Doorn, E., Liu, H., and Hak, E. (2016). Meta-analysis on randomized controlled trials of vaccines with QS-21 or IScomatrix adjuvant: safety and tolerability. PLoS One 11, e0154757. doi:10.1371/journal.pone.0154757
Borriello, F., Poli, V., Shrock, E., Spreafico, R., Liu, X., Pishesha, N., et al. (2022). An adjuvant strategy enabled by modulation of the physical properties of microbial ligands expands antigen immunogenicity. Cell. 185, 614–629.e21. doi:10.1016/J.CELL.2022.01.009
Chea, E. K., Fernández-Tejada, A., Damani, P., Adams, M. M., Gardner, J. R., Livingston, P. O., et al. (2012). Synthesis and preclinical evaluation of QS-21 variants leading to simplified vaccine adjuvants and mechanistic probes. J. Am. Chem. Soc. 134, 13448–13457. doi:10.1021/ja305121q
Cibulski, S., de Souza, T. A., Raimundo, J. P., Nascimento, Y. M., Abreu, L. S., Suarez, N., et al. (2022). ISCOM-matrices nanoformulation using the raw aqueous extract of quillaja lancifolia (Q. Brasiliensis). Bionanoscience 12, 1166–1171. doi:10.1007/S12668-022-01023-8
Cibulski, S., Rivera-Patron, M., Suárez, N., Pirez, M., Rossi, S., Yendo, A. C., et al. (2018). Leaf saponins of Quillaja brasiliensis enhance long-term specific immune responses and promote dose-sparing effect in BVDV experimental vaccines. Vaccine 36, 55–65. doi:10.1016/j.vaccine.2017.11.030
Cibulski, S. P., Mourglia-Ettlin, G., Teixeira, T. F., Quirici, L., Roehe, P. M., Ferreira, F., et al. (2016a). Novel ISCOMs from Quillaja brasiliensis saponins induce mucosal and systemic antibody production, T-cell responses and improved antigen uptake. Vaccine 34, 1162–1171. doi:10.1016/j.vaccine.2016.01.029
Cibulski, S. P., Silveira, F., Mourglia-Ettlin, G., Teixeira, T. F., dos Santos, H. F., Yendo, A. C., et al. (2016b). Quillaja brasiliensis saponins induce robust humoral and cellular responses in a bovine viral diarrhea virus vaccine in mice. Comp. Immunol. Microbiol. Infect. Dis. 45, 1–8. doi:10.1016/J.CIMID.2016.01.004
Coccia, M., Collignon, C., Hervé, C., Chalon, A., Welsby, I., Detienne, S., et al. (2017). Cellular and molecular synergy in AS01-adjuvanted vaccines results in an early IFNγ response promoting vaccine immunogenicity. NPJ Vaccines 2, 25. doi:10.1038/S41541-017-0027-3
Copland, M. J., Rades, T., Davies, N. M., and Baird, M. A. (2005). Lipid based particulate formulations for the delivery of antigen. Immunol. Cell. Biol. 83, 97–105. doi:10.1111/J.1440-1711.2005.01315.X
Cox, J. C., Coulter, A. R., Morein, B., Lovgren-Bengtsson, K., and Sundquist, B. (1995). Saponin preparations and use thereof in iscoms. United States Patent, US 6,352,697 B1.
Dalsgaard, K. (1974). Saponin adjuvants - III. Isolation of a substance from Quillaja saponaria molina with adjuvant activity in foot-and-mouth disease vaccines. Arch. Gesamte Virusforsch 44, 243–254. doi:10.1007/bf01240612
Dalsgaard, K. (1977). Saponin adjuvants. V. Precipitation of Serum components by non-purified saponin adjuvants in agar gel diffusion. Acta Vet. Scand. 18, 361–366. doi:10.1186/bf03548433
Demana, P. H., Berger, B., Vosgerau, U., Rades, T., and Davies, N. M. (2010). A comparison of pseudo-ternary diagrams of aqueous mixtures of Quil A, cholesterol and phospholipid prepared by lipid-film hydration and dialysis. J. Pharm. Pharmacol. 56, 573–580. doi:10.1211/0022357023259
Didierlaurent, A. M., Laupèze, B., Di Pasquale, A., Hergli, N., Collignon, C., and Garçon, N. (2017). Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expert Rev. Vaccines 16, 55–63. doi:10.1080/14760584.2016.1213632
Di Pasquale, A., Preiss, S., Da Silva, F. T., and Garçon, N. (2015). Vaccine adjuvants: from 1920 to 2015 and beyond. Vaccines (Basel) 3, 320–343. doi:10.3390/vaccines3020320
Espinet, E. (1951). Nouveau vaccin antiaphteux a complexe glucoviral. Gac. Vet. (B. Aires) 13, 268–272.
Fleck, J. D., Betti, A. H., Pereira da Silva, F., Troian, E. A., Olivaro, C., Ferreira, F., et al. (2019). Saponins from quillaja saponaria and quillaja brasiliensis: particular chemical characteristics and biological activities. Molecules 24, 171. doi:10.3390/MOLECULES24010171
Fleck, J. D., Kauffmann, C., Spilki, F., Lencina, C. L., Roehe, P. M., and Gosmann, G. (2006). Adjuvant activity of Quillaja brasiliensis saponins on the immune responses to bovine herpesvirus type 1 in mice. Vaccine 24, 7129–7134. doi:10.1016/J.VACCINE.2006.06.059
Fossum, C., Bergström, M., Lövgren, K., Watson, D. L., and Morein, B. (1990). Effect of iscoms and their adjuvant moiety (matrix) on the initial proliferation and IL-2 responses: comparison of spleen cells from mice inoculated with iscoms and/or matrix. Cell. Immunol. 129, 414–425. doi:10.1016/0008-8749(90)90217-F
Gao, Y., Dong, Q., Zhao, S., Zhao, Y., Zhang, Y., Wang, H., et al. (2022). Efficient ultrasound-assisted enzymatic method for extraction of immunostimulant QS-21 from Quillaja saponaria Molina. Ind. Crops Prod. 189, 115807. doi:10.1016/J.INDCROP.2022.115807
Garçon, N., and Di Pasquale, A. (2017). From discovery to licensure, the Adjuvant System story. Hum. Vaccin Immunother. 13, 19–33. doi:10.1080/21645515.2016.1225635
Guo, Y., Feinberg, H., Conroy, E., Mitchell, D. A., Alvarez, R., Blixt, O., et al. (2004). Structural basis for distinct ligand-binding and targeting properties of the receptors DC-SIGN and DC-SIGNR. Nat. Struct. Mol. Biol. 11, 591–598. doi:10.1038/nsmb784
Hu, K. F., Elvander, M., Merza, M., Åkerblom, L., Brandenburg, A., and Morein, B. (1998). The immunostimulating complex (ISCOM) is an efficient mucosal delivery system for respiratory syncytial virus (RSV) envelope antigens inducing high local and systemic antibody responses. Clin. Exp. Immunol. 113, 235–243. doi:10.1046/J.1365-2249.1998.00650.X
Jolly, A., Hour, Y., and Lee, Y. C. (2024). An outlook on the versatility of plant saponins: a review. Fitoterapia 174, 105858. doi:10.1016/J.FITOTE.2024.105858
Kensil, C. R., Patel, U., Lennick, M., and Marciani, D. (1991). Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J. Immunol. 146, 431–437. doi:10.4049/jimmunol.146.2.431
Lacaille-Dubois, M. A. (2019). Updated insights into the mechanism of action and clinical profile of the immunoadjuvant QS-21: a review. Phytomedicine 60, 152905. doi:10.1016/J.PHYMED.2019.152905
Lacaille-Dubois, M. A., and Wagner, H. (2017). New perspectives for natural triterpene glycosides as potential adjuvants. Phytomedicine 37, 49–57. doi:10.1016/J.PHYMED.2017.10.019
Lenart, K., Arcoverde Cerveira, R., Hellgren, F., Ols, S., Sheward, D. J., Kim, C., et al. (2024). Three immunizations with Novavax’s protein vaccines increase antibody breadth and provide durable protection from SARS-CoV-2. NPJ Vaccines 9, 17. doi:10.1038/s41541-024-00806-2
Lendemans, D. G., Myschik, J., Hook, S., and Rades, T. (2005). Cationic cage-like complexes formed by DC-cholesterol, Quil-A, and phospholipid. J. Pharm. Sci. 94, 1794–1807. doi:10.1002/JPS.20394
Lendemans, D. G., Myschik, J., Hook, S., and Rades, T. (2010). Immuno-stimulating complexes prepared by ethanol injection. J. Pharm. Pharmacol. 57, 729–733. doi:10.1211/0022357056280
Liu, G., Anderson, C., Scaltreto, H., Barbon, J., and Kensil, C. R. (2002). QS-21 structure/function studies: effect of acylation on adjuvant activity. Vaccine 20, 2808–2815. doi:10.1016/S0264-410X(02)00209-8
Lorent, J. H., Quetin-Leclercq, J., and Mingeot-Leclercq, M. P. (2014). The amphiphilic nature of saponins and their effects on artificial and biological membranes and potential consequences for red blood and cancer cells. Org. Biomol. Chem. 12, 8803–8822. doi:10.1039/C4OB01652A
Lövgren Bengtsson, K., Morein, B., and Osterhaus, A. D. (2011). ISCOM technology-based Matrix MTM adjuvant: success in future vaccines relies on formulation. Expert Rev. Vaccines 10, 401–403. doi:10.1586/ERV.11.25
Luebert, F. (2013). Taxonomy and distribution of the genus quillaja molina (Quillajaceae). Feddes Repert. 124, 157–162. doi:10.1002/FEDR.201400029
Lv, X., Martin, J., Hoover, H., Joshi, B., Wilkens, M., Ullisch, D. A., et al. (2024). Chemical and biological characterization of vaccine adjuvant QS-21 produced via plant cell culture. iScience 27, 109006. doi:10.1016/j.isci.2024.109006
Magedans, Y. V. S., Yendo, A. C. A., Costa, F. D., Gosmann, G., and Fett-Neto, A. G. (2019). Foamy matters: an update on quillaja saponins and their use as immunoadjuvants. Future Med. Chem. 11, 1485–1499. doi:10.4155/FMC-2018-0438
Marciani, D. J. (2018). Elucidating the mechanisms of action of saponin-derived adjuvants. Trends Pharmacol. Sci. 39, 573–585. doi:10.1016/J.TIPS.2018.03.005
Marciani, D. J. (2022). Effects of N-acylation on the immune adjuvanticity of analogs of the Quillaja saponins derivative GPI-0100. Vaccine 40, 4169–4173. doi:10.1016/J.VACCINE.2022.05.084
Marciani, D. J. (2024). Vaccine adjuvants: from empirical to a more rational drug design. Explor. Res. Hypothesis Med. 000, 000–208. doi:10.14218/ERHM.2024.00002
Martin, L. B. B., Kikuchi, S., Rejzek, M., Owen, C., Reed, J., Orme, A., et al. (2024). Complete biosynthesis of the potent vaccine adjuvant QS-21. Nat. Chem. Biol. 20, 493–502. doi:10.1038/s41589-023-01538-5
Marty-Roix, R., Vladimer, G. I., Pouliot, K., Weng, D., Buglione-Corbett, R., West, K., et al. (2016). Identification of QS-21 as an inflammasome-activating molecular component of saponin adjuvants. J. Biol. Chem. 291, 1123–1136. doi:10.1074/JBC.M115.683011
Moghimipour, E., and Handali, S. (2015). Saponin: properties, methods of evaluation and applications. Annu. Res. Rev. Biol. 5, 207–220. doi:10.9734/ARRB/2015/11674
Morein, B., Sundquist, B., Höglund, S., Dalsgaard, K., and Osterhaus, A. (1984). Iscom, a novel structure for antigenic presentation of membrane proteins from enveloped viruses. Nature 308, 457–460. doi:10.1038/308457a0
Myschik, J., Lendemans, D. G., McBurney, W. T., Demana, P. H., Hook, S., and Rades, T. (2006). On the preparation, microscopic investigation and application of ISCOMs. Micron 37, 724–734. doi:10.1016/J.MICRON.2006.03.016
Pedersen, G. K., Sjursen, H., Nøstbakken, J. K., Jul-Larsen, Å., Hoschler, K., and Cox, R. J. (2014). Matrix MTM adjuvanted virosomal H5N1 vaccine induces balanced Th1/Th2 CD4+ T cell responses in man. Hum. Vaccin Immunother. 10, 2408–2416. doi:10.4161/HV.29583
Petrovsky, N. (2015). Comparative safety of vaccine adjuvants: a summary of current evidence and future needs. Drug Saf. 38, 1059–1074. doi:10.1007/s40264-015-0350-4
Pham, H. L., Shaw, P. N., and Davies, N. M. (2006). Preparation of immuno-stimulating complexes (ISCOMs) by ether injection. Int. J. Pharm. 310, 196–202. doi:10.1016/J.IJPHARM.2005.11.011
Pons, M., Foradada, M., and Estelrich, J. (1993). Liposomes obtained by the ethanol injection method. Int. J. Pharm. 95, 51–56. doi:10.1016/0378-5173(93)90389-W
Qi, Y., and Fox, C. B. (2021). A two-step orthogonal chromatographic process for purifying the molecular adjuvant QS-21 with high purity and yield. J. Chromatogr. A 1635, 461705. doi:10.1016/J.CHROMA.2020.461705
Ragupathi, G., Gardner, J. R., Livingston, P. O., and Gin, D. Y. (2011). Natural and synthetic saponin adjuvant QS-21 for vaccines against cancer. Expert Rev. Vaccines 10, 463–470. doi:10.1586/ERV.11.18
Rai, S., Acharya-Siwakoti, E., Kafle, A., Devkota, H. P., and Bhattarai, A. (2021). Plant-derived saponins: a review of their surfactant properties and applications. Sci 3, 44. doi:10.3390/sci3040044
Rivera-Patron, M., Cibulski, S. P., Miraballes, I., and Silveira, F. (2022). Formulation of IMXQB: nanoparticles based on quillaja brasiliensis saponins to be used as vaccine adjuvants. Methods Mol. Biol. 2469, 183–191. doi:10.1007/978-1-0716-2185-1_15
Rivera-Patron, M., Moreno, M., Baz, M., Roehe, P. M., Cibulski, S. P., and Silveira, F. (2021). Iscom-like nanoparticles formulated with quillaja brasiliensis saponins are promising adjuvants for seasonal influenza vaccines. Vaccines (Basel) 9, 1350. doi:10.3390/vaccines9111350
Schlotterbeck, T., Castillo–Ruiz, M., Cañon–Jones, H., and Martín, R. S. (2015). The use of leaves from young trees of quillaja saponaria (molina) plantations as a new source of saponins. Econ. Bot. 69, 262–272. doi:10.1007/S12231-015-9320-0
Shen, L., Luo, H., Fan, L., Tian, X., Tang, A., Wu, X., et al. (2023). Potential immunoregulatory mechanism of plant saponins: a review. Molecules 29, 113. doi:10.3390/MOLECULES29010113
Shi, S., Zhu, H., Xia, X., Liang, Z., Ma, X., and Sun, B. (2019). Vaccine adjuvants: understanding the structure and mechanism of adjuvanticity. Vaccine 37, 3167–3178. doi:10.1016/j.vaccine.2019.04.055
Shinde, V., Cho, I., Plested, J. S., Agrawal, S., Fiske, J., Cai, R., et al. (2022). Comparison of the safety and immunogenicity of a novel Matrix-M-adjuvanted nanoparticle influenza vaccine with a quadrivalent seasonal influenza vaccine in older adults: a phase 3 randomised controlled trial. Lancet Infect. Dis. 22, 73–84. doi:10.1016/S1473-3099(21)00192-4
Sjölander, A., Van ’t Land, B., and Bengtsson, K. L. (1997). Iscoms containing purified Quillaja saponins upregulate both Th1-like and Th2-like immune responses. Cell. Immunol. 177, 69–76. doi:10.1006/cimm.1997.1088
Soltysik, S., Wu, J. Y., Recchia, J., Wheeler, D. A., Newman, M. J., Coughlin, R. T., et al. (1995). Structure/function studies of QS-21 adjuvant: assessment of triterpene aldehyde and glucuronic acid roles in adjuvant function. Vaccine 13, 1403–1410. doi:10.1016/0264-410X(95)00077-E
Sparg, S. G., Light, M. E., and Van Staden, J. (2004). Biological activities and distribution of plant saponins. J. Ethnopharmacol. 94, 219–243. doi:10.1016/J.JEP.2004.05.016
Stertman, L., Palm, A.-K. E., Zarnegar, B., Carow, B., Lunderius Andersson, C., Magnusson, S. E., et al. (2023). The Matrix-MTM adjuvant: a critical component of vaccines for the 21 century. Hum. vaccines and Immunother. 19, 2189885. doi:10.1080/21645515.2023.2189885
Sun, H. X., Xie, Y., and Ye, Y. P. (2009). Advances in saponin-based adjuvants. Vaccine 27, 1787–1796. doi:10.1016/J.VACCINE.2009.01.091
Underwood, E., Dunkle, L. M., Madhi, S. A., Gay, C. L., Heath, P. T., Kotloff, K. L., et al. (2023). Safety, efficacy, and immunogenicity of the NVX-CoV2373 vaccine. Expert Rev. Vaccines 22, 501–517. doi:10.1080/14760584.2023.2218913
Vandepapeliere, P. (2013). Vaccine compositions comprising a saponin adjuvant. European Patent EP2 364 721B1.
Verma, S. K., Mahajan, P., Singh, N. K., Gupta, A., Aggarwal, R., Rappuoli, R., et al. (2023). New-age vaccine adjuvants, their development, and future perspective. Front. Immunol. 14, 1043109. doi:10.3389/fimmu.2023.1043109
Vincken, J. P., Heng, L., de Groot, A., and Gruppen, H. (2007). Saponins, classification and occurrence in the plant kingdom. Phytochemistry 68, 275–297. doi:10.1016/J.PHYTOCHEM.2006.10.008
Wallace, F., Fontana, C., Ferreira, F., and Olivaro, C. (2022). Structure elucidation of triterpenoid saponins found in an immunoadjuvant preparation of quillaja brasiliensis using mass spectrometry and 1H and 13C NMR spectroscopy. Molecules 27, 2402. doi:10.3390/MOLECULES27082402
Wang, P. (2021). Natural and synthetic saponins as vaccine adjuvants. Vaccines (Basel) 9, 222. doi:10.3390/vaccines9030222
Wang, P., Škalamera, D., Sui, X., Zhang, P., and Michalek, S. M. (2019). Synthesis and evaluation of a QS-17/18-based vaccine adjuvant. J. Med. Chem. 62, 1669–1676. doi:10.1021/acs.jmedchem.8b01997
Yendo, A. C. A., de Costa, F., Kauffmann, C., Fleck, J. D., Gosmann, G., and Fett-Neto, A. G. (2017). Purification of an immunoadjuvant saponin fraction from quillaja brasiliensis leaves by reversed-phase silica gel chromatography. Methods Mol. Biol. 1494, 87–93. doi:10.1007/978-1-4939-6445-1_6
Keywords: Quillaja saponaria, Quillaja brasiliensis, saponin, adjuvant, nanoparticle, QS-21, iscom, matrix
Citation: Morais V, Suarez N and Silveira F (2025) Methods of saponin purification from Quillaja sp. for vaccine adjuvant production. Front. Nat. Produc. 3:1524624. doi: 10.3389/fntpr.2024.1524624
Received: 13 November 2024; Accepted: 16 December 2024;
Published: 07 January 2025.
Edited by:
Mostafa Rateb, University of the West of Scotland, United KingdomReviewed by:
Himanshi Tanwar, University of Maryland, United StatesCopyright © 2025 Morais, Suarez and Silveira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fernando Silveira, ZnNpbHZlaXJhQGhpZ2llbmUuZWR1LnV5; Victor Morais, dm1vcmFpc0BoaWdpZW5lLmVkdS51eQ==
 Victor Morais
Victor Morais Norma Suarez
Norma Suarez Fernando Silveira
Fernando Silveira