- 1Department of Zoology, Babasaheb Bhimrao Ambedkar University, Lucknow, Uttar Pradesh, India
- 2Botany Department, Rashtriya PG College, Jaunpur, Uttar Pradesh, India
- 3Department of Botany, University of Delhi, Delhi, India
- 4Amity Institute of Phytochemistry and Phytomedicine, Amity University, Noida, Uttar Pradesh, India
- 5Department of Environmental Studies, North Eastern Hill University, Shillong, Meghalaya, India
- 6Reserach and Development, Helix Biosciences, Delhi, India
The increasing prevalence of viral infections and the emergence of drug-resistant or mutant strains necessitate the exploration of novel antiviral strategies. Accumulating evidence suggests that natural plant products have significant potential to enhance the human antiviral response. Various plant natural products (PNPs) known for their antiviral properties have been evaluated for their ability to modulate immune responses and inhibit viral infections. Research has focused on understanding the mechanisms by which these PNPs interact with the human immune system and their potential to complement existing antiviral therapies. PNPs control compounds such as alkaloids, flavonoids, terpenoids, and polyphenols to promote antiviral cytokine synthesis, increase T-cell and macrophage activity, and activate antiviral genes. Studies have investigated the molecular interactions between PNPs, viruses, and host cells, exploring the potential of combining PNPs with conventional antiviral drugs to enhance efficacy. However, several challenges remain, including identifying, characterizing, and standardizing PNP extracts, optimizing dosages, improving bioavailability, assessing long-term safety, and navigating regulatory approval. The promising potential of PNPs is being explored to develop new, effective, and natural antiviral therapies. This review outlines a framework for an integrative approach to connect the full potential of PNPs in combating viral infections and improving human health. By combining natural plant products with conventional antiviral treatments, more effective and sustainable management of viral diseases can be achieved.
Introduction
Infectious diseases have shaped human civilization, with urbanization and trade increasing zoonotic pathogen spread. Major pandemics like SARS-CoV, MERS-CoV, and COVID-19 have highlighted the global health burden and socioeconomic conditions of viral infections, with COVID-19 alone causing over 180 million cases and 4 million deaths (World Health Organization, 2021), Despite advancements in immunization and drug development, many viruses still lack preventive vaccines and effective antiviral treatments, often undermined by viral escape mutants (Lin L.-T. et al., 2014). Therefore, discovering new antiviral drugs is crucial, and natural products offer a valuable resource for these discoveries. Natural products from plants, animals, or microbes offer pharmacological benefits beyond nutrition and have long been used as medicines, hallucinogens, food additives, and fragrances.
Plants are the primary source of natural products that serve as a key source of human medicines and play a pivotal role in drug discovery and design. Various traditional medicinal practices, such as Ayurveda, traditional Chinese medicine, Kampo, traditional Korean medicine, and Unani medicine, have enlisted several kinds of plant species used for treating human diseases. Identifying active compounds in these medicines remains a source of modern drug discovery. Interestingly, the FDA approved 1,562 drugs till 2019, of which 586 (37%) are natural products or their derivatives (Newman and Cragg, 2020). Of these approved drugs, only 53 are antiviral drugs, classified into biological molecules, natural product derivatives, and mimics of natural compounds (Newman and Cragg, 2020). However, antiviral drug discovery acts slowly compared to antibiotics, probably due to the “one drug one virus” dogma. Viruses are obligatory parasites, and most naturally derived antiviral compounds inhibit viral replication and target virus-host interactions. Some natural compounds can inhibit the growth of multiple viruses that utilize a similar pathway or host factor, acting as a source for the “one drug multiple virus” consortium. Thus, the pharmaceutical industry’s focus on natural products can accelerate antiviral drug discovery. In addition to aiding in drug discovery, plant natural products (PNPs), can improve human immunity. Consuming PNPs as probiotics and functional foods helps combat infections due to their bioactive compounds. These foods, rich in flavonoids, phenolics, carotenoids, vitamins, and minerals, can eliminate or inactivate viruses, boost immunity, and improve overall health. Regular intake may prevent viral replication, reduce symptom severity, low mortality rate, and speed recovery. Furthermore, PNPs function as probiotics to prevent viral infections by adhering to them and hindering host-cell communication (Singh S. et al., 2021; Saddiqa et al., 2024).
PNPs are involved in innate immunity response modulation, B-lymphocyte proliferation, and the generation of cytotoxic chemicals such as nitric oxide free radicals, T-lymphocytes, and phagocytosis activation (Vieira et al., 2024). Various ex-vivo and in vivo measures have proven that consuming PNPs enhances immunity against viruses (Elshafie et al., 2023). In this review, we discussed the role of bioactive natural products in antiviral drug discovery and highlighted their action against viral invasion based on insights from previously published studies.
Plant natural products (PNPs): an insight
PNPs have high structural diversity and unique pharmacological or biological activities due to the natural selection and evolutionary processes that have shaped their utility over hundreds of thousands of years. PNPs include a wide range of compounds, for example, Alkaloids (Nicotine, morphine, quinine), Phenolics (Flavonoids, tannins, lignans), Terpenoids (Essential oils, carotenoids, saponins), Glycosides (Cardiac glycosides, cyanogenic glycosides), Polysaccharides (Beta-glucans, pectins) (Kaur et al., 2021; Prakash et al., 2021; Thomas et al., 2021; Kumar A. et al., 2022; Al-Khayri et al., 2023; Bhatla and Lal, 2023). PNP compounds are often divided into two major classes: primary and secondary metabolites (Figure 1). Primary metabolites are vital for survival, forming key macromolecules like nucleic acids, amino acids, and sugars. Secondary metabolites, though not essential for survival, aid in the organism’s competitiveness within its environment. (Bhardwaj et al., 2021; Elshafie et al., 2023). Plants produce a wide array of secondary metabolites with complex chemical compositions. These compounds are synthesized in response to various stresses and serve crucial physiological functions (Srivastava et al., 2014; Pandey et al., 2016; Srivastava et al., 2018; Pandey et al., 2019; Mishra et al., 2020; Bajpai et al., 2023; Elshafie et al., 2023). Plant secondary metabolites are classified into four major classes depending on their biosynthetic pathway: (i) phenolic groups; (ii) terpenes and steroids (iii) nitrogen-containing compounds; and (iv) sulfur-containing compounds (Prakash et al., 2021; Kumar A. et al., 2022; Al-Khayri et al., 2023; Bhatla and Lal, 2023). Due to their bioactive qualities, which can have toxicological or pharmacological impacts on humans as well as animals, many of these secondary metabolites are relevant to the drug manufacturing sector. Plant secondary metabolites have various health benefits, including immune system support, antioxidant, anti-inflammatory, neutralizing free radicals, allergy relief, cardiovascular health, neurodegenerative diseases, pain-relieving effects, and anti-carcinogenic properties (Elshafie et al., 2023).
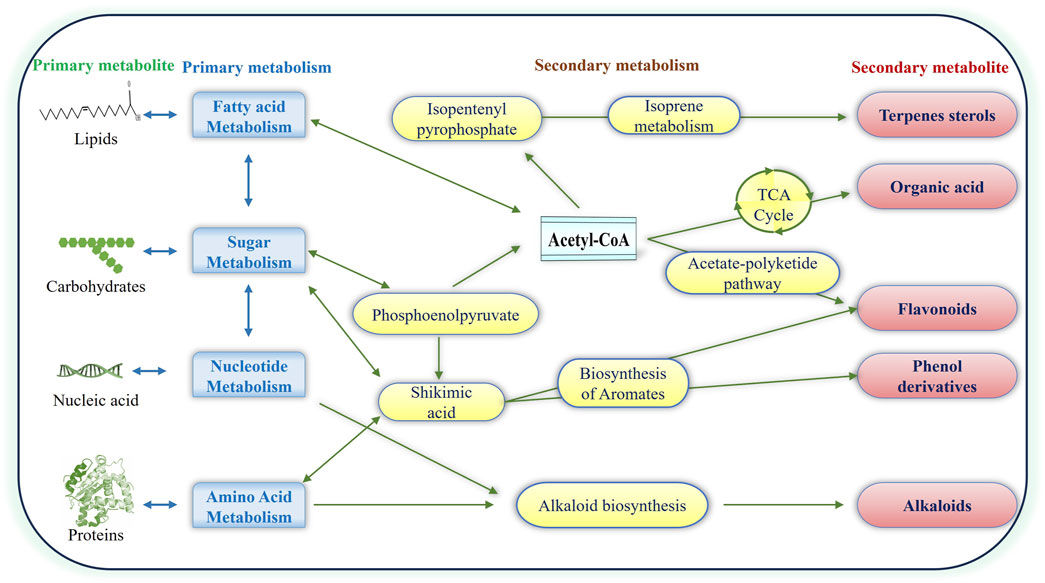
Figure 1. Illustration of Plant Natural Products: primary and secondary metabolites. Pathways from primary metabolites contribute to the production of terpenes, flavonoids, phenolics, alkaloids and organic acids, forming distinctive major classes of secondary metabolites.
Viral infections and human immunity
Human viral infections can seriously negatively influence society and the economy. Human viral diseases are a major global health concern due to their high morbidity and mortality rates, frequency, and potential for outbreaks (Luo and Gao, 2020; Baker et al., 2022). Viruses are tiny entities with either RNA or DNA as their genetic material, and they cannot exist without a host. Structurally, a typical virus has a lipid membrane called the viral envelope, covering a protein coat called a capsid that surrounds the genetic material (MW, 2014; Luo and Gao, 2020). Viruses reproduce by inserting their genome into the host cell, making multiple copies, and assembling new viral components within the infected cell (Roossinck, 2011; Hull, 2014). The etiologies vary due to distinct viruses, such as RNA viruses (e.g., influenza, coronaviruses) and DNA viruses (e.g., poxviruses, herpesviruses). The dsDNA (double standard DNA) viruses that infect humans have genome sizes ranging from 5 to 1,180 kb, linked to the stability of dsDNA and a low replication error rate (Payne, 2017). At the same time, ssDNA (single standard DNA) viruses have smaller genomes (2–7 Kb) and are generally non-enveloped with icosahedral capsids. Retroviruses, which include families like Retroviridae (e.g., HIV) and Hepadnaviridae (e.g., Hep B), challenge the Central Dogma of human biology (Gelderblom, 1996; Tramontano et al., 2019). Human viral infections can present with a wide variety of symptoms, from mild flu-like signs to severe respiratory distress, neurological disorders, and potentially premature death. These viral infections may be progressive, cancer-causing, latent, revived, acute, or chronic. The specific health problems vary based on the infection’s progression, cellular affinity, and host cell resistance. Because of their high rates of morbidity and mortality, high frequency, and potential for outbreaks and pandemics, human viral diseases have become a major global health concern (Morales-Sánchez and Fuentes-Pananá, 2014; Prasad et al., 2017). The illnesses’ etiologies differ because of distinct viruses, such as RNA viruses (e.g., G. influenza, and coronaviruses) and DNA viruses (e.g., G. Poxviruses, Herpesviruses, etc.) (Payne, 2017; Rampersad and Tennant, 2018).
The defense against pathogens is broadly classified into innate and adaptive immunity. Innate immunity is non-specific and serves as the first line of defense such as inflammatory responses and the complement system (Smith et al., 2019; Pal and Chakravarty, 2020). Adaptive immunity is specific and acquired over time, involving antibodies, lymphocytes, antigen presentation and immunological memory. Human viral infections can range from mild symptoms, like the flu, to severe respiratory issues, neurological disorders, or even death. The health impact of viral infections depends on factors like infection progression, cellular affinity, and host resistance, with infections potentially being acute, chronic, latent, or cancer-causing. (Silva et al., 2022; Brown et al., 2023).
Multiple consequences of viruses on human immunity frequently result in a compromised immune system and increased vulnerability to new infections. For example, HIV targets and destroys CD4+ T cells, causing immunosuppression, while the measles virus induces “immune amnesia” by depleting memory T and B cells (Mueller and Rouse, 2008; McNab et al., 2015; Sette and Crotty, 2021). Through antigenic variation and latency, viruses have developed evasive ways to avoid the immune system being recognized. For instance, Viruses like HIV and influenza evade immunity through antigenic variation, continually mutating their surface proteins. Herpesviruses, such as HSV and EBV, enter latency within host cells, reactivating periodically to avoid detection by the immune system (Yewdell and Hill, 2002; Paludan et al., 2011; White et al., 2012; Silva et al., 2024).
Certain viruses generate proteins that directly disrupt the host’s immune system, resulting in the suppression of immunological responses. Hepatitis C and other viruses produce proteins that inhibit interferon synthesis. In chronic infections like Hepatitis B and C, viruses also disrupt immune checkpoints to suppress effective T-cell responses. (Gokhale et al., 2014; Karamichali et al., 2022; Zheng et al., 2023). Prolonged viral infections result in chronic or persistent immunological activation, which could have negative consequences. Persistent infections, like Hepatitis B and C, leading to liver damage and cirrhosis (Marcellin and Boyer, 2003; Baseke et al., 2015; Ferrari, 2015). Persistent immune activation in HIV is linked deterioration of immune function (Gobran et al., 2021). Some viral infections may induce the immunopathology condition where the immune system causes tissue destruction. For example, Severe COVID-19 can trigger a “cytokine storm,” causing widespread inflammation and tissue damage (Gour et al., 2021; Que et al., 2022; Panteleev et al., 2023).
Viral infections can impact various components of both the innate and adaptive immune systems. For instance, Viruses like HIV and dengue impair dendritic cell function, disrupting antigen presentation and adaptive immunity (St. John and Rathore, 2019; Constant et al., 2022; Lee et al., 2022). Some also reduce natural killer (NK) cell activity by downregulating activating ligands. HIV and Hepatitis C cause T cell exhaustion, lowering functionality, while Epstein-Barr virus (EBV) infects B cells, increasing lymphoma risk (Rouse and Sehrawat, 2010; Carty et al., 2021; Singh H. et al., 2021).
Mechanisms of plant-natural products on human immunity
Plant-based natural products have been used in traditional medicine for centuries to address various health issues, including viral infections. Plant natural products (PNPs) have a significant impact on human immunity through their various bioactive compounds (Hu et al., 2024). Recently, scientific research has delved into understanding how these natural compounds boost the human antiviral response. Those mechanisms can be broadly categorized in direct antiviral activity, immune system modulation, antioxidant properties, anti-inflammatory effects, enhancement of interferon response, and anti-thrombocytopenic properties, as depicted in Figure 2 and Table 1. Firstly, direct antiviral activity involves plant compounds that inhibit viral replication. For instance, flavonoids, alkaloids, and terpenoids have been shown to interfere with viral enzymes and proteins crucial for the virus’s lifecycle (Table 1). Secondly, immune system modulation is achieved by plant-derived compounds that enhance the immune system’s ability to fight off infections. Some PNPs can modulate immune responses by enhancing or suppressing specific immune system components. PNPs can enhance the adaptive immune response by promoting the production and activity of T-cells and B-cells. Green tea catechins improve the proliferation and differentiation of T-cells. For example, Plant Ginseng whose active component is Ginsenosides enhances both innate and adaptive immunity and increases the activity of macrophages, NK cells, and T-cells (Ferrucci et al., 2024; Nikiema et al., 2024).
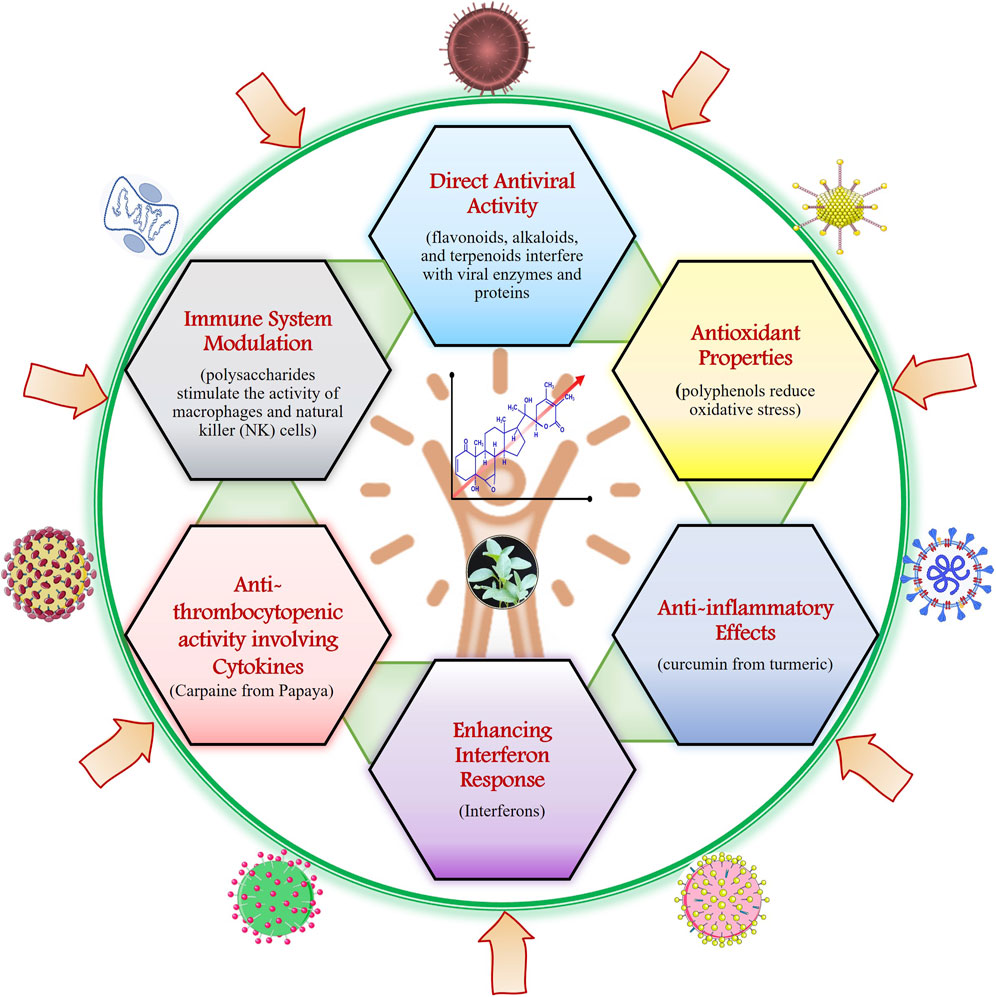
Figure 2. The image illustrates various mechanisms by which natural products contribute to antiviral activity and enhance immune function in the human.
Polysaccharides from plants such as Echinacea, for example, can stimulate macrophages and natural killer (NK) cells. PNPs play a crucial role in modulating the immune system through their anti-inflammatory, antioxidant, immunomodulatory, antimicrobial, and adaptive immunity-enhancing properties (Ferrucci et al., 2024; Nikiema et al., 2024). Plant antioxidants, such as polyphenols, can mitigate oxidative stress and protect cells from viral damage. Quercetin, found in apples and onions, is a potent antioxidant that enhances the function of the immune system. Anti-inflammatory effects also play a role, as chronic inflammation can weaken immune responses. The PNPs exhibit anti-inflammatory properties by modulating the production of pro-inflammatory cytokines and enzymes, which remain the main mechanisms of their action (Alarabei et al., 2024; Wu et al., 2024). Compounds like curcumin from turmeric help maintain immune balance by reducing excessive inflammation. The active component of Curcumin from turmeric shows anti-inflammatory, antioxidant, and enhances antibody responses. For example, curcumin from turmeric inhibits the activity of nuclear factor-kappa B (NF-κB), a key regulator of inflammation. PNPs, particularly flavonoids and phenolics, scavenge free radicals, reducing oxidative stress and protecting immune cells from damage (Hooda et al., 2024). Furthermore, some plant compounds enhance the production and activity of interferons, which are essential for the antiviral immune response. Carpaine, an alkaloid from papaya leaves, has demonstrated notable anti-thrombocytopenic activity, offering potential for managing Dengue Virus (DENV) by modulating cytokine responses and platelet levels. Studies indicate that carpaine significantly increases blood platelet counts in DENV-infected individuals by upregulating the expression of platelet-activating factor receptor and arachidonate 12-lipoxygenase genes (Zunjar et al., 2016; Anjum et al., 2017; Kapoor, 2017; Sarker et al., 2021; Munir et al., 2022). Advanced phytochemical analyses have identified several metabolites in Carica papaya leaf extract, including quinic acid, malic acid, caffeoyl malate, quercetin, p-coumaroyl malate, clitorin, rutin, feruloyl malate, nicotiflorin, and carpaine (Kasture et al., 2016; Ayodipupo Babalola et al., 2024). The diverse bioactive compounds of PNPs interact with various components of the immune system, making them valuable in promoting overall health and resilience against diseases (Frazzoli et al., 2023; Gasmi et al., 2023; Kussmann et al., 2023). However, it is crucial to note that the PNP dosage intake, effectiveness, and safety can all vary, and further investigation is needed to understand their processes and possible therapeutic uses properly.
Application of PNP to control human viral infection
Plant-derived natural products play a crucial role not only in drug discovery but also in enhancing human immunity against pathogens. As challenges in developing chemical-based antiviral treatments continue, plant extract or fraction are increasingly recognized as safe and affordable alternatives to traditional antiviral medications (Elshafie et al., 2023). These compounds offer a range of antiviral properties and contribute to bolstering the immune system. Several studies highlight the potential of plant-derived compounds in the prevention and treatment of viral infection (Pebam et al., 2022). For instance, according to Chassagne et al. (2021), natural compounds that have been extracted from a variety of plants may help improve the development flow and yield novel drugs (Chassagne et al., 2021). Studies have shown that several medicinal plants with antiviral properties, including Andrographis paniculata, Lindera chunii, Dioscorea bulbifera, Wisteria floribunda, Xanthoceras sorbifolia, and Aegle marmelos, exhibit significant anti-HIV activity (Kaur et al., 2020). Additionally, various plant-derived compounds from different chemical groups have demonstrated potential anti-HBV activity (Wu, 2016). Unlike conventional antivirals and antibiotics that target pathogens broadly, plant-based medications may offer more specific mechanisms of action against viruses. For instance, quercetin, a well-known flavonoid available as a dietary supplement, is commonly used to boost immunity, manage allergies, and improve general health. It has been shown to inhibit the replication of several viruses, including influenza, herpes simplex, and hepatitis C viruses (Agrawal et al., 2020).
Despite the availability of antiviral medications and vaccines, effectively controlling infections remains challenging due to the unique characteristics of each virus and the limited number of approved antiviral drugs (Adamson et al., 2021; Cheung et al., 2024; Mahmoudieh et al., 2024). This has driven increased interest in plant-based treatments, as PNPs exhibit diverse bioactive properties, including antiviral effects. Research supports the use of plant-based medications in treating viral infections (Chassagne et al., 2021). Advances in genetic engineering and molecular breeding in plantations have facilitated the development of potential treatments for viruses such as SARS-CoV-2. Recent studies indicate that plant extracts possess therapeutic potential against the COVID-19 strain (Jalal et al., 2021; Mukherjee et al., 2024), suggesting that the therapeutic effects of plant extracts on COVID-19 highlight their significance in managing viral infections.
Even though, the presence of over 220 identified human viruses and the limited number of clinical approvals for antiviral drugs are major concerns (Adamson et al., 2021; Cheung et al., 2024). Each virus’s unique characteristics and behaviors require customized medications or therapies, which can be challenging. Additionally, rapid viral genome evolution contributes to the emergence of several mutants in the virus leading to antiviral resistance and complicating treatment efforts (Ghaebi et al., 2020). Research on compounds such as K22, which has demonstrated strong anti-CoV activity by reducing endoplasmic reticulum zippering, offers promising insights into overcoming these challenges (Bills et al., 2023).
Humans have used herbs and supplements to treat illnesses since ancient times. Even today, influenza and coronavirus vaccinations are not 100 percent effective, so the immune system can use all the help it can get from antiviral herbs. Some of the best antiviral herbs and supplements have been used therapeutically to manage symptoms of coronavirus (Saddiqa et al., 2024). Some prevalent plant natural products that help ameliorate the effects of viral infections in humans include quercetin, resveratrol, echinacea, allicin, and epigallocatechin gallate (Lin L. T. et al., 2014; Adeosun and Loots, 2024) (Table 1).
Polyphenols prevent viral infection of host cells by disturbing virus adsorption and attachment and by suppressing reverse transcriptase and RNA polymerase activity in HIV and influenza virus attacks (Chojnacka et al., 2021). Resveratrol, is a potent polyphenolic compound, found in grapes and red wine, exhibits antiviral properties against several viruses, including influenza and herpes simplex virus, by inhibiting viral protein synthesis (Abba et al., 2015). Baicalein and luteolin are two flavones, a family of polyphenolic compounds, whose antiviral properties have also been well studied. Baicalein substantially effect viral DNA synthesis and reduced human cytomegalovirus (HCMV) early and late protein levels (Croft, 1998). Luteolin exhibits antiviral activity against viruses like the poliovirus and coxsackievirus and has antimicrobial properties that help fight bacterial and viral infections (Zakaryan et al., 2017). Polyphenols Epigallocatechin gallate, derived from green tea, has potent antiviral effects against viruses such as hepatitis B and C, influenza, and herpes, partly through inhibiting viral entry and replication (Wang et al., 2021). Echinacea is widely used in traditional medicine and contains various secondary metabolites, primarily phenolics (such as caffeic acid derivatives) and polysaccharides, which can boost immune function. It has also been shown to reduce the duration and severity of colds and other respiratory infections (Karsch-Völk et al., 2014). Curcumin, a natural polyphenolic compound and the primary ingredient in turmeric, is known for its ability to eliminate human viruses such as H5N1, SARS-CoV-2, HIV-1 & HIV-2, influenza, HSV-1 & HSV-2, coxsackievirus, hepatitis B, and other pathogens. (Babaei et al., 2020; Bormann et al., 2021; Sahoo et al., 2021; Srivastava et al., 2022). The basil (Ocimum sanctum), or tulsi, contains many flavonoids such as orientin, vicenin, eugenol, rosmarinic acid, and luteolin, which contribute to its medicinal properties. Tulsi’s antiviral properties make it a valuable herbal remedy for managing and preventing viral infections by inhibiting viral replication, modulating the immune system, reducing inflammation, and providing antioxidant support including Influenza-A, flu A subtype H9N2, HSV1, HSV2, ADV-8, CVB1, EV71, ADV-3, ADV-II, HIV-1, HIV-2, HPV, HCV, DEN-1 & 2, DNA, and RNA viruses, and SARS-CoV-2 (Bhattacharya et al., 2024; Jayashankar et al., 2024; Rani, 2024; Sao et al., 2024). Coumarins from Calophyllum lanigerum and C. inophyllum have been shown to inhibit reverse transcriptase and are effective against HIV-1 (Sharapov et al., 2023). Black tea phenolics such as tannic acid, 3-isotheaflavin-3-gallate, and theaflavin-3,3′-digallate, as well as phenolics from Isatis indigotica like hesperetin, and have exhibited inhibitory effects against various viruses (Salasc et al., 2022b; Sezer et al., 2022; Gamil and Abeer, 2023). Strychnobiflavone is a bioactive flavonoid compound derived from the bark of Strychnos pseudoquina, known for its ability to inhibit HSV-1 virus and its associated disease (Thomas et al., 2021).
Different alkaloids like Isoquinoline alkaloid thalimonine, berberine, Camptothecin, Harman, Gingeronone A, alkaloid isolated from Zanthoxylum chalybeum, Thalictrum Simplex L, Berberis vulgaris, Camptotheca acuminata, Ophiorrhiza nicobarica, Camellia sinensis (L.) Kuntze, Zingiber officinale Roscoe, used to control the viral diseases like Swartz and Edmonston measles virus, Influenza A virus, IAV, EV71, Herpes virus (Olila et al., 2002; Serkedjieva and Velcheva, 2003; Wu et al., 2004; Song et al., 2005; Xu et al., 2017; Botwina et al., 2020; Pandey et al., 2021; Gurjar and Pal, 2024). Lycorine is a natural alkaloid from Lycoris radiata L., showed anti-SARS-CoV activity (Li et al., 2005). Sanguinarine, alkaloid derived from the bloodroot plant (Sanguinaria canadensis), has potential antiviral effects against hepatitis C virus (HCV) and herpes simplex virus (HSV) by inhibiting viral RNA synthesis and blocking viral protein expression, enhancing the host’s immune response to viral infections (Croaker et al., 2016; Wink, 2020).
Artemisinin, a terpenoid derived from Artemisia annua, is used in treating malaria and has shown antiviral activity against hepatitis B and C viruses (Uzun and Toptas, 2020). Oregano (Origanum vulgare), a popular herb from the mint family, has medicinal properties. Its oil’s active components, such as carvacrol and thymol, possess antiviral properties and disrupt the lipid envelopes of viruses, leading to the inactivation of the virus, allowing it to treat murine norovirus (MNV) (Gilling et al., 2014; Solis-Sanchez et al., 2020; Mohanty and Murhekar, 2024). Andrographolide, a diterpene lactone found in Andrographis paniculata, has shown antiviral properties against influenza, hepatitis C, and dengue viruses (Kaushik et al., 2021). Components like Andrographolide, Oleanolic acid, Phorbol ester, hop-8, etc., isolated from the leaf of A. paniculata, Justicia adhatoda L., Achyranthes aspera L., Ostodes katharinae., protect against viruses like HSV-I, HIV, SARS-CoV-2, and EBV (Jayakumar et al., 2013; Mukherjee et al., 2013; Chavan and Chowdhary, 2014; Chen et al., 2017; Tshilanda et al., 2020; Bachar et al., 2021; Ghosh et al., 2021). Bel (Aegle marmelos), plant is an important ethanobotanical use in Indian culture. Seselin, a compound isolated from A. marmelos (L.) Corrêa showed effective against SARS-CoV-2 (Bachar et al., 2021; Nivetha et al., 2022). Licorice, known as “sweet grass,” has been used in traditional Chinese medicine. The plant’s root is the primary source of its antiviral and antibacterial properties. Licorice, frequently used in folk food systems during cold and cough, contains glycyrrhizin from Glycyrrhiza uralensis. Glycyrrhizin, a triterpenoid saponin compound derived from the licorice plant (Glycyrrhiza glabra), has shown activity against a range of viruses, including herpes simplex, hepatitis C, HIV, and SARS-CoV-2, by interfering with virus replication and boosting the immune response (Cinatl et al., 2003; Rizzato et al., 2017; Tseng et al., 2017; Gomaa and Abdel-Wadood, 2021). Celastrol, a quinone methide triterpene obtained from Tripterygium wilfordii root extracts, has shown promise to prevent HCV replication by targeting on the JNK/Nrf2/HO-1 pathway, providing a viable strategy to fight HCV infection (Tseng et al., 2017).
Garlic (Allium sativum L.) has been used for centuries in food and medicine and has been shown to heal viral infections in humans, animals, and plants (Tesfaye, 2021). Studies indicate that garlic can help treat the common cold, flu, viral hepatitis, and even warts (Sasi et al., 2021). Allicin, a sulfur-containing compound derived from garlic, has broad-spectrum antiviral activity, inhibiting viral RNA synthesis and boosting immune cell activity. Bulb of A. sativum L., produces garlic oil, garlicin, and lectin, etc., protects against L. ADV-3, SARS-CoV-2, HSV-I, H1N1, HIV-1 (Rouf et al., 2020; Bachar et al., 2021). Another sulfur-containing compound Progoitrin, a glucosinolate, isolated from the root of I. indigotica protect against H1N1 (Nie et al., 2020).
Essential oils, concentrated extracts from plants, contain various bioactive complex mixture of terpenes, phenolics, and other secondary metabolites that can exert antiviral effects. Some essential oils can directly inactivate viruses by disrupting their lipid envelopes, denaturing proteins, or interfering with viral entry into host cells. They can also inhibit viral replication within host cells by interfering with viral RNA or DNA synthesis and disrupting viral enzyme activity. Additionally, essential oils can enhance the immune response and reduce inflammation associated with viral infections. Different plant oils, such as those from lavender, camphor, peppermint, cinnamon, eucalyptus, tea, and thyme, contain compounds used for antiviral activity (Mohammed Ail, 2021). Specific essential oils with antiviral properties include tea tree oil (Melaleuca alternifolia), which contains compounds like terpinen-4-ol and alpha-terpineol that disrupt viral envelopes and inhibit viral replication of HSV, influenza, and HPV. Eucalyptus oil (Eucalyptus globulus) contains eucalyptol (1,8-cineole), which inhibits viral replication and enhances immune responses against influenza, HSV, and RSV (Garozzo et al., 2009). Peppermint oil (Mentha piperita) has menthol and other compounds that exhibit antiviral activity against HSV, influenza, and adenovirus. Lavender oil (Lavandula angustifolia) contains linalool and linalyl acetate, which can inactivate viruses and reduce inflammation during HSV and influenza infections (Abou Baker et al., 2021). Oregano oil (O. vulgare) is rich in carvacrol and thymol, which have strong antiviral, antibacterial, and antifungal properties, disrupting viral envelopes and inhibiting replication of HSV, rotavirus, and norovirus. Lemon balm oil (Melissa officinalis) part of the mint family, possesses antioxidant, antihistamine, anti-cancer, antibacterial, antifungal, and antiviral properties. Contains compounds like citral and citronellal, which have antiviral effects by inhibiting viral attachment and entry of HSV and enterovirus. Studies indicate that lemon balm essential oil helps treat the influenza virus (Behzadi et al., 2023; de Sousa et al., 2023). Lemon balm also relieves muscle spasms and may slow down HSV-1 (Mazzanti et al., 2008; Astani et al., 2012; Gurjar and Pal, 2024). Different oils viz., Sandalwood oil, Eucalyptus oil, Essential oil (Humulene epoxide, and caryophyllene oxide) Tea tree oil and Terpinen-4-ol from different plants like Cyperus rotundus L. Fortunella margarita, Tea tree (M. alternifolia), Santalum album Linn use against different viral disease. Eucalyptus species are used to control the disease of SARS, HAV, HSV, etc (Garozzo et al., 2011; Ibrahim et al., 2015; Battistini et al., 2019; Bellavite and Donzelli, 2020; Goyal et al., 2020; Samra et al., 2020; Amparo et al., 2021; Khan Yusuf and Sen Das, 2023).
More than 200 extracts from different plants, such as L. radiata, A. annua, Pyrrosia lingua, and Lindera aggregate, have been analyzed and found to have anti-SARS-CoV effects (Omrani et al., 2021; Perera et al., 2021; Salasc et al., 2022a; Gamil and Abeer, 2023; Pal and Lal, 2024; Sezer et al., 2024). Aqueous extract of Carica papaya leaves to treat Dengue fever (Shrivastava et al., 2022). Extracts from folk medicinal plants like Heracleum maximum, Plantago major Linn., and Sambucus nigra L. possess antiviral effects by stimulating macrophage activation (Barak et al., 2001; Chiang et al., 2003; Webster et al., 2006; Mukhtar et al., 2008). Similarly, anti-HCV activity has been observed in methanolic and aqueous extracts of Boswellia carterii, Acacia nilotica L., Embelia schimperi, Trachyspermum ammi L., Quercus infectoria, Piper cubeba L., and Syzygium aromaticum L. (Mukhtar et al., 2008). Astragalus root, a medicinal plant from Asia, is an antiviral agent used to treat avian influenza H9 (Shkondrov et al., 2023). Plant-derived zinc components boost the immune system and have effective antiviral properties, helping to protect the body from HPV, HIV, Picornavirus, Togavirus (Chikungunya), flu, coronavirus, and herpes (Murakami et al., 2007; Read et al., 2019; Khabour and Hassanein, 2021) Ginger (Z. officinale) is a spice packed with antioxidants beneficial to the human immune system’s health. It possesses antimicrobial properties and can fight off various human viruses in diseases such as Chikungunya, Dengue (DENV), SARS-CoV-2, and the human respiratory syncytial virus (HRSV) (Chang et al., 2013; Aboubakr et al., 2016; Kaushik et al., 2020; Mukherjee et al., 2024); Black elderberry (S. nigra), a popular medicinal shrub in Europe, possesses antioxidant properties and boosts the immune system. In vitro studies have shown that black elderberry can slow the spread of influenza A and B, as well as some bacterial lung (Charlebois et al., 2010; Hawkins et al., 2019; Torabian et al., 2019; Seymenska et al., 2023). The tubers of various Dioscorea species have been used to treat different viruses, including herpesvirus, poxvirus, and picornavirus. These extracts work against viruses by binding to the virion particles, preventing their penetration into cells, modifying the cell wall surface to prevent the release of viral replicates, and interfering with the intracellular replication of viruses (Ganjhu et al., 2015). Leaf extracts of Azadirachta indica have shown antiviral activity against several RNA and DNA viruses (Gurjar and Pal, 2024). Aqueous extract from the roots of Carissa edulis (Forssk.) V showed anti-HSV activity (Tolo et al., 2006), while the ethanolic extract of Phyllanthus (Phyllanthus nanus) showed anti-HBV activity (Lam et al., 2006). Whole plants of Cynodon dactylon L. and the leaf of Rosa centifolia L. show inhibitors of viruses like BCoV and HIV respectively (Nalanagula, 2020; Palshetkar et al., 2020; Bachar et al., 2021). Supplemented liquid fermented broth of Ganoderma lucidum with aqueous extract of Radix Sophorae flavescentis strongly showed anti-hepatitis B virus activity (Mukhtar et al., 2008). Hot water extracts of Stevia rebaudiana L. blocked the entry of various infectious HRV viruses (Takahashi et al., 2001).
Not only higher plant products but lower plant-like products of blue-green algae and red algae also help in managing the human attacking viruses. Cyanovirin N (CV-N), an 11-kDa protein product of cyanobacterium Nostoc ellipsosporum, and Griffithsin a red marine algae (Griffithisia sp.) product can develop anti-HIV-1 effect. These molecules neutralize the HIV viruses by inhibiting their infection and antibody based HIV particle neutralization processes (Boyd et al., 1997; Emau et al., 2007; Millet et al., 2016). DL-galactan hybrid C2S-3 derived from different algae Cryptonemia crenulata help to protect the human body from Dengue viruses (Talarico et al., 2007).
Peptides like Meliacine and Hydrolysed peptides AIHIILI and LIAVSTNIIFIVV isolated from Melia azedarach and Q. infectoria leaf and fruit part protect the human body from Foot and mouth disease virus VSV, and HSV-I, HIV-1 viruses (Wachsman et al., 1998; Alché et al., 2003; Seetaha et al., 2021). Lectins of S. nigra, which can administer either orally or parenterally in liquid composition inhibit the activity of several enveloped viruses (Ganjhu et al., 2015). Rhizome of Bletilla striata produce Phenanthrene to inhibit virus H3N2 propagation (Shi et al., 2017). Fruits of Mangifera indica L. and Forsythia suspensa produce Mangiferin and Forsythoside A which work as antiviral compounds against HSV, HIV and H1N1 (Law et al., 2017; Al-rawi et al., 2019).
These plant-derived substances have provided opportunities for creating novel antiviral treatments. However, it is crucial to remember that while many of these substances have shown antiviral activity in laboratory conditions, further clinical research is required to determine their safety and effectiveness in humans.
Limitation and toxicity of PNPs
PNPs have shown promise in antiviral drug discovery, but they also have several limitations during viral infections (Figure 3). PNPs often exist as complex mixtures with multiple active compounds. The chemical composition of PNPs can vary significantly depending on the source, growing conditions, and extraction methods. Isolating and identifying the specific components responsible for antiviral activity can be challenging, making standardization difficult. This variability can affect the consistency and reliability of the therapeutic effects (Raskin et al., 2002; Kusumawati, 2021). Many PNPs suffer from poor bioavailability, as they are not easily absorbed, distributed, or maintained in effective concentrations within the body (Kumar S. et al., 2022). This can limit their therapeutic effectiveness. For example, the pharmacokinetics of quercetin in humans showed a low oral bioavailability (∼2%) after a single dosage (Li et al., 2016). The approval process for natural products as therapeutic agents is indeed intricate and lengthy. Regulatory authorities require extensive evidence to validate the efficacy, safety, and quality of PNPs, which can significantly delay their clinical availability. One major challenge is the limited scientific data on the safety profiles of many PNPs, especially concerning long-term use and their effects on vulnerable populations such as pregnant women, children, and the elderly. This lack of comprehensive safety information increases uncertainty about potential toxicity. Furthermore, while numerous PNPs demonstrate promising antiviral effects in vitro, the transition to robust clinical trials is often insufficient. This gap between preclinical findings and clinical validation hinders the acceptance of PNPs as mainstream antiviral therapies.
The potential for toxicity in PNPs is an important consideration when using these substances for therapeutic purposes. Many PNPs are safe at low doses but can become toxic when consumed in larger quantities or when used over long periods. For instance, high doses of raw garlic extract given over an extended period of time could potentially interfere with blood coagulation, cause liver toxicity, and create gastrointestinal problems (Banerjee and Maulik, 2002). Several studies indicate that coumarin-induced hepatotoxicity is relatively infrequent in humans. Clinical research, however, suggests that coumarin therapy may be associated with liver damage, which is frequently seen as high transaminase levels (Pitaro et al., 2022). The safety profile of these compounds must be thoroughly evaluated to avoid adverse effects during treatment. Some individuals may have allergic reactions to specific PNPs. These reactions can range from mild symptoms like rashes or itching to severe anaphylactic reactions such as sesquiterpene lactones, found in plants like chamomile and arnica, these compounds can cause contact dermatitis in sensitive individuals (Paulsen, 2017). Addressing these limitations will enhance the credibility and therapeutic potential of PNPs, paving the way for their integration into modern antiviral treatments.
Conclusion and perspective
Plant-extract products with antiviral activity are gaining attention as safe and affordable alternatives to traditional antiviral medications. These plant-extract products, which include flavonoids, alkaloids, terpenoids, and phenolic compounds, have the ability to inhibit viral lifecycles and stimulate cellular immune responses, making them useful in fighting viral infections. Despite their potential, therapeutic use of PNPs faces several challenges: low natural concentrations, difficulty in identifying active components, slow plant growth rates, environmental dependence, and extinction risks. Addressing these limitations is essential, particularly in the current scenario of rapid viral infections. Biotechnological platforms, such as plant cell and tissue culture technologies, offer crucial solutions for producing large volumes of plant-derived compounds. Enhancing our understanding of biosynthetic pathways and improving supply chains for commercial production are key steps in this process.
Standardization and rigorous quality control are essential for ensuring the consistency and safety of plant-derived products in clinical applications. Research into these compounds not only aids in the development of new antiviral drugs but also helps design more effective treatments by understanding their mechanisms. Combining PNPs with conventional antiviral medications can enhance efficacy and reduce the risk of resistance, offering a synergistic approach to therapy. In-depth studies and clinical trials are necessary to fully explore the antiviral potential of these compounds. Researchers are investigating the relationship between phytochemical structures and antiviral activity through bioassays, but identifying active components in complex natural extracts remains challenging. Effectiveness may also vary within the human body, emphasizing the need for further validation. The ongoing exploration of PNPs holds significant promise for developing innovative antiviral therapies. By enhancing human antiviral responses and providing new pharmacological options, PNPs could play a crucial role in improving public health. Their nutraceutical and therapeutic properties position them as valuable candidates for combating a range of viruses, making continued research into their mechanisms, efficacy, and safety vital for the future of antiviral medicine.
Author contributions
RS (1st author): Resources, Writing–original draft, Data curation, Methodology, Visualization. ND: Data curation, Methodology, Visualization, Writing–original draft. MS: Data curation, Writing–original draft. HK: Writing–review and editing. RB: Writing–review and editing, Conceptualization. RS (6th author): Conceptualization, Data curation, Visualization, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
Author RS (6th author) was employed by Helix Biosciences.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abarova, S., Alexova, R., Dragomanova, S., Solak, A., Fagone, P., Mangano, K., et al. (2024). Emerging therapeutic potential of polyphenols from Geranium sanguineum L. In viral infections, including SARS-CoV-2. Biomolecules 14, 130. doi:10.3390/biom14010130
Abba, Y., Hassim, H., Hamzah, H., and Noordin, M. M. (2015). Antiviral activity of resveratrol against human and animal viruses. Adv. Virology 2015, 184241–184247. doi:10.1155/2015/184241
Abou Baker, D. H., Amarowicz, R., Kandeil, A., Ali, M. A., and Ibrahim, E. A. (2021). Antiviral activity of Lavandula angustifolia L. and Salvia officinalis L. essential oils against avian influenza H5N1 virus. J. Agric. Food Res. 4, 100135. doi:10.1016/j.jafr.2021.100135
Aboubakr, H. A., Nauertz, A., Luong, N. T., Agrawal, S., El-Sohaimy, S. A.A., Youssef, M. M., et al. (2016). In vitro antiviral activity of clove and ginger aqueous extracts against feline calicivirus, a surrogate for human norovirus. J. Food Prot. 79, 1001–1012. doi:10.4315/0362-028x.jfp-15-593
Adamson, C. S., Chibale, K., Goss, R. J. M., Jaspars, M., Newman, D. J., and Dorrington, R. A. (2021). Antiviral drug discovery: preparing for the next pandemic. Chem. Soc. Rev. 50, 3647–3655. doi:10.1039/d0cs01118e
Adeosun, W. B., and Loots, D. T. (2024). Medicinal plants against viral infections: a review of metabolomics evidence for the antiviral properties and potentials in plant sources. Viruses 16, 218. doi:10.3390/v16020218
Agrawal, P. K., Agrawal, C., and Blunden, G. (2020). Quercetin: antiviral significance and possible COVID-19 integrative considerations. Nat. Product. Commun. 15, 1934578X20976293. doi:10.1177/1934578x20976293
Alarabei, A. A., Abd Aziz, N. A.L., Ab Razak, N. I., Abas, R., Bahari, H., Abdullah, M. A., et al. (2024). Immunomodulating phytochemicals: an insight into their potential use in cytokine storm situations. Adv. Pharm. Bull. 14, 105–119. doi:10.34172/apb.2024.001
Alché, L. E., Ferek, G. A., Meo, M., Coto, C. E., and Maier, M. S. (2003). An antiviral meliacarpin from leaves of Melia azedarach L. Z Naturforsch C J. Biosci. 58, 215–219. doi:10.1515/znc-2003-3-413
Al-Khayri, J. M., Rashmi, R., Toppo, V., Chole, P. B., Banadka, A., Sudheer, W. N., et al. (2023). Plant secondary metabolites: the weapons for biotic stress management. Metabolites 13, 716. doi:10.3390/metabo13060716
Al-Rawi, A., Dulaimi, H., and Rawi, M. (2019). Antiviral activity of Mangifera extract on influenza virus cultivated in different cell cultures. J. Pure Appl. Microbiol. 13, 455–458. doi:10.22207/jpam.13.1.50
Alzohairy, M. A. (2016). Therapeutics role of Azadirachta indica (neem) and their active constituents in diseases prevention and treatment. Evid. Based Complement. Altern. Med. 2016, 7382506. doi:10.1155/2016/7382506
Amirzadeh, N., Moghadam, A., Niazi, A., and Afsharifar, A. (2023). Recombinant anti-HIV MAP30, a ribosome inactivating protein: against plant virus and bacteriophage. Sci. Rep. 13, 2091. doi:10.1038/s41598-023-29365-7
Amparo, T. R., Seibert, J. B., Silveira, B. M., Costa, F. S. F., Almeida, T. C., Braga, S. F. P., et al. (2021). Brazilian essential oils as source for the discovery of new anti-COVID-19 drug: a review guided by in silico study. Phytochemistry Rev. Proc. Phytochemical Soc. Eur. 20, 1013–1032. doi:10.1007/s11101-021-09754-4
Anjum, V., Arora, P., Ansari, S. H., Najmi, A. K., and Ahmad, S. (2017). Antithrombocytopenic and immunomodulatory potential of metabolically characterized aqueous extract of Carica papaya leaves. Pharm. Biol. 55, 2043–2056. doi:10.1080/13880209.2017.1346690
Arthan, D., Svasti, J., Kittakoop, P., Pittayakhachonwut, D., Tanticharoen, M., and Thebtaranonth, Y. (2002). Antiviral isoflavonoid sulfate and steroidal glycosides from the fruits of Solanum torvum. Phytochemistry 59, 459–463. doi:10.1016/s0031-9422(01)00417-4
Asres, K., Seyoum, A., Veeresham, C., Bucar, F., and Gibbons, S. (2005). Naturally derived anti-HIV agents. Phytother. Res. 19, 557–581. doi:10.1002/ptr.1629
Astani, A., Reichling, J., and Schnitzler, P. (2012). Melissa officinalis extract inhibits attachment of herpes simplex virus in vitro. Chemotherapy 58, 70–77. doi:10.1159/000335590
Ayodipupo Babalola, B., Ifeolu Akinwande, A., Otunba, A. A., Ebenezer Adebami, G., Babalola, O., and Nwufo, C. (2024). Therapeutic benefits of Carica papaya: a review on its pharmacological activities and characterization of papain. Arabian J. Chem. 17, 105369. doi:10.1016/j.arabjc.2023.105369
Babaei, F., Nassiri-Asl, M., and Hosseinzadeh, H. (2020). Curcumin (a constituent of turmeric): new treatment option against COVID-19. Food Sci. & Nutr. 8, 5215–5227. doi:10.1002/fsn3.1858
Bachar, S. C., Mazumder, K., Bachar, R., Aktar, A., and Al Mahtab, M. (2021). A review of medicinal plants with antiviral activity available in Bangladesh and mechanistic insight into their bioactive metabolites on SARS-CoV-2, HIV and HBV. Front. Pharmacol. 12, 732891. doi:10.3389/fphar.2021.732891
Bajpai, R., Srivastava, R., and Upreti, U. K. (2023). Unraveling the ameliorative potentials of native lichen Pyxine cocoes (Sw.) Nyl., during COVID 19 phase. Int. J. Biometeorology 67, 67–77. doi:10.1007/s00484-022-02386-z
Baker, R. E., Mahmud, A. S., Miller, I. F., Rajeev, M., Rasambainarivo, F., Rice, B. L., et al. (2022). Infectious disease in an era of global change. Nat. Rev. Microbiol. 20, 193–205. doi:10.1038/s41579-021-00639-z
Banerjee, S. K., and Maulik, S. K. (2002). Effect of garlic on cardiovascular disorders: a review. Nutr. J. 1, 4. doi:10.1186/1475-2891-1-4
Barak, V., Halperin, T., and Kalickman, I. (2001). The effect of Sambucol, a black elderberry-based, natural product, on the production of human cytokines: I. Inflammatory cytokines. Eur. Cytokine Netw. 12, 290–296.
Baseke, J., Musenero, M., and Mayanja-Kizza, H. (2015). Prevalence of hepatitis B and C and relationship to liver damage in HIV infected patients attending joint clinical research centre clinic (JCRC), kampala, Uganda. Afr. Health Sci. 15, 322–327. doi:10.4314/ahs.v15i2.3
Battistini, R., Rossini, I., Ercolini, C., Goria, M., Callipo, M. R., Maurella, C., et al. (2019). Antiviral activity of essential oils against hepatitis A virus in soft fruits. Food Environ. Virol. 11, 90–95. doi:10.1007/s12560-019-09367-3
Bedows, E., and Hatfield, G. M. (1982). An investigation of the antiviral activity of Podophyllum peltatum. J. Nat. Prod. 45, 725–729. doi:10.1021/np50024a015
Behzadi, A., Imani, S., Deravi, N., Mohammad Taheri, Z., Mohammadian, F., Moraveji, Z., et al. (2023). Antiviral potential of melissa officinalis L.: a literature review. Nutr. Metab. Insights 16, 11786388221146683. doi:10.1177/11786388221146683
Bekhit, A., and Bekhit, A. (2014). ChemInform abstract: natural antiviral compounds. ChemInform 45. doi:10.1002/chin.201444282
Bellavite, P., and Donzelli, A. (2020). Hesperidin and SARS-CoV-2: new light on the healthy function of citrus fruits. Antioxidants (Basel) 9, 742. doi:10.3390/antiox9080742
Bhardwaj, K., Silva, A. S., Atanassova, M., Sharma, R., Nepovimova, E., Musilek, K., et al. (2021). Conifers phytochemicals: a valuable forest with therapeutic potential. Molecules 26, 3005. doi:10.3390/molecules26103005
Bhatla, S. C., and Lal, M. A. (2023). “Secondary metabolites,” in Plant physiology, development and metabolism (Singapore: Springer Nature Singapore), 765–808.
Bhattacharya, R., Bose, D., Maqsood, Q., Gulia, K., and Khan, A. (2024). Recent advances on the therapeutic potential with Ocimum species against COVID-19: a review. South Afr. J. Bot. 164, 188–199. doi:10.1016/j.sajb.2023.11.034
Bills, C., Xie, X., and Shi, P. Y. (2023). The multiple roles of nsp6 in the molecular pathogenesis of SARS-CoV-2. Antivir. Res. 213, 105590. doi:10.1016/j.antiviral.2023.105590
Bormann, M., Alt, M., Schipper, L., Van De Sand, L., Le-Trilling, V. T. K., Rink, L., et al. (2021). Turmeric root and its bioactive ingredient curcumin effectively neutralize SARS-CoV-2 in vitro. Viruses 13 (1914), 1914. doi:10.3390/v13101914
Botwina, P., Owczarek, K., Rajfur, Z., Ochman, M., Urlik, M., Nowakowska, M., et al. (2020). Berberine hampers influenza A replication through inhibition of MAPK/ERK pathway. Viruses 12, 344. doi:10.3390/v12030344
Bourjot, M., Leyssen, P., Eydoux, C., Guillemot, J. C., Canard, B., Rasoanaivo, P., et al. (2012). Flacourtosides A-F, phenolic glycosides isolated from Flacourtia ramontchi. J. Nat. Prod. 75, 752–758. doi:10.1021/np300059n
Boyd, M. R., Gustafson, K. R., Mcmahon, J. B., Shoemaker, R. H., O'keefe, B. R., Mori, T., et al. (1997). Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob. Agents Chemother. 41, 1521–1530. doi:10.1128/aac.41.7.1521
Brown, B., Ojha, V., Fricke, I., Al-Sheboul, S. A., Imarogbe, C., Gravier, T., et al. (2023). Innate and adaptive immunity during SARS-CoV-2 infection: biomolecular cellular markers and mechanisms. Vaccines 11, 408. doi:10.3390/vaccines11020408
Buckheit, R. W., White, E. L., Fliakas-Boltz, V., Russell, J., Stup, T. L., Kinjerski, T. L., et al. (1999). Unique anti-human immunodeficiency virus activities of the nonnucleoside reverse transcriptase inhibitors calanolide A, costatolide, and dihydrocostatolide. Antimicrob. Agents Chemother. 43, 1827–1834. doi:10.1128/aac.43.8.1827
Caamal-Herrera, I., Muñoz-Rodriguez, D., Madera-Santana, T. J., and Azamar, A. (2016). Identification of volatile compounds in essential oil and extracts of Ocimum micranthum Willd leaves using GC/MS. Int. J. Appl. Res. Nat. Prod. 9, 31.
Cadman, C. H. (1959). Some properties of an inhibitor of virus infection from leaves of raspberry. Microbiology 20, 113–128. doi:10.1099/00221287-20-1-113
Carty, M., Guy, C., and Bowie, A. G. (2021). Detection of viral infections by innate immunity. Biochem. Pharmacol. 183, 114316. doi:10.1016/j.bcp.2020.114316
Chang, J. S., Wang, K. C., Yeh, C. F., Shieh, D. E., and Chiang, L. C. (2013). Fresh ginger (Zingiber officinale) has anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J. Ethnopharmacol. 145, 146–151. doi:10.1016/j.jep.2012.10.043
Charlebois, D., Byers, P. L., Finn, C. E., and Thomas, A. L. (2010). Elderberry: botany, horticulture, potential. Hortic. Rev. 37 (37), 213–280. doi:10.1002/9780470543672.ch4
Chassagne, F., Samarakoon, T., Porras, G., Lyles, J. T., Dettweiler, M., Marquez, L., et al. (2021). A systematic review of plants with antibacterial activities: a taxonomic and phylogenetic perspective. Front. Pharmacol. 11, 586548. doi:10.3389/fphar.2020.586548
Chattopadhyay, D., and Naik, T. N. (2007). Antivirals of ethnomedicinal origin: structure-activity relationship and scope. Mini Rev. Med. Chem. 7, 275–301. doi:10.2174/138955707780059844
Chavan, R., and Chowdhary, A. (2014). In vitro inhibitory activity of Justicia adhatoda extracts against influenza virus infection and hemagglutination. Int. J. Pharm. Sci. Rev. Res. 25, 231–236.
Chen, H., Zhang, R., Luo, R.-H., Yang, L.-M., Wang, R.-R., Hao, X.-J., et al. (2017). Anti-HIV activities and mechanism of 12-O-Tricosanoylphorbol-20-acetate, a novel Phorbol ester from Ostodes katharinae. Molecules 22, 1498. doi:10.3390/molecules22091498
Chen, J. L., Blanc, P., Stoddart, C. A., Bogan, M., Rozhon, E. J., Parkinson, N., et al. (1998). New iridoids from the medicinal plant Barleria prionitis with potent activity against respiratory syncytial virus. J. Nat. Prod. 61, 1295–1297. doi:10.1021/np980086y
Chen, M. H., Lee, M. Y., Chuang, J. J., Li, Y. Z., Ning, S. T., Chen, J. C., et al. (2012). Curcumin inhibits HCV replication by induction of heme oxygenase-1 and suppression of AKT. Int. J. Mol. Med. 30, 1021–1028. doi:10.3892/ijmm.2012.1096
Cheng, H.-Y., Lin, T.-C., Yang, C.-M., Shieh, D.-E., and Lin, C.-C. (2005). In vitro anti-HSV-2 activity and mechanism of action of proanthocyanidin A-1 from Vaccinium vitis-idaea. J. Sci. Food Agric. 85, 10–15. doi:10.1002/jsfa.1958
Cheung, Y. Y. H., Lau, E. H. Y., Yin, G., Lin, Y., Cowling, B. J., and Lam, K. F. (2024). Effectiveness of vaccines and antiviral drugs in preventing severe and fatal COVID-19, Hong Kong. Emerg. Infect. Dis. 30, 70–78. doi:10.3201/eid3001.230414
Chiang, L. C., Chiang, W., Chang, M. Y., and Lin, C. C. (2003). In vitro cytotoxic, antiviral and immunomodulatory effects of Plantago major and Plantago asiatica. Am. J. Chin. Med. 31, 225–234. doi:10.1142/s0192415x03000874
Choi, J. G., Lee, H., Kim, Y. S., Hwang, Y. H., Oh, Y. C., Lee, B., et al. (2019). Aloe vera and its components inhibit influenza A virus-induced autophagy and replication. Am. J. Chin. Med. 47, 1307–1324. doi:10.1142/s0192415x19500678
Chojnacka, K., Skrzypczak, D., Izydorczyk, G., Mikula, K., Szopa, D., and Witek-Krowiak, A. (2021). Antiviral properties of polyphenols from plants. Foods 10, 2277. doi:10.3390/foods10102277
Cinatl, J., Morgenstern, B., Bauer, G., Chandra, P., Rabenau, H., and Doerr, H. W. (2003). Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet 361, 2045–2046. doi:10.1016/s0140-6736(03)13615-x
Constant, O., Maarifi, G., Blanchet, F. P., Van De Perre, P., Simonin, Y., and Salinas, S. (2022). Role of dendritic cells in viral brain infections. Front. Immunol. 13, 862053. doi:10.3389/fimmu.2022.862053
Croaker, A., King, G. J., Pyne, J. H., Anoopkumar-Dukie, S., and Liu, L. (2016). Sanguinaria canadensis: traditional medicine, phytochemical composition, biological activities and current uses. Int. J. Mol. Sci. 17, 1414. doi:10.3390/ijms17091414
Croft, K. D. (1998). The chemistry and biological effects of flavonoids and phenolic acidsa. Ann. N. Y. Acad. Sci. 854, 435–442. doi:10.1111/j.1749-6632.1998.tb09922.x
De Sousa, D. P., Damasceno, R. O. S., Amorati, R., Elshabrawy, H. A., De Castro, R. D., Bezerra, D. P., et al. (2023). Essential oils: chemistry and pharmacological activities. Biomolecules 13, 1144. doi:10.3390/biom13071144
El-Ansari, M., Ibrahim, L., and Sharaf, M. (2020). Anti-HIV activity of some natural phenolics. Herba Pol. 66, 34–43. doi:10.2478/hepo-2020-0010
Elshafie, H. S., Camele, I., and Mohamed, A. A. (2023). A comprehensive review on the biological, agricultural and pharmaceutical properties of secondary metabolites based-plant origin. Int. J. Mol. Sci. 24, 3266. doi:10.3390/ijms24043266
Emau, P., Tian, B., O'keefe B, R., Mori, T., Mcmahon, J. B., Palmer, K. E., et al. (2007). Griffithsin, a potent HIV entry inhibitor, is an excellent candidate for anti-HIV microbicide. J. Med. Primatol. 36, 244–253. doi:10.1111/j.1600-0684.2007.00242.x
Erdelmeier, C. A., Cinatl, J., Rabenau, H., Doerr, H. W., Biber, A., and Koch, E. (1996). Antiviral and antiphlogistic activities of Hamamelis virginiana bark. Planta Med. 62, 241–245. doi:10.1055/s-2006-957868
Ferrucci, V., Miceli, M., Pagliuca, C., Bianco, O., Castaldo, L., Izzo, L., et al. (2024). Modulation of innate immunity related genes resulting in prophylactic antimicrobial and antiviral properties. J. Transl. Med. 22, 574. doi:10.1186/s12967-024-05378-2
Frazzoli, C., Grasso, G., Husaini, D. C., Ajibo, D. N., Orish, F. C., and Orisakwe, O. E. (2023). Immune system and epidemics: the role of african indigenous bioactive substances. Nutrients 15, 273. doi:10.3390/nu15020273
Gamil, S. G. Z., and Abeer, M. A. (2023). “Antiviral plant extracts: a treasure for treating viral diseases,” in Antiviral strategies in the treatment of human and animal viral infections. Editor P. Arli Aditya (London, United Kingdom: Rijeka: IntechOpen).
Ganjhu, R. K., Mudgal, P. P., Maity, H., Dowarha, D., Devadiga, S., Nag, S., et al. (2015). Herbal plants and plant preparations as remedial approach for viral diseases. Virusdisease 26, 225–236. doi:10.1007/s13337-015-0276-6
Garozzo, A., Timpanaro, R., Bisignano, B., Furneri, P. M., Bisignano, G., and Castro, A. (2009). In vitro antiviral activity of Melaleuca alternifolia essential oil. Lett. Appl. Microbiol. 49 (6), 806–808. doi:10.1111/j.1472-765x.2009.02740.x
Garozzo, A., Timpanaro, R., Stivala, A., Bisignano, G., and Castro, A. (2011). Activity of Melaleuca alternifolia (tea tree) oil on Influenza virus A/PR/8: study on the mechanism of action. Antivir. Res. 89, 83–88. doi:10.1016/j.antiviral.2010.11.010
Gasmi, A., Shanaida, M., Oleshchuk, O., Semenova, Y., Mujawdiya, P. K., Ivankiv, Y., et al. (2023). Natural ingredients to improve immunity. Pharmaceuticals 16, 528. doi:10.3390/ph16040528
Gelderblom, H. R. (1996). “Structure and classification of viruses,” in Medical microbiology. Editor S. Baron (Galveston (TX): University of Texas Medical Branch at Galveston).
Ghaebi, M., Osali, A., Valizadeh, H., Roshangar, L., and Ahmadi, M. (2020). Vaccine development and therapeutic design for 2019-nCoV/SARS-CoV-2: challenges and chances. J. Cell Physiol. 235, 9098–9109. doi:10.1002/jcp.29771
Ghosh, R., Chakraborty, A., Biswas, A., and Chowdhuri, S. (2021). Identification of alkaloids from Justicia adhatoda as potent SARS CoV-2 main protease inhibitors: an in silico perspective. J. Mol. Struct. 1229, 129489. doi:10.1016/j.molstruc.2020.129489
Gilling, D. H., Kitajima, M., Torrey, J. R., and Bright, K. R. (2014). Antiviral efficacy and mechanisms of action of oregano essential oil and its primary component carvacrol against murine norovirus. J. Appl. Microbiol. 116, 1149–1163. doi:10.1111/jam.12453
Gobran, S. T., Ancuta, P., and Shoukry, N. H. (2021). A tale of two viruses: immunological insights into HCV/HIV coinfection. Front. Immunol. 12, 726419. doi:10.3389/fimmu.2021.726419
Gokhale, N. S., Vazquez, C., and Horner, S. M. (2014). Hepatitis C virus. Strategies to evade antiviral responses. Future Virol. 9, 1061–1075. doi:10.2217/fvl.14.89
Gomaa, A. A., and Abdel-Wadood, Y. A. (2021). The potential of glycyrrhizin and licorice extract in combating COVID-19 and associated conditions. Phytomedicine plus 1, 100043. doi:10.1016/j.phyplu.2021.100043
Gosse, B., Gnabre, J., Bates, R. B., Dicus, C. W., Nakkiew, P., and Huang, R. C. (2002). Antiviral saponins from Tieghemella heckelii. J. Nat. Prod. 65, 1942–1944. doi:10.1021/np020165g
Gour, A., Manhas, D., Bag, S., Gorain, B., and Nandi, U. (2021). Flavonoids as potential phytotherapeutics to combat cytokine storm in SARS-CoV -2. Phytotherapy Res. 35, 4258–4283. doi:10.1002/ptr.7092
Goyal, R. K., Majeed, J., Tonk, R., Dhobi, M., Patel, B., Sharma, K., et al. (2020). Current targets and drug candidates for prevention and treatment of SARS-CoV-2 (COVID-19) infection. Rev. Cardiovasc Med. 21, 365–384. doi:10.31083/j.rcm.2020.03.118
Gurjar, V. K., and Pal, D. (2024). “Classification of medicinal plants showing anti-viral activity, classified by family and viral infection types,” in Anti-viral metabolites from medicinal plants. Editor D. Pal (Cham: Springer International Publishing), 97–195.
Hassan, M. Z., Osman, H., Ali, M. A., and Ahsan, M. J. (2016). Therapeutic potential of coumarins as antiviral agents. Eur. J. Med. Chem. 123, 236–255. doi:10.1016/j.ejmech.2016.07.056
Hawkins, J., Baker, C., Cherry, L., and Dunne, E. (2019). Black elderberry (Sambucus nigra) supplementation effectively treats upper respiratory symptoms: a meta-analysis of randomized, controlled clinical trials. Complementary Ther. Med. 42, 361–365. doi:10.1016/j.ctim.2018.12.004
He, J., Qi, W. B., Wang, L., Tian, J., Jiao, P. R., Liu, G. Q., et al. (2013). Amaryllidaceae alkaloids inhibit nuclear-to-cytoplasmic export of ribonucleoprotein (RNP) complex of highly pathogenic avian influenza virus H5N1. Influenza Other Respir. Viruses 7, 922–931. doi:10.1111/irv.12035
Hegde, V. R., Pu, H., Patel, M., Das, P. R., Butkiewicz, N., Arreaza, G., et al. (2003). Two antiviral compounds from the plant Stylogne cauliflora as inhibitors of HCV NS3 protease. Bioorg Med. Chem. Lett. 13, 2925–2928. doi:10.1016/s0960-894x(03)00584-5
Hooda, P., Malik, R., Bhatia, S., Al-Harrasi, A., Najmi, A., Zoghebi, K., et al. (2024). Phytoimmunomodulators: a review of natural modulators for complex immune system. Heliyon 10, e23790. doi:10.1016/j.heliyon.2023.e23790
Hu, R., Teng, X., and Li, Y. (2024). Unleashing plant synthetic capacity: navigating regulatory mechanisms for enhanced bioproduction and secondary metabolite discovery. Curr. Opin. Biotechnol. 88, 103148. doi:10.1016/j.copbio.2024.103148
Hull, R. (2014). “Chapter 6 - genome composition, organization, and expression,” in Plant virology. Editor R. Hull Fifth Edition (Boston: Academic Press), 247–339.
Husson, G. P., Vilagines, P., Sarrette, B., and Vilagines, R. (1994). Study of antiviral action of total alkaloids from Haemanthus albiflos. Ann. Pharm. Fr. 52, 311–322.
Ibrahim, N. A., Mohammed, M. M. D., Farid, M. A., Abdelwahed, N. A. M., Ali, M. A., and El-Abd, E. A. W. (2015). Chemical Composition, Antiviral against avian Influenza (H5N1) Virus and Antimicrobial activities of the Essential Oils of the Leaves and Fruits of Fortunella margarita, Lour. Swingle, Growing in Egypt. J. Appl. Pharm. Sci. 5 (1), 006–012. doi:10.7324/JAPS.2015.50102
Ishida, J., Wang, H. K., Oyama, M., Cosentino, M. L., Hu, C. Q., and Lee, K. H. (2001). Anti-AIDS agents. 46. Anti-HIV activity of harman, an anti-HIV principle from Symplocos setchuensis, and its derivatives. J. Nat. Prod. 64, 958–960. doi:10.1021/np0101189
Jalal, Z., Bakour, M., and Lyoussi, B. (2021). Medicinal plants and zinc: impact on COVID-19 pandemic. ScientificWorldJournal 2021, 9632034. doi:10.1155/2021/9632034
Jayakumar, T., Hsieh, C. Y., Lee, J. J., and Sheu, J. R. (2013). Experimental and clinical pharmacology of Andrographis paniculata and its major bioactive phytoconstituent Andrographolide. Evid. Based Complement. Altern. Med. 2013, 1–16. doi:10.1155/2013/846740
Jayashankar, J., Ningaraju, G. N., Nanjundaswamy, S., Rajabathar, J. R., Karnan, M., Karthik, C. S., et al. (2024). An in-silico investigation of volatile compounds in Tulsi and Ginger as a potent inhalant for SARS-CoV-2 treatment. J. Iran. Chem. Soc. 21, 479–502. doi:10.1007/s13738-023-02939-y
Jennings, M. R., and Parks, R. J. (2020). Curcumin as an antiviral agent. Viruses 12, 1242. doi:10.3390/v12111242
Jiang, Z. Y., Liu, W. F., Zhang, X. M., Luo, J., Ma, Y. B., and Chen, J. J. (2013). Anti-HBV active constituents from Piper longum. Bioorg Med. Chem. Lett. 23, 2123–2127. doi:10.1016/j.bmcl.2013.01.118
Jin, Y. H., Min, J. S., Jeon, S., Lee, J., Kim, S., Park, T., et al. (2021). Lycorine, a non-nucleoside RNA dependent RNA polymerase inhibitor, as potential treatment for emerging coronavirus infections. Phytomedicine 86, 153440. doi:10.1016/j.phymed.2020.153440
Kapoor, P. (2017). Dengue: differently conceptualized. Indian Pediatr. 54, 505. doi:10.1007/s13312-017-1057-7
Karamichali, E., Foka, P., Papadopoulou, G., Loukaki-Gkountara, D., Andresaki, K., Koskinas, I., et al. (2022). Hepatitis viruses control host immune responses by modifying the exosomal biogenesis pathway and cargo. Int. J. Mol. Sci. 23, 10862. doi:10.3390/ijms231810862
Karsch-Völk, M., Barrett, B., Kiefer, D., Bauer, R., Ardjomand-Woelkart, K., and Linde, K. (2014). Echinacea for preventing and treating the common cold. Cochrane Database Syst. Rev. 2014, Cd000530. doi:10.1002/14651858.cd000530.pub3
Kasture, P. N., Nagabhushan, K. H., and Kumar, A. (2016). A multi-centric, double-blind, placebo-controlled, randomized, prospective study to evaluate the efficacy and safety of Carica papaya leaf extract, as empirical therapy for thrombocytopenia associated with dengue fever. J. Assoc. Physicians India 64, 15–20.
Kaur, G., Prakash, P., Srivastava, R., and Verma, P. C. (2021). Enhanced secondary metabolite production in hairy root cultures through biotic and abiotic elicitors. Springer Nat. Switz. Ag. 2020 (0), 625–660. doi:10.1007/978-3-030-30185-9_38
Kaur, R., Sharma, P., Gupta, G. K., Ntie-Kang, F., and Kumar, D. (2020). Structure-activity-relationship and mechanistic insights for anti-HIV natural products. Molecules 25, 2070. doi:10.3390/molecules25092070
Kaushik, S., Dar, L., Kaushik, S., and Yadav, J. P. (2021). Identification and characterization of new potent inhibitors of dengue virus NS5 proteinase from Andrographis paniculata supercritical extracts on in animal cell culture and in silico approaches. J. Ethnopharmacol. 267, 113541. doi:10.1016/j.jep.2020.113541
Kaushik, S., Jangra, G., Kundu, V., Yadav, J. P., and Kaushik, S. (2020). Anti-viral activity of Zingiber officinale (Ginger) ingredients against the Chikungunya virus. VirusDisease 31, 270–276. doi:10.1007/s13337-020-00584-0
Kaushik-Basu, N., Bopda-Waffo, A., Talele, T. T., Basu, A., Costa, P. R., Da Silva, A. J., et al. (2008). Identification and characterization of coumestans as novel HCV NS5B polymerase inhibitors. Nucleic Acids Res. 36, 1482–1496. doi:10.1093/nar/gkm1178
Khabour, O. F., and Hassanein, S. F. M. (2021). Use of vitamin/zinc supplements, medicinal plants, and immune boosting drinks during COVID-19 pandemic: a pilot study from Benha city, Egypt. Heliyon 7, e06538. doi:10.1016/j.heliyon.2021.e06538
Khan Yusuf, S., and Sen Das, S. (2023). Aromatherapy of Santalum album Linn. and its antiviral activity. 44, 12–20.
Kim, H. J., Yoo, H. S., Kim, J. C., Park, C. S., Choi, M. S., Kim, M., et al. (2009). Antiviral effect of Curcuma longa Linn extract against hepatitis B virus replication. J. Ethnopharmacol. 124, 189–196. doi:10.1016/j.jep.2009.04.046
Kumar, A., Sharma, P., Srivastava, R., and Verma, P. C. (2022a). “Demystifying the role of transcription factors in plant terpenoid biosynthesis,” in Plant transcription factors (Academic Press, Elsevier), 233–249.
Kumar, S., Saini, R., Suthar, P., Kumar, V., and Sharma, R. (2022b). “Plant secondary metabolites: their food and therapeutic importance,” in Plant secondary metabolites: physico-chemical properties and therapeutic applications. Editors A. K. Sharma, and A. Sharma (Singapore: Springer Nature Singapore), 371–413.
Kumari, P. S., and Priya, N. C. (2017). Antiviral activities and cytotoxicity assay of seed extracts of Piper longum and Piper nigrum on human cell lines, 42, 197–202.
Kussmann, M., Abe Cunha, D. H., and Berciano, S. (2023). Bioactive compounds for human and planetary health. Front. Nutr. 10, 1193848. doi:10.3389/fnut.2023.1193848
Kusumawati, I. (2021). A great challenge on the reproducibility of therapeutic results of phytopharmaceuticals. Phytopharmaceuticals, 1–18. doi:10.1002/9781119682059.ch1
Lam, W. Y., Leung, K. T., Law, P. T., Lee, S. M., Chan, H. L., Fung, K. P., et al. (2006). Antiviral effect of Phyllanthus nanus ethanolic extract against hepatitis B virus (HBV) by expression microarray analysis. J. Cell Biochem. 97, 795–812. doi:10.1002/jcb.20611
Law, A. H., Yang, C. L., Lau, A. S., and Chan, G. C. (2017). Antiviral effect of forsythoside A from Forsythia suspensa (Thunb.) Vahl fruit against influenza A virus through reduction of viral M1 protein. J. Ethnopharmacol. 209, 236–247. doi:10.1016/j.jep.2017.07.015
Lee, M. F., Voon, G. Z., Lim, H. X., Chua, M. L., and Poh, C. L. (2022). Innate and adaptive immune evasion by dengue virus. Front. Cell Infect. Microbiol. 12, 1004608. doi:10.3389/fcimb.2022.1004608
Li, B. Q., Fu, T., Dongyan, Y., Mikovits, J. A., Ruscetti, F. W., and Wang, J. M. (2000). Flavonoid baicalin inhibits HIV-1 infection at the level of viral entry. Biochem. Biophys. Res. Commun. 276, 534–538. doi:10.1006/bbrc.2000.3485
Li, S. Y., Chen, C., Zhang, H. Q., Guo, H. Y., Wang, H., Wang, L., et al. (2005). Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antivir. Res. 67, 18–23. doi:10.1016/j.antiviral.2005.02.007
Li, Y., Yao, J., Han, C., Yang, J., Chaudhry, M. T., Wang, S., et al. (2016). Quercetin, inflammation and immunity. Nutrients 8, 167. doi:10.3390/nu8030167
Likhitwitayawuid, K., Sritularak, B., Benchanak, K., Lipipun, V., Mathew, J., and Schinazi, R. F. (2005). Phenolics with antiviral activity from Millettia erythrocalyx and Artocarpus lakoocha. Nat. Prod. Res. 19, 177–182. doi:10.1080/14786410410001704813
Lin, L.-T., Hsu, W.-C., and Lin, C.-C. (2014a). Antiviral natural products and herbal medicines. J. Traditional Complementary Med. 4, 24–35. doi:10.4103/2225-4110.124335
Lin, L. T., Hsu, W. C., and Lin, C. C. (2014b). Antiviral natural products and herbal medicines. J. Tradit. Complement. Med. 4, 24–35. doi:10.4103/2225-4110.124335
Luo, G. G., and Gao, S. J. (2020). Global health concerns stirred by emerging viral infections. J. Med. Virol. 92, 399–400. doi:10.1002/jmv.25683
Mahmoudieh, M., Naghavi, M. R., Sobri, Z. M., Azzeme, A. M., Abd-Aziz, N., Nik Abd Rahman, N. M. A., et al. (2024). Biotechnological approaches in the production of plant secondary metabolites for treating human viral diseases: prospects and challenges. Biocatal. Agric. Biotechnol. 59, 103249. doi:10.1016/j.bcab.2024.103249
Marcellin, P., and Boyer, N. (2003). Chronic viral hepatitis. Best Pract. & Res. Clin. Gastroenterology 17, 259–275. doi:10.1016/s1521-6918(03)00014-3
Mazzanti, G., Battinelli, L., Pompeo, C., Serrilli, A. M., Rossi, R., Sauzullo, I., et al. (2008). Inhibitory activity of Melissa officinalis L. extract on Herpes simplex virus type 2 replication. Nat. Prod. Res. 22, 1433–1440. doi:10.1080/14786410802075939
Mcnab, F., Mayer-Barber, K., Sher, A., Wack, A., and O'garra, A. (2015). Type I interferons in infectious disease. Nat. Rev. Immunol. 15, 87–103. doi:10.1038/nri3787
Mieres-Castro, D., Ahmar, S., Shabbir, R., and Mora-Poblete, F. (2021). Antiviral activities of Eucalyptus essential oils: their effectiveness as therapeutic targets against human viruses. Pharmaceuticals 14, 1210. doi:10.3390/ph14121210
Millet, J. K., Séron, K., Labitt, R. N., Danneels, A., Palmer, K. E., Whittaker, G. R., et al. (2016). Middle East respiratory syndrome coronavirus infection is inhibited by griffithsin. Antivir. Res. 133, 1–8. doi:10.1016/j.antiviral.2016.07.011
Mishra, J., Srivastava, R., Trivedi, P. K., and Verma, P. C. (2020). Effect of virus infection on the secondary metabolite production and phytohormone biosynthesis in plants. 3 Biotech. 10, 547. doi:10.1007/s13205-020-02541-6
Mohamed, F. F., Anhlan, D., Schöfbänker, M., Schreiber, A., Classen, N., Hensel, A., et al. (2022). Hypericum perforatum and its ingredients hypericin and pseudohypericin demonstrate an antiviral activity against SARS-CoV-2. Pharm. (Basel) 15, 530. doi:10.3390/ph15050530
Mohammed Ail, A. A.-G, Thaer, A. H., and Marwa Mohammed, A. J. (2020). Inhibition of viral infection by using of natural herbal remedies as alternative treatment. System. Revie. Pharmacy. 11 (6), 416–419. doi:10.31838/srp.2020.6.66
Mohanty, M. C., and Murhekar, M. M. (2024). “Gastrointestinal viral diseases and the assessment of effectiveness of herbal drugs in prevention and treatment,” in Anti-viral metabolites from medicinal plants. Editor D. Pal (Cham: Springer International Publishing), 429–460.
Morales-Sánchez, A., and Fuentes-Pananá, E. M. (2014). Human viruses and cancer. Viruses 6, 4047–4079. doi:10.3390/v6104047
Mpiana, P. T., Ngbolua, K. T., Tshibangu, D. S. T., Kilembe, J. T., Gbolo, B. Z., Mwanangombo, D. T., et al. (2020). Identification of potential inhibitors of SARS-CoV-2 main protease from Aloe vera compounds: a molecular docking study. Chem. Phys. Lett. 754, 137751. doi:10.1016/j.cplett.2020.137751
Mueller, S. N., and Rouse, B. T. (2008). Immune responses to viruses. Clin. Immunol., 421–431. doi:10.1016/B978-0-323-04404-2.10027-2
Mukherjee, A. G., Bradu, P., Biswas, A., Wanjari, U. R., Renu, K., Kannampuzha, S., et al. (2024). “Exploring medicinal plant resources for combating viral diseases, including COVID-19,” in Medicinal plants and antimicrobial therapies. Editors V. Kumar, V. Shriram, and A. Dey (Singapore: Springer Nature Singapore), 125–141.
Mukherjee, H., Ojha, D., Bag, P., Chandel, H. S., Bhattacharyya, S., Chatterjee, T. K., et al. (2013). Anti-herpes virus activities of Achyranthes aspera: an indian ethnomedicine, and its triterpene acid. Microbiol. Res. 168, 238–244. doi:10.1016/j.micres.2012.11.002
Mukhtar, M., Arshad, M., Ahmad, M., Pomerantz, R. J., Wigdahl, B., and Parveen, Z. (2008). Antiviral potentials of medicinal plants. Virus Res. 131, 111–120. doi:10.1016/j.virusres.2007.09.008
Müller, C., Schulte, F. W., Lange-Grünweller, K., Obermann, W., Madhugiri, R., Pleschka, S., et al. (2018). Broad-spectrum antiviral activity of the eIF4A inhibitor silvestrol against corona- and picornaviruses. Antivir. Res. 150, 123–129. doi:10.1016/j.antiviral.2017.12.010
Munir, S., Liu, Z.-W., Tariq, T., Rabail, R., Kowalczewski, P. Ł., Lewandowicz, J., et al. (2022). Delving into the therapeutic potential of Carica papaya leaf against thrombocytopenia. Molecules 27, 2760. doi:10.3390/molecules27092760
Murakami, Y., Koyabu, T., Kawashima, A., Kakibuchi, N., Kawakami, T., Takaguchi, K., et al. (2007). Zinc supplementation prevents the increase of transaminase in chronic hepatitis C patients during combination therapy with pegylated interferon.ALPHA.-2b and ribavirin. J. Nutr. Sci. Vitaminol. (Tokyo) 53, 213–218. doi:10.3177/jnsv.53.213
Mw, T. (2014). What is a virus? Viruses Man A Hist. Interact. 22, 23–40. doi:10.1007/978-3-319-07758-1_2
Nalanagula, M. (2020). Cynodon dactylon against SARS-CoV-2 (COVID-19): exploratory considerations for quick-fix pandemic speed. doi:10.13140/RG.2.2.28950.98889
Nesari, T. M., Bhardwaj, A., Shrikrishna, R., Ruknuddin, G., Ghildiyal, S., Das, A., et al. (2021). Neem (azadirachta indica A. Juss) capsules for prophylaxis of COVID-19 infection: a pilot, double-blind, randomized controlled trial. Altern. Ther. Health Med. 27, 196–203.
Newman, D. J., and Cragg, G. M. (2020). Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 83, 770–803. doi:10.1021/acs.jnatprod.9b01285
Nie, L. X., Wu, Y. L., Dai, Z., and Ma, S. C. (2020). Antiviral activity of Isatidis Radix derived glucosinolate isomers and their breakdown products against influenza A in vitro/ovo and mechanism of action. J. Ethnopharmacol. 251, 112550. doi:10.1016/j.jep.2020.112550
Nikiema, W. A., Ouédraogo, M., Ouédraogo, W. P., Fofana, S., Ouédraogo, B. H. A., Delma, T. E., et al. (2024). Systematic review of chemical compounds with immunomodulatory action isolated from african medicinal plants. Molecules 29, 2010. doi:10.3390/molecules29092010
Nivetha, R., Bhuvaragavan, S., Muthu Kumar, T., Ramanathan, K., and Janarthanan, S. (2022). Inhibition of multiple SARS-CoV-2 proteins by an antiviral biomolecule, seselin from Aegle marmelos deciphered using molecular docking analysis. J. Biomol. Struct. Dyn. 40, 11070–11081. doi:10.1080/07391102.2021.1955009
Notka, F., Meier, G., and Wagner, R. (2004). Concerted inhibitory activities of Phyllanthus amarus on HIV replication in vitro and ex vivo. Antivir. Res. 64, 93–102. doi:10.1016/s0166-3542(04)00129-9
Olila, D., Olwa, O., and Opuda-Asibo, J. (2002). Screening extracts of Zanthoxylum chalybeum and Warburgia ugandensis for activity against measles virus (Swartz and Edmonston strains) in vitro. Afr. Health Sci. 2, 2–10.
Omrani, M., Keshavarz, M., Nejad Ebrahimi, S., Mehrabi, M., Mcgaw, L. J., Ali Abdalla, M., et al. (2021). Potential natural products against respiratory viruses: a perspective to develop anti-COVID-19 medicines. Front. Pharmacol. 11, 586993. doi:10.3389/fphar.2020.586993
Ooi, L. S., Sun, S. S., and Ooi, V. E. (2004). Purification and characterization of a new antiviral protein from the leaves of Pandanus amaryllifolius (Pandanaceae). Int. J. Biochem. Cell Biol. 36, 1440–1446. doi:10.1016/j.biocel.2004.01.015
Pal, A., and Chakravarty, A. K. (2020). “Chapter 4 - basic concepts of immunogenetics,” in Genetics and breeding for disease resistance of livestock. Editors A. Pal, and A. K. Chakravarty (Academic Press), 95–100.
Pal, D., and Lal, P. (2024). “Plants showing anti-viral activity with emphasis on secondary metabolites and biological screening,” in Anti-viral metabolites from medicinal plants. Editor D. Pal (Cham: Springer International Publishing), 29–95.
Palshetkar, A., Pathare, N., Jadhav, N., Pawar, M., Wadhwani, A., Kulkarni, S., et al. (2020). In vitro anti-HIV activity of some Indian medicinal plant extracts. BMC Complement. Med. Ther. 20, 69. doi:10.1186/s12906-020-2816-x
Paludan, S. R., Bowie, A. G., Horan, K. A., and Fitzgerald, K. A. (2011). Recognition of herpesviruses by the innate immune system. Nat. Rev. Immunol. 11, 143–154. doi:10.1038/nri2937
Panda, S. K., Padhi, L., Leyssen, P., Liu, M., Neyts, J., and Luyten, W. (2017). Antimicrobial, anthelmintic, and antiviral activity of plants traditionally used for treating infectious disease in the similipal biosphere reserve, odisha, India. Front. Pharmacol. 8, 658. doi:10.3389/fphar.2017.00658
Pandey, B., Prakash, P., Verma, P. C., and Srivastava, R. (2019). Regulated gene expression by synthetic modulation of the promoter architecture in plants. Curr. Dev. Biotechnol. Bioeng. Synthetic Biol. Cell Eng. Bioprocess. Technol., 235–255. doi:10.1016/B978-0-444-64085-7.00010-1
Pandey, P., Singhal, D., Khan, F., and Arif, M. (2021). An in silico screening on piper nigrum, Syzygium aromaticum and zingiber officinale roscoe derived compounds against SARS-CoV-2: a drug repurposing approach. Biointerface Res. Appl. Chem. 11, 11122–11134. doi:10.33263/BRIAC114.1112211134
Pandey, V., Srivastava, R., Akhtar, N., Mishra, J., Mishra, P., and Verma, P. C. (2016). Expression of Withania somnifera steroidal glucosyltransferase gene enhances withanolide content in hairy roots. Plant Mol. Biol. Report. 34, 681–689. doi:10.1007/s11105-015-0955-x
Panteleev, M. A., Sveshnikova, A. N., Shakhidzhanov, S. S., Zamaraev, A. V., Ataullakhanov, F. I., and Rumyantsev, A. G. (2023). The ways of the virus: interactions of platelets and red blood cells with SARS-CoV-2, and their potential pathophysiological significance in COVID-19. Int. J. Mol. Sci. 24, 17291. doi:10.3390/ijms242417291
Panthong, P., Bunluepuech, K., Boonnak, N., Chaniad, P., Pianwanit, S., Wattanapiromsakul, C., et al. (2015). Anti-HIV-1 integrase activity and molecular docking of compounds from Albizia procera bark. Pharm. Biol. 53, 1861–1866. doi:10.3109/13880209.2015.1014568
Parhira, S., Yang, Z. F., Zhu, G. Y., Chen, Q. L., Zhou, B. X., Wang, Y. T., et al. (2014). In vitro anti-influenza virus activities of a new lignan glycoside from the latex of Calotropis gigantea. PLoS One 9, e104544. doi:10.1371/journal.pone.0104544
Park, J. C., Hur, J. M., Park, J. G., Hatano, T., Yoshida, T., Miyashiro, H., et al. (2002). Inhibitory effects of Korean medicinal plants and camelliatannin H from Camellia japonica on human immunodeficiency virus type 1 protease. Phytother. Res. 16, 422–426. doi:10.1002/ptr.919
Paulsen, E. (2017). Systemic allergic dermatitis caused by sesquiterpene lactones. Contact Dermat. 76, 1–10. doi:10.1111/cod.12671
Payne, S. (2017). “Chapter 28 - introduction to DNA viruses,” in Viruses. Editor S. Payne (Academic Press), 231–236.
Pebam, M., Sushma, M. V., Sankaranarayanan, S. A., Thanekar, A. M., Koyande, N., and Rengan, A. K. (2022). Antiviral perspectives of economically important Indian medicinal plants and spices. Proc. Indian Natl. Sci. Acad. 88, 392–416. doi:10.1007/s43538-022-00099-w
Perera, W. P. R. T., Liyanage, J. A., Dissanayake, K. G. C., Gunathilaka, H., Weerakoon, W. M. T. D. N., Wanigasekara, D. N., et al. (2021). Antiviral potential of selected medicinal herbs and their isolated natural products. BioMed Res. Int. 2021, 1–18. doi:10.1155/2021/7872406
Pitaro, M., Croce, N., Gallo, V., Arienzo, A., Salvatore, G., and Antonini, G. (2022). Coumarin-induced hepatotoxicity: a narrative review. Molecules 27, 9063. doi:10.3390/molecules27249063
Prakash, P., Srivastava, R., and Verma, P. C. (2021). “Transcription factor and microRNA-mediated manipulation of the tropane alkaloids biosynthesis,” in Tropane alkaloids (Singapore: Springer), 157–172.
Prasad, S., Lownik, E., and Ricco, J. (2017). “Viral infections of the respiratory tract,” in Family medicine: principles and practice. Editors P. M. Paulman, R. B. Taylor, A. A. Paulman, and L. S. Nasir (Cham: Springer International Publishing), 507–517.
Que, Y., Hu, C., Wan, K., Hu, P., Wang, R., Luo, J., et al. (2022). Cytokine release syndrome in COVID-19: a major mechanism of morbidity and mortality. Int. Rev. Immunol. 41, 217–230. doi:10.1080/08830185.2021.1884248
Rampersad, S., and Tennant, P. (2018). Replication and expression strategies of viruses. Viruses, 55–82. doi:10.1016/b978-0-12-811257-1.00003-6
Rani, K. (2024). Ocimum Basilicum (basil/tulsi): a herbal sustainable treasure and its therapeutic potency. J. Bacteriol. & Mycol. Open Access 12, 36–38. doi:10.15406/jbmoa.2024.12.00370
Rao, V. B., and Yeturu, K. (2020). Possible Anti-viral effects of Neem (Azadirachta indica) on Dengue virus. bioRxiv 2020, 069567. doi:10.1101/2020.04.29.069567
Raskin, I., Ribnicky, D. M., Komarnytsky, S., Ilic, N., Poulev, A., Borisjuk, N., et al. (2002). Plants and human health in the twenty-first century. Trends Biotechnol. 20, 522–531. doi:10.1016/s0167-7799(02)02080-2
Read, S. A., Obeid, S., Ahlenstiel, C., and Ahlenstiel, G. (2019). The role of zinc in antiviral immunity. Adv. Nutr. 10, 696–710. doi:10.1093/advances/nmz013
Rimando, A. M., Pezzuto, J. M., Farnsworth, N. R., Santisuk, T., Reutrakul, V., and Kawanishi, K. (1994). New lignans from Anogeissus acuminata with HIV-1 reverse transcriptase inhibitory activity. J. Nat. Prod. 57, 896–904. doi:10.1021/np50109a004
Rizzato, G., Scalabrin, E., Radaelli, M., Capodaglio, G., and Piccolo, O. (2017). A new exploration of licorice metabolome. Food Chem. 221, 959–968. doi:10.1016/j.foodchem.2016.11.068
Roossinck, M. J. (2011). The good viruses: viral mutualistic symbioses. Nat. Rev. Microbiol. 9, 99–108. doi:10.1038/nrmicro2491
Rouf, R., Uddin, S. J., Sarker, D. K., Islam, M. T., Ali, E. S., Shilpi, J. A., et al. (2020). Antiviral potential of garlic (Allium sativum) and its organosulfur compounds: a systematic update of pre-clinical and clinical data. Trends Food Sci. Technol. 104, 219–234. doi:10.1016/j.tifs.2020.08.006
Rouse, B. T., and Sehrawat, S. (2010). Immunity and immunopathology to viruses: what decides the outcome? Nat. Rev. Immunol. 10, 514–526. doi:10.1038/nri2802
Saddiqa, A., Shazad, U., and Arshad, S. (2024). The effectiveness of common household spices and herbs in combating the COVID-19: a review based on experimental and virtual screening. J. Herb. Med. 46, 100887. doi:10.1016/j.hermed.2024.100887
Sahoo, J. P., Behera, L., Praveena, J., Sawant, S., Mishra, A., Sharma, S. S., et al. (2021). The golden spice turmeric (Curcuma longa) and its feasible benefits in prospering human health—a review. Am. J. Plant Sci. 12, 455–475. doi:10.4236/ajps.2021.123030
Salasc, F., Lahlali, T., Laurent, E., Rosa-Calatrava, M., and Pizzorno, A. (2022a). Treatments for COVID-19: lessons from 2020 and new therapeutic options. Curr. Opin. Pharmacol. 62, 43–59. doi:10.1016/j.coph.2021.11.002
Salasc, F., Lahlali, T., Laurent, E., Rosa-Calatrava, M., and Pizzorno, A. (2022b). Treatments for COVID-19: lessons from 2020 and new therapeutic options. Curr. Opin. Pharmacol. 62, 43–59. doi:10.1016/j.coph.2021.11.002
Samra, R., Soliman, A., Zaki, A., El Gendy, A. E. N., Hassan, M., and Zaghloul, A. (2020). Chemical composition, antiviral and cytotoxic activities of essential oil fromCyperus rotundusgrowing in Egypt: evidence from chemometrics analysis. J. Essent. Oil-Bearing Plants 23, 648–659. doi:10.1080/0972060x.2020.1823892
Sao, A., Nimbekar, T., Venkateswarlu, G., Mishra, M., Kate, A., Chauhan Kanwar, M., et al. (2024). Exploring the efficacy of traditional herbs in combating COVID-19: a comprehensive review. Coronaviruses 5, 8–21. doi:10.2174/0126667975253901231108092829
Sarker, M. M. R., Khan, F., and Mohamed, I. N. (2021). Dengue fever: therapeutic potential of Carica papaya L. Leaves. Front. Pharmacol. 12, 610912. doi:10.3389/fphar.2021.610912
Sasi, M., Kumar, S., Kumar, M., Thapa, S., Prajapati, U., Tak, Y., et al. (2021). Garlic (Allium sativum L.) bioactives and its role in alleviating oral pathologies. Antioxidants 10, 1847. doi:10.3390/antiox10111847
Schwarz, S., Sauter, D., Wang, K., Zhang, R., Sun, B., Karioti, A., et al. (2014). Kaempferol derivatives as antiviral drugs against the 3a channel protein of coronavirus. Planta Med. 80, 177–182. doi:10.1055/s-0033-1360277
Seetaha, S., Hannongbua, S., Rattanasrisomporn, J., and Choowongkomon, K. (2021). Novel peptides with HIV-1 reverse transcriptase inhibitory activity derived from the fruits of Quercus infectoria. Chem. Biol. Drug Des. 97, 157–166. doi:10.1111/cbdd.13770
Serkedjieva, J., and Velcheva, M. (2003). In vitro anti-influenza virus activity of the pavine alkaloid (-)-thalimonine isolated from Thalictrum simplex L. Antivir. Chem. Chemother. 14, 75–80. doi:10.1177/095632020301400202
Sette, A., and Crotty, S. (2021). Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 184, 861–880. doi:10.1016/j.cell.2021.01.007
Seymenska, D., Shishkova, K., Hinkov, A., Benbassat, N., Teneva, D., and Denev, P. (2023). Comparative study on phytochemical composition, antioxidant, and anti-HSV-2 activities of Sambucus nigra L. And Sambucus ebulus L. Extracts. Appl. Sci. 13, 12593. doi:10.3390/app132312593
Sezer, A., Halilović-Alihodžić, M., Vanwieren, A. R., Smajkan, A., Karić, A., Djedović, H., et al. (2022). A review on drug repurposing in COVID-19: from antiviral drugs to herbal alternatives. J. Genet. Eng. Biotechnol. 20, 78. doi:10.1186/s43141-022-00353-0
Sezer, Z., Pavel, S. T. I., Inal, A., Yetiskin, H., Kaplan, B., Uygut, M. A., et al. (2024). Long-term immunogenicity and safety of a homologous third dose booster vaccination with TURKOVAC: phase 2 clinical study findings with 32-week post-booster follow-up. Vaccines 12, 140. doi:10.3390/vaccines12020140
Sharapov, A. D., Fatykhov, R. F., Khalymbadzha, I. A., Zyryanov, G. V., Chupakhin, O. N., and Tsurkan, M. V. (2023). Plant coumarins with anti-HIV activity: isolation and mechanisms of action. Int. J. Mol. Sci. 24, 2839. doi:10.3390/ijms24032839
Sharifi, S., Fathi, N., Memar, M. Y., Hosseiniyan Khatibi, S. M., Khalilov, R., Negahdari, R., et al. (2020). Anti-microbial activity of curcumin nanoformulations: new trends and future perspectives. Phytother. Res. 34, 1926–1946. doi:10.1002/ptr.6658
Shi, Y., Zhang, B., Lu, Y., Qian, C., Feng, Y., Fang, L., et al. (2017). Antiviral activity of phenanthrenes from the medicinal plant Bletilla striata against influenza A virus. BMC Complement. Altern. Med. 17, 273. doi:10.1186/s12906-017-1780-6
Shkondrov, A., Hinkov, A., Cvetkov, V., Shishkova, K., Todorov, D., Shishkov, S., et al. (2023). Astragalus glycyphyllos L.: antiviral activity and tablet dosage formulation of a standardized dry extract. Biotechnol. & Biotechnol. Equip. 37, 2221752. doi:10.1080/13102818.2023.2221752
Shrivastava, N., Alagarasu, K., Cherian, S., and Parashar, D. (2022). Antiviral & platelet-protective properties of Carica papaya in dengue. Indian J. Med. Res. 156, 459–463. doi:10.4103/ijmr.ijmr_2406_21
Silva, J. D. M., Alves, C. E. D. C., and Pontes, G. S. (2024). Epstein-Barr virus: the mastermind of immune chaos. Front. Immunol. 15, 1297994. doi:10.3389/fimmu.2024.1297994
Silva, M. J. A., Ribeiro, L. R., Lima, K. V. B., and Lima, L. (2022). Adaptive immunity to SARS-CoV-2 infection: a systematic review. Front. Immunol. 13, 1001198. doi:10.3389/fimmu.2022.1001198
Singh, H., Koury, J., and Kaul, M. (2021a). Innate immune sensing of viruses and its consequences for the central nervous system. Viruses 13, 170. doi:10.3390/v13020170
Singh, S., Kola, P., Kaur, D., Singla, G., Mishra, V., Panesar, P. S., et al. (2021b). Therapeutic potential of nutraceuticals and dietary supplements in the prevention of viral diseases: a review. Front. Nutr. 8, 679312. doi:10.3389/fnut.2021.679312
Smith, N. C., Rise, M. L., and Christian, S. L. (2019). A comparison of the innate and adaptive immune systems in cartilaginous fish, ray-finned fish, and lobe-finned fish. Front. Immunol. 10, 2292. doi:10.3389/fimmu.2019.02292
Solis-Sanchez, D., Rivera-Piza, A., Lee, S., Kim, J., Kim, B., Choi, J. B., et al. (2020). Antiviral effects of Lindera obtusiloba leaf extract on murine norovirus-1 (MNV-1), a human norovirus surrogate, and potential application to model foods. Antibiotics 9, 697. doi:10.3390/antibiotics9100697
Song, J. M., Lee, K. H., and Seong, B. L. (2005). Antiviral effect of catechins in green tea on influenza virus. Antivir. Res. 68, 66–74. doi:10.1016/j.antiviral.2005.06.010
Srivastava, B. B. L., Ripanda, A. S., and Mwanga, H. M. (2022). Ethnomedicinal, phytochemistry and antiviral potential of turmeric (Curcuma longa). Compounds 2, 200–221. doi:10.3390/compounds2030017
Srivastava, R., Rai, K. M., and Srivastava, R. (2018). “Plant biosynthetic engineering through transcription regulation: an insight into molecular mechanisms during environmental stress,” in Biosynthetic technology and environmental challenges (Springer Nature Singapore Pte Ltd), 51–72.
Srivastava, R., Srivastava, R., and Singh, U. M. (2014). “Understanding the patterns of gene expression during climate change,” in Climate change effect on crop productivity (Boca Raton, Floida, United States: CRC Press, Taylor & Francis Group), 279–328.
St. John, A. L., and Rathore, A. P. S. (2019). Adaptive immune responses to primary and secondary dengue virus infections. Nat. Rev. Immunol. 19, 218–230. doi:10.1038/s41577-019-0123-x
Su, H.-X., Yao, S., Zhao, W.-F., Li, M.-J., Liu, J., Shang, W.-J., et al. (2020). Anti-SARS-CoV-2 activities in vitro of Shuanghuanglian preparations and bioactive ingredients. Acta Pharmacol. Sin. 41, 1167–1177. doi:10.1038/s41401-020-0483-6
Takahashi, K., Matsuda, M., Ohashi, K., Taniguchi, K., Nakagomi, O., Abe, Y., et al. (2001). Analysis of anti-rotavirus activity of extract from Stevia rebaudiana. Antivir. Res. 49, 15–24. doi:10.1016/s0166-3542(00)00134-0
Talarico, L. B., Duarte, M. E., Zibetti, R. G., Noseda, M. D., and Damonte, E. B. (2007). An algal-derived DL-galactan hybrid is an efficient preventing agent for in vitro dengue virus infection. Planta Med. 73, 1464–1468. doi:10.1055/s-2007-990241
Tan, W. C., Jaganath, I. B., Manikam, R., and Sekaran, S. D. (2013). Evaluation of antiviral activities of four local Malaysian Phyllanthus species against herpes simplex viruses and possible antiviral target. Int. J. Med. Sci. 10, 1817–1829. doi:10.7150/ijms.6902
Tesfaye, A. (2021). Revealing the therapeutic uses of garlic (Allium sativum) and its potential for drug discovery. Sci. World J. 2021, 1–7. doi:10.1155/2021/8817288
Thimmulappa, R. K., Mudnakudu-Nagaraju, K. K., Shivamallu, C., Subramaniam, K. J. T., Radhakrishnan, A., Bhojraj, S., et al. (2021). Antiviral and immunomodulatory activity of curcumin: a case for prophylactic therapy for COVID-19. Heliyon 7, e06350. doi:10.1016/j.heliyon.2021.e06350
Thomas, E., Stewart, L. E., Darley, B. A., Pham, A. M., Esteban, I., and Panda, S. S. (2021). Plant-based natural products and extracts: potential source to develop new antiviral drug candidates. Molecules 26, 6197. doi:10.3390/molecules26206197
Tiwari, P., Jena, S., and Sahu, P. (2019). Butea monosperma: phytochemistry and. Pharmacology 3, 19–26.
Tolo, F. M., Rukunga, G. M., Muli, F. W., Njagi, E. N. M., Njue, W., Kumon, K., et al. (2006). Anti-viral activity of the extracts of a Kenyan medicinal plant Carissa edulis against herpes simplex virus. J. Ethnopharmacol. 104, 92–99. doi:10.1016/j.jep.2005.08.053
Torabian, G., Valtchev, P., Adil, Q., and Dehghani, F. (2019). Anti-influenza activity of elderberry (Sambucus nigra). J. Funct. foods 54, 353–360. doi:10.1016/j.jff.2019.01.031
Tramontano, E., Corona, A., and Menéndez-Arias, L. (2019). Ribonuclease H, an unexploited target for antiviral intervention against HIV and hepatitis B virus. Antivir. Res. 171, 104613. doi:10.1016/j.antiviral.2019.104613
Tseng, C. K., Hsu, S. P., Lin, C. K., Wu, Y. H., Lee, J. C., and Young, K. C. (2017). Celastrol inhibits hepatitis C virus replication by upregulating heme oxygenase-1 via the JNK MAPK/Nrf2 pathway in human hepatoma cells. Antivir. Res. 146, 191–200. doi:10.1016/j.antiviral.2017.09.010
Tshilanda, D., Ngoyi, E., Kabengele, C., Matondo, A., Bongo, G. N., Inkoto, C., et al. (2020). Ocimum species as potential bioresources against COVID-19: a review of their phytochemistry and antiviral activity. Int. J. Pathogen Res., 42–54. doi:10.9734/ijpr/2020/v5i430143
Uzun, T., and Toptas, O. (2020). Artesunate: could be an alternative drug to chloroquine in COVID-19 treatment? Chin. Med. 15, 54. doi:10.1186/s13020-020-00336-8
Verma, H., Patil, P. R., Kolhapure, R. M., and Gopalkrishna, V. (2008). Antiviral activity of the Indian medicinal plant extract Swertia chirata against herpes simplex viruses: a study by in-vitro and molecular approach. Indian J. Med. Microbiol. 26, 322–326. doi:10.1016/s0255-0857(21)01807-7
Vicidomini, C., Roviello, V., and Roviello, G. N. (2021). Molecular basis of the therapeutical potential of clove (Syzygium aromaticum L.) and clues to its anti-COVID-19 utility. Molecules 26, 1880. doi:10.3390/molecules26071880
Vieira, S. F., Reis, R. L., Ferreira, H., and Neves, N. M. (2024). Plant-derived bioactive compounds as key players in the modulation of immune-related conditions. Phytochem. Rev. doi:10.1007/s11101-024-09955-7
Wachsman, M. B., Castilla, V., and Coto, C. E. (1998). Inhibition of foot and mouth disease virus (FMDV) uncoating by a plant-derived peptide isolated from Melia azedarach L leaves. Arch. Virol. 143, 581–590. doi:10.1007/s007050050314
Wahyuni, T., Azmi, D., Permanasari, A., Adianti, M., Tumewu, L., Widiandani, T., et al. (2019). Anti-viral activity of phyllanthus niruri against hepatitis c virus. Malays. Appl. Biol. 48, 105–111.
Wang, G. C., Li, T., Deng, F. Y., Li, Y. L., and Ye, W. C. (2013). Five new phenolic glycosides from Hedyotis scandens. Bioorg Med. Chem. Lett. 23, 1379–1382. doi:10.1016/j.bmcl.2012.12.077
Wang, L., Yang, R., Yuan, B., Liu, Y., and Liu, C. (2015). The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharm. Sin. B 5, 310–315. doi:10.1016/j.apsb.2015.05.005
Wang, Y. Q., Li, Q. S., Zheng, X. Q., Lu, J. L., and Liang, Y. R. (2021). Antiviral effects of green tea EGCG and its potential application against COVID-19. Molecules 26, 3962. doi:10.3390/molecules26133962
Webster, D., Taschereau, P., Lee, T. D., and Jurgens, T. (2006). Immunostimulant properties of Heracleum maximum bartr. J. Ethnopharmacol. 106, 360–363. doi:10.1016/j.jep.2006.01.018
White, D. W., Suzanne Beard, R., and Barton, E. S. (2012). Immune modulation during latent herpesvirus infection. Immunol. Rev. 245, 189–208. doi:10.1111/j.1600-065x.2011.01074.x
Wilder-Smith, A., Ooi, E.-E., Horstick, O., and Wills, B. (2019). Dengue. Lancet 393, 350–363. doi:10.1016/s0140-6736(18)32560-1
Wink, M. (2020). Potential of DNA intercalating alkaloids and other plant secondary metabolites against SARS-CoV-2 causing COVID-19. Diversity 12, 175. doi:10.3390/d12050175
Wu, P. L., Lin, F. W., Wu, T. S., Kuoh, C. S., Lee, K. H., and Lee, S. J. (2004). Cytotoxic and anti-HIV principles from the rhizomes of Begonia nantoensis. Chem. Pharm. Bull. (Tokyo) 52, 345–349. doi:10.1248/cpb.52.345
Wu, T.-Y., Chen, C.-C., and Lin, J.-Y. (2024). Anti-inflammatory in vitro activities of eleven selected caffeic acid derivatives based on a combination of pro−/anti-inflammatory cytokine secretions and principal component analysis – a comprehensive evaluation. Food Chem. 458, 140201. doi:10.1016/j.foodchem.2024.140201
Wu, Y. H. (2016). Naturally derived anti-hepatitis B virus agents and their mechanism of action. World J. Gastroenterol. 22, 188–204. doi:10.3748/wjg.v22.i1.188
World Health Organization (2021). WHO Coronavirus Disease (COVID-19) Dashboard. Available at: https://covid19.who.int/.
Xu, H. X., Zeng, F. Q., Wan, M., and Sim, K. Y. (1996). Anti-HIV triterpene acids from Geum japonicum. J. Nat. Prod. 59, 643–645. doi:10.1021/np960165e
Xu, J., Xu, Z., and Zheng, W. (2017). A review of the antiviral role of green tea catechins. Molecules 22, 1337. doi:10.3390/molecules22081337
Yang, C. M., Cheng, H. Y., Lin, T. C., Chiang, L. C., and Lin, C. C. (2007). The in vitro activity of geraniin and 1,3,4,6-tetra-O-galloyl-β-d-glucose isolated from Phyllanthus urinaria against herpes simplex virus type 1 and type 2 infection. J. Ethnopharmacol. 110, 555–558. doi:10.1016/j.jep.2006.09.039
Yewdell, J. W., and Hill, A. B. (2002). Viral interference with antigen presentation. Nat. Immunol. 3, 1019–1025. doi:10.1038/ni1102-1019
Yoshida, T., Ito, H., Hatano, T., Kurata, M., Nakanishi, T., Inada, A., et al. (1996). Tannins and related polyphenols from elaeagnaceous plants. Part 2. New hydrolyzable tannins, shephagenins A and B, from shepherdia argentea as HIV-1 reverse transcriptase inhibitors. Chem. Pharm. Bull. (Tokyo) 44, 1436–1439. doi:10.1248/cpb.44.1436
Zakaryan, H., Arabyan, E., Oo, A., and Zandi, K. (2017). Flavonoids: promising natural compounds against viral infections. Archives Virology 162, 2539–2551. doi:10.1007/s00705-017-3417-y
Zhang, H. J., Tan, G. T., Hoang, V. D., Hung, N. V., Cuong, N. M., Soejarto, D. D., et al. (2003). Natural anti-HIV agents. Part IV. Anti-HIV constituents from Vatica cinerea. J. Nat. Prod. 66, 263–268. doi:10.1021/np020379y
Zheng, P., Dou, Y., and Wang, Q. (2023). Immune response and treatment targets of chronic hepatitis B virus infection: innate and adaptive immunity. Front. Cell. Infect. Microbiol. 13, 1206720. doi:10.3389/fcimb.2023.1206720
Zhou, N. J., Geng, C. A., Huang, X. Y., Ma, Y. B., Zhang, X. M., Wang, J. L., et al. (2015). Anti-hepatitis B virus active constituents from Swertia chirayita. Fitoterapia 100, 27–34. doi:10.1016/j.fitote.2014.11.011
Keywords: secondary metabolite, human pathogen, virus, antiviral response, immunity, plants
Citation: Srivastava R, Dubey NK, Sharma M, Kharkwal H, Bajpai R and Srivastava R (2025) Boosting the human antiviral response in conjunction with natural plant products. Front. Nat. Produc. 3:1470639. doi: 10.3389/fntpr.2024.1470639
Received: 25 July 2024; Accepted: 11 December 2024;
Published: 07 January 2025.
Edited by:
Abdel Nasser B. Singab, Ain Shams University, EgyptReviewed by:
Ahmed Elissawy, Ain Shams University, EgyptHimanshi Tanwar, University of Maryland, United States
Copyright © 2025 Srivastava, Dubey, Sharma, Kharkwal, Bajpai and Srivastava. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rakesh Srivastava, cmFrZXNoc3JpdmFzdGF2YV9yc0B5YWhvby5jb20=; Rajesh Bajpai, YmFqcGFpZW52aXJvQGdtYWlsLmNvbQ==
†ORCID: Rashmi Srivastava, orcid.org/0000-0001-9617-0757; Neeraj Kumar Dubey, orcid.org/0000-0002-2992-7171; Harsha Kharkwal, orcid.org/0000-0002-9615-6223; Rajesh Bajpai, orcid.org/0000-0002-1806-0939; Rakesh Srivastava, orcid.org/0000-0002-6640-1216
 Rashmi Srivastava
Rashmi Srivastava Neeraj Kumar Dubey
Neeraj Kumar Dubey Megha Sharma
Megha Sharma Harsha Kharkwal
Harsha Kharkwal Rajesh Bajpai
Rajesh Bajpai Rakesh Srivastava
Rakesh Srivastava