- Department of Chemical, Biological, and Materials Engineering, TECO Clean Energy Research Center, University of South Florida - Tampa, Tampa, FL, United States
Concentration of CO2 in an indoor environment can be four to five times higher than the outdoor air. This higher indoor concentration of CO2 reduces the work efficiency of individuals working indoors and negatively impacts human health. However, the elevated concentration also makes it easier to capture CO2 from indoor air. This study examines the performance of monoethanolamine (MEA) and L-arginine (Arg) solutions for indoor carbon dioxide (CO2) capture through experimental screening. Key parameters evaluated include CO2 absorption and desorption capacity, absorption kinetics, and the impact on relative humidity (RH) and total volatile organic compound (TVOC) concentrations. Two solvent formulations were employed in this study: one utilizing pure water as the solvent and the other incorporating a water-glycol mixture. The aqueous Arg solution demonstrated minimal to no detectable increase in VOC levels and exhibited lower evaporation rates than the benchmark aqueous MEA solution. Microwave (MW) heating was utilized to facilitate rapid CO2 desorption from saturated solutions. The regeneration efficiency, solvent loss, and energy consumption were found to be dependent on the MW desorption time. Optimizing the desorption resulted in faster and almost complete regeneration, minimized solvent loss, and reduced overall energy consumption. The incorporation of glycol minimized evaporation during absorption, decreased the likelihood of complete drying during desorption, and improved solution regeneration. Cyclic absorption-desorption experiments were conducted to evaluate the long-term stability and kinetic performance of the solutions. While the aqueous MEA solution experienced significantly larger declines of 54.3% in CO2 absorption capacity and 34.24% in absorption kinetics, the water-PG-based Arg solution demonstrated promising performance, with a smaller reduction of 31.24% in CO2 absorption and a 2.13% decrease in kinetics after ten cycles. Additionally, the water-PG-based Arg solution resulted in lower volatile organic compound (VOC) levels and provided more effective control over relative humidity. These findings underscore the potential of the water-PG-based Arg solution for cyclic CO2 absorption and microwave-assisted regeneration processes.
1 Introduction
The fight against rising CO2 emissions has become a global race, with researchers focusing on two key fronts: reducing dependence on fossil fuels by making renewable energy more accessible and efficient, and advancing direct air capture (DAC) and carbon capture and storage (CCS) technologies to remove CO2 from the atmosphere. These efforts are not just about slowing emissions but also about reversing their impact—aiming to achieve net-zero CO2 emissions by 2050 and preventing global temperatures from exceeding the critical 1.5°C threshold (Ma et al., 2022; Chen et al., 2023). According to the Global Monitoring Laboratory of the National Oceanic and Atmospheric Administration, the average global CO2 concentration in 2023 was 420 ppm (0.042%), which is considered safe for humans (Global Monitoring Laboratory-NOAA, 2023). Nevertheless, indoor CO2 concentrations, especially in workplaces like offices and conference halls, often surpass recommended limits due to occupant’s exhalation, placing increased demands on heating, ventilation, and air conditioning (HVAC) systems to maintain indoor air quality (Young et al., 2024). Prolonged exposure to elevated CO2 levels can lead to health issues such as respiratory acidosis, increased heart rate, cognitive decline, and hypercapnia (Azuma et al., 2018; Jacobson et al., 2019). The Occupational Safety and Health Administration (OSHA) has established a maximum permissible exposure limit of 5,000 ppm over an 8-h workday (Young et al., 2024). However, relying solely on ventilation to meet this standard can significantly increase the system’s carbon footprint (Young et al., 2024). Additionally, research indicates that keeping CO2 levels below specific thresholds enhances the occupants’ cognitive functions and productivity (Kuramochi et al., 2023). Therefore, capturing CO2 is the only viable option to keep the CO2 level within the acceptable limit in indoor spaces. Since the concentration of CO2 in indoor air can be four to five times higher than that in outdoor air, capturing CO2 directly from indoor air can be easier than from outdoor air. The benefits of capturing CO2 from indoor air are twofold: first, it removes CO2 from the atmosphere, and second, it improves the health and work efficiency of individuals working indoors.
Chemical absorption is one of the most promising technologies for CO2 capture due to its high selectivity and scalability opportunities (Tsubaki et al., 2020). This process employs various absorbing agents, including aqueous alkali solutions (Zeman, 2007), amines (Kim et al., 2013), ionic liquids (Zhao and Baker, 2023), amine-functionalized adsorbents (Lee and Park, 2015), and amino acid solutions (Aronu et al., 2010; Wei et al., 2023), to chemically extract CO2 from air or gas streams. Among these methods, amine-based technology remains the most widely used. Researchers have shown that the interaction between CO2 and aqueous amines follows the zwitterionic mechanism. The nucleophilic lone pair on the nitrogen atom of the amine attacks the electrophilic carbon of CO2, forming zwitterionic adduct (R-NH2+-COO-), which stabilizes into carbamate (R-NH-COO-) and an alkylammonium ion (R-NH2-H+) upon deprotonation (Reaction 1) (Masiren and Harun, 2017; Lv et al., 2015; Said et al., 2020). As absorption progresses, the pH and absorption rate decrease due to the depletion of the CO2 scrubbing agent (CSA), weakening Reaction 1 (Lv et al., 2015). This favors hydration reactions (Reactions 2–5), resulting in the formation of bicarbonate (HCO₃⁻) and carbonate (CO₃2⁻) (Lv et al., 2015). Additionally, as the pH drops, carbamate formed in Reactions 1 and 2 decomposes into bicarbonate, as shown in Reaction 6 (Lv et al., 2015; Said et al., 2020). This process is reversible during desorption, as shown in Reactions 7–10 (Lv et al., 2015). The full sequence of absorption and desorption reactions is outlined in Figure 1:
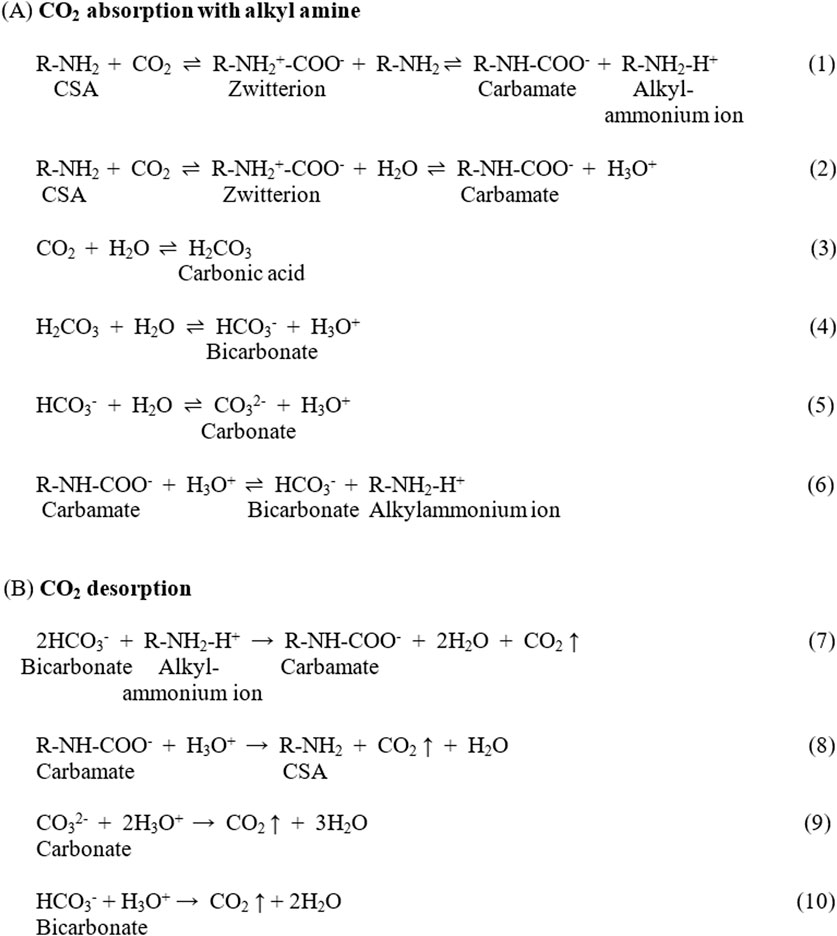
Figure 1. (A) Reaction between CO₂ and amine, presence and absence of water (Lv et al., 2015) (said et al., 2020). (B) Regeneration of amine (Lv et al., 2015) (said et al., 2020).
Alkanolamines are preferred over alkylamines for CO2 capture due to their higher boiling points and lower volatility (National Library of Medicine–Ethylamine, 2024; National Library of Medicine–Monoethanolamine, 2024), which reduces the emission of VOCs during absorption and desorption processes. Monoethanolamine (MEA) is used as a benchmark solvent due to its affordability, high water solubility, and rapid CO2 absorption rate (Chai et al., 2022). However, it still has drawbacks, such as volatility that can lead to higher TVOC emissions and may limit indoor use, along with a high energy demand for regeneration. Furthermore, it is prone to thermal and oxidative degradation, which results in increased viscosity, solution fouling, and corrosion of downstream equipment (Chai et al., 2022; Custelcean, 2022). These challenges highlight the need for alternative CSA for indoor CO2 capture. Aqueous amino acids are emerging as promising alternatives. Known for their high surface tension, they offer comparable CO2 absorption capacity with negligible vapor pressure and greater resistance to degradation (Mai et al., 2023). These properties make them well-suited for capturing CO2 in indoor applications (Mai et al., 2023; Sang Safidi and Luis, 2019), providing a viable path forward in overcoming the limitations of conventional absorbents like MEA. Arginine (Arg) specifically offers several advantages over traditional absorbents like MEA, including greater resistance to thermal and oxidative degradation. This allows the Arg solution to undergo multiple absorption-desorption cycles, reducing costs associated with frequent solution replacement. Its higher surface tension also minimizes evaporation losses during both CO2 capture and release, and its biodegradability makes Arg a more environmentally sustainable option (Guo et al., 2018). However, despite these promising attributes, only a limited number of studies have explored the role of Arg in CO2 capture (Mahmud et al., 2017; Wei et al., 2023). To the best of our knowledge, no research to date has specifically addressed CO2 capture from indoor air while simultaneously controlling TVOC and humidity levels. Maintaining optimal levels of both TVOCs and humidity is essential for safeguarding respiratory health and ensuring thermal comfort for occupants. This study aims to develop efficient and affordable CO2 capture solutions specifically designed for indoor use, with added functionality for managing TVOCs and humidity to enhance overall indoor air quality.
This study explores the potential of various CO2 capture solutions, including aqueous MEA, aqueous Arg, and water-glycol-Arg solutions, to enhance indoor air quality by regulating CO2 levels, RH, and TVOC concentrations. Through experimental evaluations, the solutions’ desorption capabilities, including regeneration efficiency, solvent stability, and visual integrity post-desorption, were analyzed using microwave irradiation at varying exposure times. A subsequent cyclic study was performed to assess the stability, safety, and long-term performance of aqueous MEA and water-PG-Arg solutions. This investigation advances our understanding of CO2 capture kinetics and positions Arg as a promising, sustainable alternative to traditional absorbents like MEA.
2 Materials and methods
2.1 Reagents
MEA (purity ≥98%), L-arginine (purity ≥98%), triethylene glycol (purity ≥98%), dipropylene glycol (purity ≥99%), diethylene glycol (purity ≥99%), and ethylene glycol (purity ≥99%) were procured from ThermoFisher Scientific. Propylene glycol (purity ≥99.8%) was sourced from Millipore Sigma. All the solutions were prepared using Milli-Q water, and the CO2 gas (purity ≥99.999 mol%) was obtained from the Airgas company.
2.2 Experimental setup and procedure
The CO2 scrubbing solutions (CSS) were formulated by adding 0.05 mol of CSA to 50 mL of solvent. For the aqueous CSS, Milli-Q water was used as the solvent, while the water-glycol-based CSS was prepared by mixing water and glycol in a 1:1 volumetric ratio based on the solubility of Arg in water.
The CO2 absorption setup consisted of a 100 mL two-neck round-bottom flask filled with the prepared CSS and connected to a condenser to minimize the loss of water and amine vapors. This setup was placed inside a sealed steel chamber measuring 32 × 33 × 36 inches (Supplementary Figure S1A), equipped with a 12 V fan to ensure thorough mixing of air within. CO2 was introduced from the top of the chamber until the concentration stabilized between 1,000 and 1,100 ppm.
The air inside was bubbled through the solution using a micro air pump during the CO2 absorption process. The experiments were performed under laboratory conditions at atmospheric pressure, with an initial relative humidity of 54% ± 5%. Changes in CCO2 (ppm), RH (%), and TVOCs (ppb) were continuously monitored using Graywolf IQ-610 sensors and logged at 30-s intervals with an advanced sense meter. As per the guidelines from Graywolf Sensing Solutions, the TVOC sensor operates accurately within a relative humidity (RH) range of 0%–90%, becoming unreliable beyond this threshold. Consequently, in our study, TVOC data were recorded until the RH reached 90%. The VOCs generated inside the chamber were collected by passing the air through 20 mL of Milli-Q water for 60 min. The resulting solution (referred to as the TVOC solution) was analyzed using Triple Quadrupole Liquid Chromatography-Mass Spectrometry (LC-QqQ-MS) and Headspace Solid-Phase Microextraction Gas Chromatography-Mass Spectrometry (HS-SPME-GC-MS), with the instruments shown in Supplementary Figure S13, S14, respectively. The operating conditions of both instruments are provided in Supplementary Figure S15, S16.
Once the CO2 absorption process was complete, water was added to replenish the solution that had evaporated due to vaporization. The solution was then transferred to a 500 mL conical flask, and a few small porcelain boiling chips were added to ensure smooth boiling and prevent bumping during the regeneration process. MW-assisted regeneration of the saturated scrubbing solution was performed using the setup shown in Supplementary Figure S2B. Initially, the temperature of the solution, along with the CCO₂, RH, and TVOC values, were recorded. The solution was then heated using a Panasonic 1200 W MW oven at heating power level 10 for varying time periods. The temperature and volume of the solution were recorded right after heating, whereas other parameters, such as TVOC and RH, were measured 5 minutes later after they had stabilized. Rather than using one prolonged MW heating duration, the desorption process was broken into shorter sessions to ensure that the solution did not dry out completely.
2.3 Quantitative analysis and kinetics
The CO₂ loading (αabs) is defined as the moles of CO₂ absorbed per unit moles of CSA, while the CO₂ unloading (αdes) represents the reverse process (Song et al., 2012). These values were calculated using the following formula based on the initial and final CO₂ concentrations.
Where ΔC is the concentration gradient, MCO₂ = 44 g/mol and ρCO₂ = 1.96 × 10−3 g/mL are the molecular weight and density of CO₂, respectively, and Vc = 623 L is the chamber volume. The amount of CO₂ absorbed or desorbed (in mg) depends on the moles of CO₂ absorbed or desorbed (nCO₂) and can be calculated using the following formula:
Additionally, based on the CO₂ absorption experimental data, the integrated rate law equation for a second-order reaction was used to model the kinetics of gas-liquid absorption reactions. The reaction rate constant (K″) was determined from the slope of the linear regression plot of 1/CCO₂ versus time.
3 Results and discussion
The performances of aqueous MEA and aqueous Arg solutions in terms of CO₂ absorption, kinetics, and their impact on RH levels and TVOC concentrations were evaluated. Although the aqueous Arg solution showed a lower capacity for CO₂ absorption (α = 0.31 mol/mol) and slower kinetics (K ˝ = 1.80 × 10−6 ppm−1 min−1) compared to the aqueous MEA solution (α = 0.40 mol/mol and K ˝ = 2.57 × 10−6 ppm−1 min−1) (Figure 2A; Supplementary Figure S3), presumably due to Arg’s larger molecular size (Suleman et al., 2020) affecting solution viscosity and hindering CO₂ diffusion, it still presents notable advantages over MEA, particularly concerning VOC emissions and RH behavior. The aqueous MEA solution caused a significant increase in TVOC levels during the absorption process, as shown in Figure 2B, whereas the aqueous Arg solution produced minimal to no detectable rise in TVOCs. Qualitative analysis of the VOCs emitted from the aqueous MEA solution using LC-QqQ-MS identified MEA as the primary volatile component (Supplementary Figure S4), as its higher vapor pressure likely contributed to elevated TVOC levels, highlighting a potential drawback of using MEA for CO₂ absorption. In contrast, the aqueous Arg solution demonstrated lower vapor pressure, as evidenced by the RH profiles. Analysis of the RH slopes revealed that the Arg solution took longer to reach 90% RH compared to the MEA solution, indicating a lower vaporization rate (Supplementary Figure S5). These findings indicate that, while the aqueous Arg solution has slightly lower CO₂ absorption capacity and slower kinetics, it is a more environmentally sustainable option due to its minimal VOC emissions, especially indoors. To further evaluate the practical application of these solutions, MW regeneration experiments were conducted to assess their sustainability for continuous absorption-desorption cycles.
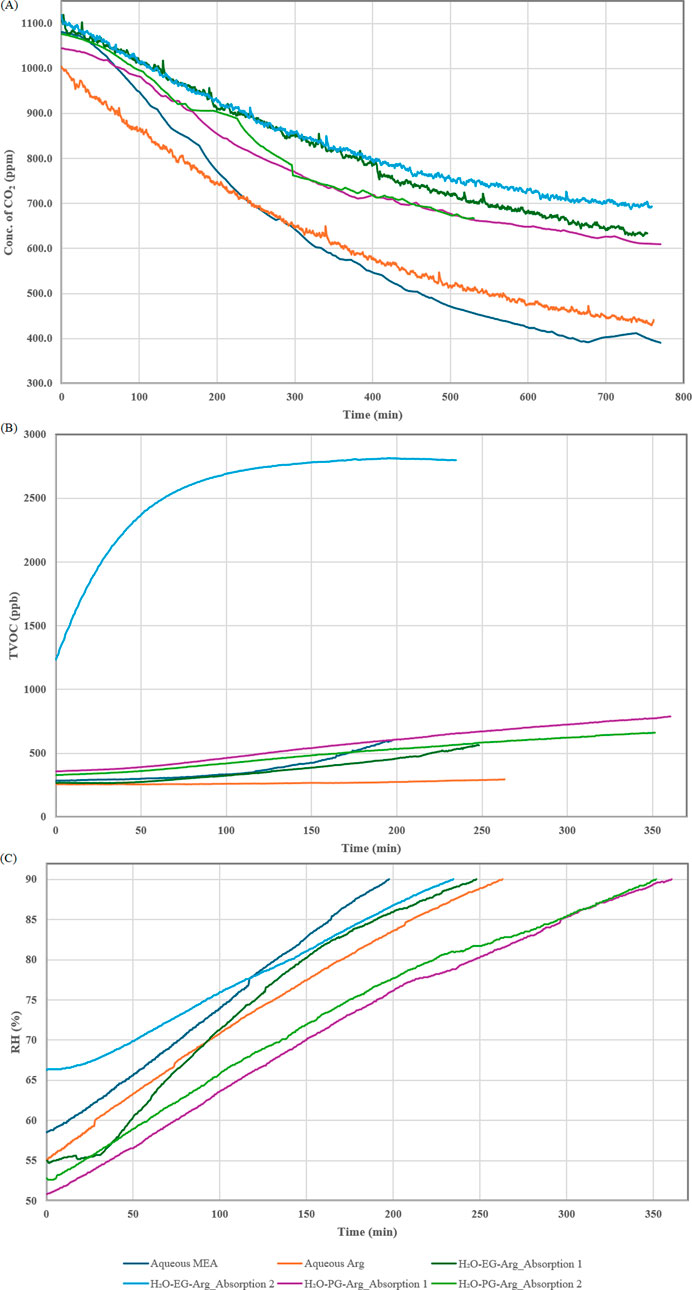
Figure 2. (A) CO₂ absorption, (B) TVOC concentration and (C) RH levels during the CO₂ absorption process for aqueous MEA, aqueous L-arginine, H₂O-EG-Arg, and H₂O-PG-Arg solution. Absorption 2 refers to the second cycle after the initial absorption-desorption cycle.
Microwave heating was employed for CO₂ desorption due to its faster process, lower energy consumption, and simpler setup compared to conventional heating (McGurk et al., 2017). The irradiation process was optimized to achieve full solution regeneration with minimal microwave irradiation time and solvent loss; to this end, a series of absorption-desorption studies were conducted using aqueous MEA solutions. The first MW irradiation time was adjusted by careful inspection of the solution during the MW session to avoid excessive boiling and bumping of the solution inside the MW chamber, while achieving maximum CO₂ desorption possible. As shown in Figures 3A, B, it was observed that increasing the first MW session beyond 150 s led to excessive solvent loss and worse CO₂ desorption outcome. The optimum MW irradiation profile was found to be an initial MW session of 150 s followed by another session of 60 s, achieving near-complete regeneration by desorbing 589 mg (99.5%) of CO₂ from 592 mg absorbed, while limiting solvent loss to just 37 mL.
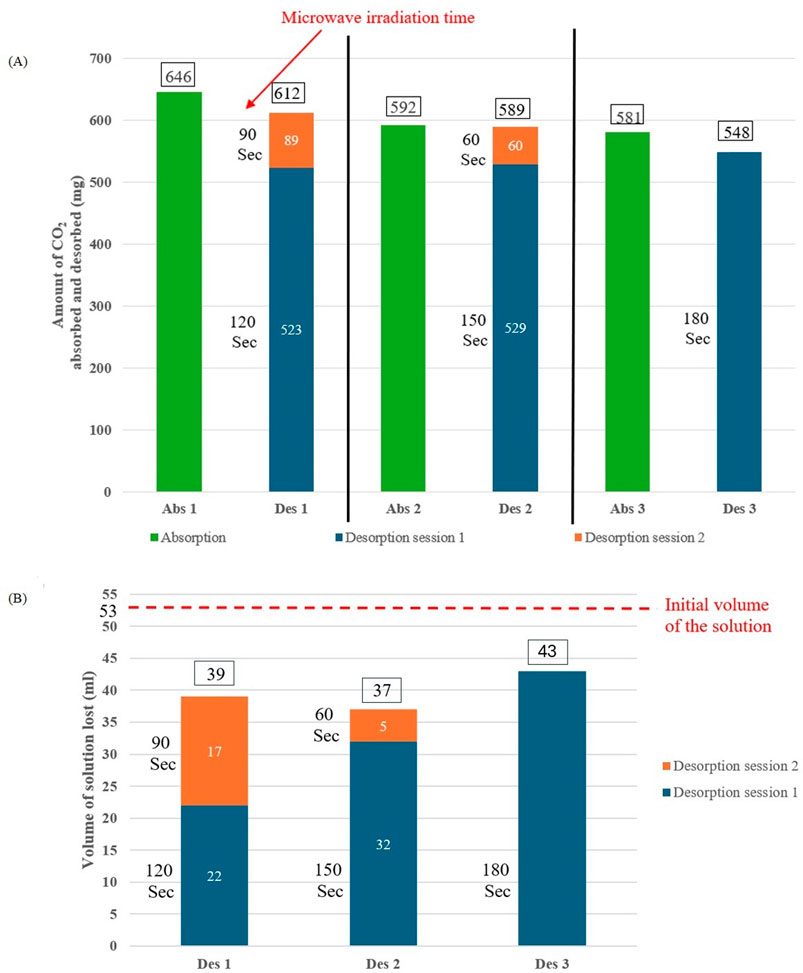
Figure 3. (A) Effect of MW irradiation time on CO2 desorption for aqueous MEA solution. (B) Effect of MW Desorption Time on volume of the solution for aqueous MEA solution.
While the optimal MW irradiation times of 150 s for the first session and 60 s for the second session were effective for aqueous MEA, they proved insufficient for aqueous Arg solution. Only 436 mg (63.5%) of CO₂ was desorbed from the 687 mg absorbed in the first session. Extending the second session to 90 s released an additional 82 mg, totaling 518 mg (75.4%), showing that the desorption remained incomplete. Prolonged MW irradiation also caused complete solvent evaporation, leaving behind solid Arg crystals, as shown in Supplementary Figure S6. To address this issue and maintain the solution in liquid form, a water-glycol-based Arg solution was formulated.
The choice of glycol with a higher boiling point was intended to manage the RH during the absorption process and to reduce complete evaporation of the solution during the desorption process. To formulate the water-glycol-based CSS, water and glycol were mixed in an optimized 1:1 volumetric ratio. A comprehensive evaluation of some commonly used glycol compounds (Taylor et al., 2024; Garg et al., 2019) was conducted, focusing on key properties such as low vapor pressure, appropriate viscosity, and high molecular weight, as shown in Supplementary Table S1. Among these, the water-EG-Arg and water-PG-Arg solutions exhibited better Arg solubility (Supplementary Figure S2) and were thus chosen for further studies.
While the water-PG-Arg solution initially showed slightly lower CO₂ absorption capacity (α = 0.24 mol/mol, K ˝ = 1.15 × 10−6 ppm−1 min−1) than the water-EG-Arg solution (α = 0.26 mol/mol, K ˝ = 0.95 × 10−6 ppm−1 min−1) (Table 1), it exhibited comparable kinetics and offered better RH control, taking longer to reach 90% RH during absorption (Figure 2C). Qualitative analysis using LC-QqQ-MS and HS-SPME-GC-MS identified only propylene glycol among the emitted VOCs, with no trace of Arg in the case of water-PG-Arg solution (Supplementary Figures S9, S10). It is worth mentioning that PG, as a VOC, is odorless, non-toxic, and has a Workplace Environmental Exposure Limit (WEEL) of 3,200 ppb, averaged over an 8-h work shift, as set by the American Industrial Hygiene Association (AIHA) (New Jersey Department of Health, 2009). This limit is at least five times higher than the levels detected in our experiment. During desorption, the water-PG-Arg solution achieved ∼90% regeneration in a shorter time, reducing energy consumption by 29% (Supplementary Table S2). Additionally, no visible color change was observed in the water-PG-Arg solution after the first desorption process (Supplementary Figure S7), whereas the water-EG-Arg solution exhibited a shift from transparent to amber, indicating possible degradation (Supplementary Figure S8).
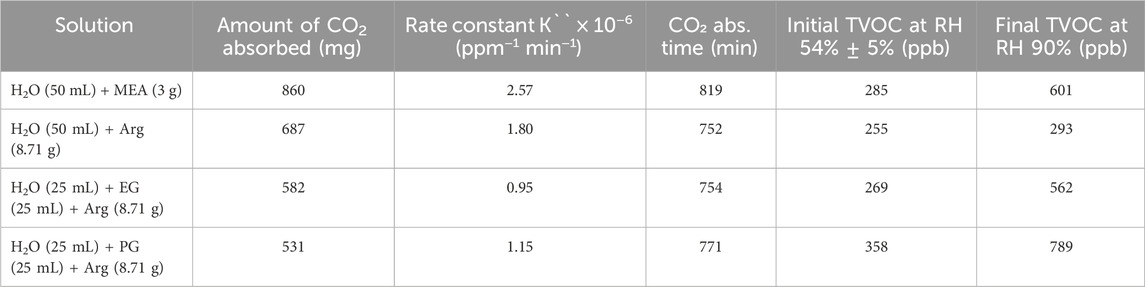
Table 1. CO₂ absorption and reaction kinetics of aqueous MEA and aqueous Arg solutions. Rate Constant (K ˝ ) is based on second-order kinetics.
In subsequent cyclic test, the water-PG-Arg solution demonstrated improved stability with consistently lower TVOC emissions during absorption (Figure 2B) and no color change (Supplementary Figure S7) after desorption processes. The water-EG-Arg solution, however, emitted notably higher TVOCs with an unpleasant pungent odor throughout the cyclic study, and its color gradually deepened to a darker red after each desorption process (Supplementary Figure S8). This degradation was likely driven by elevated temperatures during desorption processes, reaching 156°C in the first cycle and 176°C in the second, suggesting potential side reactions and thermal degradation (Supplementary Tables S4, S5). In contrast, the water-PG-Arg solution exhibited lower temperature increases during desorption (Supplementary Tables S6–S15), attributed to the higher specific heat capacity of PG (Supplementary Table S3). Additionally, PG’s lower polarity and dielectric constant limited the absorption of MW energy, reducing excessive heat generation (Sengwa, 2003; Gabriel et al., 1998). Thus, the thermal stability, improved absorption performance, and reduced energy consumption of the water-PG-Arg solution highlight its potential for long-term CO₂ capture applications. These findings support further investigation into its cyclic performance over multiple absorption-desorption processes.
The cyclic performances of aqueous MEA and water-PG-Arg solutions were evaluated over ten absorption-desorption cycles, as shown in Figures 4A, B. The reaction rate constants for both solutions across these cycles are presented in Figures 5A, B. Although the aqueous MEA solution initially exhibited higher CO₂ absorption, its performance declined significantly over time, with a 54.3% reduction in CO₂ absorption after ten cycles. In contrast, the water-PG-Arg solution demonstrated greater stability, with only a 31.24% decline over the same period, becoming increasingly more consistent as our study progressed. The steeper downward trend observed in the MEA solution (Figure 4B) highlights its limitations for long-term CO₂ capture applications. The effectiveness of the aqueous MEA solution declines over time because of oxidative and thermal degradation, leading to a reduced concentration of MEA available for CO₂ absorption. The degradation products, such as ammonia, acetaldehyde, and acetone (Chanchey et al., 2011), may pose potential risks to indoor environments. As a result, the reaction rate constant of the aqueous MEA solution decreased by 34.24% after ten cycles, compared to only a 2.13% reduction for the water-PG-Arg solution. These findings highlight the superior stability and long-term viability of the water-PG-Arg solution for indoor CO₂ capture applications.
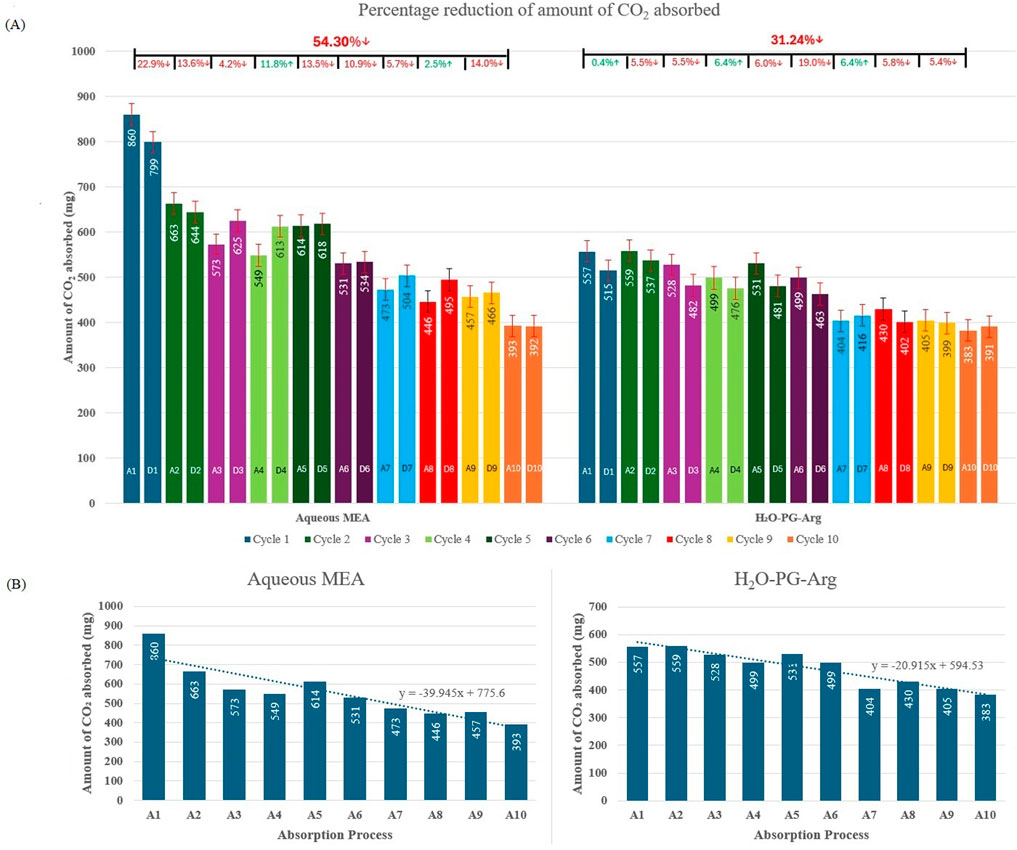
Figure 4. (A) Absorption-desorption performance over ten cycles of aqueous MEA and H2O-PG-Arg solution. The percentage reduction of the amount of CO2 absorbed after each absorption process, as well as the cumulative reduction after ten cycles, is shown at the top of the figure. (B) Absorption-desorption performance over ten cycles of aqueous MEA and H2O-PG-Arg solution with trendline.
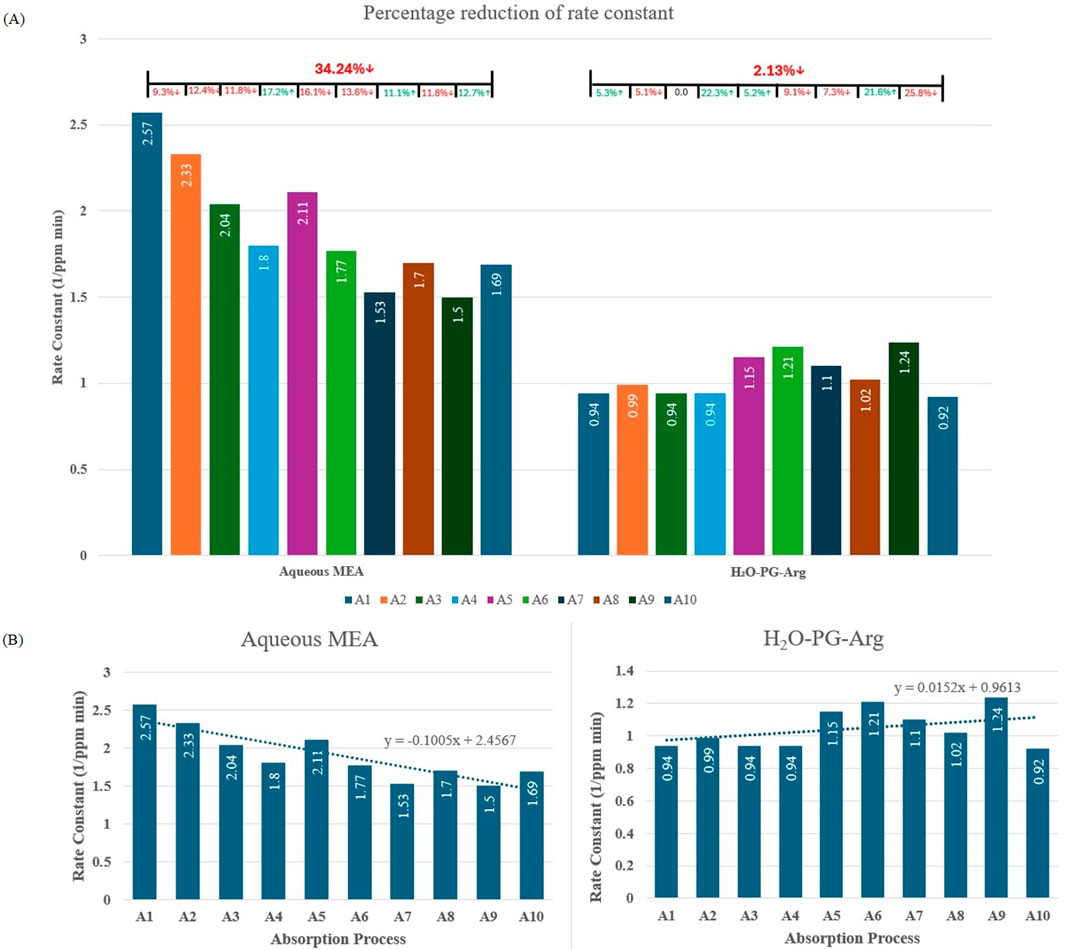
Figure 5. (A) Reaction rate constant over ten cycles of aqueous MEA and H2O-PG-Arg solution. The percentage decrease in the rate constant in each absorption process, as well as the cumulative reduction after ten cycles, is shown at the top of the figure. (B) Reaction rate constant over ten cycles of aqueous MEA and H2O-PG-Arg solution with trendlines.
4 Conclusion and future works
This study evaluated seven CSS, focusing on their appearance, CO₂ absorption-desorption performance, reaction kinetics, and effects on RH levels and TVOC concentrations. Aqueous MEA showed high CO₂ absorption and faster reaction kinetics but proved less suitable for indoor air capture due to elevated VOC emissions compared to aqueous Arg. Although the aqueous Arg solution performed better than aqueous MEA in terms of TVOC and RH control, it faced challenges with incomplete regeneration and significant solvent vaporization, limiting its use in continuous cycles. To address these issues, we introduced a water-PG-Arg solution, which demonstrated promising results by outperforming aqueous MEA in stability, safety, and long-term efficiency. The inclusion of PG effectively regulated RH during absorption and maintained the solution’s liquid state during desorption. Overall, the water-PG-Arg solution exhibited consistent CO₂ absorption and reliable cyclic performance, positioning it as a viable and sustainable solution for indoor CO₂ capture. In our preliminary testing, the integration of an activated charcoal canister within the absorption setup further stabilized RH levels and reduced TVOC concentrations, as illustrated in Supplementary Figures S11A, 11B. As we progressed through the cyclic study, the water-PG-Arg solution’s capacity to control RH was slowly impacted (Supplementary Figure S12A), presumably due to the loss of PG to evaporation primarily during the desorption processes, leading to a reduced mole fraction of PG in the resulting solutions, which can be attributed to a gradually lower TVOC generation (Supplementary Figure S12B) during the subsequent absorption processes. Future studies will investigate the use of alternative CSA and less viscous solvents to develop improved CSS that can effectively manage relative humidity across multiple cycles while achieving even lower TVOC emissions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
KM: Conceptualization, Investigation, Methodology, Writing–original draft, Writing–review and editing, Formal Analysis, Validation. SB: Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing–original draft, Writing–review and editing. SW: Validation, Writing–review and editing. DG: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation, Writing–review and editing, Writing–original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Funding for this research was provided by the TECO Clean Energy Research Center (CERC) at the University of South Florida (USF).
Acknowledgments
We would like to thank the following staff and students at the University of South Florida (USF) for their assistance: Dr. Laurent Calcul for assistance and training on Liquid Chromatography-Mass Spectrometry Tripple Quad (LC-MS-QqQ) and Headspace-Solid Phase Micro Extraction-Gas Chromatography-Mass Spectroscopy (HS-SPME-GC-MS); Mr. Timothy Mead for preparing the required experimental setup.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnano.2025.1525106/full#supplementary-material
References
Aronu, U. E., Svendsen, H. F., and Hoff, K. A. (2010). Investigation of amine amino acid salts for carbon dioxide absorption. Int. J. Greenhouse Gas. Control 4, 771–775. doi:10.1016/j.ijggc.2010.04.003
Azuma, K., Kagi, N., Yanagi, U., and Osawa, H. (2018). Effects of low-level inhalation exposure to carbon dioxide in indoor environments: a short review on human health and psychomotor performance. Environ. Int. 121, 51–56. doi:10.1016/j.envint.2018.08.059
Chai, S. Y. W., Ngu, L. H., and How, B. S. (2022). Review of carbon capture absorbents for CO2utilization. Greenhouse Gases Sci. Technol. 12 (3), 394–427. doi:10.1002/ghg.2151
Chanchey, A., Saiwan, C., Supap, T., Idem, R., and Tontiwachwuthikul, P. (2011). Off-gas emission in CO2 capture process using aqueous monoethanolamine solution. Energy Procedia 4, 504–511. doi:10.1016/j.egypro.2011.01.081
Chen, H., Lu, J., and Obobisa, E. S. (2023). Striving towards 2050 net zero CO₂ emissions: how critical are clean energy and financial sectors? Heliyon 9 (12), e22705. doi:10.1016/j.heliyon.2023.e22705
Custelcean, R. (2022). Direct air capture of CO2Using solvents. Annu. Rev. Chem. Biomol. Eng. 13 (1), 217–234. doi:10.1146/annurev-chembioeng-092120-023936
Gabriel, C., Gabriel, S., Grant, E. H., Halstead, B. S. J., Mingos, D. M. P., and Michael P. Mingos, D. (1998). Dielectric parameters relevant to microwave dielectric heating. Chem. Soc. Rev. 27 (3), 213–223. doi:10.1039/a827213z
Garg, U., Lowry, J., and Algren, D. A. (2019). “Ethylene glycol and other glycols: analytical and interpretation issues,” in Critical issues in alcohol and drugs of abuse testing (Elsevier), 59–69. doi:10.1016/B978-0-12-815607-0.00005-8
Global Monitoring Laboratory – NOAA (2023). Carbon cycle greenhouse gases. Available at: https://gml.noaa.gov/ccgg/trends/global.html (Accessed September 6, 2023).
Guo, H., Li, H., and Shen, S. (2018). CO2 capture by water-lean amino acid salts: absorption performance and mechanism. Energy fuels. 32 (6), 6943–6954. doi:10.1021/acs.energyfuels.8b01012
Jacobson, T. A., Kler, J. S., Hernke, M. T., Braun, R. K., Meyer, K. C., and Funk, W. E. (2019). Direct human health risks of increased atmospheric carbon dioxide. Nat. Sustain. 2 (8), 691–701. doi:10.1038/s41893-019-0323-1
Kim, Y. E., Lim, J. A., Jeong, S. K., Yoon, Y. il, Bae, S. T., and Nam, S. C. (2013). Comparison of carbon dioxide absorption in aqueous MEA, DEA, TEA, and AMP solutions. Bull. Korean Chem. Soc. 34 (3), 783–787. doi:10.5012/bkcs.2013.34.3.783
Kuramochi, H., Tsurumi, R., and Ishibashi, Y. (2023). Meta-analysis of the effect of ventilation on intellectual productivity. Int. J. Environ. Res. Public Health 20 (8), 5576. doi:10.3390/ijerph20085576
Lee, S. Y., and Park, S. J. (2015). A review on solid adsorbents for carbon dioxide capture. J. Ind. Eng. Chem. 23, 1–11. doi:10.1016/j.jiec.2014.09.001
Lv, B., Guo, B., Zhou, Z., and Jing, G. (2015). Mechanisms of CO2 capture into monoethanolamine solution with different CO2 loading during the absorption/desorption processes. Environ. Sci. Technol. 49, 10728–10735. doi:10.1021/acs.est.5b02356
Ma, J., Li, L., Wang, H., Du, Y., Ma, J., Zhang, X., et al. (2022). Carbon capture and storage: history and the road ahead. Engineering 14, 33–43. doi:10.1016/j.eng.2021.11.024
Mahmud, N., Benamor, A., Nasser, M. S., Al-Marri, M. J., Qiblawey, H., and Tontiwachwuthikul, P. (2017). Reaction kinetics of carbon dioxide with aqueous solutions of L-Arginine, Glycine and Sarcosine using the stopped flow technique. Int. J. Greenhouse Gas. Control 63, 47–58. doi:10.1016/j.ijggc.2017.05.012
Mai, B., Halfdan, E., Mai Lerche, B., Stenby, E. H., and Thomsen, K. (2023) “CO₂ capture from flue gas using amino acid salt solutions,” in Downloaded from orbit. Available at: http://www.risoe.dtu.dk/Knowledge_base/publications/Reports/ris-r-1712.aspx.
Masiren, E. E., and Harun, N. (2017). Comparison of different types of carbamate amine for stripping process. Chem. Eng. Trans. 56, 721–726. doi:10.3303/CET1756121
McGurk, S. J., Martín, C. F., Brandani, S., Sweatman, M. B., and Fan, X. (2017). Microwave swing regeneration of aqueous monoethanolamine for post-combustion CO2 capture. Appl. Energy 192, 126–133. doi:10.1016/j.apenergy.2017.02.012
National Library of Medicine – Ethylamine (2024). C2H5NH2 | CID 6341 - PubChem. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/Ethylamine#section=Nikkaji-Number (Accessed October 27, 2024).
National Library of Medicine – Monoethanolamine (2024). C2H7NO | CID 700 - PubChem. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/Monoethanolamine (Accessed October 27, 2024).
New Jersey Department of Health (2009). Right to know hazardous substance fact sheet. Available at: https://nj.gov/health/eoh/rtkweb/documents/fs/3595.pdf (Accessed October 31, 2024).
Said, R. ben, Kolle, J. M., Essalah, K., Tangour, B., and Sayari, A. (2020). A unified approach to CO2–amine reaction mechanisms. ACS Omega 5 (40), 26125–26133. doi:10.1021/acsomega.0c03727
Sang Sefidi, V., and Luis, P. (2019). Advanced amino acid-based technologies for CO2 capture: a review. Ind. Eng. Chem. Res. 58 (44), 20181–20194. doi:10.1021/acs.iecr.9b01793
Sengwa, R. J. (2003). A comparative dielectric study of ethylene glycol and propylene glycol at different temperatures. J. Mol. Liq. 108 (1–3), 47–60. doi:10.1016/S0167-7322(03)00173-9
Song, H. J., Park, S., Kim, H., Gaur, A., Park, J. W., and Lee, S. J. (2012). Carbon dioxide absorption characteristics of aqueous amino acid salt solutions. Int. J. Greenhouse Gas. Control 11, 64–72. doi:10.1016/j.ijggc.2012.07.019
Suleman, H., Maulud, A. S., Syalsabila, A., and Shahid, M. Z. (2020). The contribution of aqueous L-arginine salts to equilibrium carbon dioxide absorption in a co-promoter role at high pressure. Fluid Phase Equilib. 524, 112743. doi:10.1016/j.fluid.2020.112743
Taylor, C. D. L., Klemm, A., Al-Mahbobi, L., Bradford, B. J., Gurkan, B., and Pentzer, E. B. (2024). Ionic liquid-glycol mixtures for direct air capture of CO2: decreased viscosity and mitigation of evaporation via encapsulation. ACS Sustain. Chem. Eng. 12 (20), 7882–7893. doi:10.1021/acssuschemeng.4c01265
Tsubaki, S., Furusawa, K., Yamada, H., Kato, T., Higashii, T., Fujii, S., et al. (2020). Insights into the dielectric-heating-enhanced regeneration of CO2-rich aqueous amine solutions. ACS Sustain. Chem. Eng. 8 (36), 13593–13599. doi:10.1021/acssuschemeng.0c05342
Wei, D., Kotwica, D., Nardi, G., Moazezbarabadi, A., Spannenberg, A., Ludwig, R., et al. (2023). A lysine-based system for CO2 capture and catalytic utilization to produce formates: irreproducibility and conflicting reactivity. ChemistrySelect 8 (32). doi:10.1002/slct.202302307
Young, A. S., Parikh, S., Dedesko, S., Bliss, M., Xu, J., Zanobetti, A., et al. (2024). Home indoor air quality and cognitive function over one year for people working remotely during COVID-19. Build. Environ. 257, 111551. doi:10.1016/j.buildenv.2024.111551
Zeman, F. (2007). Energy and material balance of CO2 capture from ambient air. Environ. Sci. Technol. 41 (21), 7558–7563. doi:10.1021/es070874m
Zhao, H., and Baker, G. A. (2023). Functionalized ionic liquids for CO 2 capture under ambient pressure. Green Chem. Lett. Rev. 16 (1), 2149280. doi:10.1080/17518253.2022.2149280
Nomenclature
HVAC Heating, Ventilation, and Air Conditioning system
OSHA Occupational Safety and Health Administration
NOAA National Oceanic and Atmospheric Administration
CCO₂ Concentration of CO₂
CSS CO₂ scrubbing solution
CSA CO₂ scrubbing agent
MW Microwave
TVOCs Total Volatile Organic Compounds
RH Relative Humidity
ppm parts per million
Ppb parts per billion
LC-MS-QqQ Liquid Chromatography Mass Spectroscopy triple quadruple
HS-GC-MS Headspace Gas Chromatography Mass Spectroscopy
Arg L-arginine
MEA Monoethanolamine
PG Propylene Glycol
EG Ethylene Glycol
Keywords: CO2 capture, indoor air, monoethanolamine, L-arginine, microwave regeneration, relative humidity, total volatile organic compounds
Citation: Maity K, Bhadirke SD, Wijewardane S and Goswami DY (2025) CO2 capture from indoor air for human comfort and sequestration or reuse: a promising step toward sustainable CO2 removal. Front. Nanotechnol. 7:1525106. doi: 10.3389/fnano.2025.1525106
Received: 08 November 2024; Accepted: 03 January 2025;
Published: 24 February 2025.
Edited by:
Rajeev Kumar, Panjab University, IndiaReviewed by:
Rahul R. Bhosale, University of Tennessee at Chattanooga, United StatesRakesh Kumar Soni, Chaudhary Charan Singh University, India
Shankarjit Singh, Punjab Pollution Control Board, India
Copyright © 2025 Maity, Bhadirke, Wijewardane and Goswami. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: D. Yogi Goswami, Z29zd2FtaUB1c2YuZWR1
 Krishnendu Maity
Krishnendu Maity Shreyash D. Bhadirke
Shreyash D. Bhadirke Samantha Wijewardane
Samantha Wijewardane D. Yogi Goswami*
D. Yogi Goswami*