
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nanotechnol. , 22 January 2025
Sec. Nanomaterials
Volume 6 - 2024 | https://doi.org/10.3389/fnano.2024.1513683
This article is part of the Research Topic Nanomaterial and Nanostructures for Cancer and Pathogenic Infection Diagnosis and Therapy View all 7 articles
 Hai Jiang1†
Hai Jiang1† Ranil Vikraman Kumarasamy2†
Ranil Vikraman Kumarasamy2† JinJin Pei1
JinJin Pei1 Kamaraj Raju3
Kamaraj Raju3 Gopalakrishnan Velliyur Kanniappan4*
Gopalakrishnan Velliyur Kanniappan4* Chella Perumal Palanisamy5*
Chella Perumal Palanisamy5* Ion Dan Mironescu6*
Ion Dan Mironescu6*Extracellular vesicles (EVs), natural membrane-bound structures released by cells, offer a promising platform for precise and targeted delivery of therapeutic payloads, including drugs and nanoparticles. This comprehensive review explores the integration of engineered nanomaterials with EVs to advance drug delivery systems. It covers various aspects of this integration, including techniques for synthesizing, loading, characterizing, and applying nanomaterials within EVs. Methodologies for integrating diverse nanocarriers like nanoparticles, liposomes, and quantum dots into EVs are discussed, along with characterization methods such as electron microscopy and light scattering. Attention is also given to considerations of biological compatibility, safety assessments, and strategies for surface modification to enhance targeting precision. Highlighting various applications, including targeted drug delivery, cancer therapy, and vaccine development, the review underscores the potential of EV-nanomaterial hybrids to leverage EVs’ innate targeting capabilities and the versatility of nanomaterials. However, it also addresses challenges such as scalability, standardization, and safety that must be overcome to facilitate the clinical translation of these promising biomedical solutions.
Extracellular vesicles (EVs) are gaining considerable attention in advanced biomedical applications due to their pivotal role in intercellular communication. EVs are naturally occurring nanoscale vesicles, secreted by various cell types and provide a unique platform for transporting bioactive cargo, such as proteins, nucleic acids, and lipids, across biological barriers (Song et al., 2022). However, realizing the full therapeutic potential of EVs presents challenges like limited cargo capacity, rapid clearance from circulation, and difficulties in achieving targeted delivery. This is where nanomaterials come into play a versatile class of engineered nanoparticles and structures at the nanoscale, designed with specific properties and functions (Xu M. et al., 2023). Integrating nanomaterials with EVs forms a synergistic partnership that offers promising solutions to these challenges, unlocking advanced possibilities in biomedical applications (Tian et al., 2022).
Understanding the fundamental characteristics of nanomaterials and EVs are essential before exploring their synergistic integration. An EV is a membrane-bound structure released by a cell, such as an exosome, nanovesicles, or apoptotic bodies. Physiological processes rely on them for transporting biologically active molecules (Zinger et al., 2020). Furthermore to the endosomal sorting complex required for transport (ESCRT) machinery, lipid rafts, and protein sorting pathways play a significant role in their formation and cargo loading (Rani et al., 2023). Meanwhile, nanomaterials have different physical and chemical properties, including nanoparticles, liposomes, and polymers. Size, shape, surface chemistry, and cargo-loading capacity can all be precisely controlled using top-down and bottom-up manufacturing methods (Gong et al., 2023). The integration of nanomaterials with EVs addresses the challenge of enhancing their cargo capacity. The increased cargo capacity of EVs is one of the key advantages of this integration (Liu C. et al., 2022). A more substantial cargo of drugs, nucleic acids, or imaging agents can be delivered to target cells or tissues using EVs loaded with nanomaterials that contain therapeutic agents. As cancer therapy requires high drug doses, systemic toxicity is often a concern, which makes this feature particularly valuable. Integration of EVs and nanomaterials can be used to enhance drug delivery efficiency while minimizing off-target effects (Zhang et al., 2021).
Additionally, the incorporation of nanomaterials into EVs enhances the vesicles’ stability. The EVs may be degraded by enzymes or cleared by the immune system if administered directly. By extending the circulation time of EVs and ensuring a more sustained therapeutic effect, nanomaterials can protect them against external factors (Manandhar et al., 2022). Furthermore, nanomaterials can be customized with targeting ligands to enable precise site-specific delivery. This active targeting capability addresses the challenge of achieving precision medicine by directing therapeutic cargo exclusively to the intended cells or tissues while sparing healthy ones. This develops particularly significant in the context of diseases like cancer, where the precise delivery of drugs to tumor sites is crucial (Luo et al., 2020). Besides, nanomaterials combined with EVs present novel opportunities for regenerative medicine. A substantial amount of attention has been paid to EVs derived from stem cells because of their regenerative potential. It is possible to engineer these EVs to repair and regenerate tissues by combining them with nanomaterials. The nanomaterials not only offer structural support but can also be designed to mimic the extracellular matrix, facilitating cell adhesion and tissue regeneration. This approach holds promise in various applications, such as repairing damaged cardiac tissue, addressing neural injuries, and treating musculoskeletal disorders (Beetler et al., 2023).
In this review, we explore the transformative synergy between nanomaterials and EVs, including implications for diagnostics, drug delivery, and regenerative medicine. EVs, once overlooked, have now come to the forefront as crucial mediators of intercellular communication, proficient in transporting biomolecules across biological barriers. Nanomaterials, engineered on the nanoscale, effectively address the limitations of EVs, enhancing their cargo capacity, stability, and targeted delivery capabilities. This comprehensive review navigates through various sources of EVs, elucidates their biogenesis, and outlines the regulatory mechanisms involved. Subsequently, it explores the multifaceted advantages and innovative strategies associated with the integration of nanomaterials and EVs, unveiling advanced biomedical applications. Therefore, this review aims to provide a comprehensive analysis of the synergistic potential between extracellular vesicles and nanomaterials for advanced biomedical applications.
In this review, we conducted a systematic literature search across PubMed, Scopus, Web of Science, and Google Scholar to identify studies on integrating extracellular vesicles with nanomaterials for targeted drug delivery and biomedical applications. Using keywords like “extracellular vesicles,” “nanomaterials,” and “targeted therapy,” we included peer-reviewed articles in English published within the last decade, prioritizing those that directly addressed EV-nanomaterial hybrids in therapeutic or diagnostic applications. Studies were selected based on relevance, methodological quality, and robustness of findings, with exclusions applied to non-medical uses, opinion pieces, and theoretical-only models. This approach ensures a focused and comprehensive review of recent advances in EV-nanomaterial integration for biomedical applications.
EVs signify a fascinating and diverse class of biological nanoparticles that have garnered substantial attention in the fields of cell biology, immunology, and biomedical research (Mansoori et al., 2022). These small membranous structures are buried by virtually all cell types and play pivotal roles in intercellular communication, both in physiological and pathological contexts (Patil and Soekmadji, 2021).
Extracellular vesicles (EVs) are tiny structures secreted by various cell types, serving as crucial messengers in intercellular communication. In animals, diverse sources of EVs (Figure 1). On the other hand, in plants (Figure 2), EVs are produced by variety of cells which are described in Table 1. Both animal and plant-derived EVs carry diverse cargo and play crucial roles in physiology, health, and disease. Research into EVs continues to expand, offering promising applications in various fields (Silachev, 2023).
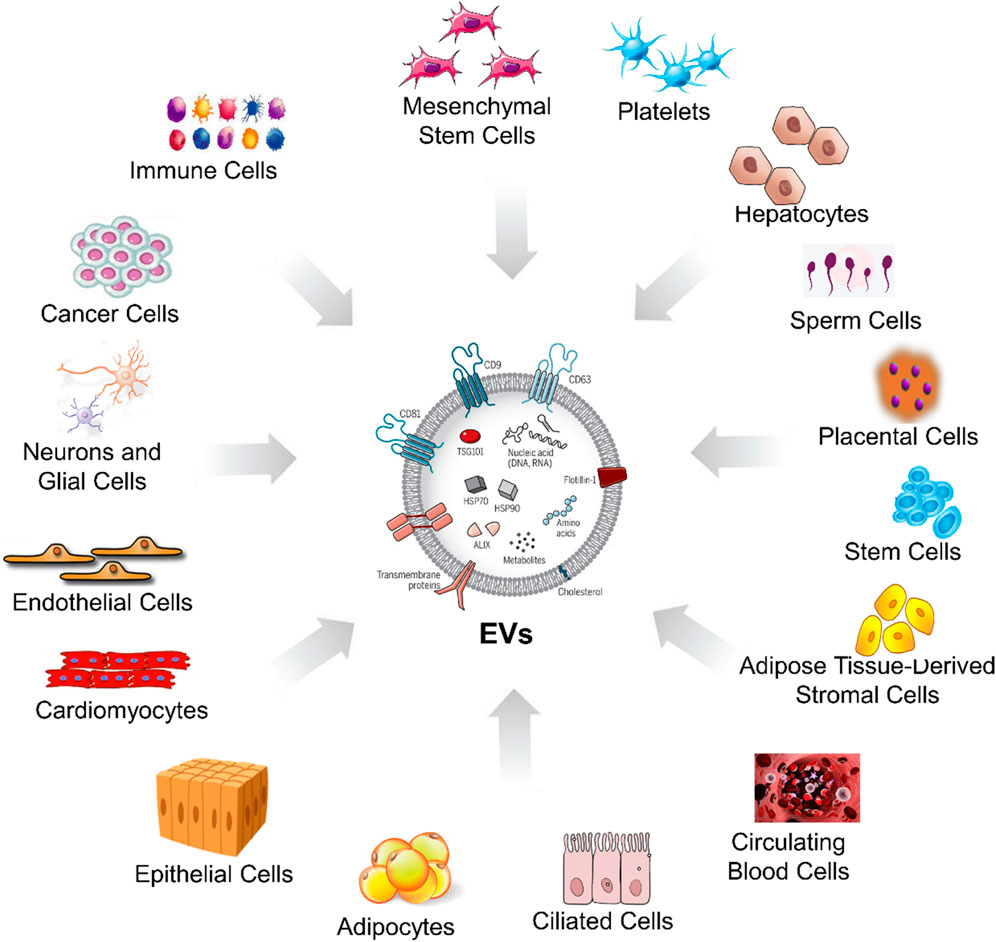
Figure 1. Sources of EVs from the different animal cells. This figure visually bears the concept that EVs are released by animal cells, originating from various cellular sources, and they could play various roles in the advanced biomedical applications.
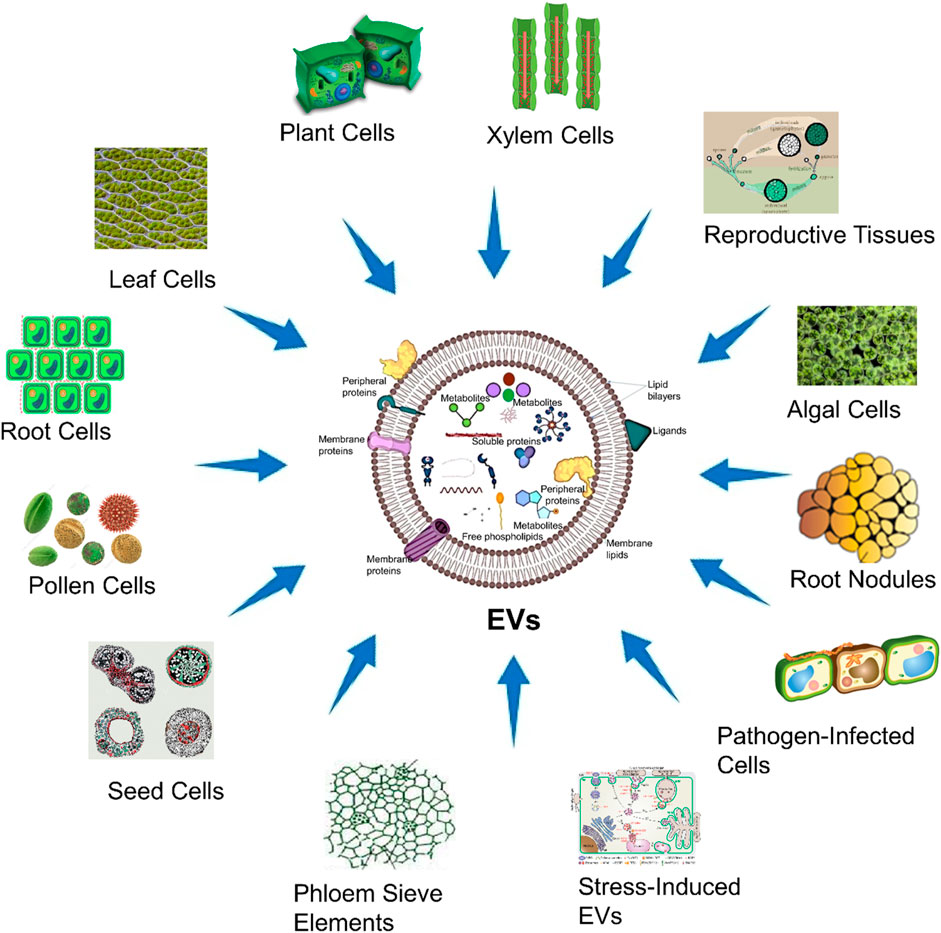
Figure 2. Sources of EVs from plant cells. This figure visually conveys the concept that EVs are released by plant cells, originating from several cellular sources within the plant cells, and they could play diverse roles in biomedical applications.
EVs are produced through a complex biogenesis process, involving budding from the plasma membrane or intracellular compartments. They come in three types (Table 2): exosomes, originating from the endosomal system; microvesicles, budding directly from the cell membrane; and apoptotic bodies formed during cell apoptosis. EV biogenesis is tightly regulated and includes the sorting of cargo molecules like proteins and nucleic acids, facilitating their vital roles in intercellular communication and physiology (Kang et al., 2021).
Extracellular vesicles (EVs) are small, membrane-bound particles secreted by cells into the extracellular environment, serving as key mediators of intercellular communication. Based on characteristics such as size, biogenesis pathways, and molecular composition, EVs are generally classified into three main types: exosomes, microvesicles, and apoptotic bodies.
Exosomes are the smallest type of EVs, typically ranging from 30 to 150 nm in diameter. They are formed within endosomal compartments called multivesicular bodies (MVBs), which fuse with the plasma membrane to release exosomes into the extracellular space. Exosomes carry a diverse array of bioactive molecules, including proteins, lipids, and nucleic acids (e.g., miRNAs, mRNAs), which play essential roles in cellular signaling and immune modulation. Key surface markers for exosomes include tetraspanins such as CD63, CD81, and CD9. Exosomes are involved in processes such as immune responses, tissue regeneration, and tumor progression, and can originate from various cell types like dendritic cells, mesenchymal stem cells, and cancer cells.
Microvesicles, also known as ectosomes or microparticles, vary widely in size, generally ranging from 100 to 1,000 nm. Unlike exosomes, microvesicles are formed through the outward budding and fission of the plasma membrane. Their molecular composition is highly dependent on the originating cell type, encompassing surface markers and cargos that reflect the cell’s physiological state. They often express phosphatidylserine on their surface, which serves as a distinguishing marker. Microvesicles are implicated in hemostasis, inflammation, and cellular repair processes and are commonly secreted by platelets, endothelial cells, and immune cells.
Apoptotic bodies are the largest type of EVs, typically ranging from 1 to 5 µm in diameter. These vesicles are produced during apoptosis, a regulated form of cell death, and contain fragments of the parent cell, including DNA, cytoplasmic components, and intact organelles. Apoptotic bodies are cleared by phagocytic cells, contributing to immune tolerance and inflammation regulation. Their composition often includes phosphatidylserine and markers like histones, which are indicative of their apoptotic origin.
Beyond these primary classifications, EVs can also be categorized into subpopulations based on specific markers, cargo contents, and the cell types from which they originate. For example, EVs derived from cancer cells often contain oncogenic proteins and nucleic acids that support tumor growth and metastasis, while EVs from mesenchymal stem cells are rich in regenerative factors that aid in tissue repair. Additionally, EVs secreted under different physiological conditions, such as oxidative stress or hypoxia, display unique molecular profiles that reflect these environmental cues. (Palanisamy et al., 2023).
EVs serve diverse physiological roles in intercellular communication (Table 3). They transport bioactive molecules, including proteins, lipids, and nucleic acids, influencing processes like immune regulation, tissue repair, and cell signaling. EVs are also implicated in disease pathogenesis and hold promise as diagnostic and therapeutic tools in various medical fields. There are several physiological processes to be understood and novel biomedical applications to be developed, which depend on EVs, and these vesicles represent a versatile and dynamic way to communicate between cells. Their biogenesis, classification, and roles continue to be areas of intense research, represents promising avenues for future discoveries and therapeutic interferences (Cheng and Hill, 2022).
The formation and secretion of EVs are complex processes regulated by numerous cellular mechanisms (Table 4). These pathways vary by cell type and are tailored to meet specific cellular needs. Key pathways, such as those mediated by the Endosomal Sorting Complex Required for Transport (ESCRT) machinery, lipid rafts, and calcium signaling, orchestrate EV biogenesis, ensuring the correct cargo is sorted, enclosed, and released. Below, we outline the principal mechanisms that regulate EV formation and secretion, with examples of their roles in producing different EV types, including exosomes, microvesicles, and apoptotic bodies (Zhang et al., 2023).
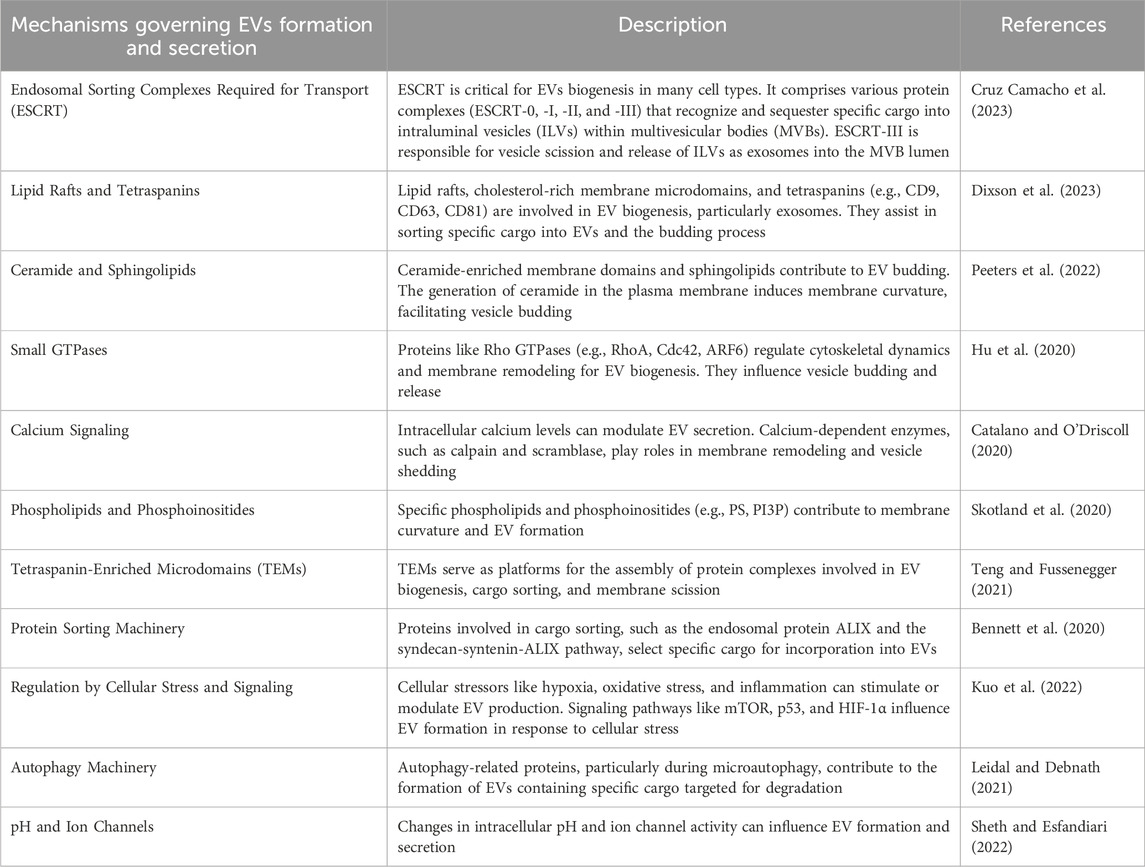
Table 4. The various cellular pathways and processes involved in the formation and secretion of EVs.
Nanomaterials are engineered or naturally occurring substances with structures at the nanoscale, typically ranging from 1 to 100 nm. Their unique and enhanced physical, chemical, and biological properties arise from their small size, high surface area, and quantum effects, making them ideal for diverse applications (Han and Choi, 2021). Broadly, nanomaterials can be classified into four primary types based on their morphology and composition: Nanoparticles: These are the most commonly studied nanomaterials and include metallic (e.g., gold, silver), metal oxide (e.g., titanium dioxide, zinc oxide), polymeric, and carbon-based particles. Nanoparticles are widely applied in drug delivery, imaging, and antibacterial treatments due to their capacity for targeted delivery and surface functionalization. Nanotubes: Carbon nanotubes (CNTs) are a prominent example, known for their mechanical strength and electrical conductivity. They are utilized in electronic devices, as well as in tissue engineering and biosensing due to their flexibility and biocompatibility. Nanowires: Metallic and semiconducting nanowires, such as those made from silicon or gold, find applications in nanoelectronics, sensors, and photodetectors. Their ability to conduct electrons efficiently makes them valuable in energy storage and conversion devices. Quantum Dots: These are semiconductor nanocrystals that exhibit unique optical properties, such as fluorescence, which vary with their size. They are used in imaging, drug delivery, and as fluorescent markers in biological research and diagnostics.
In terms of contextual applications, nanomaterials are pivotal in medicine, particularly for targeted drug delivery and regenerative medicine, where their controlled release and interaction with biological systems can enhance therapeutic efficacy. In materials science, they improve the properties of composites, making them stronger, lighter, or more flexible. In electronics, nanomaterials facilitate advancements in microelectronics, energy storage, and solar cells. Environmental applications include water treatment, air purification, and environmental sensing. However, their small size also raises concerns regarding toxicity and environmental persistence, making it critical to evaluate their safety and regulatory requirements (Albalawi et al., 2021).
Nanomaterials can be classified into several categories based on their composition, structure, and properties, each with distinct applications and characteristics that make them valuable across various fields.
These are solid particles with at least one dimension within the nanoscale (1–100 nm). Examples include: Gold nanoparticles (AuNPs), commonly used in diagnostics and drug delivery due to their biocompatibility and ease of functionalization. Silver nanoparticles (AgNPs), known for their antimicrobial properties and widely used in medical devices and coatings. Titanium dioxide (TiO₂) nanoparticles, frequently used in sunscreens, cosmetics, and photocatalysis applications for environmental remediation.
These materials consist of a bulk matrix embedded with nanoscale inclusions to enhance their mechanical, thermal, or electrical properties. Polymer-matrix nanocomposites, where polymers are reinforced with nanoparticles like clay, carbon nanotubes, or graphene, are used in automotive and aerospace components for their strength and light weight. Metal-matrix nanocomposites, such as aluminum reinforced with silicon carbide nanoparticles, are applied in structural materials needing high strength and durability.
These materials contain nanoscale pores, which make them suitable for applications in adsorption, catalysis, and filtration. Zeolites, microporous aluminosilicate minerals used in catalysis, adsorption, and ion-exchange processes. Metal-organic frameworks (MOFs), a class of nanoporous materials with high surface area and tunable pore sizes, are used in gas storage, separation, and drug delivery. Mesoporous silica (MCM-41), which is utilized in catalysis and as a drug delivery vehicle due to its high surface area and stability.
Bulk materials with nanoscale features throughout the structure exhibit enhanced mechanical, optical, and electrical properties. Nanostructured metals, like nanocrystalline copper, are used in wear-resistant coatings and conductive films due to their enhanced hardness and ductility. Nanostructured ceramics, such as nanocrystalline zirconia, are used in dental and orthopedic implants for their strength and biocompatibility. Graphene, a single layer of carbon atoms with a two-dimensional nanostructure, is applied in flexible electronics, sensors, and composite materials due to its high conductivity and strength.
These materials are categorized based on their chemical makeup. Carbon-based nanomaterials, including carbon nanotubes and fullerenes, are valued in electronics and composite materials for their electrical conductivity and tensile strength. Metal nanoparticles, like silver, gold, and iron oxide nanoparticles, are used in biomedical applications for imaging, drug delivery, and hyperthermia treatment. Semiconductor nanocrystals, such as quantum dots made from cadmium selenide (CdSe), are utilized in displays, solar cells, and bioimaging for their tunable optical properties.
Nanomaterials can also be grouped based on their primary applications, facilitating targeted research and development. Medical applications: Gold nanoparticles and iron oxide nanoparticles are used in diagnostics, drug delivery, and imaging. Electronics: Carbon nanotubes and graphene are key in creating flexible electronics, transistors, and sensors. Energy storage and conversion: Nanostructured silicon and lithium iron phosphate nanoparticles are utilized in batteries and fuel cells for improved energy capacity and efficiency. Environmental applications: Titanium dioxide nanoparticles and silver nanoparticles are used in water treatment, antimicrobial coatings, and air purification.
These classifications help to understand and leverage the unique properties of nanomaterials, enabling their tailored use across disciplines, including medicine, electronics, materials science, and environmental sustainability (Han et al., 2020).
This section highlights the significant impact of nanomaterials on medical advancements. It possess unique properties that make them highly advantageous for diverse medical applications. In the realm of drug delivery, nanomaterials serve as carriers for pharmaceutical compounds, safeguarding them from degradation and enabling precise targeting. This enhances therapeutic efficacy while reducing side effects, as drugs can be delivered directly to affected tissues or cells while sparing healthy ones (Lai et al., 2022). Examples of drug delivery platforms employing nanomaterials include liposomes, polymer nanoparticles, and lipid-based nanocarriers (Nasrollahzadeh et al., 2020).
Cancer therapy, in particular, has benefited significantly from nanomaterials, allowing the targeted delivery of chemotherapy drugs to cancer cells while sparing healthy tissue. Additionally, nanoparticles can be engineered to release drugs in response to specific triggers within tumor microenvironments (Meng et al., 2021). Targeted therapies like photodynamic therapy and hyperthermia, where nanomaterials generate heat or light to destroy cancer cells, have shown promise. The field of vaccines has also been revolutionized by nanomaterials, as they can effectively carry antigens to enhance immune responses, potentially leading to more effective vaccines against infectious diseases (Makabenta et al., 2021).
Nanomaterials have made substantial contributions to medical imaging, serving as contrast agents in various imaging modalities such as magnetic resonance imaging (MRI), computed tomography (CT), and ultrasound. Their ability to enhance image quality aids in early and accurate disease detection, assisting clinicians in making informed decisions (Chen et al., 2021).
In regenerative medicine, nanomaterials offer scaffolds that mimic the extracellular matrix, supporting tissue rejuvenation. They can also carry growth factors and bioactive molecules, promoting tissue repair in applications ranging from bone regeneration to wound healing (Tan et al., 2023). Nanomaterials have shown promise in delivering therapeutic agents to the brain, potentially offering new avenues for treating neurodegenerative diseases like Alzheimer’s and Parkinson’s disease, which are notoriously difficult to address due to the blood-brain barrier (Soufi and Iravani, 2020).
Nanomaterials with antibacterial properties have emerged as promising tools in the fight against antibiotic-resistant bacteria and infections (Meng et al., 2023). They can be incorporated into wound dressings, medical implants, or device coatings to prevent infections and improve patient outcomes (Naskar and Kim, 2019). Cardiovascular therapies have also benefited from nanomaterials in the development of drug-eluting stents and as carriers for medications targeting heart diseases (Cassani et al., 2020).
Furthermore, nanomaterials have played a crucial role in gene therapy by facilitating the delivery of nucleic acids for the treatment of genetic disorders, offering hope for previously untreatable conditions (Ma et al., 2021). They have also advanced the field of personalized medicine, enabling therapies tailored to an individual’s genetic or disease profile, maximizing treatment effectiveness (dos Santos et al., 2021).
While the potential of nanomaterials in therapeutics is undeniable, challenges related to safety, scalability, and cost-effectiveness need to be addressed through rigorous testing and regulation (Mundekkad and Cho, 2022). Despite these challenges, nanomaterials have ushered in a new era of therapeutic innovation, offering precision, versatility, and efficiency in the treatment of a wide range of medical conditions. Continued research and development in this field hold great promise for improving patient outcomes and reshaping the healthcare landscape (Pusta et al., 2023).
This section explores the emerging area of research that combines nanomaterials with EVs for potential therapeutic applications in medicine, drug delivery, and diagnostics. EVs are small membranous structures released by cells, which play a crucial role in intercellular communication by carrying bioactive molecules like proteins, nucleic acids, and lipids (Rani et al., 2023). In contrast, nanomaterials are engineered materials with unique properties at the nanoscale that can be tailored for various applications. The integration of nanomaterials with EVs aims to merge the properties and functions of both entities to enhance their therapeutic or diagnostic potential (Niu et al., 2021). This integration holds several promising aspects.
The integration of nanomaterials with EVs significantly enhances drug delivery systems by providing a robust platform for improved precision and efficiency in targeting specific cells or tissues. Nanomaterials can serve as ideal carriers for EVs, protecting them from enzymatic degradation and environmental factors that may compromise their therapeutic efficacy. The surface properties of nanomaterials can also be engineered to facilitate the binding of EVs to target receptors, ensuring that therapeutic molecules are delivered directly to disease sites.
For example, in cancer treatment, EVs loaded with therapeutic molecules, such as small interfering RNAs (siRNAs) or proteins, can be delivered precisely to tumor cells. Nanomaterials like liposomes, gold nanoparticles, and carbon nanotubes can be employed to enhance the stability and drug-loading capacity of EVs, facilitating the co-delivery of multiple therapeutic agents. This ability to co-load various drugs or biomolecules allows for synergistic effects, improving the efficacy of treatment regimens, minimizing the risk of drug resistance, and enabling the treatment of multifactorial diseases. As shown in studies by Meng et al. (2020) and Mondal et al. (2023), the use of nanomaterials for loading EVs can help target hard-to-reach areas and improve overall bioavailability (Meng et al., 2020; Mondal et al., 2023).
One of the key advantages of integrating nanomaterials with EVs is the ability to control the loading of specific therapeutic cargo during EV biogenesis. This can be achieved by incorporating nanomaterials into the EV membrane or internalizing them within the EV lumen, allowing for the targeted delivery of nucleic acids (such as mRNAs or small RNAs), proteins, and other bioactive compounds. By precisely engineering nanomaterials to interact with EVs, researchers can enhance the specificity and efficiency of therapeutic cargo delivery.
For example, gold nanoparticles and magnetic nanoparticles can be used to guide the EVs to desired locations within the body, ensuring that they carry out their therapeutic function only at the target site. Moreover, the use of nanomaterials in the engineering of EVs allows for the co-delivery of several different therapeutic agents, providing a multi-pronged approach to therapy. This method not only enhances the efficacy of treatments but also reduces off-target effects, as shown by Yom-Tov et al. (2022) and Tripathi et al. (2023), where custom-engineered EVs facilitated controlled drug release and minimized systemic toxicity (Yom-Tov et al., 2022; Tripathi et al., 2023).
A major limitation of EV-based therapeutics is their susceptibility to degradation during storage, transport, and in vivo circulation. Nanomaterials can address this challenge by providing protective barriers for EVs, enhancing their stability and longevity. By coating or encapsulating EVs with nanomaterials, it is possible to shield them from harsh environmental conditions such as high temperatures, enzymatic degradation, and oxidative stress.
Nanomaterials, such as silica nanoparticles, lipid nanoparticles, and polymeric nanocarriers, have been shown to enhance the shelf life and stability of EVs, ensuring that they retain their therapeutic cargo’s integrity until they reach their target site. Additionally, nanomaterials help to prevent aggregation, which can occur when EVs are exposed to external stresses, improving their therapeutic efficacy upon delivery. As reported by Paolillo et al. (2021) and Yaraki et al. (2022), this integration significantly extends the shelf-life of EVs and ensures that their therapeutic potential remains intact over time (Paolillo et al., 2021; Yaraki et al., 2022).
Nanomaterials offer powerful tools for the imaging and tracking of EVs in vivo, enabling real-time monitoring of their distribution and therapeutic effects. By integrating nanomaterials such as quantum dots, gold nanoparticles, or superparamagnetic iron oxide nanoparticles (SPIONs) into EVs, researchers can enhance the visualization and detectability of EVs using various imaging modalities, including fluorescence imaging, magnetic resonance imaging (MRI), and computed tomography (CT).
This capability is especially valuable in clinical diagnostics and biomedical research, where tracking the biodistribution of EVs can provide insights into their therapeutic efficacy and potential side effects. For instance, Liu Q. et al. (2022) demonstrated the use of fluorescent nanomaterials to track EVs in cancer models, offering a non-invasive approach to monitor EV delivery in real-time. The ability to visualize EVs can also aid in determining the best time and dosage for EV-based therapies, improving treatment outcomes (Liu Q. et al., 2022).
Nanomaterials integrated with EVs can be utilized to modulate the immune system’s response, offering a potential strategy for therapeutic interventions in autoimmune diseases, inflammation, and cancer immunotherapy. Nanomaterials can be engineered to enhance the immunomodulatory properties of EVs, such as boosting the ability of EVs to interact with immune cells or modulating their immunosuppressive effects.
For example, the incorporation of gold nanoparticles or lipid-based nanomaterials into EVs has been shown to enhance their ability to stimulate immune responses, thereby promoting antitumor immunity or enhancing vaccine efficacy. Conversely, EVs can be engineered with nanomaterials to carry immunosuppressive cargo, which could be beneficial in conditions where dampening the immune response is desired, such as in autoimmune diseases. Cheng et al. (2021) and Liu Z. et al. (2023) demonstrated how the combination of nanomaterials and EVs could be used to control immune responses with high precision, opening up new avenues for immunotherapies (Cheng et al., 2021; Liu Z. et al., 2023).
The integration of nanomaterials with EVs has significant potential in tissue engineering and regenerative medicine, particularly for promoting tissue repair and regeneration. Nanomaterials can serve as scaffolds or delivery systems for EVs, ensuring that bioactive molecules are efficiently delivered to damaged tissues, thereby accelerating the healing process. For example, the combination of nanomaterials such as hydrogels, nanofibers, and nanoparticles with EVs can provide both structural support and bioactive cues for cell proliferation and tissue regeneration.
Trubiani et al. (2019) and Ju et al. (2022) have explored how EV-loaded nanomaterials can be used in bone and cartilage regeneration, where nanomaterials help to direct the EVs to the site of injury, enhancing the regenerative potential of EVs. This synergistic approach not only promotes tissue repair but also improves the overall efficacy of regenerative therapies (Trubiani et al., 2019; Ju et al., 2022).
While the integration of nanomaterials with EVs holds great promise, it is essential to thoroughly assess the safety and biocompatibility of these systems. The long-term effects of nanomaterials on human health and the environment must be carefully evaluated to mitigate potential risks. Toxicological studies are needed to understand the potential cytotoxicity, immunogenicity, and biodistribution of the nanomaterials when integrated with EVs.
Researchers are exploring methods to design biocompatible nanomaterials that are easily excreted from the body and do not accumulate in vital organs. In addition, the impact of these integrated systems on immune function, organ health, and overall system toxicity should be systematically studied. Cheng et al. (2018), El Nahrawy et al. (2021), and Herrmann et al. (2021) highlight the importance of safety profiles in nanomaterial-EV therapeutics, which will be essential for translating these systems into clinical applications (Cheng et al., 2018; El Nahrawy et al., 2021; Herrmann et al., 2021).
The synergistic integration of nanomaterials with EVs represents an exciting frontier in biomedical research, with the potential to revolutionize various aspects of medicine and healthcare. However, further research and development are needed to fully unlock the clinical potential of this approach (Rufino-Ramos et al., 2017).
Characterizing the integration of nanomaterials with EVs is essential for understanding their properties, behaviour, and potential applications in various fields. Numerous characterization approaches could be employed to assess these integrated systems which are given below.
TEM is an authoritative imaging technique for characterizing nanomaterial-EVs complexes with exceptional detail. By using a attentive beam of electrons, TEM gives the ultra-high resolution images that unveil the intricate features of these integrated structures. Researchers could exactly determine the size, shape, and arrangement of nanomaterials within EVs, shedding light on their morphology and distribution. This microscopic vision is precious for understanding how nanomaterials and EVs interact at the nanoscale, providing crucial information that underpins the development of novel therapeutic strategies and biomedical applications (Murugesan, 2021).
SEM is a valuable tool for characterizing EVs-nanomaterial complexes, particularly when it comes to assessing their surface properties. SEM produces high-resolution images by scanning an electron beam across a sample’s surface. Nanomaterial-EV complexes can be characterized based on their surface morphology, texture, and features by this technique. Scientists can gain a deeper understanding of EV stability and cellular uptake by studying their surface characteristics. In a variety of biomedical applications, SEM’s ability to provide detailed surface information is crucial for optimizing design and functionality. (Noble et al., 2020).
In solution, DLS is an effective technique for characterizing nanomaterial-EV complexes. DLS provides crucial information about particle size distribution and hydrodynamic diameter by analysing the fluctuations in scattered light caused by Brownian motion. As nanomaterials are integrated with EVs, DLS assists researchers in determining the dimensions of these integrated structures. Physiological and experimental settings require dynamic data to understand their stability, dispersibility, and interactions. DLS enables precise size measurements and aids in optimizing the design of these complexes for targeted therapeutic delivery and other biomedical applications (Tiwari et al., 2021).
Zeta Potential Analysis is a valuable method for assessing the surface charge properties of nanomaterial-EVs complexes. By quantifying the electrical charge at the particle’s surface when suspended in a solution, it exposes vibrant information about their stability and propensity for aggregation. A high positive or negative zeta potential proposes repulsion between particles, hindering aggregation and promoting stability. Equally, a low zeta potential can indicate the potential for particles to aggregate or clump together. This characterization method is precious for forecasting the behaviour of nanomaterial-EVs complexes in various environments, aiding researchers in optimizing their design for targeted drug delivery, imaging, and other biomedical applications (Midekessa et al., 2020).
Fourier Transform Infrared Spectroscopy (FTIR) is a prevailing analytical technique used to find the chemical composition of nanomaterial-EVs complexes. By subjecting these complexes to infrared radiation, FTIR perceives the specific vibrational frequencies of chemical bonds and functional groups existing in the sample. It provides a complete molecular fingerprint, allowing researchers to identify and quantify the numerous constituents within the nanomaterial-EVs complexes. FTIR aids in discriminating critical information about the structural modifications, surface functionalization, or chemical interactions which may occur during the integration of nanomaterials with EVs (Di Santo et al., 2022).
X-ray Diffraction (XRD) is a powerful technique for probing the crystallographic characteristics of nanomaterials encapsulated EVs. By exposing the sample to X-rays and analysing the resultant diffraction pattern, XRD could precisely determine the arrangement and spacing of atoms within crystalline structures. This information is precious in understanding the structural integrity and phase composition of nanomaterial-EVs complexes. Researchers could assess whether nanomaterials maintain their crystalline properties post-integration, which is critical for predicting their stability and functionality in various applications, from drug delivery to catalysis and beyond. XRD contributes crucial insights into the behaviour of nanomaterial-EVs complexes at the atomic and molecular level (Paisrisarn et al., 2022).
NMR Spectroscopy is a adaptable analytical technique employed to gain perceptions into the chemical structure, dynamics, and interactions within nanomaterial-EVs complexes. NMR exploits the magnetic properties of certain atomic nuclei, exposing their specific environments within a sample. In the integration of nanomaterials with EVs, NMR could identify and characterize the chemical constituents of both components. It could elucidate how nanomaterials and EVs interrelate on a molecular level, provides data on binding affinities, conformational changes, and intermolecular forces. NMR is essential for considerate the intricacies of these integrated systems, aiding researchers in enhancing their design for diverse applications in biomedicine, materials science, and beyond (Nielsen et al., 2021).
Fluorescence and Confocal Microscopy are essential tools for examining the behaviour of nanomaterial-EVs complexes within biological systems. These techniques control the intrinsic or fluorescently labelled properties of the nanomaterials or EVs to visualize their uptake and intracellular localization in real-time. By enlightening the interactions between these complexes and living cells or tissues, researchers could track their journey by cellular compartments, discerning how they are internalized, trafficked, and whether they reach specific target sites. This information is essential for optimizing the design of nanomaterial-EV systems for drug delivery, imaging, or therapeutic applications, eventually enhancing our understanding of their behaviour within complex biological environments (Shimomura et al., 2021).
MS is a vital analytical technique hired to delve into the cargo content of EVs and monitor any alterations resulting from their integration with nanomaterials. Through ionizing and separating molecules based on their mass-to-charge ratios, MS could precisely identify and quantify the proteins, lipids, nucleic acids, and metabolites carried by EVs. This technique allows researchers to examine the cargo composition and assess whether nanomaterial integration induces any changes, shedding light on potential modifications or cargo loading efficiency. MS plays a vital role in interpreting the functional and therapeutic potential of nanomaterial-EVs complexes, guiding their optimization for various biomedical applications (Jalaludin et al., 2023).
Biological assays are crucial for assessing the functional aspects and safety profile of nanomaterial-EVs complexes within biologically relevant contexts. Cell viability assays provides critical insights into the biological activity and possible toxicity of these integrated systems. By exposing cells to nanomaterial-EVs complexes and measuring their impact on cell viability and proliferation, researchers could assess whether these complexes encourage cytotoxicity or adversely affect cellular health. Such assays are essential for defining the biocompatibility of these complexes and guiding their safe use in various biomedical applications, from drug delivery to regenerative medicine, confirming that they have a positive impact on biological systems (Nguyen et al., 2020).
Spectroscopic techniques, together with ultra violet -visible (UV-Vis) spectroscopy, Raman spectroscopy, and fluorescence spectroscopy, provides valuable insights into the optical properties and interactions of nanomaterial with EVs. UV-Vis spectroscopy dealings the absorption of light by these complexes, exposing the information about their electronic structure and composition. On the other hand, Raman spectroscopy provides information about molecular vibrations within complexes, allowing chemical bonds and structural changes to be identified. After excitation, fluorescence spectroscopy utilizes the emitted light to determine fluorescence properties and biomolecular interactions. A variety of biomedical and materials science applications demand the use of these spectroscopic methods to characterize and optimize nanomaterial-EV systems (Imanbekova et al., 2022).
In order to evaluate the stability and electrophoretic mobility of nanomaterial-EV complexes, electrophoretic techniques, with gel electrophoresis, are indispensable. When subjected to an electric field, gel electrophoresis separates these complexes according to their size and charge, providing vital information about their structure and dispersion. Researchers could determine whether nanomaterial-EVs complexes have aggregated or degraded by analyzing their migration patterns in a gel matrix. Nanomaterial-EV complexes are essential for targeted drug delivery, diagnostics, and other biomedical applications (Yang et al., 2020).
qPCR is a powerful molecular biology technique used to investigate how nanomaterial-EV complexes alter gene expression. Through the measurement of gene levels, qPCR provides valuable insight into the biological effects of these integrated systems. Nanomaterial-EV complexes are shown to influence cellular processes, including signaling pathways, immune responses, and other essential functions. Using this information, we can develop tailored biomedical interventions and advance our understanding of their interactions with biological systems as well as understand the therapeutic or regulatory impact of these complexes (Fauth et al., 2019).
A proteomic and lipidomic analysis is a sophisticated method of dissecting EVs molecular composition and examining the changes caused by nanomaterial integration. To conduct proteomics, it is necessary to identify and quantify the proteins present in EVs. Additionally, lipidomics focuses on determining the lipid content of these vesicles. By encapsulating nanomaterials with EVs, these techniques can detect changes in protein and lipid compositions. In order to understand what effects nanomaterial integration may have on therapeutic potential, EV stability, or functionality, this is crucial. Our understanding of the complex interactions between nanomaterials and EVs is improved by proteomic and lipidomic analyses, which guide the optimization of EVs for various biomedical applications (Wang T. et al., 2023).
Characterizing EVs proteins is crucial for understanding their composition and functions. Common methods include Western Blotting, which uses specific antibodies to detect proteins; Enzyme-Linked Immunosorbent Assay (ELISA) for quantification; Mass Spectrometry, which identifies and quantifies proteins through mass analysis of peptides; Flow Cytometry, allowing analysis of EVs based on size and surface proteins; Immunoelectron Microscopy for nanoscale visualization of EVs and their protein content; Nanoparticle Tracking Analysis (NTA) for measuring size and concentration; Transmission Electron Microscopy (TEM) for high-resolution imaging; Protein Arrays for simultaneous screening of multiple proteins; Immunoprecipitation (IP) for isolating target proteins; Size-Exclusion Chromatography (SEC) for EV subpopulation separation; Surface Plasmon Resonance (SPR) for analyzing protein interactions; and Proteomic Analysis (e.g., LC-MS) for comprehensive proteome identification. Researchers often combine these techniques based on research goals and equipment availability to comprehensively characterize EV protein composition.
The synergistic integration of nanomaterials with EVs are emerging as an innovative and dynamic approach that has been profound implications for advanced biomedical application (Table 5) (Figure 3) (Yang et al., 2022). This synergistic integration epitomizes a growing field at the intersection of biomedicine, drug delivery, and nanotechnology, promising transformative solutions to a variety of healthcare challenges (Li et al., 2021). Accordingly, this approach harnesses their combined potential. Nanomaterials, including nanoparticles, liposomes, quantum dots, and other engineered carriers offers multifunctionality and versatility, while EVs provides natural targeting competences and a biocompatible delivery system. Moreover, this approach amplifies therapeutic and diagnostic potential of both components, leading to improved consequences in the advanced biomedical applications (Wang et al., 2021).
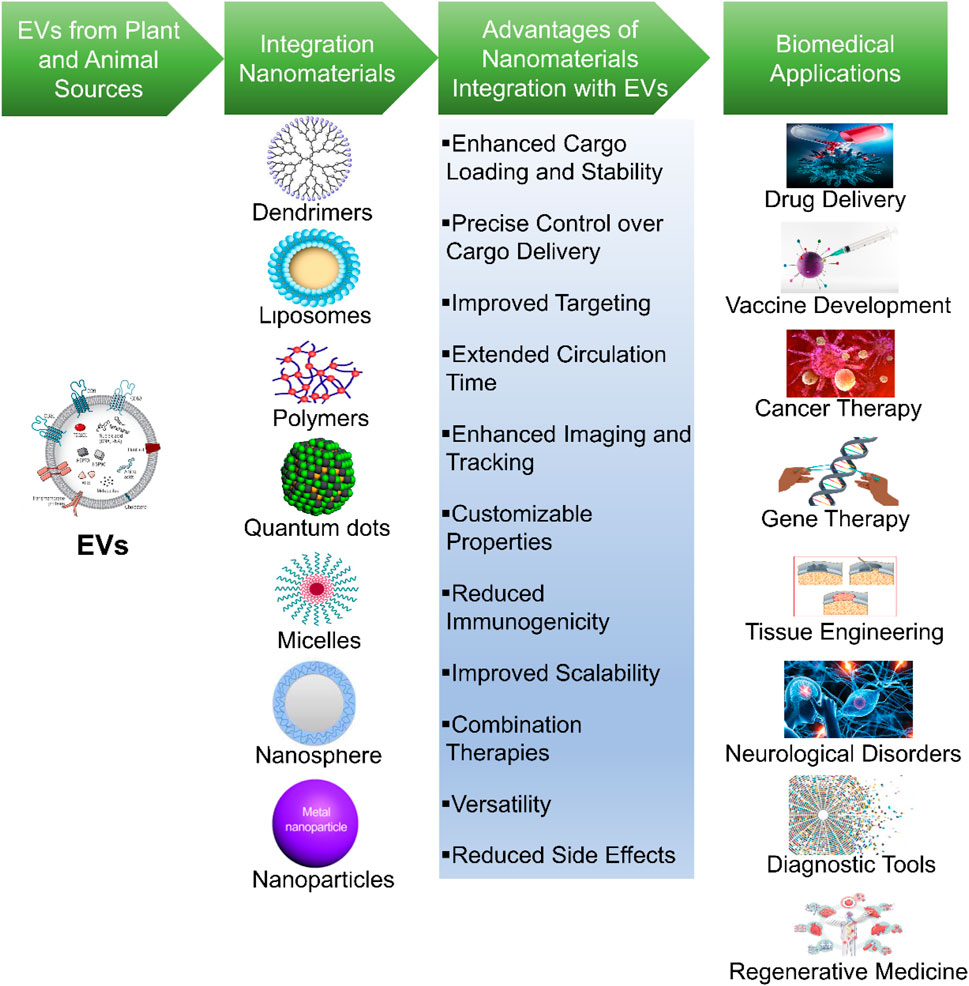
Figure 3. Synergistic of integration of nanomaterials with EVs. This figure depicts the integration of nanomaterials with EVs for advanced biomedical applications. It illustrates the synergy between these two components to achieve various biomedical and therapeutic applications.
Research and development (R&D) in this field encompass various stages, starting with the isolation of EVs from various cell sources (both animal and plant cells) and biological fluids. Numerous techniques employed to obtain pure and functional EVs which includes ultracentrifugation, filtration, size exclusion chromatography, and immunoaffinity capture (Sidhom et al., 2020). Consequently, the loading of nanomaterials into EVs is an essential step, involving several strategies to professionally encapsulate these carriers within EVs. This process confirms that the therapeutic payloads are safely loaded into EVs and ready for targeted delivery. Evaluation of integration success, researchers utilized several characterization methods such as transmission electron microscopy, dynamic light scattering, and Western blotting which techniques are enabling the validation of nanomaterial integration with EVs and offer insights into their structure and stability. Besides, biological compatibility and safety considerations are supreme, necessitating comprehensive assessments of toxicity, immunogenicity, and stability in relevant biological environments (Wang Z. et al., 2023).
Surface modification approaches are also discovered to improve the targeting specificity of EVs-nanomaterial complexes for various biomedical applications. By engineering the surface properties of these hybrids, researchers can tailor their interactions with specific cell types or tissues, thereby enhancing their therapeutic efficacy and reducing off-target effects (Tian et al., 2022). The applications of EV-nanomaterial hybrids are extensive and continue to expand. They encompass targeted drug delivery, in vivo imaging, regenerative medicine, cancer therapy, and vaccine development. By leveraging the natural targeting abilities of EVs and the multifunctionality of nanomaterials, these hybrid systems hold the potential to revolutionize the treatment and diagnosis of various diseases (Liu R. et al., 2023). Nonetheless, several challenges, such as scalability, standardization, and safety, must be addressed for the clinical translation of these promising innovations. Despite these hurdles, the synergistic integration of nanomaterials with EVs represents a rapidly evolving and highly promising field in biomedical research, offering new avenues for precision medicine and improved healthcare outcomes (Table 1) (Wu Q. et al., 2023).
The research by Elkhoury et al. (2022) explores a groundbreaking approach to enhance the potential of additive manufacturing for tissue engineering. Traditional 3D-printed tissues have limitations when it comes to influencing the behavior of the cells they contain, especially in terms of delivering crucial gene expression regulators like microRNAs (miRNAs). To overcome this challenge, the researchers incorporated extracellular vesicles (EVs), which are naturally occurring nanovesicles, into bioinks used in 3D bioprinting. miRNA could be delivered to cells without impairment through EVs due to their excellent biocompatibility, rapid endocytosis capabilities, and low immunogenicity. In order to improve the control and release of these EVs, liposomes combined with them. EV-liposome hybrid nanovesicles (hEL) compressed in gelatin-based hydrogels molded bioinks that effectively encapsulated and delivered miRNA to their target sites. This breakthrough permissible for the accurate regulation of gene expression within a 3D bioprinted matrix, confirming not only excellent shape fidelity but also high cell viability in the engineered tissues.
The integration of these innovative regulatory factor-loaded bioinks has the possibilities to intensify the development of new bioprinting applications in the field of tissue engineering. It describes an synergistic integration of nanomaterials, such as EVs and liposomes, with bioinks to overcome venerable challenges in delivering gene expression regulators, eventually advancing the possibilities for effective tissue regeneration and biomedical applications. In 2021, Niu et al. (2021) established an advanced glioma therapy drug delivery approach. They integrated grapefruit EVs with doxorubicin-loaded heparin-based nanoparticles (DNs) to create biomimetic EV-DNs. This strategy, known as “patching,” improved drug loading capability by four-fold compared to conventional methods. Biomimetic EV-DNs effectively crossed the blood-brain barrier (BBB) and penetrated glioma tissues, thanks to receptor-mediated transcytosis and membrane fusion. This approach improved cellular uptake, anti-proliferative effects, and circulation time. In vivo results demonstrated significant accumulation in glioma tissues, promising a breakthrough in challenging glioma therapy by overcoming critical delivery barriers.
Khongkow et al. (2019) introduced an innovative biomedical approach focusing on enhancing gold nanoparticles’ (AuNPs) ability to breach the blood-brain barrier for precise brain cell targeting. They combined the unique characteristics of extracellular vesicle-derived membranes with synthetic AuNPs, creating a novel nanomaterial for brain cell delivery. Brain-targeted exosomes, obtained from genetically engineered mammalian cells, were integrated onto synthetic AuNPs’ surfaces using mechanical or extrusion methods. This led to specialized nanomaterial development with enhanced targeting capabilities, as demonstrated by binding to brain cells under laminar flow conditions. The modified AuNPs also showed improved blood-brain barrier transport, signifying a significant achievement in brain drug delivery. In vivo imaging confirmed their brain accumulation, promising advanced neurological therapies and diagnostics through brain-targeted exosome-coated AuNPs. This research offers an effective and innovative strategy for efficient brain targeting in biomedical applications (Khongkow et al., 2019).
Jia et al. (2018) tackled glioma treatment challenges by introducing engineered EVs as targeted drug delivery carriers. Early glioma detection and overcoming the blood-brain barrier (BBB) for drug delivery are critical issues. Exosomes, with their cargo capacity and BBB-crossing ability, serve as ideal candidates. Researchers loaded superparamagnetic iron oxide nanoparticles (SPIONs) and curcumin into EVs, modifying their membrane with a neuropilin-1-targeted peptide. This engineered EV system effectively crossed the BBB, providing targeted imaging and therapy for glioma. Combining SPION-mediated magnetic flow hyperthermia and Cur therapy demonstrated potent synergistic antitumor effects, improving glioma management while minimizing side effects. This innovative approach offers promise for glioma diagnosis and treatment enhancement (Jia et al., 2018).
Kooijmans et al. (2016) tackled the limitations of using extracellular vesicles (EVs) for drug delivery by developing a method to modify EVs with targeting ligands. They used nanobodies specific for the epidermal growth factor receptor (EGFR) conjugated to polyethylene glycol (PEG) and introduced them to EVs through 'post-insertion.’ This process didn't alter EV characteristics but enhanced their cell specificity. Unmodified EVs cleared rapidly from the bloodstream, while modified EVs remained detectable for over 60 min post-injection. This innovation offers improved EV targeting and extended circulation times, promising enhanced drug delivery for biomedical applications without the need to modify EV-secreting cells (Kooijmans et al., 2016).
Gaurav et al. (2023) combat citrus canker, caused by Xanthomonas axonopodis, using an eco-friendly approach. They synthesized silver nanoparticles with Phyllanthus niruri leaf extract (GS-AgNP-LEPN), eliminating the need for toxic reagents. To enhance their efficacy, these nanoparticles were encapsulated in extracellular vesicles (EVs) from leaf apoplastic fluid (APF-EV-GS-AgNP-LEPN). This hybrid was tested against Xanthomonas axonopodis pv., outperforming ampicillin. The treatment works by inhibiting flavin adenine dinucleotide-flavodoxin NADP(H) reductase (FAD-FNR) and Xanthomonas outer protein AI (XopAI) through nirurinetin. This eco-friendly approach offers a promising solution to combat citrus canker, a significant threat to the citrus industry (Gaurav et al., 2023).
Yang Y. et al. (2021) address tongue squamous cell carcinoma (TSCC) treatment challenges by using extracellular vesicles (EVs) loaded with chrysin, a flavonoid with anti-tumor properties. They combine this with gold nanoparticles (AuNPs) for photothermal therapy. EVs enhance specificity for TSCC cells. Au-EVs, with near-infrared irradiation, induce TSCC cell apoptosis. RNA sequencing reveals upregulated tumor suppressor miRNAs, particularly let-7a-3p, in EVs-chrysin. Au-EVs combined with near-infrared radiation (NIR) irradiation inhibit tumor growth in vivo by increasing let-7a-3p expression, offering a promising nanomaterial-based approach for TSCC treatment (Yang Z. et al., 2021).
Li J. et al. (2023) enhance tumor immunotherapy using engineered extracellular vesicles (EVs), addressing limitations in tumor antigen presentation to dendritic cells (DCs). They create Te@EVsHSP70 by upregulating heat shock protein 70 (HSP70) expression in tumor cells, leading to engineered EVs with tellurium nanoparticles (Te) for photothermal therapy. Te@EVsHSP70 induce immunogenic cell death and DC maturation, resulting in superior antitumor efficacy in vitro and in vivo. This study offers a practical method to boost tumor immunotherapy by combining photothermal therapy with engineered EVs for enhanced antigen presentation, highlighting an innovative approach in oncology (Li X. et al., 2023).
Naskar et al. (2022) developed an innovative approach to combat Staphylococcus aureus using a nanocomposite coated with EVs from Lactobacillus paracasei (MZ-LPEV). This bioinspired nanoantibiotic exposed considerably progressive antibacterial activity against S. aureus, outperforming other nanoparticles. Its mechanisms included reactive oxygen species production and bacterial membrane disruption. MZ-LPEV also demonstrated enhanced biocompatibility and selectivity for S. aureus. This innovative approach offers promise in addressing antibiotic-resistant strains and represents a novel strategy in the battle against infectious diseases (Naskar et al., 2022).
Cheng et al. (2018) developed a biomimetic nanoparticle platform for efficient systemic and intracellular protein delivery. Guest proteins with high loading content are encapsulated in metal-organic framework (MOF) nanoparticles and protected from degradation. These nanoparticles, coated with an EVs membrane, selectively target tumors, avoid the immune system’s detection, and improve cellular uptake. By using therapeutic protein gelonin, the platform accomplished a 14-fold advances in therapeutic efficacy, inhibiting tumor growth in vivo. This novel approach provides promise in enhanced protein delivery for therapeutic interventions in various diseases, overcoming critical obstacles in current strategies (Cheng et al., 2018).
Qiao et al. (2022) developed EV-Pd-Pt, a biomimetic nanoplatform for synergetic anti-infective therapy. The integration of Pd-Pt nanosheets with ginger-derived EVs, this nanoplatform shows prolonged circulation, exact accumulation at infection sites, and the capability to enter bacteria cells. It also generates sustainable reactive oxygen species (ROS) for enhanced therapy. Notably, EV-Pd-Pt employs both electrodynamic and photothermal therapy, enhancing its anti-infective efficacy. With excellent biocompatibility and biosafety, this nanoplatform presents a promising approach for combating infections in complex biological environments (Qiao et al., 2022).
Li et al. (2021) explored an novel photochemotherapy nanoplatform through macrophage-derived EVs. These EVs were altered with folate for boosted tumor targeting and employed an in situ biosynthetic approach to produce photosensitizer protoporphyrin X (PpIX) and chemotherapy drug doxorubicin (DOX) within the EVs. This approach accomplished high drug-loading efficiency, enhanced tumor accumulation and penetration, and triggered a strengthened immune response for efficacious cancer treatment. The smart nanoplatform’s versatility permits for possible applications in numerous biomedical contexts. This research highlights an well-organized strategy for EVs-based drug delivery, mainly in photochemotherapy, providing potential biomedicine for cancer (Li et al., 2021).
Dumonte et al. (2019) established Trojan nano-horses (TNHs) as a nanotherapeutic approach by encapsulating zinc oxide (ZnO) nanocrystals within EVs. In cancer theranostics, TNHs address issues such as aggregation, degradation, and clearance. In this study, ZnO nanocrystals were carried in EVs, which are known to be stable and biocompatible. Researchers demonstrated TNHs’ biomimetic nanotheranostic potential in this study by verifying their efficient encapsulation, biostability, and cytotoxicity in cells. TNHs are a promising avenue for cancer therapy due to their biomimetic nature and cellular uptake of ZnO nanocrystals (Dumontel et al., 2019).
Shang et al. (2023) investigated into the communications between cationic polymer nanoparticles (cNPs) and cells, converging on their transport mechanisms and possible as biomimetic drug delivery systems. Their study exposed that cNPs could be excreted as cNP@EVs, enveloped by EVs, and travel across several cellular compartments, assisted by autophagy. Nanoparticles can be efficiently transported between cells via EVs, enhancing deep penetration and the effectiveness of antitumor therapies. These results provide insights into nanoparticle transport within and between cells, accenting the role of EVs in facilitating this process. This knowledge possess potential for the advancement of EVs-based drug delivery systems in cancer therapy (Shang et al., 2023).
Mansur et al. (2023) developed a scalable approach for producing EVs loaded with quercetin-iron complex nanoparticles. This novel method was done by direct flow filtration, resulted in improved EVs yield compared to conventional ultracentrifugation. Characterization studies confirmed EVs morphology and standard protein markers, including ALIX, tumor susceptibility gene 101 (TSG101), CD63, and CD81. Cellular uptake experiments specified that, nanoparticle-loaded EVs had greater internalization and uniform distribution in MDA-MB-231br cells when compared with free nanoparticles. In this study, researcher’s, demonstrated the potential of direct flow filtration for scalable nanoparticle-loaded EVs production and suggests their efficiency in targeted drug delivery for advanced biomedical applications, mainly in cancer therapy (Mansur et al., 2023).
Wu M.-S. et al. (2023) delved an novel strategy to improve the therapeutic potential of nanomaterials for cancer treatment. In this study, researchers engineered EVs from modified cancer cells, changing their surface properties and microRNA-21 (miR-21) expression to improve the cancer targeting. These improved EVs encapsulated with gold-polydopamine (PDA) core-shell nanoparticles loaded with doxorubicin (Dox), glucose oxidase (GOx), and miR-21-indicative DNA tags. Upon cellular uptake, the nanoreactors released Dox, triggered GOx-catalyzed hydrogen peroxide (H2O2) generation, and consumed glucose due to the acidic pH and photothermal effect. This cascade treatment strategy, facilitated by EV-assisted delivery, offers a promising approach for improving cancer therapy, addressing biocompatibility and targeting challenges associated with nanomaterial-based treatments (Wu M.-S. et al., 2023).
In their 2018 study, Kim et al. addressed limitations in using human mesenchymal stem cell (hMSC)-derived exosomes for spinal cord injury treatment. To enhance their therapeutic potential, researchers created exosome-mimetic nanovesicles (NV-IONP) by incorporating iron oxide nanoparticles (IONPs) into exosomes from hMSCs treated with IONPs. NV-IONP acted as magnet-guided navigation tools, containing higher therapeutic growth factor levels than exosomes without IONPs. Systemic injection with magnetic guidance increased NV-IONP accumulation in the injured spinal cord, promoting blood vessel formation, reducing inflammation, and improving spinal cord function. This study represents progress in enhancing therapeutic extracellular nanovesicles for spinal cord injury therapy (Kim et al., 2018).
In their 2021 study, Jhan et al. address the challenge of creating effective combination therapies for cancer treatment. They use extracellular vesicles (EVs) as a delivery platform, engineering lipid-hybridized EVs (eEVs) to load small-interfering RNA (siRNA) and then creating an outer polyelectrolyte shell incorporating the chemotherapeutic agent doxorubicin (DOX) through layer-by-layer (LbL) assembly. These findings exposed that, stable EVs-polymer complexes (LbL-eEVs) that efficiently enters cancer cells, delivering siRNA into the cytoplasm and DOX into the cell nuclei, inducing cell death. In addition to being selective for cancer cells, LbL-eEVs minimized harm to normal tissues, making them a auspicious platform for accurate cancer treatment (Jhan et al., 2021).
Pan et al. (2023) developed siS100A4-loaded EVs modified with internalizing arginylglycylaspartic acid (iRGD), a tumor-penetrating peptide, to target S100A4 and inhibit postoperative breast cancer metastasis. These integrated nanoparticles confirmed the protection of siRNA, improved cellular uptake, and compatibility in vitro. In the in vivo model, iRGD-modified EVs exposed enhanced siRNA accumulation in lung metastatic sites, leading to a significant reduction in lung metastases and improved survival rates. In this study, researchers highlights the potential of siS100A4-iRGD-EV nanoparticles as a compelling approach to combat postoperative breast cancer metastasis, provides the hope for better treatment outcomes in breast cancer patients at risk of metastasis after surgery (Pan et al., 2023).
Wu et al. (2021) investigated the hepatocellular carcinoma (HCC)-derived EVs as nanocarriers for sequential nanocatalysts, creating glucose oxidase (GOD)-extremely small-sized iron oxide nanoparticles (ESIONs)@EVs (GE@EVs) for targeted nanocatalytic therapy against HCC. GE@EVs exploited RGD targeting for efficient intracellular delivery. These nanocatalysts encouraged a cascade of reactions in the tumor microenvironment, producing toxic hydroxyl radicals that triggered HCC cell death. In this study, researchers effectively suppressed HCC in vitro and in vivo and doubled as a contrast agent for MRI, offering valuable imaging capabilities. The study highlights the possible of EVs-based nanocatalytic therapy for HCC and validates the versatility of EVs in advanced biomedical applications (Wu et al., 2021).
Xu C. et al. (2023) examined the bone marrow mesenchymal stem cell-derived EVs (BMSC-EVs) loaded on magnetic nanoparticles for diabetic osteoporosis (DO). They identify miR-150-5p as a key player in osteoporosis and use gold-coated magnetic nanoparticles (GMNPs) to enhance BMSC-EV isolation. GMNPs combined with anti-CD63, known as GMNPE, enrich EVs in DO rat bone tissues. BMSC-EVs deliver miR-150-5p to osteoblasts, targeting MMP14 and activating the Wnt/β-catenin pathway. This activation enhances osteoblast proliferation and maturation, making GMNP-BMSC-EVs a promising strategy for diabetic osteoporosis treatment (Xu C. et al., 2023).
In their 2022 study, Li et al. tackle chemotherapy challenges in lung cancer treatment by utilizing EVs for simultaneous delivery of the anticancer drug doxorubicin (DOX) and the chemosensitizer lonidamine (LND). Two types of EVs, 16k EVs and 120k EVs, were prepared and co-delivered DOX and LND, leading to enhanced cytotoxicity and reduced drug doses in vitro. The direct fusion of EVs with cancer cell membranes enabled efficient drug delivery. In vivo studies in a mouse lung cancer model exhibited substantial anticancer activity, with smaller EVs representing improved efficiency. This co-delivery strategy possess efficiency lung cancer chemotherapy outcomes (Li H. et al., 2022).
Kim et al. (2020) addressed the challenge of targeting ischemic brain lesions in stroke treatment by emerging magnetic nanovesicles (MNVs) derived from mesenchymal stem cells (MSCs). These MNVs, carrying iron oxide nanoparticles (IONPs), were exposed to prompt developed levels of therapeutic growth factors when compared with untreated nanovesicles. MNVs are 5.1 times more effective when delivered systemically and guided to the lesion using magnetic navigation in rats with ischemic stroke. This targeted method encouraged an anti-inflammatory response, angiogenesis, and anti-apoptosis, reducing infarction volume and enhanced motor function. MNVs provides a promising strategy for attractive stroke treatment efficacy (Kim et al., 2020).
Yang Y. et al. (2021) addressed a significant challenge in osteoarthritis (OA) therapy through mesenchymal stem cell-derived small EVs (MSC-sEVs). DAHP (Diels-Alder crosslinked hyaluronic acid/PEG) hydrogel was developed as a delivery platform for MSC-sEVs, in order to improve the bioavailability and therapeutic effectiveness. The DAHP hydrogel was exposed to sustain the release of MSC-sEVs without compromising their therapeutic function in vitro and in vivo. This approach provides a promising solution for improving OA treatment by gorgeous the delivery and efficacy of MSC-sEVs, potentially advancing cell-free therapies in regenerative medicine (Yang Y. et al., 2021).
Sancho-Albero et al. (2019) discovered an novel approach in bioorthogonal catalysis, leveraging cancer-derived EVs as carriers for ultrathin palladium (Pd) nanosheets. Bioorthogonal chemistries permit the non-natural synthesis of bioactive compounds in vivo, with Pd catalysts playing a important role. In this study researchers addressed the challenge of selectively delivering catalysts to specific cell types by developing a bio-artificial device consisting of exosomes loaded with Pd catalysts. These EVs enable the controlled assembly of Pd nanosheets inside EVs. This integrated system facilitates Pd-triggered reactions in vitro and within specific cancer cells, opening new possibilities for targeted therapy, including “EVs-directed catalyst prodrug therapy” for accurate and effective cancer treatment (Sancho-Albero et al., 2019).
Li C. et al. (2022) examined the stability and bioavailability challenges of astaxanthin (AST), a potent antioxidant and anticancer compound. They developed AST@PLGA@BEVs by using broccoli-derived extracellular vesicles (BEVs) to coat AST-loaded poly (lactic-co-glycolic acid) (PLGA) nanoparticles. The optimized AST@PLGA@BEVs had a size of 191.60 ± 2.23 nm, a polydispersity index of 0.166, and a zeta potential of −15.85 ± 0.92 mV. Importantly, in vitro experiments demonstrated significantly enhanced anticancer activity compared to AST alone. This innovative approach offers improved stability and bioavailability for astaxanthin, making it a potential functional food with enhanced health benefits (Li C. et al., 2022).
In their 2019 study, Mol et al. explored the potential of injectable supramolecular ureidopyrimidinone (UPy) hydrogels as a delivery platform for extracellular vesicles (EVs). EVs, small vesicles secreted by cells, have garnered interest as promising vehicles for drug delivery and cell-free therapeutics in regenerative medicine. One challenge is achieving optimal therapeutic effects by prolonging EV exposure to target organs, and this study investigates the use of UPy-based hydrogels to address this challenge. The UPy-hydrogel is designed to undergo a solution-to-gel transition when transitioning from a high to neutral pH, enabling rapid gelation upon administration into physiological systems. The researchers demonstrate sustained release of EVs from the UPy-hydrogel over a period of 4 days, during which EVs retain their functional capacity. Importantly, in an in vivo experiment involving the local administration of fluorescently labeled EVs incorporated into a UPy-hydrogel, the EVs are still detected within the hydrogel after 3 days. In contrast, when administered without a hydrogel, the EVs are internalized by surrounding fat and skin tissue near the injection site. These findings highlight the potential of UPy-hydrogels for providing sustained release of EVs and enhancing local retention in vivo. This approach has the potential to improve therapeutic efficacy when using local delivery methods and may open up new applications in the field of regenerative medicine (Mol et al., 2019).
In their 2022 study, Zhu et al. developed a composite hydrogel-based approach for diabetic wound healing. They used gelatin methacrylate (GelMA) and silk fibroin glycidyl methacrylate (SFMA) to create a versatile hydrogel with suitable properties for sustained drug release. Resveratrol (RES) was loaded into mesoporous silica nanoparticles (MSNs) to enhance drug release, and platelet-derived extracellular vesicles (PDEVs) were added to promote cell proliferation and angiogenesis. These hydrogels demonstrated low toxicity, excellent biocompatibility, and the ability to inhibit inflammation and enhance tube formation in vitro. In diabetic mice, they reduced pro-inflammatory factors, boosted anti-inflammatory factors, stimulated angiogenesis, and accelerated wound healing. This approach shows promise for diabetic wound healing by addressing both inflammation and angiogenesis synergistically (Zhu et al., 2022).
In their 2019 study, Han et al. tackled aging-related vascular dysfunction, a complex problem with limited solutions. They uncovered microRNA-675 (miR-675) as a key player in aging-induced vascular issues. MiR-675 levels were reduced in aging muscle tissue, and its mimic reduced markers of aging in senescent cells. They confirmed miR-675’s role by targeting transforming growth factor-β1 (TGF-β1) through dual-luciferase assays. EVs from umbilical cord stem cells loaded with miR-675 were effective in rejuvenating senescent cells. These EVs were embedded in a silk fibroin hydrogel for controlled release. This approach improved blood flow in ischemic limbs and extended EVs retention. It offers promise for addressing aging-related vascular problems and other age-related complications (Han et al., 2019).
In their 2018 study, Zhang et al. aimed to boost the therapeutic potential of MSC-derived EVs for hindlimb ischemia, a condition characterized by reduced blood flow to the legs. They integrated human placenta-derived MSC-derived EVs into a chitosan hydrogel to enhance their stability, retention, and therapeutic impact. The chitosan hydrogel significantly improved EVs stability, crucial for maintaining therapeutic cargo. It also extended EVs retention in target tissues, potentially prolonging their therapeutic effects. In vitro, these EVs showed endothelium-protective and proangiogenic properties. In a murine model, the hydrogel-incorporated exosomes improved angiogenesis. This strategy may advance cell-free treatments for ischemic conditions and other diseases by enhancing EVs stability, retention, and efficacy (Zhang et al., 2018).
In their 2019 study, Pizzicannella et al. explored bone tissue regeneration through 3D printing and biomaterials enriched with gingival stem cells (GSCs) and extracellular vesicles (EVs). They used poly (lactide) (PLA) for 3D printing, creating a scaffold (3D-PLA) enriched with human gingival mesenchymal stem cells (hGMSCs) and their EVs. In vitro, they found increased expression of osteogenic and angiogenic markers in the living construct. In rats, implantation of 3D-PLA/hGMSCs/EVs stimulated bone regeneration and vascularization, confirmed by histology and microCT imaging. Upregulation of miR-2861 and miR-210, associated with osteoangiogenesis, was observed. This approach offers promise for bone tissue regeneration, driven by specific microRNAs and regenerative biomaterials (Pizzicannella et al., 2019).
In their 2018 study, Li et al. developed a novel approach to accelerate bone regeneration by creating cell-free tissue-engineered bone. They immobilized EVs from human adipose-derived stem cells (hASCs) onto poly (lactic-co-glycolic acid) (PLGA) scaffolds coated with polydopamine (pDA). These EVs, nanoscale vesicles crucial for cell communication, enhanced osteogenesis, cell proliferation, and migration of mesenchymal stem cells in vitro. Optimizing their osteoinductive effects further boosted bone formation. In mouse calvarial defects, this cell-free system significantly improved bone regeneration, attributed to EVs-induced osteoinduction and enhanced mesenchymal stem cell migration. This research offers a promising therapeutic avenue for bone tissue engineering and defect repair (Li et al., 2018).
In their 2020 study, Hamada et al. tackled the challenges of cardiac repair in chronic heart failure by developing a controlled release system using a photocurable adhesive polymer called poly (glycerol-co-sebacate) acrylate ethylene glycol (PGSA-g-EG). This polymer adheres to the heart’s surface, enabling local and sustained delivery of EVs, which play a crucial role in tissue regeneration. Importantly, the study showed that the polymer didn't compromise EV bioactivity. In rat experiments, the polymer proved biocompatible and stayed attached to the heart surface for over a month. The release kinetics of EVs from the polymer were improved, confirming the sustained delivery of bioactive EVs for at least 14 days. This novel approach provides a promising solution for controlled EVs delivery in cardiac repair, potentially improving therapy for chronic heart failure (Hamada et al., 2020).
Lv et al. (2019) introduced an innovative therapeutic approach for myocardial infarction (MI) using sEVs derived from bone marrow MSCs. In this study, researchers developed a sodium alginate hydrogel system with sEVs incorporated into it (sEVs-Gel) in order to overcome the challenges of limited retention and short-term effects of MSC-derived sEVs in the heart. This optimized system improved sEV release and rheological properties. Ex vivo imaging confirmed enhanced sEV retention in heart tissue. Functional analysis revealed reduced cardiac cell apoptosis, improved macrophage polarization, increased scar thickness, and enhanced angiogenesis after MI. Cardiac function and infarct size assessments showed superior outcomes compared to sEV treatment alone. This approach represents a promising cell-free therapy for MI (Lv et al., 2019).
In their 2019 research, Mardpour and colleagues aimed to improve hepatic regeneration in chronic liver failure using MSC-EVs. These EVs hold therapeutic potential but are rapidly cleared when systemically administered, reducing their efficacy. To address this, they developed a novel approach involving sustained EV release into the peritoneum. Clickable polyethylene glycol (PEG) macromeres were mixed with MSC-EVs to form EV-encapsulated PEG hydrogels. These hydrogels gradually released EVs as they biodegraded, extending EV accumulation in the liver over a month. In a rat model of liver fibrosis, Gel-EV treatment outperformed Free-EV, reducing fibrosis, inflammation, apoptosis, and improving liver regeneration. This study suggests that sustained EV delivery through hydrogel-mediated release could enhance their therapeutic potential for chronic liver failure (Mardpour et al., 2019).
In their 2020 study, Liu et al. investigated the enhanced therapeutic potential of MSC-EVs for acute kidney injury (AKI). They encapsulated EVs from human placental MSCs (hP-MSC-EVs) in a collagen matrix to address retention and stability issues post-transplantation. The collagen matrix significantly improved EV stability, promoted sustained release, and extended EV retention in an AKI model, as shown by Gaussia luciferase imaging. It also stimulated renal tubular epithelial cell proliferation and enhanced therapeutic effects, including angiogenesis, reduced fibrosis, and apoptosis. The study revealed that EVs partly exerted their therapeutic effects by inhibiting endoplasmic reticulum stress. This strategy holds promise for advancing cell-free regenerative medicine in AKI treatment (Liu et al., 2020).
In their 2020 study, Zhang et al. sought to enhance the therapeutic effectiveness of MSC-EVs for treating acute kidney injury (AKI). MSC-EVs offer a cell-free AKI therapy, but their limited stability and retention have been obstacles. To address this, researchers developed hydrogels with Arg-Gly-Asp (RGD) peptides, which bind to MSC-EV membranes’ integrins. RGD hydrogels improved EV retention and stability, confirmed through in vivo tracking and integrin gene knockdown experiments. When EV-RGD hydrogels were injected intrarenal into mouse models with AKI, enhanced kidney function, damage was reduced, and cell proliferation was improved. MicroRNA let-7a-5p also improved the MSC-EV’s antifibrotic effects during chronic AKI phases when RGD hydrogels were applied. Cell-free renal repair is conceivable with MSC-EVs and insights into regenerative medicine are exposed in this study (Zhang et al., 2020).
The integration of nanomaterials with EVs for biomedical applications, while promising, faces several significant limitations that need to be addressed before clinical translation can be achieved. These limitations span issues related to standardization, scalability, safety, understanding of underlying mechanisms, regulatory hurdles, and ethical considerations. Addressing these challenges is essential for optimizing the efficacy of EV-nanomaterial hybrids and advancing their use in various therapeutic and diagnostic applications.
A fundamental challenge in the integration of EVs with nanomaterials is the lack of standardized protocols. There is no consensus on optimal methods for isolating EVs or selecting nanomaterials, and differences in experimental protocols—ranging from EV extraction methods to nanomaterial properties—result in considerable variability across studies. EVs are derived from various sources such as stem cells, tumor cells, and platelets, each with distinct biological compositions and functionalities. Nanomaterials, including liposomes, nanoparticles, and polymers, also vary widely in size, surface charge, and chemical composition, further complicating the integration process. This diversity in both EVs and nanomaterials leads to inconsistent results in terms of loading efficiency, stability, and therapeutic outcomes. Without uniform protocols for EV-nanomaterial hybridization, comparing results across different studies becomes challenging, making it difficult to establish reliable conclusions about the most effective combinations and methods (Nieuwland et al., 2022).
One of the most significant barriers to the clinical translation of EV-nanomaterial hybrids is scaling up production. While small-scale studies have demonstrated the potential of these systems, there are challenges in translating these findings to clinical settings. The large-scale production of EVs with consistent quality is still in its nascent stages. The isolation of EVs from biological fluids often involves ultracentrifugation or filtration methods, which can be time-consuming, costly, and not easily scaled for clinical use. Moreover, the therapeutic properties of EVs—such as their ability to efficiently deliver cargo and maintain functionality—can degrade during large-scale production processes. Ensuring that the high yield of EVs retains its therapeutic properties is critical for maintaining their efficacy in clinical applications. Furthermore, maintaining the functionality of the hybrid system during large-scale manufacturing, particularly the stability of the nanomaterials integrated with EVs, remains a significant challenge. This makes the standardization of large-scale production methods essential for the successful clinical translation of these therapies (Paganini et al., 2019).
The safety of EV-nanomaterial hybrids is a major concern that needs to be addressed before their clinical application. While EVs are generally considered biocompatible due to their natural origin, the incorporation of nanomaterials can introduce risks related to toxicity, immune responses, and long-term side effects. Nanomaterials, particularly metallic nanoparticles, have been shown to elicit immune responses, cause oxidative stress, and potentially lead to tissue toxicity. The combination of nanomaterials with EVs could exacerbate these effects, as the nanomaterials may induce inflammation, alter cell signaling, or affect the stability of the EVs themselves. Moreover, the potential for accumulation of nanomaterials in non-target organs over time raises concerns about chronic toxicity. Understanding the dose-response relationships, tissue distribution, and long-term effects of these hybrid systems is crucial to assess their safety. Additionally, ensuring that the hybrid systems do not provoke unwanted immune responses, such as hypersensitivity or autoimmune reactions, is critical for their clinical success (Worthington and Hagood, 2020).
Another limitation is the incomplete understanding of the mechanisms underlying EV-nanomaterial interactions. The efficiency with which nanomaterials are loaded into EVs, their stability within the vesicles, and the ability of the hybrid system to efficiently deliver therapeutic cargo to target tissues depend on a complex interplay of biochemical and physical factors. For example, the surface properties of nanomaterials, such as charge and size, play a significant role in determining the efficiency of cargo loading into EVs and their cellular uptake. However, the molecular mechanisms governing these interactions remain poorly understood. Factors such as the affinity between the nanomaterial and the EV membrane, the loading capacity of different nanomaterials, and the potential for disrupting the native properties of EVs are areas that require further investigation. A deeper understanding of these mechanisms will allow for better design and optimization of EV-nanomaterial hybrids, improving their therapeutic efficacy (van Niel et al., 2022).
The lack of clear regulatory guidelines for EV-nanomaterial hybrids remains a major obstacle to their clinical translation. Regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have yet to establish comprehensive frameworks for the approval of nanomaterial-based therapies. This creates uncertainty regarding the requirements for preclinical and clinical testing, safety evaluations, and manufacturing standards. The complexity of EV-nanomaterial hybrids—being a combination of biologically derived materials and engineered nanomaterials—adds an extra layer of complexity to the regulatory process. Regulatory agencies must determine the appropriate criteria for assessing the quality, safety, and efficacy of these systems, considering both their biological and engineered components. Furthermore, ensuring consistency in batch production, controlling for variability in both EVs and nanomaterials, and establishing reproducible safety profiles are essential for obtaining regulatory approval. As the field progresses, there is an urgent need for clear, standardized guidelines for the clinical development of EV-nanomaterial hybrid therapies (Chua et al., 2022).
While EV-nanomaterial hybrids show great promise for targeted drug delivery, their therapeutic potential is often limited by the short half-life of EVs in circulation. EVs are naturally cleared from the body quickly, primarily through interactions with the mononuclear phagocyte system (MPS), which reduces their efficacy for systemic drug delivery. Although surface modifications, such as PEGylation or the incorporation of stabilizing nanomaterials, can prolong the circulation time of EVs, achieving sustained therapeutic efficacy remains challenging. Strategies to enhance the persistence of EVs in circulation, such as modifying their surface properties to reduce immune recognition or designing nanomaterial-based carriers to shield EVs from clearance, are actively being explored. However, these strategies are still in early stages, and the long-term effects of such modifications need thorough investigation to ensure that they do not compromise the EVs’ natural properties or induce adverse immune reactions (Pirisinu et al., 2022).
As with any novel therapeutic approach, ethical considerations must be carefully addressed when developing EV-nanomaterial hybrid therapies. These therapies involve modifying biological materials, which raises questions about informed consent, patient privacy, and the long-term implications of using genetically modified or engineered materials in humans. Ethical concerns also extend to the collection and use of human-derived EVs, particularly in terms of privacy and consent for their use in research or clinical applications. Additionally, the introduction of nanomaterials into the body, particularly those that may accumulate over time, raises potential concerns about unintended consequences. Regulatory bodies will need to consider these ethical issues in developing frameworks that ensure the responsible use of EV-nanomaterial hybrids in clinical settings (Hagey and El Andaloussi, 2023).
These limitations underscore the complexity of integrating nanomaterials with EVs for advanced biomedical applications and highlight areas requiring further research and collaboration to overcome these challenges.
Emerging trends in EV-nanomaterial research indicate significant advancements in the integration of AI and machine learning (ML) to accelerate the design and optimization of hybrid systems. AI-driven computational models are increasingly being used to predict the behavior of EVs and nanomaterials at the molecular level, enabling more efficient selection of nanomaterials based on specific properties such as size, surface charge, and targeting capacity. Machine learning algorithms can analyze large datasets to identify patterns that may not be immediately apparent, helping to streamline the discovery of novel nanomaterial-EV combinations.
Furthermore, AI has the potential to revolutionize the customization of EV-based drug delivery systems. By combining AI with high-throughput screening techniques, researchers can rapidly identify the most effective formulations for therapeutic delivery, based on factors like cargo loading efficiency, stability, and cellular uptake. In addition, AI can assist in predicting and mitigating the potential toxicity or immunogenicity of these hybrid systems, which is a major concern in their clinical translation.
Additionally, the future of EV-nanomaterial hybrids may involve the incorporation of advanced nanofabrication techniques, such as 3D printing, to create more complex, multifunctional systems. These advances could pave the way for more personalized and scalable therapeutic options, particularly in areas such as cancer treatment, regenerative medicine, and gene therapy.
By integrating AI and other cutting-edge technologies, the future of EV-nanomaterial hybrids looks promising, offering enhanced efficiency, precision, and safety in drug delivery and biomedical applications. We believe this addition significantly enriches the manuscript by providing insight into the exciting potential of AI in shaping the next-generation of EV-based therapies.
The integration of nanomaterials with EVs offers exhilarating potentials for advanced biomedical applications. As a result of this synergy, healthcare can be transformed and innovative advances made. One of the most promising future prospects is the expansion of highly targeted therapies using EVs-nanomaterial hybrids. Researchers are actively developing the engineered EVs to carry specific cargo molecules, like drugs or nucleic acids, and functionalizing nanomaterials to enhance targeting. This innovative approach could be transformed into cancer treatment, delivering drugs precisely to tumors while minimizing side effects. It may also possess efficacy treatments for other diseases, such as neurodegenerative disorders and cardiovascular conditions. Personalized medicine is another boulevard that, this integration could unlock. By leveraging the diversity of EVs and tailoring nanomaterials properties, clinicians might create modified therapies for individual patients. This could involve selecting EVs from specific cell sources, engineering them with patient-specific cargo, and optimizing nanomaterials for improved targeting, resulting in more operative and safer treatments.
EVs have exposed promise as vehicles for vaccines. In the future, EVs-based vaccines using nanomaterials to enhance the stability and immune responses. EVs could carry viral antigens, making them ideal for vaccine delivery. Nanomaterials could optimize the vaccine formulation and delivery, possibly leading to more operative and easily administered immunization strategies. Regenerative medicine can be benefited significantly from EVs-nanomaterial hybrids. Researchers are discovering these hybrids to excite tissue repair and regeneration. By designing nanomaterials that mimic the extracellular matrix and loading EVs with regenerative cargo, this method could encourage the healing of damaged tissues, such as cartilage, bone, and nerves, providing new hope for treating injuries and degenerative diseases. The integration of nanomaterials with EVs also provides new potentials in diagnostics and medical imaging. Researchers are emerging the EVs-based biosensors and imaging agents for real-time disease monitoring. This innovative approach revolutionize early disease detection, leading to more effective and personalized healthcare.
Future research will likely focus on refining the use of EV-nanomaterial hybrids for drug delivery. Improving cargo loading efficiency, extending EV circulation time, and enhancing stability are key objectives. Nanomaterials can play a pivotal role in achieving these goals through various approaches, ultimately leading to more efficient and safer drug delivery systems for a wide range of diseases. Combining the unique properties of EVs and nanomaterials may enable the development of multimodal therapies. These therapies could incorporate various treatment strategies into a single platform. EVs, as versatile carriers, and engineered nanomaterials could facilitate multiple therapeutic approaches simultaneously, particularly valuable for complex diseases requiring a multifaceted approach. Addressing scalability is a crucial future perspective for this field. Researchers are actively exploring innovative manufacturing techniques to produce EVs and nanomaterials at larger scales while maintaining quality and reproducibility. Scalable production methods are essential to translating EV-nanomaterial therapies from the lab to clinical practice effectively. As EV-nanomaterial-based therapies progress toward clinical use, regulatory and ethical considerations will become increasingly important. Regulatory agencies will need to establish clear guidelines for evaluating the safety and efficacy of these novel treatments. Ethical discussions should continue to ensure responsible development and equitable access to these advanced biomedical applications.
The synergistic integration of nanomaterials with extracellular vesicles represents a promising frontier in biomedical research and applications. Future prospects include highly targeted therapies, personalized medicine, EV-based vaccines, regenerative medicine, diagnostics, drug delivery optimization, multimodal therapies, advancements in manufacturing, and regulatory and ethical considerations. Collaborative research energies among researchers, clinicians, industry partners, and regulatory bodies are essential to realizing the full potential of this novel field. With continued research and innovation, EVs-nanomaterials hybrids possess the promise of revolutionizing healthcare and improving patients’ lives worldwide.
The integration of nanomaterials with EVs signifies a highly promising frontier in advanced biomedical research. This synergistic approach provides exciting prospects, including highly targeted therapies, personalized medicine, regenerative medicine, improved diagnostics, efficient drug delivery, multimodal therapies, scalable production methods, and ethical regulatory considerations. The possible impact of EVs-nanomaterials hybrids on healthcare is substantial. They have the capability to revolutionize treatment strategies, efficacy patient outcomes, and allow the tailored therapies for a range of diseases. Nevertheless, addressing existing limitations such as scalability, cargo loading efficiency, and regulatory challenges is crucial to realizing these probabilities. Research collaboration among researchers, clinicians, industry partners, and regulatory bodies will be crucial for the responsible development and equitable access to these innovative biomedical applications. With ongoing research and innovation, EVs-nanomaterials hybrids possess the promise of transforming the landscape of healthcare, enlightening patient wellbeing, and advancing the field of medicine.
HJ: Conceptualization, Investigation, Writing–original draft. RK: Conceptualization, Investigation, Writing–original draft. JP: Formal Analysis, Investigation, Resources, Validation, Writing–original draft. KR: Formal Analysis, Investigation, Resources, Validation, Writing–original draft. GV: Conceptualization, Supervision, Validation, Writing–review and editing. CP: Conceptualization, Formal Analysis, Investigation, Supervision, Writing–review and editing. IDM: Conceptualization, Formal Analysis, Investigation, Supervision, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by Lucian Blaga University of Sibiu & Hasso Plattner Foundation research grants LBUS-IRG-2021-07.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Albalawi, F., Hussein, M. Z., Fakurazi, S., and Masarudin, M. J. (2021). Engineered nanomaterials: the challenges and opportunities for nanomedicines. Int. J. nanomedicine 16, 161–184. doi:10.2147/ijn.s288236
Ali, W., Deng, K., Bian, Y., Liu, Z., and Zou, H. (2023). Spectacular role of epididymis and bio-active cargo of nano-scale exosome in sperm maturation: a review. Biomed. and Pharmacother. 164, 114889. doi:10.1016/j.biopha.2023.114889
Allan, D., Tieu, A., Lalu, M., and Burger, D. (2020). Mesenchymal stromal cell-derived extracellular vesicles for regenerative therapy and immune modulation: progress and challenges toward clinical application. Stem Cells Transl. Med. 9, 39–46. doi:10.1002/sctm.19-0114
Bayat, F., Afshar, A., and Baghban, N. (2021). Algal cells-derived extracellular vesicles: a review with special emphasis on their antimicrobial effects. Front. Microbiol. 12, 785716. doi:10.3389/fmicb.2021.785716
Beetler, D. J., Di Florio, D. N., Law, E. W., Groen, C. M., Windebank, A. J., Peterson, Q. P., et al. (2023). The evolving regulatory landscape in regenerative medicine. Mol. Aspects Med. 91, 101138. doi:10.1016/j.mam.2022.101138
Bennett, A. P., De La Torre-Escudero, E., and Robinson, M. W. (2020). Helminth genome analysis reveals conservation of extracellular vesicle biogenesis pathways but divergence of RNA loading machinery between phyla. Int. J. Parasitol. 50, 655–661. doi:10.1016/j.ijpara.2020.04.004
Bhattacharya, S., and Kundu, A. (2020). “Sugars and sugar polyols in overcoming environmental stresses,” in Protective chemical agents in the amelioration of plant abiotic stress: biochemical and molecular perspectives, 71–101.
Boilard, E., and Bellio, M. (2022). Platelet extracellular vesicles and the secretory interactome join forces in health and disease. Immunol. Rev. 312, 38–51. doi:10.1111/imr.13119
Borniego, M. L., and Innes, R. W. (2023). Extracellular RNA: mechanisms of secretion and potential functions. J. Exp. Bot. 74, 2389–2404. doi:10.1093/jxb/erac512
Buzas, E. I. (2023). The roles of extracellular vesicles in the immune system. Nat. Rev. Immunol. 23, 236–250. doi:10.1038/s41577-022-00763-8
Cai, Q., He, B., Wang, S., Fletcher, S., Niu, D., Mitter, N., et al. (2021). Message in a bubble: shuttling small RNAs and proteins between cells and interacting organisms using extracellular vesicles. Annu. Rev. plant Biol. 72, 497–524. doi:10.1146/annurev-arplant-081720-010616
Cassani, M., Fernandes, S., Vrbsky, J., Ergir, E., Cavalieri, F., and Forte, G. (2020). Combining nanomaterials and developmental pathways to design new treatments for cardiac regeneration: the pulsing heart of advanced therapies. Front. Bioeng. Biotechnol. 8, 323. doi:10.3389/fbioe.2020.00323
Catalano, M., and O’driscoll, L. (2020). Inhibiting extracellular vesicles formation and release: a review of EV inhibitors. J. Extracell. vesicles 9, 1703244. doi:10.1080/20013078.2019.1703244
Chen, J., Zhang, X., Millican, R., Sherwood, J., Martin, S., Jo, H., et al. (2021). Recent advances in nanomaterials for therapy and diagnosis for atherosclerosis. Adv. drug Deliv. Rev. 170, 142–199. doi:10.1016/j.addr.2021.01.005
Cheng, G., Li, W., Ha, L., Han, X., Hao, S., Wan, Y., et al. (2018). Self-assembly of extracellular vesicle-like metal–organic framework nanoparticles for protection and intracellular delivery of biofunctional proteins. J. Am. Chem. Soc. 140, 7282–7291. doi:10.1021/jacs.8b03584
Cheng, H. W., Tsao, H. Y., Chiang, C. S., and Chen, S. Y. (2021). Advances in magnetic nanoparticle-mediated cancer immune-theranostics. Adv. Healthc. Mater. 10, 2001451. doi:10.1002/adhm.202001451
Cheng, L., and Hill, A. F. (2022). Therapeutically harnessing extracellular vesicles. Nat. Rev. Drug Discov. 21, 379–399. doi:10.1038/s41573-022-00410-w
Chua, J. K. E., Lim, J., Foong, L. H., Mok, C. Y., Tan, H. Y., Tung, X. Y., et al. (2022). Mesenchymal stem cell-derived extracellular vesicles: progress and remaining hurdles in developing regulatory compliant quality control assays. Cell Biol. Transl. Med. 17, 191–211. doi:10.1007/5584_2022_728
Cruz Camacho, A., Alfandari, D., Kozela, E., and Regev-Rudzki, N. (2023). Biogenesis of extracellular vesicles in protozoan parasites: the ESCRT complex in the trafficking fast lane? PLoS Pathog. 19, e1011140. doi:10.1371/journal.ppat.1011140
Dicenzo, G. C., Tesi, M., Pfau, T., Mengoni, A., and Fondi, M. (2020). Genome-scale metabolic reconstruction of the symbiosis between a leguminous plant and a nitrogen-fixing bacterium. Nat. Commun. 11, 2574. doi:10.1038/s41467-020-16484-2
Di Santo, R., Vaccaro, M., Romanò, S., Di Giacinto, F., Papi, M., Rapaccini, G. L., et al. (2022). Machine learning-assisted FTIR analysis of circulating extracellular vesicles for cancer liquid biopsy. J. Personalized Med. 12, 949. doi:10.3390/jpm12060949
Dixson, A. C., Dawson, T. R., Di Vizio, D., and Weaver, A. M. (2023). Context-specific regulation of extracellular vesicle biogenesis and cargo selection. Nat. Rev. Mol. Cell Biol. 24, 454–476. doi:10.1038/s41580-023-00576-0
Dos Santos, J., De Oliveira, R. S., De Oliveira, T. V., Velho, M. C., Konrad, M. V., Da Silva, G. S., et al. (2021). 3D printing and nanotechnology: a multiscale alliance in personalized medicine. Adv. Funct. Mater. 31, 2009691. doi:10.1002/adfm.202009691
Dumontel, B., Susa, F., Limongi, T., Canta, M., Racca, L., Chiodoni, A., et al. (2019). ZnO nanocrystals shuttled by extracellular vesicles as effective Trojan nano-horses against cancer cells. Nanomedicine 14, 2815–2833. doi:10.2217/nnm-2019-0231
Elkhoury, K., Chen, M., Koçak, P., Enciso-Martínez, E., Bassous, N. J., Lee, M. C., et al. (2022). Hybrid extracellular vesicles-liposome incorporated advanced bioink to deliver microRNA. Biofabrication 14, 045008. doi:10.1088/1758-5090/ac8621
El Nahrawy, A. M., Hemdan, B. A., Mansour, A., Elzwawy, A., and Abou Hammad, A. B. (2021). Integrated use of nickel cobalt aluminoferrite/Ni2+ nano-crystallites supported with SiO2 for optomagnetic and biomedical applications. Mater. Sci. Eng. B 274, 115491. doi:10.1016/j.mseb.2021.115491
Fauth, M., Hegewald, A. B., Schmitz, L., Krone, D. J., and Saul, M. J. (2019). Validation of extracellular miRNA quantification in blood samples using RT-qPCR. FASEB bioAdvances 1, 481–492. doi:10.1096/fba.2019-00018
Gaurav, I., Thakur, A., Kumar, G., Long, Q., Zhang, K., Sidu, R. K., et al. (2023). Delivery of apoplastic extracellular vesicles encapsulating Green-synthesized silver nanoparticles to treat citrus canker. Nanomaterials 13, 1306. doi:10.3390/nano13081306
Girija, A., Hacham, Y., Dvir, S., Panda, S., Lieberman-Lazarovich, M., and Amir, R. (2023). Cystathionine γ-synthase expression in seeds alters metabolic and DNA methylation profiles in Arabidopsis. Plant Physiol. 193, 595–610. doi:10.1093/plphys/kiad330
Gong, D., Sun, L., Li, X., Zhang, W., Zhang, D., and Cai, J. (2023). Micro/nanofabrication, assembly, and actuation based on microorganisms: recent advances and perspectives. Small Struct. 4, 2200356. doi:10.1002/sstr.202370026
Hagey, D. W., and El Andaloussi, S. (2023). “The promise and challenges of extracellular vesicles in the diagnosis of neurodegenerative diseases,” in Handbook of clinical neurology (Elsevier), 227–241.
Hamada, T., Dubois, J. L., Bellamy, V., Pidial, L., Hagège, A., Pereira, M. N., et al. (2020). In vitro controlled release of extracellular vesicles for cardiac repair from poly (glycerol sebacate) acrylate-based polymers. Acta Biomater. 115, 92–103. doi:10.1016/j.actbio.2020.08.015
Han, C., Zhou, J., Liu, B., Liang, C., Pan, X., Zhang, Y., et al. (2019). Delivery of miR-675 by stem cell-derived exosomes encapsulated in silk fibroin hydrogel prevents aging-induced vascular dysfunction in mouse hindlimb. Mater. Sci. Eng. C 99, 322–332. doi:10.1016/j.msec.2019.01.122
Han, H. S., and Choi, K. Y. (2021). Advances in nanomaterial-mediated photothermal cancer therapies: toward clinical applications. Biomedicines 9, 305. doi:10.3390/biomedicines9030305
Han, X., Mitchell, M. J., and Nie, G. (2020). Nanomaterials for therapeutic RNA delivery. Matter 3, 1948–1975. doi:10.1016/j.matt.2020.09.020
Haque, N., Widera, D., Govindasamy, V., Soesilawati, P., and Abu Kasim, N. H. (2022). Extracellular vesicles from stem and progenitor cells for cell-free regenerative therapy. Curr. Mol. Med. 22, 120–131. doi:10.2174/1566524021666210125114828
Herrmann, I. K., Wood, M. J. A., and Fuhrmann, G. (2021). Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 16, 748–759. doi:10.1038/s41565-021-00931-2
Hu, W., Liu, C., Bi, Z.-Y., Zhou, Q., Zhang, H., Li, L.-L., et al. (2020). Comprehensive landscape of extracellular vesicle-derived RNAs in cancer initiation, progression, metastasis and cancer immunology. Mol. Cancer 19, 102–123. doi:10.1186/s12943-020-01199-1
Imanbekova, M., Suarasan, S., Lu, Y., Jurchuk, S., and Wachsmann-Hogiu, S. (2022). Recent advances in optical label-free characterization of extracellular vesicles. Nanophotonics 11, 2827–2863. doi:10.1515/nanoph-2022-0057
Jalaludin, I., Lubman, D. M., and Kim, J. (2023). A guide to mass spectrometric analysis of extracellular vesicle proteins for biomarker discovery. Mass Spectrom. Rev. 42, 844–872. doi:10.1002/mas.21749
Jhan, Y.-Y., Palou Zuniga, G., Singh, K. A., Gaharwar, A. K., Alge, D. L., and Bishop, C. J. (2021). Polymer-coated extracellular vesicles for selective codelivery of chemotherapeutics and siRNA to cancer cells. ACS Appl. Bio Mater. 4, 1294–1306. doi:10.1021/acsabm.0c01153
Jia, G., Han, Y., An, Y., Ding, Y., He, C., Wang, X., et al. (2018). NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials 178, 302–316. doi:10.1016/j.biomaterials.2018.06.029
Jia, Z., Zhang, S., and Li, W. (2023). Harnessing stem cell-derived extracellular vesicles for the regeneration of degenerative bone conditions. Int. J. Nanomedicine Vol. 18, 5561–5578. doi:10.2147/ijn.s424731
Ju, Y., Hu, Y., Yang, P., Xie, X., and Fang, B. (2022). Extracellular vesicle-loaded hydrogels for tissue repair and regeneration. Mater. Today Bio 18, 100522. doi:10.1016/j.mtbio.2022.100522
Kalininskiy, A., Kittel, J., Nacca, N. E., Misra, R. S., Croft, D. P., and Mcgraw, M. D. (2021). E-cigarette exposures, respiratory tract infections, and impaired innate immunity: a narrative review. Pediatr. Med. Hong. Kong, China 4, 5. doi:10.21037/pm-20-97
Kang, T., Atukorala, I., and Mathivanan, S. (2021). Biogenesis of extracellular vesicles. New Front. Extracell. Vesicles 97, 19–43. doi:10.1007/978-3-030-67171-6_2
Khongkow, M., Yata, T., Boonrungsiman, S., Ruktanonchai, U. R., Graham, D., and Namdee, K. (2019). Surface modification of gold nanoparticles with neuron-targeted exosome for enhanced blood–brain barrier penetration. Sci. Rep. 9, 8278. doi:10.1038/s41598-019-44569-6
Kim, H. Y., Kim, T. J., Kang, L., Kim, Y.-J., Kang, M. K., Kim, J., et al. (2020). Mesenchymal stem cell-derived magnetic extracellular nanovesicles for targeting and treatment of ischemic stroke. Biomaterials 243, 119942. doi:10.1016/j.biomaterials.2020.119942
Kim, H. Y., Kumar, H., Jo, M.-J., Kim, J., Yoon, J.-K., Lee, J.-R., et al. (2018). Therapeutic efficacy-potentiated and diseased organ-targeting nanovesicles derived from mesenchymal stem cells for spinal cord injury treatment. Nano Lett. 18, 4965–4975. doi:10.1021/acs.nanolett.8b01816
Kooijmans, S., Fliervoet, L., Van Der Meel, R., Fens, M., Heijnen, H., En Henegouwen, P. V. B., et al. (2016). PEGylated and targeted extracellular vesicles display enhanced cell specificity and circulation time. J. Control. Release 224, 77–85. doi:10.1016/j.jconrel.2016.01.009
Kuo, C.-L., Ponneri Babuharisankar, A., Lin, Y.-C., Lien, H.-W., Lo, Y. K., Chou, H.-Y., et al. (2022). Mitochondrial oxidative stress in the tumor microenvironment and cancer immunoescape: foe or friend? J. Biomed. Sci. 29, 74. doi:10.1186/s12929-022-00859-2
Lai, J., Meng, Q.-F., Tian, M., Zhuang, X., Pan, P., Du, L., et al. (2022). A decoy microrobot that removes SARS-CoV-2 and its variants in wastewater. Cell Rep. Phys. Sci. 3, 101061. doi:10.1016/j.xcrp.2022.101061
Leidal, A. M., and Debnath, J. (2021). Emerging roles for the autophagy machinery in extracellular vesicle biogenesis and secretion. FASEB BioAdvances 3, 377–386. doi:10.1096/fba.2020-00138
Li, C., Song, Q., Yin, X., Song, R., and Chen, G. (2022a). Preparation, characterization, and in vitro anticancer activity evaluation of broccoli-derived extracellular vesicle-coated astaxanthin nanoparticles. Molecules 27, 3955. doi:10.3390/molecules27123955
Li, H., Xu, W., Li, F., Zeng, R., Zhang, X., Wang, X., et al. (2022b). Amplification of anticancer efficacy by co-delivery of doxorubicin and lonidamine with extracellular vesicles. Drug Deliv. 29, 192–202. doi:10.1080/10717544.2021.2023697
Li, J., Zhang, Y., Dong, P.-Y., Yang, G.-M., and Gurunathan, S. (2023a). A comprehensive review on the composition, biogenesis, purification, and multifunctional role of exosome as delivery vehicles for cancer therapy. Biomed. and Pharmacother. 165, 115087. doi:10.1016/j.biopha.2023.115087
Li, R., Gong, X., Hong, C., Wang, H., Chen, Y., Tan, K., et al. (2021). An efficient photochemotherapy nanoplatform based on the endogenous biosynthesis of photosensitizer in macrophage-derived extracellular vesicles. Biomaterials 279, 121234. doi:10.1016/j.biomaterials.2021.121234
Li, W., Liu, Y., Zhang, P., Tang, Y., Zhou, M., Jiang, W., et al. (2018). Tissue-engineered bone immobilized with human adipose stem cells-derived exosomes promotes bone regeneration. ACS Appl. Mater. and interfaces 10, 5240–5254. doi:10.1021/acsami.7b17620
Li, X., He, S., Luo, B., Li, P., Chen, X., Wu, M., et al. (2023b). Engineered extracellular vesicles to enhance antigen presentation for boosting light-driven tumor immunotherapy. Small 19, 2303541. doi:10.1002/smll.202303541
Li, Y., Tang, X., Gu, Y., and Zhou, G. (2023c). Adipocyte-derived extracellular vesicles: small vesicles with big impact. Front. Bioscience-Landmark 28, 149. doi:10.31083/j.fbl2807149
Li, Y., Wu, J., Liu, R., Zhang, Y., and Li, X. (2022c). Extracellular vesicles: catching the light of intercellular communication in fibrotic liver diseases. Theranostics 12, 6955–6971. doi:10.7150/thno.77256
Liu, C., Wang, Y., Li, L., He, D., Chi, J., Li, Q., et al. (2022a). Engineered extracellular vesicles and their mimetics for cancer immunotherapy. J. Control. Release 349, 679–698. doi:10.1016/j.jconrel.2022.05.062
Liu, Q., Huang, J., Xia, J., Liang, Y., and Li, G. (2022b). Tracking tools of extracellular vesicles for biomedical research. Front. Bioeng. Biotechnol. 10, 943712. doi:10.3389/fbioe.2022.943712
Liu, R., Luo, C., Pang, Z., Zhang, J., Ruan, S., Wu, M., et al. (2023a). Advances of nanoparticles as drug delivery systems for disease diagnosis and treatment. Chin. Chem. Lett. 34, 107518. doi:10.1016/j.cclet.2022.05.032
Liu, Y., Cui, J., Wang, H., Hezam, K., Zhao, X., Huang, H., et al. (2020). Enhanced therapeutic effects of MSC-derived extracellular vesicles with an injectable collagen matrix for experimental acute kidney injury treatment. Stem cell Res. and Ther. 11, 161. doi:10.1186/s13287-020-01668-w
Liu, Z., Zhuang, Y., Fang, L., Yuan, C., Wang, X., and Lin, K. (2023b). Breakthrough of extracellular vesicles in pathogenesis, diagnosis and treatment of osteoarthritis. Bioact. Mater. 22, 423–452. doi:10.1016/j.bioactmat.2022.10.012
Luo, Y., Yang, H., Zhou, Y.-F., and Hu, B. (2020). Dual and multi-targeted nanoparticles for site-specific brain drug delivery. J. Control. Release 317, 195–215. doi:10.1016/j.jconrel.2019.11.037
Lv, K., Li, Q., Zhang, L., Wang, Y., Zhong, Z., Zhao, J., et al. (2019). Incorporation of small extracellular vesicles in sodium alginate hydrogel as a novel therapeutic strategy for myocardial infarction. Theranostics 9, 7403–7416. doi:10.7150/thno.32637
Ma, W., Zhan, Y., Zhang, Y., Mao, C., Xie, X., and Lin, Y. (2021). The biological applications of DNA nanomaterials: current challenges and future directions. Signal Transduct. Target. Ther. 6, 351. doi:10.1038/s41392-021-00727-9
Makabenta, J. M. V., Nabawy, A., Li, C.-H., Schmidt-Malan, S., Patel, R., and Rotello, V. M. (2021). Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 19, 23–36. doi:10.1038/s41579-020-0420-1
Manandhar, B., Paudel, K. R., Panth, N., Hansbro, P., Oliver, B. G., and Dua, K. (2022). Applications of extracellular vesicles as a drug-delivery system for chronic respiratory diseases. Future Med. 17, 817–820. doi:10.2217/nnm-2021-0384
Mansoori, B., Baradaran, B., Nazari, A., Gaballu, F. A., Cho, W.C.-S., and Mansoori, B. (2022). MicroRNAs in the cancer cell-to-cell communication: an insight into biological vehicles. Biomed. and Pharmacother. 153, 113449. doi:10.1016/j.biopha.2022.113449
Mansur, S., Habib, S., Hawkins, M., Brown, S. R., Weinman, S. T., and Bao, Y. (2023). Preparation of nanoparticle-loaded extracellular vesicles using direct flow filtration. Pharmaceutics 15, 1551. doi:10.3390/pharmaceutics15051551
Mardpour, S., Ghanian, M. H., Sadeghi-Abandansari, H., Mardpour, S., Nazari, A., Shekari, F., et al. (2019). Hydrogel-mediated sustained systemic delivery of mesenchymal stem cell-derived extracellular vesicles improves hepatic regeneration in chronic liver failure. ACS Appl. Mater. and interfaces 11, 37421–37433. doi:10.1021/acsami.9b10126
Meng, Q.-F., Tai, W., Tian, M., Zhuang, X., Pan, Y., Lai, J., et al. (2023). Inhalation delivery of dexamethasone with iSEND nanoparticles attenuates the COVID-19 cytokine storm in mice and nonhuman primates. Sci. Adv. 9, eadg3277. doi:10.1126/sciadv.adg3277
Meng, Q. F., Zhao, Y., Dong, C., Liu, L., Pan, Y., Lai, J., et al. (2021). Genetically programmable fusion cellular vesicles for cancer immunotherapy. Angew. Chem. Int. Ed. 60, 26320–26326. doi:10.1002/anie.202108342
Meng, W., He, C., Hao, Y., Wang, L., Li, L., and Zhu, G. (2020). Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv. 27, 585–598. doi:10.1080/10717544.2020.1748758
Midekessa, G., Godakumara, K., Ord, J., Viil, J., LäTtekivi, F., Dissanayake, K., et al. (2020). Zeta potential of extracellular vesicles: toward understanding the attributes that determine colloidal stability. ACS omega 5, 16701–16710. doi:10.1021/acsomega.0c01582
Mittal, S., Gupta, P., Chaluvally-Raghavan, P., and Pradeep, S. (2020). Emerging role of extracellular vesicles in immune regulation and cancer progression. Cancers 12, 3563. doi:10.3390/cancers12123563
Mol, E. A., Lei, Z., Roefs, M. T., Bakker, M. H., Goumans, M. J., Doevendans, P. A., et al. (2019). Injectable supramolecular ureidopyrimidinone hydrogels provide sustained release of extracellular vesicle therapeutics. Adv. Healthc. Mater. 8, 1900847. doi:10.1002/adhm.201900847
Mondal, J., Pillarisetti, S., Junnuthula, V., Saha, M., Hwang, S. R., Park, I.-K., et al. (2023). Hybrid exosomes, exosome-like nanovesicles and engineered exosomes for therapeutic applications. J. Control. Release 353, 1127–1149. doi:10.1016/j.jconrel.2022.12.027
Mundekkad, D., and Cho, W. C. (2022). Nanoparticles in clinical translation for cancer therapy. Int. J. Mol. Sci. 23, 1685. doi:10.3390/ijms23031685
Murugesan, R. (2021). “Advanced characterization and techniques,” in 2D functional nanomaterials: synthesis, characterization and applications, 141–156.
Nair, S., and Salomon, C. (2020). Extracellular vesicles as critical mediators of maternal-fetal communication during pregnancy and their potential role in maternal metabolism. Placenta 98, 60–68. doi:10.1016/j.placenta.2020.06.011
Naskar, A., Cho, H., and Kim, K.-S. (2022). A nanocomposite with extracellular vesicles from Lactobacillus paracasei as a bioinspired nanoantibiotic targeting Staphylococcus aureus. Pharmaceutics 14, 2273. doi:10.3390/pharmaceutics14112273
Naskar, A., and Kim, K.-S. (2019). Nanomaterials as delivery vehicles and components of new strategies to combat bacterial infections: advantages and limitations. Microorganisms 7, 356. doi:10.3390/microorganisms7090356
Nasrollahzadeh, M., Sajjadi, M., Soufi, G. J., Iravani, S., and Varma, R. S. (2020). Nanomaterials and nanotechnology-associated innovations against viral infections with a focus on coronaviruses. Nanomaterials 10, 1072. doi:10.3390/nano10061072
Natale, F., Fusco, S., and Grassi, C. (2022). Dual role of brain-derived extracellular vesicles in dementia-related neurodegenerative disorders: cargo of disease spreading signals and diagnostic-therapeutic molecules. Transl. Neurodegener. 11, 50. doi:10.1186/s40035-022-00326-w
Nguyen, V. V., Witwer, K. W., Verhaar, M. C., Strunk, D., and Van Balkom, B. W. (2020). Functional assays to assess the therapeutic potential of extracellular vesicles. J. Extracell. vesicles 10, e12033. doi:10.1002/jev2.12033
Nielsen, J. E., Maltesen, R. G., Havelund, J. F., Færgeman, N. J., Gotfredsen, C. H., Vestergård, K., et al. (2021). Characterising Alzheimer's disease through integrative NMR-and LC-MS-based metabolomics. Metab. open 12, 100125. doi:10.1016/j.metop.2021.100125
Nieuwland, R., Siljander, P.R.-M., Falcón-Pérez, J. M., and Witwer, K. W. (2022). Reproducibility of extracellular vesicle research. Eur. J. cell Biol. 101, 151226. doi:10.1016/j.ejcb.2022.151226
Niu, W., Xiao, Q., Wang, X., Zhu, J., Li, J., Liang, X., et al. (2021). A biomimetic drug delivery system by integrating grapefruit extracellular vesicles and doxorubicin-loaded heparin-based nanoparticles for glioma therapy. Nano Lett. 21, 1484–1492. doi:10.1021/acs.nanolett.0c04753
Noble, J. M., Roberts, L. M., Vidavsky, N., Chiou, A. E., Fischbach, C., Paszek, M. J., et al. (2020). Direct comparison of optical and electron microscopy methods for structural characterization of extracellular vesicles. J. Struct. Biol. 210, 107474. doi:10.1016/j.jsb.2020.107474
Notaguchi, M., Pallas, V., Qiu, J., and Wang, X. (2022). Editorial: systemic RNA signalling in plants. Front. plant Sci. 13, 878728. doi:10.3389/fpls.2022.878728
Paganini, C., Capasso Palmiero, U., Pocsfalvi, G., Touzet, N., Bongiovanni, A., and Arosio, P. (2019). Scalable production and isolation of extracellular vesicles: available sources and lessons from current industrial bioprocesses. Biotechnol. J. 14, 1800528. doi:10.1002/biot.201800528
Paisrisarn, P., Yasui, T., Zhu, Z., Klamchuen, A., Kasamechonchung, P., Wutikhun, T., et al. (2022). Tailoring ZnO nanowire crystallinity and morphology for label-free capturing of extracellular vesicles. Nanoscale 14, 4484–4494. doi:10.1039/d1nr07237d
Palanisamy, C. P., Pei, J., Alugoju, P., Anthikapalli, N. V. A., Jayaraman, S., Veeraraghavan, V. P., et al. (2023). New strategies of neurodegenerative disease treatment with extracellular vesicles (EVs) derived from mesenchymal stem cells (MSCs). Theranostics 13, 4138–4165. doi:10.7150/thno.83066
Pan, R., He, T., Zhang, K., Zhu, L., Lin, J., Chen, P., et al. (2023). “Tumor-targeting extracellular vesicles loaded with siS100A4 for suppressing postoperative breast cancer metastasis,” in Cellular and molecular bioengineering, 1–9.
Paolillo, M., Comincini, S., and Schinelli, S. (2021). Fostering “education”: do extracellular vesicles exploit their own delivery code? Cells 10, 1741. doi:10.3390/cells10071741
Patil, K. C., and Soekmadji, C. (2021). Extracellular vesicle-mediated bone remodeling and bone metastasis: implications in prostate cancer. New Front. Extracell. Vesicles 97, 297–361. doi:10.1007/978-3-030-67171-6_12
Peeters, B. W., Piët, A. C., and Fornerod, M. (2022). Generating membrane curvature at the nuclear pore: a lipid point of view. Cells 11, 469. doi:10.3390/cells11030469
Pirisinu, M., Pham, T. C., Zhang, D. X., Hong, T. N., Nguyen, L. T., and Le, M. T. (2022). “Extracellular vesicles as natural therapeutic agents and innate drug delivery systems for cancer treatment: recent advances, current obstacles, and challenges for clinical translation,” in Seminars in cancer biology (Elsevier), 340–355.
Pizzicannella, J., Diomede, F., Gugliandolo, A., Chiricosta, L., Bramanti, P., Merciaro, I., et al. (2019). 3D printing PLA/gingival stem cells/EVs upregulate miR-2861 and-210 during osteoangiogenesis commitment. Int. J. Mol. Sci. 20, 3256. doi:10.3390/ijms20133256
Pusta, A., Tertis, M., Crăciunescu, I., Turcu, R., Mirel, S., and Cristea, C. (2023). Recent advances in the development of drug delivery applications of magnetic nanomaterials. Pharmaceutics 15, 1872. doi:10.3390/pharmaceutics15071872
Qiao, Z., Zhang, K., Liu, J., Cheng, D., Yu, B., Zhao, N., et al. (2022). Biomimetic electrodynamic nanoparticles comprising ginger-derived extracellular vesicles for synergistic anti-infective therapy. Nat. Commun. 13, 7164. doi:10.1038/s41467-022-34883-5
Rani, S., Lai, A., Nair, S., Sharma, S., Handberg, A., Carrion, F., et al. (2023). Extracellular vesicles as mediators of cell-cell communication in ovarian cancer and beyond–A lipids focus. Cytokine and Growth Factor Rev. 73, 52–68. doi:10.1016/j.cytogfr.2023.06.004
Ritter, A., Kreis, N.-N., Hoock, S. C., Solbach, C., Louwen, F., and Yuan, J. (2022). Adipose tissue-derived mesenchymal stromal/stem cells, obesity and the tumor microenvironment of breast cancer. Cancers 14, 3908. doi:10.3390/cancers14163908
Rogo, U., Fambrini, M., and Pugliesi, C. (2023). Embryo rescue in plant breeding. Plants 12, 3106. doi:10.3390/plants12173106
Rufino-Ramos, D., Albuquerque, P. R., Carmona, V., Perfeito, R., Nobre, R. J., and De Almeida, L. P. (2017). Extracellular vesicles: novel promising delivery systems for therapy of brain diseases. J. Control. Release 262, 247–258. doi:10.1016/j.jconrel.2017.07.001
Sancho-Albero, M., Rubio-Ruiz, B., Pérez-López, A. M., Sebastián, V., Martín-Duque, P., Arruebo, M., et al. (2019). Cancer-derived exosomes loaded with ultrathin palladium nanosheets for targeted bioorthogonal catalysis. Nat. Catal. 2, 864–872. doi:10.1038/s41929-019-0333-4
Schubert, A., and Boutros, M. (2021). Extracellular vesicles and oncogenic signaling. Mol. Oncol. 15, 3–26. doi:10.1002/1878-0261.12855
Shaimardanova, A. A., Solovyeva, V. V., Chulpanova, D. S., James, V., Kitaeva, K. V., and Rizvanov, A. A. (2020). Extracellular vesicles in the diagnosis and treatment of central nervous system diseases. Neural Regen. Res. 15, 586. doi:10.4103/1673-5374.266908
Shang, L., Xie, Q., Yang, C., Kong, L., and Zhang, Z. (2023). Extracellular vesicles facilitate the transportation of nanoparticles within and between cells for enhanced tumor therapy. ACS Appl. Mater. and Interfaces 15, 42378–42394. doi:10.1021/acsami.3c10237
Sheth, M., and Esfandiari, L. (2022). Bioelectric dysregulation in cancer initiation, promotion, and progression. Front. Oncol. 12, 846917. doi:10.3389/fonc.2022.846917
Shimomura, T., Seino, R., Umezaki, K., Shimoda, A., Ezoe, T., Ishiyama, M., et al. (2021). New lipophilic fluorescent dyes for labeling extracellular vesicles: characterization and monitoring of cellular uptake. Bioconjugate Chem. 32, 680–684. doi:10.1021/acs.bioconjchem.1c00068
Sidhom, K., Obi, P. O., and Saleem, A. (2020). A review of exosomal isolation methods: is size exclusion chromatography the best option? Int. J. Mol. Sci. 21, 6466. doi:10.3390/ijms21186466
Silachev, D. N. (2023). Study of the molecular mechanisms of the therapeutic properties of extracellular vesicles Int. J. Mol. Sci. 24 (8), 7093. doi:10.3390/ijms24087093
Skotland, T., Sagini, K., Sandvig, K., and Llorente, A. (2020). An emerging focus on lipids in extracellular vesicles. Adv. drug Deliv. Rev. 159, 308–321. doi:10.1016/j.addr.2020.03.002
Song, J., Yu, S., Yang, R., Xiao, J., and Liu, J. (2023). Opportunities for the use of selenium nanoparticles in agriculture. NanoImpact 31, 100478. doi:10.1016/j.impact.2023.100478
Song, M., Cui, M., Fang, Z., and Liu, K. (2022). Advanced research on extracellular vesicles based oral drug delivery systems. J. Control. Release 351, 560–572. doi:10.1016/j.jconrel.2022.09.043
Song, T., Lv, M., Zhang, L., Zhang, X., Song, G., Huang, M., et al. (2020). The protective effects of tripeptides VPP and IPP against small extracellular vesicles from angiotensin II-induced vascular smooth muscle cells mediating endothelial dysfunction in human umbilical vein endothelial cells. J. Agric. Food Chem. 68, 13730–13741. doi:10.1021/acs.jafc.0c05698
Soufi, G. J., and Iravani, S. (2020). Eco-friendly and sustainable synthesis of biocompatible nanomaterials for diagnostic imaging: current challenges and future perspectives. Green Chem. 22, 2662–2687. doi:10.1039/d0gc00734j
Tan, D., Zhu, W., Liu, L., Pan, Y., Xu, Y., Huang, Q., et al. (2023). In situ formed scaffold with royal jelly-derived extracellular vesicles for wound healing. Theranostics 13, 2811–2824. doi:10.7150/thno.84665
Teng, F., and Fussenegger, M. (2021). Shedding light on extracellular vesicle biogenesis and bioengineering. Adv. Sci. 8, 2003505. doi:10.1002/advs.202003505
Thangaraju, K., Neerukonda, S. N., Katneni, U., and Buehler, P. W. (2020). Extracellular vesicles from red blood cells and their evolving roles in health, coagulopathy and therapy. Int. J. Mol. Sci. 22, 153. doi:10.3390/ijms22010153
Tian, T., Qiao, S., and Tannous, B. A. (2022). Nanotechnology-inspired extracellular vesicles theranostics for diagnosis and therapy of central nervous system diseases. ACS Appl. Mater. and Interfaces 15, 182–199. doi:10.1021/acsami.2c07981
Tiwari, S., Kumar, V., Randhawa, S., and Verma, S. K. (2021). Preparation and characterization of extracellular vesicles. Am. J. Reproductive Immunol. 85, e13367. doi:10.1111/aji.13367
Tripathi, D., Hajra, K., and Maity, D. (2023). Recent advancement of bio-inspired nanoparticles in cancer theragnostic. J. Nanotheranostics 4, 299–322. doi:10.3390/jnt4030014
Trubiani, O., Marconi, G. D., Pierdomenico, S. D., Piattelli, A., Diomede, F., and Pizzicannella, J. (2019). Human oral stem cells, biomaterials and extracellular vesicles: a promising tool in bone tissue repair. Int. J. Mol. Sci. 20, 4987. doi:10.3390/ijms20204987
Van Niel, G., Carter, D. R., Clayton, A., Lambert, D. W., Raposo, G., and Vader, P. (2022). Challenges and directions in studying cell–cell communication by extracellular vesicles. Nat. Rev. Mol. Cell Biol. 23, 369–382. doi:10.1038/s41580-022-00460-3
Wagner, K. T., Nash, T. R., Liu, B., Vunjak-Novakovic, G., and Radisic, M. (2021). Extracellular vesicles in cardiac regeneration: potential applications for tissues-on-a-chip. Trends Biotechnol. 39, 755–773. doi:10.1016/j.tibtech.2020.08.005
Wang, J., Zhu, M., and Nie, G. (2021). Biomembrane-based nanostructures for cancer targeting and therapy: from synthetic liposomes to natural biomembranes and membrane-vesicles. Adv. drug Deliv. Rev. 178, 113974. doi:10.1016/j.addr.2021.113974
Wang, T., Koukoulis, T. F., Vella, L. J., Su, H., Purnianto, A., Nie, S., et al. (2023a). The proteome and lipidome of extracellular vesicles from Haemonchus contortus to underpin explorations of host–parasite cross–talk. Int. J. Mol. Sci. 24, 10955. doi:10.3390/ijms241310955
Wang, Z., Wang, X., Xu, W., Li, Y., Lai, R., Qiu, X., et al. (2023b). Translational challenges and prospective solutions in the implementation of biomimetic delivery systems. Pharmaceutics 15, 2623. doi:10.3390/pharmaceutics15112623
Worthington, E. N., and Hagood, J. S. (2020). Therapeutic use of extracellular vesicles for acute and chronic lung disease. Int. J. Mol. Sci. 21, 2318. doi:10.3390/ijms21072318
Wu, H., Xing, H., Wu, M.-C., Shen, F., Chen, Y., and Yang, T. (2021). Extracellular-vesicles delivered tumor-specific sequential nanocatalysts can be used for MRI-informed nanocatalytic Therapy of hepatocellular carcinoma. Theranostics 11, 64–78. doi:10.7150/thno.46124
Wu, M.-S., Zhou, Z.-R., Wang, X.-Y., Lv, J., Li, D.-W., and Qian, R.-C. (2023a). Engineered extracellular vesicle-encapsuled nanoreactors for effective targeting and cascade killing of cancer cells. ACS Appl. Bio Mater. 6, 1479–1487. doi:10.1021/acsabm.2c01019
Wu, Q., Fu, S., Xiao, H., Du, J., Cheng, F., Wan, S., et al. (2023b). Advances in extracellular vesicle nanotechnology for precision theranostics. Adv. Sci. 10, 2204814. doi:10.1002/advs.202204814
Xu, C., Wang, Z., Liu, Y., Wei, B., Liu, X., Duan, K., et al. (2023a). Extracellular vesicles derived from bone marrow mesenchymal stem cells loaded on magnetic nanoparticles delay the progression of diabetic osteoporosis via delivery of miR-150-5p. Cell Biol. Toxicol. 39, 1257–1274. doi:10.1007/s10565-022-09744-y
Xu, M., Han, X., Xiong, H., Gao, Y., Xu, B., Zhu, G., et al. (2023b). Cancer nanomedicine: emerging strategies and therapeutic potentials. Molecules 28, 5145. doi:10.3390/molecules28135145
Xu, Y., Feng, K., Zhao, H., Di, L., Wang, L., and Wang, R. (2022). Tumor-derived extracellular vesicles as messengers of natural products in cancer treatment. Theranostics 12, 1683–1714. doi:10.7150/thno.67775
Yang, L., Patel, K. D., Rathnam, C., Thangam, R., Hou, Y., Kang, H., et al. (2022). Harnessing the therapeutic potential of extracellular vesicles for biomedical applications using multifunctional magnetic nanomaterials. Small 18, 2104783. doi:10.1002/smll.202104783
Yang, M., Liu, X., Luo, Q., Xu, L., and Chen, F. (2020). An efficient method to isolate lemon derived extracellular vesicles for gastric cancer therapy. J. nanobiotechnology 18, 100–112. doi:10.1186/s12951-020-00656-9
Yang, Y., Zhu, Z., Gao, R., Yuan, J., Zhang, J., Li, H., et al. (2021a). Controlled release of MSC-derived small extracellular vesicles by an injectable Diels-Alder crosslinked hyaluronic acid/PEG hydrogel for osteoarthritis improvement. Acta biomater. 128, 163–174. doi:10.1016/j.actbio.2021.04.003
Yang, Z., Liu, D., Zhou, H., Tao, B., Chang, L., Liu, H., et al. (2021b). A new nanomaterial based on extracellular vesicles containing chrysin-induced cell apoptosis through let-7a in tongue squamous cell carcinoma. Front. Bioeng. Biotechnol. 9, 766380. doi:10.3389/fbioe.2021.766380
Yaraki, M. T., Tukova, A., and Wang, Y. (2022). Emerging SERS biosensors for the analysis of cells and extracellular vesicles. Nanoscale 14, 15242–15268. doi:10.1039/d2nr03005e
Yom-Tov, N., Guy, R., and Offen, D. (2022). Extracellular vesicles over adeno-associated viruses: advantages and limitations as drug delivery platforms in precision medicine. Adv. drug Deliv. Rev. 190, 114535. doi:10.1016/j.addr.2022.114535
Yu, T.-Y., Xu, C.-X., Li, W.-J., Wang, B., Cai, G., Takeuchi, H., et al. (2023). SPECIALTY SECTION this article was submitted to plant cell biology, a section of the journal frontiers in plant science, II. Regulation of Pollen Tube Growth, 59.
Zhang, C., Shang, Y., Chen, X., Midgley, A. C., Wang, Z., Zhu, D., et al. (2020). Supramolecular nanofibers containing arginine-glycine-aspartate (RGD) peptides boost therapeutic efficacy of extracellular vesicles in kidney repair. ACS nano 14, 12133–12147. doi:10.1021/acsnano.0c05681
Zhang, K., Zhao, X., Chen, X., Wei, Y., Du, W., Wang, Y., et al. (2018). Enhanced therapeutic effects of mesenchymal stem cell-derived exosomes with an injectable hydrogel for hindlimb ischemia treatment. ACS Appl. Mater. and interfaces 10, 30081–30091. doi:10.1021/acsami.8b08449
Zhang, L., Liu, J., and Zhou, C. (2023). Current aspects of small extracellular vesicles in pain process and relief. Biomaterials Res. 27, 78. doi:10.1186/s40824-023-00417-3
Zhang, X., Zhang, H., Gu, J., Zhang, J., Shi, H., Qian, H., et al. (2021). Engineered extracellular vesicles for cancer therapy. Adv. Mater. 33, 2005709. doi:10.1002/adma.202005709
Zhou, Q., Ma, K., Hu, H., Xing, X., Huang, X., and Gao, H. (2022a). Extracellular vesicles: their functions in plant–pathogen interactions. Mol. Plant Pathol. 23, 760–771. doi:10.1111/mpp.13170
Zhou, Q., Yan, Y., Li, Y., Fu, H., Lu, D., Li, Z., et al. (2022b). Tumor-derived extracellular vesicles in melanoma immune response and immunotherapy. Biomed. and Pharmacother. 156, 113790. doi:10.1016/j.biopha.2022.113790
Zhu, W., Dong, Y., Xu, P., Pan, Q., Jia, K., Jin, P., et al. (2022). A composite hydrogel containing resveratrol-laden nanoparticles and platelet-derived extracellular vesicles promotes wound healing in diabetic mice. Acta Biomater. 154, 212–230. doi:10.1016/j.actbio.2022.10.038
Zinger, A., Brozovich, A., Pasto, A., Sushnitha, M., Martinez, J. O., Evangelopoulos, M., et al. (2020). Bioinspired extracellular vesicles: lessons learned from nature for biomedicine and bioengineering. Nanomaterials 10, 2172. doi:10.3390/nano10112172
Zonneveld, M. I., Van Herwijnen, M. J., Fernandez-Gutierrez, M. M., Giovanazzi, A., De Groot, A. M., Kleinjan, M., et al. (2021). Human milk extracellular vesicles target nodes in interconnected signalling pathways that enhance oral epithelial barrier function and dampen immune responses. J. Extracell. Vesicles 10, e12071. doi:10.1002/jev2.12071
Keywords: engineered nanomaterials, extracellular vesicles, characterization, targeted drug delivery, biomedical applications
Citation: Jiang H, Kumarasamy RV, Pei J, Raju K, Kanniappan GV, Palanisamy CP and Mironescu ID (2025) Integrating engineered nanomaterials with extracellular vesicles: advancing targeted drug delivery and biomedical applications. Front. Nanotechnol. 6:1513683. doi: 10.3389/fnano.2024.1513683
Received: 18 October 2024; Accepted: 11 December 2024;
Published: 22 January 2025.
Edited by:
Mona Khafaji, Sharif University of Technology, IranReviewed by:
Gopal Niraula, Iowa State University, United StatesCopyright © 2025 Jiang, Kumarasamy, Pei, Raju, Kanniappan, Palanisamy and Mironescu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gopalakrishnan Velliyur Kanniappan, dmtnb3BhbGFrcmlzaG5hbkBnbWFpbC5jb20=; Chella Perumal Palanisamy, cGVydW1hbGJpb2luZm9AZ21haWwuY29t; Ion Dan Mironescu, aW9uLm1pcm9uZXNjdUB1bGJzaWJpdS5ybw==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.