- 1Department of Chemistry, International University of Business Agriculture and Technology (IUBAT), Dhaka, Bangladesh
- 2Genetic Engineering and Biotechnology Research Laboratory (GEBRL), Centre for Advanced Research in Sciences, University of Dhaka, Dhaka, Bangladesh
Innovative hybrid nanostructures have revolutionized modern therapy by combining different materials at the nanoscale, offering unique synergistic properties that enhance their functionality. These advancements are pivotal in areas such as targeted drug delivery, photothermal and photodynamic therapy, and gene delivery, where they significantly improve therapeutic outcomes. The article discusses the synthesis methods and characterization techniques of HNSs, providing a comprehensive analysis of their mechanisms of action in various therapeutic applications. Highlighted case studies demonstrate their efficacy in treating conditions such as cancer, neurodegenerative diseases, and cardiovascular disorders, underscoring their potential to bridge existing gaps in medical treatments. The review also emphasizes the need for continued innovation and interdisciplinary collaboration to optimize these nanostructures for clinical applications. Future research should focus on enhancing biocompatibility, targeting capabilities, and integrating advanced imaging techniques and AI-driven applications to further improve the precision and efficacy of HNSs in therapy. These advancements herald a new era in medical treatment, offering transformative solutions for complex diseases.
1 Introduction
Nanotechnology enables precise interventions at the molecular level, improving targeted drug delivery, reducing side effects, and enhancing therapeutic efficacy (Cheng et al., 2023). Hybrid nanostructures (HNSs) combine different materials at the nanoscale, exhibiting synergistic properties that enhance their functionality for various therapeutic applications (Desouky et al., 2023). For instance, HNSs can integrate the benefits of both organic and inorganic materials, leading to improved stability, biocompatibility, and multifunctionality in treatments ranging from cancer to cardiovascular diseases (Yang et al., 2022). Foundational studies such as the work by Tang et al. (2012) on mesoporous silica nanoparticles have been pivotal in demonstrating the synthesis, biocompatibility, and drug delivery capabilities of these structures. This article provides a comprehensive analysis of HNS synthesis methods and characterization techniques, identifying effective approaches for creating multifunctional nanostructures. It explores mechanisms of action in therapies such as drug delivery, photothermal and photodynamic therapy, and gene delivery through specific case studies. Additionally, the article reviews clinical applications across various diseases, offering insights into recent advancements and future research directions that can guide clinical practice and further innovation in the field.
2 Fundamentals of HNSs
HNSs are advanced materials that combine two or more distinct components at the nanoscale to exploit their synergistic properties for enhanced functionality. These components can vary widely in nature, including organic and inorganic materials, metals, polymers, or a combination of these. The integration of different materials at the nanoscale allows for the creation of novel structures with unique physical, chemical, and biological properties, making them highly versatile for various applications, especially in modern therapy (Povolotskaya et al., 2015).
2.1 Types of HNSs
Organic-inorganic HNSs, for instance, involve combining organic molecules like polymers with inorganic materials such as metals or semiconductors (Le et al., 2024). These hybrids enhance mechanical strength, electrical conductivity, and biocompatibility, making them ideal for drug delivery systems where stability and targeted delivery are crucial. Metallic-polymeric hybrids integrate metallic nanoparticles with polymers, used in photothermal therapy (PTT) for cancer treatment. The metallic part provides thermal response, while the polymer ensures biocompatibility and controlled therapeutic release (Silva et al., 2019). Inorganic-inorganic hybrids, combining different inorganic materials like metal oxides and nanoparticles, are used for antibacterial coatings (Tanveer et al., 2022). Titanium dioxide with silver nanoparticles, for example, provides photocatalytic activity and antimicrobial properties (Chand et al., 2020; Harun-Ur-Rashid et al., 2024). Organic-organic hybrids combine different organic materials, such as polymer-based nanocarriers with peptides for targeted drug delivery, enhancing targeting and therapeutic efficacy (Ribeiro et al., 2019). Hybrid quantum dot nanostructures involve the integration of quantum dots (QDs) with various materials to enhance their photophysical properties. Graphene quantum dots (GQDs) incorporated into polymer nanocomposites enhance fluorescence for bioimaging applications. The quantum dots provide superior optical properties, while the polymer matrix offers biocompatibility and mechanical support (Mansur et al., 2019).
2.2 Synthetic routes of HNSs
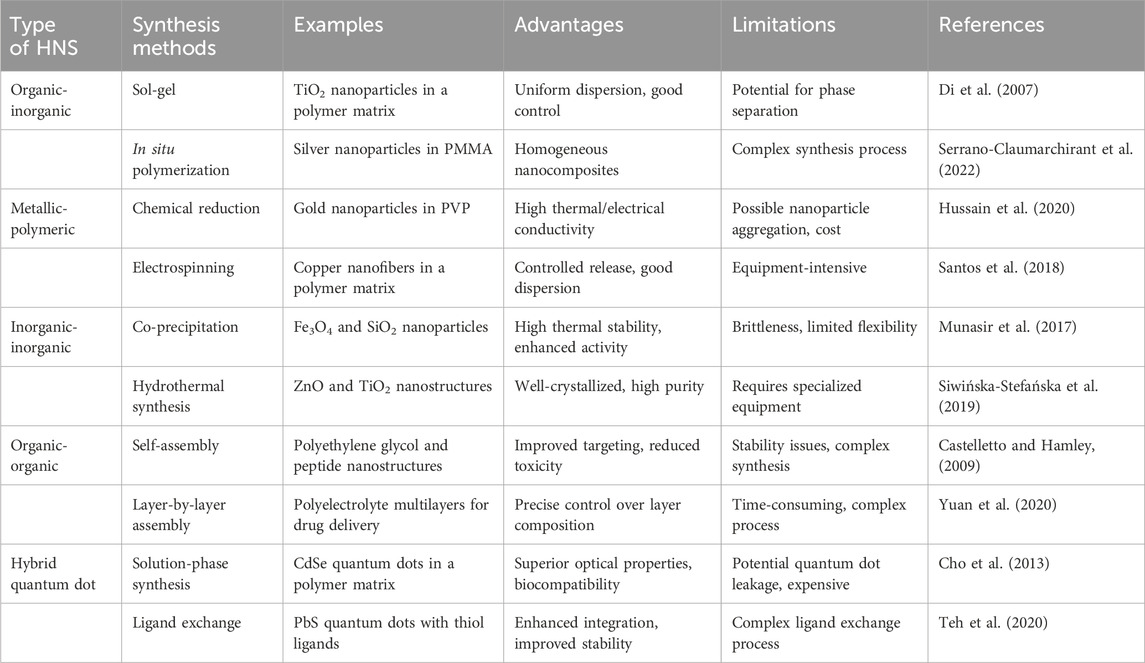
The synthesis of HNSs involves various methods tailored to combine distinct materials at the nanoscale effectively. For organic-inorganic hybrids, the sol-gel method transitions a system from a liquid “sol” to a solid “gel” phase, ensuring uniform nanoparticle dispersion in a polymer matrix, while in situ polymerization disperses inorganic nanoparticles in a monomer solution followed by polymerization, creating homogeneous nanocomposites. Metallic-polymeric hybrids are often created using chemical reduction, where metallic nanoparticles form within a polymer matrix via reduction of metal ions, and electrospinning, which produces nanofibers embedded with metallic nanoparticles from a solution containing both polymer and metal precursors. Inorganic-inorganic hybrids can be synthesized through co-precipitation, simultaneously precipitating two or more inorganic materials from a solution, or hydrothermal synthesis, which employs high-pressure and high-temperature conditions to create well-crystallized nanocomposites. Organic-organic hybrids are formed using self-assembly, where organic molecules and polymers organize into structures through non-covalent interactions, or layer-by-layer assembly, which sequentially adsorbs oppositely charged polymers to fabricate multilayered structures. Hybrid quantum dot nanostructures can be synthesized via solution-phase synthesis, forming nanoparticles in a solution followed by their incorporation into polymers, or ligand exchange, replacing quantum dot surface ligands with molecules that facilitate polymer integration. Table 1 providing a classification of different methods to synthesize HNSs for modern therapies, including specific examples.
2.3 Classification and applications of nanostructures in modern therapies
Nanostructures can be classified based on their dimensionality into zero-dimensional (0D), one-dimensional (1D), two-dimensional (2D), and three-dimensional (3D) (Tiwari et al., 2012). Each category possesses unique characteristics that make them suitable for specific therapeutic applications, including PTT, PDT, and gene therapy.
0D nanostructures, such as nanoparticles and quantum dots, have all three spatial dimensions confined to the nanoscale. These structures are extensively utilized in various therapies due to their high surface area and unique optical properties. Gold nanoparticles (AuNPs) are widely used for PTT. Their strong absorption in the near-infrared (NIR) region allows them to convert light into heat efficiently, leading to the localized ablation of cancer cells (Dheyab et al., 2023). Quantum dots (QDs) can act as photosensitizers, generating reactive oxygen species (ROS) upon light activation. This makes them effective in targeting and destroying cancer cells (Uprety and Abrahamse, 2022). Polymeric nanoparticles are designed for gene delivery, protecting genetic material from degradation and facilitating efficient cellular uptake (Khan et al., 2024).
1D nanostructures, including nanorods, nanotubes, and nanowires, have one dimension significantly larger than the other two. Their high aspect ratio provides unique mechanical and electrical properties beneficial for therapeutic applications. Gold nanorods (AuNRs) are preferred for their efficient light-to-heat conversion capabilities, making them ideal for targeted thermal ablation of tumors (Deinavizadeh et al., 2024). Carbon nanotubes (CNTs) can be functionalized with photosensitizers to enhance ROS production upon light activation, improving PDT efficacy (Naief et al., 2023). Nanowires are used as delivery vehicles for nucleic acids, enhancing transfection efficiency and promoting targeted gene editing (Inal et al., 2022).
2D nanostructures, such as nanosheets and nanoplates, have a thickness much smaller than their length and width, providing a large surface area for functionalization. Graphene oxide (GO) nanosheets exhibit excellent photothermal conversion efficiency and are used for thermal ablation of tumors (Lim et al., 2018). Molybdenum disulfide (MoS2) nanosheets can be loaded with photosensitizers to enhance ROS production and improve treatment efficacy (Zhao et al., 2024). Layered double hydroxides (LDHs) are utilized for gene delivery due to their biocompatibility and ability to protect nucleic acids from degradation (Yu et al., 2020).
3D nanostructures encompass bulk materials with nanoscale features in three dimensions, including nanocages, nanoflowers, and nanospheres. These structures offer structural stability and multifunctionality. Gold nanocages are used for their hollow structure, which allows for efficient light absorption and heat generation, making them suitable for cancer treatment (Hu et al., 2021). Porous silicon nanospheres can be loaded with photosensitizers and drugs, providing a combination of PDT and chemotherapy (Kang et al., 2022). 3D DNA nanostructures, such as DNA origami, are used for precise gene delivery and editing, offering high loading capacity and targeted delivery (Tang et al., 2023).
2.4 Recent developments in HNSs
Recent advancements highlight the emergence of nanobubbles as eco-friendly HNSs with promising biomedical applications. Jannesari et al. have developed reduced graphene oxide/copper peroxide (rGO/CuO2) nanocomposites that deliver in situ oxygen nanobubbles (O2 NBs) to combat bacterial infections (Jannesari et al., 2020). The presence of rGO allows for the decomposition of CuO2 into O2 NBs in a controllable and prolonged manner, effectively battling methicillin-resistant Staphylococcus aureus (MRSA) and other bacteria by enhancing reactive oxygen species (ROS) generation and inducing mechanical disruptions via near-infrared laser irradiation. Another study by Jannesari et al. introduced oxygen-rich rGO/ZnO2-Ag nanoframeworks as suppliers of O2 NBs with dual pH-and-temperature-sensitive behavior (Jannesari et al., 2023b). These nanoframeworks demonstrated significant antibacterial properties against MRSA by facilitating the generation and controlled release of O2 NBs, thereby enhancing ROS production and utilizing the inherent photothermal properties of rGO to combat bacterial infections more efficiently. These recent developments underscore the potential of nanobubbles in biomedical applications, particularly in hybrid forms, offering a new avenue for eco-friendly and efficient therapeutic strategies.
Modern developments in HNSs reveal the promise of triboelectric nanogenerator (TENG) devices as sustainable HNSs for biomedical applications. These devices are particularly effective in converting waste energy for treatments and minimizing long-term side effects. Jannesari et al. developed a polypyrrole-graphene oxide (PPy-GO) composite TENG with superior electrical outputs and bactericidal activity (Jannesari et al., 2023a). The PPy-GO TENG, fabricated through a facile electrodeposition route, exhibited high electrical outputs with an open-circuit voltage of 413.2 V and a short-circuit current of ∼41 μA. This device demonstrated marked bactericidal activity against S. aureus, attributed to enhanced ROS generation and cellular membrane rupture. Another study by Jannesari et al. (2024) investigated the antibacterial properties of TENGs by examining the effects of charge polarity and host material composition. They found that positively charged substrates inhibited bacterial growth through electrostatic interactions, while negatively charged substrates, particularly graphene oxide (GO), exhibited strong antibacterial properties by generating ROS. The study demonstrated that TENGs could harness waste energy to provide self-powered antibacterial treatments, highlighting their potential for advanced wearable healthcare and biomedical applications. This eco-friendly technique offers a promising approach to reducing long-term side effects associated with traditional nanostructures.
2.5 Characterization of HNSs
Characterization of HNSs is vital for understanding their properties and optimizing their performance in therapeutic applications. For organic-inorganic hybrids, Transmission Electron Microscopy (TEM) provides detailed images of internal structures, while Fourier Transform Infrared Spectroscopy (FTIR) identifies chemical bonds and confirms material integration. Metallic-polymeric hybrids are examined using Scanning Electron Microscopy (SEM) for surface morphology and X-ray Diffraction (XRD) for crystalline structure analysis. Inorganic-inorganic hybrids benefit from Energy Dispersive X-ray Spectroscopy (EDS) for elemental composition and Thermogravimetric Analysis (TGA) for thermal stability. Organic-organic hybrids are characterized using Nuclear Magnetic Resonance (NMR) Spectroscopy for molecular structure and Gel Permeation Chromatography (GPC) for molecular weight distribution. Hybrid quantum dot nanostructures are analyzed with Photoluminescence (PL) Spectroscopy for optical properties and Dynamic Light Scattering (DLS) for size distribution and stability.
3 Mechanisms of action in therapy
3.1 Drug delivery systems
HNSs have revolutionized drug delivery systems by providing innovative mechanisms for drug loading and release, as well as precise targeting strategies. The mechanisms of drug loading typically involve encapsulating therapeutic agents within the nanostructure or attaching them to the surface via covalent or non-covalent bonds. This encapsulation ensures the stability and bioavailability of the drugs (Huang et al., 2019; Harun-Ur-Rashid et al., 2023a; Harun-Ur-Rashid et al., 2023b). For drug release, stimuli-responsive strategies are often employed, where the nanostructure responds to specific triggers such as pH, temperature, or light to release the drug in a controlled manner.
Targeting strategies for HNSs are designed to increase the efficacy of drug delivery by directing the therapeutic agents precisely to the diseased cells while minimizing impact on healthy tissues. Active targeting involves modifying the nanostructure surface with ligands, antibodies, or peptides that recognize and bind to specific receptors overexpressed on target cells. This approach enhances cellular uptake and improves therapeutic outcomes. Passive targeting exploits the enhanced permeability and retention (EPR) effect, which allows nanostructures to accumulate in tumor tissues due to their leaky vasculature (Subhan et al., 2021). Both strategies have shown significant efficacy in preclinical and clinical studies, offering promising avenues for cancer therapy and beyond.
3.2 Photothermal and photodynamic therapy
Photothermal therapy (PTT) and photodynamic therapy (PDT) are cutting-edge approaches that utilize light to activate HNSs for therapeutic purposes (Asadian et al., 2023). PTT is a minimally invasive therapeutic approach that utilizes light energy to generate heat for the ablation of cancer cells. The principle behind PTT involves the use of photothermal agents, such as gold nanoparticles, carbon nanotubes, or HNSs, which can absorb near-infrared (NIR) light and convert it into heat. This localized heating effect leads to the destruction of cancer cells through hyperthermia, which disrupts cellular structures and induces apoptosis. Zhang et al. enhanced the bactericidal performance of polymeric nanopillars by integrating PTT (Zhang, et al., 2023). Using a cost-effective anodized aluminum oxide (AAO) template and Layer-by-Layer (LbL) assembly with tannic acid (TA) and iron ion (Fe3+), they fabricated hybrid nanopillars. These HNSs achieved over 99% bactericidal efficacy against Gram-negative Pseudomonas aeruginosa and Gram-positive S. aureus.
PDT involves the use of photosensitizing agents that, upon activation by specific wavelengths of light, produce reactive oxygen species (ROS) that induce cellular damage and apoptosis. Imanparast et al. designed and fabricated a microfluidic chip to synthesize PpIX-loaded micelles, transforming PpIX into photo-protoporphyrin (PPP) and integrating it with bovine serum albumin (BSA) and hollow gold nanoshells (HGN) (Imanparast et al., 2023). The resulting HGN-BSA-CTAB-PPP HNSs, sized at 120 nm with a strong absorption peak at 670 nm, showed significant cytotoxicity against MDA-MB-231 and 4T1 cancer cells at low radiation doses (<10 J/cm2). This study highlights the potential of albumin-based HNSs for enhancing PDT efficacy.
The integration of nanotechnology has significantly advanced the efficiency and specificity of both PTT and PDT. Nanomaterials such as HNSs combine the benefits of multiple materials, enhancing their functionality and therapeutic outcomes. For instance, Özkan et al. developed HNSs composed of a red-emitting conjugated oligomer (COL) and gold nanoparticles (Au-NPs) using a one-pot synthetic method (Özkan et al., 2020). The conjugated oligomer served as both a reducing agent and a matrix for the Au-NPs. These HNSs (COL-Au-NPs) demonstrated both photodynamic and photothermal activity against Gram-positive and Gram-negative bacterial strains. They exhibited high photostability and thermal reversibility. The dark cytotoxicity of COL-Au-NPs towards pathogens and mammalian breast cancer cells (MCF-7) was significantly reduced upon complexation with cucurbit [7]uril, while their light-induced cytotoxicity was preserved when irradiated with a 915 nm laser for PTT and white light for PDT.
3.3 Gene delivery and editing
HNSs offer groundbreaking techniques for gene delivery and editing, addressing challenges associated with conventional methods. These nanostructures protect genetic material from degradation and facilitate efficient cellular uptake (Tang et al., 2023b). Polymeric nanoparticles and lipid-based hybrids are widely used to deliver nucleic acids such as DNA, RNA, and CRISPR-Cas9 components. Success stories in gene delivery include the use of lipid nanoparticles for mRNA vaccines, which have proven highly effective in recent applications like COVID-19 vaccines (Lee et al., 2023). However, challenges remain, including potential immunogenicity and the need for precise control over gene expression. HNSs are being continuously refined to overcome these hurdles, with ongoing research focusing on enhancing delivery efficiency and minimizing side effects.
4 HNSs in modern therapy
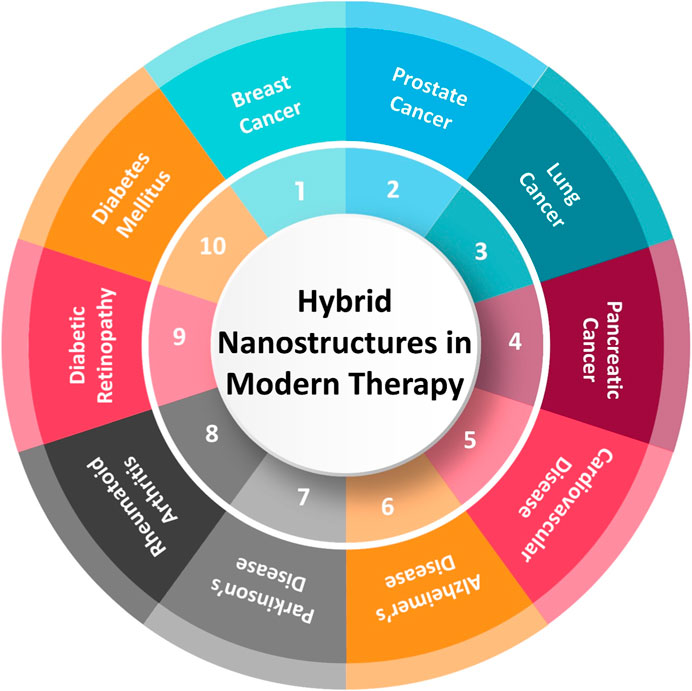
HNSs represent a cutting-edge advancement in the field of modern therapy, offering multifaceted approaches to diagnose, treat, and manage various diseases. By combining different materials at the nanoscale, these nanostructures exhibit unique properties that enhance their therapeutic potential. They can target specific cells or tissues, improve drug delivery efficiency, overcome biological barriers, and reduce side effects, making them highly effective in treating complex conditions. HNSs have shown significant promise in treating various diseases, including cancer and cardiovascular disorders. The foundational work by Medintz et al. (2008) on the intracellular delivery of quantum dot-protein cargos mediated by cell-penetrating peptides is a key example of how HNSs can be effectively utilized for targeted therapy. Additionally, the work by Silva et al. (2012) on the cellular transfer of magnetic nanoparticles provides crucial insights into the mechanisms of action and the impact of these nanoparticles on cell tracking by magnetic resonance imaging. Figure 1 visually summarizes the therapeutic applications of hybrid nanostructures across various medical fields, including cancer therapy, cardiovascular diseases, neurodegenerative diseases, autoimmune diseases, diabetic retinopathy, and gestational diabetes mellitus.
4.1 Targeted drug delivery and enhanced efficacy
HNSs have revolutionized the field of targeted drug delivery by combining multiple materials to optimize therapeutic outcomes. These structures can be engineered to possess specific surface modifications, such as ligands, antibodies, or peptides, which allow for precise targeting of diseased cells while sparing healthy tissues. This specificity enhances the efficacy of the drug delivery system, as demonstrated in various clinical applications including cancer therapy. For instance, plasmonic platinum nanoclusters (PtNC) with a protein scaffold have shown superior efficacy in treating breast cancer by enhancing tumor delivery and reducing side effects. Similarly, luminescent-superparamagnetic hybrid nanoparticles have proven effective in prostate cancer treatment by combining PDT and PTT, resulting in significant cell death with minimal toxicity. These examples highlight the transformative potential of HNSs in improving drug bioavailability, reducing systemic toxicity, and ensuring targeted therapeutic action.
4.2 Multifunctional therapeutic applications
The multifunctionality of HNSs extends their application beyond drug delivery to include diagnostic and therapeutic roles in various diseases. This versatility is particularly evident in the treatment of neurodegenerative diseases and cardiovascular conditions. For example, lipid-polymer hybrid nanoparticles have been developed for the intranasal delivery of rivastigmine, significantly improving amyloid inhibition and brain bioavailability for Alzheimer’s treatment. In cardiovascular therapy, biomimetic nanomaterials, such as noble metal-based nanozymes, offer enzymatic catalytic activity and targeted drug delivery, enhancing the diagnosis and treatment of conditions like atherosclerosis and myocardial infarction. Additionally, boron nitride nanotubes (BNNT) have shown promise in destabilizing α-synuclein proteins in Parkinson’s disease, highlighting their potential in neurodegenerative disease management. These multifunctional applications underscore the importance of HNSs in providing comprehensive therapeutic solutions that integrate treatment, diagnosis, and monitoring, thereby paving the way for innovative approaches in modern therapy.
Table 2 provides a comprehensive comparison of the clinical applications and case studies of various HNSs, highlighting their mechanisms of action, applications, efficacy, safety, advantages, and limitations. These innovative solutions showcase the transformative impact of nanotechnology in healthcare and underscore the importance of continued research and development in this promising field.
5 Future perspectives and directions
5.1 Emerging trends
The field of HNSs is poised for significant advancements, with several emerging trends shaping its future. One promising trend is the development of multifunctional nanostructures capable of simultaneous diagnosis and therapy, known as theranostics. These nanostructures can target specific disease sites, deliver therapeutic agents, and monitor treatment responses in real-time, providing a comprehensive approach to disease management. For example, polydopamine-based multifunctional HNSs are particularly suitable for treating cancer and neurodegenerative diseases, offering benefits such as improved targeting, biocompatibility, and minimized side effects (Battaglini et al., 2024). Polydopamine-based HNSs have superior biocompatibility and targeting efficiency compared to other multifunctional nanostructures.
Thermal ablation treats hepatocellular carcinoma but often leaves tumor residues that cause recurrence. The biodegradable polymeric nanoparticle@(131I-Hyp) targets and eliminates these residues, offering fluorescence imaging, SPECT imaging, and necrosis-targeted radiotherapy (Bao et al., 2024). This nanoparticle is composed of iodine-131-labeled hypericin (131I-Hyp) as the core and an amphiphilic copolymer shell of poly (ethylene glycol)-block-poly (ε-caprolactone) (PEG–PCL). This material is able to show its multifunctional capabilities, including imaging and targeted radiotherapy, which are not commonly combined in other nanostructures.
Another innovation is the use of biodegradable and biocompatible materials to minimize long-term toxicity and environmental impact. Researchers are also exploring the potential of HNSs in immunotherapy, where they can modulate immune responses to enhance the efficacy of treatments for cancer and autoimmune diseases (Angelopoulou, 2024; Moon et al., 2024; Patra et al., 2024). Additionally, the integration of nanostructures with advanced imaging techniques is enabling more precise and early detection of diseases. For instance, 50-nm gas-filled protein nanostructures enable access to lymphatic cells by ultrasound technologies, providing a novel method for early disease detection and monitoring (Shen et al., 2024). These nanostructures were selected for their innovative approach to enabling access to lymphatic cells by ultrasound technologies.
5.2 Technological advancements
Significant strides in synthesis and characterization technologies are driving the evolution of HNSs. Advanced synthesis methods, such as microfluidic systems and self-assembly techniques, allow for the precise control over size, shape, and composition of nanostructures, enhancing their functionality and effectiveness (Petrova et al., 2024; Du et al., 2024). Characterization technologies, including high-resolution electron microscopy and spectroscopy, provide detailed insights into the structural and functional properties of nanostructures, facilitating their optimization for specific applications (Jagadeesh et al., 2024; Gupta et al., 2024). Furthermore, the integration of HNSs with emerging technologies such as artificial intelligence (AI) and personalized medicine is opening new frontiers in healthcare. AI can analyze large datasets to predict the behavior of nanostructures and personalize treatments based on individual patient profiles, improving outcomes and reducing adverse effects (Chugh et al., 2024).
5.3 Strategic recommendations
For researchers and clinicians, several strategic recommendations can guide the future development and application of HNSs. Researchers should focus on interdisciplinary collaboration, combining expertise from materials science, biology, medicine, and engineering to develop innovative solutions. Clinicians should be involved in the early stages of research to ensure that the developed nanostructures meet clinical needs and can be seamlessly integrated into existing treatment protocols. Additionally, it is crucial to invest in robust preclinical and clinical studies to validate the safety and efficacy of HNSs before their widespread adoption. Policymakers and funding agencies should support collaborative research efforts and create frameworks that facilitate the translation of laboratory findings into clinical practice.
6 Conclusion
HNSs have significant potential in modern therapy, demonstrated by their multifunctionality for diagnosis and therapy, reduced toxicity through biodegradable materials, and enhanced treatment efficacy in immunotherapy. They show promise in treating residual tumors post-thermal ablation and advancing therapies for cancer, neurodegenerative diseases, and cardiovascular conditions. Future research should refine HNSs for better biocompatibility and targeted delivery, explore their use in personalized medicine and AI-driven applications, and validate their safety and efficacy through comprehensive studies. Integrating advanced imaging techniques with HNSs could also improve early disease detection and monitoring. The continuous evolution of this technology, supported by interdisciplinary collaboration and strategic research, can revolutionize healthcare and provide innovative solutions for complex medical challenges.
Author contributions
MH-U-R: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. IJ: Conceptualization, Data curation, Formal Analysis, Methodology, Validation, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The authors conducted the study using resources and facilities provided by the International University of Business Agriculture and Technology (IUBAT), Dhaka, Bangladesh, and the Centre for Advanced Research in Sciences, Genetic Engineering and Biotechnology Research Laboratory (GEBRL), University of Dhaka, Dhaka, Bangladesh. The funding bodies had no role in the design of the study, data collection, analysis, interpretation of data, or writing of the manuscript.
Acknowledgments
The authors would like to thank the International University of Business Agriculture and Technology (IUBAT) and the University of Dhaka for providing the necessary resources and support for this research. Special thanks to the colleagues and staff of the Department of Chemistry, IUBAT for their invaluable assistance and contributions throughout this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Angelopoulou, A. (2024). Nanostructured biomaterials in 3D tumor tissue engineering scaffolds: regenerative medicine and immunotherapies. Int. J. Mol. Sci. 25 (10), 5414. doi:10.3390/ijms25105414
Asadian, E., Jannesari, M., and Mohammad-Ali, S. (2023). “Chapter 4 - application of infrared waves in cancer therapy,” in Electromagnetic waves-based cancer diagnosis and therapy. Editors M. Khafaji,, and O. Bavi (Cambridge, Massachusetts: Academic Press), 151–237.
Aydindogan, E., Koc, I., Onbasli, K., and Acar, H. Y. (2023). Imaging guided PTT-PDT combination therapy of prostate cancer utilizing Ag2S-Fe3O4 hybrid nanoparticles and 5-ALA. Photodiagnosis Photodyn. Ther. 41, 103381. doi:10.1016/j.pdpdt.2023.103381
Bao, H., Wang, N., Chen, S., Wang, Y., Shao, H., Ni, Y., et al. (2024). Multimodal theranostic nanoparticles for necrosis targeting, fluorescence/SPECT imaging, and radiotherapy of residual tumors after hepatocellular carcinoma ablation. Mol. Pharm. 21 (4), 1729–1744. doi:10.1021/acs.molpharmaceut.3c01081
Battaglini, M., Emanet, M., Carmignani, A., and Ciofani, G. (2024). Polydopamine-based nanostructures: a new generation of versatile, multi-tasking, and smart theranostic tools. Nano Today 55, 102151. doi:10.1016/j.nantod.2024.102151
Castelletto, V., and Hamley, I. W. (2009). Self assembly of a model amphiphilic phenylalanine peptide/polyethylene glycol block copolymer in aqueous solution. Biophys. Chem. 141 (2), 169–174. doi:10.1016/j.bpc.2009.01.008
Chand, K., Cao, D., Fouad, D. E., Shah, A. H., Lakhan, M. N., Abdul, Q. D., et al. (2020). Photocatalytic and antimicrobial activity of biosynthesized silver and titanium dioxide nanoparticles: a comparative study. J. Mol. Liq. 316, 113821. doi:10.1016/j.molliq.2020.113821
Cheng, X., Xie, Q., and Sun, Y. (2023). Advances in nanomaterial-based targeted drug delivery systems. Front. Bioeng. Biotechnol. 11, 1177151. doi:10.3389/fbioe.2023.1177151
Cho, S., Kwag, J., Jeong, S., Baek, Y., and Kim, S. (2013). Highly fluorescent and stable quantum dot-polymer-layered double hydroxide composites. Chem. Mater. 25 (7), 1071–1077. doi:10.1021/cm3040505
Chugh, V., Basu, A., Kaushik, A., Manshu, S. B., and Basu, A. K. (2024). Employing nano-enabled artificial intelligence (AI)-based smart technologies for prediction, screening, and detection of cancer. Nanoscale 16 (11), 5458–5486. doi:10.1039/D3NR05648A
Deinavizadeh, M., Kiasat, A. R., Shafiei, M., Sabaeian, M., Mirzajani, R., Zahraei, S. M., et al. (2024). Synergistic chemo-photothermal therapy using gold nanorods supported on thiol-functionalized mesoporous silica for lung cancer treatment. Sci. Rep. 14 (1), 4373. doi:10.1038/s41598-024-54778-3
Desouky, F. G.El, Saadeldin, M. M., and Eisa, W. H. (2023). Synergistic influence of SnFe2O4 on ZnSnO3 hybrid nanostructures and optimizations optical, photoluminescence, and magnetic properties for multifunction application. Surfaces Interfaces 38, 102749. doi:10.1016/j.surfin.2023.102749
Dheyab, M. A., Aziz, A. A., Khaniabadi, P. M., Jameel, M. S., Oladzadabbasabadi, N., Rahman, A. A., et al. (2023). Gold nanoparticles-based photothermal therapy for breast cancer. Photodiagnosis Photodyn. Ther. 42, 103312. doi:10.1016/j.pdpdt.2023.103312
Di, G. A., Trabelsi, S., Rizza, G., Sangermano, M., Althues, H., Kaskel, S., et al. (2007). Hyperbranched polymer/TiO2 hybrid nanoparticles synthesized via an in situ sol-gel process. Macromol. Chem. Phys. 208 (1), 76–86. doi:10.1002/macp.200600431
Ding, X., Chen, C., Liu, Q., Yang, Y., Wang, G., and Qian, H. (2023). Reverse engineering of DNA and RNA hybrid origami structures as targeted nanomedicine for KRAS-mutated lung cancer therapy. ACS Appl. Polym. Mater. 5 (8), 5880–5887. doi:10.1021/acsapm.3c00535
Du, J., Shi, L.-Li, Jiang, W.-W., Liu, X.-Ai, Wu, X.-H., Huang, X.-X., et al. (2024). Crafting docetaxel-loaded albumin nanoparticles through a novel thermal-driven self-assembly/microfluidic combination technology: formulation, process optimization, stability, and bioavailability. Int. J. Nanomedicine 19 (null), 5071–5094. doi:10.2147/IJN.S457482
Gong, Y., Liu, H., Ke, S., Zhuo, Li, and Wang, H. (2023). Latest advances in biomimetic nanomaterials for diagnosis and treatment of cardiovascular disease. Front. Cardiovasc. Med. 9, 1037741. doi:10.3389/fcvm.2022.1037741
Gupta, P., Rai, N., Verma, A., and Gautam, V. (2024). Microscopy based methods for characterization, drug delivery, and understanding the dynamics of nanoparticles. Med. Res. Rev. 44 (1), 138–168. doi:10.1002/med.21981
Harun-Ur-Rashid, M., Jahan, I., Foyez, T., and Imran, A. B. (2023a). Bio-inspired nanomaterials for micro/nanodevices: a new era in biomedical applications. Micromachines 14 (9), 1786. doi:10.3390/mi14091786
Harun-Ur-Rashid, M., Jahan, I., and Imran, A. B. (2023b). “Recent advancement of nanostructured materials for clinical challenges in vaccinology,” in Nanovaccinology: clinical application of nanostructured materials research to translational medicine. Editor K. Pal (Cham: Springer International Publishing), 135–160.
Harun-Ur-Rashid, M., Pal, K., and Abu, B. I. (2024). Hybrid nanocomposite fabrication of nanocatalyst with enhanced and stable photocatalytic activity. Top. Catal. 67, 17–45. doi:10.1007/s11244-023-01809-4
Hu, Y., Huang, S., Zhao, X., Chang, L., Ren, X., Mei, X., et al. (2021). Preparation of photothermal responsive and ROS generative gold nanocages for cancer therapy. Chem. Eng. J. 421, 129744. doi:10.1016/j.cej.2021.129744
Huang, W.-T., Chang, M.-C., Chu, C.-Yi, Chang, C.-C., Li, M.-C., and Liu, D.-Mo (2019). Self-assembled amphiphilic chitosan: a time-dependent nanostructural evolution and associated drug encapsulation/elution mechanism. Carbohydr. Polym. 215, 246–252. doi:10.1016/j.carbpol.2019.03.083
Hussain, M. H., Bakar, N. F. A., Najwa Mustapa, A., Low, K.-F., Othman, N. H., and Adam, F. (2020). Synthesis of various size gold nanoparticles by chemical reduction method with different solvent polarity. Nanoscale Res. Lett. 15 (1), 140. doi:10.1186/s11671-020-03370-5
Imanparast, A., Shaegh, S. A. M., Attaran, N., Ameri, A. R., and Sazgarnia, A. (2023). Opto-microfluidic assisted synthesis of photo-protoporphyrin (pPP) conjugated to hollow gold-albumin hybrid nanoshells to enhance the efficiency of photodynamic therapy of triple negative breast cancer cells. Photodiagnosis Photodyn. Ther. 43, 103632. doi:10.1016/j.pdpdt.2023.103632
Inal, O., Badilli, U., Sibel Ozkan, A., and Mollarasouli, F. (2022). “Chapter 11 - bioactive hybrid nanowires for drug delivery,” in Hybrid nanomaterials for drug delivery. Editors P. Kesharwani,, and N. K. Jain (Sawston, Cambridge: Woodhead Publishing), 269–301.
Jagadeesh, P., Rangappa, S. M., and Siengchin, S. (2024). Advanced characterization techniques for nanostructured materials in biomedical applications. Adv. Industrial Eng. Polym. Res. 7 (1), 122–143. doi:10.1016/j.aiepr.2023.03.002
Jannesari, M., Akhavan, O., Madaah Hosseini, H. R., and Bakhshi, B. (2020). Graphene/CuO2 nanoshuttles with controllable release of oxygen nanobubbles promoting interruption of bacterial respiration. ACS Appl. Mater. and Interfaces 12 (32), 35813–35825. doi:10.1021/acsami.0c05732
Jannesari, M., Akhavan, O., Madaah Hosseini, H. R., and Bakhshi, B. (2023b). Oxygen-Rich Graphene/ZnO2-Ag nanoframeworks with pH-Switchable Catalase/Peroxidase activity as O2 Nanobubble-Self generator for bacterial inactivation. J. Colloid Interface Sci. 637, 237–250. doi:10.1016/j.jcis.2023.01.079
Jannesari, M., Asadian, E., Ejehi, F., English, N. J., Mohammadpour, R., and Sasanpour, P. (2023a). Boosting on-demand antibacterial activity using electrical stimulations from polypyrrole-graphene oxide triboelectric nanogenerator. Nano Energy 112, 108463. doi:10.1016/j.nanoen.2023.108463
Jannesari, M., Ejehi, F., English, N. J., Mohammadpour, R., Akhavan, O., and Shams, S. (2024). Triggering triboelectric nanogenerator antibacterial Activities: effect of charge polarity and host material correlation. Chem. Eng. J. 486, 150036. doi:10.1016/j.cej.2024.150036
Jeong, S., Kwon, Y. W., Lee, Y., Song, T.-K., and Lee, J. (2024). A Platinum-Protein hybrid nanostructure enables both targeted cancer theragnosis and platinum clearance. Chem. Eng. J. 494, 152957. doi:10.1016/j.cej.2024.152957
Kang, R. H., Kim, Y., Ji Um, H., Kim, J., Bang, E.-K., Yeo, S. G., et al. (2022). Glioblastoma homing photodynamic therapy based on multifunctionalized porous silicon nanoparticles. ACS Appl. Nano Mater. 5 (4), 5387–5397. doi:10.1021/acsanm.2c00368
Khan, I. N., Navaid, S., Waqar, W., Hussein, D., Ullah, N., Khan, M. U., et al. (2024). Chitosan-based polymeric nanoparticles as an efficient gene delivery system to cross blood brain barrier: in vitro and in vivo evaluations. Pharmaceuticals 17 (2), 169. doi:10.3390/ph17020169
Le, H. N., Wang, R., Hou, B., Kim, S., and Kim, J. (2024). Preparation of low-temperature solution-processed high-κ gate dielectrics using organic–inorganic TiO2 hybrid nanoparticles. Nanomaterials 14 (6), 488. doi:10.3390/nano14060488
Lee, Y., Jeong, M., Park, J., Jung, H., and Lee, H. (2023). Immunogenicity of lipid nanoparticles and its impact on the efficacy of mRNA vaccines and therapeutics. Exp. and Mol. Med. 55 (10), 2085–2096. doi:10.1038/s12276-023-01086-x
Lim, J. H., Kim, Da E., Kim, E.-J., Ahrberg, C. D., and Chung, B. G. (2018). Functional graphene oxide-based nanosheets for photothermal therapy. Macromol. Res. 26 (6), 557–565. doi:10.1007/s13233-018-6067-3
Mansur, A. A. P., Caires, A. J., Carvalho, S. M., Capanema, N. S. V., Carvalho, I. C., and Mansur, H. S. (2019). Dual-functional supramolecular nanohybrids of quantum dot/biopolymer/chemotherapeutic drug for bioimaging and killing brain cancer cells in vitro. Colloids Surfaces B Biointerfaces 184, 110507. doi:10.1016/j.colsurfb.2019.110507
Medintz, I. L., Thomas, P., Delehanty, J. B., Susumu, K., Brunel, F. M., Dawson, P. E., et al. (2008). Intracellular delivery of quantum Dot−Protein cargos mediated by cell penetrating peptides. Bioconjugate Chem. 19 (9), 1785–1795. doi:10.1021/bc800089r
Moon, Y., Cho, H., and Kim, K. (2024). Nano-delivery of immunogenic cell death inducers and immune checkpoint blockade agents: single-nanostructure strategies for enhancing immunotherapy. Pharmaceutics 16 (6), 795. doi:10.3390/pharmaceutics16060795
Munasir, , Dewanto, A. S., Yulianingsih, A., Saadah, I. K. F., Supardi, Z. A. I., Mufid, A., et al. (2017). Composites of Fe3O4/SiO2 from natural material synthesized by Co-precipitation method. IOP Conf. Ser. Mater. Sci. Eng. 202 (1), 012057. doi:10.1088/1757-899X/202/1/012057
Naief, M. F., Mohammed, S. N., Hadil, J. M., and Ahmed, M. M. (2023). A review of the role of carbon nanotubes for cancer treatment based on photothermal and photodynamic therapy techniques. J. Organomet. Chem. 999, 122819. doi:10.1016/j.jorganchem.2023.122819
Nikita, S. H., Kathuria, H., and Pandey, M. M. (2023). Rivastigmine-DHA ion-pair complex improved loading in hybrid nanoparticles for better amyloid inhibition and nose-to-brain targeting in Alzheimer’s. Eur. J. Pharm. Biopharm. 190, 131–149. doi:10.1016/j.ejpb.2023.06.007
Ou, Z., Li, X., You, Y., Liu, D., and Wang, J. (2023). Interpreting the therapeutic efficiency of multifunctional hybrid nanostructure against glioblastoma. ACS Omega 8 (13), 12259–12267. doi:10.1021/acsomega.2c08265
Özkan, M., Hadi, S. E., Tunç, İ., Midilli, Y., Ortaç, B., and Tuncel, D. (2020). Cucurbit[7]uril-Capped hybrid conjugated oligomer-gold nanoparticles for combined photodynamic-photothermal therapy and cellular imaging. ACS Appl. Polym. Mater. 2 (9), 3840–3849. doi:10.1021/acsapm.0c00540
Pandey, S., Kim, S., Kim, Y. S., Kumar, D., and Kang, M. (2024). Fabrication of next-generation multifunctional LBG-s-AgNPs@ g-C3N4 NS hybrid nanostructures for environmental applications. Environ. Res. 240, 117540. doi:10.1016/j.envres.2023.117540
Patra, P., Upadhyay, T. K., Alshammari, N., Saeed, M., and Kesari, K. K. (2024). Alginate-Chitosan biodegradable and biocompatible based hydrogel for breast cancer immunotherapy and diagnosis: a comprehensive review. ACS Appl. Bio Mater. 7 (6), 3515–3534. doi:10.1021/acsabm.3c00984
Petrova, S. L., Sincari, V., Pavlova, E., Pokorný, V., Lobaz, V., and Hrubý, M. (2024). Microfluidic controlled self-assembly of polylactide (PLA)-Based linear and graft copolymers into nanoparticles with diverse morphologies. ACS Polym. Au 4, 331–341. doi:10.1021/acspolymersau.4c00033
Povolotskaya, A. V., Povolotskiy, A. V., and Manshina, A. A. (2015). Hybrid nanostructures: synthesis, morphology and functional properties. Russ. Chem. Rev. 84 (6), 579–600. doi:10.1070/RCR4487
Ribeiro, L. N. M., Alcantara, A. C. S., Franz-Montan, M., Couto, V. M., Nista, S. V. G., and de Paula, E. (2019). “Chapter 13 - nanostructured organic-organic bio-hybrid delivery systems,” in Biomedical applications of nanoparticles. Editor A. M. Grumezescu (Norwich, New York: William Andrew Publishing), 341–374.
Santos, J. P. F., Silva, A. B. da, Arjmand, M., Sundararaj, U., and Bretas, R. E. S. (2018). Nanofibers of poly(vinylidene fluoride)/copper nanowire: microstructural analysis and dielectric behavior. Eur. Polym. J. 101, 46–55. doi:10.1016/j.eurpolymj.2018.02.017
Serrano-Claumarchirant, J. F., Seijas-Da Silva, A., Sánchez-Royo, J. F., Culebras, M., Cantarero, A., Gómez, C. M., et al. (2022). In situ synthesis of polythiophene and silver nanoparticles within a pmma matrix: a nanocomposite approach to thermoelectrics. ACS Appl. Energy Mater. 5 (9), 11067–11076. doi:10.1021/acsaem.2c01701
Sharma, D. S., Wadhwa, S., Gulati, M., Kumar, B., Chitranshi, N., Gupta, V. K., et al. (2023). Chitosan modified 5-fluorouracil nanostructured lipid carriers for treatment of diabetic retinopathy in rats: a new dimension to an anticancer drug. Int. J. Biol. Macromol. 224, 810–830. doi:10.1016/j.ijbiomac.2022.10.168
Shen, Q., Li, Z., Wang, Y., Meyer, M. D., De Guzman, M. T., Lim, J. C., et al. (2024). 50-nm gas-filled protein nanostructures to enable the access of lymphatic cells by ultrasound technologies. Adv. Mater. n/a 36 (n/a), 2307123. doi:10.1002/adma.202307123
Silva, A. K. A., Wilhelm, C., Kolosnjaj-Tabi, J., Luciani, N., and Gazeau, F. (2012). Cellular transfer of magnetic nanoparticles via cell microvesicles: impact on cell tracking by magnetic resonance imaging. Pharm. Res. 29 (5), 1392–1403. doi:10.1007/s11095-012-0680-1
Silva, C. O., Pinho, J. O., Lopes, J. M., Almeida, A. J., Gaspar, M. M., and Reis, C. (2019). Current trends in cancer nanotheranostics: metallic, polymeric, and lipid-based systems. Pharmaceutics 11 (1), 22. doi:10.3390/pharmaceutics11010022
Siwińska-Stefańska, K., Kubiak, A., Piasecki, A., Dobrowolska, A., Czaczyk, K., Motylenko, M., et al. (2019). Hydrothermal synthesis of multifunctional TiO2-ZnO oxide systems with desired antibacterial and photocatalytic properties. Appl. Surf. Sci. 463, 791–801. doi:10.1016/j.apsusc.2018.08.256
Smida, K., Albedah, M. A., Rashid, R. F., and Al-Qawasmi, A.-R. (2023). Molecular dynamics method for targeting α-synuclein aggregation induced Parkinson's disease using boron nitride nanostructures. Eng. Analysis Bound. Elem. 146, 89–95. doi:10.1016/j.enganabound.2022.10.016
Subhan, Md A., Yalamarty, S. S., Filipczak, N., Parveen, F., and Torchilin, V. P. (2021). Recent advances in tumor targeting via EPR effect for cancer treatment. J. Personalized Med. 11 (6), 571. doi:10.3390/jpm11060571
Sudheer, P., Sogali, B. S., and Sogali, B. S. (2024). Nanostructured lipid carrier-mediated transdermal delivery system of glibenclamide for gestational diabetes: pharmacokinetic and pharmacodynamic evaluation. Curr. Drug Deliv. 21 (10), 1386–1407. doi:10.2174/0115672018274038231212105440
Tang, F., Li, L., and Chen, D. (2012). Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv. Mater. 24 (12), 1504–1534. doi:10.1002/adma.201104763
Tang, W., Liu, J., and Ding, B. (2023). Nucleic acid nanostructure for delivery of CRISPR/Cas9-based gene editing system. Interdiscip. Med. 1 (1), e20220014. doi:10.1002/INMD.20220014
Tang, W., Tong, T., Wang, H., Lu, X., Yang, C., Wu, Y., et al. (2023). A DNA origami-based gene editing system for efficient gene therapy in vivo. Angew. Chem. Int. Ed. 62 (51), e202315093. doi:10.1002/anie.202315093
Tanveer, M., Imran, M., Latif, S., Hussain, N., and Bilal, M. (2022). “Structural design of organic–inorganic nanohybrids,” in Hybrid nanomaterials: biomedical, environmental and energy applications. Editors K. Rizwan, M. Bilal, T. Rasheed, and T. A. Nguyen (Singapore: Springer Nature Singapore), 29–36.
Teh, Z.Li, Hu, L., Zhang, Z., Gentle, A. R., Chen, Z., Gao, Y., et al. (2020). Enhanced power conversion efficiency via hybrid ligand exchange treatment of p-type PbS quantum dots. ACS Appl. Mater. and Interfaces 12 (20), 22751–22759. doi:10.1021/acsami.9b23492
Tiwari, J. N., Tiwari, R. N., and Kim, K. S. (2012). Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Prog. Mater. Sci. 57 (4), 724–803. doi:10.1016/j.pmatsci.2011.08.003
Uprety, B., and Abrahamse, H. (2022). Semiconductor quantum dots for photodynamic therapy: recent advances. Front. Chem. 10, 946574. doi:10.3389/fchem.2022.946574
Yang, C., Lin, Z.-I., Chen, J.-An, Xu, Z., Gu, J., Wing-Cheung, L., et al. (2022). Organic/inorganic self-assembled hybrid nano-architectures for cancer therapy applications. Macromol. Biosci. 22 (2), 2100349. doi:10.1002/mabi.202100349
Yu, Z., Hu, P., Xu, Y., Bao, Q., Ni, D., Wei, C., et al. (2020). Efficient gene therapy of pancreatic cancer via a peptide nucleic acid (PNA)-Loaded layered double hydroxides (LDH) nanoplatform. Small 16 (23), 1907233. doi:10.1002/smll.201907233
Yuan, W., Weng, G.-M., Lipton, J., Li, C. M., Van Tassel, P. R., and Taylor, A. D. (2020). Weak polyelectrolyte-based multilayers via layer-by-layer assembly: approaches, properties, and applications. Adv. Colloid Interface Sci. 282, 102200. doi:10.1016/j.cis.2020.102200
Zamborlin, A., Sarogni, P., Frusca, V., Gonnelli, A., Giannini, N., Ermini, M. L., et al. (2023). Drug-free hybrid nanoarchitecture modulation of the metastatic behavior of pancreatic ductal adenocarcinoma in alternative in vivo models. ACS Appl. Nano Mater. 6 (24), 22532–22544. doi:10.1021/acsanm.3c05299
Zhang, M., Wen, Y., Huang, Z., Qin, X., Zhou, Mi, Xiao, D., et al. (2023). Targeted therapy for autoimmune diseases based on multifunctional frame nucleic acid system: blocking TNF-α-NF-κB signaling and mediating macrophage polarization. Chem. Eng. J. 454, 140399. doi:10.1016/j.cej.2022.140399
Zhang, X., Zhang, J., Han, X., Wang, S., Hao, L., Zhang, C., et al. (2023). A photothermal therapy enhanced mechano-bactericidal hybrid nanostructured surface. J. Colloid Interface Sci. 645, 380–390. doi:10.1016/j.jcis.2023.04.148
Keywords: hybrid nanostructures (HNSs), photothermal and photodynamic therapy, multifunctional nanostructures, gene delivery and editing, cancer therapy, cardiovascular diseases, neurodegenerative diseases, autoimmune diseases
Citation: Harun-Ur-Rashid M and Jahan I (2024) Innovative hybrid nanostructures: pioneering advances in modern therapy. Front. Nanotechnol. 6:1458894. doi: 10.3389/fnano.2024.1458894
Received: 03 July 2024; Accepted: 16 August 2024;
Published: 29 August 2024.
Edited by:
Mona Khafaji, Sharif University of Technology, IranReviewed by:
Marziyeh Jannesari, University College Dublin, IrelandMariana Tasso, National Scientific and Technical Research Council (CONICET), Argentina
Ashish Tiwari, University of Delaware, United States
Copyright © 2024 Harun-Ur-Rashid and Jahan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Harun-Ur-Rashid, bXJhc2hpZEBpdWJhdC5lZHU=
 Mohammad Harun-Ur-Rashid
Mohammad Harun-Ur-Rashid Israt Jahan
Israt Jahan