- 1Department of Pharmaceutical Biotechnology, School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 2Faculty of Pharmacy, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran
The liver and kidneys are vital organs responsible for essential metabolic and excretory functions, and their protection is a cornerstone of therapeutic innovation. This review highlights the emerging role of eco-friendly, bioengineered silver (AgNPs) and gold nanoparticles (AuNPs) as protective agents for liver and kidney health, based on evidence from animal studies. The discussion emphasizes green synthesis approaches, which offer sustainable and biocompatible routes for nanoparticle production. Key findings reveal the effects of these nanoparticles (NPs) on hepatic enzymes—Aspartate aminotransferase (AST), Alanine transaminase (ALT), and Alkaline phosphatase (ALP)—and renal function markers, including urea and creatinine levels, under both healthy and pathological conditions. In diseased animal models, biosynthesized NPs significantly reduced ALT, AST, ALP, urea, and creatinine levels, demonstrating their protective effects. Conversely, in healthy animals, lower nanoparticle concentrations exhibited no statistically significant impact on these parameters, suggesting their safety at therapeutic doses. This review presents the novelty of bioengineered NPs as potential therapeutic agents for hepatic and renal protection while highlighting the need for further research into their pharmacokinetics and pharmacodynamics to advance clinical translation.
1 Introduction
1.1 Bridging the gap between nanotechnology and biotechnology: an introduction to nanobiofabrication
Nanotechnology is a novel multi-disciplinary field that has attracted researchers from various areas over the past few decades. Due to the significant attention towards nanotechnology, novel investigations, and innovative approaches have been implemented this twenty-first-century area of research. However, it is still in its infancy, and much remains to be uncovered. Nanoscience is a field of study that encompasses the analysis of the structure and features of elements with sizes ranging from 1 to 100 nm, while nanotechnology employs nanoscience for practical applications. By taking control of the novel characteristics of nanosized materials, nanotechnology aims to improve existing technologies or develop new ones. Nanotechnology has made beneficial advancements in fields, such as engineering, medicine, and stem sciences (Bayda et al., 2019; Ahire et al., 2022; Khan et al., 2022). Researchers have discovered unique properties at the nanoscale, which have been utilized in various fields like engineering, biology, physics, medicine, and agriculture. The emergence of beneficial physicochemical properties at the nanoscale has led to the production of different NPs through various methods. The size reduction in nanomaterials and increased surface area result in mechanical, magnetic, optical, thermal properties and changes in substance reactivity (Mekuye and Abera, 2023; Baig et al., 2021). Combining the principles of nanotechnology with biotechnology has created the field of nanobiotechnology, which can benefit from the advantages of both areas. Nanobiotechnology involves employing nanotechnology potentials in biological areas, with significant applications in the medical field, such as targeted drug delivery, novel dosage forms, diagnosis, and treatment processes (Fakruddin et al., 2012).
Nanobiotechnology has shown great potential in the healthcare field by synthesizing beneficial NPs for targeted drug delivery, gene therapy, and treating various diseases in different body organs (Anjum et al., 2021). Fabrication of NPs is one of the particular and leading emphases of nanotechnology and nanobiotechnology disciplines; among numerous NPs, metallic NPs have attracted considerable attention because of their novel practical properties (Chopra et al., 2022). AuNPs and AgNPs have indicated beneficial characteristics that led to applications like targeted drug delivery, drug analysis, and biomedical uses such as bio-imaging and bio-detection. The synthesis of AuNPs and AgNPs can be achieved through physical, chemical, and biological approaches (Alaqad and Saleh, 2016). Each of these approaches has its advantages and disadvantages but, due to its profitable nature, the biological approach, known for its green synthesis method, has garnered more attention in recent decades. In the green synthesis process, metallic NPs are synthesized using biological resources like bacteria, fungi, algae, and various parts of plants (Chopra et al., 2022; Harinisri et al., 2023). Utilizing natural resources for metallic NPs fabrication is more environmentally friendly and cost-effective. Given the current global environmental challenges and increasing population, these factors are crucial. When comparing the different approaches, the biological approach emerges as the most sustainable method for synthesizing AuNPs and AgNPs, making it the least harmful process for both current society and future generation. This review offer a detailed comparison of metallic NPs biosynthesis methods and their characteristics. Nanobiotechnology, particularly in the formation of nanomaterials, has significantly impacted various fields (Chopra et al., 2022; Harinisri et al., 2023; Shahcheraghi et al., 2022). This review focuses on the green synthesis of AuNPs and AgNPs and their applications in protecting hepatic and renal function profiles.
1.2 Metallic nanomaterials: an overview of synthesis approaches
1.2.1 Traditional approach: advantages and disadvantages
As mentioned earlier, metallic NPs can be synthesized through traditional approaches, such as physical, chemical, and biological methods. Each method provides unique NPs with specific sizes and morphologies that can be tailored for different purposes. The physical and chemical techniques are considered traditional approaches, while the biological approach is known for its green synthesis. The acquisition of nanomaterials typically involves two main techniques: the bottom-up and top-down approaches. The top-down approach involves obtaining nanoscaled objects from bulk materials through destructive strategies. Usually, some physical methods that employ techniques such as milling, laser fragmentation, chemical etching, and electro-explosion can be classified under the top-down approach. On the other hand, the bottom-up technique involvesmanufacturing nanomaterials by assembling their elemental constituents through a precise process. This method includes processes like atomic or molecular condensation, laser pyrolysis, vapor deposition, and more. The bottom-up approach is also utilized in chemical and biological methods (Khan et al., 2019; Kumar et al., 2023). It is important to discuss the advantages and drawbacks of each method of metallic NPs synthesis in order to compare them effectively. Firstly, the physical process of laser ablation and evaporation-condensation are the most significant practices compared to others, but they also have their own potential and shortcomings. For example, one of the essential benefits of physical approach over the chemical one is the absence of chemical residuals in the produced nanoparticle-containing solvent. Additionally, achieving more uniform distribution among NPs through the physical method is an advantage compared to the chemical process. However, this metallic NPs formation technique has drawbacks such as being time-consuming, reuiring high temperatures, and consuming energy that is harmful to the environment (Iravani et al., 2014). Chemical methods are the most common technique for metallic NPs synthesis and involve using reducing and stabilization agents as two key steps. Chemicals like formaldehyde, sodium borohydride, and ethylene glycol are used as reducing agents to obtain AuNPs and AgNPs. Stabilization agents such as peptide-biphenyl hybrids (PBHs) and trisodium citrate are also utilized. Unfortunately, the chemical reagents used in the synthesis process can be expensive and harmful to the environment. Moreover, the toxicity of chemical residues could limit the use of the produced NPs in biomedical fields due to potential health risks (Alaqad and Saleh, 2016; Herizchi et al., 2016). In conclusion, the main shortcomings of the traditional approach include its hazardous impact on living entities and the environment due to toxic chemicals and energy-consuming procedures, as well as its high cost and the need for labor-intensive supplies. As a result, several researchers have attempted to find an alternative method of nanoparticle development that does not have the limitations of physicochemical techniques (Iravani et al., 2014; Akter and Huq, 2020; Jamkhande et al., 2019).
1.2.2 Green approach: advantages and disadvantages
Nowadays, the list of worldwide issues that need immediate solutions is immeasurable. Among them, addressing environmental concerns is essential. Therefore, the exploration of alternatives to conventional methods, which have been harmful to the environment, has become more prevalent in recent decades. Due to the shortcomings of traditional methods of metallic nanoparticle synthesis, particularly the economic and environmental drawbacks, introducing a novel green approach has been a more sustainable process for obtaining NPs. This approach has garnered significant attention among researchers (Srivastava et al., 2022). In the aforementioned approach, biological resources such as fungi, bacteria, plants, and algae are natural entities that can be utilized for nanoparticle production. There are numerous advantages to employing the biological approach over traditional methods. Firstly, there is no need for toxic chemicals in the synthesis process and it does not require energy-intensive procedures, making nanoparticle development more environmentally friendly (Jamkhande et al., 2019; Srivastava et al., 2022; Chen et al., 2003; Abu Hajleh et al., 2021). Moreover, this approach is cost-effective because the existing bioactives in biological resources act as reducing agents and stabilizers, eliminating the need for expensive chemicals (Huq et al., 2022). In addition, other significant benefits of the environmentally friendly approach include the possibility of better nanoparticle stabilization due to the slower reaction rate and increased biocompatibility (Barabadi et al., 2023). Researchers have successfully fabricated metallic NPs with different sizes and morphologies using various bioresources, including plant extracts from flowers, roots, leaves, fruit, fungi, algae, and bacteria. Figure 1A provides a schematic presentation of the biological fabrication of metal-based nanomaterials using natural agents (kazemi et al., 2023). Additionally, Figure 1B illustrates the potential bioactive agents in biological resources responsible for the fabrication of metallic NPs as reducing and stabilizing agents (Garg et al., 2021). NPs with different characteristics, such as shape and size, can be achieved by using various bioresources. The variations in the developed NPs result from synthesis conditions and the active chemicals present in the selected living organism (Prasad et al., 2021). NPs produced through the green approach are typically characterized using standard tests, including UV-visible (UV-vis) spectroscopy, dynamic light scattering (DLS) also known as photon correlation spectroscopy, fourier transform infrared (FT-IR) spectroscopy, electron microscope (such as transmission electron microscope (TEM) and scanning electron microscope (SEM)), and X-ray diffraction (XRD) (Choi et al., 2007). Moreover, the biological fabrication of NPs is generally categorized into two main categories: extracellular and intracellular (Singh H. et al., 2018).
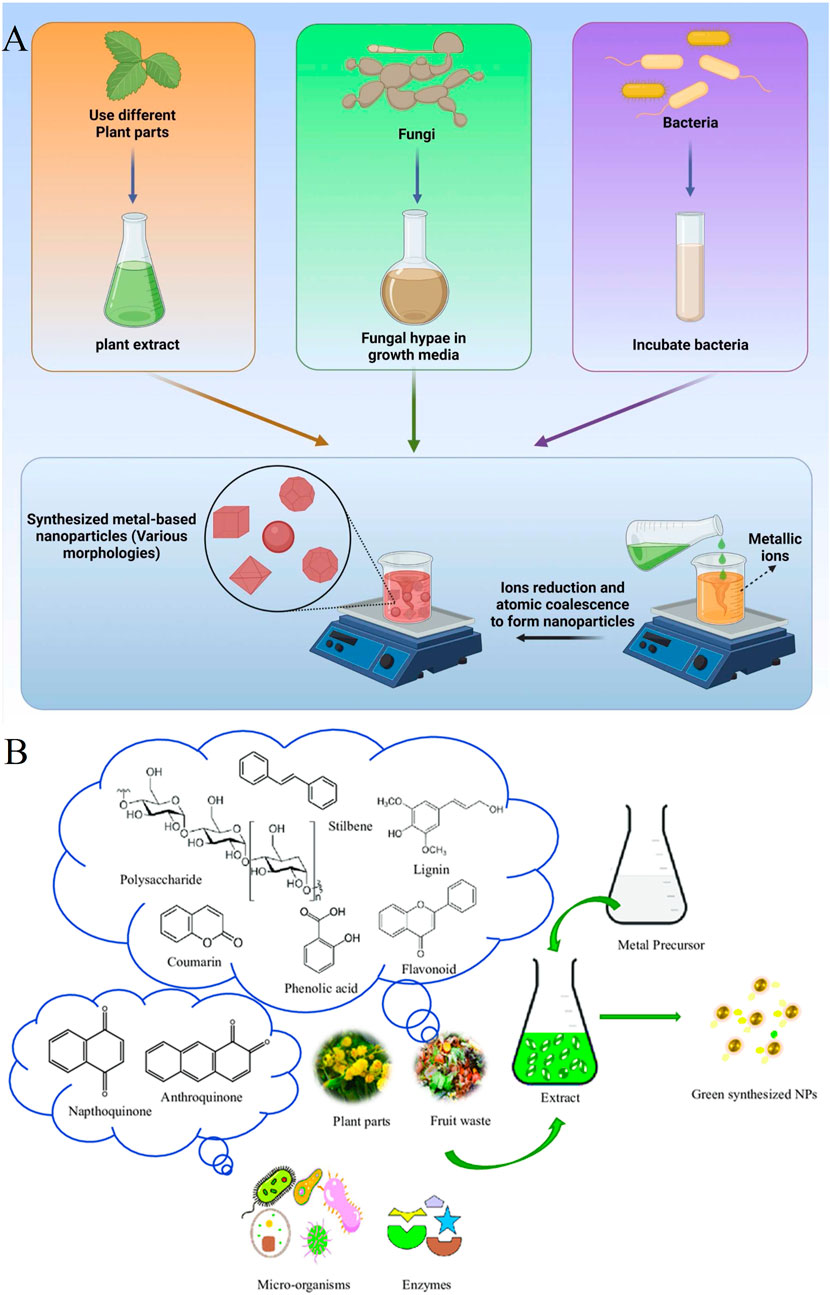
Figure 1. (A) An illustration of the biological fabrication of metal-based nanomaterials by employing natural agents (kazemi et al., 2023) (Copyright 2023 The Author(s). Published by Elsevier Ltd. Open access article distributed under a Creative Commons Attribution 4.0 International License); (B) A schematic illustration of the probable bioactive agents in the biological resources that are responsible for the fabrication of metallic NPs as reducing and stabilizing agents (Garg et al., 2021) (Copyright 2021 Copyright TÜBİTAK. Open access article distributed under a Creative Commons Attribution 4.0 International License).
1.3 Eco-friendly and sustainable fabrication of silver/gold nanostructures
1.3.1 Mycosynthesis of silver/gold nanostructures
Among the implemented biological resources for the fabrication of AuNPs and AgNPs, each has unique properties and beneficial attributes, and fungi are no exception. Fungi can assist in the biological synthesis of AuNPs and AgNPs through the extracellular and/or intracellular routes. However, extracellular methods are more straightforward due to the easier extraction process, making them more accessible for scaling up (Sastry et al., 2003). Fungi cultivation in various conditions, including industrial settings or laboratories results in the presence of mycelia, which can withstand harsh conditions. Notably, one of the unique feature of fungi is their ability to secrete large quantities of beneficial enzymes for the formation of metallic NPs (Joshi et al., 2017). As a result, many researchers have used different species of fungi in green nanotechnology for the development of AgNPs and AuNPs, producing NPs of varying sizes and morphologies. For example, research has shown that by utilizing Aspergillus fumigatus DSM819, extracellular synthesis of AgNPs can be achieved, resulting in spherical NPs with an average size of 84.4 nm (Othman et al., 2019). Similarly, Bhainsa et al. used A. fumigatus for the biosynthesis of AgNPs through an extracellular process obtaining AgNPs ranging in size from 5–25 nm, characterized by UV-vis, TEM, and XRD (Bhainsa and D’souza, 2006). A separate study reported the fabrication of AgNPs using fungal biomass from the Verticillium fungus was reported (Mukherjee et al., 2001). Besides, research by Kathiresan et al. demonstrated the high-rate synthesis of stable AgNPs using Penicillium fellutanum, a marine fungus, confirmed by TEM (Kathiresan et al., 2009). Likewise, Mohanta et al. found that Ganoderma sessiliforme mushroom could synthesize AgNPs, characterized by UV-vis spectroscopy, TEM, and DLS (Mohanta et al., 2018). G. sessiliforme is known for producing various physiologically active proteins with applications in nanoparticle fabrication. However, the use of these valuable mushrooms for metallic NPs synthesis is limited due to disadvantages such as the required cultivation process and inconsistency in nanoparticle sizes (Chopra et al., 2021; Li et al., 2011). Moreover, Madhanraj et al. conducted research showing the potential of Basidiomycetes fungi extract in providing AuNPs and AgNPs, confirmed through color change and UV-vis spectroscopy (Madhanraj et al., 2017). A separate example of fungi-assisted AgNPs obtaining study involved utilizing endophytic Fusarium oxysporum-NFW16 extract. In this study, spherical AgNPs with an approximate size of 30–36.1 nm were quickly acquired by optimizing the presented synthesis factors. Characterization tests, including XRD, UV-vis, and SEM, demonstrated the production of AgNPs and their properties. FT-IR illustrated that the phenolic and primary amine-containing components in F. oxysporum-NFW16 were responsible for biosynthesis (Ilahi et al., 2022). In a separate biologically-mediated AgNPs synthesis research, using biomass of A. fumigatus KIBGE-IB33 demonstrated a high ability to reduce Ag ions in silver nitrate solution to obtain AgNPs. The fabricated NPs were initially confirmed by UV-vis analysis followed by other usual analysis tests, which revealed an approximate spherical morphology of AgNPs with a size of less than 100 nm (Raza et al., 2021). Alternatively, according to an AgNPs green synthesis approach implemented by Wang and coworkers, Aspergillus sydowii fungus assisted in acquiring spherical AgNPs with a size of 1–24 nm by an extracellular method. The researchers optimized the biosynthesis process by discovering the best condition, which were a pH value of 8.0, 50°C, and 1.5 mM substrate concentration. After mixing silver nitrate with fungal cell filtrate, the color transformation, followed by UV-vis spectroscopy, approved the nanoparticle development. Further analysis with examinations such as TEM and XRD indicated more results of fabricated AgNP attributes (Wang et al., 2021). Moreover, an example of fungi-mediated AgNP fabrication is a study utilizing Letendraea sp. WZ07. In this study, by adding silver nitrate solution to the achieved mycelial-free filtrate of fungus under certain circumstances, the color transformation was detected, and the subsequent UV-vis test verified the occurrence of AgNPs fabrication. The following usual characterization analysis indicated the formation of 33.8 nm-sized AgNPs, which were face-centred cubic (Qiao et al., 2022). Moreover, an example of exploiting fungi for providing AuNPs is a recent study that used 29 fungal strains of thermophilic filamentous. In this investigation, different strains’ capability for the biosynthesis of AuNPs was assessed by different methods containing extracellular and intracellular extract and fungus autolysate. The results demonstrated that various strains and synthesis methods led to the fabrication of AuNPs with different characteristics. The available bioactive components in the fungi, which were responsible for the reducing process through the intracellular synthesis method, were probably identified as amino acids, cofactors, and glucose. The size of active metabolites acting as reducing agents is assumably less than 3 kDa, and biocompounds functioning as capping agents are more extensive than 3 kDa, such as proteins, capable of stabilizing the fabricated NPs (Molnár et al., 2018). In addition, another example of fungal-generated AuNPs and AgNPs is a research using Aspergillus flavus. The fabricated AuNPs had spherical and hexagonal morphology, and their size was between 37 and 62 nm; also, the manufactured AgNPs were spherical-shaped with a 5–37 nm size (Sheikh and Awad, 2022).
1.3.2 Bacteriogenesis of silver/gold nanostructures
Bacteria have been extensively utilized as bioresources in green nanotechnology, with a longer history of use compared to other biological resources. Like other biological-mediated nanoparticle synthesis methods, utilizing bacteria is cost effective and environmentally friendly. Bacteria-assisted nanoparticle production offers an energy-efficient and controllable method for converting metallic ions into non-toxic metallic NPs (Rai and Duran, 2011; Fang et al., 2019; Garole et al., 2018). Over the past few decades, researchers have identified numerous bacterial microorganisms capable of green-synthesizing AuNPs and AgNPs with varying morphologies and characteristics. Recently, researchers have taken an interest in certain strains of marine-isolated bacteria for the biosynthesis of AuNPs and AgNPs (Liu et al., 2018; Gupta and Padmanabhan, 2018). Several examples of using bacteria to obtain these metallic NPs have been documented. For example, the bacterium Lysinibacillus xylanilyticus MAHUQ-40 was used in a study to produce AgNPs. UV-vis spectroscopy results confirmed the synthesis of NPs, with their characteristics further analyzed using TEM, XRD, and DLS techniques. The fabricated AgNPs were found to be spherical in shape, with crystalline structures and sizes ranging from 8–30 nm size (Huq, 2020). In a separate research conducted by Akter and Huq, AgNPs ranging from 7–22 nm in size with spherical shapes were successfully synthesized by assisting Sphingobium sp. MAH-11. The researchers observed a color change when adding the supernatant of the bacterial culture to a silver nitrate solution under specific conditions followed by conventional characterization analysis (Akter and Huq, 2020). Furthermore, a study suggests a bacterium-mediated route for providing AgNPs by employing Terrabacter humi MAHUQ-38. This environmentally-friendly approach involves mixing an AgNO3 solution with the bacterium culture supernatant leading to a color change as an initial sign of nanoparticle formation. Characterization tests using TEM indicated a spherical morphology with sizes ranging from 6–24 nm (Akter et al., 2020). In a study by John et al., AgNPs with different sizes were fabricated using a biological approach with the assistance of Pseudomonas sp. biomass. SEM results showed spherical morphology and variation in size with SEM and TEM confirming sizes ranging from 20 to 70 nm. FT-IR analysis suggested that bacterial proteins are likely responsiblefor biosynthesis, specifically carbonyl and free amine groups (John et al., 2020). Besides, a study attempted to obtain AgNPs through bacteriogenic synthesis using Arthrobacter Bangladeshi sp. nov. Adding bacterial supernatant to a silver nitrate solution produced AgNPs with sizes ranging from 12–50 nm, confirmed by color change and UV-vis spectroscopy. Characterization tests using TEM, DLS, and XRD were conducted and revealed crystalline spherical-shaped AgNPs (Huq and Akter, 2021). The use of bacteria for synthesizing AuNPs also has numerous examples. For instance, Shunmugam and coworkers utilized the Vibrio alginolyticus bacterium to manufacture AuNPs extracellularly through a synthesis process involving aqueous chloroauric acid and bacterial supernatant. The absorption peak on UV-vis confirmed the formation of the expected NPs. Moreover, FT-IR indicated the presence of secondary metabolites like aldehydes, flavonoids, and ketones which likely act as synthesis agents. In addition, SEM and TEM revealed irregular shaped AuNPs with sizes between 100 and 150 nm (Shunmugam et al., 2021). In a separate study, Enterococcus sp. RMAA, a marine bacterium, was used for the intracellular fabrication of AuNPs. Adding gold (III) chloride to the culture broth led to a color variation, indicating nanoparticle development. Subsequently, UV-vis confirmed AuNPs biosynthesis, and TEM showed their physical properties with an average particle size of 7 nm and spherical shape (Nandhini et al., 2021). Additionally, a group of researchers conducted a study using the Paracoccus haeundaensis BC74171 bacterium for the biosynthesis of AuNPs. This marine bacterium produced AuNPs through an extracellular method, and the synthesized NPs underwent precise characterization analysis. Specifically, the solution containing bacterial supernatant and chloroauric acid was incubated, and the visual indicator of ruby-red color emergence demonstrated the formation of AuNPs. Moreover, in this study, the effects of various parameters, such as time, temperature, and precursor and supernatant concentration, were investigated to determine the optimal synthesis conditions. Under the optimized conditions, TEM results showed spherical AuNPs, and DLS revealed a mean size of 20.93 ± 3.46 nm (Patil et al., 2019).
1.3.3 Phytosynthesis of silver/gold nanostructures
Plants have shown great potential for nanoparticle biosynthesis compared to microbial bioresources due to their active metabolites, leading to an efficient synthesis process. Another advantage of using plants over other biological resources is that it is not necessary to eliminate existing cell cultures in the final solution, which can enhance the rate of nanoparticle synthesis. Additionally, utilizing plants for obtaining metallic NPs is a scalable method. Various studies have suggested using different parts of plants, such as roots, leaves, and gum, for green synthesis of metallic NPs (Ahmad et al., 2019; Shankar et al., 2004). Flavonoids, polysaccharides, ketones, proteins, and polyphenols are active metabolites in plants that act as reducing, capping, and stabilizing agents for metallic NPs fabrication (Siddiqi and Husen, 2017). For more than a decade, plants have been commonly used for the synthesis of AuNPs and AgNPs. There are examples of using different parts of plants to obtain these valuable metallic NPs in this section. For example, a study reported the synthesis of AuNPs using an extract of cinnamon bark. The mixture of prepared cinnamon bark extract with HAuCl4 solution under specific conditions resulted in the synthesis of AuNPs, likely due to biochemicals in the extract acting as reducing and stabilizing agents. UV-vis spectroscopy confirmed the biosynthesis process, and TEM showed that the green-synthesized AuNPs were approximately 35 nm in size and spherical in shape (ElMitwalli et al., 2020). In a separate research, Curcuma pseudomontana rhizomes were used to obtain AuNPs through a green metallic NPs approach. The synthesis involved isolating curcumin from the plant’s rhizomes and creating a solution containing curcumin, which was then mixed with the HAuCl4 solution. The color change and UV-vis spectroscopy confirmed the formation of AuNPs, which were found to have a mean size of 20 nm and a spherical morphology as demonstrated by TEM and SEM (Muniyappan et al., 2021).
A group of researchers utilized Tecoma capensis plant leaves to facilitate the green-mediated synthesis of AuNPs. In this study, the addition of HAuCl4.3H2O solution to the plant’s extract resulted in a color transition, indicating the production of AuNPs, which was later confirmed by UV-vis spectroscopy. FT-IR analysis revealed potential chemicals such as terpenoids, flavonoids, and alkaloids that may play a role in the synthesis of metallic NPs. Additionally, TEM showed characteristics of AuNPs, including a size range of 10–35 nm and a spherical shape (Hosny et al., 2022). Alternatively, Onitsuka and colleagues conducted a study to produce AuNPs and AgNPs using Camellia sinensis plant leaves. By employing two methods, precursor solutions were added to the plant extract and the plant extract with NaHCO3 solution to control the pH, respectively. Subsequently, the NPs obtained from the second method were found to be more stable and less time-consuming. By adjusting the conditions, the optimal circumstances for obtaining desired AgNPs and AuNPs were identified using the second method. Ultimately, AgNPs with an average size of approximately 30 nm and smaller spherical AuNPs with a size of ∼10 nm were successfully obtained by utilizing tea leaf extracts (Onitsuka et al., 2019). Another example of using a plant for the synthesis of AgNPs is a study suggesting the potential of Ziziphus mauritiana extract. In this research, combining the aerial parts extract of the plant with a silver nitrate solution resulted in the production of AgNPs, characterized by FT-IR, TEM, UV-vis, SEM, and XRD revealing their irregular shape and size range of 10–45 nm with a crystalline structure. Moreover, the influence of factors such as pH value, contact time, and temperature was analyzed and confirmed by UV-vis, showing a direct relationship between these factors and the phytofabrication of AgNPs; this means that higher temperature among the examined ones, basic pH, and more contact time had better synthesis consequences (Sameem et al., 2022a). In addition, Rajiv Gandhi and his team conducted a study conducted a study using Lonicera japonica plant extract to obtain AgNPs. Mixing the plant leaf extract with a silver nitrate solution led to a color change, confirming the fabrication of AgNPs. SEM and TEM results indicated the ball-like spherical shape of the plant-mediated synthesized AgNPs (Rajivgandhi et al., 2022). In a recent study, the stem extract of Cissus quadrangularis was utilized to assist in the biosynthesis of AgNPs with an average size of 24 nm. The combination of silver nitrate solution with the plant extract resulted in the desired NPs, characterized by analytical methods including XRD, FT-IR, UV-vis, and SEM. SEM results showed that the produced AgNPs were spherical, while FT-IR demonstrated the presence of functional groups related to secondary metabolites that could act as reducing agents or stabilizers, and XRD confirmed the crystalline structure of the synthesized AgNPs (Kanimozhi et al., 2022).
1.3.4 Phycosynthesis of silver/gold nanostructures
Algae have been indicated to have numerous beneficial applications in biotechnology. To be more specific, by implementing recent advancements in technology, distinguishing different present phenotypes of algae and even producing new microalgal genotypes has been made possible. Subsequently, various industrial and agricultural benefits can be supplied, creating a considerable impact (Fabris et al., 2020). Among various utilizations of algae, using different types of them as a natural entity for generating nanomaterials is worthwhile. Phyconanotechnology, a word constructed by combining nanotechnology and phycology, refers to utilizing nanotechnology by mediating algae species. Phyconanotechnology has made fabricating metallic NPs such as AuNPs and AgNPs possible by coupling the technology and existing bioactive components in various algae species. In recent decades, algae as a biological resource for obtaining AgNPs and AuNPs has been an environmentally friendly and low-cost approach. Algae are mainly categorized into four classes including Phaeophyta, Rhodophyta, Chlorophyta, and Cyanophyta which refers to brown algae, red algae, green algae, and cyanobacteria, respectively. Various researchers examined the mentioned different species for nanoparticle fabrication. Another classification categorizes these autotrophic organisms living in various habitats worldwide under two groups, the unicellular organisms, called microalgae, and the multicellular group, known as macroalgae, which both illustrate beneficial properties for obtaining metallic NPs (Lee, 2008; Chan et al., 2022). One of the beneficial characteristics of algae is their remarkable potential to accumulate metal, leading to a higher-speed metallic NPs synthesis process. Furthermore, this sustainable approach is less energy-consuming and easy to cultivate, which is another advantage of exploiting it (Sharma et al., 2016). In recent years, by utilizing different species of algae, AuNPs, and AgNPs in various shapes and sizes were synthesized extracellularly or intracellularly. In a study, cyanobacterium alga, Plectonema boryanum UTEX 485, and two solutions containing gold were used to synthesize the AuNPs in different shapes. The synthesis process utilizing gold (I) thiosulphate solution and gold (III) chloride solution led to cubic <10–25 nm sized AuNPs and octahedral <10 nm sized AuNPs, respectively (Lengke et al., 2006). According to research developed by Lengke et al., spherical and octahedral AgNPs were fabricated by confronting AgNO3 precursor in an incubator for up to 28 days with an alga from the Cyanophyta class, P. boryanum UTEX 485. The intracellularly-produced AgNPs in this process were less than 10 nm, and the extracellular synthesized one had a size between 1 and 200 nm (Lengke et al., 2007). In a study performed by Chakraborty and coworkers, Lyngbya majuscula and Spirulina subsalsa as Cyanobacteria algae and Rhizoclonium hieroglyphicum as green algae produced spherical AuNPs with the size of <20 nm noticed at intra and extracellular parts of the organisms. The visual color change revealed the occurring synthesis process in the study, and TEM aided the NPs’ characterization (Chakraborty et al., 2009). A separate group of researchers performed a study assisting Trichodesmium erythraeum, a marine microalga, to acquire AgNPs. By an extracellular method, the algal supernatant mixture with silver nitrate resulted in color variation into brown, an optical indicator of AgNP formation, verified by UV-vis spectroscopy. FT-IR analyzed the active chemicals on developed AgNPs that could have played a role in biosynthesis. Moreover, other characterization tests, including XRD and SEM, revealed that the acquired AgNPs through this green approach were cubical-formed and had crystalline structures and 26.5 mean sizes (Sathishkumar et al., 2019). Due to research conducted by Karimi and his coworker, Chaetomorpha sp. mediated the biosynthesis of AgNPs. UV-vis, TEM, XRD, and DLS aided the verification and characterization of produced AgNPs. Henceforward, the algal-mediated synthesized AgNPs were approximately 15 nm and spherical-shaped (Karimi and Samimi, 2019). According to a separate study, AuNPs were fabricated with the aid of Sargassum cymosum alga. The research mentioned that macroalga extract was added to a tetrachloroauric acid solution, resulting in rapid color transformation. Afterward, the UV-vis and DLS analysis verified the fabrication of AuNPs, demonstrated earlier by the observable sign. TEM results indicated that the developed AuNP shape was spherical, illustrating that their mean size was 7–20 nm. Moreover, researchers examined the influence of factors such as temperature, pH value, and mass ratio of tetrachloroauric acid that appeared to affect the obtained AuNPs stability and yield (Costa et al., 2020). An example of the algal-mediated synthesis of metallic NPs is utilizing Gelidium corneum, a red alga, for fabricating AgNPs. In this study, the combination of optimized concentration of AgNO3 solution and algal extract led to color change detectable by the naked eye, followed by confirmation via UV-vis spectroscopy. The size of the produced NPs was between 20 and 50 nm, determined by TEM. Besides, FT-IR revealed the functional groups of active components liable for biosynthesis, and XRD indicated the crystalline structure of AgNPs attained from green biofabrication (Öztürk et al., 2020).
1.4 Bridging the gap between biogenic silver/gold nanostructures and hepatic and/or renal function: What does this review aim to address?
Despite significant progress in the synthesis and characterization of metallic NPs, particularly AgNPs and AuNPs, there remains a notable gap in the literature regarding their specific role in protecting hepatic and renal functions. While numerous studies have explored the general biological properties of these NPs, such as their antimicrobial, antioxidant, and anticancer activities, fewer have focused on their potential therapeutic applications for organ protection, particularly in the context of liver and kidney function. This review aims to address this gap by consolidating animal-based evidence supporting the protective activity of these bioengineered NPs, with a particular emphasis on their potential to preserve and enhance the function of vital organs such as the liver and kidneys in non-healthy animal models as well as evaluating the influence of these NPs on healthy animal models. Hence, this review aims to provide novel insights into the therapeutic implications of bioengineered NPs, offering a unique perspective that highlights their potential to preserve hepatic and renal functions. This focus not only addresses a critical knowledge gap but also presents emerging applications that could redefine the role of nanomaterials in biomedical research and therapy.
2 Methods
This study is a narrative review discussing the influence of biosynthesized silver/gold nanostructures on hepatic and/or renal function profile parameters in animal models. The following databases were searched: PubMed, Web of Science, Scopus, ProQuest, EBSCO and Google scholar. The language was restricted to English in the literature search and the literature was searched using a different combinations of several keywords: “biosynthesis”, “synthesis”, “fabrication”, “biofabrication”, “fungal”, “bacterial”, “green”, “bioreduction”, “microbial”, “biological”, “myco*”, “plant*”, “phyto*”, “herbal”, “algal”, “biogenic”, “biomimetic”, and “nanoparticle*”, “nano-silver”, “nanostructure*”, “colloidal”, “silver”, “Ag”, “gold”, “Au”, “nano-gold”, “liver”, “hepatic”, “kidney”, and “renal”. Publications that evaluated the influence of eco-friendly green fabricated silver/gold nanostructures on hepatic and/or renal function profile parameters in animal models were included, whereas those that reported only biosynthesis of silver/gold nanostructures were excluded from the study.
3 Results and discussion
3.1 Protective activity of eco-friendly green fabricated silver/gold nanostructures on hepatic function profile parameters in non-healthy animal models
As shown in Table 1, most of the laboratory studies support the protective effects of biosynthesized AgNPs and AuNPs on liver parameters in non-healthy animal models. For instance, in a study, eco-friendly phytofabricated AgNPs were tested to evaluate their effects on hepatic enzyme levels in Wistar rats at concentrations of 50 and 200 mg kg−1. In this study, animals were categorized into three-member groups, and streptozotocin (STZ) was administered to induce diabetes with elevated liver parameters. In this study, one group of rats was healthy, a separate group had STZ-induced diabetes without any additional intervention, and the remaining three groups were diabetic rats that received intravenous glibenclamide, and AgNPs at the above-mentioned doses, respectively. As a result of receiving NPs, the levels of hepatic function profile parameters of ALT, ALP, and AST dropped significantly and approached normal levels in both study groups (P < 0.01). The strengths of this study include the use of a green synthesis method, appropriate controls, and significant results (P < 0.01). However, limitations include small sample size, lack of long-term toxicity data, and no mechanistic explanation. While Wistar rats are suitable for this model, further research is needed to explore safety and mechanisms of action in more detail (Berlin Grace et al., 2021). Likewise, in a separate study, the phytofabricated AgNPs were tested to evaluate their effects on hepatic enzymes level in rats at concentrations of 20 and 30 mg kg−1. To climb up the liver parameters in rats, they were injected intraperitoneally with diethylnitrosamine which caused liver cancer. The rats were categorized into six groups, with one group receiving no therapeutic intervention, one receiving the plant extract, one receiving silymarin as a control, and the other two groups receiving NPs orally. In contrast to the group that developed cancer with no therapeutic intervention, the levels of hepatic function profile parameters of ALT, ALP, and AST dropped significantly in these two groups (P < 0.001). The strengths of this study include the use of eco-friendly NPs, a reliable control (silymarin), and significant therapeutic effects. However, limitations include the absence of long-term toxicity data, no mechanistic explanation, and a limited dosage range. Despite promising results, further research is required to explore safety and underlying mechanisms (Singh D. et al., 2018).
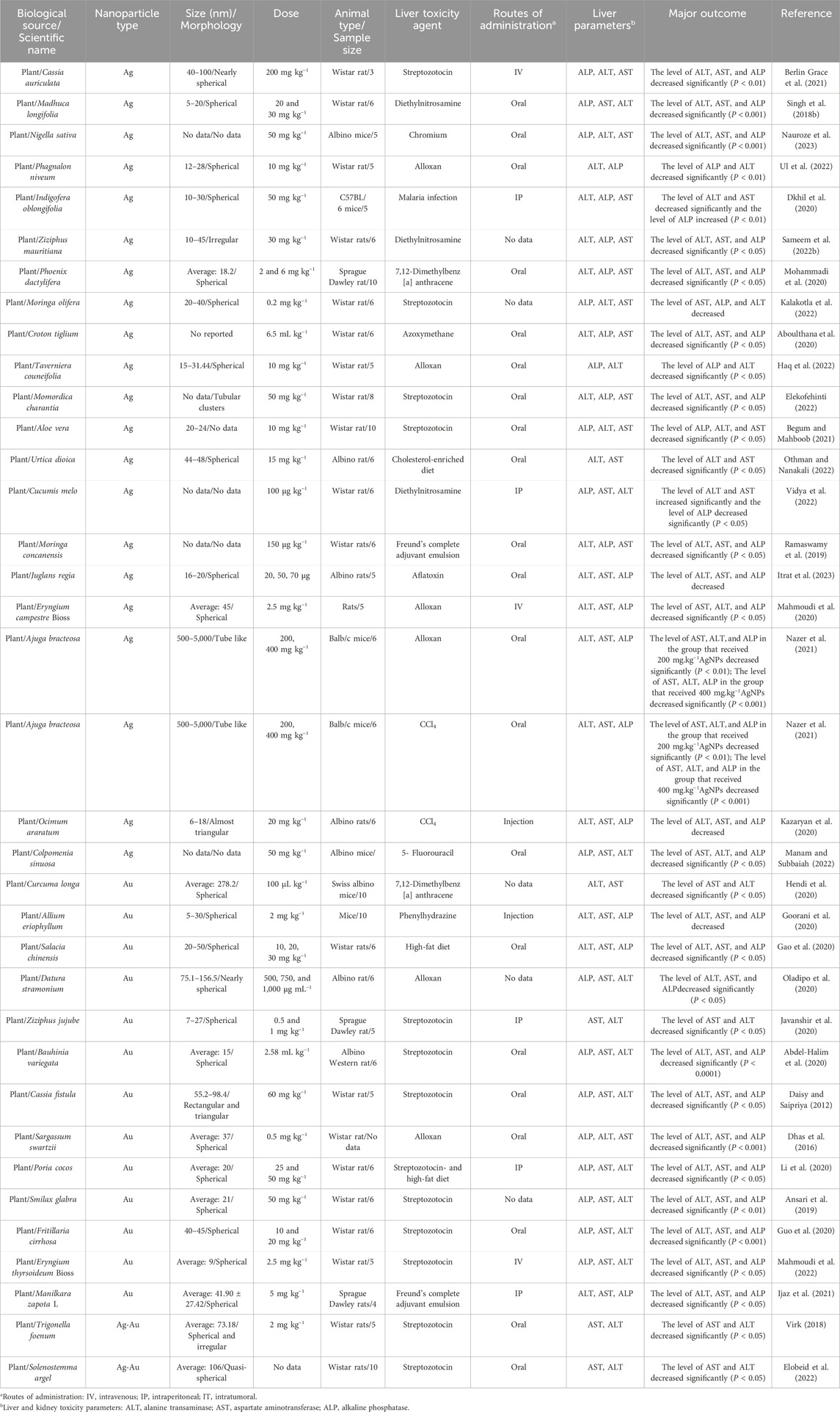
Table 1. The results of protective activity of eco-friendly green fabricated silver/gold nanostructures on hepatic function profile parameters in non-healthy animal models.
Alternatively, in a study, the seed extract of Nigella sativa was used for the phytofabrication of AgNPs to evaluate their effects on hepatic enzymes level in albino mice at a concentration of 50 mg kg−1. In this study, chromium-perturbed mice caused a rise in liver parameters. The mice in this study were categorized into five-member groups, one of which consisted of healthy mice, a separate group of disordered mice that received no therapeutic intervention, and a final group of disordered mice received biosynthesized NPs. This study demonstrated that NPs can substantially reduce the levels of hepatic function profile parameters of ALT, ALP, and AST in comparison to the group that received no therapeutic intervention (P < 0.001). The study highlights the strong hepatoprotective potential of these NPs. However, limitations include a small sample size and lack of long-term toxicity data (Nauroze et al., 2023). Besides, in a study, Momordica charantia leaf extract was used to fabricate AgNPs, and their effects on hepatic enzymes were evaluated in STZ-induced diabetic Wistar rats. The rats were divided into six groups, including untreated controls, those treated with AgNPs (50 mg kg−1), silver nitrate, metformin, and plant extract (100 mg kg−1). The biosynthesized AgNP group showed a significant reduction in liver enzyme levels (ALT, ALP, AST) compared to the untreated diabetic group (P < 0.05) (Figure 2). While the study demonstrated promising hepatoprotective effects, limitations include the need for mechanistic insight and long-term safety assessment (Elekofehinti, 2022).
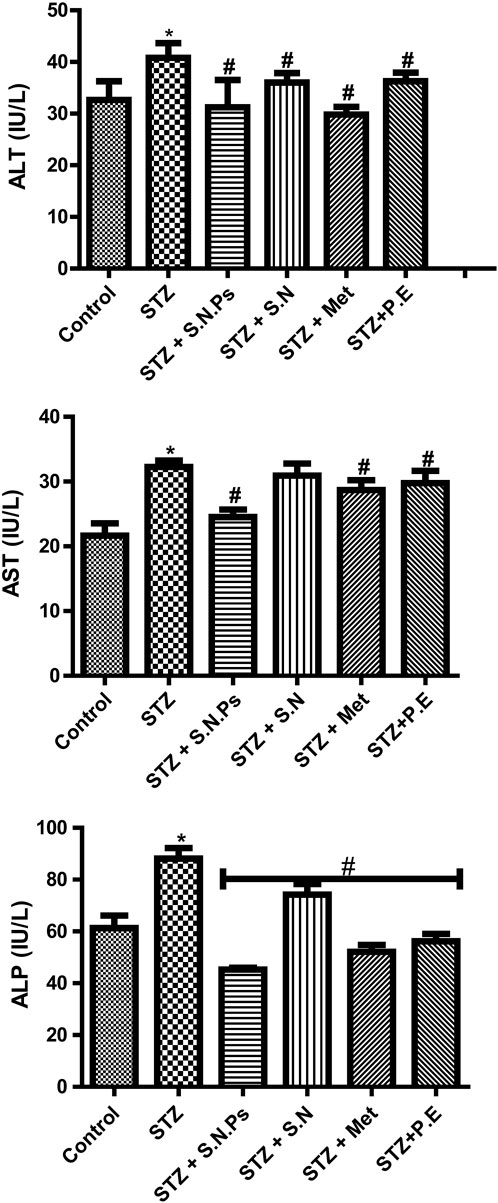
Figure 2. The amount of the levels of hepatic function profile parameters of ALT, ALP, and AST in different groups of Wistar: STZ (streptozotocin), P.E (plant extract), Met (metformin), S.N (silver nitrate), and S.N.Ps (silver nanoparticles) (Elekofehinti, 2022). (Copyright 2022 The Author(s). Published by Springer Nature. Open access article distributed under a Creative Commons Attribution 4.0 International License).
In a separate study, the Phagnalon niveum herbal extract-derived AgNPs were tested to evaluate their effects on hepatic enzyme levels in Wistar rats at a concentration of 10 mg kg−1. In this study, alloxan was used to induce diabetes in Wistar rats, and their liver parameters were altered. In this study, rats were categorized into five-member groups; the first group consisted of healthy rats, the second of diabetic rats that did not receive any therapeutic intervention, and the last three groups of diabetic rats that were administered plant extract, glibenclamide, and NPs orally. The liver parameters (ALP and ALT) were significantly reduced in groups that received biosynthesized AgNPs (P < 0.01) (Figure 3A). While the study shows promising hepatoprotective effects, limitations include a narrow dosage range and lack of long-term safety evaluation or mechanistic understanding. Further studies are needed to confirm these results (Ul et al., 2022).
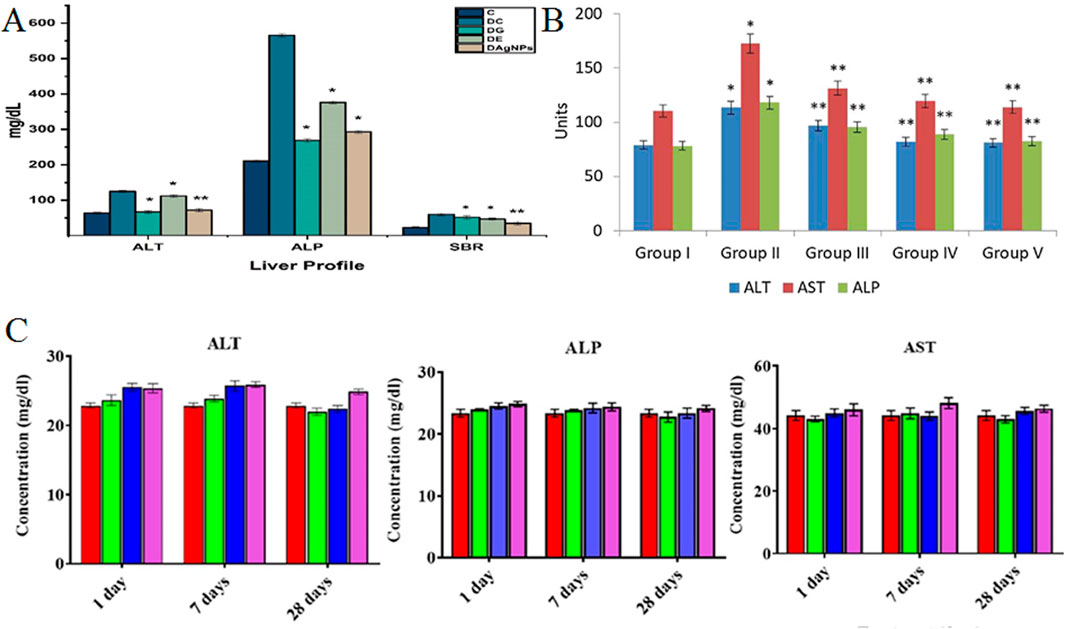
Figure 3. (A) The number of liver parameters in different groups: Group C (control), Group DC (diabetic without treatment), Group DE (diabetic treated with extract), Group DG (diabetic treated with glibenclamide), and Group DAgNPs (diabetic treated with AgNPs). *P ≤ 0.05 and **P ≤ 0.01 were statistically significant (Ul et al., 2022). [Copyright 2022 The Author(s). Licensee MDPI, Basel, Switzerland. Open access article distributed under a Creative Commons Attribution 4.0 International License]; (B) The amount of the levels of hepatic function profile parameters of ALT, ALP, and AST in different groups of Wistar rats: group I (healthy rats), group II (rats with diabetes by STZ), group III (diabetic rats treated with AuNPs in 10 mg kg−1 dose), group IV (diabetic rats treated with AuNPs at a dose of 20 mg kg−1), and group V (diabetic rats treated with glibenclamide). *P ≤ 0.05 and **P ≤ 0.01 are statistically significant. Units = IU/L (Li et al., 2020). (Copyright 2020 The Author(s). Published by Elsevier (B).V. on behalf of King Saud University. Open access article distributed under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)); (C) Level of kidney parameters in different groups of Wistar rats. Red (Control), Green (5 mg kg−1), Blue (10 mg kg−1), and Purple (50 mg kg−1) (Mahmud et al., 2022). [Copyright 2022 The Author(s). Published by American Chemical Society. Open access article distributed under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)].
Alternatively, AgNPs synthesized from Indigofera oblongifolia leaf extract were evaluated at 50 mg kg−1 in C57BL/6 mice infected with Plasmodium chabaudi malaria. Mice were categorized into groups, including healthy controls, healthy mice treated with AgNPs, infected mice without intervention, and infected mice receiving AgNPs intraperitoneally. The results showed a significant decrease in hepatic enzymes AST and ALT, while ALP levels increased and approached normal levels (P < 0.01) in treated mice. While the study highlights the potential of AgNPs in managing liver function during malaria infection, limitations include the lack of long-term follow-up and mechanistic insights into how these NPs exert their effects. Further research is necessary to validate these findings (Dkhil et al., 2020). Moreover, the extract of Z. mauritiana was used for the eco-friendly fabrication of AgNPs with irregular morphology in the range of 10–45 nm. In this study, diethylnitrosamine induced hepatic cancer in these Wistar rats, which was one of the effects of increasing liver parameters. A group of these rats received no therapeutic substance, a group received silymarin as a control, three groups received plant extract at three different doses of 300 mg kg−1 and lowers, and the final group was treated with AgNPs biosynthesized at a concentration of 30 mg kg−1. These NPs were capable of substantially lowering liver parameters such as ALT, AST, and ALP and bringing them closer to the normal range (P < 0.05). While the findings suggest potential therapeutic benefits, limitations include a lack of mechanistic insights and the need for long-term toxicity assessments (Sameem et al., 2022b). Similarly, the Phoenix dactylifera seed extract-derived AgNPs were tested to evaluate their effects on hepatic enzyme levels in Sprague-Dawley rats at the concentrations of 2 and 6 mg kg−1. In this study, rats exposed to 7,12-dimethylbenz [a]anthracene developed mammary gland carcinoma, leading to a rise in liver parameters. In this study, rats were categorized into several groups; the first group consisted of healthy rats, the second group had cancer but was not treated, and the last two groups were afflicted with biosynthesized NPs orally. After receiving both concentrations of NPs, the hepatic parameters ALT, AST, and ALP dropped significantly (P < 0.05). While the study indicates the therapeutic potential of these NPs, limitations include a lack of long-term safety data and mechanistic insights, necessitating further investigation to confirm these findings (Mohammadi et al., 2020). In addition, AgNPs derived from Croton tiglium seed extract were evaluated at 6.5 mL kg−1 in Wistar rats with azoxymethane-induced colon cancer. The groups included untreated controls, those treated with AgNPs, and cancer-affected rats receiving NPs. Results showed a significant reduction in hepatic enzymes (ALT, AST, ALP) in the AgNP-treated group (P < 0.05), indicating potential hepatoprotective effects. The strengths of the study include the eco-friendly synthesis of AgNPs and the demonstration of significant therapeutic efficacy. The limitations involve the small sample size (six rats per group), lack of mechanistic insights into how AgNPs exert their effects, and the absence of long-term safety evaluations. Future prospects should focus on exploring the underlying mechanisms of action, potential side effects, and broader applications of AgNPs in cancer therapy and liver protection (Aboulthana et al., 2020). In a separate study, AgNPs synthesized from Taverniera couneifolia extract were evaluated at a concentration of 10 mg kg−1 in Wistar rats with alloxan-induced diabetes. The rats were divided into groups: healthy controls, untreated diabetic rats, and those treated with glibenclamide, plant extract, or biosynthesized AgNPs. The group receiving AgNPs demonstrated a significant reduction in hepatic enzymes ALT and ALP (P < 0.05), indicating potential therapeutic benefits. The strengths of the study include the use of an eco-friendly synthesis method and the significant therapeutic impact observed in the AgNP group. However, the limitations include a small sample size (five rats per group) and a lack of mechanistic insights into the action of AgNPs (Haq et al., 2022). Alternatively, AgNPs synthesized from Urtica dioica extract were evaluated at a concentration of 15 mg kg−1 in albino mice subjected to a high-cholesterol diet, which induced dyslipidemia and elevated liver parameters. The mice were divided into groups, including healthy controls, untreated high-cholesterol diet mice, and those receiving various treatments: plant aqueous extract, plant ethanol extract, biosynthesized AgNPs, and atorvastatin as a positive control. The results indicated a significant reduction in liver enzymes (AST and ALT) in the AgNP-treated group (P < 0.05), suggesting hepatoprotective effects. The strengths of the study include the comprehensive experimental design with multiple treatment comparisons and the use of a natural extract for NP synthesis. However, the limitations involve the small group size (six mice per group) and the lack of mechanistic explanations for the observed effects (Othman and Nanakali, 2022). Furthermore, AgNPs were sustainably fabricated using Cucumis melo fruit extract and evaluated in Wistar rats grouped into six, including healthy controls and groups with diethylnitrosamine-induced liver cancer. The treatment groups included untreated controls, those receiving the plant extract, rats administered 100 μg kg−1 of biosynthesized AgNPs, and a cyclophosphamide control group. Results indicated that AgNPs significantly improved liver parameters, reducing elevated ALP levels and normalizing ALT and AST levels, suggesting hepatoprotective effects. The strengths of the study include the sustainable synthesis approach and the clear demonstration of therapeutic efficacy in improving liver function. However, the limitations consist of the small group sizes and a lack of detailed mechanistic insights into how AgNPs mediate their protective effects (Vidya et al., 2022). In a separate investigation, AgNPs synthesized from Moringa concanensis aqueous leaf extract were tested at a concentration of 150 μg kg−1 in Wistar rats with arthritis induced by Freund’s complete adjuvant (FCA). The rats were divided into groups, including healthy controls, untreated FCA-induced rats, rats receiving AgNPs, and those treated with diclofenac (10 mg kg−1) as a positive control. The results showed that AgNPs significantly reduced hepatic enzyme levels (ALT, AST, ALP), bringing them closer to normal levels compared to the untreated group (P < 0.05), indicating hepatoprotective effects. The strengths of this study include the use of a natural extract for AgNP synthesis and clear demonstration of therapeutic benefits in a relevant disease model. However, the limitations include the small group size and lack of mechanistic insights into how AgNPs exert their effects. Future prospects should focus on exploring the mechanisms underlying the observed liver protection, evaluating long-term safety, and investigating the potential of AgNPs in treating other inflammatory conditions (Ramaswamy et al., 2019). Likewise, AgNPs derived from Juglans regia leaf extract were evaluated for their effects on hepatic enzyme levels in albino rats exposed to aflatoxin-induced hepatotoxicity. The rats were divided into five groups: healthy controls receiving normal saline, aflatoxin-only controls, and groups receiving aflatoxin with AgNPs at dosages of 20 μg, 50 μg, and 70 μg. The group treated with AgNPs at 50 μg showed the most significant reduction in liver parameters (ALT, AST, ALP), while all treatment groups exhibited lower enzyme levels compared to untreated controls. The strengths of the study include the demonstration of dose-dependent therapeutic effects of AgNPs and the use of a natural extract for NP synthesis. However, the limitations involve the small sample size (five rats per group) and the unclear mechanisms by which AgNPs mediate hepatoprotection (Itrat et al., 2023). Moreover, in a study, the Eryngium campestre-derived AgNPs were tested to evaluate their effects on hepatic enzymes level in mice at a concentration of 2.5 mg kg−1. In this research, mice were categorized into groups of five, each subjected to a distinct treatment regimen. Specifically, one group of mice underwent alloxan injection to induce diabetes, leading to an elevation in liver parameters. Another group of diabetic mice, induced by alloxan, received intravenous administration of AgNPs. This intervention resulted in a significant reduction in ALT, AST, and ALP, indicating a potential hepatoprotective role for AgNPs on the liver (P < 0.05). The strengths of the study include the identification of a natural source for AgNP synthesis and the clear demonstration of its therapeutic potential in a diabetes model. However, the limitations include the small sample size and the lack of mechanistic insights into how AgNPs exert their protective effects. Future prospects should focus on elucidating the underlying mechanisms, exploring optimal dosing strategies, and evaluating long-term safety and efficacy in broader animal models (Mahmoudi et al., 2020). In an interesting study, AgNPs synthesized from Ajuga bracteosa root extract were evaluated at concentrations of 200 and 400 mg kg−1 in Balb/c mice subjected to two different physiological disruptions: alloxan-induced diabetes and CCl4-induced liver cancer. Mice were categorized into groups, including healthy controls, untreated diabetic mice, and diabetic mice treated with AgNPs, as well as healthy controls, untreated cancer-affected mice, and cancer-affected mice treated with AgNPs. Results showed that both concentrations of AgNPs provided significant protective effects on liver function, with the 200 mg kg−1 dosage reducing liver parameters (P < 0.01) and the 400 mg kg−1 dosage achieving an even more substantial reduction (P < 0.001). The strengths of the study include the dual assessment of AgNPs in both diabetic and cancer models, representing their broad therapeutic potential. However, the limitations involve the relatively small group sizes and lack of detailed mechanistic insights into how AgNPs mediate liver protection. Future prospects should focus on elucidating the mechanisms of action, optimizing dosing strategies for maximum efficacy, and conducting long-term safety assessments to explore the potential of AgNPs in clinical applications for liver-related diseases (Nazer et al., 2021). Additionally, Ocimum araratum leaf extract-derived AgNPs were tested to evaluate their effects on hepatic enzyme levels in albino mice at a concentration of 20 mg kg−1. In this research, mice were stratified into four groups, each comprising six individuals. Group A represented healthy rodents administered normal saline, and Group B received only CCl4, resulting in disturbances such as a rise in liver parameters. Group C was subjected to CCl4 exposure but received treatment with an ethanol extract of the plant, while Group D, also exposed to CCl4, underwent treatment with AgNPs. The investigation revealed that liver parameters, including ALT, AST, and ALP, in Group D exhibited a significant reduction compared to Group B and even fell below the levels observed in Group A. The strengths of the study include the dual assessment of AgNPs in both diabetic and cancer models, indicating their broad therapeutic potential. However, the limitations involve the relatively small group sizes and lack of detailed mechanistic insights into how AgNPs mediate liver protection (Kazaryan et al., 2020). In a study, Curcuma longa extract-derived AgNPs were tested to evaluate their effects on hepatic enzyme levels in Swiss albino mice. The experimental design involved three groups, each comprising ten mice. The first group consisted of healthy mice receiving normal saline, while the second group comprised mice induced with breast cancer through 7,12-dimethylbenz [a]anthracene. Contrarily, the mice in the final group developed breast cancer despite being treated with biosynthesized AgNPs. The liver parameters, specifically ALT and AST, were altered in response to breast cancer. The study results indicated that these NPs exhibited a noteworthy protective effect, leading to a significant reduction in liver parameters (P < 0.05). The strengths of the study include the clear demonstration of AgNPs’ hepatoprotective properties in a cancer model and the use of a natural extract for nanoparticle synthesis. However, the limitations involve the small sample size and the absence of detailed mechanistic insights into how AgNPs exert their protective effects. Future prospects should focus on further elucidating the mechanisms of action, optimizing dosages for maximum efficacy, and exploring the therapeutic potential of AgNPs in various cancer models and liver conditions (Hendi et al., 2020). Moreover, Allium eriophyllum leaf extract-derived AuNPs were tested to evaluate their effects on hepatic enzyme levels in mice at a concentration of 2 mg kg−1. This investigation comprised five groups, each consisting of ten mice, where hemolytic anemia was induced using phenylhydrazine, subsequently leading to alterations in liver parameters. The first group encompassed healthy mice, the second group received phenylhydrazine without any additional therapeutic intervention, and the third, fourth, and fifth groups all exhibited anemic conditions and were treated with HAuCl4, plant extract, and AuNPs, respectively. The findings revealed that the administration of 2 mg kg−1 of these NPs exerted a protective influence on the liver, resulting in a significant reduction in hepatic parameters. The strengths of the study include the innovative use of plant-derived NPs to address liver damage in an anemia model and the clear comparison among treatment groups. However, the limitations encompass the small sample size and the need for further exploration of the mechanisms by which AuNPs confer hepatoprotection. Future prospects should involve investigating the optimal dosing, understanding the underlying protective mechanisms, and evaluating the long-term safety and efficacy of AuNPs in various models of liver injury (Goorani et al., 2020). Likewise, Gau et al. documented the synthesis of spherical AuNPs utilizing leaf extract derived from Salacia chinensis with particle sizes ranging from 20 to 50 nm. In this research, a total of 30 Wistar rats were categorized into six groups. The first group consisted solely of healthy rats. Subsequent groups were subjected to a high-fat diet, inducing dyslipidemia. The second group received no therapeutic intervention, while the third, fourth, and fifth groups were treated with AuNPs at concentrations of 10, 20, and 30 mg kg−1, respectively. The levels of hepatic function profile parameters of ALT, AST, and ALP exhibited a significant reduction in all three groups treated with AuNPs, approaching levels close to normal in comparison to the untreated group (P < 0.05). The strengths of this study include the dose-dependent investigation of AuNPs’ hepatoprotective effects and the well-defined control groups, making the results robust. However, the limitations involve the absence of long-term safety data and detailed mechanistic insights into how AuNPs mediate these liver improvements (Gao et al., 2020). Additionally, AuNPs synthesized from Datura stramonium seed extract were tested for their effects on hepatic enzyme levels in albino rats at varying concentrations. The study involved seven groups of six rats each. Group A consisted of healthy rats, while Groups B through F were subjected to alloxan injection to induce diabetes. Group B received no treatment, Group C was treated with plant extract, and Groups D, E, and F were administered AuNPs at concentrations of 500, 750, and 1,000 μg mL−1, respectively. Group G received glibenclamide as a control. Alloxan-induced diabetes led to elevated liver parameters (ALT, AST, ALP), and treatment with AuNPs resulted in a significant reduction in all three liver parameters across the three nanoparticle-treated groups (P < 0.05). The strengths of this study include the use of multiple concentrations of AuNPs, which allows for dose-response analysis, and the inclusion of a pharmaceutical control (glibenclamide). However, the limitations are the relatively short follow-up period and lack of insight into the long-term impact of NP administration on diabetic complications. Future prospects should focus on understanding the mechanisms behind the hepatoprotective effects of AuNPs, exploring their effects in other diabetic complications, and evaluating their long-term safety and efficacy (Oladipo et al., 2020). Moreover, AuNPs synthesized from Bauhinia variegata extract were tested for their impact on hepatic enzyme levels in STZ-induced diabetic albino rats. The study included three groups: healthy rats, diabetic rats without treatment, and diabetic rats treated with AuNPs at a concentration of 2.58 mL kg−1. Diabetes induction via STZ caused an elevation in liver parameters (ALT, AST, and ALP). The group treated with biosynthesized AuNPs exhibited a significant reduction in these liver parameters compared to the untreated diabetic group (P < 0.000). The strengths include the well-defined control and experimental groups and the highly significant findings, which strongly suggest the potential hepatoprotective effect of AuNPs. However, the limitations include the lack of additional dosage variations or long-term studies to assess prolonged effects and possible toxicity. Future prospects could involve exploring the precise mechanisms through which these NPs exert their protective effects, testing multiple dosage ranges, and extending studies to different metabolic disorders to evaluate broader therapeutic applicability (Abdel-Halim et al., 2020). In a separate study, AuNPs synthesized from Cassia fistula stem bark extract were evaluated for their effects on hepatic enzyme levels in STZ-induced diabetic Wistar rats. The rats were divided into five-member groups, including healthy controls, diabetic rats without treatment, and diabetic rats treated with either 60 60 mg kg−1 of extract, 60 mg kg−1 of AuNPs, or insulin (3 IU kg−1). STZ-induced diabetes resulted in elevated ALT, AST, and ALP levels, while the group treated with biosynthesized AuNPs showed a significant reduction in these liver parameters compared to the untreated diabetic group (P < 0.05). The strengths include the use of multiple treatment groups, allowing for comparison between traditional treatments (insulin) and plant-based NPs. The study shows the potential of AuNPs as a viable therapeutic alternative. However, the limitations are the absence of varying AuNP doses, which could provide insight into dose-dependent effects, and the lack of long-term analysis to assess any lasting benefits or adverse effects. Future prospects could include exploring dose optimization and testing AuNPs across different disease models, along with elucidating the underlying mechanisms of their hepatoprotective actions (Daisy and Saipriya, 2012). Alternatively, Poria cocos extract-derived AuNPs were tested to evaluate their effects on hepatic enzyme levels in Wistar rats at the concentrations of 25 and 50 mg kg−1. Rats were grouped into healthy controls, high-fat diet, STZ-induced diabetes, and two groups with both high-fat diet and diabetes treated with AuNPs. Both AuNP doses significantly reduced ALT, AST, and ALP levels compared to the untreated diabetic group (P < 0.05) (Figure 3B). The strengths include mimicking complex disease conditions with diabetes and high-fat diet, and testing different AuNP dosages. However, the limitations involve short treatment duration and no long-term follow-up. Future prospects include exploring molecular pathways, long-term efficacy, and NP safety (Li et al., 2020). Likewise, Fritillaria cirrhosa extract-derived AuNPs were tested for their effects on hepatic enzyme levels in Wistar rats at 10 and 20 mg kg−1. The rats were divided into five groups: healthy controls, untreated diabetic rats, two groups treated with AuNPs at the specified concentrations, and a group treated with glibenclamide. Both AuNP-treated groups exhibited a significant reduction in ALT, AST, and ALP levels compared to untreated diabetic rats, indicating a protective effect on liver function (P < 0.001). The strengths include testing multiple AuNP doses and comparing them with a standard drug. However, the limitations involve the absence of long-term evaluation of the NP’s safety and effects. Future prospects include deeper exploration of molecular mechanisms, prolonged studies, and the potential for clinical trials (Guo et al., 2020). Overall, the above-mentioned laboratory studies supported the protective effects of biosynthesized AgNPs and AuNPs on liver parameters in non-healthy animal models. The studies consistently demonstrate that these NPs exhibit hepatoprotective effects by effectively reducing liver enzyme levels such as ALT, AST, and ALP, suggesting a therapeutic promise in managing conditions like malaria, diabetes, and cancer. However, despite the encouraging results, the research is hindered by several limitations, notably the small sample sizes, the absence of long-term follow-up, and a significant lack of mechanistic insights into the pathways through which these NPs exert their effects. These gaps highlight the necessity for further investigations that not only validate these preliminary findings but also elucidate the molecular mechanisms underlying the observed hepatoprotection. Future research should prioritize rigorous long-term safety assessments, optimal dosing strategies, and the exploration of broader applications of these NPs in clinical settings. This multi-faceted approach will be essential for transitioning from bench to bedside, ultimately enhancing the therapeutic repertoire available for liver-related diseases.
3.2 The influence of eco-friendly green fabricated silver/gold nanostructures on hepatic function profile parameters in healthy animal models
As shown in Table 2, most of the laboratory studies support no statistically significant variances in liver parameters in healthy animal models treated with biosynthesized AgNPs and AuNPs. For instance, N. sativa seed extract-derived AgNPs were tested to evaluate their effects on hepatic enzyme levels in albino mice at a concentration of 50 mg kg−1. The impact of orally administered biosynthesized AgNPs was evaluated in a group of five mice. Although there was an observed rise in levels of hepatic function profile parameters of ALT, ALP, and AST, in these mice, the rise was not deemed statistically significant. The strengths of this study include the use of a specific concentration of AgNPs and the focused examination of hepatic enzyme levels. However, the limitations include the small sample size and the lack of a control group, which hinders the ability to draw definitive conclusions. Future prospects may involve exploring larger sample sizes and varying dosages to better understand the relationship between AgNPs and liver function (Nauroze et al., 2023). Similarly, I. oblongifolia leaf extract-derived AgNPs were administered at a dosage of 50 mg kg−1 to groups of five mice, including a group of healthy mice, to evaluate their effects on hepatic enzyme levels. While the changes in liver parameters, specifically ALT and ALP, were not statistically significant, a notable finding was the significant reduction in AST levels (P < 0.01), indicating a potential hepatoprotective effect of the biosynthesized NPs. The strengths of this study include the focused examination of specific hepatic enzymes and the differentiation between healthy and treated groups. However, the limitations involve the lack of statistically significant findings for some parameters, which could suggest that the dosage or treatment duration needs optimization. Future prospects may involve further studies with varying dosages or additional animal models to clarify the therapeutic potential of AgNPs derived from I. oblongifolia (Dkhil et al., 2020). Besides, C. tiglium seed extract-derived AgNPs were administered at a concentration of 6.5 mL kg−1 to six Wistar rats to assess their effects on hepatic enzyme levels. Compared to the control group receiving only distilled water, the treatment did not result in statistically significant changes in the hepatic function profile parameters, including ALT, AST, and ALP. The strengths of this study include the direct evaluation of the biosynthesized NPs on liver enzymes, providing insights into their potential therapeutic role. However, the limitations arise from the small sample size and the lack of significant results, which may indicate that further research is needed to establish efficacy. Future prospects could involve exploring different dosages or longer treatment periods, as well as investigating additional biochemical markers for a more comprehensive understanding of the hepatic effects of AgNPs derived from C. tiglium (Aboulthana et al., 2020). Moreover, Acorus calamus rhizome extract-derived AgNPs were tested to evaluate their effects on hepatic enzyme levels in Wistar rats at the concentrations of 5 and 10 mg kg−1. In this research, the rats were categorized into three groups, each comprising six individuals. The first group received normal saline, the second group was administered biosynthesized AgNPs at a dosage of 5 mg kg−1, and the third group received biosynthesized AgNPs at a dosage of 10 mg kg−1. After 89 days, there was no statistically significant alteration observed in any of the liver parameters. However, after 29 days, both groups that received AgNPs exhibited a significant climbed up in the levels of AST and ALT compared to the group without NPs, while the levels of ALP did not exhibit a significant change (P < 0.05). The strengths of this study include its long duration, allowing for the observation of both immediate and delayed effects of AgNPs on liver function. However, the limitations include the lack of significant alterations in liver parameters over the longer term and the absence of more detailed histological or biochemical analyses. Future prospects could focus on investigating different time frames for observations, higher dosages, or exploring combinations with other therapeutic agents to better understand the hepatoprotective potential of AgNPs derived from A. calamus (Nakkala et al., 2018). Furthermore, Ashajyothi and Chandrakanth biofabricated core-shell AgNPs using Enterococcus faecalis, yielding particles sized between 9 and 130 nm. Wistar rats were divided into four groups of six: one group received normal saline via intravenous injection, another via intraperitoneal injection, while two groups received biosynthesized AgNPs through the same routes at concentrations of 124–132 μg kg−1 and 97–105 μg kg−1, respectively. Following treatment, liver parameters were assessed, revealing that both groups administered NPs exhibited increased levels of ALT, AST, and ALP compared to the control group. However, these increases were not statistically significant, suggesting that the AgNPs were non-toxic to the rats. The strengths of this study include the innovative biofabrication method and the thorough assessment of hepatic enzyme levels, which provides insight into the safety profile of the AgNPs. However, the limitations may involve the small sample size and the lack of long-term monitoring of potential effects (Ashajyothi and Kelmani Chandrakanth, 2019). In addition, Ginkgo biloba extract-derived AgNPs were tested to evaluate their effects on hepatic enzymes level in Wistar rats at a concentration of 50 mg kg−1. The experimental design included three groups: the first group, serving as the control, received no substances; the second group received intraperitoneal injections of biosynthesized AgNPs at a concentration of 50 mg kg−1, and the third group received both AgNPs and oral administration of G. biloba extract at a concentration of 100 mg kg−1. The findings of this study demonstrated a significant rise in the levels of hepatic function profile parameters of ALT, AST, and ALP at the specified concentration of AgNPs (P < 0.05). The strengths of this study include the comparative approach of combining G. biloba extract with AgNPs, potentially highlighting synergistic effects. However, the limitations may involve the short duration of treatment and the lack of long-term monitoring of liver function. Future prospects could focus on exploring varying dosages, the effects of longer treatment durations, and the underlying mechanisms of AgNPs on liver health (Abd El-Maksoud et al., 2019). Alternatively, the B. variegata extract-AuNPs were tested to evaluate their effects on hepatic enzyme levels in albino Western rats at a concentration of 2.58 mL kg−1. The research focused on examining the impact and toxicity of the biosynthesized NPs on six healthy rats. The administration of orally delivered AuNPs was investigated, and the ensuing effects were systematically recorded. The study outcomes indicated that, at the specified dosage, these NPs did not induce statistically significant alterations in the levels of hepatic function profile parameters of ALT, AST, and ALP. The strengths of this study include its focus on the biosafety of AuNPs, indicating their potential non-toxicity at the tested dosage. However, the limitations may arise from the small sample size and the short duration of the observation. Future prospects could explore the effects of varying dosages, longer administration periods, and potential combinations with other therapeutic agents to better understand the NPs’ effects on liver health (Abdel-Halim et al., 2020). The study on Ficus religiosa leaf extract-derived AgNPs investigated their effects on hepatic enzyme levels in Wistar rats at concentrations of 5 mg kg−1 and 10 mg kg−1. The experimental design involved categorizing the rats into three groups: the first group served as a control, while the second and third groups received oral administrations of AgNPs at the specified concentrations. Findings showed that after 28 days, levels of AST and ALT increased, although ALP levels did not change significantly. However, after a longer observation period of 89 days, the alterations in all three parameters were considered insignificant. The strengths of this study include the systematic approach to evaluating the effects of AgNPs over different durations. However, the limitations involve the lack of detailed mechanistic insights and the relatively small sample size (Nakkala et al., 2017). Additionally, the Syzigyum cymosum leaf extract-derived AgNPs were tested to evaluate their effects on hepatic enzymes level in Wistar rats at the concentration of 5, 10, and 50 mg kg−1. The experimental design included the division of rats into four groups, each comprising four individuals. The first group received normal saline (serving as the control), the second, third, and fourth groups received AgNPs at the above mentioned concentrations. The examination of the rodents in this study indicated that there were no statistically significant alterations in the levels of hepatic function profile parameters of ALT, AST, and ALP in any of the three groups (Figure 3C). The strengths of this study include a clear control group and a systematic approach to evaluating multiple concentrations. However, the limitations involve the small sample size and the lack of observed effects, which may necessitate further investigation with larger groups or alternative dosages. Future prospects could include exploring different exposure durations, investigating potential long-term effects, and examining additional liver parameters to fully understand the hepatotoxicity or hepatoprotective potential of AgNPs derived from S. cymosum (Mahmud et al., 2022). Moreover, C. fistula stem bark extract-derived AuNPs were tested to evaluate their effects on hepatic enzyme levels in Wistar rats at a concentration of 60 mg kg−1. In this research, healthy rats were treated with phytofabricated AuNPs orally. The comparison group involved healthy rodents who received a normal saline solution. The evaluation of the levels of hepatic function profile parameters of ALT, AST, and ALP in the group receiving NPs revealed no statistically significant changes. The strengths of this study include a clear control group and the use of a defined concentration of AuNPs. However, the limitations may involve the short duration of the study and the exclusive focus on healthy rats, which limits insights into the potential effects in diseased states. Future prospects could involve extending the treatment duration, testing on animals with induced liver conditions, and exploring various concentrations to comprehensively assess the hepatotoxicity or hepatoprotective properties of AuNPs derived from C. fistula (Daisy and Saipriya, 2012). Alternatively, the E. thyrsoideum-derived AuNPs were tested to evaluate their effects on hepatic enzyme levels in Wistar rats at a concentration of 2.5 mg kg−1. In this investigation, a group of 5 rats was intravenously administered NPs. The examination of levels of hepatic function profile parameters of ALT, AST, and ALP, did not reveal any statistically significant changes in the group of healthy rats that received normal saline, as evidenced by clinical assessments. The strengths of this study include the intravenous administration method, which can provide more direct absorption of the NPs. However, the limitations may involve the small sample size and the focus on healthy rats, which limits understanding of potential effects in pathological conditions. Future prospects could include expanding the sample size, testing various concentrations, and evaluating the effects in disease models to better understand the safety and efficacy of AuNPs derived from E. thyrsoideum (Mahmoudi et al., 2022). Likewise, the Leptadenia hastata extract-derived AuNPs were tested to evaluate their effects on hepatic enzyme levels in mice. The experimental design involved the categorization of mice into three groups, each comprising ten individuals. One group consisted of healthy mice, while the other two groups were inoculated with a conidia suspension of A. fumigatus. One group of these mice underwent no therapeutic intervention, whereas the other group received a dosage of 0.5 μg g−1 of biosynthesized AuNPs. The fungal infection did not lead to an elevation in liver parameters, and in the third group treated with AuNPs, there were no noteworthy alterations in liver parameters. While the study’s strengths include the use of a well-defined experimental design and the relevance of the mouse model for studying liver function, limitations include the small sample size and the lack of long-term follow-up to assess potential delayed effects (Abdallah and Ali, 2022).
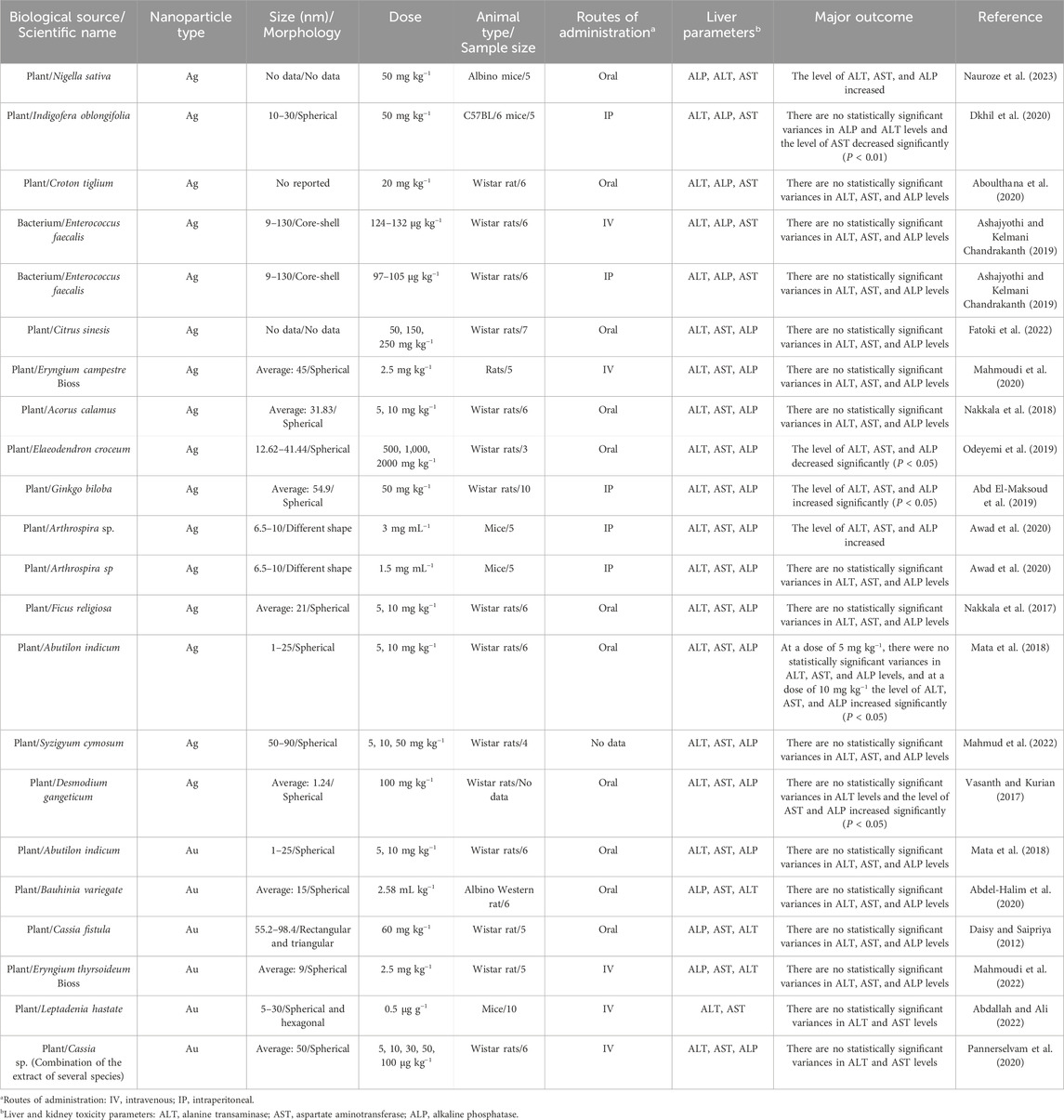
Table 2. The results of the influence of eco-friendly green fabricated silver/gold nanostructures on hepatic function profile parameters in healthy animal models.
Overall, while several studies reported increases in hepatic function parameters, such as ALT, AST, and ALP, these changes were often statistically insignificant, suggesting that the therapeutic potential of these NPs may not be as straightforward as initially posited. The strengths of the investigations included the methodological rigor, such as well-defined dosages and control groups, which provide a clearer picture of the interactions between NPs and liver function. However, the limitations are equally pronounced, particularly concerning the small sample sizes, lack of comprehensive control groups, and limited treatment durations, which severely restrict the generalizability of the results. The potential hepatoprotective effects indicated in some studies, particularly regarding the I. oblongifolia-derived AgNPs, warrant further exploration, yet they also highlight the necessity for optimizing dosages and treatment durations to elicit a clearer therapeutic benefit. Furthermore, the overall trend in the studies suggests a critical need for more extensive and methodologically robust research. Future investigations should not only focus on larger sample sizes but also explore varying dosages, longer treatment periods, and the incorporation of pathological models to fully elucidate the safety and efficacy of these NPs. Without addressing these critical gaps, conclusions drawn from the current body of work remain tentative, representing the complexity of NP interactions within biological systems and the imperative for further research to establish a definitive understanding of their potential roles in liver health.
3.3 Protective activity of eco-friendly green fabricated silver/gold nanostructures on renal function profile parameters in non-healthy animal models
As shown in Table 3, most of the laboratory studies support the protective effects of biosynthesized AgNPs and AuNPs on kidney parameters in non-healthy animal models. For instance, In a study evaluating the renal protective effects of Cassia auriculata flower extract-derived AgNPs on Wistar rats, diabetic rats induced with streptozotocin (STZ) showed elevated creatinine and urea levels, indicating impaired renal function. The rats were divided into groups receiving either AgNPs at concentrations of 50 and 200 mg kg−1 or glibenclamide as a control. Results demonstrated a significant reduction in both urea and creatinine levels in the AgNP-treated groups, approaching normal levels (P < 0.01). The strengths of the study include the use of a well-established diabetic rat model, which effectively simulates human renal impairment, and the promising therapeutic potential of AgNPs. However, the limitations involve the small sample size per group, which may affect the generalizability of the findings, and the lack of long-term follow-up to assess the sustained effects of treatment. Overall, while the findings are encouraging, further studies with larger sample sizes and extended observation periods are necessary to fully understand the efficacy and safety of AgNPs in renal protection (Berlin Grace et al., 2021). In addition, the N. sativa seed extract-derived AgNPs were tested to evaluate their effects on renal function profile parameters in albino mice at a concentration of 50 mg kg−1. In this investigation, the mice were categorized into groups of five individuals, and they were subjected to chromium treatment, leading to physiological disturbances. Subsequently, a subset of these mice exposed to chromium received oral administration of biosynthesized AgNPs, with their clinical outcomes being assessed. The study focused on examining the creatinine level, revealing a significant drop (P < 0.05). The strengths of this study include the use of a specific toxin (chromium) to model renal impairment, which highlights the potential of AgNPs as a therapeutic intervention. However, the limitations include the small group size and the focus solely on creatinine without evaluating additional renal function parameters, which could provide a more comprehensive understanding of AgNPs’ efficacy. Additionally, the short duration of the intervention calls for further studies to confirm long-term effects and safety profiles, emphasizing the need for larger, more diverse animal models to validate findings (Nauroze et al., 2023). Alternatively, the P. niveum herbal extract-derived AgNPs were tested to evaluate their effects on renal function profile parameters in Wistar rats at a concentration of 10 mg kg−1. Following the induction of diabetes through alloxan in this investigation, Wistar rats were randomly categorized into groups of five, each receiving a distinct therapeutic intervention. One group received no medicinal substance, the other group received glibenclamide as a control, a third group received plant extract, and the final group received orally administered biosynthesized AgNPs at a concentration of 10 mg kg−1. These AgNPs exhibited a substantial reduction in blood urea levels, bringing them close to normal values (P < 0.05). The measurement of urea is a pivotal indicator in assessing kidney function (Figure 4A). The study’s strengths include the use of a specific diabetic rat model that closely mimics human diabetes and the clear demonstration of AgNPs’ therapeutic potential in improving renal parameters. However, the limitations include a relatively small group size, which may limit the robustness of the findings, and the focus on urea as the primary measure of renal function, neglecting other important parameters such as creatinine or histological assessments. Further research with larger sample sizes and comprehensive evaluations of renal function is essential to validate these promising results and explore the long-term effects of AgNPs in diabetic nephropathy (Ul et al., 2022).
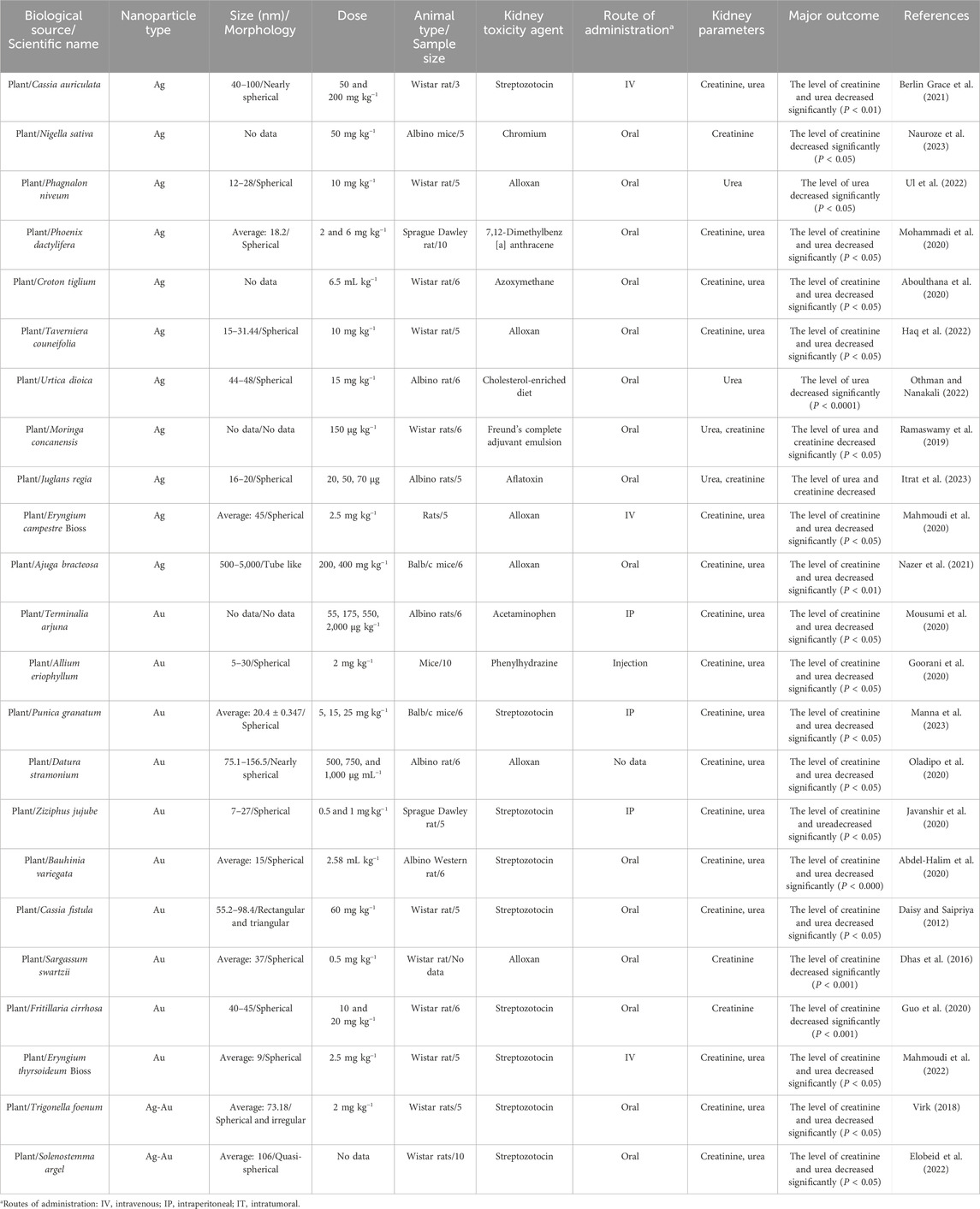
Table 3. The results of the protective activity of eco-friendly green fabricated silver/gold nanostructures on renal function profile parameters in non-healthy animal models.
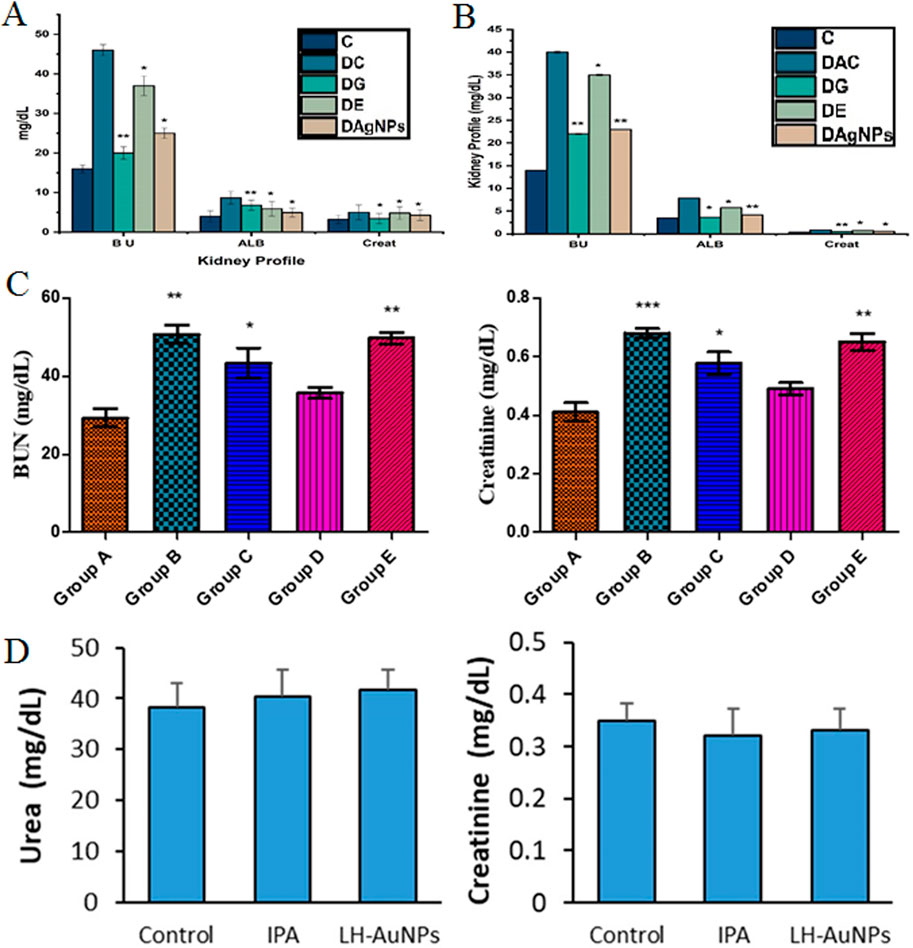
Figure 4. (A) The level of kidney parameters (creatinine, blood urine, and albumin) in different groups of Wistar rats: Group C (Healthy rat), Group DC (Diabetic without any intervention), Group DG (Diabetic rats treated with glibenclamide), Group DE (Diabetic treated with plant extract), and Group DAgNPs (Diabetic treated with AgNPs). *P ≤ 0.05 and **P ≤ 0.01 are statistically significant (Ul et al., 2022). [Copyright 2022 The Author(s). Licensee MDPI, Basel, Switzerland. Open access article distributed under a Creative Commons Attribution 4.0 International License]; (B) The amount of albumin, blood urea, and creatinine in various groups of Wistar rats: Group C (Healthy rat), Group DC (Diabetic without any intervention), Group DG (Diabetic rats treated with glibenclamide), Group DE (Diabetic treated with plant extract), and Group DAgNPs (Diabetic treated with AgNPs). *P < 0.05 and **P < 0.01 are statistically significant (Haq et al., 2022). [Copyright 2022 The Author(s). Licensee MDPI, Basel, Switzerland. Open access article distributed under a Creative Commons Attribution 4.0 International License]; (C) Blood urea and creatinine (two important kidney factors) in different groups: Group A (Healthy rat), Group B (Infected with aflatoxin without any treatment), Group C (Infected with aflatoxin treated with AgNPs at a dose of 20 μg), Group D (Infected with aflatoxin treated with AgNPs at a dose of 50 μg), and Group E (Infected with aflatoxin treated with AgNPs at a dose of 70 μg) *P < 0.05, **P < 0.01 and ***P < 0.001 are statistically significant (Itrat et al., 2023). [Copyright 2023 The Author(s). Licensee MDPI, Basel, Switzerland. Open access article distributed under a Creative Commons Attribution 4.0 International License]; (D) Evaluation of kidney parameters (urea and creatinine) in different groups of IPA mice: Invasive pulmonary aspergillosis (IPA), L. hastata extract-derived AuNPs (LH-AuNPs) (Abdallah and Ali, 2022). (Copyright 2022 The Author(s). Licensee MDPI, Basel, Switzerland. Open access article distributed under a Creative Commons Attribution 4.0 International License).
Furthermore, the P. dactylifera seed extract-derived AgNPs were tested to evaluate their effects on renal function profile parameters in Sprague-Dawley rats at the concentrations of 2 and 6 mg kg−1. In this investigation, the rats, induced with 7,12-Dimethylbenz [a] anthracene, developed mammary gland carcinogenesis, leading to the subsequent division of these rats into several groups. One group of these rats remained healthy without any therapeutic intervention, the other group was ill without the administration of a therapeutic substance, two groups of ailing rats were injected with plant extracts at different concentrations, and the remaining two groups of diseased rats were treated with AgNPs at the above-mentioned concentrations. Consequently, the renal function profile parameters level of creatinine and urea in the blood of rodents treated with AgNPs exhibited a significant drop in both groups (P < 0.05). The strengths of the study include its innovative approach to assessing AgNPs in a cancer model, highlighting their potential dual role in renal protection and cancer treatment. However, the limitations include the small number of rats per group, which could affect the statistical power, and the lack of long-term assessment of renal function post-treatment (Mohammadi et al., 2020). Alternatively, in a study investigating the renal protective effects of C. tiglium seed extract-derived AgNPs on Wistar rats, the rats were induced with colon cancer using azoxymethane, resulting in elevated renal parameters such as creatinine and blood urea. The rats were divided into groups of six, with a subgroup receiving oral administration of AgNPs at a concentration of 6.5 mL kg−1. The results demonstrated that AgNPs significantly reduced blood levels of creatinine and urea (P < 0.05), indicating improved renal function despite the presence of cancer. The strengths of this study include its relevance to cancer models, which highlight the potential for AgNPs to provide renal protection in compromised physiological conditions. However, the limitations consist of the small sample size per group, which may limit the statistical robustness, and a narrow focus on only creatinine and urea as indicators of renal function, potentially overlooking other critical parameters. Additionally, long-term effects of AgNPs on renal health were not evaluated. Further research involving larger sample sizes and a comprehensive analysis of various renal markers is essential to validate these promising findings and assess the long-term efficacy of AgNPs in cancer-induced renal impairment (Aboulthana et al., 2020). Similarly, the T. couneifolia extract-derived AgNPs were tested to evaluate their effects on renal function profile parameters in Wistar rats at a concentration of 10 mg kg−1. Alloxan induction was employed to induce diabetes in rats in this study, causing alterations in kidney parameters, including creatinine and blood urea. The rats were categorized into groups of five individuals each. One group comprised healthy rats, while the other group consisted of diabetic rats without any treatment. Glibenclamide-treated diabetic rats constituted the other group, diabetic rats treated with plant extract formed the subsequent group, and the final group comprised diabetic rats treated with AgNPs at the above-mentioned concentration. The administration involved an oral concentration of 10 mg kg−1. Subsequently, both creatinine and blood urea levels witnessed a significant reduction in these rodents (P < 0.05) (Figure 4B). The strengths of the study lie in its well-structured design, utilizing a diabetic rat model that is relevant to human diabetes and effectively demonstrating the potential of AgNPs for renal protection. However, the limitations include the small sample size, which could affect the robustness of statistical analysis, and the absence of long-term follow-up to evaluate sustained effects (Haq et al., 2022). In a study evaluating the renal protective effects of U. dioica extract-derived AgNPs on albino mice, the subjects were fed a cholesterol-rich diet, resulting in altered urea levels. The experimental design involved groups of six mice each: one group received only the high-cholesterol diet, while others received the diet combined with either an aqueous extract, an ethanolic extract, AgNPs at a concentration of 15 mg kg−1, or atorvastatin as a therapeutic control. The results demonstrated that AgNPs significantly reduced blood urea levels (P < 0.0001), indicating effective renal protection against the adverse effects of a high-cholesterol diet. The study’s strengths include the clear focus on a dietary model relevant to metabolic disorders, representing the therapeutic potential of AgNPs in mitigating diet-induced renal impairment. However, the limitations include the small sample size per group, which may influence the generalizability of the findings, and the lack of assessment of other important renal function markers or long-term effects of treatment. Further research with larger cohorts and comprehensive evaluations of renal parameters is essential to fully elucidate the efficacy and safety of AgNPs in managing renal dysfunction associated with high cholesterol intake (Othman and Nanakali, 2022). Additionally, in a study examining the renal protective effects of M. concanensis aqueous leaf extract-derived AgNPs on Wistar rats, arthritis was induced using FCA, leading to complications in kidney function. The rats were divided into groups of six: one group comprised healthy controls, another consisted of arthritic rats without treatment, a third group received AgNPs at a dosage of 150 μg kg−1, and the final group was treated with diclofenac. The findings indicated that AgNPs significantly reduced blood urea and creatinine levels, restoring them to near-normal levels (P < 0.05), suggesting effective renal protection in the context of arthritis. The strengths of this study include the exploration of AgNPs in a relevant model of inflammatory disease, highlighting their potential therapeutic application in managing renal complications associated with arthritis. However, the limitations include the small sample size, which may restrict the robustness of the conclusions. Further studies with larger cohorts and more comprehensive renal function analyses are necessary to validate these promising results and assess the long-term safety and efficacy of AgNPs in treating arthritis-related renal dysfunction (Ramaswamy et al., 2019). In a separate study, the J. regia leaf extract-derived AgNPs were tested to evaluate their effects on renal function profile parameters in albino rats at different concentrations. The research involved infecting rats with aflatoxin, a toxin known to induce symbiotic complications in the liver and kidneys. The rodents were systematically organized into three groups, each comprising five members, and were subjected to aflatoxin along with AgNPs at concentrations of 20, 50, and 70 μg, respectively. A fourth group solely received aflatoxin without any NP intervention. Intriguingly, in the group that orally received AgNPs at a concentration of 50 μg, blood urea and creatinine levels exhibited a substantial drop, aligning closely with normal levels (Figure 4C). The study’s strengths lie in its focus on a clinically relevant model of toxin-induced renal injury, representing the potential of AgNPs as a therapeutic intervention. However, the limitations include the small group size, which may limit statistical power, and the absence of long-term follow-up to evaluate the sustained effects of treatment (Itrat et al., 2023). Moreover, the E. campestre-derived AgNPs were tested to evaluate their effects on renal function profile parameters in mice at a concentration of 2.5 mg kg−1. In this investigation, diabetic rats induced by alloxan at a concentration of 150 mg kg−1 underwent administration of both biosynthesized NPs and chemically synthesized NPs. The mice were systematically grouped into sets of five; one group comprised diabetic rats receiving no treatment, the other group consisted of healthy mice, and the remaining two groups consisted of diabetic mice receiving intravenous concentrations of 2.5 mg kg−1 of AgNPs, distinguished by their synthesis method (biosynthesized or chemically synthesized). Notably, the biosynthesized AgNPs demonstrated a substantial capability to reduce blood creatinine and blood urea levels, effectively restoring them to levels closely resembling the norm. The strengths of this study include the comparative analysis of biosynthesized versus chemically synthesized AgNPs, which provides insights into the potential advantages of natural synthesis methods. However, the limitations include the small group size, which may limit the statistical significance of the findings, and the short duration of treatment, necessitating further studies to evaluate long-term effects and safety (Mahmoudi et al., 2020). Likewise, in a study assessing the renal protective effects of A. bracteosa root extract-derived AgNPs on Balb/c mice, diabetes was induced using alloxan injections. The mice were divided into groups of six, including healthy controls, untreated diabetic mice, two groups receiving plant extract at concentrations of 200 and 400 mg kg−1, and two groups treated with AgNPs at the same concentrations, alongside a final group treated with glibenclamide. Results indicated that both AgNP-treated groups showed a significant reduction in creatinine and blood urea levels, approaching those of the healthy mice (P < 0.01), suggesting effective renal function restoration. The strengths of this study include its comprehensive design, allowing for comparisons between various treatment modalities and the use of a well-established diabetic model relevant to human diabetes. However, the limitations include the small sample size, which may affect the statistical reliability of the results, and the absence of long-term follow-up to assess the durability of the therapeutic effects (Nazer et al., 2021). In addition, the Terminalia arjuna bark extract-derived AuNPs were tested to evaluate their effects on renal function profile parameters in albino mice at different concentrations. In this study, the kidney toxicity induced by acetaminophen led to elevated levels of creatinine and urea in mice. These mice were systematically categorized into groups of six, each following a unique treatment regimen. The initial group comprised healthy rodents, the second group exclusively exhibited renal toxicity induced by acetaminophen, without any therapeutic intervention, and the subsequent groups were administered concentrations of 55, 175, 550, and 2,000 μg kg−1, respectively. The study ascertained a significant drop in the levels of both renal function profile parameters in all groups treated with biosynthesized NPs compared to the group without any therapeutic substance (P < 0.05), with the most substantial reduction observed in the group treated with AuNPs at a concentration of 175 μg kg−1. The strengths of this study include its focus on acetaminophen-induced renal toxicity, a common clinical model of drug-induced nephrotoxicity, and the use of multiple AuNP concentrations, allowing for a dose-dependent analysis of efficacy. However, the limitations include the small sample size, which may affect the statistical power, and the limited focus on only two renal parameters without considering other markers of kidney injury or histopathological evaluations. Additionally, the lack of long-term data on the effects of AuNPs necessitates further research with larger cohorts and more comprehensive assessments to validate the findings and ensure the safety and efficacy of AuNPs in treating drug-induced renal damage (Mousumi et al., 2020). In a separate study, A. eriophyllum leaf extract-derived AuNPs were tested to evaluate their effects on renal function profile parameters in mice at a concentration of 2 mg kg−1. This study induced hemolytic anemia in mice using phenylhydrazine, followed by their categorization into distinct groups. Each group consisted of ten mice, where the first group comprised healthy mice, the second group included anemic mice without any therapeutic intervention, and the last three groups involved anemic mice treated with plant extract, HAuCl4, and biosynthesized AuNPs at a dosage of 2 mg kg−1. Mice treated with NPs exhibited a significant drop in creatinine and blood urea levels compared to those that received no treatment. The study’s strengths include its focus on a model of hemolytic anemia, highlighting the potential renal complications of this condition and demonstrating the efficacy of AuNPs in mitigating kidney dysfunction. However, limitations include the relatively small sample size, without conducting long-term evaluations. Further research with larger groups and more comprehensive renal function analyses would be necessary to fully validate these findings and assess the broader therapeutic potential of AuNPs in managing anemia-related kidney impairment (Goorani et al., 2020). Similarly, the Punica granatum aqueous peel extract-derived AuNPs were tested to evaluate their effects on renal function profile parameters in Balb/c mice at the concentrations of 5, 15, and 25 mg kg−1. In this research, STZ was employed to induce diabetes in mice. These mice were systematically categorized into groups, with the initial group comprising healthy mice, the second group encompassing diabetic mice without any therapeutic intervention, and the latter three groups involving diabetic mice treated with AuNPs at the above-mentioned concentrations. The administration of a 5 mg kg−1 concentration did not yield a significant impact on creatinine and blood urea levels, while concentrations of 15 and 25 mg kg−1 markedly dropped the concentrations of these two parameters (P < 0.05). The study’s strengths include its use of a well-established diabetic model relevant to human diabetes and the exploration of different AuNP dosages, allowing for dose-dependent insights into their therapeutic effects. However, the limitations include the lack of long-term observation and a narrow focus on only two renal function markers, which may not fully capture the extent of kidney protection. Additionally, the small sample sizes could limit the statistical robustness of the findings. Further studies with larger groups and a broader set of renal function evaluations are necessary to confirm these results and explore the full therapeutic potential of AuNPs in managing diabetes-induced renal dysfunction (Manna et al., 2023). Besides, the D. stramonium seed extract-derived AuNPs were tested to evaluate their effects on renal function profile parameters in albino rats at the concentrations of 500, 750, and 1,000 μg mL−1. The investigation focused on exploring the anti-diabetic effects of the biosynthesized alloxan NPs. The rats were categorized into six groups, with the initial group comprising healthy rats, the second group involving diabetic rats without any therapeutic intervention, the third group consisting of diabetic rats treated with herbal extract, and the subsequent three groups encompassing diabetic rats treated with AuNPs. The study unveiled that, across all three AuNP doses, a significant drop in creatinine and blood urea levels was observed indicating a kidney-protective influence (P < 0.05). The strengths of the study include the use of multiple AuNP concentrations, which allows for a dose-response analysis, and the focus on alloxan-induced diabetes, a model closely related to human diabetic nephropathy. However, the study’s limitations include the small sample size, which may weaken the statistical significance. Moreover, the absence of long-term follow-up or histological kidney evaluations leaves the broader impact of AuNPs unaddressed. Future research with larger sample sizes and more comprehensive renal function assessments is required to confirm these findings and better understand the therapeutic potential of AuNPs in managing diabetes-related kidney dysfunction (Oladipo et al., 2020). Furthermore, Ziziphus jujube fruit extract-derived AuNPs were tested to evaluate their effects on renal function profile parameters in Sprague-Dawley rats at the concentrations of 0.5 and 1 mg kg−1. STZ was employed to induce diabetes in rats, leading to elevated blood creatinine and urea levels. The rats were systematically organized into groups of five. The initial group comprised healthy rats, the second group encompassed diabetic rats induced by STZ without any therapeutic intervention, and the final two groups received AuNPs. In both AuNP-administered groups, a significant drop in the levels of both parameters was recorded (P < 0.05). The study’s strengths include its focus on diabetes-induced nephropathy, a model relevant to human kidney disease, and the investigation of two different AuNP doses, allowing for a comparison of efficacy at varying concentrations. However, the small group size may reduce the depth of the findings. The absence of long-term follow-up and additional renal function indicators further limits the scope of the study. Future research with larger sample sizes, extended treatment durations, and a more comprehensive assessment of kidney health is needed to validate the therapeutic potential of AuNPs in treating diabetic kidney damage (Javanshir et al., 2020). In a separate study, the C. fistula stem bark extract-derived AuNPs were tested to evaluate their effects on renal function profile parameters in Wistar rats at a concentration of 60 mg kg−1. The investigation focused on evaluating the renal protective effects of the biosynthesized AuNPs in diabetic rats induced by STZ. Diabetic conditions often cause elevated levels of creatinine and blood urea. The rats were systematically organized into 5-member groups, comprising healthy rats in the first group, diabetic rats receiving only distilled water in the second group, and the last three groups consisting of diabetic rats treated with plant extract, AuNPs, and insulin, respectively. In this study, the AuNPs were orally administered at a concentration of 60 mg kg−1, resulting in a noteworthy fall in creatinine and blood urea levels compared to the untreated group (P < 0.05). The study’s strengths include the comparison of multiple treatment strategies—AuNPs, plant extract, and insulin—offering a broader context for evaluating the effectiveness of AuNPs. Additionally, the use of STZ-induced diabetes in Wistar rats provides a relevant model for studying diabetic nephropathy. However, the lack of long-term observations and a broader renal function profile limits the understanding of AuNPs’ full protective potential. Further research is needed with larger groups, extended treatment periods, and more comprehensive renal assessments to confirm these findings and optimize therapeutic strategies for managing diabetes-related kidney dysfunction (Daisy and Saipriya, 2012). Similarly, in a study assessing the renal protective effects of Sargassum swartzii-derived AuNPs on Wistar rats, diabetes was induced using alloxan, which elevated creatinine levels, signaling kidney dysfunction. The experimental groups included healthy rats given distilled water, untreated diabetic rats, diabetic rats treated with glibenclamide at 0.5 mg kg−1, and diabetic rats treated with AuNPs at 0.5 mg kg−1. The results demonstrated that the AuNP-treated group exhibited a significant reduction in creatinine levels (P < 0.001), approaching normal values, indicating an improvement in renal function. The study’s strengths lie in the use of a well-established diabetic model and the comparison with glibenclamide, a standard anti-diabetic drug, providing a useful benchmark for evaluating the efficacy of AuNPs. However, the focus solely on creatinine as a marker of renal function is a limitation, as other renal parameters such as urea or electrolyte levels were not assessed, potentially overlooking other aspects of kidney health. The short-term nature of the study and the relatively small group sizes may also limit the generalizability of the results. Further studies with larger sample sizes, longer observation periods, and a more comprehensive set of kidney function markers are needed to fully understand the therapeutic potential of AuNPs in managing diabetic kidney damage (Dhas et al., 2016). In summary, the use of biosynthesized AgNPs and AuNPs derived from various bioresources has demonstrated significant potential in preserving kidney function in several disease-induced animal models, including cancer, diabetes, arthritis, and chemically-induced nephrotoxicity. Across multiple studies, these NPs have been shown to notably reduce elevated levels of key renal function markers, such as creatinine and urea, suggesting their ability to alleviate kidney damage and restore normal physiological function. Moreover, these findings highlight the NPs’ promising role in mitigating renal dysfunction caused by various pathological conditions, ranging from diabetic nephropathy to toxin-induced kidney injury. Despite their potential, many of these studies are limited by small sample sizes, short observation periods, and a narrow focus on just a few renal biomarkers, leaving several aspects of long-term kidney health unexplored. Consequently, while the results provide a strong foundation for the kidney-protective effects of biosynthesized NPs, more extensive and prolonged investigations are needed to comprehensively validate their therapeutic efficacy, safety, and broader impact on overall kidney health.
3.4 The influence of eco-friendly green fabricated silver/gold nanostructures on renal function profile parameters in healthy animal models
As shown in Table 4, most of the laboratory studies support no statistically significant variances in kidney parameters in healthy animal models treated with biosynthesized AgNPs and AuNPs. For instance, a study on Citrus sinensis seed extract-derived AgNPs tested doses of 50, 150, and 250 mg kg−1 in Wistar rats, showing a slight reduction in creatinine and urea at 50 mg kg−1, with no significant effect at 250 mg kg−1. The strengths of this study include the use of varying doses for comparative analysis and a clear focus on renal function. However, the limitations involve a small sample size, and no long-term follow-up, which restricts conclusions about efficacy and safety across different dosages. Hence, further research with extended parameters is required (Fatoki et al., 2022). Alternatively, a study on Elaeodendron croceum extract-derived AgNPs tested doses of 500–2000 mg kg−1 in Wistar rats, with 27 rats divided into seven groups, including controls and groups receiving plant extract or AgNPs. The AgNPs reduced renal parameters, including creatinine and urea, below normal levels at all concentrations. The strengths of the study include the use of multiple dosages and comprehensive group categorization for comparative analysis. However, the limitations include the absence of long-term evaluation (Odeyemi et al., 2019). In a study on Theobroma cacao L. pod husk extract-derived AgNPs, albino rats were divided into three groups: a control group receiving distilled water and two groups receiving AgNPs at 50 and 100 mg kg−1. Both doses resulted in increased creatinine levels, indicating reduced glomerular clearance and potential kidney impairment. The strengths of the study include the controlled experimental design and the examination of two different dosages, allowing for initial insights into the dose-response relationship. However, the limitations include a small sample size of four rats per group, which may limit the statistical power and generalizability of the findings. Additionally, the lack of long-term follow-up raises concerns about the chronic effects of AgNPs on kidney function (Salau et al., 2022). Likewise, the E. campestre-derived AgNPs were tested to evaluate their effects on renal function profile parameters in mice at a concentration of 2.5 mg kg−1. The study investigated the impact of the biosynthesized AgNPs on a group of seven Wistar rats, specifically examining creatinine and blood urea levels. The findings indicated that these biosynthesized NPs had no observable effect on these two parameters. The strengths of the study include a focused investigation of specific biomarkers related to kidney function, contributing to understanding the potential renal safety of AgNPs. However, the limitations consist of the small sample size of only seven rats, which restricts the reliability and generalizability of the findings. Additionally, the absence of observed effects does not provide insights into the long-term safety or potential benefits of AgNPs on renal health. Future research should involve larger populations and consider a broader range of renal function indicators and longer evaluation periods to enhance the understanding of AgNPs’ impact on kidney function (Mahmoudi et al., 2020). In contrast, in a study evaluating the effects of Desmodium gangeticum extract-derived AgNPs at a concentration of 100 mg kg−1, Wistar rats were divided into three groups: a control group receiving distilled water and two groups receiving either biosynthesized or chemically synthesized AgNPs. The results indicated that the administration of biosynthesized AgNPs led to increased creatinine and blood urea levels, suggesting a potential risk of renal injury. The strengths of this study include a comparative analysis between biosynthesized and chemically synthesized AgNPs, providing insights into their differential effects on kidney function. The study’s design allows for a clearer understanding of how the synthesis method may influence the biocompatibility of the NPs. However, the limitations involve a relatively small sample size, which may undermine the robustness of the findings, and the short duration of the study, which does not capture long-term renal effects (Vasanth and Kurian, 2017). In addition, in a study assessing the effects of L. hastata extract-derived AuNPs on renal function, invasive pulmonary aspergillosis was induced in mice through injection of A. fumigatus conidia. The research compared the blood levels of creatinine and urea between infected mice and healthy controls, finding no significant differences. Additionally, intravenous administration of biosynthesized AuNPs at a concentration of 0.5 μg g−1 to groups of ten mice did not result in changes in renal parameters. The strengths of this study include its investigation of the impact of AuNPs in a context of infectious disease, providing valuable insights into their safety profile under pathological conditions. The absence of significant renal parameter changes suggests a potential biocompatibility of these NPs in this model. However, the limitations arise from the small sample size, which limits statistical power, and the focus on a specific infection model that may not represent other pathological states. Moreover, the lack of observed effects does not exclude the possibility of longer-term toxicity or other subtle renal alterations that were not measured. Future studies should explore varying dosages, treatment durations, and broader pathological contexts to comprehensively evaluate the safety and efficacy of AuNPs in renal health (Abdallah and Ali, 2022). In a study evaluating the effects of C. fistula stem bark extract-derived AuNPs on renal function, Wistar rats were administered AuNPs at a concentration of 60 mg kg−1. The experimental design included three groups: one receiving distilled water, another receiving the plant extract, and the third receiving AuNPs, with each group consisting of five rats. The primary focus was on measuring kidney parameters, specifically blood urea and creatinine levels. Results indicated no significant differences in these parameters between the AuNP-treated group and the distilled water control group, suggesting that the AuNPs did not adversely affect renal function in this model. The strengths of this study include its clear experimental design and the use of multiple treatment groups, allowing for comparative analysis. The focus on key renal function indicators enhances the relevance of the findings. However, the limitations include the small sample size, which may limit the ability to detect subtle effects, and the short duration of the study, which does not account for potential long-term effects of AuNPs on renal health (Daisy and Saipriya, 2012). Alternatively, the alga S. swartzii-derived AuNPs were tested to evaluate their effects on renal function profile parameters in Wistar rats at a concentration of 0.5 mg kg−1. The experimental design included two groups: one group of healthy rats received distilled water as a control, while the other group received the AuNPs. The primary outcome measured was creatinine levels, a key indicator of renal function. Results indicated that the biosynthesized AuNPs had no observable effect on creatinine levels, demonstrating statistical significance (P < 0.001). The strengths of this study include the use of a clear control group and the focus on a relevant biomarker for renal health, enhancing the credibility of the findings. The specific concentration of AuNPs tested also provides a targeted approach to understanding their effects. However, the limitations include a lack of information on long-term effects and potential cumulative toxicity of AuNPs, as the study primarily focused on short-term outcomes. Future studies should incorporate a broader range of renal function tests and explore various dosages and treatment durations to better understand the therapeutic potential and safety profile of AuNPs (Dhas et al., 2016). Additionally, in a study assessing the effects of Cassia sp. extract-derived AuNPs on renal function, Wistar rats were administered varying concentrations of AuNPs (5–100 μg kg−1). The rats were divided into groups of six, each receiving a different concentration of AuNPs via intravenous injection. Following treatment, the impact on kidney parameters, particularly creatinine and blood urea levels, was evaluated. The findings revealed that there were no significant differences in kidney parameters among the groups receiving AuNPs compared to the healthy control group, suggesting that the administered concentrations did not adversely affect renal function. The strengths of this study include a comprehensive evaluation across multiple concentrations, which helps to determine a dose-response relationship, and the use of a control group for comparison, enhancing the validity of the results. However, the limitations include a relatively small group size, which may reduce the statistical power of the findings. The study also does not provide long-term effects or potential cumulative toxicity associated with the repeated use of AuNPs. Furthermore, additional renal function indicators could be assessed for a more thorough evaluation. Future research should explore broader dosing regimens and long-term exposure effects to better understand the therapeutic potential and safety of AuNPs derived from Cassia sp. extracts (Pannerselvam et al., 2020). Overall, most of the above mentioned laboratory studies supported no statistically significant variances in kidney parameters in healthy animal models treated with biosynthesized AgNPs and AuNPs. While several studies have shown promising results in reducing markers of kidney damage, such as creatinine and blood urea levels, limitations like small sample sizes, short observation periods, and a narrow focus on a few biomarkers highlight the need for further investigation. Future research should focus on larger, more diverse populations and longer treatment durations to comprehensively assess the long-term safety and efficacy of these NPs. Additionally, exploring a broader range of renal function parameters will provide a more complete understanding of their impact on kidney health. Investigating the mechanisms of action and biodistribution of these NPs will be crucial for optimizing their therapeutic applications and ensuring their safe use in clinical settings.
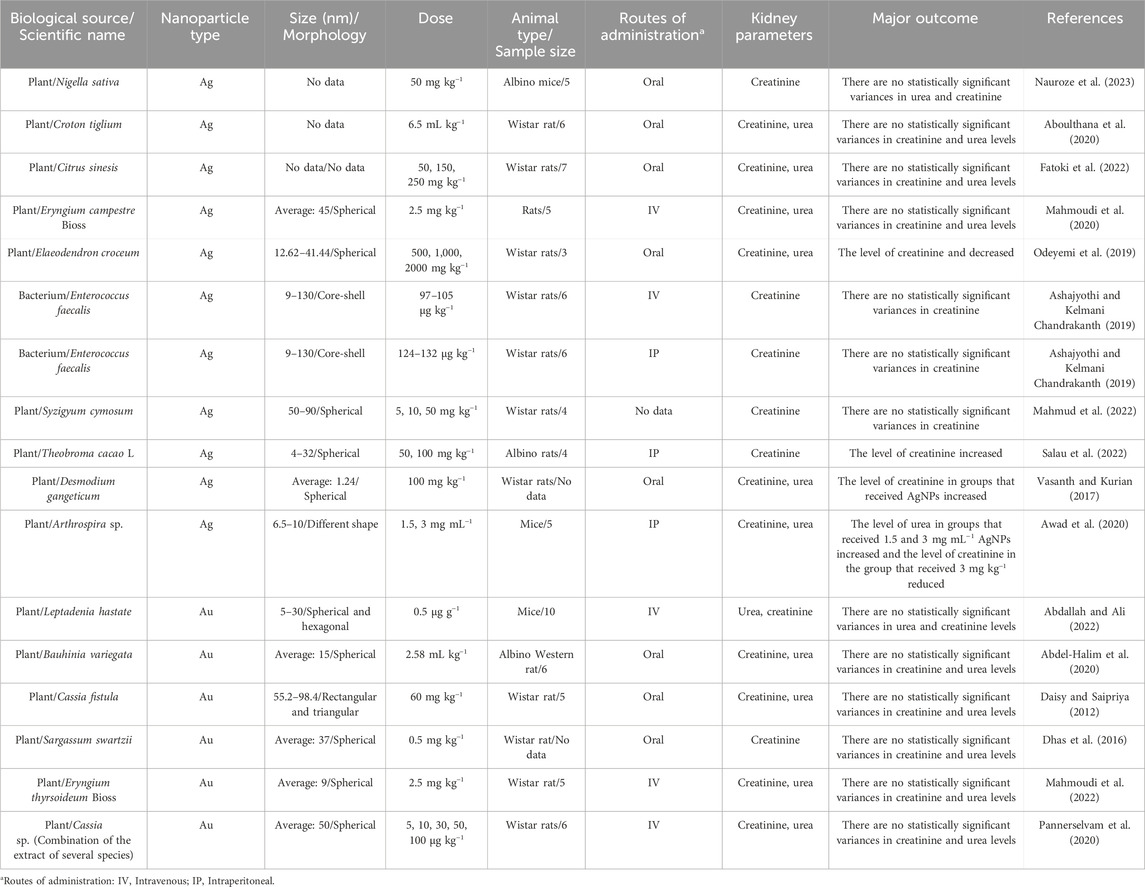
Table 4. The results of the influence of eco-friendly green fabricated silver/gold nanostructures on renal function profile parameters in healthy animal models.
3.5 Bridging the gap: from animal models to clinical translation of bioengineered nanoparticles
The growing body of evidence from animal studies demonstrates the remarkable therapeutic potential of AgNPs and AuNPs in protecting against liver and kidney damage. These NPs, due to their unique size, shape, and surface properties, have shown promise in mitigating the harmful effects of various diseases and toxicities, offering a potential alternative to conventional treatments. However, despite the encouraging preclinical results, the path to translating these findings into clinical applications remains fraught with challenges. To bridge this critical gap, several significant steps must be undertaken, involving both scientific and regulatory advancements. Firstly, while animal models provide valuable insights into the biological interactions of these NPs, they often do not fully represent the complexity of human biology, posing a significant hurdle in predicting clinical outcomes. Therefore, it is essential to refine the NP characteristics to ensure they align with the requirements for human therapies, including uniformity in size, shape, and surface functionalization. Furthermore, one of the most pressing issues in NP therapeutics is the optimization of dosage and the administration route. The dosages used in animal studies, which are often based on body weight or specific disease models, may not be directly applicable to humans, requiring careful dose scaling and adjustment in human trials. Additionally, the safety and biocompatibility of bioengineered NPs are important considerations that cannot be overlooked. While many animal studies have demonstrated the therapeutic efficacy of these NPs, long-term safety data, especially regarding toxicity, immunogenicity, and the potential for unintended side effects, are still limited. Rigorous preclinical testing and careful monitoring in clinical trials will be necessary to evaluate the long-term effects of NP exposure in humans. Regulatory approval for NP-based therapeutics also presents a major challenge, with existing frameworks for drug approval needing to be adapted to account for the unique properties and behavior of nanomaterials in biological systems. Last but not least, the scalability of NP production remains another obstacle. While laboratory-scale synthesis methods can produce bioengineered NPs with high efficiency, the processes must be refined and scaled up for commercial production. Ensuring cost-effective, reproducible, and large-scale production of NPs that meet the required purity and quality standards is a significant challenge that will require innovation in manufacturing technologies and processes. Overall, the NP optimization, safety assessment, and regulatory considerations are challenging issues highlighting the need for further research and collaborative efforts between scientists, clinicians, and regulatory bodies to successfully transition these promising therapeutic agents from the laboratory to the clinic.
4 Conclusion
Green synthesized metallic NPs, such as AgNPs and AuNPs have attracted significant attention owing to their wide range of biomedical performance. These NPs are formulated by employing biological resources like plant extracts and microbes to convert the metal ions to their nanoforms. In the present review study, the green process of preparation of AgNPs and AuNPs by different natural resources was reviewed. The findings from the majority of the investigations reviewed reveal a significant decline in the levels of hepatic and renal function markers among the non-healthy animal groups that were administered the green-synthesized AgNPs and AuNPs. This substantial reduction indicates a protective influence of these NPs against organ damage, suggesting their potential therapeutic benefits in managing hepatic and renal dysfunction. Conversely, in healthy animal models, the studies indicated no statistically significant changes in liver and kidney parameters when subjected to lower concentrations of these NPs. This lack of significant variance points to the possibility that, at minimal dosages, AgNPs and AuNPs may not adversely affect the physiological functions of healthy organs, highlighting their potential safety profile. Although the studies included in this review provide valuable insights into the protective effects of bioengineered NPs on liver and kidney function, the molecular mechanisms underlying these effects remain largely unexplored. Most studies focused on evaluating the therapeutic outcomes in animal models, without investigating the specific signaling pathways or cellular processes that might contribute to the observed effects. As a result, our review does not provide a detailed mechanistic analysis, as such data was not available in the reviewed literature. Future studies should aim to address this gap by exploring the molecular interactions of these NPs with key cellular targets and signaling pathways, such as oxidative stress pathways, inflammatory responses, and cell survival mechanisms. Overall, his review provides compelling evidence from animal studies that highlight the protective effects of biosynthesized AgNPs and AuNPs on hepatic and renal function. However, to fully understand the implications of these findings, further research is imperative. Future studies should aim to investigate both the acute and chronic toxicity of these NPs, as well as their pharmacokinetics and pharmacodynamics through robust in vivo models. Such comprehensive investigations will be essential for establishing the safety, efficacy, and therapeutic potential of biogenic AgNPs and AuNPs in clinical applications.
Author contributions
HB: Conceptualization, Project administration, Supervision, Visualization, Writing–original draft, Writing–review and editing. HN: Writing–original draft, Writing–review and editing. MS: Writing–original draft, Writing–review and editing. AK: Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by a grant from Shahid Beheshti University of Medical Sciences, Tehran, Iran (Grant Number 43009915).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdallah, B. M., and Ali, E. M. (2022). Therapeutic potential of green synthesized gold nanoparticles using extract of Leptadenia hastata against invasive pulmonary aspergillosis. J. Fungi 8 (5), 442. doi:10.3390/jof8050442
Abdel-Halim, A. H., Fyiad, A. A. A., Aboulthana, W. M., El-Sammad, N. M., Youssef, A. M., and Ali, M. M. (2020). Assessment of the anti-diabetic effect of Bauhinia variegata gold nano-extract against streptozotocin induced diabetes mellitus in rats. J. Appl. Pharm. Sci. 10 (5), 77–91. doi:10.7324/JAPS.2020.10511
Abd El-Maksoud, E. M., Lebda, M. A., Hashem, A. E., Taha, N. M., and Kamel, M. A. (2019). Ginkgo biloba mitigates silver nanoparticles-induced hepatotoxicity in Wistar rats via improvement of mitochondrial biogenesis and antioxidant status. Environ. Sci. Pollut. Res. 26 (25), 25844–25854. doi:10.1007/s11356-019-05835-2
Aboulthana, W. M., Ibrahim, N. E. S., Osman, N. M., Seif, M. M., Hassan, A. K., Youssef, A. M., et al. (2020). Evaluation of the biological efficiency of silver nanoparticles biosynthesized using Croton tiglium L. Seeds extract against azoxymethane induced colon cancer in rats. Asian Pac. J. Cancer Prev. 21 (5), 1369–1389. doi:10.31557/apjcp.2020.21.5.1369
Abu Hajleh, M. N., Abu-Huwaij, R., AL-Samydai, A., Al-Halaseh, L. K., and Al-Dujaili, E. A. (2021). The revolution of cosmeceuticals delivery by using nanotechnology: a narrative review of advantages and side effects. J. Cosmet. Dermatology 20 (12), 3818–3828. doi:10.1111/jocd.14441
Ahire, S. A., Bachhav, A. A., Pawar, T. B., Jagdale, B. S., Patil, A. V., and Koli, P. B. (2022). The Augmentation of nanotechnology era: a concise review on fundamental concepts of nanotechnology and applications in material science and technology. Results Chem. 4, 100633. doi:10.1016/j.rechem.2022.100633
Ahmad, S., Munir, S., Zeb, N., Ullah, A., Khan, B., Ali, J., et al. (2019). Green nanotechnology: a review on green synthesis of silver nanoparticles—an ecofriendly approach. Int. J. nanomedicine Vol. 14, 5087–5107. doi:10.2147/ijn.s200254
Akter, S., and Huq, M. A. (2020). Biologically rapid synthesis of silver nanoparticles by Sphingobium sp. MAH-11T and their antibacterial activity and mechanisms investigation against drug-resistant pathogenic microbes. Artif. Cells, Nanomedicine, Biotechnol. 48 (1), 672–682. doi:10.1080/21691401.2020.1730390
Akter, S., Lee, S.-Y., Siddiqi, M. Z., Balusamy, S. R., Ashrafudoulla, M., Rupa, E. J., et al. (2020). Ecofriendly synthesis of silver nanoparticles by Terrabacter humi sp. nov. and their antibacterial application against antibiotic-resistant pathogens. Int. J. Mol. Sci. 21 (24), 9746. doi:10.3390/ijms21249746
Alaqad, K., and Saleh, T. A. (2016). Gold and silver nanoparticles: synthesis methods, characterization routes and applications towards drugs. J. Environ. Anal. Toxicol. 6 (4), 525–2161. doi:10.4172/2161-0525.1000384
Anjum, S., Ishaque, S., Fatima, H., Farooq, W., Hano, C., Abbasi, B. H., et al. (2021). Emerging applications of nanotechnology in healthcare systems: grand challenges and perspectives. Pharmaceuticals 14 (8), 707. doi:10.3390/ph14080707
Ansari, S. A., Bari, A., Ullah, R., Mathanmohun, M., Veeraraghavan, V. P., and Sun, Z. (2019). Gold nanoparticles synthesized with Smilax glabra rhizome modulates the anti-obesity parameters in high-fat diet and streptozotocin induced obese diabetes rat model. J. Photochem. Photobiol. B Biol. 201, 111643-. doi:10.1016/j.jphotobiol.2019.111643
Ashajyothi, C., and Kelmani Chandrakanth, R. (2019). A pilot toxicology study of biogenic silver nanoparticles: in vivo by intraperitoneal and intravenous infusion routes in rats. J. Exp. Nanosci. 14 (1), 89–106. doi:10.1080/17458080.2018.1502479
Awad, O. A., Abou Shady, A. M., El Deeb, N. M., and Abo eleneen, M. A. (2020). Safety assays of biogenic silver nanoparticles synthesized by of Arthrospira sp, in vivo study. Delta J. Sci. 41 (1), 57–65. doi:10.21608/djs.2020.139229
Baig, N., Kammakakam, I., and Falath, W. (2021). Nanomaterials: a review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2 (6), 1821–1871. doi:10.1039/d0ma00807a
Barabadi, H., Mobaraki, K., Ashouri, F., Noqani, H., Jounaki, K., and Mostafavi, E. (2023). Nanobiotechnological approaches in antinociceptive therapy: animal-based evidence for analgesic nanotherapeutics of bioengineered silver and gold nanomaterials. Adv. Colloid Interface Sci. 316, 102917. doi:10.1016/j.cis.2023.102917
Bayda, S., Adeel, M., Tuccinardi, T., Cordani, M., and Rizzolio, F. (2019). The history of nanoscience and nanotechnology: from chemical-physical applications to nanomedicine. Molecules 25 (1), 112. doi:10.3390/molecules25010112
Begum, Q., and Mahboob, T. (2021). Silver nanoparticles protects streptozotocin-induced hepatotoxicity: a biochemical and histopathological approach. Int. J. Nano Res. 4, 01–09.
Berlin Grace, V. M., Wilson, D. D., and Siddikuzzaman, G. R (2021). A new silver nano-formulation of cassia auriculata flower extract and its anti-diabetic effects. Recent Pat Nanotechnol. 16 (2):160–169. doi:10.2174/1872210515666210329160523
Bhainsa, K. C., and D'souza, S. (2006). Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids surfaces B Biointerfaces 47 (2), 160–164. doi:10.1016/j.colsurfb.2005.11.026
Chakraborty, N., Banerjee, A., Lahiri, S., Panda, A., Ghosh, A. N., and Pal, R. (2009). Biorecovery of gold using cyanobacteria and an eukaryotic alga with special reference to nanogold formation–a novel phenomenon. J. Appl. Phycol. 21, 145–152. doi:10.1007/s10811-008-9343-3
Chan, S. S., Low, S. S., Chew, K. W., Ling, T. C., Rinklebe, J., Juan, J. C., et al. (2022). Prospects and environmental sustainability of phyconanotechnology: a review on algae-mediated metal nanoparticles synthesis and mechanism. Environ. Res. 212, 113140. doi:10.1016/j.envres.2022.113140
Chen, J., Lin, Z., and Ma, X. (2003). Evidence of the production of silver nanoparticles via pretreatment of Phoma sp. 3.2883 with silver nitrate. Lett. Appl. Microbiol. 37 (2), 105–108. doi:10.1046/j.1472-765x.2003.01348.x
Choi, Y., Ho, N. H., and Tung, C. H. (2007). Sensing phosphatase activity by using gold nanoparticles. Angew. Chem. 119 (5), 721–723. doi:10.1002/ange.200603735
Chopra, H., Bibi, S., Singh, I., Hasan, M. M., Khan, M. S., Yousafi, Q., et al. (2022). Green metallic nanoparticles: biosynthesis to applications. Front. Bioeng. Biotechnol. 10, 874742. doi:10.3389/fbioe.2022.874742
Chopra, H., Mishra, A. K., Baig, A. A., Mohanta, T. K., Mohanta, Y. K., and Baek, K.-H. (2021). Narrative review: bioactive potential of various mushrooms as the treasure of versatile therapeutic natural product. J. Fungi 7 (9), 728. doi:10.3390/jof7090728
Costa, L., Hemmer, J., Wanderlind, E. H., Gerlach, O., Santos, A., Tamanaha, M., et al. (2020). Green synthesis of gold nanoparticles obtained from algae Sargassum cymosum: optimization, characterization and stability. BioNanoScience 10, 1049–1062. doi:10.1007/s12668-020-00776-4
Daisy, P., and Saipriya, K. (2012). Biochemical analysis of Cassia fistula aqueous extract and phytochemically synthesized gold nanoparticles as hypoglycemic treatment for diabetes mellitus. Int. J. Nanomedicine 7, 1189–1202. doi:10.2147/ijn.s26650
Dhas, T. S., Kumar, V. G., Karthick, V., Vasanth, K., Singaravelu, G., and Govindaraju, K. (2016). Effect of biosynthesized gold nanoparticles by Sargassum swartzii in alloxan induced diabetic rats. Enzyme Microb. Technol. 95, 100–106. doi:10.1016/j.enzmictec.2016.09.003
Dkhil, M. A., Abdel-Gaber, R., Alojayri, G., Al-Shaebi, E. M., Qasem, M. A. A., Murshed, M., et al. (2020). Biosynthesized silver nanoparticles protect against hepatic injury induced by murine blood-stage malaria infection. Environ. Sci. Pollut. Res. 27 (15), 17762–17769. doi:10.1007/s11356-020-08280-8
Elekofehinti, O. O. (2022). Momordica charantia nanoparticles potentiate insulin release and modulate antioxidant gene expression in pancreas of diabetic rats. Egypt. J. Med. Hum. Genet. 23 (1), 63. doi:10.1186/s43042-022-00282-0
ElMitwalli, O. S., Barakat, O. A., Daoud, R. M., Akhtar, S., and Henari, F. Z. (2020). Green synthesis of gold nanoparticles using cinnamon bark extract, characterization, and fluorescence activity in Au/eosin Y assemblies. J. Nanoparticle Res. 22, 309–9. doi:10.1007/s11051-020-04983-8
Elobeid, M., Awad, M., Virk, P., Ortashi, K., Merghani, N., Asiri, A., et al. (2022). Synthesis and characterization of noble metal/metal oxide nanoparticles and their potential antidiabetic effect on biochemical parameters and wound healing. Green Process. Synthesis 11, 106–115. doi:10.1515/gps-2022-0010
Fabris, M., Abbriano, R. M., Pernice, M., Sutherland, D. L., Commault, A. S., Hall, C. C., et al. (2020). Emerging technologies in algal biotechnology: toward the establishment of a sustainable, algae-based bioeconomy. Front. plant Sci. 11, 279. doi:10.3389/fpls.2020.00279
Fakruddin, M., Hossain, Z., and Afroz, H. (2012). Prospects and applications of nanobiotechnology: a medical perspective. J. nanobiotechnology 10 (1), 31–38. doi:10.1186/1477-3155-10-31
Fang, X., Wang, Y., Wang, Z., Jiang, Z., and Dong, M. (2019). Microorganism assisted synthesized nanoparticles for catalytic applications. Energies 12 (1), 190. doi:10.3390/en12010190
Fatoki, J. O., Badmus, J. A., Ajilore, B. S., Faokunla, O., and Alabi, S. O. (2022). Biological synthesis of silver nanoparticles using Citrus sinesis seeds: effects on hepatic and renal functional integrities and antioxidant activities. Res. J. Health Sci. 10 (2), 66–79. doi:10.4314/rejhs.v10i2.1
Gao, L., Hu, Y., Hu, D., Li, Y., Yang, S., Dong, X., et al. (2020). Anti-obesity activity of gold nanoparticles synthesized from Salacia chinensis modulates the biochemical alterations in high-fat diet-induced obese rat model via AMPK signaling pathway. Arabian J. Chem. 13 (8), 6589–6597. doi:10.1016/j.arabjc.2020.06.015
Garg, R., Rani, P., Garg, R., and Eddy, N. O. (2021). Study on potential applications and toxicity analysis of green synthesized nanoparticles. Turkish J. Chem. 45 (6), 1690–1706. doi:10.3906/kim-2106-59
Garole, D. J., Choudhary, B. C., Paul, D., and Borse, A. U. (2018). Sorption and recovery of platinum from simulated spent catalyst solution and refinery wastewater using chemically modified biomass as a novel sorbent. Environ. Sci. Pollut. Res. 25, 10911–10925. doi:10.1007/s11356-018-1351-5
Goorani, S., Koohi, M. K., Morovvati, H., Hassan, J., Ahmeda, A., and Zangeneh, M. M. (2020). Application of natural compounds–based gold nanoparticles for the treatment of hemolytic anemia in an anemic mouse model: formulation of a novel drug from relationship between the nanotechnology and hematology sciences. Appl. Organomet. Chem. 34 (4), 1–14. doi:10.1002/aoc.5475
Guo, Y., Jiang, N., Zhang, L., and Yin, M. (2020). Green synthesis of gold nanoparticles from Fritillaria cirrhosa and its anti-diabetic activity on Streptozotocin induced rats. Arabian J. Chem. 13 (4), 5096–5106. doi:10.1016/j.arabjc.2020.02.009
Gupta, R., and Padmanabhan, P. (2018). Biogenic synthesis and characterization of gold nanoparticles by a novel marine bacteria Marinobacter algicola: progression from nanospheres to various geometrical shapes. J. Microbiol. Biotechnol. Food Sci. 8 (1), 732–737. doi:10.15414/jmbfs.2018.8.1.732-737
Haq, M. N. U., Shah, G. M., Menaa, F., Ali Khan, R., Althobaiti, N. A., Albalawi, A. E., et al. (2022). Green silver nanoparticles synthesized from Taverniera couneifolia elicits effective anti-diabetic effect in alloxan-induced diabetic wistar rats. Nanomaterials 12 (7), 1035. doi:10.3390/nano12071035
Harinisri, K., Jayanthi, N., and Kumar, R. S. (2023). Diverse application of green nanotechnology–A review. Mater. Today Proc. (in press). doi:10.1016/j.matpr.2023.06.085
Hendi, A. A., El-Nagar, D. M., Awad, M. A., Ortashi, K. M., Alnamlah, R. A., and Merghani, N. M. (2020). Green nanogold activity in experimental breast carcinoma in vivo. Biosci. Rep. 40 (11), BSR20200115–12. doi:10.1042/bsr20200115
Herizchi, R., Abbasi, E., Milani, M., and Akbarzadeh, A. (2016). Current methods for synthesis of gold nanoparticles. Artif. cells, nanomedicine, Biotechnol. 44 (2), 596–602. doi:10.3109/21691401.2014.971807
Hosny, M., Fawzy, M., El-Badry, Y. A., Hussein, E. E., and Eltaweil, A. S. (2022). Plant-assisted synthesis of gold nanoparticles for photocatalytic, anticancer, and antioxidant applications. J. Saudi Chem. Soc. 26 (2), 101419. doi:10.1016/j.jscs.2022.101419
Huq, M. A. (2020). Biogenic silver nanoparticles synthesized by Lysinibacillus xylanilyticus MAHUQ-40 to control antibiotic-resistant human pathogens Vibrio parahaemolyticus and Salmonella Typhimurium. Front. Bioeng. Biotechnol. 8, 597502. doi:10.3389/fbioe.2020.597502
Huq, M. A., and Akter, S. (2021). Characterization and genome analysis of Arthrobacter bangladeshi sp. nov., applied for the green synthesis of silver nanoparticles and their antibacterial efficacy against drug-resistant human pathogens. Pharmaceutics 13 (10), 1691. doi:10.3390/pharmaceutics13101691
Huq, M. A., Ashrafudoulla, M., Rahman, M. M., Balusamy, S. R., and Akter, S. (2022). Green synthesis and potential antibacterial applications of bioactive silver nanoparticles: a review. Polymers 14 (4), 742. doi:10.3390/polym14040742
Ijaz, M., Fatima, M., Anwar, R., and Uroos, M. (2021). Green synthesis of gold nanoparticles from: manilkara zapota L. extract and the evaluation of its intrinsic in vivo antiarthritic potential. RSC Adv. 11 (44), 27092–27106. doi:10.1039/d1ra03186d
Ilahi, N., Haleem, A., Iqbal, S., Fatima, N., Sajjad, W., Sideeq, A., et al. (2022). Biosynthesis of silver nanoparticles using endophytic Fusarium oxysporum strain NFW16 and their in vitro antibacterial potential. Microsc. Res. Tech. 85 (4), 1568–1579. doi:10.1002/jemt.24018
Iravani, S., Korbekandi, H., Mirmohammadi, S. V., and Zolfaghari, B. (2014). Synthesis of silver nanoparticles: chemical, physical and biological methods. Res. Pharm. Sci. 9 (6), 385–406.
Itrat, S., Naqvi, Z., Kausar, H., Afzal, A., Hashim, M., Mujahid, H., et al. (2023). Antifungal activity of juglans-regia -mediated silver nanoparticles (AgNPs) against aspergillus-ochraceus -induced toxicity in in vitro and in vivo settings. J. Funct. Biomater. 14, 221. doi:10.3390/jfb14040221
Jamkhande, P. G., Ghule, N. W., Bamer, A. H., and Kalaskar, M. G. (2019). Metal nanoparticles synthesis: an overview on methods of preparation, advantages and disadvantages, and applications. J. drug Deliv. Sci. Technol. 53, 101174. doi:10.1016/j.jddst.2019.101174
Javanshir, R., Honarmand, M., Hosseini, M., and Hemmati, M. (2020). Anti-dyslipidemic properties of green gold nanoparticle: improvement in oxidative antioxidative balance and associated atherogenicity and insulin resistance. Clin. Phytoscience 6 (1), 74. doi:10.1186/s40816-020-00224-6
John, M. S., Nagoth, J. A., Ramasamy, K. P., Mancini, A., Giuli, G., Natalello, A., et al. (2020). Synthesis of bioactive silver nanoparticles by a Pseudomonas strain associated with the antarctic psychrophilic protozoon Euplotes focardii. Mar. drugs 18 (1), 38. doi:10.3390/md18010038
Joshi, C. G., Danagoudar, A., Poyya, J., Kudva, A. K., Dhananjaya, B., and Bl, D. (2017). Biogenic synthesis of gold nanoparticles by marine endophytic fungus-Cladosporium cladosporioides isolated from seaweed and evaluation of their antioxidant and antimicrobial properties. Process Biochem. 63, 137–144. doi:10.1016/j.procbio.2017.09.008
Kalakotla, S. P. G., Banu, A., and Shaik, S. (2022). Development of plant-mediated silver nanoparticles and their pharmacological evaluation.
Kanimozhi, S., Durga, R., Sabithasree, M., Kumar, A. V., Sofiavizhimalar, A., Kadam, A. A., et al. (2022). Biogenic synthesis of silver nanoparticle using Cissus quadrangularis extract and its invitro study. J. King Saud University-Science. 34 (4), 101930. doi:10.1016/j.jksus.2022.101930
Karimi, S., and Samimi, T. (2019). Green and simple synthesis route of Ag@ AgCl nanomaterial using green marine crude extract and its application for sensitive and selective determination of mercury. Spectrochimica Acta Part A Mol. Biomol. Spectrosc. 222, 117216. doi:10.1016/j.saa.2019.117216
Kathiresan, K., Manivannan, S., Nabeel, M., and Dhivya, B. (2009). Studies on silver nanoparticles synthesized by a marine fungus, Penicillium fellutanum isolated from coastal mangrove sediment. Colloids surfaces B Biointerfaces 71 (1), 133–137. doi:10.1016/j.colsurfb.2009.01.016
Kazaryan, S., Petrosyan, M., Rshtuni, L., Dabaghyan, V., and Hovhannisyan, A. (2020). Effects of green silver nanoparticles on CCL4 injured albino rats’ liver. Springer International Publishing, 127–131.
kazemi, S., Hosseingholian, A., Gohari, S. D., Feirahi, F., Moammeri, F., Mesbahian, G., et al. (2023). Recent advances in green synthesized nanoparticles: from production to application. Mater. Today Sustain. 24, 100500. doi:10.1016/j.mtsust.2023.100500
Khan, I., Saeed, K., and Khan, I. (2019). Nanoparticles: properties, applications and toxicities. Arabian J. Chem. 12 (7), 908–931. doi:10.1016/j.arabjc.2017.05.011
Khan, S., Mansoor, S., Rafi, Z., Kumari, B., Shoaib, A., Saeed, M., et al. (2022). A review on nanotechnology: properties, applications, and mechanistic insights of cellular uptake mechanisms. J. Mol. Liq. 348, 118008. doi:10.1016/j.molliq.2021.118008
Kumar, R., Kumar, M., and Luthra, G. (2023). Fundamental approaches and applications of nanotechnology: a mini review. Mater. Today Proc. doi:10.1016/j.matpr.2022.12.172
Lengke, M. F., Fleet, M. E., and Southam, G. (2006). Morphology of gold nanoparticles synthesized by filamentous cyanobacteria from gold (I)− thiosulfate and gold (III)− chloride complexes. Langmuir 22 (6), 2780–2787. doi:10.1021/la052652c
Lengke, M. F., Fleet, M. E., and Southam, G. (2007). Biosynthesis of silver nanoparticles by filamentous cyanobacteria from a silver (I) nitrate complex. Langmuir 23 (5), 2694–2699. doi:10.1021/la0613124
Li, W., Wan, H., Yan, S., Yan, Z., Chen, Y., Guo, P., et al. (2020). Gold nanoparticles synthesized with Poria cocos modulates the anti-obesity parameters in high-fat diet and streptozotocin induced obese diabetes rat model. Arabian J. Chem. 13 (7), 5966–5977. doi:10.1016/j.arabjc.2020.04.031
Li, X., Xu, H., Chen, Z.-S., and Chen, G. (2011). Biosynthesis of nanoparticles by microorganisms and their applications. J. Nanomater. 2011, 1–16. doi:10.1155/2011/270974
Liu, W., Wang, L., Wang, J., Du, J., and Jing, C. (2018). New insights into microbial-mediated synthesis of Au@ biolayer nanoparticles. Environ. Sci. Nano 5 (7), 1757–1763. doi:10.1039/c8en00104a
Madhanraj, R., Eyini, M., and Balaji, P. (2017). Antioxidant assay of gold and silver nanoparticles from edible basidiomycetes mushroom fungi. Free Radicals Antioxidants 7 (2), 137–142. doi:10.5530/fra.2017.2.20
Mahmoudi, F., Mahmoudi, F., Gollo, K. H., and Amini, M. M. (2020). Biosynthesis of novel silver nanoparticles using Eryngium thyrsoideum boiss extract and comparison of their antidiabetic activity with chemical synthesized silver nanoparticles in diabetic rats. Biol. Trace Elem. Res. 199, 1967–1978. doi:10.1007/s12011-020-02315-4
Mahmoudi, F., Mahmoudi, F., Gollo, K. H., and Amini, M. M. (2022). Novel gold nanoparticles: green synthesis with Eryngium thyrsoideum boiss extract, characterization, and in vivo investigations on inflammatory gene expression and biochemical parameters in type 2 diabetic rats. Biol. Trace Elem. Res. 200 (5), 2223–2232. doi:10.1007/s12011-021-02819-7
Mahmud, K. M., Hossain, M. M., Polash, S. A., Takikawa, M., Shakil, M. S., Uddin, M. F., et al. (2022). Investigation of antimicrobial activity and biocompatibility of biogenic silver nanoparticles synthesized using Syzigyum cymosum extract. ACS Omega 7 (31), 27216–27229. doi:10.1021/acsomega.2c01922
Manam, V. K., and Subbaiah, M. (2022). Silver nanoparticles bio-genesis from colpomenia sinuosa and its in-vivo anti-tumor efficacy on DLA inoculated tumor in albino mice. Int. J. Pharm. Sci. Nanotechnol. 15 (5), 6161–6168. doi:10.37285/ijpsn.2022.15.5.8
Manna, K., Mishra, S., Saha, M., Mahapatra, S., Saha, C., Yenge, G., et al. (2023). Amelioration of diabetic nephropathy using pomegranate peel extract-stabilized gold nanoparticles: assessment of NF-ΚB and Nrf2 signaling system. Int. J. Nanomedicine 14, 1753–1777. doi:10.2147/IJN.S176013
Mata, R., Nakkala, J. R., Chandra, V. K., Raja, K., and Sadras, S. R. (2018). In vivo bio-distribution, clearance and toxicity assessment of biogenic silver and gold nanoparticles synthesized from Abutilon indicum in Wistar rats. J. Trace Elem. Med. Biol. 48, 157–165. doi:10.1016/j.jtemb.2018.03.015
Mekuye, B., and Abera, B. (2023). Nanomaterials: an overview of synthesis, classification, characterization, and applications. Nano Sel. 4 (8), 486–501. doi:10.1002/nano.202300038
Mohammadi, G., Zangeneh, M. M., Zangeneh, A., and Haghighi, Z. M. S. (2020). Chemical characterization and anti-breast cancer effects of silver nanoparticles using Phoenix dactylifera seed ethanolic extract on 7,12-Dimethylbenz[a] anthracene-induced mammary gland carcinogenesis in Sprague Dawley male rats. Appl. Organomet. Chem. 34 (1). doi:10.1002/aoc.5136
Mohanta, Y. K., Nayak, D., Biswas, K., Singdevsachan, S. K., Abd_Allah, E. F., Hashem, A., et al. (2018). Silver nanoparticles synthesized using wild mushroom show potential antimicrobial activities against food borne pathogens. Molecules 23 (3), 655. doi:10.3390/molecules23030655
Molnár, Z., Bódai, V., Szakacs, G., Erdélyi, B., Fogarassy, Z., Sáfrán, G., et al. (2018). Green synthesis of gold nanoparticles by thermophilic filamentous fungi. Sci. Rep. 8 (1), 3943. doi:10.1038/s41598-018-22112-3
Mousumi, M., Amit, B., Gouriprasad, D., and Dilip, K. N. (2020). Nephroprotective effect of green synthesised gold nanoparticles using bark extract of Terminalia arjuna on acetaminophen induced nephrotoxicity in male albino rat. Int. J. Life Sci. Pharma Res. 10 (4), 22–30. doi:10.22376/ijpbs/lpr.2020.10.4.P22-30
Mukherjee, P., Ahmad, A., Mandal, D., Senapati, S., Sainkar, S. R., Khan, M. I., et al. (2001). Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: a novel biological approach to nanoparticle synthesis. Nano Lett. 1 (10), 515–519. doi:10.1021/nl0155274
Muniyappan, N., Pandeeswaran, M., and Amalraj, A. (2021). Green synthesis of gold nanoparticles using Curcuma pseudomontana isolated curcumin: its characterization, antimicrobial, antioxidant and anti-inflammatory activities. Environ. Chem. Ecotoxicol. 3, 117–124. doi:10.1016/j.enceco.2021.01.002
Nakkala, J. R., Mata, R., Raja, K., Khub Chandra, V., and Sadras, S. R. (2018). Green synthesized silver nanoparticles: catalytic dye degradation, in vitro anticancer activity and in vivo toxicity in rats. Mater. Sci. Eng. C 91, 372–381. doi:10.1016/j.msec.2018.05.048
Nakkala, J. R., Mata, R., and Sadras, S. R. (2017). Green synthesized nano silver: synthesis, physicochemical profiling, antibacterial, anticancer activities and biological in vivo toxicity. J. Colloid Interface Sci. 499, 33–45. doi:10.1016/j.jcis.2017.03.090
Nandhini, J. T., Ezhilarasan, D., and Rajeshkumar, S. (2021). An ecofriendly synthesized gold nanoparticles induces cytotoxicity via apoptosis in HepG2 cells. Environ. Toxicol. 36 (1), 24–32. doi:10.1002/tox.23007
Nauroze, T., Ali, S., Kanwal, L., Ara, C., Akbar Mughal, T., and Andleeb, S. (2023). Ameliorative effect of Nigella sativa conjugated silver nanoparticles against chromium-induced hepatotoxicity and renal toxicity in mice. Saudi J. Biol. Sci. 30 (3), 103571. doi:10.1016/j.sjbs.2023.103571
Nazer, S., Andleeb, S., Ali, S., Gulzar, N., Raza, A., Khan, H., et al. (2021). Cytotoxicity, anti-diabetic, and hepato-protective potential of Ajuga bracteosa-conjugated silver nanoparticles in balb/c mice. Curr. Pharm. Biotechnol. 23 (3), 318–336. doi:10.2174/1389201022666210421101837
Odeyemi, S. W., De La Mare, J., Edkins, A. L., and Afolayan, A. J. (2019). In vitro and in vivo toxicity assessment of biologically synthesized silver nanoparticles from Elaeodendron croceum. J. Complementary Integr. Med. 16, 1–14. doi:10.1515/jcim-2018-0184
Oladipo, I. C., Lateef, A., Azeez, M. A., Asafa, T. B., Yekeen, T. A., Ogunsona, S. B., et al. (2020). Antidiabetic properties of phytosynthesized gold nanoparticles (AuNPs) from Datura stramonium seed. IOP Conf. Ser. Mater. Sci. Eng. 805 (1), 012035. doi:10.1088/1757-899x/805/1/012035
Onitsuka, S., Hamada, T., and Okamura, H. (2019). Preparation of antimicrobial gold and silver nanoparticles from tea leaf extracts. Colloids surfaces B Biointerfaces 173, 242–248. doi:10.1016/j.colsurfb.2018.09.055
Othman, A. M., Elsayed, M. A., Al-Balakocy, N. G., Hassan, M. M., and Elshafei, A. M. (2019). Biosynthesis and characterization of silver nanoparticles induced by fungal proteins and its application in different biological activities. J. Genet. Eng. Biotechnol. 17, 8–13. doi:10.1186/s43141-019-0008-1
Othman, B. A., and Nanakali, N. M. (2022). The preventive effects of Urtica dioica Extract and nanoparticle on oxidative stress and lipid profile in hyperlipidemic male Rats. Cihan University-Erbil Sci. J. 6 (2), 125–132. doi:10.24086/cuesj.v6n2y2022.pp125-132
Öztürk, B. Y., Gürsu, B. Y., and Dağ, İ. (2020). Antibiofilm and antimicrobial activities of green synthesized silver nanoparticles using marine red algae Gelidium corneum. Process Biochem. 89, 208–219. doi:10.1016/j.procbio.2019.10.027
Pannerselvam, B., Devanathadesikan, V., Alagumuthu, T. S., Kanth, S. V., and Thangavelu, K. P. (2020). Assessment of in-vivo biocompatibility evaluation of phytogenic gold nanoparticles on Wistar albino male rats. IET Nanobiotechnology 14 (4), 314–324. doi:10.1049/iet-nbt.2019.0116
Patil, M. P., Kang, M.-j., Niyonizigiye, I., Singh, A., Kim, J.-O., Seo, Y. B., et al. (2019). Extracellular synthesis of gold nanoparticles using the marine bacterium Paracoccus haeundaensis BC74171T and evaluation of their antioxidant activity and antiproliferative effect on normal and cancer cell lines. Colloids Surfaces B Biointerfaces 183, 110455. doi:10.1016/j.colsurfb.2019.110455
Prasad, S., Teli, S., Ghosh, J., Prasad, N., Shaikh, V., Nazeruddin, G., et al. (2021). A review on bio-inspired synthesis of silver nanoparticles: their antimicrobial efficacy and toxicity. Eng. Sci. 16, 90–128. doi:10.30919/es8d479
Qiao, Z.-P., Wang, M.-Y., Liu, J.-F., and Wang, Q.-Z. (2022). Green synthesis of silver nanoparticles using a novel endophytic fungus Letendraea sp. WZ07: characterization and evaluation of antioxidant, antibacterial and catalytic activities (3-in-1 system). Inorg. Chem. Commun. 138, 109301. doi:10.1016/j.inoche.2022.109301
Rai, M., and Duran, N. (2011). Metal nanoparticles in microbiology. Springer Science and Business Media.
Rajivgandhi, G. N., Chackaravarthi, G., Ramachandran, G., Manoharan, N., Ragunathan, R., Siddiqi, M. Z., et al. (2022). Synthesis of silver nanoparticle (Ag NPs) using phytochemical rich medicinal plant Lonicera japonica for improve the cytotoxicity effect in cancer cells. J. King Saud University-Science. 34 (2), 101798. doi:10.1016/j.jksus.2021.101798
Ramaswamy, M., Solaimuthu, C., and Duraikannu, S. (2019). Antiarthritic activity of synthesized silver nanoparticles from aqueous extract of Moringa concanensis Nimmo leaves against FCA induced rheumatic arthritis in rats. J. Drug Deliv. Ther. 9 (3), 66–75. doi:10.22270/jddt.v9i3.2707
Raza, S., Ansari, A., Siddiqui, N. N., Ibrahim, F., Abro, M. I., and Aman, A. (2021). Biosynthesis of silver nanoparticles for the fabrication of non cytotoxic and antibacterial metallic polymer based nanocomposite system. Sci. Rep. 11 (1), 10500. doi:10.1038/s41598-021-90016-w
Salau, A. K., Osineye, S. O., and Lateef, A. (2022). Nanotoxicological investigations of cocoa pod husk extract-mediated silver nanoparticles in selected tissues of albino rats. Toxicol. Environ. Health Sci. 14 (2), 193–202. doi:10.1007/s13530-022-00129-6
Sameem, S., Neupane, N. P., Ansari, S. M. S., Khan, M. M. U., Kumar, V., Pathak, P., et al. (2022a). Phyto-fabrication of silver nanoparticles from Ziziphus mauritiana against hepatic carcinoma via modulation of Rho family-alpha serine/threonine protein kinase. J. Drug Deliv. Sci. Technol. 70, 103227. doi:10.1016/j.jddst.2022.103227
Sameem, S., Neupane, N. P., Ansari, S. M. S., Khan, M. M. U., Kumar, V., Pathak, P., et al. (2022b). Phyto-fabrication of silver nanoparticles from Ziziphus mauritiana against hepatic carcinoma via modulation of Rho family-alpha serine/threonine protein kinase. J. Drug Deliv. Sci. Technol. 70, 103227. doi:10.1016/j.jddst.2022.103227
Sastry, M., Ahmad, A., Khan, M. I., and Kumar, R. (2003). Biosynthesis of metal nanoparticles using fungi and actinomycete. Curr. Sci. 85 (2), 162–170.
Sathishkumar, R., Sundaramanickam, A., Srinath, R., Ramesh, T., Saranya, K., Meena, M., et al. (2019). Green synthesis of silver nanoparticles by bloom forming marine microalgae Trichodesmium erythraeum and its applications in antioxidant, drug-resistant bacteria, and cytotoxicity activity. J. Saudi Chem. Soc. 23 (8), 1180–1191. doi:10.1016/j.jscs.2019.07.008
Shahcheraghi, N., Golchin, H., Sadri, Z., Tabari, Y., Borhanifar, F., and Makani, S. (2022). Nano-biotechnology, an applicable approach for sustainable future. 3 Biotech. 12 (3), 65. doi:10.1007/s13205-021-03108-9
Shankar, S. S., Rai, A., Ahmad, A., and Sastry, M. (2004). Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J. colloid interface Sci. 275 (2), 496–502. doi:10.1016/j.jcis.2004.03.003
Sharma, A., Sharma, S., Sharma, K., Chetri, S. P., Vashishtha, A., Singh, P., et al. (2016). Algae as crucial organisms in advancing nanotechnology: a systematic review. J. Appl. Phycol. 28, 1759–1774. doi:10.1007/s10811-015-0715-1
Sheikh, H., and Awad, M. F. (2022). Biogenesis of nanoparticles with inhibitory effects on aflatoxin B1 production by Aspergillus flavus. Electron. J. Biotechnol. 60, 26–35. doi:10.1016/j.ejbt.2022.09.003
Shunmugam, R., Balusamy, S. R., Kumar, V., Menon, S., Lakshmi, T., and Perumalsamy, H. (2021). Biosynthesis of gold nanoparticles using marine microbe (Vibrio alginolyticus) and its anticancer and antioxidant analysis. J. King Saud University-Science. 33 (1), 101260. doi:10.1016/j.jksus.2020.101260
Siddiqi, K. S., and Husen, A. (2017). Recent advances in plant-mediated engineered gold nanoparticles and their application in biological system. J. Trace Elem. Med. Biol. 40, 10–23. doi:10.1016/j.jtemb.2016.11.012
Singh, D., Singh, M., Yadav, E., Falls, N., Komal, U., Dangi, D. S., et al. (2018b). Amelioration of diethylnitrosamine (DEN)-induced hepatocellular carcinogenesis in animal models: via knockdown oxidative stress and proinflammatory markers by Madhuca longifolia embedded silver nanoparticles. RSC Adv. 8 (13), 6940–6953. doi:10.1039/c7ra12775h
Singh, H., Du, J., Singh, P., and Yi, T. H. (2018a). Extracellular synthesis of silver nanoparticles by Pseudomonas sp. THG-LS1. 4 and their antimicrobial application. J. Pharm. Analysis 8 (4), 258–264. doi:10.1016/j.jpha.2018.04.004
Srivastava, S., Bhargava, A., Srivastava, S., and Bhargava, A. (2022). Green nanotechnology: an overview. Green nanoparticles: the future of nanobiotechnology, 1–13.
Ul, H. M. N., Shah, G. M., Gul, A., Foudah, A. I., Alqarni, M. H., Yusufoglu, H. S., et al. (2022). Biogenic synthesis of silver nanoparticles using Phagnalon niveum and its in vivo anti-diabetic effect against alloxan-induced diabetic wistar rats. Nanomaterials 12 (5), 830. doi:10.3390/nano12050830
Vasanth, S. B., and Kurian, G. A. (2017). Toxicity evaluation of silver nanoparticles synthesized by chemical and green route in different experimental models. Nanomedicine Biotechnol. 45 (8), 1721–1727. doi:10.1080/21691401.2017.1282500
Vidya, R., Kalaivani, K., and Amudha, P. (2022). Therapeutic potential of Cucumis melo (L.) fruit extract and its silver nanopartciles against DEN-induced hepatocellular cancer in rats. Appl. Biochem. Biotechnol. 194 (1), 368–381. doi:10.1007/s12010-021-03765-9
Virk, P. (2018). Antidiabetic activity of green gold-silver nanocomposite with trigonella foenum graecum L. Seeds extract on streptozotocin-induced diabetic rats. Pak. J. Zoology 50 (2), 711–718. doi:10.17582/journal.pjz/2018.2.711.718
Keywords: silver nanoparticles, gold nanoparticles, biological synthesis, liver protection, kidney protection
Citation: Barabadi H, Noqani H, Soltani M and Sabbagh Kashani A (2025) Animal-based evidence supports protective activity of bioengineered silver and gold nanomaterials on hepatic and renal function profile parameters. Front. Nanotechnol. 6:1424562. doi: 10.3389/fnano.2024.1424562
Received: 28 April 2024; Accepted: 30 December 2024;
Published: 28 January 2025.
Edited by:
Hassan Waqas, Government College University, PakistanReviewed by:
Van-An Duong, University of Texas Health Science Center at Houston, United StatesSatyajit Tripathy, Amity University, India
Abdulhammed Hamzat, Wichita State University, United States
Copyright © 2025 Barabadi, Noqani, Soltani and Sabbagh Kashani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hamed Barabadi, YmFyYWJhZGlAc2JtdS5hYy5pcg==, YmFyYWJhZGkuODdAZ21haWwuY29t
 Hamed Barabadi
Hamed Barabadi Hesam Noqani
Hesam Noqani Maha Soltani
Maha Soltani Ayeh Sabbagh Kashani
Ayeh Sabbagh Kashani