- University Institute of Pharma Sciences, Chandigarh University, Mohali, India
Nanodiamonds have emerged as a powerful tool in the field of drug delivery, offering exciting recent advancements and promising future prospects. Recent research has focused on enhancing drug loading efficiency, targeted delivery, and precision medicine using nanodiamonds. Strategies such as surface functionalization, polymer coatings, and encapsulation techniques have been optimized to improve drug loading, stability, and controlled release. Nanodiamonds have shown potential in targeted drug delivery through the incorporation of targeting ligands, enabling site-specific drug delivery and improved therapeutic outcomes. Additionally, nanodiamonds allow for combination therapy by carrying multiple drugs simultaneously, opening avenues for synergistic treatments. Stimuli-responsive nanodiamonds have been developed for precise and controlled drug release. Overcoming biological barriers, such as the blood-brain barrier, has been investigated by modifying nanodiamonds’ surfaces. Recent studies have highlighted innovative strategies for loading a variety of therapeutic cargoes onto nanodiamond platforms, including small molecules, proteins, nucleic acids, and even gene-editing tools. Future prospects include the integration of nanodiamonds with emerging technologies and addressing biocompatibility and safety concerns. Despite the need for further research and clinical studies, the recent advancements and future prospects of nanodiamonds in drug delivery suggest their potential to revolutionize the field and enhance patient care.
Introduction
Nanodiamonds have emerged as a promising and versatile platform in the field of drug delivery, ushering in a new era of advancements and offering exciting future prospects. These nanoscale diamond particles possess unique properties that make them ideal for various biomedical applications, particularly in the realm of targeted drug delivery (Zhang X-Q. et al., 2011). Over the past decade, significant progress has been made in harnessing the potential of nanodiamonds to overcome challenges associated with conventional drug delivery systems. This review article provides an overview of recent advancements in the field of nano-diamond-based drug delivery and explores the promising future prospects that lie ahead. The remarkable features of nanodiamonds, such as their high surface area, biocompatibility, and tunable surface chemistry, make them well-suited for drug delivery application (Chow et al., 2011). Recent research has focused on improving drug loading efficiency, enhancing stability, and enabling controlled release of therapeutics. Surface modifications and functionalization strategies have been explored to tailor the properties of nanodiamonds for specific applications, including the attachment of targeting ligands or antibodies for site-specific delivery. One of the significant recent advancements lies in the development of combination therapy using nanodiamonds. By carrying multiple drugs or therapeutic agents simultaneously, nanodiamonds offer the potential for synergistic treatments, opening up new avenues for improved therapeutic outcomes. Moreover, stimuli-responsive nanodiamonds have been engineered to enable precise and controlled drug release triggered by specific environmental cues or external stimuli (Liu et al., 2007).
Recent research has directed its attention toward exploiting the capacity of nanodiamonds to surmount biological obstacles, notably the formidable blood-brain barrier. Strategies involving surface modifications and functionalization techniques have been harnessed to amplify the ability of nanodiamonds to traverse these barriers, thereby opening avenues for precise drug delivery to anatomical locales that were once impervious. Looking towards the future, nanodiamonds hold immense promise in the field of drug delivery. Integration with emerging technologies, such as gene therapy, immunotherapy, or regenerative medicine, could lead to groundbreaking advancements (Schrand et al., 2007). Furthermore, addressing biocompatibility and safety concerns will be crucial for successful translation into clinical practice.
This review article aims to provide a comprehensive overview of recent advancements in nano-diamond-based drug delivery, highlighting the significant strides made and the promising prospects that lie ahead. By examining the current state of the field, we hope to inspire further research, foster innovation, and ultimately pave the way for the widespread utilization of nanodiamonds as a transformative tool in drug delivery, leading to improved patient care and therapeutic outcomes.
Nanodiamonds: a brief appraisal
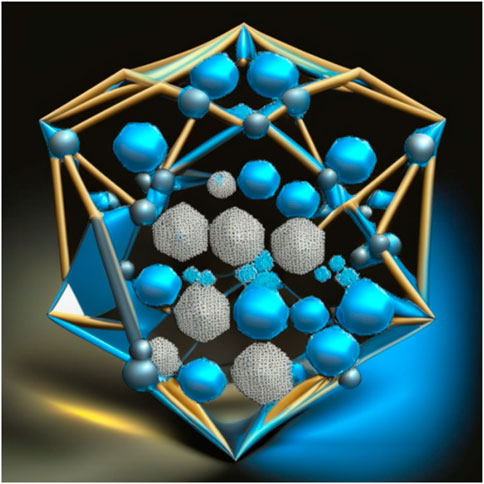
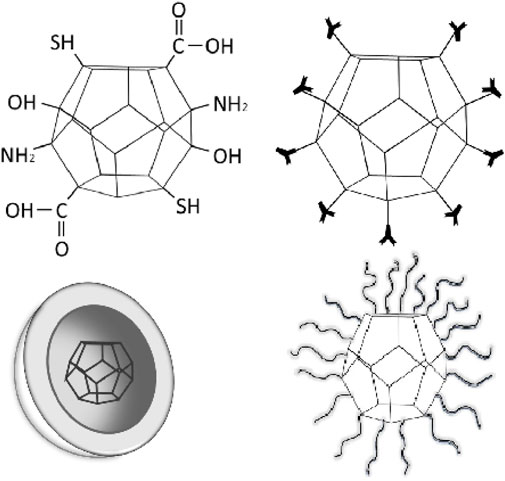
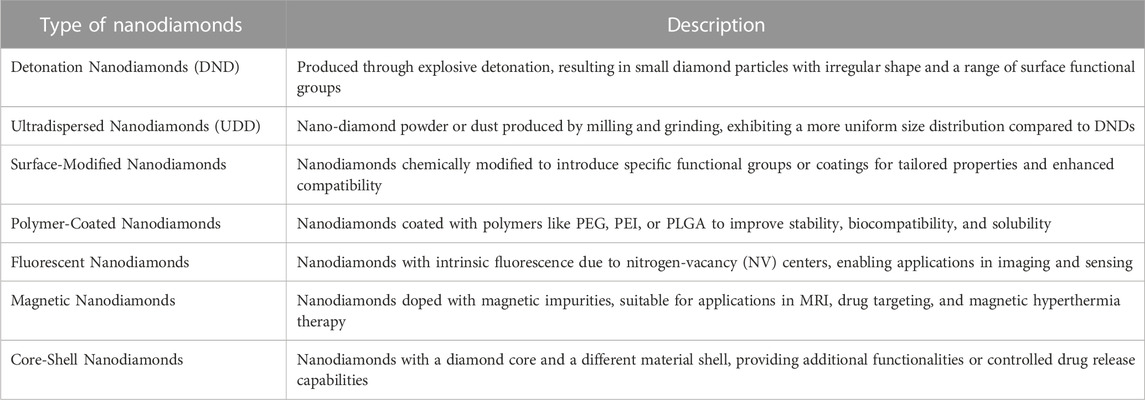
Nanodiamonds are tiny particles composed of carbon atoms arranged in a diamond lattice structure. They are tiny, typically measuring less than 10 nm in size (Figure 1). These nanoscale diamonds possess unique properties that make them useful in various fields, including medicine and drug delivery. One of the key advantages of nanodiamonds is their biocompatibility, meaning they are generally well-tolerated by biological systems and have low toxicity. This makes them suitable for biomedical applications. Nanodiamonds can be easily modified on their surface by attaching specific molecules or drugs, allowing for targeted drug delivery (Zhang Q. et al., 2011). This means that drugs can be precisely delivered to specific cells or tissues, increasing their effectiveness and reducing side effects. Furthermore, nanodiamonds provide a protective environment for drugs, shielding them from degradation and increasing their stability. They can be engineered to release drugs in a controlled manner, ensuring a sustained therapeutic effect over a desired timeframe. This controlled release feature reduces the need for frequent dosing. Nanodiamonds also offer the potential for multifunctionality. They can be combined with imaging agents or targeting ligands, enabling simultaneous drug delivery and real-time monitoring of therapeutic response (Alhaddad et al., 2017a). The small size of nanodiamonds allows for efficient cellular uptake, ensuring that the drugs reach their intended target sites within cells. Figure 2 provides different structural orientation of nanodiamond morphology. Table 1 enlists different types of Nanodiamonds available for therapeutic use.
Surface modification strategies in nanodiamonds
Surface modifications in nanodiamonds
Surface modification plays a crucial role in tailoring the properties of nanodiamonds for specific applications, including drug delivery. Here’s an overview of surface modification techniques used in nanodiamonds.
1. Chemical Functionalization: Chemical functionalization involves the attachment of various functional groups to the surface of nanodiamonds. Common functional groups include carboxyl (-COOH), amino (-NH2), hydroxyl (-OH), and thiol (-SH) groups. Functionalization can be achieved through oxidation, hydrogenation, or covalent bonding reactions. This process allows for the introduction of specific chemical moieties on the nano-diamond surface, enabling subsequent attachment of drugs, targeting ligands, or other biomolecules (Zhang et al., 2007).
2. Polymer Coating: Nanodiamonds can be coated with polymers to modify their surface properties. Polymers such as polyethylene glycol (PEG), polyethyleneimine (PEI), or poly (lactic-co-glycolic acid) (PLGA) can be used to create a protective layer around the nanodiamonds. Polymer coatings can improve the stability, biocompatibility, and colloidal stability of nanodiamonds, as well as provide functional groups for further bioconjugation.
3. Biomolecule Conjugation: Nanodiamonds ' surfaces can be functionalized with biomolecules, including antibodies, peptides, or aptamers, to confer specific targeting abilities. This involves the attachment of biomolecules to the nano-diamond surface through covalent bonding or affinity interactions. The biomolecules act as targeting ligands that recognize and bind to specific receptors on target cells or tissues, facilitating site-specific drug delivery.
4. Surface Charge Modification: Nanodiamonds can be modified to control their surface charge, which can influence their interactions with biological systems. This can be achieved by altering the type and density of functional groups on the nano-diamond surface. Controlling the surface charge allows for optimized drug loading, stability, and cellular uptake based on the specific requirements of the drug delivery system (Huang et al., 2007).
5. Encapsulation: Nanodiamonds can also be utilized for drug encapsulation, where drugs are trapped within the voids or interstitial spaces of the nano-diamond structure. This can be achieved by modifying the surface chemistry of nanodiamonds to create hydrophobic or hydrophilic environments, which can facilitate drug entrapment.
6. Intracellular Drug Action: Once released, the drugs exert their therapeutic effect within the target cells. The precise delivery facilitated by the nanodiamonds increases the efficacy of the drugs and reduces the potential for off-target effects (Chen et al., 2009a). Table 2 provides different surface modification strategies for Nanodiamonds in drug delivery.
Stability aspects of nanodiamonds
Nanodiamonds exhibit remarkable stability, both in terms of their physical structure and their ability to protect drugs. The diamond lattice structure of nanodiamonds provides exceptional mechanical robustness, allowing them to withstand a range of environmental conditions encountered during drug formulation, storage, and administration. This inherent stability enables nanodiamonds to retain their integrity under harsh physiological conditions, including changes in temperature, pH, and mechanical stress (Mochalin et al., 2012). Consequently, nanodiamonds can maintain their structural integrity and functionality, ensuring their effectiveness as drug carriers. In addition to their physical stability, nanodiamonds offer a unique protective environment for drugs. When drugs are loaded onto or encapsulated within nanodiamonds, they benefit from the diamond’s protective properties. Nanodiamonds act as a shield, preventing direct exposure of the drugs to external factors that may cause degradation, such as light, moisture, or enzymatic activity (Alhaddad et al., 2017b). This protective effect significantly enhances the stability of the loaded drugs, preserving their chemical integrity and therapeutic activity over extended periods. The stability provided by nanodiamonds is particularly advantageous for drugs that are prone to degradation or have a limited shelf life. By encapsulating these drugs within nanodiamonds or attaching them to the nano-diamond surface, their stability can be greatly enhanced, reducing the risk of loss in potency or therapeutic efficacy. Moreover, the stability of nanodiamonds allows for long-term storage of drug-loaded formulations without compromising the drugs’ stability, which is essential for commercialization and widespread use (Li Y. et al., 2019).
Strategies to improve drug loading efficiency and controlled release using nanodiamonds
Surface Functionalization: Nanodiamonds can be functionalized with various chemical groups to enhance their compatibility with different drug molecules. Functionalization can be achieved through methods like oxidation or plasma treatment to introduce functional groups like carboxyl, amine, or hydroxyl groups on the nanodiamond surface. These functional groups can form strong chemical bonds with drug molecules, increasing drug loading efficiency. Additionally, specific functional groups can be added to target specific cells or tissues, enabling targeted drug delivery.
Coating and Encapsulation: Coating or encapsulating nanodiamonds with biocompatible polymers, lipids, or other materials can protect the loaded drug molecules and control their release. Polymers like polyethylene glycol (PEG) can form a protective layer around nanodiamonds, preventing premature drug release. The encapsulating material can be designed to degrade or respond to specific triggers, such as changes in pH or temperature, allowing controlled release over time.
pH-Responsive Systems: pH-responsive nanodiamonds are designed to release drugs in response to the pH environment of a specific location in the body. For instance, in cancer therapy, the slightly acidic tumor microenvironment can be exploited. Nanodiamonds can be functionalized with pH-sensitive linkers that break down in the acidic environment, leading to the release of the drug payload selectively within the tumor.
Temperature-Responsive Systems: Temperature-responsive polymers, such as thermosensitive hydrogels, can be coated onto nanodiamonds. These polymers undergo conformational changes in response to temperature shifts, allowing the encapsulated drug to be released when exposed to a certain temperature. This approach is particularly useful for localized drug release, such as in hyperthermia-based cancer treatment.
Magnetic Targeting: Nanodiamonds can be coated with magnetic nanoparticles, allowing for external magnetic guidance of the drug-loaded particles to specific target sites. Once the nanodiamonds accumulate at the desired location, the drug release can be triggered by a specific stimulus, such as a change in temperature or pH, ensuring controlled release at the intended site.
Electric Field-Responsive Systems: Nanodiamonds can be functionalized with electrically responsive materials that change their properties under the influence of an electric field. This approach enables on-demand drug release by applying an electric field externally, which triggers changes in the nanodiamond structure or the surrounding encapsulation layer, leading to drug release.
Ultrasound-Triggered Release: By incorporating ultrasound-responsive materials onto nanodiamond surfaces, drug release can be triggered using focused ultrasound waves. The energy from the ultrasound causes mechanical stress, leading to the disruption of the encapsulation layer and controlled drug release. This technique offers non-invasive and precise control over drug delivery.
Multi-Drug Loading: Nanodiamonds’ high surface area allows for the loading of multiple types of drugs, making them ideal for combination therapies. Different drugs can be loaded onto the nanodiamonds simultaneously, allowing for synergistic effects and improved therapeutic outcomes.
Size and Surface Charge Optimization: The size of nanodiamonds affects their drug loading capacity and release kinetics. Smaller nanodiamonds have larger surface area-to-volume ratios, potentially leading to higher drug loading. Additionally, optimizing the surface charge through functionalization can influence interactions with drug molecules and cellular uptake, affecting drug release profiles.
In vivo Studies and Safety: Rigorous in vivo studies are essential to validate the safety, biocompatibility, and efficacy of nanodiamond-based drug delivery systems. These studies involve assessing factors such as nanoparticle distribution, clearance, potential toxicity, and therapeutic efficacy in living organisms.
Method of preparation of nanodiamonds
Nanodiamonds are synthesized using diverse methods that manipulate carbon atoms at the nanoscale, yielding unique structures with applications across various fields. One common approach involves subjecting carbon sources to extreme conditions of high pressure and temperature, akin to natural diamond formation, resulting in nanodiamonds. Another method employs chemical vapor deposition, where carbon-containing gases are broken down into atoms and deposited onto a substrate to form diamond structures. Detonation of carbon-containing mixtures and shock compression are explosive methods that induce the transformation of carbon into nanodiamonds through rapid pressure changes. These versatile techniques cater to specific applications, and continual research ensures the evolution of nanodiamond synthesis for innovative uses. The general depiction of nanodiamond formation is given in Figure 3.
Recent advancements in nanodiamonds
Improved drug loading capacity
Researchers have been exploring novel methods to enhance the drug loading capacity of nanodiamonds. For example, surface modifications with specific functional groups or the use of polymer coatings have been investigated to increase the loading efficiency and stability of drugs on nanodiamonds.
Targeted drug delivery
Advancements have been made in the development of targeted drug delivery systems using nanodiamonds. Researchers have been working on functionalizing nanodiamonds with targeting ligands, such as antibodies or peptides, to enable specific interactions with target cells or tissues. This allows for more precise drug delivery, reducing off-target effects and improving therapeutic outcomes (Shenderova and Zhirnov, 2013).
Controlled drug release
Recent research has focused on developing strategies for controlled drug release from nanodiamonds. By designing nanodiamonds with responsive properties, such as pH or temperature sensitivity, researchers aim to achieve on-demand drug release at the target site. This controlled release approach can enhance the therapeutic efficacy and reduce the frequency of drug administration.
Multimodal imaging and therapy
Nanodiamonds have been explored for their potential in combined imaging and therapy. By incorporating imaging agents, such as fluorescent dyes or magnetic nanoparticles, onto the nano-diamond surface, researchers can monitor drug delivery and therapeutic response in real-time. This multimodal approach allows for personalized medicine and improved treatment outcomes (Yuan and Liu, 2018).
Bioimaging applications
Nanodiamonds have shown promise as contrast agents for bioimaging techniques, such as magnetic resonance imaging (MRI) and fluorescence imaging. Their unique optical and magnetic properties make them suitable for enhancing imaging resolution, providing better visualization of tissues or specific cell populations.
Translational features of nanodiamonds
Nanodiamonds hold significant translational potential across multiple fields, including medicine, diagnostics, and biotechnology. Ongoing research and development are exploring their practical applications and paving the way for their translation into real-world use. Here are some areas where nanodiamonds show promising translational potential.
1. Drug Delivery Systems: Nanodiamonds have the potential to revolutionize drug delivery systems by enhancing drug stability, enabling targeted delivery, and providing controlled release of therapeutics. Ongoing research aims to optimize nano-diamond-based drug delivery platforms for specific diseases, improving treatment efficacy and reducing side effects (Alhaddad et al., 2018a).
2. Imaging and Diagnostics: The unique optical and magnetic properties of nanodiamonds make them attractive for various imaging modalities and diagnostic applications. Ongoing studies are focused on harnessing nanodiamonds as contrast agents for advanced imaging techniques, including MRI, fluorescence imaging, and Raman spectroscopy. The development of nano-diamond-based imaging agents holds promise for early disease detection, personalized medicine, and improved diagnostic accuracy.
3. Biosensors and Bioassays: Nanodiamonds are being explored as building blocks for biosensors and bioassays due to their exceptional surface chemistry and biocompatibility. Ongoing research is focused on utilizing nanodiamonds for ultrasensitive detection of biomarkers, environmental pollutants, and disease-specific molecules. Their high surface area and robustness make nano-diamond-based biosensors promising tools for rapid and sensitive diagnostic applications (Krueger, 2019).
4. Tissue Engineering and Regenerative Medicine: Nanodiamonds have shown potential in tissue engineering and regenerative medicine applications. Ongoing research is investigating the use of nanodiamonds as scaffolds or additives in biomaterials for promoting cell adhesion, proliferation, and differentiation. The unique properties of nanodiamonds, such as their biocompatibility and ability to support cellular functions, make them valuable tools for tissue engineering and regenerative medicine approaches (Perevedentseva et al., 2013).
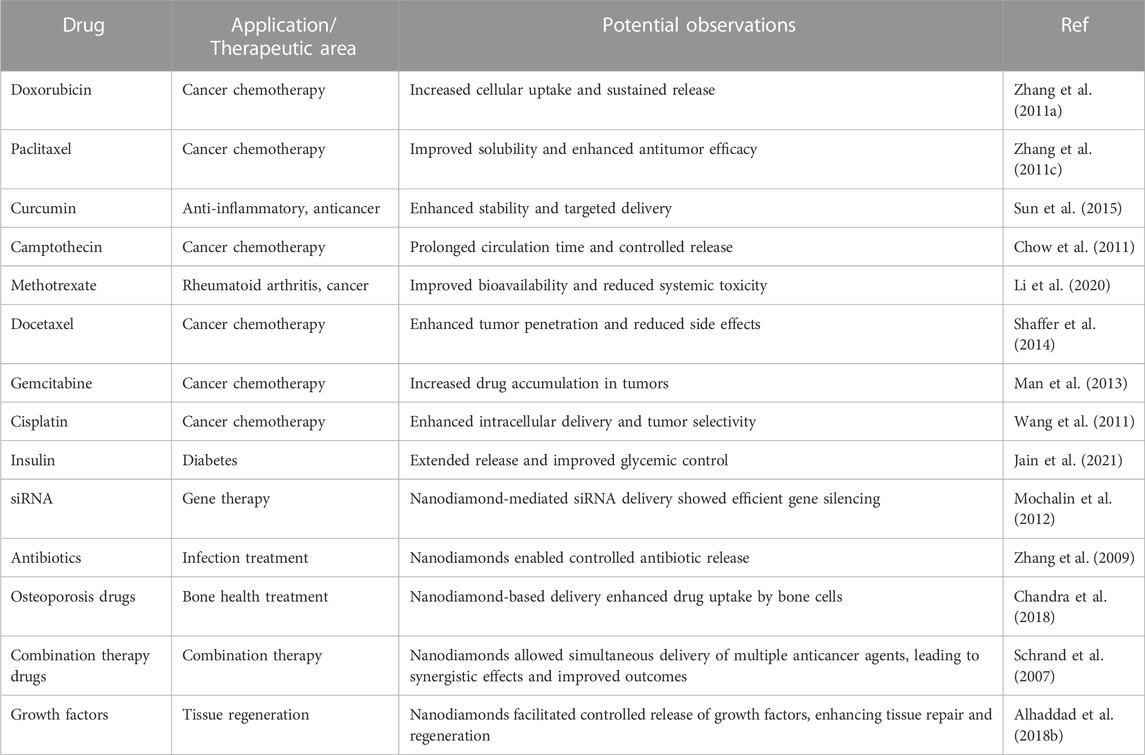
5. Theranostics: Nanodiamonds have the potential to integrate therapeutic and diagnostic functions into a single platform, known as theranostics. Ongoing research is exploring the development of nano-diamond-based theranostic systems that can simultaneously deliver therapeutics to the target site and provide real-time monitoring of treatment response. This integrated approach holds promise for personalized medicine, allowing for tailored therapies based on individual patient needs. Table 3 provides critical assessment of various drugs utilized in nanodiamonds to improve therapeutic efficacy.
Emerging technologies integrated with nanodiamonds in drug delivery
Several emerging technologies have the potential to synergize with nanodiamonds and further amplify their capabilities in drug delivery. These technologies offer innovative ways to address existing limitations and improve the precision, efficiency, and control of drug release from nanodiamond-based systems. Some of these technologies include:
Gene Editing and Therapy: Integrating nanodiamonds with gene editing tools like CRISPR-Cas9 could enable targeted delivery of genetic materials for therapeutic purposes. Nanodiamonds could serve as carriers for CRISPR components, enhancing their delivery to specific cells or tissues, and improving the efficiency of gene editing therapies (Chen et al., 2009b).
Nanotechnology-Enabled Drug Targeting: Combining nanodiamonds with advanced targeting strategies, such as ligand-receptor interactions or antibody conjugation, can enhance the specificity of drug delivery. By attaching targeting ligands to nanodiamond surfaces, drug-loaded nanoparticles can be guided precisely to diseased cells or tissues, minimizing off-target effects (Chow et al., 2011).
Responsive Nanomaterials: Developing responsive nanomaterials that can be triggered by external stimuli, such as light, heat, or magnetic fields, can provide on-demand drug release from nanodiamonds. By incorporating these stimuli-responsive elements into the nanodiamond-based systems, researchers can achieve precise spatiotemporal control over drug release (Haziza et al., 2015).
Microfluidics and Lab-on-a-Chip Devices: Microfluidic technologies can be integrated with nanodiamonds to create lab-on-a-chip devices for real-time monitoring of drug release. These devices allow for the continuous monitoring of drug concentration, enabling feedback-driven control of drug delivery based on therapeutic needs (Krueger, 2013).
Biosensors and Imaging Modalities: Nanodiamonds possess unique optical properties that make them ideal candidates for biosensing and imaging applications. By engineering nanodiamonds to carry specific biomarkers or imaging agents, they can be used to monitor drug release, assess treatment efficacy, and provide real-time feedback (Bumb et al., 2009).
3D Printing and Personalized Medicine: Nanodiamonds can be incorporated into 3D-printed drug delivery matrices, enabling the creation of personalized implantable devices. Such devices can be tailored to patient-specific needs, allowing for controlled and localized drug release over extended periods.
Artificial Intelligence (AI) and Machine Learning: AI-driven approaches can optimize drug delivery systems by analyzing large datasets to predict the behavior of nanodiamond-based carriers in different biological environments. AI can help design personalized drug delivery strategies based on patient-specific characteristics and disease profiles.
Nanodiamonds for Combination Therapies: Integrating nanodiamonds with multiple therapeutic agents, including drugs, nucleic acids, and nanoparticles, can enable synergistic combination therapies. These therapies can target multiple aspects of a disease simultaneously, improving treatment outcomes (Yang et al., 2013).
Bioinformatics and Systems Biology: Leveraging bioinformatics and systems biology approaches can enhance the understanding of nanodiamond interactions with biological systems. This knowledge can guide the design of more effective drug delivery systems and predict potential adverse effects.
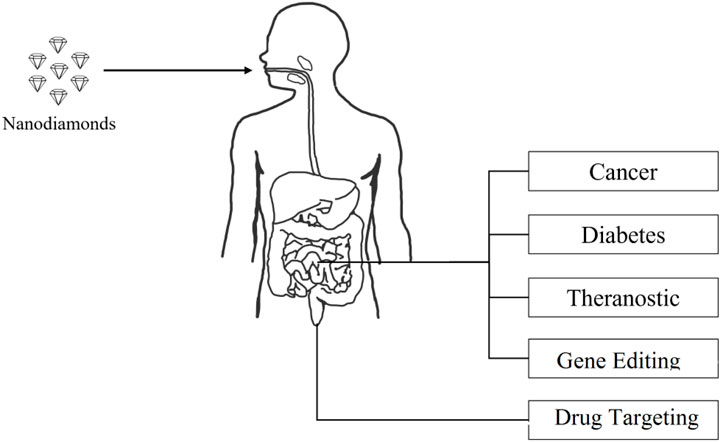
Miniaturized Sensors and Wearable Devices: Nanodiamond-based drug delivery systems can be integrated into wearable devices equipped with sensors. These devices can monitor physiological parameters and adjust drug release rates in real time, offering personalized and adaptable therapeutic interventions (Perevedentseva et al., 2009). The compiled assembly of various pharmaceutical applications of nanodiamonds is given in Figure 4.
Current limitations or safety concerns of nanodiamonds in clinical applications
The use of nanodiamonds in clinical applications holds significant promise, yet it is accompanied by several limitations and safety concerns that must be addressed for their successful integration into medical practice. Despite their generally favorable biocompatibility, concerns about potential toxicity persist (Alhaddad et al., 2018c). While nanodiamonds have shown promise in preclinical studies, the long-term effects of their accumulation in tissues and organs remain inadequately understood, necessitating in-depth investigations into potential adverse effects. Moreover, the modifications and coatings used to functionalize nanodiamonds can influence their biocompatibility and trigger immune responses, potentially leading to inflammation, immune system activation, or even rejection (Perevedentseva and Chang, 2013). The biodistribution and clearance mechanisms of nanodiamonds within the body require detailed characterization to ensure that they do not accumulate in specific organs or tissues, which could result in localized toxicity or unintended effects. Furthermore, the impact of factors like nanodiamond size, surface charge, and functional groups on their interactions with cells, proteins, and biomolecules must be thoroughly explored to mitigate potential safety risks (Neugart et al., 2007). As a relatively novel area of research, the long-term effects of nanodiamond-based therapies on human health remain a subject of ongoing investigation. The consistency of nanodiamond manufacturing processes is also a challenge, as variations can impact their safety and efficacy. Regulatory approval processes, while essential for ensuring patient safety, can pose delays in translating promising nanodiamond research into clinical applications (Sotoma et al., 2015). Additionally, concerns about nanodiamond aggregation, interactions with biomolecules, and potential environmental impact must be carefully addressed through comprehensive research and collaboration among various stakeholders. In sum, while nanodiamonds hold immense potential, a comprehensive understanding of their safety profile and thorough research is imperative to harness their benefits and mitigate any potential risks in clinical settings (Schrand et al., 2014).
Ethical concerns and environmental impact of nanodiamonds
The integration of nanodiamonds into drug delivery systems presents a promising avenue for medical advancements, yet it is accompanied by a range of ethical concerns that necessitate careful consideration. Foremost among these concerns is the long-term safety of nanodiamonds within the human body. While initial studies have indicated their biocompatibility, the potential consequences of prolonged exposure remain largely uncharted territory. Ensuring that nanodiamonds do not accumulate in tissues over time and that they do not pose latent health risks is a paramount ethical obligation (Lee et al., 2014). Additionally, the possible toxicity of nanodiamonds and their biodegradability warrant thorough examination. As foreign nanoparticles, their interactions with biological systems need comprehensive assessment to ascertain whether they trigger unexpected cellular responses or unwanted immune reactions (Mo et al., 2012). These unintended consequences could have far-reaching implications for the safety and efficacy of nanodiamond-based drug delivery systems. Moreover, ethical considerations extend beyond human health to the environment (Li J-L. et al., 2019). The manufacturing, utilization, and disposal of nanodiamonds could potentially release these nanoparticles into ecosystems, raising concerns about their impact on wildlife and natural ecosystems. Regulatory oversight must be agile enough to keep pace with rapid nanodiamond research developments to ensure rigorous evaluation and comprehensive testing before introducing these therapies into clinical practice. Equitable access to nanodiamond-based therapies also emerges as a concern, guarding against potential disparities in healthcare delivery (Zhang et al., 2020). To navigate these ethical complexities, interdisciplinary collaboration among researchers, clinicians, ethicists, regulatory bodies, and policymakers is indispensable. A responsible approach that emphasizes risk assessment, comprehensive research, transparent communication, and equitable distribution is imperative to harness the potential of nanodiamonds for drug delivery while safeguarding human health and the environment (Yoon et al., 2020).
Future prospects
Nanodiamonds have emerged as a promising tool in the field of drug delivery, offering significant advancements and holding promising future prospects. Recent research has contributed to their development and understanding, and ongoing studies continue to uncover new possibilities. Here are some recent advancements and future prospects in the field of nano-diamond-based drug delivery.
1. Enhanced Drug Loading and Delivery Efficiency: Recent advancements have focused on improving the loading capacity and delivery efficiency of nanodiamonds. Strategies such as surface functionalization, polymer coatings, and encapsulation techniques have been optimized to enhance drug loading, stability, and controlled release. These advancements enable more efficient and targeted delivery of therapeutics to the desired sites of action (Lewinski et al., 2008).
2. Targeted Delivery and Precision Medicine: Nanodiamonds have shown promise in targeted drug delivery. Recent developments include the incorporation of targeting ligands, such as antibodies or peptides, onto nano-diamond surfaces for specific interactions with target cells or tissues. This targeted approach allows for site-specific drug delivery, reducing off-target effects and improving therapeutic outcomes. The future prospects involve the customization of nanodiamonds to match specific patient profiles, enabling personalized medicine approaches (Zhang W. et al., 2011).
3. Combination Therapy and Multifunctionality: Nanodiamonds offer the potential for combination therapy by carrying multiple drugs or therapeutic agents simultaneously. Recent studies have explored the co-loading of different drugs or combining therapeutic agents with diagnostic capabilities, such as imaging agents. This multifunctionality of nanodiamonds opens avenues for synergistic treatments and improved therapeutic outcomes (Nel et al., 2006).
4. Stimuli-Responsive Drug Release: Advancements in nano-diamond-based drug delivery systems include the development of stimuli-responsive nanodiamonds. These nanodiamonds can release drugs in response to specific triggers such as pH, temperature, light, or enzymes. This controlled drug release feature allows for precise temporal and spatial control over drug administration, maximizing therapeutic efficacy while minimizing side effects (Liu et al., 2008).
5. Overcoming Biological Barriers: Nanodiamonds show promise in overcoming biological barriers, such as the blood-brain barrier (BBB), which limits drug delivery to the central nervous system. Recent research has explored surface modifications and functionalization strategies to enhance the transport of nanodiamonds across biological barriers, opening up opportunities for the treatment of neurological disorders and diseases. (Lin et al., 2006).
6. Biocompatibility and Safety Considerations: Ongoing research aims to improve the biocompatibility and safety profiles of nano-diamond-based drug delivery systems. Recent advancements have focused on understanding the long-term effects and potential immune responses associated with nanodiamonds. By addressing biocompatibility and safety concerns, nanodiamonds can be further optimized for clinical translation (Duncan, 2006).
Conclusion
While significant progress has been made in understanding the potential of nanodiamonds, there is still a need for further research, optimization, and safety evaluations to translate these advancements into clinical and commercial applications. Nonetheless, the ongoing translational potential of nanodiamonds across these diverse areas holds promise for addressing critical challenges in healthcare and biotechnology, with the potential to improve patient outcomes and advance medical practices.
Author contributions
DS: Investigation, Methodology, Validation, Visualization, Writing–original draft, Writing–review and editing. SR: Writing–review and editing. All authors contributed to the article and approved the submitted version.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alhaddad, A., Das, G., and Ercius, P. (2017b). Multifunctional nanodiamonds in regenerative medicine: recent advances and future directions. Adv. Healthc. Mater. 6 (7), 1601110.
Alhaddad, A., Adam, M. P., Botsoa, J., Marangon, I., Boudou, T., Bertrand, J. R., et al. (2017a). Functionalizing detonation nanodiamonds for biomedical applications: a survey of chemical approaches. Nanomaterials 7 (2), 39.
Alhaddad, A., Adamcik, J., Mezzenga, R., and Glinel, K. (2018b). Nanodiamond-based materials for drug delivery: functionalization, characterization, and biocompatibility. Biomacromolecules 19 (3), 810–821.
Alhaddad, A., Das, G., and Cai, L. (2018a). Detonation nanodiamonds as platform for the multi-modal drug delivery system: loading, release and visualization. J. Control. Release 279, 169–177.
Alhaddad, A., Durand, M., and Adamcik, J. (2018c). Nanodiamond-based therapeutic delivery agents: detonation nanodiamonds– liposome complexes for controlled drug release. Nanoscale 10 (38), 18066–18075.
Bumb, A., Sarkar, S., and Prasanth Kumar, S. (2009). Nanodiamonds as a platform for fluorescence imaging and drug delivery. ACS Nano 3 (12), 3955–3961.
Chandra, S., Barui, A. K., Hasegawa, D., Hasani, M., Zheng, T., and Schirhagl, R. (2018). Nanodiamonds and their applications in cells. Small 14 (21), 1704263. doi:10.1002/smll.201704263
Chen, M., Pierstorff, E., Lam, R., Li, S. Y., Huang, H., Osawa, E., et al. (2009b). Nanodiamond-mediated delivery of water-insoluble therapeutics. ACS Nano 3 (7), 2016–2022. doi:10.1021/nn900480m
Chen, M., Zhang, X. Q., and Man, H. B. (2009a). Nanodiamond vectors functionalized with polyethyleneimine for siRNA delivery. J. Control. Release 139 (1), 67–73.
Chow, E. K. H., Zhang, X-Q., Chen, M., Lam, R., Robinson, E., Huang, H., et al. (2011). Nanodiamond therapeutic delivery agents mediate enhanced chemoresistant tumor treatment. Sci. Transl. Med. 3 (73), 73ra21. doi:10.1126/scitranslmed.3001713
Cui, H., Webber, M. J., and Stupp, S. I. (2010). Self-assembly of peptide amphiphiles: from molecules to nanostructures to biomaterials. Biopolymers 94 (1), 1–18. doi:10.1002/bip.21328
Duncan, R. (2006). Polymer conjugates as anticancer nanomedicines. Nat. Rev. Cancer 6 (9), 688–701. doi:10.1038/nrc1958
Fang, C-Y., Vaijayanthimala, V., Cheng, Y-H., Yeh, C-C., Chang, C-C., and Li, C-L. (2013). Noncytotoxic nanodiamonds as calcium carriers for gene delivery. ACS Nano 7 (6), 5770–5780.
Ghosh, D., Bagley, A. F., Na, Y. J., Birrer, M. J., Bhatia, S. N., and Belcher, A. M. (2014). Deep, noninvasive imaging and surgical guidance of submillimeter tumors using targeted M13-stabilized single-walled carbon nanotubes. Proc. Natl. Acad. Sci. 111 (38), 13948–13953. doi:10.1073/pnas.1400821111
Haziza, S., Mohan, N., and Loe-Mie, Y. (2015). Nanodiamonds for in vivo applications. Molecules 20 (4), 6728–6740.
Huang, H., Pierstorff, E., Osawa, E., and Ho, D. (2007). Active nanodiamond hydrogels for chemotherapeutic delivery. Nano Lett. 7 (11), 3305–3314. doi:10.1021/nl071521o
Jain, A., Thareja, S., and Singh, N. (2021). Nanodiamonds: emerging delivery system for therapeutics and imaging. Nano Rev. Exp. 12 (1), 1975784.
Krueger, A. (2013). Diamond nanocrystals and graphene for biomedical applications. Nanomedicine 8 (12), 1991–2010.
Krueger, A., and Lang, D. (2012). Functionality is key: recent progress in the surface modification of nanodiamond. Adv. Funct. Mater. 22 (5), 890–906. doi:10.1002/adfm.201102670
Krueger, A. (2019). Nanodiamond: a high impact nanomaterial for multifaceted applications in drug delivery. J. Control. Release 307, 383–395.
Lee, S., Huang, H., and Choi, Y. (2014). Nanodiamond–Graphene nanocomposites as highly efficient materials for photothermal cancer therapy. ACS Appl. Mater Interfaces 6 (12), 10268–10277.
Lewinski, N., Colvin, V., and Drezek, R. (2008). Cytotoxicity of nanoparticles. Small 4 (1), 26–49. doi:10.1002/smll.200700595
Li, J-L., Goh, C. C., and Wong, A. S. W. (2019b). Nanodiamonds mediate oral delivery of chemotherapeutic agents. Sci. Rep. 9 (1), 15148.
Li, Y., Liu, X., Ji, T., and Niu, Q. (2020). Inhibition of miR-217 protects against myocardial ischemia-reperfusion injury through inactivating NF-κB and MAPK pathways. Curr. Drug Targets 21 (3), 219–227. doi:10.1007/s13239-019-00452-z
Li, Y., Wen, T., Zhao, R., Liu, X., Ji, T., and Wang, X. (2019a). Recent developments in nanodiamond-based delivery systems for cancer therapy. Drug Deliv. 26 (1), 446–460.
Liang, Y-C., Chiu, Y-C., Shieh, Y-A., Lin, C-W., Huang, C-C., Chuang, Y-J., et al. (2013). Size-dependent modulation of graphene oxide-aptamer interactions for an amplified fluorescence-based detection of aflatoxin B1. ACS Appl. Mater Interfaces 5 (17), 8934–8941.
Lin, W., Huang, Y-W., Zhou, X-D., and Ma, Y. (2006). Toxicity of cerium oxide nanoparticles in human lung cancer cells. Int. J. Toxicol. 25 (6), 451–457. doi:10.1080/10915810600959543
Liu, K. K., Zheng, W. W., Wang, C. C., Chiang, W. H., Han, C. C., Liu, T. M., et al. (2007). Endocytic carboxylated nanodiamond for the labeling and tracking of cell division and differentiation in cancer and stem cells. Biomaterials 28 (27), 4259–4267.
Liu, Z., Davis, C., Cai, W., He, L., Chen, X., and Dai, H. (2008). Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopy. Proc. Natl. Acad. Sci. U. S. A. 105 (5), 1410–1415. doi:10.1073/pnas.0707654105
Man, H. B., Kim, D., and Miller, J. D. (2013). Near-infrared fluorescence imaging of cancer cells and tumors through specific biosynthesis of silver nanoclusters. Sci. Rep. 3, 1394.
Mo, J., Liang, H., and Su, S. (2012). Engineered nanodiamonds for delivery of siRNA to neuronal cells. Adv. Mater 24 (46), 6285–6290.
Mochalin, V. N., Shenderova, O., Ho, D., and Gogotsi, Y. (2012). The properties and applications of nanodiamonds. Nat. Nanotechnol. 7 (1), 11–23. doi:10.1038/nnano.2011.209
Nel, A., Xia, T., Mädler, L., and Li, N. (2006). Toxic potential of materials at the nanolevel. Science 311 (5761), 622–627. doi:10.1126/science.1114397
Németh, C. L., Gali, A., and Szász, K. (2017). Nanodiamond-enhanced MRI via in situ hyperpolarization. Nat. Commun. 8, 12401.
Neugart, F., Zappe, A., Jelezko, F., Tietz, C., Boudou, J. P., Krueger, A., et al. (2007). Dynamics of diamond nanoparticles in solution and cells. Nano Lett. 7 (12), 3588–3591. doi:10.1021/nl0716303
Perevedentseva, E., and Chang, H-C. (2013). Nanodiamond: a promising platform for biomedical applications. Nanomedicine 8 (13), 2043–2060.
Perevedentseva, E., Lee, C., Cheng, C-Y., Chang, C-C., and Yeh, C-C. (2013). Functionalized nanodiamonds for biological and medical applications. J. Mater. Chem. B 1 (29), 3491–3502.
Perevedentseva, E., Lin, Y-C., Jani, M., Ponomarev, A., Lugovtsov, A., and Cheng, C-L. (2009). Noninvasive fluorescent imaging of human oral epithelial carcinoma cells by using nonfunctionalized nanodiamonds. Adv. Funct. Mater 19 (17), 2979–2984.
Prabhakar, N., Zhang, J., Deshmukh, M., and Nitin, N. (2014). Nanodiamonds: the intersection of nanotechnology, drug development, and personalized medicine. Wiley Interdiscip. Rev. Nanomed Nanobiotechnol 6 (6), 619–633.
Schrand, A. M., Huang, H., Carlson, C., Schlager, J. J., Ōsawa, E., Hussain, S. M., et al. (2007). Are diamond nanoparticles cytotoxic? J. Phys. Chem. B 111 (1), 2–7. doi:10.1021/jp066387v
Schrand, A. M., Lin, J. B., and Hens, S. C. (2014). Characterization of nanodiamond-based anti-amyloidogenic compounds for alzheimer’s disease. ACS Nano 8 (12), 11760–11769.
Shaffer, M. S. P., Gordeev, G., and Gupta, S. (2014). A general synthetic approach to ordered mesoporous polymers with high surface areas. J. Am. Chem. Soc. 136 (6), 2048–2051.
Shenderova, O. A., and Zhirnov, V. V. (2013). Nanotoxicity: challenging the myth of nano-specific toxicity. Curr. Opin. Biotechnol. 24 (4), 724–734. doi:10.1016/j.copbio.2013.05.003
Sotoma, S., Tobita, S., and Hiraoka, Y. (2015). Nanodiamond-enhanced neurotrophic factor delivery and release from fibrin matrices for neural tissue engineering. Biomaterials 53, 370–381.
Sun, Y., Chen, J., and Liu, H. (2015). Active targeting theranostic iron oxide nanoparticles for MRI and magnetic resonance-guided focused ultrasound ablation of lung cancer. ACS Appl. Mater Interfaces 7 (39), 21530–21539.
Wang, X., Liu, J., and Song, L. (2011). High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 6 (9), 553–557.
Yang, X., Liu, Y., and Cao, W. (2013). Application of nanodiamonds in cancer therapy. Expert Opin. Drug Deliv. 10 (4), 369–381.
Yoon, H., Youn, H., Kim, S., Kim, B., Kim, D., and Lee, N. (2020). Ultrasound-triggered on-demand antibiotic release from nanodiamonds decorated with liposomes for treatment of bacterial infection. J. Control Release 321, 638–648.
Yuan, Y., and Liu, B. (2018). Recent advances in the development of nanodiamond-based drug delivery systems. Expert Opin. Drug Deliv. 15 (10), 987–1002.
Zhang, X. Q., Chen, M., Lam, R., Xu, X., Osawa, E., Ho, D., et al. (2011c). Nanodiamond therapeutic delivery agents mediate enhanced chemoresistant tumor treatment. Sci. Transl. Med. 3 (73), 73ra21. doi:10.1126/scitranslmed.3001713
Zhang, Q., Mochalin, V. N., Neitzel, I., Hazeli, K., and Niesman, M. (2011b). Antibody binding and fluorescence imaging of nanodiamonds with nitrogen-vacancy centers. Nano Lett. 11 (3), 977–981.
Zhang, Q., Zhou, C., and Dang, R. (2020). Nanodiamond-based pH-responsive drug delivery systems for enhanced cancer therapy. ACS Appl. Mater Interfaces 12 (7), 7897–7906.
Zhang, W., Yao, Y., Sullivan, N., and Chen, Y. (2011d). Modeling the primary size effects of citrate-coated silver nanoparticles on their ion release kinetics. Environ. Sci. Technol. 45 (9), 4422–4428. doi:10.1021/es104205a
Zhang, X-Q., Chen, M., Lam, R., Xu, X., Osawa, E., and Ho, D. (2007). Non-covalent cellular uptake and intracellular fate of functionalized nanodiamonds. Nano Lett. 7 (12), 3714–3721.
Zhang, X-Q., Chen, M., Lam, R., Xu, X., Osawa, E., and Ho, D. (2009). Polymer-functionalized nanodiamond platforms as vehicles for gene delivery. ACS Nano 3 (9), 2609–2616. doi:10.1021/nn900865g
Zhang, X-Q., Chen, M., Lam, R., Xu, X., Osawa, E., and Ho, D. (2011a). Polymer-functionalized nanodiamond platforms as vehicles for gene delivery. ACS Nano 5 (7), 5322–5329.
Keywords: nanodiamonds, structural features, surface modification, drug targeting, translational potential
Citation: Singh D and Ray S (2023) A short appraisal of nanodiamonds in drug delivery and targeting: recent advancements. Front. Nanotechnol. 5:1259648. doi: 10.3389/fnano.2023.1259648
Received: 16 July 2023; Accepted: 17 August 2023;
Published: 30 August 2023.
Edited by:
Shahla Rezaei, Tehran University of Medical Sciences, IranReviewed by:
Sobia Noreen, University of Innsbruck, AustriaCopyright © 2023 Singh and Ray. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dilpreet Singh, ZGlscHJlZXQuZGFtYW5AZ21haWwuY29t
 Dilpreet Singh
Dilpreet Singh Supriya Ray
Supriya Ray