- 1School of Pharmacy, National Forensic Sciences University, Gandhinagar, India
- 2Dubai Pharmacy College, Dubai, United Arab Emirates
Editorial on the Research Topic
Advances in self-assembled nanocarriers for drug delivery
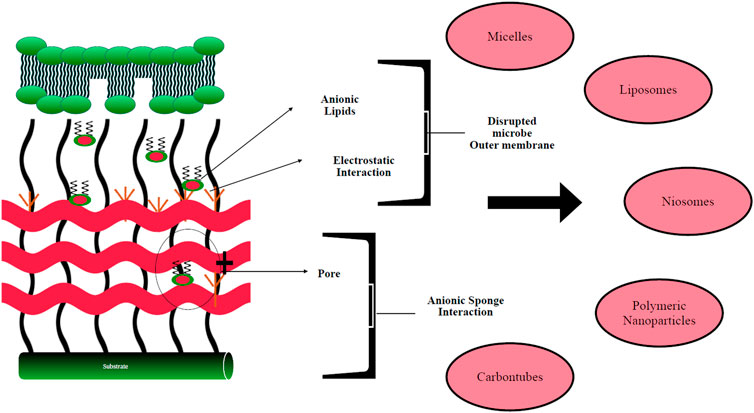
Self-assembled nanocarriers are attracting increasing attention from drug delivery scientists due to their ability to provide higher efficacy with fewer side effects. Creating such a system requires carefully selecting atoms or molecules that can be assembled spontaneously by hydrogen bonding, electrostatic, hydrophobic, and van der Waal interactions to form a stable, well-defined structure with the desired physicochemical and biological properties. Self-assembled nanocarriers comprise a wide range of systems, including surfactant-based nanoparticles such as micelles, liposomes, niosomes, polymeric nanoparticles, carbon nanotubes, and metal nanoclusters (Figure 1). Biomedical applications of these systems include drug, gene, and vaccine delivery, as well as tissue engineering. Combined with advanced materials science and the latest technologies, such systems could provide promising solutions to many unmet clinical needs.
The Research Topic Advances in Self-Assembled Nanocarriers for Drug Delivery includes four articles in total: two reviews and two original research papers. The review by Paliwal et al. discusses the current state of, and new trends in, self-assembled nanocarriers (Paliwal et al.). They highlight some of the innovations in these nanocarriers and their applications, such as in prolonging drug action, improving bioavailability, avoiding drug resistance, and enhancing cellular uptake. In addition to being interesting to read, this review also provides the most up-to-date status of the self-assembled nanocarriers in the preclinical, clinical, and market phases.
Despite the availability of small-molecule antiviral treatments, there was a clear unmet clinical need for them during the COVID pandemic caused by the SARS-CoV-2 virus. Nanoviricide’s Biopolymer (NV-CoV-2) with covalently attached polyethylene glycol and alkyl pendants is a platform technology developed by NanoViricides, Inc. NV-CoV-2 are unique micelles with up to 1,200 chemically attached ligands on their surface that can bind and inactivate viruses. A mini-review published in this Research Topic by Chakraborty et al. proposed a new antiviral regimen against SARS-CoV-2 based on NV-CoV-2 (Chakraborty et al.). The authors suggested that ligands like the SARS-CoV-2 S-protein could be designed by molecular modelling and attached to the nanoviricide at the same binding site of the cognate cellular receptor, ACE2, to achieve a competitive binding inhibition. Combining NV-CoV-2 with other anti-coronavirus regimens like remdesivir (NV-CoV-2-R) could have a stronger, synergistic antiviral effect. Encapsulating antiviral compounds in nanoviricide protects them from serum-mediated degradation in vivo, making the antiviral compounds available for longer to interact with RNA polymerase and inhibit it. Altogether, this manuscript describes a multipoint antiviral efficacy by the nanoviricide NV-CoV-2.
Among the different cationic polymers, polyethylenimines (PEIs) have been widely explored as a vector in gene delivery. The charge on the surface of the particles is an important criterion for gene binding and transfection efficiency, and efforts have been made to modify the surface of PEIs to improve these latter two qualities. Yadav et al. reported on the synthesis of tetramethylguanidine (TMG) bearing the amphiphilic cationic linker propyl tetramethylguanidinium (PTMG), and having a hydrophobic alkyl chain and a positively charged N, N, N, N-tetrametylguanidinium group, for tethering onto the backbone of linear polyethylenimine (lPEI) (Yadav et al.). A series of amphiphilic LP-PTMG polymers with varying amounts of PTMG linker was prepared, physicochemically characterized, and assessed for their cellular toxicity and transfection efficiency compared to native lPEI (25 kDa) and widely used branched PEI (bPEI). Unlike the high transfection efficiency with low toxicity reported in the earlier studies with TMG-modified branched PEIs, linear PEI modified with TMG resulted in a strong binding with pDNA, with lower pDNA release and transfection efficiency. The authors concluded that the functionalization or modification of the polymers for gene delivery does not necessarily result in a better vector than the native ones. Their findings will be useful for better understanding the structure–activity relationship of these polymers.
A study by Lee et al. aimed to assess drug–polymer compatibility as a determinant of encapsulation efficiency (EE) using Hansen solubility parameters (HSPs), Hansen interaction spheres (Ra), and Flory Huggins interaction parameters (χsp) (Lee et al.). The authors reported a relationship between drug–polymer compatibility and concentration ratios on the sizes and encapsulation efficiency of four different drugs (i.e., coumarin 6, DEX, SAHA, and lutein) in two different self-assembled block copolymers (i.e., poly [caprolactone]-b-poly [ethylene oxide] [PCL-b-PEO] and poly [styrene]-b-poly [ethylene oxide] [PS-b-PEO]). Nanoparticles were synthesized using batch sonication, continuous flow flash nanoprecipitation (FNP), and electrohydrodynamic mixing-mediated nanoprecipitation (EM-NP). The findings of this study are consistent with earlier studies that suggested that solubility parameters are unsuitable for predicting EE because they do not include entropic contributions or concentration effects in their calculations, and solubility parameters should be augmented with more complex modelling approaches to capture the relationship. The authors suggested that the polymer:drug ratio is an important determinant of EE and needs careful optimization during nanocarrier design. As this work is limited to only two polymers and four drugs, with only one drug used in polymer:drug ratio studies, additional combinations should be explored to strengthen the findings of this study.
We believe that scientists working in the fields of drug/gene delivery and tissue engineering will benefit from the studies in this Research Topic and will direct them to pursue further research in the area of self-assembled nanodrug delivery. We are thankful to the authors for their contributions to this topic, and we are also grateful to the reviewers for their thorough and timely review that made the high-quality output of this Research Topic possible.
Author contributions
DR supervised the project, AS has prepared and revised the Editorial, PP prepared Figure.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: self-assembled, nanocarriers, drug delivery, biopolymers, encapsulation
Citation: Rawtani D, Shahiwala A and Prajapati P (2022) Editorial: Advances in self-assembled nanocarriers for drug delivery. Front. Nanotechnol. 4:1020414. doi: 10.3389/fnano.2022.1020414
Received: 16 August 2022; Accepted: 07 September 2022;
Published: 12 October 2022.
Edited by:
Ajeet Kaushik, Florida Polytechnic University, United StatesReviewed by:
Raj Kumar, University of Nebraska Medical Center, United StatesCopyright © 2022 Rawtani, Shahiwala and Prajapati. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aliasgar Shahiwala, YWxpc2hhaGl3YWxhQGdtYWlsLmNvbQ==
 Deepak Rawtani
Deepak Rawtani Aliasgar Shahiwala
Aliasgar Shahiwala Prajesh Prajapati
Prajesh Prajapati