- Indian Institute of Technology Bombay, Mumbai, India
With growing interest, a large number of researches have been conducted on carbon-based nanomaterials (CBNs). However, their uses are limited due to comprehensive potential environmental and human health effects. It is often confusing for researchers to make an informed choice regarding the versatile carbon-based nanocarrier system and its potential applications. This review has highlighted emerging applications and cutting-edge progress of CBNs in drug delivery. Some critical factors like enzymatic degradation, surface modification, biological interactions, and bio-corona have been discussed here. These factors will help to fabricate CBNs for effective drug delivery. This review also addresses recent advancements in carbon-based target specific and release controlled drug delivery to improve disease treatment. The scientific community has turned their research efforts into the development of novel production methods of CBNs to make their production more attractive to the industrial sector. Due to the nanosize and diversified physical properties, these CBNs have demonstrated distinct biological interaction. Thus long-term preclinical toxicity study is recommended before finally translating to clinical application.
Introduction
Carbon is the primary key component of deoxyribonucleic acid (DNA) and provides the origin of life on the planet. It can be available in different forms. Due to special electron configuration (1s2, 2s2, 2p2), carbon can persist in a variety of crystalline and amorphous forms. Carbon is an extremely flexible component, categorized by diversifying allotropes and arrangements. The type of allotrope depends on the hybridization of carbon (sp3, sp2, and sp1) and its bonding around the atoms. For example, diamond is formed due to sp3 hybridization, whereas graphite is formed due to sp2 hybridization. This carbon has many technological applications, including the delivery of drugs and synthetic materials. The main reason behind this is the ability of carbon to bind itself and almost all elements (Kumar and Kumbhat, 2016). Carbon-based nanomaterials (CBNs) have drawn significant interest in diverse areas due to their distinctive structural dimensions and physicochemical properties (Rauti et al., 2019). Based on structure, CBNs are categorized as carbon nanotubes, graphene, mesoporous carbon, nanodiamonds, and fullerenes. With excellent optical activity and considerable multifunctional surface area, these materials have demonstrated higher drug-loading capacity, improved biocompatibility, and lower immunogenicity (Mohajeri et al., 2018). Functionalized CBNs have extensively explored the development of biocompatible scaffolds and nanomedicines. Chemically modification to specific moieties (e.g., functional groups, molecules, and polymers) of CBNs found as suitable for biomedical applications. Most of the materials under CBNs have been extensively studied for cancer therapy because of their excellent supramolecular π-π stacking, high adsorption ability, and photothermal conversion ability (Wang et al., 2020). Therapy can be improved by chemical functionalization coupled with adaptive properties (Chen et al., 2013). However, all nanocarriers come with numerous side effects or toxic effects that reduce their potential application. Additionally, the presence of bio-corona on the surface of CBNs may alter their activity, bio-distribution, pharmacokinetics, cellular uptake, toxicity, and clearance (Shannahan, 2017). In this current review, we classified CBNs and highlighted their biomedical applications. The physicochemical properties of CBNs described here will help to fabricate modified nanocarrier systems for better health management. Usefulness of different functionalization techniques for CBNs has been explored here. This section will help to select the best functionalization technique as per the need or industrial scale-up. CBNs are not only used as a carrier but also employed for targeted delivery. This section will help researchers to intervene in novel targeted delivery based on CBNs.
Classification of Carbon-Based Nanomaterials and Biomedical Application
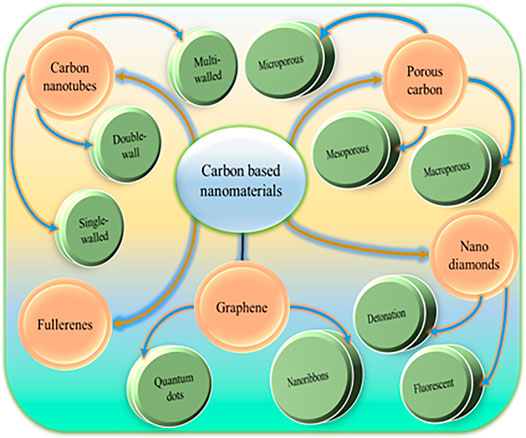
The core component of CBNs is carbon atoms. They are classified according to their shape and geometrical structure (Figure 1). The most frequently used carbon-nanomaterials are nanotubes (tube), graphene, nanodiamonds, fullerenes (spherical or ellipsoidal), porous carbon, and carbon dots.
Carbon Nanotubes
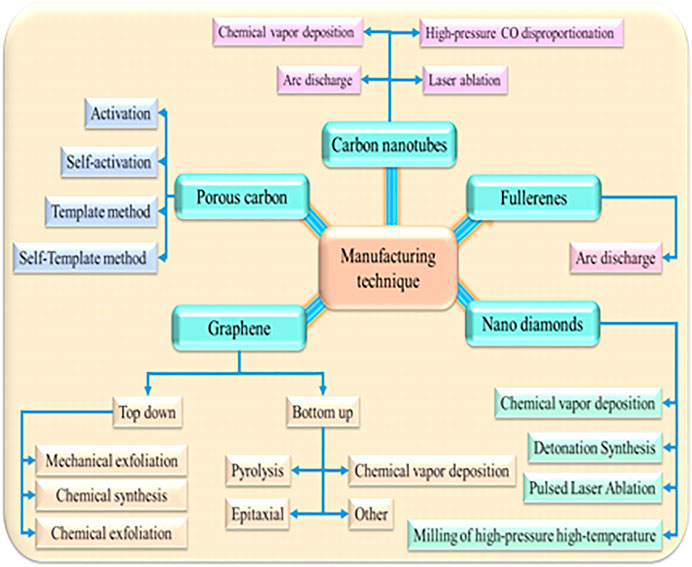
Carbon nanotubes (CNTs) are mainly tube or cylindrical shapes. They may differ in terms of length, diameters, number of layers, and chirality. Due to the unique combination of elasticity, strength, and rigidity, they showed many attractive properties. Due to varying structural configurations, CNTs can be categorized as single, double, multi-walled, and functionalized. There are several methodologies adopted for the synthesis of CNTs like chemical vapor deposition, arc discharge, laser ablation, high-pressure carbon monoxide disproportionation (Figure 2).
Single-Walled Nanotubes
Single-walled nanotubes (SWCNTs) are an elongated enfolded graphene sheet. The diameter and C-C orientation determine the patterns of the graphene sheet (Bepete and Coleman, 2019). Here, the length to diameter ratio is about 1,000:1. They are characterized by one-dimensional hollow and cylindrical shapes constructed with sp2 hybridization. The diameter of these types of CNTs is about 1 nm and may multiply thousands of times in length. They are often used in many nanotechnological applications. But the main obstacle is the high manufacturing cost (Raval et al., 2018). Thus more research is needed to develop an affordable synthesis technique. The modified form of SWCNTs is nanohorns. They have a single hexagonal ring of carbon. Due to the unique feature and increased diameter with an increase in length, they are used in drug delivery and targeting drug molecules to malignant cancer (Beg et al., 2018). In the biomedical field, SWCNTs are extensively used as scaffolds for cell culture growth and diagnostic devices. SWCNTs possess low solubility and are difficult to disperse in the aqueous phase due to strong π-π interaction. Chemical modification has suggested to solubilize and disperse SWCNTs in water to increase its biomedical and pharmaceutical interest (Liang and Chen, 2009).
Double-Wall Carbon Nanotubes
Double-wall carbon nanotubes (DWCNTs) possess similar characteristics and morphology as SWNTs. Coupling this property to the nanotube is crucial for supplementing new functionality (Bhatt et al., 2016). DWCNTs consist of two carbon nanotubes and a double-layer structure comprising two graphene sheets folded one upon another. It is an emerging class of CBNs. In comparison with SWCNTs, DWCNTs have higher mechanical strength, thermal stability, and chemical resistance. This class also shows interesting optical and electronic properties (Shen et al., 2011). They are also used in the development of biosensors. In this area, an immunosensor was developed for adiponectin (an obesity biomarker) by grafting antibodies on the DWCNTs surface. In this fabrication, DWCNTs bundles were used as an electrode on which anti-salmonella was impregnated on the surface (Punbusayakul et al., 2013). In tissue engineering, DWCNTs provide favorable surface texture to grow the neuron cells rather than the SiO2 surface. Improved cell differentiation was observed after the neuron growth on DWCNT (Beduer et al., 2012).
Multi-Walled Carbon Nanotubes
Multi-walled carbon nanotubes (MWCNTs) are composed of multiple layers of graphene sheets and possess more complicated electronic properties. The diameters of this class of nanotubes are varying between 5 and 50 nm. Wrapping one outside of another with an interlayer dispersing of 3.4 Å makes the structural complexity and varieties in this class. Thus MWCNTs are less well understood. A slight modification in structure may reduce its desired material properties (Ganji and Kachapi, 2015). The structure of MWCNTs can well explain using the Russian-Doll and Parchment model (Bhatt et al., 2016). They are found to be more superior in terms of signal/noise ratio compared to DWCNT and SWCNT. However, the sensitivity depends on the properties of the biological matrix. MWCNTs were used to develop scaffolds for pancreatic cancer cells (Matta-Domjan et al., 2018). Validated materials support fibers/sheet-based growth and proliferate that demonstrates the higher potential in cancer cell destruction.
Graphene
Graphene is consisting of a single layer of carbon below and above the plane with partially filled sp2-orbits. It serves a large surface area, excellent thermal conductivity, optical transparency, high mechanical and elastic strength. Often it is employed as a semiconductor. Due to the interaction of electrons and graphene, new quasi-particles are formed. In this class, the two most important components are graphene nanoribbons and quantum dots. These particles show ballistic transport where electrons can travel up to micrometre without scattering (Crevillen et al., 2019). Graphene shows good electrical conductivity. It possesses lower aqueous solubility. Therefore, several modifications have been made to develop chemically changed graphene, graphene oxide, and layered graphene-oxide. Graphene oxide and its derivatives are extensively used in sol-gel chemistry and are found to be suitable in the fabrication of biocompatible nanocomposites.
Graphene Nanoribbons
Graphene nanoribbons (GNRs) are finite, planar, and quasi-one-dimensional graphene structures. GNRs are prepared by slicing the graphene sheets into fine tiles with a higher aspect ratio. A fixed bandgap of less than 10 nm wide is observed. With the increase in width, these materials change their behaviour from semiconductors to semimetals (Crevillen et al., 2019). GNRs show higher thermal and electrical conductivity. As a result, it is considered an alternative to copper for an integrated circuit. They produce a bandgap that can be adjusted to the edge and width structure. The zigzag form of GNRs is metallic, whereas armchair GNRs may be semiconductor or metallic. GNRs can be prepared by chemical synthesis either by a liquid phase of exfoliation or by etching graphene with high-resolution electron-beam lithography. They are generally less than 1 µm in length and ∼10 nm in width (Hernandez et al., 2012). GNRs functionalized with the oxide have a high potential use in the medical field like drug delivery, cancer therapy, and DNA applications. These materials are almost non-toxic to human health and the environment (Mousavi et al., 2019). GNRs are the perfect substitution of silicon-based transistors and are widely used in biomedicine and diagnostics due to higher mammalian cell uptake.
Graphene Quantum Dots
Graphene quantum dots (GQDs) can be manufactured by slicing the graphene into a piece in the range of 2–20 nm. GQDs are less toxic and eco-friendly compared with conventional quantum dots (Crevillen et al., 2019). They are zero-dimensional nanomaterials. They serve as several biomedical applications due to their non-toxicity, ultra-small size, excellent photo-stability, and high water solubility. GQDs overcome the problems associated with different types of fluorophores as they have provided strong photoluminescence, photo-stability, and improved renal clearance. Thus they are successfully used in bio-imaging. GQDs show fluorescent activity. As a result, they were used to develop a fluorescent probe for observing the in-vivo and in-vitro tumor imaging and cellular dynamics (Pan et al., 2010). A redox fluorescent probe was developed using GQDs that is helpful to monitor the real-time oxidative stress-induced dynamic changes (Li et al., 2016).
Nanodiamonds
Nanodiamonds (NDs) contain tetrahedrally bonded carbon atoms in the form of a 3D cubic lattice. They possess the properties of diamond. Depending upon the synthesis process and size, nanodiamonds can be classified into two categories: detonation nanodiamonds (DNDs) and fluorescent nanodiamonds. DNDs possess monodispersing with a size near 5 nm and are commonly manufactured by shocks with trinitrotoluene-like explosives and hexogen. On the other hand, FNDs show wider size distribution and are manufactured from high pressure and temperature (Bondon et al., 2020). The flexibility in the sp2/sp3 bonds is observed in this type of material. NDs can adhere easily to different ligands, chemical compounds, and drug molecules. These NDs are extensively studied due to their high surface area and ease of fabrication and doping (Reina et al., 2015). Nitrogen-vacancy (NV) defect centres of NDs emit fluorescence at 550–800 nm. This property was successfully employed in making fluorescent probes for single-particle tracking in a heterogeneous environment. Due to these unique spectroscopic and optical properties, NDs have been adopted in the bio-imaging (Tinwala and Wairkar, 2019). NDs are also employed for drug delivery for low soluble materials like G9a inhibitors through conjugation (Gu et al., 2019). This composite is found to be suitable for intravenous administration, and the release of drug was pH-responsive. Hence, NDs have been successfully employed in drug delivery. Upon functionalization, this class becomes more superior in terms of biocompatibility rather than CNTs (Patel et al., 2019). The interest in nano-diamonds is growing gradually in the field of theranostics. Additionally, they are used to fabricate biodegradable bone surgery, scaffold, tissue engineering, and delivery of genetic materials to cells (Turcheniuk and Mochalin, 2017). Carbon nanoonions (CNOs) are derived from the carbon nanodiamond particles under vacuum. CNOs have smaller fullerenes demonstrated as cage in cage structure (Plonska-Brzezinska, 2019). They are polyhedral- and quasi-spherical shaped graphitic layers close to one another. These materials possess unique physical and chemical properties those not observed in any other types of CBNs. They are found as potential carriers for drugs (Bhinge, 2017). Through several in-vitro, in-vivo, and ex-vivo studies, the biocompatibility of CNOs has been confirmed. They show some additional benefits than other types of CBNs like easy fabrication, high purity with narrow polydispersibility index, and higher stability thus do not require any specialized condition for storage. Additionally, covalent and non-covalent functionalization is possible with CNOs that enables the attachment of more functional moieties (Bartkowski and Giordani, 2021).
Fullerenes
Fullerenes are the allotropic modification of carbon and are often termed as a molecular form of carbon. The fullerene family includes several atomic Cn clusters (n > 20) and is composed of carbon atoms on a spherical surface. Carbon atoms present on the surface of the sphere at the vertices form pentagons and hexagons. In fullerenes, carbon atoms are present in the sp2-hybrid form and linked together by covalent bonds. Fullerene C60 is the most common and best-investigated fullerene (Zaytseva and Neumann, 2016). Improving the water solubility of fullerenes is possible with the help of chemical functionalization. This technique enables the development of new materials or a bioactive redox drug (Hirsch, 2010). Pure C60 does not show any acute or chronic toxicity and improves human health. With growing interest, C60 and its derivatives stand alone as a unique class and are exploring continuously in biomedical field (Moussa, 2018). The scope of functionalized fullerenes is increasing continuously in therapy and diagnostics. They are mainly focused on reactive oxygen species quenching, drug delivery, and targeted imaging with the help of functionalized derivatives (Streicher et al., 2007).
Porous Carbon
Porous carbon materials are gaining interest due to their high surface area and physicochemical properties. This type of carbon is also known as activated carbons. They can be classified based on their pore size as microporous (<2 nm), mesoporous (2–50 nm), and macroporous (>50 nm). These pores are not well organized and can be arranged as ordered carbons span over at specific intervals of macro-, meso-, and micro-pore range.
Conventionally, they are manufactured by pyrolysis and chemical or physical activation of organic precursors at elevated temperatures. It has been observed that the mixed porous carbon (a combination of the three types) has the largest surface area among all types of carbons (Yang and Ionescu, 2017). They can be produced in large quantities and extensively used in adsorption, separation, and many more (Liang et al., 2008). In the 3D bulk form, micro and mesoporous forms may develop mass transfer resistance (Liese and Hilterhaus, 2013). Mesoporous carbon nanomaterials (MCNs) are extensively used in drug delivery.
Carbon Dots
Carbon dots (CDs) are the distinct and newest member of CBNs. These are quasi-spherical carbon nanomaterials with a particle size of less than 10 nm. Using light energy, these materials are able to kill the nearby pathogens through the production of reactive oxygen species (ROS). CDs based photodynamic therapy or photocatalytic disinfection open a new scope to destroy pathogens even the initiation of infection (Knoblauch and Geddes, 2020). These materials often mimic the morphology and functions of naturally occurring enzymes. Due to these unique properties, they are often employed in biosensing (Garg and Bisht, 2016). CDs are zero-dimensional spherical allotropes and show several interesting properties like water solubility, eco-friendly, conductivity, optical properties, low toxicity, biocompatibility, and theranostics (Cohen et al., 2020). The bottom-up approach has been used to synthesize CDs mainly through the hydrothermal treatment of various molecular precursors. CDs are also prepared by simple and specific reactions. Template methods are also employed to synthesize spherical polymer due to its easy operation and easily accessible equipment (Tian and Yin, 2019).
Impact of Physicochemical Properties in Drug Delivery Using Carbon-Based Nanomaterials
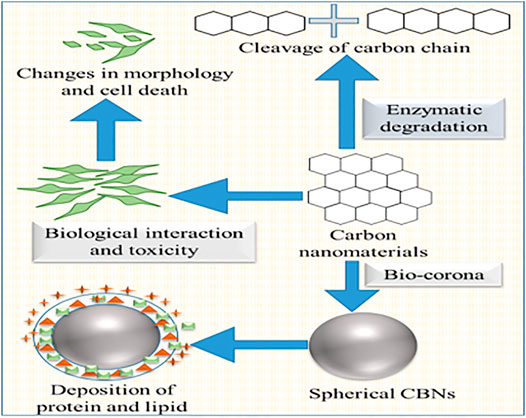
Their physicochemical characterization is essential to assure the product quality (purity, defects, chemical species on the surface, etc.) and elucidate their structure. In this sense, Raman spectroscopy, electron microscopy, and atomic force microscopy are the most useful techniques. Different physicochemical properties like enzymatic degradation, biocompatibility, biological interaction, bio-corona, and toxicity have been discussed in detail (Figure 3).
Enzymatic Degradation
Enzymatic degradation of CBNs is crucial for immune-competent cells as they serve a special case of biological interactions in the medical application of these CBNs. It was observed that biodegradation of SWCNTs occurs via natural enzymatic catalysis (Allen et al., 2008). This degradation was increased in the presence of natural horseradish peroxidase (HRP) and a low concentration of H2O2 for 12 weeks. A combination technique of transmission electron microscope (TEM), Raman, and Ultraviolet-near-infrared (UV-NIR) spectroscopy demonstrated the disappearance of discriminating D- and G-bands. The carboxyl group present on the surface formed interaction between CNTs and oxidizing agents. Layer-by-layer degradation was also observed in MWCNTs (Zhao Y. et al., 2011). Functionalization of MWCNTs by doping with the help of nitrogen altered the degradation rate in these MWCNTs. Here, the degree of carboxylation depended on the time of oxidative acid treatment. And the overall rate of degradation of MWCNTs was directly associated with carboxylation. It also observed that the hydrophilic interaction between HRPs and oxygen content in the defective sites causes the oxidation and degradation of CNTs. MWCNTs take a longer time to degrade than the SWCNTs as MWCNTs are more resistant to HRP degradation. In a separate study, SWCNTs degradation was influenced by myeloperoxidase (MPO) present in the neutrophils extrude neutrophil extracellular traps (NETs) (Farrera et al., 2014). This degradation was facilitated by the addition of NaBr and H2O2. Acellular and MPO mediated degradation of SWCNTs work similarly as body immune systems offending nanomaterials and microorganisms. CDs possess distinct properties rather than other classes of CBNs. They undergo peroxide catalyzed-degradation in the presence of a lipase. It was observed that the different charged CDs exhibit unique degradation kinetics through enzyme oxidation (Srivastava et al., 2019).
Biocompatibility and Biological Interactions
CBNs possess several interesting physicochemical properties that raise their demands in different biomedical fields. CNTs, graphene, fullerenes, and nanodiamonds have drawn remarkable attention in the last couple of years. However, the potential toxicity due to non-compatibility with a biological system of CBNs restricts its wider acceptance. Thus it is highly essential to assess the biocompatibility of CBNs. Thus surface modification has been introduced to make these materials more compatible and susceptible to enzymatic degradation. CBNs can interact with several mammalian cells and enter into them via cytoplasmic translocation. Generally, CNTs interact with blood via two specific events: direct exposure due to the presence of CNTs in blood and interaction with blood leukocytes via antigen-presenting cells. Leukocyte activation markers may be useful to understand the direct and indirect interaction between blood and immune response (Medepalli et al., 2011). However, CDs have little influence on the human serum albumin (HSA) and γ-globulins. Apparent dissociation constants of the CDs to bind with HAS and γ-globulins were the same (Xu Z. Q. et al., 2016).
Additionally, CDs exhibit higher cell viability and well tolerance with serum in comparison with commercially available polyethyleneimine (He et al., 2019). The sub-classes of CBNs possess different physicochemical properties like aspect ratio, surface functionalization, purity, etc., and exert different toxicological profiles (Bhattacharya et al., 2016). Therefore, a rigorous assessment of the biological interaction of individual nanomaterials is a highly prerequisite element to explore for medical application. The innate immune system of the body is composed of sensors or inflammatory cells and soluble mediators like cytokines, chemokines, and complement factors (Bhattacharya et al., 2013). The inflammatory cells contain macrophages and phagocytic cells which are different from monocytes. The monocytes are mainly responsible for phagocytosis, cytokine production, and antigen presentation. F-CNTs activate immune-related pathways, and trigger inflammation activation and monocyte-derived macrophages that promote the secretion of interleukin 1β and pro-inflammatory cytokine (Meunier et al., 2012; Pescatori et al., 2013). Graphene materials interfere with the metabolic activity of macrophages, damaging the mitochondrial membrane and increase the reactive oxygen species (ROS) level. Upon uptake by the cancer cells, CBNs lead to DNA and lipid damage, followed by cell death through ROS excitation (Patel et al., 2019). The mechanism by which CNs enter cells is unclear. The evaluated processes are passive diffusion of CNs through the lipid bilayers of the cell membrane or attachment of CNs to the external cell membrane resulting in absorption by the cell using an energy-dependent process. Small CNs with a length of up to 400 nm are internalized by a diffusion mechanism, whereas the others are internalized by endocytosis. In this regard, CBNs with improved bio-function enhance delivery efficiency by increasing retention time and reducing clearance.
Bio-Corona
Nanomaterials rapidly are coated with biomolecules, lipid, and protein. Afterward, they present themselves with a new biological identity (Docter et al., 2015). This form is known as bio-corona which is responsible for several biological and toxicological interactions with nanomaterials in the living system (Fadeel et al., 2013). Albumin was successfully adsorbed on the surface of SWCNTs. This bio-corona played a crucial role in cellular uptake in RAW264.7 macrophages. Damaged albumin interacts with scavenger receptors and clears from the systemic circulation (Dutta et al., 2007). The interaction of four major serum proteins (bovine serum albumin, transferrin, immunoglobulin, and bovine fibrinogen) with SWCNTs was analysed (Ge et al., 2011). Prepared serum protein-coated SWCNTs produced less cytotoxicity than the uncoated SWCNTs in human umbilical vein endothelial cells and leukemia cells. Cell line study also demonstrated that serum binding on SWCNTs altered the cellular interaction pathways. A competitive binding was established between them with different adsorption capacities. The π-π interaction between the SWCNTs and aromatic residue was observed. This interaction plays a crucial role in tissue binding. It was observed that serum proteins responsible for the Toll-like receptor-dependent signaling in macrophages. GO showed a higher loading capacity for protein. Cytotoxicity of GO was altered in the presence of 10% fetal bovine serum (FBS). However, GO showed concentration-dependent cytotoxicity at the lower concentration FBS due to the physical damage of the cell membrane. It was also suggested to coat GO with BSA for reducing the cytotoxicity towards A549 cell lines (Bhattacharya et al., 2016). Bio-corona is also observed in other body compartments like the lung, gastrointestinal tract, etc. The formation of different types of bio-corona may depend upon the environment. In a mice model, lung surfactant protein and lipid were absorbed by the pharyngeal aspiration of SWCNTs (Kapralov et al., 2012). Remarkably, phosphatidylglycerol and phosphatidylcholines were absorbed more on the SWCNTs surface with varying binding affinity. Phospholipids formed a coating over the CWCNTs whereas, hydrophobic alkyl chains of the phospholipids adsorbed on the surface. These types of surface coating significantly increase the cellular uptake of SWCNTs. Bio-corona may alter the orientation and conformational changes that may lead to an immune response through cell surface receptors. Protein adsorption on the nanomaterials may inhibit the enzyme activity. The research found SWCNTs and GO can inhibit drug-metabolizing enzymes named CYP3A4. This inhibition efficiency decreases when nanomaterials pre-coated with BSA.
Toxicity
CBNs have a promising approach in the biomedical domain like image processing, sensor, tissue engineering, and therapeutics. However, they are still struggling with their unwanted toxic effect on biological systems. The potential toxicity of CBNs become a growing concern from the past decade due to fiber-like materials that were assumed to be bio-persistent and may cause asbestos-like pathogenicity. Several factors equally contribute to elicit the toxicity of CBNs (Aldieri et al., 2013; Kobayashi et al., 2017). Additionally, the inclusion of metal impurities during synthesis has remarkable toxicity on cells. The physical structure of CBNs, especially length, has a substantial influence on their toxicity through cellular internalization. Researchers found the long-lasting lengthy CNTs initiate inflammation and progress to fibrosis (Murphy et al., 2011). Apart from the length of CNT, a higher diameter of CNT also elicits substantial toxicity. Variation in the structure, size, and chemical surface between MWCNT and SWCNT show altered toxicity on cells (Di Giorgio et al., 2011). With the introduction of functionalization, this problem has been managed significantly. Surface modification is growing opportunistically to increase the biocompatibility of CNTs. However, cellular phagocytosis and cell apoptosis were detected with acid-treated MWCNTs and taurine-functionalized MWCNTs (Wang et al., 2012). Different types of macrophages respond to MWCNTs in different ways, and they are markedly dependent on the concentration of MWCNTs. For example, MWCNTs induced cell death of murine bone marrow at the concentration of 3–30 μg/ml but produced no cytotoxicity on the RAW264.7 cell line at 300 μg/ml (Dutta et al., 2007). Fullerene did not show cytotoxic effect in alveolar macrophages during in-vitro analysis and showed a lower cytotoxic effect against human macrophages (Yuan et al., 2019). In a separate study, CBNs influenced cell death was investigated. The exposure of RAW264.7 and human alveolar macrophages was characterized by cell membrane rupture, cell enlargement, lactate dehydrogenase leakage IL-1β, caspase-1 activation (Reisetter et al., 2011). Pristine graphene triggered apoptosis, necrosis, and cell death in RAW264.7 macrophages (Reisetter et al., 2011). Thus toxicity of different CBNs was analyzed and compared the extracellular LDH release. CNTs and nanographite treated groups showed production of higher LDH, and NG showed extensive cellular toxicity (Figarol et al., 2015). In vitro toxicity of CDs was conducted on standard mouse fibroblasts (NIH/3T3). No abnormality in the cell morphology, cell cycle, and intracellular trafficking was detected for neutral CDs-PEG (up to the concentration of 300 µg/ml). Thus CDs may be demanded as a promising material for biomedical application. However, negative-charged pristine-CDs stimulated the proliferation and produced higher oxidative stress without entering the cell nucleus. In contrast, polyethyleneimine-coated dots can enter the nucleus and cause severe toxicity at lower concentrations (100 μg/ml) (Havrdova et al., 2016). Effect of carbonization degree of CDs on cytotoxicity and photo-induced toxicity was observed in cells (Esfandiari et al., 2019). Negative-charged CDs (fabricated at 160°C for 50 min) with smaller than 5 nm showed a significantly higher quantum yield. These materials showed the toxicity of 20–30% at breast cell lines at higher concentrations of 1.5 mg/ml and 2.0 mg/ml. Most of the materials under CBNs are susceptible to enzymatic degradation. Thus it is crucial to analyze the harmful-potential effects of CBNs using long-term toxicity study.
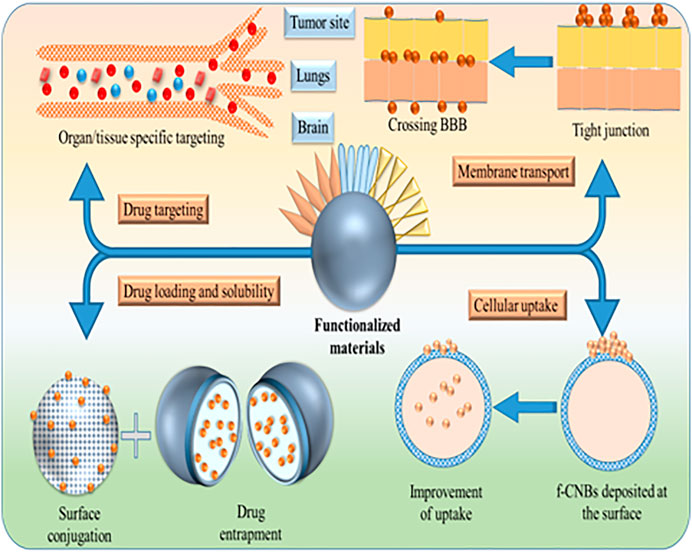
Functionalization of Carbon Based Nanomaterials for Drug Delivery
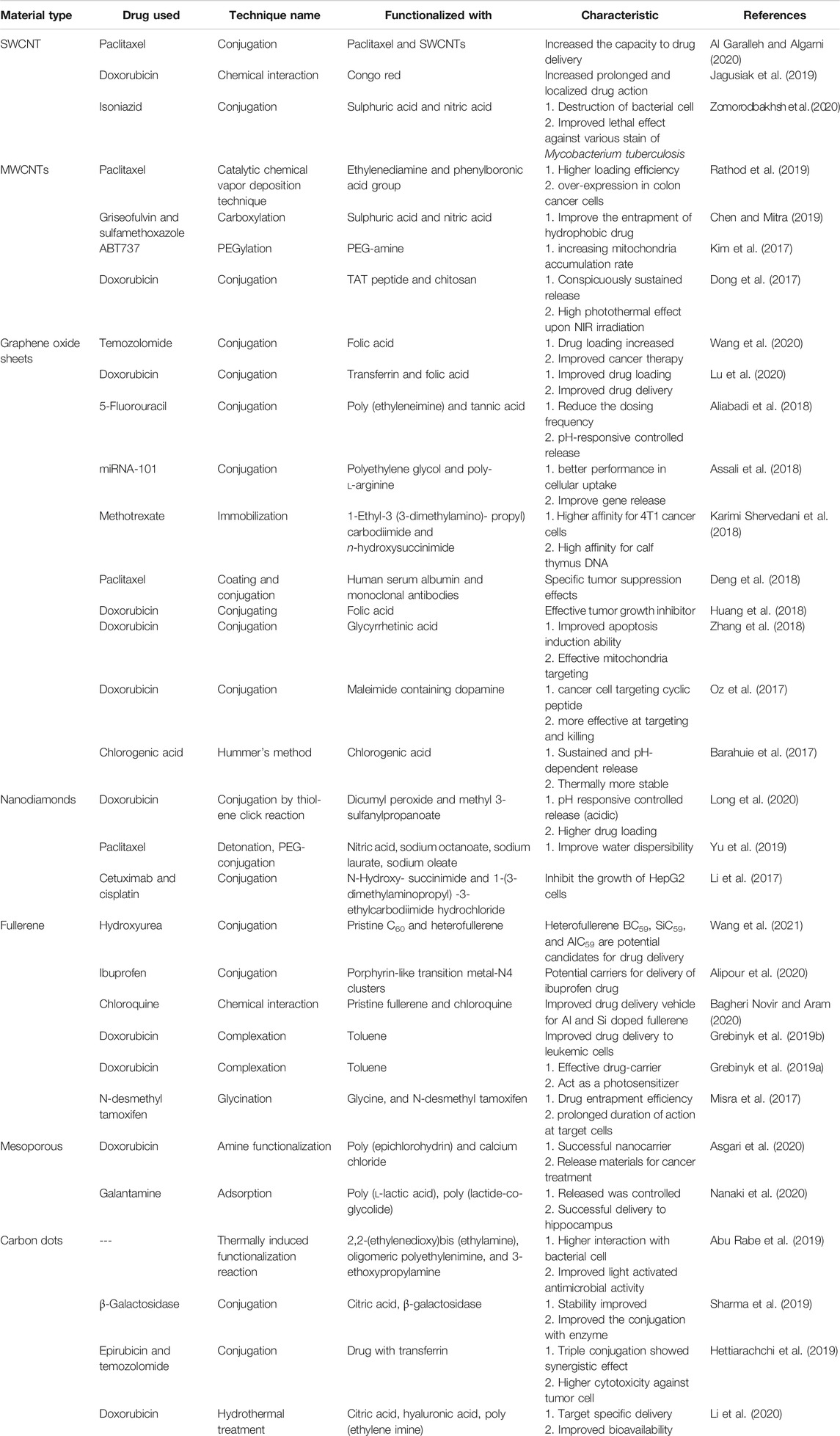
Several sub-group of CBNs possess as a carrier for effective delivery of a wide range of drugs. In recent years, these materials are extensively used for cancer therapy. Concurrently, the scope of CBNs towards other diseases is also exploring gradually. Here, the main focus has been given on the functionalized CNTs, graphene, nanodiamonds, fullerene, and mesoporous carbon materials in order to improve drug delivery (Table 1).
Carbon Nanotubes
Functionalized carbon nanotubes (f-CNTs) are an especial class of CNTs. CNTs show several interesting properties. However, the outer layer of CNTs is chemically inert. Tagging functional groups or therapeutic molecules on the surface of CNTs shows improved solubility, biocompatibility, and reduces toxicity. Accordingly, f-CNTs have drawn significant importance in the area of drug delivery (Beg et al., 2018). In this contrast, surface modification of covalent and non-covalent are two promising approaches to modify CNTs. In the non-covalent modification method, long-chain polymers (polystyrene sulfonate) are enveloped around the CNTs. The covalent or chemical modification has been used to functionalize the CNTs by forming a covalent bonding between CNTs and coupling agents. This modification may hamper the potential mechanical strength of f-CNTs. The purification process increased significantly depending on oxidation conditions (Merum et al., 2017). Functionalized MWCNTs were investigated for cell labeling in T1-weighted MRI applications (Servant et al., 2016). Here, MWCNTs were covalently bound with gadolinium (Gd) chelating agent and assisting a tight attachment of Gd atoms onto the nanotube surface. These materials were found stable for 2 weeks in mouse serum and water. The r1 relaxivity of the material was found more superior than the clinically approved T1 of Magnevist. Notably, f-CNT shows numerous biomedical applications without eliciting any side effects (Saito et al., 2008). Collagen and polymer-based f-CNT are used as scaffolds in tissue engineering (MacDonald et al., 2005). F-CNT supports the production of osteoblastic cells which is an essential prerequisite for bone. The in-vivo study suggested superior bone-tissue compatibility and effective periosteal tissue refurbishment. Additionally, this material is compatible with blood and other tissues. Oxygen-containing functional groups of CNTs enhance the adhesive affinity towards platelets, promote anti-coagulation activity, and improve implants (Vardharajula et al., 2012). The three basic approaches for the fabrication of CNTs are π-conjugation of the group by covalent bond, wrapping of functional molecules, and endohedral filling of the inner cavity (Tasis et al., 2006). The CNTs can be produced by three major techniques: electric arc discharge, catalytic chemical vapor deposition (CCVD), and laser ablation of carbon. Some other techniques like flame pyrolysis, plasma torch, and condensed-phase electrolysis are also employed in the synthesis of CNTs. Out of all these techniques, CCVD (thermal or plasma-enhanced) is commonly employed due to higher production output (Patila et al., 2020). Biomolecules, polymers, and macrocycles can be conjugated with the CNTs using the adoption of irradiation, isotopic labeling, pulsed streamer discharge, and microwave techniques (Kharisov et al., 2009). The solubility of CNTs in an aqueous environment is a prerequisite for the drug’s kinetics in the body. Similarly, uniform dispersion of CNTs is equally important to deliver therapeutic materials. The first generation SWCNTs were not suitable for drug delivery due to adequate water solubility. Several hypotheses have been adopted in order to disperse the CNTs in aqueous. They are solvent dispersion, surface-assisted dispersion, biomolecular dispersion, and sidewall functionalization (Zhang et al., 2011). Among them, functionalization is the most promising approach. The introduction of non-covalent surface modifications with natural (Yuan et al., 2006) and synthetic polymers (Abarrategi et al., 2008) solve the solubility issue with CNTs. Carbon nanotubes act as nano-containers for the effective delivery of biological molecules. Functional modifications were carried out in several molecules, metallic elements, or ionic species that can conjugate on the surface of CNTs. These materials can be employed as nano-vehicles in cancer treatment and other diverse areas of biomedical without triggering the toxicity to normal tissue while promoting prolonged drug release. Out of various carbon nanotubes, SWCNTs have gained considerable interest that provides potential advantages in metal nanoparticles. Additionally, they allow bulk drug loading, structural flexibility, intrinsic stability, improved circulation time, and bioavailability (Kumar et al., 2013). Functionalized-SWCNTs would able to entrap low molecular weight agents and antibodies that demonstrated higher cellular penetration (Chen et al., 2008; Zeineldin et al., 2009). This system provides higher drug loading and allows the conjugation of biological molecules without triggering an immune response (Mu et al., 2009).
Graphene
Graphene and its derivatives are extensively used in drug delivery and the field of advanced medicine. Normally, surface modification of graphene nanomaterials with polymer helps to make them compatible with the biological system (Bai and Husseini, 2019). Graphene nanomaterials react with the change of several stimuli like a magnetic field, electric field, radiation, temperature, pH, etc. Based on the internal and external stimuli, they controlled the drug release (Yang K. et al., 2016). These stimuli also help to improve drug bioavailability, cross biological barriers, and reduce side effects. Among graphene nanomaterials, GOs have drawn significant interest due to the availability of functional groups on the side walls. Using hydrophobic interaction and π-π stacking graphene was able to entrap DOX and camptothecin. Hydroxyl and carboxyl groups available on the surface of GOs facilitate binding with hydroxyl and amino groups of DOX (Pattnaik et al., 2020). Drug release was higher at the acidic pH compared with other physiological pH of 6.5 and 7.4. Alginate conjugated GO-DOX did not produce any cell cytotoxicity by MTT assay on NIH-3T3 and HeLa cell lines. Methotrexate (an anticancer drug) was immobilized on the graphene surface to evaluate the drug-carrying capacity of graphene nanomaterials (Karimi Shervedani et al., 2018). A study on 4T1 cancer cell lines demonstrated successful carrier capacity and cellular capacity through the electrochemical method. 5-fluorouracil loaded GO was developed based on pH-stimuli drug delivery (Aliabadi et al., 2018). This formulation released the anticancer drug in a controlled manner in acidic pH of 5.8 (tumor environment) whereas, the release was decreased significantly in normal physiological pH of 7.4.
Nanodiamonds
Nanodiamonds are another promising approach in the drug delivery system. Notable, they are non-toxic and never interfere with the immune system of the body. Nanodiamonds are able to bind a wide range of molecules and deliver them to the tumor site (Pattnaik et al., 2020). Dual-ligand-functionalized nanodiamonds were prepared to target cancer-cell-specific mitochondria to circumvent drug resistance. Here, folic acid and mitochondrial sequence peptide were used for surface functionalization. These NDs were able to penetrate the plasma membrane of tumor cells. They induced cytotoxicity in mitochondria and initiated higher cell death in comparison with free DOX. Cetuximab-NDs-cisplatin bio-conjugated material was fabricated to enhance target specific and improved efficacy (Li et al., 2017). It was a multicomponent NDs-based drug targeting system that inhibited the growth of HepG2 cells. The introduction of the ester group on the surface of NDs through thiol-ene reaction (Long et al., 2020) improved the water dispersibility and higher loading capacity. Through this reaction, DOX formed a link with CHO-PEG through hydrazine bonds. This composite successfully released DOX in the acidic environment in a controlled release fashion. A reversible addition-fragmentation transfer polymerization technique was used to graft two different polymers onto the surface of amine-functionalized nanodiamonds (Lu et al., 2016). Gemcitabine (anti-cancer drug) was physically adsorbed on the surface of ND-PEG (Nanodiamonds-Polyethylene glycol) to produce ND-PEG (Gem). Although the polymer coating couldn’t improve the activity of gemcitabine, this carrier successfully delivered gemcitabine. pH-responsive controlled release of DOX was observed from poly [N (4-aminophenyl)methacrylamide)]- carbon nanoonions (Mamidi et al., 2020). In this study, DOX released was controlled over 15 days and found 99.2% release in pH 4.5 compared with 59.3% in pH 6.5. It was observed that π-π stacking of f-CNOs and DOX responsible for this release kinetics. Non-covalent functionalization of CNOs with hyaluronic acid-phospholipid (HA-DMPE) was developed for targeted drug delivery for cancer (d’Amora et al., 2020). Due to this functionalization, the solubility of CNOs was improved significantly. Additionally, targeting CD44 cancerous cells is also possible in human breast carcinoma cells. Cellular uptake of these functionalized CNOs were observed in different types of cancerous cells.
Fullerene
In the field of biomedicine, it is crucial to select appropriate nanomaterials that are compatible with biomolecules. From this point of view, C-60 fullerene is a promising material whose energy and structure properties are palatable with cells. However, the safety issue and higher drug loading are the key challenging part of it. To overcome these issues, several approaches have been taken like stabilization with the OH group, functionalization with the OH group, and super-positioning the hydrogen bonds. Fullerene was used to deliver the docetaxel to the tumor site. Delay released formulations are employed to release the drug after a specific time interval. Self-assembly hollow nano-vesicles were prepared from functionalized fullerenes (C60R5Cl) (Lin et al., 2017). Depending upon the methyl ester of 4-aminobutyric/glutamic acid or phenylalanine, the vesicle size differs. These materials were found as potential delayed-release carriers for cyclophosphamide, cisplatin, and 5-fluorouracil. Fullerene was modified with magnetic surfactant CTAF (C60@CTAF) via hydrophobic interaction to increase the solubility of fullerenes (Wang et al., 2017). DNA was introduced in these nanovesicles as a scaffold to entrap doxorubicin and modified the C60@CTAF to C60@CTAF/DNA. A further modification was done by coating with HA-SS-COOH to reduce the agglomeration and regulation of its morphology. Final conjugated materials reduced extra-tumoral environment using cleavage of disulphide linkage mediated by glutathione. Functionalization of C60-fullerenes with glycine found as beneficial for drug entrapment and drug delivery (Misra et al., 2017). A further conjugation of N-desmethyl tamoxifen on glycine-tethered C60-fullerenes showed higher drug entrapment and drug loading efficiency. In a pharmacokinetic study, the area under the curve was enhanced by three-time after a single intravenous injection. In a separate study, DOX was conjugated covalently with fullerene nano-aggregates using a ROS –sensitive thioketal linker (Shi et al., 2016). A hydrophilic shell was conjugated to control the release of DOX in the acidic environment. ROS-sensitive linkers were broken by ROS generated from fullerene and enabled the burst release of DOX. This composite showed higher anticancer activity with lower toxicity to a normal cell. It demonstrated a combined therapeutic effect with controlled drug release property.
Mesoporous Carbon Nanomaterials
The notable drawback of the hydrophobic drug in clinical practice is the poor solubility and low bioavailability after conventional administration (Mahapatra et al., 2013). Mesoporous materials are promising carriers for poorly soluble drugs. Mesoporous silica, mesoporous hydroxyapatite, mesoporous metal oxides, and mesoporous carbon are mostly employed to entrap the poorly soluble drug. MCNs were found as more suitable due to their unique properties like higher porosity, higher loading capacity, lower density, and higher adsorption ability (Pattnaik et al., 2020). Nitrogen-doped hydrophilic mesoporous carbons were prepared for poorly soluble hydroxycamptothecin (Wang et al., 2019). Spray drying and carbonizing techniques were employed with different carbon-to-silicon ratios. In this fabrication, tetraethyl orthosilicate and triblock copolymer were used as template agents. Biocompatible chitosan was employed as a source of carbon and nitrogen. This material showed satisfactory hydrophilicity that was confirmed with the increasing content of nitrogen. Additionally, higher adsorption capacity (1,013.51 mg/g) and higher dissolution (93.75%) was observed. The sustained-release formulation can maintain drug concentration for an extended period that is not possible for the immediately released formulation. Sustained-release formulations could reduce dosing frequency, stabilize plasma drug concentration, reduce side effects, and provide better patient compliance. MCNs have been explored for the sustained release of drug delivery. The drug release mechanism from the MCNs follows three different factors: pore length and structure, interaction force between MCNs with the drug, and diffusion hindrance effect. The promising approach in CBNs is the modification of “gatekeeper” by covalent bonds or physical adsorption that prevent leakage of the drug unless exposing to certain stimuli like redox, pH, temperature, redox potential, and enzyme (Pattnaik et al., 2020). Gated channel-interconnected MCN were fabricated to understand the stimuli-responsive controlled release of rhodamine 6G as a model drug (Huang et al., 2016). Here, the gatekeeper was N-(3-Tri-methoxy-silyl-propyl) ethylene-diamine triacetate-factionalized ZnO quantum dots that bound with carboxylated-MCNs using covalent linkage. This conjugated material encapsulated the drug via a dual amide linkage. The controlled-release of the drug was achieved by lowering the pH followed by elevation of temperature. Under NIR, MCNs generated heat and raised the local temperature that further assists the drug release. Amine functionalities were introduced to MCNs in order to facilitate the poly (epichlorohydrin) chains. In the fabrication of these novel hollow MCNs, calcium chloride was employed as a capping agent. These materials successfully carry doxycycline and release pH sensitive manner for cancer treatment (Asgari et al., 2020).
Carbon Dots
CDs are one of the ideal choices for targeted drug delivery. Through surface functionalization, significant improvement of drug efficiency with reduced toxicity has been observed. Thus these materials are successfully employed in gene-, chemo- and antibiotic therapy (Cohen et al., 2020). Charges on CDs are varied depending upon the type of materials used during the surface functionalization: carboxylic groups show negative charge, neutral changes observe with poly-ethylene glycol, and positive charge with a poly-ethylenimine coat (Havrdova et al., 2016). Surface functionalization of CDs was performed with 2,2-(ethylenedioxy)bis (ethylamine) (EDA) and 3-ethoxy-propylamine (EPA) (Abu Rabe et al., 2019). EDA-CDs demonstrated the positive charges due to the presence of –NH2 group and are found to be more favourable to counter-attack the bacterial cells. CDs functionalized with oligomeric poly-ethylenimine also demonstrated improved antibacterial activities. Nitrogen and oxygen co-doped CDs were prepared from 1,3,6-trinitropyrine and N- containing polymers of branched polyethyleneimine (Geng et al., 2018). These materials were found to be improved biocompatibility and higher photo-stability. These materials provide the dual activity of fluorescence imaging and photothermal therapy. To improve the CD44 targeted delivery, CDs were modified with hyaluronic acid (Li et al., 2020). This hyaluronic acid contains the hydrophilic group that was successfully employed to entrap doxorubicin and also a targeting ligand system. An in-vitro study of these conjugated materials demonstrated higher serum stability, hemocompatibility and exhibit higher cytotoxicity on 4T1 cells.
Potential Applications of Carbon Based Nanomaterials in the Delivery of Biological Molecules
CBNs are found to be potentially useful and gain interest in medicine including therapeutics, diagnosis, and regenerative medicine due to their unique properties. Here the potential application of CBNs includes gene delivery, cancer therapy, peptide delivery. Among the wide range of carbon allotropes, CNTs have drawn significant attention in transporting many drugs into the living cells. Its natural morphology helps to penetrate biological membranes in a non-invasive manner (Pattnaik et al., 2016).
CBNs in Gene Delivery
Sp2-hybridized with 1D CNTs with a cylindrical nanostructure and 2D graphene has been identified as a promising carrier for gene delivery. The basic adaptable technique to introduce a new gene into the desired cells is based on replacing and knocking down a mutated gene. The major obstacle in this gene delivery is the lack of suitable carriers that protect the gene from being degraded and higher membrane permeability with low immunogenicity (Taghavi et al., 2020). The direct intracellular delivery of drugs and biological molecules is restricted due to the lipophilic biological-barrier. Thus intracellular transport is the key challenging part of gene therapy. SWCNTs are found as good carriers of bio-molecules like small interfering RNAs (siRNAs). Chemically functionalized SWCNTs with the help of poly (di-allyl-dimethyl-ammonium) chloride and hexamethylene-diamine were fabricated (Krajcik et al., 2008). These materials were able to bind with negatively charged siRNA through electrostatic interactions. After loading with extracellular signal-regulated kinase, these materials were able to penetrate the cell membrane to suppress the ERK target proteins. The authors also suggested a passive “needled-like” mechanism that was observed for cellular entry of CNTs. In this context, bio-corona formed on the surface of CNTs also plays a vital role in cellular recognition followed by uptake. Efficient delivery of siRNA was observed in mice and rats, where MWCNTs were administered through stereotactic for neuroprotection (Bhattacharya et al., 2016). It was observed that CNTs functionalized with ammonium could conjugate with plasmid DNA using electrostatic interaction (Pantarotto et al., 2004). These f-CNTs could penetrate the cell membranes and successfully up-taken by the cell. This material was able to deliver cells efficiently and 10-fold higher gene expression was observed. Drug targeting to the specific tissue or cell is a more challenging part. Exogenous DNA transport into mammalian cells stands alone as a unique methodology to manipulate signal transduction. Nanotube spearing is based on nickel-embedded nanotubes that can cross the cell membrane using the magnetic field. The enhanced green fluorescent protein sequence coupling with DNA plasmids was immobilized on the surface of nanotubes which was spread on the targeted cells (Cai et al., 2005). These nanotubes showed high transduction efficiency in ex-vivo, Bal17-B-lymphoma, and primary neurons. Surface modification of CBNs with polymer acts as novel carriers with reduced toxicity. Intraarticular delivery of antisense oligonucleotides using PEG-SWCNTs in mice model showed no side effects (Karjalainen et al., 2007). Chitosan-carboxylated graphene oxide was developed to deliver the therapeutic gene, pDNA-TNF-α (Jaleel et al., 2019). After conjugation with folic acid, these materials would able to target the folate receptors for overexpressing the cancer cells. Additionally, these materials protect the vector from the mononuclear phagocyte system that increased the circulation half-life.
CBNs in Cancer Therapy
CNTs possess strong NIR light absorption capability. Therefore, they can be used as efficient photothermal agents. Functionalized MWCNT conjugated with candesartan was assembled with plasmid AT-2. This complex was used to target αvβ3-integrin, AT1R of tumor endothelium (Maiti et al., 2019). The inner hollow cavity of CNT serves as perfect protection of the drug from the outside environment and increases the drug stability. Shin group analyse the temperature-responsive biomolecule release of bovine serum albumin (BSA) from chitosan-functionalized CNT with thermosensitive polymers like 1-butyl-3-21-vinyl imidazolium and poly-N-isopropylacryl amide. BSA release was observed at body temperature (Jena et al., 2017). The combinational effect of NIR and temperature was observed for MWCNT-carrageenan hydrogel on cancerous cells. The release of methylene blue was well controlled from this complex (Estrada et al., 2013). The efficiency of in-vitro and in-vivo antitumor efficiency was tested on different drugs including doxorubicin (DOX) (Huang et al., 2011), paclitaxel (Singh et al., 2016), docetaxel (Jain et al., 2015), oxaliplatin (Lee et al., 2016), etc. The targeting tendency of DOS loaded with folic acid and estrone-ligand functionalized MWCNTs were investigated on the MCF-7 tumor-infected mice model (Zeglis et al., 2015). A longer survival span (more than 43 days) was observed with the mice treated with DOX/ES-PEG-MWCNTs compared to the group treated with free-DOX (18 days) or PBS (12 days). Zhou group fabricated MWCNT-magneto fluorescent carbon quantum dot loaded with DOX for dual chemo- and photo-thermal therapy (Zhang et al., 2017). The positive charged DOX was successfully bound to the negative surface charge of GdN@CQDs-MWCNTs. This material showed a higher ability to absorb NIR light. During photothermal therapy, this conjugate material raised the temperature to 51.8°C on the tumor site of the mice model with a power density at 2 W/cm2 for 5 min. In contrast, not many changes were observed in the treatment group. This dual therapy successfully suppresses the tumor volume. Dual therapy was also studied with DOX-loaded TAT-chitosan functionalized MWCNT nanosystem (Dong et al., 2017). Improved apoptosis was observed with PEG-coated CNT-ABT737 nano-drug when they target mitochondria (Kim et al., 2017). Graphene oxide (GO) was synthesized using Hummer’s method to explore these CBNs as a potential nanocarrier for targeting the chlorogenic acid (CA) to cancer cells (Barahuie et al., 2017). In this method, a nano-composite was produced from the GO and a drug that can control the release of both materials based on the pH of the microenvironment. This fabricated material showed less toxicity for normal cell lines. Concurrently, it was more toxic for cancer cell lines.
Photothermal therapy has drawn sufficient interest as an alternative cancer therapy. However, the development of highly efficient and safe photothermal agents has become a challenging task. SWCNTs were fabricated with infrared fluorescent cyanines to treat breast cancer (Liang et al., 2016). Tumors were successfully localized using NIR imaging. This material was found as low toxic and high stability. With the introduction of PEG, the blood circulation time of this conjugated material improved. The therapeutic performance was improved with the localized heating effect under NIR radiation. Chen group fabricated carbon nanotube rings (CNTRs) coated with gold nanoparticles that showed higher Raman and optical signal properties, improved photoacoustic signal, and photothermal conversion characteristics (Song et al., 2016). The thermo-sensitive hydrogel was prepared using the SWCNTs (Zhou et al., 2015). DOX incorporated in this gel showed higher tumor suppression with the NIR radiation in mice xenograft gastric tumor models. This DOX-loaded SWNT hydrogel improved the efficiency of DOX and concurrently reduced the systemic adverse effects. Fullerene derivatives are broadly useful for the delivery of different cargos due to their unique structure. Modified fullerene (DMA-C60-NGR) was explored as an antitumor drug carrier for targeted delivery. Synergistic enhancement of cancer therapy was studied using photodynamic therapy-induced by DMA-C60-NGR and 2-methoxyestradiol. This tumor-targeted delivery could efficiently penetrate the cancerous cell membrane, induced intracellular reactive oxygen species and damaged DNA (Shi et al., 2013). For a combined chemotherapy and photodynamic therapy, doxorubicin (DOX) loaded fullerene (C60-PEI-DOX) was investigated. DOX was conjugated covalently with C60-PEI using the hydrazine linkage (Shi et al., 2014). Here, DOX release from C60-PEI-DOX was dependent on the pH of the microenvironment. This loaded complex was further conjugated with CdSe/ZnS that showed higher tumor targeting efficiency compared with quantum dots alone or free DOX. 2.4-fold higher DOX-released was observed in the tumor site rather than the normal tissue. MWCNT-based drug delivery was developed by covalently bonding the CNTs with 10-hydroxycamptothecin (HCPT-an antitumor agent) to form a hydrophilic diaminotriethylene glycol between drug and nanotubes (Wu et al., 2009). The further surface modification increased the carboxylic group, followed by the covalently linking hydrophilic diaminotiethylene glycol using the amidation reaction. Later, HCPT was conjugated with MWNT using cleavable ester linkage. This conjugated material was found to be superior in antitumor activity in-vivo. GNRs also are considered as promising drug delivery for tumor therapy. Due to its aromatic nature, it facilitates the loading of anticancer drugs. To improve the anti-cancer activity, copper sulphide nanoparticles were capped with DOX-loaded mesoporous carbon nano-spheres (Zhang et al., 2015). Here the conjugation was established due to the π-π interactions. DOX uptake in MCN was prolonged in the physiological environments, and DOX release was influenced by the acidic environment. Paclitaxel was coupled with nitrogen-doped carbon dots through a labile ester bond. The water solubility of this conjugate was improved significantly. Additionally, this complex induced apoptosis in cancer cells with improved anticancer activity compared to the drug (Gomez et al., 2018). Photosensitizers are light-sensitive molecules that are difficult to deliver due to high hydrophobicity. Folic acid-functionalized CDs were developed to deliver such type of material named zinc phthalocyanine for dual activity: targeted photodynamic therapy and bio-imaging (Choi et al., 2014). This targeted therapy opens a new scope of photodynamic therapy to treat cancers.
CBNs for Peptide Delivery
CBNs are considered suitable molecules in medicinal chemistry and are mostly explored for peptide delivery. The most challenging part for the delivery of peptides is the solubility of CBNs. The organic modification in the nanotubes serves multiple sites of conjugation for biological molecules like peptides. Biologically active peptides can bond covalently with CBNs. The amine group of the CNTs was bound covalently with the foot-mouth disease virus (FMDV) mainly to the viral envelope protein (VP1-region 141-159) with a bifunctional linker. The structural integrity of CNTs was retained the same, and they were well recognized by mon- and polyclonal-antibodies (Pantarotto et al., 2003a). The B cell epitope was neutralized from the FMDM to mono- and bis-derivatized CNTs (Pantarotto et al., 2003b). These peptide-CNs triggered anti-peptide antibody responses in mice models without eliciting the adverse effects. These conjugate CNTs prompted higher virus-neutralizing antibodies. In a study, the interaction between the human immune system and CNTs was analyzed (Salvador-Morales et al., 2006). It was observed that the C1q directly binds to CNTs and the protein binding to CNTs is highly selective. Fibrinogen and apolipoproteins (AI, AIV, and CIII) bound to CNTs with higher affinity (Bianco et al., 2005). Carbon dots-Sulfosuccinimidyl-4-(N-maleimidomethyl) cyclohexane-1-carboxylate were synthesized by crosslinking method (Liu et al., 2019). This complex successfully bound and delivered small interfering RNA. Due to the conjugation with carbon dots, this material can be preferred more over commercially available poly-ethyleneimine. These materials successfully target the inflammatory cytokine tumor necrosis factor-α in the stem cell-based therapy of cartilage defects.
Considerations for Target-specific Drug Delivery
The concept of drug targeting is gradually improving to fabricate a novel formulation to manage the disease condition. This concept is based on three primary elements: Explore the target disease, an effective drug, and a cargo-carrier to transport drugs. Before step forward to novel CBNs for targeted delivery, the following points need to consider.
Mechanism of Drug Targeting
The unique feature of CNTs is large expanded surface area, higher stability, and ability to bind a wide variety of therapeutics, including DNA, enzymes, therapeutic antibodies, and drugs. Additionally, CNTs can release the loaded materials to the target side. CNTs carry the drugs either by attaching them to the surface or loading them into the matrix. The internalization method is generally recommended for effective surface attachment. After entering into the cells, the microenvironment of the cellular matrix destructs the binding and liberates the therapeutic content inside the cells. Whereas, in other types of surface attachment methods, drug releases before entering into the cells. There are several methods reported to pass the CNTs through cells. They are endocytosis, diffusion, or insertion (Rajakumar et al., 2020).
In targeting cancer cells, drug-loaded CNTs are bound with the antibody. The selection and specificity of antibodies depend on the antigen present on the surface of cancer cells. There is a possibility of antibody driven drug-CNTs mechanism in targeting tumor cells. However, this method has not been explored well. An alternative approach in tumor targeting is the coating of CNTs with a layer of magnetite (Fe3O4) nanoparticles (Rajakumar et al., 2020). In this targeting, an external magnet is required to drive this fabricated complex to reach target cells. Fabrication of ligand-anchored MWCNTs using folic acid (FA) is another opportunistic way for targeting breast cancer (Kaur et al., 2017). Functionalized FA-PEG bis-amine was further processed for carboxylation. The In-vivo and in-vitro studies showed increasing 5-fluorouracil accumulation in the target tissue. The ligand FA improved the delivery efficiency of 5-FA. Additionally, FA enhanced the antitumor activity to MCF-7 breast cancer. Another ligand named estrone (ES) was also introduced in addition to FA to improve the drug targeting to cancer (Mehra and Jain, 2015). In this study, DOX loaded ligand anchored PEGylated MWCNTs was fabricated. Estrone anchored nanotube showed higher uptake rather than free for of DOX and other formulations. Whereas combinational ligand (FA and ER) showed higher antitumor activities and elevated breast cancer targeting tendency. Hyaluronic acid (HA) was also explored to check the targeting efficiency of MWCNTs (Cao et al., 2015). In this investigation, DOX was loaded into MWCNTs and further modified with polyethyleneimine (PEI). This complex formed conjugation by a covalent bond with fluorescein isothiocyanate (FI) and HA to target cancer cells. Overexpression of CD44 receptors signified that this conjugated form successfully delivered DOX to the cancer cells. Thus HA-modified MWCNTs is a promising approach for efficiently targeting drugs to cancer cells. Functionalized MWCNTs are able to entrap cisplatin (a chemotherapeutic drug) or inner platinum (Li et al., 2014). The presence of platinum did not influence the distribution of cisplatin. However, significant lung tissue accumulation was observed for both the materials. Concurrently, kidney and tissue accumulation was reduced significantly, resulting in decreased nephrotoxicity. Light-activated cancer-targeting therapy can be feasible with SWCNTs (Yang Y. et al., 2016). Stimuli-activated targeted delivery provides an accurate treatment of tumors. In this therapy, the DNA sequence was hybridized, and mask sgc8 aptamers were conjugated on the surface of SWCNTs. Upon NIR lesser, localized photothermal heating de-hybridized the DNA and uncaged the aptamer sequence for cancer targeted therapy. DOX loaded SWCNTs enabled tissue-specific and efficient therapy. Carbon dots were also employed successfully as drug carriers and targeted drug delivery. Utilizing the fluorescent behaviour, these materials are used for drug monitoring in rea-time. Fluorescence resonance energy transfer-based drug delivery was developed (Tang et al., 2013). Using the same system, drug monitoring was done. Doxorubicin was incorporated in this matrix and observed satisfactory biocompatibility in the deep tissue environment. Antibiotics were conjugated with CDs to controlled the drug-releasing and enhanced antimicrobial activity.
Drug-Laden Nanocarrier Fabrication
CNTs are facing several challenges before they are considered as effective drug delivery. Low drug loading and rapid release profile restrict its vast application. Thus it is highly required to improve the drug-landing capacity of CNTs. Drug loading can be performed using three different methods: adsorption equilibrium, melting, and solvent evaporation. The loading efficiency depends on the type of drugs used. Out of these methods, the melting technique serves as a higher drug-loading approach. In this method, drugs cannot enter into pores but are eventually distributed throughout the carrier. However, drugs are not stable in this form as they don’t bind or conjugate with carbon materials and turn into a crystalline form. In the physical adsorption method, CBNs are soaked into a drug solution. The drugs eventually penetrate and distribute until equilibrium. Drug laded CNMs could be separated from the solution by centrifugation. After adsorption, the solvent needs to evaporate in the solvent evaporation technique. This method serves higher loading efficiency compared to the melting method (Pattnaik et al., 2020). Cisplatin was loaded into MWCNTs followed by grafting with high molecular weight polyethylene glycol to partially block the exit path for drugs (Sui et al., 2014). A remarkable increase in loading efficiency and inhibition effect was observed. Fabrication of CNT-based hydrogel from nanocomposite material was performed using biodegradable hydrogel and DWCNTs (Guillet et al., 2017). This material improved mechanical properties and electrical conductivity. Ex-vivo mouse skin model represented the successful transdermal delivery of the nanocomposite hydrogel. The excellent drug loading efficiency of CNOs has been reported for several drugs like DOX (Mamidi et al., 2020), 5-fluorouracil (5-FU) (Mamidi et al., 2019), ibuprofen, and paracetamol (Miriyala et al., 2020). The large surface area, porosity, drug molecules-CNOs interaction (π-π stacking), and attachment with polymer matrix increase the loading efficiency of CNOs. Carbon dots are also employed to improve the drug loading capacity. Hollow CDs were prepared by hydrothermal method with l-ascorbic acid and urea. This hollow structure increase the loading capacity of doxorubicin (Zhang et al., 2019).
Cellular Uptake of Carbon Nanotubes
F-CNTs possess a high propensity to cross the cell membranes. F-CNTs are uptake by the cell through passive and endocytosis-independent processes. In the presence of endocytosis inhibitors, the cells did not show any influence on cell permeability. This influence was observed in both low temperature and after incubation. Labelled-CNT would be helpful to internalize and track within the cytoplasm or the nucleus of fibroblasts either by confocal microscopy or fluorescence. Conjugated oxidized-CNT with fluorescent streptavidin were detected within the cells (Kam and Dai, 2005). The sidewalls of SWCNTs were acid-oxidised. The binding was non-specific and noncovalent protein-nanotube conjugation. The protein was readily transported inside different mammalian cells, where nanotubes served as a transporter acting through the endocytosis pathway. It was observed that the conjugated materials penetrated successfully into the cytoplasm of cells and initiated the biological activity. A model membrane was developed from molecular dynamics simulations to study the lipid-assisted mechanism for passive insertion (Lopez et al., 2004). Generic nanotube functionalized with hydrophilic termini was able to penetrate the membrane using trans-leaflet lipid flips. It was observed that hydrophobic-hydrophilic matching is required for the insertion mechanism. The uptake of pristine SWCNT into macrophages was analyzed using nanotubes fluorescence spectra (Cherukuri et al., 2004). The fluorescence intensities were increased exponentially with the incubation time and concentration of nanotube. The phagocytosis process was involved in these nanotube’s uptake. Functionalized mesoporous carbon sphere loaded with doxorubicin and verapamil significantly increased cell apoptosis and cell cytotoxicity. This study signified higher anticancer activity with lower systemic toxicity (Wan et al., 2016). Recently, the presence of CDs has been observed in the blood plasma. It was believed that the metabolism of beverages and foods in the human body is the main source of it. The size, shape, functionalities, and concentration of CDNs regulate the activities and health conditions of the human body (Mandal et al., 2019).
Methods to Augment Drug Solubility
Surface modification is feasible between drug molecules and functionalized CNT to improve the solubility either by the covalent or non-covalent bond. However, each process has its advantages and disadvantages. Covalent bond based drug-loaded CNT produces a stable form of CNT in both intra- and extracellular compartments. However, this type of CNT retards the release of drugs in a sustained manner within the cancer cell. Most of all materials under this class are possessing low-solubility and unequal dispersion due to hydrophobic structure. Therefore, CBNs need to purify and solubilize in biological fluid before being used as drug carriers in nanomedicine. The most commonly employed method for purification is the oxidative acid treatment like sonicating or refluxing the CBNs with concentrated HNO3/H2SO4. Generally, covalent and non-covalent methods have been suggested to improve solubility and biocompatibility (Tan et al., 2014). Covalent functionalization is more superior if a strong bond is required between biomolecules with CBNs. It helps to graft chemically reactive molecules onto the surface of the sp2 carbon structure. It can be possible by functionalization of the pristine CNTs with hydrophilic polymers like oligomers, biomolecules, or PEG. However, loss of materials has been observed during the acid-oxidation process. Generally, this issue is less concerned with drug delivery (Peretz and Regev, 2012). Surface functionalization is often useful to improve the performance of the material. Surface modification of PEGylation of CNTs was performed using the combination of Michael addition reaction and mussel-inspired chemistry. The intracellular biomedical evaluation was performed by incorporating Doxorubicin. Prepared PEGylated CNTs were found to be good biocompatibility with cancer cells and dispersed well in the aqueous solution (Xu H. et al., 2016). Oxygenated functionalities like hydroxyl, carboxylic, and carbonyl groups have been introduced to decorate the sidewall of CNTs. Carboxylic acid functionalization is commonly employed to bind the amine site of biomolecules with CNTs. In this process, several cross-linking agents like carbo-di-imides, thionyl, oxalyl chloride, or active esters are used to activate the carboxylic acids. Some other approaches are also available to functionalize the sidewall of CNTs. They are hydrogenation, electrophilic reaction, radical additions, and ozonolysis. In comparison with covalent functionalization, non-covalent functionalization does not rupture the sp2 bonding.
Non-covalent binding enables the release of drugs in a controlled manner in the acidic environment of the cancer cell. However, extracellular pH level interferes with the stability in this type of CNT (Maiti et al., 2019). Thus it retains the structure and functional properties in a more effective way. However, surface doping effects were observed due to electronic transitions (Fernando et al., 2004). Additionally, the non-covalent bond is vulnerable to salt concentration. Due to strong Van-der Waals forces, CBNs are difficult to disperse in the solution, and they readily get aggregate. To reduce the self-aggregation, many dispersing agents (peptides, biomolecules, hyaluronic acid, surfactants) are used to make homogeneous dispersion. Amphiphobic drug delivery is a big challenge as neither they are soluble in water nor oil. SNX-2112 (a novel inhibitor of Hsp90) was extensively explored in various types of cancer. Thus chitosan-functionalized SWCNTs were fabricated to deliver the SNX-2112 to a cancer cell (Zheng et al., 2016). With this functionalized modification, drug-loading capability increased significantly. SWCNTs-chitosan increased the ROS in comparison with SWCNTs and leads to higher mitogen-activated protein kinase and cell apoptosis. This complex successfully inhibits the K562 cells and become a promising approach for cancer therapy. The surface modification of these materials with the drugs can deliver a large amount of drugs to the target site in an accurate and precise manner. Surface modifications are also feasible with ligand or polymer to obtain tumor-targeted or stimulus-responsive drug delivery. Controllable hollow mesoporous carbon labeled with fluorescence (Eu3+/Gd3+-EDTA) was developed to improve the oral bioavailability of insoluble drugs (carvedilol) (Liu et al., 2016). Eu3+/Gd3+-EDTA significantly improved the oral bioavailability and showed longer residency in the gastrointestinal tract which was confirmed by the bio-image test. It was observed that the rate of dissolution increased with the mesoporous shell. Paracetamol was incorporated into the wrinkled mesoporous carbons (Goscianska et al., 2021). The hard template method was employed to entrap paracetamol in mesoporous carbons. Zero-ordered kinetic profile was observed in simulated gastric fluid over 24 h.
Penetration of Blood Brain Barrier
Permeation is crucial for the effective delivery of drugs to the specific tissue. The blood-brain barrier (BBB) is a tight junction composed of endothelial cells which are highly specific for drug molecules. It is quite hard to penetrate this for small and big therapeutic/biological molecules to cross BBB. Many invasive procedures like injections and surgery are employed to administer drugs to the brain. Intracranial drug delivery is a promising approach to overcome this problem. However, this technique may cause edema and infection (Guo et al., 2017). PEG-CNTs were functionalized with immune-adjuvant CpG oligodeoxynucleotides for the brain tumor model (Zhao D. et al., 2011). This material was injected intracranially for anti-glioma effects. CpG uptake was improved significantly in GL261 gliomas cells with the help of PEG-CNTs. After uptake, pro-inflammatory cytokines were released from this conjugated material to inhibit the tumor growth. However, the actual reason for unfavourable effects and inflammatory response was not revealed. Levodopa-loaded COOH-conjugated SWCNTs were fabricated to check the drug delivery to the nervous system (Tan et al., 2015). This synthesized nanohybrid was found as safe in PC12 cell lines and exhibited a slow and sustained release profile for more than 20 h. One group demonstrated the lysosomes and mitochondria are the primary site (organelles) for cytotoxicity which can be targeted by SWCNTs (Yang et al., 2010). It can penetrate the mitochondria at large doses. Phosphoinositide-3-kinase and membrane protein-2 were involved in organelle preference. After administration, SWCNTs collapsed the mitochondrial membrane and raised the overproduction of ROS that caused mitochondrial damage. SWCNTs delivered acetylcholine into the brain in Alzheimer disease-induced mice. Improved learning and memory capabilities were observed at the dose level of 5 mg/kg. To improve the brain targeted delivery, different ligands (angiopep-2, lactoferrin, endothelial growth factor) were investigated. With the modification of angiopep-2, PEGylated oxidized MWCNTs able to target the anticancer drugs to brain glioma (Ren et al., 2012). Angiopep-2 can bind with low-density receptor-related protein receptors and over-express the BBB and glioma cells. Fullerenes possess additional activities like antioxidant, anti-aggregation which can be employed for the generation of new drugs for neurodegenerative disease. Fullerene and graphene quantum dots also demonstrate the anti-amyloid action which opens a new path in the management of neurodegenerative disorder. Several other carbon nanostructures like CNTs, carbon dots, and nanodiamonds are able to interact with several biological including amyloid molecules and proteins. They can cross the BBB due to the smaller size and able to repair or heal neurons (Henna et al., 2020). Galantamine was adsorbed on the surface of hierarchical porous carbon (HPC) which was further incorporated into the poly (l-lactic acid) and poly (lactide-co-glycolide) to form hybrid nanoparticles (Nanaki et al., 2020). In an animal experiment, this material successfully delivered to the hippocampus, the brain area. Tryptophan is one of the 20 essential amino acids and is involved in several biological processes. It is a key component of the brain and crosses the BBB through a specialized transporter. Due to this reason, tryptophan-CDs were prepared from tryptophan and 1,2-ethylenediamine (Mintz et al., 2019). The presence of tryptophan on the surface of CDs will help to cross the BBB. Thus no need for any conjugation to receptors as this conjugation may possess low-yield. Triple conjugated CDs (epirubicin, temozolomide, and transferrin were developed for malignant brain tumors (Hettiarachchi et al., 2019). CDs This conjugated system showed a synergistic effect with higher cytotoxicity to brain tumor cell lines.
Discussion and Prospects
In recent years, CBNs have drawn special interest in biomedical and drug delivery applications. These are only possible due to their unique properties and large surface areas which are missing in other types of non-carbon materials. Specially, functionalized CBNs successfully improve the drug loading, targeting, cellular uptake, and membrane transportation of drugs (Figure 4). Out of the wider scope, these CBNs came with few drawbacks and limitations, especially feasibility in the industrial scale-up. Taking the example of graphene oxide: its introduction was in 1898. However, it’s industrial manufacturing on a large scale has faced several challenges. Likewise, all other CBNs have some drawbacks. Extensive examinations and thoroughly understanding need to perform to address these challenges (Figure 3). More advanced applications of these materials are in nanomedicine for improving different types of diseases like cancer, HIV, etc. Lack of safety, efficacy standardization, and formulation prospects, etc., are some major problems that need the development of the new clinical protocol and translation methods. Newer anticancer drugs have come to market through tight regulation and rigorous clinical trials. However, CBNs based anticancer drugs could not overcome this stage yet. Another promising challenging issue is the long term toxicity of CBNs. To mitigate the adverse physiological response, surface modification of biomaterials is required to enhance hemo- and bio-compatibility. Several reports suggested the surface modification is an effective approach to reduce toxicity. Most of the animal experiments have been performed on lower rodents that are different from primates and humans. Additionally, a long-term toxicity study should not restrict to six months. A 6 month’s toxicity study is not sufficient to assume the potential toxicity of CBNs. The influence of CBNs on the reproductive, nerve and immune system has not been explored systematically. Careful evaluation of biological interaction and bio-compatibility of each CBNs is needed to explore for medicine. Thus more preclinical toxicity needs to perform before finally translating to clinical application. With this in-vivo study, oxidative stress, chemical modification, and biodegradability studies also need to be performed. Long-time effects, mass production, and cost-effectiveness should be considered to improve treatment at the mass level. Sophisticated techniques need to be employed using analytical chemistry, computation sciences, and biophysics for structural elucidations and production of CBNs (Saleem et al., 2018). Drug targeting to infection sites is crucial for any type of drug delivery that provides a boost in treatment efficacy, reducing side effects, and multidrug resistance. This drug targeting can be achieved by two different mechanisms: passive and active. Passive targeting is possible by reducing the size of particles between 50 and 200nm that have potential access to penetrate cells, which is also termed as enhanced permeability and retention (ERP) effect. Active targeting is possible by conjugating the ligand or antibiotics on the carrier. Hydrophilicity and particle size is crucial to target the drugs to the tumor tissue. In comparison with organic macromolecules, CBNs may not have recognizable benefits if they are commonly employed as drug carriers as they hardly degrade in the bio-environment. Better preparation and synthesis techniques need to develop with optimum physiological properties. However, dual therapy like a combination of drug therapy with different cancer therapy (photothermal, RE ablation, or photoacoustic) may overcome the multi-drug resistance problems in current cancer chemotherapies. The growing interest in zero-dimension carbon explores a new scope for better targeting to tissues or cells. Due to the smaller size (<10nm) they can easily cross the tight cellular junctions (e.g. blood-brain barrier) without any ligand or receptors. Additionally, they demonstrate higher bio-compatibility with negligible toxicity.
Conclusion
The present review highlights the different roles of CBNs in medical and biomedical applications. Targeting delivery is possible with CBNs that have been explained in this review. Several subcategories of CBN possess opportunistic carrier materials for drug delivery. The specific surface-association or functionalized surface properties enable CBNs a more promising candidate for drug delivery. A higher drug loading efficiency, modified release kinetics, improved solubility, and improved biocompatibility with reduced toxicity are prerequisites in different target-specific drug delivery systems. More advanced application of CBNs has been explored in cancer therapy. Several toxicities are associated with CBNs that restrict their wider acceptance. Covalent and non-covalent modifications can decrease such toxicity. However, every alternation of the surface may trigger the body's immune response that can verify by animal experiments. But short-term preclinical studies are not sufficient to trace. Thus long-term toxicity studies for more than six months are suggested here. As CBNs can cross the BBB, more research needs to focus on neurodegenerative disorders like Alzheimer's disease and Parkinson's disease.
Author Contributions
SD wrote this paper. RS supervised this work and revised the manuscript. Both authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abarrategi, A., Gutiérrez, M. C., Moreno-Vicente, C., Hortigüela, M. J., Ramos, V., López-Lacomba, J. L., et al. (2008). Multiwall carbon nanotube scaffolds for tissue engineering purposes. Biomaterials 29, 94–102. doi:10.1016/j.biomaterials.2007.09.021
Abu Rabe, D. I., Al Awak, M. M., Yang, F., Okonjo, P. A., Dong, X., Teisl, L. R., et al. (2019). The dominant role of surface functionalization in carbon dots' photo-activated antibacterial activity. Int. J. Nanomed. 14, 2655–2665. doi:10.2147/IJN.S200493
Aldieri, E., Fenoglio, I., Cesano, F., Gazzano, E., Gulino, G., Scarano, D., et al. (2013). The role of iron impurities in the toxic effects exerted by short multiwalled carbon nanotubes (MWCNT) in murine alveolar macrophages. J. Toxicol. Environ. Health Part. A. 76, 1056–1071. doi:10.1080/15287394.2013.834855
Al Garalleh, H., and Algarni, A. (2020). Modelling of paclitaxel conjugated with carbon nanotubes as an antitumor agent for cancer therapy. J. Biomed. Nanotechnol. 16, 224–234. doi:10.1166/jbn.2020.2886
Aliabadi, M., Yunessnia lehi, A., Shagholani, H., and Gerayeli, A. (2018). Planar polymer-graphene oxide nanohybrid as a 5-fluorouacil carrier in pH-responsive controlled release. J. Drug Deliv. Sci. Tech. 43, 103–106. doi:10.1016/j.jddst.2017.09.020
Alipour, E., Alimohammady, F., Yumashev, A., and Maseleno, A. (2020). Fullerene C60 containing porphyrin-like metal center as drug delivery system for ibuprofen drug. J. Mol. Model. 26, 7. doi:10.1007/s00894-019-4267-1
Allen, B. L., Kichambare, P. D., Gou, P., Vlasova, I. I., Kapralov, A. A., Konduru, N., et al. (2008). Biodegradation of single-walled carbon nanotubes through enzymatic catalysis. Nano Lett. 8, 3899–3903. doi:10.1021/nl802315h
Asgari, S., Pourjavadi, A., Hosseini, S. H., and Kadkhodazadeh, S. (2020). A pH-sensitive carrier based-on modified hollow mesoporous carbon nanospheres with calcium-latched gate for drug delivery. Mater. Sci. Eng. C 109, 110517. doi:10.1016/j.msec.2019.110517
Assali, A., Akhavan, O., Adeli, M., Razzazan, S., Dinarvand, R., Zanganeh, S., et al. (2018). Multifunctional core-shell nanoplatforms (gold@graphene oxide) with mediated NIR thermal therapy to promote miRNA delivery. Nanomed. Nanotech. Biol. Med. 14, 1891–1903. doi:10.1016/j.nano.2018.05.016
Bai, R. G., and Husseini, G. A. (2019). “Graphene-based drug delivery systems,” in biomimetic nanoengineered Materials for advanced drug delivery. Editors A. R. Unnithan, A. R. K. Sasikala, C. H. Park, and C. S. Kim (Amsterdam, Netherlands: Elsevier), 149–168. doi:10.1016/B978-0-12-814944-7.00011-4
Bagheri Novir, S., and Aram, M. R. (2020). Quantum mechanical simulation of chloroquine drug interaction with C60 fullerene for treatment of COVID-19. Chem. Phys. Lett. 757, 137869. doi:10.1016/j.cplett.2020.137869
Barahuie, F., Saifullah, B., Dorniani, D., Fakurazi, S., Karthivashan, G., Hussein, M. Z., et al. (2017). Graphene oxide as a nanocarrier for controlled release and targeted delivery of an anticancer active agent, chlorogenic acid. Mater. Sci. Eng. C Mater. Biol. Appl. 74, 177–185. doi:10.1016/j.msec.2016.11.114
Bartkowski, M., and Giordani, S. (2021). Carbon nano-onions as potential nanocarriers for drug delivery. Dalton Trans. 50, 2300–2309. doi:10.1039/d0dt04093b
Béduer, A., Seichepine, F., Flahaut, E., Loubinoux, I., Vaysse, L., and Vieu, C. (2012). Elucidation of the role of carbon nanotube patterns on the development of cultured neuronal cells. Langmuir 28, 17363–17371. doi:10.1021/la304278n
Beg, S., Rahman, M., Jain, A., Saini, S., Hasnain, M. S., Swain, S., et al. (2018). “Emergence in the functionalized carbon nanotubes as smart nanocarriers for drug delivery applications,” in Fullerenes, Graphenes and nanotubes: a pharmaceutical approach. Editor A. M. Grumezescu (Amsterdam, Netherlands: Elsevier), 105–133. doi:10.1016/B978-0-12-813691-1.00004-X
Bepete, G., and Coleman, K. S. (2019). “Carbon nanotubes: electronic structure and spectroscopy,” in Comprehensive Nanoscience and nanotechnology. Editors D. L. Andrews, R. H. Lipson, and T. Nann (Amsterdam, Netherlands: Elsevier), 205–218. doi:10.1016/B978-0-12-803581-8.11401-8
Bhatt, A., Jain, A., Gurnany, E., Jain, R., Modi, A., and Jain, A. (2016). “Carbon nanotubes: a promising carrier for drug delivery and targeting,” in Nanoarchitectonics for smart Delivery and drug targeting. Editors A. M. Holban, and A. M. Grumezescu (Amsterdam, Netherlands: Elsevier), 465–501. doi:10.1016/B978-0-323-47347-7.00017-3
Bhattacharya, K., Andón, F. T., El-Sayed, R., and Fadeel, B. (2013). Mechanisms of carbon nanotube-induced toxicity: focus on pulmonary inflammation. Adv. Drug Deliv. Rev. 65, 2087–2097. doi:10.1016/j.addr.2013.05.012
Bhattacharya, K., Mukherjee, S. P., Gallud, A., Burkert, S. C., Bistarelli, S., Bellucci, S., et al. (2016). Biological interactions of carbon-based nanomaterials: from coronation to degradation. Nanomedicine 12, 333–351. doi:10.1016/j.nano.2015.11.011
Bhinge, S. D. (2017). Carbon nano-onions – an overview. J. Pharm. Chem. Chem. Sci. 1, 1–2. Available at: http://www.alliedacademies.org/journal-pharmaceutical-chemistry-chemical-science/Editorial (Accessed January 30, 2021).
Bianco, A., Kostarelos, K., and Prato, M. (2005). Applications of carbon nanotubes in drug delivery. Curr. Opin. Chem. Biol. 9, 674–679. doi:10.1016/j.cbpa.2005.10.005
Bondon, N., Raehm, L., Charnay, C., Boukherroub, R., and Durand, J. O. (2020). Nanodiamonds for bioapplications, recent developments. J. Mater. Chem. B 8, 10878–10896. doi:10.1039/d0tb02221g
Cai, D., Mataraza, J. M., Qin, Z. H., Huang, Z., Huang, J., Chiles, T. C., et al. (2005). Highly efficient molecular delivery into mammalian cells using carbon nanotube spearing. Nat. Methods 2, 449–454. doi:10.1038/nmeth761
Cao, X., Tao, L., Wen, S., Hou, W., and Shi, X. (2015). Hyaluronic acid-modified multiwalled carbon nanotubes for targeted delivery of doxorubicin into cancer cells. Carbohydr. Res. 405, 70–77. doi:10.1016/j.carres.2014.06.030
Chen, J., Chen, S., Zhao, X., Kuznetsova, L. V., Wong, S. S., and Ojima, I. (2008). Functionalized single-walled carbon nanotubes as rationally designed vehicles for tumor-targeted drug delivery. J. Am. Chem. Soc. 130, 16778–16785. doi:10.1021/ja805570f
Chen, K., and Mitra, S. (2019). Incorporation of functionalized carbon nanotubes into hydrophobic drug crystals for enhancing aqueous dissolution. Colloids Surfaces B Biointer. 173, 386–391. doi:10.1016/j.colsurfb.2018.09.080
Chen, Y. C., Huang, X. C., Luo, Y. L., Chang, Y. C., Hsieh, Y. Z., and Hsu, H. Y. (2013). Non-metallic nanomaterials in cancer theranostics: a review of silica- and carbon-based drug delivery systems. Sci. Technol. Adv. Mater. 14, 044407. doi:10.1088/1468-6996/14/4/044407
Cherukuri, P., Bachilo, S. M., Litovsky, S. H., and Weisman, R. B. (2004). Near-infrared fluorescence microscopy of single-walled carbon nanotubes in phagocytic cells. J. Am. Chem. Soc. 126, 15638–15639. doi:10.1021/ja0466311
Choi, Y., Kim, S., Choi, M.-H., Ryoo, S.-R., Park, J., Min, D.-H., et al. (2014). Highly biocompatible carbon nanodots for simultaneous bioimaging and targeted photodynamic therapy in vitro and in vivo. Adv. Funct. Mater. 24, 5781–5789. doi:10.1002/adfm.201400961
Cohen, E. N., Kondiah, P. P. D., Choonara, Y. E., du Toit, L. C., and Pillay, V. (2020). Carbon dots as nanotherapeutics for biomedical application. Curr. Pharm. Des. 26, 2207–2221. doi:10.2174/1381612826666200402102308
Crevillen, A. G., Escarpa, A., and Garcia, C. D. (2019). “Carbon-based nanomaterials in analytical chemistry,” in Carbon-based Nanomaterials in analytical chemistry. Editors A. G. Crevillen, A. Escarpa, and C. D. García (Cambridge, United Kingdom: Royal Society of Chemistry), 1–36. doi:10.1039/9781788012751-00001
d'Amora, M., Camisasca, A., Boarino, A., Arpicco, S., and Giordani, S. (2020). Supramolecular functionalization of carbon nano-onions with hyaluronic acid-phospholipid conjugates for selective targeting of cancer cells. Colloids Surf. B Biointerfaces 188, 110779. doi:10.1016/j.colsurfb.2020.110779
Deng, W., Qiu, J., Wang, S., Yuan, Z., Jia, Y., Tan, H., et al. (2018). Development of biocompatible and VEGF-targeted paclitaxel nanodrugs on albumin and graphene oxide dual-carrier for photothermal-triggered drug delivery in vitro and in vivo. Int. J. Nanomed. 13, 439–453. doi:10.2147/IJN.S150977
Di Giorgio, M. L., Bucchianico, S. D., Di, Ragnelli, A. M., Ragnelli, P., Santucci, S., and Poma, A. (2011). Effects of single and multi walled carbon nanotubes on macrophages: cyto and genotoxicity and electron microscopy. Mutat. Res./Genet. Toxicol. Environ. Mutagenesis 722, 20–31. doi:10.1016/j.mrgentox.2011.02.008
Docter, D., Westmeier, D., Markiewicz, M., Stolte, S., Knauer, S. K., and Stauber, R. H. (2015). The nanoparticle biomolecule corona: lessons learned - challenge accepted?. Chem. Soc. Rev. 44, 6094–6121. doi:10.1039/c5cs00217f
Dong, X., Sun, Z., Wang, X., and Leng, X. (2017). An innovative MWCNTs/DOX/TC nanosystem for chemo-photothermal combination therapy of cancer. Nanomedicine 13, 2271–2280. doi:10.1016/j.nano.2017.07.002
Dutta, D., Sundaram, S. K., Teeguarden, J. G., Riley, B. J., Fifield, L. S., Jacobs, J. M., et al. (2007). Adsorbed proteins influence the biological activity and molecular targeting of nanomaterials. Toxicol. Sci. 100, 303–315. doi:10.1093/toxsci/kfm217
Esfandiari, N., Bagheri, Z., Ehtesabi, H., Fatahi, Z., Tavana, H., and Latifi, H. (2019). Effect of carbonization degree of carbon dots on cytotoxicity and photo-induced toxicity to cells. Heliyon 5, e02940. doi:10.1016/j.heliyon.2019.e02940
Estrada, A. C., Daniel-Da-Silva, A. L., and Trindade, T. (2013). Photothermally enhanced drug release by κ-carrageenan hydrogels reinforced with multi-walled carbon nanotubes. RSC Adv. 3, 10828–10836. doi:10.1039/c3ra40662h
Fadeel, B., Feliu, N., Vogt, C., Abdelmonem, A. M., and Parak, W. J. (2013). Bridge over troubled waters: understanding the synthetic and biological identities of engineered nanomaterials. Wiley Interdiscip. Rev. Nanomed Nanobiotechnol 5, 111–129. doi:10.1002/wnan.1206
Farrera, C., Bhattacharya, K., Lazzaretto, B., Andón, F. T., Hultenby, K., Kotchey, G. P., et al. (2014). Extracellular entrapment and degradation of single-walled carbon nanotubes. Nanoscale 6, 6974–6983. doi:10.1039/c3nr06047k
Fernando, K. A., Lin, Y., Wang, W., Kumar, S., Zhou, B., Xie, S. Y., et al. (2004). Diminished band-gap transitions of single-walled carbon nanotubes in complexation with aromatic molecules. J. Am. Chem. Soc. 126, 10234–10235. doi:10.1021/JA047691+
Figarol, A., Pourchez, J., Boudard, D., Forest, V., Akono, C., Tulliani, J. M., et al. (2015). In vitro toxicity of carbon nanotubes, nano-graphite and carbon black, similar impacts of acid functionalization. Toxicol. Vitro 30, 476–485. doi:10.1016/j.tiv.2015.09.014
Ganji, D. D., and Kachapi, S. H. H. (2015). “Semi nonlinear analysis in carbon nanotube,” in Application of nonlinear Systems in Nanomechanics and nanofluids. Editors D. D. Ganji, and S. H. H. Kachapi (Amsterdam, Netherlands: Elsevier), 13–70. doi:10.1016/b978-0-323-35237-6.00002-9
Garg, B., and Bisht, T. (2016). Carbon nanodots as peroxidase nanozymes for biosensing. Molecules 21, 1653. doi:10.3390/molecules21121653
Ge, C., Du, J., Zhao, L., Wang, L., Liu, Y., Li, D., et al. (2011). Binding of blood proteins to carbon nanotubes reduces cytotoxicity. Proc. Natl. Acad. Sci. USA 108, 16968–16973. doi:10.1073/pnas.1105270108
Geng, B., Yang, D., Pan, D., Wang, L., Zheng, F., Shen, W., et al. (2018). NIR-responsive carbon dots for efficient photothermal cancer therapy at low power densities. Carbon 134, 153–162. doi:10.1016/j.carbon.2018.03.084
Gomez, I. J., Arnaiz, B., Cacioppo, M., Arcudi, F., and Prato, M. (2018). Nitrogen-doped carbon nanodots for bioimaging and delivery of paclitaxel. J. Mater. Chem. B 6, 5540–5548. doi:10.1039/c8tb01796d
Goscianska, J., Olejnik, A., Ejsmont, A., Galarda, A., and Wuttke, S. (2021). Overcoming the paracetamol dose challenge with wrinkled mesoporous carbon spheres. J. Colloid Interf. Sci. 586, 673–682. doi:10.1016/j.jcis.2020.10.137
Grebinyk, A., Prylutska, S., Chepurna, O., Grebinyk, S., Prylutskyy, Y., Ritter, U., et al. (2019a). Synergy of chemo- and photodynamic therapies with C60 fullerenedoxorubicin nanocomplex. Nanomaterials 9, 1540. doi:10.3390/nano9111540
Grebinyk, A., Prylutska, S., Grebinyk, S., Prylutskyy, Y., Ritter, U., Matyshevska, O., et al. (2019b). Complexation with C60 fullerene increases doxorubicin efficiency against leukemic cells in vitro. Nanoscale Res. Lett. 14, 61. doi:10.1186/s11671-019-2894-1
Gu, M., Toh, T. B., Hooi, L., Lim, J. J., Zhang, X., and Chow, E. K. (2019). Nanodiamond-mediated delivery of a G9a inhibitor for hepatocellular carcinoma therapy. ACS Appl. Mater. Inter. 11, 45427–45441. doi:10.1021/acsami.9b16323
Guillet, J. F., Flahaut, E., and Golzio, M. (2017). A hydrogel/carbon-nanotube needle-free device for electrostimulated skin drug delivery. ChemPhysChem 18, 2715–2723. doi:10.1002/cphc.201700517
Guo, Q., Shentao, X. Li., Li, Y., and Xuqing, S. (2017). Carbon nanotubes-based drug delivery to cancer and brain. J. Huazhong Univ. Sci. Technol. Med. Sci. 37, 635–641. doi:10.1007/s11596-017-1783-z
Havrdova, M., Hola, K., Skopalik, J., Tomankova, K., Petr, M., Cepe, K., et al. (2016). Toxicity of carbon dots - effect of surface functionalization on the cell viability, reactive oxygen species generation and cell cycle. Carbon 99, 238–248. doi:10.1016/j.carbon.2015.12.027
He, X., Chen, P., Zhang, J., Luo, T. Y., Wang, H. J., Liu, Y. H., et al. (2019). Cationic polymer-derived carbon dots for enhanced gene delivery and cell imaging. Biomater. Sci. 7, 1940–1948. doi:10.1039/c8bm01578c
Henna, T. K., Raphey, V. R., Sankar, R., Ameena Shirin, V. K., Gangadharappa, H. V., and Pramod, K. (2020). Carbon nanostructures: the drug and the delivery system for brain disorders. Int. J. Pharm. 587, 119701. doi:10.1016/j.ijpharm.2020.119701
Hernandez, Y., Pang, S., Feng, X., and Müllen, K. (2012). “Graphene and its synthesis,,” in Polymer science: a comprehensive reference, 10 volume set. Editors K. Matyjaszewski, and M. Moller (Amsterdam, Netherlands: Elsevier), 415–438. doi:10.1016/B978-0-444-53349-4.00216-8
Hettiarachchi, S. D., Graham, R. M., Mintz, K. J., Zhou, Y., Vanni, S., Peng, Z., et al. (2019). Triple conjugated carbon dots as a nano-drug delivery model for glioblastoma brain tumors. Nanoscale 11, 6192–6205. doi:10.1039/C8NR08970A
Huang, C., Wu, J., Jiang, W., Liu, R., Li, Z., and Luan, Y. (2018). Amphiphilic prodrug-decorated graphene oxide as a multi-functional drug delivery system for efficient cancer therapy. Mater. Sci. Eng. C 89, 15–24. doi:10.1016/j.msec.2018.03.017
Huang, H., Yuan, Q., Shah, J. S., and Misra, R. D. (2011). A new family of folate-decorated and carbon nanotube-mediated drug delivery system: synthesis and drug delivery response. Adv. Drug Deliv. Rev. 63, 1332–1339. doi:10.1016/j.addr.2011.04.001
Huang, X., Wu, S., and Du, X. (2016). Gated mesoporous carbon nanoparticles as drug delivery system for stimuli-responsive controlled release. Carbon 101, 135–142. doi:10.1016/j.carbon.2016.01.094
Jagusiak, A., Chłopaś, K., Zemanek, G., Jemioła-Rzemińska, M., Piekarska, B., Stopa, B., et al. (2019). Self-assembled supramolecular ribbon-like structures complexed to single walled carbon nanotubes as possible anticancer drug delivery systems. Int. J. Mol. Sci. 20, 2064. doi:10.3390/ijms20092064
Jain, S., Spandana, G., Agrawal, A. K., Kushwah, V., and Thanki, K. (2015). Enhanced antitumor efficacy and reduced toxicity of docetaxel loaded estradiol functionalized stealth polymeric nanoparticles. Mol. Pharm. 12, 3871–3884. doi:10.1021/acs.molpharmaceut.5b00281
Jaleel, J. A., Ashraf, S. M., Rathinasamy, K., and Pramod, K. (2019). Carbon dot festooned and surface passivated graphene-reinforced chitosan construct for tumor-targeted delivery of TNF-α gene. Int. J. Biol. Macromol. 127, 628–636. doi:10.1016/j.ijbiomac.2019.01.174
Jena, P. V., Roxbury, D., Galassi, T. V., Akkari, L., Horoszko, C. P., Iaea, D. B., et al. (2017). A carbon nanotube optical reporter maps endolysosomal lipid flux. ACS Nano 11, 10689–10703. doi:10.1021/acsnano.7b04743
Kam, N. W., and Dai, H. (2005). Carbon nanotubes as intracellular protein transporters: generality and biological functionality. J. Am. Chem. Soc. 127, 6021–6026. doi:10.1021/ja050062v
Kapralov, A. A., Feng, W. H., Amoscato, A. A., Yanamala, N., Balasubramanian, K., Winnica, D. E., et al. (2012). Adsorption of surfactant lipids by single-walled carbon nanotubes in mouse lung upon pharyngeal aspiration. ACS Nano 6, 4147–4156. doi:10.1021/nn300626q
Karimi Shervedani, R., Mirhosseini, H., Samiei Foroushani, M., Torabi, M., Rahsepar, F. R., and Norouzi-Barough, L. (2018). Immobilization of methotrexate anticancer drug onto the graphene surface and interaction with calf thymus DNA and 4T1 cancer cells. Bioelectrochemistry 119, 1–9. doi:10.1016/j.bioelechem.2017.08.004
Karjalainen, P. P., Porela, P., Ylitalo, A., Vikman, S., Nyman, K., Vaittinen, M. A., et al. (2007). Safety and efficacy of combined antiplatelet-warfarin therapy after coronary stenting. Eur. Heart J. 28, 726–732. doi:10.1093/eurheartj/ehl488
Kaur, S., Mehra, N. K., Jain, K., and Jain, N. K. (2017). Development and evaluation of targeting ligand-anchored CNTs as prospective targeted drug delivery system. Artif. Cell Nanomed Biotechnol 45, 242–250. doi:10.3109/21691401.2016.1146728
Kharisov, B. I., Kharissova, O. V., Leija Gutierrez, H., and Ortiz Méndez, U. (2009). Recent advances on the soluble carbon nanotubes. Ind. Eng. Chem. Res. 48, 572–590. doi:10.1021/ie800694f
Kim, S. W., Kyung Lee, Y., Yeon Lee, J., Hee Hong, J., and Khang, D. (2017). PEGylated anticancer-carbon nanotubes complex targeting mitochondria of lung cancer cells. Nanotechnology 28, 465102. doi:10.1088/1361-6528/aa8c31
Knoblauch, R., and Geddes, C. D. (2020). Carbon nanodots in photodynamic antimicrobial therapy: a review. Materials 13, 4004. doi:10.3390/ma13184004
Kobayashi, N., Izumi, H., and Morimoto, Y. (2017). Review of toxicity studies of carbon nanotubes. J. Occup. Health 59, 394–407. doi:10.1539/joh.17-0089-RA
Krajcik, R., Jung, A., Hirsch, A., Neuhuber, W., and Zolk, O. (2008). Functionalization of carbon nanotubes enables non-covalent binding and intracellular delivery of small interfering RNA for efficient knock-down of genes. Biochem. Biophys. Res. Commun. 369, 595–602. doi:10.1016/j.bbrc.2008.02.072
Kumar, N., and Kumbhat, S. (2016). “Carbon‐based nanomaterials,” in Essentials in Nanoscience and nanotechnology. New York, US: Wiley, 189–236. doi:10.1002/9781119096122.ch5
Kumar, S., Kushwaha, S., Ghoshal, S., Rai, A. K., and Singh, S. (2013). Carbon nanotubes as a novel drug delivery system for anticancer therapy: a review. Artic. Braz. J. Pharm. Sci. 49, 629–643. doi:10.1590/S1984-82502013000400002
Lee, P. C., Lin, C. Y., Peng, C. L., and Shieh, M. J. (2016). Development of a controlled-release drug delivery system by encapsulating oxaliplatin into SPIO/MWNT nanoparticles for effective colon cancer therapy and magnetic resonance imaging. Biomater. Sci. 4, 1742–1753. doi:10.1039/c6bm00444j
Li, D., Chen, X., Wang, H., Liu, J., Zheng, M., Fu, Y., et al. (2017). Cetuximab-conjugated nanodiamonds drug delivery system for enhanced targeting therapy and 3D Raman imaging. J. Biophoton. 10, 1636–1646. doi:10.1002/jbio.201700011
Li, J., Li, M., Tian, L., Qiu, Y., Yu, Q., Wang, X., et al. (2020). Facile strategy by hyaluronic acid functional carbon dot-doxorubicin nanoparticles for CD44 targeted drug delivery and enhanced breast cancer therapy. Int. J. Pharm. 578, 119122. doi:10.1016/j.ijpharm.2020.119122
Li, J., Pant, A., Chin, C. F., Ang, W. H., Ménard-Moyon, C., Nayak, T. R., et al. (2014). In vivo biodistribution of platinum-based drugs encapsulated into multi-walled carbon nanotubes. Nanomedicine 10, 1465–1475. doi:10.1016/j.nano.2014.01.004
Li, N., Than, A., Sun, C., Tian, J., Chen, J., Pu, K., et al. (2016). Monitoring dynamic cellular redox homeostasis using fluorescence-switchable graphene quantum dots. ACS Nano 10, 11475–11482. doi:10.1021/acsnano.6b07237
Liang, C., Li, Z., and Dai, S. (2008). Mesoporous carbon materials: synthesis and modification. Angew. Chem. Int. Ed. Engl. 47, 3696–3717. doi:10.1002/anie.200702046
Liang, F., and Chen, B. (2009). A review on biomedical applications of single-walled carbon nanotubes. Curr. Med. Chem. 17, 10–24. doi:10.2174/092986710789957742
Liang, X., Shang, W., Chi, C., Zeng, C., Wang, K., Fang, C., et al. (2016). Dye-conjugated single-walled carbon nanotubes induce photothermal therapy under the guidance of near-infrared imaging. Cancer Lett. 383, 243–249. doi:10.1016/j.canlet.2016.09.006
Liese, A., and Hilterhaus, L. (2013). Evaluation of immobilized enzymes for industrial applications. Chem. Soc. Rev. 42, 6236–6249. doi:10.1039/c3cs35511j
Lin, M. S., Chen, R. T., Yu, N. Y., Sun, L. C., Liu, Y., Cui, C. H., et al. (2017). Fullerene-based amino acid ester chlorides self-assembled as spherical nano-vesicles for drug delayed release. Colloids Surf. B Biointerfaces 159, 613–619. doi:10.1016/j.colsurfb.2017.08.007
Liu, J., Zhao, Y., Cui, Y., Yue, Y., Gao, Y., Zhao, Q., et al. (2016). A Eu(3+)/Gd(3+)-EDTA-doped structurally controllable hollow mesoporous carbon for improving the oral bioavailability of insoluble drugs and in vivo tracing. Nanotechnology 27, 315101. doi:10.1088/0957-4484/27/31/315101
Liu, J., Jiang, T., Li, C., Wu, Y., He, M., Zhao, J., et al. (2019). Bioconjugated carbon dots for delivery of siTnfα to enhance chondrogenesis of mesenchymal stem cells by suppression of inflammation. Stem Cell Transl. Med. 8, 724–736. doi:10.1002/sctm.18-0289
Long, W., Ouyang, H., Wan, W., Yan, W., Zhou, C., Huang, H., et al. (2020). "Two in one": simultaneous functionalization and DOX loading for fabrication of nanodiamond-based pH responsive drug delivery system. Mater. Sci. Eng. C 108, 110413. doi:10.1016/j.msec.2019.110413
Lopez, C. F., Nielsen, S. O., Moore, P. B., and Klein, M. L. (2004). Understanding nature's design for a nanosyringe. Proc. Natl. Acad. Sci. USA 101, 4431–4434. doi:10.1073/pnas.0400352101
Lu, M., Wang, Y. K., Zhao, J., Lu, H., Stenzel, M. H., and Xiao, P. (2016). PEG grafted-nanodiamonds for the delivery of gemcitabine. Macromol. Rapid Commun. 37, 2023–2029. doi:10.1002/marc.201600344
MacDonald, R. A., Laurenzi, B. F., Viswanathan, G., Ajayan, P. M., and Stegemann, J. P. (2005). Collagen-carbon nanotube composite materials as scaffolds in tissue engineering. J. Biomed. Mater. Res. A. 74, 489–496. doi:10.1002/jbm.a.30386
Mahapatra, A., Murthy, P., Patra, R., and Pattnaik, S. (2013). Solubility enhancement of modafinil by complexation with β-cyclodextrin and hydroxypropyl β-cyclodextrin: a response surface modeling approach. Drug Delivery Lett. 3, 210–219. doi:10.2174/22103031113039990005
Maiti, D., Tong, X., Mou, X., and Yang, K. (2018). Carbon-based nanomaterials for biomedical applications: a recent study. Front. Pharmacol. 9, 1401. doi:10.3389/fphar.2018.01401
Mamidi, N., González-Ortiz, A., Lopez Romo, I., and V. Barrera, E. E. (2019). Development of functionalized carbon nano-onions reinforced zein protein hydrogel interfaces for controlled drug release. Pharmaceutics 11, 621. doi:10.3390/pharmaceutics11120621
Mamidi, N., Velasco Delgadillo, R., Gonzáles Ortiz, A., and Barrera, E. (2020). Carbon nano-onions reinforced multilayered thin film system for stimuli-responsive drug release. Pharmaceutics 12, 1208. doi:10.3390/pharmaceutics12121208
Mandal, S. M., Sinha, T. K., Katiyar, A. K., Das, S., Mandal, M., and Ghosh, S. (2019). Existence of carbon nanodots in human blood. J. Nanosci. Nanotechnol. 19, 6961–6964. doi:10.1166/jnn.2019.16628
Matta-Domjan, B., King, A., Totti, S., Matta, C., Dover, G., Martinez, P., et al. (2018). Biophysical interactions between pancreatic cancer cells and pristine carbon nanotube substrates: potential application for pancreatic cancer tissue engineering. J. Biomed. Mater. Res. 106, 1637–1644. doi:10.1002/jbm.b.34012
Medepalli, K., Alphenaar, B., Raj, A., and Sethu, P. (2011). Evaluation of the direct and indirect response of blood leukocytes to carbon nanotubes (CNTs). Nanomedicine 7, 983–991. doi:10.1016/j.nano.2011.04.002
Mehra, N. K., and Jain, N. K. (2015). One platform comparison of estrone and folic acid anchored surface engineered MWCNTs for doxorubicin delivery. Mol. Pharm. 12, 630–643. doi:10.1021/mp500720a
Merum, S., Veluru, J. B., and Seeram, R. (2017). Functionalized carbon nanotubes in bio-world: applications, limitations and future directions. Mater. Sci. Eng. B 223, 43–63. doi:10.1016/j.mseb.2017.06.002
Meunier, E., Coste, A., Olagnier, D., Authier, H., Lefèvre, L., Dardenne, C., et al. (2012). Double-walled carbon nanotubes trigger IL-1β release in human monocytes through Nlrp3 inflammasome activation. Nanomedicine 8, 987–995. doi:10.1016/j.nano.2011.11.004
Mintz, K. J., Mercado, G., Zhou, Y., Ji, Y., Hettiarachchi, S. D., Liyanage, P. Y., et al. (2019). Tryptophan carbon dots and their ability to cross the blood-brain barrier. Colloids Surf. B Biointerfaces 176, 488–493. doi:10.1016/j.colsurfb.2019.01.031
Miriyala, N., Kirby, D. J., Cumont, A., Zhang, R., Shi, B., Ouyang, D., et al. (2020). Synthesis of carbon onion and its application as a porous carrier for amorphous drug delivery. Crystals 10, 281. doi:10.3390/cryst10040281
Misra, C., Kumar, M., Sharma, G., Kumar, R., Singh, B., Katare, O. P., et al. (2017). Glycinated fullerenes for tamoxifen intracellular delivery with improved anticancer activity and pharmacokinetics. Nanomedicine (Lond) 12, 1011–1023. doi:10.2217/nnm-2016-0432
Mohajeri, M., Behnam, B., and Sahebkar, A. (2018). Biomedical applications of carbon nanomaterials: drug and gene delivery potentials. J. Cel. Physiol. 234, 298–319. doi:10.1002/jcp.26899
Mousavi, S. M., Soroshnia, S., Hashemi, S. A., Babapoor, A., Ghasemi, Y., Savardashtaki, A., et al. (2019). Graphene nano-ribbon based high potential and efficiency for DNA, cancer therapy and drug delivery applications. Drug Metab. Rev. 51, 91–104. doi:10.1080/03602532.2019.1582661
Moussa, F. (2018). “Fullerene and derivatives for biomedical applications,” in Nanobiomaterials: nanostructured Materials for biomedical applications. Editor R. Narayan (Amsterdam, Netherlands: Elsevier), 113–136. doi:10.1016/B978-0-08-100716-7.00005-2
Mu, Q., Broughton, D. L., and Yan, B. (2009). Endosomal leakage and nuclear translocation of multiwalled carbon nanotubes: developing a model for cell uptake. Nano Lett. 9, 4370–4375. doi:10.1021/nl902647x
Murphy, F. A., Poland, C. A., Duffin, R., Al-Jamal, K. T., Ali-Boucetta, H., Nunes, A., et al. (2011). Length-dependent retention of carbon nanotubes in the pleural space of mice initiates sustained inflammation and progressive fibrosis on the parietal pleura. Am. J. Pathol. 178, 2587–2600. doi:10.1016/j.ajpath.2011.02.040
Nanaki, S. G., Spyrou, K., Bekiari, C., Veneti, P., Baroud, T. N., Karouta, N., et al. (2020). Hierarchical porous carbon-PLLA and PLGA hybrid nanoparticles for intranasal delivery of galantamine for Alzheimer's disease therapy. Pharmaceutics 12, 227. doi:10.3390/pharmaceutics12030227
Oz, Y., Barras, A., Sanyal, R., Boukherroub, R., Szunerits, S., and Sanyal, A. (2017). Functionalization of reduced graphene oxide via thiol-maleimide “click” chemistry: Facile fabrication of targeted drug delivery vehicles. ACS Appl. Mater. Interf. 9, 34194–34203. doi:10.1021/acsami.7b08433
Pan, D., Zhang, J., Li, Z., and Wu, M. (2010). Hydrothermal route for cutting graphene sheets into blue-luminescent graphene quantum dots. Adv. Mater. Weinheim 22, 734–738. doi:10.1002/adma.200902825
Pantarotto, D., Partidos, C. D., Graff, R., Hoebeke, J., Briand, J. P., Prato, M., et al. (2003a). Synthesis, structural characterization, and immunological properties of carbon nanotubes functionalized with peptides. J. Am. Chem. Soc. 125, 6160–6164. doi:10.1021/ja034342r
Pantarotto, D., Partidos, C. D., Hoebeke, J., Brown, F., Kramer, E., Briand, J. P., et al. (2003b). Immunization with peptide-functionalized carbon nanotubes enhances virus-specific neutralizing antibody responses. Chem. Biol. 10, 961–966. doi:10.1016/j.chembiol.2003.09.011
Pantarotto, D., Singh, R., McCarthy, D., Erhardt, M., Briand, J. P., Prato, M., et al. (2004). Functionalized carbon nanotubes for plasmid DNA gene delivery. Angew. Chem. Int. Ed. Engl. 43, 5242–5246. doi:10.1002/anie.200460437
Patel, K. D., Singh, R. K., and Kim, H.-W. (2019). Carbon-based nanomaterials as an emerging platform for theranostics. Mater. Horiz. 6, 434–469. doi:10.1039/c8mh00966j
Patila, M., Chalmpes, N., Dounousi, E., Stamatis, H., and Gournis, D. (2020). “Use of functionalized carbon nanotubes for the development of robust nanobiocatalysts,” in Methods in enzymology. Editor C. V Kumar (Cambridge, Massachusetts, United States: Academic Press), 630, 263–301. doi:10.1016/bs.mie.2019.10.015
Pattnaik, S., Swain, K., and Lin, Z. (2016). Graphene and graphene-based nanocomposites: biomedical applications and biosafety. J. Mater. Chem. B 4, 7813–7831. doi:10.1039/C6TB02086K
Pattnaik, S., Surendra, Y., Rao, J. V., and Swain, K. (2020). “Carbon family nanomaterials for drug delivery applications,” in Nanoengineered Biomaterials for advanced drug delivery. Editor M. Mozafari (Amsterdam, Netherlands: Elsevier Ltd)), 421–445. doi:10.1016/b978-0-08-102985-5.00018-8
Peretz, S., and Regev, O. (2012). Carbon nanotubes as nanocarriers in medicine. Curr. Opin. Colloid Interf. Sci. 17, 360–368. doi:10.1016/j.cocis.2012.09.001
Pescatori, M., Bedognetti, D., Venturelli, E., Ménard-Moyon, C., Bernardini, C., Muresu, E., et al. (2013). Functionalized carbon nanotubes as immunomodulator systems. Biomaterials 34, 4395–4403. doi:10.1016/j.biomaterials.2013.02.052
Plonska-Brzezinska, M. E. (2019). Carbon nano-onions: a review of recent progress in synthesis and applications. ChemNanoMat 5, 568–580. doi:10.1002/cnma.201800583
Punbusayakul, N., Talapatra, S., Ajayan, P. M., and Surareungchai, W. (2013). Label-free as-grown double wall carbon nanotubes bundles for Salmonella typhimurium immunoassay. Chem. Cent. J. 7, 102–108. doi:10.1186/1752-153X-7-102
Rajakumar, G., Zhang, X.-H., Gomathi, T., Wang, S.-F., Azam Ansari, M., Mydhili, G., et al. (2020). Current use of carbon-based materials for biomedical applications-A prospective and review. Processes 8, 355. doi:10.3390/PR8030355
Rathod, V., Tripathi, R., Joshi, P., Jha, P. K., Bahadur, P., and Tiwari, S. (2019). Paclitaxel encapsulation into dual-functionalized multi-walled carbon nanotubes. AAPS PharmSciTech 20, 51. doi:10.1208/s12249-018-1218-6
Rauti, R., Musto, M., Bosi, S., Prato, M., and Ballerini, L. (2019). Properties and behavior of carbon nanomaterials when interfacing neuronal cells: how far have we come?. Carbon 143, 430–446. doi:10.1016/j.carbon.2018.11.026
Raval, J. P., Joshi, P., Chejara, D. R., Inamuddin, A. M., and Mohammad, A. (2018). “Carbon nanotube for targeted drug delivery,” in Applications of nanocomposite Materials in drug delivery (Amsterdam, Netherlands: Elsevier), 203–216. doi:10.1016/B978-0-12-813741-3.00009-1
Reina, G., Orlanducci, S., Cairone, C., Tamburri, E., Lenti, S., Cianchetta, I., et al. (2015). Rhodamine/nanodiamond as a system model for drug carrier. J. Nanosci. Nanotechnol. 15, 1022–1029. doi:10.1166/jnn.2015.9736
Reisetter, A. C., Stebounova, L. V., Baltrusaitis, J., Powers, L., Gupta, A., Grassian, V. H., et al. (2011). Induction of inflammasome-dependent pyroptosis by carbon black nanoparticles. J. Biol. Chem. 286, 21844–21852. doi:10.1074/jbc.M111.238519
Ren, J., Shen, S., Wang, D., Xi, Z., Guo, L., Pang, Z., et al. (2012). The targeted delivery of anticancer drugs to brain glioma by PEGylated oxidized multi-walled carbon nanotubes modified with angiopep-2. Biomaterials 33, 3324–3333. doi:10.1016/j.biomaterials.2012.01.025
Saito, N., Usui, Y., Aoki, K., Narita, N., Shimizu, M., Ogiwara, N., et al. (2008). Carbon nanotubes for biomaterials in contact with bone. Curr. Med. Chem. 15, 523–527. doi:10.2174/092986708783503140
Saleem, J., Wang, L., and Chen, C. (2018). Carbon-based nanomaterials for cancer therapy via targeting tumor microenvironment. Adv. Healthc. Mater. 7, e1800525. doi:10.1002/adhm.201800525
Salvador-Morales, C., Flahaut, E., Sim, E., Sloan, J., Green, M. L., and Sim, R. B. (2006). Complement activation and protein adsorption by carbon nanotubes. Mol. Immunol. 43, 193–201. doi:10.1016/j.molimm.2005.02.006
Servant, A., Jacobs, I., Bussy, C., Fabbro, C., Da Ros, T., Pach, E., et al. (2016). Gadolinium-functionalised multi-walled carbon nanotubes as a T 1 contrast agent for MRI cell labelling and tracking. Carbon 97, 126–133. doi:10.1016/j.carbon.2015.08.051
Shannahan, J. (2017). The biocorona: a challenge for the biomedical application of nanoparticles. Nanotechnol. Rev. 6, 345–353. doi:10.1515/ntrev-2016-0098
Sharma, S. K., Micic, M., Li, S., Hoar, B., Paudyal, S., Zahran, E. M., et al. (2019). Conjugation of carbon dots with β-galactosidase enzyme: Surface chemistry and use in biosensing. Molecules 24, 3275. doi:10.3390/molecules24183275
Shen, C., Brozena, A. H., and Wang, Y. (2011). Double-walled carbon nanotubes: challenges and opportunities. Nanoscale 3, 503–518. doi:10.1039/c0nr00620c
Shi, J., Wang, Z., Wang, L., Wang, H., Li, L., Yu, X., et al. (2013). Photodynamic therapy of a 2-methoxyestradiol tumor-targeting drug delivery system mediated by Asn-Gly-Arg in breast cancer. Int. J. Nanomed. 8, 1551–1562. doi:10.2147/IJN.S40011
Shi, J., Liu, Y., Wang, L., Gao, J., Zhang, J., Yu, X., et al. (2014). A tumoral acidic pH-responsive drug delivery system based on a novel photosensitizer (fullerene) for in vitro and in vivo chemo-photodynamic therapy. Acta Biomater. 10, 1280–1291. doi:10.1016/j.actbio.2013.10.037
Shi, J., Wang, B., Wang, L., Lu, T., Fu, Y., Zhang, H., et al. (2016). Fullerene (C60)-based tumor-targeting nanoparticles with "off-on" state for enhanced treatment of cancer. J. Control Release 235, 245–258. doi:10.1016/j.jconrel.2016.06.010
Singh, S., Mehra, N. K., and Jain, N. K. (2016). Development and characterization of the paclitaxel loaded riboflavin and thiamine conjugated carbon nanotubes for cancer treatment. Pharm. Res. 33, 1769–1781. doi:10.1007/s11095-016-1916-2
Song, J., Wang, F., Yang, X., Ning, B., Harp, M. G., Culp, S. H., et al. (2016). Gold nanoparticle coated carbon nanotube ring with enhanced Raman scattering and photothermal conversion property for theranostic applications. J. Am. Chem. Soc. 138, 7005–7015. doi:10.1021/jacs.5b13475
Srivastava, I., Sar, D., Mukherjee, P., Schwartz-Duval, A. S., Huang, Z., Jaramillo, C., et al. (2019). Enzyme-catalysed biodegradation of carbon dots follows sequential oxidation in a time dependent manner. Nanoscale 11, 8226–8236. doi:10.1039/c9nr00194h
Streicher, R. M., Schmidt, M., and Fiorito, S. (2007). Nanosurfaces and nanostructures for artificial orthopedic implants. Nanomedicine (Lond) 2, 861–874. doi:10.2217/17435889.2.6.861
Sui, L., Yang, T., Gao, P., Meng, A., Wang, P., Wu, Z., et al. (2014). Incorporation of cisplatin into PEG-wrapped ultrapurified large-inner-diameter MWCNTs for enhanced loading efficiency and release profile. Int. J. Pharm. 471, 157–165. doi:10.1016/j.ijpharm.2014.05.022
Taghavi, S., Abnous, K., Taghdisi, S. M., Ramezani, M., and Alibolandi, M. (2020). Hybrid carbon-based materials for gene delivery in cancer therapy. J. Control Release 318, 158–175. doi:10.1016/j.jconrel.2019.12.030
Tan, J. M., Arulselvan, P., Fakurazi, S., Ithnin, H., and Hussein, M. Z. (2014). A review on characterizations and biocompatibility of functionalized carbon nanotubes in drug delivery design. J. Nanomater. 2014, 1. doi:10.1155/2014/9170242014
Tan, J. M., Foo, J. B., Fakurazi, S., and Hussein, M. Z. (2015). Release behaviour and toxicity evaluation of levodopa from carboxylated single-walled carbon nanotubes. Beilstein J. Nanotechnol. 6, 243–253. doi:10.3762/bjnano.6.23
Tang, J., Kong, B., Wu, H., Xu, M., Wang, Y., Wang, Y., et al. (2013). Carbon nanodots featuring efficient FRET for real-time monitoring of drug delivery and two-photon imaging. Adv. Mater. Weinheim 25, 6569–6574. doi:10.1002/adma.201303124
Tasis, D., Tagmatarchis, N., Bianco, A., and Prato, M. (2006). Chemistry of carbon nanotubes. Chem. Rev. 106, 1105–1136. doi:10.1021/cr050569o
Tian, X. T., and Yin, X. B. (2019). Carbon dots, unconventional preparation strategies, and applications beyond photoluminescence. Small 15, e1901803. doi:10.1002/smll.201901803
Tinwala, H., and Wairkar, S. (2019). Production, surface modification and biomedical applications of nanodiamonds: a sparkling tool for theranostics. Mater. Sci. Eng. C 97, 913–931. doi:10.1016/j.msec.2018.12.073
Turcheniuk, K., and Mochalin, V. N. (2017). Biomedical applications of nanodiamond (Review). Nanotechnology 28, 252001. doi:10.1088/1361-6528/aa6ae4
Vardharajula, S., Ali, S. Z., Tiwari, P. M., Eroğlu, E., Vig, K., Dennis, V. A., et al. (2012). Functionalized carbon nanotubes: biomedical applications. Int. J. Nanomedicine 7, 5361–5374. doi:10.2147/IJN.S35832
Wan, L., Jiao, J., Cui, Y., Guo, J., Han, N., Di, D., et al. (2016). Hyaluronic acid modified mesoporous carbon nanoparticles for targeted drug delivery to CD44-overexpressing cancer cells. Nanotechnology 27, 135102. doi:10.1088/0957-4484/27/13/135102
Wang, P., Yan, G., Zhu, X., Du, Y., Chen, D., and Zhang, J. (2021). Heterofullerene MC59 (M = B, Si, Al) as potential carriers for hydroxyurea drug delivery. Nanomaterials 11, 115. doi:10.3390/nano11010115
Wang, X., Guo, J., Chen, T., Nie, H., Wang, H., Zang, J., et al. (2012). Multi-walled carbon nanotubes induce apoptosis via mitochondrial pathway and scavenger receptor. Toxicol. Vitro 26, 799–806. doi:10.1016/j.tiv.2012.05.010
Wang, L., Wang, Y., Hao, J., and Dong, S. (2017). Magnetic fullerene-DNA/hyaluronic acid nanovehicles with magnetism/reduction dual-responsive triggered release. Biomacromolecules 18, 1029–1038. doi:10.1021/acs.biomac.6b01939
Wang, X., Pan, H., Lin, Q., Wu, H., Jia, S., and Shi, Y. (2019). One-step synthesis of nitrogen-doped hydrophilic mesoporous carbons from chitosan-based triconstituent system for drug release. Nanoscale Res. Lett. 14, 259. doi:10.1186/s11671-019-3075-y
Wang, S. Y., Hu, H. Z., Qing, X. C., Zhang, Z. C., and Shao, Z. W. (2020). Recent advances of drug delivery nanocarriers in osteosarcoma treatment. J. Cancer 11, 69–82. doi:10.7150/jca.36588
Wu, W., Li, R., Bian, X., Zhu, Z., Ding, D., Li, X., et al. (2009). Covalently combining carbon nanotubes with anticancer agent: preparation and antitumor activity. ACS Nano 3, 2740–2750. doi:10.1021/nn9005686
Xu H., H., Liu, M., Lan, M., Yuan, H., Yu, W., Tian, J., et al. (2016). Mussel-inspired PEGylated carbon nanotubes: biocompatibility evaluation and drug delivery applications. Toxicol. Res. (Camb) 5, 1371–1379. doi:10.1039/c6tx00094k
Xu, Z. Q., Yang, Q. Q., Lan, J. Y., Zhang, J. Q., Peng, W., Jin, J. C., et al. (2016). Interactions between carbon nanodots with human serum albumin and γ-globulins: the effects on the transportation function. J. Hazard. Mater. 301, 242–249. doi:10.1016/j.jhazmat.2015.08.062
Yang, D., and Ionescu, M. I. (2017). ““Metal oxide–carbon hyhybrid materials for application in supercapacitors,” in Metal Oxides in supercapacitors. Editors D. P. Dubal, and P. Gomez-Romero (Amsterdam, Netherlands: Elsevier), 193–218. doi:10.1016/b978-0-12-810464-4.00008-5
Yang, Z., Zhang, Y., Yang, Y., Sun, L., Han, D., Li, H., et al. (2010). Pharmacological and toxicological target organelles and safe use of single-walled carbon nanotubes as drug carriers in treating Alzheimer disease. Nanomedicine 6, 427–441. doi:10.1016/j.nano.2009.11.007
Yang, K., Feng, L., and Liu, Z. (2016). Stimuli responsive drug delivery systems based on nano-graphene for cancer therapy. Adv. Drug Deliv. Rev. 105, 228–241. doi:10.1016/j.addr.2016.05.015
Yang, Y., Liu, J., Sun, X., Feng, L., Zhu, W., Liu, Z., et al. (2016). Near-infrared light-activated cancer cell targeting and drug delivery with aptamer-modified nanostructures. Nano Res. 9, 139–148. doi:10.1007/s12274-015-0898-4
Yu, Y., Yang, X., Liu, M., Nishikawa, M., Tei, T., and Miyako, E. (2019). Amphipathic nanodiamond supraparticles for anticancer drug loading and delivery. ACS Appl. Mater. Interf. 11, 18978–18987. doi:10.1021/acsami.9b04792
Yuan, W. Z., Sun, J. Z., Dong, Y., Häussler, M., Yang, F., Xu, H. P., et al. (2006). Wrapping carbon nanotubes in pyrene-containing poly(phenylacetylene) chains: solubility, stability, light emission, and surface photovoltaic properties. Macromolecules 39, 8011–8020. doi:10.1021/ma061856c
Yuan, X., Zhang, X., Sun, L., Wei, Y., and Wei, X. (2019). Cellular toxicity and immunological effects of carbon-based nanomaterials. Part. Fibre Toxicol. 16, 18–27. doi:10.1186/s12989-019-0299-z
Zaytseva, O., and Neumann, G. (2016). Carbon nanomaterials: production, impact on plant development, agricultural and environmental applications. Chem. Biol. Technol. Agric. 3, 17. doi:10.1186/s40538-016-0070-8
Zeglis, B. M., Brand, C., Abdel-Atti, D., Carnazza, K. E., Cook, B. E., Carlin, S., et al. (2015). Optimization of a pretargeted strategy for the PET imaging of colorectal carcinoma via the modulation of radioligand pharmacokinetics. Mol. Pharm. 12, 3575–3587. doi:10.1021/acs.molpharmaceut.5b00294
Zeineldin, R., Al-Haik, M., and Hudson, L. G. (2009). Role of polyethylene glycol integrity in specific receptor targeting of carbon nanotubes to cancer cells. Nano Lett. 9, 751–757. doi:10.1021/nl8033174
Zhang, C., Liu, Z., Zheng, Y., Geng, Y., Han, C., Shi, Y., et al. (2018). Glycyrrhetinic acid functionalized graphene oxide for mitochondria targeting and cancer treatment in vivo. Small 14, 1703306. doi:10.1002/smll.201703306
Zhang, L., Li, Y., Jin, Z., Chan, K. M., and Yu, J. C. (2015). Mesoporous carbon/CuS nanocomposites for pH-dependent drug delivery and near-infrared chemo-photothermal therapy. RSC Adv. 5, 93226–93233. doi:10.1039/c5ra19458j
Zhang, W., Zhang, Z., and Zhang, Y. (2011). The application of carbon nanotubes in target drug delivery systems for cancer therapies. Nanoscale Res. Lett. 6, 555. doi:10.1186/1556-276X-6-555
Zhang, D. Y., Zheng, Y., Tan, C. P., Sun, J. H., Zhang, W., Ji, L. N., et al. (2017). Graphene oxide decorated with Ru(II)-Polyethylene glycol complex for lysosome-targeted imaging and photodynamic/photothermal therapy. ACS Appl. Mater. Inter. 9, 6761–6771. doi:10.1021/acsami.6b13808
Zhang, Z., Lei, Y., Yang, X., Shi, N., Geng, L., Wang, S., et al. (2019). High drug-loading system of hollow carbon dots-doxorubicin: preparation, in vitro release and pH-targeted research. J. Mater. Chem. B 7, 2130–2137. doi:10.1039/c9tb00032a
Zhao, D., Alizadeh, D., Zhang, L., Liu, W., Farrukh, O., Manuel, E., et al. (2011). Carbon nanotubes enhance CpG uptake and potentiate antiglioma immunity. Clin. Cancer Res. 17, 771–782. doi:10.1158/1078-0432.CCR-10-2444
Zhao, Y., Allen, B. L., and Star, A. (2011). Enzymatic degradation of multiwalled carbon nanotubes. J. Phys. Chem. A. 115, 9536–9544. doi:10.1021/jp112324d
Zheng, L., Wu, S., Tan, L., Tan, H., and Yu, B. (2016). Chitosan-functionalised single-walled carbon nanotube-mediated drug delivery of SNX-2112 in cancer cells. J. Biomater. Appl. 31, 379–386. doi:10.1177/0885328216651183
Keywords: carbon nanomaterials, carbon-based nanomaterials, nanocarrier, therapeutic, surface functionalization, biological interaction, drug delivery
Citation: Debnath SK and Srivastava R (2021) Drug Delivery With Carbon-Based Nanomaterials as Versatile Nanocarriers: Progress and Prospects. Front. Nanotechnol. 3:644564. doi: 10.3389/fnano.2021.644564
Received: 21 December 2020; Accepted: 15 February 2021;
Published: 14 April 2021.
Edited by:
Yiqun Zhou, University of Miami, United StatesReviewed by:
Monika Nehra, Panjab University, IndiaAmitabha Acharya, Institute of Himalayan Bioresource Technology (CSIR), India
Copyright © 2021 Debnath and Srivastava. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sujit Kumar Debnath, c2tkLm1waGFybWFAcmVkaWZmbWFpbC5jb20=; Rohit Srivastava, cnNyaXZhc3RhQGlpdGIuYWMuaW4=
 Sujit Kumar Debnath
Sujit Kumar Debnath Rohit Srivastava
Rohit Srivastava