
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nanotechnol. , 19 November 2020
Sec. Biomedical Nanotechnology
Volume 2 - 2020 | https://doi.org/10.3389/fnano.2020.595352
This article is part of the Research Topic National Conference on Nano/Bio-Technology 2019, India View all 12 articles
 Bhawna1,2
Bhawna1,2 Ashish Kumar Choudhary3
Ashish Kumar Choudhary3 Akanksha Gupta4*
Akanksha Gupta4* Sanjeev Kumar1,2
Sanjeev Kumar1,2 Pramod Kumar5
Pramod Kumar5 R. P. Singh4
R. P. Singh4 Prashant Singh6
Prashant Singh6 Vinod Kumar1,7*
Vinod Kumar1,7*This work represented the synthesis of Ce doped SnO2 nanoparticles by a wet chemical method and was characterized by various characterization techniques. PXRD confirmed the presence of the rutile phase for Ce doped SnO2 nanoparticles. SEM image and elemental mapping showed agglomerated irregular shaped particles and uniform distribution of 5% Ce ions within the SnO2 lattice, respectively. Ce doped SnO2 nanoparticles showed antimicrobial activity against E. coli and prevented the growth of bacteria. The nanoparticles were found photocatalytic active and photocatalytic behavior was elucidated by the degradation of Malachite Green dye under UV light irradiation.
There has been a continuous threat to health, food packaging, cosmetics, and many more industries due to microbes and these industries highly depend on various antimicrobial agents (Ananpattarachai et al., 2009). Contaminated surfaces, colonization, subsequent biofilm formation, improper cleaning of equipments are found to be the primary carriers of microorganisms leading to several foodborne and other outbreaks (Swaminathan and Smidt, 2007; Yemmireddy and Hung, 2017). Nanotechnology has its impacts on all fields of science related to nanomedicine, biomedical, biosensor, development of smart cities, energy, environment, etc. (Kumar et al., 2019a; Bhawna et al., 2020; Gupta et al., 2020a,b).
Nanoparticles have been long known for their antimicrobial behavior against gram-positive and gram-negative bacteria, pathogens, and other microbes (Azam et al., 2012; Vargas-Reus et al., 2012). Metal oxide nanoparticles serve as antimicrobial agents owing to their large surface area (Raghunath and Perumal, 2017). Out of several metal oxide nanomaterials, scientists have more interest in SnO2 nanoparticles because of their novel properties such as high chemical stability, high transparency, and low electrical sheet resistance, etc. (Jarzebski and Marton, 1976a,b; Jarzebski and Morton, 1976). The modified SnO2 also has great technical and scientific interests because of its diverse applications, e.g., transparent conducting electrodes, gas sensors, as electrodes in lithium-ion batteries, electronic devices, dye-based solar cells, H2 generation, etc. (Jiang et al., 2017, 2018; Park et al., 2017; Xie et al., 2017; Wang et al., 2018; Bhawna et al., 2020). Other than these applications, SnO2 has been seeking attention as an antimicrobial agent and has played an essential role against the growth of various bacterial strains like Staphylococcus aureus, E. coli (Kumari and Philip, 2015; Vidhu and Philip, 2015; Phukan et al., 2017). Green synthesis of SnO2 nanoparticles using Aloe barbadensis miller showed antibacterial and antifungal activities (Ayeshamariam et al., 2013). Apart from antimicrobial activities, other important biological properties like anticancer, antitumor, and antioxidant activities have also been reported using green synthesized SnO2 nanoparticles (Kamaraj et al., 2014; Khan et al., 2018). However, when doped with transition metal ions, SnO2 disinfects microbes with good efficiency, i.e., Co-doped SnO2 and Ag-doped SnO2 nanoparticles have shown potent antibacterial activities (Chandran et al., 2015; Nasir et al., 2017; Qamar et al., 2017; Ali et al., 2018). Only a few reports are available on antimicrobial activities of Ce doped metal oxide NPs. Ce-doped ZnO NPs showed antimicrobial activity against both gram-negative and gram-positive bacteria (Rooshde et al., 2020). Similarly, Ce doped CuO NPs completely eradicated the E. coli and S. aureus bacteria (Jan et al., 2014) and Ce-doped ZrO2 NPs showed high antibacterial property against gram-positive bacteria than gram-negative bacteria (Mekala et al., 2018).
Water is a crucial factor for the existence of life on earth and clean water is the necessity of the hour. Consumption of water by a rapid increasing population is leading to the depletion of major aquifers. On the other hand, organic manufacturing industries have been the target for disposing of their chemical wastes into water bodies. According to research, the world's dye production of about 0.7 million tons (>11%) is annually released as industrial wastewater (Samadi et al., 2019). Among various known dyes, Malachite green dye has its extensive uses worldwide. Besides its use as a dye in silk, jute, leather, wool and paper industries; it is also used as a food additive, coloring agent and as a disinfectant. However, due to its carcinogenic effects on human health and aquatic life, it has now become a controversial compound and has been banned in many countries. Continuous efforts are being made to recycle contaminated water containing bacteria, toxic chemicals, dyes, heavy metals, etc. to make it safe for drinking and other purposes. Some conventional methods are- photocatalysis, ozonation, Fenton's reagent, electrochemical routes, membrane filtration, coagulation, adsorption, ion-exchange, irradiation, anaerobic and aerobic degradation, etc. (Gusain et al., 2019). Though, metal oxides such as TiO2, SnO2, ZnO have been found as better photocatalysts for the degradation of organic dyes in aquoues solution. SnO2 as an n-type semiconductor has also been reported for the degradation of various azo dyes. Besides antimicrobial activities, doped SnO2 finds improved results in photocatalytic activities. Ce doping has been known for bandgap tailoring as well as lattice distortion in SnO2. There are various methods reported in literature for the synthesis of Ce doped SnO2 such as- sol-gel (Shide et al., 2010; Ahmed et al., 2019), hydrothermal (Lian et al., 2017), co-precipitation (Bharathi et al., 2017; Kumar et al., 2018), wet-chemical (Kumar et al., 2019b), flame spray method (Kotchasak et al., 2018), etc. Kumar et al. showed degradation of dyes such as methylene blue and methyl orange using Ce doped SnO2 nanoparticles (Kumar et al., 2019b) whereas Wu et al. degraded methyl orange dye using Ce doped SnO2 (Shide et al., 2010).
To the best of our knowledge, until now, no work has been reported on antimicrobial behavior using Ce doped SnO2. This work involves the facile synthesis of Ce doped SnO2 nanoparticles and reports its antimicrobial behavior against microbes. It also represents photocatalytic degradation of malachite green dye using Ce doped SnO2.
Ce doped SnO2 nanoparticles were synthesized by a wet-chemical method using hydrogen peroxide, as mentioned in our previous report (Kumar et al., 2019b). Solutions of SnCl2.2H2O (Merck, 18 ml of 0.5 M) and CeCl3 (Merck, 6 mL of 40 mM) were mixed and 30 mL hydrogen peroxide was added into the mixture. Then, the mixture was refluxed at 100°C for 14 h. The white suspension was cooled to room temperature, centrifuged, and was dried after washing several times to remove dissolved impurities.
The powder X-ray diffraction (PXRD) pattern was recorded using Rigaku, Miniflex 600 X-ray diffractometer employing monochromatized Cu Kα radiation. The Field Emission Scanning Electron Microscope (FESEM) image of the SnO2 NPs was recorded on a ZEISS Gemini SEM 500.
The Antimicrobial activity of Ce doped SnO2 nanoparticles was carried out using Diffusion Susceptibility Test method (Bauer et al., 1966). The bacterial strain, E. coli was inoculated in 5 ml LB Media (Luria-Bertani; HiMedia Laboratories) and was kept at 37°C and 180 rpm for overnight incubation. The overnight incubated bacterial culture was diluted in 1:100 ratios with fresh LB media. A zone of inhibition experiment was analyzed using an LB Agar plate well-diffusion method. Then, the sterilized well cutter was used for boring the LB Agar plate. The diluted overnight bacterial culture of E. coli was spread on LB agar plate. Thereafter, seven concentrations of Ce: SnO2 NPs, namely, 0.25, 0.50, 1, 2, 3, 4, and 5 mg were poured into LB Agar wells. The well in the center of LB agar plate did not contain Ce doped SnO2 NPs and was used as a control. Thereafter, the LB agar plate with Ce doped SnO2 NP was incubated at 37°C for 16 h.
Photocatalytic degradation of dye was performed in an in-house fabricated solar reactor under UV (λ <400 nm) light by high vapor pressure mercury lamp 125 W (Osram, India) (Kumar et al., 2011). In the photocatalytic activity, 0.1 g of Ce: SnO2 NPs were suspended into an aqueous solution of 100 mL of 15 μM MG dye, which was taken in the photoreactor. The dye solution suspended with the catalyst was stirred for 30 min in the dark to attain the equilibrium and then the light was irradiated over the solution. Each time, five mL volume were pipetted out timely, centrifuged and the absorbance was noted using the UV-visible spectrometer.
The Powder X-ray diffraction pattern of the synthesized Ce doped SnO2 nanoparticles has been shown in the Figure 1. It shows a rutile structure with tetragonal symmetry space group P42/mnm [a = 4.680 (4) Å and c = 3.167 (4) Å] and shows clear reflection at (110), (101), (200), and (211) crystallographic planes corresponding to JCPDS file no. 41-1445 (Kumar et al., 2019b). The absence of any other characteristic peaks rule out possibilities of impurities or other species within the lattice represents high phase purity. The broadness of the diffracted peaks depicts a small size of crystals and the average crystallite size determined using the Scherrer formula was found to be ~6 nm (Scherrer, 1912).
The morphology and elemental mapping of Ce doped SnO2 nanoparticles was investigated through FESEM (Figure 2). Irregularly shaped particles distributed unevenly over the lattice surface has been shown through SEM imaging. Elemental mapping shows the spatial distribution of elements within the lattice and provides the evidence that Ce (yellow), Sn (purple), and O (green) were homogeneously distributed within the crystal lattice.
After elucidation of the phase formation and morphology of the formed nanoparticles, the concentration of Ce ions was found to be 5% as determined through X-ray photoelectron spectroscopy in our previous report (Kumar et al., 2019b). Also, the presence of Ce3+ and Ce4+ ions was confirmed into SnO2 lattice, which caused charge imbalance and hence disorderness in the lattice (Kumar et al., 2019b).
Antibacterial activity of Ce doped SnO2 NPs was observed on LB Agar well-diffusion method (Dil and Sadeghi, 2018). Antibacterial activity of the NPs was compared with the control well (without NPs). The antibacterial activity of Ce doped SnO2 NPs was not observed at concentration 0.25–3 mg (Figure 3). Figure 3 suggested that the zones of inhibition were prominent at two concentrations namely, 4 and 5 mg, respectively and was highest at 5 mg concentration. The antibacterial activities have been assessed through the diameter of the zone of inhibition. At a concentration of 4 mg or above, Ce doped SnO2 NPs showed potent antibacterial activities (Table 1). Previously, other metal ions doped with SnO2 like Co-doped SnO2, Cu-doped SnO2, Fe-doped SnO2, and Ag-doped SnO2 nanoparticles have also been reported for their antibacterial activities (Chandran et al., 2015; Nasir et al., 2017; Ali et al., 2018; Baig et al., 2020; Sathishkumar and Geethalakshmi, 2020). Generally, nanoparticles kill the bacteria through cell membrane disruption, free radical formation causing reactive oxygen species responsible for antibacterial action (Sirelkhatim et al., 2015).
The degradation of malachite green was performed photocatalytically using Ce: SnO2 nanoparticles (Figure 4) under UV light irradiation. It degrades malachite green dye ~50% in 120 min of light irradiation. When compared with other metal oxides, it is found that undoped TiO2 NPs and F doped TiO2 NPs photocatalytically degraded 99.9 and 54.26% MG dye in 240 min and 120 min, respectively (Chen et al., 2007; Panahian and Arsalani, 2017). Sn doped TiO2 has been reported degrading 85% MG in 340 min under light irradiation (Sayilkan et al., 2007), while SnO2 NPs degraded 27% MG in 180 min under UV light irradiation (Kumar et al., 2016).
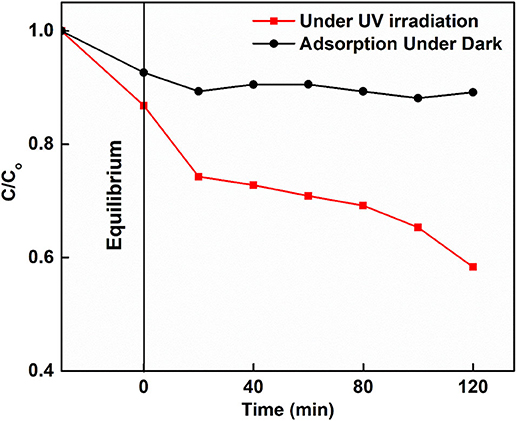
Figure 4. Adsorption of MG in dark and photocatalytic degradation of MG in the presence of Ce doped SnO2 under UV irradiation.
The probable mechanism for the degradation of malachite green dye using Ce doped SnO2 NPs has been revealed in Figure 5. Electrons were excited into the conduction band of Ce doped SnO2 nanoparticles from its valence band on light irradiation [bandgap = 3.80 eV (Kumar et al., 2019b)]. Electrons were also injected into the conduction band of photocatalyst after transfer from HOMO to LUMO of malachite green dye (Helaïli et al., 2017). These electrons from two different sites then move to the surface for surface reactions. The electrons at the surface react with adsorbed/dissolved oxygen to produce radical. The concentration of the O2 molecule is responsible for the efficiency of degradation as these molecules scavenge the electrons in the conduction band, preventing electron-hole recombination. Moreover, holes in the valence band react with water molecules or hydroxide ions to produce hydroxyl radicals (OH.) (Kumar et al., 2016; Ma et al., 2018). The generated oxidizing agents (superoxide radical anions and hydroxyl radicals) contributed to the oxidative degradation of malachite green, which was then converted into simple and less harmful products. The high stability of Ce doped SnO2 NPs mentioned in the previous report and hence, these nanoparticles can be reused without undergoing any change in structure (Kumar et al., 2019b).
Facile and economical synthesis of Ce doped SnO2 NPs showed potent antimicrobial properties so far. Also, nanomaterials were able to degrade toxic organic pollutants like malachite green. These nanomaterials could be used against bacterial infection as well as for multidrug-resistant bacteria along with wastewater treatment purposes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Bhawna and SK thanks UGC and CSIR for Junior Research Fellowship, respectively. The authors also thanks USIC, Department of Chemistry, University of Delhi and SCNS, JNU for various characterizations. The authors also thank Dr. V. K. Singh for valuable discussion and suggestions.
Ahmed, A., Siddique, M. N., Ali, T., and Tripathi, P. (2019). Defect assisted improved room temperature ferromagnetism in Ce doped SnO2 nanoparticles. Appl. Surf. Sci. 483: p. 463–471. doi: 10.1016/j.apsusc.2019.03.209
Ali, T., Ahmed, A., Alam, U., Uddin, I., Tripathi, P., and Muneer, M. (2018). Enhanced photocatalytic and antibacterial activities of Ag-doped TiO2 nanoparticles under visible light. Mater. Chem. Phys. 212, 325–335. doi: 10.1016/j.matchemphys.2018.03.052
Ananpattarachai, J., Kajitvichyanukul, P., and Seraphin, S. (2009). Visible light absorption ability and photocatalytic oxidation activity of various interstitial N-doped TiO2 prepared from different nitrogen dopants. J. Hazard. Mater. 168, 253–261. doi: 10.1016/j.jhazmat.2009.02.036
Ayeshamariam, A., Meera, T., Jayachandran, M., Kumar, P., and Bououdina, M. (2013). Green synthesis of nanostructured materials for antibacterial and antifungal activities. Int. J. Bioassays 2, 304–311.
Azam, A., Ahmed, A. S., Oves, M., Khan, M. S., Habib, S. S., and Memic, A. (2012). Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: a comparative study. Int. J. Nanomed. 7:6003. doi: 10.2147/IJN.S35347
Baig, A. B. A., Rathinam, V., and Ramya, V. (2020). Synthesis and investigation of Fe doped SnO2 nanoparticles for improved photocatalytic activity under visible light and antibacterial performances. Mater. Technol. 2020, 1–13. doi: 10.1080/10667857.2020.1786781
Bauer, A., Kirby, W. M., Sherris, J. C., and Turck, M. (1966). Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 45, 493–496. doi: 10.1093/ajcp/45.4_ts.493
Bharathi, P. M. R. M., Amutha, T., Rameshbabu, M., and Prabha, K. (2017). Synthesis and Investigation of Ce doped Tinoxide (SnO2) nanoparticles. Int. Res. J. Eng. Technol. 4:334–337.
Bhawna Gupta, A., Kumar, P., Tyagi, A., Kumar, R., Kumar, A., Singh, P., et al. (2020). Facile synthesis of N-Doped SnO2 nanoparticles: a cocatalyst-free promising photocatalyst for hydrogen generation. ChemistrySelect 5, 7775–7782. doi: 10.1002/slct.202001301
Chandran, D., Nair, L. S., Balachandran, S., Babu, K. R., and Deepa, M. (2015). Structural, optical, photocatalytic, and antimicrobial activities of cobalt-doped tin oxide nanoparticles. J. Sol Gel Sci. Technol. 76, 582–591. doi: 10.1007/s10971-015-3808-z
Chen, C., Lu, C. S., Chung, Y. C., and Jan, J. L. (2007). UV light induced photodegradation of malachite green on TiO2 nanoparticles. J. Hazard. Mater. 141, 520–528. doi: 10.1016/j.jhazmat.2006.07.011
Dil, N. N., and Sadeghi, M. (2018). Free radical synthesis of nanosilver/gelatin-poly (acrylic acid) nanocomposite hydrogels employed for antibacterial activity and removal of Cu (II) metal ions. J. Hazard. Mater. 351, 38–53. doi: 10.1016/j.jhazmat.2018.02.017
Gupta, A., Kumar, S., Kumar, R., Choudhary, A. K., Kumari, K., Singh, P., et al. (2020a). COVID-19: emergence of infectious diseases, nanotechnology aspects, challenges, and future perspectives. ChemistrySelect 5:7521. doi: 10.1002/slct.202001709
Gupta, A., Kumar, V., Ahmed, S., and Gautam, S. (2020b). “Impact of nanotechnology in the development of smart cities,” in Smart Cities-Opportunities and Challenges, Smart Cities-Opportunities and Challenges. Lecture Notes in Civil Engineering, eds S. Ahmed, S. Abbas, and H. Zia (Singapore: Springer), 845–857. doi: 10.1007/978-981-15-2545-2_68
Gusain, R., Gupta, K., Joshi, P., and Khatri, O. P. (2019). Adsorptive removal and photocatalytic degradation of organic pollutants using metal oxides and their composites: a comprehensive review. Adv. Colloid Interface Sci. 272:102009. doi: 10.1016/j.cis.2019.102009
Helaïli, N., Boudjamaa, A., Kebir, M., and Bachari, K. (2017). Efficient photo–catalytic degradation of malachite green using nickel tungstate material as photo–catalyst. Environ. Sci. Pollut. Res. 24, 6481–6491. doi: 10.1007/s11356-016-8296-3
Jan, T., Iqbql, J., Mansoor, Q., Ismail, M., Naqvi, M. S. H., Gul, A., et al. (2014). Synthesis, physical properties and antibacterial activity of Ce doped CuO: a novel nanomaterial. J. Phys. D Appl. Phys. 47:355301. doi: 10.1088/0022-3727/47/35/355301
Jarzebski, Z., and Marton, J. (1976a). Physical properties of SnO2 materials: I. preparation and defect structure. J. Electrochem. Soc. 123:199C. doi: 10.1149/1.2133010
Jarzebski, Z., and Marton, J. (1976b). Physical properties of SnO2 materials: II. Electrical properties. J. Electrochem. Soc. 123:299C. doi: 10.1149/1.2133090
Jarzebski, Z., and Morton, J. (1976). Physical properties of SnO2 materials: III. Optical properties. J. Electrochem. Soc., 123, 333C. doi: 10.1149/1.2132647
Jiang, B., He, Y., Li, B., Zhao, S., Wang, S., He, Y. B., et al. (2017). Polymer-templated formation of polydopamine-coated SnO2 nanocrystals: anodes for cyclable lithium-ion batteries. Angew. Chem. Int. Ed. 56, 1869–1872. doi: 10.1002/anie.201611160
Jiang, Q., Zhang, X., and You, J. (2018). SnO2: a wonderful electron transport layer for perovskite solar cells. Small 14:1801154. doi: 10.1002/smll.201801154
Kamaraj, P., Vennila, R., Arthanareeswari, M., and Devikala, S. (2014). Biological activities of tin oxide nanoparticles synthesized using plant extract. World J. Pharm Pharm Sci. 3, 382–388.
Khan, S. A., Kanwal, S., Rizwan, K., and Shahid, S. (2018). Enhanced antimicrobial, antioxidant, in vivo antitumor and in vitro anticancer effects against breast cancer cell line by green synthesized un-doped SnO2 and Co-doped SnO2 nanoparticles from clerodendrum inerme. Microb. Pathog. 125: p. 366–384. doi: 10.1016/j.micpath.2018.09.041
Kotchasak, N., Wisitsoraat, A., Tuantranont, A., Phanichphant, S., Yordsri, V., and Liewhiran, C. (2018). Highly sensitive and selective detection of ethanol vapor using flame-spray-made CeOx-doped SnO2 nanoparticulate thick films. Sens. Actuators B Chem. 255, 8–21. doi: 10.1016/j.snb.2017.08.027
Kumar, A., Rout, L., Achary, L. S. K., Mohanty, A., Marpally, J., Chand, P. K., et al. (2016). “Design of binary SnO2-CuO nanocomposite for efficient photocatalytic degradation of malachite green dye,” in AIP Conference Proceeding AIP Publishing LLC (Rajasthan). doi: 10.1063/1.4945147
Kumar, M., Bhatt, V., Abhyankar, A. C., Kim, J., Kumar, A., Patil, S. H., et al. (2018). New insights towards strikingly improved room temperature ethanol sensing properties of p-type Ce-doped SnO2 sensors. Sci. Rep. 8, 1–12. doi: 10.1038/s41598-018-26504-3
Kumar, V., Bhawna, Y., adav, S. K., Gupta, A., Dwivedi, B., Kumar, A., Singh, P., et al. (2019a). Facile synthesis of Ce–doped SnO2 nanoparticles: a promising Photocatalyst for hydrogen evolution and dyes degradation. ChemistrySelect 4, 3722–3729. doi: 10.1002/slct.201900032
Kumar, V., Choudhary, A. K., Kumar, P., and Sharma, S. (2019b). Nanotechnology: nanomedicine, nanotoxicity and future challenges. Nanosci. Nanotechnol. Asia 9, 64–78. doi: 10.2174/2210681208666180125143953
Kumar, V., Govind, A., and Nagarajan, R. Optical photocatalytic properties of heavily F—doped SnO2 nanocrystals by a novel single-source precursor approach. Inorg. Chem. (2011). 50, 5637–5645. doi: 10.1021/ic2003436
Kumari, M. M., and Philip, D. (2015). Synthesis of biogenic SnO2 nanoparticles and evaluation of thermal, rheological, antibacterial and antioxidant activities. Powder Technol. 270, 312–319. doi: 10.1016/j.powtec.2014.10.034
Lian, X., Li, Y., Tong, X., Zou, Y., Liu, X., An, D., et al. (2017). Synthesis of Ce-doped SnO2 nanoparticles and their acetone gas sensing properties. Appl. Surf. Sci. 407:447–455. doi: 10.1016/j.apsusc.2017.02.228
Ma, Y., Ni, M., and Li, S. (2018). Optimization of malachite green removal from water by TiO2 nanoparticles under UV irradiation. Nanomaterials 8:428. doi: 10.3390/nano8060428
Mekala, R., Rajendran, V., and Deepa, B. (2018). Effect of cerium doped Baddeleyite on their antibacterial activity by co-precipitation method. Mater. Sci. Eng., 360:012014. doi: 10.1088/1757-899X/360/1/012014
Nasir, Z., Shakir, M., Wahab, R., Shoeb, M., Alam, P., Khan, R. H., et al. (2017). Co-precipitation synthesis and characterization of Co doped SnO2 NPs, HSA interaction via various spectroscopic techniques and their antimicrobial and photocatalytic activities. Int. J. Biol. Macromol. 94, 554–565. doi: 10.1016/j.ijbiomac.2016.10.057
Panahian, Y., and Arsalani, N. (2017). Synthesis of hedgehoglike F-TiO2 (B)/CNT nanocomposites for sonophotocatalytic and photocatalytic degradation of malachite green (MG) under visible light: kinetic study. J. Phys. Chem. A 121, 5614–5624. doi: 10.1021/acs.jpca.7b02580
Park, G. D., Lee, J. K., and Kang, Y. C. (2017). Synthesis of uniquely structured SnO2 hollow nanoplates and their electrochemical properties for Li-ion storage. Adv. Funct. Mater. 27:1603399. doi: 10.1002/adfm.201603399
Phukan, A., Bhattacharjee, R. P., and Dutta, D. K. (2017). Stabilization of SnO2 nanoparticles into the nanopores of modified montmorillonite and their antibacterial activity. Adv. Powder Technol. 28, 139–145. doi: 10.1016/j.apt.2016.09.005
Qamar, M., Shahid, S., Khan, S. A., Zaman, S., and Sarwar, M. N. (2017). Synthesis characterization, optical and antibacterial studies of Co-doped SnO2 nanoparticles. Dig. J. Nanomater. Biostruct. 12, 1127–1135. doi: 10.13140/RG.2.2.29282.22720
Raghunath, A., and Perumal, E. (2017). Metal oxide nanoparticles as antimicrobial agents: a promise for the future. Int. J. Antimicrob. Agents 49, 137–152. doi: 10.1016/j.ijantimicag.2016.11.011
Rooshde, M. S., Abdullah, W. R. W., Amran, A. Z., Ibrahim, N. F., Ariffin, F., Sabri, M., et al. (2020). Antimicrobial Activity of Photoactive Cerium Doped Zinc Oxide. in Solid State Phenomena. Trans. Tech. Publ. 307, 217–222. doi: 10.4028/www.scientific.net/SSP.307.217
Samadi, M., Zirak, M., Naseri, A., Kheirabadi, M., Ebrahimi, M., and Moshfegh, A. Z. (2019). Design and tailoring of one-dimensional ZnO nanomaterials for photocatalytic degradation of organic dyes: a review. Res. Chem. Intermed. 45, 2197–2254. doi: 10.1007/s11164-018-03729-5
Sathishkumar, M., and Geethalakshmi, S. (2020). Enhanced photocatalytic and antibacterial activity of Cu: SnO2 nanoparticles synthesized by microwave assisted method. Mater. Today 20, 54–63. doi: 10.1016/j.matpr.2019.08.246
Sayilkan, F., Asiltürk, M., Tatar, P., Kiraz, N., Arpa,ç, E., and Sayilkan, H. (2007). Photocatalytic performance of Sn-doped TiO2 nanostructured mono and double layer thin films for malachite green dye degradation under UV and vis-lights. J. Hazard. Mater. 144, 140–146. doi: 10.1016/j.jhazmat.2006.10.011
Scherrer, P. (1912). “Bestimmung der inneren struktur und der Größe von kolloidteilchen mittels röntgenstrahlen,” in kolloidchemie Ein Lehrbuch. (Berlin; Heidelberg: Springer), 387–409. doi: 10.1007/978-3-662-33915-2_7
Shide, W., Chao, L., Wei, W., Huanxin, W., and Youqi, Z. (2010). Synthesis and photocatalytic property of Ce-doped SnO2. J. Rare Earths 28:168–170. doi: 10.1016/S1002-0721(10)60312-2
Sirelkhatim, A., Mahmud, S., Seeni, A., Kaus, N. H. M., Ann, L. C., Bakhori, S. K. M., et al. (2015). Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nanomicro Lett., 7, 219–242. doi: 10.1007/s40820-015-0040-x
Swaminathan, B., and Smidt, P. G. (2007). The epidemiology of human listeriosis. Microb Infect. 9, 1236–1243. doi: 10.1016/j.micinf.2007.05.011
Vargas-Reus, M. A., Memarzadeh, K., Huang, J., Ren, G. G., and Allaker, R. P. (2012). Antimicrobial activity of nanoparticulate metal oxides against peri-implantitis pathogens. Int. J. Antimicrob. Agents 40, 135–139. doi: 10.1016/j.ijantimicag.2012.04.012
Vidhu, V., and Philip, D. (2015). Phytosynthesis and applications of bioactive SnO2 nanoparticles. Mater. Charact. 101, 97–105. doi: 10.1016/j.matchar.2014.12.027
Wang, Z., Han, T., Fei, T., Liu, S., and Zhang, T. (2018). Investigation of microstructure effect on NO2 sensors based on SnO2 nanoparticles/reduced graphene oxide hybrids. ACS Appl. Mater. Interf. 10, 41773–41783. doi: 10.1021/acsami.8b15284
Xie, J., Huang, K., Yu, X., Yang, Z., Xiao, K., Qiang, Y., et al. (2017). Enhanced electronic properties of SnO2 via electron transfer from graphene quantum dots for efficient perovskite solar cells. Acs Nano 11, 9176–9182. doi: 10.1021/acsnano.7b04070
Keywords: cerium, E. coli, malachite green, photocatalyst, doping
Citation: Bhawna, Choudhary AK, Gupta A, Kumar S, Kumar P, Singh RP, Singh P and Kumar V (2020) Synthesis, Antimicrobial Activity, and Photocatalytic Performance of Ce Doped SnO2 Nanoparticles. Front. Nanotechnol. 2:595352. doi: 10.3389/fnano.2020.595352
Received: 16 August 2020; Accepted: 13 October 2020;
Published: 19 November 2020.
Edited by:
Ajeet Kaushik, Florida Polytechnic University, United StatesReviewed by:
Rajan Patel, Jamia Millia Islamia, IndiaCopyright © 2020 Bhawna, Choudhary, Gupta, Kumar, Kumar, Singh, Singh and Kumar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Akanksha Gupta, YWthbmtzaGFjaGVtMDVAZ21haWwuY29t; Vinod Kumar, dmlub2Q3Njc0QGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.