- 1School of Advanced Science, Vellore Institute of Technology, Vellore, India
- 2Centre for Bioseparation Technology (CBST), Vellore Institute of Technology, Vellore, India
- 3Centre for Nanobiotechnology, Vellore Institute of Technology, Vellore, India
COVID-19 outbreak in the Hubei province of China as of December 2019 has caused massive global fatalities. Recommended preventive strategies that include the use of sanitizers and masks caused a global shortage of protective gear for the frontline health workers who were at a higher risk of infection. Implementing effective containment strategies and promoting large scale diagnosis of the general public thus forms the first line of defense in controlling the spread of the contagion as of now. The understanding of the biological identity of the SARS-CoV-2 virus has helped the researchers figure out the mechanism of infection and also helped them design efficient diagnostic kits. This review primarily provides comprehensive details of the gold standard viral diagnostic methods. With nanoparticles playing a vital role in healthcare platforms, its mode of action against other viruses and its application in COVID-19 has been elaborated. The relevance of incorporating nanoparticles in surface coatings, sanitizers, masks, and air filters have been highlighted. NP-based biosensors that can be utilized as point-of-care tests and multiplexed assay have also been discussed. Considering the ongoing threat, new research studies focus on strategically combining different diagnostic systems to enhance the sensitivity and accuracy of the detection kits. In this regard, the key details of the patents that encompass new methodologies for virus detection have been summarized.
Introduction
In 1960, coronavirus was first identified as a cause of common cold and was considered as a mild and non-fatal virus until 2002 (Yang et al., 2020). However, a mutated form of the coronavirus caused severe acute respiratory syndrome (SARS) in 2002 in Guangdong, China, and also spread to various parts of the world (Xu et al., 2004). In 2012, the virus once again reappeared in Middle East countries, where it was named Middle East Respiratory Syndrome coronavirus (MERS-CoV) (Rao et al., 2020).
In December 2019, there was an outbreak of the novel SARS virus in Wuhan, China. Beginning of this year, the world was appalled by this outbreak, and the virus was officially named as Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 or COVID-19) by the World Health Organization (Coronaviridae Study Group of the International Committee on Taxonomy of Viruses, 2020). This virus has created havoc throughout the world and currently, the world has witnessed over 211,000 deaths globally (as of June 27, 2020). Identifying asymptomatic cases remains one of the major challenges. A case study from diamond Princess cruise ship, Yokohama, Japan, 2020 pointed out that asymptomatic cases are as infectious as symptomatic cases for spreading COVID-19 infection (Mizumoto et al., 2020). Except patients having comorbidities such as hypertension, diabetes, and heart disease otherwise symptoms against COVID-19 very non-specific in nature (Udugama et al., 2020) and cannot be used for an accurate diagnosis (Pulia et al., 2020). Timely diagnosis and effective treatment are therefore very essential.
Over the last few months, healthcare personnel and researchers from different fields have been actively engaged in finding solutions to vanquish the virus A simple PubMed search on MERS-CoV which shares ~50% genome similarity with SARS-CoV-2 show a gradual increase in publication over the last 5 years. However, an enormous spike in the publications related to SARS-CoV and SARS-CoV-2 was seen in the year 2020 (Supplementary Figure 1). Considering the inherent challenges associated with the development of vaccines, it may take a while before we can eliminate the deadly virus from our day-to-day lifestyle. Thus, it is necessary to focus our research on efficient preventive measures and advanced diagnostic techniques. COVID-19 or viral infections were diagnosed using nucleotide detection (Polymerase chain reaction), gene sequencing, and antigen and antibody detection (ELISA, Immunofluorescence). These methods are time-consuming and need skilled labor. In recent years, nanotechnology-based solutions have been immensely helpful in battling several viral infections. A wide range of nanoparticles (NPs) has been deployed for the treatment and detection of viruses like HIV, HSV, and Influenza (Chen and Liang, 2020). Efficient preventive strategies and robust detection kits can be developed based on the physio-chemical and anti-viral properties of NPs.
In this article, we aim to provide an overview of the mechanistic details of the gold standard viral diagnostic methods. The other key takeaways are the intervening strategies from the viewpoint of nanotechnology that can improve the current preventive and detection platforms. A strong groundwork about NP-based biosensors and patents that involve the detection of COVID-19 have been highlighted. Summarizing the mechanistic details of different prevention and detection methods can create better awareness about the ongoing pandemic.
Current Diagnostic Modes: Recent Advances, Advantages, and Limitations
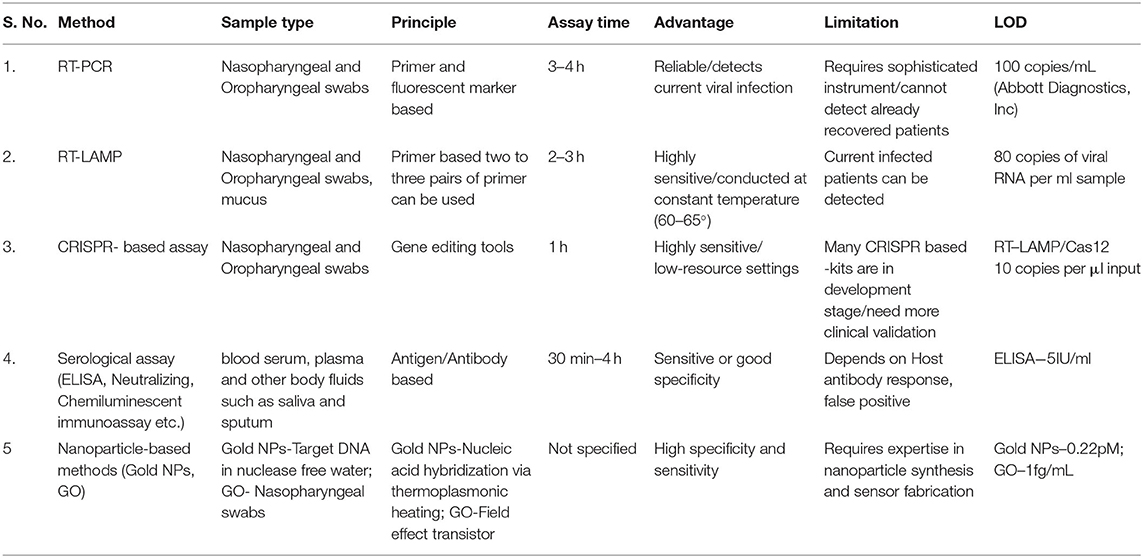
Currently, the detection of COVID-19 is based on the contact and travel history of patients from the affected area as well as clinical symptoms (Kumar et al., 2020). Unavailability of a patient's contact history at all-time makes laboratory testing more difficult in the current situation. COVID-19 mainly infects the respiratory tract. Nasopharyngeal, oropharyngeal, and/or sputum or bronchoalveolar specimens are taken for diagnosing the viral load (Udugama et al., 2020). A rapid and quick diagnostic technique is still unavailable for the detection of COVID-19 although various methods have been used in the current scenario to detect with its advantages and disadvantages (Carter et al., 2020). Table 1 compares and highlights the key features of all the diagnostic techniques that are used for COVID-19 detection.
RT-PCR (Reverse Transcriptase Polymerase Chain Reaction)
RT-PCR is the most reliable and gold standard method for the identification of COVID-19 infection. As of now, various COVID-19 RT-PCR based kits available in the market covert viral genomic RNA to complementary DNA (cDNA) by using RNA dependent DNA polymerase or reverse transcriptase enzyme and further use it for the amplification. The RNA probes that are designed from the COVID-19 genetic sequence are retrieved from the Global Initiative on Sharing All Influenza Data (GISAID) database. Probes against different target sequences of COVID-19, like spike protein, RNA dependent RNA polymerase, nucleocapsid protein are designed. In real-time RT- PCR, viral sequence-specific DNA probes are labeled with a fluorescent tag and a quencher molecule is used and the progress of the reaction can be easily monitored by observing the fluorescent tag intensity. The automated RT-PCR runs through repeated cycles of annealing and extension. While one-step RT-PCR is carried out in a single tube, where reaction mix contains forward and reverses primers together. In a two-step procedure, the forward and reverses PCR reactions are carried out in separate tubes. The one-step method is simple and quick and therefore is the preferred procedure for COVID-19 detection (Carter et al., 2020). Although it is a gold standard for the detection of COVID-19 it has the disadvantages, of requiring high equipped laboratory and skilled labor.
Reverse Transcription Loop-Mediated Isothermal Amplification (RT-LAMP)
RT-LAMP uses isothermal amplification technique at constant temperature and avoids the use of thermal cyclers, unlike conventional RT-PCR. Sensitivity is enhanced by using two to three pairs of primers against the viral gene sequence. The amount of DNA amplified in a patient specimen is measured by the turbidity formed due to the production of magnesium pyrophosphate. The approach of this method simply requires an isothermal water bath. The main drawbacks of RT-LAMP include optimizing the primer and sample run which is limited to one (Carter et al., 2020).
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) Based Assay
CRISPR represents a family of nucleotide sequences originally inferred from the prokaryotic system such as bacteria and archaea. CRISPR associated enzymes such as Cas9, Cas12, and Cas13 can recognize and cut these nucleotide sequences. Recently two companies Mammoth Biosciences and Sherlock Biosciences developed CRISPR-based technology for the detection of COVID-19 (Broughton et al., 2020). Mammoth bioscience used the Cas12 enzyme for the detection of COVID-19. In this method, guide RNA (gRNA) can be designed that recognizes one of the genes found in the COVID-19. Cas12 and gRNA will search for RNA present in the sample and Cas12 will cut it without stopping. For the detection, there is an additional RNA molecule tag with fluorescein amidite and FAM which generates color. The CRISPR based assay does not require complex instrumentation for diagnosis.
Serological Assay
Serological assay means the detection of antigen and antibody present in the serum of patients. Serological assays utilize body fluids such as blood serum, plasma, saliva, and sputum. During any infection or encounter with antigen, our body immune system generates specific antibodies. Due to specificity or limited cross-reactivity, antibody detection is one of the most reliable methods to detect any infectious agents. Serological test plays a significant role in understanding antibody response, amount of antibody formation, and diversity generated by the body. The presence of immunoglobulins such as IgM indicates an early stage of infection, whereas the presence of IgG indicates the stage of current or prior infection. Some of the most commonly utilized serological test during COVID-19 include ELISA (Enzyme-Linked Immunosorbent Assay), Neutralizing test, chemiluminescent immunoassay (CLIA), and Rapid diagnostic test (RDT).
ELISA (Enzyme-Linked Immunosorbent Assay)
An ELISA is a microwell plate-based assay designed for detecting antigen or antibody. The indirect ELISA used for COVID-19 determines the total antibody concentration in a sample. For microwell plate coating, different proteins of SARS-CoV-2 mainly the structural proteins N and S (highly immunogenic and prolifically expressed during infection) are used. Viral antibodies present in the samples bind to the antigen and forms an antibody-protein complex, later detected by secondary antibody and colorimetric method. The ELISA-based serological tests entirely depend on host antibody response to the virus and it is suggested to do tests after 1-week post symptom (Carter et al., 2020).
Neutralizing Test
Plaque reduction neutralizing tests use a subset of antibodies produced against a virus that independently block viral entry. Performed under the BSL3 facility, it requires the use of live viruses. In this test, the patient's serum is mixed with a suspension of the infectious virus here COVID-19 particles. Affected patient serum containing antibody against COVID-19 will bind to virus particles and prevent them from further invading the cells in the culture, finally neutralizing the virus (Carter et al., 2020). Limitations of this test include its restriction to the BSL3 facility only and it takes 3–5 days for the viral neutralization so it is very time-consuming.
Chemiluminescent Immunoassay
Chemiluminescent immunoassay is a variation of the standard enzyme immunoassay (EIA) work. This test is a quantitative, lab-based technique where antigens can be identified in an affected sample by using an enzyme that converts a substrate to a reaction product, which emits a photon of light. Mixing the COVID-19 patient sample and a specific enzyme-linked antibody gives a light-based luminescent signal. Antibodies present in the sample against COVID-19 react with the viral protein and forms a complex inducing a chemical reaction that produces light which is directly proportional to the amount of antibodies in the sample (Vashist, 2020). The disadvantages of chemiluminescent immunoassay are high cost, less sensitivity, and requirement of a closed analytical system.
Rapid Diagnostic Test (RDT)
RDT is typically a qualitative lateral flow assay that detects antigens/antibody (IgM or IgG) in patients sample (blood sample, saliva, and nasal swab fluids). Herein, COVID-19 viral antigen is present in the sample binds to specific antibody encoded in the paper strip providing results within 15 to 30 min. False-negative results may occur due to cross-reactivity from other endemic infections. Compared to molecular diagnostic tests they are fast and easy to perform and can serve for point of care testing (POC).
Role of Nanotechnology in COVID-19 Prevention and Detection
NPs due to their high surface-area-to volume-ratio easily binds either with the viral membrane proteins or to the viral replication system (DNA/RNA) eventually hampering it's potential to infect the cells. Metal NPs such as gold, silver, and copper have been strategically used for inhibition of viral growth (Ravishankar Rai, 2011). Copper being a cheaper alternative to silver and gold has often been examined for its wide anti-microbial activities. Inactivation of the virus on copper surfaces after 4 h suggests the possible application of copper in destructing the virus (van Doremalen et al., 2020). CuI NPs destructs the influenza A virus (Fujimori et al., 2012) and also reduce the infectivity of Feline calicivirus (FCV) (Shionoiri et al., 2012). Copper composite NPs were also reported to inactivate 50% of virus-like particles (VLPs) in 10 min (Broglie et al., 2015). Supplementary Table 1 summarizes a few of the applications of NPs against a range of viruses.
Scope of Nanotechnology in the Prevention of COVID-19
Since the COVID-19 pandemic, extensive research has been focused mainly on the fabrication of efficient vaccines and drugs. However, the first line of safety from the viral infection mainly depends on an individual's awareness and hygiene practices. In this section, we highlight a few of the aspects wherein, the use of nanomaterials to manage COVID-19 can be beneficial (Kaushik, 2019; Mujawar et al., 2020). Surface contact and/or droplet-based transmission of the virus can be carefully eliminated by incorporating anti-viral NPs in surface coatings, sanitizers, filters, and respiratory cloth masks.
Silver and copper-based NPs have long been used in surface coatings, paints for their anti-microbial properties. ROS generation and viral cell destruction by these metal NPs can be put into use by incorporating them in a different matrix. PEI-coated model surfaces with AgNPs and CuNPs successfully reduced the viral titer by changing the viral membrane permeability and/or by electrostatically binding the virus to the cationic surface (Sinclair et al., 2019). In another study perhydrolase-immobilized carbon nanotubes (CNTs) paints generated peracetic acid (PAA) effectively reducing influenza A titer values (Grover et al., 2013). NPs can also be incorporated in disinfectants. Currently, most of the disinfectants contain either hypochlorite or 70–80% alcohol. Recently, a disinfectant formulation containing nano-TiO2 and AgNPs were used for sanitizing public places in Milan, Italy (Nanotech Surface Coronavirus, 2020). In another interesting study, mesoporous silica protected AgNP-based composite was synthesized as an efficient disinfectant with controlled Ag+ release and easy magnetic removal post-treatment (Wang et al., 2019). At this time, dentists are at a much higher risk due to the inherent nature of the practice that is involved in the treatment of the patients. The use of positively charged imidazolium-based ionic liquid-protected silver nanoparticles serves as a promising anti-bacterial disinfectant. Considering the involvement of AgNPs, the concept can be extended for viral inhibition (Abbaszadegan et al., 2015).
Introduction of NP-based high-efficiency particulate air-filters (HEPA) in health-care centers, airports, and other closed places can effectively eliminate the viral spread. SiO2-Ag NPs coated antiviral air filters with a coating density higher than 2.0 × 108 cm2 were reported to reduce the viral infection by 99.9% (Joe et al., 2014). AgNPs-coated filters with nano-TiO2 were established as an advanced air purifying system in hospitals (Le et al., 2015). A hybrid composite comprising AgNPs and CNTs synthesized using aerosol nebulization and thermal evaporation/condensation processes were used as antimicrobial air filter (Jung et al., 2011). Anti-viral NPs embedded onto fabrics can resolve the issue of viral contamination related to masks and PPE kits (Adams and Walls, 2020). Anti-viral textile was manufactured by a Swiss company named HeiQ by combining vesicle technology and the anti-viral property of AgNPs. The vesicle system entraps the virus and the AgNPs inhibit the viral replication (Materials, 2020). The impregnation of CuO NPs in respiratory masks can efficiently reduce the risk of viral contamination (Iyigundogdu et al., 2017). The problems of improper handling of PPE can be solved by treating them with graphene oxide (GO) grafted-metal NPs (Perreault et al., 2015). Interestingly, fluorescent virus-like NPs with a size range nearly that of SARS-CoV-2 can track the filtration efficiency of different fabrics that are currently in use (Lustig et al., 2020). Preventive measures in combination with nanotechnology can serve as a productive platform for research studies related to virology in the future. Supplementary Scheme 1 summarizes the role of nanotechnology in the prevention of COVID-19.
Nanotechnology-Based Viral Diagnostics in the Era of COVID-19
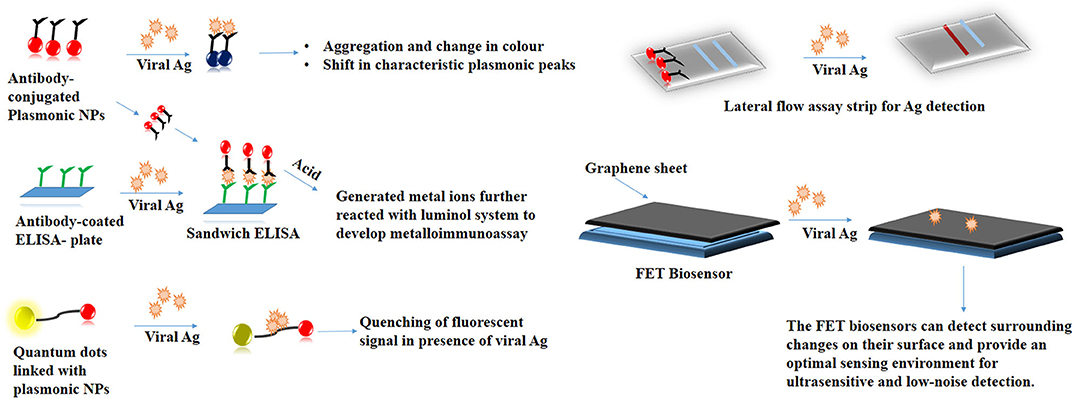
NPs with their tunable physiological properties have served as a promising tool in several biosensing applications (Mejía-Salazar and Oliveira, 2018). It is necessary to address the problems associated with RT-PCR wherein, the genetic drift of the viral strains that tend to compromise the assay performance. Earlier, mutations in the highly conserved region of the Influenza A virus had reduced the sensitivity of numerous RT-PCR kits (Stellrecht, 2018). Bottlenecks in such classical detection techniques can be solved with the help of nanotechnology. Colorimetric assays based on the principle of aggregation of antibody-conjugated plasmonic NPs in the presence of viral proteins can serve as an inexpensive tool for viral detection (Yadavalli and Shukla, 2017). Virus-mediated fluorescence quenching of peptide-linked nanocomposite consisting of QDs and AuNPs achieved a detection limit of 17.02 fg/mL (Nasrin et al., 2020). AgNPs-based chemiluminescent Did you mean: radioimmunoassay metalloimmunoassay has high sensitivity toward the detection of H1N1 Influenza virus without involving the complexity of antigen amplification (Li et al., 2013). The colorimetric changes in these NPs following their interaction with the target analyte can be easily tracked by cameras in smartphones. Such an approach makes sensitive diagnostic platforms more portable and user-friendly (Dong et al., 2017). Photothermal effect (PTT) and localized surface plasmon resonance (LSPR) was utilized to synthesize a sensitive dual-functional gold nanoisland (AuNIs)-based sensor with a detection limit of 0.22 pM. The sensor showed a precise detection of a specific gene sequence (Qiu et al., 2020). Fabric-based sensor synthesized by incorporating antibody-coated GO has been used to detect the presence of the Influenza virus with a detection limit of 10 ng/mL (Kinnamon et al., 2018). A graphene-based field-effect transistor (FET) has been for on-site detection of the SARS-CoV-2 virus (Seo et al., 2020). NP-based point-of-care detection kits mainly the lateral flow assays (LFA) with enhanced sensitivity can be explored for detection for several viral analytes (Soh et al., 2020). Scheme 1 summarizes the NP-based detection strategies that are employed in viral diagnostics.
Current Patents Involving Detection of COVID-19
Tremendous effort has been put forward by several research groups to find a possible solution for the ongoing pandemic, more than 500 patents have been filed during this time that discusses the potential strategies of preventing and treating the infection (Liu et al., 2020). In this section, we discuss a few of the patents related to COVID-19 diagnosis. Detection methods based on the principle of antigen binding to antibody-coated NPs were used for biosensor fabrication. For instance, a colloidal gold-based immunochromatography device (LOD-1000 pg/mL) (CN111239400A, 2020) and a dual antigen kit (CN111239394A, 2020) was patented. Europium microspheres coated with specific novel coronavirus NP protein mAb (LOD-10 pg/mL) (CN111060691A, 2020) was patented. Fluorescent nanostrips-based bedside detection technique was fabricated using immunoglobulin conjugated Up-Conversion Nanoparticles (UCNPs) (CN111190012A, 2020). Other nucleic acid-based detection techniques include the use of RT-PCR in combination with RPA reaction on the reversed cDNA (CN111074008A, 2020). RPA reaction system in combination with multi-component deoxyribozyme (MNAzyme) was used for the generation of non-specific linear DNA that can emit light for a relatively long time. The method tends to overcome weak signals generated by target nucleic acids (CN111187863A, 2020). Digital PCR-based technique that involves designing a primer pair and probe for ORF1ab region of COVID-19 has been patented which claims to have high sensitivity and eliminates signal interference (CN111270017A, 2020). Inventions such as these that can benefit the public health at this unforeseen emergency situation.
Discussion
The current COVID-19 pandemic has created a global emergency crippling both the developed and the developing nations. With the unavailability of the vaccine and the virus spreading rapidly it is necessary to develop robust, sensitive, and advanced, detection tools. Nanomaterials can be readily applied to modify the available detection platforms to improve their sensitivity. The review aimed to brief about the methodologies of viral diagnostics that are in use and also other NP-based sensing systems. Disinfectant formulations and textiles with anti-viral NPs can be utilized to reduce the long-term viability of the SARS-CoV-2. Advanced smartphone and/or LFA strip-based detection techniques that can be performed at the bedside can prove to be indispensable for the healthcare community who are already overworked. The present situation establishes scope for new research developments, helping us handle similar pandemics in the future in a much-controlled fashion.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
AM conceived the original idea and designed the review paper. SK gave conceptual ideas. DC wrote the manuscript in consultation with AM and SK. NC gave critical inputs. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnano.2020.588795/full#supplementary-material
References
Abbaszadegan, A., Nabavizadeh, M., Gholami, A., Aleyasin, Z., Dorostkar, S., Saliminasab, M., et al. (2015). Positively charged imidazolium-based ionic liquid-protected silver nanoparticles: a promising disinfectant in root canal treatment. Int. Endod. J. 48, 790–800. doi: 10.1111/iej.12377
Adams, J. G., and Walls, R. M. (2020). Supporting the health care workforce during the COVID-19 global epidemic. JAMA 323, 1439–1440. doi: 10.1001/jama.2020.3972
Broglie, J. J., Alston, B., Yang, C., Ma, L., Adcock, A. F., Chen, W., et al. (2015). Antiviral activity of gold/copper sulfide core/shell nanoparticles against human norovirus virus-like particles. PLoS ONE 10:e0141050. doi: 10.1371/journal.pone.0141050
Broughton, J. P., Deng, X., Yu, G., Fasching, C. L., Servellita, V., Singh, J., et al. (2020). CRISPR-Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 38, 870–874. doi: 10.1038/s41587-020-0513-4
Carter, L. J., Garner, L. V., Smoot, J. W., Li, Y., Zhou, Q., Saveson, C. J., et al. (2020). Assay techniques and test development for COVID-19 diagnosis. ACS Cent. Sci. 6, 591–605. doi: 10.1021/acscentsci.0c00501
Chen, L., and Liang, J. (2020). An overview of functional nanoparticles as novel emerging antiviral therapeutic agents. Mater. Sci. Eng. C 2020:110924. doi: 10.1016/j.msec.2020.110924
CN111060691A (2020). Fluorescence Immunochromatography Device for Detecting COVID-19 and Using Method Thereof .
CN111074008A (2020). COVID-19 Novel Coronavirus Nucleic Acid Detection Method Capable of Improving Accuracy.
CN111187863A (2020). Kit and Method for Detecting COVID-19 by Double-Enzyme Method Isothermal Amplification.
CN111190012A (2020). Rare Earth Up-Conversion Fluorescent Nano Test Strip for Novel Coronavirus Detection and Preparation Method Thereof .
CN111239400A (2020). Colloidal Gold Immunochromatographic Device for Detecting COVID-19 and Use Method Thereof.
CN111270017A (2020). Primer Probe Combination for Detecting Novel Coronavirus Based on Digital PCR and Application Thereof .
Coronaviridae Study Group of the International Committee on Taxonomy of Viruses (2020). The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 5, 536–544. doi: 10.1038/s41564-020-0695-z
Dong, C., Wang, Z., Zhang, Y., Ma, X., Iqbal, M. Z., Miao, L., et al. (2017). High-performance colorimetric detection of thiosulfate by using silver nanoparticles for smartphone-based analysis. ACS Sensors 2, 1152–1159. doi: 10.1021/acssensors.7b00257
Fujimori, Y., Sato, T., Hayata, T., Nagao, T., Nakayama, M., Nakayama, T., et al. (2012). Novel antiviral characteristics of nanosized copper (I) iodide particles showing inactivation activity against 2009 pandemic H1N1 influenza virus. Appl. Environ. Microbiol. 78, 951–955. doi: 10.1128/AEM.06284-11
Grover, N., Douaisi, M. P., Borkar, I. V., Lee, L., Dinu, C. Z., Kane, R. S., et al. (2013). Perhydrolase-nanotube paint composites with sporicidal and antiviral activity. Appl. Microbiol. Biotechnol. 97, 8813–8821. doi: 10.1007/s00253-012-4573-3
Iyigundogdu, Z. U., Demir, O., Asutay, A. B., and Sahin, F. (2017). Developing novel antimicrobial and antiviral textile products. Appl. Biochem. Biotechnol. 181, 1155–1166. doi: 10.1007/s12010-016-2275-5
Joe, Y. H., Woo, K., and Hwang, J. (2014). Fabrication of an anti-viral air filter with SiO2–Ag nanoparticles and performance evaluation in a continuous airflow condition. J. Hazard. Mater. 280, 356–363. doi: 10.1016/j.jhazmat.2014.08.013
Jung, J. H., Hwang, G. B., Lee, J. E., and Bae, G. N. (2011). Preparation of airborne Ag/CNT hybrid nanoparticles using an aerosol process and their application to antimicrobial air filtration. Langmuir 27, 10256–10264. doi: 10.1021/la201851r
Kaushik, A. (2019). Biomedical nanotechnology related grand challenges and perspectives. Front. Nanotechnol. 1:1. doi: 10.3389/fnano.2019.00001
Kinnamon, D. S., Krishnan, S., Brosler, S., Sun, E., and Prasad, S. (2018). Screen printed graphene oxide textile biosensor for applications in inexpensive and wearable point-of-exposure detection of influenza for at-risk populations. J. Electrochem. Soc. 165:B3084. doi: 10.1149/2.0131808jes
Kumar, R., Nagpal, S., Kaushik, S., and Mendiratta, S. (2020). COVID-19 diagnostic approaches: different roads to the same destination. Virusdisease 31, 97–105. doi: 10.1007/s13337-020-00599-7
Le, T. S., Dao, T. H., Nguyen, D. C., Nguyen, H. C., and Balikhin, I. (2015). Air purification equipment combining a filter coated by silver nanoparticles with a nano-TiO2 photocatalyst for use in hospitals. Adv. Nat. Sci. Nanosci. Nanotechnol. 6:015016. doi: 10.1088/2043-6262/6/1/015016
Li, Y., Hong, M., Qiu, B., Lin, Z., Cai, Z., Chen, Y., et al. (2013). A highly sensitive chemiluminescent metalloimmunoassay for H1N1 influenza virus detection based on a silver nanoparticle label. Chem. Commun. 49, 10563–10565. doi: 10.1039/c3cc45329d
Liu, C., Qiongqiong, Z., Yingzhu, L., Garner, L. V., Watkins, S. P., Carter, L. J., et al. Research development on therapeutic agents vaccines for COVID-19 related human coronavirus diseases. ACS Cent Sci. (2020) 6:315–31. doi: 10.1021/acscentsci.0c00272
Lustig, S., Biswakarma, J. J., Rana, D., Tilford, S. H., Hu, W., Su, M., et al. (2020). Effectiveness of common fabrics to block aqueous aerosols of virus-like nanoparticles. ACS Nano 14, 7651–7658. doi: 10.1021/acsnano.0c03972
Materials, H. (2020). HeiQ Viroblock NPJ03 Antiviral Textile Technologyn Tested Effective Against Coronavirus. Available online at: https://heiq.com/2020/03/16/heiq-viroblock-antiviral
Mejía-Salazar, J., and Oliveira, O. N. Jr. (2018). Plasmonic biosensing: focus review. Chem. Rev 118, 10617–10625. doi: 10.1021/acs.chemrev.8b00359
Mizumoto, K., Kagaya, K., Zarebski, A., and Chowell, G. (2020). Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 25:2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180
Mujawar, M. A., Gohel, H., Bhardwaj, S. K., Srinivasan, S., Hickman, N., and Kaushik, A. (2020). Aspects of nano-enabling biosensing systems for intelligent healthcare; towards COVID-19 management. Mater. Today Chem. 17:100306. doi: 10.1016/j.mtchem.2020.100306
Nanotech Surface Coronavirus (2020). Nanotech Surface Sanitizes Milan with Nanomaterials Remaining Self-Sterilized for Years|STATNANO. Available online at: https://statnano.com//news/67531/Coronavirus-Nanotech-Surface-Sanitizes-Milan-with-Nanomaterials-Remaining-Self-sterilized-for-Years
Nasrin, F., Chowdhury, A. D., Takemura, K., Kozaki, I., Honda, H., Adegoke, O., et al. (2020). Fluorometric virus detection platform using quantum dots-gold nanocomposites optimizing the linker length variation. Anal Chim Acta. 1109, 148–157. doi: 10.1016/j.aca.2020.02.039
Perreault, F., de Faria, A. F., Nejati, S., and Elimelech, M. (2015). Antimicrobial properties of graphene oxide nanosheets: why size matters. ACS Nano 9, 7226–7236. doi: 10.1021/acsnano.5b02067
Pulia, M. S., O'brien, T. P., Hou, P. C., Schuman, A., and Sambursky, R. (2020). Multi-tiered screening and diagnosis strategy for COVID-19: a model for sustainable testing capacity in response to pandemic. Ann. Med. 52, 207–214. doi: 10.1080/07853890.2020.1763449
Qiu, G., Gai, Z., Tao, Y., Schmitt, J., Kullak-Ublick, G. A., and Wang, J. (2020). Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano 14, 5268–5277. doi: 10.1021/acsnano.0c02439
Rao, K., Verma, P., Kumar, K., Verma, M. K., Siddiqui, A. H., Singh, S., et al. (2015). Review on newly identified coronavirus and its genomic organization. SSR Inst. Int. J. Life Sci. 6, 2509–2519. doi: 10.21276/SSR-IIJLS.2020.6.2.5
Ravishankar Rai, V. (2011). “Nanoparticles and their potential application as antimicrobials” in Science Against Microbial Pathogens: Communicating Current Research and Technological Advances (Badajoz: Formatex Research Center).
Seo, G., Lee, G., Kim, M. J., Baek, S.-H., Choi, M., Ku, K. B., et al. (2020). Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano 14, 5135–5142. doi: 10.1021/acsnano.0c02823
Shionoiri, N., Sato, T., Fujimori, Y., Nakayama, T., Nemoto, M., Matsunaga, T., et al. (2012). Investigation of the antiviral properties of copper iodide nanoparticles against feline calicivirus. J. Biosci. Bioeng. 113, 580–586. doi: 10.1016/j.jbiosc.2011.12.006
Sinclair, T., Patil, A., Raza, B., Reurink, D., van Den Hengel, S., Rutjes, S., et al. (2019). Cationically modified membranes using covalent layer-by-layer assembly for antiviral applications in drinking water. J. Memb. Sci. 570, 494–503. doi: 10.1016/j.memsci.2018.10.081
Soh, J. H., Chan, H.-M., and Ying, J. Y. (2020). Strategies for developing sensitive and specific nanoparticle-based lateral flow assays as point-of-care diagnostic device. Nano Today 30:100831. doi: 10.1016/j.nantod.2019.100831
Stellrecht, K. A. (2018). The drift in molecular testing for influenza: mutations affecting assay performance. J. Clin. Microbiol. 56, e01531–e01517. doi: 10.1128/JCM.01531-17
Udugama, B., Kadhiresan, P., Kozlowski, H. N., Malekjahani, A., Osborne, M., Li, V. Y. C., et al. (2020). Diagnosing COVID-19: the disease and tools for detection. Acs Nano. 14, 3822–3835. doi: 10.1021/acsnano.0c02624
van Doremalen, N., Bushmaker, T., Morris, D. H., Holbrook, M. G., Gamble, A., Williamson, B. N., et al. (2020). Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 382, 1564–1567. doi: 10.1056/NEJMc2004973
Vashist, S. K. (2020). In vitro diagnostic assays for COVID-19: recent advances and emerging trends. Diagnostics 10:202. doi: 10.3390/diagnostics10040202
Wang, X., Sun, W., Yang, W., Gao, S., Sun, C., and Li, Q. (2019). Mesoporous silica-protected silver nanoparticle disinfectant with controlled Ag+ ion release, efficient magnetic separation, and effective antibacterial activity. Nanoscale Adv. 1, 840–848. doi: 10.1039/C8NA00275D
Xu, R.-H., He, J.-F., Evans, M. R., Peng, G.-W., Field, H. E., Yu, D.-W., et al. (2004). Epidemiologic clues to SARS origin in China. Emerg. Infect. Dis. 10, 1030–1037. doi: 10.3201/eid1006.030852
Yadavalli, T., and Shukla, D. (2017). Role of metal and metal oxide nanoparticles as diagnostic and therapeutic tools for highly prevalent viral infections. Nanomed. Nanotechnol. Biol. Med. 13, 219–230. doi: 10.1016/j.nano.2016.08.016
Keywords: COVID 19, SARS-CoV-2, diagnostic, biosensor, patents
Citation: Chakraborty D, Kumar S, Chandrasekaran N and Mukherjee A (2020) Viral Diagnostics and Preventive Techniques in the Era of COVID-19: Role of Nanoparticles. Front. Nanotechnol. 2:588795. doi: 10.3389/fnano.2020.588795
Received: 29 July 2020; Accepted: 14 September 2020;
Published: 23 October 2020.
Edited by:
Ajeet Kaushik, Florida Polytechnic University, United StatesReviewed by:
Jong-Hoon Kim, Washington State University Vancouver, United StatesParshant Kumar Sharma, Kwangwoon University, South Korea
Copyright © 2020 Chakraborty, Kumar, Chandrasekaran and Mukherjee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amitava Mukherjee, YW1pdC5tb29rZXJqZWFAZ21haWwuY29t; YW1pdGF2QHZpdC5hYy5pbg==
 Debolina Chakraborty
Debolina Chakraborty Sanjit Kumar
Sanjit Kumar N. Chandrasekaran
N. Chandrasekaran Amitava Mukherjee
Amitava Mukherjee