
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Musculoskelet. Disord. , 11 March 2025
Sec. Spine Conditions
Volume 3 - 2025 | https://doi.org/10.3389/fmscd.2025.1532965
Introduction: Decompressive surgery is considered a practical option for patients with progressive degenerative cervical myelopathy (DCM), nearly 40% of patients with moderate and severe DCM report partial recovery post-surgery (e.g., <50% improvement).
Research question: To examine the impact of decompression surgery on cervical muscle morphology and strength in DCM patients and the relationship between preoperative muscle conditions and postoperative outcomes.
Material and methods: A total of 10 DCM patients underwent surgery and were followed for 2 years. Among 10 patients, 7 underwent posterior fusion surgery, and 3 underwent anterior cervical discectomy and fusion (ACDF). Cervical muscle strength and MRI measurements were taken before and after surgery. Metrics included cross-sectional area (CSA), functional CSA (FCSA), fatty infiltration, and asymmetry of multifidus and semispinalis cervicis (MF + Scer) muscles. Functional outcomes were assessed using the modified Japanese Orthopedic Association, Neck Disability Index, and SF−12 health survey post-surgery.
Results: No significant differences in isometric cervical muscle strength were found between the ACDF and posterior fusion groups at the two-year follow-up. Posterior fusion resulted in decreased MF + Scer muscle CSA (p = 0.01), FCSA (p = 0.027), and increased asymmetry (p = 0.003). The entire cervical extensor muscle CSA also decreased (p < 0.03) with posterior fusion. ACDF led to decreased CSA (p = 0.001) and FCSA (p < 0.001) of all cervical muscles. No significant correlations were observed between pre-surgery muscle measures and functional score changes in posterior fusion.
Conclusion: Contrary to our hypothesis, cervical muscle strength did not improve two years post-surgery in either surgical group. Additionally, no significant correlations were observed between pre-surgical muscle strength or fat infiltration and postoperative functional outcomes. Posterior fusion surgery had a more pronounced effect on cervical musculature compared to ACDF, with greater reductions in muscle CSA and increases in asymmetry.
Degenerative cervical myelopathy (DCM) is a major cause of disability in the adult and elderly population (1). Common anatomical features of the aging spine include degeneration of facet joints, intervertebral discs, and vertebral bodies, hypertrophy of the ligamentum flavum, and ossification of the posterior longitudinal ligament. These changes contribute to persistent spinal cord compression, triggering ischemia, neuronal damage, and inflammation, which underlie the pathogenesis of DCM. Clinically, DCM presents with symptoms ranging from neck stiffness and hand numbness to gait impairment and tetraplegia. As a leading cause of spinal cord dysfunction, DCM significantly impacts healthcare systems through its association with long-term disability, surgical costs, and the growing demands of an aging population (2, 3). While surgery can help prevent the progression of DCM and improve neurological outcomes, functional status, and quality of life (1, 3–5), whether surgical decompression is equally successful and safe in elderly individuals as it is in younger ones is a point of disagreement (1).
Recent studies have demonstrated that the deep extensor neck muscles, especially the cervical multifidus (MF) and semispinalis cervicis (Scer) are often impaired in patients with cervical disorders (6–8) and atrophied in patients with whiplash-type injury or chronic neck pain (7, 9). However, few studies have evaluated the deep extensor neck muscles of patients with DCM (10–12); the presence, extent, and clinical implications of morphologic muscle changes in patients with DCM warrants further attention. Fortin et al. (11) reported an association between greater fatty infiltration and lower functional scores in patients with DCM. A significant correlation between the deep cervical extensor muscle morphology, clinical signs, and symptoms as well as cervical muscle strength was also observed (10). Indeed, the MF and Scer play a critical role in maintaining normal cervical curvature, cervical spinal stability, and activity through their deep attachments to the cervical spine (10, 13). The deep cervical extensor muscles are innervated by the cervical plexus (C1-C4), cranial nerves, or dorsal rami of upper cervical nerves (11, 13), and previous evidence suggested that muscle denervation may progress at the same level, or level below the spinal cord compression in patients with DCM (8, 10). However, further research is needed to fully understand the relationship between cervical muscle morphology, muscular strength, clinical symptom, and functional status to truly comprehend the clinical significance of imaging-defined features of cervical muscle morphology and their impact on muscle function (e.g., strength). Improving our current knowledge regarding the characteristics and implications of cervical muscle morphology in DCM patients might provide useful insights for more effective surgical approaches (anterior vs. posterior) and comprehensive rehabilitation. Therefore, the purpose of this study was to investigate the effect of decompression surgery on cervical muscle morphology and strength in patients with DCM. The secondary purpose was to examine the correlation between preoperative cervical muscle morphology, cervical muscle strength and postoperative functional outcomes in patients with DCM. Based on previous findings (14), we hypothesized that cervical muscle strength will increase at 2-year post-surgery. Also, greater cervical muscle strength and lower fat infiltration pre-surgery would be associated with better functional outcome post-surgery.
The current study involved the enrollment of 20 patients diagnosed with symptomatic DCM, as confirmed by an orthopedic spine surgeon through MRI scans. All patients were scheduled for decompression surgery. Among the 20 DCM patients, only 10 patients were subsequently monitored post-surgery, as the majority of those who underwent surgery expressed satisfaction and did not feel the necessity to return for further follow-up appointments. Consequently, no post-surgery data on muscle measurements were available for the patients who did not return for follow-up. This monitoring included 7 patients who underwent posterior fusion and 3 who underwent anterior cervical discectomy and fusion (ACDF) (Figure 1) and were recruited from the McGill University Orthopedic Clinic, based on the following inclusion criteria: (1) ≥18 years of age, (2) diagnosed with degenerative condition of the cervical spine, (3) present with symptom(s) of cervical myelopathy, (4) non-traumatic origin, (5) underwent MRI of the cervical spine (e.g., MRIs were obtained in different centers), (6) no previous cervical spine surgery. All patients signed informed consent forms agreeing that their information will be utilized for studies aimed at better understanding and describing DCM and this study was approved by the Ethics Research Board of McGill University Health Centre (Study Code: 13-436-GEN).
This cohort study was followed for 2 years and outcomes were obtained post-surgery (e.g., 6 weeks, 12 months, and 24 months) following surgical treatment. Magnetic resonance imaging (MRI) and cervical strength measurements were collected at baseline and 2-years post-surgery. Clinical signs of myelopathy were collected at the time of recruitment and the following clinical and functional scores were used to assess prognosis and functional recovery post-surgery at each time point: modified Japanese Orthopedic Association (mJOA), Neck Disability Index (NDI) and SF-12 health survey. The mJOA is an 18-point scale which quantitatively assesses upper and lower extremity motor and sensory function and which has been previously validated (15, 16). The NDI is a self-reported questionnaire used to measure related pain and disability; higher scores (out of 100) are indicative of greater disability. This questionnaire has previously demonstrated good levels of reliability and validity for neck pain (17, 18). The SF-12 health survey is a reliable and valid questionnaire, consisting of 8 classified scores to measure health-related quality of life. Both physical and mental components of health are assessed in SF-12 health survey. The scores of all questions are finally summed together to calculate the final score, which is between 0 and 100, with higher score reflecting the best health of life (19, 20).
A micro FET2 dynamometer was used to manually measure the isometric neck muscular strength in flexion, extension, right- and left-side bending at the time of recruitment and two years after operation. Patients were asked for to exert a maximal force against the hand-held dynamometer and maintain the head and neck position for 3 s (21, 22). The patients' heads were maintained in a neutral position while they were lying down (prone or supine) to maximize patient stability and isolate the neck musculature (21–23). All patients had a practice round in each position before testing. The examiner's resistance was equal to the highest force exerted by the patients. Patients were positioned supine on a treatment table with the dynamometer on their foreheads, and resistance was given when they lifted their heads. The dynamometer was positioned centrally above the ear for side-bending. Patients were examined in a prone position on a treatment table with a pillow under their chest/shoulder area to assess extensor muscle strength. As they lifted their heads, the dynamometer was positioned over their backs and resistance was applied. Measurements were collected 3 times in each direction with 30- to 60-second rest periods in between, and the average will be used in the analysis. When compared to the gold standard isokinetic testing, hand-held dynamometry has been proved to be a viable instrument, and it has been suggested as a feasible standard for clinical settings (24). Previous studies have found that hand-held dynamometry is reliable for measuring neck muscle strength, with intra-rater reliability ICCs ranging from 0.80 to 0.97 (21, 23, 25, 26), inter-rater reliability ICCs ranging from 0.81 to 0.87 (26).
Pre and post quantitative measurements of the deep extensor neck cervical muscles acquired from axial T2-weighted MR images at the C2 to C7 using ImageJ imaging software (version 1.43;National Institutes of Health, Bethesda, Maryland, downloadable at http://rsbweb.nih.gov/ij/download.html) after multiplanar reconstruction (3D MPR) using the 32-bitOsiriX software program (version 3.8.1; Pixmeo, Geneva, Switzerland) to position the image slices perpendicular to the muscle mass, when required. Cervical muscle measurements of interest, including CSA, FCSA (fat free area), ratio of FCSA/CSA (fatty infiltration) and CSA asymmetry for the MF + SCer together, and deep extensor muscles as a group (e.g., MF, SCer, semispinalis capitis, splenius capitis) were obtained bilaterally at mid-disc (Figures 2A,B). Due to the instrumentation/surgery, muscle measurements were only acquired at the cervical level without instrumentation and thus from C2-C7 levels in 3 patients, C2-C5 levels in 1 patient, C2-C3 and C5-C7 in 1 patient, C2-C3 and C6-C7 in 1 patient, C2-C3 in 1 patient and C6-C7 in 1 patient. Muscle FCSA was measured using a highly reliable thresholding technique described in detail elsewhere (27) (Figure 2B). The relative percent asymmetry of the paraspinal muscles on axial view was calculated as follows: the relative asymmetry rate=[(L−S)/L)] × 100, where L is the larger side and S is the smaller side (8). The mean value of the sum of the muscle CSAs or FCSAs on right and left side at each level, and the means for the FCSA/CSA ratio were calculated for each level of interest and used in the statistical analysis.
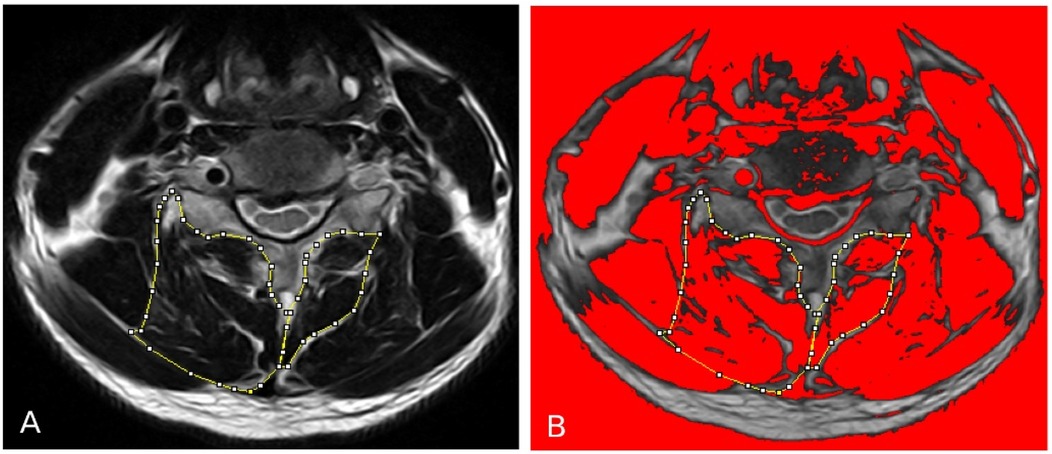
Figure 2. (A) Measurements of the total CSA of the MF + Scer muscles and extensor muscles group on axial T2-weighted images at the C4-C5 level. (B) The image shows the application of a signal threshold filter (ImageJ) to highlight the fat-free muscle area and obtain the FCSA muscle measurements.
Statistical analysis was performed using IBM SPSS (version 29.0). In our study, we meticulously conducted distinct analyses for the posterior fusion vs. ACDF approach of surgery to gain a comprehensive understanding of the outcomes associated with each surgical approach. Means and standard deviations were calculated for patients' characteristics. Kolmogorov–Smirnov test was applied to assess the normal distribution of data. An evaluation of the primary and secondary outcome measures, specifically examining the changes in cervical muscle strength and MRI muscle measurements from the pre-surgery to 2-year post-surgery phases was conducted. To analyze normally distributed variables, we employed paired samples t-tests. Similarly, Wilcoxon signed-rank test was used to make comparison between pre and post cervical muscle and strength measurements for those variables were not normally distributed. Of note, all participants had C2-C3 level available, the pre and post operation comparison was performed twice. The first analysis compared pre- to post-surgery “total” muscle measurements at levels available between C2-C7, which was the sum of measurements at each level. While the second analysis compared pre- to post-surgery muscle measurements at C2-C3 only. All analyses were performed separately for patients that had a posterior fusion vs. ACDF. Pearson correlations were used to assess the relationship between pre-surgical muscle measurements and post-op muscle strength, and pre-surgery muscle measurements with the changes in functional outcomes (mJOA, NDI, SF12-PCS and SF12-MCS) from baseline to 6 weeks, 12- and 24-months post-surgery in posterior fusion group of surgery. A p-value of <0.05 was considered statistically significant in all analysis. Due to the limited number of participants (only 3) in the ACDF surgical approach group, correlation analysis was not conducted within this specific group.
The mean age of patients that underwent posterior fusion and ACDF was 66.86 ± 8.03 years and 53.66 ± 9.07, respectively (Table 1). Only one participant had single level surgery (C3C4), while the remaining participants had multi-level surgery (Table 1). Patients' clinical characteristics are presented in Table 1. Pre- and post-surgery measurements for cervical muscle strength and cervical muscle MRI measurements of interest are presented through Tables 2–4.
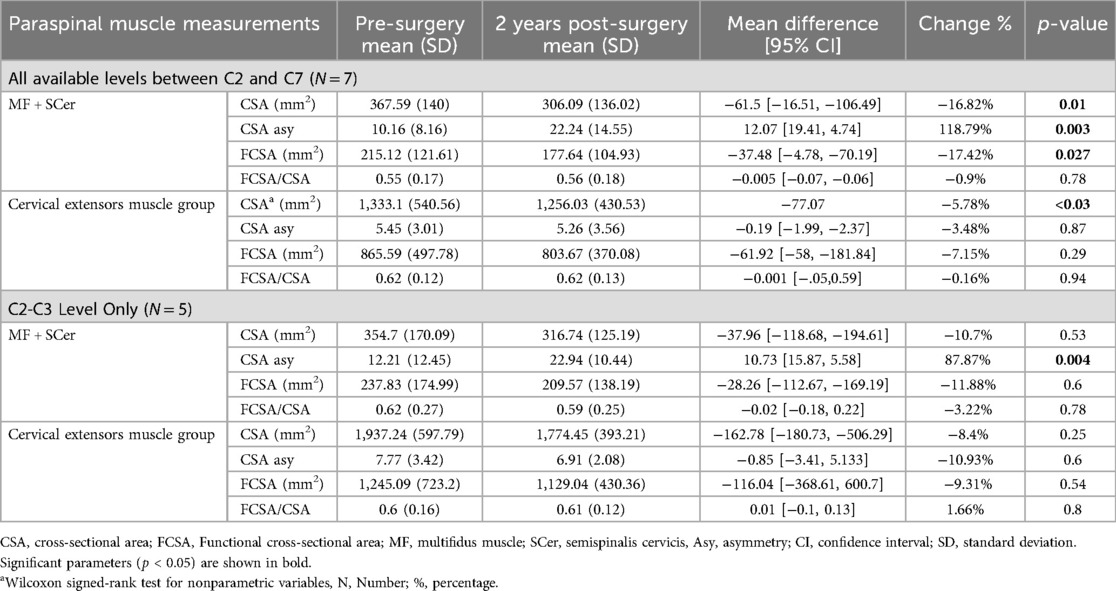
Table 3. MRI cervical paraspinal muscle measurements pre- and post-surgery for patients that underwent posterior fusion.
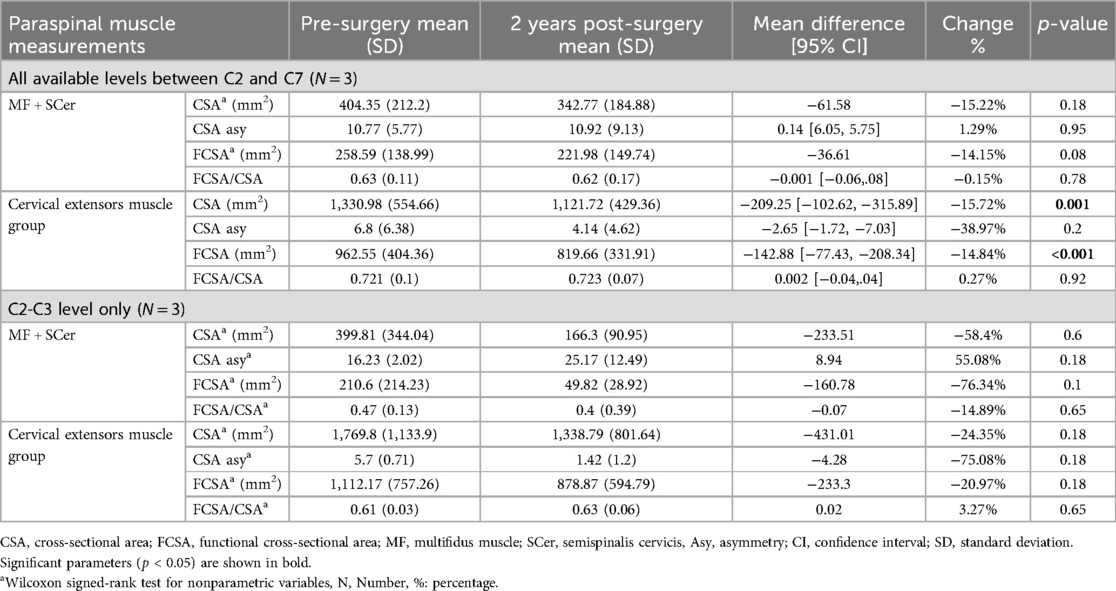
Table 4. MRI cervical paraspinal muscle measurements pre- and post-surgery for patients that underwent ACDF.
Our findings revealed no significant difference in isometric cervical muscle strength in flexion, extension, right- and left-side bending at 2 years follow up after surgery for both ACDF and posterior fusion approach (Table 2) (Figure 3). With regards to patients that underwent posterior fusion, our finding showed a significant decrease in MF + Scer CSA (p-value = 0.01) (Figure 4) and MF + Scer FCSA (p-value = 0.027), with a significant increase in MF + Scer CSA asymmetry (p-value = 0.003) (Table 3). Notably, the CSA of the entire cervical extensor muscle showed a significant decrease (p-value < 0.03) at 2- year post-surgery (Table 3) (Figure 5). Our analysis looking at C2C3 level only revealed a significant increase in MF + SCer CSA asymmetry (p-value = 0.004) post-surgery (Table 3). There were no significant correlations between pre-surgery muscle strength or pre-surgery cervical muscle morphology with changes in functional score including mJOA, NDI, SF12-PCS and SF12-MCS (Table 5) in patients that had a posterior fusion.
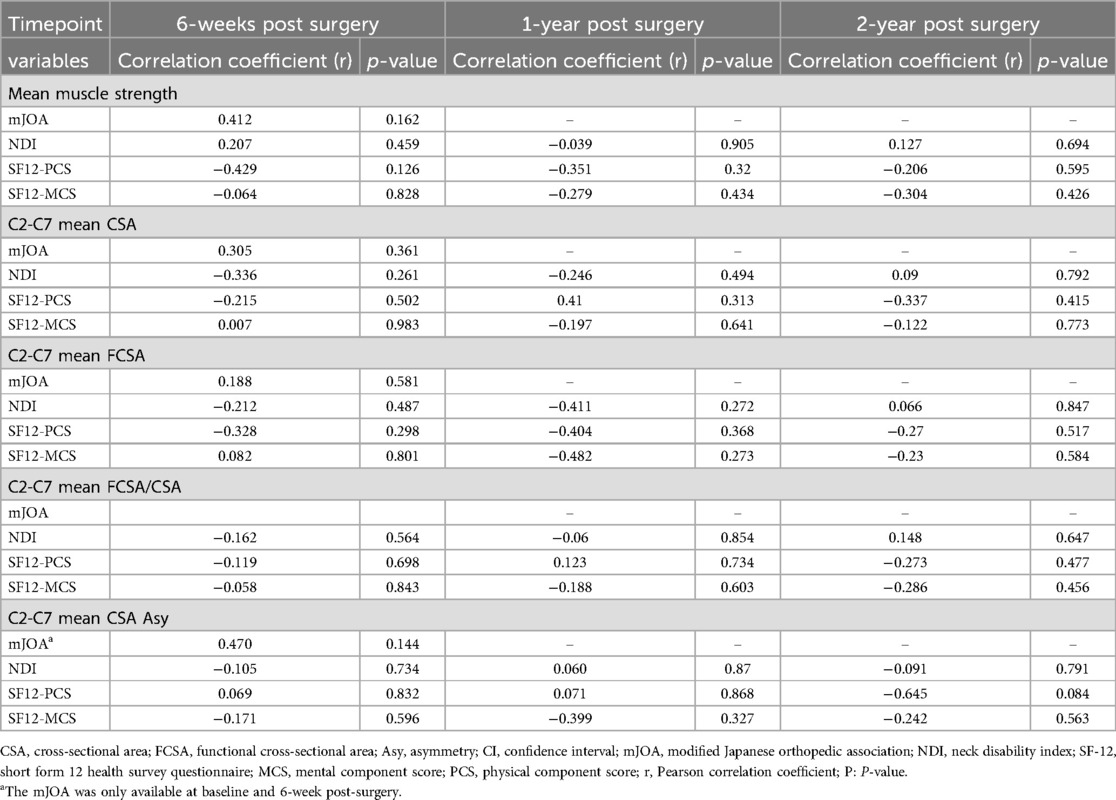
Table 5. Associations between muscle strength and MRI muscle parameters with functional outcomes for posterior fusion group.
Our analysis for patients that underwent ACDF revealed a significant decrease in the CSA of the entire muscle (p-value = 0.001) and FCSA (p-value = <0.001) (Table 4) post-surgery when comparing cervical muscle morphology at all available levels (e.g., C2-C7). However, when examining the C2-C3 level only, no significant changes in muscle morphology were observed (Table 4).
DCM is a progressive spine disorder and the most common cause of spinal cord dysfunction in adults' population globally (3, 10, 28). The use of surgery as a preferred treatment approach for patients with DCM is growing, as it not only effectively stops the progression of the disease but also leads to substantial improvements in function and quality of life. Nevertheless, almost 40% of patients experience only partial recovery following surgical treatment, with less than 50% improvement reported (8, 28–30). As a result, identifying patients that are more likely to benefit from surgery is critical to help guide the clinical decision-making process and manage patients’ expectations. Surgical decisions regarding whether to approach a procedure anteriorly or posteriorly are intricate and currently lack a thorough evaluation of the posterior cervical musculature (31). Additionally, both early and late complications, including post-operative neck pain, adjacent segment disease (ASD), and proximal junctional kyphosis (PJK), may be adversely influenced by the chosen surgical approach (32–34).
Patients with whiplash injury and chronic neck pain are frequently associated with abnormalities in the paraspinal muscles (7, 35, 36). Based on prior research, we had initially hypothesized that surgical treatment would lead to an increase in muscular strength (14). Contrary to our hypothesis, cervical muscle strength did not show significant improvement at the two-year post-surgery follow-up for either surgical group. However, our findings do not corroborate with Fujibayashi et al. (14), who examined the progressive changes in neck muscular strength before and after cervical laminoplasty in a population with cervical spondylotic myelopathy. Indeed, Fujibayashi et al.'s study (14) examined cervical muscle strength based on Visual Analog Scale (VAS) scores at 3-month and 12-month post-surgery (e.g., non-pain group vs. pain group). They reported that cervical muscle strength was recovered by 3-month post-surgery, with a further increase up to 120% of the preoperative value at 12-month mark in the non-pain group (e.g., post-op VAS score <3). However, in the pain group (VAS score ≥3), neck muscle strength remained 60% below the preoperative baseline level at the 3-month mark and did not show any signs of recovery. These disparate findings may be attributed to the differences in surgical approaches between our study and Fujibayashi et al.'s study (14), as laminoplasty was used as a decompressive surgery in their study without fusion which generally leads to muscle atrophy across joints. Additionally, our study had a limited sample size in comparison to theirs, with only 7 DCM participants that underwent a posterior fusion and 3 participants that had an ACDF, whereas their study included 19 participants. Furthermore, previous reports have indicated that, in normal volunteers, men tend to exhibit approximately double the cervical muscle strength of women (32, 37). In their study, the non-pain group at the 3-month mark comprised 11 males and 2 females, and at the 12-month mark, it consisted of 11 males and 5 females. In our study, out of the 7 participants, 6 were females. These sex differences may also contribute to the variations observed in muscle strength outcomes between both studies.
Our findings reveal a significant decrease in MF + Scer CSA and a corresponding significant decrease in MF + Scer FCSA in patients who undertaken posterior fusion surgical approach. Furthermore, there was a noteworthy increase (118.79%) in MF + Scer CSA asymmetry two years after this surgical procedure. Also, when assessing changes in muscle morphology at C2C3 only, CSA asymmetry of the MF + Scer significantly increased post-surgery in patients who had a posterior fusion. Notable findings also emerged in our ACDF subgroup analyses, which meticulously compared cervical muscle morphology before and after surgery across all available levels from C2 to C7. Our results revealed a substantial decrease in the CSA and FCSA of the entire muscle post-surgery. This observation suggests that ACDF also had a notable impact on the overall cervical muscle structure, with a generalized reduction in muscle size. This is attributed to the fusion process, where muscles crossing a fused level no longer contribute to the motion segment, leading to atrophy. This observation emphasizes the intricate relationship between ACDF and its effect on cervical muscle morphology. Interestingly, when specifically examining the C2C3 level, a distinctive pattern emerged, as no significant changes in cervical muscle characteristics post-surgery were observed. Cervical muscle sparing morphology at the C2C3 level prompts further exploration and consideration of potential anatomical or biomechanical variations at this specific vertebral level. The absence of significant changes in this segment could signify unique characteristics or resilience within the C2C3 region in response to the ACDF surgical intervention, warranting additional investigation. These findings contribute valuable insights into the nuanced effects of ACDF cervical surgery on muscle morphology, emphasizing the importance of level-specific analyses to unveil differential impacts across the cervical spine.
The observed muscle atrophy (e.g., decrease in muscle size) suggests that the surgical procedures likely had an impact on the structural integrity of the cervical musculature (28–30, 38–40). We noticed that there were no significant alterations in the MF + Scer within the ACDF approach when compared to the posterior fusion approach. The results of our study provide valuable insights regarding the effect surgical treatment on overall cervical muscle morphology in patients with DCM. The lack of significant changes in MF + Scer in ACDF, in comparison to the posterior fusion, suggests that ACDF may not exert a pronounced impact on that muscle group. In contrast, patients that received a posterior fusion exhibited significant changes in MF + Scer, suggesting that the surgical approach from the posterior aspect may have more substantial effects on this specific muscle group. These findings underscore the importance of considering the differential impacts of surgical approaches on muscle structures, potentially influencing postoperative outcomes and rehabilitation strategies in patients undergoing cervical spine surgeries. Additionally, the significant increase in MF + Scer CSA asymmetry is a noteworthy finding in patients who had posterior fusion surgery as compared to ACDF. This asymmetry may indicate an uneven distribution of muscle size or changes in muscle composition between the left and right sides of the cervical spine. Such asymmetry can have implications for neck stability and function, potentially affecting patient outcomes. Furthermore, the observed significant decrease in CSA of the entire extensors muscle group in both surgical approaches emphasize the overall impact of this treatment on cervical muscle health. This decline in muscle size is likely related to a combination of muscle atrophy, scarring, and increased in fatty infiltration (41). These changes can have functional implications, including potential effects on neck mobility and strength (14, 41).
Previous literature on ACDF and posterior fusion cervical spine surgeries has provided valuable insights into their respective impacts on musculature. Studies focusing on ACDF have highlighted its efficacy in addressing cervical disc pathology, with favorable outcomes in terms of pain relief and functional improvement (32, 34). However, concerns have been raised regarding potential muscle-related complications, such as dysphagia and alterations in cervical spine biomechanics, specifically with fusion surgery (32, 34). In contrast, literature on posterior cervical spine surgeries, including laminectomy and fusion, has explored their effectiveness in decompressing neural structures and stabilizing the spine (42, 43). Some studies have emphasized the importance of preserving posterior musculature to mitigate postoperative muscle-related complications (42, 43). Cervical spine fusion may lead to two common post-operative complications: ASD and PJK. ASD involves degeneration in adjacent segments, managed conservatively or surgically, while PJK causes abnormal curvature above the fusion site, and likely requires additional interventions. Careful patient selection and monitoring are vital for optimal outcomes in cervical spine fusion (33). While both surgical approaches have demonstrated efficacy, the current findings suggesting greater changes in the MF + SCer muscle following posterior surgery add a nuanced layer to the existing literature, highlighting the need for further investigation into the differential impacts of these procedures on overall cervical muscle quality.
It is important to consider the clinical relevance of these findings. While the observed changes in muscle morphology were statistically significant, their clinical significance may vary among individuals. The functional implications of these morphological changes should be explored in future research, as they may provide insights into the long-term outcomes and quality of life of patients who undergo similar surgical procedures. Moreover, the timing of the assessments is critical. The two-year post-surgery period represents a specific point in the recovery process, and longer-term follow-up studies may be needed to fully understand the trajectory of muscle changes and their impact on patients' quality of life. The impact on the posterior musculature in cervical spine surgery is significantly influenced by the number of levels and the type of procedure (3, 22, 29, 30). Single-level surgeries generally result in less disruption to the posterior musculature, contributing to lower impact on muscle function and stability. In contrast, multi-level surgeries may necessitate more extensive manipulation of muscle tissue, potentially leading to increased trauma and affecting muscle strength (3, 22, 29, 30). Indeed, 70% (n = 7) of the patients included in our study had a posterior fusion, and all except one patient, had a multi-level surgery. The latter likely explain the detrimental cervical muscle changes that we observed. Posterior-based surgeries, such as laminectomy or posterior cervical fusion, directly impact the posterior muscles, with the extent of dissection depending on the specific technique. Anterior-based surgeries, like ACDF or cervical disc replacement, typically involve less disruption to the posterior muscles, but indirect effects may occur due to changes in spinal alignment or biomechanics (3, 22) which corroborates with our findings.
Given the profound understanding that myelopathy significantly affects cervical musculature, coupled with the acknowledged atrophy of these muscles following fusion surgery—whether through an anterior or posterior approach—it is imperative to delve into the specific ramifications of disrupting posterior muscles with a posterior cervical approach as opposed to an anterior one. This nuanced exploration is crucial for comprehending the potential added impact on post-operative muscle morphology and function. Such insight is essential for anticipating and addressing surgical outcomes, both in the short term and over an extended period (beyond 2 years), encompassing factors like ASD, PJK, and neck pain. Moreover, recognizing the intricacies of how the disruption of posterior muscles influences post-operative recovery can inform the development of tailored rehabilitation programs and interventions. Different patient groups may benefit distinctively from specific rehabilitation approaches, such as isometric strengthening exercises, aimed at mitigating the impact on muscle structure and function. In the context of this project, it is paramount to acknowledge the inherent limitations stemming from its size. Subsequently, the next phase of investigation should delve into the correlation between the size and levels of fusion performed and their subsequent impact on musculature. As the pre- and postoperative rehabilitation process undoubtedly plays a pivotal role in ameliorating the negative consequences of surgery on musculature, it is incumbent upon surgeons and patients to engage in comprehensive discussions. These dialogues should encompass treatment options, considerations for overall health, and alignment of surgery goals with a keen focus on optimizing post-operative outcomes. Ongoing advancements in surgical techniques offer evolving options for minimizing musculature impact during cervical spine procedures (29, 30). Surgery posteriorly is clearly disrupting the normal muscles of the posterior cervical spine based on the quantification of these muscles volume pre- and post-operatively (30). The lack of functional change following posterior cervical spine surgery, despite disruption to normal muscles, may be attributed to pre-existing muscle dysfunction from spinal stenosis, adaptive changes in muscle function, incomplete recovery time, surgical technique, neurological adaptations, and the absence of targeted rehabilitation (43). A comprehensive exploration of these factors is crucial for understanding the complexities of post-operative outcomes in the posterior cervical spine.
Furthermore, there was no significant correlation between pre-surgery cervical muscle strength or lower fat infiltration and functional outcomes, as measured by mJOA, NDI, or SF-12 scores. The lack of correlation between pre-surgery neck muscle parameters and changes in functional scores, as observed in this study, aligns with some existing literature in the field (10, 12, 17). It is important to note that the relationship between cervical muscle morphology or strength and functional outcomes in patients undergoing cervical surgery is complex and multifactorial (10, 14). While several studies have explored the impact of cervical muscle characteristics on postoperative outcomes, findings remain contradictory (8, 11, 12, 28). Such inconsistency may be attributed to several factors; functional outcomes after cervical surgery are influenced by a myriad of variables, including surgical technique, disease severity, patient age, and comorbidities. These factors can often overshadow the influence of cervical muscle parameters in predicting functional changes. Variations in the methods used to measure muscle strength and morphology, as well as differences in functional score assessments, can also contribute to disparities in study results. Standardization of measurement techniques and functional assessments is crucial for meaningful comparisons. The timing of postoperative assessments can also play a significant role. Muscle recovery and functional improvement may occur at different rates, and a longer follow-up period might be necessary to detect potential associations. The absence of significant associations could be due to limitations in sample size or statistical power. A larger and more diverse sample may reveal subtle relationships that were not evident in the current study. Given these considerations, the fact that our findings revealed no significant correlations between pre-surgery neck muscle parameters and changes in functional scores does not necessarily imply that cervical muscle health is unrelated to postoperative outcomes. Our findings likely underscore the complexity of these relationships. More comprehensive analysis, possibly incorporating multiple variables and a longer follow-up, is needed to fully elucidate the role of cervical muscles health in post-surgery outcomes. Future research efforts should continue to explore this area to provide a clearer understanding of the intricate interplay between cervical muscle characteristics and patient outcomes.
Our study has certain limitations, including the small number of participants, which makes it difficult to determine how muscle strength, morphology and functional outcomes could be affected by surgical approach. Baseline T2-weighted images were acquired from different institutions, and therefore, the imaging scanner parameters were not standardized. Our analysis focused on CSA and FCSA rather than volume, which might provide a more comprehensive understanding of the three-dimensional morphology of cervical muscles. Future studies incorporating volume analysis could offer deeper insights into the structural changes associated with surgical interventions. Since degenerative muscle changes have primarily been observed in the extensor muscles compartment in previous studies examining the relationship between various cervical spine pathologies and cervical muscle morphology (3, 8, 12), we restricted our muscle quantitative MRI assessment to this compartment and did not consider the difference between upper vs. lower cervical level flexion/extension. The accuracy of measuring muscle strength in the population may have been influenced by reduced physical activity, discomfort, and fear of movement. To address this, incorporating a load cell would have provided a more precise assessment of the overall strength of the cervical muscles. Furthermore, only MRI assessment of muscle morphology/composition was performed, additional measures of cervical muscle function should be considered in future work. Additionally, it is important to mention that there have been significant advancements in deep learning automatic segmentation techniques, like convolutional neural networks. These methods have been applied in clinical studies involving patients with DCM (44) and whiplash (45), enabling quick and precise assessment of the cervical muscles. Besides, the small sample size and the lack of data from non-returning participants warrant caution when interpreting the findings and generalizing them to a broader population. To address these limitations, future research should focus on larger, standardized longitudinal studies that integrate advanced imaging techniques, such as volume analysis and deep learning segmentation, to better evaluate the morphological and functional changes in cervical muscles post-surgery.
In conclusion, our study aimed to investigate the impact of cervical fusion surgery on both cervical muscle strength and morphology. Notably, while our findings did not reach statistically significant, there was a clear trend for a decrease in cervical muscle strength two years after surgery in all patients, irrespective if the surgical approach. However, the surgical intervention revealed significant alterations in cervical muscle morphology, resulting in reductions in CSA and FCSA, along with an increase in CSA asymmetry. While we found significant changes in both groups, our results do suggest that greater degenerative muscle changes occurred in patients that had a posterior surgical approach. Importantly, we did not find any significant bivariate associations between pre-surgery measurements of neck muscle strength and neck muscle MRI measurements. These findings suggest that, within the scope of this study, pre-surgery neck muscle characteristics do not appear to directly correlate with postoperative changes in functional scores. It is crucial to note that our study was conducted with a limited sample size, and we did not control the number of levels fused. As a result, our conclusions should be interpreted with caution, and we acknowledge the exploratory nature of this study.
Our findings highlight the importance of assessing and monitoring cervical muscle health in patients undergoing such procedures and suggest the need for further research with larger sample sizes, variable fusion construct length and longer follow-up periods to explore the functional consequences of these morphological and functional changes. As surgical treatment has a strong implication in the management of the DCM, a better understanding of the characteristics and implications of this treatment on the cervical muscle morphology and function in patients with DCM may provide valuable insight for more effective surgery and targeted pre- or post-surgery rehabilitation strategies.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Ethics Research Board of McGill University Health Centre (Study Code: 13-436-GEN). The studies were conducted in accordance with the local legislation and institutional requirements. All participants provided written informed consent prior to participation.
NN: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. MW: Conceptualization, Investigation, Project administration, Writing – review & editing. MF: Conceptualization, Investigation, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. MF is supported by the Fonds de Recherche en Santé du Quebec (FRQS).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Nakashima H, Tetreault LA, Nagoshi N, Nouri A, Kopjar B, Arnold PM, et al. Does age affect surgical outcomes in patients with degenerative cervical myelopathy? Results from the prospective multicenter AOSpine international study on 479 patients. J Neurol Neurosurg Psychiatry. (2016) 87(7):734–40. doi: 10.1136/jnnp-2015-311074
2. Kubaszewski Ł, Wojdasiewicz P, Rożek M, Słowińska IE, Romanowska-Próchnicka K, Słowiński R, et al. Syndromes with chronic non-bacterial osteomyelitis in the spine. Reumatologia. (2015) 53(6):328–36. doi: 10.5114/reum.2015.57639
3. Davies BM, Mowforth OD, Smith EK, Kotter MR. Degenerative cervical myelopathy. Br Med J. (2018) 360:k186. doi: 10.1136/bmj.k186
4. Tykocki T, Poniatowski ŁA, Czyz M, Wynne-Jones G. Oblique corpectomy in the cervical spine. Spinal Cord. (2018) 56(5):426–35. doi: 10.1038/s41393-017-0008-4
5. Singh A, Tetreault L, Casey A, Laing R, Statham P, Fehlings MG. A summary of assessment tools for patients suffering from cervical spondylotic myelopathy: a systematic review on validity, reliability and responsiveness. Eur Spine J. (2015) 24(Suppl 2):209–28. doi: 10.1007/s00586-013-2935-x
6. Chae SH, Lee SJ, Kim MS, Kim TU, Hyun JK. Cervical Multifidus muscle atrophy in patients with unilateral cervical radiculopathy. J Korean Academy of Rehabil Med. (2010) 34(6):743–51.
7. Elliott J, Jull G, Noteboom JT, Darnell R, Galloway G, Gibbon WW. Fatty infiltration in the cervical extensor muscles in persistent whiplash-associated disorders: a magnetic resonance imaging analysis. Spine (Phila Pa 1976). (2006) 31(22):E847–55. doi: 10.1097/01.brs.0000240841.07050.34
8. Naghdi N, Elliott JM, Weber MH, Fehlings MG, Fortin M. Morphological changes of deep extensor neck muscles in relation to the maximum level of cord compression and canal compromise in patients with degenerative cervical myelopathy. Global Spine J. (2022) 14:21925682221136492. doi: 10.1177/21925682221136492
9. Rezasoltani A, Ali-Reza A, Khosro KK, Abbass R. Preliminary study of neck muscle size and strength measurements in females with chronic non-specific neck pain and healthy control subjects. Man Ther. (2010) 15(4):400–3. doi: 10.1016/j.math.2010.02.010
10. Fortin M, Wilk N, Dobrescu O, Martel P, Santaguida C, Weber MH. Relationship between cervical muscle morphology evaluated by MRI, cervical muscle strength and functional outcomes in patients with degenerative cervical myelopathy. Musculoskelet Sci Pract. (2018) 38:1–7. doi: 10.1016/j.msksp.2018.07.003
11. Fortin M, Dobrescu O, Courtemanche M, Sparrey CJ, Santaguida C, Fehlings MG, et al. Association between paraspinal muscle morphology, clinical symptoms, and functional Status in patients with degenerative cervical myelopathy. Spine (Phila Pa 1976). (2017) 42(4):232–9. doi: 10.1097/BRS.0000000000001704
12. Naghdi N, Elliott JM, Weber MH, Fehlings MG, Fortin M. Cervical muscle morphometry and composition demonstrate prognostic value in degenerative cervical myelopathy outcomes. Front Neurol. (2023) 14:1209475. doi: 10.3389/fneur.2023.1209475
13. Hou X, Lu S, Wang B, Kong C, Hu H. Morphologic characteristics of the deep cervical paraspinal muscles in patients with single-level cervical spondylotic myelopathy. World Neurosurg. (2020) 134:e166–e71. doi: 10.1016/j.wneu.2019.09.162
14. Fujibayashi S, Neo M, Yoshida M, Miyata M, Takemoto M, Nakamura T. Neck muscle strength before and after cervical laminoplasty: relation to axial symptoms. J Spinal Disord Tech. (2010) 23(3):197–202. doi: 10.1097/BSD.0b013e3181a1a73e
15. Kopjar B, Tetreault L, Kalsi-Ryan S, Fehlings M. Psychometric properties of the modified Japanese orthopaedic association scale in patients with cervical spondylotic myelopathy. Spine. (2015) 40(1):E23–E8. doi: 10.1097/BRS.0000000000000648
16. Tetreault L, Kopjar B, Nouri A, Arnold P, Barbagallo G, Bartels R, et al. The modified Japanese orthopaedic association scale: establishing criteria for mild, moderate and severe impairment in patients with degenerative cervical myelopathy. Eur Spine J. (2017) 26(1):78–84. doi: 10.1007/s00586-016-4660-8
17. Vernon H, Mior S. The neck disability Index: a study of reliability and validity. J Manipulative Physiol Ther. (1991) 14:409–15.1834753
18. Pietrobon R, Coeytaux RR, Carey TS, Richardson WJ, DeVellis RF. Standard scales for measurement of functional outcome for cervical pain or dysfunction: a systematic review. Spine. (2002) 27(5):515–22. doi: 10.1097/00007632-200203010-00012
19. Findler M, Cantor J, Haddad L, Gordon W, Ashman T. The reliability and validity of the SF-36 health survey questionnaire for use with individuals with traumatic brain injury. Brain Inj. (2001) 15(8):715–23. doi: 10.1080/02699050010013941
20. Ware JE Jr. SF-36 health survey update. Spine (Phila Pa 1976). (2000) 25(24):3130–9. doi: 10.1097/00007632-200012150-00008
21. Cibulka MT, Herren J, Kilian A, Smith S, Mahmutovic F, Dolles C. The reliability of assessing sternocleidomastoid muscle length and strength in adults with and without mild neck pain. Physiother Theory Pract. (2017) 33(4):323–30. doi: 10.1080/09593985.2017.1302539
22. Nakama S, Nitanai K, Oohashi Y, Endo T, Hoshino Y. Cervical muscle strength after laminoplasty. J Orthop Sci. (2003) 8(1):36–40. doi: 10.1007/s007760300006
23. Versteegh T, Beaudet D, Greenbaum M, Hellyer L, Tritton A, Walton D. Evaluating the reliability of a novel neck-strength assessment protocol for healthy adults using self-generated resistance with a hand-held dynamometer. Physiother Can. (2015) 67(1):58–64. doi: 10.3138/ptc.2013-66
24. Stark T, Walker B, Phillips JK, Fejer R, Beck R. Hand-held dynamometry correlation with the gold standard isokinetic dynamometry: a systematic review. PM R. (2011) 3(5):472–9. doi: 10.1016/j.pmrj.2010.10.025
25. Geary K, Green BS, Delahunt E. Intrarater reliability of neck strength measurement of rugby union players using a handheld dynamometer. J Manipulative Physiol Ther. (2013) 36(7):444–9. doi: 10.1016/j.jmpt.2013.05.026
26. Silverman JL, Rodriquez AA, Agre JC. Quantitative cervical flexor strength in healthy subjects and in subjects with mechanical neck pain. Arch Phys Med Rehabil. (1991) 72(9):679–81.1859264
27. Fortin M, Dobrescu O, Jarzem P, Ouellet J, Weber MH. Quantitative magnetic resonance imaging analysis of the cervical spine extensor muscles: intrarater and interrater reliability of a novice and an experienced rater. Asian Spine J. (2018) 12(1):94–102. doi: 10.4184/asj.2018.12.1.94
28. Cloney M, Smith AC, Coffey T, Paliwal M, Dhaher Y, Parrish T, et al. Fatty infiltration of the cervical multifidus musculature and their clinical correlates in spondylotic myelopathy. J Clin Neurosci. (2018) 57:208–13. doi: 10.1016/j.jocn.2018.03.028
29. Rhee J, Tetreault LA, Chapman JR, Wilson JR, Smith JS, Martin AR, et al. Nonoperative versus operative management for the treatment degenerative cervical myelopathy: an updated systematic review. Global Spine J. (2017) 7(3_suppl):35S–41. doi: 10.1177/2192568217703083
30. Jannelli G, Nouri A, Molliqaj G, Grasso G, Tessitore E. Degenerative cervical myelopathy: review of surgical outcome predictors and need for multimodal approach. World Neurosurg. (2020) 140:541–7. doi: 10.1016/j.wneu.2020.04.233
31. Sattari SA, Ghanavatian M, Feghali J, Rincon-Torroella J, Yang W, Xu R, et al. Anterior cervical discectomy and fusion versus posterior decompression in patients with degenerative cervical myelopathy: a systematic review and meta-analysis. Journal of Neurosurgery: Spine. (2023) 38(6):631–43. doi: 10.3171/2023.1.SPINE221244
32. Badhiwala JH, Ellenbogen Y, Khan O, Nouri A, Jiang F, Wilson JR, et al. Comparison of the inpatient complications and health care costs of anterior versus posterior cervical decompression and fusion in patients with multilevel degenerative cervical myelopathy: a retrospective propensity score–matched analysis. World Neurosurg. (2020) 134:e112–e9. doi: 10.1016/j.wneu.2019.09.132
33. Zou L, Liu J, Lu H. Characteristics and risk factors for proximal junctional kyphosis in adult spinal deformity after correction surgery: a systematic review and meta-analysis. Neurosurg Rev. (2019) 42:671–82. doi: 10.1007/s10143-018-1004-7
34. Kato S, Ganau M, Fehlings MG. Surgical decision-making in degenerative cervical myelopathy–anterior versus posterior approach. J Clin Neurosci. (2018) 58:7–12. doi: 10.1016/j.jocn.2018.08.046
35. Elliott J, Jull G, Noteboom JT, Galloway G. MRI Study of the cross-sectional area for the cervical extensor musculature in patients with persistent whiplash associated disorders (WAD). Man Ther. (2008) 13(3):258–65. doi: 10.1016/j.math.2007.01.012
36. Elliott JM. Are there implications for morphological changes in neck muscles after whiplash injury? Spine (Phila Pa 1976). (2011) 36(25 Suppl):S205–10. doi: 10.1097/BRS.0b013e3182387f57
37. Vasavada AN, Li S, Delp SL. Three-dimensional isometric strength of neck muscles in humans. Spine. (2001) 26(17):1904–9. doi: 10.1097/00007632-200109010-00018
38. Hiyama A, Katoh H, Sakai D, Sato M, Tanaka M, Nukaga T, et al. Correlation analysis of sagittal alignment and skeletal muscle mass in patients with spinal degenerative disease. Sci Rep. (2018) 8(1):15492. doi: 10.1038/s41598-018-33867-0
39. Badran A, Davies BM, Bailey H-M, Kalsi-Ryan S, Kotter MR. Is there a role for postoperative physiotherapy in degenerative cervical myelopathy? A systematic review. Clin Rehabil. (2018) 32(9):1169–74. doi: 10.1177/0269215518766229
40. Fehlings MG, Santaguida C, Tetreault L, Arnold P, Barbagallo G, Defino H, et al. Laminectomy and fusion versus laminoplasty for the treatment of degenerative cervical myelopathy: results from the AOSpine North America and international prospective multicenter studies. Spine J. (2017) 17(1):102–8. doi: 10.1016/j.spinee.2016.08.019
41. Tetreault LA, Karpova A, Fehlings MG. Predictors of outcome in patients with degenerative cervical spondylotic myelopathy undergoing surgical treatment: results of a systematic review. Eur Spine J. (2015) 24(Suppl 2):236–51. doi: 10.1007/s00586-013-2658-z
42. Asher AL, Devin CJ, Kerezoudis P, Chotai S, Nian H, Harrell Jr FE, et al. Comparison of outcomes following anterior vs posterior fusion surgery for patients with degenerative cervical myelopathy: an analysis from quality outcomes database. Neurosurgery. (2019) 84(4):919–26. doi: 10.1093/neuros/nyy144
43. El-Ghandour NM, Soliman MA, Ezzat AA, Mohsen A, Zein-Elabedin M. The safety and efficacy of anterior versus posterior decompression surgery in degenerative cervical myelopathy: a prospective randomized trial. Journal of Neurosurgery: Spine. (2020) 33(3):288–96. doi: 10.3171/2020.2.SPINE191272
44. Paliwal M, Weber KA 2nd, Smith AC, Elliott JM, Muhammad F, Dahdaleh NS, et al. Fatty infiltration in cervical flexors and extensors in patients with degenerative cervical myelopathy using a multi-muscle segmentation model. PLoS One. (2021) 16(6):e0253863. doi: 10.1371/journal.pone.0253863
Keywords: extensor neck muscles, degenerative cervical myelopathy, magnetic resonance images, decompressive surgery, total cross-sectional area, muscle fat infiltration
Citation: Naghdi N, Weber MH and Fortin M (2025) Postoperative assessment of cervical muscle morphology, strength, and functional outcomes in patients with degenerative cervical myelopathy. Front. Musculoskelet. Disord. 3:1532965. doi: 10.3389/fmscd.2025.1532965
Received: 22 November 2024; Accepted: 24 February 2025;
Published: 11 March 2025.
Edited by:
Maruti Gudavalli, Keiser University, United StatesReviewed by:
Łukasz A. Poniatowski, Medical University of Warsaw, PolandCopyright: © 2025 Naghdi, Weber and Fortin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maryse Fortin, bWFyeXNlLmZvcnRpbkBjb25jb3JkaWEuY2E=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.