
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Musculoskelet. Disord. , 13 February 2025
Sec. Musculoskeletal Diagnostic Imaging Techniques
Volume 3 - 2025 | https://doi.org/10.3389/fmscd.2025.1528162
Objective: We present a rare case of lumbosacral plexopathy secondary to alcohol-induced rhabdomyolysis.
Case description: A 58-year-old male patient was admitted to the intensive care unit (ICU). He reported a sensation of weakness in both legs and a tingling sensation in his feet upon awakening post binge drinking. Magnetic resonance imaging (MRI) of the thoracic and lumbar spine did not reveal any intraspinal abnormalities. However, an MRI showed lesions with abnormal intensities in both paraspinal muscles. Serum creatine phosphokinase level was markedly elevated. A diagnosis of lumbosacral plexopathy following alcohol-induced rhabdomyolysis was determined by clinical findings, MRI data and an electrophysiological evaluation. Upon discharge, lower limb strength was significantly improved.
Discussion: In severe rhabdomyolysis, the resulting kidney injury and electrolyte imbalances can lead to systemic complications, including peripheral neuropathy or plexopathy. The exact mechanisms by which this occurs are not fully understood, however it is believed that the accumulation of toxic substances, electrolyte imbalances, and metabolic disturbances can contribute to nerve damage and dysfunction. Therefore, lumbosacral plexopathy should be included in the differential diagnosis of such cases, presenting with sudden leg weakness.
Lumbosacral plexopathy (LSP) commonly results from nerve entrapment or trauma, with the most prevalent etiologies being pelvic fractures and neoplasms, which may compress the lumbosacral plexus nerves. Notably, the incidence of LSP is identified in approximately 0.7% of cases following traumatic pelvic fractures, with an increased incidence of 2% following sacral fractures (1). Additionally, metabolic, inflammatory, and autoimmune conditions can also contribute to LSP. Specifically, diabetic amyotrophy, particularly among individuals with type II diabetes mellitus, exhibits an incidence rate of 4.2 per 100,000 annually, affecting around 0.8% of the diabetic population (2). Other potential etiologies include radiation, vascular causes, and infectious, and infiltrative disorders (3). LSP is more frequently observed in females due to the predisposing risk factors of pregnancy and gynecological cancers.
The lumbar and sacral plexuses collectively constitute the lumbosacral plexus, which is formed from the anterior rami of the L1 through S4 nerve roots. The rami then pass downward and laterally along the psoas major muscle. The posterior branches of the anterior rami exit from the lateral aspect of the psoas, travelling through the iliacus and under the inguinal ligament to the anterior thigh. The LSP most frequently presents with asymmetric, focal weakness, numbness, dysesthesia, and/or paresthesia in multiple contiguous lumbosacral nerve root distributions (4).
The gold standard for diagnosing LSP is magnetic resonance imaging (MRI) with gadolinium contrast, as it provides the most comprehensive evaluation of the lumbosacral plexus and surrounding structures. Nerve conduction study (NCS) and electromyography (EMG) are often required to confirm a plexus lesion alongside MRI findings, and determine the extent of injury and prognosis (5).
We present a case of rhabdomyolysis complicated by lumbosacral plexopathy in which MRI and electrodiagnostic studies contributed to the diagnosis.
A 58-year-old male was admitted to the ICU. He complained of weakness in both legs, a tingling sensation in his feet, and pain upon palpation of both hips and the lower back. These symptoms were attributed to alcohol abuse over the previous 48 h, resulting in recurrent falls. Subsequently, he remained motionless for several hours. Additionally, he reported no urine output. The patient was a former boxer with a history of multiple bone fractures.
On admission, the patient had a body temperature of 36.7°C, a heart rate of 85 beats/min, a respiratory rate of 20 breaths/min, and a blood pressure of 127/58 mmHg.
The patient was alert and oriented to person, place, and time with normal speech. The neurological assessment revealed asymmetric weakness of both legs, and diminished pain and touch sensation in both L5 and S1 dermatomes. Additionally, there was an absence of knee and ankle reflexes.
On laboratory studies, serum creatine phosphokinase (64,222 IU/L) and serum myoglobin (8,713 ng/ml) were markedly elevated. The white blood cell count was elevated to 16.2 ul but hemoglobin was normal. Electrolyte profile abnormalities were noted, including decreased chloride, calcium and sodium levels. The biochemical blood test showed significant abnormalities, including blood urea nitrogen: 10.21 μmol/L, creatinine: 228.01 μmol/L, Alanine aminotransferase: 506.55 IU/L, Aspartate aminotransferase: 1,189.74 IU/L, C-reactive protein: 133.3 mg/L. Urine myoglobin was positive and 200 red blood cells were observed per high- power field on urine microscopic examination. All other laboratory tests yielded normal results.
The patient presented with weakness in both legs, sensory disturbances and anuria (no urine output) which necessitated a differential diagnosis related to potential spinal cord or cauda equina lesions.
• MRI of the thoracic and lumbar spine showed no evidence of intraspinal abnormalities. However, MRI revealed the presence of multiple irregular nodular and geographic lesions in both paraspinal muscles and the iliopsoas muscle, which showed mild hypointensity on T1-weighted images, hyperintensity on T2-weighted images (Figures 1A,B), and high signal intensity on short tau inversion recovery (STIR) images (Figure 1C). STIR sequences provide excellent differentiation between affected (hyperintense signal) and unaffected tissue with greater sensitivity than other imaging techniques and without radiation exposure.
• A bone scan (bone scintigraphy): The technetium-99m hydroxydiphosphonate (Tc-99m HDP) whole-body bone scintigraphy revealed an anomalous increase in soft tissue uptake in the lumbar region on the left and right, the sacral region on the left, the lower half of the right thigh, and the lower half of the left thigh (Figure 2).
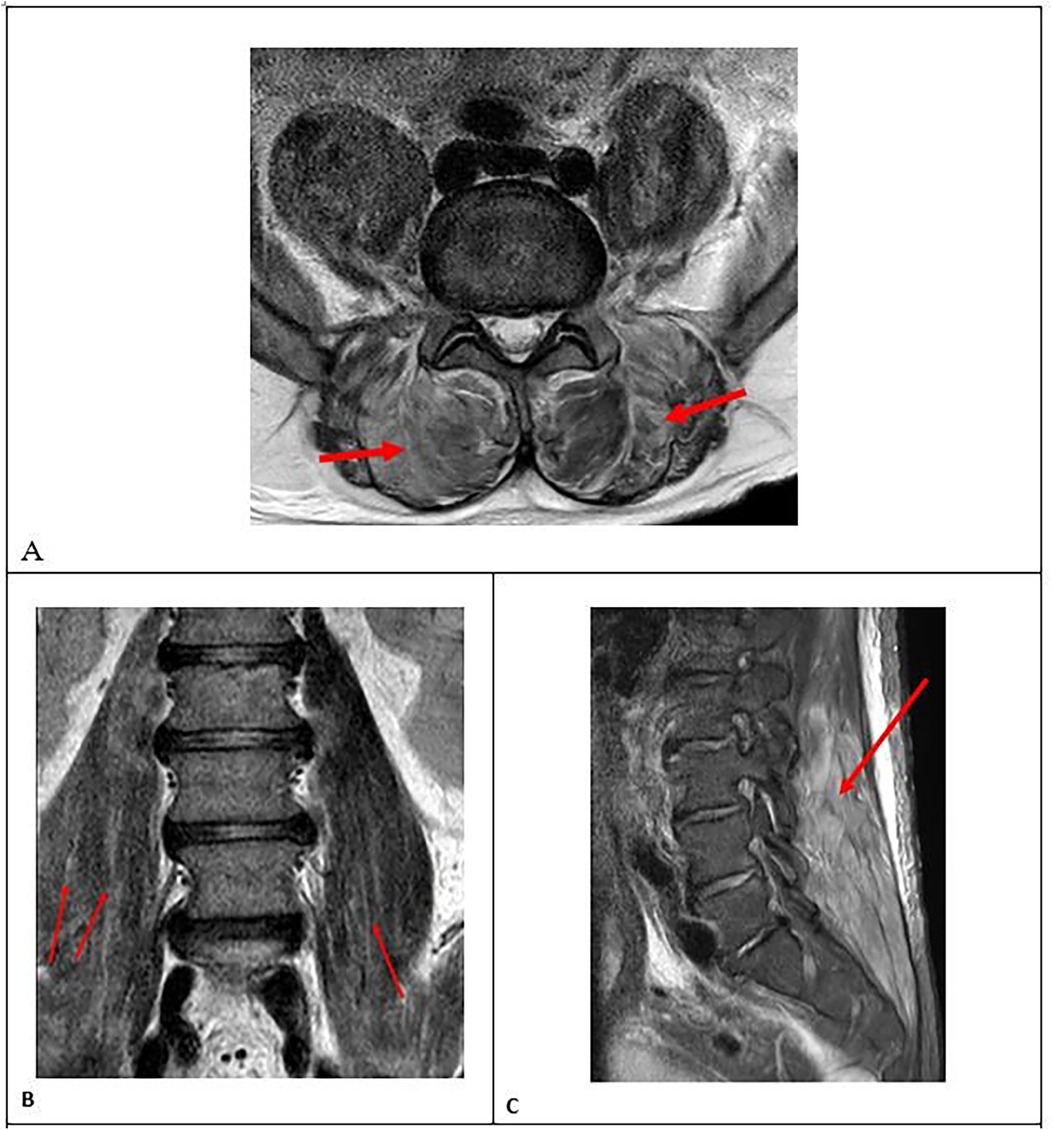
Figure 1. (A) axial MRI scans showing multiple irregular nodular and geographic lesions: hyperintense areas in the paraspinal muscles on T2-weighted images (red arrows), (B) coronal MRI scans showing hyperintense areas in the m.iliopsoas on T2-weighted images (red arrows), (C) sagittal MRI scan showing high signal intensity in the paraspinal muscles on short tau inversion recovery (STIR) images (red arrow).
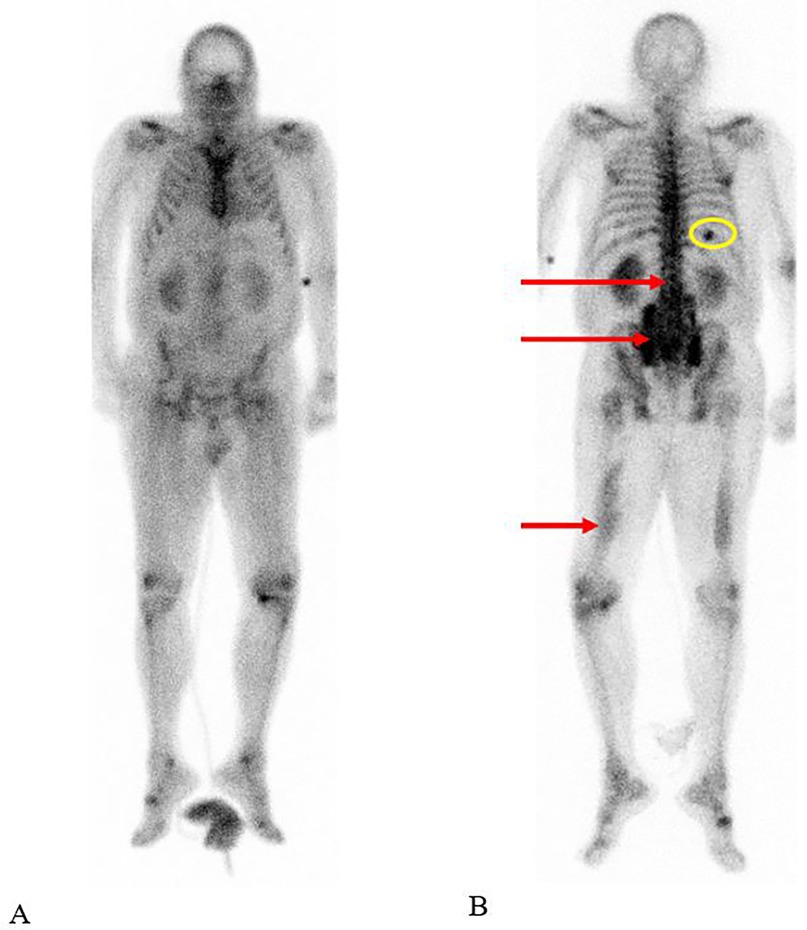
Figure 2. The technetium-99m hydroxydiphosphonate (Tc-99m HDP) whole-body bone scan demonstrated increased soft tissue uptake in both the lumbar and sacral regions, as well as in the mid to distal segments of both thighs, visible on the anterior (A) and posterior (B) views (red arrows). Additionally, there was focal uptake noted in a rib on the right, corresponding to a fracture (circled in yellow). High background activity may be attributed to poor renal function associated with rhabdomyolysis.
Electrodiagnostic studies were performed using a Nicolet EDX® System. The nerve conduction studies (NCS) did not reveal any abnormalities, indicating that the conduction velocities were within the normal range for the affected nerves. However, the electromyography (EMG) revealed significant myopathic changes in the paraspinal muscles), characterized by increased insertional activity and spontaneous activity (Figure 3A, decreased amplitude and duration of motor unit action potentials (MUAPs). These findings are consistent with muscle damage due to rhabdomyolysis. Conversely, the EMG assessment of the bilateral quadriceps, bilateral biceps femoris, and bilateral gluteus medius revealed the presence of spontaneous activities, including fibrillations and positive sharp waves (Figures 3B,C). Additionally, neuropathic MUAPs were observed. These findings, in conjunction with the clinical and imaging data, substantiated the diagnosis of LSP.

Figure 3. Electromyography (EMG) findings: spontaneous activity in lumbar paraspinal muscles (A), m. Rectus femoris (B), m. Gluteus medius (C). (A) Yellow arrows indicate positive sharp waves, which are a type of spontaneous activity that can be detected in muscle denervation or myopathy. (C) Red arrows indicate fibrillation potentials (fill the screen: ++++), which are a type of spontaneous activity that can be detected in muscle denervation or myopathy.
Given the potential overlap of symptoms between lumbosacral plexopathy, myelopathy and peripheral neuropathy, the differential diagnosis can complicate the overall assessment and requires a comprehensive evaluation.
The patient was diagnosed with rhabdomyolysis associated with LSP. A range of advanced therapeutic interventions were available for consideration, including plasma exchange, prolonged haemodialysis and hyperbaric oxygen therapy. With supportive care, pain in both the hip and low back, as well as voiding difficulty, improved after seven days. The serum creatine phosphokinase decreased to 330.5 IU/L. The patient was discharged after a 23-day period, exhibiting improvement in the strength of the bilateral legs, with a rating of 3/4.
Alcohol-induced rhabdomyolysis is a condition characterized by severe muscle damage resulting from the toxic effects of alcohol on the skeletal muscle (6). The precise pathophysiology of alcohol intoxication remains unclear. However, there are notable differences between the short-term and long-term effects, which have been the subject of much research. In the case of short-term alcohol intoxication, the primary causative factors of rhabdomyolysis are immobilization and a comatose state (7).
Rhabdomyolysis is defined as the breakdown of skeletal muscle tissue, which thus results in the release of various substances into the bloodstream. These include myoglobin, creatine kinase, and electrolytes. The aforementioned substances, particularly myoglobin, have the potential to accumulate and cause damage to the kidneys, resulting in acute kidney injury.
Electrolyte imbalances and metabolic disturbances associated with rhabdomyolysis can also occur. In severe cases of rhabdomyolysis, the resulting kidney injury and electrolyte imbalances can lead to systemic complications, including peripheral neuropathy or plexopathy. The precise mechanisms by which this occurs remain unclear; however, it is postulated that the accumulation of toxic substances, electrolyte imbalances and metabolic disturbances may contribute to the damage and dysfunction of the nerves. Furthermore, the inflammation of the psoas muscle may also be a contributing factor in the development of LSP. In some cases, the inflammatory process and subsequent swelling can exert pressure on the lumbosacral plexus in the vicinity. Such compression or irritation of the nerves can result in LSP, which presents as pain, weakness, numbness, or tingling in the affected areas. It is important to note that although psoasitis may potentially contribute to LSP, it is not the sole cause.
It is important to note that LSP as a result of rhabdomyolysis is relatively rare and typically occurs in severe cases with significant systemic complications (8). A single case report by Jeon H.J. et al. describes a male patient of approximately the same age who presented with similar neurological symptoms, namely flaccid inferior paraparesis and sensory disturbances, following alcohol abuse (9). However, in our case, the patient exhibited urinary disturbances in the form of anuria, which necessitated a differential diagnosis with a spinal cord or cauda equina lesion. MRI and whole-body bone scintigraphy demonstrated that the muscle changes had a different localization and were present in the paraspinal muscles. In their report, Jeon H.J. et al. stated that EMG revealed no abnormalities.
However, the authors did not specify which muscles were examined or the timing of the examination in relation to the onset. In the present case, EMG was performed on both the paraspinal muscles and the distal and proximal muscles of the lower extremities on the 14th day of the disease. The timing of the test plays a crucial role, as abnormalities may appear later in the illness. A decrease in the amplitude of motor and sensory responses can be reliably recorded from day 10–12 of the disease, after the completion of Wallerian degeneration.
Rhabdomyolysis can usually be diagnosed, based on elevation of serum creatine kinase level. A TC-99m HDP whole-body bone scan may also be employed to detect muscle injury and reveal increased soft tissue uptake in the corresponding areas. MRI has been shown to be useful for diagnosis and localization of rhabdomyolysis and more sensitive than computed tomography or ultrasonography. It has been documented that T2-weighted images demonstrate rhabdomyolysis lesions as areas of increased signal intensity (10).
In considering differential diagnoses for conditions that may present with symptoms similar to lumbosacral plexopathy, such as myelopathy and polyneuropathy, the following key distinctions can be made. NCS and EMG can indicate whether there is evidence of generalized nerve involvement. It is of the utmost importance to differentiate between these conditions in order to ensure an accurate diagnosis and subsequent treatment plan.
In instances where patients present with a tingling sensation and sudden weakness of the legs, a differential diagnosis that should be considered includes LSP due to rhabdomyolysis. MRI and EMG appear to be valuable tools for diagnosing LSP in cases of alcohol-induced rhabdomyolysis.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the study was approved by the Ethics Committee of Sklifosovsky Research Institute for Emergency Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
ES: Conceptualization, Data curation, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. MS: Methodology, Supervision, Validation, Writing – review & editing. AG: Methodology, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was sponsored by the Moscow Center for Innovative Technologies in Healthcare.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kutsy RL, Robinson LR, Routt ML. Lumbosacral plexopathy in pelvic trauma. Muscle Nerve. (2000) 23(11):1757–60. doi: 10.1002/1097-4598(200011)23:11%3C1757::AID-MUS13%3E3.0.CO;2-M
2. Ng PS, Dyck PJ, Laughlin RS, Thapa P, Pinto MV, Dyck PJB. Lumbosacral radiculoplexus neuropathy: incidence and the association with diabetes mellitus. Neurology. (2019) 2(11):e1188–94. doi: 10.1212/WNL.0000000000007020
3. Dydyk AM, Hameed S. Lumbosacral plexopathy. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing (2023).
4. Miniato MA, Black AC, Varacallo M. Anatomy, back, lumbosacral trunk. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing (2023).
5. Vazquez Do Campo R. Brachial and lumbosacral plexopathies. Semin Neurol. (2024) 45(01):049–62. doi: 10.1055/s-0044-1791664
6. Htet Z. Alcohol-induced rhabdomyolysis: a disease with potential pitfalls. Acute Med. (2018) 17(4):226–8. doi: 10.52964/AMJA.0735
7. Qiu LL, Nalin P, Huffman Q, Sneed JB, Renshaw S, Hartman SW. Nontraumatic rhabdomyolysis with long-term alcohol intoxication. J Am Board Fam Pract. (2004) 17(1):54–8. doi: 10.3122/jabfm.17.1.54
8. Chandrasekhar V, Singh H, Subramanian A, Dhibar DP, Suri V, Bhalla A. Rhabdomyolysis due to acute alcohol intoxication. Am J Med. (2024) 137(8):e151–2. doi: 10.1016/j.amjmed.2024.04.022
9. Jeon HJ, Cho BM, Oh SM, Park SH. Lumbosacral plexopathy, complicating rhabdomyolysis in a 57-year-old man, presented with sudden weakness in both legs. J Korean Neurosurg Soc. (2007) 42(6):481–3. doi: 10.3340/jkns.2007.42.6.481
Keywords: rhabdomyolysis, lumbosacral plexopathy, magnetic resonance imaging, electromyography, leg weakness, paraspinal muscles
Citation: Seliverstova EG, Sinkin MV and Grin AA (2025) Lumbosacral plexopathy following alcohol-induced rhabdomyolysis: a case report. Front. Musculoskelet. Disord. 3:1528162. doi: 10.3389/fmscd.2025.1528162
Received: 5 December 2024; Accepted: 30 January 2025;
Published: 13 February 2025.
Edited by:
Hyungseok Jang, University of California, Davis, United StatesReviewed by:
Sam Sedaghat, Heidelberg University Hospital, GermanyCopyright: © 2025 Seliverstova, Sinkin and Grin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: E. G. Seliverstova, ZS5zZWxpdmVyc3RvdmEubWRAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.