- 1Department of Neurological Surgery, University of Pittsburgh, Pittsburgh, PA, United States
- 2VA Medical Center, United States Department of Veterans Affairs, Hunter Holmes McGuire, Richmond, VA, United States
- 3Department of Neurological Surgery, Virginia Commonwealth University, Richmond, VA, United States
- 4Veterans Health Administration, United States Department of Veterans Affairs, VA Ann Arbor Healthcare System, Ann Arbor, MI, United States
- 5Department of Neurological Surgery, University of Michigan, Ann Arbor, MI, United States
- 6Department of Radiology, Emory University, Atlanta, GA, United States
- 7United States Department of Veterans Affairs, Veterans Health Administration, Atlanta VA Health Care System, Decatur, GA, United States
- 8Department of Physical Medicine and Rehabilitation, University of Colorado, Boulder, CO, United States
- 9Veterans Health Administration, United States Department of Veterans Affairs, VA Eastern Colorado Health Care System, Denver, CO, United States
- 10Department of Internal Medicine, University of Michigan, Ann Arbor, MI, United States
- 11Veterans Health Administration, United States Department of Veterans Affairs, VA San Diego Healthcare System, San Diego, CA, United States
- 12Department of Medicine and Orthopedic Surgery, Harvard Medical School, Boston, MA, United States
- 13T.H. Chan Center for Work, Health, and Well-being, School of Public Health, Harvard University, Boston, MA, United States
- 14Department of Medicine and Orthopedic Surgery, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, United States
- 15Department of Medicine, University of Pittsburgh, Pittsburgh, PA, United States
- 16Department of Biostatistics, University of Pittsburgh, Pittsburgh, PA, United States
- 17Department of Psychiatry, University of Pittsburgh, Pittsburgh, PA, United States
- 18Department of Anesthesiology, University of Pittsburgh, Pittsburgh, PA, United States
- 19Geriatric Research, Education and Clinical Center, VA Pittsburgh Health Care System, Pittsburgh, PA, United States
Background/context: Decompressive laminectomy (DL) for lumbar spinal stenosis (LSS) is the most common spinal surgery for older adults. Biopsychosocial factors are associated with 1-year outcomes in these patients. While most surgical failures occur within 12 months, some are delayed, and factors responsible for delayed surgical failure are poorly understood.
Purpose: We sought to identify preoperative factors associated with long-term surgical success as defined by the Brigham Spinal Stenosis (BSS) questionnaire.
Study design/setting/patient sample: Within this prospective cohort study, we used logistic regression modeling to identify preoperative biopsychosocial factors that predict 4-year DL success in 110 prospectively evaluated veterans who underwent DL without fusion for LSS.
Outcome measures/methods: A questionnaire was used to evaluate BSS outcomes at 4 years post-DL.
Results: Overall, 69 participants (63%) demonstrated 4-year surgical success—sustained improvement in at least two of the three BSS domains (symptoms, function, and satisfaction). Greater catastrophizing [OR for 2 points 0.92 (0.84–1.00); p = 0.0512] and longer symptom duration [OR for 12 months 0.96 (0.93–0.99); p = 0.0231] were associated with lower likelihood of success, while presence of moderate/severe stenosis (OR 7.16–7.39; p = 0.0195–0.0260), college education [OR 2.93 (1.27–6.77); p = 0.0120], and greater treatment credibility [OR for 10 points 1.35 (1.10–1.66); p = 0.0048] were associated with greater likelihood of success in bivariate analyses. Symptom duration [OR 0.96 (0.92–0.99); p = 0.0208], treatment credibility [OR 1.51 (1.15–1.98); p = 0.0031], and stenosis severity (OR 14.4–17.4; p = 0.0045–0.0055) constituted a parsimonious set of factors in multivariable modeling.
Conclusions: Further work is needed to definitively identify preoperative factors that predict long-term outcomes. This may facilitate more accurate patient selection and counseling for patients undergoing DL for LSS.
Introduction
Lumbar spinal stenosis (LSS) is a narrowing of the central spinal canal or lateral canal(s) caused by bone or soft tissue encroachment that causes mobility limitation because of pain and/or lower extremity paresthesias, i.e., neurogenic claudication. Lumbar stenosis impacts over 10% of older adults leading to millions of hospital visits annually (1–3). Decompressive laminectomy (DL) for LSS is the most common indication for spine surgery in older adults (1, 2). While DL provides symptom relief (i.e., reduction of pain and paresthesias) in some patients, nearly one-half of veterans undergoing DL will not achieve functional improvement at 12 months (4). Few studies have evaluated longer-term outcomes following DL, beyond 1 year, although some indicate that DL failure rates increase with extended follow-up beyond 1 year (5–7). As many as one in three DL for LSS patients undergo additional surgery within 5 years of the original procedure (8). While failed DL is often followed by more complex surgical procedures such as fusion, outcomes associated with these procedures are not superior (9).
A comprehensive understanding of factors associated with outcomes beyond 1 year is needed to optimize patient selection, surgical decision-making, and associated utilization of healthcare resources. Preliminary data raise the possibility that the more extensive the pathology (i.e., the greater the number of spinal levels decompressed), especially in the setting of altered spinal biomechanics, the greater the risk of DL failure (10). Others suggest that selecting patients with severe anatomical stenosis, no back pain, symptoms for less than 4 years, and no other conditions that impair walking will have superior DL outcomes (7). The burden of medical comorbidity also has been highlighted as a risk factor for worse long-term DL outcomes (11). Studies that examine a comprehensive set of predictors, including biopsychosocial factors, of long-term outcomes in patients undergoing DL for LSS are lacking (12). Such studies may inform patient selection and help identify patients likely to benefit from prehabilitation prior to DL for LSS.
We recently published the results of a prospective cohort study of Veterans with LSS who underwent DL. Among a comprehensive set of factors evaluated preoperatively, opioid use, apparent leg length inequality, and low self-efficacy were the strongest predictors of outcome 1 year following DL (3). We now examine predictors of 4-year DL success.
Methods
Participant cohort
The study protocol was approved by the Department of Veterans Affairs (VA) Central Institutional Review Board and at enrollment sites as described in Weiner et al. (3). All participants underwent DL without fusion at baseline.
Inclusion criteria were (1) preoperative neurogenic claudication for at least 3 months defined as pain, weakness, numbness, or tingling in the legs that are precipitated by walking or standing and relieved by sitting; (2) MRI evidence of lumbar spinal stenosis; and (3) failure of non-surgical management. Exclusion criteria included (1) prior lumbar surgery; (2) spondylolisthesis with ≥4 mm translation or ≥10° of angular motion; and (3) cognitive impairment that undermines the capacity to complete questionnaires (3, 13).
Outcomes
The Brigham Spinal Stenosis (BSS) score was the main outcome. It consists of three domains: physical function, symptom severity, and surgical satisfaction (14–16). Each domain is scored from one to five for symptom severity and one to four for physical function and satisfaction, with a higher score signifying a worse disability. Meaningful improvements from baseline are noted as reductions in a score of ≥0.42 in physical function, ≥0.46 in symptom severity, and ≥2.42 in satisfaction. Surgical success is defined as a meaningful improvement in at least two of three categories (3, 15).
Baseline variables
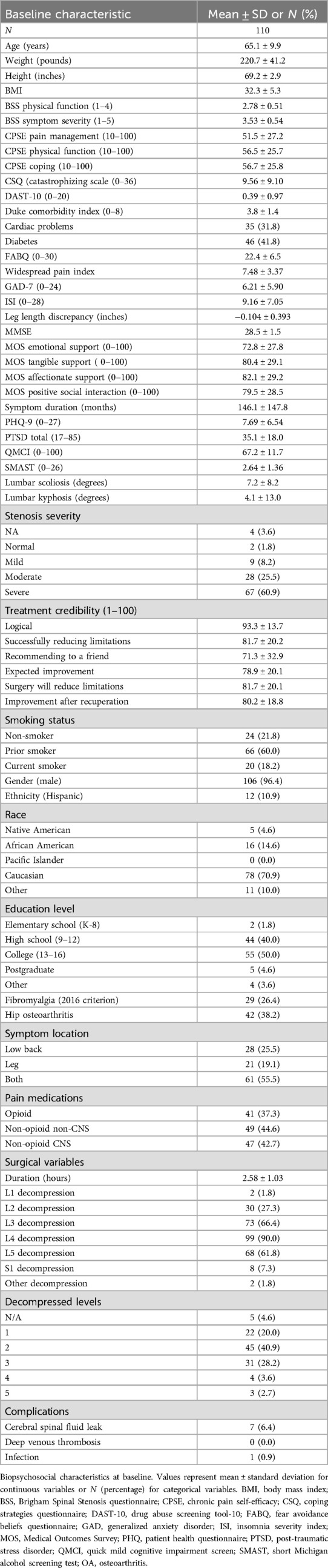
Table 1 summarizes baseline characters of the patient cohort.
1. Demographics—age, gender, race, ethnicity, education.
2. Physical characteristics—height, weight, pain characteristics (location, severity, duration).
3. Hip osteoarthritis (OA): participants reporting hip pain underwent x-rays with OA diagnosed by study radiologists according to American College of Rheumatology criteria (17).
4. Scoliosis/kyphosis: all participants underwent standing spine x-rays with lateral and anterior/posterior view projections. A radiologist who was blinded to outcomes reported Cobb's angle (scoliosis) and kyphosis from the uppermost tilted vertebra to the lowermost tilted vertebra (18).
5. Leg length discrepancy: measured as the right vs. left difference in umbilicus to medial malleolus while laying supine. Each measurement was performed twice with the average reported (19).
6. Comorbidities: medical comorbidities were based on participant self-report and calculated according to the Duke comorbidity index (20).
7. Pain medication data: we categorized preoperative pain medication as opioid; central nervous system active non-opioid (gabapentin, pregabalin, muscle relaxants, tricyclic antidepressants, other antidepressants); and non-central nervous system active medications (salicylate, NSAID, acetaminophen, topical).
8. Additional specific conditions including generalized pain disorders (21), fibromyalgia (14), mild cognitive impairment (22), and insomnia were evaluated using standardized instruments (23).
Psychosocial factors
1. Depressive symptoms were evaluated using the patient health questionnaire (PHQ−9), a 10-item questionnaire widely used for depression screening (24).
2. Anxiety was evaluated with two measures: (a) generalized anxiety was evaluated using the seven-item generalized anxiety disorder (GAD) scale, and (b) post-traumatic stress disorder (PTSD) was evaluated using the PTSD checklist (25).
3. Pain coping skills were evaluated using three scales: (a) cognitive strategies questionnaire (26), chronic pain self-efficacy (CPSE) scale (27), and (c) fear avoidance beliefs questionnaire (28).
4. Substance use was evaluated using the short Michigan alcoholism screening test (SMAST-13) for alcoholism, drug use questionnaire (DAST-10) for illicit drug use, and previous and active smoking status for tobacco use (29).
5. Social support was evaluated using the Medical Outcomes Survey (MOS) Social Support Scale (30).
6. Treatment credibility/expectations were measured using the methods of Borkovec (31).
Anatomic and surgical factors
1. Severity of central canal stenosis was quantified by a blinded radiologist, using a validated scoring system (32).
2. Surgical variables including location and number of decompressive levels, duration of surgery, presence of postoperative complications, and revision surgeries were collected from participant medical records and surgical follow-up evaluations, as reported by surgeons.
Participant follow-up
Participant flow is depicted in Figure 1. Two hundred thirty-nine Veterans signed informed consent, 8 dropped out, and 231 underwent baseline testing. Thirty-eight did not proceed further with the study; 11 dropped out, and 27 were withdrawn by the site principal investigator for reasons listed in Figure 1. Participants were telephoned 1 and 4 years after DL, and the BSS was completed during each of these follow-ups. The original consent indicated a 1-year participation period. Subsequently, a waiver of the need for additional consent was obtained, and participants were sent a letter indicating that they would be telephoned 4 years after their DL and that they would be asked questions about their pain and function. The letter indicated that participation was not required. One hundred ninety-three underwent 1-year post-DL follow-up assessment, and 110 underwent 4-year post-DL follow-up assessment. The present report focuses on 4-year post-DL data. Previous reports have endorsed a delayed decline in surgical success rate post 2 years in participants undergoing isolated DL without fusion (33, 34). We chose 4 years to enable sufficient time to capture delayed failure. All participants included in this study were derived from the original 1-year outcomes study by Weiner et al. (2021). We rigorously compared participants with non-participants, and there were no significant differences.
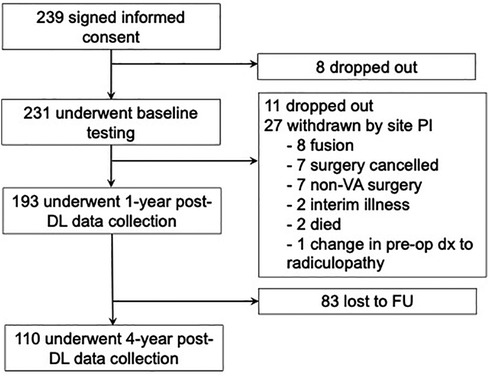
Figure 1. Study participant follow-up to 4-year outcomes. A total of 239 participants were originally enrolled in the study with 231 undergoing initial baseline follow-up. Of the 193 participants that completed 1-year outcomes, 83 were lost to follow-up resulting in 110 participants for 4-year follow-up. PI, principal investigator; dx, diagnosis; DL, decompressive laminectomy; FU, follow-up.
Statistical analysis
We used independent samples t-test, Wilcoxon rank sum, chi-square, and Fisher's exact tests to compare characteristics of participants within the 1-year cohort who were and were not included in the present 4-year analysis. Surgical success at 1 and 4 years was compared using the McNemar test for paired data. We fitted a series of logistic regression models. Dependent variables included participants who met the criteria for a successful DL at 4 years (yes/no). Independent variables included demographic and other pre-DL variables, each evaluated one at a time. Next, to obtain a parsimonious set of pre-DL measures independently associated with the likelihood of a successful DL with an exploratory view, we fitted a multivariable logistic model for each dependent variable with all the baseline measures as independent variables and a stepwise variable selection procedure. Odds ratios were rescaled to provide an intuitively relevant magnitude without altering their statistical significance. For example, odds ratios for weight were expressed per 10 pounds (a more meaningful magnitude) rather than per 1 pound (raw estimate by a regression coefficient; see Tables 3, 4). We used SAS software version 9.4 (SAS Institute, Inc., Cary, North Carolina, USA) for statistical analyses.
Results
This prospectively collected cohort of United States Veterans included 193 participants with symptomatic spinal stenosis who underwent elective DL surgery with 1-year follow-up of whom 110 (focused herein) had 4-year follow-up. Table 1 summarizes the baseline participant characteristics. Overall, the average age was 65.1 ± 9.9, BMI 32.3 ± 5.3 with 70.9% being Caucasian. The majority (60.0%) of participants previously smoked. Only 18.2% reported smoking currently. All participants exhibited neurogenic claudication with the majority (55.5%) endorsing concurrent back and leg pain with imaging respectively confirming moderate (25.5%) or severe (60.9%) stenosis. Those included in the 4-year analysis had greater stenosis, symptom severity, comorbidity, poorer self-efficacy, poorer mood, and lower treatment credibility ratings (all p < 0.05; Supplementary Table S1).
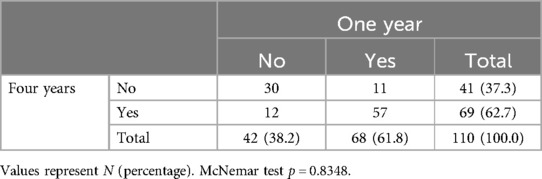
Of the 68 participants demonstrating surgical success at 1 year, 11 did not sustain surgical success at 4 years (16%). Of the 69 participants demonstrating surgical success at 4 years, 12 did not demonstrate initial surgical success at 1 year (17%). Comparing surgical success rates at 1 and 4 years, there was no statistically significant difference (p = 0.8348; Table 2). In addition, 1- vs. 4-year success rates in BSS physical function (50.9 vs. 49.1%; p = 0.6831), BSS symptoms severity (65.5 vs. 67.3%; p = 0.6171), and BSS satisfaction (68.2 vs. 71.8%; p = 0.2850) were not statistically different (Supplementary Tables S2–S4).
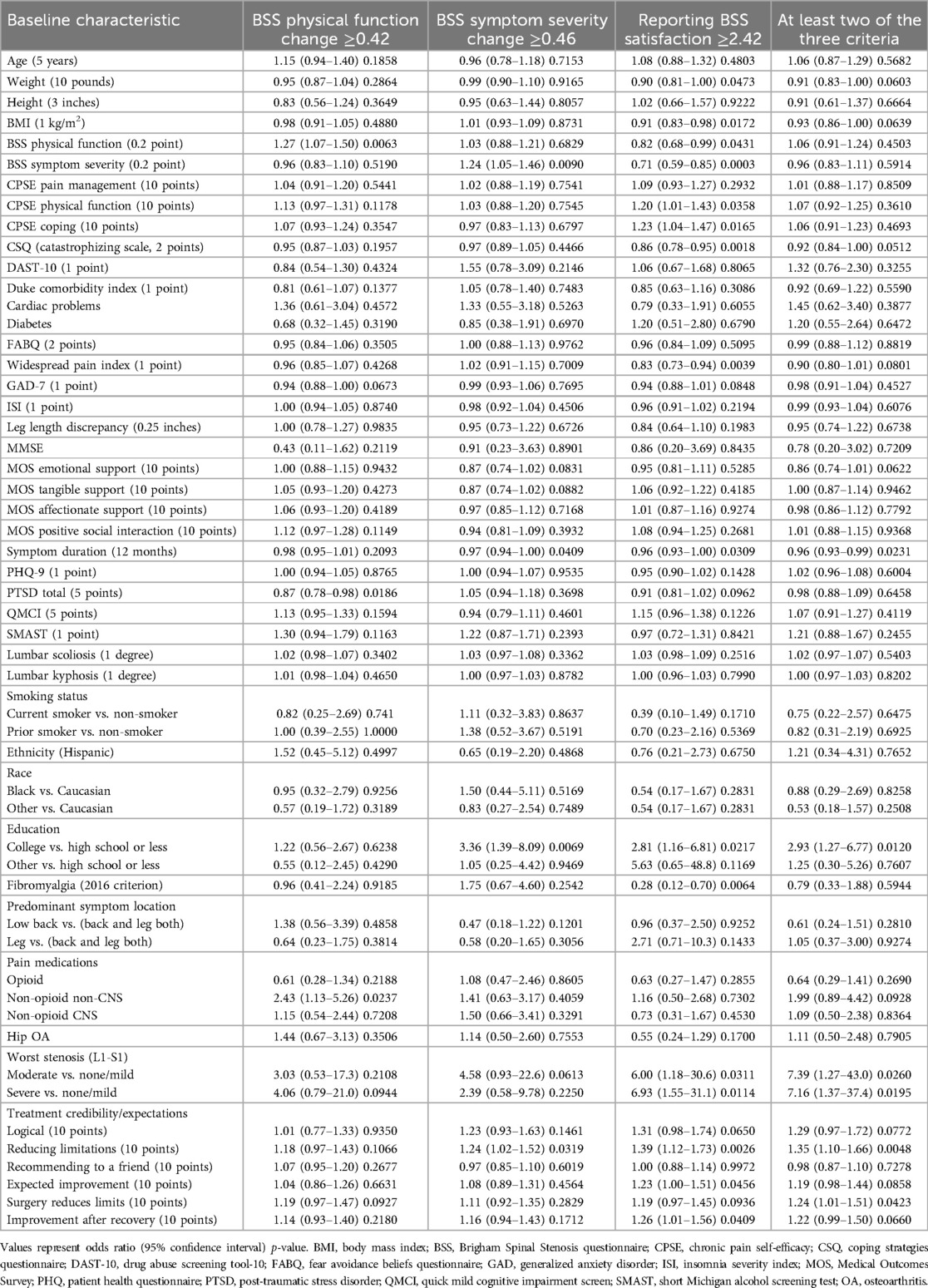
Figure 2 highlights the BSS metric associations at 4 years. Table 3 summarizes the bivariate associations between surgical success and preoperative factors. A 0.2-point greater (worse) baseline BSS physical function score was associated with a 27% increase in odds of physical function improvement [OR 1.27 (1.07–1.51); p = 0.0063]. In addition, being on a non-CNS pain medication conferred over twice the odds of improving BSS physical function [OR 2.43 (1.13–5.26); p = 0.0237], while 5 points in the PTSD scale decreased the odds by 13% [OR 0.87 (0.78–0.98); p = 0.0186].
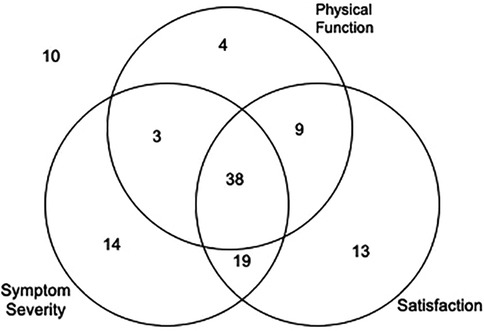
Figure 2. Decompressive laminectomy success rates by different BSS criteria. 10/110 (9.1%) did not improve by any of the three criteria. 31/110 (28.2%) improved by only one of the three criteria. 31/110 (28.2%) improved by only two of the three criteria. 38/110 (34.5%) improved by all three criteria.
Having a 0.2-point greater baseline BSS symptom severity score (i.e., worse symptoms) was associated with 24% increased odds of improving BSS symptom severity post-DL [OR 1.24 (1.05–1.46); p = 0.0090]. A 10-point difference in treatment credibility was associated with a 24% increase in odds of symptom improvement [OR 1.24 (1.02–1.52); p = 0.0319]. In addition, having a college education was associated with an over threefold increase in odds of improvement [OR 3.36 (1.39–8.09); p = 0.0069]. Conversely, an additional year of symptom duration was associated with a 3% decrease in odds [OR 0.97 (0.94–1.00); p = 0.0409] of an improvement in BSS symptom severity post-DL.
Many variables demonstrated an association with BSS satisfaction. A 10-point greater CPSE physical function score (i.e., higher physical function disability) was associated with 20% greater odds [OR 1.20 (1.01–1.43); p = 0.0358] of BSS satisfaction. A 10-point greater CPSE coping score (i.e., better coping skills) was associated with a 23% increase in odds [OR 1.23 (1.04–1.47); p = 0.0165], a college education with an over twofold increase in odds [OR 2.81 (1.16–6.81); p = 0.0217], severe stenosis with an almost sevenfold increase in odds [OR 6.93 (1.55–31.1); p = 0.0114], and a 10-point increase in the preoperative expectations of postsurgical improvement score associated with a 23% increase in odds [OR 1.23 (1.00–1.51); p = 0.0456] of achieving BSS satisfaction. Conversely, each 1 kg/m2 increase in BMI was associated with a 9% reduction in satisfaction [OR 0.91 (0.83–0.98); p = 0.0172], a 10-point greater BSS physical function score lowered the odds by 18% [OR 0.82 (0.68–0.99); p = 0.0431], and a 10-point greater BSS symptom severity resulted in a decrease of 29% [OR 0.71 (0.59–0.85); p = 0.0003]. Other factors associated with lower satisfaction included a 2 point greater coping strategies questionnaire (CSQ) score, which reduced the odds of achieving satisfaction by 14% [OR 0.86 (0.78–0.95); p = 0.0018], a year longer symptom duration with a decrease of 4% [OR 0.96 (0.93–1.00); p = 0.0309], 1 point in the widespread pain index with a 17% reduction [OR 0.83 (0.73–0.94); p = 0.0039]; and fibromyalgia, which decreased the odds of achieving satisfaction by 72% [OR 0.28 (0.12–0.70); p = 0.0064].
Factors demonstrating an association with improvements in at least two of three criteria, and thus overall surgical success at 4 years, include having some college education [OR 2.93 (1.27–6.77); p = 0.0120], moderate or severe stenosis on imaging (OR 7.16–7.39; p = 0.0195–0.0260), and having greater expectations for improvement and reduced limitations (OR for 10 points 1.24–1.35; p = 0.0048–0.0423). Prolonged symptom duration of an additional year [OR 0.96 (0.93–0.99); p = 0.0231] was associated with reduced odds of surgical success.
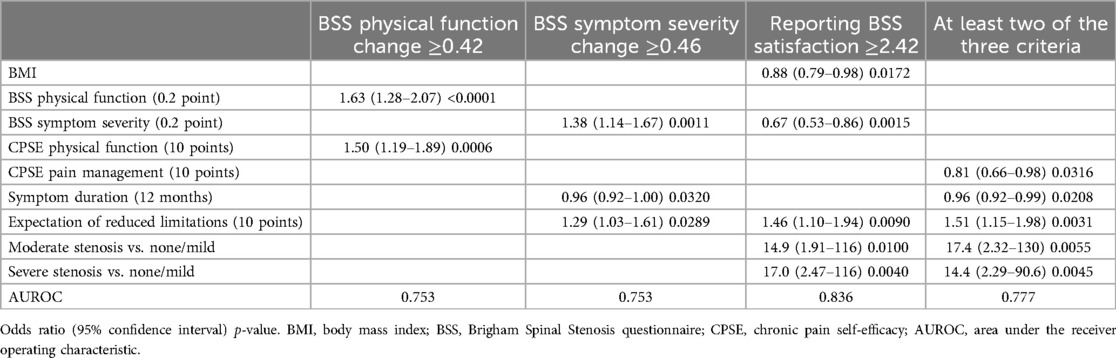
Multivariable models showed that a combination of several preoperative biopsychosocial factors could be used to predict the likelihood of 4-year surgical success (Table 4). CPSE pain management [adjusted OR or AOR for 10 points 0.81 (0.66–0.98); 0.0316], symptom duration [AOR for 12 months 0.96 (0.92–0.99); p = 0.0208], expectation of reduced limitations [AOR for 10 points 1.51 (1.15–1.98); p = 0.0031], and moderate or severe stenosis (AOR 14.4–17.4; p = 0.0045–0.0055) constituted a parsimonious subset of predictors of overall surgical success with an accuracy of 0.777 as indicated by the area under receiver operator characteristic curve (AUROC). Other criteria for improvement had similar levels of accuracy (AUROC 0.753–0.836).
Discussion
In United States Veterans with symptomatic LSS, 63% of participants demonstrated sustained DL success at 4 years, similar to the success rate at 1 year (3). Noteworthy preoperative characteristics associated with a higher likelihood of sustained, 4-year, surgical success included lower self-efficacy for pain management, shorter duration of symptoms, greater treatment credibility, and having moderate to severe anatomical stenosis.
Similar to the 1-year success rate, only about 50% of participants experienced significant functional improvement, a key outcome for older adults. Having worse baseline physical function and higher baseline self-efficacy for function were associated with a higher likelihood of significant functional improvement. Studies have highlighted the potential benefits of cognitive and physical prehabilitation prior to and immediately following spinal surgery (35–39), although heterogeneous methods prevent definitive conclusions from being drawn. Our findings suggest the potential value of future studies designed to evaluate the impact on DL outcomes of prehabilitation focused on enhancing self-efficacy.
Four-year improvements in symptom severity (e.g., pain and paresthesias) mirrored those at 1 year (3). Approximately 2/3 of participants had sustained a reduction in symptom severity. Significant predictors of 4-year symptom severity reduction were greater preoperative symptom severity, shorter symptom duration, and greater treatment credibility.
Satisfaction at 4 years post-DL also mirrors those reported at 1 year post-DL (3), with approximately 69% reporting satisfaction at 1 year and 72% reporting satisfaction at 4 years. Approximately 13% of participants at 1 year and 12% at 4 years post-DL reported being satisfied despite experiencing no significant improvement in pain or function. Predictors of significant satisfaction included lower BMI, lesser preoperative symptom severity, greater treatment credibility, and having moderate to severe anatomical stenosis. It has been highlighted in non-surgical patients with chronic pain that symptomatic improvement and satisfaction are not necessarily correlated and that the patient–provider relationship plays a prominent role in determining satisfaction with care (40). Future studies should include such contextual factors as a part of preoperative assessment.
Our findings suggest the importance of preoperative counseling that emphasizes personalized goals. If functional improvement is the patient's main goal, then preoperative efforts to optimize self-efficacy for function may be important. If reduction of symptoms is the patient's main goal, encouraging patients with more severe symptoms and anatomical pathology toward surgery may need to be considered. Future studies that include more participants are needed to validate our observations and evaluate the impact on patient outcomes of a personalized approach to care.
We note that approximately 20% of participants who demonstrated surgical success at 1 year did not demonstrate success at 4 years and vice versa. Despite most revision surgeries for simple DL being performed prior to 1 year (41), our data suggest that continued observation (vs. operative intervention) may be prudent for some participants with potentially delayed improvement. Factors that predict deterioration following initial success also require identification. While a more detailed analysis of participants with delayed improvement and delayed deterioration is beyond the scope of the present study, further investigation is warranted.
The main strength of our study was the rigorous assessment of a comprehensive set of biopsychosocial predictors, and additional preoperative factors, relevant to older adults. To our knowledge, we included the most comprehensive set of preoperative factors that have been studied in patients with LSS undergoing DL. Several study limitations also should be noted. The 4-year cohort represented approximately 57% of the original 1-year cohort, and there were some differences in baseline characteristics as noted earlier. However, the direction of the differences does not indicate the same informative censoring in typical longitudinal studies on aging, where the frailest participant dropout. In addition, our study participants were predominantly male veterans; thus, our findings may not generalize to the larger community of individuals undergoing DL for LSS. Although we observed acceptable predictive accuracy in multivariable models, there are very likely key extra-skeletal predictive factors that we did not collect such as muscle health, genetic profiles, and other biological parameters. Future studies should be conducted on a larger cohort with an even more comprehensive set of preoperative factors to optimize predictive accuracy. Estimates of predictive accuracy from an independent validation sample are more credible and necessary before definitive prediction rules can be considered.
Conclusion
Preoperative characteristics may predict long-term DL surgical success at 4 years. Further study is needed to not only establish predictive accuracy but also the impact of modifying presurgical risk factors on surgical outcomes.
Data availability statement
Due to a patient protection of veterans, the authors are limited in their capacity to provide raw data.
Ethics statement
The studies involving humans were approved by the Department of Veterans Affairs (VA) Central Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
DF: Writing – review & editing, Writing – original draft, Validation, Methodology, Investigation, Formal Analysis, Data curation. KH: Writing – review & editing, Investigation, Formal Analysis. EL: Writing – review & editing, Investigation. HK: Writing – review & editing, Investigation. SN: Writing – review & editing, Investigation. SK: Writing – review & editing, Investigation, Funding acquisition, Formal Analysis. AG: Writing – review & editing, Investigation. JK: Writing – review & editing, Investigation, Formal Analysis. SP: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal Analysis, Data curation, Conceptualization. DW: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal Analysis, Data curation, Conceptualization.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. This material is based on work supported by the Department of Veterans Affairs (VA), Veterans Health Administration, Office of Research and Development, grant number RX000798. Dr. Krein is supported by the VA Health Services Research & Development Research Career Scientist Award (RCS 11-222). The contents of this report do not represent the views of the Department of Veterans Affairs or the United States government.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmscd.2024.1493642/full#supplementary-material
References
1. Katz JN, Zimmerman ZE, Mass H, Makhni MC. Diagnosis and management of lumbar spinal stenosis: a review. JAMA. (2022) 327(17):1688–99. doi: 10.1001/jama.2022.5921
2. Long CR, Lisi AJ, Vining RD, Wallace RB, Salsbury SA, Shannon ZK, et al. Veteran response to dosage in chiropractic therapy (VERDICT): study protocol of a pragmatic randomized trial for chronic low back pain. Pain Med. (2020) 21(Supplement_2):S37–44. doi: 10.1093/pm/pnaa289
3. GBD. Disease and injury incidence and prevalence collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354, diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2018) 392(10159):1789–858. doi: 10.1016/S0140-6736(18)32279-7
4. Weiner DK, Holloway K, Levin E, Keyserling H, Epstein F, Monaco E, et al. Identifying biopsychosocial factors that impact decompressive laminectomy outcomes in veterans with lumbar spinal stenosis: a prospective cohort study. Pain. (2021) 162(3):835–45. doi: 10.1097/j.pain.0000000000002072
5. Atlas SJ, Keller RB, Wu YA, Deyo RA, Singer DE. Long-term outcomes of surgical and nonsurgical management of lumbar spinal stenosis: 8 to 10 year results from the maine lumbar spine study. Spine. (2005) 30(8):936–43. doi: 10.1097/01.brs.0000158953.57966.c0
6. Javid MJ, Hadar EJ. Long-term follow-up review of patients who underwent laminectomy for lumbar stenosis: a prospective study. J Neurosurg. (1998) 89(1):1–7. doi: 10.3171/jns.1998.89.1.0001
7. Jönsson B, Annertz M, Sjöberg C, Strömqvist B. A prospective and consecutive study of surgically treated lumbar spinal stenosis. Part II: five-year follow-up by an independent observer. Spine. (1997) 22(24):2938–44. doi: 10.1097/00007632-199712150-00017
8. Lang Z, Li JS, Yang F, Yu Y, Khan K, Jenis LG, et al. Reoperation of decompression alone or decompression plus fusion surgeries for degenerative lumbar diseases: a systematic review. Eur Spine J. (2019) 28(6):1371–85. doi: 10.1007/s00586-018-5681-2
9. Martin BI, Mirza SK, Comstock BA, Gray DT, Kreuter W, Deyo RA. Are lumbar spine reoperation rates falling with greater use of fusion surgery and new surgical technology? Spine (Phila Pa 1976). (2007) 32(19):2119–26. doi: 10.1097/BRS.0b013e318145a56a
10. Iguchi T, Kurihara A, Nakayama J, Sato K, Kurosaka M, Yamasaki K. Minimum 10-year outcome of decompressive laminectomy for degenerative lumbar spinal stenosis. Spine. (2000) 25(14):1754–9. doi: 10.1097/00007632-200007150-00003
11. Foulongne E, Derrey S, Ould Slimane M, Levèque S, Tobenas A, Dujardin F, et al. Lumbar spinal stenosis: which predictive factors of favorable functional results after decompressive laminectomy? Neurochirurgie. (2013) 59(1):23–9. doi: 10.1016/j.neuchi.2012.09.005
12. McIlroy S, Walsh E, Sothinathan C, Stovold E, Norwitz D, Norton S, et al. Pre-operative prognostic factors for walking capacity after surgery for lumbar spinal stenosis: a systematic review. Age Ageing. (2021) 50(5):1529–45. doi: 10.1093/ageing/afab150
13. Folstein MF, Folstein SE, McHugh PR. Mini-mental state. J Psychiatr Res. (1975) 12(3):189–98. doi: 10.1016/0022-3956(75)90026-6
14. Fokter SK, Yerby SA. Patient-based outcomes for the operative treatment of degenerative lumbar spinal stenosis. Eur Spine J. (2006) 15(11):1661–9. doi: 10.1007/s00586-005-0033-4
15. Tuli SK, Yerby SA, Katz JN. Methodological approaches to developing criteria for improvement in lumbar spinal stenosis surgery. Spine. (2006) 31(11):1276–80. doi: 10.1097/01.brs.0000217615.20018.6c
16. Watters WC, Baisden J, Gilbert TJ, Kreiner S, Resnick DK, Bono CM, et al. Degenerative lumbar spinal stenosis: an evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spinal stenosis. Spine J. (2008) 8(2):305–10. doi: 10.1016/j.spinee.2007.10.033
17. Airaksinen O, Herno A, Turunen V, Saari T, Suomlainen O. Surgical outcome of 438 patients treated surgically for lumbar spinal stenosis. Spine. (1997) 22(19):2278–82. doi: 10.1097/00007632-199710010-00016
18. Winter RB, Lonstein JE, Denis F. Sagittal spinal alignment: the true measurement, norms, and description of correction for thoracic kyphosis. J Spinal Disord Tech. (2009) 22(5):311–4. doi: 10.1097/BSD.0b013e31817dfcc3
19. Sabharwal S, Kumar A. Methods for assessing leg length discrepancy. Clin Orthop. (2008) 466(12):2910–22. doi: 10.1007/s11999-008-0524-9
20. Rigler SK, Studenski S, Wallace D, Reker DM, Duncan PW. Co-morbidity adjustment for functional outcomes in community-dwelling older adults. Clin Rehabil. (2002) 16(4):420–8. doi: 10.1191/0269215502cr515oa
21. Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz RL, et al. 2016 revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. (2016) 46(3):319–29. doi: 10.1016/j.semarthrit.2016.08.012
22. O’Caoimh R, Svendrovski A, Johnston BC, Gao Y, McGlade C, Eustace J, et al. The quick mild cognitive impairment screen correlated with the standardized Alzheimer’s disease assessment scale-cognitive section in clinical trials. J Clin Epidemiol. (2014) 67(1):87–92. doi: 10.1016/j.jclinepi.2013.07.009
23. Bastien CH, Vallières A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. (2001) 2(4):297–307. doi: 10.1016/S1389-9457(00)00065-4
24. Weiner DK, Rudy TE, Morrow L, Slaboda J, Lieber S. The relationship between pain, neuropsychological performance, and physical function in community-dwelling older adults with chronic low back pain. Pain Med. (2006) 7(1):60–70. doi: 10.1111/j.1526-4637.2006.00091.x
25. McDonald SD, Calhoun PS. The diagnostic accuracy of the PTSD checklist: a critical review. Clin Psychol Rev. (2010) 30(8):976–87. doi: 10.1016/j.cpr.2010.06.012
26. Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain. (1983) 17(1):33–44. doi: 10.1016/0304-3959(83)90125-2
27. Anderson KO, Dowds BN, Pelletz RE, Edwards TW, Peeters-Asdourian C. Development and initial validation of a scale to measure self-efficacy beliefs in patients with chronic pain. Pain. (1995) 63(1):77–83. doi: 10.1016/0304-3959(95)00021-J
28. George SZ, Valencia C, Beneciuk JM. A psychometric investigation of fear-avoidance model measures in patients with chronic low back pain. J Orthop Sports Phys Ther. (2010) 40(4):197–205. doi: 10.2519/jospt.2010.3298
29. Yudko E, Lozhkina O, Fouts A. A comprehensive review of the psychometric properties of the drug abuse screening test. J Subst Abuse Treat. (2007) 32(2):189–98. doi: 10.1016/j.jsat.2006.08.002
30. Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. (1991) 32(6):705–14. doi: 10.1016/0277-9536(91)90150-b
31. Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry. (2000) 31(2):73–86. doi: 10.1016/S0005-7916(00)00012-4
32. Jarvik JJ, Hollingworth W, Heagerty P, Haynor DR, Deyo RA. The longitudinal assessment of imaging and disability of the back (LAIDBack) study: baseline data. Spine. (2001) 26(10):1158–66. doi: 10.1097/00007632-200105150-00014
33. Weinstein JN, Lurie JD, Tosteson TD, Hanscom B, Tosteson AN, Blood EA, et al. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med. (2007) 356(22):2257–70. doi: 10.1056/NEJMoa070302
34. Park DK, An HS, Lurie JD, Zhao W, Tosteson A, Tosteson TD, et al. Does multilevel lumbar stenosis lead to poorer outcomes? A subanalysis of the SPORT lumbar stenosis study. Spine. (2010) 35(4):439–46. doi: 10.1097/BRS.0b013e3181bdafb9
35. Parrish JM, Jenkins NW, Parrish MS, Cha ED, Lynch CP, Massel DH, et al. The influence of cognitive behavioral therapy on lumbar spine surgery outcomes: a systematic review and meta-analysis. Eur Spine J. (2021) 30(5):1365–79. doi: 10.1007/s00586-021-06747-x
36. Punnoose A, Claydon-Mueller LS, Weiss O, Zhang J, Rushton A, Khanduja V. Prehabilitation for patients undergoing orthopedic surgery: a systematic review and meta-analysis. JAMA Netw Open. (2023) 6(4):e238050. doi: 10.1001/jamanetworkopen.2023.8050
37. McGregor AH, Probyn K, Cro S, Doré CJ, Burton AK, Balagué F, et al. Rehabilitation following surgery for lumbar spinal stenosis. A Cochrane review. Spine. (2014) 39(13):1044–54. doi: 10.1097/BRS.0000000000000355
38. Archer KR, Devin CJ, Vanston SW, Koyama T, Phillips SE, Mathis SL, et al. Cognitive-behavioral-based physical therapy for patients with chronic pain undergoing lumbar spine surgery: a randomized controlled trial. J Pain. (2016) 17(1):76–89. doi: 10.1016/j.jpain.2015.09.013
39. Özden F. The effect of exercise interventions after lumbar decompression surgery: a systematic review and meta-analysis. World Neurosurg. (2022) 167:e904–21. doi: 10.1016/j.wneu.2022.08.103
40. Yamashita K, Hayashi J, Ohzono K, Hiroshima K. Correlation of patient satisfaction with symptom severity and walking ability after surgical treatment for degenerative lumbar spinal stenosis. Spine. (2003) 28(21):2477–81. doi: 10.1097/01.BRS.0000090888.63860.4F
Keywords: lumbar spinal stenosis, decompressive laminectomy, long-term follow-up, function, symptom, veterans, lumbar stenosis
Citation: Fields DP, Holloway KL, Levin E, Keyserling H, Nortman S, Krein SL, Gentili A, Katz JN, Perera S and Weiner DK (2025) Preoperative factors and four-year decompressive laminectomy success in symptomatic lumbar spinal stenosis. Front. Musculoskelet. Disord. 2:1493642. doi: 10.3389/fmscd.2024.1493642
Received: 9 September 2024; Accepted: 19 November 2024;
Published: 7 January 2025.
Edited by:
Maruti Gudavalli, Keiser University, United StatesReviewed by:
Łukasz A. Poniatowski, Medical University of Warsaw, PolandTalgat Kerimbayev, National Center for Neurosurgery, Kazakhstan
Copyright: © 2025 Fields, Holloway, Levin, Keyserling, Nortman, Krein, Gentili, Katz, Perera and Weiner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Debra K. Weiner, ZHdlaW5lckBwaXR0LmVkdQ==
 Daryl P. Fields
Daryl P. Fields Kathryn Lois Holloway
Kathryn Lois Holloway Emily Levin
Emily Levin Harold Keyserling
Harold Keyserling Samuel Nortman
Samuel Nortman Sarah L. Krein4,10
Sarah L. Krein4,10 Debra K. Weiner
Debra K. Weiner