
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Mol. Neurosci., 08 January 2025
Sec. Molecular Signalling and Pathways
Volume 17 - 2024 | https://doi.org/10.3389/fnmol.2024.1482015
This article is part of the Research TopicMechanisms of Ferroptosis, Necroptosis, and Pyroptosis in Neurological Disorders, Brain Injuries, and Brain-Related DiseasesView all articles
 He-Yan Tian1
He-Yan Tian1 Yun-Xing Lei1
Yun-Xing Lei1 Jing-Tao Zhou1
Jing-Tao Zhou1 Long-Jun Liu1
Long-Jun Liu1 Tong Yang2
Tong Yang2 Yue Zhou2
Yue Zhou2 Jin-Wen Ge2,3*
Jin-Wen Ge2,3* Chen Xu1*
Chen Xu1* Zhi-Gang Mei2*
Zhi-Gang Mei2*PANoptosis is a novelly defined mode of programmed cell death that involves the activation of multiple cellular death pathways, including pyroptosis, apoptosis, and necroptosis, triggering robust inflammatory reactions. Autophagy is a crucial cellular process that maintains cellular homeostasis and protects cells from various stresses. PANoptosis and autophagy, both vital players in the intricate pathological progression of ischemic stroke (IS), a brain ailment governed by intricate cell death cascades, have garnered attention in recent years for their potential interplay. While mounting evidence hints at a crosstalk between these two processes in IS, the underlying mechanisms remain elusive. Therefore, this review delves into and dissects the intricate mechanisms that underpin the intersection of PANoptosis and autophagy in this devastating condition. In conclusion, the crosstalk between PANoptosis and autophagy in IS presents a promising target for the development of novel stroke therapies. Understanding the interplay between these two pathways offers a much-needed insight into the underlying mechanisms of IS and opens the possibility for new therapeutic strategies.
Stroke, a leading culprit of mortality and disability in China, causes 5.5 million deaths and 44 million physical disabilities around the world per year (Collaborators, 2019), which is a disease of immense public health importance with serious economic and social consequences (Barthels and Das, 2020; Zhou M. et al., 2019). IS comprises 70–80% of all stroke incidents globally, characterized by disrupted blood flow to the brain tissues. This interruption triggers a complex cascade of pathophysiological events that ultimately leads to various forms of cell death, notably necroptosis, apoptosis, and pyroptosis, among others (Davidson et al., 2020). PANoptosis is a newly described form of programmed cell death that involves the activation of multiple cellular death pathways, including pyroptosis, apoptosis and necroptosis, resulting in a potent inflammatory response. Autophagy is a crucial cellular process that maintains cellular homeostasis and protects cells from various stresses. Recent studies have highlighted the dual role of PANoptosis and autophagy in both initiating and exacerbating brain tissue damage in the aftermath of cerebral ischemia and cerebral ischemia/reperfusion injury (CIRI), emphasizing their intricate involvement in disease progression (Chen et al., 2019; Degterev et al., 2005; Zhang et al., 2020). Tissue-type plasminogen activator (tPA) stands as the sole thrombolytic agent currently approved for clinical application in the management of IS. However, owing to the narrow therapeutic window (<4.5 h) and hemorrhagic transformation, its usage in the treatment of IS has been limited (Alawieh et al., 2018; Henderson et al., 2018). Given the aforementioned reasons, research has delved into interventions that specifically target distinct forms of programmed cell death, aiming to offer innovative therapeutic avenues for IS (Chen et al., 2018; Liu D. et al., 2020). Nonetheless, a growing body of evidence suggests that a substantial interplay exists between PANoptosis and autophagy in the aftermath of IS (Gonzalez-Rodriguez and Fernandez-Lopez, 2023; Wang J. F. et al., 2018; Xu et al., 2017; Yang et al., 2022). This review aims to consolidate the understanding of the intricate mechanisms underlying the crosstalk between PANoptosis and autophagy in IS. Uncovering these mechanisms could potentially unveil novel therapeutic targets, thereby advancing the treatment landscape for IS (Table 1).
Regulated cell death holds a pivotal role in safeguarding the host. PANoptosis, a newly recognized modality of programmed cell death, encompasses the orchestration of diverse cell death pathways, such as pyroptosis, apoptosis, and necroptosis. Research underscores the existence of an intricate, dynamic molecular interplay among these processes, indicating that their individual cell death mechanisms are not mutually exclusive. Extensive literature has already discussed the distinctions between pyroptosis, apoptosis, and necroptosis. In the following discussion, our primary focus lies in examining the interplay and regulatory mechanisms that govern the relationships between pyroptosis, apoptosis, and necroptosis.
Both pyroptosis and apoptosis involve the activation of Caspase family members, hinting at a shared evolutionary ancestry. Caspases can be categorized into inflammatory caspases (including caspase-1, 4, 5, and 11) and apoptosis-specific caspases (encompassing caspase-3, 6, 7, 8, 9, and 10), further underscoring their distinct yet interconnected roles (Husain, 2022; Kesavardhana et al., 2020a). Nevertheless, Research has revealed that caspase-3 and caspase-8 play pivotal roles in mediating pyroptosis, demonstrating their expanded functional spectrum beyond the realm of apoptosis (Sarhan et al., 2018; Wang C. P. et al., 2017). Caspase-8 is recognized as a versatile molecular switch that governs cell fate, capable of orchestrating apoptosis, pyroptosis, or necroptosis, underscoring its pivotal role in regulating diverse cell death pathways (Fritsch et al., 2019; Jiang et al., 2021).
Gasdermin D (GSDMD) plays a pivotal role in the activation of pyroptosis by cleavage and activation of the protein (Shi et al., 2015). When cells encounter danger signals such as bacterial lipopolysaccharides (LPS) or extracellular ATP, they activate pattern recognition receptors (PRRs) that trigger the formation of inflammasomes. The inflammasomes then activate caspase-1, which cleaves GSDMD at a specific site to release an N-terminal fragment that contains an oligomerization domain. This domain assembles into pore-like structures made up of several GSDMD subunits, causing the cells to lyse and release pro-inflammatory cytokines (Ding et al., 2016).
RIP3 protein (Receptor Interacting Protein Kinase 3) plays a key role in the regulation of necroptosis, which is activated in response to several stimuli, including the binding of certain ligands to TNFR1 (tumor necrosis factor receptor-1), a cell surface receptor involved in inflammation and immunity (Humphries et al., 2015). Upon activation, RIP3 phosphorylates and activates another protein called MLKL (Mixed Lineage Kinase domain-like), which mediates the downstream signaling events that lead to necroptosis.
MLKL, a crucial player in necroptosis, has also been implicated in activating the NLRP3 inflammasome, highlighting its multifaceted role in the immune response and cell death machinery (Frank and Vince, 2019). The study has showed that MLKL can directly activate the NLRP3 inflammasome in response to certain stimuli, leading to the release of mature IL-1β and IL-18 cytokines and the induction of pyroptosis (Sanchez-Fernandez et al., 2019). MLKL can also indirectly activate the NLRP3 inflammasome by promoting the release of mitochondrial DNA (mtDNA) from damaged mitochondria. This mtDNA release triggers the assembly of the NLRP3 inflammasome and the subsequent induction of pyroptosis (Han et al., 2019).
ZBP1 is an essential cytoplasmic protein involved in a range of cellular functions, including immune responses, cell death, and RNA metabolism. When activated, ZBP1 triggers a complex sequence of events that can lead to various forms of programmed cell death, namely necroptosis, apoptosis, and pyroptosis, collectively known as PANoptosis. This activation process engages key signaling molecules, such as receptor-interacting protein kinase 3 (RIP3), caspase-8, and the NLRP3 inflammasome (Kesavardhana et al., 2020b; Zheng et al., 2020; Zheng and Kanneganti, 2020). ZBP1 is recognized as a vital component of the PANoptosome, a multiprotein complex that regulates these cell death pathways. The PANoptosome is composed of various receptors, including ZBP1 and inflammasome components, along with adapters like ASC (apoptosis-associated speck-like protein containing a CARD) and FADD (Fas-associated protein with death domain), as well as catalytic effectors such as caspase-1, RIP3, RIP1, and caspase-8. This assembly acts as a central hub for integrating signals that determine cell fate under stress conditions. A key aspect of ZBP1 is its Zα2 domain, which is crucial for activating NLRP3 and initiating PANoptosis. This underscores ZBP1’s pivotal role in the regulatory framework governing these cell death processes. When ZBP1 or its Zα2 domain is lost or impaired, significant consequences arise: activation of NLRP3 is markedly reduced, leading to decreased pyroptosis, which is characterized by the release of pro-inflammatory cytokines. Additionally, there is a reduction in the cleavage of executioner caspases, such as caspase-3, caspase-7, and caspase-8, which are vital for the apoptotic process. Moreover, the phosphorylation of MLKL (mixed lineage kinase domain-like protein), an important event in necroptosis, is also diminished. These findings highlight the critical role of ZBP1 and its Zα2 domain in the regulation of PANoptosis. By activating essential pathways involved in cellular stress responses, ZBP1 contributes to maintaining cellular homeostasis and plays a significant role in the immune response to pathogens and cellular injury. A deeper understanding of ZBP1’s multifaceted functions and its interactions within the PANoptosome could offer valuable insights for developing therapeutic strategies targeting diseases associated with dysregulated cell death.
RIP1 Regulation in PANoptosis: Essential for Cell Death and Inflammatory Responses (Lalaoui et al., 2020; Tao et al., 2020). In the context of PANoptosis, RIP1 undergoes intricate regulation, exhibiting dualistic roles that are highly context-dependent. Its function alternates between fostering cellular survival and inducing cell death, contingent upon the specific cellular milieu and the presence of regulatory molecules. The loss of RIP1 can trigger RIP3-mediated PANoptosis via caspase-8 and FADD, ultimately resulting in cell death (Dillon et al., 2014). The functionality of RIP1 is further influenced by phosphorylation at multiple sites, which triggers distinct cellular outcomes. Under specific conditions, the phosphorylation of RIP1 promotes the formation of the necrosome, a necessary step for initiating necroptosis. This modification serves as a signal for recruiting additional proteins involved in necroptosis, leading to a cascade of events that ultimately result in cell death. Conversely, dephosphorylated RIP1 enhances its interaction with caspases, guiding the cell toward apoptosis. This dual role highlights the complexity of RIP1’s involvement in determining cell fate. Additionally, various regulatory proteins are essential for modulating RIP1’s activity. Cellular inhibitors of apoptosis proteins (cIAPs) ubiquitinate RIP1, enhancing its pro-survival effects by preventing degradation and promoting survival signaling pathways. In contrast, the deubiquitinating enzyme CYLD removes ubiquitin chains from RIP1, thereby activating mechanisms that lead to cell death. This dynamic interaction between cIAPs and CYLD is crucial for balancing cell survival and death, underscoring the finely tuned regulatory networks that control RIP1’s function. In essence, RIP1’s intricate regulation in PANoptosis underscores the complexity of this programmed cell death and emphasizes the imperative for deeper research to unravel its signaling pathways and potential therapeutic avenues.
RIP3 is a serine/threonine kinase that plays a vital role in regulating programmed cell death, particularly necroptosis. High levels of RIP3 expression lead cells toward necroptosis, while lower levels favor apoptosis. Additionally, RIP3 promotes the release of IL-1β through the NLRP3-caspase-1 pathway (Frank and Vince, 2019). These findings suggest that RIP3 is crucial in balancing pyroptosis, apoptosis, and necroptosis. RIP3 is activated by the phosphorylation of RIP1, which facilitates the recruitment of other proteins necessary for necroptosis—a form of inflammatory cell death characterized by cell swelling and membrane rupture. This process differs from apoptosis and is associated with various pathological conditions, including neurodegenerative diseases, ischemic injury, and certain inflammatory disorders.
Inflammasomes, intricate multiprotein complexes, are central in regulating PANoptosis, a comprehensive cell death process (Oh et al., 2023). These assemblies consist of pattern recognition receptors (PRRs), an adapter protein, and caspase-1, which, when activated by pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), initiate pyroptosis. Activated caspase-1 cleaves and releases pro-inflammatory cytokines IL-1β and IL-18, as well as gasdermin D (GSDMD), facilitating pyroptotic cell death. In the context of PANoptosis, inflammasomes act as key integrators, enabling communication between pyroptosis and other cell death pathways. The activation of caspase-1, along with various effectors, orchestrates the simultaneous activation of multiple death mechanisms, resulting in PANoptosis.
There are five identified inflammasome sensors: NLRP1, NLRP3, absent in melanoma 2 (AIM2), NLRC4, and Pyrin, with the latter four playing direct roles in PANoptosis. NLRP3 activation and GSDMD-mediated pyroptosis are particularly important in the relationship between apoptosis and necroptosis. Upon activation of the NLRP3 inflammasome, inflammatory cells engage in PANoptosis. Although the knockdown of NLRP3 or GSDMD reduces early cell death, the overall incidence of inflammatory cell death rises over time, driven by caspase-8 and RIP3. This suggests that in the absence of NLRP3 or pyroptosis, cell death becomes dependent on caspase-8 and RIP3 (Zheng et al., 2020). During P. aeruginosa infection, the lack of NLRC4 results in the activation of alternative cell death pathways involving RIP1 and MLKL, while the activation of caspases 1, 3, 7, and 8 is reduced. This highlights the critical role of NLRC4 in the PANoptosis process (Sundaram et al., 2022).
Overall, inflammasomes are essential regulators of PANoptosis, with their orchestration of pyroptosis being vital for maintaining immune balance and influencing pathological conditions. Ongoing research is focused on elucidating their complex mechanisms and exploring their therapeutic potential in the context of PANoptosis.
PANoptosis, a term coined to encompass a broader spectrum of cell death modalities beyond the classical apoptosis, autophagy-dependent cell death, and necroptosis, refers to a concept that encompasses multiple regulated cell death pathways that can be triggered simultaneously or sequentially under certain stress conditions. This holistic view of cell death recognizes that cells, particularly in response to severe insults or pathological states like ischemic stroke, may engage in a complex interplay of molecular mechanisms leading to their demise. In the context of ischemic stroke, a sudden interruption of blood flow to a part of the brain, PANoptosis becomes particularly relevant as it highlights the intricate interplay of various cell death mechanisms that contribute to tissue damage and neurological dysfunction. Ischemic stroke triggers a cascade of events, including energy depletion, oxidative stress, excitotoxicity, and inflammation, which ultimately lead to neuronal and glial cell death (Figure 1).
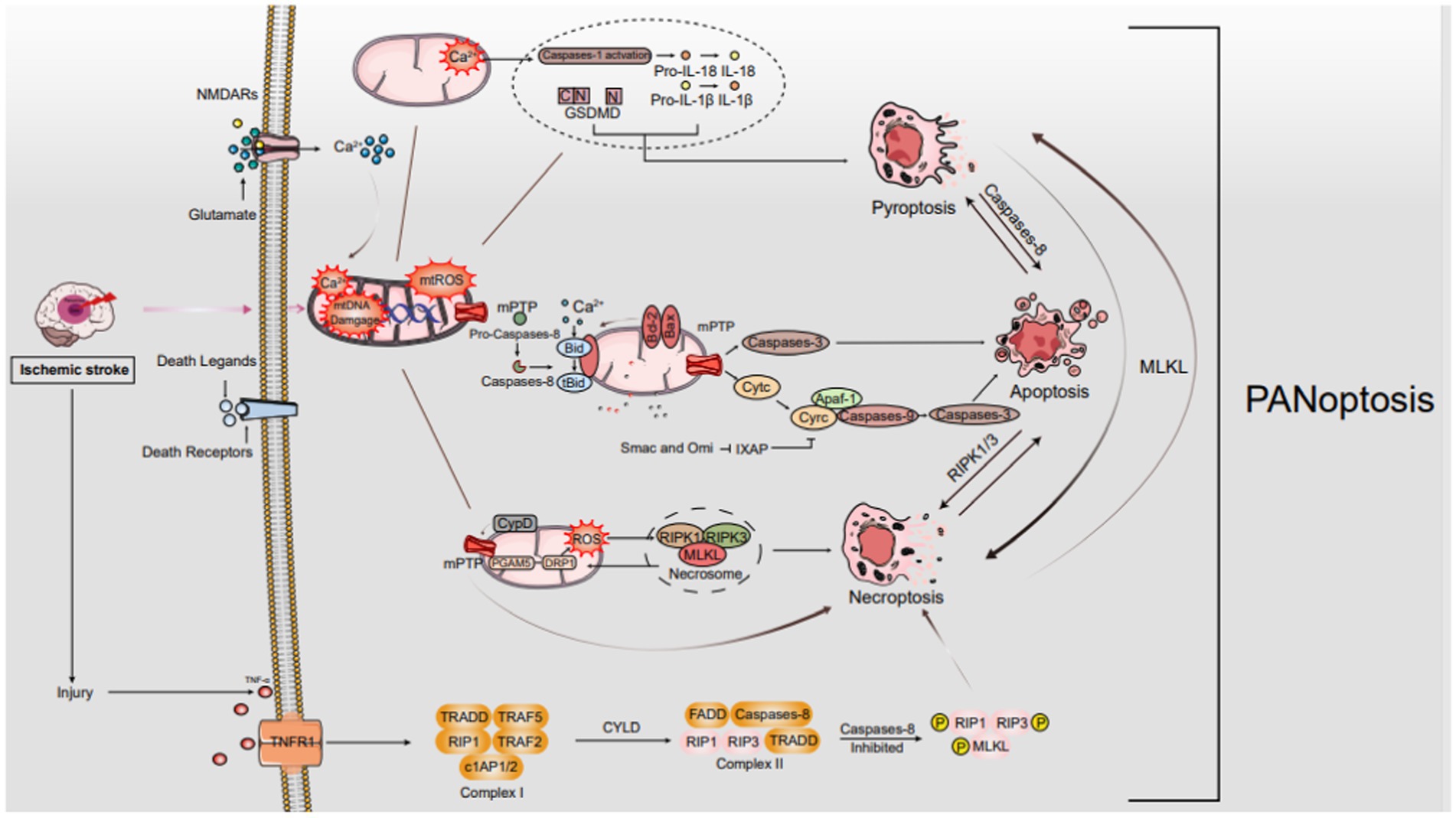
Figure 1. The mechanisms of PANoptosis in IS. In ischemic stroke, oxygen–glucose deprivation sparks a cascade leading to programmed cell death, notably PANoptosis. It starts with ATP depletion and death ligand binding to membrane receptors. As ischemia intensifies, Na+/K+ ATPase failure causes neuronal depolarization, unleashing glutamate, ROS, free radical damage, Ca2+ imbalance, and EAA toxicity. Death ligand-receptor interactions trigger signaling cascades that selectively engage FADD for apoptosis or TRADD for necroptosis. These cascades disrupt mitochondria, manifesting as mPTP formation, mtROS surge, Ca2+ overload, and mtDNA damage. These mitochondrial perturbations are crucial in orchestrating diverse cell death pathways, encompassing apoptosis, necroptosis, and possibly other PANoptosis modalities.
The mechanisms of PANoptosis in IS. In ischemic stroke, oxygen–glucose deprivation sparks a cascade leading to programmed cell death, notably PANoptosis. It starts with ATP depletion and death ligand binding to membrane receptors. As ischemia intensifies, Na+/K+ ATPase failure causes neuronal depolarization, unleashing glutamate, ROS, free radical damage, Ca2+ imbalance, and EAA toxicity. Death ligand-receptor interactions trigger signaling cascades that selectively engage FADD for apoptosis or TRADD for necroptosis. These cascades disrupt mitochondria, manifesting as mPTP formation, mtROS surge, Ca2+ overload, and mtDNA damage. These mitochondrial perturbations are crucial in orchestrating diverse cell death pathways, encompassing apoptosis, necroptosis, and possibly other PANoptosis modalities.
Recent discoveries on PCD pathways’ intricate interplay reveal multifaceted signaling platforms. Pyroptosis, apoptosis, and necroptosis often coexist in cellular stress, contributing to neuroinflammation with health implications. PANoptosis’s components linked to neurological disorders underscore the potential of unraveling its mechanisms for innovative therapies (Malireddi et al., 2020). PANoptosis is often associated with inflammatory reactions in various central nervous system (CNS) diseases (Lunemann et al., 2021; McKenzie et al., 2020; Yan et al., 2021). Core components of the PANoptosome, such as the inflammasome, caspase-8, RIPK1, and others, have been linked to neuronal death in various contexts (Degterev et al., 2019; Fricker et al., 2018). Inflammation and activation of the immune system are frequently implicated in the pathophysiology of IS, which can result in severe brain damage (Zhang F. et al., 2022). Existing studies on PANoptosis have observed similarities in the expression of cell death and the underlying pathophysiological mechanisms related to inflammation in IS. These findings provide fundamental evidence supporting the potential existence of PANoptosis and PANoptosomes (Yan et al., 2022). Furthermore, it has been reported that glial cells can modulate these three forms of cell death following injury, which aligns with the findings in existing studies of PANoptosis that are related to inflammation and the immune system (Liu X. et al., 2022). Additionally, research has indicated that certain molecules can concurrently affect two components of PANoptosis during IS. Notably, RIPK3, a key mediator of necroptosis, engages in crosstalk with the Jun N-terminal kinase-driven inflammatory signaling cascade, which is intimately tied to neuronal apoptosis processes (Hu et al., 2020). Inhibiting thromboxane A synthase/thromboxane A2/thromboxane prostanoid signaling has been demonstrated to concurrently suppress both apoptosis and pyroptosis (Chueh et al., 2020). Furthermore, the nucleotide oligomerization domain-like receptors with caspase activation and recruitment domain 4 (NLRC4) inflammasome complex has the capacity to simultaneously modulate both apoptosis and pyroptosis (Poh et al., 2019). Therefore, it is possible to simultaneously regulate and intervene in PANoptosis induced by IS.
Although PANoptosome’s direct role in IS is unexplored, its components are abundant in the brain. Research shows inhibiting TAK1 reduces neuronal death from these conditions, hinting at PANoptosome’s potential involvement (Wang J. X. et al., 2019; Wu W. X. et al., 2020). Moreover, TAK1 plays a crucial role in modulating microglial function and its interaction with an inflammatory pathway that triggers neuronal apoptosis and pyroptosis (Zeyen et al., 2020). Furthermore, it plays a critical role in the crosstalk between necroptosis and apoptosis in neurons during IS (Naito et al., 2020). These findings hint at TAK1’s regulatory role in PANoptosomes during IS. Yan et al.’s study shows PANoptosis-like death in R28 retinal cells exposed to oxygen–glucose deprivation/recovery, a model mimicking ischemia–reperfusion. Combined inhibitors targeting apoptosis, pyroptosis, and necroptosis effectively reduced cell death, especially when combined, confirming PANoptosis’s presence in R28 cells (Yan et al., 2023).
Introduced by Christian de Duve in 1963, “autophagy” stems from Greek roots for “self-eating.” (De Duve and Wattiaux, 1966). This conserved mechanism in eukaryotes maintains cellular balance by degrading organelles, proteins, lipids, and nucleic acids, recycling their components, especially during nutrient deprivation. Autophagy also eliminates pathogens, damaged organelles, and abnormal proteins. Dysregulation of autophagy is associated with diseases like neurodegeneration, infections, inflammation, metabolic disorders, and aging (Dikic and Elazar, 2018).
Autophagy falls into two main types: bulk/non-selective and selective. Bulk autophagy degrades various cytoplasmic components indiscriminately. In contrast, selective autophagy targets specific cargos, often those posing threats or requiring removal. It encompasses diverse pathways, each targeting distinct cellular components like mitochondria, lysosomes, protein/RNA aggregates, pathogens, ER, peroxisomes, ribosomes, ferritin, glycogen, lipid droplets, and fluid. Effective degradation of these cargos is crucial for physiological functions, and failure can lead to diseases. These cargos are intricately tied to cellular health and function (Jiang and Mizushima, 2014). Both autophagy types involve autophagosome formation, spherical double-membranes engulfing cellular content for degradation. This process is tightly regulated by Atg proteins, conserved from yeast to mammals. Over 40 Atg genes exist, with core Atg1-18 crucial for both autophagy types. These proteins orchestrate autophagosome formation and function, vital for maintaining cellular homeostasis and eliminating unwanted/damaged components.
Over 30 autophagy-related (Atg) proteins participate in autophagy, categorized into four key functional groups: (1) Initiation Complex: This comprises unc-51-like kinase 1 (ULK1), Atg13, and Atg17, which collaboratively initiate the autophagy process. (2) Class III PI3K Complex for Autophagy Activation: Constituted by phosphatidylinositol 3-kinase (PI3K), Beclin1, Atg14, and AMBRA1, this complex fosters phagophore formation, a crucial step in autophagy. (3) Atg12 Conjugation System: Involving Atg12, Atg5, Atg16L1, and Atg10, this system orchestrates the conjugation reactions essential for autophagy progression. (4) The LC3 (Light Chain 3) conjugation and lipidation system:LC3 exists as LC3-I (cytosolic, inactive) and LC3-II (lipidated, active). The conversion from LC3-I to LC3-II involves activation by ATG7, conjugation with ATG3, and lipidation by the ATG5-ATG12 complex. LC3B-I levels indicate autophagy activity, while LC3B-II marks autophagosome formation and interacts with autophagy receptors for selective substrate degradation (Yoshii and Noboru, 2017). LC3-II and p62 (SQSTM1) are autophagy markers. LC3-II decorates autophagosome membranes, while p62 tags cargo for degradation. High LC3-II levels (more autophagosomes) and low p62 (efficient cargo degradation) signify increased autophagy flux. Flux covers autophagosome formation, fusion with lysosomes, and cargo degradation. Fusion disruption or lysosomal dysfunction elevates both markers. Thus, autophagy studies should employ multiple markers and techniques for clarity (Klionsky et al., 2021).
Multiple signaling pathways, responsive to both internal and external cues, coordinate autophagy. Here, we focus on pathways that may explain gender-specific autophagy differences in stroke (Martens, 2018). mTOR, a serine/threonine kinase, dually regulates autophagy via ULK1 phosphorylation. Central to cell processes, mTOR comprises mTORC1 and mTORC2. Rapamycin-sensitive mTORC1 drives anabolism and inhibits autophagy, especially in the brain. Rapamycin-insensitive mTORC2 contributes to Akt activation and diverse signaling pathways (Shi et al., 2019). Asiaticoside, in a rat model of dementia, effectively diminishes autophagic activity and enhances memory performance by regulating the mTOR signaling pathway (Guo et al., 2021). During ischemia and reperfusion processes, mTOR plays a crucial role in the regulation of autophagy through two primary pathways: the AMPK-mTOR pathway and the Akt–mTOR pathway (Wang et al., 2020).
The 5′-AMP-activated protein kinase (AMPK) is a serine/threonine kinase that becomes activated in response to a decrease in the ATP/AMP ratio, a condition often observed after an IS. AMPK exerts its effect by inhibiting mTOR, leading to the subsequent activation of autophagy (Ke et al., 2018). Once activated, AMPK initiates the activation of the ULK1 complex, which subsequently undergoes autophosphorylation. This autophosphorylation event is essential for phosphorylating specific autophagy-initiating protein complexes, thereby initiating the process of autophagy (Thapa et al., 2021). Autophagy, which is mediated by AMPK, plays a crucial role in the neuroprotection afforded by ischemia preconditioning. This suggests that AMPK holds promise as a potential therapeutic target for IS. Evidence further indicates that the AMPK-mTOR signaling pathway effectively regulates autophagy activation through the coordinated phosphorylation of ULK1 (Wang Z. et al., 2018). Simultaneously, PI3K enzymes play a role in the modulation of the Akt–mTOR pathway. PI3K serves as a critical regulator of autophagy and is involved in phagosome maturation. There are three main classes of PI3K: Class I, II, and III. Class III PI3K(Vps34), takes part in complex formation with beclin-1, contributing to the regulation of autophagy. In contrast, class I PI3Ks are primarily involved in the activation of Akt (Noorolyai et al., 2019).
The MAPK signaling pathway comprises p38 extracellular regulated protein kinases (p38-ERK), ERK, and C-Jun N-terminal kinase (JNK) (Kim and Choi, 2015). Early stroke, p38 MAPK activation supports neuronal survival, anti-inflammation, and anti-apoptosis via Elk1, CHOP10, LEF2C, and MAPKK2/3. Later, p38 overactivation triggers target gene expression, activating transcription factors and caspases (Ferrer et al., 2003; Li et al., 2015; Song et al., 2016). indorsing neuronal apoptosis. It is evident that interventions for IS should be tailored to target specific p38 MAPK signal molecules. Notably, p38 inhibitors enhance cell survival routes, such as ERK, and alleviate ischemic mitochondrial fragmentation and autophagy. This, in turn, reduces the volume of ischemic cerebral infarction and preserves nerve function (Zhang et al., 2015). This evidence supports the notion that ERK, JNK, and p38 MAPK are involved in mediating autophagy in IS, with distinctive roles in modulating this process.
NF-κB, a transcription factor, governs the expression of numerous genes (Chen et al., 2011). In ischemic conditions, IκappaB kinase (IKK) becomes activated and subsequently phosphorylates IκBa. This leads to the release of NF-κB, which is then translocated into the nucleus to facilitate autophagy (Jiang et al., 2012). Notably, in mouse models, the knockout of the NF-κB p50 subunit has been observed to promote autophagy by inhibiting the mTOR pathway after cerebral ischemia (Li et al., 2013). This insight sheds light on the complex regulatory mechanisms at play during ischemic conditions and their impact on autophagy processes. Also, it has been demonstrated that the NF-κB-dependent p53 signaling pathway is involved in the cellular response after ischemic events (Cui et al., 2013). In the context of brain ischemia/reperfusion, it has been observed that p53-dependent nuclear factor NF-κB expression is induced. Simultaneously, the damage-regulated autophagy modulator (DRAM) serves as a crucial regulator of p53-dependent autophagy. This DRAM-mediated NF-κB/p53 pathway plays an active role during the ischemia/reperfusion process, contributing to both apoptosis and autophagic cell death (Gupta et al., 2019). Moreover, it’s worth noting that autophagy and apoptotic pathways may interplay to influence programmed cell death by modulating the p53 pathway. This complex interplay suggests that targeting autophagy via mTOR to mediate the NF-κB-p53 signaling molecule could offer a promising approach for stroke treatment (Sun B. et al., 2018).
HIF-1α, activated under hypoxia, regulates autophagy post-stroke. It’s a heterodimer with HIF-1β. HIF-1α targets BNIP3, which triggers autophagy by displacing Bcl-2 from beclin-1 (Mo et al., 2020). Recent reports indicate that Hydroxysafflor Yellow A exerts neuroprotective effects by activating neuronal autophagy after oxygen–glucose deprivation/reoxygenation (OGD/R) through the HIF-1α/BNIP3 pathway (Wei et al., 2022). Furthermore, there are indications that BNIP3L/NIX is implicated in mitochondrial autophagy induced by cerebral ischemia–reperfusion, suggesting that BNIP3L could be a potential therapeutic target for addressing IS (Yuan et al., 2017). BNIP3 also has the capacity to inhibit Rheb, which results in the reduction of mTOR activity and the subsequent initiation of autophagy. Nevertheless, the expression of HIF-1 is associated with the upregulation of mitochondrial autophagy through mTOR pathway inhibition. However, further verification is required to confirm whether this effect is mediated by BNIP3 (Sun Y. et al., 2018).
PPAR-γ, a member of the hormone receptor superfamily, functions as a ligand-activated transcription factor. PPARγ agonists have demonstrated anti-inflammatory and antioxidant properties in stroke (Li Q. et al., 2019). Additionally, Li and colleagues revealed that luteoloside, a flavonoid, significantly suppressed the activation of the NF-κB signaling, elevated PPARγ protein expression, and enhanced nuclear accumulation of Nrf2 in rats subjected to MCAO. Furthermore, the anti-inflammatory impact of luteoloside was closely associated with the actions of both PPARγ and Nrf2 (Li Q. et al., 2019). Furthermore, Zhao and colleagues concluded that Berberine, an alkaloid compound derived from herbs, plays a beneficial role in providing neuroprotection against cerebral ischemia/reperfusion (I/R) injury. It achieves this by up-regulating PPAR-γ to inhibit NF-κB-mediated pyroptosis (Zhao et al., 2021).
The UPR, an intracellular signaling cascade, responds to unfolded/misfolded ER proteins. In cerebral ischemia, it’s a double-edged sword, initially promoting survival but potentially causing cell death with persistent activation. Regulated by ATF6, IRE1, and PERK, these sensors detect ER stress, triggering signaling for protein folding, degradation, and ER biogenesis gene activation (Xiaowei et al., 2023). This coordinated response aims to restore ER function and mitigate the damage caused by ischemia. However, the precise regulation of the UPR in this context is complex, involving crosstalk with other signaling pathways and feedback loops that fine-tune its activation.
The UPR’s effects in cerebral ischemia vary, depending on context. Initially, it promotes survival by enhancing protein folding and reducing synthesis. However, prolonged activation may induce apoptosis and necrosis, worsening ischemia damage. The balance between protective and harmful effects is influenced by ischemia severity, duration, and cellular/molecular environment.
The ROS pathway, critical in cellular metabolism and signaling, produces signaling molecules under normal conditions, regulating growth, differentiation, and apoptosis. Oxidative stress arises when ROS outpaces antioxidant defenses, damaging cells. Mitochondria are primary ROS sources, with contributions from other compartments. NADPH oxidases and nitric oxide synthases facilitate ROS production. In cerebral ischemia, ROS have dual roles: signaling for adaptation and survival, but excessive levels cause oxidative stress, damaging proteins, lipids, and DNA. This damage triggers cell death pathways, exacerbating neuronal injury and stroke pathophysiology.
Cerebral ischemia, characterized by inadequate brain blood flow, causes neuronal damage and dysfunction. Reperfusion, vital for tissue survival, paradoxically triggers Cerebral Ischemia–Reperfusion Injury (CIRI). Autophagy, a cellular self-cleaning process, has garnered attention in neurology due to its dual roles in these contexts. Autophagy, a tightly regulated process, degrades and recycles cellular components to maintain homeostasis. Early in ischemia, autophagy activates as a neuroprotective response, eliminating damaged proteins and organelles. However, prolonged ischemia and CIRI during reperfusion can lead to excessive autophagy, degrading essential components and exacerbating brain damage (Figure 2).Thus, autophagy’s role in ischemic stroke is intricate, influenced by context, timing, and intensity. Researchers are investigating autophagy modulation as a potential therapeutic strategy. Pharmacological interventions targeting autophagy could enhance neuroprotection during ischemia’s acute phase or mitigate neuronal injury during reperfusion.

Figure 2. The mechanisms of autophagy in IS. Under ischemic conditions, autophagy is orchestrated by the ULK1 and PtdIns3K complexes, facilitated by Atg genes. The process unfolds in phases: induction initiates phagophore formation, which matures into a double-membraned autophagosome. Fusion.
The mechanisms of autophagy in IS. Under ischemic conditions, autophagy is orchestrated by the ULK1 and PtdIns3K complexes, facilitated by Atg genes. The process unfolds in phases: induction initiates phagophore formation, which matures into a double-membraned autophagosome. Fusion with lysosomes forms an autolysosome, where lysosomal enzymes degrade its contents, completing the autophagy cycle.
Autophagy holds a pivotal role in ischemic stroke’s pathological and physiological processes, engaging various pathways. Multiple studies underscore its activation as a response to ischemia/reperfusion injury, emphasizing its complexity and significance in stroke management (Hou et al., 2019). Autophagy activation was confirmed through electron microscopy, where an increased number of autophagosomes were observed. Specifically, in mice subjected to middle cerebral artery occlusion (MCAO) followed by reperfusion, elevated levels of LC3 protein were detected in the ischemic penumbra (Nitatori et al., 1995). The research findings indicate that following 1 h of ischemia, there was a significant increase in LC3 expression within the ischemic penumbra. This heightened expression persisted for a duration of 5 h in the absence of reperfusion (Yan et al., 2011). Meaningfully, the presence of cleaved caspase-3 was detected, and its temporal pattern mirrored that of autophagy. This observation suggests that both autophagy and apoptosis coexist within the cerebral ischemic penumbra (Sodhi et al., 2021). However, the question of whether autophagy activation promotes neuronal survival or leads to cell death remains a subject of debate. Some researchers argue that autophagy induction following a stroke serves to supply the cell with energy by removing damaged cellular components. On the contrary, there is an opposing viewpoint suggesting that excessive autophagy can worsen stroke injury. This is because it can lead to the destruction of healthy cells and trigger autophagic cell death, in addition to apoptosis (Wang, et al., 2020). Hence, evaluating the timing and extent of autophagy’s induction or modulation can elucidate its role in cerebral ischemia. While additional mechanistic insights into the autophagy pathway are crucial, it emerges as a promising therapeutic target for stroke. Based on experimental evidence, two opposing perspectives emerge regarding autophagy’s potential influence on ischemic neurons (Hou et al., 2019).
Following ischemia and subsequent reperfusion, a range of environmental disruptions, including ER stress, oxidative stress, mitochondrial dysfunction, and cellular death, have the potential to exacerbate neurological dysfunction (Khan et al., 2020). It has been reported that Lomitapide demonstrated significant benefits in terms of enhancing survival rates, decreasing neuronal tissue loss, and improving neurological function following MCAO. Moreover, lomitapide exhibited the capacity to enhance LC3-II expression, decrease P62 and LAMP2 expression, thereby facilitating autophagic flux. Additionally, it suppressed apoptosis by upregulating the expression of the anti-apoptotic protein Bcl-2 and downregulating the expression of the pro-apoptotic protein Bax. This suggests that Lomitapide facilitates autophagy (Zheng et al., 2022). Furthermore, Zhang et al. reported that astragaloside IV has the potential to reduce neuronal apoptosis in HT22 cells following OGD/R (oxygen–glucose deprivation and reoxygenation) by stimulating autophagy (Zhang D. M. et al., 2019). Additionally, Ezetimibe has demonstrated its efficacy in reducing infarct volume and improving neurobehavioral deficits in rats subjected to MCAO. However, the neuroprotective and antiapoptotic benefits of ezetimibe were found to diminish when an autophagy inhibitor, 3-MA, was introduced as an intervention (Jing et al., 2018). These studies indicate that the activation of autophagy in response to ischemia and reperfusion may have a neuroprotective effect by reducing brain damage through the inhibition of apoptosis. The study has demonstrated that rapamycin, an inhibitor of mTOR known for its role in regulating energy balance and suppressing autophagy, effectively reduces infarction volumes and enhances neurological outcomes (Wu et al., 2018). This protective effect of rapamycin has been consistently observed in both clinical and pre-clinical studies involving IS. To delve into the specifics, the restoration of autophagy, achieved by increasing NAD+: NADH levels or elevating Beclin1 expression, has shown significant benefits. These interventions improved mitochondrial function and contributed to a reduction in stroke development, particularly in stroke-prone spontaneously hypertensive rats (Forte et al., 2020). In addition, the overexpression of BAG3 triggers autophagy activation while concurrently suppressing apoptosis, thus serving as a potential therapeutic strategy for mitigating IS and hypoxia/reoxygenation injuries. This suggests a promising therapeutic role for BAG3 expression in cerebral ischemia (Liu M. et al., 2023). Also, Wang and his colleagues confirmed that the long non-coding RNA (lncRNA) Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) additionally triggers autophagy and offers protection against cerebral ischemia. This protective effect is achieved through its interaction with miR-200c-3p, leading to the upregulation of Sirt1 expression (Wang L. et al., 2019).
Accumulated evidence indicates that autophagy activation may worsen neurological dysfunction post-ischemia/reperfusion. During ischemia, a sophisticated interplay of interconnected pathways initiates autophagy, highlighting its intricate involvement (Wang et al., 2020). One such example is the association observed between elevated LC3-II levels (indicative of an increased number of autophagosomes) and the extent of cortical infarction in rat models subjected to MCAO (Acaz-Fonseca et al., 2020).
The administration of tetrahydroxystilbene glucoside (TSG), a constituent of Fallopia multiflora, similarly reduced infarct volume and improved neurobehavioral deficits in ischemia/reperfusion mice by suppressing autophagy (Yu et al., 2019). The study further illuminates TIGAR’s (TP53-induced glycolysis and apoptosis regulator) role as a fructose-2,6-bisphosphatase in modulating autophagy post-ischemia/reperfusion. TIGAR-transgenic mice exhibited reduced autophagy, whereas TIGAR-knockout mice showed enhanced autophagy, accompanied by larger infarcts and heightened neurological deficits. Notably, 3-MA administration post-ischemia/reperfusion mitigated these adverse effects, suggesting TIGAR’s neuroprotective role partly stems from inhibiting autophagy (Zhang Y. et al., 2019). Puerarin, a traditional Chinese herb, has been shown to mitigate brain dysfunction following ischemia/reperfusion by inhibiting the expression of autophagy proteins through modulation of the AMPK-mTOR-ULK1 signaling pathway (Wang J. F. et al., 2018). Correspondingly, Liu and his colleagues confirmed that Activin A enhanced neurological function and decreased infarct size in mice afflicted by MCAO/R-induced IS through the inhibition of autophagy (Liu X. et al., 2023). Moreover, Calycosin alleviates CIRI by attenuating autophagy through the STAT3/FOXO3a signaling pathway (Xu et al., 2023). Zhang et al. have uncovered that Danhong injection mitigates cerebral ischemia–reperfusion injury by restraining autophagy via the miRNA-132-3p/ATG12 signaling axis (Zhang H. et al., 2023). These findings indicate that suppressing autophagy provides neuroprotection.
Following an IS, the activation of autophagy remains a subject of ongoing debate. The presence of autophagy in cells undergoing ischemic injury at different stages raises the question of whether it plays a role in neuroprotection or contributes to apoptosis (Hou et al., 2019).
PANoptosis and autophagy are different forms of cell death, but numerous lines of structural, functional, and mechanical evidence show that crosstalk ensues between them.
Autophagy and apoptosis, two fundamental cellular processes, govern cell fate under stress. Recently, attention has shifted towards dual regulators that interface between these two pathways. Despite observed interactions among autophagy- and apoptosis-related proteins, their underlying regulatory mechanisms remain elusive. Notably, autophagy and apoptosis display a dual role, exhibiting both protective and detrimental effects at distinct stages of IS progression (Figure 3).
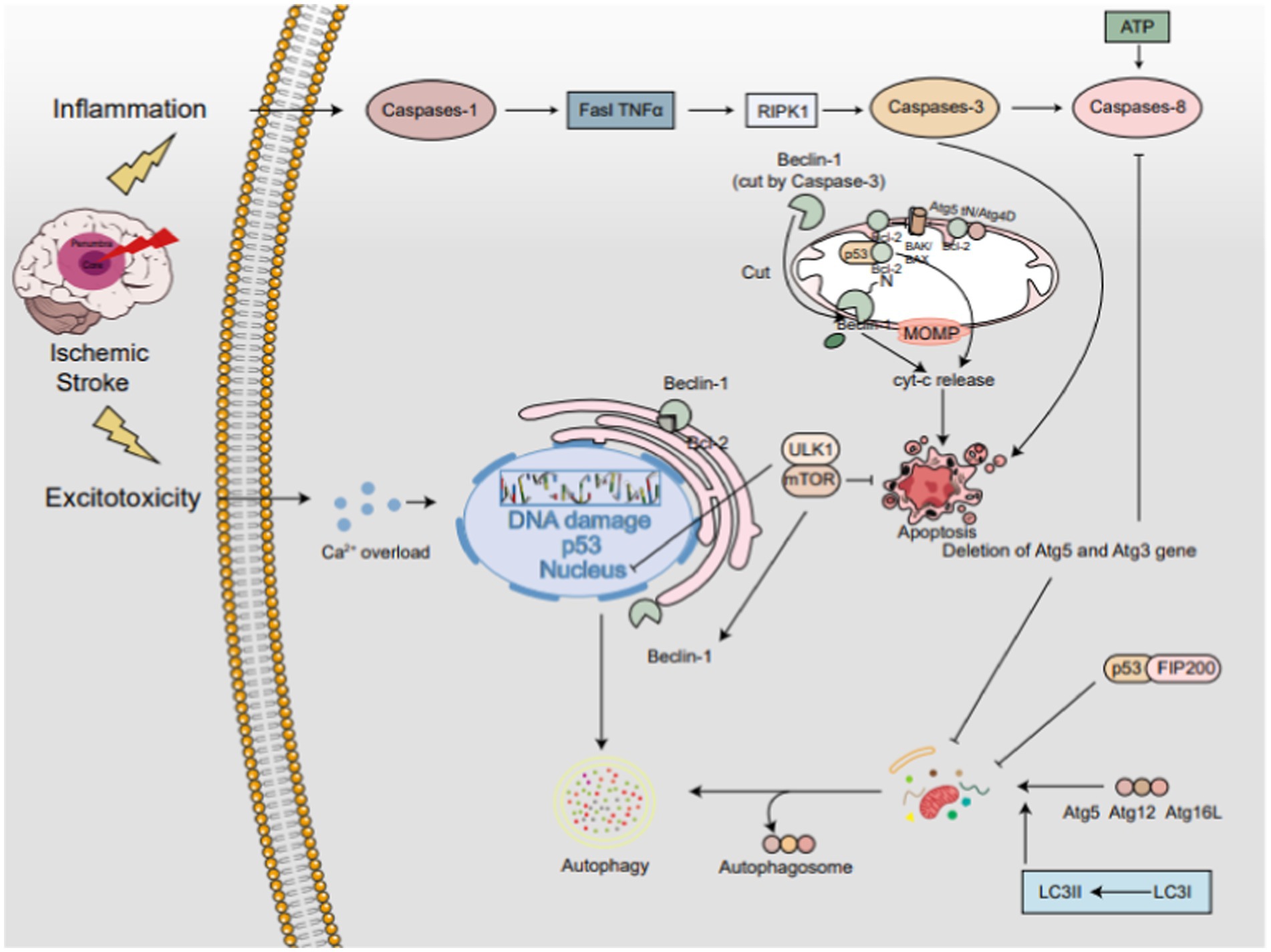
Figure 3. The interplay between autophagy and apoptosis in IS. The PI3K/AKT/mTOR axis serving as a central hub. Beclin-1, residing in the endoplasmic reticulum, acts as a bifunctional player, promoting autophagy independently but inhibiting it when bound to Bcl-2. Caspase-3-mediated cleavage of Beclin-1 redirects it to mitochondria, triggering apoptosis. The Atg5-Atg12-Atg16L complex activates LC3-I to LC3-II, pivotal for autophagy progression. Conversely, calpain-cleaved Atg5tN enters mitochondria, interacting with BCL-2 proteins to unleash Cyt-c and foster apoptosis. Atg4D variants further fine-tune the autophagy-apoptosis balance. p53 exhibits a dual-edged sword, promoting apoptosis by associating with Bcl-2 in mitochondria but enhancing autophagy via the mTOR pathway in the nucleus. In the cytoplasm, p53 engages with FIP200 to restrain autophagy. Notably, ATG5 and ATG3 deletions impede both autophagy and apoptosis, whereas caspase-3-mediated Beclin-1 cleavage propels apoptosis.
The interplay between autophagy and apoptosis in IS. The PI3K/AKT/mTOR axis serving as a central hub. Beclin-1, residing in the endoplasmic reticulum, acts as a bifunctional player, promoting autophagy independently but inhibiting it when bound to Bcl-2. Caspase-3-mediated cleavage of Beclin-1 redirects it to mitochondria, triggering apoptosis. The Atg5-Atg12-Atg16L complex activates LC3-I to LC3-II, pivotal for autophagy progression. Conversely, calpain-cleaved Atg5tN enters mitochondria, interacting with BCL-2 proteins to unleash Cyt-c and foster apoptosis. Atg4D variants further fine-tune the autophagy-apoptosis balance. p53 exhibits a dual-edged sword, promoting apoptosis by associating with Bcl-2 in mitochondria but enhancing autophagy via the mTOR pathway in the nucleus. In the cytoplasm, p53 engages with FIP200 to restrain autophagy. Notably, ATG5 and ATG3 deletions impede both autophagy and apoptosis, whereas caspase-3-mediated Beclin-1 cleavage propels apoptosis.
The PI3K/AKT/mTOR pathway, a quintessential signaling cascade, functions to bolster cell viability, deter apoptosis, and impede autophagy. In an environment with sufficient nutrients, signals like growth factors, glucose, and amino acids engage with the serine/threonine phosphorylation-mediated mTOR complex 1 (mTORC1) signaling pathway, resulting in the suppression of autophagy (Russell et al., 2014). In IS, mTOR activation is bifurcated: it supports cell survival via protein synthesis and metabolism, yet unchecked activity may drive dysfunction, contributing to stroke pathogenesis. mTOR centrally regulates autophagy, and under ischemia, its inhibition triggers autophagy. The intricate balance of mTOR-mediated pathways intersects multiple cellular processes. Intriguingly, inhibiting mTOR has shown potential to mitigate apoptosis and necroptosis in ischemia-mimicking cellular models (Jiang et al., 2024). This finding suggests that modulating mTOR activity might hold promise as a therapeutic strategy to mitigate apoptosis in stroke. Conversely, excessive mTOR activation could promote apoptosis by enhancing cellular stress and dysfunction.
Beclin-1, a vital regulatory protein in autophagosome formation, performs multiple functions. Free Beclin-1 enhances autophagy, while its binding to Bcl-2 suppresses it (Li Z. G. et al., 2019). As a novel Caspase substrate, Beclin-1 is cleaved by Caspases 3–10, losing its autophagy-inducing capability. The cleaved C-terminal fragment relocates to mitochondria, triggering Cyt-c release and apoptosis. Thus, Beclin-1 plays a dual role in apoptosis and autophagy.
Current findings emphasize Atg’s pivotal regulatory function in autophagy and apoptosis. Early in autophagosome genesis, the Atg12-Atg5-Atg16L complex catalyzes LC3-I to LC3-II, which integrates into the autophagosome membrane, facilitating its expansion. Beyond complex formation, Atg proteins modulate autophagy through alternate pathways. Additionally, Atg proteins regulate autophagy via complex assembly and apoptosis by cleaving specific targets. In apoptosis, calpain-mediated cleavage of non-conjugated Atg5 releases Atg5tN, which enters mitochondria and interacts with Bcl-2 proteins to release cytochrome c. Notably, Atg5 knockdown in mice subjected to MCAO-induced ischemia increased beclin1 and LC3-II expression, suggesting attenuated autophagy-mediated ferroptosis (Zhu et al., 2024). In addition, research has corroborated that indobufen exerts protective effects against OGD/R-induced injury in SH-SY5Y cells by inhibiting autophagy, oxidative stress, and apoptosis. This protective mechanism involves the modulation of the transcription factor NRF2 and the suppression of Atg5 expression, thereby mitigating IS damage (Wang R. et al., 2024).
Atg3 is crucial for autophagosome biogenesis, featuring a Caspase-8 cleavage site within its sequence. Introducing mutations to this site significantly inhibits Caspase-8 proteolytic activity in vitro. Studies reveal that the absence of Atg5 or Atg3 disrupts autophagosome formation, thereby suppressing Caspase-8 activation and apoptosis. Similarly, Caspase-3 can cleave autophagy-related proteins, with Beclin-1 harboring two such sites, implicating its role in regulating autophagy and apoptosis. Following Caspase-3 cleavage, the unveiled BH3 domain of Beclin-1 may intensify apoptosis by engaging with anti-apoptotic Bcl-2 family members. Furthermore, research indicates that morphine preconditioning mitigates ischemia/reperfusion-induced Caspase 8-mediated neuronal apoptosis (Huang et al., 2024). In addition, As the study demonstrates, Astragaloside-IV suppressed the upregulation of Caspase-8 and Bax/Bcl-2 mRNAs, as well as the protein levels of apoptosis cytokines Caspase-8, cleaved Caspase-3, and Cyto C following ischemia reperfusion. This suggests that Astragaloside-IV mitigates ischemia reperfusion-induced apoptosis by inhibiting the activation of key factors in both the death receptor and mitochondrial pathways (Yin et al., 2020).
P53, a key apoptosis regulator, triggers apoptosis via two distinct mechanisms. Firstly, acting as a transcription factor, p53 can upregulate pro-apoptotic genes while suppressing anti-apoptotic genes. Additionally, p53 can relocate to the mitochondria and engage with Bcl-2 family proteins, resulting in mitochondrial membrane permeability and the release of Cyt-c (Castrogiovanni et al., 2018). Under normal conditions, p53 is typically found in the cytoplasm, but upon DNA damage, it is translocated to the nucleus. Recent research indicates that nuclear p53 promotes autophagy by modulating the mTOR pathway in a transcription-dependent manner (Denisenko et al., 2018). However, in the cytoplasm, p53 exhibits opposing effects, inhibiting autophagy by interacting with FIP200. This interaction prevents the activation of the ulk1-fip200-Atg13-Atg101 complex, thus impeding autophagosome formation. The research concludes that hyperglycemia can exacerbate brain injury stemming from I/R by potentially stimulating excessive autophagy. Additionally, it is postulated that hyperglycemia activates the intricate p53-Sesn2-AMPK signaling cascade, further contributing to the pathophysiological processes (Wang et al., 2024a).
The interplay between autophagy and pyroptosis in IS is intricate. Autophagy, by degrading damaged cellular components, can foster cell survival and dampen pyroptosis triggers. Nevertheless, exaggerated autophagy may conversely lead to cell demise, which may, in turn, amplify pyroptosis. On the other hand, pyroptosis unleashes inflammatory cytokines, which can ignite autophagy in adjacent cells, fostering a detrimental cycle of cell loss and inflammation (Figure 4).
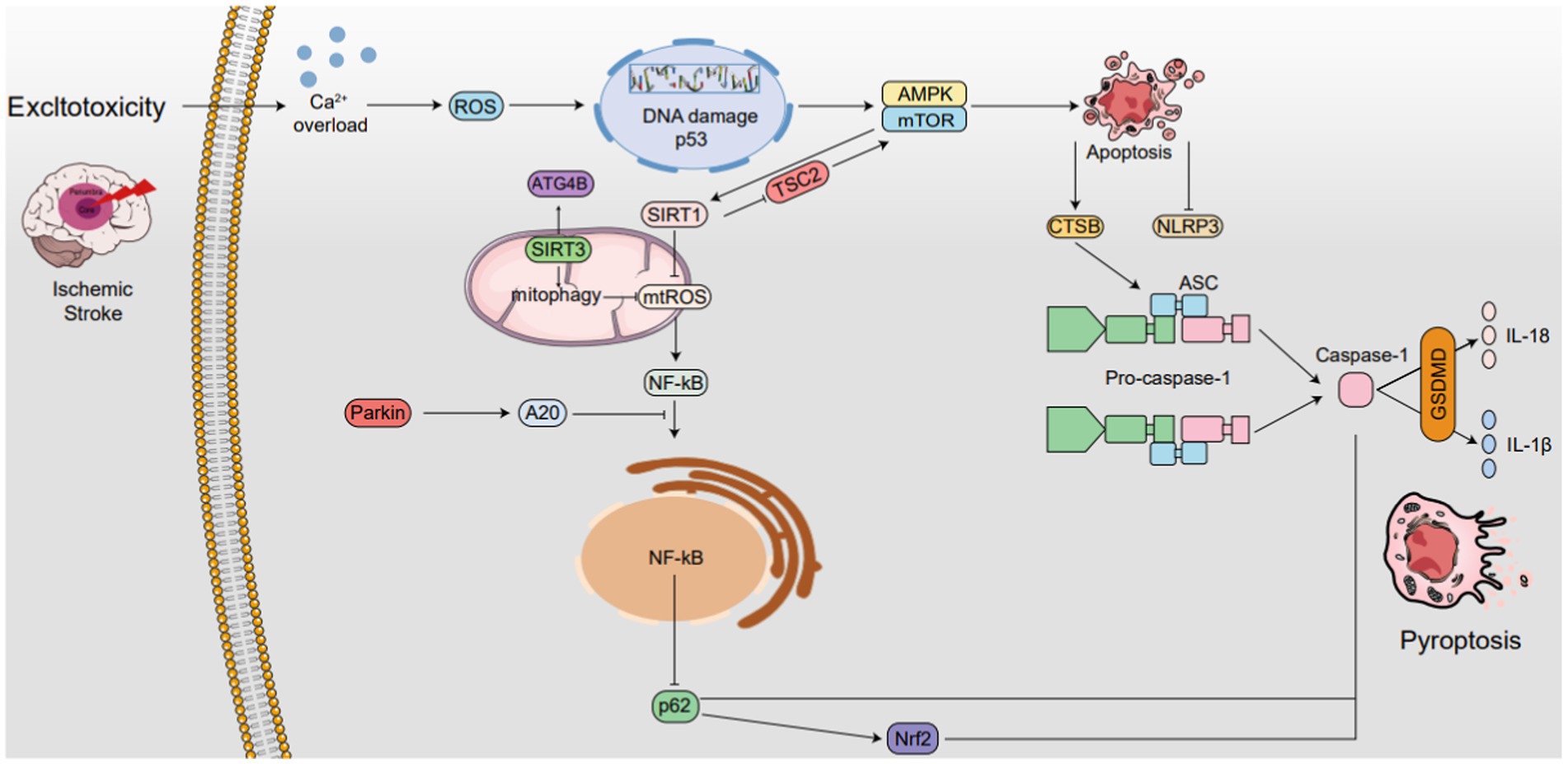
Figure 4. The interplay between autophagy and pyroptosis in IS. SIRT1 is not only induced by mTOR, it also induces mTOR by inhibiting TSC2 activity, while SIRT3 also activates ATG4B. SIRT1 suppresses ROS in mitochondria, while SIRT3 enhances mitophagy and also reduces ROS levels, thereby inhibiting NLRP3 inflammasome activation. Additionally, NLRP3 expression reciprocally regulates mitochondrial damage and ROS production. Autophagy boosts CTSB, accelerating NLRP3 activation and pyroptosis. Parkin triggers mitophagy and upregulates A20, inhibiting NF-κB and NLRP3. NF-κB, via p62 and Nrf2/ARE, activates Caspase-1, accelerating pyroptosis. AMPK phosphorylation. Degrades TXNIP, activating NLRP3. The AMPK/mTOR pathway upregulates SIRT1, downregulates ROS, and inhibits NLRP3 ↑ induce; ⟂ inhibit.
The interplay between autophagy and pyroptosis in IS. SIRT1 is not only induced by mTOR, it also induces mTOR by inhibiting TSC2 activity, while SIRT3 also activates ATG4B. SIRT1 suppresses ROS in mitochondria, while SIRT3 enhances mitophagy and also reduces ROS levels, thereby inhibiting NLRP3 inflammasome activation. Additionally, NLRP3 expression reciprocally regulates mitochondrial damage and ROS production. Autophagy boosts CTSB, accelerating NLRP3 activation and pyroptosis. Parkin triggers mitophagy and upregulates A20, inhibiting NF-κB and NLRP3. NF-κB, via p62 and Nrf2/ARE, activates Caspase-1, accelerating pyroptosis. AMPK phosphorylation. Degrades TXNIP, activating NLRP3. The AMPK/mTOR pathway upregulates SIRT1, downregulates ROS, and inhibits NLRP3 ↑ induce; ⟂ inhibit.
The accumulation of mtROS has been intricately linked to the activation of the NLRP3 inflammasome, a component of the NLR family containing the pyrin domain (Liu Z. et al., 2022; Wang et al., 2022). Mitophagy, an autophagic process, specifically targets dysfunctional and potentially harmful mitochondria for degradation. However, when mitophagy is impeded, damaged mitochondria fail to be cleared, leading to the release of mtDNA and mtROS. This, in turn, triggers the activation of the NLRP3 inflammasome. Subsequently, the activated NLRP3 inflammasome can cause mitochondrial membrane rupture, further releasing mtROS and exacerbating mitochondrial damage and inflammation (Dominic et al., 2022). This interaction between mtROS and the NLRP3 inflammasome forms a feedback loop, indicating mtROS’s regulatory function in activating the NLRP3 Inflammasome. Additionally, recent studies indicate the involvement of novel substances in modulating mtROS’s effect on pyroptosis. The study found that Quercetin prevents neuronal injury via inhibition of mtROS-mediated NLRP3 inflammasome activation in microglia through promoting mitophagy (Han et al., 2021).
In recent years, the intricate mechanism underlying the initiation of mitophagy via the PINK1-Parkin pathway has been unveiled (Eckl et al., 2021). Parkin plays a crucial role in suppressing the activation of inflammasomes. Specifically, it enhances the expression of anti-apoptotic signaling protein 20 (A20), subsequently impeding the nuclear entry and activation of nuclear factor kappa-B (NF-κB) (Liu H. et al., 2021; Mouton-Liger et al., 2018). This results in a reduction in NLRP3 inflammasome activity. Additionally, the NF-κB-p62 mitophagy pathway functions to restrain Caspase-1 and mitigate pyroptosis, establishing a reciprocal regulatory mechanism between autophagy and pyroptosis. Recent research has also revealed that Nrf2, a nuclear factor that is activated by p62, is implicated in macrophage pyroptosis. Studies have shown that Glycosides hinder inflammation by inhibiting pyroptosis and p62, while boosting PINK1 and Parkin to activate mitophagy. This results in neuroprotective effects against cerebral ischemia–reperfusion, showing their potential in reducing neurological damage (Jiao et al., 2024).
CTSB, a key intracellular cysteine protease primarily localized in lysosomes, exhibits a close link to the autophagic flux within the cytoplasm. Notably, icaritin has been found to alleviate neuroinflammation in brain tissue by intricately modulating the TLR4, MAPK, and NLRP3 inflammasome signaling cascades, with CTSB serving as a pivotal target in this regulatory process (Sun et al., 2023).
The AMPK/mTOR signaling axis plays a pivotal role in regulating autophagy. Recent studies have demonstrated that eugenol pretreatment mitigates I/R injury by promoting autophagy through the intricate modulation of the AMPK/mTOR/P70S6K signaling pathway (Sun et al., 2020). Moreover, research has elucidated the neuroprotective efficacy of CK against neural autophagy and apoptosis induced by OGD/R, which is achieved through the strategic regulation of the AMPK and mTOR signaling pathways (Huang et al., 2020). Besides, research underscores schaftoside’s neuroprotective capacity against cerebral ischemia/reperfusion injury (CI/RI), both in vitro and in vivo, by modulating the autophagy-mediated AMPK/mTOR pathway (Zhang L. et al., 2022).
Also, the study has demonstrated that Pien-Tze-Huang inhibited NLRP3 inflammasome-mediated neuroinflammation, which was associated with enhanced autophagy via AMPK/mTOR/ULK1 pathway in vitro and in vivo (Huang et al., 2021).
The interplay between autophagy and necroptosis in ischemic stroke is characterized by a delicate balance. Autophagy can function as a protective mechanism against necroptosis by eliminating harmful stimuli and promoting cell survival. However, when autophagy is insufficient or overwhelmed, necroptosis may be triggered as a backup mechanism to eliminate severely damaged cells, thereby limiting tissue damage and promoting tissue homeostasis (Figure 5).
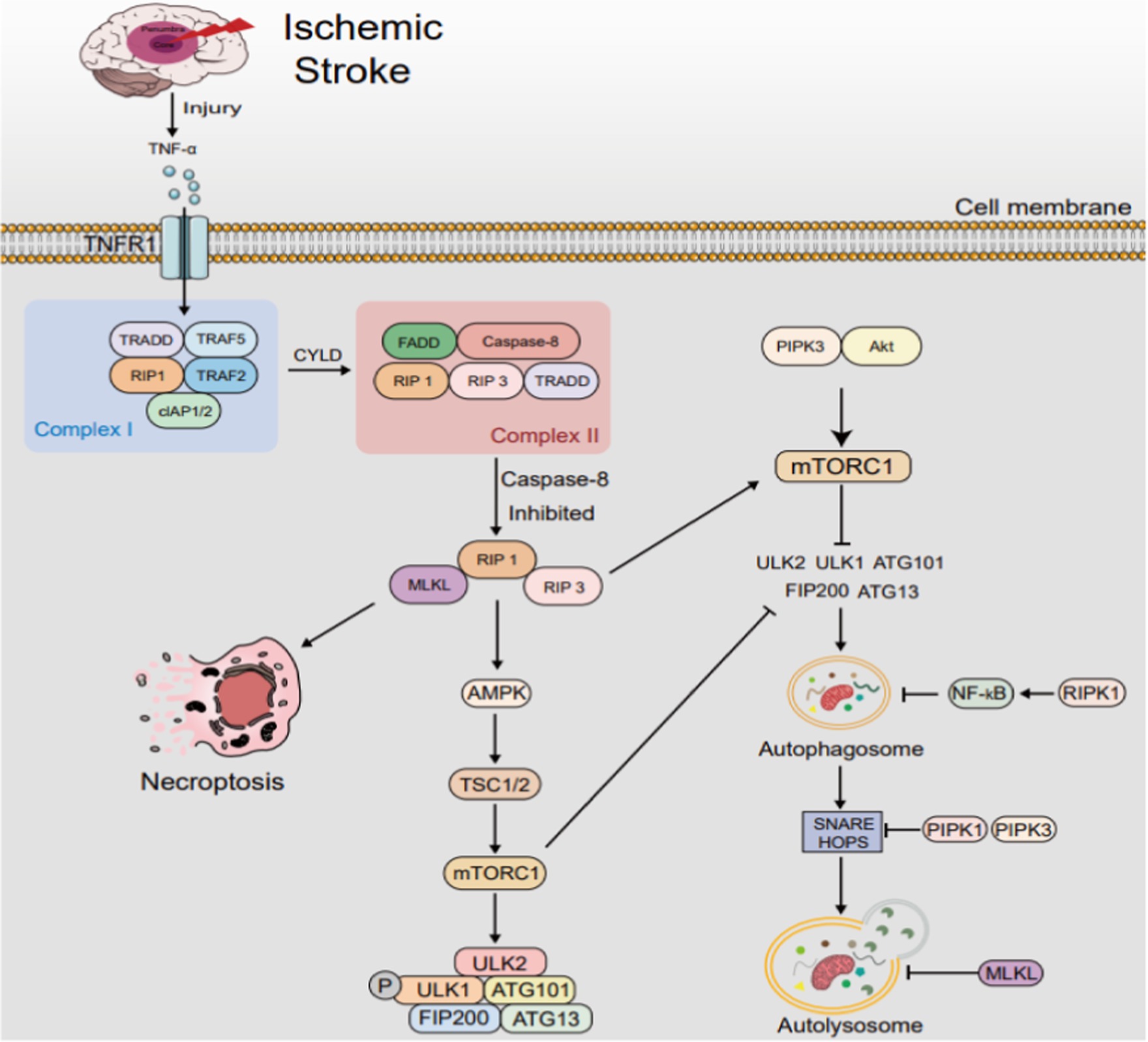
Figure 5. The interplay between autophagy and necroptosis in IS. After IS, TNF-α elevation triggers complex I/II formation, transforming into necrosome under caspase-8 inhibition. This prompts RIP1 activation, RIP3 and MLKL phosphorylation, accelerating necroptosis, which modulates autophagy. RIPK1 and RIPK3 stimulate AMPK, fostering autophagy via the AMPK/TSC/mTORC1 pathway. RIPK3 also phosphorylates ULK1 for alternate autophagy. Conversely, RIPK1 hinders autophagy by activating NF-κB and disrupting autophagosome-lysosome fusion via SNARE complex interference. MLKL inhibits autophagy by activating PI3K/AKT/mTOR signaling and damaging autophagosomal membranes, disrupting autophagic flux. TRADD: TNF receptor-associated death domain; TRAF: TNF receptor-associated factor; FADD: Fas-associating with death domain; cIAP: cellular inhibitors of apoptosis protein. mTOR, mechanistic target of rapamycin; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor.
The interplay between autophagy and necroptosis in IS. After IS, TNF-α elevation triggers complex I/II formation, transforming into necrosome under caspase-8 inhibition. This prompts RIP1 activation, RIP3 and MLKL phosphorylation, accelerating necroptosis, which modulates autophagy. RIPK1 and RIPK3 stimulate AMPK, fostering autophagy via the AMPK/TSC/mTORC1 pathway. RIPK3 also phosphorylates ULK1 for alternate autophagy. Conversely, RIPK1 hinders autophagy by activating NF-κB and disrupting autophagosome-lysosome fusion via SNARE complex interference. MLKL inhibits autophagy by activating PI3K/AKT/mTOR signaling and damaging autophagosomal membranes, disrupting autophagic flux. TRADD: TNF receptor-associated death domain; TRAF: TNF receptor-associated factor; FADD: Fas-associating with death domain; cIAP: cellular inhibitors of apoptosis protein. mTOR, mechanistic target of rapamycin; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor.
Recent studies have illuminated the pivotal role that RIPK1 and RIPK3 plays in regulating cellular processes, particularly in the context of amino acid availability, autophagy, and programmed cell death (Tian et al., 2022; Wu X. et al., 2020). RIPK1 and RIPK3 emerge as pivotal players, not merely initiating necroptosis but also intricately intertwining with autophagy signaling pathways to preserve cellular homeostasis. Specifically, they regulate the availability of asparagine, a crucial amino acid that directly influences AMPK (AMP-Activated Protein Kinase) activation—a master switch for autophagy. By modulating asparagine levels, RIPK1 and RIPK3 subtly impact AMPK’s activity, thereby indirectly governing the initiation and progression of autophagy. Autophagy, being a fundamental cellular process, ensures the degradation and recycling of damaged or redundant cellular constituents, thereby maintaining cellular integrity and survival.
Furthermore, RIPK1 and RIPK3 engages in multifaceted interactions with autophagy signaling pathways. It modulates the expression or activity of autophagy-related genes and proteins at multiple levels, including the well-known P13K/AKT/mTOR signaling cascade. Likewise, RIPK1 assumes a pivotal role in cellular adaptation to low energy states, intricately orchestrating the AMPK-mTORC1 signaling cascade (Najafov et al., 2021). This intricate crosstalk between RIPK1 and autophagy signaling underscores the complexity of cellular regulatory networks and highlights the importance of RIPK1 in maintaining these networks’ delicate balance. Beyond its role in autophagy regulation, RIPK1 serves as a critical initiator of necroptosis, a highly regulated form of necrosis that occurs when apoptosis (another form of programmed cell death) is compromised. Upon activation, RIPK1 forms a complex with RIPK3, leading to MLKL phosphorylation and subsequent activation, ultimately triggering necroptosis—a distinct form of cell death marked by membrane disruption and the release of intracellular contents.
Essentially, RIPK1 and RIPK3 function as a gatekeeper, meticulously balancing the intricate equilibrium between inflammation and cell death. Their absence may shift cellular fate towards apoptosis or alternative cell death pathways, potentially jeopardizing tissue integrity and function. Conversely, unchecked RIPK1 activity can spur unchecked inflammation and tissue damage, as necroptosis amplifies inflammatory signals, attracting immune cells to the site of cell demise. Notably, research demonstrates that catalytically inactive RIP1 can mitigate infarct size and enhance neurological recovery following middle cerebral artery occlusion/reperfusion (MCAO/R), highlighting its therapeutic potential in managing the delicate balance between inflammation and cell death (Zhang et al., 2020). Additionally, Oridonin potently halts RIPK3-mediated excessive mitophagy, consequently salvaging neuronal loss at the onset of IS, highlighting its therapeutic potential (Li L. et al., 2023). Furthermore, the study has indicated that RIP3 regulates early reperfusion injury via oxidative stress and mitochondrial activity-related effects, rather than cell loss due to necroptosis (Horvath et al., 2021).
Upon its phosphorylation by RIPK3, MLKL undergoes a profound conformational shift. This transformation empowers MLKL to translocate to the plasma membrane, where it disrupts its integrity, ultimately triggering cell death (Fan et al., 2024). This orchestrated sequence underscores the intricate interplay between MLKL’s structural dynamics and its functional role in necroptosis. The activation of MLKL is meticulously controlled by the interplay between RIPK1 and RIPK3, ensuring that necroptosis is initiated only in response to specific stimuli and when apoptotic pathways are impaired. This tight regulation underscores the cell’s meticulous balancing act between life and death decisions. Moreover, necroptosis, orchestrated by RIPK1 and MLKL, has far-reaching consequences beyond mere cell demise. By releasing Damage-Associated Molecular Patterns (DAMPs) and activating immune cells, necroptosis fuels inflammation, highlighting MLKL’s pivotal role in orchestrating the inflammatory cascade associated with this form of cell death.
Intriguingly, while MLKL’s direct involvement in autophagy remains elusive, its function in necroptosis may indirectly influence autophagy processes. By modulating cellular homeostasis and stress responses, necroptosis can potentially alter the cellular landscape in ways that impact autophagy, the cellular ‘recycling’ mechanism. Thus, the story of MLKL extends beyond its primary role in necroptosis, inviting further exploration into its broader implications for cellular physiology and pathology. The investigation reveals that necrosulfonamide safeguards against focal ischemia/reperfusion injury by inhibiting astrocytic necroptosis. This protection occurs through preventing the upregulation of necroptotic kinases and impeding the co-localization of phosphorylated MLKL (p-MLKL) and RIP3K (p-RIP3K) within the nucleus and nuclear envelope. Notably, the translocation of both p-MLKL and p-RIP3K to these compartments is crucial in MLKL-mediated necroptosis under ischemic conditions (Zhou et al., 2023).
Currently, the clinical management of IS primarily revolves around interventional approaches to restore blood flow, including drug-based or mechanical thrombolysis techniques. Nevertheless, these strategies have encountered limitations in their effectiveness, and there remains a dearth of efficacious interventions or treatments that can safeguard the brain from cellular demise (Zhou R. P. et al., 2019).
Therapeutic strategies aimed at modulating autophagy present a promising avenue in the management of IS. These strategies encompass both enhancing adaptive autophagy and inhibiting excessive autophagy that occurs post-IS, thereby modulating distinct autophagy processes. Several compounds have demonstrated their ability to induce adaptive autophagy. Notably, rapamycin, extensively studied for its mTOR inhibition properties, has shown potential in managing IS. Recent rodent studies employing middle cerebral artery occlusion (MCAO) models have reported reduced infarct volumes, mitigated neuronal damage, and improved neurological outcomes upon rapamycin administration (Wang S. et al., 2019). Furthermore, resveratrol, a naturally occurring polyphenol, has garnered attention for its ability to extend the therapeutic window of recombinant tissue plasminogen activator (r-tPA) in stroke patients (Chen et al., 2016). Research by He et al. underscores resveratrol’s efficacy in alleviating cerebral ischemia–reperfusion (I/R) injury and reducing infarct size (He et al., 2017). Regarding the inhibition of excessive autophagy, dexmedetomidine (DEX) has emerged as a neuroprotective agent in IS. In both in vitro neuronal cultures subjected to oxygen–glucose deprivation (OGD) and in vivo transient middle cerebral artery occlusion (tMCAO) models, DEX has demonstrated neuroprotective effects by suppressing autophagy. This protection is mediated through the upregulation of HIF-1α, effectively inhibiting neuronal autophagy and mitigating ischemia–reperfusion injury in the mouse brain (Luo et al., 2017). Propofol administration has been found to significantly reduce infarct size and enhance the prognosis of acute IS patients (Sun B. et al., 2018). Recent research insights indicate that propofol’s neuroprotective effects against CIRI stem from its ability to suppress excessive autophagy, accomplished through the regulation of either the mTOR/S6K1 pathway or the long noncoding RNA SNHG14 (Sun et al., 2021). In parallel, Icariside II, a key bioactive component derived from Epimedii, has shown remarkable neuroprotective potential in OGD and rodent MCAO models. Its mechanism involves curbing excessive autophagy, achieved by modulating the PKG/GSK-3β signaling cascade (Gao et al., 2020). Moreover, Gomisin N (GN) has recently garnered attention for its novel neuroprotective role in mitigating cerebral ischemia injury. This protective effect is postulated to be intimately linked to GN’s ability to inhibit autophagy. Notably, studies suggest that GN’s neuroprotective actions may be mediated through the PI3K/AKT/mTOR signaling pathway, further underlining its therapeutic potential (Li R. Q. et al., 2023).
Therapeutic approaches that modulate pyroptosis, necroptosis, and apoptosis hold great promise in the treatment of IS. Regarding pyroptosis, recent findings have illuminated that Chrysophanol postconditioning protects against cerebral ischemia–reperfusion injury (CIRI) by inhibiting NLRP3-related pyroptosis, a process that is contingent on TRAF6 (Xia et al., 2022). Notably, isoquercetin post-treatment has been shown to mitigate infarct size, reduce the number of apoptotic cells, and alleviate oxidative stress and inflammatory responses following ischemia and reperfusion (Wang J. et al., 2017). Moreover, berberine has been discovered to safeguard rats from cerebral ischemia–reperfusion (I/R) injury by upregulating PPAR-γ, which curbs NF-κB-mediated pyroptosis (Zhao et al., 2021). In a novel finding, we have identified the involvement of pyroptosis in brain microvascular endothelial cells in the pathogenesis of IS. Notably, Medioresinol ameliorates this pyroptosis and reduces ischemic brain injury, potentially through decreasing mitochondrial reactive oxygen species (mtROS) via the PPARα/GOT1 axis in endothelial cells (Wang et al., 2021). Dendrobium has also demonstrated its protective effects against CIR damage by inhibiting pyroptosis-mediated neuronal death (Liu J. et al., 2020). Furthermore, remimazolam has been shown to effectively improve neurological function, reduce infarct volume, and alleviate cortical neuronal damage post-I/R injury. Importantly, the downregulation of the NLRP3 inflammasome pathway suggests that remimazolam exerts its protective effects by suppressing pyroptosis and reducing the expression and release of inflammatory factors (Shi et al., 2022). In the context of necroptosis, the RIP1 inhibitor, necrostatin-1 (Nec-1), effectively decreases RIP1 and RIP3 protein levels, thereby suppressing necroptosis and enhancing cognitive function (Deng et al., 2019; Zhang et al., 2016). Necrosulfonamide, a potent small-molecule inhibitor targeting MLKL, binds to its N-terminal CC region, diminishing MLKL expression and consequently inhibiting necroptosis. This leads to a reduction in infarct volume and improvement in neurological outcomes (Zhou et al., 2017). Dabrafenib, an RIP3 inhibitor at micromolar concentrations, reduces TNF-α mRNA levels and dampens TNF-α activation in macrophages, contributing to a decrease in infarct size and neuronal protection post-focal cerebral ischemic injury (Cruz et al., 2018). Infliximab, a monoclonal antibody commonly utilized in inflammatory diseases, has been shown to mitigate mitochondrial damage, limit cytoplasmic transparency, reduce blood–brain barrier (BBB) permeability, and inhibit necroptosis formation in ischemic regions, ultimately ameliorating neurological deficits (Chen et al., 2019). Furthermore, the inhibition of necroptosis-related gene expression is being actively explored as a therapeutic strategy for IS. For instance, the combination of Gsk′872 (an RIP3 inhibitor) and RIP3 siRNA reduces the levels of RIP1, RIP3, MLKL, and phosphorylated MLKL, offering neuroprotective benefits. Chinese herbs and their active ingredients have also garnered attention for their ability to decrease necroptosis-related proteins (RIP3, MLKL, and phosphorylated MLKL), suppressing necroptosis and enhancing neurological function in rats subjected to middle cerebral artery occlusion (MCAO) (Zhang D. M. et al., 2019). Notably, Panax notoginseng saponins, isolated from the plant’s roots, inhibit necroptosis by downregulating the expression of these necroptosis-associated proteins (Hu et al., 2022). In the realm of apoptosis, studies have unveiled that Salidroside exerts a protective effect against CIRI-induced microglial NLRP3 inflammasome activation and apoptosis by inhibiting the TLR4/NF-κB signaling cascade (Liu et al., 2021). Meanwhile, Astilbin safeguards against CIRI by modulating the MAPK pathway (suppression) and AKT pathway (activation), thereby inhibiting apoptosis and inflammation (Li et al., 2020). Furthermore, myricetin has been shown to significantly reduce neuronal apoptosis both in vivo and in vitro following ischemic events. Notably, myricetin alleviates various apoptotic factors associated with IS, including excitotoxicity, oxidative stress, and inflammation-mediated apoptosis (Zhang L. et al., 2023).
Additional pharmacotherapeutics aimed at modulating pyroptosis, apoptosis, necroptosis and autophagy in IS have garnered substantial research attention. Specifically, regarding autophagy and pyroptosis, Astragaloside IV demonstrates efficacy in reducing cerebral infarct volume and neurological deficit scores, while enhancing cell viability and regulating autophagy, with an inhibitory effect on pyroptosis (Xiao et al., 2021; Zhang Y. Y. et al., 2019). Spautin-1, on the other hand, is postulated to alleviate cerebral ischemia–reperfusion injury via modulation of the autophagy/pyroptosis pathway (Liu et al., 2021a,b). When it comes to autophagy and necroptosis, URB597 exhibits a dose-dependent improvement in neurological function and reduction in brain infarct volume and edema post-ischemia. It also enhances autophagic flux and mitigates neuronal necroptosis (Yuan et al., 2023). Cyclosporine-A, another compound, effectively diminishes autophagy-related proteins while reducing necroptosis markers like RIP1 and RIP3 (Fakharnia et al., 2017). Baf-A1 treatment is found to mitigate necroptosis and block autophagy, while global cerebral ischemia/reperfusion (I/R) induces necroptosis, possibly augmented by autophagy, and inversely influences caspase-3-mediated apoptosis (Ryan et al., 2018). Regarding autophagy and apoptosis, melatonin administration post-cerebral ischemia (CI) reduces infarct area and induces autophagic proteins like Beclin-1, LC3, and p62 by inhibiting the apoptotic caspase-3 protein (Yilmaz et al., 2023). Resveratrol pretreatment, too, extends the therapeutic window in cerebral ischemic mice, modulating autophagy and inhibiting apoptosis (He et al., 2017; Hou et al., 2018). 6-Gingerol exerts its anti-apoptotic and anti-inflammatory effects via TRPV1/FAF1 complex dissociation-mediated autophagy during cerebral I/R injury (Luo et al., 2021). Furthermore, Stilbene glycoside promotes mitochondrial autophagy in ischemic neurons by upregulating SIRT3/AMPK expression, thereby inhibiting apoptosis (Li et al., 2021). Notably, the study has demonstrated neuroprotective potential of safinamide via anti-oxidant, anti-inflammatory, anti-apoptotic, and autophagy inducing properties (Wasan et al., 2021). Table 2 presents data pertaining to pharmacotherapeutic targets aimed at modulating PANoptosis and autophagy in the context of cerebral ischemia or IS, offering insights into potential therapeutic strategies.
Stroke is assuming a pivotal role as a major concern for individuals in developing nations, particularly in China, where IS stands as the foremost cause of mortality due to its staggeringly high morbidity, mortality, and disability rates. Multiple cell death pathways have been identified, and extensive research has illuminated their significance in maintaining biological homeostasis, as well as the intricate interplay between them. Elucidating the molecular mechanisms underlying this crosstalk will not only enhance our comprehension of the cell death machinery but also offer potential novel therapeutic targets for related illnesses. The intricate crosstalk between PANoptosis and autophagy in the context of IS has garnered significant attention in recent years, owing to its profound implications in neuronal survival and recovery. PANoptosis, as a novel form of cell death, encompasses necroptosis, apoptosis, and pyroptosis, each with unique mechanisms and downstream effects. Autophagy, on the other hand, serves as a crucial cellular mechanism for the degradation and recycling of damaged components, playing a pivotal role in maintaining cellular homeostasis.
The current understanding of the interplay between these two processes in IS suggests that they are intricately linked and mutually influential. On one hand, PANoptosis can be triggered by ischemia-induced stress, leading to neuronal cell death and tissue damage. On the other hand, autophagy can either promote cell survival by clearing damaged proteins and organelles or contribute to cell death if.
excessive or deregulated. The identification of novel therapeutic targets that can modulate this crosstalk holds immense promise for the development of effective treatments for IS. For instance, targeting specific PANoptosis pathways or enhancing autophagic flux may offer neuroprotective effects, reducing infarct size and improving functional recovery. However, the complex nature of these pathways necessitates a cautious and thorough approach, as modulation of one process may inadvertently affect the other. Future research should focus on elucidating the precise molecular mechanisms underlying the crosstalk between PANoptosis and autophagy in IS. This will not only provide a deeper understanding of the disease but also aid in the identification of novel therapeutic targets. Furthermore, preclinical and clinical studies are essential to validate the efficacy and safety of potential therapies.
In conclusion, the intersection between PANoptosis and autophagy represents a fertile ground for the development of novel therapeutic strategies in IS. With continued research and innovation, we may soon witness a paradigm shift in the treatment of this debilitating disease.
H-YT: Funding acquisition, Methodology, Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Investigation, Project administration, Resources, Software, Supervision, Validation, Visualization. Y-XL: Formal analysis, Investigation, Writing – original draft. J-TZ: Conceptualization, Supervision, Writing – original draft. L-JL: Formal analysis, Supervision, Writing – review & editing. TY: Methodology, Supervision, Writing – review & editing. YZ: Formal analysis, Methodology, Supervision, Writing – original draft. J-WG: Methodology, Writing – review & editing. CX: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. Z-GM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Shenzhen Polytechnic University Research Fund (6024310022K), the National Natural Science Foundation of China (82174167); the Key Project of Hunan Province Education Department (20A366), the Project of Natural Science Foundation of Hunan Province (2023JJ30464), and the Young Qihuang Scholar Support Project of National Administration of Traditional Chinese Medicine (2022).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acaz-Fonseca, E., Castelló-Ruiz, M., Burguete, M. C., Aliena-Valero, A., Salom, J. B., Torregrosa, G., et al. (2020). Insight into the molecular sex dimorphism of ischaemic stroke in rat cerebral cortex: focus on neuroglobin, sex steroids and autophagy. Eur. J. Neurosci. 52, 2756–2770. doi: 10.1111/ejn.14731
Alawieh, A., Andersen, M., Adkins, D. L., and Tomlinson, S. (2018). Acute complement inhibition potentiates neurorehabilitation and enhances tPA-mediated neuroprotection. J. Neurosci. 38, 6527–6545. doi: 10.1523/JNEUROSCI.0111-18.2018
Allocca, M., Corrigan, J. J., Mazumder, A., Fake, K. R., and Samson, L. D. (2019). Inflammation, necrosis, and the kinase RIP3 are key mediators of AAG-dependent alkylation-induced retinal degeneration. Sci. Signal. 12:eaau9216. doi: 10.1126/scisignal.aau9216
Barthels, D., and Das, H. (2020). Current advances in ischemic stroke research and therapies. Biochim. Biophys. Acta Mol. basis Dis. 1866:165260. doi: 10.1016/j.bbadis.2018.09.012
Cao, Y., Chen, J., Ren, G., Zhang, Y., Tan, X., and Yang, L. (2019). Punicalagin prevents inflammation in LPS-induced RAW264.7 macrophages by inhibiting FoxO3a/autophagy signaling pathway. Nutrients 11:2794. doi: 10.3390/nu11112794
Castrogiovanni, C., Waterschoot, B., De Backer, O., and Dumont, P. (2018). Serine 392 phosphorylation modulates p53 mitochondrial translocation and transcription-independent apoptosis. Cell Death Differ. 25, 190–203. doi: 10.1038/cdd.2017.143
Chen, A. C. H., Arany, P. R., Huang, Y.-Y., Tomkinson, E. M., Sharma, S. K., Kharkwal, G. B., et al. (2011). Low-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. PLoS One 6:e22453. doi: 10.1371/journal.pone.0022453
Chen, J., Bai, Q., Zhao, Z., Sui, H., and Xie, X. (2016). Resveratrol improves delayed r-tPA treatment outcome by reducing MMPs. Acta Neurol. Scand. 134, 54–60. doi: 10.1111/ane.12511
Chen, A. Q., Fang, Z., Chen, X. L., Yang, S., Zhou, Y. F., Mao, L., et al. (2019). Microglia-derived TNF-α mediates endothelial necroptosis aggravating blood brain-barrier disruption after ischemic stroke. Cell Death Dis. 10:487. doi: 10.1038/s41419-019-1716-9
Chen, Q., Kang, J., and Fu, C. (2018). The independence of and associations among apoptosis, autophagy, and necrosis. Sig. Trans. Target. Therapy 3:18. doi: 10.1038/s41392-018-0018-5
Chueh, T. H., Cheng, Y. H., Chen, K. H., and Chien, C. T. (2020). Thromboxane A2 synthase and thromboxane receptor deletion reduces Ischaemia/reperfusion-evoked inflammation, apoptosis. Autophagy and pyroptosis. Thromb Haemost. 120, 329–343. doi: 10.1055/s-0039-3400304
Collaborators, G. B. D. S. (2019). Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 18, 439–458. doi: 10.1016/S1474-4422(19)30034-1
Costa Pereira, L. M., Thongkittidilok, C., Lopes, M. D., and Songsasen, N. (2018). Effect of anti-apoptotic drug Z-VAD-FMK on in vitro viability of dog follicles. Theriogenology 122, 124–129. doi: 10.1016/j.theriogenology.2018.09.012
Cruz, S. A., Qin, Z., Stewart, A. F. R., and Chen, H. H. (2018). Dabrafenib, an inhibitor of RIP3 kinase-dependent necroptosis, reduces ischemic brain injury. Neural Regen. Res. 13, 252–256. doi: 10.4103/1673-5374.226394
Cui, D. R., Wang, L., Jiang, W., Qi, A. H., Zhou, Q. H., and Zhang, X. L. (2013). Propofol prevents cerebral ischemia-triggered autophagy activation and cell death in the rat hippocampus through the NF-κB/p53 signaling pathway. Neuroscience 246, 117–132. doi: 10.1016/j.neuroscience.2013.04.054
Davidson, S. M., Adameova, A., Barile, L., Cabrera-Fuentes, H. A., Lazou, A., Pagliaro, P., et al. (2020). Mitochondrial and mitochondrial-independent pathways of myocardial cell death during ischaemia and reperfusion injury. J. Cell. Mol. Med. 24, 3795–3806. doi: 10.1111/jcmm.15127
De Duve, C., and Wattiaux, R. (1966). Functions of lysosomes. Annu. Rev. Physiol. 28, 435–492. doi: 10.1146/annurev.ph.28.030166.002251
Degterev, A., Huang, Z., Boyce, M., Li, Y., Jagtap, P., Mizushima, N., et al. (2005). Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 1, 112–119. doi: 10.1038/nchembio711
Degterev, A., Ofengeim, D., and Yuan, J. (2019). Targeting RIPK1 for the treatment of human diseases. Proc. Natl. Acad. Sci. USA 116, 9714–9722. doi: 10.1073/pnas.1901179116
Deng, X. X., Li, S. S., and Sun, F. Y. (2019). Necrostatin-1 prevents necroptosis in brains after ischemic stroke via inhibition of RIPK1-mediated RIPK3/MLKL signaling. Aging Dis. 10, 807–817. doi: 10.14336/AD.2018.0728
Denisenko, T. V., Pivnyuk, A. D., and Zhivotovsky, B. (2018). p53-Autophagy-Metastasis Link. Cancer 10:148. doi: 10.3390/cancers10050148
Dikic, I., and Elazar, Z. (2018). Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 19, 349–364. doi: 10.1038/s41580-018-0003-4
Dillon, C. P., Weinlich, R., Rodriguez, D. A., Cripps, J. G., Quarato, G., Gurung, P., et al. (2014). RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell 157, 1189–1202. doi: 10.1016/j.cell.2014.04.018
Ding, J. J., Wang, K., Liu, W., She, Y., Sun, Q., Shi, J. J., et al. (2016). Pore-forming activity and structural autoinhibition of the gasdermin family (vol 535, pg 111, 2016). Nature 540, 150–116. doi: 10.1038/nature20106
Dominic, A., Le, N. T., and Takahashi, M. (2022). Loop between NLRP3 Inflammasome and reactive oxygen species. Antioxid. Redox Signal. 36, 784–796. doi: 10.1089/ars.2020.8257
Eckl, E. M., Ziegemann, O., Krumwiede, L., Fessler, E., and Jae, L. T. (2021). Sensing, signaling and surviving mitochondrial stress. Cell. Molecular Life Sci. 78, 5925–5951. doi: 10.1007/s00018-021-03887-7
Fakharnia, F., Khodagholi, F., Dargahi, L., and Ahmadiani, A. (2017). Prevention of Cyclophilin D-mediated mPTP opening using cyclosporine-a alleviates the elevation of necroptosis, autophagy and apoptosis-related markers following global cerebral ischemia-reperfusion. J. Mol. Neurosci. 61, 52–60. doi: 10.1007/s12031-016-0843-3
Fan, X., Lin, F., Chen, Y., Dou, Y., Li, T., Jin, X., et al. (2024). Luteolin-7-O-β-d-glucuronide ameliorates cerebral ischemic injury: involvement of RIP3/MLKL signaling pathway. Molecules 29:1665. doi: 10.3390/molecules29071665
Ferrer, I., Friguls, B., Dalfó, E., and Planas, A. M. (2003). Early modifications in the expression of mitogen-activated protein kinase (MAPK/ERK), stress-activated kinases SAPK/JNK and p38, and their phosphorylated substrates following focal cerebral ischemia. Acta Neuropathol. 105, 425–437. doi: 10.1007/s00401-002-0661-2
Forte, M., Bianchi, F., Cotugno, M., Marchitti, S., De Falco, E., Raffa, S., et al. (2020). Pharmacological restoration of autophagy reduces hypertension-related stroke occurrence. Autophagy 16, 1468–1481. doi: 10.1080/15548627.2019.1687215
Frank, D., and Vince, J. E. (2019). Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Differ. 26, 99–114. doi: 10.1038/s41418-018-0212-6
Fricker, M., Tolkovsky, A. M., Borutaite, V., Coleman, M., and Brown, G. C. (2018). Neuronal cell death. Physiol. Rev. 98, 813–880. doi: 10.1152/physrev.00011.2017
Fritsch, M., Gunther, S. D., Schwarzer, R., Albert, M. C., Schorn, F., Werthenbach, J. P., et al. (2019). Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 575, 683–687. doi: 10.1038/s41586-019-1770-6
Gao, J., Long, L., Xu, F., Feng, L., Liu, Y., Shi, J., et al. (2020). Icariside II, a phosphodiesterase 5 inhibitor, attenuates cerebral ischaemia/reperfusion injury by inhibiting glycogen synthase kinase-3beta-mediated activation of autophagy. Br. J. Pharmacol. 177, 1434–1452. doi: 10.1111/bph.14912
Gonzalez-Rodriguez, P., and Fernandez-Lopez, A. (2023). PANoptosis: new insights in regulated cell death in ischemia/reperfusion models. Neural Regen. Res. 18, 342–343. doi: 10.4103/1673-5374.343910
Guo, M., Xu, J., Wang, S., and Dong, B. (2021). Asiaticoside reduces autophagy and improves memory in a rat model of dementia through mTOR signaling pathway regulation. Mol. Med. Rep. 24:645. doi: 10.3892/mmr.2021.12284
Gupta, A., Shah, K., Oza, M. J., and Behl, T. (2019). Reactivation of p53 gene by MDM2 inhibitors: a novel therapy for cancer treatment. Biomed. Pharmacotherapy 109, 484–492. doi: 10.1016/j.biopha.2018.10.155
Han, X., Xu, T., Fang, Q., Zhang, H., Yue, L., Hu, G., et al. (2021). Quercetin hinders microglial activation to alleviate neurotoxicity via the interplay between NLRP3 inflammasome and mitophagy. Redox Biol. 44:102010. doi: 10.1016/j.redox.2021.102010
Han, J., Zhong, C. Q., and Zhang, D. W. (2019). Programmed necrosis: backup to and competitor with apoptosis in the immune system. Nat. Immunol. 12, 1143–1149. doi: 10.1038/ni.2159
He, Q., Li, Z. Y., Wang, Y. T., Hou, Y. H., Li, L. Y., and Zhao, J. (2017). Resveratrol alleviates cerebral ischemia/reperfusion injury in rats by inhibiting NLRP3 inflammasome activation through Sirt1-dependent autophagy induction. Int. Immunopharmacol. 50, 208–215. doi: 10.1016/j.intimp.2017.06.029
Henderson, S. J., Weitz, J. I., and Kim, P. Y. (2018). Fibrinolysis: strategies to enhance the treatment of acute ischemic stroke. J. Thromb. Haemost. 16, 1932–1940. doi: 10.1111/jth.14215
Horvath, C., Young, M., Jarabicova, I., Kindernay, L., Ferenczyova, K., Ravingerova, T., et al. (2021). Inhibition of cardiac RIP3 mitigates early reperfusion injury and calcium-induced mitochondrial swelling without altering Necroptotic Signalling. Int. J. Mol. Sci. 22:7983. doi: 10.3390/ijms22157983
Hou, Y. Y., Wang, K., Wan, W. J., Cheng, Y., Pu, X., and Ye, X. F. (2018). Resveratrol provides neuroprotection by regulating the JAK2/STAT3/PI3K/AKT/mTOR pathway after stroke in rats. Genes Dis. 5, 245–255. doi: 10.1016/j.gendis.2018.06.001
Hou, K., Xu, D., Li, F., Chen, S., and Li, Y. (2019). The progress of neuronal autophagy in cerebral ischemia stroke: mechanisms, roles and research methods. J. Neurol. Sci. 400, 72–82. doi: 10.1016/j.jns.2019.03.015
Hu, Y., Lei, H., Zhang, S., Ma, J., Kang, S., Wan, L., et al. (2022). Panax notoginseng Saponins protect brain microvascular endothelial cells against oxygen-glucose deprivation/resupply-induced necroptosis via suppression of RIP1-RIP3-MLKL signaling pathway. Neurochem. Res. 47, 3261–3271. doi: 10.1007/s11064-022-03675-0
Hu, W., Wu, X., Yu, D., Zhao, L., Zhu, X., Li, X., et al. (2020). Regulation of JNK signaling pathway and RIPK3/AIF in necroptosis-mediated global cerebral ischemia/reperfusion injury in rats. Exp. Neurol. 331:113374. doi: 10.1016/j.expneurol.2020.113374
Huang, Y., Chi, W., Li, Y., Zhang, C., Li, J., and Meng, F. (2024). Morphine preconditioning alleviates ischemia/reperfusion-induced Caspase-8-dependent neuronal apoptosis through cPKCγ-NF-κB-cFLIPL pathway. J. Neurosurg. Anesthesiol. 37, 75–87. doi: 10.1097/ana.0000000000000963
Huang, Q., Lou, T., Wang, M., Xue, L., Lu, J., Zhang, H., et al. (2020). Compound K inhibits autophagy-mediated apoptosis induced by oxygen and glucose deprivation/reperfusion via regulating AMPK-mTOR pathway in neurons. Life Sci. 254:117793. doi: 10.1016/j.lfs.2020.117793
Huang, Z., Zhou, X., Zhang, X., Huang, L., Sun, Y., Cheng, Z., et al. (2021). Pien-Tze-Huang, a Chinese patent formula, attenuates NLRP3 inflammasome-related neuroinflammation by enhancing autophagy via the AMPK/mTOR/ULK1 signaling pathway. Biomed. Pharmacother. 141:111814. doi: 10.1016/j.biopha.2021.111814
Humphries, F., Yang, S., Wang, B., and Moynagh, P. N. (2015). RIP kinases: key decision makers in cell death and innate immunity. Cell Death Differ. 22, 225–236. doi: 10.1038/cdd.2014.126
Husain, M. (2022). Identifying caspases and their motifs that cleave proteins during influenza a virus infection. J. Vis. Exp. 185:e64189. doi: 10.3791/64189-v
Jiang, P., and Mizushima, N. (2014). Autophagy and human diseases. Cell Res. 24, 69–79. doi: 10.1038/cr.2013.161
Jiang, J., Qi, T., Li, L., Pan, Y., Huang, L., Zhu, L., et al. (2024). MRPS9-mediated regulation of the PI3K/Akt/mTOR pathway inhibits neuron apoptosis and protects ischemic stroke. J. Mol. Neurosci. 74:23. doi: 10.1007/s12031-024-02197-4
Jiang, M. X., Qi, L., Li, L. S., Wu, Y. M., Song, D. F., and Li, Y. J. (2021). Caspase-8: a key protein of cross-talk signal way in "PANoptosis" in cancer. Int. J. Cancer 149, 1408–1420. doi: 10.1002/ijc.33698
Jiang, Y., Zhu, J., Wu, L., Xu, G., Dai, J., and Liu, X. (2012). Tetracycline inhibits local inflammation induced by cerebral ischemia via modulating autophagy. PLoS One 7:e48672. doi: 10.1371/journal.pone.0048672
Jiao, K., Lai, Z., Cheng, Q., Yang, Z., Liao, W., Liao, Y., et al. (2024). Glycosides of Buyang Huanwu decoction inhibits inflammation associated with cerebral ischemia-reperfusion via the PINK1/Parkin mitophagy pathway. J. Ethnopharmacol. 325:117766. doi: 10.1016/j.jep.2024.117766
Jing, Y., Xue, L., Nathanael, M., Devin, M. B., Jiping, T., Min, Y., et al. (2018). Ezetimibe, a NPC1L1 inhibitor, attenuates neuronal apoptosis through AMPK dependent autophagy activation after MCAO in rats. Exp. Neurol. 307, 12–23. doi: 10.1016/j.expneurol.2018.05.022
Ke, R., Xu, Q., Li, C., Luo, L., and Huang, D. (2018). Mechanisms of AMPK in the maintenance of ATP balance during energy metabolism. Cell Biol. Int. 42, 384–392. doi: 10.1002/cbin.10915
Kesavardhana, S., Malireddi, R. K. S., Burton, A. R., Porter, S. N., Vogel, P., Pruett-Miller, S. M., et al. (2020a). The Z?2 domain of ZBP1 is a molecular switch regulating influenza-induced PANoptosis and perinatal lethality during development. J. Biol. Chem. 295, 8325–8330. doi: 10.1074/jbc.RA120.013752
Kesavardhana, S., Malireddi, R. K. S., and Kanneganti, T. D. (2020b). Caspases in cell death, inflammation, and Pyroptosis. Annu. Rev. Immunol. 38, 567–595. doi: 10.1146/annurev-immunol-073119-095439
Khan, H., Kashyap, A., Kaur, A., and Singh, T. G. (2020). Pharmacological postconditioning: a molecular aspect in ischemic injury. J. Pharm. Pharmacol. 72, 1513–1527. doi: 10.1111/jphp.13336
Kim, E. K., and Choi, E. J. (2015). Compromised MAPK signaling in human diseases: an update. Arch. Toxicol. 89, 867–882. doi: 10.1007/s00204-015-1472-2
Klionsky, D. J., Abdel-Aziz, A. K., Abdelfatah, S., Abdellatif, M., Abdoli, A., Abel, S., et al. (2021). Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy 17, 1–382. doi: 10.1080/15548627.2020.1797280
Lalaoui, N., Boyden, S. E., Oda, H., Wood, G. M., Stone, D. L., Chau, D., et al. (2020). Mutations that prevent caspase cleavage of RIPK1 cause autoinflammatory disease. Nature 577, 103–108. doi: 10.1038/s41586-019-1828-5
Li, Y., Hu, K., Liang, M., Yan, Q., Huang, M., Jin, L., et al. (2021). Stilbene glycoside upregulates SIRT3/AMPK to promotes neuronal mitochondrial autophagy and inhibit apoptosis in ischemic stroke. Adv. Clin. Exp. Med. 30, 139–146. doi: 10.17219/acem/130608
Li, Z. G., Li, Q. J., Lv, W., Jiang, L. P., Geng, C. Y., Yao, X. F., et al. (2019). The interaction of Atg4B and Bcl-2 plays an important role in cd-induced crosstalk between apoptosis and autophagy through disassociation of Bcl-2-Beclin1 in A549 cells. Free Radical Bio. Med. 130, 576–591. doi: 10.1016/j.freeradbiomed.2018.11.020
Li, L., Song, J.-J., Zhang, M.-X., Zhang, H.-W., Zhu, H.-Y., Guo, W., et al. (2023). Oridonin ameliorates caspase-9-mediated brain neuronal apoptosis in mouse with ischemic stroke by inhibiting RIPK3-mediated mitophagy. Acta Pharmacol. Sin. 44, 726–740. doi: 10.1038/s41401-022-00995-3
Li, W., and Teng, J. (2023). circCELF1 induces the apoptosis and autophagy of astrocytes in ischemic stroke via upregulating NFAT5. Cerebrovasc. Dis. 52, 306–317. doi: 10.1159/000526359
Li, Q., Tian, Z., Wang, M., Kou, J., Wang, C., Rong, X., et al. (2019). Luteoloside attenuates neuroinflammation in focal cerebral ischemia in rats via regulation of the PPARγ/Nrf2/NF-κB signaling pathway. Int. Immunopharmacol. 66, 309–316. doi: 10.1016/j.intimp.2018.11.044
Li, Y., Wang, R., Xue, L., Yang, Y., and Zhi, F. (2020). Astilbin protects against cerebral ischaemia/reperfusion injury by inhibiting cellular apoptosis and ROS-NLRP3 inflammasome axis activation. Int. Immunopharmacol. 84:106571. doi: 10.1016/j.intimp.2020.106571
Li, W. L., Yu, S. P., Chen, D., Yu, S. S., Jiang, Y. J., Genetta, T., et al. (2013). The regulatory role of NF-κB in autophagy-like cell death after focal cerebral ischemia in mice. Neuroscience 244, 16–30. doi: 10.1016/j.neuroscience.2013.03.045
Li, R. Q., Zheng, Y. Y., Zhang, J. X., Zhou, Y., and Fan, X. (2023). Gomisin N attenuated cerebral ischemia-reperfusion injury through inhibition of autophagy by activating the PI3K/AKT/mTOR pathway. Phytomedicine 110:154644. doi: 10.1016/j.phymed.2023.154644
Li, H., Zhou, S., Wu, L., Liu, K., Zhang, Y., Ma, G., et al. (2015). The role of p38MAPK signal pathway in the neuroprotective mechanism of limb postconditioning against rat cerebral ischemia/reperfusion injury. J. Neurol. Sci. 357, 270–275. doi: 10.1016/j.jns.2015.08.004
Liu, D., Dong, Z., Xiang, F., Liu, H., Wang, Y., Wang, Q., et al. (2020). Dendrobium alkaloids promote neural function after cerebral ischemia-reperfusion injury through inhibiting Pyroptosis induced neuronal death in both in vivo and in vitro models. Neurochem. Res. 45, 437–454. doi: 10.1007/s11064-019-02935-w
Liu, J., Guo, Z. N., Yan, X. L., Huang, S., Ren, J. X., Luo, Y., et al. (2020). Crosstalk between autophagy and Ferroptosis and its putative role in ischemic stroke. Front. Cell. Neurosci. 14:577403. doi: 10.3389/fncel.2020.577403
Liu, M., Li, Y., Han, S., Wang, H., and Li, J. (2023). Activin a alleviates neuronal injury through inhibiting cGAS-STING-mediated autophagy in mice with ischemic stroke. J. Cereb. Blood Flow Metab. 43, 736–748. doi: 10.1177/0271678X221147056
Liu, X., Lv, X., Liu, Z., Zhang, M., and Leng, Y. (2022). MircoRNA-29a in astrocyte-derived extracellular vesicles suppresses brain ischemia reperfusion injury via TP53INP1 and the NF-kappaB/NLRP3 Axis. Cell. Mol. Neurobiol. 42, 1487–1500. doi: 10.1007/s10571-021-01040-3
Liu, J., Ma, W., Zang, C. H., Wang, G. D., Zhang, S. J., Wu, H. J., et al. (2021a). Salidroside inhibits NLRP3 inflammasome activation and apoptosis in microglia induced by cerebral ischemia/reperfusion injury by inhibiting the TLR4/NF-κB signaling pathway. Ann. Transl. Med. 9:1694. doi: 10.21037/atm-21-5752
Liu, J., Wang, C., Li, J., Yu, Y., Liu, Y., Liu, H., et al. (2021b). Autophagy blockage promotes the pyroptosis of ox-LDL-treated macrophages by modulating the p62/Nrf2/ARE axis. J. Physiol. Biochem. 77, 419–429. doi: 10.1007/s13105-021-00811-2
Liu, Z., Wang, M., Wang, X., Bu, Q., Wang, Q., Su, W., et al. (2022). XBP1 deficiency promotes hepatocyte pyroptosis by impairing mitophagy to activate mtDNA-cGAS-STING signaling in macrophages during acute liver injury. Redox Biol. 52:102305. doi: 10.1016/j.redox.2022.102305
Liu, X., Ye, Q., Huang, Z., Li, X., Zhang, L., Liu, X., et al. (2023). BAG3 overexpression attenuates ischemic stroke injury by activating autophagy and inhibiting apoptosis. Stroke 54, 2114–2125. doi: 10.1161/STROKEAHA.123.041783
Liu, H., Zhao, Z., Wu, T., Zhang, Q., Lu, F., Gu, J., et al. (2021). Inhibition of autophagy-dependent pyroptosis attenuates cerebral ischaemia/reperfusion injury. J. Cell. Mol. Med. 25, 5060–5069. doi: 10.1111/jcmm.16483
Long, J., Sun, Y., Liu, S., Chen, C., Yan, Q., Lin, Y., et al. (2024). Ginsenoside Rg1 treats ischemic stroke by regulating CKLF1/CCR5 axis-induced neuronal cell pyroptosis. Phytomedicine 123:155238. doi: 10.1016/j.phymed.2023.155238
Lunemann, J. D., Malhotra, S., Shinohara, M. L., Montalban, X., and Comabella, M. (2021). Targeting Inflammasomes to treat neurological diseases. Ann. Neurol. 90, 177–188. doi: 10.1002/ana.26158
Luo, J., Chen, J., Yang, C., Tan, J., Zhao, J., Jiang, N., et al. (2021). 6-Gingerol protects against cerebral ischemia/reperfusion injury by inhibiting NLRP3 inflammasome and apoptosis via TRPV1 / FAF1 complex dissociation-mediated autophagy. Int. Immunopharmacol. 100:108146. doi: 10.1016/j.intimp.2021.108146
Luo, C., Ouyang, M. W., Fang, Y. Y., Li, S. J., Zhou, Q., Fan, J., et al. (2017). Dexmedetomidine protects mouse brain from ischemia-reperfusion injury via inhibiting neuronal autophagy through up-regulating HIF-1α. Front. Cell. Neurosci. 11:197. doi: 10.3389/fncel.2017.00197
Ma, X., Hao, J., Wu, J., Li, Y., Cai, X., and Zheng, Y. (2022). Prussian blue Nanozyme as a Pyroptosis inhibitor alleviates neurodegeneration. Adv. Materials (Deerfield Beach, Fla.) 34:e2106723. doi: 10.1002/adma.202106723
Malireddi, R. K. S., Tweedell, R. E., and Kanneganti, T. D. (2020). PANoptosis components, regulation, and implications. Aging (Albany NY) 12, 11163–11164. doi: 10.18632/aging.103528
Martens, S. (2018). A division of labor in mTORC1 signaling and autophagy. Sci. Signal. 11:eaav3530. doi: 10.1126/scisignal.aav3530
McKenzie, B. A., Dixit, V. M., and Power, C. (2020). Fiery cell death: Pyroptosis in the central nervous system. Trends Neurosci. 43, 55–73. doi: 10.1016/j.tins.2019.11.005
Mei, Z. G., Tan, L. J., Wang, J. F., Li, X. L., Huang, W. F., and Zhou, H. J. (2017). Fermented Chinese formula Shuan-Tong-Ling attenuates ischemic stroke by inhibiting inflammation and apoptosis. Neural Regen. Res. 12, 425–432. doi: 10.4103/1673-5374.202946
Mo, Y., Sun, Y. Y., and Liu, K. Y. (2020). Autophagy and inflammation in ischemic stroke. Neural Regen. Res. 15, 1388–1396. doi: 10.4103/1673-5374.274331
Mouton-Liger, F., Rosazza, T., Sepulveda-Diaz, J., Ieang, A., Hassoun, S. M., Claire, E., et al. (2018). Parkin deficiency modulates NLRP3 inflammasome activation by attenuating an A20-dependent negative feedback loop. Glia 66, 1736–1751. doi: 10.1002/glia.23337
Naito, M. G., Xu, D., Amin, P., Lee, J., Wang, H., Li, W., et al. (2020). Sequential activation of necroptosis and apoptosis cooperates to mediate vascular and neural pathology in stroke. Proc. Natl. Acad. Sci. USA 117, 4959–4970. doi: 10.1073/pnas.1916427117
Najafov, A., Luu, H. S., Mookhtiar, A. K., Mifflin, L., Xia, H. G., Amin, P. P., et al. (2021). RIPK1 promotes energy sensing by the mTORC1 pathway. Mol. Cell 81, 370–385.e7. doi: 10.1016/j.molcel.2020.11.008
Nitatori, T., Sato, N., Waguri, S., Karasawa, Y., Araki, H., Shibanai, K., et al. (1995). Delayed neuronal death in the CA1 pyramidal cell layer of the gerbil hippocampus following transient ischemia is apoptosis. Journal of neuroscience the official journal of the society for. Neuroscience 15, 1001–1011. doi: 10.1523/JNEUROSCI.15-02-01001.1995
Noorolyai, S., Shajari, N., Baghbani, E., Sadreddini, S., and Baradaran, B. (2019). The relation between PI3K/AKT signalling pathway and cancer. Gene 698, 120–128. doi: 10.1016/j.gene.2019.02.076
Oh, S., Lee, J., Oh, J., Yu, G., Ryu, H., Kim, D., et al. (2023). Integrated NLRP3, AIM2, NLRC4, pyrin inflammasome activation and assembly drive PANoptosis. Cell. Mol. Immunol. 20, 1513–1526. doi: 10.1038/s41423-023-01107-9
Poh, L., Kang, S.-W., Baik, S.-H., Ng, G. Y. Q., She, D. T., Balaganapathy, P., et al. (2019). Evidence that NLRC4 inflammasome mediates apoptotic and pyroptotic microglial death following ischemic stroke. Brain Behav. Immun. 75, 34–47. doi: 10.1016/j.bbi.2018.09.001
Russell, R. C., Yuan, H. X., and Guan, K. L. (2014). Autophagy regulation by nutrient signaling. Cell Res. 24, 42–57. doi: 10.1038/cr.2013.166
Ryan, F., Khodagholi, F., Dargahi, L., Minai-Tehrani, D., and Ahmadiani, A. (2018). Temporal pattern and crosstalk of necroptosis markers with autophagy and apoptosis associated proteins in ischemic Hippocampus. Neurotox. Res. 34, 79–92. doi: 10.1007/s12640-017-9861-3
Sanchez-Fernandez, A., Skouras, D. B., Dinarello, C. A., and Lopez-Vales, R. (2019). OLT1177 (Dapansutrile), a selective NLRP3 Inflammasome inhibitor, ameliorates experimental autoimmune encephalomyelitis pathogenesis. Front. Immunol. 10:2578. doi: 10.3389/fimmu.2019.02578
Sarhan, J., Liu, B. C., Muendlein, H. I., Li, P., Nilson, R., Tang, A. Y., et al. (2018). Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc. Natl. Acad. Sci. USA 115, E10888–E10897. doi: 10.1073/pnas.1809548115
Seglen, P. O., and Gordon, P. B. (1982). 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc. Natl. Acad. Sci. USA 79, 1889–1892. doi: 10.1073/pnas.79.6.1889
Shi, M., Chen, J., Liu, T., Dai, W., Zhou, Z., Chen, L., et al. (2022). Protective effects of Remimazolam on cerebral ischemia/reperfusion injury in rats by inhibiting of NLRP3 Inflammasome-dependent Pyroptosis. Drug Des. Devel. Ther. 16, 413–423. doi: 10.2147/dddt.s344240
Shi, B., Ma, M., Zheng, Y., Pan, Y., and Lin, X. (2019). mTOR and Beclin1: two key autophagy-related molecules and their roles in myocardial ischemia/reperfusion injury. J. Cell. Physiol. 234, 12562–12568. doi: 10.1002/jcp.28125
Shi, J. J., Zhao, Y., Wang, K., Shi, X. Y., Wang, Y., Huang, H. W., et al. (2015). Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665. doi: 10.1038/nature15514
Sodhi, R. K., Grewal, A. K., Madan, J., Jhajj, T. G. S., and Kumar, R. (2021). Recent approaches to target apoptosis in neurological disorders. Clini. Persp. Target. Therap. Apopt., 8, 217–283. doi: 10.1016/B978-0-12-815762-6.00008-1
Song, Y. Q., Zou, H. L., Zhao, Y. J., Yu, L. Q., Tan, Z. X., and Kong, R. (2016). Activation of p38-mitogen-activated protein kinase contributes to ischemia reperfusion in rat brain. Genet. Mol. Res. 15, 1–3. doi: 10.4238/gmr.15038492
Sun, C., Cao, N., Wang, Q., Liu, N., Yang, T., Li, S., et al. (2023). Icaritin induces resolution of inflammation by targeting cathepsin B to prevents mice from ischemia-reperfusion injury. Int. Immunopharmacol. 116:109850. doi: 10.1016/j.intimp.2023.109850
Sun, B., Ou, H., Ren, F., Guan, Y., Huan, Y., and Cai, H. (2021). Propofol protects against cerebral ischemia/reperfusion injury by Down-regulating Long noncoding RNA SNHG14. ACS Chem. Neurosci. 12, 3002–3014. doi: 10.1021/acschemneuro.1c00059
Sun, B., Ou, H., Ren, F., Huan, Y., Zhong, T., Gao, M., et al. (2018). Propofol inhibited autophagy through ca/CaMKKβ/AMPK/mTOR pathway in OGD/R-induced neuron injury. Mol. Med. 24, 1–11. doi: 10.1186/s10020-018-0054-1
Sun, X., Wang, D., Zhang, T., Lu, X., Duan, F., Ju, L., et al. (2020). Eugenol attenuates cerebral ischemia-reperfusion injury by enhancing autophagy via AMPK-mTOR-P70S6K pathway. Front. Pharmacol. 11:84. doi: 10.3389/fphar.2020.00084
Sun, Y., Zhu, Y., Zhong, X., Chen, X., Wang, J., and Ying, G. (2018). Crosstalk between autophagy and cerebral ischemia. Front. Neurosci. 12:1022. doi: 10.3389/fnins.2018.01022
Sundaram, B., Karki, R., and Kanneganti, T. D. (2022). NLRC4 deficiency leads to enhanced phosphorylation of MLKL and necroptosis. Immunohorizons 6, 243–252. doi: 10.4049/immunohorizons.2100118
Tanemura, M., Ohmura, Y., Deguchi, T., Machida, T., Tsukamoto, R., Wada, H., et al. (2012). Rapamycin causes upregulation of autophagy and impairs islets function both in vitro and in vivo. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transplant Surg. 12, 102–114. doi: 10.1111/j.1600-6143.2011.03771.x
Tao, P., Sun, J., Wu, Z., Wang, S., Wang, J., Li, W., et al. (2020). A dominant autoinflammatory disease caused by non-cleavable variants of RIPK1. Nature 577, 109–114. doi: 10.1038/s41586-019-1830-y
Thapa, K., Singh, T. G., and Kaur, A. (2021). Cyclic nucleotide phosphodiesterase inhibition as a potential therapeutic target in renal ischemia reperfusion injury. Life Sci. 282:119843. doi: 10.1016/j.lfs.2021.119843
Tian, J., Zhang, Y. Y., Peng, Y. W., Liu, B., Zhang, X. J., Hu, Z. Y., et al. (2022). Polymyxin B reduces brain injury in ischemic stroke rat through a mechanism involving targeting ESCRT-III machinery and RIPK1/RIPK3/MLKL pathway. J. Cardiovasc. Transl. Res. 15, 1129–1142. doi: 10.1007/s12265-022-10224-1
Wang, Y., Bai, G., Mu, S., Zhang, F., and Wang, Y. (2024a). Indobufen alleviates ischemic stroke injury by regulating transcription factor NRF2 and inhibiting ATG5 expression. J. Pharm. Pharmacol. 76, 842–850. doi: 10.1093/jpp/rgae038
Wang, Y. P., Gao, W. Q., Shi, X. Y., Ding, J. J., Liu, W., He, H. B., et al. (2017). Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547, 99–103. doi: 10.1038/nature22393
Wang, Y., Guan, X., Gao, C. L., Ruan, W., Zhao, S., Kai, G., et al. (2021). Medioresinol as a novel PGC-1α activator prevents pyroptosis of endothelial cells in ischemic stroke through PPARα-GOT1 axis. Pharmacol. Res. 169:105640. doi: 10.1016/j.phrs.2021.105640
Wang, Z., Guo, L. M., Wang, Y., Zhou, H. K., Wang, S. C., Chen, D., et al. (2018). Inhibition of HSP90alpha protects cultured neurons from oxygen-glucose deprivation induced necroptosis by decreasing RIP3 expression. J. Cell. Physiol. 233, 4864–4884. doi: 10.1002/jcp.26294
Wang, S., Han, X., Mao, Z., Xin, Y., Maharjan, S., and Zhang, B. (2019). MALAT1 lncRNA induces autophagy and protects brain microvascular endothelial cells against oxygen-glucose deprivation by binding to miR-200c-3p and upregulating SIRT1 expression. Neuroscience 397, 116–126. doi: 10.1016/j.neuroscience.2018.11.024
Wang, M., Lee, H., Elkin, K., Bardhi, R., Guan, L., Chandra, A., et al. (2020). Detrimental and beneficial effect of autophagy and a potential therapeutic target after ischemic stroke. Evid. Based Complement. Alternat. Med. 2020, 1–10. doi: 10.1155/2020/8372647
Wang, J. X., Lin, X. J., Mu, Z. H., Shen, F. X., Zhang, L. Y., Xie, Q., et al. (2019). Rapamycin increases collateral circulation in rodent brain after focal ischemia as detected by multiple modality dynamic imaging. Theranostics 9, 4923–4934. doi: 10.7150/thno.32676
Wang, J., Liu, Y., Li, X. H., Zeng, X. C., Li, J., Zhou, J., et al. (2017). Curcumin protects neuronal cells against status-epilepticus-induced hippocampal damage through induction of autophagy and inhibition of necroptosis. Can. J. Physiol. Pharmacol. 95, 501–509. doi: 10.1139/cjpp-2016-0154
Wang, H., Lv, D., Jiang, S., Hou, Q., Zhang, L., Li, S., et al. (2022). Complement induces podocyte pyroptosis in membranous nephropathy by mediating mitochondrial dysfunction. Cell Death Dis. 13:281. doi: 10.1038/s41419-022-04737-5
Wang, J. F., Mei, Z. G., Fu, Y., Yang, S. B., Zhang, S. Z., Huang, W. F., et al. (2018). Puerarin protects rat brain against ischemia/reperfusion injury by suppressing autophagy via the AMPK-mTOR-ULK1 signaling pathway. Neural Regen. Res. 13, 989–998. doi: 10.4103/1673-5374.233441
Wang, C. P., Shi, Y. W., Tang, M., Zhang, X. C., Gu, Y., Liang, X. M., et al. (2017). Isoquercetin ameliorates cerebral impairment in focal ischemia through anti-oxidative, anti-inflammatory, and anti-apoptotic effects in primary culture of rat hippocampal neurons and hippocampal CA1 region of rats. Mol. Neurobiol. 54, 2126–2142. doi: 10.1007/s12035-016-9806-5
Wang, R., Wang, M., Fan, Y. C., Wang, W. J., Zhang, D. H., Andy Li, P., et al. (2024). Hyperglycemia exacerbates cerebral ischemia/reperfusion injury by up-regulating autophagy through p53-Sesn2-AMPK pathway. Neurosci. Lett. 821:137629. doi: 10.1016/j.neulet.2024.137629
Wang, L., Wu, D., and Xu, Z. (2019). USP10 protects against cerebral ischemia injury by suppressing inflammation and apoptosis through the inhibition of TAK1 signaling. Biochem. Biophys. Res. Commun. 516, 1272–1278. doi: 10.1016/j.bbrc.2019.06.042
Wang, Y., Yuan, H., Shen, D., Liu, S., Kong, W., Zheng, K., et al. (2024b). Artemisinin attenuated ischemic stroke induced pyroptosis by inhibiting ROS/TXNIP/NLRP3/Caspase-1 signaling pathway. Biomed. Pharmacotherapy 177:116894. doi: 10.1016/j.biopha.2024.116894
Wasan, H., Singh, D., Joshi, B., Sharma, U., Dinda, A. K., and Reeta, K. H. (2021). Post stroke safinamide treatment attenuates neurological damage by modulating autophagy and apoptosis in experimental model of stroke in rats. Mol. Neurobiol. 58, 6121–6135. doi: 10.1007/s12035-021-02523-6
Wei, R., Song, L., Miao, Z., Liu, K., Han, G., Zhang, H., et al. (2022). Hydroxysafflor yellow a exerts neuroprotective effects via HIF-1ɑ/BNIP3 pathway to activate neuronal autophagy after OGD/R. Cells 11:3726. doi: 10.3390/cells11233726
Wu, X., Lin, L., Qin, J. J., Wang, L., Wang, H., Zou, Y., et al. (2020). CARD3 promotes cerebral ischemia-reperfusion injury via activation of TAK1. J. Am. Heart Assoc. 9:e014920. doi: 10.1161/JAHA.119.014920
Wu, W. X., Wang, X. J., Berleth, N., Deitersen, J., Wallot-Hieke, N., Böhler, P., et al. (2020). The autophagy-initiating kinase ULK1 controls RIPK1-mediated cell death. Cell Rep. 31:107547. doi: 10.1016/j.celrep.2020.107547
Wu, M., Zhang, H., Kai, J., Zhu, F., Dong, J., Xu, Z., et al. (2018). Rapamycin prevents cerebral stroke by modulating apoptosis and autophagy in penumbra in rats. Ann. Clin. Transl. Neurol. 5, 138–146. doi: 10.1002/acn3.507
Xia, P., Marjan, M., Liu, Z., Zhou, W., Zhang, Q., Cheng, C., et al. (2022). Chrysophanol postconditioning attenuated cerebral ischemia-reperfusion injury induced NLRP3-related pyroptosis in a TRAF6-dependent manner. Exp. Neurol. 357:114197. doi: 10.1016/j.expneurol.2022.114197
Xiao, L., Dai, Z., Tang, W., Liu, C., and Tang, B. (2021). Astragaloside IV alleviates cerebral ischemia-reperfusion injury through NLRP3 Inflammasome-mediated Pyroptosis inhibition via activating Nrf2. Oxidative Med. Cell. Longev. 2021:9925561. doi: 10.1155/2021/9925561
Xiaowei, X., Qian, X., and Dingzhou, Z. (2023). Sirtuin-3 activates the mitochondrial unfolded protein response and reduces cerebral ischemia/reperfusion injury. Int. J. Biol. Sci. 19, 4327–4339. doi: 10.7150/ijbs.86614
Xu, S., Huang, P., Yang, J., Du, H., Wan, H., and He, Y. (2023). Calycosin alleviates cerebral ischemia/reperfusion injury by repressing autophagy via STAT3/FOXO3a signaling pathway. Phytomedicine 115:154845. doi: 10.1016/j.phymed.2023.154845
Xu, Y., Ma, H. B., Fang, Y. L., Zhang, Z. R., Shao, J., Hong, M., et al. (2017). Cisplatin-induced necroptosis in TNFalpha dependent and independent pathways. Cell. Signal. 31, 112–123. doi: 10.1016/j.cellsig.2017.01.004
Yan, W. T., Lu, S., Yang, Y. D., Ning, W. Y., Cai, Y., Hu, X. M., et al. (2021). Research trends, hot spots and prospects for necroptosis in the field of neuroscience. Neural Regen. Res. 16, 1628–1637. doi: 10.4103/1673-5374.303032
Yan, W. T., Yang, Y. D., Hu, X. M., Ning, W. Y., Liao, L. S., Lu, S., et al. (2022). Do pyroptosis, apoptosis, and necroptosis (PANoptosis) exist in cerebral ischemia? Evidence from cell and rodent studies. Neural Regen. Res. 17, 1761–1768. doi: 10.4103/1673-5374.331539
Yan, W., Zhang, H., Bai, X., Lu, Y., Dong, H., and Xiong, L. (2011). Autophagy activation is involved in neuroprotection induced by hyperbaric oxygen preconditioning against focal cerebral ischemia in rats. Brain Res. 1402, 109–121. doi: 10.1016/j.brainres.2011.05.049
Yan, W. T., Zhao, W. J., Hu, X. M., Ban, X. X., Ning, W. Y., Wan, H., et al. (2023). PANoptosis-like cell death in ischemia/reperfusion injury of retinal neurons. Neural Regen. Res. Publish Ahead of Print, 357–363. doi: 10.4103/1673-5374.346545
Yang, Y. D., Li, Z. X., Hu, X. M., Wan, H., Zhang, Q., Xiao, R., et al. (2022). Insight into crosstalk between Mitophagy and apoptosis/necroptosis: mechanisms and clinical applications in ischemic stroke. Curr. Med. Sci. 42, 237–248. doi: 10.1007/s11596-022-2579-3
Yang, X. S., Yi, T. L., Zhang, S., Xu, Z. W., Yu, Z. Q., Sun, H. T., et al. (2017). Hypoxia-inducible factor-1 alpha is involved in RIP-induced necroptosis caused by in vitro and in vivo ischemic brain injury. Sci. Rep. 7:5818. doi: 10.1038/s41598-017-06088-0
Yilmaz, U., Tanbek, K., Gul, S., Gul, M., Koc, A., and Sandal, S. (2023). Melatonin attenuates cerebral ischemia/reperfusion injury through inducing autophagy. Neuroendocrinology 113, 1035–1050. doi: 10.1159/000531567
Yin, F., Zhou, H., Fang, Y., Li, C., He, Y., Yu, L., et al. (2020). Astragaloside IV alleviates ischemia reperfusion-induced apoptosis by inhibiting the activation of key factors in death receptor pathway and mitochondrial pathway. J. Ethnopharmacol. 248:112319. doi: 10.1016/j.jep.2019.112319
Yoshii, S. R., and Noboru, M. (2017). Monitoring and measuring autophagy. Int. J. Molecular Ences 18:1865. doi: 10.3390/ijms18091865
Yu, F., Xue, W., Dong, L., Hu, X., Huang, D., and Wang, K. (2019). Tetrahydroxystilbene glucoside suppresses NAPDH oxidative stress to mitigate apoptosis and autophagy induced by cerebral ischemia/reperfusion injury in mice. Evid. Based Complement. Alternat. Med. 2019, 3913981–3913989. doi: 10.1155/2019/3913981
Yuan, X., Ye, W., Chen, L., Luo, D., Zhou, L., Qiu, Y., et al. (2023). URB597 exerts neuroprotective effects against transient brain ischemia injury in mice by regulating autophagic flux and necroptosis. Eur. J. Pharmacol. 957:175982. doi: 10.1016/j.ejphar.2023.175982
Yuan, Y., Zheng, Y., Zhang, X., Chen, Y., Wu, X., Wu, J., et al. (2017). BNIP3L/NIX-mediated mitophagy protects against ischemic brain injury independent of PARK2. Autophagy 13, 1754–1766. doi: 10.1080/15548627.2017.1357792
Zang, R., Ling, F., Wu, Z., Sun, J., Yang, L., Lv, Z., et al. (2023). Ginkgo biloba extract (EGb-761) confers neuroprotection against ischemic stroke by augmenting autophagic/lysosomal signaling pathway. J. Neuroimmunol. 382:578101. doi: 10.1016/j.jneuroim.2023.578101
Zeyen, T., Noristani, R., Habib, S., Heinisch, O., Slowik, A., Huber, M., et al. (2020). Microglial-specific depletion of TAK1 is neuroprotective in the acute phase after ischemic stroke. J. Mol. Med. 98, 833–847. doi: 10.1007/s00109-020-01916-9
Zhang, Y., Li, M., Li, X., Zhang, H., Wang, L., Wu, X., et al. (2020). Catalytically inactive RIP1 and RIP3 deficiency protect against acute ischemic stroke by inhibiting necroptosis and neuroinflammation. Cell Death Dis. 11:565. doi: 10.1038/s41419-020-02770-w
Zhang, Y. Y., Liu, W. N., Li, Y. Q., Zhang, X. J., Yang, J., Luo, X. J., et al. (2019). Ligustroflavone reduces necroptosis in rat brain after ischemic stroke through targeting RIPK1/RIPK3/MLKL pathway. N-S Arch. Pharmacol. 392, 1085–1095. doi: 10.1007/s00210-019-01656-9
Zhang, F., Ran, Y., Tahir, M., Li, Z., Wang, J., and Chen, X. (2022). Regulation of N6-methyladenosine (m6A) RNA methylation in microglia-mediated inflammation and ischemic stroke. Front. Cell. Neurosci. 16:955222. doi: 10.3389/fncel.2022.955222
Zhang, H., Wang, X., Chen, W., Yang, Y., Wang, Y., Wan, H., et al. (2023). Danhong injection alleviates cerebral ischemia-reperfusion injury by inhibiting autophagy through miRNA-132-3p/ATG12 signal axis. J. Ethnopharmacol. 300:115724. doi: 10.1016/j.jep.2022.115724
Zhang, S., Wang, Y., Li, D., Wu, J., Si, W., and Wu, Y. (2016). Necrostatin-1 attenuates inflammatory response and improves cognitive function in chronic ischemic stroke mice. Medicines (Basel) 3:16. doi: 10.3390/medicines3030016
Zhang, L., Wu, M., and Chen, Z. (2022). Schaftoside improves cerebral ischemia-reperfusion injury by enhancing autophagy and reducing apoptosis and inflammation through the AMPK/mTOR pathway. Adv. Clin. Exp. Med. 31, 1343–1354. doi: 10.17219/acem/152207
Zhang, Y., Zhang, Y., Jin, X. F., Zhou, X. H., Dong, X. H., Yu, W. T., et al. (2019). The role of Astragaloside IV against cerebral ischemia/reperfusion injury: suppression of apoptosis via promotion of P62-LC3-autophagy. Molecules 24:1838. doi: 10.3390/molecules24091838
Zhang, X. M., Zhang, L., Wang, G., Niu, W., He, Z., Ding, L., et al. (2015). Suppression of mitochondrial fission in experimental cerebral ischemia: the potential neuroprotective target of p38 MAPK inhibition. Neurochem. Int. 90, 1–8. doi: 10.1016/j.neuint.2015.06.010
Zhang, D. M., Zhang, T., Wang, M. M., Wang, X. X., Qin, Y. Y., Wu, J., et al. (2019). TIGAR alleviates ischemia/reperfusion-induced autophagy and ischemic brain injury. Free Radic. Biol. Med. 137, 13–23. doi: 10.1016/j.freeradbiomed.2019.04.002
Zhang, L., Zhou, T., Ji, Q., He, L., Lan, Y., Ding, L., et al. (2023). Myricetin improves apoptosis after ischemic stroke via inhibiting MAPK-ERK pathway. Mol. Biol. Rep. 50, 2545–2557. doi: 10.1007/s11033-022-08238-8
Zhao, Y., Li, Z., Lu, E., Sheng, Q., and Zhao, Y. (2021). Berberine exerts neuroprotective activities against cerebral ischemia/reperfusion injury through up-regulating PPAR-γ to suppress NF-κB-mediated pyroptosis. Brain Res. Bull. 177, 22–30. doi: 10.1016/j.brainresbull.2021.09.005
Zheng, Y., Hu, Y., Han, Z., Yan, F., Zhang, S., Yang, Z., et al. (2022). Lomitapide ameliorates middle cerebral artery occlusion-induced cerebral ischemia/reperfusion injury by promoting neuronal autophagy and inhibiting microglial migration. CNS Neurosci. Ther. 28, 2183–2194. doi: 10.1111/cns.13961
Zheng, M., and Kanneganti, T. D. (2020). The regulation of the ZBP1-NLRP3 inflammasome and its implications in pyroptosis, apoptosis, and necroptosis (PANoptosis). Immunol. Rev. 297, 26–38. doi: 10.1111/imr.12909
Zheng, M., Williams, E. P., Malireddi, R. K. S., Karki, R., Banoth, B., Burton, A., et al. (2020). Impaired NLRP3 inflammasome activation/pyroptosis leads to robust inflammatory cell death via caspase-8/RIPK3 during coronavirus infection. J. Biol. Chem. 295, 14040–14052. doi: 10.1074/jbc.RA120.015036
Zhou, R. P., Leng, T. D., Yang, T., Chen, F. H., Hu, W., and Xiong, Z. G. (2019). β-Estradiol protects against acidosis-mediated and ischemic neuronal injury by promoting ASIC1a (acid-sensing Ion Channel 1a) protein degradation. Stroke 50, 2902–2911. doi: 10.1161/STROKEAHA.119.025940
Zhou, X. Y., Lin, B., Chen, W., Cao, R. Q., Guo, Y., Said, A., et al. (2023). The brain protection of MLKL inhibitor necrosulfonamide against focal ischemia/reperfusion injury associating with blocking the nucleus and nuclear envelope translocation of MLKL and RIP3K. Front. Pharmacol. 14:1157054. doi: 10.3389/fphar.2023.1157054
Zhou, M., Wang, H., Zeng, X., Yin, P., Zhu, J., Chen, W., et al. (2019). Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 394, 1145–1158. doi: 10.1016/S0140-6736(19)30427-1
Zhou, Y. L., Zhou, B. Q., Tu, H., Tang, Y., Xu, C., Chen, Y. B., et al. (2017). The degradation of mixed lineage kinase domain-like protein promotes neuroprotection after ischemic brain injury. Oncotarget 8, 68393–68401. doi: 10.18632/oncotarget.19416
Keywords: ischemic stroke, PANoptosis, autophagy, pyroptosis, apoptosis, necroptosis, therapeutics
Citation: Tian H-Y, Lei Y-X, Zhou J-T, Liu L-J, Yang T, Zhou Y, Ge J-W, Xu C and Mei Z-G (2025) Insight into interplay between PANoptosis and autophagy: novel therapeutics in ischemic stroke. Front. Mol. Neurosci. 17:1482015. doi: 10.3389/fnmol.2024.1482015
Received: 17 August 2024; Accepted: 19 December 2024;
Published: 08 January 2025.
Edited by:
Junke Song, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaReviewed by:
Marius Baeken, Johannes Gutenberg University Mainz, GermanyCopyright © 2025 Tian, Lei, Zhou, Liu, Yang, Zhou, Ge, Xu and Mei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi-Gang Mei, bWVpemhpZ2FuZ0BobnVjbS5lZHUuY24=; Chen Xu, eHVjaGVuQHN6cHUuZWR1LmNu; Jin-Wen Ge, MDAxMjY3QGhudWNtLmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.