- 1Department of Neurobiology and Behavior, University of California, Irvine, Irvine, CA, United States
- 2Center for the Neurobiology of Learning and Memory, University of California, Irvine, Irvine, CA, United States
- 3Institute for Memory Impairments and Neurological Disorders, University of California, Irvine, Irvine, CA, United States
- 4The Oxford Research Centre in the Humanities, University of Oxford, Oxford, United Kingdom
Olfactory loss accompanies at least 139 neurological, somatic, and congenital/hereditary conditions. This observation leads to the question of whether these associations are correlations or whether they are ever causal. Temporal precedence and prospective predictive power suggest that olfactory loss is causally implicated in many medical conditions. The causal relationship between olfaction with memory dysfunction deserves particular attention because this sensory system has the only direct projection to memory centers. Mechanisms that may underlie the connections between medical conditions and olfactory loss include inflammation as well as neuroanatomical and environmental factors, and all 139 of the medical conditions listed here are also associated with inflammation. Olfactory enrichment shows efficacy for both prevention and treatment, potentially mediated by decreasing inflammation.
1 Introduction
1.1 Observations linking olfactory loss and medical conditions: correlation, precedence, and prediction
1.1.1 Olfactory loss is associated with many medical conditions
First, a strikingly large number of medical conditions are accompanied by olfactory dysfunction (Tables 1–3). The remarkably long and diverse list of medical conditions that co-occur with olfactory loss raises the possibility that there is something deeper to these relationships.
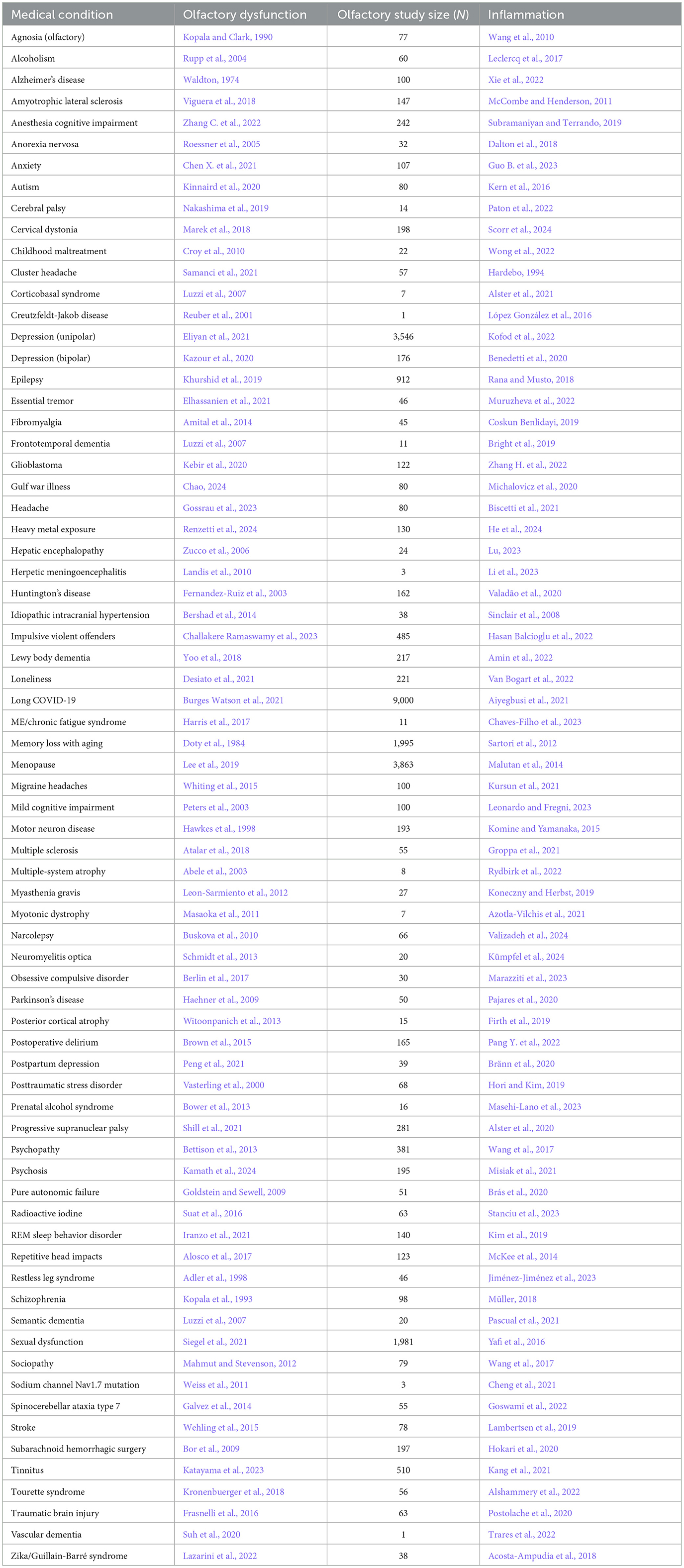
Table 1. Neurological condition/disorder, the reference for accompanying olfactory dysfunction, study size of olfactory study, and reference for inflammation.
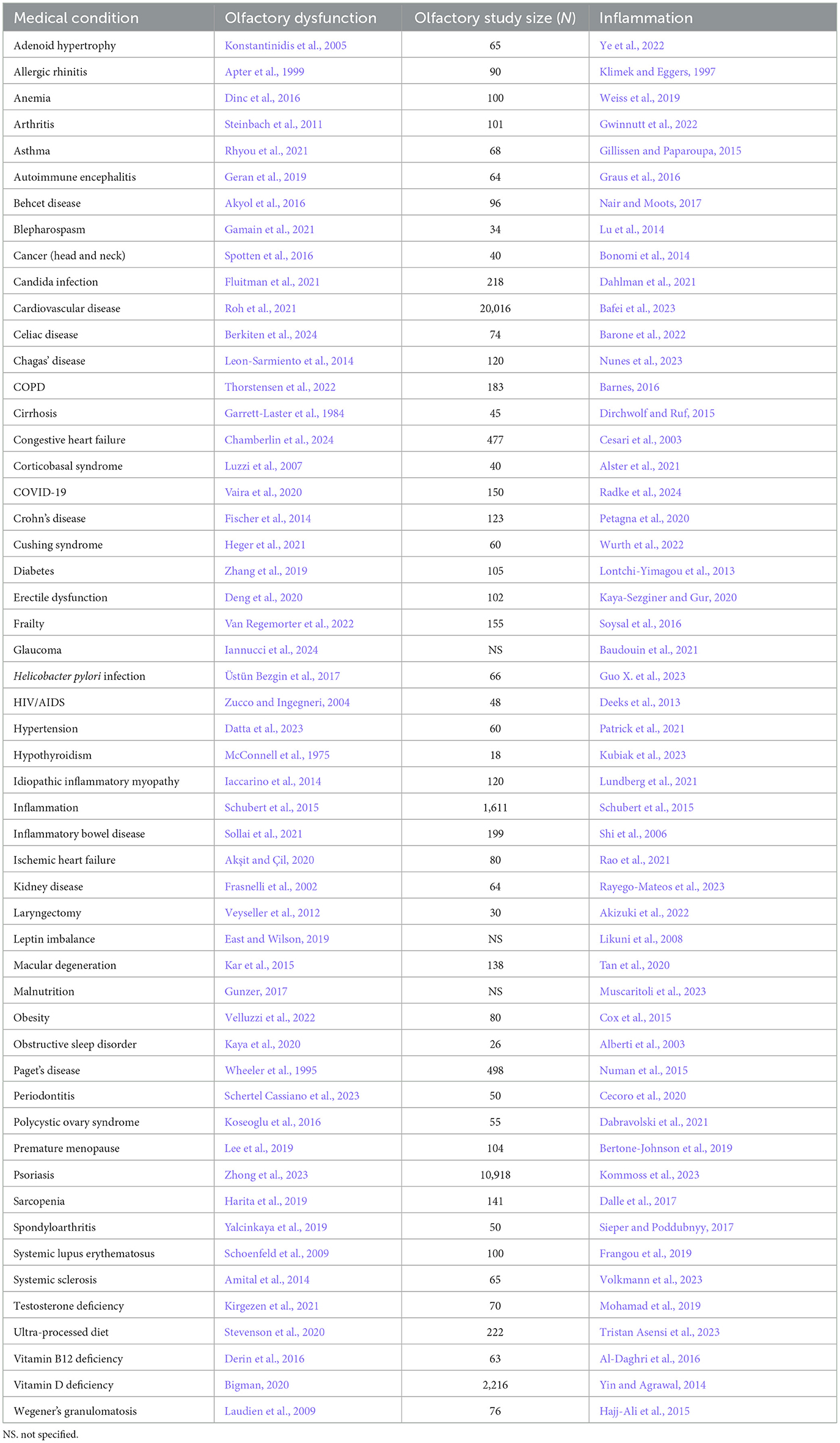
Table 2. Somatic condition/disorder, the reference for accompanying olfactory dysfunction, study size of olfactory study, and reference for inflammation.
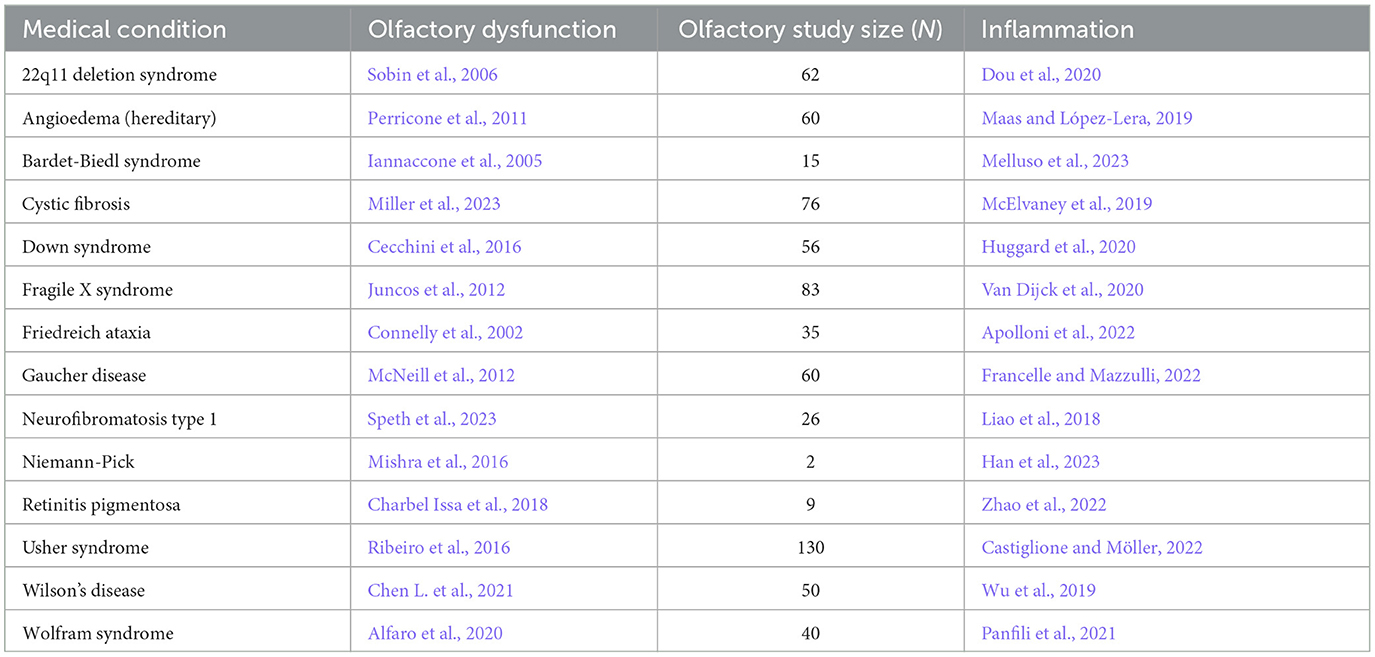
Table 3. Congenital/hereditary disorder, the reference for accompanying olfactory dysfunction, study size of olfactory study, and reference for inflammation.
Many of the associations between olfactory loss and medical conditions are supported by a single study. However, there are several conditions that have been studied extensively and there is strong support that has been reviewed for the relationship between these conditions and olfactory dysfunction: COVID-19 (Las Casas Lima et al., 2022), Alzheimer's disease (McLaren and Kawaja, 2024), Parkinson's disease (Bagherieh et al., 2023), depression (Kohli et al., 2016), and rhinitis (Ahmed and Rowan, 2020).
1.1.2 Olfactory dysfunction occurs early in the development of some medical conditions
To show that olfactory loss increases the risk of developing symptoms of medical conditions, one would need to show that olfactory dysfunction arises before the medical condition. The relevant experiments are quite difficult to do because one must evaluate the olfactory ability of many individuals and then follow them for years to determine whether poor olfactory ability precedes the medical condition. Despite the challenge, several such studies have been conducted. Olfactory loss appears well before any other Parkinson's symptoms (Walker et al., 2021), and similarly, an early symptom of Alzheimer's disease is the loss of olfaction (Serby et al., 1991), with the first part of the brain to deteriorate in that disease being the olfactory pathway (Peters et al., 2003). Schizophrenia is associated with olfactory dysfunction and such dysfunction can be seen in youths who eventually develop schizophrenia (Kamath et al., 2012). Olfactory loss also precedes depression (Kamath et al., 2024), major cardiac events (Chamberlin et al., 2024), and multiple sclerosis (Constantinescu et al., 1994); olfactory dysfunction therefore appears to be a prodromal symptom of these conditions.
1.1.3 Olfactory dysfunction prospectively predicts cognitive loss and all-cause mortality
In men, significant correlations are found in measurements of olfactory thresholds and language index score, along with correlations with executive function. On the other hand, women had correlations for olfactory discrimination and olfactory identification with a visuospatial index score (Masala et al., 2024). In young adults, olfactory ability is correlated with cognitive performance as assessed by verbal fluency, word list learning, word list recall, and the Trail Making Tests, even when the outcomes were adjusted for age, sex, education, and depression symptoms (Yahiaoui-Doktor et al., 2019). Challakere Ramaswamy and Schofield (2022) reviewed 54 studies and found a variety of cognitive abilities that correlated with olfactory ability, including: impulsivity, processing speed, inhibitory control, verbal fluency, working memory, mental flexibility, decision-making, visuospatial processing, planning, and executive function.
If olfactory loss has a causal relationship with at least some medical conditions, one might expect that the loss of olfaction would predict the incidence of those conditions. Indeed, one can predict the probability that older adults will later develop mild cognitive impairment (MCI) based on their olfactory ability (Wheeler and Murphy, 2021). Furthermore, of those individuals who have MCI, one can predict which individuals will develop Alzheimer's disease, as well as which individuals will descend rapidly into their dementia, based on their olfactory ability (Wheeler and Murphy, 2021). Parkinson's patients have both a loss of olfactory function and a loss of executive function (Solla et al., 2023). There are now a number of large prospective cohort studies showing that olfactory ability is a strong predictive factor for all-cause mortality up to 17 years later (Wilson et al., 2011; Gopinath et al., 2012; Pinto et al., 2014; Devanand et al., 2015; Ekström et al., 2017; Schubert et al., 2017; Fuller-Thomson and Fuller-Thomson, 2019; Kamath and Leff, 2019; Liu et al., 2019; Choi et al., 2021; Pinto, 2021; Xiao et al., 2021; Pang N. Y. et al., 2022), with higher accuracy than predictions based on heart disease (Pinto et al., 2014).
1.2 Mechanisms linking olfactory loss and medical conditions: inflammation, neuroanatomy, environmental stressors
1.2.1 Mechanism for triggering olfactory system damage
There are several possibilities for the mechanism underlying the many associations between olfaction and disease. One possibility is that there is a common mechanism that affects both the olfactory system and various neurological and somatic targets. Another possibility is that the neurological and somatic conditions produce something that degrades the olfactory system. A third possibility is that the olfactory system produces something that puts the brain and the body at risk either for contracting diseases or for expressing the symptoms of those diseases. One common product of disease is inflammation, and there is a strong relationship between olfactory dysfunction and elevated inflammation. As can be seen in Tables 1–3, at least 139 conditions that are associated with olfactory loss are also associated with increased inflammatory responses. These conditions have been subdivided into three separate categories: neurological, somatic, and congenital/hereditary conditions (Tables 1–3, respectively). Although the conditions could have been further subdivided into many other more specific categories, and some of the conditions may fall under two different categories, for simplicity, each medical condition was included in only one of the three categories.
1.2.2 Inflammation may be causing the olfactory dysfunction
Perhaps the olfactory system is particularly sensitive to inflammation that reaches it either from other parts of the brain or through the peripheral bloodstream. Alternatively, inflammation in the olfactory system may be triggered by agents that enter through the nose, such as air pollution (Ajmani et al., 2017) or unpleasant odors (Anja Juran et al., 2022). In addition, olfactory dysfunction associated with SARS-CoV-2 (COVID-19) infection is thought to be mediated in part via inflammation (Chang et al., 2024). The olfactory system may be uniquely sensitive to damage inflicted by other sources of inflammation (brain or body) that arise from various diseases because it is already sustaining high levels of inflammation from exposure to volatile agents from the air.
Poor ability to sniff contributes to the olfactory dysfunction of Parkinson's patients (Sobel et al., 2001). The ability to sniff predicted performance on olfactory tasks and increasing sniff vigor improved olfactory ability. The problems with sniffing may be due to increased inflammation that may prevent the respiratory system from compensating for the olfactory dysfunction (Huxtable et al., 2011).
Murphy et al. (2024) found that the efficacy of olfactory training for those individuals who had lost their olfactory ability after a COVID-19 infection was quite variable, with large differences in outcomes for different age groups. They surveyed more than 5,500 patients who had olfactory dysfunction following COVID-19 and compared the efficacy of various treatments including steroids and olfactory training. They found that nasal steroid use, given to reduce inflammation, was most effective for those 25–39 years old, with their effectiveness at about 25%, while oral steroid use was most effective for 18–24-year-olds, nearing 50%. Nasal steroids were most effective for treating hyposmia (poor olfactory ability), while oral steroids were most effective for phantosmia (imagined odors). Olfactory training was most effective for 18–24-year-olds, with effectiveness nearing 50%, while 40–60-year-olds had very poor effectiveness scores. Olfactory training was most effective for hyposmia.
Interestingly, several scents have been shown to have anti-inflammatory action in animal models, including: eucalyptol (Juergens et al., 2003), 1,8-cineol (Pries et al., 2023), lavender (Ueno-Iio et al., 2014), ginger (Aimbire et al., 2007), carvacrol (Alavinezhad et al., 2018), Shirazi thyme (Alavinezhad et al., 2017), farnesol (Ku and Lin, 2016), thymoquinone (El Gazzar et al., 2006, thymol (Gholijani et al., 2016), limonene (Hirota et al., 2012), citronellol (Pina et al., 2019), α-terpineol (Pina et al., 2019), Mentha piperita (Hudz et al., 2023), and mango (Rivera et al., 2011; see Ramsey et al., 2020 and Gandhi et al., 2020 for reviews).
The links between olfaction and inflammation seem also to be mediated by diet. Transgenic mice with high levels of the apolipoprotein E gene APOE4 (a risk factor for Alzheimer's disease) and given a diet with low docosahexaenoic acid (an omega-3 fatty acid) had olfactory loss and memory loss along with an increase in IBA-1, an inflammatory factor, in the olfactory bulb. The mice given a diet high in docosahexaenoic acid experienced no olfactory loss, cognitive loss, or elevated inflammation (González et al., 2023). Humans who have a diet low in monosaturated and polyunsaturated fats have an increased risk of both cognitive loss and olfactory loss (Vohra et al., 2023).
Although the list of conditions in which olfactory loss and inflammation co-occur is long, there do exist medical conditions that involve olfactory loss, without reports of inflammation. One example is Kallmann syndrome, in which olfactory bulb development is disordered. Individuals with this condition have olfactory loss as well as deterioration in various brain areas, but it is unclear whether the neurological differences arise from olfactory dysfunction or from the other aspects of the syndrome (Manara et al., 2014; Ottaviano et al., 2015). It certainly is possible that this condition involves an increase in inflammation, even though no one has reported it.
1.2.3 Olfactory loss results in damage to brain regions central to memory function
Given the predictive nature of olfactory loss for memory impairment in dementia, the question arises as to how olfactory loss could play a role in memory loss specifically. In fact, the olfactory system is anatomically unique among the senses, in that it has a “superhighway” that bypasses the thalamus and projects directly to regions of the brain involved in memory processing (Gottfried, 2006). Multiple studies now show that loss of olfaction is associated with deterioration of several brain regions (Bitter et al., 2010a,b; Eckert et al., 2024; Han et al., 2023; Kovalová et al., 2024; Peter et al., 2023; Seubert et al., 2020; Whitcroft et al., 2023; Yao et al., 2018), including the regions of the brain integral to memory acquisition and processing. While the deterioration of brain areas may be due to olfactory loss, it is also possible that the factor that produced the olfactory dysfunction also produced the damage in the other brain areas.
1.2.4 Environmental challenges compromise both olfaction and memory
Having identified inflammation as a possible global mediating factor in the links between olfactory loss and medical conditions and mortality, as well as neuroanatomical factors creating a tighter fit between olfactory loss and memory loss specifically, we can proceed to ask whether specific life experiences may activate such connections. There are indeed experiences that are known to cause both loss of olfactory ability and loss of memory, as well as the more diffuse impairments often referred to as “brain fog”. These include: smoking (Ajmani et al., 2017; Lewis et al., 2021), air pollution (Calderón-Garcidueñas and Ayala, 2022; Wang X. et al., 2021), a wide range of medications (Schiffman, 2018; Chavant et al., 2011), stress (Hoenen et al., 2017; Shields et al., 2017), childhood maltreatment (Maier et al., 2020; O'Shea et al., 2021), illiteracy (Dong et al., 2021; Arce Rentería et al., 2019), menopause (Lee et al., 2019; Maki, 2015), toxins (Upadhyay and Holbrook, 2004; Guan et al., 2022), alcoholism (Maurage et al., 2014; Pitel et al., 2014), respiratory infections (Potter et al., 2020; Matsui et al., 2003), nasal passage blockage (Mohamed et al., 2019; Arslan et al., 2018), head trauma (Lötsch et al., 2016; McInnes et al., 2017), highly processed food (Makhlouf et al., 2024; Gomes Gonçalves et al., 2023), and COVID-19 (Doty, 2022).
In one longitudinal study (Douaud et al., 2022), imaging was used to examine the effects of COVID-19 on the brain for individuals who had contracted a mild case of COVID-19 during the time between two brain scans. The second scan was completed approximately 141 days after testing positive for COVID-19, with an average time of 3 years between scans. Comparisons were made with brain scans from individuals who had not tested positive between scans. In the group who had contracted COVID-19, the researchers found significant damage in the regions of the brain involved in olfaction and memory, including the anterior cingulate cortex, orbitofrontal cortex, ventral striatum, amygdala, hippocampus, and parahippocampal gyrus, and the extent of olfactory loss predicted the extent of the brain damage (Campabadal et al., 2023). These individuals also continued to experience cognitive loss.
1.2.5 Olfactory dysfunction and cognitive loss
Compared to our ancestors, most humans in the affluent world experience a narrower range of evolutionarily relevant odors. In addition, people typically have experiences that damage their olfactory system: air pollution, stress, toxins, anatomical blockage, smoking, various medications, adverse childhood experiences, menopause, and even chronic sinusitis, all of which also trigger memory loss (Eimer and Vassar, 2013). As people age, the deterioration of their olfactory ability accompanies the deterioration of their cognitive ability (Leon and Woo, 2022; Doty et al., 1984), perhaps because olfactory loss results in a significant loss of both gray matter and white matter in the cognitive areas of human brains (Schaie et al., 2004; Bitter et al., 2010a,b).
1.2.5.1 Olfactory loss accompanies dementia
Olfactory dysfunction predicts cognitive dysfunction in humans (Schubert et al., 2008) and the loss of olfactory function precedes or parallels the initiation of a wide variety of cognitive disorders such as: AD, MCI, Parkinson's disease, Lewy body dementia, frontotemporal dementia, Creutzfeldt-Jakob disease, alcoholism, and schizophrenia (Wang Q. et al., 2021; Conti et al., 2013; Adams et al., 2018; Ponsen et al., 2004; Birte-Antina et al., 2018).
1.2.5.2 COVID-19 links olfactory loss and dementia
COVID-19 typically produces olfactory loss, and comparisons of MRI scans from individuals both pre-infection and post-infection have revealed neural deterioration that resembles a decade of aging in the cognitive brain regions that receive olfactory-system projections, along with damage to those areas involved in olfaction (Kollndorfer et al., 2015; Segura et al., 2013). Kay (2022) made the case that COVID-19 infections that produce olfactory loss may foster the cognitive loss that is seen in Alzheimer's disease. In fact, Wang et al. (2022) did a retrospective study of 6,245,282 older adults and showed that people with COVID-19 were at significantly increased risk for new diagnosis of Alzheimer's disease within 360 days after the initial COVID-19 diagnosis. Rahmati et al. (2023) went on to do a meta-analysis of twelve studies tracking over 33 million individuals who either had contracted COVID-19 or did not contract the virus. The pooled analyses showed a significant association between COVID-19 infection and subsequent increased risk for new-onset Alzheimer's disease. Given the remarkable number of physiological systems that were affected by the disease (Nasserie et al., 2021), there is no reason to believe that the olfactory loss was the sole factor in increasing the risk of Alzheimer's, but it may be that the loss of olfaction contributed to the degradation of regions in the brain integral to normal memory functioning, as mentioned previously (Kovalová et al., 2024).
1.3 Efficacy of olfactory enrichment
1.3.1 Olfactory enrichment improves symptoms of cognitive impairment
Shi et al. (2023) reviewed a number of studies examining the effects of exposure to essential oils and found a wide range of benefits to the brain and behavior. The benefits included normalizing neurotransmitter levels, decreasing inflammatory factors, decreasing oxidation, increasing neuroprotective factors, improving memory, decreasing neuronal loss, and suppressing beta amyloid levels.
1.3.2 Olfactory enrichment results in memory benefits for healthy adults
From a preventive perspective, about 20 studies have now been performed showing that increasing olfactory stimulation can improve memory (Vance et al., 2024).
For example, olfactory enrichment improves cognition in older adults. Birte-Antina et al. (2018) provided olfactory enrichment with 4 essential-oil odorants twice a day for 5 months, while controls solved daily Sudoku puzzles. The olfactory-enriched group had a significant improvement of olfactory function, improved verbal function, and decreased depression symptoms. Oleszkiewicz et al. (2022) exposed 68 older adults either to 9 odorants twice a day or to no new odorants for 3–6 months, and found the enriched olfactory experience produced improvements in cognitive abilities, dementia status, and olfactory function relative to controls. Specifically, the Montreal Cognitive Assessment revealed a significant improvement in the olfactory-enriched group relative to controls, and the AD8 Dementia Screening Interview showed that enriched participants had no increase in dementia symptoms over the trial period, while control participants had an increase in symptoms.
Increased complexity of olfactory enrichment also improves dementia symptoms. Cha et al. (2022) exposed 34 older adults with dementia to 40 odorants twice a day for 15 days. The control group consisting of 31 individuals with dementia received no such stimulation. There were no initial differences between groups, and all had a Mini-Mental Status Examination score of at least 10. The results were remarkable, as the olfactory-enriched group showed highly significant improvements in memory, olfactory identification, depression symptoms, attention, and language skills. Olfactory-enriched individuals improved their olfactory identification, while controls did not. The Verbal Fluency Test also showed significant improvements for the enriched group relative to the controls. Similarly, the Boston Naming Test revealed a significant improvement in the enriched subjects relative to controls. The Word-List Memory Test, the Word-List Recall Test, the Word List Recognition Test, and the Geriatric Depression Scale all improved in the enriched group relative to controls.
Lin and Li (2022) exposed older adults with mild-to-moderate dementia to 15 essential oils/essences twice a week for 30-min sessions over a 12-week randomized clinical trial. Participants in the olfactory enrichment group also were asked to relate each scent to a matching photo of the scent source. The olfactory enrichment group showed significant cognitive improvement on the Loewenstein Occupational Therapy Cognitive Assessment-Geriatric test. In addition, olfactory enrichment prevented the increase in plasma beta amyloid seen in the control group.
In an effort to minimize burden and increase compliance, we tested the idea that we could get enhanced neural and cognitive outcomes after even minimal olfactory enrichment at night (Woo et al., 2023). The limitations of the available diffusion devices at the time forced us to use this minimal level of olfactory enrichment. Therefore, we gave olfactory enrichment or control exposures to older adults (60–85 years old) for 2 h every night for 6 months, using a single odorant each night, rotating through seven scents a week (Woo et al., 2023). There were statistically significant differences between enriched and control older adults in their cognitive ability using the Rey Auditory Verbal Learning Test, with enriched individuals scoring 226% better than controls. We also found a statistically significant change in mean diffusivity in the uncinate fasciculus of the enriched group compared to controls.
1.4 Mechanisms of olfactory enrichment: inflammation, neuroanatomy, and cognitive reserve
1.4.1 Reduction of inflammation may be the mechanism by which olfactory enrichment improves neurological symptoms
A range of correlational and causal relationships connect inflammation with olfactory loss. Olfactory loss is associated with an increase in Interleukin-6 (IL-6), which increases both inflammation and the maturation of B cells (Henkin et al., 2013) and is also correlated with an increase in C-reactive protein, which increases in the presence of inflammation as indicated by IL-6 (Ekström et al., 2021). Chronic inflammation is associated with olfactory dysfunction (LaFever and Imamura, 2022). As noted earlier, a proinflammatory diet for older adults with low levels of polyunsaturated fatty acids and monosaturated fatty acids is associated with elevated inflammation, olfactory dysfunction, and cognitive decline (Vohra et al., 2023). Moreover, such a diet increases the risk of dementia (Simopoulos, 2002). The association between olfactory dysfunction and frailty varies with the level of inflammation, as measured by circulating levels of the pro-inflammatory cytokine IL-6 (Laudisio et al., 2019). Hahad et al. (2020) found that inflammation mediated the loss of cognition in those exposed to high levels of pollution.
Turning to causal links, unpleasant odors activate the inflammatory response by increasing tumor necrosis factor alpha (TNFα) and decreasing secretory immunoglobulin A (slgA) in saliva (Anja Juran et al., 2022). Imamura and Hasegawa-Ishii (2016) found that toxins can activate the immune response in the olfactory mucosa. Conversely, smelling pleasant odors suppresses immune activity, and more strikingly, even the act of imagining pleasant odors suppresses the immune response, specifically circulating interleukin-2 (IL-2; Matsunaga et al., 2013; Shibata et al., 1991). Casares et al. (2023) found that 6 months of exposure to menthol odor improved both the memory of young mice and the memory of mice that were modified to model Alzheimer's disease. This odor exposure also suppressed inflammation (IL-1β; Casares et al., 2023). Equally, pharmaceutical suppression of inflammation in those mice improved their memory (Casares et al., 2023).
The suppression of the inflammatory response may therefore underlie the finding that olfactory enrichment can improve memory (Cha et al., 2022; Woo et al., 2023). In addition, olfactory enrichment may improve symptoms of other neurological conditions through a similar mechanism.
1.4.2 Olfactory enrichment creates functional and structural changes in the brain
Increased olfactory stimulation, as experienced daily by master perfumers and sommeliers, who sample many odors each day for months and years, results in increased volume of brain regions that receive olfactory projections (Royet et al., 2013; Filiz et al., 2022). A longitudinal study showed that after a year and a half of olfactory training, sommeliers in training, who sampled dozens of odors every day for months to be able to identify those odors in fine wines, increased the thickness of their entorhinal cortex, a brain region critical for memory formation and consolidation (Filiz et al., 2022; Takehara-Nishiuchi, 2014). This structural change may well have functional benefits. Daily olfactory training for 6 weeks resulted in improved olfactory functioning as well as increased cortical thickness of olfactory processing regions of the brain (Al Aïn et al., 2019), and multiple scents presented daily improved cognition in both adults and older adults (Oleszkiewicz et al., 2021, 2022). Additionally, reversal of some medical issues, such as removing an anatomical blockade in the nasal passages, can result in improved cognition and attention, as measured using neuropsychological assessments and event-related auditory evoked potentials (P300; Arslan et al., 2018). In the memory study with healthy older adults described above (Woo et al., 2023), the enriched group that showed improvement in memory performance also had a statistically significant change in mean diffusivity in the uncinate fasciculus, a brain pathway involved with maintaining cognitive processes.
1.4.3 Electrical stimulation of the olfactory system
One mechanism by which olfactory enrichment may be working is by stimulating specific brain areas. Beta amyloid (Aβ) is elevated in Alzheimer's disease (Pignataro and Middei, 2017). In a rat model of Alzheimer's disease, electrical stimulation of the olfactory bulb reversed the accumulation of beta amyloid (Aβ) plaque formation in the prefrontal cortex, the entorhinal cortex, the dorsal hippocampus, and the ventral hippocampus. It also blocked the impairments in working memory in these rats (Salimi et al., 2024). In addition, electrical stimulation of the olfactory bulb also increased functional connectivity in the brain during a working memory task. It should be noted that transethmoid electrical stimulation of the human olfactory bulb induced olfactory perceptions (Holbrook et al., 2019). Olfactory enrichment may therefore act to stimulate the areas to which the olfactory input projects (Gottfried, 2006). Conversely, intrabulbar injections of Aβ in rats decreased olfactory function, a phenomenon that was more easily triggered in older rats (Alvarado-Martínez et al., 2013).
1.4.4 Making a distinction between contracting a disease vs. experiencing symptoms of a disease
It is important in a discussion regarding causality to consider whether something can differentially change the risk of contracting a disease or the risk of experiencing the symptoms of the disorder. This distinction may be important for our understanding of the relationships between olfaction, cognition, and disease. Typically, the symptoms of the disease accompany the disease itself, but there are exceptions. Some people who contracted the COVID-19 virus, for instance, did not show any symptoms of the disease (Rasmussen and Popescu, 2021). In the phenomenon called cognitive reserve, an individual can develop the neuropathology of Alzheimer's disease, indicating that they had contracted the disease, but show none of the symptoms of severe memory loss (Stern, 2012).
1.4.5 Olfactory ability and cognitive reserve
In mice, long-term olfactory enrichment improves olfactory ability, and it also improves learning and memory for tasks that do not involve odors (Terrier et al., 2024). This effect may represent a form of cognitive reserve in mice, here mediated by an increase in noradrenergic innervation and resulting in the remodeling of brain connectivity in older mice. These data suggest a causal association between olfactory enrichment and cognition. In humans, odor threshold correlates with a measure of cognitive reserve that involves education, while odor discrimination ability correlates with career experiences and leisure experiences. Women had significant correlations between odor threshold, discrimination and identification, and leisure experiences, while men had a significant association between odor threshold and educational experiences (Masala et al., 2023).
1.4.6 Olfactory enrichment may induce cognitive reserve in humans
Cognitive reserve in humans comes from leading a life filled with environmental enrichment, with a high level of education, a cognitively engaging career, and a high level of socializing (Stern, 2012). Conversely, illiterate individuals have the highest probability of developing Alzheimer's disease (Dong et al., 2021), and they have little of the enrichment that seems to protect those with cognitive reserve (Brucki, 2010). Perhaps the uniquely direct connections of the olfactory system to the regions of the brain that are critical for memory functioning allow the olfactory system to rapidly induce what may be called cognitive reserve in humans.
2 Discussion
There is reason to believe that the relationship between olfactory loss and medical conditions may be more than coincidental. First, there are many instances where both are present, with at least 139 medical conditions showing associations with olfactory dysfunction. Second, olfactory loss precedes the expression of the medical condition, raising the possibility that olfactory loss makes the brain or body vulnerable to expressing the symptoms of these medical conditions. Third, olfactory loss prospectively predicts both memory loss and all-cause mortality.
Inflammation could be a key mechanism underlying a causal relationship between olfaction and memory; neuroanatomical and environmental factors also play a role. While the causal arrow may go either way, it is possible that for some conditions, it is the olfactory loss that raises the risk of expressing the symptoms of those conditions.
If olfactory loss increases the risk of either developing these medical conditions or having the symptoms of the conditions, then it may be possible to prevent the onset of symptoms from these conditions. Studies show that olfactory enrichment improves memory performance in healthy adults and there are even greater improvements found for adults with dementia. These benefits may be mediated via reduction of inflammation.
A suggestive notion underlying many of these observations is that neuropathology is not always symptomatic, thanks to phenomena such as cognitive reserve. For instance, people with cognitive reserve have the neuropathology of Alzheimer's disease, but they don't have the memory-loss symptoms. The olfactory system may be involved in generating protective cognitive reserve especially for memory-related conditions. More widely, since pleasant scents can decrease harmful inflammation, it seems possible that olfactory enrichment may reduce the symptoms of other medical conditions.
Future directions for research in this area would include simultaneously studying both olfaction and inflammation in specific medical conditions, studying more conditions in individuals who have olfactory dysfunction, and studying these variables over time. It also would be interesting to block inflammation in specific medical conditions to determine the effects on olfaction.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
ML: Writing – original draft. ET: Conceptualization, Writing – review & editing. CW: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank Dr. Tom Lane for his insightful comments on the manuscript.
Conflict of interest
ML holds equity in Science Lab 3, which is developing Memory Air®, a system that automatically delivers olfactory enrichment.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abele, M., Riet, A., Hummel, T., Klockgether, T., and Wüllner, U. (2003). Olfactory dysfunction in cerebellar ataxia and multiple system atrophy. J. Neurol. 250, 1453–1455. doi: 10.1007/s00415-003-0248-4
Acosta-Ampudia, Y., Monsalve, D. M., Castillo-Medina, L. F., Rodríguez, Y., Pacheco, Y., Halstead, S., et al. (2018). Autoimmune neurological conditions associated with Zika virus infection. Front. Mol. Neurosci. 11:116. doi: 10.3389/fnmol.2018.00116
Adams, D. R., Kern, D. W., Wroblewski, K. E., McClintock, M. K., Dale, W., and Pinto, J. M. (2018). Olfactory dysfunction predicts subsequent dementia in older U.S. Adults. J. Am. Geriatr. Soc. 66, 140–144. doi: 10.1111/jgs.15048
Adler, C. H., Gwinn, K. A., and Newman, S. (1998). Olfactory function in restless legs syndrome. Mov. Disord. 13, 563–565. doi: 10.1002/mds.870130332
Ahmed, O. G., and Rowan, N. R. (2020). Olfactory dysfunction and chronic rhinosinusitis. Immunol. Allergy Clin. North Am. 40, 223–232. doi: 10.1016/j.iac.2019.12.013
Aimbire, F., Penna, S. C., Rodrigues, M., Rodrigues, K. C., Lopes-Martins, R. A., and Sertié, J. A. (2007). Effect of hydroalcoholic extract of Zingiber officinalis rhizomes on LPS-induced rat airway hyperreactivity and lung inflammation. Prostagland. Leukot. Essent. Fatty Acids 77, 129–138. doi: 10.1016/j.plefa.2007.08.008
Aiyegbusi, O. L., Hughes, S. E., Turner, G., Rivera, S. C., McMullan, C., Chandan, J. S., et al. (2021). Symptoms, complications and management of long COVID: a review. J. R. Soc. Med. 114, 428–442. doi: 10.1177/01410768211032850
Ajmani, G. S., Suh, H. H., Wroblewski, K. E., and Pinto, J. M. (2017). Smoking and olfactory dysfunction: a systematic literature review and meta-analysis. Laryngoscope 127, 1753–1761. doi: 10.1002/lary.26558
Akizuki, H., Wada, T., and Tabuchi, K. (2022). Inflammation-based score (combination of platelet count and neutrophil-to-lymphocyte ratio) predicts pharyngocutaneous fistula after total laryngectomy. Laryngoscope 132, 1582–1587. doi: 10.1002/lary.29970
Akşit, E., and Çil, Ö. Ç. (2020). Olfactory dysfunction in patients with ischemic heart failure. Acta Cardiol. Sin. 36, 133–139. doi: 10.6515/ACS.202003_36(2).20190812B
Akyol, L., Günbey, E., Karlı, R., Önem, S., Özgen, M., and Sayarlioglu, M. (2016). Evaluation of olfactory function in Behçet's disease. Eur. J. Rheumatol. 3, 153–156. doi: 10.5152/eurjrheum.2016.017
Al Aïn, S., Poupon, D., Hétu, S., Mercier, N., Steffener, J., and Frasnelli, J. (2019). Smell training improves olfactory function and alters brain structure. Neuroimage 189, 45–54. doi: 10.1016/j.neuroimage.2019.01.008
Alavinezhad, A., Hedayati, M., and Boskabady, M. H. (2017). The effect of Zataria multiflora and carvacrol on wheezing, FEV1 and plasma levels of nitrite in asthmatic patients. Avicenna J. Phytomed. 7, 531–541.
Alavinezhad, A., Khazdair, M. R., and Boskabady, M. H. (2018). Possible therapeutic effect of carvacrol on asthmatic patients: a randomized, double blind, placebo-controlled, Phase II clinical trial. Phytother. Res. 32, 151–159. doi: 10.1002/ptr.5967
Alberti, A., Sarchielli, P., Gallinella, E., Floridi, A., Floridi, A., Mazzotta, et al. (2003). Plasma cytokine levels in patients with obstructive sleep apnea syndrome: a preliminary study. J. Sleep Res. 12, 305–311. doi: 10.1111/j.1365-2869.2003.00361.x
Al-Daghri, N. M., Rahman, S., Sabico, S., Yakout, S., Wani, K., Al-Attas, O. S., et al. (2016). Association of vitamin B12 with pro-inflammatory cytokines and biochemical markers related to cardiometabolic risk in Saudi subjects. Nutrients 8:460. doi: 10.3390/nu8090460
Alfaro, R., Doty, R. T., Narayanan, A., Lugar, H., Hershey, T., and Pepino, M. Y. (2020). Taste and smell function in Wolfram syndrome. Orphanet J. Rare Dis. 15:57. doi: 10.1186/s13023-020-1335-7
Alosco, M. L., Jarnagin, J., Tripodis, Y., Platt, M., Martin, B., Chaisson, C. E., et al. (2017). Olfactory function and associated clinical correlates in former National Football League players. J. Neurotrauma 34, 772–780. doi: 10.1089/neu.2016.4536
Alshammery, S., Patel, S., Jones, H. F., Han, V. X., Gloss, B. S., Gold, W. A., et al. (2022). Common targetable inflammatory pathways in brain transcriptome of autism spectrum disorders and Tourette syndrome. Front. Neurosci. 16:999346. doi: 10.3389/fnins.2022.999346
Alster, P., Madetko, N., and Friedman, A. (2021). Neutrophil-to-lymphocyte ratio (NLR) at boundaries of progressive supranuclear palsy syndrome (PSPS). and corticobasal syndrome (CBS). Neurol. Neurochir. Pol. 55, 97–101. doi: 10.5603/PJNNS.a2020.0097
Alster, P., Madetko, N., Koziorowski, D., and Friedman, A. (2020). microglial activation and inflammation as a factor in the pathogenesis of progressive supranuclear palsy (PSP). Front. Neurosci. 14:893. doi: 10.3389/fnins.2020.00893
Alvarado-Martínez, R., Salgado-Puga, K., and Peña-Ortega, F. (2013). Amyloid beta inhibits olfactory bulb activity and the ability to smell. PLoS ONE 8:e75745. doi: 10.1371/journal.pone.0075745
Amin, J., Erskine, D., Donaghy, P. C., Surendranathan, A., Swann, P., Kunicki, A. P., et al. (2022). Inflammation in dementia with Lewy bodies. Neurobiol. Dis. 168:105698. doi: 10.1016/j.nbd.2022.105698
Amital, H., Agmon-Levin, N., Shoenfeld, N., Arnson, Y., Amital, D., Langevitz, P., et al. (2014). Olfactory impairment in patients with the fibromyalgia syndrome and systemic sclerosis. Immunol. Res. 60, 201–207. doi: 10.1007/s12026-014-8573-5
Anja Juran, S., Tognetti, A., Lundström, J. N., Kumar, L., Stevenson, R. J., Lekander, M., et al. (2022). Disgusting odors trigger the oral immune system. Evol. Med. Public Health 11, 8–17. doi: 10.1093/emph/eoac042
Apolloni, S., Milani, M., and D'Ambrosi, N. (2022). Neuroinflammation in Friedreich's ataxia. Int. J. Mol. Sci. 23:6297. doi: 10.3390/ijms23116297
Apter, A. J., Gent, J. F., and Frank, M. (1999). Fluctuating olfactory sensitivity and distorted odor perception in allergic rhinitis. Arch. Otolaryngol. Head Neck Surg. 125, 1005–1010. doi: 10.1001/archotol.125.9.1005
Arce Rentería, M., Vonk, J. M. J., Felix, G., Avila, J. F., Zahodne, L. B., Dalchand, E., et al. (2019). Illiteracy, dementia risk, and cognitive trajectories among older adults with low education. Neurology 93, e2247–e2256. doi: 10.1212/WNL.0000000000008587
Arslan, F., Tasdemir, S., Durmaz, A., and Tosun, F. (2018). The effect of nasal polyposis related nasal obstruction on cognitive functions. Cogn. Neurodyn. 12, 385–390. doi: 10.1007/s11571-018-9482-4
Atalar, A. Ç., Erdal, Y., Tekin, B., Yildiz, M., Akdogan, Ö., and Emre, U. (2018). Olfactory dysfunction in multiple sclerosis. Mult. Scler. Relat. Disord. 21, 92–96. doi: 10.1016/j.msard.2018.02.032
Azotla-Vilchis, C. N., Sanchez-Celis, D., Agonizantes-Juárez, L. E., Suárez-Sánchez, R., Hernández-Hernández, J. M., Peña, J., et al. (2021). Transcriptome analysis reveals altered inflammatory pathway in an inducible glial cell model of myotonic dystrophy type 1. Biomolecules 11:159. doi: 10.3390/biom11020159
Bafei, S. E. C., Zhao, X., Chen, C., Sun, J., Zhuang, Q., Lu, X., et al. (2023). Interactive effect of increased high sensitive C-reactive protein and dyslipidemia on cardiovascular diseases: a 12-year prospective cohort study. Lipids Health Dis. 22:113. doi: 10.1186/s12944-023-01894-0
Bagherieh, S., Arefian, N. M., Ghajarzadeh, M., Tafreshinejad, A., Zali, A., Mirmosayyeb, O., et al. (2023). Olfactory dysfunction in patients with Parkinson's disease: a systematic review and meta-analysis. Curr. J. Neurol. 22, 249–254. doi: 10.18502/cjn.v22i4.14530
Barnes, P. J. (2016). Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 138, 16–27. doi: 10.1016/j.jaci.2016.05.011
Barone, M. V., Auricchio, R, Nanayakkara, M., Greco, L., Troncone, R., and Auricchio, S. (2022). Pivotal role of inflammation in celiac disease. Int. J. Mol. Sci. 23:7177. doi: 10.3390/ijms23137177
Baudouin, C., Kolko, M., Melik-Parsadaniantz, S., and Messmer, E. M. (2021). Inflammation in glaucoma: from the back to the front of the eye, and beyond. Prog. Retin. Eye Res. 83:100916. doi: 10.1016/j.preteyeres.2020.100916
Benedetti, F., Aggio, V., Pratesi, M. L., Greco, G., and Furlan, R. (2020). Neuroinflammation in bipolar depression. Front. Psychiatry 11:71. doi: 10.3389/fpsyt.2020.00071
Berkiten, G., Tutara, B., G?¨kdenb, Y., Sengiz, S., Karaketir, S., Sari?am, S. S., et al. (2024). Does celiac disease affect smell sensation, mucociliary clearance and nasal smear? J. Ear Nose Throat Head Neck Surg. 3, 23–29. doi: 10.24179/kbbbbc.2023-99179
Berlin, H. A., Stern, E. R., Ng, J., Zhang, S., Rosenthal, D., Turetzky, R., et al. (2017). Altered olfactory processing and increased insula activity in patients with obsessive-compulsive disorder: an fMRI study. Psychiatry Res. Neuroimaging 262, 15–24. doi: 10.1016/j.pscychresns.2017.01.012
Bershad, E. M., Urfy, M. Z., Calvillo, E., Tang, R., Cajavilca, C., Lee, A. G., et al. (2014). Marked olfactory impairment in idiopathic intracranial hypertension. J. Neurol. Neurosurg. Psychiatr. 85, 959–964. doi: 10.1136/jnnp-2013-307232
Bertone-Johnson, E. R., Manson, J. E., Purdue-Smithe, A. C., Hankinson, S. E., Rosner, B. A., and Whitcomb, B. W. (2019). A prospective study of inflammatory biomarker levels and risk of early menopause. Menopause 26, 32–38. doi: 10.1097/GME.0000000000001162
Bettison, T. M., Mahmut, M. K., and Stevenson, R. J. (2013). The relationship between psychopathy and olfactory tasks sensitive to orbitofrontal cortex function in a non-criminal student sample. Chemosens. Percept. 6, 198–210. doi: 10.1007/s12078-013-9157-9
Bigman, G. (2020). Age-related smell and taste impairments and vitamin D associations in the U.S. Adults National Health and Nutrition Examination Survey. Nutrients 12:984. doi: 10.3390/nu12040984
Birte-Antina, W., Ilona, C., Antje, H., and Thomas, H. (2018). Olfactory training with older people. Int. J. Geriatr. Psychiatry 33, 212–220. doi: 10.1002/gps.4725
Biscetti, L., De Vanna, G., Cresta, E., Corbelli, I., Gaetani, L., Cupini, L., et al. (2021). Headache and immunological/autoimmune disorders: a comprehensive review of available epidemiological evidence with insights on potential underlying mechanisms. J. Neuroinflamm. 18:259. doi: 10.1186/s12974-021-02229-5
Bitter, T., Bruderle, J., Gudziol, H., Burmeister, H. P., Gaser, C., and Guntinas-Lichius, O. (2010a). Gray and white matter reduction in hyposmic subjects - a voxel-based morphometry study. Brain Res. 1347, 42–47. doi: 10.1016/j.brainres.2010.06.003
Bitter, T., Gudziol, H., Burmeister, H. P., Mentzel, H. J., Guntinas-Lichius, O., and Gaser, C. (2010b). Anosmia leads to a loss of gray matter in cortical brain areas. Chem. Senses 35, 407–415. doi: 10.1093/chemse/bjq028
Bonomi, M., Patsias, A., Posner, M., and Sikora, A. (2014). The role of inflammation in head and neck cancer. Adv. Exp. Med. Biol. 816, 107–127. doi: 10.1007/978-3-0348-0837-8_5
Bor, A. S., Niemansburg, S. L., Wermer, M. J., and Rinkel, G. J. (2009). Anosmia after coiling of ruptured aneurysms: prevalence, prognosis, and risk factors. Stroke 40, 2226–2228. doi: 10.1161/STROKEAHA.108.539445
Bower, E., Szajer, J., Mattson, S. N., Riley, E. P., and Murphy, C. (2013). Impaired odor identification in children with histories of heavy prenatal alcohol exposure. Alcohol 47, 275–278. doi: 10.1016/j.alcohol.2013.03.002
Bränn, E., Fransson, E., White, R. A., Papadopoulos, F. C., Edvinsson, Å., Kamali-Moghaddam, M., et al. (2020). Inflammatory markers in women with postpartum depressive symptoms. J. Neurosci. Res. 98, 1309–1321. doi: 10.1002/jnr.24312
Brás, I. C., Xylaki, M., and Outeiro, T. F. (2020). Mechanisms of alpha-synuclein toxicity: an update and outlook. Prog. Brain Res. 252, 91–129. doi: 10.1016/bs.pbr.2019.10.005
Bright, F., Werry, E. L., Dobson-Stone, C., Piguet, O., Ittner, L. M., Halliday, G. M., et al. (2019). Neuroinflammation in frontotemporal dementia. Nat. Rev. Neurol. 15, 540–555. doi: 10.1038/s41582-019-0231-z
Brown, C. H. 4th, Morrissey, C., Ono, M., Yenokyan, G., Selnes, O. A., Walston, J., et al. (2015). Impaired olfaction and risk of delirium or cognitive decline after cardiac surgery. J. Am. Geriatr. Soc. 63, 16–23. doi: 10.1111/jgs.13198
Brucki, S. M. D. (2010). Illiteracy and dementia. Dement. Neuropsychol. 4, 153–157. doi: 10.1590/S1980-57642010DN40300002
Burges Watson, D. L., Campbell, M., Hopkins, C., Smith, B., Kelly, C., and Deary, V. (2021). Altered smell and taste: anosmia, parosmia and the impact of long COVID-PLoS ONE 16:e0256998. doi: 10.1371/journal.pone.0256998
Buskova, J., Klaschka, J., Sonka, K., and Nevsimalova, S. (2010). Olfactory dysfunction in narcolepsy with and without cataplexy. Sleep Med. 11, 558–561. doi: 10.1016/j.sleep.2010.01.009
Calderón-Garcidueñas, L., and Ayala, A. (2022). Air pollution, ultrafine particles, and your brain: are combustion nanoparticle emissions and engineered nanoparticles causing preventable fatal neurodegenerative diseases and common neuropsychiatric outcomes? Environ. Sci. Tech. 56, 6847–6856. doi: 10.1021/acs.est.1c04706
Campabadal, A., Oltra, J., Junqué, C., Guillen, N., Botí, M. Á., Sala-Llonch, R., et al. (2023). Structural brain changes in post-acute COVID-19 patients with persistent olfactory dysfunction. Ann. Clin. Transl. Neurol. 10, 195–203. doi: 10.1002/acn3.51710
Casares, N., Alfaro, M., Cuadrado-Tejedor, M., Lasarte-Cia, A., Navarro, F., Vivas, I., et al. (2023). Improvement of cognitive function in wild-type and Alzheimer's disease mouse models by the immunomodulatory properties of menthol inhalation or by depletion of T regulatory cells. Front. Immunol. 14:1130044. doi: 10.3389/fimmu.2023.1130044
Castiglione, A., and Möller, C. (2022). Usher syndrome. Audiol. Res. 12, 42–65. doi: 10.3390/audiolres12010005
Cecchini, M. P., Viviani, D., Sandri, M., Hähner, A., Hummel, T., and Zancanaro, C. (2016). Olfaction in people with Down syndrome: a comprehensive assessment across four decades of age. PLoS ONE 11:e0146486. doi: 10.1371/journal.pone.0146486
Cecoro, G., Annunziata, M., Iuorio, M. T., Nastri, L., and Guida, L. (2020). Periodontitis, low-grade inflammation and systemic health: a scoping review. Medicina 56:272. doi: 10.3390/medicina56060272
Cesari, M., Penninx, B. W., Newman, A. B., Kritchevsky, S. B., Nicklas, B. J., Sutton-Tyrrell, K., et al. (2003). Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation 108, 2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC
Cha, H., Kim, S., Kim, H., Kim, G., and Kwon, K. Y. (2022). Effect of intensive olfactory training for cognitive function in patients with dementia. Geriatr. Gerontol. Int. 22, 5–11. doi: 10.1111/ggi.14287
Challakere Ramaswamy, V. M., Butler, T., Ton, B., Wilhelm, K., Mitchell, P. B., Knight, L., et al. (2023). Neuropsychiatric correlates of olfactory identification and traumatic brain injury in a sample of impulsive violent offenders. Front. Psychol. 14, 1254574. doi: 10.3389/fpsyg.2023.1254574
Challakere Ramaswamy, V. M., and Schofield, P. W. (2022). Olfaction and executive cognitive performance: a systematic review. Front. Psychol. 13:871391. doi: 10.3389/fpsyg.2022.871391
Chamberlin, K. W., Yuan, Y., Li, C., Luo, Z., Reeves, M., Kucharska-Newton, A., et al. (2024). Olfactory impairment and the risk of major adverse cardiovascular outcomes in older adults. J. Am. Heart Assoc. 13:e033320. doi: 10.1161/JAHA.123.033320
Chang, K., Zaikos, T., Kilner-Pontone, N., and Ho, C. Y. (2024). Mechanisms of COVID-19-associated olfactory dysfunction. Neuropath. Appl. Neurobiol. 50:e12960. doi: 10.1111/nan.12960
Chao, L. L. (2024). Olfactory and cognitive decrements in 1991 Gulf War veterans with gulf war illness/chronic multisymptom illness. Environ. Health 23, 14–23. doi: 10.1186/s12940-024-01058-2
Charbel Issa, P., Reuter, P., Kühlewein, L., Birtel, J., Gliem, M., Tropitzsch, A., et al. (2018). Olfactory dysfunction in patients with CNGB1-associated retinitis pigmentosa. JAMA Ophthalmol. 136, 761–769. doi: 10.1001/jamaophthalmol.2018.162
Chavant, F., Favrelière, S., Lafay-Chebassier, C., Plazanet, C., and Pérault-Pochat, M. C. (2011). Memory disorders associated with consumption of drugs: updating through a case/noncase study in the French PharmacoVigilance Database. Br. J. Clin. Pharmacol. 72, 898–904. doi: 10.1111/j.1365-2125.2011.04009.x
Chaves-Filho, A. M., Braniff, O., Angelova, A., Deng, Y., and Tremblay, M. È. (2023). Chronic inflammation, neuroglial dysfunction, and plasmalogen deficiency as a new pathobiological hypothesis addressing the overlap between post-COVID-19 symptoms and myalgic encephalomyelitis/chronic fatigue syndrome. Brain Res. Bull. 201:110702. doi: 10.1016/j.brainresbull.2023.110702
Chen, L., Wang, X., Doty, R. L., Cao, S., Yang, J., Sun, F., et al. (2021). Olfactory impairment in Wilson's disease. Brain Behav. 11:e02022. doi: 10.1002/brb3.2022
Chen, X., Guo, W., Yu, L., Luo, D., Xie, L., and Xu, J. (2021). Association between anxious symptom severity and olfactory impairment in young adults with generalized anxiety disorder: a case-control study. Neuropsychiatr. Dis. Treat. 17, 2877–2883. doi: 10.2147/NDT.S314857
Cheng, X., Choi, J. S., Waxman, S. G., and Dib-Hajj, S. D. (2021). Sodium channels and beyond in peripheral nerve disease: modulation by cytokines and their effector protein kinases. Neurosci. Lett. 741:135446. doi: 10.1016/j.neulet.2020.135446
Choi, J. S., Jang, S. S., Kim, J., Hur, K., Ference, E., and Wrobel, B. (2021). Association between olfactory dysfunction and mortality in US adults. JAMA Otolaryngol. Head Neck Surg. 147, 49–55. doi: 10.1001/jamaoto.2020.3502
Connelly, T., Farmer, J. M., Lynch, D. R., and Doty, R. L. (2002). Olfactory dysfunction in degenerative ataxias. J. Neurol. Neurosurg. Psychiatr. 74, 1435–1437. doi: 10.1136/jnnp.74.10.1435
Constantinescu, C. S., Raps, E. C., Cohen, J. A., West, S. E., and Doty, R. L. (1994). Olfactory disturbances as the initial or most prominent symptom of multiple sclerosis. J. Neurol. Neurosurg. Psychiatr. 57, 1011–1012. doi: 10.1136/jnnp.57.8.1011
Conti, M. Z., Vicini-Chilovi, B., Riva, M., Zanetti, M., Liberini, P., Padovani, A., et al. (2013). Odor identification deficit predicts clinical conversion from mild cognitive impairment to dementia due to Alzheimer's disease. Arch. Clin. Neuropsychol. 28, 391–399. doi: 10.1093/arclin/act032
Coskun Benlidayi, I. (2019). Role of inflammation in the pathogenesis and treatment of fibromyalgia. Rheumatol. Int. 39, 781–791. doi: 10.1007/s00296-019-04251-6
Cox, A. J., West, N. P., and Cripps, A. W. (2015). Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 3, 207–215. doi: 10.1016/S2213-8587(14)70134-2
Croy, I., Schellong, J., Gerber, J., Joraschky, P., Iannilli, E., and Hummel, T. (2010). Women with a history of childhood maltreatment exhibit more activation in association areas following non-traumatic olfactory stimuli: a fMRI study. PLoS ONE 5:e9362. doi: 10.1371/journal.pone.0009362
Dabravolski, S. A., Nikiforov, N. G., Eid, A. H., Nedosugova, L. V., Starodubova, A. V., Popkova, T. V., et al. (2021). Mitochondrial dysfunction and chronic inflammation in polycystic ovary syndrome. Int. J. Mol. Sci. 22:3923. doi: 10.3390/ijms22083923
Dahlman, A., Puthia, M., Petrlova, J., Schmidtchen, A., and Petruk, G. (2021). Thrombin-derived c-terminal peptide reduces Candida-induced inflammation and infection in vitro and in vivo. Antimicrob. Agents Chemother. 65:e0103221. doi: 10.1128/AAC.01032-21
Dalle, S., Rossmeislova, L., and Koppo, K. (2017). The role of inflammation in age-related sarcopenia. Front. Physiol. 8:1045. doi: 10.3389/fphys.2017.01045
Dalton, B., Campbell, I. C., Chung, R., Breen, G., Schmidt, U., and Himmerich, H. (2018). Inflammatory markers in anorexia nervosa: an exploratory study. Nutrients 10:1573. doi: 10.3390/nu10111573
Datta, S., Jha, K., Ganguly, A., and Kumar, T. (2023). Olfactory dysfunction as a marker for essential hypertension in a drug-naive adult population: A hospital-based study. Cureus 15:e41920. doi: 10.7759/cureus.41920
Deeks, S. G., Tracy, R., and Douek, D. C. (2013). Systemic effects of inflammation on health during chronic HIV infection. Immunity 39, 633–645. doi: 10.1016/j.immuni.2013.10.001
Deng, H. Y., Feng, J. R., Zhou, W. H., Kong, W. F., Ma, G. C., Hu, T. F., et al. (2020). Olfactory sensitivity is related to erectile function in adult males. Front. Cell Devel. Biol. 8:93. doi: 10.3389/fcell.2020.00093
Derin, S., Koseoglu, S., Sahin, C., and Sahan, M. (2016). Effect of vitamin B12 deficiency on olfactory function. Int. Forum Allergy Rhinol. 6, 1051–1055. doi: 10.1002/alr.21790
Desiato, V. M., Soler, Z. M., Nguyen, S. A., Salvador, C., Hill, J. B., Lamira, J., et al. (2021). Evaluating the relationship between olfactory function and loneliness in community-dwelling individuals: a cross-sectional study. Am. J. Rhinol. Allergy 35, 334–340. doi: 10.1177/1945892420958365
Devanand, D. P., Lee, S., Manly, J., Andrews, H., Schupf, N., Masurkar, A., et al. (2015). Olfactory identification deficits and increased mortality in the community. Ann. Neurol. 78, 401–411. doi: 10.1002/ana.24447
Dinc, M. E., Dalgic, A., Ulusoy, S., Dizdar, D., Develioglu, O., and Topak, M. (2016). Does iron deficiency anemia affect olfactory function? Acta Otolaryngol. 136, 754–757. doi: 10.3109/00016489.2016.1146410
Dirchwolf, M., and Ruf, A. E. (2015). Role of systemic inflammation in cirrhosis: from pathogenesis to prognosis. World J. Hepatol. 7, 1974–1981. doi: 10.4254/wjh.v7.i16.1974
Dong, Y., Wang, Y., Liu, K., Liu, R., Tang, S., Zhang, Q., et al. (2021). Olfactory impairment among rural-dwelling Chinese older adults: prevalence and associations with demographic, lifestyle, and clinical factors. Front. Aging Neurosci.13:621619. doi: 10.3389/fnagi.2021.621619
Doty, R. L. (2022). Olfactory dysfunction in COVID-19: pathology and long-term implications for brain health. Trends Mol. Med. 28, 781–794. doi: 10.1016/j.molmed.2022.06.005
Doty, R. L., Shaman, P., Applebaum, S. L., Giberson, R., Siksorski, L., and Rosenberg, L. (1984). Smell identification ability: changes with age. Science 226, 1441–1443. doi: 10.1126/science.6505700
Dou, Y., Blaine Crowley, T., Gallagher, S., Bailey, A., McGinn, D., Zackai, E., et al. (2020). Increased T-cell counts in patients with 22q11.2 deletion syndrome who have anxiety. Am. J. Med. Genet. A. 182, 1815–1818. doi: 10.1002/ajmg.a.61588
Douaud, G., Lee, S., Alfaro-Almagro, F., Arthofer, C., Wang, C., McCarthy, P., et al. (2022). SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 604, 697–707. doi: 10.1038/s41586-022-04569-5
East, B. S., and Wilson, D. A. (2019). A hunger for odour: Leptin modulation of olfaction. Acta Physiol. 227:e13363. doi: 10.1111/apha.13363
Eckert, M. A., Benitez, A., Soler, Z. M., Dubno, J. R., and Schlosser, R. J. (2024). Gray matter and episodic memory associations with olfaction in middle-aged to older adults. Int. Forum Allergy Rhinol. 14, 961–971. doi: 10.1002/alr.23290
Eimer, W. A., and Vassar, R. (2013). Neuron loss in the 5XFAD mouse model of Alzheimer's disease correlates with intraneuronal Aβ42 accumulation and Caspase-3 activation. Mol. Neurodegener. 8:2. doi: 10.1186/1750-1326-8-2
Ekström, I., Sjölund, S., Nordin, S., Nordin Adolfsson, A., Adolfsson, R., Nilsson, L. G., et al. (2017). Smell loss predicts mortality risk regardless of dementia conversion. J. Am. Geriatr. Soc. 65, 1238–1243. doi: 10.1111/jgs.14770
Ekström, I., Vetrano, D. L., Papenberg, G., and Laukka, E. J. (2021). Serum C-reactive protein is negatively associated with olfactory identification ability in older adults. Iperception 12:20416695211009928. doi: 10.1177/20416695211009928
El Gazzar, M., El Mezayen, R., Nicolls, M. R., Marecki, J. C., and Dreskin, S. C. (2006).Downregulation of leukotriene biosynthesis by thymoquinone attenuates airway inflammation in a mouse model of allergic asthma. Biochim. Biophys. Acta 1760, 1088–1095. doi: 10.1016/j.bbagen.2006.03.006
Elhassanien, M. E. M., Bahnasy, W. S., El-Heneedy, Y. A. E., Kishk, A. M., Tomoum, M. O., Ramadan, K. M., et al. (2021). Olfactory dysfunction in essential tremor versus tremor dominant Parkinson disease. Clin. Neurol. Neurosurg. 200:106352. doi: 10.1016/j.clineuro.2020.106352
Eliyan, Y., Wroblewski, K. E., McClintock, M. K., and Pinto, J. M. (2021). Olfactory dysfunction predicts the development of depression in older US adults. Chem. Senses 46:bjaa075. doi: 10.1093/chemse/bjaa075
Fernandez-Ruiz, J., Diaz, R., Hall-Haro, C., Vergara, P., Fiorentini, A., Nunez, L., et al. (2003). Olfactory dysfunction in hereditary ataxia and basal ganglia disorders. Neuroreport 14, 1339–1341. doi: 10.1097/00001756-200307180-00011
Filiz, G., Poupon, D., Banks, S., Fernandez, P., and Frasnelli, J. (2022). Olfactory bulb volume and cortical thickness evolve during sommelier training. Hum. Brain Mapp. 43, 2621–2633. doi: 10.1002/hbm.25809
Firth, N. C., Primativo, S., Marinescu, R. V., Shakespeare, T. J., Suarez-Gonzalez, A., Lehmann, M., et al. (2019). Longitudinal neuroanatomical and cognitive progression of posterior cortical atrophy. Brain 142, 2082–2095. doi: 10.1093/brain/awz136
Fischer, M., Zopf, Y., Elm, C., Pechmann, G., Hahn, E. G., Schwab, D., et al. (2014). Subjective and objective olfactory abnormalities in Crohn's disease. Chem. Senses 39, 529–538. doi: 10.1093/chemse/bju022
Fluitman, K. S., van den Broek, T. J., Nieuwdorp, M., Visser, M., IJzerman, R. G., and Keijser, B. J. F. (2021). Associations of the oral microbiota and Candida with taste, smell, appetite and undernutrition in older adults. Sci. Rep. 11:23254. doi: 10.1038/s41598-021-02558-8
Francelle, L., and Mazzulli, J. R. (2022). Neuroinflammation in Gaucher disease, neuronal ceroid lipofuscinosis, and commonalities with Parkinson's disease. Brain Res. 1780:147798. doi: 10.1016/j.brainres.2022.147798
Frangou, E., Vassilopoulos, D., Boletis, J., and Boumpas, D. T. (2019). An emerging role of neutrophils and NETosis in chronic inflammation and fibrosis in systemic lupus erythematosus (SLE) and ANCA-associated vasculitides (AAV): Implications for the pathogenesis and treatment. Autoimmun. Rev. 18, 751–760. doi: 10.1016/j.autrev.2019.06.011
Frasnelli, J., Laguë-Beauvais, M., LeBlanc, J., Alturki, A. Y., Champoux, M. C., Couturier, C., et al. (2016). Olfactory function in acute traumatic brain injury. Clin. Neurol. Neurosurg. 140, 68–72. doi: 10.1016/j.clineuro.2015.11.013
Frasnelli, J. A., Temmel, A. F., Quint, C., Oberbauer, R., and Hummel, T. (2002). Olfactory function in chronic renal failure. Am. J. Rhinol. 16, 275–279.
Fuller-Thomson, E. R., and Fuller-Thomson, E. G. (2019). Relationship between poor olfaction and mortality. Ann. Intern. Med. 171, 525–526. doi: 10.7326/L19-0467
Galvez, V., Diaz, R., Hernandez-Castillo, C. R., Campos-Romo, A., and Fernandez-Ruiz, J. (2014). Olfactory performance in spinocerebellar ataxia type 7 patients. Parkinsonism Rel. Disord. 20, 499–502. doi: 10.1016/j.parkreldis.2014.01.024
Gamain, J., Herr, T., Fleischmann, R., Stenner, A., Vollmer, M., Willert, C., et al. (2021). Smell and taste in idiopathic blepharospasm. J. Neural Transm. 128, 1215–1224. doi: 10.1007/s00702-021-02366-4
Gandhi, G. R., Leão, G. C. S., Calisto, V. K. D. S., Vasconcelos, A. B. S., Almeida, M. L. D., Quintans, J. S. S., et al. (2020). Modulation of interleukin expression by medicinal plants and their secondary metabolites: A systematic review on anti-asthmatic and immunopharmacological mechanisms. Phytomedicine 70:153. doi: 10.1016/j.phymed.2020.153229
Garrett-Laster, M., Russell, R. M., and Jacques, P. F. (1984). Impairment of taste and olfaction in patients with cirrhosis: the role of vitamin A. Hum. Nutr. Clin. 38, 203–214.
Geran, R., Uecker, F. C., Prüss, H., Haeusler, K. G., Paul, F., Ruprecht, K., et al. (2019). Olfactory and gustatory dysfunction in patients with autoimmune encephalitis. Front. Neurol. 10:480. doi: 10.3389/fneur.2019.00480
Gholijani, N., Gharagozloo, M., Farjadian, S., and Amirghofran, Z. (2016). Modulatory effects of thymol and carvacrol on inflammatory transcription factors in lipopolysaccharide-treated macrophages. J. Immunotoxicol. 13, 157–164. doi: 10.3109/1547691X.2015.1029145
Gillissen, A., and Paparoupa, M. (2015). Inflammation and infections in asthma. Clin. Respir. J. 9, 257–269. doi: 10.1111/crj.12135
Goldstein, D. S., and Sewell, L. (2009). Olfactory dysfunction in pure autonomic failure: implications for the pathogenesis of Lewy body diseases. Parkinson. Relat. Disord. 1, 516–520. doi: 10.1016/j.parkreldis.2008.12.009
Gomes Gonçalves, N., Vidal Ferreira, N., Khandpur, N., Martinez Steele, E., Bertazzi Levy, R., Andrade Lotufo, P., et al. (2023). Association between consumption of ultraprocessed foods and cognitive decline. JAMA Neurol. 80, 142–150. doi: 10.1001/jamaneurol.2022.4397
González, L. M., Bourissai, A., Lessard-Beaudoin, M., Lebel, R., Tremblay, L., Lepage, M., et al. (2023). Amelioration of cognitive and olfactory system deficits in APOE4 transgenic mice with DHA treatment. Mol. Neurobiol. 60, 5624–5641. doi: 10.1007/s12035-023-03401-z
Gopinath, B., Sue, C. M., Kifley, A., and Mitchell, P. (2012). The association between olfactory impairment and total mortality in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 67, 204–209. doi: 10.1093/gerona/glr165
Gossrau, G., Zaranek, L., Klimova, A., Sabatowski, R., Koch, T., Richter, M., et al. (2023). Olfactory training reduces pain sensitivity in children and adolescents with primary headaches. Front. Pain Res. 4:1091984. doi: 10.3389/fpain.2023.1091984
Goswami, R., Bello, A. I., Bean, J., Costanzo, K. M., Omer, B., Cornelio-Parra, D., et al. (2022). The molecular basis of spinocerebellar ataxia type 7. Front. Neurosci. 16:818757. doi: 10.3389/fnins.2022.818757
Gottfried, J. A. (2006). Smell: central nervous processing. Adv. Otorhinolaryngol. 63, 44–69. doi: 10.1159/000093750
Graus, F., Titulaer, M. J., Balu, R., Benseler, S., Bien, C. G., Cellucci, T., et al. (2016). A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 15, 391–404. doi: 10.1016/S1474-4422(15)00401-9
Groppa, S., Gonzalez-Escamilla, G., Eshaghi, A., Meuth, S. G., and Ciccarelli, O. (2021). Linking immune-mediated damage to neurodegeneration in multiple sclerosis: could network-based MRI help? Brain Commun. 3:fcab237. doi: 10.1093/braincomms/fcab237
Guan, R., Wang, T., Dong, X., Du, K., Li, J., Zhao, F., et al. (2022). Effects of co-exposure to lead and manganese on learning and memory deficits. J. Environ. Sci. 121, 65–76. doi: 10.1016/j.jes.2021.09.012
Gunzer, W. (2017). Changes of olfactory performance during the process of aging-psychophysical testing and its relevance in the fight against malnutrition. J. Nutr. Health Aging 21, 1010–1015. doi: 10.1007/s12603-017-0873-8
Guo, B., Zhang, M., Hao, W., Wang, Y., Zhang, T., and Liu, C. (2023). Neuroinflammation mechanisms of neuromodulation therapies for anxiety and depression. Transl. Psychiatry 13:5. doi: 10.1038/s41398-022-02297-y
Guo, X., Tang, P., Zhang, X., and Li, R. (2023). Causal associations of circulating Helicobacter pylori antibodies with stroke and the mediating role of inflammation. Inflamm. Res. 72,1193–1202. doi: 10.1007/s00011-023-01740-0
Gwinnutt, J. M., Norton, S., Hyrich, K. L., Lunt, M., Combe, B., Rincheval, N., et al. (2022). Exploring the disparity between inflammation and disability in the 10-year outcomes of people with rheumatoid arthritis. Rheumatology 61, 4687–4701. doi: 10.1093/rheumatology/keac137
Haehner, A., Hummel, T., and Reichmann, H. (2009). Olfactory dysfunction as a diagnostic marker for Parkinson's disease. Expert Rev. Neurother. 9, 1773–1779. doi: 10.1586/ern.09.115
Hahad, O., Lelieveld, J., Birklein, F., Lieb, K., Daiber, A., and Münzel, T. (2020). Ambient air pollution increases the risk of cerebrovascular and neuropsychiatric disorders through induction of inflammation and oxidative stress. Int. J. Mol. Sci. 21:4306. doi: 10.3390/ijms21124306
Hajj-Ali, R. A., Major, J., Langford, C., Hoffman, G. S., Clark, T., Zhang, L., et al. (2015). The interface of inflammation and subclinical atherosclerosis in granulomatosis with polyangiitis (Wegener's): a preliminary study. Transl. Res. 166, 366–374. doi: 10.1016/j.trsl.2015.04.001
Han, S., Wang, Q., Song, Y., Pang, M., Ren, C., Wang, J., et al. (2023). Lithium ameliorates Niemann-Pick C1 disease phenotypes by impeding STING/SREBP2 activation. iScience 26:106613. doi: 10.1016/j.isci.2023.106613
Hardebo, J. E. (1994). How cluster headache is explained as an intracavernous inflammatory process lesioning sympathetic fibers. Headache 34, 125–131. doi: 10.1111/j.1526-4610.1994.hed3403125.x
Harita, M., Miwa, T., Shiga, H., Yamada, K., Sugiyama, E., Okabe, Y., et al. (2019). Association of olfactory impairment with indexes of sarcopenia and frailty in community-dwelling older adults. Geriatr. Gerontol. Int. 19, 384–391. doi: 10.1111/ggi.13621
Harris, S., Gilbert, M., Beasant, L., Linney, C., Broughton, J., and Crawley, E. (2017). A qualitative investigation of eating difficulties in adolescents with chronic fatigue syndrome/myalgic encephalomyelitis. Clin. Child Psychol. Psychiatry 22, 128–139. doi: 10.1177/1359104516646813
Hasan Balcioglu, Y., Kirlioglu Balcioglu, S. S., Oncu, F., Turkcan, A., and Coskun Yorulmaz, A. (2022). Impulsive and aggressive traits and increased peripheral inflammatory status as psychobiological substrates of homicide behavior in schizophrenia. Euro. J. Psychiatry 36, 207–214. doi: 10.1016/j.ejpsy.2022.01.004
Hawkes, C. H., Shephard, B. C., Geddes, J. F., Body, G. D., and Martin, J. E. (1998). Olfactory disorder in motor neuron disease. Exp. Neurol. 150, 248–253. doi: 10.1006/exnr.1997.6773
He, Y. S., Cao, F., Musonye, H. A., Xu, Y. Q., Gao, Z. X., Ge, M., et al. (2024). Serum albumin mediates the associations between heavy metals and two novel systemic inflammation indexes among U.S. adults. Ecotoxicol. Environ. Safety 270:115863. doi: 10.1016/j.ecoenv.2023.115863
Heger, E., Rubinstein, G., Braun, L. T., Zopp, S., Honegger, J., Seidensticker, M., et al. (2021). Chemosensory dysfunction in Cushing's syndrome. Endocrine 73, 674–681. doi: 10.1007/s12020-021-02707-z
Henkin, R. I., Schmidt, L., and Velicu, I. (2013). Interleukin 6 in hyposmia. JAMA Otolaryngol. Head Neck Surg. 139, 728–734. doi: 10.1001/jamaoto.2013.3392
Hirota, R., Nakamura, H., Bhatti, S. A., Ngatu, N. R., Muzembo, B. A., Dumavibhat, N., et al. (2012). Limonene inhalation reduces allergic airway inflammation in Dermatophagoides farinae-treated mice. Inhal. Toxicol. 24, 373–381. doi: 10.3109/08958378.2012.675528
Hoenen, M., Wolf, O. T., and Pause, B. M. (2017). The impact of stress on odor perception. Perception 46, 366–376. doi: 10.1177/0301006616688707
Hokari, M., Uchida, K., Shimbo, D., Gekka, M., Asaoka, K., and Itamoto, K. (2020). Acute systematic inflammatory response syndrome and serum biomarkers predict outcomes after subarachnoid hemorrhage. J. Clin. Neurosci. 78, 108–113. doi: 10.1016/j.jocn.2020.05.055
Holbrook, E. H., Puram, S. V., See, R. B., Tripp, A. G., and Nair, D. G. (2019). Induction of smell through transethmoid electrical stimulation of the olfactory bulb. Int. Forum Allergy Rhinol. 9, 158–164. doi: 10.1002/alr.22237
Hori, H., and Kim, Y. (2019). Inflammation and post-traumatic stress disorder. Psychiatry Clin. Neurosci. 73, 143–153. doi: 10.1111/pcn.12820
Hudz, N., Kobylinska, L., Pokajewicz, K., Horčinová Sedláčkov,á, V., Fedin, R., Voloshyn, M., et al. (2023). Mentha piperita: essential oil and extracts, their biological activities, and perspectives on the development of new medicinal and cosmetic products. Molecules 28:7444. doi: 10.3390/molecules28217444
Huggard, D., Kelly, L., Ryan, E., McGrane, F., Lagan, N., Roche, E., et al. (2020). Increased systemic inflammation in children with Down syndrome. Cytokine 127:154938. doi: 10.1016/j.cyto.2019.154938
Huxtable, A. G., Vinit, S., Windelborn, J. A., Crader, S. M., Guenther, C. H., Watters, J. J., et al. (2011). Systemic inflammation impairs respiratory chemoreflexes and plasticity. Respir. Physiol. Neurobiol. 178, 482–489. doi: 10.1016/j.resp.2011.06.017
Iaccarino, L., Shoenfeld, N., Rampudda, M., Zen, M., Gatto, M., Ghirardello, A., et al. (2014). The olfactory function is impaired in patients with idiopathic inflammatory myopathies. Immunol. Res. 60, 247–252. doi: 10.1007/s12026-014-8581-5
Iannaccone, A., Mykytyn, K., Persico, A. M., Searby, C. C., Baldi, A., Jablonski, M. M., et al. (2005). Clinical evidence of decreased olfaction in Bardet-Biedl syndrome caused by a deletion in the BBS4 gene. Am. J. Med. Genet. A. 132, 343–346. doi: 10.1002/ajmg.a.30512
Iannucci, V., Bruscolini, A., Iannella, G., Visioli, G., Alisi, L., Salducci, M., et al. (2024). Olfactory dysfunction and glaucoma. Biomedicines 12:1002. doi: 10.3390/biomedicines12051002
Imamura, F., and Hasegawa-Ishii, S. (2016). Environmental toxicants-induced immune responses in the olfactory mucosa. Front. Immunol. 7:475. doi: 10.3389/fimmu.2016.00475
Iranzo, A., Marrero-González, P., Serradell, M., Gaig, C., Santamaria, J., and Vilaseca, I. (2021). Significance of hyposmia in isolated REM sleep behavior disorder. J. Neurol. 268, 963–966. doi: 10.1007/s00415-020-10229-3
Jiménez-Jiménez, F. J., Alonso-Navarro, H., García-Martín, E., and Agúndez, J. A. G. (2023). Inflammatory factors and restless legs syndrome: a systematic review and meta-analysis. Sleep Med. Rev. 68:101744. doi: 10.1016/j.smrv.2022.101744
Juergens, U. R., Dethlefsen, U., Steinkamp, G., Gillissen, A., Repges, R., and Vetter, H. (2003). Anti-inflammatory activity of 1.8-cineol (eucalyptol) in bronchial asthma: a double-blind placebo-controlled trial. Respir. Med. 97, 250–256. doi: 10.1053/rmed.2003.1432
Juncos, J. L., Lazarus, J. T., Rohr, J., Allen, E. G., Shubeck, L., Hamilton, D., et al. (2012). Olfactory dysfunction in fragile X tremor ataxia syndrome. Mov. Disord. 27, 1556–1559. doi: 10.1002/mds.25043
Kamath, V., Jiang, K., Manning, K. J., Mackin, R. S., Walker, K. A., Powell, D., et al. (2024). Olfactory dysfunction and depression trajectories in community-dwelling older adults. J. Gerontol. A. Biol. Sci. Med. Sci. 79:glad139. doi: 10.1093/gerona/glad139
Kamath, V., and Leff, B. (2019). Mortality risk in older adults: what the nose knows. Ann. Intern. Med. 170, 722–723. doi: 10.7326/M19-1013
Kamath, V., Moberg, P. J., Calkins, M. E., Borgmann-Winter, K., Conroy, C. G., Gur, R. E., et al. (2012). An odor-specific threshold deficit implicates abnormal cAMP signaling in youths at clinical risk for psychosis. Schizophr. Res. 138, 280–284. doi: 10.1016/j.schres.2012.03.029
Kang, D. W., Kim, S. S., Park, D. C., Kim, S. H., and Yeo, S. G. (2021). Objective and measurable biomarkers in chronic subjective tinnitus. Int. J. Mol. Sci. 22:6619. doi: 10.3390/ijms22126619
Kar, T., Yildirim, Y., Altundag, A., Sonmez, M., Kaya, A., Colakoglu, K., et al. (2015). The relationship between age-related macular degeneration and olfactory function. J. Neuro-degener. Dis. 15, 219–224. doi: 10.1159/000381216
Katayama, N., Yoshida, T., Nakashima, T., Ito, Y., Teranishi, M., Iwase, T., et al. (2023). Relationship between tinnitus and olfactory dysfunction: audiovisual, olfactory, and medical examinations. Front. Pub. Health 11:1124404. doi: 10.3389/fpubh.2023.1124404
Kay, L. M. (2022). COVID-19 and olfactory dysfunction: a looming wave of dementia? J. Neurophysiol. 128, 436–444. doi: 10.1152/jn.00255.2022
Kaya, K. S., Akpinar, M., Turk, B., Seyhun, N., Cankaya, M., and Coskun, B. U. (2020). Olfactory function in patients with obstructive sleep apnea using positive airway pressure. Ear, Nose Throat J. 99, 239–244. doi: 10.1177/0145561319878949
Kaya-Sezginer, E., and Gur, S. (2020). The inflammation network in the pathogenesis of erectile dysfunction: attractive potential therapeutic targets. Curr. Pharm. Des. 26, 3955–3972. doi: 10.2174/1381612826666200424161018
Kazour, F., Richa, S., Char, C. A., Atanasova, B., and El-Hage, W. (2020). Olfactory memory in depression: state and trait differences between bipolar and unipolar disorders. Brain Sci. 10:189. doi: 10.3390/brainsci10030189
Kebir, S., Hattingen, E., Niessen, M., Rauschenbach, L., Fimmers, R., Hummel, T., et al. (2020). Olfactory function as an independent prognostic factor in glioblastoma. Neurology 94, e529–e537. doi: 10.1212/WNL.0000000000008744
Kern, J. K., Geier, D. A., Sykes, L. K., and Geier, M. R. (2016). Relevance of neuroinflammation and encephalitis in autism. Front. Cell. Neurosci. 9:519. doi: 10.3389/fncel.2015.00519
Khurshid, K., Crow, A. J. D., Rupert, P. E., Minniti, N. L., Carswell, M. A., Mechanic-Hamilton, D. J., et al. (2019). A quantitative meta-analysis of olfactory dysfunction in epilepsy. Neuropsychol. Rev. 29, 328–337. doi: 10.1007/s11065-019-09406-7
Kim, R., Jun, J. S., Kim, H. J., Jung, K. Y., Shin, Y. W., Yang, T. W., et al. (2019). Peripheral blood inflammatory cytokines in idiopathic REM sleep behavior disorder. Mov. Disord. 34, 1739–1744. doi: 10.1002/mds.27841
Kinnaird, E., Stewart, C., and Tchanturia, K. (2020). The relationship of autistic traits to taste and olfactory processing in anorexia nervosa. Mol. Autism 11:25. doi: 10.1186/s13229-020-00331-8
Kirgezen, T., Yücetaş, U., Server, E. A., Övünç, O., and Yigit, Ö. (2021). Possible effects of low testosterone levels on olfactory function in males. Braz. J. Otorhinolaryngol. 87, 702–710. doi: 10.1016/j.bjorl.2020.03.001
Klimek, L., and Eggers, G. (1997). Olfactory dysfunction in allergic rhinitis is related to nasal eosinophilic inflammation. J. Allergy Clin. Immunol. 100, 158–164. doi: 10.1016/s0091-6749(97)70218-5
Kofod, J., Elfving, B., Nielsen, E. H., Mors, O., and Köhler-Forsberg, O. (2022). Depression and inflammation: correlation between changes in inflammatory markers with antidepressant response and long-term prognosis. Eur. Neuropsychopharmacol. 54, 116–125. doi: 10.1016/j.euroneuro.2021.09.006
Kohli, P., Soler, Z. M., Nguyen, S. A., Muus, J. S., and Schlosser, R. J. (2016). The association between olfaction and depression: a systematic review. Chem. Senses 41, 479–486. doi: 10.1093/chemse/bjw061
Kollndorfer, K., Jakab, A., Mueller, C. A., Trattnig, S., and Schöpf, V. (2015). Effects of chronic peripheral olfactory loss on functional brain networks. Neuroscience 310, 589–599. doi: 10.1016/j.neuroscience.2015.09.045
Komine, O., and Yamanaka, K. (2015). Neuroinflammation in motor neuron disease. Nagoya J. Med. Sci. 77, 537–549.
Kommoss, K. S., Enk, A., Heikenwälder, M., Waisman, A., Karbach, S., and Wild, J. (2023). Cardiovascular comorbidity in psoriasis - psoriatic inflammation is more than just skin deep. J. Dtsch. Dermatol. Ges. 21, 718–725. doi: 10.1111/ddg.15071
Koneczny, I., and Herbst, R. (2019). Myasthenia gravis: Pathogenic effects of autoantibodies on neuromuscular architecture. Cells 8:671. doi: 10.3390/cells8070671
Konstantinidis, I., Triaridis, S., Triaridis, A., Petropoulos, I., Karagiannidis, K., and Kontzoglou, G. (2005). How do children with adenoid hypertrophy smell and taste? Clinical assessment of olfactory function pre- and post-adenoidectomy. Int. J. Pediatr. Otorhinolaryngol. 69, 1343–1349. doi: 10.1016/j.ijporl.2005.03.022
Kopala, L., and Clark, C. (1990). Implications of olfactory agnosia for understanding sex differences in schizophrenia. Schizophr. Bull. 16, 255–261. doi: 10.1093/schbul/16.2.255
Kopala, L. C., Clark, C., and Hurwitz, T. (1993). Olfactory deficits in neuroleptic naive patients with schizophrenia. Schizophr. Res. 8, 245–250. doi: 10.1016/0920-9964(93)90022-B
Koseoglu, S. B., Koseoglu, S., Deveer, R., Derin, S., Kececioglu, M., and Sahan, M. (2016). Impaired olfactory function in patients with polycystic ovary syndrome. Kaohsiung J. Med. Sci. 32, 313–316. doi: 10.1016/j.kjms.2016.04.015
Kovalová, M., Gottfriedová, N., Mrázková, E., Janout, V., and Janoutová, J. (2024). Cognitive impairment, neurodegenerative disorders, and olfactory impairment: a literature review. Polish Otolaryngol. 78, 1–17. doi: 10.5604/01.3001.0053.6158
Kronenbuerger, M., Belenghi, P., Ilgner, J., Freiherr, J., Hummel, T., and Neuner, I. (2018). Olfactory functioning in adults with Tourette syndrome. PLoS ONE 13:e0197598. doi: 10.1371/journal.pone.0197598
Ku, C. M., and Lin, J. Y. (2016). Farnesol, a sesquiterpene alcohol in essential oils, ameliorates serum allergic antibody titres and lipid profiles in ovalbumin-challenged mice. Allergol Immunopathol. 44, 149–159. doi: 10.1016/j.aller.2015.05.009
Kubiak, K., Szmidt, M. K., Kaluza, J., Zylka, A., and Sicinska, E. (2023). Do dietary supplements affect inflammation, oxidative stress, and antioxidant status in adults with hypothyroidism or Hashimoto's disease? A systematic review of controlled trials. Antioxidants 12:1798. doi: 10.3390/antiox12101798
Kümpfel, T., Giglhuber, K., Aktas, O., Ayzenberg, I., Bellmann-Strobl, J., Häußler, V., et al. (2024). Update on the diagnosis and treatment of neuromyelitis optica spectrum disorders (NMOSD). - revised recommendations of the Neuromyelitis Optica Study Group (NEMOS). Part II: Attack therapy and long-term management. J. Neurol. 271, 141–176. doi: 10.1007/s00415-023-11910-z
Kursun, O., Yemisci, M., van den Maagdenberg, A. M. J. M., and Karatas, H. (2021). Migraine and neuroinflammation: the inflammasome perspective. J. Headache Pain 22:55. doi: 10.1186/s10194-021-01271-1
LaFever, B. J., and Imamura, F. (2022). Effects of nasal inflammation on the olfactory bulb. J. Neuroinflamm. 19:294. doi: 10.1186/s12974-022-02657-x
Lambertsen, K. L., Finsen, B., and Clausen, B. H. (2019). Post-stroke inflammation-target or tool for therapy? Acta Neuropathol. 137, 693–714. doi: 10.1007/s00401-018-1930-z
Landis, B. N., Vodicka, J., and Hummel, T. (2010). Olfactory dysfunction following herpetic meningoencephalitis. J. Neurol. 257, 439–443. doi: 10.1007/s00415-009-5344-7
Las Casas Lima, M. H., Cavalcante, A. L. B., and Leão, S. C. (2022). Pathophysiological relationship between COVID-19 and olfactory dysfunction: a systematic review. Braz. J. Otorhinolaryngol. 88, 794–802. doi: 10.1016/j.bjorl.2021.04.001
Laudien, M., Lamprecht, P., Hedderich, J., Holle, J., and Ambrosch, P. (2009). Olfactory dysfunction in Wegener's granulomatosis. Rhinology 47, 254–259. doi: 10.4193/Rhin08.159
Laudisio, A., Navarini, L., Margiotta, D. P. E., Fontana, D. O., Chiarella, I., Spitaleri, D., et al. (2019). The association of olfactory dysfunction, frailty, and mortality is mediated by inflammation: results from the InCHIANTI Study. J. Immunol. Res. 2019:3128231. doi: 10.1155/2019/3128231
Lazarini, F., Lannuzel, A., Cabi,é, A., Michel, V., Madec, Y., Chaumont, H., et al. (2022). Olfactory outcomes in Zika virus-associated Guillain-Barré syndrome. Eur. J. Neurol. 29, 2823–2831. doi: 10.1111/ene.15444
Leclercq, S., de Timary, P., Delzenne, N. M., and Stärkel, P. (2017). The link between inflammation, bugs, the intestine and the brain in alcohol dependence. Transl. Psychiatry 7:e1048. doi: 10.1038/tp.2017.15
Lee, K., Choi, I. H., Lee, S. H., and Kim, T. H. (2019). Association between subjective olfactory dysfunction and female hormone-related factors in South Korea. Sci. Rep. 9:20007. doi: 10.1038/s41598-019-56565-x
Leon, M., and Woo, C. C. (2022). Olfactory loss is a predisposing factor for depression, while olfactory enrichment is an effective treatment for depression. Front. Neurosci. 16:1013363. doi: 10.3389/fnins.2022.1013363
Leonardo, S., and Fregni, F. (2023). Association of inflammation and cognition in the elderly: a systematic review and meta-analysis. Front. Aging Neurosci. 15:1069439. doi: 10.3389/fnagi.2023.1069439
Leon-Sarmiento, F. E., Bayona, E. A., Bayona-Prieto, J., Osman, A., and Doty, R. L. (2012). Profound olfactory dysfunction in myasthenia gravis. PLoS ONE 7:e45544. doi: 10.1371/journal.pone.0045544
Leon-Sarmiento, F. E., Bayona, E. A., Rizzo-Sierra, C. V., Garavito, A., Campos, M. F., and Doty, R. (2014). Olfactory dysfunction in Chagas' disease. Neurology 82 (Suppl. 10), P3–027. doi: 10.1212/WNL.82.10_supplement.P3.027
Lewis, C. R., Talboom, J. S., De Both, M. D., Schmidt, A. M., Naymik, M. A., Håberg, A. K., et al. (2021). Smoking is associated with impaired verbal learning and memory performance in women more than men. Sci. Rep. 11:10248. doi: 10.1038/s41598-021-88923-z
Li, F., Wang, Y., and Zheng, K. (2023). Microglial mitophagy integrates the microbiota-gut-brain axis to restrain neuroinflammation during neurotropic herpesvirus infection. Autophagy 19, 734–736. doi: 10.1080/15548627.2022.2102309
Liao, C. P., Booker, R. C., Brosseau, J. P., Chen, Z., Mo, J., Tchegnon, E., et al. (2018). Contributions of inflammation and tumor microenvironment to neurofibroma tumorigenesis. J. Clin. Invest. 128, 2848–2861. doi: 10.1172/JCI99424
Likuni, N., Lam, Q. L., Lu, L., Matarese, G., and La Cava, A. (2008). Leptin and inflammation. Curr. Immunol. Rev. 4, 70–79. doi: 10.2174/157339508784325046
Lin, L.-J., and Li, K.-Y. (2022). Comparing the effects of olfactory-based sensory 223. stimulation and board game training on cognition, emotion, and blood biomarkers among individuals with dementia: a pilot randomized controlled trial. Front. Psychol. 13:1003325. doi: 10.3389/fpsyg.2022.1003325
Liu, B., Luo, Z., and Chen, H. (2019). Relationship between poor olfaction and mortality. Ann. Intern. Med. 171:526. doi: 10.7326/L19-0468
Lontchi-Yimagou, E., Sobngwi, E., Matsha, T. E., and Kengne, A. P. (2013). Diabetes mellitus and inflammation. Curr. Diab. Rep. 13, 435–444. doi: 10.1007/s11892-013-0375-y
López González, I., Garcia-Esparcia, P., Llorens, F., and Ferrer, I. (2016). Genetic and transcriptomic profiles of inflammation in neurodegenerative diseases: Alzheimer, Parkinson, Creutzfeldt-Jakob and tauopathies. Int. J. Mol. Sci. 17:206. doi: 10.3390/ijms17020206
Lötsch, J., Ultsch, A., Eckhardt, M., Huart, C., Rombaux, P., and Hummel, T. (2016). Brain lesion-pattern analysis in patients with olfactory dysfunctions following head trauma. Neuroimage Clin. 11, 99–105. doi: 10.1016/j.nicl.2016.01.011
Lu, K. (2023). Cellular pathogenesis of hepatic encephalopathy: an update. Biomolecules 13:396. doi: 10.3390/biom13020396
Lu, R., Huang, R., Li, K., Zhang, X., Yang, H., Quan, Y., et al. (2014). The influence of benign essential blepharospasm on dry eye disease and ocular inflammation. Am. J. Ophthalmol. 157, 591–597. doi: 10.1016/j.ajo.2013.11.014
Lundberg, I. E., Fujimoto, M., Vencovsky, J., Aggarwal, R., Holmqvist, M., Christopher-Stine, L., et al. (2021). Idiopathic inflammatory myopathies. Nature Rev. Dis. Prim. 7:86. doi: 10.1038/s41572-021-00321-x
Luzzi, S., Snowden, J. S., Neary, D., Coccia, M., Provinciali, L., and Lambon Ralph, M. A. (2007). Distinct patterns of olfactory impairment in Alzheimer's disease, semantic dementia, frontotemporal dementia, and corticobasal degeneration. Neuropsychologia 45, 1823–1831. doi: 10.1016/j.neuropsychologia.2006.12.008
Maas, C., and López-Lera, A. (2019). Hereditary angioedema: Insights into inflammation and allergy. Mol. Immunol. 112, 378–386. doi: 10.1016/j.molimm.2019.06.017
Mahmut, M. K., and Stevenson, R. J. (2012). Olfactory abilities and psychopathy: Higher psychopathy scores are associated with poorer odor discrimination and identification. Chemosens. Percept. 5, 300–307. doi: 10.1007/s12078-012-9135-7
Maier, A., Heinen-Ludwig, L., Güntürkün, O., Hurlemann, R., and Scheele, D. (2020). Childhood maltreatment alters the neural processing of chemosensory stress signals. Front. Psychiatry 11:783. doi: 10.3389/fpsyt.2020.00783
Makhlouf, M., Souza, D. G., Kurian, S., Bellaver, B., Ellis, H., Kuboki, A., et al. (2024). Short-term consumption of highly processed diets varying in macronutrient content impair the sense of smell and brain metabolism in mice. Mol. Metab. 79:101837. doi: 10.1016/j.molmet.2023.101837
Maki, P. M. (2015). Verbal memory and menopause. Maturitas 82, 288–290. doi: 10.1016/j.maturitas.2015.07.023
Malutan, A. M., Dan, M., Nicolae, C., and Carmen, M. (2014). Proinflammatory and anti-inflammatory cytokine changes related to menopause. Menopause Rev. 13, 162–168. doi: 10.5114/pm.2014.43818
Manara, R., Salvalaggio, A., Favaro, A., Palumbo, V., Citton, V., Elefante, A., et al. (2014). Brain changes in Kallmann syndrome. Am. J. Neuroradiol. 35, 1700–1706. doi: 10.3174/ajnr.A3946
Marazziti, D., Palermo, S., Arone, A., Massa, L., Parra, E., Simoncini, M., et al. (2023). Obsessive-compulsive disorder, PANDAS, and Tourette syndrome: immuno-inflammatory disorders. Adv. Exp. Med. Biol. 1411, 275–300. doi: 10.1007/978-981-19-7376-5_13
Marek, M., Linnepe, S., Klein, C., Hummel, T., and Paus, S. (2018). High prevalence of olfactory dysfunction in cervical dystonia. Parkinsonism Relat. Disord. 53, 33–36. doi: 10.1016/j.parkreldis.2018.04.028
Masala, C., Loy, F., Pinna, I., Manis, N. A., Ercoli, T., and Solla, P. (2024). Olfactory function as a potential predictor of cognitive impairment in men and women. Biology 13:503. doi: 10.3390/biology13070503
Masala, C., Solla, P., and Loy, F. (2023). Gender-related differences in the correlation between odor threshold, discrimination, identification, and cognitive reserve index in healthy subjects. Biology 12:586. doi: 10.3390/biology12040586
Masaoka, Y., Kawamura, M., Takeda, A., Kobayakawa, M., Kuroda, T., Kasai, H., et al. (2011). Impairment of odor recognition and odor-induced emotions in type 1 myotonic dystrophy. Neurosci. Lett. 503, 163–166. doi: 10.1016/j.neulet.2011.08.006
Masehi-Lano, J. J., Deyssenroth, M., Jacobson, S. W., Jacobson, J. L., Molteno, C. D., Dodge, N. C., et al. (2023). Alterations in placental inflammation-related gene expression partially mediate the effects of prenatal alcohol consumption on maternal iron homeostasis. Nutrients 15:4105. doi: 10.3390/nu15194105
Matsui, T., Arai, H., Nakajo, M., Maruyama, M., Ebihara, S., Sasaki, H., et al. (2003). Role of chronic sinusitis in cognitive functioning in the elderly. J. Am. Geriatr. Soc. 51, 1818–1819. doi: 10.1046/j.1532-5415.2003.51572_5.x
Matsunaga, M., Bai, Y., Yamakawa, K., Toyama, A., Kashiwagi, M., Fukuda, K., et al. (2013). Brain-immune interaction accompanying odor-evoked autobiographic memory. PLoS ONE 8:e72523. doi: 10.1371/journal.pone.0072523
Maurage, P., Rombaux, P., and de Timary, P. (2014). Olfaction in alcohol-dependence: a neglected yet promising research field. Front. Psychol. 4:1007. doi: 10.3389/fpsyg.2013.01007
McCombe, P. A., and Henderson, R. D. (2011). The role of immune and inflammatory mechanisms in ALS. Curr. Mol. Med. 11, 246–254. doi: 10.2174/156652411795243450
McConnell, R. J., Menendez, C. E., Smith, F. R., Henkin, R. I., and Rivlin, R. S. (1975). Defects of taste and smell in patients with hypothyroidism. Am. J. Med. 59, 354–364. doi: 10.1016/0002-9343(75)90394-0
McElvaney, O. J., Wade, P., Murphy, M., Reeves, E. P., and McElvaney, N. G. (2019). Targeting airway inflammation in cystic fibrosis. Expert Rev. Respir. Med. 13, 1041–1055. doi: 10.1080/17476348.2019.1666715
McInnes, K., Friesen, C. L., MacKenzie, D. E., Westwood, D. A., and Boe, S. G. (2017). Mild traumatic brain injury (mTBI) and chronic cognitive impairment: a scoping review. PLoS ONE 12:e0174847. doi: 10.1371/journal.pone.0174847
McKee, A. C., Daneshvar, D. H., Alvarez, V. E., and Stein, T. D. (2014). The neuropathology of sport. Acta Neuropathol. 127, 29–51. doi: 10.1007/s00401-013-1230-6
McLaren, A. M. R., and Kawaja, M. D. (2024). Olfactory dysfunction and Alzheimer's disease: a review. J. Alzheimers Dis. 99, 811–827. doi: 10.3233/JAD-231377
McNeill, A., Duran, R., Proukakis, C., Bras, J., Hughes, D., Mehta, A., et al. (2012). Hyposmia and cognitive impairment in Gaucher disease patients and carriers. Mov. Disord. 27, 526–532. doi: 10.1002/mds.24945
Melluso, A., Secondulfo, F., Capolongo, G., Capasso, G., and Zacchia, M. (2023). Bardet-Biedl syndrome: current perspectives and clinical outlook. Ther. Clin. Risk Manag. 19, 115–132. doi: 10.2147/TCRM.S338653
Michalovicz, L. T., Kelly, K. A., Sullivan, K., and O'Callaghan, J. P. (2020). Acetylcholinesterase inhibitor exposures as an initiating factor in the development of Gulf War Illness, a chronic neuroimmune disorder in deployed veterans. Neuropharmacology 171:108073. doi: 10.1016/j.neuropharm.2020.108073
Miller, J. E., Liu, C. M., Zemanick, E. T., Woods, J. C., Goss, C. H., Taylor-Cousar, J. L., et al. (2023). Olfactory loss in people with cystic fibrosis: community perceptions and impact. J. Cys. Fibros. doi: 10.1016/j.jcf.2023.11.006. [Epub ahead of print].
Mishra, S., Karan, K., Nag, D., and Sengupta, P. (2016). Adult onset Niemann–Pick type C disease: two different presentations. Neurol. India 64, 1044–1047. doi: 10.4103/0028-3886.190242
Misiak, B., Bartoli, F., Carr,à, G., Stańczykiewicz, B., Gładka, A., Frydecka, D., et al. (2021). Immune-inflammatory markers and psychosis risk: a systematic review and meta-analysis. Psychoneuroendocrinology 127:105200. doi: 10.1016/j.psyneuen.2021.105200
Mohamad, N. V., Wong, S. K., Wan Hasan, W. N., Jolly, J. J., Nur-Farhana, M. F., Ima-Nirwana, S., et al. (2019). The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male 22, 129–140. doi: 10.1080/13685538.2018.1482487
Mohamed, S., Emmanuel, N., and Foden, N. (2019). Nasal obstruction: a common presentation in primary care. Br. J. Gen. Pract. 69, 628–629. doi: 10.3399/bjgp19X707057
Müller, N. (2018). Inflammation in schizophrenia: pathogenetic aspects and therapeutic considerations. Schizophr. Bull. 44, 973–982. doi: 10.1093/schbul/sby024
Murphy, C., Dalton, P., Boateng, K., Hunter, S., Silberman, P., Trachtman, J., et al. (2024). Integrating the patient's voice into the research agenda for treatment of chemosensory disorders. Chem. Senses 49:bjae020. doi: 10.1093/chemse/bjae020
Muruzheva, Z. M., Ivleva, I. S., Traktirov, D. S., Zubov, A. S., and Karpenko, M. N. (2022). The relationship between serum interleukin-1β, interleukin-6, interleukin-8, interleukin-10, tumor necrosis factor-α levels and clinical features in essential tremor. Int. J. Neurosci. 132, 1143–1149. doi: 10.1080/00207454.2020.1865952
Muscaritoli, M., Imbimbo, G., Jager-Wittenaar, H., Cederholm, T., Rothenberg, E., di Girolamo, F. G., et al. (2023). Disease-related malnutrition with inflammation and cachexia. Clin. Nutr. 42, 1475–1479. doi: 10.1016/j.clnu.2023.05.013
Nair, J. R., and Moots, R. J. (2017). Behçet's disease. Clin. Med. 17, 71–77. doi: 10.7861/clinmedicine.17-1-71
Nakashima, T., Katayama, N., Sugiura, S., Teranishi, M., Suzuki, H., Hirabayashi, M., et al. (2019). Olfactory function in persons with cerebral palsy. J. Policy Pract. Intellect. Disabil. 16, 217–222. doi: 10.1111/jppi.12284
Nasserie, T., Hittle, M., and Goodman, S. N. (2021). Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw. Open 4:e2111417. doi: 10.1001/jamanetworkopen.2021.11417
Numan, M. S., Amiable, N., Brown, J. P., and Michou, L. (2015). Paget's disease of bone: an osteoimmunological disorder? Drug Des. Devel. Ther. 9, 4695–4707. doi: 10.2147/DDDT.S88845
Nunes, J. P. S., Roda, V. M. P., Andrieux, P., Kalil, J., Chevillard, C., and Cunha-Neto, E. (2023). Inflammation and mitochondria in the pathogenesis of chronic Chagas disease cardiomyopathy. Exp. Biol. Med. 248, 2062–2071. doi: 10.1177/15353702231220658
Oleszkiewicz, A., Abriat, A., Doelz, G., Azema, E., and Hummel, T. (2021). Beyond olfaction: Beneficial effects of olfactory training extend to aging-related cognitive decline. Behav. Neurosci. 135, 732–740. doi: 10.1037/bne0000478
Oleszkiewicz, A., Bottesi, L., Pieniak, M., Fujita, S., Krasteva, N., Nelles, G., et al. (2022). Olfactory training with aromastics: olfactory and cognitive effects. Head Neck Surg. 279, 225–232. doi: 10.1007/s00405-021-06810-9
O'Shea, B. Q., Demakakos, P., Cadar, D., and Kobayashi, L. C. (2021). Adverse childhood experiences and rate of memory decline from mid to later life: evidence from the English longitudinal study of ageing. Am. J. Epidemiol. 190, 1294–1305. doi: 10.1093/aje/kwab019
Ottaviano, G., Cantone, E., D'Errico, A., Salvalaggio, A., Citton, V., Scarpa, B., et al. (2015). Sniffin' Sticks and olfactory system imaging in patients with Kallmann syndrome. Int. Forum Allergy Rhinol. 5, 855–861. doi: 10.1002/alr.21550
Pajares, M. I., Rojo, A., Manda, G., Boscá, L., and Cuadrado, A. (2020). Inflammation in Parkinson's disease: mechanisms and therapeutic implications. Cells 9:1687. doi: 10.3390/cells9071687
Panfili, E., Mondanelli, G., Orabona, C., Belladonna, M. L., Gargaro, M., Fallarino, F., et al. (2021). Novel mutations in the WFS1 gene are associated with Wolfram syndrome and systemic inflammation. Hum. Mol. Genet. 30, 265–276. doi: 10.1093/hmg/ddab040
Pang, N. Y., Song, H. J. J., Tan, B. K. J., Tan, J. X., Chen, A. S. R., See, A., et al. (2022). Association of olfactory impairment with all-cause mortality: a systematic review and meta-analysis. JAMA Otolaryngol. Head Neck Surg. 148, 436–445. doi: 10.1001/jamaoto.2022.0263
Pang, Y., Li, Y., Zhang, Y., Wang, H., Lang, J., Han, L., et al. (2022). Effects of inflammation and oxidative stress on postoperative delirium in cardiac surgery. Front. Cardiovasc. Med. 9:1049600. doi: 10.3389/fcvm.2022.1049600
Pascual, B., Funk, Q., Zanotti-Fregonara, P., Cykowski, M. D., Veronese, M., Rockers, E., et al. (2021). Neuroinflammation is highest in areas of disease progression in semantic dementia. Brain 144, 1565–1575. doi: 10.1093/brain/awab057
Paton, M. C. B., Finch-Edmondson, M., Dale, R. C., Fahey, M. C., Nold-Petry, C. A., Nold, M. F., et al. (2022). Persistent inflammation in cerebral palsy: pathogenic mediator or comorbidity? A scoping review. J. Clin. Med. 11:7368. doi: 10.3390/jcm11247368
Patrick, D. M., Van Beusecum, J. P., and Kirabo, A. (2021). The role of inflammation in hypertension: novel concepts. Curr. Opin. Physiol. 19, 92–98. doi: 10.1016/j.cophys.2020.09.016
Peng, M., Potterton, H., Chu, J. T. W., and Glue, P. (2021). Olfactory shifts linked to postpartum depression. Sci. Rep. 11:14947. doi: 10.1038/s41598-021-94556-z
Perricone, C., Agmon-Levin, N., Shoenfeld, N., de Carolis, C., Guarino, M. D., Gigliucci, G., et al. (2011). Evidence of impaired sense of smell in hereditary angioedema. Allergy 66, 149–154. doi: 10.1111/j.1398-9995.2010.02453.x
Petagna, L., Antonelli, A., Ganini, C., Bellato, V., Campanelli, M., Divizia, A., et al. (2020). Pathophysiology of Crohn's disease inflammation and recurrence. Biol. Direct. 15:23. doi: 10.1186/s13062-020-00280-5
Peter, M. G., Darki, F., Thunell, E., Mårtensson, G., Postma, E. M., Boesveldt, S., et al. (2023). Lifelong olfactory deprivation-dependent cortical reorganization restricted to orbitofrontal cortex. Hum. Brain Mapp. 44, 6459–6470. doi: 10.1002/hbm.26522
Peters, J. M., Hummel, T., Kratzsch, T., Lötsch, J., Skarke, C., and Frölich, L. (2003). Olfactory function in mild cognitive impairment and Alzheimer's disease: an investigation using psychophysical and electrophysiological techniques. Am. J. Psychiatry 160, 1995–2002. doi: 10.1176/appi.ajp.160.11.1995
Pignataro, A., and Middei, S. (2017). Trans-synaptic spread of amyloid-β in Alzheimer's disease: paths to β-amyloidosis. Neural Plast. 2017:5281829. doi: 10.1155/2017/5281829
Pina, L. T. S., Ferro, J. N. S., Rabelo, T. K., Oliveira, M. A., Scotti, L., Scotti, M. T., et al. (2019). Alcoholic monoterpenes found in essential oil of aromatic spices reduce allergic inflammation by the modulation of inflammatory cytokines. Nat. Prod. Res. 33, 1773–1777. doi: 10.1080/14786419.2018.1434634
Pinto, J. M. (2021). The specter of olfactory impairment: lessons about mortality in older US adults. JAMA Otolaryngol. Head Neck Surg. 147, 56–57. doi: 10.1001/jamaoto.2020.3745
Pinto, J. M., Wroblewski, K. E., Kern, D. W., Schumm, L. P., and McClintock, M. K. (2014). Olfactory dysfunction predicts 5-year mortality in older adults. PLoS ONE 9:e107541. doi: 10.1371/journal.pone.0107541
Pitel, A. L., Eustache, F., and Beaunieux, H. (2014). Component processes of memory in alcoholism: pattern of compromise and neural substrates. Handb. Clin. Neurol. 125, 211–225. doi: 10.1016/B978-0-444-62619-6.00013-6
Ponsen, M. M., Stoffers, D., Booij, J., van Eck-Smit, B. L., Wolters, E. C., and Berendse, H. W. (2004). Idiopathic hyposmia as a preclinical sign of Parkinson's disease. Ann. Neurol. 56, 173–181. doi: 10.1002/ana.20160
Postolache, T. T., Wadhawan, A., Can, A., Lowry, C. A., Woodbury, M., Makkar, H., et al. (2020). Inflammation in traumatic brain injury. J. Alzheimers. Dis. 74, 1–28. doi: 10.3233/JAD-191150
Potter, M. R., Chen, J. H., Lobban, N. S., and Doty, R. L. (2020). Olfactory dysfunction from acute upper respiratory infections: relationship to season of onset. Int. Forum Allergy Rhinol. 10, 706–712. doi: 10.1002/alr.22551
Pries, R., Jeschke, S., Leichtle, A., and Bruchhage, K. L. (2023). Modes of Action of 1,8-Cineol in Infections and Inflammation. Metabolites 13:751. doi: 10.3390/metabo13060751
Radke, J., Meinhardt, J., Aschman, T., Chua, R. L., Farztdinov, V., Lukassen, S., et al. (2024). Proteomic and transcriptomic profiling of brainstem, cerebellum and olfactory tissues in early- and late-phase COVID-19. Nature Neurosci. 27, 409–420. doi: 10.1038/s41593-024-01573-y
Rahmati, M., Yon, D. K., Lee, S. W., Soysal, P., Koyanagi, A., Jacob, L., et al. (2023). New-onset neurodegenerative diseases as long-term sequelae of SARS-CoV-2 infection: a systematic review and meta-analysis. J. Med. Virol. 95, e28909. doi: 10.1002/jmv.28909
Ramsey, J. T., Shropshire, B. C., Nagy, T.R., Chambers, K. D., Li, Y., and Korach, K. S. Essential oils and health. Yale J. Biol. Med. (2020) 93:291.
Rana, A., and Musto, A. E. (2018). The role of inflammation in the development of epilepsy. J. Neuroinflamm. 15:144. doi: 10.1186/s12974-018-1192-7
Rao, M., Wang, X., Guo, G., Wang, L., Chen, S., Yin, P., et al. (2021). Resolving the intertwining of inflammation and fibrosis in human heart failure at single-cell level. Basic Res. Cardiol. 116:55. doi: 10.1007/s00395-021-00897-1
Rasmussen, A. L., and Popescu, S. V. (2021). SARS-CoV-2 transmission without symptoms. Science 371, 1206–1207. doi: 10.1126/science.abf9569
Rayego-Mateos, S., Rodrigues-Diez, R. R., Fernandez-Fernandez, B., Mora-Fernández, C., Marchant, V., Donate-Correa, J., et al. (2023). Targeting inflammation to treat diabetic kidney disease: the road to 2Kidney Int. 103, 282–296. doi: 10.1016/j.kint.2022.10.030
Renzetti, S., van Thriel, C., Lucchini, R. G., Smith, D. R., Peli, M., Borgese, L., et al. (2024). A multi-environmental source approach to explore associations between metals exposure and olfactory identification among school-age children residing in northern Italy. J. Expos. Sci. Environ. Epidemiol. 34, 699–708. doi: 10.1038/s41370-024-00687-6
Reuber, M., Al-Din, A. S., Baborie, A., and Chakrabarty, A. (2001). New variant Creutzfeldt-Jakob disease presenting with loss of taste and smell. J. Neurol. Neurosurg. Psychiatr. 71, 412–413. doi: 10.1136/jnnp.71.3.412
Rhyou, H. I., Bae, W. Y., and Nam, Y. H. (2021). Association between olfactory function and asthma in adults. J. Asthma Allergy 14, 309–316. doi: 10.2147/JAA.S299796
Ribeiro, J. C., Oliveiros, B., Pereira, P., António, N., Hummel, T., Paiva, A., et al. (2016). Accelerated age-related olfactory decline among type 1 Usher patients. Sci. Rep. 6:28309. doi: 10.1038/srep28309
Rivera, D. G., Hernández, I., Merino, N., Luque, Y., Álvarez, A., Martín, Y., et al. (2011). Mangifera indica L. extract (Vimang) and mangiferin reduce the airway inflammation and Th2 cytokines in murine model of allergic asthma. J. Pharm. Pharmacol. 63, 1336–1345. doi: 10.1111/j.2042-7158.2011.01328.x
Roessner, V., Bleich, S., Banaschewski, T., and Rothenberger, A. (2005). Olfactory deficits in anorexia nervosa. Eur. Arch. Psychiatry Clin. Neurosci. 255, 6–9. doi: 10.1007/s00406-004-0525-y
Roh, D., Lee, D. H., Kim, S. W., Kim, S. W., Kim, B. G., Kim, D. H., et al. (2021). The association between olfactory dysfunction and cardiovascular disease and its risk factors in middle-aged and older adults. Sci. Rep. 11:1248. doi: 10.1038/s41598-020-80943-5
Royet, J. P., Plailly, J., Saive, A. L., Veyrac, A., and Delon-Martin, C. (2013). The impact of expertise in olfaction. Front. Psychol. 4:928. doi: 10.3389/fpsyg.2013.00928
Rupp, C. I., Fleischhacker, W. W., Hausmann, A., Mair, D., Hinterhuber, H., and Kurz, M. (2004). Olfactory functioning in patients with alcohol dependence: Impairments in odor judgements. Alcohol 39, 514–519. doi: 10.1093/alcalc/agh100
Rydbirk, R., Østergaard, O., Folke, J., Hempel, C., DellaValle, B., Andresen, T. L., et al. (2022). Brain proteome profiling implicates the complement and coagulation cascade in multiple system atrophy brain pathology. Cell. Mol. Life Sci. 79:336. doi: 10.1007/s00018-022-04378-z
Salimi, M., Nazari, M., Shahsavar, P., Dehghan, S., Javan, M., Mirnajafi-Zadeh, J., et al. (2024). Olfactory bulb stimulation mitigates Alzheimer-like disease progression. bioRxiv. doi: 10.1101/2024.03.03.583116
Samanci, B., Sahin, E., Sen, C., Samanci, Y., Sezgin, M., Emekli, S., et al. (2021). Olfactory dysfunction in patients with cluster headache. Eur. Arch. Otorhinolaryngol. 278, 4361–4365. doi: 10.1007/s00405-021-06738-0
Sartori, A. C., Vance, D. E., Slater, L. Z., and Crowe, M. (2012). The impact of inflammation on cognitive function in older adults: Implications for healthcare practice and research. J. Neurosci. Nurs. 44, 206–217. doi: 10.1097/JNN.0b013e3182527690
Schaie, K. W., Willis, S. L., and Caskie, G. I. (2004). The Seattle longitudinal study: relationship between personality and cognition. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 11, 304–324. doi: 10.1080/13825580490511134
Schertel Cassiano, L., Ribeiro, A. P., Peres, M. A., Lopez, R., Fjaeldstad, A., Marchini, L., et al. (2023). Self-reported periodontitis association with impaired smell and taste: a multicenter survey. Oral Dis. 30, 1516–1524. doi: 10.1111/odi.14601
Schiffman, S. S. (2018). Influence of medications on taste and smell. World J. Otorhinolaryngol. Head Neck Surg. 4, 84–91. doi: 10.1016/j.wjorl.2018.02.005
Schmidt, F., Göktas, O., Jarius, S., Wildemann, B., Ruprecht, K., Paul, F., et al. (2013). Olfactory dysfunction in patients with neuromyelitis optica. Mult. Scler. Int. 2013, 654501. doi: 10.1155/2013/654501
Schoenfeld, N., Agmon-Levin, N., Flitman-Katzevman, I., Paran, D., Katz, B. S., Kivity, S., et al. (2009). The sense of smell in systemic lupus erythematosus. Arthritis Rheum. 60, 1484–1487. doi: 10.1002/art.24491
Schubert, C. R., Carmichael, L. L., Murphy, C., Klein, B. E., Klein, R., and Cruickshanks, K. J. (2008). Olfaction and the 5-year incidence of cognitive impairment in an epidemiological study of older adults. J. Am. Geriatr. Soc. 56, 1517–1521. doi: 10.1111/j.1532-5415.2008.01826.x
Schubert, C. R., Cruickshanks, K. J., Fischer, M. E., Klein, B. E., Klein, R., and Pinto, A. A. (2015). Inflammatory and vascular markers and olfactory impairment in older adults. Age Ageing 44, 878–882. doi: 10.1093/ageing/afv075
Schubert, C. R., Fischer, M. E., Pinto, A. A., Klein, B. E. K., Klein, R., Tweed, T. S., et al. (2017). Sensory impairments and risk of mortality in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 72, 710–715. doi: 10.1093/gerona/glw036
Scorr, L. M., Kilic-Berkmen, G., Sutcliffe, D. J., Dinasarapu, A. R., McKay, J. L., Bagchi, P., et al. (2024). Exploration of potential immune mechanisms in cervical dystonia. Parkinsonism Relat. Disord. 122:106036. doi: 10.1016/j.parkreldis.2024.106036
Segura, B., Baggio, H. C., Solana, E., Palacios, E. M., Vendrell, P., Bargalló, N., et al. (2013). Neuroanatomical correlates of olfactory loss in normal aged subjects. Behav. Brain Res. 246, 148–153. doi: 10.1016/j.bbr.2013.02.025
Serby, M., Larson, P., and Kalkstein, D. (1991). The nature and course of olfactory deficits in Alzheimer's disease. Am. J. Psychiatry 148, 357–360. doi: 10.1176/ajp.148.3.357
Seubert, J., Kalpouzos, G., Larsson, M., Hummel, T., Bäckman, L., and Laukka, E. J. (2020). Temporolimbic cortical volume is associated with semantic odor memory performance in aging. Neuroimage 211:116600. doi: 10.1016/j.neuroimage.2020.116600
Shi, A., Long, Y., Ma, Y., et al. (2023). Natural essential oils derived from herbal medicines: a promising therapy strategy for treating cognitive impairment. Front. Aging Neurosci. 15:1104269. doi: 10.3389/fnagi.2023.1104269
Shi, D., Das, J., and Das, G. (2006). Inflammatory bowel disease requires the interplay between innate and adaptive immune signals. Cell Res. 16, 70–74. doi: 10.1038/sj.cr.7310009
Shibata, H., Fujiwara, R., Iwamoto, M., Matsuoka, H., and Yokoyama, M. M. (1991). Immunological and behavioral effects of fragrance in mice. Int. J. Neurosci. 57, 151–159. doi: 10.3109/00207459109150355
Shields, G. S., Doty, D., Shields, R. H., Gower, G., Slavich, G. M., and Yonelinas, A. P. (2017). Recent life stress exposure is associated with poorer long-term memory, working memory, and self-reported memory. Stress 20, 598–607. doi: 10.1080/10253890.2017.1380620
Shill, H. A., Zhang, N., Driver-Dunckley, E., Mehta, S., Adler, C. H., and Beach, T. G. (2021). Olfaction in neuropathologically defined progressive supranuclear palsy. Mov. Disord. 36, 1700–1704. doi: 10.1002/mds.28568
Siegel, J. K., Kung, S. Y., Wroblewski, K. E., Kern, D. W., McClintock, M. K., and Pinto, J. M. (2021). Olfaction is associated with sexual motivation and satisfaction in older men and women. J. Sex. Med. 18, 295–302. doi: 10.1016/j.jsxm.2020.12.002
Sieper, J., and Poddubnyy, D. (2017). Axial spondyloarthritis. Lancet 390, 73–84. doi: 10.1016/S0140-6736(16)31591-4
Simopoulos, A. P. (2002). Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 21, 495–505. doi: 10.1080/07315724.2002.10719248
Sinclair, A. J., Ball, A. K., Burdon, M. A., Clarke, C. E., Stewart, P. M., Curnow, S. J., et al. (2008). Exploring the pathogenesis of IIH: an inflammatory perspective. J. Neuroimmunol. 201–202, 212–220. doi: 10.1016/j.jneuroim.2008.06.029
Sobel, N., Thomason, M. E., Stappen, I., Tanner, C. M., Tetrud, J. W., Bower, J. M., et al. (2001). An impairment in sniffing contributes to the olfactory impairment in Parkinson's disease. Proc. Natl. Acad. Sci. U. S. A. 98, 4154–4159. doi: 10.1073/pnas.071061598
Sobin, C., Kiley-Brabeck, K., Dale, K., Monk, S. H., Khuri, J., and Karayiorgou, M. (2006). Olfactory disorder in children with 22q11 deletion syndrome. Pediatrics 118, e697–e703. doi: 10.1542/peds.2005-3114
Solla, P., Masala, C., Ercoli, T., Frau, C., Bagella, C., Pinna, I., et al. (2023). Olfactory impairment correlates with executive functions disorders and other specific cognitive dysfunctions in Parkinson's disease. Biology 12:112. doi: 10.3390/biology12010112
Sollai, G., Melis, M., Mastinu, M., Paduano, D., Chicco, F., Magri, S., et al. (2021). Olfactory function in patients with inflammatory bowel disease (IBD) is associated with their body mass index and polymorphism in the odor binding-protein (OBPIIa) gene. Nutrients 13:703. doi: 10.3390/nu13020703
Soysal, P., Stubbs, B., Lucato, P., Luchini, C., Solmi, M., Peluso, R., et al. (2016). Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res. Rev. 31, 1–8. doi: 10.1016/j.arr.2016.08.006
Speth, U. S., König, D., Burg, S., Gosau, M., and Friedrich, R. E. (2023). Evaluation of the sense of taste and smell in patients with neurofibromatosis type 1. J. Stomatol. Oral Maxillofac. Surg. 124:101271. doi: 10.1016/j.jormas.2022.08.014
Spotten, L., Corish, C., Lorton, C., Dhuibhir, P. U., O'Donoghue, N., O'Connor, B., et al. (2016). Subjective taste and smell changes in treatment-naive people with solid tumours. Support. Care Cancer 24, 3201–3208. doi: 10.1007/s00520-016-3133-2
Stanciu, A. E., Hurduc, A., Stanciu, M. M., Gherghe, M., Gheorghe, D. C., Prunoiu, V. M., et al. (2023). Portrait of the inflammatory response to radioiodine therapy in female patients with differentiated thyroid cancer with/without type 2 diabetes mellitus. Cancers 15:3793. doi: 10.3390/cancers15153793
Steinbach, S., Proft, F., Schulze-Koops, H., Hundt, W., Heinrich, P., Schulz, S., et al. (2011). Gustatory and olfactory function in rheumatoid arthritis. Scand. J. Rheum. 40, 169–177. doi: 10.3109/03009742.2010.517547
Stern, Y. (2012). Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol. 11, 1006–1012. doi: 10.1016/S1474-4422(12)70191-6
Stevenson, R. J., Mahmut, M. K., Horstmann, A., and Hummel, T. (2020). The aetiology of olfactory dysfunction and its relationship to diet quality. Brain Sci. 10:769. doi: 10.3390/brainsci10110769
Suat, B., Deniz Tuna, E., Ozgur, Y., Muhammet, Y., and Tevfik Fikret, C. (2016). The effects of radioactive iodine therapy on olfactory function. Am. J. Rhinol. Allergy 30, 206–210. doi: 10.2500/ajra.2016.30.4384
Subramaniyan, S., and Terrando, N. (2019). Neuroinflammation and perioperative neurocognitive disorders. Anesth. Analg. 128, 781–788. doi: 10.1213/ANE.0000000000004053
Suh, K. D., Kim, S. M., Han, D. H., Min, H. J., and Kim, K. S. (2020). Olfactory function test for early diagnosis of vascular dementia. Korean J. Fam. Med. 41, 202–204. doi: 10.4082/kjfm.18.0202
Takehara-Nishiuchi, K. (2014). Entorhinal cortex and consolidated memory. Neurosci. Res. 84, 27–33. doi: 10.1016/j.neures.2014.02.012
Tan, W., Zou, J., Yoshida, S., Jiang, B., and Zhou, Y. (2020). The role of inflammation in age-related macular degeneration. Int. J. Biol. Sci. 16, 2989–3001. doi: 10.7150/ijbs.49890
Terrier, C., Greco-Vuilloud, J., Cavelius, M., Thevenet, M., Mandairon, N., Didier, A., et al. (2024). Long-term olfactory enrichment promotes non-olfactory cognition, noradrenergic plasticity and remodeling of brain functional connectivity in older mice. Neurobiol. Aging 136, 133–156. doi: 10.1016/j.neurobiolaging.2024.01.011
Thorstensen, W. M., Oie, M. R., Dahlslett, S. B., Sue-Chu, M., Steinsvag, S. K., and Helvik, A. S. (2022). Olfaction in COPD. Rhinology 60, 47–55. doi: 10.4193/Rhin21.037
Trares, K., Bhardwaj, M., Perna, L., Stocker, H., Petrera, A., Hauck, S. M., et al. (2022). Association of the inflammation-related proteome with dementia development at older age: results from a large, prospective, population-based cohort study. Alzheimers. Res. Ther. 14:128. doi: 10.1186/s13195-022-01063-y
Tristan Asensi, M., Napoletano, A., Sofi, F., and Dinu, M. (2023). Low-grade inflammation and ultra-processed foods consumption: a review. Nutrients 15:1546. doi: 10.3390/nu15061546
Ueno-Iio, T., Shibakura, M., Yokota, K., Aoe, M., Hyoda, T., Shinohata, R., et al. (2014). Lavender essential oil inhalation suppresses allergic airway inflammation and mucous cell hyperplasia in a murine model of asthma. Life Sci. 108, 109–115. doi: 10.1016/j.lfs.2014.05.018
Upadhyay, U. D., and Holbrook, E. H. (2004). Olfactory loss as a result of toxic exposure. Otolaryngol. Clin. North Am. 37, 1185–1207. doi: 10.1016/j.otc.2004.05.003
Üstün Bezgin, S., Çakabay, T., Irak, K., Koçyigit, M., Serin Keskinege, B., Cevizci, R., et al. (2017). Association of Helicobacter pylori infection with olfactory function using smell identification screening test. Eur. Arch. Otorhinolaryngol. 274, 3403–3405. doi: 10.1007/s00405-017-4656-y
Vaira, L. A., Hopkins, C., Petrocelli, M., Lechien, J. R., Chiesa-Estomba, C. M., Salzano, G., et al. (2020). Smell and taste recovery in coronavirus disease 2019 patients: a 60-day objective and prospective study. J. Laryngol. Otol. 134, 703–709. doi: 10.1017/S0022215120001826
Valadão, P. A. C., Santos, K. B. S., Ferreira Vieira, T. H., Macedo E Cordeiro, T., Teixeira, A. L., Guatimosim, C., et al. (2020). Inflammation in Huntington's disease: a few new twists on an old tale. J. Neuroimmunol. 348:577380. doi: 10.1016/j.jneuroim.2020.577380
Valizadeh, P., Momtazmanesh, S., Plazzi, G., and Rezaei, N. (2024). Connecting the dots: an updated review of the role of autoimmunity in narcolepsy and emerging immunotherapeutic approaches. Sleep Med. 113, 378–396. doi: 10.1016/j.sleep.2023.12.005
Van Bogart, K., Engeland, C. G., Sliwinski, M. J., Harrington, K. D., Knight, E. L., Zhaoyang, R., et al. (2022). The association between loneliness and inflammation: findings from an older adult sample. Front. Behav. Neurosci. 15:801746. doi: 10.3389/fnbeh.2021.801746
Van Dijck, A., Barbosa, S., Bermudez-Martin, P., Khalfallah, O., Gilet, C., Martinuzzi, E., et al. (2020). Reduced serum levels of pro-inflammatory chemokines in fragile X syndrome. BMC Neurol. 20:138. doi: 10.1186/s12883-020-01715-2
Van Regemorter, V., Dollase, J., Coulie, R., Stouffs, A., Dieu, A., de Saint-Hubert, M., et al. (2022). Olfactory dysfunction predicts frailty and poor postoperative outcome in older patients scheduled for elective non-cardiac surgery. J. Nutr. Health Aging. 26, 981–986. doi: 10.1007/s12603-022-1851-3
Vance, D. E., Del Bene, V. A., Kamath, V., Frank, J. S., Billings, R., Cho, D. Y., et al. (2024). Does olfactory training improve brain function and cognition? A systematic review. Neuropsychol. Rev. 34, 155–191. doi: 10.1007/s11065-022-09573-0
Vasterling, J. J., Brailey, K., and Sutker, P. B. (2000). Olfactory identification in combat-related posttraumatic stress disorder. J. Trauma. Stress 13, 241–253. doi: 10.1023/A:1007754611030
Velluzzi, F., Deledda, A., Onida, M., Loviselli, A., Crnjar, R., and Sollai, G. (2022). Relationship between olfactory function and BMI in normal weight healthy subjects and patients with overweight or obesity. Nutrients 14:1262. doi: 10.3390/nu14061262
Veyseller, B., Ozucer, B., Aksoy, F., Yildirim, Y. S., Gürbüz, D., Balikçi, H. H., et al. (2012). Reduced olfactory bulb volume and diminished olfactory function in total laryngectomy patients: a prospective longitudinal study. Am. J. Rhinol. Allergy 26, 191–193. doi: 10.2500/ajra.2012.26.3768
Viguera, C., Wang, J., Mosmiller, E., Cerezo, A., and Maragakis, N. J. (2018). Olfactory dysfunction in amyotrophic lateral sclerosis. Ann. Clin. Transl. Neurol. 5, 976–981. doi: 10.1002/acn3.594
Vohra, V., Assi, S., Kamath, V., Soler, Z. M., and Rowan, N. R. (2023). Potential role for diet in mediating the association of olfactory dysfunction and cognitive decline: a nationally representative study. Nutrients 15:3890. doi: 10.3390/nu15183890
Volkmann, E. R., Andréasson, K., and Smith, V. (2023). Systemic sclerosis. Lancet 401, 304–318. doi: 10.1016/S0140-6736(22)01692-0
Waldton, S. (1974). Clinical observations of impaired cranial nerve function in senile dementia. Acta Psychiatr. Scand. 50, 539–547. doi: 10.1111/j.16000447.1974.tb09714.x
Walker, I. M., Fullard, M. E., Morley, J. F., and Duda, J. E. (2021). Olfaction as an early marker of Parkinson's disease and Alzheimer's disease. Handb. Clin. Neurol. 182, 317–329. doi: 10.1016/B978-0-12-819973-2.00030-7
Wang, H. J., Zakhari, S., and Jung, M. K. (2010). Alcohol, inflammation, and gut-liver-brain interactions in tissue damage and disease development. World J. Gastroenterol. 16, 1304–1313. doi: 10.3748/wjg.v16.i11.1304
Wang, L., Davis, P. B., Volkow, N. D., Berger, N. A., Kaelber, D. C., and Xu, R. (2022). Association of COVID-19 with new-onset Alzheimer's disease. J. Alzheimers Dis. 89, 411–414. doi: 10.3233/JAD-220717
Wang, Q., Chen, B., Zhong, X., Zhou, H., Zhang, M., Mai, N., et al. (2021). Olfactory dysfunction is already present with subjective cognitive decline and deepens with disease severity in the Alzheimer's disease spectrum. J. Alzheimers Dis. 79, 585–595. doi: 10.3233/JAD-201168
Wang, T. Y., Lee, S. Y., Hu, M. C., Chen, S. L., Chang, Y. H., Chu, C. H., et al. (2017). More inflammation but less brain-derived neurotrophic factor in antisocial personality disorder. Psychoneuroendocrinology 85, 42–48. doi: 10.1016/j.psyneuen.2017.08.006
Wang, X., Younan, D., Petkus, A. J., Beavers, D. P., Espeland, M. A., Chui, H. C., et al. (2021). Ambient air pollution and long-term trajectories of episodic memory decline among older women in the WHIMS-ECHO Cohort. Environ. Health Perspect. 129:97009. doi: 10.1289/EHP7668
Wehling, E., Naess, H., Wollschlaeger, D., Hofstad, H., Bramerson, A., Bende, M., et al. (2015). Olfactory dysfunction in chronic stroke patients. BMC Neurol. 15:199. doi: 10.1186/s12883-015-0463-5
Weiss, G., Ganz, T., and Goodnough, L. T. (2019). Anemia of inflammation. Blood 133, 40–50. doi: 10.1182/blood-2018-06-856500
Weiss, J., Pyrski, M., Jacobi, E., Bufe, B., Willnecker, V., Schick, B., et al. (2011). Loss-of-function mutations in sodium channel Nav1.7 cause anosmia. Nature 472, 186–190. doi: 10.1038/nature09975
Wheeler, P. L., and Murphy, C. (2021). Olfactory measures as predictors of conversion to mild cognitive impairment and Alzheimer's disease. Brain Sci. 11, 1391. doi: 10.3390/brainsci11111391
Wheeler, T. T., Alberts, M. A., Dolan, T. A., and McGorray, S. P. (1995). Dental, visual, auditory and olfactory complications in Paget's disease of bone. J. Am. Geriatr. Soc. 43, 1384–1391. doi: 10.1111/j.1532-5415.1995.tb06618.x
Whitcroft, K. L., Mancini, L., Yousry, T., Hummel, T., and Andrews, P. J. (2023). Functional septorhinoplasty alters brain structure and function: Neuroanatomical correlates of olfactory dysfunction. Front. Allergy 4, 1079945. doi: 10.3389/falgy.2023.1079945
Whiting, A. C., Marmura, M. J., Hegarty, S. E., and Keith, S. W. (2015). Olfactory acuity in chronic migraine: A cross-sectional study. Headache 55, 71–75. doi: 10.1111/head.12462
Wilson, R. S., Yu, L., and Bennett, D. A. (2011). Odor identification and mortality in old age. Chem. Senses 36, 63–67. doi: 10.1093/chemse/bjq098
Witoonpanich, P., Cash, D. M., Shakespeare, T. J., Yong, K. X., Nicholas, J. M., Omar, R., et al. (2013). Olfactory impairment in posterior cortical atrophy. J. Neurol. Neurosurg. Psychiatr. 84, 588–590. doi: 10.1136/jnnp-2012-304497
Wong, K. E., Wade, T. J., Moore, J., Marcellus, A., Molnar, D. S., O'Leary, D. D., et al. (2022). Examining the relationships between adverse childhood experiences (ACEs), cortisol, and inflammation among young adults. Brain Behav. Immun. Health 25, 100516. doi: 10.1016/j.bbih.2022.100516
Woo, C. C., Miranda, B., Sathishkumar, M., Dehkordi-Vakil, F., Yassa, M. A., and Leon, M. (2023). Overnight olfactory enrichment using an odorant diffuser improves memory and modifies the uncinate fasciculus in older adults. Front. Neurosci. 17, 1200448. doi: 10.3389/fnins.2023.1200448
Wu, P., Dong, J., Cheng, N., Yang, R., Han, Y., and Han, Y. (2019). Inflammatory cytokines expression in Wilson's disease. Neurol. Sci. 40, 1059–1066. doi: 10.1007/s10072-018-3680-z
Wurth, R., Rescigno, M., Flippo, C., Stratakis, C. A., and Tatsi, C. (2022). Inflammatory biomarkers in the evaluation of pediatric endogenous Cushing syndrome. Eur. J. Endocrinol. 186, 503–510. doi: 10.1530/EJE-21-1199
Xiao, Z., Zhao, Q., Liang, X., Wu, W., Cao, Y., and Ding, D. (2021). Poor odor identification predicts mortality risk in older adults without neurodegenerative diseases: The Shanghai Aging Study. J. Am. Med. Dir. Assoc. 22, 2218-2219.e1. doi: 10.1016/j.jamda.2021.05.026
Xie, J., Van Hoecke, L., and Vandenbroucke, R. E. (2022). The impact of systemic inflammation on Alzheimer's disease pathology. Front. Immunol. 12, 796867. doi: 10.3389/fimmu.2021.796867
Yafi, F. A., Jenkins, L., Albersen, M., Corona, G., Isidori, A. M., Goldfarb, S., et al. (2016). Erectile dysfunction. Nature Rev. Dis. Primers 2, 16003. doi: 10.1038/nrdp.2016.3
Yahiaoui-Doktor, M., Luck, T., Riedel-Heller, S. G., Loeffler, M., Wirkner, K., and Engel, C. (2019). Olfactory function is associated with cognitive performance: results from the population-based LIFE-Adult-Study. Alzheimers. Res. Ther. 11, 43. doi: 10.1186/s13195-019-0494-z
Yalcinkaya, E., Basaran, M. M., Erdem, H., Kocyigit, M., Altundag, A., and Hummel, T. (2019). Olfactory dysfunction in spondyloarthritis. Eur. Arch. Oto-rhino-laryngol. 276, 1241–1245. doi: 10.1007/s00405-019-05364-1
Yao, L., Yi, X., Pinto, J. M., Yuan, X., Guo, Y., Liu, Y., et al. (2018). Olfactory cortex and olfactory bulb volume alterations in patients with post-infectious olfactory loss. Brain Imag. Behav. 12, 1355–1362. doi: 10.1007/s11682-017-9807-7
Ye, C., Guo, X., Wu, J., Wang, M., Ding, H., and Ren, X. (2022). Mediated macrophage activation and polarization can promote adenoid epithelial inflammation in adenoid hypertrophy. J. Inflamm. Res. 15, 6843–6855. doi: 10.2147/JIR.S390210
Yin, K., and Agrawal, D. K. (2014). Vitamin D and inflammatory diseases. J. Inflamm. Res. 7, 69–87. doi: 10.2147/JIR.S63898
Yoo, H. S., Jeon, S., Chung, S. J., Yun, M., Lee, P. H., Sohn, Y. H., et al. (2018). Olfactory dysfunction in Alzheimer's disease- and Lewy body-related cognitive impairment. Alzheimers. Dement. 14, 1243–1252. doi: 10.1016/j.jalz.2018.05.010
Zhang, C., Han, Y., Liu, X., Tan, H., Dong, Y., Zhang, Y., et al. (2022). Odor enrichment attenuates the anesthesia/surgery-induced cognitive impairment. Ann. Surg. 277, e1387–e1396. doi: 10.1097/SLA.0000000000005599
Zhang, H., Wang, Y., Zhao, Y., Liu, T., Wang, Z., Zhang, N., et al. (2022). PTX3 mediates the infiltration, migration, and inflammation-resolving-polarization of macrophages in glioblastoma. CNS Neurosci. Therap. 28, 1748–1766. doi: 10.1111/cns.13913
Zhang, Z., Zhang, B., Wang, X., Zhang, X., Yang, Q. X., Qing, Z., et al. (2019). Olfactory dysfunction mediates adiposity in cognitive impairment of type 2 diabetes: Insights from clinical and functional neuroimaging studies. Diabetes Care 42, 1274–1283. doi: 10.2337/dc18-2584
Zhao, L., Hou, C., and Yan, N. (2022). Neuroinflammation in retinitis pigmentosa: therapies targeting the innate immune system. Front. Immunol. 13:1059947. doi: 10.3389/fimmu.2022.1059947
Zhong, P. X., Chen, Y. H., Li, I. H., Wen, Y. L., Kao, H. H., Chiang, K. W., et al. (2023). Increased risk of olfactory and taste dysfunction in the United States psoriasis population. Eur. Arch. Otorhinolaryngol. 280, 695–702. doi: 10.1007/s00405-022-07530-4
Zucco, G. M., Amodio, P., and Gatta, A. (2006). Olfactory deficits in patients affected by minimal hepatic encephalopathy: a pilot study. Chem. Senses 31, 273–278. doi: 10.1093/chemse/bjj029
Keywords: olfaction, inflammation, medical conditions, causation, correlation, olfactory dysfunction, olfactory enrichment
Citation: Leon M, Troscianko ET and Woo CC (2024) Inflammation and olfactory loss are associated with at least 139 medical conditions. Front. Mol. Neurosci. 17:1455418. doi: 10.3389/fnmol.2024.1455418
Received: 27 June 2024; Accepted: 16 September 2024;
Published: 11 October 2024.
Edited by:
Jolanta Dorszewska, Poznan University of Medical Sciences, PolandReviewed by:
Donald A. Wilson, New York University, United StatesBrigit High, National Institutes of Health (NIH), United States
Carla Masala, University of Cagliari, Italy
Leslie M. Kay, The University of Chicago, United States
Copyright © 2024 Leon, Troscianko and Woo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Leon, bWxlb25AdWNpLmVkdQ==
†Present address: Emily T. Troscianko, Department of English, University of California, Santa Barbara, Santa Barbara, CA, United States
 Michael Leon
Michael Leon Emily T. Troscianko
Emily T. Troscianko Cynthia C. Woo
Cynthia C. Woo