- 1Department of Pain Medicine, The First Affiliated Hospital of Soochow University, Suzhou, China
- 2Department of Pain Medicine, Suzhou Xiangcheng People's Hospital, Suzhou, China
Background: Zoster-associated neuralgia (ZAN) is recognized as a challenging neuralgia that often leads to poor prognosis in patients receiving interventional pain management. Identifying risk factors early can enable clinicians to develop personalized treatment plans; however, research in this area is limited.
Methods: We retrospectively screened all patients with ZAN who received interventional therapy in the Pain Department of Soochow University First Affiliated Hospital from January 1, 2022 to August 31, 2023. Data on patient demographics, medical history, neutrophil-to-lymphocyte ratio (NLR), clinical scoring, and treatment methods were collected. Interventional therapy included short-term nerve electrical stimulation (st-NES), pulsed radiofrequency (PRF) and radiofrequency thermocoagulation (RF-TC). Patients were categorized into poor prognosis and control groups based on outcomes 3 months post-discharge. Multivariate logistic regression was used to identify risk factors for poor prognosis.
Results: The final analysis included 282 patients. The rate of poor prognosis was 32.6% (92/282). Multivariate logistic regression analysis revealed that age ≥ 65 years (odds ratio, 2.985; 95% confidence interval, 1.449–6.148; p = 0.003), disease duration >3 months (odds ratio, 3.135; 95% confidence interval, 1.685–5.832; p < 0.001), head and face pain (odds ratio, 3.140; 95% confidence interval, 1.557–6.330; p = 0.001), use of immunosuppressants (odds ratio, 2.737; 95% confidence interval, 1.168–6.416; p = 0.021), higher NLR (odds ratio, 1.454; 95% confidence interval, 1.233–1.715; p < 0.001), PRF (st-NES as reference) (odds ratio, 2.324; 95% confidence interval, 1.116–4.844; p = 0.024) and RF-TC (st-NES as reference) (odds ratio, 5.028; 95% confidence interval, 2.139–11.820; p < 0.001) were found to be independent risk factors for poor prognosis in patients with ZAN who underwent interventional pain management.
Conclusion: Age ≥ 65 years (odds ratio, 2.985; 95% confidence interval, 1.449–6.148; p = 0.003), disease duration >3 months (odds ratio, 3.135; 95% confidence interval, 1.685–5.832; p < 0.001), head and face pain (odds ratio, 3.140; 95% confidence interval, 1.557–6.330; p = 0.001), immunosuppressants use (odds ratio, 2.737; 95% confidence interval, 1.168–6.416; p = 0.021), higher NLR (odds ratio, 1.454; 95% confidence interval, 1.233–1.715; p < 0.001), PRF (odds ratio, 2.324; 95% confidence interval, 1.116–4.844; p = 0.024) and RF-TC (odds ratio, 5.028; 95% confidence interval, 2.139–11.820; p < 0.001) were identified as independent risk factors for poor prognosis in patients with ZAN who underwent interventional pain management.
1 Introduction
Herpes zoster-associated neuralgia (ZAN) is the most common complication of herpes zoster (HZ) and leads to chronic pain manifestations in up to 30% of affected patients (Kawai et al., 2014). Despite the various available treatments, satisfactory outcomes have not been achieved (Johnson et al., 2015). The severity and persistence of pain, in addition to causing distress, significantly impair patients’ quality of life, daily functions, work capabilities, and mental well-being (Drolet et al., 2010). Epidemiological data shows that globally, approximately one-third of the population will develop herpes zoster during their lifetime, with an estimated incidence of 3–5 cases per 1,000 person-years. The burden of ZAN significantly impacts healthcare resources and costs (Kawai et al., 2014; Harvey et al., 2020; Cebrian-Cuenca et al., 2011). The complexity of ZAN pain management continues to constitute a clinical dilemma that perplexes both healthcare providers and patients alike (Drolet et al., 2010).
The underlying mechanisms of ZAN syndrome, potentially linked to neuroinflammation and neuroplasticity, have yet to be fully understood (Baron and Wasner, 2006; Wood, 2002). Medication serves as the foundational treatment approach, yet the efficacy of pharmacotherapy is generally modest and dose dependent (Finnerup et al., 2021). For patients who exhibit resistance to conventional therapies, a combination of interventional treatments offered additional pain relief (van Wijck et al., 2011; Jang et al., 2015). However, the response to interventional pain management, including neuromodulation and denervation techniques, varies significantly among individuals, and the overall success rates have not been entirely satisfactory (Whitley et al., 2010). Therefore, it is crucial to identify high-risk patients early and develop personalized treatment strategies. Currently, such researches on risk factors for poor prognosis in ZAN patients receiving interventional pain management are lacking.
This study aimed to compile and analyze the incidence and risk factors for poor prognosis in ZAN patients receiving interventional pain management. Thus, we are able to identify high-risk patients early and develop personalized treatment strategies, effectively reducing the incidence of poor prognosis in ZAN patients receiving interventional pain management.
2 Materials and methods
2.1 Ethics and patients
This observational retrospective case–control study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University (agreement number: 2023545). Informed consent was waived for this study after a waiver was approved by the ethics committee. Patients with ZAN who underwent interventional therapy at the Department of Pain Medicine, the First Affiliated Hospital of Soochow University, from January 1, 2022 to August 31, 2023 were selected as the research objects. The inclusion criteria for patients were as follows: (1) was at least 18 years old; (2) had any period of herpes zoster-associated pain; and (3) underwent the first instance of inpatient interventional pain management. The exclusion criteria for patients were as follows: (1) had intellectual disability or cognitive impairment; (2) had disease such as other pain disorders that confounded the ZAN assessment; and (3) patients with incomplete data.
2.2 Diagnosis
Drawing on the source (Watson et al., 1991), this study categorizes the prognosis of ZAN patients as poor if any of the following three conditions manifest within 3 months post-discharge: (1) moderate-to-severe pain, as indicated by an NRS pain score of 4 or higher on a 0 to 10 scale, where higher scores denote worse pain; (2) nighttime awakenings or difficulty initiating sleep due to pain; and (3) the presence of pain-related depression or anxiety necessitated medication. Furthermore, patients who required a second session or multiple sessions of interventional pain management within 3 months post-discharge were also classified as having a poor prognosis in this study.
2.3 Data collection
Researchers meticulously reviewed the electronic medical records on an individual basis to gather patient data, which included age (years), sex, BMI (kg/m2), disease duration (months), vaccination, antiviral treatment status, pain score (NRS), prodromal pain, breakthrough pain, allodynia, head and face pain, hypertension, diabetes, tumors, immunosuppressant use, and intervention type (including st-NES, PRF, and RF-TC).
Patient information was obtained by direct interview or telephone follow-up after discharge and recorded in the follow-up record system. Exposure years were defined as the number of years since the initial diagnosis of herpes zoster. Significant familial or personal events, such as severe illness, accidents, or the death of a loved one, were also recorded.
After the data collection, a thorough review of the electronic medical records system and the follow-up records system was conducted to ensure the accuracy of the data, and the finalized dataset was used for analysis.
2.4 Evaluation scale
Numerical Rating Scale (NRS): The NRS comprises 11 scores ranging from 0 to 10. The patients used a scale that included 11 numbers from 0 to 10 to describe the intensity of the pain, and the greater the number was, the greater the severity of the pain (Bijur et al., 2003).
2.5 Statistical analyses
The sample size was estimated using PASS version 15. All available samples were utilized in this study. SPSS 25.0 (IBM) software was used for data processing and analysis. The Kolmogorov–Smirnov test was used to determine whether continuous data were normally distributed. Continuous variables are expressed as the mean ± standard deviation, and the independent sample t test was used to compare normally distributed continuous data. Categorical variables were compared using the χ2 test. Univariate logistic regression was used to screen risk factors through univariate analysis (p < 0.1), which were then subjected to multivariate logistic regression analysis, and p < 0.05 was considered to indicate statistical significance. The independent variables in the model were analyzed for collinearity. The discrimination and calibration of the multivariable logistic regression model were evaluated using the C-index and Hosmer-Lemeshow test.
3 Results
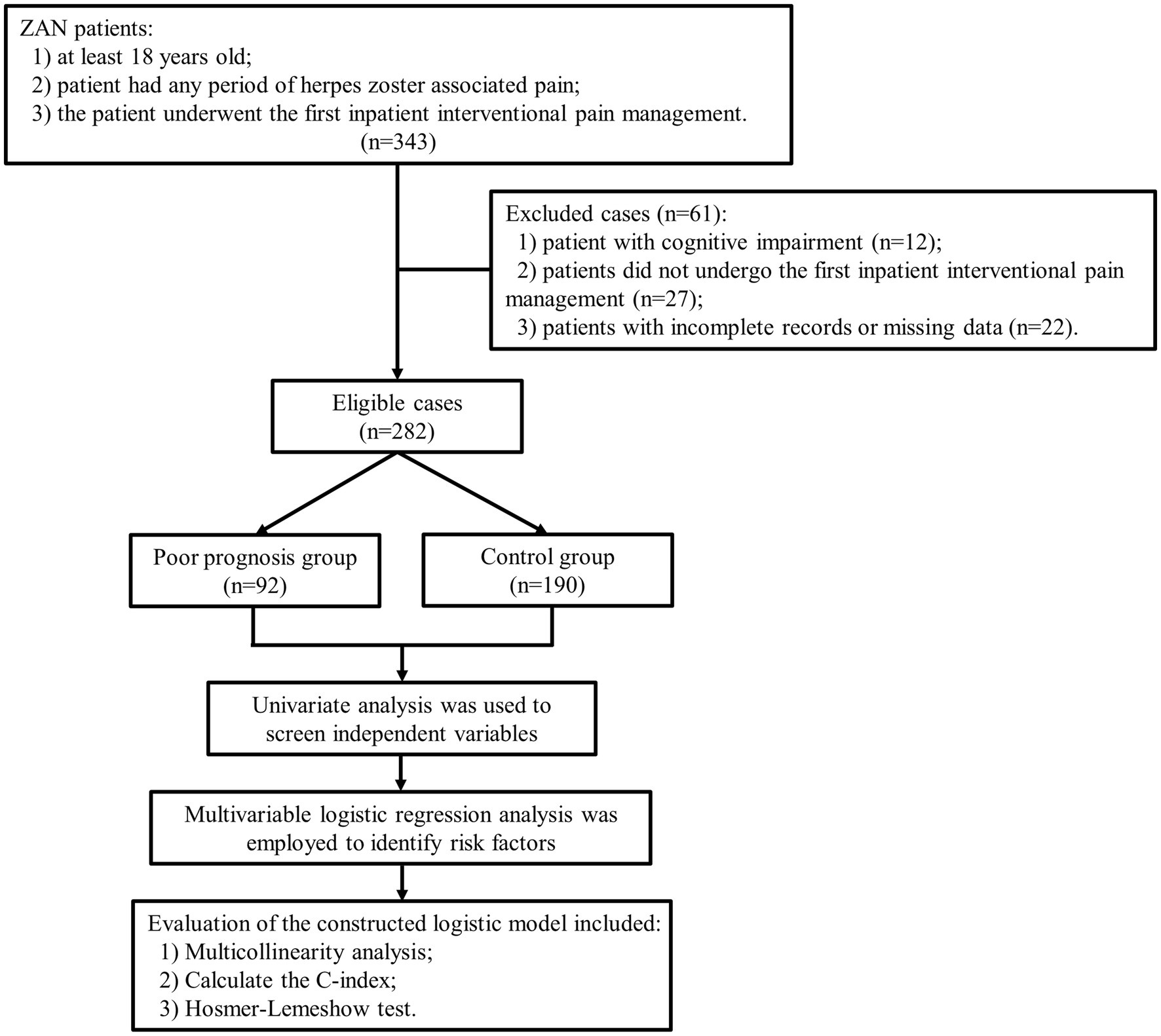
The final analysis included 282 patients. The poor prognosis group included 92 patients, and the control group included 190 patients, as shown in Figure 1. The rate of poor prognosis was 32.6% (92/282). There was no significant difference in the years of exposure between case patients and controls (p = 0.121). No major family or personal events (such as a car accident, serious illness, or death) occurred in either group.
Univariate logistic regression analysis revealed that age ≥ 65 years, disease duration >3 months, head and face pain, diabetes status, immunosuppressant use, NLR, and interventional therapy were the 7 independent variables that needed to be included in the multivariate logistic regression analysis (p < 0.1), as shown in Table 1.
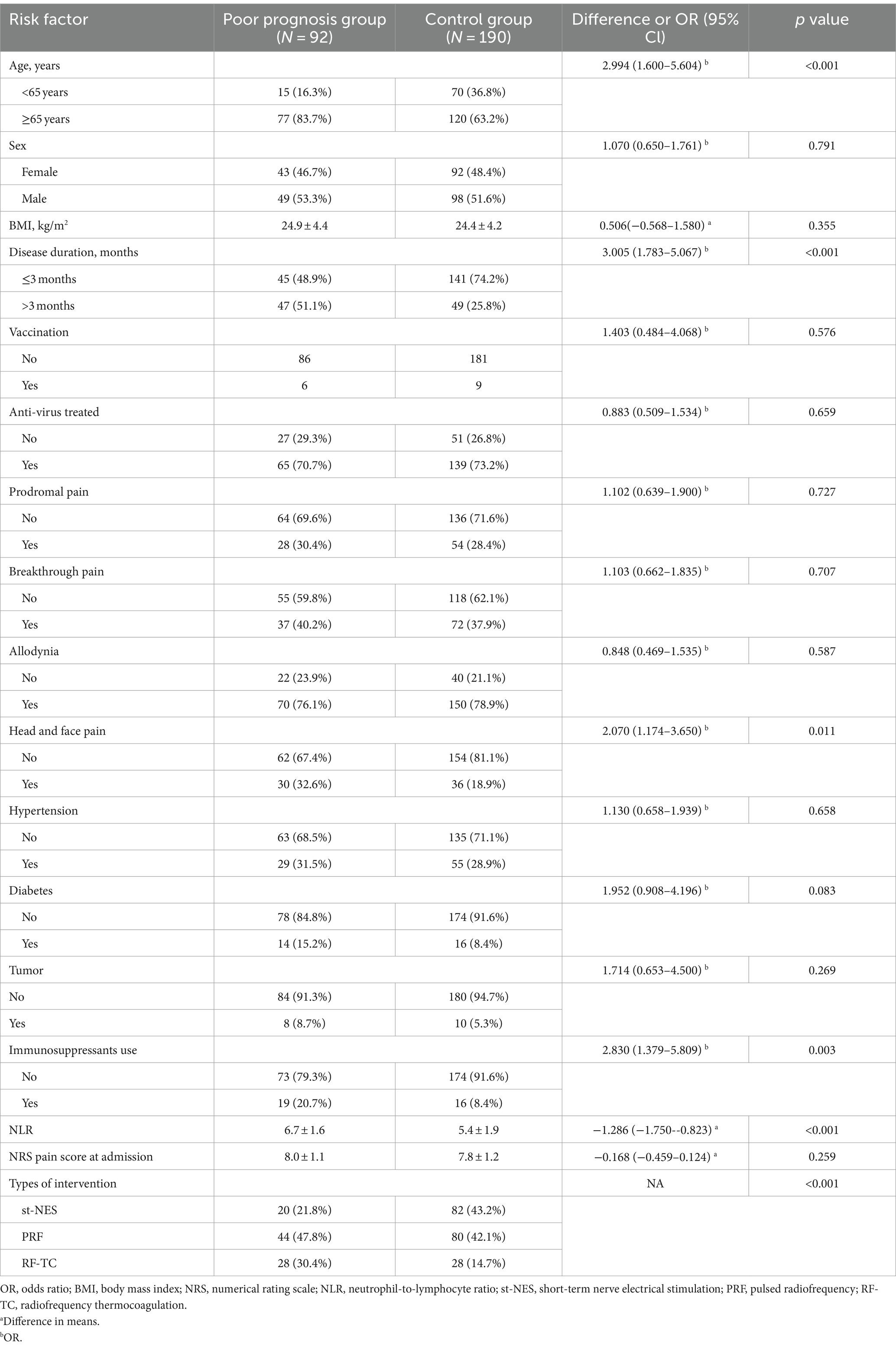
Table 1. Univariate analysis of risk factors for poor prognosis in patients with ZAN who underwent interventional pain management.
Multivariate logistic regression analysis revealed that age ≥ 65 years (odds ratio, 2.985; 95% confidence interval, 1.449–6.148; p = 0.003), disease duration >3 months (odds ratio, 3.135; 95% confidence interval, 1.685–5.832; p < 0.001), head and face pain (odds ratio, 3.140; 95% confidence interval, 1.557–6.330; p = 0.001), use of immunosuppressants (odds ratio, 2.737; 95% confidence interval, 1.168–6.416; p = 0.021), higher NLR (odds ratio, 1.454; 95% confidence interval, 1.233–1.715; p < 0.001), PRF (st-NES as reference) (odds ratio, 2.324; 95% confidence interval, 1.116–4.844; p = 0.024) and RF-TC (st-NES as reference) (odds ratio, 5.028; 95% confidence interval, 2.139–11.820; p < 0.001) were identified as independent risk factors for poor prognosis in patients with ZAN who underwent interventional pain management, as shown in Table 2 and Figure 2.

Table 2. Multivariate analysis of risk factors for poor prognosis in ZAN patients who underwent interventional pain management.

Figure 2. Risk factors for poor prognosis in ZAN patients receiving interventional therapy. NLR, neutrophil-to-lymphocyte ratio; st-NES, short-term nerve electrical stimulation; PRF, pulsed radiofrequency; RF-TC, radiofrequency thermocoagulation. aPatients aged <65 years were used as the reference. bDisease duration <3 months was used as the reference. cPatients without head or face pain were used as the reference. dPatients without diabetes were used as the reference. ePatients who did not receive immunosuppressive therapy were used as the reference.
The constructed multifactorial logistic regression model was subjected to multicollinearity analysis, which indicated that the tolerance values were all greater than 0.1 and that the variance inflation factors (VIFs) were all less than 10, indicating no significant collinearity among the independent variables (Table 3). The C-index was 0.812 with a 95% confidence interval of 0.759–0.866, indicating good discrimination of the model. The Hosmer-Lemeshow test yielded a chi-square value of 8.799 with a p-value of 0.360, suggesting a satisfactory calibration of the model.
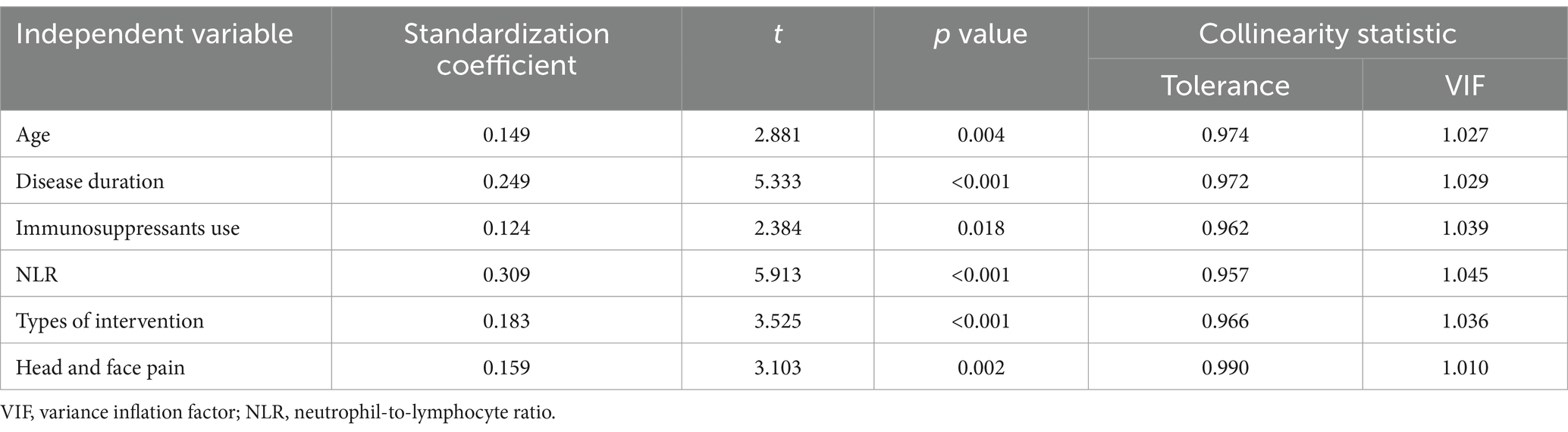
Table 3. Multiple collinearity analysis of independent variables in the multivariate logistic regression model.
4 Discussion
In this research, we conducted a multivariate logistic regression analysis of clinical data from 282 patients. These findings indicate that several factors, including age ≥ 65 years, longer disease duration, head and face pain, use of immunosuppressants, higher NLR, PRF (st-NES as reference), and RF-TC (st-NES as reference) are associated with a greater risk of poor prognosis in patients with ZAN who are receiving interventional therapy. This study evaluated the constructed multivariable logistic regression model using the C-index and Hosmer-Lemeshow test, demonstrating that both the discriminative ability and calibration of the predictive model are at a high level. Notably, the NLR is an inflammatory marker and was highlighted for the first time in ZAN treatment. This finding is in accordance with the findings of Schutzer-Weissmann and Farquhar-Smith (2017), who underscore the vital role of inflammatory markers in the management of pain.
The risk factors identified in this study can be categorized into modifiable and non-modifiable factors. Non-modifiable factors, such as age ≥ 65 and head and facial pain, cannot be altered. However, early identification of these high-risk patients is crucial for developing personalized intervention strategies. For such patients, in addition to conventional neuro-modulation therapies, adjunct treatments such as physical therapy can be employed to improve outcomes. Studies suggest that phototherapy may promote scab formation and healing of facial herpes zoster lesions, potentially aiding in facial esthetics recovery (Teitelbaum et al., 2020). However, further large-scale randomized controlled trials are needed to verify the exact efficacy and long-term effect of this neuromodulation therapy combined with physical therapy. On the other hand, modifiable factors, including elevated NLR, the use of immunosuppressants, and early healthcare engagement, offer clear targets for clinical intervention. Optimizing immunosuppressant use and controlling inflammation, along with patient education to encourage early intervention, can significantly reduce the risk of chronic pain.
In our study, 32.6% of patients with ZAN experienced poor outcomes at 3 months post-discharge. Previous research has shown that the rate of poor outcomes following interventional therapy for ZAN varies between 17.2 and 50% (Aggarwal et al., 2020; Isagulyan et al., 2023). The frequency of poor prognosis observed in this study was moderate. In this study, poor prognosis was assessed through a multidimensional approach, adhering to the definition of poor prognosis for ZAN as outlined by Watson et al. (1991). The incorporation of evaluation criteria such as pain score, sleep condition, and emotional state has bolstered the scientific rigor of the outcome endpoint.
This study demonstrated that a higher NLR indicates an increased risk of poor prognosis. The NLR is the ratio of the number of neutrophils to the number of lymphocytes in peripheral blood and is related to the inflammatory state and immune status of the human body (Grivennikov et al., 2010). The NLR has been used to evaluate the outcome of nervous system diseases, and studies have shown that a higher NLR is associated with a poor prognosis in patients with Ramsay Hunt syndrome (Soh et al., 2019). The basic lesions of ZAN are secondary to the inflammatory response of the sensory nervous system and are characterized by neuroinflammatory lesions such as congestion, hemorrhage, inflammatory cell infiltration and demyelination in the ganglion and nerve fibers according to histopathological examination (Gershon et al., 2015). An increase in neutrophils indicates aggravation of the systemic inflammatory response and predicts more severe nerve damage (Bowers et al., 2014). Pain, poor sleep and negative emotions put the body in a high stress state, which causes lymphopenia (Bombeiro et al., 2020; Zhu et al., 2020). An imbalance caused by a strong inflammatory response and immunosuppression is not conducive to viral clearance or neural repair (Amulic et al., 2012). A higher NLR indicates more significant nerve damage and diminished nerve repair capabilities, potentially resulting in heightened pain severity.
This study revealed that pain involving the head and face is a risk factor for poor prognosis, possibly because of the following reasons. First, research indicates that viral invasion of cranial nerves is more likely to lead to persistent pain sequelae (Tsau et al., 2020). Second, HZ infection affecting cranial nerves often co-occurs with complications such as facial paralysis, vestibular dysfunction, eye pain, visual impairment, and vertigo, which can amplify the patient’s feelings of tension, anxiety, and other negative emotions (Liesegang, 2008). Third, facial herpes and hyperpigmentation can further exacerbate anxiety and depression (Tontodonati et al., 2012). Given that the involvement of cranial nerves often leads to significant functional imbalances and facial esthetic concerns, which are difficult to fully recover from even after intervention, patients experiencing these conditions are more susceptible to a poor prognosis.
This study identified age ≥ 65 years, disease duration >3 months, immunosuppressant use, PRF (st-NES as reference) and RF-TC (st-NES as reference) as risk factors for poor prognosis in ZAN patients after interventional therapy. As people age, their immune system gradually weakens, increasing the vulnerability of elderly patients to attacks by the HZ virus, leading to more severe neurological damage (Arvin, 2005). A prolonged disease course can result in sensitization of both the peripheral and central nervous systems, with 3 months considered a critical threshold for sensitization (Fields et al., 1998; Block, 2016). This study revealed that the use of immunosuppressants significantly increases the risk of a poor prognosis. Immunosuppressants diminish the body’s immune response to viruses by inhibiting functional lymphocytes, which also reduces the efficiency of nerve repair (Ehrenstein, 2020). Differences in treatment approaches have led to variations in prognosis. Previous research has demonstrated that short-term nerve electrical stimulation (st-NES) significantly outperforms pulsed radiofrequency (PRF) in treating ZAN (Moriyama, 2009; Liu et al., 2022). RF-TC alleviates pain by destroying nerve fibers, but this can result in numbness and impaired motor function (Mehta et al., 2015). Additionally, a study has indicated that RF-TC might exacerbate pain in nerve fibers that already have pathological neuropathic pain (Chen et al., 2022), suggesting that this destructive treatment should be used with caution.
Both the NLR and the use of immunosuppressants are related to immune status. Our study conducted a collinearity analysis and found no evidence of multicollinearity. This may be due to the wide variety of immunosuppressants used, which can affect neutrophils and lymphocytes differently depending on the stage of the disease, either individually or in combination. The dose–response relationships between different types and doses of immunosuppressants and their effects on neutrophils and lymphocytes also vary.
In this study, the majority of patients were hospitalized for further interventional pain management treatment due to failure of conventional medical treatment. Although our progressive collinearity analysis of all independent variables showed that: There was no significant linear relationship between acute pain and intervention mode, but it is still possible that some patients who experience severe pain in the early stage, without conventional drug treatment, receive interventional treatment. For such patients, early pain and drug treatment may affect the final decision of interventional treatment. PRF and RF-TC may not be considered as independent risk factors for poor prognosis.
The findings of this study offer valuable insights for the personalized management of ZAN. High-risk groups, such as those experiencing head and facial pain or elderly patients, can be identified early, enabling the timely initiation of comprehensive treatment plans, including the use of Chinese medicine treatment or physical therapy (Teitelbaum et al., 2020; Pan et al., 2022). For patients with elevated NLR, early immunomodulation and inflammation control are crucial, while regular follow-up and timely interventions can help reduce the risk of chronic pain. Patients using immunosuppressive agents should have their immune function closely monitored, with medication adjustments made to minimize complications. For those with a prolonged disease course, emphasis should be placed on prevention through increased public awareness and education, helping patients understand the importance of early treatment and timely intervention.
5 Limitations
This study has several limitations. Firstly, the retrospective design may introduce information and selection biases. Secondly, being a single-center study, the sample size was relatively small, which limits the generalizability of the findings. Larger, multicenter, prospective studies are needed to validate our results. Thirdly, while research suggests that herpes zoster (HZ) involving the ocular nerves can increase the risk of adverse outcomes (Liesegang, 2008), our limited sample size precluded the inclusion of ocular nerve involvement as a variable. Fourthly, to enhance the study’s rigor, we employed strict screening criteria, which, however, might constrain the external validity of our results. We focused on patients undergoing their first interventional pain treatment to minimize overlap and collinearity among independent variables. Fifth, neural blockade treatment was excluded from our study due to its primary use in outpatient settings and limited effectiveness in treating ZAN (Niv and Maltsman-Tseikhin, 2005).
6 Conclusion
Our findings demonstrated that age ≥ 65 years, disease duration >3 months, head and face pain, use of immunosuppressants, higher NLR, PRF (st-NES as reference), and RF-TC (st-NES as reference) were identified as independent risk factors for poor prognosis in patients with ZAN who were receiving interventional pain management.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the First Affiliated Hospital of Soochow University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
JY: Writing – original draft, Data curation, Formal analysis. YY: Writing – original draft, Data curation, Formal analysis, Funding acquisition. HL: Writing – original draft, Data curation, Formal analysis, Funding acquisition. HX: Writing – original draft, Data curation, Formal analysis, Investigation, Software. YL: Writing – review & editing, Funding acquisition, Supervision. XJ: Writing – review & editing, Funding acquisition, Resources, Supervision.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The construction of clinical key specialties was funded by the “science and education to strengthen health” project in Suzhou.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aggarwal, A., Suresh, V., Gupta, B., and Sonthalia, S. (2020). Post-herpetic neuralgia: a systematic review of current interventional pain management strategies. J Cutan Aesthet Surg 13, 265–274. doi: 10.4103/jcas.Jcas_45_20
Amulic, B., Cazalet, C., Hayes, G. L., Metzler, K. D., and Zychlinsky, A. (2012). Neutrophil function: from mechanisms to disease. Annu. Rev. Immunol. 30, 459–489. doi: 10.1146/annurev-immunol-020711-074942
Arvin, A. (2005). Aging, immunity, and the varicella-zoster virus. N. Engl. J. Med. 352, 2266–2267. doi: 10.1056/NEJMp058091
Baron, R., and Wasner, G. (2006). Prevention and treatment of postherpetic neuralgia. Lancet 367, 186–188. doi: 10.1016/S0140-6736(06)68010-0
Bijur, P. E., Latimer, C. T., and Gallagher, E. J. (2003). Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad. Emerg. Med. 10, 390–392. doi: 10.1197/aemj.10.4.390
Block, L. (2016). Glial dysfunction and persistent neuropathic postsurgical pain. Scand. J. Pain 10, 74–81. doi: 10.1016/j.sjpain.2015.10.002
Bombeiro, A. L., Pereira, B. T. N., Bonfanti, A. P., and Oliveira, A. L. R. (2020). Immunomodulation by dimethyl fumarate treatment improves mouse sciatic nerve regeneration. Brain Res. Bull. 160, 24–32. doi: 10.1016/j.brainresbull.2020.04.005
Bowers, N. L., Helton, E. S., Huijbregts, R. P., Goepfert, P. A., Heath, S. L., and Hel, Z. (2014). Immune suppression by neutrophils in HIV-1 infection: role of PD-L1/PD-1 pathway. PLoS Pathog. 10:e1003993. doi: 10.1371/journal.ppat.1003993
Cebrian-Cuenca, A. M., Diez-Domingo, J., San-Martin-Rodriguez, M., Puig-Barbera, J., and Navarro-Perez, J.Herpes zoster research Group of the Valencian C (2011). Epidemiology and cost of herpes zoster and postherpetic neuralgia among patients treated in primary care centres in the Valencian community of Spain. BMC Infect. Dis. 11:302. doi: 10.1186/1471-2334-11-302
Chen, L., Li, J., Liu, H., Yang, P., Zuo, Y., and Ye, L. (2022). Interventions for zoster-associated pain: a retrospective study based on the clinical database. Front. Neurol. 13:1056171. doi: 10.3389/fneur.2022.1056171
Drolet, M., Brisson, M., Schmader, K. E., Levin, M. J., Johnson, R., Oxman, M. N., et al. (2010). The impact of herpes zoster and postherpetic neuralgia on health-related quality of life: a prospective study. CMAJ 182, 1731–1736. doi: 10.1503/cmaj.091711
Ehrenstein, B. (2020). Diagnosis, treatment and prophylaxis of herpes zoster. Z. Rheumatol. 79, 1009–1017. doi: 10.1007/s00393-020-00915-y
Fields, H. L., Rowbotham, M., and Baron, R. (1998). Postherpetic neuralgia: irritable nociceptors and deafferentation. Neurobiol. Dis. 5, 209–227. doi: 10.1006/nbdi.1998.0204
Finnerup, N. B., Kuner, R., and Jensen, T. S. (2021). Neuropathic pain: from mechanisms to treatment. Physiol. Rev. 101, 259–301. doi: 10.1152/physrev.00045.2019
Gershon, A. A., Breuer, J., Cohen, J. I., Cohrs, R. J., Gershon, M. D., Gilden, D., et al. (2015). Varicella zoster virus infection. Nat. Rev. Dis. Primers 1:15016. doi: 10.1038/nrdp.2015.16
Grivennikov, S. I., Greten, F. R., and Karin, M. (2010). Immunity, inflammation, and cancer. Cell 140, 883–899. doi: 10.1016/j.cell.2010.01.025
Harvey, M., Prosser, L. A., Rose, A. M., Ortega-Sanchez, I. R., and Harpaz, R. (2020). Aggregate health and economic burden of herpes zoster in the United States: illustrative example of a pain condition. Pain 161, 361–368. doi: 10.1097/j.pain.0000000000001718
Isagulyan, E., Tkachenko, V., Semenov, D., Asriyants, S., Dorokhov, E., Makashova, E., et al. (2023). The effectiveness of various types of electrical stimulation of the spinal cord for chronic pain in patients with Postherpetic neuralgia: a literature review. Pain Res. Manag. 2023, 6015680–6015688. doi: 10.1155/2023/6015680
Jang, Y. H., Lee, J. S., Kim, S. L., Chi, S. G., Lee, W. J., Lee, S. J., et al. (2015). Do interventional pain management procedures during the acute phase of herpes zoster prevent Postherpetic neuralgia in the elderly?: a Meta-analysis of randomized controlled trials. Ann. Dermatol. 27, 771–774. doi: 10.5021/ad.2015.27.6.771
Johnson, R. W., Alvarez-Pasquin, M. J., Bijl, M., Franco, E., Gaillat, J., Clara, J. G., et al. (2015). Herpes zoster epidemiology, management, and disease and economic burden in Europe: a multidisciplinary perspective. Ther. Adv. Vac. 3, 109–120. doi: 10.1177/2051013615599151
Kawai, K., Gebremeskel, B. G., and Acosta, C. J. (2014). Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open 4:e004833. doi: 10.1136/bmjopen-2014-004833
Liesegang, T. J. (2008). Herpes zoster ophthalmicus natural history, risk factors, clinical presentation, and morbidity. Ophthalmology 115, S3–S12. doi: 10.1016/j.ophtha.2007.10.009
Liu, L., Zhang, W. J., Xu, S. X., Guo, W. S., Yan, R. R., Jiang, X. H., et al. (2022). Propensity score matching comparing short-term nerve electrical stimulation to pulsed radiofrequency for herpes zoster-associated pain: a retrospective study. Front. Mol. Neurosci. 15:1069058. doi: 10.3389/fnmol.2022.1069058
Mehta, P., Maher, P., and Singh, J. R. (2015). Treatment of postherpetic neuralgia using a thoracic transforaminal epidural steroid injection. PM R 7, 443–446. doi: 10.1016/j.pmrj.2014.11.009
Moriyama, K. (2009). Effect of temporary spinal cord stimulation on postherpetic neuralgia in the thoracic nerve area. Neuromodulation 12, 39–43. doi: 10.1111/j.1525-1403.2009.00186.x
Niv, D., and Maltsman-Tseikhin, A. (2005). Postherpetic neuralgia: the never-ending challenge. Pain Pract. 5, 327–340. doi: 10.1111/j.1533-2500.2005.00035.x
Pan, Z., Huang, S., Ma, T., Yuan, R., Wang, M., Luo, R., et al. (2022). Comparative effectiveness of external therapies of traditional Chinese medicine and surgical treatments in pain management of postherpetic neuralgia: a protocol for a systematic review and network meta-analysis. Medicine 101:e31517. doi: 10.1097/MD.0000000000031517
Schutzer-Weissmann, J., and Farquhar-Smith, P. (2017). Post-herpetic neuralgia - a review of current management and future directions. Expert. Opin. Pharmacother. 18, 1739–1750. doi: 10.1080/14656566.2017.1392508
Soh, Y., Lee, J. H., Jung, J., Dong, S. H., Byun, J. Y., Park, M. S., et al. (2019). A high neutrophil-to-lymphocyte ratio is associated with recovery from Ramsay hunt syndrome. ORL J. Otorhinolaryngol. Relat. Spec. 81, 130–137. doi: 10.1159/000499471
Teitelbaum, S., Azevedo, L. H., and Bernaola-Paredes, W. E. (2020). Antimicrobial photodynamic therapy used as first choice to treat herpes zoster virus infection in younger patient: a case report. Photobiomodul. Photomed. Laser Surg. 38, 232–236. doi: 10.1089/photob.2019.4725
Tontodonati, M., Ursini, T., Polilli, E., Vadini, F., Di Masi, F., Volpone, D., et al. (2012). Post-herpetic neuralgia. Int. J. Gen. Med. 5, 861–871. doi: 10.2147/ijgm.S10371
Tsau, P. W., Liao, M. F., Hsu, J. L., Hsu, H. C., Peng, C. H., Lin, Y. C., et al. (2020). Clinical presentations and outcome studies of cranial nerve involvement in herpes zoster infection: a retrospective single-center analysis. J. Clin. Med. 9:946. doi: 10.3390/jcm9040946
van Wijck, A. J., Wallace, M., Mekhail, N., and van Kleef, M. (2011). Evidence-based interventional pain medicine according to clinical diagnoses. 17. Herpes zoster and post-herpetic neuralgia. Pain Pract. 11, 88–97. doi: 10.1111/j.1533-2500.2010.00428.x
Watson, P. N. C., Watt, V. R., Chipman, M., Birkett, N., and Evans, R. J. (1991). The prognosis with postherpetic neuralgia. Pain 46, 195–199. doi: 10.1016/0304-3959(91)90076-A
Whitley, R. J., Volpi, A., McKendrick, M., Wijck, A., and Oaklander, A. L. (2010). Management of herpes zoster and post-herpetic neuralgia now and in the future. J. Clin. Virol. 48, S20–S28. doi: 10.1016/s1386-6532(10)70005-6
Wood, M. (2002). Understanding pain in herpes zoster: an essential for optimizing treatment. J. Infect. Dis. 186 Suppl 1, S78–S82. doi: 10.1086/342958
Keywords: risk factors, zoster-associated neuralgia, interventional pain management, poor prognosis, herpes zoster
Citation: Yuan J, Yu Y, Liu H, Xu H, Li Y and Jin X (2024) Risk factors for poor prognosis in patients with zoster-associated neuralgia who underwent interventional pain management. Front. Mol. Neurosci. 17:1393219. doi: 10.3389/fnmol.2024.1393219
Edited by:
Wolfgang B. Liedtke, Regeneron Pharmaceuticals, Inc., United StatesCopyright © 2024 Yuan, Yu, Liu, Xu, Li and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohong Jin, amlueGlhb2hvbmdAc3VkYS5lZHUuY24=; Yan Li, c3p4Y2xpeWFuQHNpbmEuY29t
†These authors have contributed equally to this work
 Junpeng Yuan1†
Junpeng Yuan1† Xiaohong Jin
Xiaohong Jin