- 1Department of Anesthesiology, The Second Hospital of Hebei Medical University, Shijiazhuang, Hebei, China
- 2The Key Laboratory of Neurology, Ministry of Education, Shijiazhuang, Hebei, China
Clemastine, a Food and Drug Administration (FDA)-approved compound, is recognized as a first-generation, widely available antihistamine that reduces histamine-induced symptoms. Evidence has confirmed that clemastine can transport across the blood–brain barrier and act on specific neurons and neuroglia to exert its protective effect. In this review, we summarize the beneficial effects of clemastine in various central nervous system (CNS) disorders, including neurodegenerative disease, neurodevelopmental deficits, brain injury, and psychiatric disorders. Additionally, we highlight key cellular links between clemastine and different CNS cells, in particular in oligodendrocyte progenitor cells (OPCs), oligodendrocytes (OLs), microglia, and neurons.
1. Introduction
Clemastine, known as 2-[2-[1-(4-chlorophenyl)-1-phenylethoxy]ethyl]-1 -methylpyrrolidine, is a Food and Drug Administration (FDA)-approved compound. It is a first-generation, widely available histamine H1 receptor antagonist that prevents the symptoms caused by high histamine levels (Simons, 2004). Clemastine is often produced as clemastine fumarate to improve its solubility and bioavailability. The molecular structure of clemastine and clemastine fumarate are shown in Figure 1. Although the initial research on clemastine was primary mainly focused on its antihistamine properties and side effects, following a high-throughput screen it has been identified, as a potential treatment for multiple sclerosis (MS) or more specifically as a drug promoting remyelination (Mei et al., 2014), in an attempt aimed at repurposing known molecules. Then various preclinical and clinical researches have investigated the therapeutic potential of clemastine in neurological disorders, including neurodegenerative disease, neurodevelopmental deficits, brain injury, and psychiatric disorders (Mei et al., 2016; Cree et al., 2018; Pan et al., 2020; Shimizu et al., 2020; Chen J. F. et al., 2021; Chen L. et al., 2021; Leng and Edison, 2021; Wu et al., 2021b; Hong et al., 2022; Bohlen et al., 2023). However, a full appreciation of therapeutic potential of clemastine in neurological disorders needs to reveal the underlying mechanisms.
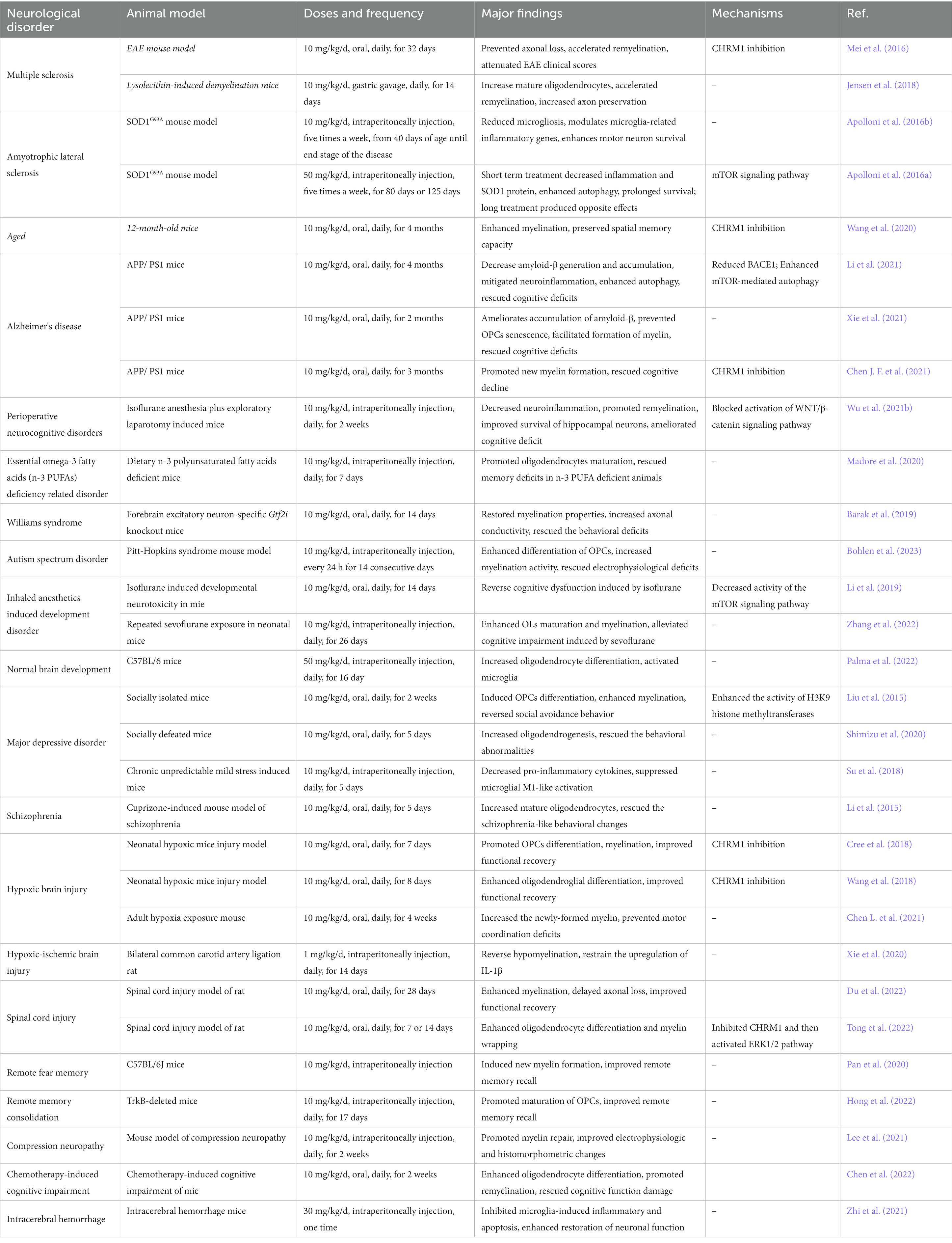
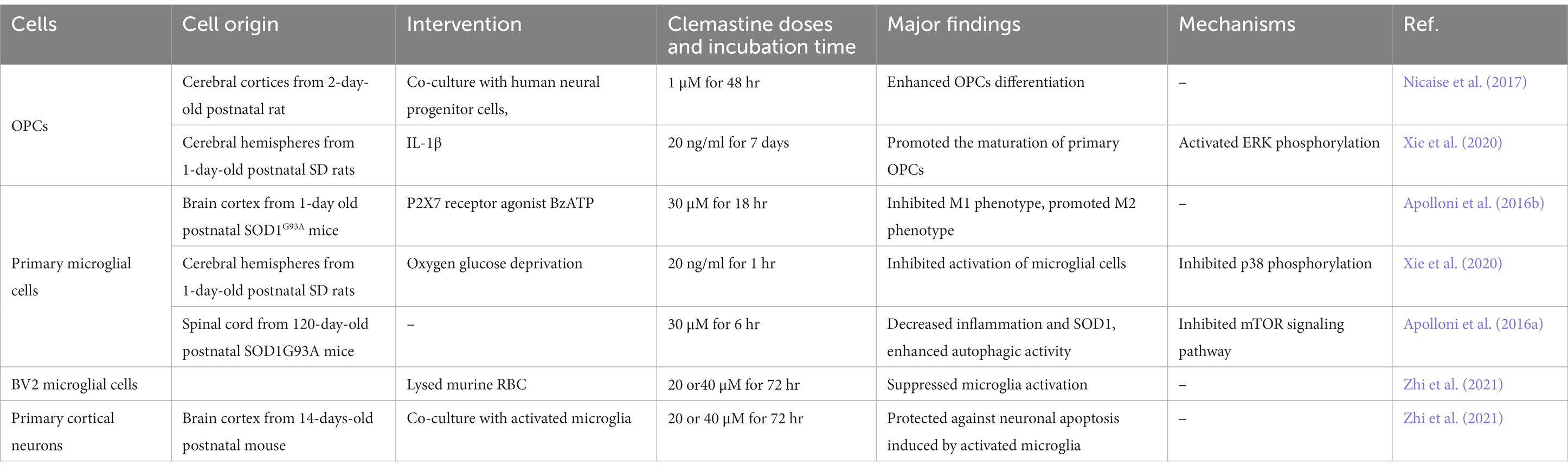
Oligodendrocytes (OLs) are the myelin-forming glial cells of the central nervous system (CNS), which derive from the differentiation of oligodendrocyte progenitor cells (OPCs) throughout the lifespan (Young et al., 2013; Cristobal and Lee, 2022). The balance of myelination, demyelination, and remyelination is often regulated by cell–cell interactions with other glia (Molina-Gonzalez et al., 2022). Microglia are known to undergo significant transcriptional changes during demyelination and remyelination. On one hand microglia facilitate developmental myelination and remyelination through engulfing excessive unneeded myelin and myelin debris (Xu et al., 2022). However, in pathological conditions of neurological disorders, microglia can be activated and produce pro-inflammatory cytokines, which impair myelination and result in progressive demyelination (Kent and Miron, 2023). In this review, we present preclinical and clinical evidence to illustrate the beneficial effects of clemastine in neurological disorders. We highlight that clemastine’s actions are mainly associated with promoting oligodendrocyte differentiation and maturation, inhibiting microglia induced neuroinflammation, and decreasing the apoptosis of neurons. Additionally, we summarize related molecular mechanisms underlying therapeutic potential of clemastine in animal models of neurological disorders (Table 1) and cell experiments (Table 2).
2. Effects of clemastine in experimental studies of neurodegenerative diseases
Ageing is the primary risk factor for a majority of neurodegenerative diseases. Unfortunately, effective strategies for ageing-related neurodegenerative diseases are limited. Recent evidence has indicated that clemastine showed beneficial effects in neurodegenerative diseases by promoting myelination, enhancing autophagy, and mitigating neuroinflammation.
2.1. Multiple sclerosis
Multiple sclerosis (MS) is a chronic autoimmune disease characterized by chronic demyelination. The disease pathogenesis involves in oligodendrocyte cell death, myelin sheath destruction, and axonal injury (Jäkel et al., 2019). Almost three million people are estimated to suffer from MS worldwide (Walton et al., 2020). A high-throughput in vitro screening system has identified clemastine as a promising candidate for MS therapy (Mei et al., 2014).
Anterior visual pathway is demyelinated in almost all MS patients (Toosy et al., 2014), and visual evoked potentials has been identified as a preclinical, quantitative biomarker for remyelination efficacy in MS patients (Cordano et al., 2022). A clinical phase II, 150 days, double-blind, randomized, placebo-controlled, crossover trial (ReBUILD) in patients with relapsing MS has been completed (NCT02040298). In this trail, patients received either clemastine (5.36 mg orally twice daily) for 90 days followed by a placebo for 60 days, or vice versa. Clemastine treatment reduced the visual-evoked potentials latency delay by 1.7 ms/eye when analyzing the trial as a crossover (Green et al., 2017). Recently the group of the ReBUILD trial (NCT02040298) further verified the therapeutic effects of clemastine in MS patients. Neurofilament light chain (NfL), a marker of neuroaxonal injury, is elevated in patients with MS (Cantó et al., 2019; Kuhle et al., 2019, 2020). Clemastine was able to reduce blood NfL in MS patients, suggesting that therapeutic remyelination acted on neuroprotection (Abdelhak et al., 2022). To test whether clemastine treatment is effective in patients with acute MS, another phase II double-blind placebo-controlled clinical trial (ReCOVER study; NCT02521311) is in the recruiting stage. Meanwhile, a phase II, randomized, double-blind, placebo-controlled trial of the ability of the combination of metformin and clemastine to promote remyelination in people with relapsing–remitting multiple sclerosis already on disease-modifying therapy (NCT05131828) is also at the recruiting stage. In addition, there is another clinical trial intended to assess clinical evidence of clemastine fumarate as a myelin repair therapy in patients with chronic inflammatory injury-causing demyelination as measured by multi-parametric MRI assessments (NCT05359653).
In mouse model studies, experimental autoimmune encephalomyelitis (EAE) and toxin-induced demyelination are the two major animal models used in MS research (Sutiwisesak et al., 2021). Expression of acetylcholine receptors has been reported in oligodendrocytes. M1 muscarinic acetylcholine receptor (CHRM1) is one of the main subtypes expressed in OPCs that negatively regulates cell differentiation and myelination (De Angelis et al., 2012). In the EAE mouse model, CHRM1 on OPCs was identified as the target of anti-muscarinic treatment for remyelination. Clemastine could accelerate remyelination, prevent axonal loss, and improve functional recovery specifically by targeting CHRM1 (Mei et al., 2016). In EAE rat model, clemastine showed protective role against neuroinflammation, oxidation, and demyelination by hindering p38 MAPK/NLRP3 signaling and Nrf2/HO-1/NLRP3/caspase-1/IL-1β pathway (Motawi et al., 2023). Glutathione S-transferase 4α (Gsta4) is highly expressed during adult oligodendrocytes differentiation. Clemastine activated Gsta4, and then restricted oligodendrocytes apoptosis and enhanced myelination, suggesting Gsta4 as a potential target of clemastine for MS therapies (Carlström et al., 2020). In lysolecithin-induced demyelination mice, exercise enhances oligodendrogenesis, remyelination. Moreover, exercise combined with clemastine additively enhance remyelination (Jensen et al., 2018), which provide evidence that combinations of clemastine with other different approaches may achieve a better therapeutic effect in MS.
In vitro, the neural progenitor cells from blood samples of MS patients were treated with or without clemastine for two consecutive days, and then the conditioned media was collected to culture primary rat OPCs. Clemastine in conditioned media enhanced OPCs differentiation and improved OPC maturation, further indicating the therapeutic potential of clemastine for patients with progressive MS (Nicaise et al., 2017).
2.2. Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is a progressive, paralytic neurodegenerative disease that characterized by degeneration of motor neurons and axonal degeneration in the brain and spinal cord (Feldman et al., 2022). The standardized global incidence of ALS is 1.68 per 100,000 persons each year (Marin et al., 2017). Cu/Zn SOD1 mutations are the most frequent types in ALS. Transgenic mouse model SOD1G93A overexpresses the human protein with glycine for alanine substitution in position 93 and has been widely used for preclinical studies (Bonifacino et al., 2021). In SOD1G93A mice, chronic clemastine administration reduced microgliosis, modulated inflammatory genes, and enhanced motor neuron survival. Moreover, in vitro, clemastine reduced activation of CD68-positive macrophages/microglia and inhibited pro-inflammatory reactions (Apolloni et al., 2016b). Interestingly, short treatment (from 40 days to 120 days, last for 80 days) and long treatment (from 40 days to the end of life, last for about 125 days) by clemastine in SOD1G93A mouse showed opposite effects. Short treatment with clemastine could decrease inflammatory parameters and stimulate autophagic flux via the mTOR signaling pathway, thereby prolonging survival in SOD1G93A mice. However, long treatment with clemastine failed to ameliorate ALS disease progression. Instead, long treatment with clemastine increased microgliosis and SOD1 protein levels in the late phases of the diseases. These differences in phenotypic outcomes may due to clemastine possesses complex and dual functions in the pathogenesis of ALS (Apolloni et al., 2016a). As the SOD1G93A mouse model is just one of the genetic mouse models of ALS, therefore, further investigation is necessary to test the effects of clemastine on other ALS rodent models, such as TDP-43 mouse models, FUS mice, and C9orf72 knock-out mice (Bonifacino et al., 2021), and to identify suitable dose that can protect against ALS damage.
2.3. Alzheimer’s disease
Alzheimer’s disease (AD) is the most common cause of dementia in the old. By 2050, the prevalence of dementia will triple worldwide based on a biological definition of AD (Scheltens et al., 2021). The memory impairments of AD correlate with the accumulation of amyloid-β plaques and Tau protein (Bloom, 2014; Puzzo et al., 2020). Myelin injury, neuroinflammation, synaptic and neuronal loss, and other neuropathological changes (Yang et al., 2017; Mathys et al., 2019; Leng and Edison, 2021; Pereira et al., 2021; Ashleigh et al., 2022; Blanchard et al., 2022) have also present in patients and animal models of AD. Animal studies suggest that clemastine has neuroprotective effects on AD-related memory deficits. In APP/PS1 transgenic mice, clemastine could decrease amyloid-β generation and accumulation by reducing beta-site amyloid precursor protein cleaving enzyme 1 (BACE1), which requires for the monomeric forms of amyloid-β. In addition, clemastine mitigated neuroinflammation and enhanced autophagy by suppressing mTOR signaling, ultimately attenuating AD-like pathology (Li et al., 2021). Clemastine also prevented OPCs from entering the state of cellular senescence, which facilitating myelin formation in AD mice (Xie et al., 2021). It is worthy to mention that another research showed that clemastine did not change the number of amyloid-β plaques in different brain regions of APP/PS1 brains, implying that clemastine rescued cognitive decline in AD mice mainly through enhancing myelin renewal instead of altering amyloid-β deposition or clearance (Chen J. F. et al., 2021). Currently, the pharmacological mechanisms of clemastine in AD are limited, further researches are required to fully understand the potential therapeutic targets of clemastine in this disease.
2.4. Perioperative neurocognitive disorders
With the worldwide increase in lifespan, surgical patients are becoming older. Perioperative neurocognitive disorder (PND) is the most common complication experienced by older individuals undergoing anesthesia and surgery. PND is an umbrella term including preexisting cognitive impairment, preoperative delirium, delirium occurring up to 7 days after surgery, delayed neurocognitive recovery, and postoperative neurocognitive disorders that last for 30 days and 12 months after surgical procedures (Evered et al., 2018). Next-generation sequencing and bioinformatics predicted clemastine had therapeutic potential on PND (Wu et al., 2021a). Clemastine was given at 10 mg/kg per day for 2 weeks to evaluate its effects on PND in aged mice. As expected, clemastine blocked the overactivation of the Wnt/β-catenin signaling pathway, further enhancing OLs differentiation and remyelination. Additionally, clemastine also reduced neuroinflammation, improved synaptic plasticity, and prevented the loss of hippocampal mature neurons (Wu et al., 2021b). The forementioned findings provide some insight into the positive effects of clemastine in PND.
3. Experimental studies of clemastine in environmental and genetic induced brain development disorders
Environment and genetics are major contributors to affect neurodevelopment in the early life (Champagne, 2010). Throughout early neurodevelopment, myelination helps provide the foundation for brain connectivity and supports the maturation of cognitive functioning. If myelination progress is affected by nutritional deficiencies, environmental neurotoxins, or genetic abnormality, this may lead to permanent alterations in brain function, and have detrimental consequences for long-term behavior (Li et al., 2019; McCann and Soriano, 2019; Raper et al., 2021). Clemastine can alleviate environmental and genetic induced brain development disorders by promoting myelination.
3.1. Omega-3 fatty acids (n-3 PUFAs) deficiency related disorder
Lipids are one of the main constituents of the CNS. Docosahexaenoic acid (DHA, omega-3) is one of the principal form of the long chain polyunsaturated fatty acid of the white matter (Martinez, 1992). Low maternal intake of n-3 PUFAs leads to neurodevelopmental disorders and defects in brain functional connectivity (Madore et al., 2020). Recent data suggested that decreasing n-3 PUFA dietary intake led to deficits in postnatal oligodendrocytes maturation and myelination processes in mice. N-3 PUFA defiieny then resulted in cognitive and emotional disorders in adult life. Clemastine could enhanceoligodendrocyte precursor cell differentiation, promote myelination, and rescue memory function in n-3 PUFA deficiency mice (Leyrolle et al., 2022). Since n-3 PUFAs are lipids possess anti-inflammatory function, n-3 PUFAs deficiency may lead to pro-inflammatory condition in CNS, Madore et al. (2020). It is plausible that clemastine promoted OLs maturation by modulating brain inflammation.
3.2. Anesthetics induced development disorder
Isoflurane and sevoflurane are commonly used anesthetics. Studies showed that isoflurane induced apoptosis of OLs and impaired neural function in neonatal primate and rodent brains (Brambrink et al., 2012; Creeley et al., 2014; Li et al., 2019). After exposure to isoflurane, mice were fed with clemastine (10 mg/kg/day) from postnatal days 21 to 35, the critical period of myelin development. Data revealed that clemastine enhanced myelination and could reverse cognitive dysfunction induced by isoflurane (Li et al., 2019). Similarly, clemastine injected intraperitoneally (10 mg/kg/day) in mice on postnatal days 6 to 8 enhanced OLs maturation and myelination, and thus alleviated sevoflurane-induced cognitive impairment (Zhang et al., 2022), indicating that although doses differed, long-term and short-term treatments with clemastine could rescue developmental cognitive impairment induced by anesthetics.
3.3. Williams syndrome
Williams syndrome, caused by a heterozygous microdeletion on chromosome 7q11.23, is a multisystemic neurodevelopmental disorder characterized by hypersociability with unique cognitive and personality profiles (Kozel et al., 2021). General transcription factor IIi (Gtf2i) is located on adjacent loci at the telomeric end of the Williams syndrome critical region, which linked to typical WS behavior and development. In Gtf2i cKO mice, clemastine treatment normalized the number of oligodendrocytes in the cortex and the corpus callosum, and increased myelin thickness. These results in rescuing the myelination deficit further normalize the behavioral deficits (Barak et al., 2019). These data imply that targeting myelination deficits by clemastine might be a beneficial therapeutic strategy in Williams syndrome.
3.4. Autism spectrum disorder
Pitt-Hopkins syndrome, a syndromic form of autism spectrum disorder (ASD), is a neurodevelopmental disorder characterized by intellectual disability, specific facial features, deficits in motor learning, and marked autonomic nervous system dysfunction (Phan et al., 2020). Pitt-Hopkins syndrome is caused by haploinsufficiency of the transcription factor 4 gene (TCF4) on chromosome 18q21. Recent study found that clemastine is benificial at restoring myelination in a Pitt-Hopkins syndrome mouse model. Clemastine treatment normalized OPCs and oligodendrocyte density in vivo and in vitro. Importantly, clemastine helped functional recovery by improving electrophysiology and behavior. The preclinical evidence indicated that clemastine may be beneficial in Pitt-Hopkins syndrome (Bohlen et al., 2023).
However, a recent study found clemastine-induced impairment in developmental myelination in healthy mice (Palma et al., 2022). C57BL/6 wild-type mice were treated with clemastine (50 mg/kg/day) by intraperitoneal injections from postnatal day 5 to postnatal day 21. This literature found that clemastine resulted in the decreasement of conduction velocity of myelinated fibers in mice, despite the increase of oligodendrocyte differentiation. These data implied that clemastine’s impact on neurodevelopment brain is complex. It seems like that clemastine protects the developing brain from damage, but has little effect or is even detrimental to normal neural development.
4. Effects of clemastine in experimental studies of brain injury
Preclinical and clinical evidence illustrates the beneficial effects of clemastine in brain injury, especially in hypoxic brain injury, hypoxic–ischemic brain injury, and spinal cord injury.
4.1. Hypoxic brain injury
Hypoxia, or insufficient supply of oxygen (O2) with respect to the demand, afflicts millions of people worldwide. Chronic hypoxia impairs myelination and synaptogenesis in developing brains, which leading to cognitive deficits in adolescents (Wang et al., 2018; Koehn et al., 2020).
Clinical treatments supported the therapeutical effect of clemastine on hypoxia-related cognitive dysfunction. White matter injury and demyelination following necrosis of vulnerable oligodendrocytes is one of the pathophysiology of delayed post-hypoxic leukoencephalopathy, a unique clinical condition that presents with cognitive impairment occurring following an episode of acute hypoxic brain injury (Hamlin et al., 2020). A 45-year-old male patient who developed delayed post hypoxic leukoencephalopathy was given clemastine, 5.36 mg, twice daily for 10 months. After the treatment, magnetic resonance imaging (MRI) showed an improvement and nearly complete resolution of the diffuse white matter hyperintensity, and the cognitive function of this patient had markedly improved as well (Cree et al., 2018).
In neonatal hypoxic injury mice model, clemastine promoted OPC differentiation, myelination, and improved cognitive functional recovery. The action of clemastine in hypoxia was oligodendroglial specific via the CHRM1 (Cree et al., 2018). Consistent with this study, another study also found that clemastine administration during or after hypoxia exposure could enhance oligodendroglial differentiation and facilitate functional recovery in neonatal hypoxic injury mice (Wang et al., 2018). Not only that, clemastine also protected adult mice brain functions from hypoxia. 4-month-old mice suffered from hypoxia were given clemastine for 4 weeks. As a result, clemastine increased the newly-formed myelin in the motor cortex and corpus callosum (Chen L. et al., 2021). As mentioned, glial cell interactions regulate myelination processes and affect myelin functions. In addition to improving myelin repairment directly, clemastine mediated its therapeutic effect by inhibiting the microglia-induced inflammatory response (Zhi et al., 2021). In hypoxic–ischemic brain injury, IL-1β was released by activated microglia, which resulting in hypomyelination. Further, co-culture of microglia and OPCs cell experiment illustrated that clemastine could inhibit the production of IL-1β by inhibition of the p38 MAPK/NLRP3 pathway in microglia cells, and then promote the maturation of OPCs. These data indicated that clemastine might be a viable strategy to promote myelination in hypoxic–ischemic brain injury models (Xie et al., 2020).
4.2. Spinal cord injury
Spinal cord injury (SCI) is a traumatic injury that affects the normal function of the spinal cord (Anderson et al., 2022). Loss of OLs and demyelination of spared axons lead to slower or blocked conduction through the lesion site caused by SCI. In SCI rat model, clemastine preserved myelin integrity, and thus improved functional recovery (Du et al., 2022). One mechanism was that clemastine enhanced OPCs differentiation and myelin wrapping (Tong et al., 2022). Clemastine could activate ERK1/2 via CHRM1 in OPCs, and promote cell differentiation (Tong et al., 2022). Clemastine also negated cell death and improved remyelination and recovery of cognitive function (Myatich et al., 2023). Thus clemastine potentially serves as an efficient drug treatment in the recovery from SCI.
5. Experimental studies of clemastine in psychiatric disorders
Mylin damage and neuroinflammation occurs in psychiatric disorders. Recently evidence has indicated that clemastine also presented therapeutic potential in animal models of major depressive disorder and schizophrenia.
5.1. Major depressive disorder
Depressive disorder is one of the most prevalent and debilitating psychiatric disorders. In 2019, depressive disorders were the second leading cause of disability worldwide (GBD 2019 Mental Disorders Collaborators, 2022). Characterized by impairments in cognition, emotional regulation, and neurovegetative symptoms, the major depressive disorder can cause severe disability (Park and Zarate, 2019). The pathophysiology of depressive disorders includes increased inflammation, decreased neurogenesis and neuroplasticity, and abnormal myelination (Fries et al., 2022). Several studies have shown that clemastine ameliorated depressive-like behaviors by targeting different pathological changes. On the one hand, clemastine enhanced the process of myelination. Epigenetic modification of repressive histone methylation is critical for gene repression during oligodendrocyte differentiation (Liu et al., 2015). Clemastine could induce OPCs differentiation by enhancing the activity of H3K9 histone methyltransferases and favoring chromatin compaction, thereby reversing depressive-like social behavior (Liu et al., 2016). It has also been observed that clemastine rescued the behavioral abnormalities accompanied by increasing oligodendrogenesis in stress model of social defeat (Shimizu et al., 2020). On the other hand, clemastine alleviated depressive-like behavior by inhibiting microglia-related pro-inflammatory response (Su et al., 2018). It is well-known that purinergic ligand-gated ion channel 7 receptor (P2X7R) is present on activated microglia (Bhattacharya and Biber, 2016). Evidence suggested that clemastine suppressed the expression of P2X7R and restrained the microglia activation (Su et al., 2018).
5.2. Schizophrenia
The systematic analysis for the Global Burden of Disease Study reported that the prevalence of schizophrenia was 23.6 per million worldwide in 2019 (GBD 2019 Mental Disorders Collaborators, 2022). Clinical observation found that schizophrenia patients exhibited white matter volume consistent with microstructural disorganization and axonal dysfunction (Park et al., 2014; Caprihan et al., 2015). To date, few or no effective drug is available for the myelination related deficits that may underly cognitive deficits in schizophrenia. Animal study revealed that targeting oligodendrocytes and myelin repair might be beneficial to cure schizophrenia like behaviors (Yu et al., 2022). Mice exposed to cuprizone displayed schizophrenia-like behavioral changes, clemastine enhanced myelin repairment in demyelinated regions of the brain and rescued memory and anxiety related behavioral deficits (Li et al., 2015). However, other cognitive domains need to be investigated in more schizophrenia-related animal models.
6. Conclusion and future perspectives
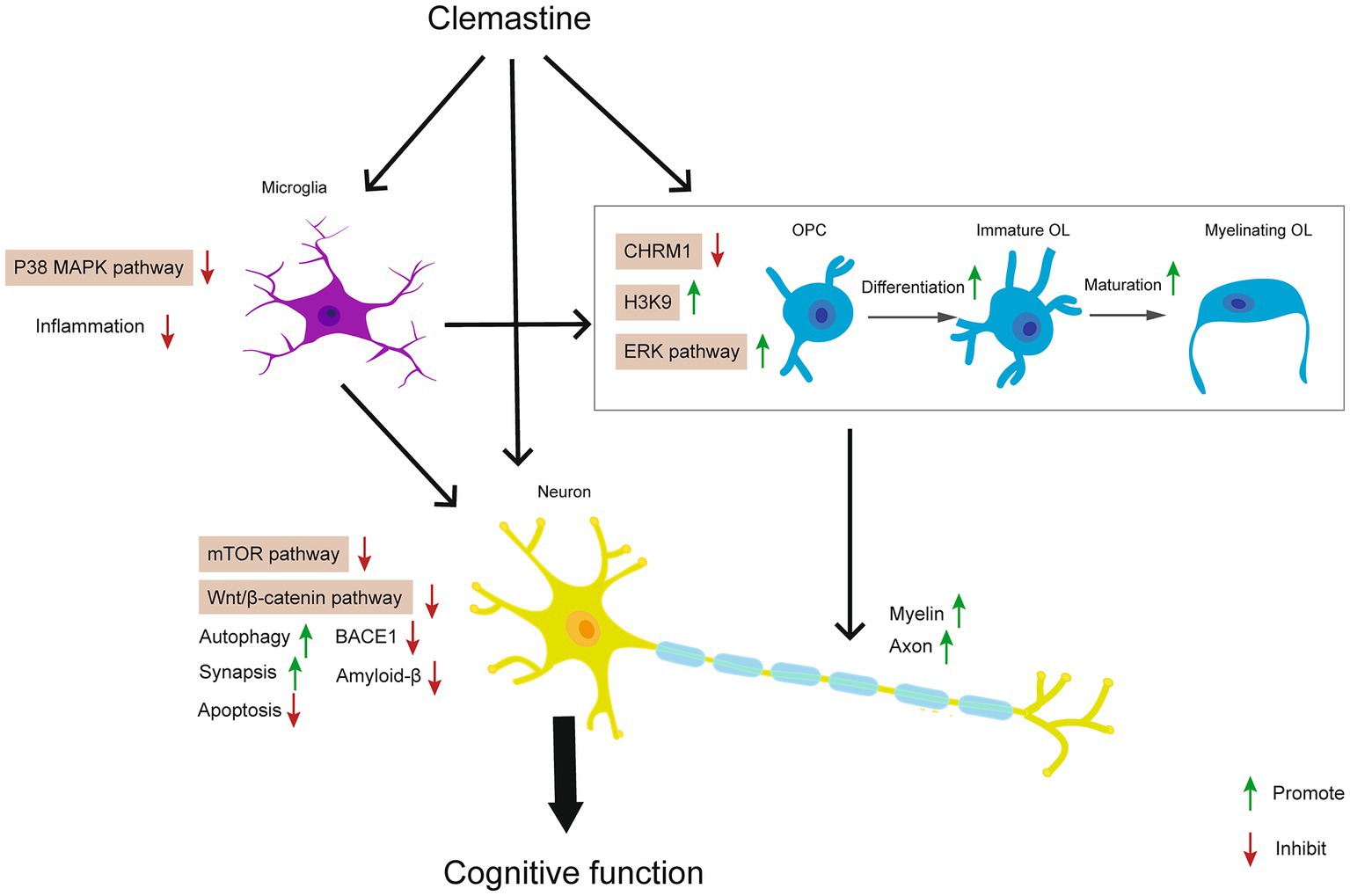
In conclusion, we summarized current evidence about the neuroprotective effects of clemastine in neurodegenerative diseases, neurodevelopmental deficits, brain injury, and psychiatric disorders. In these processes, clemastine regulates CHRM1, p38 MAPK, ERK, mTOR, and Wnt/β-catenin signaling pathways in OPCs, OLs, microglia, and neurons. The underlying mechanisms of action of clemastine are generally associated with promoting oligodendrocytes differentiation and maturation, inhibiting microglia induced neuroinflammation, and decreasing the apoptosis of neurons (Figure 2). Therefore clemastine might be a promising candidate compound and repurposing for various neurological disorders.
Nevertheless, only a few clinical trials assessed the efficacy and safety of clemastine as a therapy for patients with MS. Until now, there is still lacking confirmed clinical trials about the amelioration of clemastine in patients with other CNS disorders. Besides, clemastine shows remyelination and functional repair over short timescales in phase II clinical trials. It needs to explore whether clemastine still has beneficial effects beyond clinical study. Furthermore, the dose of clemastine selected in the clinical trials only achieves partial saturation of the target muscarinic receptor. Future investigations aims at the optimal dosage may hold promise as potential therapeutic strategies for clinical effect of treatment (Green et al., 2017). Experiments have implied that clemastine may cause immune suppression (Peternel et al., 2006; Johansen et al., 2011). C57BL/6 mice have a high resistance to listeria monocytogenes, but clemastine enhanced the susceptibility of mice to listeria monocytogenes infection, augmented listerial caused liver abscesses and disruption of splenic architecture, leading to death. In human monocytes, clemastine inhibited the potential of cells to produce antibacterial cytokines in response to listeria or lipopolysaccharide (Johansen et al., 2011). Besides clemastine has antihistamine property with anticholinergic and sedative side effects. Generally, neurological disorders require long-term therapy, thus whether clemastine long treatment causes immune suppression or other pathological change, like its effects in ALS, are not clear. More clinical trials with long-term follow-ups are needed to validate the efficacy of clemastine for CNS diseases. Additionally, the mechanisms underlying the positive impact of clemastine during neuropathological disorders remain unclear. However, preclinical studies mainly focused on one signaling pathway of clemastine in specific cell types. Therefore, further investigations that aimed at unraveling the detailed molecular mechanisms and identifying therapeutic targets for CNS damage are necessary.
Author contributions
SJ: Writing – review & editing, Writing – original draft. XW: Writing – original draft, Writing – review & editing. TC: Writing – review & editing. RK: Writing – review & editing, Supervision, Visualization. LH: Supervision, Visualization, Writing – review & editing, Funding acquisition, Project administration.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82071901).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelhak, A., Cordano, C., Boscardin, W. J., Caverzasi, E., Kuhle, J., Chan, B., et al. (2022). Plasma neurofilament light chain levels suggest neuroaxonal stability following therapeutic remyelination in people with multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 93, 972–977. doi: 10.1136/jnnp-2022-329221
Anderson, M. A., Squair, J. W., Gautier, M., Hutson, T. H., Kathe, C., Barraud, Q., et al. (2022). Natural and targeted circuit reorganization after spinal cord injury. Nat. Neurosci. 25, 1584–1596. doi: 10.1038/s41593-022-01196-1
Apolloni, S., Fabbrizio, P., Amadio, S., and Volonte, C. (2016a). Actions of the antihistaminergic clemastine on presymptomatic SOD1-G93A mice ameliorate ALS disease progression. J. Neuroinflammation 13:191. doi: 10.1186/s12974-016-0658-8
Apolloni, S., Fabbrizio, P., Parisi, C., Amadio, S., and Volonte, C. (2016b). Clemastine confers neuroprotection and induces an anti-inflammatory phenotype in SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Mol. Neurobiol. 53, 518–531. doi: 10.1007/s12035-014-9019-8
Ashleigh, T., Swerdlow, R. H., and Beal, M. F. (2022). The role of mitochondrial dysfunction in Alzheimer's disease pathogenesis. Alzheimers Dement. 19, 333–342. doi: 10.1002/alz.12683
Barak, B., Zhang, Z., Liu, Y., Nir, A., Trangle, S. S., Ennis, M., et al. (2019). Neuronal deletion of Gtf2i, associated with Williams syndrome, causes behavioral and myelin alterations rescuable by a remyelinating drug. Nat. Neurosci. 22, 700–708. doi: 10.1038/s41593-019-0380-9
Bhattacharya, A., and Biber, K. (2016). The microglial ATP-gated ion channel P2X7 as a CNS drug target. Glia 64, 1772–1787. doi: 10.1002/glia.23001
Blanchard, J. W., Akay, L. A., Davila-Velderrain, J., von Maydell, D., Mathys, H., Davidson, S. M., et al. (2022). APOE4 impairs myelination via cholesterol dysregulation in oligodendrocytes. Nature 611, 769–779. doi: 10.1038/s41586-022-05439-w
Bloom, G. S. (2014). Amyloid-beta and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 71, 505–508. doi: 10.1001/jamaneurol.2013.5847
Bohlen, J. F., Cleary, C. M., das, D., Sripathy, S. R., Sadowski, N., Shim, G., et al. (2023). Promyelinating drugs promote functional recovery in an autism spectrum disorder mouse model of Pitt-Hopkins syndrome. Brain 146, 3331–3346. doi: 10.1093/brain/awad057
Bonifacino, T., Zerbo, R. A., Balbi, M., Torazza, C., Frumento, G., Fedele, E., et al. (2021). Nearly 30 years of animal models to study amyotrophic lateral sclerosis: a historical overview and future perspectives. Int. J. Mol. Sci. 22:12236. doi: 10.3390/ijms222212236
Brambrink, A. M., Back, S. A., Riddle, A., Gong, X., Moravec, M. D., Dissen, G. A., et al. (2012). Isoflurane-induced apoptosis of oligodendrocytes in the neonatal primate brain. Ann. Neurol. 72, 525–535. doi: 10.1002/ana.23652
Cantó, E., Barro, C., Zhao, C., Caillier, S. J., Michalak, Z., Bove, R., et al. (2019). Association between serum neurofilament light chain levels and long-term disease course among patients with multiple sclerosis followed up for 12 years. JAMA Neurol. 76, 1359–1366. doi: 10.1001/jamaneurol.2019.2137
Caprihan, A., Jones, T., Chen, H., Lemke, N., Abbott, C., Qualls, C., et al. (2015). The paradoxical relationship between white matter, psychopathology and cognition in schizophrenia: a diffusion tensor and proton spectroscopic imaging study. Neuropsychopharmacology 40, 2248–2257. doi: 10.1038/npp.2015.72
Carlström, K. E., Zhu, K., Ewing, E., Krabbendam, I. E., Harris, R. A., Falcão, A. M., et al. (2020). Gsta4 controls apoptosis of differentiating adult oligodendrocytes during homeostasis and remyelination via the mitochondria-associated Fas-Casp8-bid-axis. Nat. Commun. 11:4071. doi: 10.1038/s41467-020-17871-5
Champagne, F. A. (2010). Early adversity and developmental outcomes: interaction between genetics, epigenetics, and social experiences across the life span. Perspect. Psychol. Sci. 5, 564–574. doi: 10.1177/1745691610383494
Chen, J. F., Liu, K., Hu, B., Li, R. R., Xin, W., Chen, H., et al. (2021). Enhancing myelin renewal reverses cognitive dysfunction in a murine model of Alzheimer's disease. Neuron 109:e5, 2292–2307.e5. doi: 10.1016/j.neuron.2021.05.012
Chen, L., Ren, S. Y., Li, R. X., Liu, K., Chen, J. F., Yang, Y. J., et al. (2021). Chronic exposure to hypoxia inhibits myelinogenesis and causes motor coordination deficits in adult mice. Neurosci. Bull. 37, 1397–1411. doi: 10.1007/s12264-021-00745-1
Chen, Y., Sheng, J., Tang, X., Zhao, Y., Zhu, S., and Liu, Q. (2022). Clemastine Rescues Chemotherapy-Induced Cognitive Impairment by Improving White Matter Integrity. Neuroscience. 484, 66–79. doi: 10.1016/j.neuroscience.2022.01.001
Cordano, C., Sin, J. H., Timmons, G., Yiu, H. H., Stebbins, K., Guglielmetti, C., et al. (2022). Validating visual evoked potentials as a preclinical, quantitative biomarker for remyelination efficacy. Brain 145, 3943–3952. doi: 10.1093/brain/awac207
Cree, B. A. C., Niu, J., Hoi, K. K., Zhao, C., Caganap, S. D., Henry, R. G., et al. (2018). Clemastine rescues myelination defects and promotes functional recovery in hypoxic brain injury. Brain 141, 85–98. doi: 10.1093/brain/awx312
Creeley, C. E., Dikranian, K. T., Dissen, G. A., Back, S. A., Olney, J. W., and Brambrink, A. M. (2014). Isoflurane-induced apoptosis of neurons and oligodendrocytes in the fetal rhesus macaque brain. Anesthesiology 120, 626–638. doi: 10.1097/ALN.0000000000000037
Cristobal, C. D., and Lee, H. K. (2022). Development of myelinating glia: an overview. Glia 70, 2237–2259. doi: 10.1002/glia.24238
De Angelis, F., Bernardo, A., Magnaghi, V., Minghetti, L., and Tata, A. M. (2012). Muscarinic receptor subtypes as potential targets to modulate oligodendrocyte progenitor survival, proliferation, and differentiation. Dev. Neurobiol. 72, 713–728. doi: 10.1002/dneu.20976
Du, W., Deng, Y., Jiang, R., Tong, L., Li, R., and Jiang, X. (2022). Clemastine enhances myelination, delays axonal loss and promotes functional recovery in spinal cord injury. Neurochem. Res. 47, 503–515. doi: 10.1007/s11064-021-03465-0
Evered, L., Silbert, B., Knopman, D. S., Scott, D. A., DeKosky, S. T., Rasmussen, L. S., et al. (2018). Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br. J. Anaesth. 121, 1005–1012. doi: 10.1016/j.bja.2017.11.087
Feldman, E. L., Goutman, S. A., Petri, S., Mazzini, L., Savelieff, M. G., Shaw, P. J., et al. (2022). Amyotrophic lateral sclerosis. Lancet 400, 1363–1380. doi: 10.1016/S0140-6736(22)01272-7
Fries, G. R., Saldana, V. A., Finnstein, J., and Rein, T. (2022). Molecular pathways of major depressive disorder converge on the synapse. Mol. Psychiatry 28, 284–297. doi: 10.1038/s41380-022-01806-1
GBD 2019 Mental Disorders Collaborators (2022). Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Psychiatry 9, 137–150. doi: 10.1016/S2215-0366(21)00395-3
Green, A. J., Gelfand, J. M., Cree, B. A., Bevan, C., Boscardin, W. J., Mei, F., et al. (2017). Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial. Lancet 390, 2481–2489. doi: 10.1016/S0140-6736(17)32346-2
Hamlin, D. W., Hussain, N., and Pathare, A. (2020). Storms and silence: a case report of catatonia and paroxysmal sympathetic hyperactivity following cerebral hypoxia. BMC Psychiatry 20:473. doi: 10.1186/s12888-020-02878-5
Hong, J., Jeong, Y., and Heo, W. D. (2022). The Neurotrophic receptor tyrosine kinase in MEC-mPFC neurons contributes to remote memory consolidation. J. Neurosci. 42, 6605–6619. doi: 10.1523/JNEUROSCI.2433-21.2022
Jäkel, S., Agirre, E., Mendanha Falcão, A., van Bruggen, D., Lee, K. W., Knuesel, I., et al. (2019). Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature 566, 543–547. doi: 10.1038/s41586-019-0903-2
Jensen, S. K., Michaels, N. J., Ilyntskyy, S., Keough, M. B., Kovalchuk, O., and Yong, V. W. (2018). Multimodal enhancement of remyelination by exercise with a pivotal role for oligodendroglial PGC1alpha. Cell Rep. 24, 3167–3179. doi: 10.1016/j.celrep.2018.08.060
Johansen, P., Weiss, A., Bünter, A., Waeckerle-Men, Y., Fettelschoss, A., Odermatt, B., et al. (2011). Clemastine causes immune suppression through inhibition of extracellular signal-regulated kinase-dependent proinflammatory cytokines. J. Allergy Clin. Immunol. 128, 1286–1294. doi: 10.1016/j.jaci.2011.06.023
Kent, S. A., and Miron, V. E. (2023). Microglia regulation of central nervous system myelin health and regeneration. Nat. Rev. Immunol. doi: 10.1038/s41577-023-00907-4. [Epub ahead of print].
Koehn, L. M., Chen, X., Logsdon, A. F., Lim, Y. P., and Stonestreet, B. S. (2020). Novel neuroprotective agents to treat neonatal hypoxic-ischemic encephalopathy: inter-alpha inhibitor proteins. Int. J. Mol. Sci. 21:9193. doi: 10.3390/ijms21239193
Kozel, B. A., Barak, B., Kim, C. A., Mervis, C. B., Osborne, L. R., Porter, M., et al. (2021). Williams syndrome. Nat. Rev. Dis. Primers. 7:42. doi: 10.1038/s41572-021-00276-z
Kuhle, J., Kropshofer, H., Haering, D. A., Kundu, U., Meinert, R., Barro, C., et al. (2019). Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 92, e1007–e1015. doi: 10.1212/WNL.0000000000007032
Kuhle, J., Plavina, T., Barro, C., Disanto, G., Sangurdekar, D., Singh, C. M., et al. (2020). Neurofilament light levels are associated with long-term outcomes in multiple sclerosis. Mult. Scler. 26, 1691–1699. doi: 10.1177/1352458519885613
Lee, J. I., Park, J. W., Lee, K. J., and Lee, D. H. (2021). Clemastine improves electrophysiologic and histomorphometric changes through promoting myelin repair in a murine model of compression neuropathy. Sci. Rep. 11:20886. doi: 10.1038/s41598-021-00389-1
Leng, F., and Edison, P. (2021). Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat. Rev. Neurol. 17, 157–172. doi: 10.1038/s41582-020-00435-y
Leyrolle, Q., Decoeur, F., Dejean, C., Brière, G., Leon, S., Bakoyiannis, I., et al. (2022). N-3 PUFA deficiency disrupts oligodendrocyte maturation and myelin integrity during brain development. Glia 70, 50–70. doi: 10.1002/glia.24088
Li, Z. Y., Chen, L. H., Zhao, X. Y., Chen, H., Sun, Y. Y., Lu, M. H., et al. (2021). Clemastine attenuates AD-like pathology in an AD model mouse via enhancing mTOR-mediated autophagy. Exp. Neurol. 342:113742. doi: 10.1016/j.expneurol.2021.113742
Li, Z., He, Y., Fan, S., and Sun, B. (2015). Clemastine rescues behavioral changes and enhances remyelination in the cuprizone mouse model of demyelination. Neurosci. Bull. 31, 617–625. doi: 10.1007/s12264-015-1555-3
Li, Q., Mathena, R. P., Xu, J., Eregha, O. N., Wen, J., and Mintz, C. D. (2019). Early postnatal exposure to isoflurane disrupts oligodendrocyte development and myelin formation in the mouse hippocampus. Anesthesiology 131, 1077–1091. doi: 10.1097/ALN.0000000000002904
Liu, J., Dupree, J. L., Gacias, M., Frawley, R., Sikder, T., Naik, P., et al. (2016). Clemastine enhances myelination in the prefrontal cortex and rescues behavioral changes in socially isolated mice. J. Neurosci. 36, 957–962. doi: 10.1523/JNEUROSCI.3608-15.2016
Liu, J., Magri, L., Zhang, F., Marsh, N. O., Albrecht, S., Huynh, J. L., et al. (2015). Chromatin landscape defined by repressive histone methylation during oligodendrocyte differentiation. J. Neurosci. 35, 352–365. doi: 10.1523/JNEUROSCI.2606-14.2015
Madore, C., Leyrolle, Q., Morel, L., Rossitto, M., Greenhalgh, A. D., Delpech, J. C., et al. (2020). Essential omega-3 fatty acids tune microglial phagocytosis of synaptic elements in the mouse developing brain. Nat. Commun. 11:6133. doi: 10.1038/s41467-020-19861-z
Marin, B., Boumédiene, F., Logroscino, G., Couratier, P., Babron, M. C., Leutenegger, A. L., et al. (2017). Variation in worldwide incidence of amyotrophic lateral sclerosis: a meta-analysis. Int. J. Epidemiol. 46, dyw061–dyw074. doi: 10.1093/ije/dyw061
Martinez, M. (1992). Tissue levels of polyunsaturated fatty acids during early human development. J. Pediatr. 120, S129–S138. doi: 10.1016/s0022-3476(05)81247-8
Mathys, H., Davila-Velderrain, J., Peng, Z., Gao, F., Mohammadi, S., Young, J. Z., et al. (2019). Single-cell transcriptomic analysis of Alzheimer's disease. Nature 570, 332–337. doi: 10.1038/s41586-019-1195-2
McCann, M. E., and Soriano, S. G. (2019). Does general anesthesia affect neurodevelopment in infants and children? BMJ 367:l6459. doi: 10.1136/bmj.l6459
Mei, F., Fancy, S. P. J., Shen, Y. A., Niu, J., Zhao, C., Presley, B., et al. (2014). Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nat. Med. 20, 954–960. doi: 10.1038/nm.3618
Mei, F., Lehmann-Horn, K., Shen, Y. A., Rankin, K. A., Stebbins, K. J., Lorrain, D. S., et al. (2016). Accelerated remyelination during inflammatory demyelination prevents axonal loss and improves functional recovery. elife 5:e18246. doi: 10.7554/eLife.18246
Molina-Gonzalez, I., Miron, V. E., and Antel, J. P. (2022). Chronic oligodendrocyte injury in central nervous system pathologies. Commun. Biol. 5:1274. doi: 10.1038/s42003-022-04248-1
Motawi, T. K., El-Maraghy, S. A., Kamel, A. S., Said, S. E., and Kortam, M. A. (2023). Modulation of p38 MAPK and Nrf2/HO-1/NLRP3 inflammasome signaling and pyroptosis outline the anti-neuroinflammatory and remyelinating characters of clemastine in EAE rat model. Biochem. Pharmacol. 209:115435. doi: 10.1016/j.bcp.2023.115435
Myatich, A., Haque, A., Sole, C., and Banik, N. L. (2023). Clemastine in remyelination and protection of neurons and skeletal muscle after spinal cord injury. Neural Regen. Res. 18, 940–946. doi: 10.4103/1673-5374.355749
Nicaise, A. M., Banda, E., Guzzo, R. M., Russomanno, K., Castro-Borrero, W., Willis, C. M., et al. (2017). iPS-derived neural progenitor cells from PPMS patients reveal defect in myelin injury response. Exp. Neurol. 288, 114–121. doi: 10.1016/j.expneurol.2016.11.012
Palma, A., Chara, J. C., Montilla, A., Otxoa-de-Amezaga, A., Ruíz-Jaén, F., Planas, A. M., et al. (2022). Clemastine induces an impairment in developmental myelination. Front. Cell Dev. Biol. 10:841548. doi: 10.3389/fcell.2022.841548
Pan, S., Mayoral, S. R., Choi, H. S., Chan, J. R., and Kheirbek, M. A. (2020). Preservation of a remote fear memory requires new myelin formation. Nat. Neurosci. 23, 487–499. doi: 10.1038/s41593-019-0582-1
Park, J. Y., Park, H. J., Kim, D. J., and Kim, J. J. (2014). Positive symptoms and water diffusivity of the prefrontal and temporal cortices in schizophrenia patients: a pilot study. Psychiatry Res. 224, 49–57. doi: 10.1016/j.pscychresns.2014.07.003
Park, L. T., and Zarate, C. A. Jr. (2019). Depression in the primary care setting. N. Engl. J. Med. 380, 559–568. doi: 10.1056/NEJMcp1712493
Pereira, J. B., Janelidze, S., Ossenkoppele, R., Kvartsberg, H., Brinkmalm, A., Mattsson-Carlgren, N., et al. (2021). Untangling the association of amyloid-beta and tau with synaptic and axonal loss in Alzheimer's disease. Brain 144, 310–324. doi: 10.1093/brain/awaa395
Peternel, L., Černe, M., Drevenšek, G., Štempelj, M., Urleb, U., Stanovnik, L., et al. (2006). Inhibition of mast cell degranulation-induced drop of blood pressure with clemastine, cromolyn and compound 48/80 pretreatment. Inflamm. Res. 55, S07–S08. doi: 10.1007/s00011-005-0016-2
Phan, B. N., Bohlen, J. F., Davis, B. A., Ye, Z., Chen, H. Y., Mayfield, B., et al. (2020). A myelin-related transcriptomic profile is shared by Pitt-Hopkins syndrome models and human autism spectrum disorder. Nat. Neurosci. 23, 375–385. doi: 10.1038/s41593-019-0578-x
Puzzo, D., Argyrousi, E. K., Staniszewski, A., Zhang, H., Calcagno, E., Zuccarello, E., et al. (2020). Tau is not necessary for amyloid-beta-induced synaptic and memory impairments. J. Clin. Invest. 130, 4831–4844. doi: 10.1172/JCI137040
Raper, J., Simon, H. K., and Kamat, P. P. (2021). Long-term evidence of neonatal anaesthesia neurotoxicity linked to behavioural phenotypes in monkeys: where do we go from here? Br. J. Anaesth. 127, 343–345. doi: 10.1016/j.bja.2021.06.005
Scheltens, P., de Strooper, B., Kivipelto, M., Holstege, H., Chételat, G., Teunissen, C. E., et al. (2021). Alzheimer's disease. Lancet 397, 1577–1590. doi: 10.1016/S0140-6736(20)32205-4
Shimizu, T., Ishida, A., Hagiwara, M., Ueda, Y., Hattori, A., Tajiri, N., et al. (2020). Social defeat stress in adolescent mice induces depressive-like behaviors with reduced oligodendrogenesis. Neuroscience 443, 218–232. doi: 10.1016/j.neuroscience.2020.07.002
Simons, F. E. (2004). Advances in H1-antihistamines. N. Engl. J. Med. 351, 2203–2217. doi: 10.1056/NEJMra033121
Su, W. J., Zhang, T., Jiang, C. L., and Wang, W. (2018). Clemastine alleviates depressive-like behavior through reversing the imbalance of microglia-related pro-inflammatory state in mouse hippocampus. Front. Cell. Neurosci. 12:412. doi: 10.3389/fncel.2018.00412
Sutiwisesak, R., Burns, T. C., Rodriguez, M., and Warrington, A. E. (2021). Remyelination therapies for multiple sclerosis: optimizing translation from animal models into clinical trials. Expert Opin. Investig. Drugs 30, 857–876. doi: 10.1080/13543784.2021.1942840
Tong, L. Y., Deng, Y. B., Du, W. H., Zhou, W. Z., Liao, X. Y., and Jiang, X. (2022). Clemastine promotes differentiation of oligodendrocyte progenitor cells through the activation of ERK1/2 via muscarinic receptors after spinal cord injury. Front. Pharmacol. 13:914153. doi: 10.3389/fphar.2022.914153
Toosy, A. T., Mason, D. F., and Miller, D. H. (2014). Optic neuritis. Lancet Neurol. 13, 83–99. doi: 10.1016/S1474-4422(13)70259-X
Walton, C., King, R., Rechtman, L., Kaye, W., Leray, E., Marrie, R. A., et al. (2020). Rising prevalence of multiple sclerosis worldwide: insights from the atlas of MS, third edition. Mult. Scler. 26, 1816–1821. doi: 10.1177/1352458520970841
Wang, F., Yang, Y. J., Yang, N., Chen, X. J., Huang, N. X., Zhang, J., et al. (2018). Enhancing oligodendrocyte myelination rescues synaptic loss and improves functional recovery after chronic hypoxia. Neuron 99:e5, 689–701.e5. doi: 10.1016/j.neuron.2018.07.017
Wang, F., Ren, S. Y., Chen, J. F., Liu, K., Li, R. X., Li, Z. F., et al. (2020). Myelin degeneration and diminished myelin renewal contribute to age-related deficits in memory. Nat. Neurosci. 23, 481–486. doi: 10.1038/s41593-020-0588-8
Wu, W., Peng, Y., Zhou, J., Zhang, X., Cao, L., Lin, W. J., et al. (2021a). Identification of the potential gene regulatory networks and therapeutics in aged mice with postoperative neurocognitive disorder. Front. Neurosci. 15:689188. doi: 10.3389/fnins.2021.689188
Wu, W., Zhang, X., Zhou, J., Yang, H., Chen, J., Zhao, L., et al. (2021b). Clemastine ameliorates perioperative neurocognitive disorder in aged mice caused by anesthesia and surgery. Front. Pharmacol. 12:738590. doi: 10.3389/fphar.2021.738590
Xie, D., Ge, X., Ma, Y., Tang, J., Wang, Y., Zhu, Y., et al. (2020). Clemastine improves hypomyelination in rats with hypoxic-ischemic brain injury by reducing microglia-derived IL-1beta via P38 signaling pathway. J. Neuroinflammation 17:57. doi: 10.1186/s12974-019-1662-6
Xie, Y. Y., Pan, T. T., Xu, D. E., Huang, X., Tang, Y., Huang, W., et al. (2021). Clemastine ameliorates myelin deficits via preventing senescence of oligodendrocytes precursor cells in Alzheimer's disease model mouse. Front. Cell Dev. Biol. 9:733945. doi: 10.3389/fcell.2021.733945
Xu, T., Liu, C., Deng, S., Gan, L., Zhang, Z., Yang, G. Y., et al. (2022). The roles of microglia and astrocytes in myelin phagocytosis in the central nervous system. J. Cereb. Blood Flow Metab. 43, 325–340. doi: 10.1177/0271678X221137762
Yang, Q., Song, D., and Qing, H. (2017). Neural changes in Alzheimer's disease from circuit to molecule: perspective of optogenetics. Neurosci. Biobehav. Rev. 79, 110–118. doi: 10.1016/j.neubiorev.2017.05.015
Young, K. M., Psachoulia, K., Tripathi, R. B., Dunn, S. J., Cossell, L., Attwell, D., et al. (2013). Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron 77, 873–885. doi: 10.1016/j.neuron.2013.01.006
Yu, G., Su, Y., Guo, C., Yi, C., Yu, B., Chen, H., et al. (2022). Pathological oligodendrocyte precursor cells revealed in human schizophrenic brains and trigger schizophrenia-like behaviors and synaptic defects in genetic animal model. Mol. Psychiatry 27, 5154–5166. doi: 10.1038/s41380-022-01777-3
Zhang, Z., Liu, H., Jia, S., Zhao, J., Wang, J., Liu, Y., et al. (2022). Spatial and temporal alterations of developing oligodendrocytes induced by repeated sevoflurane exposure in neonatal mice. Biochem. Biophys. Res. Commun. 640, 12–20. doi: 10.1016/j.bbrc.2022.11.105
Zhi, C., Zeng, S., Chen, Y., Liao, D., Lai, M., Wang, Z., et al. (2021). Clemastine promotes recovery of neural function and suppresses neuronal apoptosis by restoring balance of pro-inflammatory mediators in an experimental model of intracerebral hemorrhage. Int. J. Med. Sci. 18, 639–645. doi: 10.7150/ijms.51150
Keywords: clemastine, central nervous system diseases, neuroprotective, myelination, cognition
Citation: Jiang S, Wang X, Cao T, Kang R and Huang L (2023) Insights on therapeutic potential of clemastine in neurological disorders. Front. Mol. Neurosci. 16:1279985. doi: 10.3389/fnmol.2023.1279985
Edited by:
Xianghui Zhao, Air Force Medical University, ChinaReviewed by:
Lin Xiao, South China Normal University, ChinaYubo Xie, The First Affiliated Hospital of Guangxi Medical University, China
Copyright © 2023 Jiang, Wang, Cao, Kang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lining Huang, MTU4MDMyMTM3MzBAMTYzLmNvbQ==
 Sufang Jiang1
Sufang Jiang1 Lining Huang
Lining Huang