- 1AlzeCure Pharma AB, Huddinge, Sweden
- 2Department of Neurobiology, Care Sciences, and Society, Division of Neurogeriatrics, Center for Alzheimer Research, Karolinska Institutet, Stockholm, Sweden
- 3Sinfonia Biotherapeutics AB, Huddinge, Sweden
Recent clinical data with three therapeutic anti-Aβ antibodies have demonstrated that removal of Aβ-amyloid plaques in early Alzheimer's disease (AD) can attenuate disease progression. This ground-breaking progress in AD medicine has validated both the amyloid cascade hypothesis and Aβ-amyloid as therapeutic targets. These results also strongly support therapeutic approaches that aim to reduce the production of amyloidogenic Aβ to prevent the formation of Aβ-pathology. One such strategy, so-called gamma-secretase modulators (GSM), has been thoroughly explored in preclinical settings but has yet to be fully tested in clinical trials. Recent scientific progress has shed new light on the role of Aβ in Alzheimer's disease and suggests that GSMs exhibit specific pharmacological features that hold great promise for the prevention and treatment of Alzheimer's disease. In this short review, we discuss the data that support why it is important to continue to progress in this class of compounds.
Introduction
Alzheimer's disease (AD) is the most common form of dementia, affecting millions of people worldwide. AD therapies have recently been limited to symptomatic treatments, with Memantine representing the latest approved treatment for symptoms ~20 years ago (Witt et al., 2004). However, since 2021, the AD field has experienced a rebirth (Vellas and Aisen, 2021), highlighted by positive clinical data with three monoclonal antibodies (mAbs), Aducunumab (Haeberlein et al., 2022), Lecanemab (Dyck et al., 2022), and Donanemab (Sims et al., 2023).
In large phase 3 trials, these mAbs targeted the amyloid-beta (Aβ) component of AD and demonstrated Aβ amyloid clearance, along with significant disease-modifying effects in early AD. Together, these studies have proven the Aβ amyloid-cascade hypothesis in AD and shown that the course of AD can be treated therapeutically. Unfortunately, in a subset of patients, these therapies cause amyloid-related image abnormalities (ARIA), such as micro-hemorrhages (ARIA-H) and oedemas (ARIA-E), which is an important safety concern.
Encouraged by the progress, current drug discovery efforts steer toward more effective and safe treatments that ultimately could prevent Aβ amyloidogenesis and AD. This could be achieved with small-molecule treatments, providing cost-effective, patient/user-friendly oral therapies that would be fit for purpose as a chronic preventive treatment paradigm in people with emerging amyloidosis who are otherwise unaffected by the disease.
Extracellular Aβ-amyloid plaques, so-called “senile plaques”, are key neuropathological hallmarks of AD, originally described by Alois Alzheimer (Alzheimer, 1907). These extracellular proteinaceous deposits contain aggregates of the amyloid-beta peptide (Glenner and Wong, 1984; Surguchov et al., 2023). Seminal genetic discoveries in the 1990s linked early-onset familial AD (FAD) to three genes: the amyloid precursor protein (APP) and the presenilin (PS) 1 and 2 encoding genes (Bagyinszky et al., 2014). These genes were soon demonstrated to be directly involved in Aβ generation and accelerate the development of Aβ-amyloid pathology, indicating a pivotal role for Aβ in AD pathogenesis. To date, more than 200 disease-causing mutations in the APP and PS genes have been identified (see https://www.alzforum.org/mutations). Owing to the development of sensitive biochemical and imaging biomarker technologies, it is possible to monitor the process of Aβ amyloidosis during disease progression. It appears that the process of Aβ-amyloidosis begins ~10–15 years prior to the onset of symptoms in both sporadic AD and FAD. These findings could be viewed as optimistic since they provide opportunities to detect and treat AD early, many years before the overt symptomatic phase of the disease.
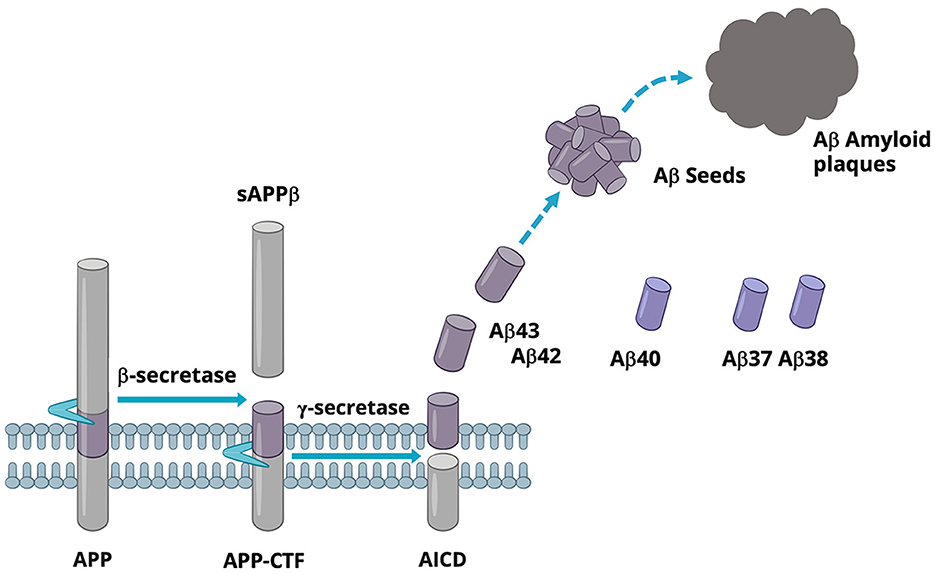
Aβ is a family of secreted peptides generated from the sequential cleavages of the type 1 membrane protein APP by beta-secretase (BACE) and gamma-secretase (GSEC), respectively. BACE cleaves APP in the luminal domain, releasing the N-terminal soluble APPβ domain and leaving the C-terminal fragment, APP-CTF, which remains in the membrane. Subsequently, the APP-CTF is recruited to GSEC, a complex comprising four subunits, including PS, which harbors the active site. GSEC first cuts APP-CTF at the epsilon-cleavage site located close to the inner leaflet of the membrane. This cleavage event produces either Aβ48 or Aβ49 and the APP intracellular domain (AICD). The membrane-retained Aβ48 or Aβ49 is then further processed by GSEC in a continuous cascade of proteolytical events at every third of fourth amino acid, where the N-terminal product of each reaction becomes the substrate for the next GSEC cleavage event. Accordingly, GSEC processes APP-CTF along two main product lines, Aβ49 → 46 → 43 → 40 → 37… and Aβ48 → 45 → 42 → 38…, respectively (Takami et al., 2009; Matsumura et al., 2014; Olsson et al., 2014). During this processing cascade, Aβ43 and shorter Aβ peptides stochastically escape further processing by GSEC and are released into the extracellular space. As a result, Aβ peptides varying from 30 to 43 amino acids in length are secreted into the extracellular space. Among all secreted Aβ, Aβ40 is the most abundant in human CSF, followed by Aβ38, Aβ42, and Aβ37 (Liu et al., 2022). In cognitively normal individuals, Aβ42 and Aβ43 represent a smaller portion of the total secreted Aβ (Liu et al., 2022). These longer forms of Aβ seed the formation of Aβ-amyloid aggregates, a key step in the formation of amyloid plaques (Veugelen et al., 2016), as illustrated in Figure 1. Aβ42, which is produced in higher amounts than Aβ43, is the most abundant Aβ in amyloid plaques (Welander et al., 2009).
In FAD, the disease-causing mutations in PS appear to shift the Aβ product formation toward longer, more amyloidogenic forms of Aβ at the expense of shorter forms of Aβ (Sun et al., 2017). The preference of the FAD mutants for the generation of the more aggregation-prone Aβ42 and Aβ43 has been shown to be due to a destabilization of GSEC and incomplete processing through the Aβ product lines (Chávez-Gutiérrez et al., 2012; Szaruga et al., 2017). Interestingly, some PS1 mutations show an unaltered or only marginal increase in Aβ42/43 but are accompanied by a significant reduction in shorter Aβ, resulting in a decrease in total Aβ produced (Bentahir et al., 2006). But still, these Aβ profiles lead to accelerated amyloidosis, implicating that not only are the absolute levels of amyloidogenic Aβ critical for amyloidosis but also a decrease in shorter forms of Aβ may promote Aβ-amyloidosis.
Indeed, a growing body of scientific data support the pathogenic significance of an altered ratio between the shorter and longer forms of Aβ in AD. Liu et al. (2022) made the important observation that the actual ratio between Aβ37 or Aβ38 and Aβ42 or Aβ43 in human CSF correlates with the age of onset and the Mini-Mental State Exam (MMSE) in a range of FAD mutant carriers. Similarly, Petit et al. (2022a) found a correlation between the age of onset in familial AD caused by different PS1 mutants and the ratio of short peptides (Aβ37 + 38 + 40) over long peptides (Aβ42 + 43) produced, as studied in cell culture models. Indeed, the higher the (Aβ42 + 43)/(Aβ37 + 38 + 40) ratio, the earlier the age of onset of disease. Congruent with these findings, Cullen et al. (2022) have recently demonstrated a positive correlation between CSF Aβ38 levels and protection from developing AD. Although the mechanism by which shorter Aβ affects Aβ-amyloidosis is not fully understood, several studies have demonstrated that the shorter peptides Aβ37, Aβ38, and Aβ40 can individually and cooperatively inhibit the aggregation of Aβ42 (Kim et al., 2007; Moore et al., 2018; Nordvall et al., 2018; Braun et al., 2022), supporting an anti-amyloidogenic role of short forms of Aβ. Collectively, these data reveal the key importance of functional GSEC and complete Aβ product processing in maintaining a low Aβ long/Aβ short ratio, which could prevent the development of AD.
The pivotal role of Aβ-amyloid in AD has, for the last 25 years, engaged an enormous amount of drug discovery efforts targeting existing Aβ pathology, Aβ clearance, and Aβ production.
For the scope of this review, we will focus on the major therapeutic strategies explored to date to reduce Aβ production, with a special emphasis on GSMs.
Several approaches to reducing Aβ production have been explored in clinical studies. Inhibitors of gamma-secretase (GSIs) produced robust Aβ lowering in animals and were tested in clinical trials (Semagacestat Phase 3, Avagacestat Phase 2) (Doody et al., 2013; Coric et al., 2015). However, the inhibition of GSEC was associated with severe side effects, including cognitive worsening. These side effects were mainly mechanism-related due to the inhibition of other GSEC-dependent signaling events. More than 150 different GSEC substrates have so far been identified (Güner and Lichtenthaler, 2020). Many of these substrates, including the Notch family of receptors, mediate pivotal signaling both during development and in adults, and many GSI-associated side effects have indeed been linked to disturbed Notch signaling (Milano et al., 2004).
Another approach to reduce Aβ production that was tested in the clinic was to inhibit beta-secretase (BACE1). Five different BACE1 inhibitors were tested in late-stage clinical testing in mild-to-moderate AD, prodromal AD, and in people at risk of developing AD (for review see Imbimbo and Watling, 2019). Despite a large reduction in CSF Aβ42 levels, these compounds failed to show clinical benefit or were terminated due to futility and, somewhat unexpectedly, impaired cognitive abilities in the patients (Wessels et al., 2020). The explanation for these side effects is still a matter of debate. Clearly, like GSEC, BACE1 plays a pivotal role in neurobiology and has more than 40 substrates, some of which are involved in various synaptic functions like axonal guidance, neuronal plasticity, and LTP such as seizure protein 6 (SEZ6), CHL1, and neuregulin-1 (Munro et al., 2016; Yan, 2017; Müller et al., 2023). Therefore, it is likely that the safety liabilities discovered in the clinic with BACE1 inhibitors are mechanism-related.
Gamma-secretase modulators
In light of the unsuccessful clinical outcomes of GSIs and BACE1 inhibitors, the alternative way to reduce Aβ production using gamma-secretase modulators needs to be further assessed clinically. In 2001, a seminal article by Weggen et al. described the first GSMs as an alternative mechanism to modulate gamma-secretase-mediated Aβ production. It was discovered that certain non-steroidal anti-inflammatory drugs (NSAIDs, e.g., Ibuprofen, Indomethacin, and Sulindac sulfide) could modulate GSEC to lower the production of Aβ42 and concomitantly increase Aβ38 without affecting the total amount of Aβ (Weggen et al., 2001; Eriksen et al., 2003).
These encouraging findings led to clinical phase 3 trials in AD patients using the (R)-enantiomer of the NSAID flurbiprofen (Tarenflurbil, Flurizan® from Myriad) (Green et al., 2009). However, this compound was unable to demonstrate effects on cognitive function, likely due to its very low potency (in vitro IC50 in high μM) and poor CNS penetration (Wan et al., 2009). Another NSAID derivative that was clinically tested was the GSM Itanapraced (CHF5074) from Chiesi Farmaceutici, which was tested in a phase 1 study but did not affect CSF Aβ42 levels (Ross et al., 2012), most likely due to its low potency. Several carboxylic acid derivatives with improved potency and physicochemical profiles were later developed (Peng et al., 2011; Rogers et al., 2012) but were never clinically tested. In parallel, attempts were made to identify new types of gamma-secretase modulators, first identified by Neurogenetics (Cheng et al., 2004), but several other pharmaceutical companies followed and identified non-carboxylic acid series of compounds, with most molecules containing an aryl-imidazole moiety—the “second generation” GSMs (Xia, 2019; Mekala et al., 2020; Wolfe, 2021; Hur, 2022; Luo and Li, 2022). Recently, the structure of the gamma-secretase complex co-crystallized with the second-generation GSM E2012 developed by Eisai was determined (Yang et al., 2021). An in silico model supported by mutational data suggests that imidazole-based GSMs interact at the interface between GSEC and APP-C99, potentially providing new opportunities for drug design (Petit et al., 2022b). A similar structure has not been determined for the carboxylic acid class of GSMs, and how this class of GSMs modulates Aβ remains obscure. The binding sites for the two classes of GSMs are likely different since they do not show competitive binding, and they affect the processing of APP differently, resulting in different Aβ profiles (Borgegård et al., 2012; Olsson et al., 2014). There is evidence suggesting that the carboxylic acid class of GSMs interacts with APP rather than GSEC (Kukar et al., 2008), a finding coherent with the fact that both classes of GSMs have synergistic properties in reducing longer forms of Aβ (Robertson et al., 2017; Luo et al., 2022).
No GSMs of the “second generation” have reached phase 2 clinical trials yet, but several have demonstrated impressive activity in preclinical studies (Kounnas et al., 2010; Wanngren et al., 2012; Toyn et al., 2014, 2016; Brendel et al., 2015; Ratni et al., 2020; Rynearson et al., 2021), and some have been tested in phase 1 clinical trials. The first (non-NSAID) GSM tested in human phase 1 trials was E2012, which produced a ~50% reduction of plasma Aβ42 (Nagy et al., 2010). However, this compound showed some unacceptable side effects by affecting cholesterol metabolism, leading to lenticular opacity (Nakano-Ito et al., 2014). This side-effect was absent in phase 1 trials with the follow-up compound E2212, which robustly lowered plasma Aβ42 and did not display any serious adverse events (Yu et al., 2014). Still, E2212 was not further developed for undisclosed reasons. BMS demonstrated that their GSM BMS-932481 produced a large increase in Aβ37 and a reduction of Aβ42 CSF levels in healthy volunteers (Soares et al., 2016). However, compound-related adverse liver findings were seen after repeated dosing (Zhuo et al., 2023), which led to the termination of further studies. Neurogenetics performed a small phase 1 study with NGP 555 (Kounnas et al., 2017), which increased the Aβ37/Aβ42 ratio and appeared well tolerated, but no further development has been reported. Pfizer showed promising phase 1 SAD and MAD data with PF-06648671 demonstrating reductions of Aβ42 and Aβ40, together with increases in Aβ37 in healthy volunteers (Ahn et al., 2019). No major side effects were reported, but the compound did not progress further, potentially due to Pfizer's decision to leave the CNS therapeutic area. Currently, only a limited number of GSM programs appear to be active, including UCSD-776890 from the group of Steven Wagner that received NIH funding for a Phase 1 study (Rynearson et al., 2021). Roche has recently completed a phase 1 study with their GSM RG6289 (Ratni et al., 2020; Sturm et al., 2023), and AlzeCure Pharma is developing GSMs within their Alzstatin platform (Sandin et al., 2022). The key requirements for an effective GSM are high potency, good CNS exposure, and PK properties to provide robust Aβ42 reductions at reasonable doses. The safety of the compound is of paramount importance, as many GSMs have suffered from insufficient margins between efficient Aβ42-lowering effects and compound-related side effects. This is probably a consequence of the binding site requiring compounds with high logP, flat structures with high aromatic content, and an imidazole moiety, potentially leading to poor selectivity. Recent examples have shown that it is indeed possible to develop GSMs with reduced aromaticity and planarity, as well as to avoid an imidazole moiety (Ratni et al., 2020).
The pharmacology of GSMs provides a number of key features that hold great promise as a preferred treatment to prevent amyloidogenic Aβ production. First, it appears to be a safe, tolerable mechanism. In contrast to GSIs and BACE inhibitors, GSMs do not inhibit any enzyme but rather modulate the activity of GSEC. Thus, neither Notch processing nor other important signaling pathways dependent on GSEC appear to be affected by GSMs (Weggen et al., 2001; Wanngren et al., 2012). Furthermore, several tested GSMs have been shown to be selective for APP processing and Aβ modulation, demonstrating that it is feasible to design GSMs tailored for Aβ modulation (Wanngren et al., 2012; Weber et al., 2021). These are critical attributes of GSMs and minimize the safety liabilities that have been associated with both BACE and GSEC inhibitors. In fact, currently, no mechanism-related toxicity has been assigned to GSMs, which is promising considering their potential use as an early, preventive, chronic treatment in individuals at risk of developing AD.
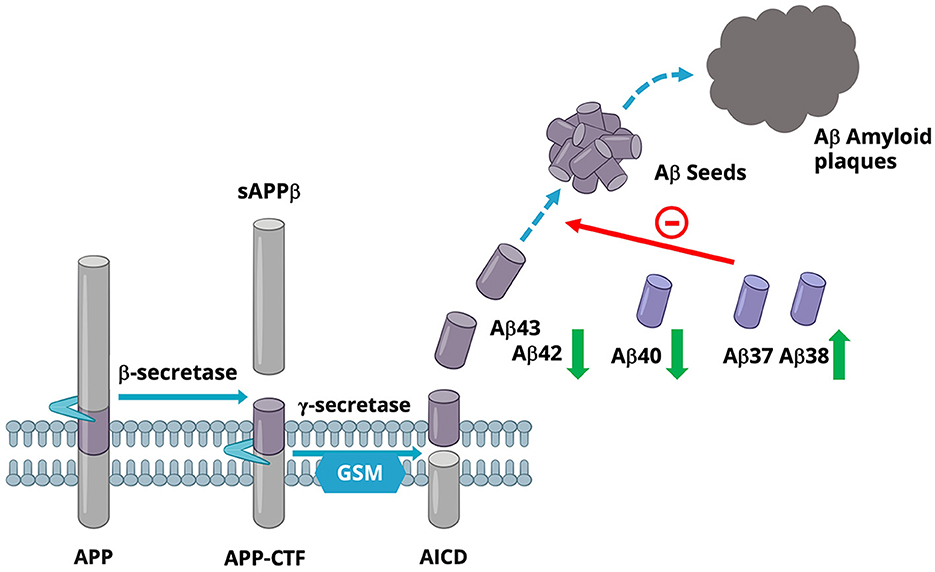
Second, GSMs are effective anti-amyloidogenic agents. GSMs do not change the total amount of Aβ formed but rather decrease the production of amyloidogenic Aβ while increasing the production of shorter Aβ. This is the opposite effect on Aβ generation as compared to the situation with several FAD-causing mutations in the PS genes (which accelerate amyloidosis). Indeed, GSMs reduce the production of the aggregation-prone Aβ42 and most likely Aβ43, as well as Aβ40 (Olsson et al., 2014), while the levels of Aβ37 and Aβ38 are increased. Studies have shown that GSMs appear to stabilize the GSEC/APP-CTF complex, allowing GSEC to continue processing Aβ43/Aβ42/Aβ40 into the shorter forms Aβ37 and Aβ38, thus increasing the turnover of the longer forms of Aβ (Olsson et al., 2014; Szaruga et al., 2017). Interestingly, the increase of the shorter Aβ37 and Aβ38 could have several beneficial effects, including attenuation of Aβ42-mediated toxicity (Moore et al., 2018; Quartey et al., 2021) and/or reduced Aβ42 aggregation (Nordvall et al., 2018; Braun et al., 2022). These effects would lead to decreased formation of Aβ pathology, and would represent an inverted Aβ pattern to that observed in FAD; see Figure 2. Indeed, higher levels of Aβ38 have been shown to be associated with a lower risk of AD-related changes in clinical studies (Cullen et al., 2022).
Finally, an increasing body of data suggests that Aβ itself may play an important physiological role in normal cellular processes (Sturchio et al., 2022). Since GSMs do not affect the total amount of Aβ peptides produced but only alter the ratio between longer and shorter Aβ forms, the potential signaling function of Aβ in the presence of GSMs may not be disturbed.
In light of the recent progress with Aβ immunotherapies in the clinic, combined with our increased understanding of the pathogenic mechanisms resulting in amyloidosis and FAD, GSMs hold great promise as a novel anti-amyloidogenic therapy. Based on our current knowledge, a GSM is unlikely to produce any major mechanism-related side effects, and with a profile that is the reverse of the familial mutations in PS, a GSM would be an excellent choice for the primary prevention of Alzheimer's disease (Voytyuk et al., 2018). This could be the ultimate goal when diagnostic and prognostic biomarkers have evolved even further to efficiently select and monitor the target population, which could include risk groups such as APOE4-positive individuals (Leonenko et al., 2021). Such a treatment needs to start early, well before amyloid deposition in the brain is initiated. In this case, it is conceivable that an early treatment with a GSM would provide a superior anti-amyloidogenic effect.
A secondary prevention approach could also be considered with a GSM. An increase in Aβ pathology as detected by PET is the first pathological change in AD, which in turn appears to subsequently drive the tau pathology (Zhang et al., 2021). Therefore, using a GSM prior to the rapid increase in tau pathology driven by Aβ could serve as an alternative strategy (Karran and Strooper, 2022).
Clinical evaluation of either primary or secondary prevention would probably require extended clinical trials. Therefore, evaluating a GSM as a maintenance therapy after Aβ-clearance with an anti-Aβ antibody with the aim of preventing the buildup of new amyloid aggregates could be an attractive option as a first step. The antibody treatment aims to clear plaques until amyloid levels are no longer detectable in PET scans (~20 centiloid). Once this is achieved, the treatment is stopped. Therefore, these patients would be “reset” to an approximate common starting level with no or low levels of amyloid plaques, and the buildup of plaques would then commence again. A GSM treatment at this stage, reducing the production of aggregation-prone Aβ species, would be a clinically feasible and suitable treatment option to reduce the buildup of new plaques.
Alzheimer's disease is a complex disease to treat and prevent. Anti-Aβ antibodies have reinvigorated the field by showing significant clinical benefits with treatment. We believe that GSMs will be an essential addition to the treatment toolbox for Alzheimer's disease, and one that is likely necessary for its ultimate prevention.
Author contributions
GN: Writing—original draft, Writing—review and editing. JL: Writing—original draft, Writing—review and editing. JS: Writing—original draft, Writing—review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
GN and JS are employees of AlzeCure Pharma AB. JL is a consultant to AlzeCure Pharma AB and an employee of Sinfonia Biotherapeutics AB.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahn, J. E., Carrieri, C., Cruz, F. D., Fullerton, T., Hajos-Korcsok, E., He, P., et al. (2019). Pharmacokinetic and pharmacodynamic effects of a γ-secretase modulator, PF-06648671, on CSF amyloid-β peptides in randomized phase I studies. Clin. Pharmacol. Ther. 107, 211–220. doi: 10.1002/cpt.1570
Alzheimer, A. (1907). Über eine eigenartige Erkrankung der Hirnrinde. Allg. Zschr. Psychiatr. Psych. Gerichtl. Med. 64, 146–148.
Bagyinszky, E., Youn, Y. C., An, S. S. A., and Kim, S. (2014). The genetics of Alzheimer's disease. Clin. Interv. Aging 9, 535–551. doi: 10.2147/CIA.S51571
Bentahir, M., Nyabi, O., Verhamme, J., Tolia, A., Horré, K., Wiltfang, J., et al. (2006). Presenilin clinical mutations can affect γ-secretase activity by different mechanisms. J. Neurochem. 96, 732–742. doi: 10.1111/j.1471-4159.2005.03578.x
Borgegård, T., Gustavsson, S., Nilsson, C., Parpal, S., Klintenberg, R., Berg, A.-L., et al. (2012). Alzheimer's disease: presenilin 2-sparing γ-secretase inhibition is a tolerable Aβ peptide-lowering strategy. J. Neurosci. 32, 17297–17305. doi: 10.1523/JNEUROSCI.1451-12.2012
Braun, G. A., Dear, A. J., Sanagavarapu, K., Zetterberg, H., and Linse, S. (2022). Amyloid-β peptides 37, 38, and 40 individually and cooperatively inhibit amyloid-β 42 aggregation. Chem. Sci. 13, 2423–2439. doi: 10.1039/D1SC02990H
Brendel, M., Jaworska, A., Herms, J., Trambauer, J., Tzer, C. R., Gildehaus, F.-J., et al. (2015). Amyloid-PET predicts inhibition of de novo plaque formation upon chronic gamma;-secretase modulator treatment. Mol. Psychiatr. 20, 1179–1187. doi: 10.1038/mp.2015.74
Chávez-Gutiérrez, L., Bammens, L., Benilova, I., Vandersteen, A., Benurwar, M., Borgers, M., et al. (2012). The mechanism of γ-Secretase dysfunction in familial Alzheimer's disease. Embo. J. 31, 2261–2274. doi: 10.1038/emboj.2012.79
Cheng, S., Comer, D. D., Balow, G. P., and Pleynet, D. (2004). Compounds and Uses Thereof in Modulating Amyloid Beta, Patent WO2004/110350.
Coric, V., Salloway, S., Dyck, C. H., van Dubois, B., Andreasen, N., Brody, M., et al. (2015). Targeting prodromal Alzheimer disease with avagacestat: a randomized clinical trial. JAMA Neurol. 72, 1324–1333. doi: 10.1001/jamaneurol.2015.0607
Cullen, N., Janelidze, S., Palmqvist, S., Stomrud, E., Mattsson-Carlgren, N., and Hansson, O. (2022). Association of CSF Aβ38 levels with risk of Alzheimer disease–related decline. Neurology 98, e958–e967. doi: 10.1212/WNL.0000000000013228
Doody, R. S., Raman, R., Farlow, M., Iwatsubo, T., Vellas, B., Joffe, S., et al. (2013). A phase 3 trial of semagacestat for treatment of Alzheimer's disease. N. Engl. J. Med. 369, 341–350. doi: 10.1056/NEJMoa1210951
Dyck, C. H., van Swanson, C. J., Aisen, P., Bateman, R. J., Chen, C., Gee, M., et al. (2022). Lecanemab in early Alzheimer's disease. New Engl. J. Med. 388, 9–21. doi: 10.1056/NEJMoa2212948
Eriksen, J. L., Sagi, S. A., Smith, T. E., Weggen, S., Das, P., McLendon, D. C., et al. (2003). NSAIDs and enantiomers of flurbiprofen target γ-secretase and lower Aβ42 in vivo. J. Clin. Investig. 112, 440–449. doi: 10.1172/JCI18162
Glenner, G. G., and Wong, C. W. (1984). Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem. Biophys. Res. Commun. 122, 1131–1135. doi: 10.1016/0006-291X(84)91209-9
Green, R. C., Schneider, L. S., Amato, D. A., Beelen, A. P., Wilcock, G., Swabb, E. A., et al. (2009). Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial. JAMA 302, 2557–2564. doi: 10.1001/jama.2009.1866
Güner, G., and Lichtenthaler, S. F. (2020). The substrate repertoire of γ-secretase/presenilin. Semin. Cell Dev. Biol. 105, 27–42. doi: 10.1016/j.semcdb.2020.05.019
Haeberlein, S. B., Aisen, P. S., Barkhof, F., Chalkias, S., Chen, T., Cohen, S., et al. (2022). Two randomized phase 3 studies of aducanumab in early Alzheimer's disease. J. Prev. Alzheimers Dis. 9, 197–210. doi: 10.14283/jpad.2022.30
Hur, J.-Y. (2022). γ-Secretase in Alzheimer's disease. Exp. Mol. Med. 54, 433–446. doi: 10.1038/s12276-022-00754-8
Imbimbo, B. P., and Watling, M. (2019). Investigational BACE inhibitors for the treatment of Alzheimer's disease. Expert Opin. Investig. Drugs 28, 967–975. doi: 10.1080/13543784.2019.1683160
Karran, E., and Strooper, B. D. (2022). The amyloid hypothesis in Alzheimer disease: new insights from new therapeutics. Nat. Rev. Drug Discov. 21, 306–318. doi: 10.1038/s41573-022-00391-w
Kim, J., Onstead, L., Randle, S., Price, R., Smithson, L., Zwizinski, C., et al. (2007). Abeta40 inhibits amyloid deposition in vivo. J. Neurosci. 27, 627–633. doi: 10.1523/JNEUROSCI.4849-06.2007
Kounnas, M. Z., Danks, A. M., Cheng, S., Tyree, C., Ackerman, E., Zhang, X., et al. (2010). Modulation of γ-secretase reduces β-amyloid deposition in a transgenic mouse model of Alzheimer's disease. Neuron 67, 769–780. doi: 10.1016/j.neuron.2010.08.018
Kounnas, M. Z., Lane-Donovan, C., Nowakowski, D. W., Herz, J., and Comer, W. T. (2017). NGP 555, a γ-secretase modulator, lowers the amyloid biomarker, Aβ42, in cerebrospinal fluid while preventing Alzheimer's disease cognitive decline in rodents. Alzheimers Demen. Transl. Res. Clin. Interv. 3, 65–73. doi: 10.1016/j.trci.2016.09.003
Kukar, T. L., Ladd, T. B., Bann, M. A., Fraering, P. C., Narlawar, R., Maharvi, G. M., et al. (2008). Substrate-targeting γ-secretase modulators. Nature 453, 925–929. doi: 10.1038/nature07055
Leonenko, G., Baker, E., Stevenson-Hoare, J., Sierksma, A., Fiers, M., Williams, J., et al. (2021). Identifying individuals with high risk of Alzheimer's disease using polygenic risk scores. Nat. Commun. 12, 4506. doi: 10.1038/s41467-021-24082-z
Liu, L., Lauro, B. M., He, A., Lee, H., Bhattarai, S., Wolfe, M. S., et al. (2022). Identification of the Aβ37/42 peptide ratio in CSF as an improved Aβ biomarker for Alzheimer's disease. Alzheimers Dementia. 19, 79–96. doi: 10.1002/alz.12646
Luo, J. E., and Li, Y.-M. (2022). Turning the tide on Alzheimer's disease: modulation of γ-secretase. Cell Biosci. 12, 2. doi: 10.1186/s13578-021-00738-7
Luo, J. E., Wagner, S. L., and Li, Y. (2022). Mechanism of gamma-secretase modulators. Alzheimers Dement. 18, 064014. doi: 10.1002/alz.064014
Matsumura, N., Takami, M., Okochi, M., Wada-Kakuda, S., Fujiwara, H., Tagami, S., et al. (2014). γ-secretase associated with lipid rafts. Multiple interactive pathways in the stepwise processing of β-carboxyl-terminal fragment. J. Biol. Chem. 289, 5109–5121. doi: 10.1074/jbc.M113.510131
Mekala, S., Nelson, G., and Li, Y.-M. (2020). Recent developments of small molecule γ-secretase modulators for Alzheimer's disease. RSC Med. Chem. 11, 1003–1022. doi: 10.1039/D0MD00196A
Milano, J., McKay, J., Dagenais, C., Foster-Brown, L., Pognan, F., Gadient, R., et al. (2004). Modulation of notch processing by γ-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol. Sci. 82, 341–358. doi: 10.1093/toxsci/kfh254
Moore, B. D., Martin, J., Mena, L., de Sanchez, J., Cruz, P. E., Ceballos-Diaz, C., et al. (2018). Short Aβ peptides attenuate Aβ42 toxicity in vivo. J. Exp. Med. 215, 283–301. doi: 10.1084/jem.20170600
Müller, S. A., Shmueli, M. D., Feng, X., Tüshaus, J., Schumacher, N., Clark, R., et al. (2023). The Alzheimer's disease-linked protease BACE1 modulates neuronal IL-6 signaling through shedding of the receptor gp130. Mol. Neurodegener. 18, 13. doi: 10.1186/s13024-023-00596-6
Munro, K. M., Nash, A., Pigoni, M., Lichtenthaler, S. F., and Gunnersen, J. M. (2016). Functions of the Alzheimer's disease protease BACE1 at the synapse in the central nervous system. J. Mol. Neurosci. 60, 305–315. doi: 10.1007/s12031-016-0800-1
Nagy, C., Schuck, E., Ishibashi, A., Nakatani, Y., Rege, B., and Logovinsky, V. (2010). P3-415: E2012, a novel gamma-secretase modulator, decreases plasma amyloid-beta (Aβ) levels in humans. Alzheimers Dementia 6, S574–S574. doi: 10.1016/j.jalz.2010.05.1958
Nakano-Ito, K., Fujikawa, Y., Hihara, T., Shinjo, H., Kotani, S., Suganuma, A., et al. (2014). E2012-Induced cataract and its predictive biomarkers. Toxicol. Sci. 137, 249–258. doi: 10.1093/toxsci/kft224
Nordvall, G., Yan, P., Lundkvist, J., Sandin, J., Winblad, B., Zetterberg, H., et al (2018). Poster 32 AD/PD 2018: Gamma-Secretase Modulation Results In Multiple Anti-Amyloidogenic Effects In Vivo. Available online at: https://www.alzecurepharma.se/sv/wp-content/uploads/sites/3/2020/04/adpd-poster-final.pdf
Olsson, F., Schmidt, S., Althoff, V., Munter, L. M., Jin, S., Rosqvist, S., et al. (2014). Characterization of intermediate steps in amyloid beta (Aβ) production under near-native conditions. J. Biol. Chem. 289, 1540–1550. doi: 10.1074/jbc.M113.498246
Peng, H., Talreja, T., Xin, Z., Cuervo, J. H., Kumaravel, G., Humora, M. J., et al. (2011). Discovery of BIIB042, a potent, selective, and orally bioavailable γ-secretase modulator. ACS Med. Chem. Lett. 2, 786–791. doi: 10.1021/ml200175q
Petit, D., Fernández, S. G., Zoltowska, K. M., Enzlein, T., Ryan, N. S., O'Connor, A., et al. (2022a). Aβ profiles generated by Alzheimer's disease causing PSEN1 variants determine the pathogenicity of the mutation and predict age at disease onset. Mol. Psychiatr. 27, 2821–2832. doi: 10.1038/s41380-022-01518-6
Petit, D., Hitzenberger, M., Koch, M., Lismont, S., Zoltowska, K. M., Enzlein, T., et al. (2022b). Enzyme–substrate interface targeting by imidazole-based γ-secretase modulators activates γ-secretase and stabilizes its interaction with APP. EMBO J. 41, e111084. doi: 10.15252/embj.2022111084
Quartey, M. O., Nyarko, J. N. K., Maley, J. M., Barnes, J. R., Bolanos, M. A. C., Heistad, R. M., et al. (2021). The Aβ(1–38) peptide is a negative regulator of the Aβ(1–42) peptide implicated in Alzheimer disease progression. Sci. Rep. 11, 431. doi: 10.1038/s41598-020-80164-w
Ratni, H., Alker, A., Bartels, B., Bissantz, C., Chen, W., Gerlach, I., et al. (2020). Discovery of RO7185876, a highly potent γ-secretase modulator (GSM) as a potential treatment for Alzheimer's disease. ACS Med. Chem. Lett. 11, 1257–1268. doi: 10.1021/acsmedchemlett.0c00109
Robertson, A. S., Iben, L. G., Wei, C., Meredith, J. E., Drexler, D. M., Banks, M., et al. (2017). Synergistic inhibition of Aβ production by combinations of γ-secretase modulators. Eur. J. Pharmacol. 812, 104–112. doi: 10.1016/j.ejphar.2017.07.019
Rogers, K., Felsenstein, K. M., Hrdlicka, L., Tu, Z., Albayya, F., Lee, W., et al. (2012). Modulation of γ-secretase by EVP-0015962 reduces amyloid deposition and behavioral deficits in Tg2576 mice. Mol. Neurodegen. 7, 61. doi: 10.1186/1750-1326-7-61
Ross, J., Sharma, S., Winston, J., Nunez, M., Bottini, G., Franceschi, M., et al. (2012). CHF5074 reduces biomarkers of neuroinflammation in patients with mild cognitive impairment: a 12-week, double-blind, placebo-controlled study. Curr. Alzheimer Res. 10, 742–753. doi: 10.2174/13892037113149990144
Rynearson, K. D., Ponnusamy, M., Prikhodko, O., Xie, Y., Zhang, C., Nguyen, P., et al. (2021). Preclinical validation of a potent γ-secretase modulator for Alzheimer's disease prevention. J. Exp. Med. 218, 2560. doi: 10.1084/jem.20202560
Sandin, J., Dahlström, M., Lidell, V., Rasti, A., Forsell, P., Juric, S., et al. (2022). AC-0027875, a novel gamma-secretase modulator for the treatment of Alzheimers's disease. J. Preven. Alzheimers Dis. 9(Suppl. 1), S82, LP21. doi: 10.14283/jpad.2022.97
Sims, J. R., Zimmer, J. A., Evans, C. D., Lu, M., Ardayfio, P., Sparks, J., et al. (2023). Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA 330, 512. doi: 10.1001/jama.2023.13239
Soares, H. D., Gasior, M., Toyn, J. H., Wang, J.-S., Hong, Q., Berisha, F., et al. (2016). The γ-secretase modulator, BMS-932481, modulates Aβ peptides in the plasma and cerebrospinal fluid of healthy volunteers. J. Pharmacol. Exp. Therap. 358, 138–150. doi: 10.1124/jpet.116.232256
Sturchio, A., Dwivedi, A. K., Malm, T., Wood, M. J. A., Cilia, R., Sharma, J. S., et al. (2022). High soluble amyloid-β42 predicts normal cognition in amyloid-positive individuals with Alzheimer's disease-causing mutations. J. Alzheimer Dis. 90, 333–348. doi: 10.3233/JAD-220808
Sturm, S., Portron, A., Vogt, A., Poirier, A., Yang, T., Abdi, A. M., et al. (2023). OC31-RG6289, a New-Secretase Modulator for the Treatment of Alzheimer's Disease: Results from a Phase I Healthy Volunteer Study. Oral communication at CTAD October 23–27 2023 (Boston, MA).
Sun, L., Zhou, R., Yang, G., and Shi, Y. (2017). Analysis of 138 pathogenic mutations in presenilin-1 on the in vitro production of Aβ42 and Aβ40 peptides by γ-secretase. Proc. Natl. Acad. Sci. 114, E476–E485. doi: 10.1073/pnas.1618657114
Surguchov, A., Emamzadeh, F. N., Titova, M., and Surguchev, A. A. (2023). Controversial properties of amyloidogenic proteins and peptides: new data in the COVID era. Biomedicines 11, 1215. doi: 10.3390/biomedicines11041215
Szaruga, M., Munteanu, B., Lismont, S., Veugelen, S., Horré, K., Mercken, M., et al. (2017). Alzheimer's-causing mutations shift Aβ length by destabilizing γ-secretase-Aβn interactions. Cell 170, 443–456.e14. doi: 10.1016/j.cell.2017.07.004
Takami, M., Nagashima, Y., Sano, Y., Ishihara, S., Morishima-Kawashima, M., Funamoto, S., et al. (2009). Gamma-secretase: successive tripeptide and tetrapeptide release from the transmembrane domain of -carboxyl terminal fragment. J. Neurosci. 29, 13042–13052. doi: 10.1523/JNEUROSCI.2362-09.2009
Toyn, J. H., Boy, K. M., Raybon, J., Meredith, J. E., Robertson, A. S., Guss, V., et al. (2016). Robust translation of γ-secretase modulator pharmacology across preclinical species and human subjects. J. Pharmacol. Exp. Therap. 358, 125–137. doi: 10.1124/jpet.116.232249
Toyn, J. H., Thompson, L. A., Lentz, K. A., Meredith, J. E., Burton, C. R., Sankaranararyanan, S., et al. (2014). Identification and preclinical pharmacology of the γ-secretase modulator BMS-869780. Int. J. Alzheimers Dis. 2014, 431858. doi: 10.1155/2014/431858
Vellas, B., and Aisen, P. (2021). New hope for Alzheimer's disease. J. Prev. Alzheimers Dis. 8, 238–239. doi: 10.14283/jpad.2021.26
Veugelen, S., Saito, T., Saido, T. C., Chávez-Gutiérrez, L., and De Strooper, B. (2016). Familial Alzheimer's disease mutations in presenilin generate amyloidogenic Aβ peptide seeds. Neuron 90, 410–416. doi: 10.1016/j.neuron.2016.03.010
Voytyuk, I., Strooper, B. D., and Chávez-Gutiérrez, L. (2018). Modulation of γ- and β-secretases as early prevention against Alzheimer's disease. Biol. Psychiatry 83, 320–327. doi: 10.1016/j.biopsych.2017.08.001
Wan, H. I., Jacobsen, J. S., Rutkowski, J. L., and Feuerstein, G. Z. (2009). Translational medicine lessons from Flurizan's failure in Alzheimer's disease (AD) trial: implication for future drug discovery and development for AD. Clin. Transl. Sci. 2, 242–247. doi: 10.1111/j.1752-8062.2009.00121.x
Wanngren, J., Ottervald, J., Parpal, S., Portelius, E., Strömberg, K., Borgegård, T., et al. (2012). Second generation γ-secretase modulators exhibit different modulation of notch β and Aβ production. J. Biol. Chem. 287, 32640–32650. doi: 10.1074/jbc.M112.376541
Weber, T. A., Lundkvist, J., Wanngren, J., Kvartsberg, H., Jin, S., Larssen, P., et al. (2021). γ-Secretase modulators show selectivity for γ-secretase–mediated amyloid precursor protein intramembrane processing. J. Cell Mol. Med. 26, 880–892. doi: 10.1111/jcmm.17146
Weggen, S., Eriksen, J. L., Das, P., Sagi, S. A., Wang, R., Pietrzik, C. U., et al. (2001). A subset of NSAIDs lower amyloidogenic Aβ42 independently of cyclooxygenase activity. Nature 414, 212–216. doi: 10.1038/35102591
Welander, H., Frånberg, J., Graff, C., Sundström, E., Winblad, B., and Tjernberg, L. O. (2009). Aβ43 is more frequent than Aβ40 in amyloid plaque cores from Alzheimer disease brains. J. Neurochem. 110, 697–706. doi: 10.1111/j.1471-4159.2009.06170.x
Wessels, A. M., Lines, C., Stern, R. A., Kost, J., Voss, T., Mozley, L. H., et al. (2020). Cognitive outcomes in trials of two BACE inhibitors in Alzheimer's disease. Alzheimers Dement. 16, 1483–1492. doi: 10.1002/alz.12164
Witt, A., Macdonald, N., and Kirkpatrick, P. (2004). Memantine hydrochloride. Nat. Rev. Drug Discov. 3, 109–110. doi: 10.1038/nrd1311
Wolfe, M. S. (2021). Targeting γ-secretase for familial Alzheimer's disease. Med. Chem. Res. 30, 1321–1327. doi: 10.1007/s00044-021-02744-3
Xia, W. (2019). γ-Secretase and its modulators: twenty years and beyond. Neurosci. Lett. 701, 162–169. doi: 10.1016/j.neulet.2019.02.011
Yan, R. (2017). Physiological functions of the β-site amyloid precursor protein cleaving enzyme 1 and 2. Front. Mol. Neurosci. 10, 97. doi: 10.3389/fnmol.2017.00097
Yang, G., Zhou, R., Guo, X., Yan, C., Lei, J., and Shi, Y. (2021). Structural basis of γ-secretase inhibition and modulation by small molecule drugs. Cell 184, 521–533.e14. doi: 10.1016/j.cell.2020.11.049
Yu, Y., Logovinsky, V., Schuck, E., Kaplow, J., Chang, M., Miyagawa, T., et al. (2014). Safety, tolerability, pharmacokinetics, and pharmacodynamics of the novel γ-secretase modulator, E2212, in healthy human subjects. J. Clin. Pharmacol. 54, 528–536. doi: 10.1002/jcph.249
Zhang, H., Wei, W., Zhao, M., Ma, L., Jiang, X., Pei, H., et al. (2021). Interaction between Aβ and tau in the pathogenesis of Alzheimer's disease. Int. J. Biol. Sci. 17, 2181–2192. doi: 10.7150/ijbs.57078
Keywords: gamma-secretase, gamma-secretase modulator, GSM, Alzheimer's disease, APP, amyloid-beta
Citation: Nordvall G, Lundkvist J and Sandin J (2023) Gamma-secretase modulators: a promising route for the treatment of Alzheimer's disease. Front. Mol. Neurosci. 16:1279740. doi: 10.3389/fnmol.2023.1279740
Received: 18 August 2023; Accepted: 15 September 2023;
Published: 16 October 2023.
Edited by:
Andrei Surguchov, University of Kansas Medical Center, United StatesReviewed by:
Irina G. Sourgoutcheva, University of Kansas Medical Center, United StatesMoore Arnold, Biogen Idec, United States
Copyright © 2023 Nordvall, Lundkvist and Sandin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gunnar Nordvall, Z3VubmFyLm5vcmR2YWxsQGFsemVjdXJlcGhhcm1hLmNvbQ==
 Gunnar Nordvall
Gunnar Nordvall Johan Lundkvist
Johan Lundkvist Johan Sandin1,2
Johan Sandin1,2