
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mol. Neurosci. , 15 July 2022
Sec. Pain Mechanisms and Modulators
Volume 15 - 2022 | https://doi.org/10.3389/fnmol.2022.960460
This article is part of the Research Topic Molecular Mechanism of Neuroimmune Modulation and Synaptic Plasticity in Acute and Chronic Pain View all 19 articles
Screening serum biomarkers for acute and subacute pain is important for precise pain management. This study aimed to examine serum levels of angiogenic factors in patients with acute and subacute pain as potential biomarkers. Serum samples were collected from 12 healthy controls, 20 patients with postherpetic neuralgia (PHN), 4 with low back pain (LBP), and 1 with trigeminal neuralgia (TN). Pain intensity in these patients was evaluated using the visual analog scale (VAS). The serum concentrations of 11 angiogenic biomarkers were examined by Milliplex Map Human Angiogenesis Magnetic Bead Panel 2. The pain assessment from VAS showed that all patients showed moderate and severe pain. Among 11 angiogenic factors, osteopontin (OPN), thrombospondin-2 (TSP-2), soluble platelet endothelial cell adhesion molecule-1 (sPECAM-1), soluble urokinase-type plasminogen activator receptor (suPAR), and soluble epidermal growth factor receptors (sErbB2) were up-regulated and soluble interleukin-6 receptor α (sIL-6Rα) were down-regulated in patients with pain compared to the healthy participants (all P-values were < 0.005). Moreover, a linear regression model showed that the serum OPN concentration was correlated with pain intensity in patients with PHN (P = 0.03). There was no significant difference between the serum concentration of soluble epidermal growth factor receptors, sErbB3, soluble AXL, tenascin, and soluble neuropilin-1 in patients with acute and subacute pain and that of healthy controls. The results of this study provided new valuable insights into our understanding of angiogenic factors that may contribute to as mechanistic biomarkers of pain, and reveal the pathophysiological mechanism of pain.
Clinical Trial Registration: www.chictr.org.cn, identifier ChiCTR2200061775.
Pain may be conceived as a disease in conditions, such as fibromyalgia or non-specific low back pain (LBP) (Reckziegel et al., 2019; Treede et al., 2019). More popularly, pain is a leading complaint in some diseases such as postherpetic neuralgia (PHN), trigeminal neuralgia (TN), peripheral nerve injury, painful polyneuropathy, etc. (Scholz et al., 2019; Treede et al., 2019). The cost of acute or subacute pain due to direct medical treatment and productivity lost represents a heavy economic burden. Moreover, therapeutic management of pain is challenging, and complete relief is uncommon, owing to efficacy limitations of current treatments (Scholz et al., 2019). It is also intricately related to opiate addiction and has a significant impact on disability and overdose deaths. To combat these massive societal burdens, more effort is required to develop novel tools and therapies for combating chronic pain and reducing reliance on opiates for managing chronic pain (Reckziegel et al., 2019). This effort has emphasized the urgent need to develop validated biomarkers, with the assumption that such biomarkers would, in turn, facilitate mechanically driven development of novel therapies (Reckziegel et al., 2019). The current study aim to identify new biomarkers for acute and subacute pain by examining blood samples of patients with pain, and to identify clues for pain pathogenesis.
Angiogenesis, which is the formation of new blood vessels from the endothelium of the existing vasculature, is a fundamental process that occurs in physiological and pathophysiological conditions (Sajib et al., 2018). Insufficient or excessive blood vessel growth underlies many diseases, including cardiovascular and cerebrovascular diseases, osteoarthritis and cancer (Lawton et al., 2015; Viallard and Larrivée, 2017; Sajib et al., 2018). Normal angiogenesis and angiogenic signaling in pathological conditions are mediated by soluble growth factors, membrane-bound receptors, and cell-cell and cell-matrix interactions (Sajib et al., 2018). These factors may also play a role in pain pathogenesis by enhancing inflammation and inappropriate sensory innervation of local tissues (Mapp and Walsh, 2012). Angiogenesis-related signaling factors such as interleukin-1β (IL-1β), IL-6, tumor necrosis factor-α, and cyclooxygenase 2 are known to contribute to pain hypersensitivity by inducing the production of prostaglandins and other proalgesic agents, which activate nociceptors (Mafu et al., 2018). Moreover, previous studies have shown that angiogenesis contributes to pain in osteoarthritis patients and shoulder pain of breast cancer survivors (Mapp and Walsh, 2012; Mafu et al., 2018). All of the above studies shed light on the role of angiogenesis in pain pathogenesis.
We examined the serum levels of 11 angiogenic factors in patients with PHN, LBP, or TN using the Millipore Milliplex Map Human Angiogenesis Magnetic Bead Panel 2 assay and evaluated the correlations of these factors with pain intensity in PHN patients. These 11 angiogenic factors included osteopontin (OPN), thrombospondin-2 (TSP-2), soluble platelet endothelial cell adhesion molecule-1 (sPECAM-1), soluble urokinase-type plasminogen activator receptor (suPAR), soluble epidermal growth factor receptors (sEGFR/sHER1/sErbB1), soluble human epidermal growth factor receptor 2 (sEGFR2/sErbB2s/HER2), sErbB3 (sEGFR3/sHER3), soluble interleukin-6 receptor α (sIL-6Rα), soluble AXL (sAXL), tenascin C (TN-C), and soluble neuropilin-1 (sNRP-1). We will observe the expression levels of their homologous genes in the dorsal root ganglion (DRG), spinal cord, and the key parts of the sensory nervous system using a mouse neuropathic pain model in the next step.
All the participants were recruited from the Tianjin Union Medical Centre, Tianjin, China. There were 20 patients with PHN, 4 with LBP, and 1 with TN. All the patients were diagnosed by clinically qualified doctors. Twelve age-matched healthy participants without pain-related diseases or conditions were enrolled from the physical examination center of the Tianjin Union Medical Centre. Baseline characteristics (age and sex) of the participants are listed in Table 1. All the patients were notified of no analgesic medication 24 h before the study visit. Ethical approval for this research was approved by the ethical committee of Tianjin Union Medical Centre (approval number: 2016-B08).
All the patients and healthy participants completed a visual analog scale (VAS) questionnaire for pain. The participants were asked to rate their current pain intensity on a VAS, ranging between “0–10.” A VAS of “0” represents “no pain”; “1–3” represents mild tolerable pain; “4–6” represents moderate tolerable pain and sleep was disturbed. A VAS pain score from of “7–10” represents intense intolerable pain and both sleep and diet were disturbed.
Blood samples from the participants were collected into 10 mL sterile tubes containing no additives. The samples were immediately centrifuged at 1,000 × g for 10 min and sediment-free serum samples were obtained, aliquoted, and frozen at –80°C until further analysis.
Serum concentrations of the 11 angiogenic factors (OPN, TSP-2, sPECAM-1, suPAR, sHER2, sIL-6Rα, sEGFR, sHER3, sAXL, TN-C, and sNRP-1) were measured by commercially available Milliplex Map Human Angiogenesis Magnetic Bead Panel 2 assay (EMD Millipore, Darmstadt, Germany). The serum samples were diluted to 1:5 before use. All procedures were carried out according to manufacturer’s the instructions and all the samples were tested along with the quality controls and standard samples provided in the kit. After all steps were completed, the 96-well plate was placed onto Luminex® 200™ and analyzed using the Bio-Plex Manager software. The equipment settings were provided in the instruction book. Results were expressed in nanogram per milliliter (ng/mL) for the serum levels.
Serum levels of the angiogenic factors were evaluated using absolute concentration values. Data are reported as mean ± standard error of the mean (SEM). Comparisons of each angiogenic factor between the patients with pain and healthy controls was performed using the Student’s t-test.
Correlations between the concentration of the angiogenic factors and pain intensity or duration in patients with PHN were determined using linear regression analysis. The F-test was used to determine statistical significance, with P < 0.05 considered as significant. Spearman’s order correlation was performed to examine the correlation and calculate the correlation coefficients.
All analyses were performed using Statistical Package for the Social Sciences (SPSS; Chicago, IL, United States). Statistical significance was set at P < 0.05.
Descriptive characteristics of the patients are shown in Table 1. Male and female patients were enrolled randomly in each group (Table 1). All the patients were aged > 55 years. Pain duration varied among the patients (Table 2). According to the classification of acute, subacute and chronic pain, most patients with PHN were classified as having acute pain with a duration less than 3 months. There was only one patient with subacute pain and two with chronic pain in the PHN patients. There was one LBP patient with acute pain and three LBP patients with subacute pain. One patient with TN was classified as having subacute pain. All the patients experienced medium to severe pain (Figure 1). The mean VAS was 8.7 in the PHN patients and 6.3 in the LBP patients, respectively. The mean VAS score of the TN patient was 10.

Figure 1. Comparison of visual analog scale (VAS) in healthy controls and patients with postherpetic neuralgia (PHN), low back pain (LBP), or trigeminal neuralgia (TN). ***P < 0.001.
The mean ± S.E.M. levels of the serum angiogenic factors are shown in Table 3. Serum OPN, TSP-2, sPECAM-1, suPAR, and sErbB2 levels were significantly higher in patients with PHN (Figures 2A–E), while the serum sIL-6Rα levels were lower in the PHN patients than in the healthy controls (Figure 2F). Similarly, in patients with LBP, serum levels of TSP-2, sPECAM-1, sHER2, and suPAR were significantly increased, while serum sIL-6Rα levels were significantly decreased compared to those in the healthy control group (Figures 2B–F). However, the serum OPN level in patients with LBP didn’t differ from those in the healthy controls (Figure 2A). The serum concentrations of OPN, TSP-2, sPECAM-1, sErbB2, and suPAR in TN patients were higher than those in the healthy controls (Figures 2A–E), while serum sIL-6Rα in the TN patient was lower than the mean value of the healthy controls (Figure 2F). The other five angiogenic factors, including sEGFR, sErbB3, sAXL, TN-C, and sNRP-1 didn’t show any significant differences in patients with pain compared to the healthy controls (Figures 2G–K).
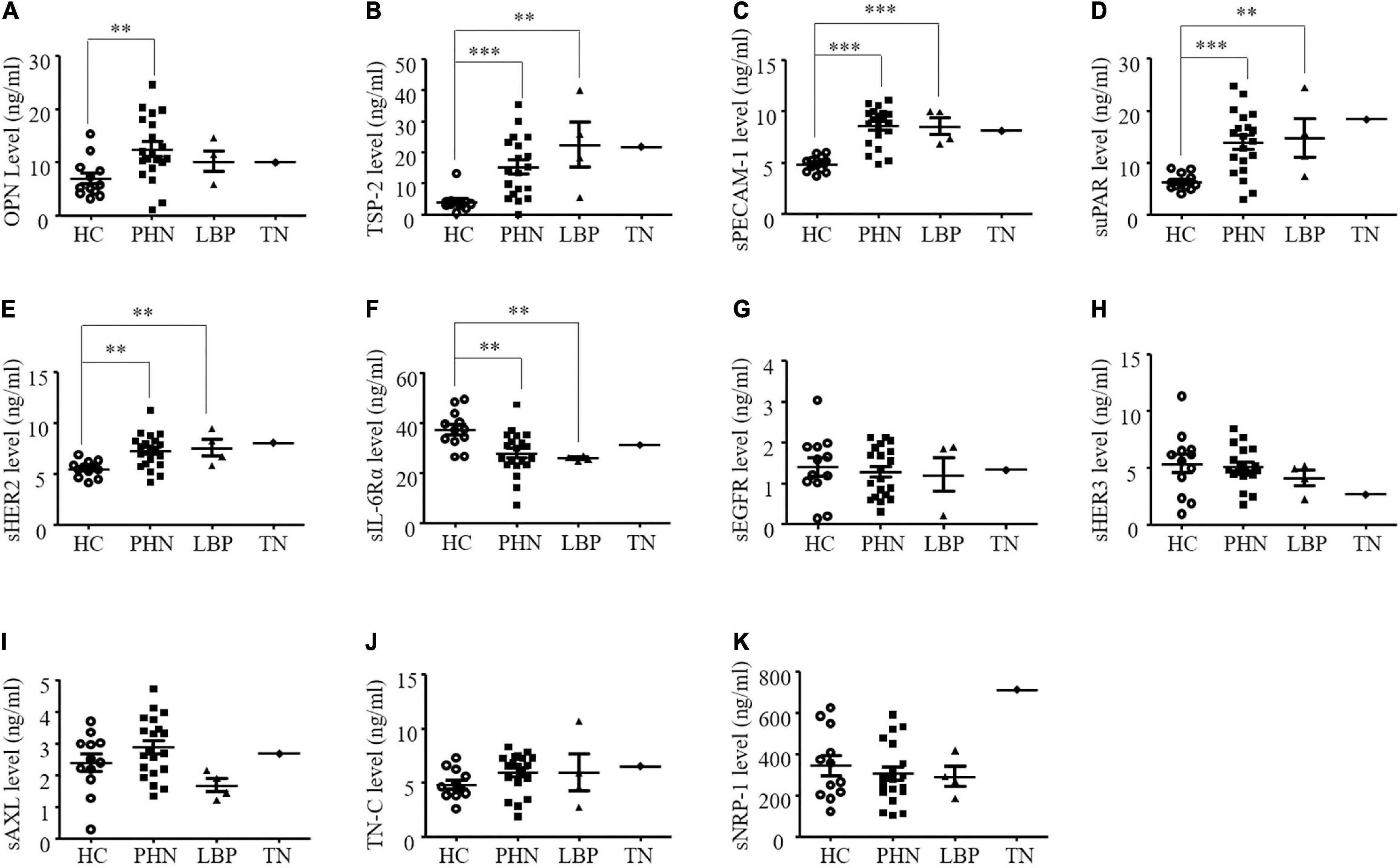
Figure 2. (A-K) Comparison of serum angiogenic factors in healthy controls (HC) and patients with postherpetic neuralgia (PHN), low back pain (LBP), or trigeminal neuralgia (TN). **P < 0.01; ***P < 0.001. OPN, osteopontin; TSP-2, thrombospondin-2; sPECAM-1, soluble platelet endothelial cell adhesion molecule-1; suPAR, soluble urokinase-type plasminogen activator receptor; sErbB2, soluble human epidermal growth factor receptor 2; sIL-6Rα, soluble interleukin-6 receptor α; sEGFR, soluble epidermal growth factor receptors; sErbB3, soluble human epidermal; sAXL, soluble AXL; TN-C, tenascin C; sNRP-1, solubleneuropilin-1.
The altered angiogenic factors including OPN, TSP-2, sPECAM-1, sHER2, suPAR, and sIL-6Rα in the PHN patients were further analyzed according to pain intensity and duration (Figure 3). We found that only serum OPN level was significantly correlated with VAS scores in the patients with PHN (P = 0.03, Figure 3A). The correlation coefficient between serum OPN level and VAS scores in patients with PHN was 0.49. Other factors were not significantly correlated with VAS in the PHN patients although higher levels of TSP-2, sPECAM-1 sErbB2, suPAR and a lower level of sIL-6Rα were found in the serum of PHN patients (Figures 3B–F). None of these angiogenic factors were significantly correlated with pain duration (Figures 4A–F).
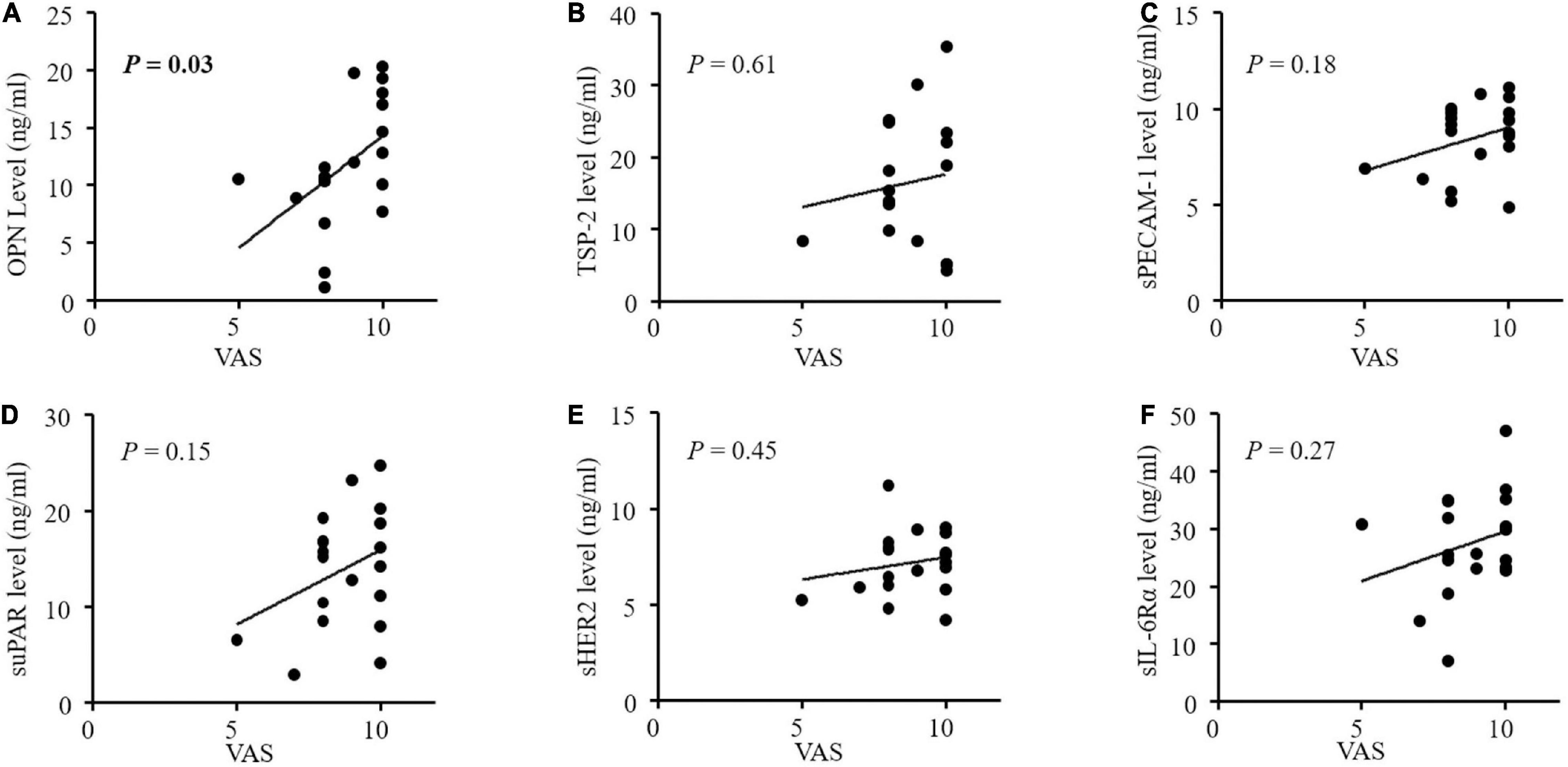
Figure 3. (A-F) Correlations between concentrations of angiogenic factors and pain intensity by VAS in patients with postherpetic neuralgia (PHN). Only serum OPN levels were significantly correlated with VAS in PHN patients (P = 0.03). OPN, osteopontin; TSP-2, thrombospondin-2; sPECAM-1, soluble platelet endothelial cell adhesion molecule-1; suPAR, soluble urokinase-type plasminogen activator receptor; s ErbB2, soluble human epidermal growth factor receptor 2; sIL-6Rα, soluble interleukin-6 receptor α; VAS, visual analog scale.
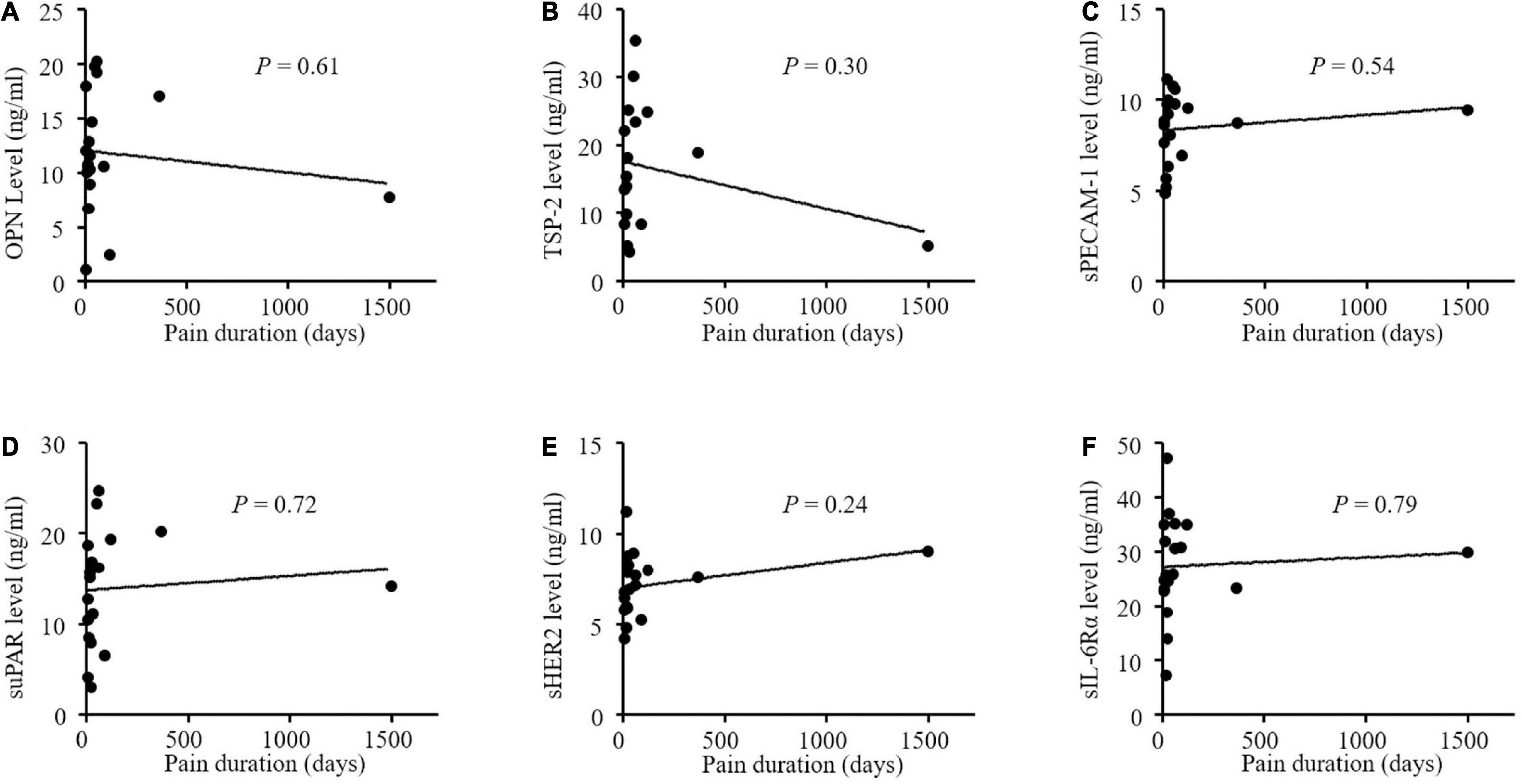
Figure 4. (A-F) No correlations between concentrations of angiogenic factors and pain duration in patients with postherpetic neuralgia (PHN). OPN indicates osteopontin; TSP-2, thrombospondin-2; sPECAM-1, soluble platelet endothelial cell adhesion molecule-1; suPAR, soluble urokinase-type plasminogen activator receptor; s ErbB2, soluble human epidermal growth factor receptor 2; sIL-6Rα, soluble interleukin-6 receptor α; VAS, visual analog scale.
The objective of this study was to compare the levels of angiogenic factors in the sera of patients with pain and healthy controls. We found that higher levels of OPN, TSP-2, sPECAM-1, sErbB2, and suPAR and lower levels of sIL-6Rα were up-regulated and sIL-6Rα were down-regulated in those patients with pain compared to the healthy participants. Moreover, serum OPN levels were correlated with pain intensity in patients with PHN. These angiogenic factors may be involved in the pathogenesis of pain, and their soluble part in the blood can serve as biochemical indicators of acute or subacute pain.
The biological functions of OPN are diverse as it can be beneficial for wound healing, bone homeostasis, and extracellular matrix function, and deleterious in cardiovascular diseases, cancer, diabetes, and kidney stone diseases (Icer and Gezmen-Karadag, 2018; Lamort et al., 2019). Circulating OPN has also been indicated in some pain conditions as elevated plasma OPN levels have been found in patients with pain and unstable angina or multiple sclerosis (Soejima et al., 2006; Agah et al., 2018). A statistically significant correlation between OPN in synovial fluid and pain has been found in symptomatic patients with primary knee osteoarthritis and in patients who underwent anterior cruciate ligament reconstruction surgery (Yamaga et al., 2012; Calvet et al., 2018). Osteopontin has been reported to upregulate the expression of IL-6 and IL-8 cytokines in chondrocytes isolated from human osteoarthritis knee cartilage (Yang et al., 2014). Moreover, OPN in the peripheral DRG may contribute to the mechanisms of neuropathic pain. In the DRG, 25% of neurons were immunoreactive for OPN. These neurons were mostly large and exhibited parvalbumin-immunoreactivity, but not calcitonin gene-related peptide immunoreactivity (Ichikawa et al., 2000). Nerve injury induced an increase in OPN in DRG sensory neurons, and OPN knockout reversed mechanical hypersensitivity in mice with neuropathic pain (Ichikawa et al., 2000; Marsh et al., 2007). In the present study, we found an increased OPN levels in the sera of patients with PHN, which was positively associated with pain intensity in the PHN patients. This strongly supports that OPN exerts a role in pain pathogenesis, which may serve as a potential target for pain management.
Platelet endothelial cell adhesion molecule-1, also called CD31, is a 130-kDa protein belonging to the Ig superfamily; it has distinct variants and therefore different isoforms which allow diversity of PECAM-1 functions, including inflammation, angiogenesis and vascular development (Kalinowska and Losy, 2006). Splicing of exon 9, which encodes the transmembrane domain, results in the formation sPECAM-1, a soluble form of PECAM-1 (Goldberger et al., 1994) may result in PECAM-1 inhibition (Kalinowska and Losy, 2006). Thus, PECAM-1 is also involved in pain. For example, the sevenfold higher density of PECAM-1-positive capillaries in the cell body-rich area of mouse DRG than in the cell fiber-rich area of the DRG or the sciatic nerve facilitated many potentially neurotoxic agents by preferentially accumulating and injuring cells within the DRG, thereby inducing peripheral sensory neuropathy (Jimenez-Andrade et al., 2008). Moreover, PECAM-1 may also contribute to spinal mechanisms of pain because peripheral nerve injury induces an increase in PECAM-1 immunoreactivity in related spinal cord (Rutkowski et al., 2002; Sweitzer et al., 2002). In the blood circulation, PECAM-1 may mediate the analgesic effect of endomorphin on inflammatory pain by recruiting immunocytes containing β-endorphin to sites of painful inflammation (Sweitzer et al., 2002). The above studies have suggested various roles of PECAM-1 in multilevel pain transmission and various pain conditions. Herein, we found an increase in serum sPECAM-1 levels in patients with pain. Soluble PECAM-1, which may originate from endothelial cells, along with PECAM-1, may result in altered immune cell-endothelial cell interactions and thus, affect pain behaviors under pain conditions (Goldberger et al., 1994; Onore et al., 2012).
ErbB2 is a member of the EGFR family of receptor tyrosine kinases, which comprises ErbB1/EGFR/HER1, ErbB2/HER2, ErbB3/HER3, and ErbB4/HER4 (Moasser, 2007; Ho et al., 2017). ErbB2 is thought to be an oncogene and has become a target for a number of targeted anti-cancer drugs (Moasser, 2007; Al-Dasooqi et al., 2009). Soluble ErbB2, generated from ErbB2 proteolytic cleavage, includes only the extracellular domain of ErbB2 (Perrier et al., 2018). Soluble HER2 has been detected in the blood of cancer patients, and anticancer strategies targeting it are being developed (Dokala and Thakur, 2017; Perrier et al., 2018). In the present study, we found an increase in sErbB2 but not in sErbB1 or sErbB3 in patients with pain. Clinical and experimental studies have shown that EGFR/ErbB1 inhibitors can relieve neuropathic and breast cancer-related pain (Kaufman et al., 2010; Kersten and Cameron, 2012; Kersten et al., 2013, 2015, 2019; Martin et al., 2017; Wang et al., 2019). Moreover, EGFR/ErbB1 has been implicated in peripheral mechanisms of neuropathic pain (Martin et al., 2017; Wang et al., 2019). Therefore, ErbB2 signaling may be involved in the pathogenesis of pain. However, further studies are required to support this hypothesis.
Interleukin-6 is a key cytokine in many inflammatory and autoimmune diseases and IL-6, as well as IL-6 signaling, have been implicated in pain pathogenesis (Kawasaki et al., 2008; Assier et al., 2010; Boettger et al., 2010; Rendina et al., 2018). The IL-6 receptor is a heterodimer composed of an α chain (IL-6Rα) and a transmembrane chain gp130. Interleukin-6 can also induce cell signaling via sIL-6Rα which is generated by proteolysis of the transmembrane form of IL-6Rα, or by alternative splicing of the messenger RNAs for the α chain. Soluble IL-6Rα allows cells that do not express IL-6Rα to respond to IL-6 (Assier et al., 2010). Increased sIL-6R levels have been found in the sera of Paget disease patients with LBP, suggesting an enhanced transmission of IL-6 signaling in the specialized neural system linked to irregular perception in these patients (Rendina et al., 2018). In DRGs, IL-6 and IL-6R mRNA are expressed in DRG neurons and satellite glial cells, and are significantly elevated in response to peripheral nerve injury (Brázda et al., 2013). Moreover, IL-6, as well as sIL-6Rα, induced long-lasting robust sensitization of joint nociceptors to mechanical stimuli in an experimental arthritis model (Boettger et al., 2010). Spinal injection of sIL-6Rα produces heat hyperalgesia and sIL-6Rα and IL-6 enhance spinal central sensitization (Kawasaki et al., 2008). All these studies provide strong evidence of the close correlation between IL-6 signaling and pathological pain development. Currently, we observed decreased levels of sIL-6Rα in the sera of patients with pain. As a high level of sIL-6Rα is indicative of inflammation and increased nociception, sIL-6Rα levels in these patients with acute or subacute pain conflicts with our expectation. A study on a monoclonal antibody (tocilizumab) to transmembrane and sIL-6Rα showed that tocilizumab was effective in patients with rheumatoid arthritis although tocilizumab increased serum levels of IL-6 and sIL-6Rα. It has been suggested that sIL-6Rα is taken up by immune complexes which might increase the half-life of IL-6Rα and sIL-6Rα (Nishimoto et al., 2008). Therefore, an unidentified complex may be responsible for the decrease in sIL-6Rα levels in the blood of patients with pain observed in the current study.
Urokinase-type plasminogen activator receptor is a glycosyl-phosphatidylinositol-anchored membrane glycoprotein belonging to the plasminogen activator system (Irigoyen et al., 1999; Thunø et al., 2009). Soluble uPAR, a free soluble receptor, is cleaved from uPAR. Soluble uPAR levels can be determined in the blood and have been used as biomarkers for chronic inflammatory conditions (Thunø et al., 2009). Elevated serum suPAR levels have been found in patients with chest pain osteoarthritis and migraine with attacks and aura (Tang et al., 2010; Lyngbæk et al., 2013; Yılmaz et al., 2017). Similarly, we postulate that suPAR and its related plasminogen activator system may be involved in the pathogenesis of pain. Urokinase-type plasminogen activator receptor mRNA was found in small and large DRG neurons (Hayden and Seeds, 1996; Siconolfi and Seeds, 2001). Sciatic nerve crush leads to elevated expression of uPAR, tissue-type plasminogen activator (tPA), and urokinase plasminogen activator (uPA) in DRG neurons (Siconolfi and Seeds, 2001). In the future, it will be important to determine the underlying mechanisms for the release of suPAR into circulation under acute or subacute pain conditions and to investigate the role of the plasminogen activator system in acute pain or subacute pain development.
Thrombospondin-2, a matricellular glycoprotein of the thrombospondin family, regulates multiple biological functions, including proliferation, angiogenesis, cell adhesion, and extracellular matrix modeling (Bornstein et al., 2000; Adams and Lawler, 2011). High TSP-2 levels in human sera and tissues have been reported in various patients (Neuschwander-Tetri et al., 2006; Farrow et al., 2009; Naumnik et al., 2015; Liu et al., 2018). Herein, we demonstrated a high serum TSP-2 level in patients with pain. Although the exact mechanisms linking serum TSP-2 and pain conditions are not well-understood, it has been found that TSP-4, another member of the thrombospondin family, contributes to spinal centralization by regulating the calcium channel Cavα2δ-1 in the spinal dorsal horn (Park et al., 2016). Therefore, investigation of TSP-2 expression and the local effects of TSP-2 in the pain pathway may provide clues on how TSP-2 is involved in pain pathogenesis.
We found significant changes in OPN, TSP-2, sPECAM-1, sErbB2, suPAR, and sIL-6Rα levels in the serum of patients with pain. Moreover, the serum OPN concentration may indicate pain severity. The results from this study provide new valuable insights into our understanding of angiogenic factors that may contribute to mechanistic biomarkers of pain to reveal the pathophysiological mechanisms of acute and subacute pain.
The present study showed significant changes in OPN, TSP-2, sPECAM-1, sErbB2, suPAR, and sIL-6Rα levels in the serum of patients with pain, especially with PHN. Moreover, the serum OPN concentration may indicate pain severity. Based on our findings, we hypothesized that elevated levels of circulating angiogenic factors contribute to the development of pain pathogenesis. Further studies should focus on their local effects and functions in the pain pathway, such as in the dorsal root ganglion and spinal cord, to determine their contribution to pain pathogenesis. To date, the expression of genes encoding these altered angiogenic factors has been found to be increased in the injured DRG in an experimental neuropathic pain model based on RNA-sequencing analysis (Wu et al., 2016). However, it needs to be noted that the correlation of circulating angiogenic factors and their local expression is not always consistent. For example, investigation of EGFR and AXL in DRG sensory neurons showed that they are involved in the peripheral mechanism of neuropathic pain although we didn’t observe a significant change in sEGFR and sAXL in the sera of patients with pain (Martin et al., 2017; Wang et al., 2019). Therefore, serum indices unquestionably enlighten some ideas on pain pathogenesis, but further experimental animal studies need to be performed to explore precise mechanisms.
A limitation of our study was the study group selection. Healthy controls were chosen from among age-matched elderly individuals who may have various other diseases, which may have affected serum angiogenic factor concentrations. Another limitation of this study was the inadequate number of patients, especially those with LBP and TN. We recruited only one patient with TN; therefore, the data could not be statistically analyzed. Further large-scale studies are required to define the role of these markers in patients with pain.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethical Committee of Tianjin Union Medical Center. The patients/participants provided their written informed consent to participate in this study.
XY and JZ: conceptualization. HW and YW: methodology. HW and ML: formal analysis. XY and CY: writing—original draft preparation. JZ and ZL: writing—review and editing. CY: visualization. XY: supervision. JZ and XY: project administration and funding acquisition. All authors have read and agreed to the published version of the manuscript.
This work was supported by the Applied Basic Research Joint Project of Tianjin (Item No. 21JCYBJC00030) and Tianjin Health Research Project (No. ZC20035).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We are grateful to the study participants for their continued participation.
Adams, J. C., and Lawler, J. (2011). The thrombospondins. Cold Spring Harb. Perspect. Biol. 3:a009712. doi: 10.1101/cshperspect.a009712
Agah, E., Zardoui, A., Saghazadeh, A., Ahmadi, M., Tafakhori, A., and Rezaei, N. (2018). Osteopontin (OPN) as a CSF and blood biomarker for multiple sclerosis: a systematic review and meta-analysis. PLoS One 13:e0190252. doi: 10.1371/journal.pone.0190252
Al-Dasooqi, N., Gibson, R., Bowen, J., and Keefe, D. (2009). HER2 targeted therapies for cancer and the gastrointestinal tract. Curr. Drug Targets 10, 537–542. doi: 10.2174/138945009788488440
Assier, E., Boissier, M. C., and Dayer, J. M. (2010). Interleukin-6: from identification of the cytokine to development of targeted treatments. Joint Bone Spine 77, 532–536. doi: 10.1016/j.jbspin.2010.07.007
Boettger, M. K., Leuchtweis, J., Kümmel, D., Gajda, M., Bräuer, R., and Schaible, H. G. (2010). Differential effects of locally and systemically administered soluble glycoprotein 130 on pain and inflammation in experimental arthritis. Arthritis Res. Ther. 12:R140. doi: 10.1186/ar3079
Bornstein, P., Armstrong, L. C., Hankenson, K. D., Kyriakides, T. R., and Yang, Z. (2000). Thrombospondin 2, a matricellular protein with diverse functions. Matrix Biol. 19, 557–568. doi: 10.1016/s0945-053x(00)00104-9
Brázda, V., Klusáková, I., Hradilová Svíženská, I., and Dubový, P. (2013). Dynamic response to peripheral nerve injury detected by in situ hybridization of IL-6 and its receptor mRNAs in the dorsal root ganglia is not strictly correlated with signs of neuropathic pain. Mol. Pain 9:42. doi: 10.1186/1744-8069-9-42
Calvet, J., Orellana, C., Albiñana Giménez, N., Berenguer-Llergo, A., Caixàs, A., García-Manrique, M., et al. (2018). Differential involvement of synovial adipokines in pain and physical function in female patients with knee osteoarthritis. Cross-sectional Study Osteoarthr. Cartil. 26, 276–284. doi: 10.1016/j.joca.2017.11.010
Dokala, A., and Thakur, S. S. (2017). Extracellular region of epidermal growth factor receptor: a potential target for anti-EGFR drug discovery. Oncogene 36, 2337–2344. doi: 10.1038/onc.2016.393
Farrow, B., Berger, D. H., and Rowley, D. (2009). Tumor-derived pancreatic stellate cells promote pancreatic cancer cell invasion through release of thrombospondin-2. J. Surg. Res. 156, 155–160. doi: 10.1016/j.jss.2009.03.040
Goldberger, A., Middleton, K. A., Oliver, J. A., Paddock, C., Yan, H. C., DeLisser, H. M., et al. (1994). Biosynthesis and processing of the cell adhesion molecule PECAM-1 includes production of a soluble form. J. Biol. Chem. 269, 17183–17191.
Hayden, S. M., and Seeds, N. W. (1996). Modulated expression of plasminogen activator system components in cultured cells from dissociated mouse dorsal root ganglia. J. Neurosci. 16, 2307–2317. doi: 10.1523/jneurosci.16-07-02307.1996
Ho, J., Moyes, D. L., Tavassoli, M., and Naglik, J. R. (2017). The Role of ErbB receptors in infection. Trends Microbiol. 25, 942–952. doi: 10.1016/j.tim.2017.04.009
Icer, M. A., and Gezmen-Karadag, M. (2018). The multiple functions and mechanisms of osteopontin. Clin. Biochem. 59, 17–24. doi: 10.1016/j.clinbiochem.2018.07.003
Ichikawa, H., Itota, T., Nishitani, Y., Torii, Y., Inoue, K., and Sugimoto, T. (2000). Osteopontin-immunoreactive primary sensory neurons in the rat spinal and trigeminal nervous systems. Brain Res. 863, 276–281. doi: 10.1016/s0006-8993(00)02126-0
Irigoyen, J. P., Muñoz-Cánoves, P., Montero, L., Koziczak, M., and Nagamine, Y. (1999). The plasminogen activator system: biology and regulation. Cell Mol. Life Sci. 56, 104–132. doi: 10.1007/pl00000615
Jimenez-Andrade, J. M., Herrera, M. B., Ghilardi, J. R., Vardanyan, M., Melemedjian, O. K., and Mantyh, P. W. (2008). Vascularization of the dorsal root ganglia and peripheral nerve of the mouse: implications for chemical-induced peripheral sensory neuropathies. Mol. Pain 4:10. doi: 10.1186/1744-8069-4-10
Kalinowska, A., and Losy, J. (2006). PECAM-1, a key player in neuroinflammation. Eur. J. Neurol. 13, 1284–1290. doi: 10.1111/j.1468-1331.2006.01640.x
Kaufman, B., Wu, Y., Amonkar, M. M., Sherrill, B., Bachelot, T., Salazar, V., et al. (2010). Impact of lapatinib monotherapy on QOL and pain symptoms in patients with HER2+ relapsed or refractory inflammatory breast cancer. Curr. Med. Res. Opin. 26, 1065–1073. doi: 10.1185/03007991003680323
Kawasaki, Y., Zhang, L., Cheng, J. K., and Ji, R. R. (2008). Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J. Neurosci. 28, 5189–5194. doi: 10.1523/jneurosci.3338-07.2008
Kersten, C., and Cameron, M. G. (2012). Cetuximab alleviates neuropathic pain despite tumour progression. BMJ Case Rep. 2012, bcr1220115374. doi: 10.1136/bcr.12.2011.5374
Kersten, C., Cameron, M. G., Bailey, A. G., Fallon, M. T., Laird, B. J., Paterson, V., et al. (2019). Relief of neuropathic pain through epidermal growth factor receptor inhibition: a randomized proof-of-concept trial. Pain Med. 20, 2495–2505. doi: 10.1093/pm/pnz101
Kersten, C., Cameron, M. G., Laird, B., and Mjåland, S. (2015). Epidermal growth factor receptor-inhibition (EGFR-I) in the treatment of neuropathic pain. Br. J. Anaesth. 115, 761–767. doi: 10.1093/bja/aev326
Kersten, C., Cameron, M. G., and Mjåland, S. (2013). Erratum to “Epithelial growth factor receptor (EGFR)-inhibition for relief of neuropathic pain-A case series” [Scand. J. Pain 4 (2013) 3-7]. Scand. J. Pain 4:125. doi: 10.1016/j.sjpain.2013.02.001
Lamort, A. S., Giopanou, I., Psallidas, I., and Stathopoulos, G. T. (2019). Osteopontin as a link between inflammation and cancer: the thorax in the spotlight. Cells 8:815. doi: 10.3390/cells8080815
Lawton, M. T., Rutledge, W. C., Kim, H., Stapf, C., Whitehead, K. J., Li, D. Y., et al. (2015). Brain arteriovenous malformations. Nat. Rev. Dis. Primers 1:15008. doi: 10.1038/nrdp.2015.8
Liu, J. F., Lee, C. W., Tsai, M. H., Tang, C. H., Chen, P. C., Lin, L. W., et al. (2018). Thrombospondin 2 promotes tumor metastasis by inducing matrix metalloproteinase-13 production in lung cancer cells. Biochem. Pharmacol. 155, 537–546. doi: 10.1016/j.bcp.2018.07.024
Lyngbæk, S., Andersson, C., Marott, J. L., Møller, D. V., Christiansen, M., Iversen, K. K., et al. (2013). Soluble urokinase plasminogen activator receptor for risk prediction in patients admitted with acute chest pain. Clin. Chem. 59, 1621–1629. doi: 10.1373/clinchem.2013.203778
Mafu, T. S., September, A. V., and Shamley, D. (2018). The potential role of angiogenesis in the development of shoulder pain, shoulder dysfunction, and lymphedema after breast cancer treatment. Cancer Manag. Res. 10, 81–90. doi: 10.2147/cmar.S151714
Mapp, P. I., and Walsh, D. A. (2012). Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat. Rev. Rheumatol. 8, 390–398. doi: 10.1038/nrrheum.2012.80
Marsh, B. C., Kerr, N. C., Isles, N., Denhardt, D. T., and Wynick, D. (2007). Osteopontin expression and function within the dorsal root ganglion. Neuroreport 18, 153–157. doi: 10.1097/WNR.0b013e328010d4fa
Martin, L. J., Smith, S. B., Khoutorsky, A., Magnussen, C. A., Samoshkin, A., Sorge, R. E., et al. (2017). Epiregulin and EGFR interactions are involved in pain processing. J. Clin. Invest. 127, 3353–3366. doi: 10.1172/jci87406
Moasser, M. M. (2007). The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene 26, 6469–6487. doi: 10.1038/sj.onc.1210477
Naumnik, W., Ossolińska, M., Płońska, I., Chyczewska, E., and Nikliński, J. (2015). Circulating thrombospondin-2 and FGF-2 in Patients with advanced non-small cell lung cancer: correlation with survival. Adv. Exp. Med. Biol. 833, 9–14. doi: 10.1007/5584_2014_78
Neuschwander-Tetri, B. A., Talkad, V., and Otis Stephen, F. (2006). Induced thrombospondin expression in the mouse pancreas during pancreatic injury. Int. J. Biochem. Cell Biol. 38, 102–109. doi: 10.1016/j.biocel.2005.08.008
Nishimoto, N., Terao, K., Mima, T., Nakahara, H., Takagi, N., and Kakehi, T. (2008). Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood 112, 3959–3964. doi: 10.1182/blood-2008-05-155846
Onore, C. E., Nordahl, C. W., Young, G. S., Van de Water, J. A., Rogers, S. J., and Ashwood, P. (2012). Levels of soluble platelet endothelial cell adhesion molecule-1 and P-selectin are decreased in children with autism spectrum disorder. Biol. Psychiatry 72, 1020–1025. doi: 10.1016/j.biopsych.2012.05.004
Park, J., Yu, Y. P., Zhou, C. Y., Li, K. W., Wang, D., Chang, E., et al. (2016). Central mechanisms mediating thrombospondin-4-induced pain states. J Biol Chem 291, 13335–13348. doi: 10.1074/jbc.M116.723478
Perrier, A., Gligorov, J., Lefèvre, G., and Boissan, M. (2018). The extracellular domain of Her2 in serum as a biomarker of breast cancer. Lab Invest 98, 696–707. doi: 10.1038/s41374-018-0033-8
Reckziegel, D., Vachon-Presseau, E., Petre, B., Schnitzer, T. J., Baliki, M. N., and Apkarian, A. V. (2019). Deconstructing biomarkers for chronic pain: context- and hypothesis-dependent biomarker types in relation to chronic pain. Pain 160 Suppl 1, (Suppl. 1), S37–S48. doi: 10.1097/j.pain.0000000000001529
Rendina, D., De Filippo, G., Postiglione, L., Covelli, B., Ricciardone, M., Guillaume, S., et al. (2018). Interleukin-6 trans-signaling and pathological low back pain in patients with Paget disease of bone. Pain 159, 1664–1673. doi: 10.1097/j.pain.0000000000001260
Rutkowski, M. D., Winkelstein, B. A., Hickey, W. F., Pahl, J. L., and DeLeo, J. A. (2002). Lumbar nerve root injury induces central nervous system neuroimmune activation and neuroinflammation in the rat: relationship to painful radiculopathy. Spine 27, 1604–1613. doi: 10.1097/00007632-200208010-00003
Sajib, S., Zahra, F. T., Lionakis, M. S., German, N. A., and Mikelis, C. M. (2018). Mechanisms of angiogenesis in microbe-regulated inflammatory and neoplastic conditions. Angiogenesis 21, 1–14. doi: 10.1007/s10456-017-9583-4
Scholz, J., Finnerup, N. B., Attal, N., Aziz, Q., Baron, R., Bennett, M. I., et al. (2019). The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain 160, 53–59. doi: 10.1097/j.pain.0000000000001365
Siconolfi, L. B., and Seeds, N. W. (2001). Induction of the plasminogen activator system accompanies peripheral nerve regeneration after sciatic nerve crush. J. Neurosci. 21, 4336–4347. doi: 10.1523/jneurosci.21-12-04336.2001
Soejima, H., Irie, A., Fukunaga, T., Sugamura, K., Kojima, S., Sakamoto, T., et al. (2006). Elevated plasma osteopontin levels were associated with osteopontin expression of CD4+ T cells in patients with unstable angina. Circ. J. 70, 851–856. doi: 10.1253/circj.70.851
Sweitzer, S. M., White, K. A., Dutta, C., and DeLeo, J. A. (2002). The differential role of spinal MHC class II and cellular adhesion molecules in peripheral inflammatory versus neuropathic pain in rodents. J. Neuroimmunol. 125, 82–93. doi: 10.1016/s0165-5728(02)00036-x
Tang, Y. L., Zhu, G. Q., Hu, L., Zheng, M., Zhang, J. Y., Shi, Z. D., et al. (2010). Effects of intra-articular administration of sodium hyaluronate on plasminogen activator system in temporomandibular joints with osteoarthritis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 109, 541–547. doi: 10.1016/j.tripleo.2009.11.007
Thunø, M., Macho, B., and Eugen-Olsen, J. (2009). suPAR: the molecular crystal ball. Dis. Markers 27, 157–172. doi: 10.3233/dma-2009-0657
Treede, R. D., Rief, W., Barke, A., Aziz, Q., Bennett, M. I., Benoliel, R., et al. (2019). Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the international classification of diseases (ICD-11). Pain 160, 19–27. doi: 10.1097/j.pain.0000000000001384
Viallard, C., and Larrivée, B. (2017). Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis 20, 409–426. doi: 10.1007/s10456-017-9562-9
Wang, S., Liu, S., Xu, L., Zhu, X., Liu, W., Tian, L., et al. (2019). The upregulation of EGFR in the dorsal root ganglion contributes to chronic compression of dorsal root ganglions-induced neuropathic pain in rats. Mol. Pain 15:1744806919857297. doi: 10.1177/1744806919857297
Wu, S., Marie Lutz, B., Miao, X., Liang, L., Mo, K., Chang, Y. J., et al. (2016). Dorsal root ganglion transcriptome analysis following peripheral nerve injury in mice. Mol. Pain 12:1744806916629048. doi: 10.1177/1744806916629048
Yamaga, M., Tsuji, K., Miyatake, K., Yamada, J., Abula, K., Ju, Y. J., et al. (2012). Osteopontin level in synovial fluid is associated with the severity of joint pain and cartilage degradation after anterior cruciate ligament rupture. PLoS One 7:e49014. doi: 10.1371/journal.pone.0049014
Yang, Y., Gao, S. G., Zhang, F. J., Luo, W., Xue, J. X., and Lei, G. H. (2014). Effects of osteopontin on the expression of IL-6 and IL-8 inflammatory factors in human knee osteoarthritis chondrocytes. Eur. Rev. Med. Pharmacol. Sci. 18, 3580–3586.
Keywords: angiogenic factors, acute pain, postherpetic neuralgia, low back pain, trigeminal neuralgia, TSP-2
Citation: Yang X, Yuan C, Wang H, Wang Y, Liu M, Li Z and Zhang J (2022) Changes in serum angiogenic factors among patients with acute pain and subacute pain. Front. Mol. Neurosci. 15:960460. doi: 10.3389/fnmol.2022.960460
Received: 03 June 2022; Accepted: 27 June 2022;
Published: 15 July 2022.
Edited by:
Yize Li, Tianjin Medical University General Hospital, ChinaReviewed by:
Kai Mo, Southern Medical University, ChinaCopyright © 2022 Yang, Yuan, Wang, Wang, Liu, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Zhang, MzAxMTkwMzBAbmFua2FpLmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.