
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Mol. Neurosci. , 13 July 2022
Sec. Neuroplasticity and Development
Volume 15 - 2022 | https://doi.org/10.3389/fnmol.2022.934222
This article is part of the Research Topic New Challenges and Future Perspectives in Molecular Neuroscience View all 4 articles
The neuropeptide pigment-dispersing factor (Pdf) is critically involved in the regulation of circadian rhythms in various insects. The function of Pdf in circadian rhythms has been best studied in the fruitfly, i.e., Drosophila melanogaster. Drosophila Pdf is produced in a small subset of circadian clock neurons in the adult brain and functions as a circadian output signal. Recently, however, Pdf has been shown to play important roles not only in regulating circadian rhythms but also in innate and learned behaviors in Drosophila. In this mini-review, we will focus on the current findings that Pdf signaling and Pdf-producing neurons are essential for consolidating and maintaining long-term memory induced by the courtship conditioning in Drosophila and discuss the mechanisms of courtship memory processing through Pdf-producing neurons.
Animals acquire temporary memories through their experience. Under certain conditions, an acquired memory is consolidated into a stable long-term memory (LTM). Once LTM is established in the brain, it is maintained until recall. The fruitfly Drosophila melanogaster uses various genetic techniques that has been used to clarify the molecular mechanisms of learning and memory. Many memory genes are expressed in the mushroom body (MB), which is considered to be the Drosophila memory center (Davis, 2005; Griffith and Ejima, 2009). Interestingly, the Drosophila circadian clock gene period (per) also plays a vital role in memory consolidation to establish LTM, although per is not expressed in MB neurons (Sakai et al., 2004; Donlea et al., 2009; Chen et al., 2012; Suzuki et al., 2022). Thus, per-expressing clock neurons should also be essential for Drosophila LTM (Suzuki et al., 2022). However, little is known about how clock neurons modulate LTM formed and maintained in MB.
In the Drosophila brain, there are about 150 clock neurons (Peschel and Helfrich-Forster, 2011). They are anatomically divided into seven groups as follows: dorsal neurons 1 (DN1), DN2, and DN3, large ventral lateral neurons (l-LNvs), small ventral lateral neurons (s-LNvs), 5th small ventral lateral neurons (5th s-LNvs), and dorsal lateral neurons (LNds) (Peschel and Helfrich-Forster, 2011). Drosophila Pigment-dispersing factor (Pdf ) encoding a neuropeptide, which is well conserved in insect species, is specifically expressed in s-LNvs and l-LNvs (Renn et al., 1999; Helfrich-Forster, 2005; Peschel and Helfrich-Forster, 2011). Pdf functions in the brain have been well studied in Drosophila. Pdf was initially identified as a neuropeptide required to generate circadian behavioral rhythms (Renn et al., 1999). Subsequent studies revealed that Pdf plays a vital role in the circadian network as an intercellular messenger from Pdf-expressing clock neurons (hereafter referred to as Pdf neurons) to other clock neurons (Shafer and Yao, 2014; Yoshii et al., 2016). Thus, Pdf is widely known as a circadian neuromodulator.
Pdf is essential not only for circadian rhythms but also for other behavioral phenomena. A null mutation of Pdf (Pdf01) induces a defective geotaxis, which is restored by Pdf expression in Pdf neurons (Mertens et al., 2005). Pdf receptor (Pdfr) mutant flies also show the Pdf01-like phenotype (Mertens et al., 2005), indicating that Pdf/Pdfr signaling is essential for the Drosophila geotaxis. Pdf/Pdfr signaling is also indispensable for behavioral plasticity. When wild-type males are housed together with rivals for 5 d before mating, their mating duration is extended compared with the socially isolated males (Kim et al., 2012). Pdf/Pdfr signaling is also required for the experience-dependent extension of mating duration, and this behavioral plasticity is regulated by centrally expressing Pdf and Pdfr in a circadian-clock-independent manner (Kim et al., 2012). Furthermore, Pdf01 flies show a decreased ability to establish short-term aversive olfactory memory (aversive STM), although a null mutation of Pdfr, which induces arrhythmic locomotor activity, has no effect on aversive STM (Flyer-Adams et al., 2020), suggesting that Pdf signaling has roles different from those in modifying circadian rhythms.
In this article, we summarize our current knowledge about the novel functions of Pdf signaling and Pdf neurons that are identified in Drosophila courtship memory (Inami et al., 2020, 2021).
The courtship conditioning paradigm has been used to measure Drosophila memory (Siegel and Hall, 1979). In this paradigm, a virgin male and a mated female were placed in a small chamber. In this situation, the males receive stresses such as physical rejection and male-courtship-inhibiting cues from mated females (conditioning). After conditioning, males show courtship suppression even toward virgin females. Conditioning-dependent male courtship suppression is based on memory formation because many memory mutants isolated by olfactory classical conditioning do not show courtship suppression (Griffith and Ejima, 2009). Based on the retention time, courtship memory is classified into at least two phases. When males are conditioned with mated females for 1 h (1 h of conditioning), they establish a short-term memory (STM). Although STM lasts at least for 8 h, it disappears 24 h after 1 h of conditioning (Inami et al., 2021). On the other hand, when single males were conditioned for 7 h (7 h of conditioning), they form LTM, which lasts for at least 5 d (Sakai et al., 2004, 2012). Since 2004, many genes related to LTM in Drosophila courtship memory have been identified (Table 1). Similar to Drosophila aversive olfactory memory (Margulies et al., 2005; Davis, 2011), it is considered that MB neurons are responsible for courtship LTM because many LTM genes identified in MB neurons were found to play essential roles in consolidating and maintaining courtship LTM (Table 1). On the other hand, the circadian clock does not affect courtship LTM because LTM in mutant flies with a defective circadian clock (e.g., timeless01, cycle0, and ClockJrk) is intact (Sakai et al., 2004).
The cAMP signaling pathways and the transcription factor cAMP response element-binding protein (CREB) are evolutionarily conserved in the vertebrates and invertebrates, and they play critical roles in memory consolidation to establish LTM (Yin and Tully, 1996; Davis, 2005; Kandel, 2012). Thus, synthesis of newly proteins is essential for memory consolidation in vertebrates and invertebrates (Kandel, 2012). In Drosophila, synthesis of newly proteins via CREB-dependent transcription in MB neurons is indispensable for consolidating and maintaining LTM induced by olfactory classical conditioning and courtship conditioning (Yin and Tully, 1996; Sakai et al., 2004; Ishimoto et al., 2009; Hirano et al., 2016; Inami et al., 2020). In the adult brain, MB neurons comprise of at least three types (α/β, α′/β′, and γ), and each type extends into axonal lobes (α/β, α′/β′, and γ lobes) (Davis, 2005; Mabuchi et al., 2016). Although CREB activity in α′/β′ neurons do not affect the consolidation and maintenance of courtship LTM, CREB activity in α/β and γ neurons during courtship conditioning is necessary for LTM, suggesting that α/β and γ neurons play an essential role in memory consolidation to establish courtship LTM (Inami et al., 2020). However, CREB activity in only α/β neurons, but not that in α′/β′ and γ neurons, is necessary for keeping courtship LTM for more than 2 d (Inami et al., 2020), indicating that the early phase of courtship LTM, which lasts for at least 1 d after conditioning, is formed in α/β and γ neurons, whereas the late phase of courtship LTM, which persists for more than 2 d, is maintained in only α/β neurons (Figure 1A). Thus, courtship memory seems to be consolidated within at least 1 day after conditioning, and the maintenance phase of courtship LTM appears to begin at least 2 d after the courtship conditioning (Figure 1A). However, it still remains unclarified exactly when LTM consolidation ends and how the memory consolidation phase transitions to the LTM maintenance phase.
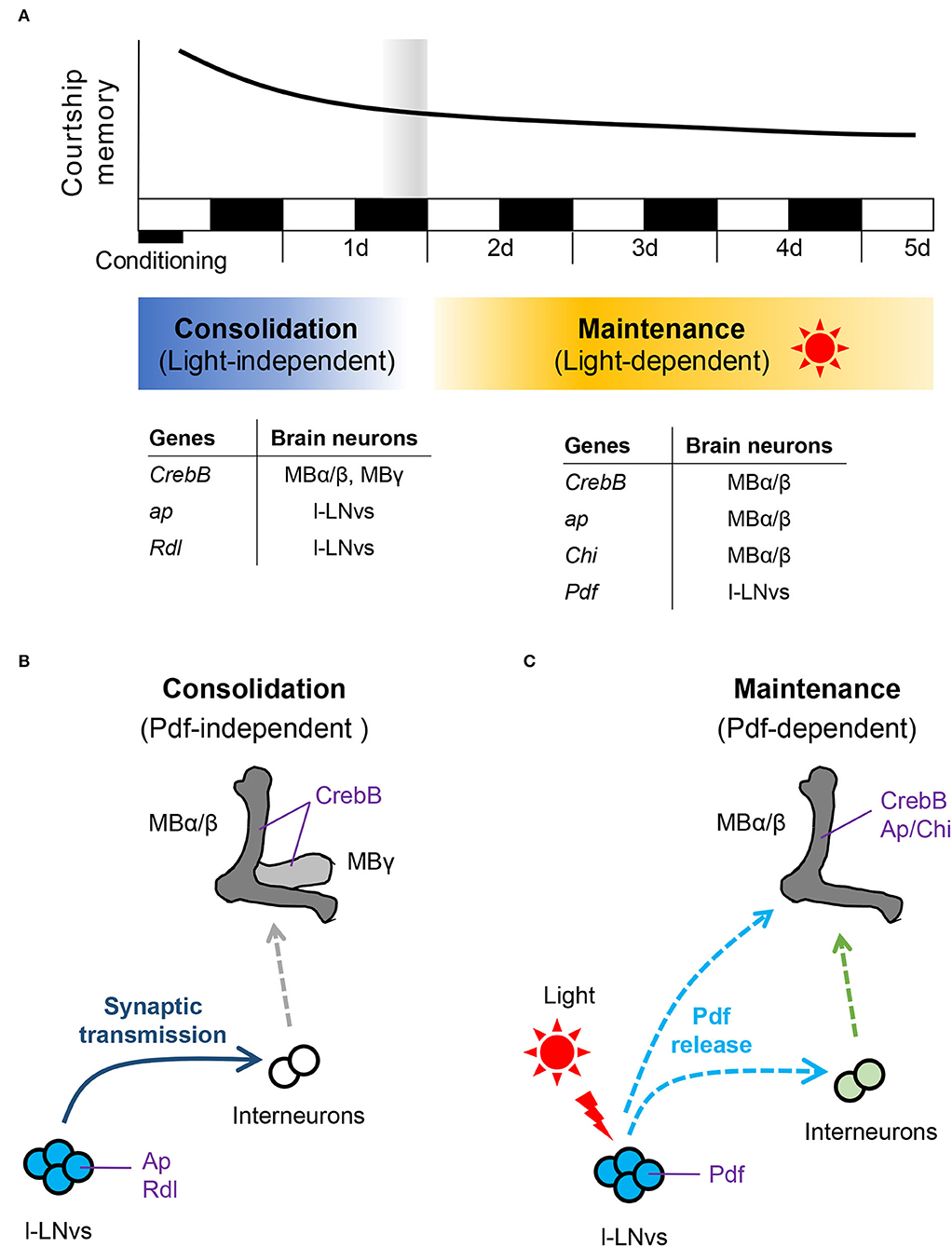
Figure 1. Molecular and cellular basis of courtship memory in Drosophila. (A) Schematic diagram of courtship memory processing and genes regulating consolidation and maintenance of courtship LTM. ap, apterous; Rdl, Resistant to dieldrin; Chi, Chip; Pdf, Pigment-dispersing factor. (B) Pdf-independent LTM consolidation. Synaptic transmission from Pdf-positive l-LNvs modulates the consolidation of courtship LTM. (C) Pdf-dependent LTM maintenance. Pdf release driven by light is essential for the maintenance of courtship LTM.
Various research studies on Drosophila chronobiology support the idea that s-LNvs are essential for sustaining circadian locomotor rhythms in constant darkness (Grima et al., 2004; Stoleru et al., 2004, 2005; Helfrich-Forster, 2005; Rieger et al., 2006). Unlike the s-LNvs, l-LNvs mainly contribute to sleep and arousal regulation (Parisky et al., 2008; Shang et al., 2008; Sheeba et al., 2008b; Chung et al., 2009; Shimada et al., 2016). Cryptochrome (Cry), a blue-light-sensitive photopigment, is expressed in many clock neurons containing s-LNvs and l-LNvs, and it acts as a circadian photoreceptor in Drosophila (Stanewsky et al., 1998; Emery et al., 2000; Yoshii et al., 2016). Similarly, Rhodopsin 7 (Rh7) also contributes to the light sensitivity of s-LNvs and l-LNvs (Ni et al., 2017). Furthermore, Pdf neurons can sense environmental light directly via the circadian photoreceptors Cry and Rh7 (Sheeba et al., 2008a; Fogle et al., 2011; Ni et al., 2017) or indirectly via one of the light-sensing organs, the Hofbauer–Buchner (H–B) eyelets (Yoshii et al., 2016; Li et al., 2018). Thus, it is considered that the l-LNvs induce light-dependent Pdf secretion, which regulates light-mediated arousal in Drosophila (Shang et al., 2008; Sheeba et al., 2008b).
The rhythmic light–dark (LD) cycles on the Earth significantly affect animal behavior and physiology (Inami et al., 2020). In animals, light is not only essential for acquiring information for image-forming vision in nature but also acts as a potent modulator of brain functions such as circadian entrainment, hormone secretion, sleep–wake cycles, mood, and cognitive functions (Vandewalle et al., 2009; Crocker et al., 2016; Fernandez et al., 2018; Inami et al., 2020). We have recently found that environmental light affects courtship LTM maintenance, but not memory consolidation (Inami et al., 2020). Regardless of whether flies are conditioned in light or darkness, 5-d memory after courtship conditioning is detected. Thus, courtship memory is consolidated into LTM regardless of the presence or absence of light. Unlike memory consolidation, when flies are kept in constant darkness (DD) after the courtship conditioning and before the test, their LTM disappears. Furthermore, DD for 2 d after the conditioning is sufficient to impair LTM. Thus, light is essential for LTM maintenance. Although the amount of daytime sleep in DD is slightly but significantly smaller than that in LD, the decreased sleep amount has no effect on LTM maintenance (Inami et al., 2020). Furthermore, Pdf expression is also critical for the maintenance of courtship LTM (Inami et al., 2020). Temporal activation of Pdf neurons compensates for the DD-inducible LTM impairment. In contrast, l-LNv-specific electrical silencing using the inwardly rectifying Kir2.1 channel impairs LTM maintenance in LD (Inami et al., 2020). Considering these findings, it is most likely that light-inducible Pdf secretion from l-LNvs regulates the light-dependent maintenance of courtship LTM.
A null mutation of Pdf does not affect 1-d memory, whereas it impairs 2-d memory or 5-d memory (Inami et al., 2020). These findings support the idea that Pdf release is required for only the maintenance of LTM. If LTM maintenance is light-dependent in Drosophila, is CREB activity in MB neurons also light-dependent during the memory maintenance phase? A bioluminescent reporter assay revealed that CREB-dependent transcription in α/β neurons is also light-dependent, but that in α′/β′ and γ neurons is not (Inami et al., 2020). These findings also support the idea that courtship LTM is maintained in α/β neurons in a light-dependent manner.
A null mutation of Pdfr also impairs 5-d memory and markedly attenuates a light-dependent increase in the CREB activity in α/β neurons (Inami et al., 2020). Similarly, flies that are kept in DD for 2 d also do not show a light-dependent increase in CREB activity in α/β neurons (Inami et al., 2020). Thus, it is considered that environmental light triggers CREB-dependent transcription in α/β neurons via Pdf/Pdfr signaling, and this system is essential for the maintenance of courtship LTM.
The circadian clock drives the rhythmic expression of hundreds of genes in MB neurons, including Pka-C1, which encodes a regulatory subunit of cAMP-dependent protein kinase A (PKA) (Almeida et al., 2021). Since CREB phosphorylated by PKA is transcriptionally active (Kandel, 2012), the circadian clock may also regulate CREB activity in MB neurons in DD. However, since CREB activity in MB neurons in LD is markedly higher than that in DD (Inami et al., 2020), the effect of light on CREB activity may outweigh that of the circadian clock.
The LIM homeodomain protein Apterous (Ap), which acts as a transcription factor, is well conserved in vertebrates and invertebrates (Hobert and Westphal, 2000). Ap and its cofactor Chip (Chi) are essential for the neuro developmental events (Lundgren et al., 1995; O'Keefe et al., 1998; van Meyel et al., 2000). However, Ap continues to be expressed in the brain neurons including MB α/β neurons, s-LNvs, and l-LNvs (Shimada et al., 2016; Inami et al., 2021). We have recently found that Ap and Chi in MB α/β neurons are indispensable for maintaining courtship LTM (Figure 1A) (Inami et al., 2021). Since Ap/Chi regulates the transcription of Ap target genes (Hobert and Westphal, 2000; Inami et al., 2021), Ap/Chi in MB α/β neurons should be necessary for providing proteins required to maintain courtship LTM (Inami et al., 2021). As was observed in Ap/Chi, CREB-dependent transcription in MB α/β neurons is also essential for the maintenance of courtship LTM. Thus, courtship LTM is likely maintained in MB α/β neurons from the second day after conditioning, and proteins required for maintaining LTM for more than 2 d should be provided via transcriptions by CREB and Ap/Chi. However, the molecular interactions between CREB and Ap/Chi still remain unclarified.
Unlike Ap in MB α/β neurons, Ap in l-LNvs, but not in s-LNvs, is essential for memory consolidation to establish courtship LTM in a Chi-independent manner (Inami et al., 2021) (Figure 1B). In addition, Ap in l-LNvs plays a vital role in preventing over-responses to the inhibitory neurotransmitter GABA. The induction of the Drosophila ionotropic GABAA receptor on the Pdf neurons compensates for the impaired memory consolidation in ap null mutant flies (Inami et al., 2021). These findings indicate that the excitability of Pdf neurons plays a crucial role in memory consolidation to establish LTM.
Drosophila shibire (shi) encodes Dynamin regulating synaptic vesicle recycling (Vanderbliek and Meyerowitz, 1991). Induction of the temperature-sensitive shi allele (shits1) can inhibit synaptic transmission in a temperature-dependent manner (Kitamoto, 2001; Suzuki et al., 2022). Although Shits1 functions as normal Dynamin at the permissive temperature, it is dysfunctional at the restrictive temperature. Thus, the targeted expression of shits1 can spatially and temporally inhibit synaptic transmission through a temperature shift (Kasuya et al., 2009). Disruption of synaptic transmission in PDF neurons using shits1 impairs memory consolidation. However, it does not affect LTM maintenance or recall. These findings indicate that synaptic transmission in Pdf neurons mainly contributes to memory consolidation (Figure 1B) (Inami et al., 2021). Why does disruption of synaptic transmission in Pdf neurons impair memory consolidation, although the Pdf neuropeptide does not affect memory consolidation? We previously reported that disruption of synaptic transmission in Pdf neurons using shits1 has little impact on locomotor activity rhythms (Mabuchi et al., 2016). This finding suggests that disruption of the Dynamin function cannot inhibit Pdf release. Thus, it is likely that neurotransmitters other than Pdf released from Pdf neurons are involved in the consolidation of courtship LTM.
The current research studies using Drosophila courtship conditioning reveal that Pdf neurons have two distinct functions and modify two different memory processes. First, dynamin-dependent neurotransmission from Pdf neurons during courtship conditioning is essential for memory consolidation to establish courtship LTM (Figure 1B). Since Pdf neuropeptide release seems to be dynamin-independent, other neurotransmitters such as the classical neurotransmitters should be released from Pdf neurons. However, it remains unknown whether neurotransmission from Pdf neurons is driven in a conditioning-dependent manner or endogenously occurs in Pdf neurons. Since, to the best of our knowledge, there is no direct evidence that l-LNvs synaptically project to MB α/β or γ neurons directly, intercellular communication from l-LNvs to MB α/β and/or γ neurons via interneurons may play a crucial role in the establishment of courtship LTM (Figure 1B). Second, the light-dependent release of the Pdf neuropeptide from l-LNvs plays a critical role in the courtship LTM maintenance (Figure 1C). Environmental light induces Pdf release and activates the transcription factor CREB in MB α/β neurons. Moreover, the light dependent CREB activation in MB α/β neurons occurs via Pdfr. Chronobiological research studies using Pdfr-GAL4 lines or an anti-Pdfr antibody did not indicate Pdfr expression in MB neurons (Mertens et al., 2005; Im and Taghert, 2010). In contrast, RNA sequencing analysis has revealed that Pdfr is expressed in MB neurons (Crocker et al., 2016). Furthermore, Flyer-Adams et al. have recently shown using a LexA knock-in fly strain, Pdfr-2A-LexA that Pdfr is expressed in at least one of the MB neurons (Flyer-Adams et al., 2020). Although it remains to be clarified whether activated Pdfr directly or indirectly increases CREB activity in MB α/β neurons, the light-dependent Pdf/Pdfr/CREB pathway is found to be essential for courtship LTM maintenance (Inami et al., 2020).
In Drosophila, the LTM maintenance phase has been defined conceptually as the time after LTM is fully formed and consolidated, and it is generally believed that memory consolidation is completed within 1 d after conditioning (Davis, 2005; Margulies et al., 2005; Inami et al., 2021). The recent LTM research studies using Drosophila courtship conditioning identified interesting mutants or transgenic flies with intact 1-d memory but are defective 2-d memory (Inami et al., 2020, 2021). This finding indicates that there are genetically manipulated flies that can consolidate LTM but cannot maintain it. Furthermore, the recent studies showed the vital roles of Pdf neurons in modulating LTM processes in a Pdf-dependent or Pdf-independent manner (Inami et al., 2020, 2021). Considering these findings, the consolidation and maintenance phases in the courtship LTM seem to be molecularly and cellularly separate (Figure 1A). Although it will be necessary to determine whether this model can be extended to other memory paradigms in Drosophila, the clock neuron network and the memory center may, in general, cooperatively work in establishing and maintaining Drosophila LTM.
SI, TSt, and TSk contributed to conception and design of the study. TSk wrote the first draft of the manuscript. SI and TSt wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
This work was supported by a JSPS KAKENHI (grant number 15J06303) to SI, JSPS KAKENHI (grant numbers 16H04816 and 21H02528) to TSk, and a Grant-in-Aid for Scientific Research on Innovative Areas, Singularity Biology (grant number 21H00434) to TSk.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abou Tayoun, A. N., Pikielny, C., and Dolph, P. J. (2012). Roles of the Drosophila SK channel (dSK) in courtship memory. PLoS ONE 7, e34665. doi: 10.1371/journal.pone.0034665
Almeida, P. M., Solis, B. L., Feidler, A., Nagoshi, E., and Stickley, L. (2021). Neurofibromin 1 in mushroom body neurons mediates circadian wake drive through activating cAMP-PKA signaling. Nat. Commun. 12, 5758. doi: 10.1038/s41467-021-26031-2
Chen, C. C., Wu, J. K., Lin, H. W., Pai, T. P., Fu, T. F., Wu, C. L., et al. (2012). Visualizing long-term memory formation in two neurons of the Drosophila brain. Science 335, 678–685. doi: 10.1126/science.1212735
Chung, B. Y., Kilman, V. L., Keath, J. R., Pitman, J. L., and Allada, R. (2009). The GABA(A) receptor RDL acts in peptidergic PDF neurons to promote sleep in Drosophila. Curr. Biol. 19, 386–390. doi: 10.1016/j.cub.2009.01.040
Crocker, A., Guan, X. J., Murphy, C. T., and Murthy, M. (2016). Cell-type-specific transcriptome analysis in the Drosophila mushroom body reveals memory-related changes in gene expression. Cell Rep. 15, 1580–1596. doi: 10.1016/j.celrep.2016.04.046
Davis, R. L. (2005). Olfactory memory formation in Drosophila: from molecular to systems neuroscience. Annu. Rev. Neurosci. 28, 275–302. doi: 10.1146/annurev.neuro.28.061604.135651
Davis, R. L. (2011). Traces of Drosophila memory. Neuron 70, 8–19. doi: 10.1016/j.neuron.2011.03.012
Donlea, J. M., Ramanan, N., and Shaw, P. J. (2009). Use-dependent plasticity in clock neurons regulates sleep need in Drosophila. Science 324, 105–108. doi: 10.1126/science.1166657
Emery, P., Stanewsky, R., Helfrich-Forster, C., Emery-Le, M., Hall, J. C., and Rosbash, M. (2000). Drosophila CRY is a deep brain circadian photoreceptor. Neuron 26, 493–504. doi: 10.1016/S0896-6273(00)81181-2
Fernandez, D. C., Fogerson, P. M., Ospri, L. L., Thomsen, M. B., Layne, R. M., Severin, D., et al. (2018). Light affects mood and learning through distinct retina-brain pathways. Cell 175, 71–84. doi: 10.1016/j.cell.2018.08.004
Fitzsimons, H. L., Schwartz, S., Given, F. M., and Scott, M. J. (2013). The histone deacetylase HDAC4 regulates long-term memory in Drosophila. PLoS ONE 8, e83903. doi: 10.1371/journal.pone.0083903
Fitzsimons, H. L., and Scott, M. J. (2011). Genetic modulation of Rpd3 expression impairs long-term courtship memory in Drosophila. PLoS ONE 6, e29171. doi: 10.1371/journal.pone.0029171
Flyer-Adams, J. G., Rivera-Rodriguez, E. J., Yu, J., Mardovin, J. D., Reed, M. L., and Griffith, L. C. (2020). Regulation of olfactory associative memory by the circadian clock output signal Pigment-Dispersing Factor (PDF). J. Neurosci. 40, 9066–9077. doi: 10.1523/JNEUROSCI.0782-20.2020
Fogle, K. J., Parson, K. G., Dahm, N. A., and Holmes, T. C. (2011). CRYPTOCHROME is a blue-light sensor that regulates neuronal firing rate. Science 331, 1409–1413. doi: 10.1126/science.1199702
Griffith, L. C., and Ejima, A. (2009). Courtship learning in Drosophila melanogaster: diverse plasticity of a reproductive behavior. Learn. Mem. 16, 743–750. doi: 10.1101/lm.956309
Grima, B., Chelot, E., Xia, R. H., and Rouyer, F. (2004). Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature 431, 869–873. doi: 10.1038/nature02935
Helfrich-Forster, C. (2005). Neurobiology of the fruit fly's circadian clock. Genes Brain Behav. 4, 65–76. doi: 10.1111/j.1601-183X.2004.00092.x
Hirano, Y., Ihara, K., Masuda, T., Yamamoto, T., Iwata, I., Takahashi, A., et al. (2016). Shifting transcriptional machinery is required for long-term memory maintenance and modification in Drosophila mushroom bodies. Nat. Commun. 7, 13471. doi: 10.1038/ncomms13471
Hobert, O., and Westphal, H. (2000). Functions of LIM-homeobox genes. Trends Genet. 16, 75–83. doi: 10.1016/S0168-9525(99)01883-1
Im, S. H., and Taghert, P. H. (2010). PDF receptor expression reveals direct interactions between circadian oscillators in Drosophila. J. Comp. Neurol. 518, 1925–1945. doi: 10.1002/cne.22311
Inami, S., Sato, S., Kondo, S., Tanimoto, H., Kitamoto, T., and Sakai, T. (2020). Environmental light is required for maintenance of long-term memory in Drosophila. J. Neurosci. 40, 1427–1439. doi: 10.1523/JNEUROSCI.1282-19.2019
Inami, S., Sato, T., Kurata, Y., Suzuki, Y., Kitamoto, T., and Sakai, T. (2021). Consolidation and maintenance of long-term memory involve dual functions of the developmental regulator Apterous in clock neurons and mushroom bodies in the Drosophila brain. PLoS Biol. 19, e3001459. doi: 10.1371/journal.pbio.3001459
Ishimoto, H., Sakai, T., and Kitamoto, T. (2009). Ecdysone signaling regulates the formation of long-term courtship memory in adult Drosophila melanogaster. Proc. Natl. Acad. Sci. U. S. A. 106, 6381–6386. doi: 10.1073/pnas.0810213106
Kandel, E. R. (2012). The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol. Brain 5, 14. doi: 10.1186/1756-6606-5-14
Kasuya, J., Ishimoto, H., and Kitamoto, T. (2009). Neuronal mechanisms of learning and memory revealed by spatial and temporal suppression of neurotransmission using shibire, a temperature-sensitive dynamin mutant gene in Drosophila melanogaster. Front. Mol. Neurosci. 2, 11. doi: 10.3389/neuro.02.011.2009
Keleman, K., Kruttner, S., Alenius, M., and Dickson, B. J. (2007). Function of the Drosophila CPEB protein Orb2 in long-term courtship memory. Nat. Neurosci. 10, 1587–1593. doi: 10.1038/nn1996
Kim, W. J., Jan, L. Y., and Jan, Y. N. (2012). Contribution of visual and circadian neural circuits to memory for prolonged mating induced by rivals. Nat. Neurosci. 15, 876–883. doi: 10.1038/nn.3104
Kitamoto, T. (2001). Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J. Neurobiol. 47, 81–92. doi: 10.1002/neu.1018
Kruttner, S., Traunmuller, L., Dag, U., Jandrasits, K., Stepien, B., Iyer, N., et al. (2015). Synaptic Orb2A bridges memory acquisition and late memory consolidation in Drosophila. Cell Rep. 11, 1953–1965. doi: 10.1016/j.celrep.2015.05.037
Lee, S. S., and Adams, M. E. (2021). Regulation of Drosophila long-term courtship memory by ecdysis triggering hormone. Front. Neurosci. 15, 670322. doi: 10.3389/fnins.2021.670322
Li, M. T., Cao, L. H., Xiao, N., Tang, M., Deng, B., Yang, T., et al. (2018). Hub-organized parallel circuits of central circadian pacemaker neurons for visual photoentrainment in Drosophila. Nat. Commun. 9, 4247. doi: 10.1038/s41467-018-06506-5
Lundgren, S. E., Callahan, C. A., Thor, S., and Thomas, J. B. (1995). Control of neuronal pathway selection by the Drosophila LIM homeodomain gene apterous. Development 121, 1769–1773. doi: 10.1242/dev.121.6.1769
Mabuchi, I., Shimada, N., Sato, S., Ienaga, K., Inami, S., and Sakai, T. (2016). Mushroom body signaling is required for locomotor activity rhythms in Drosophila. Neurosci Res 111, 25–33. doi: 10.1016/j.neures.2016.04.005
Margulies, C., Tully, T., and Dubnau, J. (2005). Deconstructing memory in Drosophila. Curr. Biol. 15, R700– R 713. doi: 10.1016/j.cub.2005.08.024
Mertens, I., Vandingenen, A., Johnson, E. C., Shafer, O. T., Li, W., Trigg, J. S., et al. (2005). PDF receptor signaling in Drosophila contributes to both circadian and geotactic behaviors. Neuron 48, 213–219. doi: 10.1016/j.neuron.2005.09.009
Ni, J. D., Baik, L. S., Holmes, T. C., and Montell, C. (2017). A rhodopsin in the brain functions in circadian photoentrainment in Drosophila. Nature 545, 340–344. doi: 10.1038/nature22325
O'Keefe, D. D., Thor, S., and Thomas, J. B. (1998). Function and specificity of LIM domains in Drosophila nervous system and wing development. Development 125, 3915–3923. doi: 10.1242/dev.125.19.3915
Parisky, K. M., Agosto, J., Pulver, S. R., Shang, Y., Kuklin, E., Hodge, J. J., et al. (2008). PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron 60, 672–682. doi: 10.1016/j.neuron.2008.10.042
Peschel, N., and Helfrich-Forster, C. (2011). Setting the clock–by nature: circadian rhythm in the fruitfly Drosophila melanogaster. FEBS Lett. 585, 1435–1442. doi: 10.1016/j.febslet.2011.02.028
Presente, A., Boyles, R. S., Serway, C. N., de Belle, J. S., and Andres, A. J. (2004). Notch is required for long-term memory in Drosophila. Proc. Natl. Acad. Sci. U. S. A. 101, 1764–1768. doi: 10.1073/pnas.0308259100
Renn, S. C. P., Park, J. H., Rosbash, M., Hall, J. C., and Taghert, P. H. (1999). A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell 99, 791–802. doi: 10.1016/S0092-8674(00)81676-1
Rieger, D., Shafer, O. T., Tomioka, K., and Helfrich-Forster, C. (2006). Functional analysis of circadian pacemaker neurons in Drosophila melanogaster. J. Neurosci. 26, 2531–2543. doi: 10.1523/JNEUROSCI.1234-05.2006
Sakai, T., Sato, S., Ishimoto, H., and Kitamoto, T. (2012). Significance of the centrally expressed TRP channel painless in Drosophila courtship memory. Learn. Mem. 20, 34–40. doi: 10.1101/lm.029041.112
Sakai, T., Tamura, T., Kitamoto, T., and Kidokoro, Y. (2004). A clock gene, period, plays a key role in long-term memory formation in Drosophila. Proc. Natl. Acad. Sci. U. S. A. 101, 16058–16063. doi: 10.1073/pnas.0401472101
Shafer, O. T., and Yao, Z. (2014). Pigment-dispersing factor signaling and circadian rhythms in insect locomotor activity. Curr. Opin. Insect Sci. 1, 73–80. doi: 10.1016/j.cois.2014.05.002
Shang, Y., Griffith, L. C., and Rosbash, M. (2008). Light-arousal and circadian photoreception circuits intersect at the large PDF cells of the Drosophila brain. Proc. Natl. Acad. Sci. U. S. A. 105, 19587–19594. doi: 10.1073/pnas.0809577105
Sheeba, V., Fogle, K. J., Kaneko, M., Rashid, S., Chou, Y. T., Sharma, V. K., et al. (2008b). Large ventral lateral neurons modulate arousal and sleep in Drosophila. Curr. Biol. 18, 1537–1545. doi: 10.1016/j.cub.2008.08.033
Sheeba, V., Gu, H., Sharma, V. K., O'Dowd, D. K., and Holmes, T. C. (2008a). Circadian- and light-dependent regulation of resting membrane potential and spontaneous action potential firing of Drosophila circadian pacemaker neurons. J. Neurophysiol. 99, 976–988. doi: 10.1152/jn.00930.2007
Shimada, N., Inami, S., Sato, S., Kitamoto, T., and Sakai, T. (2016). Modulation of light-driven arousal by LIM-homeodomain transcription factor Apterous in large PDF-positive lateral neurons of the Drosophila brain. Sci. Rep. 6, 37255. doi: 10.1038/srep37255
Siegel, R. W., and Hall, J. C. (1979). Conditioned-responses in courtship behavior of normal and mutant Drosophila. Proc. Natl. Acad. Sci. U. S. A. 76, 3430–3434. doi: 10.1073/pnas.76.7.3430
Stanewsky, R., Kaneko, M., Emery, P., Beretta, B., Wager-Smith, K., Kay, S. A., et al. (1998). The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell 95, 681–692. doi: 10.1016/S0092-8674(00)81638-4
Stoleru, D., Peng, Y., Agosto, J., and Rosbash, M. (2004). Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature 431, 862–868. doi: 10.1038/nature02926
Stoleru, D., Peng, Y., Nawathean, P., and Rosbash, M. (2005). A resetting signal between Drosophila pacemakers synchronizes morning and evening activity. Nature 438, 238–242. doi: 10.1038/nature04192
Suzuki, Y., Kurata, Y., and Sakai, T. (2022). Dorsal-lateral clock neurons modulate consolidation and maintenance of long-term memory in Drosophila. Genes Cells 27, 266–279. doi: 10.1111/gtc.12923
van Meyel, D. J., O'Keefe, D. D., Thor, S., Jurata, L. W., Gill, G. N., and Thomas, J. B. (2000). Chip is an essential cofactor for apterous in the regulation of axon guidance in Drosophila. Development 127, 1823–1831. doi: 10.1242/dev.127.9.1823
Vanderbliek, A. M., and Meyerowitz, E. M. (1991). Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature 351, 411–414. doi: 10.1038/351411a0
Vandewalle, G., Maquet, P., and Dijk, D. J. (2009). Light as a modulator of cognitive brain function. Trends Cogn. Sci. 13, 429–438. doi: 10.1016/j.tics.2009.07.004
Yin, J. C., and Tully, T. (1996). CREB and the formation of long-term memory. Curr. Opin. Neurobiol. 6, 264–268. doi: 10.1016/S0959-4388(96)80082-1
Keywords: pigment-dispersing factor, clock neurons, long-term memory, courtship conditioning, memory consolidation, memory maintenance, Drosophila
Citation: Inami S, Sato T and Sakai T (2022) Circadian Neuropeptide-Expressing Clock Neurons as Regulators of Long-Term Memory: Molecular and Cellular Perspectives. Front. Mol. Neurosci. 15:934222. doi: 10.3389/fnmol.2022.934222
Received: 02 May 2022; Accepted: 13 June 2022;
Published: 13 July 2022.
Edited by:
Clive R. Bramham, University of Bergen, NorwayReviewed by:
Chunghun Lim, Ulsan National Institute of Science and Technology, South KoreaCopyright © 2022 Inami, Sato and Sakai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takaomi Sakai, c2FrYWktdGFrYW9taUB0bXUuYWMuanA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.