- 1Hunan Provincial Key Laboratory of Clinical Epidemiology, Department of Epidemiology and Health Statistics, Xiangya School of Public Health, Central South University, Changsha, China
- 2Department of Geriatric Rehabilitation, Hunan Provincial Rehabilitation Hospital, Changsha, China
- 3Department of Neurology, The First Affiliated Hospital of Jishou University, Xiangxi, China
- 4Department of Planned Immunity, Xiangxi Center for Disease Prevention and Control, Xiangxi, China
- 5Jishou University School of Medicine, Xiangxi, China
China has had explosive growth in ischemic stroke (IS) burden with significant ethnic and geographic disparities. The aim of this study was to explore the possible combination effect between gut microbiota and traditional potentially modifiable risk factors for IS among two ethnic minorities (Tujia and Miao) and the Han population. Herein, we first used the 16 S rRNA sequencing to compare the gut microbial compositions of 82 patients with first-ever IS vs. 82 normal controls (NCs) among Han, Tujia, and Miao people between 1 May 2018 and 30 April 2019, from Xiangxi Tujia and Miao Autonomous Prefecture in China. An additive model was used to study the interaction between traditional risk factors and gut microbiota with R software. Linear discriminant analysis (LDA) and LDA effect size (LEfSe) results showed that the identified key gut microbiota's taxonomic composition varied in different ethnicity between the IS patients and NCs. Furthermore, families Lactobacillaceae, Enterococcaceae, Streptococcaceae, and Enterobacteriaceae were found to be positively correlated with high-risk factors and negatively correlated with preventive factors in the IS patients, but families Ruminococcaceae and Lachnospiraceae were just the opposite in the NCs. There were additive interactions between traditional risk factors (systolic blood pressure, diastolic blood pressure, and high-sensitive C-reactive protein) and family Enterococcaceae for first-ever IS with the attributable proportion due to the interaction was 0.74, 0.71, and 0.85, respectively; and the synergy index was 4.45, 3.78, and 7.01, respectively. This preliminary but promising study showed that the gut microbiota disturbances may potentially interact to IS with different ethnic host's traditional risk factors.
Introduction
Ischemic stroke (IS) occurs when a vessel supplying blood to the brain is obstructed, and it constitutes 69.6% and 77.8%, respectively, in total stroke incidence and prevalence in China (Wang et al., 2017). The INTERSTROKE investigators found ten potentially modifiable risk factors, including previous history of hypertension or blood pressure of 140/90 mmHg or higher, regular physical activity, apolipoprote in (Apo) B/ApoA1 ratio, diet, waist-to-hip ratio, phychosocial factors, current smoking, cardiac causes, alcohol consumption, and diabetes mellitus, that were collectively associated with about 91.5% of the population attributable risk (PAR) of IS in each major region of the world, among ethnic groups, in men and women, and in all ages (O'Donnell et al., 2016). Despite significant progress in the prevention and treatment strategies in the past two decades, the Global Burden of Disease 2017 Study reported that stroke is still the leading cause of death and disability-adjusted life years at the national level in China and the third cause of global years of life lost (O'Donnell et al., 2016; GBD 2017 Causes of Death Cllaborators., 2018; Zhou et al., 2019). Hence, IS has become one of the most important disease burdens and a major public health problem in China, even in the world.
Accumulating evidence demonstrated that gut microbiota can exert considerate influence over the gastrointestinal physiology, immune function, and even mental wellbeing through the microbiota-gut-brain axis (Sharon et al., 2016; Dinan and Cryan, 2017; Fung et al., 2017). Although the functional significance of the symbiotic relationship between host and microbe especially in the context of brain health has not been fully elucidated, several tools and animal models have been invaluable in allowing the scientific research community to constantly narrow the gaps in our understanding of the microbiota-gut-brain axis. Gut permeability, gastrointestinal motility, and microbiota composition are all drastically altered in mouse models of stroke (Houlden et al., 2016; Singh et al., 2016; Stanley et al., 2016). Furthermore, fecal microbiota transplantation (FMT) into germ-free (GF) mice from a cerebral ischemia model transmitted functional deficits of proinflammatory T-lymphocyte (Th1 and Th17 Thelper phenotype) trafficking and the ischemia-induced cerebral lesion volume (Singh et al., 2016). To date, only a few studies have preliminarily explored the gut microbiota in IS patients. In a Chinese patient group presenting with stroke or a transient ischemic attack, fecal abundance of three major commensal microbes (Bacteroides, Prevotella, and Faecalibacterium) were severely depleted, with a concomitant enhancement in abundance of opportunistic pathogens such as Enterobacter, Megasphaera, and Desulfovibrio (Yin et al., 2015). Moreover, patients presenting with severe stroke had a greater abundance of Proteobacteria over those with milder stroke.
The INTERSTROKE investigators also found that there are regional and ethnic variations in the effect of risk factors, which was related to differences in risk factors prevalence among regions that may be contribute to worldwide variations in the frequency of IS and support the development population-specific measures to prevent IS (O'Donnell et al., 2016). China is a unified multi-ethnic country with 56 ethnic groups, of which the Han is the largest group, and the other 55 ethnic groups are referred to as ethnic minorities. According to the sixth national population census in 2010, the Miao (9.43 million) and Tujia (8.35 million) minorities were ranked as the fifth and seventh largest minority groups, respectively. Our previous epidemiological studies have found that there was an additive interaction between the ApoB/ApoA1 ratio and ethnicity in the Tujia and Miao populations with first-ever ischemic stroke (the relative excess risk due to the interaction was 5.75) (Zhang et al., 2021), but few reliable data are available to identify the differences in the gut microbiota of IS among the people of Tujia, Miao, and Han. As the data were limited in previous studies, this study aims to study the differences among Tujia, Miao, and Han populations in the gut microbiota of IS patients in Xiangxi Tujia and Miao Autonomous Prefecture (Xiangxi), and to study the association between gut microbiota and traditional risk factors of IS patients for providing new insights into the risk factors, prevention, and management strategies of IS among the people of Tujia, Miao, and Han ethnicities.
Materials and methods
Study population
Participants were recruited between 1 May 2018 and 30 April 2019, from Xiangxi, located in western Hunan Province and adjacent to Hubei and Guizhou Provinces, where people of Tujia and Miao constitute 80% of the total population of 2.98 million. All the participants are residents who had lived in Xiangxi more than 5 years. All patients who admitted to the First Affiliated Hospital of Jishou University and all the eight county people's hospitals of Xiangxi, within 5 days of symptoms onset and 72 h of hospital admission, with a discharge diagnosis of first-ever IS, were selected as a case group. IS was diagnosed with the WHO clinical criteria (Hatano, 1976). A CT or MRI of the brain was completed within 1 week of presentation. All patients also underwent a range of blood tests, a chest radiograph, and an electrocardiography (ECG) at admission. According to the Trial of Org 10,172 in Acute Stroke Treatment (TOAST) criteria (Adams et al., 1993), IS was divided into the following: (1) large-artery atherosclerosis, (2) cardioembolism, (3) small-vessel occlusion, (4) stroke of other determined etiology, and (5) stroke of undetermined etiology. The disease severity was estimated by National Institutes of Health Stroke Scale (NIHSS) score on admission by the physicians. For patients unable to communicate adequately, proxy respondents were used. We defined a valid proxy respondent as a spouse or first-degree relative who was living in the same home or was aware of the participant's previous medical history and present treatments. Exclusion criteria: (1) patients unable to communicate and without a valid surrogate respondent; (2) subdural hemorrhage, tumor, and brain abscess (i.e., non-vascular causes); (3) current hospitalization for acute coronary syndrome/myocardial infarction; and (4) unable to get consent from the subject or the surrogate. Community-based normal controls (NCs) were all from the eight counties and had no history of stroke. Each control was matched for sex, ethnicity, and age (±5 years) for every case. Exclusion criteria for controls are identical to those described for cases. Species accumulation boxplot was used to validate the sample size (Colwell et al., 2004). Interviewer bias may result when knowledge of case or control status influences the manner questions asked or indirectly influences interviewee response. To overcome this potential bias, interviewers are trained to obtain information in a standardized fashion. Recall bias may result when the presence or absence of a medical condition may influence patient's or caregiver's ability to recall events. The objective evidence of risk factors (e.g., history of allergies and history of antibiotic administration) is sought, whenever possible.
Sample collection and processing
Fasting blood samples (10 mL) were taken from IS patients and NCs within 72 h of recruitment, separated by centrifugation, and frozen at −80°C immediately after processing. Samples were shipped in packaging incorporating dry ice-cooling agents by courier from every site to a blood storage site, where they were stored at −80°C. Total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), apolipoprotein B (ApoB), ApoA1, fasting plasma glucose (FPG), high-sensitive C-reactive protein (hs-CRP), and homocysteine (Hcy) concentrations were measured in the laboratory of the Dian Diagnostics Group Co. Ltd with Beckman Coulter AU680 Clinical Chemistry Analyzer and Beckman reagents (Beckman Coulter, Brea, CA, USA). All the participants have not taken antibiotics, probiotics, and prebiotics in the 3 months prior to the feces collection. Feces were collected at hospital or home. The feces were collected according to the instruction and delivered immediately at low temperatures. The frozen feces were shipped using dry ice to Xiangxi Center for Disease Prevention and Control. Once received, fecal samples were divided into three parts of 200 mg and stored at −80°C until extraction.
DNA extraction, PCR amplification, and Ion S5TM XL sequencing
CTAB method was used to extract total genome DNA from samples according to the DNA extraction kit (#DP328, Tiangen Company, Beijing, China). The concentration and purity of the extracted bacterial DNA were detected using Qubit® 2.0 Fluorometer (Thermo Scientific, USA). The 16S rRNA gene V4 regions were amplified used specific primers 515F (5'-GTGCCAGCMGCCGCGGTAA-3') and 806R (5'-GGACTACHVGGGTWTCTA AT-3') with the barcode. All PCR reactions were carried out in 30 μL reactions with 15 μL of Phusion® High-Fidelity PCR Master Mix (New England Biolabs). The mixture of PCR products was purified with GeneJETTM Gel Extraction Kit (Thermo Scientific). Sequencing libraries were generated using Ion Plus Fragment Library Kit 48 rxns (Thermo Scientific) following manufacture's recommendations. The library quality was assessed on the Qubit® 2.0 Fluorometer (Thermo Scientific). At last, the library was sequenced on the Ion S5TM XL platform and 400 bp/600 bp single-end reads were generated.
16S rRNA gene sequence analysis
Quality filtering on the raw reads was performed under specific filtering conditions to obtain the high-quality clean reads according to the Cutadapt (V1.9.1, https://cutadapt.readthedocs.io/en/stable/) quality-controlled process (Martin, 2011). The reads were compared with the reference database (Silva Database, https://www.arb-silva.de/) using UCHIME algorithm (http://www.drive5.com/usearch/manual/uchime_algo.html) to detect chimera sequences (Edgar et al., 2011; Quast et al., 2013), and then the chimera sequences were removed. Then, the Clean Reads finally obtained.
Sequence analyses were performed by Uparse software (Uparse v7.0.1001, http://drive5.com/uparse/) (Edgar, 2013). Sequences with ≥97% similarity were assigned to the same operational taxonomic units (OTUs). Representative sequence for each OTU was screened for further annotation. For each representative sequence, the Silva 138.1 was used based on Mothur algorithm to annotate taxonomic information (Quast et al., 2013). In order to study phylogenetic relationship of different OTUs and the difference of the dominant species in different samples (groups), multiple sequence alignments were conducted using the MUSCLE software (V3.8.31, http://www.drive5.com/muscle/) (Edgar, 2004).
Participants' metadata was obtained about ethnicity, sex, age, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), TC, TG, LDL-C, HDL-C, ApoB/ApoA1 ratio, FPG, hs-CRP, and Hcy. All alpha diversity indices in our samples were calculated with QIIME (V1.7.0) and displayed with R. Wilcoxon rank sum test was used to perform hypothesis test of alpha diversity. Principal coordinates analysis (PCoA) was displayed by WGCNA package, STAT packages, and ggplot2 package in R. Analysis of similarities (ANOSIM) was displayed by function anosim of vegan package in R. The key bacterial taxa responsible for discrimination between NCs and IS patients were identified using linear discriminant analysis (LDA) and LDA effect size (LEfSe). LEfSe is available as a Galaxy module, a Conda formula, a Docker image, and included in bioBakery (VM and cloud) (Segata et al., 2011).
Statistical analysis
Continuous variables were described as mean ± standard deviation (SD) if they were normally distributed or median (interquartile range, IQR) if not normally distributed, and compared using independent samples t-test or Mann-Whitney U-test. Spearman's rank correlation analysis between gut microbiota and traditional risk factors was constructed by corr.test in the psych package from R, after which the visualization work was done by the pheatmap function in pheatmap package. Three measures of biological interaction—relative excess risk of interaction (RERI), attributable proportion due to interaction (API), and synergy index (SI)—with corresponding 95% confidence intervals (CIs) were calculated in R (Andersson et al., 2005; Källberg et al., 2006). The RERI or API equal to 0 or SI equal to 1 were defined as no interaction (Andersson et al., 2005). Statistical analyses and graphics were produced with IBM SPSS Statistics for Windows, V23.0 (Armonk, NY: IBM Corp, Released 2015) and R for Windows (Version 4.0.3). All statistical tests of hypotheses are two-sided. Statistical significance level was set at P < 0.05.
Results
Basic information of recruited subjects
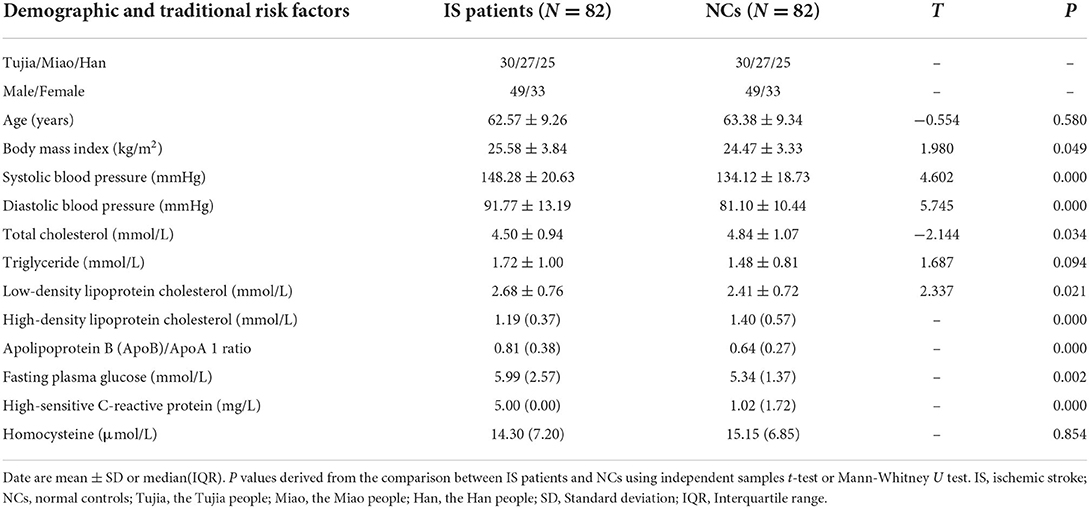
The study included 82 IS patients and 82 NCs, age ranged from 41 to 79 years old. The TOAST classification of IS patients are: large-artery atherosclerosis (n = 34, 41.5%); cardioembolism (n = 1, 1.2%); and small-vessel occlusion (n = 47, 57.3%). The NIHSS score of the IS patients were: 0 as no stroke symptoms (n = 10, 12.2%); 1–4 as minor stroke (n = 48, 58.5%); 5–15 as moderate stroke (n = 24, 29.3%). The mean values of BMI, SBP, DBP, TC, TG, LDL-C, HDL-C, ApoB/ApoA1 ratio, FPG, hs-CRP, and Hcy were 25.58 ± 3.84 kg/m2, 148.28 ± 20.63 mmHg, 91.77 ± 13.19 mmHg, 4.50 ± 0.94 mmol/L, 1.72 ± 1.00 mmol/L, 2.68 ± 0.76 mmol/L, 1.19 (0.37) mmol/L, 0.81 (0.38), 5.99 (2.57) mmol/L, 5.00 (0.00) mg/L, and 14.30 (7.20) μmol/L, respectively, in IS patients. The mean values of the same clinical indexes were 24.47 ± 3.33 kg/m2, 134.12 ± 18.73 mmHg, 81.10 ± 10.44 mmHg, 4.84 ± 1.07 mmol, 1.48 ± 0.81 mmol/L, 2.41 ± 0.72 mmol/L, 1.40 (0.57) mmol/L, 0.64 (0.27), 5.34 (1.37) mmol/L, 1.02 (1.72) mg/L, and 15.15 (6.85) μmol/L, respectively, in NCs. Differences of BMI, SBP, DBP, TC, LDL-C, HDL-C, ApoB/ApoA1 ratio, FPG, and hs-CRP between the two groups were both statistically significant. The detailed ethnicity and sex characteristics of the two groups are shown in Table 1.
Differences of gut microbiota composition between normal controls and ischemic stroke patients
In total, we identified 12,808,213 high-quality reads in 164 fecal samples with an average length of 253. The species accumulation boxplot is an effective method to evaluate whether the sample size is sufficient or not. The boxplot is tended to be flat (Figure 1A), indicating that the sequencing sample size was ample, and the results were reliable. The top 10 in relative abundance of gut microbiota (in the level of phylum, class, order, family, genus, and species, respectively) between NCs and IS patients among Tujia, Miao, and Han ethnicity are shown in Supplementary Figure 1. Detailed information about alpha diversity indexes between NCs and IS patients among Tujia, Miao, and Han ethnicity are shown in Supplementary Figure 2. A PCoA was performed to investigate the extent of the similarity of the microbial communities between NCs and IS patients based on unweighted UniFrac distance metrics (Figure 1B); the analysis indicated that the microbiota composition of IS patients was more heterogeneous and significantly different from NCs. These reads could be annotated and classified into 3,536 OTUs in the entire population by the Silva 138.1 Database, and 90.27% of OTUs were annotated in the level of family. So the follow-up analysis and discussion were based on the family level of gut microbiota. In total, 2,647 OTUs were shared between the NCs and IS groups, while 598 and 291 OTUs were unique to NCs and IS patients, respectively (Figure 1C). As shown in Figure 1D, Venn diagram displayed 637 unique OTUs in the NCs and 426 unique OTUs in the IS patients, and 1,940 OTUs were shared by both groups of the Tujia people. In the Miao people, 1,994 OTUs were shared between NCs and IS patients, while 548 and 390 OTUs were unique to NCs and IS patients, respectively (Figure 1E). In the Han people, 1,834 OTUs were shared between NCs and IS patients, while 550 and 544 OTUs were unique to NCs and IS patients, respectively (Figure 1F).

Figure 1. The differences of OTUs between normal controls (NCs) and ischemic stroke (IS) patients in the entire population and for each ethnic people (Tujia, Miao, and Han) according to the 16S rRNA data. The IS patients of the Tujia, Miao, and Han people were indicated with IS1, IS2, and IS3, respectively. The NCs of the Tujia, Miao, and Han people were indicated with NC1, NC2, and NC3, respectively. (A) Species accumulation boxplot of the entire population. (B) Principal coordinate analysis (PCoA) of the microbiota based on the unweighted UniFrac distance metrics for NCs and IS patients. ANOSIM, R = 0.067, P = 0.001. (C–F) Venn diagram of the observed OTUs in NCs and IS patients in the entire population and for each ethnic people (Tujia, Miao, and Han).
The microbial signatures responsible for distinguishing ischemic stroke patients from normal controls
LDA and LEfSe were used to identify the key gut microbes responsible for distinguishing the IS patients from NCs. In the entire population, 13 differential OTUs were identified (Figure 2A), compared to NCs, patients with IS were characterized by eight enriched OTUs mainly belonging to classes Bacilli and Gammaproteobacteria, and five depleted OTUs are mainly belonging to class Clostridia. Lactobacillaceae, Streptococcaceae, and Enterobacteriaceae families were more abundant in IS patients, while Lachnospiraceae and Ruminococcaceae families were more abundant in NCs (Figure 2B). We further assessed the ethnicity-related microbial signatures responsible for distinguishing IS patients from NCs. However, only 11 differential OTUs were identified in the Tujia people (Figure 2C), compared to NCs, patients with IS were characterized by nine enriched OTUs mainly belonging to classes Bacilli, Gammaproteobacteria, Actinobacteria, Rubrobacteria, and Coriobacteriia, and only two depleted OTUs are belonging to class Clostridia. Lactobacillaceae, Streptococcaceae, and Enterobacteriaceae families were more abundant in IS patients, while only Ruminococcaceae family was more abundant in NCs (Figure 2D). And only six differential OTUs were identified in the Miao people (Figure 2E), compared to NCs, patients with IS were characterized by only six enriched OTUs mainly belonging to class Bacilli but not depleted OTUs; Lactobacillaceae and Streptococcaceae families were more abundant in IS patients (Figure 2F). In the Han population, 13 differential OTUs were identified (Figure 2G), compared to NCs, patients with IS were characterized by nine enriched OTUs mainly belonging to classes Bacilli and Gammaproteobacteria, and four depleted OTUs are mainly belonging to class Clostridia. Lactobacillaceae, Enterococcaceae, and Enterobacteriaceae families were more abundant in IS patients, while only Ruminococcaceae family was more abundant in NCs (Figure 2H).

Figure 2. The differences of gut microbiota between normal controls (NCs) and ischemic stroke (IS) patients in the entire population and for each ethnic people(Tujia, Miao, and Han). The IS patients of the Tujia, Miao, and Han people were indicated with IS1, IS2, and IS3, respectively. The NCs of the Tujia, Miao, and Han people were indicated with NC1, NC2, and NC3, respectively. (A–H) Histograms of the LDA scores for the bacterial taxa differentially abundant between NCs (green bars) and IS patients (red bars) (LDA score >4.0, P < 0.05), and cladograms generated by LEfSe indicating differences in the bacterial taxa between NCs (green dots) and IS patients (red dots) in the entire population and for each ethnic people(Tujia, Miao, and Han).
Traditional risk factors correlate with gut microbiota in ischemic stroke patients
To explore the potential associations between gut microbiota and traditional risk factors in IS patients, regardless of ethnicity differences, we constructed a correlation analysis using Spearman's rank correlation coefficients (Figure 3). BMI was negatively correlated with the abundance of families Veillonellaceae and X.Eubacterium, coprostanoligenes_group. SBP was positively correlated with the abundance of Enterococcaceae, Enterobacteriaceae, Streptococcaceae, Erysipelotrichaceae, Fusobacteriaceae, Lactobacillaceae, and Succinivibrionaceae families, while negatively correlated with Bacteroidaceae, Lachnospiraceae, Ruminococcaceae, and Sutterellaceae families, respectively (P < 0.05). DBP was positively correlated with the abundance of Enterobacteriaceae, Streptococcaceae, Fusobacteriaceae, and Succinivibrionaceae families, while negatively correlated with Ruminococcaceae and Sutterellaceae families, respectively (P < 0.05). However, LDL-C was positively correlated with only the abundance of Barnesiellaceae family (P < 0.05). Moreover, HDL-C was positively correlated with the abundance of Peptostreptococcaceae and Veillonellaceae families, while negatively correlated with Enterococcaceae, Barnesiellaceae, and Synergistaceae families (P < 0.05). The abundance of Enterococcaceae, Barnesiellaceae, Tannerellaceae, and Succinivibrionaceae families wase positively correlated, while Lachnospiraceae, Ruminococcaceae, and Pasteurellaceae families were negatively correlated with ApoB/ApoA1 ratio (P < 0.05). FPG was positively correlated with only the abundance of Synergistaceae family, while negatively correlated with Bacteroidaceae, Ruminococcaceae, and Pasteurellaceae families (P < 0.05). In addition, hs-CRP was positively correlated with the abundance of Enterococcaceae, Enterobacteriaceae, Streptococcaceae, Erysipelotrichaceae, Fusobacteriaceae, Lactobacillaceae, Succinivibrionaceae, and Synergistaceae, but negatively correlated with families Lachnospiraceae, Ruminococcaceae, and Sutterellaceae (P < 0.05).

Figure 3. Association between gut microbiota and traditional risk factors of ischemic stroke. The heat map of Spearman's rank correlation coefficients of top 30 gut microbiota in family and 9 traditional risk factors (BMI, SBP, DBP, TC, LDL-C, HDL-C, ApoB/ApoA1 ratio, FPG, and hs-CRP, respectively, just as differences of these risk factors between normal controls and ischemic stroke patients were all statistically significant in Table 1). The correlation effect was indicated by a color gradient from blue (negative correlation) to red (positive correlation). The statistical significance was denoted on the squares (*P < 0.05, **P < 0.01). BMI indicates body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; LDLC, low-density lipoprotein cholesterol; HDLC, high-density lipoprotein cholesterol; ApoBvsA1, apolipoprotein B (ApoB)/ApoA1 ratio; FPG, fasting plasma glucose; hsCRP, high-sensitive C-reactive protein.
Additive interaction analysis between traditional risk factors and gut microbiota for first-ever ischemic stroke
To further explore the influence between traditional risk factors and gut microbiota on first-ever IS, the additive interaction analyses (6 × 9 = 54) were performed with six families of gut microbiota and nine traditional risk factors. The LEfSe results showed that six families, Lactobacillaceae, Streptococcaceae, Enterobacteriaceae, Enterococcaceae, Lachnospiraceae, and Ruminococcaceae, were enriched in NCs or IS patients (Figure 2). Nine traditional risk factors were BMI, SBP, DBP, TC, LDL-C, HDL-C, ApoB/ApoA1 ratio, FPG and hs-CRP, respectively, just as the differences of these risk factors between NCs and IS patients were all statistically significant in Table 1. Only the additive interaction analysis results with the statistical significance were listed in this paper.
As previous studies on microbioal biosphere usually set 0.01% as the relative abundance threshold for rare taxa (Galand et al., 2009; Anderson et al., 2015), and the median of relative abundance of family Enterococcaceae was 0.01%, so set 0.01% as the relative abundance threshold for dichotomous variable in this paper. For the interaction analysis, the relative abundance is as follows of family Enterococcaceae ≥0.01% = 1 and <0.01% = 0, SBP ≥ 140 mmHg = 1 and <140 mmHg = 0, DBP ≥ 90 mmHg = 1 and <90 mmHg = 0, h-CRP ≥ 5.0 mg/L = 1 and <5.0 mg/L = 0, and OR00 = 1. In the R program, the function used a logistic regression model to estimate ORs with 95% CIs. Then, we used the epi.interaction function to calculate additive interactions by means of RERI, API, and SI with 95% CIs. The results of the interaction analysis showed that there were additive interactions between traditional risk factors (SBP, DBP, and hs-CRP) and family Enterococcaceae for first-ever IS. The risks of IS related to SBP, DBP, and hs-CRP were significantly higher in the enriched family Enterococcaceae (OR 21.00: 95% CI 7.31–69.67, 27.52: 9.00–99.15, 205.00: 50.81–1187.07, respectively) than in the depleted family Enterococcaceae (2.80: 0.93–9.24, 3.82: 1.25–12.39, 29.29: 7.52–153.62, respectively). Although adjusted for age, sex, and ethnicity, the risks of IS related to SBP, DBP, and hs-CRP remained significantly higher in the enriched family Enterococcaceae than in the depleted family Enterococcaceae, ORs are shown in the Tables 2–4, respectively.

Table 2. Additive interaction analysis between SBP and family Enterococcaceae for first-ever ischemic stroke.

Table 3. Additive interaction analysis between DBP and family Enterococcaceae for first-ever ischemic stroke.

Table 4. Additive interaction analysis between hs-CRP and family Enterococcaceae for first-ever ischemic stroke.
Discussion
In this case–control study, we examined gut microbiome composition between NCs and IS patients among Han, Tujia, and Miao ethnicity, and then analyzed correlations between gut microbes and traditional risk factors. Furthermore, we explored the additive interaction between traditional risk factors and gut microbiota for first-ever IS. We found that in the entire population Lactobacillaceae, Enterococcaceae, Streptococcaceae, and Enterobacteriaceae families enriched in IS patients; however, Ruminococcaceae and Lachnospiraceae families enriched in NCs, so the following discussion will focus on these six families enriched in IS patients or NCs.
Furthermore, we found that Lactobacillaceae, Enterococcaceae, Streptococcaceae, and Enterobacteriaceae families enriched in IS patients were positively correlated with high-risk factors (SBP, DBP, ApoB/ApoA1 ratio, FPG, and hs-CRP) and negatively correlated with the preventive factor (HDL-C) in the IS patients, respectively. However, Ruminococcaceae and Lachnospiraceae families enriched in NCs were negatively correlated with high-risk factors (SBP, DBP, ApoB/ApoA1 ratio, FPG, and hs-CRP). Lactobacillus rhamnosus GG and Lactobacillus casei DN-114001 are mostly recognized as probiotics and beneficial microbes (Dietrich et al., 2014); however, increased abundance of family Lactobacillaceae was observed among acute IS patients in a Chinese study (Tan et al., 2021). Lactobacillus was found to be enriched in the high-risk group compared to the low-risk group in a study of 141 participants aged ≥60 years without prior history of stroke (Zeng et al., 2019). A recent study using a middle cerebral artery occlusion model to explore gut dysbiosis post-stroke in mice revealed a reciprocal relationship between stroke and gut dysbiosis; ischemic stroke rapidly triggers gut microbiome dysbiosis with Enterobacteriaceae overgrowth that in turn exacerbates brain infarction (Xu et al., 2021). The interaction analysis also showed that there were additive interactions between traditional risk factors (SBP, DBP, and hs-CRP) and family Enterococcaceae for first-ever IS. Few human clinical studies evaluating the efficacy of interventions targeting the gut–brain axis have been published (Vendrik et al., 2020; Yuan et al., 2021). FMT is a novel technique that is beginning to gain traction in the realm of recovery from neurologic injury; however, the lack of human studies on the efficacy of this intervention limits its current applicability in the clinical setting (Panther et al., 2022). Our findings suggest that gut microbiota disturbances could potentially contribute to IS pathogenesis by modulating the host's traditional risk factors, which provides a new avenue by which to understand the prevention of IS.
Consistent with other studies that reported important regional variations in the relative importance of most individual risk factors for stroke may relate to differences in the magnitude of association by ethnic origin (Hyun et al., 2013; Eastwood et al., 2015). Our results also demonstrated that the gut microbiota's taxonomic composition between NCs and IS patients varies in different ethnicity; Streptococcaceae family was only more abundant in IS patients of Tujia and Miao people; Enterococcaceae and Enterobacteriaceae families were only more abundant in IS patients of Han people, and Ruminococcaceae family was only more abundant in NCs of Tujia and Han people. This suggests the possibility that regional and ethnic variations in the effect of traditional risk factors for IS may be linked to the differences of gut microbiota in different ethnic people, which suggests the necessity to more deeply understand the ethnic differences of risk factors as they pertain to prevention and treatment of IS.
However, 16S RNA amplicon sequencing represents a comparatively cheap way to measure the relative abundance of microbes present in a sample using next-generation sequencing technology, there are also important limitations. First, 16S sequencing cannot identify novel microbial species nor account for intraspecies variation and mutations as the technique is restricted to genetic reference sequences that must be defined in a database a priori. Second, PCR introduces a bias in 16S tables, as some amplicon sequences will inevitably be amplified more efficiently than others (Acinas et al., 2005). Besides the cheaper cost, because 16S sequencing relies on the 16S subunit ribosomal RNA, which is unique to prokaryotes, most host contamination is not an issue. Also, the data were cross-section rather than longitudinal, and dietary considerations were not included. It would also be interesting to perform dynamic metagenomic and metabolomic analyses to uncover how the gut microbiome and its modulated metabolic pathways dynamically change in IS.
Conclusion
In conclusion, we first found that gut microbiome were associated with first-ever IS and had ethnic variations of this relationship in the Xiangxi autonomous prefecture, and the gut microbiota disturbances could potentially contribute to IS pathogenesis by modulating the host's traditional risk factors. Race/ethnicity categories may help interpret health-related data and are important in ensuring that prevention programs of IS remain generalizable to diverse populations.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found in the Genome Sequence Archive in BIG Data Center, Beijing Institute of Genomics (BIG), Chinese Academy of Sciences, under accession numbers CRA006722, the shared URL is https://ngdc.cncb.ac.cn/search/?dbId=gsa&q=CRA006722.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committees of the Xiangya School of Public Health Central South University (XYGW-2018-26). The patients/participants provided their written informed consent to participate in this study.
Author contributions
HT, NZ, and HW designed the study. NZ, XWa, MT, YT, QL, CL, and XP recruited participants, collected basic data and samples. NZ and XWu analyzed the data and wrote the manuscript. HT, JD, and XWu contributed to discussion and reviewed/edited the manuscript. HT and XWu supervised the study and the guarantor of this work. All authors read and approved the final manuscript.
Funding
This study was supported by National Natural Science Foundation of China (81773535); Key Research and Development Program of Hunan Province (2018SK2061); the Fundamental Research Funds for Central Universities of the Central South University (2019zzts328); Scientific Research Project of Hunan Provincial Health Commission (C2014-17, 202212053368); Changsha Municipal Social Science Foundation (kh200502).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnmol.2022.922399/full#supplementary-material
References
Acinas, S. G., Sarma-Rupavtarm, R., Klepac-Ceraj, V., and Polz, M. F. (2005). PCR-induced sequence artifacts and bias: insights from comparison of two 16S rRNA clone libraries constructed from the same sample. Appl. Environ. Microbiol. 71, 8966–8969. doi: 10.1128/AEM.71.12.8966-8969.2005
Adams, H. P. Jr, Bendixen, B. H., Kappelle, L. J., Biller, J., Love, B. B., Gordon, D. L., et al. (1993). Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. trial of org 10172 in acute stroke treatment. Stroke. 24, 35–41. doi: 10.1161/01.STR.24.1.35
Anderson, R. E., Sogin, M. L., and Baross, J. A. (2015). Biogeography and ecology of the rare and abundant microbial lineages in deep-sea hydrothermal vents. FEMS. Microbiol. Ecol. 91, 1–11. doi: 10.1093/femsec/fiu016
Andersson, T., Alfredsson, L., Källberg, H., Zdravkovic, S., and Ahlbom, A. (2005). Calculating measures of biological interaction. Eur. J. Epidemiol. 20, 575–579. doi: 10.1007/s10654-005-7835-x
Colwell, R. K., Mao, C. X., and Chang, J. (2004). Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology 85, 2717–2727. doi: 10.1890/03-0557
Dietrich, C. G., Kottmann, T., and Alavi, M. (2014). Commercially available probiotic drinks containing lactobacillus casei DN-114001 reduce antibiotic-associated diarrhea. World. J. Gastroenterol. 20, 15837–15844. doi: 10.3748/wjg.v20.i42.15837
Dinan, T. G., and Cryan, J. F. (2017). Gut-brain axis in 2016: Brain-gut-microbiota axis-mood, metabolism and behaviour. Nat. Rev. Gastroenterol. Hepatol. 14, 69–70. doi: 10.1038/nrgastro.2016.200
Eastwood, S. V., Tillin, T., Chaturvedi, N., and Hughes, A. D. (2015). Ethnic differences in associations between blood pressure and stroke in South Asian and European men. Hypertension 66, 481–488. doi: 10.1161/HYPERTENSIONAHA.115.05672
Edgar, R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic. Acids. Res. 32, 1792–1797. doi: 10.1093/nar/gkh340
Edgar, R. C. (2013). UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods.10, 996–998. doi: 10.1038/nmeth.2604
Edgar, R. C., Haas, B. J., Clemente, J. C., Quince, C., and Knight, R. (2011). UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 27, 2194–2200. doi: 10.1093/bioinformatics/btr381
Fung, T. C., Olson, C. A., and Hsiao, E. Y. (2017). Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 20, 145–155. doi: 10.1038/nn.4476
Galand, P. E., Casamayor, E. O., Kirchman, D. L., and Lovejoy, C. (2009). Ecology of the rare microbial biosphere of the Arctic Ocean. Proc. Natl. Acad. Sci. USA. 106, 22427–22432. doi: 10.1073/pnas.0908284106
GBD 2017 Causes of Death Cllaborators. (2018). Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the global burden of disease study 2017. Lancet. 392, 1736–1788. doi: 10.1016/S0140-6736(18)32203-7
Hatano, S. (1976). Experience from a multicentre stroke register: a preliminary report. Bull. World. Health. Organ. 54, 541–553.
Houlden, A., Goldrick, M., Brough, D., Vizi, E. S., Lénárt, N., Martinecz, B., et al. (2016). Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain. Behav. Immun. 57, 10–20. doi: 10.1016/j.bbi.2016.04.003
Hyun, K. K., Huxley, R. R., Arima, H., Woo, J., Lam, T. H., Ueshima, H., et al. (2013). A comparative analysis of risk factors and stroke risk for Asian and non-Asian men: the Asia pacific cohort studies collaboration. Int J Stroke. 8, 606–611. doi: 10.1111/ijs.12166
Källberg, H., Ahlbom, A., and Alfredsson, L. (2006). Calculating measures of biological interaction using R. Eur. J. Epidemiol. 21, 571–573. doi: 10.1007/s10654-006-9037-6
Martin, M. (2011). CUTADAPT removes adapter sequences from high-throughput sequencing reads. EM Bnet. J. 17, 10–12. doi: 10.14806/ej.17.1.200
O'Donnell, M. J., Chin, S. L., Rangarajan, S., Xavier, D., Liu, L., Zhang, H., et al. (2016). Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet 388, 761–775. doi: 10.1016/S0140-6736(16)30506-2
Panther, E. J., Dodd, W., Clark, A., and Lucke-Wold, B. (2022). Gastrointestinal microbiome and neurologic injury. Biomedicines 10:500. doi: 10.3390/biomedicines10020500
Quast, C., Pruesse, E., Yilmaz, P., Gerken, J., Schweer, T., Yarza, P., et al. (2013). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic. Acids. Res. 41, D590–D596. doi: 10.1093/nar/gks1219
Segata, N., Izard, J., Waldron, L., Gevers, D., Miropolsky, L., Garrett, W. S., et al. (2011). Metagenomic biomarker discovery and explanation. Genome. Biol. 12, R60. doi: 10.1186/gb-2011-12-6-r60
Sharon, G., Sampson, T. R., Geschwind, D. H., and Mazmanian, S. K. (2016). The central nervous system and the gut microbiome. Cell 167, 915–932. doi: 10.1016/j.cell.2016.10.027
Singh, V., Roth, S., Llovera, G., Sadler, R., Garzetti, D., Stecher, B., et al. (2016). Microbiota dysbiosis controls the neuroinflammatory response after stroke. J Neurosci. 36, 7428–7440. doi: 10.1523/JNEUROSCI.1114-16.2016
Stanley, D., Mason, L. J., Mackin, K. E., Srikhanta, Y. N., Lyras, D., Prakash, M. D., et al. (2016). Translocation and dissemination of commensal bacteria in post-stroke infection. Nat. Med. 22, 1277–1284. doi: 10.1038/nm.4194
Tan, C., Wu, Q., Wang, H., Gao, X., Xu, R., Cui, Z., et al. (2021). Dysbiosis of gut microbiota and short-chain fatty acids in acute ischemic stroke and the subsequent risk for poor functional outcomes. JPEN. J. Parenter. Enteral. Nutr. 45, 518–529. doi: 10.1002/jpen.1861
Vendrik, K., Ooijevaar, R. E., de Jong, P., Laman, J. D., van Oosten, B. W., van Hilten, J. J., et al. (2020). Fecal microbiota transplantation in neurological disorders. Front. Cell. Infect. Microbiol. 10:98. doi: 10.3389/fcimb.2020.00098
Wang, W., Jiang, B., Sun, H., Ru, X., Sun, D., Wang, L., et al. (2017). Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480 687 adults. Circulation. 135, 759–771. doi: 10.1161/CIRCULATIONAHA.116.025250
Xu, K., Gao, X., Xia, G., Chen, M., Zeng, N., Wang, S., et al. (2021). Rapid gut dysbiosis induced by stroke exacerbates brain infarction in turn. Gut. gutjnl-2020-323263. doi: 10.1136/gutjnl-2020-323263. [Epub ahead of print].
Yin, J., Liao, S. X., He, Y., Wang, S., Xia, G. H., Liu, F. T., et al. (2015). Dysbiosis of gut microbiota with reduced trimethylamine-n-oxide level in patients with large-artery atherosclerotic stroke or transient ischemic attack. J Am. Heart. Assoc.4:e002699. doi: 10.1161/JAHA.115.002699
Yuan, B., Lu, X. J., and Wu, Q. (2021). Gut microbiota and acute central nervous system injury: a new target for therapeutic intervention. Front. Immunol. 12:800796. doi: 10.3389/fimmu.2021.800796
Zeng, X., Gao, X., Peng, Y., Wu, Q., Zhu, J., Tan, C., et al. (2019). Higher risk of stroke is correlated with increased opportunistic pathogen load and reduced levels of butyrate-producing bacteria in the gut. Front. Cell. Infect. Microbiol. 9:4. doi: 10.3389/fcimb.2019.00004
Zhang, N., Wu, X., Tian, M., Wang, X., Ding, J., Tian, Y., et al. (2021). Additive interaction between potentially modifiable risk factors and ethnicity among individuals in the Han, Tujia and Miao populations with first-ever ischaemic stroke. BMC. Public. Health. 21, 1059. doi: 10.1186/s12889-021-11115-x
Keywords: ischemic stroke, gut microbiota, additive interaction, risk factor, prevention
Citation: Zhang N, Wang H, Wang X, Tian M, Tian Y, Li Q, Liang C, Peng X, Ding J, Wu X and Tan H (2022) Combination effect between gut microbiota and traditional potentially modifiable risk factors for first-ever ischemic stroke in Tujia, Miao and Han populations in China. Front. Mol. Neurosci. 15:922399. doi: 10.3389/fnmol.2022.922399
Received: 17 April 2022; Accepted: 15 August 2022;
Published: 25 October 2022.
Edited by:
Chan Chen, Sichuan University, ChinaReviewed by:
Frederik Denorme, The University of Utah, United StatesBrandon Peter Lucke-Wold, University of Florida, United States
Copyright © 2022 Zhang, Wang, Wang, Tian, Tian, Li, Liang, Peng, Ding, Wu and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongzhuan Tan, dGFuaHo5OUBxcS5jb20=; Jian Ding, ODA5ODQzMTIzQHFxLmNvbQ==; Xinrui Wu, NjE5MTY1NDM3QHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share senior authorship
 Na Zhang1,2†
Na Zhang1,2† Xinrui Wu
Xinrui Wu Hongzhuan Tan
Hongzhuan Tan