
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mol. Neurosci. , 13 October 2022
Sec. Brain Disease Mechanisms
Volume 15 - 2022 | https://doi.org/10.3389/fnmol.2022.1015906
One major factor regulating the strength of GABAB receptor signaling and thereby neuronal excitability is the dynamic control of their cell surface expression. GABAB receptors are constitutively internalized and recycled back to the plasma membrane to maintain a stable number of receptors at cell surface for appropriate signaling. Protein phosphatase 2A (PP2A) dependent dephosphorylation of serine 783 (S783) in the GABAB2 subunit is a key event for downregulating GABAB receptor cell surface expression particularly under conditions associated with excitotoxicity. Here, we investigated the role of PP2A in regulating GABAB receptor cell surface expression under physiological and excitotoxic conditions. For this purpose, we developed an interfering peptide (PP2A-Pep) that inhibits the interaction of GABAB receptors with PP2A. Using cultured cortical neurons, we found that PP2A downregulates GABAB receptor cell surface expression by inhibiting recycling of the receptors and thereby promoting degradation of the receptors. Inhibition of the GABAB receptor/PP2A interaction by PP2A-Pep in cultured cortical neurons restored GABAB receptor cell surface expression after excitotoxic stress and inhibited progressing neuronal death even when added 48 h after the insult. To explore the therapeutic potential of PP2A-Pep, we further analyzed effect of PP2A-Pep in the middle cerebral artery occlusion (MCAO) mouse model of cerebral ischemia. Incubation of brain slices prepared from MCAO-treated mice with PP2A-Pep restored normal GABAB receptor expression and GABAB receptor-mediated inhibition, reduced ischemic-induced overexcitability of neurons, and prevented neuronal death in the ischemic penumbra. This data illustrates the crucial role of regulating GABAB receptor phosphorylation by PP2A for controlling neuronal inhibition and excitability. The results further suggest that interfering with the GABAB receptor/PP2A interaction is a promising strategy for the development of specific therapeutic interventions to treat neurological diseases associated with a disturbed excitation/inhibition balance and downregulation of GABAB receptors.
The GABAB receptor is one of the key regulators of neuronal excitability by mediating sustained inhibition (Chalifoux and Carter, 2011). They are expressed at pre- and postsynaptic sites in most neurons and are involved in virtually all fundamental brain functions. GABAB receptors are G protein-coupled receptors (GPCRs) composed of the two subunits, GABAB1 and GABAB2 (Bettler et al., 2004; Gassmann and Bettler, 2012; Terunuma, 2018). Binding of GABA to the receptors activates Gi/o proteins which in turn regulate several downstream effectors. GABAB receptor-induced neuronal inhibition is mainly mediated by hyperpolarization of the neuronal plasma membrane via enhancing potassium influx through G protein-coupled inwardly rectifying potassium (GIRK or Kir3) channels (Gähwiler and Brown, 1985; Luscher et al., 1997; Chalifoux and Carter, 2011) and by inhibition of neurotransmitter release via suppression of voltage-gated Ca2+ channels (Mintz and Bean, 1993; Santos et al., 1995; Chen and van den Pol, 1998; Bussieres and El Manira, 1999; Chalifoux and Carter, 2011).
A crucial determinant for the strength of GABAB receptor-mediated inhibition is the number of receptors available at the cell surface for signaling. This is regulated by a precise balance of distinct trafficking mechanisms including receptor exocytosis, endocytosis, recycling, and degradation (Benke, 2010). GABAB receptors are constitutively internalized via clathrin- and dynamin-dependent endocytosis. Internalized receptors are rapidly recycled back to the cell surface or targeted to lysosomes for degradation. Under pathological conditions associated with neuronal overexcitation, the balance of recycling and degradation of the receptors is shifted toward degradation, thereby downregulating cell surface expression of GABAB receptors (Guetg et al., 2010; Maier et al., 2010; Terunuma et al., 2010; Kantamneni et al., 2014). This is a very detrimental mechanism since one fundamental function of GABAB receptors is to counter-balance neuronal hyperexcitability to prevent neurons to enter a state of overexcitation that may lead to excitotoxic death.
Two key events have been identified causing the aberrant sorting of the receptors to lysosomes for degradation. (1) Sustained activation of glutamate receptors induces Ca2+/calmodulin-dependent protein kinase II (CaMKII)-mediated phosphorylation of serine 867 of GABAB1 (Guetg et al., 2010). This phosphorylation event serves as a signal for K63-linked ubiquitination of the receptor by the E3-ligase Mind-bomb 2 (MIB2) and sorts the receptors to lysosomes for degradation (Zemoura et al., 2019). (2) Activation of protein phosphatase 2A (PP2A) dephosphorylates serine 783 of GABAB2. This most likely affects constitutive internalization and recycling of the receptors (Terunuma et al., 2010).
There is mounting evidence that PP2A-mediated dephosphorylation of GABAB2(S783) and the associated reduction of cell surface GABAB receptors plays, beside in cerebral ischemia, an important role in neurological diseases such as addiction (Maeda et al., 2006; Padgett et al., 2012; Hearing et al., 2013) and depression (Lecca et al., 2016). In either case, blocking PP2A activity with specific inhibitors reduced disease symptoms in mouse models of the diseases, indicating PP2A as promising drug target. However, systemic inhibition of PP2A activity is not a favored option for a therapeutic intervention because PP2A is involved in a variety of physiological functions and global inhibition of PP2A is expected to be associated with severe side effects. Therefore, a more targeted strategy addressing specifically the GABAB receptor/PP2A interaction might be a promising solution to this problem.
In this study, we aimed at clarifying in greater detail the trafficking pathway(s) affected by enhanced PP2A-dependent dephosphorylation of GABAB2(S783) and exploring the potential therapeutic benefit of targeting specifically the GABAB receptor/PP2A interaction. For this, we developed a short interfering peptide (PP2A-Pep) able to block the interaction of PP2A with GABAB receptors. We used PP2A-Pep for analyzing the pathway affected by PP2A in untreated and glutamate-stressed cultured neurons and tested it for its effects on GABAB receptor expression, function, and neuroprotective activity in the MCAO mouse model of cerebral ischemia.
Animal experiments were performed according to the national guidelines of the Swiss Federal act on animal protection. All animal experiments were approved by the Zurich cantonal veterinary office, Zurich, Switzerland (license ZH152/16, ZH011/19, and ZH031/16).
Middle cerebral artery occlusion experiments were performed unblinded using 9–12 weeks old C57BL/6J male mice. Animals were housed up to five per cage with a standard 12 h light/12 h dark cycle. Food and water were available ad libitum. The mice were randomly assigned to MCAO or sham operation. Primary neuron/glia cultures were prepared from E18 embryos of Wistar rats. All animals used in this study were purchased from ENVIGO (Netherlands).
The following primary antibodies were used in this study: mouse anti-EEA1 (1:25, BD Biosciences Cat# 610456), mouse anti-GABAB2 (1:400, Abcam ab181736), rabbit anti-GABAB2 (1:400, Abcam ab75838), rabbit anti-GABAB2N (1:250, custom-made by GeneScript, Benke et al., 2002), mouse anti-HA-tag (1:400, Sigma Aldrich, H9658), rabbit anti-NeuN (1:400, Millipore ABN78), rabbit anti-PP2A (1:400, MyBioSource MBS858915), mouse anti-Rab4 (1:25, BD Biosciences Cat# 610888) and rabbit anti-Rab7 (1:50, Abcam ab137029). Secondary antibodies: donkey anti-rabbit AlexaFluor Plus 488 (1:2000, Thermo Fisher Scientific A32790), donkey anti-mouse AlexaFluor Plus 555 (1:2000, Thermo Fisher Scientific A32773), goat anti-rabbit AlexaFluor Plus 800 (1:2000, Thermo Fisher Scientific A32735), and AlexaFluor 488-conjugated streptavidin (1:500, Jackson ImmunoResearch 016-540-084). All normal laboratory chemicals used were purchased from Sigma-Aldrich.
The interfering peptide (PP2A-Pep) contains a sequence derived from the intracellularly located C-terminal domain of GABAB2 (FQFTQNQKKEDSKTSTSV). For controls, a peptide (Ctrl-Pep) containing the same amino acids but in a random sequence (QKFSVNTFQEKDTKSQTS) was used. The peptides were rendered cell-permeable by tagging them at their N-terminus with a peptide sequence derived from the Rabies virus glycoprotein followed by 9 arginine residues (YTIWMPENPRPGTPCDIFTNSRGKRASNGGGG-RRRRRRRRR) (Kumar et al., 2007). Peptides were custom-synthesized by Pepmic Co., Ltd, Suzhou, China with an N-terminal biotin for detection and used at a concentration of 10 μg/ml throughout the experiments.
Cortical neurons were prepared from E18 Wistar rat embryos as described previously (Buerli et al., 2007). Briefly, cortices from embryos were dissected and washed with 5 ml PBGA buffer [PBS, 10 mM glucose, antibiotic-antimycotic solution (1:100, Invitrogen)]. The tissue was digested in papain solution (0.5 mg/ml papain, 1 mg/ml BSA, 10 μg/ml DNAse I and 10 mM glucose in PBS) for 15 min at 37°C and thereafter washed twice with 2 ml Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal calf serum (FCS) and 1:100 antibiotic-antimycotic solution (complete DMEM). Tissue was triturated with a fire-polished Pasteur pipette and cells were plated at a density of 60,000 cells/ml onto poly-L-lysine coated 18 mm coverslips placed in a 12 well culture plate. After incubation overnight in complete DMEM at 37°C and 5% CO2, the complete DMEM medium was exchanged for NU-medium (MEM, supplemented with 15% Nu-serum, 2% B27 supplement, 15 mM HEPES pH 7.1, 0.45% glucose, 1 mM sodium pyruvate, 2 mM GlutaMAX) and cells were incubated at 37°C and 5% CO2 for further 11–15 days.
For expression of recombinant proteins, cultured neurons were transfected with 1 μg of the respective plasmid DNA by magnetofection using Lipofectamine 2000 (Invitrogen) and CombiMag (OZ Biosciences) at 7–12 DIV following a previously described protocol (Buerli et al., 2007). Neurons were analyzed 48–96 h after transfection. The following cDNAs in expression vectors were used: EGFP (gift from Dr. Shiva Tyagarajan), GABAB1 (Kaupmann et al., 1997), HA-tagged GABAB2 (Kaupmann et al., 1998), HA-tagged GABAB2(S783A) (mutation inserted by Genescript), HA-tagged GABAB2(S783D) (mutation inserted by Genescript) and GABAB2(BBS) tagged with the HA-tag and the minimum α-bungarotoxin binding site (BBS) (attached to the extracellularly located N-terminus using standard cloning techniques), HA-tagged PP2A-C (catalytic subunit of PP2A, Evans et al., 1999), and HA-tagged PP2A-C(L199P) (inactive mutant of PP2A-C, Evans et al., 1999).
Coverslips were rinsed in PBS and transferred to 4% PFA/4% sucrose solution for 15 min at room temperature. After fixation, cells were washed with PBS and permeabilized by incubation for 12 min in PBS containing 0.2% Triton X-100. Then, cultures were incubated with primary antibodies diluted in PBS supplemented with 10% normal donkey serum (NDS) in a humidity chamber overnight at 4°C. After incubation, cells were washed three times with PBS and incubated with secondary antibody diluted in PBS, 10% NDS for 1h at room temperature. Finally, coverslips were washed with PBS, dried, and mounted upside down on glass slides (SuperFrost Plus, Thermo Scientific) with DAKO fluorescent mounting medium (Agilent Technologies).
Coverslips were washed twice with pre-cooled Krebs solution (2 mM CaCl2, 2 mM MgCl2, 5 mM KCl, 30 mM glucose, 25 mM, 119 mM NaCl, HEPES pH 7.4) and then incubated on ice for 2 h with GABAB2N antibody directed against the extracellularly located N-terminus of GABAB2 (1:250 dilution in Krebs solution containing 10% NDS). The coverslips were washed three times for 5 min with ice cold Krebs solution and incubated for 1 h with donkey anti-rabbit secondary antibody in PBS/10% NDS. Cells were fixated with 4% PFA/4% sucrose for 15 min at room temperature. After fixation, the cells were washed twice with PBS, dried and mounted on glass slides with DAKO fluorescent mounting medium.
We used the highly sensitive in situ proximity ligation assay (PLA) (Soderberg et al., 2006; Leuchowius et al., 2010) to test for the interaction of GABAB receptors with PP2A and for their colocalization with the endosomal marker proteins EEA1, Rab4, and Rab7. Cultures were washed with PBS, fixated with 4% PFA/4% sucrose for 20 min at room temperature and permeabilized with 0.2% Triton X-100 in PBS for 10 min. Neurons were then incubated overnight at 4°C with the appropriate pair of antibodies for GABAB2 and either PP2A, EEA1, Rab4 or Rab7. Subsequently, in situ PLA was performed using the Duolink kit (Sigma Aldrich) exactly according to the manufacturer’s instructions. AlexaFluor Plus 488-conjugated secondary antibodies were added during the final polymerization step to stain for total GABAB receptor expression.
Excitotoxic conditions were induced by incubating cultures with 50 μM glutamate (Sigma Aldrich) for 1 h. For this, half of the medium (1 ml) was removed from the cultures and stored at 37°C and 5% CO2. Then, cultures were treated with 50 μM glutamate (Sigma Aldrich) for 1 h at 37°C and 5% CO2, washed thereafter with PBS and further incubated for 12–16 h in the saved original conditioned culture medium supplemented with PP2A-Pep (10 μg/ml) or with Ctrl-Pep (10 μg/ml) before testing.
To determine the neuroprotective activity of PP2A-Pep, PP2A-Pep, or Ctrl-Pep were added at different timepoints after the glutamate stress (3, 6, 9, 12, 24, and 48 h) and cells were analyzed 24 h following each timepoint. Hence, neurons were exposed to the peptides for equal duration.
To test for neuron survival, cultures were stained with an antibody directed against the neuron-specific marker protein NeuN, followed by staining with AlexaFluor Plus 488-conjugated secondary antibodies. The total number of cells was determined by counting the cell nuclei stained with DAPI (1:2000) added with the secondary antibody. Surviving neurons were quantified by counting the number of neurons in relation to the number of DAPI positive nuclei (mainly glia cells) using ImageJ software.
Monitoring GABAB receptor internalization by life cell imaging of GABAB receptors tagged with the α-bungarotoxin binding site (BBS) was performed as described previously by Hannan et al. (2013) with minor modifications. The minimum sequence for the α-bungarotoxin binding site (BBS, WRYYESSLEPYPD) along with two HA-tag sequences (YPYDVPDYA-YPYDVPDYA) were attached to the extracellularly located N-terminus of GABAB2 [GABAB2(BBS)] using standard cloning techniques. Cultures were transfected at DIV 7–9 with GABAB2(BBS) together with EGFP (for fast identification of transfected neurons) and cultured for further 72–96 h. For live cell imaging, cultures were washed with ice-cold Krebs solution (140 mM NaCl, 4.7 mM KCl, 1.2 mM MgCl2, 2.5 mM CaCl2, 11 mM glucose, and 5 mM HEPES pH 7.4), preincubated with 1 mM D-tubocurarin (Sigma-Aldrich) for 5 min to block endogenous nicotinic acetylcholine receptors containing the α7 subunit and then incubated with 3 μg/ml AlexaFlour 555-conjugated α-bungarotoxin (BTX, Invitrogen, B35451) for 15 min on ice to label all GABAB receptors containing GABAB2(BBS) expressed at the cell surface (incubation at low temperature inhibited internalization of the receptors). Following three washes with ice-cold Krebs solution to remove excess BTX, neurons were transferred to the imaging chamber with warm Krebs solution at room temperature to permit internalization of the receptors. Cells expressing EGFP were located as quickly as possible, and settings were optimized for acquiring BTX florescence at time zero (t0). Imaging of a single plane was performed at Nyquist sampling and eight times averaging to increase the signal/noise ratio. Glutamate (50 μM) and PP2A-Pep (10 μg/ml) were added after adjusting the imaging settings directly to the imaging chamber. Cells were captured at the indicated timepoints without modifying the acquisition setting.
The rate of GABAB receptor internalization was assessed by measuring the mean fluorescence intensity at the cell surface at every timepoint. Measurements were normalized to the mean cell surface membrane fluorescence obtained at t0 timepoint.
Middle cerebral artery occlusion (MCAO) was performed on naïve adult male C57Bl/6J mice as described previously (Hleihil et al., 2021). Briefly, mice were anesthetized with isoflurane and analgesia was provided by injection of buprenorphine and lidocaine. The left common carotid artery was exposed through a midline incision in the neck and unilateral MCAO was conducted by inserting a 7-0 silicone rubber-coated monofilament (Doccol Corp., Sharon, MA, United States, 701956PK5Re) to occlude the middle cerebral artery. During the occlusion, the mice were kept at 37°C in a ventilated recovery chamber. After exactly 60 min, the filament was withdrawn to allow reperfusion. After additional 60 min in the recovery chamber the mice were euthanized for the ex vivo analysis. In sham operated mice, the filament was inserted into left middle cerebral artery and immediately withdrawn to allow instant reperfusion. The Bederson score for testing impaired neurological/motor function (Schaar et al., 2010) was used to verify successful MCAO surgery.
Patch clamp electrophysiology was performed on acute brain slices obtained from MCAO or sham operated mice. After 60 min reperfusion, brains were extracted and cut into 300 μm coronal cortical slices using a vibratome with a horizontally oscillating blade (HM 650, Microm). Slices were then incubated in oxygenated artificial cerebrospinal fluid (ACSF, 125 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 25 mM NaHCO3, 1 mM MgCl2, 2 mM CaCl2, and 25 mM glucose, pH 7.4, osmolarity 315 mOsm) supplemented with 10 μg/ml PP2A-Pep or Ctrl-Pep at 34°C for 30 min and then at room temperature until analysis. Recordings were performed 2–6 h after peptide treatment at 32–34°C from cortical pyramidal neurons, which were identified by their shape, large soma and firing patterns.
To measure baclofen-induced currents, borosilicate glass patch pipettes (3.5–4.5 mΩ) were filled with potassium gluconate based intracellular solution consisting of 135 mM potassium gluconate, 2 mM NaCl, 4 mM KCl, 4 mM EGTA, 10 mM HEPES, 4 mM Mg-ATP, and 0.3 mM Na3GTP, pH 7.3, 295 mOsm. Neurons were held at –50 mV and GABAB receptor-mediated currents were measured as a response to bath application of 100 μM baclofen (Tocris Bioscience). For some neurons, GABAB receptor-mediated GIRK currents were confirmed by application of the GABAB receptor antagonist CGP36742 (10 μM, Sigma Aldrich).
Neuronal excitability was determined in current-clamp experiments using 135 mM potassium gluconate intracellular solution. Throughout all experiments, cells were maintained at their original membrane potential. To measure the current-voltage (I–V) curve, a series of 50 pA current steps were performed (from –350 to 450 pA, 250 ms duration). The input resistance was calculated from the voltage responses after applying a 150 pA hyperpolarizing current pulse in the current clamp mode. The resting membrane potential (Vm) was determined immediately after acquiring the whole cell mode and the firing threshold (Vth) was determined by injection of depolarization current steps (5 pA, 250 ms) until the first action potential was generated.
Recordings were excluded from the analysis if either the series resistance (Rs) varied by more than 20% throughout the experiment or the resting membrane potential was depolarized more than -55 mV. Currents were filtered at 5 kHz and digitized at 20 kHz. All recordings were performed using a Multiclamp 700B amplifier, acquired with Digidata 1550A, and analyzed offline using Clampfit 10.5 (Molecular Devices).
Brain sections obtained from MCAO or sham operated mice were incubated with PP2A-Pep to test its neuroprotective activity and its ability to restore GABAB receptor expression. Brains were handled and cut similar to acute brain slice preparation procedure for electrophysiology (see above) with small modifications. Brains were sliced into 90 μm thick coronal using a vibrotome, followed by incubation in a modified 12-well culture plate containing ACSF with constant oxygenation (95% O2 and 5% CO2). Peptides (10 μg/ml) were added to each well simultaneously and slices were incubated at room temperature for 6 h with constant oxygenation. Subsequently, the slices were fixated with 4% PFA for 60 min at room temperature. Staining was performed with free-floating sections. Sections were incubated with rabbit anti-NeuN and mouse anti-GABAB2, diluted in Tris-Triton solution (50 mM Tris pH 7.4, 150 mM NaCl, 0.2% Triton X-100) containing 2% NDS, overnight at 4°C. After three washes for 10 min with Tris-Triton, sections were incubated with secondary antibodies diluted in Tris-Triton/2% NDS for 1 h at room temperature [donkey anti-mouse AlexaFluor Plus 555, donkey anti-rabbit AlexaFluor Plus 647, and AlexaFluor 488-conjugated streptavidin (to stain for biotin-tagged peptides)]. After five washes with Tris-Triton, sections were placed onto glass slides (SuperFrost Plus, Thermo Scientific), dried at room temperature and coversliped with DAKO fluorescence mounting medium.
Stained brain sections and neuron/glia cultures were analyzed by confocal laser scanning microscopy (LSM 700 or LSM 800, Zeiss) using 40x (1.4 NA) or 63x (1.4 NA) plan-apochromat oil differential interference contrast objectives and sampled sequentially in 4–5 z-planes according to the Nyquist criterions. For some experiments an optical zoom was applied, and sampling parameters were optimized accordingly. For quantitative analysis, all images belonging to an experiment were recorded with identical settings. Analysis was performed after merging the optical planes into a single image.
Images were recorded using the Zen 2.6 software (Zeiss) and further data analysis was conducted with ImageJ Fiji (Schindelin et al., 2012). For analysis of cell surface staining, the outer and the inner border of the plasma membrane were outlined, and the mean fluorescence intensity was measured. Then the value of the inner surface was subtracted from the value of the outer surface. For analysis of total staining, the outer border of each cell was outlined, and the mean fluorescence intensity was measured. The quantification of in situ PLA signals was performed using the ImageJ plug-in Analyze Particles. A Gaussian blur filter was applied (sigma = 1), followed by background subtraction (Rolling Ball radius = 30). The build-in Moments algorithm was applied to determine the signal threshold for all images. For each experiment, images were reviewed separately, and the noise tolerance was set to ensure that all PLA signals were counted. The PLA signals of neurons were normalized to the total expression level of GABAB2 and to the area analyzed.
The data are depicted as means ± SD. Statistical evaluation of data sets were performed with either one-way or two-way analysis of variance (ANOVA) followed by Tukey’s post hoc tests using GraphPad Prism 8. D’Agostino-Pearson test and QQ plots were employed to test for normal or lognormal distributions. In case of significant deviation from homoscedasticity Welch and Brown Forsythe variations of ANOVA was used. Differences were considered statistically significant when p < 0.05. The statistical test used is indicated in the respective figure legends.
It was previously shown that dephosphorylation of GABAB2 at S783 by PP2A is involved in glutamate and NMDA receptor-mediated downregulation of GABAB receptors (Terunuma et al., 2010). To confirm that the phosphorylation state of S783 in GABAB2 regulates GABAB receptor expression, we transfected primary cortical neurons with either wild type HA-tagged GABAB2, a HA-tagged GABAB2 mutant containing a serine-to-alanine substitution at position 783 (S783A) or with serine-to-aspartate substitution (S783D) to mimic the persistent dephosphorylation and phosphorylation status of the receptor, respectively (Figure 1A). As expected, mimicking dephosphorylation of GABAB(S783) (S783A) significantly reduced GABAB receptor expression, while mimicking phosphorylation (S783D) enhanced GABAB receptor expression as compared to neurons transfected with wild type HA-tagged GABAB2 (Figure 1A).
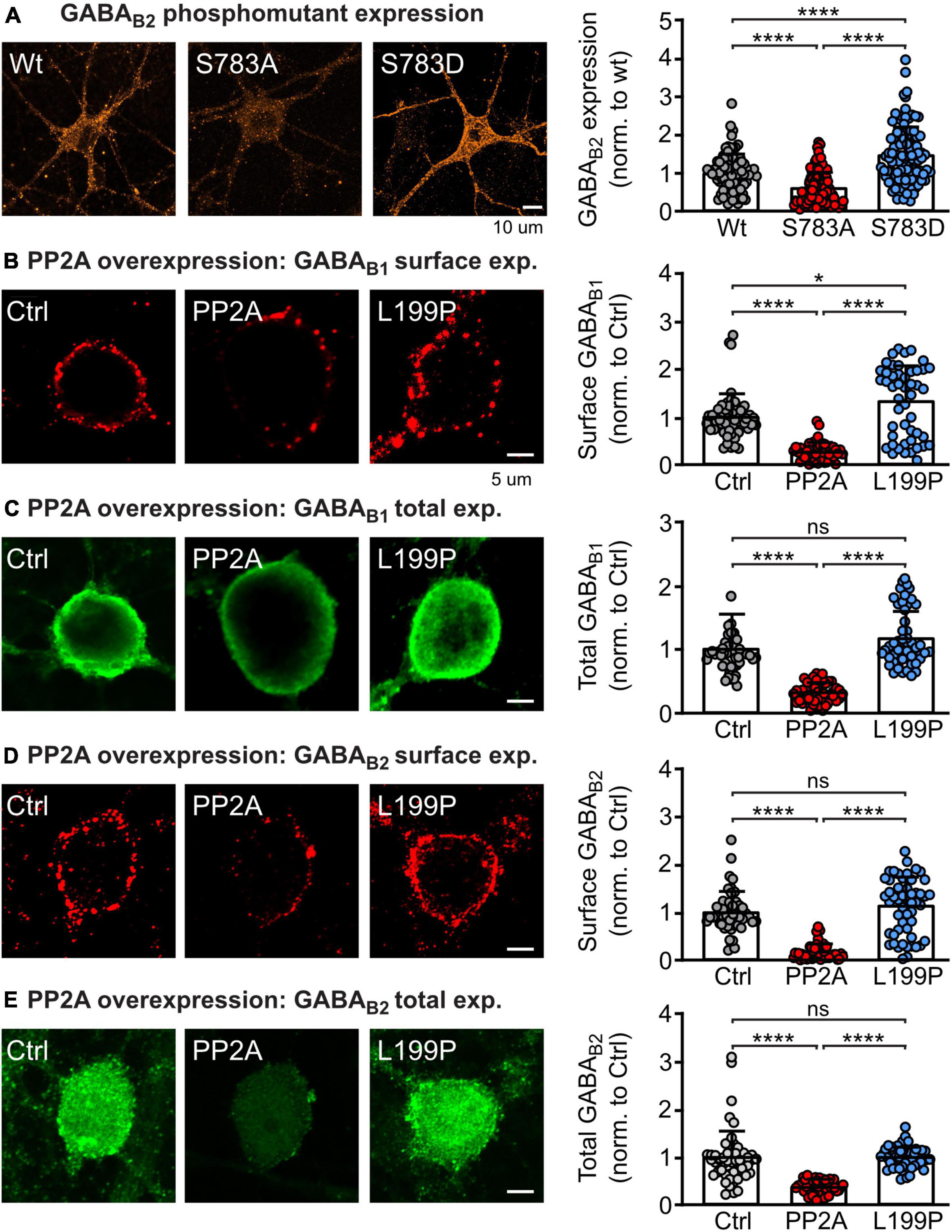
Figure 1. PP2A regulates GABAB receptor expression. (A) Phosphorylation/dephosphorylation of GABAB2(S783) affects expression of GABAB receptors. Neurons were transfected either with wild type HA-tagged GABAB2, a HA-tagged GABAB2 mutant with inactivated S783 phosphorylation site 783 (S783A) or with a mutation mimicking phosphorylation of this site (S783D) along with wild type GABAB1. Expression of HA-tagged GABAB receptors was determined after 2 days by staining with antibodies directed against the HA-tag. Left: Representative images of total GABAB receptor expression (scale bar: 10 μm). Right: Quantification of GABAB2 staining. Expression levels were normalized to the control (wild type HA-GABAB2). The data represent the mean ± SD of 87–144 neurons from three independent. Brown–Forsythe and Welch ANOVA with Games Howell’s multiple comparison test (****p < 0.0001). (B–E) Overexpression of the catalytic subunit of PP2A (PP2A-C) downregulates total and cell surface expression of GABAB receptors in neurons. Neurons were transfected with EGFP and either PP2A-C or an inactive mutant of PP2A-C (L199P). Cell surface and total endogenous GABAB1 (B,C) and endogenous GABAB2 (D,E) was determined after 2 days via staining with GABAB1 and GABAB2 antibodies. Left: Representative images (scale bar: 5 μm). Right: Quantification of GABAB receptor staining. Signals were normalized to the control (only transfected with EGFP). The data represent the mean ± SD of 87–144 neurons from three independent. Brown–Forsythe and Welch ANOVA with Games Howell’s multiple comparison test (ns, p > 0.05, *p < 0.05, ****p < 0.0001).
To confirm that PP2A is involved in the regulation of GABAB receptor expression, we transfected cortical neurons with the catalytic subunit of PP2A (PP2A-C, constantly active) or with a dominant negative mutant thereof (L199P) and determined endogenous total and cell surface expression of GABAB1 and GABAB2 (Figures 1B–E). Overexpressing PP2A-C reduced total and cell surface expression of both GABAB receptor subunits. By contrast, overexpressing the inactive mutant of the catalytic subunit of PP2A (L199P) did largely not affect receptor expression.
These results strengthen previous data indicating that dephosphorylation of GABAB2(S783) by PP2A is crucial for determining the expression level of GABAB receptor (Terunuma et al., 2010).
A prerequisite for the ability of PP2A to dephosphorylate GABAB2(S783) is its interaction with GABAB receptors (Terunuma et al., 2010; Li et al., 2020). To interfere with the PP2A-mediated dephosphorylation of GABAB receptors, we aimed to develop a targeted approach to specifically inhibit the PP2A/GABAB receptor interaction. Most likely the C-terminal domain of GABAB2 is involved in constituting the interaction site with PP2A. We therefore screened several small peptide sequences of the C-terminal domain of GABAB2 for their ability to increase cell surface expression of GABAB receptors. The effect of three candidate peptides (R2C1, R2C2, and R2C3) located upstream of the coiled-coil domain of GABAB2, adjacent or partially overlapping with the S783 phosphorylation site targeted by PP2A (Figure 2A), is shown in Figure 2B. Among these peptides, R2C1 and R2C2 mediated enhancement cell surface GABAB receptor expression in cultured neurons under control conditions and after glutamate stress, which downregulated receptor expression (Figures 2B,B’). R2C3 did not affect GABAB receptor expression and therefore the sequence of R2C3 appears not to contribute to the GABAB receptor/PP2A interaction site. We selected R2C2 for all further experiments because it most reliably increased receptor expression. This peptide (designated PP2A-Pep) was tagged at the N-terminus with a peptide sequence derived from the Rabies virus glycoprotein to render it cell-permeable (Kumar et al., 2007). A control peptide (Ctrl-Pep), consisting of the same amino acids but in a random sequence, was used to verify the specificity of PP2A-Pep in main experiments.
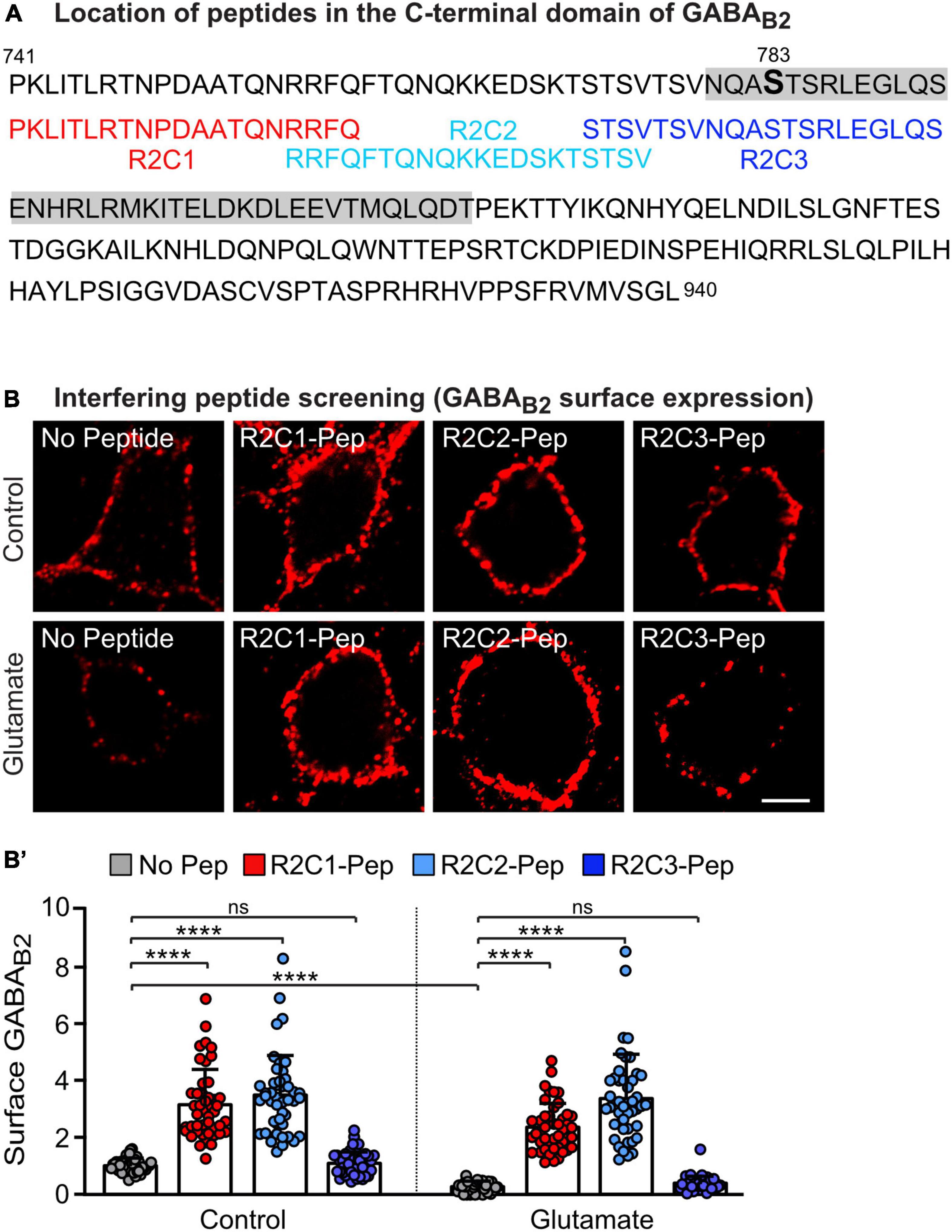
Figure 2. Screening for a peptide sequence present in the C-terminal domain of GABAB2 that blocks glutamate-induced downregulation of GABAB receptors. (A) Sequence of the C-terminal domain of GABAB2 with the coiled-coil domain indicated in gray and the overlapping peptides R2C1, R2C2 and R2C3 used in (B) shown below. (B,B’) Peptides R2C1 and R2C2 increased expression of GABAB receptors and restored glutamate-induced downregulated receptor cell surface levels. Cultures were stressed for 60 min with glutamate (50 μM) and immediately thereafter treated for 16 h with the peptides indicated in (A). Neurons were then tested for cell surface expression of endogenous GABAB receptors using antibodies directed against the N-terminus of GABAB2. (B) Representative images of GABAB2 cell surface staining (scale bar: 5 μm). (B’) Quantification of the fluorescence intensities. The data were normalized to cultures not treated with glutamate and peptides and represent the mean ± SD of 45 neurons derived from three independent experiments. Two-way ANOVA followed by Tukey’s multiple comparison test (ns, p > 0.05, ****p < 0.0001.
Next, we tested whether PP2A-Pep inhibits the interaction of PP2A with GABAB receptors in primary cortical cultures using the in situ PLA. We detected PLA signals in neurons, suggesting that PP2A is involved in regulating GABAB receptor expression under basal conditions (Figure 3A). The PLA signals were largely inhibited by the treatment with PP2A-Pep, indicating that PP2A-Pep largely prevented the interaction of PP2A with GABAB receptors. As expected, the control peptide (Ctrl-Pep) had no effect (Figure 3A).
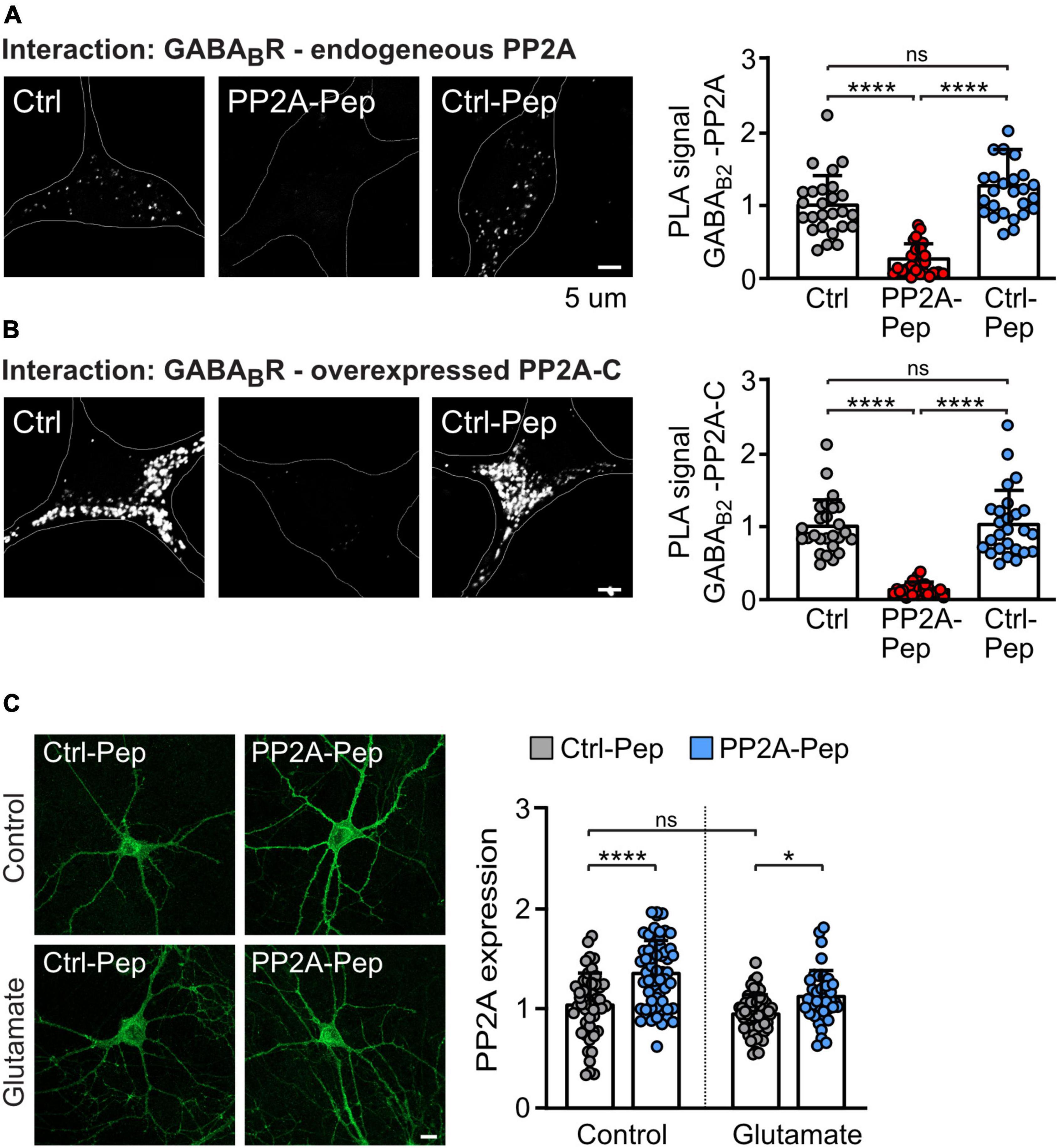
Figure 3. PP2A-Pep inhibits the interaction of GABAB receptors with PP2A and does not downregulate PP2A expression. (A,B) PP2A-Pep prevents the PP2A/GABAB receptor interaction. (A) The interaction of endogenous PP2A with endogenous total (cell surface and intracellular) GABAB receptors in the presence or absence of PP2A-Pep (10 μM) was analyzed by in situ PLA using antibodies directed against PP2A and GABAB2. (B) The interaction of GABAB receptors and the overexpressed catalytic subunit of PP2A (PP2A-C) in the presence or absence of PP2A or Ctrl-Pep was analyzed by in situ PLA. Neurons were co-transfected with EGFP for identification of PP2A-C overexpressing neurons. Neurons transfected only with EGFP and not treated with peptides served as controls. Left: representative images (white dots represent interactions, scale bar: 5 μm). Right: quantification of the in situ PLA signals. The data were normalized to control cultures not treated with peptides and represent the mean ± SD of 26 neurons derived from three independent experiments. Brown–Forsythe and Welch ANOVA with Games Howell’s multiple comparison test (ns, p > 0.05, *p < 0.05, ****p > 0.0001). (C) PP2A-Pep does not downregulate PP2A expression. Glutamate-stressed and unstressed neurons were treated with PP2A-Pep (10 μM) and analyzed for PP2A expression. Left: representative images (scale bar: 10 μm). Right: quantification of the immunofluorescence. The data were normalized to control cultures not treated with glutamate and peptide and represent the mean ± SD of 38–60 neurons derived from three independent experiments. Two-way ANOVA followed by Tukey’s multiple comparison test (ns, p > 0.05; *p < 0.05, ****p < 0.0001).
Furthermore, overexpression of the catalytic subunit of PP2A (PP2A-C) in neurons strongly enhanced PLA signals, indicating an increased interaction of PP2A with GABAB receptors (Figure 3B). In addition, this result suggests that the catalytic subunit of PP2A is involved in the interaction with GABAB receptors. In contrast to the inactive Ctrl-Pep, PP2A-Pep largely abolished the interaction (Figure 3B). These results indicate that PP2A-Pep prevents the interaction of GABAB receptor with PP2A.
Next, we tested whether PP2A-Pep alters the expression of PP2A as it most likely binds to PP2A to prevent its binding to GABAB receptors which might induce the degradation of this complex. However, treatment of neurons with PP2A-Pep resulted in a slight (but statistically significant) enhancement of PP2A expression in both control and glutamate stressed neurons, a condition which aberrantly downregulates GABAB receptors (Guetg et al., 2010; Maier et al., 2010; Terunuma et al., 2010; Figure 3C).
The dephosphorylation of GABAB2(S783) by PP2A downregulates receptor expression most likely via aberrant postendocytic sorting after sustained NMDA receptor activation (Terunuma et al., 2010). However, the underlying mechanism is largely unclear. Here, we hypothesized that blocking PP2A-mediated dephosphorylation of GABAB receptors using PP2A-Pep enhances cell surface expression by reducing receptor internalization. To test this, we tagged GABAB2 at the extracellularly located N-terminus with the binding site for α-bungarotoxin [GABAB2(BBS)], transfected it into cultured neurons and labeled cell surface receptors containing GABAB2(BBS) with AF555-conjugated α-bungarotoxin followed by live cell imaging with or without glutamate (Figure 4).
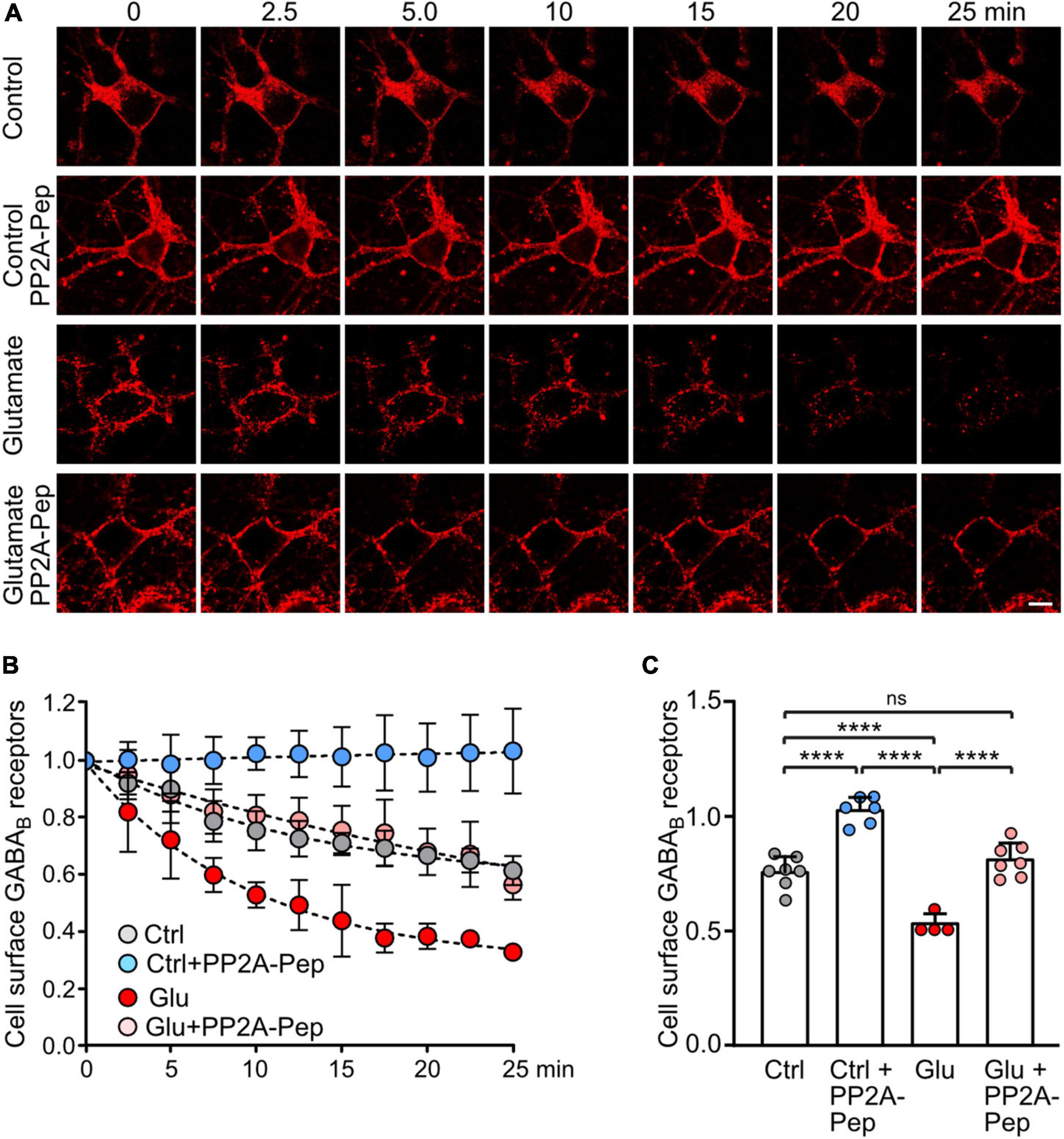
Figure 4. PP2A-Pep prevents constitutive internalization of GABAB receptors and normalizes glutamate-induced loss of cell surface receptors. Neurons were transfected with GABAB2 tagged with the binding site for α-bungarotoxin [GABAB2(BBS)] along with EGFP for quick identification of transfected neurons and with wild type GABAB1. After labeling cell surface receptors containing GABAB2(BBS) with AlexaFluor 555 conjugated α-bungarotoxin at 4°C, neurons were analyzed at room temperature by life cell imaging for internalization of labeled receptors at the indicated time points and treatments. (A) Representative images (scale bar: 10 μm). (B) Quantification of the immunofluorescence signal. (C) Statistical evaluation of data 10 min after initiation of internalization. The data represent the mean ± SD of 4–7 neurons derived from three independent experiments. Two-way ANOVA followed by Tukey’s multiple comparison test (ns, p > 0.05, ****p < 0.0001).
Cell surface GABAB receptors were reduced to 75 ± 7% of initial receptors 10 min after initiating internalization, indicating rapid internalization of receptors (Figures 4B,C). Treatment of neurons with PP2A-Pep completely prevented loss of cell surface receptors (102 ± 6% of initial receptors), suggesting that PP2A is involved in regulating constitutive endocytosis of GABAB receptors. Application of glutamate induced a pronounced reduction in cell surface receptors 10 min after initiating endocytosis (58 ± 4% of initial receptors). Treatment of neurons with PP2A completely prevented aberrant glutamate-induced downregulation of GABAB receptors back to normal control conditions (81 ± 7%, Figures 4B,C).
To further analyze the pathway underlying PP2A-induced downregulation of GABAB receptors, we quantitatively determined the co-localization of the receptors with marker proteins for early endosomes (EEA1) (Mu et al., 1995; Wilson et al., 2000), fast recycling endosomes (Rab4) (van der Sluijs et al., 1992; Daro et al., 1996) and late endosomes (Rab7) (Feng et al., 1995) using in situ PLA. Treatment of neurons with PP2A-Pep enhanced colocalization of the receptors with early endosomes and fast recycling endosomes but reduced colocalization with late endosomes (Figure 5). These results suggest that PP2A regulates internalization and fast recycling of the receptors and thereby sorting of the receptors to lysosomal degradation.
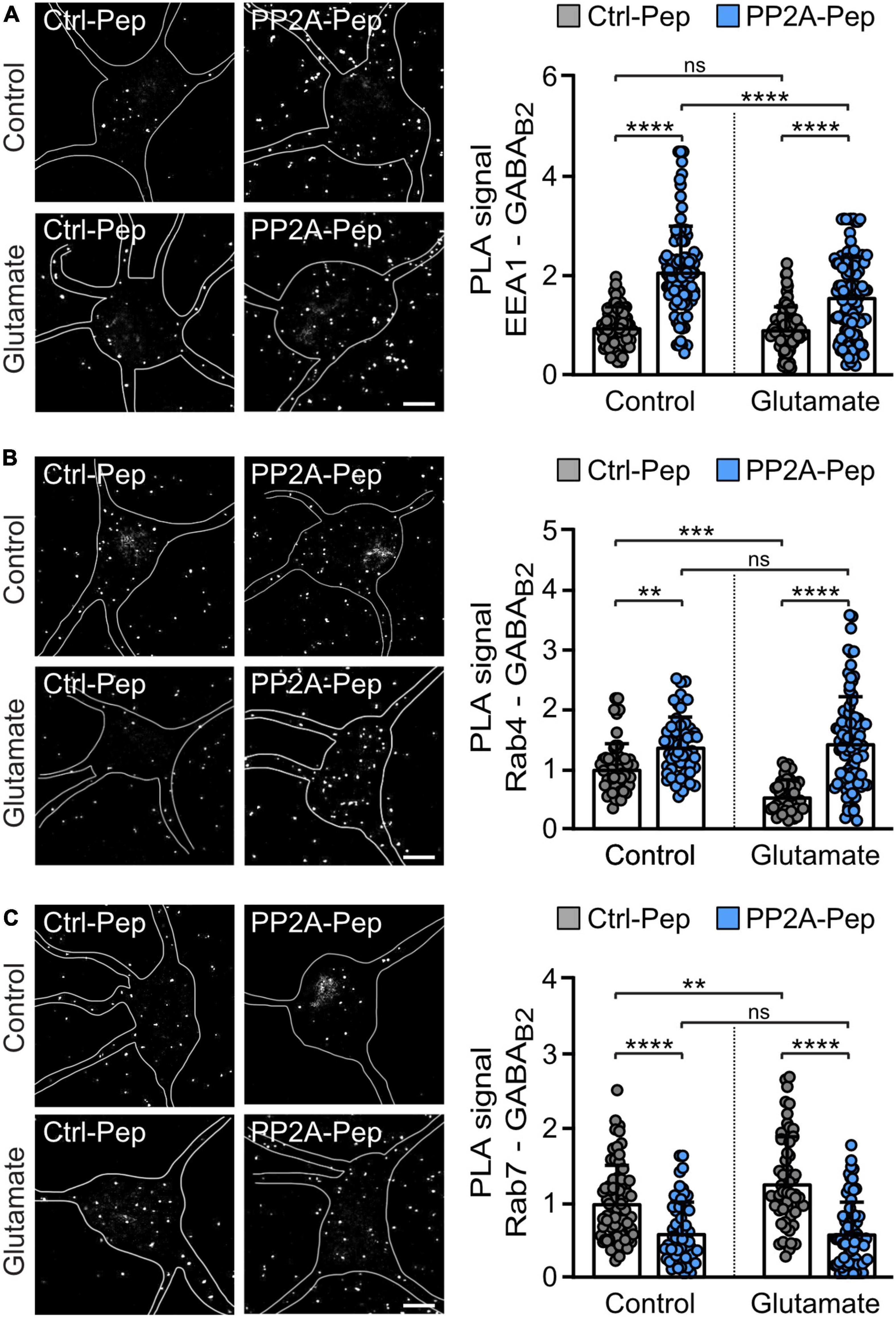
Figure 5. PP2A-Pep increased fast recycling of GABAB receptors and reduced their sorting to lysosomal degradation. Cultured neurons were stressed or not (control) with glutamate (50 μM for 1 h) followed by incubation with PP2A-Pep. Neurons were then analyzed for the colocalization of GABAB receptors with endosomal markers by in situ PLA using antibodies directed against GABAB2 and EEA1 (A), Rab4 (B), and Rab7 (C). The location and shape of neurons analyzed in the representative images are outlined. Please note that PLA signals outside of the outlined neurons are associated with dendrites of adjacent neurons, which were not marked. (A) PP2A-Pep treatment increased the colocalization of GABAB receptors with the early endosome marker EEA1. Left: representative images (scale bar, 10 μm). Right: quantification of PLA signals (white dots). The data represent the mean ± SD of 94–114 neurons per condition from five independent experiments. Two-way ANOVA followed by Tukey’s multiple comparison test (ns, p > 0.05; ****p < 0.0001). (B) PP2A-Pep treatment increased the colocalization of GABAB receptors with the fast-recycling endosome marker Rab4. Left: representative images (scale bar, 10 μm). Right: quantification of PLA signals. The data represent the mean ± SD of 42–93 neurons per condition from three independent experiments. Two-way ANOVA followed by Tukey’s multiple comparison test (ns, p > 0.05, **p < 0.005, ***p < 0.0005, ****p < 0.0001). (C) PP2A-Pep treatment reduced colocalization of GABAB receptors with the late endosome marker Rab7. Left: representative images (scale bar, 10 μm). Right: quantification of PLA signals. The data represent as mean ± SD of 58–74 neurons per condition from three independent experiments. Two-way ANOVA followed by Tukey’s multiple comparison test (ns, p < 0.05, **p < 0.01, ****p < 0.0001).
Under excitotoxic conditions (treatment of neurons with glutamate), GABAB receptors are rapidly downregulated by inhibiting recycling of the receptors and sorting them to lysosomes for degradation (Guetg et al., 2010; Maier et al., 2010; Terunuma et al., 2010). This is reflected by reduced colocalization of the receptors with fast recycling endosomes (Rab4 positive) and enhanced colocalization with late endosomes (Rab7 positive) after treatment of neurons for one hour with 50 μM glutamate (Figure 5). Treatment of glutamate-stressed neurons with PP2A-Pep normalized the aberrant sorting of the receptors back to control levels by restoring fast recycling (colocalization with Rab4-positive endosomes and EEA1-positive endosomes), which reduced lysosomal targeting of the receptors (colocalization with Rab7 positive endosomes) (Figure 5).
Our previous work showed that stabilization of cell surface GABAB receptors by its persistent activation with the agonist baclofen or restoring GABAB receptor expression using interfering peptides targeting the interaction of GABAB receptors with CaMKII or CHOP, respectively, mediates neuroprotection after ischemic stress in vitro and ex vivo (Hleihil et al., 2021; Balakrishnan et al., 2022; Bhat et al., 2022). We therefore tested if treatment with PP2A-Pep limits glutamate induced neuronal death. Cortical neurons were stressed for 60 min with glutamate and treated with PP2A-Pep at different time intervals thereafter (0, 3, 6, 12, 24, and 48 h). The number of surviving neurons was determined 24 h after treatment with the peptide at each timepoint. As expected, treatment with PP2A-Pep following glutamate stress inhibited progressing neuronal death at virtually all time points tested (Figure 6). Notably, treatment of neurons with PP2A-Pep 48 h after the excitotoxic stress still limited progressing neuronal death, indicating a very wide time window for the neuroprotective activity of PP2A-Pep. This finding further supports our hypothesis that restoring GABAB receptor expression by blocking the PP2A/GABAB receptor interaction limits neuronal death under excitotoxic conditions.
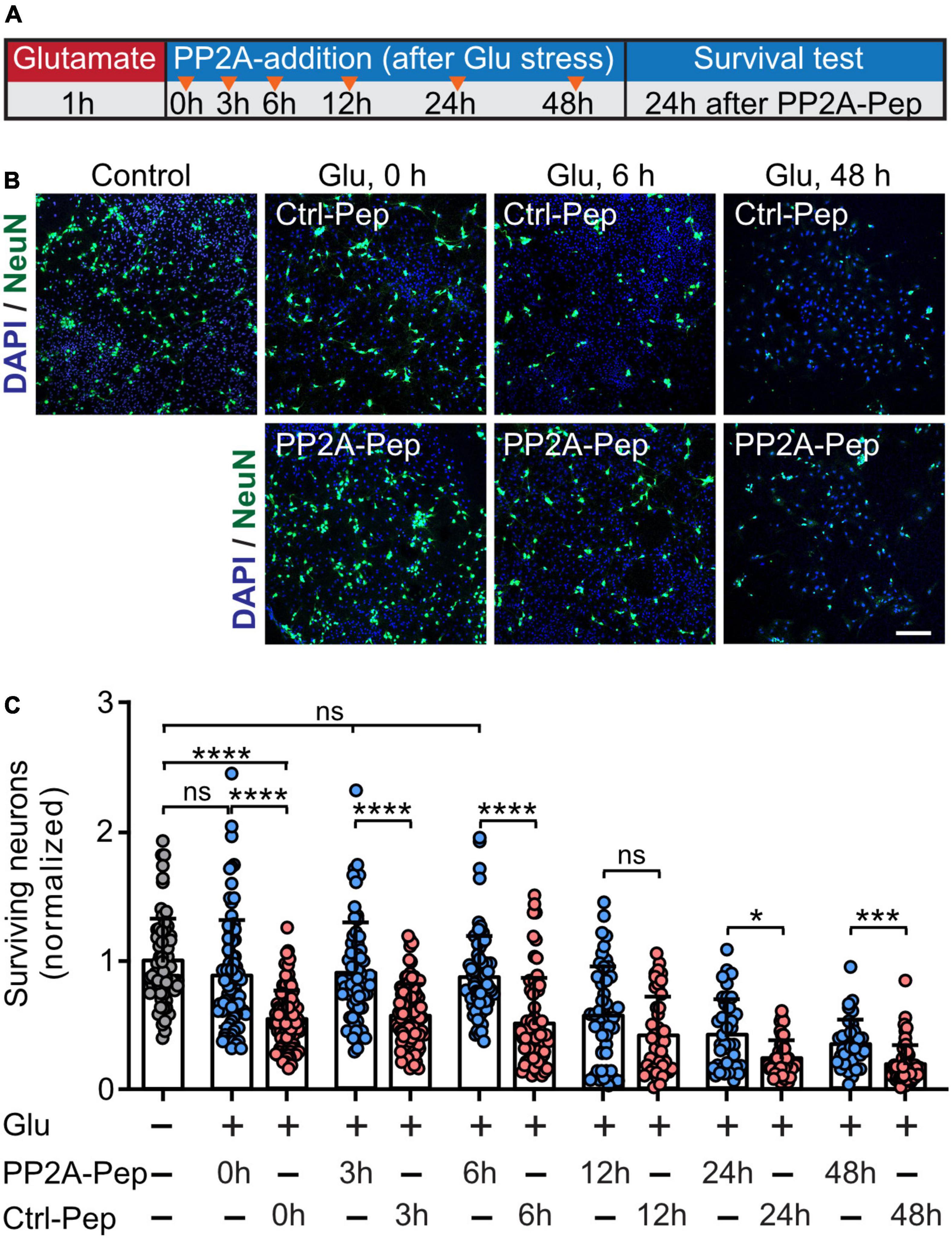
Figure 6. PP2A-Pep inhibits progressing excitotoxic neuronal death. Neurons were stressed for 60 min with 50 μM glutamate and treated with no peptide (control), PP2A-Pep (10 μg/ml) or Ctrl-Pep (10 μg/ml) 0, 3, 6, 12, 24, and 48 h after the glutamate stress. After additional 24 h of incubation, the cultures were stained with DAPI for total cells (glia plus neurons) and with an antibody directed against NeuN for neurons. (A) Schematic representation of the experimental design. (B) Representative images (scale bar: 100 μm) are shown for the untreated control and for the condition where the peptides were immediately administered after the glutamate stress (0 h), or after 6 and 48 h. (C) Quantification of neuronal loss. Number of neurons were normalized to the untreated control cultures. The data represent the mean ± SD of 35–112 frames (fields of view) per experimental condition from three independent experiments. Brown–Forsythe and Welch ANOVA with Games Howell’s multiple comparison test (ns, p > 0.05, *p < 0.05, ***p < 0.0005, ****p < 0.0001).
Our in vitro data indicate a potent neuroprotective activity of PP2A-Pep caused by inhibiting the interaction of GABAB receptors with PP2A, which restores GABAB receptor expression after excitotoxic stress. We therefore tested the effects of PP2A-Pep in the MCAO mouse model of cerebral ischemia. Mice were subjected to 60 min MCAO and the effect of PP2A-Pep was tested on brain slices containing the somatosensory cortex ex vivo 6 h after the insult (this time span ensures healthy ex vivo brain slices). The somatosensory cortex was selected because it belongs to the ischemic penumbra that is responsive to neuroprotective treatments.
Brain slices obtained from MCAO mice treated with Ctrl-Pep exhibited a reduced expression of GABAB receptors as compared to sham operated mice treated with Ctrl-Pep or PP2A-Pep as monitored by GABAB2 antibodies (Figures 7A–C,E). The reduced GABAB receptor expression was accompanied by significant neuronal death as monitored by the loss of NeuN positive cells (Figures 7A–C,F). Treatment of slices with PP2A-Pep for 6 h restored GABAB receptor expression to normal levels in brain slices of MCAO mice and prevented the loss of neurons (Figures 7D–F). In addition, we observed a robust colocalization of peptide staining with NeuN-positive cells, confirming the predominantly neuron-specific uptake of peptides tagged with Rabis virus glycoprotein sequences (Figure 7). These data illustrate that treatment of ex vivo brain slices with PP2A-Pep about 1 h after MCAO restored GABAB receptor expression and inhibited progressing neuronal death.
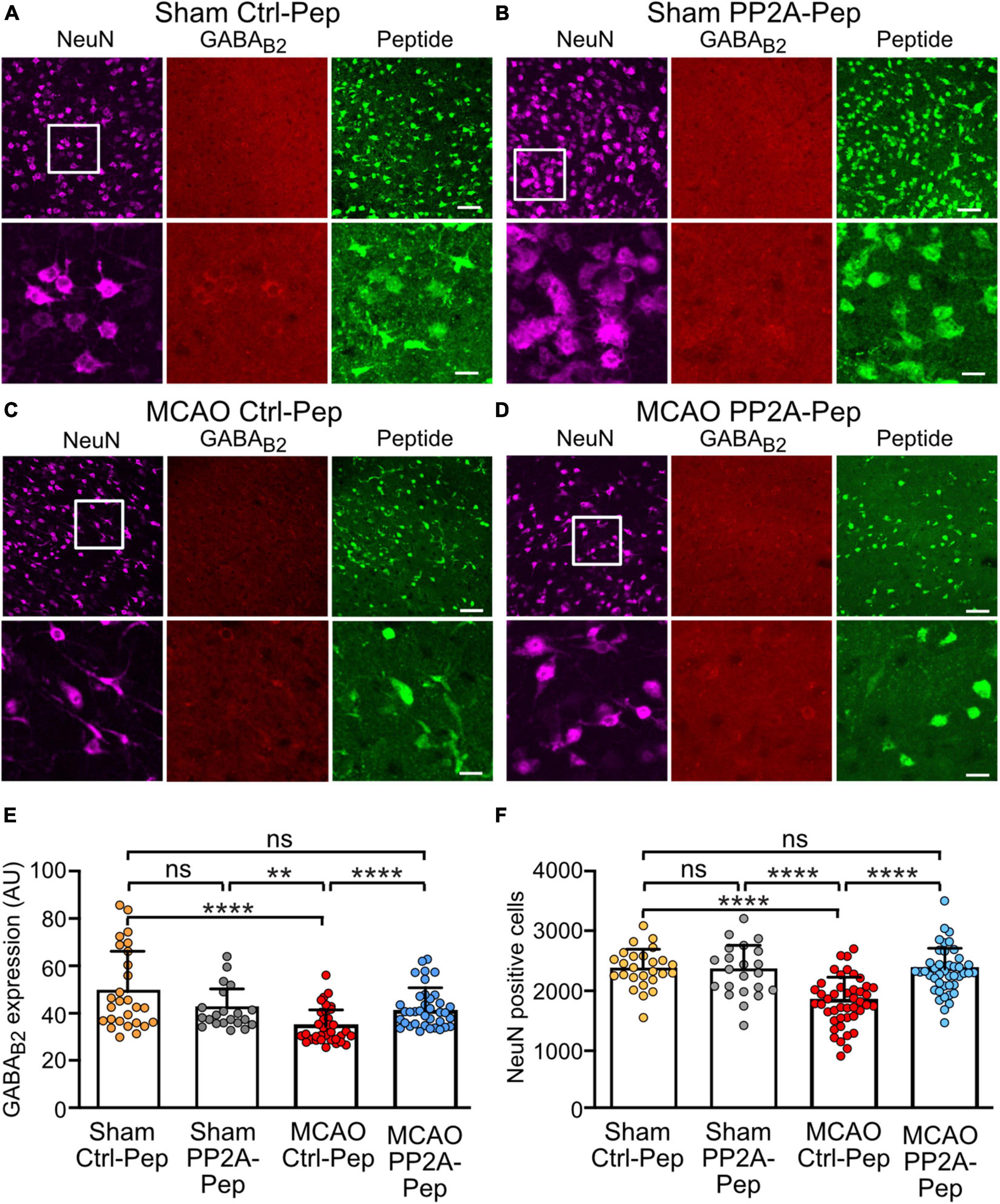
Figure 7. PP2A-Pep normalized receptor expression and inhibited neuronal death after MCAO-induced cerebral ischemia. Brain slices containing the somatosensory cortex were prepared from sham and MCAO operated mice and incubated for 6 h with 10 μg/ml PP2A-Pep followed by staining for GABAB receptors (red), neurons (magenta, NeuN positive cells) or PP2A-Pep (green). Representative images are shown for sections from sham operated mice treated with Ctrl-Pep (A) or PP2A-Pep (B) and for section from MCAO operated mice treated with Ctrl-Pep (C) or PP2A-Pep (D). Images on top of each panel are of low magnification (scale bar: 40 μm) and those on the bottom are of higher magnification (scale bars 10 μm). (E) Quantification of GABAB receptor expression. The data represent the mean ± SD of 21–37 frames (fields of view) derived from 4 to 5 mice per condition. Two-way ANOVA with Tukey’s multiple comparison test (ns, p > 0.05, **p < 0.005, ****p < 0.0001). (F) Quantification of neuronal loss. The data represent the mean ± SD of 22–44 frames (fields of view) derived from 4 to 6 mice per condition. Two-way ANOVA with Tukey’s multiple comparison test (ns, p > 0.05, ****p < 0.0001.
Since PP2A-Pep restored GABAB receptor expression in brain slices from MCAO-treated mice, we next tested if exposure to PP2A-Pep also restored normal GABAB receptor-mediated inhibition using whole-cell patch-clamp recordings. In line with the downregulation of GABAB receptors in MCAO brain slices (Figure 7), GABAB receptor-mediated currents were strongly reduced (Figures 8A,A’). However, incubation of the slices with PP2A-Pep restored GABAB receptor-mediated currents to levels observed in brain slices obtained from sham-operated mice. By contrast, treatment with Ctrl-Pep did not affect GABAB receptor-mediated currents in MCAO brain slices, documenting the specificity of the PP2A-Pep effect (Figures 8A,A’).

Figure 8. PP2A-Pep restored GABAB receptor-mediated inhibition and reduced neuronal excitability after MCAO-induced cerebral ischemia. Brain slices containing the somatosensory cortex of sham and MCAO operated mice were analyzed using whole-cell patch-clamp recordings 2–6 h after treatment with PP2A-Pep, Ctrl-Pep or saline. (A) Representative traces of baclofen-evoked currents. (A’) Quantification of baclofen-evoked current traces. The data represent the mean ± SD of 6–10 neurons. One-way ANOVA, Tukey’s multiple comparison test (ns, p > 0.05, *p < 0.05, **p < 0.01). (B–B”) Neuronal excitability was determined by injecting increasing current steps. (B) Representative current traces. (B’) Quantification of action potential firing evoked by increasing current injections. (B”) Bar graph and statistical evaluation of data depicted in the gray box in (B’). The data represent the mean ± SD of 10–16 neurons. Brown–Forsythe and Welch ANOVA with Games Howell’s multiple comparison test (ns, p > 0.05, *p < 0.05, **p < 0.01). (C) Determination of input resistance. (D) Determination of resting membrane potential. (E) Determination of action potential threshold. The data represent the mean ± SD of 10–16 neurons. One-way ANOVA with Tukey’s multiple comparison test (ns, p > 0.05, *p < 0.05, **p < 0.01).
Since GABAB receptor-mediated inhibition is a key player regulating neuronal excitability, we test whether the reduction of GABAB receptor-mediated inhibition is accompanied with increased neuronal excitability. As expected, neurons in slices from MCAO mice exhibited enhanced firing frequency as compared to neurons from sham-operated mice (Figure 8B). This was reflected by an enhanced input resistance (Figure 8C), a depolarized resting membrane potential (Figure 8D), and a hyperpolarized threshold for action potential firing (Figure 8E). Incubation of the slices with PP2A-Pep after ischemia normalized the ischemia-induced alterations in passive neuronal properties, resulting in a reduced neuronal firing rate (Figures 8B–E).
These data indicate that blocking the PP2A/GABAB receptor interaction restored normal GABAB receptor inhibition which resulted in reduced neuronal excitability after cerebral ischemia.
In this study, we developed a small interfering peptide that inhibits the interaction of GABAB receptors with PP2A to analyze in more detail the trafficking pathways affected by PP2A and to explore the therapeutic potential of specifically interfering with the GABAB receptor/PP2A interaction using cerebral ischemia as a model for a severely disturbed excitation/inhibition balance.
GABAB receptors are downregulated under pathological conditions by a mechanism involving CaMKII-mediated phosphorylation of GABAB1(S867) (Guetg et al., 2010; Zemoura et al., 2019; Balakrishnan et al., 2022) and PP2A-mediated dephosphorylation of GABAB2(S783) (Terunuma et al., 2010; Li et al., 2020). This regulation of the GABAB receptor phosphorylation status aberrantly sorts constitutive internalized receptors to lysosomal degradation instead of recycling them back to the plasma membrane. Severely elevated intracellular Ca2+ levels by sustained activation of glutamate receptors enhance phosphorylation of GABAB1(S867) by CaMKII, which promotes K68-linked ubiquitination of GABAB receptors at multiple sites by the ubiquitin E3 ligase MIB2 and thereby targets the receptors to the ubiquitin-dependent lysosomal degradation pathway (Zemoura et al., 2016). In this pathway, CaMKII acts after the receptors are internalized, most likely at the level of early endosomes where the decision for recycling or degradation of the receptors is made.
Under the same conditions, GABAB receptors are initially phosphorylated by AMPK at GABAB2(S783). This appears to be a response to stabilize the receptors at the cell surface (Kuramoto et al., 2007; Terunuma et al., 2010). However, GABAB2(S783) then gets dephosphorylated by PP2A, which contributes to the aberrant sorting of the receptors to lysosomal degradation (Terunuma et al., 2010). Both events, phosphorylation of GABAB1(S867) and dephosphorylation of GABAB2(S783) are key steps in the pathological downregulation of GABAB receptors since blocking CaMKII or PP2A activity with specific inhibitors blocks this detrimental process (Guetg et al., 2010; Terunuma et al., 2010; Zemoura et al., 2019).
To analyze the detailed trafficking pathways affected by PP2A, we identified an interfering peptide (PP2A-Pep) that blocks the GABAB receptor/PP2A interaction by screening a small library of synthetic peptides comprising the intracellularly located C-terminal domain of GABAB2. PP2A-Pep is composed of an amino acid sequence located close to S783 of GABAB2. PP2A-Pep effectively blocked the interaction of PP2A with GABAB receptors, upregulated cell surface expression of the receptors cultured in neurons under normal physiological conditions and after excitotoxic stress. A PP2A/GABAB2 interaction site close to GABAB2(S783) is not surprising as this site is the substrate of the dephosphorylating activity of PP2A. Interestingly, Li et al. (2020) identified another interaction site of PP2A in the C-terminal domain of GABAB1. As PP2A-Pep, a peptide comprising the amino acid sequence of the GABAB1 interaction site inhibited the interaction of PP2A with GABAB receptors (Li et al., 2020). Therefore, it is very likely that PP2A interacts with GABAB receptors at least via two distinct sites for proper dephosphorylation of GABAB2(S783).
The number of cell surface receptors is determined by a precise balance of receptor exocytosis, endocytosis, recycling, and degradation. Phosphorylation of GABAB1(S867), most likely at the level of early endosomes, serves as a signal for sorting the receptors to late endosomes by promoting K63-linked ubiquitination of the receptors via MIB2 (Zemoura et al., 2019). The data of this study suggests that PP2A-dependent dephosphorylation of GABAB2(S783) takes place upstream of CaMKII phosphorylation of GABAB1(S867). Inhibition of the GABAB receptor/PP2A interaction in neurons under physiological conditions completely prevented constitutive reduction of cell surface receptors. This suggests that PP2A may regulate endocytosis of the receptors. However, it is very unlikely that GABAB2(S783) dephosphorylation directly serves as a signal for receptors endocytosis since in glutamate-stressed neurons PP2A-Pep did not completely block removal of cell surface receptors but restored it to normal levels of constitutive internalization. In addition, our previous findings indicate that glutamate stress, which induces GABAB2(S783) dephosphorylation, did not enhance constitutive internalization of GABAB receptor (Maier et al., 2010). Therefore, GABAB2(S783) dephosphorylation most likely inhibits recycling of the receptors. This view is in line with our finding that preventing the interaction of GABAB receptor with PP2A resulted in enhanced colocalization of the receptors with EEA1-positive early endosomes and Rab4-positive fast recycling endosomes, which originate from early endosomes (Sheff et al., 1999). The enhanced recycling of the receptors should stabilize the number of receptors at the cell surface. Thus, the most likely scenario for the sequence of events in glutamate-induced downregulation of GABAB receptors is that PP2A-dependent dephosphorylation of GABAB2(S783) inhibits recycling of the receptors and subsequently CaMKII-dependent phosphorylation of GABAB1(S867) promotes K63-linked ubiquitination of the receptors by MIB2, which then serves as sorting signals for the lysosomal degradation pathway.
A main purpose of this study was to test the therapeutic potential of specifically blocking the interaction of GABAB receptors with PP2A using an interfering peptide. Because PP2A is involved in regulating a multitude of pathways, globally blocking PP2A activity with specific inhibitors is expected to be associated with severe side effects. Therefore, a targeted approach specifically blocking the interaction of PP2A with GABAB receptors might be a valid alternative. Since GABAB receptors are downregulated under excitotoxic/ischemic conditions occurring in cerebral ischemia (Guetg et al., 2010; Maier et al., 2010; Terunuma et al., 2010; Kim et al., 2011; Kantamneni et al., 2014; Zhu et al., 2015; Huang et al., 2017; Hleihil et al., 2021; Balakrishnan et al., 2022; Bhat et al., 2022), we tested the ability of PP2A-Pep to normalize GABAB receptor expression/function and its neuroprotective activity in vitro using glutamate-stressed neuronal cultures and in the MCAO mouse model of cerebral ischemia ex vivo. In cultured glutamate-stressed neurons, PP2A-Pep normalized GABAB receptor expression and inhibited progressing neuronal death even when applied 48 h after the insult. This is a very promising finding in view of the development of a therapeutic application for inhibiting progressing neuronal death in acute stroke patients. Most importantly, application of PP2A-Pep to brain slices prepared from MCAO-treated mice restored GABAB receptor expression and function to normal levels, reduced ischemia-induced over-excitability of neurons, and prevented neuronal death in the ischemic penumbra, which is the main target for neuroprotection. Unfortunately, we could test PP2A-Pep in MCAO-treated mice only ex vivo because of the poor pharmacokinetics of the current peptide. This limited the time for analysis to 6 hours after the insult. Future optimization of PP2A-Pep for systemic application in vivo is required to explore its full neuroprotective potential in vivo.
These promising results mirror our recent findings targeting the CaMKII/GABAB receptor interaction, which is involved in this pathway apparently downstream of PP2A-dependent dephosphorylation of GABAB2(S783) (Balakrishnan et al., 2022). In addition to the glutamate-induced aberrant lysosomal sorting of GABAB receptors involving CaMKII and PP2A, ischemic conditions further cause downregulation of cell surface receptors via interaction with the pro-apoptotic transcription factor CHOP (Maier et al., 2014). The severe endoplasmic stress associated with ischemic conditions upregulates CHOP, which, in addition to inducing apoptosis, interacts with GABAB receptors in the ER and prevents their export to the cell surface. Inhibition of the CHOP/GABAB receptor interaction with an interfering peptide also normalized GABAB receptor expression after ischemic stress, reduced stress-induced neuronal overexcitation and neuronal death in cultured neurons (Bhat et al., 2022). Since interfering with any of these detrimental GABAB receptor interactions restored GABAB receptor-inhibition and eventually inhibited progressing neuronal death, it would be very interesting to test the neuroprotective activity of a combination of all three interfering peptides targeting the distinct trafficking events in this pathway downregulating GABAB receptors. In addition, it would be interesting to analyze whether the GABAB receptor agonist baclofen can potentiate the neuroprotective activity of PP2A-Pep. In a recent study we showed that baclofen stabilizes GABAB receptor expression under ischemic conditions by enhancing fast recycling of the receptors (Hleihil et al., 2021). If GABAB receptors in the ischemic penumbra are not fully activated by enhanced release of GABA, co-treatment of PP2A-Pep with baclofen is expected to exert a higher neuroprotective activity than treatment of PP2A or baclofen alone.
Apart from cerebral ischemia, which is an extreme pathological situation, interfering with the GABAB receptor/PP2A interaction might also be effective for the treatment of less severe neurological diseases associated with downregulation of GABAB receptors and a disturbed excitation/inhibition balance such as addiction, anxiety, and depression (Maeda et al., 2006; Padgett et al., 2012; Hearing et al., 2013; Lecca et al., 2016). Therefore, targeting the GABAB receptor/PP2A interaction is a promising strategy for the development of a novel therapeutic intervention.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by Zurich cantonal veterinary office, Zurich, Switzerland (license: ZH152/16, ZH011/19, and ZH031/16).
MH and KB performed the experimental work and analyzed the data. MH, KB, and DB designed the study. DB supervised the experimental work and analyzed the data. MH and DB wrote the manuscript. All authors discussed the results and commented on the manuscript.
This work was funded by the Swiss National Science Foundation grants 31003A_156648 and 31003A_182325 to DB.
The authors thank Thomas Grampp for excellent technical assistance and Louis Scheurer for constructing GABAB2(BBS).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Balakrishnan, K., Hleihil, M., Bhat, M. A., Ganley, R. P., Vaas, M., Klohs, J., et al. (2022). Targeting the interaction of GABAB receptors with CaMKII with an interfering peptide restores receptor expression after cerebral ischemia and inhibits progressive neuronal death in mouse brain cells and slices. Brain Pathol. e13099. doi: 10.1111/bpa.13099
Benke, D. (2010). Mechanisms of GABAB receptor exocytosis, endocytosis, and degradation. Adv. Pharmacol. 58, 93–111. doi: 10.1016/S1054-3589(10)58004-9
Benke, D., Michel, C., and Mohler, H. (2002). Structure of GABAB receptors in rat retina. J. Recept. Signal Transduc. 22, 253–266. doi: 10.1081/rrs-120014600
Bettler, B., Kaupmann, K., Mosbacher, J., and Gassmann, M. (2004). Molecular structure and physiological functions of GABAB receptors. Physiol. Rev. 84, 835–867. doi: 10.1152/physrev.00036.2003
Bhat, M. A., Esmaeili, A., Neumann, E., Balakrishnan, K., and Benke, D. (2022). Targeting the interaction of GABAB receptors with CHOP after an ischemic insult restores receptor expression and inhibits progressive neuronal death. Front. Pharmacol. 13:870861. doi: 10.3389/fphar.2022.870861
Buerli, T., Pellegrino, C., Baer, K., Lardi-Studler, B., Chudotvorova, I., Fritschy, J. M., et al. (2007). Efficient transfection of DNA or shRNA vectors into neurons using magnetofection. Nat. Protoc. 2, 3090–3101. doi: 10.1038/nprot.2007.445
Bussieres, N., and El Manira, A. (1999). GABAB receptor activation inhibits N- and P/Q-type calcium channels in cultured lamprey sensory neurons. Brain Res. 847, 175–185. doi: 10.1016/s0006-8993(99)02002-8
Chalifoux, J. R., and Carter, A. G. (2011). GABAB receptor modulation of synaptic function. Curr. Opin. Neurobiol. 21, 339–344. doi: 10.1016/j.conb.2011.02.004
Chen, G., and van den Pol, A. N. (1998). Presynaptic GABAB autoreceptor modulation of P/Q-type calcium channels and GABA release in rat suprachiasmatic nucleus neurons. J. Neurosci. 18, 1913–1922. doi: 10.1523/JNEUROSCI.18-05-01913.1998
Daro, E., van der Sluijs, P., Galli, T., and Mellman, I. (1996). Rab4 and cellubrevin define different early endosome populations on the pathway of transferrin receptor recycling. Proc. Natl. Acad. Sci U.S.A. 93, 9559–9564. doi: 10.1073/pnas.93.18.9559
Evans, D. R., Myles, T., Hofsteenge, J., and Hemmings, B. A. (1999). Functional expression of human PP2Ac in yeast permits the identification of novel C-terminal and dominant-negative mutant forms. J. Biol. Chem. 274, 24038–24046. doi: 10.1074/jbc.274.34.24038
Feng, Y., Press, B., and Wandinger-Ness, A. (1995). Rab 7: An important regulator of late endocytic membrane traffic. J. Cell Biol. 131, 1435–1452. doi: 10.1083/jcb.131.6.1435
Gähwiler, B. H., and Brown, D. A. (1985). GABAB-receptor-activated K+ current in voltage-clamped CA3 pyramidal cells in hippocampal cultures. Proc. Natl. Acad. Sci. U.S.A. 82, 1558–1562. doi: 10.1073/pnas.82.5.1558
Gassmann, M., and Bettler, B. (2012). Regulation of neuronal GABAB receptor functions by subunit composition. Nat. Rev. Neurosci. 13, 380–394. doi: 10.1038/nrn3249
Guetg, N., Aziz, S. A., Holbro, N., Turecek, R., Rose, T., Seddik, R., et al. (2010). NMDA receptor-dependent GABAB receptor internalization via CaMKII phosphorylation of serine 867 in GABAB1. Proc. Natl. Acad. Sci. U.S.A. 107, 13924–13929. doi: 10.1073/pnas.1000909107
Hannan, S., Wilkins, M. E., Thomas, P., and Smart, T. G. (2013). Tracking cell surface mobility of GPCRs using α-bungarotoxin-linked fluorophores. Methods Enzymol. 521, 109–129. doi: 10.1016/b978-0-12-391862-8.00006-5
Hearing, M., Kotecki, L., Marron Fernandez de Velasco, E., Fajardo-Serrano, A., Chung, H. J., Lujan, R., et al. (2013). Repeated cocaine weakens GABA-Girk signaling in layer 5/6 pyramidal neurons in the prelimbic cortex. Neuron 80, 159–170. doi: 10.1016/j.neuron.2013.07.019
Hleihil, M., Vaas, M., Bhat, M. A., Balakrishnan, K., and Benke, D. (2021). Sustained baclofen-induced activation of GABAB receptors after cerebral ischemia restores receptor expression and function and limits progressing loss of neurons. Front. Mol. Neurosci. 14:726133. doi: 10.3389/fnmol.2021.726133
Huang, L., Li, Q., Wen, R., Yu, Z., Li, N., Ma, L., et al. (2017). Rho-kinase inhibitor prevents acute injury against transient focal cerebral ischemia by enhancing the expression and function of GABA receptors in rats. Eur. J. Pharmacol. 797, 134–142. doi: 10.1016/j.ejphar.2017.01.021
Kantamneni, S., Gonzalez-Gonzalez, I. M., Luo, J., Cimarosti, H., Jacobs, S. C., Jaafari, N., et al. (2014). Differential regulation of GABAB receptor trafficking by different modes of N-methyl-D-aspartate (n.d.) receptor signaling. J. Biol. Chem. 289, 6681–6694. doi: 10.1074/jbc.M113.487348
Kaupmann, K., Huggel, K., Heid, J., Flor, P. J., Bischoff, S., Mickel, S. J., et al. (1997). Expression cloning of GABAB receptors uncovers similarity to metabotropic glutamate receptors. Nature 386, 239–246. doi: 10.1038/386239a0
Kaupmann, K., Malitschek, B., Schuler, V., Heid, J., Froestl, W., Beck, P., et al. (1998). GABAB receptor subtypes assemble into functional heteromeric complexes. Nature 396, 683–687. doi: 10.1038/25360
Kim, J. Y., Kim, N., Yenari, M. A., and Chang, W. (2011). Mild hypothermia suppresses calcium-sensing receptor (CaSR) induction following forebrain ischemia while increasing GABAB receptor 1 (GABAB-R1) expression. Transl. Stroke Res. 2, 195–201. doi: 10.1007/s12975-011-0082-4
Kumar, P., Wu, H., McBride, J. L., Jung, K. E., Kim, M. H., Davidson, B. L., et al. (2007). Transvascular delivery of small interfering RNA to the central nervous system. Nature 448, 39–43. doi: 10.1038/nature05901
Kuramoto, N., Wilkins, M. E., Fairfax, B. P., Revilla-Sanchez, R., Terunuma, M., Tamaki, K., et al. (2007). Phospho-dependent functional modulation of GABAB receptors by the metabolic sensor AMP-dependent protein kinase. Neuron 53, 233–247. doi: 10.1016/j.neuron.2006.12.015
Lecca, S., Pelosi, A., Tchenio, A., Moutkine, I., Lujan, R., Herve, D., et al. (2016). Rescue of GABAB and GIRK function in the lateral habenula by protein phosphatase 2A inhibition ameliorates depression-like phenotypes in mice. Nat. Med. 22, 254–261. doi: 10.1038/nm.4037
Leuchowius, K. J., Jarvius, M., Wickstrom, M., Rickardson, L., Landegren, U., Larsson, R., et al. (2010). High content screening for inhibitors of protein interactions and post-translational modifications in primary cells by proximity ligation. Mol. Cell. Proteom. 9, 178–183. doi: 10.1074/mcp.M900331-MCP200
Li, X., Terunuma, M., Deeb, T. G., Wiseman, S., Pangalos, M. N., Nairn, A. C., et al. (2020). Direct interaction of PP2A phosphatase with GABAB receptors alters functional signaling. J. Neurosci. 40, 2808–2816. doi: 10.1523/jneurosci.2654-19.2020
Luscher, C., Jan, L. Y., Stoffel, M., Malenka, R. C., and Nicoll, R. A. (1997). G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron 19, 687–695.
Maeda, T., Yoshimatsu, T., Hamabe, W., Fukazawa, Y., Kumamoto, K., Ozaki, M., et al. (2006). Involvement of serine/threonine protein phosphatases sensitive to okadaic acid in restraint stress-induced hyperlocomotion in cocaine-sensitized mice. Br. J. Pharmacol. 148, 405–412. doi: 10.1038/sj.bjp.0706769
Maier, P. J., Marin, I., Grampp, T., Sommer, A., and Benke, D. (2010). Sustained glutamate receptor activation down-regulates GABAB receptors by shifting the balance from recycling to lysosomal degradation. J. Biol. Chem. 285, 35606–35614. doi: 10.1074/jbc.M110.142406
Maier, P. J., Zemoura, K., Acuna, M. A., Yevenes, G. E., Zeilhofer, H. U., and Benke, D. (2014). Ischemia-like oxygen and glucose deprivation mediates down-regulation of cell surface γ-aminobutyric acidB receptors via the endoplasmic reticulum (ER) stress-induced transcription factor CCAAT/enhancer-binding protein (C/EBP)-homologous protein (CHOP). J. Biol. Chem. 289, 12896–12907. doi: 10.1074/jbc.M114.550517
Mintz, I. M., and Bean, B. P. (1993). GABAB receptor inhibition of P-type Ca2+ channels in central neurons. Neuron 10, 889–898.
Mu, F. T., Callaghan, J. M., Steele-Mortimer, O., Stenmark, H., Parton, R. G., Campbell, P. L., et al. (1995). EEA1, an early endosome-associated protein. EEA1 is a conserved alpha-helical peripheral membrane protein flanked by cysteine “fingers” and contains a calmodulin-binding IQ motif. J. Biol. Chem. 270, 13503–13511. doi: 10.1074/jbc.270.22.13503
Padgett, C. L., Lalive, A. L., Tan, K. R., Terunuma, M., Munoz, M. B., Pangalos, M. N., et al. (2012). Methamphetamine-evoked depression of GABAB receptor signaling in GABA neurons of the VTA. Neuron 73, 978–989. doi: 10.1016/j.neuron.2011.12.031
Santos, A. E., Carvalho, C. M., Macedo, T. A., and Carvalho, A. P. (1995). Regulation of intracellular [Ca2+] and GABA release by presynaptic GABAB receptors in rat cerebrocortical synaptosomes. Neurochem. Int. 27, 397–406.
Schaar, K. L., Brenneman, M. M., and Savitz, S. I. (2010). Functional assessments in the rodent stroke model. Exp. Transl. Stroke Med. 2:13. doi: 10.1186/2040-7378-2-13
Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., et al. (2012). Fiji: An open-source platform for biological-image analysis. Nat. Methods 9, 676–682. doi: 10.1038/nmeth.2019
Sheff, D. R., Daro, E. A., Hull, M., and Mellman, I. (1999). The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J. Cell Biol. 145, 123–139. doi: 10.1083/jcb.145.1.123
Soderberg, O., Gullberg, M., Jarvius, M., Ridderstrale, K., Leuchowius, K. J., Jarvius, J., et al. (2006). Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat. Methods 3, 995–1000. doi: 10.1038/nmeth947
Terunuma, M. (2018). Diversity of structure and function of GABAB receptors: A complexity of GABAB-mediated signaling. Proc. Jpn. Acad. Ser. B 94, 390–411. doi: 10.2183/pjab.94.026
Terunuma, M., Vargas, K. J., Wilkins, M. E., Ramirez, O. A., Jaureguiberry-Bravo, M., Pangalos, M. N., et al. (2010). Prolonged activation of NMDA receptors promotes dephosphorylation and alters postendocytic sorting of GABAB receptors. Proc. Natl. Acad. Sci. U.S.A. 107, 13918–13923. doi: 10.1073/pnas.1000853107
van der Sluijs, P., Hull, M., Webster, P., Mâle, P., Goud, B., and Mellman, I. (1992). The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell 70, 729–740. doi: 10.1016/0092-8674(92)90307-x
Wilson, J. M., de Hoop, M., Zorzi, N., Toh, B. H., Dotti, C. G., and Parton, R. G. (2000). EEA1, a tethering protein of the early sorting endosome, shows a polarized distribution in hippocampal neurons, epithelial cells, and fibroblasts. Mol. Biol. Cell 11, 2657–2671. doi: 10.1091/mbc.11.8.2657
Zemoura, K., Balakrishnan, K., Grampp, T., and Benke, D. (2019). Ca2+/Calmodulin-dependent protein kinase II (CaMKII) β-dependent phosphorylation of GABAB1 triggers lysosomal degradation of GABAB Receptors via mind bomb-2 (MIB2)-mediated Lys-63-linked ubiquitination. Mol. Neurobiol. 56, 1293–1309. doi: 10.1007/s12035-018-1142-5
Zemoura, K., Trumpler, C., and Benke, D. (2016). Lys-63-linked ubiquitination of γ-aminobutyric acid (GABA), Type B1, at multiple sites by the E3 ligase Mind Bomb-2 targets GABAB receptors to lysosomal degradation. J. Biol. Chem. 291, 21682–21693. doi: 10.1074/jbc.M116.750968
Keywords: protein phosphatase 2A, GABAB receptor, interfering peptide, cerebral ischemia, excitotoxicity, neuroprotection
Citation: Hleihil M, Balakrishnan K and Benke D (2022) Protein phosphatase 2A regulation of GABAB receptors normalizes ischemia-induced aberrant receptor trafficking and provides neuroprotection. Front. Mol. Neurosci. 15:1015906. doi: 10.3389/fnmol.2022.1015906
Received: 10 August 2022; Accepted: 26 September 2022;
Published: 13 October 2022.
Edited by:
Bernhard Luscher, The Pennsylvania State University (PSU), United StatesReviewed by:
Stephen Moss, Tufts University, United StatesCopyright © 2022 Hleihil, Balakrishnan and Benke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dietmar Benke, YmVua2VAcGhhcm1hLnV6aC5jaA==
†Present address: Karthik Balakrishnan, Dewpoint Therapeutics GmbH, Dresden, Germany
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.