- 1Department of Radiology, Stanford University, Stanford, CA, United States
- 2Stanford University Mass Spectrometry Laboratory, Stanford University, Stanford, CA, United States
Fragile X syndrome (FXS) is the leading monogenetic cause of autism spectrum disorder and inherited cause of intellectual disability that affects approximately one in 7,000 males and one in 11,000 females. In FXS, the Fmr1 gene is silenced and prevents the expression of the fragile X mental retardation protein (FMRP) that directly targets mRNA transcripts of multiple GABAA subunits. Therefore, FMRP loss adversely impacts the neuronal firing of the GABAergic system which creates an imbalance in the excitatory/inhibitory ratio within the brain. Current FXS treatment strategies focus on curing symptoms, such as anxiety or decreased social function. While treating symptoms can be helpful, incorporating non-invasive imaging to evaluate how treatments change the brain’s biology may explain what molecular aberrations are associated with disease pathology. Thus, the GABAergic system is suitable to explore developing novel therapeutic strategies for FXS. To understand how the GABAergic system may be affected by this loss-of-function mutation, GABA concentrations were examined within the frontal cortex and thalamus of 5-day-old wild type and Fmr1 knockout mice using both 1H magnetic resonance imaging (1H-MRS) and liquid chromatography-tandem mass spectrometry (LC-MS/MS). Our objective was to develop a reliable scanning method for neonatal mice in vivo and evaluate whether 1H-MRS is suitable to capture regional GABA concentration differences at the front end of the critical cortical period where abnormal neurodevelopment occurs due to FMRP loss is first detected. 1H-MRS quantified GABA concentrations in both frontal cortex and thalamus of wild type and Fmr1 knockout mice. To substantiate the results of our 1H-MRS studies, in vitro LC-MS/MS was also performed on brain homogenates from age-matched mice. We found significant changes in GABA concentration between the frontal cortex and thalamus within each mouse from both wild type and Fmr1 knockout mice using 1H-MRS and LC-MS/MS. Significant GABA levels were also detected in these same regions between wild type and Fmr1 knockout mice by LC-MS/MS, validating that FMRP loss directly affects the GABAergic system. Thus, these new findings support the need to develop an effective non-invasive imaging method to monitor novel GABAergic strategies aimed at treating patients with FXS.
Introduction
Fragile X syndrome (FXS) affects approximately one in 7,000 males and one in 11,000 females (Hunter et al., 2014) and is the leading monogenetic cause of autism spectrum disorder (ASD) and inherited cause of intellectual disability (Lozano et al., 2014; Hagerman et al., 2017). Some features of FXS include attention-deficit/hyperactivity disorder (ADHD), social anxiety, and seizures (Hagerman et al., 2017). FXS is caused when there is an expansion of over 200 CGG-trinucleotide repeats in the 5′ region on the FMR1 gene and hypermethylation of the gene occurs. This hypermethylation silences the FMR1 gene, inhibiting the production of fragile X mental retardation protein (FMRP; Tabolacci et al., 2016). FMRP is an RNA-binding protein that is associated with translation of various mRNA relating to neuronal development (Darnell et al., 2011; Ascano et al., 2012; Maurin et al., 2018), regulation of ribosome stalling, neural circuit function, and synaptic plasticity (Gatto and Broadie, 2009; Zukin et al., 2009; Darnell et al., 2011). The loss of FMRP has also been associated with a downregulation of gamma-aminobutyric acid (GABA) membrane transporters and receptor scaffolding proteins (Gao et al., 2018). Furthermore, dysregulation of synaptic proteins is thought to disrupt synapse maturation (Remmers and Contractor, 2018) leading to long, thin dendritic spines seen in FXS (Irwin et al., 2000).
FMRP loss has been shown to affect many systems including the glutamatergic, NMDA, and GABAergic systems in the brain (Bear et al., 2004; Darnell et al., 2011; Davidovic et al., 2011; Paluszkiewicz et al., 2011), thereby impacting neuronal firing. This imbalance of the major excitatory and inhibitory neurotransmitter systems is thought to contribute to phenotypes in FXS cognitive and functional impairment and epilepsy (Paluszkiewicz et al., 2011). In the critical cortical period of development, a phase characterized by heightened neuronal plasticity beginning at birth and closing around postnatal day 14 (Lo et al., 2017), the switch of GABA neurons from depolarizing to hyperpolarizing is delayed in fragile X mice (He et al., 2014). During this period, the excitatory connection of the thalamocortical synapse is fundamentally disrupted in Fmr1 KO mice in early postnatal development (Harlow et al., 2010). FMRP has been identified to directly target mRNA transcripts of multiple GABAA subunits and GABA transporter Slc6a1 (Miyashiro et al., 2003; Darnell et al., 2011; Ascano et al., 2012; Braat and Kooy, 2015; Braat et al., 2015), including lowered GABAA receptor subunit mRNA and protein levels in both FXS mouse and Drosophila models (El Idrissi et al., 2005; D’Hulst et al., 2006; Gantois et al., 2006; Adusei et al., 2010). Levels of the GABA synthesizing enzyme, glutamic acid decarboxylase, have also been found to be affected in Fmr1 knockout (KO) Drosophila. Lastly, recent studies reveal changes in GABAergic signaling at both pre- and post-synaptic domains of inhibitory synapses in an FXS mouse model (Kramvis et al., 2020). Hence, alterations in GABAA receptor distribution and GABA production and metabolism are pertinent to FXS and make the GABAergic system an attractive path to study.
FXS researchers have used preclinical FXS models, like the Fmr1 KO mouse (The Dutch-Belgian Fragile X Consortium, 1994; Dahlhaus, 2018) to develop novel therapeutic strategies that have found shown promise for clinical translation, however, many clinical trials employing these strategies, unfortunately, do not pass Phase 3 (Gross et al., 2015; Ligsay and Hagerman, 2016). A newly published preclinical study using gaboxadol (OV101), a GABA agonist, has shown reversal of FXS-related behavior such as hyperactivity, anxiety, and cognition (Cogram et al., 2019), and an OV101 phase 2 clinical trial has succeeded with positive results including improved anxiety and social withdrawal (NCT03697161). These studies indicate that a therapeutic approach addressing GABA in FXS patients may be a molecular approach to reducing many symptoms for patients with FXS. Additionally, there are both decreased GABAA1 subunit expression in neonatal mouse brains, as early as postnatal day 5 (Adusei et al., 2010) and decreased GABA concentrations were also found in multiple brain regions of neonatal and young Fmr1 KO mice (Davidovic et al., 2011; Braat et al., 2015) further substantiating the investigation of the GABAergic system for FXS treatment. Integrating multimodal imaging such as, MRI and positron emission tomography (PET), into preclinical FXS research could enable the visualization of chemical and protein changes within the GABAergic system of FXS subjects that may afford insight towards new treatment strategies. Moreover, utilizing non-invasive imaging as a tool to monitor and evaluate treatment efficacy can improve the accuracy of study outcome measures by observing real-time changes in the brain, rather than relying on treatment response questionnaires filled out by patients and caregivers.
1H-magnetic resonance spectroscopy (1H-MRS) is an MRI technique clinically used to study brain disease (Öz et al., 2014; Wilson et al., 2019), including the measurement of GABA in respect to autism spectrum disorder (Harada et al., 2011; Horder et al., 2018; Fung et al., 2020). 1H-MRS is capable of non-invasively measuring neurotransmitters and metabolites, in vivo and is typically utilized in conjunction with structural MRI (sMRI) to assess neurochemical composition within brain anatomical regions (Faghihi et al., 2017). Furthermore, 1H-MRS is the only non-invasive method capable of measuring in vivo GABA concentrations, with the most common method in humans being a spectral-editing approach known as MEGA-PRESS (Cochran et al., 2015). In animal models studied at higher magnetic fields, non-edited approaches, such as PRESS, are also viable (Horder et al., 2018). Employing this non-invasive analytical technique in the clinic to study brain disease and abnormal cognitive development brings new information via quantifying neurotransmitters and metabolites in tissues of interest.
To date, clinical studies using 1H-MRS to study GABA in ASD have found a decrease in GABA concentration in the frontal lobe of children with ASD (Harada et al., 2011) while, alternatively found an increase in the dorsal lateral prefrontal cortex in adults with ASD (Fung et al., 2020). PET imaging studies in participants with FXS have found a structurally-dependent decrease in GABAA receptor binding potential primarily for the thalamus (D’Hulst et al., 2015) using 11C-Flumazenil (FMZ), a GABAA antagonist that binds to the post-synaptic benzodiazepine site of the GABAA receptor, between the α and γ subunits. This decrease in binding potential suggests a decreased receptor expression, consistent with D’Hulst et al.’s (2006) findings of decreased mRNA expression for several α and γ subunits in the cortex. Clinical 1H-MRS has demonstrated its feasibility to begin understanding the relationship between PET data indicating a decrease in GABAA receptor density vs. the changes in free GABA neurotransmitter concentration in FXS. However, the previously mentioned confounding clinical 1H-MRS data for ASD warrants preclinical investigation of how the GABAergic system is affected by FXS concerning GABA concentrations. Increasing the use of non-invasive imaging in preclinical research can aid in determining the effectiveness of FXS therapy on a molecular level by being able to visualize changes in endogenous GABA levels via 1H-MRS. 1H-MRS can also be paired with PET to study how the GABAA receptors may be altered by implementing novel FXS therapies. An advantage to utilizing preclinical non-invasive imaging for novel therapeutic validation is the ability to compare imaging results, particularly in longitudinal studies, with previously collected and validated in vitro and ex vivo data to gain a comprehensive understanding and correlation with functional molecular biology.
Since 1H-MRS can non-invasively measure GABA concentrations in both clinical and animal subjects, we sought to employ this technique to allow for future cross-species comparisons with in vitro/ex vivo experiments that are easily performed in relevant disease models. In particular, this study examined GABA levels in the thalamus and frontal cortex of 5-day-old Fmr1 KO compared to WT mice. Aberrant neurodevelopment was exhibited in both regions of young children with FXS (Hoeft et al., 2010) and in the excitatory thalamocortical synapses in the somatosensory cortex of Fmr1 KO mice during the critical cortical period (Harlow et al., 2010). Though it is reported that the perturbation in the GABAergic system is more substantial around day 12 toward the end of the critical cortical window (Adusei et al., 2010; Davidovic et al., 2011), our study concentrates on the earliest age (5-days-old) that GABAergic alteration has been detected (Adusei et al., 2010). Our strategy focuses on utilizing non-invasive imaging to capture the front end of the critical cortical period where abnormal development occurs due to loss of FMRP, to understand the changes in the brain that precedes the more substantial atypical development seen around day 12. The 1H-MRS GABA measurements were then compared to GABA levels in brain homogenates as quantified using liquid chromatography-tandem mass spectrometry (LC-MS/MS) to determine whether 1H-MRS is suitable for studying GABA levels in FXS. Validating 1H-MRS as a method to non-invasively assess GABA concentrations during early development would also provide a powerful tool to elucidate the role GABA plays in FXS and evaluate novel therapeutic strategies for clinical translation.
Materials and Methods
General
Unless stated otherwise, all supplies and equipment were purchased from commercial sources and used for these preclinical studies without modification.
Animals
All animal experiments were approved by Stanford IACUC, with animals having access to food and water ad libitum while being kept under a 12-h light/dark cycle. Experiments were carried out using 5-day-old Wild type (FVB.129P2-Pde6b+ Tyrc-ch/AntJ, Jackson Labs) and Fmr1 knockout (FVB.129P2-Pde6b+ Tyrc-ch Fmr1tm1Cgr/J, Jackson Labs) male mice weighing 2–4 g.
Magnetic Resonance Imaging
MRI at the Stanford Center for Innovation in in vivo Imaging (SCi3) includes an actively-shielded Bruker 7 Tesla horizontal bore scanner (Bruker Corp., Billerica, MA, USA), with International Electric Company (IECO) gradient drivers, a 120 mm inner diameter (ID) shielded gradient insert (600 mT/m, 1,000 T/m/s), AVANCE III electronics; eight-channel multi-coil RF and multinuclear capabilities; and the supporting Paravision 6.0.1 platform. Acquisitions were performed with an 86 mm ID actively de-couplable volume radiofrequency (RF) coil with a four-channel cryo-cooled receive-only RF coil.
1H-Magnetic Resonance Spectroscopy (1H-MRS)
Before each scan, WT (N = 10) and Fmr1 KO (N = 5) mice were anesthetized using ketamine (45 mg/kg) and dexmedetomidine (0.9 mg/kg). 1H-MRS scans were then performed using a Bruker 7T animal MR scanner using a cryogenically cooled RF coil (CryoProbe, Bruker Instruments). Specifically, the 5-day-old mice were placed in a custom scanning bed that was inserted directly into CryoProbe before placing the probe into the scanner (Figure 1). A localizer is used to verify the mouse brain is in the isocenter of the probe. The mice were given subcutaneous saline before the scan and kept warm through heated airflow maintained at 37°C for the duration of the scan. Heart rate monitoring was not possible during the scan due to the small size of the mice used.
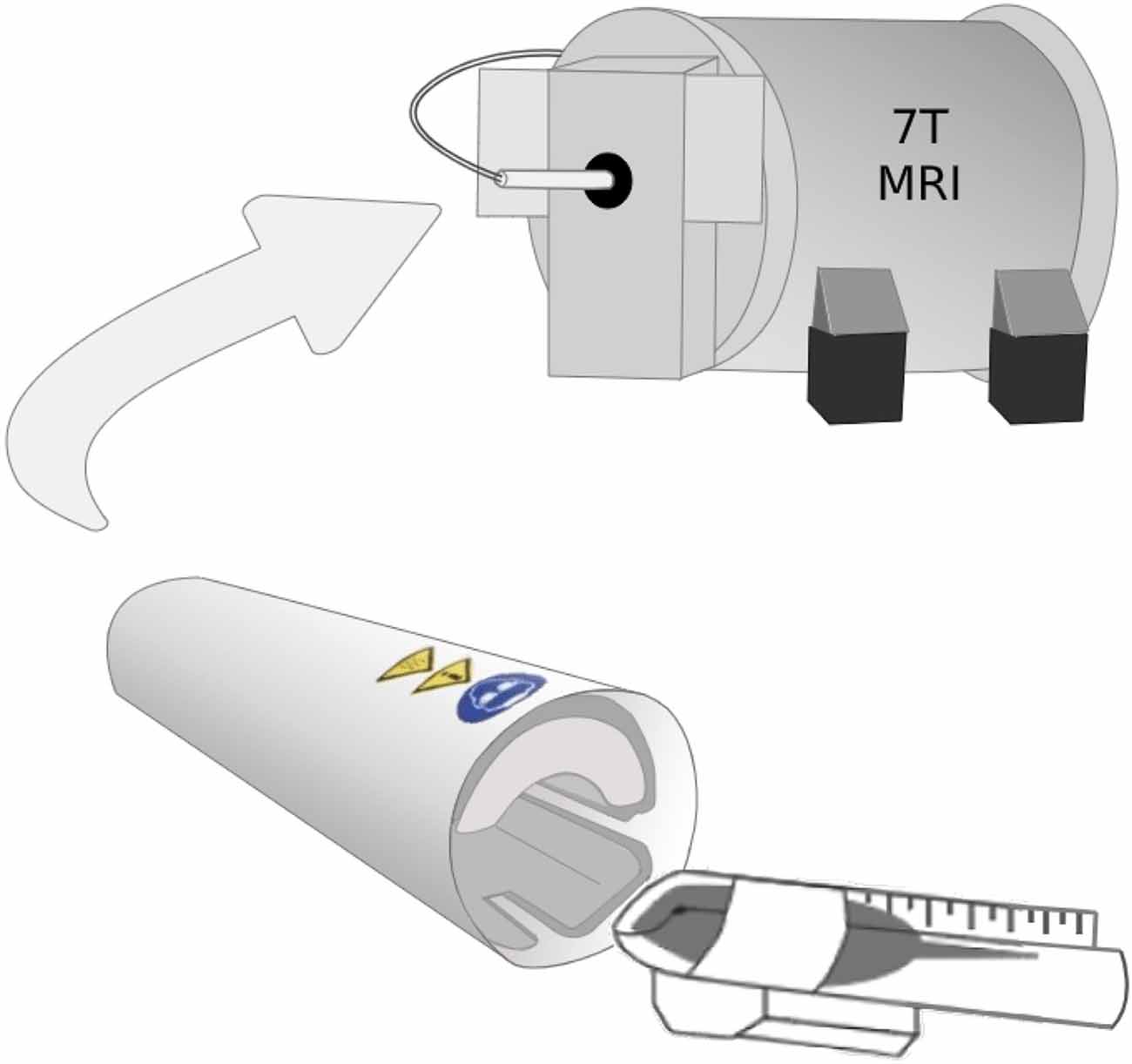
Figure 1. The 5-day-old mice were placed in a custom scanning bed that was created by cutting a 15 ml falcon tube lengthwise. After the mice were secured in the scanning bed with their nose in the conical portion of the tube to allow for airflow, the mice were inserted directly into CryoProbe and held in place by a hand-shaped piece of packing foam. The probe was inserted into the scanner with the mouse brain already in the isocenter of the probe.
In vivo single-voxel spectroscopy using the PRESS sequence was then performed on thalamus-rich and frontal cortex-rich regions-of-interest in the mouse brain. Voxels were manually placed on thalamus-rich and frontal cortex-rich regions on a T2-weighted MRI anatomy scan with the following parameters: 2 × 2 × 2 mm3 voxels, TE = 16 ms, TR = 3,000 ms, spectral bandwidth = 5,000 Hz and 256 averages. The transmit gain was adjusted manually, and both field-mapped and localized voxel shimming were performed. Water suppression using the Vapor method in combination with outer volume suppression was also applied (Henning, 2018; Muñoz-Hernández and García-Martín, 2018). Finally, an unsuppressed-water spectrum was acquired from each voxel for magnetic field drifts and eddy current corrections. Figure 2 shows representative 1H-MRS spectra from wild type and Fmr1 KO mouse brains. Spectral quantification and determination of metabolite ratios to total creatine (creatine + phosphocreatine, CR + PCR) were achieved using LCModel (Provencher, 2001). Basis sets were generated based on density matrix simulations of the sequence using values for chemical shifts and J-couplings of metabolites. LCModel fitting %SD values for our metabolite of interest GABA was ≤12%. LCModel is a commercial software that quantifies metabolites from an MRS spectrum using a least-squares combination of basis spectra. The level of confidence was reported as %SD with 20% or less considered acceptable in general. The basis set is simulated for TE = 16 ms at 7T and contains metabolites of interest.

Figure 2. Representative in vivo 1H magnetic resonance imaging (1H-MRS) spectra of thalamus-rich and frontal cortex-rich voxels and corresponding fits of wild type and Fmr1 KO 5-day-old mouse brain obtained at 7T with a Bruker CryoProbe and analyzed by LC Model. (A) WT thalamus region, (B) WT frontal cortex region, (C) Fmr1 KO thalamus region, and (D) Fmr1 KO frontal cortex region. Scan parameters: 2 × 2 × 2 mm3 voxels, TE = 16 ms, TR = 3,000 ms, spectral bandwidth = 5,000 Hz and 256 averages.
Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS)
Brains from a separate group of mice were harvested from WT (N = 5) and Fmr1 KO (N = 5) mice following decapitation. The frontal cortex and thalamus were collected in microfuge tubes using the Palkovits Punch technique and immediately frozen on dry ice (Palkovits, 1973). The tissue was then homogenized in PBS at a concentration of 150 μl PBS per gram of tissue, and the analyte was extracted from the tissue homogenate using cold 0.1% formic acid in acetonitrile. The sample extracts were derivatized according to Ez:faast™ Amino Acid Analysis protocol according to the manufacturer’s instructions (Phenomenex, Torrance, CA, USA), dried under nitrogen, and reconstituted in LC-MS/MS buffer before the analysis. The LC-MS/MS system consisted of an HP1100 HPLC stack (Agilent, Santa Clara, CA, USA) coupled to a Quattro Premier triple quadrupole mass spectrometer (Waters, Milford, MA, USA). The LC system includes a binary pump, degasser, temperature-controlled column compartment, and autosampler. The mass spectrometer was equipped with an electrospray ionization (ESI) source. The LC separation was achieved on EZ:faast™ AAA-MS column (250 × 3 mm, KH0-7338, Phenomenex, Torrance, CA, USA) at 35°C. Mobile phase A (10 mM ammonium formate) and mobile phase B (10 mM ammonium formate in methanol) were used for a gradient elution from 68 to 83% B at a flow rate of 250 μl/min with a total LC-MS/MS run of 17 min. The mass spectrometer was operated in positive mode using selected reaction monitoring (SRM) scanning mode. The protonated molecules of GABA (m/z 232.0, A2129, Sigma–Aldrich, St. Louis, MO, USA) and GABA-d6 (m/z 237.9, D-1828, C/D/N Isotopes Inc., Quebec, QC, Canada) were used as precursor ions for collision-induced dissociation (CID) for MS/MS analysis. A unique precursor ion-fragment ion SRM transition was select for GABA (232.0 > 130.0, 172.1) and GABA-d6 (237.9 > 135.7, 178.1) which was used as internal standard (IS). Obtained data were processed using QuanLynx software (Waters, Milford, MA, USA). Data were then normalized to protein concentration for each sample using a protein concentration assay (Pierce™ BCA Assay, Thermo Fisher Scientific, Waltham, MA, USA, Supplementary Table 1). Calibration curve standards covered the range of concentrations from 10 nM to 10 μM and resulted in a linear response over the tested range. The low limit of quantitation for GABA was 50 fmols.
Statistical Analysis
GABA was measured in the thalamus and cortex of wild type (N = 10 1H-MRS, N = 5 LC-MS/MS) and Fmr1 knockout (N = 5 1H-MRS, 5 LC-MS/MS) mice, using either 1H-MRS or LC-MS/MS methods. For each method a separate linear regression of GABA on factors of type (Fmr1 KO vs. WT) and region (cortex vs. thalamus) was done, using a robust Huber-White (Huber, 1967; White, 1980) variance estimator to adjust for clustering within the same animal.
Results
Endogenous GABA levels were measured in vivo and normalized to total creatine (CR + PCR) using 1H-MRS. Thalamic and frontal cortical GABA concentrations were compared cross-structurally (within each mouse) and between the same structure across WT and Fmr1 KO mouse groups. To validate the preclinical model used, we employed LC-MS/MS to provide another method for measuring the GABA concentrations in vitro and demonstrate a change in GABA concentration between animal cohorts. Specific dissections for the thalamus and frontal cortex from both WT and Fmr1 KO mice were made and processed for LC-MS/MS analysis. Endogenous GABA concentration measurements were obtained and subsequently compared between both regions within the same animal and for each region across both groups.
For both LC-MS/MS and 1H-MRS methods, both WT and Fmr1 KO mice demonstrated within in each animal statistically significantly higher GABA levels (p < 0.001, Figure 3) in the thalamus-rich region compared to frontal cortical-rich regions. With LC-MS/MS methods, WT mice had statistically significantly higher GABA concentration than Fmr1 KO mice (p = 0.028, Figure 3A), but we were unable to detect a statistically significant difference in GABA concentration between WT and Fmr1 KO groups by 1H-MRS methods (p = 0.92, Figure 3B).
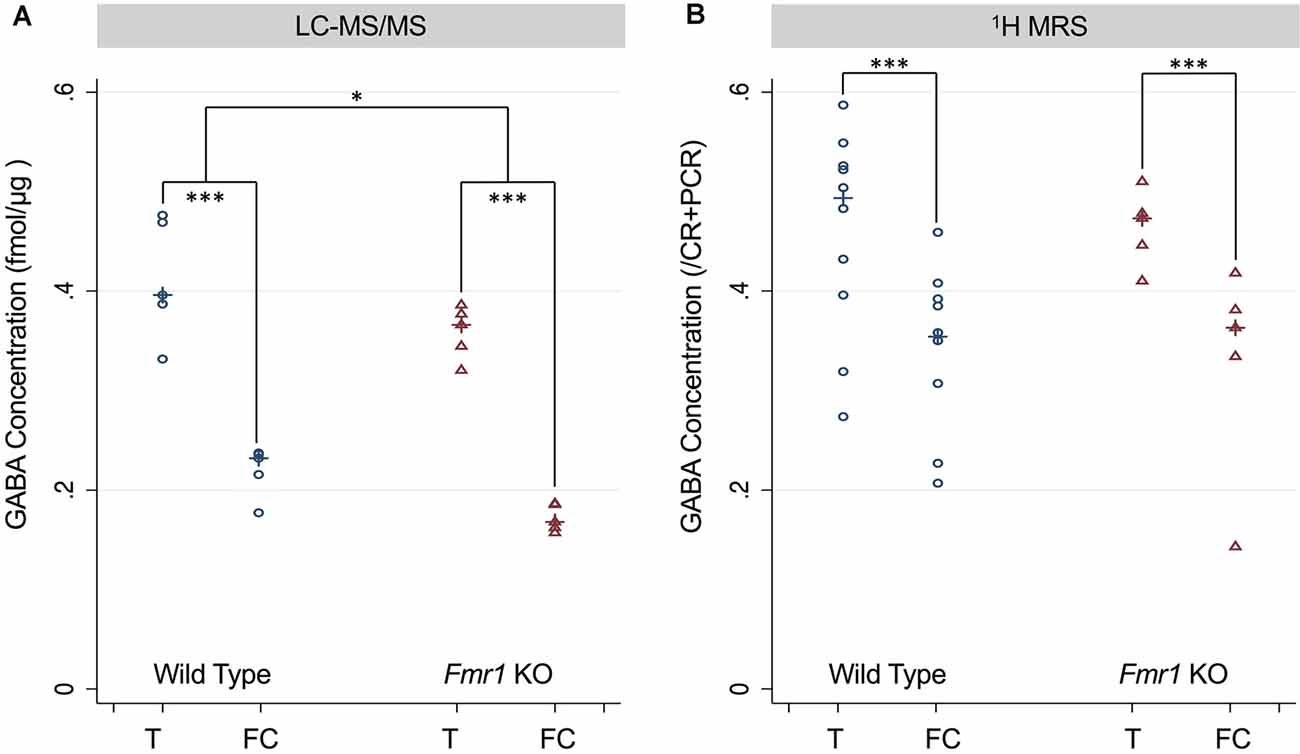
Figure 3. (A) GABA concentration measured in the thalamus and frontal cortex of P5 wild type (WT) and Fmr1 KO mice measured using LC-MS/MS and normalized to the protein concentration of the sample. (B) GABA concentration measured using 1H-MRS GABA/CR + PCR ratios from thalamus-rich and frontal cortex-rich regions of P5 wild type and Fmr1 KO mice analyzed by LC Model. Statistical significance was determined using a robust Huber-White variance estimator. *p < 0.05, ***P < 0.001.
Discussion
Current treatment methods for children and adults with FXS focus on alleviating the symptoms of FXS, such as ADHD and decreased social function, vs. treating the root cause (i.e., loss of FMRP and how the brain is affected). In a clinical trial, social functioning was improved by the use of intranasal oxytocin (Hall et al., 2012) and symptoms of ADHD were improved with the use of pharmaceutical stimulants, such as methylphenidate (Hagerman et al., 1988). While improvements in symptomatic molecular treatments are valuable, a new molecular target may provide an opportunity to improve the quality of life for patients and their families and novel approaches to preclinical FXS research are imperative. Exploring non-invasive imaging strategies [i.e., PET, sMRI, functional MRI (fMRI), diffusion tensor imaging (DTI), MRS] to study biological systems (i.e., GABAergic) may empower researchers to see how novel therapies change the brain early in development and understand the underlying mechanisms leading to disease phenotypes (i.e., FXS).
This study, to the best of our knowledge, is the first 1H-MRS and LC-MS/MS GABA study performed on 5-day-old mouse brains from the Fmr1 KO mouse model to investigate the impact FMRP loss has on endogenous GABA concentration in the frontal cortex and thalamus. Our 1H-MRS study detected significantly decreased GABA in the frontal cortex relative to that observed in the thalamus of both the wild type and Fmr1 KO mice and confirmed these measurements using LC-MS/MS. The development of our method to measure these extremely small animals in a cryogenically cooled RF coil (Bruker CryoProbe) on a 7T MRI required the reliable placement of the neonatal brain in the isocenter of the scanner. Moreover, positioning the neonates on the heated ceramic casing within the CryoProbe ensures consistent warmed body temperature during the MRI scan. Additionally, the use of the CryoProbe increases SNR by reducing the noise from the coil itself, caused by resistance in the coil’s wiring (Ratering et al., 2008; Baltes et al., 2009). Our novel scanning technique can be applied to any mouse model for MRI scanning, including sMRI, 1H-MRS, fMRI, and DTI. Furthermore, our technique uses a homemade scanning bed constructed from common laboratory materials (Figure 1), making this method of scanning neonates using a CryoProbe easily reproducible.
Although 1H-MRS was unable to detect the same-structure differences within the frontal cortices or thalami between Fmr1 KO mice and WT mice, LC-MS/MS was able to detect significantly lower GABA in the frontal cortex and thalamus of Fmr1 KO mice compared to age-matched WT mice. This is the first report of lowered GABA concentration in the frontal cortex and thalamus in a 5-day-old FXS mouse model. Alterations within these structures are supported by previous clinical imaging studies showing abnormal activation patterns in the supplementary motor area of the cerebral cortex and thalamus in female FXS patients via fMRI (Menon et al., 2004) and significantly decreased binding potential in the thalamus of male FXS patients found with 11C-FMZ PET (D’Hulst et al., 2015). Overall, the findings of these imaging studies in combination with our detection of decreased GABA in the thalamus supports that FMRP loss affects multiple components of the thalamus, including the GABAergic system. Recent studies have also found that inhibitory deficits in the medial prefrontal cortex in a prepubescent FXS mouse model (Kramvis et al., 2020). Multiple clinical 1H-MRS studies measuring GABA in various adult populations have found that decreased GABA concentrations in the prefrontal cortex, dorsolateral prefrontal cortex, or frontal cerebrum are correlated with decreased cognitive function and working memory task performance (Yoon et al., 2016; Porges et al., 2017; Kim et al., 2019). Another 1H-MRS clinical study found a positive correlation between visual intelligence and GABA concentration in the primary visual cortex (Cook et al., 2016). These results are consistent with our studies showing lowered GABA concentration in the frontal cortex, as decreased cognition and lowered IQ are characteristic of FXS.
This study confirmed that in vivo 1H-MRS is a promising non-invasive imaging technique for detecting GABA-regional brain differences within 5-day-old mice of each respective cohort, despite not detecting structural differences between Fmr1 KO vs. WT mice in this study. To use similar 1H-MRS methods to detect smaller differences in GABA concentration between Fmr1 KO and WT mice detected by LC-MS/MS improved MRI sensitivity (using techniques such as increased averaging, larger voxels, or higher magnetic gradients) to increase the signal to noise ratio (SNR) or more animals would be needed for the study (i.e., increased sample size). Based on the linear regression models’ marginal effect of type, the standard error for LC-MS/MS mice is 0.019, while that for 1H-MRS mice is twice as large, 0.039, even though the sample size is 50% larger in number. To achieve a similar standard error to that of the LC-MS/MS mice, the 1H MRS study would require a sample size of 65 animals. The apparent variability in the 1H-MRS GABA measurements, particularly for each animal in the WT group (Figure 3B), may contribute to the lack of significant difference seen in same-structural comparisons between WT and Fmr1 KO mice. Since decreased GABA transmission in either the medial prefrontal cortex or basolateral amygdala has been linked with decreased sociability, this observed variability may be due to a wider range in social function in control animals when compared to animals with known social deficits. Changes in GABA signaling is seen in conditions, such as autism spectrum disorder, may mediate the social withdrawal aspect of these conditions (Paine et al., 2017). Additionally, our voxel size and cubic shape of 2 × 2 × 2 are larger than the volume for the frontal cortex and thalamus of our 5-day-old mice, thus increasing the variability in the 1H-MRS data. In particular to our study of GABA, we chose to use separate animals for each technique because the animals that underwent 1H-MRS scanning were subjected to anesthesia. Anesthesia has a known effect on both circadian rhythms (Poulsen et al., 2018) and the GABAA receptor’s binding to 18F-FMZ (Palner et al., 2016) therefore, we chose to eliminate anesthesia from our in vitro LC-MS/MS analysis. Another factor to consider is that GABA exists in different states in vivo including bound, free, and within vesicles. These different pools of GABA could have varying relaxation times and, hence, may have differential sensitivity to detection using 1H-MRS. Conversely, for LC-MS/MS studies, the brain sections are homogenized, likely releasing all available GABA.
If higher SNR can be achieved, 1H-MRS could be a powerful non-invasive imaging tool for measuring endogenous FXS GABA levels, which could potentially be paired with 11C- or 18F-FMZ PET to image the postsynaptic benzodiazepine site on the GABAA receptor. Such experiments would allow the assessment of correlations between regional GABA concentrations and receptor densities between WT and Fmr1 KO mice. Having a better understanding of how neuronal GABA varies between WT and Fmr1 KO mice could provide new information into the mechanisms of FXS’s impact on early-stage brain development. Implementing 1H-MRS into pre-clinical therapeutic evaluation studies in an FXS model can be particularly valuable for longitudinal studies.
Our study is the first known 1H-MRS and LC-MS/MS measurements of GABA in 5-day-old mice. We demonstrated that a Bruker 7T MRI with a cryogenically cooled RF coil provides sufficient sensitivity to measure regional significant differences in GABA concentration between the frontal cortex and thalamus within individual 5-day-old mice. However, the sensitivity to see significant differences within the same structures between WT and Fmr1 KO mice did not measure up to the LC-MS/MS being able to detect a significant decrease in both structures in the Fmr1 KO mice compared to WT. This in vitro data elucidates that alterations in the GABAergic system of 5-day-old FXS mice extend further than the previously reported subunit alterations (Adusei et al., 2010). Although LC-MS/MS is a valuable in vitro technique that can be used to complement in vivo data, it is not ideal to analyze brain samples from animals studied over many time points. Exploring novel FXS therapy approaches tailored to the critical window of cortical development may have promising outcomes due to addressing changes in the GABAergic system at their earliest occurrence. For 1H-MRS to be routinely utilized in preclinical research and development of novel FXS molecular therapeutics, our studies suggest an increased field strength or increased scan time can be used to improve SNR while employing a cryogenically cooled RF coil. 1H-MRS is non-invasive and does not require contrast agents or radiotracers, making it an attractive modality to use for longitudinal studies. 1H-MRS can be an incredibly useful tool to monitor the effectiveness of novel therapies at addressing how to normalize the concentration of free neurotransmitters, such as GABA in the brain.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by Stanford Institutional Care and Use Committee (IACUC).
Author Contributions
FC and DS conceived the experiments. FC, DS, SR, SM and LP designed the 1H-MRS experiment. FC, DS, SR and KK designed the LC-MS/MS experiment. All authors performed the experiments and/or analyzed the data. SR wrote the manuscript with the support of all authors. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by NIH R01 HD084214 (FC), Ben and Catherine Ivy Foundation (FC), Stanford Bio-X Summer Undergraduate Research Grant (SG), NIH R01 MH110683 (DS), NIH P41 EB015891 (DS), and NIH S10 Shared Instrumentation Grant (S10 RR026917-01, PI Michael Moseley, Ph.D.).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Stanford University Mass Spectrometry Facility, Stanford Center for Innovations in in vivo Imaging (SCi3) small animal imaging center.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnmol.2020.612685/full#supplementary-material.
References
Adusei, D. C., Pacey, L. K. K., Chen, D., and Hampson, D. R. (2010). Early developmental alterations in GABAergic protein expression in fragile X knockout mice. Neuropharmacology 59, 167–171. doi: 10.1016/j.neuropharm.2010.05.002
Ascano, M., Mukherjee, N., Bandaru, P., Miller, J. B., Nusbaum, J. D., Corcoran, D. L., et al. (2012). FMRP targets distinct mRNA sequence elements to regulate protein expression. Nature 492, 382–386. doi: 10.1038/nature11737
Baltes, C., Radzwill, N., Bosshard, S., Marek, D., and Rudin, M. (2009). Micro MRI of the mouse brain using a novel 400 MHz cryogenic quadrature RF probe. NMR Biomed. 22, 834–842. doi: 10.1002/nbm.1396
Bear, M. F., Huber, K. M., and Warren, S. T. (2004). The mGluR theory of fragile X mental retardation. Trends Neurosci. 27, 370–377. doi: 10.1016/j.tins.2004.04.009
Braat, S., D’Hulst, C., Heulens, I., Rubeis, S., Mientjes, E., Nelson, D. L., et al. (2015). The GABAA receptor is an FMRP target with therapeutic potential in fragile X syndrome. Cell Cycle 14, 2985–2995. doi: 10.4161/15384101.2014.989114
Braat, S., and Kooy, R. F. (2015). The GABAA receptor as a therapeutic target for neurodevelopmental disorders. Neuron 86, 1119–1130. doi: 10.1016/j.neuron.2015.03.042
Cochran, D. M., Sikoglu, E. M., Hodge, S. M., Edden, R. A. E., Foley, A., Kennedy, D. N., et al. (2015). Relationship among glutamine, γ-aminobutyric acid and social cognition in autism spectrum disorders. J. Child Adolesc. Psychopharmacol. 25, 314–322. doi: 10.1089/cap.2014.0112
Cogram, P., Deacon, R. M. J., Warner-Schmidt, J., von Schimmelmann, M. J., Abrahams, B. S., and During, M. J. (2019). Gaboxadol normalizes behavioral abnormalities in a mouse model of fragile X syndrome. Front. Behav. Neurosci. 13:141. doi: 10.3389/fnbeh.2019.00141
Cook, E., Hammett, S. T., and Larsson, J. (2016). GABA predicts visual intelligence. Neurosci. Lett. 632, 50–54. doi: 10.1016/j.neulet.2016.07.053
Dahlhaus, R. (2018). Of men and mice: modeling the fragile X syndrome. Front. Mol. Neurosci. 11:41. doi: 10.3389/fnmol.2018.00041
Darnell, J. C., Van Driesche, S. J., Zhang, C., Sharon Hung, K. Y., Mele, A., Fraser, C. E., et al. (2011). FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 146, 247–261. doi: 10.1016/j.cell.2011.06.013
Davidovic, L., Navratil, V., Bonaccorso, C. M., Catania, M. V., Bardoni, B., and Dumas, M.-E. (2011). A metabolomic and systems biology perspective on the brain of the fragile X syndrome mouse model. Genome Res. 21, 2190–2202. doi: 10.1101/gr.116764.110
D’Hulst, C., De Geest, N., Reeve, S. P., Van Dam, D., De Deyn, P. P., Hassan, B. A., et al. (2006). Decreased expression of the GABAA receptor in fragile X syndrome. Brain Res. 1121, 238–245. doi: 10.1016/j.brainres.2006.08.115
D’Hulst, C., Heulens, I., Van der Aa, N., Goffin, K., Koole, M., Porke, K., et al. (2015). Positron emission tomography (PET) quantification of GABAA receptors in the brain of fragile X patients. PLoS One 10:e0131486. doi: 10.1371/journal.pone.0131486
El Idrissi, A., Ding, X.-H., Scalia, J., Trenkner, E., Ted Brown, W., and Dobkin, C. (2005). Decreased GABAA receptor expression in the seizure-prone fragile X mouse. Neurosci. Lett. 377, 141–146. doi: 10.1016/j.neulet.2004.11.087
Faghihi, R., Zeinali-Rafsanjani, B., Mosleh-Shirazi, M.-A., Saeedi-Moghadam, M., Lotfi, M., Jalli, R., et al. (2017). Magnetic resonance spectroscopy and its clinical applications: a review. J. Med. Imaging Radiat. Sci. 48, 233–253. doi: 10.1016/j.jmir.2017.06.004
Fung, L. K., Flores, R. E., Gu, M., Sun, K. L., James, D., Schuck, R. K., et al. (2020). Thalamic and prefrontal GABA concentrations but not GABAA receptor densities are altered in high-functioning adults with autism spectrum disorder. Mol. Psychiatry doi: 10.1038/s41380-020-0756-y [Epub ahead of print].
Gantois, I., Vandesompele, J., Speleman, F., Reyniers, E., D’Hooge, R., Severijnen, L.-R., et al. (2006). Expression profiling suggests underexpression of the GABAA receptor subunit δ in the fragile X knockout mouse model. Neurobiol. Dis. 21, 346–357. doi: 10.1016/j.nbd.2005.07.017
Gao, F., Qi, L., Yang, Z., Yang, Z., Zhang, Y., Xu, H., et al. (2018). Impaired GABA neural circuits are critical for fragile X syndrome. Neural Plast. 2018, 1–7. doi: 10.1155/2018/8423420
Gatto, C. L., and Broadie, K. (2009). The fragile X mental retardation protein in circadian rhythmicity and memory consolidation. Mol. Neurobiol. 39, 107–129. doi: 10.1007/s12035-009-8057-0
Gross, C., Hoffmann, A., Bassell, G. J., and Berry-Kravis, E. M. (2015). Therapeutic strategies in fragile X syndrome: from bench to bedside and back. Neurotherapeutics 12, 584–608. doi: 10.1007/s13311-015-0355-9
Hagerman, R. J., Berry-Kravis, E., Berry-Kravis, E., Cody Hazlett, H., Bailey, D. B., Moine, H., et al. (2017). Fragile X syndrome. Nat. Rev. Dis. Primers 3:17065. doi: 10.1038/nrdp.2017.65
Hagerman, R. J., Murphy, M. A., and Wittenberger, M. D. (1988). A controlled trial of stimulant medication in children with the fragile X syndrome. Am. J. Med. Genet. 30, 377–392. doi: 10.1002/ajmg.1320300138
Hall, S. S., Lightbody, A. A., McCarthy, B. E., Parker, K. J., and Reiss, A. L. (2012). Effects of intranasal oxytocin on social anxiety in males with fragile X syndrome. Psychoneuroendocrinology 37, 509–518. doi: 10.1016/j.psyneuen.2011.07.020
Harada, M., Taki, M. M., Nose, A., Kubo, H., Mori, K., Nishitani, H., et al. (2011). Non-invasive evaluation of the GABAergic/glutamatergic system in autistic patients observed by MEGA-editing proton mr spectroscopy using a clinical 3 tesla instrument. J. Autism. Dev. Disord. 41, 447–454. doi: 10.1007/s10803-010-1065-0
Harlow, E. G., Till, S. M., Russell, T. A., Wijetunge, L. S., Kind, P., and Contractor, A. (2010). Critical period plasticity is disrupted in the barrel cortex of Fmr1 knockout mice. Neuron 65, 385–398. doi: 10.1016/j.neuron.2010.01.024
He, Q., Nomura, T., Xu, J., and Contractor, A. (2014). The developmental switch in GABA polarity is delayed in fragile X mice. J. Neurosci. 34, 446–450. doi: 10.1523/JNEUROSCI.4447-13.2014
Henning, A. (2018). Proton and multinuclear magnetic resonance spectroscopy in the human brain at ultra-high field strength: a review. NeuroImage 168, 181–198. doi: 10.1016/j.neuroimage.2017.07.017
Hoeft, F., Carter, J. C., Lightbody, A. A., Cody Hazlett, H., Piven, J., and Reiss, A. L. (2010). Region-specific alterations in brain development in 1- to 3-year-old boys with fragile X syndrome. Proc. Natl. Acad. Sci. U S A 107, 9335–9339. doi: 10.1073/pnas.1002762107
Horder, J., Petrinovic, M. M., Mendez, M. A., Bruns, A., Takumi, T., Spooren, W., et al. (2018). Glutamate and GABA in autism spectrum disorder—a translational magnetic resonance spectroscopy study in man and rodent models. Transl. Psychiatry 8:106. doi: 10.1038/s41398-018-0155-1
Huber, P. J. (1967). “The behavior of maximum likelihood estimates under nonstandard conditions,” in Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability, (Berkeley: Statistical Laboratory of the University of California), 221–233.
Hunter, J., Rivero-Arias, O., Angelov, A., Kim, E., Fotheringham, I., and Leal, J. (2014). Epidemiology of fragile X syndrome: a systematic review and meta-analysis. Am. J. Med. Genet. A 164, 1648–1658. doi: 10.1002/ajmg.a.36511
Irwin, S. A., Galvez, R., and Greenough, W. T. (2000). Dendritic spine structural anomalies in fragile-X mental retardation syndrome. Cereb. Cortex 10, 1038–1044. doi: 10.1093/cercor/10.10.1038
Kim, G. H., Kang, I., Jeong, H., Park, S., Hong, H., Kim, J., et al. (2019). Low prefrontal GABA levels are associated with poor cognitive functions in professional boxers. Front. Hum. Neurosci. 13:193. doi: 10.3389/fnhum.2019.00193
Kramvis, I., van Westen, R., Lammertse, H. C. A., Riga, D., Heistek, T. S., Loebel, A., et al. (2020). Dysregulated prefrontal cortex inhibition in prepubescent and adolescent fragile X mouse model. Front. Mol. Neurosci. 13:88. doi: 10.3389/fnmol.2020.00088
Ligsay, A., and Hagerman, R. J. (2016). Review of targeted treatments in fragile X syndrome. Intractable Rare Dis. Res. 5, 158–167. doi: 10.5582/irdr.2016.01045
Lo, S. Q., Sng, J. C. G., and Augustine, G. J. (2017). Defining a critical period for inhibitory circuits within the somatosensory cortex. Sci. Rep. 7:7271. doi: 10.1038/s41598-017-07400-8
Lozano, R., Hare, E. B., and Hagerman, R. J. (2014). Modulation of the GABAergic pathway for the treatment of fragile X syndrome. Neuropsychiatr. Dis. Treat. 10, 1769–1779. doi: 10.2147/NDT.S42919
Maurin, T., Lebrigand, K., Castagnola, S., Paquet, A., Jarjat, M., Popa, A., et al. (2018). HITS-CLIP in various brain areas reveals new targets and new modalities of RNA binding by fragile X mental retardation protein. Nucleic Acids Res. 46, 6344–6355. doi: 10.1093/nar/gky267
Menon, V., Leroux, J., White, C. D., and Reiss, A. L. (2004). Frontostriatal deficits in fragile X syndrome: relation to FMR1 gene expression. Proc. Natl. Acad. Sci. U S A 101, 3615–3620. doi: 10.1073/pnas.0304544101
Miyashiro, K. Y., Beckel-Mitchener, A., Purk, T. P., Becker, K. G., Barret, T., Liu, L., et al. (2003). RNA cargoes associating with FMRP reveal deficits in cellular functioning in Fmr1 null mice. Neuron 37, 417–431. doi: 10.1016/s0896-6273(03)00034-5
Muñoz-Hernández, M. C., and García-Martín, M. L. (2018). “In vivo 1H magnetic resonance spectroscopy,” in Preclinical MRI: Methods and Protocols, eds M. L. García Martín and P. López Larrubia (New York, NY: Springer), 151–167.
Öz, G., Alger, J. R., Barker, P. B., Bartha, R., Bizzi, A., Boesch, C., et al. (2014). Clinical proton MR spectroscopy in central nervous system disorders. Radiology 270, 658–679. doi: 10.1148/radiol.13130531
Paine, T. A., Swedlow, N., and Swetschinski, L. (2017). Decreasing GABA function within the medial prefrontal cortex or basolateral amygdala decreases sociability. Behav. Brain Res. 317, 542–552. doi: 10.1016/j.bbr.2016.10.012
Palkovits, M. (1973). Isolated removal of hypothalamic or other brain nuclei of the rat. Brain Res. 59, 449–450. doi: 10.1016/0006-8993(73)90290-4
Palner, M., Beinat, C., Banister, S., Zanderigo, F., Park, J. H., Shen, B., et al. (2016). Effects of common anesthetic agents on [18F]flumazenil binding to the GABAA receptor. EJNMMI Res. 6:80. doi: 10.1186/s13550-016-0235-2
Paluszkiewicz, S. M., Martin, B. S., and Huntsman, M. M. (2011). Fragile X syndrome: the gabaergic system and circuit dysfunction. Dev. Neurosci. 33, 349–364. doi: 10.1159/000329420
Porges, E. C., Woods, A. J., Edden, R. A. E., Puts, N. A. J., Harris, A. D., Chen, H., et al. (2017). Frontal gamma-aminobutyric acid concentrations are associated with cognitive performance in older adults. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2, 38–44. doi: 10.1016/j.bpsc.2016.06.004
Poulsen, R. C., Warman, G. R., Sleigh, J., Ludin, N. M., and Cheeseman, J. F. (2018). How does general anaesthesia affect the circadian clock? Sleep Med. Rev. 37, 35–44. doi: 10.1016/j.smrv.2016.12.002
Provencher, S. W. (2001). Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 14, 260–264. doi: 10.1002/nbm.698
Ratering, D., Baltes, C., Nordmeyer-Massner, J., Marek, D., and Rudin, M. (2008). Performance of a 200-MHz cryogenic RF probe designed for MRI and MRS of the murine brain. Magn. Reson. Med. 59, 1440–1447. doi: 10.1002/mrm.21629
Remmers, C. L., and Contractor, A. (2018). Development of GABAergic inputs is not altered in early maturation of adult born dentate granule neurons in fragile X mice. eNeuro 5:ENEURO.0137-18.2018. doi: 10.1523/ENEURO.0137-18.2018
Tabolacci, E., Palumbo, F., Nobile, V., and Neri, G. (2016). Transcriptional reactivation of the FMR1 gene. A possible approach to the treatment of the fragile X syndrome. Genes 7:49. doi: 10.3390/genes7080049
The Dutch-Belgian Fragile X Consortium. (1994). Fmr1 knockout mice: a model to study fragile X mental retardation. Cell 78, 23–33.
White, H. (1980). A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica 48, 817–838. doi: 10.2307/1912934
Wilson, M., Andronesi, O., Barker, P. B., Bartha, R., Bizzi, A., Bolan, P. J., et al. (2019). Methodological consensus on clinical proton MRS of the brain: review and recommendations. Magn. Reson. Med. 82, 527–550. doi: 10.1002/mrm.27742
Yoon, J. H., Grandelis, A., and Maddock, R. J. (2016). Dorsolateral prefrontal cortex GABA concentration in humans predicts working memory load processing capacity. J. Neurosci. 36, 11788–11794. doi: 10.1523/JNEUROSCI.1970-16.2016
Keywords: fragile X syndrome, Fmr1 knockout, mouse, neonatal, GABA, 1H-MRS, LC-MS/MS
Citation: Reyes ST, Mohajeri S, Krasinska K, Guo SG, Gu M, Pisani L, Rosenberg J, Spielman DM and Chin FT (2020) GABA Measurement in a Neonatal Fragile X Syndrome Mouse Model Using 1H-Magnetic Resonance Spectroscopy and Mass Spectrometry. Front. Mol. Neurosci. 13:612685. doi: 10.3389/fnmol.2020.612685
Received: 30 September 2020; Accepted: 20 November 2020;
Published: 18 December 2020.
Edited by:
Barbara Bardoni, UMR7275 Institut de Pharmacologie Moléculaire et Cellulaire (IPMC), FranceReviewed by:
Randi Jenssen Hagerman, UC Davis, United StatesSimona D’Antoni, National Research Council (CNR), Italy
Copyright © 2020 Reyes, Mohajeri, Krasinska, Guo, Gu, Pisani, Rosenberg, Spielman and Chin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Frederick T. Chin, Y2hpbmZAc3RhbmZvcmQuZWR1
 Samantha T. Reyes1
Samantha T. Reyes1 Frederick T. Chin
Frederick T. Chin