
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Mol. Neurosci. , 07 January 2021
Sec. Molecular Signalling and Pathways
Volume 13 - 2020 | https://doi.org/10.3389/fnmol.2020.612439
This article is part of the Research Topic Modulation of Microglia Transition Activity in Neuroinflammation following CNS Injury View all 12 articles
Ischemic stroke is one of the most common causes of death and disability worldwide. Neuroinflammation is a major pathological event involved in the process of ischemic injury and repair. In particular, microglia play a dual role in neuroinflammation. During the acute phase of stroke onset, M2 microglia are the dominant phenotype and exert protective effects on neuronal cells, whereas permanent M1 microglia contribute to prolonged inflammation and are detrimental to brain tissue. Emerging evidence indicates that microRNAs (miRNAs) may have regulatory effects on microglia-associated inflammation. Thus, we briefly reviewed the dynamic response of microglia after a stroke and assessed how specific miRNAs affect the behavior of reactive microglia. We concluded that miRNAs may be useful novel therapeutic targets to improve stroke outcomes and modulate neuroinflammation.
Ischemic stroke is one of the most common causes of death and disability worldwide (Chen et al., 2019). The only effective therapeutic approach to salvage the penumbra in the acute phase of stroke is reperfusion therapy (Knecht et al., 2017; Dela Pena et al., 2018). With improved understanding of the mechanism underlying ischemic stroke, researchers have discovered that several pathogenic events can be triggered by ischemic cell stress, such as excitotoxicity (Lai et al., 2014), inflammation, oxidative stress (Li P. et al., 2018), and mitochondrial disturbances (Narne et al., 2017). These stroke-associated pathogenic events can expand the ischemic core and influence the post-stroke recovery as well as the brain regenerative responses (Kriz and Lalancette-Hébert, 2009).
Among the multiple events leading to short-term mortality and long-term disability, post-stroke inflammation plays an important role in brain tissue damage and repair (Tobin et al., 2014; Yong et al., 2019). Glial cells, especially microglia, are primarily involved in the immune response following an ischemic attack and are activated within hours after the ischemic stroke, thereby maintaining the immunologic homeostasis (Iadecola and Anrather, 2011; Macrez et al., 2011). Moreover, reactive microglia play a dual role in ischemic injuries, which is directly related to the microglial phenotypes. More specifically, the pro-inflammatory M1 phenotype exerts harmful effects, while the anti-inflammatory M2 phenotype plays protective role following ischemic attacks (Michell-Robinson et al., 2015). The phenotypic shift between M1 and M2 in microglia is similar to that of macrophages. An imbalance between M1 and M2 microglia can induce prolonged neuroinflammation and worsen the prognosis. Due to the impact of microglial M1/M2 polarization on neuroinflammation, several studies have proposed microglial polarization modification as a potential treatment for ischemic stroke. More importantly, compared to thrombolytic therapies, therapies targeted at neuroinflammation provide a broader treatment window (Tobin et al., 2014; Yong et al., 2019).
Emerging evidence suggests that microRNAs (miRNAs), the small non-coding RNAs of 18–22 nucleotides play essential roles in stroke pathogenesis (Kosik, 2006; Kabekkodu et al., 2018). Following cerebral ischemia, the expression levels of many miRNAs (i.e., miR-126, miR-145, and miR-320) are significantly altered in rat models of middle cerebral artery occlusion (MCAO) as well as in stroke patients (Dewdney et al., 2018). Furthermore, changes in miRNA expression play key roles in several aspects of stroke pathophysiology, such as excitotoxicity, oxidative stress, mitochondrial disturbances, and inflammation as well as in aspects of post-stroke recovery such as neurogenesis and angiogenesis (Majdi et al., 2016; Li G. et al., 2018). Furthermore, certain miRNAs are potential prognostic, diagnostic, and therapeutic biomarkers for stroke (Vijayan and Reddy, 2016; Mirzaei et al., 2018). The modulation of miRNAs contributes to the post-stroke pathology, including neuroinflammation. Neuroinflammation is strongly associated with stroke, occurs within minutes of focal ischemia, and is involved in all stages of the ischemic cascade (Iadecola and Anrather, 2011). Studies have shown that miRNAs are key mediators in the processes of cerebral ischemia and are modulated in stroke-associated immune cells (i.e., microglia, macrophages, astrocytes, neutrophils, innate lymphocytes, and mast cells) (Karthikeyan et al., 2016; Khoshnam et al., 2017; Gaudet et al., 2018). Furthermore, miRNAs regulating microglia-mediated inflammatory events have been investigated (Freilich et al., 2013; Amici et al., 2017; Wang S. W. et al., 2018; Rodríguez-Gómez et al., 2020). For instance, miRNA-155 acts as a proinflammatory mediator in microglia. MiR-155 inhibition suppresses microglia activation and expression of M1-related inflammatory cytokines (Thounaojam et al., 2014; Guo et al., 2019). In contrast, miR-181c inhibits neuroinflammation by suppressing toll-like-receptor (TLR)-4 expression, thereby inhibiting NF-κB activation and production of downstream cytokines (Zhang et al., 2015).
Therefore, this review describes microglial behaviors such as activation and polarization following ischemic stroke as well as the relationship between miRNAs and the microglial behavior. This will provide further insights into the regulatory roles of miRNAs in microglia-associated inflammation. Further, this review will provide a novel perspective for the future basic as well as clinical research and clinical management of ischemic stroke.
Microglia, the resident immune cells of the central nervous system (CNS), are highly plastic the primary cells involved in neuroinflammation caused by ischemia (Iadecola and Anrather, 2011; Gaire et al., 2019). Microglia react to CNS insults and change their phenotypes to clear the neuronal damage through phagocytosis and repair (Nimmerjahn et al., 2005; Hanisch and Kettenmann, 2007). Microglia phenotypes change in response to changes in the brain microenvironment. The ratio of pro- and anti-inflammatory microglia plays the pivotal role in determining the course and severity of neuroinflammation (Yenari et al., 2010). Excessive or inappropriate activation of microglia promotes chronic inflammation and leads to neuropathological progression (Hu et al., 2012).
Reactive microglia can be divided into two phenotypes according to their function: the pro-inflammatory phenotype M1 and the anti-inflammatory phenotype M2. The activation of the M1 or M2 phenotype is closely related to the environment around the microglia at the time of the ischemic stroke onset (Ponomarev et al., 2013). M1 microglia, induced by lipopolysaccharides (LPS) or interferon gamma (INF-γ), enhance the inflammatory response by producing pro-inflammatory mediators (i.e., IFN-γ, interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and IL-6), reactive oxygen species, nitric oxide, proteolytic enzymes (i.e., metalloproteinase 9 and 3), and chemokines (i.e., chemokine interferon-γ inducible protein). The pathological events caused by pro-inflammatory mediators lead to further brain damage and deterioration. In contrast, the M2 microglia stimulated by IL-4 and IL-13, produce anti-inflammatory cytokines (i.e., IL-10, transforming growth factor-beta(TGF-β), IL-4, IL-13, and insulin-like growth factor 1) and participate in the anti-inflammatory responses that repair damaged brain tissue and exert neuroprotective effects following ischemic insults. Furthermore, previous studies have shown that there are multiple subclasses of M2 phenotypes, and exposure to different stimuli may induce different forms of the M2 phenotype (Mantovani et al., 2004). M2a is induced by IL-4 or IL-13 and is involved in regeneration and repair. M2b exerts immunoregulatory effects and is activated by immune complexes such as Fcγ receptors, TLRs, or IL-1R. Chhor et al. (2013) suggested that the pro-inflammatory stimuli, such as LPS, IL-1β, TNF-α, and IFN-γ induced activation of M1 and M2b. M2c represents an acquired-deactivating phenotype produced in response to IL-10, TGF-β, and glucocorticoids (Ransohoff and Perry, 2009; Chhor et al., 2013; Orihuela et al., 2016; Lam et al., 2017). In addition, new signature genes have been reported to distinguish microglia from infiltrating macrophages during neuroinflammation using RNA sequencing (DePaula-Silva et al., 2019). These data showed that Sall1 and SLC2a5 are specifically expressed in reactive microglia, Fcrls and Slc2a5 are expressed in hemostatic microglia, while Ly6c2 and Ccr2 are expressed in infiltrating macrophages. Moreover, TREM-1 protein was confirmed as an important cell surface marker for infiltrating macrophages at the peak of neuroinflammation (DePaula-Silva et al., 2019). Furthermore, Schmid et al. (2009) investigated the differentially expressed genes between microglia and macrophages and identified 15 genes including Loc620695, GPR84, and Sal3 enriched in cultured neonatal microglia compared to peritoneal macrophages. However, only three of these 15 genes (C1qA, Trem2, and CXCL14) were differentially expressed in the adult microglia isolated from LPS/IFN-γ-injected CNS compared to the infiltrating peripheral macrophages. Similarly, Lund et al. (2006) highlighted that the CNS microenvironment may contribute to the different phenotypes of both CNS-resident microglia as well as CNS-infiltrating macrophages. Certain genes associated with inflammatory responses are differentially expressed in ex vivo and in vitro cultured microglia. For instance, CX3CR1 and P2RY12 are downregulated in microglia cultured in vitro (Gosselin et al., 2017). The findings described by Gosselin et al. (2017) indicated the limitations that steer microglial phenotypes in vitro. Thus, the M1/M2 phenotype transition may not have the same molecular mechanism in vitro as that in vivo. In summary, fully polarized M1 and M2 microglia/macrophages in neuroinflammation, both in vivo and in vitro, represent the extremes of a phenotype continuum. Here, we used the terms M1 and M2 to refer to the two extremes of the microglia/macrophage phenotype spectrum. Due to the dual function of microglia, targeting the M1/M2 polarization is postulated as an important therapeutic strategy for cerebral ischemia. The M1 and M2 phenotype ratio is also considered essential in determining whether the brain suffers from secondary injuries following cerebral ischemia (Iadecola and Anrather, 2011; Patel et al., 2013; Barakat and Redzic, 2016; Wan et al., 2016; Wang et al., 2018a; Gaire et al., 2019).
Owing to the distinct functional phenotypes of microglia, researchers have attempted to identify methods to amplify their beneficial results while suppressing negative effects (Xia et al., 2015; Wang et al., 2018a). Acute ischemic injury is believed to trigger the induction of the M2 phenotype in resting microglia during the early stage of the ischemic stroke, while the left infarcted tissue, following incomplete treatment, stimulates microglia activation and promotes the M1 phenotype (Hu et al., 2012). Thus, we are interested in the dynamic changes of microglial phenotypes depending on the post-stroke different stimulatory environments.
Ischemic stroke is caused by focal reduction in cerebral blood flow, and result in an ischemic core and the surrounding penumbra. The location of microglia in the ischemic brain affects their activation and cell fate. Microglia respond rapidly to the cell stress signals and migrate to the ischemic area, where they modify their morphology and function within minutes to hours following the onset of ischemia. During expansion of the ischemic lesion, microglia were activated in the surrounding intact area. Resting type ramified microglia were first detected 6 h following MCAO and lasted for 7 d in the ischemic hemisphere. Meanwhile, at 16 and 24 h after MCAO, activated microglia were observed in the infarcted tissue as well as in the area adjacent to infarction (Mabuchi et al., 2000). Simultaneously, peripheral monocytes/macrophages and granulocytes enter the CNS and macrophages cluster at the edge of the infarcted tissue, while granulocytes infiltrate in the center (Mabuchi et al., 2000; del Zoppo, 2009). Several studies have focused on the spatiotemporal features of microglial activation and not the dynamic changes in microglial phenotypes. Recent studies detected M2 microglia/macrophages, which are characterized by Ym1 and CD206, to be exclusively expressed in the ischemic core at 24 h and 7 d after permanent middle cerebral artery occlusion (pMCAO). Furthermore, M2 microglia were the dominant phenotype during the early phase of infarction in the ischemic core. The dominant phenotype gradually switches to the M1 phenotype characterized by CD68 expression within the following 2 weeks (Perego et al., 2011; Hu et al., 2012; Taylor and Sansing, 2013). Perego et al. (2011) provided a detailed description of the microglia phenotype within 2 weeks after stroke. The M1 phenotype surface marker CD68 was expressed 6 h after ischemia and the expression progressively increased during the following 2 weeks, with a dramatic increase on day 7. CD68 appeared to be strongly concentrated in the border zone, and rarely present in the ischemic core during the early phase. However, as the CD68 expression increased gradually, it appeared in the border as well as the core areas. The M2 phenotype markers Ym1 was detected starting from 12 h, reached maximum expression at 24 h post-infarction, and decreased subsequently. The expression of CD206, a M2 phenotype marker, was observed 6 h after pMCAO and reached its peak approximately 24 h following pMCAO. Furthermore, Ym1 and CD206 were expressed exclusively in the ischemic core (Perego et al., 2011). The increase in M1 microglia in the penumbra area may prevent the expansion of the ischemic lesion. During later stages, M1 microglia become dominant and release pro-inflammatory factors, thereby exacerbating neuronal death. M2 microglia that accumulate in the ischemic core may play a role in engulfing debris and promoting wound healing. Similar to the data on the surface markers CD68, Ym1, and CD206, other studies have shown that the expression of M2 phenotype related genes, including Arg1, CCL22, Ym1/2, IL-10, and TNF-α, increased progressively within 24 h after MCAO and reached a peak after 3–5 d (Hu et al., 2012) (In contradiction with the previously presented data, this study indicated that the M2 related-gene have a different expression pattern than previously described). The M2 microglia/macrophage gene expression decreased after 1 week followed by a return to the baseline expression at 14 d (Mabuchi et al., 2000; Perego et al., 2011; Hu et al., 2012; Ma et al., 2017). However, other studies have indicated that the expression of M2 phenotype related genes will revert to baseline expression 28 d after the ischemia. Parallel to the change in the expression of M2 phenotype related genes, inflammatory genes (such as CD11b, iNOS, and CD16) in M1 phenotype of microglia, which were stimulated by the enlarging infarcted tissue, were expressed on day 1 and gradually increased until 14 d after MCAO (Liu et al., 2018). Notably, the real-time PCR results for inflammatory or anti-inflammatory genes suggested that the changes in gene expression showed a similar trend regarding the surface markers of M1 or M2 microglia (see Figure 1 for detailed information).
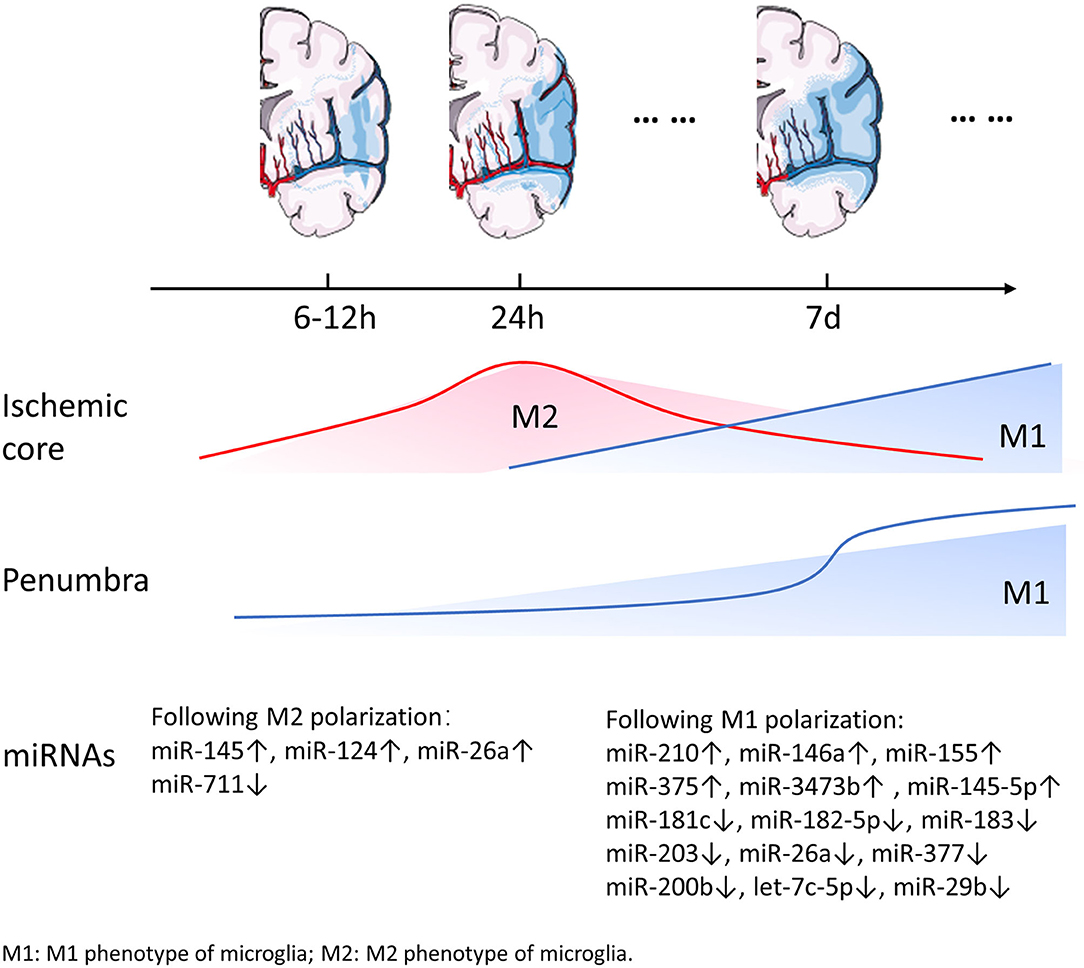
Figure 1. Dynamic changes of miRNAs and microglia following ischemic stroke. Along with the expansion of the ischemic lesions, reactive microglia change dynamically with time and in space. M1 microglia appear to be strongly concentrated in the penumbra and rarely occur in ischemic core within 24 h after stroke. At a later time point M1 microglia appear both in the penumbra and the ischemic core. It is worth noting that the number of M1 microglia increases dramatically 7 d after stroke. M2 microglia are exclusively expressed in the ischemic core in the acute phase of stroke and then gradually decrease. The expression of several miRNAs involved in microglial polarization changes over time.
Understandably, the data obtained from a permanent ischemia model and a transient ischemic stroke attack with reperfusion paradigm may not be the same. Kim and Cho (2016) showed that microglia are present in the injured core only in an event of an ischemic/reperfusion injury. In the case of no reperfusion (i.e., pMCAO), microglia appear in the penumbra and their levels decrease gradually (Kim and Cho, 2016). Thus, this is not in agreement with previous findings of Perego et al. (2011) indicating that reactive microglia appeared in the ischemic core as well as in penumbra regardless of their phenotype.
Additionally, reactive astrocytes began to appear around the infarcted area 24 h after MCAO, followed by a variety of glial cells, including microglia, oligodendrocytes, and astrocytes, which are recruited to the edge of the infarction area at the 7 d to promote the formation of glial scars (Zhou et al., 2018). Collectively, microglia dynamically respond to ischemic injury by first inducing the “helpful” M2 phenotype and then inducing the “harmful” M1 phenotype. The future studies should focus on adjusting the balance between M2 and M1 phenotypes to maintain the inflammatory defense and promote neuroprotection, rather than simply reducing the damage caused by M1-related chronic inflammation.
Microglia are the main mediators of neuroinflammation and are involved in the progressive brain damage as well as repair following ischemic stroke (Holtman et al., 2017; Wolf et al., 2017). During the complex pathological processes associated with ischemic stroke, a large number of miRNAs regulate the behavior of microglia (Li S. H. et al., 2015; Xiao et al., 2015; He et al., 2016). Animal as well as cellular models have been developed to simulate human cerebral ischemia reperfusion for investigating how these miRNAs affect the role of M1/M2 phenotypes in the damage and repair of brain cells.
LPS and IFN-γ stimulate microglia to exhibit the M1 phenotype, which is a result of activation of many inflammation-related transcription factors (Chhor et al., 2013; Freilich et al., 2013; Orihuela et al., 2016), such as nuclear factor (NF)-κB, activated protein 1 (AP-1), signal transducers and transcriptional activators (STAT1-4), and interferon regulatory factors (IRF 1, 3, 7, and 8). In addition to the pro-inflammatory transcription factors, overexpression of pro-inflammatory cytokines, including iNOS, IL-1β, IL-6, CXCL1, CCL5, and TNF-α, is the characteristic feature of the M1 phenotype (Chhor et al., 2013). Previous studies assessed the differential expression of miRNAs (such as miR-210, miR-155, and miR-375) in LPS-stimulated cells to get a better understanding on the role of miRNAs in regulating expression of inflammatory transcription factors and pro-inflammatory cytokines (Freilich et al., 2013; Cunha et al., 2016). Similarly, certain miRNAs, including miR-182-5p (Wang et al., 2018a), miR-183 (Xiang et al., 2019), and miR-146a (Chu et al., 2018) were shown to be differentially expressed in the plasma of ischemic stroke patients and in the plasma and brains of mice subjected to experimental stroke. Since miRNAs regulate the inflammatory cascades in microglia after classical activation by modulating inflammation-associated pathways (Chu et al., 2018; Li et al., 2020), miRNAs may be a potential therapeutic target for ischemic stroke (see Table 1 for detailed information).
NF-κB is one of the major regulators of the inflammatory response, and the NF-κB pathway is activated by multiple inflammatory signals, leading to the expression of a variety of inflammatory and immune-related genes (Bonizzi and Karin, 2004; Vallabhapurapu and Karin, 2009). Previous studies investigated the molecular mechanism underlying the miRNA-induced regulation of the NF-κB signaling in microglia. Li et al. (2020) determined that inhibition of miR-210 reduced expression of pro-inflammatory cytokines, thereby reducing brain damage and ameliorating behavioral deficits induced by MCAO. Furthermore, miR-210 was shown to play an important role in the regulation of the SIRT1/NF-κB pathway (Li et al., 2020). Other in vivo studies further supported the finding that the inhibition of miR-210 suppresses the expression of microglial pro-inflammatory factors (Huang et al., 2018). However, Lou et al. (2012), showed that the upregulation of miR-210 may promote angiogenesis following cerebral ischemia, which is beneficial for patient prognosis. Zhang et al. (2019b) intravenously administered exosomes loaded with miR-210 to the ischemic brain and showed that miR-210 loaded exosomes promote VEGF expression and angiogenesis. Moreover, a higher miR-210 level in patients' blood was associated with a good patient outcome following ischemic stroke (Zeng et al., 2011). However, the results related to the role of miR-210 remain inconsistent and require further validation.
Several miRNAs regulate the expression of M1-related inflammatory factors via the NF-κB pathway. For instance, miR-181c, miR-182-5p, and miR-183 regulate the expression of inflammatory factors by inhibiting the activation of the NF-κB signaling pathway. Previous studies have shown that miR-181c and miR-182-5p downregulate TLR4, thereby inhibiting the NF-κB pathway and reducing the production of pro-inflammatory cytokines (Zhang et al., 2015; Wang et al., 2018a). In addition, the levels of miR-181c, miR-182-5p, and miR-183 are downregulated in oxygen-glucose deprivation (OGD)-activated microglia and in the brain tissue of MCAO rat models, and the administration of these miRNAs exerted a protective effect against cerebral ischemia reperfusion injury (Zhang et al., 2015; Wang et al., 2018a; Xiang et al., 2019). Previous studies have shown that the expression of miR-146a is upregulated following LPS stimulation (Juknat et al., 2019). Furthermore, the inhibition of miR-146a triggers an inflammatory response by promoting the activation of the IRAK1/NF-κB signaling pathway, thereby further enhancing the overproduction of TNF-α, IL-1β, and IL-6. Thus, the upregulation of miR-146a exerts a protective effect (Chu et al., 2018). Furthermore, miR-203 regulates the NF-κB signaling via negative feedback by directly targeting the myeloid differentiation primary response gene 88, which leads to the activation of microglia, and promotes the release of inflammatory factors. Previous studies investigating the roles of miR-203 in rats showed its downregulation in the ischemia/reperfusion brain tissue, and that it can inhibit the inflammatory response as well as improve neurological symptoms and brain function following ischemia (Yang et al., 2015; Zhong et al., 2020).
In addition to the differential expression of the miRNAs mentioned earlier, upregulated expression of miR-155 (Freilich et al., 2013), miR-375 (Tang et al., 2016), miR-3473b (Wang X. et al., 2018) and the downregulated expression of miR-26a (Kumar et al., 2015), miR-377 (Fan et al., 2018) and miR-200b (Jadhav et al., 2014) were observed in LPS-stimulated primary microglia, BV2 microglia, and N9 microglia cellular models. In addition to the NF-κB pathway, several other pathways are regulated by miRNAs to regulate expression of inflammatory factors.
In microglia are exposed to LPS, miR-155 was shown to exert a pro-inflammatory effect. It also participated in the inflammatory response regulated by suppressor of cytokine signaling molecules-1 (SOCS-1). Cardoso et al. (2012) showed that miR-155 inhibition induces a neuroprotective effect. However, the regulatory role of miR-155 in microglia is complex. In a recent study, a miR-155 inhibitor was injected for three consecutive days starting 48 h after a distal middle cerebral artery occlusion (dMCAO) and the data was analyzed after 7 d post-dMCAO. The results showed that miR-155 inhibition may deactivate STAT-3 and inhibit the JAK/STAT signaling cascade via its direct targets SOCS-1 and src homology 2 domain-containing inositol 5-phosphatase 1(SHIP-1), thereby suppressing the early transient harmful effects of activated microglia/macrophages after 7 d. Subsequently, 14 d after the dMCAO onset, the inhibition of miR-155 most likely triggered the IL-10/STAT-3-mediated anti-inflammatory response, which may have facilitated the upregulation of CCAAT/enhancer-binding protein beta. Finally, miR-155 inhibition enhanced the protective and reparative role of microglia/macrophages 14 d after dMCAO (Pena-Philippides et al., 2016). Differential regulation of neuroinflammation by miR-155 inhibition indicates the complexity of miRNA-mediated regulation of microglial inflammation following ischemic stroke.
Similar to miR-155, miR-375 plays a complex role in regulating the role of microglia following ischemia. A previous study showed that LPS treatment increased N-myc downstream regulated gene 2 (NDRG2) expression in N9 microglia, which positively regulated miR-375. Notably, the IL-4 treatment also suppressed the NDRG2 expression, which suggests that NDRG2 controls miR-375 expression across different environments. However, in contrast to the upregulated expression of miR-375 in LPS-stimulated N9 cells, miR-375 expression decreased after cerebral ischemia reperfusion in rats (Tang et al., 2016). Nevertheless, further research is required to gain a better understanding of the influence of microenvironment on miRNA expression in vivo and in vitro.
Certain miRNAs, such as miR-26a (Kumar et al., 2015), miR-377 (Fan et al., 2018), miR-200b (Jadhav et al., 2014), miR-3473b (Wang et al., 2018b), miR-101a (Saika et al., 2017), let-7c-5p (Ni et al., 2015), miR-29b (Wang et al., 2019a), and miR-145-5p (Xie et al., 2017) were differentially expressed following ischemia and were shown to work through specific proteins and signaling pathways. Downregulation of miR-26a increased the expression of activating transcription factor 2 (ATF2), which led to increased production of IL-6. However, the increased production of TNF-α was not associated with the expression of ATF2. miR-26a increased the expression of TNF-α through a mechanism that was independent of ATF2 (Kumar et al., 2015). Microglia under OGD or LPS treatment showed reduced expression of miR-377 and increased expression of early growth response 2 gene (EGR2). The changes in miR-377 and EGR2 were also present in rats following MCAO. Additionally, Fan et al. (2018) showed that miR-377 can directly regulate EGR2 expression by suppressing the secretion of inflammatory cytokines following OGD. On the other hand, miR-200b expression decreased in activated BV2 microglia, which negatively regulated the cJun/MAPK pathway; thus suppressing the inflammatory response (Jadhav et al., 2014). miR-3473b was shown to participate in post-stroke neuroinflammation by targeting SOCS3 (Wang et al., 2018b). Similar to the previously mentioned targets of miR-155, both SOCS1 and SOCS3 play a role in inhibiting the JAK/STAT1/3 signaling pathway, while SOCS3 mainly inhibits the IL-6 family of cytokines (Baker et al., 2009). Alternatively, miR-101a can increase the ability of microglia to produce IL-6 and TNF-α by inhibiting mitogen-activated protein kinase phosphatase 1 (Saika et al., 2017). Furthermore, the let-7c-5p expression was shown to be reduced in activated microglia, while let-7c-5p overexpression was shown to attenuate LPS-induced iNOS, COX-2, TNF-α, and IL-6 expression. Additionally, the overexpression of let-7c-5p inhibited caspase 3 expression in microglia. A previous study suggested that let-7c-5p levels were decreased in patients with ischemic stroke and in mouse models of MCAO. Its overexpression inhibited post-ischemic neuroinflammation (Ni et al., 2015). In reactive microglia stimulated by OGD, miR-29b is downregulated. Previous studies have shown that the upregulation of miR-29b in microglia inhibits the JAK-2/STAT3 signaling pathway by promoting the expression of SOCS-1; thus suppressing the production of IL-1β. Furthermore, miR-29b inhibition can regulate microglial-induced apoptosis of hippocampal neurons via the JAK-2/STAT3 pathway (Wang et al., 2019a). In an oxygen-glucose deprivation/reperfusion injury model or MCAO model, miR-145-5p was significantly upregulated in microglia. miR-145-5p decreased nuclear receptor related-1 protein (Nurr1) while increasing the TNF-α expression and promoting TNF-α-induced neuronal injury. Thus, blocking the abnormal activation of miR-145-5p/Nurr1/TNF-α axis signaling might improve the neuroinflammatory outcomes and represent an effective therapeutic strategy (Xie et al., 2017).
The complex interactions of miRNAs throughout the neuroinflammation regulatory network are complemented by their complicated regulatory relationships with other regulatory molecules such as long non-coding RNAs (lncRNAs) (Paraskevopoulou and Hatzigeorgiou, 2016). Interestingly, lncRNA can act as a sponge for miRNAs, which reduces the regulatory effect of miRNAs on mRNA (Paraskevopoulou and Hatzigeorgiou, 2016). Additionally, suppression of miR-145-5p has been proven to reduce the pro-inflammatory response, which indicates that lncRNAs could represent another method that can be used regulate miR-145-5p for therapeutic purposes (i.e., SNHG14). Qi et al. (2017) showed that negative regulation of the SNHG14/miR-145-5p/PLA2G4A axis reduces the production of TNF-α.
IL-4 and IL-13 can induce the M2 phenotype in microglia (Chhor et al., 2013; Hamzei Taj et al., 2018). In previous studies, the anti-inflammatory cytokine IL-4 was the only stimulating factor shown to increase the expression of the M2a repair/regeneration phenotypic marker gene. Following IL-4 stimulation, the microglial phenotype transitioned from M0 to M2, leading to a significant upregulation of miR-145 and miR-124 as well as a significant downregulation of miR-711 (Freilich et al., 2013).
Previous studies have shown activated microglia trigger an inflammatory response that may lead to the production of exosomes containing cytokines or pro-inflammatory miRNAs. LPS-stimulated M1 microglia were shown to release miR-155-containing exosomes; thus, promoting neuroinflammation through exosomal transport (Cunha et al., 2016; Li et al., 2019). On the other hand M2 microglia-derived exosomes containing miR-124 were shown to be absorbed by neurons and further boost neuronal survival by targeting the ubiquitin-specific protease 14 (Song et al., 2019). In addition, IL-4-stimulated microglia was suggested to promote angiogenesis by secreting exosomes containing miR-26a, thereby reducing the damage caused by ischemic stroke (Tian et al., 2019). These studies indicate that M2 microglia play a protective role against ischemic stroke through miRNA-mediated regulation.
Microglia exhibit microenvironment-dependent phenotypes which play either an anti-inflammatory or pro-inflammatory role following cerebral ischemia/reperfusion. The M2 phenotype has neuroprotective effects and works by promoting brain tissue regeneration and repair after ischemia. On the other hand, the M1 phenotype is associated with the release of inflammatory cytokines that aggravate the detrimental effects of the stroke on the brain tissue. miRNAs play essential roles in microenvironmental regulation; therefore, it is necessary to understand the mechanisms through which miRNAs influence the M1/M2 phenotypic transformation. A better understanding of the role of miRNAs in the M1/M2 transformation might provide a new paradigm for stroke treatment.
Resting microglia are activated by LPS or IL-4 and a pro- or anti-inflammatory phenotype is induced. Several miRNAs have a significant role in regulating microglial activation. In this section, we highlight some interesting miRNAs that are known to participate in microglial activation.
The TLR/NF-κB signaling pathway is known to be associated with inflammatory responses (Chen et al., 2018; Zhao et al., 2018). By stimulating microglia with LPS, it has been shown that TLR4 translocated to the microglial membrane and TLR2 expression was increased. Considering that the translocation of NF-κB into the nucleus and the activation of the inflammatory cascade are associated with M1 polarization, the M1 microglia might be mediated via the TLR2/TLR4/NF-kB signaling cascade (Cunha et al., 2016). Thus, miRNAs which regulate inflammatory cytokine production through the TLR/NF-κB signaling pathway, such as miR-210, might regulate microglial polarization. For instance, Li et al. (2020) showed that miR-210 overexpression in neonatal rat primary microglia let to an increase in M1 marker expression (including CD11b, CD45, CD80, and CD86).
miR-9 has also been shown to play a role in microglial transformation. Primary mouse microglia were transfected with lentiviruses expressing the miR-9 precursor to identify the role of miR-9 in mediating the transition of microglia from resting state to the M1 state. Furthermore, miR-9 was shown to be involved in microglial activation via the MCPIP1/NF-κB signaling pathway (Yao et al., 2014; Liu et al., 2016).
Recent studies have suggested that miR-124 plays an important role in regulating the M2 phenotype. Hamzei Taj et al. (2016b) showed that miR-124 increased the expression of arginase-1 (Arg-1), a surface marker of the M2 microglia/macrophage phenotype, and this change was associated with neuroprotection and functional improvement. Furthermore, administration of miR-124 following stroke lead to a significant increase in the M2 and decrease in the M1 microglia/macrophage phenotype. Previous studies indicated that most of the newly recruited microglia and macrophages in the acute phase of neurological diseases are M2; however, after 1 week the M1 phenotype starts to become dominant (Wang et al., 2013; Kumar et al., 2016). miR-124 extended the M2 phase and shortened the M1 phase in following stroke. Hamzei Taj et al. (2016a) reported that treatment with miR-124 intracranial injections both early and delayed contributed to the M1/M2 balance. However, miR-124 administration achieved a maximum impact before the anti-inflammatory phase of the M2 phenotype became dominant (Hamzei Taj et al., 2016a). While the M1 phenotype was not dominant, the effect of the early miR-124 intervention was shown to play a partial role in changing the patterns of M0 to M1 and M0 to M2. Hence, early miRNA administration following stroke leads to maximal increase in protective M2 microglia and a decrease in the pro-inflammatory M1 microglia.
Ingenuity canonical pathway analysis (IPA) and enrichment analysis suggested that upregulation of miR-155 might contribute to the establishment of the M1 state, while upregulation of miR-145 might contribute to the establishment of the M2a state (Freilich et al., 2013). Furthermore, p53-family proteins are notable molecules that play a crucial role in regulating inflammation (Aloi et al., 2015; Agupitan et al., 2020). Studies have shown that the M1 activation in p53−/− microglia was inhibited, while the expression of genes associated with M2 activation was increased. p53 negatively regulated Twist2 through miR-145 and miR-34a, which modulated c-Maf expression in microglia; thus, inhibiting the anti-inflammatory and tissue-repairing effects of c-Maf. (Jayadev et al., 2011; Su et al., 2014; Aloi et al., 2015).
Another possible approach can be based on the phenotypic switching between the M1 and M2 when the pro-inflammatory M1 phenotype has already become dominant after the stroke. If such a phenotype conversion can be achieved, it will result in a significant improvement in stroke treatment. To investigate this approach, previous studies used sequential cytokine-stimulated microglia to assess microglia repolarization and cell functions. The microglial stimulation with LPS or IFN-γ combined with TNF-α (L + T or I + T), followed by IL-4 or IL-10 showed that secondary IL-4 or IL-10 treatment generally suppressed the L + T or I + T-induced M1 response and further skewed microglia toward an anti-inflammatory M2a state (Siddiqui et al., 2016; Lively and Schlichter, 2018). The repolarization between M1 and M2a states supports the possibility to reprogram the inflammatory response in vivo.
Numerous studies have shown that exosomal release from mesenchymal stem cells represents a new type of cell-to-cell communication pathway, which plays a critical role in signal regulation. These membrane-like structures transmit exogenous functional mRNA and miRNAs to target cells and contribute to the development and treatment of disease (Jaimes et al., 2017; Qing et al., 2018). In this review, we mentioned that microglia play a role in signal transduction by releasing miRNA-containing exosomes to mediate the inflammatory response or additional secondary effects of cells. Similarly, the phenotypic transition between M1 and M2 involves signal transduction as well. Zheng et al. (2019) polarized BV2 cells from M0 to M1 phenotype by incubating them with LPS and treated M1 microglia with exosomes secreted from LPS-stimulated macrophages. By quantifying the expression of iNOS (an M1 marker) and Arg1 (an M2 marker), it has been shown that LPS-stimulated macrophages might skew microglial functional polarity from M1 to M2 phenotype (Zheng et al., 2019). This finding showed that exosomes play a role in microglial phenotype conversion. Furthermore, the relationship between the miR-30d-5p-overexpressing exosomes and microglial phenotype was investigated in another study (Jiang et al., 2018). Exosomes derived from miR-30d-5p-overexpressing cells can more effectively inhibit the expression of inflammatory factors and switch microglial phenotype from OGD-stimulated M1 phenotype into the M2 phenotype. Additionally, miR-30d-5p targeted both Beclin-1 and autophagy-related gene 5 (Atg5), thereby inhibiting autophagy-mediated microglial polarization to M1, and promoting the conversion of M1 to M2 (Jiang et al., 2018).
As one of the mechanisms by which miRNAs modulate M1/M2 phenotypes and the lncRNA pattern was also investigated. Studies have shown that taurine upregulated gene 1 (TUG1) gene knockout can promote microglial M1 to M2 phenotypic transformation, and further studies have shown that this transformation will be reversed when miR-145a-5p was inhibited. Thus, these results suggest that TUG1/miR-145a-5p plays a role in microglial polarization (Wang et al., 2019b). A previous study reported the use of the sponge couple GAS5/miR-146a-5p and used isosteviol sodium to break the sponge pair, thus breaking the negative regulation among GAS5/miR-146a-5p/Notch1, and finally inhibiting the expression of pro-inflammatory cytokines as well as promoting the polarization of M1 to M2 phenotype (Zhang et al., 2019a). This regulatory network is regulated by lncRNAs and provides a sophisticated framework for further research and identify the genes involved in the inflammatory response of microglia and their corresponding regulatory miRNAs.
Certain miRNAs have been shown to regulate the transition from M1 to M2 phenotypes in other neurological diseases (i.e., amyotrophic lateral sclerosis, Japanese encephalitis virus, spinal cord injury, and neuropathic pain), thus providing the possibility of studying miRNAs in stroke-related neuroinflammation (Parisi et al., 2016; Yang et al., 2017; Hazra et al., 2019; Liu W. et al., 2020). For instance, miR-128 overexpression significantly decreased the expression of M1 phenotypic marker CD86 as well as CD32 and increased the expression of the M2 phenotypic markers Arg1 and CD206 after compressive spinal cord injury. Furthermore, miR-128 overexpression led to a decrease in the expression of TNF-α, IL-1β, and IL-6 (Yang et al., 2017).
miRNA-based therapeutics can be divided into two categories: miRNA mimics and miRNA inhibitors. Previous studies have investigated the therapeutic values of certain miRNA mimics and inhibitors following stroke. For example, the inhibition of miR-9 activity via microinjection of lenti-anti-miR-9 into the hippocampus/subtantia nigra pars reticulata of mice followed by LPS injection resulted in a dampened inflammatory response (Åkerblom et al., 2013; Yao et al., 2014). The intracerebral injection of miR-124 in a stroke mouse model modulated the microglia/macrophage activation of the M2 phenotype, thus contributing to the restoration of brain cell function (Hamzei Taj et al., 2016a).
Pharmaceutical therapies with immunomodulatory properties could be beneficial in treating ischemic stroke (Tili and Michaille, 2016; Martinez and Peplow, 2017). Pharmaceutical studies have identified that several immunomodulators, including sulforaphane (Eren et al., 2018), triptolide (Feng et al., 2019), and sulfasalazine (Cetin et al., 2017), differentially regulated microglia-associated miRNAs, thereby regulating the expression of inflammatory factors and the shift in microglial polarization.
Sulforaphane is a molecule that exerts antioxidant, anti-inflammatory, and cytoprotective effects and can cross the blood-brain barrier (Tarozzi et al., 2013). Sulforaphane treatment inhibited the expression of M1-related genes after LPS stimulation and reversed the LPS-induced increase in miR-155 expression and decrease in miR-223 expression. Furthermore, the effect of sulforaphane on miR-223 expression was shown to be nuclear factor erythroid 2-related factor 2-dependent (Brandenburg et al., 2010; Eren et al., 2018). Triptolide, a monomeric component isolated from the Chinese herb Tripterygium Wilfordii Hook F, suppressed M1 microglial activation by targeting the miR-155-5p/SHIP1 pathway (Feng et al., 2019). Resveratrol, another representative immunomodulator with an anti-inflammatory role, is capable of regulating the expression of miRNAs underlying inflammation (Tili and Michaille, 2016). For instance, resveratrol can reduce the expression of miR-204 and increase the expression of miR-146a-5p, thereby inhibiting LPS-induced inflammation (Li L. et al., 2015; Ge et al., 2019).
Multiple studies have shown that isosteviol sodium has neuroprotective effects in experimental models (Zhang et al., 2017, 2018). Zhang et al. (2019a) showed that isosteviol sodium disrupted the GAS5/miR-146a-5p sponge and suppressed the expression of Notch1 underlying stroke both in vivo and in vitro, thus shifting the M1 phenotype to the M2 phenotype and inhibiting the expression of pro-inflammatory cytokines. Sulfasalazine, an immune-modulating drug, was shown to exhibit a protective role following cerebral ischemia/reperfusion injury in rats (Cetin et al., 2017) and block microglia from switching to the M1 phenotype both in vivo and in vitro by upregulating the expression miR-136-5p (Duan et al., 2018).
Most studies on microglial phenotypes have focused on the process through which miRNAs reduce pro-inflammatory responses, while less attention has been paid to the anti-inflammatory effects shown by the M2 phenotype. This may be due to the M2 phenotype which is expressed transiently during stroke progression, and thus, does not completely alleviate the consequences of the overall inflammation cascade. On the other hand, although more attention has been directed to the phenotypic transformation, there are fewer studies on the role of miRNAs in this field. Thus, the regulatory function of miRNAs in the inflammatory network requires further research. It can be hypothesized that shifting our attention to miRNAs involved in the neuroinflammatory network will provide a new perspective on neuroinflammation regulation. Furthermore, numerous stroke studies do not provide a clear distinction between microglia and macrophages and the difference in the microglial behaviors in vivo and in vitro. Distinguishing these two cell types and clarifying the effect of the microenvironment on microglial phenotypes might bring new breakthroughs in neuroinflammation regulation.
Microglia play a key role following stroke, and the M1 phenotype typically releases pro-inflammatory mediators, while the M2 phenotype suppresses the harmful processes associated with ischemia. Numerous studies have shown that the interaction of various cells (i.e., astrocytes, oligodendrocytes, neurons), specific cytokines, drugs, and other extracellular components can promote microglia phenotype conversion (Kim and Cho, 2016; Pepe et al., 2017; Zhang et al., 2019a; Liu L. R. et al., 2020). Furthermore, a large number of studies have focused on the combined intervention of miRNAs and microglia; however, the role of miRNAs in the phenotypic transformation of microglia might not be limited to reducing the release of pro-inflammatory factors (Kim and Cho, 2016). The expected treatment should be able to balance the M1 and M2 phenotypes as well as inhibit the inflammation (Liu et al., 2018). Therefore, in many studies, the ratio of M1/M2 is used as an indicator of the treatment effect and success.
A better understanding of the effects of miRNAs on microglia-related neuroinflammation might uncover novel therapeutic targets. For instance, modulating the protein expression of key factors associated with specific diseases through miRNA delivery systems might represent a new approach (Zhang et al., 2015). Currently, miRNA-based therapies involve the use of miRNA mimics and miRNA inhibitors (Wahid et al., 2014; Sun et al., 2018). Certain immunomodulators have been shown to regulate microglia neuroinflammation by interfering with miRNAs (Martinez and Peplow, 2017).
Conceptualization: LLian, YZ, and SX. Review of literature: LLian, LLiu, LY, and YC. Writing—original draft preparation: LLian. Writing—review and editing: YZ and SX. Supervision: JZ and SX. All authors have read and agreed to the published version of the manuscript.
This research was funded by the National Natural Science Foundation of China (Grant Nos. 81774059 and 81503505) and the Tianjin Natural Science Foundation (Grant No. 19JCZDJC37100).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Åkerblom, M., Sachdeva, R., Quintino, L., Wettergren, E. E., Chapman, K. Z., Manfre, G., et al. (2013). Visualization and genetic modification of resident brain microglia using lentiviral vectors regulated by microRNA-9. Nat. Commun. 4:1770. doi: 10.1038/ncomms2801
Agupitan, A. D., Neeson, P., Williams, S., Howitt, J., Haupt, S., and Haupt, Y. (2020). P53: a guardian of immunity becomes its saboteur through mutation. Int. J. Mol. Sci. 21:3452. doi: 10.3390/ijms21103452
Aloi, M. S., Su, W., and Garden, G. A. (2015). The p53 transcriptional network influences microglia behavior and neuroinflammation. Crit. Rev. Immunol. 35, 401–415. doi: 10.1615/critrevimmunol.v35.i5.40
Amici, S. A., Dong, J., and Guerau-de-Arellano, M. (2017). Molecular mechanisms modulating the phenotype of macrophages and microglia. Front. Immunol. 8:1520. doi: 10.3389/fimmu.2017.01520
Baker, B. J., Akhtar, L. N., and Benveniste, E. N. (2009). SOCS1 and SOCS3 in the control of CNS immunity. Trends Immunol. 30, 392–400. doi: 10.1016/j.it.2009.07.001
Barakat, R., and Redzic, Z. (2016). The role of activated microglia and resident macrophages in the neurovascular unit during cerebral ischemia: is the jury still out?. Med. Princ. Pract. 25(Suppl 1or Suppl 1), 3–14. doi: 10.1159/000435858
Bonizzi, G., and Karin, M. (2004). The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 25, 280–288. doi: 10.1016/j.it.2004.03.008
Brandenburg, L. O., Kipp, M., Lucius, R., Pufe, T., and Wruck, C. J. (2010). Sulforaphane suppresses LPS-induced inflammation in primary rat microglia. Inflamm. Res. 59, 443–450. doi: 10.1007/s00011-009-0116-5
Cardoso, A. L., Guedes, J. R., Pereira de Almeida, L., and Pedroso de Lima, M. C. (2012). miR-155 modulates microglia-mediated immune response by down-regulating SOCS-1 and promoting cytokine and nitric oxide production. Immunology 135, 73–88. doi: 10.1111/j.1365-2567.2011.03514.x
Cetin, C., Erdogan, A. M., Dincel, G. C., Bakar, B., and Kisa, U. (2017). Effects of sulphasalazine in cerebral ischemia reperfusion injury in rat. Arch. Med. Res. 48, 247–256. doi: 10.1016/j.arcmed.2017.06.004
Chen, J., Yang, C., Xu, X., Yang, Y., and Xu, B. (2018). The effect of focal cerebral ischemia-reperfusion injury on TLR4 and NF-kappaB signaling pathway. Exp. Ther. Med. 15, 897–903. doi: 10.3892/etm.2017.5463
Chen, R., Xu, X., Huang, L., Zhong, W., and Cui, L. (2019). The regulatory role of long non-coding RNAs in different brain cell types involved in ischemic stroke. Front. Mol. Neurosci. 12:61. doi: 10.3389/fnmol.2019.00061
Chhor, V., Le Charpentier, T., Lebon, S., Oré, M. V., Celador, I. L., and Josserand, J.. (2013). Characterization of phenotype markers and neuronotoxic potential of polarised primary microglia in vitro. Brain Behav. Immun. 32, 70–85. doi: 10.1016/j.bbi.2013.02.005
Chu, B., Zhou, Y., Zhai, H., Li, L., Sun, L., and Li, Y. (2018). The role of microRNA-146a in regulating the expression of IRAK1 in cerebral ischemia-reperfusion injury. Can. J. Physiol. Pharmacol. 96, 611–617. doi: 10.1139/cjpp-2017-0586
Cunha, C., Gomes, C., Vaz, A. R., and Brites, D. (2016). Exploring new inflammatory biomarkers and pathways during LPS-induced M1 polarization. Mediators Inflamm. 2016:6986175. doi: 10.1155/2016/6986175
del Zoppo, G. J. (2009). Inflammation and the neurovascular unit in the setting of focal cerebral ischemia. Neuroscience 158, 972–982. doi: 10.1016/j.neuroscience.2008.08.028
Dela Pena, I. C., Yang, S., Shen, G., Fang Liang, H., Solak, S., and Borlongan, C. V. (2018). Extension of tissue plasminogen activator treatment window by granulocyte-colony stimulating factor in a thromboembolic rat model of stroke. Int. J. Mol. Sci. 19:1635. doi: 10.3390/ijms19061635
DePaula-Silva, A. B., Gorbea, C., Doty, D. J., Libbey, J. E., Sanchez, J. M. S., Hanak, T. J., et al. (2019). Differential transcriptional profiles identify microglial- and macrophage-specific gene markers expressed during virus-induced neuroinflammation. J. Neuroinflammation 16:152. doi: 10.1186/s12974-019-1545-x
Dewdney, B., Trollope, A., Moxon, J., Thomas Manapurathe, D., Biros, E., and Golledge, J. (2018). Circulating microRNAs as biomarkers for acute ischemic stroke: a systematic review. J. Stroke Cerebrovasc. Dis. 27, 522–530. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.058
Duan, C., Liu, Y., Li, Y., Chen, H., Liu, X., Chen, X., et al. (2018). Sulfasalazine alters microglia phenotype by competing endogenous RNA effect of miR-136-5p and long non-coding RNA HOTAIR in cuprizone-induced demyelination. Biochem. Pharmacol. 155, 110–123. doi: 10.1016/j.bcp.2018.06.028
Eren, E., Tufekci, K. U., Isci, K. B., Tastan, B., Genc, K., and Genc, S. (2018). Sulforaphane inhibits lipopolysaccharide-induced inflammation, cytotoxicity, oxidative stress, and miR-155 expression and switches to mox phenotype through activating extracellular signal-regulated kinase 1/2-nuclear factor erythroid 2-related factor 2/antioxidant response element pathway in murine microglial cells. Front. Immunol. 9:36. doi: 10.3389/fimmu.2018.00036
Fan, Y., Ding, S., Sun, Y., Zhao, B., Pan, Y., and Wan, J. (2018). MiR-377 regulates inflammation and angiogenesis in rats after cerebral ischemic injury. J. Cell Biochem. 119, 327–337. doi: 10.1002/jcb.26181
Feng, Y., Zheng, C., Zhang, Y., Xing, C., Cai, W., Li, R., et al. (2019). Triptolide inhibits preformed fibril-induced microglial activation by targeting the MicroRNA155-5p/SHIP1 pathway. Oxid. Med. Cell Longev. 2019:6527638. doi: 10.1155/2019/6527638
Freilich, R. W., Woodbury, M. E., and Ikezu, T. (2013). Integrated expression profiles of mRNA and miRNA in polarized primary murine microglia. PLoS ONE 8:e79416. doi: 10.1371/journal.pone.0079416
Gaire, B. P., Bae, Y. J., and Choi, J. W. (2019). S1P(1) Regulates M1/M2 Polarization toward brain injury after transient focal cerebral ischemia. Biomol. Ther. 27, 522–529. doi: 10.4062/biomolther.2019.005
Gaudet, A. D., Fonken, L. K., Watkins, L. R., Nelson, R. J., and Popovich, P. G. (2018). MicroRNAs: roles in regulating neuroinflammation. Neuroscientist 24, 221–245. doi: 10.1177/1073858417721150
Ge, Y. T., Zhong, A. Q., Xu, G. F., and Lu, Y. (2019). Resveratrol protects BV2 mouse microglial cells against LPS-induced inflammatory injury by altering the miR-146a-5p/TRAF6/NF-κB axis. Immunopharmacol. Immunotoxicol. 41, 549–557. doi: 10.1080/08923973.2019.1666406
Gosselin, D., Skola, D., Coufal, N. G., Holtman, I. R., Schlachetzki, J. C. M., Sajti, E., et al. (2017). An environment-dependent transcriptional network specifies human microglia identity. Science 356:6344. doi: 10.1126/science.aal3222
Guo, Y., Hong, W., Wang, X., Zhang, P., Körner, H., Tu, J., et al. (2019). MicroRNAs in microglia: how do microRNAs affect activation, inflammation, polarization of microglia and mediate the interaction between microglia and glioma?. Front. Mol. Neurosci. 12:125. doi: 10.3389/fnmol.2019.00125
Hamzei Taj, S., Kho, W., Aswendt, M., Collmann, F. M., Green, C., Adamczak, J., et al. (2016a). Dynamic modulation of microglia/macrophage polarization by miR-124 after focal cerebral ischemia. J. Neuroimmune Pharmacol. 11, 733–748. doi: 10.1007/s11481-016-9700-y
Hamzei Taj, S., Kho, W., Riou, A., Wiedermann, D., and Hoehn, M. (2016b). MiRNA-124 induces neuroprotection and functional improvement after focal cerebral ischemia. Biomaterials 91, 151–165. doi: 10.1016/j.biomaterials.2016.03.025
Hamzei Taj, S., Le Blon, D., Hoornaert, C., Daans, J., Quarta, A., Praet, J., et al. (2018). Targeted intracerebral delivery of the anti-inflammatory cytokine IL13 promotes alternative activation of both microglia and macrophages after stroke. J. Neuroinflammation 15:174. doi: 10.1186/s12974-018-1212-7
Hanisch, U. K., and Kettenmann, H. (2007). Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 10, 1387–1394. doi: 10.1038/nn1997
Hazra, B., Chakraborty, S., Bhaskar, M., Mukherjee, S., Mahadevan, A., and Basu, A. (2019). miR-301a regulates inflammatory response to Japanese encephalitis virus infection via suppression of NKRF activity. J. Immunol. 203, 2222–2238. doi: 10.4049/jimmunol.1900003
He, W., Chen, S., Chen, X., Li, S., and Chen, W. (2016). Bioinformatic analysis of potential microRNAs in ischemic stroke. J. Stroke Cerebrovasc. Dis. 25, 1753–1759. doi: 10.1016/j.jstrokecerebrovasdis.2016.03.023
Holtman, I. R., Skola, D., and Glass, C. K. (2017). Transcriptional control of microglia phenotypes in health and disease. J. Clin. Invest. 127, 3220–3229. doi: 10.1172/jci90604
Hu, X., Li, P., Guo, Y., Wang, H., Leak, R. K., Chen, S., et al. (2012). Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 43, 3063–3070. doi: 10.1161/STROKEAHA.112.659656
Huang, L., Ma, Q., Li, Y., Li, B., and Zhang, L. (2018). Inhibition of microRNA-210 suppresses pro-inflammatory response and reduces acute brain injury of ischemic stroke in mice. Exp. Neurol. 300, 41–50. doi: 10.1016/j.expneurol.2017.10.024
Iadecola, C., and Anrather, J. (2011). The immunology of stroke: from mechanisms to translation. Nat. Med. 17, 796–808. doi: 10.1038/nm.2399
Jadhav, S. P., Kamath, S. P., Choolani, M., Lu, J., and Dheen, S. T. (2014). microRNA-200b modulates microglia-mediated neuroinflammation via the cJun/MAPK pathway. J. Neurochem. 130, 388–401. doi: 10.1111/jnc.12731
Jaimes, Y., Naaldijk, Y., Wenk, K., Leovsky, C., and Emmrich, F. (2017). Mesenchymal stem cell-derived microvesicles modulate lipopolysaccharides-induced inflammatory responses to microglia cells. Stem Cells 35, 812–823. doi: 10.1002/stem.2541
Jayadev, S., Nesser, N. K., Hopkins, S., Myers, S. J., Case, A., Lee, R. J., et al. (2011). Transcription factor p53 influences microglial activation phenotype. Glia 59, 1402–1413. doi: 10.1002/glia.21178
Jiang, M., Wang, H., Jin, M., Yang, X., Ji, H., Jiang, Y., et al. (2018). Exosomes from MiR-30d-5p-ADSCs reverse acute ischemic stroke-induced, autophagy-mediated brain injury by promoting M2 microglial/macrophage polarization. Cell Physiol. Biochem. 47, 864–878. doi: 10.1159/000490078
Juknat, A., Gao, F., Coppola, G., Vogel, Z., and Kozela, E. (2019). miRNA expression profiles and molecular networks in resting and LPS-activated BV-2 microglia-Effect of cannabinoids. PLoS ONE 14:e0212039. doi: 10.1371/journal.pone.0212039
Kabekkodu, S. P., Shukla, V., Varghese, V. K., Souza, J. D., Chakrabarty, S., and Satyamoorthy, K. (2018). Clustered miRNAs and their role in biological functions and diseases. Biol. Rev. Camb. Philos. Soc. 93, 1955–1986. doi: 10.1111/brv.12428
Karthikeyan, A., Patnala, R., Jadhav, S. P., Eng-Ang, L., and Dheen, S. T. (2016). MicroRNAs: key players in microglia and astrocyte mediated inflammation in CNS pathologies. Curr. Med. Chem. 23, 3528–3546. doi: 10.2174/0929867323666160814001040
Khoshnam, S. E., Winlow, W., and Farzaneh, M. (2017). The interplay of MicroRNAs in the inflammatory mechanisms following ischemic stroke. J. Neuropathol. Exp. Neurol. 76, 548–561. doi: 10.1093/jnen/nlx036
Kim, E., and Cho, S. (2016). Microglia and monocyte-derived macrophages in stroke. Neurotherapeutics 13, 702–718. doi: 10.1007/s13311-016-0463-1
Knecht, T., Story, J., Liu, J., Davis, W., Borlongan, C. V., and Dela Pena, I. C. (2017). Adjunctive therapy approaches for ischemic stroke: innovations to expand time window of treatment. Int. J. Mol. Sci. 18:2756. doi: 10.3390/ijms18122756
Kosik, K. S. (2006). The neuronal microRNA system. Nat. Rev. Neurosci. 7, 911–920. doi: 10.1038/nrn2037
Kriz, J., and Lalancette-Hébert, M. (2009). Inflammation, plasticity and real-time imaging after cerebral ischemia. Acta Neuropathol. 117, 497–509. doi: 10.1007/s00401-009-0496-1
Kumar, A., Alvarez-Croda, D. M., Stoica, B. A., Faden, A. I., and Loane, D. J. (2016). Microglial/macrophage polarization dynamics following traumatic brain injury. J. Neurotrauma 33, 1732–1750. doi: 10.1089/neu.2015.4268
Kumar, A., Bhatia, H. S., de Oliveira, A. C., and Fiebich, B. L. (2015). microRNA-26a modulates inflammatory response induced by toll-like receptor 4 stimulation in microglia. J. Neurochem. 135, 1189–1202. doi: 10.1111/jnc.13364
Lai, T. W., Zhang, S., and Wang, Y. T. (2014). Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog. Neurobiol. 115, 157–188. doi: 10.1016/j.pneurobio.2013.11.006
Lam, D., Lively, S., and Schlichter, L. C. (2017). Responses of rat and mouse primary microglia to pro- and anti-inflammatory stimuli: molecular profiles, K(+) channels and migration. J. Neuroinflammation. 14:166. doi: 10.1186/s12974-017-0941-3
Li, B., Dasgupta, C., Huang, L., Meng, X., and Zhang, L. (2020). MiRNA-210 induces microglial activation and regulates microglia-mediated neuroinflammation in neonatal hypoxic-ischemic encephalopathy. Cell Mol. Immunol. 17, 976–991. doi: 10.1038/s41423-019-0257-6
Li, G., Morris-Blanco, K. C., Lopez, M. S., Yang, T., Zhao, H., Vemuganti, R., et al. (2018). Impact of microRNAs on ischemic stroke: from pre- to post-disease. Prog. Neurobiol. 163–164, 59–78. doi: 10.1016/j.pneurobio.2017.08.002
Li, H., Luo, Y., Zhu, L., Hua, W., Zhang, Y., Zhang, H., et al. (2019). Glia-derived exosomes: promising therapeutic targets. Life Sci. 239:116951. doi: 10.1016/j.lfs.2019.116951
Li, L., Sun, Q., Li, Y., Yang, Y., Yang, Y., Chang, T., et al. (2015). Overexpression of SIRT1 induced by resveratrol and inhibitor of miR-204 suppresses activation and proliferation of microglia. J. Mol. Neurosci. 56, 858–867. doi: 10.1007/s12031-015-0526-5
Li, P., Stetler, R. A., Leak, R. K., Shi, Y., Li, Y., Yu, W., et al. (2018). Oxidative stress and DNA damage after cerebral ischemia: potential therapeutic targets to repair the genome and improve stroke recovery. Neuropharmacology 134, 208–217. doi: 10.1016/j.neuropharm.2017.11.011
Li, S. H., Su, S. Y., and Liu, J. L. (2015). Differential regulation of microRNAs in patients with ischemic stroke. Curr. Neurovasc. Res. 12, 214–221. doi: 10.2174/1567202612666150605121709
Liu, L., Liu, X., Wang, R., Yan, F., Luo, Y., Chandra, A., et al. (2018). Mild focal hypothermia regulates the dynamic polarization of microglia after ischemic stroke in mice. Neurol. Res. 40, 508–515. doi: 10.1080/01616412.2018.1454090
Liu, L. R., Liu, J. C., Bao, J. S., Bai, Q. Q., and Wang, G. Q. (2020). Interaction of microglia and astrocytes in the neurovascular Unit. Front. Immunol. 11:1024. doi: 10.3389/fimmu.2020.01024
Liu, W., Rong, Y., Wang, J., Zhou, Z., Ge, X., Ji, C., et al. (2020). Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. J. Neuroinflammation 17:47. doi: 10.1186/s12974-020-1726-7
Liu, W., Wang, X., Zheng, Y., Shang, G., Huang, J., Tao, J., et al. (2016). Electroacupuncture inhibits inflammatory injury by targeting the miR-9-mediated NF-kappaB signaling pathway following ischemic stroke. Mol. Med. Rep. 13, 1618–1626. doi: 10.3892/mmr.2015.4745
Lively, S., and Schlichter, L. C. (2018). Microglia responses to Pro-inflammatory stimuli (LPS, IFNγ+TNFα) and reprogramming by resolving cytokines (IL-4, IL-10). Front. Cell Neurosci. 12:215. doi: 10.3389/fncel.2018.00215
Lou, Y. L., Guo, F., Liu, F., Gao, F. L., Zhang, P. Q., Niu, X., et al. (2012). miR-210 activates notch signaling pathway in angiogenesis induced by cerebral ischemia. Mol. Cell. Biochem. 370, 45–51. doi: 10.1007/s11010-012-1396-6
Lund, S., Christensen, K. V., Hedtjärn, M., Mortensen, A. L., Hagberg, H., Falsig, J., et al. (2006). The dynamics of the LPS triggered inflammatory response of murine microglia under different culture and in vivo conditions. J. Neuroimmunol. 180, 71–87. doi: 10.1016/j.jneuroim.2006.07.007
Ma, Q., Zhao, H., Tao, Z., Wang, R., Liu, P., Han, Z., et al. (2016). MicroRNA-181c exacerbates brain injury in acute ischemic stroke. Aging. Dis. 7, 705–714. doi: 10.14336/AD.2016.0320
Ma, Y., Wang, J., Wang, Y., and Yang, G. Y. (2017). The biphasic function of microglia in ischemic stroke. Prog. Neurobiol. 157, 247–272. doi: 10.1016/j.pneurobio.2016.01.005
Mabuchi, T., Kitagawa, K., Ohtsuki, T., Kuwabara, K., Yagita, Y., Yanagihara, T., et al. (2000). Contribution of microglia/macrophages to expansion of infarction and response of oligodendrocytes after focal cerebral ischemia in rats. Stroke 31, 1735–1743. doi: 10.1161/01.str.31.7.1735
Macrez, R., Ali, C., Toutirais, O., Le Mauff, B., Defer, G., Dirnagl, U., et al. (2011). Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurology 10, 471–480. doi: 10.1016/s1474-4422(11)70066-7
Majdi, A., Mahmoudi, J., Sadigh-Eteghad, S., Farhoudi, M., and Shotorbani, S. S. (2016). The interplay of microRNAs and post-ischemic glutamate excitotoxicity: an emergent research field in stroke medicine. Neurol. Sci. 37, 1765–1771. doi: 10.1007/s10072-016-2643-5
Mantovani, A., Sica, A., Sozzani, S., Allavena, P., Vecchi, A., and Locati, M. (2004). The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686. doi: 10.1016/j.it.2004.09.015
Martinez, B., and Peplow, P. V. (2017). Immunomodulators and microRNAs as neurorestorative therapy for ischemic stroke. Neural Regen. Res. 12, 865–874. doi: 10.4103/1673-5374.208540
Michell-Robinson M. A. Touil H. Healy L. M. Owen D. R. Durafourt B. A. Bar-Or A. . (2015). Roles of microglia in brain development, tissue maintenance and repair. Brain 138, 1138–1159. doi: 10.1093/brain/awv066
Mirzaei, H., Momeni, F., Saadatpour, L., Sahebkar, A., Goodarzi, M., Masoudifar, A., et al. (2018). MicroRNA: relevance to stroke diagnosis, prognosis, and therapy. J. Cell Physiol. 233, 856–865. doi: 10.1002/jcp.25787
Narne, P., Pandey, V., and Phanithi, P. B. (2017). Interplay between mitochondrial metabolism and oxidative stress in ischemic stroke: an epigenetic connection. Mol. Cell Neurosci. 82, 176–194. doi: 10.1016/j.mcn.2017.05.008
Ni, J., Wang, X., Chen, S., Liu, H., Wang, Y., Xu, X., et al. (2015). MicroRNA let-7c-5p protects against cerebral ischemia injury via mechanisms involving the inhibition of microglia activation. Brain Behav. Immun. 49, 75–85. doi: 10.1016/j.bbi.2015.04.014
Nimmerjahn, A., Kirchhoff, F., and Helmchen, F. (2005). Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318. doi: 10.1126/science.1110647
Orihuela, R., McPherson, C. A., and Harry, G. J. (2016). Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 173, 649–665. doi: 10.1111/bph.13139
Paraskevopoulou, M. D., and Hatzigeorgiou, A. G. (2016). Analyzing MiRNA-LncRNA interactions. Methods Mol. Biol. 1402, 271–286. doi: 10.1007/978-1-4939-3378-5_21
Parisi, C., Napoli, G., Pelegrin, P., and Volonté, C. (2016). M1 and M2 functional imprinting of primary microglia: role of P2X7 activation and miR-125b. Mediat. Inflamm. 2016:2989548. doi: 10.1155/2016/2989548
Patel, A. R., Ritzel, R., McCullough, L. D., and Liu, F. (2013). Microglia and ischemic stroke: a double-edged sword. Int. J. Physiol. Pathophysiol. Pharmacol. 5, 73–90.
Pena-Philippides, J. C., Caballero-Garrido, E., Lordkipanidze, T., and Roitbak, T. (2016). In vivo inhibition of miR-155 significantly alters post-stroke inflammatory response. J. Neuroinflammation 13:287. doi: 10.1186/s12974-016-0753-x
Pepe, G., De Maglie, M., Minoli, L., Villa, A., Maggi, A., and Vegeto, E. (2017). Selective proliferative response of microglia to alternative polarization signals. J. Neuroinflammation 14:236. doi: 10.1186/s12974-017-1011-6
Perego, C., Fumagalli, S., and De Simoni, M. G. (2011). Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J. Neuroinflammation 8:174. doi: 10.1186/1742-2094-8-174
Ponomarev, E. D., Veremeyko, T., and Weiner, H. L. (2013). MicroRNAs are universal regulators of differentiation, activation, and polarization of microglia and macrophages in normal and diseased CNS. Glia 61, 91–103. doi: 10.1002/glia.22363
Qi, X., Shao, M., Sun, H., Shen, Y., Meng, D., and Huo, W. (2017). Long non-coding RNA SNHG14 promotes microglia activation by regulating miR-145-5p/PLA2G4A in cerebral infarction. Neuroscience 348, 98–106. doi: 10.1016/j.neuroscience.2017.02.002
Qing, L., Chen, H., Tang, J., and Jia, X. (2018). Exosomes and Their MicroRNA cargo: new players in peripheral nerve regeneration. Neurorehabil. Neural Repair 32, 765–776. doi: 10.1177/1545968318798955
Ransohoff, R. M., and Perry, V. H. (2009). Microglial physiology: unique stimuli, specialized responses. Annu. Rev. Immunol. 27, 119–145. doi: 10.1146/annurev.immunol.021908.132528
Rodríguez-Gómez, J. A., Kavanagh, E., Engskog-Vlachos, P., Engskog, M. K. R., Herrera, A. J., Espinosa-Oliva, A. M., et al. (2020). Microglia: agents of the CNS pro-inflammatory response. Cells 9:1717. doi: 10.3390/cells9071717
Saika, R., Sakuma, H., Noto, D., Yamaguchi, S., Yamamura, T., and Miyake, S. (2017). MicroRNA-101a regulates microglial morphology and inflammation. J. Neuroinflammation 14:109. doi: 10.1186/s12974-017-0884-8
Schmid, C. D., Melchior, B., Masek, K., Puntambekar, S. S., Danielson, P. E., Lo, D. D., et al. (2009). Differential gene expression in LPS/IFNgamma activated microglia and macrophages: in vitro versus in vivo. J. Neurochem. 109 (Suppl. 1), 117–125. doi: 10.1111/j.1471-4159.2009.05984.x
Siddiqui, T. A., Lively, S., and Schlichter, L. C. (2016). Complex molecular and functional outcomes of single vs sequential cytokine stimulation of rat microglia. J. Neuroinflammation 13:66. doi: 10.1186/s12974-016-0531-9
Song, Y., Li, Z., He, T., Qu, M., Jiang, L., Li, W., et al. (2019). M2 microglia-derived exosomes protect the mouse brain from ischemia-reperfusion injury via exosomal miR-124. Theranostics 9, 2910–2923. doi: 10.7150/thno.30879
Su, W., Hopkins, S., Nesser, N. K., Sopher, B., Silvestroni, A., Ammanuel, S., et al. (2014). The p53 transcription factor modulates microglia behavior through microRNA-dependent regulation of c-Maf. J. Immunol. 192, 358–366. doi: 10.4049/jimmunol.1301397
Sun, P., Liu, D. Z., Jickling, G. C., Sharp, F. R., and Yin, K. J. (2018). MicroRNA-based therapeutics in central nervous system injuries. J. Cereb. Blood Flow Metab. 38, 1125–1148. doi: 10.1177/0271678x18773871
Tang, L. L., Wu, Y. B., Fang, C. Q., Qu, P., and Gao, Z. L. (2016). NDRG2 promoted secreted miR-375 in microvesicles shed from M1 microglia, which induced neuron damage. Biochem. Biophys. Res. Commun. 469, 392–398. doi: 10.1016/j.bbrc.2015.11.098
Tarozzi, A., Angeloni, C., Malaguti, M., Morroni, F., Hrelia, S., and Hrelia, P. (2013). Sulforaphane as a potential protective phytochemical against neurodegenerative diseases. Oxid. Med. Cell. Longev. 2013:415078. doi: 10.1155/2013/415078
Taylor, R. A., and Sansing, L. H. (2013). Microglial responses after ischemic stroke and intracerebral hemorrhage. Clin. Dev. Immunol. 2013:746068. doi: 10.1155/2013/746068
Thounaojam, M. C., Kundu, K., Kaushik, D. K., Swaroop, S., Mahadevan, A., Shankar, S. K., et al. (2014). MicroRNA 155 regulates Japanese encephalitis virus-induced inflammatory response by targeting Src homology 2-containing inositol phosphatase 1. J. Virol. 88, 4798–4810. doi: 10.1128/jvi.02979-13
Tian, Y., Zhu, P., Liu, S., Jin, Z., Li, D., Zhao, H., et al. (2019). IL-4-polarized BV2 microglia cells promote angiogenesis by secreting exosomes. Adv. Clin. Exp. Med. 28, 421–430. doi: 10.17219/acem/91826
Tili, E., and Michaille, J. J. (2016). Promiscuous effects of some phenolic natural products on inflammation at least in part arise from their ability to modulate the expression of global regulators, namely microRNAs. Molecules 21:1263. doi: 10.3390/molecules21091263
Tobin, M. K., Bonds, J. A., Minshall, R. D., Pelligrino, D. A., Testai, F. D., and Lazarov, O. (2014). Neurogenesis and inflammation after ischemic stroke: what is known and where we go from here. J. Cereb. Blood Flow Metab. 34, 1573–1584. doi: 10.1038/jcbfm.2014.130
Vallabhapurapu, S., and Karin, M. (2009). Regulation and function of NF-kappaB transcription factors in the immune system. Annu. Rev. Immunol. 27, 693–733. doi: 10.1146/annurev.immunol.021908.132641
Vijayan, M., and Reddy, P. H. (2016). Peripheral biomarkers of stroke: focus on circulatory microRNAs. Biochim. Biophys. Acta 1862, 1984–1993. doi: 10.1016/j.bbadis.2016.08.003
Wahid, F., Khan, T., and Kim, Y. Y. (2014). MicroRNA and diseases: therapeutic potential as new generation of drugs. Biochimie 104, 12–26. doi: 10.1016/j.biochi.2014.05.004
Wan, S., Cheng, Y., Jin, H., Guo, D., Hua, Y., Keep, R. F., et al. (2016). Microglia activation and polarization after intracerebral hemorrhage in Mice: the role of protease-activated receptor-1. Transl. Stroke Res. 7, 478–487. doi: 10.1007/s12975-016-0472-8
Wang, G., Zhang, J., Hu, X., Zhang, L., Mao, L., Jiang, X., et al. (2013). Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J. Cereb. Blood Flow Metab. 33, 1864–1874. doi: 10.1038/jcbfm.2013.146
Wang, H., Li, Z., Gao, J., and Liao, Q. (2019a). Circular RNA circPTK2 regulates oxygen-glucose deprivation-activated microglia-induced hippocampal neuronal apoptosis via miR-29b-SOCS-1-JAK2/STAT3-IL-1beta signaling. Int. J. Biol. Macromol. 129, 488–496. doi: 10.1016/j.ijbiomac.2019.02.041
Wang, H., Liao, S., Li, H., Chen, Y., and Yu, J. (2019b). Long non-coding RNA TUG1 sponges Mir-145a-5p to regulate microglial polarization after oxygen-glucose deprivation. Front. Mol. Neurosci. 12, 215. doi: 10.3389/fnmol.2019.00215
Wang, J., Xing, H., Wan, L., Jiang, X., Wang, C., and Wu, Y. (2018a). Treatment targets for M2 microglia polarization in ischemic stroke. Biomed. Pharmacother. 105, 518–525. doi: 10.1016/j.biopha.2018.05.143
Wang, J., Xu, Z., Chen, X., Li, Y., Chen, C., Wang, C., et al. (2018b). MicroRNA-182-5p attenuates cerebral ischemia-reperfusion injury by targeting Toll-like receptor 4. Biochem. Biophys. Res. Commun. 505, 677–684. doi: 10.1016/j.bbrc.2018.09.165
Wang, S. W., Liu, Z., and Shi, Z. S. (2018). Non-Coding RNA in acute ischemic stroke: mechanisms, biomarkers and therapeutic targets. Cell Transplant. 27, 1763–1777. doi: 10.1177/0963689718806818
Wang, X., Chen, S., Ni, J., Cheng, J., Jia, J., and Zhen, X. (2018). miRNA-3473b contributes to neuroinflammation following cerebral ischemia. Cell Death. Dis. 9:11. doi: 10.1038/s41419-017-0014-7
Wolf, S. A., Boddeke, H. W., and Kettenmann, H. (2017). Microglia in physiology and disease. Annu. Rev. Physiol. 79, 619–643. doi: 10.1146/annurev-physiol-022516-034406
Xia, C. Y., Zhang, S., Gao, Y., Wang, Z. Z., and Chen, N. H. (2015). Selective modulation of microglia polarization to M2 phenotype for stroke treatment. Int. Immunopharmacol. 25, 377–382. doi: 10.1016/j.intimp.2015.02.019
Xiang, B., Zhong, P., Fang, L., Wu, X., Song, Y., and Yuan, H. (2019). miR-183 inhibits microglia activation and expression of inflammatory factors in rats with cerebral ischemia reperfusion via NF-kappaB signaling pathway. Exp. Ther. Med. 18, 2540–2546. doi: 10.3892/etm.2019.7827
Xiao, S., Ma, Y., Zhu, H., Sun, H., Yin, Y., and Feng, G. (2015). miRNA functional synergistic network analysis of mice with ischemic stroke. Neurol. Sci. 36, 143–148. doi: 10.1007/s10072-014-1904-4
Xie, X., Peng, L., Zhu, J., Zhou, Y., Li, L., Chen, Y., et al. (2017). miR-145-5p/Nurr1/TNF-α Signaling-induced microglia activation regulates neuron injury of acute cerebral ischemic/reperfusion in rats. Front. Mol. Neurosci. 10:383. doi: 10.3389/fnmol.2017.00383
Yang, Z., Xu, J., Zhu, R., and Liu, L. (2017). Down-regulation of miRNA-128 contributes to neuropathic pain following spinal cord injury via activation of P38. Med. Sci. Monit. 23, 405–411. doi: 10.12659/msm.898788
Yang, Z., Zhong, L., Zhong, S., Xian, R., and Yuan, B. (2015). miR-203 protects microglia mediated brain injury by regulating inflammatory responses via feedback to MyD88 in ischemia. Mol. Immunol. 65, 293–301. doi: 10.1016/j.molimm.2015.01.019
Yao, H., Ma, R., Yang, L., Hu, G., Chen, X., Duan, M., et al. (2014). MiR-9 promotes microglial activation by targeting MCPIP1. Nat. Commun. 5:4386. doi: 10.1038/ncomms5386
Yenari, M. A., Kauppinen, T. M., and Swanson, R. A. (2010). Microglial activation in stroke: therapeutic targets. Neurotherapeutics 7, 378–391. doi: 10.1016/j.nurt.2010.07.005
Yong, H. Y. F., Rawji, K. S., Ghorbani, S., Xue, M., and Yong, V. W. (2019). The benefits of neuroinflammation for the repair of the injured central nervous system. Cell Mol. Immunol. 16, 540–546. doi: 10.1038/s41423-019-0223-3
Zeng, L., Liu, J., Wang, Y., Wang, L., Weng, S., Tang, Y., et al. (2011). MicroRNA-210 as a novel blood biomarker in acute cerebral ischemia. Front. Biosci. 3, 1265–1272. doi: 10.2741/e330
Zhang, H., Lu, M., Zhang, X., Kuai, Y., Mei, Y., Tan, Q., et al. (2019a). Isosteviol sodium protects against ischemic stroke by modulating microglia/macrophage polarization via disruption of GAS5/miR-146a-5p sponge. Sci Rep. 9:12221. doi: 10.1038/s41598-019-48759-0
Zhang, H., Sun, X., Xie, Y., Tian, F., Hu, H., and Tan, W. (2018). Isosteviol sodium inhibits astrogliosis after cerebral ischemia/reperfusion injury in rats. Biol. Pharm. Bull. 41, 575–584. doi: 10.1248/bpb.b17-00921
Zhang, H., Sun, X., Xie, Y., Zan, J., and Tan, W. (2017). Isosteviol sodium protects against permanent cerebral ischemia injury in Mice via inhibition of NF-κB-mediated inflammatory and apoptotic responses. J. Stroke Cerebrovasc. Dis. 26, 2603–2614. doi: 10.1016/j.jstrokecerebrovasdis.2017.06.023
Zhang, H., Wu, J., Wu, J., Fan, Q., Zhou, J., Wu, J., et al. (2019b). Exosome-mediated targeted delivery of miR-210 for angiogenic therapy after cerebral ischemia in Mice. J. Nanobiotechnology 17:29. doi: 10.1186/s12951-019-0461-7
Zhang, L., Li, Y. J., Wu, X. Y., Hong, Z., and Wei, W. S. (2015). MicroRNA-181c negatively regulates the inflammatory response in oxygen-glucose-deprived microglia by targeting toll-like receptor 4. J. Neurochem. 132, 713–723. doi: 10.1111/jnc.13021
Zhao, H., Chen, Z., Xie, L. J., and Liu, G. F. (2018). Suppression of TLR4/NF-kappaB signaling pathway improves cerebral ischemia-reperfusion injury in rats. Mol. Neurobiol. 55, 4311–4319. doi: 10.1007/s12035-017-0552-0
Zheng, Y., He, R., Wang, P., Shi, Y., Zhao, L., and Liang, J. (2019). Exosomes from LPS-stimulated macrophages induce neuroprotection and functional improvement after ischemic stroke by modulating microglial polarization. Biomater. Sci 7, 2037–2049. doi: 10.1039/c8bm01449c
Zhong, H., Chen, H., and Gu, C. (2020). Sevoflurane post-treatment upregulated miR-203 expression to attenuate cerebral ischemia-reperfusion-induced neuroinflammation by targeting MyD88. Inflammation 43, 651–663. doi: 10.1007/s10753-019-01147-2
Keywords: microRNAs, microglia, neuroinflammation, ischemic stroke, polarization
Citation: Lian L, Zhang Y, Liu L, Yang L, Cai Y, Zhang J and Xu S (2021) Neuroinflammation in Ischemic Stroke: Focus on MicroRNA-mediated Polarization of Microglia. Front. Mol. Neurosci. 13:612439. doi: 10.3389/fnmol.2020.612439
Received: 30 September 2020; Accepted: 30 November 2020;
Published: 07 January 2021.
Edited by:
Midori A. Yenari, University of California, San Francisco, United StatesReviewed by:
Li Tian, University of Tartu, EstoniaCopyright © 2021 Lian, Zhang, Liu, Yang, Cai, Zhang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shixin Xu, c2hpeGlueHUxOTczQG91dGxvb2suY29t
†These authors contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.