
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Mol. Neurosci., 25 October 2018
Sec. Brain Disease Mechanisms
Volume 11 - 2018 | https://doi.org/10.3389/fnmol.2018.00391
Background: Various studies have reported associations between synuclein alpha (SNCA) polymorphisms and Parkinson's disease (PD) risk. However, the results are inconsistent. We conducted a comprehensive meta-analysis of the associations between SNCA single-nucleotide polymorphisms (SNPs) and PD risk in overall populations and subpopulations by ethnicity.
Methods: Standard meta-analysis was conducted according to our protocol with a cutoff point of p < 0.05. To find the most relevant SNCA SNPs, we used a cutoff point of p < 1 × 10−5 in an analysis based on the allele model. In the subgroup analysis by ethnicity, we divided the overall populations into five ethnic groups. We conducted further analysis on the most relevant SNPs using dominant and recessive models to identify the contributions of heterozygotes and homozygotes regarding each SNP.
Results: In our comprehensive meta-analysis, 24,075 cases and 22,877 controls from 36 articles were included. We included 16 variants in the meta-analysis and found 12 statistically significant variants with p < 0.05. After narrowing down the variants using the p < 1 × 10−5 cutoff, in overall populations, seven SNPs increased the risk of PD (rs2736990, rs356220, rs356165, rs181489, rs356219, rs11931074, and rs2737029, with odds ratios [ORs] of 1.22–1.38) and one SNP decreased the risk (rs356186, with an OR of 0.77). In the East Asian group, rs2736990 and rs11931074 increased the risk (with ORs of 1.22–1.34). In the European group, five SNPs increased the risk (rs356219, rs181489, rs2737029, rs356165, and rs11931074, with ORs of 1.26–1.37) while one SNP decreased the risk (rs356186, with an OR of 0.77). The heterozygotes and homozygotes contributed differently depending on the variant.
Conclusions: In summary, we found eight SNCA SNPs associated with PD risk, which had obvious differences between ethnicities. Seven SNPs increased the risk of PD and one SNP decreased the risk in the overall populations. In the East Asian group, rs2736990 and rs11931074 increased the risk. In the European group, rs356219, rs181489, rs2737029, rs356165, and rs11931074 increased the risk while rs356186 decreased the risk. Variants with the highest ORs and allele frequencies in our analysis should be given priority when carrying out genetic screening.
Parkinson's disease (PD) is a common neurodegenerative disease. Bradykinesia, resting tremor, rigidity and postural instability are the prominent motor features of PD, and they are caused by dopamine-containing neuron loss in the substantia nigra. Another pathological characteristic is Lewy body accumulation in surviving neurons (Coppede, 2012; Rochet et al., 2012), the major component of which is synuclein alpha (SNCA) (Chartier-Harlin et al., 2004). The mechanism of PD remains elusive. Genetics, environmental factors, aging and their interactions are considered to be major factors that influence how the disease develops. Regarding the genetic factors, SNCA, LRRK2, and DJ-1 have been found to be associated with PD risk (Tang et al., 2006; Guo et al., 2010; Wang et al., 2010; Lv et al., 2012).
SNCA was first found to be associated with familial autosomal dominant PD (ADPD) in 1997 (Coppede, 2012). In addition to pathogenic SNCA point mutations or multiplications (Chartier-Harlin et al., 2004), single-nucleotide polymorphisms (SNPs) can affect disease risks by affecting gene expression (Deng and Yuan, 2014). Since the Rep1 263-bp allele was shown to be associated with PD risk, SNPs such as rs356219, rs356165, rs11931074, rs7684318 etc., have been shown to be associated with PD risks in Caucasian or Asian populations (Mizuta et al., 2006; Ross et al., 2007; Myhre et al., 2008). Heterogeneities were observed among risk variants of SNCA from various populations. For example, rs356221, rs3822086, and rs11931074 were risk variants in East Asian populations while rs356186, rs2736990 were risk variants in European populations (Chung et al., 2011; Trotta et al., 2012; Wu-Chou et al., 2013; Chen et al., 2015). Genome-wide association studies (GWAS) had discovered multiple risk loci in PD and SNCA was among the top hits associated with PD risk (Davis et al., 2016). Several variants such as rs8180209, rs3756063, rs356165 had been found in GWAS of PD cohorts (Wei et al., 2016; Foo et al., 2017). In a large-scale meta-analysis of GWAS data, polymorphisms rs7681154 in SNCA also been proved to be related to risk of PD (Nalls et al., 2014). However, the results of these original studies in specific geographical areas were inconsistent and they were based on limited sample sizes. In 2015, a meta-analysis demonstrated that SNCA SNPs such as rs356186, rs356219, rs894278, rs2583988, and rs2619363 were common risk variants (Chartier-Harlin et al., 2004). However, the meta-analysis set a cutoff point of p < 0.05, which was too high to evaluate many variants at the same time without adjusting p-values by the numbers of variants. Moreover, the ethnic differences between different areas were not clearly stated. With the publication of more articles, the role of these variants plus other candidate variants in the risk of PD needed to be clarified, so we conducted a comprehensive analysis on the association between SNCA SNPs and the risk of PD in the overall populations and subpopulations by ethnicity.
We performed our meta-analysis based on PICOS (participants, interventions, controls, outcomes and studies) rules. The following inclusion criteria were used:
Participants: all PD patients were diagnosed based on the UK Parkinson's Disease Society Brain Bank (UKBB) Clinical Diagnostic Criteria or other accepted criteria (Hughes et al., 1992).
Interventions: genetic analysis was carried out by PCR-based methods or other accepted methods.
Controls: all controls were reported controls that had no obvious neurological diseases.
Outcomes: all participants were clearly reported the status of responsive genotypes, including homozygotes and heterozygotes.
Studies: all studies were case-control or cohort studies.
Three English language databases were used: Medline in PubMed, Embase in Ovid and Cochrane databases. In addition, two Chinese databases were used: CNKI and Wanfang databases. The date of the search was Dec 1, 2017. The keywords were listed separately as follows: “SNCA,” “PARK1,” “α-synuclein,” “PD,” “Parkinso*/$,” “polymorphism,” “SNP,” “variant,” “genetic.”
The data extraction was carried out by two researchers. A third researcher was asked to resolve any disputes. The first author, publication year, ethnicity, country, gene, variants, number of cases, and controls and responsive genotypes' carriers among the cases and controls were retrieved, and they are shown in Table 1 and Supplementary Table 1. The ethnic groups comprised five groups: European, East Asian, Latino, West Asian and Mixed (i.e., at least two ethnicities) (Risch et al., 2002). Newcastle-Ottawa Scale (NOS) scores (Stang, 2010) were used to evaluate the quality of all the studies.
Meta-analyses were carried out using Revman 5.3 software. Pooled analyses of variants (involving at least four original articles) were conducted in the overall populations or subpopulations by ethnicity. The pooled analyses were conducted using the allele model. P < 0.05 was considered to represent statistically significant differences in the allele model. Dominant and recessive models were applied to analyze the contributions of heterozygotes and homozygotes concerning each variant that had a significant difference in the allele model (p < 1 × 10−5). The significance of each of the dominant and recessive models was also reflected by p < 1 × 10−5. Allele frequencies (AFs) were calculated in the overall populations and subpopulations by ethnicity. Pooled odds ratios (ORs) and 95% CIs were calculated to assess the significance of the results. Q and I2 statistics were used to demonstrate the heterogeneity of the analysis. If Q statistic p > 0.1 or I2 ≤ 50%, a fixed-effects model were used for the analysis. Otherwise, a random-effects model was applied. Sensitivity analysis was carried out by deleting each original article one at a time. Publication bias was assessed based on the symmetry of the funnel plot.
As can be shown in the flowchart in Figure 1, 2,756 articles were retrieved in the databases search. We excluded 889 overlapping studies and 2,549 articles after reviewing the titles and abstracts. The last step involved excluding 120 articles after full-text review because of lack of controls, functional studies and so on. Finally, 36 articles involving 24,075 cases and 22,877 controls were included in the meta-analysis (Table 1 and Supplementary Table 1).
As can be seen in Table 2, 16 SNPs were included in the meta-analysis. Among them, 12 variants were significant at p < 0.05 (rs181489, rs356165, rs356186, rs356219, rs356220, rs2583988, rs2619363, rs2619364, rs2736990, rs2737029, rs7684318, and rs11931074) (Supplementary Figure 1), which we defined as recommended SNCA SNPs for genetic screening (Figure 2). We narrowed down the SNPs to the most relevant based on p < 1 × 10−5. Eight SNPs (rs181489, rs356165, rs356186, rs356219, rs356220, rs2736990, rs2737029, and rs11931074) met this standard and we defined them as the most recommended SNCA SNPs (Figure 2). Of these eight SNPs, seven increased the risk of PD (rs2736990, rs356220, rs356165, rs181489, rs356219, rs11931074, and rs2737029 (with ORs of 1.22–1.38 and AFs of 0.6092, 0.4865, 0.5117, 0.3486, 0.4723, 0.2825, and 0.5205, separately) and one decreased the risk of PD (rs356186, with an OR of 0.77 and an AF of 0.1629) in the overall populations. In the subgroup analysis by ethnicity, rs2736990 and rs11931074 increased the risk of PD (with ORs of 1.22 and 1.34, and AFs of 0.6618 and 0.6023) in the East Asian group. Most of the research on SNCA variants in East Asians was conducted in China. We carried out a pooled analysis in the Chinese group and found similar results to that in the East Asian group (Supplementary Table 2). In the European group, five SNPs (rs356219, rs181489, rs2737029, rs356165, and rs11931074) increased the risk of PD (with ORs of 1.26–1.37, and AFs of 0.4367, 0.3479, 0.4451, 0.4098, and 0.0917, respectively) while one SNP decreased the risk of PD (rs356186, with an OR of 0.77 and an AF of 0.1629).
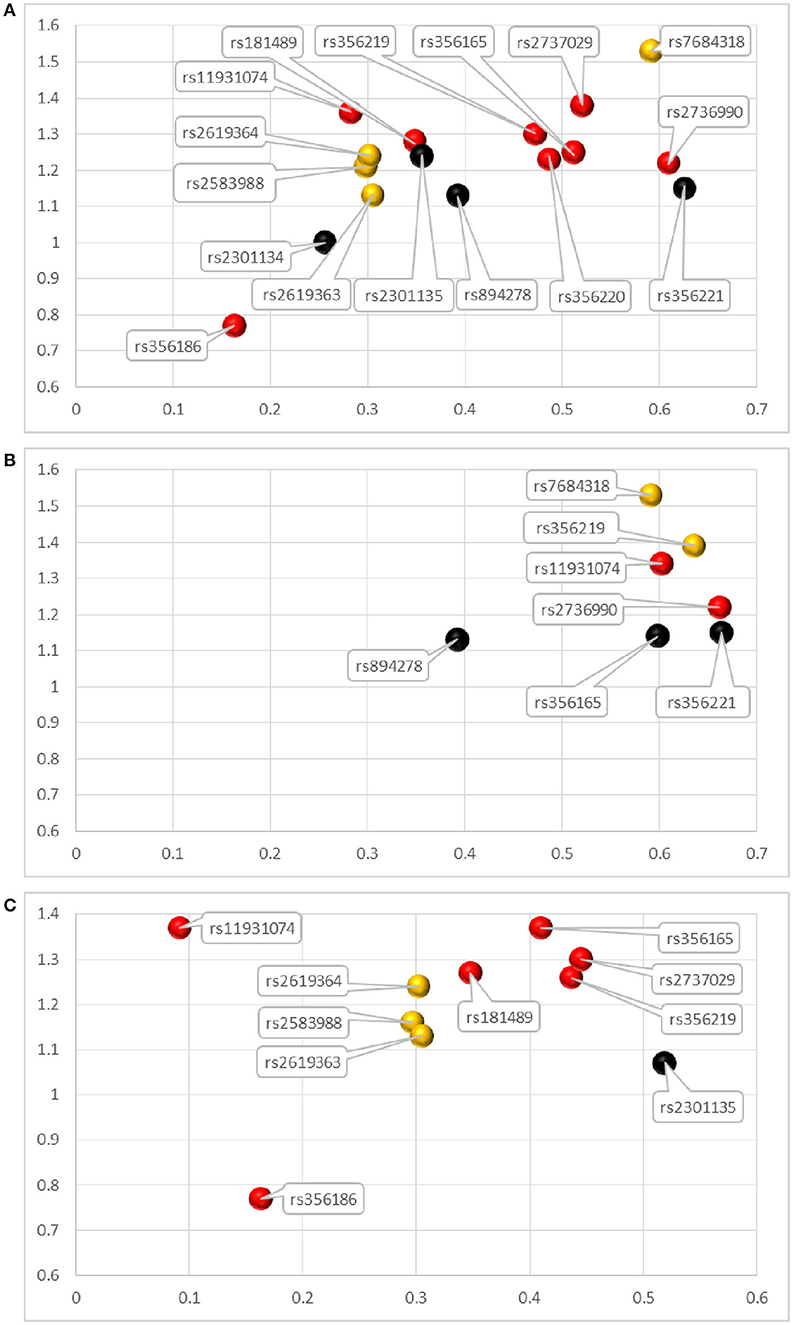
Figure 2. Attributions for SNCA variants analyzed in the meta-analysis. (A–C) represent the SNCA variants conducted pooled analysis in the overall populations, East Asian group and European group, respectively. The horizonal axis represents the allele frequencies (AFs) of variants and vertical axis represents the odds ratios (ORs). The red bubbles represent the most recommended SNPs for the genetic screening of SNCA, with p< 1 × 10−5. The orange bubbles represent the recommended SNPs, with p < 0.05. The black bubbles represent SNPs not recommended for genetic screening of SNCA, with no meaningful p-values.
Regarding the relevant eight SNCA SNPs, we explored the contributions of heterozygotes and homozygotes concerning each variant using dominant and recessive models (Table 3 and Supplementary Figures 2, 3). In the overall populations, there were significant differences in the dominant models for rs356186 and rs2737029, which indicated that heterozygotes contributed to the importance of these variants (ORs: 0.74 and 1.52, separately). In the recessive models, rs2736990, rs356220, rs356165, and rs181489 had significant differences, which demonstrated the contributions of homozygotes concerning these variants (ORs: 1.3–1.58). Rs356219 and rs11931074 had significant differences in both models, which demonstrated the importance of both heterozygotes and homozygotes (ORs: 1.34, 1.52; 1.43, and 1.53, separately).
Regarding the ethnic groups, there were also different results in the dominant and recessive models concerning each variant, which indicated the different contributions of heterozygotes and homozygotes. In the European groups, rs11931074 had a significant difference in the dominant model while rs181489 had a significant difference in the recessive model (ORs: 1.39 and 1.58 separately). Rs356165 and rs356219 had significance differences in both models (ORs: 1.4, 1.71; 1.3 and 1.47). In the East Asian groups, rs356186 had a significant difference in the dominant model while rs356165 had a significant difference in the recessive model (ORs: 0.74 and 1.42). Rs356219 and rs11931074 had significant differences in both models (ORs: 1.55, 1.6; 1.43 and 1.52).
The sensitivity analyses were carried out by sequentially deleting each original article. No obvious changes were demonstrated by the pooled OR of each meta-analysis, which indicated that the results were stable.
Publication bias was investigated using funnel plots in Revman 5.3. Most of the meta-analysis had no publication bias based on the symmetrical shapes of the plots (Supplementary Figures 4–6).
In our meta-analysis, we systematically analyzed the relationship between SNCA SNPs and the risk of PD. Our meta-analysis was a comprehensive pooled analysis of SNCA SNPs associated with PD risk in the overall populations and subpopulations by ethnicity.
Several SNPs with significant differences were found in our meta-analysis. We found seven SNPs increased the risk of PD (rs2736990, rs356220, rs356165, rs181489, rs356219, rs11931074, and rs2737029) and one decreased the risk of PD (rs356186) in the overall populations. Significant ethnic differences were observed in our meta-analysis. In the East Asian group, rs2736990 and rs11931074 increased the risk of PD. In the European group, five SNPs (rs356219, rs181489, rs2737029, rs356165, and rs11931074) increased the risk of PD while one (rs356186) decreased the risk of PD.
In 2005, genome-wide association studies (GWASs) identified genes such as SNCA and MAPT as genes involved in PD. In GWAS, p < 5 × 10−8 was always considered to be the appropriate statistically significant threshold in genome-wide analyses (Nalls et al., 2014). In routine meta-analysis, the statistically significant p-value has been defined as < 0.05. Using this cutoff point, we identified 12 significant SNCA variants associated with PD risk, which we defined as recommended SNCA variants for genetic screening (Figure 2). Just like GWASs that adjusted the p-values using the number of whole-genome bases, we considered that when evaluating the PD risks of variants simultaneously, it is wise to use a lower p-value as a cutoff point to reduce the false-positive rate and declare variants of a gene to be statistically significant. Therefore, we narrowed down the 12 positive variants using the cutoff point of p < 1 × 10−5 which was the minimum p-value offered in the Revman 5.3 software. Using this cutoff point, we identified eight SNPs as being the most important SNCA variants, which we defined as the most recommended SNCA variants for genetic screening (Figure 2). Additionally, we explored the contributions of heterozygotes and homozygotes concerning each variant, which could provide evidence for genotype targeting in genetic screening.
In the overall populations, the eight most recommended SNPs (based on our analysis defined by p-values) ranked from smallest to largest contribution to PD risk were rs2736990, rs356220, rs356165, rs181489, rs356219, rs11931074, and rs2737029 (with ORs of 1.22–1.38). In the East Asian group, rs2736990 and rs11931074 increased the risk of PD (with ORs of 1.22 and 1.34, separately). In the European group, rs356219, rs181489, rs2737029, rs356165, and rs11931074 increased the risk of PD (with ORs of 1.26–1.37). Rs356186 decreased the risk of PD in the overall populations and the European group (with an OR of 0.77). The ORs were convincing measures of effect size estimates of variants, which represented the variants' contributions to PD risk. In our analysis, our most recommended SNCA SNPs only have moderate effect size estimates (with OR < 1.5), which reflected their relatively low contributions to the risk of PD when compared with GBA variants (Lill, 2016). Although the ORs associated with SNCA SNPs were relatively low, previous research has suggested that functional SNCA SNPs can affect SNCA expression through epigenetic modification (Soldner et al., 2016), which may allow them to be used as potential therapeutic targets and to contribute to precise treatment strategy design (Carlson, 1990). These polymorphisms in SNCA may also possibly affect the risk of PD by damaging the normal function of α-synuclein by affecting synaptic activity through modulating the release of synaptic vesicle (Surguchev and Surguchov, 2017). Substitution of amino acids ofα-synuclein changed the gene expression levels including genes functioning in apoptosis, transcription process, membrane proteins, etc. (Baptista et al., 2003). Further functional researches were needed to find the molecular mechanisms in our recommended risk variants of SNCA.
When developing genetic screening strategies in the future, not only do we need to focus on the most recommended SNCA variants in our analysis, but we should also give priority to screening for important risk SNPs that have the highest ORs and AFs in our analysis (Figure 2). From the Figure 2, we can clearly see that among the most recommended SNPs, rs356165, rs2737029, and rs2736990 in the overall populations, rs356165, rs356219, and rs2737029 in the European group, and rs2736990 and rs11931074 in the Asian group had both ORs and AFs that ranked high in our analysis. Risk variants with low AFs should also be paid attention to because these variants are not easily screened out without large-sample research. Additionally, in developing screening strategies, we should also pay attention to SNPs that share the same haplotype in a specific ethnic group. We performed haplotype analysis for the 8 statistically significant variants with p < 1 × 10−5 in our results. Because we could not extract specific data for both patients and controls from the publications included in our manuscript, public data from 1000 Genomes project (http://www.internationalgenome.org/home) and Haploview software were used for haplotype analysis. The 8 variants could be divided into 2 blocks (Figure 3): the first block was variants rs2737029 and rs356186, the second block was variants rs2736990, rs356165, rs356220, rs356219, and rs181489, which indicate that any of the two variants exposed LD. The haplotype analysis demonstrated a kind of interaction between variants that the variants were in LD and the interaction commonly existed in the same gene and in the close distance. The tagging variants in the haplotype can represent the other variants. For example, a haplotype in the 3′ region of SNCA contains rs356219, rs356220, rs356165, and rs356203 in the Caucasian population (Pankratz et al., 2009). When we choose SNPs as markers for genetic screening, we should select relevant representative SNPs that are found in different haplotypes across the entire gene (Myhre et al., 2008).
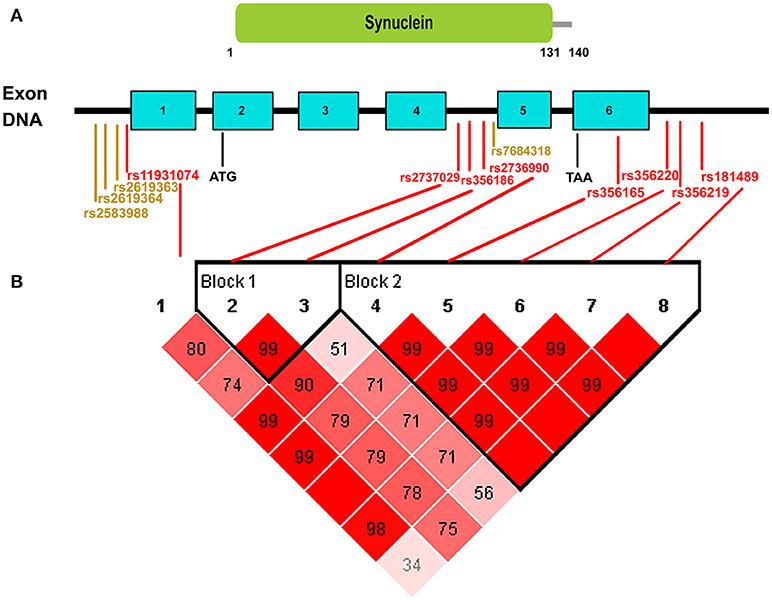
Figure 3. Schematic of the distribution of positive variants in our meta-analysis across the whole SNCA gene. (A) ATG indicates the start codon and TAA indicates the end codon. SNCA contains six exons and all of the positive SNPs (red markers represent the SNPs with p < 1 × 10−5 and orange markers represent the SNPs with p < 0.05) in our analysis were in non-coding regions of SNCA. (B) In terms of the haplotype analysis with public data from 1000 Genomes Project, the 8 red significant SNPs could be divided into two blocks: the first block contained variants rs2737029 and rs356186, the second block contained variants rs2736990, rs356165, rs356220, rs356219, and rs181489. The variants in the same block indicated that any of the two variants exposed LD.
It has been reported that there were other common interactions among variants in different genes associated with the risk of developing PD. In 2015, Guo et al. (2015) analyzed 16 SNPs in eight genes and/or loci in a large Chinese cohort and found Rep1, rs356165, and rs11931074 in SNCA gene, G2385R in LRRK2 gene, rs4698412 in BST1 gene, rs1564282 in PARK17, and L444P in GBA gene have an independent and combined significant relationship with PD. Wang et al. (2012) reported that Rep1 and rs356219 in SNCA, rs242562 and rs2435207 in MAPT, L444P in GBA, rs4273468 in BST1, rs823144 in PARK16 significantly modified the LRRK2-related risk for PD and the patients' ages at onset (AAOs) in a Chinese cohort consisting of 2013 sporadic PD patients and 1971 controls. Moreover, clinical research has demonstrated that patients with SNCA variants had deteriorated cognitive functions (Myhre et al., 2008). In some SNCA variant carriers, a more tremor-predominant phenotype and a slower rate of motor progression were also shown to be distinct features (Cooper et al., 2017). When selecting SNCA variants for genetic screening, it is important to pay attention to the associated unique clinical features in carriers in order to facilitate the estimation of disease prognosis, the selection of optimum symptomatic treatments and the stratification of patients in clinical trials.
Limitations in our pooled analysis were inevitable. First, some unadjusted factors may have caused bias. For instance, differences among original articles in methods, onset age and gender of cases and controls might have confounded the pooled results. With more original articles, we could deal with this problem using detailed subgroup analysis. Second, the original studies in our analysis were cross-sectional studies. Due to the lack of longitudinal and multicenter studies, it is hard to define SNCA variants as independent risk factors of PD. Third, the sample sizes of some SNP analyses were not large enough to reach precise results. More research is needed in the future.
In summary, we observed eight SNPs that were most associated with PD risk, and there were obvious ethnic differences. Seven SNPs (rs2736990, rs356220, rs356165, rs181489, rs356219, rs11931074, and rs2737029) increased the risk of PD and one decreased the risk of PD in the overall populations. In the East Asian group, rs2736990 and rs11931074 increased the risk of PD. In the European group, five SNPs (rs356219, rs181489, rs2737029, rs356165, and rs11931074) increased the risk of PD while one decreased the risk of PD. Variants with relatively high ORs and AFs in our analysis should be given priority when carrying out genetic screening.
YZ, LS, and BT conceived and designed the experiments and wrote the manuscript. YZ, LS, and QS performed the experiments. YZ, LS, QS, and BT analyzed the data. HP and JG reference collection and data management.
This work was supported by grants from the National Natural Science Foundation of China (No. 81430023, No. 81401059), the National Key Plan for Scientific Research and Development of China (NO. 2016YFC1306000, 2017YFC0909100) and Hunan Provincial Innovation Foundation For Postgraduate (NO. CX2017B066).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnmol.2018.00391/full#supplementary-material
Baptista, M. J., O'Farrell, C., Daya, S., Ahmad, R., Miller, D. W., Hardy, J., et al. (2003). Co-ordinate transcriptional regulation of dopamine synthesis genes by alpha-synuclein in human neuroblastoma cell lines. J. Neurochem. 85, 957–968. doi: 10.1046/j.1471-4159.2003.01742.x
Campelo, C. L. C., Cagni, F. C., de Siqueira Figueredo, D., Oliveira, L. G. Jr., Silva-Neto, A. B., Macedo, P. T., et al. (2017). Variants in SNCA gene are associated with Parkinson's disease risk and cognitive symptoms in a brazilian sample. Front. Aging Neurosci. 9:198. doi: 10.3389/fnagi.2017.00198
Cardo, L. F., Coto, E., de Mena, L., Ribacoba, R., Lorenzo-Betancor, O., Pastor, P., et al. (2012). A search for SNCA 3' UTR variants identified SNP rs356165 as a determinant of disease risk and onset age in Parkinson's disease. J. Mol. Neurosci. 47, 425–430. doi: 10.1007/s12031-011-9669-1
Carlson, M. J. (1990). Signs of the times. More physicians and hospitals jump on the smoke-free bandwagon. Ohio Med. 86, 382–386.
Chang, X. L., Mao, X. Y., Li, H. H., Zhang, J. H., Li, N. N., Burgunder, J. M., et al. (2011). Association of GWAS loci with PD in China. Am. J. Med. Genet. B Neuropsychiatr. Genet. 156B, 334–339. doi: 10.1002/ajmg.b.31167
Chartier-Harlin, M. C., Kachergus, J., Roumier, C., Mouroux, V., Douay, X., Lincoln, S., et al. (2004). Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet 364, 1167–1169. doi: 10.1016/S0140-6736(04)17103-1
Chen, W. J., Hu, F. Y., Dong, Y. T., Wang, M., Han, B., and Chang, X. L. (2017) Correlation between polymorphic site of synuclein gene rs356165 Parkinson's disease of Han population in Shanxi province. Chin. Remed. Clin. 17, 341–343. doi: 10.11655/zgywylc2017.03.010
Chen, Y., Wei, Q. Q., Ou, R., Cao, B., Chen, X., Zhao, B., et al. (2015). Genetic variants of SNCA are associated with susceptibility to parkinson's disease but not amyotrophic lateral sclerosis or multiple system atrophy in a chinese population. PLoS ONE 10:e0133776. doi: 10.1371/journal.pone.0133776
Chung, S. J., Armasu, S. M., Biernacka, J. M., Lesnick, T. G., Rider, D. N., Lincoln, S. J., et al. (2011). Common variants in PARK loci and related genes and Parkinson's disease. Mov. Disord. 26, 280–288. doi: 10.1002/mds.23376
Cooper, C. A., Jain, N., Gallagher, M. D., Weintraub, D., Xie, S. X., Berlyand, Y., et al. (2017). Common variant rs356182 near SNCA defines a Parkinson's disease endophenotype. Ann. Clin. Transl. Neurol. 4, 15–25. doi: 10.1002/acn3.371
Coppede, F. (2012). Genetics and epigenetics of Parkinson's disease. Sci. World J. 2012:489830. doi: 10.1100/2012/489830
Davis, A. A., Andruska, K. M., Benitez, B. A., Racette, B. A., Perlmutter, J. S., and Cruchaga, C. (2016). Variants in GBA, SNCA, and MAPT influence Parkinson disease risk, age at onset, and progression. Neurobiol. Aging 37, 209 e201.e7–e209.e7. doi: 10.1016/j.neurobiolaging.2015.09.014
Deng, H., and Yuan, L. (2014). Genetic variants and animal models in SNCA and Parkinson disease. Ageing Res. Rev. 15, 161–176. doi: 10.1016/j.arr.2014.04.002
Elbaz, A., Ross, O. A., Ioannidis, J. P., Soto-Ortolaza, A. I., Moisan, F., Aasly, J., et al. (2011). Independent and joint effects of the MAPT and SNCA genes in Parkinson disease. Ann. Neurol. 69, 778–792. doi: 10.1002/ana.22321
Emelyanov, A., Andoskin, P., Yakimovskii, A., Usenko, T., Nuzhnyi, E., Nikolaev, M., et al. (2013). SNCA, LRRK2, MAPT polymorphisms and Parkinson's disease in Russia. Parkinsonism Relat. Disord. 19, 1064–1065. doi: 10.1016/j.parkreldis.2013.06.003
Fang, J., Yi, K., Guo, M., An, X., Qu, H., Lin, Q., et al. (2016). Analysis of LRRK2, SNCA, and ITGA8 gene variants with sporadic Parkinson's disease susceptibility in chinese han population. Parkinsons Dis. 2016:3474751. doi: 10.1155/2016/3474751
Foo, J. N., Tan, L. C., Irwan, I. D., Au, W. L., Low, H. Q., Prakash, K. M., et al. (2017). Genome-wide association study of Parkinson's disease in East Asians. Hum. Mol. Genet. 26, 226–232. doi: 10.1093/hmg/ddw379
Gan, R., Zhang, Y. H., Nie, K., and Wang, L. J. (2012). Relation of synuclein gene polymorphisms with Parkinson's disease and its cognitive impairment. Chin. J. Geriatr. Heart Brain Vessel Dis. 8, 788–791. doi: 10.3969/j.issn.1009-0126.2012.08.002
Goris, A., Williams-Gray, C. H., Clark, G. R., Foltynie, T., Lewis, S. J., Brown, J., et al. (2007). Tau and alpha-synuclein in susceptibility to, and dementia in, Parkinson's disease. Ann. Neurol. 62, 145–153. doi: 10.1002/ana.21192
Guo, J. F., Li, K., Yu, R. L., Sun, Q. Y., Wang, L., Yao, L. Y., et al. (2015). Polygenic determinants of Parkinson's disease in a Chinese population. Neurobiol. Aging 36, 1765 e1761–1765 e1766. doi: 10.1016/j.neurobiolaging.2014.12.030
Guo, J. F., Zhang, X. W., Nie, L. L., Zhang, H. N., Liao, B., Li, J., et al. (2010). Mutation analysis of Parkin, PINK1 and DJ-1 genes in Chinese patients with sporadic early onset parkinsonism. J. Neurol. 257, 1170–1175. doi: 10.1007/s00415-010-5485-8
Guo, X. Y., Chen, Y. P., Song, W., Zhao, B., Cao, B., Wei, Q. Q., et al. (2014). SNCA variants rs2736990 and rs356220 as risk factors for Parkinson's disease but not for amyotrophic lateral sclerosis and multiple system atrophy in a Chinese population. Neurobiol. Aging 35, 2882 e2881.e6–e2882.e6. doi: 10.1016/j.neurobiolaging.2014.07.014
Hu, F. Y., Hu, W. B., Liu, L., Yu, L. H., Xi, J., He, X. H., et al. (2010). Lack of replication of a previously reported association between polymorphism in the 3'UTR of the alpha-synuclein gene and Parkinson's disease in Chinese subjects. Neurosci. Lett. 479, 31–33. doi: 10.1016/j.neulet.2010.05.022
Hu, Y., Tang, B., Guo, J., Wu, X., Sun, Q., Shi, C., et al. (2012). Variant in the 3' region of SNCA associated with Parkinson's disease and serum alpha-synuclein levels. J. Neurol. 259, 497–504. doi: 10.1007/s00415-011-6209-4
Hughes, A. J., Daniel, S. E., Kilford, L., and Lees, A. J. (1992). Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 55, 181–184. doi: 10.1136/jnnp.55.3.181
Lee, P. C., Bordelon, Y., Bronstein, J., Sinsheimer, J. S., Farrer, M., and Ritz, B. (2015). Head injury, alpha-synuclein genetic variability and Parkinson's disease. Eur. J. Neurol. 22, 874–878. doi: 10.1111/ene.12585
Li, N. N., Mao, X. Y., Chang, X. L., Zhao, D. M., Zhang, J. H., Liao, Q., et al. (2013). SNCA rs356219 variant increases risk of sporadic Parkinson's disease in ethnic Chinese. Am. J. Med. Genet. B Neuropsychiatr. Genet. 162B, 452–456. doi: 10.1002/ajmg.b.32143
Lill, C. M. (2016). Genetics of Parkinson's disease. Mol. Cell. Probes 30, 386–396. doi: 10.1016/j.mcp.2016.11.001
Liu, B., Zhang, L. H., Xu, Z., Ji, C., Pang, A. L., Zheng, Y., et al. (2013) Association of SNCA gene polymorphism with Parkinson disease by SNapshot micro sequencing. Chin. J. Med. 48, 58–60. doi: 10.3969/j.issn.1008-1070.2013.10.024
Liu, J., Xiao, Q., Wang, Y., Xu, Z. M., Wang, Y., Yang, Q., et al. (2013). Analysis of genome-wide association study-linked loci in Parkinson's disease of Mainland China. Mov. Disord. 28, 1892–1895. doi: 10.1002/mds.25599
Lv, Z., Guo, J., Sun, Q., Li, K., Yu, R., Tian, J., et al. (2012). Association between PLA2G6 gene polymorphisms and Parkinson's disease in the Chinese Han population. Parkinsonism Relat. Disord. 18, 641–644. doi: 10.1016/j.parkreldis.2012.02.015
Mata, I. F., Shi, M., Agarwal, P., Chung, K. A., Edwards, K. L., Factor, S. A., et al. (2010). SNCA variant associated with Parkinson disease and plasma alpha-synuclein level. Arch. Neurol. 67, 1350–1356. doi: 10.1001/archneurol.2010.279
Miyake, Y., Tanaka, K., Fukushima, W., Kiyohara, C., Sasaki, S., Tsuboi, Y., et al. (2012). SNCA polymorphisms, smoking, and sporadic Parkinson's disease in Japanese. Parkinsonism Relat. Disord. 18, 557–561. doi: 10.1016/j.parkreldis.2012.02.016
Mizuta, I., Satake, W., Nakabayashi, Y., Ito, C., Suzuki, S., Momose, Y., et al. (2006). Multiple candidate gene analysis identifies alpha-synuclein as a susceptibility gene for sporadic Parkinson's disease. Hum. Mol. Genet. 15, 1151–1158. doi: 10.1093/hmg/ddl030
Mizuta, I., Tsunoda, T., Satake, W., Nakabayashi, Y., Watanabe, M., Takeda, A., et al. (2008). Calbindin 1, fibroblast growth factor 20, and alpha-synuclein in sporadic Parkinson's disease. Hum. Genet. 124, 89–94. doi: 10.1007/s00439-008-0525-5
Myhre, R., Toft, M., Kachergus, J., Hulihan, M. M., Aasly, J. O., Klungland, H., et al. (2008). Multiple alpha-synuclein gene polymorphisms are associated with Parkinson's disease in a Norwegian population. Acta Neurol. Scand. 118, 320–327. doi: 10.1111/j.1600-0404.2008.01019.x
Nalls, M. A., Pankratz, N., Lill, C. M., Do, C. B., Hernandez, D. G., Saad, M., et al. (2014). Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson's disease. Nat. Genet. 46, 989–993. doi: 10.1038/ng.3043
Pan, F., Ding, H., Dong, H., Ye, M., Liu, W., Cui, G., et al. (2013). Association of polymorphism in rs2736990 of the alpha-synuclein gene with Parkinson's disease in a Chinese population. Neurol. India 61, 360–364. doi: 10.4103/0028-3886.117595
Pan, F., Dong, H., Ding, H., Ye, M., Liu, W., Wu, Y., et al. (2012). SNP rs356219 of the alpha-synuclein (SNCA) gene is associated with Parkinson's disease in a Chinese Han population. Parkinsonism Relat. Disord. 18, 632–634. doi: 10.1016/j.parkreldis.2012.01.025
Pankratz, N., Nichols, W. C., Elsaesser, V. E., Pauciulo, M. W., Marek, D. K., Halter, C. A., et al. (2009). Alpha-synuclein and familial Parkinson's disease. Mov. Disord. 24, 1125–1131. doi: 10.1002/mds.22524
Parsian, A. J., Racette, B. A., Zhao, J. H., Sinha, R., Patra, B., Perlmutter, J. S., et al. (2007). Association of alpha-synuclein gene haplotypes with Parkinson's disease. Parkinsonism Relat. Disord. 13, 343–347. doi: 10.1016/j.parkreldis.2006.12.006
Risch, N., Burchard, E., Ziv, E., and Tang, H. (2002). Categorization of humans in biomedical research: genes, race and disease. Genome Biol. 3:comment2007. doi: 10.1186/gb-2002-3-7-comment2007
Rochet, J. C., Hay, B. A., and Guo, M. (2012). Molecular insights into Parkinson's disease. Prog. Mol. Biol. Transl. Sci. 107, 125–188. doi: 10.1016/B978-0-12-385883-2.00011-4
Ross, O. A., Gosal, D., Stone, J. T., Lincoln, S. J., Heckman, M. G., Irvine, G. B., et al. (2007). Familial genes in sporadic disease: common variants of alpha-synuclein gene associate with Parkinson's disease. Mech. Ageing Dev. 128, 378–382. doi: 10.1016/j.mad.2007.04.002
Shahmohammadibeni, N., Rahimi-Aliabadi, S., Jamshidi, J., Emamalizadeh, B., Shahmohammadibeni, H. A., Zare Bidoki, A., et al. (2016). The analysis of association between SNCA, HUSEYO and CSMD1 gene variants and Parkinson's disease in Iranian population. Neurol. Sci. 37, 731–736. doi: 10.1007/s10072-015-2420-x
Soldner, F., Stelzer, Y., Shivalila, C. S., Abraham, B. J., Latourelle, J. C., Barrasa, M. I., et al. (2016). Parkinson-associated risk variant in distal enhancer of alpha-synuclein modulates target gene expression. Nature 533, 95–99. doi: 10.1038/nature17939
Stang, A. (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25, 603–605. doi: 10.1007/s10654-010-9491-z
Surguchev, A. A., and Surguchov, A. (2017). Synucleins and gene expression: ramblers in a crowd or cops regulating traffic? Front. Mol. Neurosci. 10:224. doi: 10.3389/fnmol.2017.00224
Tang, B., Xiong, H., Sun, P., Zhang, Y., Wang, D., Hu, Z., et al. (2006). Association of PINK1 and DJ-1 confers digenic inheritance of early-onset Parkinson's disease. Hum. Mol. Genet. 15, 1816–1825. doi: 10.1093/hmg/ddl104
Trotta, L., Guella, I., Solda, G., Sironi, F., Tesei, S., Canesi, M., et al. (2012). SNCA and MAPT genes: independent and joint effects in Parkinson disease in the Italian population. Parkinsonism Relat. Disord. 18, 257–262. doi: 10.1016/j.parkreldis.2011.10.014
Wang, C. D., Cai, Y. N., Zheng, Z., Tang, B. S., Xu, Y. M., Wang, T., et al. (2012). Penetrance of LRRK2 G2385R and R1628P is modified by common PD-associated genetic variants. Parkinsonism Relat. Disord. 18, 958–963. doi: 10.1016/j.parkreldis.2012.05.003
Wang, L., Guo, J. F., Nie, L. L., Xu, Q., Zuo, X., Sun, Q. Y., et al. (2010). A novel LRRK2 mutation in a mainland Chinese patient with familial Parkinson's disease. Neurosci. Lett. 468, 198–201. doi: 10.1016/j.neulet.2009.10.080
Wei, Y., Yang, N., Xu, Q., Sun, Q., Guo, J., Li, K., et al. (2016). The rs3756063 polymorphism is associated with SNCA methylation in the Chinese Han population. J. Neurol. Sci. 367, 11–14. doi: 10.1016/j.jns.2016.05.037
Westerlund, M., Belin, A. C., Anvret, A., Hakansson, A., Nissbrandt, H., Lind, C., et al. (2008). Cerebellar alpha-synuclein levels are decreased in Parkinson's disease and do not correlate with SNCA polymorphisms associated with disease in a Swedish material. FASEB J. 22, 3509–3514. doi: 10.1096/fj.08-110148.
Winkler, S., Hagenah, J., Lincoln, S., Heckman, M., Haugarvoll, K., Lohmann-Hedrich, K., et al. (2007). alpha-Synuclein and Parkinson disease susceptibility. Neurology 69, 1745–1750. doi: 10.1212/01.wnl.0000275524.15125.f4
Wu, G. P., Wang, D. Y., Kang, W. Y., and Liu, J. (2015). Association study of α-synuclein gene polymorphism and non motor symptoms in Parkinson's disease. Chin. J. Neurol. 10, 870–875. doi: 10.3760/cma.j.issn.1006-7876.2015.10.011
Wu-Chou, Y. H., Chen, Y. T., Yeh, T. H., Chang, H. C., Weng, Y. H., Lai, S. C., et al. (2013). Genetic variants of SNCA and LRRK2 genes are associated with sporadic PD susceptibility: a replication study in a Taiwanese cohort. Parkinsonism Relat. Disord. 19, 251–255. doi: 10.1016/j.parkreldis.2012.10.019
Keywords: Parkinson's disease, SNCA, Asian, European, meta-analysis
Citation: Zhang Y, Shu L, Sun Q, Pan H, Guo J and Tang B (2018) A Comprehensive Analysis of the Association Between SNCA Polymorphisms and the Risk of Parkinson's Disease. Front. Mol. Neurosci. 11:391. doi: 10.3389/fnmol.2018.00391
Received: 31 March 2018; Accepted: 05 October 2018;
Published: 25 October 2018.
Edited by:
Robert J. Harvey, University of the Sunshine Coast, AustraliaReviewed by:
Félix Javier Jiménez-Jiménez, Hospital Universitario del Sureste, SpainCopyright © 2018 Zhang, Shu, Sun, Pan, Guo and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Beisha Tang, YnN0YW5nNzM5OEAxNjMuY29t
†These authors have contributed equally to this work and share co-first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.