
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mol. Neurosci. , 12 March 2018
Sec. Molecular Signalling and Pathways
Volume 11 - 2018 | https://doi.org/10.3389/fnmol.2018.00065
 Zhuoran Yin1,2†
Zhuoran Yin1,2† Divya D. Raj2†
Divya D. Raj2† Wandert Schaafsma2‡
Wandert Schaafsma2‡ Roel A. van der Heijden3‡
Roel A. van der Heijden3‡ Susanne M. Kooistra2‡
Susanne M. Kooistra2‡ Aaffien C. Reijne4,5,6
Aaffien C. Reijne4,5,6 Xiaoming Zhang2
Xiaoming Zhang2 Jill Moser7
Jill Moser7 Nieske Brouwer2
Nieske Brouwer2 Peter Heeringa3
Peter Heeringa3 Chun-Xia Yi8
Chun-Xia Yi8 Gertjan van Dijk5,6,9
Gertjan van Dijk5,6,9 Jon D. Laman2
Jon D. Laman2 Erik W. G. M. Boddeke2
Erik W. G. M. Boddeke2 Bart J. L. Eggen2*
Bart J. L. Eggen2*Rodent models of both aging and obesity are characterized by inflammation in specific brain regions, notably the corpus callosum, fornix, and hypothalamus. Microglia, the resident macrophages of the central nervous system, are important for brain development, neural support, and homeostasis. However, the effects of diet and lifestyle on microglia during aging are only partly understood. Here, we report alterations in microglia phenotype and functions in different brain regions of mice on a high-fat diet (HFD) or low-fat diet (LFD) during aging and in response to voluntary running wheel exercise. We compared the expression levels of genes involved in immune response, phagocytosis, and metabolism in the hypothalamus of 6-month-old HFD and LFD mice. We also compared the immune response of microglia from HFD or LFD mice to peripheral inflammation induced by intraperitoneal injection of lipopolysaccharide (LPS). Finally, we investigated the effect of diet, physical exercise, and caloric restriction (40% reduction compared to ad libitum intake) on microglia in 24-month-old HFD and LFD mice. Changes in diet caused morphological changes in microglia, but did not change the microglia response to LPS-induced systemic inflammation. Expression of phagocytic markers (i.e., Mac-2/Lgals3, Dectin-1/Clec7a, and CD16/CD32) in the white matter microglia of 24-month-old brain was markedly decreased in calorically restricted LFD mice. In conclusion, LFD resulted in reduced activation of microglia, which might be an underlying mechanism for the protective role of caloric restriction during aging-associated decline.
Aging and obesity are worldwide health issues affecting millions of people (Han et al., 2011). Both aging and obesity are linked to chronic low-grade inflammation that is associated with multisystem diseases (Lumeng and Saltiel, 2011; Woods et al., 2012). Aging is considered as a pro-obesogenic factor, associated with age-related metabolic decline, which is characterized by changes in fat distribution, obesity, and insulin resistance (Gabriely et al., 2002). Concurrently, obesity can aggravate age-related decline in physical function and cause frailty (Villareal et al., 2005). A number of obesity-associated comorbidities such as type 2 diabetes (Eckel et al., 2011), hypertension (Kotsis et al., 2010), and cardiovascular disease (Poirier et al., 2006) may ultimately contribute to premature aging and shortened lifespan. Importantly, obesity not only affects the function of peripheral organs, but also influences the CNS. Previous studies reported that obesity is associated with synapse loss (Bocarsly et al., 2015), hypothalamic gliosis, and cognitive deficits (Thaler et al., 2013; Kälin et al., 2015). It was also proposed that obesity and its direct comorbidities act as a facilitator and predictor of neurodegenerative diseases (Cai, 2013; van Dijk et al., 2015). Metabolic dysfunction and obesity are associated with learning and memory impairment in early old age (Sabia et al., 2009; Singh-Manoux et al., 2012). It has been suggested that a HFD promotes the progression of obesity (Golay and Bobbioni, 1997). Thus, investigating the pathological alterations resulting from HFD in the aging brain will help to better estimate the role of obesity in neurodegeneration in the elderly.
The brain has long been viewed as an immune-privileged organ with limited access for peripheral immune signals. However, the differently structured blood–brain barrier in the hypothalamic ARC-ME complex allows more blood-borne signals to enter the brain (Gross, 1992). Microglia, the tissue macrophages of the CNS, play a pivotal role in the chronic inflammation observed in aging (Norden and Godbout, 2013; Galatro et al., 2017a,b) as well as metabolic diseases such as obesity (Kälin et al., 2015). Increased inflammation in the aging brain is most predominant in the white matter, and includes loss of myelinated fibers and malformation of myelin sheaths (Gunning-Dixon et al., 2009). In addition to aging, an HFD induces brain inflammation (Zhang et al., 2008). Young wild-type mice on a short-term HFD showed an increase in microglia numbers and pro-inflammatory factors in ARC-ME areas (Thaler et al., 2012; Gao et al., 2014), even before body weight was affected (Gao et al., 2014). HFD may cause central leptin (Münzberg et al., 2004; Enriori et al., 2007) and insulin resistance (De Souza et al., 2005), which underlies increased food intake, body fat content (Rosenbaum and Leibel, 2010), and adaptive thermogenic responses aimed at maintaining the increased body fat content (Koch et al., 2014). Moreover, this condition in the CNS leads to increased hepatic glucose production (Pocai et al., 2005), and collectively this underlies an increased risk toward obesity, diabetes, and cardiovascular disease (Thaler and Schwartz, 2010). In contrast, a prolonged period of HFD was recently reported to induce mixed pro- and anti-inflammatory responses in the hypothalamus (Baufeld et al., 2016). Summarizing these observations, our understanding of the impact of HFD on inflammation and the role of microglia in different brain regions is incomplete.
Lifestyle, e.g., diet pattern and physical exercise, considerably impacts aging phenotypes (Woods et al., 2012; Akbaraly et al., 2013), and indeed, caloric restriction has a preventive effect on age-related chronic diseases (Heilbronn and Ravussin, 2003) and accelerated aging (Vermeij et al., 2016). For example, MRI experiments show that caloric restriction can attenuate age-related deterioration in white matter (Bendlin et al., 2011). The neuroprotective mechanisms of caloric restriction may include: (1) upregulation of BDNF (Stranahan et al., 2009) and heat shock proteins, such as HSP70 (Yu and Mattson, 1999); (2) increased neuronal resistance to excitotoxic damage (Bruce-Keller et al., 1999); and (3) inhibition of the increase of age-related lipid peroxidation (Sohal and Weindruch, 1996; Sharma and Kaur, 2005). Besides reduced intake of calories, there is also growing evidence suggesting that physical exercise induces loss of adipose tissue, decreases the expression of systemic markers of inflammation (Woods et al., 2012), upregulates the level of BDNF (Stranahan et al., 2009), and increases hippocampal neurogenesis (Van Praag et al., 1999; Van der Borght et al., 2009).
The dynamic communication between microglia and all other cell types in the CNS helps them perform both their immune and non-immune functions under homeostasis. Moreover, a variety of activation states has been described for microglia upon disease and injury, where the context dictates whether the outcome for the CNS is tissue-supportive or detrimental (Li and Barres, 2017). Although the knowledge of microglia responses to CNS disease is rapidly increasing, the function and phenotype of microglia under conditions of caloric restriction and physical exercise are still poorly understood. Hence, the aim of this study was to investigate the effects of diet, aging, caloric restriction, and physical exercise on mouse microglia in a basal and inflammatory state induced by LPS. In different regions of the brain, microglia were characterized according to their gene expression pattern, expression of phagocytosis markers and morphology.
All adult mice of different ages were used and kept under specified pathogen-free conditions on a 12-h light/dark cycle and lights switched on at 7:00 am. Animals were sacrificed in the morning, between 9:00 am and 11:00 am. Details on experimental design per mouse strain are described below and in Table 1 (i.e., animal strain, supplier, age, exercise, and diet). Throughout the experiments, mice were kept under close observation and regularly weighted. All animal work was approved by the Animal Ethics Committee of University of Groningen, and adhered to the European Directive (2010/63/EU) on the protection of animals used for scientific purposes.
C57BL/6 OlaHsd mice were obtained from Harlan and subsequently bred in-house at the University of Groningen.
Male C57BL/6 OlaHsd mice were subjected to both low-fat (LFD; 6.5% fat, ab Diets 2141 AM-II, Supplementary Data Sheet 1) and high-fat (HFD; 42% fat, ab Diets 4031.09, Supplementary Data Sheet 1) diets. The mice were exposed to the different diets and/or restriction from weaning until sacrifice. Weaning was performed at day 28 (day 0 was day of birth), after which mice were individually housed with ad libitum access to food and water. For the caloric restriction group, mice were exposed to 40% caloric restriction. The restricted mice received their food 3 h before lights were turned off (7:00 pm); they consumed the food within these 3 h, mostly faster.
Mice had free access to the running wheels. Voluntary wheel-running activity was recorded throughout the lifetime of the mice. The passing of a magnet embedded in the running wheel past the sensor on the cage would signal a wheel revolution. These revolutions were collected continuously and stored in 1 min bins by a Circadian Activity Monitor System (CAMS, by H. M. Cooper and J. A. Cooper, INSERM U846, Department of Chronobiology, Bon, France). Raw data were imported into a custom-made excel macro package (ACTOVIEW, C. K. Mulder, Department of Molecular Neurobiology, Groningen, Netherlands). Initial imports condensed the data to 60-min bins, allowing for easy visual inspection of long-term recordings.
For the cohorts of C57BL/6J animals from Charles River, the mice were treated as described previously (van der Heijden et al., 2015a). Male C57BL/6J mice at the age of 6 weeks (20 ± 2 g) were individually housed with ad libitum access to food and water. After arrival, all mice received a low-fat control diet (LFD; 10% lard; Research Diets Inc., New Brunswick, NJ, United States; D12450HY, Supplementary Data Sheet 1) for 6 weeks after which mice were either switched to a HFD (45% lard; Research Diets; D12451, Supplementary Data Sheet 1) or kept on the low-fat control diet. All animals were kept on their respective diets until time of sacrifice.
High-fat diet and LFD mice (6 months) were injected intraperitoneally (i.p.) with 1 mg/kg LPS (Sigma-Aldrich, L4391) or PBS. After 3 h, animals were anesthetized and perfused with 0.9% saline. The brains were sagittally separated and used for two purposes: (1) from one half, microglia were acutely isolated and the RNA extracted (n = 5); (2) from the other half, the hypothalamus was dissected and RNA isolated (n = 5).
Microglia were isolated as described in Galatro et al. (2017b) Brain tissue (n = 5) was mechanically homogenized in HBSS with 0.6% glucose and 7.5 mM HEPES followed by centrifugation at 220 RCF, 4°C for 10 min. In order to remove myelin, the pellet was re-suspended in a mixture of 22% Percoll (GE Healthcare, 17-0891-01) and 77% myelin gradient buffer (5.6 mM NaH2PO4.2H2O, 20 mM Na2HPO4.2H2O, 140 mM NaCl, 5.4 mM KCl, 11 mM glucose) and 40 mM NaCl, and centrifuged for 20 min at 950 RCF at 4°C. The remaining cell pellet was incubated with phycoerythrin (PE)-coupled rat anti-mouse CD11b (Clone M1/70, eBiosciences), FITC-coupled rat anti-mouse CD45 (Clone 30-F11, eBiosciences). Forward and side scatter parameters were used to identify single cells and the exclusion of DAPI was used to select live cells. Microglia were defined as CD11bposCD45intDAPIneg and sorted on a BD FACSAria II (BD Biosciences).
Primary microglia cultures were prepared from brains of postnatal day-1 wild-type male C57Bl/6J mice as previously described (Schaafsma et al., 2015). Briefly, after the removal of the meninges and brain stem, the brains were triturated and washed in medium A (HBSS with 0.6% glucose [D-(+)-glucose solution, Sigma, G8769], 15 mM HEPES buffer (Lonza BE17-737E), 1% penicillin/streptomycin). The minced brains were incubated in 2.5% trypsin at 37°C for 20 min. The enzymatic reaction was stopped by adding trypsin inhibitor medium, followed by washing with medium containing medium A, 10% FCS, and 0.5 μg/ml DNaseI. Cells were triturated using a glass pipette in 25 ml normal medium (Dulbecco’s Modified Eagle Medium, Lonza, BE12-707F, 10% FCS, 1 mM sodium pyruvate, Gibco, 11360-070, and 1% penicillin/streptomycin), and centrifuged at 960 rpm at 12°C for 12 min. The supernatant was removed, and then the pellet was resuspended and plated as 1.5 brains per T75 culture flask in DMEM supplemented with 10% FCS. After 7 days of culture, 5 ml L929 fibroblast-conditioned medium was added to 10 ml culture medium per flask to stimulate microglia proliferation. After around 10 days of culture, astrocytes reached 100% confluence, and microglia were harvested at the 14th day by orbital shaking for 1 h at 150 rpm at 37°C. Microglia were seeded into 3.5 cm 6-well plates, and cultured in the above-mentioned normal medium and medium collected from the mixed glial cultures in a ratio of 1:1 at 37°C with 5% CO2. Male and female pups were separated based on anogenital distance. The sex of the pups used for the cultures was confirmed by PCR on genomic DNA using primers for gene loci on X- and Y-chromosomes.
Cultured microglia were stimulated with recombinant mouse leptin (1 μg/ml, R&D, CF 498-OB-01M) for 24 h, followed by 100 ng/ml LPS stimulation for 3 h.
RNA from sorted microglia and cultured microglia was extracted using the RNeasy Plus Micro Kit (Qiagen, 74034) and RNA from hypothalamus was extracted using RNeasy Plus Mini Kit (Qiagen, 74134). After RNA isolation, reverse-transcription PCR (RT-PCR) was performed using Applied Biosystems Gene Amp 9700 thermal cycler, and quantitative PCR was performed as previously described (Raj et al., 2014). See primer information in Supplementary Table 1.
Animals (details are shown in Table 1) were sacrificed and transcardially perfused with saline. Brains were separated, then snap-frozen in liquid nitrogen or fixed in 4% paraformaldehyde (PFA) in PBS fixed for 1 day (details are shown in Table 1), dehydrated with 25% sucrose in PBS overnight at 4°C, and then frozen at -50°C. All samples were stored at -80°C.
Brain sections (30 μm) from 4% PFA-fixed mouse brains were pre-incubated in 0.3% H2O2 at room temperature (RT) for 30 min, and then in 10% serum at RT for 1 h. Sections were incubated with the primary antibody at 4°C overnight: Iba1 (1:1000, WAKO, 019-19741) or Mac-2 (1:1000, Cedarlane, CL8942AP). After PBS washing, sections were incubated with the secondary antibody: biotinylated goat anti-rabbit IgG (1:400, Vector Laboratories, BA-1000) or biotinylated rabbit anti-rat IgG (1:400, Vector Laboratories, BA-4001) at RT for 1 h, then with avidin-biotin-peroxidase complex (Vector Laboratories, PK-6100) at RT for 30 min, and finally visualized with 3, 3′-diaminobenzidine (DAB, Sigma, D-5637).
Brain sections (14 μm) from snap-frozen mouse brains were acetone-fixed for 10 min, pre-incubated in Peroxidase Blocking Reagent (DAKO, K4009) for 15 min, and blocked in 5% fetal bovine serum for 30 min (RT). Sections were incubated with primary antibodies at RT for 2 h: Dectin-1 (1:100, AbDSerotec, MCA2289) or CD16/CD32 (1:100, eBioscience, 14-0161-82). Next, sections were incubated with unconjugated rabbit anti-rat IgG (Vector Laboratories, AI-4001) at RT for 1 h. Then sections were incubated with labeled polymer-HRP anti-rabbit (DAKO, K4009) at RT for 30 min. The complex was visualized after 10 min of incubation with 3-amino-9-ethylcarbazole (AEC) substrate-chromogen solution (DAKO, K4009) and counterstained with hematoxylin. The visualization and counterstaining were both done at RT.
Immunohistochemically stained sections were imaged using a Hamamatsu Nanozoomer (Hamamatsu Photonics). The images of AEC-stained sections (Figure 5) were analyzed with the positive pixel count algorithm (Imagescope). 5–10 images (20× magnification) from the internal capsule were quantified for each sample (n = 4–7). The average number of positive pixels was compared between groups. The images of DAB-stained sections (Figure 1) were analyzed using Image J. Microglia cell numbers were determined by binary thresholding in ImageJ followed by ‘analyze particles’ (pixels 10–infinity) and ‘cell count’ (n = 3–4 mice/condition).
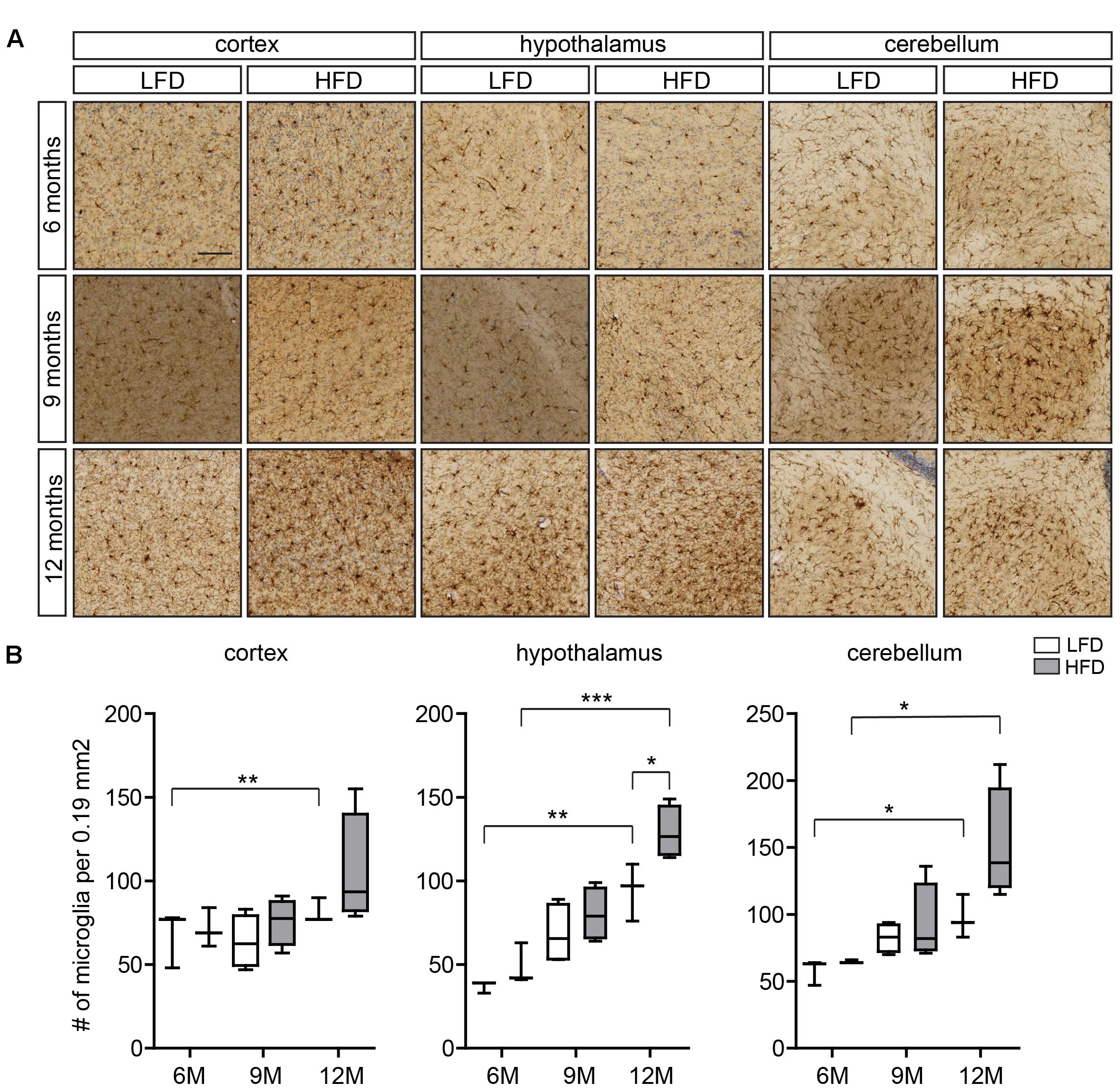
FIGURE 1. Microglia number in the cortex, hypothalamus, and cerebellum of HFD and LFD mice. (A) Representative images of brain sections of HFD and LFD mice at the indicated ages immunostained for Iba1, and counterstained with Cresyl violet. Iba1+ microglia in the cortex, hypothalamus, and cerebellum were analyzed. (B) The number of microglia was compared between HFD animals and LFD animals at the indicated ages. In both HFD and LFD mice, the number of microglia increased during aging. The number of microglia was significantly increased in the hypothalamus of 12-month-old HFD animals (n = 3 mice, gray boxes depict HFD samples, white boxes depict LFD samples, Student’s t-test, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001). Scale bar: (A) = 100 μm.
Data were analyzed using GraphPad Prism software. For comparison of two different groups, a two-tailed Student’s t-test was used. Comparisons of multiple groups were analyzed by one-way analysis of variance (ANOVA) followed by post hoc analysis using Bonferroni’s multiple-comparison test. Asterisks indicate: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Here we evaluated regional differences in the number of microglia of 6-, 9-, and 12-month-old HFD (45% fat) and LFD (10% fat) mice using Iba1 immunohistochemistry (Figure 1A). Microglia numbers were determined in the cortex, hypothalamus, and cerebellum (Figure 1B). As described previously, Iba1 reactivity of microglia in the white matter of the CNS increases with age (Raj et al., 2017). In the cortex, no significant differences in the number of microglia between LFD and HFD mice were detected at these respective ages (Figure 1B). In the hypothalamus, microglia numbers were significantly higher in both HFD and LFD mice at 12 months of age compared to 6-month-old mice; and at 12 months microglia numbers were significantly increased in HFD compared to LFD mice (Figure 1B). In the cerebellum, microglia numbers were significantly increased in 12-month-old mice compared to 6-month-old mice for both HFD and LFD mice (Figure 1B).
To assess if HFD affected the microglia response to an inflammatory challenge, microglia were isolated from the brain of 6-month-old LFD and HFD mice, 3 h after an intraperitoneal (i.p.) injection with PBS or LPS (1 mg/kg). The mRNA expression levels of pro-inflammatory, phagocytic, and metabolic genes were determined. The basal expression of pro-inflammatory genes Il-1β, Tnf-α, and Il-6 and other immune-related genes Spp1 and Cybb was not significantly different between HFD and LFD microglia. As expected, i.p. injection with LPS resulted in a significant increase in proinfammatory and immune gene expression. However, this response was not significantly different between HFD and LFD microglia (Figures 2A,B). Expression levels of the phagocytic markers Axl and Lgals3 (Mac-2) were induced by LPS but not significantly different between LFD and HFD conditions (Figure 2C). Apoe, a gene involved in lipid metabolism, was induced by LPS, in both LFD and HFD microglia (Figure 2D). Collectively, these data indicate that a 6-month HFD did not result in generalized microglial activation or an altered responsiveness to an inflammatory stimulus such as LPS.
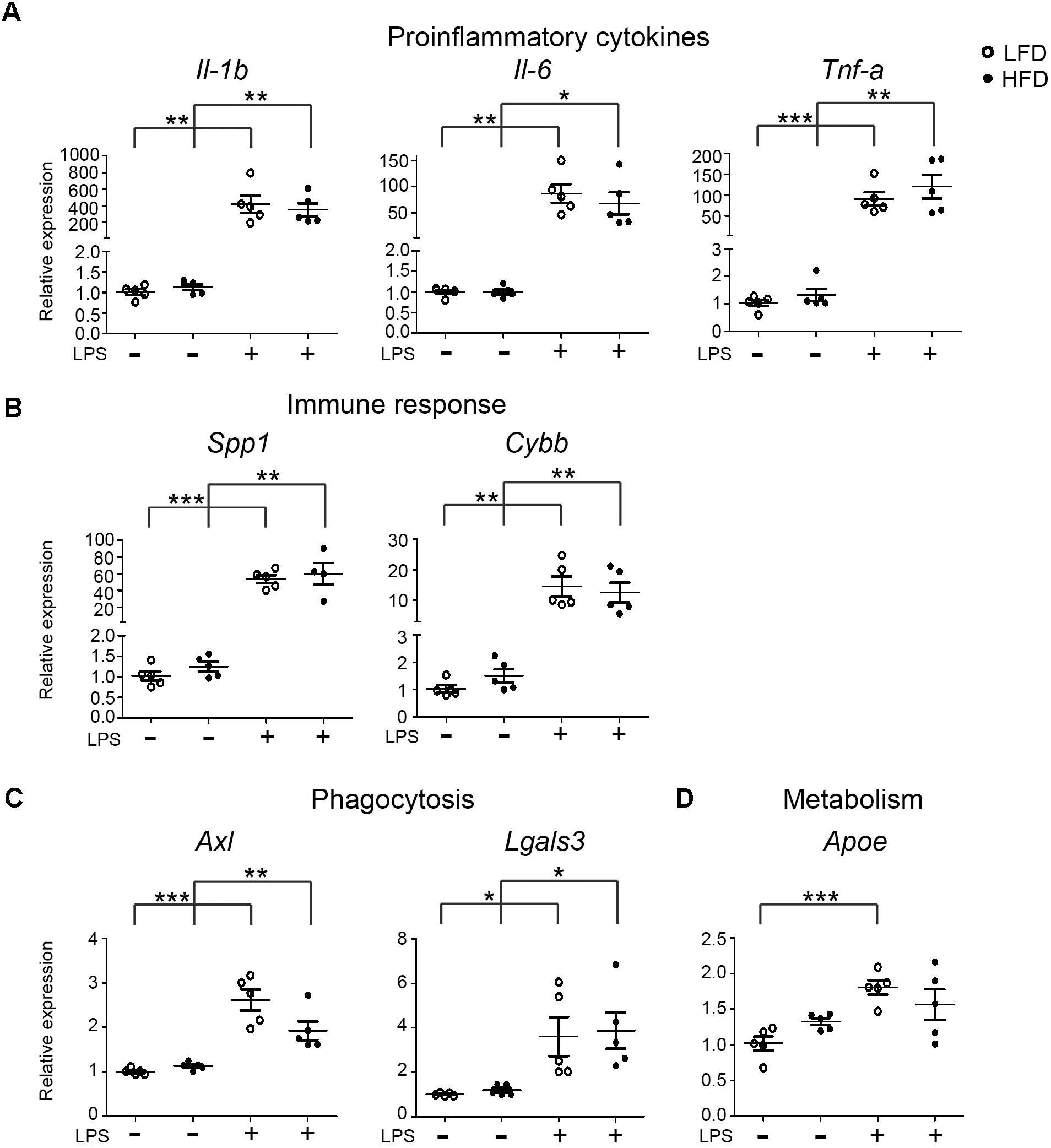
FIGURE 2. Expression of inflammatory, phagocytic and metabolism genes in LFD and HFD microglia. 6-month-old HFD and LFD animals were i.p. injected with LPS or PBS. Microglia were FACS isolated and RNA was extracted and quantified using RT-qPCR. RNA expression levels were normalized to Hmbs levels as in internal control and the expression levels in PBS-injected LFD mice were set at 1. Gene expression levels were compared between both PBS- and LPS-injected mice and between HFD and LFD animals. (A) proinflammatory cytokines (Il-1β, Il-6, and Tnf-α), (B) immune response (Spp1 and Cybb), (C) phagocytosis (Axl, Lgals3), and (D) metabolism (Apoe) genes were significantly upregulated after LPS injection, but no significant difference between HFD+LPS and LFD+LPS samples was detected. Open circles depict LFD and closed circles depict HFD samples (n = 5 mice, mean ± SEM is depicted, Student’s t-test, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001).
In microglia isolated from total mouse brain, no significant changes in LPS-induced inflammatory responses were observed under LFD and HFD conditions. However, it is possible that regional effects of LFD/HFD on microglia were obscured when analyzing total brain microglia. The hypothalamus for example is substantially affected by HFD (Thaler et al., 2012; Gao et al., 2014). Hence, gene-expression levels of microglia-associated genes were determined in the hypothalamus of 6-month-old LFD and HFD mice, which received an i.p. injection of PBS or LPS (1 mg/kg). LPS induced a significant increase in expression of the proinflammatory cytokine genes Il-1β and Tnf-α in the hypothalamus of both HFD and LFD mice, but their expression was not significantly different between HFD and LFD mice (Figure 3A). With the exception of Ifitm2, no significant effect of HFD on the expression of several immune-response genes, CD44, Cryab, Csf-1, Sirpa, Spp1, and Ifitm3 was detected. LPS significantly induced CD44, Csf-1, and Ifitm3 expression levels in both LFD and HFD hypothalamus (Figure 3B). No substantial differences in the hypothalamus were observed in the expression of phagocytic markers CD36, Axl, Clec7a (Dectin-1), and Lgals3 between LFD and HFD hypothalamus was detected. LPS significantly increased expression of CD36, Axl, and Lgals3 in LFD and HFD hypothalamus (Figure 3C). No significant effect of HFD on Apoe, Lpl, and Lrp12 was detected (Figure 3D). In summary, these data indicate that LPS treatment resulted in increased expression of a range of immune-, phagocytosis-, and metabolism-associated genes in the hypothamalus, but no significant differences were detected between LFD and HFD samples.
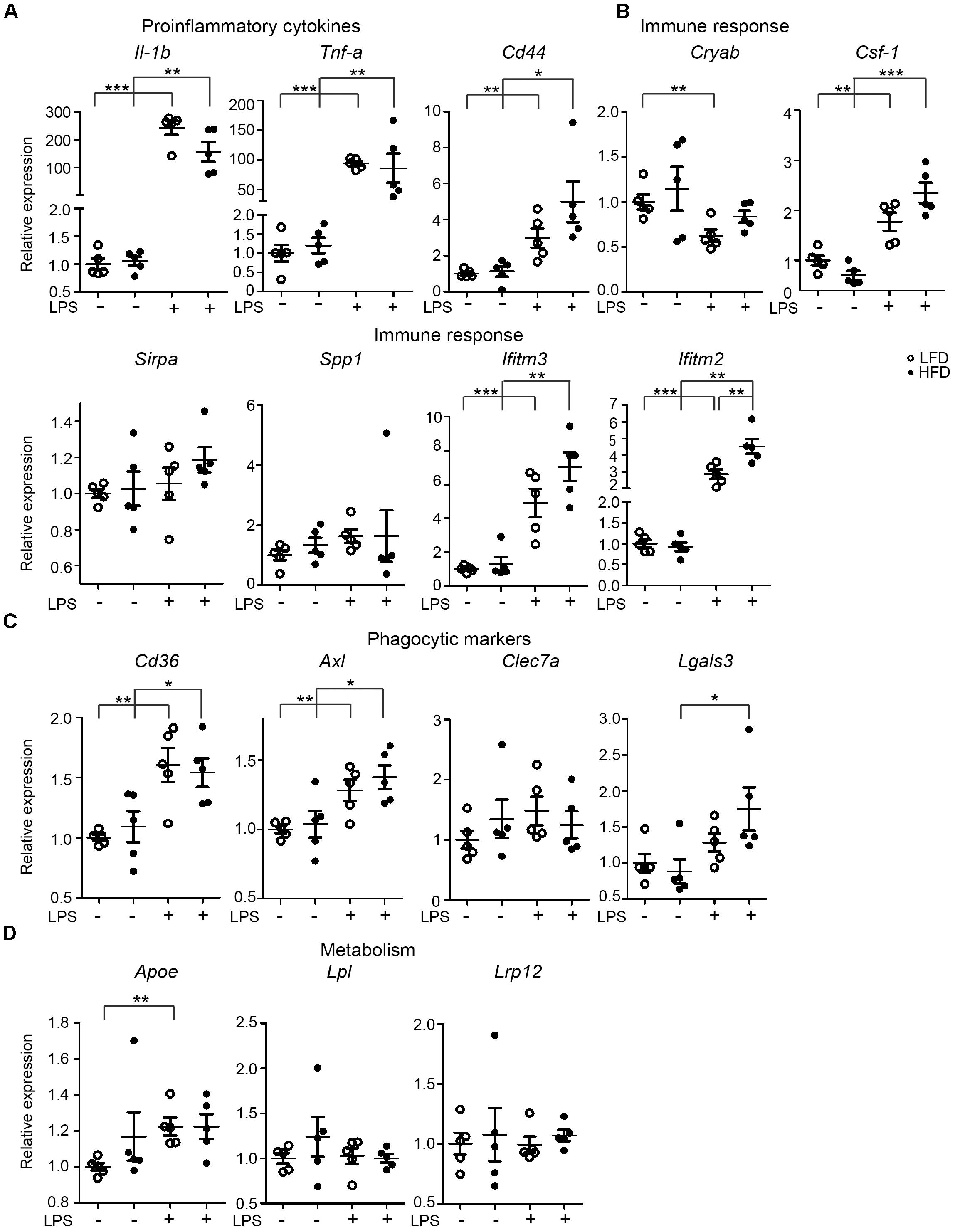
FIGURE 3. Expression of immune-related genes in the hypothalamus of PBS/LPS-treated LFD and HFD mice. 6-month-old HFD and LFD animals were i.p. injected with LPS or PBS, total RNA was extracted from the hypothalamus and analyzed using RT-qPCR. The gene expression of (A) proinflammatory cytokines (Il-1β and Tnf-α), (B) genes relating to immune response (CD44, Cryab, Sirpa, Spp1, Ifitm3, and Ifitm2), (C) phagocytic markers (Axl, Lgals3, CD36, and Clec7a), and (D) genes relating to lipid metabolism (Apoe, Csf-1, Lpl, and Lrp12) were compared between HFD and LFD animals, but also between samples with or without LPS injection. Open circles depict LFD and closed circles depict HFD samples (n = 5 mice, mean ± SEM is depicted, Student’s t-test, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001).
The effects of HFD or LFD on serum leptin levels and body weight in the C57BL/6J mouse cohort were reported earlier (van der Heijden et al., 2015a,b). In mice on a HFD (45% fat), leptin plasma levels strongly increased from 10 to 40 ng/ml, peaked around 28 weeks and remained elevated. Previous data indicated that in vitro pretreatment of rat microglia with leptin for 24 h, was capable of potentiating a subsequent LPS response, resulting in approximately 2-fold higher Il-1β release (Pinteaux et al., 2007; Lafrance et al., 2010). However, a concomitant increase in Il-1β mRNA was not detected (Lafrance et al., 2010). As described above, we did not observe an effect on LPS responsiveness in vivo. Therefore, we next evaluated if leptin (1 μg/ml) altered the inflammatory response of male primary microglia in vitro. In primary microglia, LPS induced a significant upregulation of the pro-inflammatory genes Il-1β and Tnf-α. However, in agreement with the data from rat microglia (Lafrance et al., 2010), pretreatment with leptin for 24 h did not alter the microglia response to LPS at the mRNA level (Figure 4). These data show that leptin preconditioning of microglia in vitro did not result in increased inflammatory gene expression nor in enhanced LPS sensitivity, which is in contrast to aging, where this microglial hyperreactivity was observed (Henry et al., 2009).
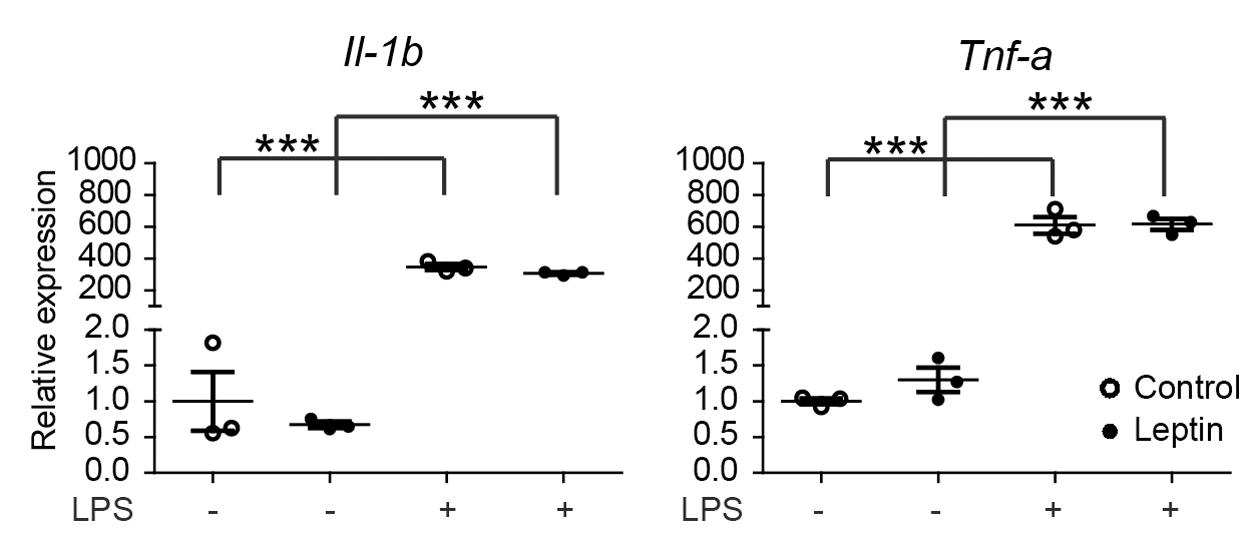
FIGURE 4. Leptin does not alter microglia responsiveness to LPS. Male primary mouse microglia were incubated with leptin (1 μg/ml) for 24 h, followed by an LPS stimulation (100 ng/ml) for 3 h. Gene expression of proinflammatory cytokines (Il-1β and Tnf-α) was compared between control and leptin-treated cells with or without LPS stimulation. Leptin treatment had no significant effect on the LPS-induced expression of proinflammatory cytokines. Open circles depict untreated and closed circles depict leptin-treated microglia (n = 3 microglia cultures, mean ± SEM is depicted, one-way ANOVA with Bonferroni post hoc comparison, ∗∗∗p < 0.001).
Increased inflammation in the aging human brain is most predominantly observed in the white matter (Gunning-Dixon et al., 2009). However, the effect of long-term diet pattern and exercise on the aging brain and white matter inflammation has not been assessed. We therefore evaluated microglia numbers and activation in white matter regions in 6- and 24-month-old LFD and HFD mice. In addition, 24-month-old mice were subjected either to a lifelong voluntary running wheel paradigm or lifelong caloric restriction (Figure 5). At the age of 24 months, both HFD and LFD mice showed increased expression of Iba1. Clustering of microglia was also observed in fimbriae (Figures 5A,B). Remarkably, in LFD mice, caloric restriction completely prevented this increase in Iba1 expression, whereas in HFD mice, caloric restriction had little effect (Figure 5C). Microglial expression of the phagocytic marker Mac-2 (Lgals3) in the fimbria was increased in both 24-month-old HFD and LFD mice when compared to 6-month-old mice (Figures 5A,B). Similar to Iba1, the expression of the phagocytic marker Mac-2 was completely absent in 24-month-old LFD mice with caloric restriction, indicating that LFD plus caloric restriction reduces microglia activation (Figure 5C). In another prominent white-matter brain region, the internal capsule, we quantified the expression of phagocytic markers Dectin-1 and CD16/CD32 in 24-month-old HFD- and LFD mice. These mice either had access to a running wheel or were subjected to caloric restriction (Figures 5D,E). Remarkably, significant expression of Dectin-1 and CD16/CD32 was observed in the white matter bundle in all experimental groups except in caloric-restricted LFD mice (Figures 5D,E).
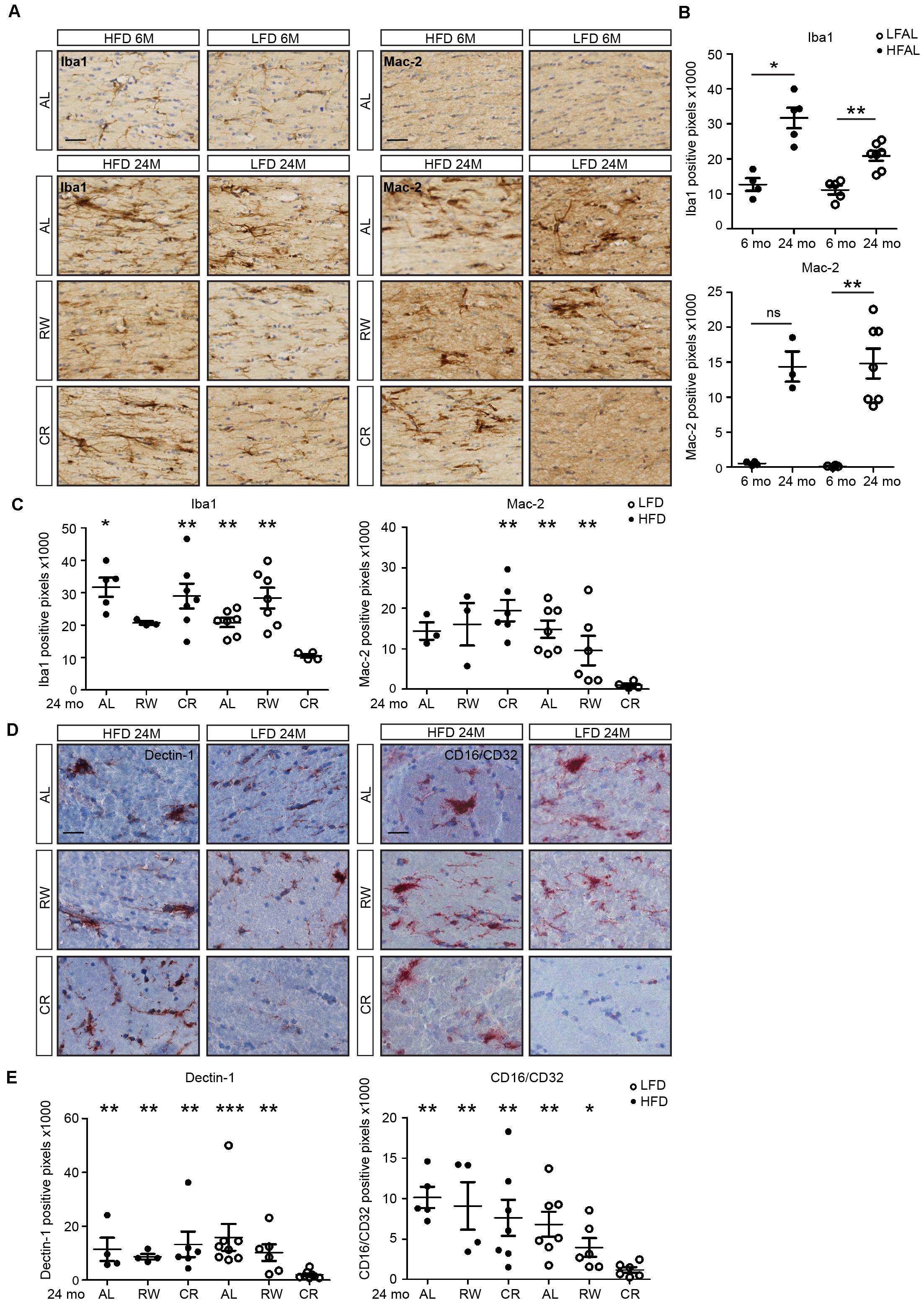
FIGURE 5. Low-fat diet with caloric restriction reduces white matter microglia activation during aging. (A) Brain sections of 6- and 24-month-old mice on different diets and regimes were immunostained for Iba1 and Mac-2, and counterstained with cresyl violet. LFAL, low-fat diet ad libitum; HFAL, high-fat diet ad libitum; LFRW, low-fat diet with running wheel; HFRW, high-fat diet with running wheel; LFCR, low-fat diet with caloric restriction; HFCR, high-fat diet with caloric restriction. (B) Quantification of conditions depicted in the top panel of (A). The expression of Iba1 and Mac-2 increased during aging in both HFAL and LFAL groups (n = 3–7 mice, mean ± SEM is depicted, Mann–Whitney test, ns, not significant, ∗p < 0.05, ∗∗p < 0.01). (C) Quantification of conditions depicted in the bottom panel of (A). Compared to the LFCR group, the expression of Iba1 was significantly higher in HFAL, HFCR, LFAL, and LFRW mice. Mac-2 was significantly higher expressed in HFCR, LFAL, and LFRW mice (n = 3–7, Mean ± SEM, Mann–Whitney test, ∗p < 0.05, ∗∗p < 0.01). (D) Tissues from animals on different diets and regimes (LFAL, HFAL, LFRW, HFRW, LFCR, and HFCR) were immunostained for Dectin 1 and CD16/CD32 and counterstained with hematoxylin. (E) Dectin 1 and CD16/CD32 were significantly lower expressed in LFCR mice exclusively (n = 4–7 mice, mean ± SEM is depicted, Mann–Whitney test, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001). Scale bar: (A,D) = 40 μm.
In this study, we observed a marked effect of aging on microglia numbers in the hypothalamus and cerebellum. In HFD mice, the cell number of microglia increased compared to their age-matched LFD littermates, especially at the age of 9 and 12 months. These results are in accordance with earlier studies. An increase in microglia cell number has been observed previously in the hypothalamus of mice subjected to 20-week HFD (Valdearcos et al., 2014; Baufeld et al., 2016). The expression of inflammatory genes increased significantly in the cerebellum after a 16-week HFD, while those genes in cortex remained unchanged (Guillemot-Legris et al., 2016).
Previous data has indicated that neuroinflammation can be influenced by caloric intake, where not only fat content, but also the source of the fat and carbohydrate content have been reported to influence microglia in the hypothalamus (Maric et al., 2014; Valdearcos et al., 2014; André et al., 2017; Gao et al., 2017). In particular, saturated fatty acids have been connected with microglia-induced inflammation (Valdearcos et al., 2014). More recently, it was shown that carbohydrates are mainly responsible for microgliosis in the hypothalamus of mice on a diet with high caloric content (Gao et al., 2017). However, the fact that we observed increased microglia numbers in mice subjected to caloric restriction in combination with HFD suggests that under our experimental conditions, it is mainly the fat content and/or composition that dictated microglia activation.
Basal expression levels of pro-inflammatory, phagocytosis, immune response, and energy metabolism markers did not differ in microglia isolated from the total brain tissue of HFD and LFD mice. Furthermore, microglial responsiveness to LPS did not differ between HFD and LFD mice, in agreement with earlier results (Baufeld et al., 2016). In rats on an 8-week high-saturated-fat diet, hypothalamic inflammation markers were increased but no effect of diet on the change in core body temperature after a peripheral LPS challenge was observed (Maric et al., 2014).
Previous experiments in HFD mice showed an increased expression of pro-inflammatory cytokines and activation of NF-κB in the hypothalamus (De Souza et al., 2005). Microglia have been proposed to be important players in this inflammatory cascade, perpetuating hypothalamic injury (Zhang et al., 2008; Milanski et al., 2009; Posey et al., 2009; Thaler et al., 2012). Inflammation in the hypothalamus disrupts feeding-related pathways, and promotes insulin and leptin resistance, thereby favoring an elevated body weight (De Souza et al., 2005; Posey et al., 2009; Spencer and Tilbrook, 2011). However, the exact role of microglia in HFD-induced neuroinflammation is still poorly understood. A recent study showed that HFD induced an acute inflammatory response in the hypothalamus after 3 days, but this increased expression of proinflammatory genes was no longer observed after 4 and 8 weeks. In contrast, at these time points a more pronounced anti-inflammatory gene expression profile was observed (Baufeld et al., 2016). However, it has been clearly demonstrated that microglia play critical roles in neuroinflammation in response to HFD. Depletion of microglia resulted in reduced hypothalamic inflammation (Valdearcos et al., 2014) and a similar effect was seen when proliferation of microglia was inhibited (André et al., 2017).
The detrimental effect of HFD on the brain predominantly occurs in the hypothalamus. The hypothalamus has an important role in feeding behavior and neuroendocrine/autonomic outflow, where leptin and insulin play key roles in regulating hunger signals and energy expenditure, mainly by acting through neuronal subpopulations in the arcuate nucleus (Yi et al., 2012). In the hypothalamus, the expression of most genes involved in pro-inflammatory cytokine signaling, immune response, phagocytosis, and metabolism in HFD and LFD mice were not significantly different. However, a significant difference was observed in the expression of interferon-induced transmembrane protein 2 (Ifitm2), where HFD mice showed a significant higher expression compared to LFD mice. In addition, the expression of Ifitm3 showed a strong tendency to being increased in HFD mice compared to LFD mice after LPS injection, although the difference did not reach statistical significance. Ifitm proteins are important players in the type I interferon antiviral response. Deletion of Ifitm genes in mice results in an obese phenotype and metabolic dysfunction. The microglia in the hypothalamus of IfitmDel mice have an activated phenotype and an exaggerated pro-inflammatory response to Poly I:C, a viral mimetic and TLR3 agonist (Wee et al., 2015).
Leptin, a satiety hormone secreted by adipose tissue, is essential in regulating body weight and can enter the brain (Rivest, 2002). Since leptin is a prominent factor in the detrimental effects of HFD (Lin et al., 2000), the effect of leptin on microglia was analyzed both in vivo and in vitro. We observed that high peripheral levels of leptin in HFD mice were not accompanied by an altered responsiveness of microglia to a systemic inflammatory stimulus in comparison to microglia from LFD mice. Previous studies reported that in obesity, the ability of leptin to cross the blood–brain barrier is reduced (Burguera et al., 2000; Banks et al., 2006). In order to test the effect of leptin on microglia directly, we pretreated primary microglia with leptin, which did not change basal inflammatory gene-expression levels or the response to an LPS challenge. In summary, neither HFD nor leptin induced changes in the inflammatory responses of microglia in aging mice.
Although no major differences were observed in cytokine expression levels in microglia isolated from total brain tissue and hypothalamic tissue from HFD and LFD mice, we observed very profound differences in neuroinflammation and phagocytic markers in prominent white matter bundles between HFD and LFD mice at different ages in combination with caloric restriction. Previous studies showed that dietary intervention, such as caloric restriction, slows down the aging process (Johnson et al., 2006; Colman et al., 2014). Indeed a lifelong reduction in caloric intake reduces the oxidative damage in the brain (Mattson et al., 2001), preserves long-term potentiation of synaptic transmission (Hori et al., 1992), and ameliorates cognitive decline (Pitsikas and Algeri, 1992). In peripheral nerves, caloric restriction maintains the nerve structure, improves nerve function, and attenuates the age-associated reduction of myelin proteins and widening of the nodes of Ranvier (Rangaraju et al., 2009). The underlying mechanisms might be the expression of protein chaperones and activation of the autophagy-lysosomal pathway (Rangaraju et al., 2009). Here, the reduction of phagocytic markers in white matter microglia of LFD with caloric restriction might reflect decreased axonal stress in the CNS. However, the pathology of axons at LFD vs. HFD combined with caloric restriction conditions has not been examined yet. Physical exercise, which was provided by a running wheel in our study, reduced the risk of cardiovascular disease, type 2 diabetes, obesity, stroke, and is protective against age-related cognitive decline (McKee et al., 2014). In our study, the expression of Mac-2 and Dectin-1 remained high in the running-wheel HFD group, only the expression of CD16/CD32 was partially decreased in LFD mice in the running-wheel experiment. However, the expression of CD16/CD32 was still significantly higher in HFD mice than in LFD mice with caloric restriction. Although previous studies reported that physical exercise plays an equivalent role in terms of energy balance (Redman et al., 2007) and regulating insulin resistance (Coker et al., 2009), our data suggest that in mice, caloric restriction might be a more effective intervention in protecting white matter structures than physical exercise as expression of markers of microglia activation and phagocytosis (CD16/CD32, Mac-2, and Dectin-1) was notably absent in the white matter of caloric restriction LFD mice.
In this study, we show that HFD increased the number of microglia in the hypothalamus and both number and soma size of microglia were increased in the cerebellum during aging in HFD mice. Under basal- or LPS-induced inflammatory conditions, gene expression analysis of the total brain microglia population or hypothalamus tissue showed similar findings in HFD and LFD mice. Caloric restriction in LFD mice prevented the increased expression of phagocytic markers in white matter microglia with aging, and this protective effect of caloric restriction was not observed in HFD mice. Because running wheel access did not affect white matter microglia activation in either diet, dietary fat as well as caloric content may play an important role in the inflammatory process in brain aging.
ZY and DR designed and conducted the RNA experiments. ZY conducted the immunohistochemistry experiments and finalized the figures. ZY and WS analyzed the data and wrote the manuscript. RvdH, AR, JM, PH, and GvD provided the animals. XZ and WS conducted the in vitro leptin experiments. NB and C-XY provided technical support. JL, SK, EB, and BE supervised, wrote the manuscript, and provided funding for the study.
This work was supported by the China Scholarship Council (Grant No. 201206160050), Graduate School of Medical Sciences, UMCG, Disease models and mechanisms travel fellowship to DR (Grand No. DMMTF-141208).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank Ietje Mantingh-Otter for technical support.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnmol.2018.00065/full#supplementary-material
ARC-ME, arcuate nucleus-median eminence; BDNF, brain-derived neurotrophic factor; CNS, central nervous system; FCS, fetal calf serum; FITC, fluorescein isothiocyanate; HFD, high-fat diet; i.p., intraperitoneal; LFD, low-fat diet; LPS, lipopolysaccharide; MRI, magnetic resonance imaging; PBS, phosphate buffered saline; PE, phycoerythrin; RT-PCR, reverse transcription PCR.
Akbaraly, T., Sabia, S., Hagger-Johnson, G., Tabak, A. G., Shipley, M. J., Jokela, M., et al. (2013). Does overall diet in midlife predict future aging phenotypes? A cohort study. Am. J. Med. 126, 411.e3–419.e3. doi: 10.1016/j.amjmed.2012.10.028
André, C., Guzman-Quevedo, O., Rey, C., Rémus-Borel, J., Clark, S., Castellanos-Jankiewicz, A., et al. (2017). Inhibiting microglia expansion prevents diet-induced hypothalamic and peripheral inflammation. Diabetes 66, 908–919. doi: 10.2337/db16-0586
Banks, W. A., Farr, S. A., and Morley, J. E. (2006). The effects of high fat diets on the blood-brain barrier transport of leptin: Failure or adaptation? Physiol. Behav. 88, 244–248. doi: 10.1016/j.physbeh.2006.05.037
Baufeld, C., Osterloh, A., Prokop, S., Miller, K. R., and Heppner, F. L. (2016). High-fat diet-induced brain region-specific phenotypic spectrum of CNS resident microglia. Acta Neuropathol. 132, 361–375. doi: 10.1007/s00401-016-1595-4
Bendlin, B. B., Canu, E., Willette, A., Kastman, E. K., McLaren, D. G., Kosmatka, K. J., et al. (2011). Effects of aging and calorie restriction on white matter in rhesus macaques. Neurobiol. Aging 32, 2319.e1–2319.e11. doi: 10.1016/j.neurobiolaging.2010.04.008
Bocarsly, M. E., Fasolino, M., Kane, G. A., LaMarca, E. A., Kirschen, G. W., Karatsoreos, I. N., et al. (2015). Obesity diminishes synaptic markers, alters microglial morphology, and impairs cognitive function. Proc. Natl. Acad. Sci. U.S.A. 112, 15731–15736. doi: 10.1073/pnas.1511593112
Bruce-Keller, A. J., Umberger, G., McFall, R., and Mattson, M. P. (1999). Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Ann. Neurol. 45, 8–15. doi: 10.1002/1531-8249(199901)45:1<8::AID-ART4>3.0.CO;2-V
Burguera, B., Couce, M. E., Curran, G. L., Jensen, M. D., Lloyd, R. V., Cleary, M. P., et al. (2000). Obesity is associated with a decreased leptin transport across the blood-brain barrier in rats. Diabetes 49, 1219–1223. doi: 10.2337/diabetes.49.7.1219
Cai, D. (2013). Neuroinflammation and neurodegeneration in overnutrition-induced diseases. Trends Endocrinol. Metab. 24, 40–47. doi: 10.1016/j.tem.2012.11.003
Coker, R. H., Williams, R. H., Yeo, S. E., Kortebein, P. M., Bodenner, D. L., Kern, P. A., et al. (2009). The impact of exercise training compared to caloric restriction on hepatic and peripheral insulin resistance in obesity. J. Clin. Endocrinol. Metab. 94, 4258–4266. doi: 10.1210/jc.2008-2033
Colman, R. J., Beasley, T. M., Kemnitz, J. W., Johnson, S. C., Weindruch, R., and Anderson, R. M. (2014). Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat. Commun. 5:3557. doi: 10.1038/ncomms4557
De Souza, C. T., Araujo, E. P., Bordin, S., Ashimine, R., Zollner, R. L., Boschero, A. C., et al. (2005). Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 146, 4192–4199. doi: 10.1210/en.2004-1520
Eckel, R. H., Kahn, S. E., Ferrannini, E., Goldfine, A. B., Nathan, D. M., Schwartz, M. W., et al. (2011). Obesity and type 2 diabetes: what can be unified and what needs to be individualized? Diabetes Care 34, 1424–1430. doi: 10.2337/dc11-0447
Enriori, P. J., Evans, A. E., Sinnayah, P., Jobst, E. E., Tonelli-Lemos, L., Billes, S. K., et al. (2007). Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 5, 181–194. doi: 10.1016/j.cmet.2007.02.004
Gabriely, I., Ma, H. X., Yang, X. M., Rossetti, L., and Barzilai, N. (2002). Leptin resistance during aging is independent of fat mass. Diabetes 51, 1016–1021. doi: 10.2337/diabetes.51.4.1016
Galatro, T. F., Holtman, I. R., Lerario, A. M., Vainchtein, I. D., Brouwer, N., Sola, P. R., et al. (2017a). Transcriptomic analysis of purified human cortical microglia reveals age-associated changes. Nat. Neurosci. 20, 1162–1171. doi: 10.1038/nn.4597
Galatro, T. F., Vainchtein, I. D., Brouwer, N., Boddeke, E. W., and Eggen, B. J. (2017b). Isolation of microglia and immune infiltrates from mouse and primate central nervous system. Methods Mol. Biol. 1559, 333–342. doi: 10.1007/978-1-4939-6786-5_23
Gao, Y., Bielohuby, M., Fleming, T., Grabner, G. F., Foppen, E., Bernhard, W., et al. (2017). Dietary sugars, not lipids, drive hypothalamic inflammation. Mol. Metab. 6, 897–908. doi: 10.1016/j.molmet.2017.06.008
Gao, Y., Ottaway, N., Schriever, S. C., Legutko, B., García-Cáceres, C., de la Fuente, E., et al. (2014). Hormones and diet, but not body weight, control hypothalamic microglial activity. Glia 62, 17–25. doi: 10.1002/glia.22580
Golay, A., and Bobbioni, E. (1997). The role of dietary fat in obesity. Int. J. Obes. Relat. Metab. Disord. 21(Suppl. 3), S2–S11.
Gross, P. M. (1992). Circumventricular organ capillaries. Prog. Brain Res. 91, 219–233. doi: 10.1016/S0079-6123(08)62338-9
Guillemot-Legris, O., Masquelier, J., Everard, A., Cani, P. D., Alhouayek, M., and Muccioli, G. G. (2016). High-fat diet feeding differentially affects the development of inflammation in the central nervous system. J. Neuroinflammation 13:206. doi: 10.1186/s12974-016-0666-8
Gunning-Dixon, F. M., Brickman, A. M., Cheng, J. C., and Alexopoulos, G. S. (2009). Aging of cerebral white matter: a review of MRI findings. Int. J. Geriatr. Psychiatry 24, 109–117. doi: 10.1002/gps.208
Han, T. S., Tajar, A., and Lean, M. E. J. (2011). Obesity and weight management in the elderly. Br. Med. Bull. 97, 169–196. doi: 10.1093/bmb/ldr002
Heilbronn, L. K., and Ravussin, E. (2003). Calorie restriction and aging: review of the literature and implications for studies in humans. Am. J. Clin. Nutr. 78, 361–369. doi: 10.1093/ajcn/78.3.361
Henry, C. J., Huang, Y., Wynne, A. M., and Godbout, J. P. (2009). Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav. Immun. 23, 309–317. doi: 10.1016/j.bbi.2008.09.002
Hori, N., Hirotsu, I., Davis, P. J., and Carpenter, D. O. (1992). Long-term potentiation is lost in aged rats but preserved by calorie restriction. Neuroreport 3, 1085–1088. doi: 10.1097/00001756-199212000-00013
Johnson, J. B., Laub, D. R., and John, S. (2006). The effect on health of alternate day calorie restriction: eating less and more than needed on alternate days prolongs life. Med. Hypotheses 67, 209–211. doi: 10.1016/j.mehy.2006.01.030
Kälin, S., Heppner, F. L., Bechmann, I., Prinz, M., Tschöp, M. H., and Yi, C.-X. (2015). Hypothalamic innate immune reaction in obesity. Nat. Rev. Endocrinol. 11, 339–351. doi: 10.1038/nrendo.2015.48
Koch, M., Borggrefe, J., Barbaresko, J., Groth, G., Jacobs, G., Siegert, S., et al. (2014). Dietary patterns associated with magnetic resonance imaging-determined liver fat content in a general population study. Am. J. Clin. Nutr. 99, 369–377. doi: 10.3945/ajcn.113.070219
Kotsis, V., Stabouli, S., Papakatsika, S., Rizos, Z., and Parati, G. (2010). Mechanisms of obesity-induced hypertension. Hypertens. Res. 33, 386–393. doi: 10.1038/hr.2010.9
Lafrance, V., Inoue, W., Kan, B., and Luheshi, G. N. (2010). Leptin modulates cell morphology and cytokine release in microglia. Brain Behav. Immun. 24, 358–365. doi: 10.1016/j.bbi.2009.11.003
Li, Q., and Barres, B. A. (2017). Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol. doi: 10.1038/nri.2017.125 [Epub ahead of print].
Lin, S., Thomas, T. C., Storlien, L. H., and Huang, X. F. (2000). Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. Int. J. Obes. Relat. Metab. Disord. 24, 639–646. doi: 10.1038/sj.ijo.0801209
Lumeng, C. N., and Saltiel, A. R. (2011). Inflammatory links between obesity and metabolic disease. J. Clin. Invest. 121, 2111–2117.
Maric, T., Woodside, B., and Luheshi, G. N. (2014). The effects of dietary saturated fat on basal hypothalamic neuroinflammation in rats. Brain Behav. Immun. 36, 35–45. doi: 10.1016/j.bbi.2013.09.011
Mattson, M. P., Duan, W., Lee, J., and Guo, Z. (2001). Suppression of brain aging and neurodegenerative disorders by dietary restriction and environmental enrichment: molecular mechanisms. Mech. Ageing Dev. 122, 757–778. doi: 10.1016/S0047-6374(01)00226-3
McKee, A. C., Daneshvar, D. H., Alvarez, V. E., and Stein, T. D. (2014). The neuropathology of sport. Acta Neuropathol. 127, 29–51. doi: 10.1007/s00401-013-1230-6
Milanski, M., Degasperi, G., Coope, A., Morari, J., Denis, R., Cintra, D. E., et al. (2009). Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J. Neurosci. 29, 359–370. doi: 10.1523/jneurosci.2760-08.2009
Münzberg, H., Flier, J. S., and Bjørbaek, C. (2004). Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 145, 4880–4889. doi: 10.1210/en.2004-0726
Norden, D. M., and Godbout, J. P. (2013). Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol. Appl. Neurobiol. 39, 19–34. doi: 10.1111/j.1365-2990.2012.01306.x
Pinteaux, E., Inoue, W., Schmidt, L., Molina-Holgado, F., Rothwell, N. J., and Luheshi, G. N. (2007). Leptin induces interleukin-1beta release from rat microglial cells through a caspase 1 independent mechanism. J. Neurochem. 102, 826–833. doi: 10.1111/j.1471-4159.2007.04559.x
Pitsikas, N., and Algeri, S. (1992). Deterioration of spatial and nonspatial reference and working memory in aged rats: protective effect of life-long calorie restriction. Neurobiol. Aging 13, 369–373. doi: 10.1016/0197-4580(92)90110-J
Pocai, A., Lam, T. K. T., Gutierrez-Juarez, R., Obici, S., Schwartz, G. J., Bryan, J., et al. (2005). Hypothalamic K(ATP) channels control hepatic glucose production. Nature 434, 1026–1031. doi: 10.1038/nature03439
Poirier, P., Giles, T. D., Bray, G. A., Hong, Y., Stern, J. S., Pi-Sunyer, F. X., et al. (2006). Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American heart association scientific statement on obesity and heart disease from the obesity committee of the council on nutrition, physical activity, and metabolism. Circulation 113, 898–918. doi: 10.1161/CIRCULATIONAHA.106.171016
Posey, K. A., Clegg, D. J., Printz, R. L., Byun, J., Morton, G. J., Vivekanandan-giri, A., et al. (2009). Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am. J. Physiol. Metab. 296, E1003–E1012. doi: 10.1152/ajpendo.90377.2008
Raj, D., Yin, Z., Breur, M., Doorduin, J., Holtman, I. R., Olah, M., et al. (2017). Increased white matter inflammation in aging- and Alzheimer’s disease Brain. Front. Mol. Neurosci. 10:206. doi: 10.3389/fnmol.2017.00206
Raj, D. D., Jaarsma, D., Holtman, I. R., Olah, M., Ferreira, F. M., Schaafsma, W., et al. (2014). Priming of microglia in a DNA-repair deficient model of accelerated aging. Neurobiol. Aging 35, 2147–2160. doi: 10.1016/j.neurobiolaging.2014.03.025
Rangaraju, S., Hankins, D., Madorsky, I., Madorsky, E., Lee, W. H., Carter, C. S., et al. (2009). Molecular architecture of myelinated peripheral nerves is supported by calorie restriction with aging. Aging Cell 8, 178–191. doi: 10.1111/j.1474-9726.2009.00460.x
Redman, L. M., Heilbronn, L. K., Martin, C. K., Alfonso, A., Smith, S. R., Ravussin, E., et al. (2007). Effect of calorie restriction with or without exercise on body composition and fat distribution. J. Clin. Endocrinol. Metab. 92, 865–872. doi: 10.1210/jc.2006-2184
Rivest, S. (2002). Editorial: Does circulating leptin have the ability to cross the blood-brain barrier and target neurons directly? Endocrinology 143, 3211–3213. doi: 10.1210/en.2002-220655
Rosenbaum, M., and Leibel, R. L. (2010). Adaptive thermogenesis in humans. Int. J. Obes. 34, S47–S55. doi: 10.1038/ijo.2010.184
Sabia, S., Kivimaki, M., Shipley, M. J., Marmot, M. G., and Singh-Manoux, A. (2009). Body mass index over the adult life course and cognition in late midlife: the Whitehall II Cohort study. Am. J. Clin. Nutr. 89, 601–607. doi: 10.3945/ajcn.2008.26482
Schaafsma, W., Zhang, X., van Zomeren, K. C., Jacobs, S., Georgieva, P. B., Wolf, S. A., et al. (2015). Long-lasting pro-inflammatory suppression of microglia by LPS-preconditioning is mediated by RelB-dependent epigenetic silencing. Brain Behav. Immun. 48, 205–221. doi: 10.1016/j.bbi.2015.03.013
Sharma, S., and Kaur, G. (2005). Neuroprotective potential of dietary restriction against kainate-induced excitotoxicity in adult male Wistar rats. Brain Res. Bull. 67, 482–491. doi: 10.1016/j.brainresbull.2005.07.015
Singh-Manoux, A., Czernichow, S., Elbaz, A., Dugravot, A., Sabia, S., Hagger-Johnson, G., et al. (2012). Obesity phenotypes in midlife and cognition in early old age: the Whitehall II cohort study. Neurology 79, 755–762. doi: 10.1212/wnl.0b013e3182661f63
Sohal, R. S., and Weindruch, R. (1996). Oxidative stress, caloric restriction, and aging. Science 273, 59–63. doi: 10.1126/science.273.5271.59
Spencer, S. J., and Tilbrook, A. (2011). The glucocorticoid contribution to obesity. Stress 14, 233–246. doi: 10.3109/10253890.2010.534831
Stranahan, A. M., Lee, K., Martin, B., Maudsley, S., Golden, E., Cutler, R. G., et al. (2009). Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus 19, 951–961. doi: 10.1002/hipo.20577
Thaler, J. P., Guyenet, S. J., Dorfman, M. D., Wisse, B. E., and Schwartz, M. W. (2013). Hypothalamic inflammation: marker or mechanism of obesity pathogenesis? Diabetes 62, 2629–2634. doi: 10.2337/db12-1605
Thaler, J. P., and Schwartz, M. W. (2010). Minireview: Inflammation and obesity pathogenesis: the hypothalamus heats up. Endocrinology 151, 4109–4115. doi: 10.1210/en.2010-0336
Thaler, J. P., Yi, C.-X., Schur, E. A., Guyenet, S. J., Hwang, B. H., Dietrich, M. O., et al. (2012). Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest. 122, 153–162. doi: 10.1172/JCI59660
Valdearcos, M., Robblee, M. M., Benjamin, D. I., Nomura, D. K., Xu, A. W., and Koliwad, S. K. (2014). Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. 9, 2124–2138. doi: 10.1016/j.celrep.2014.11.018
Van der Borght, K., Kóbor-Nyakas, D. É, Klauke, K., Eggen, B. J. L., Nyakas, C., Van Der Zee, E. A., et al. (2009). Physical exercise leads to rapid adaptations in hippocampal vasculature: temporal dynamics and relationship to cell proliferation and neurogenesis. Hippocampus 19, 928–936. doi: 10.1002/hipo.20545
van der Heijden, R. A., Bijzet, J., Meijers, W. C., Yakala, G. K., Kleemann, R., Nguyen, T. Q., et al. (2015a). Obesity-induced chronic inflammation in high fat diet challenged C57BL/6J mice is associated with acceleration of age-dependent renal amyloidosis. Sci. Rep. 5:16474. doi: 10.1038/srep16474
van der Heijden, R. A., Sheedfar, F., Morrison, M. C., Hommelberg, P. P. H., Kor, D., Kloosterhuis, N. J., et al. (2015b). High-fat diet induced obesity primes inflammation in adipose tissue prior to liver in C57BL/6j mice. Aging 7, 256–268. doi: 10.18632/aging.100738
van Dijk, G., van Heijningen, S., Reijne, A. C., Nyakas, C., van der Zee, E. A., and Eisel, U. L. (2015). Integrative neurobiology of metabolic diseases, neuroinflammation, and neurodegeneration. Front. Neurosci. 9:173. doi: 10.3389/fnins.2015.00173
Van Praag, H., Christie, B. R., Sejnowski, T. J., and Gage, F. H. (1999). Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl. Acad. Sci. U.S.A. 96, 13427–13431. doi: 10.1073/pnas.96.23.13427
Vermeij, W. P., Reiling, E., Jaarsma, D., Payan-Gomez, C., Bombardieri, C. R., Wu, H., et al. (2016). Restricted diet delays accelerated ageing and genomic stress in DNA-repair-deficient mice. Nature 537, 427–431. doi: 10.1038/nature19329
Villareal, D. T., Apovian, C. M., Kushner, R. F., and Klein, S. (2005). Obesity in older adults: technical review and position statement of the American society for nutrition and NAASO, the obesity society. Am. J. Clin. Nutr. 82, 923–934. doi: 10.1038/oby.2005.228
Wee, Y. S., Weis, J. J., Gahring, L. C., Rogers, S. W., and Weis, J. H. (2015). Age-related onset of obesity corresponds with metabolic dysregulation and altered microglia morphology in mice deficient for Ifitm proteins. PLoS One 10:e0123218. doi: 10.1371/journal.pone.0123218
Woods, J. A., Wilund, K. R., Martin, S. A., and Kistler, B. M. (2012). Exercise, inflammation and aging. Aging Dis. 3, 130–140.
Yi, C.-X., Tschop, M. H., Woods, S. C., and Hofmann, S. M. (2012). High-fat-diet exposure induces IgG accumulation in hypothalamic microglia. Dis. Model. Mech. 5, 686–690. doi: 10.1242/dmm.009464
Yu, Z. F., and Mattson, M. P. (1999). Dietary restriction and 2-deoxyglucose administration reduce focal ischemic brain damage and improve behavioral outcome: evidence for a preconditioning mechanism. J. Neurosci. Res. 57, 830–839. doi: 10.1002/(SICI)1097-4547(19990915)57:6<830::AID-JNR8>3.0.CO;2-2
Keywords: aging, high-fat diet, low-fat diet, caloric restriction, physical exercise, microglia, neuroinflammation
Citation: Yin Z, Raj DD, Schaafsma W, van der Heijden RA, Kooistra SM, Reijne AC, Zhang X, Moser J, Brouwer N, Heeringa P, Yi C-X, van Dijk G, Laman JD, Boddeke EWGM and Eggen BJL (2018) Low-Fat Diet With Caloric Restriction Reduces White Matter Microglia Activation During Aging. Front. Mol. Neurosci. 11:65. doi: 10.3389/fnmol.2018.00065
Received: 21 June 2017; Accepted: 15 February 2018;
Published: 12 March 2018.
Edited by:
Julie A. Chowen, Hospital Infantil Universitario Niño Jesús, SpainReviewed by:
Daniela Cota, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceCopyright © 2018 Yin, Raj, Schaafsma, van der Heijden, Kooistra, Reijne, Zhang, Moser, Brouwer, Heeringa, Yi, van Dijk, Laman, Boddeke and Eggen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bart J. L. Eggen, Yi5qLmwuZWdnZW5AdW1jZy5ubA==
†Shared first authors
‡Shared second authors
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.