
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Mol. Neurosci. , 04 August 2017
Sec. Molecular Signalling and Pathways
Volume 10 - 2017 | https://doi.org/10.3389/fnmol.2017.00246
This article is part of the Research Topic Gap Junctions, Hemichannels and Pannexons: New Implications in Neuroplasticity and Neuroinflammation View all 19 articles
Brain microvascular endothelial cells (BMECs) separate the peripheral blood from the brain. These cells, which are surrounded by basal lamina, pericytes and glial cells, are highly interconnected through tight and gap junctions. Their permeability properties restrict the transfer of potentially useful therapeutic agents. In such a hermetic system, the gap junctional exchange of small molecules between cerebral endothelial and non-endothelial cells is crucial for maintaining tissue homeostasis. MicroRNA were shown to cross gap junction channels, thereby modulating gene expression and function of the recipient cell. It was also shown that, when altered, BMEC could be regenerated by endothelial cells derived from pluripotent stem cells. Here, we discuss the transfer of microRNA through gap junctions between BMEC, the regeneration of BMEC from induced pluripotent stem cells that could be engineered to express specific microRNA, and how such an innovative approach could benefit to the treatment of glioblastoma and other neurological diseases.
Human brain microvascular endothelial cells (BMECs) interact with astrocytes and pericytes to form a functional “neurovascular unit” called the blood–brain barrier (BBB), which protects the central nervous system by preventing the transfer of circulating molecules from the bloodstream to the brain parenchyma. A drawback of this efficient barricade is its ability to restrict the transfer of potentially useful neurotherapeutic agents. In the recent years, microvascular endothelial cells derived from induced pluripotent stem cells (iPSC) were used to further explore BBB development and maintenance by co-culture with neural cells. These iPSC appeared also as a biological tools to screen neuropharmaceuticals (Lippmann et al., 2013, 2014a; Cecchelli et al., 2014; Minami et al., 2015; Katt et al., 2016; Appelt-Menzel et al., 2017; Yamamizu et al., 2017). Meanwhile, analysis of gap junction channels between heterotypic cells suggested promising applications by blocking or promoting microRNA transfer and delivery (Valiunas et al., 2005; Lemcke et al., 2015). This short review focuses on how we could deal with these specific aspects of the BBB biology to transfer therapeutic microRNAs to the brain.
As compared to other endothelial cells, the highly polarized BMEC demonstrate specific features. They form circumferential tight junction complexes, establishing a high-resistance paracellular barrier to small hydrophilic molecules and ions. The rate of transcytosis is low, although this remains the preferred pathway for the selective transport of plasma macromolecules. The expression of selective influx/efflux transporters such as ATP binding cassette (ABC) efflux transporters against concentration gradients is another characteristic aspect of these cells. Finally, the absence of leukocyte adhesion molecules, together with tight junctions, prevents the entry of peripheral immune cells in the absence of trauma or disease (Abbott et al., 2006; Chow and Gu, 2015). These BMEC specific features are a physical challenge and rate-limiting step for therapeutically targeting brain cells (Joshi et al., 2017). Importantly, the microvascular network of the brain is dense and so intricate that every neuron or glial cell is less than 20 μm from a blood capillary. In other words, a molecule that would cross the BBB will be immediately delivered to every neuron within the brain (Pardridge, 2002). The protective function of the BBB can be severely impaired during neurodegenerative and neuroinflammatory disorders, ischemic stroke and central nervous system (CNS) tumor development. An altered BBB may influence the treatment efficacy of these diseases with drugs that may not traverse the BBB to reach their target in the diseased brain while the delivery of others is hampered by disturbed transport mechanisms (Schenk and de Vries, 2016; Reinhold and Rittner, 2017). In brain tumors, vasogenic edema, elevated intracranial pressure, hypoxia, and neo-angiogenesis also contribute to create a chaotic situation that affects drug bioavailability (Saunus et al., 2017).
Small, lipophilic compounds and some hydrophobic molecules can cross the BBB. In contrast, biologic drugs such as nucleic acids, recombinant proteins, antibodies, and peptides are usually too large to be transferred through the BBB. This delivery could be promoted by re-engineering these large molecules into brain-penetrating neuropharmaceuticals (Pardridge, 2015a,b). Their delivery to the brain after intravenous injection could be also slightly improved by co-administration of low doses of a hyperosmolar solution (Gray et al., 2010; Kwon et al., 2010) or by disrupting the BBB with microbubble-enhanced ultrasound (Tan et al., 2016). Gene vectors have been injected directly into the brain to circumvent the BBB (Do Thi et al., 2004; Yang et al., 2013) but the innate difficulty of the method and the risks induced by such an approach make it hardly applicable over long-term clinical trials (Joshi et al., 2017).
Another strategy would be to control the functions of BMEC into the intact endothelium. These cells have a short time life when compared to other cerebral cell types (i.e., of about 2 months) and this life time could be further reduced by inflammation. The renewal of BMEC is ensured within few hours, either by cell division of neighboring endothelial cells or by the cell differentiation of circulating blood cells. Embryonic stem cells can differentiate into any cell type including endothelial cells (Levenberg et al., 2002; Wang et al., 2007; Kane et al., 2010; Nourse et al., 2010) and their properties are recapitulated by iPSC, which hold great promise for regenerative medicine (Kane et al., 2011; Kimbrel and Lanza, 2016). Such iPSC-derived endothelial cells have been combined with cardiomyocytes, and smooth muscle cells to improve cardiac function after acute myocardial infarction in a porcine model (Ye et al., 2014). It remains to be determined if they could modify the BBB composition, which would allow to engineer these cells, e.g., to express a specific microRNA or small silencing RNA (siRNA), before using them to repair an altered BBB while introducing new functions that facilitate drug delivery to the brain.
The renewal of microvascular endothelial cells is followed by the rapid re-establishment of intercellular junctions. The gap junctional intercellular communication (GJIC) can only be established if the cells are closely joined by tight junctions [and especially express adhesion proteins such as zonulae occludens ZO-1 (Zhang et al., 2003; Sin et al., 2012; Lippmann et al., 2014b; Minami et al., 2015)]. For instance, adhesion of cells to an endothelial monolayer is usually achieved in less than 1 h, cell–cell communication is established in 1–2 h and the gap junctional shuttling of microRNA observed within 3 h in vitro (Thuringer et al., 2015b, 2016a). The gap junction proteins, namely connexins (Cx) Cx37, Cx40, and Cx43 are expressed in BMEC (Vis et al., 1998; De Bock et al., 2011, 2017; Kaneko et al., 2015; Bader et al., 2017) as well as in iPSC which display intercellular dye transfer as expected for GJIC (see below). Interestingly, the glioblastoma microenvironment up-regulates the gene expression of tight junction proteins in iPSC-derived BMEC (Minami et al., 2015). Healthy and diseased glial cells separated by 3–4 nm also form functional gap junctions (Baker et al., 2014; Stamatovic et al., 2016), mostly composed of Cx30 and Cx43 (Wallraff et al., 2006; De Bock et al., 2017). In all cases, remodeling of gap junctions occurs constantly with a high turnover rate; i.e., Cx typically have short half-life of about 1.5–6 h in mammalian cells (Laird, 2006; Herve et al., 2007). Heterocellular gap junctions formed by Cx43 are largely described between BMEC and glial cells in vitro, and induce barrier properties in non-brain blood vessels in transplantation studies (Janzer and Raff, 1987; Abbott et al., 2006). In vivo, the basal lamina may limit the formation of gap junctions in most of the brain vasculature but pathological situations such as glioblastoma cell invasion that degrade the basal lamina, increase the probability of developing such communications (Bart et al., 2000; Vajkoczy and Menger, 2004; Alves et al., 2011).
The GJIC established between cancer and healthy cells permits the direct transfer of cytosolic messengers, including single strand of 22-nucleotide non-coding RNA that modify gene expression and functions of recipient cells (Valiunas et al., 2005; Lemcke et al., 2015). The GJIC-mediated intercellular transfer of mature microRNA was reported to be Cx-dependent (Zong et al., 2016). The permeability of Cx-formed gap junctions to microRNAs, responds to the following order: Cx43 > Cx26/30 > Cx26 > Cx31 > Cx30 = Cx-null with Cx43 having high permeability and Cx30 being poorly permeable to microRNAs (Zong et al., 2016). This is consistent with previous reports that Cx30 channels are impermeable to negatively charged molecules; i.e., all nucleotides including microRNA being anionic at physiological pH. We have detected in vitro microRNA exchanges between human microvascular endothelial (HMEC) and colon cancer cells through Cx43-formed gap junctions (Thuringer et al., 2016b). More specifically, the transfer of miR-145 from HMECs to tumor cells was observed to inhibit angiogenesis and tumor growth. Similar exchanges were observed between HMECs and glioblastoma cells, highlighting the crucial role of Cx43-formed gap junctions in the regulation of BBB genes (Thuringer et al., 2016a).
The opening of gap junction channels can also lead to the transfer of pathologic microRNAs such as miR-5096 that promotes glioblastoma cell invasion (Hong et al., 2015; Thuringer et al., 2016a, 2017). We evidenced such a transfer between HMEC and glioblastoma cell lines, indicating that microRNAs have to be carefully selected and evaluated for their ability to favor tumor regression. Of note, miR-5096 was shown to down-regulate Cx43 expression in glioma cells. More specifically, in co-culture experiments, membrane GJIC plaques disappeared in glioma cells while they drastically increased in HMEC (Thuringer et al., 2016a), maybe explaining why BBB remains hermetic to glioma metastasis (Blecharz et al., 2015; Steeg, 2016; Saunus et al., 2017). Despite of the absence of GJIC, glioma cells were still able to transfer miR-5096 to HMEC through the release of exosomes (Thuringer et al., 2017). Whether other microRNAs could be transferred from glioma cells to BMEC or reciprocally and how it could interfere with invasion process remains to be determined. Additionally to forming GJIC, hemichannels also participate in cell–cell communication (De Bock et al., 2017): firstly, by behaving as docking sites for exosomes, allowing therefore direct transfer of exosomal microRNA to neighboring cells (Soares et al., 2015); and secondly, by secreting microRNA into the intercellular spaces, compensating therefore the loss of glial endfeet of proliferating cancer cells and the increase in the perivascular space observed in tumor satellites (Noell et al., 2012).
Thus, the GJIC could be used to transfer therapeutic microRNA from the iPSC to neighboring cells in order to block the invasiveness and clinical aggravation of different forms of cancer.
Glioblastomas are the most prevalent and aggressive brain cancer and arise from glial cells. The diffuse invasive nature of glioblastoma precludes its complete surgical resection, which inevitably leads to tumor recurrence and patient death. Since the first histological observations of Hans Scherer (Scherer, 1940), the occurrence of perivascular invasion has been described in multiple experimental tumor models (Farin et al., 2006; Winkler et al., 2009). This invasion is more important in vascular endothelial growth factor (VEGF)-deficient glioblastoma cells (Blouw et al., 2003; Du et al., 2008) and brain tumor xenografts treated with anti-VEGF blocking antibodies such as bevacizumab (Rubenstein et al., 2000; Kunkel et al., 2001; de Groot et al., 2010; Carbonell et al., 2013; Baker et al., 2014). Clinical glioblastomas resistant to the anti-VEGF bevacizumab therapy also show a tendency toward increased perivascular invasion (Clark et al., 2012). Altogether, neoangiogenesis is dispensable for brain tumor progression and antiangiogenic drugs fail to meaningfully extend survival of patients with a glioblastoma. Conversely, this perivascular invasion could offer an opportunity to deliver effective drugs to cancer cells and our hypothesis is that engineered BMEC could be used for that purpose and deliver microRNA that limit cancer cell invasion and tumor growth (Figure 1).
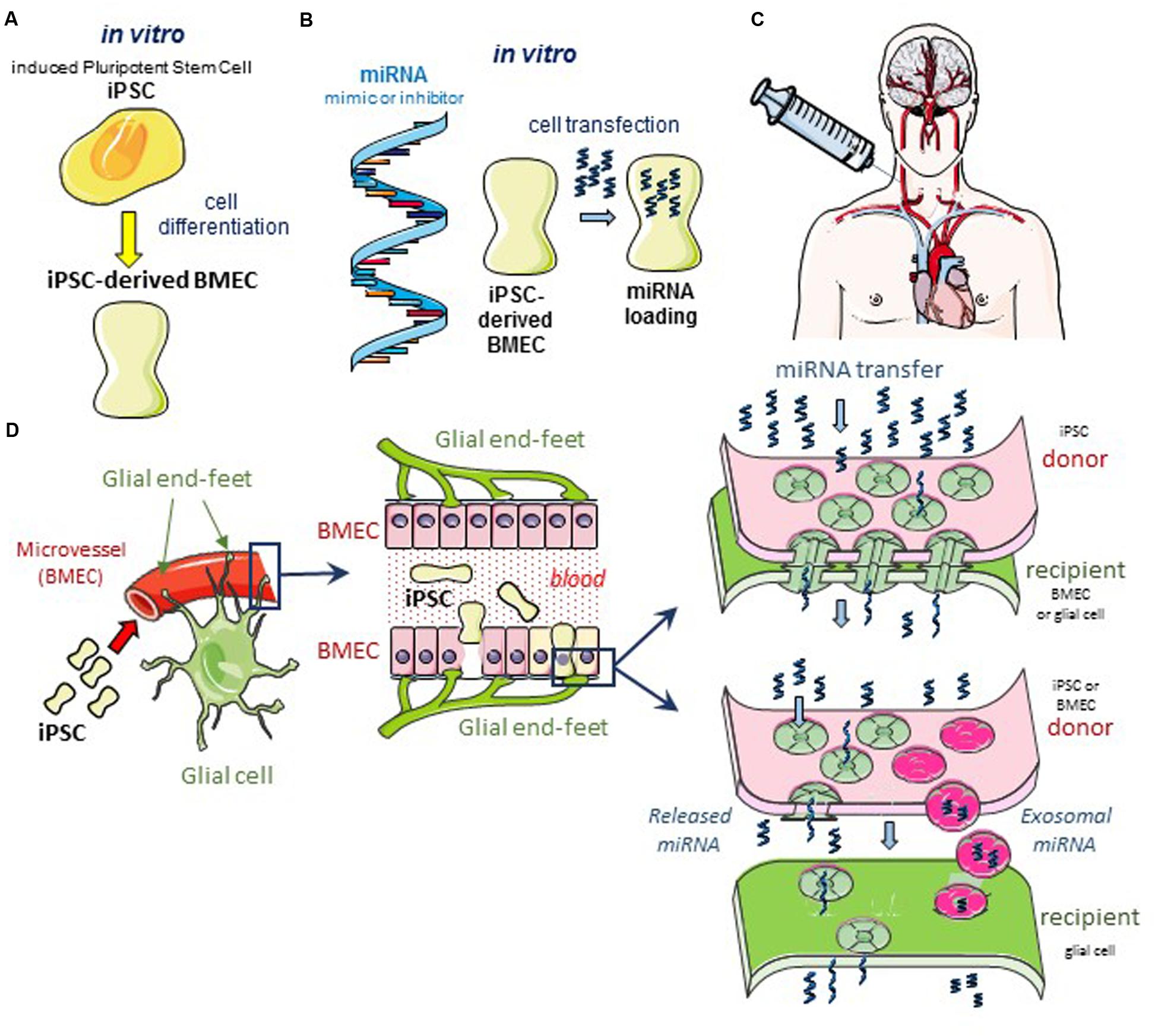
FIGURE 1. Hypothetical cell based therapeutic intervention in glioblastoma. (A) Induced pluripotent stem cells (iPSC) of human origin (i.e., umbilical cord, bone marrow, biopsied tissue sample from diseased patient, or the immortalized cell line hBMEC/D3) are pre-differentiated with neural cells and retinoic acid in vitro (Lippmann et al., 2013, 2014a). After 2 days in co-culture, cells sorting with anti-von Willebrand Factor (vWF) antibody are iPSC-derived BMEC. These cells can be sub-cultured as a pure monolayer expressing typical endothelial and BBB markers. (B) In the following day, iPSC-derived BMEC are loaded with the microRNA (miRNA) of interest, using the lipofectamine transfection procedure as previously described (Thuringer et al., 2016a). (C) After 12 h in culture, transfected cells are dissociated and suspended in a conventional infusion medium then injected directly into the patient’s carotid artery. (D) Schematic diagrams of the GJ-mediated shuttling of microRNA at the microvascular level in situ. The blue box areas are enlarged in the scheme below. Note the expected diffusion of miRNA from the iPSC-derived BMEC (yellow) to a large number of cells (bystander effect). Two modes of miRNA transfer from BMEC to glioblastoma cells are proposed: direct via gap-junction channels (upper panel), and indirect via the release of miRNA either free or contained in exosomes, to the intercellular space (lower panel). In the two modes, the Cx43 expression is required at the plasma membrane of cells, forming gap junction or hemichannel (Soares et al., 2015; De Bock et al., 2017).
To develop such a therapeutic strategy, the first parameter to deal with is biocompatibility. A first approach consists of isolating and culturing primary BMEC collected from patient tissue samples. Adult BMEC have been cultured successfully by many laboratories but rapidly lose their phenotype (Kniesel and Wolburg, 2000; Roux and Couraud, 2005; Lyck et al., 2009). In addition, given that brain vasculature comprises only 0.1% of the brain by volume, such techniques require a significant amount of brain material to achieve a reasonable yield of BMEC, limiting high throughput applications. A scalable alternative is the use of immortalized brain endothelial cell lines such as the hCMEC/D3 human brain endothelial cell line (Weksler et al., 2005). While these cells maintain many aspects of their primary BMEC counterparts and represent very useful tools for certain applications, they lack significant barrier function (Ogunshola, 2011; Naik and Cucullo, 2012).
Induced pluripotent stem cells may be another alternative. These cells are currently explored in the treatment of a wide variety of diseases, given their ability to differentiate into every cell type (Kimbrel and Lanza, 2016). Lippmann colleagues have established the method to generate BMEC from human iPSC by co-differentiation with neural cells and retinoic acid, leading to differentiated cells which exhibit properties similar to those of tissue-derived BMEC (Lippmann et al., 2012, 2013, 2014a; Hollmann et al., 2017). Some projects aim to use iPSC to recapitulate 3D human neuron/neurovasculature interactions ‘on a chip’ in vitro and reconstitute the neurovascular unit, allowing pharmacological drug testing on cells derived from patients of different ages, metabolic conditions or neuro-pathologies (Brown et al., 2015; Walter et al., 2016).
Undifferentiated human embryonic stem cells express mRNA for almost all known Cx subtypes and display intercellular dye transfer, which is characteristic of GJIC (Huettner et al., 2006). iPSC obtained through reprogramming somatic fibroblasts cells also express most Cx subtypes (Oyamada et al., 2013) and stem cells of various sources express Cx43 (Kar et al., 2012). In undifferentiated cord-blood-derived iPSC, the gap junction plaques mostly contain Cx43 (Beckmann et al., 2016). Cx43 is upregulated during the reprogramming process and Cx43 knockdown via short interfering RNA significantly impairs reprogramming efficiency (Sharovskaya et al., 2012; Ke et al., 2013).
The expression of Cx43 in reprogrammed cells suggests that circulating iPSC may adhere and form Cx43 gap junction plaques with BMEC, then integrate the microvascular endothelium. We have reported that dissociated HMEC (donor), pre-loaded with a fluorescent dye, then plated onto unlabeled HMEC monolayer (acceptor), were rapidly integrated into the monolayer and formed functional gap junctions with HMEC within 2 h (Thuringer et al., 2016a). Actually, all the cells expressing Cx43 proteins, such as SW480 colon cancer cells and human monocytes, communicate with the microvascular endothelium and may pass through the monolayer (Thuringer et al., 2015a,b). Of note, glioma cells also establish Cx43 gap junction with HMEC, thereby reinforcing the barrier (Thuringer et al., 2016a).
Another question regarding the delivering of iPSC to reconstitute BMEC is the route of administration. Until now, gene vectors have been injected directly into the brain to circumvent the BBB (Do Thi et al., 2004; Yang et al., 2013); but the innate risk due to direct intra-brain administration makes this method hardly applicable in clinics. Convection-enhanced delivery, in which one or more catheters are carefully placed in the brain parenchyma for therapeutic delivery, may be a solution, this technology is currently tested in phase III clinical trial in patients with a glioblastoma (Debinski and Tatter, 2009). If validated, this approach could be used also for future iPSC-based therapies. A less complex although still tricky strategy would be to inject iPSC directly into the carotid artery, knowing that the arterial pressure is strong enough to prevent the cell adhesion in its duct.
A third question is the identification of the microRNA to transfer to diseased cells through iPSC-derived BMEC (Lopez-Ramirez et al., 2016; Shea et al., 2016). The transfer of mature miR-4519 and miR-5096 from glioma cells to astrocytes enhance the glioma pro-invasive potential (Hong et al., 2015), e.g., several microRNAs associated with survival and chemotherapy resistance passed through gap junctions formed between astrocytes and lung tumor cells in vitro (Menachem et al., 2016). Conversely, the transfer of miR-124-3p between transfected and non-transfected glioma cells has anti-proliferative effects (Suzhi et al., 2015), and the transfer of miR-145-5p to glioma cells has anti-tumor properties (Thuringer et al., 2016a,b). Additionally, some microRNAs regulate expression and/or function of Cx for several types of cancer (Calderon and Retamal, 2016). These observations suggest that iPSC will have to be engineered to express and transfer not only selected microRNA with antitumor effects but also inhibitors of specific pro-tumorigenic microRNAs (anti-miRs) (Gurwitz, 2016).
Finally, the mode of transfer between engineered iPSC and tumor cells will have to be determined as GJIC-mediated microRNA transfer may be more efficient than microvesicle/exosome-based intercellular transport (Zong et al., 2016). As indicated above, Cx43, which is highly expressed in many cell types, is involved in both GJIC-mediated and exosome-mediated transfer of microRNA. The bystander effect described with suicide gene therapeutic approaches involves both GJIC formation and exosome delivery (Yamasaki and Katoh, 1988a,b; Elshami et al., 1996; Mesnil et al., 1996; Dilber and Smith, 1997; Vrionis et al., 1997; Duflot-Dancer et al., 1998; Touraine et al., 1998; Yang et al., 1998). The GJIC-mediated bystander effect can be amplified by treatments, such as intraperitoneal injection of retinoic acid, that increase GJIC (Stahl and Sies, 1998; Touraine et al., 1998; Bertram and Vine, 2005; Kong et al., 2016). Strategies that promote the formation of GIJC between iPSC-derived BMEC and target cells in the brain may therefore increase the efficacy of this therapeutic approach.
The use of iPSC is an emerging strategy that remains to be validated in the treatment of various diseases. Because the BBB is a limitation to the treatment of neurological diseases including brain cancer, iPSC-derived BMEC engineered to transfer microRNA, anti-miR or siRNA to target cells could be one of these new therapeutic applications. The transfer of selected single strand RNA through Cx43-containing gap junction channels between iPSC-derived BMEC and neuronal target cells could potentially improve the control of neurological diseases through modulation of gene expression and function in target cells. An unresolved issue is the best small RNA to transfer via GJIC, as it has to respect Cx43 expression and limit target cell development. Several practical issues have now to be solved to develop this approach to treat glioblastoma and potentially other neurological diseases.
DT: designed, drafted and revised the manuscript; ES: drafted and revised the manuscript; CG: revised the final manuscript.
This research was funded by Centre National de la Recherche Scientifique [https://doi.org/10.13039/501100004794], Institut National de la Santé et de la Recherche Médicale [https://doi.org/10.13039/501100001677], and Ligue Contre le Cancer [https://doi.org/10.13039/501100004099].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbott, N. J., Ronnback, L., and Hansson, E. (2006). Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 7, 41–53. doi: 10.1038/nrn1824
Alves, T. R., Lima, F. R., Kahn, S. A., Lobo, D., Dubois, L. G., Soletti, R., et al. (2011). Glioblastoma cells: a heterogeneous and fatal tumor interacting with the parenchyma. Life Sci. 89, 532–539. doi: 10.1016/j.lfs.2011.04.022
Appelt-Menzel, A., Cubukova, A., Gunther, K., Edenhofer, F., Piontek, J., Krause, G., et al. (2017). Establishment of a human blood-brain barrier co-culture model mimicking the neurovascular unit using induced pluri- and multipotent stem cells. Stem Cell Rep. 8, 894–906. doi: 10.1016/j.stemcr.2017.02.021
Bader, A., Bintig, W., Begandt, D., Klett, A., Siller, I. G., Gregor, C., et al. (2017). Adenosine receptors regulate gap junction coupling of the human cerebral microvascular endothelial cells hCMEC/D3 by Ca2+ influx through cyclic nucleotide-gated channels. J. Physiol. 595, 2497–2517. doi: 10.1113/JP273150
Baker, G. J., Yadav, V. N., Motsch, S., Koschmann, C., Calinescu, A. A., Mineharu, Y., et al. (2014). Mechanisms of glioma formation: iterative perivascular glioma growth and invasion leads to tumor progression, VEGF-independent vascularization, and resistance to antiangiogenic therapy. Neoplasia 16, 543–561. doi: 10.1016/j.neo.2014.06.003
Bart, J., Groen, H. J., Hendrikse, N. H., van der Graaf, W. T., Vaalburg, W., and de Vries, E. G. (2000). The blood-brain barrier and oncology: new insights into function and modulation. Cancer Treat. Rev. 26, 449–462. doi: 10.1053/ctrv.2000.0194
Beckmann, A., Schubert, M., Hainz, N., Haase, A., Martin, U., Tschernig, T., et al. (2016). Ultrastructural demonstration of Cx43 gap junctions in induced pluripotent stem cells from human cord blood. Histochem. Cell Biol. 146, 529–537. doi: 10.1007/s00418-016-1469-9
Bertram, J. S., and Vine, A. L. (2005). Cancer prevention by retinoids and carotenoids: independent action on a common target. Biochim. Biophys. Acta 1740, 170–178. doi: 10.1016/j.bbadis.2005.01.003
Blecharz, K. G., Colla, R., Rohde, V., and Vajkoczy, P. (2015). Control of the blood-brain barrier function in cancer cell metastasis. Biol. Cell 107, 342–371. doi: 10.1111/boc.201500011
Blouw, B., Song, H., Tihan, T., Bosze, J., Ferrara, N., Gerber, H. P., et al. (2003). The hypoxic response of tumors is dependent on their microenvironment. Cancer Cell 4, 133–146. doi: 10.1016/S1535-6108(03)00194-6
Brown, J. A., Pensabene, V., Markov, D. A., Allwardt, V., Neely, M. D., Shi, M., et al. (2015). Recreating blood-brain barrier physiology and structure on chip: a novel neurovascular microfluidic bioreactor. Biomicrofluidics 9:054124. doi: 10.1063/1.4934713
Calderon, J. F., and Retamal, M. A. (2016). Regulation of connexins expression levels by microRNAs, an update. Front. Physiol. 7:558. doi: 10.3389/fphys.2016.00558
Carbonell, W. S., DeLay, M., Jahangiri, A., Park, C. C., and Aghi, M. K. (2013). beta1 integrin targeting potentiates antiangiogenic therapy and inhibits the growth of bevacizumab-resistant glioblastoma. Cancer Res. 73, 3145–3154. doi: 10.1158/0008-5472.CAN-13-0011
Cecchelli, R., Aday, S., Sevin, E., Almeida, C., Culot, M., Dehouck, L., et al. (2014). A stable and reproducible human blood-brain barrier model derived from hematopoietic stem cells. PLoS ONE 9:e99733. doi: 10.1371/journal.pone.0099733
Chow, B. W., and Gu, C. (2015). The molecular constituents of the blood-brain barrier. Trends Neurosci. 38, 598–608. doi: 10.1016/j.tins.2015.08.003
Clark, A. J., Lamborn, K. R., Butowski, N. A., Chang, S. M., Prados, M. D., Clarke, J. L., et al. (2012). Neurosurgical management and prognosis of patients with glioblastoma that progresses during bevacizumab treatment. Neurosurgery 70, 361–370. doi: 10.1227/NEU.0b013e3182314f9d
De Bock, M., Culot, M., Wang, N., Bol, M., Decrock, E., De Vuyst, E., et al. (2011). Connexin channels provide a target to manipulate brain endothelial calcium dynamics and blood-brain barrier permeability. J. Cereb. Blood Flow Metab. 31, 1942–1957. doi: 10.1038/jcbfm.2011.86
De Bock, M., Leybaert, L., and Giaume, C. (2017). Connexin channels at the glio-vascular interface: gatekeepers of the brain. Neurochem. Res. doi: 10.1007/s11064-017-2313-x [Epub ahead of print].
de Groot, J. F., Fuller, G., Kumar, A. J., Piao, Y., Eterovic, K., Ji, Y., et al. (2010). Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro Oncol. 12, 233–242. doi: 10.1093/neuonc/nop027
Debinski, W., and Tatter, S. B. (2009). Convection-enhanced delivery for the treatment of brain tumors. Expert Rev. Neurother. 9, 1519–1527. doi: 10.1586/ern.09.99
Dilber, M. S., and Smith, C. I. (1997). Suicide genes and bystander killing: local and distant effects. Gene Ther. 4, 273–274. doi: 10.1038/sj.gt.3300389
Do Thi, N. A., Saillour, P., Ferrero, L., Dedieu, J. F., Mallet, J., and Paunio, T. (2004). Delivery of GDNF by an E1,E3/E4 deleted adenoviral vector and driven by a GFAP promoter prevents dopaminergic neuron degeneration in a rat model of Parkinson’s disease. Gene Ther. 11, 746–756. doi: 10.1038/sj.gt.3302222
Du, R., Lu, K. V., Petritsch, C., Liu, P., Ganss, R., Passegue, E., et al. (2008). HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell 13, 206–220. doi: 10.1016/j.ccr.2008.01.034
Duflot-Dancer, A., Piccoli, C., Rolland, A., Yamasaki, H., and Mesnil, M. (1998). Long-term connexin-mediated bystander effect in highly tumorigenic human cells in vivo in herpes simplex virus thymidine kinase/ganciclovir gene therapy. Gene Ther. 5, 1372–1378. doi: 10.1038/sj.gt.3300734
Elshami, A. A., Saavedra, A., Zhang, H., Kucharczuk, J. C., Spray, D. C., Fishman, G. I., et al. (1996). Gap junctions play a role in the ‘bystander effect’ of the herpes simplex virus thymidine kinase/ganciclovir system in vitro. Gene Ther. 3, 85–92.
Farin, A., Suzuki, S. O., Weiker, M., Goldman, J. E., Bruce, J. N., and Canoll, P. (2006). Transplanted glioma cells migrate and proliferate on host brain vasculature: a dynamic analysis. Glia 53, 799–808. doi: 10.1002/glia.20334
Gray, S. J., Blake, B. L., Criswell, H. E., Nicolson, S. C., Samulski, R. J., McCown, T. J., et al. (2010). Directed evolution of a novel adeno-associated virus (AAV) vector that crosses the seizure-compromised blood-brain barrier (BBB). Mol. Ther. 18, 570–578. doi: 10.1038/mt.2009.292
Gurwitz, D. (2016). MicroRNAs as CNS drug targets. Drug Dev. Res. 77, 331–335. doi: 10.1002/ddr.21336
Herve, J. C., Derangeon, M., Bahbouhi, B., Mesnil, M., and Sarrouilhe, D. (2007). The connexin turnover, an important modulating factor of the level of cell-to-cell junctional communication: comparison with other integral membrane proteins. J. Membr. Biol. 217, 21–33. doi: 10.1007/s00232-007-9054-8
Hollmann, E. K., Bailey, A. K., Potharazu, A. V., Neely, M. D., Bowman, A. B., and Lippmann, E. S. (2017). Accelerated differentiation of human induced pluripotent stem cells to blood-brain barrier endothelial cells. Fluids Barriers CNS 14:9. doi: 10.1186/s12987-017-0059-0
Hong, X., Sin, W. C., Harris, A. L., and Naus, C. C. (2015). Gap junctions modulate glioma invasion by direct transfer of microRNA. Oncotarget 6, 15566–15577. doi: 10.18632/oncotarget.3904
Huettner, J. E., Lu, A., Qu, Y., Wu, Y., Kim, M., and McDonald, J. W. (2006). Gap junctions and connexon hemichannels in human embryonic stem cells. Stem Cells 24, 1654–1667. doi: 10.1634/stemcells.2005-0003
Janzer, R. C., and Raff, M. C. (1987). Astrocytes induce blood-brain barrier properties in endothelial cells. Nature 325, 253–257. doi: 10.1038/325253a0
Joshi, C. R., Labhasetwar, V., and Ghorpade, A. (2017). Destination brain: the past, present, and future of therapeutic gene delivery. J. Neuroimmune Pharmacol. 12, 51–83. doi: 10.1007/s11481-016-9724-3
Kane, N. M., Meloni, M., Spencer, H. L., Craig, M. A., Strehl, R., Milligan, G., et al. (2010). Derivation of endothelial cells from human embryonic stem cells by directed differentiation: analysis of microRNA and angiogenesis in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 30, 1389–1397. doi: 10.1161/ATVBAHA.110.204800
Kane, N. M., Xiao, Q., Baker, A. H., Luo, Z., Xu, Q., and Emanueli, C. (2011). Pluripotent stem cell differentiation into vascular cells: a novel technology with promises for vascular re(generation). Pharmacol. Ther. 129, 29–49. doi: 10.1016/j.pharmthera.2010.10.004
Kaneko, Y., Tachikawa, M., Akaogi, R., Fujimoto, K., Ishibashi, M., Uchida, Y., et al. (2015). Contribution of pannexin 1 and connexin 43 hemichannels to extracellular calcium-dependent transport dynamics in human blood-brain barrier endothelial cells. J. Pharmacol. Exp. Ther. 353, 192–200. doi: 10.1124/jpet.114.220210
Kar, R., Batra, N., Riquelme, M. A., and Jiang, J. X. (2012). Biological role of connexin intercellular channels and hemichannels. Arch. Biochem. Biophys. 524, 2–15. doi: 10.1016/j.abb.2012.03.008
Katt, M. E., Xu, Z. S., Gerecht, S., and Searson, P. C. (2016). Human brain microvascular endothelial cells derived from the BC1 iPS cell line exhibit a blood-brain barrier phenotype. PLoS ONE 11:e0152105. doi: 10.1371/journal.pone.0152105
Ke, Q., Li, L., Cai, B., Liu, C., Yang, Y., Gao, Y., et al. (2013). Connexin 43 is involved in the generation of human-induced pluripotent stem cells. Hum. Mol. Genet. 22, 2221–2233. doi: 10.1093/hmg/ddt074
Kimbrel, E. A., and Lanza, R. (2016). Pluripotent stem cells: the last 10 years. Regen. Med. 11, 831–847. doi: 10.2217/rme-2016-0117
Kniesel, U., and Wolburg, H. (2000). Tight junctions of the blood-brain barrier. Cell Mol. Neurobiol. 20, 57–76. doi: 10.1023/A:1006995910836
Kong, H., Liu, X., Yang, L., Qi, K., Zhang, H., Zhang, J., et al. (2016). All-trans retinoic acid enhances bystander effect of suicide gene therapy in the treatment of breast cancer. Oncol. Rep. 35, 1868–1874. doi: 10.3892/or.2015.4535
Kunkel, P., Ulbricht, U., Bohlen, P., Brockmann, M. A., Fillbrandt, R., Stavrou, D., et al. (2001). Inhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor-2. Cancer Res. 61, 6624–6628.
Kwon, D. Y., Seo, W. K., Yoon, H. K., Park, M. H., Koh, S. B., and Park, K. W. (2010). Transcranial brain sonography in Parkinson’s disease with restless legs syndrome. Mov. Disord. 25, 1373–1378. doi: 10.1002/mds.23066
Laird, D. W. (2006). Life cycle of connexins in health and disease. Biochem. J. 394(Pt 3), 527–543. doi: 10.1042/BJ20051922
Lemcke, H., Steinhoff, G., and David, R. (2015). Gap junctional shuttling of miRNA–A novel pathway of intercellular gene regulation and its prospects in clinical application. Cell. Signal. 27, 2506–2514. doi: 10.1016/j.cellsig.2015.09.012
Levenberg, S., Golub, J. S., Amit, M., Itskovitz-Eldor, J., and Langer, R. (2002). Endothelial cells derived from human embryonic stem cells. Proc. Natl. Acad. Sci. U.S.A. 99, 4391–4396. doi: 10.1073/pnas.032074999
Lippmann, E. S., Al-Ahmad, A., Azarin, S. M., Palecek, S. P., and Shusta, E. V. (2014a). A retinoic acid-enhanced, multicellular human blood-brain barrier model derived from stem cell sources. Sci. Rep. 4:4160. doi: 10.1038/srep04160
Lippmann, E. S., Al-Ahmad, A., Palecek, S. P., and Shusta, E. V. (2013). Modeling the blood-brain barrier using stem cell sources. Fluids Barriers CNS 10:2. doi: 10.1186/2045-8118-10-2
Lippmann, E. S., Azarin, S. M., Kay, J. E., Nessler, R. A., Wilson, H. K., Al-Ahmad, A., et al. (2012). Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nat. Biotechnol. 30, 783–791. doi: 10.1038/nbt.2247
Lippmann, E. S., Estevez-Silva, M. C., and Ashton, R. S. (2014b). Defined human pluripotent stem cell culture enables highly efficient neuroepithelium derivation without small molecule inhibitors. Stem Cells 32, 1032–1042. doi: 10.1002/stem.1622
Lopez-Ramirez, M. A., Reijerkerk, A., de Vries, H. E., and Romero, I. A. (2016). Regulation of brain endothelial barrier function by microRNAs in health and neuroinflammation. FASEB J. 30, 2662–2672. doi: 10.1096/fj.201600435RR
Lyck, R., Ruderisch, N., Moll, A. G., Steiner, O., Cohen, C. D., Engelhardt, B., et al. (2009). Culture-induced changes in blood-brain barrier transcriptome: implications for amino-acid transporters in vivo. J. Cereb. Blood Flow Metab. 29, 1491–1502. doi: 10.1038/jcbfm.2009.72
Menachem, A., Makovski, V., Bodner, O., Pasmanik-Chor, M., Stein, R., Shomron, N., et al. (2016). Intercellular transfer of small RNAs from astrocytes to lung tumor cells induces resistance to chemotherapy. Oncotarget 7, 12489–12504. doi: 10.18632/oncotarget.7273
Mesnil, M., Piccoli, C., Tiraby, G., Willecke, K., and Yamasaki, H. (1996). Bystander killing of cancer cells by herpes simplex virus thymidine kinase gene is mediated by connexins. Proc. Natl. Acad. Sci. U.S.A. 93, 1831–1835. doi: 10.1073/pnas.93.5.1831
Minami, H., Tashiro, K., Okada, A., Hirata, N., Yamaguchi, T., Takayama, K., et al. (2015). Generation of brain microvascular endothelial-like cells from human induced pluripotent stem cells by co-culture with C6 glioma cells. PLoS ONE 10:e0128890. doi: 10.1371/journal.pone.0128890
Naik, P., and Cucullo, L. (2012). In vitro blood-brain barrier models: current and perspective technologies. J. Pharm. Sci. 101, 1337–1354. doi: 10.1002/jps.23022
Noell, S., Wolburg-Buchholz, K., Mack, A. F., Ritz, R., Tatagiba, M., Beschorner, R., et al. (2012). Dynamics of expression patterns of AQP4, dystroglycan, agrin and matrix metalloproteinases in human glioblastoma. Cell Tissue Res. 347, 429–441. doi: 10.1007/s00441-011-1321-4
Nourse, M. B., Halpin, D. E., Scatena, M., Mortisen, D. J., Tulloch, N. L., Hauch, K. D., et al. (2010). VEGF induces differentiation of functional endothelium from human embryonic stem cells: implications for tissue engineering. Arterioscler. Thromb. Vasc. Biol. 30, 80–89. doi: 10.1161/ATVBAHA.109.194233
Ogunshola, O. O. (2011). In vitro modeling of the blood-brain barrier: simplicity versus complexity. Curr. Pharm. Des. 17, 2755–2761. doi: 10.2174/138161211797440159
Oyamada, M., Takebe, K., Endo, A., Hara, S., and Oyamada, Y. (2013). Connexin expression and gap-junctional intercellular communication in ES cells and iPS cells. Front. Pharmacol. 4:85. doi: 10.3389/fphar.2013.00085
Pardridge, W. M. (2002). Targeting neurotherapeutic agents through the blood-brain barrier. Arch. Neurol. 59, 35–40. doi: 10.1001/archneur.59.1.35
Pardridge, W. M. (2015a). Blood-brain barrier endogenous transporters as therapeutic targets: a new model for small molecule CNS drug discovery. Expert Opin. Ther. Targets 19, 1059–1072. doi: 10.1517/14728222.2015.1042364
Pardridge, W. M. (2015b). Targeted delivery of protein and gene medicines through the blood-brain barrier. Clin. Pharmacol. Ther. 97, 347–361. doi: 10.1002/cpt.18
Reinhold, A. K., and Rittner, H. L. (2017). Barrier function in the peripheral and central nervous system-a review. Pflugers Arch. 469, 123–134. doi: 10.1007/s00424-016-1920-8
Roux, F., and Couraud, P. O. (2005). Rat brain endothelial cell lines for the study of blood-brain barrier permeability and transport functions. Cell Mol. Neurobiol. 25, 41–58. doi: 10.1007/s10571-004-1376-9
Rubenstein, J. L., Kim, J., Ozawa, T., Zhang, M., Westphal, M., Deen, D. F., et al. (2000). Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia 2, 306–314. doi: 10.1038/sj.neo.7900102
Saunus, J. M., McCart Reed, A. E., Lim, Z. L., and Lakhani, S. R. (2017). Breast cancer brain metastases: clonal evolution in clinical context. Int. J. Mol. Sci. 18:152. doi: 10.3390/ijms18010152
Schenk, G. J., and de Vries, H. E. (2016). Altered blood-brain barrier transport in neuro-inflammatory disorders. Drug Discov. Today Technol. 20, 5–11. doi: 10.1016/j.ddtec.2016.07.002
Scherer, H. J. (1940). A critical review: the pathology of cerebral gliomas. J. Neurol. Psychiatry 3, 147–177. doi: 10.1136/jnnp.3.2.147
Sharovskaya, Y. Y., Philonenko, E. S., Kiselev, S. L., and Lagarkova, M. A. (2012). De novo reestablishment of gap junctional intercellular communications during reprogramming to pluripotency and differentiation. Stem Cells Dev. 21, 2623–2629. doi: 10.1089/scd.2011.0707
Shea, A., Harish, V., Afzal, Z., Chijioke, J., Kedir, H., Dusmatova, S., et al. (2016). MicroRNAs in glioblastoma multiforme pathogenesis and therapeutics. Cancer Med. 5, 1917–1946. doi: 10.1002/cam4.775
Sin, W. C., Crespin, S., and Mesnil, M. (2012). Opposing roles of connexin43 in glioma progression. Biochim. Biophys. Acta 1818, 2058–2067. doi: 10.1016/j.bbamem.2011.10.022
Soares, A. R., Martins-Marques, T., Ribeiro-Rodrigues, T., Ferreira, J. V., Catarino, S., Pinho, M. J., et al. (2015). Gap junctional protein Cx43 is involved in the communication between extracellular vesicles and mammalian cells. Sci. Rep. 5:13243. doi: 10.1038/srep13243
Stahl, W., and Sies, H. (1998). The role of carotenoids and retinoids in gap junctional communication. Int. J. Vitam. Nutr. Res. 68, 354–359.
Stamatovic, S. M., Johnson, A. M., Keep, R. F., and Andjelkovic, A. V. (2016). Junctional proteins of the blood-brain barrier: new insights into function and dysfunction. Tissue Barriers 4:e1154641. doi: 10.1080/21688370.2016.1154641
Suzhi, Z., Liang, T., Yuexia, P., Lucy, L., Xiaoting, H., Yuan, Z., et al. (2015). Gap junctions enhance the antiproliferative effect of microRNA-124-3p in glioblastoma cells. J. Cell. Physiol. 230, 2476–2488. doi: 10.1002/jcp.24982
Tan, J. K., Pham, B., Zong, Y., Perez, C., Maris, D. O., Hemphill, A., et al. (2016). Microbubbles and ultrasound increase intraventricular polyplex gene transfer to the brain. J. Control. Release 231, 86–93. doi: 10.1016/j.jconrel.2016.02.003
Thuringer, D., Berthenet, K., Cronier, L., Jego, G., Solary, E., and Garrido, C. (2015a). Oncogenic extracellular HSP70 disrupts the gap-junctional coupling between capillary cells. Oncotarget 6, 10267–10283. doi: 10.18632/oncotarget.3522
Thuringer, D., Berthenet, K., Cronier, L., Solary, E., and Garrido, C. (2015b). Primary tumor- and metastasis-derived colon cancer cells differently modulate connexin expression and function in human capillary endothelial cells. Oncotarget 6, 28800–28815. doi: 10.18632/oncotarget.4894
Thuringer, D., Boucher, J., Jego, G., Pernet, N., Cronier, L., Hammann, A., et al. (2016a). Transfer of functional microRNAs between glioblastoma and microvascular endothelial cells through gap junctions. Oncotarget 7, 73925–73934. doi: 10.18632/oncotarget.12136
Thuringer, D., Chanteloup, G., Boucher, J., Pernet, N., Boudesco, C., Jego, G., et al. (2017). Modulation of the inwardly rectifying potassium channel Kir4.1 by the pro-invasive miR-5096 in glioblastoma cells. Oncotarget 8, 37681–37693. doi: 10.18632/oncotarget.16949
Thuringer, D., Jego, G., Berthenet, K., Hammann, A., Solary, E., and Garrido, C. (2016b). Gap junction-mediated transfer of miR-145-5p from microvascular endothelial cells to colon cancer cells inhibits angiogenesis. Oncotarget 7, 28160–28168. doi: 10.18632/oncotarget.8583
Touraine, R. L., Ishii-Morita, H., Ramsey, W. J., and Blaese, R. M. (1998). The bystander effect in the HSVtk/ganciclovir system and its relationship to gap junctional communication. Gene Ther. 5, 1705–1711. doi: 10.1038/sj.gt.3300784
Vajkoczy, P., and Menger, M. D. (2004). Vascular microenvironment in gliomas. Cancer Treat. Res. 117, 249–262. doi: 10.1007/978-1-4419-8871-3_15
Valiunas, V., Polosina, Y. Y., Miller, H., Potapova, I. A., Valiuniene, L., Doronin, S., et al. (2005). Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions. J. Physiol. 568(Pt 2), 459–468. doi: 10.1113/jphysiol.2005.090985
Vis, J. C., Nicholson, L. F., Faull, R. L., Evans, W. H., Severs, N. J., and Green, C. R. (1998). Connexin expression in Huntington’s diseased human brain. Cell Biol. Int. 22, 837–847. doi: 10.1006/cbir.1998.0388
Vrionis, F. D., Wu, J. K., Qi, P., Waltzman, M., Cherington, V., and Spray, D. C. (1997). The bystander effect exerted by tumor cells expressing the herpes simplex virus thymidine kinase (HSVtk) gene is dependent on connexin expression and cell communication via gap junctions. Gene Ther. 4, 577–585. doi: 10.1038/sj.gt.3300438
Wallraff, A., Kohling, R., Heinemann, U., Theis, M., Willecke, K., and Steinhauser, C. (2006). The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J. Neurosci. 26, 5438–5447. doi: 10.1523/JNEUROSCI.0037-06.2006
Walter, A., Saric, T., Hescheler, J., and Papadopoulos, S. (2016). Calcium imaging in pluripotent stem cell-derived cardiac myocytes. Methods Mol. Biol. 1353, 131–146. doi: 10.1007/7651_2015_267
Wang, Z. Z., Au, P., Chen, T., Shao, Y., Daheron, L. M., Bai, H., et al. (2007). Endothelial cells derived from human embryonic stem cells form durable blood vessels in vivo. Nat. Biotechnol. 25, 317–318. doi: 10.1038/nbt1287
Weksler, B. B., Subileau, E. A., Perriere, N., Charneau, P., Holloway, K., Leveque, M., et al. (2005). Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 19, 1872–1874. doi: 10.1096/fj.04-3458fje
Winkler, F., Kienast, Y., Fuhrmann, M., Von Baumgarten, L., Burgold, S., Mitteregger, G., et al. (2009). Imaging glioma cell invasion in vivo reveals mechanisms of dissemination and peritumoral angiogenesis. Glia 57, 1306–1315. doi: 10.1002/glia.20850
Yamamizu, K., Iwasaki, M., Takakubo, H., Sakamoto, T., Ikuno, T., Miyoshi, M., et al. (2017). In vitro modeling of blood-brain barrier with human iPSC-derived endothelial cells, pericytes, neurons, and astrocytes via notch signaling. Stem Cell Rep. 8, 634–647. doi: 10.1016/j.stemcr.2017.01.023
Yamasaki, H., and Katoh, F. (1988a). Further evidence for the involvement of gap-junctional intercellular communication in induction and maintenance of transformed foci in BALB/c 3T3 cells. Cancer Res. 48, 3490–3495.
Yamasaki, H., and Katoh, F. (1988b). Novel method for selective killing of transformed rodent cells through intercellular communication, with possible therapeutic applications. Cancer Res. 48, 3203–3207.
Yang, C., Yang, W. H., Chen, S. S., Ma, B. F., Li, B., Lu, T., et al. (2013). Pre-immunization with an intramuscular injection of AAV9-human erythropoietin vectors reduces the vector-mediated transduction following re-administration in rat brain. PLoS ONE 8:e63876. doi: 10.1371/journal.pone.0063876
Yang, L., Chiang, Y., Lenz, H. J., Danenberg, K. D., Spears, C. P., Gordon, E. M., et al. (1998). Intercellular communication mediates the bystander effect during herpes simplex thymidine kinase/ganciclovir-based gene therapy of human gastrointestinal tumor cells. Hum. Gene Ther. 9, 719–728. doi: 10.1089/hum.1998.9.5-719
Ye, L., Chang, Y. H., Xiong, Q., Zhang, P., Zhang, L., Somasundaram, P., et al. (2014). Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell 15, 750–761. doi: 10.1016/j.stem.2014.11.009
Zhang, W., DeMattia, J. A., Song, H., and Couldwell, W. T. (2003). Communication between malignant glioma cells and vascular endothelial cells through gap junctions. J. Neurosurg. 98, 846–853. doi: 10.3171/jns.2003.98.4.0846
Keywords: targeted therapy, microRNA, gap junction, blood capillary, connexin43, neurological disorders, glioblastoma
Citation: Thuringer D, Solary E and Garrido C (2017) The Microvascular Gap Junction Channel: A Route to Deliver MicroRNAs for Neurological Disease Treatment. Front. Mol. Neurosci. 10:246. doi: 10.3389/fnmol.2017.00246
Received: 31 May 2017; Accepted: 24 July 2017;
Published: 04 August 2017.
Edited by:
Juan Andrés Orellana, Pontificia Universidad Católica de Chile, ChileReviewed by:
Andrei B. Belousov, University of Kansas School of Medicine, United StatesCopyright © 2017 Thuringer, Solary and Garrido. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dominique Thuringer, ZG9taW5pcXVlLnRodXJpbmdlckB1LWJvdXJnb2duZS5mcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.