
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CORRECTION article
Front. Mol. Biosci. , 28 November 2024
Sec. Structural Biology
Volume 11 - 2024 | https://doi.org/10.3389/fmolb.2024.1517143
This article is a correction to:
Shedding light on the DICER1 mutational spectrum of uncertain significance in malignant neoplasms
A Corrigendum on
Shedding light on the DICER1 mutational spectrum of uncertain significance in malignant neoplasms
by Bug DS, Moiseev IS, Porozov YB and Petukhova NV (2024). Front. Mol. Biosci. 11:1441180. doi: 10.3389/fmolb.2024.1441180
In the published article, there was an error in Figure 7 as published. The Figures 7, 8 were mixed up. The corrected Figure 7 and its caption appear below.
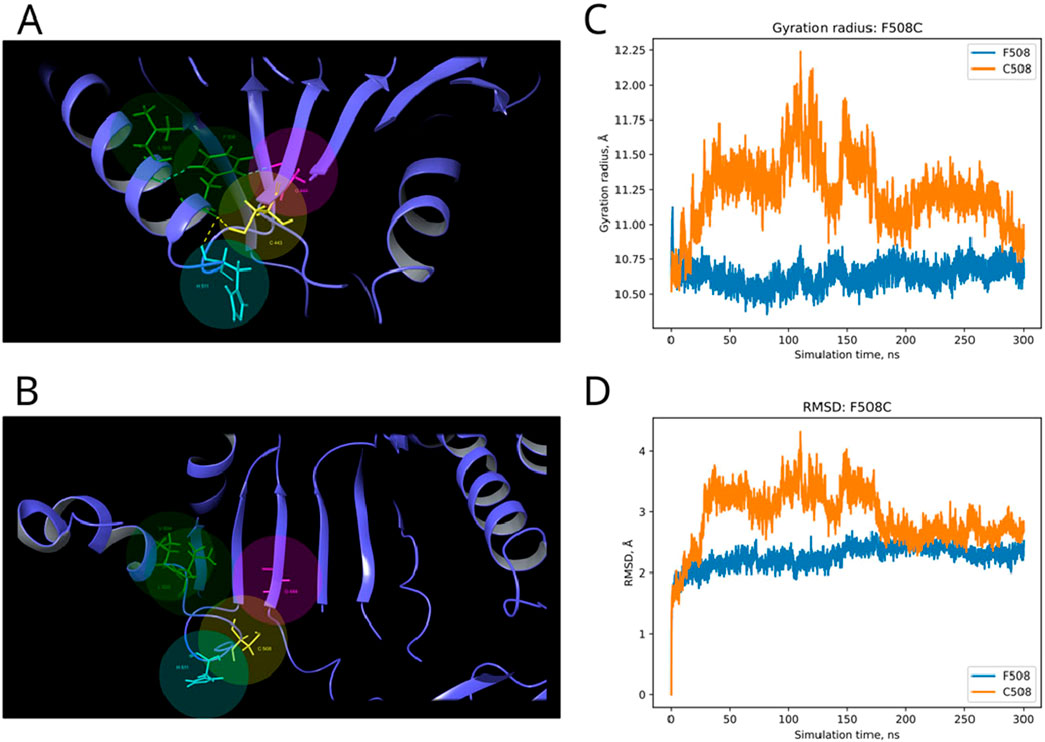
Figure 7. Structural alterations of Dicer1 variant F508C. (A) Interactions formed by wild-type amino acid F508. (B) Interactions formed by mutation C508. Amino acids taking part in bond formation are marked by spheres. H-bonds are indicated by dashed yellow lines, and aromatic H-bonds are indicated by dashed blue lines. Protein secondary structural elements (α-helixes, β-strands, and disordered loops) are shown in blue by cartoon representation. The radius of gyration (C) and RMSD (D) fluctuations of the 10 Å region around the wild-type amino acid and corresponding mutation through a 300-ns MD simulation.
In the published article, there was an error in Figure 8 as published. The Figures 7, 8 were mixed up. The corrected Figure 8 and its caption appear below.
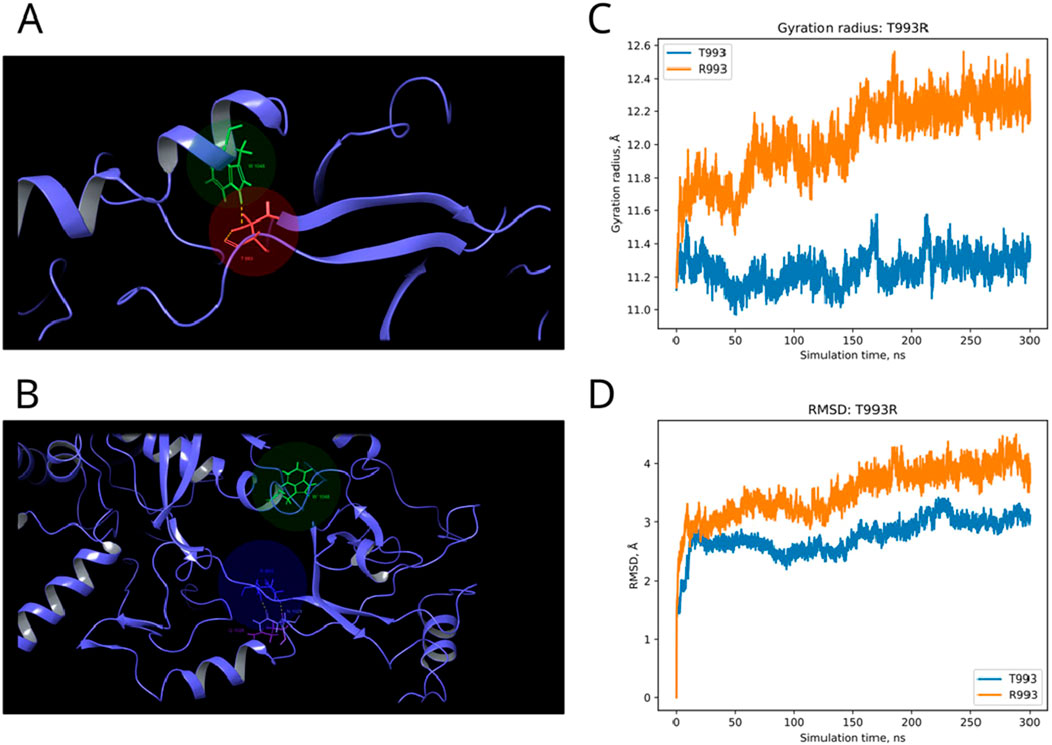
Figure 8. Structural alterations of Dicer1 variant T993R. (A) Interactions formed by wild-type amino acid T993. (B) Interactions formed by mutation R993. Amino acids taking part in bond formation are marked by spheres. H-bonds are indicated by dashed yellow lines, and aromatic H-bonds are indicated by dashed blue lines. Protein secondary structural elements (α-helixes, β-strands, and disordered loops) are shown in blue by cartoon representation. The radius of gyration (C) and RMSD (D) fluctuations of the 10 Å region around the wild-type amino acid and corresponding mutation through a 300-ns MD simulation.
The authors apologize for these error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: Dicer1, variant of uncertain significance, variant effect prediction, gene evolution, oncology, molecular dynamics
Citation: Bug DS, Moiseev IS, Porozov YB and Petukhova NV (2024) Corrigendum: Shedding light on the DICER1 mutational spectrum of uncertain significance in malignant neoplasms. Front. Mol. Biosci. 11:1517143. doi: 10.3389/fmolb.2024.1517143
Received: 25 October 2024; Accepted: 11 November 2024;
Published: 28 November 2024.
Edited and reviewed by:
Annalisa Pastore, King’s College London, United KingdomCopyright © 2024 Bug, Moiseev, Porozov and Petukhova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: N. V. Petukhova, cGV0dWhvdmFudkAxc3BiZ211LnJ1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.