- Department of Biology, Andrews University, Berrien Springs, MI, United States
Inactive enzyme homologs, or pseudoenzymes, are proteins, found within most enzyme families, that are incapable of performing catalysis. Rather than catalysis, they are involved in protein-protein interactions, sometimes regulating the activity of their active enzyme cousins, or scaffolding protein complexes. Pseudoenzymes found within metallopeptidase families likewise perform these functions. Pseudoenzymes within the M14 carboxypeptidase family interact with collagens within the extracellular space, while pseudopeptidase members of the M12 “a disintegrin and metalloprotease” (ADAM) family either discard their pseudopeptidase domains as unnecessary for their roles in sperm maturation or utilize surface loops to enable assembly of key complexes at neuronal synapses. Other metallopeptidase families contain pseudopeptidases involved in protein synthesis at the ribosome and protein import into organelles, sometimes using their pseudo-active sites for these interactions. Although the functions of these pseudopeptidases have been challenging to study, ongoing work is teasing out the secret lives of these proteins.
1 Introduction
Enzyme families expand through the duplication and subsequent mutation of progenitor genes. While this process often results in the degeneration of duplicate genes to form pseudogenes, lacking functional open reading frames, duplicate genes may continue to produce functional proteins (Conant and Wolfe, 2008; Copley, 2020). These proteins may retain their original functions, restricted to subsets of tissues in which the progenitors were expressed (subfunctionalization), or at different developmental times or places. In yet other cases, proteins acquire new functions through changes in enzyme substrate specificities or activation mechanisms (neofunctionalization), or through loss of enzymatic activity but maintenance of interactions previously involved in enzyme function or intracellular signaling. Occasionally, these inactive enzymes (pseudoenzymes) acquire novel interactions and therefore novel functions.
Recently it has been appreciated that as many as 10% of proteins within any enzyme family may consist of pseudoenzymes (Ribeiro et al., 2019). Pseudoenzymes are more difficult to study than their enzyme relatives, lacking clear activities to track. Therefore, we are just beginning to learn of their biological and biochemical functions (Zaru et al., 2020). Work to date has shown these functions to include regulation of active enzymes, integration of molecular signals, interaction with protein complexes, and competition for enzyme substrates or enzyme complex assembly (Reynolds and Fischer, 2015; Murphy et al., 2017; Goldberg and Sreelatha, 2023). In some cases, it has been found that certain pseudoenzymes are not pseudoenzymes at all, as new enzymatic functions have been discovered (Sreelatha et al., 2018; Black et al., 2019; Park et al., 2022). Some pseudoenzymes have been implicated in diseases, highlighting the need to understand more about their mechanisms of action (Fukata et al., 2006; Adrain et al., 2012; Reiterer et al., 2014; Cui et al., 2015; Dulloo et al., 2019).
Recent reviews have highlighted the functions of pseudoenzymes from the kinase and phosphatase families (Reiterer et al., 2020; Mace and Murphy, 2021; Mattei et al., 2021; O'Boyle et al., 2022; Goldberg and Sreelatha, 2023). Pseudokinases have co-opted kinase activation mechanisms to initiate allosteric interactions through molecular switch conformational changes and the formation of signaling scaffolds (Mace and Murphy, 2021). Pseudophosphatases function in similar ways (Reiterer et al., 2014; Mattei et al., 2021; Goldberg and Sreelatha, 2023). Less has been said about pseudoenzymes from peptidase families, although some studies have investigated the functions of serine and cysteine pseudopeptidases (Reynolds and Fischer, 2015). Serine pseudopeptidases have been shown to play important roles in fertility, development, and immune responses, and these have been reviewed elsewhere (Reynolds and Fischer, 2015; Zupanic et al., 2023). The iRhoms are inactive members of the rhomboid protease family. While the rhomboid proteases cleave transmembrane substrates for intercellular signaling, the iRhoms bind to transmembrane domains without cleaving them, modifying the trafficking of these membrane proteins from the endoplasmic reticulum (Adrain and Freeman, 2012). Pseudopeptidases within caspase and metacaspase families have also been identified. Metacaspases 1 and 4 within Trypanosoma brucei are inactive enzymes that interact with and regulate the functions of their active cousins in parasitic virulence. C-FLIPL is a mammalian caspase 8 homolog that regulates the extrinsic apoptosis pathway through interaction with caspases 8 and 10 (Reynolds and Fischer, 2015).
While the above illustrates the importance of serine and cysteine pseudopeptidases, the largest family of human peptidases is the metallopeptidase family (Puente et al., 2003). Here I review current knowledge regarding pseudoenzymes within the metallopeptidase family, a number of which have been shown to have significant roles in health and disease (Table 1). I consider their biochemical and biological functions, and the specific modifications that have led to their becoming pseudoenzymes (Table 2). Since the majority of pseudometallopeptidases are found within the M12 and M14 families (MEROPS classification), I highlight these.
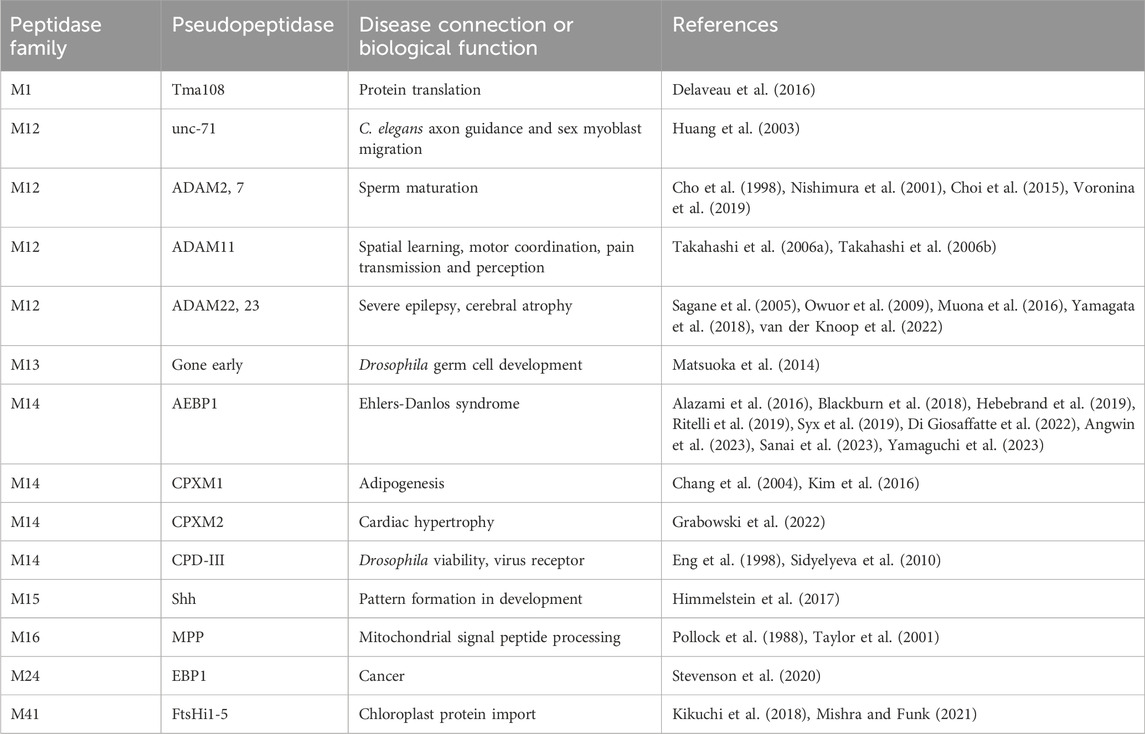
Table 1. Pseudopeptidase members of the metallopeptidase family and their known biological functions.
2 M12 ADAMs
The ADAM (a disintegrin and metalloprotease) family is a large family of single-pass transmembrane proteins, found within the larger metzincin family of metalloproteases. Metzincins include the matrix metalloproteases (MMPs), ADAMs, ADAM proteases with thrombospondin motifs (ADAMTSs), astacins, and others, all of which contain a zinc within their catalytic sites and a conserved methionine within an active site loop, the Met-turn (Gomis-Rüth, 2009). ADAMs typically have extracellular metalloprotease and disintegrin domains, followed by cysteine-rich and EGF repeat regions, a transmembrane segment, and a C-terminal cytosolic tail (Figure 1A) (Seals and Courtneidge, 2003; Alfandari et al., 2009). The disintegrin domain is typically involved in protein-protein interactions, often as a ligand for integrin receptors during cell adhesion (Bridges et al., 2005). The metalloprotease domain functions in the shedding of a variety of cell surface proteins such as the ephrins and cadherins (Seals and Courtneidge, 2003). A number of pseudopeptidases exist in the ADAM family (Figure 1B) with primary roles in neuronal migration, sperm maturation, and CNS function. These pseudopeptidases have many substitutions of both zinc-binding and general base active site residues (Table 2), although they typically conserve the “Met-turn” characteristic of the enzyme family that appears to play an important structural role (Tallant et al., 2010).
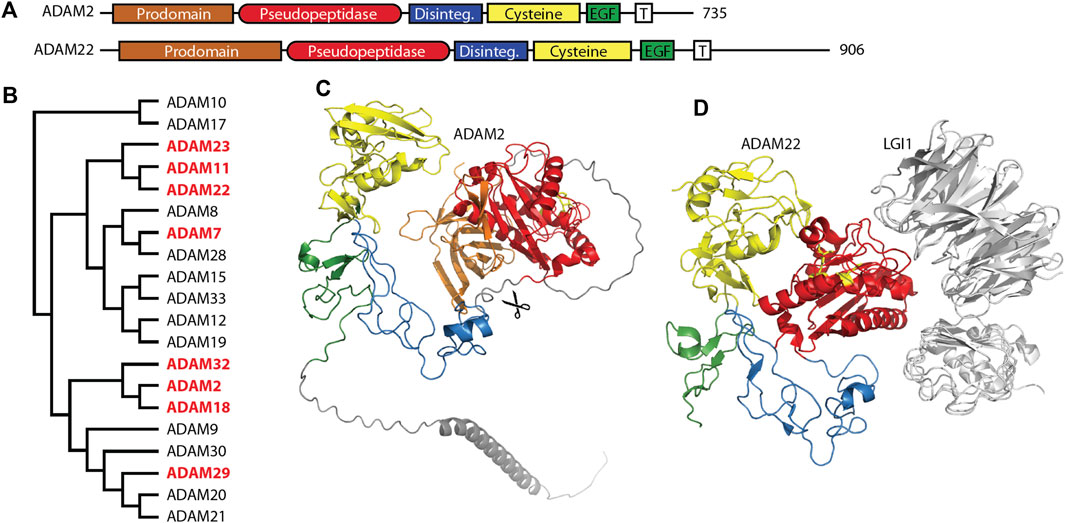
Figure 1. Many members of the M12 ADAM family of metallopeptidases are pseudopeptidases. (A) ADAM family members have a very similar domain structure, with an N-terminal prodomain and pseudopeptidase domain followed by a disintegrin domain, cysteine-rich domain, EGF-like domain, and a transmembrane segment leading to a cytosolic C-terminus. (B) Protein sequences for all human ADAMs were aligned with Clustal Omega and a midpoint-rooted cladogram prepared with Iqtree2 and Dendroscope. Pseudopeptidase members are shown in red. (C) The predicted structure of human ADAM2, as modeled by Alphafold (AF-Q99965-F1-model_v4). Domains are color-coded as shown in (A), scissors indicate the location of proteolytic cleavage, and pseudoactive site residues are shown as yellow sticks. (D) The structure of human ADAM22 in complex with LGI1 (5y31), showing that the surface of ADAM22, not the pseudoactive site (yellow sticks), is involved in this interaction. Domains are color-coded as shown in (A).
2.1 Unc-71/ADAM14, a C. elegans pseudoenzyme involved in axon and cell guidance
The C. elegans genome contains four ADAM genes (adm-1, adm-2, adm-4, and sup-17) and one secreted ADAM-like gene (mig-17). The adm-1 gene, also known as unc-71 or ADAM-14, was identified in the original C. elegans screen as an allele that resulted in uncoordinated movement (Brenner, 1974; Podbilewicz, 1996). It was thought to be an inactive enzyme due to the identity of key active site amino acids (Table 2). A number of loss-of-function mutations were identified in the disintegrin domain that resulted in motor axon guidance and sex myoblast migration defects; a version of UNC-71 that lacked the metalloprotease domain was unable to rescue unc-71, suggesting a necessary, although currently unknown, role for this pseudopeptidase in the guidance of cells and growth cones within C. elegans (Huang et al., 2003).
2.2 ADAM1, ADAM2, ADAM3, ADAM6, ADAM7, ADAM18, ADAM29 and ADAM32, pseudopeptidase-containing proteins necessary for sperm maturation
Many ADAM genes, including many that encode pseudopeptidases, are solely or primarily expressed in the testes and play a role either in sperm maturation and/or fertilization (Cho, 2012). Early work showed the pseudopeptidase-containing proteins ADAM1 and ADAM2 (also known as fertilin α and fertilin β) to form a heterodimer necessary for sperm-egg fusion in mice (Primakoff et al., 1987). Targeted deletion of ADAM2, ADAM3, and ADAM6 resulted in infertile mice (Cho et al., 1998; Nishimura et al., 2001; Voronina et al., 2019). Humans lack functional ADAM1, ADAM3, and ADAM6 genes, and so rely on the remaining ADAM2 gene, which is primarily expressed in the testes (proteinatlas.org).
Molecular studies have shed light on the role of these mouse and human ADAMs. Recombinant forms of ADAM1 that correspond to either the disintegrin-like domain or the cysteine-rich and EGF-like repeat can interfere with sperm-egg binding (Wong et al., 2001). A short 8-amino acid segment of the disintegrin domain of ADAM1 can inhibit the binding of both ADAM1 and ADAM2 to eggs, suggesting the critical component of these proteins for sperm adhesion is found within the disintegrin domain (Wong et al., 2001). Many experiments have shown interactions between ADAMs and integrin heterodimers, including interactions with ADAMs 1, 2, and 3, and have been reviewed elsewhere (Lu et al., 2007). It appears that the disintegrin domain is necessary and sufficient for sperm-egg interactions, as the metalloprotease domains of ADAM1, ADAM2, and ADAM3 are proteolytically removed during sperm development (Figure 1C) (Lum and Blobel, 1997; Eto et al., 2002; Marcello and Evans, 2010). This suggests that the metalloprotease domain is dispensable for the functions of these ADAMs.
While ADAM2 is known to have an important role in human fertility, as do ADAMs 1, 3, and 6 in mice, four additional human ADAM pseudopeptidase genes, ADAMs 7, 18, 29 and 32, are also expressed primarily in the human testes. There have been no experiments reported on the function of ADAM18. Experiments in the mouse model suggest that ADAM32 may not be necessary for fertility, as Adam32-mutant mice had normal fertility, testicular integrity, and sperm characteristics (Lee et al., 2020). Abnormally high expression of ADAM32 was recently observed in hepatoblastoma (Fukazawa et al., 2022); experiments in HepG2 and primary hepatoblastoma cells showed that colony formation, cell migration and invasion, and cell viability were increased upon overexpression of ADAM32 and decreased upon knockdown. Together with other experiments it was suggested that ADAM32 may play a role in apoptosis signaling in these cells. ADAM29, although normally expressed in the testes, has been implicated in a variety of cancers, including colon cancer (Alrubie et al., 2024), esophageal cancer (Song et al., 2014; Wang et al., 2020), breast cancer (Purrington et al., 2014; Zhao et al., 2016), melanoma (Wei et al., 2011), and chronic lymphocytic leukemia (Oppezzo et al., 2005; Kienle et al., 2010).
ADAM7 is closely related to ADAM28 (see Figure 1B), an enzymatically active ADAM highly expressed in the epididymis and lymphocytes (Howard et al., 2001). Cell adhesion assays showed that ADAM7 was recognized by the leukocyte integrins α4β1, α4β7, and α9β1; however, this interaction required only the disintegrin domain (Bridges et al., 2005). Early studies showed that ADAM7 was expressed primarily in the mouse and human epididymis and in the anterior pituitary (Cornwall and Hsia, 1997; Lin et al., 2001). In the mouse epididymis, ADAM7 protein was found to be transferred without proteolytic processing to the surface of sperm, where it functioned as an integral plasma membrane protein (Oh et al., 2005; Oh et al., 2009). Proteomics analysis showed that Adam7 formed sperm membrane complexes with the protein chaperones calnexin and heat shock protein 5, and with the integral membrane protein Itm2b within detergent-resistant membranes (Han et al., 2011). It was proposed that these interactions may play a role in sperm capacitation. Indeed, targeted disruption of the Adam7 gene in the mouse showed decreased male fertility due to histological changes in the epididymal epithelium and abnormal sperm function and morphology (Choi et al., 2015). It is not clear, however, what domains of the ADAM7 protein are necessary for its epididymal and sperm function. While the normal function of ADAM7 is in the epididymis, a study of somatic ADAM mutations present in human cutaneous metastatic melanoma found that ADAM7 was frequently mutated and many of these mutations led to decreased cell adhesion to collagen IV and laminin and increased cell migration (Wei et al., 2011). A number of these mutations were present within the metallopeptidase-like domain, with one occurring at the Met-Turn, suggesting a role for the pseudopeptidase domain in this system.
2.3 ADAM11, ADAM22 and ADAM23 in CNS function
ADAM11/MDC, ADAM22/MDC2, and ADAM23/MDC3 contain inactive metallopeptidase domains (Figures 1A, D) and are highly expressed in the brain (Sagane et al., 1998). These three proteins have common roles at the neuronal synapse where they interact with LGI proteins, PSD-95 and voltage-gated potassium (Kv) channels. It is likely that minor differences between these three ADAM proteins lie in differences in interaction affinity and expression patterns. A number of recent reviews have considered their functions in detail (Hsia et al., 2019; Yamagata and Fukai, 2020; Fukata et al., 2021b).
ADAM11 was first identified as a potential tumor suppressor gene, that was subsequently found to be expressed primarily in neurons in the mouse CNS and PNS (Katagiri et al., 1995; Rybnikova et al., 2002). While ADAM11-deficient mice were largely normal, they exhibited some deficits in spatial learning, motor coordination, and pain transmission and perception (Takahashi et al., 2006a; Takahashi et al., 2006b). ADAM22 was found expressed only in the nervous system, and a knockout mouse model showed peripheral neuron hypomyelination with severe ataxia and convulsions (Sagane et al., 2005). ADAM23 was also highly expressed in the CNS and mouse knockout resulted in lethal epilepsy (Owuor et al., 2009).
The primary roles for these ADAM proteins appear to be as membrane-bound receptors for the secreted neuronal leucine-rich glioma inactivated (LGI) proteins (Figure 1D). ADAM11, ADAM22, and ADAM23 all interact with both LGI1 and LGI4 at the neuronal synapse (Sagane et al., 2008; Owuor et al., 2009). The ADAM22-LGI1 receptor-ligand pair, scaffolded at the synapse with stargazin by PSD-95, enhanced AMPA receptor-mediated synaptic transmission in hippocampal slices (Fukata et al., 2006). Biallelic mutations in ADAM22 prevented protein expression, LGI1 binding, and/or interaction with PSD-95, and, most importantly, resulted in cerebral atrophy leading to infantile-onset epilepsy with profound delay in intellectual and motor development (Muona et al., 2016; van der Knoop et al., 2022). While one study showed that a mutation of ADAM22 within its disintegrin domain prevented binding of LGI1 (Fukata et al., 2006), recent structural characterization of the ADAM22-LGI1 interaction showed that the interaction involved specific residues on the surface of the ADAM22 metalloprotease domain (Figure 1D) (Yamagata et al., 2018). Mutation of these residues in both ADAM22 and ADAM23 disturbed the interaction with LGI1, while mutation of a nearby cysteine was identified in a patient with progressive encephalopathy, cortical atrophy, and severe epilepsy (Muona et al., 2016; Yamagata et al., 2018).
The details of the interactions mediated by ADAM11, 22, and 23 at the synapse have been explored through mouse models. Kole et al. (2015) showed through immunofluorescence and immunoprecipitation that ADAM11 was highly expressed in cerebellar basket cell terminals where it associated with ADAM22, PSD-95, and Shaker type Kv1 potassium channels. An Adam11Δ12-18 mutant mouse lacked expression of these proteins at the basket cell termini that impinged on Purkinje cell initial segments and also lacked the rapid ephaptic inhibition of Purkinje cells normally performed by these basket cell interactions, suggesting that ADAM11 played a key role in organizing synaptic proteins (Kole et al., 2015). Similar results were shown for ADAM22 and ADAM23. ADAM22-interacting proteins were identified in the mouse brain using immunoprecipitation and mass spectrometry, and included LGI family members, ADAM22 family members, presynaptic and postsynaptic MAGUKs such as PSD-95, presynaptic Kv1 channels, and 14-3-3 proteins (Ogawa et al., 2010; Fukata et al., 2021a). Loss of the C-terminal PDZ-interacting motif from ADAM22 via a knock-in approach resulted in lethal epilepsy, and super-resolution microscopy indicated a loss of alignment between pre- and post-synaptic proteins. A careful study of ADAM22 and LGI1 hypomorphic mice showed that 50% of LGI1 and 10% of ADAM22 was sufficient to prevent lethal epilepsy. Importantly, this study demonstrated that levels of ADAM22 could be maintained through increased interaction with 14-3-3 following phosphorylation of ADAM22 by protein kinase A (Yokoi et al., 2021). Other studies have continued to show functional interactions between these ADAM proteins and Kv1 channels and LGI family proteins leading to regulation of axon physiology and synaptic plasticity (Lovero et al., 2015; Hivert et al., 2019; Kozar-Gillan et al., 2023; Miyazaki et al., 2024). ADAM11 has also been shown to play a role in Xenopus neural tube closure and cranial neural crest cell migration through its interaction with Wnt and BMP4 pathway proteins, and to serve as a ligand for integrin alpha4, thus supporting cell adhesion as do other ADAM proteins (Wang et al., 2018; Pandey et al., 2023). A variety of reports have implicated ADAM22 and ADAM23 in cancer as well. It appears that ADAM11, ADAM22, and ADAM23 utilize both their disintegrin and pseudopeptidase domains in a variety of protein-protein interactions.
3 M14 carboxypeptidases
The M14 family of metallocarboxypeptidases is a large family of enzymes (Figure 2B) responsible for cleaving C-terminal amino acids from substrate proteins and peptides (Reznik and Fricker, 2001; Arolas et al., 2007; Gomis-Ruth, 2008; Fernandez et al., 2010). These enzymes are commonly known to be involved in dietary protein digestion and neuropeptide processing. The family contains four pseudopeptidase members within vertebrate organisms, and one characterized pseudopeptidase in the yeast Saccharomyces cerevisiae, the only member of the M14 family in yeast (Figure 2A).
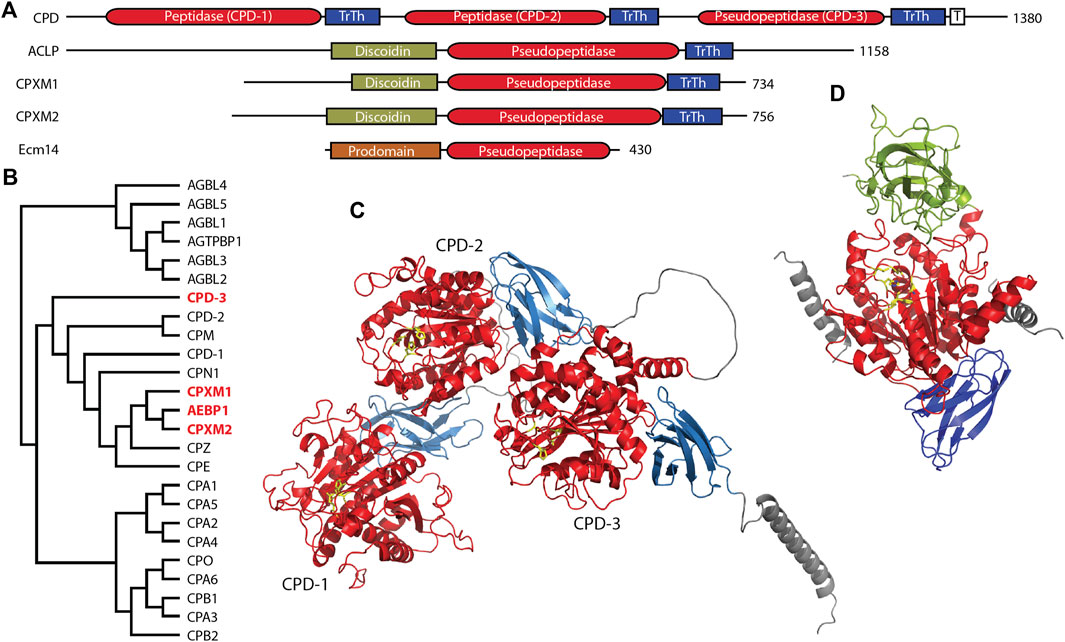
Figure 2. Four members of the human M14 metallocarboxypeptidase family are pseudopeptidases. (A) The domain structure of M14 pseudopeptidases. CPD contains 3 peptidase/transthyretin domain units and a C-terminal transmembrane segment; ACLP, CPXM1, CPXM2 all have an N-terminal discoidin domain; Ecm14 from S. cerevisiae is closely related to the digestive A/B carboxypeptidases with an N-terminal prodomain. (B) Protein sequences for human M14 metallopeptidase domains were aligned with Clustal Omega and a midpoint-rooted cladogram prepared with Iqtree2 and Dendroscope. These fall into three major groups, the cytosolic carboxypeptidases (top), the regulatory carboxypeptidases (middle) and the digestive carboxypeptidases (bottom). Pseudopeptidase members are shown in red. (C) The predicted structure of human CPD, as modeled by Alphafold (AF-O75976-F1-model_v4). Domains are color-coded as shown in (A), and pseudoactive site residues are shown as yellow sticks. (D) The predicted structure of human AEBP1/ACLP as modeled by Alphafold (AF-Q8IUX7-F1-model_v4) with pseudoactive site residues shown in yellow; disordered N- and C-termini are not included. AlphaFold shows two helices (gray) derived from the N-terminal repetitive and disordered region on either side of the pseudopeptidase domain. Domains are color-coded as shown in (A). Note also that the arrangement of the discoidin and carboxypeptidase domains relative to each other is predicted by AlphaFold and requires experimental validation.
3.1 CPD, an inactive domain working with two active domains to process bioactive proteins and peptides
Carboxypeptidase D (CPD) is a protein with important roles in bioactive peptide processing (Dong et al., 1999; Varlamov et al., 1999; Zhang et al., 2008). It was discovered in a search for enzymes with carboxypeptidase E (CPE)-like activity (Song and Fricker, 1995). Both CPD and CPE are involved in the maturation of neuroendocrine peptides formed by the cleavage of long precursor proproteins by proprotein convertases (e.g., furin, PC1/3, PC2, etc.) (Zhang et al., 2008; Wardman et al., 2010). Proprotein convertases cleave at dibasic sites, often with the consensus sequence R-x-K/R-R. C-terminal basic amino acids are then removed through the action of CPE to form the fully mature peptide. Even in the absence of CPE, however, C-terminal neuroendocrine peptide processing activity remains, due to the activity of CPD (Dong et al., 1999; Fricker and Leiter, 1999; Zhang et al., 2008).
CPD is a large protein consisting of an N-terminal signal peptide, three carboxypeptidase/transthyretin domain units, and a C-terminal transmembrane segment and cytosolic tail (Figures 2A, C) (Fricker et al., 1996; Fricker, 2004). While CPD contains three tandem carboxypeptidase domains, only the first two of these are active (Tan et al., 1997); the third domain has substitutions at nearly all active-site residues (Table 2). The second domain has had its structure characterized by X-ray crystallography (Gomis-Ruth et al., 1999; Aloy et al., 2001), and AlphaFold has predicted the structure of the entire protein (Figure 2C). While this AlphaFold prediction requires experimental validation, it does present a model of how the three domains may associate. CPD expression is widely distributed throughout the body (Song and Fricker, 1996; Xin et al., 1997).
The three domains of CPD appear to have complementary functions. The active first and second domains have different specificities, with the first domain preferring C-terminal arginine and a pH of 6.3–7.5, and the second domain preferring C-terminal lysine and a pH of 5.0–6.5 (Novikova et al., 1999; Sidyelyeva and Fricker, 2002; Garcia-Pardo et al., 2017). Prior to the characterization of CPD as an enzyme, it was identified as a duck glycoprotein of 180 kDa (gp180) that bound the preS envelope protein of duck hepatitis B virus, enabling viral entry (Kuroki et al., 1995). While deletion of the first and second domains eliminated enzymatic activity, only deletion of the third domain eliminated preS binding (Eng et al., 1998). It has been suggested that this third domain may play a role in the binding of proteins and/or the presentation of substrates to the other active domains (Aloy et al., 2001), and this possibility is supported by the AlphaFold prediction (Figure 2C). Whatever this binding function may be, it has been shown to be crucial for Drosophila survival. A variety of transgenes expressing various domains of CPD were placed in an embryonic lethal Drosophila mutant, svrPG33 (Sidyelyeva et al., 2010). It was found that both of the two active domains were necessary for viability; however, lack of the third (inactive) domain dramatically reduced this viability, when considering both survival from embryo to adult and from pupa to adult. This supports the possibility that the third domain may assist in the function of the active domains, perhaps through presentation of substrates.
3.2 AEBP1/ACLP, a regulator of cellular differentiation and collagen fiber properties within connective tissue
Adipocyte enhancer binding protein 1 (AEBP1) and aortic carboxypeptidase-like protein (ACLP) are both encoded by the AEBP1 gene. Both ACLP and AEBP1 contain a discoidin domain and a carboxypeptidase/transthyretin domain unit; C-terminal repetitive sequences enable AEBP1 DNA binding (Figures 2A, D) (Lyons et al., 2005; Lyons et al., 2006). Although AEBP1 was initially characterized as a transcriptional repressor that required an intact and enzymatically-active carboxypeptidase domain for this function (He et al., 1995; Muise and Ro, 1999), enzymatic activity was not detected in subsequent studies (Song and Fricker, 1997; Reznik and Fricker, 2001; Lyons et al., 2006); analysis of amino acid sequence shows that all active site amino acids are substituted except for two zinc-binding amino acids (Table 2). The nuclear AEBP1 protein isoform is derived from an alternatively-spliced or truncated mRNA (Layne et al., 1998; Ro et al., 2001), while the widely-expressed and predominant protein isoform, ACLP, contains additional repetitive amino acid sequence N-terminal to the discoidin-like domain (Figure 2A) and a signal peptide that results in its secretion and function in the extracellular matrix (Layne et al., 1998). The expression of two different proteins (AEBP1 and ACLP) with different localizations, and thus different functions, from one gene (AEBP1), presents some confusion in terminology and interpretation of experimental results, and therefore requires careful examination of the experimental details in any particular study.
The intracellular variant, AEBP1, was first identified as a transcriptional repressor that bound the AE-1 enhancer element upstream of the adipose P2 gene in preadipocytes and was downregulated upon differentiation of these cells (He et al., 1995). A similar AEBP1 cDNA was identified in an osteoblastic cell line where expression was also turned off upon final differentiation (Ohno et al., 1996). Subsequent work implicated AEBP1 in obesity and inflammation through a variety of protein-protein interactions. The gamma5 subunit of a heterotrimeric G protein was found to interact with AEBP1 via a yeast two-hybrid screen, and this interaction regulated the transcriptional repressor activity of AEBP1 at the onset of adipocyte differentiation (Park et al., 1999). Another yeast two-hybrid screen identified PTEN as an interacting partner for AEBP1 (Gorbenko et al., 2004). Co-immunoprecipitation experiments supported an interaction via the carboxypeptidase domain of AEBP1; this interaction stimulated PTEN degradation and correlated with enhanced adipose apoptosis and resistance to diet-induced obesity in AEBP1 knockout mice (Ro et al., 2007). The opposite, massive obesity, was observed in female transgenic mice, with AEBP1 overexpression in adipose tissue and macrophages (Zhang et al., 2005). AEBP1 was found to interact with the mitogen-activated protein kinases ERK1 and ERK2 through its discoidin-like domain, preventing dephosphorylation of ERK1/2 and correlating with a decreased ability of 3T3-L1 cells to undergo adipogenesis (Kim et al., 2001). Finally, in vitro and ex vivo studies identified a role for AEBP1 in regulating cholesterol efflux from macrophages and promoting foam cell formation and therefore likely playing a role in atherosclerosis (Majdalawieh et al., 2006; Majdalawieh and Ro, 2009; Huang et al., 2012). As similar results were not observed for a DNA binding-deficient mutant of AEBP1, these results were suggested to be through a transcriptional mechanism (Majdalawieh et al., 2006); subsequent work suggested that the interaction of the discoidin-like domain of AEBP1 with IκBα was also responsible (Majdalawieh et al., 2007). Further in vivo analysis of AEBP1 knockout mice and transgenic mice with macrophage-specific overexpression of AEBP1 showed a role for AEBP1 in the development of atherosclerotic lesions in the proximal aorta (Bogachev et al., 2011). Altogether, a variety of results have suggested a role for the intracellular AEBP1 variant in the control of cellular differentiation through the regulation of intracellular signals and transcription.
While the above data suggest possible roles for a shorter AEBP1 variant in cellular differentiation, current gene expression databases show that the primary mRNA expressed by the AEBP1 gene is the longer variant producing the ACLP protein. ACLP is highly expressed in smooth muscle cells of the aorta (Layne et al., 1998; Layne et al., 2002) and other major arteries (GTex). It is broadly expressed in collagen-rich tissues, including vasculature, dermal, and skeletal tissues, primarily in the extracellular matrix (Layne et al., 2001; Ith et al., 2005). Like AEBP1, ACLP was found to be expressed in 3T3-L1 preadipocytes and downregulated upon stimulation of differentiation (Gagnon et al., 2002). Although overexpression of ACLP in 3T3-L1 cells did not impact adipogenesis, the overexpression of ACLP in 3T3-F442A cells inhibited adipogenesis and promoted transdifferentiation into smooth muscle-like cells (Abderrahim-Ferkoune et al., 2004). Treatment of mouse and human lung fibroblasts with recombinant ACLP stimulated the fibroblast to myofibroblast transition through an upregulation of smooth muscle α-actin (SMA) and collagen expression (Tumelty et al., 2014); a similar result was obtained with mouse 10T1/2 cells and in this setting ACLP was connected with adipose tissue fibrosis (Jager et al., 2018). Other studies have implicated the AEBP1 gene and the ACLP protein in fibrosis, including lung fibrosis (Schissel et al., 2009), liver fibrosis and nonalcoholic steatohepatitis (Lou et al., 2017; Teratani et al., 2018; Gerhard et al., 2019), and renal fibrosis (Liu et al., 2023).
It is likely that ACLP interacts with collagen through its discoidin-like domain (see Figures 2A, D). Although no experiment has directly shown this, the related CPXM1 interacts with collagen in this way (Kim et al., 2015), and other discoidin domains have been shown to interact directly with collagens (Ichikawa et al., 2007). Several studies have shown the importance of ACLP-collagen interactions. When 3T3-L1 preadipocytes overexpressing ACLP were grown on collagen I-coated plates, differentiation was inhibited (Gusinjac et al., 2011). ACLP-null lung fibroblasts exhibited reduced cell spreading and proliferation when cultured on collagen; these phenotypes could be reversed upon extracellular addition of the ACLP discoidin-like domain (Schissel et al., 2009). In vitro experiments demonstrated that ACLP had a direct mechanical effect on collagen fibers, with collagen ACLP composite fibers exhibiting increased stiffness, toughness, and tensile strength (Vishwanath et al., 2020).
Animal models for the study of AEBP1/ACLP function, together with recent discoveries of disease connections, confirm the importance of the AEBP1 gene. Disruption of the AEBP1 gene, resulting in elimination of ACLP expression, led to the perinatal death of most homozygous null pups due to gastroschisis, the disruption of the abdominal wall (Layne et al., 2001). This gastroschisis was shown though immunohistochemistry to be a neuromuscular defect (Danzer et al., 2010). Those that survived birth had indications of defective wound healing as seen in skin lesions and reduced healing following a dermal punch (Layne et al., 2001). In another knockout mouse model, in which the gene deletion was similar, but smaller (disruption of exons 7–12, rather than exons 7–16 in the previous knockout), null offspring of heterozygous crosses were about half the expected numbers, corroborating an important role in embryonic development. In this case, however, the primary defect seen in knockouts was slower growth and decreased mass of white adipose fat pads (Ro et al., 2007). This AEBP1-null mouse also exhibited premature involution of mammary glands at late pregnancy that could be rescued by adipose-specific expression of an AEBP1 transgene (Zhang et al., 2011). It is unclear why different phenotypes were observed in these two knockout models of AEBP1 function. Although an early targeted search for AEBP1 mutations in infants affected by gastroschisis did not clearly support a connection (Feldkamp et al., 2012), a large number of recent publications have shown that mutations in the AEBP1 gene result in a subtype of autosomal recessive Ehlers-Danlos syndrome (EDS) (Alazami et al., 2016; Blackburn et al., 2018; Hebebrand et al., 2019; Ritelli et al., 2019; Syx et al., 2019; Di Giosaffatte et al., 2022; Angwin et al., 2023; Sanai et al., 2023; Yamaguchi et al., 2023). EDS is a heterogenous condition that usually presents with joint hypermobility, skin elasticity and fragility, poor wound healing, and risk of vascular rupture. EDS patients have an increased risk for abdominal wall herniation (Harrison et al., 2016), reminiscent of the gastroschisis observed in mutant mice. These disease phenotypes most closely match the observed functions for the extracellular ACLP protein isoform. The AEBP1 gene has additionally been implicated in a variety of cancers (Majdalawieh et al., 2020).
In summary, much has been published regarding AEBP1 and ACLP function. However, it is not clear what the relationship is between the various structural domains. Evidence points to a role for the discoidin domain in collagen interaction, and some studies of AEBP1 have suggested a role for the carboxypeptidase domain in other interactions. It is notable that the N-terminal unstructured region of ACLP is predicted to interact via two helices on either side of the carboxypeptidase domain, suggestive of a yet-uncharacterized regulatory function (Figure 2D). This, of course, is currently a predicted model and will need further experimental validation.
3.3 CPXM1 and CPXM2, collagen binding and cardiac hypertrophy
While much has been reported on the functions of AEBP1/ACLP, much less is known about the closely related proteins CPXM1 and CPXM2. Both CPXM1 and CPXM2 proteins were first identified through bioinformatics and shown to be inactive following expression in Sf9 cells and incubated with a number of artificial substrates (Xin et al., 1998; Lei et al., 1999). Sequence analysis shows that CPXM1 has a carboxypeptidase domain with a pseudo-active site very similar to the active sites of its active relatives, but for the change of the substrate-binding arginine 145 to histidine, while CPXM2 has two additional substitutions (Table 2). Similar to ACLP, both CPXM1 and CPXM2 have an inactive carboxypeptidase domain preceded by a discoidin domain and a signal peptide, so are secreted and have functions in the extracellular matrix. Both the AEBP1 and CPXM1 genes are duplicated in a wide range of fish species, while CPXM2 is not. This may be due to the nearly ten-fold larger size of the CPXM2 gene in comparison to AEBP1 and CPXM1 (Fajardo et al., 2023).
Expression of CPXM1 was found to be broad in adult tissues and high in developing mouse cartilage and skeletal structures (Lei et al., 1999); current expression databases show a preponderance of expression in fibroblasts. Using the RAW264.7 osteoclastogenesis model cell line, Chang et al. (2004) showed expression of CPXM1 in pre-osteoclasts that was downregulated upon maturation to osteoclasts. Overexpression of CPXM1 in these cells inhibited the differentiation process. Interestingly, investigation of the role of CPXM1 in adipocyte differentiation suggested an opposite role to that of AEBP1. CPXM1 was identified as a downstream effector of FGF-1 mediated proliferation and differentiation of human preadipocytes (Kim et al., 2016). Upon induction of adipocyte differentiation, CPXM1 expression increased (in contrast to the decrease of AEBP1 and ACLP expression upon 3T3-L1 differentiation (He et al., 1995; Gagnon et al., 2002; Abderrahim-Ferkoune et al., 2004)). Likewise, knockdown of CPXM1 inhibited differentiation as well as collagen expression (Kim et al., 2016). An increase in CPXM1 expression in the adipose tissue of obese mice and humans suggested a key role in adipogenesis (Kim et al., 2016), while increased CPXM1 expression in a mouse model of polycystic ovary syndrome suggested a role in the ovary as well (Pervaz et al., 2022). Further in vitro work through a collagen pull-down assay and mutagenesis of key amino acids within the discoidin domain showed that CPXM1 directly interacted with collagen III (Kim et al., 2015). The specific function of the pseudopeptidase domain is unknown.
CPXM2 is expressed in most tissues examined, with notably high expression in the choroid plexus (proteinatlas.org). Expression was detected by in situ hybridization in most regions of the mouse brain, consistent with current data from the human brain dataset (proteinatlas.com) (Xin et al., 1998). CPXM2, expressed in the Sf9 insect cell system, was able to bind to an Arg-Sepharose resin (Xin et al., 1998). It was not washed off with 200 mM NaCl, but could be eluted with 10 mM arginine, suggesting a specific interaction via its pseudo active site. This contrasted with CPXM1, which did not bind specifically to Arg-Sepharose (Lei et al., 1999). Several diseases have been connected with CPXM2, with varying levels of experimental support. The headbobber mouse contained a recessive mutation that resulted in headbobbing, circling, and deafness. This headbobber mutation was a result of transgene insertion that deleted a 648-kb segment of chromosome 7 encompassing three protein-coding genes, G protein-coupled receptor 26 (Gpr26), carboxypeptidase X2 (Cpxm2) and carbohydrate (N-acetylgalactosamine 4-sulfate 6-O) sulfotransferase 15 (Chst15), all expressed within the postnatal mouse inner ear and so candidates for the mutant phenotype (Somma et al., 2012; Buniello et al., 2013). Interestingly, similar mutations involving CPXM2 have been reported in humans further implicating CPXM2 and/or nearby genes in inner ear development (Miller et al., 2009; Buniello et al., 2013).
The CPXM2 gene has been associated with several diseases, many through genome-wide studies and with SNPs of unknown significance. For example, an exome sequencing and genotyping study of over 400 multiple sclerosis patients found a CPXM2 intronic polymorphism significantly associated with aggressive multiple sclerosis (Gil-Varea et al., 2018). Another genome-wide association study found a number of intronic CPXM2 SNPs associated with cognitive decline in schizophrenia (Hashimoto et al., 2013), and another found weak association with Alzheimer’s disease (Chen et al., 2016). Several studies have found association between overexpression of CPXM2 and cancers, gastric (Niu et al., 2019), hepatic (Ye et al., 2020), and osteosarcoma (Zhao et al., 2019).
Most interesting is the recent discovery of a role for CPXM2 in left ventricular cardiac hypertrophy (Grabowski et al., 2022). This study, initiated with a transcriptome analysis of the left ventricle, found an increased expression of CPXM2 in a rat hypertensive model system compared to controls. CPXM2 was upregulated in both cardiofibroblasts and cardiomyocytes, where it was found along the t-tubule network. Gene knockout in mice had a protective effect against left ventricular hypertrophy and fibrosis upon deoxycorticosterone acetate (DOCA) treatment and following 8 weeks of treatment no significant difference in left ventricular internal systolic diameter and ejection fraction was observed between DOCA-treated knockouts and sham groups. While over 1,000 transcripts were differentially expressed between wild-type sham and DOCA-treated mice, no differentially expressed transcripts were identified between knockout sham and DOCA-treated mice, suggesting an important, although currently unknown, role for CPXM2 in the mechanisms of cardiac hypertrophy.
3.4 Ecm14, a fungal pseudopeptidase with unknown function
The commonly used yeast model system, S. cerevisiae, contains one member of the M14 metallocarboxypeptidase family. The ECM14 gene (extracellular mutant 14) was initially identified in a screen for mutations that imparted sensitivity to calcofluor white, suggesting a cell wall defect (Lussier et al., 1997). The ECM14 mutant also exhibited sensitivity to hygromycin B and papulacandin, an increased ratio of mannose to glucose (Lussier et al., 1997), and sensitivity to sonication (Ruiz et al., 1999). The Ecm14 protein is homologous to the A/B subfamily of M14 metallocarboxypeptidases (see Figure 2B). It contains a prodomain, which can be cleaved by the action of trypsin or chymotrypsin, and a domain with homology to the carboxypeptidase domain, but with a substitution of the general base glutamate for a lysine (Table 2). Likely due to this substitution, no enzymatic activity has been detected, as determined by standard assays (Figure 1A) (McDonald et al., 2020). A synthetic lethal screen in the S. cerevisiae system was unable to identify a function (McDonald et al., 2020). However, other studies have found Ecm14 to be involved in aggregate invasive growth in C. albicans and possibly in invasion by the plant pathogen Verticillium dahlia (Chow et al., 2019; Zhang et al., 2020). There is still much to be learned about the function of this fungal pseudopeptidase; however, the fact that the prodomain can be cleaved suggests an activation mechanism for a yet-to-be-determined function.
4 Other pseudometallopeptidases
Most inactive members of mammalian peptidase families are found in the M12 and M14 families. There are, however, a few reported pseudopeptidases within other peptidase families.
Tma108, a member of the M1 aminopeptidase family, was first identified as a translation machinery-associated factor in the yeast S. cerevisiae (Fleischer et al., 2006). Further work showed that Tma108 interacted with the nascent protein chain as it emerged from the ribosome (Delaveau et al., 2016). Interestingly, Tma108 only interacted with a small subgroup of about 200 nascent proteins, this group being specified by an unknown motif within the first 35 amino acids of the nascent protein. Mutagenesis of a key residue in Tma108, known to be responsible for binding of substrate N-termini in related aminopeptidases, eliminated this interaction with nascent proteins, suggesting that Tma108 utilized its substrate binding pocket to bind the nascent protein. While it was not clear if Tma108 functioned as an active enzyme [it retains all active site residues except one involved in substrate binding (Table 2)], it was shown that Tma108 remained associated with its target protein until translation was completed, suggesting that it may play a non-enzymatic role (Delaveau et al., 2016).
ErbB3-binding protein 1 (Ebp1, or proliferation-associated 2G4, PA2G4) is another aminopeptidase homolog, within the M24 methionine aminopeptidase (MetAP) family, with relatively low sequence identity to the active MetAPs and retaining only two of five zinc-binding residues (Table 2) (Kowalinski et al., 2007). Ebp1 is best known for its role in cell growth and differentiation and therefore in the progression of many cancers. Depending on the context, Ebp1 can function as a tumor suppressor or as an oncogene (Stevenson et al., 2020). Ebp1 was shown to interact with a variety of RNAs, most strongly to ribosomal 5S RNA, suggesting an interaction with mature ribosomes in this manner (Kowalinski et al., 2007; Monie et al., 2007). Recently cryoEM structures of Ebp1 bound to 60S and 80S ribosomes have been reported showing Ebp1 bound to the ribosomal exit tunnel, excluding the interaction of modifying enzymes such as active MetAPs, chaperones, and targeting factors (Figure 3A) (Wild et al., 2020; Kraushar et al., 2021). The implications of this are not entirely clear, although it is likely that the presence of Ebp1 regulates the production of the nascent protein (Wild et al., 2020). The orientation of Ebp1 with the pseudoactive site toward the ribosomal exit site (Figure 3A) suggests that the MetAP active site has been repurposed for a role in guiding nascent proteins out of the ribosome, possibly playing a similar role as Tma108 (see above); however, high resolution details of the interaction between a nascent protein and Ebp1 are yet to be reported.
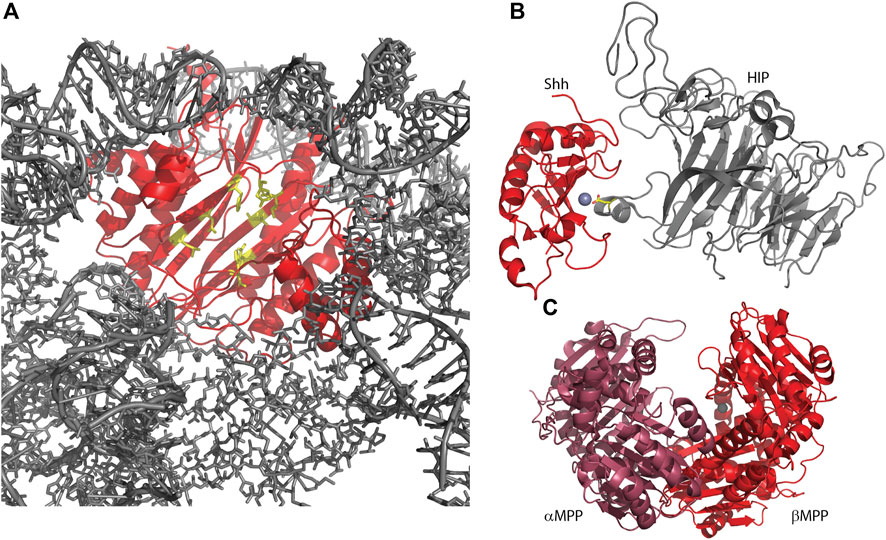
Figure 3. Several metallopeptidase homologs utilize their pseudo-active sites for protein interactions. (A) Human Ebp1, in complex with a human 80S ribosome (6SXO). The view shown is looking outwards through the ribosomal exit tunnel, surrounded by ribosomal components (gray) and capped by Ebp1 (red). Pseudo-active site residues are shown in yellow, available to capture the nascent protein N-terminus. (B) Sonic hedgehog protein (Shh) in complex with hedgehog-interacting protein (HIP; 2WG4) shows HIP directly interacting with the Shh-bound zinc ion via an aspartic acid (yellow sticks). (C) Yeast mitochondrial processing peptidase (MPP; 1HR6) is a heterodimer consisting of a beta subunit with bound zinc and an alpha subunit, lacking the necessary zinc-binding residues, but contributing other necessary residues for a functional enzyme.
Gone early (Goe) is an inactive pseudopeptidase member of the M13 neprilysin family in Drosophila (Matsuoka et al., 2014), with substitutions in all key active site positions (Table 2). Goe is a type II transmembrane protein that was detected on germ cell membranes that interfaced with other germ cells, but not on neighboring somatic cells. Overexpression of Goe on germ cells resulted in a decrease in the number of primordial germ cells entering the gametogenic pathway, while decreasing Goe expression increased primordial germ cell differentiation. It was suggested that, since Goe was expressed in EGF-producing cells and was able to suppress EGFR signaling in the wing imaginal disc, Goe may have been involved in trapping EGF ligands prior to their binding of a receptor. The extracellular portion of Goe, containing the pseudopeptidase domain, was necessary for this function, while the cytosolic portion was dispensable. Interestingly, this is reminiscent of the role of the iRhom pseudopeptidases in regulating EGF signaling in Drosophila (see Introduction) (Zettl et al., 2011).
Hedgehog (Hh) protein in Drosophila and its mammalian homologs Sonic hedgehog (Shh), Indian hedgehog (Ihh) and Desert hedgehog (Dhh) function as secreted ligands of the Patched1 (Ptc1) transmembrane protein. This prevents interaction of Ptc1 and Smoothened (Smo) so that Smo can translocate to cilia and activate the Gli transcription factors leading to a host of developmental responses. The structure of the N-terminal signaling domain of Shh determined by X-ray crystallography showed a fold and catalytic site homologous to that of the M15 metallopeptidases (Hall et al., 1995; Bosanac et al., 2009) (Figure 3B; Table 2). However, mutation of putative catalytic residues H135 and E177 did not affect the cellular signaling mediated by Shh as measured via cultured cells, nor its biophysical characteristics, leading to the conclusion that Shh was a pseudopeptidase (Day et al., 1999; Fuse et al., 1999). This was further supported by crystal structures showing that hedgehog interacting protein (HIP) bound to Shh directly through the pseudoactive site of Shh (Figure 3B), while binding studies showed that peptides derived from Ptc1 and HIP, and inhibitory antibodies, bound to the pseudoactive site as well (Bishop et al., 2009; Bosanac et al., 2009; Maun et al., 2010). Recent studies, however, have called this conclusion into question, suggesting that Shh, but not Drosophila Hh, may in fact be an active metallopeptidase. Himmelstein et al. (2017) examined the signaling capacity of Shh and the E177A mutant in vivo and found that the expression of Shh-E177A at endogenous sites resulted in patterning defects similar to those seen in Shh null mice. Subsequently, Jagers and Roelink showed that zinc coordination and E177 were required for Shh association with extracellular matrix and non-cell autonomous signaling (Jägers and Roelink, 2021). They suggested that Shh might be a proteoglycan peptidase that is required for the release of Shh into the ECM; other activities of Shh in vitro or in cell culture systems may not require this proteolytic function.
The mitochondrial processing peptidase, MPP, is a member of the M16B peptidase family. MPP is a dimeric protein, composed of a proteolytically active ∼500 residue beta subunit with a zinc-binding HXXEH motif, and a homologous, yet inactive, ∼500 residue alpha subunit that lacks the zinc binding motif (Figure 3C) (Pollock et al., 1988; Taylor et al., 2001). This inactive alpha subunit is essential for MPP function, as it assists in forming the clamshell active center and contains a glycine-rich loop important for substrate binding (Table 2). In related M16A and M16C enzymes, these two subunits are combined in one ∼1,000 residue polypeptide; hence, these enzymes also have a pseudopeptidase domain that is critically important for the enzymatic function of the whole (Li et al., 2006). Prokaryotic homologs of MPP have also been identified, produced from a two-gene operon reflecting the alpha/beta subunit arrangement seen in eukaryotic MPP (Aleshin et al., 2009; Maruyama et al., 2011; Ishida et al., 2022). It is notable that the Core I and II proteins of the bc1 mitochondrial respiratory complex are homologous to alpha and beta MPP; in some cases these respiratory proteins are pseudopeptidases (Braun and Schmitz, 1995; Taylor et al., 2001).
In addition to mitochondrial function, pseudometalloproteases have also found roles within chloroplasts. The FtsHi proteins are inactive FtsH enzymes of the M41 AAA metallopeptidase family, with very few active site residues conserved in these members (Table 2). Active FtsH enzymes are found in both inner mitochondrial membranes and chloroplast thylakoid membranes where they assemble into hexameric complexes and use their AAA + domains to hydrolyze ATP for the translocation of substrates into a proteolytic processing chamber. In contrast, all five of the FtsHi proteins (FtsHi1-5) were found to interact with the chloroplast inner membrane translocon complex, FtsHi3 within a 1 megadalton complex, and the others within a 2 megadalton complex, where they facilitated ATP-driven protein translocation (Kikuchi et al., 2018). Although their precise roles are not known, FtsHi proteins appear to be most important in seedling development, with FtsHi1 and FtsHi4 null mutants being embryonic lethal (Mishra and Funk, 2021).
5 Conclusion
As might be expected, pseudopeptidases within the metallopeptidase family have taken on a diversity of biological roles in lieu of enzymatic activity. In some cases, the pseudopeptidases make use of the pseudoactive site for interaction rather than catalytic purposes (Tma108, Shh, possibly Ebp1 and Cpxm2). In other cases, the pseudopeptidase domain is used for interactions via other surface regions (ADAM22, ADAM23, possibly gone early). Some proteins containing pseudopeptidase domains appear to primarily use other domains in interactions, leaving the pseudopeptidase domain seemingly superfluous (ADAMs involved in sperm maturation, using their disintegrin domains for interactions; AEBP1, CPXM1, CPXM2, likely interacting with collagen via their discoidin domains). αMPP is inactive on its own but is directly involved in the enzymatic activity of its partner, βMPP. In other cases, the jury is still out on the function of the pseudopeptidase domain. In at least one case, sonic hedgehog, there remains a possibility that it is not actually a pseudopeptidase. It is likely that future work will reveal more secrets of these superficially “dead” enzymes.
Author contributions
PL: Conceptualization, Investigation, Visualization, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Funding for this work was provided by Andrews University.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abderrahim-Ferkoune, A., Bezy, O., Astri-Roques, S., Elabd, C., Ailhaud, G., and Amri, E. Z. (2004). Transdifferentiation of preadipose cells into smooth muscle-like cells: role of aortic carboxypeptidase-like protein. Exp. Cell Res. 293 (2), 219–228. doi:10.1016/j.yexcr.2003.10.020
Adrain, C., and Freeman, M. (2012). New lives for old: evolution of pseudoenzyme function illustrated by iRhoms. Nat. Rev. Mol. Cell Biol. 13 (8), 489–498. doi:10.1038/nrm3392
Adrain, C., Zettl, M., Christova, Y., Taylor, N., and Freeman, M. (2012). Tumor necrosis factor signaling requires iRhom2 to promote trafficking and activation of TACE. Science 335 (6065), 225–228. doi:10.1126/science.1214400
Alazami, A. M., Al-Qattan, S. M., Faqeih, E., Alhashem, A., Alshammari, M., Alzahrani, F., et al. (2016). Expanding the clinical and genetic heterogeneity of hereditary disorders of connective tissue. Hum. Genet. 135 (5), 525–540. doi:10.1007/s00439-016-1660-z
Aleshin, A. E., Gramatikova, S., Hura, G. L., Bobkov, A., Strongin, A. Y., Stec, B., et al. (2009). Crystal and solution structures of a prokaryotic M16B peptidase: an open and shut case. Structure 17 (11), 1465–1475. doi:10.1016/j.str.2009.09.009
Alfandari, D., McCusker, C., and Cousin, H. (2009). ADAM function in embryogenesis. Semin. Cell Dev. Biol. 20 (2), 153–163. doi:10.1016/j.semcdb.2008.09.006
Aloy, P., Companys, V., Vendrell, J., Aviles, F. X., Fricker, L. D., Coll, M., et al. (2001). The crystal structure of the inhibitor-complexed carboxypeptidase D domain II and the modeling of regulatory carboxypeptidases. J. Biol. Chem. 276 (19), 16177–16184. doi:10.1074/jbc.M011457200
Alrubie, T. M., Shaik, J. P., Alamri, A. M., Alanazi, M., Alshareeda, A. T., Alqarni, A., et al. (2024). FTHL17, PRM2, CABYR, CPXCR1, ADAM29, and CABS1 are highly expressed in colon cancer patients and are regulated in vitro by epigenetic alterations. Heliyon 10 (1), e23689. doi:10.1016/j.heliyon.2023.e23689
Angwin, C., Ghali, N., and van Dijk, F. S. (2023). Case report: two individuals with AEBP1-related classical-like EDS: further clinical characterisation and description of novel AEBP1 variants. Front. Genet. 14, 1148224. doi:10.3389/fgene.2023.1148224
Arolas, J. L., Vendrell, J., Aviles, F. X., and Fricker, L. D. (2007). Metallocarboxypeptidases: emerging drug targets in biomedicine. Curr. Pharm. Des. 13 (3), 349–366. doi:10.2174/138161207780162980
Barrett, A. J., Rawlings, N. D., and Woessner, J. F. (2012). Handbook of proteolytic enzymes. Elsevier Science.
Bishop, B., Aricescu, A. R., Harlos, K., O'Callaghan, C. A., Jones, E. Y., and Siebold, C. (2009). Structural insights into hedgehog ligand sequestration by the human hedgehog-interacting protein HHIP. Nat. Struct. Mol. Biol. 16 (7), 698–703. doi:10.1038/nsmb.1607
Black, M. H., Osinski, A., Gradowski, M., Servage, K. A., Pawlowski, K., Tomchick, D. R., et al. (2019). Bacterial pseudokinase catalyzes protein polyglutamylation to inhibit the SidE-family ubiquitin ligases. Science 364 (6442), 787–792. doi:10.1126/science.aaw7446
Blackburn, P. R., Xu, Z., Tumelty, K. E., Zhao, R. W., Monis, W. J., Harris, K. G., et al. (2018). Bi-Allelic alterations in AEBP1 Lead to defective collagen assembly and connective tissue structure resulting in a variant of Ehlers-Danlos syndrome. Am. J. Hum. Genet. 102 (4), 696–705. doi:10.1016/j.ajhg.2018.02.018
Bogachev, O., Majdalawieh, A., Pan, X., Zhang, L., and Ro, H. S. (2011). Adipocyte enhancer-binding protein 1 (AEBP1) (a novel macrophage proinflammatory mediator) overexpression promotes and ablation attenuates atherosclerosis in ApoE (-/-) and LDLR (-/-) mice. Mol. Med. 17 (9-10), 1056–1064. doi:10.2119/molmed.2011.00141
Bosanac, I., Maun, H. R., Scales, S. J., Wen, X., Lingel, A., Bazan, J. F., et al. (2009). The structure of SHH in complex with HHIP reveals a recognition role for the Shh pseudo active site in signaling. Nat. Struct. Mol. Biol. 16 (7), 691–697. doi:10.1038/nsmb.1632
Braun, H. P., and Schmitz, U. K. (1995). Are the 'core' proteins of the mitochondrial bc1 complex evolutionary relics of a processing protease? Trends Biochem. Sci. 20 (5), 171–175. doi:10.1016/s0968-0004(00)88999-9
Brenner, S. (1974). The genetics of Caenorhabditis elegans. Genetics 77 (1), 71–94. doi:10.1093/genetics/77.1.71
Bridges, L. C., Sheppard, D., and Bowditch, R. D. (2005). ADAM disintegrin-like domain recognition by the lymphocyte integrins alpha4beta1 and alpha4beta7. Biochem. J. 387 (Pt 1), 101–108. doi:10.1042/bj20041444
Buniello, A., Hardisty-Hughes, R. E., Pass, J. C., Bober, E., Smith, R. J., and Steel, K. P. (2013). Headbobber: a combined morphogenetic and cochleosaccular mouse model to study 10qter deletions in human deafness. PLoS One 8 (2), e56274. doi:10.1371/journal.pone.0056274
Chang, E. J., Kwak, H. B., Kim, H., Park, J. C., Lee, Z. H., and Kim, H. H. (2004). Elucidation of CPX-1 involvement in RANKL-induced osteoclastogenesis by a proteomics approach. FEBS Lett. 564 (1-2), 166–170. doi:10.1016/S0014-5793(04)00338-2
Chen, Y. C., Hsiao, C. J., Jung, C. C., Hu, H. H., Chen, J. H., Lee, W. C., et al. (2016). Performance Metrics for Selecting single Nucleotide polymorphisms in late-onset Alzheimer's disease. Sci. Rep. 6, 36155. doi:10.1038/srep36155
Cho, C. (2012). Testicular and epididymal ADAMs: expression and function during fertilization. Nat. Rev. Urol. 9 (10), 550–560. doi:10.1038/nrurol.2012.167
Cho, C., Bunch, D. O., Faure, J. E., Goulding, E. H., Eddy, E. M., Primakoff, P., et al. (1998). Fertilization defects in sperm from mice lacking fertilin beta. Science 281 (5384), 1857–1859. doi:10.1126/science.281.5384.1857
Choi, H., Han, C., Jin, S., Kwon, J. T., Kim, J., Jeong, J., et al. (2015). Reduced fertility and altered epididymal and sperm integrity in mice lacking ADAM7. Biol. Reprod. 93 (3), 70. doi:10.1095/biolreprod.115.130252
Chow, J., Dionne, H. M., Prabhakar, A., Mehrotra, A., Somboonthum, J., Gonzalez, B., et al. (2019). Aggregate Filamentous growth responses in yeast. mSphere 4 (2), e00702. doi:10.1128/mSphere.00702-18
Conant, G. C., and Wolfe, K. H. (2008). Turning a hobby into a job: how duplicated genes find new functions. Nat. Rev. Genet. 9 (12), 938–950. doi:10.1038/nrg2482
Copley, S. D. (2020). Evolution of new enzymes by gene duplication and divergence. FEBS J. 287 (7), 1262–1283. doi:10.1111/febs.15299
Cornwall, G. A., and Hsia, N. (1997). ADAM7, a member of the ADAM (a disintegrin and metalloprotease) gene family is specifically expressed in the mouse anterior pituitary and epididymis. Endocrinology 138 (10), 4262–4272. doi:10.1210/endo.138.10.5468
Cui, J., Xiao, J., Tagliabracci, V. S., Wen, J., Rahdar, M., and Dixon, J. E. (2015). A secretory kinase complex regulates extracellular protein phosphorylation. Elife 4, e06120. doi:10.7554/eLife.06120
Danzer, E., Layne, M. D., Auber, F., Shegu, S., Kreiger, P., Radu, A., et al. (2010). Gastroschisis in mice lacking aortic carboxypeptidase-like protein is associated with a defect in neuromuscular development of the eviscerated intestine. Pediatr. Res. 68 (1), 23–28. doi:10.1203/PDR.0b013e3181e17c75
Day, E. S., Wen, D., Garber, E. A., Hong, J., Avedissian, L. S., Rayhorn, P., et al. (1999). Zinc-dependent structural stability of human Sonic hedgehog. Biochemistry 38 (45), 14868–14880. doi:10.1021/bi9910068
Delaveau, T., Davoine, D., Jolly, A., Vallot, A., Rouvière, J. O., Gerber, A., et al. (2016). Tma108, a putative M1 aminopeptidase, is a specific nascent chain-associated protein in Saccharomyces cerevisiae. Nucleic Acids Res. 44 (18), 8826–8841. doi:10.1093/nar/gkw732
Di Giosaffatte, N., Ferraris, A., Gaudioso, F., Lodato, V., Savino, E., Celletti, C., et al. (2022). Congenital defects in a patient Carrying a novel homozygous AEBP1 variant: further expansion of the phenotypic Spectrum of Ehlers-Danlos syndrome classical-like type 2? Genes (Basel) 13 (12), 2358. doi:10.3390/genes13122358
Dong, W., Fricker, L. D., and Day, R. (1999). Carboxypeptidase D is a potential candidate to carry out redundant processing functions of carboxypeptidase E based on comparative distribution studies in the rat central nervous system. Neuroscience 89 (4), 1301–1317. doi:10.1016/s0306-4522(98)00381-9
Dulloo, I., Muliyil, S., and Freeman, M. (2019). The molecular, cellular and pathophysiological roles of iRhom pseudoproteases. Open Biol. 9 (3), 190003. doi:10.1098/rsob.190003
Eng, F. J., Novikova, E. G., Kuroki, K., Ganem, D., and Fricker, L. D. (1998). gp180, a protein that binds duck hepatitis B virus particles, has metallocarboxypeptidase D-like enzymatic activity. J. Biol. Chem. 273 (14), 8382–8388. doi:10.1074/jbc.273.14.8382
Eto, K., Huet, C., Tarui, T., Kupriyanov, S., Liu, H. Z., Puzon-McLaughlin, W., et al. (2002). Functional classification of ADAMs based on a conserved motif for binding to integrin alpha 9beta 1: implications for sperm-egg binding and other cell interactions. J. Biol. Chem. 277 (20), 17804–17810. doi:10.1074/jbc.M200086200
Fajardo, D., Saint Jean, R., and Lyons, P. J. (2023). Acquisition of new function through gene duplication in the metallocarboxypeptidase family. Sci. Rep. 13 (1), 2512. doi:10.1038/s41598-023-29800-9
Feldkamp, M. L., Bowles, N. E., and Botto, L. D. (2012). AEBP1 gene variants in infants with gastroschisis. Birth Defects Res. A Clin. Mol. Teratol. 94 (9), 738–742. doi:10.1002/bdra.23041
Fernandez, D., Pallares, I., Vendrell, J., and Aviles, F. X. (2010). Progress in metallocarboxypeptidases and their small molecular weight inhibitors. Biochimie 92 (11), 1484–1500. doi:10.1016/j.biochi.2010.05.002
Fleischer, T. C., Weaver, C. M., McAfee, K. J., Jennings, J. L., and Link, A. J. (2006). Systematic identification and functional screens of uncharacterized proteins associated with eukaryotic ribosomal complexes. Genes Dev. 20 (10), 1294–1307. doi:10.1101/gad.1422006
Fricker, L. D. (2004). “Metallocarboxypeptidase D,” in Handbook of proteolytic enzymes. Editors A. J. Barrett, N. D. Rawlings, and J. F. Woessner 2 ed (San Diego: Academic Press), 848–851.
Fricker, L. D., Berman, Y. L., Leiter, E. H., and Devi, L. A. (1996). Carboxypeptidase E activity is deficient in mice with the fat mutation. Effect on peptide processing. J. Biol. Chem. 271 (48), 30619–30624. doi:10.1074/jbc.271.48.30619
Fricker, L. D., and Leiter, E. H. (1999). Peptides, enzymes and obesity: new insights from a 'dead' enzyme. Trends Biochem. Sci. 24 (10), 390–393. doi:10.1016/s0968-0004(99)01448-6
Fukata, Y., Adesnik, H., Iwanaga, T., Bredt, D. S., Nicoll, R. A., and Fukata, M. (2006). Epilepsy-related ligand/receptor complex LGI1 and ADAM22 regulate synaptic transmission. Science 313 (5794), 1792–1795. doi:10.1126/science.1129947
Fukata, Y., Chen, X., Chiken, S., Hirano, Y., Yamagata, A., Inahashi, H., et al. (2021a). LGI1-ADAM22-MAGUK configures transsynaptic nanoalignment for synaptic transmission and epilepsy prevention. Proc. Natl. Acad. Sci. U. S. A. 118 (3), e2022580118. doi:10.1073/pnas.2022580118
Fukata, Y., Hirano, Y., Miyazaki, Y., Yokoi, N., and Fukata, M. (2021b). Trans-synaptic LGI1–ADAM22–MAGUK in AMPA and NMDA receptor regulation. Neuropharmacology 194, 108628. doi:10.1016/j.neuropharm.2021.108628
Fukazawa, T., Tanimoto, K., Yamaoka, E., Kojima, M., Kanawa, M., Hirohashi, N., et al. (2022). Oncogenic role of ADAM32 in hepatoblastoma: a potential molecular target for Therapy. Cancers (Basel) 14 (19), 4732. doi:10.3390/cancers14194732
Fuse, N., Maiti, T., Wang, B., Porter, J. A., Hall, T. M., Leahy, D. J., et al. (1999). Sonic hedgehog protein signals not as a hydrolytic enzyme but as an apparent ligand for patched. Proc. Natl. Acad. Sci. U. S. A. 96 (20), 10992–10999. doi:10.1073/pnas.96.20.10992
Gagnon, A., Abaiian, K. J., Crapper, T., Layne, M. D., and Sorisky, A. (2002). Down-regulation of aortic carboxypeptidase-like protein during the early phase of 3T3-L1 adipogenesis. Endocrinology 143 (7), 2478–2485. doi:10.1210/endo.143.7.8875
Garcia-Pardo, J., Tanco, S., Diaz, L., Dasgupta, S., Fernandez-Recio, J., Lorenzo, J., et al. (2017). Substrate specificity of human metallocarboxypeptidase D: comparison of the two active carboxypeptidase domains. PLoS One 12 (11), e0187778. doi:10.1371/journal.pone.0187778
Gerhard, G. S., Hanson, A., Wilhelmsen, D., Piras, I. S., Still, C. D., Chu, X., et al. (2019). AEBP1 expression increases with severity of fibrosis in NASH and is regulated by glucose, palmitate, and miR-372-3p. PLoS One 14 (7), e0219764. doi:10.1371/journal.pone.0219764
Gil-Varea, E., Urcelay, E., Vilarino-Guell, C., Costa, C., Midaglia, L., Matesanz, F., et al. (2018). Exome sequencing study in patients with multiple sclerosis reveals variants associated with disease course. J. Neuroinflammation 15 (1), 265. doi:10.1186/s12974-018-1307-1
Goldberg, T., and Sreelatha, A. (2023). Emerging functions of pseudoenzymes. Biochem. J. 480 (10), 715–728. doi:10.1042/bcj20220373
Gomis-Ruth, F. X. (2008). Structure and mechanism of metallocarboxypeptidases. Crit. Rev. Biochem. Mol. Biol. 43 (5), 319–345. doi:10.1080/10409230802376375
Gomis-Rüth, F. X. (2009). Catalytic domain architecture of metzincin metalloproteases. J. Biol. Chem. 284 (23), 15353–15357. doi:10.1074/jbc.R800069200
Gomis-Ruth, F. X., Companys, V., Qian, Y., Fricker, L. D., Vendrell, J., Aviles, F. X., et al. (1999). Crystal structure of avian carboxypeptidase D domain II: a prototype for the regulatory metallocarboxypeptidase subfamily. EMBO J. 18 (21), 5817–5826. doi:10.1093/emboj/18.21.5817
Gorbenko, O., Kuznetsov, V., Kukharenko, O., Zhyvoloup, A., Panasyuk, G., Nemazanyy, I., et al. (2004). Identification of a novel binding partners for tumor suppressor PTEN by a yeast two-hybrid approach. Eksp. Onkol. 26 (1), 15–19.
Grabowski, K., Herlan, L., Witten, A., Qadri, F., Eisenreich, A., Lindner, D., et al. (2022). Cpxm2 as a novel candidate for cardiac hypertrophy and failure in hypertension. Hypertens. Res. 45 (2), 292–307. doi:10.1038/s41440-021-00826-8
Gusinjac, A., Gagnon, A., and Sorisky, A. (2011). Effect of collagen I and aortic carboxypeptidase-like protein on 3T3-L1 adipocyte differentiation. Metabolism 60 (6), 782–788. doi:10.1016/j.metabol.2010.07.028
Hall, T. M., Porter, J. A., Beachy, P. A., and Leahy, D. J. (1995). A potential catalytic site revealed by the 1.7-A crystal structure of the amino-terminal signalling domain of Sonic hedgehog. Nature 378 (6553), 212–216. doi:10.1038/378212a0
Han, C., Park, I., Lee, B., Jin, S., Choi, H., Kwon, J. T., et al. (2011). Identification of heat shock protein 5, calnexin and integral membrane protein 2B as Adam7-interacting membrane proteins in mouse sperm. J. Cell Physiol. 226 (5), 1186–1195. doi:10.1002/jcp.22444
Harrison, B., Sanniec, K., and Janis, J. E. (2016). Collagenopathies-implications for abdominal wall Reconstruction: a Systematic review. Plast. Reconstr. Surg. Glob. Open 4 (10), e1036. doi:10.1097/GOX.0000000000001036
Hashimoto, R., Ikeda, M., Ohi, K., Yasuda, Y., Yamamori, H., Fukumoto, M., et al. (2013). Genome-wide association study of cognitive decline in schizophrenia. Am. J. Psychiatry 170 (6), 683–684. doi:10.1176/appi.ajp.2013.12091228
He, G. P., Muise, A., Li, A. W., and Ro, H. S. (1995). A eukaryotic transcriptional repressor with carboxypeptidase activity. Nature 378 (6552), 92–96. doi:10.1038/378092a0
Hebebrand, M., Vasileiou, G., Krumbiegel, M., Kraus, C., Uebe, S., Ekici, A. B., et al. (2019). A biallelic truncating AEBP1 variant causes connective tissue disorder in two siblings. Am. J. Med. Genet. A 179 (1), 50–56. doi:10.1002/ajmg.a.60679
Himmelstein, D. S., Cajigas, I., Bi, C., Clark, B. S., Van Der Voort, G., and Kohtz, J. D. (2017). SHH E176/E177-Zn(2+) conformation is required for signaling at endogenous sites. Dev. Biol. 424 (2), 221–235. doi:10.1016/j.ydbio.2017.02.006
Hivert, B., Marien, L., Agbam, K. N., and Faivre-Sarrailh, C. (2019). ADAM22 and ADAM23 modulate the targeting of the Kv1 channel-associated protein LGI1 to the axon initial segment. J. Cell Sci. 132 (2), jcs219774. doi:10.1242/jcs.219774
Howard, L., Zheng, Y., Horrocks, M., Maciewicz, R. A., and Blobel, C. (2001). Catalytic activity of ADAM28. FEBS Lett. 498 (1), 82–86. doi:10.1016/s0014-5793(01)02506-6
Hsia, H.-E., Tüshaus, J., Brummer, T., Zheng, Y., Scilabra, S. D., and Lichtenthaler, S. F. (2019). Functions of ‘A disintegrin and metalloproteases (ADAMs)’ in the mammalian nervous system. Cell. Mol. Life Sci. 76 (16), 3055–3081. doi:10.1007/s00018-019-03173-7
Huang, X., Huang, P., Robinson, M. K., Stern, M. J., and Jin, Y. (2003). UNC-71, a disintegrin and metalloprotease (ADAM) protein, regulates motor axon guidance and sex myoblast migration in C. elegans. Development 130 (14), 3147–3161. doi:10.1242/dev.00518
Huang, Z., Dong, F., Li, S., Chu, M., Zhou, H., Lu, Z., et al. (2012). Berberine-induced inhibition of adipocyte enhancer-binding protein 1 attenuates oxidized low-density lipoprotein accumulation and foam cell formation in phorbol 12-myristate 13-acetate-induced macrophages. Eur. J. Pharmacol. 690 (1-3), 164–169. doi:10.1016/j.ejphar.2012.07.009
Ichikawa, O., Osawa, M., Nishida, N., Goshima, N., Nomura, N., and Shimada, I. (2007). Structural basis of the collagen-binding mode of discoidin domain receptor 2. EMBO J. 26 (18), 4168–4176. doi:10.1038/sj.emboj.7601833
Ishida, K., Nakamura, A., and Kojima, S. (2022). Crystal structure of the AlbEF complex involved in subtilosin A biosynthesis. Structure 30 (12), 1637–1646.e3. doi:10.1016/j.str.2022.10.002
Ith, B., Wei, J., Yet, S. F., Perrella, M. A., and Layne, M. D. (2005). Aortic carboxypeptidase-like protein is expressed in collagen-rich tissues during mouse embryonic development. Gene Expr. Patterns 5 (4), 533–537. doi:10.1016/j.modgep.2004.11.002
Jager, M., Lee, M. J., Li, C., Farmer, S. R., Fried, S. K., and Layne, M. D. (2018). Aortic carboxypeptidase-like protein enhances adipose tissue stromal progenitor differentiation into myofibroblasts and is upregulated in fibrotic white adipose tissue. PLoS One 13 (5), e0197777. doi:10.1371/journal.pone.0197777
Jägers, C., and Roelink, H. (2021). Association of Sonic Hedgehog with the extracellular matrix requires its zinc-coordination center. BMC Mol. Cell Biol. 22 (1), 22. doi:10.1186/s12860-021-00359-5
Katagiri, T., Harada, Y., Emi, M., and Nakamura, Y. (1995). Human metalloprotease/disintegrin-like (MDC) gene: exon-intron organization and alternative splicing. Cytogenet. Cell Genet. 68 (1-2), 39–44. doi:10.1159/000133884
Kienle, D., Benner, A., Läufle, C., Winkler, D., Schneider, C., Bühler, A., et al. (2010). Gene expression factors as predictors of genetic risk and survival in chronic lymphocytic leukemia. Haematologica 95 (1), 102–109. doi:10.3324/haematol.2009.010298
Kikuchi, S., Asakura, Y., Imai, M., Nakahira, Y., Kotani, Y., Hashiguchi, Y., et al. (2018). A Ycf2-FtsHi Heteromeric AAA-ATPase complex is required for chloroplast protein import. Plant Cell 30 (11), 2677–2703. doi:10.1105/tpc.18.00357
Kim, S. W., Muise, A. M., Lyons, P. J., and Ro, H. S. (2001). Regulation of adipogenesis by a transcriptional repressor that modulates MAPK activation. J. Biol. Chem. 276 (13), 10199–10206. doi:10.1074/jbc.M010640200
Kim, Y. H., Barclay, J. L., He, J., Luo, X., O'Neill, H. M., Keshvari, S., et al. (2016). Identification of carboxypeptidase X (CPX)-1 as a positive regulator of adipogenesis. FASEB J. 30 (7), 2528–2540. doi:10.1096/fj.201500107R
Kim, Y. H., O'Neill, H. M., and Whitehead, J. P. (2015). Carboxypeptidase X-1 (CPX-1) is a secreted collagen-binding glycoprotein. Biochem. Biophys. Res. Commun. 468 (4), 894–899. doi:10.1016/j.bbrc.2015.11.053
Kole, M. J., Qian, J., Waase, M. P., Klassen, T. L., Chen, T. T., Augustine, G. J., et al. (2015). Selective loss of presynaptic potassium channel Clusters at the cerebellar basket cell terminal Pinceau in Adam11 mutants reveals their role in ephaptic control of Purkinje cell firing. J. Neurosci. 35 (32), 11433–11444. doi:10.1523/jneurosci.1346-15.2015
Kowalinski, E., Bange, G., Bradatsch, B., Hurt, E., Wild, K., and Sinning, I. (2007). The crystal structure of Ebp1 reveals a methionine aminopeptidase fold as binding platform for multiple interactions. FEBS Lett. 581 (23), 4450–4454. doi:10.1016/j.febslet.2007.08.024
Kozar-Gillan, N., Velichkova, A., Kanatouris, G., Eshed-Eisenbach, Y., Steel, G., Jaegle, M., et al. (2023). LGI3/2-ADAM23 interactions cluster Kv1 channels in myelinated axons to regulate refractory period. J. Cell Biol. 222 (4), e202211031. doi:10.1083/jcb.202211031
Kraushar, M. L., Krupp, F., Harnett, D., Turko, P., Ambrozkiewicz, M. C., Sprink, T., et al. (2021). Protein synthesis in the developing Neocortex at Near-atomic resolution reveals ebp1-mediated neuronal proteostasis at the 60S tunnel exit. Mol. Cell 81 (2), 304–322.e16. doi:10.1016/j.molcel.2020.11.037
Kuroki, K., Eng, F., Ishikawa, T., Turck, C., Harada, F., and Ganem, D. (1995). gp180, a host cell glycoprotein that binds duck hepatitis B virus particles, is encoded by a member of the carboxypeptidase gene family. J. Biol. Chem. 270 (25), 15022–15028. doi:10.1074/jbc.270.25.15022
Layne, M. D., Endege, W. O., Jain, M. K., Yet, S. F., Hsieh, C. M., Chin, M. T., et al. (1998). Aortic carboxypeptidase-like protein, a novel protein with discoidin and carboxypeptidase-like domains, is up-regulated during vascular smooth muscle cell differentiation. J. Biol. Chem. 273 (25), 15654–15660. doi:10.1074/jbc.273.25.15654
Layne, M. D., Yet, S. F., Maemura, K., Hsieh, C. M., Bernfield, M., Perrella, M. A., et al. (2001). Impaired abdominal wall development and deficient wound healing in mice lacking aortic carboxypeptidase-like protein. Mol. Cell Biol. 21 (15), 5256–5261. doi:10.1128/MCB.21.15.5256-5261.2001
Layne, M. D., Yet, S. F., Maemura, K., Hsieh, C. M., Liu, X., Ith, B., et al. (2002). Characterization of the mouse aortic carboxypeptidase-like protein promoter reveals activity in differentiated and dedifferentiated vascular smooth muscle cells. Circ. Res. 90 (6), 728–736. doi:10.1161/01.res.0000013289.97650.c8
Lee, S., Hong, S. H., and Cho, C. (2020). Normal fertility in male mice lacking ADAM32 with testis-specific expression. Reprod. Biol. 20 (4), 589–594. doi:10.1016/j.repbio.2020.09.001
Lei, Y., Xin, X., Morgan, D., Pintar, J. E., and Fricker, L. D. (1999). Identification of mouse CPX-1, a novel member of the metallocarboxypeptidase gene family with highest similarity to CPX-2. DNA Cell Biol. 18 (2), 175–185. doi:10.1089/104454999315565
Li, P., Kuo, W. L., Yousef, M., Rosner, M. R., and Tang, W. J. (2006). The C-terminal domain of human insulin degrading enzyme is required for dimerization and substrate recognition. Biochem. Biophys. Res. Commun. 343 (4), 1032–1037. doi:10.1016/j.bbrc.2006.03.083
Lin, Y. C., Sun, G. H., Lee, Y. M., Guo, Y. W., and Liu, H. W. (2001). Cloning and characterization of a complementary DNA encoding a human epididymis-associated disintegrin and metalloprotease 7 protein. Biol. Reprod. 65 (3), 944–950. doi:10.1095/biolreprod65.3.944
Liu, N., Liu, D., Cao, S., and Lei, J. (2023). Silencing of adipocyte enhancer-binding protein 1 (AEBP1) alleviates renal fibrosis in vivo and in vitro via inhibition of the β-catenin signaling pathway. Hum. Cell 36, 972–986. doi:10.1007/s13577-023-00859-w
Lou, Y., Chen, Y. D., Sun, F. R., Shi, J. P., Song, Y., and Yang, J. (2017). Potential regulators driving the transition in nonalcoholic fatty liver disease: a stage-based view. Cell Physiol. Biochem. 41 (1), 239–251. doi:10.1159/000456061
Lovero, K. L., Fukata, Y., Granger, A. J., Fukata, M., and Nicoll, R. A. (2015). The LGI1-ADAM22 protein complex directs synapse maturation through regulation of PSD-95 function. Proc. Natl. Acad. Sci. U. S. A. 112 (30), E4129–E4137. doi:10.1073/pnas.1511910112
Lu, X., Lu, D., Scully, M. F., and Kakkar, V. V. (2007). Structure-activity relationship studies on ADAM protein-integrin interactions. Cardiovasc Hematol. Agents Med. Chem. 5 (1), 29–42. doi:10.2174/187152507779315822
Lum, L., and Blobel, C. P. (1997). Evidence for distinct serine protease activities with a potential role in processing the sperm protein fertilin. Dev. Biol. 191 (1), 131–145. doi:10.1006/dbio.1997.8609
Lussier, M., White, A. M., Sheraton, J., di Paolo, T., Treadwell, J., Southard, S. B., et al. (1997). Large scale identification of genes involved in cell surface biosynthesis and architecture in Saccharomyces cerevisiae. Genetics 147 (2), 435–450. doi:10.1093/genetics/147.2.435
Lyons, P. J., Mattatall, N. R., and Ro, H. S. (2006). Modeling and functional analysis of AEBP1, a transcriptional repressor. Proteins 63 (4), 1069–1083. doi:10.1002/prot.20946
Lyons, P. J., Muise, A. M., and Ro, H. S. (2005). MAPK modulates the DNA binding of adipocyte enhancer-binding protein 1. Biochemistry 44 (3), 926–931. doi:10.1021/bi0480178
Mace, P. D., and Murphy, J. M. (2021). There's more to death than life: noncatalytic functions in kinase and pseudokinase signaling. J. Biol. Chem. 296, 100705. doi:10.1016/j.jbc.2021.100705
Majdalawieh, A., and Ro, H. S. (2009). LPS-induced suppression of macrophage cholesterol efflux is mediated by adipocyte enhancer-binding protein 1. Int. J. Biochem. Cell Biol. 41 (7), 1518–1525. doi:10.1016/j.biocel.2009.01.003
Majdalawieh, A., Zhang, L., Fuki, I. V., Rader, D. J., and Ro, H. S. (2006). Adipocyte enhancer-binding protein 1 is a potential novel atherogenic factor involved in macrophage cholesterol homeostasis and inflammation. Proc. Natl. Acad. Sci. U. S. A. 103 (7), 2346–2351. doi:10.1073/pnas.0508139103
Majdalawieh, A., Zhang, L., and Ro, H. S. (2007). Adipocyte enhancer-binding protein-1 promotes macrophage inflammatory responsiveness by up-regulating NF-kappaB via IkappaBalpha negative regulation. Mol. Biol. Cell 18 (3), 930–942. doi:10.1091/mbc.e06-03-0217
Majdalawieh, A. F., Massri, M., and Ro, H. S. (2020). AEBP1 is a novel oncogene: mechanisms of action and signaling pathways. J. Oncol. 2020, 8097872. doi:10.1155/2020/8097872
Marcello, M. R., and Evans, J. P. (2010). Multivariate analysis of male reproductive function in Inpp5b-/- mice reveals heterogeneity in defects in fertility, sperm-egg membrane interaction and proteolytic cleavage of sperm ADAMs. Mol. Hum. Reprod. 16 (7), 492–505. doi:10.1093/molehr/gaq029
Maruyama, Y., Chuma, A., Mikami, B., Hashimoto, W., and Murata, K. (2011). Heterosubunit composition and crystal structures of a novel bacterial M16B metallopeptidase. J. Mol. Biol. 407 (1), 180–192. doi:10.1016/j.jmb.2011.01.038
Matsuoka, S., Gupta, S., Suzuki, E., Hiromi, Y., and Asaoka, M. (2014). Gone early, a novel germline factor, ensures the proper size of the stem cell precursor pool in the Drosophila ovary. PLoS One 9 (11), e113423. doi:10.1371/journal.pone.0113423
Mattei, A. M., Smailys, J. D., Hepworth, E. M. W., and Hinton, S. D. (2021). The roles of pseudophosphatases in disease. Int. J. Mol. Sci. 22 (13), 6924. doi:10.3390/ijms22136924
Maun, H. R., Wen, X., Lingel, A., de Sauvage, F. J., Lazarus, R. A., Scales, S. J., et al. (2010). Hedgehog pathway antagonist 5E1 binds hedgehog at the pseudo-active site. J. Biol. Chem. 285 (34), 26570–26580. doi:10.1074/jbc.M110.112284
McDonald, R. C., Schott, M. J., Idowu, T. A., and Lyons, P. J. (2020). Biochemical and genetic analysis of Ecm14, a conserved fungal pseudopeptidase. BMC Mol. Cell Biol. 21 (1), 86. doi:10.1186/s12860-020-00330-w
Miller, N. D., Nance, M. A., Wohler, E. S., Hoover-Fong, J. E., Lisi, E., Thomas, G. H., et al. (2009). Molecular (SNP) analyses of overlapping hemizygous deletions of 10q25.3 to 10qter in four patients: evidence for HMX2 and HMX3 as candidate genes in hearing and vestibular function. Am. J. Med. Genet. A 149A (4), 669–680. doi:10.1002/ajmg.a.32705
Mishra, L. S., and Funk, C. (2021). The FtsHi enzymes of Arabidopsis thaliana: pseudo-proteases with an important function. Int. J. Mol. Sci. 22 (11), 5917. doi:10.3390/ijms22115917
Miyazaki, Y., Otsuka, T., Yamagata, Y., Endo, T., Sanbo, M., Sano, H., et al. (2024). Oligodendrocyte-derived LGI3 and its receptor ADAM23 organize juxtaparanodal Kv1 channel clustering for short-term synaptic plasticity. Cell Rep. 43 (1), 113634. doi:10.1016/j.celrep.2023.113634
Monie, T. P., Perrin, A. J., Birtley, J. R., Sweeney, T. R., Karakasiliotis, I., Chaudhry, Y., et al. (2007). Structural insights into the transcriptional and translational roles of Ebp1. EMBO J. 26 (17), 3936–3944. doi:10.1038/sj.emboj.7601817
Muise, A. M., and Ro, H. S. (1999). Enzymic characterization of a novel member of the regulatory B-like carboxypeptidase with transcriptional repression function: stimulation of enzymic activity by its target DNA. Biochem. J. 343 (Pt 2), 341–345. doi:10.1042/bj3430341
Muona, M., Fukata, Y., Anttonen, A. K., Laari, A., Palotie, A., Pihko, H., et al. (2016). Dysfunctional ADAM22 implicated in progressive encephalopathy with cortical atrophy and epilepsy. Neurol. Genet. 2 (1), e46. doi:10.1212/nxg.0000000000000046
Murphy, J. M., Mace, P. D., and Eyers, P. A. (2017). Live and let die: insights into pseudoenzyme mechanisms from structure. Curr. Opin. Struct. Biol. 47, 95–104. doi:10.1016/j.sbi.2017.07.004
Nishimura, H., Cho, C., Branciforte, D. R., Myles, D. G., and Primakoff, P. (2001). Analysis of loss of adhesive function in sperm lacking cyritestin or fertilin beta. Dev. Biol. 233 (1), 204–213. doi:10.1006/dbio.2001.0166
Niu, G., Yang, Y., Ren, J., Song, T., Hu, Z., Chen, L., et al. (2019). Overexpression of CPXM2 predicts an unfavorable prognosis and promotes the proliferation and migration of gastric cancer. Oncol. Rep. 42 (4), 1283–1294. doi:10.3892/or.2019.7254
Novikova, E. G., Eng, F. J., Yan, L., Qian, Y., and Fricker, L. D. (1999). Characterization of the enzymatic properties of the first and second domains of metallocarboxypeptidase D. J. Biol. Chem. 274 (41), 28887–28892. doi:10.1074/jbc.274.41.28887
O'Boyle, B., Shrestha, S., Kochut, K., Eyers, P. A., and Kannan, N. (2022). Computational tools and resources for pseudokinase research. Methods Enzymol. 667, 403–426. doi:10.1016/bs.mie.2022.03.040
Ogawa, Y., Oses-Prieto, J., Kim, M. Y., Horresh, I., Peles, E., Burlingame, A. L., et al. (2010). ADAM22, a Kv1 channel-interacting protein, recruits membrane-associated guanylate kinases to juxtaparanodes of myelinated axons. J. Neurosci. 30 (3), 1038–1048. doi:10.1523/jneurosci.4661-09.2010
Oh, J., Woo, J. M., Choi, E., Kim, T., Cho, B. N., Park, Z. Y., et al. (2005). Molecular, biochemical, and cellular characterization of epididymal ADAMs, ADAM7 and ADAM28. Biochem. Biophys. Res. Commun. 331 (4), 1374–1383. doi:10.1016/j.bbrc.2005.04.067
Oh, J. S., Han, C., and Cho, C. (2009). ADAM7 is associated with epididymosomes and integrated into sperm plasma membrane. Mol. Cells 28 (5), 441–446. doi:10.1007/s10059-009-0140-x
Ohno, I., Hashimoto, J., Shimizu, K., Takaoka, K., Ochi, T., Matsubara, K., et al. (1996). A cDNA cloning of human AEBP1 from primary cultured osteoblasts and its expression in a differentiating osteoblastic cell line. Biochem. Biophys. Res. Commun. 228 (2), 411–414. doi:10.1006/bbrc.1996.1675
Oppezzo, P., Vasconcelos, Y., Settegrana, C., Jeannel, D., Vuillier, F., Legarff-Tavernier, M., et al. (2005). The LPL/ADAM29 expression ratio is a novel prognosis indicator in chronic lymphocytic leukemia. Blood 106 (2), 650–657. doi:10.1182/blood-2004-08-3344
Owuor, K., Harel, N. Y., Englot, D. J., Hisama, F., Blumenfeld, H., and Strittmatter, S. M. (2009). LGI1-associated epilepsy through altered ADAM23-dependent neuronal morphology. Mol. Cell. Neurosci. 42 (4), 448–457. doi:10.1016/j.mcn.2009.09.008
Pandey, A., Cousin, H., Horr, B., and Alfandari, D. (2023). ADAM11 a novel regulator of Wnt and BMP4 signaling in neural crest and cancer. Front. Cell Dev. Biol. 11, 1271178. doi:10.3389/fcell.2023.1271178
Park, G. J., Osinski, A., Hernandez, G., Eitson, J. L., Majumdar, A., Tonelli, M., et al. (2022). The mechanism of RNA capping by SARS-CoV-2. Nature 609 (7928), 793–800. doi:10.1038/s41586-022-05185-z
Park, J. G., Muise, A., He, G. P., Kim, S. W., and Ro, H. S. (1999). Transcriptional regulation by the gamma5 subunit of a heterotrimeric G protein during adipogenesis. EMBO J. 18 (14), 4004–4012. doi:10.1093/emboj/18.14.4004
Pervaz, S., Ullah, A., Adu-Gyamfi, E. A., Lamptey, J., Sah, S. K., Wang, M. J., et al. (2022). Role of CPXM1 in impaired glucose metabolism and ovarian dysfunction in polycystic ovary syndrome. Reprod. Sci. 30, 526–543. doi:10.1007/s43032-022-00987-y
Podbilewicz, B. (1996). ADM-1, a protein with metalloprotease- and disintegrin-like domains, is expressed in syncytial organs, sperm, and sheath cells of sensory organs in Caenorhabditis elegans. Mol. Biol. Cell 7 (12), 1877–1893. doi:10.1091/mbc.7.12.1877
Pollock, R. A., Hartl, F. U., Cheng, M. Y., Ostermann, J., Horwich, A., and Neupert, W. (1988). The processing peptidase of yeast mitochondria: the two co-operating components MPP and PEP are structurally related. Embo J. 7 (11), 3493–3500. doi:10.1002/j.1460-2075.1988.tb03225.x
Primakoff, P., Hyatt, H., and Tredick-Kline, J. (1987). Identification and purification of a sperm surface protein with a potential role in sperm-egg membrane fusion. J. Cell Biol. 104 (1), 141–149. doi:10.1083/jcb.104.1.141
Puente, X. S., Sánchez, L. M., Overall, C. M., and López-Otín, C. (2003). Human and mouse proteases: a comparative genomic approach. Nat. Rev. Genet. 4 (7), 544–558. doi:10.1038/nrg1111
Purrington, K. S., Slager, S., Eccles, D., Yannoukakos, D., Fasching, P. A., Miron, P., et al. (2014). Genome-wide association study identifies 25 known breast cancer susceptibility loci as risk factors for triple-negative breast cancer. Carcinogenesis 35 (5), 1012–1019. doi:10.1093/carcin/bgt404
Reiterer, V., Eyers, P. A., and Farhan, H. (2014). Day of the dead: pseudokinases and pseudophosphatases in physiology and disease. Trends Cell Biol. 24 (9), 489–505. doi:10.1016/j.tcb.2014.03.008
Reiterer, V., Pawłowski, K., Desrochers, G., Pause, A., Sharpe, H. J., and Farhan, H. (2020). The dead phosphatases society: a review of the emerging roles of pseudophosphatases. Febs J. 287 (19), 4198–4220. doi:10.1111/febs.15431
Reynolds, S. L., and Fischer, K. (2015). Pseudoproteases: mechanisms and function. Biochem. J. 468 (1), 17–24. doi:10.1042/BJ20141506
Reznik, S. E., and Fricker, L. D. (2001). Carboxypeptidases from A to z: implications in embryonic development and Wnt binding. Cell Mol. Life Sci. 58 (12-13), 1790–1804. doi:10.1007/PL00000819
Ribeiro, A. J. M., Das, S., Dawson, N., Zaru, R., Orchard, S., Thornton, J. M., et al. (2019). Emerging concepts in pseudoenzyme classification, evolution, and signaling. Sci. Signal 12 (594), eaat9797. doi:10.1126/scisignal.aat9797
Ritelli, M., Cinquina, V., Venturini, M., Pezzaioli, L., Formenti, A. M., Chiarelli, N., et al. (2019). Expanding the clinical and mutational spectrum of recessive AEBP1-related classical-like ehlers-danlos syndrome. Genes (Basel) 10 (2), 135. doi:10.3390/genes10020135
Ro, H. S., Kim, S. W., Wu, D., Webber, C., and Nicholson, T. E. (2001). Gene structure and expression of the mouse adipocyte enhancer-binding protein. Gene 280 (1-2), 123–133. doi:10.1016/s0378-1119(01)00771-5
Ro, H. S., Zhang, L., Majdalawieh, A., Kim, S. W., Wu, X., Lyons, P. J., et al. (2007). Adipocyte enhancer-binding protein 1 modulates adiposity and energy homeostasis. Obes. (Silver Spring) 15 (2), 288–302. doi:10.1038/oby.2007.569
Ruiz, C., Cid, V. J., Lussier, M., Molina, M., and Nombela, C. (1999). A large-scale sonication assay for cell wall mutant analysis in yeast. Yeast 15 (10B), 1001–1008. doi:10.1002/(SICI)1097-0061(199907)15:10B<1001::AID-YEA400>3.0.CO;2-T
Rybnikova, E., Kärkkäinen, I., Pelto-Huikko, M., and Huovila, A. P. (2002). Developmental regulation and neuronal expression of the cellular disintegrin ADAM11 gene in mouse nervous system. Neuroscience 112 (4), 921–934. doi:10.1016/s0306-4522(02)00124-0
Sagane, K., Hayakawa, K., Kai, J., Hirohashi, T., Takahashi, E., Miyamoto, N., et al. (2005). Ataxia and peripheral nerve hypomyelination in ADAM22-deficient mice. BMC Neurosci. 6, 33. doi:10.1186/1471-2202-6-33
Sagane, K., Ishihama, Y., and Sugimoto, H. (2008). LGI1 and LGI4 bind to ADAM22, ADAM23 and ADAM11. Int. J. Biol. Sci. 4 (6), 387–396. doi:10.7150/ijbs.4.387
Sagane, K., Ohya, Y., Hasegawa, Y., and Tanaka, I. (1998). Metalloproteinase-like, disintegrin-like, cysteine-rich proteins MDC2 and MDC3: novel human cellular disintegrins highly expressed in the brain. Biochem. J. 334 (Pt 1), 93–98. doi:10.1042/bj3340093
Sanai, H., Nakamura, Y., Koike, Y., Murota, H., Kosho, T., and Sase, M. (2023). The detailed obstetric course of the first Japanese patient with AEBP1-related Ehlers-Danlos syndrome (classical-like EDS, type 2). J. Obstet. Gynaecol. Res. 49 (3), 1043–1047. doi:10.1111/jog.15541
Schissel, S. L., Dunsmore, S. E., Liu, X., Shine, R. W., Perrella, M. A., and Layne, M. D. (2009). Aortic carboxypeptidase-like protein is expressed in fibrotic human lung and its absence protects against bleomycin-induced lung fibrosis. Am. J. Pathol. 174 (3), 818–828. doi:10.2353/ajpath.2009.080856
Seals, D. F., and Courtneidge, S. A. (2003). The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev. 17 (1), 7–30. doi:10.1101/gad.1039703
Sidyelyeva, G., and Fricker, L. D. (2002). Characterization of Drosophila carboxypeptidase D. J. Biol. Chem. 277 (51), 49613–49620. doi:10.1074/jbc.M209652200
Sidyelyeva, G., Wegener, C., Schoenfeld, B. P., Bell, A. J., Baker, N. E., McBride, S. M., et al. (2010). Individual carboxypeptidase D domains have both redundant and unique functions in Drosophila development and behavior. Cell Mol. Life Sci. 67 (17), 2991–3004. doi:10.1007/s00018-010-0369-8
Somma, G., Alger, H. M., McGuire, R. M., Kretlow, J. D., Ruiz, F. R., Yatsenko, S. A., et al. (2012). Head bobber: an insertional mutation causes inner ear defects, hyperactive circling, and deafness. J. Assoc. Res. Otolaryngol. 13 (3), 335–349. doi:10.1007/s10162-012-0316-5
Song, L., and Fricker, L. D. (1995). Purification and characterization of carboxypeptidase D, a novel carboxypeptidase E-like enzyme, from bovine pituitary. J. Biol. Chem. 270 (42), 25007–25013. doi:10.1074/jbc.270.42.25007
Song, L., and Fricker, L. D. (1996). Tissue distribution and characterization of soluble and membrane-bound forms of metallocarboxypeptidase D. J. Biol. Chem. 271 (46), 28884–28889. doi:10.1074/jbc.271.46.28884
Song, L., and Fricker, L. D. (1997). Cloning and expression of human carboxypeptidase Z, a novel metallocarboxypeptidase. J. Biol. Chem. 272 (16), 10543–10550. doi:10.1074/jbc.272.16.10543
Song, Y., Li, L., Ou, Y., Gao, Z., Li, E., Li, X., et al. (2014). Identification of genomic alterations in oesophageal squamous cell cancer. Nature 509 (7498), 91–95. doi:10.1038/nature13176
Sreelatha, A., Yee, S. S., Lopez, V. A., Park, B. C., Kinch, L. N., Pilch, S., et al. (2018). Protein AMPylation by an evolutionarily conserved pseudokinase. Cell 175 (3), 809–821. doi:10.1016/j.cell.2018.08.046
Stevenson, B. W., Gorman, M. A., Koach, J., Cheung, B. B., Marshall, G. M., Parker, M. W., et al. (2020). A structural view of PA2G4 isoforms with opposing functions in cancer. J. Biol. Chem. 295 (47), 16100–16112. doi:10.1074/jbc.REV120.014293
Syx, D., De Wandele, I., Symoens, S., De Rycke, R., Hougrand, O., Voermans, N., et al. (2019). Bi-allelic AEBP1 mutations in two patients with Ehlers-Danlos syndrome. Hum. Mol. Genet. 28 (11), 1853–1864. doi:10.1093/hmg/ddz024
Takahashi, E., Sagane, K., Nagasu, T., and Kuromitsu, J. (2006a). Altered nociceptive response in ADAM11-deficient mice. Brain Res. 1097 (1), 39–42. doi:10.1016/j.brainres.2006.04.043
Takahashi, E., Sagane, K., Oki, T., Yamazaki, K., Nagasu, T., and Kuromitsu, J. (2006b). Deficits in spatial learning and motor coordination in ADAM11-deficient mice. BMC Neurosci. 7, 19. doi:10.1186/1471-2202-7-19
Tallant, C., García-Castellanos, R., Baumann, U., and Gomis-Rüth, F. X. (2010). On the relevance of the Met-turn methionine in metzincins. J. Biol. Chem. 285 (18), 13951–13957. doi:10.1074/jbc.M109.083378
Tan, F., Rehli, M., Krause, S. W., and Skidgel, R. A. (1997). Sequence of human carboxypeptidase D reveals it to be a member of the regulatory carboxypeptidase family with three tandem active site domains. Biochem. J. 327 (Pt 1), 81–87. doi:10.1042/bj3270081
Taylor, A. B., Smith, B. S., Kitada, S., Kojima, K., Miyaura, H., Otwinowski, Z., et al. (2001). Crystal structures of mitochondrial processing peptidase reveal the mode for specific cleavage of import signal sequences. Structure 9 (7), 615–625. doi:10.1016/s0969-2126(01)00621-9
Teratani, T., Tomita, K., Suzuki, T., Furuhashi, H., Irie, R., Nishikawa, M., et al. (2018). Aortic carboxypeptidase-like protein, a WNT ligand, exacerbates nonalcoholic steatohepatitis. J. Clin. Invest. 128 (4), 1581–1596. doi:10.1172/JCI92863
Tumelty, K. E., Smith, B. D., Nugent, M. A., and Layne, M. D. (2014). Aortic carboxypeptidase-like protein (ACLP) enhances lung myofibroblast differentiation through transforming growth factor β receptor-dependent and -independent pathways. J. Biol. Chem. 289 (5), 2526–2536. doi:10.1074/jbc.M113.502617
van der Knoop, M. M., Maroofian, R., Fukata, Y., van Ierland, Y., Karimiani, E. G., Lehesjoki, A. E., et al. (2022). Biallelic ADAM22 pathogenic variants cause progressive encephalopathy and infantile-onset refractory epilepsy. Brain 145 (7), 2301–2312. doi:10.1093/brain/awac116
Varlamov, O., Eng, F. J., Novikova, E. G., and Fricker, L. D. (1999). Localization of metallocarboxypeptidase D in AtT-20 cells. Potential role in prohormone processing. J. Biol. Chem. 274 (21), 14759–14767. doi:10.1074/jbc.274.21.14759
Vishwanath, N., Monis, W. J., Hoffmann, G. A., Ramachandran, B., DiGiacomo, V., Wong, J. Y., et al. (2020). Mechanisms of aortic carboxypeptidase-like protein secretion and identification of an intracellularly retained variant associated with Ehlers-Danlos syndrome. J. Biol. Chem. 295 (28), 9725–9735. doi:10.1074/jbc.RA120.013902
Voronina, V. A., Harris, F. M., Schmahl, J., Galligan, C., Oristian, D., Zamfirova, R., et al. (2019). Deletion of Adam6 in Mus musculus leads to male subfertility and deficits in sperm ascent into the oviduct. Biol. Reprod. 100 (3), 686–696. doi:10.1093/biolre/ioy210
Wang, L., Hoggard, J. A., Korleski, E. D., Long, G. V., Ree, B. C., Hensley, K., et al. (2018). Multiple non-catalytic ADAMs are novel integrin α4 ligands. Mol. Cell Biochem. 442 (1-2), 29–38. doi:10.1007/s11010-017-3190-y
Wang, T., Lv, X., Jiang, S., Han, S., and Wang, Y. (2020). Expression of ADAM29 and FAM135B in the pathological evolution from normal esophageal epithelium to esophageal cancer: their differences and clinical significance. Oncol. Lett. 19 (3), 1727–1734. doi:10.3892/ol.2020.11272
Wardman, J. H., Zhang, X., Gagnon, S., Castro, L. M., Zhu, X., Steiner, D. F., et al. (2010). Analysis of peptides in prohormone convertase 1/3 null mouse brain using quantitative peptidomics. J. Neurochem. 114 (1), 215–225. doi:10.1111/j.1471-4159.2010.06760.x
Wei, X., Moncada-Pazos, A., Cal, S., Soria-Valles, C., Gartner, J., Rudloff, U., et al. (2011). Analysis of the disintegrin-metalloproteinases family reveals ADAM29 and ADAM7 are often mutated in melanoma. Hum. Mutat. 32 (6), E2148–E2175. doi:10.1002/humu.21477
Wild, K., Aleksić, M., Lapouge, K., Juaire, K. D., Flemming, D., Pfeffer, S., et al. (2020). MetAP-like Ebp1 occupies the human ribosomal tunnel exit and recruits flexible rRNA expansion segments. Nat. Commun. 11 (1), 776. doi:10.1038/s41467-020-14603-7
Wong, G. E., Zhu, X., Prater, C. E., Oh, E., and Evans, J. P. (2001). Analysis of fertilin alpha (ADAM1)-mediated sperm-egg cell adhesion during fertilization and identification of an adhesion-mediating sequence in the disintegrin-like domain. J. Biol. Chem. 276 (27), 24937–24945. doi:10.1074/jbc.M101637200
Xin, X., Day, R., Dong, W., Lei, Y., and Fricker, L. D. (1998). Identification of mouse CPX-2, a novel member of the metallocarboxypeptidase gene family: cDNA cloning, mRNA distribution, and protein expression and characterization. DNA Cell Biol. 17 (10), 897–909. doi:10.1089/dna.1998.17.897
Xin, X., Varlamov, O., Day, R., Dong, W., Bridgett, M. M., Leiter, E. H., et al. (1997). Cloning and sequence analysis of cDNA encoding rat carboxypeptidase D. DNA Cell Biol. 16 (7), 897–909. doi:10.1089/dna.1997.16.897
Yamagata, A., and Fukai, S. (2020). Insights into the mechanisms of epilepsy from structural biology of LGI1–ADAM22. Cell. Mol. Life Sci. 77 (2), 267–274. doi:10.1007/s00018-019-03269-0
Yamagata, A., Miyazaki, Y., Yokoi, N., Shigematsu, H., Sato, Y., Goto-Ito, S., et al. (2018). Structural basis of epilepsy-related ligand-receptor complex LGI1-ADAM22. Nat. Commun. 9 (1), 1546. doi:10.1038/s41467-018-03947-w
Yamaguchi, T., Hayashi, S., Nagai, S., Uchiyama, A., Motegi, S. I., Fujikawa, T., et al. (2023). Case report: further delineation of AEBP1-related Ehlers-Danlos Syndrome (classical-like EDS type 2) in an additional patient and comprehensive clinical and molecular review of the literature. Front. Genet. 14, 1102101. doi:10.3389/fgene.2023.1102101
Ye, Q. Z., Xie, S. X., Ma, Z. Q., Huang, M., and Hanzlik, R. P. (2006). Structural basis of catalysis by monometalated methionine aminopeptidase. Proc. Natl. Acad. Sci. U. S. A. 103 (25), 9470–9475. doi:10.1073/pnas.0602433103
Ye, Y., An, Y., Wang, M., Liu, H., Guan, L., Wang, Z., et al. (2020). Expression of carboxypeptidase X M14 family member 2 accelerates the progression of hepatocellular carcinoma via regulation of the gp130/JAK2/stat1 pathway. Cancer Manag. Res. 12, 2353–2364. doi:10.2147/CMAR.S228984
Yokoi, N., Fukata, Y., Okatsu, K., Yamagata, A., Liu, Y., Sanbo, M., et al. (2021). 14-3-3 proteins stabilize LGI1-ADAM22 levels to regulate seizure thresholds in mice. Cell Rep. 37 (11), 110107. doi:10.1016/j.celrep.2021.110107
Zaru, R., Magrane, M., Orchard, S., and UniProt, C. (2020). Challenges in the annotation of pseudoenzymes in databases: the UniProtKB approach. FEBS J. 287 (19), 4114–4127. doi:10.1111/febs.15100
Zettl, M., Adrain, C., Strisovsky, K., Lastun, V., and Freeman, M. (2011). Rhomboid family pseudoproteases use the ER quality control machinery to regulate intercellular signaling. Cell 145 (1), 79–91. doi:10.1016/j.cell.2011.02.047
Zhang, L., Reidy, S. P., Bogachev, O., Hall, B. K., Majdalawieh, A., and Ro, H. S. (2011). Lactation defect with impaired secretory activation in AEBP1-null mice. PLoS One 6 (11), e27795. doi:10.1371/journal.pone.0027795
Zhang, L., Reidy, S. P., Nicholson, T. E., Lee, H. J., Majdalawieh, A., Webber, C., et al. (2005). The role of AEBP1 in sex-specific diet-induced obesity. Mol. Med. 11 (1-12), 39–47. doi:10.2119/2005-00021.Ro
Zhang, X., Che, F. Y., Berezniuk, I., Sonmez, K., Toll, L., and Fricker, L. D. (2008). Peptidomics of Cpe(fat/fat) mouse brain regions: implications for neuropeptide processing. J. Neurochem. 107 (6), 1596–1613. doi:10.1111/j.1471-4159.2008.05722.x
Zhang, X., Cheng, W., Feng, Z., Zhu, Q., Sun, Y., Li, Y., et al. (2020). Transcriptomic analysis of gene expression of Verticillium dahliae upon treatment of the cotton root exudates. BMC Genomics 21 (1), 155. doi:10.1186/s12864-020-6448-9
Zhao, M., Jia, W., Jiang, W. G., Wang, P., Du, G., Cheng, S., et al. (2016). ADAM29 expression in human breast cancer and its effects on breast cancer cells in vitro. Anticancer Res. 36 (3), 1251–1258.
Zhao, X., Li, R., Wang, Q., Wu, M., and Wang, Y. (2019). Overexpression of carboxypeptidase X M14 family member 2 predicts an unfavorable prognosis and promotes proliferation and migration of osteosarcoma. Diagn Pathol. 14 (1), 118. doi:10.1186/s13000-019-0887-0
Keywords: metallopeptidase, protease, pseudoenzyme, carboxypeptidase, ADAM, AEBP1, mitochondrial processing peptidase, sonic hedgehog
Citation: Lyons PJ (2024) Inactive metallopeptidase homologs: the secret lives of pseudopeptidases. Front. Mol. Biosci. 11:1436917. doi: 10.3389/fmolb.2024.1436917
Received: 22 May 2024; Accepted: 25 June 2024;
Published: 10 July 2024.
Edited by:
Sofia R. Pauleta, New University of Lisbon, PortugalReviewed by:
F. Xavier Gomis-Rüth, Higher Scientific Research Council (CSIC), SpainSlawomir Potocki, University of Wrocław, Poland
Copyright © 2024 Lyons. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter J. Lyons, lyons@andrews.edu
 Peter J. Lyons
Peter J. Lyons