
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mol. Biosci. , 02 May 2024
Sec. Protein Biochemistry for Basic and Applied Sciences
Volume 11 - 2024 | https://doi.org/10.3389/fmolb.2024.1346259
 Juho Choi1
Juho Choi1 Yerin Jeon1
Yerin Jeon1 Youngin Roh1
Youngin Roh1 Jeongyun Jang1
Jeongyun Jang1 Eunbin Lee1
Eunbin Lee1 Luigie Villamante1
Luigie Villamante1 Minjae Kim2
Minjae Kim2 Myung-Hee Kwon1,2*
Myung-Hee Kwon1,2*Introduction: The CH1 domain of IgG antibodies controls assembly and secretion, mediated by the molecular chaperone BiP via the endoplasmic reticulum protein quality control (ERQC) mechanism. However, it is not clear whether the variable domains are necessary for this process.
Methods: Here, we generated IgG1 antibodies in which the V domain (VH and/or VL) was either removed or replaced, and then assessed expression, assembly, and secretion in HEK293 cells.
Results: All Ig variants formed a covalent linkage between the Cγ1 and Cκ, were successfully secreted in an assembled form. Replacement of the cognate Vκ with a non-secretory pseudo Vκ (ψVκ) hindered secretion of individual or assembled secretion of neither heavy chains (HCs) nor light chains (LCs). The ψLC (ψVκ-Cκ) exhibited a less folded structure compared to the wild type (wt) LC, as evidenced by enhanced stable binding to the molecular chaperone BiP and susceptibility to proteolytic degradation. Molecular dynamics simulation demonstrated dramatic alterations in overall structure of ψFab (Fd-ψLC) from wt Fab.
Discussion: These findings suggest that V domains do not initiate HC:LC assembly and secretion; instead, the critical factor governing IgG assembly and secretion is the CH-CL pairing. Additionally, the structural integrity of the VL domain is crucial for IgG secretion. These data offer valuable insight into the design of bioactive molecules based on an IgG backbone.
Antibodies (Abs) comprise two heavy chains (HCs) and two light chains (LCs), the proper folding and assembly of which are prerequisite for secretion by Ab-producing cells. The endoplasmic reticulum quality control (ERQC) mechanism in these cells ensures that only correctly assembled Ig molecules are secreted, thereby preventing secretion of misfolded and misassembled immunoglobulin (Ig) proteins (Feige et al., 2010). The ERQC mechanism has been examined most extensively in the context of the IgG isotype.
Newly synthesized Ig chains assemble first as gamma H chain (γHC) dimers in the ER through interchain disulfide bonds. The Cγ1 domain of these HC dimers remains unfolded and is retained in the ER by the molecular chaperon BiP until it binds covalently to the two folded LCs through interaction with the CL domain (Bole et al., 1986; Hendershot et al., 1987). Interaction of the folded CL domain with the Cγ1 domains releases BiP and induces complete folding and oxidization of the Cγ1 domain, thereby enabling assembly of the HC and LC into IgG (H2L2) structures prior to secretion (Lee et al., 1999; Vanhove et al., 2001; Feige et al., 2009).
The Cγ1 domain of the HC cannot fold or form intradomain disulfide bonds in the absence of LC expression; it therefore remains a substrate for BiP. BiP-bound HCs retained in the ER eventually undergo degradation in the proteasome compartment, unless they assemble with LCs (Mains and Sibley, 1983). Thus, full-size HCs are typically not secreted without first being assembled with LCs. Deletion of Cγ1 from IgG results in secretion of short HC dimers (VH-Cγ2-3), independent of LC expression. This is likely due to bypassing of BiP-mediated ER retention (Hendershot et al., 1987; Janssens et al., 2006; Zou et al., 2007; Drabek et al., 2016; Zhang et al., 2019). HC dimers secreted without first assembling with LC, often referred to as HC-only Abs (HCAbs), can exist as CH1-deleted forms or as full-size HC forms. HCAbs are not commonly found in healthy mammals; one exception is the camel, which naturally expresses CH1-lacking HC dimers (VHH-Cγ2-3) because it lacks the genes encoding the CH1 domain and LC (Hamers-Casterman et al., 1993). In human heavy chain disease (HCD), abnormal short HC dimers are overproduced by malignant B cells due to VH gene mutations during somatic hypermutation; such mutations often cause partial or complete loss of the CH1 domain (Biewenga et al., 1980; Corcos et al., 2011). Certain humanized HCs bearing modified VH sequences can be secreted as full-size HC dimers (Stoyle et al., 2017; Mieczkowski et al., 2020).
Conversely, most LCs can be secreted in free form without the need for HC assembly (Ambrosino et al., 1991; Dul et al., 1996; Leitzgen et al., 1997; Skowronek et al., 1998). While certain LCs require dimerization for proper folding and secretion (Leitzgen et al., 1997), many LCs can be secreted as monomers (Ambrosino et al., 1991; Dul et al., 1996; Skowronek et al., 1998). Most free LCs bind transiently to BiP through their VL domain, in contrast to HCs where BiP associates stably with unfolded and reduced Cγ1. However, LCs with specific sequences in the VL form stable BiP/LC complexes, which prevents secretion of free LCs (Dul and Argon, 1990; Ma et al., 1990; Knittler et al., 1995; Skowronek et al., 1998).
Studies involving point mutations demonstrate that the VH and/or VL domain sequences have a marked impact on the efficiency of Ig assembly and secretion (Wu et al., 1983; Dul and Argon, 1990; Dul et al., 1992; Rocca et al., 1993; Chen et al., 1994; Horwitz et al., 1994; Martin et al., 1996; Wiens et al., 2001; Whitcomb et al., 2003; Stoyle et al., 2017; Mieczkowski et al., 2020); however, the pivotal role of VH and VL domains during the IgG assembly process remains uncertain, particularly regarding whether the pairing of VH/VL provides a signal that initiates covalent assembly of HC and LC.
The aim of the present study was to investigate the intrinsic significance and role of the VH and/or VL domains during the process of IgG secretion and assembly by deleting V domain (s), rather than focusing on mutational effects. Plasmid vectors encoding various Ig fragments were designed based on the IgG1/κ molecule, some lacking VH or Vκ, or both, while others contained pseudo Vκ (ψVκ). We then assessed expression and secretion of these Ig fragments by HEK293 cells. We also investigated the BiP binding and resistance to proteolytic degradation of ψVκ-harboring LC (ψLC), and the influence of ψVκ domain on the overall structure of Fab format by molecular dynamics simulation. The results show that the VH or VL domain, or pairing of VH/VL, are not necessary for the IgG assembly and secretion process. Instead, the decisive factor governing this process is the pairing between CH and CL, while the structural integrity of the VL domain is crucial.
Plasmid vectors expressing 6C407 IgG1 and its variants were generated from plasmids KV10 and KV12, which differ only in terms of the restriction enzyme sites flanking the HC genes (Kim et al., 2019; Seo et al., 2020). 6C407 IgG1, a chimeric Ab bearing V regions (VH and Vκ) of mouse origin and C regions (Cγ and Cκ) of human origin, is specific for the KIFC1 antigen (Seo et al., 2020). In the KV10 plasmid, the VH and CH chain genes are flanked by MluI/NheI and NheI/BamHI restriction enzyme sites, respectively, while the VL and CL genes were flanked by BglII/BsiWI and BsiWI/EcoRI, respectively. In KV12, the VH and CH chains are flanked by MluI/NheI and NheI/HindIII restriction enzyme sites, respectively. These plasmids act as a cassette vector that permits cloning of the HC and LC genes harboring upstream leader sequences into specific cloning sites, followed by simultaneous expression of HC and LC under the control of two individual CMV promoters (PCMV). By replacing or deleting specific domain genes from the pre-existing KV10-6C407 IgG1 (Seo et al., 2020), a diverse set of plasmids, including KV10-[HC + Cκ], KV10-[Cγ1-3 + LC], KV10-[HC + ψLC], KV10-[Cγ1-3 + ψLC], KV10-[Cγ1-3 + LC], KV12-[HC], KV10-[Cγ1-3], and KV10-[Fc]. KV10-[Cγ1-3 + Cκ], also known as KV10-IgCw-γκ, was generated (Kim et al., 2019). The ψVκ sequence (here designated as 2C281 ψVκ) is available from GenBank (accession no. MH638370.1).
Human embryonic kidney 293 (HEK293) cells (ATCC; cat# CRL-1573) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Welgene; cat# LM 001-05) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin in a 5% CO2 humidified incubator at 37°C.
Cells were seeded in 60 mm dishes at a density of 1 × 106 cells/dish 24 h prior to transfection with plasmids. Plasmid DNA (4 μg) was pre-incubated at room temperature for 10 min with polyethylenimine (PEI) reagent (12 μg), and then added to the seeded cells in 200 μL of Opti-MEM (ThermoFisher Scientific; cat# 31985-070). Dishes were incubated at 37°C for 48 h.
At 48 h post-transfection, the cell culture was collected and a clear supernatant obtained by centrifugation. Transfected cells were washed three times with cold PBS and then harvested in cold PBS using a scraper. The collected cells were centrifuged at 850 g at 4°C for 5 min, and then lysed with IP Lysis Buffer (0.025 M Tris, 0.15 M NaCl, 0.001 M EDTA, 1% NP-40, 5% glycerol, pH 7.4) containing a protease inhibitor cocktail (Roche; cat# 11697498001). Supernatants were obtained by centrifugation at 16,000 × g at 4°C for 10 min. The protein concentration in the cell lysates was measured using a BCA Protein assay kit (Thermo Fisher Scientific; cat# 23227).
Aliquots of cell lysate (250 μg) prepared as described above were subjected to immunoprecipitation at 4°C for 18 h using anti-HA agarose (Thermo Fisher Scientific; cat# 26181) and KappaXP-Agarose (Thermo Fisher Scientific; cat# 2943212005). After washing the resin, immunoprecipitated proteins were eluted by heating at 100°C for 10 min, separated by SDS-PAGE, and then analyzed by immunoblotting.
SDS-PAGE was performed on 4%–20% gradient gels under reducing and non-reducing conditions, and resolved proteins were transferred to polyvinylidene fluoride (PVDF) membranes. Goat anti-human IgG-Fc (abcam; cat# ab97221) and goat anti-human Cκ (Thermo Fisher Scientific; cat# 31129) were used to detect human IgG-Fc and κ LC, respectively. A rabbit anti-goat IgG-HRP Ab was used to detect the bound primary Abs. GAPDH was detected by probing the membrane with primary mouse anti-GAPDH (Santa Cruz Biotechnology; cat# sc-322330) Ab, followed by horse anti-mouse IgG-horseradish peroxidase (Cell Signaling; cat# 7076). Immunoreactive proteins were visualized using an ECL Kit (GE Healthcare, Cat. No. RPN2106).
An indirect sandwich enzyme-linked immunosorbent assay (ELISA) was performed to assess the association between HC and LC in cell lysates. Briefly, 96-well polystyrene plates (Thermo Fisher Scientific; cat# 439454) were coated overnight at 4°C with 100 μL (2 μg/mL) of goat anti-human IgG-Fc Ab (abcam; cat# ab97221) as the capture Ab. After three washes with TBST, the wells were blocked for 1 h at room temperature with 3% BSA. Next, the wells were incubated for 1 h at room temperature with lysates of transfected cells (40 μg/well), followed by rabbit anti-human IgG κ light chain Ab (abcam; cat# ab125919) as the detection Ab, and an alkaline phosphatase-conjugated goat anti-rabbit IgG Ab (Thermo Fisher Scientific; cat# 31341). After each incubation step, the wells were washed three times with TBST (20 mM Tris-HCl, 150 mM NaCl, 0.05% Tween 20, pH 7.4). Color development was achieved by adding p-nitrophenyl phosphate substrate solution (1 mg/mL prepared in 0.1 M glycine, 1 mM ZnCl2, and 1 mM MgCl2, pH 10.3) to each well. Absorbance at 405 nm was measured in an Epoch2 microplate reader (BioTek).
DNA encoding HA-tagged BiP was cloned into the lentiviral vector pCDH-CMV-MCS-EF1-Puro (System Biosciences; cat# CD510B-1). Lentivirus particles were obtained by co-transfecting HEK293T packaging cells (2
Lysates from HEK293 cells transfected with LCs were prepared as described above in the ‘Preparation of supernatant and cell lysates’ section. Aliquots (500 μg) of lysate underwent IP at 4°C for 18 h using KappaXP-Agarose. After washing the resin, IP proteins were eluted with 0.1 M glycine (pH 3.0) and the pH of eluted proteins was adjusted to 7 with 1 M Tris (pH 9.5). Neutralized eluates (20 μL) were treated with trypsin (5 mg/mL) to achieve a final concentration of 0.2 mg/mL. Trypsin digestion was performed at 37°C for 0.5–10 min and quenched immediately on ice after each reaction. Samples were resolved by SDS-PAGE, followed by immunoblotting with an anti-Cκ antibody. Band intensity was quantified using ImageJ software and normalized to the undigested protein.
Protein structure modeling of Fab fragments (wild-type (wt) Fab and ψFab) was performed using Ab modeler embedded in Biovia Discovery Studio 2020 (Modeler ver. 9.22). The amino acid sequences of two HCs and LCs were annotated by the ImMunoGeneTics information system (IMGT) numbering scheme. Crystal structures M2177 (PDB ID: 5TL5) and 13A9 (PDB ID: 6DDR) were used as templates for the overall structures of the wt Fab and ψFab, respectively. The LC structures of Abs 14.1 (PDB ID: 5FB8) and 13A9 (PDB ID: 6DDR) were used as templates for the LC portions of wt and ψFab, while the HC structure of Ab 48G7 (PDB ID: 1AJ7) served as the template for generating HC structures for wt Fab and ψFab. Template structures were selected based on sequence similarity. The “Identify Framework Templates,” “Model Ab Framework,” and “Model Ab Loop” protocols in Discovery Studio were applied sequentially to generate structures for both Fabs. The generated models were ranked according to their probability density function (PDF) total energy.
The model with the lowest total potential energy determined by PDF analysis was subjected to explicit-water molecular dynamics (MD) simulation using the Discovery Studio 2020 software, which incorporates the CHARMm force field. Solvation and charge neutralization of the Fabs were followed by energy minimization by the built-in Smart Minimizer module within the Discovery Studio. The energy minimized protein–solvent system was gradually heated from 0 to 300 K at 1 atm pressure over a period of 100 ps. Then equilibration simulation of the energy minimized protein–solvent system run at 300 K for 200 ps. Finally, production simulation of the equilibrated protein–solvent system was conducted, running for 1 ns in the NVT (constant number of particles, volume, and temperature) ensemble. During this phase, structural coordinates of the system were saved at intervals of 2 ps. The accuracy of the predicted Fab models was validated using Ramachandran plots.
From the predicted Fab structure, we calculated the Fab elbow angle and visually presented it using the PyMOL software (Schrödinger LLC. 2021. the PyMOL molecular graphics system, version 2.5.0, https://www.pymolwiki.org/index.php/Elbow_angle), which allows for the calculation of the Fab elbow angle and simultaneous visualization of the two pseudo-dyad axes on the Fab structure.
First, we investigated whether the presence of the VH and/or VL domains of IgG affects secretion of fully assembled Ig. We did this by generating domain-deleted variants using a single-vector strategy to co-express HC and LCs (Figure 1A). Subsequently, lysates and supernatants of transfected cells were analyzed under both reducing and non-reducing denaturing conditions. The results revealed that compared with wt IgG HC, expression levels of HCs and LCs were similar in the absence of the VH, VL, or both domains (Figure 1B, lanes 1–4). Analysis of the supernatants revealed that Igs lacking the VH, VL, or both domains were secreted in their fully assembled form (Figure 1C, lanes 1–4). This indicates that the VH and VL chains, or their paired form (VH/VL), are dispensable for the secretory competence of IgG. Rather, the Cγ1 and Cκ domains are the determining factor.
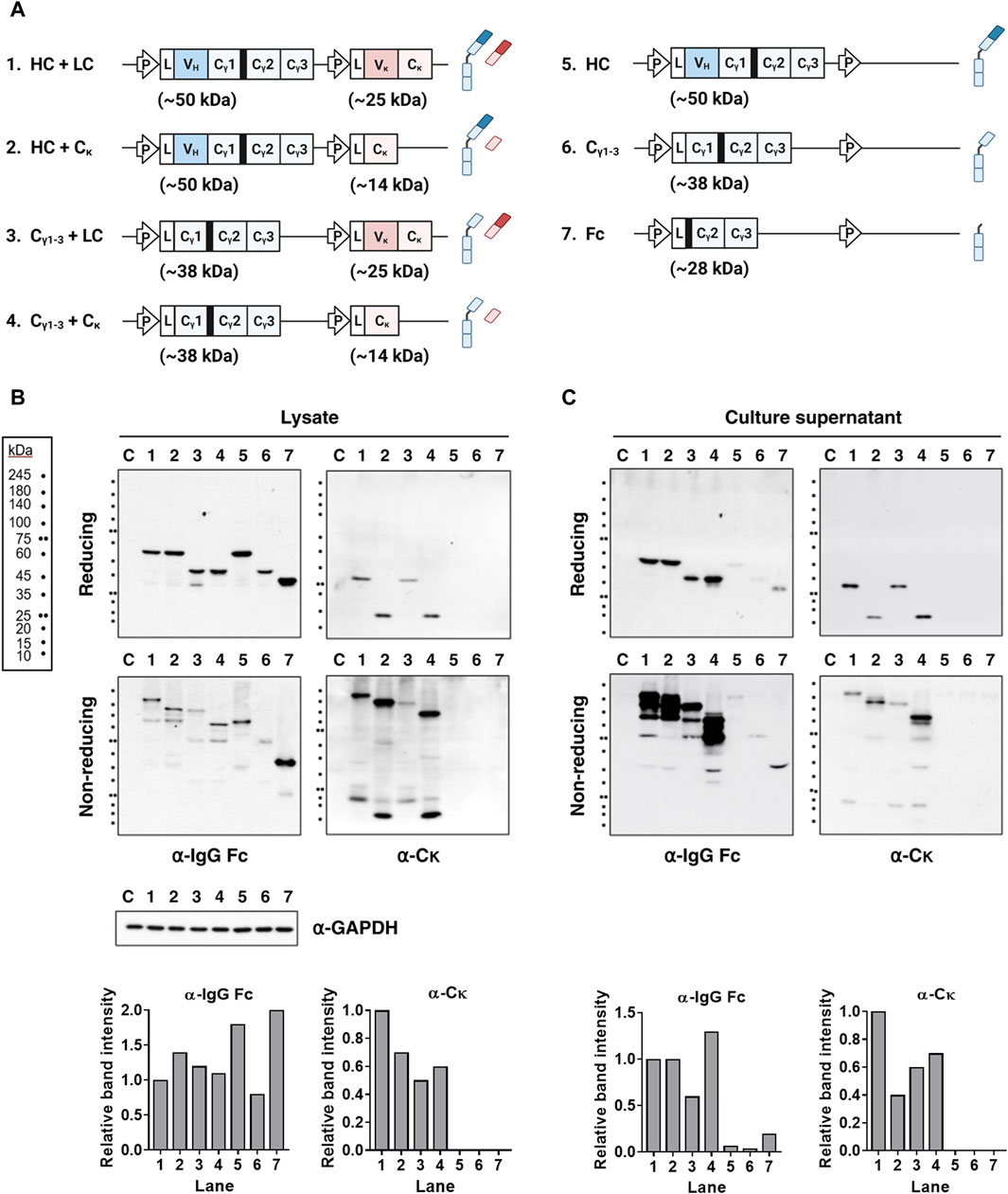
Figure 1. Covalent association between the Cγ1 and Cκ domains is sufficient for secretion of fully assembled Igs, regardless of the presence of the V domains. (A) Schematic representation of the plasmid vectors and Ig chain (or domain) proteins encoded by the corresponding Ig genes. The expected molecular weights of the individual subunits are indicated. The hinge region of human γ heavy chain is shown as a thick black bar upstream of the Cγ2 region. L, leader sequence; P, cytomegalovirus promoter. (B,C) Immunoblot analysis of Ig subunits. The lysates (B) and supernatant (C) of HEK293 transfectants were resolved in reducing and non-reducing SDS-PAGE gels. The resolved Ig proteins were probed with antibodies specific for human IgG-Fc and Cκ region. The bar graph below each gel image displays the relative intensity of bands detected by the specified antibodies under reducing conditions. Lanes 1–7 contain cell lysates obtained after lysis if cells transfected with each plasmids labeled as in (A). Lane C represents the non-transfected control. GAPDH was used as a loading control for protein normalization.
Previously, it was thought that full-size HC proteins could not be secreted independently of the LC. However, we found that they were secreted at very low, nearly undetectable levels, rather than not at all. The secreted HC-only protein appeared as a multimer (>180 kDa), in contrast to the monomers and HC dimers detected in cell lysates (Figures 1B, C, lane 5). In the presence of the Cκ chain, the Cγ1-3 chains were secreted in a covalently assembled form (as 2Cγ1-32Cκ tetramers), at a level almost equivalent to that of IgG (based on protein band intensity); however, the Cγ1-3 chains expressed in the absence of LC were secreted as dimers (∼86 kDa), but at a very low level (Figure 1C, lanes 4 and 6). This aligns with our earlier discovery that co-expression of Cγ1-3 and Cκ chains in HEK293F cells produces fully assembled IgG-like structures, at concentrations 30 times greater than that of Cγ1-3 alone (Kim et al., 2019). The Cγ2-3 chain (an Fc fragment containing the hinge region) was secreted as a dimer (∼56 kDa) at a significantly higher level than the Cγ1-3 chain (Figure 1C, lane 7). The difference in secretion of Cγ2-3 and Cγ1-3 is likely due to the ability of the Cγ2-3 chain to bypass Cγ1-mediated ERQC, which involves Cκ domains.
While our observations indicate that the VH/VL pairing is not critical for assembly and secretion of IgG (Figure 1), we sought to further investigate the impact of improper Vκ domain on IgG secretion, spurred by reports of VH-induced alterations in Cγ1 structure that led to HC-only antibody secretion through prevention of BiP binding (Stoyle et al., 2017; Mieczkowski et al., 2020). We hypothesized a similar scenario where distinct Vκ variants could induce changes in Cκ structure and potentially impact IgG secretion. To investigate this, we substituted the native Vκ domain with a pseudo type Vκ (ψVκ) derived from 2C281 hybridoma clone, which is characterized by the absence of defined FR4 segment resulting from a frame shift mutation in CDR3 (Figures 2A, B). Given FR4 is positioned just upstream of the Cκ domain, it is presumed to exert significant influence on Cκ structure. We designated LC harboring ψVκ as ψLC. We then investigated their secretion into the supernatant of four HEK293 cell transfectants: [HC + LC], [Cγ1-3 chain + LC], [HC + ψLC], and [Cγ1-3 chain + ψLC] (Figures 2C, D).
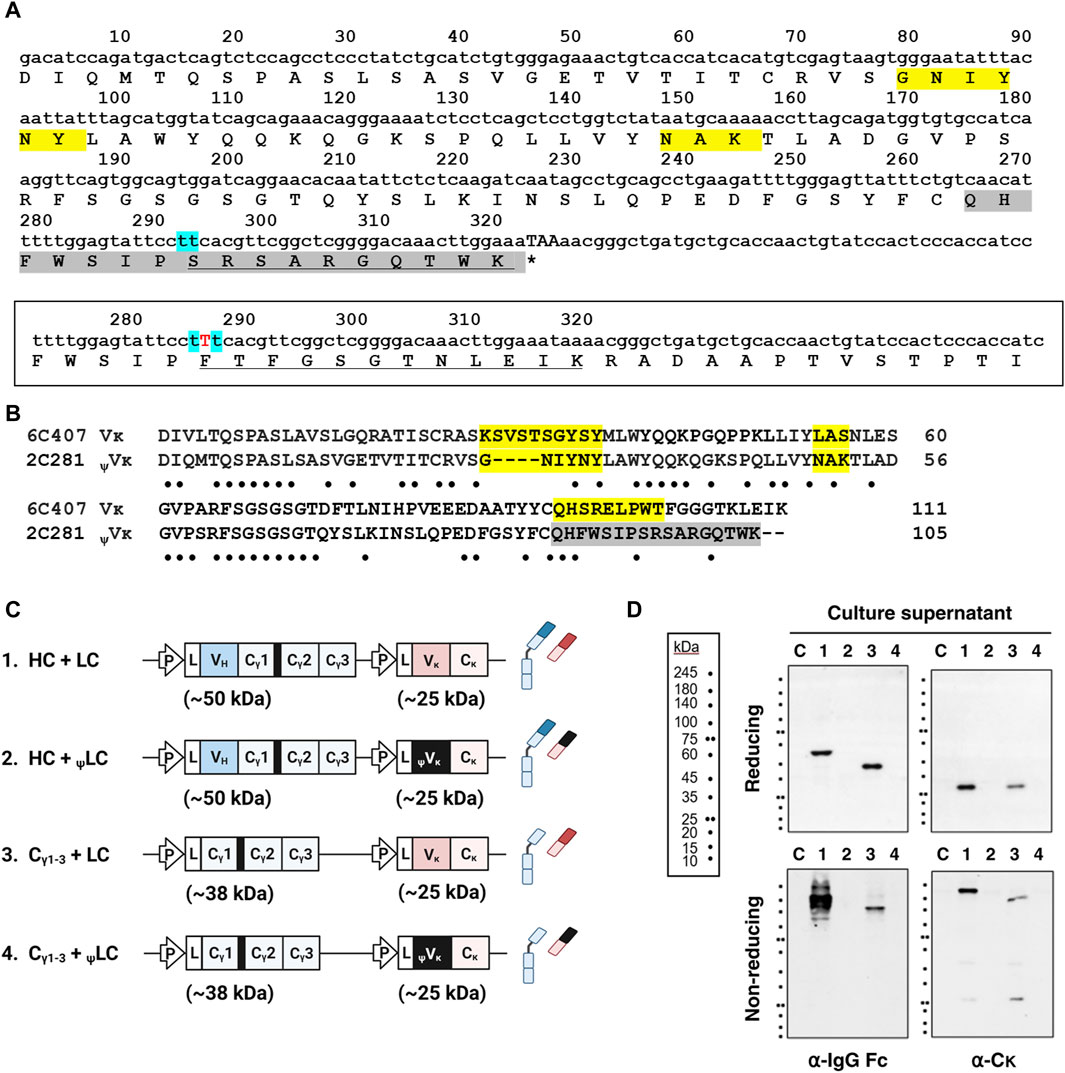
Figure 2. Replacement of Vκ with the ψVκ domain disrupts secretion of Ig molecules. (A) Nucleotide and amino acid sequences of 2C281 ψVκ. CDR residues according to the IMGT (International ImMunoGeneTics) information system shaded yellow. Due to a frame shift mutation in CDR3, the IMGT system does not define CDR3 of 2C281 ψVκ. Consequently, regions beyond FR3 are shaded in grey. Mint-colored shading marks the site with presumed base deletion (s). Amino acid sequence affected by base (s) deletion-induced reading-frame shift is underlined. Asterisk indicates stop codon. (B) Vκ sequence alignment. Sequences of 2C281 ψVκ and true Vκ regions from 6C407 clone aligned using the Clustal Omega server (https://www.ebi.ac.uk/Tools/msa/clustalo/). Absent residues denoted by dash. Dots indicate the positions of conserved residues between the sequences. (C) Schematic representation of the plasmid vectors and genes encoding the corresponding Ig chain (or domain) proteins. The hinge region of the human γ1 heavy chain is always present upstream of the Cγ2 region, and is shown here as a thick black bar. L, leader sequence; P, cytomegalovirus promoter. (D) Immunoblot analysis of culture supernatants. Lanes 1–4 contain supernatants obtained from cells transfected with each plasmid labeled as in (C). Lane C represents the non-transfected control.
When HCs and ψLCs were co-expressed, neither were secreted into the culture supernatant, whether individually, as assembly intermediates, or in their fully assembled form (Figure 2D, lane 2). Surprisingly, the same outcome was observed when co-expressing the Cγ1-3 chain with ψLCs (Figure 2D, lane 4). The reason why the Cγ1-3 chain did not get secreted when co-expressed with ψLC, in contrast to its successful expression in assembled form with wt LC (as shown in Figure 1), is likely because the ψVκ domain induces unfavorable changes in the downstream Cκ structure. The altered Cκ domain may fail to trigger BiP dissociation from Cγ1 and proper Cγ1 folding, leading to ultimate failure of secretion as an assembled Ig.
To determine whether the expressed aberrant ψLC can associate with HC, we assessed expression as well as assembly of HC-LC in lysates of four HEK293 cell transfectants: [HC + LC], [Cγ1-3 chain + LC], [HC + ψLC], and [Cγ1-3 chain + ψLC] (Figure 3A). When HCs and ψLCs were co-expressed, both chains were expressed within the cells to a degree similar to that of IgG, as seen in the input analysis under reducing conditions (Figure 3B). However, efficiency of correct and covalent HC-ψLC assembly within the cells was lower than that of wt IgG, as observed in the input analysis under non-reducing conditions. This was evident from a decrease in the size of IgG band (150 kDa), and the appearance of multiple smeared protein bands that were larger than the molecular weight of IgG, in the input analysis under non-reducing conditions (Figure 3B, lower panels, lane 2). The same observation was made when the Cγ1-3 chain, rather than full-size HC, was co-expressed with ψLCs (Figure 3B, lower panels, lane 4). The reduced efficiency of HC-ψLC assembly was confirmed by IP-immunoblotting and ELISA. The quantity of HC (as well as Cγ1-3 chain) pulled down by LC Cκ exceeded that pulled down by ψLC Cκ. Protein band intensity was normalized to IgG HC (Figure 3C, lane 1), and presented as a graph alongside the gel image. The same lysates used in Figure 3B were utilized for the ELISA. For the ELISA, the wells were coated with an anti-Fc Ab for capture, followed by incubation of cell lysates with an anti-Cκ Ab for detection. We noted a 52% reduction in HC and ψLC assembly compared with full-size HC and LC. In addition, assembly of the Cγ1-3 chain and ψLC decreased by 40% in comparison to Cγ1-3 chain and the LC (Figure 3D). Taken together, the data suggest that ψLC associates less with the HC or Cγ1-3 chain, despite both being expressed within cells.
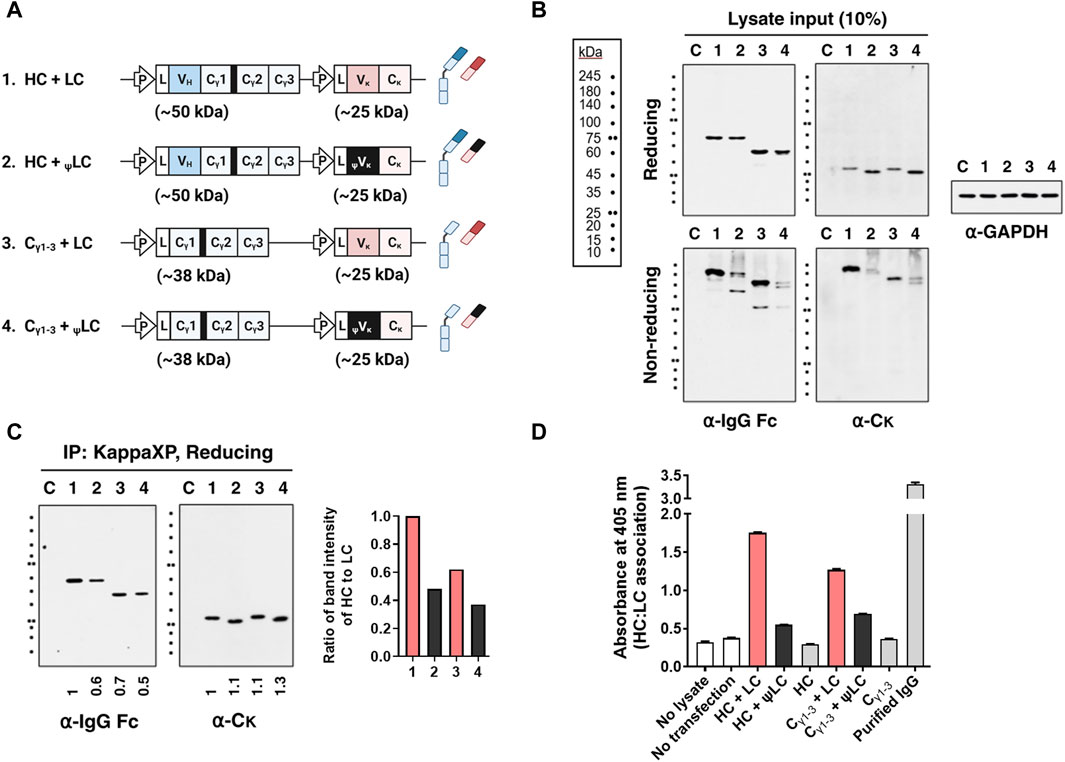
Figure 3. The presence of an improper Vκ domain hinders assembly of HC and LC. (A) Schematic representation of the plasmid vectors and genes encoding the corresponding Ig chain (or domain) proteins. The hinge region of human γ1 heavy chain is always present upstream of the Cγ2 region, and is shown here as a thick black bar. L, leader sequence; P, cytomegalovirus promoter. (B,C) Immunoblot and immunoprecipitation (IP) analyses of Ig subunits in cell lysates. Lysates of HEK293 transfectants were immunoprecipitated with KappaXP-Agarose, which captures the Cκ domain. Input samples and IP samples were separated by reducing and/or non-reducing SDS-PAGE, and subsequently analyzed by immunoblotting. (B) The input represents 10% of the total amount of lysate used for IP. GAPDH was used as an internal loading control. (C) Co-IP analysis of HC and LC in lysates from HEK293 transfectants. Numbers below the Western blot images represent protein band intensity, which was analyzed with ImageJ software and normalized to lane 1. The bar graph shows the ratio of the HC band intensity to that of the pulled-down LC. (D) Evaluation of H:L chain association by sandwich ELISA. Lysates of transfectants were placed in wells coated with goat anti-human IgG/Fc, and bound LCs were detected with rabbit anti-human Cκ followed by an AP-conjugated anti-rabbit IgG Ab. The data are expressed as the mean ± standard deviation of triplicate samples, and are representative of a single experiment from a series of three independent experiments.
Most LCs can be secreted on their own, independently of their association with HCs; these are designated as secretory-competent LCs. They then associate transiently with BiP through their VL domain, and can be secreted as monomers or homodimers. However, certain secretory-incompetent LCs bind irreversibly to BiP and cannot be secreted due to factors such as aggregation, incomplete folding, the presence of free thiol groups, and an inability to form homodimers; all of these characteristics are determined by the VL amino acid sequence (Reddy et al., 1996; Leitzgen et al., 1997; Skowronek et al., 1998). Therefore, we asked whether diminished association efficiency between ψLC and HC stemmed from the inability of the ψLC protein to achieve complete folding, causing BiP to retain ψLC within the cells. To investigate this, we conducted a study examining secretion, expression, and BiP binding within HEK293 cells. This cell line stably expressed BiP fused to an HA tag, and was transfected with genes encoding wt LC, ψLC, and Cκ (as depicted in Figure 4A). The HEK293 cells transduced with the BiP-HA lentiviral particles exhibited a 1.5-fold increase in BiP expression (both endogenous BiP and BiP-HA) compared to wild-type HEK293 cells (containing only endogenous BiP) (Figure 4B).
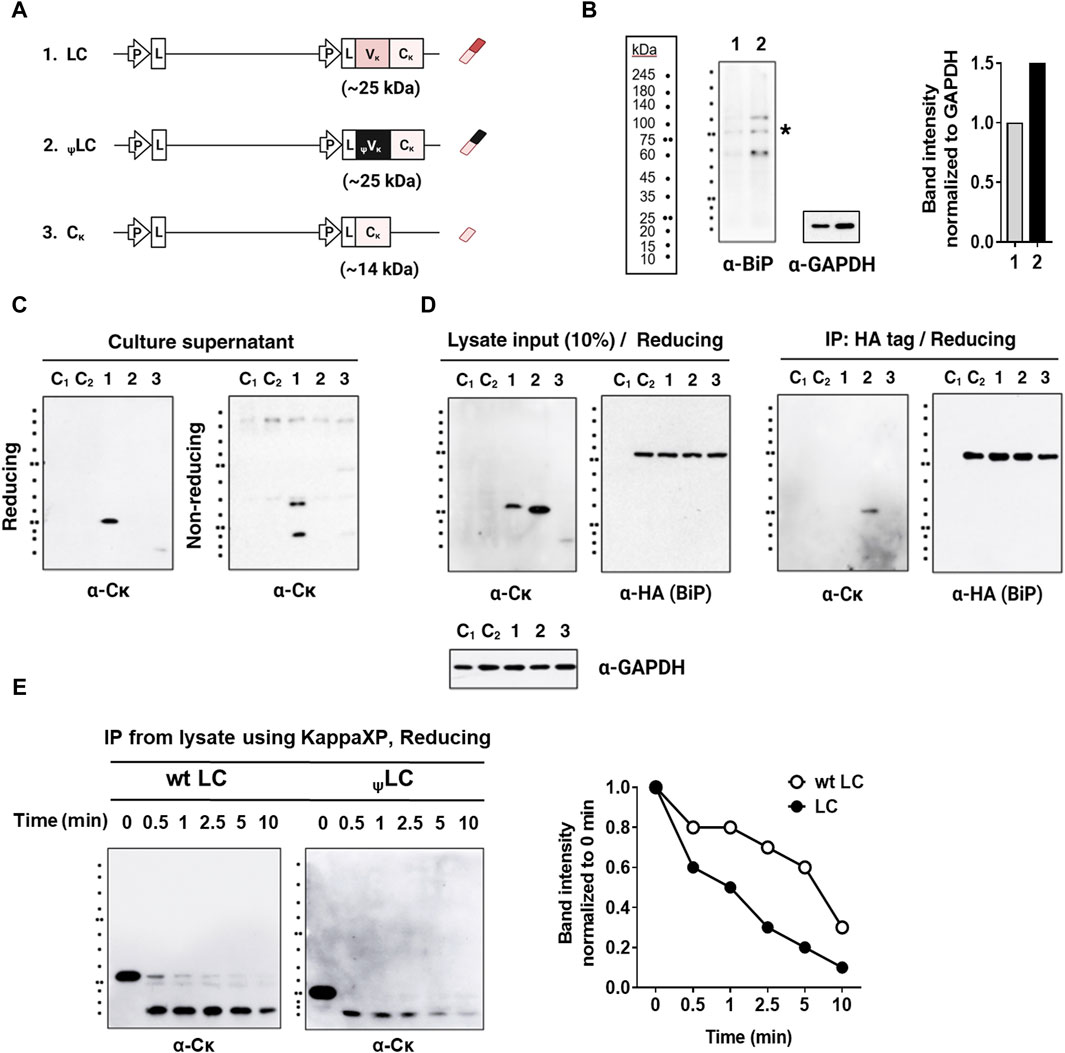
Figure 4. Interaction of LCs with BiP in HEK293 cells stably expressing HA-tagged BiP (HEK293-BiP). (A) Schematic representation of the plasmid vectors and genes encoding the corresponding Ig chain (or domain) proteins. (B) BiP detected using anti-BiP antibody in wild-type HEK293 cells (lane 1) and HEK293 cells transduced with the BiP-HA lentiviral particles (lane 2). The asterisk indicates the band corresponding to the size of BiP (C) Immunoblot analysis of LCs in the culture supernatant of HEK293-BiP. Forty-eight post-transfection culture supernatants from HEK293 transfectants were resolved in reducing and non-reducing SDS-PAGE gels. (D) Co-IP of LC and BiP in lysates from HEK293-BiP cells transfected with LCs. Co-immunoprecipitation was performed using an anti-HA agarose. Proteins in the extract (input = 10% of the lysate) and pull-down fractions (IP) were resolved by reducing SDS-PAGE and probed with Abs specific for human IgG-Fc, the Cκ region, and the HA tag. Lane C1, non-transfected HEK293 control; Lane C2, non-transfected HEK293-BiP control. (E) Protease susceptibility assay. wt LCs and ψLCs were enriched by IP of lysates from the respective HEK293 transfectants using KappaXP-Agarose. The eluates were treated with protease trypsin for the indicated times and resolved by SDS-PAGE, followed by immunoblotting with an anti-Cκ antibody. The intensity of bands from wt LC and ψLC proteins treated with trypsin was normalized to the untreated lane (0 min) and displayed as a graph alongside the gel image. The graph was generated from the cumulative intensity sum of three protein bands in each lane.
Notably, ψLCs were not secreted into the culture supernatant, in stark contrast to wt LC (Figure 4C). This observation, in conjunction with previous findings (shown in Figure 2), suggests that ψLC is inherently secretion-incompetent, regardless of the presence or absence of HC. We also observed that wt LC was secreted in both monomeric and dimeric forms, while Cκ was secreted as a dimer (Figure 4C, lanes 1). It is intriguing that wt LC is secreted as a mixture of these two forms, contrary to previous reports that some LCs are secreted as dimers (Leitzgen et al., 1997) while others are monomers (Ambrosino et al., 1991; Dul et al., 1996; Skowronek et al., 1998). Secretion of single Cκ domains was observed at a level barely detectable in the absence of HC expression, unlike wt LC (Vκ-Cκ). Regarding cellular expression, ψLCs exhibited higher expression than wt LC, as observed in the input analysis (Figure 4D, left panel). BiP binding to all three chains was confirmed through immunoprecipitation. Only ψLC was pulled down by the HA tag, indicating that ψLCs are stably bound to BiP within cells (Figure 4D, right panel). Our findings align with those of a previous report indicating that VL domains dictate the physical stability of BiP/LC complexes (Skowronek et al., 1998). Indeed, several mutant LCs bind BiP more avidly than their wt counterparts (Ma et al., 1990; Knittler et al., 1995). Stable binding of BiP to ψLC suggests that the ψLC structure possesses an incomplete conformation that cannot be secreted. In our experimental setup, we could not ascertain whether BiP binds exclusively to the ψVκ domain of ψLC, or if it also interacts with the Cκ domain, which might undergo structural changes via the Vκ/Cκ interface.
We conducted a protease susceptibility assay to compare the folding states of wt LC and ψLC in cells. Lysates from HEK293 transfectants were immunoprecipitated with KappaXP-Agarose. The eluates treated with the broad specificity protease trypsin. Subsequently, immunoblotting was conducted using an anti-Cκ antibody for detection (Figure 4E). ψLCs were degraded more rapidly than wt LC, indicating its more unstructured state compared to wt LC.
To better understand why neither ψLC was secreted, either individually or in assembled form, despite a partial covalent association between HC and ψLC in cell lysates (Figures 3, 4), we used a computer-assisted approach to explore the structural impact of replacing the cognate Vκ with ψVκ in the Fab format, designating it as ψFab. Discovery Studio 2020 software was used to generate three-dimensional structures of both the wt Fab and ψFab proteins; it then analyzed their structural dissimilarity by measuring the root mean square deviation (RMSD) and Fab elbow angle (Figure 5). The RMSD is a measure of the average distance between the backbone C-alpha atoms of superimposed molecules. Two identical structures yield an RMSD value of zero (a distant unit), while differing structures present values proportionate to their dissimilarity. The RMSD based on superimposition of wt Fab and ψFab structures was calculated as 18.3115 Å. The Fab elbow angle is the angle between the two pseudo-dyad axes relating the variable domains (VH and Vκ) and the constant domains (Cγ1 and Cκ) It is a valuable indicator of the overall topology of the Fab fragment. The distributions of elbow angles vary distinctly depending on the type of Fab light chain. Kappa Fab typically ranges from 125° to 195°, with a prevalence of 135°–145°, while lambda Fab ranges from 115° to 225°, favoring 185°–195° (Stanfield et al., 2006). In this study, the kappa Fab elbow angle measured 146° for wt Fab and 194° for ψFab, showing notable distinction in the relative positioning of V and C domains. Essentially, this reflects a difference in overall arrangement of the HC and LC domains of the Fab.
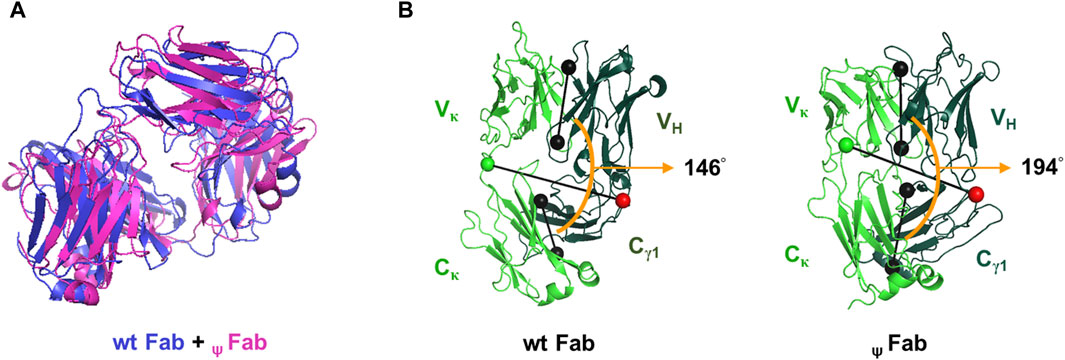
Figure 5. Ribbon diagrams illustrating the predicted Fab structures. (A) Superposition of wt Fab and ψFab structures, modeled using Discovery Studio 2020 (Modeler ver. 9.22). The Fab molecules are represented by blue (wt Fab) and pink (ψFab) ribbons. (B) The Fab elbow angle was calculated and represented visually by PyMOL software (version 2.5.0). Black dumbbells pass through the center of mass of the V and C domains of each Fab. The orange arc denotes the Fab elbow angles. Green and red dumbbells represent residues used to separate the V and C domains, with a green ball representing the LC and a red ball representing the HC.
Taken together, we suggest that the ψVκ domain might distort the downstream Cκ structure, making the altered Cκ domain lose the ability to release BiP from the Cγ1 domain, resulting in failure of complete CH folding and secretion as an assembled IgG. The modified Cκ domain may also lead to loss of LC secretion and hinder covalent binding with Cγ1. We ruled out the possibility that the ψVκ domain has a detrimental effect on the VH structure at the interface, which would then impact downstream Cγ1, maintaining BiP binding and preventing secretion of assembled Ig. This is supported by the fact that co-expression of the ψLC and Cγ1-3 chains did not result in the secretion of assembled Ig (Figure 2), and ψLC exhibited poor association with Cγ1-3 chains (Figure 3). Thus, it seems that BiP-bound ψLCs might have disrupted the entire IgG secretion process.
While covalent assembly between CH1 and CL, as well as the functions of BiP-mediated ERQC mechanisms during the IgG assembly and secretion process, have been studied comprehensively at the molecular level, our understanding of the role of the V domains in this process remains insufficient. It is suggested that assembly of HC and LC is initiated by non-covalent interactions between the VH and VL domains, which may trigger dissociation from BiP, thereby allowing formation of disulfide bonds between the CH1 and CL domains (Dul and Argon, 1990; Haas, 1991). Dorrington and others demonstrated that non-covalent pairing of VH and VL precedes formation of interchain disulfide bonds between HC and LC, thereby playing a pivotal role in regulating Ig chain assembly (Chothia et al., 1985; Hamel et al., 1986); however, our findings challenge this notion. Instead, our results indicate that VH-VL pairing is not required for assembly of HC and LC. Rather, the critical factor governing IgG assembly and secretion is the CH-CL pairing within the IgG molecule.
Full-size HCs were secreted effectively, and in a completely assembled form, when expressed along with either the Vκ-Cκ chain or the Cκ-only domain; this also seems to be the case for the VH-deficient Cγ1-3 chain. The Cγ1-3 chains were secreted efficiently in their assembled form when co-expressed with LCs in either the Vκ-Cκ chain or Cκ-only domain (Figure 1). Our previous findings underscored the importance of the interaction between the Cγ1 and Cκ domains for Ig secretion, as co-expressing Cγ1-3 and Cκ chains in HEK293F resulted in a production yield approximately 30-fold higher than that of Cγ1-3-only, and 5-fold higher than that of the Cκ-only domains (Kim et al., 2019). Moreover, full-size LC (Vκ-Cκ chains) was secreted efficiently in both free and assembled forms, depending on the presence of the HC. Notably, the Cκ domain showed limited independent secretion, but was released effectively when co-expressed with HCs in an assembled form (Figures 1, 4). This implies that the association between the Cγ1 and Cκ domains may induce complete folding of the Cκ domain, which lacks the Vκ region. To the best of our knowledge, this is the first study to demonstrate this mutual facilitation between the Cγ1 and Cκ domains.
With respect to secretion of the HC and LC as individual entities, we found that the presence of the V domain had a markedly different effect on secretion. In the absence of the LC, the HC was not secreted, regardless of the presence/absence of the VH domain (full-size HC and Cγ1-3 chain) (Figure 1). By contrast, in the absence of HC expression, secretion of LC (Vκ-Cκ) was markedly higher than that of the Cκ chain, which was undetectable (Figure 4). In this context, during cellular IgG expression, it is probable that an unfolded VH structure within HCs is inherently preferrable to maintain the unfolded state of the Cγ1 domain of IgG, thereby aiding BiP-mediated ER retention until the CL domain associates with the Cγ1 domain. By contrast, for LCs, a folded VL domain is inherently favored to facilitate CL folding, although experimental confirmation is necessary.
While VH and VL pairing is not mandatory for Ig secretion, it is clear that the primary sequence of the V domains, when present in an Ig molecule, influences the process. This conclusion is supported by our observation that introducing an aberrant ψVκ domain into the Ig molecule impeded Ig secretion completely (Figure 2). Furthermore, several studies show that the VH and VL domain sequences have a significant impact on Ig assembly and secretion (Wu et al., 1983; Dul and Argon, 1990; Dul et al., 1992; Rocca et al., 1993; Chen et al., 1994; Horwitz et al., 1994; Martin et al., 1996; Wiens et al., 2001; Whitcomb et al., 2003; Stoyle et al., 2017; Mieczkowski et al., 2020). Single-point mutations in VL can disrupt Ig assembly and secretion completely, as exemplified by the FS62 (Phe62 to Ser) mutation in the λ1 chain (Dul and Argon, 1990), the GR15 (Gly15 to Arg) mutation in the λ2 chain (Wu et al., 1983), and the YH87 (Tyr87 to His) mutation in the κ chain, in MOPC 21 myeloma cells (Dul et al., 1992). Some point mutations in VL only partially block secretion (Rocca et al., 1993). LC secretion levels vary depending on their VL sequence (Horwitz et al., 1994). Studies on murine T15 IgG revealed that point mutation(s) in the VH-complementarity-determining region 2 (CDR2) impeded assembly with LCs and subsequent secretion (Chen et al., 1994; Wiens et al., 2001). Deletion of specific residues in T15 VH-CDR3 restored secretion of HCs with the T15 VH-CDR2 mutation (Martin et al., 1996). Mutations in the framework regions (FRs) of T15 VL, which could not be secreted alone, restored secretion competency and enabled secretion-defective HCs with the T15 VH-CDR2 mutation to be secreted as assembled IgG, illustrating the compensatory effect of LC mutations on harmful HC mutations (Whitcomb et al., 2003). Yet, the exact biochemical mechanism underlying the outcomes of V domain sequence changes remains unclear. Recently, the cognate pairing preference of VH and VL, which is a crucial factor in achieving high yields of bispecific IgG1, could be dictated by specific residues in the CDR3 loops (Fernandez-Quintero et al., 2022). Low secretion of some IgG molecules was attributed to reduced recognition of incompletely folded VK by protein disulfide isomerase (PDI), which impairs disulfide bond formation within LC, ultimately leading to proteasomal degradation (Mathias et al., 2020).
Given that a previous study suggests that differences in IgG secretion levels associated with VH and VL sequences, it is reasonable to assume that expression of Ig comprising Cγ1-3 and Cκ domains (previously designated as IgCw-γκ of 98 kDa) (Kim et al., 2019) could serve as a reference point for secretion efficiency. Further research on production of IgG-based recombinant Abs should be undertaken to investigate whether specific VH and VL sequences increase or reduce IgG secretion, using IgCw-γκ as a reference.
Some reports support the idea that the folding state of the Cγ1 domain can be influenced by its upstream domain structure. Certain humanized HCs with modified VH-CDR3 sequences were secreted as full-size HC dimers, often referred to as HC-only Abs. These HC dimers, whether full-length or Fd dimers, were produced by CHO cells (even in the presence of cognate LCs) instead of fully assembled Ig forms (Stoyle et al., 2017; Mieczkowski et al., 2020). This phenomenon was due to promotion of Fd-Fd dimer formation by intrinsic VH sequences, leading to Cγ1 folding and formation of intrachain disulfide bonds through the robust VH/Cγ1 interface. Consequently, folded Cγ1 avoids binding to BiP and circumvents ER retention mechanisms. In another study, chimeric HCs comprising CD40-Cγ1-3, in which the VH region of the HC was replaced by human CD40, were secreted even in the absence of LCs (Capon et al., 1989). It is plausible that a specific protein located upstream of the Cγ1 domain (whether or not it is VH) induces complete folding of the Cγ1 domain, thereby bypassing ER retention mechanisms in the absence of LCs. Here, we put forth the idea that a particular protein (in our investigation, the ψVκ domain) upstream of the Cκ domain may induce structural changes in Cκ, followed by unfavorable interactions with Cγ1, thereby impeding IgG secretion. This, in turn, underscores the intricate interplay of antibody domains.
We only examined IgG1-based fragments to study their assembly and secretion patterns. Abs of different isotypes are likely to exhibit distinct assembly mechanisms, with IgE being a noteworthy example. In a previous study of an IgE-based fragment, we proposed the potential involvement of alternative assembly mechanisms, and chaperones other than BiP. Co-expressing Cε1-4 and Cκ chains in HEK293F cells led to secretion of individual chains without covalent assembly (Kim et al., 2022), whereas co-expressing the Cγ1-3 and Cκ chains resulted in assembly and secretion of IgCw-γκ (Kim et al., 2019). Substituting the Cε1 domain in the Cε1-4 chain with the Cγ1 domain restored secretion of assembled IgE-like molecule, designated as IgCw-γεκ, with a size of ∼130 kDa (Kim et al., 2022). Exploring the assembly process of IgE, which remains largely uncharted, would be highly fascinating.
Recombinant Ab formats based on IgG1 have been diversified for biopharmaceutical research. Our study increases our understanding of their assembly and secretion, highlighting the role of the V domains. Our data will inform the design of alternative molecular formats, potentially incorporating non-cognate HCs and LCs, for therapeutic applications.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
JC: Writing–original draft, Conceptualization, Formal Analysis, Investigation. YJ: Investigation, Writing–original draft. YR: Validation, Writing–original draft. JJ: Formal Analysis, Validation, Writing–original draft. EL: Validation, Writing–original draft. LV: Validation, Writing–original draft. MK: Conceptualization, Writing–original draft. M-HK: Funding acquisition, Project administration, Supervision, Writing–original draft, Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Mid-Career Researcher Program (NRF-22020R1A2C2008258) from the National Research Foundation of Korea (NRF).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ig, immunoglobulin; HC, heavy chain; LC, light chain; VH, variable region of the heavy chain; VL, variable region of the light chain; Cγ, constant region of gamma chain; Cκ, constant region of light chain; ERQC, endoplasmic reticulum quality control; IP, immunoprecipitation; ELISA, enzyme-linked immunosorbent assay.
Ambrosino, D. M., Kanchana, M. V., Delaney, N. R., and Finberg, R. W. (1991). Human B cells secrete predominantly lambda L chains in the absence of H chain expression. J. Immunol. 146 (2), 599–602. doi:10.4049/jimmunol.146.2.599
Biewenga, J., Frangione, B., Franklin, E. C., and van Loghem, E. (1980). A gamma l heavy-chain disease protein *EST lacking the entire VH and CHl domains. Scand. J. Immunol. 11 (6), 601–607. doi:10.1111/j.1365-3083.1980.tb00028.x
Bole, D. G., Hendershot, L. M., and Kearney, J. F. (1986). Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J. Cell. Biol. 102 (5), 1558–1566. doi:10.1083/jcb.102.5.1558
Capon, D. J., Chamow, S. M., Mordenti, J., Marsters, S. A., Gregory, T., Mitsuya, H., et al. (1989). Designing CD4 immunoadhesins for AIDS therapy. Nature 337 (6207), 525–531. doi:10.1038/337525a0
Chen, C., Martin, T. M., Stevens, S., and Rittenberg, M. B. (1994). Defective secretion of an immunoglobulin caused by mutations in the heavy chain complementarity determining region 2. J. Exp. Med. 180 (2), 577–586. doi:10.1084/jem.180.2.577
Chothia, C., Novotny, J., Bruccoleri, R., and Karplus, M. (1985). Domain association in immunoglobulin molecules. The packing of variable domains. J. Mol. Biol. 186 (3), 651–663. doi:10.1016/0022-2836(85)90137-8
Corcos, D., Osborn, M. J., and Matheson, L. S. (2011). B-cell receptors and heavy chain diseases: guilty by association? Blood 117 (26), 6991–6998. doi:10.1182/blood-2011-02-336164
Drabek, D., Janssens, R., de Boer, E., Rademaker, R., Kloess, J., Skehel, J., et al. (2016). Expression cloning and production of human heavy-chain-only antibodies from murine transgenic plasma cells. Front. Immunol. 7, 619. doi:10.3389/fimmu.2016.00619
Dul, J. L., and Argon, Y. (1990). A single amino acid substitution in the variable region of the light chain specifically blocks immunoglobulin secretion. Proc. Natl. Acad. Sci. U. S. A. 87 (20), 8135–8139. doi:10.1073/pnas.87.20.8135
Dul, J. L., Aviel, S., Melnick, J., and Argon, Y. (1996). Ig light chains are secreted predominantly as monomers. J. Immunol. 157 (7), 2969–2975. doi:10.4049/jimmunol.157.7.2969
Dul, J. L., Burrone, O. R., and Argon, Y. (1992). A conditional secretory mutant in an Ig L chain is caused by replacement of tyrosine/phenylalanine 87 with histidine. J. Immunol. 149 (6), 1927–1933. doi:10.4049/jimmunol.149.6.1927
Feige, M. J., Groscurth, S., Marcinowski, M., Shimizu, Y., Kessler, H., Hendershot, L. M., et al. (2009). An unfolded CH1 domain controls the assembly and secretion of IgG antibodies. Mol. Cell. 34 (5), 569–579. doi:10.1016/j.molcel.2009.04.028
Feige, M. J., Hendershot, L. M., and Buchner, J. (2010). How antibodies fold. Trends Biochem. Sci. 35 (4), 189–198. doi:10.1016/j.tibs.2009.11.005
Fernandez-Quintero, M. L., Kroell, K. B., Grunewald, L. J., Fischer, A. M., Riccabona, J. R., and Liedl, K. R. (2022). CDR loop interactions can determine heavy and light chain pairing preferences in bispecific antibodies. MAbs 14 (1), 2024118. doi:10.1080/19420862.2021.2024118
Haas, I. G. (1991). BiP--a heat shock protein involved in immunoglobulin chain assembly. Curr. Top. Microbiol. Immunol. 167, 71–82. doi:10.1007/978-3-642-75875-1_4
Hamel, P. A., Klein, M. H., and Dorrington, K. J. (1986). The role of the VL- and VH-segments in the preferential reassociation of immunoglobulin subunits. Mol. Immunol. 23 (5), 503–510. doi:10.1016/0161-5890(86)90113-6
Hamers-Casterman, C., Atarhouch, T., Muyldermans, S., Robinson, G., Hamers, C., Songa, E. B., et al. (1993). Naturally occurring antibodies devoid of light chains. Nature 363 (6428), 446–448. doi:10.1038/363446a0
Hendershot, L., Bole, D., Kohler, G., and Kearney, J. F. (1987). Assembly and secretion of heavy chains that do not associate posttranslationally with immunoglobulin heavy chain-binding protein. J. Cell. Biol. 104 (3), 761–767. doi:10.1083/jcb.104.3.761
Horwitz, A. H., Nadell, R., Preugschat, F., and Better, M. (1994). Chimeric immunoglobulin light chains are secreted at different levels: influence of framework-1 amino acids. Mol. Immunol. 31 (9), 683–692. doi:10.1016/0161-5890(94)90178-3
Janssens, R., Dekker, S., Hendriks, R. W., Panayotou, G., van Remoortere, A., San, J. K., et al. (2006). Generation of heavy-chain-only antibodies in mice. Proc. Natl. Acad. Sci. U. S. A. 103 (41), 15130–15135. doi:10.1073/pnas.0601108103
Kim, M., Choi, J., Seo, Y., and Kwon, M. H. (2019). Applications of the immunoglobulin Cw fragment (IgCw) composed of the constant regions of heavy and light (CH and CL) chains. Biochem. Biophys. Res. Commun. 512 (3), 571–576. doi:10.1016/j.bbrc.2019.03.108
Kim, M., Lee, J., Choi, J., Seo, Y., Park, G., Jeon, J., et al. (2022). A recombinant Ig fragment (IgCw-γεκ) comprising the cγ1-cε2-4 and Cκ domains is an alternative reagent to human IgE. J. Immunol. 208 (3), 772–779. doi:10.4049/jimmunol.2100576
Knittler, M. R., Dirks, S., and Haas, I. G. (1995). Molecular chaperones involved in protein degradation in the endoplasmic reticulum: quantitative interaction of the heat shock cognate protein BiP with partially folded immunoglobulin light chains that are degraded in the endoplasmic reticulum. Proc. Natl. Acad. Sci. U. S. A. 92 (5), 1764–1768. doi:10.1073/pnas.92.5.1764
Lee, Y. K., Brewer, J. W., Hellman, R., and Hendershot, L. M. (1999). BiP and immunoglobulin light chain cooperate to control the folding of heavy chain and ensure the fidelity of immunoglobulin assembly. Mol. Biol. Cell. 10 (7), 2209–2219. doi:10.1091/mbc.10.7.2209
Leitzgen, K., Knittler, M. R., and Haas, I. G. (1997). Assembly of immunoglobulin light chains as a prerequisite for secretion. A model for oligomerization-dependent subunit folding. J. Biol. Chem. 272 (5), 3117–3123. doi:10.1074/jbc.272.5.3117
Ma, J., Kearney, J. F., and Hendershot, L. M. (1990). Association of transport-defective light chains with immunoglobulin heavy chain binding protein. Mol. Immunol. 27 (7), 623–630. doi:10.1016/0161-5890(90)90004-j
Mains, P. E., and Sibley, C. H. (1983). The requirement of light chain for the surface deposition of the heavy chain of immunoglobulin M. J. Biol. Chem. 258 (8), 5027–5033. doi:10.1016/s0021-9258(18)32532-8
Martin, T. M., Kowalczyk, C., Stevens, S., Wiens, G. D., Stenzel-Poore, M. P., and Rittenberg, M. B. (1996). Deletion in HCDR3 rescues T15 antibody mutants from a secretion defect caused by mutations in HCDR2. J. Immunol. 157 (10), 4341–4346. doi:10.4049/jimmunol.157.10.4341
Mathias, S., Wippermann, A., Raab, N., Zeh, N., Handrick, R., Gorr, I., et al. (2020). Unraveling what makes a monoclonal antibody difficult-to-express: from intracellular accumulation to incomplete folding and degradation via ERAD. Biotechnol. Bioeng. 117 (1), 5–16. doi:10.1002/bit.27196
Mieczkowski, C., Bahmanjah, S., Yu, Y., Baker, J., Raghunathan, G., Tomazela, D., et al. (2020). Crystal structure and characterization of human heavy-chain only antibodies reveals a novel, stable dimeric structure similar to monoclonal antibodies. Antibodies (Basel) 9 (4), 66. doi:10.3390/antib9040066
Reddy, P., Sparvoli, A., Fagioli, C., Fassina, G., and Sitia, R. (1996). Formation of reversible disulfide bonds with the protein matrix of the endoplasmic reticulum correlates with the retention of unassembled Ig light chains. EMBO J. 15 (9), 2077–2085. doi:10.1002/j.1460-2075.1996.tb00561.x
Rocca, A., Khamlichi, A. A., Aucouturier, P., Noel, L. H., Denoroy, L., Preud'homme, J. L., et al. (1993). Primary structure of a variable region of the V kappa I subgroup (ISE) in light chain deposition disease. Clin. Exp. Immunol. 91 (3), 506–509. doi:10.1111/j.1365-2249.1993.tb05932.x
Seo, Y., Lee, Y., Kim, M., Park, H., and Kwon, M. H. (2020). Assembly and folding properties of cytosolic IgG intrabodies. Sci. Rep. 10 (1), 2140. doi:10.1038/s41598-020-58798-7
Skowronek, M. H., Hendershot, L. M., and Haas, I. G. (1998). The variable domain of nonassembled Ig light chains determines both their half-life and binding to the chaperone BiP. Proc. Natl. Acad. Sci. U. S. A. 95 (4), 1574–1578. doi:10.1073/pnas.95.4.1574
Stanfield, R. L., Zemla, A., Wilson, I. A., and Rupp, B. (2006). Antibody elbow angles are influenced by their light chain class. J. Mol. Biol. 357 (5), 1566–1574. doi:10.1016/j.jmb.2006.01.023
Stoyle, C. L., Stephens, P. E., Humphreys, D. P., Heywood, S., Cain, K., and Bulleid, N. J. (2017). IgG light chain-independent secretion of heavy chain dimers: consequence for therapeutic antibody production and design. Biochem. J. 474 (18), 3179–3188. doi:10.1042/BCJ20170342
Vanhove, M., Usherwood, Y. K., and Hendershot, L. M. (2001). Unassembled Ig heavy chains do not cycle from BiP in vivo but require light chains to trigger their release. Immunity 15 (1), 105–114. doi:10.1016/s1074-7613(01)00163-7
Whitcomb, E. A., Martin, T. M., and Rittenberg, M. B. (2003). Restoration of Ig secretion: mutation of germline-encoded residues in T15L chains leads to secretion of free light chains and assembled antibody complexes bearing secretion-impaired heavy chains. J. Immunol. 170 (4), 1903–1909. doi:10.4049/jimmunol.170.4.1903
Wiens, G. D., Lekkerkerker, A., Veltman, I., and Rittenberg, M. B. (2001). Mutation of a single conserved residue in VH complementarity-determining region 2 results in a severe Ig secretion defect. J. Immunol. 167 (4), 2179–2186. doi:10.4049/jimmunol.167.4.2179
Wu, G. E., Hozumi, N., and Murialdo, H. (1983). Secretion of a lambda 2 immunoglobulin chain is prevented by a single amino acid substitution in its variable region. Cell. 33 (1), 77–83. doi:10.1016/0092-8674(83)90336-7
Zhang, T., Cheng, X., Yu, D., Lin, F., Hou, N., Cheng, X., et al. (2019). Corrigendum: genetic removal of the CH1 exon enables the production of heavy chain-only IgG in mice. Front. Immunol. 10, 398. doi:10.3389/fimmu.2019.00398
Keywords: IgG, assembly, secretion, endoplasmic reticulum quality control, VH domain, VL domain
Citation: Choi J, Jeon Y, Roh Y, Jang J, Lee E, Villamante L, Kim M and Kwon M-H (2024) The dispensability of VH-VL pairing and the indispensability of VL domain integrity in the IgG1 secretion process. Front. Mol. Biosci. 11:1346259. doi: 10.3389/fmolb.2024.1346259
Received: 29 November 2023; Accepted: 09 April 2024;
Published: 02 May 2024.
Edited by:
Martin Wear, University of Edinburgh, United KingdomReviewed by:
Harald Kolmar, Darmstadt University of Technology, GermanyCopyright © 2024 Choi, Jeon, Roh, Jang, Lee, Villamante, Kim and Kwon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Myung-Hee Kwon, a3dvbm1oQGFqb3UuYWMua3I=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.