
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mol. Biosci., 10 April 2024
Sec. Molecular Evolution
Volume 11 - 2024 | https://doi.org/10.3389/fmolb.2024.1286824
Adaptation in an environment can either be beneficial, neutral or disadvantageous in another. To test the genetic basis of pleiotropic behaviour, we evolved six lines of E. coli independently in environments where glucose and galactose were the sole carbon sources, for 300 generations. All six lines in each environment exhibit convergent adaptation in the environment in which they were evolved. However, pleiotropic behaviour was observed in several environmental contexts, including other carbon environments. Genome sequencing reveals that mutations in global regulators rpoB and rpoC cause this pleiotropy. We report three new alleles of the rpoB gene, and one new allele of the rpoC gene. The novel rpoB alleles confer resistance to Rifampicin, and alter motility. Our results show how single nucleotide changes in the process of adaptation in minimal media can lead to wide-scale pleiotropy, resulting in changes in traits that are not under direct selection.
Populations adapt to an environment by accumulating beneficial genetic changes. The effects of mutations depend on the genetic background (GxG interactions) and this dependency is called epistasis. Due to unknown widespread genetic interactions that lead to epistasis, predicting the effects of mutations, and hence adaptation, is a challenge (Johnson et al., 2023). Despite sequence level dependencies, examples of evolution of populations to show convergence in phenotypes—convergent evolution—have been reported from laboratory experiments (Cunningham et al., 1997; Nakatsu et al., 1998; Cooper et al., 2001; Cooper et al., 2003; Woods et al., 2006; Wong and Kassen, 2011; Herron and Doebeli, 2013; Scribner et al., 2020; Mulvey et al., 2023; Pearl Mizrahi et al., 2023) and nature (Jaekel and Wake, 2007; Chen et al., 2020; Fukushima and Pollock, 2023). Such phenotypic convergence may often be a result of remarkable genetic parallelism, where adaptation is a result of mutations in a specific set of target genes.
Mutational effects also depend on the environment the population is in, due to GxE interactions. When populations well-adapted to an environment are shifted to another, the effects of the mutations they have accumulated may change due to their by-product or pleiotropic effects (MacLean et al., 2004; He and Zhang, 2006; Paulander et al., 2009; Sivakumaran et al., 2011; McGee et al., 2016; Utrilla et al., 2016; Deatherage et al., 2017; Chesmore et al., 2018; Li et al., 2018; Noda-Garcia et al., 2019; Jerison et al., 2020; Kinsler et al., 2020; Ruelens et al., 2023). Such effects can lead to ecological specialization (Schluter, 2009; Forister et al., 2012) and help maintain diversity (Mitchell-Olds et al., 2007; Savolainen et al., 2013). Pleiotropy was thought to operate antagonistically, however, recent ecological evidences show that pleiotropic effects can also be beneficial (Anderson et al., 2011; Remold, 2012; Savolainen et al., 2013; Bono et al., 2017; Elena, 2017). Experiments in laboratory show that while antagonistic pleiotropic effects are possible (Cooper and Lenski, 2000; Turner and Elena, 2000; MacLean et al., 2004; Zhong et al., 2004; Duffy et al., 2006; Bennett and Lenski, 2007; Dettman et al., 2007; Lee et al., 2009; Wenger et al., 2011; Jasmin et al., 2012; Hietpas et al., 2013; Hong and Nielsen, 2013; Jasmin and Zeyl, 2013; Yi and Dean, 2013; Schick et al., 2015; Fraebel et al., 2017), they are not universal (Ostrowski et al., 2005; Bennett and Lenski, 2007; Lee et al., 2009; Jasmin and Zeyl, 2013; Leiby and Marx, 2014; Rodriguez-Verdugo et al., 2014; Li and Zhang, 2018).
Global epistasis provides a framework to predict the effect of a mutation based on the genetic background that it occurs on Diaz-Colunga et al. (2023), and we now know that the effect of a beneficial mutation decreases with increase in the fitness of the background genome it occurs on. However, there exists no such understanding of pleiotropy, rendering its effects unpredictable. Given that environments rarely remain constant or predictable, an understanding of pleiotropy is vital. In this study, we propose to provide an understanding of the link between adaptation and pleiotropy. Specifically, we seek to identify if convergent genetic (and phenotypic) changes lead to predictable pleiotropic effects.
Our experimental setup consists of Escherichia coli populations evolving in two environments—glucose and galactose. The utilization of glucose and galactose occur via well-defined routes in E. coli. Glucose is broken down into pyruvate via glycolysis. Subsequently, under aerobic conditions, pyruvate enters the TCA cycle, and produce 32 molecules of ATP per molecule of glucose. Galactose, on the other hand, cannot be used for glycolysis directly. Leloir pathway converts it into glucose-6-phosphate, a version convertible into pyruvate molecules. It is then metabolised via glycolysis and TCA cycle (Sellick et al., 2008).
After letting these populations adapt for three hundred generations in their “home” (glucose or galactose) environments, we test the effects of adaptive mutations in each of these populations in “similar” and “dissimilar” non-home environments. Genome sequencing reveals that the genetic basis of adaptation exhibits remarkable convergence, with most lines acquiring mutations in rpoB or rpoC. Our results show that different alleles of these global regulators fixed in the bacterial populations, leading to statistically identical fitness increases in the evolution environment. Despite conferring this remarkable convergence in adaptive response, the exact pleiotropic effects (in the “away” environment) of the mutations fixed in these populations are unpredictable. Our results show that single nucleotide changes in a global regulator can exhibit divergent pleiotropic effects.
Escherichia coli K-12 (MG1655) strain was used as the parent strain in our experiment. Six independent lines, starting from the ancestor, were started and propagated for 300 generations in minimal M9 media in a specific sugar [0.2% glucose (Sigma Aldrich, Catalogue GA270) or 0.2% galactose (Himedia, Catalogue GRM101)] environment. Cultures were grown to mid-log phase and transferred via a 1:100 dilution to a tube containing 5 mL fresh media. The lines evolved in glucose are referred to as glu1 to glu6; and the lines evolved in galactose are referred to as gal1 to gal6. Minimal media (M9) was used as per the following composition (per litre): Na2HPO4 (Himedia, Catalogue GRM3961), 6.78g: KH2PO4 (Himedia, Catalogue TC011), 3g: NaCl (Himedia, Catalogue MB023), 0.5g; NH4Cl (Himedia, Catalogue GRM717), 1g; to which filter sterilized 1M MgSO4 (Himedia, Catalogue GRM1281) and 0.1M CaCl2 (Himedia, Catalogue GRM710) solutions were added.
Freezer stock of a strain was revived by streaking on an LB plate and allowed to grow overnight at 37°C. A single colony from the plate was then used to inoculate 2 mL LB liquid media, and allowed to grow overnight at 37°C and at 250 rpm. The overnight culture was then sub-cultured 1:100 in M9 media containing the carbon resource of interest at 0.2%, and 150 μL of the culture allowed to grow in a 96-well plate in a microplate (Tecan 1000Pro) reader, with shaking. OD600 readings were taken every 30 min.
Genomic DNA was isolated by inoculating a single colony of the strain of interest on an LB plate into 2 mL LB and allowed to grow for 6–8 h at 37°C and 250 rpm. Genomic DNA was isolated from the culture as per instructions provided with FavorPrep Tissue Genomic DNA Extraction Mini Kit. Genomic DNA was sequenced by Eurofins Genomics India Pvt. Ltd., Bangalore, on a NextSeq500 platform.
Cultures were revived from freezer stock in LB (Himedia, Catalogue M575) and incubated overnight at 37°C and 250rpm. Rifampicin (Himedia, Catalogue CMS1889) stock of 50 mg/mL was made in DMSO. A 1:10 dilution of the cultures was made in LB and different concentrations (200, 100, 50, 25, 12.5, 6.25, 3.125 μg/mL) of Rifampicin were added to it. 150 µL of each of the culture was then transferred in triplicate into a 96-well plate. The minimum inhibitory concentration was checked after 24 h of incubation at 37°C by measuring the optical density.
The galP promoter was cloned upstream of green fluorescent protein (gfp) in pPROBE-NT plasmid (Miller et al., 2000). The galP promoter sequence was amplified using the primers (5′ AG CGG ATC CCG ATG CTG CCG GTC TGA AGT 3′) and (5′ GG GAA TTC GAT GCC CTC CAA TAT GGT TA 3′) (Miller et al., 2000).
The ΔgalP knockout was generated using the λ-Red based recombination assay as described in Datsenko and Wanner (2000), using the primers (5′ ATG GCT GAC GCT AAA AAA CAG GGG CGG TCA AAC AAG GCA AGT GTA GGC TGG AGC TGC TTC 3′) and (5′ TTA ATC GTG ACC GCC TAT TTC GCG CAG TTT ACG ACC ATA TGA ATA TCC TCC TTA 3′). The knockout was checked using the primers (5′ CGT GGG AAA AAA CCG ACA AAG C 3′) and (5′ TAC GGT AAG CTG ATG CTC CTG G 3′).
The mutant rpoB alleles in the strains gal1, gal5, and glu5 were transformed into the wild-type ancestor by amplifying the mutant RpoB allele, and replacing the ancestral allele using a λ-Red based recombination. The transformed population was plated on LB plates containing Rifampicin (25 μg/mL), to select for the rpoB allele replacement. The PCR product for recombination was amplified using the primers (5′ TTG GCC TGG TAC GTG TAG AGC GTG CGG TGA AAG AGC 3′) and (5′ AAG TTA CCA GGT CTT CTA CGA AGT GGC CTT CTT CAT CCA A 3′).
Cultures were revived from −80°C freezer stocks in tryptone broth [1.5% tryptone (Amresco, Catalogue J859), 0.5% sodium chloride] and incubated at 37°C, 250 rpm shaking for 12 h. Motility plates [1.5% tryptone, 0.5% sodium chloride, 0.2% agar (SRM, Catalogue 19661)] were poured fresh and 1 µL of the culture was stabbed in the centre of the plate. The plates were incubated at 30°C overnight and imaged using gel doc.
All the error-bars shown in the plots correspond to standard deviations, unless specified otherwise. Y-tests (one or two-tailed) were used to compare means of two data sets and in all cases, the significance level (p) was set to 0.05. In cases where multiple hypothesis tests were performed, Bonferroni corrections were performed to avoid false positives.
Genomic DNA of ancestral and all 12 evolution lines was isolated using DNA Mini Kit (Qiagen). Cells from each line were spread on an LB plate for single colonies. A single colony was picked and grown for 6 h in LB at 37°C for DNA isolation. DNA quality and concentration were measured immediately after DNA isolation using Nanodrop Spectrophotometer, and were also confirmed by gel electrophoresis.
All 13 samples (six lines in each environment, and the ancestor) were sent for paired-end sequencing using Illumina NovaSeq 6000, with an average read-depth of 151 bp.
Based on the quality report of fastq files, sequences were trimmed to retain only high-quality sequences for analysis and low-quality sequences were removed. The adapter trimmed reads were aligned to Escherichia coli (ATCC 47076) reference genome. Each sample had a minimum coverage of more than ×30. Variant calling was done for the samples using GATK and further annotated using SnpEff. Variants that were present in the ancestral strain were filtered out manually. Raw sequencing data for all 13 samples is available at https://www.ncbi.nlm.nih.gov/sra/PRJNA1022868.
The six lines evolved in each of the two environments were tested for growth kinetics in the conditions in which they were evolved at three time points. For glucose-evolved lines, the growth kinetics were characterized after 120, 180, and 300 generations; and for galactose-evolved lines, the growth kinetics were characterized after 60, 180, and 300 generations.
As shown in Figure 1 (and Supplementary Figures S1, S2), the adaptive response of the six evolved lines after 300 generations is statistically similar (for all pairwise comparisons of growth rates in the exponential phase, p > 0.05).
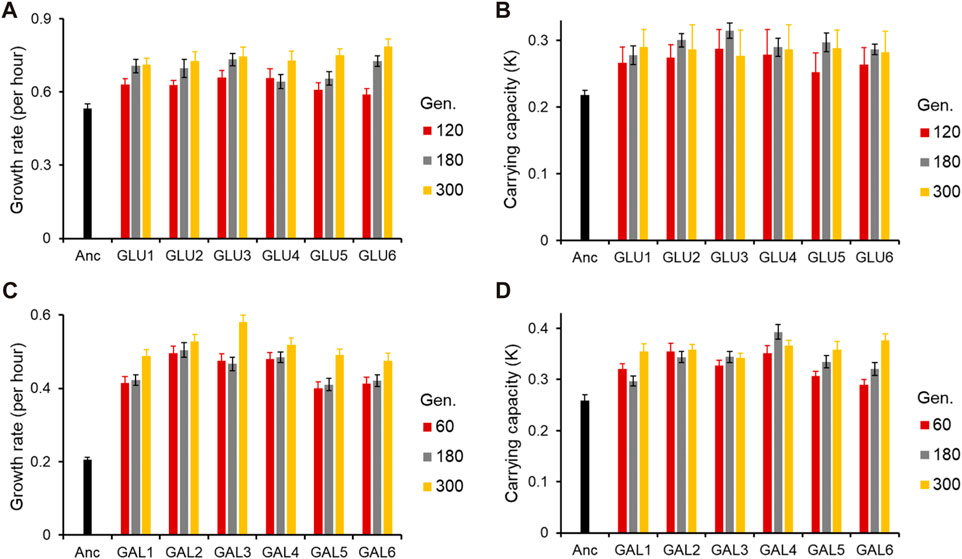
Figure 1. Adaptive response of the evolved lines. (A) Growth rate (r) and (B) Carrying capacity (K) of glucose-evolved lines, at 120, 180, and 300 generations, in M9 media containing glucose (home environment). (C) Growth rate (r) and (D) Carrying capacity (K) of galactose-evolved lines, at 60, 180, and 300 generations, in M9 media containing galactose (home environment). All experiments were performed three independent times. The average and standard deviation is reported.
All six lines evolved in glucose and galactose exhibit exponential phase growth kinetics which is statistically different from the ancestor (p < 0.05 and p < 0.01, respectively). Thus, phenotypic adaptation after 300 generations of evolution, in each of the two environments, was found to be highly convergent.
At 120 generations, all six glucose-evolved lines exhibited a growth rate higher than that of the ancestor (for all pairwise comparisons, p < 0.05). Between the six evolved lines, glu6, when compared to lines glu3 and glu4, exhibited a lower growth rate (for both pairwise comparisons, p < 0.05). All other pairwise comparisons between the glucose-evolved lines yielded insignificant differences (p > 0.05). At 180 and 300 generations, no pairs of evolved lines exhibited significant difference in growth rate (for all pairwise comparisons, p > 0.05).
Comparisons of biomass yield in glucose reveals that after 120 generations, no significant differences were observed between any two evolved lines (for all pairwise comparisons, p > 0.05). However, all evolved populations exhibited biomass yield which was significantly greater than that of the ancestor (for all pairwise comparisons, p < 0.05). After 180 and 300 generations of adaptation, the evolved lines continued to exhibit no difference in biomass yield between themselves (for all pairwise comparisons, p > 0.05) and accumulated greater biomass as compared to the ancestor (for all pairwise comparisons, p < 0.05).
Similar analysis was also done for the galactose-evolved populations at 60 and 180 generations after adaptation. The galactose-evolved lines at 60 generations exhibit a significantly higher growth rate, when compared against the ancestor (p < 0.001). The differences in growth rates between the six lines, however, are statistically insignificant (for all pairwise comparisons, p > 0.05). The same pattern was observed at 180 generations, where the growth rate of the evolved lines was statistically significantly higher than that of the ancestor (p < 0.001), and the growth rate differences between the evolved lines were statistically insignificant (for all pairwise comparisons, p > 0.05).
However, the growth pattern after the exponential phase exhibits a change from 60 to 300 generations. After adaptation for 60 generations, all six lines accumulate biomass which is significantly greater than the ancestor (for all pairwise comparisons, p < 0.05). However, unlike growth rate, biomass accumulation exhibits divergence between the evolved lines. Among all pairwise comparisons, only four pairs (gal1 with gal4, gal5 and gal6, and gal3–gal5) were found to have similar biomass yield (for all four pairwise comparisons, p > 0.05). All other pairwise comparisons between the evolved lines yield p < 0.05. After 300 generations of adaptation, however, only line gal3 exhibits a different biomass yield (as compared to any of the other five evolved lines, p < 0.05). All other lines exhibit accumulate biomass similarly (for all pairwise comparisons, p > 0.05).
In Figures 1, 2, we demonstrate that the glucose- and galactose-adapted populations exhibit convergent growth dynamics, when grown in their respective environments, galactose, and glucose. We next tested for growth of the glucose-evolved and galactose-evolved populations in an away environment, that differed in the source of carbon (glucose, galactose, or lactose) and report the results in Figures 2, 3 and Supplementary Figure S3.
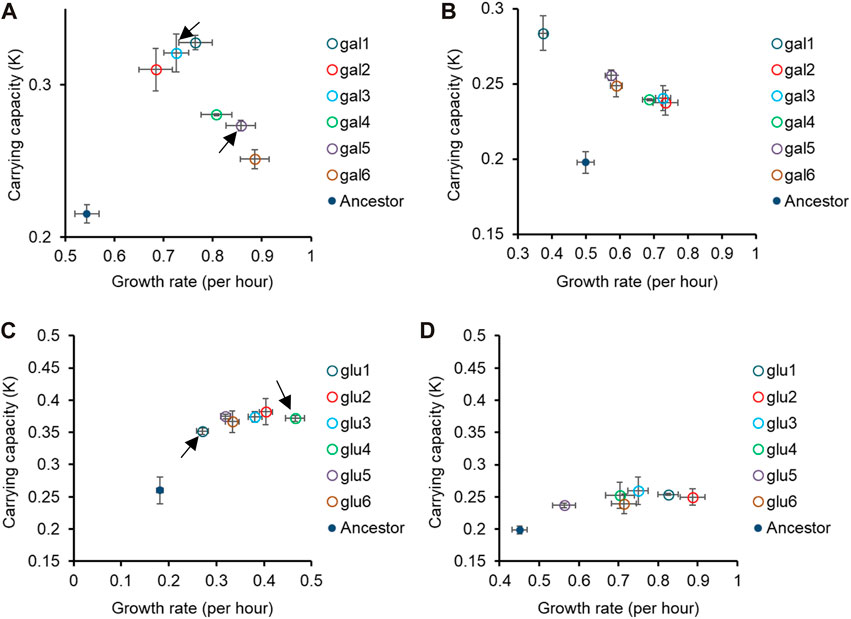
Figure 2. Pleiotropic effects of adaptation in home carbon environments. Growth rate (r) and carrying capacity (K) of glucose-evolved lines at 300 generations, in M9 media containing (A) galactose and (B) lactose. Growth rate (r) and carrying capacity (K) of glucose-evolved lines at 300 generations, in M9 media containing (C) galactose and (D) lactose. In (A,C), arrows indicate the two glucose- and galactose-evolved lines selected for further analysis. All experiments were performed three independent times. The average and standard deviation is reported.
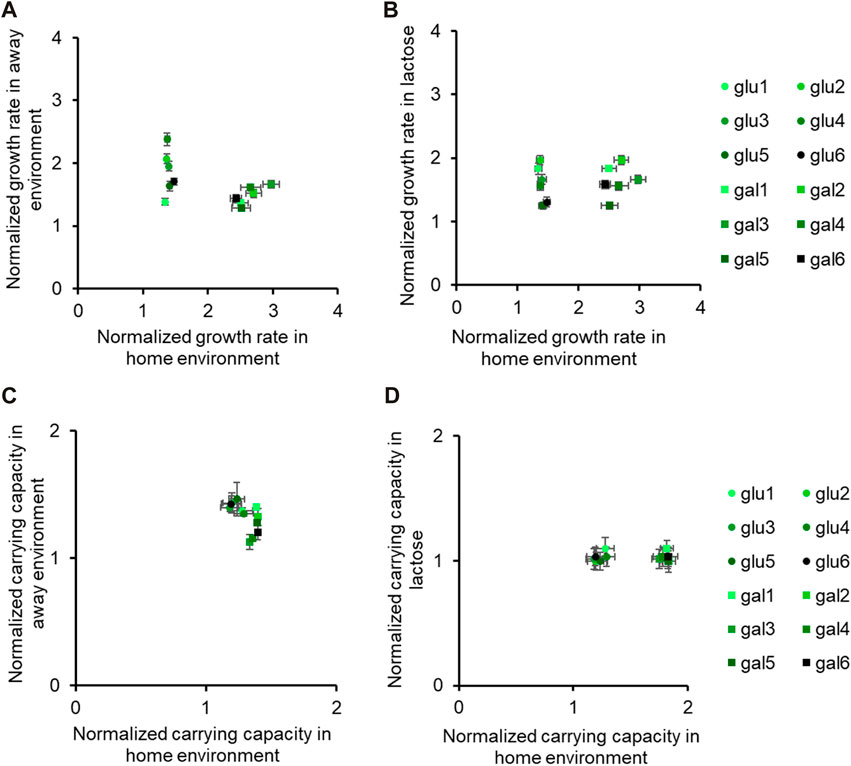
Figure 3. Fitness effects in “similar” non-home environments are not predictable based on fitness in home environment. All six glucose-evolved and galactose-evolved populations were tested for growth in M9 minimal media containing alternative sources of carbon. Growth rates and carrying capacities were normalized with that of the ancestor in the environment tested. Away environment for glucose-evolved lines is galactose, and that for galactose-evolved lines is glucose. (A,B) are used to compare the normalized growth rates of the twelve populations in their home environment with that in the away environments. Similarly, (C,D) show how the normalized carrying capacity changes in different environments.
While all the evolved populations had accumulated mutations that were beneficial in these environments as well, their exact fitnesses were not predictable based on growth in the home environment.
We compared the growth rates of these evolved populations and found that the lines gal1 and gal5 had dissimilar growth rates in glucose (p < 0.001), but identical growth rates in lactose (p > 0.05). This observation presents as evidence of pleiotropic effects of adaptation.
Similar effects of pleiotropy were seen in glucose-evolved lines, when grown in galactose and lactose. The growth rate of lines glu4 and glu5, when grown in galactose, was statistically significantly different from each other (p < 0.001). On the other hand, the growth rate and optical density of the same two lines, when grown in lactose, was statistically identical (p > 0.05).
Since the above-discussed two pairs of lines—gal1 and gal5 and glu4 and glu5—exhibit the most diverse phenotypes when tested in glucose and galactose, respectively, we analyse the pleiotropic effects of adaptation in glucose and galactose in these lines in two other contexts. Before explaining the effects of pleiotropy in these four lines, we first identified the genetic basis of adaptation in these twelve evolution lines.
To test the genetic basis of the adaptive response described above, we sequenced the genomes of all twelve evolved lines, and compared it with the ancestor. The list of the mutations in the evolved lines are as shown in Table 1. The sequencing results demonstrate that, as has been previously observed (Conrad et al., 2010a; LaCroix et al., 2015; Wannier et al., 2018; Bakerlee et al., 2021), rpoB and rpoC are major mutational targets of adaptation in minimal media. For glucose-adapted lines, four out of the six lines acquired mutation in either rpoB or rpoC. On the other hand, out of the six galactose-adapted lines, five acquired mutation in either rpoB or rpoC.
Mutations in rpoB and rpoC have previously been isolated, during adaptation in minimal media (Conrad et al., 2010a; LaCroix et al., 2015). In a single experiment, as many as 37 out of 45 lines adapted in minimal media acquired a mutation in rpoB or rpoC (Conrad et al., 2010a). Interestingly, these mutations include not just SNPs, but also deletions of specific regions of rpoC (Herring et al., 2006).
As discussed above, we focus on two lines evolved in glucose (glu4 and glu5) and two lines evolved in galactose (gal1 and gal5). The choice of these lines for sequencing was dictated by the observation that their growth kinetics in the foreign environment was most different from each other’s (as indicated in Figure 2 and Supplementary Figure S3).
The glucose-adapted lines have mutation in only a single gene: rpoC in glu4 line and rpoB in glu5 line. Both the lines carry one non-synonymous mutation each. The galactose-evolved lines (gal1 and gal5), on the other hand, had more than one mutation each. Each of the two galactose-evolved line, however, also had a non-synonymous mutation in rpoB. Thus, three of the four evolved lines analysed further in this work have a mutation in rpoB. Mutations in RNA Polymerase (RNAP) components have been demonstrated to be adaptive under nutrient, thermal, radiation, and antibiotic stresses (Severinov et al., 1993; Herring et al., 2006; Conrad et al., 2010b; Tenaillon et al., 2012; Degen et al., 2014; Harden et al., 2015; Bruckbauer et al., 2019; Lennen et al., 2023).
In addition to mutations in rpoB, the galactose-evolved lines gal1 and gal5 also have mutations in sspA (related to starvation response), galP promoter region, and rRNA genes. Genes associated with these mutations are known to be associated with cellular response to growth in stressful conditions (Abo et al., 2002; Christensen and Gerdes, 2003; Moore and Sauer, 2007; Ranquet and Gottesman, 2007; Ono et al., 2009; Li et al., 2013).
To test the role of the rpoB alleles in dictating aspects of cellular physiology, we shifted the rpoB alleles to the ancestral background. The rpoB mutants isolated from adaptation in minimal media are known to confer antibiotic resistance to Rifampicin (Alifano et al., 2015; Li MC. et al., 2021). Hence, the rpoB alleles were amplified and transformed in an ancestor carrying the plasmid pKD46 (Datsenko and Wanner, 2000). The transformed cells were selected on Rifampicin plates, which contained 40 μg/mL of the antibiotic. All three rpoB alleles identified in this study were transferred to the ancestor genetic background in this fashion, and the constructs were verified via sequencing. The pleiotropic effects of the mutant alleles were characterized as described below.
Many rpoB alleles have been previously demonstrated to alter the sensitivity of the cell to the antibiotic, rifampicin (Ezekiel and Hutchins, 1968). The minimum concentration of Rifampicin which permitted growth was established as the MIC associated with each of the three isolated rpoB allele. As shown in Table 2, the MIC associated with all three rpoB alleles reported in this study is greater than of the ancestral rpoB allele.
Mutations in rpoB which confer resistance to Rifampicin are classified into four groups—N terminal cluster (143–148), cluster I (505–537), cluster II (562–575) and cluster III (684–690) (Campbell et al., 2001). Two of the three rpoB alleles we report have single amino acid changes in cluster I. One of the mutants (H526Y) has been reported before (Lisitsyn et al., 1984) and is said to alter the rifampicin binding pocket in Mycobacterium tuberculosis (Molodtsov et al., 2017). The crystal structure of Rif-RNAP reveals that rifampicin touches closely the base of a loop which is flanked by the cluster I amino acids (Campbell et al., 2001). As a result, due to steric hindrance caused by mutations in this region, rifampicin fails to bind to RNAP, yielding the cell insensitive to the antibiotic. The third mutation (N613H) does not lie in any of these clusters, and little is known about how such a “distant” change can confer resistance.
It has been suggested previously that the increase in fitness in minimal media can be due to a reduction/loss in motility (Fong et al., 2005; Leatham et al., 2005). Since rpoB alleles confer changes to cellular physiology and gene expression in a global sense (Conrad et al., 2010a), we tested how the three rpoB alleles change the ability of E. coli to swim in liquid media. Alleles of rpoB have previously been shown to lead to a decrease in the gene expression of genes associated with flagellar biosynthesis, and as a result, reduced motility on plates (Conrad et al., 2010a; Meenakshi and Munavar, 2018).
As shown in Figures 4A–E, the swimming ability of the strains carrying any of the three rpoB alleles identified in this study was compromised, as compared to the ancestral strain. Flagellar biosynthesis and the expression of the chemotaxis proteins are under the control of a single transcriptional regulatory complex, FlhD4C2 (Neidhardt, 1996). Escherichia coli “decides” to be motile or not, based on integration of environmental and cellular signals at the flhDC promoter and transcript (Bertin et al., 1994; Shin and Park, 1995; Soutourina et al., 1999; Ko and Park, 2000; Lehnen et al., 2002; Sperandio et al., 2002; Francez-Charlot et al., 2003). FlhD4C2 activates the expression of the flagella-specific sigma factor, FliA. FliA, in turn, activates its own expression and that of several genes, including fliC, which encodes for the flagellar filament (Chilcott and Hughes, 2000). The promoter activity in the strains carrying the three mutant rpoB alleles, the PfliA and PfliC promoter activity was reduced as compared to the ancestor (Figure 4F). All four evolved strains exhibit statistically significantly lower class 2 and class 3 gene expression, compared to the ancestor (p < 0.01 for glu4, p < 0.001 for glu5, p < 0.001 for gal1, and p < 0.001 for gal5).
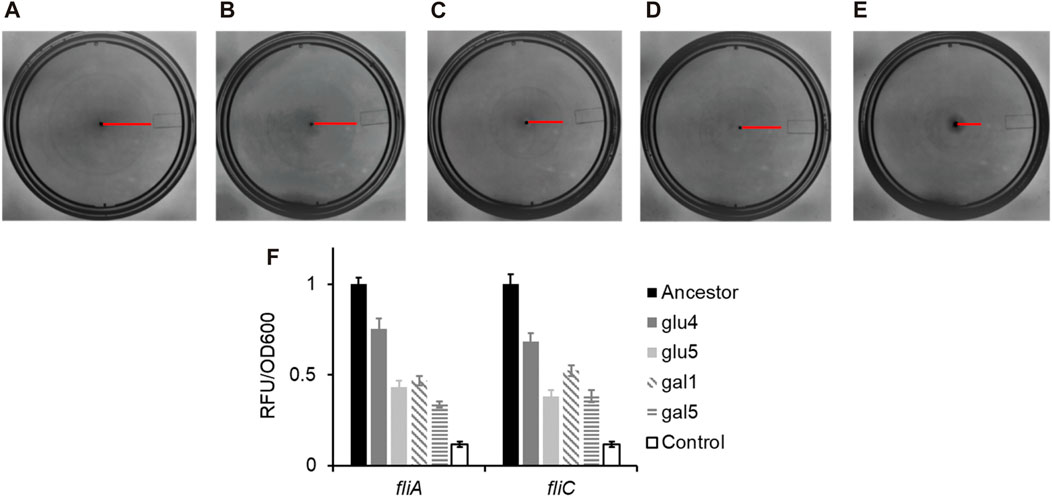
Figure 4. RpoB alleles reduce the ability of Escherichia coli to swim in liquid media, and lower gene expression from the flagellar promoters fliA and fliC. (A–E) Motility rings of ancestor (A), GLU4 (B), GLU5 (C), GAL1 (D), and GAL5 (E) after 8 h of growth on Tryptone Broth motility plates at 30°C. (F) Expression from the PfliA and PfliC promoters in the strains with ancestral and the mutant rpoB alleles. All experiments were performed in triplicate. The average values and standard deviation are reported.
Consistent with the observations reported in the past, the alleles that fixed in our evolving populations also exhibit decreased motility, via a downregulation of the class 2/3 promoter (PfliA) and a class 3 promoter (PfliC) in the flagellar cascade.
One of the mutations in the gal5 line was in the promoter region of the gene galP (Table 1). GalP is responsible for transport of galactose from the extracellular environment into the cell (Macpherson et al., 1983). From the perspective of adaptation in galactose, an increased expression of the galactose transporter should lead to an increase in fitness. To test whether the mutation in the galP promoter increases rate of transcription, we fused the ancestral and the evolved galP promoter with gfp and tested for fluorescence levels in the presence of galactose.
Our results showed that the mutated galP promoter exhibits a significantly higher level of fluorescence, as compared to the ancestral galP promoter (Figure 5). Thus, a part of increase in fitness of the galactose-evolved lines in galactose environment is contributed to by the increased levels of the galP protein.
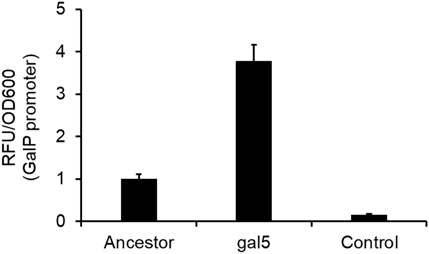
Figure 5. PgalP promoter activity is increased by the mutation in the promoter region. galP promoter activity, as measured by the promoter fusion with gfp, shows that the promoter mutation increases expression from the promoter ∼4-fold, as compared to the ancestral galP promoter (p < 0.01). The experiment was performed in triplicate. The average value and standard deviation are reported.
Populations adapt to an environment under the action of evolutionary forces like mutations, natural selection, and genetic drift. While natural selection acts to increase the frequency of beneficial mutations, drift randomly alters the frequency of alleles. In such a context, it is the most beneficial alleles that survive drift. Despite the role of chance events in dictating the trajectory of evolving populations (Lee et al., 2009; Jasmin and Zeyl, 2013; Bono et al., 2017), adaptive convergence, at a phenotypic and genotypic level, has been observed in several microbial evolution experiments (Cunningham et al., 1997; Nakatsu et al., 1998; Cooper et al., 2001; Cooper et al., 2003; Wong and Kassen, 2011; Herron and Doebeli, 2013; Scribner et al., 2020; Lambros et al., 2021; Mulvey et al., 2023; Pearl Mizrahi et al., 2023; Tjendra et al., 2023). Such experimental evidences show that evolution in certain conditions, given a genetic background and environment, could be predictable, even at the genetic level.
In the absence of information about genetic background and environment, it becomes impossible to predict the exact effect of a mutation because of epistasis (GxG) and pleiotropy (GxE). Genetic interactions (GxG) are pervasive, and lead to widespread epistasis across the genome (Diaz-Colunga et al., 2023). These epistatic relations also change depending on the environment, leading to another layer of complexity in the form of GxGxE interactions. Using theoretical and empirical approaches, we now know from global epistasis patterns that despite sequence-level unpredictability, beneficial effects of a mutation decrease with an increase in background fitness (Wiser et al., 2013; Kryazhimskiy et al., 2014). Although a mechanistic explanation to such an observation does not exist, a statistical framework based on the distribution of fitness effects enables prediction of evolutionary trajectories (Sarah et al., 2023). However, no such model exists to understand GxE interactions, or pleiotropic effects of mutations. Several experimental studies have reported pleiotropy in a wide-range of cases (Chen et al., 2020; Jerison et al., 2020; Bakerlee et al., 2021). Recent laboratory evolution-based studies to understand pleiotropy have commented on the role of initial fitness, ploidy, and environment (Jerison et al., 2017; Bakerlee et al., 2021; Chen et al., 2023). While it is agreed that several by-product effects of mutations are deleterious, antagonism is not universal, and the prediction of pleiotropy remains a challenge. We ask if adaptive trajectories in a particular environment can help predict pleiotropy, in “similar” or “dissimilar” non-home environments.
In an attempt to identify if pleiotropic effects can be predicted based on adaptation, we evolve E. coli in an environment consisting of one sugar source (0.2% glucose or galactose) in M9 minimal media, for a period of 300 generations. By using a simple organism such as E. coli, we eliminate the complexities that come with eukaryotic model organisms. We know from previous works that evolution of E. coli in stressful conditions leads to genetic changes in global regulators (Rudolph et al., 2010; Weinstein and Zaman, 2019; Li F. et al., 2021). Specifically, different alleles of global regulators like rpoB and rpoC are shown to be adaptive in environments where temperature is elevated, or an antibiotic is present (Rudolph et al., 2010; Weinstein and Zaman, 2019). We investigate whether the mutational targets in our evolving populations were nutrient utilization genes, or global regulators, or both. After evolving for 300 generations, nine out of these twelve populations accumulated mutations in the rpo genes. In fact, seven of these nine populations did not have any other mutations. Other mutational targets included genes involved in starvation response, ribosome synthesis, and nutrient uptake, as expected.
Despite the sequence level differences, cells evolving in an environment showed extremely similar speeds of adaptation, and fitness change at the end of 300 generations. This was a classic case of convergent evolution, both at phenotypic and genotypic levels.
Since adaptive mutations occurred in global regulators, we checked if the fitness of the evolved populations, in two types of environments (non-home environments), was identical to that in the home environment. First, we tested how changing the sugar source in the environment could alter fitness. This constituted our test of pleiotropy in a highly “similar” environment. Given that the evolution of these populations occurred in an environment where selection acted on nutrient utilization, we expected insignificant changes in the fitness of these populations when shifted to environments with other sugars.
Growth assays showed that no mutation that fixed in these evolving populations was deleterious in the away environments. In our experiments, some evolved cells showed diverse responses in non-home environments as observed in literature (Travisano et al., 1995)—two glucose-evolved lines (glu4 and glu5) grew similarly in lactose but differently in galactose, and two galactose-evolved lines (gal1 and gal5) grew similarly in lactose but differently in glucose. Three of these populations had one SNP each in rpoB, while the fourth one had a SNP in a rpoC. Despite all these populations having adapted identically in minimal nutrient media, they do not show identical pleiotropic effects in environments containing different sugar sources. Therefore, our results show that extending the implications of adaptation in one sugar environment to another should be done with caution.
Second, we probed the fitness of these evolved populations in environments that posed a non-nutrient stress, i.e., in highly “dissimilar” environments. Since rpoB mutations have been reported to confer resistance to Rifampicin, we quantified the minimum inhibitory concentration of the evolved glu4, gal1, and gal5 populations. Again, the fitness of these evolved cells in the presence of Rifampicin was not predictable based on the fitness in the home environment—all three populations had different minimum inhibitory concentrations of the antibiotic. We report three new alleles of the rpoB gene, and show that quantitative pleiotropic effects of adaptation are not gene-dependent, but change with mutations at the nucleotide level. Therefore, an attempt to quantify pleiotropy in a high-throughput fashion using deletion collections, like done in the past (Dudley et al., 2005), could yield highly inaccurate predictions of quantitative fitness effects in away environments.
Mutations in global regulators facilitate adaptation by altering gene regulation patterns (Fong et al., 2005; Saxer et al., 2014; Rodriguez-Verdugo et al., 2016; Gonzalez-Gonzalez et al., 2017; Iyer et al., 2021; Mahilkar et al., 2021). Altered motility is one of the outcomes of change in gene regulation (Conrad et al., 2010a; Perez-Varela et al., 2017; Cutugno et al., 2020). None of the populations which harboured a mutation in the rpoB gene showed similar motility.
Pleiotropic effects are known to slow down or speed up adaptation, depending on the precise relation between the genotype and selection acting in the environment (Hamala et al., 2020). Empirical investigations to ascertain the nature of pleiotropic effects of adaptation show that pleiotropy could be both deterministic or idiosyncratic (Jerison et al., 2020; Bakerlee et al., 2021). We have tested pleiotropic effects of evolution in a simple environment in different environments—one in which a nutrient source was changed, and another in which an antibiotic was present. Overall, in the two contexts, pleiotropic effects were only beneficial, making possible the prediction of the qualitative nature of pleiotropic effect. However, the exact by-product fitness effects, even of single mutations in global regulators, were not predictable based on adaptation (in four out of twelve populations) that resulted in identical mutational targets. In the small set of environments that we tested, we also observed that pleiotropy emerges early, as reported in Bakerlee et al. (2021), and that the generalist or specialist behaviour of a population depends on the pair of environments in which pleiotropy is being investigated, even if the pairs imposed “similar” selection pressures on the population. Given that unpredictability is not ruled out, our results highlight that in order to build a statistical understanding of pleiotropic effects, sequence-level mapping of mutations with several environmental pairs, of various types, is necessary.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
PV: Formal Analysis, Investigation, Methodology, Writing–original draft, Writing–review and editing. PN: Investigation, Methodology, Writing–original draft, Writing–review and editing. NA: Formal Analysis, Investigation, Methodology, Writing–original draft. SS: Conceptualization, Methodology, Project administration, Supervision, Validation, Writing–review and editing. RGB: Conceptualization, Data curation, Investigation, Methodology, Writing–original draft.
The authors declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by a DBT/Wellcome Trust (India Alliance) grant (Award No. IA/S/19/2/504632) to SS. PV was supported by Prime Minister’s Research Fellowship (PMRF ID 1302050). NA was supported as an Institute Post-Doctoral Fellow by Indian Institute of Technology Bombay, Mumbai.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmolb.2024.1286824/full#supplementary-material
Abo, T., Ueda, K., Sunohara, T., Ogawa, K., and Aiba, H. (2002). SsrA-mediated protein tagging in the presence of miscoding drugs and its physiological role in Escherichia coli. Genes Cells. 7 (7), 629–638. doi:10.1046/j.1365-2443.2002.00549.x
Alifano, P., Palumbo, C., Pasanisi, D., and Tala, A. (2015). Rifampicin-resistance, rpoB polymorphism and RNA polymerase genetic engineering. J. Biotechnol. 202, 60–77. doi:10.1016/j.jbiotec.2014.11.024
Anderson, J. T., Willis, J. H., and Mitchell-Olds, T. (2011). Evolutionary genetics of plant adaptation. Trends Genet. 27 (7), 258–266. doi:10.1016/j.tig.2011.04.001
Bakerlee, C. W., Phillips, A. M., Nguyen, Ba A. N., and Desai, M. M. (2021). Dynamics and variability in the pleiotropic effects of adaptation in laboratory budding yeast populations. Elife 10, e70918. doi:10.7554/eLife.70918
Bennett, A. F., and Lenski, R. E. (2007). An experimental test of evolutionary trade-offs during temperature adaptation. Proc. Natl. Acad. Sci. U. S. A. 104 (1), 8649–8654. doi:10.1073/pnas.0702117104
Bertin, P., Terao, E., Lee, E. H., Lejeune, P., Colson, C., Danchin, A., et al. (1994). The H-NS protein is involved in the biogenesis of flagella in Escherichia coli. J. Bacteriol. 176 (17), 5537–5540. doi:10.1128/jb.176.17.5537-5540.1994
Bono, L. M., Smith, L. B., Pfennig, D. W., and Burch, C. L. (2017). The emergence of performance trade-offs during local adaptation: insights from experimental evolution. Mol. Ecol. 26 (7), 1720–1733. doi:10.1111/mec.13979
Bruckbauer, S. T., Trimarco, J. D., Martin, J., Bushnell, B., Senn, K. A., Schackwitz, W., et al. (2019). Experimental evolution of extreme resistance to ionizing radiation in Escherichia coli after 50 cycles of selection. J. Bacteriol. 201 (8), e00784-18. doi:10.1128/JB.00784-18
Campbell, E. A., Korzheva, N., Mustaev, A., Murakami, K., Nair, S., Goldfarb, A., et al. (2001). Structural mechanism for rifampicin inhibition of bacterial rna polymerase. Cell 104 (6), 901–912. doi:10.1016/s0092-8674(01)00286-0
Chen, V., Johnson, M. S., Herissant, L., Humphrey, P. T., Yuan, D. C., Li, Y., et al. (2023). Evolution of haploid and diploid populations reveals common, strong, and variable pleiotropic effects in non-home environments. Elife 12, 12. doi:10.7554/elife.92899
Chen, Y. C., Kuo, H. C., Lo, W. S., and Hung, C. M. (2020). Avian phenotypic convergence is subject to low genetic constraints based on genomic evidence. BMC Evol. Biol. 20 (1), 147. doi:10.1186/s12862-020-01711-7
Chesmore, K., Bartlett, J., and Williams, S. M. (2018). The ubiquity of pleiotropy in human disease. Hum. Genet. 137 (1), 39–44. doi:10.1007/s00439-017-1854-z
Chilcott, G. S., and Hughes, K. T. (2000). Coupling of flagellar gene expression to flagellar assembly in Salmonella enterica serovar typhimurium and Escherichia coli. Microbiol. Mol. Biol. Rev. 64 (4), 694–708. doi:10.1128/mmbr.64.4.694-708.2000
Christensen, S. K., and Gerdes, K. (2003). RelE toxins from bacteria and Archaea cleave mRNAs on translating ribosomes, which are rescued by tmRNA. Mol. Microbiol. 48 (5), 1389–1400. doi:10.1046/j.1365-2958.2003.03512.x
Conrad, T. M., Frazier, M., Joyce, A. R., Cho, B. K., Knight, E. M., Lewis, N. E., et al. (2010a). RNA polymerase mutants found through adaptive evolution reprogram Escherichia coli for optimal growth in minimal media. Proc. Natl. Acad. Sci. U. S. A. 107 (47), 20500–20505. doi:10.1073/pnas.0911253107
Conrad, T. M., Frazier, M., Joyce, A. R., Cho, B. K., Knight, E. M., Lewis, N. E., et al. (2010b). RNA polymerase mutants found through adaptive evolution reprogram Escherichia coli for optimal growth in minimal media. Proc. Natl. Acad. Sci. U. S. A. 107 (47), 20500–20505. doi:10.1073/pnas.0911253107
Cooper, T. F., Rozen, D. E., and Lenski, R. E. (2003). Parallel changes in gene expression after 20,000 generations of evolution in Escherichiacoli. Proc. Natl. Acad. Sci. U. S. A. 100 (3), 1072–1077. doi:10.1073/pnas.0334340100
Cooper, V. S., and Lenski, R. E. (2000). The population genetics of ecological specialization in evolving Escherichia coli populations. Nature 407 (6805), 736–739. doi:10.1038/35037572
Cooper, V. S., Schneider, D., Blot, M., and Lenski, R. E. (2001). Mechanisms causing rapid and parallel losses of ribose catabolism in evolving populations of Escherichia coli B. J. Bacteriol. 183 (9), 2834–2841. doi:10.1128/JB.183.9.2834-2841.2001
Cunningham, C. W., Jeng, K., Husti, J., Badgett, M., Molineux, I. J., Hillis, D. M., et al. (1997). Parallel molecular evolution of deletions and nonsense mutations in bacteriophage T7. Mol. Biol. Evol. 14 (1), 113–116. doi:10.1093/oxfordjournals.molbev.a025697
Cutugno, L., Mc Cafferty, J., Pane-Farre, J., O'Byrne, C., and Boyd, A. (2020). rpoB mutations conferring rifampicin-resistance affect growth, stress response and motility in Vibrio vulnificus. Microbiol. Read. 166 (12), 1160–1170. doi:10.1099/mic.0.000991
Datsenko, K. A., and Wanner, B. L. (2000). One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97 (12), 6640–6645. doi:10.1073/pnas.120163297
Deatherage, D. E., Kepner, J. L., Bennett, A. F., Lenski, R. E., and Barrick, J. E. (2017). Specificity of genome evolution in experimental populations of Escherichia coli evolved at different temperatures. Proc. Natl. Acad. Sci. U. S. A. 114 (10), E1904–E12. doi:10.1073/pnas.1616132114
Degen, D., Feng, Y., Zhang, Y., Ebright, K. Y., Ebright, Y. W., Gigliotti, M., et al. (2014). Transcription inhibition by the depsipeptide antibiotic salinamide A. Elife 3, e02451. doi:10.7554/eLife.02451
Dettman, J. R., Sirjusingh, C., Kohn, L. M., and Anderson, J. B. (2007). Incipient speciation by divergent adaptation and antagonistic epistasis in yeast. Nature 447 (7144), 585–588. doi:10.1038/nature05856
Diaz-Colunga, J., Skwara, A., Gowda, K., Diaz-Uriarte, R., Tikhonov, M., Bajic, D., et al. (2023). Global epistasis on fitness landscapes. Philos. Trans. R. Soc. Lond B Biol. Sci. 378 (1877), 20220053. doi:10.1098/rstb.2022.0053
Dudley, A. M., Janse, D. M., Tanay, A., Shamir, R., and Church, G. M. (2005). A global view of pleiotropy and phenotypically derived gene function in yeast. Mol. Syst. Biol. 1, 0001. doi:10.1038/msb4100004
Duffy, S., Turner, P. E., and Burch, C. L. (2006). Pleiotropic costs of niche expansion in the RNA bacteriophage phi 6. Genetics 172 (2), 751–757. doi:10.1534/genetics.105.051136
Elena, S. F. (2017). Local adaptation of plant viruses: lessons from experimental evolution. Mol. Ecol. 26 (7), 1711–1719. doi:10.1111/mec.13836
Ezekiel, D. H., and Hutchins, J. E. (1968). Mutations affecting RNA polymerase associated with rifampicin resistance in Escherichia coli. Nature 220 (5164), 276–277. doi:10.1038/220276a0
Fong, S. S., Joyce, A. R., and Palsson, B. O. (2005). Parallel adaptive evolution cultures of Escherichia coli lead to convergent growth phenotypes with different gene expression states. Genome Res. 15 (10), 1365–1372. doi:10.1101/gr.3832305
Forister, M. L., Dyer, L. A., Singer, M. S., Stireman, J. O., and Lill, J. T. (2012). Revisiting the evolution of ecological specialization, with emphasis on insect-plant interactions. Ecology 93 (5), 981–991. doi:10.1890/11-0650.1
Fraebel, D. T., Mickalide, H., Schnitkey, D., Merritt, J., Kuhlman, T. E., and Kuehn, S. (2017). Environment determines evolutionary trajectory in a constrained phenotypic space. Elife 6, e24669. doi:10.7554/eLife.24669
Francez-Charlot, A., Laugel, B., Van Gemert, A., Dubarry, N., Wiorowski, F., Castanie-Cornet, M. P., et al. (2003). RcsCDB His-Asp phosphorelay system negatively regulates the flhDC operon in Escherichia coli. Mol. Microbiol. 49 (3), 823–832. doi:10.1046/j.1365-2958.2003.03601.x
Fukushima, K., and Pollock, D. D. (2023). Detecting macroevolutionary genotype-phenotype associations using error-corrected rates of protein convergence. Nat. Ecol. Evol. 7 (1), 155–170. doi:10.1038/s41559-022-01932-7
Gonzalez-Gonzalez, A., Hug, S. M., Rodriguez-Verdugo, A., Patel, J. S., and Gaut, B. S. (2017). Adaptive mutations in RNA polymerase and the transcriptional terminator rho have similar effects on Escherichia coli gene expression. Mol. Biol. Evol. 34 (11), 2839–2855. doi:10.1093/molbev/msx216
Hamala, T., Gorton, A. J., Moeller, D. A., and Tiffin, P. (2020). Pleiotropy facilitates local adaptation to distant optima in common ragweed (Ambrosia artemisiifolia). PLoS Genet. 16 (3), e1008707. doi:10.1371/journal.pgen.1008707
Harden, M. M., He, A., Creamer, K., Clark, M. W., Hamdallah, I., Martinez, K. A., et al. (2015). Acid-adapted strains of Escherichia coli K-12 obtained by experimental evolution. Appl. Environ. Microbiol. 81 (6), 1932–1941. doi:10.1128/AEM.03494-14
He, X., and Zhang, J. (2006). Toward a molecular understanding of pleiotropy. Genetics 173 (4), 1885–1891. doi:10.1534/genetics.106.060269
Herring, C. D., Raghunathan, A., Honisch, C., Patel, T., Applebee, M. K., Joyce, A. R., et al. (2006). Comparative genome sequencing of Escherichia coli allows observation of bacterial evolution on a laboratory timescale. Nat. Genet. 38 (12), 1406–1412. doi:10.1038/ng1906
Herron, M. D., and Doebeli, M. (2013). Parallel evolutionary dynamics of adaptive diversification in Escherichia coli. PLoS Biol. 11 (2), e1001490. doi:10.1371/journal.pbio.1001490
Hietpas, R. T., Bank, C., Jensen, J. D., and Bolon, D. N. A. (2013). Shifting fitness landscapes in response to altered environments. Evolution 67 (12), 3512–3522. doi:10.1111/evo.12207
Hong, K. K., and Nielsen, J. (2013). Adaptively evolved yeast mutants on galactose show trade-offs in carbon utilization on glucose. Metab. Eng. 16, 78–86. doi:10.1016/j.ymben.2013.01.007
Iyer, M. S., Pal, A., Srinivasan, S., Somvanshi, P. R., and Venkatesh, K. V. (2021). Global transcriptional regulators fine-tune the translational and metabolic efficiency for optimal growth of Escherichia coli. mSystems 6 (2), e00001-21. doi:10.1128/mSystems.00001-21
Jaekel, M., and Wake, D. B. (2007). Developmental processes underlying the evolution of a derived foot morphology in salamanders. Proc. Natl. Acad. Sci. U. S. A. 104 (51), 20437–20442. doi:10.1073/pnas.0710216105
Jasmin, J. N., Dillon, M. M., and Zeyl, C. (2012). The yield of experimental yeast populations declines during selection. Proc. Biol. Sci. 279 (1746), 4382–4388. doi:10.1098/rspb.2012.1659
Jasmin, J. N., and Zeyl, C. (2013). Evolution of pleiotropic costs in experimental populations. J. Evol. Biol. 26 (6), 1363–1369. doi:10.1111/jeb.12144
Jerison, E. R., Kryazhimskiy, S., Mitchell, J. K., Bloom, J. S., Kruglyak, L., and Desai, M. M. (2017). Genetic variation in adaptability and pleiotropy in budding yeast. Elife 6, e27167. doi:10.7554/eLife.27167
Jerison, E. R., Nguyen, Ba A. N., Desai, M. M., and Kryazhimskiy, S. (2020). Chance and necessity in the pleiotropic consequences of adaptation for budding yeast. Nat. Ecol. Evol. 4 (4), 601–611. doi:10.1038/s41559-020-1128-3
Johnson, M. S., Reddy, G., and Desai, M. M. (2023). Epistasis and evolution: recent advances and an outlook for prediction. BMC Biol. 21 (1), 120. doi:10.1186/s12915-023-01585-3
Kinsler, G., Geiler-Samerotte, K., and Petrov, D. A. (2020). Fitness variation across subtle environmental perturbations reveals local modularity and global pleiotropy of adaptation. Elife 9, e61271. doi:10.7554/eLife.61271
Ko, M., and Park, C. (2000). H-NS-Dependent regulation of flagellar synthesis is mediated by a LysR family protein. J. Bacteriol. 182 (16), 4670–4672. doi:10.1128/jb.182.16.4670-4672.2000
Kryazhimskiy, S., Rice, D. P., Jerison, E. R., and Desai, M. M. (2014). Microbial evolution. Global epistasis makes adaptation predictable despite sequence-level stochasticity. Science 344 (6191), 1519–1522. doi:10.1126/science.1250939
LaCroix, R. A., Sandberg, T. E., O'Brien, E. J., Utrilla, J., Ebrahim, A., Guzman, G. I., et al. (2015). Use of adaptive laboratory evolution to discover key mutations enabling rapid growth of Escherichia coli K-12 MG1655 on glucose minimal medium. Appl. Environ. Microbiol. 81 (1), 17–30. doi:10.1128/AEM.02246-14
Lambros, M., Pechuan-Jorge, X., Biro, D., Ye, K., and Bergman, A. (2021). Emerging adaptive strategies under temperature fluctuations in a laboratory evolution experiment of Escherichia coli. Front. Microbiol. 12, 724982. doi:10.3389/fmicb.2021.724982
Leatham, M. P., Stevenson, S. J., Gauger, E. J., Krogfelt, K. A., Lins, J. J., Haddock, T. L., et al. (2005). Mouse intestine selects nonmotile flhDC mutants of Escherichia coli MG1655 with increased colonizing ability and better utilization of carbon sources. Infect. Immun. 73 (12), 8039–8049. doi:10.1128/IAI.73.12.8039-8049.2005
Lee, M. C., Chou, H. H., and Marx, C. J. (2009). Asymmetric, bimodal trade-offs during adaptation of Methylobacterium to distinct growth substrates. Evolution 63 (11), 2816–2830. doi:10.1111/j.1558-5646.2009.00757.x
Lehnen, D., Blumer, C., Polen, T., Wackwitz, B., Wendisch, V. F., and Unden, G. (2002). LrhA as a new transcriptional key regulator of flagella, motility and chemotaxis genes in Escherichia coli. Mol. Microbiol. 45 (2), 521–532. doi:10.1046/j.1365-2958.2002.03032.x
Leiby, N., and Marx, C. J. (2014). Metabolic erosion primarily through mutation accumulation, and not tradeoffs, drives limited evolution of substrate specificity in Escherichia coli. PLoS Biol. 12 (2), e1001789. doi:10.1371/journal.pbio.1001789
Lennen, R. M., Lim, H. G., Jensen, K., Mohammed, E. T., Phaneuf, P. V., Noh, M. H., et al. (2023). Laboratory evolution reveals general and specific tolerance mechanisms for commodity chemicals. Metab. Eng. 76, 179–192. doi:10.1016/j.ymben.2023.01.012
Li, C., and Zhang, J. (2018). Multi-environment fitness landscapes of a tRNA gene. Nat. Ecol. Evol. 2 (6), 1025–1032. doi:10.1038/s41559-018-0549-8
Li, F., Xiong, X. S., Yang, Y. Y., Wang, J. J., Wang, M. M., Tang, J. W., et al. (2021b). Effects of NaCl concentrations on growth patterns, phenotypes associated with virulence, and energy metabolism in Escherichia coli BW25113. Front. Microbiol. 12, 705326. doi:10.3389/fmicb.2021.705326
Li, J., Ji, L., Shi, W., Xie, J., and Zhang, Y. (2013). Trans-translation mediates tolerance to multiple antibiotics and stresses in Escherichia coli. J. Antimicrob. Chemother. 68 (11), 2477–2481. doi:10.1093/jac/dkt231
Li, M. C., Lu, J., Lu, Y., Xiao, T. Y., Liu, H. C., Lin, S. Q., et al. (2021a). rpoB mutations and effects on rifampin resistance in Mycobacterium tuberculosis. Infect. Drug Resist 14, 4119–4128. doi:10.2147/IDR.S333433
Li, Y., Venkataram, S., Agarwala, A., Dunn, B., Petrov, D. A., Sherlock, G., et al. (2018). Hidden complexity of yeast adaptation under simple evolutionary conditions. Curr. Biol. 28 (4), 515–525. doi:10.1016/j.cub.2018.01.009
Lisitsyn, N. A., Gur'ev, S. O., Sverdlov, E. D., Moiseeva, E. P., and Nikiforov, V. G. (1984). Nucleotide substitutions in the rpoB gene leading to rifampicin resistance of E. coli RNA polymerase. Bioorg Khim 10 (1), 127–128.
MacLean, R. C., Bell, G., and Rainey, P. B. (2004). The evolution of a pleiotropic fitness tradeoff in Pseudomonas fluorescens. Proc. Natl. Acad. Sci. U. S. A. 101 (21), 8072–8077. doi:10.1073/pnas.0307195101
Macpherson, A. J., Jones-Mortimer, M. C., Horne, P., and Henderson, P. J. (1983). Identification of the GalP galactose transport protein of Escherichia coli. J. Biol. Chem. 258 (7), 4390–4396. doi:10.1016/s0021-9258(18)32635-8
Mahilkar, A., Venkataraman, P., Mall, A., and Saini, S. (2021). Experimental evolution of anticipatory regulation in Escherichia coli. Front. Microbiol. 12, 796228. doi:10.3389/fmicb.2021.796228
McGee, L. W., Sackman, A. M., Morrison, A. J., Pierce, J., Anisman, J., and Rokyta, D. R. (2016). Synergistic pleiotropy overrides the costs of complexity in viral adaptation. Genetics 202 (1), 285–295. doi:10.1534/genetics.115.181628
Meenakshi, S., and Munavar, M. H. (2018). Evidence for up and down regulation of 450 genes by rpoB12 (rif) mutation and their implications in complexity of transcription modulation in Escherichia coli. Microbiol. Res. 212-213, 80–93. doi:10.1016/j.micres.2018.04.009
Miller, W. G., Leveau, J. H., and Lindow, S. E. (2000). Improved gfp and inaZ broad-host-range promoter-probe vectors. Mol. Plant Microbe Interact. 13 (11), 1243–1250. doi:10.1094/MPMI.2000.13.11.1243
Mitchell-Olds, T., Willis, J. H., and Goldstein, D. B. (2007). Which evolutionary processes influence natural genetic variation for phenotypic traits? Nat. Rev. Genet. 8 (11), 845–856. doi:10.1038/nrg2207
Molodtsov, V., Scharf, N. T., Stefan, M. A., Garcia, G. A., and Murakami, K. S. (2017). Structural basis for rifamycin resistance of bacterial RNA polymerase by the three most clinically important RpoB mutations found in Mycobacterium tuberculosis. Mol. Microbiol. 103 (6), 1034–1045. doi:10.1111/mmi.13606
Moore, S. D., and Sauer, R. T. (2007). The tmRNA system for translational surveillance and ribosome rescue. Annu. Rev. Biochem. 76, 101–124. doi:10.1146/annurev.biochem.75.103004.142733
Mulvey, K., Brosnan, K., Galvin, M., Mohr, S., Muldowney, L., Oser, M., et al. (2023). Parallel evolution in predatory bdellovibrio sp. NC01 during long-term coculture with a single prey strain. Appl. Environ. Microbiol. 89 (1), e0177622. doi:10.1128/aem.01776-22
Nakatsu, C. H., Korona, R., Lenski, R. E., de Bruijn, F. J., Marsh, T. L., and Forney, L. J. (1998). Parallel and divergent genotypic evolution in experimental populations of Ralstonia sp. J. Bacteriol. 180 (17), 4325–4331. doi:10.1128/JB.180.17.4325-4331.1998
Neidhardt, F. C. (1996). Escherichia coli and Salmonella: cellular and molecular biology. United States: ASM Press.
Noda-Garcia, L., Davidi, D., Korenblum, E., Elazar, A., Putintseva, E., Aharoni, A., et al. (2019). Chance and pleiotropy dominate genetic diversity in complex bacterial environments. Nat. Microbiol. 4 (7), 1221–1230. doi:10.1038/s41564-019-0412-y
Ono, K., Kutsukake, K., and Abo, T. (2009). Suppression by enhanced RpoE activity of the temperature-sensitive phenotype of a degP ssrA double mutant in Escherichia coli. Genes Genet. Syst. 84 (1), 15–24. doi:10.1266/ggs.84.15
Ostrowski, E. A., Rozen, D. E., and Lenski, R. E. (2005). Pleiotropic effects of beneficial mutations in Escherichia coli. Evolution 59 (11), 2343–2352. doi:10.1554/05-116.1
Paulander, W., Maisnier-Patin, S., and Andersson, D. I. (2009). The fitness cost of streptomycin resistance depends on rpsL mutation, carbon source and RpoS (sigmaS). Genetics 183 (2), 539–546. 1SI-2SI. doi:10.1534/genetics.109.106104
Pearl Mizrahi, S., Goyal, A., and Gore, J. (2023). Community interactions drive the evolution of antibiotic tolerance in bacteria. Proc. Natl. Acad. Sci. U. S. A. 120 (3), e2209043119. doi:10.1073/pnas.2209043119
Perez-Varela, M., Corral, J., Vallejo, J. A., Rumbo-Feal, S., Bou, G., Aranda, J., et al. (2017). Mutations in the β-subunit of the RNA polymerase impair the surface-associated motility and virulence of acinetobacter baumannii. Infect. Immun. 85 (8), e00327-17. doi:10.1128/IAI.00327-17
Ranquet, C., and Gottesman, S. (2007). Translational regulation of the Escherichia coli stress factor RpoS: a role for SsrA and Lon. J. Bacteriol. 189 (13), 4872–4879. doi:10.1128/JB.01838-06
Remold, S. (2012). Understanding specialism when the Jack of all trades can be the master of all. Proc. Biol. Sci. 279 (1749), 4861–4869. doi:10.1098/rspb.2012.1990
Rodriguez-Verdugo, A., Carrillo-Cisneros, D., Gonzalez-Gonzalez, A., Gaut, B. S., and Bennett, A. F. (2014). Different tradeoffs result from alternate genetic adaptations to a common environment. Proc. Natl. Acad. Sci. U. S. A. 111 (33), 12121–12126. doi:10.1073/pnas.1406886111
Rodriguez-Verdugo, A., Tenaillon, O., and Gaut, B. S. (2016). First-Step mutations during adaptation restore the expression of hundreds of genes. Mol. Biol. Evol. 33 (1), 25–39. doi:10.1093/molbev/msv228
Rudolph, B., Gebendorfer, K. M., Buchner, J., and Winter, J. (2010). Evolution of Escherichia coli for growth at high temperatures. J. Biol. Chem. 285 (25), 19029–19034. doi:10.1074/jbc.M110.103374
Ruelens, P., Wynands, T., and de Visser, J. (2023). Interaction between mutation type and gene pleiotropy drives parallel evolution in the laboratory. Philos. Trans. R. Soc. Lond B Biol. Sci. 378 (1877), 20220051. doi:10.1098/rstb.2022.0051
Sarah, A., Alena, M., Milo, S. J., and Sergey, K. (2023). Environment-independent distribution of mutational effects emerges from microscopic epistasis. bioRxiv, 2023. 11.18.567655.
Savolainen, O., Lascoux, M., and Merila, J. (2013). Ecological genomics of local adaptation. Nat. Rev. Genet. 14 (11), 807–820. doi:10.1038/nrg3522
Saxer, G., Krepps, M. D., Merkley, E. D., Ansong, C., Deatherage Kaiser, B. L., Valovska, M. T., et al. (2014). Mutations in global regulators lead to metabolic selection during adaptation to complex environments. PLoS Genet. 10 (12), e1004872. doi:10.1371/journal.pgen.1004872
Schick, A., Bailey, S. F., and Kassen, R. (2015). Evolution of fitness trade-offs in locally adapted populations of Pseudomonas fluorescens. Am. Nat. 186 (1), S48–S59. doi:10.1086/682932
Schluter, D. (2009). Evidence for ecological speciation and its alternative. Science 323 (5915), 737–741. doi:10.1126/science.1160006
Scribner, M. R., Santos-Lopez, A., Marshall, C. W., Deitrick, C., and Cooper, V. S. (2020). Parallel evolution of tobramycin resistance across species and environments. mBio 11 (3), e00932-20. doi:10.1128/mBio.00932-20
Sellick, C. A., Campbell, R. N., and Reece, R. J. (2008). Galactose metabolism in yeast-structure and regulation of the leloir pathway enzymes and the genes encoding them. Int. Rev. Cell Mol. Biol. 269, 111–150. doi:10.1016/S1937-6448(08)01003-4
Severinov, K., Soushko, M., Goldfarb, A., and Nikiforov, V. (1993). Rifampicin region revisited. New rifampicin-resistant and streptolydigin-resistant mutants in the beta subunit of Escherichia coli RNA polymerase. J. Biol. Chem. 268 (20), 14820–14825. doi:10.1016/s0021-9258(18)82407-3
Shin, S., and Park, C. (1995). Modulation of flagellar expression in Escherichia coli by acetyl phosphate and the osmoregulator OmpR. J. Bacteriol. 177 (16), 4696–4702. doi:10.1128/jb.177.16.4696-4702.1995
Sivakumaran, S., Agakov, F., Theodoratou, E., Prendergast, J. G., Zgaga, L., Manolio, T., et al. (2011). Abundant pleiotropy in human complex diseases and traits. Am. J. Hum. Genet. 89 (5), 607–618. doi:10.1016/j.ajhg.2011.10.004
Soutourina, O., Kolb, A., Krin, E., Laurent-Winter, C., Rimsky, S., Danchin, A., et al. (1999). Multiple control of flagellum biosynthesis in Escherichia coli: role of H-NS protein and the cyclic AMP-catabolite activator protein complex in transcription of the flhDC master operon. J. Bacteriol. 181 (24), 7500–7508. doi:10.1128/JB.181.24.7500-7508.1999
Sperandio, V., Torres, A. G., and Kaper, J. B. (2002). Quorum sensing Escherichia coli regulators B and C (QseBC): a novel two-component regulatory system involved in the regulation of flagella and motility by quorum sensing in E. coli. Mol. Microbiol. 43 (3), 809–821. doi:10.1046/j.1365-2958.2002.02803.x
Tenaillon, O., Rodriguez-Verdugo, A., Gaut, R. L., McDonald, P., Bennett, A. F., Long, A. D., et al. (2012). The molecular diversity of adaptive convergence. Science 335 (6067), 457–461. doi:10.1126/science.1212986
Tjendra, J., Storesund, J. E., Dahle, H., Sandaa, R. A., and Vage, S. (2023). Molecular evidence of parallel evolution in a cyanophage. PLoS One 18 (2), e0281537. doi:10.1371/journal.pone.0281537
Travisano, M., Vasi, F., and Lenski, R. E. (1995). Long-Term experimental evolution in Escherichia coli. Iii. Variation among replicate populations in correlated responses to novel environments. Evolution 49 (1), 189–200. doi:10.1111/j.1558-5646.1995.tb05970.x
Turner, P. E., and Elena, S. F. (2000). Cost of host radiation in an RNA virus. Genetics 156 (4), 1465–1470. doi:10.1093/genetics/156.4.1465
Utrilla, J., O'Brien, E. J., Chen, K., McCloskey, D., Cheung, J., Wang, H., et al. (2016). Global rebalancing of cellular resources by pleiotropic point mutations illustrates a multi-scale mechanism of adaptive evolution. Cell Syst. 2 (4), 260–271. doi:10.1016/j.cels.2016.04.003
Wannier, T. M., Kunjapur, A. M., Rice, D. P., McDonald, M. J., Desai, M. M., and Church, G. M. (2018). Adaptive evolution of genomically recoded Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 115 (12), 3090–3095. doi:10.1073/pnas.1715530115
Weinstein, Z. B., and Zaman, M. H. (2019). Evolution of rifampin resistance in Escherichia coli and Mycobacterium smegmatis due to substandard drugs. Antimicrob. Agents Chemother. 63 (1), e01243-18. doi:10.1128/AAC.01243-18
Wenger, J. W., Piotrowski, J., Nagarajan, S., Chiotti, K., Sherlock, G., and Rosenzweig, F. (2011). Hunger artists: yeast adapted to carbon limitation show trade-offs under carbon sufficiency. PLoS Genet. 7 (8), e1002202. doi:10.1371/journal.pgen.1002202
Wiser, M. J., Ribeck, N., and Lenski, R. E. (2013). Long-term dynamics of adaptation in asexual populations. Science 342 (6164), 1364–1367. doi:10.1126/science.1243357
Wong, A., and Kassen, R. (2011). Parallel evolution and local differentiation in quinolone resistance in Pseudomonas aeruginosa. Microbiol. Read. 157 (4), 937–944. doi:10.1099/mic.0.046870-0
Woods, R., Schneider, D., Winkworth, C. L., Riley, M. A., and Lenski, R. E. (2006). Tests of parallel molecular evolution in a long-term experiment with Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 103 (24), 9107–9112. doi:10.1073/pnas.0602917103
Yi, X., and Dean, A. M. (2013). Bounded population sizes, fluctuating selection and the tempo and mode of coexistence. Proc. Natl. Acad. Sci. U. S. A. 110 (42), 16945–16950. doi:10.1073/pnas.1309830110
Keywords: E. coli, adaptation, minimal media, RpoB, RpoC
Citation: Venkataraman P, Nagendra P, Ahlawat N, Brajesh RG and Saini S (2024) Convergent genetic adaptation of Escherichia coli in minimal media leads to pleiotropic divergence. Front. Mol. Biosci. 11:1286824. doi: 10.3389/fmolb.2024.1286824
Received: 31 August 2023; Accepted: 15 February 2024;
Published: 10 April 2024.
Edited by:
Riddhiman Dhar, Indian Institute of Technology Kharagpur, IndiaReviewed by:
Pouria Dasmeh, University of Marburg, GermanyCopyright © 2024 Venkataraman, Nagendra, Ahlawat, Brajesh and Saini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Supreet Saini, c2FpbmlAY2hlLmlpdGIuYWMuaW4=
†Present address: Raman G. Brajesh, Department of Biomedical Engineering and Bioinformatics, Chhatisgarh Swami Vivekanand Technical University, Durg, India
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.