
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Mol. Biosci. , 12 August 2022
Sec. Molecular Diagnostics and Therapeutics
Volume 9 - 2022 | https://doi.org/10.3389/fmolb.2022.976705
This article is part of the Research Topic New Strategies for the Diagnosis and Treatment of Multi-Drug Resistant Bacteria or Fungi in Wounds View all 5 articles
The antimicrobial resistance (AMR) crisis from bacterial pathogens is frequently emerging and rapidly disseminated during the sustained antimicrobial exposure in human-dominated communities, posing a compelling threat as one of the biggest challenges in humans. The frequent incidences of some common but untreatable infections unfold the public health catastrophe that antimicrobial-resistant pathogens have outpaced the available countermeasures, now explicitly amplified during the COVID-19 pandemic. Nowadays, biotechnology and machine learning advancements help create more fundamental knowledge of distinct spatiotemporal dynamics in AMR bacterial adaptation and evolutionary processes. Integrated with reliable diagnostic tools and powerful analytic approaches, a collaborative and systematic surveillance platform with high accuracy and predictability should be established and implemented, which is not just for an effective controlling strategy on AMR but also for protecting the longevity of valuable antimicrobials currently and in the future.
The increasing prevalence of antimicrobial resistance (AMR) among bacterial pathogens poses one of the greatest threats to public health all over the world (Yue et al., 2021; Resistance, 2016), mainly because of the overuse of broad-spectrum antibiotics and potential antimicrobial-bacterium mismatch in empirical selections (Pan Hang et al., 2018; Jiang et al., 2019; Wang et al., 2019). Scientific reports indicate that death due to ineffective treatment of multidrug-resistant bacteria reaches over 100 cases daily in the European Union and the United States of America. Meanwhile, the increasing resistance to the “last-resort” antibiotics continuously concerns public health worldwide (Biswas et al., 2019; Elbediwi et al., 2019; Elbediwi et al., 2020).
To identify common AMR and guide diagnostics and effective treatment for bacterial infections, a gold-standard laboratory test used to determine the antimicrobial sensitivity of AMR organisms, termed antimicrobial susceptibility tests (AST), has been adopted in clinics for over 50 years. Traditional AST involves broth microdilution and disc diffusion on agar to determine the minimum inhibitory concentration (MIC). The MIC value indicates the lowest concentration of antibiotics where bacterial growth stops, and the growth level is usually measured by absorbance or light scattering. Before and during testing, multiple turnarounds of culturing the bacteria in the sample and isolating colonies and growth needed to be accomplished before AST. The whole pipeline takes at least 48 h, which usually provides the results after using an empirical prescription of antibiotics that might be misused. It has been estimated that around one-fifth of prescribed antibiotics from 2013 to 2015 were inappropriate in the United Kingdom (Smieszek et al., 2018). Also, currently, with over 500 million infected cases, COVID-19 has increased the setbacks in fighting against AMR (Knight et al., 2021; Ma et al., 2021). As many patients suffering from COVID-19 are being prescribed inappropriate antibiotics, this scenario might surge drug-resistant infections during the pandemic (Kariyawasam et al., 2022), particularly in developing countries where people lack antimicrobial stewardship programs and reliable diagnostics. Numerous AMR-associated outbreaks have been reported during the COVID-19 pandemic, such as carbapenemase-producing Enterobacteriaceae (Farfour et al., 2020; Tiri et al., 2020), highlighting the urgent demand for an efficient AMR surveillance system.
With decreasing sequencing costs and advancements in bioinformatics toolkits, whole-genome sequencing (WGS) for AMR diagnosing has been considered an efficient avenue for combating AMR (Schurch and van Schaik, 2017). The genotype-based alternative promises a quicker AMR detection than phenotypic AST by bypassing routine culturing for early detection and providing genomic insights into the mechanisms of AMR action, as well as critical clinical information, including pathogen species and virulence factors (Li et al., 2022). Utilizing the increasing WGS data and robust computerized analysis, machine learning has emerged to address the global AMR increase (Ren et al., 2021). It is a subfield of artificial intelligence focusing on developing algorithms to provide accurate and reliable predictions for clinical decisions. Conceptually, the genotype-based machine learning pipeline primarily requires the following steps: data selection (e.g., single-nucleotide polymorphisms and MIC value); encoding to be interpreted by algorithms; data training for model construction; evaluation and optimization by other dataset (Ren et al., 2021). The key challenge of these genotypic strategies that rely on the detection of AMR genes or SNPs to predict AMR phenotypes is the prevalent discrepancy between genotype and phenotype, which may produce detrimental effects on patients by prescribing inappropriate or unnecessary broad-spectrum antimicrobials (Yee et al., 2021). The phenotype of an organism is the observed expression of its genotype but is affected by multiple environmental variables (Davis et al., 2011; Rapun Mas et al., 2019; Urmi et al., 2020). The significance of the genotype–phenotype relationship emphasizes both their inherent correlation and the intricate uncertainty that should not be ignored but evaluated especially upon fighting AMR. Furthermore, AMR strains like Pseudomonas aeruginosa exhibit high plasticity of resistance phenotype driven by environmental changes (Dotsch et al., 2015). Overall, these phenomena suggest that bacteria may prefer different survival strategies under pressure circumstances. Various environmental signals, including antimicrobial and non-antimicrobial compounds, could significantly affect various susceptible phenotypes (Paudyal and Yue, (2019); Berendonk et al., 2015). AMR bacteria is an essential evolutionary outcome under selection pressure, and numerous unknown factors are involved, which implies limitations of single-dimension surveillance and diagnostic approaches. Therefore, more information on specific bacteria-host or -environment dynamics should also be considered to predict AMR.
The COVID-19 pandemic has seriously affected global efforts against AMR (Tomczyk et al., 2021), magnifying the insufficiency of available AMR surveillance capabilities. Accurate and rapid identification of antimicrobial resistance is still highly demanded, and a fine-tuned surveillance system remains the top priority of global public health. Since the culture-dependent traditional approaches cannot cope with the new challenge, various emerging novel methods have shown potential to solve the dilemma. Herein, we summarize these methods and propose an informed database/platform of resistant strains coordinated with reliable rapid molecular assays, multi-omics analysis, and machine learning to improve the performance of the established AMR surveillance system.
Traditional phenotypical diagnostics require isolating the initial bacterial colony from a clinical sample after incubation on a solid medium overnight. For the following broth microdilution assay, the monomicrobial cell is suspended under serial 2-fold dilutions with testing antibiotics in micro-wells. The lowest concentration of antibiotics in which bacteria cannot grow is recorded as the MIC value. For disc diffusion assay, the bacteria is spread onto an agar plate containing a fixed antibiotic concentration. After culturing overnight, the diameter of the circle zone of inhibition is measured. These two results are usually the basis for selecting effective antibiotics for bacterial infections. These traditional diagnoses of antimicrobial susceptibility of AMR strains have long relied on bacteria culturing, which is a time-consuming and labor-intensive process (Burnham et al., 2017). Moreover, the time lag of bacteria growth required for the conventional standard test may change the susceptibility to antimicrobials, particularly for important pathogens such as Yersinia pestis (Inglesby et al., 2000) and Mycobacterium tuberculosis (Votintseva et al., 2017). Although the culture-based antimicrobial susceptibility test used in clinical laboratories produces reproducible results, these assays in vitro cannot approximate the complicated interaction between microorganisms and the host in the tissue (Burnham et al., 2017), which may ultimately direct wrong drug selection (Wang et al., 2019; Elbediwi et al., 2021; Xu et al., 2021; Chen et al., 2022).
To tackle the dilemma of traditional phenotypical diagnostics, microbiologists have made novel efforts on establishing effective AST methodologies. Thore et al. (1977) found that ampicillin treatment induces a significant decrease of intracellular ATP, and an increase in inhibitory zone diameters, according to which they developed the luciferase assay-aided AST by measuring intracellular ATP of bacteria. For antifungal drug testing on Candida albicans, Pore established a flow cytofluorometric susceptibility test (FCST) by determining the cellular fluorescence intensity owing to antibiotic-caused membrane damage and the resulting uptake of propidium iodide or rose Bengal (Pore, 1990). To rapidly detect antibiotic resistance of M. tuberculosis, Riska et al. (1999) used phage with the luciferase reporter to infect the bacteria, and the causal detectable light indicates the infection quantitatively. Fredborg et al. (2013) introduced a real-time optical detection system to image bacterial growth and antimicrobial susceptibility. The automatically generated graphs supported by imaging material showed the antibiotic effects on bacteria, and it was able to screen 96 bacteria–antibiotic combinations at once. Idelevich et al. (2017) developed a methodology based on the BacterioScanTM216R laser scattering technology and following statistical analysis to rapidly discriminate the resistant and susceptible phenotypes of Gram-positive bacteria. Kang et al. (2019) built a droplet microfluidic device consisting of four integrated microdroplet arrays with each holding over 8,000 docking sites, which was capable of screening four antibiotics/pathogens simultaneously and assessing antibiotic sensitivity in 15–30 min. Greater flexibility can be achieved by operating microdroplet arrays individually or jointly. Combined with different aptamers, it has been divided into droplet microfluidics active microfluidics, paper microfluidics, and capillary-driven microfluidics (Han et al., 2021). But it has not been fully applied in clinical settings because of fabrication and operational complexity and less portability (Needs et al., 2020). More phenotypical diagnostics methods are summarized in Table 1.
Offering speed and accuracy advantages compared with the traditional gold-standard phenotypic assay, molecular detection on the genetic determinants of resistance phenotype has been widely used in clinical settings. These DNA-based molecular assays can be primarily classified into three major platforms according to their underlying mechanics: polymerase chain reaction (PCR) or RT-PCR (Waseem et al., 2019), microfluidics-supported (Bai et al., 2022), and clustered regularly interspaced short palindromic repeats (CRISPR)-based methodologies (Kaminski et al., 2021). PCR has been extensively used today as a laboratory routine to identify bacteria from multiple environments and resistance genes (Rohde et al., 2017). Multiplex PCR, an optimized PCR by adding several primers, was described to detect nine clinically antibiotic resistance genes of Staphylococcus aureus in a single run including mecA (encoding methicillin resistance), aacA-aphD (aminoglycoside resistance), tetK, tetM (tetracycline resistance), erm(A), erm(C) (macrolide-lincosamide-streptogramin B resistance), vat(A), vat(B), and vat(C) (streptogramin A resistance) (Strommenger et al., 2003). With the high-throughput trait of microfluidic technology and rapid amplification at low temperature (37–42°C) of recombinase polymerase amplification (RPA), RPA-based microfluidics has been reported to identify M. tuberculosis by targeting 16 S rRNA with a sensitivity of 11 CFU/ml in 25 min (Tsaloglou et al., 2018); 10 copies of methicillin-resistant S. aureus DNA mixed with human whole blood were detected in 30 min by probing related SNP (Yeh et al., 2017); the major pathogenic bacteria in urinary tract infections Escherichia coli, Proteus mirabilis, P. aeruginosa, and S. aureus were successfully detected with detection limits of 100 CFU/ml from urine samples within 40 min by similar assay (Chen et al., 2018). The two leading platforms CRISPR-Cas13 based SHERLOCK (specific high-sensitivity enzymatic reporter unlocking) and CRISPR-Cas12 based DETECTR (DNA endonuclease-targeted CRISPR trans reporter) have realized that pathogen detections such as Zika and dengue viruses, bacterial isolates (E. coli; Klebsiella pneumoniae; P. aeruginosa; M. tuberculosis; S. aureus), AMR genes (K. pneumoniae carbapenemase and New Delhi metallo-beta-lactamase 1 (NDM-1), and even could detect cancer mutations with attomolar sensitivity and single-base mismatch specificity (Gootenberg et al., 2017; Chen et al., 2018). This CRISPR-based diagnostics pipeline involves pre-amplification of the target sequence, target recognizing, and cleaving by Cas nuclease, and result visualization. Once the target sequence is base-paired with the guide RNA of the CRISPR complex, the Cas protein cleaves the surrounding reporters (described as “collateral cleavage”), resulting in a detectable signal as a positive detection. CRISPR-Cas12 has already been used to diagnose M. tuberculosis with a sensitivity of two copies (Ai et al., 2019) and identify subspecies of the bacterium by targeting rpoB and erm genes (Xiao et al., 2020). Curti et al. (2020) successfully applied CRISPR-Cas12a to identify carbapenem-resistant genes such as blaKPC, blaNDM, and blaOXA of K. pneumoniae at a pM level within 30 min. Shen et al. (2020) proposed a Cas13a-based system termed as APC-Cas by integrating an allosteric probe, and it was able to detect Salmonella in milk samples with single-cell sensitivity. A brief introduction of other different molecular assays is summarized in Table 2.
WGS emerges as a powerful tool for understanding the genetic makeup of bacteria AMR. As the high-throughput sequencing technology developed in the mid-2000 s, it can illustrate a landscape of the whole resistome in a couple of days with a sophisticated downstream bioinformatic pipeline (Chan, 2016; Goodwin et al., 2016). The new advancement in sequencing technology shortens the time of the whole procedure from days to a few hours (Shendure et al., 2017). According to these known genetic determinants, mass software tools have been designed to detect and predict drug resistance (Henry et al., 2014; Sallet et al., 2019). An immense amount of sequencing data generated annually is used to build a global genotype–phenotype database contributing to a worldwide surveillance system on the AMR strains (Gardy and Loman, 2017). Once the public-health-threatening “criminal” is identified by fingerprinting with a genetic test, its AMR profile will be provided for treatment by the database in time. The Antibiotic Resistance Monitoring, Analysis, and Diagnostics Alliance (https://joinarmada.org/), a nonprofit global organization combatting superbugs with a database of bacterial genomes, has been established to create a specific “criminal database” of AMR pathogens, collecting an unprecedented amount of bacterial strains and details of their antibiotic resistance profiles, genetic identity and epidemiology from a global network of hospitals, veterinarians, scientists, and other advocates. Alcock et al. (2020) developed the Comprehensive Antibiotic Resistance Database providing data of curated reference sequences and SNPs, models, and algorithms, helping users with genotype analysis and phenotype prediction of AMR. The notable AMR databases also include ResFinder (Zankari et al., 2012), ARG-ANNOT (Gupta et al., 2014), the and National Center for Biotechnology Information Pathogen Detection Reference Gene catalog (Sayers et al., 2022). These primary AMR databases curate information from the scientific research into the collection for further sequence analysis and knowledge integration. WGS has also been applied for the risk assessment of probiotic lactic acid bacteria (Peng et al., 2022). However, to detect the genetic determinants of AMR, knowledge or prior research regarding which gene is responsible for the resistance is required for the method before diagnostics. Since complex features and mechanisms of AMR remain obscure, the genotype–phenotype method seems less valuable than expected for AMR identification (Wu et al., 2021; Hu et al., 2022).
Learning essential characteristics directly from the data of genomic sequences, machine learning obviates the need for prior knowledge of resistant strains caused by unknown mechanisms. It has been proven valuable for predicting AMR with an unbiased method (Martens et al., 2016; Her and Wu, 2018; Wheeler et al., 2018). Unlike direct detection of AMR genes, machine learning often takes more extensive features indiscriminately into AMR identification, such as SNP, K-mer, and pan-genome (Her and Wu, 2018; Nguyen et al., 2019; Ren et al., 2021), and even some parameters affecting the performance and reliability of machine learning-based antibiotic susceptibility tests need to be evaluated (Hicks et al., 2019). Different from the traditional DNA alignment approach, machine learning is mathematically oriented by algorithms including logistic regression (LR), support vector machine (SVM), random forest (RF), and convolutional neural network (CNN) (Her and Wu, 2018). In order to confirm the WGS data to the valid format of existing classification algorithms, these data are usually encoded before analysis by the algorithms as mentioned above for model construction, and standard encoding methods include Label, One-Hot, and frequency matrix chaos game representation (FCGR), etc., (Her and Wu, 2018). The final prediction model will be tested and evaluated by other datasets, and the model established might vary for different coding methods and algorithms/classifiers involved. Leveraged with big data from WGS or other NGS technologies, machine learning facilitates potential AMR prediction and helps direct informed drug decisions (Moradigaravand et al., 2018; Ren et al., 2021). Recent literature in the field is presented in Table 3.
Clinical application of machine learning for AMR diagnosing, preventing, and understanding still stands in the infancy stage because of the complexity of AMR itself and the deployment of machine learning for clinical practice. That genomic-based machine learning aiming to predict the MIC value is the foremost strategy for AMR prediction (Coelho et al., 2013; Pesesky et al., 2016; Nguyen et al., 2019). But challenges/limitations remain to be overcome. Training data, (e.g., MIC value) might not be available for all laboratories and sections, which would affect the clinical effectiveness of the model, which suggests unified standardization of operating protocols or guidelines is highly demanded. For algorithms used in the model, different ones reach different outcomes, indicating a comparative study of algorithms should be conducted before applying for better accuracy. In addition, the prediction result should be understandable to intended users. For the breadth of machine learning prediction, studies have primarily predicted the most common antibiotics used on a few common bacterial pathogens, but the clinical encountered pathogens far outrange the prediction covering. Special effort should also be taken on the highly clinically significant organisms, including vancomycin-non-susceptible S. aureus (Shariati et al., 2020) and the ones with AMR phenotypic plasticity, including P. aeruginosa (Khaledi et al., 2020). Furthermore, data on AMR to new antibiotics (e.g., eravacycline, ceftazidime-avibactam, and cefiderocol) are also required to examine in clinical settings. With the exponential increase of available biological data, massive investments in computational power, critical advancements in algorithm performance, and increasing global involvement worldwide, machine learning will accelerate the clinical diagnosis of AMR to a new level.
The DNA-based method only detects the existence of these potential resistance DNA traits without providing information regarding efficient transcription, expression, and antimicrobial susceptibility. The feedback in bacterial transcriptome and proteome to antibiotics exposure offers real-time dynamic information of relevant genes. Their expression change in mRNA and protein levels is endowed with key diagnostic values for AMR detection. Some efforts have also been made in transcriptome and proteome, but remain limited. Steinberger-Levy et al. (2016) established a research model by exposing 53 strains of Y. pestis to the growth-inhibiting concentrations of ciprofloxacin for different time periods and investigates the expression changes of specific marker genes by transcriptomic DNA microarray analysis. Eleven transcripts with significant change were identified as the potential biomarkers, of which four mRNAs (recA, pla2, recN, and dinI) were selected to determine the susceptibility by performing quantitative RT-PCR, and the results are consistent with MIC values. Rohde et al. (2016) used fluorescence in situ hybridization and immunofluorescence tests to locate and quantify the mRNA and protein existence of the TEM β-lactamase, conferring ampicillin resistance in the E. coli. Chen et al. (2020) performed a shotgun proteomics assay and WGS on four isolates of Campylobacter jejuni and analyzed the data in the Comprehensive Antibiotic Resistance Database for AMR detection. It was found that both genomic and proteomic approaches can identify molecular determinants responsible for resistance to tetracycline and ciprofloxacin, in line with their phenotypes. These methods require several steps and analyses, which are more suitable for basic research, but not feasible for the field application. For rapid RNA detection, the RNA-targeting CRISPR-associated enzyme Cas13a directly binds to the target RNA and releases the positive signal (Shinoda et al., 2021). Combined with RT-RPA that transforms the RNA to cDNA and amplifies DNA with primers, the DNA-targeting enzyme Cas12a can also be applied to the RNA detections (Malci et al., 2022). In clinical practice, rapid molecular diagnostics help distinguish viral infections from bacterial infections, preventing unnecessary treatment of antibiotics. Only molecular-based methods cannot differentiate which bacteria contain the AMR element (plasmids containing AMR gene) in the mixed bacterial infection of clinical use. If the carrying AMR genes among various bacterial populations can be identified specifically, we might use a more targeting or effective strategy against AMR increase, suggesting this field might need further investigation.
A comprehensive analysis has suggested more factors to make more precision diagnostics and informed preclinical decisions (Sommer et al., 2017). To deal with this compiling problem, a full-scale assessment system should be set up to predict AMR precisely. Establishing a database on genotype–phenotype data is a good start, but a more comprehensive platform of AMR strain is highly needed for the precision diagnostics of AMR. Utilizing the multi-dimension data collected from the genome, transcriptome, and proteome profiles of AMR bacteria isolated from hospitals, doctor offices, clinical laboratories, communities, and veterinary sources, a ‘criminal database’ of AMR pathogens can be geographically and phylogenetically established. After fingerprinting those known genetic makers by rapid molecular test and sampling information, the database will report with the best match containing current and historical essential details, including WGS report, pathogen, MIC phenotype, drug use and treatment outcomes, clinical population, and other accessory information. With the data collected from multiple sources, bacterial AMR patterns will be well analyzed by machine learning, enabling reliable digital models of a particular drug, pathogen, and clinical population. Machine learning will act as an alternative to pinpoint that unknown resistance. Surveillance and diagnostic interaction significantly improve antimicrobial selection and epidemiological monitoring in the pipeline. Once database standardization is well-established, this constantly updated global detection platform combining diagnostics, surveillance, and prediction of AMR would improve proactive identification and mitigate the emerging crisis of AMR (Figure 1).
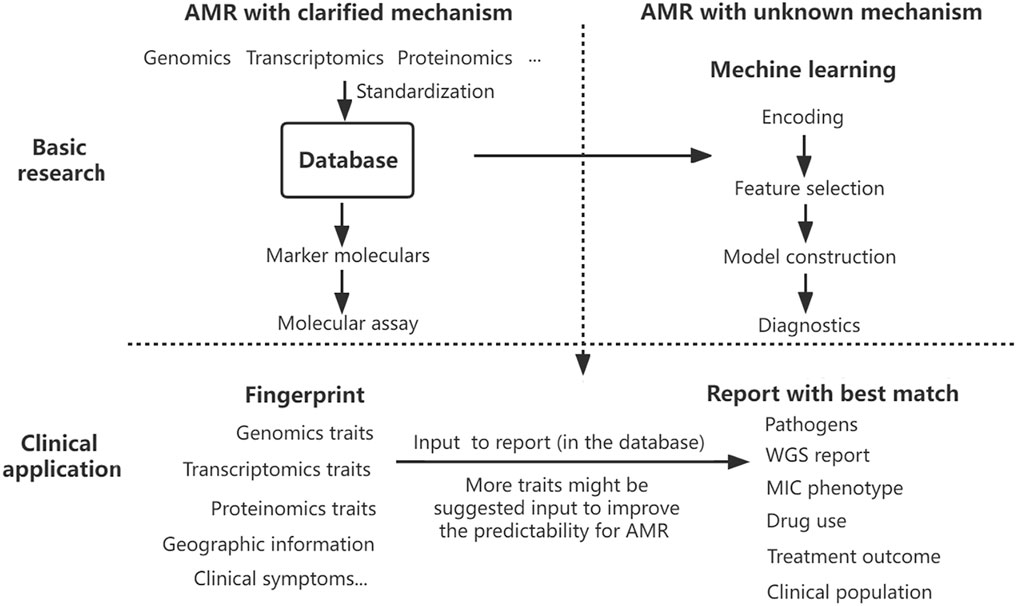
FIGURE 1. Proposed AMR surveillance model. Various data on antibiotic resistance have been collected into the database, which provides marker molecules to detect AMR with clarified mechanism and fuels the model training of machine learning to predict the AMR with an unknown mechanism. The results of rapid molecular detection on multi-traits and other information help fingerprint the pathogens, and the database interface will report the best matched or advised treatment decision directly. Taking advantage of the rapidity of molecular assays and the precision of machine learning fueled by a constant flow of multi-dimensional data, the AMR surveillance platform optimizes drug selection, antimicrobial stewardship, and epidemiological monitoring.
From the clinical perspective, the priority is confirming whether antimicrobial therapies are needed in a particular case; the second is which drug is suitable for an optimized treatment if a bacterial infection is already present. Apparently, the most clinical urgency for patients and physicians is not knowing which bacteria is resistant to what antimicrobial but determining which narrow-spectrum drug can eliminate the pathogen. Most diagnostic methods aim to identify drug-resistant determinants of superbugs, but few studies explored if there are any drug-susceptible determinants or other multi-omics traits in AMR strains. These drug-susceptible feature data can be used for model training in machine learning, and molecular-based assays can detect drug-susceptible markers directly. More innovative efforts are needed in this area in the future.
In the coming decade, advancements in technical and computational tools for multi-omics approaches are continually improving knowledge regarding the diverse AMR mechanisms and integrating a deep mechanistic understanding of AMR determinants with a broader systematic analysis of microorganisms. The ultimate goal will lead to a revolutionary change in AMR diagnostics, significantly guiding the surveillance of AMR threats and finally slowing down the rising crisis.
HW, CJ, HL, and RY wrote the manuscript; JC, YL, and MY revised and confirmed the manuscript.
This study was supported by the National Program on Key Research Project of China (2019YFE0103900 and 2017YFC1600103) as well as the European Union’s Horizon 2020 Research and Innovation Programme under Grant Agreement No. 861917—SAFFI, National Natural Science Foundation of China (31872837 & 32150410374), Zhejiang Provincial Natural Science Foundation of China (LR19C180001), and Zhejiang Provincial Key R&D Program of China (2022C02024, 2021C02008, and 2020C02032).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdou Mohamed, M. A., Kozlowski, H. N., Kim, J., Zagorovsky, K., Kantor, M., Feld, J. J., et al. (2021). Diagnosing antibiotic resistance using nucleic acid enzymes and gold nanoparticles. ACS Nano 15 (6), 9379–9390. doi:10.1021/acsnano.0c09902
Ai, J. W., Zhou, X., Xu, T., Yang, M., Chen, Y., He, G. Q., et al. (2019). CRISPR-based rapid and ultra-sensitive diagnostic test for Mycobacterium tuberculosis. Emerg. Microbes Infect. 8 (1), 1361–1369. doi:10.1080/22221751.2019.1664939
Alcock, B. P., Raphenya, A. R., Lau, T. T. Y., Tsang, K. K., Bouchard, M., Edalatmand, A., et al. (2020). CARD 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 48 (D1), D517–D525. doi:10.1093/nar/gkz935
Bai, Y., Ji, J., Ji, F., Wu, S., Tian, Y., Jin, B., et al. (2022). Recombinase polymerase amplification integrated with microfluidics for nucleic acid testing at point of care. Talanta 240, 123209. doi:10.1016/j.talanta.2022.123209
Barry, A. L., Joyce, L. J., Adams, A. P., and Benner, E. J. (1973). Rapid determination of antimicrobial susceptibility for urgent clinical situations. Am. J. Clin. Pathol. 59 (5), 693–699. doi:10.1093/ajcp/59.5.693
Berendonk, T. U., Manaia, C. M., Christophe, M., Despo, F. K., Eddie, C., Fiona, W., et al. (2015). Tackling antibiotic resistance: the environmental framework. Nat. Rev. Microbiol. 13 (5), 310–317. doi:10.1038/nrmicro3439
Biswas, S., Li, Y., Elbediwi, M., and Yue, M. (2019). Emergence and dissemination of mcr-carrying clinically relevant Salmonella typhimurium monophasic clone ST34. Microorganisms 7 (9), E298. doi:10.3390/microorganisms7090298
Burnham, C. A. D., Leeds, J., Nordmann, P., O'Grady, J., and Patel, J. (2017). Diagnosing antimicrobial resistance. Nat. Rev. Microbiol. 15 (11), 697–703. doi:10.1038/nrmicro.2017.103
Chan, K. G. (2016). Whole-genome sequencing in the prediction of antimicrobial resistance. Expert Rev. anti. Infect. Ther. 14 (7), 617–619. doi:10.1080/14787210.2016.1193005
Chen, C. Y., Clark, C. G., Langner, S., Boyd, D. A., Bharat, A., McCorrister, S. J., et al. (2020). Detection of antimicrobial resistance using proteomics and the comprehensive antibiotic resistance database: A case study. Proteomics. Clin. Appl. 14 (4), e1800182. doi:10.1002/prca.201800182
Chen, J. G., Xu, Y. C., Yan, H., Zhu, Y. Z., Wang, L., Zhang, Y., et al. (2018a). Sensitive and rapid detection of pathogenic bacteria from urine samples using multiplex recombinase polymerase amplification. Lab. Chip 18 (16), 2441–2452. doi:10.1039/c8lc00399h
Chen, J. S., Ma, E., Harrington, L. B., Da Costa, M., Tian, X., Palefsky, J. M., et al. (2018b). CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 360 (6387), 436–439. doi:10.1126/science.aar6245
Chen, Q., Zhao, L., Liu, H., Ding, Q., Jia, C., Liao, S., et al. (2022). Nanoporous silver nanorods as surface-enhanced Raman scattering substrates. Biosens. Bioelectron. 202, 114004. doi:10.1016/j.bios.2022.114004
Choi, J., Jung, Y. G., Kim, J., Kim, S., Jung, Y., Na, H., et al. (2013). Rapid antibiotic susceptibility testing by tracking single cell growth in a microfluidic agarose channel system. Lab. Chip 13 (2), 280–287. doi:10.1039/c2lc41055a
Chowdhury, A. S., Call, D. R., and Broschat, S. L. (2020). PARGT: a software tool for predicting antimicrobial resistance in bacteria. Sci. Rep. 10 (1), 11033. doi:10.1038/s41598-020-67949-9
Coelho, J. R., Carrico, J. A., Knight, D., Martinez, J. L., Morrissey, I., Oggioni, M. R., et al. (2013). The use of machine learning methodologies to analyse antibiotic and biocide susceptibility in Staphylococcus aureus. PLoS One 8 (2), e55582. doi:10.1371/journal.pone.0055582
Curti, L. A., Pereyra-Bonnet, F., Repizo, G. D., Fay, J. V., Salvatierra, K., Blariza, M. J., et al. (2020). CRISPR-based platform for carbapenemases and emerging viruses detection using Cas12a (Cpf1) effector nuclease. Emerg. Microbes Infect. 9 (1), 1140–1148. doi:10.1080/22221751.2020.1763857
Davis, M. A., Besser, T. E., Orfe, L. H., Baker, K. N., Lanier, A. S., Broschat, S. L., et al. (2011). Genotypic-phenotypic discrepancies between antibiotic resistance characteristics of Escherichia coli isolates from calves in management settings with high and low antibiotic use. Appl. Environ. Microbiol. 77 (10), 3293–3299. doi:10.1128/AEM.02588-10
Descours, G., Desmurs, L., Hoang, T. L. T., Ibranosyan, M., Baume, M., Ranc, A. G., et al. (2018). Evaluation of the Accelerate Pheno system for rapid identification and antimicrobial susceptibility testing of Gram-negative bacteria in bloodstream infections. Eur. J. Clin. Microbiol. Infect. Dis. 37 (8), 1573–1583. doi:10.1007/s10096-018-3287-6
Dotsch, A., Schniederjans, M., Khaledi, A., Hornischer, K., Schulz, S., Bielecka, A., et al. (2015). The Pseudomonas aeruginosa transcriptional landscape is shaped by environmental heterogeneity and genetic variation. mBio 6 (4), e00749. doi:10.1128/mBio.00749-15
Elbediwi, M., Beibei, W., Pan, H., Jiang, Z., Biswas, S., Li, Y., et al. (2020). Genomic characterization of mcr-1-carrying Salmonella enterica serovar 4, [5], 12:i:- ST 34 clone isolated from pigs in China. Front. Bioeng. Biotechnol. 8, 663. doi:10.3389/fbioe.2020.00663
Elbediwi, M., Li, Y., Paudyal, N., Pan, H., Li, X., Xie, S., et al. (2019). Global burden of colistin-resistant bacteria: Mobilized colistin resistance genes study (1980-2018). Microorganisms 7 (10), E461. doi:10.3390/microorganisms7100461
Elbediwi, M., Shi, D., Biswas, S., Xu, X., and Yue, M. (2021). Changing patterns of Salmonella enterica serovar rissen from humans, food animals, and animal-derived foods in China, 1995-2019. Front. Microbiol. 12, 702909. doi:10.3389/fmicb.2021.702909
Farfour, E., Lecuru, M., Dortet, L., Le Guen, M., Cerf, C., Karnycheff, F., et al. (2020). Carbapenemase-producing Enterobacterales outbreak: Another dark side of COVID-19. Am. J. Infect. Control 48 (12), 1533–1536. doi:10.1016/j.ajic.2020.09.015
Fredborg, M., Andersen, K. R., Jorgensen, E., Droce, A., Olesen, T., Jensen, B. B., et al. (2013). Real-time optical antimicrobial susceptibility testing. J. Clin. Microbiol. 51 (7), 2047–2053. doi:10.1128/JCM.00440-13
Gardy, J. L., and Loman, N. J. (2017). Towards a genomics-informed, real-time, global pathogen surveillance system. Nat. Rev. Genet. 19 (1), 9–20. doi:10.1038/nrg.2017.88
Goodwin, S., McPherson, J. D., and McCombie, W. R. (2016). Coming of age: ten years of next-generation sequencing technologies. Nat. Rev. Genet. 17 (6), 333–351. doi:10.1038/nrg.2016.49
Gootenberg, J. S., Abudayyeh, O. O., Lee, J. W., Essletzbichler, P., Dy, A. J., Joung, J., et al. (2017). Nucleic acid detection with CRISPR-Cas13a/C2c2. Science 356 (6336), 438–442. doi:10.1126/science.aam9321
Gupta, S. K., Padmanabhan, B. R., Diene, S. M., Lopez-Rojas, R., Kempf, M., Landraud, L., et al. (2014). ARG-ANNOT, a new bioinformatic tool to discover antibiotic resistance genes in bacterial genomes. Antimicrob. Agents Chemother. 58 (1), 212–220. doi:10.1128/Aac.01310-13
Han, X., Liu, Y., Yin, J., Yue, M., and Mu, Y. (2021). Microfluidic devices for multiplexed detection of foodborne pathogens. Food Res. Int. 143, 110246. doi:10.1016/j.foodres.2021.110246
Henry, V. J., Bandrowski, A. E., Pepin, A. S., Gonzalez, B. J., and Desfeux, A. (2014). OMICtools: an informative directory for multi-omic data analysis. Oxford: Database. doi:10.1093/database/bau069
Her, H. L., and Wu, Y. W. (2018). A pan-genome-based machine learning approach for predicting antimicrobial resistance activities of the Escherichia coli strains. Bioinformatics 34 (13), i89–i95. doi:10.1093/bioinformatics/bty276
Hicks, A. L., Wheeler, N., Sanchez-Buso, L., Rakeman, J. L., Harris, S. R., and Grad, Y. H. (2019). Evaluation of parameters affecting performance and reliability of machine learning-based antibiotic susceptibility testing from whole genome sequencing data. PLoS Comput. Biol. 15 (9), e1007349. doi:10.1371/journal.pcbi.1007349
Hu, B., Hou, P., Teng, L., Miao, S., Zhao, L., Ji, S., et al. (2022). Genomic investigation reveals a community typhoid outbreak caused by contaminated drinking water in China, 2016. Front. Med. 9, 753085. doi:10.3389/fmed.2022.753085
Idelevich, E. A., Hoy, M., Gorlich, D., Knaack, D., Grunastel, B., Peters, G., et al. (2017). Rapid phenotypic detection of microbial resistance in gram-positive bacteria by a real-time laser scattering method. Front. Microbiol. 8, 1064. doi:10.3389/fmicb.2017.01064
Idelevich, E. A., Sparbier, K., Kostrzewa, M., and Becker, K. (2018). Rapid detection of antibiotic resistance by MALDI-TOF mass spectrometry using a novel direct-on-target microdroplet growth assay. Clin. Microbiol. Infect. 24 (7), 738–743. doi:10.1016/j.cmi.2017.10.016
Inglesby, T. V., Dennis, D. T., Henderson, D. A., Bartlett, J. G., Ascher, M. S., Eitzen, E., et al. (2000). Plague as a biological weapon: Medical and public health management. Working group on civilian biodefense. JAMA 283 (17), 2281–2290. doi:10.1001/jama.283.17.2281
Jiang, Z., Paudyal, N., Xu, Y., Deng, T., Li, F., Pan, H., et al. (2019). Antibiotic resistance profiles of Salmonella recovered from finishing pigs and slaughter facilities in henan, China. Front. Microbiol. 10, 1513. doi:10.3389/fmicb.2019.01513
Kaminski, M. M., Abudayyeh, O. O., Gootenberg, J. S., Zhang, F., and Collins, J. J. (2021). CRISPR-based diagnostics. Nat. Biomed. Eng. 5 (7), 643–656. doi:10.1038/s41551-021-00760-7
Kang, W., Sarkar, S., Lin, Z. S., McKenney, S., and Konry, T. (2019). Ultrafast parallelized microfluidic platform for antimicrobial susceptibility testing of gram positive and negative bacteria. Anal. Chem. 91 (9), 6242–6249. doi:10.1021/acs.analchem.9b00939
Kariyawasam, R. M., Julien, D. A., Jelinski, D. C., Larose, S. L., Rennert-May, E., Conly, J. M., et al. (2022). Antimicrobial resistance (AMR) in COVID-19 patients: a systematic review and meta-analysis (november 2019-june 2021). Antimicrob. Resist Infect. Control 11 (1), 45. doi:10.1186/s13756-022-01085-z
Khaledi, A., Weimann, A., Schniederjans, M., Asgari, E., Kuo, T. H., Oliver, A., et al. (2020). Predicting antimicrobial resistance in Pseudomonas aeruginosa with machine learning-enabled molecular diagnostics. EMBO Mol. Med. 12 (3), e10264. doi:10.15252/emmm.201910264
Kim, H., Lee, S., Seo, H. W., Kang, B., Moon, J., Lee, K. G., et al. (2020). Clustered regularly interspaced short palindromic repeats-mediated surface-enhanced Raman scattering assay for multidrug-resistant bacteria. ACS Nano 14, 17241–17253. doi:10.1021/acsnano.0c07264
Knight, G. M., Glover, R. E., McQuaid, C. F., Olaru, I. D., Gallandat, K., Leclerc, Q. J., et al. (2021). Antimicrobial resistance and COVID-19: Intersections and implications. Elife 10, e64139. doi:10.7554/eLife.64139
Li, B., Zhou, X., Liu, H., Deng, H., Huang, R., and Xing, D. (2018). Simultaneous detection of antibiotic resistance genes on paper-based chip using [Ru(phen)2dppz](2+) turn-on fluorescence probe. ACS Appl. Mat. Interfaces 10 (5), 4494–4501. doi:10.1021/acsami.7b17653
Li, Y., Kang, X., Ed-Dra, A., Zhou, X., Jia, C., Müller, A., et al. (2022) Genome-based assessment of antimicrobial resistance and virulence potential for non-Pullorum/Gallinarum Salmonella serovars recovered from dead poultry in China. Microbiol. Spectr., e0096522. doi:10.1128/spectrum.00965-22
Li, X., Lin, J., Hu, Y., and Zhou, J. (2020). PARMAP: A pan-genome-based computational framework for predicting antimicrobial resistance. Front. Microbiol. 11, 578795. doi:10.3389/fmicb.2020.578795
Ma, E. S. K., Kung, K. H., and Chen, H. (2021). Combating antimicrobial resistance during the COVID-19 pandemic. Hong Kong Med. J. 27 (6), 396–398. doi:10.12809/hkmj215124
Malci, K., Walls, L. E., and Rios-Solis, L. (2022). Rational design of CRISPR/Cas12a-RPA based one-pot COVID-19 detection with design of experiments. ACS Synth. Biol. 11 (4), 1555–1567. doi:10.1021/acssynbio.1c00617
Martens, K., Hallin, J., Warringer, J., Liti, G., and Parts, L. (2016). Predicting quantitative traits from genome and phenome with near perfect accuracy. Nat. Commun. 7, 11512. doi:10.1038/ncomms11512
Moradigaravand, D., Palm, M., Farewell, A., Mustonen, V., Warringer, J., and Parts, L. (2018). Prediction of antibiotic resistance in Escherichia coli from large-scale pan-genome data. PLoS Comput. Biol. 14 (12), e1006258. doi:10.1371/journal.pcbi.1006258
Needs, S. H., Donmez, S. I., Bull, S. P., McQuaid, C., Osborn, H. M. I., and Edwards, A. D. (2020). Challenges in microfluidic and point-of-care phenotypic antimicrobial resistance tests. Front. Mech. Eng. 6. doi:10.3389/fmech.2020.00073
Nguyen, M., Long, S. W., McDermott, P. F., Olsen, R. J., Olson, R., Stevens, R. L., et al. (2019). Using machine learning to predict antimicrobial MICs and associated genomic features for nontyphoidal Salmonella. J. Clin. Microbiol. 57 (2), e01260–18. doi:10.1128/JCM.01260-18
Pan, H., Li, X., Fang, W., and Yue, M. (2018). Analysis of major human and foodborne pathogens and their resistance to antimicrobials in the USA in the past two decades: Implications for surveillance and control of antimicrobial resistance in China. J. Zhejiang Univ. Agric. Life Sci. 44 (2), 237–246. doi:10.3785/j.issn.1008-9209.2018.03.124
Paudyal, N., and Yue, M. (2019) Antibiotic resistance in the “dark matter”. Clin. Infect. Dis. 69 (2), 379–380. doi:10.1093/cid/ciz007
Peng, X., Ed-Dra, A., and Yue, M. (2022). Whole genome sequencing for the risk assessment of probiotic lactic acid bacteria. Crit. Rev. Food Sci. Nutr., 1–19. doi:10.1080/10408398.2022.2087174,
Pesesky, M. W., Hussain, T., Wallace, M., Patel, S., Andleeb, S., Burnham, C. D., et al. (2016). Evaluation of machine learning and rules-based approaches for predicting antimicrobial resistance profiles in gram-negative bacilli from whole genome sequence data. Front. Microbiol. 7, 1887. doi:10.3389/fmicb.2016.01887
Pore, R. S. (1990). Antibiotic susceptibility testing of Candida albicans by flow cytometry. Curr. Microbiol. 20 (5), 323–328. doi:10.1007/BF02091913
Quan, J., Langelier, C., Kuchta, A., Batson, J., Teyssier, N., Lyden, A., et al. (2019). FLASH: a next-generation CRISPR diagnostic for multiplexed detection of antimicrobial resistance sequences. Nucleic Acids Res. 47 (14), e83. doi:10.1093/nar/gkz418
Rapun Mas, L., Angulo Lopez, I., Cecilio Irazola, A., and Betran Escartin, A. I. (2019). Discrepancy in the genotypic versus phenotypic testing for resistance to rifampicin in Mycobacterium tuberculosis. A case report. Enferm. Infecc. Microbiol. Clin. 37 (3), 212–213. doi:10.1016/j.eimc.2018.03.015
Ren, Y., Chakraborty, T., Doijad, S., Falgenhauer, L., Falgenhauer, J., Goesmann, A., et al. (2021). Prediction of antimicrobial resistance based on whole-genome sequencing and machine learning. Bioinformatics 38, 325–334. doi:10.1093/bioinformatics/btab681
Resistance, R. o. A. (2016). “Tackling drug-resistant infections globally: final report and recommendations,” in Review on antimicrobial resistance.
Riska, P. F., Su, Y., Bardarov, S., Freundlich, L., Sarkis, G., Hatfull, G., et al. (1999). Rapid film-based determination of antibiotic susceptibilities of Mycobacterium tuberculosis strains by using a luciferase reporter phage and the Bronx Box. J. Clin. Microbiol. 37 (4), 1144–1149. doi:10.1128/JCM.37.4.1144-1149.1999
Rohde, A., Hammerl, J. A., Boone, I., Jansen, W., Fohler, S., Klein, G., et al. (2017). Overview of validated alternative methods for the detection of foodborne bacterial pathogens. Trends Food Sci. Technol. 62, 113–118. doi:10.1016/j.tifs.2017.02.006
Rohde, A., Hammerl, J. A., and Dahouk, S. A. (2016). Rapid screening for antibiotic resistance elements on the RNA transcript, protein and enzymatic activity level. Ann. Clin. Microbiol. Antimicrob. 15 (1), 55. doi:10.1186/s12941-016-0167-8
Sallet, E., Gouzy, J., and Schiex, T. (2019). EuGene: An automated integrative gene finder for eukaryotes and prokaryotes. Methods Mol. Biol. 1962, 97–120. doi:10.1007/978-1-4939-9173-0_6
Sayers, E. W., Bolton, E. E., Brister, J. R., Canese, K., Chan, J., Comeau, D. C., et al. (2022). Database resources of the national center for biotechnology information. Nucleic Acids Res. 50 (D1), D20–D26. doi:10.1093/nar/gkab1112
Schoepp, N. G., Schlappi, T. S., Curtis, M. S., Butkovich, S. S., Miller, S., Humphries, R. M., et al. (2017). Rapid pathogen-specific phenotypic antibiotic susceptibility testing using digital LAMP quantification in clinical samples. Sci. Transl. Med. 9 (410), eaal3693. doi:10.1126/scitranslmed.aal3693
Schurch, A. C., and van Schaik, W. (2017). Challenges and opportunities for whole-genome sequencing-based surveillance of antibiotic resistance. Ann. N. Y. Acad. Sci. 1388 (1), 108–120. doi:10.1111/nyas.13310
Shariati, A., Dadashi, M., Moghadam, M. T., van Belkum, A., Yaslianifard, S., and Darban-Sarokhalil, D. (2020). Global prevalence and distribution of vancomycin resistant, vancomycin intermediate and heterogeneously vancomycin intermediate Staphylococcus aureus clinical isolates: a systematic review and meta-analysis. Sci. Rep. 10 (1), 12689. doi:10.1038/s41598-020-69058-z
Shen, J., Zhou, X., Shan, Y., Yue, H., Huang, R., Hu, J., et al. (2020). Sensitive detection of a bacterial pathogen using allosteric probe-initiated catalysis and CRISPR-Cas13a amplification reaction. Nat. Commun. 11 (1), 267. doi:10.1038/s41467-019-14135-9
Shendure, J., Balasubramanian, S., Church, G. M., Gilbert, W., Rogers, J., Schloss, J. A., et al. (2017). DNA sequencing at 40: past, present and future. Nature 550 (7676), 345–353. doi:10.1038/nature24286
Shinoda, H., Taguchi, Y., Nakagawa, R., Makino, A., Okazaki, S., Nakano, M., et al. (2021). Amplification-free RNA detection with CRISPR-Cas13. Commun. Biol. 4 (1), 476. doi:10.1038/s42003-021-02001-8
Smieszek, T., Pouwels, K. B., Dolk, F. C. K., Smith, D. R. M., Hopkins, S., Sharland, M., et al. (2018). Potential for reducing inappropriate antibiotic prescribing in English primary care. J. Antimicrob. Chemother. 73 (2), ii36–ii43. doi:10.1093/jac/dkx500
Sommer, M. O. A., Munck, C., Toftkehler, R. V., and Dan, I. A. (2017). Prediction of antibiotic resistance: time for a new preclinical paradigm? Nat. Rev. Microbiol. 15 (11), 689–696. doi:10.1038/nrmicro.2017.75
Steinberger-Levy, I., Shifman, O., Zvi, A., Ariel, N., Beth-Din, A., Israeli, O., et al. (2016). A rapid molecular test for determining Yersinia pestis susceptibility to ciprofloxacin by the quantification of differentially expressed marker genes. Front. Microbiol. 7, 763. doi:10.3389/fmicb.2016.00763
Strommenger, B., Kettlitz, C., Werner, G., and Witte, W. (2003). Multiplex PCR assay for simultaneous detection of nine clinically relevant antibiotic resistance genes in Staphylococcus aureus. J. Clin. Microbiol. 41 (9), 4089–4094. doi:10.1128/Jcm.41.9.4089-4094.2003
Sun, L., Talarico, S., Yao, L., He, L., Self, S., You, Y., et al. (2018). Droplet digital PCR-based detection of clarithromycin resistance in Helicobacter pylori isolates reveals frequent heteroresistance. J. Clin. Microbiol. 56 (9), e00019–18. doi:10.1128/JCM.00019-18
Sun, X., Wang, Y., Zhang, L., Liu, S., Zhang, M., Wang, J., et al. (2020). CRISPR-Cas9 triggered two-step isothermal amplification method for E. coli O157:H7 detection based on a metal-organic framework platform. Anal. Chem. 92 (4), 3032–3041. doi:10.1021/acs.analchem.9b04162
Thore, A., Nilsson, L., Hojer, H., Ansehn, S., and Brote, L. (1977). Effects of ampicillin on intracellular levels of adenosine triphosphate in bacterial cultures related to antibiotic susceptibility. Acta Pathol. Microbiol. Scand. B 85 (2), 161–166. doi:10.1111/j.1699-0463.1977.tb01690.x
Tiri, B., Sensi, E., Marsiliani, V., Cantarini, M., Priante, G., Vernelli, C., et al. (2020). Antimicrobial stewardship program, COVID-19, and infection control: Spread of carbapenem-resistant Klebsiella pneumoniae colonization in ICU COVID-19 patients. What did not work? J. Clin. Med. 9 (9), E2744. doi:10.3390/jcm9092744
Tomczyk, S., Taylor, A., Brown, A., de Kraker, M. E. A., El-Saed, A., Alshamrani, M., et al. (2021). Impact of the COVID-19 pandemic on the surveillance, prevention and control of antimicrobial resistance: a global survey. J. Antimicrob. Chemother. 76 (11), 3045–3058. doi:10.1093/jac/dkab300
Tsaloglou, M. N., Nemiroski, A., Camci-Unal, G., Christodouleas, D. C., Murray, L. P., Connelly, J. T., et al. (2018). Handheld isothermal amplification and electrochemical detection of DNA in resource-limited settings. Anal. Biochem. 543, 116–121. doi:10.1016/j.ab.2017.11.025
Urmi, U. L., Nahar, S., Rana, M., Sultana, F., Jahan, N., Hossain, B., et al. (2020). Genotypic to phenotypic resistance discrepancies identified involving beta-lactamase genes, blaKPC, blaIMP, blaNDM-1, and blaVIM in uropathogenic Klebsiella pneumoniae. Infect. Drug Resist. 13, 2863–2875. doi:10.2147/IDR.S262493
Votintseva, A. A., Bradley, P., Pankhurst, L., Del Ojo Elias, C., Loose, M., Nilgiriwala, K., et al. (2017). Same-day diagnostic and surveillance data for tuberculosis via whole-genome sequencing of direct respiratory samples. J. Clin. Microbiol. 55 (5), 1285–1298. doi:10.1128/JCM.02483-16
Wang, X., Biswas, S., Paudyal, N., Pan, H., Li, X., Fang, W., et al. (2019). Antibiotic resistance in Salmonella typhimurium isolates recovered from the food chain through national antimicrobial resistance monitoring system between 1996 and 2016. Front. Microbiol. 10, 985. doi:10.3389/fmicb.2019.00985
Waseem, H., Jameel, S., Ali, J., Saleem Ur Rehman, H., Tauseef, I., Farooq, U., et al. (2019). Contributions and challenges of high throughput qPCR for determining antimicrobial resistance in the environment: A critical review. Molecules 24 (1), E163. doi:10.3390/molecules24010163
Wheeler, N. E., Gardner, P. P., and Barquist, L. (2018). Machine learning identifies signatures of host adaptation in the bacterial pathogen Salmonella enterica. PLoS Genet. 14 (5), e1007333. doi:10.1371/journal.pgen.1007333
Wu, B., Ed-Dra, A., Pan, H., Dong, C., Jia, C., and Yue, M. (2021). Genomic investigation of Salmonella isolates recovered from a pig slaughtering process in hangzhou, China. Front. Microbiol. 12, 704636. doi:10.3389/fmicb.2021.704636
Xiao, G., Zhang, S., Liang, Z., Li, G., Fang, M., Liu, Y., et al. (2020). Identification of Mycobacterium abscessus species and subspecies using the Cas12a/sgRNA-based nucleic acid detection platform. Eur. J. Clin. Microbiol. Infect. Dis. 39 (3), 551–558. doi:10.1007/s10096-019-03757-y
Xu, Y., Zhou, X., Jiang, Z., Qi, Y., Ed-Dra, A., and Yue, M. (2021). Antimicrobial resistance profiles and genetic typing of Salmonella serovars from chicken embryos in China. Antibiot. (Basel) 10 (10), 1156. doi:10.3390/antibiotics10101156
Yee, R., Dien Bard, J., and Simner, P. J. (2021). The genotype-to-phenotype dilemma: How should laboratories approach discordant susceptibility results? J. Clin. Microbiol. 59 (6), e00138–20. doi:10.1128/JCM.00138-20
Yeh, E. C., Fu, C. C., Hu, L., Thakur, R., Feng, J., and Lee, L. P. (2017). Self-powered integrated microfluidic point-of-care low-cost enabling (SIMPLE) chip. Sci. Adv. 3 (3), e1501645. ARTN e150164510. doi:10.1126/sciadv.1501645
Yue, M., Bai, L., Song, H., and Fang, W.(2021) Impacts of microbial food safety in China and beyond. Foodborne Pathog. Dis. 18 (8), 508–509. doi:10.1089/fpd.2021.29015.int
Keywords: antimicrobial resistance (AMR), AMR bacterial adaptation and evolutionary processes, advancements in biotechnology and computer science, reliable diagnostic tools, surveillance platform
Citation: Wang H, Jia C, Li H, Yin R, Chen J, Li Y and Yue M (2022) Paving the way for precise diagnostics of antimicrobial resistant bacteria. Front. Mol. Biosci. 9:976705. doi: 10.3389/fmolb.2022.976705
Received: 23 June 2022; Accepted: 19 July 2022;
Published: 12 August 2022.
Edited by:
Huan Chen, Zhejiang Chinese Medical University, ChinaReviewed by:
Mujeeb Ur Rehman, Disease Investigation Laboratory, Livestock and Dairy Development Department, PakistanCopyright © 2022 Wang, Jia, Li, Yin, Chen, Li and Yue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiang Chen, amNoZW5AY2RjLnpqLmNu; Yan Li, eWFubGkzQHpqdS5lZHUuY24=; Min Yue, bXl1ZUB6anUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.