- 1Department of Computer Science, Purdue University, West Lafayette, IN, United States
- 2Computer Engineering School, Costa Rica Institute of Technology, Cartago, Costa Rica
- 3Department of Biological Sciences, Purdue University, West Lafayette, IN, United States
An increasing number of protein complex structures are determined by cryo-electron microscopy (cryo-EM). When individual protein structures have been determined and are available, an important task in structure modeling is to fit the individual structures into the density map. Here, we designed a method that fits the atomic structures of proteins in cryo-EM maps of medium to low resolutions using Markov random fields, which allows probabilistic evaluation of fitted models. The accuracy of our method, MarkovFit, performed better than existing methods on datasets of 31 simulated cryo-EM maps of resolution 10
1 Introduction
Proteins are vital components of living cells. Among experimental technologies that are used to determine protein structures, cryogenic-electron microscopy (cryo-EM) has been used in an increasing number of cases as it has notable advantages, including its suitability for determining large macromolecular structures (Kuhlbrandt, 2014; Cheng, 2018; Renaud et al., 2018). The use of Cryo-EM was boosted by the advanced technology of improvements in direct detectors and image processing algorithms (Bai et al., 2015; Nogales, 2016; Wu and Lander, 2020).
To interpret determined cryo-EM maps, computational methods for modeling macromolecule structures play a crucial role (Esquivel-Rodriguez and Kihara, 2013; Alnabati and Kihara, 2019; Malhotra et al., 2019). If a map is determined at a relatively high resolution of up to about 3–4 Å, protein structures can be directly modeled from the density (Terashi and Kihara, 2018; Terwilliger et al., 2018; Terashi et al., 2020). For maps of medium to low resolution (∼4–10 Å and even lower resolution), the density usually does not have sufficient information for de novo full-atom modeling. However, protein secondary structures can be captured (Maddhuri Venkata Subramaniya et al., 2019; Wang et al., 2021) and known structures of individual proteins can be fitted to the density maps (Han et al., 2021).
Structure fitting methods have a long history of over two decades, partly because they have been the only option for structure interpretation in the early days when high-resolution maps were not obtained. Methods developed include EMfit (Rossmann et al., 2001), ADP_EM (Garzon et al., 2007), colores (Chacon and Wriggers, 2002), HADDOCK (van Zundert et al., 2015), gmfit (Kawabata, 2008), EMLZerD (Esquivel-Rodriguez and Kihara, 2012), and BCL EM-Fit (Woetzel et al., 2011). As the structure of proteins in the map can be slightly different from those available in an isolated condition, some methods focus more on considering the flexibility of proteins (Tama et al., 2004; Velazquez-Muriel et al., 2006; Velazquez-Muriel and Carazo, 2007; Trabuco et al., 2008; Wang and Schroder, 2012; Lopez-Blanco and Chacon, 2013). To assess the goodness of fit of a structure in a density map, conventionally, the cross-correlation coefficient (CCC) has been used. Several other metrics were also developed, such as core-weighted CCC (Wu et al., 2003), Laplacian-filtered CCC (Vasishtan and Topf, 2011; Joseph et al., 2017), mutual information (Joseph et al., 2017), overlap, and evolutionary information of interface residues (Joseph et al., 2016), and match of density gradient (Han et al., 2021).
Among all the structure fitting methods developed, there are only a few methods developed for multiple chain fitting.
Here, we developed a new method, named MarkovFit, which performs simultaneous-rigid fitting of atomic protein subunits into medium- to low-resolution cryo-EM maps. The method starts by using FFT to search the conformational space for potential positions of subunits and computes scores that quantify the goodness-of-fit between each subunit and the cryo-EM map and the interactions between the subunits. Subsequently, subunits and their physical interactions are represented using a Markov random field (MRF) graph (Besag, 1971). MRF nodes exchange information using a belief propagation algorithm, and the best conformations are extracted using a max-heap tree. Lastly, the top final conformations undergo structural refinement. Taking advantage of MRF, MarkovFit evaluates the fit of individual subunits to the map and subunit interactions efficiently in an integrated fashion. We first benchmarked MarkovFit in comparison with
We benchmarked MarkovFit on a dataset of nine high-resolution experimentally determined maps in which each subunit was shifted and rotated randomly. The average RMSD was 1.85 Å for the best model among the top 10, which was the same for the top-scored models. We further benchmarked MarkovFit on a dataset consisting of 28 experimental maps of medium to low resolution. The experimental dataset has two versions. In the first version, each subunit was shifted and rotated randomly, while the initial orientation was used in the second version. For the randomly transformed experimental dataset, the average RMSD values were 8.14 Å and 13.91 Å for the best model among the top 10 and top-scored models, respectively. For the non-transformed experimental dataset, the average RMSD values were 6.08 Å and 9.95 Å for the best model among the top 10 and top-scored models, respectively. The source code of MarkovFit was made freely available at https://github.com/kiharalab/MarkovFit.
2 Materials and Methods
2.1 Overview of MarkovFit Algorithm
The purpose of MarkovFit is to fit the atomic structures of subunits of a protein complex to a cryo-EM density map so that subunits align well with the map and subunits establish proper physical interactions. As illustrated in Figure 1, the method performs 6D exhaustive searching of the translational and orientational sampling space in the cryo-EM map to compute the candidate positions of each subunit. To accelerate the sampling of the translational space, we used fast Fourier transform (FFT) with a shifting interval of one voxel of the map (it is usually less than 2 Å). To sample the orientational space efficiently, we used a set of quaternions which covers the whole orientation space by 7,416 orientations (Karney, 2007). For each rotation, the search process shifts the rotated subunit structure with the interval and calculates a score at each position, quantifying the goodness of fit of a subunit into the cryo-EM map, which is the sum of cross-correlation and overlap.
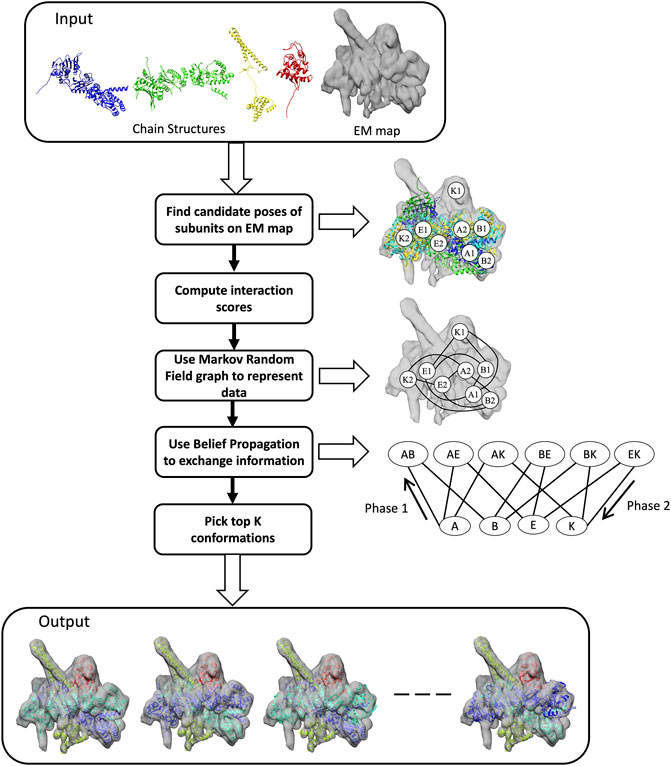
FIGURE 1. Workflow of MarkovFit. The method starts by searching the conformational space for potential positions of each subunit and computes scores which quantify the goodness-of-fit between each subunit and the cryo-EM map, and the interactions between the subunits. The right panel shows some candidate poses of subunits A, B, E, and K, which are A1, A2, B1, B2, E1, E2, K1, and K2. Subsequently, subunits and their interactions are represented using a Markov random field (MRF) graph. MRF nodes exchange information using a belief propagation algorithm. The graph at the bottom of the right panel shows single nodes, each representing the candidate poses of a subunit, and interaction/pairwise nodes, each representing the interactions between candidate poses of a pair of subunits. The last step is extracting the top conformations using a max-heap tree.
The cross-correlation coefficient (CCC) is computed as follows for experimental maps:
where
Here,
The overlap (OV) score measures the fraction of overlapping voxels between two maps, X and Y:
Any pose that has an overlap <50% with the EM map was excluded. The 50% overlap was chosen arbitrarily as we thought a pose less than 50% overlap would be safe to remove from the consideration. A careful optimization of this cutoff may be able to further improve the accuracy or efficiency of the algorithm. The shifted position of the highest score is selected per rotation. Resulted poses are clustered to remove positions within a predetermined distance (8 Å) from each other, and then they are sorted by the combined score. Then, up to the top 100 poses are selected for each subunit.
Next, for each pair of subunits, interactions are evaluated by a docking score that combines scoring terms, which are a Van der Waals potential, electrostatic interaction, hydrogen and disulfide bonds, solvation, and atom clashes, which we have used for evaluating protein-protein docking (Esquivel-Rodriguez et al., 2012).
Exploring all possible combinations of the subunits is a time-consuming process. To explore the search space efficiently, we use pairwise Markov random field (MRF) in MarkovFit. An MRF is a non-directional probabilistic graphical model where nodes represent poses of subunits in the EM map and edges represent interactions between subunits. A complex conformation is evaluated by the sum of node potentials over single random variables, representing the different conformations of each subunit, and edge potentials over pairs of variables:
where N is the number of subunits, Xi is a pose of subunit i, Φ is the node potential, which considers the goodness-of-fit of each subunit in the pose, Ψ is an edge potential, which considers the interaction of subunits, E represents all combinations of the subunits, and Z is the partition function used for normalization.
To avoid numerical issues that might arise from multiplications, we adopted a log-linear model for the scoring function:
where CC is cross-correlation, OV is overlap, Ph is the physics-based docking score, CL is the clash score, and w is the weight assigned for each score.
The four weights were briefly optimized on five experimental maps, EMDB-ID 0366, 1970, 3340, 2526, and 8475, as follows: First, we tried an equal weight, 1.0 for all the four weights, fcc, fov, fph, and fcl. Then, we compared the RMSD of the models with a weight combination of 0.5, 1.0, 1.0, and 1.0, for the four weights, respectively. Furthermore, we used fcc = 0.5, with exhaustive combinations of 0.8, 0.9, and 1.0 for fov, fph, and fcl, respectively; and finally chose to use fcc = 0.5, fov = 0.9, fph = 1.0, and fcl = 0.8 throughout the study. We did not perform extensive optimization because the results were the same for most of the combinations on these five maps.
We apply Maximum a Posteriori (MAP) inference to find values of random variables, i.e., subunit conformations that maximize the score (Eq. 5). The MAP inference is applied using max-sum belief propagation (Koller and Friedman, 2009), which is a message-passing algorithm. To apply max-sum belief propagation, we first generate a cluster graph from the MRF graph to transfer information between nodes. A cluster graph has subunit/single clusters (nodes) representing poses of subunits relative to the EM map and pairwise/interaction nodes representing interactions between subunits. There is an edge between single and pairwise nodes if the pairwise node includes the subunit (the diagram on the right in Figure 1). The max-sum belief propagation method works by assigning initial beliefs to all nodes and then sending messages in two phases (Figure 2). The initial beliefs for single nodes and pairwise nodes are the weighted sums of the goodness-of-fit scores of candidate poses for single nodes and the weighted sums of interaction scores between candidate poses of pairs of single nodes for pairwise nodes. After computing the beliefs of candidate poses, each single node sends its beliefs to all connected pairwise nodes. Subsequently, pairwise nodes send messages back to their neighboring single nodes. A message from a pairwise node (i, j) to a single node i is the maximum of the sum of the belief of the pairwise interaction and the message received from single nodes. Thus, for each pose of subunit i, the pairwise node (i, j) sends its pairwise belief with the pose of subunit i and the belief of subunit j’s pose that maximizes the sum of the beliefs. Single nodes add received messages to their beliefs. From the final beliefs of single nodes, we can extract the top-scoring pose. We check if subunits do not have a significant amount of atom clashes overall in the complex.
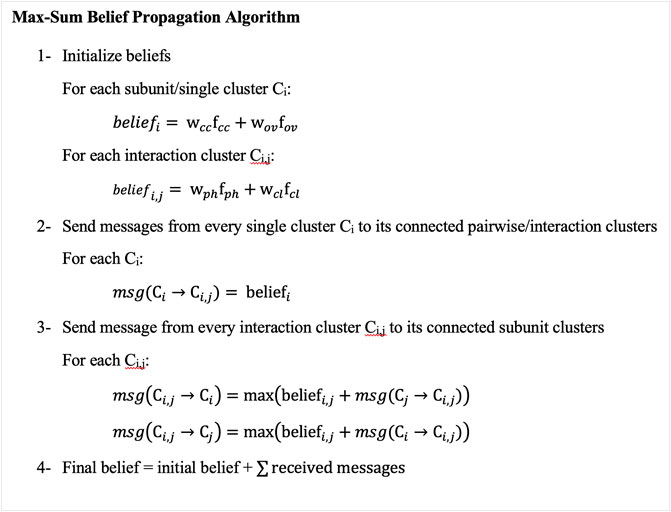
FIGURE 2. Overview of the Max-Sum Belief Propagation Algorithm. The algorithm has four main steps. 1, it initializes the beliefs of subunit/single clusters (nodes) to be the sum of the weighted goodness-of-fit scores of their candidate poses, and the beliefs of interaction/pairwise clusters (nodes) to be the sum of the weighted pairwise scores between the poses of every pair of subunits. 2, subunit clusters (nodes) send their initial beliefs to their connected pairwise clusters (nodes). 3, pairwise clusters (nodes) send messages to their connected subunit clusters (nodes) containing the sum of the pairwise initial beliefs and the subunit messages they received from subunit clusters (nodes), excluding the receiving cluster (node) messages. 4, subunit clusters (nodes) add pairwise beliefs to their initial beliefs. This algorithm corresponds to the right-bottom graph in Figure 1, which is called a cluster graph. A node in the cluster graph is also called a cluster, and thus we used the term cluster and nodes interchangeably.
Our goal is to find the top K docking conformations (in this work K = 10). To extract the top 10 conformations efficiently, we used a max-heap tree, an efficient algorithm for finding maximum values (Cormen et al., 2009) (Supplementary Figure S1).
Representing the fitting problem using MRF reduces the computational time. The brute force method of fitting subunits into a density map would be to enumerate all possible combinations of poses of all individual subunits. The advantage of MRF is that information/scores of the various conformations are exchanged in two phases: from single nodes to pairwise nodes, and then from pairwise nodes to single nodes. Thus, instead of an exhaustive search, docking conformations are considered for every pair of subunits to decide the most plausible pose of the individual subunits. After subunits receive all the information about the other subunits and interactions, extracting top conformations is performed using an efficient max-heap tree, which has a constant time for selecting the top-scoring conformation and subsequent conformations in a log time.
2.2 Evaluation Metrics
We evaluated the resultant final docked conformations in terms of the root-mean-square deviation (RMSD) from the native structure and the Assembly Placement Score (APS). The RMSD is computed between the
3 Results
3.1 Structure Fitting Results
We benchmarked the performance of MarkovFit on three datasets: a set of 31 simulated EM maps; a set of nine high-resolution experimentally determined cryo-EM maps; and a set of 28 experimentally determined cryo-EM maps of medium to low resolution. All datasets are non-redundant, e.g., any protein pairs from two maps have less than 25% global sequence identity between each other.
The simulated EM maps of 31 protein complexes were constructed as follows: From the Protein Data Bank (PDB) (Berman et al., 2000), we selected entries that contain only proteins and have two to seven subunits, which yielded 295 entries. Then, we applied the 25% sequence identity cutoff, which resulted in 22 entries. Then, we added 9 complexes that were common in datasets used in the two previous works (Pandurangan et al., 2015; Bonomi et al., 2019), which we later compared against. EM maps of these complexes were simulated using the epdb2mrc.py program of the EMAN2 package (Tang et al., 2007) at 10 Å resolution and with a voxel size of 1.0 Å.
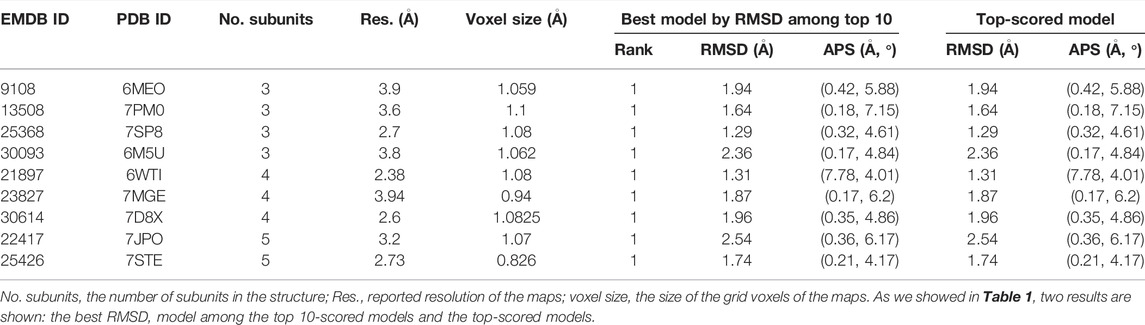
The high-resolution experimental dataset includes nine cryo-EM maps from EMDB and was selected as follows: We selected EM maps that have associated PDB entries that have three to five protein subunits and resolution less than 4 Å. Next, we applied the 25% sequence identity cutoff. After that, we checked experimental cryo-EM maps and their associated PDB entries manually using UCSF Chimera (Pettersen et al., 2004) to ensure they had sufficient overlap. The contour threshold used for this dataset was 0.5 * the recommended contour level.
The medium to low resolution experimental dataset contains 28 cryo-EM maps from EMDB and was selected as follows: we selected EM maps that have associated PDB entries containing two to seven protein subunits and resolution ranges between 6 and 20 Å, which returned 58 EM maps. Next, we applied the 25% sequence identity cutoff, which resulted in 36 entries. Last, experimental cryo-EM maps and their associated PDB entries are checked manually by UCSF Chimera to assure they have adequate overlap. The contour threshold used was 0.5 times the recommended contour level.
In addition to the simulated and experimental datasets, we have three more datasets derived from them. In the new datasets, protein subunits are randomly rotated and shifted from their original positions. The purpose of the random transformations of subunits is to objectively test the search method. Without random transformation, the resulting accuracy was substantially high (Supplementary Figure S2 shows the fitting accuracy of the simulated and medium resolution experimental datasets).
3.1.1 Structure Fitting Results for Simulated Maps
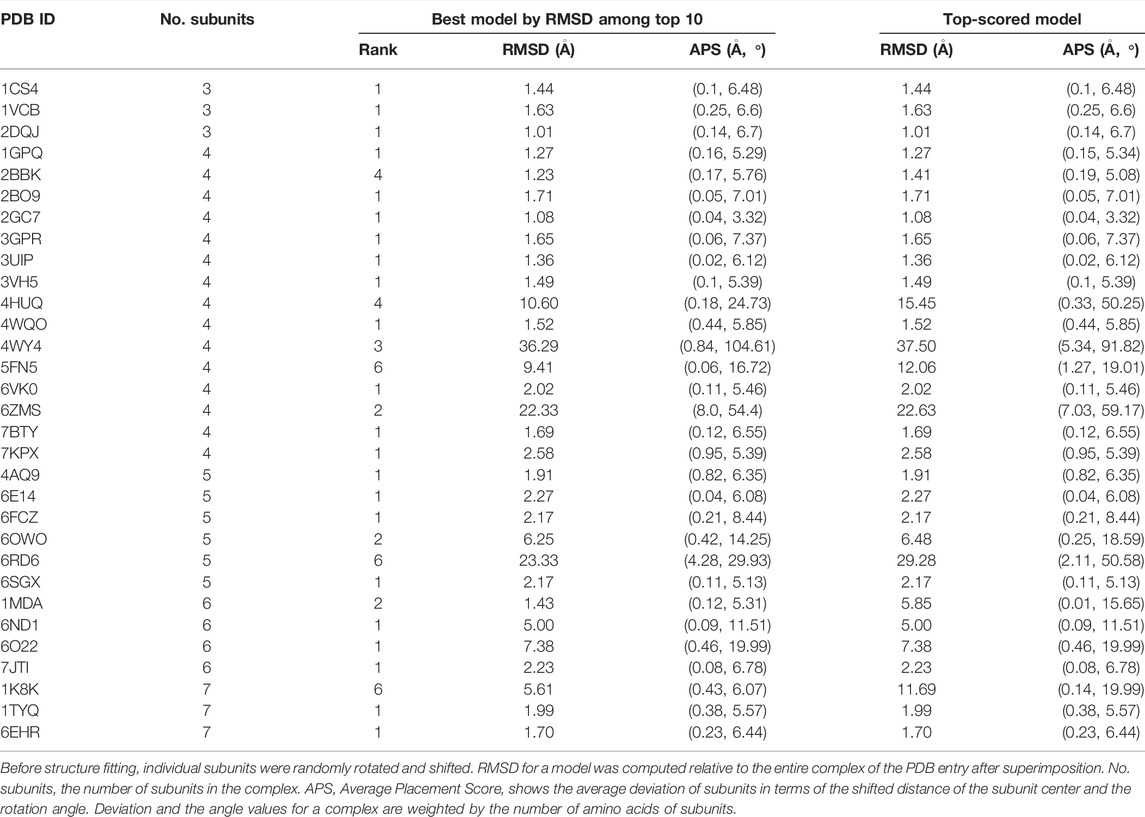
Structure fitting results for the 31 simulated map datasets are summarized in Table 1. To remove potential bias to the docked poses of subunits in the PDB files, subunits were randomly rotated and shifted before the fitting process by MarkovFit was applied. In Table 1, two results are shown for each target. On the left are the results of the lowest root-mean square deviation (RMSD) model among the top 10 scored models. On the right, we showed the top-scored model. The top-scored model is the model that had the best score among the models generated. This model does not always be the best in quality in terms of RMSD among all the models generated because often the score could not select the best quality model among the pool. The best model among the top 10-scored models is the best RMSD model among the 10 models selected by the score. Since there are 10 choices to choose from, there is a higher chance that the RMSD value of the best model among the top 10-scored models is better than the top-scored model.
Overall, fitting was very successful in this simulated map dataset. When the best model among the top 10-scored models was considered, 22 out of 31 models (71.0%) had an RMSD of less than 3.0 Å with an average RMSD of 1.69 Å. We also reported the assembly placement score (APS) (Lasker et al., 2009). APS for a complex model is the average distance and angle deviation of subunits in the complex from the reference structure, weighted by the number of amino acids of each subunit. Since APS is weighted by the subunit size, misplacement of a small subunit does not affect the score as much as that of a large subunit. The average APS for the 22 models with a less than 3.0 Å RMSD was 0.21 Å and 6.06
Turning attention now to the top-scored model results (the right column in Table 1), RMSDs were almost identical to the results shown for the best model among the top 10-scored models. Thus, the scoring function was very successful in selecting the best model. Out of the 27 targets that have a less than 10 Å RMSD model within the top 10-scored models, there were only two targets, 5FN5 and 1MDA, where the RMSD of the top-scored model dropped more than 2.0 Å from the best among the top 10.
In Figure 3, we analyzed how the placement of individual subunits affects the overall accuracy of the complex model. For each target, the largest distance and angle deviations among subunits were plotted relative to the overall RMSD of the model. Obviously, both distance and angle deviations were small when the overall RMSD was small enough, for example, less than 3 Å, while above 3 Å RMSD, the angle deviation sharply increased above 75°. Also, the distance deviation and RMSD.
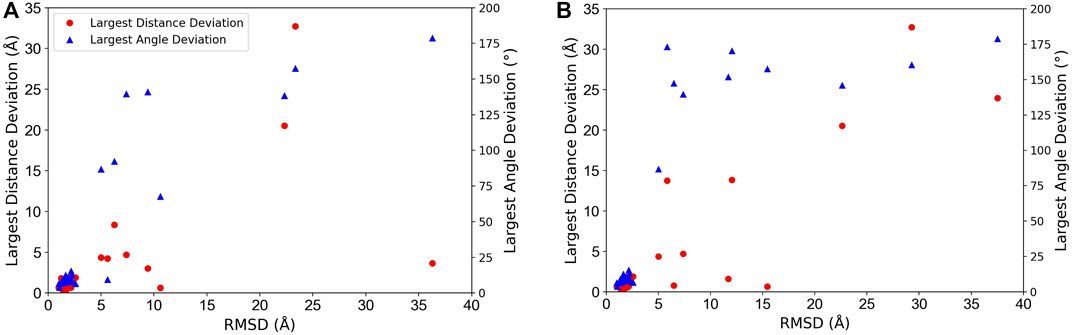
FIGURE 3. Relationship between overalls RMSD of protein complex models and distance and angle deviation of subunits. The worst (largest) deviations among subunits were plotted. Red circles, distance deviation; blue triangles, angle deviation. (A) The best model among the top 10-scored models was considered. (B) The top-scored models were considered.
We show five examples of structure fitting by MarkovFit in Figure 4. The first two targets were successful examples of MarkovFit. For these two targets, 2GC7 and 1TYQ, the best (smallest RMSD) model was ranked at the top by the score, and the RMSD was 1.08 Å and 1.99 Å, respectively. For the third example, a seven-chain complex of actin-related protein (Arp) 2/3 complex (1K8K), there was a 5.61 Å RMSD model ranked within the top 10; however, it was not selected as the top-scored model. The top-scored model had an RMSD of 11.69 Å. For this target, the main difficulty was placing Chain F (dark blue) in the correct position and orientation. In the top-scored model, Chain F was placed around at the right position (1.62 Å) but with a large rotation deviation of 151.81°. The best model among the top 10 models had an APS of (4.23 Å, 9.41
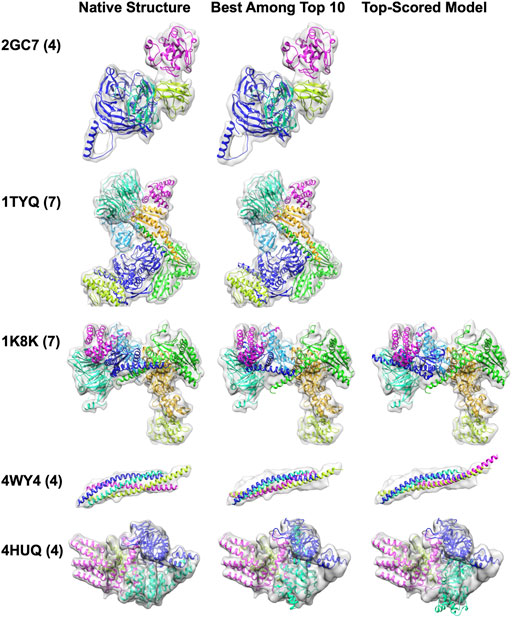
FIGURE 4. Structure fitting examples of simulated map targets. The number of chains in a complex is shown in the parentheses. The middle panel shows the best among the top 10-scored models, and the right panel shows the top-scored models. Only one fitted structure was shown for 2GC7 and 1TYQ because the top-scored model was the best among the top 10-scored models. 2GCN, the human RhoC-GDP complex. 1TYQ, Arp2/3 complex with bound ATP or ADP. 1K8K, bovine Arp2/3 complex. 4WY4, an autophagic SNARE complex. 4HUQ, folate ECF transporter from Lactobacillus brevis.
3.1.2 Structure Fitting Results for Experimental Maps
3.1.2.1 Fitting to High Resolution Maps
Although the main purpose of MarkovFit is to fit individual subunits into medium resolution density maps, we first tested the algorithm on higher resolution maps. For that, we generated a dataset of nine experimental cryo-EM maps with a resolution of less than 4 Å.
The results are summarized in Table 2. Table 2 shows two results for each target. On the left, we showed the results of the lowest RMSD model among the top 10 scored models. On the right, we showed the results of the top-scored model. Fitting was highly successful in this dataset. Looking at the lowest RMSD model among the top 10-scored models, all nine models had an RMSD of less than 3.0 Å with an average RMSD of 1.86 Å and average APS of 1.11 Å and 5.32
3.1.2.2 Fitting to Medium Resolution Maps
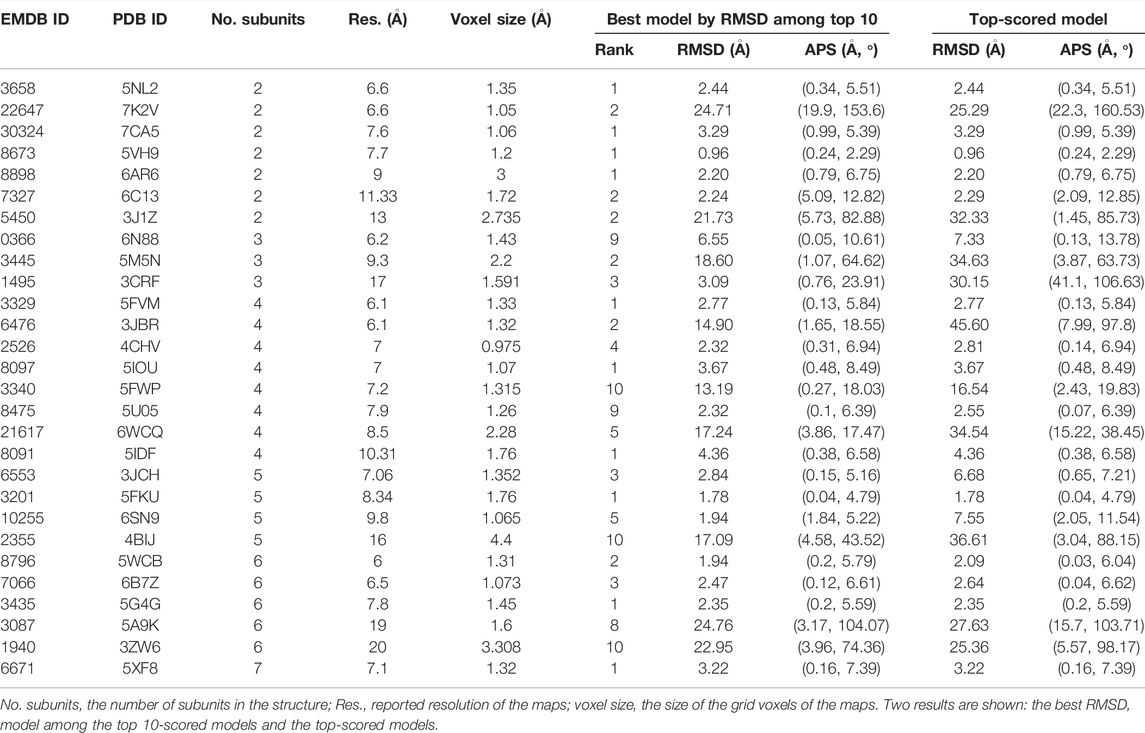
Next, we tested MarkovFit on the 28 experimental map datasets (Table 3). Here, again, we randomly rotated and shifted subunits before applying the structure fitting process to avoid bias in the initial poses of subunits in the PDB files. Similar to the simulated map cases, the resulting accuracy was substantially higher if random transformation was not applied (Supplementary Figure S3).
Clearly, structure fitting was more difficult on this dataset as compared with the previous simulated map dataset and the high-resolution experimental dataset: when the lowest RMSD model among the top 10-scored models was considered, 13 out of 28 models (46.4%) had an RMSD of less than 3.0 Å with an average RMSD of 2.20 Å. When counted on the simulated map dataset (Table 1), the fraction was 71.0%. There were six more targets with an RMSD of less than 10 Å, which had an average RMSD of 4.03 Å. We tried to refine the model structures with the Rosetta Relax protocol (Nivon et al., 2013) and a rigid-body refinement (Esquivel-Rodriguez et al., 2012) but did not observe meaningful improvement (data not shown). The conformational change that the refinement methods could make was not large. Thus, if a model’s RMSD is large, it is often beyond what the refinement methods can handle. Also, changes made by the refinement methods were not always in the right direction and made the structure worse.
The scoring function worked well on this dataset as well. Looking at the top-scored model results, RMSD values were identical or close to the best model among the top 10-scored models, especially for targets where the best model among the top 10 has an RMSD less than 10 Å. Out of the 19 targets that have a less than 10 Å RMSD model within the top 10-scores, the score was able to select the lowest RMSD model as the top for 16 of them (84.2%).
In Figure 5, we examined the correlation between the map resolution and the model accuracy. There is no clear linear correlation between the model RMSD and the map resolution observed. But we can see that the structure fitting accuracy substantially dropped when the map resolution was worse than 10 Å. For example, considering the best among the top 10 models, when the map resolution is better than 10 Å, 16 out of 21 maps (76.2%) had a model with an RMSD lower than 5 Å but it drops to 42.9% (3 out of 7 maps) when maps with a resolution worse than 10 Å are counted.
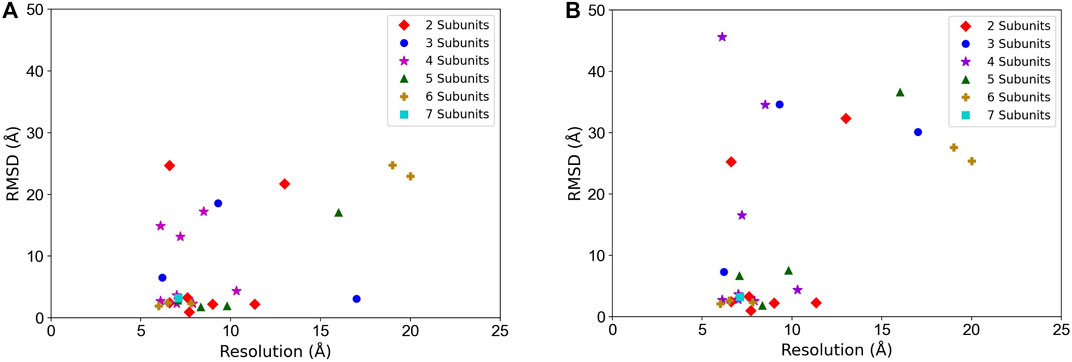
FIGURE 5. Structure fitting accuracy relative to the map resolution. RMSD of the entire model structure of complexes is plotted. (A) the best RMSD model among the top 10-scored models. (B) the top-scored models. Targets with different chain numbers are separately plotted.
Five examples of structure fitting to experimental maps are presented in Figure 6. MarkovFit worked well in the first two targets, the E. coli replicative DNA polymerase complex in DNA-free state (EMD-3201, PDB ID: 5FKU) and the Cdt1-MCM2-7 complex in the AMPPNP state (EMD-6671, PDB ID: 5XF8). Near-native complex structure was successfully ranked as the top score, which has an RMSD of 1.78 Å and 3.22 Å, respectively. For the third example, a homo five-chain complex of CorA from Thermotoga maritima in the absence of magnesium, state II (EMD-6553, PDB ID: 3JCH), the best-RMSD model among the top 10 models has an RMSD of 2.84 Å, but it was not selected as the top-scored model. The top-scored model had an RMSD of 6.68 Å. This is a membrane protein complex, and the large volume of the density in the bottom half of the map is mostly from the nanodiscs, which mimic the membrane environment. Among the top 10 models, the best model ranked third by the score placed all subunits in their correct position and orientation. However, the top-scored model had one chain (blue) in a substantially deviated orientation, which caused a larger overall RMSD of 6.68 Å. The next example is the microtubule-bound S. pombe kinesin-5 motor domain in the AMPPNP state (EMD-3445, PDB ID: 5M5N), a three-chain complex. In the best among the top 10-scored model which has an RMSD of 18.60 Å, Chain A (blue) was placed around the right position but with a large rotation deviation of 166.22°. In the top-scored models, Chain A (blue) was placed at the position of Chain B (a shift of 29.76 Å) with a large rotation deviation of 178.84°. In turn, Chain B (green) was placed in the position of Chain A (a shift of 18.68 Å) with a small rotation deviation of 2.83°, resulting in the increase in the RMSD value of the top-scored model to 34.63 Å. The last example is the Hsp90-Cdc37-Cdk4 kinase complex (EMD: 3340, PDB ID: 5FWP), which has four chains. The main difficulty with this target was Chain E (blue). For this target, both the best among the top 10-scored models and the top-scored model have an RMSD above 10 Å. In the best among the top 10-scored models, Chain E (blue) did not occupy its density well due to a large rotation of 50.45°. In the top-scored model, Chain E (blue) was shifted by 14.62 Å and has a rotation deviation of 65.91°.
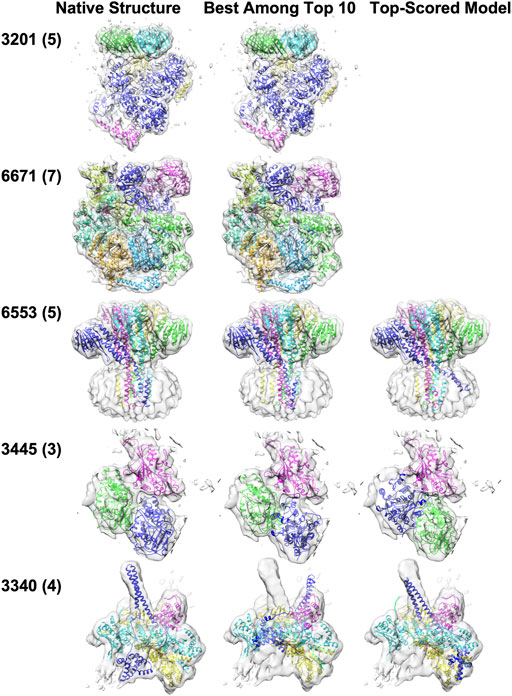
FIGURE 6. Structure fitting examples of experimental map targets. The number of chains in a complex is shown in the parentheses. The middle panel shows the best among the top 10-scored models, and the right panel shows the top-scored models. Only one fitted structure was shown for EMD-3201 and EMD-6671 because the top scored model was the best among top 10-scored models. (EMD-3201, PDB ID: 5FKU), the E. coli replicative DNA polymerase complex in DNA free state. (EMD-6671, PDB ID: 5XF8), the Cdt1-MCM2-7 complex in the AMPPNP state. (EMD-6553, PDB ID: 3JCH), the magnesium channel CorA in the magnesium-free asymmetric open state II. (EMD-3445, PDB ID: 5M5N), microtubule-bound S. pombe kinesin-5 motor domain in the AMPPNP state. (EMD: 3340, PDB ID: 5FWP), the Hsp90-Cdc37-Cdk4 kinase complex.
3.2 Comparison With Other Methods
We compared MarkovFit with two state-of-the-art methods for structure fitting. The first method is
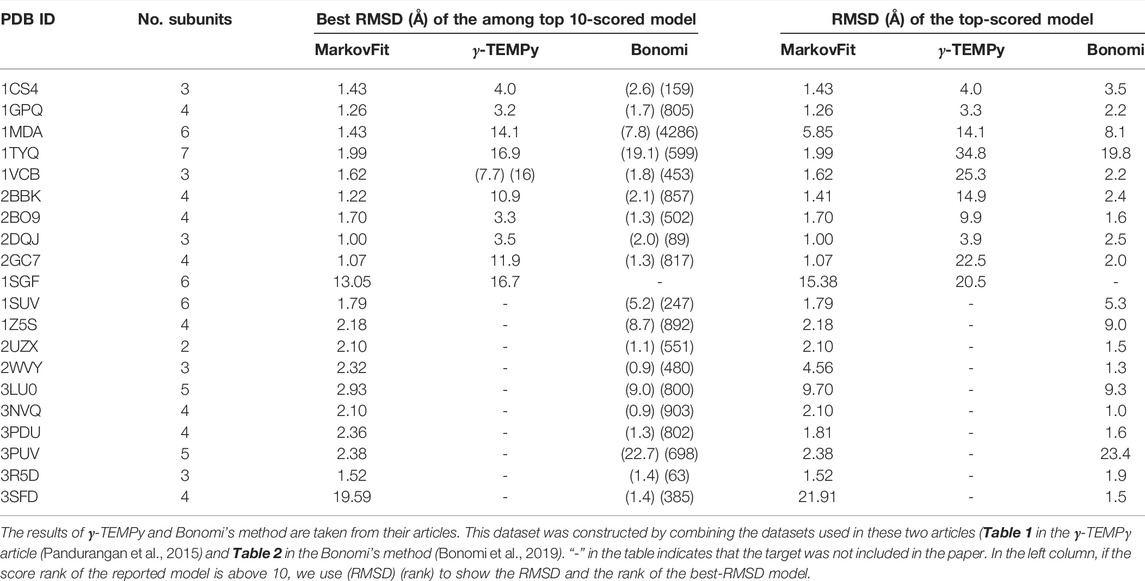
The comparison was performed on a dataset of simulated maps at 10 Å as shown in Table 4. This dataset includes nine protein complexes, which are in common with Table 1 and the datasets used in the two works (Pandurangan et al., 2015) and (Bonomi et al., 2019). In addition, from the
When the top-scored models of the nine common targets were considered, MarkovFit clearly showed the best performance. The average RMSD of these targets is 1.93 Å, 14.74 Å, and 4.92 Å for MarkovFit, γ-TEMPy, and the Bonomi’s method, respectively. MarkovFit showed the lowest RMSD for eight out of the nine targets. Our method had a substantially higher accuracy on 1TYQ, where our method had an RMSD of 1.99 Å and the other two methods had over 10 Å RMSD.
We also compare MarkovFit with each of the two methods individually in terms of the top-scored models. 1SGF is the remaining target to compare with γ-TEMPy. For this target, both MarkovFit and γ-TEMPy did not produce near-native conformation, but the former had a lower RMSD of 15.38 Å. With the Bonomi’s method, we compare the 19 targets for which both methods have results. The average RMSD values on these targets by MarkovFit and Bonomi’s method were 3.55 Å and 5.27 Å, respectively. MarkovFit had a lower RMSD than the Bonomi’s method for 12 out of the 19 targets.
In terms of the best among the top 10-scored models, MarkovFit and γ-TEMPy had an average RMSD of 2.58 Å and 9.2 Å, respectively, on the 10 targets that are common for these 2 methods. Comparing against the Bonomi’s method is not possible because the reported results in their papers are models ranked lower than the top 10.
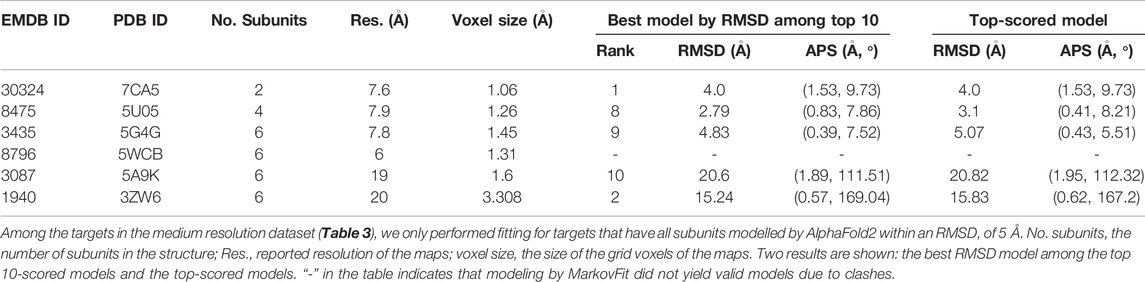
3.3 Fitting Predicted Models by AlphaFold2
To further investigate the performance of MarkovFit, we tested the method on protein structure models generated by AlphaFold2 (Jumper et al., 2021). AlphaFold2 is a deep learning-based method for predicting protein structure models from sequences that showed a substantial improvement in structure prediction accuracy in the Critical Assessment of techniques in protein Structure Prediction (CASP) (Kryshtafovych et al., 2021).
We modeled individual protein subunits in the medium-resolution experimental dataset by AlphaFold2. Among five models produced by AlphaFold2, we reported the best RMSD in Supplementary Table S1. Among the 28 targets in the dataset, interestingly, there were only six targets where AlphaFold2 modeled all their subunits within an RMSD of less than 5 Å (Supplementary Table S1). We performed structure fitting only for these six targets. Table 5 summarizes the results.
Among the six targets, only one target had an RMSD of less than 3 Å and three targets were within 10 Å RMSD when the lowest RMSD model among the top 10-scored models was considered. The map EMD-8796 (PDB: 5WCB) did not yield models as subunit poses had too many clashes among them. Thus, overall, AlphaFold2 models were not accurate enough for most of the targets in this dataset.
3.4 Computational Time
The CPU hours of running MarkovFit are shown in Figure 7. They were measured on 11 experimental map targets from the medium resolution dataset with a different number of subunits. The computational time of the entire MarkovFit process is the sum of the times for the subunit pose search process using FFT, the pairwise score computation between every pair of subunits, the belief propagation application on the MRF graph, and the step of picking the top 10 docked structures using the max-heap tree. The subunit pose search process and the pairwise score computation take most of the MarkovFit time, while the belief propagation algorithm and the picking of the top 10 conformations step take a few minutes. The search process and the pairwise score computation on the 11-target experimental subset took on average about 59% (7.7 h) and 40% (6.25 h) of the total computational time of MarkovFit, respectively.
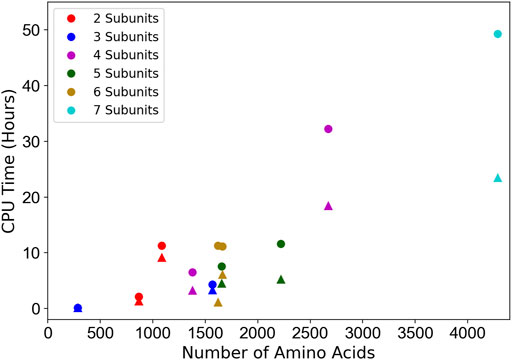
FIGURE 7. Computational time relative to the protein complex size. The CPU time of the entire process (circles) as well as the initial subunit pose search step (triangles) are shown. 11 experimental map targets with different number of chains were used: EMD-3658, EMD-7327, EMD-0366, EMD-1495, EMD- 2526, EMD-8475, EMD-6553, EMD-3201, EMD-8796, EMD-3087, and EMD-6671. The time was measured on 20 cores of AMD EPYC 7402P 24-Core @ 2.8 GHz.
In Figure 7, we see a correlation between the number of amino acids in the protein complex and the computational time. The two main factors that affect the computational time are the size of the map and the number of subunits. Apparently, a large map has a larger search space. As the search process is performed on each subunit individually, the running time increases as we have more subunits. Also, the pairwise interaction score computation increases as we have more subunits.
4 Discussion
In this work, we developed MarkovFit, a procedure to perform structure fitting to cryo-EM maps of medium to low resolution. The MRF is effective in finding correct combinations of subunit poses that are mapped in the target EM map, and the Max-heap algorithm is used to select top-scored conformations.
Although the algorithm worked well overall, there were several reasons that could lead to failure of the search process. A main cause is the small size of a subunit, e.g., ∼100 or fewer residues as observed in the target 4WY4 in the simulated map dataset (Table 1) and 6N88 and 6SN9 in the experimental map dataset (Table 3). Finding the correct pose of a small domain is difficult, especially when the other subunits are large and have a higher density in the corresponding positions in the EM map. Also, apparently, as the map resolution gets worse, density values across the cryo-EM map become undistinguishable, resulting in incorrect predictions of candidate poses for subunits (Figure 5).
Fitting existing subunit structures have been a major strategy in structure modeling for cryo-EM maps of medium to low resolution. This approach is becoming more important now as predicting structures is becoming increasingly reliable by structure prediction methods of the new generation (Jumper et al., 2021), although AlphaFold2 did not work that well in the dataset we used (Table 5, Supplementary Table S1). Structure models of dozens of organisms are precomputed and made available to the public, too (Aderinwale et al., 2022; Varadi et al., 2022). A future important direction of development would be to combine these two approaches effectively: structure prediction of individual proteins and combining and fitting them into a cryo-EM map.
Data Availability Statement
The data presented in this study can be found at https://zenodo.org/record/6509947. The MarkovFit code is freely available for academic use via https://github.com/kiharalab/MarkovFit.
Author Contributions
DK conceived the study. JE-R coded MarkovFit and did a pilot study. EA generated the benchmark datasets, updated the MarkovFit code, ran MarkovFit, collected and analyzed the results, and wrote the initial draft of the manuscript. GT coded the program to find candidate poses. DK supervised the project, analyzed data, and critically edited the manuscript. All authors approved the manuscript.
Funding
This work was partly supported by the National Institutes of Health (R01GM133840, R01GM123055, and 3R01GM133840-02S1) and the National Science Foundation (DBI2003635, DBI2146026, CMMI1825941, and MCB1925643). EA is supported by a fellowship from Umm Al-Qura University, Saudi Arabia.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmolb.2022.935411/full#supplementary-material
References
Aderinwale, T., Bharadwaj, V., and Christof, fer, C., Terashi, G., Zhang, Z., Jahandideh, R., et al. (2022). Real-time Structure Search and Structure Classification for AlphaFold Protein Models. Commun. Biol. 5 (1), 316. doi:10.1038/s42003-022-03261-8
Alnabati, E., and Kihara, D. (2019). Advances in Structure Modeling Methods for Cryo-Electron Microscopy Maps. Molecules 25 (1), 82. doi:10.3390/molecules25010082
Bai, X.-c., McMullan, G., and Scheres, S. H. W. (2015). How Cryo-EM Is Revolutionizing Structural Biology. Trends Biochem. Sci. 40 (1), 49–57. doi:10.1016/j.tibs.2014.10.005
Berman, H. M., Westbrook, J., Feng, Z., Gilliland, G., Bhat, T. N., Weissig, H., et al. (2000). The Protein Data Bank. Nucleic Acids Res. 28 (1), 235–242. doi:10.1093/nar/28.1.235
Besag, J. (1974). Spatial Interaction and the Statistical Analysis of Lattice Systems. J. R. Stat. Soc. Ser. B Methodol. 36 (2), 192–225. doi:10.1111/j.2517-6161.1974.tb00999.x
Bonomi, M., Hanot, S., Greenberg, C. H., Sali, A., Nilges, M., Vendruscolo, M., et al. (2019). Bayesian Weighing of Electron Cryo-Microscopy Data for Integrative Structural Modeling. Structure 27 (1), 175–188. doi:10.1016/j.str.2018.09.011
Chacón, P., and Wriggers, W. (2002). Multi-resolution Contour-Based Fitting of Macromolecular Structures. J. Mol. Biol. 317 (3), 375–384. doi:10.1006/jmbi.2002.5438
Cheng, Y. (2018). Single-particle Cryo-EM-How Did it Get Here and where Will it Go. Science 361 (6405), 876–880. doi:10.1126/science.aat4346
Cormen, T. H., Leiserson, C. E., Rivest, R. L., and Stein, C. (2009). Introduction to Algorithms. Cambridge, Massachusetts: The MIT Press.
Esquivel-Rodríguez, J., and Kihara, D. (2013). Computational Methods for Constructing Protein Structure Models from 3D Electron Microscopy Maps. J. Struct. Biol. 184 (1), 93–102. doi:10.1016/j.jsb.2013.06.008
Esquivel-Rodríguez, J., and Kihara, D. (2012). Fitting Multimeric Protein Complexes into Electron Microscopy Maps Using 3D Zernike Descriptors. J. Phys. Chem. B 116 (23), 6854–6861. doi:10.1021/jp212612t
Esquivel-Rodríguez, J., Yang, Y. D., and Kihara, D. (2012). Multi-LZerD: Multiple Protein Docking for Asymmetric Complexes. Proteins 80 (7), 1818–1833. doi:10.1002/prot.24079
Garzon, J. I., Kovacs, J., Abagyan, R., and Chacon, P. (2007). ADP_EM: Fast Exhaustive Multi-Resolution Docking for High-Throughput Coverage. Bioinformatics 23 (4), 427–433. doi:10.1093/bioinformatics/btl625
Han, X., Terashi, G., Christoffer, C., Chen, S., and Kihara, D. (2021). VESPER: Global and Local Cryo-EM Map Alignment Using Local Density Vectors. Nat. Commun. 12 (1), 2090. doi:10.1038/s41467-021-22401-y
Joseph, A. P., Lagerstedt, I., Patwardhan, A., Topf, M., and Winn, M. (2017). Improved Metrics for Comparing Structures of Macromolecular Assemblies Determined by 3D Electron-Microscopy. J. Struct. Biol. 199 (1), 12–26. doi:10.1016/j.jsb.2017.05.007
Joseph, A. P., Swapna, L. S., Rakesh, R., and Srinivasan, N. (2016). Use of Evolutionary Information in the Fitting of Atomic Level Protein Models in Low Resolution Cryo-EM Map of a Protein Assembly Improves the Accuracy of the Fitting. J. Struct. Biol. 195 (3), 294–305. doi:10.1016/j.jsb.2016.07.012
Jumper, J., Evans, R., Pritzel, A., Green, T., Figurnov, M., Ronneberger, O., et al. (2021). Highly Accurate Protein Structure Prediction with AlphaFold. Nature 596 (7873), 583–589. doi:10.1038/s41586-021-03819-2
Karney, C. F. F. (2007). Quaternions in Molecular Modeling. J. Mol. Graph. Model. 25 (5), 595–604. doi:10.1016/j.jmgm.2006.04.002
Kawabata, T. (2018). Gaussian-input Gaussian Mixture Model for Representing Density Maps and Atomic Models. J. Struct. Biol. 203 (1), 1–16. doi:10.1016/j.jsb.2018.03.002
Kawabata, T. (2008). Multiple Subunit Fitting into a Low-Resolution Density Map of a Macromolecular Complex Using a Gaussian Mixture Model. Biophysical J. 95 (10), 4643–4658. doi:10.1529/biophysj.108.137125
Kihara, D., Sael, L., Chikhi, R., and Esquivel-Rodriguez, J. (2011). Molecular Surface Representation Using 3D Zernike Descriptors for Protein Shape Comparison and Docking. Cpps 12 (6), 520–530. doi:10.2174/138920311796957612
Koller, D., and Friedman, N. (2009). Probabilistic Graphical Models : Principles and Techniques. Cambridge, MA: MIT Press.
Kryshtafovych, A., Schwede, T., Topf, M., Fidelis, K., and Moult, J. (2021). Critical Assessment of Methods of Protein Structure Prediction (CASP)-Round XIV. Proteins 89 (12), 1607–1617. doi:10.1002/prot.26237
Kühlbrandt, W. (2014). The Resolution Revolution. Science 343 (6178), 1443–1444. doi:10.1126/science.1251652
Lasker, K., Topf, M., Sali, A., and Wolfson, H. J. (2009). Inferential Optimization for Simultaneous Fitting of Multiple Components into a CryoEM Map of Their Assembly. J. Mol. Biol. 388 (1), 180–194. doi:10.1016/j.jmb.2009.02.031
Lopéz-Blanco, J. R., and Chacón, P. (2013). iMODFIT: Efficient and Robust Flexible Fitting Based on Vibrational Analysis in Internal Coordinates. J. Struct. Biol. 184 (2), 261–270. doi:10.1016/j.jsb.2013.08.010
Maddhuri Venkata Subramaniya, S. R., Terashi, G., and Kihara, D. (2019). Protein Secondary Structure Detection in Intermediate-Resolution Cryo-EM Maps Using Deep Learning. Nat. Methods 16 (9), 911–917. doi:10.1038/s41592-019-0500-1
Malhotra, S., Träger, S., Dal Peraro, M., and Topf, M. (2019). Modelling Structures in Cryo-EM Maps. Curr. Opin. Struct. Biol. 58, 105–114. doi:10.1016/j.sbi.2019.05.024
Nivón, L. G., Moretti, R., and Baker, D. (2013). A Pareto-Optimal Refinement Method for Protein Design Scaffolds. PLoS One 8 (4), e59004. doi:10.1371/journal.pone.0059004
Nogales, E. (2016). The Development of Cryo-EM into a Mainstream Structural Biology Technique. Nat. Methods 13 (1), 24–27. doi:10.1038/nmeth.3694
Pandurangan, A. P., Vasishtan, D., Alber, F., and Topf, M. (2015). γ-TEMPy: Simultaneous Fitting of Components in 3D-EM Maps of Their Assembly Using a Genetic Algorithm. Structure 23 (12), 2365–2376. doi:10.1016/j.str.2015.10.013
Pettersen, E. F., Goddard, T. D., Huang, C. C., Couch, G. S., Greenblatt, D. M., Meng, E. C., et al. (2004). UCSF Chimera?A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 25 (13), 1605–1612. doi:10.1002/jcc.20084
Renaud, J.-P., Chari, A., Ciferri, C., Liu, W.-t., Rémigy, H.-W., Stark, H., et al. (2018). Cryo-EM in Drug Discovery: Achievements, Limitations and Prospects. Nat. Rev. Drug Discov. 17 (7), 471–492. doi:10.1038/nrd.2018.77
Rossmann, M. G., Bernal, R., and Pletnev, S. V. (2001). Combining Electron Microscopic with X-Ray Crystallographic Structures. J. Struct. Biol. 136 (3), 190–200. doi:10.1006/jsbi.2002.4435
Tama, F., Miyashita, O., and Brooks III, C. L. (2004). Normal Mode Based Flexible Fitting of High-Resolution Structure into Low-Resolution Experimental Data from Cryo-EM. J. Struct. Biol. 147 (3), 315–326. doi:10.1016/j.jsb.2004.03.002
Tang, G., Peng, L., Baldwin, P. R., Mann, D. S., Jiang, W., Rees, I., et al. (2007). EMAN2: an Extensible Image Processing Suite for Electron Microscopy. J. Struct. Biol. 157 (1), 38–46. doi:10.1016/j.jsb.2006.05.009
Terashi, G., and Kihara, D. (2018). De Novo main-chain Modeling for EM Maps Using MAINMAST. Nat. Commun. 9 (1), 1618. doi:10.1038/s41467-018-04053-7
Terashi, G., Zha, Y., and Kihara, D. (2020). Protein Structure Modeling from Cryo-EM Map Using MAINMAST and MAINMAST-GUI Plugin. Methods Mol. Biol. 2165, 317–336. doi:10.1007/978-1-0716-0708-4_19
Terwilliger, T. C., Adams, P. D., Afonine, P. V., and Sobolev, O. V. (2018). A Fully Automatic Method Yielding Initial Models from High-Resolution Cryo-Electron Microscopy Maps. Nat. Methods 15 (11), 905–908. doi:10.1038/s41592-018-0173-1
Trabuco, L. G., Villa, E., Mitra, K., Frank, J., and Schulten, K. (2008). Flexible Fitting of Atomic Structures into Electron Microscopy Maps Using Molecular Dynamics. Structure 16 (5), 673–683. doi:10.1016/j.str.2008.03.005
van Zundert, G. C. P., Melquiond, A. S. J., and Bonvin, A. M. J. J. (2015). Integrative Modeling of Biomolecular Complexes: HADDOCKing with Cryo-Electron Microscopy Data. Structure 23 (5), 949–960. doi:10.1016/j.str.2015.03.014
Varadi, M., Anyango, S., Deshpande, M., Nair, S., Natassia, C., Yordanova, G., et al. (2022). AlphaFold Protein Structure Database: Massively Expanding the Structural Coverage of Protein-Sequence Space with High-Accuracy Models. Nucleic Acids Res. 50 (D1), D439–D444. doi:10.1093/nar/gkab1061
Vasishtan, D., and Topf, M. (2011). Scoring Functions for cryoEM Density Fitting. J. Struct. Biol. 174 (2), 333–343. doi:10.1016/j.jsb.2011.01.012
Velazquez-Muriel, J.-Á., Valle, M., Santamaría-Pang, A., Kakadiaris, I. A., and Carazo, J.-M. (2006). Flexible Fitting in 3D-EM Guided by the Structural Variability of Protein Superfamilies. Structure 14 (7), 1115–1126. doi:10.1016/j.str.2006.05.013
Velazquez-Muriel, J. A., and Carazo, J.-M. a. (2007). Flexible Fitting in 3D-EM with Incomplete Data on Superfamily Variability. J. Struct. Biol. 158 (2), 165–181. doi:10.1016/j.jsb.2006.10.014
Wang, X., Alnabati, E., Aderinwale, T. W., Maddhuri Venkata Subramaniya, S. R., Terashi, G., and Kihara, D. (2021). Detecting Protein and DNA/RNA Structures in Cryo-EM Maps of Intermediate Resolution Using Deep Learning. Nat. Commun. 12 (1), 2302. doi:10.1038/s41467-021-22577-3
Wang, Z., and Schröder, G. F. (2012). Real-space Refinement with DireX: from Global Fitting to Side-Chain Improvements. Biopolymers 97 (9), 687–697. doi:10.1002/bip.22046
Woetzel, N., Lindert, S., Stewart, P. L., and Meiler, J. (2011). BCL::EM-Fit: Rigid Body Fitting of Atomic Structures into Density Maps Using Geometric Hashing and Real Space Refinement. J. Struct. Biol. 175 (3), 264–276. doi:10.1016/j.jsb.2011.04.016
Wu, M., and Lander, G. C. (2020). Present and Emerging Methodologies in Cryo-EM Single-Particle Analysis. Biophysical J. 119 (7), 1281–1289. doi:10.1016/j.bpj.2020.08.027
Keywords: protein modeling, cryo-EM, Markov random field, structure fitting, protein structure prediction
Citation: Alnabati E, Esquivel-Rodriguez J, Terashi G and Kihara D (2022) MarkovFit: Structure Fitting for Protein Complexes in Electron Microscopy Maps Using Markov Random Field. Front. Mol. Biosci. 9:935411. doi: 10.3389/fmolb.2022.935411
Received: 03 May 2022; Accepted: 13 June 2022;
Published: 25 July 2022.
Edited by:
Yong Wang, Zhejiang University, ChinaReviewed by:
Ye Mei, East China Normal University, ChinaSheng Cui, Institute of Pathogen Biology (CAMS), China
Copyright © 2022 Alnabati, Esquivel-Rodriguez, Terashi and Kihara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daisuke Kihara, ZGtpaGFyYUBwdXJkdWUuZWR1
 Eman Alnabati
Eman Alnabati Juan Esquivel-Rodriguez
Juan Esquivel-Rodriguez Genki Terashi
Genki Terashi Daisuke Kihara
Daisuke Kihara