
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Mol. Biosci. , 25 October 2022
Sec. Molecular Diagnostics and Therapeutics
Volume 9 - 2022 | https://doi.org/10.3389/fmolb.2022.1045548
This article is part of the Research Topic Multi-Omics Dissection of Cell Plasticity and Tumor Heterogeneity for Personalized Cancer Therapy - Volume II View all 4 articles
Ferroptosis is a novel regulatory cell death, which is characterized by iron dependency and mainly caused by accumulation of intracellular lipid peroxides and reactive oxygen species. Ferroptosis plays an important role in the occurrence and development of a variety of malignant tumors, especially in anti-tumor treatment. As an emerging treatment method, the immunotherapy has been widely applied in the clinical practice, and the role of ferroptosis in tumor immunotherapy has been gradually explored. This study aims to illustrate the features of ferroptosis, and its role in anti-tumor immunotherapy and potential clinical application.
Ferroptosis, as a new form of cell death, plays an important role in many diseases, especially affecting the malignant progress of tumors and anti-tumor treatment (Liang et al., 2019; Chen et al., 2021a; Wu et al., 2020). Anti-tumor treatment is divided into drug therapy, radiation therapy, surgical treatment and so on, among which drug therapy includes immunotherapy, chemotherapy, targeted therapy, etc. Among them, anti-tumor immunotherapy, such as targeting PD-1 (programmed death-1 protein-1 (PD-1) or its ligand PD-L1 or CTLA4, aims to strengthen the immune system and exerts anti-tumor effects. Immunotherapy has been increasingly applied in the treatment of various malignant tumors, and exhibits well therapeutic effect and long-term benefit, while its curative evaluation still remains unclear. Studies have shown that targeting ferroptosis is expected to improve the therapeutic effect of anti-tumor immunotherapy, suggesting the potential relationship between ferroptosis and immunotherapy. Therefore, this review intends to summarize the role and research progress of ferroptosis in anti-tumor immunotherapy, and provide reference and hints for follow-up research.
The concept of “ferroptosis” was firstly put forward by Dixon in 2012 (Dixon et al., 2012) (Figure 1). It is a form of iron-dependent cell death, characterized by excessive oxidative stress and membrane lipid peroxidation. Ferroptosis is different from other types of cell death (such as apoptosis, necrosis, etc.) in morphological changes, biochemical characteristics and regulatory mechanisms (Chen et al., 2021). As for morphology, cell apoptosis is characterized by intact cell membrane, shrinking chromatin, and the formation of symbolic apoptotic bodies. Cell necrosis and pyroptosis showed obvious cell swelling and moderate chromatin concentration, while ferroptosis had no obvious apoptotic characteristics, mainly showing smaller mitochondria, decreased mitochondrial cristae, and increased mitochondrial membrane density. As for ferroptosis, the ultrastructural changes of mitochondrial membrane concentration and rupture are considered as the unique morphological signs (Battaglia et al., 2020).
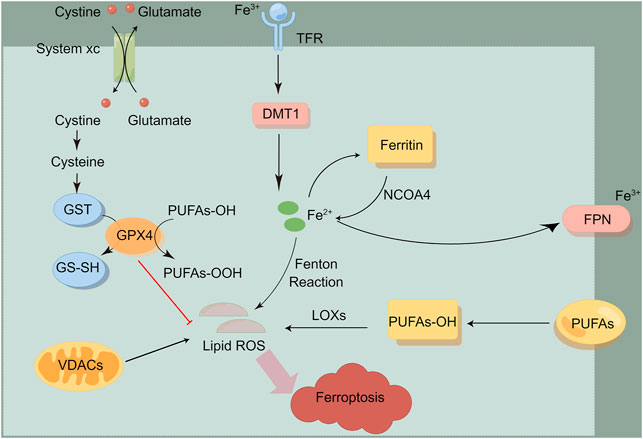
FIGURE 1. The overview of ferroptosis. Ferroptosis is mainly induced by iron metabolism, lipid metabolism, amino acid metabolism and other processes. This figure was drawn by Figdraw.
In recent years, an increasing numbers of natural and synthetic drugs related to ferroptosis have been identified, including inducers and inhibitors. The rational way to apply has become an important proposition to improve anti-tumor effectiveness. According to the mechanism of ferroptosis, Doxin et al. divided ferroptosis inducers (FINS) into four categories: targeting system Xc-, GPX4, iron and ROS respectively (Dixon et al., 2012). Currently, the drugs and chemicals used to induce and inhibit ferroptosis in cells are as followed (Tables 1, 2).
Ferroptosis is mainly induced by iron metabolism, lipid metabolism, amino acid metabolism and other processes. Previous studies have shown that the ferroptosis process can be induced by the two antagonistic processes of lipid peroxide production and elimination within cells. Many metabolic pathways include cellular respiration (i.e. mitochondrial tricarboxylic acid cycle (TCA) and electron transfer chain, ETC), lipid metabolism and amino acid metabolism, which can lead to ferroptosis by producing reactive oxygen species (ROS). In addition, iron metabolism may also induce ferroptosis through Fenton reaction, which produces lipid peroxide (Battaglia et al., 2020; Lu et al., 2018). At present, metabolic pathways related to ferroptosis are shown below.
Iron is an indispensable trace element in life body, and free ferrous ions act as the leading role of ferroptosis. Excessive ferrous ions will undergo Fenton reaction and lead to ROS accumulation, which can be oxidized with polyunsaturated fatty acids (PUFA), resulting in massive accumulation of lipid peroxide, thus causing DNA damage. Ferrous ions often exist in ferritin to form unstable iron pools. When iron ions overload and exceed the buffering capacity of ferritin, ferritin autophagy will occur, which is mediated by nuclear receptor coactivation factor 4 (NCOA4) (Latunde-Dada, 2017). Over-expression of NOCA4 will increase concentration of intracellular free iron, then promote ferroptosis. Recent studies have shown that cytoplasmic iron chaperone poly (rC) binding protein 1 (PCBP1) can inhibit the ferroptosis process mediated by iron protein phagocytosis in head and neck cancer (Lee et al., 2022). Genes related to iron metabolism, such as transferrin (TF), transferrin receptor 1 (TFR1), iron transporter (FPN), divalent metal transporter 1 (DMT1), ferritin heavy chain 1(FTH1) and ferritin light chain (FTL), are key mediators in the process of ferroptosis. As an iron transporter, transferrin mediates iron uptake or iron autophagy, and introduces iron into cells from the extracellular environment by recognizing transferrin receptor 1 (TFR1), while down-regulating the expression of transferrin receptor (TFRC) can inhibit the occurrence of ferroptosis (Bogdan et al., 2016). Ferroportin-1(FPN) is an iron transporter responsible for removing iron from cells (Dixon et al., 2012; Ma et al., 2016). Recently, it has been reported that heat shock protein β-1 (HSPB1) reduces the intracellular iron concentration by inhibiting the expression of TRF1, thus inhibiting the ferroptosis process (Battaglia et al., 2020). In addition, heme is a source of iron ions within cells, and heme oxygenase-1 (HO-1) can decompose heme and release iron ions, and regulate the process of ferroptosis (Kwon et al., 2015). Nuclear factor-erythroid 2-related factor 2 (Nrf2) dissociates from Keap1 when receiving external stimulation, transfers to the nucleus, binds to the promoter region, and activates the downstream molecule HO-1 (Dodson et al., 2019).
As for inducing cell damage, lipid peroxide can be regarded as the “executor” of ferroptosis. Lipid peroxide causes cell damage through the following ways: First, the lipid peroxide produced by Fenton reaction reacts with ferrous iron to produce ROS, which aggravates the ferroptosis cycle; Second, 4-HNE and MDA, the aldehyde degradation products of lipid peroxide, generate cytotoxicity; Last, peroxide reaction interferes with the permeability and fluidity of cell membrane (Gaschler and Stockwell, 2017). Among them, the activation of cyclooxygenase (COX), lipoxygenase (LOX), acyl-CoA synthetase long chain family member 4 (ACSL4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3) play an important role in lipid oxidation and anabolism (Liu et al., 2022). ACSL4 promotes the formation of phytosterol ester esterified by arachidonic acid (AA) and adrenaline, and participates in the ferroptosis process. According to the research, the tumor suppressor miR-424-5p directly binds to the 3′-UTR of ACSL4 to inhibit the process of ferroptosis (Cheng et al., 2020). Vitamin E acts as a potent inhibitor of ferroptosis by inhibiting LOX activity (Kagan et al., 2017).
Cystine/glutamic acid reverse transporter (system Xc-) is composed of light chain subunits (xCT,SLC7A11) and heavy chain subunits (CD98hc,SLC3A2), of which SLC7A11 is the active part. System Xc- mediates extracellular cystine to enter cells to synthesize glutathione (GSH). GSH can be converted into oxidized glutathione (GSSG) via GPX4 (Glutathione peroxidase 4), restoring active oxygen and active nitrogen, thus limiting the spread of lipid peroxidation in the membrane and alleviating the oxidative stress damage caused by ferroptosis (Dixon et al., 2012). GPX4 is a key inhibitor of ferroptosis and constitutes the defense system of ferroptosis. There are reports that RAS selective lethal small molecule 3 (RSL3) binds to GPX4 and inhibits its activity (Dixon et al., 2012; Yang et al., 2014). Erastin can target system Xc and selectively induce ferroptosis (Wang et al., 2020). Therefore, the inhibition of Systeam Xc-, the blocking of GSH synthesis and the inactivation of GPX4 may serve as an important target for inducing ferroptosis in tumor cells.
The inhibition of ferroptosis by ferroptosis inhibitor protein (FSP1) is mediated by ubiquitin (also known as coenzyme Q10, CoQ10). The reduced form of ubiquitin can capture lipid peroxidation free radicals, while FSP1 catalyzes the regeneration of CoQ10 through NAD(P)H. The FSP1-CoQ10-NAD(P)H pathway acts as an independent parallel system, which collaboratively inhibits ferroptosis together with GPX4 and glutathione (Doll et al., 2019). In addition, recent studies have confirmed GTP cyclohydrolase I (GCH1) to be another important regulator of ferroptosis.
In the process of tumor occurrence and development, it is often accompanied by the imbalance of redox environment and the high demand for iron ions, suggesting that tumor cells are highly sensitive to iron death. We have discussed the metabolic pathway, the application of inducers and inhibitors of ferroptosis, then the regulation of ferroptosis in multiple cancers will be introduced.
Ovarian cancer is a highly malignant tumor, which usually exhibit a high demand for iron. It is manifested by up-regulation of transferrin receptor and down-regulation of membrane iron transporter and ferritin, suggesting its high sensitivity to ferroptosis. The synergistic effect of Erastion and various chemotherapeutic drugs can overcome the drug resistance in ovarian cancer cells (Li et al., 2021b). According to recent studies, ferroptosis is considered to mediate the efficacy of Olapani (a classic and effective PARP inhibitor) in vitro and in vivo. Researchers believe that pharmacological inhibition of PARP or gene deletion promotes lipid peroxidation and ferroptosis in ovarian cancer cells. The efficacy and safety of the triple therapy of Olapani, FINs and DNA damage induction in BRCA wild-type ovarian cancer deserve further exploration (Hong et al., 2021).
Studies have confirmed that ferroptosis occurs when liver cancer cells are treated with sorafenib. Sigma-1 receptor (S1R) is an important negative regulator of ferroptosis in liver cancer cells, and regulates many targets of iron death, such as GPX4 (Bai et al., 2019). In addition, the activation of P62-Keap1-NRF2 antioxidant pathway can resist ferroptosis in liver cancer cells, and the inhibition of this pathway significantly enhances the anticancer activities of erastin and sorafenib in vitro and in vivo (Sun et al., 2016).
In the treatment of advanced gastric cancer, cisplatin and paclitaxel, as clinical first-line chemotherapy drugs, have increasingly serious drug resistance, and the targeted ferroptosis pathway has brought a new dawn. Arachidonic acid lipoxygenase-15 (ALOX-15) is the main producer of ROS in cells, which is obviously down-regulated in gastric cancer cells and is found to inhibit ferroptosis. While miR-522 secreted by tumor-associated fibroblasts (CAFs) regulates the expression of ALOX15, it can provide a new way to improve the sensitivity of chemotherapy in exploring the mechanism of regulating ferroptosis by exosomes (Zhang et al., 2020).
Studies have shown that the activity of polyunsaturated lipid synthase increases when colorectal cancer cells are in a highly interstitial state, indicating the potential link between interstitial state and lipid peroxidase pathway (Viswanathan et al., 2017). In addition, cetuximab is prone to drug resistance in the treatment of colon cancer, while its combination with ferroptosis inducer β-elemene can sensitize KRAS mutant colorectal cancer cells via inducing ferroptosis and inhibiting epithelial-mesenchymal transition (EMT) and is expected to provide a prospective treatment strategy for CRC patients with RAS mutant (Chen et al., 2020b).
At first, Doxin et al. found small molecules that can specifically kill RAS mutant cancer cells through ferroptosis, such as erastin and RSL3. Coincidentally, about 90% of pancreatic vessel element’s carcinoma (PDAC) has KRAS gene mutation (Mizrahi et al., 2020). The artemisinin and its derivatives can promote the occurrence of ferroptosis in PDAC by inducing ROS production (Eling et al., 2015). In addition, HSPA5 inhibits ferroptosis by directly inhibiting the degradation of GPX4 protein in human PDAC cells, and the up-regulation of HSPA5-GPX4 pathway contributes to the drug resistance of gemcitabine. And the inhibition of ATF4-HSPA5 pathway can enhance the ferroptosis induced by erastin (Zhu et al., 2017).
As the nervous system contains the highest content of polyunsaturated fatty acids in human body, which is the main substrate for peroxide production, ferroptosis in brain tumors has gradually attracted attention. The temozolomide (TMZ) has been applied in the treatment of glioblastoma (GBM), and TMZ can selectively induce glioma stem cells (GSCs) to ferroptosis during treatment (Xu et al., 2019).
Immunotherapy can act on the immune system through “passive” or “active” therapy. Passive therapy includes the application of cytokines, antibodies and immune cells, which directly acts on the immune cells within tumor micro-environment. Active therapy involves stimulating the immune system to eliminate cancer cells (such as vaccines) (Peng et al., 2019). Up to now, anti-tumor immunotherapy includes the following means.
Cytokines are protein secreted by immune cells and other cells, which restrict the tumor growth through direct anti-proliferation or pro-apoptosis activity or stimulating the cytotoxic activity of immune cells to tumor cells (Berraondo et al., 2019). Cytokines include tumor necrosis factor (TNF)-α, interferon (IFN) and IL-2, etc. IL-2 is a T cell growth factor, which participates in the growth and expansion of immune cells such as T cells, NK cells and B cells. It is one of the most widely studied cytokines and immunotherapy agents, and is used to treat cancers and other diseases. At present, IL-2 has been approved by FDA to treat melanoma and renal cell carcinoma. However, due to the serious systemic toxicity and limited curative effect of IL-2, researchers turned to explore optimized schemes, such as combination therapy. There are studies that combined treatment of aerosol IL-2 and NK cells can enhance the therapeutic effect in lung metastatic tumors (Dhupkar and Gordon, 2017).
Antibody therapy is the most mature cancer-specific immunotherapy in clinical practice at present. Rituximab is the first monoclonal antibody approved by FDA for anti-tumor immunotherapy, which targets CD20 antigen and is used to treat B-cell non-Hodgkin’s lymphoma (Sanchez-Martin et al., 2015). With the development of research, besides directly targeting tumor surface antigen, targeting key signal pathway of tumor to diminish tumor growth environment and immune checkpoint blocking therapy are also being under road (Helmy et al., 2013). As for immune checkpoint blocking therapy, the current breakthrough is mainly the recognition and targeting role in T cells by antibodies of CTLA-4, PD-1 or PD-L1 (van den Bulk et al., 2018).
The main principle of adoptive cell transfer therapy (ACT) is to inject immune cells into the patient’s body after in vivo immunization or in vitro culture activation, so as to enhance the patient’s immunity. At present, ACT is mostly carried out in hematological malignancies, while CAR-T cell technology has also been conducted in sarcoma targeting ERBB2/HER2, renal cell carcinoma targeting carbonic anhydrase, and cholangiocarcinoma targeting epidermal growth factor (Rohaan et al., 2019).
The biomarker of ferroptosis is of great significance for predicting curative effect and realizing subsequent precise molecular targeted therapy. Ferroptosis-related genes (FRGs) can be divided into genes about iron metabolism, lipid metabolism, oxidant metabolism and energy metabolism. Recent studies have shown that FRGs features can predict the prognosis in various kinds of tumors (Wan et al., 2021; Wang et al., 2021). For example, when constructing the characteristic risk model of ferroptosis in glioma, most selected FRGs were differentially expressed in patients with different pathological grades and non-tumor control groups. It is reported that in the prognosis model of hepatocellular carcinoma, the genes (NFS1, CISD1, ACSL3, NQO1, SLC7A11, GPX4) that can protect hepatocytes with ferroptosis and the genes (ACACA, CARS, G6PD, SLC1A5) that induce ferroptosis are all up-regulated in HCC, and are related to poor prognosis (Liang et al., 2020). This suggests that constructing a gene characteristic model related to ferroptosis has shown predictive value for tumor prognosis.
In the model of ferroptosis-related gene features, there is positive correlation with the enrichment scores of ICB-related positive features in the high-risk scoring group. In addition, researchers observed that immune signals were related to different risk scores of glioma, showing increase of PD-L1, PD-1, CTLA-4, IDO-1, TMB and immunogenic mutations in high-risk scoring groups (Wan et al., 2021). In the model constructed in breast cancer, the antigen presentation level and Th1 cell level of high-risk population are elevated, suggesting significant change of related genes, which may be related to the over-activation of immune system (Wang et al., 2021). Generally, these studies indicate that the development of FRGs-related risk score may help predict the anti-tumor effect of immunotherapy.
Tumors usually show low immunogenicity to escape the recognition of immune cells. Ferroptosis-related lipid peroxide can recognize dendritic cells, phagocytosis and processing of tumor antigens, and present tumor-related antigens to CD8+ lymphocytes, activating cytotoxic T lymphocytes to aid anti-tumor immunotherapy (Zhao et al., 2022). Ferroptosis plays an important role in the activity and function of tumor-associated macrophages (TAM) in TME. TAM mainly exhibits M2 subtype, which inhibits anti-tumor immunity. However, M1 subtype with higher anti-tumor activity showed higher resistance to ferroptosis caused by deletion of GPX4. There is evidence that targeting GPX4 in TAM can inhibit the survival of M2 subtype, while keeping the number of M1 unaffected, thus reversing the immunosuppressive state (Xu et al., 2021a). At the same time, some studies have found that ferroptosis promotes the release of KrasG12D, which contributes to M2 polarization and stimulates tumor growth through STAT3-dependent fatty acid oxidation pathway (Liu et al., 2021b). Therefore, the dual effects of ferroptosis on TAM need to be further studied. T-reg cells are thought to impair anti-tumor immunity. Although ferroptosis is rare in T-reg cells, recent studies have shown that targeting GPX4 can disturb the immune homeostasis, promote the production of IL-1β and Th17 cell reaction, thus enhancing the anti-tumor immune function (Xu et al., 2021b).
Recently, it has been found that CD8+T cells can enhance lipid peroxidation caused by ferroptosis in tumor cells. Meanwhile, ferroptosis improves the anti-tumor effect of immunotherapy (Wang et al., 2019). This suggests a novel therapeutic strategy. CD8+T cells activated by anti-PD-L1 immunotherapy secrete IFN-γ, which significantly down-regulates the expression of SLC3A2 and SLC7A11 in tumor cells, resulting in the decrease of cystine uptake, thus promoting the occurrence of ferroptosis. The results showed that cystine protease inhibitor could cooperate with anti-PD-L1 to induce effective anti-tumor immunity through ferroptosis (Wang et al., 2019). Based on the development of immunogenic cell death (ICD) in anti-cancer experiments, researchers have found that many cancers can produce necrotic apoptosis resistance. Recently, the research on the immunogenicity of ferroptosis made rapid progress. Cancer cells with early ferroptosis can effectively induce ICD in vivo and in vitro by activating bone marrow-derived dendritic cells BMDCs and triggering DAMP (e.g. ATP and HMGB1), resulting in protective immunity against the attack on cancer cells, which boost the development of anti-cancer immunotherapy strategies based on ferroptosis cell vaccination (Efimova et al., 2020). Recent studies have shown that RSL-3-rich nanoparticles can promote the immunogenic death of tumor cells, while the combined treatment of blocking PD-L1 further enhanced the T lymphocytes infiltration within tumors (Song et al., 2021).
Studies have shown that the synergistic effect of radiotherapy and immunotherapy is related to the increased sensitivity to ferroptosis. The anti-tumor effect of radiotherapy is not only related to DNA damage, but also lipid peroxidation caused by ferroptosis. Radiotherapy and immunotherapy can induce ferroptosis of tumor cells by activating kinases ATM and IFN-γ through DNA damage, respectively (Lang et al., 2019). In addition, inhibiting SLC7A11 or GPX4 combined with FINs can significantly improve the curative effect of radiotherapy and reverse radiation resistance (Lei et al., 2020). For those tumors that show anti-ferroptosis characteristics, combining FINs with immunotherapy and radiotherapy may be an effective strategy to sensitize such tumors. However, whether this triple therapy increases the toxicity of normal tissues remains to be determined.
In order to overcome the drug resistance of immunotherapy, researchers have focused on the emerging target of ferroptosis. Studies have shown that the expression of TYRO3 is increased in immunotherapy-resistant tumor cells, and TYRO3 signaling pathway inhibits the ferroptosis of tumor cells by up-regulating the expression of SLC3A2 and other genes. TYRO3 receptor tyrosine kinase inhibitors can effectively overcome the drug resistance of immunotherapy (Jiang et al., 2021). As mentioned above, the combination of β-elemene and cetuximab, an ferroptosis inducer, can induce ferroptosis in the colorectal cancer cell with KRAS mutation, thus relieving the drug resistance caused by cetuximab in CRC treatment (Chen et al., 2020a). In addition to inducing ferroptosis in tumor cells, ferroptosis will also occur in T cells themselves. Recent studies have shown that CD36-mediated ferroptosis of T cells inhibits the function of CD8+T cells in tumors. The combination of CD36 deletion and anti-PD-1 antibody has a better anti-tumor effect than single treatment (Ma et al., 2021). Therefore, targeting CD36 and ferroptosis may be an effective strategy to improve the anti-tumor efficacy of T cell-based immunotherapy.
In a word, different tumor cells exhibit differential sensitivity to immunotherapy and the combined therapeutic effect with ferroptosis on tumor cells is distinct. In immunoinflammatory tumors with a large number of CD8+T cells, ferroptosis inducers may diminish infiltrated T cells, weaken the maturation process and normal function of immature dendritic cells (DC), and thus reduce the efficacy of ICI immunotherapy. In some cases, ferroptosis inhibitors may be utilized to protect T cells from ferroptosis caused by specific TME. For immune-tolerant tumors, there are high levels of infiltrating myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs) or Tregs in TME. GPX4 inhibitor can eliminate M2 macrophages and Treg, while targeting system Xc- can alleviate MDSC-mediated uptake of tmecystine, thus ensuring the survival of T cells. However, for some immune-desert tumors lacking immune infiltration, the response to immunotherapy is poor, and chemotherapy and targeted drugs are used to counteract the resistance. Therefore, more attention should be paied to the research of ferroptosis in improving anti-tumor immunogenicity (Xu et al., 2021a).
In recent years, tumor immunotherapy represented by immune checkpoint inhibitors (ICIs) has received a lot of attention. However, it has little effect on some immune-desert tumors, and the application of ferroptosis may change TME state and enhance the therapeutic effect (Rosenbaum et al., 2021). The main mechanism and application progress of other cell death forms in combined immune checkpoint therapy go as follows (Table 3).
As a new way of cell death, ferroptosis is related to many physiological and pathological mechanisms, and become a hot spot in cancer treatment research. In addition, many tumors especially those with low levels of tumor infiltrating lymphocytes (TIL), are called “cold” tumors, and have poor response to ICI. Therefore, it is vital to develop new strategy to enhance anti-tumor immunity. Moreover, the defense mechanism of iron death in cells (especially mitochondria) has also been reported, which indicates the limitation and breakthrough of ferroptosis in immunotherapy (Battaglia et al., 2020). As the ferroptosis inducers/inhibitors have been widely illustrated and applied in pre-clinical study, the combined application of ferroptosis induction with tumor immunotherapy (especially ICI therapy) has shown promising prospective in clinical treatment. However, there are still some limitations of ferroptosis in immunotherapy, for example, the dual role of anti-/pro- immunity in ferroptosis, the different immune characteristics of tumor cells, and the complexity of TME all enhance the application difficulty, and more research should be conducted in the future.
JX and XL wrote the manuscript and discussed all sections of this review. XX and TL conceived of the topic and revised the review. TH, QZ, YS, and SJ collected the relevant literature and helped revise the manuscript.
This work was supported by Beijing Science and Technology Innovation Medical Development Foundation (KC2021-JX-0186-121), and Clinical Research Innovation Cultivation Fund Project (PY2018-IIA-04, Renji Hospital, Shanghai Jiao Tong University School of Medicine).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abrams, R. P., Carroll, W. L., and Woerpel, K. A. (2016). Five-membered ring peroxide selectively initiates ferroptosis in cancer cells. ACS Chem. Biol. 11 (5), 1305–1312. doi:10.1021/acschembio.5b00900
Bai, T., Lei, P., Zhou, H., Liang, R., Zhu, R., Wang, W., et al. (2019). Sigma-1 receptor protects against ferroptosis in hepatocellular carcinoma cells. J. Cell. Mol. Med. 23 (11), 7349–7359. doi:10.1111/jcmm.14594
Battaglia, A. M., Chirillo, R., Aversa, I., Sacco, A., Costanzo, F., and Biamonte, F. (2020). Ferroptosis and cancer: Mitochondria meet the "iron maiden" cell death. Cells 9 (6), 1505. doi:10.3390/cells9061505
Berraondo, P., Sanmamed, M. F., Ochoa, M. C., Etxeberria, I., Aznar, M. A., Pérez-Gracia, J. L., et al. (2019). Cytokines in clinical cancer immunotherapy. Br. J. Cancer 120 (1), 6–15. doi:10.1038/s41416-018-0328-y
Bogdan, A. R., Miyazawa, M., Hashimoto, K., and Tsuji, Y. (2016). Regulators of iron homeostasis: New players in metabolism, cell death, and disease. Trends biochem. Sci. 41 (3), 274–286. doi:10.1016/j.tibs.2015.11.012
Chen, G. Q., Benthani, F. A., Wu, J., Liang, D., Bian, Z. X., and Jiang, X. (2020a). Artemisinin compounds sensitize cancer cells to ferroptosis by regulating iron homeostasis. Cell Death Differ. 27 (1), 242–254. doi:10.1038/s41418-019-0352-3
Chen, P., Li, X., Zhang, R., Liu, S., Xiang, Y., Zhang, M., et al. (2020b). Combinative treatment of β-elemene and cetuximab is sensitive to KRAS mutant colorectal cancer cells by inducing ferroptosis and inhibiting epithelial-mesenchymal transformation. Theranostics 10 (11), 5107–5119. doi:10.7150/thno.44705
Chen, X., Kang, R., Kroemer, G., and Tang, D. (2021a). Broadening horizons: The role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 18 (5), 280–296. doi:10.1038/s41571-020-00462-0
Chen, X., Zeh, H. J., Kang, R., Kroemer, G., and Tang, D. (2021b). Cell death in pancreatic cancer: From pathogenesis to therapy. Nat. Rev. Gastroenterol. Hepatol. 18 (11), 804–823. doi:10.1038/s41575-021-00486-6
Chen, Y., Yi, X., Huo, B., He, Y., Guo, X., Zhang, Z., et al. (2022). BRD4770 functions as a novel ferroptosis inhibitor to protect against aortic dissection. Pharmacol. Res. 177, 106122. doi:10.1016/j.phrs.2022.106122
Cheng, J., Fan, Y. Q., Liu, B. H., Zhou, H., Wang, J. M., and Chen, Q. X. (2020). ACSL4 suppresses glioma cells proliferation via activating ferroptosis. Oncol. Rep. 43 (1), 147–158. doi:10.3892/or.2019.7419
Dhupkar, P., and Gordon, N. (2017). Interleukin-2: Old and new approaches to enhance immune-therapeutic efficacy. Adv. Exp. Med. Biol. 995, 33–51. doi:10.1007/978-3-319-53156-4_2
Dixon, S. J., Lemberg, K. M., Lamprecht, M. R., Skouta, R., Zaitsev, E. M., Gleason, C. E., et al. (2012). Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 149 (5), 1060–1072. doi:10.1016/j.cell.2012.03.042
Dodson, M., Castro-Portuguez, R., and Zhang, D. D. (2019). NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 23, 101107. doi:10.1016/j.redox.2019.101107
Doll, S., Freitas, F. P., Shah, R., Aldrovandi, M., da Silva, M. C., Ingold, I., et al. (2019). FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575 (7784), 693–698. doi:10.1038/s41586-019-1707-0
Doll, S., Proneth, B., Tyurina, Y. Y., Panzilius, E., Kobayashi, S., Ingold, I., et al. (2017). ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 13 (1), 91–98. doi:10.1038/nchembio.2239
Efimova, I., Catanzaro, E., Van der Meeren, L., Turubanova, V. D., Hammad, H., Mishchenko, T. A., et al. (2020). Vaccination with early ferroptotic cancer cells induces efficient antitumor immunity. J. Immunother. Cancer 8 (2), e001369. doi:10.1136/jitc-2020-001369
Eling, N., Reuter, L., Hazin, J., Hamacher-Brady, A., and Brady, N. R. (2015). Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells. Oncoscience 2 (5), 517–532. doi:10.18632/oncoscience.160
Fan, B. Y., Pang, Y. L., Li, W. X., Zhao, C. X., Zhang, Y., Wang, X., et al. (2021). Liproxstatin-1 is an effective inhibitor of oligodendrocyte ferroptosis induced by inhibition of glutathione peroxidase 4. Neural Regen. Res. 16 (3), 561–566. doi:10.4103/1673-5374.293157
Gao, R., Kalathur, R., Coto-Llerena, M., Ercan, C., Buechel, D., Shuang, S., et al. (2021). YAP/TAZ and ATF4 drive resistance to Sorafenib in hepatocellular carcinoma by preventing ferroptosis. EMBO Mol. Med. 13 (12), e14351. doi:10.15252/emmm.202114351
Gaschler, M. M., and Stockwell, B. R. (2017). Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 482 (3), 419–425. doi:10.1016/j.bbrc.2016.10.086
Guo, J., Xu, B., Han, Q., Zhou, H., Xia, Y., Gong, C., et al. (2018). Ferroptosis: A novel anti-tumor action for cisplatin. Cancer Res. Treat. 50 (2), 445–460. doi:10.4143/crt.2016.572
Helmy, K. Y., Patel, S. A., Nahas, G. R., and Rameshwar, P. (2013). Cancer immunotherapy: accomplishments to date and future promise. Ther. Deliv. 4 (10), 1307–1320. doi:10.4155/tde.13.88
Hong, T., Lei, G., Chen, X., Li, H., Zhang, X., Wu, N., et al. (2021). PARP inhibition promotes ferroptosis via repressing SLC7A11 and synergizes with ferroptosis inducers in BRCA-proficient ovarian cancer. Redox Biol. 42, 101928. doi:10.1016/j.redox.2021.101928
Jiang, Z., Lim, S. O., Yan, M., Hsu, J. L., Yao, J., Wei, Y., et al. (2021). TYRO3 induces anti-PD-1/PD-L1 therapy resistance by limiting innate immunity and tumoral ferroptosis. J. Clin. Invest. 131 (8), e139434. doi:10.1172/JCI139434
Kagan, V. E., Mao, G., Qu, F., Angeli, J. P., Doll, S., Croix, C. S., et al. (2017). Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 13 (1), 81–90. doi:10.1038/nchembio.2238
Kwon, M. Y., Park, E., Lee, S. J., and Chung, S. W. (2015). Heme oxygenase-1 accelerates erastin-induced ferroptotic cell death. Oncotarget 6 (27), 24393–24403. doi:10.18632/oncotarget.5162
Lang, X., Green, M. D., Wang, W., Yu, J., Choi, J. E., Jiang, L., et al. (2019). Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Cancer Discov. 9 (12), 1673–1685. doi:10.1158/2159-8290.CD-19-0338
Latunde-Dada, G. O. (2017). Ferroptosis: Role of lipid peroxidation, iron and ferritinophagy. Biochimica et biophysica acta. General Subj. 1861 (8), 1893–1900. doi:10.1016/j.bbagen.2017.05.019
Lee, J., You, J. H., and Roh, J. L. (2022). Poly(rC)-binding protein 1 represses ferritinophagy-mediated ferroptosis in head and neck cancer. Redox Biol. 51, 102276. doi:10.1016/j.redox.2022.102276
Lei, G., Zhang, Y., Koppula, P., Liu, X., Zhang, J., Lin, S. H., et al. (2020). The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 30 (2), 146–162. doi:10.1038/s41422-019-0263-3
Li, J., Lama, R., Galster, S. L., Inigo, J. R., Wu, J., Chandra, D., et al. (2022a). Small-molecule MMRi62 induces ferroptosis and inhibits metastasis in pancreatic cancer via degradation of ferritin heavy chain and mutant p53. Mol. Cancer Ther. 21 (4), 535–545. doi:10.1158/1535-7163.MCT-21-0728
Li, L., Jiang, M., Qi, L., Wu, Y., Song, D., Gan, J., et al. (2021a). Pyroptosis, a new bridge to tumor immunity. Cancer Sci. 112 (10), 3979–3994. doi:10.1111/cas.15059
Li, L., Qiu, C., Hou, M., Wang, X., Huang, C., Zou, J., et al. (2021b). Ferroptosis in ovarian cancer: A novel therapeutic strategy. Front. Oncol. 11, 665945. doi:10.3389/fonc.2021.665945
Li, M., Meng, Z., Yu, S., Li, J., Wang, Y., Yang, W., et al. (2022b). Baicalein ameliorates cerebral ischemia-reperfusion injury by inhibiting ferroptosis via regulating GPX4/ACSL4/ACSL3 axis. Chem. Biol. Interact. 366, 110137. doi:10.1016/j.cbi.2022.110137
Li, Y., Wei, X., Tao, F., Deng, C., Lv, C., Chen, C., et al. (2021c). The potential application of nanomaterials for ferroptosis-based cancer therapy. Biomed. Mat. 16 (4), 042013. doi:10.1088/1748-605X/ac058a10.1088/1748-605X/ac058a
Liang, C., Zhang, X., Yang, M., and Dong, X. (2019). Recent progress in ferroptosis inducers for cancer therapy. Adv. Mat. 31 (51), e1904197. doi:10.1002/adma.201904197
Liang, J. Y., Wang, D. S., Lin, H. C., Chen, X. X., Yang, H., Zheng, Y., et al. (2020). A novel ferroptosis-related gene signature for overall survival prediction in patients with hepatocellular carcinoma. Int. J. Biol. Sci. 16 (13), 2430–2441. doi:10.7150/ijbs.45050
Liu, J., Kang, R., and Tang, D. (2021a). The art of war: Ferroptosis and pancreatic cancer. Front. Pharmacol. 12, 773909. doi:10.3389/fphar.2021.773909
Liu, L., Liu, R., Liu, Y., Li, G., Chen, Q., Liu, X., et al. (2021b). Cystine-glutamate antiporter xCT as a therapeutic target for cancer. Cell biochem. Funct. 39 (2), 174–179. doi:10.1002/cbf.3581
Liu, Y., Wang, W., Li, Y., Xiao, Y., Cheng, J., and Jia, J. (2015). The 5-lipoxygenase inhibitor zileuton confers neuroprotection against glutamate oxidative damage by inhibiting ferroptosis. Biol. Pharm. Bull. 38 (8), 1234–1239. doi:10.1248/bpb.b15-00048
Liu, Y., Zhou, L., Lv, C., Liu, L., Miao, S., Xu, Y., et al. (2022). PGE <sub>2</sub> pathway mediates oxidative stress-induced ferroptosis in renal tubular epithelial cells. FEBS J. doi:10.1111/febs.16609
Lu, B., Chen, X. B., Ying, M. D., He, Q. J., Cao, J., and Yang, B. (2018). The role of ferroptosis in cancer development and treatment response. Front. Pharmacol. 8, 992. doi:10.3389/fphar.2017.00992
Ma, S., Henson, E. S., Chen, Y., and Gibson, S. B. (2016). Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells. Cell Death Dis. 7 (7), e2307. doi:10.1038/cddis.2016.208
Ma, X., Xiao, L., Liu, L., Ye, L., Su, P., Bi, E., et al. (2021). CD36-mediated ferroptosis dampens intratumoral CD8+ T cell effector function and impairs their antitumor ability. Cell Metab. 33 (5), 1001–1012.e5. doi:10.1016/j.cmet.2021.02.015
Mao, C., Liu, X., Zhang, Y., Lei, G., Yan, Y., Lee, H., et al. (2021). DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 593 (7860), 586–590. doi:10.1038/s41586-021-03539-7
Mishima, E., Ito, J., Wu, Z., Nakamura, T., Wahida, A., Doll, S., et al. (2022). A non-canonical vitamin K cycle is a potent ferroptosis suppressor. Nature 608 (7924), 778–783. doi:10.1038/s41586-022-05022-3
Mizrahi, J. D., Surana, R., Valle, J. W., and Shroff, R. T. (2020). Pancreatic cancer. Lancet (London), Engl. 395 (10242), 2008–2020. doi:10.1016/S0140-6736(20)30974-0
Rohaan, M. W., Wilgenhof, S., and Haanen, J. (2019). Adoptive cellular therapies: The current landscape. Virchows Arch. 474 (4), 449–461. doi:10.1007/s00428-018-2484-0
Peng, M., Mo, Y., Wang, Y., Wu, P., Zhang, Y., Xiong, F., et al. (2019). Neoantigen vaccine: An emerging tumor immunotherapy. Mol. cancer 18 (1), 128. doi:10.1186/s12943-019-1055-6
Rosenbaum, S. R., Wilski, N. A., and Aplin, A. E. (2021). Fueling the fire: Inflammatory forms of cell death and implications for cancer immunotherapy. Cancer Discov. 11 (2), 266–281. doi:10.1158/2159-8290.CD-20-0805
Sánchez-Martín, D., Sørensen, M. D., Lykkemark, S., Sanz, L., Kristensen, P., Ruoslahti, E., et al. (2015). Selection strategies for anticancer antibody discovery: Searching off the beaten path. Trends Biotechnol. 33 (5), 292–301. doi:10.1016/j.tibtech.2015.02.008
Shimada, K., Skouta, R., Kaplan, A., Yang, W. S., Hayano, M., Dixon, S. J., et al. (2016). Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat. Chem. Biol. 12 (7), 497–503. doi:10.1038/nchembio.2079
Song, R., Li, T., Ye, J., Sun, F., Hou, B., Saeed, M., et al. (2021). Acidity-activatable dynamic nanoparticles boosting ferroptotic cell death for immunotherapy of cancer. Adv. Mat. 33 (31), e2101155. doi:10.1002/adma.202101155
Sun, X., Ou, Z., Chen, R., Niu, X., Chen, D., Kang, R., et al. (2016). Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 63 (1), 173–184. doi:10.1002/hep.28251
Tang, R., Xu, J., Zhang, B., Liu, J., Liang, C., Hua, J., et al. (2020). Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J. Hematol. Oncol. 13 (1), 110. doi:10.1186/s13045-020-00946-7
van den Bulk, J., Verdegaal, E. M., and de Miranda, N. F. (2018). Cancer immunotherapy: Broadening the scope of targetable tumours. Open Biol. 8 (6), 180037. doi:10.1098/rsob.180037
Viswanathan, V. S., Ryan, M. J., Dhruv, H. D., Gill, S., Eichhoff, O. M., Seashore-Ludlow, B., et al. (2017). Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 547 (7664), 453–457. doi:10.1038/nature23007
Wan, R. J., Peng, W., Xia, Q. X., Zhou, H. H., and Mao, X. Y. (2021). Ferroptosis-related gene signature predicts prognosis and immunotherapy in glioma. CNS Neurosci. Ther. 27 (8), 973–986. doi:10.1111/cns.13654
Wang, D., Wei, G., Ma, J., Cheng, S., Jia, L., Song, X., et al. (2021). Identification of the prognostic value of ferroptosis-related gene signature in breast cancer patients. BMC cancer 21 (1), 645. doi:10.1186/s12885-021-08341-2
Wang, L., Liu, Y., Du, T., Yang, H., Lei, L., Guo, M., et al. (2020). ATF3 promotes erastin-induced ferroptosis by suppressing system Xc. Cell Death Differ. 27 (2), 662–675. doi:10.1038/s41418-019-0380-z
Wang, Q., Wang, F., Zhao, Y., and Tan, G. (2022). Necroptosis is related to anti-PD-1 treatment response and influences the tumor microenvironment in head and neck squamous cell carcinoma. Front. Genet. 13, 862143. doi:10.3389/fgene.2022.862143
Wang, W., Green, M., Choi, J. E., Gijón, M., Kennedy, P. D., Johnson, J. K., et al. (2019). CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 569 (7755), 270–274. doi:10.1038/s41586-019-1170-y
Wang, Z., Ding, Y., Wang, X., Lu, S., Wang, C., He, C., et al. (2018). Pseudolaric acid B triggers ferroptosis in glioma cells via activation of Nox4 and inhibition of xCT. Cancer Lett. 428, 21–33. doi:10.1016/j.canlet.2018.04.021
Wu, J., Ma, L., Wang, J., and Qiao, Y. (2020). [Mechanism of ferroptosis and its research progress in lung cancer]. Zhongguo fei ai za zhi = Chin. J. lung cancer 23 (9), 811–817. doi:10.3779/j.issn.1009-3419.2020.104.16
Xu, C., Sun, S., Johnson, T., Qi, R., Zhang, S., Zhang, J., et al. (2021a). The glutathione peroxidase Gpx4 prevents lipid peroxidation and ferroptosis to sustain Treg cell activation and suppression of antitumor immunity. Cell Rep. 35 (11), 109235. doi:10.1016/j.celrep.2021.109235
Xu, H., Ye, D., Ren, M., Zhang, H., and Bi, F. (2021b). Ferroptosis in the tumor microenvironment: Perspectives for immunotherapy. Trends Mol. Med. 27 (9), 856–867. doi:10.1016/j.molmed.2021.06.014
Xu, T., Ding, W., Ji, X., Ao, X., Liu, Y., Yu, W., et al. (2019). Molecular mechanisms of ferroptosis and its role in cancer therapy. J. Cell. Mol. Med. 23 (8), 4900–4912. doi:10.1111/jcmm.14511
Yamaguchi, Y., Kasukabe, T., and Kumakura, S. (2018). Piperlongumine rapidly induces the death of human pancreatic cancer cells mainly through the induction of ferroptosis. Int. J. Oncol. 52 (3), 1011–1022. doi:10.3892/ijo.2018.4259
Yang, C., Han, M., Li, R., Zhou, L., Zhang, Y., Duan, L., et al. (2021). Curcumin nanoparticles inhibiting ferroptosis for the enhanced treatment of intracerebral hemorrhage. Int. J. Nanomedicine 16, 8049–8065. doi:10.2147/IJN.S334965
Yang, W. S., SriRamaratnam, R., Welsch, M. E., Shimada, K., Skouta, R., Viswanathan, V. S., et al. (2014). Regulation of ferroptotic cancer cell death by GPX4. Cell 156 (1-2), 317–331. doi:10.1016/j.cell.2013.12.010
Yang, W., Wang, Y., Zhang, C., Huang, Y., Yu, J., Shi, L., et al. (2022). Maresin1 protect against ferroptosis-induced liver injury through ROS inhibition and Nrf2/HO-1/GPX4 activation. Front. Pharmacol. 13, 865689. doi:10.3389/fphar.2022.865689
Yang, Y., Luo, M., Zhang, K., Zhang, J., Gao, T., Connell, D. O., et al. (2020). Nedd4 ubiquitylates VDAC2/3 to suppress erastin-induced ferroptosis in melanoma. Nat. Commun. 11 (1), 433. doi:10.1038/s41467-020-14324-x
Ye, L., Jin, F., Kumar, S. K., and Dai, Y. (2021). The mechanisms and therapeutic targets of ferroptosis in cancer. Expert Opin. Ther. Targets 25 (11), 965–986. doi:10.1080/14728222.2021.2011206
Zhang, H., Deng, T., Liu, R., Ning, T., Yang, H., Liu, D., et al. (2020). CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol. Cancer 19 (1), 43. doi:10.1186/s12943-020-01168-8
Zhao, L., Zhou, X., Xie, F., Zhang, L., Yan, H., Huang, J., et al. (2022). Ferroptosis in cancer and cancer immunotherapy. Cancer Commun. 42 (2), 88–116. doi:10.1002/cac2.12250
Keywords: ferroptosis, anti-tumor immunotherapy, review, ferroptosis inducer and inhibitor, metabolic pathway
Citation: Xu J, Lin X, Han T, Zhou Q, Su Y, Jiang S, Xiao X and Liu T (2022) Regulation mechanism of ferroptosis and its research progress in tumor immunotherapy. Front. Mol. Biosci. 9:1045548. doi: 10.3389/fmolb.2022.1045548
Received: 15 September 2022; Accepted: 11 October 2022;
Published: 25 October 2022.
Edited by:
Deshui Jia, Shanghai General Hospital, ChinaReviewed by:
Mingxia Yan, Fudan University, ChinaCopyright © 2022 Xu, Lin, Han, Zhou, Su, Jiang, Xiao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiuying Xiao, eGlhb3hpdXlpbmcyMDAyQDE2My5jb20=; Tengfei Liu, bGl1dGVuZ2ZlaTIyN0AxMjYuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.