- 1Tumor Etiology and Screening Department of Cancer Institute and General Surgery, The First Hospital of China Medical University, Shenyang, China
- 2Key Laboratory of Cancer Etiology and Prevention in Liaoning Education Department, The First Hospital of China Medical University, Shenyang, China
- 3Key Laboratory of GI Cancer Etiology and Prevention in Liaoning Province, The First Hospital of China Medical University, Shenyang, China
Background: Dysregulated expression of TRIB3 and FABP1 have been previously observed in human cancer tissues. However, there are little information as to their expression change in dynamic gastric diseases and the functional roles.
Methods: Tissues from a total of 479 patients, including 89 GS, 102 IM-GA, 144 EGC, and 144 AGC were collected. The protein expressions of TRIB3 and FABP1 were detected by immunohistochemical staining. Meanwhile, the potential functions of TRIB3 and FABP1 in GC were further analyzed by R software and some internet public databases, such as TCGA and DAVID.
Results: During this multi-stage process that go through GS to EGC, the expression trend of TRIB3 and FABP1 protein was GS > IM-GA > EGC. Besides, the expression of TRIB3 protein continued to decrease in AGC, while the expression of FABP1 was abnormally increased. Hp infection was significantly associated with the decreased expression of TRIB3 and FABP1. In addition, the diagnostic efficiency of the combination of these two indicators to diagnose EGC was higher than that of a single indicator. Survival analysis showed that higher expression of TRIB3 or FABP1 could indicate a better prognosis of GC. The protein expressions of TRIB3 and FABP1 were significantly positively correlated. Moreover, CEACAM5 and PRAP1 were positively correlated with both TRIB3 and FABP1 expressions, while GABRP and THBS4 were negatively correlated. The macrophages M0 infiltration was positively correlated with both TRIB3 and FABP1 expressions.
Conclusion: The protein expressions of TRIB3 and FABP1 gradually decreased with the gastric disease progress, and was positively correlated. Hp infection may reduce the protein expression of TRIB3 and FABP1. Combing TRIB3 and FABP1 expressions can improve the diagnostic efficiency for EGC. Either a high expression of TRIB3 or FABP1 indicates a better prognosis for GC. TRIB3 and FABP1 may interact with CEACAM5, PRAP1, GABRP and THBS4, and affect tumor immune microenvironment by regulating immune cells, and participate in the development and progression of GC.
1 Background
Gastric cancer (GC) is one of the most common malignant tumors of the digestive tract and ranked fourth in morbidity and second in mortality among all tumor types (Topi et al., 2020). Generally, GC arises from a series of diseases such as chronic superficial gastritis (GS), intestinal metaplasia atrophic gastritis (IM-GA), and dysplasia (Valenzuela et al., 2015). With the rapid progress in molecular biology, more and more evidence has shown that some crucial genes might be involved in this multi-stage process (Joo et al., 2001). Therefore, it is very necessary to identify key molecules or prognostic biomarkers during the dynamic development of gastric diseases.
TRIB3, which belongs to the tribbles pseudokinase family, is encoded by the six exons (20p13-p12.2) located on human chromosome 20. It’s a protein of about 65 kDa containing 358 amino acids (Huang et al., 2003; Wu et al., 2003; Kiss-Toth et al., 2004). Studies have shown that TRIB3 might be an oncogene or a tumor suppressor depending on the specific tumor types (Mondal et al., 2016). The overexpression of TRIB3 in lung and colorectal cancer can regulate some important cancer signaling pathways (Wennemers et al., 2011a; Izrailit et al., 2013). Wennemer et al found that the high expression of TRIB3 was significantly associated with better prognosis in breast cancer (Wennemers et al., 2011b).
Liver fatty acid binding protein (FABP1) is a soluble protein of 14 kDa. It is widely distributed in the cytoplasm of hepatocytes, but seldom detected in the nucleus or mitochondrial outer membrane (Börchers et al., 1989; Bordewick et al., 1989; Fahimi et al., 1990). FABP1 also presents in many other cell types, such as intestinal cells, renal tubular cells, and alveolar epithelial cells (Ho and Storch, 2001; Haunerland and Spener, 2004; Chmurzynska, 2006; Furuhashi and Hotamisligil, 2008), and plays a key role in the storage and degradation of fatty acids (Georgiadi and Kersten, 2012). It has been demonstrated that FABP1 was downregulated in nearly 10% of hepatocellular carcinomas (Inoue et al., 2014). The low expression of FABP1 in adenoma was related to the occurrence and development of colorectal cancer (Lee et al., 2006). In addition, FABP1 was found significantly downregulated in kidney cancer and its low expression indicated poor disease-free survival (Wu et al., 2020).
Our previous studies have suggested that TRIB3 and FABP1 were differentially expressed in GA vs GC, and they may interact at protein level. These results suggested that they might participate in the progress of gastric diseases (Liu et al., 2020). However, the correlation between TRIB3 and FABP1 remains undefined, and the expression characteristics during gastric carcinogenesis, as well as the clinical value and potential functions of these two proteins, and remain largely unknown.
Therefore, our study detected the protein expression of TRIB3 and FABP1 in GS, IM-GA, early gastric cancer (EGC) and advanced gastric cancer (AGC) by immunohistochemical staining, aimed to clarify their correlation and roles in gastric carcinogenesis, including their associations with clinicopathological features of GC, and their diagnostic and prognostic values. We further explored the potential functions of TRIB3 and FABP1 by bioinformatics. Our study would lay firm foundations for further exploring the underlying mechanism of the involvement of TRIB3 and FABP1 in the initiation and development of GC.
2 Materials and Methods
2.1 Patients and Tissue Specimens
A total of 479 patients who were diagnosed in the First Affiliated Hospital of China Medical University from December 2012 to December 2018 were enrolled, including 89 patients with GS, 102 patients with IM-GA, 144 patients with EGC and 144 patients with AGC. Tumor tissues and their corresponding adjacent IM and distal normal (NO) tissue specimens were obtained from 47 AGC patients. The Helicobacter pylori (Hp) infection status was detected using ASSURE Hp rapid test (MP DiagnosticsTM). Histology classification was determined according to the updated Sydney System for gastritis (Dixon et al., 1996). The diagnosis of GC was based on the World Health Organization criteria (4th edition, 2010). The TNM staging of GC was determined in accordance with the NCCN Clinical Practice Guidelines (2nd Edition, 2018).
The follow-up was continued until November 2019 and the time of it ranged from 11 months to 83 months. Totally, 112 cases were included for prognosis analysis and 58 of them died at data cut-off date, with a median survival time of 30.1 months. Approval was obtained from the Research Medical Ethics Committee of the First Affiliated Hospital of China Medical University, and each subject provided written informed consent.
2.2 Immunohistochemistry Staining
IHC was performed mainly as previously described (Yang et al., 2018). 4-µm-thick tissues were mounted in poly-l-lysine-coated glass slides and baked overnight at 65°C. After deparaffinization with xylene and hydration with graded ethanol, tissue sections were heated in EDTA buffer for 20 min for antigen retrieval. The 10% normal goat serum was used for incubation for 15 min to reduce non-specific binding. Then sections were then incubated with primary antibody for 60 min at room temperature (24–27°C) and secondary antibody for 10 min. At last, the slides were stained with DAB (DAB-1031, Maxim Inc., Fujian, China) and counterstained with hematoxylin. The anti-TRIB3 (ab-137526, 1:1,000 dilution; Abcam, Cambridge, United Kingdom) and anti-FABP1 (ab-171739, 1:4,000 dilution; Abcam, Cambridge, United Kingdom) antibodies were used in our experiment.
2.3 Evaluation of IHC Staining
Two pathologists individually evaluated the protein expression of TRIB3 and FABP1 in different tissues through multiplying the staining intensity by the proportion of stained cells to get the final immunoreactivity score (IS) without knowing their clinical information. The staining intensity of cells was classified into 0 (no staining), 1 (light brown), 2 (brown staining), and 3 (heavy brown staining); the proportion of stained cells were recorded as 0 (0–5%), 1 (6–25%), 2 (26–50%), 3 (51–75%), and 4 (76–100%). We used IS = 0 (the median of immunohistochemical staining scores) to distinguish between the negative and positive expression of TRIB3 and FABP1.
2.4 Analysis of Biological Functions
R software (R 4.0.3) was applied for co-expression analysis utilizing TCGA data normalized by the log2 (TPM (Transcripts per million) +1] transformation. DAVID (https://david.ncifcrf.gov/home.jsp) website was chosen for GO and KEGG pathway analysis. Cibersort was applied to estimate the percentage of different infiltrative immunocytes of each tumor sample of TCGA STAD dataset.
2.5 Statistical Analysis
All statistical analyses were performed using SPSS software (version 26.0) or R software (R 4.0.3). χ2 test was applied to assess the differences in age, gender and Hp infection between groups. Non-parametric test was applied to evaluate the differential expression of TRIB3 and FABP1 among different gastric diseases and the relationship of their expression with clinicopathological parameters. Paired non-parametric test was selected for comparing the expression of TRIB3 and FABP1 among GC, adjacent IM-GA, and distal NO of the same person. The relationship between TRIB3 and FABP1 protein expressions was assessed by Spearman correlation analysis. Receiver operating characteristic (ROC) curves were used to evaluate the diagnostic values of these two proteins. Survival analysis was evaluated by univariate (Log-rank) and multivariate (Cox model) analysis. The p-value < 0.05 was considered statistically significant.
3 Results
3.1 The Protein Expression of TRIB3 and FABP1 in Different Gastric Diseases
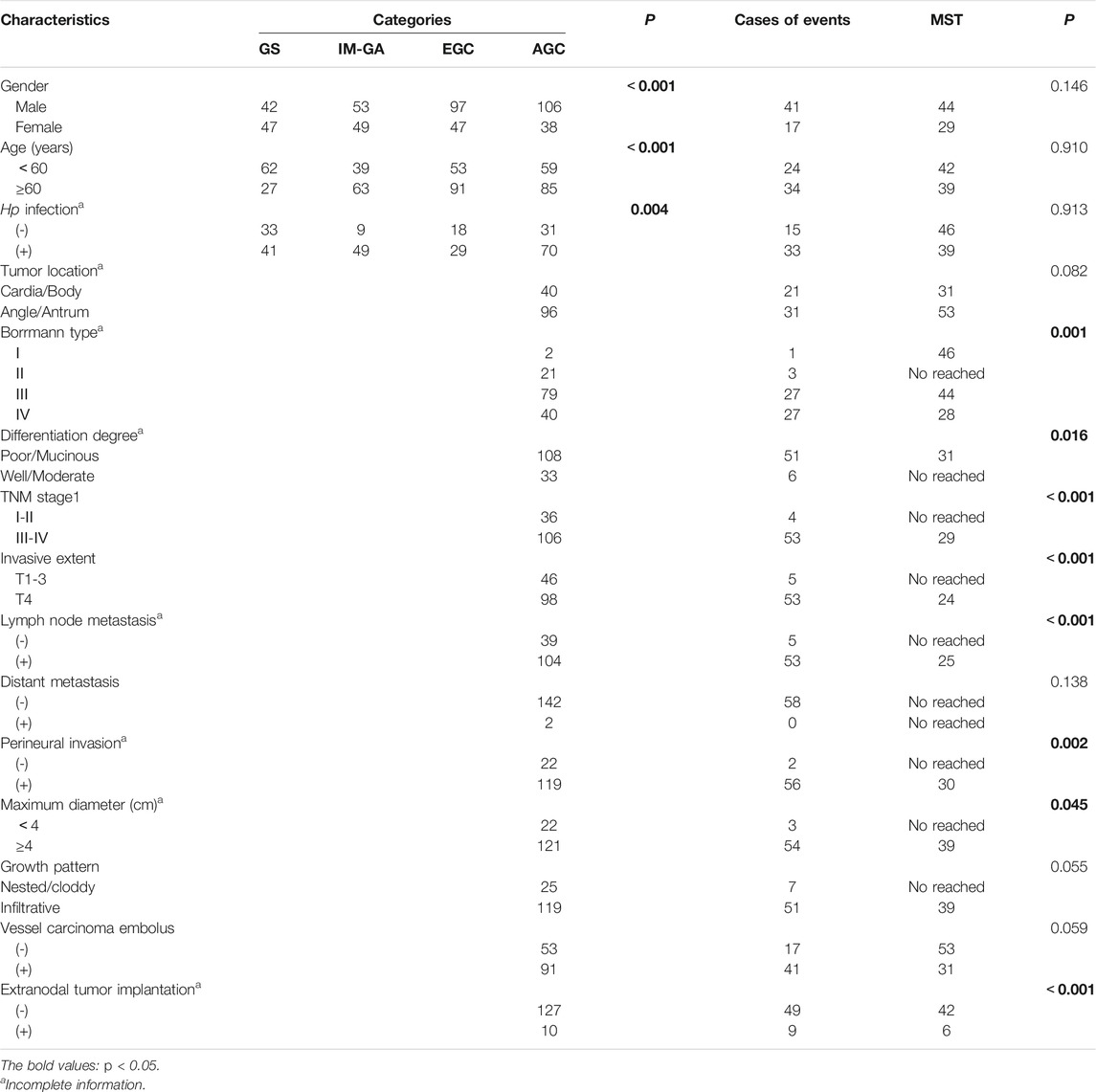
The baseline clinical characteristics of GS, IM-GA, EGC, and AGC patients were listed in Table 1. We analyzed the differential expression of TRIB3 and FABP1 in different disease groups as well as in the cancer and adjacent tissues.
3.1.1 TRIB3 Protein Expression in Different Gastric Diseases
The comparison of TRIB3 expression between different gastric diseases was shown in Figure 1. TRIB3 was mainly expressed in the epithelial cytoplasm. Representative staining images were shown in Figure 1A. Compared with GS, TRIB3 expression in the IM-GA, EGC, and AGC groups was significantly decreased (p < 0.001, p < 0.001, and p < 0.001, respectively); compared with IM-GA, TRIB3 expression was decreased in EGC and AGC (p < 0.001), though the difference in IM-GA vs EGC was not significant (p = 0.174). Specifically, TRIB3 expression in the AGC group was significantly lower than that in the EGC group (p < 0.001). Besides, the expression of TRIB3 protein in EGC + AGC group (Cancer group, CG) was significantly lower than that in GS + IM-GA group (Non cancer group, NCG) (p < 0.001). Overall, the protein expression trends of TRIB3 among different gastric disease were GS > IM-GA > EGC > AGC and NCG > CG (Figure 1B). Furthermore, the expression of TRIB3 showed a significant decrease from distal NO tissue, adjacent IM-GA to GC in the same patients (p < 0.001) (Figure 1C).

FIGURE 1. The protein expressions of TRIB3 (A–C) and FABP1 (D–F) in different gastric tissues as well as in AGC and non-tumor adjacent tissue. Magnification ×200.
3.1.2 FABP1 Protein Expression in Different Gastric Disease
The comparison of FABP1 expression between different gastric diseases was shown in Figure 1. FABP1 was also mainly expressed in the epithelial cytoplasm. Representative staining images were shown in Figure 1D. The expression pattern of FABP1 was similar with that of TRIB3 from GS, IM-GA to EGC (p < 0.001, p < 0.001, and p < 0.001, respectively). However, compared with EGC group, FABP1 expression was significantly increased in AGC group. Similarly, the protein expression of FABP1 in CG was significantly lower than that in NCG (p < 0.001). Overall, the protein expression trends of FABP1 were GS > IM-GA > EGC, EGC < AGC, and NCG > CG. (Figure 1E). Similar to TRIB3, the protein expression of FABP1 showed a significant decrease from distal NO tissue, and adjacent IM-GA to GC in the same patients (p < 0.001) (Figure 1F).
3.2 The Relation of the TRIB3 and FABP1 Expression With Clinical Parameters
We separately assessed the association of the protein expression of TRIB3 and FABP1 with clinical parameters, such as gender, age, Hp infection status, differentiation degree, and TNM stage, etc.
3.2.1 TRIB3 Protein Expression and Clinical Parameters
TRIB3 protein expression in the Hp+ group were significantly lower than that in the Hp− group (p = 0.038). This lower expression was also observed in patients with GS, IM-GA or EGC though without significance (p = 0.285, p = 0.428, and p = 0.630, respectively) (Figure 2A). We also found that among the overall or the IM-GA patients, the expression of TRIB3 was significantly different in patients with age ≥60 years and those with age <60 (p = 0.029, p = 0.049, respectively) (Figure 2B). Besides, the AGC patients with well differentiated/mucinous histology had significantly higher TRIB3 expression than those with well/moderate differentiated histology (p = 0.007) (Figure 2C). Other clinicopathological parameters didn’t indicate the significantly differential expression of TRIB3 (Supplementary Figure S1).
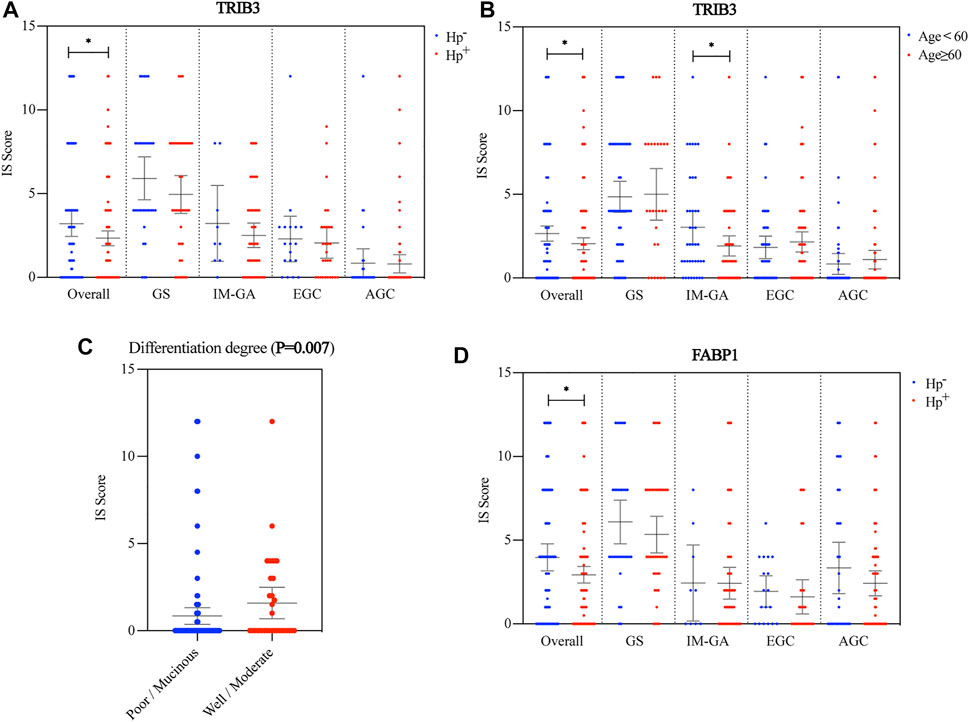
FIGURE 2. Association between the protein expressions of TRIB3 and FABP1 and clinicopathological parameters. TRIB3 was correlated with Hp infection status (A) and age (B). Besides, it was significantly correlated with the differentiation degree in AGC (C). FABP1 was also correlated with Hp infection status (D).
3.2.2 FABP1 Protein Expression and Clinical Parameters
Consistent with TRIB3, FABP1 showed significantly lower expression in the Hp+ group than in the Hp− group (p = 0.031). Likewise, this lower expression was observed in patients with GS, IM-GA or EGC though without significance (p = 0.320, p = 0.193, and p = 0.600, respectively) (Figure 2D). Other clinicopathological parameters didn’t indicate the significantly differential expression of FABP1 (Supplementary Figure S2).
3.3 The Diagnostic/Prognostic Value of TRIB3 and FABP1 for GC
We evaluated the diagnostic value of TRIB3 and FABP1 protein expressions, separately or combined, for GC and EGC. Their prognostic value in AGC, separately or combined, and was also analyzed in this study.
3.3.1 The Diagnostic/Prognostic Value of TRIB3 for GC
The ROC results showed that the expression of TRIB3 had significant diagnostic value for GC (AUC = 0.705, p < 0.001), but had no diagnostic value for EGC (AUC = 0.507, p = 0.821) (Figures 3A,B).
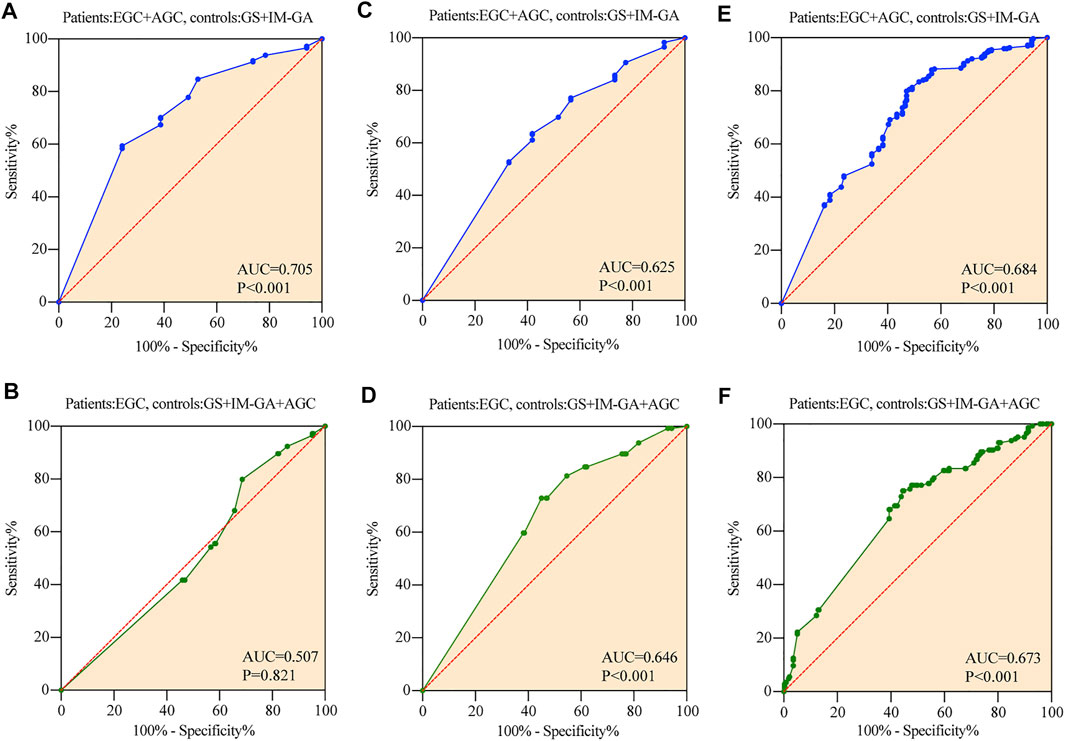
FIGURE 3. The diagnostic value of TRIB3 and FABP1 for GC. TRIB3 had significant diagnostic value for GC (A), but had no diagnostic value for EGC (B). FABP1 had significant diagnostic value for both GC (C) and EGC (D). Combined TRIB3 with FABP1 had significant diagnostic value for both GC (E) and EGC (F).
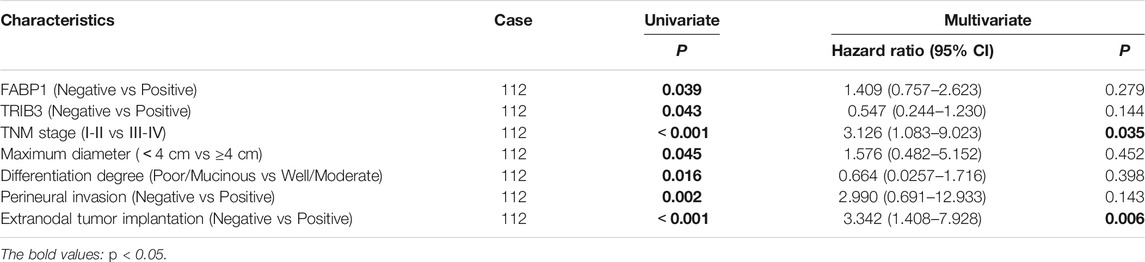
The Kaplan-Meier curve showed that AGC patients positive for TRIB3 expression tended to have better survival in comparison to those negative (p = 0.043) (Figure 4A). However, multivariate survival analysis didn’t reveal a prognostic significance considering the expression level of TRIB3 in AGC (p = 0.144) (Figure 4D; Table 2).
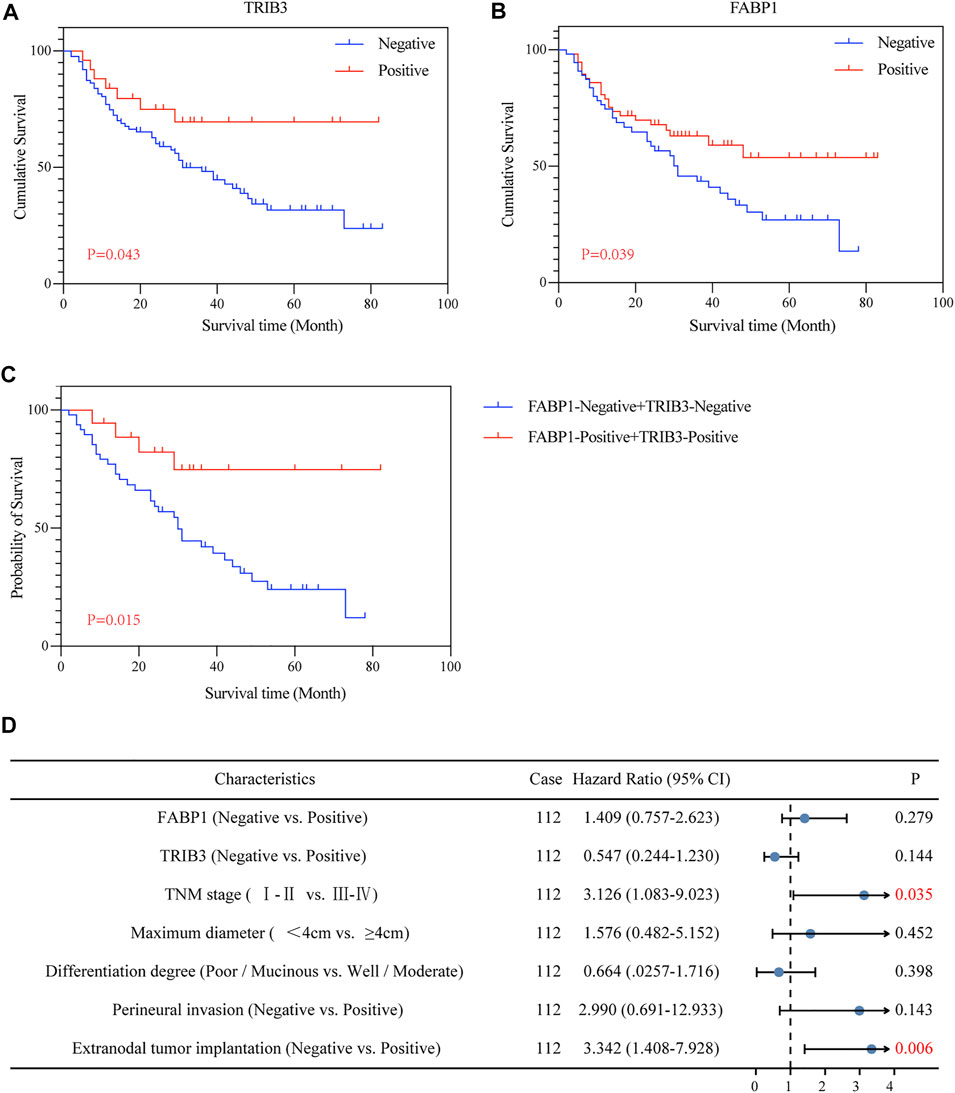
FIGURE 4. The prognostic value of TRIB3 and FABP1 for AGC. The protein expressions of TRIB3 (A) and FABP1 (B) tended to have better survival in comparison to those negative. Besides, patients with both positive expression of TRIB3 and FABP1 tended to have superior survival time compared with the both negative patients (C).
3.3.2 The Diagnostic/Prognostic Value of FABP1 for GC
The expression of FABP1 had significant diagnostic value for both GC and EGC, with an AUC of 0.646 for EGC (p < 0.001), and an AUC of 0.625 for GC (p < 0.001) (Figures 3C,D).
Moreover, AGC patients with positive FABP1 expression achieved better prognosis than those with negative FABP1 expression (p = 0.039) (Figure 4B); whereas the multivariate survival analysis suggested that FABP1 was not an independent prognostic factor of AGC (p = 0.279) (Figure 4D; Table 2).
3.3.3 Combined Diagnostic/Prognostic Value for GC
The value of TRIB3 combined with FABP1 to diagnose GC (AUC = 0.684, p < 0.001) was lower than that of TRIB3 alone (Figure 3E). However, the diagnostic efficiency of the combination of these two indicators to diagnose EGC was higher than that of a single indicator (AUC = 0.673, p < 0.001) (Figure 3F).
Interestingly, AGC patients with both positive expression of TRIB3 and FABP1 tended to have superior survival time compared with the both negative patients (p = 0.015) (Figure 4C).
3.4 Significantly Positive Correlation Between the Protein Expression of TRIB3 and FABP1
The spearman correlation analysis was used to evaluate the relationship between TRIB3 and FABP1 protein expressions (Figure 5). The protein expressions of TRIB3 and FABP1 were significantly positively correlated in the overall patients (r = 0.474, p < 0.001) (Figure 5A) as well as in each disease group. Interestingly, during the development and progression of gastric disease, and the correlation coefficient gradually decreased (GS: r = 0.806, p < 0.001; IM-GA: r = 0.533, p < 0.001; EGC: r = 0.365, p < 0.001; AGC: r = 0.265, p < 0.01) (Figures 5B–E).
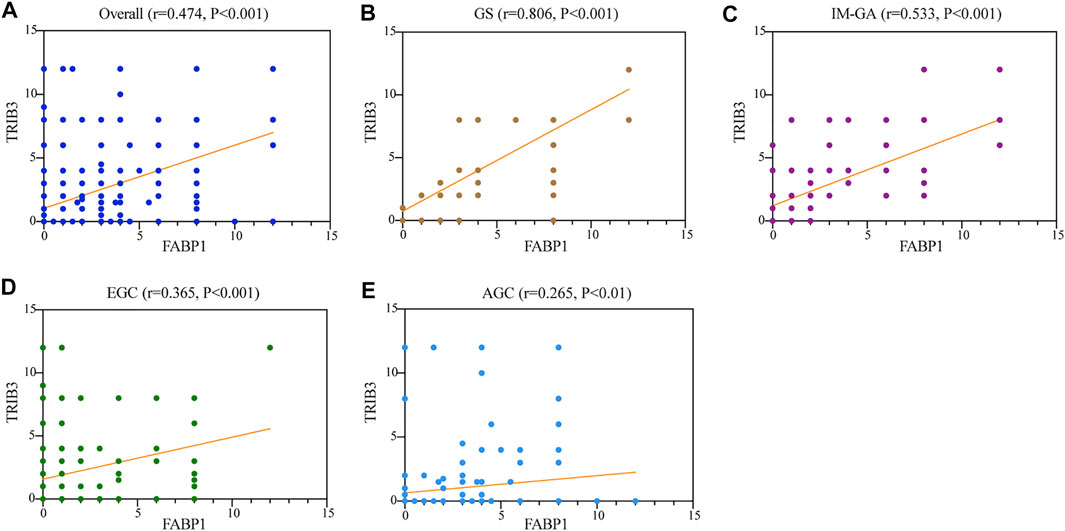
FIGURE 5. The protein expressions of TRIB3 and FABP1 were significantly positively correlated in the overall patients (A), GS (B), IM-GA (C), EGC (D), and AGC (E).
3.5 Identification of Genes Co-expressed With TRIB3 and FABP1
We searched for genes co-expressed with TRIB3 (Figure 6A) and FABP1 (Figure 6C) respectively based on |logFC| ≥ 0.5 and adjust p value ≤0.05, with the top 20 positively or negatively regulated genes of TRIB3 (Figure 6B) or FABP1 (Figure 6D) visualized by the heat plot. We found that CEACAM5 and PRAP1 were positively correlated with both TRIB3 and FABP1 expressions, while GABRP and THBS4 were negatively correlated with both TRIB3 and FABP1 expressions.
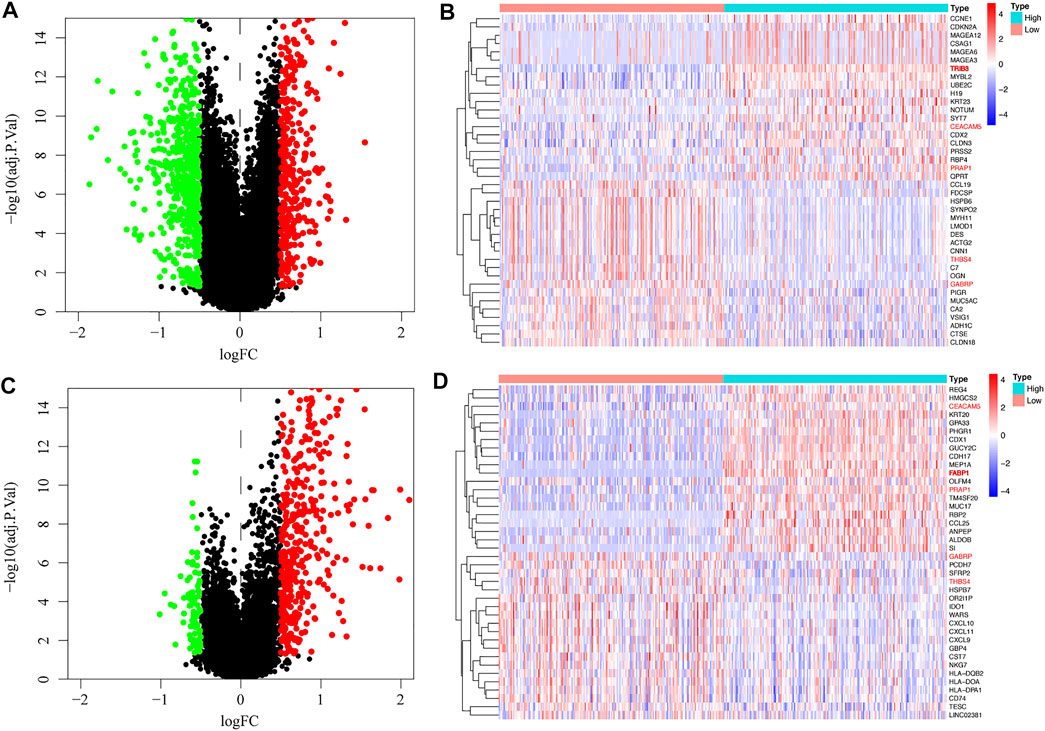
FIGURE 6. Genes co-expressed with TRIB3 (A) and FABP1 (C) were searched, with the top 20 positively or negatively regulated genes of TRIB3 (B) or FABP1 (D) visualized by the heat plot.
3.6 Potential Functions and Pathways of TRIB3 and FABP1 by GO and KEGG Analyses
The genes shared by the two co-expression analyses were used for further GO and KEGG analyses. The GO functions enriched in biological processes (BP) were antigen processing and presentation, immune response, and T cell co-stimulation, etc. The significantly enriched cell components (CC) terms were MHC class II protein complex, cell surface and extracellular exosome, etc. The molecular function (MF) significantly related to TRIB3 and FABP1 were MHC class II receptor activity, peptide antigen binding and MHC class II protein complex binding (Figure 7A). Moreover, the KEGG pathway analysis suggested that the shared co-expression genes were mainly enriched in cell adhesion molecules, inflammatory bowel disease and intestinal immune network for IgA production (Figure 7B).
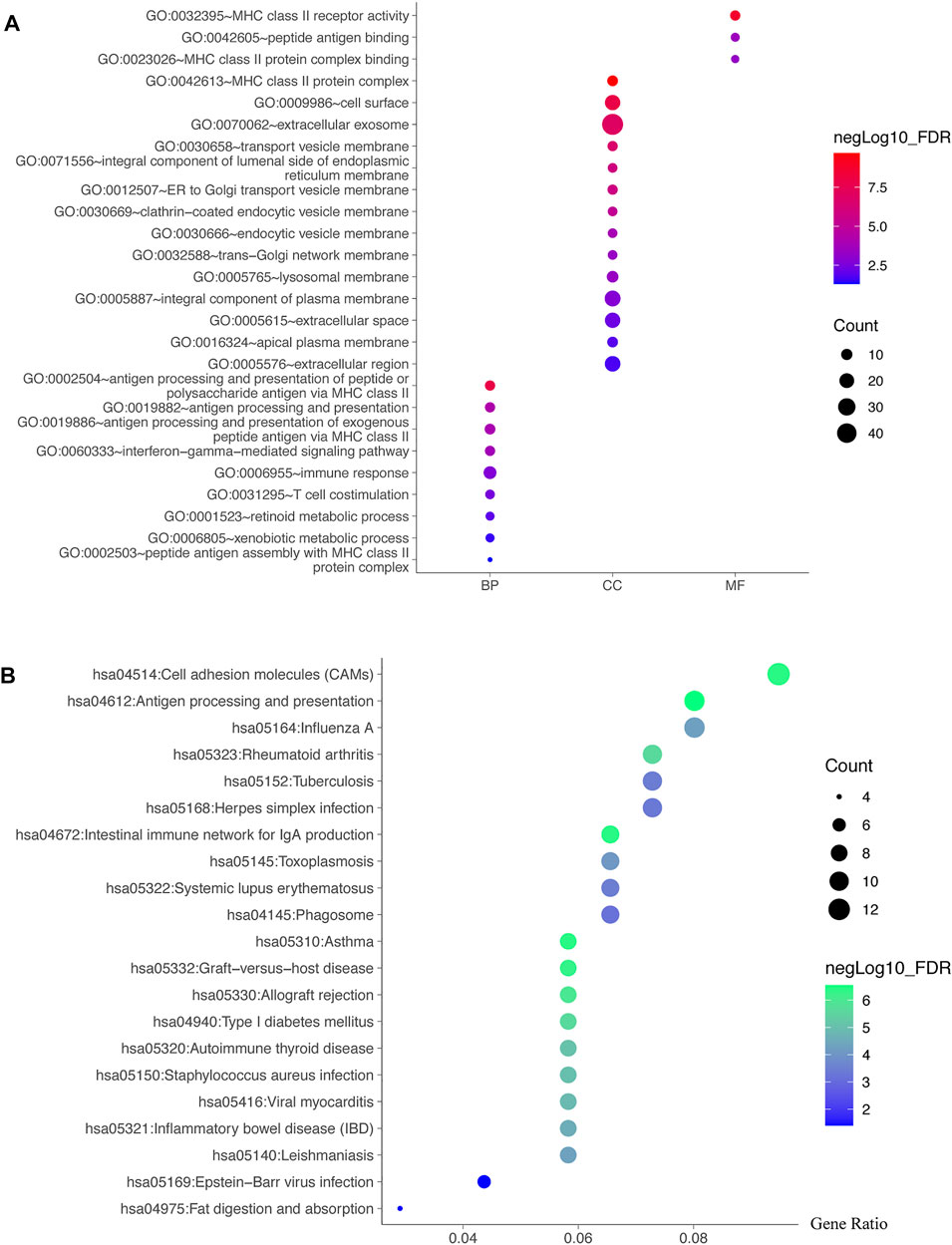
FIGURE 7. Potential functions and pathways of TRIB3 and FABP1 by GO and KEGG analyses. The genes shared by the two co-expression analyses were used for further GO (A) and KEGG (B) analyses.
3.7 Correlation Between Immune Infiltration and Expression of TRIB3 and FABP1
Due to the potential relevance of TRIB3 and FABP1 in immune-related process as suggested by the GO and KEGG analysis, we further evaluated their associations with immunocyte infiltrations in GC. TRIB3 expression was indicative of the infiltrations of T cells CD4 memory resting, NK cell resting, macrophages M0, macrophages M1, dendritic cells activated and mast cells resting (Figure 8A), while FABP1 expression was associated with the infiltrations of B cells memory, plasma cells, T cells CD8, T cells CD4 memory resting, T cells CD4 memory activated, macrophages M0, macrophages M1, and mast cells activated and neutrophils (Figure 8B). Notably, the infiltration of T cells CD4 memory resting was negatively correlated with TRIB3 but positively associated with FABP1 expression, while the infiltration of macrophages M1 was positively correlated with TRIB3 but negatively associated with FABP1 expression. Besides, the macrophages M0 infiltration was positively correlated with both TRIB3 and FABP1 expressions.
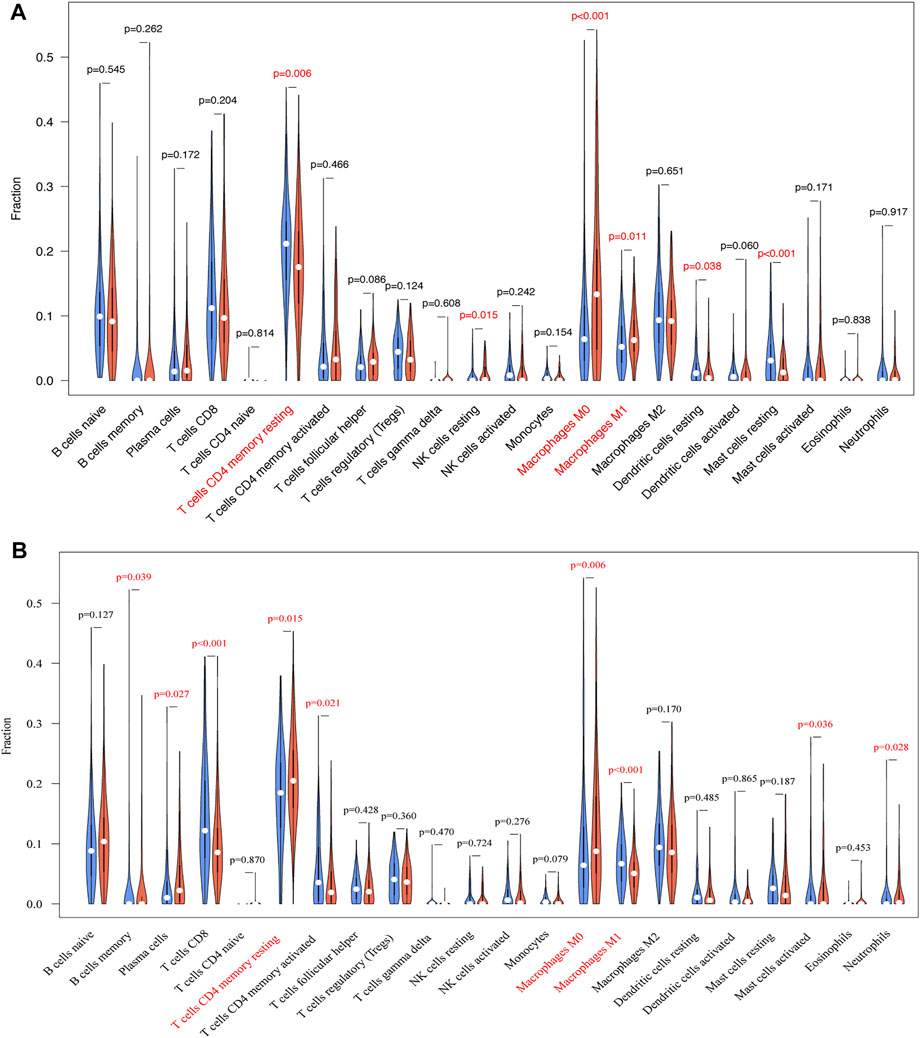
FIGURE 8. The associations of TRIB3 (A) and FABP1 (B) with immunocyte infiltrations in GC were further evaluated.
4 Discussion
In this study, we elucidated the protein expression of TRIB3 and FABP1 in different stages of gastric disease, and identified the correlation between their expressions. Their diagnostic and prognosis values, as well as their associations with clinicopathological parameters were also analyzed. Furthermore, the potential functions of TRIB3 and FABP1 were explored through TCGA and David database. As far as we know, this is the first study assessing the expression trend of TRIB3 and FABP1 in the dynamic process of gastric disease development, and also the first report correlating TRIB3 with FABP1. This study identified candidate diagnostic and prognostic biomarkers for the early detection of GC, and provided evidence and insight for further study revealing the molecular mechanism underlying the GC development and progression.
The occurrence of GC is a multi-stage process that go through GS and IM-GA. As the disease developed, EGC progressed to AGC (Liu et al., 2020). Our results showed that during this multi-stage process, the expression trend of TRIB3 and FABP1 protein was GS > IM-GA > EGC. Besides, TRIB3 and FABP1 was significantly lower expressed in GC tissues than in adjacent IM-GA or distal NO tissue. These results indicated that the expression of TRIB3 and FABP1 was downregulated when GC occurred. Some relevant studies have reported that TRIB3 played a key role in the anti-cancer activity of cannabinoids, and the gene inactivation of TRIB3 promoted the occurrence of cancer (Vara et al., 2013). Supriya Srivastava et al. observed that the protein expression of FABP1 in esophageal carcinoma was significantly lower than that in esophageal dysplasia, which could be used as a key auxiliary diagnostic index to determine the status of disease progress (Srivastava et al., 2017). Takeaki Hashimoto et al. reported that FABP1 protein was highly expressed in IM but lacking in most GC patients (Hashimoto et al., 2004). Specifically, we found that compared with EGC, the expression of TRIB3 protein continued to decrease in AGC, and while the expression of FABP1 was abnormally increased. The above results showed that TRIB3 expression was successively decreased during the process of GC initiation and development; whereas FABP1 was expressed in a two-stage pattern: its expression was decreased at first, and then increased during the progress of a disease. Previous studies based on several cancer cells and animal models had found that the inactivation of TRIB3 regulated Akt pathway and enhanced tumor development (Salazar et al., 2015), while FABP1 was significantly downregulated in some subtypes of colorectal cancer due to the influence of immune microenvironment (Wood et al., 2017). These implied the important role of TRIB3 and FABP1 in GC development. Similar to FABP1, the two-stage expression pattern of CDX2 and SOX9 during the initiation and development of GC was also reported previously (Uozaki et al., 2011; Sun et al., 2012), suggesting that these molecules might play different roles in different stages of gastric disease progression.
It’s commonly accepted that Hp infection was closely related to the occurrence of GC (Uozaki et al., 2011; Liu et al., 2020). We observed that Hp infection was significantly associated with the decreased expression of TRIB3 and FABP1, indicating that the decreased expression of TRIB3 and FABP1 in GC lesions was partly due to the Hp infection. A prior meta-analysis revealed that Hp infection probably caused hypermethylation of CDH1, which was significantly associated with the risk of GC (Zeng et al., 2015). And TRIB3 was reported to be ubiquitinated by CDH1 and then degraded (Ohoka et al., 2010). Based on these studies, we speculate that the infection of Hp may regulate TRIB3 expression through the ubiquitination-proteasome pathway mediated degradation. In addition, studies have reported that antibacterial drugs could alleviate the decrease in FABP1 expression (Brown et al., 2016). Furthermore, we explored the relationship between the expressions of TRIB3 and FABP1 and their associations with clinicopathological parameters of AGC. AGCs that were poor differentiated/mucinous presented lower TRIB3 expressions than the well/moderate differentiated ones. Our result was consistent with the previous reports that TRIB3 was significantly correlated with the degree of bone marrow differentiation in patients with acute promyelocytic leukemia (Li et al., 2017). Since differentiation is a key factor reflecting the invasiveness of tumors, the low expression of TRIB3 may indicate a higher malignant degree of GC.
In addition, we found that the expression of TRIB3 were of diagnostic value for GC, while FABP1 were of diagnostic value for both GC and EGC, and as suggested by the previous research results in renal cancer and breast cancer (Wennemers et al., 2011a; Wu et al., 2020). Moreover, combining these two proteins was proved to improve the diagnostic efficiency for EGC. These results suggested that TRIB3 can be used as a diagnostic biomarker for GC, while FABP1 is more effective in diagnosing EGC. The diagnostic efficiency for EGC was higher when combining TRIB3 and FABP1.
Studies have shown that the high expression of TRIB3 in breast cancer and oral squamous cell carcinoma indicated a better prognosis (Wennemers et al., 2011a; Zhang et al., 2011), but whether it can affect GC prognosis has not been confirmed. Takeaki Hashimoto et al. reported that the expression level of FABP1 was not associated with GC prognosis (Hashimoto et al., 2004); while another study revealed that it was a reliable prognostic indicator of GC (Satoh et al., 2012). Until now, no definite conclusion formed on the relationship between the expression of FABP1 and the prognosis of GC patients. Our study evaluated the prognostic roles of TRIB3 and FABP1 in GC by univariate and multivariate survival analyses. And the results suggested that a higher expression of TRIB3 or FABP1 could indicate a better prognosis of GC, but it was not significant in multivariate survival analyses. It had been demonstrated that TRIB3 was involved in the normal death process of tumor cells (Ohoka et al., 2005; Örd et al., 2007; Salazar et al., 2009). The high protein expression of TRIB3 could inhibit the ability of tumor immune apoptosis, cause tumor cell death and prolong the survival of patients (Wennemers et al., 2011b). Besides, our results were consistent with those of Yumiko Satoh et al., and which indicated that FABP1 was a reliable prognostic indicator of GC (Satoh et al., 2012).
We also evaluated the correlation between the expressions of TRIB3 and FABP1. The expressions of TRIB3 and FABP1 were positively correlated regardless of the diseases the patients harbored. Of note, the correlation coefficients between TRIB3 and FABP1 expressions gradually weakened as the gastric disease progressed from GS to GC, suggesting that there might be direct or indirect interactions between TRIB3 and FABP1 and the strength of this interaction can be regulated during GC development. Interestingly, the correlation between TRIB3 and FABP1 was stronger in gastric tissues that tend to be normal, and which was worthy of further study.
To further explore the potential functions of TRIB3 and FABP1, we conducted bioinformatics mining. Co-expression and correlation analyses suggested that CEACAM5 and PRAP1 expressions were positively correlated with both TRIB3 and FABP1 expressions, while GABRP and THBS4 expressions were negatively correlated with them. CEACAM5 was a human epidermal cell adhesion molecule, which could interact with Hp adhesion protein HopQ and transfer CagA protein to the host gastric epithelial cells, thus triggering inflammation (Xia et al., 2019). PRAP1 was a proline-rich acidic protein that could down-regulate MAD1 and inhibit mitotic checkpoint signaling in hepatocellular carcinoma (Sze et al., 2014). GABRP has been reported to play an immunomodulatory role in pancreatic cancer in a neurotransmitter independent manner, promoting macrophage infiltration by inducing the expression of CXCL5 and CCL20, and thereby affecting tumor growth and metastasis (Jiang et al., 2019). Studies demonstrated that THBS4, an extracellular glycoprotein, was involved in wound healing and tissue remodeling. It was found to be expressed on fibroblasts in the microenvironment of GC and was related to the metastasis of cancer cells (Kuroda et al., 2019). Our findings suggested that they might be active molecules involved in the interaction between TRIB3 and FABP1.
GO and KEGG enrichment analyses revealed the possible role of TRIB3 and FABP1 in immune related process, and further immune infiltration analysis elucidated that TRIB3 and FABP1 may play a role in the inhibition of CD4+ T cells infiltration and the differentiation of macrophages. Existing studies have found that TRIB3 participated in the differentiation of CD4+ T cells and played an important role in the apoptosis of macrophages induced by oxidized low density lipoprotein (Shang et al., 2009; Kiuchi et al., 2021). FABP1 had not been reported to be related to CD4+ T cells, but it could affect the function of tumor-associated macrophages by participating in tumor internal metabolic pathways, and thereby exerting anti-tumor effects (Xu et al., 2020). The above results suggest that TRIB3 and FABP1 may affect the tumor immune microenvironment by acting on immune cells, and then participate in the process of GC development.
However, there are still some limitations in our study. Although we have tried to reveal the functional role of TRIB3 and FABP1 by exploring co-expressed genes and immune infiltration, there is currently no direct evidence support the hypothesis, and which needs to be further investigated.
5 Conclusion
In conclusion, the protein expressions of TRIB3 and FABP1 gradually decreased with the gastric disease progress, and was positively correlated. Hp infection may reduce the protein expression of TRIB3 and FABP1. In addition, the low expression of TRIB3 protein is associated with the malignant biological behavior of GC. Combing TRIB3 and FABP1 expressions can improve the diagnostic efficiency for EGC. Either a high expression of TRIB3 or FABP1 indicates a better prognosis for GC. Bioinformatics analysis showed that TRIB3 and FABP1 may interact with CEACAM5, PRAP1, GABRP, and THBS4, and affect tumor immune microenvironment by regulating immune cells, and participate in the development and progression of GC.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Medical Science Research in the First Hospital of China Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
SL, YG, and YY performed the histological examination of the gastric cancer, and were major contributor in writing the manuscript. CN, YL, HY, and CX analyzed and interpreted the patient data. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Award No.81970501), the National Key R and D Program of China (Grant 2018YFC1311600) and Liaoning Province Science and Technology Plan Project (Award No 20180550049).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmolb.2021.790433/full#supplementary-material
Abbreviations
AGC, advanced gastric cancer; BP, biological processes; CC, cell components; CG, Cancer group; EGC, early gastric cancer; FABP1, Liver fatty acid binding protein; GC, gastric cancer; GS, superficial gastritis; Hp, Helicobacter pylori; IHC, immunohistochemistry; IM-GA, intestinal metaplasia atrophic gastritis; IS, immunoreactivity score; MF, molecular function; NCG, Non cancer group; NO, normal; ROC, Receiver operating characteristic; TCGA, The Cancer Genome Atlas; TRIB3, Tribbles Pseudokinase 3.
References
Börchers, T., Unterberg, C., Rüdel, H., Robenek, H., and Spener, F. (1989). Subcellular Distribution of Cardiac Fatty Acid-Binding Protein in Bovine Heart Muscle and Quantitation with an Enzyme-Linked Immunosorbent Assay. Biochim. Biophys. Acta (BBA) - Lipids Lipid Metab. 1002, 54–61. doi:10.1016/0005-2760(89)90063-5
Bordewick, U., Heese, M., Börchers, T., Robenek, H., and Spener, F. (1989). Compartmentation of Hepatic Fatty-Acid-Binding Protein in Liver Cells and its Effect on Microsomal Phosphatidic Acid Biosynthesis. Biol. Chem. Hoppe-Seyler 370, 229–238. doi:10.1515/bchm3.1989.370.1.229
Brown, K., Zaytsoff, S. J. M., Uwiera, R. R. E., and Inglis, G. D. (2016). Antimicrobial Growth Promoters Modulate Host Responses in Mice with a Defined Intestinal Microbiota. Sci. Rep. 6, 38377. doi:10.1038/srep38377
Chmurzynska, A. (2006). The Multigene Family of Fatty Acid-Binding Proteins (FABPs): Function, Structure and Polymorphism. J. Appl. Genet. 47, 39–48. doi:10.1007/BF03194597
Dixon, M. F., Genta, R. M., Yardley, J. H., and Correa, P. (1996). Classification and Grading of Gastritis. The Updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 20, 1161–1181. doi:10.1097/00000478-199610000-00001
Fahimi, H. D., Voelkl, A., Vincent, S. H., and Muller-Eberhard, U. (1990). Localization of the Heme-Binding Protein in the Cytoplasm and of a Heme-Binding Protein-like Immunoreactive Protein in the Nucleus of Rat Liver Parenchymal Cells: Immunocytochemical Evidence of the Subcellular Distribution Corroborated by Radioimmunoassay and Immunoblotting. Hepatology 11, 859–865. doi:10.1002/hep.1840110522
Furuhashi, M., and Hotamisligil, G. S. (2008). Fatty Acid-Binding Proteins: Role in Metabolic Diseases and Potential as Drug Targets. Nat. Rev. Drug Discov. 7, 489–503. doi:10.1038/nrd2589
Georgiadi, A., and Kersten, S. (2012). Mechanisms of Gene Regulation by Fatty Acids. Adv. Nutr. 3, 127–134. doi:10.3945/an.111.001602
Hashimoto, T., Kusakabe, T., Watanabe, K., Sugino, T., Fukuda, T., Nashimoto, A., et al. (2004). Liver-type Fatty Acid-Binding Protein Is Highly Expressed in Intestinal Metaplasia and in a Subset of Carcinomas of the Stomach without Association with the Fatty Acid Synthase Status in the Carcinoma. Pathobiology 71, 115–122. doi:10.1159/000076465
Haunerland, N. H., and Spener, F. (2004). Fatty Acid-Binding Proteins - Insights from Genetic Manipulations. Prog. Lipid Res. 43, 328–349. doi:10.1016/j.plipres.2004.05.001
Ho, S.-Y., and Storch, J. (2001). Common Mechanisms of Monoacylglycerol and Fatty Acid Uptake by Human Intestinal Caco-2 Cells. Am. J. Physiology-Cell Physiol. 281, C1106–C1117. doi:10.1152/ajpcell.2001.281.4.c1106
Huang, J., Teng, L., Liu, T., Li, L., Chen, D., Li, F., et al. (2003). Identification of a Novel Serine/threonine Kinase that Inhibits TNF-Induced NF-Κb Activation and P53-Induced Transcription. Biochem. Biophysical Res. Commun. 309, 774–778. doi:10.1016/j.bbrc.2003.08.069
Inoue, M., Takahashi, Y., Fujii, T., Kitagawa, M., and Fukusato, T. (2014). Significance of Downregulation of Liver Fatty Acid-Binding Protein in Hepatocellular Carcinoma. Wjg 20, 17541–17551. doi:10.3748/wjg.v20.i46.17541
Izrailit, J., Berman, H. K., Datti, A., Wrana, J. L., and Reedijk, M. (2013). High Throughput Kinase Inhibitor Screens Reveal TRB3 and MAPK-ERK/TGF Pathways as Fundamental Notch Regulators in Breast Cancer. Proc. Natl. Acad. Sci. 110, 1714–1719. doi:10.1073/pnas.1214014110
Jiang, S.-H., Zhu, L.-L., Zhang, M., Li, R.-K., Yang, Q., Yan, J.-Y., et al. (2019). GABRP Regulates Chemokine Signalling, Macrophage Recruitment and Tumour Progression in Pancreatic Cancer through Tuning KCNN4-Mediated Ca2+ Signalling in a GABA-independent Manner. Gut 68, 1994–2006. doi:10.1136/gutjnl-2018-317479
Joo, Y.-E., Rew, J.-S., Kim, H.-S., Choi, S.-K., Park, C.-S., and Kim, S.-J. (2001). Changes in the E-Cadherin-Catenin Complex Expression in Early and Advanced Gastric Cancers. Digestion 64, 111–119. doi:10.1159/000048849
Kiss-Toth, E., Bagstaff, S. M., Sung, H. Y., Jozsa, V., Dempsey, C., Caunt, J. C., et al. (2004). Human Tribbles, a Protein Family Controlling Mitogen-Activated Protein Kinase Cascades. J. Biol. Chem. 279, 42703–42708. doi:10.1074/jbc.m407732200
Kiuchi, M., Onodera, A., Kokubo, K., Ichikawa, T., Morimoto, Y., Kawakami, E., et al. (2021). The Cxxc1 Subunit of the Trithorax Complex Directs Epigenetic Licensing of CD4+ T Cell Differentiation. J. Exp. Med. 218, e20201690. doi:10.1084/jem.20201690
Kuroda, K., Yashiro, M., Sera, T., Yamamoto, Y., Kushitani, Y., Sugimoto, A., et al. (2019). The Clinicopathological Significance of Thrombospondin-4 Expression in the Tumor Microenvironment of Gastric Cancer. PLoS One 14, e0224727. doi:10.1371/journal.pone.0224727
Lee, S., Bang, S., Song, K., and Lee, I. (2006). Differential Expression in normal-adenoma-carcinoma Sequence Suggests Complex Molecular Carcinogenesis in colon. Oncol. Rep. 16, 747–754. doi:10.3892/or.16.4.747
Li, K., Wang, F., Cao, W.-B., Lv, X.-X., Hua, F., Cui, B., et al. (2017). TRIB3 Promotes APL Progression through Stabilization of the Oncoprotein PML-Rarα and Inhibition of P53-Mediated Senescence. Cancer Cell 31, 697–710. doi:10.1016/j.ccell.2017.04.006
Liu, S., Yin, H., Zheng, S., Chu, A., Li, Y., Xing, C., et al. (2020). Differentially Expressed mRNAs and Their Long Noncoding RNA Regulatory Network with Helicobacter Pylori-Associated Diseases Including Atrophic Gastritis and Gastric Cancer. Biomed. Res. Int. 2020, 3012193. doi:10.1155/2020/3012193
Mondal, D., Mathur, A., and Chandra, P. K. (2016). Tripping on TRIB3 at the junction of Health, Metabolic Dysfunction and Cancer. Biochimie 124, 34–52. doi:10.1016/j.biochi.2016.02.005
Ohoka, N., Sakai, S., Onozaki, K., Nakanishi, M., and Hayashi, H. (2010). Anaphase-promoting Complex/cyclosome-Cdh1 Mediates the Ubiquitination and Degradation of TRB3. Biochem. Biophysical Res. Commun. 392, 289–294. doi:10.1016/j.bbrc.2009.12.175
Ohoka, N., Yoshii, S., Hattori, T., Onozaki, K., and Hayashi, H. (2005). TRB3, a Novel ER Stress-Inducible Gene, Is Induced via ATF4-CHOP Pathway and Is Involved in Cell Death. EMBO J. 24, 1243–1255. doi:10.1038/sj.emboj.7600596
Örd, D., Meerits, K., and Örd, T. (2007). TRB3 Protects Cells against the Growth Inhibitory and Cytotoxic Effect of ATF4. Exp. Cel Res. 313, 3556–3567. doi:10.1016/j.yexcr.2007.07.017
Salazar, M., Carracedo, A., Salanueva, Í. J., Hernández-Tiedra, S., Lorente, M., Egia, A., et al. (2009). Cannabinoid Action Induces Autophagy-Mediated Cell Death through Stimulation of ER Stress in Human Glioma Cells. J. Clin. Invest. 119, 1359–1372. doi:10.1172/jci37948
Salazar, M., Lorente, M., García-Taboada, E., Pérez Gómez, E., Dávila, D., Zúñiga-García, P., et al. (2015). Loss of Tribbles Pseudokinase-3 Promotes Akt-Driven Tumorigenesis via FOXO Inactivation. Cell Death Differ 22, 131–144. doi:10.1038/cdd.2014.133
Satoh, Y., Mori, K., Kitano, K., Kitayama, J., Yokota, H., Sasaki, H., et al. (2012). Analysis for the Combination Expression of CK20, FABP1 and MUC2 Is Sensitive for the Prediction of Peritoneal Recurrence in Gastric Cancer. Jpn. J. Clin. Oncol. 42, 148–152. doi:10.1093/jjco/hyr179
Shang, Y.-y., Wang, Z.-h., Zhang, L.-p., Zhong, M., Zhang, Y., Deng, J.-t., et al. (2009). TRB3, Upregulated by Ox-LDL, Mediates Human Monocyte-Derived Macrophage Apoptosis. FEBS J. 276, 2752–2761. doi:10.1111/j.1742-4658.2009.06998.x
Srivastava, S., Kern, F., Sharma, N., McKeon, F., Xian, W., Yeoh, K. G., et al. (2017). FABP1 and Hepar Expression Levels in Barrett's Esophagus and Associated Neoplasia in an Asian Population. Dig. Liver Dis. 49, 1104–1109. doi:10.1016/j.dld.2017.06.014
Sun, M., Uozaki, H., Hino, R., Kunita, A., Shinozaki, A., Ushiku, T., et al. (2012). SOX9 Expression and its Methylation Status in Gastric Cancer. Virchows Arch. 460, 271–279. doi:10.1007/s00428-012-1201-7
Sze, K. M.-F., Chu, G. K.-Y., Mak, Q. H.-Y., Lee, J. M.-F., and Ng, I. O.-L. (2014). Proline-rich Acidic Protein 1 (PRAP1) Is a Novel Interacting Partner of MAD1 and Has a Suppressive Role in Mitotic Checkpoint Signalling in Hepatocellular Carcinoma. J. Pathol. 233, 51–60. doi:10.1002/path.4319
Topi, S., Santacroce, L., Bottalico, L., Ballini, A., Inchingolo, A. D., Dipalma, G., et al. (2020). Gastric Cancer in History: A Perspective Interdisciplinary Study. Cancers (Basel) 12, 264. doi:10.3390/cancers12020264
Uozaki, H., Barua, R. R., Minhua, S., Ushiku, T., Hino, R., Shinozaki, A., et al. (2011). Transcriptional Factor Typing with SOX2, HNF4aP1, and CDX2 Closely Relates to Tumor Invasion and Epstein-Barr Virus Status in Gastric Cancer. Int. J. Clin. Exp. Pathol. 4, 230–240.
Valenzuela, M. A., Canales, J., Corvalan, A. H., and Quest, A. F. (2015). Helicobacter Pylori-Induced Inflammation and Epigenetic Changes during Gastric Carcinogenesis. Wjg 21, 12742–12756. doi:10.3748/wjg.v21.i45.12742
Vara, D., Morell, C., Rodríguez-Henche, N., and Diaz-Laviada, I. (2013). Involvement of PPARγ in the Antitumoral Action of Cannabinoids on Hepatocellular Carcinoma. Cell Death Dis 4, e618. doi:10.1038/cddis.2013.141
Wennemers, M., Bussink, J., Grebenchtchikov, N., Sweep, F. C. G. J., and Span, P. N. (2011). TRIB3 Protein Denotes a Good Prognosis in Breast Cancer Patients and Is Associated with Hypoxia Sensitivity. Radiother. Oncol. 101, 198–202. doi:10.1016/j.radonc.2011.05.057
Wennemers, M., Bussink, J., Scheijen, B., Nagtegaal, I. D., van Laarhoven, H. W., Raleigh, J. A., et al. (2011). Tribbles Homolog 3 Denotes a Poor Prognosis in Breast Cancer and Is Involved in Hypoxia Response. Breast Cancer Res. 13, R82. doi:10.1186/bcr2934
Wood, S. M., Gill, A. J., Brodsky, A. S., Lu, S., Friedman, K., Karashchuk, G., et al. (2017). Fatty Acid-Binding Protein 1 Is Preferentially Lost in Microsatellite Instable Colorectal Carcinomas and Is Immune Modulated via the Interferon γ Pathway. Mod. Pathol. 30, 123–133. doi:10.1038/modpathol.2016.170
Wu, G., Zhang, Z., Tang, Q., Liu, L., Liu, W., Li, Q., et al. (2020). Study of FABP's Interactome and Detecting New Molecular Targets in clear Cell Renal Cell Carcinoma. J. Cel Physiol 235, 3776–3789. doi:10.1002/jcp.29272
Wu, M., Xu, L.-G., Zhai, Z., and Shu, H.-B. (2003). SINK Is a P65-Interacting Negative Regulator of NF-κb-dependent Transcription. J. Biol. Chem. 278, 27072–27079. doi:10.1074/jbc.m209814200
Xia, R., Zhang, B., Wang, X., and Jia, Q. (2019). Pathogenic Interactions between Helicobacter pylori Adhesion Protein HopQ and Human Cell Surface Adhesion Molecules CEACAMs in Gastric Epithelial Cells. Iran J. Basic Med. Sci. 22, 710–715. doi:10.22038/ijbms.2019.34237.81
Xu, L., Xia, H., Ni, D., Hu, Y., Liu, J., Qin, Y., et al. (2020). High-Dose Dexamethasone Manipulates the Tumor Microenvironment and Internal Metabolic Pathways in Anti-tumor Progression. Int. J. Mol. Sci. 21, 1846. doi:10.3390/ijms21051846
Yang, H., Liu, J., Jing, J., Wang, Z., Li, Y., Gou, K., et al. (2018). Expression of DDB2 Protein in the Initiation, Progression, and Prognosis of Colorectal Cancer. Dig. Dis. Sci. 63, 2959–2968. doi:10.1007/s10620-018-5224-z
Zeng, W., Zhu, J., Shan, L., Li, H., Han, Z., Aeixiding, P., et al. (2015). The Clinicopathological Significance of CDH1 in Gastric Cancer: a Meta-Analysis and Systematic Review. Dddt 9, 2149–2157. doi:10.2147/dddt.s75429
Keywords: TRIB3, FABP1, expression, gastric cancer, biomarker
Citation: Liu S, Ni C, Li Y, Yin H, Xing C, Yuan Y and Gong Y (2021) The Involvement of TRIB3 and FABP1 and Their Potential Functions in the Dynamic Process of Gastric Cancer. Front. Mol. Biosci. 8:790433. doi: 10.3389/fmolb.2021.790433
Received: 06 October 2021; Accepted: 18 November 2021;
Published: 09 December 2021.
Edited by:
Ismail Hosen, University of Dhaka, BangladeshReviewed by:
Zhiyuan Ke, Experimental Drug Development Centre (EDDC), SingaporeShilpita Karmakar, JAX Cancer Center-Jackson Laboratory, United States
Copyright © 2021 Liu, Ni, Li, Yin, Xing, Yuan and Gong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuan Yuan, eXVhbnl1YW5AY211LmVkdS5jbg==; Yuehua Gong, eWhnb25nQGNtdS5lZHUuY24=
 Songyi Liu
Songyi Liu Chuxuan Ni1,2,3
Chuxuan Ni1,2,3 Honghao Yin
Honghao Yin Chengzhong Xing
Chengzhong Xing Yuan Yuan
Yuan Yuan Yuehua Gong
Yuehua Gong