- 1Department of Breast Surgery, Fudan University Shanghai Cancer Center, Shanghai, China
- 2Shanghai Medical College, Fudan University, Shanghai, China
- 3Shanghai Key Laboratory of Breast Cancer, Shanghai, China
Background: The microenvironment of triple-negative breast cancer (TNBC) can be divided into three clusters based on bioinformatics-based immunogenomic analysis: the “immune-desert” cluster, the “innate immune-inactivated” cluster, and the “immune-inflamed” cluster. The immune-inflamed cluster is considered as “hot tumor” while the other two are considered as “cold tumor”.
Methods: To investigate the prognostic effect of microenvironment phenotypes on TNBC, we compared relapse-free survival (RFS) of different phenotypes in 100 patients with RNA sequencing-based expression data from the PATTERN trial (NCT01216111, published in JAMA Oncol 2020), which indicated a superior efficacy of adjuvant paclitaxel-plus-carboplatin regimen compared to the regimen of cyclophosphamide/epirubicin/fluorouracil followed by docetaxel for TNBC. We also analyzed the efficacy of the two regimens for different immune phenotypes to explore potential treatment strategies.
Results: No significant difference in RFS was observed between the “hot tumor” and the “cold tumor” (hazard ratio [HR] = 0.68, 95% confidence interval [CI] 0.28–1.66, P = 0.40). However, the “hot tumor” subtype was associated with significantly longer RFS in node-positive patients (HR = 0.27, 95%CI 0.07–0.97, P = 0.03). Consistently, a similar trend to improved RFS of the “hot tumor” phenotype was detected in patients with stage pT2-3 tumors (HR = 0.29, 95%CI 0.06–1.30, P = 0.08). Furthermore, no significant difference in RFS between the two treatment arms was observed in patients with “hot tumor” (HR = 0.39, 95% CI 0.08–2.01, P = 0.24) or “cold tumor” (HR = 1.05, 95% CI 0.39–2.82, P = 0.92).
Conclusion: The microenvironment phenotype in TNBC might have prognostic significance to patients with a high risk of recurrence. The association of the microenvironment phenotypes with the efficacy of adjuvant chemotherapy for TNBC remains to be further studied.
Introduction
Triple-negative breast cancer (TNBC) accounts for 15–20% of breast cancers that lack estrogen receptor (ER) and progesterone receptor (PR) expression and human epidermal growth factor 2 (HER2) amplification (Perou et al., 2000; Harbeck and Gnant, 2017). Higher risk of relapse and metastasis and lack of therapeutic targets are major problems in TNBC treatment at present (Bianchini et al., 2016; Denkert et al., 2017). Compared with other subtypes of breast cancer, TNBC usually has higher immunogenicity (Lehmann et al., 2011; Burstein et al., 2015). Immune infiltration in the tumor microenvironment (TME) is associated with response to treatment and prognosis of TNBC (Loi et al., 2014; Denkert et al., 2018). Therefore, efforts have been made to explore immunotherapeutic strategies for patients with TNBC. Recent research has shown that the application of immune checkpoint blockade (ICB) may benefit metastatic TNBC (Schmid et al., 2018).
To systemically characterize the impact of the TNBC microenvironment on prognosis and immunotherapy, we have classified the TNBC microenvironment phenotypes into three heterogeneous clusters taking advantage of the expression data of 386 TNBC patients from Fudan University Shanghai Cancer Center (FUSCC): the “immune-desert” cluster with low microenvironment cell infiltration; the “innate immune-inactivated” cluster with resting innate immune cells and nonimmune stromal cells infiltration; and the “immune-inflamed” cluster with abundant adaptive and innate immune cells infiltration (Xiao et al., 2019). The “immune-inflamed” cluster is considered as “hot tumor” while the other two clusters are considered as “cold tumor”.
To further investigate the prognostic effect of the TNBC microenvironment phenotypes and their association with the efficacy of different adjuvant chemotherapy regimens, we conducted a biomarker analysis of the patients with immunogenomic data on microenvironment phenotypes from the PATTERN trial (NCT01216111). The randomized multicenter phase III PATTERN trial compared six cycles of paclitaxel plus carboplatin (PCb) with a standard-dose regimen of three cycles of cyclophosphamide, epirubicin, and fluorouracil followed by three cycles of docetaxel (CEF-T) in the adjuvant setting of operable TNBC, indicating a superior efficacy of the carboplatin-containing regimen compared to the anthracycline/taxane regimen (Yu et al., 2020). A total of 100 patients in the PATTERN cohort with expression data from RNA sequencing or HTA 2.0 microarray have been involved in clustering TNBC microenvironment phenotype mentioned above. Here, we analyzed the clinical characteristics and long-term survival data of these patients to explore clues for potential treatment strategies of adjuvant chemotherapy or immunotherapy for TNBC.
Materials and Methods
Study Design
The design and conduct of the PATTERN trial were described elsewhere previously (Yu et al., 2020). In brief, between July 2011 and April 2016, 647 women with operable, primary invasive TNBC after definitive surgery at nine cancer centers and hospitals in China were randomly assigned to two treatment groups: 322 in the CEF-T group and 325 in the PCb group. The primary endpoint was disease-free survival (DFS). Secondary endpoints included overall survival distant DFS, relapse-free survival (RFS), DFS in patients with germline variants in BRCA1/2 or homologous recombination repair-related genes, and toxicity. The independent institutional review board of the participating centers approved the study protocol. We performed the study according to the International Conference on Harmonisation Good Clinical Practice guidelines and ethical principles of the Declaration of Helsinki. All patients provided written informed consent.
Patient Samples
As mentioned above, a total of 100 patients with RNA sequencing data or HTA 2.0 microarray data in the PATTERN cohort were enrolled in the previous immunogenomic analysis of TNBC microenvironment phenotypes clustering. There were 47 patients in the PCb arm and 53 patients in the CEF-T arm involved, respectively. Detailed inclusion criteria for the analysis were as follows: 1) female patients; 2) unilateral invasive ductal carcinoma; 3) pathologic examination of the ER, PR, and HER2 status performed by the Department of Pathology at FUSCC through immunochemical analysis and in situ hybridization (for HER2 status only); 4) patients with no evidence of metastasis at the time of diagnosis; and 5) sufficient frozen tissue for further research. More detailed information regarding the sample processing and sequencing data generation is described previously (Xiao et al., 2019). All data can be viewed in The National Omics Data Encyclopedia (http://www.biosino.org/node) by pasting the accession (OEP000155) into the text search box or through the URL: http://www.biosino.org/node/project/detail/OEP000155. The HTA 2.0 microarray data is also available in GSE76250 and the RNA sequencing data is available in SRP157974.
Microenvironment Phenotypes Clustering
The detail of microenvironment phenotypes clustering and relevant data processing was described elsewhere (Xiao et al., 2019). In brief, we firstly constructed a compendium of 364 genes to represent 24 microenvironment cell subsets by referring to two gene signatures, CIBERSORT (Newman et al., 2015) and MCP-Counter (Becht et al., 2016). Signatures for types 1, 2, and 17 T helper cells and myeloid-derived suppressor cells were also constructed according to a published article (Angelova et al., 2015). Then we used the “GSVA” function in R to calculate the single sample gene set enrichment analysis (ssGSEA) score to measure the abundance of each cell subset in the samples. Adjusted scores were calculated as the enrichment scores divided by the (1 - tumor purity), which was calculated by the allele-specific copy-number analysis of tumors (Van Loo et al., 2010). Subsequently, k-means clustering was performed to classify the TNBC microenvironment phenotypes into three clusters: the “immune-desert” cluster, the “innate immune-inactivated” cluster, and the “immune-inflamed” cluster. The “immune-desert” cluster and the “innate immune-inactivated” cluster were referred as “cold tumor” while the “immune-inflamed” cluster was referred as “hot tumor”.
Statistical Analysis
The primary endpoint of this analysis was RFS. The RFS events were defined as the first recurrence of locally, regionally, or distantly invasive disease, a diagnosis of contralateral breast cancer, or death from any cause. The Kaplan-Meier method was used to estimate the distributions of survival outcomes, with the log-rank test evaluating differences of survival outcome. Cox proportional hazards model was used to obtain hazard ratios (HR) and 95% confidence intervals (CI). Differences of continuous and categorical factors were assessed by the Wilcoxon rank-sum test and the χ2 test (or Fisher exact test when necessary). All statistical tests were two-tailed, with the significant level being set at p < 0.05. Data were analyzed with STATA version 16.0 and R version 3.4.2.
Results
Patient Samples and Clinical Data
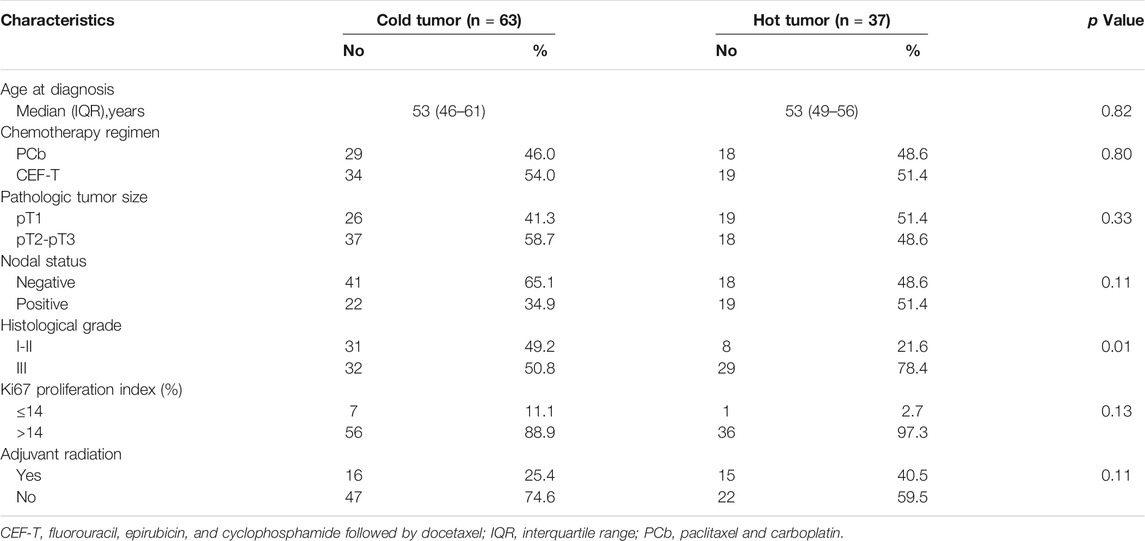
Clinicopathologic characteristics of the 100 patients involved are demonstrated in Table 1. There were 47 patients in the PCb arm and 53 patients in the CEF-T arm, respectively. The median age of these patients was 53 years (interquartile range, 47–59 years) at the time of PATTERN study entry. Among them, 43 patients belonged to the “immune-desert” cluster. Twenty and 37 patients belonged to the “innate immune-inactivated” cluster and the “immune-inflamed” cluster, respectively. Therefore, 63 patients with “cold tumor” and 37 patients with “hot tumor” were included in the analysis. Figure 1 depicted the details of the distribution of the microenvironment phenotype (Figure 1A), the FUSCC subtype (Jiang et al., 2019) (Figure 1B) and the intrinsic subtype (Parker et al., 2009) (Figure 1C) of these enrolled patients.
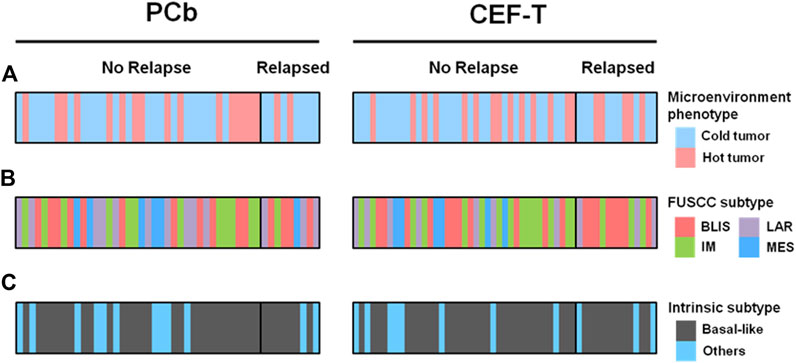
FIGURE 1. Microenvironment phenotype and other different subtypes by treatment cohorts. (A) Microenvironment phenotype, (B) FUSCC subtype, and (C) intrinsic subtype of the patients enrolled in the analysis. BLIS indicates basal-like and immune-suppressed; CEF-T, fluorouracil, epirubicin, and cyclophosphamide followed by docetaxel; FUSCC, Fudan University Shanghai Cancer Center; IM, immunomodulatory; LAR, luminal androgen receptor; MES, mesenchymal-like; PCb, paclitaxel and carboplatin.
Baseline characteristics of the patients of the two types are similar except that patients with “hot tumor” had relatively higher tumor histological grade than the patients with “cold tumor” (P = 0.01). There was no significant difference in chemotherapy regimens for patients of different microenvironment phenotypes as well. Compared with baseline characteristics of the whole PATTERN cohort, more patients enrolled in this analysis were node-positive (Table 2) in that these patients with a relatively greater tumor burden were more likely to provide sufficient frozen tissue and fulfill the criteria for further pathologic examination. 5
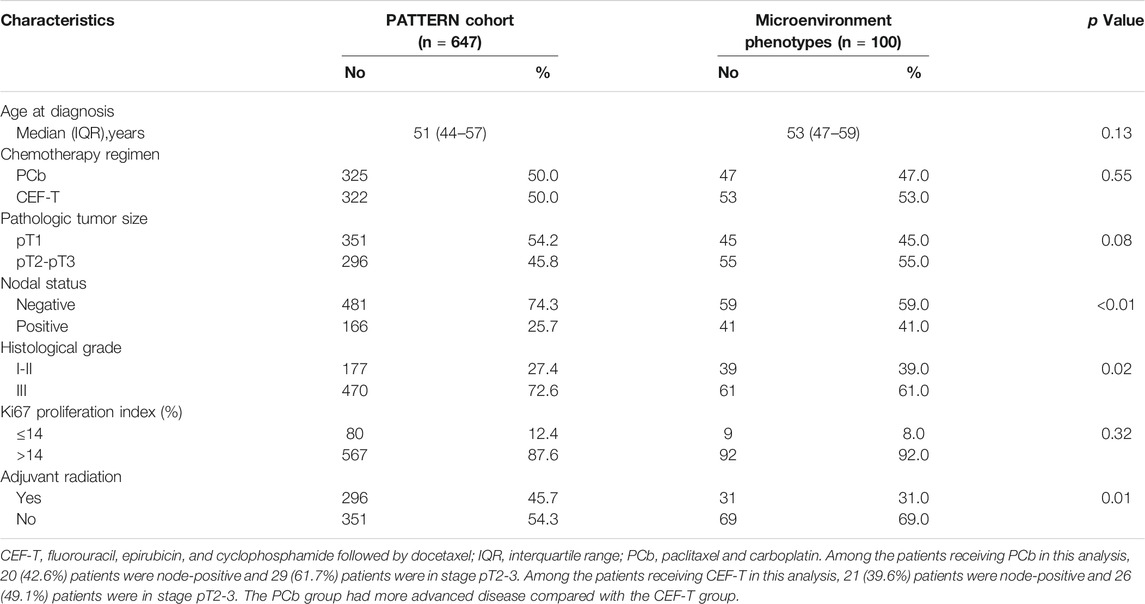
TABLE 2. Characteristics of the PATTERN cohort and the patients undergoing microenvironment phenotypes clustering.
Prognostic Significance of Microenvironment Phenotypes in TNBC
Considering the important role of TME in tumor progression, we investigated the prognostic significance of different TNBC microenvironment phenotypes taking advantage of the long-term survival data of the 100 patients from the PATTERN cohort. Firstly, we examined the association of microenvironment phenotypes with RFS status. The distribution of different types of the microenvironment in TNBC was similar between patients with different RFS statuses (P = 0.46). No significant difference in RFS was either detected between the patients with “hot tumor” and the patients with “cold tumor” (Figure 2A, HR = 0.68, 95% CI 0.28–1.66, P = 0.40).
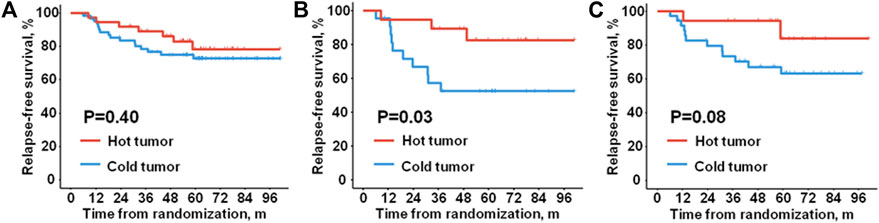
FIGURE 2. Relapse-free survival of different microenvironment phenotypes. Kaplan-Meier plots show relapse-free survival of (A) all the enrolled patients, (B) the node-positive patients, and (C) the patients with tumors in stage pT2-3.
However, we found that the TNBC microenvironment phenotypes were significantly associated with RFS status in the node-positive patients (P = 0.04). In contrast, no significant association was found between microenvironment phenotypes and RFS status in the node-negative patients (P = 0.47). Consistently, in the node-positive patients, a significantly better RFS was observed in the patients with “hot tumor” than the patients with “cold tumor” (Figure 2B, HR = 0.27, 95% CI 0.07–0.97, P = 0.03). There was no evidence of different RFS outcomes between the two phenotypes in the patients without lymph node metastasis (HR = 1.57, 95% CI 0.44–5.61, P = 0.48).
Subsequently, we investigated the prognostic relevance of microenvironment phenotypes in patients with different pathological tumor sizes. Given the limited number of cases enrolled in the analysis, a borderline significant association was indicated between the microenvironment phenotype with RFS status in the patients with tumor in stage pT2-3 (P = 0.09). Nevertheless, either of the microenvironment phenotypes was significantly related to RFS status in the patients with tumor in stage pT1 (P = 0.37). Similarly, in the patients with tumor in stage pT2-3, patients of the “hot tumor” phenotype had a borderline significantly longer RFS than the patients of the “cold tumor” phenotype (Figure 2C, HR = 0.29, 95%CI 0.06–1.30, P = 0.08). No significant difference in RFS was observed between the two microenvironment phenotypes in the patients with tumors in stage pT1 (HR = 1.76, 95% CI 0.47–6.57, P = 0.39).
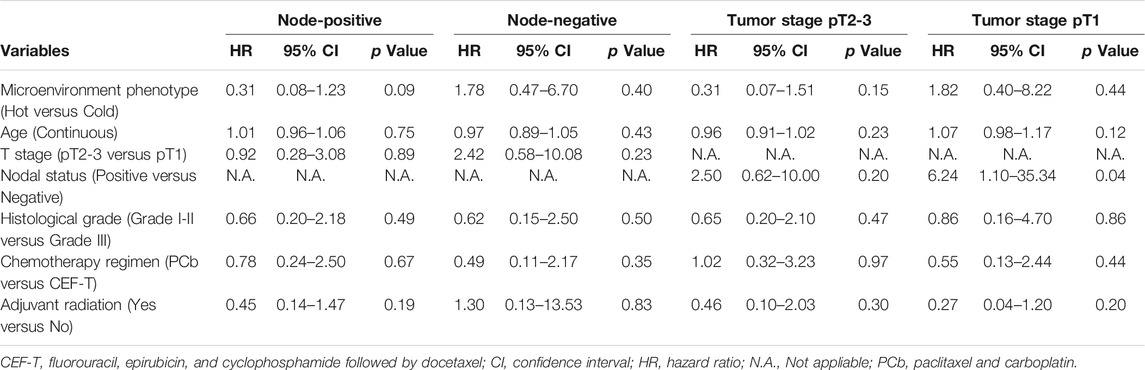
To further validate the reliability of the prognostic effect of the microenvironment phenotypes in TNBC, we conducted a multivariate analysis in patients of different nodal statuses and pathologic tumor sizes (Table 3). Prognostic relevance of the TNBC microenvironment phenotypes in patients with different status of age, histological grades, and adjuvant radiation therapy was also analyzed. The detail of the results was included in the Supplementary Contents.
Microenvironment Phenotypes Relating to the Efficacy of Adjuvant Chemotherapy
Considering the different features of genomic alteration of the microenvironment phenotypes in TNBC (Xiao et al., 2019), we further explored the association between the microenvironment phenotypes and the efficacy of adjuvant chemotherapy regimens. In the patients with “cold tumor”, the distribution of RFS was similar in the PCb cohort and the CEF-T cohort (Figure 3A, HR = 1.05, 95% CI 0.39–2.82, P = 0.92). In the patients with “hot tumor”, no significant difference in RFS was detected between the PCb cohort and the CEF-T cohort (Figure 3B, HR = 0.39, 95% CI 0.08–2.01, P = 0.24). Consistently, there was no significant difference in RFS between the patients with “hot tumor” and the patients with “cold tumor” within the PCb arm (HR = 0.39, 95% CI 0.08–1.88, P = 0.22) or the CEF-T arm (HR = 0.99, 95% CI 0.33–2.95, P = 0.98).
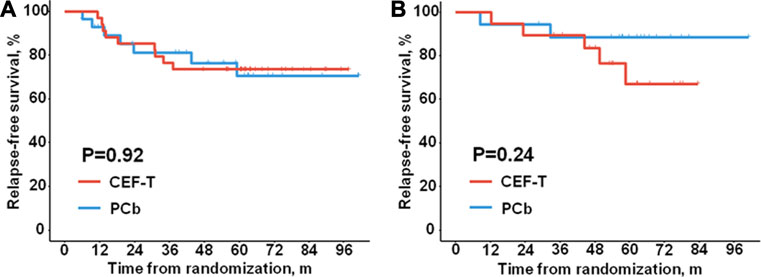
FIGURE 3. Relapse-free survival of different treatment cohorts. Kaplan-Meier plots show relapse-free survival of (A) the patients with “cold tumor”, (B) the patients with “hot tumor”. CEF-T indicates fluorouracil, epirubicin, and cyclophosphamide, followed by docetaxel; PCb, paclitaxel and carboplatin.
Given the prognostic effect of the microenvironment phenotypes in the patients with lymph node metastasis or tumors in stage pT2-3, we examined the association of the microenvironment phenotypes with adjuvant chemotherapy efficacy in these patients who had a relatively higher risk of relapse or metastasis. In the node-positive patients, no significant difference in RFS was observed between the PCb arm and the CEF-T arm no matter in the “hot tumor” subtype (HR = 2.13, 95% CI 0.19–23.63, P = 0.53) or in the “cold tumor” subtype (HR = 0.74, 95% CI 0.21–2.61, P = 0.63). A similar trend of the “cold tumor” phenotype (HR = 1.21 95% CI 0.39–3.75, P = 0.74) was also observed in the patients with tumor in stage pT2-3. RFS events in the “hot tumor” phenotype in patients with tumor in stage pT2-3 were not enough to calculate the HR and 95% CI.
Discussion
Taking advantage of the expression data of the early-stage TNBC patients from the PATTERN cohort, we investigated the prognostic significance of the microenvironment phenotype in TNBC and its association with the efficacy of adjuvant chemotherapy regimens.
Recent researches have demonstrated that different cell types in the TME are associated with response to treatment and long-term prognosis of TNBC (Denkert et al., 2010; Su et al., 2014; Denkert et al., 2015; Costa et al., 2018). In our study, we found no significant difference in RFS between the patients of the “hot tumor” phenotype and the patients of the “cold tumor” phenotype. However, in the node-positive patients enrolled in the analysis, the “hot tumor” subtype was related to a significantly better RFS compared with the “cold tumor” subtype, while the distribution of survival of the two subtypes was similar in the node-negative patients. This indicates that the “hot tumor” microenvironment phenotype with abundant adaptive and innate immune cells infiltration might be associated with a better outcome for TNBC patients with lymph node metastasis. Consistently, a borderline significantly longer RFS was observed in the “hot tumor” subtype in the patients with tumor in stage pT2-3, suggesting the prognostic effect of the microenvironment phenotypes in the patients with relatively higher tumor burden. By examining the microenvironment phenotypes in TNBC, we can better distinguish the risk of recurrence and metastasis in node-positive patients and high-risk node-negative patients. Yet, no conclusions could be drawn before further validation is conducted in the prospective study.
In addition, as the microenvironment phenotypes in TNBC have a different level of mutation load and homologous recombination deficiency (Xiao et al., 2019), we subsequently explored its association with the efficacy of different adjuvant chemotherapy for TNBC. No significant difference in RFS was observed between the patients treated by PCb and the patients treated by CEF-T in either of the two phenotypes. There was also no significant difference in RFS between the PCb cohort and the CEF-T cohort in the node-positive patients or stage pT2-3 patients of the two phenotypes. It reflects the limited power of the microenvironment phenotypes in TNBC in predicting the efficacy of a carboplatin-containing regimen.
Our research has some limitations. Firstly, the results presented here are limited by their retrospective character despite using a prospective cohort. Secondly, the limited number of cases with microenvironment phenotype data led to the insufficient statistical power of some tests involved. In addition, CEF-T is no longer a standard recommendation in the National Comprehensive Cancer Network guidelines. At present, epirubicin and cyclophosphamide followed by weekly paclitaxel (EC-wP) might be the optimal choice for TNBC (Sparano et al., 2008). Moreover, considering the limited number of cases enrolled in the analysis, larger prospective studies are necessary to determine whether carboplatin can benefit TNBC patients of certain microenvironment phenotypes.
In conclusion, our study reveals that the microenvironment phenotypes in TNBC might predict the prognosis of the node-positive patients and the high-risk node-negative patients. The association of the microenvironment phenotypes with the efficacy of adjuvant chemotherapy for TNBC remains to be further studied.
Data Availability Statement
All data can be viewed in The National Omics Data Encyclopedia (http://www.biosino.org/node) by pasting the accession (OEP000155) into the text search box or through the URL: http://www.biosino.org/node/project/detail/OEP000155. The HTA 2.0 microarray data is also available in GSE76250 and the RNA sequencing data is available in SRP157974. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Statement
The studies involving human participants were reviewed and approved by Independent institutional review board of Fudan University Shanghai Cancer Center. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This study was Supported by grants from the National Natural Science Foundation of China (grants 81672600, 81722032, and 82072916), the 2018 Shanghai Youth Excellent Academic Leader, the Fudan ZHUOSHI Project, and Chinese Young Breast Experts Research project (CYBER-2021-A01).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer (K.W.) declared a shared affiliation, with no collaboration, with the authors to the handling editor at the time of the review.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmolb.2021.752154/full#supplementary-material.
References
Angelova, M., Charoentong, P., Hackl, H., Fischer, M. L., Snajder, R., Krogsdam, A. M., et al. (2015). Characterization of the Immunophenotypes and Antigenomes of Colorectal Cancers Reveals Distinct Tumor Escape Mechanisms and Novel Targets for Immunotherapy. Genome Biol. 16, 64. doi:10.1186/s13059-015-0620-6
Becht, E., Giraldo, N. A., Lacroix, L., Buttard, B., Elarouci, N., Petitprez, F., et al. (2016). Estimating the Population Abundance of Tissue-Infiltrating Immune and Stromal Cell Populations Using Gene Expression. Genome Biol. 17, 218. doi:10.1186/s13059-016-1070-5
Bianchini, G., Balko, J. M., Mayer, I. A., Sanders, M. E., and Gianni, L. (2016). Triple-negative Breast Cancer: Challenges and Opportunities of a Heterogeneous Disease. Nat. Rev. Clin. Oncol. 13, 674–690. doi:10.1038/nrclinonc.2016.66
Burstein, M. D., Tsimelzon, A., Poage, G. M., Covington, K. R., Contreras, A., Fuqua, S. A. W., et al. (2015). Comprehensive Genomic Analysis Identifies Novel Subtypes and Targets of Triple-Negative Breast Cancer. Clin. Cancer Res. 21, 1688–1698. doi:10.1158/1078-0432.ccr-14-0432
Costa, A., Kieffer, Y., Scholer-Dahirel, A., Pelon, F., Bourachot, B., Cardon, M., et al. (2018). Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell 33, 463–479e10. doi:10.1016/j.ccell.2018.01.011,
Denkert, C., Liedtke, C., Tutt, A., and von Minckwitz, G. (2017). Molecular Alterations in Triple-Negative Breast Cancer-The Road to New Treatment Strategies. The Lancet 389, 2430–2442. doi:10.1016/s0140-6736(16)32454-0
Denkert, C., Loibl, S., Noske, A., Roller, M., Müller, B. M., Komor, M., et al. (2010). Tumor-associated Lymphocytes as an Independent Predictor of Response to Neoadjuvant Chemotherapy in Breast Cancer. Jco 28, 105–113. doi:10.1200/jco.2009.23.7370
Denkert, C., von Minckwitz, G., Brase, J. C., Sinn, B. V., Gade, S., Kronenwett, R., et al. (2015). Tumor-infiltrating Lymphocytes and Response to Neoadjuvant Chemotherapy with or without Carboplatin in Human Epidermal Growth Factor Receptor 2-positive and Triple-Negative Primary Breast Cancers. Jco 33, 983–991. doi:10.1200/jco.2014.58.1967
Denkert, C., von Minckwitz, G., Darb-Esfahani, S., Lederer, B., Heppner, B. I., Weber, K. E., et al. (2018). Tumour-infiltrating Lymphocytes and Prognosis in Different Subtypes of Breast Cancer: a Pooled Analysis of 3771 Patients Treated with Neoadjuvant Therapy. Lancet Oncol. 19, 40–50. doi:10.1016/s1470-2045(17)30904-x
Harbeck, N., and Gnant, M. (2017). Breast Cancer. The Lancet 389, 1134–1150. doi:10.1016/s0140-6736(16)31891-8
Jiang, Y.-Z., Ma, D., Suo, C., Shi, J., Xue, M., Hu, X., et al. (2019). Genomic and Transcriptomic Landscape of Triple-Negative Breast Cancers: Subtypes and Treatment Strategies. Cancer Cell 35, 428–440e5. doi:10.1016/j.ccell.2019.02.001,
Lehmann, B. D., Bauer, J. A., Chen, X., Sanders, M. E., Chakravarthy, A. B., Shyr, Y., et al. (2011). Identification of Human Triple-Negative Breast Cancer Subtypes and Preclinical Models for Selection of Targeted Therapies. J. Clin. Invest. 121, 2750–2767. doi:10.1172/jci45014
Loi, S., Michiels, S., Salgado, R., Sirtaine, N., Jose, V., Fumagalli, D., et al. (2014). Tumor Infiltrating Lymphocytes Are Prognostic in Triple Negative Breast Cancer and Predictive for Trastuzumab Benefit in Early Breast Cancer: Results from the FinHER Trial. Ann. Oncol. 25, 1544–1550. doi:10.1093/annonc/mdu112
Newman, A. M., Liu, C. L., Green, M. R., Gentles, A. J., Feng, W., Xu, Y., et al. (2015). Robust Enumeration of Cell Subsets from Tissue Expression Profiles. Nat. Methods 12, 453–457. doi:10.1038/nmeth.3337
Parker, J. S., Mullins, M., Cheang, M. C. U., Leung, S., Voduc, D., Vickery, T., et al. (2009). Supervised Risk Predictor of Breast Cancer Based on Intrinsic Subtypes. Jco 27, 1160–1167. doi:10.1200/jco.2008.18.1370
Perou, C. M., Sørlie, T., Eisen, M. B., van de Rijn, M., Jeffrey, S. S., Rees, C. A., et al. (2000). Molecular Portraits of Human Breast Tumours. Nature 406, 747–752. doi:10.1038/35021093
Schmid, P., Adams, S., Rugo, H. S., Schneeweiss, A., Barrios, C. H., Iwata, H., et al. (2018). Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 379, 2108–2121. doi:10.1056/nejmoa1809615
Sparano, J. A., Wang, M., Martino, S., Jones, V., Perez, E. A., Saphner, T., et al. (2008). Weekly Paclitaxel in the Adjuvant Treatment of Breast Cancer. N. Engl. J. Med. 358, 1663–1671. doi:10.1056/nejmoa0707056
Su, S., Liu, Q., Chen, J., Chen, J., Chen, F., He, C., et al. (2014). A Positive Feedback Loop between Mesenchymal-like Cancer Cells and Macrophages Is Essential to Breast Cancer Metastasis. Cancer Cell 25, 605–620. doi:10.1016/j.ccr.2014.03.021
Van Loo, P., Nordgard, S. H., Lingjaerde, O. C., Russnes, H. G., Rye, I. H., Sun, W., et al. (2010). Allele-specific Copy Number Analysis of Tumors. Proc. Natl. Acad. Sci. 107, 16910–16915. doi:10.1073/pnas.1009843107
Xiao, Y., Ma, D., Zhao, S., Suo, C., Shi, J., Xue, M.-Z., et al. (2019). Multi-Omics Profiling Reveals Distinct Microenvironment Characterization and Suggests Immune Escape Mechanisms of Triple-Negative Breast Cancer. Clin. Cancer Res. 25, 5002–5014. doi:10.1158/1078-0432.ccr-18-3524
Keywords: triple-negative breast cancer, microenvironment phenotype, biomarker, trial, immune
Citation: Zhu S-Y, Ma D, Shao Z-M and Yu K-D (2021) Prognostic Effect of Microenvironment Phenotype in Triple-Negative Breast Cancer: Biomarker Analysis of a Prospective Trial. Front. Mol. Biosci. 8:752154. doi: 10.3389/fmolb.2021.752154
Received: 02 August 2021; Accepted: 31 August 2021;
Published: 21 September 2021.
Edited by:
Dongqing Wei, Shanghai Jiao Tong University, ChinaReviewed by:
Kun Wang, Guangdong Provincial People’s Hospital, ChinaKejin Wu, Fudan University, China
Copyright © 2021 Zhu, Ma, Shao and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ke-Da Yu, eXVrZWRhQGZ1ZGFuLmVkdS5jbg==, WXVrZWRhQDE2My5jb20=
 Si-Yuan Zhu1,2
Si-Yuan Zhu1,2 Ke-Da Yu
Ke-Da Yu