- 1Department of Pain, The First Hospital of Jilin University, Changchun, China
- 2Department of Ophthalmology, The Second Hospital of Jilin University, Changchun, China
Increasing evidence reveals that lysophosphatidylcholine (LPC) is closely related to endothelial dysfunction. The present study aimed to investigate the mechanism of LPC in inhibiting the proangiogenesis and vascular inflammation of human endothelial progenitor cells (EPCs) derived from CD34+ cells. The early EPCs were derived from CD34+ hematopoietic stem cells whose purity was identified using flow cytometry analysis. The surface markers (CD34, KDR, CD31; VE-cadherin, vWF, eNOS) of EPCs were examined by flow cytometry analysis and immunofluorescence. RT-qPCR was used to detect the mRNA expression of inflammatory cytokines (CCL2, IL-8, CCL4) and genes associated with angiogenesis (VEGF, ANG-1, ANG-2) in early EPCs after treatment of LPC (10 μg/ml) or phosphatidylcholine (PC, 10 μg/ml, control). The angiogenesis of human umbilical vein endothelial cells (HUVECs) incubated with the supernatants of early EPCs was detected by a tube formation assay. The mRNA and protein levels of key factors on the PKC pathway (phosphorylated PKC, TGF-β1) were measured by RT-qPCR and western blot. The localization of PKC-β1 in EPCs was determined by immunofluorescence staining. We found that LPC suppressed the expression of CCL2, CCL4, ANG-1, ANG-2, promoted IL-8 expression and had no significant effects on VEGF expression in EPCs. EPCs promoted the angiogenesis of HUVECs, which was significantly inhibited by LPC treatment. Moreover, LPC was demonstrated to promote the activation of the PKC signaling pathway in EPCs. In conclusion, LPC inhibits proangiogenesis of human endothelial progenitor cells derived from CD34+ hematopoietic stem cells.
Introduction
The endothelial progenitor cells (EPCs) derived from hematopoietic stem cells of bone marrow is the precursor of endothelial cells. EPCs contribute to endothelialization and vascularization via the secretion of vasoactive and angiogenic factors including VEGF, HGF, Ang-1, SDF-1α, TGF-β, and IL-8 to repair the damaged tissues (Rosenzweig, 2003; Li and Li, 2016). EPCs have been reported to protect differentiated endothelial cells from apoptosis, while the repair mechanism of EPCs was weakened in the aging process (Wang et al., 2019). The dysfunction and loss of EPCs are dominant characteristics of many vascular pathologies. Increasing attention has been drawn in the application of EPCs as regenerative medicine in vascular pathologies such as cardiovascular diseases, atherosclerosis, and thrombosis (Liu et al., 2009; Li and Li, 2016; Kou et al., 2020). However, some limitations of EPC transplantation such as emboli formation, immunogenicity, and malignant transformation hinder the application of EPCs in stem cell therapy (Herberts et al., 2011).
Lysophosphatidylcholine (LPC) is the primary component of oxidized low-density lipoprotein (ox-LDL) which is a major contributor to many cardiovascular diseases (Itabe et al., 1994; Tsimikas et al., 2003). Previous studies have revealed that LPC promotes inflammation (Sharma et al., 2020), endothelial dysfunction (Kugiyama et al., 1990), injury and apoptosis of vascular smooth muscle cells (Hsieh et al., 2000). The expression of LPC is altered in many pathologies. For example, LPC expression is significantly elevated in the plasma of psoriatic patients (Zeng et al., 2017). LPC is a potent atherogenic molecule with an 8-fold upregulated expression levels in the intima and inner media of atherosclerotic aorta of squirrel monkeys and controls the incorporation of fatty acid into aortic phospholipid in atherosclerosis (Portman and Alexander, 1969). Rheumatoid arthritis is related to increased LPC expression that is positively correlated with cardiovascular risks (Giraud et al., 2019). Moreover, LPC and sphingolipids showed significant expression alteration in patients with acute aortic dissection and are suggested to be used as potential biomarkers for its diagnosis (Zhou et al., 2019).
The influence of oxidized low-density lipoprotein (oxLDL) on EPCs has been revealed by a previous study that oxLDL inhibits the cell growth, migration and adhesiveness of EPCs [13]. The oxLDL or LPC facilitates apoptosis and inhibits survival of EPCs by suppressing the PI3K/Akt signaling pathway and downregulating the expression of endothelial nitric oxide synthase (eNOS) (Ma et al., 2006; Tie et al., 2010; Hong et al., 2015), which may lead to various diseases (Liu et al., 2020). However, the underlying mechanisms between LPC and EPCs remained uninvestigated.
The aim of this study was to explore the function of LPC on EPCs derived from CD34+ hematopoietic stem cells in vitro. We hypothesized that LPC exerts suppressive effect on EPC angiogenesis and the underlying mechanism was further investigated, which may provide therapeutic basis for the application of EPC transplantation in the treatment of various vascular diseases.
Materials and Methods
Cell Culture and Identification
The study was approved by the ethics committee of the Second Hospital of Jilin University (approval number: 2021-061; Jilin, China). All participants gave informed consents before the study. The human hematopoietic stem cells were isolated from peripheral blood (80 ml) of healthy adult donors (n = 12). The peripheral blood was diluted with PBS in a ratio of 1:3. Subsequently, 20 ml of the peripheral blood was added into the Lymphocyte Separation Medium (Ficoll-Pague T Plus, 10 ml) and centrifuged at 400 g for 30 min. A pasteur pipette was used to transfer the middle layer of mononuclear cells into a centrifuge tube. Peripheral blood mononuclear cells were washed with 40 ml of PBS and mechanically dissociated into single cell suspension. Next, Magic™ Fc Receptor Blocker (Abace Biotechnology) and 100 μL of magnetic beads of CD34+ was added into the cell suspension and incubated for 30 min at 4°C. Finally, CD34+ cells were collected using MS Columns and MiniMACS™ Separator. The early EPCs were differentiated from CD34+ human hematopoietic stem cells. The CD34+ cells at the density of 1 × 106 cells/cm2 were seeded on fibronectin-coated dish and cultured at 37°C in 5% CO2. The FACSCalibur™ flow cytometer (Becton, Dickinson and Company, Singapore) was used to detect the labeled EPCs in the peripheral blood and a CellQuest™ software (BD Biosciences) was used for quantification. Common endothelial cell lines used for the scientific research include human umbilical vein endothelial cells (HUVECs), bovine aortic endothelial cells and microvascular endothelial cells. HUVECs have the advantage of human origin, compared with the bovine aortic endothelial cells, and are relatively easier to be cultured, compared with the microvascular endothelial cells. HUVECs were purchased from American Type Culture Collection and incubated with Dulbecco’s modified Eagle’s medium (DMEM; Thermo Fisher, Shanghai, China) containing 10% FBS at 37°C in 5% CO2. EPCs received three different treatments: 1) PBS, 2) 10 μg/ml of LPC, 3) 10 μg/ml of phosphatidylcholine (PC, the negative control).
Flow Cytometry Analysis
The suspension of EPCs (200 μL) were added with 3 ml of PBS at 4°C in a closed falcon tube. Then 10 µL of polyclonal antibodies against CD34, KDR and CD31 labeled with FITC were added into the tubes. After coincubation at 4°C for 1 h in the dark, PBS was used to wash the EPCs followed by centrifugation and removal of the supernatant. After resuspending in PBS with 0.5% paraformaldehyde, CD34+ hematopoietic stem cells and CD34+, KDR+, CD31+ EPCs were quantified in triplicate relative to the number of CD45+FShighSShigh granulocytes in the sample with a FACSCalibur™ flow cytometer by a CellQuest™ software (BD Biosciences).
Reverse Transcription Quantitative Polymerase Chain Reaction
Total RNAs in EPCs were isolated by TRIzol reagent (Invitrogen). The concentration of the extracted RNAs was assessed using the NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific), and the absorbance was read at 260 and 280 nm. Reverse transcription of total RNA (1 µg) into complementary DNA was conducted with a RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher) according to the manufacturer’s instructions. A QuantStudio five Real-Time PCR System (Thermo Fisher) was used to perform the qPCR assays under the thermocycling conditions as follows: 40 cycles at 95°C for 15 s, at 60°C for 1 min. The 2−∆∆Ct method (Livak and Schmittgen, 2001) was used to quantify the relative mRNA levels of CCL2, IL-8, CCL4, VEGF, ANG-1, ANG-2, PKC-β1, TGF-β1 normalized to GAPDH. The primer sequences are provided in Table 1.
Western Blot
Proteins in EPCs were extracted using RIPA lysis buffer (Invitrogen) followed by centrifugation at 12,000 g for 15 min at 4°C. The ultraviolet spectroscopy was used to determine the concentrations of proteins. After being isolated with 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis, 50 µg of proteins were transferred to a nitrocellulose membrane which was blocked with 5% non-fat milk at room temperature for 1 h. Then the membranes were incubated with primary antibodies including anti-CCL2 (#ab214819, 1/1,000, Abcam), anti-CCL4 (#ab45690, 1/1,000, Abcam), anti-IL-8 (#ab235584, 1/1,000, Abcam), anti-VEGF (#ab1316, 1/2000, Abcam), anti-ANG-1 (#ab8451, 1/500, Abcam), anti-ANG-2 (#ab155106, 1/500, Abcam), anti-TGF-β1 (#ab215715, 1/1,000, Abcam), anti-p-PKC (#ab109539, 1/1,000, Abcam), anti-GAPDH (#ab9485, 1/2,500, Abcam) at 4°C overnight. GAPDH served as the loading control. Next, membranes were incubated with horseradish peroxidase conjugated secondary antibodies for 1 h at room temperature. The enhanced chemiluminescence solution (Thermo Fisher Scientific) was used to visualize the targeted protein bands. The ImageJ software was used to detect densitometry analysis of the band intensity.
Immunofluorescence Staining
Immunofluorescence staining was used to detect the expression of EPC surface markers or the location of PKC-β1 in EPCs. EPCs (1 × 105 cells per well) were fixed on 8-well chamber slides with 4% paraformaldehyde for 15 min, and then blocked with 0.1% bovine serum albumin for 30 min at room temperature followed by incubation with the primary antibodies including anti-VE-cadherin (#ab225443, 1/50, Abcam), anti-vWF (#195029, 1/50, Abcam), anti-Enos (#ab76198, 1/200, Abcam), anti-PKC-β1 (#ab223452, 1/250, Abcam) at 4°C overnight. On the second day, cells were incubated with fluorescence (Alexa Fluor 488 or Alexa Fluor 647) labeled secondary antibodies for 1 h at 37°C. Subsequently, EPCs were stained with diamidino-phenyl-indole for 10 min at 37°C in the dark. A fluorescence microscope, Olympus FV 1000 (Olympus, Seoul, Korea), was used to capture the images.
Tube Formation Assay
Each well of the 96-well plates was precoated with 50 μL of Matrigel (Corning, Bedford, MA, United States). EPCs (1 × 105 cells per well), control HUVECs (2 × 104 cells per well), HUVECs (2 × 104 per well) under different treatments: 1) 100 μL of culture supernatant of EPC cultures; 2) 100 μL of culture supernatant of EPC cultures and 10 μg/ml of PC; 3) 100 μL of culture supernatant of EPC cultures and 10 μg/ml of LPC were plated into 96-well plates at 37°C with 1% O2 and 5% CO2 for 10 h. The tube counts and capillary lengths in each group were observed in five randomly chosen visual fields at ×100 magnification using ImageJ software.
Statistical Analysis
Data were shown as the mean ± standard deviation. SPSS23.0 was used for statistical analyses. Normality was tested using a Shapiro-Wilk normality test. The comparison between two groups was evaluated by Student’s t-test, and difference among multiple groups was evaluated by one-way analysis of variance followed by Tukey’s post hoc test. All experiments were performed triplicates independently. p < 0.05 was regarded as statistically significant.
Results
Identification of CD34+ Hematopoietic Stem Cells
Flow cytometry analysis was used to detect the purity of CD34+ hematopoietic stem cells. The result showed that nearly 80% of the cells expressed CD34 after positive selection process (Figure 1A). CD34+ hematopoietic stem cells were successfully expanded in vitro.
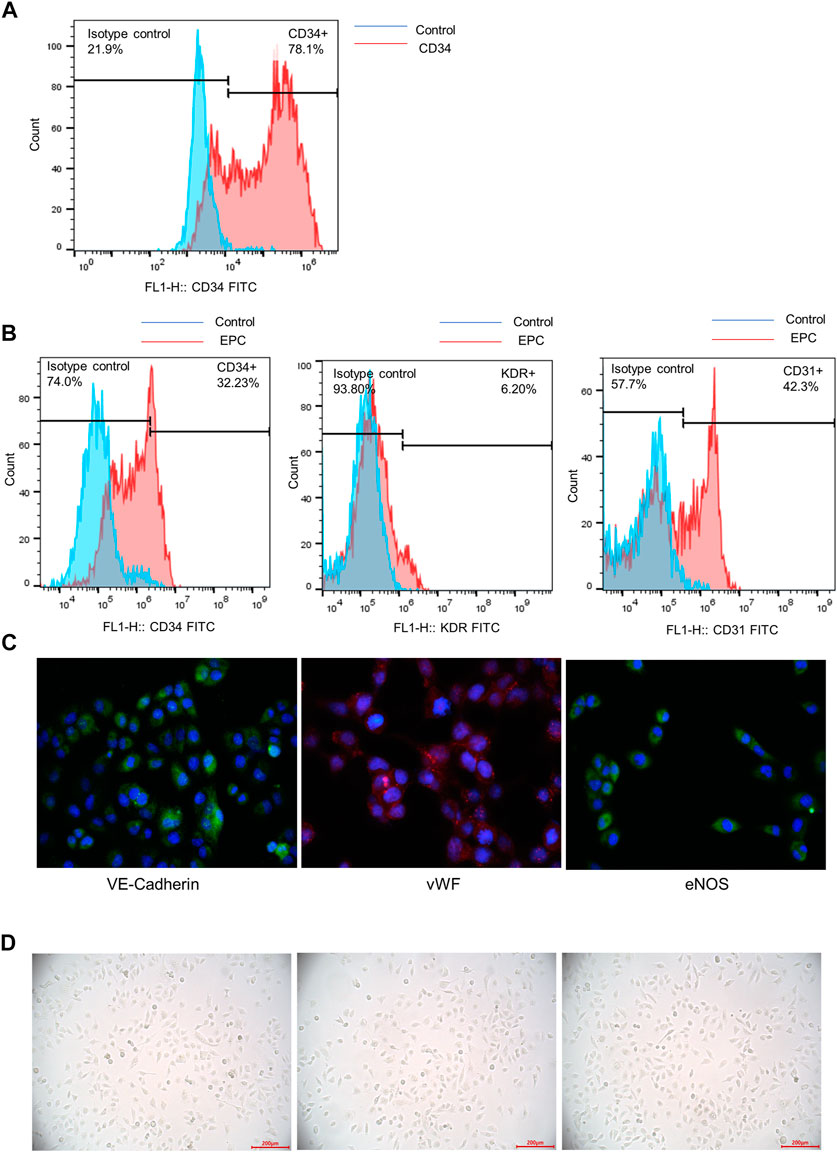
FIGURE 1. Identification of CD34+ hematopoietic stem cells and differentiation of CD34+ hematopoietic stem cells to early EPC. (A) The purity of CD34+ hematopoietic stem cells was accessed by a flow cytometry analysis. (B) Flow cytometry analysis was used to detect the expression of surface markers (CD34, KDR and CD31) of EPCs derived from CD34+ cells. (C) The immunofluorescence staining was used to examine the specific biomarkers of the endothelial cells on the cell surface (VE-Cadherin, vWF and eNOS). (D) The morphological features of EPCs derived from CD34+ hematopoietic stem cells were observed using a microscope.
Differentiation of CD34+ Hematopoietic Stem Cells to Early EPCs
Flow cytometry analysis showed the expression of surface markers of EPCs derived from CD34+ cells. The result indicated that CD34, KDR and CD31 were positively expressed in early EPCs. The expression of CD34 and KDR was relatively lower than CD31 expression (Figure 1B). The immunofluorescence staining of specific cell surface biomarkers of the endothelial cells including VE-Cadherin, vWF and eNOS were shown in Figure 1C. VE-Cadherin (green), vWF (red), eNOS (green) were expressed in early EPCs, and the cell nucleus was stained blue. The morphology of EPCs was observed under a microscope. The adherent cells were in spindle shape, which showed the morphological features of early EPCs cultured in vitro (Figure 1D). Overall, the CD34+ hematopoietic stem cells incubated in vitro were successfully differentiated into early EPCs.
The Expression of Inflammatory Cytokines After LPC Treatment in EPCs
After treatment of LPC or PC at the concentration of 10 μg/ml for 1, 5 or 24 h, the EPCs were harvested and the expression levels of inflammatory factors (CCL2, IL-8, CCL4) in EPCs were detected using RT-qPCR analysis. The result showed that the expression of CCL2 was not significantly altered after treatment for 1 h compared with that in the control group. CCL2 mRNA expression was decreased by LPC by 70% after 5 h and by 31% after 24 h. CCL2 protein expression was also decreased by LPC after 5 and 24 h (Figure 2A). The IL-8 expression at the mRNA level was significantly higher in LPC group than in control group after 1 h (17.5 fold changes), 5 h (10.7 fold changes) and 24 h (2.9 fold changes). IL-8 protein expression was also increased after LPC treatment for 1, 5, 24 h (Figure 2B). Treatment of LPC for 1 h had no significant effects on CCL4 expression. CCL4 mRNA expression was significantly decreased by LPC by 49% for 5 h, by 58% for 24 h. CCL4 protein expression was also decreased by LPC after 5 and 24 h (Figure 2C).
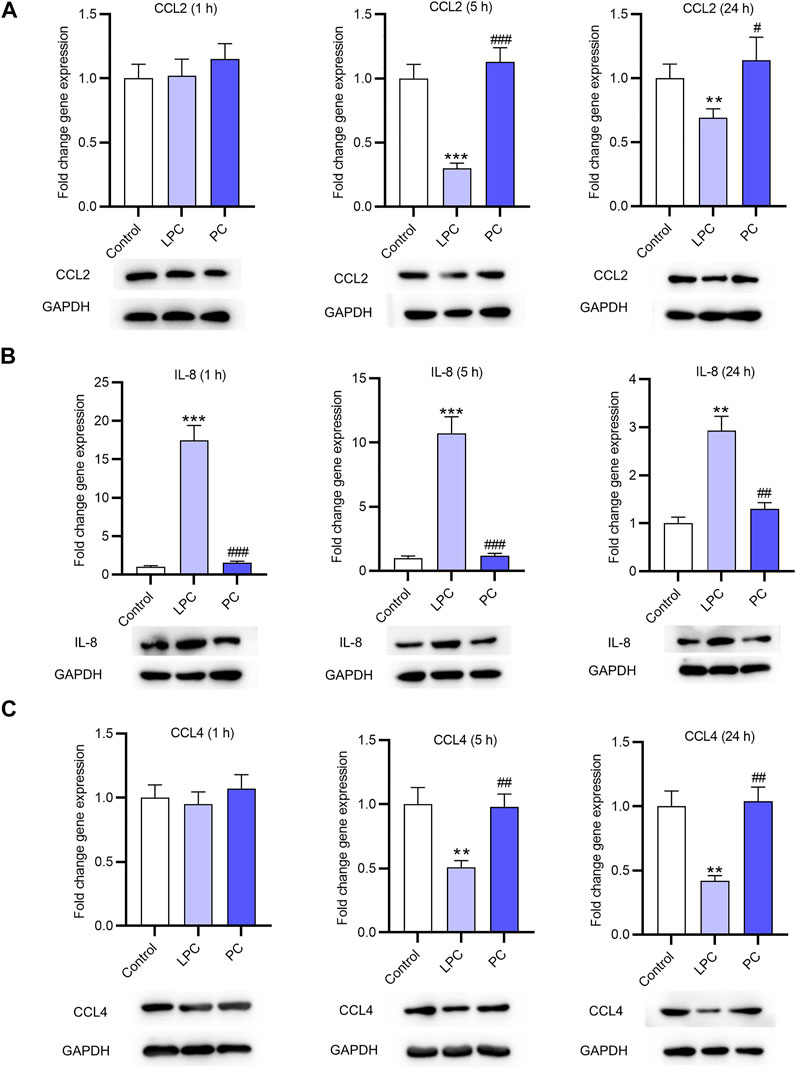
FIGURE 2. The expression of inflammatory cytokines after LPC or PC treatment in EPCs. RT-qPCR and western blotting analyses were used to detect the expression of inflammatory factors including CCL2 (A), IL-8 (B), CCL4 (C) in EPCs after 10 μg/ml of LPC or PC treatment for 1, 5 or 24 h **p < 0.01, ***p < 0.001 compared with the control, #p < 0.05, ###p < 0.001 compared with the LPC group.
The Expression of Factors Associated With Angiogenesis After LPC Treatment in EPCs
The expression of VEGF and Ang-1 in EPCs was accessed using RT-qPCR. After treatment for 1, 5 and 24 h, the expression of VEGF showed no significant changes (Figure 3A). Ang-1 and Ang-2 expression at the mRNA and protein levels was not significantly affected by LPC or PC treatment for 1 h. After treatment for 5 and 24 h, the Ang-1 and Ang-2 expression at the mRNA and protein levels was significantly lower in LPC group than in control group or PC group (Figures 3B,C). In detail, LPC treatment for 5 h decreased Ang-1 mRNA expression by 44%, and the number for 24 h is 47%. Ang-2 mRNA expression was inhibited by LPC treatment for 5 h by 57% and for 24 h by 43%.
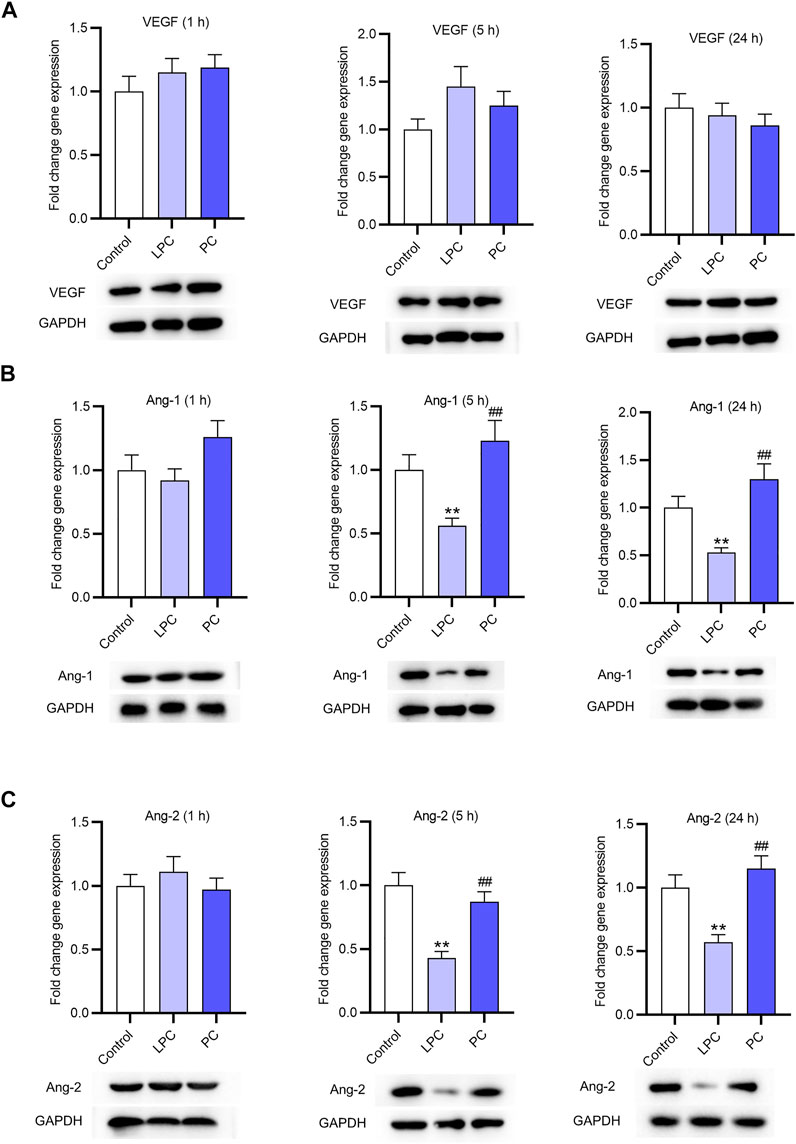
FIGURE 3. The expression of factors associated with angiogenesis after LPC or PC treatment in EPCs. RT-qPCR and western blotting was used to access the levels of angiogenesis-associated genes including VEGF (A), Ang-1 (B), Ang-2 (C) in EPCs after 10 μg/ml of LPC or PC treatment for 1, 5 and 24 h *p < 0.05, **p < 0.01 compared with the control, ##p < 0.01 compared with the LPC group.
The Suppressive Effect of LPC on the Proangiogenesis of EPCs
A tube formation assay was used to examine the angiogenesis potential of EPCs and HUVECs in vitro. The result demonstrated that EPCs alone did not independently develop tubes in vitro (Figure 4A). After incubation with supernatant of EPC cultures, HUVECs showed the increased potential of angiogenesis (Figures 4B,C). After LPC treatment, the tubes formed by HUVECs were loose and not intact compared with that after PC treatment (Figures 4D,E). These conclusions are supported by the data of quantification of tube count and capillary length, as revealed in Figures 4F,G.
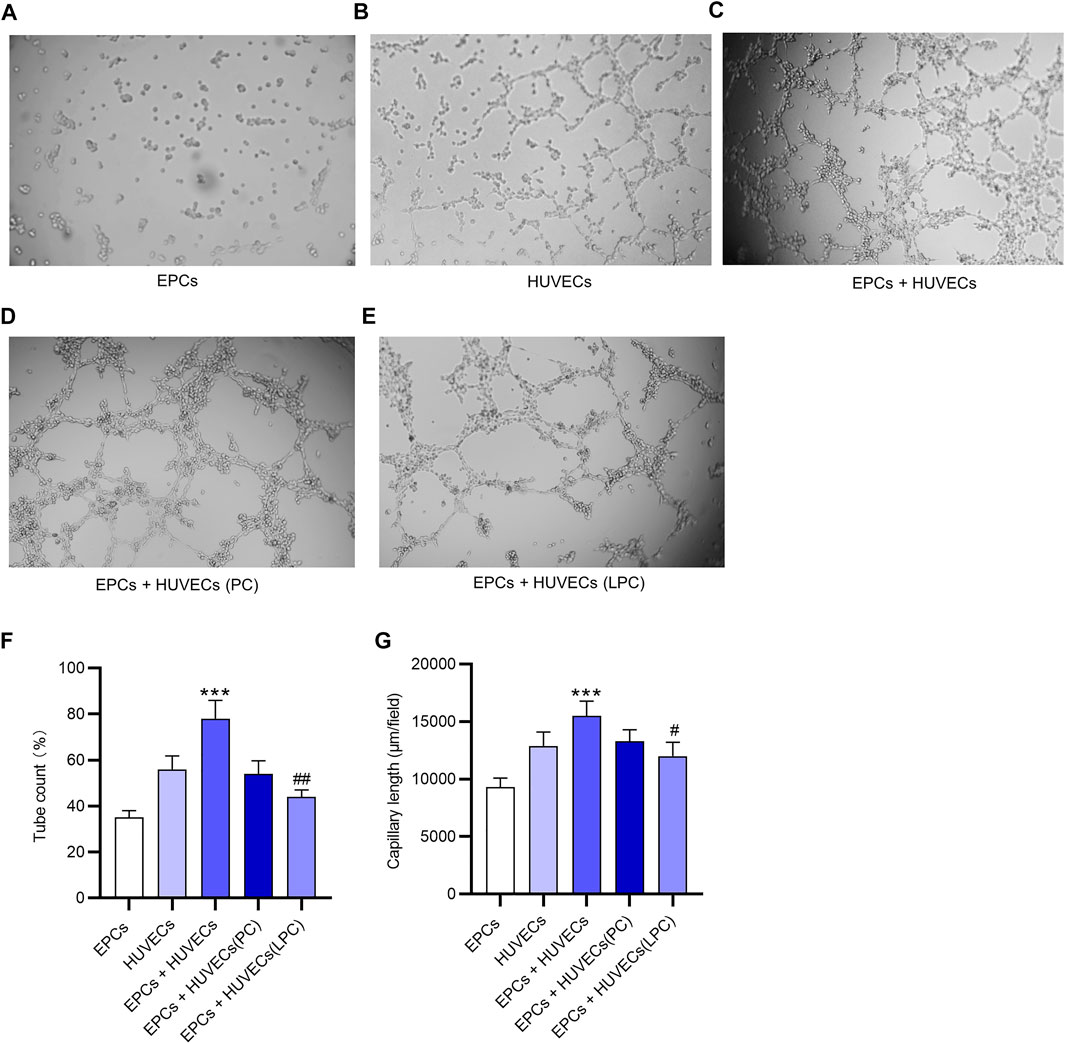
FIGURE 4. The suppressive effect of LPC on the proangiogenesis of EPCs. (A) A tube formation assay was used to examine the angiogenesis potential of EPCs in vitro. (B) Tubes developed by HUVECs in vitro. (C) Tubes developed by HUVECs cultured with the supernatant of EPCs in vitro. (D) After the PC treatment or (E) LPC treatment, the tubes formed by HUVECs that were cultured with supernatant of EPCs. (F,G) Tube count and capillary length of tubes in each group. ***p < 0.001 compared with the HUVECs group, #p < 0.05, ##p < 0.01 compared with the EPCs + HUVECs group.
The Promotive Effect of LPC on the Activation of PKC Signaling in EPCs
The mRNA levels of PKC-β1 and TGF- β1 in EPCs were both significantly increased after the LPC treatment compared with the control group (Figures 5A,B). The protein expression of p-PKC and TGF-β1 was higher in LPC group compared with the control group (Figures 5C,D). Moreover, according to results of immunofluorescence staining assay, PKC-β1 was primarily located in the cytoplasm, and the translocation of the PKC-β1 protein to the nucleus was promoted after LPC treatment in EPCs (Figure 5E).
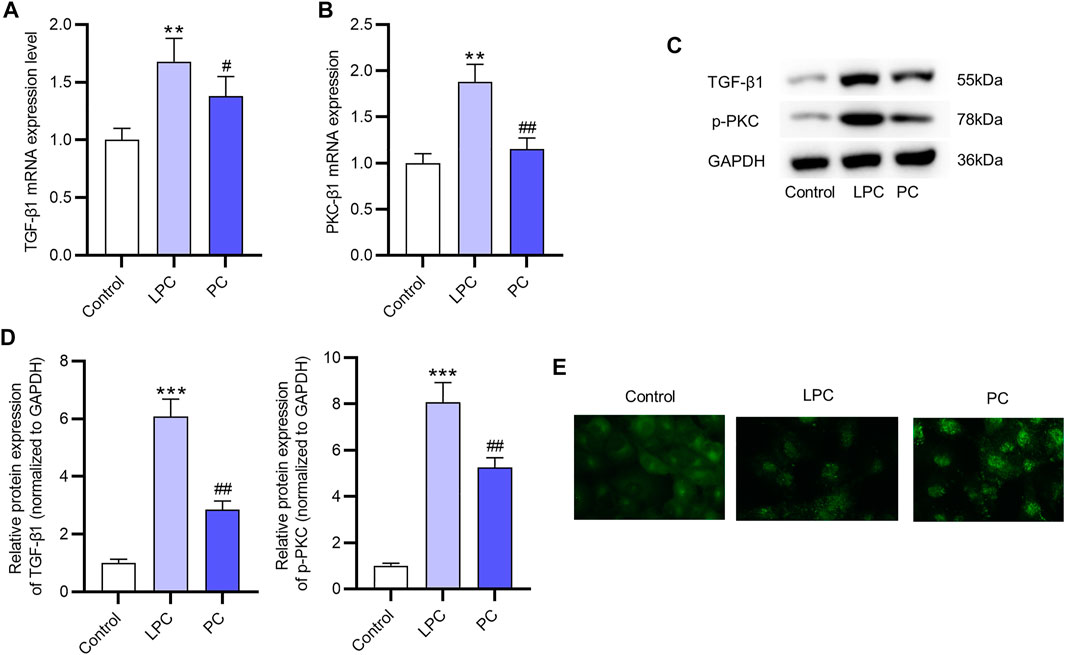
FIGURE 5. The promotive effect of LPC on the activation of the PKC signaling in EPCs. (A,B) The mRNA expression of PKC-β1 and TGF- β1 after LPC or PC treatment in EPCs were measured by RT-qPCR. (C,D) The protein levels of p-PKC and TGF-β1 were in EPCs treated with LPC or PC were accessed using western blot. (E) Immunofluorescence staining was used to determine the location of PKC-β1 after the LPC or PC treatment in EPCs (Figure 5D). **p < 0.01, ***p < 0.001 compared with the control, #p < 0.05, ##p < 0.01 compared with the LPC group.
Discussion
EPCs, smaller than 15 μm, can be detected in blood and bone marrow. Since the discovery of EPCs in 1997 (Werner et al., 2003), the exploration of their functions and application in cell therapy has achieved great progress. In vivo assays showed that EPCs promoted angiogenesis and facilitated the repair of vascular injury (Werner et al., 2003). The differentiation potential of EPCs into cardiomyocytes and smooth muscle cells has also been reported, which suggests the beneficial effect of EPCs on myocardial function (Iwasaki et al., 2006; Jujo et al., 2008; Mudyanadzo, 2018). Lower level of circulating EPCs indicates a higher risk in patients with cardiovascular diseases (Tepper et al., 2005; Iwakura et al., 2006; Westerweel et al., 2008). Dysfunctions of EPCs are found in patients with some diseases such as diabetes (Dai et al., 2017), hypertension (Garcia et al., 2020) and obesity (Peterson et al., 2019).
In the present study, we cultured CD34+ peripheral blood hematopoietic stem cells and induced their differentiation into EPCs. EPCs are divided into two categories, early EPCs and late EPCs. The early EPCs are adherent spindle-shaped with limited proliferation ability. The EPCs in this study with similar phenotypes and functions to early EPCs. After differentiation, EPCs expressed specific surface membrane markers such as CD34, CD31, KDR. Immunofluorescence staining indicated that VE-Cadherin, vWF, eNOS were all positively expressed in spindle-shaped EPCs. Previous studies have revealed that CD31, KDR, VE-Cadherin, vWF and eNOS are specific surface markers of endothelial cells and indicate the differentiation of ECs to a more mature stage (Fish and Marsden, 2006; Lagarkova et al., 2008). Additionally, the function of EPCs in this study was further explored. We demonstrated that EPCs alone did not develop into integrated tubes, while addition of supernatants of EPCs promoted the angiogenesis of HUVECs.
LPC, a bioactive lipid molecule, is implicated in various biological processes such as cell proliferation (Rikitake et al., 2000; Schaefer et al., 2004), inflammation (Gonçalves et al., 2012) and angiogenesis (Murugesan and Fox, 1996). Additionally, previous studies have revealed that LPC activates protein kinase C (PKC) in endothelial cells and platelets (Kugiyama et al., 1992). In the present study, LPC was demonstrated to inhibit the expression of angiogenesis-related factors including Ang-1 and Ang-2 at the mRNA and protein levels in vitro but had no significant effects on VEGF expression. We found that LPC treatment for 5 and 24 h suppressed the mRNA and protein expression of CCL2, CCL4, but induced the expression of IL-8. Many reports revealed that IL-8 serves as an angiogenesis promoter and induces tube formation (Bancroft et al., 2001; Dimberg, 2010; Lattanzio et al., 2013). Contradictorily, Yifat Amir Levy et al. reported that IL-8 decreases tube number or capillary outgrowth of HUVECs (Amir Levy et al., 2015). The angiogenesis of HUVECs promoted by supernatant of EPCs was demonstrated to be inhibited by the LPC treatment. Moreover, our findings revealed that LPC activated the PKC signaling pathway, which is supported by other studies (Awasthi et al., 2016; Tseng et al., 2019). IL-8 can activate the PKC signaling pathway (Xiao et al., 2018), which is closely associated with the impaired tube-forming ability of the HUVECs (Huang et al., 2015). The upregulated IL-8 expression and the diminished tubes formed by HUVECs under LPC treatment in the present study might be associated with the activation of the PKC pathway.
In conclusion, our study innovatively revealed that LPC suppressed expression of pro-inflammatory cytokines including CCL2 and CCL4, inhibited expression of proangiogenic factors including Ang-1 and Ang-2 in early EPCs differentiated from CD34+ peripheral blood hematopoietic stem cells in a PKC pathway dependent manner. Supernatant of EPCs facilitates the tube formation ability of HUVECs. The present study may provide clues for the application of EPC therapy in angiogenesis-related diseases, especially some cardiovascular diseases. To optimize its therapeutic outcomes, our understanding of the mechanisms by which EPCs contribute to tissue repair must be expanded, the methods of EPC purification, expansion, and administration need to be refined, and the techniques that overcome the scarcity and dysfunction of EPCs need to be developed. Several limitations of the current work merit mention. First, we used a traditional method for the analysis of relative quantification of PCR data in the present study, while some novel methods (Yuan et al., 2006; Steibel et al., 2009) are more applicable for the linear models. Second, in vivo studies are needed to further validate the therapeutic value of EPCs on proangiogenesis.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committee of the Second Hospital of Jilin University (Jilin, China). The patients/participants provided their written informed consents to participate in this study.
Author Contributions
HZ and YH designed the study and conducted assays. HZ assisted in figure preparation. YH analyzed the data and wrote paper.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Amir Levy, Y., Ciaraldi, T. P., Mudaliar, S. R., Phillips, S. A., and Henry, R. R. (2015). Excessive Secretion of IL-8 by Skeletal Muscle in Type 2 Diabetes Impairs Tube Growth: Potential Role of PI3K and the Tie2 Receptor. Am. J. Physiology-Endocrinology Metab. 309 (1), E22–E34. Epub 2015/05/07. PubMed PMID: 25944879. doi:10.1152/ajpendo.00513.2014
Awasthi, D., Nagarkoti, S., Kumar, A., Dubey, M., Singh, A. K., Pathak, P., et al. (2016). Oxidized LDL Induced Extracellular Trap Formation in Human Neutrophils via TLR-PKC-IRAK-MAPK and NADPH-Oxidase Activation. Free Radic. Biol. Med. 93, 190–203. Epub 2016/01/18PubMed PMID: 26774674. doi:10.1016/j.freeradbiomed.2016.01.004
Bancroft, C. C., Chen, Z., Dong, G., Sunwoo, J. B., Yeh, N., Park, C., et al. (2001). Coexpression of Proangiogenic Factors IL-8 and VEGF by Human Head and Neck Squamous Cell Carcinoma Involves Coactivation by MEK-MAPK and IKK-NF-kappaB Signal Pathways. Clin. Cancer Res. 7 (2), 435–442. Epub 2001/03/10. PubMed PMID: 11234901.
Dai, X., Yan, X., Zeng, J., Chen, J., Wang, Y., Chen, J., et al. (2017). Elevating CXCR7 Improves Angiogenic Function of EPCs via Akt/GSK-3β/Fyn-Mediated Nrf2 Activation in Diabetic Limb Ischemia. Circ. Res. 120 (5), e7–e23. Epub 2017/02/01PubMed PMID:28137917; PubMed Central PMCID: PMCPMC5336396. doi:10.1161/circresaha.117.310619
Dimberg, A. (2010). Chemokines in Angiogenesis. Curr. Top. Microbiol. Immunol. 341, 59–80. PubMed PMID: 20373091. doi:10.1007/82_2010_21
Fish, J. E., and Marsden, P. A. (2006). Endothelial Nitric Oxide Synthase: Insight into Cell-specific Gene Regulation in the Vascular Endothelium. Cell. Mol. Life Sci. 63 (2), 144–162. PubMed PMID: 16416260. doi:10.1007/s00018-005-5421-8
Garcia, V. P., Rocha, H. N. M., Rocha, M. P., Mattos, J. D., Campos, M. O., Mansur, D. E., et al. (2020). Hypertension Impairs Hypoxia-Induced Angiogenesis in Men. J. Hypertens. 38 (6), 1131–1139. PubMed PMID: 32371803. doi:10.1097/hjh.0000000000002369
Giraud, C., Tournadre, A., Pereira, B., Dutheil, F., Soubrier, M., Lhomme, M., et al. (2019). Alterations of HDL Particle Phospholipid Composition and Role of Inflammation in Rheumatoid Arthritis. J. Physiol. Biochem. 75 (4), 453–462. Epub 2019/08/09PubMed PMID: 31392628. doi:10.1007/s13105-019-00694-4
Gonçalves, I., Edsfeldt, A., Ko, N. Y., Grufman, H., Berg, K., Björkbacka, H., et al. (2012). Evidence Supporting a Key Role of Lp-PLA2-Generated Lysophosphatidylcholine in Human Atherosclerotic Plaque Inflammation. Arterioscler Thromb. Vasc. Biol. 32 (6), 1505–1512. Epub 2012/04/14PubMed PMID: 22499993. doi:10.1161/atvbaha.112.249854
Herberts, C. A., Kwa, M. S., and Hermsen, H. P. (2011). Risk Factors in the Development of Stem Cell Therapy. J. Transl Med. 9, 29. Epub 2011/03/23PubMed PMID: 21418664; PubMed Central PMCID: PMCPMC3070641. doi:10.1186/1479-5876-9-29
Hong, S. H., Jang, H. H., Lee, S. R., Lee, K. H., Woo, J. S., Kim, J. B., et al. (2015). Impact of Lysophosphatidylcholine on Survival and Function of UEA-1+acLDL+ Endothelial Progenitor Cells in Patients with Coronary Artery Disease. Heart Vessels 30 (1), 115–125. Epub 2014/02/11PubMed PMID: 24510253. doi:10.1007/s00380-014-0473-z
Hsieh, C.-C., Yen, M.-H., Liu, H.-W., and Lau, Y.-T. (2000). Lysophosphatidylcholine Induces Apoptotic and Non-apoptotic Death in Vascular Smooth Muscle Cells: in Comparison with Oxidized LDL. Atherosclerosis 151 (2), 481–491. Epub 2000/08/05PubMed PMID: 10924725. doi:10.1016/s0021-9150(00)00453-6
Huang, D., Wang, F.-B., Guo, M., Li, S., Yan, M.-L., Yu, T., et al. (2015). Effect of Combined Treatment with Rosuvastatin and Protein Kinase Cβ2 Inhibitor on Angiogenesis Following Myocardial Infarction in Diabetic Rats. Int. J. Mol. Med. 35 (3), 829–838. Epub 2014/12/20PubMed PMID: 25524396. doi:10.3892/ijmm.2014.2043
Itabe, H., Takeshima, E., Iwasaki, H., Kimura, J., Yoshida, Y., Imanaka, T., et al. (1994). A Monoclonal Antibody against Oxidized Lipoprotein Recognizes Foam Cells in Atherosclerotic Lesions. Complex Formation of Oxidized Phosphatidylcholines and Polypeptides. J. Biol. Chem. 269 (21), 15274–15279. Epub 1994/05/27. PubMed PMID: 8195164. doi:10.1016/s0021-9258(17)36602-4
Iwakura, A., Shastry, S., Luedemann, C., Hamada, H., Kawamoto, A., Kishore, R., et al. (2006). Estradiol Enhances Recovery after Myocardial Infarction by Augmenting Incorporation of Bone Marrow-Derived Endothelial Progenitor Cells into Sites of Ischemia-Induced Neovascularization via Endothelial Nitric Oxide Synthase-Mediated Activation of Matrix Metalloproteinase-9. Circulation 113 (12), 1605–1614. Epub 2006/03/15PubMed PMID: 16534014. doi:10.1161/circulationaha.105.553925
Iwasaki, H., Kawamoto, A., Ishikawa, M., Oyamada, A., Nakamori, S., Nishimura, H., et al. (2006). Dose-dependent Contribution of CD34-Positive Cell Transplantation to Concurrent Vasculogenesis and Cardiomyogenesis for Functional Regenerative Recovery after Myocardial Infarction. Circulation 113 (10), 1311–1325. Epub 2006/03/15PubMed PMID: 16534028. doi:10.1161/circulationaha.105.541268
Jujo, K., Ii, M., and Losordo, D. W. (2008). Endothelial Progenitor Cells in Neovascularization of Infarcted Myocardium. J. Mol. Cell. Cardiol. 45 (4), 530–544. Epub 2008/08/30PubMed PMID: 18755197; PubMed Central PMCID: PMCPMC2628572. doi:10.1016/j.yjmcc.2008.08.003
Kou, F., Zhu, C., Wan, H., Xue, F., Wang, J., Xiang, L., et al. (2020). Endothelial Progenitor Cells as the Target for Cardiovascular Disease Prediction, Personalized Prevention, and Treatments: Progressing beyond the State-Of-The-Art. EPMA J. 11 (4), 629–643. Epub 2020/11/27PubMed PMID: 33240451; PubMed Central PMCID: PMCPMC7680476. doi:10.1007/s13167-020-00223-0
Kugiyama, K., Kerns, S. A., Morrisett, J. D., Roberts, R., and Henry, P. D. (1990). Impairment of Endothelium-dependent Arterial Relaxation by Lysolecithin in Modified Low-Density Lipoproteins. Nature 344 (6262), 160–162. Epub 1990/03/08PubMed PMID: 2106627. doi:10.1038/344160a0
Kugiyama, K., Ohgushi, M., Sugiyama, S., Murohara, T., Fukunaga, K., Miyamoto, E., et al. (1992). Lysophosphatidylcholine Inhibits Surface Receptor-Mediated Intracellular Signals in Endothelial Cells by a Pathway Involving Protein Kinase C Activation. Circ. Res. 71 (6), 1422–1428. Epub 1992/12/01PubMed PMID: 1423937. doi:10.1161/01.res.71.6.1422
Lagarkova, M. A., Volchkov, P. Y., Philonenko, E. S., and Kiselev, S. L. (2008). Efficient Differentiation of hESCs into Endothelial Cells In Vitro Is Secured by Epigenetic Changes. Cell Cycle 7 (18), 2929–2935. Epub 2008/09/25PubMed PMID: 18814342. doi:10.4161/cc.7.18.6700
Lattanzio, L., Tonissi, F., Torta, I., Gianello, L., Russi, E., Milano, G., et al. (2013). Role of IL-8 Induced Angiogenesis in Uveal Melanoma. Invest. New Drugs 31 (5), 1107–1114. Epub 2013/08/06PubMed PMID: 23912257. doi:10.1007/s10637-013-0005-1
Li, W.-D., and Li, X.-Q. (2016). Endothelial Progenitor Cells Accelerate the Resolution of Deep Vein Thrombosis. Vasc. Pharmacol. 83, 10–16. Epub 2015/07/19PubMed PMID: 26187355. doi:10.1016/j.vph.2015.07.007
Liu, P., Zhou, B., Gu, D., Zhang, L., and Han, Z. (2009). Endothelial Progenitor Cell Therapy in Atherosclerosis: a Double-Edged Sword? Ageing Res. Rev. 8 (2), 83–93. Epub 2008/12/24PubMed PMID: 19103308. doi:10.1016/j.arr.2008.11.002
Liu, P., Zhu, W., Chen, C., Yan, B., Zhu, L., Chen, X., et al. (2020). The Mechanisms of Lysophosphatidylcholine in the Development of Diseases. Life Sci. 247, 117443. Epub 2020/02/23PubMed PMID: 32084434. doi:10.1016/j.lfs.2020.117443
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 25 (4), 402–408. Epub 2002/02/16PubMed PMID: 11846609. doi:10.1006/meth.2001.1262
Ma, F. X., Zhou, B., Chen, Z., Ren, Q., Lu, S. H., Sawamura, T., et al. (2006). Oxidized Low Density Lipoprotein Impairs Endothelial Progenitor Cells by Regulation of Endothelial Nitric Oxide Synthase. J. lipid Res. 47 (6), 1227–1237. Epub 2006/03/09PubMed PMID: 16522925. doi:10.1194/jlr.M500507-JLR200
Mudyanadzo, T. A. (2018). Endothelial Progenitor Cells and Cardiovascular Correlates. Cureus 10 (9), e3342. Epub 2018/11/27PubMed PMID: 30473975; PubMed Central PMCID: PMCPMC6248662. doi:10.7759/cureus.3342
Murugesan, G., and Fox, P. L. (1996). Role of Lysophosphatidylcholine in the Inhibition of Endothelial Cell Motility by Oxidized Low Density Lipoprotein. J. Clin. Invest. 97 (12), 2736–2744. Epub 1996/06/15PubMed PMID: 8675684; PubMed Central PMCID: PMCPMC507366. doi:10.1172/jci118728
Peterson, S. J., Shapiro, J. I., Thompson, E., Singh, S., Liu, L., Weingarten, J. A., et al. (2019). Oxidized HDL, Adipokines, and Endothelial Dysfunction: A Potential Biomarker Profile for Cardiovascular Risk in Women with Obesity. Obesity 27 (1), 87–93. Epub 2018/12/21PubMed PMID: 30569635; PubMed Central PMCID: PMCPMC6309990. doi:10.1002/oby.22354
Portman, O. W., and Alexander, M. (1969). Lysophosphatidylcholine Concentrations and Metabolism in Aortic Intima Plus Inner media: Effect of Nutritionally Induced Atherosclerosis. J. lipid Res. 10 (2), 158–165. Epub 1969/03/01. PubMed PMID: 4238547. doi:10.1016/s0022-2275(20)42662-8
Rikitake, Y., Kawashima, S., Yamashita, T., Ueyama, T., Ishido, S., Hotta, H., et al. (2000). Lysophosphatidylcholine Inhibits Endothelial Cell Migration and Proliferation via Inhibition of the Extracellular Signal-Regulated Kinase Pathway. Arterioscler Thromb. Vasc. Biol. 20 (4), 1006–1012. Epub 2000/04/15PubMed PMID: 10764665. doi:10.1161/01.atv.20.4.1006
Rosenzweig, A. (2003). Endothelial Progenitor Cells. N. Engl. J. Med. 348 (7), 581–582. Epub 2003/02/14PubMed PMID: 12584365. doi:10.1056/NEJMp020175
Schaefer, C. A., Kuhlmann, C. R. W., Gast, C., Weiterer, S., Li, F., Most, A. K., et al. (2004). Statins Prevent Oxidized Low-Density Lipoprotein- and Lysophosphatidylcholine-Induced Proliferation of Human Endothelial Cells. Vasc. Pharmacol. 41 (2), 67–73. Epub 2004/06/16PubMed PMID: 15196477. doi:10.1016/j.vph.2004.05.002
Sharma, N., Akhade, A. S., Ismaeel, S., and Qadri, A. (2020). Serum‐borne Lipids Amplify TLR‐activated Inflammatory Responses. J. Leukoc. Biol. 109, 821–831. Epub 2020/07/28PubMed PMID: 32717772. doi:10.1002/jlb.3ab0720-241rr
Steibel, J. P., Poletto, R., Coussens, P. M., and Rosa, G. J. M. (2009). A Powerful and Flexible Linear Mixed Model Framework for the Analysis of Relative Quantification RT-PCR Data. Genomics 94 (2), 146–152. Epub 2009/05/09PubMed PMID:19422910. doi:10.1016/j.ygeno.2009.04.008
Tepper, O. M., Capla, J. M., Galiano, R. D., Ceradini, D. J., Callaghan, M. J., Kleinman, M. E., et al. (2005). Adult Vasculogenesis Occurs through In Situ Recruitment, Proliferation, and Tubulization of Circulating Bone Marrow-Derived Cells. Blood 105 (3), 1068–1077. Epub 2004/09/25PubMed PMID: 15388583. doi:10.1182/blood-2004-03-1051
Tie, G., Yan, J., Yang, Y., Park, B. D., Messina, J. A., Raffai, R. L., et al. (2010). Oxidized Low-Density Lipoprotein Induces Apoptosis in Endothelial Progenitor Cells by Inactivating the Phosphoinositide 3-kinase/Akt Pathway. J. Vasc. Res. 47 (6), 519–530. Epub 2010/05/01PubMed PMID: 20431300; PubMed Central PMCID: PMCPMC2945270. doi:10.1159/000313879
Tseng, H.-C., Lin, C.-C., Hsiao, L.-D., and Yang, C.-M. (2019). Lysophosphatidylcholine-induced Mitochondrial Fission Contributes to Collagen Production in Human Cardiac Fibroblasts. J. lipid Res. 60 (9), 1573–1589. Epub 2019/08/01PubMed PMID: 31363041; PubMed Central PMCID: PMCPMC6718437. doi:10.1194/jlr.RA119000141
Tsimikas, S., Bergmark, C., Beyer, R. W., Patel, R., Pattison, J., Miller, E., et al. (2003). Temporal Increases in Plasma Markers of Oxidized Low-Density Lipoprotein Strongly Reflect the Presence of Acute Coronary Syndromes. J. Am. Coll. Cardiol. 41 (3), 360–370. Epub 2003/02/11PubMed PMID: 12575961. doi:10.1016/s0735-1097(02)02769-9
Wang, H.-H., Wu, Y.-J., Tseng, Y.-M., Su, C.-H., Hsieh, C.-L., and Yeh, H.-I. (2019). Mitochondrial Fission Protein 1 Up-Regulation Ameliorates Senescence-Related Endothelial Dysfunction of Human Endothelial Progenitor Cells. Angiogenesis 22 (4), 569–582. Epub 2019/09/05PubMed PMID: 31482366. doi:10.1007/s10456-019-09680-2
Werner, N., Junk, S., Laufs, U., Link, A., Walenta, K., Böhm, M., et al. (2003). Intravenous Transfusion of Endothelial Progenitor Cells Reduces Neointima Formation after Vascular Injury. Circ. Res. 93 (2), e17–24. Epub 2003/06/28PubMed PMID: 12829619. doi:10.1161/01.Res.0000083812.30141.74
Westerweel, P. E., Visseren, F. L. J., Hajer, G. R., Olijhoek, J. K., Hoefer, I. E., de Bree, P., et al. (2008). Endothelial Progenitor Cell Levels in Obese Men with the Metabolic Syndrome and the Effect of Simvastatin Monotherapy vs. Simvastatin/ezetimibe Combination Therapy. Eur. Heart J. 29 (22), 2808–2817. Epub 2008/10/01PubMed PMID: 18824462. doi:10.1093/eurheartj/ehn431
Xiao, P., Long, X., Zhang, L., Ye, Y., Guo, J., Liu, P., et al. (2018). Neurotensin/IL-8 Pathway Orchestrates Local Inflammatory Response and Tumor Invasion by Inducing M2 Polarization of Tumor-Associated Macrophages and Epithelial-Mesenchymal Transition of Hepatocellular Carcinoma Cells. Oncoimmunology 7 (7), e1440166, . Epub 2018/06/15PubMed PMID: 29900041; PubMed Central PMCID: PMCPMC5993481. doi:10.1080/2162402x.2018.1440166
Yuan, J. S., Reed, A., Chen, F., and Stewart, C. N. (2006). Statistical Analysis of Real-Time PCR Data. BMC Bioinformatics 7 (1), 85. doi:10.1186/1471-2105-7-85
Zeng, C., Wen, B., Hou, G., Lei, L., Mei, Z., Jia, X., et al. (2017). Lipidomics Profiling Reveals the Role of Glycerophospholipid Metabolism in Psoriasis. GigaScience 6 (10), 1–11. Epub 2017/10/20PubMed PMID: 29046044; PubMed Central PMCID: PMCPMC5647792. doi:10.1093/gigascience/gix087
Zhou, X., Wang, R., Zhang, T., Liu, F., Zhang, W., Wang, G., et al. (2019). Identification of Lysophosphatidylcholines and Sphingolipids as Potential Biomarkers for Acute Aortic Dissection via Serum Metabolomics. Eur. J. Vasc. Endovascular Surg. 57 (3), 434–441. Epub 2018/08/09PubMed PMID: 30087010. doi:10.1016/j.ejvs.2018.07.004
Keywords: LPC, EPC, CD31, angiogenesis, cell biology
Citation: Zhao H and He Y (2021) The Inhibitory Effect of Lysophosphatidylcholine on Proangiogenesis of Human CD34+ Cells Derived Endothelial Progenitor Cells. Front. Mol. Biosci. 8:682367. doi: 10.3389/fmolb.2021.682367
Received: 18 March 2021; Accepted: 28 May 2021;
Published: 10 June 2021.
Edited by:
Sandra Donnini, University of Siena, ItalyReviewed by:
Saptarshi Kar, American University of the Middle East, KuwaitThomas Mohr, Consultant, Guntramsdorf, Austria
Copyright © 2021 Zhao and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanhui He, aHloODIxMDE1QGpsdS5lZHUuY24=
 Haijun Zhao1
Haijun Zhao1 Yanhui He
Yanhui He