- 1Department of Oncology, The Affiliated Hospital of Southwest Medical University, Nuclear Medicine and Molecular Imaging Key Laboratory of Sichuan Province, Academician (Expert) Workstation of Sichuan Province, Luzhou, China
- 2Health Management Department, The Affiliated Hospital of Southwest Medical University, Luzhou, China
Background: CAP-Gly domain containing linker protein family member 4 (CLIP4) plays an important role in cancers. However, its expression, prognostic value, and biological effect in breast cancer remain unclear.
Methods: Data on patients diagnosed with breast cancer were retrieved from the TCGA-BRCA and other public omics databases. The expression profile of CLIP4 was analyzed using Oncomine, bc-GenExMiner, and TCGA. The prognostic value of CLIP4 was determined by Kaplan-Meier Plotter and Human Protein Atlas. Identification of genes co-expressed with CLIP4 and potential mechanism analyses were performed using UALCAN, STRING, Metascape, and GSEA. The epigenetic characteristics of CLIP4 were determined by DiseaseMeth and MEXPRESS.
Results: CLIP4 was downregulated and its expression was negatively correlated with estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor type 2 (HER2) status, Nottingham prognostic index (NPI), and Scarff-Bloom-Richardson (SBR) grade in breast cancer, whereas it was positively linked to basal-like and triple negative breast cancer status. Ectopic expression of CLIP4 was related with poor prognosis. In the analysis of genes co-expressed with CLIP4, GSEA showed that the Hedgehog (Hh), JAK-STAT, ERBB, Wnt signaling pathway, cell adhesion molecules, and pathways in cancer were dissimilarly enriched in the CLIP4 expression high phenotype. Analysis of the genetics and epigenetics of CLIP4 indicated that its expression was negatively correlated with DNA methylation.
Conclusion: Methylated CLIP4 may be a novel prognostic and therapeutic biomarker for breast cancer.
Introduction
Breast cancer is the most common female malignancy in China and is the main cause of mortality in Western countries (Chen et al., 2016; Siegel et al., 2018). Moreover, the incidence of breast cancer is gradually rising in most countries (DeSantis et al., 2019). Despite advances in early screening, diagnosis, and treatment of breast cancer, the overall prognosis for patients remains poor. Thus, it is urgent to find sensitive and specific biomarkers for breast cancer.
Microtubules have a dynamic structure that continuously changes during growth and shrinkage (Mitchison and Kirschner, 1984). Microtubule-associated proteins (MAPs) influence microtubule properties. One group of MAPs, the plus end binding proteins (or +TIPs), bind to and stabilize microtubule plus ends. Mammalian cytoplasmic linker protein (CLIP)-170, links microtubule plus ends to kinomeres, endocytosis vesicles, and the leading edge of migrating cells (Howard and Hyman, 2003), is a prototypical +TIP (Perez et al., 1999). The CLIP-170 family (CLIP1, CLIP2, CLIP3, and CLIP4) associates microtubules with cellular organelles through a cytoskeleton-associated protein glycine rich (CAP-Gly) domain. The CAP-Gly domain, which is conserved among organisms, is a small 80-residue protein module (Riehemann and Sorg, 1993). CAP-Gly domains are important to the function of CLIPs and many other proteins, and are implicated in cell polarity maintenance, intracellular transport, cell migration, and oncogenesis (Galjart and Perez, 2003; Akhmanova and Hoogenraad, 2005; Galjart, 2005). Recent research on CLIP4 uncovered its potential functions in cancers. However, the role of CLIP4 in breast cancer remains unknown.
Here, we first evaluated the expression profile of CLIP4 in breast cancer by data mining. The prognostic value of CLIP4 was also analyzed. To gain further insight into the molecular mechanisms involved in breast cancer-related CLIP4 regulatory networks, GSEA was used. Finally, the regulation of the expression of CLIP4 in breast cancer was investigated by genetic and epigenetic analyses.
Methods
Expression profile analysis
The expression pattern of CLIP4 was analyzed using Oncomine and TCGA. First, the expression profile of CLIP4 was analyzed with Oncomine, which facilitates investigation of cancer microarray databases and genome-wide expression analysis (Rhodes et al., 2004). The cut-off of p-value, fold change, and gene rank were defined as 0.05, 2, and 10% (Fan et al., 2019), respectively. The gene expression (1,098 cases) and corresponding clinical data were downloaded from TCGA official website for Breast Invasive Carcinoma (TCGA-BRCA). All statistical analyses were performed using R (v.3.6.3). The association with clinical features and CLIP4 was analyzed with the Wilcoxon signed-rank and logistic regression test.
bc-GenExMiner (Jezequel et al., 2012) was used to assess relationships among CLIP4 expression and clinicopathological features including age, nodal status, hormone receptor status (ER and PR), HER2, pathological subtype, NPI, and SBR grade. A value of p <0.05 was statistically significant.
Survival analysis
The Kaplan-Meier Plotter (http://kmplot.com/analysis/), an online tool established from gene expression and survival data of cancer patients originated from the GEO database (Gyorffy et al., 2010), was used to evaluate the prognostic value of CLIP4 in breast cancer. The survival plot, hazards ratio (HR), 95% confidence interval (CI), and log-rank p were displayed on the web page. The prognostic significance are available from Human Protein Atlas (https://www.proteinatlas.org/) (Uhlen et al., 2005). A log-rank p < 0.05 was statistically significant.
Analysis of Co-Expression and Protein-Protein Interaction Networks
The genes co-expressed with CLIP4 were analyzed using UALCAN (Chandrashekar et al., 2017). A total of 687 genes positively and negatively correlated with CLIP4 in breast cancer were downloaded. Genes with a Pearson’s correlation coefficient ≥0.3 (absolute value) were included. The protein-protein interaction network was established using STRING v11.0 and was based on co-expressed genes (https://string-db.org/cgi/input.pl/) (Szklarczyk et al., 2019). The confidence score was defined as 0.4 (Fan et al., 2019).
Metascape and Gene Set Enrichment Analysis
Gene ontology (GO) and pathway enrichment analysis of CLIP4-associated genes were performed using Metascape (http://metascape.org/) (Zhou et al., 2019). A computational method GSEA was used to determine whether a prior defined set of genes shows statistically significant, concordant differences between two biological states (Subramanian et al., 2005). In this study, an ordered list of genes was first generated by GSEA based on correlation with CLIP4 expression. The significant survival difference observed between high and low CLIP4 was elucidated. Gene set permutations were performed 1,000 times each analysis. The expression level of CLIP4 was used as a phenotype label. The nominal p-value and normalized enrichment score (NES) were used to classify the pathways enriched in each phenotype.
Analysis of DNA Methylation and Genetic Alterations
To further investigate the regulatory effect of DNA methylation modification on CLIP4 mRNA expression, we used DiseaseMeth, version 2.0, which was updated along with the increased DNA methylation data and associations between diseases and genes. These datasets were collected from revolutionary large international disease projects such as TCGA and GEO among others (Xiong et al., 2017). Then, 871 breast invasive carcinoma samples in MEXPRESS (https://mexpress.be/) (Koch et al., 2015) datasets were analyzed to evaluate the CLIP4 gene. The genetic alterations of CLIP4 were identified by a web portal (http://www.cbioportal.org/) (Cerami et al., 2012) evaluated from breast invasive carcinoma (TCGA, Firehose Legacy, 1108 samples).
Results
The Expression of CLIP4 in Breast Cancer
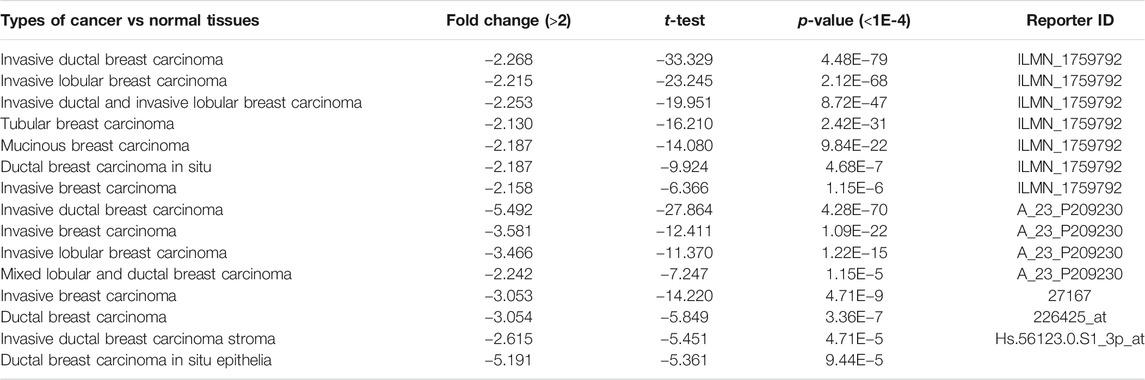
To identify the roles of CLIP4 in cancers, we searched the Oncomine dataset for CLIP4 mRNA expression in common cancer types. Comparison of cancer and normal samples was performed to analyze the expression pattern of CLIP4 in breast cancer (Figure 1A). The meta-analysis based on Oncomine was used to evaluate the integrated and median expression of CLIP4 across 15 analyses (p = 4.68E−7, <0.001) (Figure 1B). As shown in Table 1, the CLIP4 expression was significantly decreased in invasive breast carcinoma, invasive lobular breast carcinoma, invasive ductal breast carcinoma, tubular breast carcinoma, mucinous breast carcinoma, ductal breast carcinoma in situ, and mixed lobular and ductal breast carcinoma (Richardson et al., 2006; Ma et al., 2009; Curtis et al., 2012; Gluck et al., 2012). For further validation, we also investigated the expression of CLIP4 from TCGA datasets. As shown in Figure 1C, CLIP4 expression was dramatically lower in breast cancer than in normal tissues (p = 8.44E−53, <0.001). Furthermore, the paired plot for CLIP4 showed that the expression in breast cancer was significantly downregulated compared with adjacent normal samples (p = 5.07E−29, <0.001) (Figure 1D). Taken together, the combination of bioinformatic analyses demonstrated that CLIP4 was significantly downregulated in breast cancer.
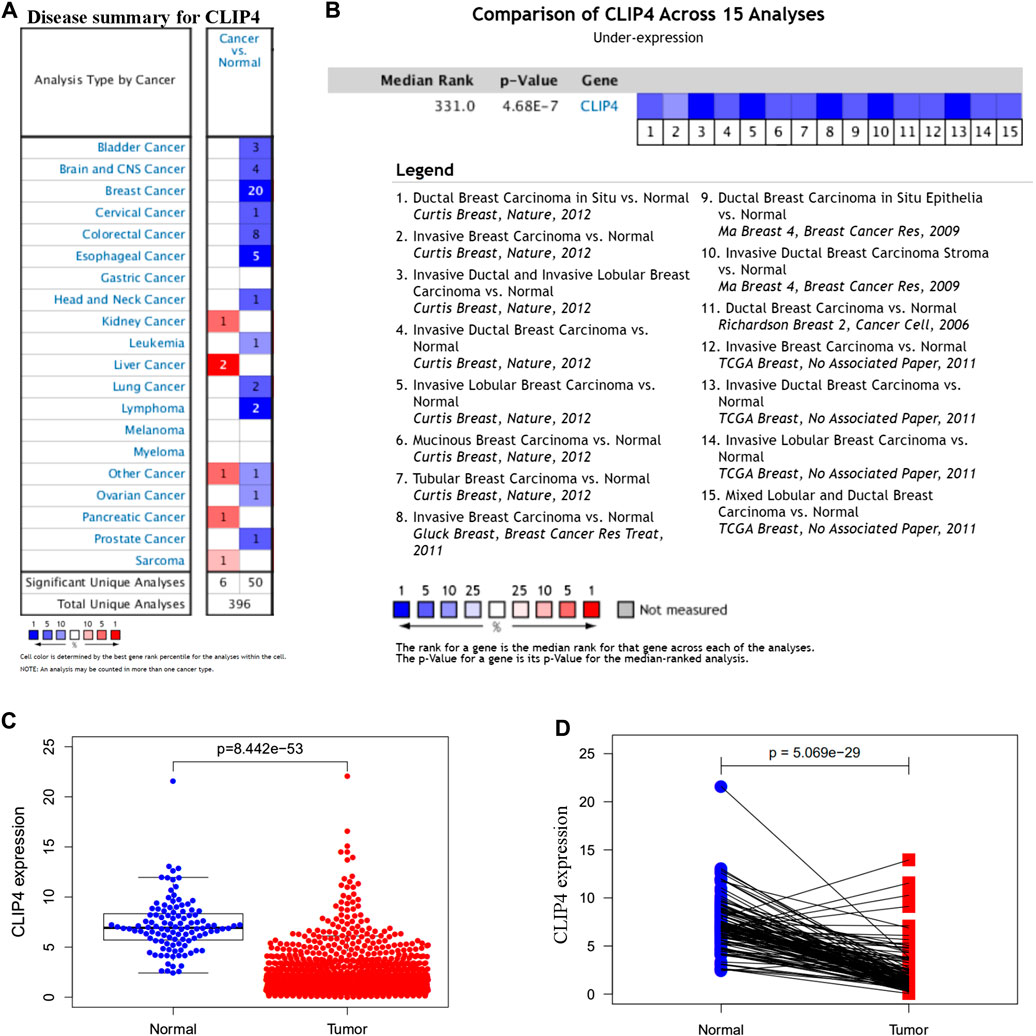
FIGURE 1. Expression pattern of CLIP4 in breast cancer. (A) Expression of CLIP4 in different human cancers by analysis of cancer and normal tissue based on Oncomine; (B) Oncomine meta-analysis for the expression of CLIP4 in breast cancer; (C) CLIP4 mRNA expression in breast cancer and normal tissue (TCGA datasets); and (D) CLIP4 mRNA expression in breast cancer and adjacent normal tissue (TCGA datasets).
We further evaluated the associations between CLIP4 expression and clinicopathological characteristics using the bc-GenExMiner tool (Figure 2). There was no significant difference of CLIP4 expression between the nodal-positive and -negative groups (p = 0.1797, >0.05). Age, ER, PR, HER2 status, NPI, and SBR grade were negatively related to CLIP4 expression (Age: p = 0.0015, <0.01; ER status: p < 0.0001; PR status: p < 0.0001; HER2 status: p < 0.0001; NPI: p = 0.0003, <0.001; SBR grade: p < 0.0001). However, the expression of CLIP4 was markedly upregulated in basal-like and triple-negative breast cancer (TNBC) (basal-like status: p < 0.0001; triple-negative status: p < 0.0001). To further evaluate the association between TNM stage and CLIP4 expression, we performed analysis using R (v.3.6.3) based on TCGA datasets. The results showed that the T, N, and stage were negatively associated with CLIP4 expression (T3-4 vs. T1-2: p = 0.008, <0.01; nodal-positive vs. -negative: p = 0.046, <0.05; stage III-IV vs. stage I-II: p = 0.027, <0.05) (Supplementary Figure S1). However, logistic regression analysis showed no significant differences between T, N, stage and CLIP4 expression (p > 0.05) (Supplementary Table S1). In short, these results indicated that CLIP4 expression may play a favorable role in breast cancer patients.
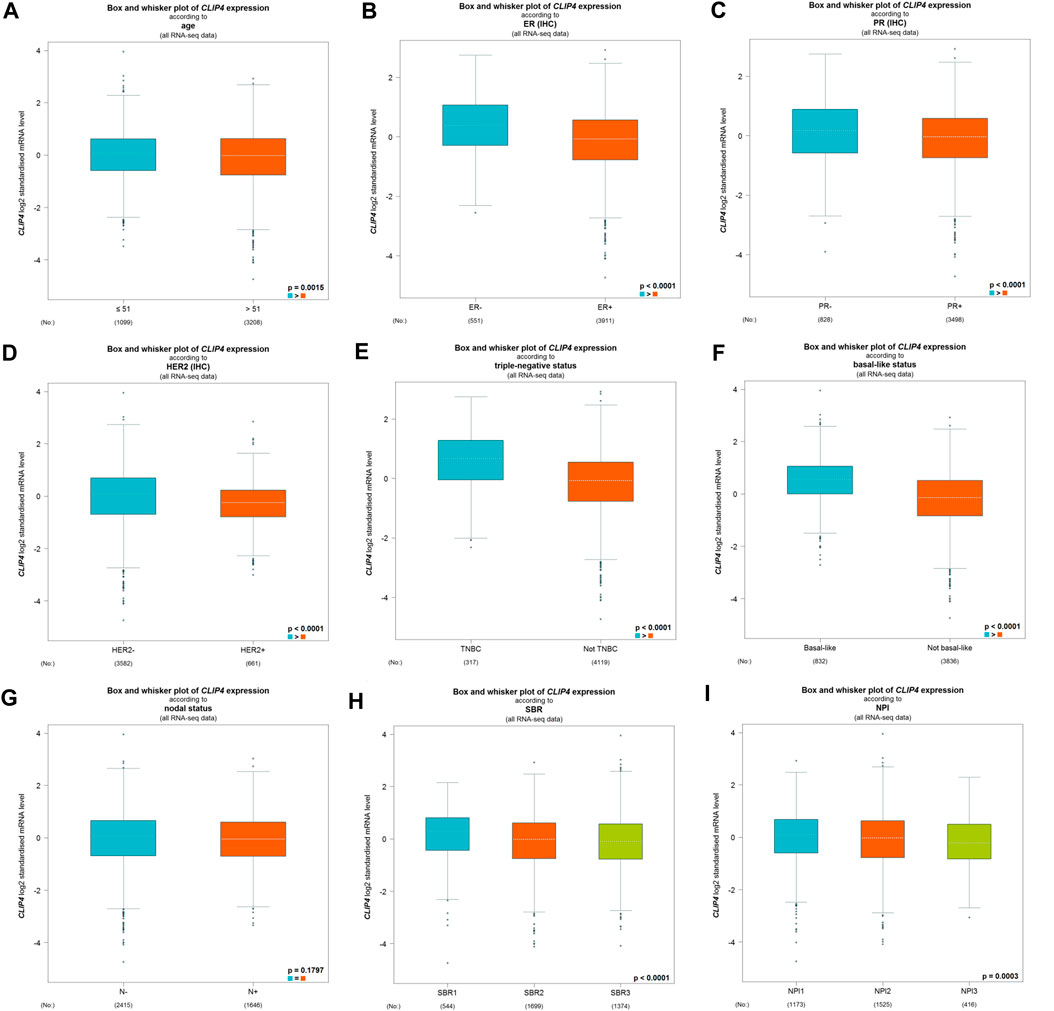
FIGURE 2. Relationships among CLIP4 expression and clinicopathological features of breast cancer based on the bc-GenExMiner database. (A) Box plot for the association of CLIP4 mRNA expression and age; (B) box plot for the association of CLIP4 mRNA expression and ER; (C) box plot for the association of CLIP4 mRNA expression and PR; (D) box plot for the association of CLIP4 mRNA expression and HER2; (E) box plot for the association of CLIP4 mRNA expression and triple-negative status; (F) box plot for the association of CLIP4 mRNA expression and basal-like status; (G) box plot for the association of CLIP4 mRNA expression and nodal status; (H) box plot for the association of CLIP4 mRNA expression and SBR; and (I) box plot for the association of CLIP4 mRNA expression and NPI.
Prognostic Significance of CLIP4 in Breast Cancer
The analysis using the Kaplan-Meier plotter based on 626 breast cancer patients showed that CLIP4 upregulation was significantly associated with better overall survival (OS) [HR: 0.71 (0.50–0.99); p = 0.043, <0.05, Figure 3A]. High expression of CLIP4 was associated with better relapse-free survival (RFS) in 1,764 breast cancer patients [HR: 0.64 (0.55–0.76); p = 1.30E-07, Figure 3B]. Furthermore, CLIP4 was also positively associated with distant metastasis-free survival (DMFS) in breast cancer, although statistical significance was not reached [HR: 0.77 (0.54–1.10); p = 0.150, >0.05, Figure 3C]. Based on TCGA dataset and the optimal cutoff value, the survival rate was significantly better in breast cancer patients (n = 726) with high expression of CLIP4 than in those with low expression (n = 349) (p = 0.020, <0.05) (https://www.proteinatlas.org/) (Figure 3D). In summary, CLIP4 may be a novel prognostic indicator in breast cancer patients. Meanwhile, the value of CLIP4 in different intrinsic subtypes of breast cancer was evaluated using the Kaplan-Meier plotter. High CLIP4 expression in three intrinsic subtypes (Luminal A, Luminal B, and HER2 positive) but not in the basal-like subtype was correlated with better OS [Luminal A: HR: 0.63 (0.37–1.07), p = 0.085; Luminal B: HR: 0.46 (0.21–1.01), p = 0.047; HER2 positive: HR: 0.46 (0.21–1.02), p = 0.050] (Supplementary Figure S2).
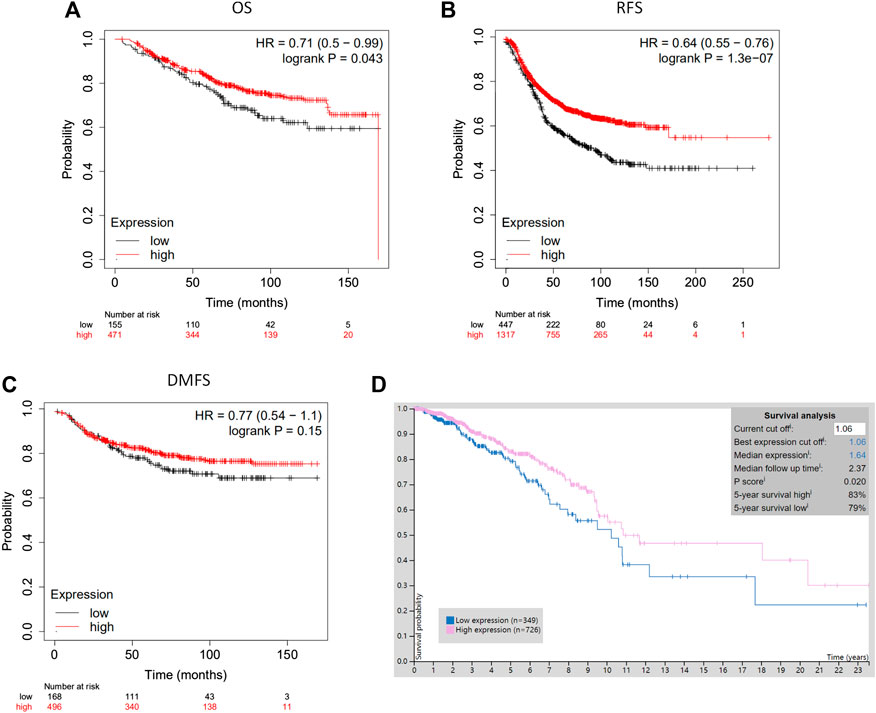
FIGURE 3. The prognostic significance of CLIP4 for breast cancer determined by Kaplan-Meier Plotter and TCGA datasets. (A) High CLIP4 expression shows a better OS for breast cancer; (B) high CLIP4 expression shows a better RFS for breast cancer; (C) high CLIP4 expression shows a better DMFS for breast cancer; (D) the 5-year survival rate for patients with breast cancer (n = 726) with greater CLIP4 mRNA expression (83%) better for patients (n = 349) than low expression (79%) (TCGA datasets).
Genes Co-Expressed with CLIP4 and Potential Biomolecular Networks
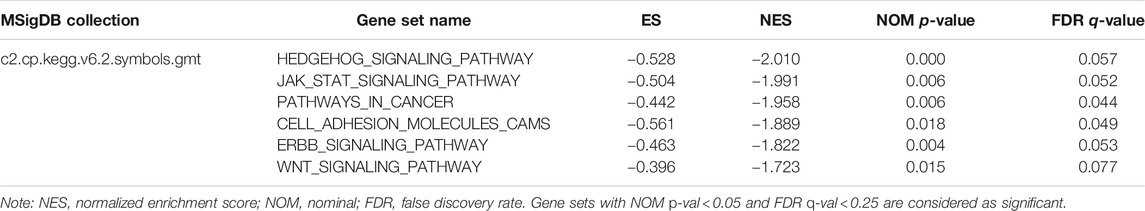
To find the potential role and regulatory mechanism of CLIP4 in breast cancer, genes co-expressed with CLIP4 were predicted using the UALCAN database, and 687 genes were identified (Supplementary Table S2). A PPI network for CLIP4 co-expressed genes, based on experimental evidence, was constructed using the STRING database (Supplementary Figure S3). We further performed GO and pathway enrichment analysis for these genes using Metascape (Figure 4). The top 20 clusters with their enriched terms, which originated from two categories, were included: GO biological process (BP) and Reactome gene sets. The representative enriched GO functions for these co-expressed genes included Wnt signaling pathway (GO: 0016055), actin cytoskeleton organization (GO: 0030036), developmental growth (GO: 0048589), transmembrane receptor protein tyrosine kinase signaling pathway (GO: 0007169), regulation of GTPase activity (GO: 0043087), toll-like receptor 2 signaling pathway (GO: 0034134), and regulation of epidermal growth factor receptor signaling pathway (GO: 0042058). To further identify molecular signaling pathways differentially activated in breast cancer, GSEA between CLIP4 low and high expression datasets was performed (Supplementary Table S3, S4). GSEA identified significant differences (FDR q-value <0.25, NOM p-value <0.05) in enrichment of the MSigDB Collection (c2.cp.kegg.v6.2.symbols.gmt). The most enriched tumor-associated signaling pathways were selected based on their NES values (Wu and Zhang, 2018) (Figure 5; Table 2). As shown in Figure 5, Hh signaling pathway, JAK-STAT signaling pathway, cell adhesion molecules, ERBB signaling pathway, Wnt signaling pathway, and pathways in cancer were differentially enriched in the CLIP4-high expression phenotype. These results demonstrated that upregulation of CLIP4 in breast cancer may involve the Wnt, ERBB, or other tumor-associated signaling pathways. Another result based on multiple GSEA analysis is shown in Supplementary Figure S4.
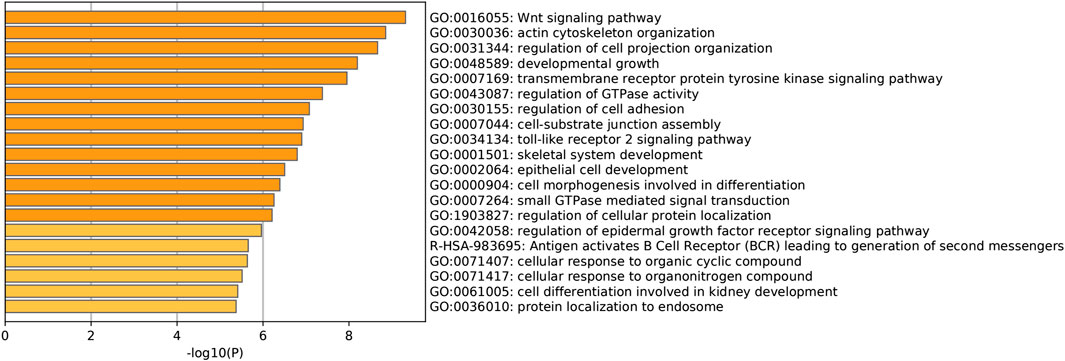
FIGURE 4. Pathway enrichment analysis of CLIP4 co-expressed genes. The top 20 pathway enrichment clusters based on Metascape analysis of CLIP4 co-expressed genes carried out with GO Biological Processes and Reactome Gene Sets. Length of bars represent log10 (p-value) determined by the best-scoring term within each cluster.
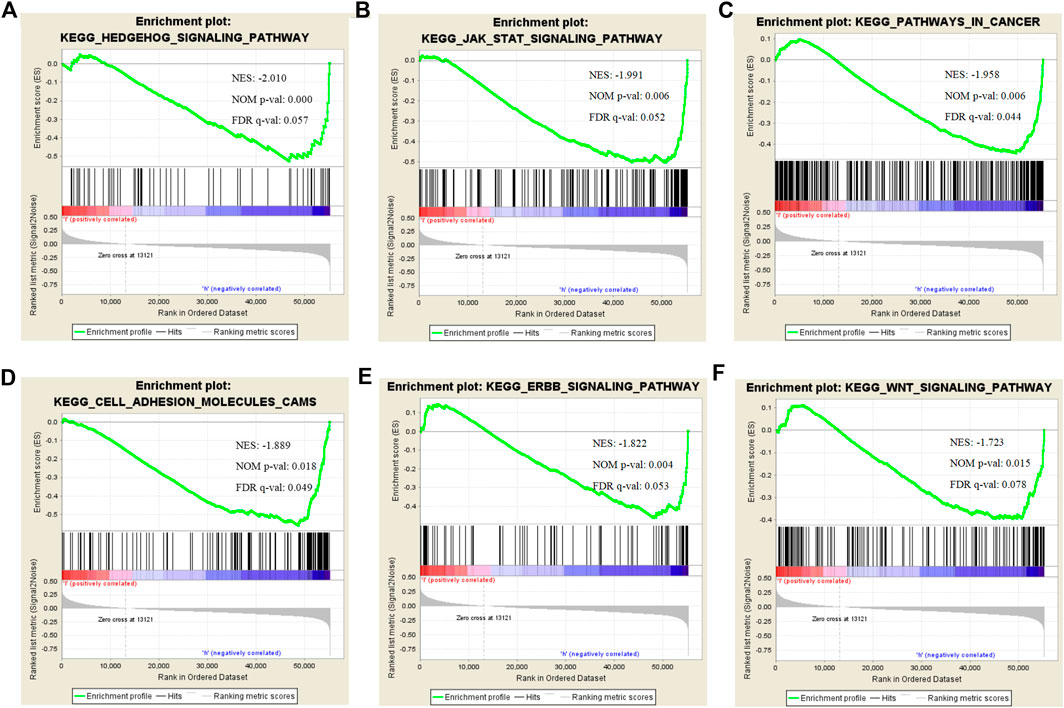
FIGURE 5. Enrichment plots for CLIP4 in breast cancer obtained by GSEA. GSEA results showing Hedgehog signaling pathway (A), JAK-STAT signaling pathway (B), pathways in cancer (C), cell adhesion molecules (D), ERBB signaling pathway (E), and Wnt signaling pathway (F) are differentially enriched in CLIP4-related breast cancer. NES, normalized ES; NOM p-value, normalized p-value; FDR q-value, false discovery rate q-value.
Promoter Methylation of CLIP4 in Breast Cancer
To assess whether CLIP4 downregulation was related with DNA methylation in breast cancer, the DiseaseMeth database was analyzed. The results showed that the CLIP4 promoter was hypermethylated in patients compared with normal controls (p = 4.533E−11, <0.001) (Figure 6A). MEXPRESS was used to confirm the promoter methylation status of CLIP4 in breast cancer. The samples were presented in ascending order according to the level of expression, and CLIP4 expression was negatively associated with DNA methylation based on Pearson’s correlation analyses (Supplementary Figure S5). The results indicated that promoter hypermethylation of CLIP4 was related to the downregulation of mRNA expression. Furthermore, we investigated whether CLIP4 downregulation was caused by genetic alterations. Copy number alterations and gene mutations of CLIP4 were analyzed using the cBioPortal online tool. Genetic alterations of CLIP4 were mutually exclusive and only found in 11 (1.1%) of 963 invasive breast carcinoma patients, of which six samples had DNA amplification, two had deep deletion, two had a truncating mutation, and one had a missense mutations (Figure 6B). These results supported the significant role of DNA methylation in the regulation of CLIP4 expression.
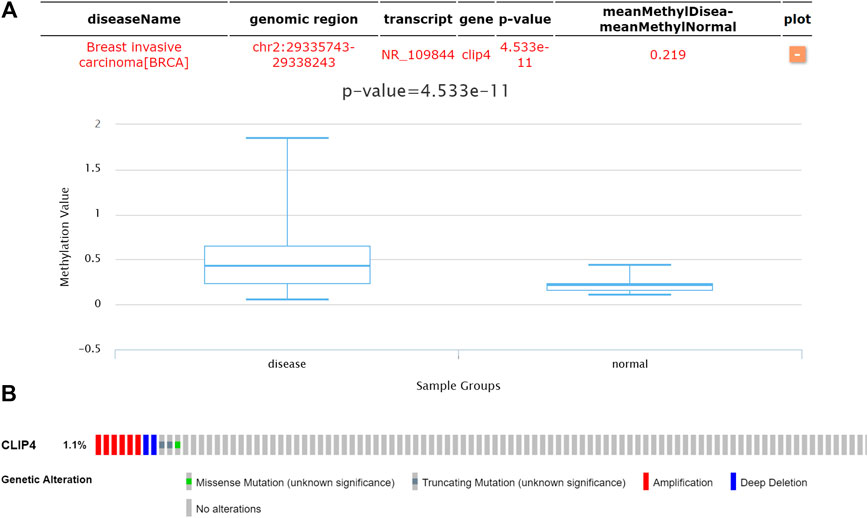
FIGURE 6. Genetic features of CLIP4 in breast cancer. (A) The DNA methylation status of CLIP4 in 1299 breast invasive carcinomas and in normal tissue obtained from DiseaseMeth datasets. (B) CLIP4 gene alterations in 963 breast cancers based on OncoPrint. Tumor tissues are shown in columns.
Discussion
In the present study, the expression of CLIP4 was investigated in breast cancer by bioinformatics analysis. Oncomine and TCGA analysis revealed mRNA expression of CLIP4 to be significantly decreased in breast cancer, when compared to normal samples. CLIP4 expression was negatively correlated with HER2 status, NPI, SBR grade, nodal status, and tumor stage. However, the basal-like and TNBC types were positively associated with CLIP4 expression. The prognostic role of CLIP4 in breast cancer was then investigated using the Kaplan-Meier Plotter, and the results demonstrated that higher expression of CLIP4 was associated with better RFS and OS, especially in Luminal B and HER2 positive breast cancers. Analysis of epigenetic and genetic alterations of CLIP4 in breast cancer indicated that promoter methylation was the main mechanism underlying the regulation of CLIP4 gene expression. These findings suggested that promoter methylation-mediated loss of CLIP4 expression may be a novel prognostic biomarker for breast cancer.
Cytoskeletal proteins play important effects in cellular functions such as opposing compression, invasion, migration, and transport. The cytoskeleton also provides an optimal scaffold for the process of signal transduction (Balikov et al., 2017). However, the role of the cytoskeleton and microtubule-associated protein CLIP4 in human cancer has not been studied extensively. The methylation of XKR6, CCDC57, MAML3, SDC2, and CLIP4 is associated with age and tumor location in gastric cancer (Chong et al., 2014). The promoter methylation of IRF4, ELMO1, CLIP4, and MSC is related with increasing seriousness from gastritis with no metaplasia to gastritis with metaplasia and gastric cancer (Pirini et al., 2017). Contrary to the results of this study, increased expression of four genes (CLIP4, NOX4, LAMP5, and MATN3) is related to poor prognosis, and the expression of these genes is higher in stromal components than in epithelial cancer cells in gastric cancer (Lee et al., 2014). CLIP4 shows high expression levels in kidney cancer cell lines compared with normal cell lines, and CLIP4 significantly increases cell migration and viability (Ahn et al., 2016). In clear cell renal cell carcinoma (ccRCC), CLIP4 mutations are three-fold higher in patients with aggressive tumors than in those without aggressive ccRCC, and high expression levels of MOCOS, BAIAP2L1, DDX11, and CLIP4 are markedly associated with poor OS (Park et al., 2020). Interestingly, potentially pathogenic mutations of CLIP4 and other genes may state a subset of lung adenocarcinoma among never-smoking women (Donner et al., 2018). In line with this study, 43 genes (including CLIP4) were downregulated in ovarian cancer tumor samples and reactivated after treatment with 5-Aza-2'-deoxycytidine (5-aza-dC); CLIP4, GULP1, BAMBI, NT5E, and TGFB2 showed a pattern of cancer-specific methylation (Maldonado et al., 2018). Three DNA methylation markers, C9orf50, KCNQ5, and CLIP4, can discriminate between the plasma from colorectal cancer patients and that of healthy individuals (Jensen et al., 2019). Furthermore, CLIP4 has been considered a promising epigenetic biomarker by analysis of differentially methylated genes and differentially expressed genes between matched tumor and non-tumor tissues of the colon (Wu et al., 2020). Of note, CLIP4 was associated with better OS in Luminal B and HER2 positive breast cancers but not TNBC type in this study, the result revealed that CLIP4 may be used as a target to overcome the drug resistance, incomplete responders, and relapsed in individualized treatment of Luminal B and HER2 breast cancer. Despite many studies providing important data and tumor-specific roles for CLIP4 in human cancer, its biological function and molecular mechanism remain unclear. Another CAP-Gly domain containing linker protein, CLIP1 is a mRNA stemness index-related key gene associated with a better lung adenocarcinoma prognosis, which is involved in tumor metastasis, relapse, and drug resistance (Zhao et al., 2020). A report showed that cells expressing FGFR2-CLIP1 fusion were sensitive to INCB054828 (a pan-FGFR inhibitor) in cholangiocarcinoma while the FGFR2 N549H mutation was resistant to this inhibitor (Krook et al., 2019).
Unlike previous reports, analyzing gastric cancer and ccRCC, this study showed that high CLIP4 expression was associated with a better prognosis, suggesting that CLIP4 had a suppressive function in breast cancer. To elucidate the molecular mechanism underlying the function of CLIP4 in breast cancer, we constructed a CLIP4-associated regulatory network. The present findings supported the important role of CLIP4 upregulation in tumor-associated signaling pathways such as Hh, JAK-STAT, Wnt, and ERBB, and suggested that it acted as a tumor suppressor in breast cancer. GSEA and Metascape analysis indicated that CLIP4 was related to the Wnt signaling pathway. Wnt signaling plays a critical role in normal development as well as tumorigenesis (Yin et al., 2018). Overactivation of Wnt signaling is implicated in human diseases including breast cancer. However, the association of CLIP4 with Wnt signaling and cytoskeletal proteins in human cancers has not been reported to date. Tankyrases, which are multifunctional poly (ADP-ribose) polymerase (PARP) superfamily members with features of both signaling and cytoskeletal proteins, antagonize the Wnt/β-catenin signaling (Kuusela et al., 2016). In addition, the Wnt/β-catenin pathway can influence the distribution of microtubules and neurofilaments (Tian et al., 2019). As a therapeutic target to overcome drug resistance in breast cancer (Tabassum et al., 2019), the JAK-STAT signaling pathway was associated with CLIP4 in this study. However, only one previous study has shown that another cytoskeletal protein, CLIP3, plays an essential role in astrocyte activation, and is associated with STAT3 pathway activation induced by spinal cord injury (Chen et al., 2018). Therefore, there is lack of other evidence in human cancers that identifies the role of CLIPs in JAK-STAT signaling. Similarly, one study reported that the cytoskeletal protein Zyxin is involved in fine tuning the neural plate patterning in Xenopus laevis embryos by modulating the activity of an effector of Hh signaling, the transcription factor glioma-associated oncogene 1 (GLI1) (Martynova et al., 2018). Hh signaling, which results in activation of GLI transcription factors and correlates with worse outcomes of breast cancer, is activated in human mammary stem cells (Bhateja et al., 2019). Although therapeutic agents such as Herceptin, which are designed to inhibit ERBB activity, have dramatically improved the survival of patients with HER2 positive breast cancer, approximately 50% of patients acquire drug resistance within 1 year (Zahnow, 2006). Therefore, it is important to identify gene targets associated with ERBB signaling. The present findings on the association between CLIP4 and ERBB signaling in breast cancer may provide a novel research direction.
In summary, the cytoplasmic linker protein CLIP4 may act as a novel prognostic and epigenetic biomarker for breast cancer patients. There were limitations to this study. First, the analysis was based on mRNA levels from public datasets, which requires confirmation at the mRNA and protein level. Second, some of the bioinformatics tools used had only limited functionality, such as hierarchical analysis without multivariable Cox regression analysis. Finally, there was a lack of in vivo and in vitro direct evidence to confirm the biological function and molecular mechanism of CLIP4 in breast cancer. Additional basic studies and clinical trials are urgently needed to validate the findings of this study. For the future, we plan to confirm and evaluate the value of CLIP4 as a potential biomarker for breast cancer.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author Contributions
Study deign: YF and LH; investigation and resources: YF; data collection: YF, SF, YH, and JF; writing-original draft preparation: YF and YW; writing-review and editing: YF and YW; data interpretation and visualization: LH and QW; supervision: YF; project administration: YF; funding acquisition: YF; statistical analysis: LH, YW, and SF. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Funded Project of Affiliated Hospital of Southwest Medical University for Doctors (grant no. 17135), and the Luzhou-Southwest Medical University Applied Basic Research Project (grant no. 2019LZXNYDJ07).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmolb.2020.616190/full#supplementary-material.
References
Ahn, J., Han, K. S., Heo, J. H., Bang, D., Kang, Y. H., Jin, H. A., et al. (2016). FOXC2 and CLIP4 : a potential biomarker for synchronous metastasis of ≤7-cm clear cell renal cell carcinomas. Oncotarget 7 (32), 51423–51434. doi:10.18632/oncotarget.9842
Akhmanova, A., and Hoogenraad, C. C. (2005). Microtubule plus-end-tracking proteins: mechanisms and functions. Curr Opin Cell Biol 17 (1), 47–54. doi:10.1016/j.ceb.2004.11.001
Balikov, D. A., Brady, S. K., Ko, U. H., Shin, J. H., De Pereda, J. M., Sonnenberg, A., et al. (2017). The nesprin-cytoskeleton interface probed directly on single nuclei is a mechanically rich system. Nucleus 8 (5), 534–547. doi:10.1080/19491034.2017.1322237
Bhateja, P., Cherian, M., Majumder, S., and Ramaswamy, B. (2019). The hedgehog signaling pathway: a viable target in breast cancer?. Cancers 11 (8), 1126. doi:10.3390/cancers11081126
Cerami, E., Gao, J., Dogrusoz, U., Gross, B. E., Sumer, S. O., Aksoy, B. A., et al. (2012). The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2 (5), 401–404. doi:10.1158/2159-8290.CD-12-0095
Chandrashekar, D. S., Bashel, B., Balasubramanya, S. A. H., Creighton, C. J., Ponce-Rodriguez, I., Chakravarthi, B. V. S. K., et al. (2017). UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia 19 (8), 649–658. doi:10.1016/j.neo.2017.05.002
Chen, W., Zheng, R., Baade, P. D., Zhang, S., Zeng, H., Bray, F., et al. (2016). Cancer statistics in China, 2015. CA Cancer J Clin 66 (2), 115–132. doi:10.3322/caac.21338
Chen, X., Chen, C., Hao, J., Zhang, J., and Zhang, F. (2018). Effect of CLIP3 upregulation on astrocyte proliferation and subsequent glial scar formation in the rat spinal cord via STAT3 pathway after injury. J Mol Neurosci 64 (1), 117–128. doi:10.1007/s12031-017-0998-6
Chong, Y., Mia-Jan, K., Ryu, H., Abdul-Ghafar, J., Munkhdelger, J., Lkhagvadorj, S., et al. (2014). DNA methylation status of a distinctively different subset of genes is associated with each histologic Lauren classification subtype in early gastric carcinogenesis. Oncol Rep 31 (6), 2535–2544. doi:10.3892/or.2014.3133
Curtis, C., Shah, S. P., Chin, S. F., Turashvili, G., Rueda, O. M., Dunning, M. J., et al. (2012). The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 486 (7403), 346–352. doi:10.1038/nature10983
DeSantis, C. E., Ma, J., Gaudet, M. M., Newman, L. A., Miller, K. D., Goding Sauer, A., et al. (2019). Breast cancer statistics, 2019. CA A Cancer J Clin. 69 (6), 438–451. doi:10.3322/caac.21583
Donner, I., Katainen, R., Sipilä, L. J., Aavikko, M., Pukkala, E., and Aaltonen, L. A. (2018). Germline mutations in young non-smoking women with lung adenocarcinoma. Lung Canc 122, 76–82. doi:10.1016/j.lungcan.2018.05.027
Fan, Y., Mu, J., Huang, M., Imani, S., Wang, Y., Lin, S., et al. (2019). Epigenetic identification of ADCY4 as a biomarker for breast cancer: an integrated analysis of adenylate cyclases. Epigenomics 11 (14), 1561–1579. doi:10.2217/epi-2019-0207
Galjart, N. (2005). CLIPs and CLASPs and cellular dynamics. Nat Rev Mol Cell Biol 6 (6), 487–98. doi:10.1038/nrm1664
Galjart, N., and Perez, F. (2003). A plus-end raft to control microtubule dynamics and function. Curr Opin Cell Biol. 15 (1), 48–53. doi:10.1016/s0955-0674(02)00007-8
Glück, S., Ross, J. S., Royce, M., McKenna, E. F., Perou, C. M., Avisar, E, et al. (2012). TP53 genomics predict higher clinical and pathologic tumor response in operable early-stage breast cancer treated with docetaxel-capecitabine ± trastuzumab. Breast Cancer Res Treat 132 (3), 781–791. doi:10.1007/s10549-011-1412-7
Györffy, B., Lanczky, A., Eklund, A. C., Denkert, C., Budczies, J., Li, Q., et al. (2010). An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat 123 (3), 725–731. doi:10.1007/s10549-009-0674-9
Howard, J., and Hyman, A. A. (2003). Dynamics and mechanics of the microtubule plus end. Nature 422 (6933), 753–758. doi:10.1038/nature01600
Jensen, S. Ø., Øgaard, N., Ørntoft, M.-B. W., Rasmussen, M. H., Bramsen, J. B., Kristensen, H., et al. (2019). Novel DNA methylation biomarkers show high sensitivity and specificity for blood-based detection of colorectal cancer-a clinical biomarker discovery and validation study. Clin Epigenet 11 (1), 158. doi:10.1186/s13148-019-0757-3
Jézéquel, P., Campone, M., Gouraud, W., Guérin-Charbonnel, C., Leux, C., Ricolleau, G., et al. (2012). bc-GenExMiner: an easy-to-use online platform for gene prognostic analyses in breast cancer. Breast Cancer Res Treat 131 (3), 765–775. doi:10.1007/s10549-011-1457-7
Koch, A., De Meyer, T., Jeschke, J., and Van Criekinge, W. (2015). MEXPRESS: visualizing expression, DNA methylation and clinical TCGA data. BMC Genom. 16, 636. doi:10.1186/s12864-015-1847-z
Krook, M. A., Bonneville, R., Chen, H. Z., Reeser, J. W., Wing, M. R., Martin, D. M., et al. (2019). Tumor heterogeneity and acquired drug resistance in FGFR2-fusion-positive cholangiocarcinoma through rapid research autopsy. Cold Spring Harb Mol Case Stud 5 (4), a004002. doi:10.1101/mcs.a004002
Kuusela, S, Wang, H, Wasik, AA, Suleiman, H, and Lehtonen, S (2016). Tankyrase inhibition aggravates kidney injury in the absence of CD2AP. Cell Death Dis 7, e2302. doi:10.1038/cddis.2016.217
Lee, J., Sohn, I., Do, I. G., Kim, K. M., Park, S. H., Park, J. O., et al. (2014). Nanostring-based multigene assay to predict recurrence for gastric cancer patients after surgery. PLoS One 9 (3), e90133. doi:10.1371/journal.pone.0090133
Ma, X. J., Dahiya, S., Richardson, E., Erlander, M., and Sgroi, D. C. (2009). Gene expression profiling of the tumor microenvironment during breast cancer progression. Breast Cancer Res 11 (1), R7. doi:10.1186/bcr2222
Maldonado, L., Brait, M., Izumchenko, E., Begum, S., Chatterjee, A., Sen, T., et al. (2018). Integrated transcriptomic and epigenomic analysis of ovarian cancer reveals epigenetically silenced GULP1. Cancer Lett 433, 242–251. doi:10.1016/j.canlet.2018.06.030
Martynova, N. Y., Parshina, E. A, Ermolina, L. V., and Zaraisky, A. G. (2018). The cytoskeletal protein Zyxin interacts with the zinc-finger transcription factor Zic1 and plays the role of a scaffold for Gli1 and Zic1 interactions during early development of Xenopus laevis. Biochem Biophys Res Commun 504 (1), 251–256. doi:10.1016/j.bbrc.2018.08.164
Mitchison, T., and Kirschner, M. (1984). Dynamic instability of microtubule growth. Nature 312 (5991), 237–242. doi:10.1038/312237a0
Park, J. S., Pierorazio, P. M., Lee, J. H., Lee, H. J., Lim, Y. S., Jang, W. S., et al. (2020). Gene expression analysis of aggressive clinical t1 stage clear cell renal cell carcinoma for identifying potential diagnostic and prognostic biomarkers. Cancers 12 (1), 222. doi:10.3390/cancers12010222
Perez, F., Diamantopoulos, G. S., Stalder, R., and Kreis, T. E. (1999). CLIP-170 highlights growing microtubule ends in vivo. Cell 96 (4), 517–527. doi:10.1016/s0092-8674(00)80656-x
Pirini, F., Noazin, S., Jahuira-Arias, M. H., Rodriguez-Torres, S., Friess, L., Michailidi, C., et al. (2017). Early detection of gastric cancer using global, genome-wide and IRF4, ELMO1, CLIP4 and MSC DNA methylation in endoscopic biopsies. Oncotarget 8 (24), 38501–38516. doi:10.18632/oncotarget.16258
Rhodes, D. R., Yu, J., Shanker, K., Deshpande, N., Varambally, R., Ghosh, D., et al. (2004). ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia 6 (1), 1–6. doi:10.1016/s1476-5586(04)80047-2
Richardson, A. L., Wang, Z. C., De Nicolo, A., Lu, X., Brown, M., Miron, A., et al. (2006). X chromosomal abnormalities in basal-like human breast cancer. Canc. Cell 9 (2), 121–132. doi:10.1016/j.ccr.2006.01.013
Riehemann, K., and Sorg, C. (1993). Sequence homologies between four cytoskeleton-associated proteins. Trends Biochem Sci 18 (3), 82–83. doi:10.1016/0968-0004(93)90159-k
Siegel, R. L., Miller, K. D., and Jemal, A. (2018). Cancer statistics, 2018. CA: A Cancer Journal for Clinicians 68 (1), 7–30. doi:10.3322/caac.21442
Subramanian, A., Tamayo, P., Mootha, V. K., Mukherjee, S., Ebert, B. L., Gillette, M. A., et al. (2005). Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102 (43), 15545–15550. doi:10.1073/pnas.0506580102
Szklarczyk, D., Gable, A. L., Lyon, D., Junge, A., Wyder, S., Huerta-Cepas, J., et al. (2019). STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 47 (D1), D607–D613. doi:10.1093/nar/gky1131
Tabassum, S., Abbasi, R., Ahmad, N., and Farooqi, A. A. (2019). Targeting of JAK-STAT signaling in breast cancer: therapeutic strategies to overcome drug resistance. Adv Exp Med Biol. 1152, 271–281. doi:10.1007/978-3-030-20301-6_14
Tian, Z., Zhang, X., Zhao, Z., Zhang, F., and Deng, T. (2019). The Wnt/β-catenin signaling pathway affects the distribution of cytoskeletal proteins in Aβ treated PC12 cells. J Integr Neurosci 18 (3), 309–312. doi:10.31083/j.jin.2019.03.168
Uhlén, M., Björling, E., Agaton, C., Szigyarto, C. A., Amini, B., Andersen, E., et al. (2005). A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol Cell Proteomics 4 (12), 1920-1932. doi:10.1074/mcp.M500279-MCP200
Pontén, H., and Zhang, J. (2018). Decreased expression of TFAP2B in endometrial cancer predicts poor prognosis: a study based on TCGA data. Gynecol Oncol 149 (3), 592–597. doi:10.1016/j.ygyno.2018.03.057
Wu, Y., Wan, X., Jia, G., Xu, Z., Tao, Y., Song, Z., et al. (2020). aberrantly methylated and expressed genes as prognostic epigenetic biomarkers for colon cancer. DNA Cell Biol 39 (11), 1961–1969. doi:10.1089/dna.2020.5591
Xiong, Y., Wei, Y., Gu, Y., Zhang, S., Lyu, J., Zhang, B., et al. (2017). DiseaseMeth version 2.0: a major expansion and update of the human disease methylation database. Nucleic Acids Res 45 (D1), D888–D895. doi:10.1093/nar/gkw1123
Yin, P., Wang, W., Zhang, Z., Bai, Y., Gao, J., and Zhao, C. (2018). Wnt signaling in human and mouse breast cancer: Focusing on Wnt ligands, receptors and antagonists. Cancer Sci 109 (11), 3368–3375. doi:10.1111/cas.13771
Zahnow, C. A. (2006). ErbB receptors and their ligands in the breast. Expert Rev. Mol. Med. 8 (23), 1–21. doi:10.1017/S146239940600010X
Zhao, M., Chen, Z., Zheng, Y., Liang, J., Hu, Z., Bian, Y., et al. (2020). Identification of cancer stem cell-related biomarkers in lung adenocarcinoma by stemness index and weighted correlation network analysis. J Cancer Res Clin Oncol 146 (6), 1463–1472. doi:10.1007/s00432-020-03194-x
Keywords: DNA methylation, CAP-Gly domain containing linker protein family member 4, breast cancer, prognosis, biomarker, integrated analysis
Citation: Fan Y, He L, Wang Y, Fu S, Han Y, Fan J and Wen Q (2021) CLIP4 Shows Putative Tumor Suppressor Characteristics in Breast Cancer: An Integrated Analysis. Front. Mol. Biosci. 7:616190. doi: 10.3389/fmolb.2020.616190
Received: 11 October 2020; Accepted: 18 December 2020;
Published: 26 January 2021.
Edited by:
Matteo Becatti, University of Florence, ItalyReviewed by:
Rifat Hamoudi, University of Sharjah, United Arab EmiratesDuanBo Shi, Shandong University, China
Zhang Fan, The People’s Hospital of Jianyang City, China
Copyright © 2021 Fan, He, Wang, Fu, Han, Fan and Wen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Fan, eXVmYW5Ac3dtdS5lZHUuY24=
 Yu Fan
Yu Fan Lijia He1
Lijia He1 Shaozhi Fu
Shaozhi Fu Yunwei Han
Yunwei Han