- 1Department of Obstetrics and Gynecology, Lanzhou University Second Hospital, Lanzhou, China
- 2Department of Clinical Laboratory, Lanzhou University Second Hospital, Lanzhou, China
- 3Department of Pathology, Lanzhou University Second Hospital, Lanzhou, China
Background: Activation of Snail and synergistic loss of E-cadherin are hallmark features of the epithelial-mesenchymal transition (EMT), which contributes to the metastasis phenotype of epithelial cancer cells. However, the prognostic impact of Snail and of its combination with E-cadherin and with other EMT prognostic markers has not yet been systematically studied in cervical carcinoma. This study aimed to explore the prognostic value of combined Snail and E-cadherin in patients with cervical carcinoma and compared it to the prognostic value of other EMT prognostic markers.
Methods: We retrospectively identified every initial diagnosis of cervical carcinoma among 203 patients treated at our hospital in China from January 2008 to March 2013. We examined the prognostic significance of Snail and other EMT protein markers, such as E-cadherin, Slug, ZEB1, Twist, Vimentin, and Survivin, by univariate and multivariate survival analyses.
Results: Multivariate analyses showed that Snail and E-cadherin were significant biomarkers for overall survival (OS) in cervical carcinoma patients (HR, hazard ratio = 1.744, P = 0.036 and HR = 1.738, P = 0.047; respectively). Moreover, a combined index including Snail and E-cadherin showed enhanced prognostic value compared to that of Snail or E-cadherin alone. The present data demonstrate that Snail shows a negative correlation with E-cadherin (P < 0.001). High Snail expression and low E-cadherin expression were also more common in high tumor stages (P = 0.044 and P = 0.036; respectively), and lymph node metastasis (both P < 0.001). Moreover, Snail was a superior prognosis factor compared to Slug, ZEB1, Twist, Vimentin, and Survivin in cervical carcinoma.
Conclusion: Based on our results, Snail and E-cadherin may be considered as independent prognosis markers, and the combination of Snail and E-cadherin might improve the OS prediction accuracy for patients with cervical carcinoma.
Introduction
As it is one of the four most common malignancies in women, cervical carcinoma threatens the health of women around the world (Bray et al., 2018). Despite the development of effective vaccines for prevention, patients with metastatic disease and advanced disease still have a poor prognosis, with a 5-year survival rate for metastatic cervical carcinoma of only 16.5% (Ferlay et al., 2013; Li H. et al., 2016). Thus, novel prognostic biomarkers for cervical carcinoma are urgently needed.
Epithelial-mesenchymal transformation (EMT) is characterized by increased cell mobility and loss of cell adhesion, which are closely related to tumor invasion and metastasis (Brabletz et al., 2018; Pastushenko and Blanpain, 2019). EMT is a complicated and multi-step process that has been found to be initiated, regulated, and maintained by several factors, including hypoxia, cytokines, signaling pathways, and transcription factors in cervical carcinoma.
Intra-tumoural hypoxia is commonly found in carcinoma, and hypoxia-induced proteome changes promote cervical carcinoma cell invasion and migration by participating in tumor necrosis factor (TGF)-β1-induced EMT (Wilson and Hay, 2011). Hypoxia can enhance ZEB1 expression and promote cervical carcinoma progression through increased CCL8 secretion and tumor-associated macrophage recruitment (Chen et al., 2019). Other EMT inducers include the inflammatory-based cytokines [i.e., interleukin (IL)-6, TNF-α, TGF-β]. IL-6, one of the key cytokines in the tumor microenvironment, and its stimulation induce the EMT program in cervical carcinoma cells (Miao et al., 2014). Given its established role in tumor carcinogenesis and metastasis, TNF-α is one of the essential EMT-promoting cytokines in a variety of tumors, including cervical carcinoma (Bohrnsen et al., 2019; Dong et al., 2019; Tan et al., 2019; Cruceriu et al., 2020). TGF-β, which is produced and secreted by tumor-infiltrating immune cells, can induce EMT in an advanced cervical tumor model by 3D printing (Sistigu et al., 2017). Thus, reactivation of EMT also links inflammation-based cytokines to cervical carcinoma. Moreover, EMT is involved in all types of signaling pathways (e.g., NF-kB, EGFR–ERK, BMP, Wnt/β-catenin, Akt/GSK-3b/Snail, EGF/EGFR, p53/TIGAR and SCO2 pathways), which are regulated by the zinc-finger family of transcription factors, including Snail, Slug, and Twist, which contribute to tumor metastasis by promoting EMT in cervical carcinoma (Qureshi et al., 2015).
E-cadherin (CDH1) is one of the most important components of adherens junctions, which are integral in cell adhesion and are principal organizers of the epithelial phenotype (van Roy, 2014). EMT contributes to enhanced mobility and the invasion of epithelial cells that results in the evolution of tumors toward migration and metastasis (Thiery, 2002). It is well established that loss of E-cadherin is involved in EMT, which induces the migration and metastasis of tumor cells, including cervical carcinoma (Schmalhofer et al., 2009; Liu et al., 2017; Jing et al., 2019). Snail, a main EMT-activating transcriptional factor, controls EMT by repressing E-cadherin expression, and upregulation of its expression is associated with a poor prognosis in many types of carcinoma (Zhang et al., 2014; Fazilaty et al., 2019). Snail was significantly upregulated in cervical squamous cell carcinoma, and high levels can contribute to the onset of EMT; however, its effectiveness in prognosis prediction of cervical carcinoma has not been fully characterized (Zhao et al., 2013; Gong et al., 2015).
Transcription factors, including Snail family members (Slug), Twist, ZEB1 and others, have been identified as critical regulators of EMT during tumor progression, embryogenesis, and metastasis (Krebs et al., 2017; Wu et al., 2019). Slug and Twist, representing basic helix–loop–helix transcription factors, act as cell–cell adhesion disrupters by inhibiting E-cadherin expression (El Ghouzzi et al., 2000; De Craene et al., 2005; Qin et al., 2012). ZEB1 has two zinc-finger domains, located at the N and C termini, through which it binds to E-box-like sequences (CACCTG) in target DNA (Ikeda and Kawakami, 1995). ZEB1 functions act as a transcriptional repressor of cell–cell adhesion by suppressing the glycoprotein E-cadherin.
EMT involves the loss of E-cadherin expression and acquisition of the expression of the mesenchymal marker Vimentin (Tiwari et al., 2012). Vimentin, which serves as an important intermediate filament protein in mesenchymal cells, plays a key role in the metastatic growth and invasion of tumor cells, including cervical carcinoma cells (Cheng et al., 2012; Lu et al., 2019). Survivin, a member of the inhibitors of apoptotic protein (IAP) family, which suppress apoptosis and enhance tumor cell proliferation and angiogenesis, is implicated in the EMT of some tumors (Mita et al., 2008; Breyer et al., 2016; Liu et al., 2018; Zhao et al., 2019).
The purpose of this pilot study was to clarify whether expression of Snail, as determined by immunohistochemistry, can help predict the long-term survival outcome of cervical carcinoma patients and to compare its value to that of an epithelial marker (E-cadherin), transcription factors (Slug, ZEB1, Twist), a mesenchymal marker (Vimentin), and another marker (Survivin). We found that although low expression of E-cadherin and high expression of Snail, Slug, Twist, Vimentin, and Survivin indicated a worse prognosis, only E-cadherin and Snail independently predicted survival after adjusting for confounding variables.
Materials and Methods
Patients
We analyzed 203 cervical carcinoma samples collected from operable cervical cases and 56 pair-matched adjacent normal tissues (peritumoral tissue more than 2 cm from cancer tissue). All clinical cervical carcinoma samples were collected from the Department of Pathology at the Second Hospital of Lanzhou University between January 2008 to March 2013. The samples were used in accordance with the principles of the Declaration of Helsinki and the guidelines of the Second Hospital of Lanzhou University Ethical Review Board for Medical Research Involving Human Subjects (approval number: 2016A-072). Written informed consent was obtained from all patients.
Patients’ clinical parameters and baseline data were collected, including International Federation of Gynecology and Obstetrics (FIGO) stage, age, lymph node metastasis status, tumor type, and histologic grade. All patients had access to their clinical follow-up data, and none had received biotherapy, chemotherapy or radiotherapy prior to surgery. The clinical stage of cervical carcinoma patients was determined according to the FIGO staging system established in 2009. The patients’ median age was 55 years (range = 28–82 years). The basic characteristics of the population are summarized in Table 1. Patients with cervical carcinoma were generally followed up via telephone or postoperative visits. Overall survival (OS) refers to the period from the date of diagnosis to the date of the last follow-up visit (June 2018) or the date of death.
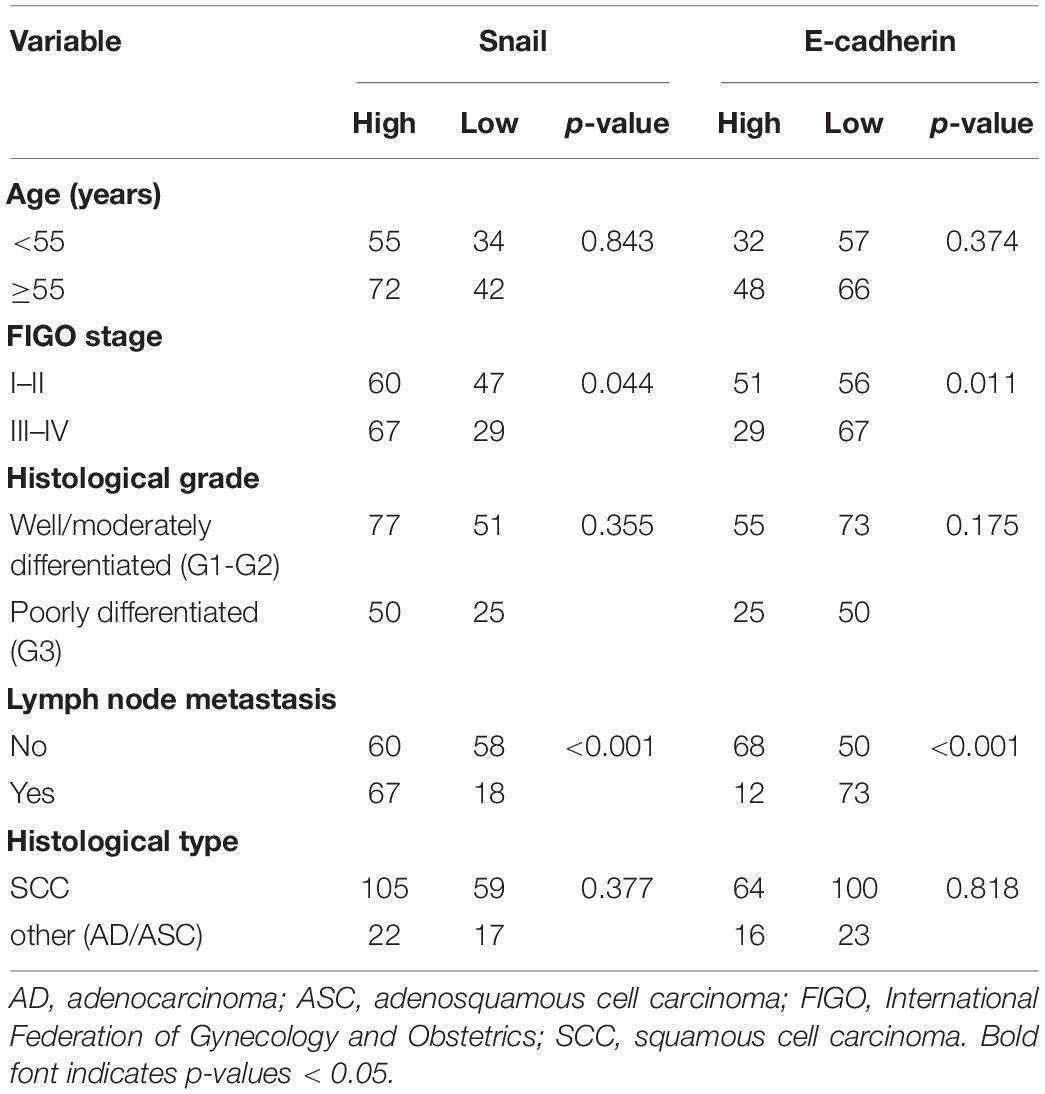
Table 1. Correlation of Snail and E-cadherin expression with the clinicopathologic characteristics of 203 cervical carcinoma patients.
Immunohistochemical Staining
Immunohistochemistry was carried out following the manufacturer’s recommendations. Tissue sections were embedded in paraffin and deparaffinized with xylene for 15 min, fixed with 100% ethanol for 10 min, and then rehydrated. The prepared slices were treated with methanol solution containing 3% hydrogen peroxide for 10 min to block endogenous peroxidase activity. Sections were washed twice with PBS and then incubated with antibodies against Snail, E-cadherin, Slug, ZEB1, Twist, Vimentin and Survivin overnight (dilutions 1:200, 1:100, 1:100, 1:50, 1:75, 1:200, and 1:100) at 4°C. All sections were subjected to a heat-induced antigen retrieval process. Sections were again washed with PBS twice and then incubated with the corresponding secondary antibody under ambient conditions for 30 min. Then, after color development with 3-amino9-ethylcarbazole for 15 min, the slides were counterstained with hematoxylin. Finally, an optical microscope was used to observe the slides. Negative and positive controls were used to optimize staining. EMT inducers (Snail, Slug, ZEB1, and Twist) and EMT markers (E-cadherin, Vimentin, and Survivin) were also used. Rabbit anti-human polyclonal antibodies against Snail (ab180714), E-cadherin (ab15148), Slug (ab180714), ZEB1 (ab87280), Twist (ab49254), Vimentin (ab45939), and Survivin (ab469) were purchased from Abcam (Cambridge, United Kingdom).
Evaluation of the Immunohistochemistry Results
The immunohistochemical staining scoring system was evaluated according to previously described criteria, with minor modifications (Li et al., 2012; Song et al., 2012). The scores from the proportion of positively stained immunoreactive cells and the staining intensity were added to the overall score.
The staining results of Snail proteins, Slug, ZEB1, Twist, Vimentin, and Survivin in the nucleus and cytoplasm were subsequently evaluated. The amounts of E-cadherin distributed in the membrane and cytoplasm were determined. The intensity scores were determined on a scale from 0 to 3, indicating negative, weak, mild and strong staining, respectively. The proportions of scores were 0 (negative); 1 (1–10% positive cells), 2 (10–70% positive cells), and 3 (>70% positive cells). The total scores ranged from 0 to 6 and were designated 0–2 (low) or 3–6 (high).
Statistical Analysis
The software used for statistical analysis was SPSS 22.0. The chi-square test was applied to analyze the correlations between E-cadherin and Snail protein expression levels and clinicopathological features. The associations between the expression levels of Snail, E-cadherin, Slug, ZEB1, Twist, Vimentin and Survivin and cervical carcinoma prognosis were analyzed using Kaplan-Meier survival analysis, and the logarithmic rank test was used in the univariate analysis. The significance levels of variables that were independently associated with OS were determined using the Cox proportional hazards regression model in the univariate and multivariate analyses. P-values lower than 0.05 were considered statistically significant.
Results
Correlation of EMT Protein Expression in Human Cervical Carcinoma Tissue
We determined Snail and E-cadherin protein expression levels in 203 cases of cervical carcinoma and 56 cases of adjacent normal tissues by IHC staining. The representative IHC results are shown in Figure 1. In total, scatter dot plot showed that the average immunostaining score of Snail protein in 203 tumor tissues was 3.59 ± 1.86; that in 56 normal tissues was 2.39 ± 1.87 (Figures 1A,B, P < 0.001). Snail showed a significant difference (high/low expression 127/203 vs. 17/56, P < 0.001) (Supplementary Table S1). In contrast, scatter dot plot showed that the average immunostaining score of E-cadherin protein in 203 tumor tissues was 2.38 ± 1.76, whereas that in 56 normal tissues was 4.09 ± 1.78 (Figures 1C,D, P < 0.001). E-cadherin protein expression was downregulated in cervical carcinoma compared with normal tissues (low/high expression 80/203 vs. 36/56, P = 0.001) (Supplementary Table S1). Moreover, Snail protein upregulation was consistent with E-cadherin downregulation, as shown in serial sections (Figure 1E, P < 0.001).
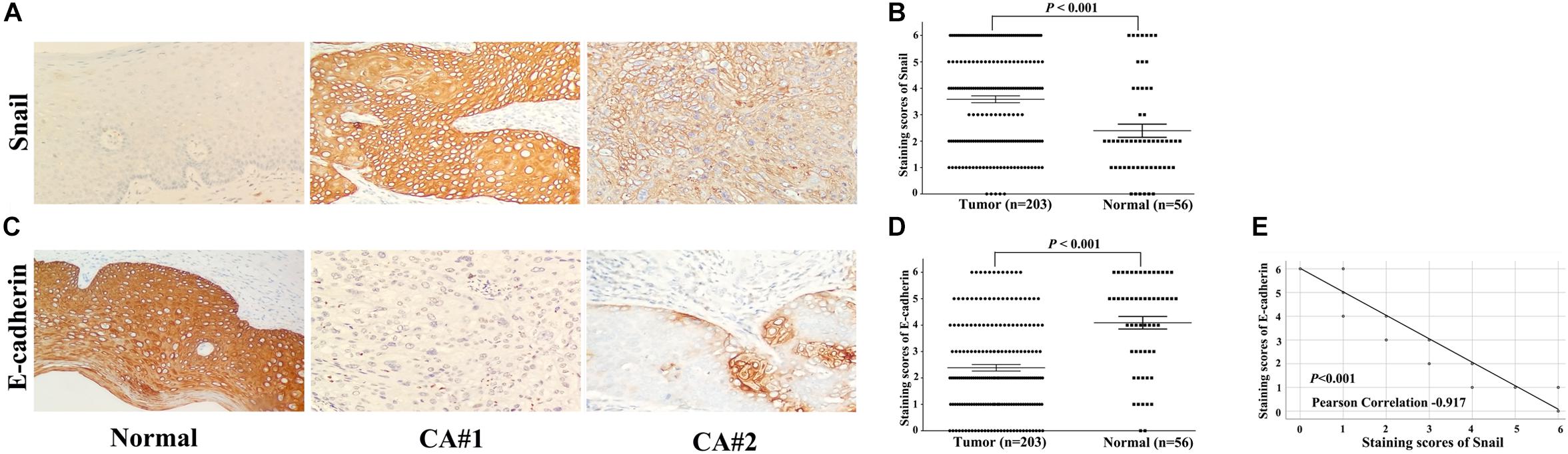
Figure 1. Snail and E-cadherin expression in cervical carcinoma tissues determined by immunohistochemical staining (original magnification × 200). (A) Representative Snail expression in tumor and normal tissues, with positive expression located in the cytoplasm. (B) Scatter dot plot showing the staining score (mean ± SEM) of Snail in tumor and normal tissues using the paired t-test. *P < 0.001; (C) Representative E-cadherin expression in tumor and normal tissues, with positive expression located in the membrane and cytoplasm. (D) Scatter dot plot showing the staining score (mean ± SEM) of E-cadherin in tumor and normal tissues using the paired t-test. *P < 0.001; (E) Snail expression was negatively correlated with E-cadherin expression in 203 patients with cervical carcinoma.
IHC staining also showed that other transcription factors and markers, including Slug, ZEB1, Twist, Vimentin, and Survivin proteins, were more highly expressed in cervical carcinoma compared with normal tissues, at 55.2% (112/203) vs. 48.2% (27/56), P = 0.355; 51.2% (104/203) vs. 42.9% (24/56), P = 0.267; 53.2% (108/203) vs. 41.1% (23/56), P = 0.108; 55.7% (113/203) vs. 32.1% (18/56), P = 0.002; and 56.7% (115/203) vs. 42.9% (24/56), P = 0.067, respectively (Supplementary Table S1). Scatter dot plot showed that the staining score (mean ± SD) of other transcription factors and markers, including Slug, ZEB1, Twist, Vimentin, and Survivin, were increased in cervical carcinoma compared with normal tissues (high/low score 3.21 ± 1.80 vs. 2.93 ± 1.74, P = 0.29; high/low score 3.14 ± 1.72 vs. 2.66 ± 1.83, P = 0.08; high/low score 3.22 ± 1.68 vs. 2.34 ± 1.94, P = 0.002; high/low score 3.12 ± 1.73 vs. 2.63 ± 1.95, P = 0.09, respectively) (Supplementary Figure S1).
Association of EMT Protein Expression With the Clinicopathological Characteristics of Cervical Carcinoma Patients
Among the 203 patients, Snail overexpression and downregulation of E-cadherin expression showed statistically significant correlations with an aggressive FIGO stage (P = 0.044 and P = 0.036; respectively), and lymph node metastasis (both P < 0.001) (Table 1). Enhanced levels of Slug and Survivin also showed statistically significant correlations with an aggressive FIGO stage (both P < 0.001) and lymph node metastasis (both P < 0.001). Furthermore, Twist and Vimentin were significantly elevated in patients who had cervical carcinoma with an aggressive FIGO stage (both P < 0.001), a high histological grade (P = 0.003 and P = 0.016; respectively), and lymph node metastasis (both P < 0.001) (Supplementary Table S2).
Univariate and Multivariate Survival Analyses of all Prognostic Parameters
We evaluated the effect of Snail, Slug, ZEB1, Twist, Vimentin, Survivin, and E-cadherin expression on OS using a Cox proportional hazard regression model (Table 2). The risk of death was assessed using univariate analysis. The results indicated that FIGO stage (HR = 2.490, P < 0.001), histological grade (HR = 1.575, P = 0.041), lymph node metastasis (HR = 3.341, P < 0.001), Snail (HR = 2.238, P = 0.001), Slug (HR = 1.785, P = 0.012), Twist (HR = 2.131, P = 0.001), Vimentin (HR = 2.226, P = 0.001), Survivin (HR = 1.791, P = 0.012), and E-cadherin (HR = 2.612, P < 0.001) were significantly associated with an increased risk of death. After adjusting for confounding variables, multivariate analysis showed that FIGO stage (HR = 1.782, P = 0.029), lymph node metastasis (HR = 1.887, P = 0.025), Snail (HR = 1.744, P = 0.036), and E-cadherin (HR = 1.738, P = 0.047) correlated obviously with OS (Table 2).
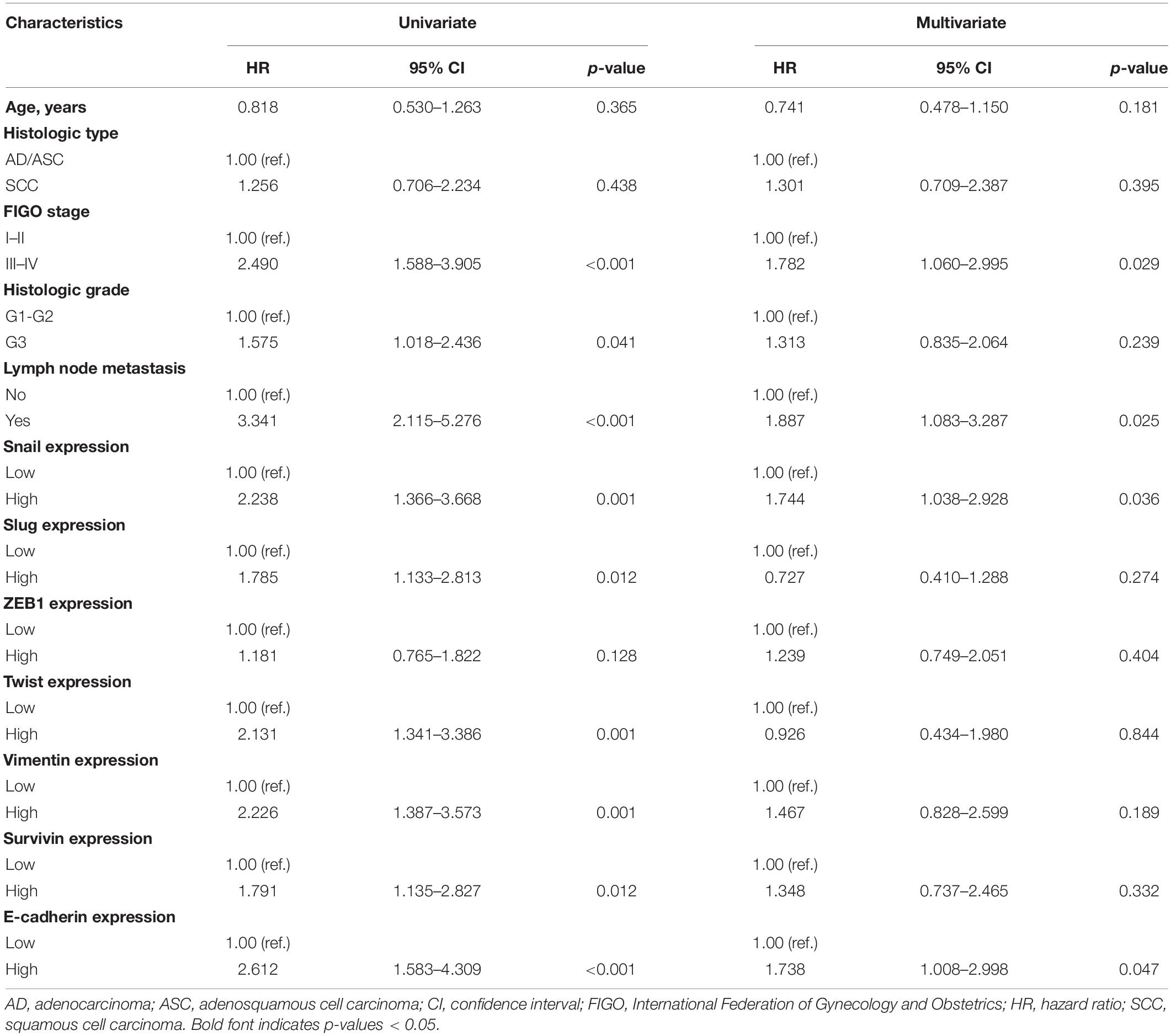
Table 2. Univariate and multivariate analyses of the characteristics associated with OS in 203 cervical carcinoma patients.
In the Kaplan-Meier analyses, increased Snail expression (Figure 2A) and decreased E-cadherin expression (Figure 2B) were unfavorable outcomes for cervical carcinoma patients (all P < 0.05). These results were supported by the estimated cumulative 5-year survival rates of cervical carcinoma patients with expression levels of Snail (62.2% high vs. 82.9% low) and E-cadherin (85.0% high vs. 60.9% low).
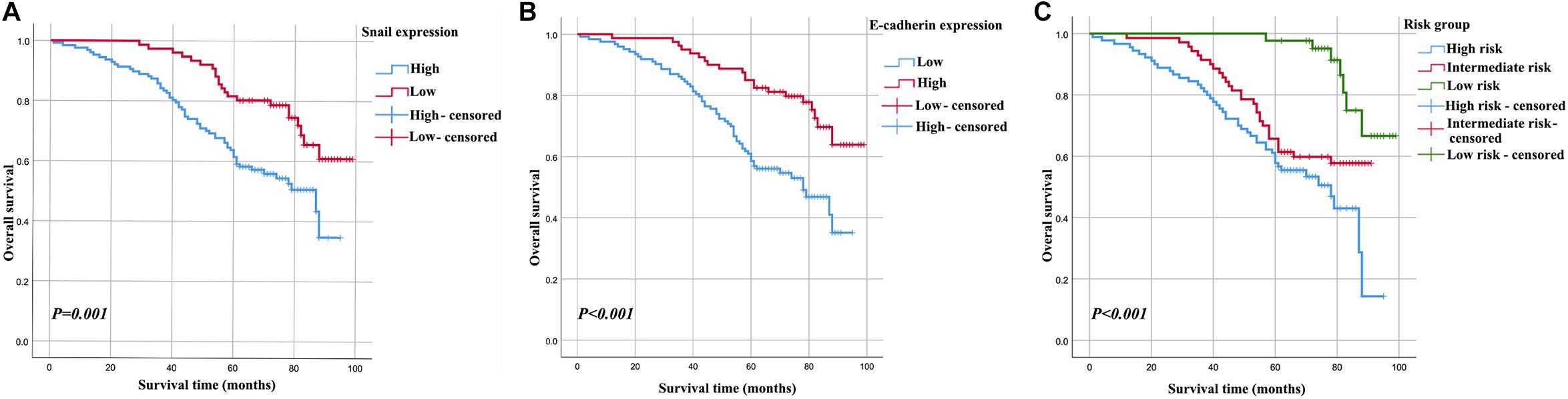
Figure 2. Kaplan–Meier curves for 5-year OS rate of patients with cervical carcinoma. OS based on Snail in cervical carcinoma patients (A), OS based on E-cadherin in cervical carcinoma patients (B), OS of combined Snail and E-cadherin-based categorisation in cervical carcinoma patients (high risk, Snail-high and E-cadherin-low; intermediate risk, Snail-high and E-cadherin-high or Snail-low and E-cadherin-high; and low risk, Snail-low and E-cadherin-high) (C).
Prognostic Value of the Combined Snail and E-cadherin
The relationship between Snail and E-cadherin was performed using Spearman’s rank correlation analysis. Methods combining Snail with E-cadherin might improve patient stratification be related to OS. Thus, the patients were divided into three groups: high risk, Snail-high and E-cadherin-low; intermediate risk, Snail-high and E-cadherin-high or Snail-low and E-cadherin-high; and low risk, Snail-low and E-cadherin-high (Figure 2C). The high-, intermediate- and low-risk groups showed 5-year OS rates corresponding to 60.0, 67.1, and 95.3%, respectively. After adjusting for other clinical variables in multivariate analysis (Table 3), both the high- and intermediate-risk groups had significantly worse prognoses compared with the low-risk group.
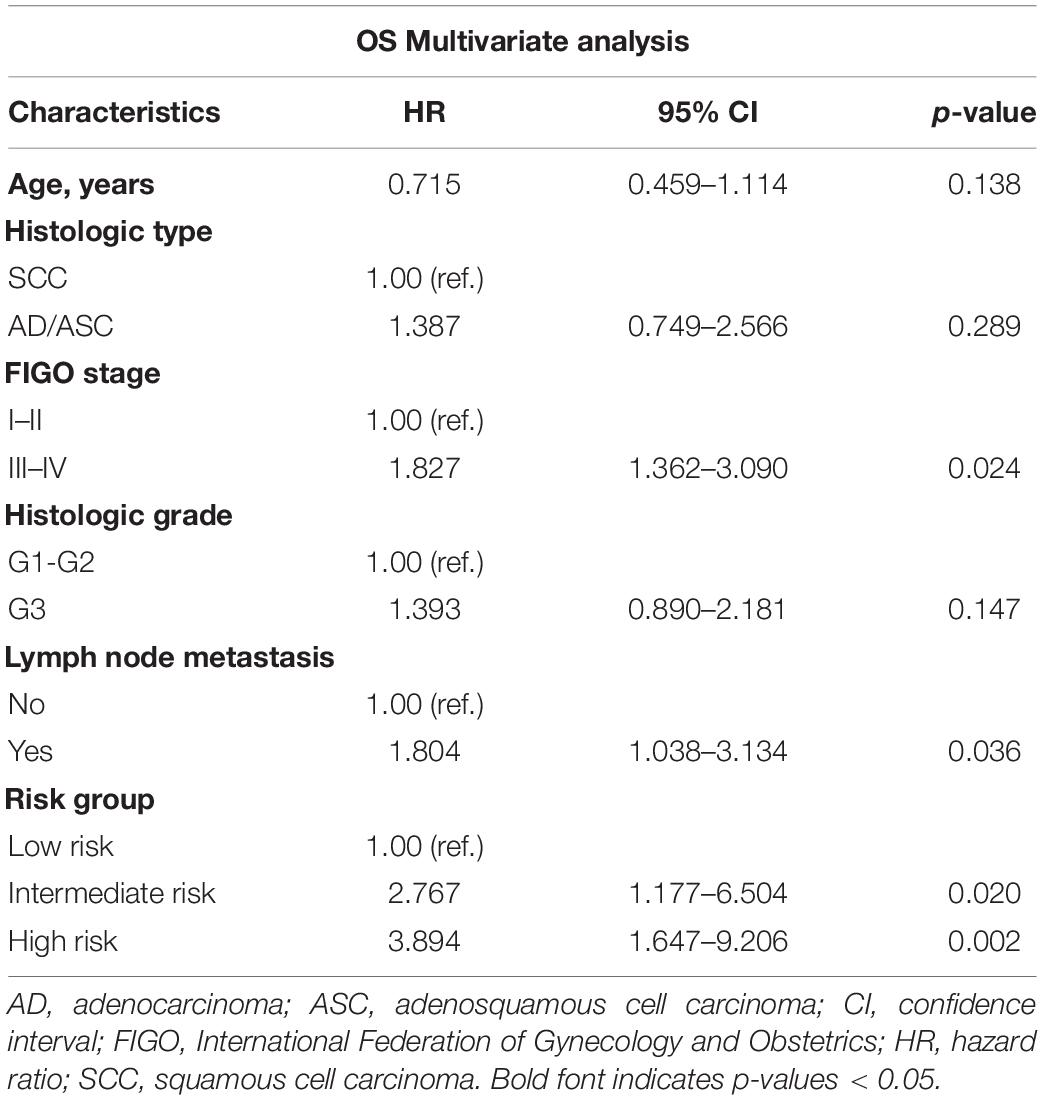
Table 3. Multivariate analyses of the characteristics associated with OS in 203 cervical carcinoma patients.
Discussion
Increasing evidence indicates that up-regulation and nuclear accumulation of Snail correlate with EMT in cervical carcinoma (Lee et al., 2008; Zhao et al., 2013). An et al. (2016) reported that in cervical carcinoma cells, α-Actinin-4 promotes EMT and tumorigenesis by regulating Snail expression and the Akt pathway. Pang et al. (2017) found that YB-1 promotes EMT and the progression of cervical carcinoma by upregulating Snail expression, which suggested that the YB-1/Snail/EMT axis may be used as a potential candidate for the diagnosis and therapy of cervical carcinoma metastasis. However, little is known about the prognosis outcome of Snail in cervical carcinoma, and the values of Snail expression in cervical carcinoma and its clinical significance have not been thoroughly explored.
In our study, the data indicated the following: (1) high Snail expression predicts a lower survival rate of cervical carcinoma patients; (2) high Snail expression is associated with a highly aggressive FIGO stage and LNM status in cervical carcinoma patients; (3) Snail expression is increased in cervical carcinoma tissue compared to normal tissue, and it correlates negatively with E-cadherin expression; (4) the Snail protein is a better prognostic factor than Slug, Twist, ZEB1, Vimentin, and Survivin in cervical carcinoma; and (5) combining Snail and E-cadherin proteins may improve the precision of OS prediction in cervical carcinoma patients.
Low E-cadherin expression is correlated with FIGO stage and lymph node metastasis, and it is a useful marked for survival outcomes in cervical carcinoma. E-cadherin immunostaining might assist in the diagnosis of cervical intraepithelial neoplasia and indicate prognosis in early stage cervical squamous cell carcinoma patients (Li B. et al., 2016). Based on this study, we suggest that E-cadherin may be considered an anti-oncogene and that downregulation of E-cadherin may contribute to tumor metastasis and poor prognosis.
The other transcription factors Slug, Twist, ZEB1 have been confirmed to serve as useful prognostic factors in many cancers. The levels of Slug, Twist, and ZEB1 expression between tumor tissues and normal tissues were not significant (Supplementary Figure S1), and multivariate analysis demonstrated that Slug, Twist, ZEB1 were not independent prognostic factors in cervical carcinoma. However, Wushou et al. (2014) and Huang et al. (2016) showed that expression of Twist and Slug is associated with worse survival in carcinoma. Our univariate analysis results showed high Twist and Slug expression levels are closely related to shorter OS, but no significant relationship was found between OS and ZEB1 expression. Therefore Snail might be a more effective EMT-related marker than Slug, Twist, and ZEB1 for predicting survival.
Increased Vimentin expression corresponded with FIGO stage, the grade of differentiation and LNM. Overexpression Vimentin clearly predicted shorter OS in univariate analysis but not in multivariate analysis. This is different from the findings of other studies. Lin et al. (2017) showed in a multivariate analysis that Vimentin overexpression in cervical cancer patients was significantly associated with poor OS. The differences may be due to differences in sample size by analysis by the log-rank test in the previous study. High Survivin expression was a prognostic factor for a short OS in univariate Cox regression analysis but not in multivariate analysis. In addition, the level of Survivin expression did not achieve a meaningful difference between tumor tissue and normal tissue. The result suggests that Survivin is a prognostic factor of OS of patients with cervical carcinoma but not an independent prognostic factor.
The present data showed a relationship between Snail and E-cadherin. Snail is a zinc finger transcription factor that mainly participates in EMT by upregulating expression of E-cadherin in epithelial tumor cells (Lin et al., 2014; Liu et al., 2019). This finding of a strong correlation between Snail and E-cadherin further suggests complex interaction between the transcription factors, with EMT biomarkers potentially influencing patient survival.
The combination of high Snail and low E-cadherin levels resulted in the lowest 5-year survival rate among the three groups. These results further support that Snail and E-cadherin are related to EMT in cervical carcinoma, leading to a subsequent adverse outcome, and further confirm that Snail correlates negatively with E-cadherin. Furthermore, our findings highlight that combining Snail and E-cadherin can discriminate patients with better prognosis in cervical carcinoma compared with Snail or E-cadherin alone.
Our study has some limitations. As this was a retrospective, single-center study, additional studies with larger patient groups are needed to evaluate the potential of these markers.
Conclusion
We demonstrate that Snail and E-cadherin act as independent factors for predicting OS in cervical carcinoma. Based on the findings of this study, combining Snail and E-cadherin expression can improve the prognostic accuracy and serve as a select criterion for risk factor-stratified patient management in cervical carcinoma.
Data Availability Statement
The datasets analyzed in this article are not publicly available. Requests to access the datasets should be directed to YT, dGlhbnlqMTRAbHp1LmVkdS5jbi4=
Ethics Statement
The studies involving human participants were reviewed and approved by the Lanzhou University Second Hospital Ethics Committee. The patients/participants provided their written informed consent to participate in this study. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
XH conceived the idea. YT, PQ, and QN performed all the experiments and wrote the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmolb.2020.00022/full#supplementary-material
References
An, H. T., Yoo, S., and Ko, J. (2016). Alpha-actinin-4 induces the epithelial-to-mesenchymal transition and tumorigenesis via regulation of snail expression and beta-catenin stabilization in cervical cancer. Oncogene 35, 5893–5904. doi: 10.1038/onc.2016.117
Bohrnsen, F., Holzenburg, J., Godek, F., Kauffmann, P., Moser, N., and Schliephake, H. (2019). Influence of tumour necrosis factor alpha on epithelial-mesenchymal transition of oral cancer cells in co-culture with mesenchymal stromal cells. Int. J. Oral Maxillofac. Surg. 49, 157–165. doi: 10.1016/j.ijom.2019.06.001
Brabletz, T., Kalluri, R., Nieto, M. A., and Weinberg, R. A. (2018). EMT in cancer. Nat. Rev. Cancer 18, 128–134. doi: 10.1038/nrc.2017.118
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global cancer statistics 2018: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424. doi: 10.3322/caac.21492
Breyer, J., Gierth, M., Shalekenov, S., Aziz, A., Schafer, J., Burger, M., et al. (2016). Epithelial-mesenchymal transformation markers E-cadherin and survivin predict progression of stage pta urothelial bladder carcinoma. World J. Urol. 34, 709–716. doi: 10.1007/s00345-015-1690-5
Chen, X. J., Deng, Y. R., Wang, Z. C., Wei, W. F., Zhou, C. F., Zhang, Y. M., et al. (2019). Hypoxia-induced ZEB1 promotes cervical cancer progression via CCL8-dependent tumour-associated macrophage recruitment. Cell Death Dis. 10:508. doi: 10.1038/s41419-019-1748-1
Cheng, Y., Zhou, Y., Jiang, W., Yang, X., Zhu, J., Feng, D., et al. (2012). Significance of E-cadherin, beta-catenin, and vimentin expression as postoperative prognosis indicators in cervical squamous cell carcinoma. Hum. Pathol. 43, 1213–1220. doi: 10.1016/j.humpath.2011.08.025
Cruceriu, D., Baldasici, O., Balacescu, O., and Berindan-Neagoe, I. (2020). The dual role of tumor necrosis factor-alpha (TNF-alpha) in breast cancer: molecular insights and therapeutic approaches. Cell. Oncol. (Dordr) 43, 1–18. doi: 10.1007/s13402-019-00489-1
De Craene, B., van Roy, F., and Berx, G. (2005). Unraveling signalling cascades for the snail family of transcription factors. Cell. Signal. 17, 535–547. doi: 10.1016/j.cellsig.2004.10.011
Dong, W., Chen, A., Chao, X., Li, X., Cui, Y., Xu, C., et al. (2019). Chrysin inhibits proinflammatory factor-induced EMT phenotype and cancer stem cell-like features in HeLa cells by blocking the NF-kappaB/Twist axis. Cell. Physiol. Biochem. 52, 1236–1250. doi: 10.33594/000000084
El Ghouzzi, V., Legeai-Mallet, L., Aresta, S., Benoist, C., Munnich, A., de Gunzburg, J., et al. (2000). Saethre-chotzen mutations cause TWIST protein degradation or impaired nuclear location. Hum. Mol. Genet. 9, 813–819. doi: 10.1093/hmg/9.5.813
Fazilaty, H., Rago, L., Kass Youssef, K., Ocana, O. H., Garcia-Asencio, F., Arcas, A., et al. (2019). A gene regulatory network to control EMT programs in development and disease. Nat. Commun. 10:5115. doi: 10.1038/s41467-019-13091-8
Ferlay, J., Steliarova-Foucher, E., Lortet-Tieulent, J., Rosso, S., Coebergh, J. W., Comber, H., et al. (2013). Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur. J. Cancer 49, 1374–1403. doi: 10.1016/j.ejca.2012.12.027
Gong, X., Tao, Y., Zhou, L., Yu, L., Wu, S., Song, W., et al. (2015). [Expressions of snail, slug and KAI1 proteins in cervical carcinoma and their clinicopathological significance]. Nan Fang Yi Ke Da Xue Xue Bao 35, 1733–1738. doi: 10.3969/j.issn.1673-4254.2015.12.14
Huang, C., Zhang, P., Zhang, D., and Weng, X. (2016). The prognostic implication of slug in all tumour patients – a systematic meta-analysis. Eur. J. Clin. Invest. 46, 398–407. doi: 10.1111/eci.12608
Ikeda, K., and Kawakami, K. (1995). DNA binding through distinct domains of zinc-finger-homeodomain protein AREB6 has different effects on gene transcription. Eur. J. Biochem. 233, 73–82. doi: 10.1111/j.1432-1033.1995.073_1.x
Jing, L., Bo, W., Yourong, F., Tian, W., Shixuan, W., and Mingfu, W. (2019). Sema4c mediates EMT inducing chemotherapeutic resistance of miR-31-3p in cervical cancer cells. Sci. Rep. 9:17727. doi: 10.1038/s41598-019-54177-z
Krebs, A. M., Mitschke, J., Lasierra Losada, M., Schmalhofer, O., Boerries, M., Busch, H., et al. (2017). The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat. Cell Biol. 19, 518–529. doi: 10.1038/ncb3513
Lee, M. Y., Chou, C. Y., Tang, M. J., and Shen, M. R. (2008). Epithelial-mesenchymal transition in cervical cancer: correlation with tumor progression, epidermal growth factor receptor overexpression, and snail up-regulation. Clin. Cancer Res. 14, 4743–4750. doi: 10.1158/1078-0432.CCR-08-0234
Li, B., Shi, H., Wang, F., Hong, D., Lv, W., Xie, X., et al. (2016). Expression of E-, P- and N-Cadherin and its clinical significance in cervical squamous cell carcinoma and precancerous lesions. PLoS One 11:e0155910. doi: 10.1371/journal.pone.0155910
Li, H., Wu, X., and Cheng, X. (2016). Advances in diagnosis and treatment of metastatic cervical cancer. J. Gynecol. Oncol. 27:e43. doi: 10.3802/jgo.2016.27.e43
Li, Y., Wang, W., Wang, W., Yang, R., Wang, T., Su, T., et al. (2012). Correlation of TWIST2 up-regulation and epithelial-mesenchymal transition during tumorigenesis and progression of cervical carcinoma. Gynecol. Oncol. 124, 112–118. doi: 10.1016/j.ygyno.2011.09.003
Lin, J., Lu, J., Wang, C., and Xue, X. (2017). The prognostic values of the expression of vimentin, TP53, and podoplanin in patients with cervical cancer. Cancer Cell Int. 17:80. doi: 10.1186/s12935-017-0450-6
Lin, Y., Dong, C., and Zhou, B. P. (2014). Epigenetic regulation of EMT: the snail story. Curr. Pharm. Des. 20, 1698–1705. doi: 10.2174/13816128113199990512
Liu, F., Sun, Y., Liu, B., Lu, J., Li, H., Zhu, H., et al. (2018). Insulin-like growth factor-1 induces epithelial-mesenchymal transition in hepatocellular carcinoma by activating survivin. Oncol. Rep. 40, 952–958. doi: 10.3892/or.2018.6516
Liu, M., Hancock, S. E., Sultani, G., Wilkins, B. P., Ding, E., Osborne, B., et al. (2019). Snail-overexpression induces epithelial-mesenchymal transition and metabolic reprogramming in human pancreatic ductal adenocarcinoma and non-tumorigenic ductal cells. J. Clin. Med. 8:822. doi: 10.3390/jcm8060822
Liu, W., Zhang, X., Zhao, J., Li, J., Cui, Z., and Mao, X. (2017). Inhibition of cervical cancer cell metastasis by benzothiazole through up-regulation of E-cadherin expression. Microb. Pathog. 111, 182–186. doi: 10.1016/j.micpath.2017.08.050
Lu, W., Luo, J. Y., Wu, M. H., Hou, J. Y., Yang, X., Chen, G., et al. (2019). Expression of vimentin in nasopharyngeal carcinoma and its possible molecular mechanism: a study based on immunohistochemistry and bioinformatics analysis. Pathol. Res. Pract. 215, 1020–1032. doi: 10.1016/j.prp.2019.02.010
Miao, J. W., Liu, L. J., and Huang, J. (2014). Interleukin-6-induced epithelial-mesenchymal transition through signal transducer and activator of transcription 3 in human cervical carcinoma. Int. J. Oncol. 45, 165–176. doi: 10.3892/ijo.2014.2422
Mita, A. C., Mita, M. M., Nawrocki, S. T., and Giles, F. J. (2008). Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin. Cancer Res. 14, 5000–5005. doi: 10.1158/1078-0432.CCR-08-0746
Pang, T., Li, M., Zhang, Y., Yong, W., Kang, H., Yao, Y., et al. (2017). Y box-binding protein 1 promotes epithelial-mesenchymal transition, invasion, and metastasis of cervical cancer via enhancing the expressions of snail. Int. J. Gynecol. Cancer 27, 1753–1760. doi: 10.1097/IGC.0000000000001066
Pastushenko, I., and Blanpain, C. (2019). EMT transition states during tumor progression and metastasis. Trends Cell Biol. 29, 212–226. doi: 10.1016/j.tcb.2018.12.001
Qin, Q., Xu, Y., He, T., Qin, C., and Xu, J. (2012). Normal and disease-related biological functions of Twist1 and underlying molecular mechanisms. Cell Res. 22, 90–106. doi: 10.1038/cr.2011.144
Qureshi, R., Arora, H., and Rizvi, M. A. (2015). EMT in cervical cancer: its role in tumour progression and response to therapy. Cancer Lett. 356(Pt B), 321–331. doi: 10.1016/j.canlet.2014.09.021
Schmalhofer, O., Brabletz, S., and Brabletz, T. (2009). E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev. 28, 151–166. doi: 10.1007/s10555-008-9179-y
Sistigu, A., Di Modugno, F., Manic, G., and Nistico, P. (2017). Deciphering the loop of epithelial-mesenchymal transition, inflammatory cytokines and cancer immunoediting. Cytokine Growth Factor Rev. 36, 67–77. doi: 10.1016/j.cytogfr.2017.05.008
Song, H., Xu, B., and Yi, J. (2012). Clinical significance of stanniocalcin-1 detected in peripheral blood and bone marrow of esophageal squamous cell carcinoma patients. J. Exp. Clin. Cancer Res. 31:35. doi: 10.1186/1756-9966-31-35
Tan, W., Luo, X., Li, W., Zhong, J., Cao, J., Zhu, S., et al. (2019). TNF-alpha is a potential therapeutic target to overcome sorafenib resistance in hepatocellular carcinoma. EBioMedicine 40, 446–456. doi: 10.1016/j.ebiom.2018.12.047
Thiery, J. P. (2002). Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2, 442–454. doi: 10.1038/nrc822
Tiwari, N., Gheldof, A., Tatari, M., and Christofori, G. (2012). EMT as the ultimate survival mechanism of cancer cells. Semin. Cancer Biol. 22, 194–207. doi: 10.1016/j.semcancer.2012.02.013
van Roy, F. (2014). Beyond E-cadherin: roles of other cadherin superfamily members in cancer. Nat. Rev. Cancer 14, 121–134. doi: 10.1038/nrc3647
Wilson, W. R., and Hay, M. P. (2011). Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 11, 393–410. doi: 10.1038/nrc3064
Wu, J., Zhang, Y., Cheng, R., Gong, W., Ding, T., Zhai, Q., et al. (2019). Expression of epithelial-mesenchymal transition regulators Twist, slug and snail in follicular thyroid tumours may relate to widely invasive, poorly differentiated and distant metastasis. Histopathology 74, 780–791. doi: 10.1111/his.13778
Wushou, A., Hou, J., Zhao, Y. J., and Shao, Z. M. (2014). Twist-1 up-regulation in carcinoma correlates to poor survival. Int. J. Mol. Sci. 15, 21621–21630. doi: 10.3390/ijms151221621
Zhang, P., Hu, P., Shen, H., Yu, J., Liu, Q., and Du, J. (2014). Prognostic role of twist or snail in various carcinomas: a systematic review and meta-analysis. Eur. J. Clin. Invest. 44, 1072–1094. doi: 10.1111/eci.12343
Zhao, G., Wang, Q., Wu, Z., Tian, X., Yan, H., Wang, B., et al. (2019). Ovarian primary and metastatic tumors suppressed by survivin knockout or a novel survivin inhibitor. Mol. Cancer Ther. 18, 2233–2245. doi: 10.1158/1535-7163.MCT-19-0118
Keywords: EMT, Snail, E-cadherin, prognosis, cervical carcinoma
Citation: Tian Y, Qi P, Niu Q and Hu X (2020) Combined Snail and E-cadherin Predicts Overall Survival of Cervical Carcinoma Patients: Comparison Among Various Epithelial-Mesenchymal Transition Proteins. Front. Mol. Biosci. 7:22. doi: 10.3389/fmolb.2020.00022
Received: 05 September 2019; Accepted: 04 February 2020;
Published: 28 February 2020.
Edited by:
Megha Agrawal, University of Illinois at Chicago, United StatesReviewed by:
Ritesh Kumar Srivastava, University of Alabama at Birmingham, United StatesChiara Giacomelli, University of Pisa, Italy
Copyright © 2020 Tian, Qi, Niu and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuejun Tian, dGlhbnlqMTRAbHp1LmVkdS5jbg==; Xuemei Hu, aHV4bWx6dUAxNjMuY29t; aHV4bUBsenUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Yuejun Tian
Yuejun Tian Ping Qi2†
Ping Qi2† Xuemei Hu
Xuemei Hu