
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 04 April 2025
Sec. Infectious Agents and Disease
Volume 16 - 2025 | https://doi.org/10.3389/fmicb.2025.1546166
This article is part of the Research TopicWomen in Infectious Agents and Disease: 2024View all 10 articles
 Yu Chen1,2†
Yu Chen1,2† Qing Li3†
Qing Li3† Liya Du4†
Liya Du4† Zhuowen Du2
Zhuowen Du2 Yixi Zhou1
Yixi Zhou1 Yanru Huang1
Yanru Huang1 Jian Zhang1
Jian Zhang1 Wenbo Wang1
Wenbo Wang1 Lutan Zhang1
Lutan Zhang1 Jieqiong Xie1
Jieqiong Xie1 Chao Xu5
Chao Xu5 Yunsheng Ge1*
Yunsheng Ge1* Xingmei Yao1*
Xingmei Yao1* Yulin Zhou1,2*
Yulin Zhou1,2*Background: Human papillomavirus (HPV) vaccination is expected to reduce the burden of cervical cancer and other HPV-related diseases. However, if competition exists among HPV types, type replacement may occur following the reduction of vaccine-targeted types. Here, we conducted the study to explore natural HPV type competition in unvaccinated women.
Methods: HPV DNA test results from cervical samples collected between January 2013 and July 2023 at Xiamen University's Women and Children's Hospital were analyzed. In cross-sectional study, first-visit HPV genotyping results were used, and logistic regression model was constructed to evaluate interactions between vaccine-targeted and other HPV types. In cohort of women with multiple visits, the risk of acquiring other HPV types was compared between women infected with vaccine-targeted types and those HPV-negative using Cox proportional hazards model.
Results: Among 159,049 women, 19.8% tested HPV-positive, with 5.1% having multiple types. Significant negative associations were observed between HPV-6 and HPV-72 (OR: < 0.01; 95%CI: < 0.01–0.03), HPV-18 and HPV-72 (OR: < 0.01; 95%CI: < 0.01–0.02), HPV-31 and HPV-83 (OR: < 0.01; 95%CI: < 0.01–0.55), HPV-33 and HPV-26 (OR: < 0.01; 95%CI: < 0.01–0.81), HPV-45 and HPV-55 (OR: < 0.01; 95%CI: < 0.01– < 0.01), HPV-56 and HPV-26 (OR: < 0.01; 95%CI: < 0.01–0.09), as well as HPV-59 and HPV-69 (OR: < 0.01; 95%CI: < 0.01–0.68), suggesting potential type competition. However, no type competition pair was found in the cohort study. Conversely, women with vaccine-targeted types had a higher risk of acquiring other types (HR > 1.0).
Conclusions: Our findings suggested that HPV-6 and HPV-72, HPV-18 and HPV-72, HPV-31 and HPV-83, HPV-33 and HPV-26, HPV-45 and HPV-55, HPV-56 and HPV-26, HPV-59 and HPV-69 were potential type competition pairs.
Human papillomavirus (HPV) is a pathogenic microorganism primarily transmitted through sexual contact. Currently, over 200 types have been identified, with more than 40 types associated with genital infections that can be transmitted through sexual contact (Chiarini et al., 2019; Nguyen et al., 2022). Currently, 13 HPV types are classified as high-risk: HPV-16, −18, −31, −33, −35, −39, −45, −51, −52, −56, −58, −59, and−68 (Kreimer et al., 2013). Up to 70% of cervical cancers are attributed to HPV-16 and HPV-18, while collectively, HPV-16, −18, −31, −33, −45, −52, and−58 account for ~90% of cervical cancer cases (Bruni et al., 2021). In recent years, the incidence and mortality rates of cervical cancer in China have shown an upward trend, rising from 3.8/100,000 person-years and 1.2/100,000 person-years in 2003 to 12.3/100,000 person-years and 3.5/100,000 person-years in 2016 (Chen et al., 2013, 2015, 2018a; Di et al., 2015; Zhang et al., 2018; Zheng et al., 2019, 2023). According to the 2023 ICO report (Bruni et al., 2023), in 2020, there were ~110,000 new cases of cervical cancer and about 59,000 deaths in China, accounting for 20% of the global total. Cervical cancer has become one of the significant public health threats to women's health in China.
There are currently six vaccines available worldwide, since the prophylactic vaccine became available in 2006, including the three bivalent vaccines, Cervarix® (GlaxoSmithKline; Wavre, Belgium), Cecolin® (Xiamen Innovax Biotech Co Ltd; Xiamen, Fujian Province, China), and Walrinvax® (Walvax Biotechnology Co; Kunming, Yunnan Province, China) which target the 2 most carcinogenic types, HPV-16 and HPV-18, two quadrivalent vaccines, Gardasil® (Merck & Co, Kenilworth, NJ, USA) and Cervavac® (Serum Institute of India (SII), Pune, India), which target HPV-16, −18, −6, and−11, the later 2 types responsible for ~90% of genital warts (condyloma acuminata), and one nonavalent vaccine Gardasil®9 (Merck & Co, Kenilworth, NJ, USA), which targets HPV-6, −11, −16, −18, −31, −33, −45, −52, and−58 (Petrosky et al., 2015; Castle, 2023). The effectiveness of HPV vaccines has been widely proven, and 141 (72.7%) countries have introduced HPV vaccine into their national immunization programs (World Health Organization, 2024). With the implementation of the HPV vaccine immunization program and high coverage, studies have shown a significant reduction in the prevalence of HPV infection and high-grade cervical precancerous lesions (Dong et al., 2021). The domestically developed nonavalent HPV vaccine by Xiamen University has successfully concluded Phase II clinical trials, showing promising immunogenicity, and Phase III clinical trials are currently in progress (Hu et al., 2023).
The preventive HPV vaccine holds the promise of ultimately reducing or even eliminating the burden of cervical cancer and other related diseases caused by HPV infection. However, the vaccine can only prevent infection and lesion related to the vaccine-targeted types, and the current vaccines do not target all high-risk HPV types. It is of great concern whether the “type replacement” would occur after the implementation of HPV vaccination (Man et al., 2019). Type replacement is defined as an increase in the infection rate of certain HPV type due to the elimination of some HPV types (Tota et al., 2017). In previous studies, type replacement has been observed in post-pneumococcal vaccination (Weinberger et al., 2011), raising concerns that a similar phenomenon could occur with HPV vaccine, potentially hindering the success of vaccination in reducing the incidence and mortality of HPV-related diseases. As the HPV vaccination rate will greatly increase with the nationwide promotion of HPV vaccination pilot programs in China, it is crucial to explore the possible of HPV type replacement to evaluate the impact of HPV vaccination on the disease burden of cervical cancer comprehensively, and thereby provide a scientific basis for the formulation of cervical cancer prevention and control strategies in China. The best way to evaluate HPV type replacement is the comparison of infection with non-vaccine-targeted types in vaccinated population vs. unvaccinated population or the comparison of those in post-vaccination vs. pre-vaccination period by long-term surveillance. However, the introduction of HPV vaccine is late and the coverage of HPV vaccine is low in China. It is difficult to directly observe whether HPV type replacement occurs after vaccination. Previous research has proposed that the potential for type replacement after HPV vaccination depends on the competitive interactions between HPV types. Therefore, it is possible to study HPV type competition by evaluating the interactions between HPV types in unvaccinated population (Tota et al., 2013). Recently, Yingying Su et al. explored HPV type competition in an unvaccinated population, and the results suggested the possibility of type competition between HPV-16 and HPV-52, HPV-18 and HPV-51, −52, −58, HPV-31 and HPV-39, −51, −52, −53, −54, −58, HPV-33 and HPV-52, −58, HPV-58 and HPV-52, HPV-6 and HPV-39, −51, −52, −53, −54, −56, −58. However, this study was based on cross-sectional data with a limited sample size (Su et al., 2024). In this study, we plan to evaluate the interactions between HPV types through cross-sectional and cohort studies with a larger sample size to assess the potential for HPV type competition and type replacement.
The subjects in the study were women who underwent HPV testing at the Women and Children's Hospital, School of Medicine, Xiamen University, China from January 2013 to July 2023. Participants lacking age and identity information were excluded from the study. Gynecologists collected cervical exfoliated cells from outpatient women strictly following standard procedures, which were then preserved in a solution for HPV DNA analysis. The study was approved by the Ethics Committee of Women and Child's Hospital, School of Medicine, Xiamen University (approval number KY-2024-025-K02).
Two commercial HPV GenoArray Diagnostic kits, HBGA-21PKG and HBGA-37PKG, were used for HPV DNA typing, detecting 21 and 37 types, respectively. The HBGA-21PKG kit (2013-2017) identified 13 HR-HPV (HPV-16, −18, −31, −33, −35, −39, −45, −51, −52, −56, −58, −59, and−68) and 8 LR-HPV (HPV-6, −11, −42, −43, −44, −53, −66, and−81) types, while the upgraded HBGA-37PKG kit (since 2018) can detect other 16 types (HPV-26, −34, −40, −54, −55, −57, −61, −67, −69, −70, −71, −72, −73, −82, −83, −84). By using polymerase chain reaction (PCR) to amplify HPV DNA extracted from cervical samples, the resulting amplicons are hybridized with specific HPV probes within the patented “HybriMem” system, utilizing our US-patented flow-through hybridization technology. The results are obtained through a colorimetric detection method using enzyme immunoassay. Both kits were approved by the National Medical Products Administration (NMPA) and have high sensitivity and specificity (>95%) compared to FDA approved HPV Genotyping assay (Liu et al., 2010; Yang et al., 2016). They are widely recognized in medical institutions and scientific research (Hybribio, 2012; Baloch et al., 2016; Li et al., 2016; Luo et al., 2020; Gao et al., 2021). The tests were performed according to the manufacturer's protocol, which has been detailed in our previous publication (Yao et al., 2024). Briefly, the experimental protocol includes DNA extraction using magnetic beads, PCR amplification, hybridization using the HPV GenoArray Diagnostic Kit, and signal processing. Extracted DNA was subjected to PCR amplification using HPV L1 consensus primers, followed by flow through hybridization on a probed membrane for the detection of HPV types. The results were manually interpreted using the provided guide, the blue dot on the probed membrane indicated a positive result, multiple dots showed multiple infections. Controls were included for quality assurance in each test (Ajenifuja et al., 2018; Wang et al., 2018; Wüstenhagen et al., 2018; Shen et al., 2023; Yao et al., 2024).
Firstly, we included HPV genotyping result from the first visit for cross-sectional study (Figure 1). Difference in positive rate of HPV infection among different age, marital status and nationality groups were analyzed using χ2 test. Binary logistic regression was constructed to evaluated the association between infection with the HPV vaccine-targeted types (Models for HPV-6, −11, −16, −18, −31, −33, −45, −52, and−58) and infection with each of other types. Of note, all women were included in the analysis of common 21 types detected by 21 and 37 genotyping kits, while women presenting from 2018 to 2023 were further selected for the analysis of 16 additional types, HPV-26, −34, −40, −54, −55, −57, −61, −67, −69, −70, −71, −72, −73, −82, −83, and −84. HPV type-type interactions were assessed by calculating the odds ratios (OR) and 95% confidence intervals (CI) using a logistic regression model, with age as a covariate.
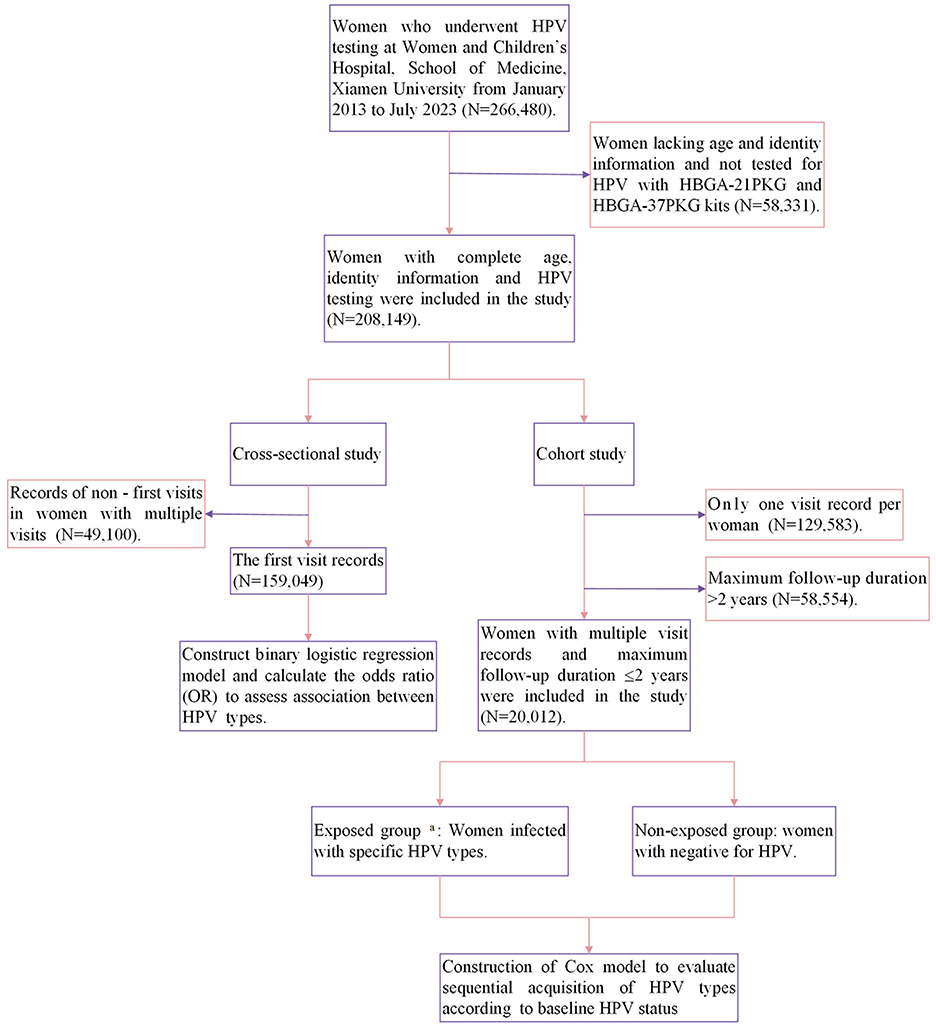
Figure 1. A flow diagram summarizing the study steps and data analysis procedures. a. Exposed group: baseline positivity for specific HPV (HPV-6/11/16/18/31/33/45/52/58/35/39/51/56/59/68).
To further explore the association between HPV vaccine-targeted types and other types, we conducted an analysis of women with multiple visits (Figure 1). Due to the low prevalence of the newly added 16 HPV types, only the 21 types from 2013 to 2023 were included in the analysis. We compared the risk of sequential acquisition of non-vaccine-targeted HPV types between women infected with HPV vaccine-targeted types and women with negative for HPV. Considering that the majority of cervical HPV infection will clear within 2 years, the study allowed for a maximum follow-up duration of 2 years to assess acquisition (Wüstenhagen et al., 2018; Zhou et al., 2019) (if the subject was persistent infection, continue to observe for 2 years from the last detection of the HPV type). A sensitivity analysis was also conducted with an extended follow-up period of up to 3 years. Cox proportional hazards regression was used to estimate hazard ratios (HR) and 95% confidence intervals (CI) for analyses of interaction of HPV vaccine-targeted types and other HPV types. Women with vaccine-targeted types as the exposed group, and women with negative for HPV as the non-exposed group, HRs of <1.0 indicate that the risk of becoming infected with a specific non-vaccine-targeted HPV types was lower among those infected with a vaccine-targeted HPV types, thus implying potential type competition between these types.
In addition, we also analyzed the interactions between the remaining six high-risk HPV types (HPV-35, −39, −51, −56, −59, −68) and other types. The significance threshold was set at P < 0.05. Statistical analyses were performed using R version 4.3.2.
Among 159,049 women, with a median age of 35 (range, 15–92), 19.8% were positive for HPV (n = 31,502). The prevalence of HPV in women aged <35 years and women aged 35–65 years were 19.7% and 19.9%, respectively, and both lower than those aged >65 years (25.0%). The HPV infection rate is relatively higher in unmarried women (23.7%) compared to married women (17.8%), and women from minority nationalities (27.2%) have a higher HPV infection rate than Han women (19.7%) (P < 0.001). Single HPV type was detected in 74.3% (n = 23,419) and multiple HPV types were detected in 25.7% (n = 8,083) of positive population. Single HPV infection was the most common pattern across different age groups, marital statuses, and nationalities (Table 1). The analysis of HPV type distribution of single and multiple infections was conducted in women who underwent testing with the HBGA-37 PKG kits (N = 83191). As we have reported previously (Yao et al., 2024), HPV-52 (3.5%), −58 (2.1%), −16 (2.0%), −51 (1.6%), and−39 (1.6%) were the five most common types and were primarily single infections (Figure 2).

Figure 2. HPV types distribution of single and multiple infections. The colors purple and pink indicate single and multiple infections.
As shown in Figure 3 and Supplementary Figure 1, in the pairwise comparisons of 15 HPV types (the current 9 vaccine-targeted types and other 6 high-risk types) and other 20 types, no significant negative association was observed. Further analysis of the association between these 15 types and the other 16 types introduced in 2018, statistically significant negative correlation were observed between several pairs of types: HPV-6 and HPV-72 (OR: < 0.01; 95%CI: < 0.01–0.03), HPV-18 and HPV-72 (OR: < 0.01; 95%CI: < 0.01–0.02), HPV-31 and HPV-83 (OR: < 0.01; 95%CI: < 0.01–0.55), HPV-33 and HPV-26 (OR: < 0.01; 95%CI: < 0.01–0.81), HPV-45 and HPV-55 (OR: < 0.01; 95%CI: < 0.01– < 0.01), HPV-56 and HPV-26 (OR: < 0.01; 95%CI: < 0.01–0.09), as well as HPV-59 and HPV-69 (OR: < 0.01; 95%CI: < 0.01–0.68).

Figure 3. Age-adjusted odds ratios (ORs) for coinfections involving vaccine-targeted HPV types and other HPV types. (a) HPV-6 and other HPV types, (b) HPV-11 and other HPV types, (c) HPV-16 and other HPV types, (d) HPV-18 and other HPV types, (e) HPV-31 and other HPV types, (f) HPV-33 and other HPV types, (g) HPV-45 and other HPV types, (h) HPV-52 and other HPV types, and (i) HPV-58 and other HPV types.
Among women with type-specific negative for HPV at baseline, 1,348 women acquired any HPV infection during follow-up, with the incidence of 41.29/1,000 person-years, and the risk of acquisition of HR-HPV and LR-HPV were 32.51/1,000 person-years and 19.99/1,000 person-years, respectively. Similarly, HPV-52 was the most commonly acquired type, with the incidence of 8.39/1,000 person-years, followed by HPV-51, −58, −39 and−16, with the incidence of 5.98/1,000 person-years, 5.71/1,000 person-years, 4.88/1,000 person-years and 4.21/1,000 person-years, respectively. Most HPV types are prone to multiple infections, while HPV-53 is less likely to be detected in co-infections with other types (Table 2).
In the subsequent analysis, women were grouped according to the presence of HPV infection, exposed group: women who were infected with 15 types, respectively, non-exposed group: women who were negative for HPV, and HPV type-specific incidence between two groups during follow-up period was compared. We found that prior infection with HPV-18 seemed to reduce the risk of acquiring HPV-6 (HR: 0.69, 95%CI: 0.10–4.96), however, the difference was not significant. Similar patterns were observed between HPV-31 and HPV-39 (HR: 0.62, 95%CI: 0.09–4.45), HPV-31 and HPV-53 (HR:0.53, 95%CI: 0.07–3.78), HPV-52 and HPV-6 (HR: 0.15, 95%CI: 0.02–1.05), HPV-58 and HPV-44 (HR: 0.32, 95%CI: 0.04–2.30), HPV-51 and HPV-6 (HR: 0.50, 95%CI: 0.07–3.58). Other results did not suggest that prior infection with any vaccine-targeted types (HPV-6, −11, −16, −18, −31, −33, −45, −52 and−58) inhibited acquisition of other types. Rather, the presence of preexisting infection with vaccine-targeted types actually increased the risk of acquiring other types (HR > 1.0, Figure 4 and Supplementary Figure 2).
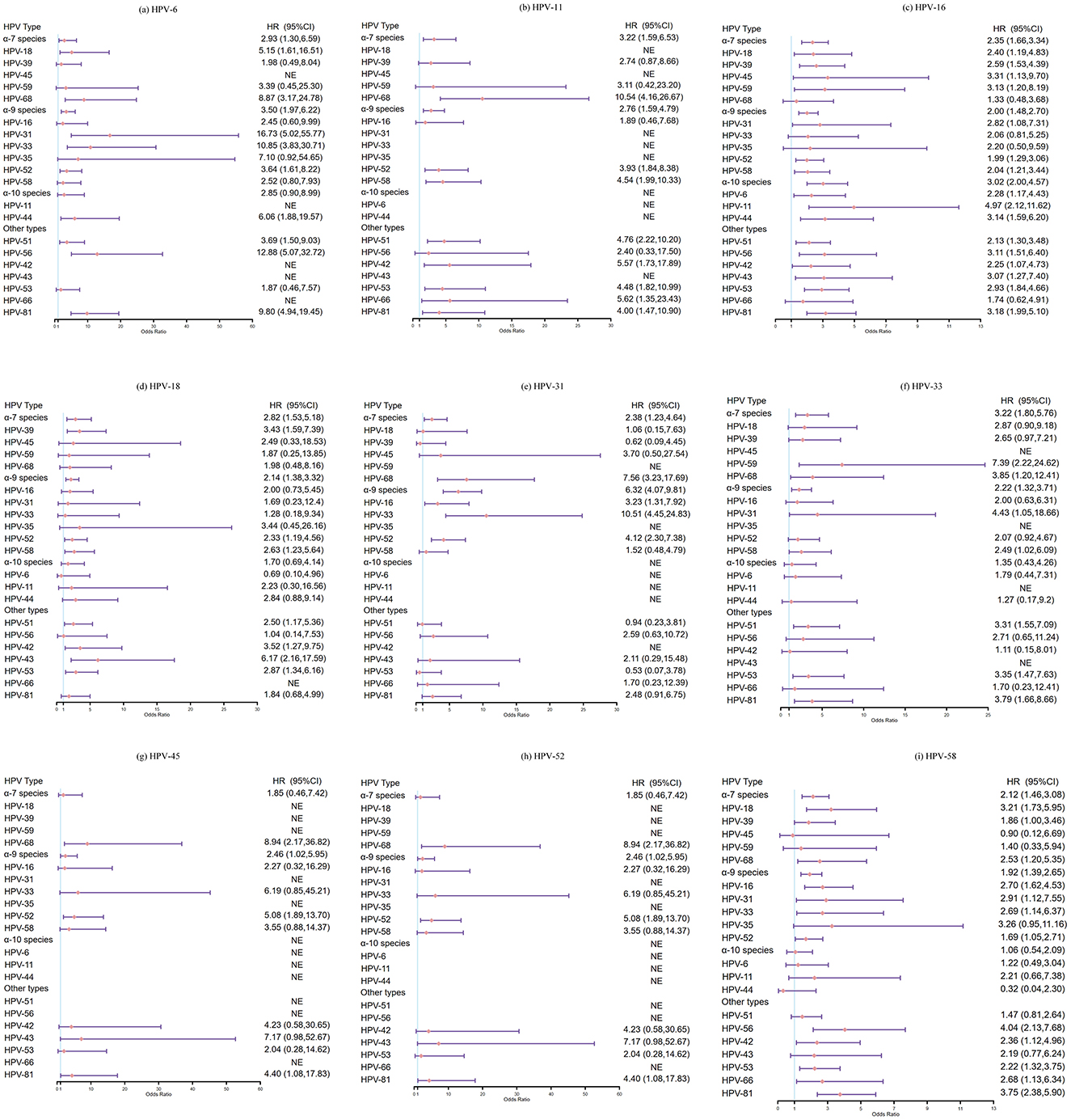
Figure 4. Hazard ratios and 95%CI for acquisition of non-vaccine-targeted HPV types in 2 years: women infected with HPV vaccine-targeted types (6, 11, 16, 18, 31, 33, 45, 52, and 58) vs. women with negative for HPV. (a) HPV-6 and other HPV types, (b) HPV-11 and other HPV types, (c) HPV-16 and other HPV types, (d) HPV-18 and other HPV types, (e) HPV-31 and other HPV types, (f) HPV-33 and other HPV types, (g) HPV-45 and other HPV types, (h) HPV-52 and other HPV types, and (i) HPV-58 and other HPV types.
To avoid underestimating the incidence of HPV infection, we further performed a sensitivity analysis by extending the follow-up period to 3 years. Although the differences were not statistically significant, we also found that prior infection with HPV-18 had reduced the risk of acquiring HPV-6 (HR: 0.70, 95%CI: 0.10–5.04), and similar trends were observed between HPV-31 and HPV-39 (HR: 0.59, 95%CI: 0.08–4.21), HPV-52 and HPV-6 (HR: 0.16, 95%CI: 0.02–1.17), HPV-58 and HPV-44 (HR: 0.58, 95%CI: 0.14–2.36), HPV-51 and HPV-44, −66 (HR: 0.62, 95%CI: 0.09–4.48; HR: 0.79, 95%CI: 0.11–5.71; respectively), HPV-68 and HPV-18(HR: 0.77, 95%CI: 0.11–5.51). In conclusion, no evidence was found that infection with HR-HPV types or HPV6/11 would reduce the risk of acquisition of other types (Supplementary Figures 3, 4).
With the wide application of HPV vaccine, a concern has been raised that it may lead to HPV type replacement. It can provide insights concerning natural HPV type competition and potential for type replacement to assess pre-vaccine epidemiological data. In this study, we studied the possibility of HPV type competition using regression and cohort approach. We observed a large number of positive associations, while a few negative associations in HPV co-infection patterns among women from Xiamen, China in cross-sectional study, and we did not observe any statistically significant HR < 1.0, conversely, several statistically significant HR > 1.0 were observed in cohort study.
In general, our results were consistent with prior studies, which suggested null or positive associations between HPV types (Plummer et al., 2007; Vaccarella et al., 2010; Rositch et al., 2012; Vaccarella et al., 2013; Su et al., 2024). Two studies based on women undergoing cervical cancer screening at New Mexico and Shengjing Hospital of China Medical University, respectively, using logistic regression, reported significant negative associations between HPV-72 and HPV-84 (Yang et al., 2014) and between HPV-16 and HPV-52 (Nie et al., 2016). Consistent with study conducted by Elizabeth Louise Dickson (Dickson et al., 2013), negative association was also observed between HPV-31 and HPV-83 in our study. Moreover, negative associations were observed between HPV-6 and HPV-72, HPV-18 and HPV-72, HPV-33 and HPV-26, HPV-45 and HPV-55, HPV-56 and HPV-26, as well as HPV-59 and HPV-69, suggesting the possibility of type competition between these paired types. Although HPV-55, −69, −72 and−83 are considered as low-risk types, research shows that their risk is increasing (Brown et al., 1999; Kantathavorn et al., 2015; Shea et al., 2020; Gao et al., 2024). According to recent studies, HPV-26 was considered as high-risk types that may play a significant role in the development of cancer (Handisurya et al., 2007; Chen et al., 2018a,b; Zhou et al., 2019; Zhong et al., 2022). With the increasing coverage of HPV vaccine, infection with HPV-26, −72, −83 and −55 need to be monitored.
Few cohort studies were performed to explore HPV type competition. Previous natural history studies have not found that prior infection with one or more HPV types can inhibit infection with other types or promote the clearance of other types, but these studies did not specifically analyze vaccine-targeted types. Tota et al. (2016) conducted a cohort study to compare the incidence and clearance of over 30 non-vaccine-targeted types between the women who were positive for HPV vaccine-targeted types and women who were negative for HPV. The results showed that women infected with HPV vaccine-targeted types had a higher risk of infection with other types (HR > 1.0). Similarly, we also found that women infected with vaccine-targeted types had a higher risk of acquiring other types compared to uninfected women (HR > 1.0). Although not statistically significant, lower HPV incidence point estimates of HPV-6/18/39/44/66 were observed in women with prior HPV-18/31/51/52/58/68 infection vs. in women with negative for HPV. However, our cohort population consisted of patients who had visited the hospital multiple times, women with negative for HPV were less likely to follow up. Moreover, the number of incident infections (especially for less common HPV types) was low. Considering the differences of follow-up interval and follow-up frequency in the study population, and the confounding factors, such as sexual behavior, the finding needs further exploration in large sample population with regular follow-up cohort.
The strength of our study is its assessment of HPV type competition using regression and cohort approach simultaneously in a large sample size of Chinese women, which provide insights concerning natural HPV type competition and HPV type replacement. However, there are some limitations: (1) We did not collect high-risk behavioral factors for confounding analysis, such as the number of sexual partners, age of first sexual activity, smoking, and other details; (2) The limited sample size in the cohort follow-up population, especially the small number of HPV-negative women, may reduce the statistical power; (3) Only 21 types were included in the cohort study due to the small number of 37 types data. However, the types (HPV-26, −55, −69, −72, −83) showing negative associations in the cross-sectional analysis were the additional types detected in the 37-type assay, whereas these types were not analyzed in the cohort study. And further accumulation of 37 type data is required to corroborate the type competition between several pairs of HPV types in cohort studies.
In summary, the large sample cross-sectional study found that there is possibility of type competition between HPV-6 and HPV-72, HPV-18 and HPV-72, HPV-31 and HPV-83, HPV-33 and HPV-26, HPV-45 and HPV-55, HPV-56 and HPV-26 and HPV-59 and HPV-69. Future prospective studies are needed to assess the long-term potential for HPV type competition.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving humans were approved by the Ethics Committee of Women and Child's Hospital, School of Medicine, Xiamen University (approval number KY-2024-025-K02). The studies were conducted in accordance with the local legislation and institutional requirements. The Ethics Committee/Institutional Review Board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because since this study is a retrospective analysis, written informed consent was waived, as per the ethics approval guidelines.
YC: Data curation, Investigation, Software, Writing – original draft. QL: Data curation, Resources, Writing – review & editing. LD: Visualization, Writing – review & editing. ZD: Investigation, Resources, Visualization, Writing – review & editing. YiZ: Data curation, Methodology, Writing – review & editing. YH: Investigation, Methodology, Visualization, Writing – review & editing. JZ: Investigation, Methodology, Software, Writing – review & editing. WW: Formal analysis, Software, Writing – review & editing. LZ: Formal analysis, Visualization, Writing – review & editing. JX: Formal analysis, Software, Writing – review & editing. CX: Formal analysis, Software, Validation, Writing – review & editing. YG: Conceptualization, Project administration, Resources, Supervision, Writing – review & editing. XY: Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – review & editing. YuZ: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Natural Science Foundation of Xiamen, China [3502Z202371049], Fujian provincial health technology project [2022QNB022], the Major health technology Projects of Xiamen [3502Z20234012], the Science and Technology Project of Xiamen [3502Z20224014], and Xiamen Municipal Health Commission [2024GZL-ZD11].
We would like to express our gratitude to all the subjects for participants in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1546166/full#supplementary-material
Ajenifuja, O. K., Ikeri, N. Z., Adeteye, O. V., and Banjo, A. A. (2018). Comparison between self sampling and provider collected samples for Human Papillomavirus (HPV) Deoxyribonucleic acid (DNA) testing in a Nigerian facility. Pan. Afr. Med. J. 30, 110. doi: 10.11604/pamj.2018.30.110.14321
Baloch, Z., Li, Y., Yuan, T., Feng, Y., Liu, Y., Tai, W., et al. (2016). Epidemiologic characterization of human papillomavirus (HPV) infection in various regions of Yunnan Province of China. BMC Infect. Dis. 16, 1–14. doi: 10.1186/s12879-016-1562-7
Brown, D. R., McClowry, T. L., Woods, K., and Fife, K. H. (1999). Nucleotide sequence and characterization of human papillomavirus type 83, a novel genital papillomavirus. Virology 260, 165–172. doi: 10.1006/viro.1999.9822
Bruni, L. A. G., Serrano, B., Mena, M., Collado, J. J., Gómez, D., Muñoz, J., et al. (2021). Human Papillomavirus and Related Diseases in the World. Summary Report 22 October 2021. Barcelona: ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre).
Bruni, L. A. G., Serrano, B., Mena, M., Collado, J. J., Gómez, D., Muñoz, J., et al. (2023). Human Papillomavirus and Related Diseases in China. Barcelona: ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre).
Castle, P. E. (2023). Prophylactic Human Papillomavirus Vaccination for Prevention of Oropharyngeal Cancer in Older Men: Is the Juice Worth the Squeeze?. Oxford: Oxford University Press.
Chen, W. Q., Li, H., Sun, K. X., Zheng, R. S., Zhang, S. W., Zeng, H. M., et al. (2018a). Report of Cancer Incidence and Mortality in China, 2014. Chin. J. Oncol. 40, 5–13. doi: 10.3760/cma.j.issn.0253-3766.2018.01.002
Chen, W. Q., Zheng, R. S., and Zhang, S. W. (2013). Report of Cancer Incidence and Mortality in China. China Cancer, 25, 1–7. doi: 10.3978/j.issn.1000-9604.2012.12.04
Chen, W. Q., Zheng, R. Z., and Zeng, H. M. (2015). Report of Cancer Incidence and Mortality in China, 2011. China Cancer, 24, 1–10. doi: 10.11735/j.issn.1004-0242.2015.01.A001
Chen, Z., Schiffman, M., Herrero, R., DeSalle, R., Anastos, K., Segondy, M., et al. (2018b). Classification and evolution of human papillomavirus genome variants: Alpha-5 (HPV26, 51, 69, 82), Alpha-6 (HPV30, 53, 56, 66), Alpha-11 (HPV34, 73), Alpha-13 (HPV54) and Alpha-3 (HPV61). Virology 516, 86–101. doi: 10.1016/j.virol.2018.01.002
Chiarini, A., Liu, D., Rassu, M., Armato, U., Eccher, C., Dal Prà, I., et al. (2019). Over expressed TKTL1, CIP-2A, and B-MYB proteins in uterine cervix epithelium scrapings as potential risk predictive biomarkers in HR-HPV-infected LSIL/ASCUS patients. Front. Oncol. 9:213. doi: 10.3389/fonc.2019.00213
Di, J., Rutherford, S., and Chu, C. (2015). Review of the cervical cancer burden and population-based cervical cancer screening in China. Asian Pac. J. Cancer Prevent. 16, 7401–7407. doi: 10.7314/APJCP.2015.16.17.7401
Dickson, E. L., Vogel, R. I., Bliss, R. L., and Downs, L. S. (2013). Multiple-type human papillomavirus (HPV) infections: a cross-sectional analysis of the prevalence of specific types in 309,000 women referred for HPV testing at the time of cervical cytology. Int. J. Gynecol. Cancer 23, 10–1097. doi: 10.1097/IGC.0b013e31829e9fb4
Dong, L., Nygård, M., and Hansen, B. T. (2021). Sociodemographic correlates of human papillomavirus vaccine uptake: opportunistic and catch-up vaccination in Norway. Cancers 13:3483. doi: 10.3390/cancers13143483
Gao, B., Liou, Y-. L., Yu, Y., Zou, L., Li, W., Huang, H., et al. (2021). The characteristics and risk factors of human papillomavirus infection: an outpatient population-based study in Changsha, Hunan. Sci. Rep. 11:15128. doi: 10.1038/s41598-021-94635-1
Gao, Y., Zhang, M. Q., Zheng, Y. Y., Huang, H., and Xie, H. L. (2024). Human papilloma virus infection and gene subtypes analysis in women undergoing physical examinations: a 2015–2020 study in Wenzhou, China. Cancer Control 31:10732748241257902. doi: 10.1177/10732748241257902
Handisurya, A., Rieger, A., Bankier, A., Koller, A., Salat, A., Stingl, G., et al. (2007). Human papillomavirus type 26 infection causing multiple invasive squamous cell carcinomas of the fingernails in an AIDS patient under highly active antiretroviral therapy. Br. J. Dermatol. 157, 788–794. doi: 10.1111/j.1365-2133.2007.08094.x
Hu, Y. M., Bi, Z. F., Zheng, Y., Zhang, L., Zheng, F. Z., Chu, K., et al. (2023). Immunogenicity and safety of an Escherichia coli-produced human papillomavirus (types 6/11/16/18/31/33/45/52/58) L1 virus-like-particle vaccine: a phase 2 double-blind, randomized, controlled trial. Sci. Bull. 68, 2448–2455. doi: 10.1016/j.scib.2023.09.020
Hybribio (2012). Product Profile. Available online at: http://hybribio.com/product/showproduct.php?id=20 (accessed June 02, 2025).
Kantathavorn, N., Mahidol, C., Sritana, N., Sricharunrat, T., Phoolcharoen, N., Auewarakul, C., et al. (2015). Genotypic distribution of human papillomavirus (HPV) and cervical cytology findings in 5906 Thai women undergoing cervical cancer screening programs. Infect Agent Cancer 10:7. doi: 10.1186/s13027-015-0001-5
Kreimer, A. R., Campbell, C. M. P., Lin, H., Fulp, W., Papenfuss, M. R., Abrahamsen, M., et al. (2013). Incidence and clearance of oral human papillomavirus infection in men: the HIM cohort study. Lancet 382, 877–887. doi: 10.1016/S0140-6736(13)60809-0
Li, X., Li, M., Yang, Y., Zhong, X., Feng, B., Xin, H., et al. (2016). Anal HPV/HIV co-infection among men who have sex with men: a cross-sectional survey from three cities in China. Sci. Rep. 6:21368. doi: 10.1038/srep21368
Liu, S. S., Leung, R. C., Chan, K. K., Cheung, A. N., and Ngan, H. Y. (2010). Evaluation of a newly developed GenoArray human papillomavirus (HPV) genotyping assay and comparison with the Roche Linear Array HPV genotyping assay. J. Clin. Microbiol. 48, 758–764. doi: 10.1128/JCM.00989-09
Luo, Q., Jiang, N., Wu, Q., Wang, J., and Zhong, J. (2020). Prevalence and genotype distribution of HPV and cervical pathological results in Sichuan Province, China: a three years surveys prior to mass HPV vaccination. Virol. J. 17, 1–10. doi: 10.1186/s12985-020-01366-2
Man, I., Auranen, K., Wallinga, J., and Bogaards, J. A. (2019). Capturing multiple-type interactions into practical predictors of type replacement following human papillomavirus vaccination. Philos. Transac. Royal Soc. B 374:20180298. doi: 10.1098/rstb.2018.0298
Nguyen, L. H., Le, T. B. T., and Le, N. Q. N. (2022). Acceptance and willingness to pay for vaccine against human papilloma virus (HPV) among parents of boys in central Vietnam. Front. Public Health 10:801984. doi: 10.3389/fpubh.2022.801984
Nie, J., Liu, J., Xie, H., Sun, Z., Zhao, J., Chen, Q., et al. (2016). Multiple human papillomavirus infections and type-competition in women from a clinic attendee population in China. J. Med. Virol. 88, 1989–1998. doi: 10.1002/jmv.24542
Petrosky, E., Bocchini Jr, J. A., Hariri, S., Chesson, H., Curtis, C. R., Saraiya, M., et al. (2015). Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb. Mortal. Wkly. Rep. 64, 300–304.
Plummer, M., Schiffman, M., Castle, P. E., Maucort-Boulch, D., and Wheeler, C. M. (2007). A 2-year prospective study of human papillomavirus persistence among women with a cytological diagnosis of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion. J. Infect. Dis. 195, 1582–1589. doi: 10.1086/516784
Rositch, A. F., Poole, C., Hudgens, M. G., Agot, K., Nyagaya, E., Moses, S., et al. (2012). Multiple human papillomavirus infections and type competition in men. J. Infect. Dis. 205, 72–81. doi: 10.1093/infdis/jir709
Shea, S., Muñoz, M., Ward, S. C., Beasley, M. B., Gitman, M. R., Nowak, M. D., et al. (2020). Human papillomavirus (HPV69/HPV73) coinfection associated with simultaneous squamous cell carcinoma of the anus and presumed lung metastasis. Viruses 12:349. doi: 10.3390/v12030349
Shen, Y., Huang, Y., Wang, W., Zhang, J., Chen, X., Zhang, L., et al. (2023). Prevalence and genotype distribution of HPV infection among women in Xiamen, China. Front. Microbiol. 14:1130226. doi: 10.3389/fmicb.2023.1130226
Su, Y. Y., Zheng, T. Q., Bi, Z. F., Jia, X. H., Li, Y. F., Kuang, X. F., et al. (2024). Pattern of multiple human papillomavirus infection and type competition: an analysis in healthy Chinese women aged 18–45 years. Human Vacc. Immunotherap. 20:2334474. doi: 10.1080/21645515.2024.2334474
Tota, J. E., Ramanakumar, A. V., Jiang, M., Dillner, J., Walter, S. D., Kaufman, J. S., et al. (2013). Epidemiologic approaches to evaluating the potential for human papillomavirus type replacement postvaccination. Am. J. Epidemiol. 178, 625–634. doi: 10.1093/aje/kwt018
Tota, J. E., Ramanakumar, A. V., Villa, L. L., Richardson, H., Burchell, A. N., Coutlée, F., et al. (2016). Cervical infection with vaccine-associated human papillomavirus (HPV) genotypes as a predictor of acquisition and clearance of other HPV infections. J. Infect. Dis. 214, 676–684. doi: 10.1093/infdis/jiw215
Tota, J. E., Struyf, F., Merikukka, M., Gonzalez, P., Kreimer, A. R., Bi, D., et al. (2017). Evaluation of type replacement following HPV16/18 vaccination: pooled analysis of two randomized trials. JNCI: J. Natl. Cancer Inst. djw300:109. doi: 10.1093/jnci/djw300
Vaccarella, S., Franceschi, S., Snijders, P. J., Herrero, R., Meijer, C. J., Plummer, M., et al. (2010). Concurrent infection with multiple human papillomavirus types: pooled analysis of the IARC HPV Prevalence Surveys. Cancer Epidemiol. Biomark. Prevent. 19, 503–510. doi: 10.1158/1055-9965.EPI-09-0983
Vaccarella, S., Söderlund-Strand, A., Franceschi, S., Plummer, M., and Dillner, J. (2013). Patterns of human papillomavirus types in multiple infections: an analysis in women and men of the high throughput human papillomavirus monitoring study. PLoS ONE 8:e71617. doi: 10.1371/journal.pone.0071617
Wang, X., Ji, Y., Li, J., Dong, H., Zhu, B., Zhou, Y., et al. (2018). Prevalence of human papillomavirus infection in women in the Autonomous Region of Inner Mongolia: a population-based study of a Chinese ethnic minority. J. Med. Virol. 90, 148–156. doi: 10.1002/jmv.24888
Weinberger, D. M., Malley, R., and Lipsitch, M. (2011). Serotype replacement in disease after pneumococcal vaccination. Lancet 378, 1962–1973. doi: 10.1016/S0140-6736(10)62225-8
World Health Organization (2024). Global HPV Programme Status. Available online at: https://app.powerbi.com/view?r=eyJrIjoiNDIxZTFkZGUtMDQ1Ny00MDZkLThiZDktYWFlYTdkOGU2NDcwIi_widCI6ImY2MTBjMGI3LWJkMjQtNGIzOS04MTBiLTNkYzI4MGFmYj_U5MCIsImMiOjh9 (accessed March 14, 2024).
Wüstenhagen, E., Boukhallouk, F., Negwer, I., Rajalingam, K., Stubenrauch, F., Florin, L., et al. (2018). The Myb-related protein MYPOP is a novel intrinsic host restriction factor of oncogenic human papillomaviruses. Oncogene 37, 6275–6284. doi: 10.1038/s41388-018-0398-6
Yang, H., Li, L-. J., Xie, L-. X., Luo, Z-. Y., Lu, M., Lin, M., et al. (2016). Clinical validation of a novel real-time human papillomavirus assay for simultaneous detection of 14 high-risk HPV type and genotyping HPV type 16 and 18 in China. Arch. Virol. 161, 449–454. doi: 10.1007/s00705-015-2673-y
Yang, Z., Cuzick, J., Hunt, W. C., and Wheeler, C. M. (2014). Concurrence of multiple human papillomavirus infections in a large US population-based cohort. Am. J. Epidemiol. 180, 1066–1075. doi: 10.1093/aje/kwu267
Yao, X., Li, Q., Chen, Y., Du, Z., Huang, Y., Zhou, Y., et al. (2024). Epidemiology of human papillomavirus infection in women from Xiamen, China, 2013 to 2023. Front. Public Health 12:1332696. doi: 10.3389/fpubh.2024.1332696
Zhang, Y. Q., Zheng, T. L., and Zhang, W. D. (2018). Report of cancer incidence and mortality in China, 2012. Adv. Modern Oncol. Res. 4, 1–7. doi: 10.30564/AMOR.V4I3.176
Zheng, R., Zhang, S., Sun, K., Chen, R., Wang, S., Li, L., et al. (2023). Cancer statistics in China, 2016. Chin. J. Oncol. 45, 212–220. doi: 10.3322/caac.21338
Zheng, R. S., Sun, K. X., Zhang, S. W., Zeng, H. M., Zou, X. N., Chen, R., et al. (2019). Report of cancer epidemiology in China, 2015. Chin. J. Oncol. 41, 19–28.
Zhong, F. F., Yu, T., Ma, X. X., Wang, S. N., Cong, Q., Tao, X., et al. (2022). Extensive HPV genotyping reveals high association between multiple infections and cervical lesions in chinese women. Dis. Mark. (2022) 10:2022:8130373. doi: 10.1155/2022/8130373
Keywords: human papillomavirus (HPV), infection, type competition, cervical cancer, HPV vaccine
Citation: Chen Y, Li Q, Du L, Du Z, Zhou Y, Huang Y, Zhang J, Wang W, Zhang L, Xie J, Xu C, Ge Y, Yao X and Zhou Y (2025) Epidemiologic evaluation of human papillomavirus type competition in unvaccinated women from Xiamen, China. Front. Microbiol. 16:1546166. doi: 10.3389/fmicb.2025.1546166
Received: 16 December 2024; Accepted: 18 March 2025;
Published: 04 April 2025.
Edited by:
Alina Maria Holban, University of Bucharest, RomaniaReviewed by:
Basem Bas Fares, Independent Researcher, Haifa, IsraelCopyright © 2025 Chen, Li, Du, Du, Zhou, Huang, Zhang, Wang, Zhang, Xie, Xu, Ge, Yao and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunsheng Ge, Z3NoZWVAMTYzLmNvbQ==; Xingmei Yao, eV94aW5nbWVpMDMwN0AxNjMuY29t; Yulin Zhou, emhvdV95dWxpbkAxMjYuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.