
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Microbiol. , 14 March 2025
Sec. Infectious Agents and Disease
Volume 16 - 2025 | https://doi.org/10.3389/fmicb.2025.1540609
This article is part of the Research Topic Women in Infectious Agents and Disease: 2024 View all 10 articles
Background: Human papillomavirus (HPV) self-sampling may be an accurate and effective alternative sampling method to conventional cervical cancer screening methods. This systematic review compares the accuracy and acceptance of self-sampling to clinician sampling for HPV testing in Asia.
Methods: The PubMed, Cochrane Library, Cumulative Index to Nursing and Allied Health, and Web of Science databases were searched for publications published from the establishment of the database to 2023. The risk of bias was assessed using the QUADAS-2 tool for studies included in this review. All studies evaluating the accuracy and acceptance of HPV self-sampling, and agreement of self- and clinician-collected samples in Asia were included. The accuracy of each study was demonstrated through the sensitivity and specificity in diagnosing cervical intraepithelial neoplasia or cancer, as well as the detection rate of HPV. The agreement between the two sampling methods was assessed based on the detection outcomes of HPV. Acceptance was indicated by women’s preferences for HPV self-sampling.
Results: Sixty-seven studies including 117,279 adult, female participants were included in this review. The type of HPV screening, other intervention components, study design, sample size, follow-up period, analysis method, numerical outcomes, results, and limitations were extracted from each study. The sensitivity and specificity of HPV self-sampling in detecting cervical intraepithelial neoplasia were higher than 80% and 70%, consistent with the results of HPV clinician sampling. The consistency between self-sampling and clinician-sampling was high in most studies, and the kappa value was more than 0.7. Women had high acceptance of self-sampling but expressed some concerns.
Conclusion: Self-sampling for HPV testing can significantly improve cervical cancer screening coverage, especially in areas with limited medical resources or reluctance to accept physician sampling. In most studies, the accuracy and acceptance of HPV self-sampling was comparable to clinician sampling. However, the diagnostic criteria and HPV detection methods still need to be adjusted due to the low sensitivity of HPV self-sampling in some studies in China and India. Targeted health education should be carried out to improve the acceptance of HPV self-sampling in women.
Systematic review registration: https://inplasy.com/?s=INPLASY202520107, INPLASY202520107.
Cervical cancer is the fourth most common cancer in women, leading to approximately 661,021 cases and 348,189 deaths in 2022 (Bray et al., 2024). Most cervical cancers develop due to persistent high-risk human papillomavirus (HR-HPV) infections (Schiffman et al., 2011). Although vaccines that protect against infections and diseases associated with specific types of HPV exist, many women in low- and middle-income countries do not have access to HPV immunization and die of this preventable cancer (Gallagher et al., 2018). Secondary prevention measures include the early detection and treatment of precancerous lesions (Arbyn et al., 2012). Population-based cervical cancer screening via Papanicolaou testing every three to 4 years has successfully reduced the incidence and mortality of cervical cancer (Bouvard et al., 2021). In organized screening programs, most new cases of cervical cancer are detected in women who have never been screened or are under-screened (Spence et al., 2007). Cervical cancer screening programs, including cervical cytology (Pap smear), visual inspection with acetic acid (VIA), and HPV testing, must be applied to reduce the occurrence of cervical cancer.
Currently, national screening programs for cervical cancer are widely provided in Asian countries including China, India, Japan, and Thailand (Aoki et al., 2020). However, the uptake rates of these programs remain low, indicating that personal barriers hamper the participation of female patients (Chorley et al., 2017; Cremer et al., 2021). It has been hypothesized that offering HR-HPV self-sampling may increase the participation rate compared to clinician sampling (Arbyn et al., 2018; Harding-Esch et al., 2017; Racey et al., 2013; Snijders et al., 2013; Verdoodt et al., 2015). HPV self-sampling may be a more acceptable option for patients in Asia who have never been screened or who are under-screened for cervical cancer. While there have been several systematic reviews on HPV self-sampling globally, there is a notable gap in the literature regarding studies focused specifically on Asian populations. Existing reviews have primarily addressed global or African cohorts, and their findings may not be fully applicable to Asian patients due to differences in cultural, economic, and healthcare factors (Sy et al., 2022). Notably, we have found only one study that has systematically reviewed HPV self-sampling outcomes within India (Hariprasad et al., 2023), but this study did not provide a comprehensive analysis of HPV self-sampling across diverse Asian countries. To our knowledge, no systematic review has reported the sensitivity, specificity, and acceptance of HPV self-sampling in Asia. This systematic review examined the accuracy, agreement, and acceptability of self-sampling for HPV DNA testing in Asian countries.
This systematic review was registered with INPLASY (INPLASY202520107, doi: 10.37766/inplasy2025.2.0107), and was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (the PRISMA checklist is supplied in Supplementary Table 1) (Page et al., 2021). No funding agency played any role in the study design, data collection, data analysis, data interpretation, or report writing. The review protocol was not registered prospectively.
Articles were included in the review if they included participants who underwent cervicovaginal self-sampling for HPV DNA testing; measured the accuracy, concordance, and acceptability of cervicovaginal self-sampling and clinician sampling for HPV; focused on Asian patients; were conducted in Asian countries and were in English. The included studies were randomized controlled trials, prospective cohort studies, cross-sectional studies, comparative studies, and other non-randomized controlled trials. Studies that did not use vaginal or cervical specimens for examination were excluded from the review. Studies that focused on non-Asian populations, or did not report relevant outcomes related to the accuracy of self-sampling, concordance with clinician-collected samples, or women’s acceptance of self-sampling, were excluded.
The PubMed, Cochrane Library, Cumulative Index to Nursing and Allied Health Library (CINHAL), and Web of Science databases were searched for studies reported from the establishment of the database to 31 October 2022. A final update of the search was completed before the final extraction and synthesis of the results on 23 February 2023. The reference lists of the included articles were also screened to identify publications that met the eligibility criteria. Database-specific Boolean operators (AND, OR, NOT) and truncation symbols (* and “ “) were used.
The following search terms were used to identify eligible studies:
1. Cervical dysplasia OR cervical intraepithelial neoplasia OR cervix neoplasms OR papillomavirus OR papillomavirus, human OR human papillomavirus OR papillomavirus, infections
AND
2. Self-collected OR self-test OR self-obtained OR self-sampling
AND
3. Asia OR Asian OR Afghanistan OR Armenia OR Azerbaijan OR Bahrain OR Bangladesh OR Bhutan OR Brunei OR Cambodia OR China OR Cyprus OR Georgia OR India OR Indonesia OR Iran OR Iraq OR Israel OR Japan OR Jordan OR Kazakhstan OR Korea, North OR Korea, South OR Kuwait OR Kyrgyzstan OR Laos OR Lebanon OR Malaysia OR Maldives OR Mongolia OR Myanmar OR Nepal OR Oman OR Pakistan OR Palestine OR Philippines OR Qatar OR Saudi Arabia OR Singapore OR Sri Lanka OR Syria OR Tajikistan OR Thailand OR Timor-Leste OR Turkmenistan OR Turkey OR United Arab Emirates OR Uzbekistan OR Vietnam OR Yemen.
The more detailed search strategies of each database were shown in Supplementary Table 2.
Descriptive data were extracted independently by two authors, and a third reviewer was consulted to resolve any differences in data collection. The citation, objectives, location, population characteristics, description of the type of HPV screening, description of any additional intervention components, study design, sample size, numerical outcomes, results, and limitations were extracted from each included study.
After finalizing the data extraction, two authors reviewed the data and the full texts to accurately classify HPV self-sampling.
The reported data regarding screening accuracy, participation, attendance, response, and compliance were combined to determine the cervical cancer screening outcomes. Conventional cytology (Pap smears), VIA, or colposcopy data were also gathered. When more than one control group was reported, the intervention group was compared to the least intensive sampling strategy group.
Two independent reviewers evaluated the risk of bias for all included studies by using the Quality Assessment Tool for Diagnostic Accuracy Studies-2 (QUADAS-2).
Heterogeneity was assessed using Cochran’s Q test and the I2 statistic. Begg’s rank correlation test was performed to further assess publication bias. A funnel plot was used to visualize publication bias.
Human papillomavirus self-sampling was defined as the process in which women insert a self-sampler into their vagina to collect isolated cells. In contrast, HPV clinician sampling involved clinicians or healthcare workers inserting a vaginal speculum into the woman’s vagina to obtain a cervical smear using a sampler.
The diagnostic test sensitivity and specificity were based on colposcopy-confirmed cases of high-grade squamous intraepithelial lesion (HSIL), previously called cervical intraepithelial neoplasia 2+ (CIN2+) or CIN3+, and detection of cervical cancer and HPV infection. The sensitivity was defined as the number of identified cases of HSIL and cervical cancer (positive for both HPV and colposcopy) divided by the total number of colposcopy-confirmed cases. Specificity was defined as the number of cases without HSIL or cervical cancer (negative on both HPV and colposcopy) divided by the total number of colposcopy-negative cases. The HPV detection rate was defined as the HPV-positive cases divided by the total number of women enrolled. Agreement was defined as the concordance between self-sampled HPV tests and clinician-sampled HPV tests (the percentage of agreement with positive test results and the percentage of agreement with negative test results). Acceptability was defined as the percentage of women willing to participate in the HPV test and their preference between HPV self-sampling and clinician sampling.
A total of 573 articles were retrieved, comprising 124 studies from PubMed, 215 from Web of Science, 26 from the Cochrane Library, and 208 from CINAHL, including 135 duplicate titles. Therefore, 438 articles were screened against the eligibility criteria. Following the exclusion of 241 articles based on their titles and abstracts, the full texts of 195 articles were read, and 67 studies were ultimately included in the systematic review (Figure 1).
Most of the included studies were cross-sectional studies (n = 62). The remaining studies were randomized controlled trials (n = 1), prospective cohort studies (n = 1), prospective population-based studies (n = 1), and prospective randomized crossover studies (n = 2) (Supplementary Table 3).
The patient populations of the included studies were women in China (28 studies) (Belinson et al., 2001; Belinson et al., 2003; Belinson et al., 2010; Belinson et al., 2012; Chang et al., 2002; Chen S. et al., 2014; Chen W. et al., 2014; Chen K. et al., 2016; Chen Q. et al., 2016; Chou et al., 2016; Du et al., 2021; Goldstein et al., 2020; Guan et al., 2012; Guan et al., 2013; He and He, 2020; Li et al., 2022; Ngu et al., 2022; Qiao et al., 2008; Qin et al., 2016; Tisci et al., 2003; Twu et al., 2011; Wang et al., 2014; Wang et al., 2017; Wong et al., 2016; Wong et al., 2018; Wong et al., 2020; Zhang et al., 2020; Zhao et al., 2013), Thailand (nine studies) (Gottschlich et al., 2019; Kittisiam et al., 2016; Nilyanimit, 2014; Nutthachote et al., 2019; Oranratanaphan et al., 2014; Phoolcharoen et al., 2018a; Phoolcharoen et al., 2018b; Ploysawang et al., 2023; Trope et al., 2013), Japan (seven studies) (Aiko et al., 2017; Hanley et al., 2016; Onuma et al., 2020; Ozawa et al., 2023; Satake et al., 2020; Terada et al., 2022; Yoshida et al., 2011), Malaysia (seven studies) (Abdullah et al., 2018; Ahmad et al., 2021; Khoo et al., 2021; Latiff et al., 2015a; Latiff et al., 2015b; Ma’som et al., 2016; Tan et al., 2021), India (six studies) (Anand et al., 2022; Asthana and Labani, 2015; Bhatla et al., 2009; Kuriakose et al., 2020; Madhivanan et al., 2021; Sowjanya et al., 2009), Korea (three studies) (Cho et al., 2019; Seo et al., 2006; Shin et al., 2019), Nepal (two studies) (Johnson et al., 2014; Shrestha et al., 2021), Singapore (Lim et al., 2022), Mongolia (Tsedenbal et al., 2022), Cambodia (Thay et al., 2019), Vietnam (Hanh, 2006), and Brunei (Chaw et al., 2022).
A total of 19 studies evaluated the sensitivity and specificity of clinician-collected and self-collected HPV testing for diagnosing CIN. A total of 35 studies reported the detection rates of HPV using both self-sampling and clinician sampling methods. A total of 29 studies examined concordance between clinician-collected and self-collected HPV testing. A total of 33 studies assessed women’s acceptance and preference rates for HPV self-sampling.
A total of 13 HPV detection methods are discussed in this review, including seven WHO-approved testing methods: HC2 (Qiagen, Germantown, MD, United States), careHPV (Qiagen, Gaithersburg, MD, United States), AmpFire (Atila BioSystems, United States), SeqHPV (BGI Shenzhen, Shenzhen, China), Cervista (Hologic, Marlborough, MA, United States), matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF, BGI Shenzhen, Shenzhen, China), and Cobas HPV test (Roche Molecular Systems, Inc., United States). Additionally, six other methods are introduced, including HPVDNA Chip™ (Biomedlab Co., Seoul, South Korea), PGMY PCR (Roche Molecular Systems, Inc., United States), Easy-Chip HPV Blot (King Car Yuanshan Research Institute, Taiwan, China), RealTime High Risk HPV assay (Abbott Molecular Inc., Abbott Park, IL), Anyplex II HPV kit assay (Seegene, Seoul, South Korea), and Linear Array HPV Genotyping test (Roche Diagnostics, United Kingdom). HC2, Cobas HPV test, and Cervista have received FDA/CE-IVD approval. HC2 test detects the presence of 13 HR-HPV types using full genome probes complementary to HPV DNA, specific antibodies, signal amplification, and chemiluminescent detection (Belinson et al., 2001). HPVDNA Chip uses HPV and β-globin primers to amplify the target HPV DNA through PCR under specific conditions, and the amplification products are labeled with Cy5-dUTP, which could contain 22 type-specific probes (15 for the high-risk group and seven the low-risk group) (Seo et al., 2006). PGMY PCR uses the PGMY09/11 L1 consensus primer system for PCR amplification and a reverse line blot detection strip that individually identifies 22 high-risk types (Bhatla et al., 2009). The careHPV assay, adapted from the HC2 assay, is a qualitative test for HR-HPV detection, targeting 14 HR-HPV types through hybridization of HR-HPV DNA with a cocktail of RNA probes and chemiluminescence signal amplification (Qiao et al., 2008). Easy-Chip HPV Blot contains 39 type-specific probes that are immobilized on a 14.4 mm × 9.6 mm nylon membrane, which is used for reverse-blot hybridization and detects HPV DNA in a single assay (Twu et al., 2011). Cervista is a signal-amplification method for the qualitative detection of 14 HR-HPV types (Belinson et al., 2012). MALDI-TOF is a mass spectrometry method that uses a multiplex primary PCR also for the same 14 HR-HPV types detected by Cervista (Belinson et al., 2012). Cobas HPV test is a real-time PCR assay that detects 14 HPV types, with HPV16 and HPV18 detected individually and the other 12 HPV types detected as a pooled group (Chen Q. et al., 2016b Terada et al., 2022). The AmpFire method is a nucleic acid amplification technique for qualitative detection of HR-HPV, using HR-HPV-specific primers and fluorescent probes to amplify the viral genomic DNA (including the E6/E7 region) under isothermal conditions. This method does not require DNA extraction or purification and can directly detect HPV from lysed clinical samples in one step (Zhang et al., 2020). The SeqHPV assay is a high-throughput HPV genotyping method based on multiplex PCR and next-generation sequencing, capable of detecting 14 HR-HPV types (Du et al., 2021). The Abbott m2000rt automatic biochemical analyzer was used for real-time fluorescence quantitative PCR detection. The detection boundary value of cycle threshold (CT) was 32.0, and the internal quality control target boundary value of CT was 35.0. Abbott HR-HPV assay could detect 14 HR HPV types (Abdullah et al., 2018; Aiko et al., 2017; Chen W. et al., 2014; Chen K. et al., 2016; Chou et al., 2016; Hariprasad et al., 2023; Li et al., 2022; Nutthachote et al., 2019; Page et al., 2021; Phoolcharoen et al., 2018a; Satake et al., 2020; Tan et al., 2021; Wong et al., 2020; Yoshida et al., 2011) simultaneously, and specifically identifies HPV16 and HPV18 (Qin et al., 2016). Anyplex^TM II HPV 28 real-time PCR test simultaneously detects 19 HR-HPV and 9 low-risk HPV types, using dual priming oligonucleotides and a melting curve analysis method of tagging oligonucleotide cleavage and extension (Cho et al., 2019). Linear Array HPV Genotyping test (Roche Diagnostics, United Kingdom) combines consensus PCR and reverse-hybridization amplification products to detect 36 genital HPV genotypes. Because it has been clearly defined and validated in research and clinical applications, it is often considered the reference method for genital HPV genotyping (Yoshida et al., 2011).
All the studies included in this systematic review were assessed for risk of bias (Figure 2). The Cohen’s kappa value between two independent reviewers was 0.839. Most of the studies included in the analysis were cross-sectional and did not employ random patient selection or allocation. As a result, the risk of bias in several domains was found to be high or unclear. Specifically, 21 studies were assessed as having a high risk of bias in the “Patient Selection” domain (Aiko et al., 2017; Chen Q. et al., 2016; Goldstein et al., 2020; Gottschlich et al., 2019; Hanh, 2006; Hanley et al., 2016; Johnson et al., 2014; Kuriakose et al., 2020; Madhivanan et al., 2021; Nilyanimit, 2014; Oranratanaphan et al., 2014; Ozawa et al., 2023; Phoolcharoen et al., 2018a; Qin et al., 2016; Seo et al., 2006; Shrestha et al., 2021; Thay et al., 2019; Twu et al., 2011; Wang et al., 2014; Wong et al., 2018; Yoshida et al., 2011). This high risk was attributed to the non-random selection of participants, which could introduce selection bias and limit the generalizability of the findings. One study was considered to have a high risk of bias in the “Index Testing” domain (Kittisiam et al., 2016), due to the use of a non-standardized or poorly validated diagnostic test, which could affect the accuracy of the results. In the “Reference Standard” domain, only one study was deemed to have an unclear risk of bias (Shrestha et al., 2021), due to a lack of detailed information regarding the reference standard used. In the “Flow and Timing” domain, four studies were assessed as having an unclear risk of bias (Hanh, 2006; He and He, 2020; Ngu et al., 2022; Shrestha et al., 2021), which was due to incomplete reporting of participant flow or unclear timing of tests, potentially leading to attrition or measurement bias. Notably, all studies were judged to have a low risk of diagnostic bias, as the diagnostic criteria were predefined prior to the availability of results, ensuring the objectivity of the assessment.
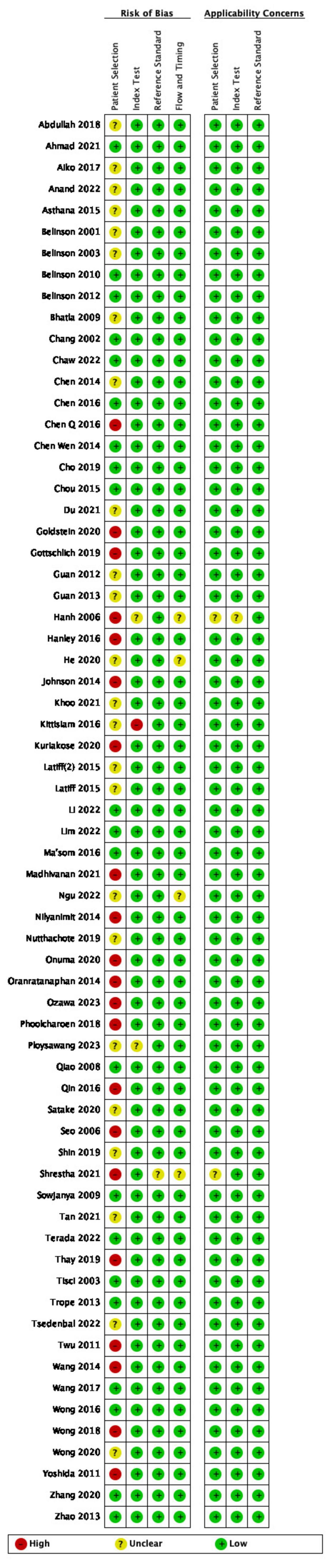
Figure 2. Quality assessment of included studies. Green, low risk of bias; red: high risk of bias; yellow, not reported/unclear risk of bias.
The Cochran’s Q statistic was highly significant (Q = 1.8 × 106, P = 0.000), indicating substantial heterogeneity among the studies. Additionally, the I2 statistic was calculated to be 100%, suggesting that nearly all of the variability in effect sizes across studies could be attributed to differences between studies rather than random error. Begg’s test yielded a significant p-value (P < 0.05), further suggesting the presence of potential publication bias. This finding implies that smaller studies or studies with non-significant results may be underrepresented or unpublished, which could have influenced the overall effect size observed in the meta-analysis. The funnel plot (Figure 3) exhibited signs of asymmetry, suggesting the presence of publication bias. Specifically, there appears to be an over-representation of studies with larger effect sizes, while smaller studies with negative or null results may be underrepresented.
Two studies found that the accuracy of HPV self-sampling is comparable to that of physician sampling (Aiko et al., 2017; Belinson et al., 2012). In the study of Belinson et al. (2012) when using the MALDI-TOF mass spectrometry system for HPV detection, the sensitivity of self-sampling for identifying CIN 3+ was equivalent to that of clinician sampling. However, when utilizing Cervista, the sensitivity for detecting CIN 3+ in self-collected specimens was only 70.9%, compared to 95.0% for clinician-collected samples (Belinson et al., 2012). In the study of Onuma et al. (2020) the sensitivity of HPV self-sampling and clinician sampling for the detection of CIN 2+ were both 100% (Aiko et al., 2017). In studies of Du et al. (2021), Zhang et al. (2020), the sensitivity for detecting CIN 2+ was higher in self-sampling than in clinician sampling. While in the other three studies of Belinson et al. (2001) the sensitivity for detecting CIN 3+ was 81% and 98% in self- and clinician-collected samples. In the remaining 12 studies, the sensitivity of HPV self-sampling was slightly lower than physician sampling, with values ranging from 59.4% to 87.5%, while the specificity of HPV self-sampling was identical to clinician sampling (Table 1).
In the detection of HPV, 17 studies reported the detection rates were higher in clinician sampling (Anand et al., 2022; Asthana and Labani, 2015; Belinson et al., 2001; Chang et al., 2002; Chen K. et al., 2016; Kuriakose et al., 2020; Latiff et al., 2015a; Madhivanan et al., 2021; Ma’som et al., 2016; Satake et al., 2020; Seo et al., 2006; Singh et al., 2023; Terada et al., 2022; Thay et al., 2019; Twu et al., 2011; Wang et al., 2014; Wang et al., 2017), while in 15 studies this rate was higher in self-collected samples (Belinson et al., 2003; Cho et al., 2019; Du et al., 2021; Latiff et al., 2015a; Nutthachote et al., 2019; Qin et al., 2016; Satake et al., 2020; Seo et al., 2006; Terada et al., 2022; Thay et al., 2019; Wong et al., 2016; Wong et al., 2018; Yoshida et al., 2011; Zhang et al., 2020; Zhao et al., 2013). Two studies evaluated that detection rates of both sampling methods were the same (Anand et al., 2022; Nilyanimit, 2014). In 13 studies, the difference in detection rates was not more than 1% (Aiko et al., 2017; Asthana and Labani, 2015; Belinson et al., 2001; Bhatla et al., 2009; Chang et al., 2002; Chen W. et al., 2014; Hanh, 2006; Kuriakose et al., 2020; Lim et al., 2022; Satake et al., 2020; Wang et al., 2017; Zhang et al., 2020; Zhao et al., 2013; Table 2). In the studies by Wong and Yoshida et al., multiple types of HPV infections were found to occur more frequently with self-sampling compared to clinician sampling (Wong et al., 2016; Yoshida et al., 2011).
A total of 29 studies reported an agreement between HPV self-sampling and clinician sampling. A total of 24 reported a high or nearly perfect agreement between self-sampling and clinician sampling for the detection of HPV DNA. Specifically, 18 studies demonstrated an agreement exceeding 90% (Anand et al., 2022; Bhatla et al., 2009; Chen K. et al., 2016; Chen Q. et al., 2016; Du et al., 2021; Johnson et al., 2014; Kuriakose et al., 2020; Latiff et al., 2015a; Madhivanan et al., 2021; Ngu et al., 2022; Nilyanimit, 2014; Nutthachote et al., 2019; Satake et al., 2020; Seo et al., 2006; Sowjanya et al., 2009; Tsedenbal et al., 2022; Wang et al., 2014; Wong et al., 2016). Three studies assessed the agreement in both collecting methods samples using two assays for the detection of HPV (Chen W. et al., 2014; Du et al., 2021; Sowjanya et al., 2009), and one study evaluated the concordance of both sampling methods in three HPV testing assays (Cho et al., 2019). Some new HPV assays such as SeqHPV and careHPV showed higher agreement in self- and clinician-collected samples. In studies of Chen W. et al. (2014), Du et al. (2021) when the same sample was tested using different detection methods, the consistency of clinician-sampled samples was higher than that of self-sampled samples.
However, three studies have reported poor agreement between self- and clinician sampling results for the detection of HPV. Twu et al. (2011) reported low agreement between vaginal and cervical specimens using the EasyChip HPV Blot (k = 0.37) (Table 3).
A total of 29 studies have assessed women’s overall acceptance of HPV self-sampling. The lowest reported acceptance was 40.3% (95% CI: 38.49%–42.11%) (Trope et al., 2013), while the highest reached 100% (Anand et al., 2022; Ploysawang et al., 2023). In 27 of the 29 studies, acceptance exceeded 60% (Abdullah et al., 2018; Ahmad et al., 2021; Aiko et al., 2017; Anand et al., 2022; Chen S. et al., 2014; Cho et al., 2019; Chou et al., 2016; Goldstein et al., 2020; Gottschlich et al., 2019; Guan et al., 2012; Hanley et al., 2016; Khoo et al., 2021; Kittisiam et al., 2016; Li et al., 2022; Ma’som et al., 2016; Ngu et al., 2022; Oranratanaphan et al., 2014; Phoolcharoen et al., 2018b; Ploysawang et al., 2023; Shrestha et al., 2021; Singh et al., 2023; Sowjanya et al., 2009; Thay et al., 2019; Tisci et al., 2003; Trope et al., 2013; Wong et al., 2016; Wong et al., 2020). A total of 13 studies indicated that women preferred self-sampling over clinician sampling (Goldstein et al., 2020; Gottschlich et al., 2019; Hanh, 2006; Khoo et al., 2021; Li et al., 2022; Lim et al., 2022; Madhivanan et al., 2021; Ploysawang et al., 2023; Shin et al., 2019; Shrestha et al., 2021; Trope et al., 2013; Wong et al., 2016; Wong et al., 2018), however, three studies found a preference for clinician sampling instead (Aiko et al., 2017; Ngu et al., 2022; Tsedenbal et al., 2022; Table 4).
When asked about their preferred location for self-sampling, four studies found that participants preferred to perform the test at the clinic rather than at home (Belinson et al., 2001; Chen K. et al., 2016; Kittisiam et al., 2016; Zhang et al., 2020). In contrast, three studies reported a preference for sampling at home (Onuma et al., 2020; Seo et al., 2006; Tan et al., 2021).
Cervical cancer remains the leading cause of cancer death in Asia, especially South-Eastern Asia. China and India account for more than 50% of new cases of cervical cancer globally (Singh et al., 2023). Given that most cervical cancers are caused by persistent infection with high-risk HPV types, increasing participation in HPV-based cervical cancer screening is essential to reduce cervical cancer incidence. As a major screening method for cervical cancer, HPV self-sampling was recommended by WHO and other organizations (Simelela, 2021). However, the participation rate of cervical cancer screening in Asian women is still far below 70% and varies widely among different regions (Ong et al., 2023).
This systematic review analyzed the accuracy, agreement, and acceptability of HPV self-sampling in Asia. Though remains slightly lower than that of clinician sampling, the sensitivity and specificity of HPV self-sampling to detect CIN2+ is high, ranging from 60% to 100%. However, in some studies, the sensitivity of self-sampling was identical to or higher than that of clinician sampling for DNA testing, especially when researchers used new collection devices, such as the “JustForMe” brush and Dacron polyester swab. There was excellent agreement between the two sampling methods in the majority of studies, which was the same to the results of two systematic reviews in Africa and low-income countries (Kamath Mulki and Withers, 2021; Nodjikouambaye et al., 2020). These observations suggest that the quantity and quality of cervicovaginal exfoliated cells obtained by patients themselves are comparable to those obtained by physicians. In a study conducted in India, the sensitivity of self-sampling was found to be only 40.6%, while the specificity was 97.3%. Besides, the concordance between the two sampling methods, were notably low in some studies. This phenomenon may be attributed to various factors, including whether women correctly understood the process of self-sampling, differences in sampling techniques, sample quality and collection methods, HPV testing methods and diagnostic thresholds. While methods such as SeqHPV, careHPV, and RealTime HR-S have demonstrated high detection rates in some studies, the EasyChip HPV Blot has shown lower detection rates in self-collected samples. We recognize that non-standardized methods may not provide the same level of reliability and performance as WHO-approved tests. Specifically, these methods can present challenges related to sensitivity, specificity, reproducibility, and ease of use. In the absence of extensive validation and standardization, such methods may exhibit significant variability in results, which can undermine diagnostic accuracy. Therefore, standardized testing methods are essential to ensure that cervical cancer screening remains both accurate and reliable across different healthcare settings. It is expected to improve the accuracy of self-sampling by enhancing sampling instruments and testing methods, as well as increasing women’s understanding of the self-sampling process.
The study participants reported broad acceptance of self-sampling, and preferred self-sampling over clinician sampling, particularly among women with higher education and greater knowledge of HPV. Asia, comprising 44 countries, is characterized by its diverse cultures, religious beliefs, economic conditions, and medical practices. Factors such as a lack of understanding of HPV, cultural barriers, and limited economic and medical resources may hinder women’s participation in screening programs. Many women expressed a lack of confidence in self-sampling at home due to concerns about the reliability of self-collected samples without a doctor’s guidance and misunderstandings regarding the results. They also emphasized the need for timely follow-up and explanations of HPV test results. By promoting awareness of HPV and cervical cancer and educating patients about the importance of cervical cancer screening, we can improve the acceptance of HPV self-sampling among patients.
Human papillomavirus self-sampling can effectively increase cervical cancer screening participation rates among women, especially among women who have never been screened or are under-screened due to feeling embarrassed. The main reasons for low acceptability were that the participants were unaware of the relationship between HPV and cervical cancer, worried that self-sampling was not reliable, did not have access to consult a doctor, or did not understand the procedure of self-sampling. Women are more willing to perform self-HPV sampling at clinics or community health centers, highlighting a significant need for professional health workers to explain the self-sampling process, interpret test results, and provide follow-up support. Nevertheless, only ten countries in Asia have reported results from studies on HPV self-sampling, indicating that the coverage of this practice remains low. In addition, HPV vaccination rates are closely associated with cervical cancer screening uptake. HPV-unvaccinated women are generally less engaged in screening compared to those who have been vaccinated (Taniguchi et al., 2019). Moreover, inadequate healthcare infrastructure remains a significant barrier to the effective implementation of cervical cancer screening, particularly in many parts of Asia (Rajkhowa et al., 2024). Increased financial support, improved HPV vaccination rates, and healthcare professionals and infrastructures are essential to advance the cervical cancer elimination plan proposed by the WHO.
Currently, the use of urine and menstrual blood self-sampling for cervical cancer screening has been explored (Martinelli Li et al., 2022; Wong et al., 2010), but the available data are still insufficient. Efforts to enhance participation in cervical cancer screening and ensure timely treatment of precancerous lesions will contribute to reducing and ultimately eliminating cervical cancer.
This review is not without limitations. The characteristics of the participants enrolled in the primary studies differed, as did the sample sizes. In addition, the methods for HPV testing and sampling devices were not described in several studies. The diagnostic accuracy of the HPV tests was not uniform across the studies, and the intervals between self-sampling and clinician sampling were also inconsistent. Another limitation of this review is that only studies conducted in East Asia, Southeast Asia, and South Asia were included. Finally, gray literature and conference abstracts were not included in this review, and the exclusion of non-English articles may have limited the comprehensiveness of the analysis. These exclusions could introduce potential bias, as studies published in languages other than English or in gray literature might have different characteristics or findings compared to those published in peer-reviewed journals. Consequently, the findings of this review may not fully represent the entire body of literature, and future research should consider including non-English studies and gray literature to provide a more comprehensive understanding of the topic.
Self-sampling for HPV detection can significantly improve cervical cancer screening coverage, especially in regions with limited medical resources or among individuals unwilling to undergo physician-collected sampling. However, its effectiveness varies across regions due to cultural, infrastructural, and healthcare factors. In rural areas of China and India, studies show that self-sampling accuracy is lower than physician-collected samples, likely due to differences in viral load capture and diagnostic thresholds. The diagnostic criteria and HPV testing methods for self-collected samples still need to be adjusted. Additionally, acceptance of self-sampling is low in China and Thailand, particularly among older women in these regions, due to concerns about procedure discomfort, infection, and reliability. To address these issues, targeted education and awareness campaigns are essential. Given these regional differences, self-sampling should be integrated into screening programs based on local contexts: in high-resource settings, physician-collected samples may remain preferred, while in low-resource areas, self-sampling can play a crucial role in expanding coverage. Policymakers should consider regional variations in healthcare infrastructure, cultural factors, and screening barriers to effectively reduce cervical cancer burden across diverse populations.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
XJ: Conceptualization, Data curation, Formal Analysis, Writing – original draft. MH: Investigation, Methodology, Writing – review and editing. YW: Investigation, Methodology, Writing – review and editing. WK: Supervision, Validation, Writing – review and editing. ZP: Supervision, Validation, Writing – review and editing. QS: Investigation, Methodology, Writing – review and editing. JM: Conceptualization, Funding acquisition, Writing – review and editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Beijing Municipal Health Commission, Capital’s Funds for Health Improvement and Research (grant number: 2022-1G-2112); Beijing Hospitals Authority’s Ascent Plan (grant number: DFL20221201); Demonstration Construction Project of Clinical Research Ward of Beijing Obstetrics and Gynecology Hospital, Capital Medical University (grant number: BCRW202109); Laboratory for Clinical Medicine, Capital Medical University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1540609/full#supplementary-material
HPV, human papillomavirus; HR-HPV, high-risk human papillomavirus; VIA, visual inspection with acetic acid; CINHAL, Cumulative Index to Nursing and Allied Health Library; CIN, cervical intraepithelial neoplasia; HSIL, high-grade squamous intraepithelial lesion; HC2, Hybrid Capture 2; PCR, polymerase chain reaction; MALDI-TOF, matrix-assisted laser desorption/ionization time-of-flight.
Abdullah, N., Daud, S., Wang, S., Mahmud, Z., Mohd Kornain, N., and Al-Kubaisy, W. (2018). Human papilloma virus (HPV) self-sampling: Do women accept it? J. Obstet. Gynaecol. 38, 402–407. doi: 10.1080/01443615.2017.1379061
Ahmad, Z., Daud, S., and Abdullah, N. (2021). Perception and knowledge of human papillomavirus (HPV) and HPV DNA self-sampling amongst women in west Malaysia. Brunei Int. Med. J. 17, 79–85.
Aiko, K., Yoko, M., Saito, O., Ryoko, A., Yasuyo, M., Mikiko, A., et al. (2017). Accuracy of self-collected human papillomavirus samples from Japanese women with abnormal cervical cytology. J. Obstet. Gynaecol. Res. 43, 710–717. doi: 10.1111/jog.13258
Anand, K., Mishra, G., and Pimple, S. (2022). Cross-sectional study of HPV self-sampling among Indian women—A way forward. Indian J. Med. Paediatr. Oncol. 43, 103–108.
Aoki, E., Yin, R., Li, K., Bhatla, N., Singhal, S., Ocviyanti, D., et al. (2020). National screening programs for cervical cancer in Asian countries. J. Gynecol. Oncol. 31:e55. doi: 10.3802/jgo.2020.31.e55
Arbyn, M., Ronco, G., Anttila, A., Meijer, C., Poljak, M., Ogilvie, G., et al. (2012). Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine 30, F88–F99. doi: 10.1016/j.vaccine.2012.06.095
Arbyn, M., Smith, S., Temin, S., Sultana, F., and Castle, P. (2018). Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: Updated meta-analyses. BMJ 363:k4823. doi: 10.1136/bmj.k4823
Asthana, S., and Labani, S. (2015). Adjunct screening of cervical or vaginal samples using careHPV testing with Pap and aided visual inspection for detecting high-grade cervical intraepithelial neoplasia. Cancer Epidemiol. 39, 104–108. doi: 10.1016/j.canep.2014.11.006
Belinson, J., Du, H., Yang, B., Wu, R., Belinson, S., Qu, X., et al. (2012). Improved sensitivity of vaginal self-collection and high-risk human papillomavirus testing. Int. J. Cancer 130, 1855–1860. doi: 10.1002/ijc.26202
Belinson, J., Hu, S., Niyazi, M., Pretorius, R., Wang, H., Wen, C., et al. (2010). Prevalence of type-specific human papillomavirus in endocervical, upper and lower vaginal, perineal and vaginal self-collected specimens: Implications for vaginal self-collection. Int. J. Cancer 127, 1151–1157. doi: 10.1002/ijc.25144
Belinson, J., Qiao, Y., Pretorius, R., Zhang, W., Elson, P., Li, L., et al. (2001). Shanxi province cervical cancer screening study: A cross-sectional comparative trial of multiple techniques to detect cervical neoplasia. Gynecol. Oncol. 83, 439–444. doi: 10.1006/gyno.2001.6370
Belinson, J., Qiao, Y., Pretorius, R., Zhang, W., Rong, S., Huang, M., et al. (2003). Shanxi Province cervical cancer screening study II: Self-sampling for high-risk human papillomavirus compared to direct sampling for human papillomavirus and liquid based cervical cytology. Int. J. Gynecol. Cancer 13, 819–826. doi: 10.1111/j.1525-1438.2003.13611.x
Bhatla, N., Dar, L., Patro, A., Kumar, P., Kriplani, A., Gulati, A., et al. (2009). Can human papillomavirus DNA testing of self-collected vaginal samples compare with physician-collected cervical samples and cytology for cervical cancer screening in developing countries? Cancer Epidemiol. 33, 446–450. doi: 10.1016/j.canep.2009.10.013
Bouvard, V., Wentzensen, N., Mackie, A., Berkhof, J., Brotherton, J., Giorgi-Rossi, P., et al. (2021). The IARC perspective on cervical cancer screening. N. Engl. J. Med. 385, 1908–1918. doi: 10.1056/NEJMsr2030640
Bray, F., Laversanne, M., Sung, H., Ferlay, J., Siegel, R., Soerjomataram, I., et al. (2024). Global cancer statistics 2022: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263. doi: 10.3322/caac.21834
Chang, C., Tseng, C., Liu, W., Jain, S., Horng, S., Soong, Y., et al. (2002). Clinical evaluation of a new model of self-obtained method for the assessment of genital human papilloma virus infection in an underserved population. Chang Gung Med. J. 25, 664–671.
Chaw, L., Lee, S., Ja’afar, N., Lim, E., and Sharbawi, R. (2022). Reasons for non-attendance to cervical cancer screening and acceptability of HPV self-sampling among Bruneian women: A cross-sectional study. PLoS One 17:e0262213. doi: 10.1371/journal.pone.0262213
Chen, K., Ouyang, Y., Hillemanns, P., and Jentschke, M. (2016). Excellent analytical and clinical performance of a dry self-sampling device for human papillomavirus detection in an urban Chinese referral population. J. Obstet. Gynaecol. Res. 42, 1839–1845. doi: 10.1111/jog.13132
Chen, Q., Du, H., Zhang, R., Zhao, J., Hu, Q., Wang, C., et al. (2016). Evaluation of novel assays for the detection of human papilloma virus in self-collected samples for cervical cancer screening. Genet. Mol. Res. 15, 1–7. doi: 10.4238/gmr.15027896
Chen, S., Hsieh, P., Chou, C., and Tzeng, Y. (2014). Determinants of women’s likelihood of vaginal self-sampling for human papillomavirus to screen for cervical cancer in Taiwan: A cross-sectional study. BMC Womens Health 14:139. doi: 10.1186/s12905-014-0139-0
Chen, W., Jeronimo, J., Zhao, F., Qiao, Y., Valdez, M., Zhang, X., et al. (2014). The concordance of HPV DNA detection by Hybrid Capture 2 and careHPV on clinician- and self-collected specimens. J. Clin. Virol. 61, 553–557. doi: 10.1016/j.jcv.2014.09.018
Cho, H., Ouh, Y., Hong, J., Min, K., So, K., Kim, T., et al. (2019). Comparison of urine, self-collected vaginal swab, and cervical swab samples for detecting human papillomavirus (HPV) with Roche Cobas HPV, Anyplex II HPV, and RealTime HR-S HPV assay. J. Virol. Methods 269, 77–82. doi: 10.1016/j.jviromet.2019.04.012
Chorley, A., Marlow, L., Forster, A., Haddrell, J., and Waller, J. (2017). Experiences of cervical screening and barriers to participation in the context of an organised programme: A systematic review and thematic synthesis. Psychooncology 26, 161–172. doi: 10.1002/pon.4126
Chou, H., Huang, H., Cheng, H., Chang, C., Yang, L., Huang, C., et al. (2016). Self-sampling HPV test in women not undergoing Pap smear for more than 5 years and factors associated with under-screening in Taiwan. J. Formos Med. Assoc. 115, 1089–1096. doi: 10.1016/j.jfma.2015.10.014
Cremer, M., Alfaro, K., and Masch, R. (2021). Cervical cancer screening in low- and middle-income countries. JAMA 325:790. doi: 10.1001/jama.2020.25214
Du, H., Duan, X., Liu, Y., Shi, B., Zhang, W., Wang, C., et al. (2021). Evaluation of cobas HPV and SeqHPV assays in the Chinese multicenter screening trial. J. Low Genit. Tract. Dis. 25, 22–26. doi: 10.1097/LGT.0000000000000577
Gallagher, K., LaMontagne, D., and Watson-Jones, D. (2018). Status of HPV vaccine introduction and barriers to country uptake. Vaccine 36, 4761–4767. doi: 10.1016/j.vaccine.2018.02.003
Goldstein, A., Goldstein, L., Lipson, R., Bedell, S., Wang, J., Stamper, S., et al. (2020). Assessing the feasibility of a rapid, high-volume cervical cancer screening programme using HPV self-sampling and digital colposcopy in rural regions of Yunnan China. BMJ Open 10:e035153. doi: 10.1136/bmjopen-2019-035153
Gottschlich, A., Nuntadusit, T., Zarins, K., Hada, M., Chooson, N., Bilheem, S., et al. (2019). Barriers to cervical cancer screening and acceptability of HPV self-testing: A cross-sectional comparison between ethnic groups in Southern Thailand. BMJ Open 9:e031957. doi: 10.1136/bmjopen-2019-031957
Guan, Y., Castle, P., Wang, S., Li, B., Feng, C., Ci, P., et al. (2012). A cross-sectional study on the acceptability of self-collection for HPV testing among women in rural China. Sex Transm. Infect. 88, 490–494. doi: 10.1136/sextrans-2012-050477
Guan, Y., Gravitt, P., Howard, R., Eby, Y., Wang, S., Li, B., et al. (2013). Agreement for HPV genotyping detection between self-collected specimens on a FTA cartridge and clinician-collected specimens. J. Virol. Methods 189, 167–171. doi: 10.1016/j.jviromet.2012.11.010
Hanh, L. (2006). Epidemiology of High-Risk HPV Infection in Northern Vietnam Among Married Women and Tolerability of Self Testing. Baltimore, MA: The Johns Hopkins University.
Hanley, S., Fujita, H., Yokoyama, S., Kunisawa, S., Tamakoshi, A., Dong, P., et al. (2016). HPV self-sampling in Japanese women: A feasibility study in a population with limited experience of tampon use. J. Med. Screen 23, 164–170. doi: 10.1177/0969141315625702
Harding-Esch, E., Hollis, E., Mohammed, H., and Saunders, J. (2017). Self-sampling and self-testing for STIs and HIV: The case for consistent nomenclature. Sex Transm. Infect. 93, 445–448. doi: 10.1136/sextrans-2016-052841
Hariprasad, R., John, A., and Abdulkader, R. (2023). Challenges in the implementation of human papillomavirus self-sampling for cervical cancer screening in India: A systematic review. JCO Glob. Oncol. 9:e2200401. doi: 10.1200/GO.22.00401
He, L., and He, J. (2020). Attitudes towards HPV self-sampling among women in Chengdu. China: A cross-sectional survey. J. Med. Screen 27, 201–206. doi: 10.1177/0969141319895543
Johnson, D., Bhatta, M., Smith, J., Kempf, M., Broker, T., Vermund, S., et al. (2014). Assessment of high-risk human papillomavirus infections using clinician- and self-collected cervical sampling methods in rural women from far western Nepal. PLoS One 9:e101255. doi: 10.1371/journal.pone.0101255
Kamath Mulki, A., and Withers, M. (2021). Human Papilloma Virus self-sampling performance in low- and middle-income countries. BMC Womens Health 21:12. doi: 10.1186/s12905-020-01158-4
Khoo, S., Lim, W., Rajasuriar, R., Nasir, N., Gravitt, P., and Woo, Y. (2021). The acceptability and preference of vaginal self-sampling for human papillomavirus (HPV) Testing among a multi-ethnic asian female population. Cancer Prev. Res. 14, 105–112. doi: 10.1158/1940-6207.CAPR-20-0280
Kittisiam, T., Tangjitgamol, S., Chaowawanit, W., Khunnarong, J., Srijaipracharoen, S., Thavaramara, T., et al. (2016). Knowledge and attitudes of bangkok metropolitan women towards HPV and self-sampled HPV testing. Asian Pac. J. Cancer Prev. 17, 2445–2451.
Kuriakose, S., Sabeena, S., Binesh, D., Abdulmajeed, J., Ravishankar, N., Ramachandran, A., et al. (2020). Diagnostic accuracy of self-collected vaginal samples for HPV DNA detection in women from South India. Int. J. Gynaecol. Obstet. 149, 219–224. doi: 10.1002/ijgo.13116
Latiff, L., Ibrahim, Z., Pei, C., Rahman, S., and Akhtari-Zavare, M. (2015a). Comparative assessment of a self-sampling device and gynecologist sampling for cytology and HPV DNA detection in a rural and low resource setting: Malaysian experience. Asian Pac. J. Cancer Prev. 16, 8495–8501. doi: 10.7314/apjcp.2015.16.18.8495
Latiff, L., Rahman, S., Wee, W., Dashti, S., Andi Asri, A., Unit, N., et al. (2015b). Assessment of the reliability of a novel self-sampling device for performing cervical sampling in Malaysia. Asian Pac. J. Cancer Prev. 16, 559–564. doi: 10.7314/apjcp.2015.16.2.559
Li, J., Wu, R., Qu, X., Huang, X., Li, L., Lin, Z., et al. (2022). Effectiveness and feasibility of self-sampling for human papillomavirus testing for internet-based cervical cancer screening. Front. Public Health 10:938272. doi: 10.3389/fpubh.2022.938272
Lim, L., Chan, M., Win, P., Shen, L., Arunachalam, I., Ng, S., et al. (2022). Self-sampling HPV DNA test for cervical cancer screening in Singapore: A prospective study. Ann. Acad. Med. Singap. 51, 733–735. doi: 10.47102/annals-acadmedsg.2022133
Madhivanan, P., Nishimura, H., Ravi, K., Pope, B., Coudray, M., Arun, A., et al. (2021). Acceptability and concordance of self- versus clinician- sampling for HPV testing among rural south Indian women. Asian Pac. J. Cancer Prev. 22, 971–976. doi: 10.31557/APJCP.2021.22.3.971
Martinelli, M., Giubbi, C., Sechi, I., Bottari, F., Iacobone, A., Musumeci, R., et al. (2022). Evaluation of BD Onclarity™ HPV assay on self-collected vaginal and first-void urine samples as compared to clinician-collected cervical samples: A pilot study. Diagnostics 12:3075. doi: 10.3390/diagnostics12123075
Ma’som, M., Bhoo-Pathy, N., Nasir, N., Bellinson, J., Subramaniam, S., Ma, Y., et al. (2016). Attitudes and factors affecting acceptability of self-administered cervicovaginal sampling for human papillomavirus (HPV) genotyping as an alternative to Pap testing among multiethnic Malaysian women. BMJ Open 6:e011022. doi: 10.1136/bmjopen-2015-011022
Ngu, S., Lau, L., Li, J., Wong, G., Cheung, A., Ngan, H., et al. (2022). Human papillomavirus self-sampling for primary cervical cancer screening in under-screened women in Hong Kong during the COVID-19 pandemic. Int. J. Environ. Res. Public Health 19:2610. doi: 10.3390/ijerph19052610
Nilyanimit, P. (2014). Comparison of detection sensitivity for human papillomavirus between self-collected vaginal swabs and physician-collected cervical swabs by electrochemical DNA chip. Asian Pac. J. Cancer Prev. 15, 10809–10812.
Nodjikouambaye, Z., Adawaye, C., Mboumba Bouassa, R., Sadjoli, D., and Bélec, L. A. (2020). systematic review of self-sampling for HPV testing in Africa. Int. J. Gynaecol. Obstet. 149, 123–129. doi: 10.1002/ijgo.13112
Nutthachote, P., Oranratanaphan, S., Termrungruanglert, W., Triratanachat, S., Chaiwongkot, A., Baedyananda, F., et al. (2019). Comparison of detection rate of high risk HPV infection between self-collected HPV testing and clinician-collected HPV testing in cervical cancer screening. Taiwan J. Obstet. Gynecol. 58, 477–481. doi: 10.1016/j.tjog.2019.05.008
Ong, S., Abe, S., Thilagaratnam, S., Haruyama, R., Pathak, R., Jayasekara, H., et al. (2023). Towards elimination of cervical cancer - human papillomavirus (HPV) vaccination and cervical cancer screening in Asian National Cancer Centers Alliance (ANCCA) member countries. Lancet Reg. Health West Pac. 39:100860. doi: 10.1016/j.lanwpc.2023.100860
Onuma, T., Kurokawa, T., Shinagawa, A., Chino, Y., and Yoshida, Y. (2020). Evaluation of the concordance in HPV type between self- and physician-collected samples using a brush-based device and a PCR-based HPV DNA test in Japanese referred patients with abnormal cytology or HPV infection. Int. J. Clin. Oncol. 25, 1854–1860. doi: 10.1007/s10147-020-01727-5
Oranratanaphan, S., Termrungruanglert, W., and Khemapech, N. (2014). Acceptability of self-sampling HPV testing among Thai women for cervical cancer screening. Asian Pac. J. Cancer Prev. 15, 7437–7441. doi: 10.7314/apjcp.2014.15.17.7437
Ozawa, N., Kurokawa, T., Hareyama, H., Tanaka, H., Satoh, M., Metoki, H., et al. (2023). Evaluation of the feasibility of human papillomavirus sponge-type self-sampling device at Japanese colposcopy clinics. J. Obstet. Gynaecol. Res. 49, 701–708. doi: 10.1111/jog.15496
Page, M., McKenzie, J., Bossuyt, P., Boutron, I., Hoffmann, T., Mulrow, C., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372:105906. doi: 10.1136/bmj.n71
Phoolcharoen, N., Kantathavorn, N., Krisorakun, W., Sricharunrat, T., Teerayathanakul, N., Taepisitpong, C., et al. (2018a). Agreement of self- and physician-collected samples for detection of high-risk human papillomavirus infections in women attending a colposcopy clinic in Thailand. BMC Res. Notes 11:136. doi: 10.1186/s13104-018-3241-9
Phoolcharoen, N., Kantathavorn, N., Krisorakun, W., Taepisitpong, C., Krongthong, W., and Saeloo, S. (2018b). Acceptability of self-sample human papillomavirus testing among thai women visiting a colposcopy clinic. J. Community Health 43, 611–615. doi: 10.1007/s10900-017-0460-2
Ploysawang, P., Pitakkarnkul, S., Kolaka, W., Ratanasrithong, P., Khomphaiboonkij, U., Tipmed, C., et al. (2023). Acceptability and preference for human papilloma virus self-sampling among thai women attending national cancer institute. Asian Pac. J. Cancer Prev. 24, 607–612. doi: 10.31557/APJCP.2023.24.2.607
Qiao, Y., Sellors, J., Eder, P., Bao, Y., Lim, J., Zhao, F., et al. (2008). A new HPV-DNA test for cervical-cancer screening in developing regions: A cross-sectional study of clinical accuracy in rural China. Lancet Oncol. 9, 929–936. doi: 10.1016/S1470-2045(08)70210-9
Qin, Y., Zhang, H., Marlowe, N., Fei, M., Yu, J., Lei, X., et al. (2016). Evaluation of human papillomavirus detection by Abbott m2000 system on samples collected by FTA Elute™ Card in a Chinese HIV-1 positive population. J. Clin. Virol. 85, 80–85. doi: 10.1016/j.jcv.2016.11.002
Racey, C., Withrow, D., and Gesink, D. (2013). Self-collected HPV testing improves participation in cervical cancer screening: A systematic review and meta-analysis. Can. J. Public Health 104, e159–e166. doi: 10.1007/BF03405681
Rajkhowa, P., Mathew, M., Fadra, R., Saha, S., Rakshitha, K., Narayanan, P., et al. (2024). A scoping review of evidence on routine cervical cancer screening in South Asia: Investigating factors affecting adoption and implementation. Cancer Causes Control. 36, 67–79. doi: 10.1007/s10552-024-01923-y
Satake, H., Inaba, N., Kanno, K., Mihara, M., Takagi, Y., Kondo, N., et al. (2020). Comparison study of self-sampled and physician-sampled specimens for high-risk human papillomavirus test and cytology. Acta Cytol. 64, 433–441. doi: 10.1159/000507342
Schiffman, M., Wentzensen, N., Wacholder, S., Kinney, W., Gage, J., and Castle, P. (2011). Human papillomavirus testing in the prevention of cervical cancer. J. Natl. Cancer Inst. 103, 368–383. doi: 10.1093/jnci/djq562
Seo, S., Song, Y., Kim, J., Park, N., Kang, S., and Lee, H. (2006). Good correlation of HPV DNA test between self-collected vaginal and clinician-collected cervical samples by the oligonucleotide microarray. Gynecol. Oncol. 102, 67–73. doi: 10.1016/j.ygyno.2005.11.030
Shin, H., Lee, B., Hwang, S., Lee, D., Sung, N., Park, J., et al. (2019). Evaluation of satisfaction with three different cervical cancer screening modalities: Clinician-collected Pap test vs. HPV test by self-sampling vs. HPV test by urine sampling. J. Gynecol. Oncol. 30:e76. doi: 10.3802/jgo.2019.30.e76
Shrestha, S., Thapa, S., Sims, P., Ardelean, A., Basu, A., Caws, M., et al. (2021). Feasibility of HPV self-sampling pathway in kathmandu valley, nepal using a human-centred design approach. Sex Reprod. Health Matters 29:2005283. doi: 10.1080/26410397.2021.2005283
Simelela, P. N. (2021). WHO global strategy to eliminate cervical cancer as a public health problem: An opportunity to make it a disease of the past. Int. J. Gynaecol. Obstet. 152, 1–3. doi: 10.1002/ijgo.13484
Singh, D., Vignat, J., Lorenzoni, V., Eslahi, M., Ginsburg, O., Lauby-Secretan, B., et al. (2023). Global estimates of incidence and mortality of cervical cancer in 2020: A baseline analysis of the WHO Global cervical cancer elimination initiative. Lancet Glob. Health 11, e197–e206. doi: 10.1016/S2214-109X(22)00501-0
Snijders, P., Verhoef, V., Arbyn, M., Ogilvie, G., Minozzi, S., Banzi, R., et al. (2013). High-risk HPV testing on self-sampled versus clinician-collected specimens: A review on the clinical accuracy and impact on population attendance in cervical cancer screening. Int. J. Cancer 132, 2223–2236. doi: 10.1002/ijc.27790
Sowjanya, A., Paul, P., Vedantham, H., Ramakrishna, G., Vidyadhari, D., Vijayaraghavan, K., et al. (2009). Suitability of self-collected vaginal samples for cervical cancer screening in periurban villages in Andhra Pradesh, India. Cancer Epidemiol. Biomark. Prev. 18, 1373–1378. doi: 10.1158/1055-9965.EPI-08-1171
Spence, A., Goggin, P., and Franco, E. (2007). Process of care failures in invasive cervical cancer: Systematic review and meta-analysis. Prev. Med. 45, 93–106. doi: 10.1016/j.ypmed.2007.06.007
Sy, F., Greuel, M., Winkler, V., Bussmann, H., Bärnighausen, T., and Deckert, A. (2022). Accuracy of HPV testing on self-collected and clinician-collected samples for different screening strategies in African settings: A systematic review and meta-analysis. Gynecol. Oncol. 166, 358–368. doi: 10.1016/j.ygyno.2022.06.012
Tan, C., Hamzah, N., Ismail, Z., Jerip, A., and Kipli, M. (2021). Self-sampling in Human Papillomavirus screening during and post-COVID-19 pandemic. Med. J. Malaysia 76, 298–303.
Taniguchi, M., Ueda, Y., Yagi, A., Ikeda, S., Endo, M., Tomimatsu, T., et al. (2019). Cervical cancer screening rate differs by HPV vaccination status: An interim analysis. Vaccine 37, 4424–4426. doi: 10.1016/j.vaccine.2019.06.064
Terada, N., Matsuura, M., Kurokawa, S., Nishimura, Y., Tamate, M., Isoyama, K., et al. (2022). Human papillomavirus testing and cytology using physician-collected uterine cervical samples vs. self-collected vaginal samples and urine samples. Int. J. Clin. Oncol. 27, 1742–1749. doi: 10.1007/s10147-022-02238-1
Thay, S., Goldstein, A., Goldstein, L., Govind, V., Lim, K., and Seang, C. (2019). Prospective cohort study examining cervical cancer screening methods in HIV-positive and HIV-negative Cambodian Women: A comparison of human papilloma virus testing, visualization with acetic acid and digital colposcopy. BMJ Open 9:e026887. doi: 10.1136/bmjopen-2018-026887
Tisci, S., Shen, Y., Fife, D., Huang, J., Goycoolea, J., Ma, C., et al. (2003). Patient acceptance of self-sampling for human papillomavirus in rural china. J. Low Genit. Tract Dis. 7, 107–116. doi: 10.1097/00128360-200304000-00007
Trope, L., Chumworathayi, B., and Blumenthal, P. (2013). Feasibility of community-based careHPV for cervical cancer prevention in rural Thailand. J. Low Genit. Tract. Dis. 17, 315–319. doi: 10.1097/LGT.0b013e31826b7b70
Tsedenbal, B., Enebish, G., Tserensodnom, B., and Saio, M. (2022). Results of self-sampling methodology impression for cervical cancer screening in mongolia. Asian Pac. J. Cancer Prev. 23, 4099–4107. doi: 10.31557/APJCP.2022.23.12.4099
Twu, N., Yen, M., Lau, H., Chen, Y., Yu, B., and Lin, C. (2011). Type-specific human papillomavirus DNA testing with the genotyping array: A comparison of cervical and vaginal sampling. Eur. J. Obstet. Gynecol. Reprod. Biol. 156, 96–100. doi: 10.1016/j.ejogrb.2010.12.023
Verdoodt, F., Jentschke, M., Hillemanns, P., Racey, C., Snijders, P., and Arbyn, M. (2015). Reaching women who do not participate in the regular cervical cancer screening programme by offering self-sampling kits: A systematic review and meta-analysis of randomised trials. Eur. J. Cancer 51, 2375–2385. doi: 10.1016/j.ejca.2015.07.006
Wang, M., Hu, S., Zhao, S., Zhang, W., Pan, Q., Zhang, X., et al. (2017). Accuracy of triage strategies for human papillomavirus DNA-positive women in low-resource settings: A cross-sectional study in China. Chin. J. Cancer Res. 29, 496–509. doi: 10.21147/j.issn.1000-9604.2017.06.04
Wang, S., Hu, S., Chen, F., Chen, W., Zhao, F., Zhang, Y., et al. (2014). Clinical evaluation of human papillomavirus detection by careHPV™ test on physician-samples and self-samples using the indicating FTA Elute® card. Asian Pac. J. Cancer Prev. 15, 7085–7089. doi: 10.7314/apjcp.2014.15.17.7085
Wong, E., Chan, P., Chor, J., Cheung, A., Huang, F., and Wong, S. (2016). Evaluation of the impact of human papillomavirus DNA self-sampling on the uptake of cervical cancer screening. Cancer Nurs. 39, E1–E11. doi: 10.1097/NCC.0000000000000241
Wong, E., Cheung, A., Huang, F., and Chor, J. (2018). Can human papillomavirus DNA self-sampling be an acceptable and reliable option for cervical cancer screening in female sex workers? Cancer Nurs. 41, 45–52. doi: 10.1097/NCC.0000000000000462
Wong, E., Cheung, A., Wong, A., and Chan, P. (2020). Acceptability and feasibility of HPV self-sampling as an alternative primary cervical cancer screening in under-screened population groups: A cross-sectional study. Int. J. Environ. Res. Public Health 17:6245. doi: 10.3390/ijerph17176245
Wong, S., Au, T., Chan, S., Chan, C., Lam, M., Zee, B., et al. (2010). Human papillomavirus DNA detection in menstrual blood from patients with cervical intraepithelial neoplasia and condyloma acuminatum. J. Clin. Microbiol. 48, 709–713. doi: 10.1128/JCM.01996-09
Yoshida, T., Sano, T., Takada, N., Kanuma, T., Inoue, H., Itoh, T., et al. (2011). Comparison of self-collected and clinician-collected materials for cervical cytology and human papillomavirus genotyping: Analysis by linear array assay. Acta Cytol. 55, 106–112. doi: 10.1159/000320924
Zhang, W., Du, H., Huang, X., Wang, C., Duan, X., Liu, Y., et al. (2020). Evaluation of an isothermal amplification HPV detection assay for primary cervical cancer screening. Infect. Agent Cancer 15:65. doi: 10.1186/s13027-020-00328-1
Keywords: human papillomavirus, clinician sampling, self-sampling, Asia, cervical cancer, screening
Citation: Ji X, Hao M, Wang Y, Kong W, Pan Z, Sun Q and Miao J (2025) Human papillomavirus self-sampling in Asia: a systematic review. Front. Microbiol. 16:1540609. doi: 10.3389/fmicb.2025.1540609
Received: 06 December 2024; Accepted: 27 February 2025;
Published: 14 March 2025.
Edited by:
Nayeli Alva-Murillo, University of Guanajuato, MexicoReviewed by:
Chung-Yao Yang, Hygeia Touch Inc., TaiwanCopyright © 2025 Ji, Hao, Wang, Kong, Pan, Sun and Miao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinwei Miao, amlud2VpbWlhb0BjY211LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.