
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Microbiol., 03 April 2025
Sec. Virology
Volume 16 - 2025 | https://doi.org/10.3389/fmicb.2025.1518275
Introduction: Cytomegalovirus (CMV) infection poses a significant threat to individuals undergoing allogeneic hematopoietic stem cell transplantation (allo-HSCT), potentially resulting in substantial morbidity and mortality. This review summarized the epidemiology, clinical outcomes, and treatment patterns of CMV infection among allo-HSCT recipients in China.
Methods: PubMed, EMBASE, the Cochrane Library, Web of Science, China National Knowledge Infrastructure (CNKI), Wanfang and Chinese Biomedical Literature Database (CBM) were systematically searched from 2013 to March 2023. All analyses were performed using R 4.1.1 software with a random effects model.
Results: Fifty-six studies, which included 13,882 patients, were reviewed. The pooled overall incidence of CMV infection was 49.99% [95% confidence interval (CI) 43.72–56.26%]. Among post allo-HSCT recipients with CMV infection, 32.03% (95% CI 22.93–41.12%) developed refractory CMV infection. The overall incidence of CMV disease was 13.30% (95% CI 8.99–19.66%). The pooled all-cause mortality rate was 29.25% (95% CI 17.96–40.55%) and the CMV-related mortality rate was 3.46% (95% CI 1.19–5.73%). Results demonstrate that management of CMV has mainly focused on pre-emptive therapy due to the treatment-limiting toxicity of anti-CMV agents. Additionally, CMV infection is continuing to occur after the discontinuation of prophylaxis, highlighting the unmet need for a more effective treatment without treatment-limiting toxicities.
Conclusion: This review underscores the urgent need for improved therapeutic strategies to effectively manage cytomegalovirus infection in allo-HSCT recipients, particularly in light of the high incidence and associated morbidity, as well as the limitations of current treatment options.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42024513908, identifier: CRD42024513908.
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a curative treatment for various hematological malignancies and disorders. Although this treatment modality offers the promise of hematopoietic system renewal, it poses unique challenges, particularly in terms of infectious complications, among which cytomegalovirus (CMV) emerges as a prominent adversary.
Globally, CMV infection is a well-documented threat in the context of allo-HSCT, influencing post-transplant outcomes (Camargo and Komanduri, 2017; Ljungman et al., 2017). Directly, CMV can initiate primary or reactive infection within recipients, leading to CMV viremia (Dziedzic et al., 2017). This uncontrolled viral replication may progress to CMV diseases, presenting as complications such as pneumonia, gastrointestinal complications and retinitis (Paris et al., 2009; Solano et al., 2013), contributing significantly to post-transplant morbidity and mortality (Mori and Kato, 2010; Giménez et al., 2019; Teira et al., 2016). Indirectly, CMV impedes the immune reconstitution process initiated by allo-HSCT, compromising the functionality of immune cells, particularly T cells. This weakened immune response heightens vulnerability to various opportunistic infections (Anderson-Smits et al., 2020; Nichols et al., 2002). Additionally, CMV is associated with an elevated risk of graft-vs.-host disease (GVHD), patient survival, non-relapse mortality and graft failure (Grigoleit et al., 2009; Teira et al., 2016; Fan et al., 2022; Boeckh and Geballe, 2011; Cantoni et al., 2010).
Since potent antivirals have been developed, CMV-related mortality and the incidence of CMV diseases have decreased (Gagelmann et al., 2018; Chen et al., 2018; Marty et al., 2019; Boeckh et al., 2015). Typically, management of CMV consists of prophylaxis or pre-emptive therapy. Unlike prophylactic therapy, which involves administering antiviral drugs to all at-risk patients regardless of their CMV status, pre-emptive therapy focuses on closely monitoring patients for early signs of CMV replication (e.g., through regular PCR testing) and initiating antiviral treatment only when a predefined viral load threshold is reached. Commonly employed antiviral agents for managing CMV worldwide encompass intravenous foscarnet, cidofovir, Ganciclovir and its valyl ester prodrug (valganciclovir), oral valganciclovir (Cho et al., 2019; Limaye et al., 2020). However, these drugs are associated with significant toxicity, including myelosuppression and nephrotoxicity (De Clercq and Li, 2016; George et al., 2010). Therefore, pre-emptive therapy is preferred to prophylaxis therapy in clinical practice to avoid the potential risk of drug-related adverse effects.
With CMV seroprevalence exceeding 90% in China's general population (Stem Cell Application Group, Chinese Society of Hematology, Chinese Medical Association, 2022), and haploidentical hematopoietic stem cell transplantation (haplo-HSCT) making up 60.1% of allo-HSCT cases in China (Xu et al., 2021), the likelihood of CMV infection following allo-HSCT in China is high, underlining the significant concern in this context. Therefore, this review aims to comprehensively summarize the epidemiology, clinical outcomes and treatment patterns of CMV infection among allo-HSCT recipients in China, including mainland China, Taiwan, Hong Kong and Macau.
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) (Page et al., 2021) extension for scoping reviews (Tricco et al., 2018). The protocol was registered on PROSPERO (registration number: CRD42024513908).
An electronic database search of PubMed, EMBASE, the Cochrane Library, Web of Science, CNKI, Wanfang, and CBM was conducted in March 2023 to search for studies published over the past 10 years (from 2013 to 2023) using the keywords “cytomegalovirus”, “stem cell transplantation”, and “China”. We applied no restrictions on language or publication status. The search strategy is provided in Supplementary material.
Studies with standard of care that investigated allo-HSCT recipients in China were included. The following outcomes were of interest:
- Epidemiology outcomes including incidence of CMV infection, breakthrough infection which is defined as the development of an CMV infection that occurs during antiviral prophylaxis (Ljungman et al., 2019), and time to CMV infection onset;
- Clinical outcomes among patients with CMV infection including incidence of resistant CMV infection, refractory CMV infection, and recurrent CMV infection; time to CMV disease onset; and incidence of CMV disease; mortality rate; and incidence of other comorbidities with CMV infection among patients with CMV infection;
- Treatment patterns;
- Treatment-limiting toxicity.
We considered observational and interventional studies that reported in English or Chinese while excluding case reports, reviews, comments, and editorials. We excluded reports that not published in English or Chinese. Two reviewers independently screened articles for eligibility. Any disagreements were resolved through discussion with a third reviewer.
Two reviewers independently extracted the following data from each study into a predefined data extraction form: study characteristics including first author's name, publication year, study design, geographic location of study, and sample size; participant characteristics including age, gender, type of transplant, and population description reported by the study, as well as outcomes. Any disagreements were resolved by discussion with a third reviewer.
Meta-analyses were performed using a random effects model with the meta package in R (version 4.1.1) when data was appropriate. For dichotomous outcomes, we extracted the reported rates and calculated as proportion and 95% confidence interval (95% CI) for each study, then pooled them by meta-analysis. When data was insufficient for meta-analysis, we described the outcomes narratively accompanied by tabulated and charted results. We used a raincloud plot to present the time to CMV infection, CMV disease onset and CMV viremia resolution when data was available from three or more studies. In a raincloud plot, each dot represents an individual study's effect estimate, the x-axis represents the measure of effects (i.e., time in days), and the box plot within each group summarizes the distribution of these estimates, with the central line indicating the median and the box showing the interquartile range. We used a pie chart to present the proportion of various agents in different therapeutic approaches. Heterogeneity of studies was assessed by the Cochrane Q-test with a significance level of 0.05 and the I2 statistic, where I2 ≥ 50% coupled with p < 0.05 from the Q-test was interpreted as evidence of substantial levels of heterogeneity. We conducted subgroup analyses to explore the potential sources of substantial heterogeneity. A funnel plot was performed for outcomes reported by 10 or more studies to assess publication bias.
Subgroup analyses were carried out on the incidence of CMV infection according to days after transplantation (within 100 days and within 200 days), prophylaxis therapy (breakthrough incidence, defined as the occurrence of CMV infection during prophylaxis therapy), patient age (adults and children), transplant type (human leukocyte antigen (HLA)-matched, HLA haploidentical, cord blood and unrelated-matched), and CMV serologic status prior to transplantation [donor-negative (D–)/recipient-negative (R–), donor-positive (D+)/recipient-positive (R+), D–/R+ and D+/R–].
The quality of included studies was assessed independently by two reviewers using Joanna Briggs Institute (JBI) critical appraisal tools for prevalence studies, case-control studies, cohort studies and randomized controlled trials (RCTs) (Barker et al., 2023; JBI, 2020). Any disagreements were resolved by discussion with a third reviewer.
The database search returned 2,684 results and provided 1,472 unique citations after duplicates were removed. A further 1,232 articles were excluded at the title- and abstract-screening stage. Full papers were sought for the remaining 240 articles. Of these, 227 full papers were retrieved and assessed for eligibility according to the inclusion and exclusion criteria. The remaining 13 articles were not successfully retrieved due to unauthorized access. Finally, 56 studies that published in 57 reports (Cao et al., 2016a,b; Gao et al., 2013; Guo et al., 2016; Han et al., 2014; He et al., 2014; Huang et al., 2022, 2013; Jin et al., 2014; Li et al., 2015a,b, 2022a, 2020, 2013b; Ma et al., 2023b; Que et al., 2014; Shi et al., 2019; Si et al., 2013; Tan et al., 2020; Wan et al., 2017; Wang et al., 2015, 2017, 2019, 2021, 2013; Wei et al., 2022; Wu et al., 2019, 2017; Xiong et al., 2023; Xu et al., 2015; Xue et al., 2019a,b; Yin et al., 2020; Zhang et al., 2016, 2014; Zhao et al., 2020; Zhao and Sun, 2021; Zhu et al., 2020; Zou et al., 2016; Bao et al., 2016; Chen et al., 2022; Cheng et al., 2022; Ding et al., 2021; Li et al., 2021, 2022b, 2013a; Lin et al., 2017; Liu et al., 2015; Meng et al., 2020; Pei et al., 2022; Tong et al., 2013; Yan et al., 2020; Yeh et al., 2022; Yin et al., 2022; Zhang et al., 2021, 2022, 2017) met the full eligibility criteria and were included in the review. The study selection process is shown in Figure 1.
We included a total of 56 studies with sample sizes ranging from 11 to 3,862 (Table 1). These studies comprised 25 cross-sectional studies, 24 cohort studies, five case-control studies, and two RCTs. The quality assessment results of the included studies are summarized in Supplementary Tables S1–S4.
The meta-analysis of 47 studies conducted on 8,442 patients revealed that the incidence of CMV infection was 49.99% (95% CI 43.72–56.26%, Figure 2). The median time for CMV infection to develop after allo-HSCT was provided in 18 studies with 3,806 patients and found to be 37 days (Figure 3). Three studies with 151 patients reported the incidence of CMV breakthrough infection during foscarnet, ganciclovir and letermovir prophylactic therapy, and the pooled result was 7.29% (95% CI 3.09–11.48%, Supplementary Figure S1).
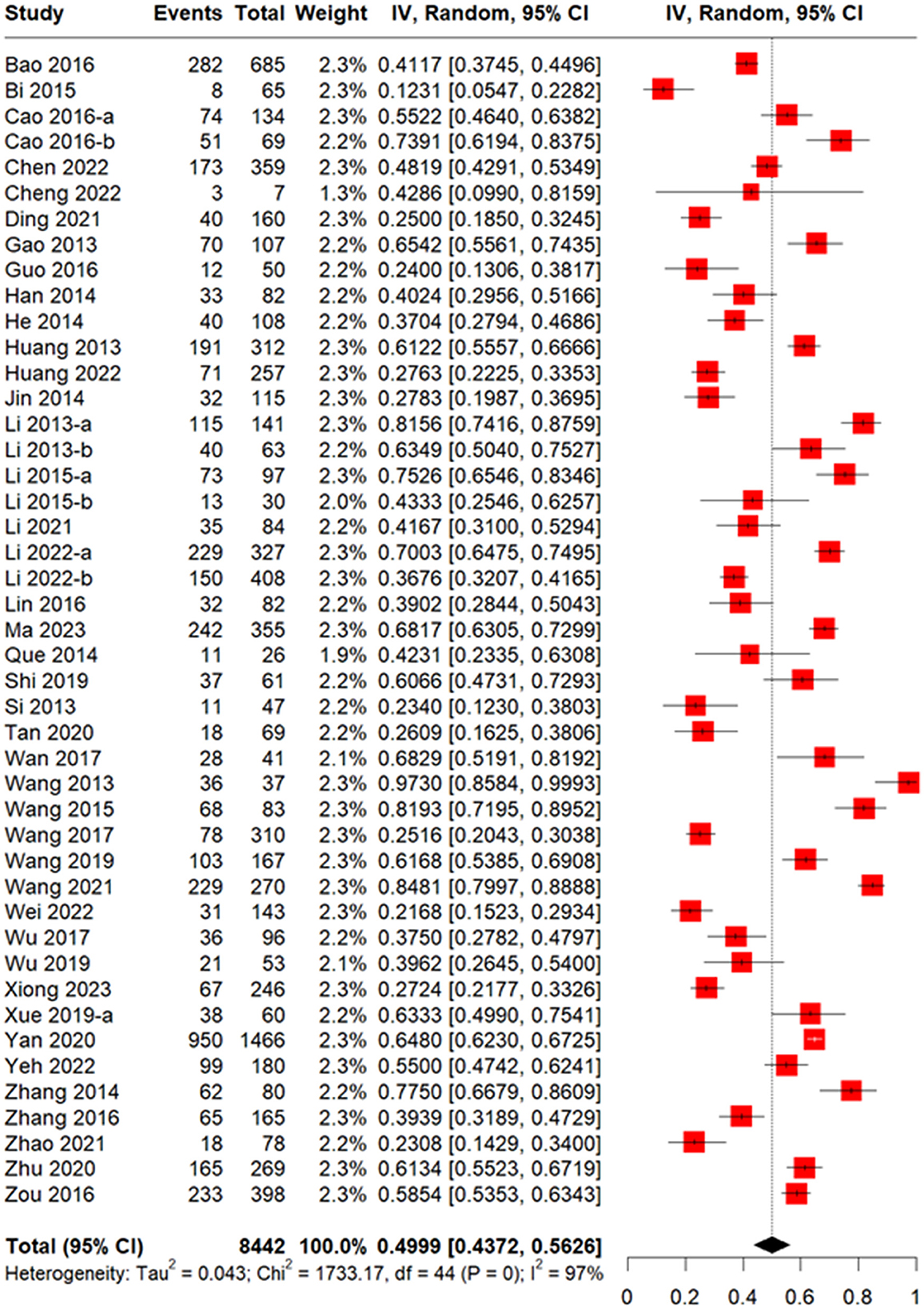
Figure 2. Forest plot of incidence of CMV infection in allogeneic hematopoietic stem cell transplantation (allo-HSCT) recipients.
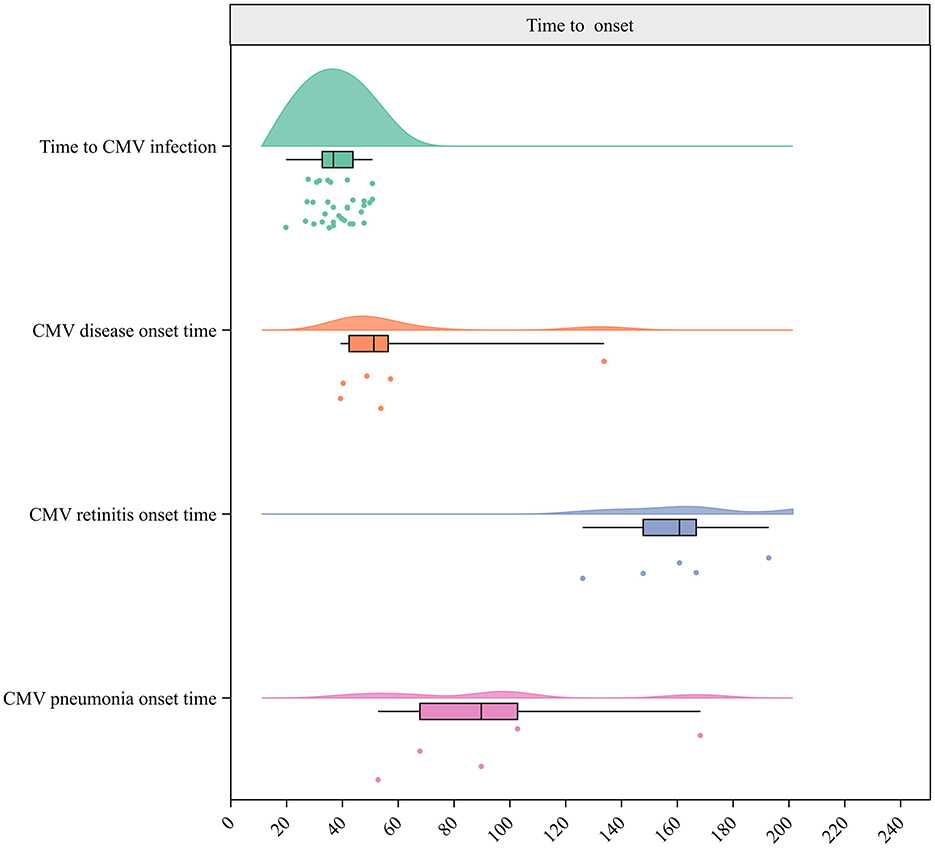
Figure 3. Raincloud plot for times to CMV infection, CMV breakthrough infection, CMV disease, CMV retinitis, and CMV pneumonia onset.
Regarding transplant types, subgroup analyses revealed that the incidence of CMV infection was 70.82% (95% CI 58.29–83.35%) in unrelated matched transplantation, 69.08% (95% CI 61.50–76.66%) in haploidentical transplantation, 55.48% (95% CI 31.29–79.67%) in cord blood transplantation and 36.15% (95% CI 22.93–49.36%) in HLA-matched transplantation (Table 2, Supplementary Figures S2–S5).
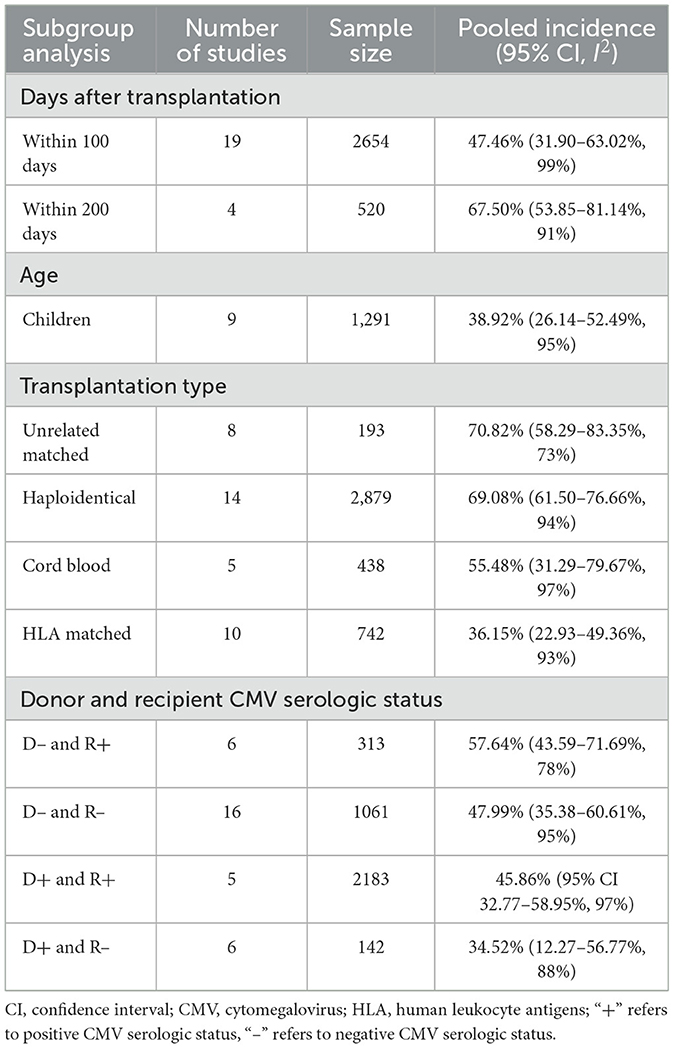
Table 2. Incidence of CMV infection based on days after transplantation, patient age, transplantation type, and donor and recipient CMV serologic status—subgroup analysis results.
The pooled incidence of CMV infection was 57.64% (95% CI 43.59–71.69%) based on the CMV serologic status of D–/R+, 47.99% (95% CI 35.38–60.61%) under the status of D–/R–, 45.86% (95% CI 32.77–58.95%) under the status of D+/R+ and 34.52% (95% CI 12.27–56.77%) under the status of D+/R– (Table 2, Supplementary Figures S6–S9).
The results showed that the incidence of cumulative CMV infection was 47.46% (95% CI 31.90–63.02%) within 100 days of transplantation and 67.50% (95% CI 53.85–81.14%) within 200 days (Table 2, Supplementary Figures S10, S11).
Two studies (Li et al., 2015a, 2021) reported incidences of CMV infection among adult patients of 70.03 and 41.67%, respectively. In addition, the pooled results revealed that the incidence of CMV infection was 38.92% (95% CI 26.14–52.49%) among pediatric patients (Table 2, Supplementary Figure S12).
After transplantation, 32.03% (95% CI 22.93–41.12%) of patients with CMV infection developed refractory CMV infection (five studies with 787 patients) and 15.42% (95% CI 7.76–30.63%) experienced CMV recurrence (12 studies with 697 patients, Supplementary Figures S13 and S14). Only one study on resistant CMV infection, which included 143 patients, was identified. Drug resistance rate seen in 0.7%.
A total of 18 studies including 1,362 patients reported the incidence of CMV disease among patients with CMV infection. The pooled incidence was 13.30% (95% CI 8.99–19.66%, Figure 4). The median CMV disease onset time was 51.5 days, based on six studies with 4,737 patients (Figure 3). Two studies (Ma et al., 2023b; Zhu et al., 2020) reported incidences of CMV disease among patients with refractory CMV infection of 3.4 and 3.3%, respectively.
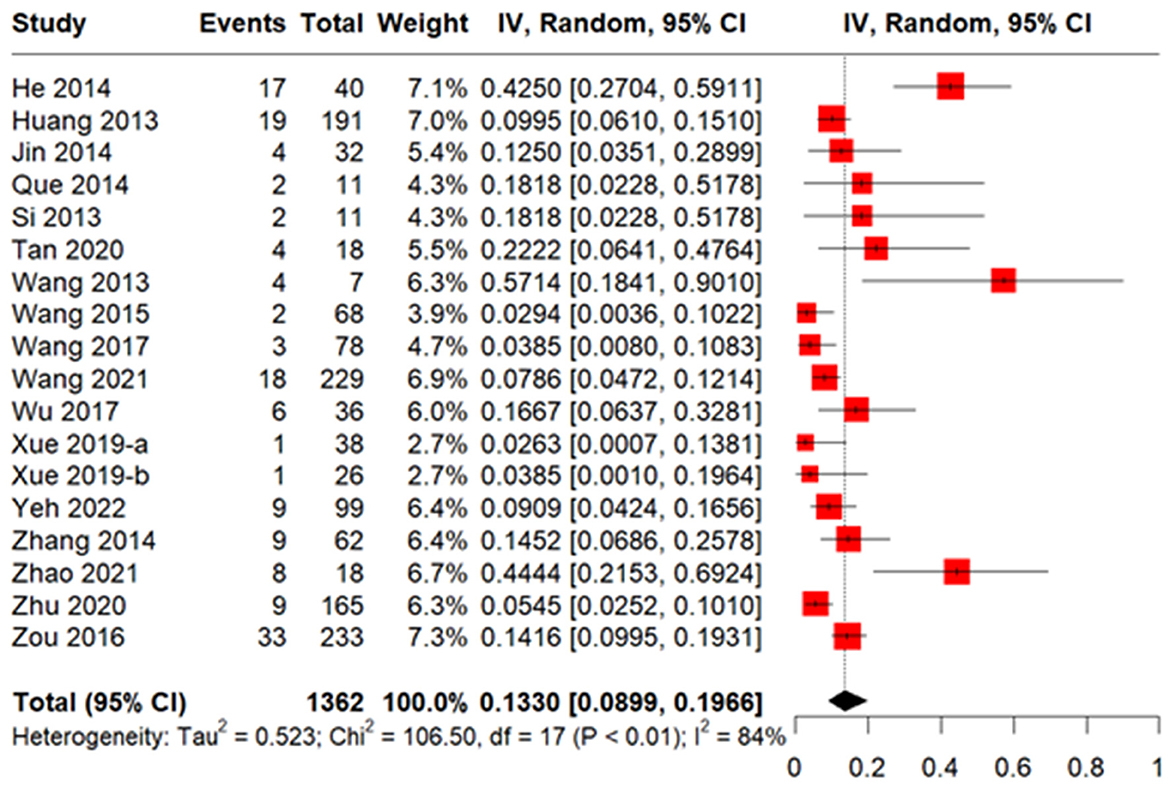
Figure 4. Forest plot of incidence of CMV disease in allogeneic hematopoietic stem cell transplantation (allo-HSCT) recipients with CMV infection.
Specifically, the incidence of CMV pneumonitis among patients with CMV infection pooled from 23 studies involving 7,434 patients was 5.96% (95% CI 4.44–8.00%, Supplementary Figure S15). The median time for CMV pneumonia onset was 90 days, based on five studies with 4,675 patients (Figure 3). Moreover, the pooled incidence was 13.48% for CMV cystitis, 7.12% for enteritis, 3.47% for retinitis, 0.59% for encephalitis, and 5% for hepatitis (He et al., 2014; Table 3, Supplementary Figures S16–S19). The median times to the onset of specific CMV diseases are shown in Table 4.
The all-cause mortality rate was reported in nine studies with 710 patients and was found to be 29.25% (95% CI 17.96–40.55%, Supplementary Figure S20), whereas the CMV-related mortality rate was found to be 3.46% (95% CI 1.19–5.73%), based on 14 studies with 632 patients (Supplementary Figure S21).
Results pooled from four studies with 283 patients indicated that 64.20% (95% CI 31.22–97.18%) of patients with CMV infection experienced GVHD (Supplementary Figure S22). Another four studies with 248 patients reported an incidence of acute GVHD of 63.97% (95% CI 17.53–100.00%, Supplementary Figure S23). Two studies (Tong et al., 2013; Huang et al., 2022) reported incidences of chronic GVHD of 14.08% and 16.00%, and two studies (Yang et al., 2022; Huang et al., 2022) reported incidences of coexisting EB virus infection of 32.75 and 29.58%, respectively. The incidence of coexisting bacterial or fungal infection was 78.13%, as reported in one study (He et al., 2014) with 32 patients.
Eleven studies provided data on the time to CMV viremia resolution, and the median time was 14.5 days. The median time to refractory CMV viremia resolution, reported in three studies, was 20.5 days (Figure 5).
Pre-emptive therapy was reported in 24 studies with 5,239 patients. Results indicated that ganciclovir was the most commonly used drug (42.19%), followed by foscarnet (20.54%) (Figure 6). For prophylaxis therapy, we identified the utilization of ganciclovir, ganciclovir in combination with acyclovir, foscarnet, foscarnet in combination with acyclovir, and letermovir.
We identified six studies that reported treatment-related adverse events associated with the use of cidofovir, foscarnet, ganciclovir, a combination of foscarnet and acyclovir, and a combination of ganciclovir and acyclovir. According to three studies, 34.90–41.20% of patients treated with ganciclovir experienced myelosuppression. Specifically, one study (He et al., 2014) reported that 34.90% patients experienced myelosuppression, one study (Tong et al., 2013) reported that 35.30% patients experienced neutrophilic leukopenia and one study (He et al., 2014) reported that 41.20% patients experienced granulocytopenia (grade III), and one study (He et al., 2014) reported that 11.40% patients experienced impaired kidney function.
The results of the publication bias assessment for other analyses are presented in Supplementary Figures S24–32. Funnel plots indicated no obvious evidence of publication bias.
This review presents a comprehensive summary of the epidemiology, clinical outcomes, and treatment patterns of CMV infection among allo-HSCT recipients in China. Our findings indicate a substantial incidence of CMV infection in Chinese recipients of allo-HSCT (48.87%), which may vary according to the follow-up period, patient's age, transplantation type, and donor and recipient's CMV serologic status.
The incidence rates of CMV infection after allo-HSCT vary globally, ranging from 24.6 to 62.6% in North America, 28.9 to 63.9% in Europe, and 24.9 to 61.2% in other countries (Bergamasco et al., 2021; Cho et al., 2023). Our review found that the pooled incidence of CMV infection in Chinese recipients aligns with global trends and is at the higher end of values. This may be attributed to the presence of high risk factors in China, including the considerable prevalence of haplo-HSCT (60.1%) (Xu et al., 2021) and the high proportion of R+ individuals indicated by the high general incidence of CMV infection (>90%) in the Chinese population (Stem Cell Application Group, Chinese Society of Hematology, Chinese Medical Association, 2022). Consistent with the findings of the 2022 Chinese consensus (Stem Cell Application Group, Chinese Society of Hematology, Chinese Medical Association, 2022) and published evidence in Europe (Huntley et al., 2020; Solano et al., 2021) and North America (Huang et al., 2019; Webb et al., 2018) regarding risk factors of CMV infection after allo-HSCT, our review found that D–/R+ serostatus (57.64%), haplo-HSCT (69.08%) and unrelated-matched HSCT (70.82%) exhibited higher incidence rates than other serostatus conditions and transplant types. This underscores the necessity for tailored and culturally sensitive strategies to effectively mitigate risks associated with CMV. Notably, the high seroprevalence of CMV infection in China indicates that most patients have been exposed to the virus. Therefore, even with antiviral prophylaxis, reactivation of the virus may still occur, leading to breakthrough infections. Our results show that the rate of breakthrough infections is 7.29%. This highlights the need for regular monitoring of patients during prophylaxis, allowing for timely pre-emptive treatment upon detection of CMV reactivation to prevent progression to CMV disease or other complications.
In addition, the pooled incidence of CMV disease among recipients with CMV infection in China of 13.40% (95% CI 9.14–19.65%) is consistent with the estimates from other countries (2.9–15.7%) (Cho et al., 2023). In contrast to previous research findings, according to which CMV pneumonitis is the most frequent and common cause of death (Ljungman et al., 2019), our results indicate that the incidence of CMV pneumonitis (5.96%) was lower than that of CMV cystitis (13.48%) and CMV enteritis (6.62%). This unexpected result may stem from methodological variations across the included studies, which may indicate differences in study quality, diagnostic criteria and immunosuppressive treatment regimens across the included studies.
Moreover, our review found that 32.03% of recipients with CMV infection experienced refractory CMV, suggesting that some patients have inadequate responses to conventional treatment (Chemaly et al., 2019). Given that refractory CMV infection is recognized as an independent risk factor for CMV diseases and non-relapse mortality (Liu et al., 2015), concurrently increasing the risk of developing GVHD (Nho et al., 2023), the relatively high incidence may emphasize the need for heightened clinical attention after allo-HSCT. The high refractory CMV infections may be influenced by a variety of factors that can be broadly categorized into patient-related factors (e.g., immune status, serological status, transplant type), virus-related factors (e.g., High viral load), and drug-related factors (e.g., antiviral resistance, drug exposure). These factors interact in complex ways, making the management of refractory CMV infections particularly challenging. Our analysis of the included studies indicates that it is challenging to distinguish CMV infection rates due to the heterogeneity of the studies and the lack of detailed information on the specific drugs and regimens used. Thus, further large-scale prospective studies are warranted. Our review also obtained a rate of 15.42% for CMV recurrence after allo-HSCT among recipients with CMV infection. The refractory and/or recurrent CMV infection may be attributed to factors including mismatched donor transplants, T-cell depletion and CMV-seropositive recipients (Gagelmann et al., 2018; Liu et al., 2015; Almyroudis et al., 2007). This information indicates to clinicians the ongoing risk of refractory and/or recurrence CMV for recipients with CMV infection after allo-HSCT. Furthermore, the absence of resistance data suggests a potential lack of emphasis on resistance in clinical practice. Limited access to resistance detection methods may lead to an underestimation of the resistance incidence. It is crucial to accumulate more resistance data to enhance understanding of CMV resistance and to guide individualized treatment strategies.
The findings of this review indicate that ganciclovir, valganciclovir and foscarnet currently remain the most commonly used drugs in both prophylaxis and pre-emptive therapy in China. Despite their widespread use, these medications are associated with treatment-limiting toxicity. Our results reveal that a notable proportion of patients treated with ganciclovir and foscarnet experience myelosuppression (34.90–41.20%) and kidney function impairment (11.4%). The treatment related myelosuppression can directly impact the generation of cytotoxic T lymphocytes (CTL), leading to a delayed immune reconstitution associated with CTL (Martín-Gandul et al., 2014). Additionally, neutropenia may increase the risk of opportunistic infections and bleeding (Qi et al., 2022; Scott et al., 2008). Furthermore, foscarnet-induced nephrotoxicity can impact drug absorption and metabolism, potentially leading to conditions such as acute kidney injury and even uraemia in severe cases (Inose et al., 2022). The findings on treatment patterns highlight the unmet medical need in China for developing more effective and well-tolerated alternatives to fill the existing treatment gaps of CMV infection. With the availability of the novel drug letermovir, the treatment pattern in China is shifting from pre-emptive therapy toward prophylaxis therapy, although data on its efficacy and safety in the Chinese population remains limited (Stem Cell Application Group, Chinese Society of Hematology, Chinese Medical Association, 2022). A recent study published in 2023 supports the potential benefits of letermovir in reducing the incidence of CMV infection after haplo-HSCT without increasing the risks of aGVHD, non-relapse mortality and myelosuppression (Ma et al., 2023a). However, it is noteworthy that 17.6% of patients in the 2023 study still experienced CMV reactivation after discontinuation of letermovir, which may due to letermovir postponing CMV-specific immune reconstitution (Gabanti et al., 2022). It is worth mentioning that the recent approval of maribavir in China may offer a significant advancement in the treatment of post-transplant refractory CMV infections or diseases (PR Newswire, 2023). In the phase 3 RCT, maribavir exhibited greater efficacy than valganciclovir/ganciclovir, foscarnet, or cidofovir in CMV viremia resolution (55.7 vs. 23.9%) (Avery et al., 2022). This was coupled with a lower incidence of treatment-related acute kidney injury compared to foscarnet (1.7 vs. 19.1%) and reduced treatment-related neutropenia compared to valganciclovir/ganciclovir (1.7% vs. 25%) (Avery et al., 2022). The favorable efficacy and reduced treatment-related adverse events have the potential to address the current unmet treatment needs in this context (2023). Additionally, the development of CMV vaccines is progressing, with an mRNA-based vaccine demonstrating strong immune responses in laboratory studies (Fierro et al., 2024). A second-generation CMV vaccine candidate, T10-F10 (Yll-Pico et al., 2024), developed using a synthetic poxvirus platform, has shown high stability and immunogenicity in preclinical studies. CMV-specific T-cell therapies are also being explored as alternative strategies (Tang et al., 2024), with recent studies highlighting their potential in managing CMV infections. Future research should focus on optimizing these existing strategies and exploring new avenues.
Our study has several limitations. Despite efforts to include studies without restrictions on publication status, publication bias may still exist, as the included studies were identified using specific keywords in bibliographical databases. The exclusion of non-English and non-Chinese articles may lead to bias. This practice, known as language bias, can limit the generalizability of the findings and potentially skew the results by omitting key data from studies published in other languages. Moreover, the substantial heterogeneity observed across outcomes underscores the need for more homogeneous study designs and reporting standards in future research. Investigating the sources of this heterogeneity is essential to better understand the factors driving variations in CMV infection rates after allo-HSCT. These rates vary widely due to patient characteristics (e.g., CMV serostatus, age, underlying diseases), transplant type, immunosuppressive regimens, monitoring methods, geographic and demographic differences, post-transplant complications (e.g., GVHD), and variations in healthcare practices. The lack of a unified threshold for pre-emptive CMV treatment further contributes to this variability. Addressing these issues is critical to improving clinical management and advancing research in this field. In addition, we conducted a proportional meta-analysis, which restricted our ability to investigate the association between subgroup factors and the incidence of CMV infection. Future research could explore these potential confounders more comprehensively to provide a more detailed understanding of their impact on outcomes. Proportional meta-analysis synthesizes proportions from multiple studies into a pooled estimate, focusing on binary outcomes like CMV infection rates (Barker et al., 2021). However, this method limits the exploration of how subgroup factors, such as patient demographics, transplant characteristics, or treatment protocols, influence CMV infection rates. Therefore, further studies are warranted to investigate whether these subgroup factors contribute to the risk of CMV infection in recipients following allo-HSCT.
This review consolidates evidence on the epidemiology, clinical outcomes, and treatment patterns in recipients following allo-HSCT in China. Our results highlight a notable incidence of CMV infection in this population, with a portion of patients developing refractory CMV infection. Current anti-CMV therapies are limited and often associated with treatment-limiting toxicities, emphasizing the need for more effective and better-tolerated treatment options. Furthermore, the development of an effective CMV vaccine could further enhance the prevention and management of CMV infections in this high-risk population.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
RL: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing. JW: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. QL: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Writing – original draft.
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by Takeda (China) International Trading Co., Ltd.
Scientific review of the manuscript was provided by Takeda Pharmaceutical Company Limited. Ms. Yang Zhang, Ms. Wenjie Zhang, and Dr. Sitong Dong from Shanghai Daotian Evidence-Based Technology Co., Ltd. provided assistance with the statistical methodology and analysis, and were funded by Takeda (China) International Trading Co., Ltd.
JW is an employee of Takeda (China) International Trading Co., Ltd. RL has received speaker/consultancy fees and research fund from Takeda. QL has received speaker/consultancy fees and research fund from Takeda.
The author(s) declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1518275/full#supplementary-material
Almyroudis, N. G., Jakubowski, A., Jaffe, D., Sepkowitz, K., Pamer, E., O'Reilly, R. J., et al. (2007). Predictors for persistent cytomegalovirus reactivation after T-cell-depleted allogeneic hematopoietic stem cell transplantation. Transpl. Infect. Dis. 9, 286–294. doi: 10.1111/j.1399-3062.2007.00235.x
Anderson-Smits, C., Baker, E. R., and Hirji, I. (2020). Coinfection rates and clinical outcome data for cytomegalovirus and Epstein-Barr virus in post-transplant patients: a systematic review of the literature. Transpl. Infect. Dis. 22:e13396. doi: 10.1111/tid.13396
Avery, R. K., Alain, S., Alexander, B. D., Blumberg, E. A., Chemaly, R. F., Cordonnier, C., et al. (2022). Maribavir for refractory cytomegalovirus infections with or without resistance post-transplant: results from a phase 3 randomized clinical trial. Clin. Infect. Dis. 75, 690–701. doi: 10.1093/cid/ciab988
Bao, X., Zhu, Q., Xue, S., Xu, Y., Ma, X., Chen, F., et al. (2016). Risk factors of clinically refractory CMV reactivation following allogeneic HSCT: a single-center study in China. Bone Marrow Transpl. 51, 1625–1627. doi: 10.1038/bmt.2016.231
Barker, T. H., Migliavaca, C. B., Stein, C., Colpani, V., Falavigna, M., Aromataris, E., et al. (2021). Conducting proportional meta-analysis in different types of systematic reviews: a guide for synthesisers of evidence. BMC Med. Res. Methodol. 21:189. doi: 10.1186/s12874-021-01381-z
Barker, T. H., Stone, J. C., Sears, K., Klugar, M., Tufanaru, C., Leonardi, -B. E. E., et al. (2023). The revised JBI critical appraisal tool for the assessment of risk of bias for randomized controlled trials. JBI Evid. Synth. 21, 494–506. doi: 10.11124/JBIES-22-00430
Bergamasco, A., Goyer, C., Arredondo-Bisono, T., Sen, R., Hirji, I., and Moride, Y. (2021). Epidemiology of resistant and refractory cytomegalovirus infection following solid organ or haematopoietic stem cell transplant: a systematic review. Bone Marrow Transpl. 56, 263–264. Available online at: https://www.webofscience.com/wos/woscc/full-record/WOS:000668928500249
Boeckh, M., and Geballe, A. P. (2011). Cytomegalovirus: pathogen, paradigm, and puzzle. J. Clin. Invest. 121, 1673–1680. doi: 10.1172/JCI45449
Boeckh, M., Nichols, W. G., Chemaly, R. F., Papanicolaou, G. A., Wingard, J. R., Xie, H., et al. (2015). Valganciclovir for the prevention of complications of late cytomegalovirus infection after allogeneic hematopoietic cell transplantation: a randomized trial. Ann. Intern. Med. 162, 1–10. doi: 10.7326/M13-2729
Camargo, J. F., and Komanduri, K. V. (2017). Emerging concepts in cytomegalovirus infection following hematopoietic stem cell transplantation. Hematol. Oncol. Stem Cell Ther. 10, 233–238. doi: 10.1016/j.hemonc.2017.05.001
Cantoni, N., Hirsch, H. H., Khanna, N., Gerull, S., Buser, A., Bucher, C., et al. (2010). Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol. Blood Marrow Transplant. 16, 1309–1314. doi: 10.1016/j.bbmt.2010.03.020
Cao, W. J., Wan, D. M., Li, L., Wang, C., Zhang, S., Liu, C. F., et al. (2016a). Clinical study of cytomegalovirus infection and preemptive therapy after allogenic hematopoie tic stem cell transplantation. J. Exp. Hematol. 24, 1143–1148.
Cao, W. J., Wan, D. M., Liu, C. F., Wang, C., and Zhang, S. P. (2016b). Clinical study of cytomegalovirus infection and preemptive therapy after haploidentical peripheral blood hematopoietic stem cell transplantation. Clin. Focus 31, 1002–1005. doi: 10.7534/j.issn.1009-2137.2016.04.034
Chemaly, R. F., Chou, S., Einsele, H., Griffiths, P., Avery, R., Razonable, R. R., et al. (2019). Definitions of resistant and refractory cytomegalovirus infection and disease in transplant recipients for use in clinical trials. Clin. Infect Dis. 68, 1420–1426. doi: 10.1093/cid/ciy696
Chen, J., Pang, A., Zhao, Y., Liu, L., Ma, R., Wei, J., et al. (2022). Risk factors for CMV infection within 100 days posttransplantation in patients with acute leukemia. Blood Sci. 4, 164–169. doi: 10.1097/BS9.0000000000000121
Chen, K., Cheng, M. P., Hammond, S. P., Einsele, H., and Marty, F. M. (2018). Antiviral prophylaxis for cytomegalovirus infection in allogeneic hematopoietic cell transplantation. Blood Adv. 2, 2159–2175. doi: 10.1182/bloodadvances.2018016493
Cheng, C. N., Li, S. S., Yeh, Y. H., Shen, C. F., and Chen, J. S. (2022). Letermovir prophylaxis for cytomegalovirus reactivation in children who underwent hematopoietic stem cell transplantation: a single-institute experience in Taiwan. J. Microbiol. Immunol. Infect. 55, 323–327. doi: 10.1016/j.jmii.2022.01.002
Cho, S. Y., Ar, M. C., Machado, C. M., Wu, D., Singh, I., Sandhu, A., et al. (2023). Epidemiology, treatment patterns, and disease burden of cytomegalovirus in hematopoietic cell transplant recipients in selected countries outside of Europe and North America: a systematic review. Transpl. Infect Dis. 25:e14083. doi: 10.1111/tid.14083
Cho, S. Y., Lee, D. G., and Kim, H. J. (2019). Cytomegalovirus infections after hematopoietic stem cell transplantation: current status and future immunotherapy. Int. J. Mol. Sci. 20:2666. doi: 10.3390/ijms20112666
De Clercq, E., and Li, G. (2016). Approved antiviral drugs over the past 50 years. Clin. Microbiol. Rev. 29, 695–747. doi: 10.1128/CMR.00102-15
Ding, Y., Ru, Y., Song, T., Guo, L., Zhang, X., Zhu, J., et al. (2021). Epstein-Barr virus and cytomegalovirus reactivation after allogeneic hematopoietic cell transplantation in patients with non-Hodgkin lymphoma: the prevalence and impacts on outcomes: EBV and CMV reactivation post allo-HCT in NHL. Ann. Hematol. 100, 2773–2785. doi: 10.1007/s00277-021-04642-5
Dziedzic, M., Sadowska-Krawczenko, I., and Styczynski, J. (2017). Risk factors for cytomegalovirus infection after allogeneic hematopoietic cell transplantation in malignancies: proposal for classification. Anticancer Res. 37, 6551–6556. doi: 10.21873/anticanres.12111
Fan, Z. Y., Han, T. T., Zuo, W., Zhao, X. S., Chang, Y. J., Lv, M., et al. (2022). CMV infection combined with acute GVHD associated with poor CD8+ T-cell immune reconstitution and poor prognosis post-HLA-matched allo-HSCT. Clin. Exp. Immunol. 208, 332–339. doi: 10.1093/cei/uxac047
Fierro, C., Brune, D., Shaw, M., Schwartz, H., Knightly, C., Lin, J., et al. (2024). Safety and immunogenicity of a messenger RNA-based cytomegalovirus vaccine in healthy adults: results from a phase 1 randomized clinical trial. J. Infect Dis. 230, e668–e678. doi: 10.1093/infdis/jiae114
Gabanti, E., Borsani, O., Colombo, A. A., Zavaglio, F., Binaschi, L., Caldera, D., et al. (2022). Human cytomegalovirus-specific T-cell reconstitution and late-onset cytomegalovirus infection in hematopoietic stem cell transplantation recipients following letermovir prophylaxis. Transpl. Cell. Ther. 28, 211.e1–211.e9. doi: 10.1016/j.jtct.2022.01.008
Gagelmann, N., Ljungman, P., Styczynski, J., and Kröger, N. (2018). Comparative efficacy and safety of different antiviral agents for cytomegalovirus prophylaxis in allogeneic hematopoietic cell transplantation: a systematic review and meta-analysis. Biol. Blood Marrow Transplant. 24, 2101–2109. doi: 10.1016/j.bbmt.2018.05.017
Gao, S., Sun, Z. M., Zhang, M., Liu, H. L., Geng, L. Q., Tong, J., et al. (2013). Clinical characteristics of patients w ith infectious complications following umbilical cord blood transpiantation. Int. J. Blood Transfus. Hematol. 36, 193–197. doi: 10.3760/cma.j.issn.1673-419X.2013.03.001
George, B., Pati, N., Gilroy, N., Ratnamohan, M., Huang, G., Kerridge, I., et al. (2010). Pre-transplant cytomegalovirus (CMV) serostatus remains the most important determinant of CMV reactivation after allogeneic hematopoietic stem cell transplantation in the era of surveillance and preemptive therapy. Transpl. Infect Dis. 12, 322–329. doi: 10.1111/j.1399-3062.2010.00504.x
Giménez, E., Torres, I., Albert, E., Piñana, J.-L., Hernández-Boluda, J.-C., Solano, C., et al. (2019). Cytomegalovirus (CMV) infection and risk of mortality in allogeneic hematopoietic stem cell transplantation (Allo-HSCT): a systematic review, meta-analysis, and meta-regression analysis. Am. J. Transpl. 19, 2479–2494. doi: 10.1111/ajt.15515
Grigoleit, G. U., Kapp, M., and Einsele, H. J. J.-I. E. O. C. I. (2009). Indirect effects of cytomegalovirus infection. Infect. Immun. 41–44. doi: 10.17925/EOH.2009.03.1.41
Guo, Z., Chen, H. R., Yang, K., Liu, X. D., Lou, J. X., He, X. P., et al. (2016). Clinical analysis of cytomegalovirus infections after haplotype allogeneic hematopoietic stem celltransplantation. Int. J. Virol. 23, 164–167. doi: 10.3760/cma.j.issn.1673-4092.2016.03.006
Han, A. Z., Zhang, T., Wang, H. J., Xia, R. X., Wang, H. J., and Zhu, H. B. (2014). Clinical analysis of cytomegalovirus infection after allogeneic hematopoietic stem cell transplantation. Chin. J. Nosocomiol. 24, 3471–3473.
He, J., Chen, B. A., Ni, M., Wu, X., and Ding, J. H. (2014). Clinical study of cytomegalovirus infection after allogeneic hematopoietic stem cell transplantation. J. Exp. Hematol. 22, 1371–1376. doi: 10.1182/blood.V124.21.5864.5864
Huang, C. W., Qu, Y. H., Nie, S. S., Liu, S., Ding, W. J., and Jiang, H. (2022). Prognostic analysis of CMV infection after allogeneic hematopoietic stem cell transplantation in children with β Thalassemia major. China Pediatr. Blood Cancer 27, 299–304.
Huang, J. J., Lu, X. Q., Yan, C. H., Zhao, X. S., Xu, L. P., Huang, X. J., et al. (2013). Comparative study on clinical features of cytomegalovirus infection after allogenic hematopoietic stem cell transplantation from HLA haploidentical related donors vs HLA-matched sibling donors. Chin. J. Organ Transplant. 34, 87–91. doi: 10.3760/cma.j.issn.0254-1785.2013.02.006
Huang, Y. T., Su, Y., Kim, S. J., Nichols, P., Burack, D., Maloy, M., et al. (2019). Cytomegalovirus infection in allogeneic hematopoietic cell transplantation managed by the preemptive approach: estimating the impact on healthcare resource utilization and outcomes. Biol. Blood Marrow Transplant. 25, 791–799. doi: 10.1016/j.bbmt.2018.11.012
Huntley, D., Giménez, E., Pascual, M. J., Hernández-Boluda, J. C., Gago, B., Vázquez, L., et al. (2020). Incidence, features, and outcomes of cytomegalovirus DNAemia in unmanipulated haploidentical allogeneic hematopoietic stem cell transplantation with post-transplantation cyclophosphamide. Transpl. Infect. Dis. 22:e13206. doi: 10.1111/tid.13206
Inose, R., Takahashi, K., Takahashi, M., Sugimoto, T., Nanno, S., Hino, M., et al. (2022). Long-term use of foscarnet is associated with an increased incidence of acute kidney injury in hematopoietic stem cell transplant patients: a retrospective observational study. Transpl. Infect Dis. 24:e13804. doi: 10.1111/tid.13804
JBI (2020). Johanna Briggs Institute. Available online at: https://jbi.global/critical-appraisal-tools (accessed November 27, 2023).
Jin, X., Tong, L. W., Xu, X. Y., Wu, H. H., Yuan, F., Jiang, X. D., et al. (2014). Clinical value of the detection of cytomegalovirus DNA in HSCT recipients. J. Mol. Diagn. Ther. 6, 79–82.
Li, G. F., Wang, L. Y., Sun, G. Y., Tang, B. L., Zhu, X. Y., Tong, J., et al. (2015a). Analysis of immune reconstruction and cytomegalovirus infection after non-related cord blood transplantation with myeloablative regimen without antithymocyte globulin. Acta Univ. Med. Anhui 50, 1128–1132. doi: 10.19405/j.cnki.issn1000-1492.2015.08.020
Li, H., Zhang, S. L., Deng, J. C., Zhang, Y., and Lou, S. F. (2015b). Regular monitoring plasma CMV-DNA level and risk factors analyzing after allogenic hematopoietic stem cell transplantation. Chon. Med. J. 4036–4038:4041. doi: 10.3969/j.issn.1671-8348.2015.29.002
Li, P. H., Lin, C. H., Lin, Y. H., Chen, T. C., Hsu, C. Y., and Teng, C. J. (2021). Cytomegalovirus prophylaxis using low-dose valganciclovir in patients with acute leukemia undergoing allogeneic hematopoietic stem-cell transplantation. Ther. Adv. Hematol. 12:2040620721998124. doi: 10.1177/2040620721998124
Li, R., Zhao, L. D., Jiang, M., Xie, L., and Cheng, Q. (2022a). Genotypes and influencing factors for CMV infection after Allo-HSCT. Chin. J. Nosocomiol. 32, 2467–2470.
Li, S., Shen, Z. H., Wan, L. P., Bao, A. H., Yang, J., Tong, Y., et al. (2020). Clinical study of 34 patients with cytomegalovirus pneumonia after allogeneic hematopoietic stem cell transplantation. Chin. J. Hematol. 41, 843–847. doi: 10.3760/cma.j.issn.0253-2727.2020.10.009
Li, S. S., Zhang, N., Jia, M., and Su, M. (2022b). Association between cytomegalovirus and Epstein-Barr virus co-reactivation and hematopoietic stem cell transplantation. Front. Cell. Infect. Microbiol. 12:818167. doi: 10.3389/fcimb.2022.818167
Li, Y., Gao, L., Wang, L. L., Ding, Y., Xu, Y. Y., Li, H. H., et al. (2013a). Surveillance of CMV infection in allo-HSCT recipients and guidance on preemptive therapy by RQ-PCR. Zhongguo Shi Yan Xue Ye Xue Za Zhi 21, 161–168. doi: 10.7534/j.issn.1009-2137.2013.01.033
Li, Y. H., Xu, C., Chen, J. L., Li, B. T., Yu, Z. Y., Wang, J., et al. (2013b). A clinicaIstudy on correlation between cytomegalovirus infection and the graft-versus-host disease occurrence in hematogenic stem cell transplantation receptors. Chin. J. Organ Transplant. 34, 84–86.
Limaye, A. P., Babu, T. M., and Boeckh, M. (2020). Progress and challenges in the prevention, diagnosis, and management of cytomegalovirus infection in transplantation. Clin. Microbiol. Rev. 34:e00043-19. doi: 10.1128/CMR.00043-19
Lin, H. C., Han, S. M., Hwang, W. L., Chou, C. W., Chang, K. H., Shi, Z. Y., et al. (2017). Cytomegalovirus infection and treatment in allogeneic hematopoietic stem cell transplantation: a retrospective study from a single institution in an endemic area. Turk. J. Haematol. 34, 159–166. doi: 10.4274/tjh.2016.0225
Liu, J., Kong, J., Chang, Y. J., Chen, H., Chen, Y. H., Han, W., et al. (2015). Patients with refractory cytomegalovirus (CMV) infection following allogeneic haematopoietic stem cell transplantation are at high risk for CMV disease and non-relapse mortality. Clin. Microbiol. Infect. 21, 1121.e9–1121.e15. doi: 10.1016/j.cmi.2015.06.009
Ljungman, P., Boeckh, M., Hirsch, H. H., Josephson, F., Lundgren, J., Nichols, G., et al. (2017). Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin. Infect Dis. 64, 87–91. doi: 10.1093/cid/ciw668
Ljungman, P., De La Camara, R., Robin, C., Crocchiolo, R., Einsele, H., Hill, J. A., et al. (2019). Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect. Dis. 19, e260–e272. doi: 10.1016/S1473-3099(19)30107-0
Ma, R., He, Y., Wang, H. F., Bai, L., Han, W., Cheng, Y. F., et al. (2023a). Clinical analysis of the usefulness of letermovir for prevention of cytomegalovirus infection after haploidentical hematopoietic stem cell transplantation Zhonghua nei ke za zhi 62, 826–832. doi: 10.3760/cma.j.cn112138-20221204-00904
Ma, R., He, Y., Xu, L. P., Zhang, X. H., Wang, Y., Liu, K. Y., et al. (2023b). Clinical analysis of the efficacies of ganciclovir plus foscarnet and a single antiviral drug for the treatment of cytomegalovirus infection after haploidentical stem cell transplantation. Chin. J. Intern. Med. 62, 76–83. doi: 10.3760/cma.j.cn112138-20220118-00058
Martín-Gandul, C., Pérez-Romero, P., González-Roncero, F. M., Berdaguer, S., Gómez, M. A., Lage, E., et al. (2014). Clinical impact of neutropenia related with the preemptive therapy of CMV infection in solid organ transplant recipients. J. Infect. 69, 500–506. doi: 10.1016/j.jinf.2014.07.001
Marty, F. M., Winston, D. J., Chemaly, R. F., Mullane, K. M., Shore, T. B., Papanicolaou, G. A., et al. (2019). A randomized, double-blind, placebo-controlled phase 3 trial of oral brincidofovir for cytomegalovirus prophylaxis in allogeneic hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 25, 369–381. doi: 10.1016/j.bbmt.2018.09.038
Meng, X. Y., Fu, H. X., Zhu, X. L., Wang, J. Z., Liu, X., Yan, C. H., et al. (2020). Comparison of different cytomegalovirus diseases following haploidentical hematopoietic stem cell transplantation. Ann. Hematol. 99, 2659–2670. doi: 10.1007/s00277-020-04201-4
Mori, T., and Kato, J. (2010). Cytomegalovirus infection/disease after hematopoietic stem cell transplantation. Int. J. Hematol. 91, 588–595. doi: 10.1007/s12185-010-0569-x
Nho, D., Lee, R., Cho, S. Y., Lee, D. G., Kim, E. J., Park, S., et al. (2023). Cytomegalovirus infection after allogeneic hematopoietic cell transplantation under 100-day letermovir prophylaxis: a real-world 1-year follow-up study. Viruses 15:1884. doi: 10.3390/v15091884
Nichols, W. G., Corey, L., Gooley, T., Davis, C., and Boeckh, M. (2002). High risk of death due to bacterial and fungal infection among cytomegalovirus (CMV)-seronegative recipients of stem cell transplants from seropositive donors: evidence for indirect effects of primary CMV infection. J. Infect. Dis. 185, 273–282. doi: 10.1086/338624
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 372:n71. doi: 10.1136/bmj.n71
Paris, C., Kopp, K., King, A., Santolaya, M. E., Zepeda, A. J., and Palma, J. (2009). Cytomegalovirus infection in children undergoing hematopoietic stem cell transplantation in Chile. Pediatr. Blood Cancer 53, 453–458. doi: 10.1002/pbc.22060
Pei, X. Y., Zhao, X. Y., Liu, X. F., Mo, X. D., Lv, M., Xu, L. P., et al. (2022). Adoptive therapy with cytomegalovirus-specific T cells for cytomegalovirus infection after haploidentical stem cell transplantation and factors affecting efficacy. Am. J. Hematol. 97, 762–769. doi: 10.1002/ajh.26535
PR Newswire (2023). Takeda's Innovative Antiviral Drug, Maribavir Tablets, has Officially Obtained Approval in China. PR Newswire. Available online at: https://www.prnasia.com/story/431806-1.shtml (accessed January 17, 2024).
Qi, J. Q., You, T., Wang, H., Han, W., Fan, Y., Chen, J., et al. (2022). Prognostic analysis and predictive model construction of bleeding events in allogeneic hematopoietic stem cell transplant patients. Zhonghua Xue Ye Xue Za Zhi 43, 481–487. doi: 10.3760/cma.j.issn.0253-2727.2022.06.007
Que, M., Xiao, J. W., Guan, X. M., Xian, Y., Su, Y. C., Wen, X. H., et al. (2014). Clinicalstudy on cytomegalovirus infection after hematopoietic stem cell transplantation in 26 patients with primary immunodeficiency diseases. Chin. J. Hematol. 35, 424–427. doi: 10.3760/cma.j.issn.0253-2727.2014.05.010
Scott, B. L., Park, J. Y., Deeg, H. J., Marr, K. A., Boeckh, M., Chauncey, T. R., et al. (2008). Pretransplant neutropenia is associated with poor-risk cytogenetic features and increased infection-related mortality in patients with myelodysplastic syndromes. Biol. Blood Marrow Transpl. 14, 799–806. doi: 10.1016/j.bbmt.2008.04.011
Shi, H. Y., Cheng, J. F., Huang, X. J., Wang, Y., Suan, P., Xu, L. P., et al. (2019). Clinical analysis of cytomegalovirus infection after haplotype hematopoietic stem cell transplantation in children. Chin. J. Hematol. 40, 426–428. doi: 10.3760/cma.j.issn.0253-2727.2019.05.015
Si, Y. J., Qin, M. Q., Zhang, C. C., Du, Z. L., Zhang, X. M., and Hu, B. (2013). Clinical analysis of cytomegalovirus infection after allogeneic hematopoietic stem cell transplantation in children. Chin. J. Postgrad. Med. 36, 275–275.
Solano, C., Muñoz-Cobo, B., Giménez, E., Remigia, M. J., Amat, P., Clari, M. A., et al. (2013). Pre-emptive antiviral therapy for active CMV infection in adult allo-SCT patients guided by plasma CMV DNAemia quantitation using a real-time PCR assay: clinical experience at a single center. Bone Marrow Transpl. 48, 1010–1012. doi: 10.1038/bmt.2012.286
Solano, C., Vázquez, L., Giménez, E., De La Cámara, R., Albert, E., Rovira, M., et al. (2021). Cytomegalovirus DNAemia and risk of mortality in allogeneic hematopoietic stem cell transplantation: analysis from the Spanish Hematopoietic Transplantation and Cell Therapy Group. Am. J. Transplant. 21, 258–271. doi: 10.1111/ajt.16147
Stem Cell Application Group Chinese Society of Hematology, Chinese Medical Association. (2022). The Chinese consensus on the management of cytomegalovirus infection in allogeneic hematopoietic stem cell transplantation patients (2022). Zhonghua Xue Ye Xue Za Zhi 43, 617–623. doi: 10.3760/cma.j.issn.0253-2727.2022.08.001
Tan, X., Zhang, X., Gao, L., Gao, L., Liu, Y., Kong, P. Y., et al. (2020). A single center, prospective randomized controlled study of ganciclovir and foscarnet in the prevention of cytomegalovirus infection after allogeneic hematopoietic stem cell transplantation. J. Clin. Hematol. 33, 618–624. doi: 10.13201/j.issn.1004-2806.2020.09.007
Tang, J., Dou, L., and Liu, D. (2024). CMV-specific TCR-T cells for the treatment of CMV reactivation after allogeneic hematopoietic stem-cell transplantation. Blood 144, 3462–3462. doi: 10.1182/blood-2024-204804
Teira, P., Battiwalla, M., Ramanathan, M., Barrett, A. J., Ahn, K. W., Chen, M., et al. (2016). Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood 127, 2427–2438. doi: 10.1182/blood-2015-11-679639
Tong, J., Sun, Z., Liu, H., Geng, L., Zheng, C., Tang, B., et al. (2013). Risk factors of CMV infection in patients after umbilical cord blood transplantation: a multicenter study in China. Chin. J. Cancer Res. 25, 695–703. doi: 10.3978/j.issn.1000-9604.2013.11.08
Tricco, A. C., Lillie, E., Zarin, W., O'Brien, K. K., Colquhoun, H., Levac, D., et al. (2018). PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann. Intern. Med. 169, 467–473. doi: 10.7326/M18-0850
Wan, L. L., Chen, J. L., Xu, C., Li, B. T., Hu, J. W., Lou, X., et al. (2017). Clinical study of virus infection after Allo-HSCT in patients with severe aplastic anemia. J. Clin. Hematol. 30, 43–46. doi: 10.13201/j.issn.1004-2806.2017.01.011
Wang, C. L., Li, C. F., He, Y. L., Peng, Z. Y., Liao, J. Y., Li, Q., et al. (2017). Current situation of cytomegalovirus infection after allogeneic stem cell transplantation under prevention and preemptive treatment. Guang. Med. J. 38, 94–96. doi: 10.13820/j.cnki.gdyx.2017.s2.038
Wang, J., Yao, J. L., Sun, Y., Liu, Z. Y., Xiao, J., and Zu, Y. (2019). Clinical characteristics of cytomegalovirus retilitis after pediatric allogeneic bone marrow hematopoietic stem cell transplantation. OphthalmoI CHN 28, 260–264. doi: 10.13281/j.cnki.issn.1004-4469.2019.04.005
Wang, L. L., Mo, W. J., Zhang, Y. P., Chen, X. W., Wang, C. X., Zhou, M., et al. (2021). Clinical analysis of CMV infection after allogeneic hematopoietic stem cell transplantation in severe aplastic anemia. J. Exp. Hematol. 29, 944–950. doi: 10.19746/j.cnki.issn.1009-2137.2021.03.046
Wang, S., Liu, D. B., Zheng, X. L., Ding, L., Han, D. M., Wang, Z. D., et al. (2015). Clinical analysis of cytomegalovirus infection after different patterns of hematopoietic stem cell transplantation. J. Exp. Hematol. 23, 1438–1444. doi: 10.7534/j.issn.1009-2137.2015.05.041
Wang, X. N., Zhang, M., He, P. C., Liu, X., Diao, J. Y., Chen, L. M., et al. (2013). Clinical study of cytomegalovirus latent infection in allogeneic peripheral blood stem cell transplantation. J. Clin. Hematol. 26, 298–300+304. doi: 10.13201/j.issn.1004-2806.2013.03.003
Webb, B. J., Harrington, R., Schwartz, J., Kammerer, J., Spalding, J., Lee, E., et al. (2018). The clinical and economic impact of cytomegalovirus infection in recipients of hematopoietic stem cell transplantation. Transpl. Infect Dis. 20:e12961. doi: 10.1111/tid.12961
Wei, Z. L., Qian, X. W., Wang, P., Jiang, W. J., Wang, H. S., Shen, C., et al. (2022). Analysis of risk factors and prognosis of cytomegalovirus infection post umbilical cord blood stem cell transplantation in children with primary immunodeficiency diseases. Chin. J. Pediatr. 60, 1019–1025. doi: 10.3760/cma.j.cn112140-20220501-00403
Wu, J., Zheng, Y. W., Huang, G., Liu, S. N., Luo, L. P., and Hou, T. Y. (2019). Clinical characteristics of human cytomegalovirus and polyomavirus infection after allogeneic hematopoietic stem cell transplantation. Chin. J. Infect. Control 18, 132–137.
Wu, Y. M., Cao, Y. B., Li, X. H., Xu, L. X., Yan, B., Li, S. W., et al. (2017). Clinical study on foscarnet prophylaxis and pre-emptive therapy for cytomegalovirus infection in hematopoietic stem cell transplantation. J. Leukem. Lymph. 26, 331–335.
Xiong, Y. Y., Liu, L., Chen, J. B., Tang, X. Q., Xiao, Q., Zhang, H. B., et al. (2023). Clinical study of cytomegalovirus infection after allogeneic hematopoietic stem cell transplantation. J. Exp. Hematol. 31, 513–521. doi: 10.19746/j.cnki.issn1009-2137.2023.02.030
Xu, L. P., Lu, P. H., Wu, D. P., Sun, Z. M., Liu, Q. F., Han, M. Z., et al. (2021). Hematopoietic stem cell transplantation activity in China 2019: a report from the Chinese Blood and Marrow Transplantation Registry Group. Bone Marrow Transpl. 56, 2940–2947. doi: 10.1038/s41409-021-01431-6
Xu, Z. L., Huang, X. J., Sun, Y. Q., Wang, F. R., Yan, C. H., Zhang, X. H., et al. (2015). Cytomegalovirus specific cytotoxic T lymphocytes for treatment of refractory cytomegalovirus infection in patients following allogeneic hematopoietic stem cell transplantation. Chin. J. Intem. Med. 54, 101–105. doi: 10.3760/cma.j.issn.0578-1426.2015.02.004
Xue, H., Feng, S. Q., Hu, Y. C., Liu, Z. B., Li, X. Y., and Gao, F. (2019a). Stratification therapy for cytomegalovirus infection after allogeneic hematopoietic stem cell transplantation. Chin. J. Tissue Eng. Res. 23, 756–760. doi: 10.3969/j.issn.2095-4344.1561
Xue, H., Hu, Y. C., Feng, S. Q., Liu, Z. B., and Gao, F. (2019b). Risk factor analysis for cytomegalovirus infection after peripheral blood allogeneic hematopoietic stem cell transplantation. J. China Med. Univ. 48, 417–420.
Yan, C. H., Wang, Y., Mo, X. D., Sun, Y. Q., Wang, F. R., Fu, H. X., et al. (2020). Incidence, risk factors, and outcomes of cytomegalovirus retinitis after haploidentical hematopoietic stem cell transplantation. Bone Marrow Transpl. 55, 1147–1160. doi: 10.1038/s41409-020-0790-z
Yang, C., Li, S., Huang, T., Lin, H., Jiang, Z., He, Y., et al. (2022). Effectiveness and safety of vonoprazan-based regimen for Helicobacter pylori eradication: a meta-analysis of randomized clinical trials. J. Clin. Pharm. Therap. 47, 897–904. doi: 10.1111/jcpt.13637
Yeh, T. J., Yang, C. I., Huang, C. T., Wang, M. H., Chuang, T. M., Ke, Y. L., et al. (2022). Revisit of the association between cytomegalovirus infection and invasive fungal infection after allogeneic hematopoietic stem cell transplantation: a real-world analysis from a high CMV seroprevalence area. J. Fungi 8:408. doi: 10.3390/jof8040408
Yin, Z., Sun, J., Yang, Y., Xu, N., Jiang, L., Fan, Z., et al. (2022). Cidofovir, a choice for salvage treatment of cytomegalovirus infection in patients with haploidentical hematopoietic stem cell transplantation. Transpl. Infect Dis. 24:e13776. doi: 10.1111/tid.13776
Yin, Z., Yu, G. P., Xu, N., Jiang, L., Huang, F., Fan, Z. P., et al. (2020). Clinical observation of cidofovir in salvage therapy for cytomegalovirus infection in patients with haploid hematopoietic stem cell transplantation. Chin. J. Hematol. 41, 326–330. doi: 10.3760/cma.j.issn.0253-2727.2020.04.013
Yll-Pico, M., Park, Y., Martinez, J., Iniguez, A., Kha, M., Kim, T., et al. (2024). Highly stable and immunogenic CMV T cell vaccine candidate developed using a synthetic MVA platform. NPJ Vaccines 9:68. doi: 10.1038/s41541-024-00859-3
Zhang, P., Yang, D., Tian, J., Feng, S., Jiang, E., and Han, M. (2021). A clinical study of lyophilized intravenous human immunoglobulin containing high-titer cytomegalovirus-neutralizing antibody for the treatment of cytomegalovirus viremia after allogeneic hematopoietic stem cell transplantation. Ann. Palliat. Med. 10, 5533–5540. doi: 10.21037/apm-21-1069
Zhang, X., Gao, L., Zhang, X., and Chen, X. H. (2016). Clinical observation of cytomegalovirus infection after allogeneic hematopoietic stem cell transplantation. Chin. J. Blood Transf. 29, 1087–1091. doi: 10.13303/j.cjbt.issn.1004-549x.2016.10.001
Zhang, Y., Liang, Y., Zhang, X., Wang, S., Cao, J., Gao, Z., et al. (2022). Pre-transplant platelet refractoriness and alternative donors are associated with cytomegalovirus retinitis in hematopoietic stem cell transplantation for severe aplastic anemia. Front. Cell Infect Microbiol. 12:870296. doi: 10.3389/fcimb.2022.870296
Zhang, Y., Ruan, X., Yang, W., Li, L., Xian, Z., Feng, Q., et al. (2017). High ocular CMV copies and mismatched receipts may predict poor visual prognosis in CMV retinitis patients following allogeneic haematopoietic stem cell transplantation. BMC Ophthalmol. 17:224. doi: 10.1186/s12886-017-0622-0
Zhang, Y. P., Hu, K. X., Sun, Q. Y., Qiao, J. H., Guo, M., Ai, S. H., et al. (2014). Risk factors analysis of cytomegalovirus infection after nonmyeloablative allogeneic peripheral blood stem cell transplantation. J. Exp. Hematol. 22, 458–463. doi: 10.7534/j.issn.1009-2137.2014.02.035
Zhao, C., Huang, X. J., Sun, Y. Q., Xu, L. P., Zhang, X. H., Liu, K. Y., et al. (2020). Impact of poor graft function on cytomegalovirus pneumonia in patients who have undergone haploidentical stem cell transplantation. Chin. J. Hematol. 41, 552–556. doi: 10.3760/cma.j.issn.0253-2727.2020.07.004
Zhao, W. J., and Sun, Y. (2021). Analysis of clinical effect of foscarnet sodium in prevention of CMV infection after allo-HSCT operation. Mod. Diagn. Treat. 32, 1535–1536.
Zhu, C. L., Chen, G. H., Zhai, Z., Yang, Q. Y., Lv, H., Li, J., et al. (2020). Clinical analysis of cytomegalovirus infection after allogeneic hematopoietic stem cell transplantation in children. J. Clin. Pediatr. 38, 641–646. doi: 10.3969/j.issn.1000-3606.2020.09.001
Keywords: cytomegalovirus, allogeneic hematopoietic stem cell transplantation, epidemiology, meta-analysis, China
Citation: Lin R, Wu J and Liu Q (2025) Epidemiology, clinical outcomes, and treatment patterns of cytomegalovirus infection after allogeneic hematopoietic stem cell transplantation in China: a scoping review and meta-analysis. Front. Microbiol. 16:1518275. doi: 10.3389/fmicb.2025.1518275
Received: 29 October 2024; Accepted: 10 March 2025;
Published: 03 April 2025.
Edited by:
Mohammed Rohaim, Lancaster University, United KingdomReviewed by:
A. Raj Kumar Patro, Kalinga Institute of Medical Sciences (KIMS), IndiaCopyright © 2025 Lin, Wu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qifa Liu, bGl1cWlmYTYyOEAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.