- 1Faculty of Biology, Institute of Environmental Sciences, Jagiellonian University, Kraków, Poland
- 2Faculty of Natural Sciences and Technology, Institute of Biology, University of Opole, Opole, Poland
- 3Department of Entomology, Faculty of Agriculture and Forestry, Phytopathology and Molecular Diagnostics, University of Warmia and Mazury in Olsztyn, Olsztyn, Poland
- 4Faculty of Geography and Spatial Management, Jagiellonian University, Kraków, Poland
- 5Department of Biology, Evolutionary Ecology of Plants, Philipps-University Marburg, Marburg, Germany
Soil microorganisms are relatively poorly studied in urban ecosystems, particularly within unmanaged woodlands that form island-like patches of vegetation. We surveyed soil bacteria on Salix spp. dominated riparian-like forest patches in Kraków, the second largest city in Poland, to find out which environmental factors influence their activities and functional diversity, measured using Biolog® ECO plates. Our results showed that soil bacterial alpha functional diversity, including substrate richness (number of substrates decomposed) and Shannon diversity, were positively correlated with patch area and number of vascular plant species in the forest floor vegetation layer. However, soil bacterial beta functional diversity (substrate use pattern, CLPP – community level physiological profiles) was primarily driven by patch area and soil physicochemical properties. Our results suggest that the positive effect of patch area (biogeographic effect) on soil bacterial functional diversity may be primarily through stabilisation of environmental conditions, as the amplitude of environmental fluctuations is reduced on larger plots compared to smaller ones. Taken together, our study provides important insights into the relationship between patch area, soil properties, vegetation characteristics, soil bacteria activity, and functional diversity in urban riparian forests, highlighting the importance of considering soil microbes when managing urban ecosystems.
Introduction
Forest ecosystems are essential for human well-being, providing multiple ecosystem services such as carbon sequestration and biodiversity conservation (Baldrian, 2017; Klimek and Niklińska, 2024). However, their extent and ecological value steadily decrease worldwide due to environmental changes and anthropogenic pressures, including urbanization (Augustynczik et al., 2020; Sabatini et al., 2020). Urban woodlands vary in size, stand age, and management intensity (Pregitzer et al., 2019), resulting in a gradual transition from dense forests to more open, park-like environments (Woźniak et al., 2025). While larger remnants of old-growth forests are preserved in only a few cities (Wang and Yang, 2022), most urban forests consist of small and micro-forests, that are highly fragmented and patchy in their spatial distribution (Picard and Tran, 2021; Ayala-Azcarraga et al., 2023). The spatiotemporal continuity of these urban forest patches is shaped by the expansion dynamic of urban areas (Doroski et al., 2022) and a range of environmental and anthropogenic factors.
Unmanaged urban green spaces are often perceived as having low biological value (Bonthoux et al., 2019), but they have also been shown to represent urban biodiversity hotspots (Hwang and Roscoe, 2017). This is particularly true for floodplains and riverbanks (Hu et al., 2019), which are linked to areas outside of towns through the riverbed as an ecological corridor (Alvey, 2006). Urban riparian forest areas are critical for maintaining ecological connectivity through the river network, acting as a corridor for species movements (Graziano et al., 2022). However, in many cities, extensive sections of rivers are heavily modified or even completely covered by artificial surfaces such as concrete. These alterations may disrupt natural habitats and ecological processes, leading to site fragmentation and loss of biodiversity (Machado and Kim, 2024).
Microbiomes are fundamental components of all ecosystems, playing essential roles in nutrient cycling, organic matter decomposition, and overall ecosystem productivity and health (Gałązka et al., 2022). In the forest ecosystem, soil microbes contribute significantly to energy flow and organic matter cycling, ecosystem biodiversity, and stability (van der Heijden et al., 2008; Baldrian, 2017; Eisenhauer et al., 2017; He et al., 2022). Due to their sensitivity to environmental changes and perturbations, soil microbial parameters (e.g., functional and structural diversity) serve as valuable indicators of ecosystem health (Azarbad et al., 2013, 2016; Fierer et al., 2021; Garg et al., 2024). However, as pointed out by Fierer et al. (2021), interpreting microbial parameters can be challenging due to the complexity and spatiotemporal variability of soil microbiomes. This highlights the importance of context-specific analysis and the integration of microbial indices with soil parameters, such as physicochemical properties, to provide a more comprehensive assessment of ecosystem functions. Urban soils are a specific environment as they suffer from amplified environmental challenges such as increased site fragmentation, soil compaction, temperature, and pollution (Godefroid and Koedam, 2003; McKinney, 2006; Zhang et al., 2020; Nugent and Allison, 2022; Wang M. et al., 2022; Teerlinck et al., 2024). These stressors may alter microbial community structure and function, with recent studies suggesting that urbanization may reduce the complexity and stability of soil microbial networks constructed using amplicon sequencing of bacterial 16S rRNA and fungal ITS genes (Liu et al., 2023) and affect soil microbial enzyme drivers, leading to soil organic carbon loss (Zhang et al., 2024). However, despite their environmental importance, soil microbes associated with urban riparian forests have received less attention (Mgelwa et al., 2019).
Urban green spaces can be highly isolated from each other, which limits the dispersal of organisms (Von Thaden et al., 2021). This may allow urban green spaces to be treated as islands, with their important environmental characteristics such as island size and isolation, which are considered the primary abiotic factors for predicting biodiversity on islands (MacArthur and Wilson, 1967). For macro-organisms, the “island biogeography theory” showed that biodiversity has positive species-area relationships (island-area effect) and negative species-isolation relationships (island-isolation effect) (MacArthur and Wilson, 1967). Some recent reports have shown that these rules can also be applied to micro-organisms (Li et al., 2020; Yang et al., 2021; Raimbaultx et al., 2024). However, the ecological differences between macro-and micro-organisms, particularly different body sizes, indicate that the soil microbial response to site size, site isolation, and site edge length are likely driven by indirect effects (Ewers and Didham, 2007; Xu et al., 2024). These effects may include the vegetation diversity, which is expected to be lower on smaller than larger plots (Olejniczak et al., 2018; Ma et al., 2023); soil properties, which are expected to be more disturbed on smaller than larger plots, i.e., soil texture (Guilland et al., 2018); and the amplitude and severity of changes in environmental fluctuations, such as temperature and moisture, as smaller plots are more susceptible to their effects than larger plots (Yang et al., 2021). Understanding how riparian forest patch area, isolation, and environmental factors shape microbial functional diversity in urban settings will help to inform strategies for preserving biodiversity and microbially-mediated ecosystem services in cities.
The functional diversity of soil microbial communities can be defined as the ability to metabolise different organic compounds (Garcia-Pausas and Paterson, 2011) and can be derived from genetic diversity (Maron et al., 2018). For this study, we chose to use the Biolog® ECO plate method, a phenotypic community-functional approach widely used to assess soil microbial functional diversity (Escalas et al., 2019). This method evaluates the metabolic potential of microbial communities by quantifying their ability to utilize a standardized array of 31 carbon sources. The advantages of the Biolog® approach include its ability to provide direct, community-level functional insights and its suitability for studying fast-growing culturable bacteria. This makes it particularly relevant for understanding functional processes in environments like riparian forest soils.
The present study addressed how the functional diversity of soil bacteria is influenced by site characteristics within willow (Salix spp.) dominated forest-like sites in urban area, with limited site connectivity provided by the river network. Our goal was to determine whether soil bacterial community catabolic characteristics are related to riparian forest patch area and/or other environmental characteristics, including vegetation and soil properties. Both vegetation characteristics and soil physical and chemical properties are among the most important factors shaping forest soil microbial communities (He et al., 2022; Khafida et al., 2024). Specifically, we aimed to examine whether the island area influences soil bacteria alpha and beta functional diversity (defined as a number of decomposed substrates and the pattern of substrate use) through affected vegetation characteristics or through soil characteristics or whether these relationships are driven by other mechanisms.
Materials and methods
Study sites, vegetation surveys, and soil sampling
Study sites were located in the city of Kraków, the second city in Poland in terms of area (327 km2) and number of inhabiting people (0.80 million in the city and 1.5 million in metropolitan area). Kraków is located in the south of Poland: latitude from 19°47′35″E to 20°13′02″ E and longitude from 49°58′04”N to 50°07′32”N. The geological structure varies in different parts of the city, as Kraków lies at the junction of three major geological units: the silesian-cracow Monocline (the Kraków-Częstochowa Upland), the Carpathian foothills (the Carpathian Foothills) and the Outer Carpathians (the Beskids). In the river valleys, the top geological layer consists mainly of Holocene sand and gravel. The climate in the region is temperate with four seasons; the mean annual average temperature (MAAT) is 10.0°C, and the mean annual average precipitation (MAAP) is 700 mm. July is the hottest (19.5°C) and wettest (120 mm) month of the year, while January is the coldest (−2.3°C) and driest (50 mm). The growing season with an average daily temperature above 5°C lasts for 220 days on average. Total forest cover in Kraków is estimated at 4% (Bank Danych Regionalnych, Główny Urząd Statystyczny, 2024), which is one of the lowest value compared to major cities in Poland.
Ten Salix spp. dominated, riparian-like forest patches were found in different parts of the city. Geographical coordinates (Supplementary Table S1) and a map of study sites (Supplementary Figure S1) are reported in Supplementary information. Only Salix spp. dominated stands were studied to reduce the number of confounding factors, as dominant tree species strongly influence soil microbial characteristics (Chodak et al., 2016). Willow is an important component of temperate riparian areas and provide multiple ecosystem services (Bita-Nicolae, 2023). The ecological continuity of the riparian forest-like vegetation on the study sites lasts up to a few decades, as the riverbanks in the Kraków area have been heavily modified, e.g., by the construction of flood protection systems. The patch area was calculated, with the tree line considered as the boundary of the patch. Despite varying sizes, all patches were ecologically linked because the city’s waterways converge into the Wisła River, which flows through the city center approximately from west to east.
On each patch, a representative 100 m2 study plot was delineated in the central part of each site. Vegetation, that is, vascular plants (N plant), including trees (N tree), shrubs (N shrub), and forest floor species (N floor), was characterized on each study plot using the Braun-Blanquet method (Braun-Blanquet, 1964). The data on plant cover in the relevés were transformed from the Brown-Blanquet scale into a 0–9 ordinal scale (Van der Maarel, 1979), and the H’plant was calculated on the basis of the Shannon-Wiener general diversity index according to the equation:
Soil physical and chemical analysis
Soil physical and chemical analyses were carried out on each collected soil sample. The dry weight (DW) of the soil samples was determined by measuring the mass loss (water) after the soil samples had been at 105°C for 24 h. The water holding capacity (WHC), which was the amount of water that a given soil can hold without leaking, was measured using a standard gravimetric method after soil was soaked for 24 h in net-ended plastic pipes immersed in water. The soil pH was measured in air-dried subsamples (2 g) shaken in deionised water (1:10 w:v) for 1 h at 200 rpm. Organic carbon (C) and total nitrogen (N) were analyzed by dry combustion of approximately 5 mg milled soil samples with an elemental analyzer (Vario El III, Elementar Analysen Systeme GmbH). The flow-injection analyzer (FIA compact, MLE) was used to analyze the total P concentration, after wet mineralization of 0.5 g DW of soil subsamples in suprapure 65% HNO3 (Merck). To assess the accuracy of the mineralization process, three blank samples and three replicates of standard certified material (CRM025-050, Sandy Loam 8, RT Corp.) were analyzed with the soil samples. The C:N ratio was subsequently calculated to capture the balance between carbon and nitrogen availability. Similarly, the C:P ratio was calculated to provide insight into the interactions between carbon and phosphorus. Particle-size distribution of mineral soil fraction was determined by laser diffraction after a 3 min ultrasound dispersion of the sample in distilled water (Mastersizer 3,000, Malvern Panalytical, United Kingdom) (Gus-Stolarczyk et al., 2022). This analysis provided data on particle size distribution, including percentages of sand, silt, and clay. Each analysis was performed in three subsamples taken from each study plots, and the results are presented as mean values with standard deviations.
Biolog® ECO plates analysis of soil bacteria
Soil bacteria activity and functional diversity was analyzed using Biolog® Eco plates (Preston-Mafham et al., 2002). The Biolog® Eco plates are 96 well microplates, that contain 3 sets of 31 common carbon sources and employ a tetrazolium redox dye as an indicator of microbial community metabolism of each individual substrate.1 The decay of the different substrates in the wells resulted in a change from colourless to purple formazan. The substrates were six compound groups: amines, amino acids, carbohydrates, carboxylic acids, polymers, and others (miscellaneous) (Campbell et al., 1997).
The soil samples (equivalent of 3 g of soil dry mass) were acclimated at 22°C at 60% of their maximal WHC for 4 days and then shaken in 30 mL of 0.9% NaCl at laboratory shaker for 30 min at 200 rpm. The supernatants containing microbes (100 μL) were diluted in 9.9 mL of 0.9% NaCl. Solutions of 100 μL per well were inoculated into the Biolog® Eco plates and the plates were incubated at 20°C in darkness. To prevent contamination, all tools used were sterile. The absorbance in particular wells was measured as light absorbance at 590 nm using a spectrophotometer Tecan with i-control software (Tecan Group Ltd., Männedorf, Switzerland). The first measurement was carried out just after inoculation and then was measured daily for 5 days. The absorbance value for each substrate was corrected by subtracting the value for the control well, which contained no substrate but only the soil suspension. Absorbance changes below 0.06 (spectrometer detection limit) were considered as 0.
Soil bacteria alpha functional diversity was expressed as the number of substrates decayed (R) and by the Shannon diversity index (H’bact), which was calculated as:
Beta functional diversity of soil bacteria was expressed as patterns of substrate use, commonly called community level physiological profiles (CLPP). The absorbance values for individual substrates were standardized to 1 for each sample to compare relative changes in substrate use pattern.
Soil bacteria activity, that is the overall rate of substrate utilization by microorganisms was expressed by the AUC (Area Under the Curve), which was calculated as follows:
Statistical analysis
Pearson correlation tests were conducted to examine the relationships between site size, vegetation and bacterial indices and soil properties and to identify independent variables for further analysis. Then, multiple regression analyses were conducted to separately assess the effects of independent factors on the R, H’bact, and AUC. The independent factors included in this analysis were patch area, vegetation coverage, number of forest floor plant species, soil pH, soil N and P content, and clay content. These variables were selected based on Pearson correlation coefficients (r < 0.6) to represent the wide range of environmental properties and to minimize collinearity. Both backward and forward stepwise selection procedures were performed for each analysis to validate the robustness of the final models. To compare relationships between vegetation diversity and structure and soil bacterial community CLPPs, dissimilarity matrices based on Euclidean distance were calculated, using either the botanical data and Biolog® data. The matrices were then compared using the Mantel test (9,999 permutations) to assess the link between the beta diversity of vegetation and soil bacteria.
Beta diversity refers to the variation in species composition within the community, represented either as a matrix of plant species occurrences or as CLPP data, which reflect substrate utilization patterns by soil bacteria on individual study plots. Next, a partial least squares path modelling (PLS-PM; Sanchez, 2013) was carried out to evaluate the direct and indirect effects of site size, vegetation properties, and soil physicochemical properties. Model was constructed based on weights on standardized manifest variables, and centroid was used for internal estimation. Correlations at significance level 0.001 and goodness of fit indices were calculated for obtained model. Multiple-variable analysis and multiple regression analysis were performed using Statgraphics Centhurion XIX software (StatPoint Technologies Inc., Warrenton VA, United States). Mantel test analysis was performed using PAST 4.10 software (Natural History Museum, University of Oslo, Norway). A partial least squares path modelling (PLS-PM) was carried out with XLSTAT (Lumivero, 2020).
Results
Site size and vegetation characteristics
Data on patch area and site characteristics are presented in Table 1. Site size ranged from 0.05 to 1.44 ha. The study plot (100 m2) vegetation coverage ranged from 110 to 220%. The number of vascular plant species per plot ranged from 13 to 24, and most of them were forest floor species (61% per plot on average). Dewberry Rubus caesius, nettle Urtica dioica and avens Geum urbanum were the most common forest floor species with the highest plot coverage. Shrubs layer was represented by 2 to 4 species per plot, and the most common were bird cherry Padus avium and black elder Sambucus nigra. Tree species number per plot varied from 2 to 4, with the predominance of Salix alba and Salix fragilis (10 and 9 plots, respectively). The vascular plant composition indicated riparian forests of the class Salicetea purpureae Moor 1958, but not all plots had the appropriate composition of diagnostic species or were characterized by a low cover of these species (Dzwonko and Loster, 1988; Dzwonko, 2015). A few so-called ancient woodland species were however recorded in some plots: Aegopodium podagraria, Athyrium filix-femina, Circaea lutetiana, Dryopteris dilatata, Dryopteris filix-mas, Festuca gigantea and Geum urbanum (Dzwonko, 2015), but most of which had low cover. Number of invasive plant species identified on all plots altogether was 13, with species number per plot ranging from 0 to 5 (15% of plant species per plot on average). Impatiens parviflora and Impatiens glandulifera were the most common invasive species (found on 4 and 6 from 10 plots, respectively). Vegetation properties on study plots were highly positively correlated (Figure 1). Plant diversity index (H’plant), which ranged from 1.04 to 1.33, was positively influenced by N floor (r = 0.84; p < 0.001) but also by the number of N invasive species (r = 0.71; p < 0.05; Figure 1).
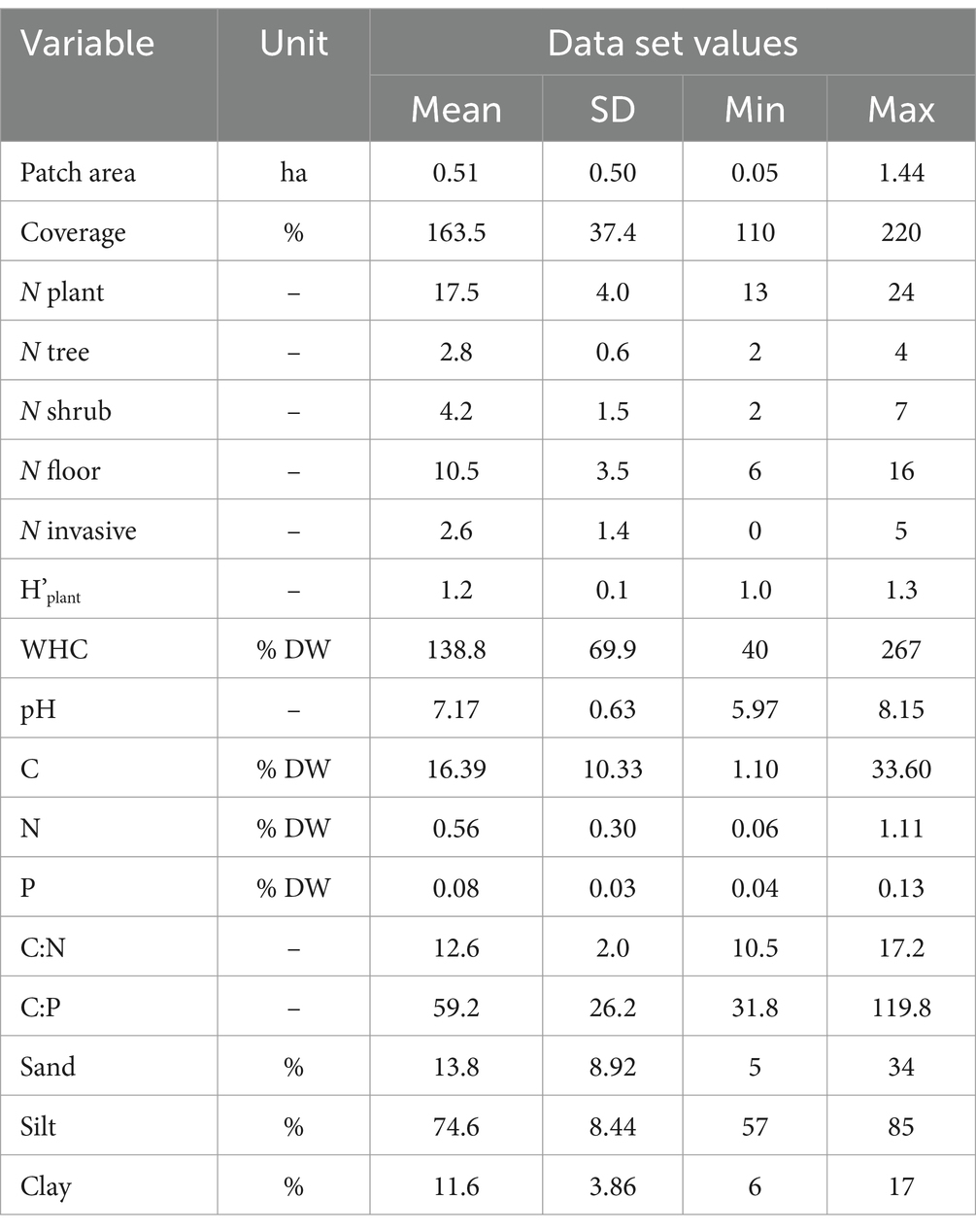
Table 1. Means, standard deviations, and minimal and maximal values (n = 10) for patch area, vegetation data: total coverage (%), total number of plant species (N plant), number of tree species (N trees), number of shrub species (N shrubs) and number of forest floor species (N floor), number of invasive species (N invasive), plant diversity index (H’plant), and soil physicochemical properties.
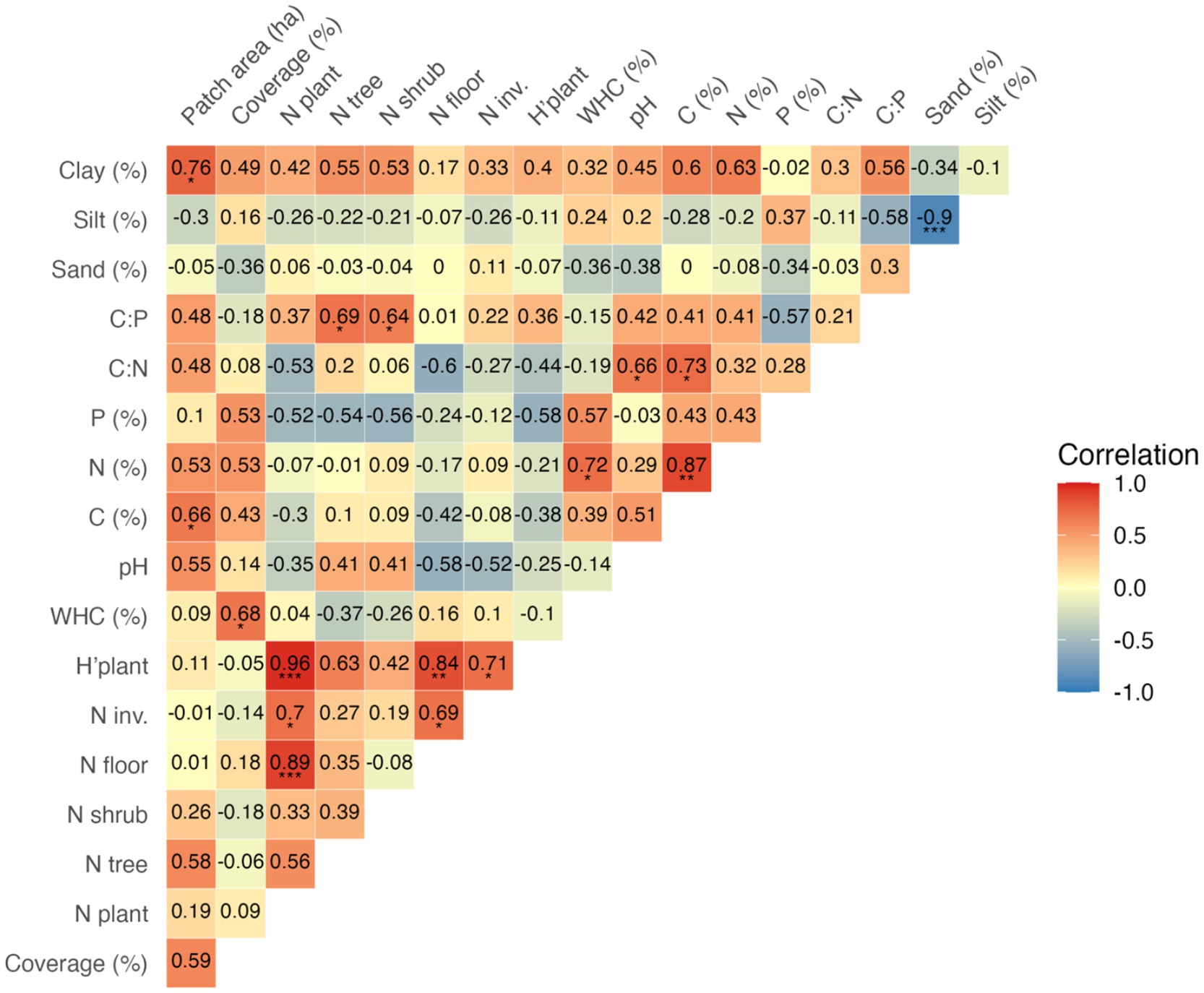
Figure 1. Pearson correlations for riparian-like forest patches data on its area, vegetation properties, and soil properties (n = 10). Within each cell, the numeric value indicates correlation strength (scaled from −1 to +1), and asterisks denote significance levels: *, **, and *** for p < 0.05, 0.01, and 0.001, respectively. Correlations are displayed in red (positive) and blue (negative); colour saturation denotes the strength of the relationship. Detailed information about soil physicochemical properties, vegetation data, and soil bacterial indices are presented in Tables 1, 2, respectively.
Soil physical and chemical properties
WHC in studied soils ranged from 60 to 127% (Table 1). The pH of the studied soils was neutral to alkaline, with a mean value of 7.17 (± 0.63). The studied soils were characterized by a low content of C, N, and P (4.29, 0.34, and 0.08% on average, respectively). The C:N ratio ranged from 10.5 to 17.2, with a mean value of 12.6 (± 2.0), while the C:P ratio was more variable, ranging from 31.8 to 119.8, with a mean of 59.2 (± 26.2). The soils were mainly composed of silt (mean: 74.6%, ± 8.44), followed by sand (13.8%, ± 8.92) and clay (11.6%, ± 3.86). Patch area was positively correlated only with soil C content (r = 0.66; p < 0.05) and clay percentage (r = 0.76, p < 0.001), as shown in Figure 1. Soil C content was, in turn, positively correlated with soil N content (r = 0.87; p < 0.01). Plot coverage was positively correlated only with soil WHC (r = 0.68; p < 0.001). C:N ratio was positively correlated with soil pH (r = 0.66; p < 0.001; Figure 1).
Soil bacteria activity and functional diversity
AUC ranged from 45.8 to 67.0 (Table 2; Supplementary Figure S2). R ranged from 18 to 28, meaning that 76% of substrates on Biolog® ECO plates was decomposed on average, indicating relatively high functional diversity in studied soils. H’bact ranged from 1.20 to 1.30. Carboxylic acids, carbohydrates and amino acids were among the most used substrate groups, for each soil representing above 70% of the response (Table 2; Supplementary Figure S2). The multiple regression analysis was performed to study the effect of patch area and plot properties on soil bacteria activity and alpha functional diversity indices (Figure 2). The output of these analyses indicated that models were significant for R, H’bact, and AUC. For R, the model explained 68.2% of the variance (p = 0.007), which was positively dependent on the patch area (p = 0.045) (Figure 2A) and the number of forest floor plant species (p = 0.006) (Figure 2B). For H’bact, the model was significant (p = 0.005) and explained 70.5% of the variance. H’bact showed a significant positive correlation with the site size (p = 0.044) (Figure 2C) and the number of forest floor plant species (p = 0.004) (Figure 2D). For AUC, the model explained 32.6% of the variance (p = 0.049), where AUC values were positively dependent only on soil P content (p = 0.049) (Figure 2E). Mantel test results revealed that plant community beta diversity was not correlated with bacteria functional beta diversity (CLPP) (p = 0.458, R = 0.017). The PLS-PM confirmed these results (Figure 3; Supplementary Figure S3), where soil bacterial alpha functional diversity indices (R, H’bact) were mainly determined by vegetation characteristics. In turn, soil bacteria beta functional diversity (CLPP) was primarily driven by site area and soil physicochemical properties.
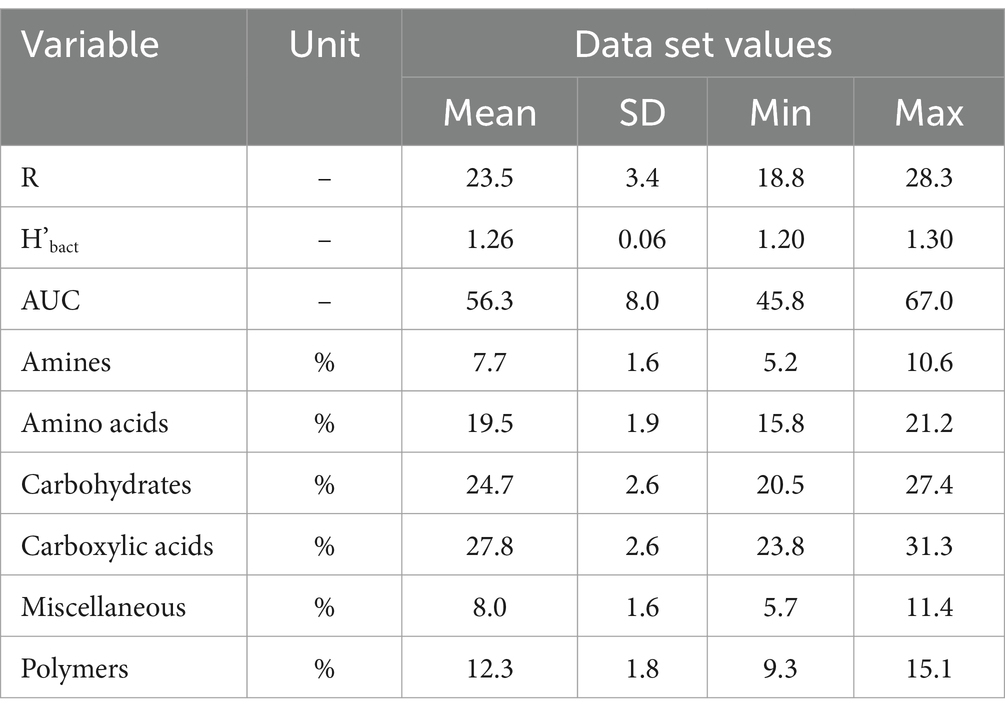
Table 2. Means, standard deviations and minimal and maximal values (n = 10) for soil bacteria alpha functional diversity, that is R (number of substrates used) and H’bact (Shannon diversity), and soil bacteria activity (AUC) and structure of substrate groups use (relative % of use).
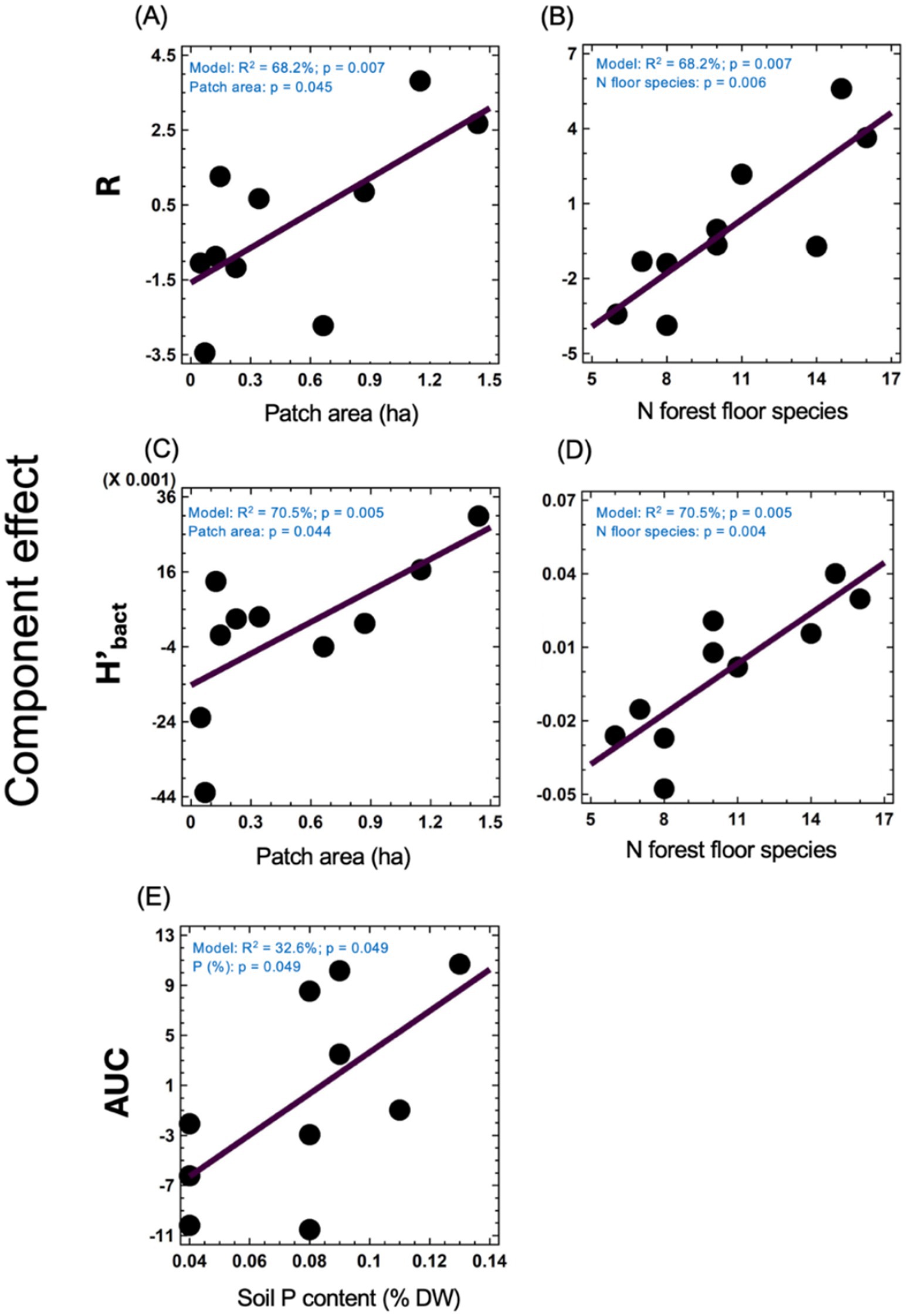
Figure 2. Component effects from multiple regression analysis on the relationships between environmental factors and bacterial parameters. Effect of patch area (A) and the number of forest floor plant species (B) on R (number of substrates decomposed by bacteria). Influence of patch area (C) and the number of forest floor plant species (D) on H’bact (Shannon diversity index of bacterial functional diversity). Effect of soil phosphorus (P) content on AUC (overall bacterial activity) (E). Dots are individual patches (n = 10). Model parameters, including adjusted R2 and overall model p-values, are indicated in blue text within each panel. Individual p-values for the factors are also provided.
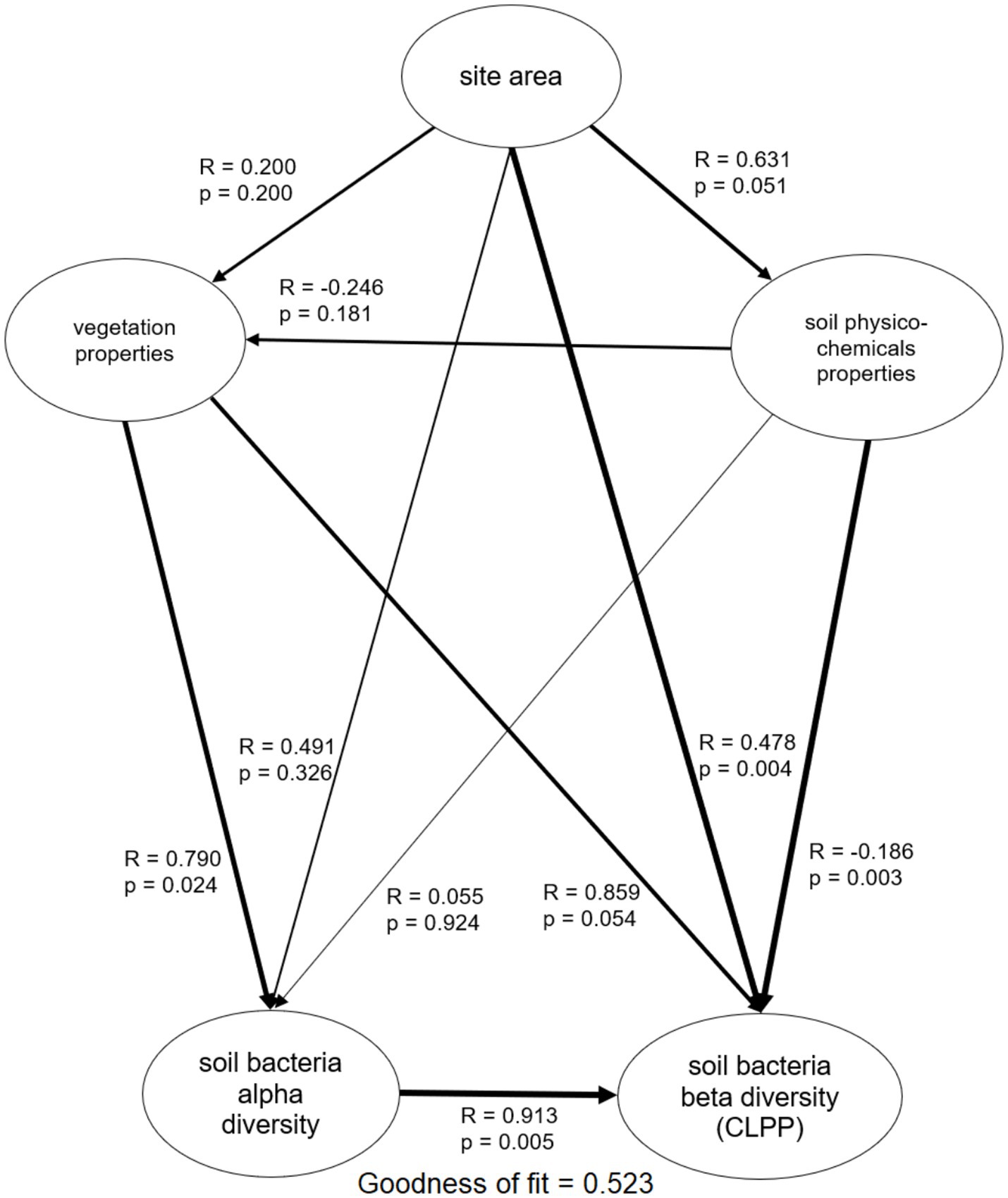
Figure 3. Partial least squares path models (PLS-PM) displaying the direct and indirect effects of the interaction of site size and vegetation effect on soil bacteria functional diversity (alpha diversity and beta diversity). Arrows width presents correlation strength, values by the individual arrows denote correlation (R) value and significance of the effect (p value).
Discussion
Soil microorganisms in urban areas provide essential ecosystem services such as those involved in nutrient cycling, erosion control, and regulation of climate change stressors (Metzler et al., 2024). Therefore, it is highly important to better understand how human-based activities influence microbial diversity and functions in urban ecosystems. Indeed, the conservation of soil biological diversity is an extremely important task in a rapidly changing world under human activity, and site fragmentation is one of the most challenging problems. Our study provides important insights into the relationship between patch area, soil properties, vegetation characteristics, soil bacterial activity, and functional diversity in urban riparian forest patches. Using Biolog® ECO plates, we showed that larger riparian forest patches exhibited higher values of the R measure, reflecting the percentage of substrates decomposed, as well as increased bacterial alpha functional diversity (H’bact). These findings suggest that larger riparian forest patches in urban environments may provide improved habitat conditions, which in turn support higher functional diversity of soil bacteria.
It is important to note that temperate riparian forests are regarded as a threatened or endangered habitat in Europe (Czortek et al., 2020; Przepióra and Ciach, 2022). This is particularly true for the vegetation, an important component that contributes to the biotic part of this unique ecosystem. On average, about 30 species of vascular plants per plot (100 m2 of phytosociological relevance) are reported in riparian forests in Poland, and in well preserved regions even 50 species per plot (Macicka and Wilczyńska, 1988; Matuszkiewicz, 2008). In our study, the mean number of plant species per 100 m2 plot was much lower and amounted to 17.1 (± 3.8). Similar results were obtained by Stefańska-Krzaczek (2013), who identified 13.7 (± 4.9) species in a single phytosociological relevé in riparian forest remnants in Wrocław, the third largest city (in terms of population) in Poland. Plant species richness and diversity are considered to be one of the most important factors shaping soil microbial communities (Toju and Sato, 2018). On the other hand, certain soil microbial groups may increase ecosystem stability, especially under stressful conditions, by facilitating nutrient acquisition for plant communities.
We used Biolog® tests to study the functional (catabolic) diversity of soil bacteria. However, the limitations of such an approach need to be taken into account while interpreting our results. For instance, this method allows the study of only those fractions of bacteria that can be extracted and cultured. In addition, direct comparisons of results obtained in different laboratories must be carefully preceded by a review of the details of the laboratory analysis, such as the degree of dilution of the inoculum (bacterial suspension) applied to the wells of the plate, which can strongly influence the final results. In general, data on the functional diversity of soil bacteria under Salix spp. stands are scarce and mostly related to post-mining sites (Kaneda et al., 2019). Soil bacterial activity and functional diversity in Salix spp. dominated riverbanks in urban areas were comparable with data obtained for other types of forests in Poland, using the same laboratory protocol. In particular, Klimek et al. (2016) found that in different nearly undisturbed temperate forest types, the mean values for AUC, R and H’bact in soil A horizon were 39.0, 24.4, and 1.10, respectively. Wasak et al. (2019), in their study conducted in a forest mountainous area near Kraków, found that averaged values for AUC, R, and H’bact were 31.1, 19.5, and 1.21, respectively. The values of soil bacterial indices measured in a current study were slightly higher than those obtained in near-natural temperate forests in Poland. This could be due to various environmental factors, such as soil pH. In natural temperate forests, which are mostly characterized by acidic pH, soil bacteria may be outcompeted by soil fungi. Soil pH was neutral to alkaline in the riparian soils, and higher soil pH may favour bacteria over fungi (Wang and Kuzyakov, 2024). Moreover, urban forest soil is becoming alkaline under rapid urbanization (Zhang et al., 2023). Soil bacteria AUC was, however, not related to soil pH, but only to soil P content. Phosphorus is an essential nutrient not only for plants, but also for microorganisms (Wang Z. et al., 2022). Although it contributes to diffuse pollution and eutrophication, riparian soils are highly effective at sorbing readily soluble forms of phosphorus (Frątczak et al., 2019). Phosphorus supports rapid plant biomass production, which in turn promotes soil microbial performance (Chen and Xiao, 2023). Willow is known for its fast growth rate (Koczorski et al., 2022). Phosphorus fertilisation has been shown to increase crop yield in willow short rotation coppice for biomass production (Kuzovkina et al., 2018), and similar effects can be expected in riparian areas.
Soil bacteria alpha functional diversity indices, both R and H’bact, increased with the number of forest floor plant species, which represents the majority of plant diversity in temperate forests (Gilliam, 2007). Vegetation influences a wide range of soil microbial parameters (Tilman et al., 1997). For example, plant species-rich communities produce more biomass and more chemically diverse litter and root exudates (rhizodeposition), which essentially contribute to higher soil C content, as we showed in this study. Higher plant species richness also promotes plant-microbes interactions, which can, in turn, lead to the establishment of more diverse microbial communities (Prober et al., 2015). Indeed, soil bacteria are effective in utilizing simple organic compounds, delivered by plant roots, which are generally more available in nutrient-rich habitats such as a rhizosphere (Wang and Kuzyakov, 2024). The chemical groups of carbon substrates most used by bacteria on Biolog® plates were carboxylic acids, carbohydrates and amino acids. These substrate chemical groups are particularly essential components of root exudates, which are known to support the growth and development of soil bacterial communities (Wang and Kuzyakov, 2024), and therefore differentiation between the experimental treatments with these substrate groups was often reported (Furtak et al., 2020). Although we did not observe a significant relationship between soil bacterial functional beta diversity (CLPP pattern) and vegetation diversity and composition as showed by Mantel test, our findings align with previous studies indicating that vegetation diversity has a greater impact on fungal communities than on bacterial communities. For instance, using amplicon sequencing of bacterial 16S and fungal internal transcribed spacer (ITS), Štursová et al. (2016) investigated bacterial and fungal communities and diversity in a regenerating temperate mountain forest in the Bohemian Forest, Central Europe. Their results revealed that fungal communities exhibited significantly higher beta diversity (variation among communities) than bacterial communities, with fungi being more strongly influenced by vegetation. In our study, another potential factor contributing to the lack of a clear relationship between soil bacterial beta functional diversification and vegetation beta diversity could be due to a relatively high proportion of invasive plant species. Previous studies have shown that cities are hotspots for alien species, with over 35˗40% of them have been recorded in cities (Pyšek, 1998; Clemants and Moore, 2003). Native plant species are generally more abundant than non-native species (Knapp et al., 2008), but in some places non-native plant species may dominate native ones. River valleys are particularly vulnerable to invasion by alien species (Pielech, 2021). In disturbed anthropogenic habitats, the number of alien plant species tends to correlate with the total number of plant species (Siwek et al., 2024). Invasive plant species can affect plant communities and soil physicochemical and microbiological properties, all of which can affect soil carbon dynamics (Raheem et al., 2024).
Multiple regression analysis confirmed that soil bacterial alpha functional diversity indices, both R and H’bact, increased with patch area, but to a greater extent from vegetation diversity, that is, forest floor species number. However, PLS-PM indicated a negligible direct effect of patch area on alpha functional diversity indices. In contrast, patch area had a significant effect on the beta diversity of soil bacteria (CLPP), which seems to confirm that the island effect on soil bacteria is driven by habitat heterogeneity. This habitat heterogeneity likely operates at the microscale within soil samples, as indicated by the significant correlation between patch area with soil clay and C contents. The role of soil texture in shaping microbial diversity is well-supported by previous studies. For instance, Biesgen et al. (2020) showed that clay content modulates bacterial community composition by increasing aggregate stability, which fosters the development of distinct microbial communities in microaggregates. In our study, the positive correlation between patch area and clay content may explain the observed influence of patch area on bacterial beta diversity, as larger patches tend to accumulate finer particles, creating heterogeneous microhabitats that enhance bacterial community differentiation. Moreover, clay content was observed to correlate with soil moisture due to the occurrence of micropores and menisci that generate capillary forces (Richert et al., 2009; Bicharanloo et al., 2022).
A larger patch area may support ecosystem functions better than a smaller one through multiple mechanisms. One such mechanism is the mitigation of direct anthropogenic disturbances such as soil compaction and soil pollution. This was demonstrated by Kostrakiewicz-Gierałt et al. (2022) in their study of urban forests and parks in Kraków, where larger patches tended to have greater resilience and functionality in the face of human-induced stressors. However, urban riparian forest, including small ones, are difficult to access due to dense vegetation cover, and direct human activity is limited. Another possible mechanism is the greater resistance of larger areas to water shortages, a factor that may be particularly relevant in riparian areas. In general, urban hydrology is drastically altered compared to agricultural and natural areas (Pickett et al., 2001). The high proportion of impervious surfaces in urban areas, combined with the vegetation structure characterized by reduced larger tree cover in urban green spaces, leads to increased rainwater runoff. Coupled with changes in the structure of precipitation under global climate change, i.e., a higher proportion of torrential rainfall, the increased runoff in urban areas changes the morphology of urban streams, which become deeply incised in their floodplains (Soboyejo et al., 2025). This hydrological modification can isolate remnant riparian vegetation from the water table, compounding the challenges posed by reduced groundwater availability during droughts. During periods of drought (reduced rainfall), water deficiency may limit soil microbial activity, both directly through reduced soil water content but also by limiting the morphological and physiological traits of plants, including a reduction in fine root biomass and their carbon contribution to the soil below ground (Hinko-Najera et al., 2015). This hypothesis can also be supported by the positive correlation observed in this study between soil water holding capacity and vegetation coverage. Furthermore, the positive correlation between plot area and soil clay content suggests that larger plots may experience less soil erosion, as clay particles, being the smallest and most easily transported by water, are more likely to be retained in these areas compared to smaller plots.
It has been shown that soil bacteria are more sensitive to drought than fungi (Azarbad et al., 2018, 2020; de Vries et al., 2018). Li et al. (2020) investigated the island biogeography of soil bacteria and fungi (via amplicon sequencing) and revealed that the diversity of soil bacteria alpha diversity is strongly influenced by soil moisture. Their study demonstrated that smaller islands, characterized by lower soil moisture and greater edge effects, exhibited reduced bacterial diversity compared to larger islands. However, it is important to note that soil moisture is a highly viable soil property that could be influenced by temporal weather conditions during sampling rather than a consistent indicator of soil property. Moving forward, future studies should focus on the potential role of larger patch areas in urban riparian forests in supporting soil health and ecosystem functions, particularly in relation to water availability and resistance to drought. Such studies should include a more detailed investigation of soil microbiomes, including bacteria and fungi, based on amplicon and metagenomic sequencing assays. Integrating microbial sequencing approaches with CLPP data helps to link phenotypic functional diversity with the underlying genetic and taxonomic drivers. Additionally, the “island effect” on soil microbial diversity and functionality may vary depending on the type of island, whether of natural or anthropogenic origin. This highlights the importance of comparative studies across different island types.
Conclusion
In conclusion, our study confirms that bigger riparian forest patches in urban areas support for higher functional diversity of soil bacteria. We showed that alpha functional diversity of soil bacteria was mainly driven by vegetation characteristics, whereas beta functional diversity was primarily by site size and soil physicochemical characteristics. Conservation of green urban areas is receiving increasing attention, as they can support important parts of the biodiversity of the geographical region, up to the national scale (Basak et al., 2021). It is particularly important to mitigate the urban heat island effect in the face of global climate change (Teerlinck et al., 2024), as it can severely impact residents in densely populated urban areas. There is a need to prevent the loss of biodiversity in disturbed landscapes, in particular in urban areas (Hansen et al., 2005). Our study may serve as an argument for preserving at least a part of urban green spaces in their natural state. It will be important to validate our findings by investigating the impact of the island effect and climate change on soil microbial community structure and functioning in urban riparian areas, with a focus on the conservation of urban green spaces to support biodiversity and mitigate the effects of urbanization.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
GK: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. MJ: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. SP: Data curation, Formal analysis, Investigation, Funding acquisition, Writing – original draft, Writing – review & editing. ŁM: Methodology, Resources, Writing – review & editing. HA: Funding acquisition, Validation, Visualization, Writing – original draft, Writing – review & editing. BK: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Jagiellonian University in Kraków, Poland (N18/DBS/000003). Open Access funding provided by the Open Access Publication Fund of Philipps-Universität Marburg with support of the DFG. Part of this work was carried out at MCBR UO (International Research and Development Center of the University of Opole), which was established as part of a project co-financed by the European Union under the European Regional Development Fund, RPO WO 2014–2020, Action 1.2 Infrastructure for R&D. Agreement No. RPOP.01.02.00-16-0001/17-00 dated January 31, 2018. Some of the results presented in the paper were obtained as part of comprehensive research financed by the University of Warmia and Mazury in Olsztyn, Faculty of Agriculture and Forestry, no. 30.610.011-110.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1517545/full#supplementary-material
Footnotes
References
Alvey, A. (2006). Promoting and preserving biodiversity in the urban forest. Urban For. Urban Green. 5, 195–201. doi: 10.1016/j.ufug.2006.09.003
Augustynczik, A., Gutsch, M., Basile, M., Suckow, F., Lasch, P., Yousefpour, R., et al. (2020). Socially optimal forest management and biodiversity conservation in temperate forests under climate change. Ecol. Econ. 169:106504. doi: 10.1016/j.ecolecon.2019.106504
Ayala-Azcarraga, C., Diaz, D., Fernandez, T., Cordova-Tapia, F., and Zambrano, L. (2023). Uneven distribution of urban green spaces in relation to marginalization in Mexico City. Sustain. For. 15:12652. doi: 10.3390/su151612652
Azarbad, H., Constant, P., Giard-Laliberté, C., Bainard, L., and Yergeau, E. (2018). Water stress history and wheat genotype modulate rhizosphere microbial response to drought. Soil Biol. Biochem. 126, 228–236. doi: 10.1016/j.soilbio.2018.08.017
Azarbad, H., Niklińska, M., van Gestel, C. A. M., van Straalen, N. M., Röling, W. F. M., and Laskowski, R. (2013). Microbial community structure and functioning along metal pollution gradients. Environ. Toxicol. Chem. 32, 1992–2002. doi: 10.1002/etc.2269
Azarbad, H., Tremblay, J., Giard-Laliberté, C., Bainard, L., and Yergeau, E. (2020). Four decades of soil water stress history together with host genotype constrain the response of the wheat microbiome to soil moisture. FEMS Microbiol. Ecol. 96:fiaa098. doi: 10.1093/femsec/fiaa098
Azarbad, H., Van Gestel, C. A. M., Niklińska, M., Laskowski, R., Röling, W. F. M., and Van Straalen, N. M. (2016). Resilience of soil microbial communities to metals and additional stressors: DNA-based approaches for assessing “stress-on-stress” responses. Int. J. Mol. Sci. 17:933. doi: 10.3390/ijms17060933
Baldrian, P. (2017). Forest microbiome: diversity, complexity and dynamics. FEMS Microbiol. Rev. 545 40–41, 109–130. doi: 10.1093/femsre/fuw04
Bank Danych Regionalnych, Główny Urząd Statystyczny . (2024) Regional data Bank, central statistical office in Poland. Available at: https://stat.gov.pl/ (accessed July 22, 2024).
Basak, S., Hossain, M., Tusznio, J., and Grodzińska-Jurczak, M. (2021). Social benefits of river restoration from ecosystem services perspective: a systematic review. Environ. Sci. Pol. 124, 90–100. doi: 10.1016/j.envsci.2021.06.005
Bicharanloo, B., Shirvan, M., and Dijkstra, F. (2022). Decoupled cycling of carbon, nitrogen, and phosphorus in a grassland soil along a hillslope mediated by clay and soil moisture. Catena 219:106648. doi: 10.1016/j.catena.2022.106648
Biesgen, D., Frindte, K., Maarastawi, S., and Knief, C. (2020). Clay content modulates differences in bacterial community structure in soil aggregates of different size. Geoderma 376:114544. doi: 10.1016/j.geoderma.2020.114544
Bita-Nicolae, C. (2023). Distribution of the riparian Salix communities in and around Romanian Carpathians. Diversity 15:397. doi: 10.3390/d15030397
Bonthoux, S., Voisina, L., Bouché-Pillon, S., and Chollet, S. (2019). More than weeds: spontaneous vegetation in streets as a neglected element of urban biodiversity. Landsc. Urban Plan. 185, 163–172. doi: 10.1016/j.landurbplan.2019.02.009
Campbell, C., Grayston, S., and Hirst, D. (1997). Use of rhizosphere carbon sources in sole carbon source tests to discriminate soil microbial communities. J. Microbiol. Methods 30, 33–41. doi: 10.1016/S0167-7012(97)00041-9
Chen, C., and Xiao, W. (2023). The global positive effect of phosphorus addition on soil microbial biomass. Soil Biol. Biochem. 176:108882. doi: 10.1016/j.soilbio.2022.108882
Chodak, M., Klimek, M., and Niklińska, M. (2016). Composition and activity of soil microbial communities in different types of temperate forests. Biol. Fertil. Soils 52, 1093–1104. doi: 10.1007/s00374-016-1144-2
Clemants, S., and Moore, G. (2003). Patterns of species richness in eight northeastern United States cities. Urban Habitat. 1, 1–16.
Czortek, P., Dyderski, M., and Jagodziński, A. (2020). River regulation drives shifts in urban riparian vegetation over three decades. Urban For. Urban Green. 47:126524. doi: 10.1016/j.ufug.2019.126524
de Vries, F., Griffiths, R., Bailey, M., Craig, H., Girlanda, M., Gweon, H., et al. (2018). Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 9:3033. doi: 10.1038/s41467-018-05516-7
Doroski, D., Bradford, M., Duguid, M., Hallett, R., Pregitzer, C., and Ashton, M. (2022). Diverging conditions of current and potential future urban forest patches. Ecosphere 13:e4001. doi: 10.1002/ecs2.4001
Dzwonko, Z. (2015). Ground cover plants as indicators of forest origin and transformation, vol. 17: Studies and Materials of the Centre for Nature and Forestry Education, 42. Rogów Experimental Forestry Station, Forest and Nature Education Centre, Rogów, Poland.
Dzwonko, Z., and Loster, S. (1988). Species richness of small woodlands on the western Carpathian foothills. Vegetatio 76, 15–27. doi: 10.1007/BF00047384
Eisenhauer, N., Lanoue, A., Strecker, T., Scheu, S., Steinauer, K., Thakur, M., et al. (2017). Root biomass and exudates link plant diversity with soil bacterial and fungal biomass. Sci. Rep. 7:44641. doi: 10.1038/srep44641
Escalas, A., Hale, L., Voordeckers, J., Yang, Y., Firestone, M., Alvarez-Cohen, L., et al. (2019). Microbial functional diversity: from concepts to applications. Ecol. Evol. 9, 12000–12016. doi: 10.1002/ece3.5670
Ewers, R., and Didham, R. (2007). Confounding factors in the detection of species responses to habitat fragmentation. Biol. Rev. 81, 117–142. doi: 10.1017/S1464793105006949
Fierer, N., Wood, S., and de Mesquita, C. (2021). How microbes can, and cannot, be used to assess soil health. Soil Biol. Biochem. 153:108111. doi: 10.1016/j.soilbio.2020.108111
Frątczak, W., Michalska-Hejduk, D., Zalewski, M., and Izydorczyk, K. (2019). Effective phosphorous reduction by a riparian plant buffer zone enhanced with a limestone-based barrier. Ecol. Eng. 130, 94–100. doi: 10.1016/j.ecoleng.2019.01.015
Furtak, K., Grządziel, J., Gałązka, A., and Niedźwiecki, J. (2020). Prevalence of unclassified bacteria in the soil bacterial community from floodplain meadows (fluvisols) under simulated flood conditions revealed by a metataxonomic approaches. Catena 188:104448. doi: 10.1016/j.catena.2019.104448
Gałązka, A., Marzec-Grządziel, A., Grządziel, J., Varsadiya, M., and Pawlik, Ł. (2022). Fungal genetic biodiversity and metabolic activity as an indicator of potential biological weathering and soil formation – case study of towards a better understanding of earth system dynamics. Ecol. Indic. 141:109136. doi: 10.1016/j.ecolind.2022.109136
Garcia-Pausas, J., and Paterson, E. (2011). Microbial community abundance and structure are determinants of soil organic matter mineralisation in the presence of labile carbon. Soil Biol. Biochem. 43, 1705–1713. doi: 10.1016/j.soilbio.2011.04.016
Garg, D., Patel, N., Rawat, A., and Rosado, A. (2024). Cutting edge tools in the field of soil microbiology. Curr. Res. Microb. Sci. 6:100226. doi: 10.1016/j.crmicr.2024.100226
Gilliam, F. (2007). The ecological significance of the herbaceous layer in temperate forest ecosystems. BioScience 57, 845–858. doi: 10.1641/B571007
Godefroid, S., and Koedam, N. (2003). Urban plant species patterns are highly driven by density and function of built-up areas. Landsc. Ecol. 22, 1227–1239. doi: 10.1007/s10980-007-9102-x
Graziano, M., Deguire, A., and Surasinghe, T. (2022). Riparian buffers as a critical landscape feature: insights for Riverscape conservation and policy renovations. Diversity 14:172. doi: 10.3390/d14030172
Guilland, C., Maron, P., Damas, O., and Ranjard, L. (2018). Biodiversity of urban soils for sustainable cities. Environ. Chem. Lett. 16, 1267–1282. doi: 10.1007/s10311-018-0751-6
Gus-Stolarczyk, M., Drewnik, M., Szymanski, W., and Stolarczyk, M. (2022). Impact of podzolization on lamellae transformation in sandy soils in a temperate climate – a case study from southern Poland. Geoderma 406:115535. doi: 10.1016/j.geoderma.2021.115535
Hansen, A., Knight, R., Marzluff, J., Powell, S., Brown, K., Gude, P., et al. (2005). Effects of exurban development on biodiversity: patterns, mechanisms, and research needs. Ecol. Appl. 15, 1893–1905. doi: 10.1890/05-5221
He, Y., Lan, Y., Zhang, H., and Ye, S. (2022). Research characteristics and hotspots of the relationship between soil microorganisms and vegetation: a bibliometric analysis. Ecol. Indic. 141:109145. doi: 10.1016/j.ecolind.2022.109145
Hinko-Najera, N., Fest, B., Livesley, S., and Arndt, S. (2015). Reduced throughfall decreases autotrophic respiration, but not heterotrophic respiration in a dry temperate broadleaved evergreen forest. Agric. For. Meteorol. 200, 66–77. doi: 10.1016/j.agrformet.2014.09.013
Hu, S., Yue, H., and Zhou, Z. (2019). Preferences for urban stream landscapes: opportunities to promote unmanaged riparian vegetation. Urban For. Urban Green. 38, 114–123. doi: 10.1016/j.ufug.2018.12.001
Hwang, Y., and Roscoe, C. (2017). Preference for site conservation in relation to on-site biodiversity and perceived site attributes: an on-site survey of unmanaged urban greenery in a tropical city. Urban For. Urban Green. 28, 12–20. doi: 10.1016/j.ufug.2017.09.011
Kaneda, S., Krištůfek, V., Baldrian, P., Malý, S., and Frouz, J. (2019). Changes in functional response of soil microbial community along Chronosequence of spontaneous succession on post mining Forest sites evaluated by biolog and SIR methods. Forests 10:1005. doi: 10.3390/f10111005
Khafida, W., Klimek, B., and Niklińska, M. (2024). The relationship between soil bacteria carbon utilization and soil physicochemical properties. E3S Web Conf. 495:02006. doi: 10.1051/e3sconf/202449502006
Klimek, B., Chodak, M., Jaźwa, M., Solak, A., Tarasek, A., and Niklińska, M. (2016). The relationship between soil bacteria substrate utilisation patterns and the vegetation structure in temperate forests. Eur. J. For. Res. 135, 179–189. doi: 10.1007/s10342-015-0929-4
Klimek, B., and Niklińska, M. (2024). Changes in temperature sensitivity of forest litter during decomposition along an altitudinal gradient in temperate mountains – a reciprocal litter transplantation study. Catena 240:107977. doi: 10.1016/j.catena.2024.107977
Knapp, S., Kühn, I., Wittig, R., Ozinga, W., Poschlod, P., and Klotz, S. (2008). Urbanization causes shifts in species’ trait state frequencies. Preslia 80, 375–388.
Koczorski, P., Furtado, B., Gołębiewski, M., Hulisz, P., Baum, C., Weih, M., et al. (2022). The effects of host plant genotype and environmental conditions on fungal community composition and phosphorus Solubilization in willow short rotation coppice. Front. Plant Sci. 12:647709. doi: 10.3389/fpls.2021.647709
Kostrakiewicz-Gierałt, K., Gmyrek, K., and Pliszko, A. (2022). The effect of the distance from a path on abiotic conditions and vascular plant species in the undergrowth of urban forests and parks. Int. J. Environ. Res. Public Health 19:5621. doi: 10.3390/ijerph19095621
Kuzovkina, Y., Schulthess, C., and Zheng, D. (2018). Influence of soil chemical and physical characteristics on willow yield in Connecticut. Biomass Bioenergy 108, 297–306. doi: 10.1016/j.biombioe.2017.11.021
Li, S., Wang, P., Chen, Y., Wilson, M., Yang, X., Ma, C., et al. (2020). Island biogeography of soil bacteria and fungi: similar patterns, but different mechanisms. ISME J. 14, 1886–1896. doi: 10.1038/s41396-020-0657-8
Liu, L., Zhang, Z., Wang, X., Zhang, R., Wang, M., Wurzburger, N., et al. (2023). Urbanization reduces soil microbial network complexity and stability in the megacity of Shanghai. Sci. Total Environ. 893:164915. doi: 10.1016/j.scitotenv.2023.164915
Lumivero . (2020). XLSTAT statistical and data analysis solution. Available at: https://www.xlstat.com/en. (accessed July 22, 2024).
Ma, Z., Zhang, P., Hu, N., Wang, G., Dong, Y., Guo, Y., et al. (2023). Understanding the drivers of woody plant diversity in urban parks in a snow climate city of China. J. For. Res. 34, 1021–1032. doi: 10.1007/s11676-022-01535-9
MacArthur, R., and Wilson, E. (1967). The theory of island biogeography. Princeton, USA: Princeton University Press.
Machado, J., and Kim, G. (2024). Ecological landscape assessment of restored urban stream to guide adaptive management. Heliyon 10:e33880. doi: 10.1016/j.heliyon.2024.e33880
Macicka, T., and Wilczyńska, W. (1988). Lasy liściaste ścinawskiego obniżenia Odry [Forest of Odra Valley]. Acta Universitatis Wratislaviensis. Prace Botaniczne 40, 131–171.
Maron, P., Sarr, A., Kaisermann, A., Lévêque, J., Mathieu, O., Guigue, J., et al. (2018). High microbial diversity promotes soil ecosystem functioning. Appl. Environ. Microbiol. 84, e02738–e02717. doi: 10.1128/AEM.02738-17
McKinney, M. (2006). Urbanization as a major cause of biotic homogenization. Biol. Conserv. 127, 247–260. doi: 10.1016/j.biocon.2005.09.005
Metzler, P., Ksiazek-Mikenas, K., and Chaudhary, V. (2024). Tracking arbuscular mycorrhizal fungi to their source: active inoculation and passive dispersal differentially affect community assembly in urban soils. New Phytol. 242, 1814–1824. doi: 10.1111/nph.19526
Mgelwa, A., Hu, Y.-L., Xu, W.-B., Ge, Z.-Q., and Yu, T.-W. (2019). Soil carbon and nitrogen availability are key determinants of soil microbial biomass and respiration in forests along urbanized rivers of southern China. Urban For. Urban Green. 43:126743:126351. doi: 10.1016/j.ufug.2019.05.013
Nugent, A., and Allison, S. (2022). A framework for soil microbial ecology in urban ecosystems. Ecosphere 13:e3968. doi: 10.1002/ecs2.3968
Olejniczak, M., Spiering, D., Potts, D., and Warren, R. (2018). Urban forests form isolated archipelagos. J. Urban Ecol. 4, 1–8. doi: 10.1093/jue/juy007
Picard, P., and Tran, T. (2021). Small urban green areas. J. Environ. Econ. Manag. 106:102418. doi: 10.1016/j.jeem.2021.102418
Pickett, S., Cadenasso, M., Grove, J., Nilon, C., Pouyat, R., Zipperer, W., et al. (2001). Urban ecological systems: linking terrestrial ecological, physical, and socioeconomic components of metropolitan areas. Annu. Rev. Ecol. Syst. 32, 127–157. doi: 10.1146/annurev.ecolsys.32.081501.114012
Pielech, R. (2021). Plant species richness in riparian forests: comparison to other forest ecosystems, longitudinal patterns, role of rare species and topographic factors. For. Ecol. Manag. 496:119400. doi: 10.1016/j.foreco.2021.119400
Pregitzer, C., Ashton, M., Charlop-Powers, S., D’Amato, A., Frey, B., Gunther, B., et al. (2019). Defining and assessing urban forests to inform management and policy. Environ. Res. Lett. 14:085002. doi: 10.1088/1748-9326/ab2552
Preston-Mafham, J., Boddy, L., and Randerson, P. (2002). Analysis of microbial community functional diversity using sole-carbon source utilisation profiles—a critique. FEMS Microbiol. Ecol. 42, 1–14. doi: 10.1111/j.1574-6941.2002.tb00990.x
Prober, S., Leff, J., Bates, S., Borer, E., Firn, J., Harpole, S., et al. (2015). Plant diversity predicts beta but not alpha diversity of soil microbes across grasslands worldwide. Ecol. Lett. 18, 85–95. doi: 10.1111/ele.12381
Przepióra, F., and Ciach, M. (2022). Tree microhabitats in natural temperate riparian forests: an ultra-rich biological complex in a globally vanishing habitat. Sci. Total Environ. 803:149881. doi: 10.1016/j.scitotenv.2021.149881
Pyšek, P. (1998). Alien and native species in central European urban floras: a quantitative comparison. J. Biogeogr. 25, 155–163. doi: 10.1046/j.1365-2699.1998.251177.x
Raheem, A., Yohanna, P., Li, G., Noh, N., Iqbal, B., Tang, J., et al. (2024). Unraveling the ecological threads: how invasive alien plants influence soil carbon dynamics. J. Environ. Manag. 356:120556. doi: 10.1016/j.jenvman.2024.120556
Raimbaultx, A., Brin, A., Manzi, S., Savoie, J., Gandois, L., Oliva, P., et al. (2024). Influence of habitat fragmentation and habitat amount on soil fungi communities in ancient forests. Landsc. Ecol. 39:19. doi: 10.1007/s10980-024-01821-3
Richert, J., Albuquerque, J., and Kaiser, D. (2009). Estimation of water retention and availability in soils of Rio Grande Do Sul. Rev. Bras. Ciênc. Solo 33, 1547–1560. doi: 10.1590/S0100-06832009000600004
Sabatini, F., Keeton, W., Lindner, M., Svoboda, M., Verkerk, P., Bauhus, J., et al. (2020). Protection gaps and restoration opportunities for primary forests in Europe. Divers. Distrib. 26, 1646–1662. doi: 10.1111/ddi.13158
Siwek, P., Jaźwa, M., Niklińska, M., and Klimek, B. (2024). Soil bacterial activity and functional diversity as indicators of recultivation of alkaline settlements of a “Solvay” process. J. Soil. Sediment. 24, 2791–2802. doi: 10.1007/s11368-024-03814-w
Soboyejo, L., Russell, K., and Fletcher, T. (2025). Predicting urban channel morphology amidst multiple complexities. Geomorphology 468:109511. doi: 10.1016/j.geomorph.2024.109511
Stefańska-Krzaczek, E. (2013). Species richness of drained riparian forests in the urban area of Wrocław. Sylwan 157, 366–375.
Štursová, M., Bárta, J., Šantrůčková, H., and Baldrian, P. (2016). Small-scale spatial heterogeneity of ecosystem properties, microbial community composition and microbial activities in a temperate mountain forest soil. FEMS Microbiol. Ecol. 92:fiw185. doi: 10.1093/femsec/fiw185
Teerlinck, J., Wittemans, K., Beele, E., Dewaelheyns, V., Steen, T., and Somers, B. (2024). What can nature-based solutions in domestic gardens contribute to climate change adaption in Western-Europe? A systematic review. Front. Environ. Sci. 12:1430739. doi: 10.3389/fenvs.2024.1430739
Tilman, D., Knops, J., Wedin, D., Reich, P., Ritchie, M., and Siemann, E. (1997). The influence of functional diversity and composition on ecosystem processes. Science 277, 1300–1302. doi: 10.1126/science.277.5330.1300
Toju, H., and Sato, H. (2018). Root-associated fungi shared between arbuscular mycorrhizal and ectomycorrhizal conifers in a temperate forest. Front. Microbiol. 9:433. doi: 10.3389/fmicb.2018.00433
Tokarska-Guzik, B., Dajdok, Z., Zając, M., Zając, A., Urbisz, A., Danielewicz, W., et al. (2012). Alien plant species in Poland with special consideration of invasive species. Warszawa: General Directorate for Environmental Protection.
van der Heijden, M., Bardgett, R., and van Straalen, N. (2008). The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 11, 296–310. doi: 10.1111/j.1461-0248.2007.01139.x
Van der Maarel, E. (1979). Transformation of cover-abundance values in phytosociology and its effects on community similarity. Vegetatio 39, 97–114. doi: 10.1007/BF00052021
Von Thaden, J., Badillo-Montaño, R., Lira-Noriega, A., García-Ramírez, A., Benítez, G., Equihua, M., et al. (2021). Contributions of green spaces and isolated trees to landscape connectivity in an urban landscape. Urban For. Urban Green. 64:127277. doi: 10.1016/j.ufug.2021.127277
Wang, C., and Kuzyakov, Y. (2024). Mechanisms and implications of bacterial–fungal competition for soil resources. ISME J. 18:wrae073. doi: 10.1093/ismejo/wrae073
Wang, Z., and Yang, J. (2022). Urbanization strengthens the edge effects on species diversity and composition of woody plants in remnant forests. For. Ecosyst. 9:100063. doi: 10.1016/j.fecs.2022.100063
Wang, M., Yu, S., Chen, X., Liu, X., Zheng, H., Wu, W., et al. (2022). Soil microbial community changes in response to the environmental gradients of urbanization in Guangzhou City. Urban Ecosyst. 25, 1865–1874. doi: 10.1007/s11252-022-01279-8
Wang, Z., Zhao, M., Yan, Z., Yang, Y., Niklas, K., Huang, H., et al. (2022). Global patterns and predictors of soil microbial biomass carbon, nitrogen, and phosphorus in terrestrial ecosystems. Catena 211:106037. doi: 10.1016/j.catena.2022.106037
Wasak, K., Klimek, B., and Drewnik, M. (2019). Rapid effects of windfall on soil microbial activity and substrate utilization patterns in the forest belt in the Tatra Mountains. J. Soil. Sediment. 20, 801–815. doi: 10.1007/s11368-019-02439-8
Woźniak, M., Radzimski, A., and Wajchman-Świtalska, S. (2025). Is more always better? Evaluating accessibility to parks and forests in 33 European cities using sustainable modes of transportation. Urban For. Urban Green. 104:128656. doi: 10.1016/j.ufug.2024.128656
Xu, M., Yang, X., Shao, J., Huang, J., Fan, W., Yang, A., et al. (2024). Biogeographic effects shape soil bacterial communities across intertidal zones on island beaches through regulating soil properties. Sci. Total Environ. 930:172785. doi: 10.1016/j.scitotenv.2024.172785
Yang, X., Wang, Y., Xu, Q., Liu, W., Liu, L., Wu, Y., et al. (2021). Soil fertility underlies the positive relationship between island area and litter decomposition in a fragmented subtropical forest landscape. Catena 204:105414. doi: 10.1016/j.catena.2021.105414
Zhang, P., Dong, Y., Guo, Y., Wang, C., Wang, G., Ma, Z., et al. (2023). Urban forest soil is becoming alkaline under rapid urbanization: a case study of Changchun, Northeast China. Catena 224:106993. doi: 10.1016/j.catena.2023.106993
Zhang, C., Li, D., Wei, J., Zhao, L., and Liu, F. (2020). Soil respiration and its responses to biotic and abiotic factors in patchy remnant forests and urban green landscapes of Tianjin, China. Urban For. Urban Green. 53:126743. doi: 10.1016/j.ufug.2020.126743
Keywords: bacterial communities, functional diversity, microorganisms biogeography, riparian forests, urban soils
Citation: Koster G, Jaźwa M, Przemieniecki SW, Musielok Ł, Azarbad H and Klimek B (2025) Size matters: larger fragments of riparian forest in urban areas support functional diversity of soil bacteria more than smaller ones. Front. Microbiol. 16:1517545. doi: 10.3389/fmicb.2025.1517545
Edited by:
Zifang Chi, Jilin University, ChinaReviewed by:
Sebastien Terrat, Université de Bourgogne, FranceZhibin Ren, Northeast Institute of Geography and Agroecology (CAS), China
Copyright © 2025 Koster, Jaźwa, Przemieniecki, Musielok, Azarbad and Klimek. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hamed Azarbad, YXphcmJhZEBzdGFmZi51bmktbWFyYnVyZy5kZQ==; YXphcmJhZC5oYW1lZEBnbWFpbC5jb20=
 Gabriela Koster1
Gabriela Koster1
 Małgorzata Jaźwa
Małgorzata Jaźwa Hamed Azarbad
Hamed Azarbad Beata Klimek
Beata Klimek