
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 25 March 2025
Sec. Microorganisms in Vertebrate Digestive Systems
Volume 16 - 2025 | https://doi.org/10.3389/fmicb.2025.1504264
This article is part of the Research TopicGut Microbiota Modulation to Mitigate Stress-Induced Functional ChangesView all 11 articles
Long photoperiods are often characterized by enhanced oxidative stress-induced damage to skeletal muscle, reduced melatonin (MT) levels and intestinal microbiota dysfunction in broilers. In this study, we aimed to investigate the association of breast muscle morphology with melatonin levels and the cecal microbiota of broilers under different photoperiods. A total of 216 healthy 5-day-old Arbor Acres (AA) male broilers were randomly assigned to 12 L:12D, 18 L:6D and 24 L:0D photoperiods for 4 weeks (L = hours of light, D = hours of darkness). The concentration of inflammatory factors and MT concentrations was measured using ELISA kits, whereas breast muscle morphology was examined through the hematoxylin (H) and eosin (E) staining, and microbiota composition was identified through 16 s rRNA analysis. Extended light exposure significantly improved the growth rate of broilers, but significantly decreased feed efficiency (FE). Furthermore, it upregulated the concentration of IL-1β, IL-6 and TNF-α and induced an abnormal breast muscle morphology. Extended light exposure significantly decreased MT levels in the hypothalamus, cecum and breast muscle, while triggering the cecal microbiota composition disorder. Specifically, there was significant alteration to the dominant bacterial phylum, following exposure to long photoperiods, with the abundance of Firmicutes decreasing and the abundance of Bacteroidota increasing. Notably, the relative abundance of Lactobacillus showed a positive correlation with MT levels and a negative correlation with inflammatory cytokines. In conclusion, the present findings indicated that extended light exposure reduced the MT levels, which were related to disturbed cecal microbiota, damaging breast muscle morphology and inducing breast muscle inflammation in broilers.
Long photoperiods, which are commonly applied in the modern and intensive broiler industry, aim to maximize the production efficiency through enhanced feed intake (Lewis and Gous, 2007). The photoperiods have also been reported to be critical factors in creating a suitable breeding environment to meet the increasing demand in the broiler chicken market. However, long photoperiods have been shown to induce detrimental effects on the quality of chicken products have been reported. For instance, it was reported that long photoperiods increased oxidative stress levels in the breast muscle of broilers (Li et al., 2010; Tuell et al., 2020), which may potentially damage the skeletal muscle morphology and induce atrophy (Zhang et al., 2023). Long photoperiods effectively increased the white striping occurrence of broilers, a muscle myopathy associated with oxidative stress (Gratta et al., 2023). Furthermore, photobiomodulation was found to enhance breast muscle atrophy in mice (Scalon et al., 2022). However, the influence of photoperiod treatments on breast muscle morphology is not completely understood.
Nighttime light exposure was demonstrated to decrease melatonin (MT) synthesis and secretion in broilers (Yang et al., 2022; Zhao et al., 2019; Saito et al., 2005). Notably, MT is a photoperiod-regulated hormone, involved in the transmission of photoperiodic signals to the peripheral organs (Tiwari et al., 2023). Understanding how the MT pathway regulates peripheral organ function in response to diverse photoperiods is a critical frontier in biological research. Some studies postulated that MT may alter skeletal muscle growth in birds and mammals: improving the myofiber formation of chick embryos (Bai et al., 2019), enhancing lipid mobilizing action in the skeletal muscle of the pigeon (John and George, 1976) and increasing the RNA content in the breast muscle of Japanese quail Coturnix coturnix japonica (Zeman et al., 1993). In mice, MT plays a role in the repair of skeletal muscle morphology injury (Su et al., 2023; Salagre et al., 2023), and, in rats, it accelerates the repair of muscle injury to promote skeletal muscle development and growth (Duan et al., 2022; Salucci et al., 2021). MT maintains the homeostasis of gut microbiota has been shown by influencing the intestinal bacterial community in broilers (Wang et al., 2020). Specifically, MT altered microbiota diversity, composition and function in geese (Li et al., 2020) and improved ileal morphology, barrier function, short-chain fatty acid (SCFA) profile, and microbial flora in laying ducks (Cui et al., 2022). Exogenous melatonin supplementation attenuated the intestinal microbiota disruption and restored normal intestinal microbiota function in mice (Li et al., 2023; Li et al., 2023).
Studies have uncovered that gut microbiota imbalance is often positively correlated with stress (Clavijo and Flórez, 2018). Exposure to different photoperiods induced alterations of the gut microbiota composition in broilers and mice (Oyola et al., 2021; Wang et al., 2018). Elsewhere, it was found that endogenous gut microbes studies have found that endogenous gut microbes regulated skeletal muscle growth in broilers (Jiang et al., 2023). In broiler, intestinal microbiota plays an important role in skeletal muscle development by modulating body metabolism and immunity (Zhang et al., 2022; Yin et al., 2024; Zhang et al., 2022). There is evidence that gut microbiota may regulate wooden breast myopathy by fine-tuning the dynamic changes in digestive metabolites in broilers (Kang et al., 2022). Moreover, breast muscle metabolites were strongly associated with various aspects of cecal microbes in broilers (Feng et al., 2022; Wen et al., 2023). However, research on the association of melatonin and cecal microbiota with the morphology of breast muscle under different photoperiods is limited. In this study, we speculated that melatonin and gut microbiota might be potential regulatory pathways mediating the effects of photoperiods. The aim of this study was to investigate the association between MT and cecal microbiota under extended light exposure and explore the potential regulatory pathways by which different photoperiods affect breast muscle morphology of broilers.
The study was approved by the Institutional Ethics Committee of Experiment Animal Welfare and Ethics at the Institute of Animal Science of Chinese Academy of Agricultural Sciences (CAAS) (permit number: IAS 2022–117), and all the methods were carried out in accordance with relevant Institutional guidelines and regulations. And this study was confirmed that all methods were carried out in accordance with the guidelines and regulations of The ARRIVE guidelines 2.0.
A total of 216 Arbor Acres (AA) male broilers (5-day old) with similar body weight (75 g ± 10) were randomly assigned to 3 photoperiod treatments (SD, LD and FD) with 6 replicates per treatment, with 12 broilers per replicate. The 3 groups (SD, LD and FD) received different photoperiods with 12 L:12D (SD, 12 h light), 18 L:6D (LD, 18 h light) and 24 L:0D (FD, 24 h light) for 4 weeks, respectively. The bird houses illuminated with LED lighting with 20 lux light intensity. All groups (SD, LD, and FD) received a standard corn and soybean meal basal diet in three feeding programs (5–7 days-old, 8–20 days-old and 21–32 days-old) (Table 2), formulated according to AA broiler recommendations (SCRIBD, 2019). The formal experimental period lasted 4 weeks (from 5 to 32 day of age). Broilers were housed in stainless steel cages without roofs (0.82 m width×0.70 m length×0.60 m height). The temperature and humidity shall be carried out according to the standard of the AA Broiler Feeding Management Manual. All broilers were farmed in different artificial climate chambers with the same size (4.08 m × 2.88 m × 2.38 m) of State Key Laboratory of Animal Nutrition and Feeding, Chinese Academy of Agricultural Sciences. Except for the photoperiods in the artificial climate chambers, other environmental parameters remained the same. Broilers were allowed free access to experimental diets and water (Table 1).
On 14 d and 28 d, one broiler from each replicate was selected with body weights close to the average after 12 h of feed deprivation. Then, the broilers from each group were killed by carbon dioxide (CO2). Tissue samples of the hypothalamus and breast muscle rinsed with sterile normal saline (NaCl 9 g/L) were immediately collected and snap-frozen in liquid nitrogen, then kept in a − 80°C freezer for measurements of gene expression and biochemical analysis. Another part of the breast muscle was immobilized by 4% paraformaldehyde to hematoxylin–eosin (HE) staining for histomorphological analysis. Cecal contents were immediately collected at d 28, and snap-frozen in liquid nitrogen, then kept in a − 80°C freezer for high-throughput sequencing and further analysis.
On 14 d and 28 d, the provided and residual feed amount, and broiler body weight of each replicate were recorded. The average body weight, average daily gain (ADG), average daily feed intake (ADFI), and feed efficiency (FE, FE = ADG/ADFI) were calculated. The right breast muscle of 1 broiler chicken from each replicate was randomly selected to determine the right breast muscle weight and right breast muscle mass-body mass ratio.
The interleukin (IL)-1β (ZB00001DU, Zhongshang Boao Biotechnology Co., Ltd., Beijing, China), IL-6 (ZB00006DU, Zhongshang Boao Biotechnology Co., Ltd., Beijing, China) and tumor necrosis factor (TNF)-α (KJEIA0018D, Kangjia Hongyuan Biotechnology Co., Ltd., Beijing, China) concentrations in breast muscle were measured using ELISA kits by the Multiskan MK3 microplate reader (Thermo Fisher Scientific, Massachusetts, United States) according to the manufacturer’s instructions. Transfer the breast muscle into a glass homogenizer and add 5–10 mL of pre-cooled PBS buffer (1: 5 mass/volume ratio of tissue to PBS buffer is recommended) for thorough grinding, centrifuge the prepared homogenate at 3500 r/min for 15 min, and then retain the supernatant for assay (Yu et al., 2024). All the kits are chicken-specific. The intra-assay coefficient and the inter-assay coefficient of variation were 5 and 10%, respectively.
4% Paraformaldehyde-fixed breast muscle were embedded in paraffin (KD-BMIV biological tissue embedding machine, KEDEE, Jinhua, China) after being trimmed, dehydrated, transparentized, and waxed. After the wax block was made, the samples were sectioned (KD-2268 paraffin microtome, KEDEE, Jinhua, China) and then stained with hematoxylin (H) and eosin (E). The sections of each tissue were observed using a panoramic scanner (PANORAMIC® 250 Flash III DX, 3DHISTECH Ltd., Budapest, Hungary) and photographed.
The concentrations of MT in the hypothalamus and cecum were measured using ELISA kits (CEA908Ge, Kangjia Hongyuan Biotechnology Co., Ltd., Beijing, China) by the Multiskan MK3 microplate reader (Thermo Fisher Scientific, Massachusetts, United States) according to the manufacturer’s instructions. The sample handling procedure was the same as described in 2.4. This kit was specifically used to detect MT and showed no obvious cross-reactivity with other similar substances. This kit was suitable for the pan-species (general) in all tissues. The intra-assay coefficient and the inter-assay coefficient of variation were 5 and 10%, respectively.
Total microbial genomic DNA was extracted from cecal content samples using the E.Z.N.A.® soil DNA Kit (Omega Bio-tek, Norcross, GA, U.S.) according to the manufacturer’s instructions. The quality and concentration of DNA were determined by 1.0% agarose gel electrophoresis and a NanoDrop2000 spectrophotometer (Thermo Scientific, United States) and kept at −80°C before further use. The hypervariable region V3-V4 of the bacterial 16S rRNA gene was amplified with primer pairs 338F (5’-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5’-GGACTACHVGGGTWTCTAAT-3′) (Liu et al., 2016) byT100 Thermal Cycler PCR thermocycler (BIO-RAD, United States). The PCR product was extracted from 2% agarose gel and purified using the PCR Clean-Up Kit (YuHua, Shanghai, China) according to manufacturer’s instructions and quantified using Qubit 4.0 (Thermo Fisher Scientific, USA). Purified amplicons were pooled in equimolar amounts and paired-end sequenced on an Illumina PE300/PE250 platform (Illumina, San Diego, USA) according to the standard protocols by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China). Raw FASTQ files were de-multiplexed using an in-house perl script, and then quality-filtered by fastp version 0.19.6 and merged by FLASH version 1.2.7. To minimize the effects of sequencing depth on alpha and beta diversity measure, the number of 16S rRNA gene sequences from each sample were rarefied to 20,000, which still yielded an average Good’s coverage of 99.09%, respectively.
All the results from the experiment were analyzed by using the 1-way ANOVA, performed using SPSS 23.0 (SPSS Inc., Chicago, IL). GraphPad Prism 8.0 (GraphPad Inc., San Diego, CA) was used for drawing. Replicate (n = 6) served as the experimental unit. The results in the tables are shown with the mean ± standard error of the mean (SEM). The p > 0.05, and p < 0.05 were deemed the statistical non-significance and significance, respectively. Bioinformatic analysis of the cecal content microbiota was carried out using the Majorbio Cloud platform.1 The taxonomy of each OTU representative sequence was analyzed by RDP Classifier version 2.2 against the 16S rRNA gene database (eg. Silva v138) using confidence threshold of 0.7. Based on the OTUs information, rarefaction curves and alpha diversity indices were calculated with Mothur v1.30.1 (Schloss et al., 2009). The similarity among the microbial communities in different samples was determined by principal coordinate analysis (PCoA) based on Bray–curtis dissimilarity using Vegan v2.5–3 package. The PERMANOVA test was used to assess the percentage of variation explained by the treatment along with its statistical significance using Vegan v2.5–3 package. A correlation between two nodes was considered to be statistically robust if the spearman’s correlation coefficient over 0.6 or less than −0.6, and the p value less than 0.01.
As shown in Table 2, long photoperiods had significant effects on the ADG and ADFI (p < 0.05). Furthermore, in the FD group, FE was significantly reduced (p < 0.05). In the 1–14 days of the experiment, the LD group and the FD group broilers had a significant increase in the ADG and ADFI compared to those in the SD group, with increases of 17.33 and 19.33%, and 18.07 and 22.37%, respectively. Additionally, compared with the SD group, the right breast muscle mass (p < 0.05) and right breast muscle mass-body mass ratio (p < 0.05) were significantly increased due to long photoperiods. However, there was a significant decrease in FE in the LD group and the FD group by 17.38 and 16.81% compared to the SD group, respectively (p < 0.05). During weeks 3 to 4 of the experiment, the LD group and the FD group had significantly higher ADG and ADFI than the SD group (p < 0.05), while the FE of the LD group and the FD group significantly decreased by 8.26 and 16.77% compared to that of the SD group, respectively. The increasing light time had a significant effect on right breast muscle growth, with a 14.90 and 20.75% increase in the LD group and the FD group, respectively (p < 0.05), while there was no significant effect on the right breast muscle growth-body growth ratio (p = 0.576). Additionally, compared with the SD group, both the LD group and the FD group significantly increased the ADG and ADFI over the 4 weeks (p < 0.05), but the LD group and the FD group had a significant decrease in the FE (p < 0.05). On day 28 of the trial, both the LD group and the FD group significantly increased the right breast muscle mass compared to the SD group (p < 0.05). However, only the right breast muscle mass-body mass ratio of the FD group significantly increased, whereas that of the LD group had no significant effect compared to the SD group (p = 0.109). These data suggest that the long photoperiods led to a substantial improvement in growth rate, with a significant decrease in the FE.
To investigate the impact of different photoperiods on melatonin, we measured the MT concentrations in the hypothalamus, cecum and breast muscle (Figure 1). As depicted in Figure 1, following treatment with extending light exposure, the concentrations of MT significantly decreased in the hypothalamus, cecum and breast muscle (p < 0.05).
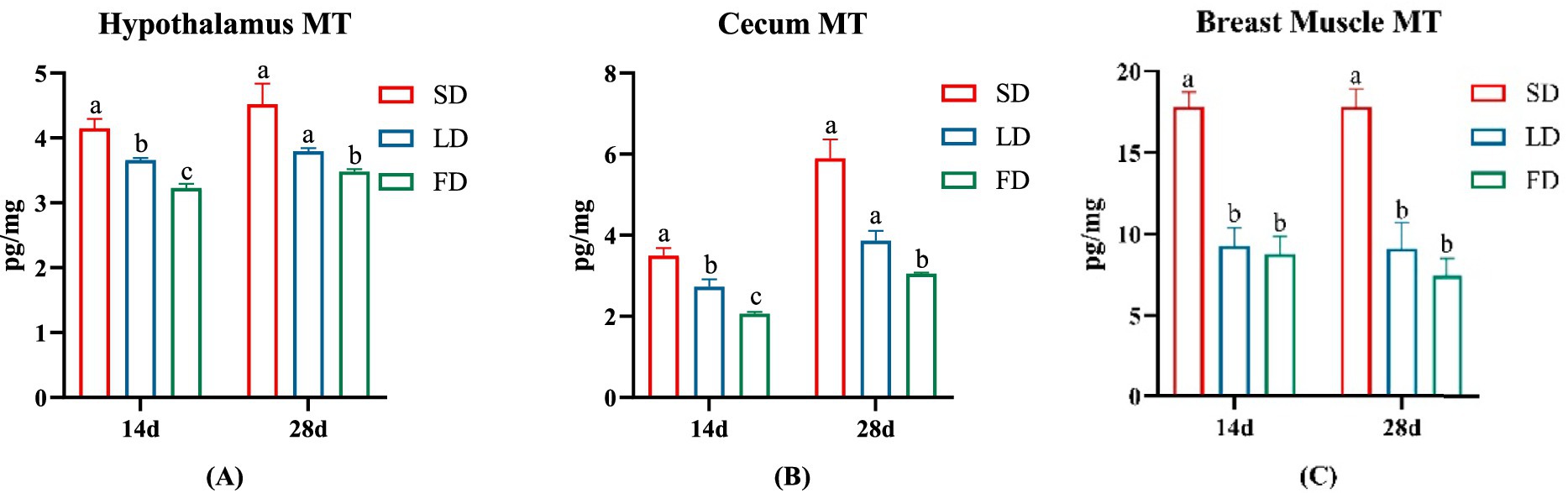
Figure 1. Effects of different photoperiods on MT concentrations of broilers. (A) Hypothalamus. (B) Cecum. (C) Breast muscle. Data are presented as Mean ± SEMs. Different superscripts (a, b, c) in each parameter indicate significance (p < 0.05).
As depicted in Figures 2A–C, we observed abnormal organizational structure in both the LD group and the FD group during 14 days. The overall structure of the breast muscle appeared normal in the 14d SD group, with no signs of muscle fiber atrophy or necrosis (black arrow indicates muscle fiber) (Figure 2A). However, the 14d LD group exhibited mild histopathological abnormalities in the breast muscle, including necrosis (black arrow indicates muscular bundle morphology lysis and disappearance) and minor myogenic injury in the muscular bundle (red arrow indicates intranuclear migration) (Figure 2B). Additionally, the 14d FD group showed injuries to the overall structure of the breast muscle, such as extensive muscular bundle injury and atrophy (black arrow indicates increasing gap, red arrows indicate intranuclear migration), muscular bundle interstitium fibrosis (yellow arrow indicates fibroblast hyperplasia), and inflammatory cell infiltration (green arrow) (Figure 2C).
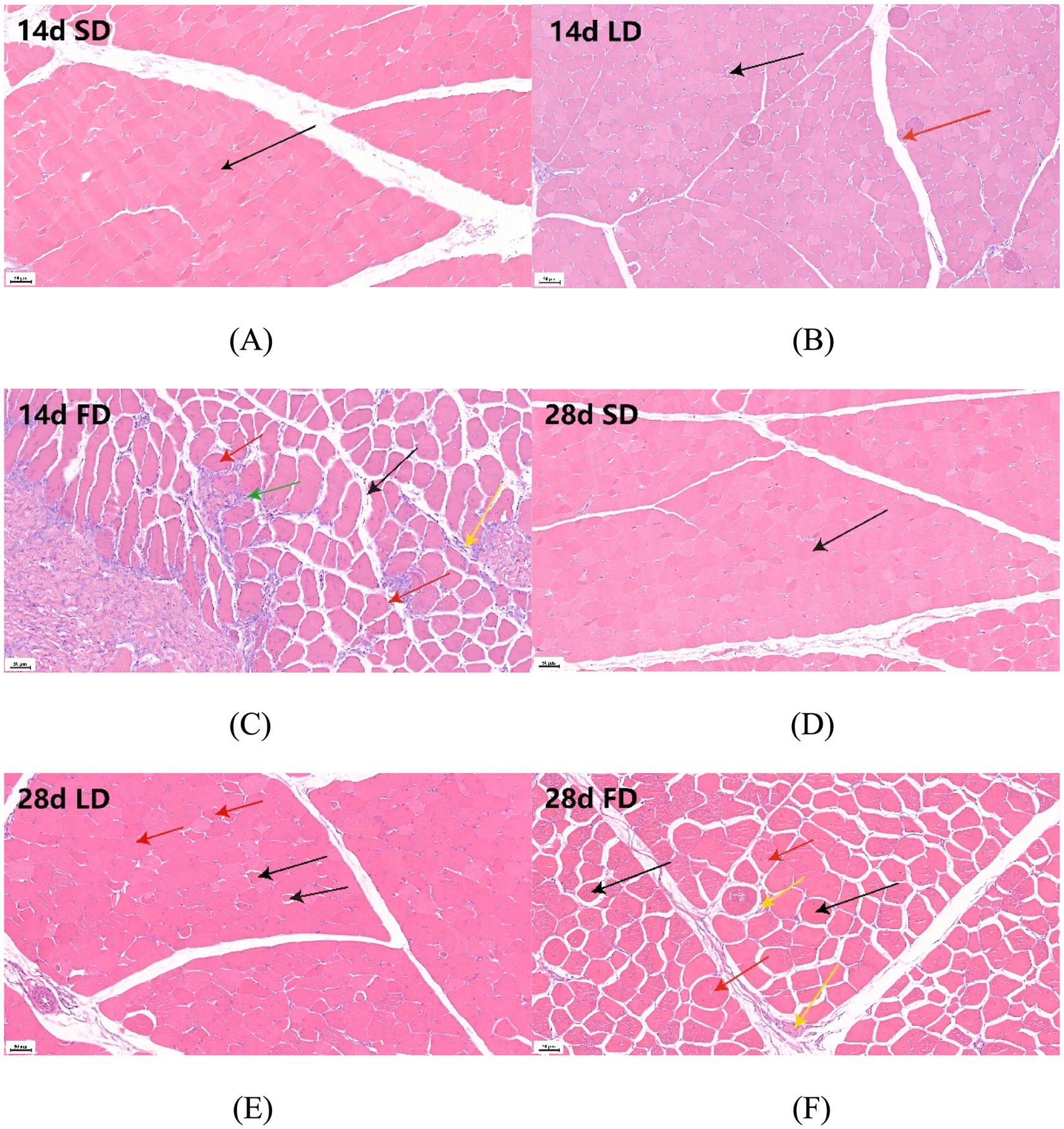
Figure 2. Effects of different photoperiods on the breast muscle morphology of broilers. (A) 14d SD group. (B) 14d LD group. (C) 14d FD group. (D) 28d SD group. (E) 28d LD group. (F) 28d FD group. Figures were 20× magnification. (A) The black arrow indicates muscle fiber. (B) The black arrow indicates muscular bundle morphology lysis and disappearance; the red arrow indicates intranuclear migration. (C) The black arrow indicates an increasing gap; the red arrows indicate intranuclear migration; the yellow arrow indicates fibroblast hyperplasia; the green arrow indicates inflammatory cell infiltration. (D) The black arrow indicates muscle fiber. (E) The black arrow indicates an increasing gap; the red arrow indicates intranuclear migration. (F) The black arrow indicates an increasing gap; the red arrows indicate intranuclear migration; the yellow arrow indicates fibroblast hyperplasia.
As also shown in Figures 2D–F, the abnormal organizational structure was observed to increase in the LD group and the FD group at 28 days. The overall structure of the breast muscle in the 28d SD group was normal, similar to that of the 14d SD group (Figure 2D). The 28-day LD group exhibited both myogenic injury and atrophy, as indicated by the increasing gap (black arrows) and intranuclear migration (red arrows) (Figure 2E). Similarly, the breast muscle in the 28-day FD group showed abnormalities throughout the day, with myogenic injury and atrophy observed in the muscular bundle, along with increasing gaps (black arrows), intranuclear migration (red arrows), and fibroblast hyperplasia in the muscular bundle interstitium (yellow arrows) (Figure 2F). The above results suggested that extended light exposure could injure the breast muscle morphology with inflammatory cell infiltration.
To further verify the inflammation levels of the breast muscles, we examined the levels of IL-1 β, IL-6 and TNF α in the breast muscle. The inflammatory factor concentrations on the breast muscle of broilers significantly increased under extending light exposure. On 14d and 28d, compared with the SD group, the concentrations of IL-1 β, IL-6 and TNF α in breast muscle significantly increased in the LD group and the FD group (p < 0.05; Figure 3).
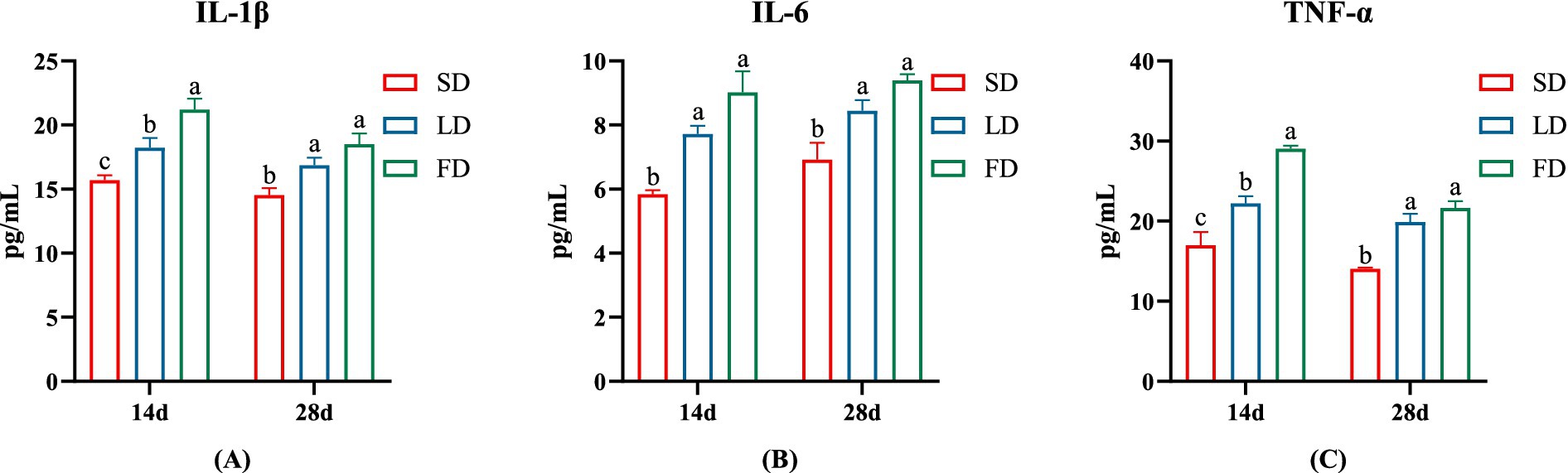
Figure 3. Effects of different photoperiods on inflammatory cytokines concentrations of broilers. (A) IL-1β. (B) IL-6. (C) TNF-α. Data are presented as Mean ± SEMs. Different superscripts (a, b, c) in each parameter indicate significance (p < 0.05).
Finally, we analyzed the composition of cecal microbiota. The ACE index (A), Chao index (B), Shannon index (C), and Simpson index (D) are shown in Figure 4. The results indicated that the indices reflecting species richness (ACE index and Chao index) had no significant effect under different photoperiods (p > 0.05). However, the indices of community diversity (Shannon index and Simpson index) were significantly affected (p < 0.05) under different photoperiods. These findings suggested that the community diversity exhibited an increase followed by a decrease with the increase of photoperiods. At the OTU level, Venn diagram showed microorganisms found in the cecal content were identified with 664, 698, and 813 OTUs, respectively. (Figure 5). Moreover, the overall structure of the cecal microbiota on the OTU level in the three groups significantly differed (R2 = 0.4282, p = 0.001), as analyzed by principal coordinate analysis (PCoA) (Figure 6).
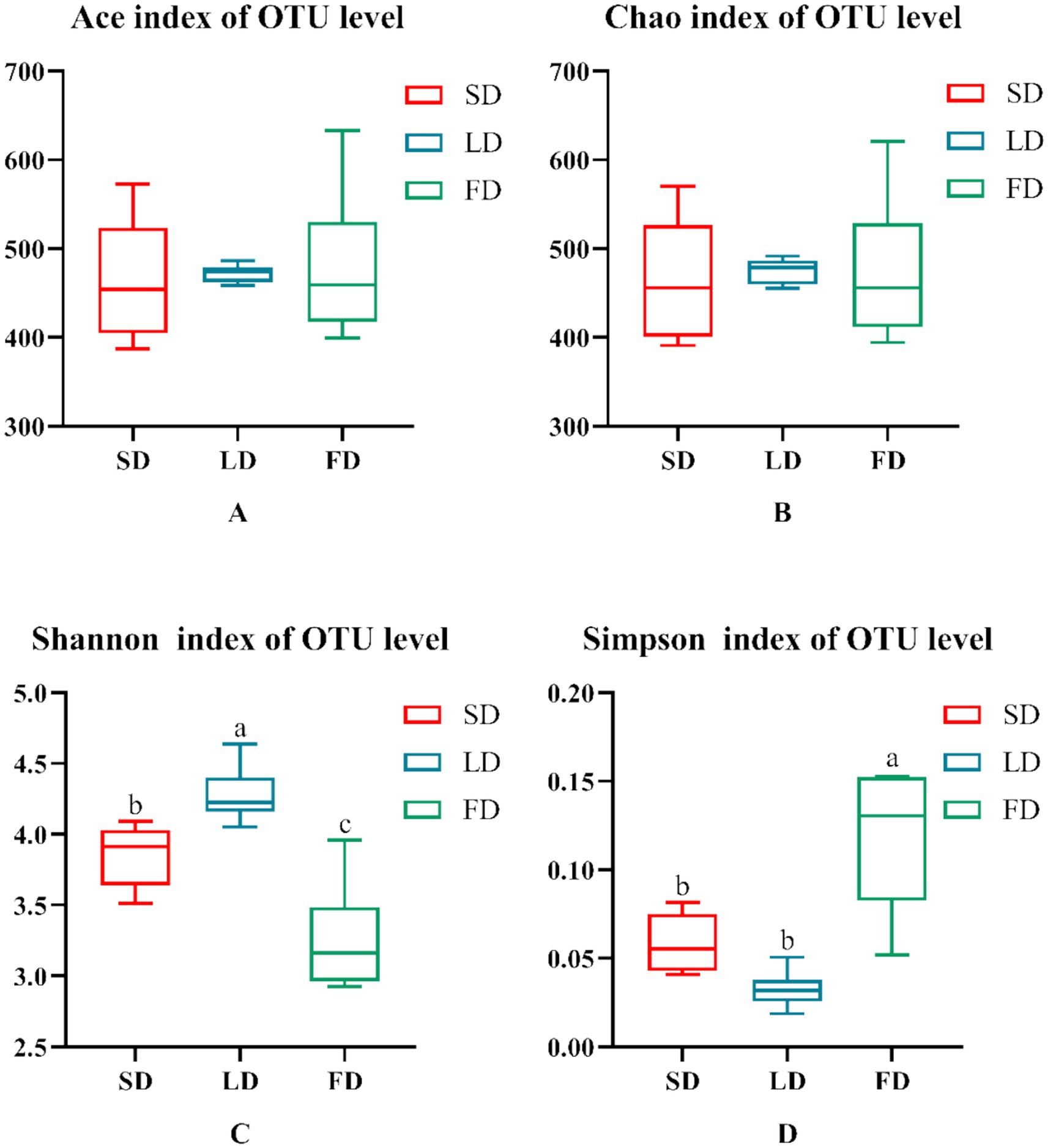
Figure 4. Indexes of alpha diversity of the microbiota. (A) Ace. (B) Chao. (C) Shannon. (D) Simpson. Data are presented as Mean ± SEMs. Different superscripts (a, b, c) in each parameter indicate significance (p < 0.05).
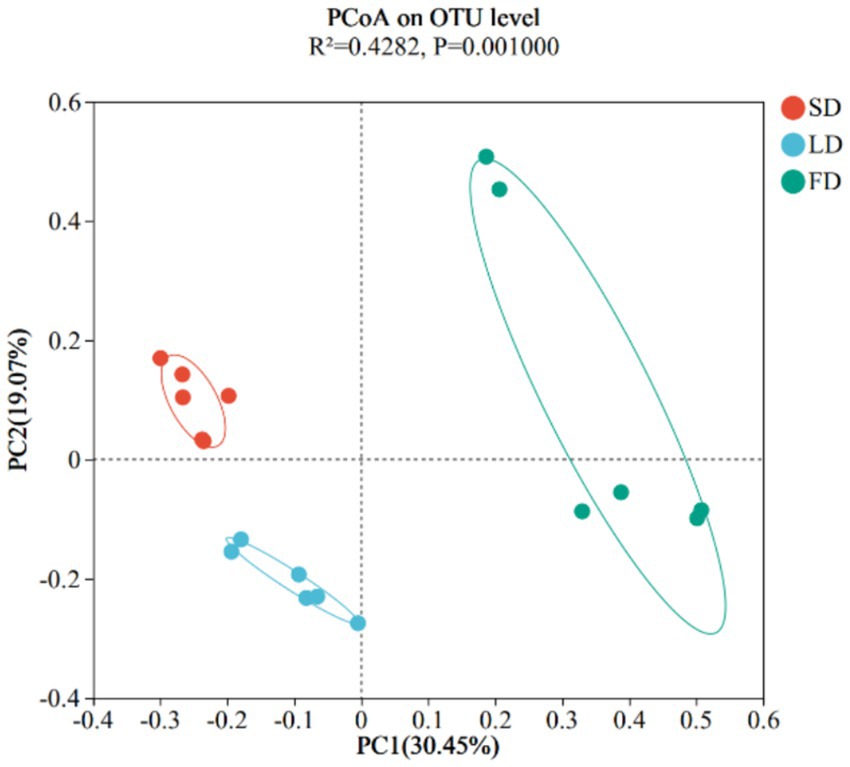
Figure 6. Beta diversity was calculated using Principle Coordinates Analysis plots based on unweighted UniFrac distance.
Taxon-based analysis showed the cecal microbial composition markedly changed on account of different light treatments (Figures 7A,B). Relative abundance analysis of the top 10 phyla in the cecal microbiota was shown in Figure 7A, it was found that Firmicutes and Bacteroidota were the dominant microbiota, with the relative abundance in the three groups 95.91, 90.75, 41.28, and 2.99%, 8.22, 52.22%, respectively. The relative abundance of Firmicutes and Bacteroidota was significantly decreased and increased in the FD group, respectively, compared with the SD group and LD group (p < 0.05). The FD group significantly increased the relative abundance of Proteobacteria in comparison with the SD group (p < 0.05). Compared with the LD group, the relative abundance of Cyanobacteria had markedly increased in the FD group (p < 0.05). Relative abundance analysis of bacteria in the cecal microbiota was shown in Figure 7B, it was found that Lactobacillus, Bacteroides, Faecalibacterium, norank_f__norank_o__Clostridia_UCG-014, norank_f__norank_o__Clostridia_vadinBB60_group, Alistipes, Barnesiella, unclassified_f__Lachnospiraceae, UCG-005 and norank_f__Ruminococcaceae was the top 10 bacteria with higher relative abundance. To evaluate the impact of different photoperiods on the taxonomic composition of microorganisms in the cecal contents, Kruskal-Wallis H test was employed to determine the genera with significant effects on the three groups. As shown in Figure 8, the results were mainly evaluated for the top 20 bacteria under three different photoperiods. The results indicated that there were 8 bacteria (Lactobacillus, norank_f__norank_o__Clostridia_UCG-014, unclassified_f__Lachnospiraceae, norank_f__Ruminococcaceae, Ruminococcus_torques_group, norank_f__Eubacterium_coprostanoligenes_group, norank_f__norank_o__RF39, unclassified_f__Oscillospiraceae) was significantly changed under different photoperiods. The relative abundance of Lactobacillus, norank_f__norank_o__Clostridia_UCG-014, norank_f__Eubacterium_coprostanoligenes_group was markedly reduced with longer photoperiods (p < 0.05; Figures 8A,B,F). As the photoperiods increased, the relative abundance of unclassified_f__Lachnospiraceae, norank_f__Ruminococcaceae, Ruminococcus_torques_group, norank_f__norank_o__RF39 and unclassified_f__Oscillospiraceae first enriched and then reduced (p < 0.05; Figures 8C–E,G,H).
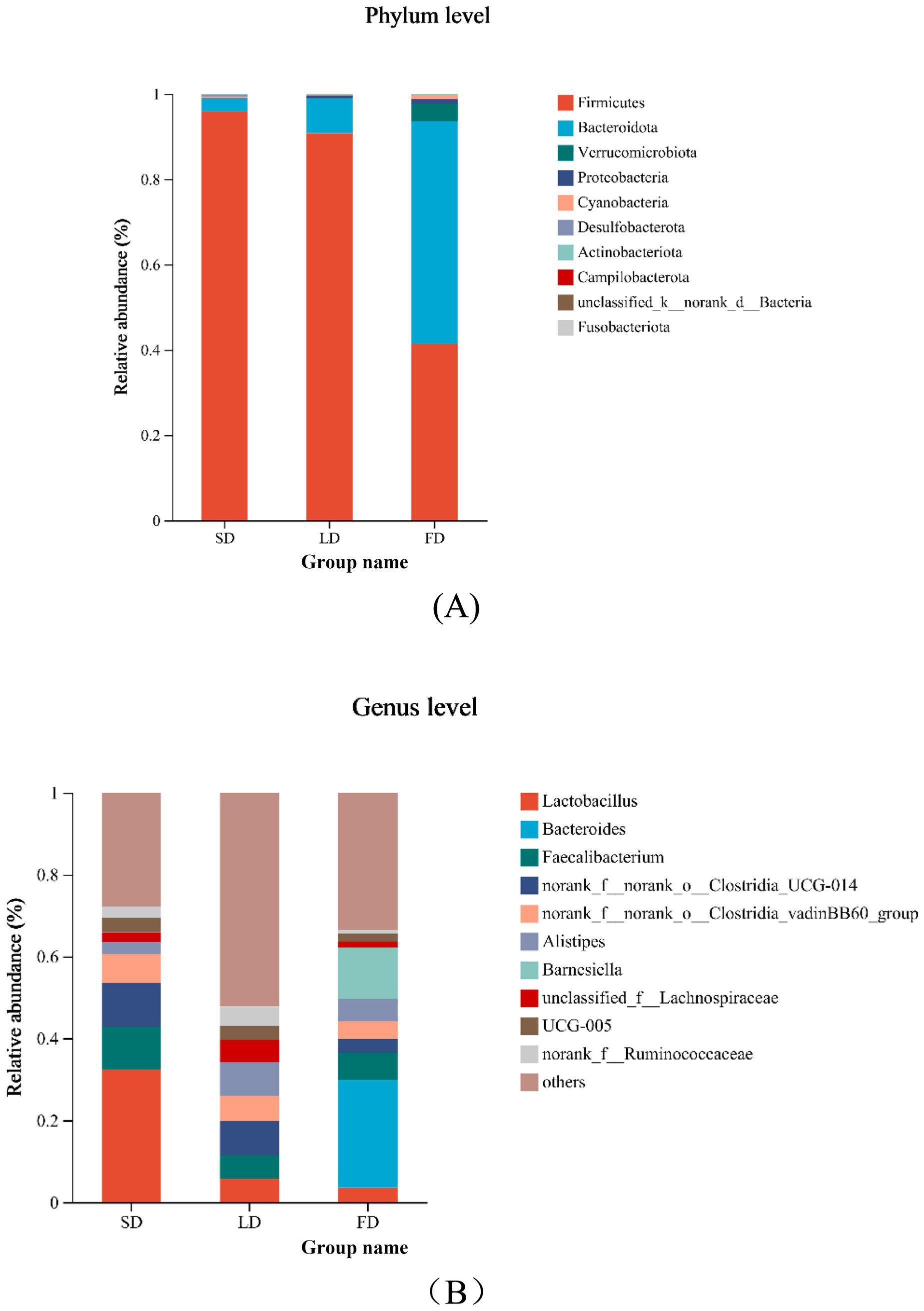
Figure 7. Relative abundance of cecal microbiology composition at the phylum and genus and species in the different photoperiods group. (A) Relative abundance of cecal microbiology at the phylum; (B) relative abundance of cecal microbiology at the genus.
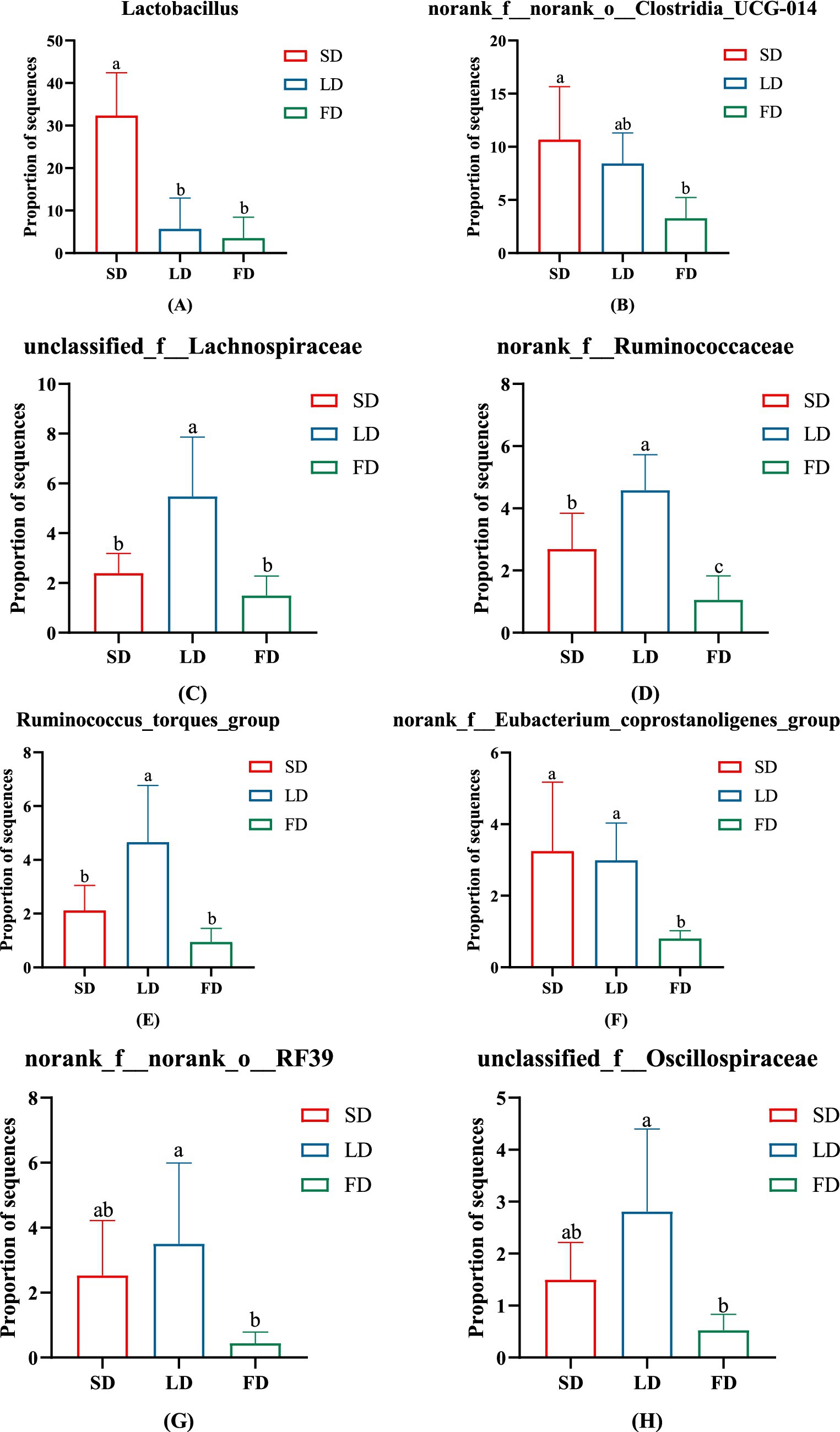
Figure 8. The significant change in the relative abundance of cecal microbiota on genus levels. (A) Lactobacillus. (B) norank_f__norank_o__Clostridia_UCG-014. (C) unclassified_f__Lachnospiraceae. (D) norank_f__Ruminococcaceae. (E) Ruminococcus_torques_group. (F) norank_f__Eubacterium_coprostanoligenes_group. (G) norank_f__norank_o__RF39. (H) unclassified_f__Oscillospiraceae. Data are presented as Mean ± SDs. Different superscripts (a, b, c) in each parameter indicate significance (p < 0.05).
To investigate the correlation between microbiota abundance with MT, IL-1β, IL-6 and TNF-α, we analyzed that by environmental factor correlation analysis based on Spearman correlation analysis (Figure 9). At the genus level, the relative abundance of Lactobacillus, norank_f__norank_o__Clostridia_UCG-014, norank_f__Eubacterium_coprostanoligenes_group and norank_f__norank_o__RF39 was a positive correlation with MT and negative correlation with IL-1β, IL-6 and TNF-α, Bacteroides and Barnesiella was negative correlation with MT and positive correlation with IL-1β, IL-6 and TNF-α. Furthermore, Prevotellaceae_NK3B31_group had a negative correlation with MT, and norank_f__norank_o__Clostridia_vadinBB60_group had a negative correlation with IL-1β.
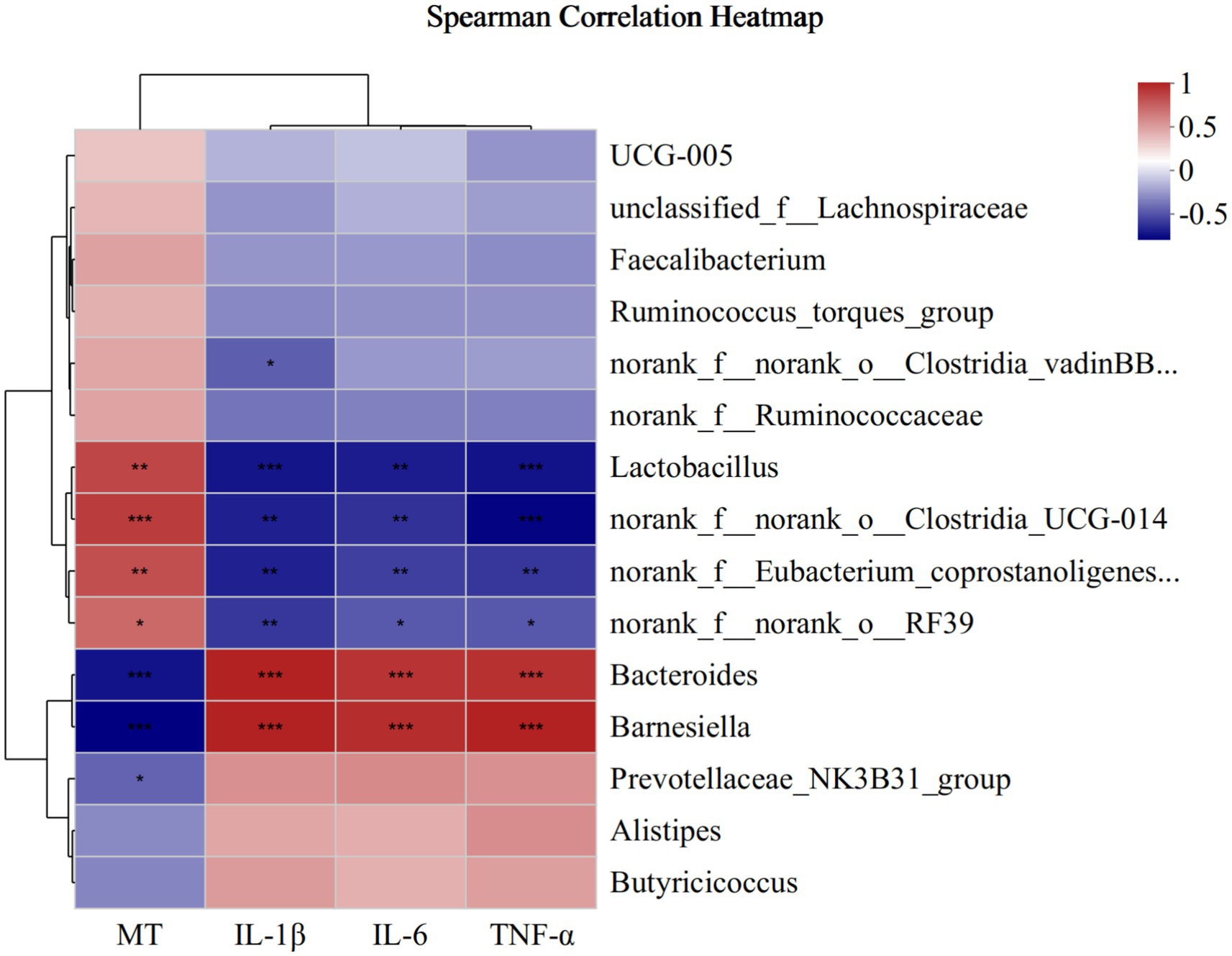
Figure 9. Correlation between microbiota abundance with cecum MT, IL-1β, IL-6 and TNF-α at the genus. * means p ≤ 0.05, ** means p ≤ 0.01, *** means p ≤ 0.001.
The main aim of this study was to explore the association between MT and cecal microbiota under extended light exposure as well as the potential regulatory pathways mediating the effects of different photoperiods on the breast muscle morphology of broilers. Recent studies have shown that continuous long photoperiods may negatively affect broiler health and welfare (Tuell et al., 2020). The results indicated that exposure to long photoperiods increased growth rate but decreased FE, impaired breast muscle morphology and significantly increased the concentrations of IL-1β, IL-6 and TNFα in breast muscle. Furthermore, MT was found to be related to cecal microbiota under different photoperiods, which also affected breast muscle morphology and inflammation of broilers.
Previous research has shown that long photoperiods enhance the growth rate and overall chicken production performance (Zhang et al., 2022; Ingram et al., 2000). Similarly, this study found LD and FD photoperiods increased the growth rate of broilers. During the 4-week trial period, the FE was significantly decreased following FD treatment. This finding was consistent with results from previous studies, which uncovered faster growth rates and higher FCR (Olanrewaju et al., 2006; Shynkaruk et al., 2022).
Notably, broilers are highly sensitive to changes in light, with studies showing that light stimulation enhances the inhibition of MT secretion from the pineal gland via the superior cervical ganglion by the suprachiasmatic nucleus of the hypothalamus under light stimulation, whereas in the absence of light stimulation at night, the pineal gland secretes MT (Shichida and Matsuyama, 2009). Nighttime light exposure decreases MT secretion (Yang et al., 2024), and in this study, we found that MT concentrations in the hypothalamus, cecum and breast muscle of broilers decreased significantly under extended light exposure. This is consistent with previous studies (Yang et al., 2024), demonstrating that extended light exposure may decrease MT levels.
Modern broilers are often genetically selected for higher breast muscle yield and faster growth rates. However, they are particularly vulnerable to oxidative stress (Xing et al., 2022; Pontes et al., 2023). Guob et al. reported that a longer photoperiod may increase oxidative stress levels (Guo et al., 2010). In this study, the concentrations of IL-1β, IL-6 and TNFα increased in the breast muscle accompanied by inflammatory cell infiltration in the breast muscle of broilers under FD treatment. Moreover, the injury to the breast muscle was positively correlated with the IL-1β, IL-6 and TNFα levels. Therefore, we speculated that the injury was induced by an inflammatory response following long photoperiod which might be related to oxidative stress. In summary, the results of this study showed that prolonged exposure to light exposure caused morphological injury and inflammation in the breast muscles of broiler chickens, especially under the FD treatment.
Cecal microbiota are important modulators of various pathological processes. The cecum harbors a wide range of symbiotic bacteria, which participate in microbial fermentation and prevent pathogen colonization (Huang et al., 2018; Guarner and Malagelada, 2003). Therefore, the diversity and composition of cecal microflora mature and stabilize as the broilers age, reaching a stable state in 21 days (Zhou et al., 2021). In this study, we characterized the cecal microbiota composition of 33-day-old broilers following exposure to different photoperiods. The alpha richness index (diversity within a community) of cecal microbiota first increased and then reduced with longer photoperiods. This was consistent with findings from previous studies exploring the effects of 12.5 L:11.5D and 16 L: 8D photoperiods, suggesting that the obtained observations were induced by the effects of long photoperiods on broiler metabolism (Wang et al., 2018). At the phylum level, Firmicutes was the dominant bacterial phylum across the three photoperiods. The dominant bacterial phylum in the FD group changed to Bacteroidota instead of Firmicutes, and Bacteroidota, Proteobacteria, and Cyanobacteria were significantly elevated in the FD group. Researchers have shown that Firmicutes are beneficial bacteria, and a decrease in their relative abundance increases the risk of systemic inflammation and development of certain diseases (Samaddar et al., 2022; Qu et al., 2022). Numerous pathogens belonging to the Proteobacteria, may cause diseases to broilers (He et al., 2022). Therefore, the observed difference in the FD group may be explained by the increased effect of the treatment on cecal microbiota, which enhanced cecal microbial disorders and increased the relative abundance of harmful bacteria. At the genus level, Lactobacillus was enriched in the SD group. The genus Lactobacillus contains probiotics which has been reported to be decreased under long photoperiods (Song et al., 2020; Zhang et al., 2022). A study found that the relative abundance of Lactobacillus influenced the development of inflammation in broilers (Yang et al., 2020; Khalique et al., 2019), suggesting that exposure to long photoperiods may suppress the abundance of probiotics and trigger cecal microbiota disorder, possibly by increasing inflammation levels. Interestingly, we found that the relative abundance of Ruminococcus_torques_group initially increased and then reduced with the prolongation of light exposure. The Ruminococcus_torques_group has been associated with inflammation and the occurrence of various health issues, including neurological diseases and abdominal fat deposition (Zheng et al., 2020; Blacher et al., 2019; Chen et al., 2022; Xiang et al., 2024; Lyu et al., 2021). However, the function of Ruminococcus_torques_group on broilers and its changes under different photoperiods has not been clarified. The decrease in Ruminococcus_torques_group in the FD treatment may be attributed to the severe disruption of cecal microbial composition. Notably, the Ruminococcus_torques_group belongs to Firmicutes, which was reduced by 46.63% in the FD treatment, accompanied by a decrease in the relative abundance of Ruminococcus_torques_group. The ratio of Ruminococcus_torques_group to Firmicutes increased, suggesting enhanced relative abundance. Altogether, our results indicated that prolonged exposure to light significantly altered the cecal microbial composition in broiler chickens by reducing beneficial bacteria and enriching the relative abundance of harmful bacteria, causing the cecal microbiota disorder.
The brain-gut axis involves the bidirectional communication between the nervous, endocrine and immune systems (Liu et al., 2018). Numerous studies have shown that MT regulates gut microbiota composition (Iesanu et al., 2022; Ma et al., 2020). Studies have reported that alterations in the intestinal microbial composition could affect inflammation (Niu et al., 2022). To further explore the correlations among MT, cecal microbiota and inflammation in breast muscle under different photoperiods, we analyzed the association of cecal microbiota with changes in MT, IL-1β, IL-6 and TNF-α levels. It was observed that the composition of cecal microbiota was significantly influenced by changes in MT, IL-1β, IL-6 and TNF-α concentrations. Lactobacillus, which is the most important probiotic in the cecum of broilers, was positively correlated with MT levels and negatively correlated with IL-1β, IL-6 and TNF-α levels. On the other hand, Bacteroides was found to enhance inflammation in mice (Li et al., 2020), and negatively correlated with MT levels and positively with IL-1β, IL-6 and TNF-α levels in this study. In comparison, Barnesiella has been reported to play dual roles in enteric infections: exerting colonization resistance against pathogenic invaders, while also inducing detrimental effects on the host (Bornet and Westermann, 2022). In this study, the relative abundance of Barnesiella increased as the concentrations of inflammatory markers increased and the MT levels decreased. This suggested that the decreased MT levels resulted in the down-regulation of the beneficial bacteria and an increased in harmful bacteria, inducing inflammation in the breast muscle of broilers.
Notably, the brain-gut axis is increasingly being recognized and studied. Here, we found that prolonged exposure of mice to light weakened the MT levels thereby reducing the number of beneficial bacteria and enriching the number of harmful bacteria, which caused morphological injury and inflammation in the breast muscle of broilers. Therefore, we speculated that the MT-gut axis may mediate the morphological injury and inflammation induced by prolonged exposure to light in the breast muscle of broilers. Furthermore, Lactobacillus was significantly reduced under extended light exposure, and closely related to MT, IL-1β, IL-6 and TNF-α levels, indicating that this bacterial genus may have an important regulator function on MT and inflammation; however, this requires further research.
In summary, this study demonstrated that prolonged exposure to light enhanced the growth rate of broilers. However, it reduced the FE, induced morphological injury and inflammation in the breast muscle of broilers, suppressed the MT levels and disrupted the cecal microbial structure. Moreover, the regulatory pathways mediating the effects of extended light exposure included inflammatory signaling in breast muscle driven by the MT-gut axis. These findings have important implications for future research into the role of MT and cecal microbiota in breast muscle morphology injury and inflammation under different photoperiods. To improve the meat quality of broilers, we recommend that the 18 L:6D photoperiod may be optimal compared with the 12 L:12D and 24 L:0D photoperiod.
The data presented in the study are deposited in NCBI/SRA repository, accession number PRJNA1190345.
The animal study was approved by the Institutional Ethics Committee of Experiment Animal Welfare and Ethics at the Institute of Animal Science of Chinese Academy of Agricultural Sciences (CAAS). The study was conducted in accordance with the local legislation and institutional requirements.
MY: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft, Methodology, Project administration, Resources, Supervision, Visualization, Writing – review & editing. MX: Writing – review & editing, Investigation, Resources. GW: Writing – review & editing. JF: Writing – review & editing. MZ: Conceptualization, Methodology, Writing – review & editing, Supervision.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was financially supported by grants from the Science and Technology Innovation Project of the Chinese Academy of Agricultural Sciences (Grant No. CAAS-ASTIP-IAS-08).
We would kindly to thank all workers in the State Key Laboratory of Animal Nutrition and Feeding for their help in completed the experiment.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Bai, X., Cao, J., Dong, Y., Wang, Z., and Chen, Y. (2019). Melatonin mediates monochromatic green light-induced satellite cell proliferation and muscle growth in chick embryo. PLoS One 14:e0216392. doi: 10.1371/journal.pone.0216392
Blacher, E., Bashiardes, S., Shapiro, H., Rothschild, D., Mor, U., Dori-Bachash, M., et al. (2019). Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature 572, 474–480. doi: 10.1038/s41586-019-1443-5
Bornet, E., and Westermann, A. J. (2022). The ambivalent role of Bacteroides in enteric infections. Trends Microbiol. 30, 104–108. doi: 10.1016/j.tim.2021.11.009
Chen, Z., Wang, Z., Li, D., Zhu, B., Xia, Y., Wang, G., et al. (2022). The gut microbiota as a target to improve health conditions in a confined environment. Front. Microbiol. 13:1067756. doi: 10.3389/fmicb.2022.1067756
Clavijo, V., and Flórez, M. J. V. (2018). The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: a review. Poult. Sci. 97, 1006–1021. doi: 10.3382/ps/pex359
Cui, Y. M., Wang, J., Zhang, H. J., Qi, G. H., Qiao, H. Z., Gan, L. P., et al. (2022). Effect of changes in photoperiods on melatonin expression and gut health parameters in laying ducks. Front. Microbiol. 13:819427. doi: 10.3389/fmicb.2022.819427
Duan, J., Cheng, M., Xu, Y., Chen, Y., Gao, T., Gu, Q., et al. (2022). Exogenous melatonin alleviates skeletal muscle wasting by regulating hypothalamic neuropeptides expression in Endotoxemia rats. Neurochem. Res. 47, 885–896. doi: 10.1007/s11064-021-03489-6
Feng, Y., Liu, D., Liu, Y., Yang, X., Zhang, M., Wei, F., et al. (2022). Host-genotype-dependent cecal microbes are linked to breast muscle metabolites in Chinese chickens. iScience 25:104469. doi: 10.1016/j.isci.2022.104469
Gratta, F., Bošković Cabrol, M., Xiccato, G., Birolo, M., Bordignon, F., and Trocino, A. (2023). Effect of light restriction on productive results and behavior of broiler chickens. Poult. Sci. 102:103084. doi: 10.1016/j.psj.2023.103084
Guarner, F., and Malagelada, J. R. (2003). Gut flora in health and disease. Lancet (London, England) 361, 512–519. doi: 10.1016/S0140-6736(03)12489-0
Guo, Y. L., Li, W. B., and Chen, J. L. (2010). Influence of nutrient density and lighting regime in broiler chickens: effect on antioxidant status and immune function. Br. Poult. Sci. 51, 222–228. doi: 10.1080/00071661003746503
He, S., Cui, S., Song, W., Jiang, Y., Chen, H., Liao, D., et al. (2022). Interleukin-17 weakens the NAFLD/NASH process by facilitating intestinal barrier restoration depending on the gut microbiota. MBio 13:e0368821. doi: 10.1128/mbio.03688-21
Huang, P., Zhang, Y., Xiao, K., Jiang, F., Wang, H., Tang, D., et al. (2018). The chicken gut metagenome and the modulatory effects of plant-derived benzylisoquinoline alkaloids. Microbiome 6:211. doi: 10.1186/s40168-018-0590-5
Iesanu, M. I., Zahiu, C. D. M., Dogaru, I. A., Chitimus, D. M., Pircalabioru, G. G., Voiculescu, S. E., et al. (2022). Melatonin-microbiome two-sided interaction in Dysbiosis-associated conditions. Antioxidants 11:2244. doi: 10.3390/antiox11112244
Ingram, D. R., Hattens, L. F., and McPherson, B. N. (2000). Effects of light restriction on broiler performance and specific body structure measurements. J. Appl. Poult. Res. 9, 501–504. doi: 10.1093/japr/9.4.501
Jiang, X., Zhang, B., Lan, F., Zhong, C., Jin, J., Li, X., et al. (2023). Host genetics and gut microbiota jointly regulate blood biochemical indicators in chickens. Appl. Microbiol. Biotechnol. 107, 7601–7620. doi: 10.1007/s00253-023-12814-8
John, T. M., and George, J. C. (1976). Diurnal variation in the effect of melatonin on plasma and muscle free fatty acid levels in the pigeon. Endocrinol. Exp. 10, 131–137
Kang, K., Zhou, N., Peng, W., Peng, F., Ma, M., Li, L., et al. (2022). Multi-omics analysis of the microbiome and metabolome reveals the relationship between the gut microbiota and wooden breast myopathy in broilers. Front. Vet. Sci. 9:922516. doi: 10.3389/fvets.2022.922516
Khalique, A., Zeng, D., Wang, H., Qing, X., Zhou, Y., Xin, J., et al. (2019). Transcriptome analysis revealed ameliorative effect of probiotic Lactobacillus johnsonii BS15 against subclinical necrotic enteritis induced hepatic inflammation in broilers. Microb. Pathog. 132, 201–207. doi: 10.1016/j.micpath.2019.05.011
Lewis, P. D., and Gous, R. M. (2007). Broilers perform better on short or step-up photoperiods. S. Afr. J. Anim. Sci. 37, 90–96. doi: 10.4314/SAJAS.V37I2.4032
Li, W.-B., Guo, Y.-L., Chen, J.-L., Wang, R., He, Y. D., and Su, D.-G. (2010). Influence of lighting schedule and nutrient density in broiler chickens: effect on growth performance, carcass traits and meat quality. Asian Australas. J. Anim. Sci. 23, 1510–1518. doi: 10.5713/ajas.2010.10087
Li, W., Lu, L., Liu, B., and Qin, S. (2020). Effects of phycocyanin on pulmonary and gut microbiota in a radiation-induced pulmonary fibrosis model. Biomed. Pharmacother. 132:110826. doi: 10.1016/j.biopha.2020.110826
Li, W., Wang, Z., Cao, J., Dong, Y., and Chen, Y. (2023). Melatonin improves the homeostasis of mice gut microbiota rhythm caused by sleep restriction. Microbes Infect. 25:105121. doi: 10.1016/j.micinf.2023.105121
Li, X., Wang, F., Gao, Z., Huang, W., Zhang, X., Liu, F., et al. (2023). Melatonin attenuates chronic intermittent hypoxia-induced intestinal barrier dysfunction in mice. Microbiol. Res. 276:127480. doi: 10.1016/j.micres.2023.127480
Li, X., Zheng, Z., Pan, J., Jiang, D., Tian, Y., and Huang, Y. (2020). Influences of melatonin and endotoxin lipopolysaccharide on goose productive performance and gut microbiota. Br. Poult. Sci. 61, 217–224. doi: 10.1080/00071668.2019.1687851
Liu, F., Horton-Sparks, K., Hull, V., Li, R. W., and Martínez-Cerdeño, V. (2018). The valproic acid rat model of autism presents with gut bacterial dysbiosis similar to that in human autism. Mol. Autism. 9:61. doi: 10.1186/s13229-018-0251-3
Liu, C., Zhao, D., Ma, W., Guo, Y., Wang, A., Wang, Q., et al. (2016). Denitrifying sulfide removal process on high-salinity wastewaters in the presence of Halomonas sp. Appl. Microbiol. Biotechnol. 100, 1421–1426. doi: 10.1007/s00253-015-7039-6
Lyu, W., Liu, X., Lu, L., Dai, B., Wang, W., Yang, H., et al. (2021). Cecal microbiota modulates fat deposition in Muscovy ducks. Front. Vet. Sci. 8:609348. doi: 10.3389/fvets.2021.609348
Ma, N., Zhang, J., Reiter, R. J., and Ma, X. (2020). Melatonin mediates mucosal immune cells, microbial metabolism, and rhythm crosstalk: a therapeutic target to reduce intestinal inflammation. Med. Res. Rev. 40, 606–632. doi: 10.1002/med.21628
Niu, W., Yang, F., Fu, Z., Dong, Y., Zhang, Z., and Ju, J. (2022). The role of enteric dysbacteriosis and modulation of gut microbiota in the treatment of inflammatory bowel disease. Microb. Pathog. 165:105381. doi: 10.1016/j.micpath.2021.105381
Olanrewaju, H. A., Thaxton, J. P., Dozier Iii, W. A., Purswell, J. L., Roush, W. B., and Branton, S. L. (2006). A review of lighting programs for broiler production. Int. J. Poult. Sci. 5, 301–308. doi: 10.3923/ijps.2006.301.308
Oyola, M. G., Johnson, R. C., Bauman, B. M., Frey, K. G., Russell, A. L., Cho-Clark, M., et al. (2021). Gut microbiota and metabolic marker alteration following dietary isoflavone-photoperiod interaction. Endocrinol. Diabetes Metab. 4:e00190. doi: 10.1002/edm2.190
Pontes, M. P., Khatlab, A. S., Del Vesco, A. P., Granzoto, G. H., Soares, M. A. M., Sousa, F. C. B., et al. (2023). The effect of light regime and time of slaughter in broiler on broiler performance, liver antioxidant status, and expression of genes related to peptide absorption in the jejunum and melatonin synthesis in the brain. J. Anim. Physiol. Anim. Nutr. 107, 607–620. doi: 10.1111/jpn.13712
Qu, C., Li, Q. P., Su, Z. R., Ip, S. P., Yuan, Q. J., Xie, Y. L., et al. (2022). Nano-Honokiol ameliorates the cognitive deficits in TgCRND8 mice of Alzheimer's disease via inhibiting neuropathology and modulating gut microbiota. J. Adv. Res. 35, 231–243. doi: 10.1016/j.jare.2021.03.012
Saito, S., Tachibana, T., Choi, Y. H., Denbow, D. M., and Furuse, M. (2005). ICV melatonin reduces acute stress responses in neonatal chicks. Behav. Brain Res. 165, 197–203. doi: 10.1016/j.bbr.2005.06.045
Salagre, D., Raya Álvarez, E., Cendan, C. M., Aouichat, S., and Agil, A. (2023). Melatonin improves skeletal muscle structure and oxidative phenotype by regulating mitochondrial dynamics and autophagy in Zücker diabetic fatty rat. Antioxidants 12:1499. doi: 10.3390/antiox12081499
Salucci, S., Taurone, S., Burattini, S., Gobbi, P., Clausi, J., and Battistelli, M. (2021). Melatonin role in skeletal muscle disorders. Eur. Rev. Med. Pharmacol. Sci. 25, 1024–1033. doi: 10.26355/eurrev_202101_24672
Samaddar, A., van Nispen, J., Armstrong, A., Song, E., Voigt, M., Murali, V., et al. (2022). Lower systemic inflammation is associated with gut firmicutes dominance and reduced liver injury in a novel ambulatory model of parenteral nutrition. Ann. Med. 54, 1701–1713. doi: 10.1080/07853890.2022.2081871
Scalon, D., Picada, J. N., de Sousa, J. T., da Silva, A. T., Colares, J. R., and Marroni, N. A. P. (2022). Photobiomodulation intervention improves oxidative, inflammatory, and morphological parameters of skeletal muscle in cirrhotic Wistar rats. Lasers Med. Sci. 37, 1973–1982. doi: 10.1007/s10103-021-03458-z
Schloss, P. D., Westcott, S. L., Ryabin, T., Hall, J. R., Hartmann, M., Hollister, E. B., et al. (2009). Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541. doi: 10.1128/AEM.01541-09
Shichida, Y., and Matsuyama, T. (2009). Evolution of opsins and phototransduction. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 364, 2881–2895. doi: 10.1098/rstb.2009.0051
Shynkaruk, T., Buchynski, K., and Schwean-Lardner, K. (2022). Lighting programme as a management tool for broilers raised without antibiotics - impact on productivity and welfare. Br. Poult. Sci. 63, 761–767. doi: 10.1080/00071668.2022.2083943
Song, E. J., Han, K., Lim, T. J., Lim, S., Chung, M. J., Nam, M. H., et al. (2020). Effect of probiotics on obesity-related markers per enterotype: a double-blind, placebo-controlled, randomized clinical trial. EPMA J. 11, 31–51. doi: 10.1007/s13167-020-00198-y
Su, C. M., Tsai, C. H., Chen, H. T., Wu, Y. S., Chang, J. W., Yang, S. F., et al. (2023). Melatonin improves muscle injury and differentiation by increasing Pax7 expression. Int. J. Biol. Sci. 19, 1049–1062. doi: 10.7150/ijbs.79169
Tiwari, J., Sur, S., Naseem, A., Rani, S., and Malik, S. (2023). Photoperiodic modulation of melatonin receptor and immune genes in migratory redheaded bunting. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 279:111381. doi: 10.1016/j.cbpa.2023.111381
Tuell, J. R., Park, J. Y., Wang, W., Cooper, B., Sobreira, T., Cheng, H. W., et al. (2020). Effects of photoperiod regime on meat quality. Oxidative stability, and metabolites of postmortem broiler fillet (M. pectoralis major) muscles. Foods 9:215. doi: 10.3390/foods9020215
Wang, J., Nesengani, L. T., Gong, Y., Yang, Y., and Lu, W. (2018). 16S rRNA gene sequencing reveals effects of photoperiod on cecal microbiota of broiler roosters. PeerJ 6:e4390. doi: 10.7717/peerj.4390
Wang, Y., Zhang, Z., Yang, P., Zhang, M., Xi, L., Liu, Q., et al. (2020). Molecular mechanism underlying the effect of illumination time on the growth performance of broilers via changes in the intestinal bacterial community. PeerJ 8:e9638. doi: 10.7717/peerj.9638
Wen, C., Gou, Q., Gu, S., Huang, Q., Sun, C., Zheng, J., et al. (2023). The cecal ecosystem is a great contributor to intramuscular fat deposition in broilers. Poult. Sci. 102:102568. doi: 10.1016/j.psj.2023.102568
Xiang, S., Chen, J., Deng, M., Wang, Z., Li, X., Lin, D., et al. (2024). Celastrol ameliorates experimental autoimmune uveitis through STAT3 targeting and gut microenvironment reprofiling. Int. Immunopharmacol. 127:111339. doi: 10.1016/j.intimp.2023.111339
Xing, T., Xu, X., Zhang, L., and Gao, F. (2022). Overexpression of heat shock protein 70 ameliorates meat quality of broilers subjected to pre-slaughter transport at high ambient temperatures by improving energy status of pectoralis major muscle and antioxidant capacity. Antioxidants 11:1468. doi: 10.3390/antiox11081468
Yang, Y., Cong, W., Liu, J., Zhao, M., Xu, P., Han, W., et al. (2022). Constant light in early life induces fear-related behavior in chickens with suppressed melatonin secretion and disrupted hippocampal expression of clock-and BDNF-associated genes. J. Anim. Sci. Biotechnol. 13:67. doi: 10.1186/s40104-022-00720-4
Yang, X., Liang, S., Guo, F., Ren, Z., Yang, X., and Long, F. (2020). Gut microbiota mediates the protective role of Lactobacillus plantarum in ameliorating deoxynivalenol-induced apoptosis and intestinal inflammation of broiler chickens. Poult. Sci. 99, 2395–2406. doi: 10.1016/j.psj.2019.10.034
Yang, Y., Liu, Q., Pan, C., Chen, J., Xu, B., Liu, K., et al. (2024). Species sensitivities to artificial light at night: a phylogenetically controlled multilevel meta-analysis on melatonin suppression. Ecol. Lett. 27:e14387. doi: 10.1111/ele.14387
Yin, H. C., Yao, W. Q., Zhang, H., Liu, S., Ma, T. Y., and Xia, C. Y. (2024). Multiomics analysis reveals that microbiota regulate fat and muscle synthesis in chickens. Poult. Sci. 103:103417. doi: 10.1016/j.psj.2023.103417
Yu, M., Xu, M., Wang, G., Feng, J., and Zhang, M. (2024). Effects of different photoperiods on peripheral 5-Hydroxytryptamine metabolism, breast muscle glucose metabolism, and myopathies in broilers. Meta 14:567. doi: 10.3390/metabo14100567
Zeman, M., Výboh, P., Juráni, M., Lamosová, D., Kostal, L., Bilcík, B., et al. (1993). Effects of exogenous melatonin on some endocrine, behavioural and metabolic parameters in Japanese quail Coturnix coturnix japonica. Comp. Biochem. Physiol. Comp. Physiol. 105, 323–328. doi: 10.1016/0300-9629(93)90215-P
Zhang, X., Akhtar, M., Chen, Y., Ma, Z., Liang, Y., Shi, D., et al. (2022). Chicken jejunal microbiota improves growth performance by mitigating intestinal inflammation. Microbiome 10:107. doi: 10.1186/s40168-022-01299-8
Zhang, X., Hu, Y., Ansari, A. R., Akhtar, M., Chen, Y., Cheng, R., et al. (2022). Caecal microbiota could effectively increase chicken growth performance by regulating fat metabolism. Microb. Biotechnol. 15, 844–861. doi: 10.1111/1751-7915.13841
Zhang, H., Qi, G., Wang, K., Yang, J., Shen, Y., Yang, X., et al. (2023). Oxidative stress: roles in skeletal muscle atrophy. Biochem. Pharmacol. 214:115664. doi: 10.1016/j.bcp.2023.115664
Zhang, Y., Wang, Z., Dong, Y., Cao, J., and Chen, Y. (2022). Effects of different monochromatic light combinations on Cecal microbiota composition and Cecal tonsil T lymphocyte proliferation. Front. Immunol. 13:849780. doi: 10.3389/fimmu.2022.849780
Zhang, J., Wang, W., Guo, D., Bai, B., Bo, T., and Fan, S. (2022). Antidiabetic effect of millet bran polysaccharides partially mediated via changes in gut microbiome. Foods 11:3406. doi: 10.3390/foods11213406
Zhao, R. X., Cai, C. H., Wang, P., Zheng, L., Wang, J. S., Li, K. X., et al. (2019). Effect of night light regimen on growth performance, antioxidant status and health of broiler chickens from 1 to 21 days of age. Asian Australas. J. Anim. Sci. 32, 904–911. doi: 10.5713/ajas.18.0525
Zheng, J., Hoffman, K. L., Chen, J. S., Shivappa, N., Sood, A., Browman, G. J., et al. (2020). Dietary inflammatory potential in relation to the gut microbiome: results from a cross-sectional study. Br. J. Nutr. 124, 931–942. doi: 10.1017/S0007114520001853
Keywords: photoperiod, melatonin, cecal microbiota, breast muscle morphology, inflammation
Citation: Yu M, Xu M, Wang G, Feng J and Zhang M (2025) Effects of different photoperiods on melatonin level, cecal microbiota and breast muscle morphology of broiler chickens. Front. Microbiol. 16:1504264. doi: 10.3389/fmicb.2025.1504264
Received: 30 September 2024; Accepted: 10 March 2025;
Published: 25 March 2025.
Edited by:
Jason W. Soares, Combat Capabilities Development Command United States Army, United StatesReviewed by:
Yafei Duan, South China Sea Fisheries Research Institute, ChinaCopyright © 2025 Yu, Xu, Wang, Feng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Minhong Zhang, emhhbmdtaW5ob25nQGNhYXMuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.