
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 26 February 2025
Sec. Terrestrial Microbiology
Volume 16 - 2025 | https://doi.org/10.3389/fmicb.2025.1503636
This article is part of the Research TopicAnthropogenic Effects on the Microbial Communities of Terrestrial EcosystemsView all 34 articles
 Bin Wang1,2†
Bin Wang1,2† Nianjie Shang3†
Nianjie Shang3† Xinwei Feng4
Xinwei Feng4 Zongling Hu2
Zongling Hu2 Pengfei Li2
Pengfei Li2 Yi Chen5
Yi Chen5 Binbin Hu5
Binbin Hu5 Mengjiao Ding6,7,8*
Mengjiao Ding6,7,8* Junju Xu1*
Junju Xu1*Understanding how soil properties and microbial communities respond to crop rotation is essential for the sustainability of agroecosystems. However, there has been limited research on how crop rotation alters below-ground microbial communities in soils with serious bacterial wilt within the karst agricultural system. This study investigated the effects of continuous planting of corn, tobacco, and tobacco–corn rotation on soil microbial communities in the karst regions of Southwestern China. High-throughput sequencing was used to evaluate the responses of the soil microbial community structure to crop monoculture and rotation patterns. As expected, the tobacco–corn rotation mitigated the negative effects of continuous cropping and reduced soil acidification. The tobacco–corn rotation also significantly altered the composition of microbial communities and promoted plant growth by fostering a higher abundance of beneficial microorganisms. The predominant bacteria genera Sphingomonas and Gaiella and the predominant fungal genera Mortierella and Saitozyma were identified as discriminant biomarkers that are critical to soil ecosystem health. pH, available potassium (AK), and available phosphorus (AP) were the primary soil factors related to the soil microbiome assembly. This study aimed to demonstrate the association between crop rotation and microbiomes, suggesting that altering cultivation patterns could enhance karst agricultural systems.
• Corn rotation increased soil bacterial diversity at the Operational Taxonomic Unit (OTU) level.
• Discriminant biomarkers were critical to soil ecosystem health.
• Corn rotation enriched the growth of beneficial microorganisms in the soil.
With the excessive pursuit of economic interests and the diminishing availability of land resources, continuous cropping patterns have become an important part of agricultural production and are widely used in China. However, this practice has led to soil nutrient imbalances and a rise in soil-borne diseases (Machado et al., 2007; Zhang M. M. et al., 2023). Previous reports have indicated that crops have strong interactions with soil, and continuous cropping of the same plant causes root rot (Duddigan et al., 2021; Chen et al., 2022). In monocropping tobacco systems, the most prevalent diseases include bacterial wilt caused by Ralstonia solanacearum, root rot caused by Fusarium oxysporum, and black shank caused by Pythium ultimum (Lee et al., 2021). Certain diseases such as stem rot, ear rot, and common rust caused by Puccinia sorghi, Fusarium subglutinans, and Pythium aphanidermatum, respectively, have become more prevalent due to the continuous cultivation of corn (Liu et al., 2022). Numerous studies have documented that the decrease in crop yield is associated with soil degradation under continuous cultivation (Fujisao et al., 2020; Arunrat et al., 2023). The composition and diversity of microbial communities in soil are critical for maintaining soil health and quality (Bai et al., 2019). Several reports have revealed that imbalances in soil microbial communities correlate with continuous cropping (Fang et al., 2018; Ding et al., 2024). Furthermore, continuous cropping has also been reported to increase the prevalence of harmful fungi (Gao et al., 2021). The ecological imbalance of microbial communities in the rhizosphere soil of plant hosts is an important mechanism for the development of plant diseases. Monoculture crop systems disrupt the soil microbial community and are associated with the lowest levels of microbial diversity (Liu et al., 2021b). The relative abundance of pathogenic fungi was found to increase synergistically with the duration of continuous tobacco cultivation (Ding et al., 2024). Furthermore, the abundance of soil-borne pathogens (e.g., Fusarium) increased significantly after cucumbers were monocropped (Jin et al., 2019). The outbreak of bacterial wilt in tomatoes was attributed to the disruption of Firmicutes and Actinobacteria in the tomato rhizosphere (Lee et al., 2021). Therefore, protecting the soil micro-ecosystem is crucial for the healthy growth of crops. However, how does the continuous cropping system change the physical and biochemical properties of soils? How is bacterial wilt caused by the imbalance in the microbial structure within soil? Currently, no definite answers to these questions are available yet.
Rotation patterns alter soil physical and biochemical properties and shift the community structure (McDaniel et al., 2014; Zhang H. F. et al., 2023). The practice of crop rotation is widely adopted for its manifold advantages, which include pest control, disease control, and the enhancement of crop yields (Behnke et al., 2021). Alkali-hydrolyzed nitrogen (AN) and available phosphorus (AP) were increased by paddy upland rotation in one study (Turmuktini et al., 2012). Compared to continuous cropping systems, rotations promote more efficient nutrient cycling (Town et al., 2022). Numerous studies have found that crop rotation increases the number of bacteria and actinomycetes, improves the ratio of bacteria to fungi, and enhances the community structure in soil (Zhang H. F. et al., 2023; Yan et al., 2024). Crop rotation alters the quantity and quality of plant residues, which serve as an energy source for soil microorganisms, leading to changes in the soil microbial community structure (Behnke et al., 2021). The bacterial community richness and Shannon index were higher in tobacco rotation systems compared to continuous cropping systems (Zheng et al., 2020). Tobacco–corn rotation has also been shown to suppress the incidences of viral diseases (Niu et al., 2016). In addition, diverse crop rotations help reduce pathogen host-plant incidence (Floc'h et al., 2020; Xie et al., 2022). However, some problems caused by crop rotation patterns cannot be ignored. For example, soil deterioration and reduced fertilizer utilization have been observed in rice-wheat crop rotation systems (Zhou et al., 2014). The abundance of plant pathogens has been reported to increase in pea rotation systems (Niu et al., 2018). In addition, phytopathogenesis results from interactions between microbiomes associated with plant hosts and pathogens, which play a central role in regulating plant health and pathogen infection (Guo et al., 2024). Therefore, a thorough understanding of changes in the microbial community structure under crop rotation patterns is important for the karst agricultural system.
Wenshan Zhuang and Miao Autonomous Prefecture (Wenshan Prefecture) has a unique geographical location, situated in the Yungui Plateau in Southwestern China, with a typical karst landform. Wenshan Prefecture enjoys sufficient sunshine, with an average annual temperature of 16–19°C and an annual rainfall of 1,075 mm, making it suitable for the growth of various crops (Feng et al., 2018). However, mountainous areas account for 97% of the total land area, with karst regions making up 53.4% (Li and Lu, 2019). The scarcity of land resources and continuous cropping obstacles have led to the outbreak of soil-borne diseases, which have seriously affected the development of industries such as grain, Panax pseudoginseng, tobacco, and chili. Crop rotation, as an effective method for preventing and controlling soil-borne diseases, is increasingly being recognized for its role in regulating soil microorganisms. By the time of writing, numerous studies have been conducted on the issue of continuous cropping in soil. However, limited research has elucidated the response of soil microbial communities to crop rotation in soils severely affected by bacterial wilt. Therefore, we employed high-throughput sequencing technology to assess the long-term adaptive differences in soil microbiome characteristics between corn and tobacco fields under monoculture and tobacco–corn rotation. The present study will help understand the effects of crop rotation on the complex interactions between host plants and soil microbial species, including soil health indicators and discriminant biomarkers. We aim to provide a theoretical basis for mitigating soil-borne diseases caused by continuous cropping barriers. The present study also attempted to explore suitable local crop rotation patterns, which are crucial for guiding agricultural practices.
The experimental site was located at the Malipo long-term continuous cropping experimental station in Wenshan Prefecture, Yunnan Province, China (23°7′43.3″N, 104°42′0.6″E). The zonal soil in this area is predominantly red soil. For sampling, we selected the following four fields as experimental sites: corn monoculture for 6 years (CO), tobacco monoculture for 6 years (TO), tobacco monoculture for 10 years with severe wilt (WI), and tobacco monoculture for 9 years, followed by 1 year of corn rotation (RO) (Figure 1). Field management practices were consistent across all experimental sites. The base fertilizer application rates were as follows: N: 90 kg·ha−1, P2O5: 90 kg·ha−1, and K2O: 120 kg·ha−1. Topdressing was applied twice, every 30 d, at rates of N: 25 kg·ha−1, P2O5: 25 kg·ha−1, and K2O: 50 kg·ha−1.
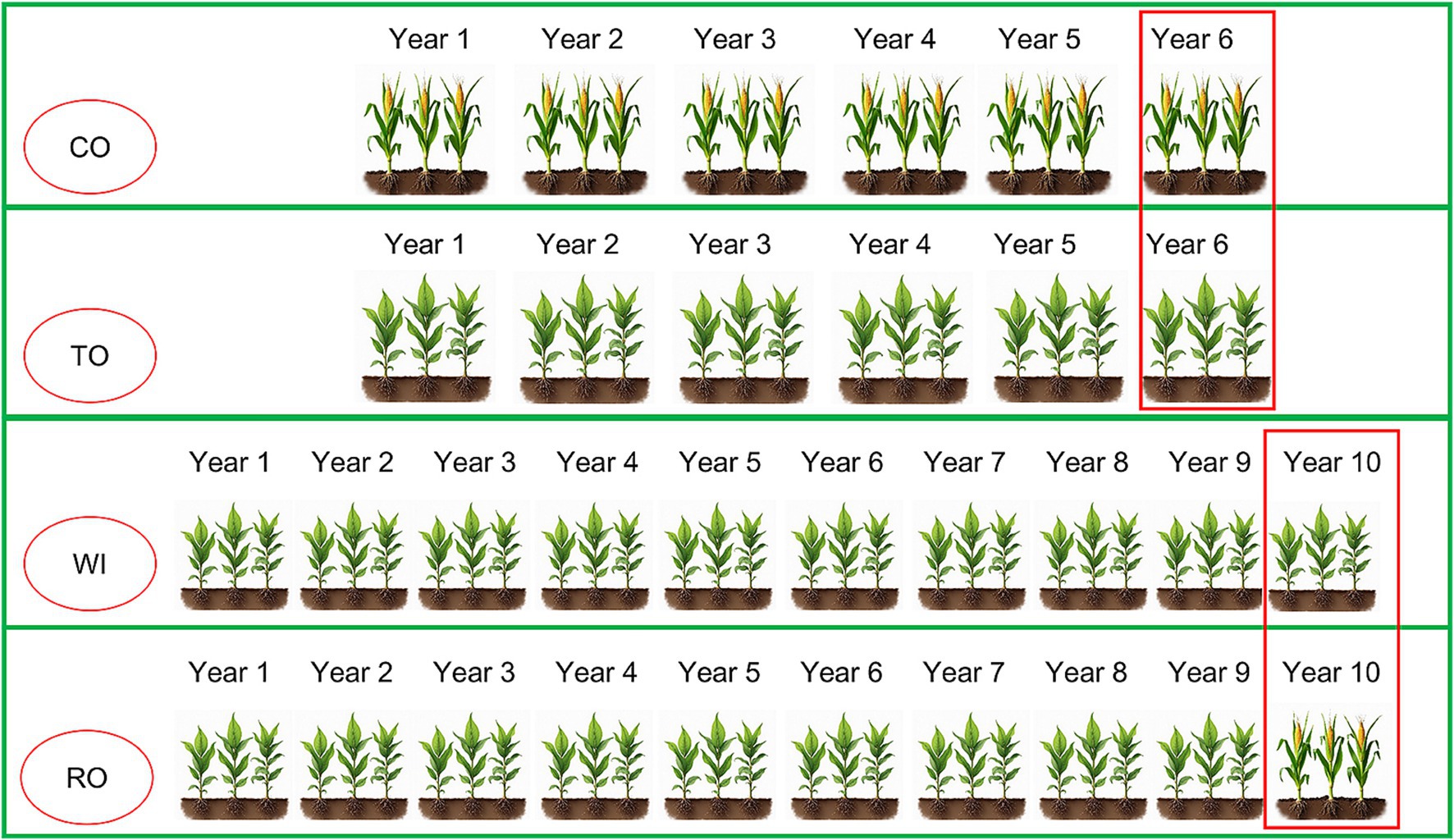
Figure 1. Schematic diagram showing the four cultivation patterns. CO, corn monoculture for 6 years; TO, tobacco monoculture for 6 years; WI, tobacco monoculture for 10 years with serious wilt; RO, tobacco monoculture for 9 years and corn rotation for 1 year. Red borders indicate the collection time of the soil samples.
Samples were collected in June 2023, marking the 10th year of the long-term experiment. The samples were collected from the four fields on the same day. Plants from each row (15 plants total per plot) were sampled using a hand trowel. Soil closely attached to the roots was collected as rhizosphere soil. Five soil samples were combined to form a single sample for each treatment. All soil samples were divided into two portions. One portion was stored at −80°C for DNA extraction, and the other was used to analyze soil physicochemical properties. The soil physicochemical properties, including soil pH, soil organic carbon (SOC), available phosphorus (AP), available potassium (AK), and alkali-hydrolyzed nitrogen (AN), were determined using previously described methods (Bao, 2005; Ali et al., 2021). The soil properties of the field experiment are shown in Table 1.
Based on the observation of typical wilt symptoms (including necrosis and leaf drooping) in corn and tobacco, a bioassay of disease incidence was conducted at 100 days. According to the methods described in “Diagnosis and Control of Maize Diseases” (Chen, 1999) and “General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Tobacco Pest and Disease Classification and Survey Methods (GB/T 23222) (Beijing: Standardization Administration, 2008)”, the disease rate was assessed by evaluating the presence of typical wilt symptoms.
Total DNA was extracted from all soil samples using a FastDNA® SPIN Kit according to the manufacturer’s instructions. The V3–V4 regions of the16S rRNA genes were amplified using the bacterial primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) (Zhang M. M. et al., 2023). The universal primers ITS1F (5′-CTTGGTCATTTAGAGGAAG TAA-3′) and ITS2R (5′-GCTG CGTTCTTCATCGATGC-3′) were used to amplify the fungal ITS1 region (Jin et al., 2022). The PCR products were checked using 2% gel electrophoresis and subsequently sent to Majorbio Co., Ltd. (Shanghai, China) for paired-end sequencing on the Illumina PE300 platform. After merging and quality checking, high-quality sequences were clustered into operational taxonomic units (OTUs) based on a 97% similarity threshold using UPARSE (Edgar, 2013; Bolyen et al., 2019). Bacterial and fungal taxonomies were assessed against the 16S rRNA database (Silva v138) and the fungal ITS database (UNITE v7.2), respectively (Liu et al., 2021c).
All statistical analyses and data presentation were conducted using R software (v4.3.2). One-way analysis of variance (ANOVA) was performed to evaluate the effects of the cultivation patterns on the soil properties. Alpha and beta diversity were calculated using QIIME (v 1.9.1). Principal coordinate analysis (PCoA), redundancy analysis (RDA), and Spearman’s rank correlation heatmap analysis were used to reveal the connection between the soil physicochemical properties and rhizosphere microbiota. The relative importance of each environmental factor in independently accounting for the total variation was quantified using the hierarchy algorithm, and the results were drawn using the “rdacca.hp” package. The Mantel test was conducted to identify the main determinants of the core microbiome in the soil. Stacked histograms showing the functional abundance of the rhizosphere microbiota were created using the ggplot2 R package. Other analyses were conducted on the Majorbio Cloud platform1 using various R packages and workflow frameworks.
The most prevalent disease affecting tobacco was bacterial wilt, while stem rot was predominant in corn at the experimental site (Supplementary Table S1). The bacterial wilt disease incidence and index of WI were significantly (p < 0.05) higher than those of TO. Compared to CO, the disease incidence and index of RO were decreased by 63.19 and 61.34%, respectively. The results indicated that long-term continuous cropping increased the incidence and severity of soil-borne diseases, while crop rotation more effectively reduced the incidence and severity of these diseases.
A total of 839,710 high-quality 16S rRNA sequences and 1,047,109 ITS sequences were obtained. At the OTU level, plant pathogenesis was correlated with the diversity of both bacterial and fungal communities. For the bacteria, the soil community diversity in CO (Shannon = 6.396) was higher than that in TO (Shannon = 6.284). However, there were no significant differences between WI and RO (Figure 2A). WI resulted in a significant increase in the number of fungal community OTUs (Shannon = 3.553) compared to RO (Shannon = 2.645) (Figure 2C). The long-term monoculture decreased the soil bacterial and fungal diversity, while the rotations of corn and tobacco increased the rhizosphere microbiota diversity (Figures 2B, D).
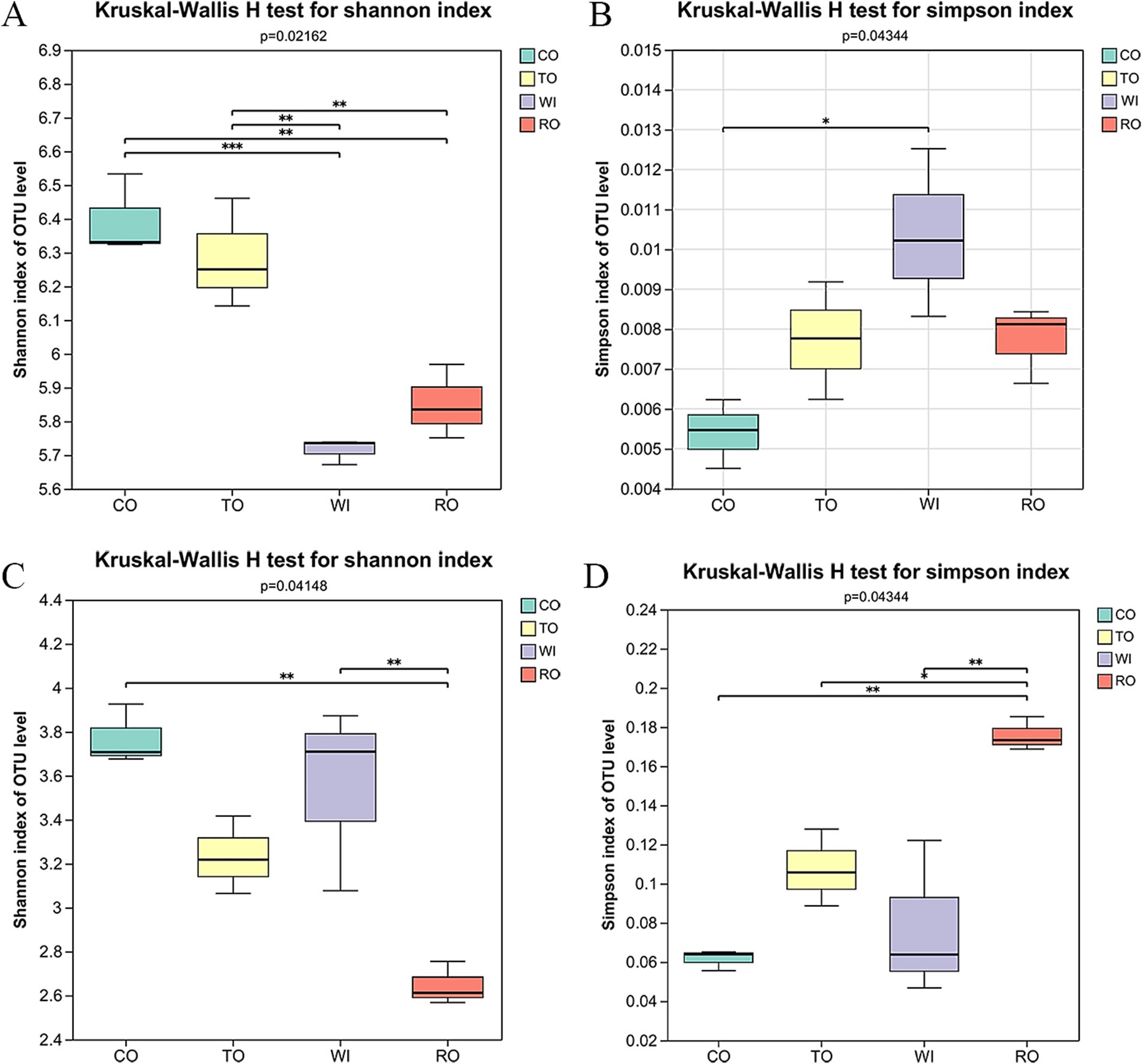
Figure 2. Alpha diversity of the bacteria and fungi in the soil. The Shannon index of the (A) bacteria and (C) fungi. The Simpson diversity index of the (B) bacteria and (D) fungi. CO, corn monoculture for 6 years; TO, tobacco monoculture for 6 years; WI, tobacco monoculture for 10 years with serious wilt; RO, tobacco monoculture for 9 years and corn rotation for 1 year. * indicate significant differences among the cultivation patterns. *p < 0.05, **p < 0.01, and ***p < 0.001.
Principal coordinate analysis based on the Bray–Curtis distance was used to analyze the rhizosphere community structure (Figure 3). For the bacteria, the first principal component (PC1) and the second principal component (PC2) contributed 61.27% of the variance, indicating that they effectively represented the characteristics of the bacterial community composition. The groups CO and TO were clustered together, indicating that their bacterial community compositions were similar but differed significantly from the other groups. The PCoA assigned the fungal communities into three groups: WI, RO, and CO/TO. There was a noticeable distance between WI and RO. The first two axes accounted for 30.59 and 26.77% of the total variability, respectively. PERMANOVA revealed that the rotations of corn and tobacco significantly affected the bacterial (F = 5.20, p = 0.001) and fungal (F = 14.63, p = 0.001) community structures.
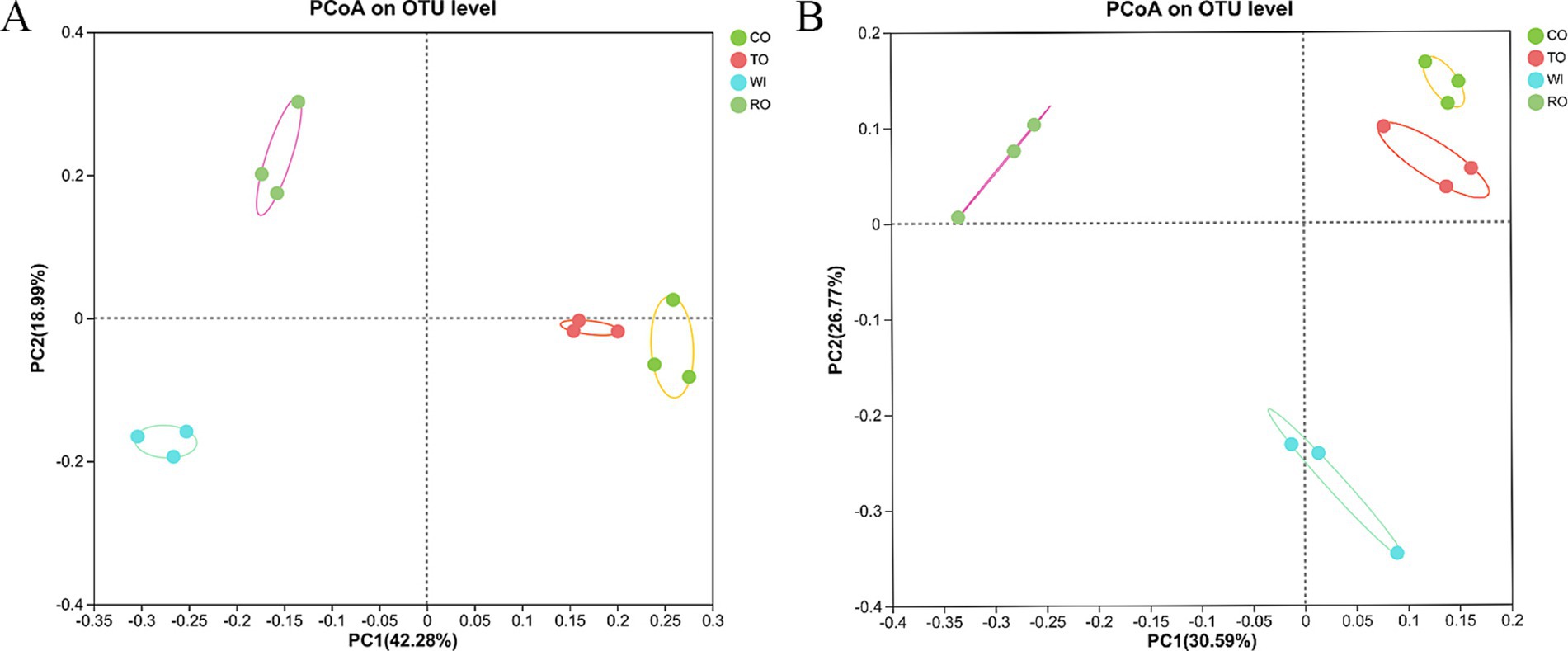
Figure 3. Principal coordinate analysis (PCoA) plots of the soil (A) bacterial and (B) fungal community structures based on the Bray–Curtis distance.
A total of 46 bacterial phyla were detected across all soil samples. Among these, Actinobacteria, Proteobacteria, and Chloroflexi were the most abundant phyla in the four groups, with Actinobacteria (35.95%) being particularly dominant in the RO group (Figure 4A). The 10 most abundant genera were JG30-KF-AS9, Sphingomonas, Gaiella, Arthrobacter, Bryobacter, Acidobacterium, Elsterales, JG30-KF-CM45, WPS-2, and Terrabacter (Figure 4C). The relative abundance of JG30-KF-AS9, Arthrobacter, and Acidobacterium in TO was higher than that in CO. In RO, the relative abundance of JG30-KF-AS9, Gaiella, Arthrobacter, Bryobacter, and Arthrobacter decreased to 7.93, 2.24, 1.44, 1.33, and 1.31%, respectively.
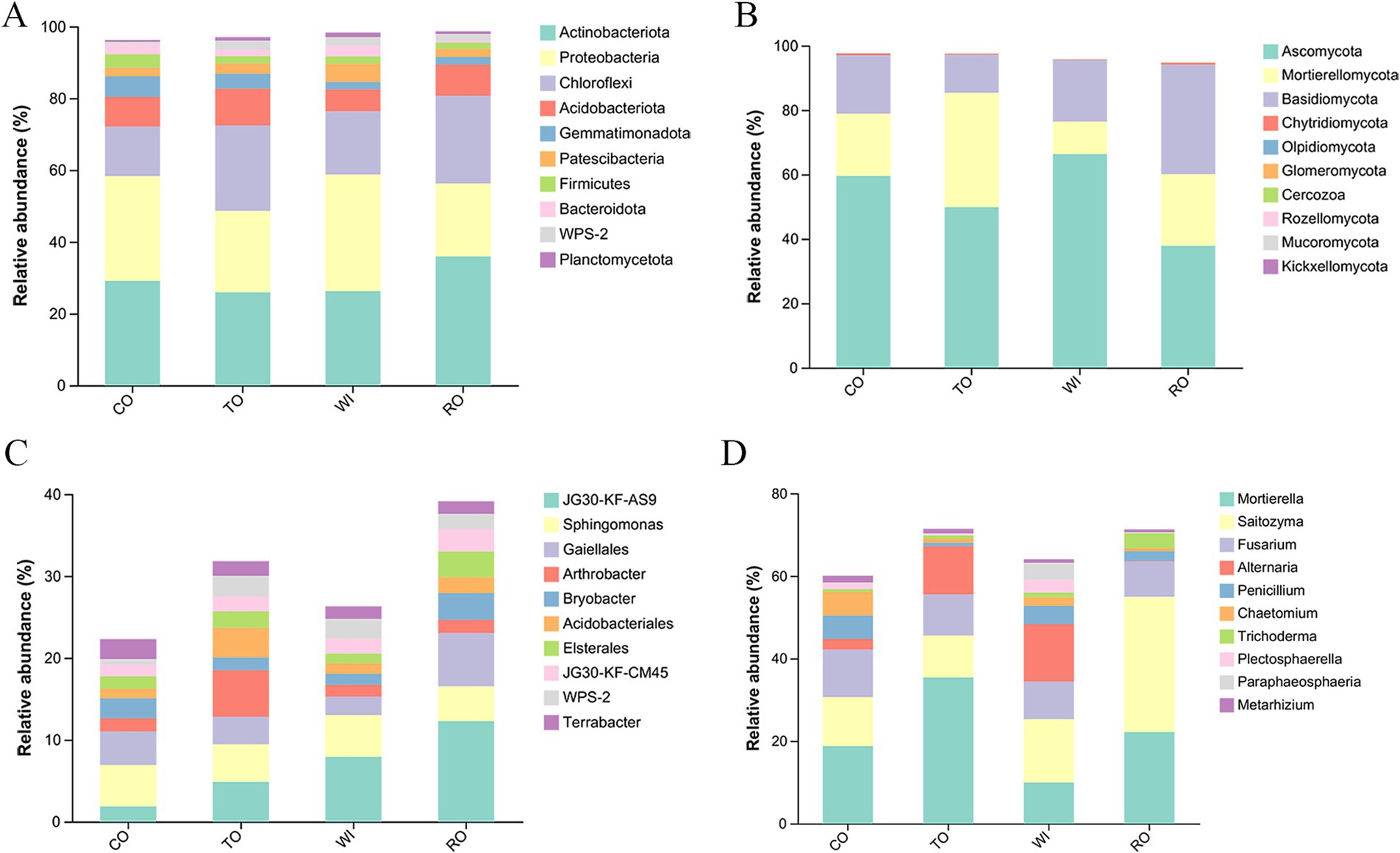
Figure 4. Bacterial and fungal community structures under different cultivation patterns. Bacterial (A) and fungal (B) community structures at the phyla level. Bacterial (C) and fungal (D) community structures at the genus level. CO, corn monoculture for 6 years; TO, tobacco monoculture for 6 years; WI, tobacco monoculture for 10 years with serious wilt; RO, tobacco monoculture for 9 years and corn rotation for 1 year.
Basidiomycota and Ascomycota were the most abundant fungal phyla across all samples. The 10 most abundant genera were Mortierella, Saitozyma, Fusarium, Alternaria, Penicillium, Trichoderma, Plectosphaerella, Paraphaeosphaeria, and Metarhizium (Figure 4B). Mortierella was more abundant in TO (35.43%) (Figure 4D). The relative abundance of Saitozyma (32.74%) and Trichoderma (3.62%) in RO was higher than that in WI. Alternaria (13.92%) was more abundant in WI.
To find differences in the bacterial and fungal communities of the soil samples, LEfSE was employed to identify discriminatory biomarkers (LDA scores >3.8) (Supplementary Figure S1). For the bacteria, two phyla—Gemmatimonadota and Myxococcota—and four genera—Roseiflexus, Gemmatimonas, Massilia, and TK10—showed higher relative abundance in CO. In contrast, three genera—Ktedonobacter, Vicinamibacter, and AD3—had higher relative abundance in TO. The genera enriched in RO included Gaiella, Acidothermus, Elsterales, Bryobacter, and Streptomyces. In contrast, the genera enriched in the WI sample included members from three phyla—Proteobacteria, Patescibacteria, and Bacteroidota and seven genera, namely Rhodanobacter, Burkholderia, Leifsonia, LWQ8, Modestobacter, Geodermatophilus, and Chujaibacter. For the fungi, five genera—Penicillium, Chaetomium, Coniophora, Clonostachys, and Aspergillus—showed higher relative abundance in CO, while the phylum Mortierellomycota and the genus Mortierella showed higher relative abundance in TO. The genera enriched in RO included the phylum Basidiomycota and genera Saitozyma and Conlarium. The genera enriched in WI included the phylum Ascomycota and genera Alternaria, Paraphaeosphaeria, Plectosphaerella, Colletotrichum, and Arthrobotrys.
For the bacterial communities, Venn analysis revealed that 1,080 OTUs, representing the core microbiome, were shared among the four groups, accounting for 7% of the total sample (Figure 5A). Sphingomonas, Arthrobacter, JG30-KF-AS9, Terrabacter, Bradyrhizobium, WPS-2, Bryobacter, Elsterales, Leifsonia, and Frankiales were the top 10 core bacterial genera in the four groups. Among the 10 OTUs, the dominant core taxon was OTU22844 Sphingomonas sp., with a relative abundance of 3.94% of the total sample. For the fungal community, a total of 183 OTUs were identified in the four groups, accounting for 18% of the total sample (Figure 5B). Saitozyma, Mortierella, Fusarium, Alternaria, Chaetomiaceae, Chaetomium, Penicillium, Trichoderma, and Plectosphaerella were the top 10 core fungal genera. The dominant core taxon was OTU572 Saitozyma sp., with a relative abundance of 17.63% of the total sample.
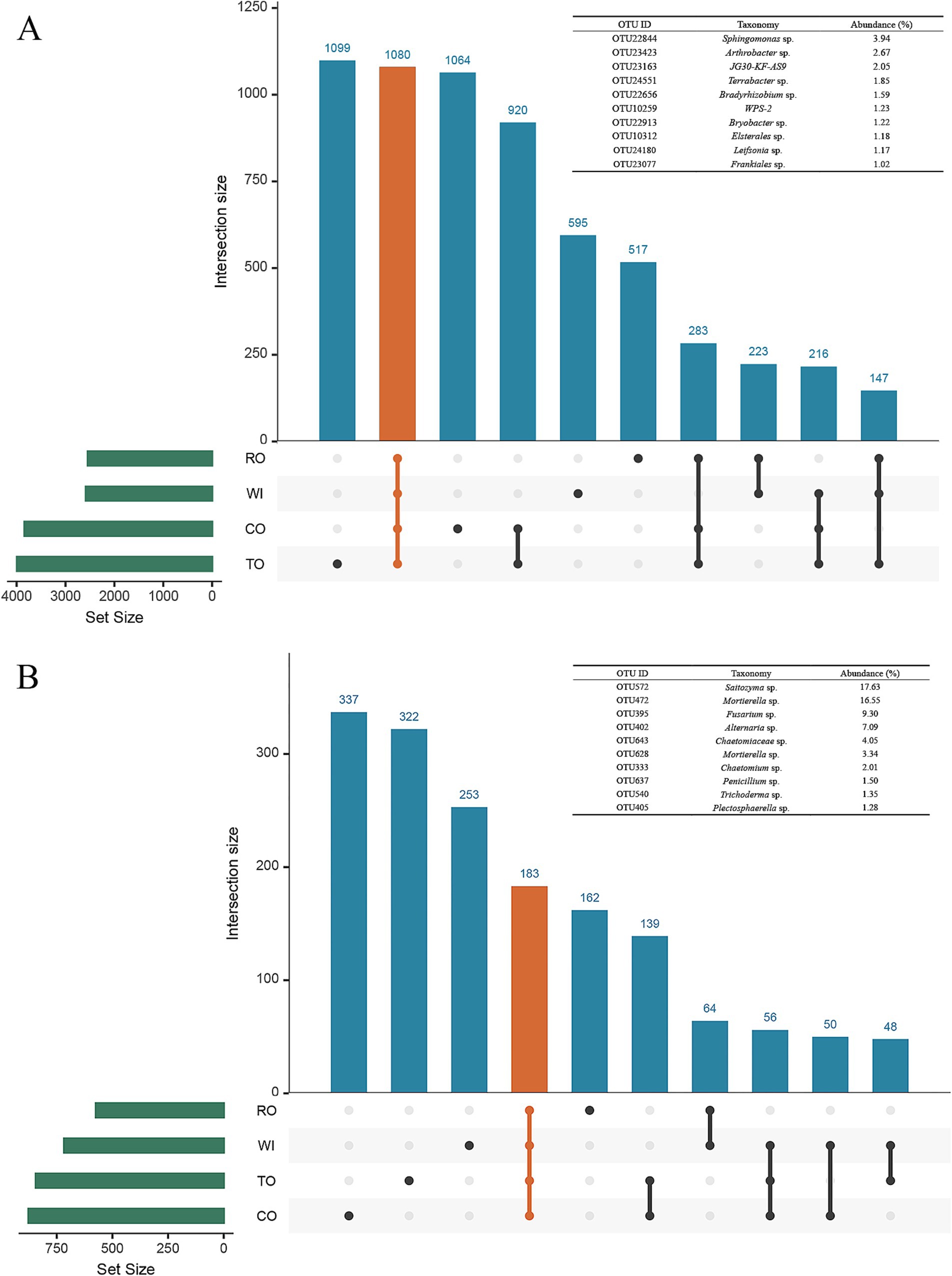
Figure 5. Taxonomic composition of the core bacterial (A) and fungal (B) microbiomes in the soil. Black dots indicate the presence of the OTUs in the Upset diagram, gray dots indicate the OTUs that were absent, and the lines between different black dots indicate where the OTUs were shared. The OTUs that were common to all five categorical groups (the core microbiome) are represented by orange dots and lines.
The relative importance of each explanatory variable in independently accounting for the total variation was quantified using the hierarchy algorithm (Supplementary Figure S2). AP had the greatest effect on the bacterial communities (24.43%), while AK significantly influenced the formation of the fungal communities (24.37%) among the five environmental factors included in this model. In Figure 6A, the first two axes of the RDA explain 34.46 and 21.83% of the total variation in the soil bacterial data, respectively. In Figure 6B, the first two axes of the RDA explain 42.30 and 24.95% of the total variation in the soil fungal data, respectively. We also used Spearman’s rank correlation to evaluate the relationships between the abundant bacterial genera and soil physicochemical properties. The dominant genus Bryobacter was positively correlated with AN (p < 0.01), while WPS-2 negatively correlated with AN (p < 0.01). For the fungal communities, the dominant genera Trichoderma and Saitozyma were positively correlated with AP (p < 0.01) and negatively correlated with AK and pH (p < 0.01). Furthermore, the variation in the core microbiome displayed significant correlations with environmental factors, as evidenced by the Mantel test (p < 0.05) (Supplementary Figure S3). We observed that the core bacterial genera were significantly impacted by AK, AP, and AN (p < 0.01). AP and AK were also found to impact the core fungal genera (p < 0.01). The relative abundance of Bryobacter and Gaiella was significantly (p < 0.001) negatively correlated with the disease index (Figure 6C). The higher abundance of these bacteria in the soil might be helpful for inhibiting bacterial wilt and stem rot. In contrast, the relative abundance of Plectosphaerella (p < 0.05) and Alternaria (p < 0.01) were significantly positively correlated with the disease index (Figure 6D). We speculated that these fungi with high relative abundance in the soil may promote the outbreak of soil-borne diseases.
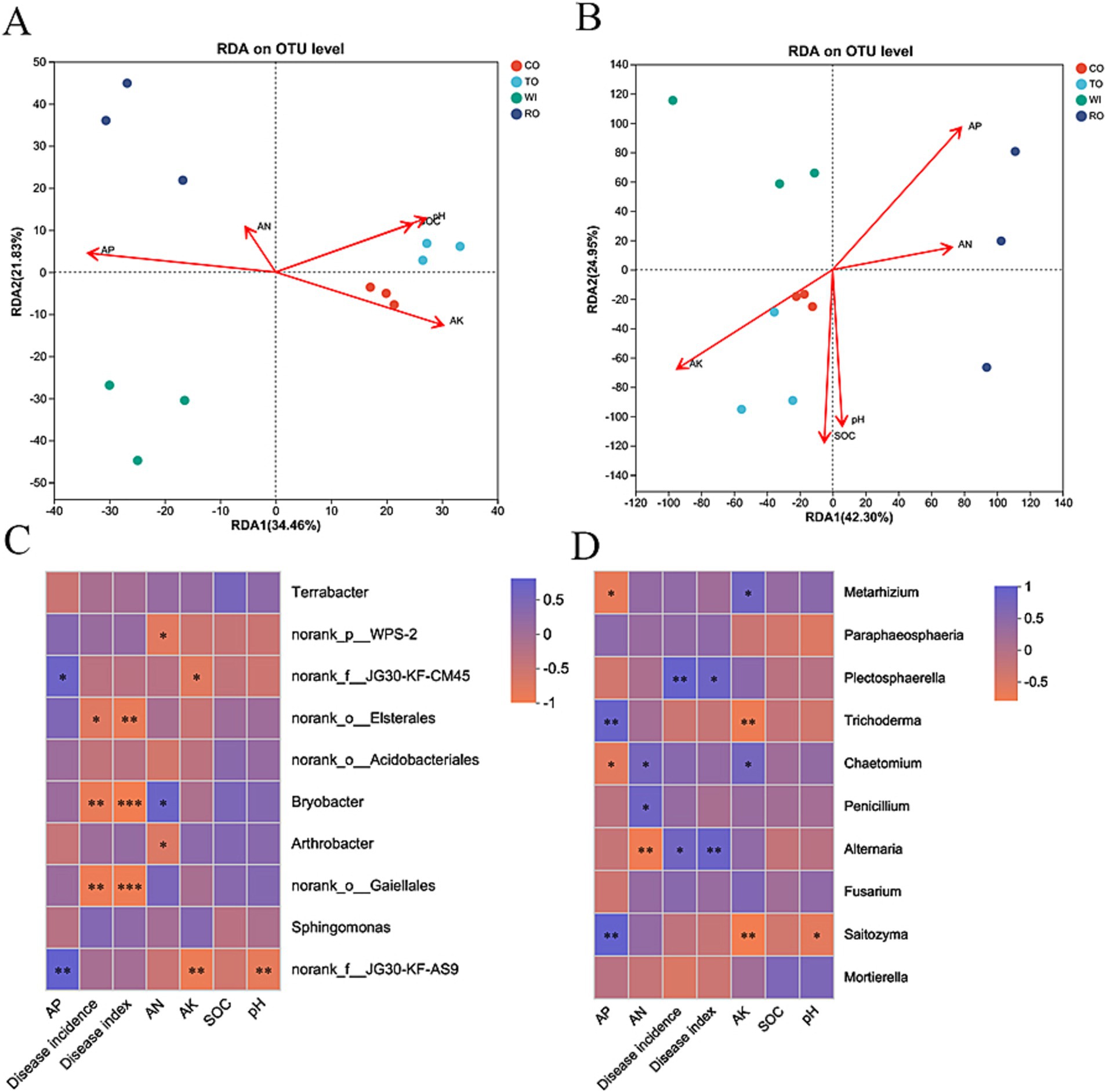
Figure 6. Redundancy analysis (RDA) based on the bacterial (A) and fungal (B) OTU data with the chemical parameters in the soils. A correlation heatmap of the top 10 bacterial (C) and fungal (D) genera with the environmental factors. The R values are indicated on the right side of the legend with different colors. *p < 0.05, **p < 0.01, and ***p < 0.001.
In this study, the differences and interactions of the soil bacterial and fungal communities were confirmed through co-occurrence networks at the OTU level (R > 0.7, p < 0.05) (Figure 7). For the bacterial communities, the number of edges (1,962) was highest in the soils from RO (Supplementary Table S2). The average degree and network density for the fungal communities were lower in CO compared to RO. Relative to WI, RO increased the average degree and network density values. For the fungal community, the network structure was significantly simpler than that of the bacterial community. The number of edges (1662) was highest in the soils from WI. Furthermore, the number of network edges in CO was fewer compared to all the other groups. Moreover, compared to WI, RO decreased the average degree and network density values. These findings showed that the fungal networks in WI were significantly more complex compared to RO.
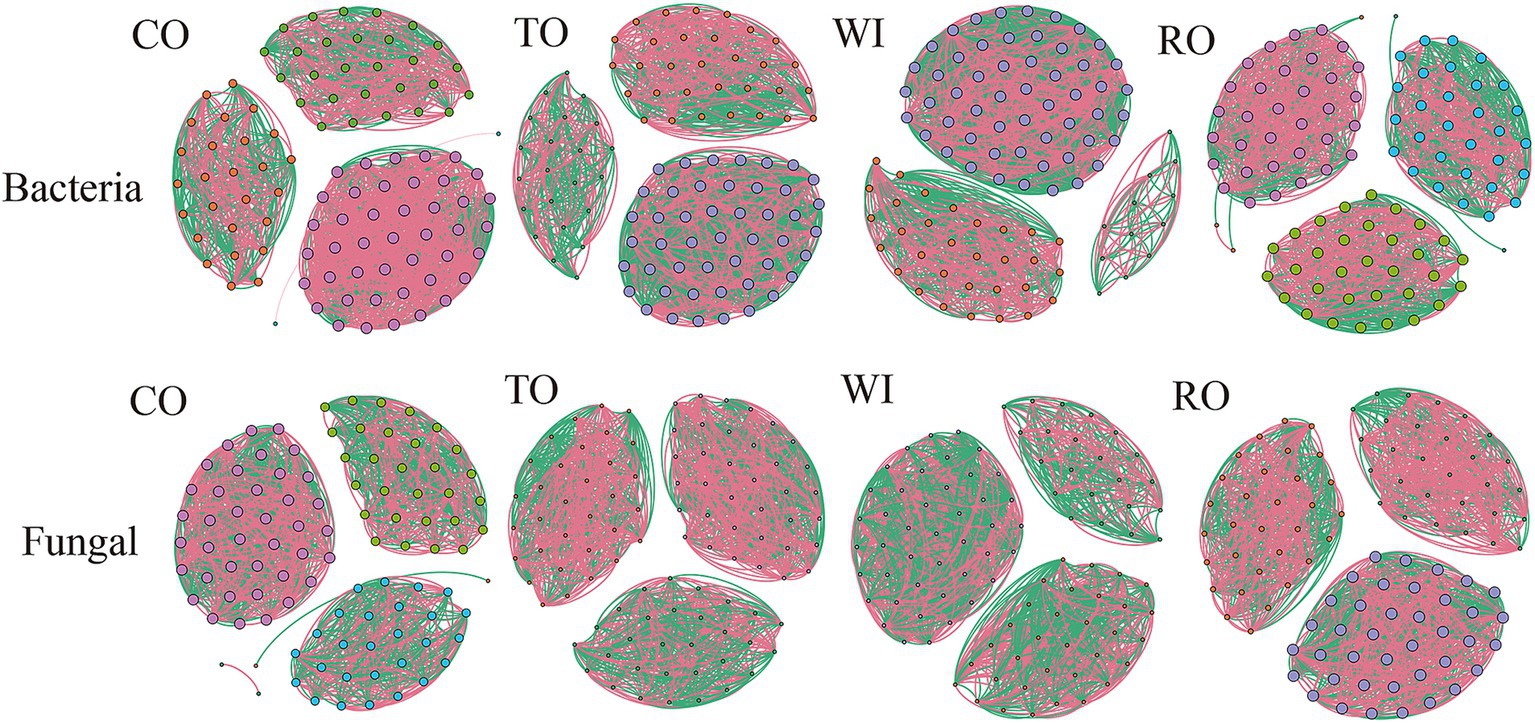
Figure 7. The co-occurrence network of the soil bacteria and fungi. CO, corn monoculture for 6 years; TO, tobacco monoculture for 6 years; WI, tobacco monoculture for 10 years with serious wilt; RO, tobacco monoculture for 9 years and corn rotation for 1 year.
Based on the FAPROTAX database, we predicted the bacterial functions and identified the top 15 functional groups (Figure 8A). RO also showed a decrease in genes related to ureolysis, hydrocarbon_degradation, and aromatic_hydrocarbon_degradation. The FUNGuild database was used to analyze the functional profiles of the fungal communities (Figure 8B). The TO group was dominated by Endophyte-Litter Saprotroph-Soil Saprotroph-Undefined Saprotroph, accounting for 35% of the total community. Animal Pathogen-Endophyte-Plant Pathogen-Wood Saprotroph was dominant in TO and WI, and their relative abundance was 11 and 14%, respectively. The saprotrophs were significantly lower (8%) in the RO group than in the WI group. In addition, animal pathogens and plant pathogens were significantly decreased in the RO group compared to the other groups.
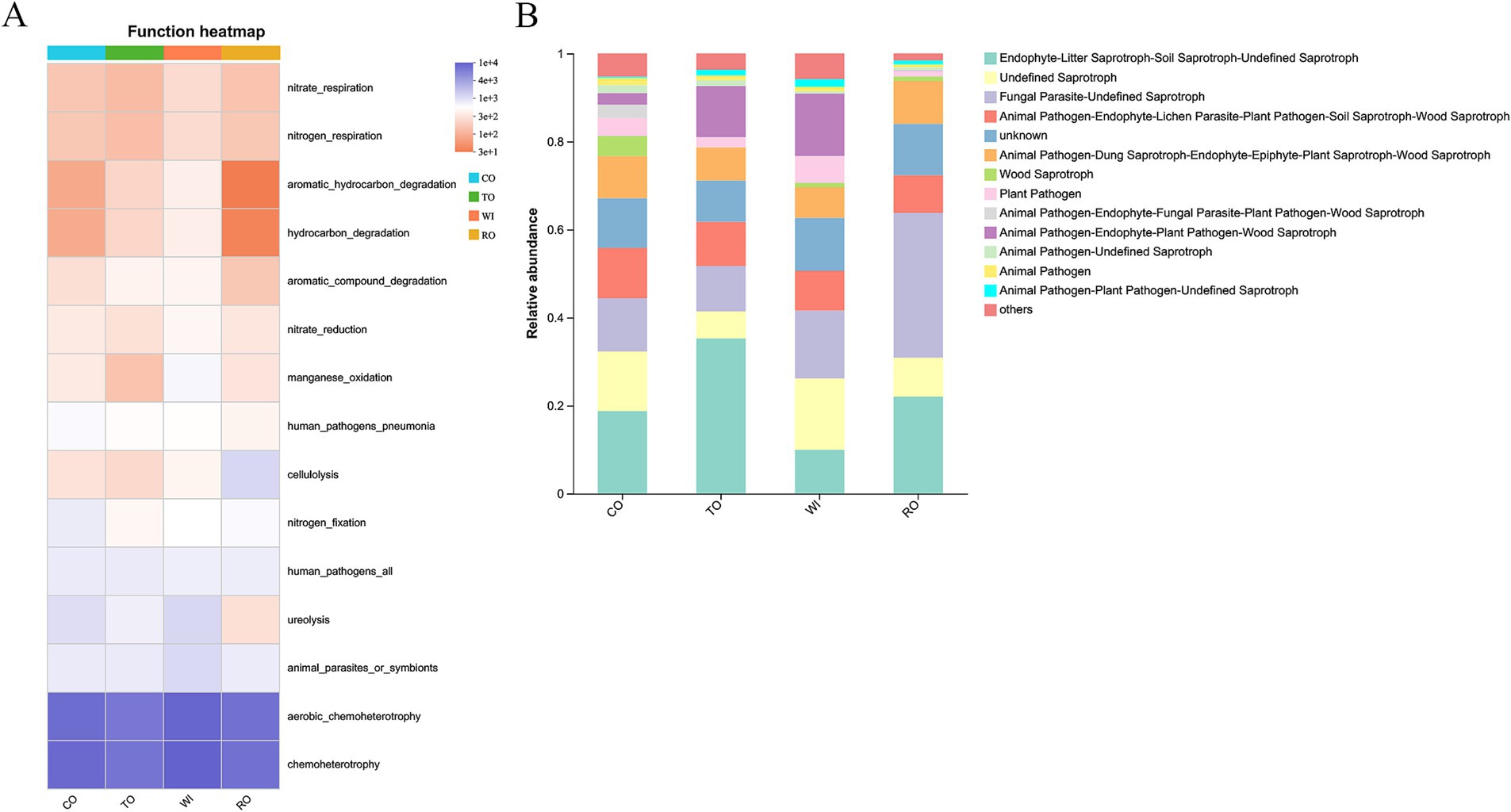
Figure 8. Changes in the functional groups based on the bacterial (A) and fungal (B) OTU data in the soil. CO, corn monoculture for 6 years; TO, tobacco monoculture for 6 years; WI, tobacco monoculture for 10 years with serious wilt; RO, tobacco monoculture for 9 years and corn rotation for 1 year.
Continuous cropping is a negative feedback mechanism for soil, which not only changes the soil environment but also promotes the progression of plant diseases (van der Putten et al., 2013). Recent studies have revealed that the stability of microbial community composition is important for a healthy host–microbe relationship and that both enrichment and imbalance in microbiota abundance are important mechanisms for disease development in plants (Schlatter et al., 2017; Berendsen et al., 2018). Our results showed that, compared to continuous tobacco cropping, the corn rotation increased the bacterial diversity. Long-term continuous cropping durations resulted in lower bacterial community diversity (Zhang et al., 2015). The soil bacterial community structure, bacterial species richness, and Shannon index values were significantly increased under crop rotation conditions (Town et al., 2023). Continuous cropping of corn and tobacco increased soil fungal community diversity, which is not completely consistent with previous studies (Zhou et al., 2017; Zhao et al., 2020). These differences may be due to variations in the duration of soil environmental conditions, crop variety, and other factors. We observed that, in the PCoA analysis, the bacterial and fungal communities of CO and TO were clustered, indicating that the soil microbial community structures were affected by the continuous cropping cultivation patterns. The rhizosphere soil microbial communities of TO and CO exhibited a certain degree of homogeneity. In particular, the communities in RO remarkably differed from those in CO, TO, and WI, suggesting that soil bacterial and fungal communities are significantly different between crop rotation and long-term continuous cultivation patterns.
Crop rotation is an important cultivation practice for crop production and the reduction of pests and diseases. Crop rotation is also considered an important measure for improving soil quality and reducing the use of mineral fertilizers (Câmara-Salim et al., 2021). Crop rotation increased the yield and oil content of canola and decreased disease pressure from Leptosphaeria and Alternaria (Town et al., 2023). Monocropped cucumber increased soil nutrient concentrations but decreased available nutrient concentrations (Chen et al., 2022). Cotton-grain-rape rotation increased the yield of cotton, maize, and wheat, as well as the above-ground dry matter weight of cotton and maize (Dong et al., 2024). Maize-wheat rotation affected the species composition of Fusarium, but no significant difference in pathogenicity was observed between wheat and rice (Dong et al., 2023). This study demonstrated that Actinobacteria, Proteobacteria, and Chloroflexi were the most abundant bacterial phyla in the four groups, with Actinobacteria (35.95%) being more abundant in RO. Similar results have been found in many previous reports (Bulgarelli et al., 2013; Delgado-Baquerizo et al., 2018). Previous studies have shown that Actinobacteria establishes disease suppression through an antagonistic effect (Weller et al., 2002). The imbalance of Actinobacteria in the tomato rhizosphere increases the incidence rate of bacterial wilt (Liu et al., 2021a). In this study, the corn rotation promoted the proliferation of Actinobacteriota. Crop rotation was associated with a more significant effect on soil fungal communities compared to bacterial communities (Kracmarova et al., 2022). The keystone taxa identified in the rotations of rice and canola were all fungal genera (Zhang H. F. et al., 2023). The fungi that were consistently associated with monocropping were known pathogens of tobacco or corn, including Alternaria and Mortierella. These fungi were enriched in CO, TO, and WI. This finding is in line with previous observations that crop rotation significantly influences the composition of the rhizosphere in canola (Town et al., 2023). The relative abundance of the multiple beneficial fungi, including Saitozyma and Trichoderma, was increased in the tobacco–corn rotation.
In this study, we focused on several typical microorganisms as these microbial taxa are commonly associated with soil function and crop productivity, directly driving important soil biological processes. At the bacterial genus level, the average relative abundance of some putative biocontrol microbes, such as Sphingomonas and Gaiella, was significantly promoted by the corn rotation. Sphingomonas has been associated with resisting the accumulation of soil-borne pathogens to protect plant health (Lan et al., 2014). It has been reported that Sphingomonas could produce carotenoids and improve stress resistance in rice (Cheng et al., 2021). Gaiella is a member of the Actinobacteriota phylum and is widely used to control soil-borne plant diseases (Liu et al., 2019). Our results showed that Sphingomonas was the core bacterial genus, and Gaiella was significantly enriched in the corn rotation. The fungal communities that were significantly enriched in the four groups also differed. Penicillium and Chaetomium, enriched in CO, have been reported to induce plant resistance to pathogens by activating multiple defense signals (Khalil et al., 2021). Mortierella was the core genus enriched in TO, which has been proven to control soil-borne pathogens and improve plant growth (Wani et al., 2017; Mares-Ponce de León et al., 2018). In addition, Alternaria, which was consistently associated with the monoculture, being enriched in WI, can cause wilt disease in various crops (Zeng et al., 2024). In addition, Saitozyma was enriched in RO, which is well known for its ability to release auxins and lipids (Gorte et al., 2020). Our results suggested that these discriminant biomarkers are critical to soil ecosystem health and induce positive or negative interactions with host plants.
Long-term continuous cropping causes the deterioration of soil chemical properties (Ding et al., 2024). Previous studies have shown that pH is one of the most important factors that affect microbial communities in soils (Zhang M. M. et al., 2023). In this study, soil pH was increased in RO and the corn rotation prevented soil acidification. pH had a significant negative impact on the bacteria in the genus norank_f_JG30-KF-AS9 (p < 0.01) and the fungi in the genera Saitozyma and Trichoderma (p < 0.01). This indicates that pH primarily regulates the abundance of multiple beneficial microorganisms in soil. Soil nutrients increased in long-term continuous cropping fields, indicating that the lack of plant nutrients may not directly cause plant diseases (Mbanyele et al., 2022). Our results also revealed that planting corn was more favorable for AN and AP accumulation in the soil. Several reports have found that environmental factors have different effects on soil microorganisms (Dong et al., 2017). Our results showed that the dominant bacterial genus Bryobacter was positively correlated with AN, while the dominant fungal genera Trichoderma and Saitozyma were positively correlated with AP. Bryobacter is considered to be a plant growth-promoting rhizosphere bacterium (PGPR) (Vasconcellos et al., 2021). Bryobacter improved the diversity and stability of the bacterial community in the rhizosphere soil of tomato, enhancing resistance to Ralstonia solanacearum (Zhang J. et al., 2023). Trichoderma enhances the absorption of P by plants and strengthens their ability to resist adversity stress (Bononi et al., 2020). Saitozyma plays a key role in promoting soil P transformation and accumulation (Li et al., 2022).
In the co-occurrence analysis, the corn rotation increased the complexity of the bacterial co-occurrence network and decreased the complexity of the fungal co-occurrence network, as indicated by the increased number of edges in the bacterial communities and the reduced average degree and network density in the fungal communities. Previous studies have shown that crop rotation improves the soil microenvironment, allowing more microorganisms to survive freely and reducing cooperation and competition (Fan et al., 2017). We observed that the ecological functional genes related to ureolysis, hydrocarbon_degradation, and aromatic_hydrocarbon_degradation were also decreased in RO, suggesting that accelerated organism decomposition, hydrocarbon, and aromatic_hydrocarbon degradation were facilitated by the improved soil properties. We found that the abundance of the animal pathogens and plant pathogens in TO and WI was higher than that in RO, which may be caused by the interactions between plant pathogens and free microorganisms. Previous studies have shown that plant pathogens secrete enzymes to inhibit the nitrogen restriction of free microorganisms, thus inhibiting organic matter decomposition (Averill, 2016). Therefore, corn rotation in continuous cropping tobacco fields could change plant–soil microbial community composition and has the potential for controlling soil-borne diseases.
Despite the robust design of our study, there are some limitations that should be taken into consideration. Each soil sample represented 15 rhizosphere soils from the plants in each plot, but it did not capture the overall temporospatial profile of the soils affected by serious bacterial wilt. The geographical position also contributed to the differences in the microbial composition over time. Notwithstanding these limitations, the results were clear, supported by a comprehensive analysis of the response of the soil properties and microbial communities under crop rotation. Future research should focus on exploring the functional responses of soil and plant microbiomes to different cultivation patterns, combining soil metabolome analysis to illustrate changes in the soil microecological environment.
This study examined the effects of continuous cropping and corn rotation cultivation patterns on soil microbial diversity and community structure in tobacco soil on the Yungui Plateau, where severe bacterial wilt is prevalent. The corn rotation altered the soil bacterial and fungal communities, increased their diversity, and improved the soil microenvironment. Furthermore, corn rotation fostered synergistic increments in the beneficial microorganisms. The cultivation patterns of corn rotation may be more conducive to the sustainable development of the karst agricultural system.
All sequence data have been deposited in NCBI Sequence Read Archive database under accession number PRJNA1166728.
BW: Conceptualization, Validation, Writing – review & editing. NS: Formal analysis, Supervision, Writing – review & editing. XF: Project administration, Supervision, Writing – review & editing. ZH: Data curation, Methodology, Writing – review & editing. PL: Methodology, Validation, Writing – review & editing. YC: Formal analysis, Investigation, Writing – review & editing. BH: Data curation, Formal analysis, Writing – review & editing. MD: Conceptualization, Data curation, Writing – original draft. JX: Funding acquisition, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (grant no. 42467007), Guizhou Provincial Basic Research Program (Natural Science) (no. QianKeHe Basic-[2024] Youth 177), Science and Technology Project of Yunnan Wenshan Tobacco Company (grant no. 20245326002), China National Tobacco Corporation Key Research and Development Project (110202402016 and 110202102037), and China National Tobacco Corporation Guizhou Provincial Branch Science and Technology Project (2024XM17 and 2024520000240027).
BW, ZH, and PL were employed by Yunnan Tobacco Company Wenshan Prefecture Company. XF was employed by Guizhou Tobacco Company Qiannan Company.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer MJ declared a shared parent affiliation with the author NS to the handling editor at the time of review.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1503636/full#supplementary-material
Ali, A., Ghani, M. I., Elrys, A. S., Ding, H. Y., Iqbal, M., Cheng, Z. H., et al. (2021). Different cropping systems regulate the metabolic capabilities and potential ecological functions altered by soil microbiome structure in the plastic shed mono-cropped cucumber rhizosphere. Agric. Ecosyst. Environ. 318:107486. doi: 10.1016/j.agee.2021.107486
Arunrat, N., Sansupa, C., Sereenonchai, S., and Hatano, R. (2023). Stability of soil bacteria in undisturbed soil and continuous maize cultivation in northern Thailand. Front. Microbiol. 14:1285445. doi: 10.3389/fmicb.2023.1285445
Averill, C. (2016). Slowed decomposition in ectomycorrhizal ecosystems is independent of plant chemistry. Soil Biol. Biochem. 102, 52–54. doi: 10.1016/j.soilbio.2016.08.003
Bai, Y., Wang, G., Cheng, Y., Shi, P., Yang, C., Yang, H., et al. (2019). Soil acidification in continuously cropped tobacco alters bacterial community structure and diversity via the accumulation of phenolic acids. Sci. Rep. 9:12499. doi: 10.1038/s41598-019-48611-5
Bao, S. (2005). Soil analysis in agricultural chemistry. 3rd Edn. Beijing: China Agricultural Press.
Behnke, G. D., Kim, N., Zabaloy, M. C., Riggins, C. W., Rodriguez-Zas, S., and Villamil, M. B. (2021). SoilMicrobial indicators within rotations and tillage systems. Microorganisms 9:1244. doi: 10.3390/microorganisms9061244
Berendsen, R. L., Vismans, G., Yu, K., Song, Y., de Jonge, R., Burgman, W. P., et al. (2018). Disease-induced assemblage of a plant-beneficial bacterial consortium. ISME J. 12, 1496–1507. doi: 10.1038/s41396-018-0093-1
Bolyen, E., Rideout, J. R., Dillon, M. R., Bokulich, N. A., Abnet, C. C., Al-Ghalith, G. A., et al. (2019). Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857. doi: 10.1038/s41587-019-0209-9
Bononi, L., Chiaramonte, J. B., Pansa, C. C., Moitinho, M. A., and Melo, I. S. (2020). Phosphorus-solubilizing Trichoderma spp. from Amazon soils improve soybean plant growth. Sci. Rep. 10:2858. doi: 10.1038/s41598-020-59793-8
Bulgarelli, D., Schlaeppi, K., Spaepen, S., Loren, V., van Themaat, E., and Schulze-Lefert, P. (2013). Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 64, 807–838. doi: 10.1146/annurev-arplant-050312-120106
Câmara-Salim, I., Almeida-García, F., Feijoo, G., Moreira, M. T., and González-García, S. (2021). Environmental consequences of wheat-based crop rotation in potato farming systems in Galicia, Spain. J. Environ. Manage. 287:112351. doi: 10.1016/j.jenvman.2021.112351
Chen, X. L., Zhang, D. L., Li, Y. M., Li, H. Y., Lou, J., Li, X. T., et al. (2022). Changes in rhizospheric microbiome structure and soil metabolic function in response to continuous cucumber cultivation. FEMS Microbiol. Ecol. 98, 1–13. doi: 10.1093/femsec/fiac129
Cheng, C., Wang, R., Sun, L., He, L., and Sheng, X. (2021). Cadmium-resistant and arginine decarboxylase-producing endophytic Sphingomonas sp. C40 decreases cadmium accumulation in host rice (Oryza sativa Cliangyou 513). Chemosphere 275:130109. doi: 10.1016/j.chemosphere.2021.130109
Delgado-Baquerizo, M., Oliverio, A. M., Brewer, T. E., Benavent-González, A., Eldridge, D. J., Bardgett, R. D., et al. (2018). A global atlas of the dominant bacteria found in soil. Science 359, 320–325. doi: 10.1126/science.aap9516
Ding, M., Dai, H., He, Y., Liang, T., Zhai, Z., Zhang, S., et al. (2024). Continuous cropping system altered soil microbial communities and nutrient cycles. Front. Microbiol. 15:374550. doi: 10.3389/fmicb.2024.1374550
Dong, F., Chen, X. X., Lei, X. Y., Wu, D. L., Zhang, Y. F., Lee, Y. W., et al. (2023). Effect of crop rotation on Fusarium mycotoxins and Fusarium species in cereals in Sichuan Province (China). Plant Dis. 107, 1060–1066. doi: 10.1094/PDIS-01-22-0024-RE
Dong, L. L., Xu, J., Zhang, L. J., Yang, J., Liao, B. S., Li, X. W., et al. (2017). High-throughput sequencing technology reveals that continuous cropping of American ginseng results in changes in the microbial community in arable soil. Chin. Med. 12:18. doi: 10.1186/s13020-017-0139-8
Dong, M., Zhang, Q., Wang, Y., Wang, S., Feng, G., Liao, Q., et al. (2024). Broadband crop rotation of cotton-grainrape improved crop yield and light utilization efficiency. Chin. J. Eco-Agric. 32, 1159–1169. doi: 10.12357/cjea.20230753
Duddigan, S., Fraser, T., Green, I., Diaz, A., Sizmur, T., and Tibbett, M. (2021). Plant, soil and faunal responses to a contrived pH gradient. Plant Soil 462, 505–524. doi: 10.1007/s11104-021-04879-z
Edgar, R. C. (2013). UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10, 996–998. doi: 10.1038/nmeth.2604
Fan, B., Li, Y. L., Li, L., Peng, X. J., Bu, C., Wu, X. Q., et al. (2017). Malonylome analysis of rhizobacterium Bacillus amyloliquefaciens FZB42 reveals involvement of lysine malonylation in polyketide synthesis and plant-bacteria interactions. J. Proteome 154, 1–12. doi: 10.1016/j.jprot.2016.11.022
Fang, Y., Wang, F., Li, Q., Lin, C., and He, C. (2018). Effect of continuous paddy-upland crop rotation on bacterial community structure in cold waterlogged paddy soil. Acta Pedol. Sin. 55, 515–525. doi: 10.11766/trxb201705110209
Feng, D., Li, D., Wang, L., and Zi, Y. (2018). Analysis on climate characteristics and risk during the field period of flue-cured tobacco in Wenshan. J. Meteorol. Res. Appl. 39, 46–50.
Floc'h, J. B., Hamel, C., Harker, K. N., and St-Arnaud, M. (2020). Fungal communities of the canola rhizosphere: keystone species and substantial between-year variation of the rhizosphere microbiome. Microb. Ecol. 80, 762–777. doi: 10.1007/s00248-019-01475-8
Fujisao, K., Khanthavong, P., Oudthachit, S., Matsumoto, N., Homma, K., Asai, H., et al. (2020). Impacts of the continuous maize cultivation on soil properties in Sainyabuli province, Laos. Sci Rep. 10:11231. doi: 10.1038/s41598-020-67830-9
Gao, Z., Hu, Y., Han, M., Xu, J., Wang, X., Liu, L., et al. (2021). Effects of continuous cropping of sweet potatoes on the bacterial community structure in rhizospheric soil. BMC Microbiol. 21:102. doi: 10.1186/s12866-021-02120-6
General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China, Standardization Administration (2008). Classification of tobacco pests and diseases and methods of investigation. In GB/T 23222–22008. (Beijing: Standardization Administration).
Gorte, O., Kugel, M., and Ochsenreither, K. (2020). Optimization of carbon source efficiency for lipid production with the oleaginous yeast Saitozyma podzolica DSM 27192 applying automated continuous feeding. Biotechnol. Biofuels 13:181. doi: 10.1186/s13068-020-01824-7
Guo, J. W., Mohamad, O. A. A., Wang, X. L., Egamberdieva, D., and Tian, B. Y. (2024). Editorial: microbiome associated with plant pathogens, pathogenesis, and their applications in developing sustainable agriculture. Front. Microbiol. 15:1423961. doi: 10.3389/fmicb.2024.1423961
Jin, L., Jin, N., Wang, S., Li, J., Meng, X., Xie, Y., et al. (2022). Changes in the microbial structure of the root soil and the yield of Chinese baby cabbage by chemical fertilizer reduction with bio-organic fertilizer application. Microbiol. Spectr. 10:e0121522. doi: 10.1128/spectrum.01215-22
Jin, X., Wang, J., Li, D., Wu, F., and Zhou, X. (2019). Rotations with Indian mustard and wild rocket suppressed cucumber fusarium wilt disease and changed rhizosphere bacterial communities. Microorganisms 7:57. doi: 10.3390/microorganisms7020057
Khalil, M. I. I., Youssef, S. A., Tartoura, K. A., and Eldesoky, A. A. (2021). Comparative evaluation of physiological and biochemical alteration in tomato plants infected by Alternaria alternata n response to Trichoderma viride and Chaetomium globosum application. Physiol. Mol. Plant Pathol. 115:101671. doi: 10.1016/j.pmpp.2021.101671
Kracmarova, M., Uhlik, O., Strejcek, M., Szakova, J., Cerny, J., Balik, J., et al. (2022). Soil microbial communities following 20 years of fertilization and crop rotation practices in the Czech Republic. Environ. Microbiome. 17:13. doi: 10.1186/s40793-022-00406-4
Lan, W. S., Lu, T. K., Qin, Z. F., Shi, X. J., Wang, J. J., Hu, Y. F., et al. (2014). Genetically modified microorganism Sphingomonas paucimobilis UT26 for simultaneously degradation of methyl-parathion and γ-hexachlorocyclohexane. Ecotoxicology 23, 840–850. doi: 10.1007/s10646-014-1224-8
Lee, S. M., Kong, H. G., Song, G. C., and Ryu, C. M. (2021). Disruption of Firmicutes and Actinobacteria abundance in tomato rhizosphere causes the incidence of bacterial wilt disease. ISME J. 15, 330–347. doi: 10.1038/s41396-020-00785-x
Li, X., and Lu, S. (2019). Regionalization of stony desertification in Yunnan Province. J. Southwest for. Univ. 39, 1–10. doi: 10.11929/j.swfu.201810220
Li, L., Xia, T., and Yang, H. (2022). Seasonal patterns of rhizosphere microorganisms suggest carbohydrate-degrading and nitrogen-fixing microbes contribute to the attribute of full-year shooting in woody bamboo Cephalostachyum pingbianense. Front. Microbiol. 13:1033293. doi: 10.3389/fmicb.2022.1033293
Liu, H., Li, J., Carvalhais, L. C., Percy, C. D., Prakash Verma, J., Schenk, P. M., et al. (2021a). Evidence for the plant recruitment of beneficial microbes to suppress soil-borne pathogens. New Phytol. 229, 2873–2885. doi: 10.1111/nph.17057
Liu, Q., Wang, S., Li, K., Qiao, J., Guo, Y., Liu, Z., et al. (2021b). Responses of soil bacterial and fungal communities to the long-term monoculture of grapevine. Appl. Microbiol. Biotechnol. 105, 7035–7050. doi: 10.1007/s00253-021-11542-1
Liu, S., Wang, Z. Y., Niu, J. F., Dang, K. K., Zhang, S. K., Wang, S. Q., et al. (2021c). Changes in physicochemical properties, enzymatic activities, and the microbial community of soil significantly influence the continuous cropping of Panax quinquefolius L. (American ginseng). Plant Soil 463, 427–446. doi: 10.1007/s11104-021-04911-2
Liu, J. L., Yao, J., Wang, F., Min, N., Gu, J. H., Li, Z. F., et al. (2019). Bacterial diversity in typical abandoned multi-contaminated nonferrous metal(loid) tailings during natural attenuation. Environ. Pollut. 247, 98–107. doi: 10.1016/j.envpol.2018.12.045
Liu, Z. Y., Zhang, W. J., He, C. G., He, R., Wei, S. L., Guo, Y., et al. (2022). Disease occurrence and environment-friendly disease control with crop–livestock integration in seed-corn production in the Hexi corridor. Pratacultural Sci. 39, 1441–1451. doi: 10.11829/j.issn.1001-0629.2022-02736
Machado, S., Petrie, S., Rhinhart, K., and Qu, A. (2007). Long-term continuous cropping in the Pacific northwest: tillage and fertilizer effects on winter wheat, spring wheat, and spring barley production. Soil Tillage Res. 94, 473–481. doi: 10.1016/j.still.2006.09.007
Mares-Ponce de León, Y., Muñoz-Castellanos, L. N., Ruiz-Cisneros, M. F., Pérez-Corral, D. A., Ornelas-Paz, J. D. J., Acosta-Muñiz, C. H., et al. (2018). Identificación morfológica y molecular de especies de Mortierella asociados a rizosfera de manzanos con síntomas de enfermedades radiculares. Rev. Mexicana Fitopatol. 36, 184–195. doi: 10.18781/R.MEX.FIT.1710-2
Mbanyele, V., Mtambanengwe, F., Nezomba, H., Rurinda, J., and Mapfumo, P. (2022). Conservation agriculture in semi-arid Zimbabwe: a promising practice to improve finger millet (Eleusine coracana Gaertn.) productivity and soil water availability in the short term. Agriculture 12:622. doi: 10.3390/agriculture12050622
McDaniel, M. D., Grandy, A. S., Tiemann, L. K., and Weintraub, M. N. (2014). Crop rotation complexity regulates the decomposition of high and low quality residues. Soil Biol. Biochem. 78, 243–254. doi: 10.1016/j.soilbio.2014.07.027
Niu, Y., Bainard, L. D., May, W. E., Hossain, Z., Hamel, C., and Gan, Y. (2018). Intensified pulse rotations buildup pea rhizosphere pathogens in cereal and pulse based cropping systems. Front. Microbiol. 9:1909. doi: 10.3389/fmicb.2018.01909
Niu, J., Rang, Z., Zhang, C., Chen, W., Tian, F., Yin, H., et al. (2016). The succession pattern of soil microbial communities and its relationship with tobacco bacterial wilt. BMC Microbiol. 16:233. doi: 10.1186/s12866-016-0845-x
Schlatter, D., Kinkel, L., Thomashow, L., Weller, D., and Paulitz, T. (2017). Disease suppressive soils: new insights from the soil microbiome. Phytopathology 107, 1284–1297. doi: 10.1094/PHYTO-03-17-0111-RVW
Town, J. R., Dumonceaux, T., Tidemann, B., and Helgason, B. L. (2023). Crop rotation significantly influences the composition of soil, rhizosphere, and root microbiota in canola (Brassica napus L.). Environ. Microbiome. 18:40. doi: 10.1186/s40793-023-00495-9
Town, J. R., Gregorich, E. G., Drury, C. F., Lemke, R., Phillips, L. A., and Helgason, B. L. (2022). Diverse crop rotations influence the bacterial and fungal communities in root, rhizosphere and soil and impact soil microbial processes. Appl. Soil Ecol. 169:104241. doi: 10.1016/j.apsoil.2021.104241
Turmuktini, T., Kantikowati, E., Natalie, B., Setiawati, M., Yuwariah, Y., Joy, B., et al. (2012). Restoring the health of paddy soil by using straw compost and biofertilizers to increase fertilizer efficiency and rice production with sobari (system of organic based aerobic rice intensification) technology. Asian J. Agric. Rural Dev. 2, 519–526.
van der Putten, W. H., Bardgett, R. D., Bever, J. D., Bezemer, T. M., Casper, B. B., Fukami, T., et al. (2013). Plant-soil feedbacks: the past, the present and future challenges. J. Ecol. 101, 265–276. doi: 10.1111/1365-2745.12054
Vasconcellos, R. L. F., Romagnoli, E. M., Taketani, R. G., Santos, S. N., Zucchi, T. D., and Melo, I. S. (2021). Impact of inoculation with Pseudomonas aestus CMAA 1215T on the non-target resident bacterial community in a saline rhizosphere soil. Curr. Microbiol. 78, 218–228. doi: 10.1007/s00284-020-02285-9
Wani, Z. A., Kumar, A., Sultan, P., Bindu, K., Riyaz-Ul-Hassan, S., and Ashraf, N. (2017). Mortierella alpina CS10E4, an oleaginous fungal endophyte of Crocus sativus L. enhances apocarotenoid biosynthesis and stress tolerance in the host plant. Sci. Rep. 7:8598. doi: 10.1038/s41598-017-08974-z
Weller, D. M., Raaijmakers, J. M., Gardener, B. B., and Thomashow, L. S. (2002). Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu. Rev. Phytopathol. 40, 309–348. doi: 10.1146/annurev.phyto.40.030402.110010
Xie, Y., Ouyang, Y., Han, S., Se, J., Tang, S., Yang, Y., et al. (2022). Crop rotation stage has a greater effect than fertilisation on soil microbiome assembly and enzymatic stoichiometry. Sci. Total Environ. 815:152956. doi: 10.1016/j.scitotenv.2022.152956
Yan, H., Wu, S., Li, P., Jin, X., Shi, D., Tu, D., et al. (2024). Tobacco crop rotation enhances the stability and complexity of microbial networks. Front. Microbiol. 15:1416256. doi: 10.3389/fmicb.2024.1416256
Zeng, T., Sha, H., Xie, Q., Lu, Y., Nong, H., Wang, L., et al. (2024). Comprehensive assessment of the microbial community structure in a typical lead-zinc mine soil. Environ. Sci. Pollut. Res. Int. doi: 10.1007/s11356-024-33377-9
Zhang, M. M., Liang, G. Y., Ren, S., Li, L. P., Li, C., Li, Y. J., et al. (2023). Responses of soil microbial community structure, potential ecological functions, and soil physicochemical properties to different cultivation patterns in cucumber. Geoderma 429:116237. doi: 10.1016/j.geoderma.2022.116237
Zhang, X. M., Liu, W., Zhang, G. M., Jiang, L., and Han, X. G. (2015). Mechanisms of soil acidification reducing bacterial diversity. Soil Biol. Biochem. 81, 275–281. doi: 10.1016/j.soilbio.2014.11.004
Zhang, H. F., Luo, G. W., Wang, Y. Z., Fei, J. C., Rong, X. M., Peng, J. W., et al. (2023). Crop rotation-driven change in physicochemical properties regulates microbial diversity, dominant components, and community complexity in paddy soils. Agric. Ecosyst. Environ. 343:108278. doi: 10.1016/j.agee.2022.108278
Zhang, J., Xing, J., Peng, L., Wu, Q., Chen, J., Xu, Q., et al. (2023). Arbuscular mycorrhizal fungi improves diversity and stability of bacterial community and abundance of beneficial bacteria genus in the rhizosphere of tomato infected with Ralstonia solanacearum. J. Plant Nutr. Fert. 29, 120–131. doi: 10.11674/zwyf.2022260
Zhao, Y. N., Mao, X. X., Zhang, M. S., Yang, W., Di, H. J., Ma, L., et al. (2020). Response of soil microbial communities to continuously mono-cropped cucumber under greenhouse conditions in a calcareous soil of North China. J. Soil Sediment. 20, 2446–2459. doi: 10.1007/s11368-020-02603-5
Zheng, J., Zhang, J., Gao, L., Kong, F., Shen, G., Wang, R., et al. (2020). The effects of tetracycline residues on the microbial community structure of tobacco soil in pot experiment. Sci. Rep. 10:8804. doi: 10.1038/s41598-020-65203-w
Zhou, X. G., Liu, J., and Wu, F. Z. (2017). Soil microbial communities in cucumber monoculture and rotation systems and their feedback effects on cucumber seedling growth. Plant Soil 415, 507–520. doi: 10.1007/s11104-017-3181-5
Keywords: crop rotation, soil microbial communities, karst agricultural system, soil physicochemical properties, microbiome assembly
Citation: Wang B, Shang N, Feng X, Hu Z, Li P, Chen Y, Hu B, Ding M and Xu J (2025) Understanding the microbiome–crop rotation nexus in karst agricultural systems: insights from Southwestern China. Front. Microbiol. 16:1503636. doi: 10.3389/fmicb.2025.1503636
Received: 29 September 2024; Accepted: 05 February 2025;
Published: 26 February 2025.
Edited by:
Yaping Lin, Minzu University of China, ChinaReviewed by:
Jian-Wei Guo, Kunming University, ChinaCopyright © 2025 Wang, Shang, Feng, Hu, Li, Chen, Hu, Ding and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mengjiao Ding, OTUyMTU2OTI5QHFxLmNvbQ==; Junju Xu, anVuanV4dTAwN0AxMjYuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.