- 1Université de Lorraine, INRAE, UMR Interactions Arbre/Micro-organismes, Centre INRAE Grand-Est Nancy, Champenoux, France
- 2The National Key Laboratory of Ecological Security and Sustainable Development in the Arid Region, Northwest Institute of Eco-Environment and Resources, Chinese Academy of Sciences, Lanzhou, China
The ascomycete Cenococcum geophilum is a cosmopolitan and ecologically significant ectomycorrhizal (ECM) fungus that forms symbiotic associations with diverse host plants worldwide. As the only known ECM species within the large class Dothideomycetes, C. geophilum exhibits several characteristics that distinguish it from other ECM fungi. This fungus significantly contributes to ecosystem stability and development as an early colonizer of primary forest succession. The capacity of this symbiont to rapidly colonize disturbed or newly formed environments promotes the development of conditions that support the growth of other plant species, thus playing a crucial role in the ecological progression and restoration of ecosystems. Several C. geophilum isolates are known to enhance the drought resistance of host plants, a trait that is becoming increasingly important in the context of climate change and frequent drought events. In this review, we examined genetic studies that have assessed the phylogenetic structure of C. geophilum populations and identified the genes associated with adaptation to environmental stress and symbiosis. The high genetic diversity of C. geophilum is particularly noteworthy, considering its putative asexual reproductive mode. Population genomic analyses have suggested that C. geophilum is not a single species but rather a species complex comprising multiple cryptic lineages. This genetic variability may contribute to its adaptability and extensive distribution across habitats from circumpolar to tropical biomes. These lineages exhibit potential host preferences, suggesting a degree of specialization within the complex. The nuclear genome of C. geophilum has been sequenced, providing valuable insights into the symbiont genetic traits. Notably, this genome encodes a large set of repeated sequences and effector-like small secreted proteins. Transcriptomics has been used to identify candidate genes related to symbiosis and adaptation to environmental stress. Additionally, we briefly discuss how C. geophilum offers potential for sustainable forestry practices by improving resilience to stress.
1 Introduction
A majority of land plants establish symbiotic relationships with mycorrhizal fungi, which play a critical role in terrestrial ecosystems by regulating nutrient and carbon cycles, influencing soil structure, and contributing to ecosystem multifunctionality (Martin and Van Der Heijden, 2024). Approximately 80% of plant N and P are provided by these mutualistic fungi, and the majority of plant species depend on them for growth and survival. An estimated 20,000 fungal species, primarily belonging to the phyla Basidiomycota and Ascomycota, establish ectomycorrhizal (ECM) associations with approximately 6,000 plant species, mostly trees and shrubs (Van Der Heijden et al., 2015). ECM fungi are present in a diverse range of terrestrial ecosystems and are responsible for colonizing 60% of the trees in temperate and boreal forest ecosystems (Baldrian et al., 2023). These tree species, belonging to the Pinaceae, Fagaceae, Betulaceae, Nothofagaceae, Myrtaceae, or Dipterocarpaceae families, play crucial ecological and economic roles in both the northern and southern hemispheres.
During symbiosis development, ECM fungi differentiate the hyphal mantle, ensheathing the rootlets and an intraradical mycelial network, the so-called Hartig net, which penetrates host roots. In numerous ECM associations, an extraradical mycelium permeating the soil environment extends from ECM roots. Mycelial networks facilitate the acquisition of water and nutrients by plants and enhance their resistance to environmental stressors. ECM symbionts secrete extracellular enzymes that degrade soil organic matter (SOM) to facilitate nitrogen acquisition in their hosts (Nicolas et al., 2019). ECM fungi from different independently evolved lineages exhibit varying capacities to degrade SOM and transfer N to their hosts (Nicolas et al., 2019). In boreal and temperate forests, ECM fungi provide 70% of N flux to their hosts (Smith and Read, 2010). Consequently, ECM plays a crucial role in C and N cycles in forest soils. In exchange for soil minerals, 10–20% of photoassimilates are allocated to fungal partners by the host plant. Plant communities allocate 9.07 Gt of atmospheric CO2 per year to their mycorrhizal symbionts (Hawkins et al., 2023).
The ascomycetous fungus Cenococcum geophilum, previously known as C. graniforme, is a cosmopolitan ECM fungus and one of the most prevalent mutualistic species found in soil fungal communities worldwide (LoBuglio, 1999; Figure 1). It forms mycorrhizal associations with over 200 trees, shrubs, and herbaceous species in boreal, temperate, and subtropical forests as well as in savannas and alpine meadows. As the only known ECM member of the class Dothideomycetes, C. geophilum exhibits several distinctive characteristics that distinguish it from other ECM fungi (LoBuglio, 1999; Obase et al., 2017). As an early colonizer of primary forest succession, C. geophilum contributes significantly to ecosystem stability and development. It is particularly important in nutrient cycling because it facilitates the transfer of nutrients, especially nitrogen (N) and phosphorus (P), from the soil to its host plants (LoBuglio, 1999). Additionally, C. geophilum enhances the drought resistance of its host plants (Coleman et al., 1989), a trait that is becoming increasingly important in the context of a warming world with an increased occurrence of drought events (Zheng et al., 2023). C. geophilum is therefore a compelling model system for research on fungal ecology, evolution, and mycorrhizal symbiosis.
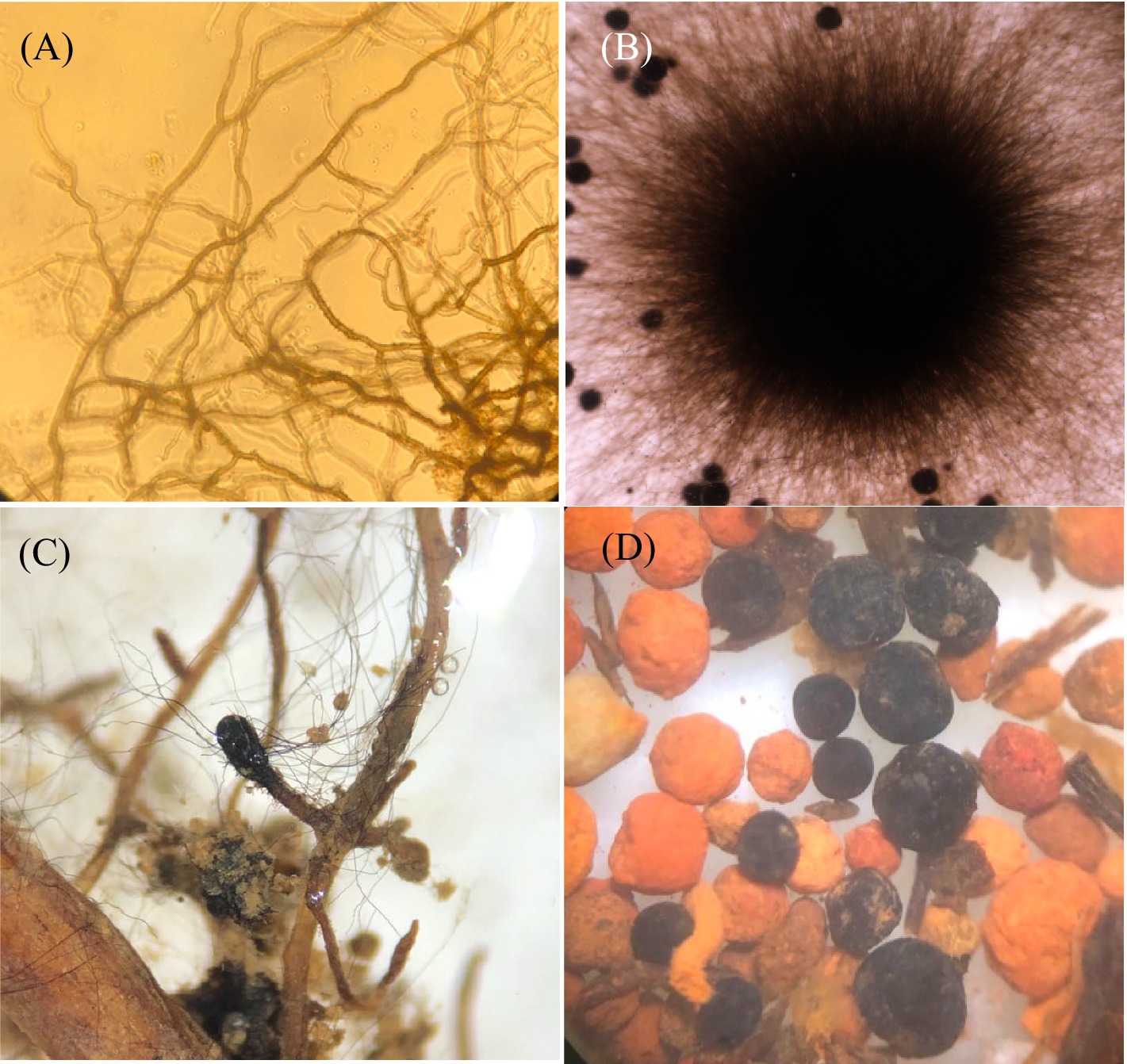
Figure 1. The ascomycete Cenococcum geophilum: (A) melanized mycelial hyphae, (B) vegetative colony of free-living mycelium, (C) an ectomycorrhizal root tip of the Fagus sylvatica-Cenocccum geophilum association with extraradical hyphae, and (D) melanized sclerotia.
The biological and ecological attributes of C. geophilum have been documented extensively (LoBuglio, 1999; Obase et al., 2017). Recently, genomics has emerged as a crucial tool for investigating the biology, evolution, and ecology of mutualistic symbionts including C. geophilum (Kohler et al., 2015; Peter et al., 2016; Miyauchi et al., 2020; Lebreton et al., 2021). This approach not only provides essential mechanistic insights but also identifies key genetic traits, such as adaptation to drought stress (Li et al., 2023; Zhang et al., 2024), which can be prioritized to select strains for the application of this mycorrhizal symbiont in forestry. This review provides a brief account of the biological and ecological attributes of C. geophilum, followed by a discussion of recent studies that have demonstrated the impact of genomics and related techniques (i.e., DNA metabarcoding, population genomics, and transcriptomics) on our understanding of this enigmatic mycorrhizal fungus. Additionally, it briefly explores the potential applications of C. geophilum in sustainable forestry and ecosystem restoration, highlighting the significance of understanding the functional traits and ecological roles of these ECM fungi in adapting to environmental changes. By consolidating the latest research findings, this review aims to identify knowledge gaps and suggest future research directions for this ubiquitous symbiont to address the global challenges in forestry and environmental sustainability.
2 Morphological features and life cycle
The black fungus C. geophilum is distinguished by its septate dematiaceous hyphae, which contain high concentrations of melanin in their cell walls (Figure 1; Fernandez and Koide, 2013). This pigmentation enables mycelia to endure various environmental challenges including UV exposure, dehydration, high temperatures, enzymatic breakdown, antimicrobial agents, and heavy metal exposure (Pal et al., 2013). The resilience of C. geophilum enables it to thrive in challenging environments for several years, where other mycorrhizal fungi may find it difficult to survive (McCormack et al., 2017). Its hyphae show various shapes according to the growth medium and the age of the mycelial colony (Trappe, 1962). Chlamydospore-like structures have been observed in both solid and liquid media (Massicotte et al., 1992). These chlamydospore-like structures are always intercalary and rarely terminal in the mycelia (Mikola, 1948). This structure also exists in the taxonomically related species Glonium spp. (Amano, 1983) and Pseudocenococcum floridanum (Obase et al., 2016).
Cenococcum geophilum can differentiate sclerotium (Figure 1), which is a compact mass of hardened fungal mycelium containing nutrient reserves, including carbohydrates and lipids. The sclerotia constitute an underestimated source of polysaccharides in forest soils, accounting for 3.6% of the total carbohydrates in subalpine forest soils (Murayama and Sugiura, 2021). These melanized sclerotia resist decomposition by soil microorganisms (Fernandez and Koide, 2014) and remain viable for up to 40 years under extreme environmental conditions (Nyamsanjaa et al., 2022). They host specific fungal and bacterial communities (Obase et al., 2014; Narisawa et al., 2021).
Although molecular evidence, such as recombination and diploidy (see below), suggests the presence of unknown sexual stages in the life cycle of C. geophilum, no sexual structures have been observed under laboratory or field conditions (Bourne et al., 2014).
The only ECM fossil related to C. geophilum is Eomelanomyces cenococcoides gen. Spec. nov., discovered in a 52-million-year-old amber specimen from a lignite mine in Gujarat State, India (Beimforde et al., 2011). This amber was produced by representatives of Dipterocarpaceae trees in the early tropical broadleaf forests. The fossil is similar to the extant Cenococcum; however, it is distinguished by high variability in the branching of ECM rootlets and by the regular formation of microsclerotia and chlamydospore-like structures (Beimforde et al., 2011).
3 Ecologically important ectomycorrhizal symbiont
The identification of C. geophilum relies on a combination of morphological and molecular techniques, as it shares soil habitats and many physical characteristics with dark septate root endophyte (DSE) fungi such as Piceirhiza bicolorata and Cadophora finlandia (Rosling et al., 2003). DNA metabarcoding surveys have shown that this ECM fungus is a major component of the soil fungal communities in most of the forest ecosystems (Figure 2). It is considered a keystone species essential for maintaining the microbial network structure and stability (Zhu et al., 2024). As an early colonizer in primary successions, C. geophilum significantly contributes to ecosystem stability and development (LoBuglio, 1999). Its rapid establishment in disturbed or newly formed habitats creates favorable conditions for other plant species, thus playing a vital role in ecological succession and ecosystem recovery. Additionally, C. geophilum can collaborate with other bacteria to establish ECM associations under varying climatic conditions. Reis et al. (2021) examined beneficial symbiotic microorganisms, including ECM fungi and mycorrhiza helper bacteria in cork oak (Quercus suber L.) forests. C. geophilum and Bacillus sp. were among the most prevalent interacting microbes. Furthermore, Kataoka et al. (2009) reported that B. subtilis can enhance C. geophilum growth during symbiosis establishment. This mutual support benefits all three partners and could play a crucial role in forest resilience to future climate change.
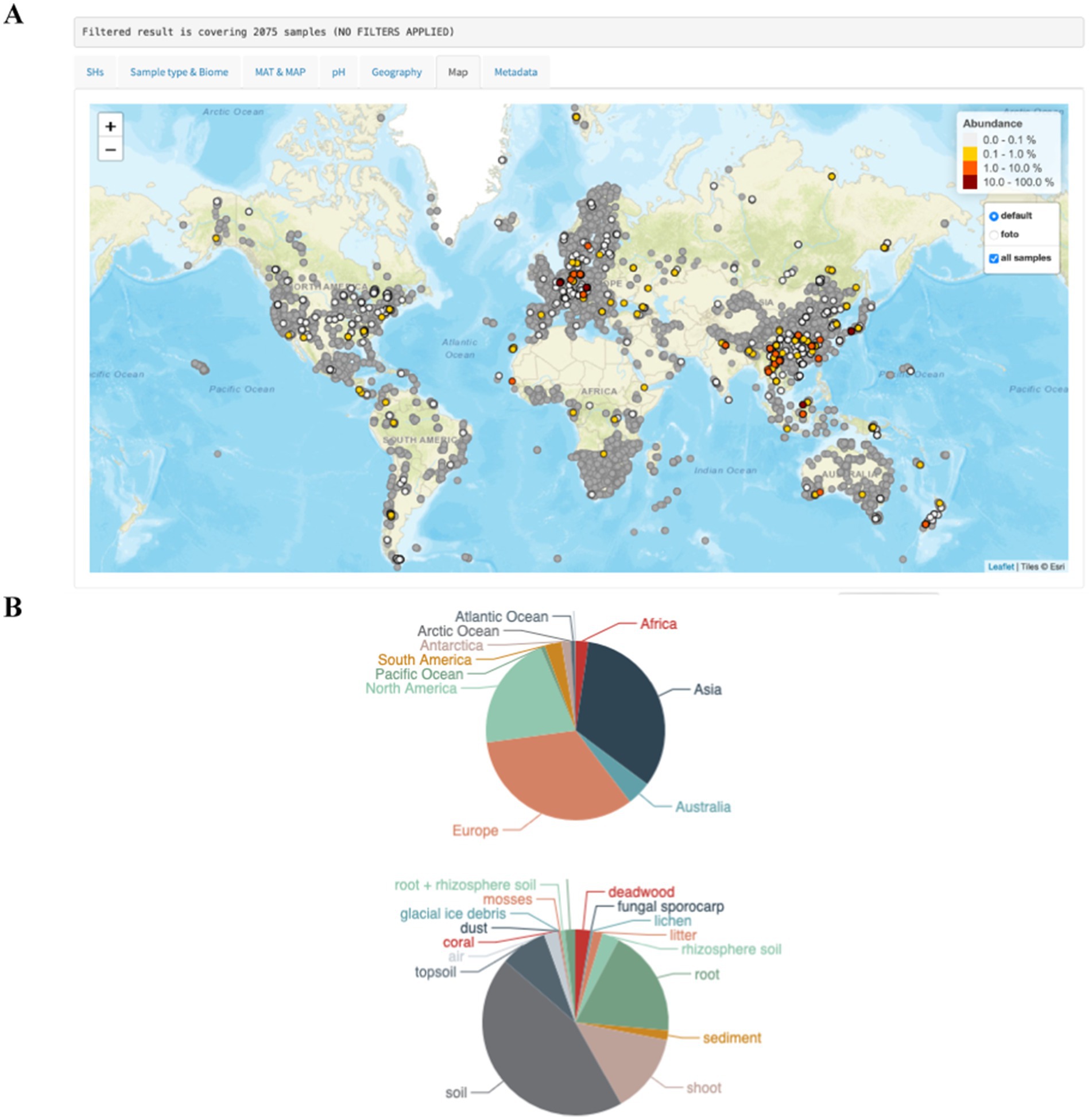
Figure 2. Assessment of the worldwide abundance and distribution of Cenococcum geophilum (A) and its distribution (% per site) among the biomes and continents (B) using the GlobalFungi database (https://globalfungi.com) (Větrovský et al., 2020).
4 The pan-global ectomycorrhizal symbiont
Cenococcum geophilum is a cosmopolitan ECM fungus and one of the most prevalent species found in soil fungal communities worldwide (Figure 2). It forms ECM or ectendomycorrhizal associations with over 200 trees, shrubs, and herbaceous species in boreal, temperate, and subtropical forests as well as in savannas. In alpine and circumpolar biomes, C. geophilum establishes ectendomycorrhizas or ECM with herbaceous plants such as sedges or shrubs (Obase et al., 2017). Its abundance in tropical ecosystems is relatively low (Tedersoo et al., 2010; Bakray et al., 2024), although high root colonization and genetic diversity have been reported in the dry deciduous forests of Thailand (Phosri et al., 2012). However, C. geophilum ECM is seldom found in African or South American tropical forests (Becerra and Zak, 2011; Bâ et al., 2012). It has also been found at the edges of deserts (Massicotte et al., 1992) and in sandy forests of Picea mongolica (Bao, 2005).
Zheng et al. (2023) employed the MaxEnt model (Phillips et al., 2017) to analyze the historical shifts in geographical distribution patterns of C. geophilum since the Last Glacial Maximum and forecast its future spread under changing climatic conditions. They showed that this geographical distribution is closely associated with climatic factors, particularly temperature and precipitation. Temperature has greater relative importance than precipitation. This is also true for majority of the ECM fungi (Bennett and Classen, 2020). C. geophilum occupied a significantly smaller area during the Last Glacial Maximum and mid-Holocene, primarily concentrated in China’s Qinling–Huaihe Line region and eastern Peninsular Malaysia. As global temperatures continue to rise, the model predicts a northward shift toward a suitable habitat for C. geophilum, resulting in an anticipated increase in suitable areas from 9 to 21%.
Cenococcum geophilum ECM rootlets are abundant in the top 0–5 cm soil layer (Rosling et al., 2003; Genney et al., 2006; Scattolin et al., 2008) but can also be found in much deeper soil layers, such as the mineral layer at a depth of 20 cm (Genney et al., 2006). C. geophilum is a pioneer species because of its propensity to partner with pioneer host trees such as Salix spp., which colonize newly exposed glacier moraines (Trappe, 1988). Moreover, the symbiont is recognized as a “multi-stage” fungus in secondary forest successions, indicating that it forms ECM associations with both seedlings and adult host plants (Visser, 1995; Danielson, 1991). In the volcanic desert of Mount Fuji, C. geophilum is present in both the early and later stages of vegetation development, colonizing young and old pioneer shrubs, such as Salix reinii, and herbaceous species, such as Polygonum cuspidatum (Nara, 2006). Interestingly, the symbiont has been found in old growth forests (Peter, 2003), although it is known to primarily colonize young trees in alpine regions near treelines (Hasselquist et al., 2005). In particular, C. geophilum is associated with seedlings and juvenile trees of Picea engelmannii and Abies lasiocarpa, with colonization rates 20 times greater for juveniles than for seedlings (Hasselquist et al., 2005). These findings suggest that this fungus plays an important role in the early stages of forest succession. However, C. geophilum has not been replaced by late-stage ECM species in older forest stands. The high prevalence of C. geophilum in mature alpine forest ecosystems, which are known for their cold climate, slow litter breakdown, and organic matter buildup in the soil, is believed to be a consequence of the substantial presence of sclerotia (approximately 3,600 kg ha−1) and synchronization of rootlet growth bursts with sclerotia germination in autumn (Vogt et al., 1982). Furthermore, C. geophilum is among the most frequent ECM symbionts following a short fire return interval (Buscardo et al., 2010).
Mineral weathering by C. geophilum can release potassium from potassium aluminosilicate minerals, such as feldspar, nepheline, biotite, muscovite, and illite (Xue et al., 2019). The symbiotic fungus can also break down the mycorrhizal necromass (Fernandez and Koide, 2014; Gray and Kernaghan, 2020), with the initial N and melanin levels strongly influencing the early decay rates and determining the remaining mass after several years.
5 Tolerance to N deposition, salt stress, and heavy metals
N addition reduced the prevalence of C. geophilum in the fungal communities of the humus and fine roots. Forsmark et al. (2024) analyzed the organic layer beneath undisturbed litter in a Norway spruce (Picea abies) forest in northern Sweden after two decades of annual N application at low (12.5 kg N ha−1 yr.−1) and high (50 kg N ha−1 yr − 1) levels. N supplementation decreased C. geophilum abundance, suggesting that decomposition linked to organic N acquisition was suppressed when inorganic forms of N were readily accessible. These community changes were associated with a decreased activity of Mn-peroxidase and peptidase and an increase in the activity of C-acquiring enzymes.
Wen et al. (2022) studied the influence of C. geophilum inoculation on the growth and nutrient uptake of Pinus thunbergii seedlings under salt stress. Their results indicated that mycorrhizal inoculation significantly increased seedling biomass, chlorophyll, and nutrient elements (such as P, N, and K) in shoots and maintained a low Na/K ratio in roots under salt stress, suggesting that inoculation with C. geophilum could assist the host in overcoming salt stress. Geographical isolates of C. geophilum have shown patterns of local adaptation to serpentine soils, with Ni concentrations having a significant effect on fitness-related traits (Gonçalves et al., 2009; Bazzicalupo et al., 2020).
6 Adaptation to water-stressed environments
C. geophilum exhibits drought tolerance and is prevalent in water-stressed environments (Pigott, 1982; McCormack et al., 2017). Several surveys of soil fungal communities have demonstrated that the proportions of C. geophilum ECM and extramatrical mycelia increase under water stress conditions and are often higher during summer in natural settings (Pigott, 1982; Querejeta et al., 2009). This tolerance has been verified through in vitro mycelial culture experiments using osmotically adjusted media (Mexal and Reid, 1973; Coleman et al., 1989), cell damage tests following desiccation (Di Pietro et al., 2007), and respiration measurements under water stress (Jany et al., 2003). The level of tolerance varies among geographical isolates (Coleman et al., 1989; Jany et al., 2003). However, the physiological mechanisms responsible for this symbiont’s success under water stress remain largely unknown. Multiple factors likely contribute to this trait, such as the accumulation of compatible osmolytes (e.g., polyols), heat shock proteins, hydrophobic proteins, and melanin in the cell walls. It has also been suggested that drought resistance in C. geophilum may be associated with the increased expression of aquaporin water channels (see below, Peter et al., 2016). Although C. geophilum is widely recognized as a drought-tolerant symbiont, this contention has recently been debated. A study utilizing Pinus seedlings colonized by C. geophilum and subjected to water shortages showed that the drought resistance of mycorrhizal plantlets was not directly correlated with that of C. geophilum isolates cultivated in liquid medium (Zhang et al., 2024). Xie et al. (2024) used inoculated Quercus mongolica and Tilia amurensis to investigate the responses of ECM fungal communities and their exploration types under drought conditions in a pot system. The relative abundance of C. geophilum in both hosts decreased. Nickel et al. (2018) examined ECM fungal community diversity changes in European beech and Norway spruce forests under drought conditions by utilizing retractable roofs to exclude rain for 3 years. The results indicated that the abundance of C. geophilum decreased irrespective of the depth, year, or host.
7 Heat and cold stresses
Laboratory experiments demonstrated that the growth inhibition of several C. geophilum isolates occurred at a temperature of 26°C (Yan et al., 2022); however, this species is capable of forming mycorrhizal associations following exposure to heat stress at approximately 70°C for a brief period or at 5°C above ambient temperature. Nevertheless, combined stress, including drought and heat stress, at a temperature of 5°C above ambient temperature and 50% precipitation can be lethal to C. geophilum (Kipfer et al., 2010; Gehring et al., 2020). Herzog et al. (2013) reported that increased temperature and water shortage can differentially affect the relative ECM abundance and exoenzyme activities of C. geophilum associated with various oak species, specifically Q. robur, Q. petraea, and Q. pubescens.
Furthermore, because of their prevalence as symbionts in arctic and alpine ecosystems, C. geophilum mycelia and ECM are likely to exhibit high tolerance to cold stress. Corbery and Le Tacon (1997) demonstrated that C. geophilum mycelium remained viable even when exposed to a freezing temperature of −80°C for a brief period, exhibiting greater resistance to cold than other ECM fungi. Additionally, studies have indicated that this fungus thrives at temperatures below 1°C (Vogt et al., 1982). This cold stress resistance may be attributed to its high mannitol synthesis rate (Martin et al., 1985) because mannitol is known to shield fungi from severe cryoenvironments (Weinstein et al., 1997).
8 Host preferences
C. geophilum is recognized as a mycorrhizal generalist species. This symbiont can form ecto-or ectendomycorrhizal associations with a broad host range. Based on the morphology and anatomy of mycorrhizal roots sampled in natural settings, three groups of host plants were identified (Trappe, 1962; LoBuglio, 1999): In Group 1, the hosts include members of the Salicaceae and Betulaceae families (excluding Corylus spp.), as well as ectotrophic genera within the Rosaceae family. The ECM root tips are typically monopodial or occasionally branched, with the mantle covering only the root tips. The Hartig net in these hosts never extends deeper than the third layer of the cortical cells, and intracellular penetration is sparse and limited to occasional cells. In Group 2, C. geophilum associates with Pinus species. The ECM root tips are monopodial, dichotomous, or occasionally irregularly branched. The mantles typically cover all short roots and have thicknesses ranging between 8 and 60 μm. The Hartig net extends inward to the innermost layer of cortical cells, and the cortex experiences strong intracellular infection. The hosts in Group 3 predominantly comprise Fagaceae, including Corylus, and Pinaceae, with the exception of Pinus spp. The root tips of these associations exhibit a range of morphologies, including monopodial, racemose, irregularly branched, long, or short structures. The mantle typically covers a significant portion or all of the short roots, and its thickness ranges from to 8 to 60 μm. The Hartig net extends to the innermost layer of the cortical cells, and intracellular infection is prevalent throughout the cortex.
Additionally, this groups includes many shrubs and herbaceous plants such as Pedicularis capitata (Kohn and Stasovski, 1990), Cistus spp. (Massicotte et al., 2010), Bistorta vivipara (Massicotte et al., 1998), Carex myosuroides (Massicotte et al., 1998), and Rhododendron spp. (Largent et al., 1980; Vohník et al., 2007). Unusual for an ECM symbiont, C. geophilum can also establish ectendomycorrhizal associations with shrubs and herbaceous plants, sharing mycelial networks with woody plants such as oak and Helianthemum bicknellii (Dickie and Reich, 2005), or the Dryas octopetala–Bistorta vivipara–Salix herbacea association (Mühlmann and Peintner, 2008). Symbiosis with herbaceous plants appears to enhance the colonization of woody plants (Dickie and Reich, 2005; Hoeksema et al., 2018). Although there is no evidence of nutrient transfer between herbaceous and woody plants sharing common mycorrhizal networks (CMNs) with C. geophilum, this structure could possibly act as a physical link between roots of herbaceous and woody plants, thereby enhancing C. geophilum colonization in sharing plants. The CMNs may also alter the bacterial communities of the hyphosphere (Vik et al., 2013).
Variations in colonization rates and/or host preferences can be attributed to genetic factors in both partners, as well as environmental factors such as soil organic matter content, total N, and available P (Wurentaoges, 2012). Zhu et al. (2024) reported that leaf photosynthesis and root morphological traits drive the topological structure of plant–fungus association networks involving Cenococcum species. Abundant plants may play a key role as reservoirs of symbiotic fungal diversity and thus contribute to the maintenance of ecosystem functions.
9 Population structure
As previously mentioned, C. geophilum is widespread and has historically posed challenges in terms of physiological and phylogenetic classification. Collections of C. geophilum isolates, both locally and globally, have shown remarkable genetic diversity. Genetic studies on C. geophilum have revealed a complex population structure, even at the soil core sample level, with evidence of both local adaptation and limited gene flow between populations (Jany et al., 2002; Douhan and Rizzo, 2005; Matsuda et al., 2015; Obase et al., 2016; Obase et al., 2017; Vélez et al., 2021). They uncovered the presence of multiple hidden clades and distinct phylogenetic groups within C. geophilum, supporting the widely held view that this species represents a highly diverse assemblage of ectomycorrhizal fungi at regional and global levels. The structure of symbiont populations is influenced by several factors, including geographic distance, environmental gradients, and host–plant associations. They are often structured according to environmental conditions such as soil type, moisture level, and temperature. For example, populations from dry nutrient-poor soils tend to be genetically distinct from those in more fertile environments, suggesting a local adaptation to specific ecological niches (Douhan and Rizzo, 2005; Lian et al., 2006). A subtle geographic structure with long-distance disjunction suggests complex alternation of sexual and asexual reproduction over space and time (Obase et al., 2016; Obase et al., 2017). However, gene flow between populations can occur through sclerotia dispersal, leading to a combination of local adaptation and genetic exchange.
The presence of cryptic species within C. geophilum has also been confirmed, with distinct genetic lineages corresponding to different ecological and geographical regions (Obase et al., 2017; Vélez et al., 2021). Obase et al. (2017) resolved seven clades with high bootstrap support among the isolates of Cenococcum derived from different geographical regions across the world using both single-and multi-locus and maximum likelihood (ML) analyses (Figure 3). All Cenococcum clades clustered together with high bootstrap support, whereas Pseudocenococcum floridanum isolates were resolved as a separate group. More recently, Vélez et al. (2021) examined a set of 200+ C. geophilum isolates obtained from the soil beneath Populus trichocarpa along an ~280 mile north–south corridor in the Pacific Northwest, United States. Additionally, they performed global phylogenetic analysis by incorporating 789 isolates with publicly accessible data from the United States, Japan, and Europe. This analysis identified 34 strongly supported clades using ML and Bayesian methods, with some clades exhibiting intra-and intercontinental distributions. These findings strongly indicate divergence within multiple cryptic species.
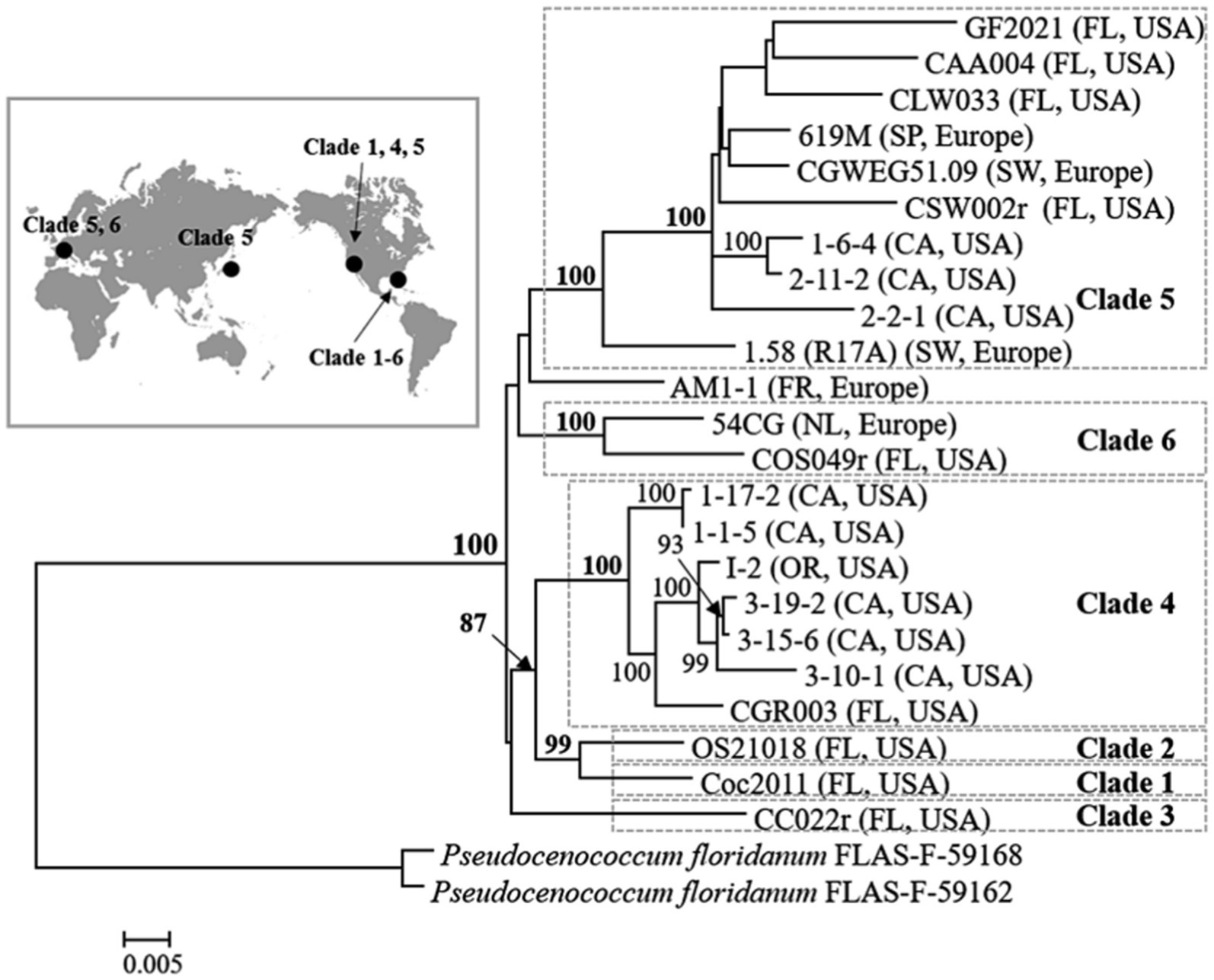
Figure 3. Phylogenetic tree of Cenococcum geophilum: major clades and their worldwide distribution (CA, California; FL, Florida; FR, France; NL, Netherlands; OR, Oregon; SP, Spain, SW, Switzerland). A maximum-likelihood phylogenetic tree was constructed using seven concatenated loci (ITS, SSU, LSU, TEF, RPB1, RPB2, and GAPDH). Isolates of Pseudocenococcum floridanum were used as outgroups (Obase et al., 2017).
Furthermore, the genetic diversity and structure of C. geophilum populations were analyzed based on the rDNA ITS2 sequences of 219 ECM root samples collected from 3 plant families (Betulaceae, Fagaceae, and Pinaceae) from 10 forest sites across China (Guo et al., 2021). Analysis of molecular variance (AMOVA) confirmed that genetic differentiation was evident within each geographical population and the population in each host plant family. The Fagaceae population was distinct from the Betulaceae and Pinaceae populations, and the haplotype composition was conspicuously different among the three plant families. These cryptic species may represent locally adapted forms of C. geophilum, which have evolved in response to specific environmental conditions. The genetic diversity of angiosperm-associated C. geophilum populations is higher than that of gymnosperm-associated populations, suggesting that angiosperm and gymnosperm hosts exert different selective pressures on their symbionts (Vélez et al., 2021). Tedersoo et al. (2024) also provided evidence for niche differentiation in tens of cryptic species of Cenococcum, many of which exhibit a preference toward particular partner plant genera.
Currently, it remains uncertain whether C. geophilum constitutes a single, highly diverse global species or whether it comprises numerous cryptic species. Subsequent studies could shed light on these local and worldwide relationships by comparing nuclear and mitochondrial genomes from a wide range of geographical isolates along with population genomics approaches.
10 Genomics, transcriptomics, and population genomics
10.1 Genomics
Within the framework of the Mycorrhizal Genome Initiative (Martin et al., 2011), the nuclear genome of C. geophilum (strain 1.58) has been sequenced and annotated by the U.S. Department of Energy Joint Genome Institute (Peter et al., 2016). This genome is the largest among the ECM fungi, with a mapped size of 178 Mbp and a total estimated size of 203 Mbp (Peter et al., 2016; Talhinhas et al., 2017). It is estimated to contain approximately 15,000 genes. In contrast, the genomes of the taxonomically related saprotrophic species Glonium stellatum and Lepidopterella palustris are approximately four times smaller than that of C. geophilum, at 41 and 46 Mbp, respectively, yet they possess similar gene counts to 14,362 and 13,870 predicted gene models, respectively. Phylogenomic analysis using single-copy conserved orthologs confirmed that C. geophilum belongs to the class Dothideomycetes, specifically in the order Mytilinidiales, and shares a close evolutionary relationship with the saprotrophic species G. stellatum and L. palustris. Despite its close taxonomic relationship with these saprotrophs, C. geophilum exhibits unique genomic features consistent with its ECM lifestyle. This is in agreement with the independent origin of ECM ability in Cenococcum within the class of otherwise saprobic Ascomycota (Dothideomycetes), with evidence that the most closely related sister group, Glonium, is likely saprobic and lacks mycorrhization ability. In their study, Obase et al. (2017) reported that Pseudocenococcum floridanum is a more closely related but distinct sister group to other Cenococcum lineages and that this new species likely lacks the ability to form ectomycorrhizas. Ongoing sequencing of several strains of P. floridanum at JGI1 will provide new insights into the evolution of the saprotrophy-to-symbiosis transition in Cenococcum clades.
The C. geophilum gene repertoire contains 2,176 species-specific genes, including effector-like small secreted proteins (SSPs). Many of these unique genes are involved in protein–protein interactions and signaling mechanisms, which are likely crucial for their symbiotic relationships with plants. Compared with its close relatives, the expanded genome of C. geophilum is attributed to its high proportion (81%) of repetitive sequences, primarily composed of transposable elements (TEs). Increased TE content is observed in numerous plant pathogenic fungi, particularly in those with (hemi-) biotrophic lifestyles. This trend is even more pronounced in symbiotic mycorrhizal fungi (Miyauchi et al., 2020; Lebreton et al., 2021). The majority of expanded gene families are associated with TEs or are involved in protein–protein interactions. These families exhibit domains typically observed in proteins related to self/non-self-recognition, which are associated with somatic incompatibility and defense mechanisms, such as HET, NACHT, and WD40 proteins. Notably, the expression of the majority of these gene families remains unregulated in functional mycorrhizas (Peter et al., 2016).
The repertoire of genes encoding Plant cell wall degrading enzymes (PCWDEs) is lower than that of the majority of saprotrophic and pathogenic Dothideomycetes but similar to that of saprotrophic and pathogenic Mycosphaerellales and Botryosphaeriales (Figure 4; Peter et al., 2016). This reduction is striking for enzymes that act on cellulose, hemicellulose, and pectin. Enzymes that act on hemicellulose, such as xylanases (GH10 and GH11), mannanases (GH26), glucuronidases (GH115), and pectin-attacking enzymes (PL1, PL3, PL4, and CE12), are also reduced from two to five members to either none or only one member. Among the sequenced ECM fungi, C. geophilum exhibited the most extensive PCWDE repertoire (43 enzymes). Notably, proteins that target crystalline cellulose (GH6, GH7, AA9, and CBM1) are found in the C. geophilum genome (Peter et al., 2016) but are frequently absent in other ECM fungi (Kohler et al., 2015; Miyauchi et al., 2020; Lebreton et al., 2021).
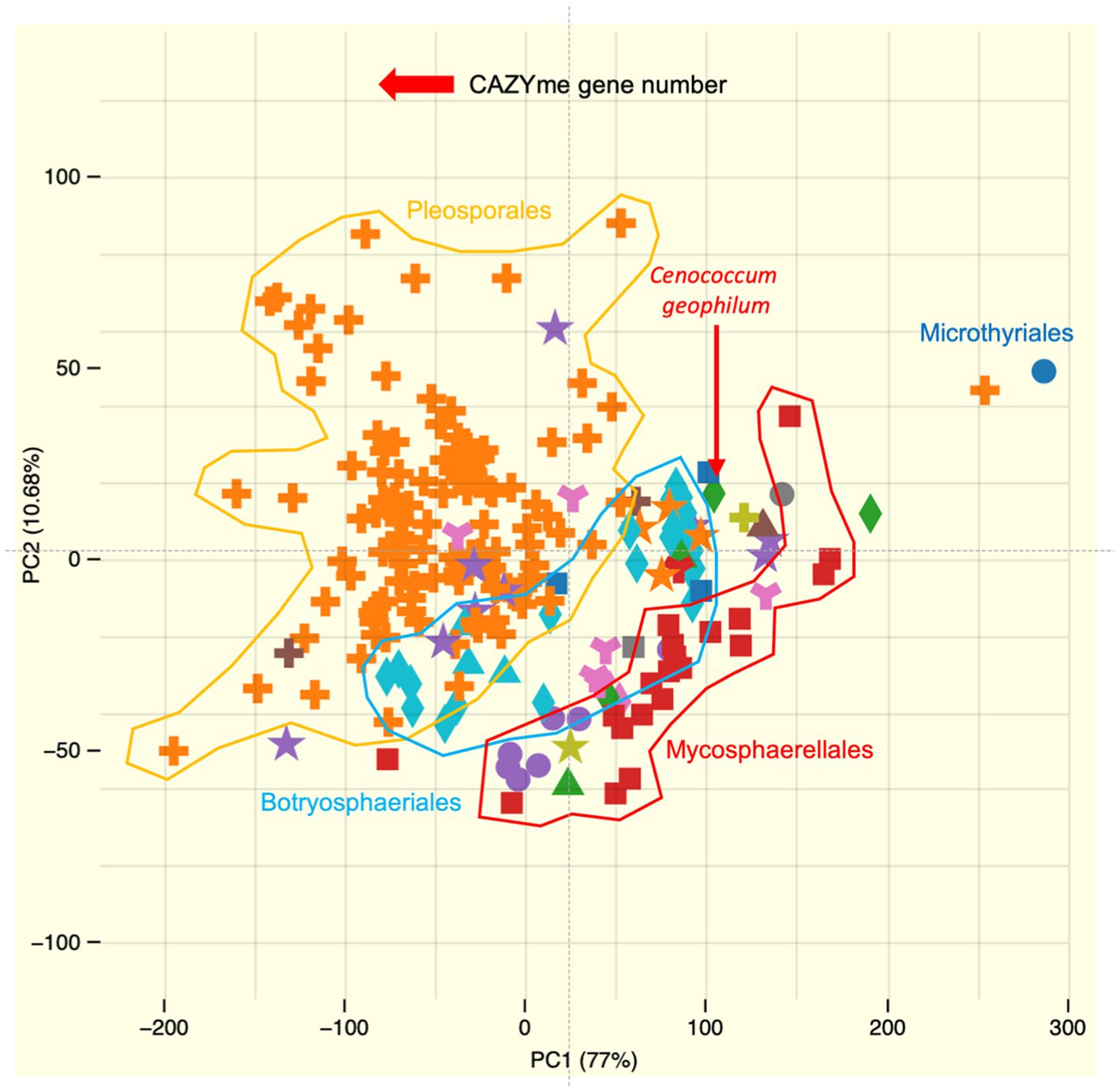
Figure 4. Principal component analysis (PCA) showing the distribution of the CAZyme repertoire in Cenococcum geophilum (red arrow) and other sequenced Dothideomycetes available in the JGI MycoCosm database (Grigoriev et al., 2014). Major orders of Dothideomycetes, such as Botryosphaeriales, Mycosphaerellales, and Pleosporales, are also indicated. These data were obtained after semi-manual curation of protein-filtered model sequences by the CAZy team (www.cazy.org) (Drula et al., 2022), and PCA was generated by MycoCosm.
With the exception of polyketide synthases (PKSs), the C. geophilum genome did not show a reduction in the number of genes associated with the biosynthesis of secondary metabolites (Figure 5; Peter et al., 2016), many of which act as antibiotics in pathogenic interactions and microbial competition in the rhizosphere. These PKSs are typically more numerous than those found in ECM basidiomycetes (Lebreton et al., 2021). In ECM root tips, the expression of the majority of secondary metabolism-related genes is suppressed, except for two non-ribosomal peptide synthases (NRPS) and a PKS (Peter et al., 2016). Notably, one of these NRPS exhibits high protein sequence similarity (42%) to Aspergillus fumigatus Pes1, which is involved in the defense against oxidative stress (Reeves et al., 2006). Oxidative stress is an unavoidable consequence of drought and is employed by plants as a defense mechanism against biotic stressors.
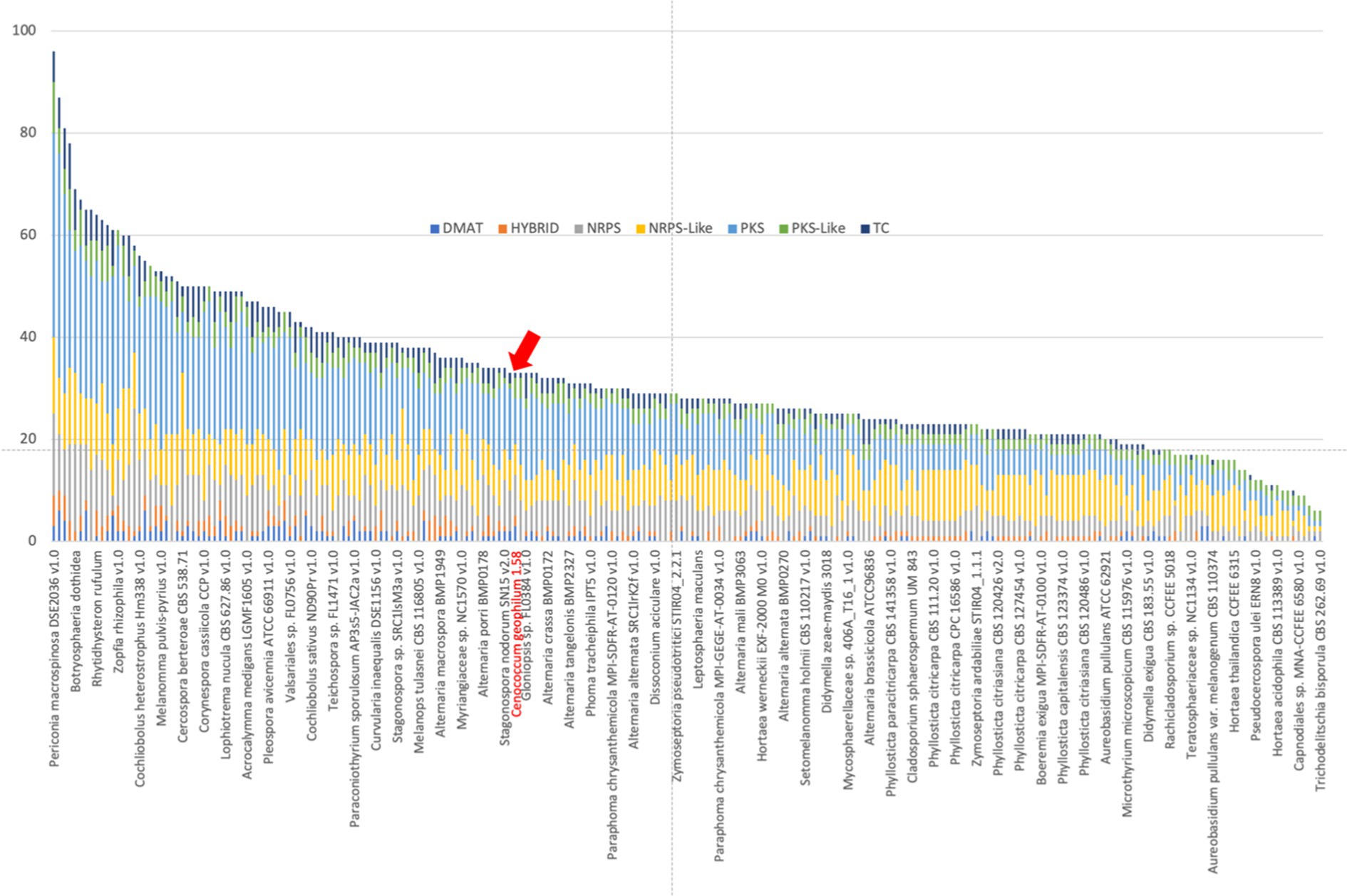
Figure 5. Number of genes coding for secondary metabolism in Cenococcum geophilum (red arrow) and other sequenced Dothideomycetes available from the JGI MycoCosm database (Grigoriev et al., 2014). DMAT, prenyltransferase; NRPS, nonribosomal peptide synthase; PKS, polyketide synthase; and TC, terpene cyclase.
10.2 Transcriptomics
Peter et al. (2016) revealed that 3% of C. geophilum genes were upregulated during symbiosis, as determined by comparing RNA sequences from mycorrhizal roots and free-living mycelia. The most highly expressed and upregulated genes in symbiosis include aquaporins, major facilitator superfamily (MFS) membrane transporters, and small secreted proteins (SSPs), which are proteins less than 300 amino acids in length with a predicted signal peptide (Table 1). Notably, 18–23% of the upregulated genes were specific to C. geophilum, with SSPs being overrepresented in these taxon-specific orphan genes compared to their proportion in the overall gene repertoire. These SSPs may function as novel symbiosis-related effectors, similar to the mycorrhiza-induced small secreted proteins (MiSSPs) in Laccaria bicolor, which regulates defense-related pathways in host roots (Martin et al., 2016).
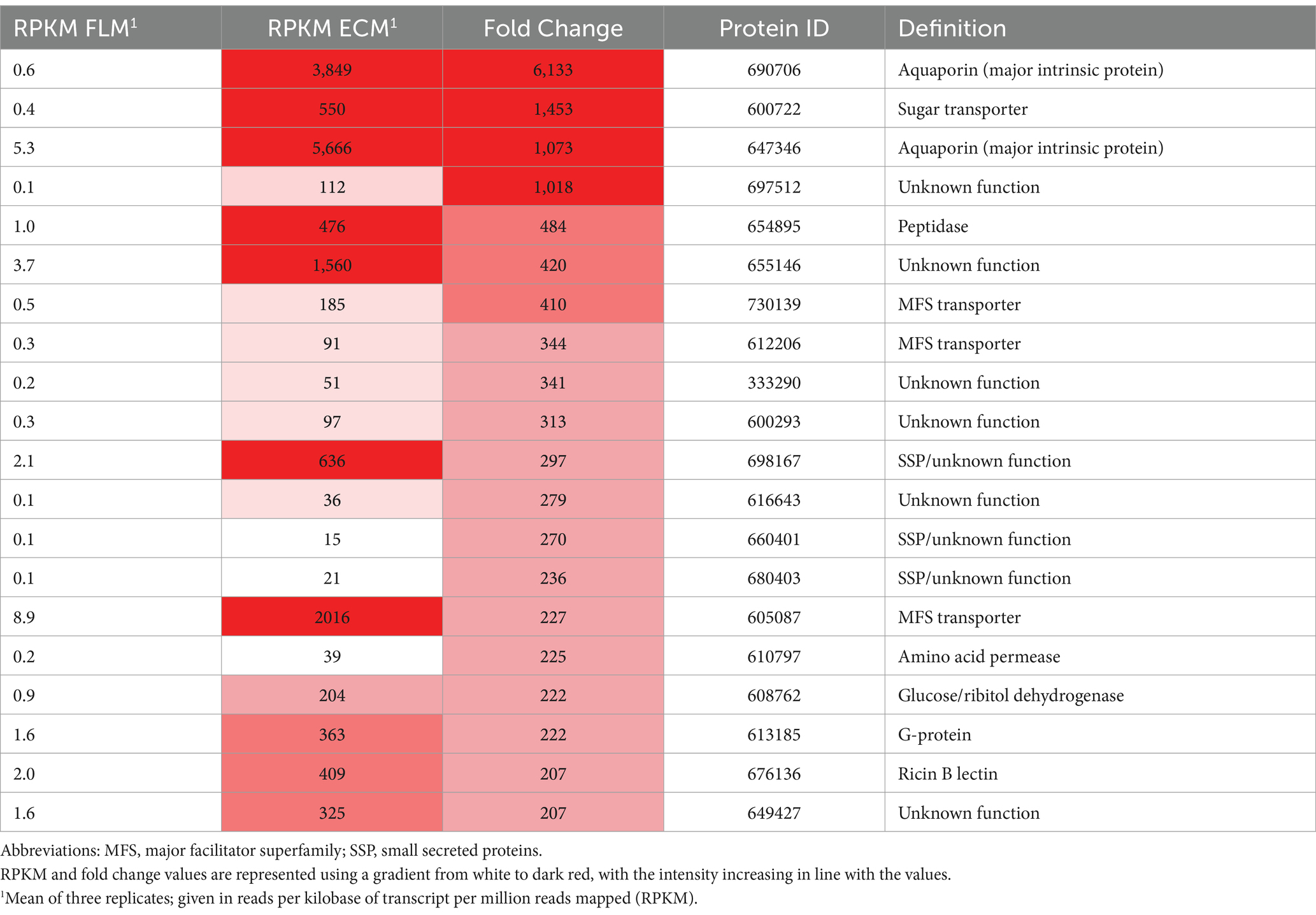
Table 1. Most highly upregulated genes in ectomycorrhizal roots (ECM) of Cenococcum geophilum/Pinus sylvestris compared to free-living mycelia (FLM) (adapted from Peter et al., 2016).
De Freitas Pereira et al. (2018) compared the secretome of C. geophilum interacting with pine and poplar trees; they reported that the levels of transcripts encoding carbohydrate-active enzymes (CAZymes) and MiSSPs were strikingly different. This may be related to the different cell wall compositions of the pine and poplar roots (Sarkar et al., 2009). Colonizing C. geophilum hyphae may require different cell wall-loosening enzymes to penetrate the roots and effectors to dampen the host immune system.
The gene expression analysis revealed significant changes in the expressions of two aquaporins (AQP) that encode water channels during symbiosis in the ECM rootlets of Pinus sylvestris (Table 1; Peter et al., 2016). The substantial increase in water-permeable AQPs in symbiotic rootlets may be triggered by the water and/or nutrient requirements of the plant during interactions. Studies on expression have shown downregulation and upregulation of these AQP genes under drought conditions (a shoot water potential of −3.5 MPa). Intriguingly, under well-watered control conditions, the transcript levels of the drought-induced classical AQP (Cenge3:604158) correlated best with the shoot water potential of their host plant. In the same drought/re-watering experiment, Peter et al. (2016) evaluated the conditions of mycorrhizal plants compared with non-mycorrhizal plants. They noted significantly higher needle N content, net photosynthesis, and water use efficiency in ECM pine seedlings than those in their non-mycorrhizal counterparts, confirming the mutually beneficial relationship between fungi and plants. However, mycorrhizal inoculation had no significant effect on drought treatment.
Zhang et al. (2024) investigated the effects of several C. geophilum ecotypes on the drought resistance of Pinus massoniana seedlings. They found that inoculation with various strains of C. geophilum improved the drought resistance of seedlings by affecting the water content, photosynthesis, osmotic adjustment substances, and antioxidant enzyme activities. The transcriptomic analysis revealed that the seedlings primarily regulate their energy metabolism and redox reactions to cope with early drought stress. The effectiveness of inoculation did not depend on the drought tolerance of the C. geophilum ecotype; that is, the drought resistance of the mycorrhizal seedlings did not correlate with the inherent drought resistance of the C. geophilum strain itself. The beneficial effects of C. geophilum inoculation on the growth of pine seedlings during the early stages of drought stress suggest that this symbiont can be used in reforestation programs in drought areas. Using 1-D gel electrophoresis and LC–MS/MS, Kerner et al. (2012) identified 12 proteins that were differentially accumulated in mycelia subjected to drought conditions compared to controls. The induced responses in C. geophilum point toward the regulation of osmotic stress, maintenance of cell integrity, and counteracting increased levels of reactive oxygen species formed during water deprivation.
The survival of C. geophilum in various environments depends on its ability to regulate stress-related gene expression. Transcriptome profiling has shown that C. geophilum can enhance the expression of numerous genes associated with stress resistance, including those associated with osmotic/drought stress (Li et al., 2023), salt stress (Li et al., 2022), oxidative stress, heat shock responses (Yan et al., 2022), and heavy metal tolerance (Shi et al., 2022). These genes, which are involved in processes such as organic acid secretion, antioxidant activity (e.g., peroxidase, superoxide dismutase, and ubiquinone), membrane transport, and sphingolipid metabolism pathways, are regulated in a coordinated manner. This suggests that their expression is controlled by transcription factors that react to environmental changes, such as heat shock factors and elements responsive to osmotic stress. Verification of the functional roles of the numerous identified stress-related genes will necessitate genetic transformation protocols to inactivate them through RNA interference silencing or CRISPR/Cas9.
Although identifying differentially expressed genes in mycelia cultivated under laboratory conditions represents a promising approach to characterizing genes involved in drought stress adaptation, it is important to consider that gene expression in natural environments may differ significantly, as demonstrated in a recent study by Pellitier et al. (2024). They investigated fungal communities inhabiting the roots of Populus trichocarpa distributed across a precipitation gradient in the Pacific Northwest, United States. These communities were analyzed using taxonomic (metabarcoding) and functional (metagenomic) approaches. Their findings revealed that fungal genes associated with drought stress tolerance and plant water uptake (including genes for melanin synthesis, hydrophobins, aquaporins, trehalose synthases, and other gene families) were not predominant in drier soils.
10.3 Population genomics
Dauphin et al. (2021) conducted a study on 16 European isolates of C. geophilum using whole-genome resequencing. Their findings revealed divergent lineages in geographically confined sampling locations, without strong geographic structuring. Genome-wide polymorphism analyses indicated species subdivisions and suggested two primary genetic groups: clonal and recombinant. The lineage phylogeny and groupings were largely corroborated by the numerous gene copy number variations (CNVs) discovered among the genomes. Although the clonal cluster contained nearly twice as many strains, gene diversity analyses showed a higher genetic diversity in the recombinant group. Based on Tajima’s D statistics, the top candidate genes potentially under positive selection differed between the two groups. The recombinant cluster exhibited more genes from lineage-specific expanded gene families involved in self/non-self-recognition, whereas the clonal cluster showed genes related to secondary metabolism. Additionally, this study confirmed C. geophilum heterothallism through chromosomal synteny analysis of the mating genes MAT1-1 and MAT1-2 idiomorphs. It also revealed significant genetic rearrangements in the surrounding coding and non-coding regions of the strains carrying both the same and opposite MAT1 idiomorphs. These results highlight the complex genome architecture of C. geophilum, possibly due to cryptic sex-and/or transposon-related mechanisms.
Lian et al. (2024) assembled five C. geophilum genomes representing different geographical regions and generated a pan-genome comprising 7,556 core gene families and 12,686 dispensable gene families. Genome resequencing of 304 isolates with worldwide distribution was performed to estimate the genetic diversity, structure, and demographic history of C. geophilum isolates. Millions of single nucleotide polymorphisms (SNPs) and 0.04–0.2% structural variations have been identified, suggesting the occurrence of several ecotypes with different drought resilience levels. Their genome-wide association and transcriptome analyses identified 161 genomic regions that were significantly associated with 9 biological and environmental adaptation traits, encompassing 2,738 potential genes, including EVM0002574, which are associated with resistance to cadmium, salt, and high-temperature stresses. These genomic resources and diversity datasets provide valuable tools and a comparative genomic framework for investigating ectomycorrhizal symbiotic relationships.
11 Applications in forestry and conservation
Cenococcum geophilum is a highly adaptable ECM fungus that demonstrates significant potential for ecological restoration and environmental remediation through microbial engineering. The symbiont forms a dense network of melanized hyphae around the roots of host plants, creating a protective sheath. This symbiotic association is particularly beneficial in water-limited environments, where C. geophilum helps the host tree maintain hydraulic conductivity and photosynthetic activity under drought stress. Additionally, the fungus has been shown to enhance salt tolerance of host plants, making it valuable for reclaiming saline soils. Finally, its extensive distribution, broad host range, and high stress tolerance make it particularly valuable for addressing desertification and adapting to climate change (Zhai et al., 2023). Through the utilization of genomics and other-omics techniques, we acquired a more comprehensive understanding of the molecular, physiological, and ecological mechanisms underlying the establishment and functioning of C. geophilum ECM under environmental stress. Candidate genes related to adaptation to these environmental stresses can be used to select appropriate strains for the mycorrhizal inoculation of tree seedlings in environments prone to drought or other abiotic stresses. Surveys of soil fungal communities using DNA metabarcoding can be used to predict the environmental conditions under which C. geophilum inoculation is beneficial for forest management and restoration. This enhanced knowledge should be leveraged to develop practical applications, such as mycorrhizal inoculation or microbial engineering, which would enhance ecosystem function and preservation, aid in alleviating climate change impacts, and maintain the sustainability of forest ecosystems.
Furthermore, C. geophilum colonizes herbaceous plants. By forming associations with both woody and non-woody plants, symbionts can contribute to the development of diverse plant communities in challenging environments. Its ability to support multiple plant species can increase soil stability, reduce erosion, and improve nutrient cycling in degraded ecosystems. In arid regions, C. geophilum colonizes both ECM trees and Cistaceae plants. The physical connection of C. geophilum mycelial networks with both tree roots and herbaceous plants could redistribute water from the deeper roots of the tree, retain a portion of the water in the upper soil layers, and facilitate enhanced nutrient acquisition by the host plants. Similarly, the mouse-tail bog sedge (Carex (Kobresia) myosuroides) can be incorporated into tree plantations in northern and alpine regions. In environments contaminated by industrial waste, C. geophilum has shown promising results in the remediation of soils affected by heavy metals and petroleum (Danielson and Visser, 1989). The fungus has also exhibited the capacity to accumulate and sequester various heavy metals, including Pb, Cd, and Zn, in its melanized cell walls (Huang et al., 2014; Azaiez et al., 2018; Shi et al., 2022; Zhang et al., 2023,). This characteristic renders C. geophilum a potential candidate for mycoremediation of polluted soils. Moreover, their association with host plants can enhance phytoremediation efforts by improving plant survival and growth at contaminated sites.
Urban environments often present challenging conditions for plant growth such as soil compaction, elevated temperatures, and air pollution. C. geophilum is frequently the most abundant ECM symbiont found in the roots of urban trees (Garbaye and Churin, 1996; Hui et al., 2017; Van Geel et al., 2018; Olchowik et al., 2021). Its ability to form symbiotic relationships can improve resilience to these stressors, potentially leading to increased tree longevity and enhanced ecosystem services in urban areas.
12 Future research
Several enduring challenges persist in utilizing genomics and other-omics approaches to enhance our understanding of the biology and ecology of C. geophilum, including its evolutionary history, developmental processes, functional aspects, and its resilience to environmental stress. We have identified several critical questions that require further investigation:
1. What molecular mechanisms underlie the genetic diversity of C. geophilum, and how does this genetic polymorphism facilitate its worldwide distribution?
2. What are the transcriptional regulators and gene networks that drive the resilience of C. geophilum to extreme environmental conditions including drought stress, heavy metal contamination, and high salinity?
3. What role do epigenetic modifications play in the ability of C. geophilum to adapt to various environments?
4. What is the significance of horizontal gene transfer (if any) in the evolutionary trajectory of C. geophilum?
5. How does the mutualistic association between C. geophilum and its plant partners fluctuate across various environmental settings?
6. What patterns have emerged in the C. geophilum population genomics across different geographical regions? How will climate change alter symbiont distribution worldwide?
7. How do the secondary metabolites produced by C. geophilum, such as melanin, influence its interactions with soil microbial communities including soil and litter decomposers?
The role of C. geophilum in ecosystem resilience is becoming increasingly important in the context of climate change. As extreme weather events and environmental stressors become more frequent, the capacity of this fungus and other mycorrhizal fungi to support plant growth and survival under adverse conditions may be crucial for maintaining ecosystem stability and biodiversity. Furthermore, their potential to enhance carbon sequestration through increased plant growth and soil organic matter accumulation may contribute to climate-change mitigation.
Author contributions
HW: Writing – original draft, Writing – review & editing. AK: Writing – original draft, Writing – review & editing. FM: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. HW was supported by scholarships from the China Scholarship Council and the Agreenium Consortium. This study was supported by the Laboratory of Excellence, ARBRE (ANR-11-LABX-0002-01) and the Northwest Institute of Eco-Environment and Resources, Chinese Academy of Sciences, Lanzhou, China (to FMM).
Acknowledgments
We would like to thank Martina Peter and Benjamin Dauphin for helpful discussions.
Conflict of interest
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Amano, N. (1983). Saprobic loculoascomycetous fungi from Japan 1. Hysteriaceous fungi. Trans. Mycol. Soc. Jpn. 24, 283–297.
Azaiez, A., Beaudoin Nadeau, M., Bertrand, A., and Khasa, D. P. (2018). In vitro selection of ecologically adapted ectomycorrhizal fungi through production of fungal biomass and metabolites for use in reclamation of biotite mine tailings. Mycologia 110, 1017–1032. doi: 10.1080/00275514.2018.1520036
Bâ, A. M., Duponnois, R., Moyersoen, B., and Diédhiou, A. G. (2012). Ectomycorrhizal symbiosis of tropical African trees. Mycorrhiza 22, 1–29. doi: 10.1007/s00572-011-0415-x
Bakray, N. A. M., Azman, A. S., Kin, T. B., Mansor, P., Mohti, A., Jamar, N. H., et al. (2024). Effects of soil nutrient availability on ectomycorrhizal communities’ distribution in two dipterocarps species under Normal and elevated atmospheric carbon dioxide. Res. Sq. 2024:828. doi: 10.21203/rs.3.rs-3780828/v1
Baldrian, P., López-Mondéjar, R., and Kohout, P. (2023). Forest microbiome and global change. Nat. Rev. Microbiol. 21, 487–501. doi: 10.1038/s41579-023-00876-4
Bao, Q. (2005). Survey of ectomycorrhizal fungus Flora Association with Picea mongolica and study on the mycelial culture condition of Cenococcum geophilum Fr. In Baiyinaobao. [Dissertation thesis]. [Hohhot (Inner Mongolia)]: Inner Mongolia Agricultural University.
Bazzicalupo, A. L., Ruytinx, J., Ke, Y.-H., Coninx, L., Colpaert, J. V., Nguyen, N. H., et al. (2020). Fungal heavy metal adaptation through single nucleotide polymorphisms and copy-number variation. Mol. Ecol. 29, 4157–4169. doi: 10.1111/mec.15618
Becerra, A. G., and Zak, M. R. (2011). “The ectomycorrhizal Symbiosis in South America: morphology, colonization, and diversity” in Soil Biology Diversity and biotechnology of Ectomycorrhizae. eds. M. Rai and A. Varma (Berlin: Springer), 19–41.
Beimforde, C., Schafer, N., Dorfelt, H., Nascimbene, P. C., Singh, H., Heinrichs, J., et al. (2011). Ectomycorrhizas from a lower Eocene angiosperm forest. New Phytol. 192, 988–996. doi: 10.1111/j.1469-8137.2011.03868.x
Bennett, A. E., and Classen, A. T. (2020). Climate change influences mycorrhizal fungal–plant interactions, but conclusions are limited by geographical study bias. Ecology 101:e02978. doi: 10.1002/ecy.2978
Bourne, E. C., Mina, D., Gonçalves, S. C., Loureiro, J., Freitas, H., and Muller, L. A. H. (2014). Large and variable genome size unrelated to serpentine adaptation but supportive of cryptic sexuality in Cenococcum geophilum. Mycorrhiza 24, 13–20. doi: 10.1007/s00572-013-0501-3
Buscardo, E., Rodríguez-Echeverría, S., Martín, M. P., De Angelis, P., Pereira, J. S., and Freitas, H. (2010). Impact of wildfire return interval on the ectomycorrhizal resistant propagules communities of a Mediterranean open forest. Fungal Biol. 114, 628–636. doi: 10.1016/j.funbio.2010.05.004
Coleman, M. D., Bledsoe, C. S., and Lopushinsky, W. (1989). Pure culture response of ectomycorrhizal fungi to imposed water stress. Can. J. Bot. 67, 29–39. doi: 10.1139/b89-005
Corbery, Y., and Le Tacon, F. (1997). Storage of ectomycorrhizal fungi by freezing. Ann. For. Sci. 54, 211–217. doi: 10.1051/forest:19970208
Danielson, R. M. (1991). Temporal changes and effects of amendments on the occurrence of sheathing (ecto-) mycorrhizas of conifers growing in oil sands tailings and coal spoil. Agric. Ecosyst. Environ. 35, 261–281. doi: 10.1016/0167-8809(91)90054-2
Danielson, R. M., and Visser, S. (1989). Host response to inoculation and behaviour of introduced and indigenous ectomycorrhizal fungi of jack pine grown on oil-sands tailings. Can. J. For. Res. 19, 1412–1421. doi: 10.1139/x89-216
Dauphin, B., De Freitas Pereira, M., Kohler, A., Grigoriev, I. V., Barry, K., Na, H., et al. (2021). Cryptic genetic structure and copy-number variation in the ubiquitous forest symbiotic fungus. Environ. Microbiol. 23, 6536–6556. doi: 10.1111/1462-2920.15752
De Freitas Pereira, M., Veneault-Fourrey, C., Vion, P., Guinet, F., Morin, E., Barry, K. W., et al. (2018). Secretome analysis from the ectomycorrhizal ascomycete Cenococcum geophilum. Front. Microbiol. 9:141. doi: 10.3389/fmicb.2018.00141
Dickie, I. A., and Reich, P. B. (2005). Ectomycorrhizal fungal communities at forest edges. J. Ecol. 93, 244–255. doi: 10.1111/j.1365-2745.2005.00977.x
Di Pietro, M., Churin, J. L., and Garbaye, J. (2007). Differential ability of ectomycorrhizas to survive drying. Mycorrhiza 17, 547–550. doi: 10.1007/s00572-007-0113-x
Douhan, G. W., and Rizzo, D. M. (2005). Phylogenetic divergence in a local population of the ectomycorrhizal fungus Cenococcum geophilum. New Phytol. 166, 263–271. doi: 10.1111/j.1469-8137.2004.01305.x
Drula, E., Garron, M. L., Dogan, S., Lombard, V., Henrissat, B., and Terrapon, N. (2022). The carbohydrate-active enzyme database: functions and literature. Nucleic Acids Res. 50, D571–D577.
Fernandez, C. W., and Koide, R. T. (2013). The function of melanin in the ectomycorrhizal fungus Cenococcum geophilum under water stress. Fungal Ecol. 6, 479–486. doi: 10.1016/j.funeco.2013.08.004
Fernandez, C. W., and Koide, R. T. (2014). Initial melanin and nitrogen concentrations control the decomposition of ectomycorrhizal fungal litter. Soil Biol. Biochem. 77, 150–157. doi: 10.1016/j.soilbio.2014.06.026
Forsmark, B., Bizjak, T., Nordin, A., Rosenstock, N. P., Wallander, H., and Gundale, M. J. (2024). Shifts in microbial community composition and metabolism correspond with rapid soil carbon accumulation in response to 20 years of simulated nitrogen deposition. Sci. Total Environ. 918:170741. doi: 10.1016/j.scitotenv.2024.170741
Garbaye, J., and Churin, J.-L. (1996). Effect of ectomycorrhizal inoculation at planting on growth and foliage quality of Tilia tomentosa. Arboric. Urban For. 22, 29–34. doi: 10.48044/jauf.1996.004
Gehring, C., Sevanto, S., Patterson, A., Ulrich, D. E. M., and Kuske, C. R. (2020). Ectomycorrhizal and dark septate fungal associations of pinyon pine are differentially affected by experimental drought and warming. Front. Plant Sci. 11:582574. doi: 10.3389/fpls.2020.582574
Genney, D. R., Anderson, I. C., and Alexander, I. J. (2006). Fine-scale distribution of pine ectomycorrhizas and their extrametrical mycelium. New Phytol. 170, 381–390. doi: 10.1111/j.1469-8137.2006.01669.x
Gonçalves, S. C., Martins-Loução, M. A., and Freitas, H. (2009). Evidence of adaptive tolerance to nickel in isolates of Cenococcum geophilum from serpentine soils. Mycorrhiza 19, 221–230. doi: 10.1007/s00572-008-0211-4
Gray, L., and Kernaghan, G. (2020). Fungal succession during the decomposition of ectomycorrhizal fine roots. Microb. Ecol. 79, 271–284. doi: 10.1007/s00248-019-01418-3
Grigoriev, I. V., Nikitin, R., Haridas, S., Kuo, A., Ohm, R., Otillar, R., et al. (2014). MycoCosm portal: gearing up for 1000 fungal genomes. Nucleic Acids Res. 42, D699–D704.
Guo, Z. X., Wang, Y. L., Wu, B. W., Xu, Y., Yao, H., Li, Z. F., et al. (2021). Population genetic diversity and structure of the ectomycorrhizal fungus Cenococcum geophilum. Mycosystema 40, 920–935. doi: 10.13346/j.mycosystema.210062
Hasselquist, N., Germino, M. J., Mcgonigle, T., and Smith, W. K. (2005). Variability of Cenococcum colonization and its ecophysiological significance for young conifers at alpine–treeline. New Phytol. 165, 867–873. doi: 10.1111/j.1469-8137.2005.01275.x
Hawkins, H.-J., Cargill, R. I. M., Van Nuland, M. E., Hagen, S. C., Field, K. J., Sheldrake, M., et al. (2023). Mycorrhizal mycelium as a global carbon pool. Curr. Biol. 33, R560–R573. doi: 10.1016/j.cub.2023.02.027
Herzog, C., Peter, M., Pritsch, K., Gunthardt-Goerg, M. S., and Egli, S. (2013). Drought and air warming affects abundance and exoenzyme profiles of Cenococcum geophilum associated with Quercus robur, Q. petraea and Q. pubescens. Plant Biol. (Stuttg.) 15 Suppl 1, 230–237. doi: 10.1111/j.1438-8677.2012.00614.x
Hoeksema, J., Roy, M., Laska, G., Sienkiewicz, A., Horning, A., Abbott, M. J., et al. (2018). Pulsatilla patens (Ranunculaceae), a perennial herb, is ectomycorrhizal in northeastern Poland and likely shares ectomycorrhizal fungi with Pinus sylvestris. Acta Soc. Bot. Pol. 87:3572. doi: 10.5586/asbp.3572
Huang, J., Nara, K., Zong, K., Wang, J., Xue, S., Peng, K., et al. (2014). Ectomycorrhizal fungal communities associated with Masson pine (Pinus massoniana) and white oak (Quercus fabri) in a manganese mining region in Hunan Province, China. Fungal Ecol. 9, 1–10. doi: 10.1016/j.funeco.2014.01.001
Hui, N., Liu, X., Kotze, D. J., Jumpponen, A., Francini, G., and Setälä, H. (2017). Ectomycorrhizal fungal communities in urban parks are similar to those in natural forests but shaped by vegetation and park age. Appl. Environ. Microbiol. 9, 1–10. doi: 10.1128/AEM.01797-17
Jany, J.-L., Garbaye, J., and Martin, F. (2002). Cenococcum geophilum populations show a high degree of genetic diversity in beech forests. New Phytol. 154, 651–659. doi: 10.1046/j.1469-8137.2002.00408.x
Jany, J.-L., Martin, F., and Garbaye, J. (2003). Respiration activity of ectomycorrhizas from Cenococcum geophilum and Lactarius sp. in relation to soil water potential in five beech forests. Plant Soil 255, 487–494. doi: 10.1023/A:1026092714340
Kataoka, R., Taniguchi, T., and Futai, K. (2009). Fungal selectivity of two mycorrhiza helper bacteria on five mycorrhizal fungi associated with Pinus thunbergii. World J. Microbiol. Biotechnol. 25, 1815–1819. doi: 10.1007/s11274-009-0082-7
Kerner, R., Delgado-Eckert, E., Del Castillo, E., Müller-Starck, G., Peter, M., Kuster, B., et al. (2012). Comprehensive proteome analysis in Cenococcum geophilum Fr. As a tool to discover drought-related proteins. J. Proteome 75, 3707–3719. doi: 10.1016/j.jprot.2012.04.039
Kipfer, T., Egli, S., Ghazoul, J., Moser, B., and Wohlgemuth, T. (2010). Susceptibility of ectomycorrhizal fungi to soil heating. Fungal Biol. 114, 467–472. doi: 10.1016/j.funbio.2010.03.008
Kohler, A., Kuo, A., Nagy, L. G., Morin, E., Barry, K. W., Buscot, F., et al. (2015). Convergent losses of decay mechanisms and rapid turnover of symbiosis genes in mycorrhizal mutualists. Nat. Genet. 47, 410–415. doi: 10.1038/ng.3223
Kohn, L. M., and Stasovski, E. (1990). The mycorrhizal status of plants at Alexandra fiord, Ellesmere Island, Canada, a high Arctic site. Mycologia 82, 23–35. doi: 10.1080/00275514.1990.12025836
Largent, D. L., Sugihara, N., and Wishner, C. (1980). Occurrence of mycorrhizae on ericaceous and pyrolaceous plants in northern California. Can. J. Bot. 58, 2274–2279. doi: 10.1139/b80-262
Lebreton, A., Zeng, Q., Miyauchi, S., Kohler, A., Dai, Y.-C., and Martin, F. M. (2021). Evolution of the mode of nutrition in symbiotic and saprotrophic Fungi in Forest ecosystems. Annu. Rev. Ecol. Evol. Syst. 52, 385–404. doi: 10.1146/annurev-ecolsys-012021-114902
Lian, C., Li, C., Yuan, C., Shi, Y., Geng, Q., Li, J., et al. (2024). Genomes and population genomics of Cenococcum geophilum highlight the genetic diversity, evolution, and stress responses of ectomycorrhizal fungi. Res. Sq. 2024:4562. doi: 10.21203/rs.3.rs-4894562/v1
Lian, C., Narimatsu, M., Nara, K., and Hogetsu, T. (2006). Tricholoma matsutake in a natural Pinus densiflora forest: correspondence between above-and below-ground genets, association with multiple host trees and alteration of existing ectomycorrhizal communities. New Phytol. 171, 825–836. doi: 10.1111/j.1469-8137.2006.01801.x
Li, J., Li, C., Tsuruta, M., Matsushita, N., Goto, S., Shen, Z., et al. (2022). Physiological and transcriptional responses of the ectomycorrhizal fungus Cenococcum geophilum to salt stress. Mycorrhiza 32, 327–340. doi: 10.1007/s00572-022-01078-1
Li, M., Yuan, C., Zhang, X., Pang, W., Zhang, P., Xie, R., et al. (2023). The transcriptional responses of ectomycorrhizal fungus Cenococcum geophilum to drought stress. J. Fungi. 9:15. doi: 10.3390/jof9010015
LoBuglio, K. F. (1999). “Cenococcum” in Ectomycorrhizal Fungi key genera in profile. eds. J. W. G. Cairney and S. M. Chambers (Berlin: Springer), 287–309.
Martin, F., Canet, D., and Marchal, J. P. (1985). 13C nuclear magnetic resonance study of mannitol cycle and trehalose synthesis during glucose utilization by the ectomycorrhizal ascomycete Cenococcum graniforme. Plant Physiol. 77, 499–502. doi: 10.1104/pp.77.2.499
Martin, F., Cullen, D., Hibbett, D., Pisabarro, A., Spatafora, J. W., Baker, S. E., et al. (2011). Sequencing the fungal tree of life. New Phytol. 190, 818–821. doi: 10.1111/j.1469-8137.2011.03688.x
Martin, F., Kohler, A., Murat, C., Veneault-Fourrey, C., and Hibbett, D. S. (2016). Unearthing the roots of ectomycorrhizal symbioses. Nat. Rev. Microbiol. 14, 760–773. doi: 10.1038/nrmicro.2016.149
Martin, F. M., and Van Der Heijden, M. G. A. (2024). The mycorrhizal symbiosis: research frontiers in genomics, ecology, and agricultural application. New Phytol. 242, 1486–1506. doi: 10.1111/nph.19541
Massicotte, H. B., Melville, L. H., Peterson, R. L., and Luoma, D. L. (1998). Anatomical aspects of field ectomycorrhizas on Polygonum viviparum (Polygonaceae) and Kobresia bellardii (Cyperaceae). Mycorrhiza 7, 287–292. doi: 10.1007/s005720050194
Massicotte, H. B., Peterson, R. L., Melville, L. H., and Tackaberry, L. E. (2010). Hudsonia ericoides and Hudsonia tomentosa: anatomy of mycorrhizas of two members in the Cistaceae from eastern Canada. Botany 88, 607–616. doi: 10.1139/b10-035
Massicotte, H. B., Trappe, J. M., Peterson, R. L., and Melville, L. H. (1992). Studies on Cenococcum geophilum. II. Sclerotium morphology, germination, and formation in pure culture and growth pouches. Can. J. Bot. 70, 125–132. doi: 10.1139/b92-017
Matsuda, Y., Takeuchi, K., Obase, K., and Ito, S.-I. (2015). Spatial distribution and genetic structure of Cenococcum geophilum in coastal pine forests in Japan. FEMS Microbiol. Ecol. 91:108. doi: 10.1093/femsec/fiv108
Mccormack, M. L., Fernandez, C. W., Brooks, H., and Pritchard, S. G. (2017). Production dynamics of Cenococcum geophilum ectomycorrhizas in response to long-term elevated CO2 and N fertilization. Fungal Ecol. 26, 11–19. doi: 10.1016/j.funeco.2016.11.001
Mexal, J., and Reid, C. P. P. (1973). The growth of selected mycorrhizal fungi in response to induced water stress. Can. J. Bot. 51, 1579–1588. doi: 10.1139/b73-201
Mikola, P. (1948). On the physiology and ecology of Cenococcum graniforme especially as a mycorrhizal fungus of birch. Inst. For. Fenn. Commun. 36, 1–104.
Miyauchi, S., Kiss, E., Kuo, A., Drula, E., Kohler, A., Sánchez-García, M., et al. (2020). Large-scale genome sequencing of mycorrhizal fungi provides insights into the early evolution of symbiotic traits. Nat. Commun. 11:5125. doi: 10.1038/s41467-020-18795-w
Mühlmann, O., and Peintner, U. (2008). Mycobionts of Salix herbacea on a glacier forefront in the Austrian Alps. Mycorrhiza 18, 171–180. doi: 10.1007/s00572-008-0169-2
Murayama, S., and Sugiura, Y. (2021). “Origin of soil polysaccharides and ectomycorrhizal fungal sclerotia as sources of forest soil polysaccharides” in Sclerotia grains in soils, Progress in soil science. ed. M. Watanabe (Singapore: Springer), 91–117.
Nara, K. (2006). Ectomycorrhizal networks and seedling establishment during early primary succession. New Phytol. 169, 169–178. doi: 10.1111/j.1469-8137.2005.01545.x
Narisawa, K., Amasya, A., Nonoyama, Y. S., and Obase, K. (2021). “Fungal communities of Sclerotia Grains from Forest soils” in Sclerotia grains in soils, Progress in soil science. ed. M. Watanabe (Singapore: Springer), 17–34.
Nickel, U. T., Weikl, F., Kerner, R., Schäfer, C., Kallenbach, C., Munch, J. C., et al. (2018). Quantitative losses vs. qualitative stability of ectomycorrhizal community responses to 3 years of experimental summer drought in a beech–spruce forest. Glob. Chang. Biol. 24, e560–e576. doi: 10.1111/gcb.13957
Nicolas, C., Martin-Bertelsen, T., Floudas, D., Bentzer, J., Smits, M., Johansson, T., et al. (2019). The soil organic matter decomposition mechanisms in ectomycorrhizal fungi are tuned for liberating soil organic nitrogen. ISME J. 13, 977–988. doi: 10.1038/s41396-018-0331-6
Nyamsanjaa, K., Oyuntsetseg, B., Takashima, Y., Sakagami, N., and Watanabe, M. (2022). Characteristics of Cenococcum geophilum sclerotia found in steppe forest soil in Mongolia. J. For. Res. 27, 76–82. doi: 10.1080/13416979.2021.2008618
Obase, K., Douhan, G. W., Matsuda, Y., and Smith, M. E. (2014). Culturable fungal assemblages growing within Cenococcum sclerotia in forest soils. FEMS Microbiol. Ecol. 90, 708–717. doi: 10.1111/1574-6941.12428
Obase, K., Douhan, G. W., Matsuda, Y., and Smith, M. E. (2016). Revisiting phylogenetic diversity and cryptic species of Cenococcum geophilum sensu lato. Mycorrhiza 26, 529–540. doi: 10.1007/s00572-016-0690-7
Obase, K., Douhan, G. W., Matsuda, Y., and Smith, M. E. (2017). “Progress and challenges in understanding the biology, diversity, and biogeography of Cenococcum geophilum” in Biogeography of mycorrhizal Symbiosis. ed. L. Tedersoo (Switzerland: Springer, Cham), 299–317.
Olchowik, J., Suchocka, M., Jankowski, P., Malewski, T., and Hilszczańska, D. (2021). The ectomycorrhizal community of urban linden trees in Gdańsk, Poland. PLoS One 16:e0237551. doi: 10.1371/journal.pone.0237551
Pal, A. K., Gajjar, D. U., and Vasavada, A. R. (2013). DOPA and DHN pathway orchestrate melanin synthesis in Aspergillus species. Med. Mycol. 52, 10–18. doi: 10.3109/13693786.2013.826879
Pellitier, P. T., Van Nuland, M., Salamov, A., Grigoriev, I. V., and Peay, K. G. (2024). Potential for functional divergence in ectomycorrhizal fungal communities across a precipitation gradient. ISME Commun. 4:31. doi: 10.1093/ismeco/ycae031
Peter, M. (2003). Volcanic deserts and primary succession – when and how do mycorrhizal fungi participate? New Phytol. 159, 534–536. doi: 10.1046/j.1469-8137.2003.00869.x
Peter, M., Kohler, A., Ohm, R. A., Kuo, A., Krützmann, J., Morin, E., et al. (2016). Ectomycorrhizal ecology is imprinted in the genome of the dominant symbiotic fungus Cenococcum geophilum. Nat. Commun. 7:12662. doi: 10.1038/ncomms12662
Phillips, S. J., Anderson, R. P., Dudík, M., Schapire, R. E., and Blair, M. E. (2017). Opening the black box: an open-source release of Maxent. Ecography 40, 887–893. doi: 10.1111/ecog.03049
Phosri, C., Põlme, S., Taylor, A. F. S., Kõljalg, U., Suwannasai, N., and Tedersoo, L. (2012). Diversity and community composition of ectomycorrhizal fungi in a dry deciduous dipterocarp forest in Thailand. Biodivers. Conserv. 21, 2287–2298. doi: 10.1007/s10531-012-0250-1
Pigott, C. D. (1982). Survival of mycorrhiza formed by Cenococcum geophilum fr. In dry soils. New Phytol. 92, 513–517. doi: 10.1111/j.1469-8137.1982.tb03409.x
Querejeta, J., Egerton-Warburton, L. M., and Allen, M. F. (2009). Topographic position modulates the mycorrhizal response of oak trees to interannual rainfall variability. Ecology 90, 649–662. doi: 10.1890/07-1696.1
Reeves, E. P., Reiber, K., Neville, C., Scheibner, O., Kavanagh, K., and Doyle, S. (2006). A nonribosomal peptide synthetase (pes 1) confers protection against oxidative stress in Aspergillus fumigatus. FEBS J. 273, 3038–3053. doi: 10.1111/j.1742-4658.2006.05315.x
Reis, F., Magalhães, A. P., Tavares, R. M., Baptista, P., and Lino-Neto, T. (2021). Bacteria could help ectomycorrhizae establishment under climate variations. Mycorrhiza 31, 395–401. doi: 10.1007/s00572-021-01027-4
Rosling, A., Landeweert, R., Lindahl, B. D., Larsson, K.-H., Kuyper, T. W., Taylor, A. F. S., et al. (2003). Vertical distribution of ectomycorrhizal fungal taxa in a podzol soil profile. New Phytol. 159, 775–783. doi: 10.1046/j.1469-8137.2003.00829.x
Sarkar, P., Bosneaga, E., and Auer, M. (2009). Plant cell walls throughout evolution: towards a molecular understanding of their design principles. J. Exp. Bot. 60, 3615–3635. doi: 10.1093/jxb/erp245
Scattolin, L., Montecchio, L., Mosca, E., and Agerer, R. (2008). Vertical distribution of the ectomycorrhizal community in the top soil of Norway spruce stands. Eur. J. For. Res. 127, 347–357. doi: 10.1007/s10342-008-0209-7
Shi, Y., Yan, T., Yuan, C., Li, C., Rensing, C., Chen, Y., et al. (2022). Comparative physiological and transcriptome analysis provide insights into the response of Cenococcum geophilum, an ectomycorrhizal fungus to cadmium stress. J Fungi (Basel) 8:724. doi: 10.3390/jof8070724
Talhinhas, P., Tavares, D., Ramos, A. P., Gonçalves, S., and Loureiro, J. (2017). Validation of standards suitable for genome size estimation of fungi. J. Microbiol. Methods 142, 76–78. doi: 10.1016/j.mimet.2017.09.012
Tedersoo, L., Drenkhan, R., Abarenkov, K., Anslan, S., Bahram, M., Bitenieks, K., et al. (2024). The influence of tree genus, phylogeny, and richness on the specificity, rarity, and diversity of ectomycorrhizal fungi. Environ. Microbiol. Rep. 16:e13253. doi: 10.1111/1758-2229.13253
Tedersoo, L., May, T. W., and Smith, M. E. (2010). Ectomycorrhizal lifestyle in fungi: global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza 20, 217–263. doi: 10.1007/s00572-009-0274-x
Trappe, J. (1962). Cenococcum graniforme: Its distribution, ecology, mycorrhiza formation and inherent variation [dissertation thesis]. [Washington]: University of Washington.
Trappe, J. M. (1988). Lessons from alpine Fungi. Mycologia 80, 1–10. doi: 10.1080/00275514.1988.12025490
Van Der Heijden, M. G., Martin, F. M., Selosse, M. A., and Sanders, I. R. (2015). Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytol. 205, 1406–1423. doi: 10.1111/nph.13288
Van Geel, M., Yu, K., Ceulemans, T., Peeters, G., Van Acker, K., Geerts, W., et al. (2018). Variation in ectomycorrhizal fungal communities associated with silver linden (Tilia tomentosa) within and across urban areas. FEMS Microbiol. Ecol. 94:207. doi: 10.1093/femsec/fiy207
Vélez, J. M., Morris, R. M., Vilgalys, R., Labbé, J., and Schadt, C. W. (2021). Phylogenetic diversity of 200+ isolates of the ectomycorrhizal fungus Cenococcum geophilum associated with Populus trichocarpa soils in the Pacific northwest, USA and comparison to globally distributed representatives. PLoS One 16:e0231367. doi: 10.1371/journal.pone.0231367
Větrovský, T., Morais, D., Kohout, P., Lepinay, C., Algora, C., Awokunle Hollá, S., et al. (2020). Global Fungi, a global database of fungal occurrences from high-throughput-sequencing metabarcoding studies. Sci. Data 7:228. doi: 10.1038/s41597-020-0567-7
Vik, U., Logares, R., Blaalid, R., Halvorsen, R., Carlsen, T., Bakke, I., et al. (2013). Different bacterial communities in ectomycorrhizae and surrounding soil. Sci. Rep. 3:3471. doi: 10.1038/srep03471
Visser, S. (1995). Ectomycorrhizal fungal succession in jack pine stands following wildfire. New Phytol. 129, 389–401. doi: 10.1111/j.1469-8137.1995.tb04309.x
Vogt, K. A., Grier, C. C., Meier, C. E., and Edmonds, R. L. (1982). Mycorrhizal role in net primary production and nutrient cycling in Abies Amabilis ecosystems in Western Washington. Ecology 63, 370–380. doi: 10.2307/1938955
Vohník, M., Fendrych, M., Albrechtová, J., and Vosátka, M. (2007). Intracellular colonization of Rhododendron and Vaccinium roots by Cenococcum geophilum, Geomyces pannorum and Meliniomyces variabilis. Folia Microbiol. 52, 407–414. doi: 10.1007/BF02932096
Weinstein, R. N., Palm, M. E., Johnstone, K., and Wynn-Williams, D. D. (1997). Ecological and physiological characterization of Humicola marvinii, a new psychrophilic fungus from fellfield soils in the maritime Antarctic. Mycologia 89, 706–711. doi: 10.1080/00275514.1997.12026836
Wen, Z., Xing, J., Liu, C., Zhu, X., Zhao, B., Dong, J., et al. (2022). The effects of ectomycorrhizal inoculation on survival and growth of Pinus thunbergii seedlings planted in saline soil. Symbiosis 86, 71–80. doi: 10.1007/s13199-021-00825-w
Wurentaoges, H. (2012). Analyze relationship between natural infection rate and vegetation, rhizosphere soil factors about Cenococcum geophilum. Chin. Agric. Sci. Bull. 28, 47–51. doi: 10.11924/j.issn.1000-6850.2012-1076 S., Yan, W
Xie, L., Yang, Y., Ma, J., Lin, G., Deng, J., Robson, T. M., et al. (2024). Variations in ectomycorrhizal exploration types parallel seedling fine root traits of two temperate tree species under extreme drought and contrasting solar radiation treatments. Plant Cell Environ. 47, 5053–5066. doi: 10.1111/pce.15093
Xue, X., Zhang, L., Peng, Y., Li, P., and Yu, J. (2019). Effects of mineral structure and microenvironment on K release from potassium Aluminosilicate minerals by Cenococcum geophilum fr. Geomicrobiol J. 36, 11–18. doi: 10.1080/01490451.2018.1485064
Yan, T., Zhang, P., Pang, W., Zhang, X., Lian, C., and Zhang, T. (2022). Effects of high temperature-triggered transcriptomics on the physiological adaptability of Cenococcum geophilum, an ectomycorrhizal fungus. Microorganisms 10:2039. doi: 10.3390/microorganisms10102039
Zhai, J., Wang, L., Liu, Y., Wang, C., and Mao, X. (2023). Assessing the effects of China’s three-north shelter Forest program over 40 years. Sci. Total Environ. 857:159354. doi: 10.1016/j.scitotenv.2022.159354
Zhang, T., Pang, W., Yan, T., Zhang, P., He, J., Rensing, C., et al. (2023). Metal-non-tolerant ecotypes of ectomycorrhizal fungi can protect plants from cadmium pollution. Front. Plant Sci. 14:1791. doi: 10.3389/fpls.2023.1301791
Zhang, X., Zhang, J., He, J., Li, M., Matsushita, N., Geng, Q., et al. (2024). Physiological and transcriptome responses of Pinus massoniana seedlings inoculated by various ecotypes of the ectomycorrhizal fungus Cenococcum geophilum during the early stage of drought stress. J. Fungi. 10:71. doi: 10.3390/jof10010071
Zheng, Y., Yuan, C., Matsushita, N., Lian, C., and Geng, Q. (2023). Analysis of the distribution pattern of the ectomycorrhizal fungus Cenococcum geophilum under climate change using the optimized max Ent model. Ecol. Evol. 13:e10565. doi: 10.1002/ece3.10565
Keywords: drought, environmental stress, forest, genomics, mycorrhizal symbiosis, population genetics
Citation: Wang H, Kohler A and Martin FM (2025) Biology, genetics, and ecology of the cosmopolitan ectomycorrhizal ascomycete Cenococcum geophilum. Front. Microbiol. 16:1502977. doi: 10.3389/fmicb.2025.1502977
Edited by:
Qiang-Sheng Wu, Yangtze University, ChinaReviewed by:
Yong Zhou, Agricultural University of Hebei, ChinaChun-Yan Liu, Yangtze University, China
Copyright © 2025 Wang, Kohler and Martin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huayong Wang, MTk5NjAyMDh3aHlAZ21haWwuY29t; Francis M. Martin, ZnJhbmNpcy5tYXJ0aW5AaW5yYWUuZnI=
 Huayong Wang
Huayong Wang Annegret Kohler
Annegret Kohler Francis M. Martin
Francis M. Martin