
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 19 February 2025
Sec. Virology
Volume 16 - 2025 | https://doi.org/10.3389/fmicb.2025.1502630
Background: Commercial foot-and-mouth disease (FMD) vaccines have several drawbacks, including a short duration of the immune response after vaccination and local adverse reactions at the vaccination site. Therefore, we developed a new vaccination strategy that simultaneously improves the health status of the host and stimulates systemic immunity by combining the oral administration of glycyrrhizic acid (GA) and intramuscular injection of the FMD vaccine.
Methods: We evaluated the efficacy of the oral immune enhancer GA in conjunction with an intramuscular injection of the FMD vaccine. After vaccination, the experimental (mice) and target animals (pigs) were orally administered GA daily for 4 weeks and once a week for the next 4 weeks. Subsequently, we evaluated safety using various biochemical serum assays, the efficacy of inducing immune responses using serological assays, and the expression of genes related to systemic immunity induction.
Results: Oral administration of GA in combination with an intramuscular injection of the FMD vaccine enhanced early, mid-term, and long-term immunity in experimental and target animals. We also confirmed that this co-administration increased the expression of secretory IgA (sIgA), an important indicator of mucosal immunity. Additionally, significant gene elevations in systemic immune markers along with T helper (Th) immune responses were observed.
Conclusion: These findings suggest that combining the oral administration of GA with the intramuscular injection of an inactivated FMD vaccine can induce a potent and sustained immune response and stimulate the systemic immune system by promoting sIgA and cytokine gene expression. Our research can be used to enhance the efficacy of existing commercial vaccines as well as control other animal diseases by improving the host’s immune system.
Foot-and-mouth disease (FMD) is a highly contagious veterinary disease listed on the World Organisation for Animal Health (WOAH) list of notifiable animal diseases. Since its identification as a viral disease in 1897, FMD has consistently caused a significant economic burden in endemic regions. In Africa, it leads to annual economic losses exceeding $2 billion, while even in regions or countries free from FMD, it causes losses of over $1.5 billion annually (Knight-Jones and Rushton, 2013). The global market for FMD vaccines, used to control FMD, is predicted to steadily increase, reaching over $2.4 billion by 2031 (Wubshet et al., 2024). It can occur in more than 70 species of hoofed animals, including cattle, pigs, goats, and sheep, and has a substantial impact on the economy and international trade of the countries where it occurs (Jamal and Belsham, 2013). Although FMD vaccination successfully induces antibody production and increases antibody titers, FMD virus (FMDV) outbreaks still occur. This is due to various reasons, such as the short-lived immunity of FMD vaccines and the diverse serotypes of FMDV, which contribute to the continued global incidence of FMD, resulting in substantial economic losses.
To overcome FMD, various studies related to antigen platform development have been conducted, including research on inactivated viruses, virus-like particles, peptides, and DNA and RNA vaccines (Kotla et al., 2016; Xiao et al., 2016; Cao et al., 2017). However, currently available FMD vaccines, formulated with a mixture of inactivated viral antigens and oil adjuvants, are intramuscularly administered. Although these oil-adjuvant-based commercial vaccines produce relatively stable immune responses in cattle, they exhibit low antibody titers in pigs compared with that in cattle and high variation in antibody titers between individuals (Lee et al., 2019). Moreover, the inclusion of oil adjuvants can lead to adverse reactions, such as abscesses and fibrosis at the injection site in pigs, resulting in substantial economic losses (Charerntantanakul, 2020). Most commercial vaccines primarily aim to stimulate a humoral immune response, making it challenging to effectively trigger a cellular immune response. If viral infection occurs before antibody titers are reached in the early stages of vaccination, a gap in host defense may occur. Therefore, it is necessary to improve the host’s immune system before the initial antibody formation after vaccination.
Although research on the route of FMDV infection is limited, studies have shown that FMDV initially enters pigs through the oropharynx and may undergo an incubation period in the tonsils or pharynx (Jamal and Belsham, 2013). Ideally, the most effective host defense against respiratory viruses would be to induce a local immune response that neutralizes or eliminates the virus at the site of infection, while simultaneously preventing entry of the virus from the primary site of infection (Mettelman et al., 2022).
The mucosa is present in major exposed areas, such as the oral cavity, respiratory system, digestive system, and urogenital tract, and serves as an important barrier between the internal and external environments in both humans and animals. Mucosa-associated lymphoid tissue (MALT), which regulates immune responses, is present in the mucosa. The MALT is divided into several regions, including gut-associated lymphoid tissue (GALT), bronchus-associated lymphoid tissue (BALT), and nasal-associated lymphoid tissue (NALT), each of which can trigger an immune response to antigens (Brandtzaeg, 2010). This surface is protected from invasion by foreign antigens by an efficient physical barrier consisting of a mucus and glycocalyx layer, and a chemical barrier containing antibacterial agents and secretory immunoglobulin A (sIgA) that responds to specific antigens (Nagashima et al., 2017). The mucosal surfaces possess an advanced immune system, and external stimuli can trigger mucosal immunity that targets specific pathogens. This immune response may also extend to systemic protection, generating antibodies and activating immune cell-mediated mechanisms (Kiyono and Azegami, 2015; Wang et al., 2015).
GALT is the largest lymphoid organ in the host, accounting for approximately 70% of all immune cells (Mörbe et al., 2021). The GALT consists of various lymphoid tissues, including Peyer’s patch (PP) in the small intestine, cecal patch, colonic patch, and isolated lymphoid follicles. These tissues are crucial for mounting an efficient protective immune response (Chen, 2000). Microfold cells (M cells), which are specialized epithelial cells found in the follicle-associated epithelium, are crucial for antigen uptake in GALT and NALT. They play a key role in monitoring immunosurveillance and immunoregulation at mucosal surfaces. M cells express cell surface receptors known as transcytosis, which are responsible for recognizing luminal antigens, actively engulfing these antigens at the apical surface, and exporting them out of the cell through the basolateral plasma membrane (Dillon and Lo, 2019). Additionally, PP constitutively generates germinal centers (GCs) in response to the uptake of microbiome- and food-derived antigens taken up by M cells. The development of GCs is essential for the somatic affinity maturation of B cells that have undergone IgA class switching within PP. Therefore, M cells play a vital role in the regulation and development of sIgA responses (Suzuki et al., 2010; Reboldi and Cyster, 2016; Kobayashi et al., 2019). Immune responses initiated by M cells can induce a diverse array of T helper cells, including Th1, Th2, Th17, and regulatory T cells (Tregs), whereas dendritic cells (DCs) present in PP direct immune responses to specific tissues. To achieve this, T and B cells can imprint homing properties, and B cells can directly control the intestinal microflora using sIgA (Li M. et al., 2020). sIgA is the front-line defense against antigens and pathogens that invade the mucosal surface. It is produced locally, secreted in large quantities, and can neutralize pathogens. Given that pathogens enter the body through the mucosal surface, they play a key role in host defense by inducing protection at the first site of contact between the host and pathogen (Pietrzak et al., 2020).
Glycyrrhizic acid (GA) is a triterpenoid saponin extracted from licorice roots, that has various pharmacological effects. Additionally, GA is non-toxic when ingested and slowly decomposes within the intestines (Wahab et al., 2021). Our previous study showed that a test vaccine with the novel adjuvant GA significantly increased both cellular and humoral immunity compared to those with a commercially available vaccine. Additionally, we confirmed the elevation of several mucosal immunity-related cytokines using qRT-PCR, although the vaccine was intramuscularly administered (Shin et al., 2023).
We aimed to address and overcome the limitations of currently available FMD vaccines by combining intramuscular (I.M.) FMD vaccination with oral administration of the immune-enhancer candidate, GA. We evaluated the efficacy of GA in stimulating mucosal and systemic immunity, and its role as an oral immune enhancer that can be used in combination with a viral vaccine.
Glycyrrhizic acid ammonium salt [from glycyrrhiza roots (licorice)] was purchased from Sigma-Aldrich (St. Louis, MO, United States).
Baby hamster kidney (BHK)-21 (ATCC, Manassas, VA, United States) and fetal porcine kidney (LF-BK, Plum Island Animal Disease Center, Oriten, NY, United States) cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; HyClone, Logan, UT, United States) supplemented with 5% fetal bovine serum (FBS) and 1% antibiotic-antimycotic (A/A; Gibco, Grand Island, NY, United States) at 37°C in 5% CO2. The fetal goat tongue epithelium (ZZ-R 127, Friedrich-Loeffler-Institut, Greifswald-Insel Riems, Germany) cell was cultured in DMEM F12 (HyClone) containing 5% FBS and 1% A/A (Gibco) at 37°C in 5% CO2.
Purified FMDV serotype O (O/PanAsia2, O PA2) and FMDV serotype A (A/SKR/YC/2017, A YC) antigens were prepared by transfecting BHK-21 cells in DMEM containing 1% A/A (Gibco). The virus was inactivated by treating with 0.003 N binary ethyleneimine (BEI) twice for 24 h. The inactivated virus was precipitated using polyethylene glycol 6000 (Sigma-Aldrich). Antigen (146S) was purified through a sucrose density gradient (15–45%), followed by ultracentrifugation at 30,000 rpm for 4 h at 4°C (SW 41Ti, Beckman Coulter, Fullerton, CA, United States) (Lee et al., 2022).
The test vaccine composition administered to mice was as follows: FMDV O PA2+A YC antigen (0.375 + 0.375 μg/dose/100 μL; 1/40 of the pig dose), ISA 206 [(50% w/w); Seppic, Paris, France)], 10% Al(OH)3 and 15 μg/dose Quil-A (InvivoGen, San Diego, CA, United States) in a total volume of 100 μL. The test vaccine composition administered to pigs was as follows: FMDV O PA2+A YC antigen (15 + 15 μg/dose/mL), ISA 206 [(50% w/w); Seppic], 10% Al(OH)3 and 150 μg/dose Quil-A (InvivoGen) in a total volume of 1 mL.
Mice (6–7 weeks old) were divided into three groups (n = 5/group): Negative Control (NC), PBS administration; the Positive Control (PC), an I.M injection of the test vaccine; and experimental (Exp) group, I.M. injection of the test vaccine and oral administration of 100 μg of GA. Pigs (8–9 weeks old) were divided into three groups (n = 5–6/group) in the same manner as the experimental mice; the Exp group was orally administered 20 mg/4 mL of GA solution. The GA solution was administered to the mice and pigs via a pipette and zonde (feeding needles), respectively. Following vaccination, GA was administered once daily until 28 days post-vaccination (dpv), and once weekly at the same time each day over a period of 4 weeks. The NC and PC groups were administered equal volumes of PBS instead of GA solution. Mice were intramuscularly injected once into the thigh with 100 μL of the test vaccine (PC and Exp) or PBS (NC). Pigs were intramuscularly injected twice into the neck with 1 mL of the test vaccine (PC and Exp) or PBS (NC) at 28 days intervals. The NC group was administered an equal volume of PBS via the same route. During the experiment, food intake was measured at scheduled times each day and body weight was recorded weekly. Food efficiency ratio (FER) was calculated using the following equation:
To determine whether the oral administration of GA affects the health (safety) of the target animals, liver and kidney function tests were performed using biochemical assays. The serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) blood urea nitrogen (BUN), creatinine (CREA), lactate dehydrogenase (LDH), total protein (TP), albumin (ALB), and albumin/globulin (A/G) ratio were assessed using HITACHI Automatic Analyzer 3100 (Hitachi High-Tech Corporation, Tokyo, Japan) with test reagents from KLS Bio-Inc. (Gyeonggi-do, Republic of Korea).
To evaluate the efficacy of GA in triggering mucosal immune responses in mice, blood samples were harvested from NC, PC, and Exp mice groups at 0, 7, 14, 21 (early), 28, 35, 42 (mid-term), 56, 70, and 84 (long-term) dpv. Blood samples were collected via retro-orbital bleeding. The serum samples were used to evaluate the antibody titer using structural protein (SP) O, A enzyme-linked immunosorbent assay (ELISA), virus neutralization (VN) titer using a VN test, and sIgA titer using immunoglobulin ELISA. The serum samples were stored at −80°C until further use.
To evaluate the protective effect of co-administration of I.M. FMD vaccination with oral GA administration, we performed challenge experiments in mice. Mice were challenged with FMDV (100 LD50 of O/VET/2013) at 84 dpv via intraperitoneal (I.P.) injection. Survival rates and changes in body weight of mice were monitored for up to 7 days post-challenge (dpc).
To evaluate the efficacy of GA in inducing mucosal immune responses in pigs, blood samples were collected at 0, 7, 14, 21 (early), 28, 35, 42 (mid-term), and 56, 70, and 84 (long-term) dpv. The obtained sera were used to evaluate the SP O, A antibody, VN, and sIgA titers. The serum samples were stored at −80°C until further use.
To assess the SP antibody levels in the serum samples, ELISA kits specific for FMDV types O and A (PrioCheck™, Prionics AG, Schlieren, Switzerland) were used, following the manufacturer’s instructions. Spectrophotometric readings were obtained at 450 nm by using a spectrophotometer (Hidex, Turku, Finland), and the data were converted to percent inhibition (PI) values. Animals were classified as antibody-seropositive if the PI value of the PrioCheck™ FMDV kit exceeded 50%.
Virus neutralization tests were performed according to WOAH guidelines. Sera from animals were heat-inactivated for 30 min at 56°C. The inactivated sera were then serially diluted 2-fold (from 1:8 to 1:1024). These diluents were exposed to FMDV type O PA2 or A YC at 100 TCID50/0.5 mL and incubated for 1 h at 37°C. After incubation, LF-BK cells were treated to all the wells. Cytopathic effects (CPE) were observed after 72 h and titers were calculated and recorded as Log10 of the reciprocal of the maximum serum dilution required to neutralize 100 TCID50 of the virus.
Serum levels of murine and porcine sIgA were assessed using commercial ELISA kits (CSB-E08413m, CSB-E12063p; Cusabio Inc, Wuhan, China), according to the manufacturer’s protocol. Spectrophotometric readings were obtained at 450 nm by using a spectrophotometer (Hidex) (Lee et al., 2022).
To elucidate the mechanisms of immune responses triggered by the stimulation of the intestinal mucosa through oral administration of GA, experiments were performed according to previously described protocols (Lee et al., 2022). Porcine PBMCs were extracted from whole blood specimens obtained from pigs (n = 5–6/group) used in the experiment at 14 dpv. Briefly, whole blood was collected in heparin-coated tubes (BD, Franklin Lakes, NJ, United States). Porcine peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using Lymphoprep (Stem Cell Technologies, Vancouver, BC, Canada). Remaining red blood cells were removed using ammonium–chloride–potassium (ACK) lysing buffer (Gibco). RNA extraction from the purified PBMCs was carried out using TRIzol reagent (Invitrogen, Carlsbad, CA, United States), followed by reverse transcription into cDNA using M-MLV RT (Promega, Madison, WI, United States). The cDNA was amplified on a CFX96 Touch Real-Time PCR system (Bio-Rad, Hercules, CA, USA) using iQ SYBR Green Supermix (Bio-Rad). Gene expression levels were quantified using the delta Ct (ΔCt) method, normalized to the housekeeping gene HPRT, and presented relative to the control values. The primers used in these assays are listed in Supplementary Table 1.
Quantitative data were presented as the mean ± SEM, unless otherwise specified. Differences between groups were evaluated using either two- or one-way analysis of variance, followed by Tukey’s or Dunnett’s post-hoc analysis. Statistical significance was indicated as *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. Parametric tests were employed to compare the various groups. Survival curves were constructed using the Kaplan–Meier technique, and the long-rank test was applied to evaluate differences between groups. All statistical analyses were performed using Prism 10.0.2 (GraphPad, San Diego, CA, United States).
To confirm the safety of the oral administration of GA, FER was measured using body weight (BW) and food intake changes. No significant changes were observed in BW or FER among the three groups (Supplementary Tables 2, 3).
To investigate the effect of oral administration of GA on adaptive immune enhancement in vaccinated mice, the early, mid-term, and long-term immunity of the mice were evaluated (Figure 1A).
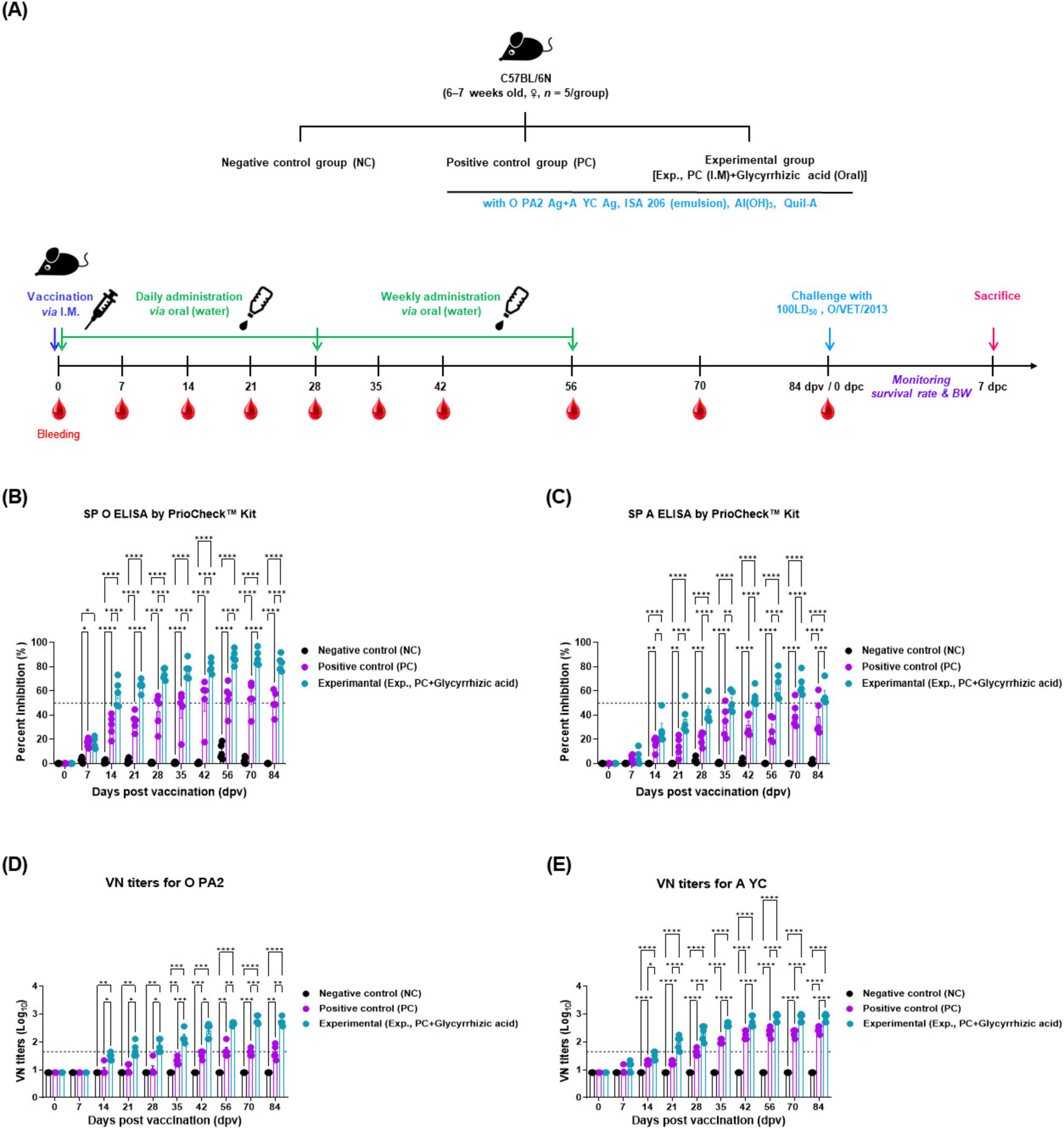
Figure 1. Oral administration of glycyrrhizic acid mediates early, and mid-term, and long-term immune responses in food-and-mouth disease (FMD)-vaccinated mice. Experiments were performed according to the mice experimental strategies described in 2.5, 2.6, 2.9, (see section “2 Materials and methods”) and Figure 1 panel (A). (A–E) Experimental strategy (A); SP O antibody titers (PrioCheck™ FMDV kit) (B); SP A antibody titers (PrioCheck™ FMDV kit) (C); VN titers for O PA2 (D); and VN titers for A YC (E). The horizontal dashed lines in panels (B,C) represent the manufacturer-specified threshold for a positive result. In panels (D,E), the dashed lines indicate a VN titer of 1:45 (equivalent to 1.65 on a Log10 scale), which is considered the minimum level required for protection against viral infection. Data are represented as the mean ± SEM of triplicate measurements (n = 5/group). Statistical analyses were performed using two-way ANOVA, followed by Tukey’s post-hoc test. *p < 0.05; **p < 0.01; ***p < 0.001; and ****p < 0.0001.
The antibody titers measured using SP O and A ELISA were significantly higher in Exp group from 14 to 84 dpv compared with that in the PC group. By 84 dpv, several mice in the PC group failed to maintain positive antibody titers and converted to negative titers. In contrast, all individuals in Exp group maintained positive antibody titers at 84 dpv. The NC group did not show any changes in antibody titers (Figures 1B, C). The animals were considered antibody-positive when the PI value was ≥ 50% for the PrioCheck™ FMDV kit.
The VN titers for O PA2 and A YC, as measured by the VN test, also significantly increased in Exp group, which received oral GA alongside the vaccine from 14 to 84 dpv, with significantly less individual variability than the PC group. The NC group did not show any changes in the VN titers (Figures 1D, E). VN titers with a Log10 value greater than or equal to 1.65 are considered capable of protecting the host from FMDV infection.
To investigate the co-administration of GA and FMD vaccine-mediated host protection against viral infections, we conducted a study as depicted in Figure 1A. The Exp group, which was orally administered GA along with vaccination, showed a 100% survival rate. The PC and NC groups showed 40 and 0% survival rates, respectively (Figure 2A). There was no change in body weight in Exp group; however, the PC and NC groups lost more than 10 and 20% of their body weight, respectively (Figure 2B).
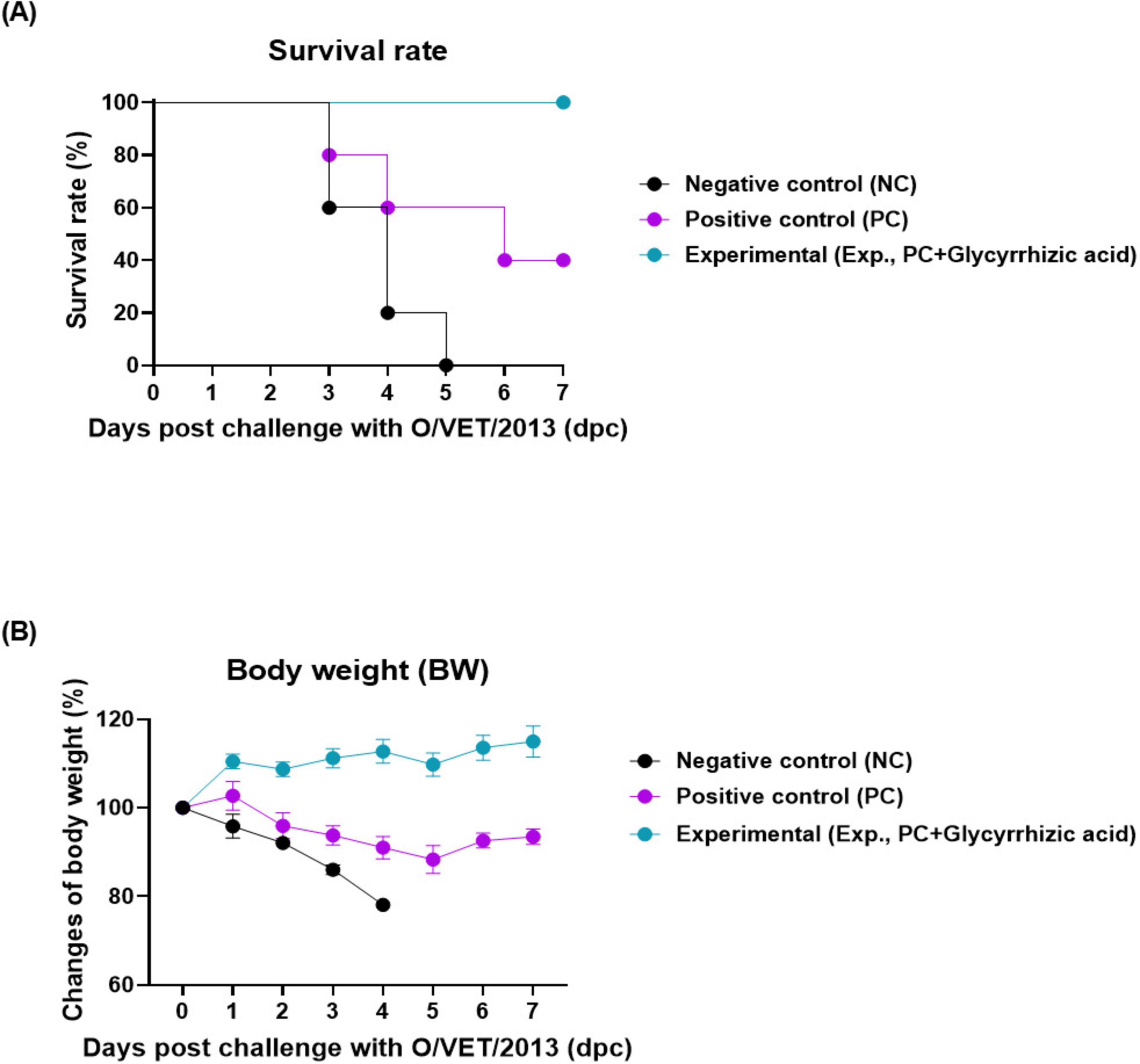
Figure 2. Oral administration of glycyrrhizic acid improves host protection against the virus in food-and-mouth disease (FMD)-vaccinated mice. Experiments were performed according to the mice experimental strategies described in 2.5, 2.7 (see section “2 Materials and methods”) and Figure 1 panel (A). (A,B) Survival rates post-challenge with O/VET/2013 (A); Changes in body weight post-challenge with O/VET/2013 (B). Data are presented as mean ± SEM of triplicate measurements (n = 5/group).
To confirm the safety of oral administration of GA, serum levels of ALT, AST, BUN, CREA, LDH, TP, and ALB and the A/G ratio were measured. Blood biochemical indices were within the normal range, and AST levels in the NC group were significantly higher than those in the Exp group. Except for AST, no significant differences were observed among the Exp, PC, and NC groups (Table 1).
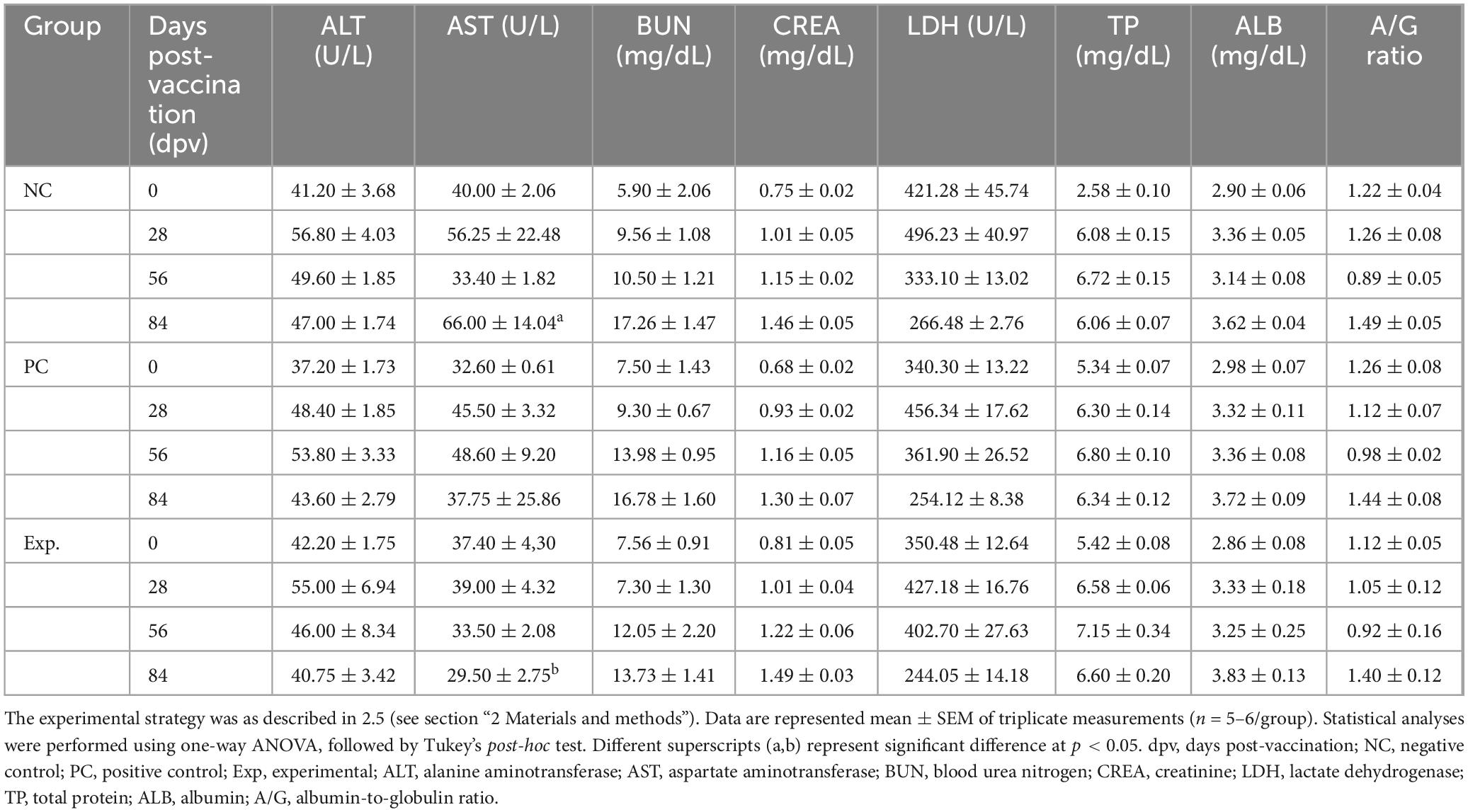
Table 1. Biochemistry assays in serum of pigs treated with glycyrrhizic acid by oral administration for 84 days post-vaccination.
To assess the effect of the oral administration of GA on vaccination-mediated humoral immunity in pigs, we monitored the early, mid-term, and long-term immune responses in pigs (Figure 3A). When the FMD test vaccine was intramuscularly injected and GA orally administered to the pigs, the antibody titers measured using SP O and A ELISA significantly increased in Exp group from 7 (SP A ELSA) or 14 (SP O ELISA) dpv to 84 dpv, compared with those in the PC group. Antibody titers in the NC group did not show any differences (Figures 3B, C). The animals were considered antibody-positive when the PI value was ≥ 50% for the PrioCheck™ FMDV kit.
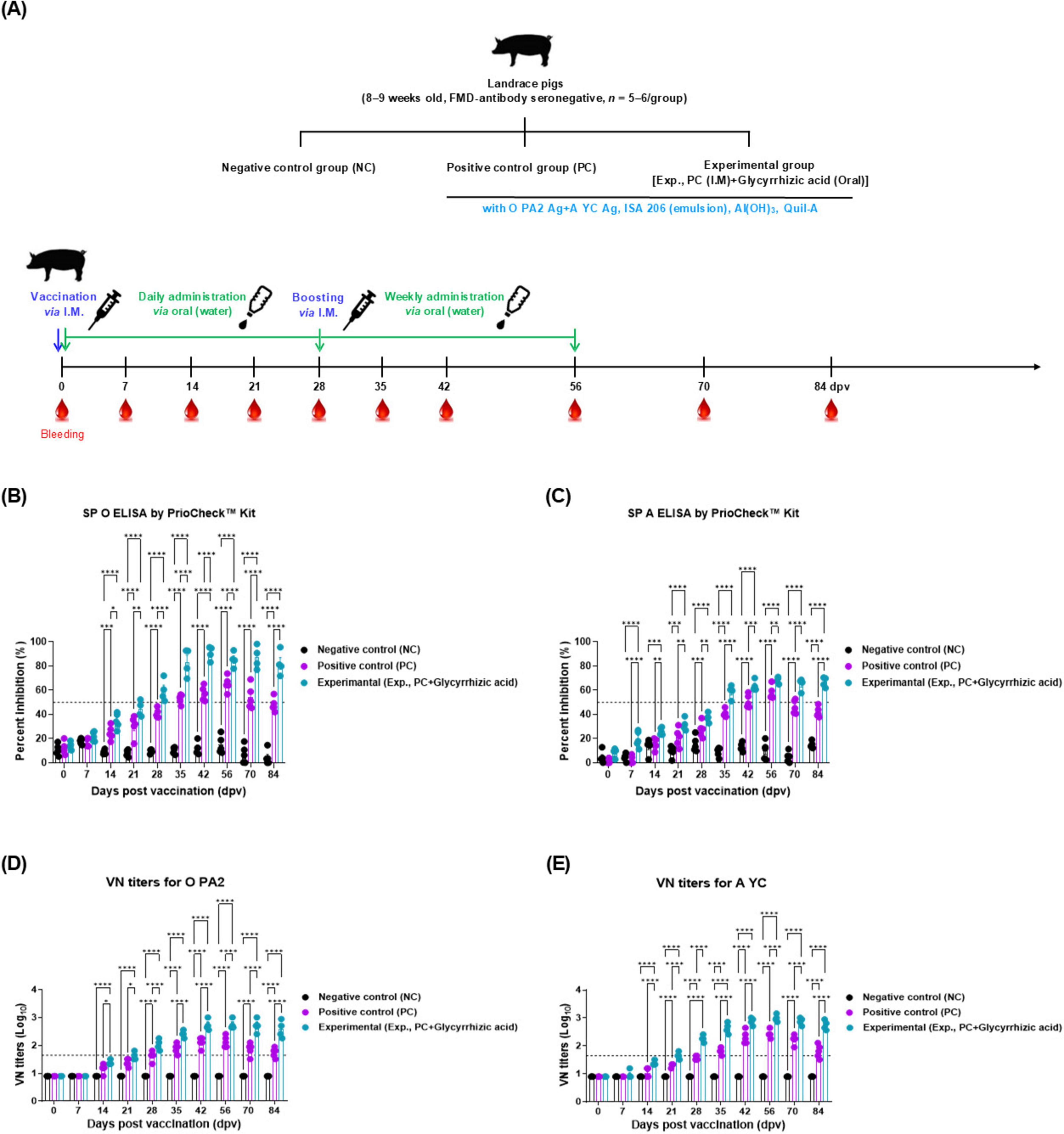
Figure 3. Oral administration of glycyrrhizic acid mediates early, and mid-term, and long-term immune responses in food-and-mouth disease (FMD)-vaccinated pigs. Experiments were performed according to the pigs experimental strategies described in 2.5, 2.8, 2.9, (see section “2 Materials and methods”) and Figure 3 panel (A). (A–E) Experimental strategy (A); structural protein (SP) O antibody titers (PrioCheck™ FMDV kit) (B); SP A antibody titers (PrioCheck™ FMDV kit) (C); virus neutralization (VN) titers for O PA2 (D); and VN titers for A YC (E). The horizontal dashed lines in panels (B,C) represent the manufacturer-specified threshold for a positive result. In panels (D,E), the horizontal dashed lines denote the VN titer of 1:45 (equivalent to 1.65 on a Log10 scale), which is considered the minimum level required for protection against viral infection. Data are represented as the mean ± SEM of triplicate measurements (n = 5/group). Statistical analyses were performed using two-way ANOVA, followed by Tukey’s post-hoc test. *p < 0.05; **p < 0.01; ***p < 0.001; and ****p < 0.0001.
The VN titers for O PA2 and A YC also significantly increased from 14 to 84 dpv in Exp group after the oral administration of GA compared with that in the PC group. From 56 dpv, the VN titers of the PC group decreased to 84 dpv, whereas those of Exp group maintained a high VN titer. VN titers in the NC group did not change (Figures 3D, E). VN titers with a Log10 value greater than or equal to 1.65 are considered capable of protecting the host from FMDV infection.
To evaluate the mucosal immunity induced by the oral administration of GA, we measured sIgA levels in serum derived from mice and pigs. The level of sIgA significantly increased at 28 and 56 dpv in Exp group compared with that in the PC and NC groups in mice and pigs (Figures 4A, B).
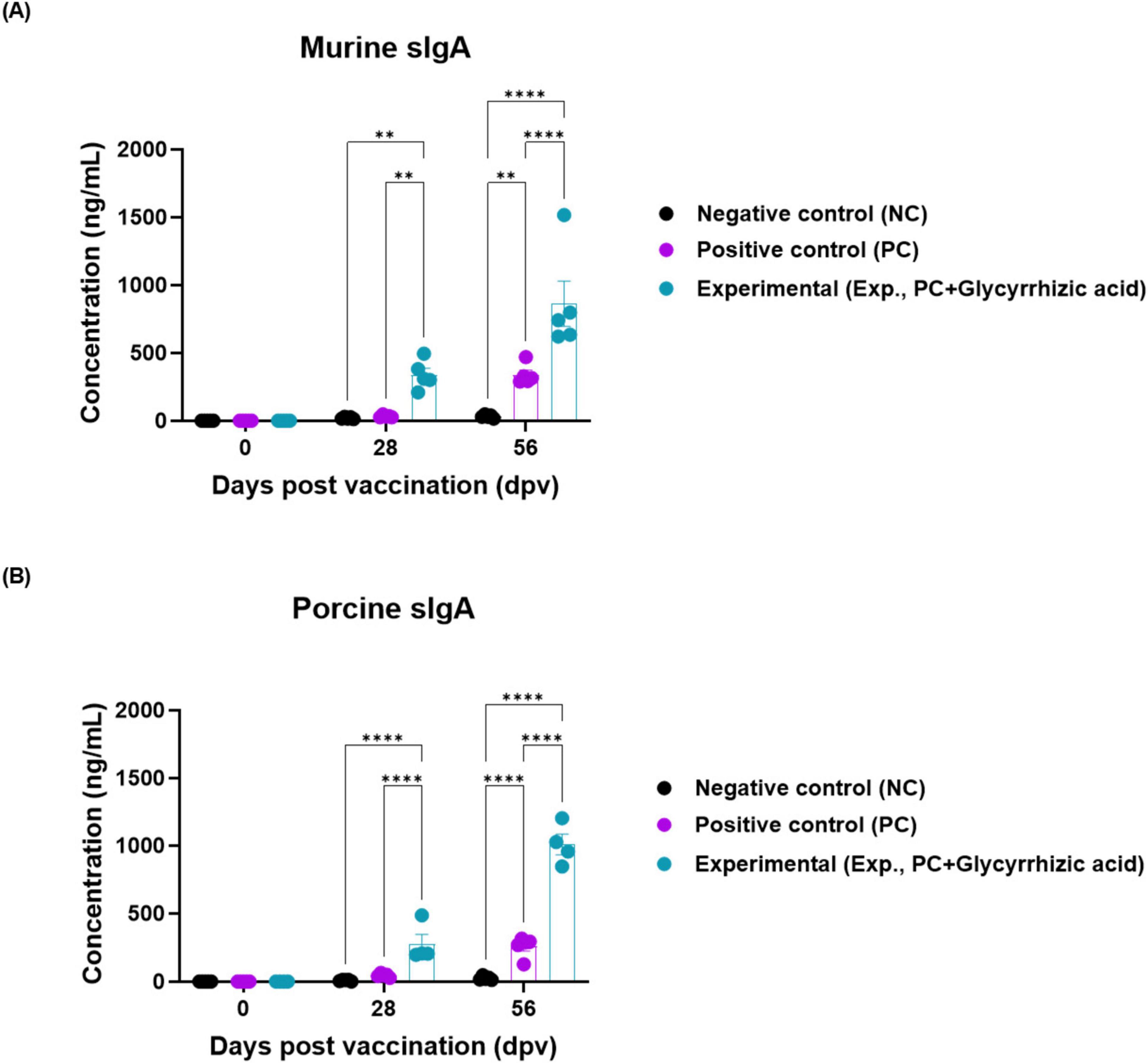
Figure 4. Oral administration of glycyrrhizic acid increases the expression of secretory IgA. Experiments were performed according to the food-and-mouth disease (FMD)-vaccinated mice and pigs experimental strategies described in 2.5, 2.6, 2.8, 2.9, (see section “2 Materials and methods”), Figures 1, 3 panel (A). The concentration of murine and porcine secretory IgA (sIgA) were assessed in serum using a secretory IgA ELISA kit. (A,B) sIgA antibody titers in mice (Cusabio secretory IgA ELISA kit) (A); sIgA antibody titers in pigs (Cusabio secretory IgA ELISA kit) (B). Statistical analyses were performed using two-way ANOVA, followed by Tukey’s post-hoc test. **p < 0.01; and ****p < 0.0001.
To elucidate the GA-mediated systemic immunity, cytokine gene expression was measured by qRT-PCR in porcine PBMCs, as shown in Figure 3A. The level of cytokines, such as IL-2, IL-4, IL-12p40, IL-17A, IL-18, IL-23p19, IL-23R, and IFNγ in the serum of the Exp group was significantly higher than that in the PC group at 14 dpv (Figures 5A–H).
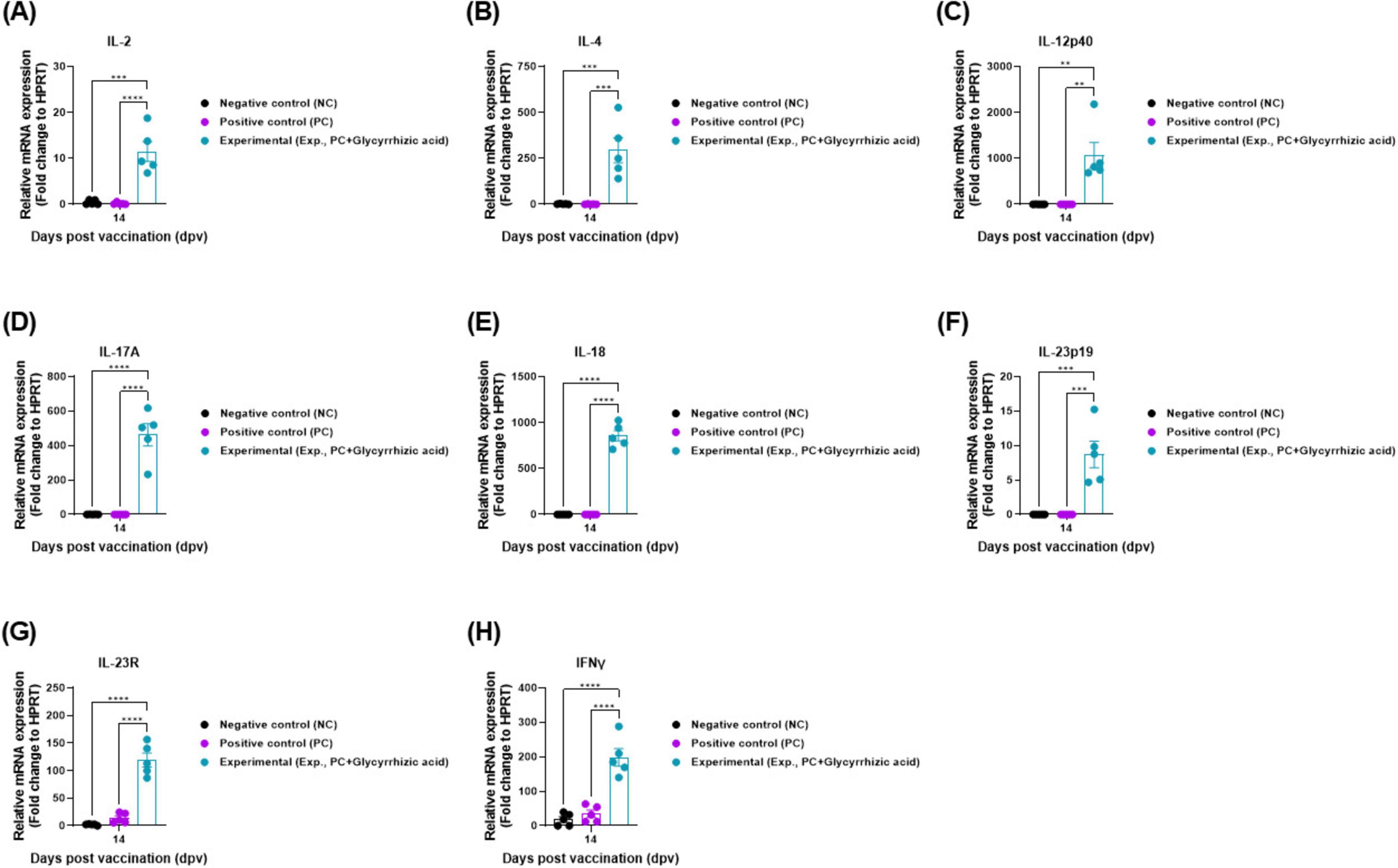
Figure 5. Oral administration of glycyrrhizic acid induces the gene expression of systemic immune-related cytokines in food-and-mouth disease (FMD)-vaccinated pigs. Experiments were performed according to the pigs experimental strategies described in 2.5, 2.8, 2.10, (see section “2 Materials and methods”) and Figure 3 panel (A). (A–H) Gene expression levels of IL-2 (A); IL-4 (B), IL-12p40 (C); IL-17A (D); IL-18 (E); IL-23p19 (F); IL-23R (G); and IFNγ (H). Statistical analyses were performed using two-way ANOVA, followed by Tukey’s post-hoc test. *p < 0.05; **p < 0.01; ***p < 0.001; and ****p < 0.0001.
The infection and spread of FMD are influenced by the interactions between the pathogen, host, and environment (Jones et al., 2008). Vaccination is widely known to be an effective preventive tool against pathogenic infections. However, to prevent infection and transmission, the health status and the innate immune system of the host must also be considered. The weakened immune status of the host and rapid changes in the environment can create new transmission patterns, allowing pathogens to invade the host, making it more susceptible to infection. To overcome this, it is essential to improve both individual and collective health, thereby preventing the spread of pathogens and controlling group infections (Engering et al., 2013).
GA, the most important active component of licorice, is known for its immunomodulatory, hepatoprotective, and pharmacological effects (Celik et al., 2012; Pastorino et al., 2018). Recent studies have indicated its potential as a drug stabilizer and anticancer agent. Owing to its hydrophilic and lipophilic properties, GA can interact with cell membranes, facilitating the intracellular entry of other drugs, making it a potential drug delivery system (Hogstrand, 2015; Zhang et al., 2023). Moreover, GA has been deemed safe for human and animal consumption at established safe dosage levels, and is a flavoring substance, allowing its unrestricted use in food products and making it suitable for research purposes (Van Gelderen et al., 2000; Selyutina and Polyakov, 2019). Therefore, we investigated whether oral GA administration in conjunction with I.M. FMD vaccination could stimulate the mucosal and systemic immune systems in the host, thereby enhancing and prolonging the efficacy of the vaccine.
We evaluated adaptive immunity to determine whether the oral administration of GA could enhance the effectiveness of an inactivated FMD vaccine by inducing mucosal immune responses in mice. There was no significant difference in the FER of mice in the Exp, PC, and NC groups, as evaluated by measuring body weight and food intake. Therefore, oral administration of GA did not have a negative effect on food intake and growth (weight gain) of the experimental animals (Supplementary Tables 1, 2). The combination of oral GA administration and I.M. FMD vaccination significantly increased SP antibody and VN titers in Exp group with less individual variation (Figure 1). Co-administration of oral GA and I.M. FMD vaccine elicited host protection against FMDV infection in mice (Figure 2). This study demonstrated that the oral administration of GA stimulated the mucosal immune system, enhanced individual immunity, and improved the efficacy of the FMD vaccine.
Subsequently, we performed follow-up experiments to evaluate the effects of oral GA administration on the adaptive immune response in pigs and determine whether it activated intestinal mucosal immunity. Biochemical analyses were performed on serum samples collected at 0, 28, 56, and 84 dpv to evaluate the safety of GA in pigs. No significant differences were found in the levels of all parameters except for AST (Table 1). AST is released into the blood when liver or muscle damage occurs and its blood concentration increases (Lala et al., 2023). These results confirmed that the oral GA administration dose used in our experiments did not have any negative effects on pigs.
The antibody and VN titers were higher in Exp group than in the PC group from the early stages, with significant differences observed from 35 to 84 dpv (Figure 3). Levels of sIgA, a marker of mucosal immunity, were also higher in the Exp group than that in the PC group (Figure 4). These results revealed that oral GA administration effectively induces adaptive immune responses through simultaneous induction of mucosal and systemic immune responses.
To define the basic mechanism of systemic immune response induced by oral GA administration, IL2, IL-4, IL-12p40, IL-18, IL-17A, IL-23p19, IL-23R, and IFNγ gene expression levels were quantified. Significantly higher gene expression was detected in Exp group than in the PC group, indicating that oral administration of GA can trigger a potent systemic immune response (Figure 5).
GALT, which is responsible for major immune responses in the gut, includes PP. PP are located in the subepithelial dome region and contain myeloid DCs that induce Th2 cell differentiation, lymphoid DCs that induce Th1 cell differentiation, and double-negative DCs. Myeloid DCs also mediate the differentiation of Th3 and Tregs after exposure to food antigens (Banchereau et al., 2000; Iwasaki and Kelsall, 2000, 2001). Moreover, M cells in PP selectively take up antigens from the external environment, transport them across the epithelial barrier, and deliver them directly to subepithelial DCs, which then present antigens locally to adjacent mucosal T-cell areas (Neutra and Kozlowski, 2006). Mucosal T cells that receive antigen presentation express IL-12, a key cytokine that initiates Th1 cell differentiation, and IL-4, which induces Th2 differentiation through STAT6 (Ruterbusch et al., 2020; Ma et al., 2021). Subsequently, differentiated Th1 cells express IFNγ and IL-2, whereas Th2 cells express IL-4 and IL-10 (Zhou et al., 2021). Previous studies have shown that IL-4 and IL-10 synergistically induce substantial changes in the IgA isotype production. Additionally, these cytokines promote the class switching of B cells to IgA and their differentiation into sIgA-producing cells (Asano et al., 2004; Li Y. et al., 2020). Therefore, IL-4 and IL-10 can be considered as critical cytokines in the generation of sIgA.
Notably, oral GA administration can activate mucosal-associated invariant T (MAIT) cells which are crucial in the initial defense against pathogenic bacteria and yeast on mucosal surfaces and play an important role in maintaining mucosal barrier homeostasis (Nel et al., 2021). Previous studies have reported that MAIT cells are found in the oral mucosa and respiratory tract and can be activated by antigen-presenting cells (APCs), such as DCs, macrophages, and monocytes (Reantragoon et al., 2013; Chen et al., 2017; Sobkowiak et al., 2019). MAIT cell activation is restricted by MR1 on APCs and triggered by microbial non-peptide ligands (Meierovics et al., 2013). Upon antigen invasion, activated APCs produce cytokines, such as IL-12 and IL-18, and the activated MAIT cells, can produce pro-inflammatory cytokines, such as IFNγ, TNFα, while MAIT cells, once activated, release pro-inflammatory cytokines such as IL-18R, IL-12R, and IL-23R, and MR1, thereby supporting local immune responses and directly stimulating innate immunity (Ussher et al., 2014). Notably, IL-17A, in conjunction with IL-23, is an important cytokine in the formation of neutrophil extracellular traps (NETs). NETs consist of extracellular DNA associated with antimicrobial proteins derived from neutrophil granules and nuclei, and capture various microbes, providing a crucial innate immune mechanism (Brinkmann et al., 2004). Therefore, NET plays a crucial role in preventing viral invasion into the host at mucosal sites.
The novel vaccination program combining oral GA administration and I.M. FMD vaccination proposed in this study demonstrated that multifaceted immune response enhancement can improve protection against FMDV. Specifically, the program increased SP antibodies and VN titers in both experimental and target animals, and enhanced the ability to rapidly capture viruses penetrating the mucosal barrier via NETs, thereby reducing the risk of host infection. Additional benefits of GA, such as economic feasibility, accessibility, and effectiveness in improving pork quality, further enhance the practicality of this program (Bazekin et al., 2023). In particular, the fact that GA can be used as a feed additive or a component of bait vaccines may increase the efficiency of disease prevention and control.
Building on the previous study that demonstrated the potential of GA as an adjuvant for I.M. vaccination, this study confirmed the efficacy of GA as an oral adjuvant (Shin et al., 2023). However, there are some limitations to this study, including the inability to observe the immune response at mucosal sites and the primary focus on humoral immunity within the adaptive immune response. Furthermore, while serum sIgA and mucosal sIgA differ significantly in their functions and roles, the increase in mucosal immune-related mRNA levels and the observed increase in serum sIgA expression are notable. Activation of the mucosal immune system may impact not only local immune responses but also systemic immunity. The increase in serum sIgA could be an indirect result of mucosal immune system activation, suggesting that plasma cells generated in the GALT may have migrated into the systemic circulation (Keppler et al., 2021).
Therefore, our hypothesis that oral administration of GA enhanced the efficacy of FMD vaccine is based on these evidences of complex immune responses. This suggests that mucosal immune stimulation may lead to enhancement of not only local defense but also systemic immune responses, which may have enhanced the overall defense capability against FMDV.
However, this interpretation requires further experimental validation. Future studies are planned to address these limitations. Specifically, we will directly observe the immune response at mucosal sites, with a particular focus on sIgA expression. Additionally, we plan to more precisely assess the efficacy of oral GA administration by using a vaccine with a reduced antigen dose or fewer vaccinations. We also aim to evaluate the preventive effects of GA as a livestock feed additive against various veterinary diseases. Through this comprehensive approach, this study demonstrates that a novel vaccination program utilizing GA could serve as an effective and practical strategy for protection against FMD, confirming its potential as an oral adjuvant for future oral FMD vaccines.
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding author.
C57BL/6 female mice (6–7 weeks old) were supplied by KOSA BIO Inc. (Gyeonggi-do, Republic of Korea). Landrace pigs (8–9 weeks old) were obtained from BARON BIO, Inc. (Gyeongsangbuk-do, Republic of Korea). All pigs used in the experiments were confirmed to be seronegative for antibodies against FMDV types O and A. During the investigation, all animals were housed in a dedicated Specific Pathogen-Free Animal Biosafety Level 3 facility of the Animal and Plant Quarantine Agency (APQA) and acclimatized for at least a week acclimatization period before the start of the experiments (Lee et al., 2022). Approval for the study and related experimental protocols was granted by the APQA Ethics Committee (IACUC 2023-719, 2024-863 and 2024-881).
SS: Investigation, Visualization, Writing – original draft. HWK: Formal analysis, Investigation, Software, Validation, Visualization, Writing – original draft, Writing – review and editing. M-KK: Investigation, Writing – original draft. SHP: Investigation, Writing – original draft. J-HP: Resources, Writing – review and editing. S-MK: Resources, Writing – review and editing. MJL: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the Animal and Plant Quarantine Agency (APQA) (B-1543386-2022-24 and M-1543386-2024-25).
We would like to thank the staff and researchers of the Center for Foot-and-Mouth Disease Vaccine Research at APQA for helping us with this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1502630/full#supplementary-material
Asano, T., Kaneko, H., Terada, T., Kasahara, Y., Fukao, T., Kasahara, K., et al. (2004). Molecular analysis of B-cell differentiation in selective or partial IgA deficiency. Clin. Exp. Immunol. 136, 284–290. doi: 10.1111/j.1365-2249.2004.02440.x
Banchereau, J., Briere, F., Caux, C., Davoust, J., Lebecque, S., Liu, Y.-J., et al. (2000). Immunobiology of dendritic cells. Annu. Rev. Immunol. 18, 767–811. doi: 10.1146/annurev.immunol.18.1.767
Bazekin, G., Gatiyatullin, I., Skovorodin, E., Chudov, I., and Ezhkov, V. (2023). Improving meat qualities of pigs after deworming against ascariasis with administered glycyrrhizic acid. J. Environ. Sci. Health B 58, 239–246. doi: 10.1080/03601234.2023.2186669
Brandtzaeg, P. (2010). Function of mucosa-associated lymphoid tissue in antibody formation. Immunol. Invest. 39, 303–355. doi: 10.3109/08820131003680369
Brinkmann, V., Reichard, U., Goosmann, C., Fauler, B., Uhlemann, Y., Weiss, D. S., et al. (2004). Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535. doi: 10.1126/science.1092385
Cao, Y., Li, D., Fu, Y., Bai, Q., Chen, Y., Bai, X., et al. (2017). Rational design and efficacy of a multi-epitope recombinant protein vaccine against foot-and-mouth disease virus serotype A in pigs. Antiviral Res. 140, 133–141. doi: 10.1016/j.antiviral.2017.01.023
Celik, M., Karakus, A., Zeren, C., Demir, M., Bayarogullari, H., Duru, M., et al. (2012). Licorice induced hypokalemia, edema, and thrombocytopenia. Hum. Exp. Toxicol. 31, 1295–1298. doi: 10.1177/0960327112446843
Charerntantanakul, W. (2020). Adjuvants for swine vaccines: Mechanisms of actions and adjuvant effects. Vaccine 38, 6659–6681. doi: 10.1016/j.vaccine.2020.08.054
Chen, H. (2000). Recent advances in mucosal vaccine development. J. Control Release 67, 117–128. doi: 10.1016/s0168-3659(00)00199-1
Chen, Z., Wang, H., D’souza, C., Sun, S., Kostenko, L., Eckle, S. B., et al. (2017). Mucosal-associated invariant T-cell activation and accumulation after in vivo infection depends on microbial riboflavin synthesis and co-stimulatory signals. Mucosal Immunol. 10, 58–68. doi: 10.1038/mi.2016.39
Dillon, A., and Lo, D. D. (2019). M cells: Intelligent engineering of mucosal immune surveillance. Front. Immunol. 10:1499. doi: 10.3389/fimmu.2019.01499
Engering, A., Hogerwerf, L., and Slingenbergh, J. (2013). Pathogen-host-environment interplay and disease emergence. Emerg. Microbes Infect. 2:e5. doi: 10.1038/emi.2013.5
Hogstrand, C. (2015). Scientific Opinion on the safety and efficacy of glycyrrhizic acid ammoniated (chemical group 30, miscellaneous substances) when used as a flavouring for all animal species. EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed). doi: 10.2903/j.efsa.2015.3971
Iwasaki, A., and Kelsall, B. L. (2000). Localization of distinct Peyer’s patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3α, MIP-3β, and secondary lymphoid organ chemokine. J. Exp. Med. 191, 1381–1394. doi: 10.1084/jem.191.8.1381
Iwasaki, A., and Kelsall, B. L. (2001). Unique functions of CD11b+, CD8α+, and double-negative Peyer’s patch dendritic cells. J. Immunol. 166, 4884–4890. doi: 10.4049/jimmunol.166.8.4884
Jamal, S. M., and Belsham, G. J. (2013). Foot-and-mouth disease: Past, present and future. Vet. Res. 44, 1–14. doi: 10.1186/1297-9716-44-116
Jones, K. E., Patel, N. G., Levy, M. A., Storeygard, A., Balk, D., Gittleman, J. L., et al. (2008). Global trends in emerging infectious diseases. Nature 451, 990–993. doi: 10.1038/nature06536
Keppler, S. J., Goess, M. C., and Heinze, J. M. (2021). The wanderings of gut-derived IgA plasma cells: Impact on systemic immune responses. Front. Immunol. 12:670290. doi: 10.3389/fimmu.2021.670290
Kiyono, H., and Azegami, T. (2015). The mucosal immune system: From dentistry to vaccine development. Proc. Jpn. Acad. Ser. B. Phys. Biol. Sci. 91, 423–439. doi: 10.2183/pjab.91.423
Knight-Jones, T. J., and Rushton, J. (2013). The economic impacts of foot and mouth disease–What are they, how big are they and where do they occur? Prev. Vet. Met. 112, 161–173. doi: 10.1016/j.prevetmed.2013.07.013
Kobayashi, N., Takahashi, D., Takano, S., Kimura, S., and Hase, K. (2019). The roles of Peyer’s patches and microfold cells in the gut immune system: Relevance to autoimmune diseases. Front. Immunol. 10:2345. doi: 10.3389/fimmu.2019.02345
Kotla, S., Vishanath, B. S., Dechamma, H., Ganesh, K., Suryanarayana, V., and Reddy, G. (2016). DNA vaccine (P1-2A-3C-pCDNA) co-administered with Bovine IL-18 gives protective immune response against foot and mouth disease in cattle. Vet. Microbiol. 193, 106–115. doi: 10.1016/j.vetmic.2016.07.007
Lala, V., Zubair, M., and Minter, D. (2023). “*Liver function tests,” in StatPearls, (Treasure Island, FL: StatPearls Publishing).
Lee, M. J., Jo, H., Shin, S. H., Kim, S.-M., Kim, B., Shim, H. S., et al. (2019). Mincle and STING-stimulating adjuvants elicit robust cellular immunity and drive long-lasting memory responses in a foot-and-mouth disease vaccine. Front. Immunol. 10:2509. doi: 10.3389/fimmu.2019.02509
Lee, M. J., Kim, H. M., Shin, S., Jo, H., Park, S. H., Kim, S.-M., et al. (2022). The C3d-fused foot-and-mouth disease vaccine platform overcomes maternally-derived antibody interference by inducing a potent adaptive immunity. npj Vaccines 7:70. doi: 10.1038/s41541-022-00496-8
Li, M., Wang, Y., Sun, Y., Cui, H., Zhu, S. J., and Qiu, H.-J. (2020). Mucosal vaccines: Strategies and challenges. Immunol. Lett. 217, 116–125. doi: 10.1016/j.imlet.2019.10.013
Li, Y., Jin, L., and Chen, T. (2020). The effects of secretory IgA in the mucosal immune system. BioMed. Res. Int. 2020:2032057. doi: 10.1155/2020/2032057
Ma, X., Nakayamada, S., and Wang, J. (2021). Multi-source pathways of T follicular helper cell differentiation. Front. Immunol. 12:621105. doi: 10.3389/fimmu.2021.621105
Meierovics, A., Yankelevich, W.-J. C., and Cowley, S. C. (2013). MAIT cells are critical for optimal mucosal immune responses during in vivo pulmonary bacterial infection. Proc. Natl. Acad. Sci. U. S. A. 110, E3119–E3128. doi: 10.1073/pnas.1302799110
Mettelman, R. C., Allen, E. K., and Thomas, P. G. (2022). Mucosal immune responses to infection and vaccination in the respiratory tract. Immunity 55, 749–780. doi: 10.1016/j.immuni.2022.04.013
Mörbe, U. M., Jørgensen, P. B., Fenton, T. M., von Burg, N., Riis, L. B., Spencer, J., et al. (2021). Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol. 14, 793–802. doi: 10.1038/s41385-021-00389-4
Nagashima, K., Sawa, S., Nitta, T., Tsutsumi, M., Okamura, T., Penninger, J. M., et al. (2017). Identification of subepithelial mesenchymal cells that induce IgA and diversify gut microbiota. Nat. Immunol. 18, 675–682. doi: 10.1038/ni.3732
Nel, I., Bertrand, L., Toubal, A., and Lehuen, A. (2021). MAIT cells, guardians of skin and mucosa? Mucosal Immunol. 14, 803–814. doi: 10.1038/s41385-021-00391-w
Neutra, M. R., and Kozlowski, P. A. (2006). Mucosal vaccines: The promise and the challenge. Nat. Rev. Immunol. 6, 148–158. doi: 10.1038/nri1777
Pastorino, G., Cornara, L., Soares, S., Rodrigues, F., and Oliveira, M. B. P. (2018). Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytother. Res. 32, 2323–2339. doi: 10.1002/ptr.6178
Pietrzak, B., Tomela, K., Olejnik-Schmidt, A., Mackiewicz, A., and Schmidt, M. (2020). Secretory IgA in intestinal mucosal secretions as an adaptive barrier against microbial cells. Int. J. Mol. Sci. 21:9254. doi: 10.3390/ijms2123925
Reantragoon, R., Corbett, A. J., Sakala, I. G., Gherardin, N. A., Furness, J. B., Chen, Z., et al. (2013). Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J. Exp. Med. 210, 2305–2320. doi: 10.1084/jem.20130958
Reboldi, A., and Cyster, J. G. (2016). Peyer’s patches: Organizing B-cell responses at the intestinal frontier. Immunol. Rev. 271, 230–245. doi: 10.1111/imr.12400
Ruterbusch, M., Pruner, K. B., Shehata, L., and Pepper, M. (2020). In vivo CD4+ T cell differentiation and function: Revisiting the Th1/Th2 paradigm. Annu. Rev. Immunol. 38, 705–725. doi: 10.1146/annurev-immunol-103019-085803
Selyutina, O. Y., and Polyakov, N. (2019). Glycyrrhizic acid as a multifunctional drug carrier–From physicochemical properties to biomedical applications: A modern insight on the ancient drug. Int. J. Pharm. 559, 271–279. doi: 10.1016/j.ijpharm.2019.01.047
Shin, S., Kim, H. W., Ko, M.-K., Park, S. H., Kim, S.-M., Park, J.-H., et al. (2023). Inactivated vaccine with glycyrrhizic acid adjuvant elicits potent innate and adaptive immune responses against foot-and-mouth disease. Front. Microbiol. 14:1289065. doi: 10.3389/fmicb.2023.1289065
Sobkowiak, M. J., Davanian, H., Heymann, R., Gibbs, A., Emgård, J., Dias, J., et al. (2019). Tissue-resident MAIT cell populations in human oral mucosa exhibit an activated profile and produce IL-17. Eur. J. Immunol. 49, 133–143. doi: 10.1002/eji.201847759
Suzuki, K., Maruya, M., Kawamoto, S., Sitnik, K., Kitamura, H., Agace, W. W., et al. (2010). The sensing of environmental stimuli by follicular dendritic cells promotes immunoglobulin A generation in the gut. Immunity 33, 71–83. doi: 10.1016/j.immuni.2010.07.003
Ussher, J. E., Bilton, M., Attwod, E., Shadwell, J., Richardson, R., de Lara, C., et al. (2014). CD 161++ CD 8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+ IL-18 in a TCR-independent manner. Eur. J. Immunol. 44, 195–203. doi: 10.1002/eji.201343509
Van Gelderen, C., Bijlsma, J., Van Dokkum, W., and Savelkoull, T. (2000). Glycyrrhizic acid: The assessment of a no effect level. Hum. Exp. Toxicol. 19, 434–439. doi: 10.1191/096032700682694251
Wahab, S., Annadurai, S., Abullais, S., Das, G., Ahmad, W., Ahmad, M., et al. (2021). “Glycyrrhiza glabra (Licorice): A comprehensive review on its phytochemistry, biological activities, clinical evidence and toxicology. Plants 10:2751. doi: 10.3390/plants10122751
Wang, S., Liu, H., Zhang, X., and Qian, F. (2015). Intranasal and oral vaccination with protein-based antigens: Advantages, challenges and formulation strategies. Protein Cell 6, 480–503. doi: 10.1007/s13238-015-0164-2
Wubshet, A. K., Werid, G. M., Teklue, T., Zhou, L., Bayasgalan, C., Tserendorj, A., et al. (2024). Foot and mouth disease vaccine efficacy in Africa: A systematic review and meta-analysis. Front. Vet. Sci. 11:1360256. doi: 10.3389/fvets.2024.1360256
Xiao, Y., Chen, H.-Y., Wang, Y., Yin, B., Lv, C., Mo, X., et al. (2016). Large-scale production of foot-and-mouth disease virus (serotype Asia1) VLP vaccine in Escherichia coli and protection potency evaluation in cattle. BMC Biotechnol. 16:56. doi: 10.1186/s12896-016-0285-6
Zhang, Y., Sheng, Z., Xiao, J., Li, Y., Huang, J., Jia, J., et al. (2023). Advances in the roles of glycyrrhizic acid in cancer therapy. Front. Pharmacol. 14:1265172. doi: 10.3389/fphar.2023.1265172
Keywords: oral administration, glycyrrhizic acid, mucosal immunity, systemic immunity, secretory IgA
Citation: Shin S, Kim HW, Ko M-K, Park SH, Park J-H, Kim S-M and Lee MJ (2025) Oral administration of glycyrrhizic acid with intramuscular injection of foot-and-mouth disease vaccine enhances the adaptive immune system. Front. Microbiol. 16:1502630. doi: 10.3389/fmicb.2025.1502630
Received: 27 September 2024; Accepted: 31 January 2025;
Published: 19 February 2025.
Edited by:
Wibke Bayer, Essen University Hospital, GermanyReviewed by:
Guo Huichen, Chinese Academy of Agricultural Sciences, ChinaCopyright © 2025 Shin, Kim, Ko, Park, Park, Kim and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Ja Lee, aGVyYjEyQGtvcmVhLmty
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.