- 1Department of Botany and Microbiology, College of Science, King Saud University, Riyadh, Saudi Arabia
- 2Infectious Disease Research Department, King Abdullah International Medical Research Center, Riyadh, Saudi Arabia
- 3Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia
- 4Ministry of the National Guard - Health Affairs, Riyadh, Saudi Arabia
- 5Department of Basic Science, College of Science and Health Professions, King Saud Bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia
Background: The rapid emergence of multidrug-resistant Klebsiella pneumoniae (MDR K. pneumoniae) is a major public health and economic burden worldwide. Various resistance mechanisms complicate treatment, leading to increased morbidity and mortality. Despite numerous studies conducted in Gulf Health Council (GHC) countries, the molecular epidemiology of MDR K. pneumoniae remains not clearly defined. This systematic review aims to analyze the emergence of antimicrobial resistance genes in MDR K. pneumoniae across GHC countries.
Methods: A systematic search was conducted using PubMed, ScienceDirect, and OpenMD for articles published up to March 15, 2023. The search strategy focused on the bacterial name, drug-resistance genotypes, and GHC countries. The review followed PRISMA guidelines, with two independent reviewers assessing the risk of bias using NIH Study Quality Assessment tools.
Results: The primary search yielded 1,663 studies, of which 67 met the inclusion criteria. Saudi Arabia contributed the most studies, with 41 (61.1%), followed by Kuwait with 7 (10.4%), and the UAE with 6 (9%) studies. Oman and Qatar each contributed 4 studies (6%), and Bahrain contributed three studies (4.5%). The remaining 4 studies (4.4%) were from multiple GHC countries. The studies exhibited considerable heterogeneity in detection methods, target genes, and resistance mechanisms. Notably, only one environmental study was conducted in the UAE, and one community-based study in Kuwait, while the remaining studies focused on clinical samples. Various resistance mechanisms and patterns were observed between countries and across different years within the same country. The review highlighted the widespread prevalence of ESBL genes, particularly blaTEM and blaCTX-M-15, and the emergence of carbapenemase genes such as blaOXA-48 and blaNDM-1 and blaKPC-2. Additionally, colistin resistance through the mcr-1 gene and mgrB mutations was reported in Saudi Arabia and the UAE, posing a significant public health challenge.
Conclusion: Data from GHC countries shows significant gaps, particularly in community and environmental and molecular epidemiology studies. Limited molecular and genome-based investigations hinder comprehensive AMR surveillance. Implementing standardized methodologies and fostering molecular and genome-based AMR surveillance programs at both national and regional levels within the GHC are essential for effectively combating the spread of MDR K. pneumoniae and improving public health outcomes in the region.
1 Introduction
In modern medicine, the emergence of Klebsiella pneumoniae strains exhibiting multidrug resistance (MDR), extensive drug resistance (XDR), and pandrug resistance (PDR), as well as the production of extended-spectrum β-lactamases (ESBL) and/or carbapenemases, represents a growing global crisis that urgently calls for the development of new antibiotics. MDR refers to bacterial strains resistant to at least one agent in three or more antimicrobial categories, XDR denotes resistance to all but one or two antimicrobial categories, and PDR signifies resistance to all agents in all antimicrobial categories, leaving no effective treatments (Magiorakos et al., 2012; Shamsuzzaman, 2015). In response to this ever-increasing threat, the World Health Organization (WHO) recently released the 2024 Bacterial Priority Pathogens List (BPPL), categorizing these pathogens into priority groups to guide research and strategies for controlling antimicrobial resistance (WHO Bacterial Priority Pathogens List, 2024). These pathogens are listed as a significant threat to public health as they can cause severe and life-threatening infections such as bloodstream infections and pneumonia. Available treatment options, including last-resort antibiotics, have become limited and ineffective due to the acquired resistance mechanisms (Hersh et al., 2012; Petrosillo et al., 2019; Rodríguez-Santiago et al., 2021; Wang et al., 2020; Pu et al., 2023; Aris et al., 2020; Cain et al., 2018).
In both humans and animals, the gastrointestinal system and oropharynx are naturally colonized by the Gram-negative bacterium K. pneumoniae. However, due to its capacity to cause community-acquired illnesses such as necrotizing pneumonia, liver abscesses, and endogenous endophthalmitis, K. pneumoniae is considered the most clinically significant species within the Klebsiella genus (Podschun and Ullmann, 1998). Additionally, K. pneumoniae contributes to hospital-acquired severe infections, such as sepsis, surgical site infections, and urinary tract infections (Yong et al., 2009). K. pneumoniae has acquired and disseminated multiple MDR genes, including ESBL variants, carbapenemase genes, and colistin-resistance genes (Al-Zahrani and Alsiri, 2018; Navon-Venezia et al., 2017). Consequently, the emergence of MDR K. pneumoniae poses significant public health challenges, complicating treatment regimens and leading to increased morbidity and mortality rates. Common antibiotics, such as third-generation cephalosporins, aminoglycosides, fluoroquinolones, and carbapenems, continuously lose their effectiveness against K. pneumoniae (Rolain et al., 2010). The rise in carbapenem resistance in K. pneumoniae is a global issue associated with higher morbidity and mortality rates, as well as increased medical expenditures in Saudi Arabia and other neighboring countries (Poirel et al., 2011; Al-Abdely et al., 2021; Alraddadi et al., 2022; Sonnevend et al., 2022; Abid et al., 2021). The Gulf Health Council (GHC) countries (Saudi Arabia, United Arab Emirates, Kuwait, Qatar, Oman, and Bahrain) are not immune to this problem. Rapid urbanization, high healthcare utilization, and extensive international travel in these regions contribute to the spread of resistant strains (Weber et al., 2017; Aiesh et al., 2023; Yu et al., 2021; Berndtson, 2020; Bokhary et al., 2021; Frost et al., 2019). Moreover, the setting of mass gatherings, such as the annual Muslim pilgrimage, Hajj and Umrah, that take place in Saudi Arabia plays an essential role in the spread of diverse antimicrobial-resistant strains (Al-Tawfiq and Memish, 2021; Leangapichart et al., 2017; Setiawaty et al., 2022; Leangapichart et al., 2016).
In the past decade, advanced molecular techniques have been employed to identify antimicrobial resistance genes and track their dissemination, providing insight into genetic mechanisms and the transmission dynamics underlying antibiotic resistance in clinically significant pathogens (Salawudeen et al., 2023; WHO, 2020). Moreover, molecular epidemiology is crucial for understanding the clonal relationships among different K. pneumoniae isolates, providing essential insights for devising effective infection control strategies and guiding appropriate antibiotic regimes (Cireșă et al., 2024; Alghoribi et al., 2018).
Recognizing the gap in knowledge regarding the molecular epidemiology of antimicrobial resistance mechanisms in the Arabian Peninsula, the 2018 position paper by Alghoribi and colleagues called for genomic epidemiology to combat AMR (Alghoribi et al., 2018). They emphasized the importance of molecular investigation of AMR as a crucial tool for identifying emerging pathogens and their resistance mechanisms. In alignment with this call, the WHO’s technical note “GLASS Whole-Genome Sequencing (WGS) for Surveillance of Antimicrobial Resistance” (2020) outlines the benefits and limitations of molecular investigation for AMR surveillance (WHO, 2020). Although significant progress has been made, comprehensive information about the molecular epidemiology of K. pneumoniae and the prevalence of its resistance genes in the GHC countries remains limited.
A comprehensive understanding of the regional epidemiological patterns and resistance mechanisms is essential for addressing the public health threat posed by this pathogen. This systematic research aims to determine the status of molecular epidemiology and the genetic distribution of MDR K. pneumoniae in the GHC countries by highlighting the prevalence of crucial resistance genes and the distribution of dominant clones. By collating and analyzing data from various studies, this review will provide a detailed overview of the current status and identify gaps in the existing literature, thereby offering a foundation for future research and intervention efforts. Understanding the regional dynamics of MDR K. pneumoniae is imperative for developing targeted strategies to curb the spread of resistance and improve patient outcomes in the GHC countries.
2 Materials and methods
While systematic reviews have extensively reported the molecular epidemiology of MDR K. pneumoniae, including ESBL, carbapenemase-producing and colistin-resistant strains globally, there is a notable lack of evidence specific to GHC countries, underscoring the need for region-specific data to understand antimicrobial resistance dynamics. The guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)1 were followed in developing the current systematic review protocol using the PRISMA 2020 checklist (Page et al., 2021). When planning a search strategy, the PICO (Population, Intervention, Comparison, and Outcome) tool was used to list terms and keywords by the main concepts in the search question as an organizing framework accessed on Oct 20, 2023.2 A comprehensive systematic review was performed using the major electronic databases of PubMed, ScienceDirect, and OpenMD for articles published from inception to March 15, 2023. Consistent keywords and search strategies were applied across these databases containing the terms (molecular epidemiology, antimicrobial resistance genotypes, AMR genotypes, genetic diversity, clones, genotyping, antibiotic resistance genes, genetic analysis, resistome, whole genome sequencing OR genomic characterization) and (Klebsiella pneumoniae) and (Gulf Cooperation Council region). Moreover, two independent reviewers selected relevant studies from the references of found studies after removing the duplicates.
2.1 Eligibility criteria
The review included accessible full-text original articles published in English before March 15, 2023, without any restrictions on the publication year of the included studies. In addition, the systematic review included clinical studies, case reports, environmental and one-health approach studies that investigated the antimicrobial resistance genes in K. pneumoniae in the GHC countries using phenotypic and genotypic methods. All studies that addressed MDR K. pneumoniae, including ESBL, carbapenemase-producing and colistin-resistant strains, were included as a sub-population of Enterobacterales. In contrast, all studies reporting antimicrobial resistance mechanisms in K. pneumoniae using phenotypic detection methods were excluded from this review. Additional exclusion criteria included reviews, conference abstracts, study protocols, and studies performed outside the GHC countries.
2.2 Selection and data extraction
All references of extracted studies were imported to EndNote (version 21.2), where duplicates were removed. Two stages of screening were performed by two independent reviewers. The first screening stage included title and abstract screening of the imported references. Afterwards, all studies that were included during the first stage of screening underwent full-text screening during the second stage. The decision of each reviewer was taken blindly, and disagreements between researchers were resolved through discussion with a third reviewer. Following the two screening stages, all articles included were carried out for data extraction based on a data collection form designed to address the aim of the current review (Supplementary Table S1) using a Microsoft Excel worksheet. The data extracted from each study included the following: the citation, the country where the study was performed, the study type, the study period in months, the sample size, the specimen sources, the genotypic detection method, detected antimicrobial resistance genes, detected multilocus sequence types, and the year(s) of specimen collection.
2.3 Risk of bias assessment
The quality of each article included in the review was assessed by two independent reviewers using the National Heart, Lung, and Blood Institute (NIH) Study Quality Assessment tools adapted for each study’s design.3 Two tools were used: (1) the NHLBI Quality Assessment Tool for Observational Cross-Sectional Studies and (2) the NHLBI Quality Assessment Tool for Case Series Studies. The quality of each study was rated as good, fair, or poor to assess the risk of bias in the study due to flaws in study design or implementation (Supplementary file S2).
2.4 Statistical analysis
Due to the nature of the current systematic review, the results are presented as frequencies and percentages. Microsoft Excel was used for the quantitative analysis of the extracted data. The study period was presented as mean ± SD. The number of studies in each country and the prevalence of antimicrobial resistance genes were presented in frequencies. GraphPad Prism (version 10.2.3) (347) was used to create graphs and figures.
3 Results
3.1 Literature search and study selection
A comprehensive systematic literature search was conducted to identify relevant studies on MDR of K. pneumoniae in the Arabian Peninsula. The search yielded a total of 1,663 studies, which were imported into the EndNote software (version 21.2). After removing duplicate studies (n = 1,039), the titles and abstracts of 624 studies were reviewed for possible inclusion in the current review. Out of 624 studies, 531 were excluded due to their irrelevance to the scope of the review. The remaining 93 studies were assessed for eligibility through full-text screening. Consequently, 26 articles were excluded for reasons detailed in Figure 1. Hence, a total of 67 articles met the eligibility criteria and were included in the present review.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow-chart describes the results of molecular epidemiological studies of MDR K. pneumoniae in the GHC countries. The flow chart was downloaded from http://www.prisma-statement.org/.
3.2 Risk of bias assessment
The quality assessment results of the 67 included studies are presented in Supplementary Table S1 based on the NHLBI study quality assessment tools for observational cross-sectional studies (Supplementary Table S1A) and case series studies (Supplementary Table S1B). The NHLBI Quality Assessment Tool for Observational Cross-Sectional Studies was used for only two studies, both of which were classified as fair quality (Moghnia et al., 2021a; Sonnevend et al., 2022). Most studies were evaluated using the NHLBI Quality Assessment Tool for Case Series Studies. Out of 67 studies, 37 (55.2%) were classified as good quality, while 30 (44.7%) were classified as fair quality, as cited and described in detail in Supplementary Table S1. None of the 67 evaluated studies were classified as poor quality.
3.3 Overview of Klebsiella pneumoniae molecular epidemiology studies in the GHC countries
Out of the 67 included studies, Saudi Arabia contributed more than 60% (41/67), followed by Kuwait and the UAE, with seven and six studies, respectively. Oman and Qatar contributed four studies in each country, while Bahrain contributed only three studies. Furthermore, researchers conducted four studies using samples collected from various countries in the area (Al-Baloushi et al., 2018; Sonnevend et al., 2015; Zowawi et al., 2014; Mouftah et al., 2021). Studies of MDR K. pneumoniae in the GHC countries, including ESBL, carbapenemase-producing and colistin-resistant strains, were conducted over an average period of 18 ± 19 months between 2006 and 2021, as shown in Figure 2. The most extended study, which was conducted over 96 months in Saudi Arabia, was on the genetic characterization of colistin-resistant isolates (Okdah et al., 2022). Most of the studies (50.7%) focused on carbapenem-resistant K. pneumoniae (CRKP), followed by ESBL-producing K. pneumoniae (29.8%), as shown in Supplementary Table S2.
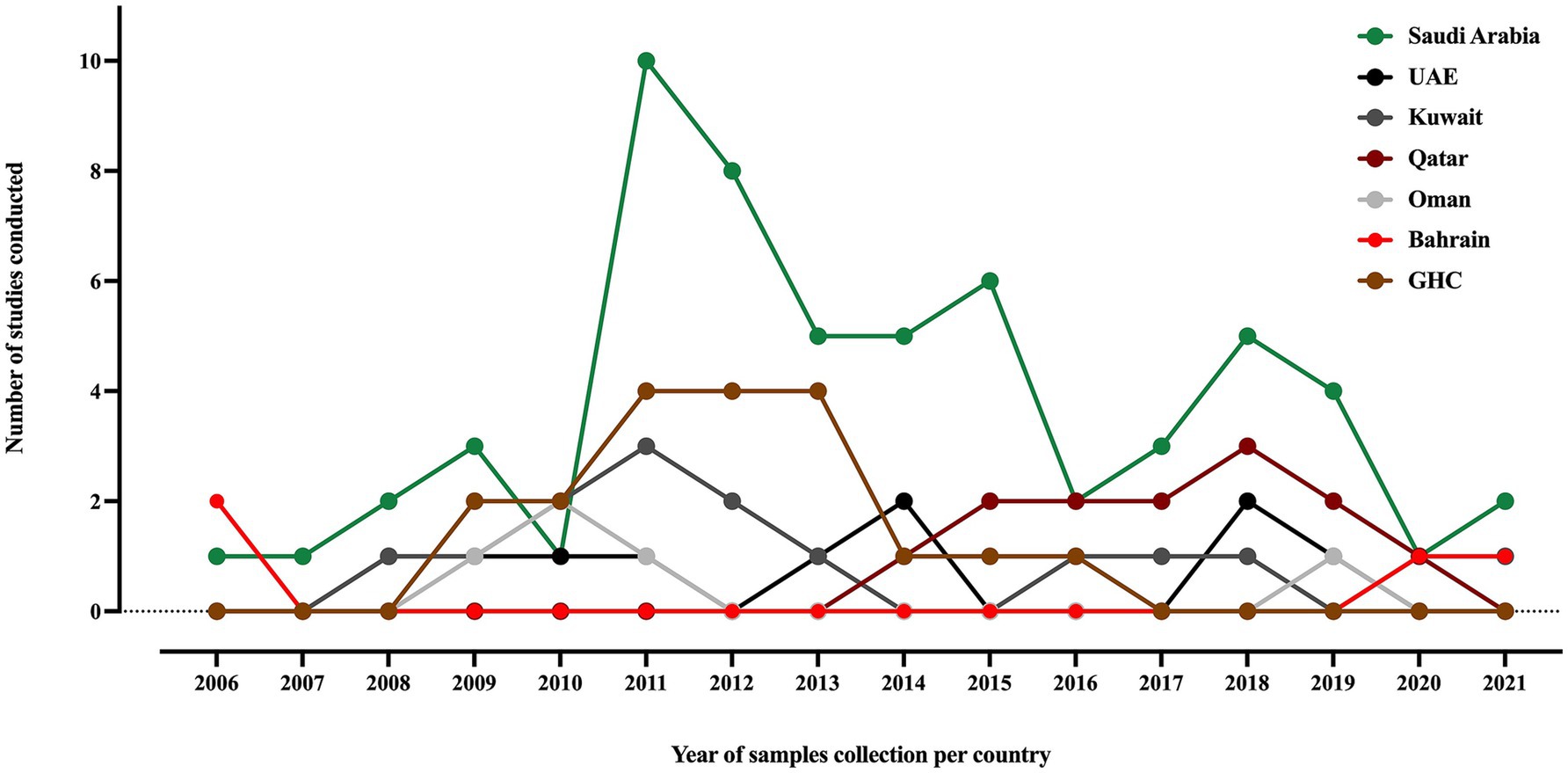
Figure 2. A timeline of K. pneumoniae studies conducted in the GHC countries, illustrating the samples collection year of each study. The label “GHC” indicates studies that involved samples collected from different GHC countries.
Furthermore, all studies were related to clinical settings, while only one study was conducted on livestock/animal samples (Sonnevend et al., 2022), and one study focused on collecting samples from the community (Moghnia et al., 2021a). The predominant specimen sources reported in the majority of studies were urine, sputum, and blood, as shown in Figure 3. The most commonly used genotypic method was PCR, followed by multiplex PCR and the WGS, as detailed in Figure 4.
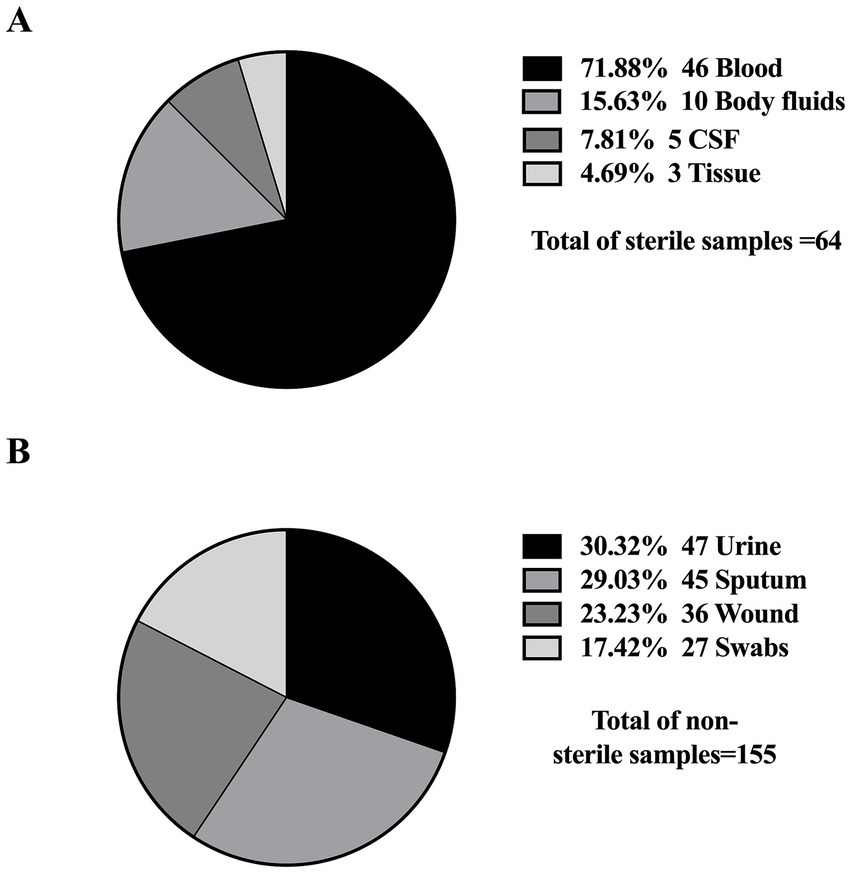
Figure 3. Distribution of sample types from which K. pneumoniae isolates were obtained. (A) Sterile samples: collected through invasive procedures (e.g., blood, CSF), often associated with life-threatening infections. (B) Non-sterile samples: obtained through non-invasive methods (e.g., urine, sputum), typically linked to less severe infections.
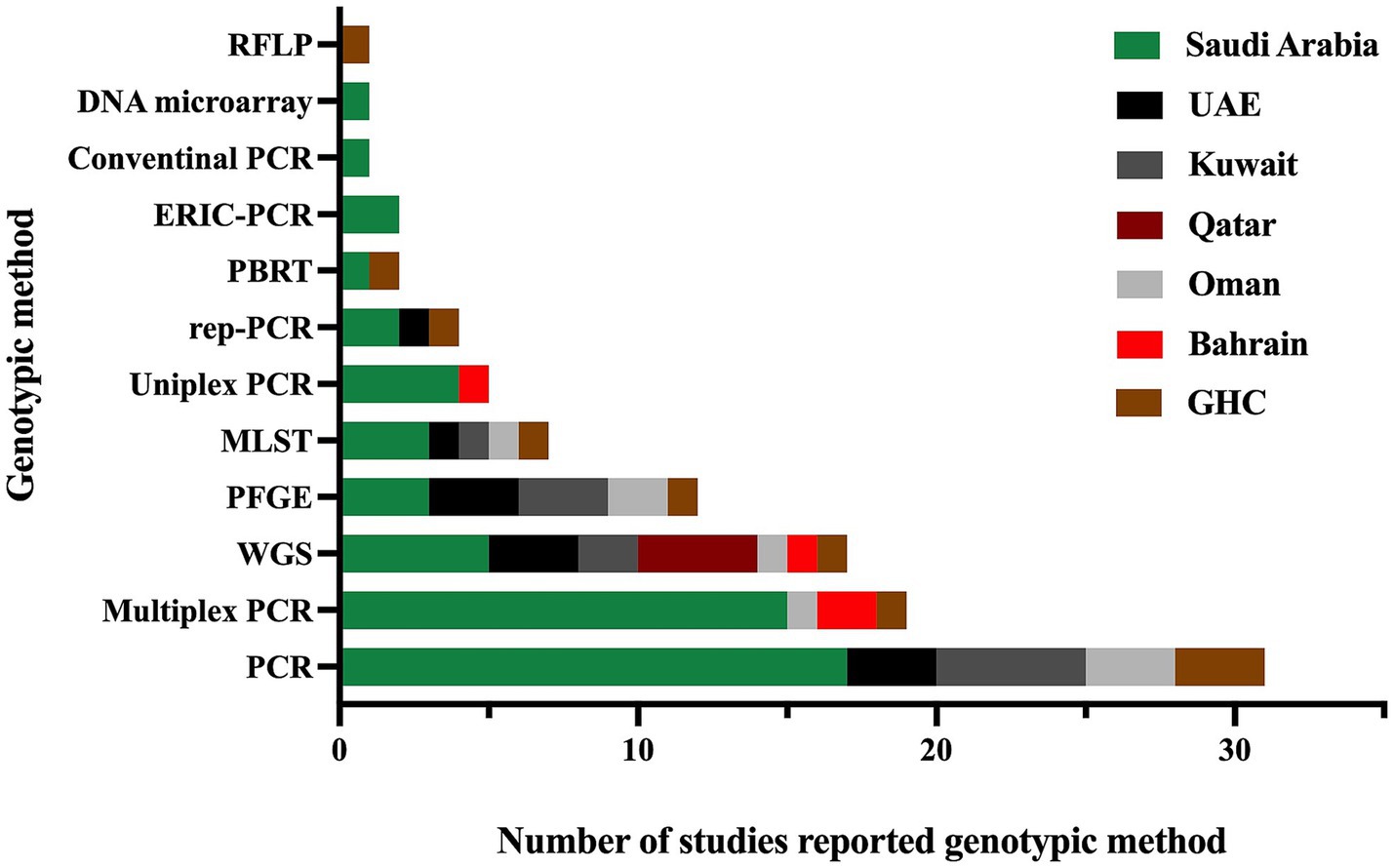
Figure 4. The genotypic methods used for the detection of antimicrobial resistance genes and identification of different sequence types in MDR K. pneumoniae were reported in studies conducted in the GHC countries. The label “GHC” reflects the studies conducted on samples obtained from different GHC countries.”
3.4 Distribution and comparison of Klebsiella pneumoniae AMR genes in the GHC countries
An extensive analysis of the studies revealed a predominant focus on MDR K. pneumoniae, including ESBL, carbapenemase-producing, and colistin-resistant strains. Among the ESBL genes, blaCTX-M was identified as the most prevalent, with two variants, blaCTX-M-14 and blaCTX-M-15, reported in most studies. The blaOXA gene was the most identified carbapenemase gene, with blaOXA-23, blaOXA-48, blaOXA-181, and blaOXA-232 as the most common variants. However, the distribution of these predominant genes varies between the GHC countries, with certain genes reported exclusively in specific countries. This variation is likely due to the differing number of studies conducted in each country, which influences the detection and reporting of particular genes. For instance, while blaCTX-M-15 was the most predominant ESBL gene in the GHC countries, blaCTX-M-14 was reported only in Saudi Arabia, Kuwait, and Qatar. Moreover, blaSHV-1 was reported in Saudi Arabia (Tawfik et al., 2011; Uz Zaman et al., 2014; Al-Qahtani et al., 2014; Alghoribi et al., 2020) and Kuwait (Jamal et al., 2015), while blaSHV-28 was identified in the UAE (Alfaresi et al., 2011), Qatar (Eltai et al., 2020), and Oman (Poirel et al., 2011). For carbapenemase genes, blaOXA and blaNDM were identified in all GHC countries, with varying distributions of their variants across different countries. Conversely, blaKPC was reported in all countries except Bahrain (Alraddadi et al., 2022; Sonnevend et al., 2022; Abid et al., 2021; Mouftah et al., 2021; Alghoribi et al., 2020; Balushi et al., 2022; Moghnia et al., 2021b; Moghnia and Al-Sweih, 2022; Azim et al., 2019; Al-Tawfiq et al., 2022; Alshahrani et al., 2022), with blaKPC-2 being the most frequently reported variant. Notably, blaKPC-2 was first reported in Saudi Arabia (Alghoribi et al., 2020). Additionally, the carbapenemase gene blaIMP was reported only in Saudi Arabia (Alraddadi et al., 2022; Azim et al., 2019; Ejaz, 2022; Badger-Emeka et al., 2021).
Furthermore, resistance to aminoglycosides and fluroquinolones has also been observed in ESBL- or carbapenemase- producing K. pneumoniae in GHC countries. Among aminoglycoside-resistance genes, aac(6′)-lb was the most frequently identified (Al-Baloushi et al., 2018; Shibl et al., 2012; Abdalhamid et al., 2017; Al-Agamy et al., 2019; Vali et al., 2015), followed by armA and rmtB (Sonnevend et al., 2022; Okdah et al., 2022; Alghoribi et al., 2020; Abdalhamid et al., 2017; Al Sheikh et al., 2014; Abdalhamid et al., 2017; Sonnevend et al., 2013). These genes have been documented in Saudi Arabia, the UAE, Kuwait, and Oman. However, no aminoglycoside-resistance genes were reported in Qatar and Bahrain. Among the fluoroquinolone-resistance genes, qnrB was reported in Saudi Arabia (Okdah et al., 2022; Shibl et al., 2012; Abdalhamid et al., 2017; Al-Agamy et al., 2019; Al-Agamy et al., 2018) and the UAE (Sonnevend et al., 2013), while qnrS was identified in Bahrain (Shahid et al., 2022) and Oman (Balushi et al., 2022). Interestingly, Kuwait stands out, as a study conducted there identified all three key fluoroquinolone-resistance genes: qnrA, qnrB, and qnrS (Vali et al., 2015). Moreover, a study conducted in Saudi Arabia identified a wide array of antimicrobial resistance genes, including aadA2, ant(3′)-lh, armA, sat-A, AmpH, aac(3′)-la, aph(3′)-Vib, strAB, oqxA, msr(E), catA, catB, sul1, tet (WHO Bacterial Priority Pathogens List, 2024), tet(A), tet(D), mcr-1, and dfrA (Al-Baloushi et al., 2018; Okdah et al., 2022; Alghoribi et al., 2020; Abdalhamid et al., 2017). Additionally, other resistance mechanisms were observed, such as mutations in gyrA, ParC, mgrB, PmrA, and PmrB (Alghoribi et al., 2020; Abdalhamid et al., 2017). Structural and functional alterations in outer membrane proteins and efflux systems, including OmpK35, OmpK36, mdtK, tolC, and acrAB (Lagha et al., 2021), were also reported. The presence of multiple resistance mechanisms, in addition to ESBL or carbapenem resistance, classifies these isolates as MDR K. pneumoniae, as they demonstrate resistance to at least one antibiotic from three or more different classes. These findings underscore the multifaceted nature of antimicrobial resistance in the region and the importance of monitoring both genetic and phenotypic mechanisms to understand resistance trends comprehensively.
Alarmingly, colistin-resistant K. pneumoniae has been documented in three studies conducted in GHC countries. Chromosome-mediated colistin resistance, driven by mgrB mutations, was identified in two studies from Saudi Arabia (Okdah et al., 2022; Zaman et al., 2018). Additionally, plasmid-mediated colistin resistance associated with the mcr-1 gene was reported in two studies, one from Saudi Arabia (Okdah et al., 2022) and the other from the UAE (Sonnevend et al., 2022).
The presence and absence of various antimicrobial resistance genes across different GHC countries are shown in Figure 5. Even though many studies did not include the analysis and identification of K. pneumoniae sequence types, some studies reported the presence of specific K. pneumoniae sequence types among their isolates. As some sequence types were reported in different geographical regions, such as ST11, ST14, ST15, ST37, ST101, ST147, ST307, ST340, ST383, and ST2096, other sequence types were specific to some regions, among others, as illustrated in (Figure 6).
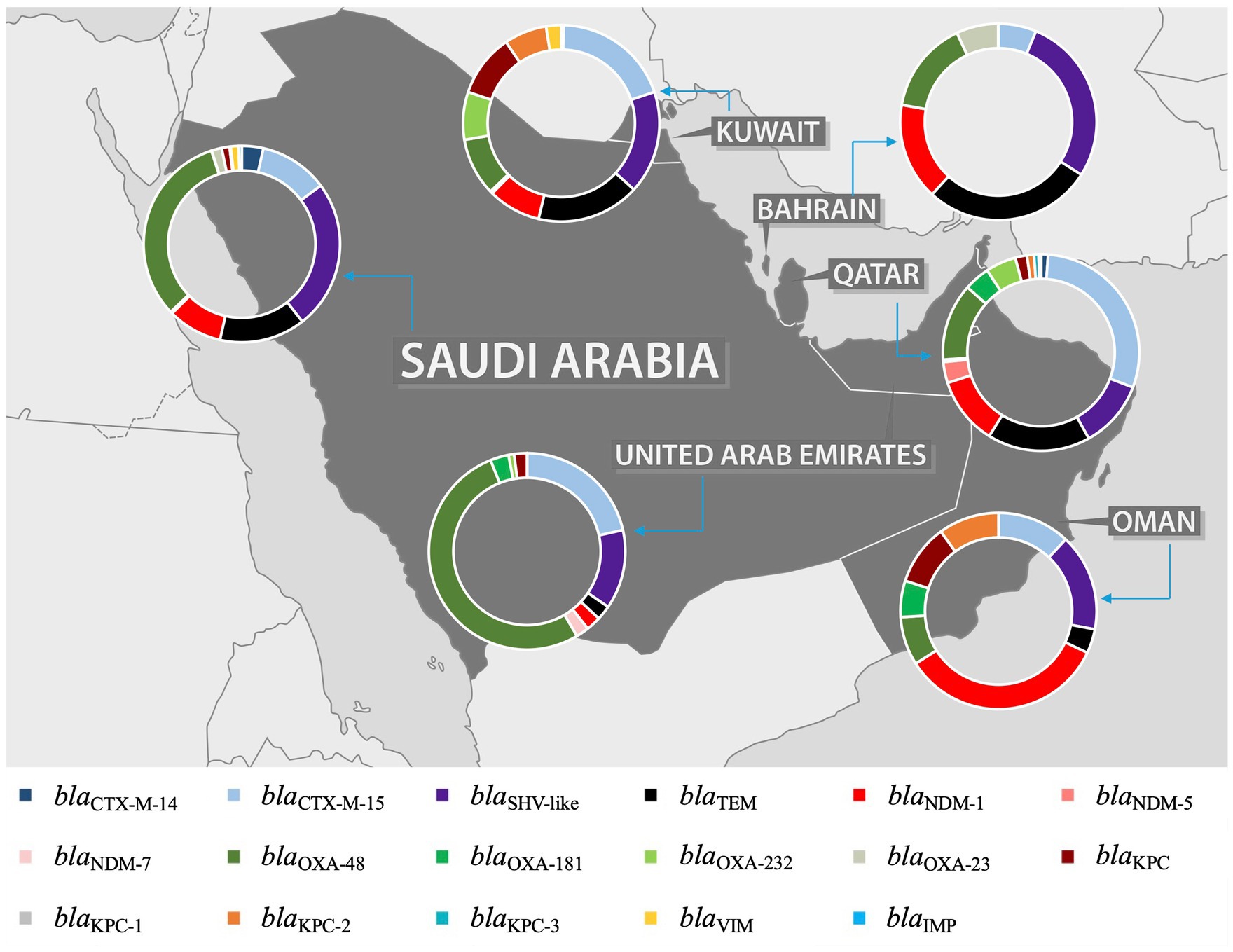
Figure 5. Distribution of most prevalent ESBL and carbapenemase genes in the GHC Countries. The map figure was licensed from Shutterstock (https://www.shutterstock.com) and modified using PowerPoint to express the prevalence of ARGs in each country of GHC.
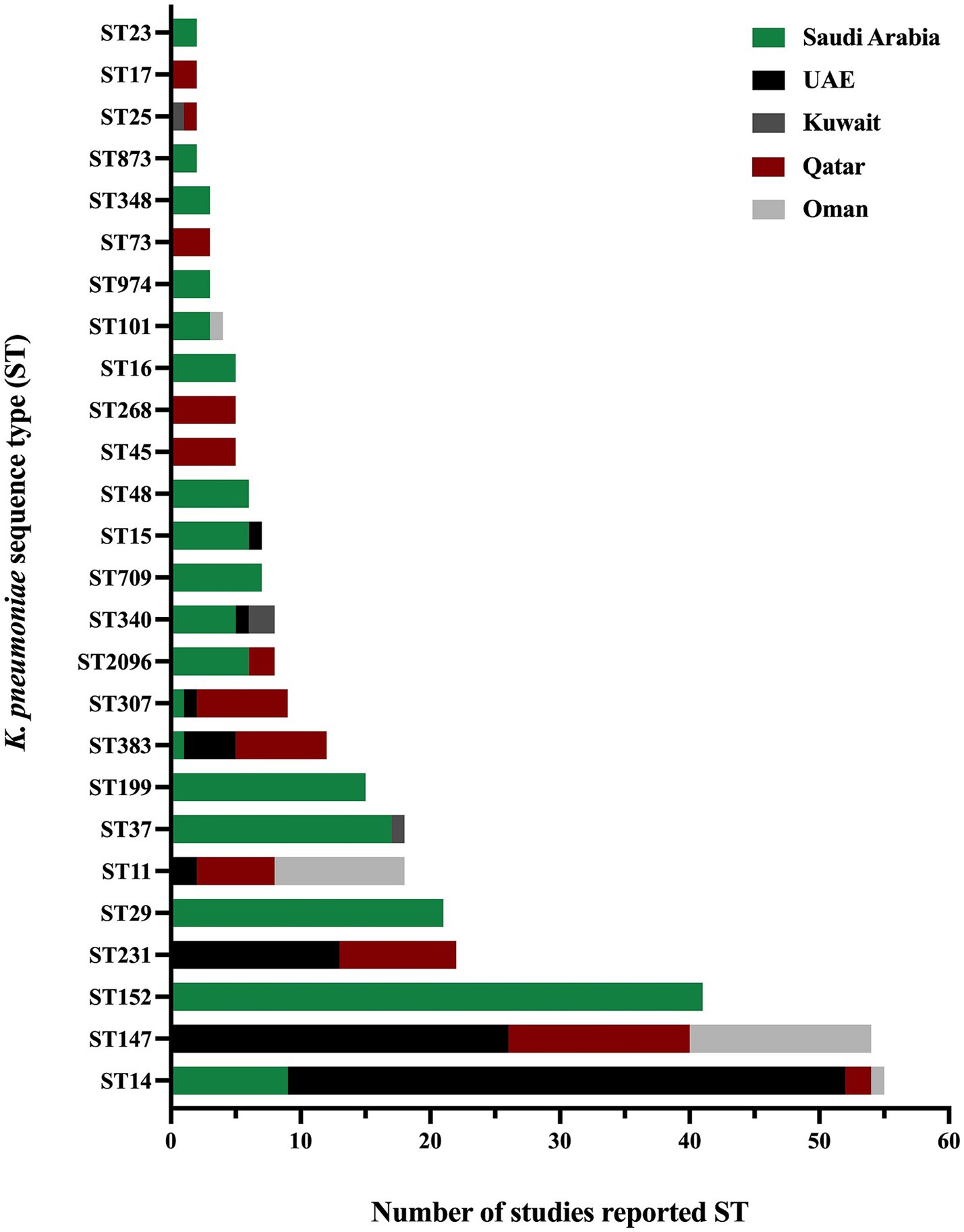
Figure 6. The distribution of K. pneumoniae sequence types in the GHC countries shows the variety of K. pneumoniae sequence types in different geographical regions.
3.5 Klebsiella pneumoniae AMR genes in the Kingdom of Saudi Arabia
The Kingdom of Saudi Arabia contributed the majority of the data from the GHC countries, with a total of 41 studies (61.1% of the total) included in this review conducted between 2010 and 2022. Most studies utilized multiplex PCR to detect ESBL and carbapenem-resistance genes, while a subset of studies incorporated WGS in their methodology. The studies demonstrated that the ESBL gene (blaCTX-M) was the most predominant resistance gene over the years, followed by β-lactamase genes (blaTEM and blaSHV), regardless of the variants. Additionally, various carbapenemase genes were detected in Saudi Arabia, including blaOXA-48, blaNDM-1, blaKPC-2, blaVIM, and blaIMP. However, blaOXA-48 and blaNDM-1 emerged as the most prevalent genes over the years. In 2013 (Shibl et al., 2013), A. Shibl and his team reported blaOXA-48 and blaNDM-1 for the first time in Saudi Arabia, while blaKPC was reported for the first time in 2019 by N. Azim and collaborators (Azim et al., 2019). In 2019, M. Khan reported the presence of triple co-producing carbapenemase genes (blaOXA-48, blaNDM-1, and blaKPC) in 80% of isolates collected from clinical samples using PCR methods (Khan et al., 2019). However, these results have not been subsequently confirmed, indicating the need for further investigation. In contrast, the triple co-producing carbapenemase genes (blaOXA-48-like, blaNDM-1, and blaKPC) were identified in 1.4% of isolates in another study by A. Alshahrani and others in 2022 (Alshahrani et al., 2022). A combination of three carbapenemase genes (blaOXA-48, blaNDM-1, and blaVIM) was reported in 21.7% of clinical isolates, while a combination of four carbapenemase genes (blaOXA-23, blaOXA-48, blaNDM-1, and blaVIM) was reported in a single clinical isolate in 2022 by R. Booq and the team (Booq et al., 2022). The emergence of ESBL and carbapenemase genes has been well-documented in Saudi Arabia. However, the use of colistin as a last-line treatment has led to the alarming emergence of colistin resistance in clinical isolates of K. pneumoniae. Multiple studies have reported colistin-resistant K. pneumoniae in clinical settings, with chromosomally mediated resistance primarily due to mutations in the mgrB, pmrA, pmrB, and phoQ genes. Furthermore, plasmid-mediated colistin resistance has been identified by acquiring the mcr-1 gene (Okdah et al., 2022; Zaman et al., 2018). The molecular typing results of K. pneumoniae isolates revealed that the most predominant clonal group is clonal complex 14 (CC14), which includes the sequence types ST14 and ST2096 in a study conducted in 2020 and 2022 (Okdah et al., 2022; Khdary et al., 2020). Several studies have analyzed the MLST of K. pneumoniae isolates, identifying various sequence types. Additionally, other sequence types reported in the literature include ST11, ST23, ST37, ST147, ST307, ST340, and ST383 (Al-Baloushi et al., 2018; Sonnevend et al., 2015; Okdah et al., 2022; Uz Zaman et al., 2014; Alghoribi et al., 2020; Al-Agamy et al., 2019; Zaman et al., 2018; Moglad et al., 2022; Uz Zaman et al., 2018; Almogbel et al., 2021). The predominance of CC14 highlights its significance in the epidemiology of K. pneumoniae in Saudi Arabia. Identifying these sequence types underscores the genetic diversity of K. pneumoniae strains circulating in clinical settings, contributing to the complexity of managing infections caused by this pathogen.
3.6 Klebsiella pneumoniae AMR genes in the United Arab Emirates
The United Arab Emirates (UAE) contributed the third-highest number of studies, following Saudi Arabia and Kuwait, with a total of six studies out of 67 (8.9%). Most of these studies focused on investigating AMR on clinical samples (Sonnevend et al., 2022; Alfaresi et al., 2011; Sonnevend et al., 2013; Sonnevend et al., 2017; Zowawi et al., 2015), with only one study conducted on livestock origin samples (Sonnevend et al., 2022). The majority reported ESBL-producing K. pneumoniae or CRKP, with one study highlighting the presence of colistin-resistant K. pneumoniae in a sample of livestock origin (Sonnevend et al., 2022). In addition, most clinical samples were recovered from various infection sites, including urine, blood, respiratory, and wound swabs. At the same time, the livestock origin specimens are comprised of fecal samples collected from birds in poultry farms. Various resistance genes were reported in the UAE, including β-lactamase genes blaTEM-1 (Sonnevend et al., 2017) and blaSHV-11 (Sonnevend et al., 2013) and ESBL genes blaSHV-12 (Sonnevend et al., 2022; Sonnevend et al., 2013), blaSHV-28 (Alfaresi et al., 2011), blaSHV-36 (Zowawi et al., 2015), and blaCTX-M-15 (Sonnevend et al., 2022; Alfaresi et al., 2011; Sonnevend et al., 2013; Sonnevend et al., 2017; Zowawi et al., 2015), with blaCTX-M-15 identified as the most predominant ESBL gene in the UAE. Carbapenemase genes were reported in four studies, including blaOXA-48 (Sonnevend et al., 2022), blaOXA-181 (Sonnevend et al., 2017; Zowawi et al., 2015), blaNDM-1 (Sonnevend et al., 2022; Sonnevend et al., 2013), blaNDM-5 (Sonnevend et al., 2017), and blaKPC (Sonnevend et al., 2022). Notably, colistin-resistant K. pneumoniae was reported in several studies within the time frame of this literature review. Chromosomal mutations were detected in clinical samples, specifically in the genes mgrB, phoP, phoQ, pmrA, and pmrB (Sonnevend et al., 2022; Sonnevend et al., 2017). The mcr-1 gene, on the other hand, was identified in fecal samples collected from birds in poultry farms (Sonnevend et al., 2022). However, no mcr genes were found in the colistin-resistant K. pneumoniae strains subjected to WGS from clinical samples. MLST analysis in the UAE indicated ST14 was the most predominant sequence type, followed by ST147 and ST231 (Sonnevend et al., 2022; Al-Baloushi et al., 2018; Sonnevend et al., 2015; Sonnevend et al., 2013; Sonnevend et al., 2017; Zowawi et al., 2015). Additional studies reported the detection of various sequence types in the UAE, including ST11, ST231, ST307, ST340, ST383, and ST1318 (Sonnevend et al., 2022; Sonnevend et al., 2022; Al-Baloushi et al., 2018; Sonnevend et al., 2015; Sonnevend et al., 2013).
3.7 Klebsiella pneumoniae AMR genes in Kuwait
Kuwait contributed the second-highest number of studies, following Saudi Arabia, with a total of seven studies reporting the ESBL producing K. pneumoniae (Vali et al., 2015; Al Sweih et al., 2011; Dashti et al., 2010), or CRKP (Jamal et al., 2015; Moghnia et al., 2021b; Moghnia and Al-Sweih, 2022; Jamal et al., 2013). Most studies were conducted on clinical samples, with only one study focusing on healthy food handlers from community settings (Moghnia et al., 2021b). In 2008, a β-lactamase gene (blaTEM-1) and an ESBL gene (blaCTX-M-15) were identified in all 14 isolates of ESBL-producing K. pneumoniae over a period of 2 months (Al Sweih et al., 2011). Genotypic studies from Kuwait revealed blaTEM-1 and blaSHV-11 as predominant β-lactamases genes, while blaCTX-M-15 was identified as the predominant ESBL genes in ESBL-producing K. pneumoniae. Interestingly, one study showed an outbreak reporting a number of isolates that were phenotypically resistant to cephalosporin antibiotics. Molecular genetics analysis showed that these isolates harbor blaSHV-112 gene, which is classified as an ESBL genes (Dashti et al., 2010). Moreover, several studies have reported the major carbapenemase genes, which include blaOXA-48, blaOXA-181, blaOXA-232, blaNDM-1, and blaKPC-2 (Jamal et al., 2015; Moghnia et al., 2021b; Moghnia and Al-Sweih, 2022; Jamal et al., 2013). Notably, other blaKPC variants, including blaKPC-18 and blaKPC-29, were first identified in Kuwait from community samples between 2016 and 2018 (Moghnia et al., 2021a). The detailed list of all ESBL and carbapenemase genes is presented in Supplementary Table S2. Results of MLST analysis of K. pneumoniae isolates obtained from food handlers reported various sequence types composed of ST10, ST38, ST295, ST1415, and ST1876 (Moghnia et al., 2021b). In addition, three new CRKP sequence types (ST1592, ST1593, and ST1594) were reported in a study conducted between 2011 and 2013 on clinical samples (Jamal et al., 2015). Other sequence types were identified in different studies including ST16, ST25, ST37, ST107, ST485, ST677, ST3495, and ST4743 (Jamal et al., 2015; Moghnia and Al-Sweih, 2022). However, all identified STs in Kuwait were reported as singleton with no observed predominance.
3.8 Klebsiella pneumoniae AMR genes in Qatar
Four studies were conducted in Qatar to detect ESBL and carbapenemase genes in K. pneumoniae isolates obtained from clinical samples such as urine, blood, respiratory, and wound swabs (Abid et al., 2021; Eltai et al., 2020; Perez-Lopez et al., 2020; Pérez-López et al., 2021). These major resistance genes were the most predominant genes in all studies conducted in Qatar between 2015 and 2019 (Eltai et al., 2020; Perez-Lopez et al., 2020; Pérez-López et al., 2021). A study conducted from 2014 to 2017 reported the presence of blaOXA-48, blaOXA-181, blaOXA-232, blaNDM-1, blaNDM-5, blaNDM-7, blaKPC-2, blaKPC-3 and blaVIM-2. In virous sequence types, including ST11, ST147, ST231 and ST383 of which ST147 being the most predominant (Abid et al., 2021). Additionally, co-occurrence of resistance genes was observed, including combinations such as (blaOXA-48 and blaNDM-5), (blaOXA-181 and blaNDM-1), and (blaOXA-181 and blaNDM-5) and (blaNDM-1 and blaKPC-3) (Abid et al., 2021). Other studies confirmed the presence of blaOXA-48, blaNDM-1, and blaNDM-5 as the most widely disseminated carbapenemase genes in Qatar (Eltai et al., 2020; Perez-Lopez et al., 2020; Pérez-López et al., 2021). The analysis highlighted blaOXA-48 and blaNDM-1 as the dominant carbapenemase genes identified in the country. The molecular typing of K. pneumoniae isolates resulted in the identification of different sequence types through several studies over the years. For instance, a study between 2015 and 2019 identified the following sequence types: ST11, ST25, ST147, ST231, ST383, ST716, ST792, and ST2096 (Eltai et al., 2020), while a study in 2018 reported the presence of ST45, ST268 and ST307 K. pneumoniae sequence types (Perez-Lopez et al., 2020). Other sequence types were identified between 2018 and 2020, composed of ST14, ST17, and ST73 (Pérez-López et al., 2021).
3.9 Klebsiella pneumoniae AMR genes in Sultanate of Oman
Four studies in Oman were eligible to be included in the current systematic review (Poirel et al., 2011; Balushi et al., 2022; Potron et al., 2011; Dortet et al., 2012). These studies employed molecular methods to investigate the presence of antimicrobial resistance genes in K. pneumoniae, primarily using PCR as the genotypic method. However, only one study incorporated WGS to further elucidate these characteristics (Balushi et al., 2022). These studies were conducted in 2011, 2012, and 2022 focused on either K. pneumoniae or CRKP. Studies have reported resistance mechanisms associated with the β-lactamase gene (blaSHV) and ESBL gene (blaCTX-M), which were identified as the predominant resistance mechanisms. These studies also identified significant carbapenemase genes, including blaNDM-1 in 2011, 2012, and 2022 (Poirel et al., 2011; Balushi et al., 2022; Dortet et al., 2012), and blaKPC-2 in 2022 (Balushi et al., 2022). Additionally, the co-occurrence carbapenemase genes, specifically blaOXA-181 and blaNDM-1, were observed in 2012 by L. Dortet and colleagues (Dortet et al., 2012). Furthermore, MLST analysis across all studies in Oman identified ST11 and ST147 as the major sequence types (Al-Baloushi et al., 2018; Sonnevend et al., 2015; Balushi et al., 2022; Potron et al., 2011; Dortet et al., 2012). These sequence types were the most predominant in the region, highlighting their significant role in the epidemiology of K. pneumoniae. Other sequence types reported as singletons include ST14, ST15, ST101, ST340, ST372, ST753, and ST754 (Poirel et al., 2011; Balushi et al., 2022).
3.10 Klebsiella pneumoniae AMR genes in the Kingdom of Bahrain
Three studies were conducted in Bahrain to evaluate the presence of antimicrobial resistance genes in K. pneumoniae obtained from clinical samples, with notable time gaps between these three studies (Shahid et al., 2022; Bindayna and Murtadha, 2011; Shahid et al., 2014). The first study investigated the prevalence of ESBL-producing K. pneumoniae over 6 months. This study analyzed clinical samples, including urine, blood, respiratory, and wound swabs, and found that nearly all samples tested positive for ESBL. The most prevalent ESBL gene identified was blaCTX-M (90%), followed by β-lactamase genes blaTEM and blaSHV (80%). An important finding was the co-occurrence of these genes, with 70% of isolates harboring all three (blaTEM, blaSHV and blaCTX-M) and only 10% containing just blaSHV and blaCTX-M (Bindayna and Murtadha, 2011). The second study reported the prevalence of ESBL-producing K. pneumoniae over 7 months, analyzing clinical samples that included urine, blood, respiratory, and wound swabs. All the K. pneumoniae samples in this study tested positive for the presence of the blaCTX-M-15 gene (Shahid et al., 2014). The third study, conducted over 7 months, focused on detecting CRKP in clinical samples, with blood being the most common sample type, followed by urine and respiratory. The study identified multiple carbapenemase genes, including blaOXA-23, blaOXA-48, blaOXA-51 and blaNDM-1. Additionally, some isolates co-produced multiple carbapenemase genes, revealing double or triple carbapenemase gene combinations, including blaOXA-48, blaOXA-51 and blaNDM-1. Notably, 100% of the isolates in this study harbored the qnrS gene alongside multiple carbapenemase genes, contributing to the multidrug-resistant profile (Shahid et al., 2022).
4 Discussion
This systematic review underscores the complex and multifaceted burden posed by MDR K. pneumoniae in the GHC countries. The majority of the studies included focused on investigating MDR K. pneumoniae on clinical samples, with limited exploration of its presence in community and environmental settings, such as food handlers (Moghnia et al., 2021b) and poultry farms (Sonnevend et al., 2022). Hospital-based studies partially reflect community health, but the One Health approach and molecular epidemiology are needed to better track and manage MDR K. pneumoniae across human, animal, and environmental interfaces. This significant gap in the data limits our understanding of the broader epidemiological landscape and potential non-clinical reservoirs of MDR K. pneumoniae, which are critical for comprehensive public health strategies.
The Kingdom of Saudi Arabia contributed the majority of research within the GHC region, followed by Kuwait and the United Arab Emirates. However, the variability in study designs, detection methods, sample sizes, and targeted genes across these studies complicates direct comparisons. It limits the potential for meta-analysis, thereby underscoring the need for standardized methodologies in future research. Despite these methodological challenges, the application of NGS in certain studies has yielded valuable insights into the molecular epidemiology of MDR K. pneumoniae, facilitating the identification and tracking of prevalent resistance genes within the region.
The widespread prevalence of resistance genes, notably the β-lactamase gene blaTEM and the ESBL gene blaCTX-M-15, emerged as a critical finding across the GHC countries. Our analysis of the prevalence of the blaSHV gene revealed regional variations. The β-lactamase gene (blaSHV-1) was exclusively found in Kuwait and Saudi Arabia. The β-lactamase gene (blaSHV-11) and ESBL genes (blaSHV-12) were identified in Saudi Arabia, UAE, and Kuwait, while the ESBL genes (blaSHV-28 and blaSHV-38) were detected in the UAE, Qatar, and Oman. This observed geographic variability in the prevalence of blaSHV suggests regional differences in disseminating these resistance mechanisms, which may reflect local healthcare practices or environmental factors. The plasmid-mediated dissemination of ESBL genes has significantly compromised the efficacy of critical antimicrobials, including penicillins and other ß-lactams, across the GHC countries, mirroring global trends in the spread of antimicrobial resistance (Paterson et al., 2003; Saravanan et al., 2018; Agyepong et al., 2019; Manandhar et al., 2020; Nordmann and Mammeri, 2007). These findings align with international trends, such as those reported in clinical settings in Africa, where high prevalence rates of blaTEM, blaSHV, and blaCTX-M have been documented, further underscoring the pervasive nature of these resistance genes.
The first identification of blaCTX-M-15 occurred in Greece during an ICU infection outbreak (Poulou et al., 2013), spreading rapidly to other countries, including Spain, France, Italy, Poland, Tunisia and Japan, underscoring its global significance (Marcade et al., 2013; Elhani et al., 2010; Robin et al., 2017; Rodrigues et al., 2014; Ochońska et al., 2021; Mrowiec et al., 2019; de Walthoffen et al., 2011). This gene, along with blaTEM and blaSHV, has become widely disseminated across regions such as Portugal, North America, Argentina, Australia, South Africa, Turkey, and the United States (Paterson et al., 2003; Rodrigues et al., 2014). These findings align with international trends observed in clinical settings, including those in Africa, where high prevalence rates of blaTEM, blaSHV, and blaCTX-M have been documented (Saravanan et al., 2018; Agyepong et al., 2019). The coexistence of these genes on a global scale mirrors the patterns observed in the GHC countries, suggesting that international travel and migration may contribute to the genetic diversity and spread of β-lactamase and ESBL genes in this region.
The widespread dissemination of ESBL-producing K. pneumoniae has necessitated the increased use of carbapenems as a last-resort treatment option [Infectious Diseases Society of America (IDSA), 2011]. Consequently, this reliance has led to the global emergence of CRKP, posing a significant public health threat (Tamma and Simner, 2018). Over 50% of the studies in the GHC region focused on CRKP, highlighting the regional concern over this growing resistance. The initial identification of blaOXA-48 and blaNDM-1 carbapenemase genes marked the beginning of CRKP’s emergence, which has since been reported in nosocomial outbreaks worldwide, including in the GHC countries.
Notably, blaOXA-48 has been the most frequently reported carbapenemase gene in the region, although specific blaOXA variants vary by geography. This aligns with findings from Tehran, where blaOXA-48 was prevalent among CRKP isolates (Solgi et al., 2020). In contrast, studies from Nepal identified blaNDM-2 as the predominant carbapenemase gene in CRKP (Manandhar et al., 2020; Pyakurel et al., 2021). Within the GHC countries, blaNDM-1 emerged as the second most common carbapenemase gene, with other variants like blaNDM-5 and blaNDM-7 being reported in Saudi Arabia, Qatar, and the UAE (Abid et al., 2021; Ejaz, 2022; Sonnevend et al., 2017).
Carbapenemase gene blaNDM-1 is particularly notable as it is the most widely distributed metallo-β-lactamase enzyme in Enterobacteriaceae across South and Southeast Asia (Hsu et al., 2017). The coexistence of multiple carbapenemase genes is a significant concern, with plasmids carrying blaNDM are frequently linked to the presence of both blaOXA-48 and blaVIM genes (Nordmann and Mammeri, 2007). Similar co-occurrence patterns, the combination of more than one carbapenemase gene, such as blaOXA-181 and blaNDM-1 or blaNDM-5, was reported among hospitalized patients in India (Castanheira et al., 2011) and Singapore (Balm et al., 2013). Additionally, blaOXA-232 was identified in the GHC countries in agreement with a previous report in South India (Shankar et al., 2019).
Recently, blaKPC was reported in different regions in the GHC countries, with blaKPC-2 being the most reported variant. The first identification of blaKPC-2 was reported in New York in 2004 (Bratu et al., 2005). Since 2004, blaKPC-2 was reported in different geographical regions globally, such as France, Columbia and China (Queenan and Bush, 2007). A combination of blaKPC gene and other carbapenemase genes, including blaOXA181 and blaNDM-1, was also reported (Moghnia et al., 2021b). The carbapenemase gene blaIMP was only reported in Saudi Arabia. Differences in the prevalence of carbapenemase genes might be due to variations in international travel and the way these genes spread within individual countries in the GHC countries. Alarmingly, the increase in CRKP prevalence worldwide has resulted in increased use of colistin with the consequence of emerging resistance (Gelband et al., 2015).
Colistin is often considered the last resort for treating infections caused by MDR gram-negative bacteria such as CRKP. However, the recent emergence and dissemination of the colistin resistance gene mcr-1 brought significant challenges to public health. Alarmingly, the colistin-resistant gene mcr-1 was identified and reported in two different countries in the GHC countries: Saudi Arabia (Okdah et al., 2022) and the UAE (Sonnevend et al., 2022) in 2022. The UAE study identified a surprising variety of mcr-producing colistin-resistant strains environmentally in the fecal specimens of broiler poultry. These findings match the data reported from neighboring countries, such as Lebanon and Egypt (Al-Mir et al., 2021; Sadek et al., 2021). A study in Saudi Arabia found colistin-resistant strains of K. pneumoniae in hospitals (Okdah et al., 2022). This comes alongside worldwide studies, such as Egypt, North India, South America, North America, Africa, and China, that reported similar antibiotic-resistant bacteria carrying a specific gene (mcr-1) on a plasmid (Singh et al., 2021; Liu et al., 2021; Attalla et al., 2023; Zurfuh et al., 2016; Arcilla et al., 2016).
Furthermore, a study in China linked the overuse of colistin in animal feed to the emergence of a colistin-resistant population. Other studies suggested that the plasmid-mediated mcr-1 gene can quickly spread from the environment and animals into key human epidemic strains (Zurfuh et al., 2016; Liu et al., 2016; Grami et al., 2016; Elmonir et al., 2021). Therefore, the Food and Agriculture Organization of the United Nations and the Codex Alimentarius Commission established the risk management options that all countries should adopt to control the spread of antimicrobial resistance in agriculture (Canton, 2021; Joint, 2015). Beyond the previously mentioned plasmid-borne mechanism, mgrB, phoP, phoQ, pmrA, and pmrB mutations have also been identified as a colistin-resistance mechanism in Saudi Arabia (Okdah et al., 2022; Zaman et al., 2018) United Arabic Emirate (Sonnevend et al., 2022; Sonnevend et al., 2017).
A study conducted using community-derived samples obtained from food handlers in Kuwait identified the presence of different carbapenemase genes with various variants such as blaOXA (blaOXA-48, blaOXA-181, and blaOXA-232), blaNDM (blaNDM-1, blaNDM-6, and blaNDM-7), and blaKPC (blaKPC-2, blaKPC-18 and blaKPC-29) (Moghnia et al., 2021b). This is in agreement with Castanheira et al. (2011) and Rojas et al. (2017) studies that reported the presence of blaOXA-181 among K. pneumoniae colonizing the gastrointestinal tract of patients admitted to the hospital in the Indian subcontinent (Castanheira et al., 2011; Rojas et al., 2017). The spread of these genes in Kuwait might be explained by the high rate of Indian food handlers working in the region. On the other hand, a study in Germany reported the identification of different genes, such as blaSHV-27, blaSHV-41, blaCTX-M, blaOXY, blaOKP, blaLEN, and mcr-1 from animals and food products (Klaper et al., 2021).
Implementing robust AMR surveillance programs at both national and regional levels within the GHC is crucial for understanding and mitigating the burden of MDR K. pneumoniae. Such programs are essential for providing timely and accurate data on the prevalence and distribution of resistance genes, which is critical for informing public health strategies and treatment guidelines. A well-coordinated AMR surveillance network across the GHC countries would enable the detection of emerging resistance trends, facilitate data sharing between nations, and support the development of targeted interventions. Without these efforts, the GHC region faces the risk of being unprepared for the rapid spread of MDR K. pneumoniae, potentially leading to increased healthcare costs, higher morbidity and mortality rates, and diminished effectiveness of available antibiotics. A comprehensive AMR surveillance program is vital for addressing current threats and safeguarding future public health in the region.
5 Conclusion
Limiting our search to English-language articles retrieved from major electronic databases allowed us to identify research published in high-impact journals. However, accurately determining the true prevalence of MDR K. pneumoniae in the region proved challenging due to the heterogeneity observed in the included studies. Due to these limitations, the findings of this systematic review are not exclusive, but they highlighted the genetic basis of increased resistance in K. pneumoniae in the area. Limited environmental and One Health studies in the GHC countries leave a gap in estimating the dissemination mechanism of MDR K. pneumoniae from the environment or food into human pathogens. The current review found a high prevalence of β-lactamase and ESBL-producing K. pneumoniae carrying blaTEM, blaSHV, and blaCTX-M genes, with significant variation between countries.
Moreover, an increased spread of CRKP was reported and found to pose a serious public health risk in the GHC countries. It is important to note that combined efforts to combat the problem of rapid dissemination of MDR K. pneumoniae in the region. In addition, a multi-disciplinary approach is needed to better understand the emergence and dissemination of MDR K. pneumoniae in the region as the one health strategy aims to reduce the rise and spread of AMR at the human-animal-environment interface across the globe.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
EI: Conceptualization, Data curation, Investigation, Methodology, Resources, Validation, Writing – review & editing, Formal analysis, Software, Visualization, Writing – original draft. MGA: Conceptualization, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – review & editing, Funding acquisition, Resources. MMA: Conceptualization, Project administration, Resources, Supervision, Writing – review & editing. MFA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This systematic literature review was funded by the King Abdullah International Medical Research Center (KAIMRC) under project approval number SP24R/011/01.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1489317/full#supplementary-material
Footnotes
1. ^accessed from https://www.prisma-statement.org.
2. ^https://mcw.libguides.com/EBM/PICO
3. ^accessed from https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
References
Abdalhamid, B., Albunayan, S., Shaikh, A., Elhadi, N., and Aljindan, R. (2017). Prevalence study of plasmid-mediated AmpC β-lactamases in Enterobacteriaceae lacking inducible ampC from Saudi hospitals. J. Med. Microbiol. 66, 1286–1290. doi: 10.1099/jmm.0.000504
Abdalhamid, B., Elhadi, N., Albunayan, S., Alsamman, K., and Aljindan, R. (2017). First description of methyltransferases in extensively drug-resistant Klebsiella pneumoniae isolates from Saudi Arabia. J. Med. Microbiol. 66, 859–863. doi: 10.1099/jmm.0.000480
Abid, F. B., Tsui, C. K. M., Doi, Y., Deshmukh, A., McElheny, C. L., Bachman, W. C., et al. (2021). Molecular characterization of clinical carbapenem-resistant Enterobacterales from Qatar. Eur. J. Clin. Microbiol. Infect. Dis. 40, 1779–1785. doi: 10.1007/s10096-021-04185-7
Agyepong, N., Govinden, U., Owusu-Ofori, A., Amoako, D. G., Allam, M., Janice, J., et al. (2019). Genomic characterization of multidrug-resistant ESBL-producing Klebsiella pneumoniae isolated from a Ghanaian teaching hospital. Int. J. Infect. Dis. 85, 117–123. doi: 10.1016/j.ijid.2019.05.025
Aiesh, B. M., Nazzal, M. A., Abdelhaq, A. I., Abutaha, S. A., SeH, Z., and Sabateen, A. (2023). Impact of an antibiotic stewardship program on antibiotic utilization, bacterial susceptibilities, and cost of antibiotics. Sci. Rep. 13:5040. doi: 10.1038/s41598-023-32329-6
Al Sheikh, Y. A., Marie, M. A., John, J., Krishnappa, L. G., and Dabwab, K. H. (2014). Prevalence of 16S rRNA methylase genes among β-lactamase-producing Enterobacteriaceae clinical isolates in Saudi Arabia. Libyan J. Med. 9:24432. doi: 10.3402/ljm.v9.24432
Al Sweih, N., Salama, M. F., Jamal, W., Al Hashem, G., and Rotimi, V. O. (2011). An outbreak of CTX-M-15-producing Klebsiella pneumoniae isolates in an intensive care unit of a teaching hospital in Kuwait. Indian J. Med. Microbiol. 29, 130–135. doi: 10.4103/0255-0857.81791
Al-Abdely, H., AlHababi, R., Dada, H. M., Roushdy, H., Alanazi, M. M., Alessa, A. A., et al. (2021). Molecular characterization of carbapenem-resistant Enterobacterales in thirteen tertiary care hospitals in Saudi Arabia. Ann. Saudi Med. 41, 63–70. doi: 10.5144/0256-4947.2021.63
Al-Agamy, M. H., Aljallal, A., Radwan, H. H., and Shibl, A. M. (2018). Characterization of carbapenemases, ESBLs, and plasmid-mediated quinolone determinants in carbapenem-insensitive Escherichia coli and Klebsiella pneumoniae in Riyadh hospitals. J. Infect. Public Health 11, 64–68. doi: 10.1016/j.jiph.2017.03.010
Al-Agamy, M. H., El-Mahdy, T. S., Radwan, H. H., and Poirel, L. (2019). Cooccurrence of NDM-1, ESBL, RmtC, AAC(6′)-Ib, and QnrB in clonally related Klebsiella pneumoniae isolates together with coexistence of CMY-4 and AAC(6′)-Ib in Enterobacter cloacae isolates from Saudi Arabia. Biomed. Res. Int. 2019, 1–7. doi: 10.1155/2019/6736897
Al-Baloushi, A. E., Pál, T., Ghazawi, A., and Sonnevend, A. (2018). Genetic support of carbapenemases in double carbapenemase producer Klebsiella pneumoniae isolated in the Arabian peninsula. Acta Microbiol. Immunol. Hung. 65, 135–150. doi: 10.1556/030.65.2018.005
Alfaresi, M. S., Elkoush, A. A., Alshehhi, H. M., and Abdulsalam, A. I. (2011). Molecular characterization and epidemiology of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae isolates in the United Arab Emirates. Med. Princ. Pract. 20, 177–180. doi: 10.1159/000319912
Alghoribi, M. F., Balkhy, H. H., Woodford, N., and Ellington, M. J. (2018). The role of whole genome sequencing in monitoring antimicrobial resistance: a biosafety and public health priority in the Arabian peninsula. J. Infect. Public Health 11, 784–787. doi: 10.1016/j.jiph.2018.08.001
Alghoribi, M. F., Binkhamis, K., Alswaji, A. A., Alhijji, A., Alsharidi, A., Balkhy, H. H., et al. (2020). Genomic analysis of the first KPC-producing Klebsiella pneumoniae isolated from a patient in Riyadh: a new public health concern in Saudi Arabia. J. Infect. Public Health 13, 647–650. doi: 10.1016/j.jiph.2020.01.003
Al-Mir, H., Osman, M., Drapeau, A., Hamze, M., Madec, J.-Y., and Haenni, M. (2021). WGS analysis of clonal and plasmidic epidemiology of colistin-resistance mediated by mcr genes in the poultry sector in Lebanon. Front. Microbiol. 12:624194. doi: 10.3389/fmicb.2021.624194
Almogbel, M., Altheban, A., Alenezi, M., Al-Motair, K., Menezes, G. A., Elabbasy, M., et al. (2021). CTX-M-15 positive Escherichia coli and Klebsiella pneumoniae outbreak in the neonatal intensive care unit of a maternity Hospital in Ha'il, Saudi Arabia. Infect. Drug Resist. 14, 2843–2849. doi: 10.2147/IDR.S317079
Al-Qahtani, A. A., Al-Agamy, M. H., Ali, M. S., Al-Ahdal, M. N., Aljohi, M. A., and Shibl, A. M. (2014). Characterization of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae from Riyadh, Saudi Arabia. J. Chemother. 26, 139–145. doi: 10.1179/1973947813Y.0000000124
Alraddadi, B. M., Heaphy, E. L. G., Aljishi, Y., Ahmed, W., Eljaaly, K., Al-Turkistani, H. H., et al. (2022). Molecular epidemiology and outcome of carbapenem-resistant Enterobacterales in Saudi Arabia. BMC Infect. Dis. 22:542. doi: 10.1186/s12879-022-07507-y
Alshahrani, A. M., Ibrahim, M. E., Aldossary, A. K., Alghamdi, M. A., Ahmed, O. B., and Bin Abdulhak, A. A. (2022). Molecular epidemiology of Carbapenem-resistant K. pneumoniae clinical isolates from the adult patients with comorbidities in a Tertiary Hospital, Southern Saudi Arabia. Antibiotics (Basel) 11:1697. doi: 10.3390/antibiotics11121697
Al-Tawfiq, J. A., and Memish, Z. A. (2021). The emergence, persistence, and dissemination of antimicrobial-resistant bacteria in environmental hajj settings and implications for public health. Trop. Med. Infect. Dis. 6:33. doi: 10.3390/tropicalmed6010033
Al-Tawfiq, J. A., Rabaan, A. A., Saunar, J. V., and Bazzi, A. M. (2022). Genotypes and prevalence of carbapenemase-producing Enterobacteriaceae and Pseudomonas aeruginosa in a hospital in Saudi Arabia. Trans. R. Soc. Trop. Med. Hyg. 116, 50–53. doi: 10.1093/trstmh/trab055
Al-Zahrani, I. A., and Alsiri, B. A. (2018). The emergence of carbapenem-resistant Klebsiella pneumoniae isolates producing OXA-48 and NDM in the southern (Asir) province, Saudi Arabia. Saudi Med. J. 39, 23–30. doi: 10.15537/smj.2018.1.21094
Arcilla, M. S., van Hattem, J. M., Matamoros, S., Melles, D. C., Penders, J., de Jong, M. D., et al. (2016). Dissemination of the mcr-1 colistin resistance gene. Lancet Infect. Dis. 16, 147–149. doi: 10.1016/S1473-3099(15)00541-1
Aris, P., Robatjazi, S., Nikkhahi, F., and Marashi, S. M. A. (2020). Molecular mechanisms and prevalence of colistin resistance of Klebsiella pneumoniae in the Middle East region: a review over the last 5 years. J. Glob. Antimicrob. Resist. 22, 625–630. doi: 10.1016/j.jgar.2020.06.009
Attalla, E. T., Khalil, A. M., Zakaria, A. S., Baker, D. J., and Mohamed, N. M. (2023). Genomic characterization of colistin-resistant Klebsiella pneumoniae isolated from intensive care unit patients in Egypt. Ann. Clin. Microbiol. Antimicrob. 22:82. doi: 10.1186/s12941-023-00632-9
Azim, N. S. A., Nofal, M. Y., AlHarbi, M. A., Al-Zaban, M. I., and Somily, A. M. (2019). Molecular-diversity, prevalence and antibiotic susceptibility of pathogenic Klebsiella Pneumoniae under Saudi condition. Pak. J. Biol. Sci. 22, 174–179. doi: 10.3923/pjbs.2019.174.179
Badger-Emeka, L. I., Al-Sultan, A. A., Bohol, M. F. F., Al-Anazi, M. R., and Al-Qahtani, A. A. (2021). Genetic analysis, population structure, and characterisation of multidrug-resistant Klebsiella pneumoniae from the Al-Hofuf region of Saudi Arabia. Pathogens 10:1097. doi: 10.3390/pathogens10091097
Balm, M., La, M.-V., Krishnan, P., Jureen, R., Lin, R., and Teo, J. (2013). Emergence of Klebsiella pneumoniae co-producing NDM-type and OXA-181 carbapenemases. Clin. Microbiol. Infect. 19, E421–E423. doi: 10.1111/1469-0691.12247
Balushi, M. A., Kumar, R., Al-Rashdi, A., Ratna, A., Al-Jabri, A., Al-Shekaili, N., et al. (2022). Genomic analysis of the emerging carbapenem-resistant Klebsiella pneumoniae sequence type 11 harbouring Klebsiella pneumoniae carbapenemase (KPC) in Oman. J. Infect. Public Health 15, 1089–1096. doi: 10.1016/j.jiph.2022.08.014
Berndtson, A. E. (2020). Increasing globalization and the movement of antimicrobial resistance between countries. Surg. Infect. 21, 579–585. doi: 10.1089/sur.2020.145
Bindayna, K. M., and Murtadha, M. (2011). High prevalence of blaCTX-M in Enterobacteriaceae isolates from the Kingdom of Bahrain. Asian Pac J Trop Med 4, 937–940. doi: 10.1016/S1995-7645(11)60222-8
Bokhary, H., Pangesti, K. N., Rashid, H., Abd El Ghany, M., and Hill-Cawthorne, G. A. (2021). Travel-related antimicrobial resistance: a systematic review. Trop. Med. Infect. Dis. 6:11. doi: 10.3390/tropicalmed6010011
Booq, R. Y., Abutarboush, M. H., Alolayan, M. A., Huraysi, A. A., Alotaibi, A. N., Alturki, M. I., et al. (2022). Identification and characterization of plasmids and genes from Carbapenemase-producing Klebsiella pneumoniae in Makkah Province, Saudi Arabia. Antibiotics (Basel) 11:1627. doi: 10.3390/antibiotics11111627
Bratu, S., Mooty, M., Nichani, S., Landman, D., Gullans, C., Pettinato, B., et al. (2005). Emergence of KPC-possessing Klebsiella pneumoniae in Brooklyn, New York: epidemiology and recommendations for detection. Antimicrob. Agents Chemother. 49, 3018–3020. doi: 10.1128/AAC.49.7.3018-3020.2005
Cain, A. K., Boinett, C. J., Barquist, L., Dordel, J., Fookes, M., Mayho, M., et al. (2018). Morphological, genomic and transcriptomic responses of Klebsiella pneumoniae to the last-line antibiotic colistin. Sci. Rep. 8:9868. doi: 10.1038/s41598-018-28199-y
Canton, H. (2021). Food and agriculture organization of the United Nations—FAO. The Europa directory of international organizations 2021. London, UK: Routledge, 297–305.
Castanheira, M., Deshpande, L. M., Mathai, D., Bell, J. M., Jones, R. N., and Mendes, R. E. (2011). Early dissemination of NDM-1-and OXA-181-producing Enterobacteriaceae in Indian hospitals: report from the SENTRY antimicrobial surveillance program, 2006-2007. Antimicrob. Agents Chemother. 55, 1274–1278. doi: 10.1128/AAC.01497-10
Cireșă, A., Tălăpan, D., Vasile, C.-C., Popescu, C., and Popescu, G.-A. (2024). Evolution of antimicrobial resistance in Klebsiella pneumoniae over 3 years (2019–2021) in a tertiary Hospital in Bucharest, Romania. Antibiotics 13:431. doi: 10.3390/antibiotics13050431
Dashti, A. A., Jadaon, M. M., Gomaa, H. H., Noronha, B., and Udo, E. E. (2010). Transmission of a Klebsiella pneumoniae clone harbouring genes for CTX-M-15-like and SHV-112 enzymes in a neonatal intensive care unit of a Kuwaiti hospital. J. Med. Microbiol. 59, 687–692. doi: 10.1099/jmm.0.019208-0
de Walthoffen, S. W., Mlynarczyk, A., Sawicka-Grzelak, A., Durlik, M., Paczek, L., Chmura, A., et al., editors. Strains of Klebsiella pneumoniae producing extended spectrum beta-lactamases, isolated from organ recipients. In: Transplantation Proceedings; (2011) New York, NY, USA: Elsevier.
Dortet, L., Poirel, L., Al Yaqoubi, F., and Nordmann, P. (2012). NDM-1, OXA-48 and OXA-181 carbapenemase-producing Enterobacteriaceae in Sultanate of Oman. Clin. Microbiol. Infect. 18, E144–E148. doi: 10.1111/j.1469-0691.2012.03796.x
Ejaz, H. (2022). Analysis of diverse β-lactamases presenting high-level resistance in association with OmpK35 and OmpK36 porins in ESBL-producing Klebsiella pneumoniae. Saudi J. Biol. Sci. 29, 3440–3447. doi: 10.1016/j.sjbs.2022.02.036
Elhani, D., Bakir, L., Aouni, M., Passet, V., Arlet, G., Brisse, S., et al. (2010). Molecular epidemiology of extended-spectrum β-lactamase-producing Klebsiella pneumoniae strains in a university hospital in Tunis, Tunisia, 1999–2005. Clin. Microbiol. Infect. 16, 157–164. doi: 10.1111/j.1469-0691.2009.03057.x
Elmonir, W., Abd El-Aziz, N. K., Tartor, Y. H., Moustafa, S. M., Abo Remela, E. M., Eissa, R., et al. (2021). Emergence of Colistin and Carbapenem resistance in extended-Spectrum β-lactamase producing Klebsiella pneumoniae isolated from chickens and humans in Egypt. Biology (Basel) 10:373. doi: 10.3390/biology10050373
Eltai, N. O., Kelly, B., Al-Mana, H. A., Ibrahim, E. B., Yassine, H. M., Al Thani, A., et al. (2020). Identification of mcr-8 in clinical isolates from Qatar and evaluation of their antimicrobial profiles. Front. Microbiol. 11:1954. doi: 10.3389/fmicb.2020.01954
Frost, I., Van Boeckel, T. P., Pires, J., Craig, J., and Laxminarayan, R. (2019). Global geographic trends in antimicrobial resistance: the role of international travel. J. Travel Med. 26:taz036. doi: 10.1093/jtm/taz036
Gelband, H., Miller, P., Molly, P. S., Gandra, S., Levinson, J., Barter, D., et al. The state of the world's antibiotics 2015. In: Wound Healing Southern Africa. (2015);8:30–34.
Grami, R., Mansour, W., Mehri, W., Bouallègue, O., Boujaâfar, N., Madec, J.-Y., et al. (2016). Impact of food animal trade on the spread of mcr-1-mediated colistin resistance, Tunisia, July 2015. Eur. Secur. 21:30144. doi: 10.2807/1560-7917.ES.2016.21.8.30144
Hersh, A. L., Newland, J. G., Beekmann, S. E., Polgreen, P. M., and Gilbert, D. N. (2012). Unmet medical need in infectious diseases. Clin. Infect. Dis. 54, 1677–1678. doi: 10.1093/cid/cis275
Hsu, L.-Y., Apisarnthanarak, A., Khan, E., Suwantarat, N., Ghafur, A., and Tambyah, P. A. (2017). Carbapenem-resistant Acinetobacter baumannii and Enterobacteriaceae in south and Southeast Asia. Clin. Microbiol. Rev. 30, 1–22. doi: 10.1128/CMR.00042-16
Infectious Diseases Society of America (IDSA) (2011). Combating antimicrobial resistance: policy recommendations to save lives. Clin. Infect. Dis. 52, S397–S428. doi: 10.1093/cid/cir153
Jamal, W. Y., Albert, M. J., Khodakhast, F., Poirel, L., and Rotimi, V. O. (2015). Emergence of new sequence type OXA-48 Carbapenemase-producing Enterobacteriaceae in Kuwait. Microb. Drug Resist. 21, 329–334. doi: 10.1089/mdr.2014.0123
Jamal, W., Rotimi, V. O., Albert, M. J., Khodakhast, F., Nordmann, P., and Poirel, L. (2013). High prevalence of VIM-4 and NDM-1 metallo-β-lactamase among carbapenem-resistant Enterobacteriaceae. J. Med. Microbiol. 62, 1239–1244. doi: 10.1099/jmm.0.059915-0
Joint, F. WHO food standards Programme. Matters arising from FAO and WHO. Recent activities on antimicrobial resistance (prepared by FAO and WHO). Codex Alimentarius Commission. 38th Session. (2015), 6–11.
Khan, M. A., Mohamed, A. M., Faiz, A., and Ahmad, J. (2019). Enterobacterial infection in Saudi Arabia: first record of Klebsiella pneumoniae with triple carbapenemase genes resistance. J. Infect. Dev. Ctries. 13, 334–341. doi: 10.3855/jidc.11056
Khdary, H. N., Almalki, A., Alkhdiri, M. H. Jr., Alhamoudi, S., Alfaleh, A., Alghoribi, M. F., et al. (2020). Investigation on the genetic signatures of antibiotic resistance in multi-drug-resistant Klebsiella Pneumoniae isolates from National Guard Hospital, Riyadh. Cureus 12:e11288. doi: 10.7759/cureus.11288
Klaper, K., Hammerl, J. A., Rau, J., Pfeifer, Y., and Werner, G. (2021). Genome-based analysis of Klebsiella spp. isolates from animals and food products in Germany, 2013–2017. Pathogens 10:573. doi: 10.3390/pathogens10050573
Lagha, R., Ben Abdallah, F., ALKhammash, A. A. H., Amor, N., MM Hassan, M. I, et al. (2021). Molecular characterization of multidrug resistant Klebsiella pneumoniae clinical isolates recovered from king Abdulaziz specialist Hospital at Taif City, Saudi Arabia. J. Infect. Public Health 14, 143–151. doi: 10.1016/j.jiph.2020.12.001
Leangapichart, T., Dia, N. M., Olaitan, A. O., Gautret, P., Brouqui, P., and Rolain, J.-M. (2016). Acquisition of extended-spectrum β-lactamases by Escherichia coli and Klebsiella pneumoniae in gut microbiota of pilgrims during the hajj pilgrimage of 2013. Antimicrob. Agents Chemother. 60, 3222–3226. doi: 10.1128/AAC.02396-15
Leangapichart, T., Rolain, J.-M., Memish, Z. A., Al-Tawfiq, J. A., and Gautret, P. (2017). Emergence of drug resistant bacteria at the hajj: a systematic review. Travel Med. Infect. Dis. 18, 3–17. doi: 10.1016/j.tmaid.2017.06.008
Liu, Y., Lin, Y., Wang, Z., Hu, N., Liu, Q., Zhou, W., et al. (2021). Molecular mechanisms of colistin resistance in Klebsiella pneumoniae in a tertiary care teaching hospital. Front. Cell. Infect. Microbiol. 11:673503. doi: 10.3389/fcimb.2021.673503
Liu, Y.-Y., Wang, Y., Walsh, T. R., Yi, L.-X., Zhang, R., Spencer, J., et al. (2016). Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect. Dis. 16, 161–168. doi: 10.1016/S1473-3099(15)00424-7
Magiorakos, A.-P., Srinivasan, A., Carey, R. B., Carmeli, Y., Falagas, M., Giske, C., et al. (2012). Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 18, 268–281. doi: 10.1111/j.1469-0691.2011.03570.x
Manandhar, S., Zellweger, R. M., Maharjan, N., Dongol, S., Prajapati, K. G., Thwaites, G., et al. (2020). A high prevalence of multi-drug resistant gram-negative bacilli in a Nepali tertiary care hospital and associated widespread distribution of extended-Spectrum Beta-lactamase (ESBL) and carbapenemase-encoding genes. Ann. Clin. Microbiol. Antimicrob. 19, 1–13. doi: 10.1186/s12941-020-00390-y
Marcade, G., Brisse, S., Bialek, S., Marcon, E., Leflon-Guibout, V., Passet, V., et al. (2013). The emergence of multidrug-resistant Klebsiella pneumoniae of international clones ST13, ST16, ST35, ST48 and ST101 in a teaching hospital in the Paris region. Epidemiol. Infect. 141, 1705–1712. doi: 10.1017/S0950268812002099
Moghnia, O. H., and Al-Sweih, N. A. (2022). Whole genome sequence analysis of multidrug resistant Escherichia coli and Klebsiella pneumoniae strains in Kuwait. Microorganisms 10:507. doi: 10.3390/microorganisms10030507
Moghnia, O. H., Rotimi, V. O., and Al-Sweih, N. A. (2021a). Monitoring antibiotic resistance profiles of faecal isolates of Enterobacteriaceae and the prevalence of carbapenem-resistant isolates among food handlers in Kuwait. J. Glob. Antimicrob. Resist. 25, 370–376. doi: 10.1016/j.jgar.2021.04.009
Moghnia, O. H., Rotimi, V. O., and Al-Sweih, N. A. (2021b). Preponderance of Bla (KPC)-carrying Carbapenem-resistant Enterobacterales among fecal isolates from community food handlers in Kuwait. Front. Microbiol. 12:737828. doi: 10.3389/fmicb.2021.737828
Moglad, E., Alanazi, N., and Altayb, H. N. (2022). Genomic study of chromosomally and plasmid-mediated multidrug resistance and virulence determinants in Klebsiella Pneumoniae isolates obtained from a tertiary Hospital in Al-Kharj, KSA. Antibiotics (Basel) 11:1564. doi: 10.3390/antibiotics11111564
Mouftah, S. F., Pál, T., Higgins, P. G., Ghazawi, A., Idaghdour, Y., Alqahtani, M., et al. (2021). Diversity of carbapenem-resistant Klebsiella pneumoniae ST14 and emergence of a subgroup with KL64 capsular locus in the Arabian peninsula. Eur. J. Clin. Microbiol. Infect. Dis. doi: 10.1007/s10096-021-04384-2
Mrowiec, P., Klesiewicz, K., Małek, M., Skiba-Kurek, I., Sowa-Sierant, I., Skałkowska, M., et al. (2019). Antimicrobial susceptibility and prevalence of extended-spectrum beta-lactamases in clinical strains of Klebsiella pneumoniae isolated from pediatric and adult patients of two polish hospitals. New Microbiol. 42, 197–204
Navon-Venezia, S., Kondratyeva, K., and Carattoli, A. (2017). Klebsiella pneumoniae: a major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol. Rev. 41, 252–275. doi: 10.1093/femsre/fux013
Nordmann, P., and Mammeri, H. (2007). Extended-spectrum cephalosporinases: Structure, detection and epidemiology. Future Microbiol. 2, 297–307. doi: 10.2217/17460913.2.3.297
Ochońska, D., Olechowska-Jarząb, A., Dobrut, A., Bulanda, M., and Brzychczy-Włoch, M. (2021). Studies on molecular epidemiology of ESβL-producing Klebsiella pneumoniae isolated from patients hospitalized in a specialist hospital in southern Poland. Postępy Higieny i Medycyny Doświadczalnej 75, 970–979. doi: 10.2478/ahem-2021-0039
Okdah, L., AlDosary, M. S., AlMazyed, A., Alkhurayb, H. M., Almossallam, M., Al Obaisi, Y. S., et al. (2022). Genomic characterization of Colistin-resistant isolates from the king Fahad Medical City, Kingdom of Saudi Arabia. Antibiotics (Basel) 11:1597. doi: 10.3390/antibiotics11111597
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. doi: 10.1136/bmj.n71
Paterson, D. L., Hujer, K. M., Hujer, A. M., Yeiser, B., Bonomo, M. D., Rice, L. B., et al. (2003). Extended-spectrum β-lactamases in Klebsiella pneumoniae bloodstream isolates from seven countries: dominance and widespread prevalence of SHV-and CTX-M-type β-lactamases. Antimicrob. Agents Chemother. 47, 3554–3560. doi: 10.1128/AAC.47.11.3554-3560.2003
Perez-Lopez, A., Sundararaju, S., Al-Mana, H., Tsui, K. M., Hasan, M. R., Suleiman, M., et al. (2020). Molecular characterization of extended-Spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae among the pediatric population in Qatar. Front. Microbiol. 11:581711. doi: 10.3389/fmicb.2020.581711
Pérez-López, A., Sundararaju, S., Tsui, K. M., Al-Mana, H., Hasan, M. R., Suleiman, M., et al. (2021). Fecal carriage and molecular characterization of Carbapenemase-producing Enterobacterales in the pediatric population in Qatar. Microbiol. Spectr. 9:e0112221. doi: 10.1128/Spectrum.01122-21
Petrosillo, N., Taglietti, F., and Granata, G. (2019). Treatment options for colistin resistant Klebsiella pneumoniae: present and future. J. Clin. Med. 8:934. doi: 10.3390/jcm8070934
Podschun, R., and Ullmann, U. (1998). Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 11, 589–603. doi: 10.1128/CMR.11.4.589
Poirel, L., Al Maskari, Z., Al Rashdi, F., Bernabeu, S., and Nordmann, P. (2011). NDM-1-producing Klebsiella pneumoniae isolated in the Sultanate of Oman. J. Antimicrob. Chemother. 66, 304–306. doi: 10.1093/jac/dkq428
Potron, A., Nordmann, P., Lafeuille, E., Al Maskari, Z., Al Rashdi, F., and Poirel, L. (2011). Characterization of OXA-181, a carbapenem-hydrolyzing class D beta-lactamase from Klebsiella pneumoniae. Antimicrob. Agents Chemother. 55, 4896–4899. doi: 10.1128/AAC.00481-11
Poulou, A., Voulgari, E., Vrioni, G., Koumaki, V., Xidopoulos, G., Chatzipantazi, V., et al. (2013). Outbreak caused by an ertapenem-resistant, CTX-M-15-producing Klebsiella pneumoniae sequence type 101 clone carrying an OmpK36 porin variant. J. Clin. Microbiol. 51, 3176–3182. doi: 10.1128/JCM.01244-13
Pu, D., Zhao, J., Chang, K., Zhuo, X., and Cao, B. (2023). “Superbugs” with hypervirulence and carbapenem resistance in Klebsiella pneumoniae: the rise of such emerging nosocomial pathogens in China. Sci. Bull. 68, 2658–2670. doi: 10.1016/j.scib.2023.09.040
Pyakurel, S., Ansari, M., Kattel, S., Rai, G., Shrestha, P., Rai, K. R., et al. (2021). Prevalence of carbapenemase-producing Klebsiella pneumoniae at a tertiary care hospital in Kathmandu, Nepal. Trop. Med. Health 49, 1–8. doi: 10.1186/s41182-021-00368-2
Queenan, A. M., and Bush, K. (2007). Carbapenemases: the versatile β-lactamases. Clin. Microbiol. Rev. 20, 440–458. doi: 10.1128/CMR.00001-07
Robin, F., Beyrouthy, R., Bonacorsi, S., Aissa, N., Bret, L., Brieu, N., et al. (2017). Inventory of extended-spectrum-β-lactamase-producing Enterobacteriaceae in France as assessed by a multicenter study. Antimicrob. Agents Chemother. 61, 01911–01916. doi: 10.1128/AAC.01911-16
Rodrigues, C., Machado, E., Ramos, H., Peixe, L., and Novais, Â. (2014). Expansion of ESBL-producing Klebsiella pneumoniae in hospitalized patients: a successful story of international clones (ST15, ST147, ST336) and epidemic plasmids (IncR, IncFIIK). Int. J. Med. Microbiol. 304, 1100–1108. doi: 10.1016/j.ijmm.2014.08.003
Rodríguez-Santiago, J., Cornejo-Juárez, P., Silva-Sánchez, J., and Garza-Ramos, U. (2021). Polymyxin resistance in Enterobacterales: overview and epidemiology in the Americas. Int. J. Antimicrob. Agents 58:106426. doi: 10.1016/j.ijantimicag.2021.106426
Rojas, L. J., Hujer, A. M., Rudin, S. D., Wright, M. S., Domitrovic, T. N., Marshall, S. H., et al. (2017). NDM-5 and OXA-181 beta-lactamases, a significant threat continues to spread in the Americas. Antimicrob. Agents Chemother. 61, 00454–00417. doi: 10.1128/AAC.00454-17
Rolain, J., Parola, P., and Cornaglia, G. (2010). New Delhi metallo-beta-lactamase (NDM-1): towards a new pandemia? Clin. Microbiol. Infect. 16, 1699–1701. doi: 10.1111/j.1469-0691.2010.03385.x
Sadek, M., Ortiz de la Rosa, J. M., Abdelfattah Maky, M., Korashe Dandrawy, M., Nordmann, P., and Poirel, L. (2021). Genomic features of MCR-1 and extended-spectrum β-lactamase-producing Enterobacterales from retail raw chicken in Egypt. Microorganisms 9:195. doi: 10.3390/microorganisms9010195
Salawudeen, A., Raji, Y. E., Jibo, G. G., Desa, M. N. M., Neoh, H.-m., Masri, S. N., et al. (2023). Epidemiology of multidrug-resistant Klebsiella pneumoniae infection in clinical setting in south-eastern Asia: a systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 12:142. doi: 10.1186/s13756-023-01346-5
Saravanan, M., Ramachandran, B., and Barabadi, H. (2018). The prevalence and drug resistance pattern of extended spectrum β–lactamases (ESBLs) producing Enterobacteriaceae in Africa. Microb. Pathog. 114, 180–192. doi: 10.1016/j.micpath.2017.11.061
Setiawaty, V., Darmawati, D., Nugraha, A. A., and Hendrati, P. M. (2022). Detection of causative agents of bacterial pneumonia in hospitalized hajj and umrah cases by multiplex real-time polymerase chain reaction. Iran. J. Microbiol. 14, 300–304. doi: 10.18502/ijm.v14i3.9759
Shahid, M., Ahmad, N., Saeed, N. K., Shadab, M., Joji, R. M., Al-Mahmeed, A., et al. (2022). Clinical carbapenem-resistant Klebsiella pneumoniae isolates simultaneously harboring Bla (NDM-1), Bla (OXA) types and qnrS genes from the Kingdom of Bahrain: resistance profile and genetic environment. Front. Cell. Infect. Microbiol. 12:1033305. doi: 10.3389/fcimb.2022.1033305
Shahid, M., Al-Mahmeed, A., Murtadha, M. M., Qareeballa, A., Eltahir, M. A., Tabbara, K. S., et al. (2014). Characterization of cephalosporin-resistant clinical Enterobacteriaceae for CTX-M ESBLs in Bahrain. Asian Pac J Trop Med 7, S212–S216. doi: 10.1016/S1995-7645(14)60234-0
Shamsuzzaman, S. (2015). Multidrug-resistant, extensively drug-resistant and Pandrug-resistant bacteria and antimicrobial therapy in combination. Bangladesh J. Med. Microbiol. 9, 1–2. doi: 10.3329/bjmm.v9i2.31348
Shankar, C., Mathur, P., Venkatesan, M., Pragasam, A. K., Anandan, S., Khurana, S., et al. (2019). Rapidly disseminating Bla OXA-232 carrying Klebsiella pneumoniae belonging to ST231 in India: multiple and varied mobile genetic elements. BMC Microbiol. 19, 1–8. doi: 10.1186/s12866-019-1513-8
Shibl, A. M., Al-Agamy, M. H., Khubnani, H., Senok, A. C., Tawfik, A. F., and Livermore, D. M. (2012). High prevalence of acquired quinolone-resistance genes among Enterobacteriaceae from Saudi Arabia with CTX-M-15 β-lactamase. Diagn. Microbiol. Infect. Dis. 73, 350–353. doi: 10.1016/j.diagmicrobio.2012.04.005
Shibl, A., Al-Agamy, M., Memish, Z., Senok, A., Khader, S. A., and Assiri, A. (2013). The emergence of OXA-48- and NDM-1-positive Klebsiella pneumoniae in Riyadh, Saudi Arabia. Int. J. Infect. Dis. 17, e1130–e1133. doi: 10.1016/j.ijid.2013.06.016
Singh, S., Pathak, A., Rahman, M., Singh, A., Nag, S., Sahu, C., et al. (2021). Genetic characterisation of colistin resistant Klebsiella pneumoniae clinical isolates from North India. Front. Cell. Infect. Microbiol. 11:666030. doi: 10.3389/fcimb.2021.666030
Solgi, H., Nematzadeh, S., Giske, C. G., Badmasti, F., Westerlund, F., Lin, Y.-L., et al. (2020). Molecular epidemiology of OXA-48 and NDM-1 producing enterobacterales species at a University Hospital in Tehran, Iran, between 2015 and 2016. Front. Microbiol. 11:529632. doi: 10.3389/fmicb.2020.00936
Sonnevend, Á., Abdulrazzaq, N., Ghazawi, A., Thomsen, J., Bharathan, G., Makszin, L., et al. (2022). The first nationwide surveillance of carbapenem-resistant Enterobacterales in the United Arab Emirates – increased association of Klebsiella pneumoniae CC14 clone with Emirati patients. Int. J. Infect. Dis. 120, 103–112. doi: 10.1016/j.ijid.2022.04.034
Sonnevend, A., Al Baloushi, A., Ghazawi, A., Hashmey, R., Girgis, S., Hamadeh, M. B., et al. (2013). Emergence and spread of NDM-1 producer Enterobacteriaceae with contribution of IncX3 plasmids in the United Arab Emirates. J. Med. Microbiol. 62, 1044–1050. doi: 10.1099/jmm.0.059014-0
Sonnevend, Á., Alali, W. Q., Mahmoud, S. A., Ghazawi, A., Bharathan, G., Melegh, S., et al. (2022). Molecular characterization of MCR-1 producing Enterobacterales isolated in poultry farms in the United Arab Emirates. Antibiotics (Basel) 11:305. doi: 10.3390/antibiotics11030305
Sonnevend, Á., Ghazawi, A., Hashmey, R., Haidermota, A., Girgis, S., Alfaresi, M., et al. (2017). Multihospital occurrence of Pan-resistant Klebsiella pneumoniae sequence type 147 with an ISEcp1-directed Bla(OXA-181) insertion in the mgrB gene in the United Arab Emirates. Antimicrob. Agents Chemother. 61:e00418-17. doi: 10.1128/AAC.00418-17
Sonnevend, Á., Ghazawi, A. A., Hashmey, R., Jamal, W., Rotimi, V. O., Shibl, A. M., et al. (2015). Characterization of Carbapenem-resistant Enterobacteriaceae with high rate of autochthonous transmission in the Arabian peninsula. PLoS One 10:e0131372. doi: 10.1371/journal.pone.0131372
Tamma, P. D., and Simner, P. J. (2018). Phenotypic detection of carbapenemase-producing organisms from clinical isolates. J. Clin. Microbiol. 56, 01140–01118. doi: 10.1128/JCM.01140-18
Tawfik, A. F., Alswailem, A. M., Shibl, A. M., and Al-Agamy, M. H. (2011). Prevalence and genetic characteristics of TEM, SHV, and CTX-M in clinical Klebsiella pneumoniae isolates from Saudi Arabia. Microb. Drug Resist. 17, 383–388. doi: 10.1089/mdr.2011.0011
Uz Zaman, T., Albladi, M., Siddique, M. I., Aljohani, S. M., and Balkhy, H. H. (2018). Insertion element mediated mgrB disruption and presence of ISKpn28 in colistin-resistant Klebsiella pneumoniae isolates from Saudi Arabia. Infect. Drug Resist. 11, 1183–1187. doi: 10.2147/IDR.S161146
Uz Zaman, T., Aldrees, M., Al Johani, S. M., Alrodayyan, M., Aldughashem, F. A., and Balkhy, H. H. (2014). Multi-drug carbapenem-resistant Klebsiella pneumoniae infection carrying the OXA-48 gene and showing variations in outer membrane protein 36 causing an outbreak in a tertiary care hospital in Riyadh, Saudi Arabia. Int. J. Infect. Dis. 28, 186–192. doi: 10.1016/j.ijid.2014.05.021
Vali, L., Dashti, A. A., Jadaon, M. M., and El-Shazly, S. (2015). The emergence of plasmid mediated quinolone resistance qnrA2 in extended spectrum β-lactamase producing Klebsiella pneumoniae in the Middle East. Daru 23:34. doi: 10.1186/s40199-015-0116-7
Wang, B., Pan, F., Wang, C., Zhao, W., Sun, Y., Zhang, T., et al. (2020). Molecular epidemiology of Carbapenem-resistant Klebsiella pneumoniae in a paediatric hospital in China. Int. J. Infect. Dis. 93, 311–319. doi: 10.1016/j.ijid.2020.02.009
Weber, A. S., Turjoman, R., Shaheen, Y., Al Sayyed, F., Hwang, M. J., and Malick, F. (2017). Systematic thematic review of e-health research in the Gulf cooperation council (Arabian gulf): Bahrain, Kuwait, Oman, Qatar, Saudi Arabia and United Arab Emirates. J. Telemed. Telecare 23, 452–459. doi: 10.1177/1357633X16647894
WHO (2020). GLASS whole-genome sequencing for surveillance of antimicrobial resistance. Geneva: World Health Organization.
WHO Bacterial Priority Pathogens List. 2024: bacterial pathogens of public health importance to guide research, development and strategies to prevent and control antimicrobial resistance. WHO; (2024).
Yong, D., Toleman, M. A., Giske, C. G., Cho, H. S., Sundman, K., Lee, K., et al. (2009). Characterization of a new metallo-β-lactamase gene, Bla NDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 53, 5046–5054. doi: 10.1128/AAC.00774-09
Yu, D., Li, X., Yu, J., Shi, X., Liu, P., and Tian, P. (2021). Whether urbanization has intensified the spread of infectious diseases—renewed question by the COVID-19 pandemic. Front. Public Health 9:699710. doi: 10.3389/fpubh.2021.699710
Zaman, T. U., Alrodayyan, M., Albladi, M., Aldrees, M., Siddique, M. I., Aljohani, S., et al. (2018). Clonal diversity and genetic profiling of antibiotic resistance among multidrug/carbapenem-resistant Klebsiella pneumoniae isolates from a tertiary care hospital in Saudi Arabia. BMC Infect. Dis. 18:205. doi: 10.1186/s12879-018-3114-9
Zowawi, H. M., Forde, B. M., Alfaresi, M., Alzarouni, A., Farahat, Y., Chong, T. M., et al. (2015). Stepwise evolution of pandrug-resistance in Klebsiella pneumoniae. Sci. Rep. 5:15082. doi: 10.1038/srep15082
Zowawi, H. M., Sartor, A. L., Balkhy, H. H., Walsh, T. R., Al Johani, S. M., AlJindan, R. Y., et al. (2014). Molecular characterization of carbapenemase-producing Escherichia coli and Klebsiella pneumoniae in the countries of the Gulf cooperation council: dominance of OXA-48 and NDM producers. Antimicrob. Agents Chemother. 58, 3085–3090. doi: 10.1128/AAC.02050-13
Zurfuh, K., Poirel, L., Nordmann, P., Nüesch-Inderbinen, M., Hächler, H., and Stephan, R. (2016). Occurrence of the plasmid-borne mcr-1 colistin resistance gene in extended-spectrum-β-lactamase-producing Enterobacteriaceae in river water and imported vegetable samples in Switzerland. Antimicrob. Agents Chemother. 60, 2594–2595. doi: 10.1128/AAC.00066-16
Keywords: MDR Klebsiella pneumoniae, molecular epidemiology, antimicrobial resistance gene, carbapenem-resistant Klebsiella pneumoniae, the GHC countries, Arabian Peninsula
Citation: Idrees EK, Aldriwesh MG, Alkhulaifi MM and Alghoribi MF (2025) Systematic review of multidrug-resistant Klebsiella pneumoniae in the Arabian Peninsula: molecular epidemiology and resistance patterns. Front. Microbiol. 16:1489317. doi: 10.3389/fmicb.2025.1489317
Edited by:
Ziad Daoud, Midland Medical Center, United StatesReviewed by:
Okon Okwong Kenneth, Federal Medical Center Makurdi, NigeriaIman Dandachi, King Fahad Medical City, Saudi Arabia
Copyright © 2025 Idrees, Aldriwesh, Alkhulaifi and Alghoribi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Majed F. Alghoribi, QWxnaG9yaWJpbWFAZ21haWwuY29t
 Enaam K. Idrees
Enaam K. Idrees Marwh G. Aldriwesh
Marwh G. Aldriwesh Manal M. Alkhulaifi
Manal M. Alkhulaifi Majed F. Alghoribi
Majed F. Alghoribi